Abstract
In rodents and nonhuman primates, sex hormones are powerful modulators of dopamine (DA) neurotransmission. Yet less is known about hormonal regulation of the DA system in the human brain. Using positron emission tomography (PET), we address this gap by comparing hormonal contraceptive users and nonusers across multiple aspects of DA function: DA synthesis capacity via the PET radioligand 6-[18F]fluoro-m-tyrosine ([18F]FMT), baseline D2/3 receptor binding potential using [11C]raclopride, and DA release using methylphenidate-paired [11C]raclopride. Participants consisted of 36 healthy women (n = 15 hormonal contraceptive users; n = 21 naturally cycling/non users of hormonal contraception), and men (n = 20) as a comparison group. A behavioral index of cognitive flexibility was assessed prior to PET imaging. Hormonal contraceptive users exhibited greater DA synthesis capacity than NC participants, particularly in dorsal caudate, and greater cognitive flexibility. Furthermore, across individuals, the magnitude of striatal DA synthesis capacity was associated with cognitive flexibility. No group differences were observed in D2/3 receptor binding or DA release. Analyses by sex alone may obscure underlying differences in DA synthesis tied to women’s hormone status. Hormonal contraception (in the form of pill, shot, implant, ring, or intrauterine device) is used by ~400 million women worldwide, yet few studies have examined whether chronic hormonal manipulations impact basic properties of the DA system. Findings from this study begin to address this critical gap in women’s health.
Keywords: cognitive flexibility, dopamine, hormonal contraception, PET imaging
Sex hormones are powerful neuromodulators of learning and memory (Taxier et al. 2020). Accumulating evidence suggests that sex hormones’ influence extends to the regulation of dopamine (DA) (Becker 1999; Barth et al. 2015; Sun et al. 2016; Yoest et al. 2018), itself a neuromodulator of higher order cognitive functions (Iversen and Iversen 2007; Cools et al. 2008; Cools and Arnsten 2022). In rodents and nonhuman primates, 17β-estradiol (E2) and progesterone (P) modulate DA synthesis and release, alter DA-D2 receptor availability, and modify the basal firing rate of dopaminergic neurons (Dluzen and Ramirez 1984, 1990; Becker and Cha 1989; Lévesque et al. 1989; Pasqualini et al. 1995; Czoty et al. 2009; Asghari et al. 2011). For example, E2 administration produces a dose-dependent increase in striatal DA (Pasqualini et al. 1995) and modulates goal-directed behavior (Uban et al. 2012) in rodents. Progesterone has a bimodal effect on striatal DA concentration, with increases in DA in the first 12 h after P perfusion, and inhibitory effects 24 h post-infusion. Furthermore, surgical removal of the ovaries reduces tyrosine hydroxylase (TH)-immunoreactive neurons in the substantia nigra (Leranth et al. 2000) and prefrontal cortex (PFC; Kritzer and Kohama 1998). Estrogen receptors are localized to regions that receive major projections from midbrain DA neurons, including PFC, dorsal striatum, and the nucleus accumbens (Björklund and Dunnett 2007). Despite the substantial literature supporting sex hormones’ role in DA neuromodulation in rodents and nonhuman primates, less is known about hormonal regulation of the DA system in the human brain.
Indirect evidence in humans suggests that estradiol modulates DA-dependent cognitive function and PFC activity (Jacobs and D’Esposito 2011; Jacobs et al. 2016, 2017; Diekhof et al. 2021). For example, Jacobs and D’Esposito (2011) found evidence that estradiol regulates PFC activity and working memory performance, and the direction of the effect depends on an individual’s basal PFC DA tone (indexed by catechol-O-methyltransferase activity). Additional evidence suggests that menstrual cycle phase influences DA-dependent motor and cognitive functions, including response time on tests of manual coordination, working memory, and cognitive flexibility (Hampson and Kimura 1988; Hidalgo and Pletzer 2017), and immediate reward selection bias (Smith et al. 2014).
Molecular positron emission tomography (PET) imaging provides a more direct assessment of dopaminergic activity in vivo in the human brain. Sex differences have been observed in DA synthesis capacity (Laakso et al. 2002), DA release (Munro et al. 2006; Riccardi et al. 2006; Manza et al. 2022), and DA transporter density (Lavalaye et al. 2000; Mozley et al. 2001). PET studies of women sampled in different phases of the menstrual cycle and menopausal transition suggest a role for sex steroid hormones in modulating aspects of DA functioning. Wong et al. (1988) observed fluctuations in DA-D2 receptor density across the menstrual cycle in healthy premenopausal women, and Pohjalainen et al. (1998) observed a greater variability in DA-D2 receptor density in premenopausal vs postmenopausal women, with the suggestion that greater variability was attributable to hormonal fluctuations across the menstrual cycle (but see Nordström et al. 1998; Smith et al. 2019; Petersen et al. 2021).
