Abstract
Poor glycemic control in type 2 diabetes has been associated with accentuated age-related cognitive decline, although the underlying neural mechanisms are not well understood. The current study sought to identify the impact of glycemic control on the neural dynamics serving working memory in adults with type 2 diabetes. Participants (n = 34, ages = 55–73) performed a working memory task while undergoing MEG. Significant neural responses were examined relative to poorer (A1c > 7.0%) or tighter glycemic control (A1c < 7.0%). Those with poorer glycemic control showed diminished responses within left temporal and prefrontal regions during encoding and showed diminished responses within right occipital cortex during maintenance but showed an enhanced activity in the left temporal, occipital, and cerebellar regions during maintenance. Notably, left temporal activity in encoding and left lateral occipital activity in maintenance significantly predicted performance on the task such that diminished temporal activity led to longer reaction times, which were driven by the poorer glycemic control group. Greater lateral occipital activity during maintenance was associated with both lower accuracy and longer reaction times across all participants. These findings suggest that glycemic control has a robust impact on the neural dynamics serving working memory, with distinct effects by subprocess (e.g. encoding vs. maintenance) and direct effects on behavior.
Keywords: MEG, hyperglycemia, complications, neuropathy, A1c
Introduction
Type 2 diabetes is characterized by chronic glycemic dysregulation and progressive insulin resistance and has been associated with many distinct health complications, including cognitive and neural deficits (Reijmer et al. 2010; Feinkohl et al. 2015; Geijselaers et al. 2015). Studies examining the acute impact of glycemic dysregulation, employing glycemic clamp and insulin administration methods, have found significant impairments across multiple cognitive domains and linked these to widespread neural dysfunction (Sommerfield et al. 2004; Tschritter et al. 2009; Kullmann et al. 2016; Backeström et al. 2021). Some of these studies have further demonstrated immediate improvements in cognition with normalized glucose levels (Ott et al. 2012; Alagiakrishnan et al. 2013; Backeström et al. 2021), suggesting a direct causal link for the neural dysfunction. In studies examining the links between chronic glycemic dysregulation and cognitive function, higher glycated hemoglobin (A1c) levels are generally associated with worse cognitive outcomes (Cukierman-Yaffe et al. 2009; Grober et al. 2011; West et al. 2014). Relatedly, current care guidelines aim for A1c levels <7.0% (Association 2021), but even then care is warranted as strict glycemic control can come at the cost of increased episodes of hypoglycemia, which have also been linked to cognitive dysfunction (Zammitt and Frier 2005; Seaquist 2015).
One cognitive domain that is differentially impacted by the disease is executive function, particularly processes like working memory (Grober et al. 2011; Palta et al. 2014). Working memory is defined as the temporary storage and/or manipulation of information to fulfill task goals and can generally be parsed into 3 distinct phases: encoding, maintenance, and retrieval. Working memory is critical to many other higher-order cognitive processes, including decision-making, language comprehension, and task switching. In healthy populations, working memory paradigms generally elicit widespread frontal–parietal, occipital, and temporal activities (Rottschy et al. 2012). For example, electrophysiological studies of working memory tasks with language-based stimuli elicit strong alpha band responses across left hemispheric language-related regions, including the left superior temporal cortex, left supramarginal gyrus, and left prefrontal cortex (Heinrichs-Graham and Wilson 2015; Proskovec et al. 2016). Further, widespread dynamic alpha activity in the occipital, parietal, and prefrontal cortices can be discerned by the phase of working memory processing (e.g. maintenance) (Brookes et al. 2011; Bonnefond and Jensen 2012; Heinrichs-Graham and Wilson 2015). Interestingly, alpha frequency activity in right hemispheric homolog regions has also been shown in studies of pathological and healthy aging (Wiesman et al. 2016; Wilson et al. 2017) and in patients with type 1 diabetes (Embury et al. 2018), with such activity broadly thought to reflect compensatory processing. Such neural dynamics during working memory processing have not yet been studied in type 2 diabetes; however, working memory deficits have been shown in type 2 diabetes using neuropsychological assessments (Palta et al. 2014) and in fMRI studies of acute hyperglycemia induced by glycemic clamp procedures (Backeström et al. 2021). Importantly, as type 2 diabetes associated with increased vascular damage, fMRI studies can be impacted by this underlying pathophysiology. MEG offers a distinct advantage in this respect as its signal is not altered by the intervening tissues, as in electroencephalography, or by alterations in the vasculature, which can have a major effect on fMRI. Further, the temporal precision of MEG enables the dynamics of these responses to be discerned and thereby enables the distinct subprocesses of working memory (e.g. encoding, maintenance, and retrieval) to be studied in relative isolation.
In the current study, we utilize a classic working memory task and a dynamic functional mapping method based on MEG to probe the chronic impact of glycemic control as evaluated by A1c on the neural dynamics underlying this critical cognitive function in patients with type 2 diabetes. We hypothesized that glycemic control level would alter the oscillatory activity across working memory relevant networks, including occipital, parietal, and left lateralized temporal and prefrontal regions, and that these effects would vary based on the phase of the task (i.e. encoding/maintenance).
