Abstract
Objective
To determine COVID-19 vaccine-related adverse events (AEs) in the seven-day post-vaccination period in patients with SLE vs autoimmune rheumatic diseases (AIRDs), non-rheumatic autoimmune diseases (nrAIDs), and healthy controls (HC).
Methods
Data were captured through the COVID-19 Vaccination in Autoimmune Diseases (COVAD) questionnaire (March–December 2021). Multivariable regression models accounted for age, gender, ethnicity, vaccine type and background treatment.
Results
Among 9462 complete respondents, 583 (6.2%) were SLE patients (mean age: 40.1 years; 94.5% females; 40.5% Asian; 42.9% Pfizer-recipients). Minor AEs were reported by 83.0% of SLE patients, major by 2.6%, hospitalization by 0.2%. AE and hospitalization frequencies were similar between patients with active and inactive SLE. Rashes were more frequent in SLE patients vs HC (OR; 95% CI: 1.2; 1.0, 1.5), chills less frequent in SLE vs AIRDs (0.6; 0.4, 0.8) and nrAIDs (0.5; 0.3, 0.8), and fatigue less frequent in SLE vs nrAIDs (0.6; 0.4, 0.9). Pfizer-recipients reported higher overall AE (2.2; 1.1, 4.2) and injection site pain (2.9; 1.6, 5.0) frequencies than recipients of other vaccines, Oxford/AstraZeneca-recipients more body ache, fever, chills (OR: 2.5, 3.0), Moderna-recipients more body ache, fever, chills, rashes (OR: 2.6, 4.3). Hospitalization frequencies were similar across vaccine types. AE frequencies were similar across treatment groups, although chills were less frequent in antimalarial users vs non-users (0.5; 0.3, 0.9).
Conclusion
While COVID-19 vaccination-related AEs were reported by four-fifths of SLE patients, those were mostly minor and comparable to AEs reported by healthy individuals, providing reassurance regarding COVID-19 vaccination safety in SLE.
Keywords: COVID-19, vaccine, adverse events, systemic lupus erythematosus, rheumatology
Rheumatology key messages.
While four-fifths of SLE patients reported COVID-19 vaccination-related adverse events (AEs), the majority were minor.
Frequencies of major AEs (2.6%) and hospitalizations (0.2%) were similar to those in healthy individuals.
Active SLE yielded increased post-vaccine fever, fatigue and tachycardia frequencies. Background therapies had no influence.
Introduction
Vaccination against SARS-CoV-2 infection (COVID-19) has at large been proven efficacious and safe for the healthy population [1]. However, data concerning populations considered vulnerable due to background diseases or ongoing immunosuppressant therapy, such as patients with SLE, are limited. Increasing knowledge and experience on the management of COVID-19 has been pivotal in addressing the controversies surrounding the use of or discontinuation of immunosuppression prior to COVID-19 vaccination [2–6]. Current recommendations encourage COVID-19 vaccination prior to B-cell depleting therapy and withholding immunosuppression post-vaccination in some circumstances, depending on agent and dose [7]. Furthermore, hesitancy for vaccination remains a concern, especially among patients with autoimmune rheumatic diseases (AIRDs) [8, 9]. Interestingly, toll-like receptor agonists used as COVID-19 vaccine adjuvants have the potential to induce SLE flares via upregulation of the type I interferon pathway [10].
We recently reported vaccine safety data from patients with idiopathic inflammatory myopathies (IIMs) from the global COVID-19 Vaccination in Autoimmune Diseases (COVAD) study initiative, which demonstrated self-reported safety and tolerance for COVID-19 vaccination, yet a higher frequency of skin rashes post-vaccination among dermatomyositis patients, especially those with active disease during vaccination [11]. Similarly, we recently reported data on COVID-19 vaccine safety in patients with RA from the same survey, wherein most of the adverse events were minor and comparable to those reported by healthy individuals. In that work, patients on methotrexate and antimalarial agents were reported to have experienced fewer minor adverse events post-vaccination compared with RA patients on other drugs [12]. The international vaccination against COVID in systemic lupus (VACOLUP) study that comprised 696 patients with SLE showed an adequate safety and tolerability profile for COVID-19 vaccination, with a minimal risk for disease flares, including following mRNA vaccines [13]. However, assessment of relative risks was not possible in that study due to the absence of a control group [13].
There is a dearth of data in terms of the impact of disease activity or use of immunosuppressant or immunomodulatory therapies on vaccine-specific adverse events (AEs) in patients with SLE. Moreover, the paucity of data on COVID-19 vaccine safety in SLE from diverse populations makes impossible their generalizability to the global population. The present study from the COVAD initiative addresses the COVID-19 vaccine safety and tolerance in the short term, that is, within seven days post-vaccination among patients with SLE compared with patients with other AIRDs, non-rheumatic autoimmune diseases (nrAIRDs), or healthy controls (HC), through a multicentre patient-reported electronic survey.
Methods
Study design and data collection
We conducted an international, online, cross-sectional, multicentre survey-based study, as a part of the COVAD initiative [14]. A comprehensive patient self-reporting electronic survey was developed, consisting of questions related to COVID-19 and AIRDs. This questionnaire included demographic details, AIRD-specific diagnosis, treatment details, current symptom status, COVID-19 infection history including symptoms, duration and complications (hospitalization and requirement of oxygen therapy), COVID-19 vaccination details, short-term (seven days) post-vaccination AEs (based on the Centre for Disease Control and Prevention criteria) and patient-reported outcome measures as per the Patient Reported Outcomes Measurement Information System (PROMIS) tool [15]. After vetting by international experts, pilot testing, revisions, validation and translation into 18 languages, the survey was hosted on an online platform (http://surveymonkey.com) and was circulated by the international COVAD study group (106 physicians) across 94 countries (Supplementary Table S1, available at Rheumatology online), as well as through numerous social media platforms and online patient support groups. Convenience sampling was used and all participants over the age of 18 years were included. Duplicate responses were removed manually. Methods have been detailed at length in the published COVAD study protocol [14].
Informed consent of the participants was obtained electronically through an initial question in the online survey, prior to the main study questionnaire. No incentives were offered for survey completion. Central approval was obtained from the Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS) ethics committee as per local guidelines [Institutional Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareli Road, Lucknow, 226014 (IEC Code: 2021–143-IP-EXP-39)] and the Checklist for Reporting results of the Internet E-Surveys (CHERRIES) was adhered to when reporting results [16, 17].
Data extraction
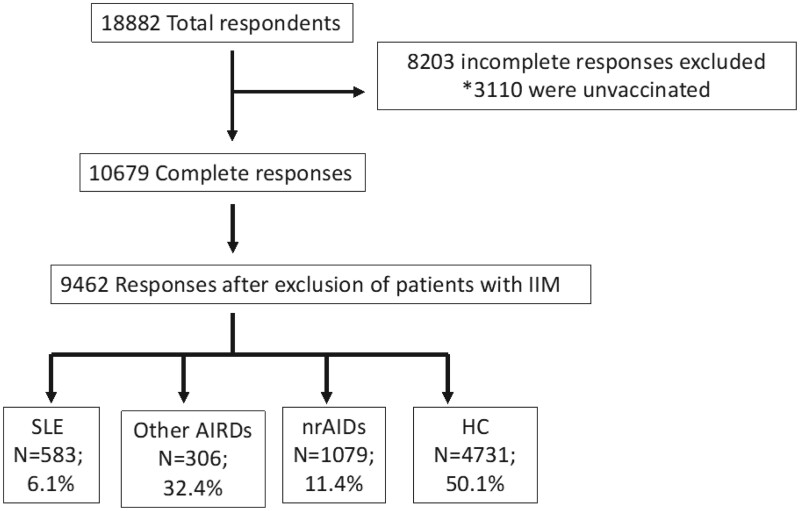
Data were retrieved at the time of survey closure on 30 December 2021. Subjects who had not received at least one dose of any COVID-19 vaccine at the time of the survey completion and subjects who had not completed the survey in full were excluded from the analysis (Fig. 1). Respondents who had received at least one dose of any COVID-19 vaccine and had completed the survey in full formed the study population that was deemed eligible for analysis. Multiple relevant variables were extracted from the survey responses, including AEs during the seven-day post-vaccination period.