An underexplored population for studying hormonal influences on DA function is women using hormonal contraception. Hormonal contraception (HC; in the form of pill, shot, implant, ring, or intrauterine device (IUD)) is used by ~400 million women worldwide (United Nations 2019), yet few studies have examined whether chronic hormone manipulations affect basic properties of the DA system. In the present analyses, we leveraged data from a well-characterized cohort (Berry et al. 2018a, 2019; Furman et al. 2020, 2021) to probe the impact of hormonal contraception on multiple properties of the DA system using molecular PET imaging techniques, offering new insights into the relationship between endocrine status and DA neurotransmission in the human brain. The study consisted of young, healthy women and men, and paired pharmacological manipulation of the DA system with PET imaging to assess synthesis capacity (radioligand [18F]fluoro-m-tyrosine, [18F]FMT), D2 receptor availability (radioligand [11C]raclopride), and DA release (radioligand [11C]raclopride paired with methylphenidate). This provides a unique opportunity to characterize differences in DA synthesis capacity, basal DA receptor occupancy, and stimulated DA release in a single cohort. Next, we investigated sex differences in DA neurotransmission. Finally, we examined whether differences in DA neurotransmission were associated with DA-dependent cognition, using a behavioral assessment of cognitive flexibility (Berry et al. 2018b; Furman et al. 2020). Leveraging an existing multimodal molecular PET imaging cohort allowed us to look at broad differences in DA neurotransmission between naturally cycling women (i.e. those who self-report not using any hormone-based medication) and those using hormonal contraception. These results offer researchers a strong rationale for designing additional imaging studies that explicitly test the breadth and depth with which hormonal contraceptives influence major neuromodulatory systems.
Materials and methods
Participants
Participants consisted of 57 adults (mean age = 21.16 years, SD = 2.37, range: 18–28 years), including 37 women and 20 men (n = 28 Asian, 10 Hispanic or Latino, 9 White (not Hispanic or Latino), 2 Black or African American, 3 more than one race or ethnicity, 2 other, and 3 declined to state). Participants were originally recruited and underwent PET and MRI imaging as part of a parent study on dopaminergic mechanisms of cognitive control (e.g. see Furman et al. 2020). PET data from this sample have previously been described in Berry et al. (2018a). This study was approved by Institutional Review Boards at the University of California, Berkeley and Lawrence Berkeley National Laboratory, and the experiments were undertaken with the understanding and written consent of each subject. Data captured regarding participants’ hormonal contraceptive use allowed for post hoc comparisons between current users and current non-users of hormonal contraception. Participants met the following eligibility criteria: (i) 18–30 years old, (ii) right-handed, (iii) current weight of at least 100 pounds, (iv) able to read and speak English fluently, (v) nondrinker or light drinker (women: <7 alcoholic drinks/week; men: <8 alcoholic drinks/week), (vi) no recent history of substance abuse, (vii) no history of neurological or psychiatric disorder as confirmed by clinician interview, (viii) no current psychoactive medication or street drug use, (ix) not pregnant, and (x) no contraindications to MRI. Most participants completed 3 PET scans over the course of 2 separate sessions: [18F]FMT, and [11C]raclopride + placebo and [11C]raclopride + methylphenidate on the same day; the exceptions were 1 participant (an NC woman) who did not complete the FMT scan due to technical issues, 1 participant (an NC woman) who did not produce reliable Raclopride scan data due to technical issues, and 2 participants (hormonal contraceptive users) who did not complete Raclopride scans.
Participants also completed a listening span test (Salthouse and Babcock 1991) and the Barratt Impulsivity Scale (Patton et al. 1995) to assess working memory capacity and trait impulsivity, respectively.
[18F]FMT sample
Women were categorized based on hormone status: naturally cycling (NC, no current reported use of hormonal contraception; n = 21, avg. age = 22.67 years, SD = 2.77) and current users of hormonal contraception (HC, n = 15, avg. age = 20.43 years, SD = 1.91). Types of hormonal contraception used included: combined oral contraception (OC, n = 10), vaginal ring (n = 1), implant (n = 2), injection (n = 1), and hormonal IUD (n = 1).
[11C]Raclopride sample
[11C]Raclopride data from 1 NC participant did not pass quality control and 2 HC users (combined OC) did not have [11C]raclopride sessions, yielding a final sample of 21 NC women (avg age = 20.67, SD = 1.91) and 13 HC users (avg age = 22.69, SD = 2.81).
In our secondary analyses, participants were grouped by self-reported sex (male, n = 20; female, n = 37), and hormone status (male, NC, and HC). Men and women did not differ significantly in age or BMI; however, HC users were older than males (p = 0.03, d = 0.85) and NC participants (p = 0.01, d = 0.94) by 25 months on average (Table 1).