Materials and methods
Participants
Fifty-four participants with type 2 diabetes were recruited from the Diabetes Center at the University of Nebraska Medical Center (UNMC; ages = 55–73, 33 females) from mid-2017 through late-2019. All procedures were performed following obtaining informed consent in accordance with the Declaration of Helsinki and full approval of UNMC’s Institutional Review Board. Formal diabetes diagnosis had to occur at least 1 year prior to entering the study. Exclusionary criteria included: (i) any medical diagnosis directly implicating brain function (e.g. psychiatric and/or neurological disease); (ii) known brain neoplasm or lesion; (iii) history of cerebrovascular events (i.e. CVA, stroke, and TIA) based on previous diagnosis and chart review; (iv) history of significant head trauma, seizures, or epilepsy; (v) current substance use disorder within the past 6 months; (vi) pregnancy or lactation; (vii) hospitalization within the previous 90 days; (viii) any type of cancer diagnosis or treatment in the past 5 years; (ix) uncontrolled hypertension, with blood pressures >140/90 or >160/100 if currently on medication treatment; (x) body mass index of ≥40; (xi) liver disease (AST or ALT >3× normal); (xii) any untreated thyroid or B12 deficiencies; (xiii) treatment with antipsychotics, antidepressants, and related medications known to affect brain function, with the exception of as-needed antidepressants following a 24-h washout period; and (xiv) ferromagnetic implants.
Participants measured their blood glucose level using a point-of-care device prior to MEG and cognitive task completion, verifying they were within the 70–200 mg/dL range. Participants who were mildly hypoglycemic (55–70 mg/dL) were asked to raise their blood sugar to the normal range, and after 1 h in the normal range, these participants started their MEG session. Participants with blood glucose levels <55 mg/dL or >200 mg/dL were rescheduled at least 1 week later, as such values equate to clinically significant hypo- and hyperglycemia.
Overall study design
Participants completed a panel of blood tests and provided key demographic and medical history data at enrollment. Full characteristics can be found in Table 1. Once blood glucose level was checked and was between 70 and 200 mg/dL, participants then completed cognitive tasks within the MEG environment. See below for task and MEG acquisition parameters. Participants’ data were analyzed groupwise relative to their glycemic control level (above and below 7.0% A1c).
Table 1.
Demographics and laboratory tests of the final sample.
| T2D with poorly controlled glycemic levels (A1c > 7.0%) | T2D with tightly controlled glycemic levels (A1c < 7.0%) | |
|---|---|---|
| N | 17 | 17 |
| Age (years) | 63.3 ± 4.9 | 62.3 ± 5.1 |
| Sex | 4 males; 13 females | 8 males; 9 females |
| Education (years) | 15.1 ± 2.9 | 14.6 ± 2.0 |
| Body mass index | 32.3 ± 4.4 | 31.7 ± 4.5 |
| Handedness | 15 right; 2 left | 16 right; 1 left |
| Disease duration (years) | 13.5 ± 8.6 | 12.0 ± 7.1 |
| A1c (%) | 8.16 ± 0.74 | 6.54 ± 0.36 |
| A1c (mmol/mol) | 66 ± 8.1 | 48 ± 3.9 |
| Creatinine (mg/dL) | 0.80 ± 0.23 | 0.85 ± 0.18 |
| Glucose (mg/dL) | 133.24 ± 34.88 | 107.65 ± 21.53 |
| Albumin/creatinine (ugAL/mgCR) | 36.82 ± 69.35 | 18.44 ± 23.51 |
| Thyroid-stimulating hormone (mcIU/mL) | 2.13 ± 0.96 | 1.96 ± 1.20 |
| B12 (pg/mL) | 394.89 ± 364.98 | 468.85 ± 285.43 |
Values depict means ± SD. T2D = Type 2 Diabetes; A1c = glycated hemoglobin.
Working memory task
During the MEG session, participants were seated in a nonmagnetic chair and were instructed to fixate on a crosshair presented centrally for 1,300 ms. Stimuli were presented at a visual angle of 17°. A grid containing 6 letters (font: Calibri, size: 80 pt) was then presented for 2,000 ms (encoding). These letters then disappeared from the grid and, 3,000 ms later, (maintenance) a single “probe” letter appeared for 900 ms (retrieval; see Fig. 1A). Participants were instructed to respond with a button press as to whether the probe letter was 1 of the 6 letters previously presented (in-set: index finger and out-of-set: middle finger). Trials were presented in pseudorandomized order, with 50% of trials having an in-set probe and 50% an out-of-set probe. Each trial lasted for 6,900 ms, and each participant completed 128 trials for a total run time of about 15 min. This same task with slightly different timing parameters has been validated by our group in several previous studies (Heinrichs-Graham and Wilson 2016; Proskovec et al. 2016, 2019; Wiesman et al. 2016; Embury et al. 2018; Killanin et al. 2022).
Fig. 1.