Figure 1.
Flow diagram of patients included in the study. *An electronic protocol terminated the survey automatically if respondents indicated not having received any COVID-19 vaccine. AIRDs: autoimmune rheumatic diseases (excluding SLE and IIM); HC: healthy controls; IIM: idiopathic inflammatory myositis; nrAIDs: non-rheumatic autoimmune diseases; SLE: systemic lupus erythematosus
Active and inactive disease
Active and inactive disease state four weeks prior to vaccination was assessed by the patient’s response to the question ‘What was the status of your autoimmune disease in the four weeks (prior to) before the first dose of COVID-19 vaccine?’. Responses of active and worsening/static/improving disease were grouped together to designate ‘active disease’. Patients who indicated ‘inactive’ as response formed the inactive disease group. Those who responded ‘I don’t know’ or ‘Other’ were assessed for activity at an individual basis based on answers to the following questions: (i) What were your symptoms four weeks prior to vaccination? (ii) If you have any swelling of your joints, how many joints are swollen? (iii) Did you require an increase in the dose of any of these [referring to previous answers] immunosuppressant medications (IS), or start a new immunosuppressant medicine within the six months prior to the first COVID-19 vaccine?
Adverse events post vaccination
Seven-day AEs were categorized into injection site pain or reaction, minor AEs, major AEs, and hospitalizations. Minor AEs included myalgia, body aches, fever, chills, nausea and vomiting, headache, rashes, fatigue, diarrhoea, abdominal pain, high pulse rate or palpitations, rise in blood pressure, fainting, difficulty in breathing, dizziness, and chest pain. Major AEs consisted of serious reactions to vaccination, requiring urgent medical attention, including anaphylaxis, a marked difficulty in breathing, throat closure (choking) and severe rashes. AEs not listed above were reported as ‘others’ in an open-ended question.
Statistical analysis
As the survey was originally designed for patients with IIMs and distributed to IIM patient groups, patients with IIM formed a disproportionately large subgroup and were therefore excluded from the other AIRDs group prior to analysis to avoid selection bias. Patients with SLE were excluded from the other AIRDs group. Comparisons were performed for vaccination-related AEs in patients with active vs inactive SLE. Also, comparisons between patients with SLE and patients with other AIRDs (IIM and SLE patients excluded) were conducted, as well as between patients with SLE and patients with nrAIRDs, and between patients with SLE and HC. Patients with SLE and autoimmune disease comorbidities were compared with those without autoimmune disease comorbidities. Autoimmune disease comorbidities included other AIRDs and nrAIDs reported by SLE patients. Chi-squared (χ2) and Mann–Whitney U tests were used for comparisons between groups for categorical and continuous variables, respectively. The variables that differed across SLE, other AIRDs, nrAIDs and HC in univariable analysis were included in multivariable binary logistic regression analysis (BLR) with adjustment for baseline factors defined a priori, that is, age, gender, ethnicity, country group defined by human development index (HDI), as well as factors with P < 0.200 in the univariable analyses. Stepwise forward regression methodology was used to build the regression models starting with age, gender, ethnicity and country group by HDI first, followed by addition of other covariates. The results for continuous variables were expressed as median (IQR) in view of the non-normal distribution of the data (by Kolmogorov–Smirnov test and Shapiro–Wilk test). In multivariable models, P < 0.05 was considered statistically significant. Statistical analysis was performed using the IBM SPSS version 26 (IBM, Armonk, NY, USA).
Results
Characteristics of study participants
Of 10 679 survey participants with complete responses, 1227 patients with IIM (11.4%) were excluded (Fig. 1; Table 1). Of 9462 respondents forming the population under study, 583 were patients with SLE (6.2%), 3069 patients with other AIRDs (32.4%), 1079 patients with nrAIDs (11.4%) and 4731 were HC (50.0%). Within the total study population, 7028 (74.3%) respondents were females, the respondents’ mean age was 42.2 ± 14.8 years, and the most frequently reported ethnicity was Caucasian (49.3%). The three most represented countries were Turkey (16.1%), India (15.3%) and Mexico (13.3%; Supplementary Table S1, available at Rheumatology online). Seventy-two per cent had taken at least two doses of COVID-19 vaccine and all had received at least one vaccine dose. Table 1 shows the type and proportion of patients with other AIRDs. The most common vaccine received was Pfizer-BioNTech (BNT162b2) in 39.3%. The most common immunosuppressants or immunomodulatory agents used by the SLE patients at the time of survey completion were antimalarial agents (62.6%), followed by glucocorticoids (55.6%) and azathioprine (14.4%; Table 1). Other population characteristics are shown in Table 1.
Table 1.
Baseline characteristics of survey respondents
| Variable | Total (n = 9462) | SLE (n = 583) | HC (n = 4731) | Other AIRDs (n = 3069) | Other nrAIDs (n = 1079) |
|---|---|---|---|---|---|
| Median age in years (IQR) | 41 (29–53) | 39 (32–49) | 34 (26–47) | 49 (39–60) | 42 (32–53) |
| Gender (Male: Female) | 2378:7028 (1:2.9) | 31:551 (1:17) | 1659:3043 (1:1.8) | 545:2508 (1:4.6) | 143: 926 (1:6.5) |
| No. of vaccine doses, n (%) | |||||
| 1 | 2617 (27.7) | 209 (35.8) | 1201 (25.3) | 858 (27.9) | 349 (32.3) |
| 2 | 6845 (72.3) | 374 (64.2) | 3530 (74.7) | 2211 (72.1) | 730 (67.6) |
| Ethnicity, n (%) | |||||
| Caucasian | 4668 (49.3) | 221 (37.9) | 1984 (41.9) | 1823 (59.4) | 640 (59.3) |
| African-American/African heritage | 66 (0.7) | 7 (1.2) | 33 (0.7) | 21 (0.7) | 5 (0.5) |
| Asian | 2516 (26.6) | 236 (40.5) | 1315 (27.7) | 750 (24.4) | 215 (19.9) |
| Hispanic | 1173 (12.4) | 73 (12.5) | 764 (16.1) | 206 (6.7) | 130 (12.0) |
| Native American/Indigenous/Pacific Islandic | 46 (0.5) | 7 (1.2) | 24 (0.5) | 11 (0.4) | 4 (0.4) |