Table 1.
Participant demographics by sex and hormone status.
| Age | BMI | |
|---|---|---|
| Men (n = 20) | 20.7 ± 2.1 | 23.8 ± 5.3 |
| Women (n = 37) | 21.4 ± 2.5 | 23.7 ± 4.2 |
| Naturally Cycling (n = 22) | 20.6 ± 2.0 | 23.0 ± 3.9 |
| Hormonal Contraception (n = 15) | 22.7 ± 2.8 | 24.7 ± 4.6 |
| NC vs HC Cohen’s d (Welch’s p) | 0.94 (0.01a) | n.s. |
| Men vs Women | n.s. | n.s. |
| Men vs NC vs HC Kruskal–Wallis p | 0.03 | n.s. |
Types of HC used (n): combined OC (10), vaginal ring (1), implant (2), injection (1), and hormonal IUD (1). NC, naturally cycling; HC, hormonal contraception.
aIndicates significance with Bonferroni correction (P < 0.0167).
As trait-like measures of working memory span, impulsivity, and smoking status have been associated with striatal DA neurotransmission (Landau et al. 2009; Smith et al. 2018; Ashok et al. 2019), we also considered whether our groups differed by these phenotypes. Neither baseline working memory (listening span score) F(2) = 0.27, p = 0.77 (HC: mean = 3.07, SD = 0.98; NC: mean = 2.91, SD = 0.81; Male: mean = 2.85, SD = 0.89), nor trait impulsivity (Barrett Impulsivity Scale score) F(2) = 0.50, p = 0.61 (HC: mean = 55.80, SD = 8.75; NC: mean = 57.86, SD = 5.86; Male: mean = 58.60, SD = 10.25) differed between groups; and a previous characterization of participants from this parent data set found a very low incidence of nicotine use (Berry et al. 2018a).
Structural MRI scan
Images were acquired using a Siemens 3T Trio Tim scanner with a 12-channel coil. Each participant was scanned on 3 occasions using a high-resolution T1-weighted magnetization prepared rapid gradient echo (MPRAGE) whole-brain scan (TR = 2,300 ms; TE = 2.98 ms; FA = 9°; matrix = 240 × 256; FOV = 256; sagittal plane; voxel size = 1 × 1 × 1 mm; 160 slices). The 3 MPRAGE scans were aligned, averaged, and segmented using FreeSurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu/) and the averaged template was used for coregistration with the PET data.
[18F]FMT PET data acquisition
Participants underwent an [18F]FMT PET scan to measure DA synthesis capacity. Detailed methods are provided in Berry et al. (2018b). PET data were acquired using a Siemens Biograph Truepoint 6 PET/CT scanner (Siemens Medical Systems, Erlangen, Germany) ~1 h after participants ingested 2.5 mg/kg of carbidopa to minimize the peripheral decarboxylation of [18F]FMT. After a short CT scan, participants were injected with ~2.5 mCi of [18F]FMT as a bolus in an antecubital vein (M ± SD; specific activity = 947.30 ± 140.26 mCi/mmol; dose = 2.43 ± 0.06 mCi). Dynamic acquisition frames were obtained over 90 min in 3D mode (25 frames total: 5 × 1, 3 × 2, 3 × 3, 14 × 5 min). Data were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation, corrected for scatter, and smoothed with a 4 mm full width at half maximum kernel.
[11C]Raclopride PET data acquisition
Participants received two [11C]raclopride PET scans an average of 21.65 days before or after the [18F]FMT scan (median = 7 days) to measure D2/3 receptor occupancy and DA release. To measure baseline D2/3 receptor occupancy, participants ingested a placebo pill ~1 h before [11C]raclopride scan 1. The placebo scan was always performed first. To measure DA release, participants ingested 30 mg (M ± SD mg/kg: 0.46 ± 0.08) of methylphenidate ~1 h before [11C]raclopride scan 2. Endogenous DA release was measured as the percent signal change (PSC) in nondisplaceable binding potential (BPND) from [11C]raclopride scans 1 to 2 ((placebo [11C]raclopride − methylphenidate [11C]raclopride)/placebo [11C]raclopride). Scans were conducted on the same day, 2 h apart and participants were blind to whether placebo or methylphenidate was administered. For both [11C]raclopride scans 1 and 2, after a short CT scan, participants were injected with ~10 mCi of [11C]raclopride as a bolus in an antecubital vein. Dynamic acquisition frames were obtained over 60 min in 3D mode (19 frames total: 5 × 1, 3 × 2, 3 × 3, 8 × 5). Reconstruction was performed as described above.