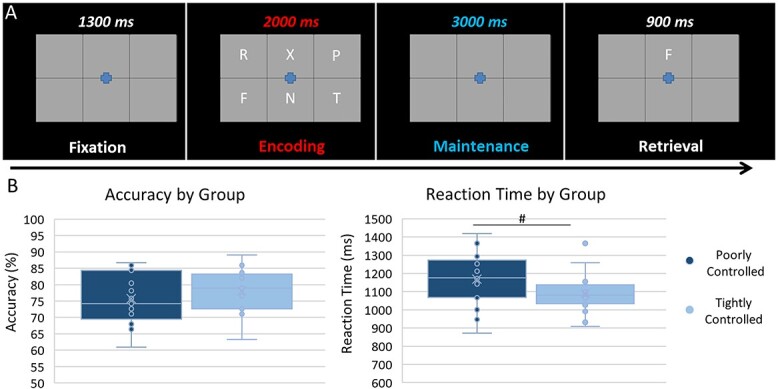
Working memory task paradigm and behavioral metrics. A) The working memory task included a 1,300 ms fixation cross, followed by the appearance of a 6-letter grid for 2,000 ms (encoding period), an empty grid for 3,000 ms (maintenance period), and finally, a probe letter within the grid for 900 ms (retrieval period). During retrieval, participants were to respond with a button press as to whether the probe letter was (index finger) or was not (middle finger) included in the previous set of 6 letters that was shown during the encoding period. B) Participants’ accuracy did not differ by group, but there was a trend for group differences in reaction time (t32 = 2.01, P = 0.053), where those with poorly controlled glycemic levels (A1c > 7.0%) had longer reaction times. # = (P = 0.053).
MEG methods and statistical analyses
Acquisition and preprocessing
MEG acquisition and analysis methodology followed standardized MEG processing pipelines that have been previously published by our group (Proskovec et al. 2016; Wiesman et al. 2016; Embury et al. 2018). Neurophysiological data were recorded using a 306-sensor Elekta/MEGIN MEG system (Helsinki, Finland) within a 1-layer magnetically shielded room with active shielding engaged. Data were sampled at 1 kHz with an acquisition bandwidth of 0.1–330 Hz. Data were corrected individually for head motion and were subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola 2006). Each participant’s functional data were coregistered with a structural T1-weighted MRI template. Blink and cardiac artifacts were modeled and extracted by signal-space projection (Uusitalo and Ilmoniemi 1997).
The continuous time series was divided into epochs of 7,200-ms duration, with 0 ms denoting the onset of the encoding grid. Epochs were rejected using a fixed threshold method, supplemented with visual inspection. To ensure analysis of task-relevant data, only correctly answered trials were included in final analyses. Additionally, participants with poor performance (i.e. near-chance accuracy) were excluded from further analyses.
Sensor space statistical analyses
Once cleared of artifactual trials, the remaining epochs were transformed into the time-frequency domain using complex demodulation, and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. Sensor-level data were normalized by dividing the power of each time-frequency bin by the mean baseline power (−1,200 to −1,000 ms) for that particular bin. Statistical analyses of the sensor-level spectrograms comparing the active window relative to the baseline period per gradiometer were then conducted to determine the time-frequency windows to be imaged.
Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model (GLM) and was then corrected for multiple comparisons in stage 2. First, paired t-tests were conducted on each data point against the mean baseline value at that frequency, and the output spectrograms of t-values was thresholded at P < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage 2, time-frequency bins that survived the threshold were clustered with other temporally and/or spectrally neighboring bins that also survived the threshold, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters were tested directly using this distribution (Maris and Oostenveld 2007). From these analyses, time-frequency windows with significant oscillatory responses across all participants were selected for imaging using a beamforming approach.
Anatomical-level statistical analyses
Significant time-frequency windows were imaged at a 4.0 × 4.0 × 4.0-mm resolution using a linearly constrained minimum variance vector beamformer (Gross et al. 2001) as implemented in the Brain Electrical Source Analysis software (version 6.1). This beamformer method uses spatial filters in the time-frequency domain to calculate the source power for the entire brain volume. Following convention, we computed noise-normalized source power per voxel in each participant using active (i.e. task) and passive (i.e. baseline) periods of equal duration and bandwidth. Resultant images are referred to as pseudo-t maps, with units that reflect noise-normalized power differences at each voxel. Each participant’s functional MEG images were transformed into standardized space using the transform that was previously applied to the structural images. Once transformed, resultant source images were resampled to enable voxel-wise statistics across the sample.
The resulting 3D maps of brain activity were averaged across all participants to assess the neuroanatomical basis of significant oscillatory responses identified through the sensor-level analysis. In addition, these images were statistically evaluated using a t-test, mass univariate approach based on the GLM. Specifically, the effect of glycemic control was determined using 2-tailed independent-sample t-tests separately for each encoding and maintenance bins between patient groups with tightly controlled and poorly controlled A1c levels. All output statistical maps were displayed as a function of alpha level, thresholded at P < 0.05, and adjusted for multiple comparisons using a spatial extent threshold (i.e. cluster restriction; k = 10 4-mm3 voxels) based on the theory of Gaussian random fields (Worsley et al. 1996). Significant peaks were extracted for post hoc analyses. Correlations with behavior metrics were also computed.
Results
Demographic, behavioral, and laboratory results
Seven participants had to be excluded for technical issues related to MEG data acquisition and/or excessively artifactual MEG data, and 1 participant had to be excluded for low blood sugar during the scan. In addition, the task itself was difficult for some participants, and 12 were excluded due to poor accuracy on the working memory task (i.e. performance at- or near-chance levels, cutoff was 60% accuracy). The final sample of 34 represented match groups of poorly controlled (A1c > 7.0%) and tightly controlled (A1c < 7.0%) glycemic levels. Groups did not differ on age (P = 0.564), sex (P = 0.151), race (P = 0.135), education (P = 0.635), handedness (P = 0.545), BMI (P = 0.711), and disease duration (P = 0.586). Participants did differ on glucose level at time of scan (P = 0.015; see Table 1), although all participants were within the normal range at time of scan. Most participants (n = 32) additionally completed the NIH Toolbox cognitive battery, achieving near-population mean standard scores (mean = 53.09 ± 9.26), which did not differ by group (P = 0.422). Thirty participants identified as White, 2 participants identified as Black, and 2 participants identified as Asian/Pacific Islander. Fifteen participants were solely treated by ≥1 oral medications (most commonly metformin), and 19 were treated with insulin with/without additional oral medications. Some participants reported additional comorbidities, including nephropathy (5), retinopathy (4), peripheral neuropathy (12), and cardiovascular (7) conditions. Seven participants from the tightly controlled group and 10 participants from the poorly controlled group reported ≥1 of these comorbid conditions. Nine participants reported ≥2 of these conditions. See Table 1 for demographics and blood panels.