| Do not wish to disclose | 559 (5.9) | 20 (3.4) | 345 (7.3) | 152 (4.9) | 42 (3.8) |
| Other | 434 (4.6) | 19 (3.3) | 266 (5.6) | 106 (3.5) | 43 (3.9) |
| Vaccine taken, n (%) | |||||
| Pfizer-BioNTech (BNT162b2) | 3723 (39.3) | 250 (42.9) | 1623 (34.3) | 1376 (44.8) | 474 (43.9) |
| Oxford/Astra Zeneca (ChAdOx1 nCoV-19) | 1292 (13.7) | 68 (11.7) | 466 (9.8) | 612 (19.9) | 146 (13.5) |
| Johnson & Johnson (J&J) (JNJ-78436735) | 89 (0.9) | 16 (2.7) | 37 (0.8) | 18 (0.6) | 18 (1.7) |
| Moderna (mRNA-1273) | 599 (6.3) | 59 (10.1) | 160 (3.4) | 261 (8.5) | 119 (11.0) |
| Novavax (NVX-CoV2373) | 10 (0.1) | – | 2 (0.0) | 5 (0.2) | 3 (0.3) |
| Covishield (ChAdOx1 nCoV-19) | 1149 (12.1) | 46 (7.8) | 677 (14.3) | 323 (10.6) | 103 (9.6) |
| Covaxin (BBV152) | 222 (2.4) | 10 (1.7) | 120 (2.6) | 75 (2.4) | 17 (1.6) |
| Sputnik (Gam-COVID-Vac) | 188 (2.0) | 7 (1.2) | 131 (2.8) | 29 (0.9) | 21 (1.9) |
| Sinopharm (BBIBP-CorV) | 1564 (16.6) | 33 (5.7) | 1244 (26.3) | 163 (5.3) | 124 (11.5) |
| Othersa | 570 (6.0) | 93 (16.0) | 238 (5.0) | 189 (6.2) | 50 (4.6) |
| I am not sure | 56 (0.6) | 1 (0.2) | 33 (0.7) | 18 (0.6) | 4 (0.4) |
| Diagnosis, n (%) | |||||
| No autoimmune disease | 4731 (44.0) | — | 4731 (44.0) | — | — |
| Rheumatoid arthritis | 1347(14.2) | — | — | 1347 (31) | — |
| SLE | 583 (5.5) | 583 (6.1) | — | — | — |
| Systemic sclerosis | 407 (3.8) | — | — | 407 (9.4) | — |
| Ankylosing spondylitis or psoriatic arthritis | 372 (3.5) | 3 (0.5) | — | 372 (8.6) | — |
| Sjögren’s syndrome | 218 (2) | — | — | 218 (5.0) | — |
| Mixed connective tissue disorder (MCTD) | 142 (1.3) | — | — | 142 (3.3) | — |
| Vasculitis | 114 (1.0) | — | — | 114 (2.6) | — |
| Crohn’s disease or ulcerative colitis (IBD) | 175 (1.6) | 3 (0.5) | — | — | 175 (16) |
| Thyroid (hypothyroid or hyperthyroid) | 498 (4.7) | 46 (7.8) | — | — | 498 (46) |
| Type 1 Diabetes | 63 (0.6) | 5 (0.8) | — | — | 63 (5.8) |
| Multiple sclerosis | 31 (0.3) | — | — | — | 31 (3.0) |
| Myasthenia gravis | 30 (0.3) | 3 (0.5) | — | — | 30 (2.8) |
| Pernicious anaemia | 10 (0.1) | 1 (0.1) | — | — | 10 (0.9) |
| Haemolytic anaemia/idiopathic thrombocytopenic purpura (ITP) | 18 (0.2) | 8 (1.3) | — | — | 18 (1.7) |
| Polymyalgia rheumatica | 18 (0.2) | 1 (0.1) | — | 18 (0.4) | — |
| Others | 705 (6.8) | 38 (6.5) | — | 451 (10.5) | 254 (23.5) |
| Treatment, n (%) | |||||
| Methotrexate | 989/4731 (21.0) | 44 (7.5) | — | 913 (29.7) | 32 (2.9) |
| Mycophenolate mofetil | 328 (7.0) | 125 (21.4) | — | 182 (5.9) | 20 (1.8) |
| Rituximab | 67 (1.4) | 7 (1.2) | — | 56 (1.8) | 4 (0.4) |
| Azathioprine | 261 (5.6) | 84 (14.4) | — | 120 (3.9) | 57 (5.3) |
| Hydroxychloroquine | 1007 (21.2) | 365 (62.6) | — | 616 (20.0) | 730 (67.6) |
| Sulfasalazine | 246 (5.2) | 1 (0.2) | — | 227 (7.4) | 18 (1.7) |
| Leflunomide | 155 (3.2) | 8 (1.4) | — | 144 (4.7) | 3 (0.3) |
| Oral tacrolimus | 49 (1.0) | 18 (3.0) | — | 24 (0.8) | 7 (0.6) |
| Ciclosporin | 24 (0.6) | 4 (0.7) | — | 16 (0.5) | 4 (0.4) |
| IVIG | 45 (1.0) | 5 (0.9) | — | 25 (0.8) | 14 (1.3) |
| Cyclophosphamide | 13 (0.2) | 4 (0.7) | — | 9 (0.3) | 0 (0.0) |
| Glucocorticoids | |||||
| No glucocorticoids | 3541 (74.8) | 258 (44.2) | — | 2309 (75.2) | 958 (88.8) |
| <10 mg prednisone (or eq.)/day | 961 (20.4) | 269 (46.1) | — | 615 (20.0) | 75 (6.9) |
| 10–20 mg prednisone (or eq.)/day | 184 (3.8) | 40 (6.8) | — | 117 (3.8) | 26 (2.4) |
| >20 mg prednisone (or eq.)/day | 63 (1.4) | 16 (2.7) | — | 27 (0.9) | 20 (1.9) |
AIDs: autoimmune diseases; AIRDs: autoimmune rheumatic diseases (other than SLE or inflammatory myopathies); HC: healthy controls; NA: not applicable.
Others included open-ended responses in different languages.
Population characteristics of SLE patients
Among the SLE patients, the mean age was 40.1 ± 12.0 years, and the majority (94.5%) were females. The most frequent ancestry was Asian (40.5%), followed by Caucasian (37,9%). Seventy-one per cent had taken two doses of COVID-19 vaccine. Pfizer-BioNTech (BNT162b2) (42.9%) and Oxford/AstraZeneca (ChAdOx1 nCoV-19) (11.7%) were the most common vaccines received. The most common co-existing other AIDs within SLE patients was thyroid disorder (n = 46; 7.8%), type 1 diabetes (n = 5; 0.8%) and haemolytic anaemia (n = 8; 1.3%).
Patients with SLE were older than HC (median: 39 vs 34 years; P < 0.001), but younger than those with other AIRDs (median: 39 vs 49 years; P < 0.001) and nrAIDs (median: 39 vs 42 years; P < 0.001) and had a higher female representation compared with all other groups (P < 0.001 for all comparisons; Table 1). Furthermore, differences were noted regarding ethnicity and the proportion of vaccines received by SLE patients (Table 1). Patients with SLE were more frequently on glucocorticoids compared with other AIRDs (OR: 3.8; 95% CI: 3.1, 4.5; P < 0.001) as well as compared with nrAIDs (OR: 9.9; 95% CI: 7.7, 12.8; P < 0.001). Also, they were more frequently on mycophenolate mofetil, azathioprine and antimalarial agents compared with patients with other AIRDs (Table 1).
Adverse events in SLE and comparisons with other subgroups
Overall, AEs within seven days following vaccination were reported by 484 (83.0%) of the SLE patients; all these patients reported minor AEs (Supplementary Table S2, available at Rheumatology online). Major AEs were reported by 15 patients (2.6%) and hospitalization by one (0.2%). Injection site pain was reported by 415 (71.2%) patients with SLE.
Among the minor AEs, the most frequently reported was fatigue (n = 162; 27.8%), followed by headache (n = 147; 25.2%) and body ache (n = 120; 20.6%; Supplementary Table S2, available at Rheumatology online). Specific major AEs reported included anaphylaxis (n = 3; 0.5%), throat closure (n = 1; 0.2%), severe rashes (n = 2; 0.3%) and others (n = 11; 1.9%). Among 11 SLE patients with self-reported coexisting antiphospholipid syndrome (APS), none reported thrombosis (stroke, deep vein thrombosis or myocardial infarction) in the seven-day period post-vaccination, and none reported chest pain, fainting, dizziness or difficulty in breathing. Only minor AEs (n = 7; 63.3%) were reported, mainly fatigue (n = 6; 54.5%) and headache (n = 3; 27.2%).