PET data analysis
PET data were preprocessed using SPM8 software (Friston et al. 2007). To correct for motion between frames, images were realigned to the middle frame. The first 5 images were summed prior to realignment to improve realignment accuracy, as these early images have relatively low signal contrast. Structural images were coregistered to PET images using the mean image of frames corresponding to the first 20 min of acquisition as a target. The mean image for the first 20 min was used rather than the mean image for the whole scan time because it provides a greater range in image contrast outside of striatum thus making it a better target for coregistration.
[18F]FMT
Graphical analysis for irreversible tracer binding was performed using Patlak plotting (Patlak and Blasberg 1985; Sossi et al. 2003) implemented using in-house software and MATLAB version 8.2 (The MathWorks, Natick, MA). Without measurement of the arterial input function, [18F]FMT PET analysis used reference region models. Cerebellar gray matter was used as the reference region because this region shows very little tracer uptake, and has an extremely low density of DA receptors and metabolites relative to striatum (Farde et al. 1986; Camps et al. 1989; Levey et al. 1993; Hall et al. 1994). The most anterior, one-fourth of cerebellar gray matter, was removed from the reference region to limit contamination of signal from the substantia nigra and ventral tegmental area. Ki images were generated from PET frames corresponding to 25–90 min (Ito et al. 2006, 2007), which represent the amount of tracer accumulated in the brain relative to the reference region.
[11C]Raclopride
For [11C]raclopride PET, reversible tracer binding was quantified using simplified reference tissue model analysis (SRTM; Lammertsma and Hume 1996). Specifically, a basis function version of the SRTM was applied as previously described (Gunn et al. 1997) with posterior cerebellar gray matter used as the reference region. The SRTM analysis was performed using in-house software provided by Dr. Roger Gunn and MATLAB version 8.2. SRTM analysis was used to determine BPND, which can be defined as: BPND = fNDBavail/KD where Bavail is the concentration of D2/3 receptors, KD is the inverse of the affinity of the radiotracer for D2/3 receptors, and fND is the free fraction of the ligand in the nondisplaceable tissue compartment (Slifstein and Laruelle 2001; Innis et al. 2007).
Regions of interest
An ROI approach was used to test relationships between hormonal status and PET measures of dopaminergic function in striatal subregions. Striatal subregions were manually drawn in native space on each participant’s averaged MPRAGE MRI scan using Mango software. The dorsal caudate, dorsal putamen, and ventral striatum were segmented as described in Mawlawi et al. (2001). Inter-rater reliability was high for manually drawn striatal subregions (see Berry et al. 2018b).
As we did not hypothesize an effect of hemisphere, ROI values for our 3 ROIs (dorsal caudate, dorsal putamen, and ventral striatum) were analyzed as voxel-weighted averages of left and right hemisphere PET values as follows:
(L value × L ROI volume + R value × R ROI volume)/Combined L + R ROI volume.
All analyses on striatal values were conducted on partial volume corrected ROIs (PVC; Rousset et al. 1998). PVC was performed in native space (non-normalized data) and corrects for between-subject differences in the inclusion of white matter and CSF in the measured volumes. To apply the PVC in native space, we used FreeSurfer-generated ROIs for gray matter cortical and subcortical regions, white matter, and cerebral spinal fluid. All statistical analyses were conducted using R (version 1.2.5001).
Cognitive paradigm
The task was an adaptation of the task-switching paradigm developed by Armbruster et al. (2012) and is described in detail in Furman et al. (2020). Briefly, on each trial, participants were required to respond quickly to digits between 1 and 9 (excluding 5) that appeared in different shades of gray against a black background. On 82% of trials, a single digit appeared above a central fixation. For these “ongoing task” trials, participants performed an operation (odd/even or low/high decisions) on the digit and responded by pressing the index finger of either their left or right hand. On the remaining 18% of trials, 2 digits appeared on the screen simultaneously: 1 above and 1 below the fixation cross. The relative brightness of the upper and lower digits varied and encoded a task cue. When the upper digit was brighter (6% of trials), participants were instructed to ignore the lower digit and continue to apply the ongoing task rule to the upper digit (“distractor trials”). When the lower digit was brighter (6% of trials), participants were signaled to switch attention to the lower digit and to apply the alternate task rule to it (“switch trials”). On the final third of these trials (6% of total trials), the difference in brightness between the upper and lower digits was reduced (“ambiguous trials”). Ambiguous trials were not considered in our analyses. Participants performed a total of 990 trials distributed across 3 blocks with brief interposed breaks. Cognitive testing occurred prior to PET imaging. Distractor cost was calculated as the difference between performance accuracy on “distractor” trials and “ongoing” trials, and is thought to reflect cognitive stability, a process sensitive to variation in prefrontal DA. Switch cost was calculated as the difference between performance accuracy on “switch” vs “ongoing” trials and is a putative behavioral marker of cognitive flexibility, reflective of striatal DA. One NC participant did not undergo cognitive testing, resulting in a final sample of 20 NC women (avg age = 20.67, SD = 1.91), 15 HC users (avg age = 22.69, SD = 2.81), and 20 men (avg age = 20.70, SD = 2.08).