Average accuracy was 76.7 ± 7.2%, and average reaction time was 1,121.99 ± 144.02 ms. The groups did not differ by accuracy (t32 = −0.76, P = 0.452), but there was a trend for reaction time differences (t32 = 2.01, P = 0.053) such that those with higher A1c levels (i.e. the poorly controlled group) had longer reaction times (see Fig. 1B).
Sensor-level results
Statistical analysis of the time-frequency spectrograms showed significant oscillatory responses in 2 distinct windows. These included a 9–18 Hz oscillatory response during encoding and a 7–11 Hz response during maintenance (P < 0.001, corrected, see Fig. 2). Imaging these responses indicated that parieto-occipital regions were engaged during early encoding and that activity spread anterior to include temporal and frontal regions during maintenance.
Fig. 2.
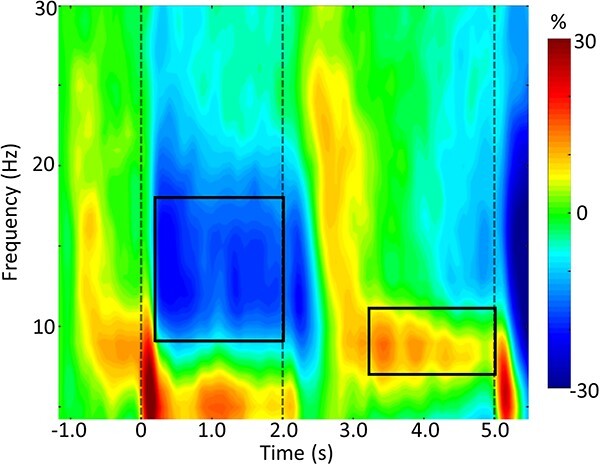
Working memory spectrogram. A grand-averaged time-frequency spectrogram is shown, which was derived from a representative parieto-occipital MEG sensor. Time is shown on the x-axis in seconds, and frequency is shown on the y-axis in Hz. The colors reflect power increases (red) and decreases (blue) relative to the baseline, and the scale bar is shown to the right. Time-frequency windows for source imaging (beamforming) were derived from the statistical analysis of the sensor-level data across all participants (Ps < 0.001, corrected). A distinct extended alpha/low-beta decrease can be seen throughout the encoding period, followed by a narrower band alpha increase (i.e. synchronization) during maintenance.
Functional mapping results
Working memory dynamics altered with level of glycemic control
To examine differences between the poorly and tightly controlled groups, a whole brain t-test approach was performed separately for the encoding and maintenance time windows. During encoding, participants in the lower A1c group (tightly controlled) exhibited stronger alpha/low-beta decreases in the left middle and inferior temporal regions compared to those in the higher A1c group (P < 0.05, corrected; see Fig. 3). Left inferior frontal and right precentral gyri also showed group differences such that those in the poorly controlled group exhibited weaker alpha/low-beta increases relative to those in the well-controlled group (P < 0.05).
Fig. 3.
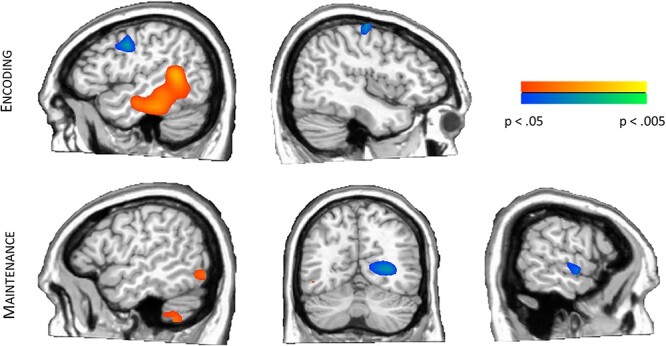
Differences in working memory neural oscillatory responses by glycemic control level. (Top) Those with tightly controlled glycemic levels (A1c < 7.0%) showed stronger alpha/low-beta decreases during encoding in left temporal regions, while those with poor glycemic control exhibited weaker alpha/low-beta increases in the left inferior frontal and right precentral regions. (Bottom) Participants with poorer glycemic control (A1c > 7.0%) had greater alpha increases in left lateral occipital and cerebellum and had greater alpha decreases in right temporal regions during the maintenance period. However, those with tightly controlled glycemic levels had greater alpha activity in right occipital regions in maintenance. Data are thresholded P < 0.05 to P < 0.005.