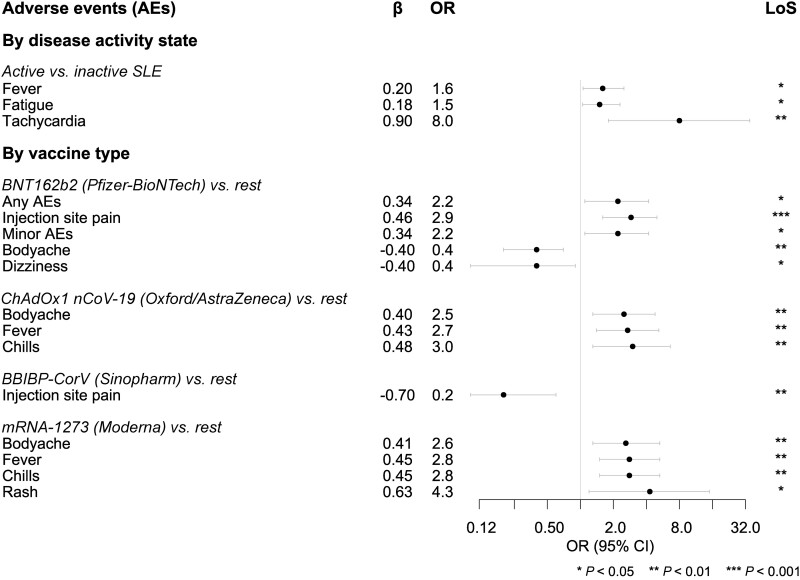
Within the SLE study population, 328 patients (56.3%) had active disease prior to vaccination. The reported post-vaccination AEs were similar between those with active and inactive disease except for higher fever (OR: 1.6; 95% CI: 1.1, 2.5; P = 0.026), fatigue (OR: 1.5; 95% CI: 1.1, 2.3; P = 0.025) and tachycardia (OR: 8.0; 95% CI: 1.8, 35.0; P = 0.006) in patients with active SLE (Table 2). Hospitalization frequencies were similar between the two groups (Table 2). A greater percentage of patients with self-reported active SLE were on glucocorticoids (64.9% vs 43.9%; P < 0.001) compared with inactive SLE patients; the differences in AEs between patients with active and patients with inactive SLE persisted upon adjustments for glucocorticoid dose and intake of other immunosuppressive or immunomodulatory agents, yielding an OR of 1.9 for fever (95% CI: 1.1, 3.2; P = 0.012), an OR of 1.7 for fatigue (95% CI: 1.1, 2.7; P = 0.007) and an OR of 8.7 for tachycardia (95% CI: 1.8, 41.4; P = 0.006).
Table 2.
Comparison of vaccination-related AEs in patients with active vs inactive SLE
| Active SLE (n = 328) n (%) | Inactive SLE (n = 255) n (%) | Univariable |
Multivariable |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| Any AEs | 277 (84.5) | 207 (81.2) | — | 0.296 | — | — |
| Injection site pain | 229 (69.8) | 186 (73.0) | — | 0.409 | — | — |
| Minor AEs | ||||||
| Any minor AEs | 277 (84.5) | 207 (81.2) | — | 0.296 | — | — |
| Myalgia | 55 (16.8) | 36 (14.1) | — | 0.382 | — | — |
| Body ache | 74 (22.6) | 46 (18.0) | — | 0.180 | — | — |
| Fever | 74 (22.6) | 38 (14.9) | 1.6 (1.1, 2.5) | 0.020 | 1.6 (1.1, 2.5) | 0.026 |
| Chills | 37 (11.3) | 29 (11.4) | — | 0.972 | — | — |
| Nausea and vomiting | 29 (8.8) | 15 (5.8) | — | 0.180 | — | — |
| Headache | 84 (25.6) | 63 (24.7) | — | 0.803 | — | — |
| Rashes | 12 (3.7) | 3 (1.2) | — | 0.060 | — | — |
| Fatigue | 102 (31) | 60 (23.5) | 1.4 (1.0, 2.1) | 0.043 | 1.5 (1.1, 2.3) | 0.025 |
| Diarrhoea | 9 (2.7) | 10 (3.9) | — | 0.427 | — | — |
| Abdominal pain | 9 (2.7) | 4 (1.6) | — | 0.340 | — | — |
| Tachycardia | 20 (6.0) | 2 (0.8) | 8.2 (1.9, 35) | 0.001 | 8.0 (1.8, 35) | 0.006 |
| Rise in blood pressure | 5 (1.5) | 3 (1.2) | — | 0.720 | — | — |
| Fainting | 0 (0.0) | 1 (0.4) | — | 0.256 | — | — |
| Difficulty in breathing | 6 (1.8) | 1 (0.4) | — | 0.114 | — | — |
| Dizziness | 26 (7.9) | 15 (5.8) | — | 0.338 | — | — |
| Chest pain | 10 (3.0) | 1 (0.4) | 7.9 (1.0, 62) | 0.019 | 7.7 (0.9, 62) | 0.054 |
| Major AEs | ||||||
| Any major AEs | 12 (3.7) | 3 (1.2) | — | 0.060 | — | — |
| Anaphylaxis | 2 (0.6) | 1 (0.4) | — | 0.716 | — | — |
| Marked dyspnoea | 1 (0.3) | 0 (0.0) | — | 0.378 | — | — |
| Throat closure | 1 (0.3) | 0 (0.0) | — | 0.378 | — | — |
| Severe rashes | 1 (0.3) | 0 (0.4) | — | 0.858 | — | — |
| Hospitalization | 0 (0.0) | 1 (0.4) | — | 0.256 | — | — |
Factors included as covariates in multivariable binary logistic regression analysis included age, gender, ethnicity, country by human development index. Bold text highlights significant P-values.
AEs: adverse events; OR: odds ratio.
Table 3, Supplementary Table S3 (available at Rheumatology online) and Fig. 2 provide an overview of vaccination-related AEs based on the type of vaccine received by patients with SLE. Overall, any AEs were reported more frequently by Pfizer-BioNTech (BNT162b2) vaccine recipients (OR: 2.2; 95% CI: 1.1, 4.2; P = 0.016) compared with the rest. Any minor AEs followed similar trends. Injection site pain was reported more frequently by Pfizer-BioNTech (BNT162b2) vaccine recipients (OR: 2.9; 95% CI: 1.6, 5.0; P < 0.001) and less frequently by Sinopharm (BBIBP-CorV) vaccine recipients (OR: 0.2; 95% CI: 0.1, 0.6; P = 0.003) compared with the rest.
Table 3.
Frequencies of vaccination-related AEs in SLE patients by vaccine type
| Pfizer-BioNTech (BNT162b2) (n = 250) | Oxford/Astra Zeneca (ChAdOx1 nCoV-19) (n = 68) | Johnson & Johnson (J&J) (JNJ-78436735) (n = 16) | Moderna (mRNA-1273) (n = 59) | Covishield (Serum Institute India) (ChAdOx1 nCoV-19) (n = 46) | Covaxin (Bharat Biotech) (BBV152) (n = 10) | Sputnik (Gam-COVID-Vac) (n = 7) | Sinopharm (BBIBP-CorV) (n = 33) | |
|---|---|---|---|---|---|---|---|---|
| Any adverse event | 219 (87.6)* | 60 (88.2) | 15 (93.8) | 55 (93.2) | 31 (67.4) | 7 (70.0) | 7 (100.0) | 28 (84.8) |
| Injection site pain | 202 (80.8)*** | 53 (77.9) | 12 (75.0) | 51 (86.4) | 19 (41.3) | 6 (60.0) | 6 (85.7) | 17 (51.5)* |
| Minor AEs | ||||||||
| Any minor AEs | 219 (87.6)* | 60 (88.2) | 15 (93.8) | 55 (93.2) | 31 (67.4) | 7 (70.0) | 7 (100.0) | 28 (84.8) |
| Myalgia | 38 (15.2) | 11 (16.2) | 3 (18.8) | 15 (25.4) | 7 (15.2) | 0 (0.0) | 1 (14.3) | 5 (15.2) |
| Body ache | 33 (13.2)*** | 25 (36.8)*** | 3 (18.8) | 19 (32.2)* | 8 (17.4) | 1 (10.0) | 2 (28.6) | 7 (21.2) |
| Fever | 38 (15.2) | 22 (32.4)** | 4 (25.0) | 23 (38.9)*** | 10 (21.7) | 0 (0.0) | 0 (0.0) | 8 (24.2) |
| Chills | 25 (10.0) | 14 (20.6)* | 2 (12.5) | 19 (32.2)*** | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea and vomiting | 19 (7.6) | 7 (10.3) | 2 (12.5) | 6 (10.2) | 0 (0.0) | 0 (0.0) | 2 (28.6) | 2 (6.1) |
| Headache | 51 (20.4) | 25 (36.8) | 8 (50.0) | 22 (37.4) | 5 (10.9) | 2 (20.0) | 3 (42.9) | 8 (24.2) |
| Rashes | 7 (2.8) | 1 (1.5) | 0 (0.0) | 5 (8.5)** | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 81 (32.4) | 21 (30.9) | 9 (56.3) | 22 (37.3) | 5 (10.9) | 0 (0.0) | 3 (42.9) | 5 (15.2) |
| Diarrhoea | 8 (3.2) | 5 (7.4) | 1 (6.3) | 2 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 4 (1.6) | 3 (4.4) | 1 (6.3) | 3 (5.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| High pulse rate | 10 (4.0) | 4 (5.9) | 1 (6.3) | 5 (8.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.0) |
| Rise in blood pressure | 3 (1.2) | 1 (1.5) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fainting | 0 (0.0) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Difficulty in breathing | 4 (1.6) | 1 (1.5) | 0 (0.0) | 2 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dizziness | 10 (4.0)* | 7 (10.3) | 3 (18.8) | 5 (8.5) | 1 (2.2) | 0 (0.0) | 1 (14.3) | 4 (12.1) |
| Chest pain | 5 (2.0) | 1 (1.5) | 0 (0.0) | 3 (5.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Others | 31 (12.4) | 7 (10.3) | 1 (6.3) | 2 (3.4) | 2 (4.3) | 1 (10.0) | 0 (0.0) | 7 (21.2) |
| Major AEs | ||||||||
| Any major AEs | 9 (3.6) | 1 (1.5) | 0 (0.0) | 3 (5.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anaphylaxis | 2 (0.8) | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Marked dyspnoea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Throat closure | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe rashes | 1 (0.4) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Others | 6 (2.4) | 0 (0.0) | 0 (0.0) | 2 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hospitalisation | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Results from binary logistic regression analysis. Comparisons are made between one drug vs the rest. Bold denotes increased risk (OR) compared with the rest, while bold underlined denotes decreased risk (OR) compared with the rest.