Statistical analysis
Impact of hormone status on DA neurotransmission
Since hormonal contraceptive (HC) users were older than NC participants, to compare markers of dopaminergic signaling between HC and NC groups, we conducted 2 × 4 ANCOVA (hormone group × bilateral region of interest, controlling for the effects of age) for measures of interest (FMT Ki, [11C]raclopride BPND and PSC in [11C]raclopride BP). We investigated significant main effects with post hoc 1-way ANCOVAs to determine which regions were driving the effect, controlling for the effects of age. Statistically significant findings that survived Bonferroni correction for multiple comparisons are noted (pBf = 0.05/3 regions = 0.0167). Partial effect sizes ( 2) are reported for statistically significant findings.
2) are reported for statistically significant findings.
Welch’s t-tests were used to compare distractor costs and switch costs between our comparison groups. One NC participant with unusable task data was omitted from these analyses. As a follow-up to observed differences between NC and HC women, switch costs were correlated with [18F]FMT Ki PVC striatal values to evaluate a relationship between performance and DA synthesis.
Sex differences in DA neurotransmission
To compare the aspects of DA signaling by sex and hormone status, we conducted 2 × 3 mixed ANCOVA (group × bilateral region of interest, controlling for age) for measures of interest (FMT Ki, [11C]raclopride BP, and PSC in [11C]raclopride BP).
Welch’s t-tests were conducted to compare distractor costs and switch costs by sex. Additionally, for reference, switch costs were correlated with [18F]FMT Ki PVC striatal values in our male sample.
Finally, to determine whether differences in hormonal status within women influenced the detection of sex differences, we conducted 3 × 3 mixed ANCOVA (group × bilateral region of interest, controlling for age) for each measure of interest (FMT Ki, [11C]raclopride BP, and PSC in [11C]raclopride BP). Significant main effects were investigated using post hoc 1-way ANCOVAs, again to control for the effects of age.
Results
DA neurotransmission differs with hormonal contraceptive use
Striatal [18F]FMT Ki
[18F]FMT PET data were obtained to assess DA synthesis capacity in the striatum. ANCOVA revealed significant main effects of age (F(1,33) = 4.844, p = 0.035,  2 = 0.13), hormone status (F((1,33) = 7.753, p = 0.009,
2 = 0.13), hormone status (F((1,33) = 7.753, p = 0.009,  2 = 0.19, Fig. 1), and region (F(2,68) = 207.859, p < 0.00001,
2 = 0.19, Fig. 1), and region (F(2,68) = 207.859, p < 0.00001,  2 = 0.86). Regional effects were expected as previously reported (Berry et al. 2018a). There was no significant interaction between hormone status and region. Results from post hoc 1-way ANCOVAs indicate that hormonal contraceptive users exhibited greater FMT Ki values compared with NC participants, with the largest effect in dorsal caudate (F(1, 33) = 9.611, pBf = 0.004). Ki values differed marginally between hormonal contraceptive users and NC participants in dorsal putamen (F(1,33) = 3.966, p = 0.055) and ventral striatum (F(1,33) = 3.754, p = 0.061) (Table 2; see Supplementary Table 2 for uncorrected values).
2 = 0.86). Regional effects were expected as previously reported (Berry et al. 2018a). There was no significant interaction between hormone status and region. Results from post hoc 1-way ANCOVAs indicate that hormonal contraceptive users exhibited greater FMT Ki values compared with NC participants, with the largest effect in dorsal caudate (F(1, 33) = 9.611, pBf = 0.004). Ki values differed marginally between hormonal contraceptive users and NC participants in dorsal putamen (F(1,33) = 3.966, p = 0.055) and ventral striatum (F(1,33) = 3.754, p = 0.061) (Table 2; see Supplementary Table 2 for uncorrected values).
Fig. 1.

Effect of hormone status on DA synthesis capacity. [18F]FMT Ki values in naturally cycling females and hormonal contraceptive users by striatal region of interest. Striatal DA synthesis capacity was greater in hormonal contraceptive users relative to naturally cycling women, with the most pronounced effects observed in dorsal caudate.
Table 2.