During the maintenance phase, groupwise effects were found in right superior temporal, right medial occipital, left lateral occipital, and left cerebellar regions (P < 0.05, see Fig. 3). Specifically, participants in the poorly controlled group exhibited significantly stronger alpha decreases in the right superior temporal cortices relative to the well-controlled group. The other 3 significant responses were alpha synchronizations, with those in the well-controlled group exhibiting more strongly increased alpha in the right medial occipital peak, while both the left lateral occipital and left cerebellar peaks reflected stronger alpha increases in the more poorly controlled group. Finally, peak differences were not significantly correlated with age (Ps of 0.072–0.571) or duration (Ps of 0.109–0.952).
Altered neural dynamics relate to behavioral metrics
Next, we evaluated neuro-behavioral effects. During encoding, both the left middle (Fisher’s Z = 2.13, P = 0.033) and inferior (Fisher’s Z = 2.16, P = 0.031) temporal peaks were significantly correlated with reaction time in those with poorly controlled A1c (middle temporal: r15 = 0.61, P = 0.009; inferior temporal: r15 = 0.69, P = 0.002) such that stronger alpha/low-beta decreases were related to faster reaction times, whereas no relationship between these variables was seen in those with tightly controlled A1c levels (middle temporal: r15 = 0.21, P = 0.419; inferior temporal: r15 = 0.14, P = 0.592; see Fig. 4).
Fig. 4.
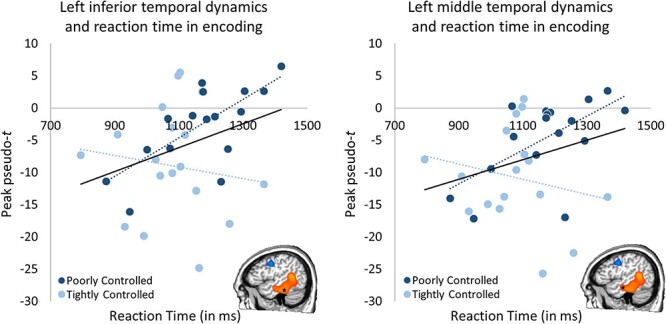
Encoding neuro-behavioral correlations. Temporal dynamics during encoding significantly related to reaction time in both the left inferior (left; Fisher’s Z = 2.16, P = 0.031, poorly controlled: r15 = 0.69, P = 0.002 tightly controlled: r15 = 0.14, P = 0.592) and left middle (right; Fisher’s Z = 2.13, P = 0.033, poorly controlled: r15 = 0.61, P = 0.009, tightly controlled: r15 = 0.21, P = 0.419) temporal regions but only in the poorly controlled group. Stronger alpha/low-beta decreases in these regions were associated with faster reaction times specifically in the poorly controlled glycemic level group (A1c > 7.0%), where overall weaker oscillatory activity during encoding in these regions led to longer reaction times. Dashed lines represent each group’s slope (light blue = tightly controlled; darker blue = poorly controlled), while the solid lines represent the overall (all subjects) slope of the relationship.
Likewise, during maintenance, left lateral occipital activity was significantly related to accuracy across both groups such that stronger alpha increases were associated with lower accuracy (r32 = 0.48, P = 0.005), but this effect was largely driven by the tightly controlled A1c group (Fisher’s Z = 2.06, P = 0.039; tightly controlled A1c: r15 = 0.79, P < 0.001, poorly controlled A1c: r15 = 0.27, P = 0.295; Fig. 5). Further, neural activity in the left lateral occipital were also significantly related to the reaction time across groups (r32 = 0.36, P = 0.043) such that stronger activity were related to slower reaction times, but this effect was not significantly different between groups (Fisher’s Z = 0.84, P = 0.401). Similarly, activity in the left cerebellar peak was significantly related to reaction time across both groups (r32 = 0.42, P = 0.017; Fig. 5) such that stronger alpha increases were associated with longer reaction times, but again this effect was not significantly different between groups (Fisher’s Z = 1.77, P = 0.077). These results highlight potential deviations in processing and neural oscillatory dynamics by A1c level.
Fig. 5.
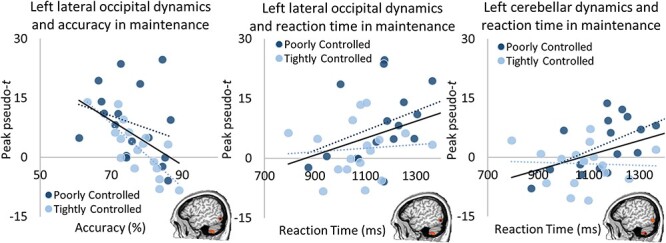
Maintenance neuro-behavioral correlations. Lateral occipital responses during maintenance were significantly related to both accuracy (left, r32 = 0.48, P = 0.005) and reaction time (right, r32 = 0.36, P = 0.043). Greater alpha increases in this region during maintenance yielded worse accuracy, driven by the tightly controlled glycemic group (Fisher’s Z = 2.06, P = 0.039; tightly controlled A1c: r15 = 0.79, P < 0.001, poorly controlled A1c: r15 = 0.27, P = 0.295), and longer reaction times across both groups (Fisher’s Z = 0.84, P = 0.401). Left cerebellar dynamics and the relationship to reaction time mirrored the effects in the left lateral occipital cortex (r32 = 0.42, P = 0.017) with a trend in the effect by group (Fisher’s Z = 1.77, P = 0.077). Dashed lines represent each group’s slope (light blue = tightly controlled; darker blue = poorly controlled), while the solid lines represent the overall (all subjects) slope of the relationship.