P < 0.05;
P < 0.005;
P < 0.001.
AEs: adverse events; OR: odds ratio.
Figure 2.
Selected vaccination-related AEs in patients with SLE. The forest plot illustrates selected results from multivariable logistic regression analysis. Circles denote odds ratios (ORs) and whiskers denote 95% confidence intervals. AEs: adverse events; LoS: level of significance; SLE: systemic lupus erythematosus
Major AEs were reported to be similar across recipients of different vaccine types. Hospitalization following vaccination was infrequent, with similar frequencies across recipients of the various vaccine types.
Systemic minor AEs such as fever, chills and body ache were more frequently reported by Oxford/AstraZeneca (ChAdOx1 nCoV-19) and Moderna (mRNA-1273) vaccine recipients (OR ranging between 2.5 and 3.0; P < 0.05 for all instances). All other minor AEs were similar across recipients of different vaccine types (Table 3 and Supplementary Table S3, available at Rheumatology online).
Supplementary Tables S4 and S5, available at Rheumatology online, provide an overview of vaccination-related AEs stratified by the type of background immunosuppressive therapy. The vaccination-related AEs were similar across background treatment categories except for lower chills in SLE patients who were on antimalarial agents (OR: 0.5; 95% CI: 0.3, 0.9; P = 0.042) compared with patients who were not on antimalarials. The hospitalization frequencies were also similar across the various background treatment groups.
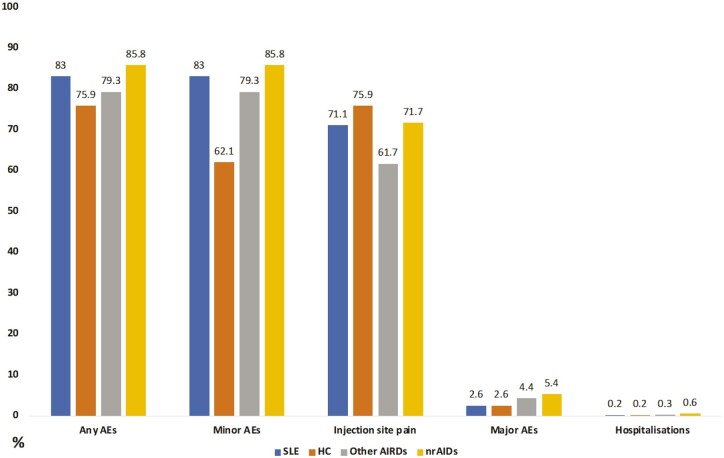
Figure 3, Supplementary Tables S2, S6 and S7, all available at Rheumatology online, provide an overview of vaccination-related AEs between patients with SLE and HC, between patients with SLE and patients with other AIRDs, and patients with SLE and patients with nrAIDs, respectively. Compared with HC, patients with SLE had similar overall AEs frequencies, frequencies of injection site pain, overall minor AEs, and hospitalization frequencies, except for higher frequencies of rashes (OR: 1.2; 95% CI: 1.0, 1.5; P = 0.038) and anaphylaxis (OR: 8.7; 95% CI: 1.3, 57.0; P = 0.024) in patients with SLE. In terms of absolute numbers, the patients with SLE who reported anaphylaxis were few (n = 3; 0.5%). When compared with other AIRD patients, patients with SLE reported similar frequencies of overall AEs, minor AEs, major AEs and hospitalizations, except for lower frequencies of chills (OR: 0.6; 95% CI: 0.4, 0.8; P = 0.005) in SLE patients. Similarly, when compared with nrAID patients, patients with SLE reported similar frequencies of overall AEs, minor AEs, major AEs and hospitalizations, except for chills (OR: 0.5; 95% CI: 0.3, 0.8; P = 0.003) and fatigue (OR: 0.6; 95% CI: 0.4, 0.9; P = 0.020) that were less frequent in the SLE population.
Figure 3.
Frequencies of vaccination-related adverse events (AEs) across patients with various rheumatic diseases and healthy controls. AEs: adverse events; AIRDs: autoimmune rheumatic diseases (excluding SLE and IIM); HC: healthy controls; nrAIDs: non-rheumatic autoimmune diseases
Supplementary Table S8, available at Rheumatology online, demonstrates that SLE patients with autoimmune comorbidities (n = 92; 15.8%) reported similar frequencies of overall AEs, minor AEs, and major AEs compared with SLE patients without autoimmune comorbidities (n = 491; 84.2%), with the exception of fatigue (OR: 2.1; 95% CI: 1.3, 3.4; P = 0.002) and abdominal pain (OR: 3.6; 95% CI: 1.1, 12.1; P = 0.033) that were more frequently reported by SLE patients with autoimmune comorbidities.
Discussion
Rapid production of vaccines against COVID-19 was necessitated by the pernicious impact of the pandemic on societies. However, the swift pace of vaccine development has resulted in hesitancy in a considerable proportion of the global population. This hesitancy has been even more prominent in patients with chronic diseases, particularly autoimmune diseases, patient groups not only considered more vulnerable to infections but also more prone to drug reactions [8, 9, 18]. Patients with SLE are often on immunosuppressant therapies and/or glucocorticoids, which may impact the biological responses to vaccines. In the present study, we evaluated patient-reported post-vaccination AEs in patients with SLE and found overall reassuring results; while 83.0% of patients reported AEs, these were typically minor and self-resolving. Nevertheless, major AEs were reported by 2.6% of SLE patients and included anaphylaxis, throat closure and severe rashes. To the best of our knowledge, this is the first study of this scale in terms of numbers, ethnic diversity and global reach, to address COVID-19 vaccination-related AEs over a seven-day post-vaccination period, with a focus on patients with SLE and comparisons with patient populations with other AIRDs, nrAIDs, as well as healthy individuals.