Dopamine synthesis capacity ([18F]FMT Ki values) by group and striatal region of interest.
| Dorsal caudate | Dorsal putamen | Ventral striatum | |
|---|---|---|---|
| Male | 0.0278 ± 0.0034 | 0.0346 ± 0.0030 | 0.0209 ± 0.0034 |
| Women | 0.0272 ± 0.0033 | 0.0343 ± 0.0037 | 0.0204 ± 0.0049 |
| Naturally Cycling | 0.0256 ± 0.0025 | 0.0331 ± 0.0033 | 0.0190 ± 0.0053 |
| Hormonal Contraception | 0.0295 ± 0.0030 | 0.0360 ± 0.0037 | 0.0224 ± 0.0037 |
HC vs NC partial  2, p-value 2, p-value |
0.23, 0.004a | 0.11, 0.055 | 0.10, 0.061 |
Men vs Women partial 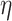 2, p-value 2, p-value |
0.02, n.s. | <0.01, n.s. | <0.01, n.s. |
Men vs NC vs HC partial 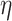 2, p-value 2, p-value |
0.18, 0.006a | 0.08, n.s. | 0.10, 0.064 |
aIndicates significance with Bonferroni correction (p < 0.0167).
n.s. indicates p > 0.10. NC, naturally cycling; HC, hormonal contraception.
Striatal [11C]raclopride BP
[11C]Raclopride PET data were obtained to measure D2/3 receptor binding potential. [11C]Raclopride BP differed significantly by region (F(2,64) = 389.281, p < 0.0001,  2 = 0.92). Regional effects were expected as previously reported (Berry et al. 2018a). There was no significant main effect of age (F(1,31) = 3.795, p = 0.061) or hormone status (F(1,31) = 0.09, p = 0.76) on [11C]raclopride BPND values, nor was there an interaction between hormone status and region (F(2,64) = 0.815, p = 0.447) (see Supplementary Table 1 for values).
2 = 0.92). Regional effects were expected as previously reported (Berry et al. 2018a). There was no significant main effect of age (F(1,31) = 3.795, p = 0.061) or hormone status (F(1,31) = 0.09, p = 0.76) on [11C]raclopride BPND values, nor was there an interaction between hormone status and region (F(2,64) = 0.815, p = 0.447) (see Supplementary Table 1 for values).
PSC in striatal [11C]raclopride BP
Methylphenidate-paired [11C]raclopride PET data were acquired to measure DA release. [11C]Raclopride BP PSC values differed significantly by region (F(2,64) = 389.281, p < 0.0001,  2 = 0.92). Again, regional effects were expected as previously reported (Berry et al. 2018a). There were no significant effects of age (F(1,31) = 3.795, p = 0.061) or hormone status (F(1,31) = 0.092, p = 0.76) on [11C]raclopride BP PSC values, nor was there an interaction between status and region (F(2,64) = 0.815, p = 0.45) (see Supplementary Table 1 for values).
2 = 0.92). Again, regional effects were expected as previously reported (Berry et al. 2018a). There were no significant effects of age (F(1,31) = 3.795, p = 0.061) or hormone status (F(1,31) = 0.092, p = 0.76) on [11C]raclopride BP PSC values, nor was there an interaction between status and region (F(2,64) = 0.815, p = 0.45) (see Supplementary Table 1 for values).
DA neurotransmission does not differ by sex
Striatal [18F]FMT Ki
We observed a main effect of region on FMT values (F(2,108) = 358.424, p < 0.0001,  2 = 0.87), no main effect of sex (F(1,53) = 0.415, p = 0.52), and no interaction between sex and region (F(2,108) = 0.032, p = 0.97).
2 = 0.87), no main effect of sex (F(1,53) = 0.415, p = 0.52), and no interaction between sex and region (F(2,108) = 0.032, p = 0.97).
Striatal [11C]raclopride BP
We observed a main effect of region on [11C]raclopride BP values (F(2,104) = 479.362, p < 0.0001, 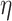 2 = 0.90), but no main effect of sex (F(1,52) = 0.084, p = 0.77), and no interaction between sex and region (F(2,104) = 1.453, p = 0.24).
2 = 0.90), but no main effect of sex (F(1,52) = 0.084, p = 0.77), and no interaction between sex and region (F(2,104) = 1.453, p = 0.24).
Striatal [11C]raclopride BP PSC
Again, we observed a main effect of region on PSC in [11C]raclopride BP values (F(2,104) = 5.383, p = 0.006,  2 = 0.09), but no main effect of sex (F(1,51) = 0.089, p = 0.77), and no interaction between sex and region (F(2, 104) = 1.488, p = 0.23).
2 = 0.09), but no main effect of sex (F(1,51) = 0.089, p = 0.77), and no interaction between sex and region (F(2, 104) = 1.488, p = 0.23).