Discussion
We found that the level of glycemic control significantly affected the neural dynamics serving encoding and maintenance phases of the working memory task. During encoding, type 2 diabetes patients with tightly controlled A1c levels (<7.0%) showed stronger left inferior frontal, right precentral, and left temporal alpha/low-beta responses than those with poorly controlled diabetes (A1c levels >7.0%). In maintenance, those with poorly controlled diabetes had greater alpha activity in right temporal, left lateral occipital, and left cerebellum and had lower activity in the right occipital cortex than those with tightly controlled diabetes. Working memory tasks, particularly those with language-based stimuli, tend to recruit left lateralized regions, beginning in occipital and spreading anterior to temporal and frontal regions throughout the encoding period, and incorporating parietal regions in maintenance to disengage from distracting information (Brookes et al. 2011; Bonnefond and Jensen 2012; Heinrichs-Graham and Wilson 2015; Proskovec et al. 2016). Poor glycemic control was associated with alterations in temporal, frontal, occipital and parietal dynamics, suggesting a widespread neural impact of chronic dysglycemia. Below, we discuss the implications of these findings for understanding the broad impact of glycemic regulation in type 2 diabetes.
Previous literature has found similar cognitive and neural decrements relative to elevated glycemic levels (McCrimmon et al. 2012; Geijselaers et al. 2015), although many of these studies used fMRI which can be affected by the vasculature damage that is known to occur with chronic dysglycemia. Previous studies similarly have shown a significant relationship between elevated A1c levels and diminished resting temporal dynamics (Xia et al. 2013), mirroring the directionality seen in the present study during encoding. Using glycemic clamp and neuropsychological methods, acute dysglycemia has been shown to yield cognitive decrements in multiple domains, including working memory (Sommerfield et al. 2004), and at least 1 glycemic clamp study showed diminished neural responses when a working memory task was completed during acute hyperglycemia (Backeström et al. 2021). In addition, a previous fMRI study of working memory in type 2 diabetes has shown increased regional recruitment in frontal–parietal regions compensating for reduced functional connectivity between these regions (Zhang et al. 2016). Further, this increased regional recruitment scaled with performance (Zhang et al. 2016).
Many of the group differences found in the current working memory study were consistent with those affected by normative aging processes (Proskovec et al. 2016). In particular, we found stronger oscillations in posterior cortices during maintenance in those with poorly controlled glycemic levels. Similarly, older healthy aging adults exhibit stronger responses than younger adults across these same brain regions during maintenance, likely reflecting the compensatory mechanisms assisting in blocking distracting stimuli and maintaining the fidelity of the encoded response during maintenance (Proskovec et al. 2016). Likewise, the increased bilateral recruitment we find throughout the maintenance period likely reflects compensation by right homologous regions to complete task goals, as also found in aging studies (Proskovec et al. 2016).
Interestingly, several of the responses in the current study also related to behavior. For example, the encoding response in both the left inferior and middle temporal regions were related to behavior, with the amplitude of these responses inversely scaling with reaction time such that stronger activity was associated with faster reaction times, and this effect was largely driven by the group with uncontrolled diabetes. This impact on behavior likely reflects a diminished neural efficiency in task-relevant regions of those, with higher A1c levels leading to worse behavioral outcomes. Conversely, increased left lateral occipital activity during maintenance was seemingly detrimental to performance, with activity in this region related to both accuracy and reaction time such that stronger responses were associated with both lower accuracy and longer reaction times. While this response was related to behavior, the effect did not differ by group, demonstrating the direct impact of glycemic control on brain–behavior relationships even in the context of controlled glycemic levels.
The mechanism thought to underlie specific deficits attributable to type 2 diabetes directly is impaired insulin signaling in the brain. Insulin is involved in a vast number of neural operations across the cortex and subcortical structures, with increased concentration in the thalamus, hippocampus, and cerebellum (Kullmann et al. 2016; Arnold et al. 2018). At the cellular level, it has a role in the regulation of key receptors (i.e. NMDA, GABA, and AMPA), the development of synapses and dendritic spines, and astrocytic inflammatory signaling (Arnold et al. 2018). Insulin levels have been linked to cognitive performance in the domains of memory, processing speed, and executive functioning (Kullmann et al. 2016). Studies of intranasal insulin administration in particular have found acute increased memory performance following administration (Kullmann et al. 2015). Insulin resistance also induces inflammatory processes through several mechanisms, including the production of advanced glycation end products (AGEs) (Yaffe et al. 2011; Chatterjee and Mudher 2018). Increased AGEs have been shown to lead to worse cognitive outcomes regardless of diabetes status (Adams et al. 2016). There is also evidence of insulin resistance contributing to the accumulation of amyloid and tau (Kullmann et al. 2016; Chatterjee and Mudher 2018), which may underlie the increased risk for Alzheimer’s disease in type 2 diabetes.
The evidence is mixed on the effects of strict glycemic control despite the clear links of hyperglycemia to cognitive decrements. Several studies, including the large ACCORD-MIND study (Cooray et al. 2011; Moheet et al. 2015), have shown modest immediate improvements in cognitive measures with tighter glycemic control, although at longer follow-up intervals, effects are not significant (Moheet et al. 2015). At least a few studies have shown negative impacts of strict glycemic control (Zammitt and Frier 2005; Launer et al. 2011; Seaquist 2015), likely due to the periodic induction of hypoglycemia when trying to overcompensate for hyperglycemic episodes.