In analysis stratifying SLE patients into patients with active and inactive disease, overall AE and hospitalization frequencies were similar between the groups. However, active SLE increased the chance for experiencing fever and fatigue by 1.6 and 1.5 times, respectively, and the chance for experiencing tachycardia by eight times. While the explanation underlying the observed association between active SLE and post-vaccination tachycardia is unclear and difficult to speculate upon, this is to the best of our knowledge the first time it is reported in the literature. The association persisted upon adjustments for glucocorticoid doses and intake of other immunosuppressive or immunomodulatory medications. Lastly, SLE patients with concurrent APS did not report thrombotic events post-vaccination, which is reassuring and concordant with previous reports on patients with APS [19–22].
In analysis stratified by vaccine type, AE frequencies reported by SLE patients were overall similar across the different vaccine types, except for Pfizer recipients who reported overall AEs and injection site pain at a 2-fold and 3-fold higher extent compared with recipients of other vaccine types, respectively, and Oxford/AstraZeneca and Moderna recipients who reported a higher probability of some minor AEs such as body ache, fever and chills. By contrast, Sinopharm recipients less frequently experienced injection site pain. Major AE and hospitalization frequencies were similar across recipients of different vaccine types. These findings were in line with those of the VACOLUP study, which reported similar frequencies of AEs across vaccine types, and irrespective of age or gender [13].
Lastly, we performed a subgroup analysis stratifying SLE patients based on background immunosuppressant or immunomodulatory therapy. In this analysis, AE frequencies did not differ across patient groups on different medications, with the exception of chills being less frequently reported by patients who were on antimalarial agents compared with patients who were not. Following conflicting initial signals, antimalarial agents were later disproved to be of efficacy in managing patients with COVID-19 infection [23, 24]. Patients with SLE who use antimalarials are recommended to continue the antimalarial treatment during a COVID-19 infection without any dose adjustment. There have been speculations about cardiotoxicity and electrocardiographic QTc prolongation with the use of antimalarials in COVID-19 infection, with a corroborating meta-analysis of several studies [5]. However, in many COVID-19 infection treatment protocols, patients were given higher doses of antimalarial agents compared with usual doses within rheumatology; for example, hydroxychloroquine loading with 800 mg [25]. Thus, SLE patients in the present study are anticipated to have received lower doses than those shown to induce cardiotoxicity; it is worth mentioning that the recommended daily hydroxychloroquine dose for patients with SLE is ≤5 mg/kg [26]. Moreover, many patients in the studies that showed hydroxychloroquine-induced cardiotoxicity also received azithromycin, another drug with potentiality to prolong the QTc [5]. Lastly, no signals were seen for B-cell depleting therapy regarding AE or hospitalization frequency.
Several limitations need to be acknowledged. Firstly, the study was entirely based on self-reported patient data, with no possibility for verification through medical charts or healthcare professionals. Secondly, online surveys introduce inherent recall bias, as well as selection bias due to an expected higher participation willingness in patients who experienced AEs, a potential lack of internet access for individuals of lower socioeconomic status, and the likely lower grade of data contribution by patients suffering severe disability. Thirdly, the focus of the study was on short-term safety of COVID-19 vaccination, thus not contributing to the knowledge on safety over a longer term. However, the study reaffirms the safety of COVID-19 vaccination in SLE patients and is in agreement with existing evidence on COVID-19 vaccine safety within SLE populations of different studies [19, 27–36]. Fourthly, it was beyond the scope of this study to explore humoral responses to vaccines, which may also have impact on the development of AEs. Nevertheless, the large number of study participants, the high frequency of complete survey responses, and the wide geographical spread of survey respondents constituted major strengths of the study. The anonymised and self-reported nature of the questionnaire may also be considered a strength, by facilitating direct and likely unbiased (without external influence) patient and HC representation.
In conclusion, the findings of the present study provide reassurance on the safety of COVID-19 vaccination for the SLE patient population, adding to the body of literature that addresses concerns and controversies around COVID-19 vaccination. Most AEs reported were minor, self-resolving, and comparable in nature and frequencies to those reported by healthy controls. Our results will hopefully allow greater confidence and better uptake of COVID-19 vaccines in this particularly vulnerable group of individuals, as advocated by European [37] and American [7] guidelines.
Supplementary Material
Acknowledgements
COVID-19 Vaccination in Autoimmune Diseases (COVAD) Study Group Author List: Bhupen Barman, Yogesh Preet Singh, Rajiv Ranjan, Avinash Jain, Sapan C Pandya, Rakesh Kumar Pilania, Aman Sharma, Manesh Manoj M, Vikas Gupta, Chengappa G Kavadichanda, Pradeepta Sekhar Patro, Sajal Ajmani, Sanat Phatak, Rudra Prosad Goswami, Abhra Chandra Chowdhury, Ashish Jacob Mathew, Padnamabha Shenoy, Ajay Asranna, Keerthi Talari Bommakanti, Anuj Shukla, Arun Kumar R Pandey, Kunal Chandwar, Sinan Kardeş, Döndü Üsküdar Cansu, Minchul Kim, Ashima Makol, Tulika Chatterjee, John D Pauling, Chris Wincup, Lorenzo Cavagna, Nicoletta Del Papa, Gianluca Sambataro, Atzeni Fabiola, Marcello Govoni, Simone Parisi, Elena Bartoloni Bocci, Gian Domenico Sebastiani, Enrico Fusaro, Marco Sebastiani, Luca Quartuccio, Franco Franceschini, Pier Paolo Sainaghi, Giovanni Orsolini, Rossella De Angelis, Maria Giovanna Danielli, Vincenzo Venerito, Marcin Milchert, Lisa S Traboco, Suryo Anggoro Kusumo Wibowo, Erick Adrian Zamora Tehozol, Jorge Rojas Serrano, Ignacio García-De La Torre, Jesús Loarce-Martos, Sergio Prieto-González, Albert Gil-Vila, Raquel Aranega Gonzalez, Masataka Kuwana, Akira Yoshida, Ran Nakashima, Shinji Sato, Naoki Kimura, Yuko Kaneko, Johannes Knitza, Stylianos Tomaras, Margarita Aleksandrovna Gromova, Or Aharonov, Tamer A Gheita, Ihsane Hmamouchi, Leonardo Santos Hoff, Margherita Giannini, François Maurier, Julien Campagne, Alain Meyer, Melinda Nagy-Vincze, Daman Langguth, Vidya Limaye, Merrilee Needham, Nilesh Srivastav, Marie Hudson, Océane Landon-Cardinal, Syahrul Sazliyana Shaharir, Wilmer Gerardo Rojas Zuleta, José António Pereira Silva, João Eurico Fonseca, Olena Zimba.
The authors are grateful to all respondents for filling out the questionnaire. The authors also thank The Myositis Association, Myositis India, Myositis UK, Myositis Support and Understanding, the Myositis Global Network, Deutsche Gesellschaft für Muskelkranke e. V. (DGM), Dutch and Swedish Myositis patient support groups, Cure JM, Cure IBM, Sjögren’s India Foundation, Patients Engage, Scleroderma India, Lupus UK, Lupus Sweden (Riksföreningen för SLE), Emirates Arthritis Foundation, EULAR PARE, ArLAR research group, AAAA patient group, APLAR myositis special interest group, Thai Rheumatism association, PANLAR, NRAS, Anti-Synthetase Syndrome support group, and various other patient support groups and organizations for their contribution in the dissemination of this survey. Finally, the authors wish to thank all members of the COVAD study group for their invaluable role in data collection.
H.C. is supported by the National Institution for Health Research Manchester Biomedical Research Centre Funding Scheme. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Contributor Information
R Naveen, Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
Elena Nikiphorou, Centre for Rheumatic Diseases, King’s College London, London, UK; Rheumatology Department, King's College Hospital, London, UK.
Mrudula Joshi, Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals, Pune, India.
Parikshit Sen, Maulana Azad Medical College, New Delhi, Delhi, India.
Julius Lindblom, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Vishwesh Agarwal, Mahatma Gandhi Mission Medical College, Navi Mumbai, Maharashtra, India.