Differences in DA neurotransmission by sex and hormone status
Striatal [18F]FMT Ki
Despite notable differences in striatal DA synthesis capacity within women based on hormone status, men did not differ appreciably from women in either hormone group. ANCOVA revealed an overall main effect of group (F(2,52) = 5.058, p = 0.010,  2 = 0.16) and region (F(2,106) = 116.5, p < 0.00001,
2 = 0.16) and region (F(2,106) = 116.5, p < 0.00001,  2 = 0.60). Post hoc Tukey’s HSD test confirmed that the main effect of hormone status was driven by significant differences between NC and hormonal contraceptive groups (p = 0.004), with no differences between males vs HC (p = 0.20) or vs NC (p = 0.12).
2 = 0.60). Post hoc Tukey’s HSD test confirmed that the main effect of hormone status was driven by significant differences between NC and hormonal contraceptive groups (p = 0.004), with no differences between males vs HC (p = 0.20) or vs NC (p = 0.12).
Striatal [11C]raclopride BP
We identified a significant effect of region (F(2,102) = 476.183, p < 0.0001, 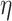 2 = 0.90); however, there was no significant main effect of age (F(1,50) = 1.330, p = 0.25) or hormone status (F(2,50) = 0.044, p = 0.96), nor an interaction between the 2 factors (F(4,102) = 1.049, p = 0.39).
2 = 0.90); however, there was no significant main effect of age (F(1,50) = 1.330, p = 0.25) or hormone status (F(2,50) = 0.044, p = 0.96), nor an interaction between the 2 factors (F(4,102) = 1.049, p = 0.39).
Striatal [11C]raclopride BP PSC
There was a significant effect of region (F(2,102) = 5.284, p = 0.007,  2 = 0.09), no significant effect of age (F(1,50) = 0.400, p = 0.53) or hormone status (F(2,50) = 0.081, p = 0.92), and no significant interaction between the two (F(4, 102) = 0.750, p = 0.56).
2 = 0.09), no significant effect of age (F(1,50) = 0.400, p = 0.53) or hormone status (F(2,50) = 0.081, p = 0.92), and no significant interaction between the two (F(4, 102) = 0.750, p = 0.56).
Individual differences in DA transmission are tied to differences in cognitive flexibility
Naturally cycling vs hormonal contraceptive users
There was no statistically significant difference in distractor cost between hormonal contraceptive users and NC participants (t(31.9) = 0.093, p = 0.926; Fig. 2a). However, hormonal contraceptive users exhibited significantly reduced switch cost compared with NC participants (t(31.0) = −2.256, p = 0.031; d = −0.74; age-adjusted) (Fig. 2b). Across female participants, switch cost was inversely correlated with [18F]FMT Ki values in the dorsal caudate (Pearson’s r(33) = −0.41, p = 0.016) and ventral striatum (r(33) = −0.34, p = 0.042), but not in the dorsal putamen (r(33) = −0.29, p = 0.089). Only the effect in the dorsal caudate was statistically significant after correcting for multiple comparisons (Fig. 2c). In contrast, there were no significant correlations between [18F]FMT Ki values and distractor cost in any ROI (all ps > 0.6). There were no significant correlations among males between [18F]FMT Ki values and switch or distractor costs in any ROI (p > 0.2 for all).
Fig. 2.
Cognitive flexibility differs between naturally cycling and hormonal contraceptive groups and correlates with DA synthesis capacity in dorsal caudate. A) Performance on a task-switching paradigm reveals no difference in cognitive stability between groups, indicated by no difference in distractor costs on distractor/ongoing trials. B) In contrast, hormonal contraceptive users exhibited greater cognitive flexibility compared with naturally cycling participants, indicated by a smaller performance cost on task-switching trials. C) We observed a significant negative correlation between performance on a task-switching paradigm and [18F]FMT Ki values in dorsal caudate across our female participants (both NC and HC).
Men vs women
We did not observe a difference in switch cost (t(46.4) = −0.11, p = 0.91) or distractor cost (t(47.9) = −0.47, p = 0.64) between men and women (Table 3).
Table 3.
Performance on task-switching paradigm.
| Distractor cost | Switch cost | |
|---|---|---|
| Men (n = 20) | 0.035 ± 0.040 | 0.125 ± 0.108 |
| Women (n = 36) | 0.029 ± 0.051 | 0.121 ± 0.132 |
| N aturally Cycling (n = 21) | 0.029 ± 0.054 | 0.160 ± 0.149 |
| H ormonal Contraception (n = 15) | 0.030 ± 0.048 | 0.070 ± 0.085 |
| NC vs HCCohen’s d (Welch’s p) | n.s. | −0.74 (0.03) |
| Men vs Women | n.s. | n.s. |
| Men vs NC vs HCKruskal–Wallis p | n.s. | n.s. |
NC, naturally cycling; HC, hormonal contraception.
Men vs naturally cycling vs hormonal contraceptive users
We did not observe significant effects of switch cost (F(2,52) = 2.428, p = 0.098) or distractor cost (F(2,52) = 0.1, p = 0.905) between groups (Table 3).