While this study made major strides in elucidating the impact of type 2 diabetes on specific neural circuits underlying higher-order cognition, some limitations should be acknowledged. First, we examined the impact of glycemic control using the coarse A1c measure, where continuous glucose monitoring may have offered a finer grain look at glucose metrics, including glycemic variability. Examining the influence of hyperglycemic peaks and hypoglycemic troughs could reveal the key details behind the deficits scaling with A1c. Further, it might reflect a multiple hit-type model, where these discrete events are specifically inducing damage rather than (or in addition to) the general background glycemic level chronically causing damage. Future studies should examine these variables more in depth to further clarify their causal links to the observed neural deficits. Second, the participants found the current task fairly difficult even though none had apparent or diagnosed neurological deficits at this stage. This may affect the generalizability of the study to the general population of those with type 2 diabetes as they age, particularly those with diagnosed neurocognitive deficits. Additionally, due to the number of exclusions, we may have introduced some selection bias into our sample. While exclusions occurred for each of the glycemic level groups, there were a greater number excluded with higher A1c values (14 poorly controlled vs. 6 within the tightly controlled group). Notably, the scope and goals of the current study were limited to those without apparent deficits in order to examine the effects more directly of diabetes rather than possible confounding conditions like dementia. Specifically, for participants that completed the NIH toolbox cognitive battery, participants generally had near-population mean standard scores and these did not differ by group. Further, no cognitive deficits were noted in the medical records of any of our participants. Future studies should examine the effects of working memory load in this population to determine whether these alterations are also found at various other smaller working memory loads and in those with apparent deficits to examine the trajectory of these changes with severity of neurocognitive effects. Notably, the current approach focused on the dynamics within the alpha and alpha/low-beta bands, similar to our approach in a previous study of individuals with type 1 diabetes. Previous studies have also found effects across the frequency spectrum, particularly in theta and gamma. Dynamics across other frequency bands should be examined in future studies across a variety of contexts and in relation to working memory processes.
Conclusion
In the current study, we found specific alterations in working memory processing by the level of glycemic control. An overall suppression of responses in task-relevant regions was found in those with poorly controlled A1c levels during working memory encoding, with distinct effects of glycemic control status on neural dynamics during working memory maintenance. In particular, participants with poorly controlled diabetes showed stronger responses in several key brain regions, with only 1 occipital cluster showing a greater response in participants with tightly controlled A1c. These findings suggest that glycemic control has a direct and major impact on cognitive and neural processing in those with type 2 diabetes.
Acknowledgments
We thank our participants for volunteering their time to the current study.
Contributor Information
Christine M Embury, Institute for Human Neuroscience, Boys Town National Research Hospital, Boys Town, NE 68010, United States; Department of Psychology, University of Nebraska, Omaha, NE 68182, United States.
Grace H Lord, Department of Internal Medicine, Division of Diabetes, Endocrinology, and Metabolism, UNMC, Omaha, NE 68198, United States.
Andjela T Drincic, Department of Internal Medicine, Division of Diabetes, Endocrinology, and Metabolism, UNMC, Omaha, NE 68198, United States.
Cyrus V Desouza, Department of Internal Medicine, Division of Diabetes, Endocrinology, and Metabolism, UNMC, Omaha, NE 68198, United States.
Tony W Wilson, Institute for Human Neuroscience, Boys Town National Research Hospital, Boys Town, NE 68010, United States; Department of Psychology, University of Nebraska, Omaha, NE 68182, United States.
CRediT authors statement
Christine M. Embury (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing), Grace H. Lord (Data curation, Project administration, Writing—review & editing), Andjela T. Drincic (Conceptualization, Investigation, Project administration, Supervision, Writing—review & editing), Cyrus V. Desouza (Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing—review & editing), and Tony W. Wilson (Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing—review & editing)
Funding
This work was supported by the National Institutes of Health (R01-MH103220, R01-MH116782, R01-MH118013, and P20-GM144641 to TWW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement: Authors CME, GHL, ATD, and TWW have no conflicts of interest to declare. CVD is a consultant with NovoNordisk, Bayer, and AstraZeneca and receives research funding from Theracos and Sanofi.
Data availability
Data are available upon reasonable request to the corresponding author.
Prior presentation
These data have been presented in abstract/poster form at the International Conference for Biomagnetism, held in Birmingham, England, United Kingdom, 28 August–1 September 2022.