James B Lilleker, Centre for Musculoskeletal Research, Division of Musculoskeletal and Dermatological Sciences, School of Biological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, Manchester, UK; Neurology, Manchester Centre for Clinical Neurosciences, Northern Care Alliance NHS Foundation Trust, Salford, UK.
Ai Lyn Tan, NIHR Leeds Biomedical Research Centre, Leeds Teaching Hospitals Trust, Leeds, UK; Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK.
Babur Salim, Rheumatology Department, Fauji Foundation Hospital, Rawalpindi, Pakistan.
Nelly Ziade, Rheumatology Department, Saint-Joseph University, Beirut, Lebanon; Rheumatology Department, Hotel-Dieu de France Hospital, Beirut, Lebanon.
Tsvetelina Velikova, Department of Clinical Immunology, Medical Faculty, University Hospital ‘Lozenetz’, Sofia University St. Kliment Ohridski, Sofia, Bulgaria.
Abraham Edgar Gracia-Ramos, Department of Internal Medicine, General Hospital, National Medical Center, ‘La Raza’, Instituto Mexicano del Seguro Social, Mexico City, Mexico.
Masataka Kuwana, Department of Allergy and Rheumatology, Nippon Medical School Graduate School of Medicine, Tokyo, Japan.
Jessica Day, Department of Rheumatology, Royal Melbourne Hospital, Parkville, VIC, Australia; Walter and Eliza Hall Institute of Medical Research, Parkville, VIC, Australia; Department of Medical Biology, University of Melbourne, Parkville, VIC, Australia.
Ashima Makol, Division of Rheumatology, Mayo Clinic, Rochester, MN, USA.
Oliver Distler, Department of Rheumatology, University Hospital Zürich, University of Zürich, Zürich, Switzerland.
Hector Chinoy, Centre for Musculoskeletal Research, Division of Musculoskeletal and Dermatological Sciences, School of Biological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, Manchester, UK; National Institute for Health Research Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, The University of Manchester, Manchester, UK; Department of Rheumatology, Salford Royal Hospital, Northern Care Alliance NHS Foundation Trust, Salford, UK.
Lisa S Traboco, Department of Medicine, Section of Rheumatology, St. Luke's Medical Center-Global City, Taguig, Philippines.
Suryo Anggoro Kusumo Wibowo, Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia/Dr Cipto Mangunkusumo General Hospital, Jakarta, Indonesia.
Erick Adrian Zamora Tehozol, Autoimmunity Division, Centro Médico Pensiones, Mérida, Yucatán, Mexico.
Jorge Rojas Serrano, Rheumatologist and Clinical Investigator, Interstitial Lung Disease and Rheumatology Unit, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico.
Ignacio García-De La Torre, Departamento de Inmunología y Reumatología, Hospital General de Occidente and University of Guadalajara, Guadalajara, Jalisco, Mexico.
Rohit Aggarwal, Division of Rheumatology and Clinical Immunology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Latika Gupta, Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India; Centre for Musculoskeletal Research, Division of Musculoskeletal and Dermatological Sciences, School of Biological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, Manchester, UK; Department of Rheumatology, Royal Wolverhampton Hospitals NHS Trust, Wolverhampton, UK; City Hospital, Sandwell and West Birmingham Hospitals NHS Trust, Birmingham, UK.
Vikas Agarwal, Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
Ioannis Parodis, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden; Department of Rheumatology, Faculty of Medicine and Health, Örebro University, Örebro, Sweden.
COVAD Study Group:
Bhupen Barman, Yogesh Preet Singh, Rajiv Ranjan, Avinash Jain, Sapan C Pandya, Rakesh Kumar Pilania, Aman Sharma, M Manesh Manoj, Vikas Gupta, Chengappa G Kavadichanda, Pradeepta Sekhar Patro, Sajal Ajmani, Sanat Phatak, Rudra Prosad Goswami, Abhra Chandra Chowdhury, Ashish Jacob Mathew, Padnamabha Shenoy, Ajay Asranna, Keerthi Talari Bommakanti, Anuj Shukla, Arun Kumar R Pandey, Kunal Chandwar, Sinan Kardeş, Döndü Üsküdar Cansu, Minchul Kim, Ashima Makol, Tulika Chatterjee, John D Pauling, Chris Wincup, Lorenzo Cavagna, Nicoletta Del Papa, Gianluca Sambataro, Atzeni Fabiola, Marcello Govoni, Simone Parisi, Elena Bartoloni Bocci, Gian Domenico Sebastiani, Enrico Fusaro, Marco Sebastiani, Luca Quartuccio, Franco Franceschini, Pier Paolo Sainaghi, Giovanni Orsolini, Rossella De Angelis, Maria Giovanna Danielli, Vincenzo Venerito, Marcin Milchert, Lisa S Traboco, Suryo Anggoro Kusumo Wibowo, Erick Adrian Zamora Tehozol, Jorge Rojas Serrano, Ignacio García-De La Torre, Jesús Loarce-Martos, Sergio Prieto-González, Albert Gil-Vila, Raquel Aranega Gonzalez, Masataka Kuwana, Akira Yoshida, Ran Nakashima, Shinji Sato, Naoki Kimura, Yuko Kaneko, Johannes Knitza, Stylianos Tomaras, Margarita Aleksandrovna Gromova, Or Aharonov, Tamer A Gheita, Ihsane Hmamouchi, Leonardo Santos Hoff, Margherita Giannini, François Maurier, Julien Campagne, Alain Meyer, Melinda Nagy-Vincze, Daman Langguth, Vidya Limaye, Merrilee Needham, Nilesh Srivastav, Marie Hudson, Océane Landon-Cardinal, Syahrul Sazliyana Shaharir, Wilmer Gerardo Rojas Zuleta, José António Pereira Silva, João Eurico Fonseca, and Olena Zimba
Supplementary material
Supplementary data are available at Rheumatology online.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Contribution statement
Conceptualisation: N.R., E.N., L.G., I.P. Data curation: All authors. Formal analysis: N.R. Funding acquisition: I.P. Investigation: N.R., E.N., L.G., I.P. Methodology: N.R., E.N., L.G., I.P. Software: L.G. Validation: V.A., R.A., J.B.L., H.C. Visualization: J.L., R.A., V.A., L.G. Writing—original draft: N.R., E.N., I.P. Writing—review and editing: All authors.
Disclaimer: No part of this manuscript is copied or published elsewhere in whole or in part.
Funding
I.P. is supported by grants from the Swedish Rheumatism Association (R-941095), King Gustaf V’s 80-year Foundation (FAI-2020–0741), Professor Nanna Svartz Foundation (2020–00368), Swedish Society of Medicine (SLS-974449), Ulla and Roland Gustafsson Foundation (2021–26), Region Stockholm (FoUI-955483) and Karolinska Institutet.
Disclosure statement: A.L.T. has received honoraria for advisory boards and speaking for Abbvie, Gilead, Janssen, Lilly, Novartis, Pfizer, UCB. E.N. has received speaker honoraria/participated in advisory boards for Celltrion, Pfizer, Sanofi, Gilead, Galapagos, AbbVie, Lilly and holds research grants from Pfizer and Lilly. H.C. has received grant support from Eli Lilly and UCB; consulting fees from Novartis, Eli Lilly, Orphazyme, Astra Zeneca; speaker for UCB, Biogen. I.P. has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly and Company, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Novartis and F. Hoffmann-La Roche AG. M.K. has received research grants and personal fees from AbbVie, AsahiKasei, Astellas, Boehringer Ingelheim, Chugai, Corbus, GlaxoSmithKline, Horizon, Kissei, MBL, Mitsubishi-Tanabe, Mochida, Nippon Shinyaku, and Ono Pharmaceuticals. J.D. has received research funding from CSL Limited. N.Z. has received speaker fees, advisory board fees and research grants from Pfizer, Roche, Abbvie, Eli Lilly, NewBridge, Sanofi-Aventis, Boehringer Ingelheim, Janssen, Pierre Fabre; none is related to this manuscript. O.D. has/had consultancy relationship with and/or has received research funding from or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three years: Abbvie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, Baecon, Blade, Bayer, Boehringer Ingelheim, ChemomAb, Corbus, CSL Behring, Galapagos, Glenmark, GSK, Horizon (Curzion), Inventiva, iQvia, Kymera, Lupin, Medac, Medscape, Mitsubishi Tanabe, Novartis, Roche, Roivant, Sanofi, Serodapharm, Topadur and UCB. Patent issued ‘mir-29 for the treatment of systemic sclerosis’ (US8247389, EP2331143). R.A. has/had a consultancy relationship with and/or has received research funding from the following companies-Bristol Myers-Squibb, Pfizer, Genentech, Octapharma, CSL Behring, Mallinckrodt, AstraZeneca, Corbus, Kezar, and Abbvie, Janssen, Alexion, Argenx, Q32, EMD-Serono, Boehringer Ingelheim, Roivant. The other authors have no conflict of interest relevant to this manuscript.
References
- 1. Chen L, Cai X, Zhao T. et al. Safety of global SARS-CoV-2 vaccines, a meta-analysis. vaccines. Vaccines 2022;10:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grainger R, Kim AHJ, Conway R, Yazdany J, Robinson PC.. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol 2022;18:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tavares A, de Melo AKG, Cruz VA. et al. Guidelines on COVID-19 vaccination in patients with immune-mediated rheumatic diseases: a Brazilian Society of Rheumatology task force. Adv Rheumatol 2022;62:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Funck-Brentano C, Nguyen LS, Salem JE.. Retraction and republication: cardiac toxicity of hydroxychloroquine in COVID-19. Lancet 2020;396:e2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tleyjeh IM, Kashour Z, AlDosary O. et al. Cardiac toxicity of chloroquine or hydroxychloroquine in patients with COVID-19: a systematic review and meta-regression analysis. Mayo Clin Proc Innov Qual Outcomes 2021;5:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mikuls TR, Johnson SR, Fraenkel L. et al. American college of rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheumatol 2020;72:1241–51. [DOI] [PubMed] [Google Scholar]
- 7. Curtis JR, Johnson SR, Anthony DD. et al. American college of rheumatology guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheumatol 2021;73:1093–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bendau A, Plag J, Petzold MB, Ströhle A.. COVID-19 vaccine hesitancy and related fears and anxiety. Int Immunopharmacol 2021;97:107724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sen P, Lilleker J, Agarwal V. et al. Vaccine hesitancy in patients with autoimmune diseases: data from the coronavirus disease-2019 vaccination in autoimmune diseases study. Indian J Rheumatol 2022;17:188. [Google Scholar]
- 10. Kayesh MEH, Kohara M, Tsukiyama-Kohara K.. An overview of recent insights into the response of TLR to SARS-CoV-2 infection and the potential of TLR agonists as SARS-CoV-2 vaccine adjuvants. Viruses 2021;13:2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gil-Vila A, Naveen R, Selva O. et al. COVID-19 Vaccination in Autoimmune Diseases (COVAD) study: vaccine safety in idiopathic inflammatory myopathies. Muscle Nerve 2022;66:426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naveen R, Parodis I, Joshi M. et al. COVID-19 Vaccination in Autoimmune Diseases (COVAD) Study: vaccine safety and tolerance in rheumatoid arthritis. Rheumatology 2023;62:2366–76. [DOI] [PubMed] [Google Scholar]
- 13. Felten R, Kawka L, Dubois M. et al. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol 2021;3:e613–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sen P, Gupta L, Lilleker JB. et al. COVID-19 vaccination in autoimmune disease (COVAD) survey protocol. Rheumatol Int 2022;42:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wahl E, Gross A, Chernitskiy V. et al. Validity and responsiveness of a 10-item patient-reported measure of physical function in a rheumatoid arthritis clinic population: PF-10a validity and responsiveness in RA. Arthritis Care Res 2017;69:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eysenbach G. Improving the quality of web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004;6:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaur PS, Zimba O, Agarwal V, Gupta L.. Reporting survey based studies – a primer for authors. J. Korean Med Sci 2020;35:e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohanasundaram K, Santhanam S, Natarajan R. et al. Covid-19 vaccination in autoimmune rheumatic diseases: a multi-center survey from southern India. Int J Rheum Dis 2022;25:1046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Signorelli F, Balbi GGM, Aikawa NE. et al. Immunogenicity, safety, and antiphospholipid antibodies after SARS-CoV-2 vaccine in patients with primary antiphospholipid syndrome. Lupus 2022;31:974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pengo V, Del Ross T, Tonello M. et al. Impact of COVID-19 and COVID-19 vaccination on high-risk patients with antiphospholipid syndrome: a nationwide survey. Rheumatology 2022;61:SI136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sciascia S, Costanzo P, Radin M. et al. Safety and tolerability of mRNA COVID-19 vaccines in people with antiphospholipid antibodies. Lancet Rheumatol 2021;3:e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan H, Tang Z, Teng J. et al. COVID-19 vaccine affects neither prothrombotic antibody profile nor thrombosis in primary antiphospholipid syndrome: a prospective study. Rheumatology 2023;62:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Das RR, Jaiswal N, Dev N. et al. Efficacy and safety of anti-malarial drugs (chloroquine and hydroxy-chloroquine) in treatment of COVID-19 infection: a systematic review and meta-analysis. Front Med 2020;7:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das RR, Behera B, Mishra B, Naik SS.. Effect of chloroquine and hydroxychloroquine on COVID-19 virological outcomes: an updated meta-analysis. Indian J Med Microbiol 2020;38:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kara E, Demirkan K, Unal S.. Recommendations for use of a hydroxychloroquine loading dose in patients with COVID-19. Int J Antimicrob Agents 2020;56:106123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fanouriakis A, Kostopoulou M, Alunno A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 27. Gerosa M, Schioppo T, Argolini LM. et al. The impact of anti-SARS-CoV-2 vaccine in patients with systemic lupus erythematosus: a multicentre cohort study. Vaccines 2022;10:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zavala-Flores E, Salcedo-Matienzo J, Quiroz-Alva A, Berrocal-Kasay A.. Side effects and flares risk after SARS-CoV-2 vaccination in patients with systemic lupus erythematosus. Clin. Rheumatol 2022;41:1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mok CC, Chan KL, Tse SM.. Hesitancy for SARS-CoV-2 vaccines and post-vaccination flares in patients with systemic lupus erythematosus. Vaccine 2022;40:5959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshida T, Tsuji H, Onishi A. et al. Medium-term impact of the SARS-CoV-2 mRNA vaccine against disease activity in patients with systemic lupus erythematosus. Lupus Sci. Med 2022;9:e000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Izmirly PM, Kim MY, Samanovic M. et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol 2022;74:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mormile I, Della Casa F, Petraroli A. et al. Immunogenicity and safety of mRNA anti-SARS-CoV-2 vaccines in patients with systemic lupus erythematosus. Vaccines 2022;10:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang W, Gartshteyn Y, Ricker E. et al. The use of COVID-19 vaccines in patients with SLE. Curr Rheumatol Rep 2021;23:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Assawasaksakul T, Lertussavavivat T, Sathitratanacheewin S. et al. Comparison of immunogenicity and safety of inactivated, adenovirus-vectored, and heterologous adenovirus-vectored/mRNA vaccines in patients with systemic lupus erythematosus and rheumatoid arthritis: a prospective cohort study. Vaccines 2022;10:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yuki EFN, Borba EF, Pasoto SG. et al. Impact of distinct therapies on antibody response to SARS-CoV-2 vaccine in systemic lupus erythematosus. Arthritis Care Res 2022;74:562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan SYS, Yee AM, Sim JJL, Lim CC.. COVID-19 vaccination in systemic lupus erythematosus: a systematic review for effectiveness, immunogenicity, flares and acceptance. Rheumatology 2023;62:1757–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Landewé RBM, Kroon FPB, Alunno A. et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis 2022;81:1628–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.