References
- Adams JN, Martelle SE, Raffield LM, Freedman BI, Langefeld CD, Hsu FC, Maldjian JA, Williamson JD, Hugenschmidt CE, Carr JJ, et al. Analysis of advanced glycation end products in the DHS Mind Study. J Diabetes Complicat. 2016:30:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P. Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer’s disease. Discov Med. 2013:16:277–286. [PubMed] [Google Scholar]
- Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang H-Y, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018:14:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AD. 6. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021:44:S73–S84. [DOI] [PubMed] [Google Scholar]
- Backeström A, Papadopoulos K, Eriksson S, Olsson T, Andersson M, Blennow K, Zetterberg H, Nyberg L, Rolandsson O. Acute hyperglycaemia leads to altered frontal lobe brain activity and reduced working memory in type 2 diabetes. PLoS One. 2021:16:e0247753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol. 2012:22:1969–1974. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Wood JR, Stevenson CM, Zumer JM, White TP, Liddle PF, Morris PG. Changes in brain network activity during working memory tasks: a magnetoencephalography study. NeuroImage. 2011:55:1804–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Mudher A. Alzheimer’s disease and type 2 diabetes: a critical assessment of the shared pathological traits. Front Neurosci. 2018:12:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray G, Nilsson E, Wahlin Å, Laukka EJ, Brismar K, Brismar T. Effects of intensified metabolic control on CNS function in type 2 diabetes. Psychoneuroendocrinology. 2011:36:77–86. [DOI] [PubMed] [Google Scholar]
- Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, Coker LH, Murray A, Sullivan MD, Marcovina SM, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009:32:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury CM, Wiesman AI, Proskovec AL, Heinrichs-Graham E, McDermott TJ, Lord GH, Brau KL, Drincic AT, Desouza CV, Wilson TW. Altered brain dynamics in patients with type 1 diabetes during working memory processing. Diabetes. 2018:67:1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinkohl I, Price JF, Strachan MWJ, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther. 2015:7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijselaers SL, Sep SJ, Stehouwer CD, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015:3:75–89. [DOI] [PubMed] [Google Scholar]
- Grober E, Hall CB, Hahn SR, Lipton RB. Memory impairment and executive dysfunction are associated with inadequately controlled diabetes in older adults. J Prim Care Community Health. 2011:2:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hämäläinen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A. 2001:98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. Spatiotemporal oscillatory dynamics during the encoding and maintenance phases of a visual working memory task. Cortex. 2015:69:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. Attention training improves aberrant neural dynamics during working memory processing in veterans with PTSD. Cogn Affect Behav Neurosci. 2016:16(6):1140–1149 PMC5154910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killanin AD, Embury CM, Picci G, Heinrichs-Graham E, Wang YP, Calhoun VD, Stephen JM, Wilson TW. Trauma moderates the development of the oscillatory dynamics serving working memory in a sex-specific manner. Cereb Cortex. 2022:32(22):5206–5215 PMC9667155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Fritsche A, Preissl H. Insulin action in the human brain: evidence from neuroimaging studies. J Neuroendocrinol. 2015:27:419–423. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring H-U. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016:96:1169–1209. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, Sullivan M, Horowitz KR, Ding J, Marcovina S, et al. Effects of randomization to intensive glucose lowering on brain structure and function in type 2 diabetes ACCORD memory in diabetes study. Lancet Neurol. 2011:10:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007:164:177–190. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012:379:2291–2299. [DOI] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Bar-Haim Y, Pine DS, Khanna MM, Heinrichs-Graham E, Wilson TW. Attention training improves aberrant neural dynamics during working memory processing in veterans with PTSD. Cogn Affect Behav Neurosci. 2016a:16(6):1140–1149 PMC5154910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Khanna MM, Heinrichs-Graham E, Wilson TW. Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing: an MEG study. J Psychiatry Neurosci. 2016b:41(4):251–260 PMC4915934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. 2015:1353:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott V, Benedict C, Schultes B, Born J, Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab. 2012:14:214–221. [DOI] [PubMed] [Google Scholar]
- Palta P, Schneider ALC, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014:20:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wilson TW. Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Hum Brain Mapp. 2016:37:2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wilson TW. Load modulates the alpha and beta oscillatory dynamics serving verbal working memory. NeuroImage. 2019:184:256–265 PMC6230485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Ruis C, Jaap Kappelle L, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010:26:507–519. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage. 2012:60:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaquist ER. The impact of diabetes on cerebral structure and function. Psychosom Med. 2015:77:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care. 2004:27:2335–2340. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006:51:1759–1768. [DOI] [PubMed] [Google Scholar]
- Tschritter O, Hennige AM, Preissl H, Grichisch Y, Kirchhoff K, Kantartzis K, Machicao F, Fritsche A, Haring HU. Insulin effects on beta and theta activity in the human brain are differentially affected by ageing. Diabetologia. 2009:52:169–171. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 1997:35:135–140. [DOI] [PubMed] [Google Scholar]
- West RK, Ravona-Springer R, Schmeidler J, Leroith D, Koifman K, Guerrero-Berroa E, Preiss R, Hoffman H, Silverman JM, Heymann A, et al. The association of duration of type 2 diabetes with cognitive performance is modulated by long-term glycemic control. Am J Geriatr Psychiatry. 2014:22:1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, McDermott TJ, Santamaria PM, Gendelman HE, Wilson TW. Quiet connections: reduced fronto-temporal connectivity in nondemented Parkinson’s disease during working memory encoding. Hum Brain Mapp. 2016:37:3224–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Proskovec AL, Heinrichs-Graham E, O’Neill J, Robertson KR, Fox HS, Swindells S. Aberrant neuronal dynamics during working memory operations in the aging HIV-infected brain. Sci Rep. 2017:7:41568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996:4:58–73. [DOI] [PubMed] [Google Scholar]
- Xia W, Wang S, Sun Z, Bai F, Zhou Y, Yang Y, Wang P, Huang Y, Yuan Y. Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology. 2013:38:2493–2501. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Schwartz AV, Vitartas C, Vittinghoff E, Satterfield S, Simonsick EM, Launer L, Rosano C, Cauley JA, et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011:77:1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005:28:2948–2961. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu S, Liu C, Zhang H, Zhou X, Ni C, Qin W, Zhang Q. Altered brain activation and functional connectivity in working memory related networks in patients with type 2 diabetes: an ICA-based analysis. Sci Rep. 2016:6:23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.