Discussion
In this study, hormonal contraceptive users exhibited greater DA synthesis capacity (as measured by [18F]FMT Ki) and greater cognitive flexibility than NC participants. No group differences in D2/3 binding potential ([11C]raclopride BP) or DA release ([11C]raclopride BP PSC) were observed. Though synthesis capacity differed significantly between NC women and women using hormonal contraception, women overall did not differ appreciably from men. This suggests that investigations into the influence of sex hormones on DA neurotransmission may be hampered if limited to comparisons between sexes. Together, these findings lay the groundwork for understanding how global manipulations of the endocrine system, e.g. via hormonal contraceptives, impact DA neurotransmission and related cognition.
DA synthesis capacity differs by hormone status
No sex differences in DA neurotransmission were observed. These findings are inconsistent with previous studies comparing DA synthesis between sexes, which report greater synthesis in females relative to males (Laakso et al. 2002; Hahn et al. 2021). However, these studies did not control for or exclude participants based on hormonal contraceptive use. In the present study (e.g. Supplementary Fig. 1), DA synthesis values for our male sample fell between values observed for HC and NC women. Thus, based on findings reported here, the magnitude and direction of a sex effect observed in DA synthesis may reflect the proportion of HC vs NC female participants in the sample rather than a sex difference, per se.
Within women, DA synthesis capacity was greater in hormonal contraceptive users compared with NC participants, whereas D2/3 receptor binding potential and stimulated DA release did not differ between groups. Findings from the preclinical literature suggest that pharmacological manipulations of the hypothalamic-pituitary-gonadal axis impact the DA system. In an ablation-replacement study in ovariectomized rats (Pasqualini et al. 1995), 17β-estradiol add-back selectively increased striatal DA synthesis but not release, as measured via local superfusion of E2 into the caudate nucleus. Similarly, Algeri et al. (1976) observed increased DA synthesis in the striatum and forebrain of intact rats after acute (4 days) and chronic (30 days) oral administration of a synthetic progestin and an estrogen.
Estradiol’s influence on DA synthesis capacity may be mediated by estradiol-induced increases in phosphorylation of tyrosine hydroxylase (TH) (Pasqualini et al. 1995), the enzyme that converts tyrosine to L-dihydroxyphenylalanine (L-DOPA). Another mechanism of action may be the hormonal regulation of aromatic L-amino acid decarboxylase (AADC) that converts L-DOPA to DA (and is the target of [18F]FMT). AADC activity is dependent on pyridoxal phosphate (PLP), or Vitamin B6 (Rahman et al. 1982; Hartvig et al. 1995), a nutrient and coenzyme with intermediate concentrations in basal ganglia (Ebadi 1985) that is reduced, in some cases to the point of deficiency, in HC users (Luhby et al. 1971; Bennink and Schreurs 1974; Wilson et al. 2011; Rios-Avila et al. 2015). If low levels of PLP are associated with reduced AADC activity (Allen et al. 2010), we would expect HC users to exhibit reduced [18F]FMT binding relative to NC women. We observed the opposite pattern. Without information regarding vitamin B6 status for participants, the relationship between PLP and [18F]FMT binding remains untested.
The selectivity of our findings to differences in AADC activity (as measured with [18F]FMT) and not DA release or D2/3 receptor binding (both measured with [11C]raclopride) also suggests the possibility that other catecholamine systems may be impacted. AADC is a critical enzyme in the formation of catecholamines in general, including serotonin (Ebadi 1985). In rodent studies, chronic treatment with oral hormonal contraceptives increases brain levels of tryptophan and serotonin (Baker et al. 1977; Daabees et al. 1981; reviewed in Porcu et al. 2019). Future investigations should clarify whether global sex steroid hormone manipulations alter DA synthesis capacity specifically, or the catecholamine system generally.
While [18F]FMT is a straightforward measure of AADC enzyme activity, which should directly reflect DA synthesis, [11C]raclopride is a more complex signal. [11C]Raclopride combines several measures, including the binding potential or number of D2/3 receptors (Bavail), and the dissociation constant or how probable the ligand–receptor complex is to dissociate (KD). One limitation of our study is that NC participants were not staged according to menstrual cycle phase. DA release and DA-D2 receptor availability vary across the estrus (Becker and Cha 1989; Lévesque et al. 1989; Yoest et al. 2018) and menstrual cycles (Czoty et al. 2009, though see Munro et al. 2006; Petersen et al. 2021). In ovariectomized rodents, 17β-estradiol administration augments striatal D2 receptor density (Bavail), but does not influence binding affinity (1/KD) (reviewed in Di Paolo 1994). Thus, it is possible that differences in DA release and baseline binding potential between HC users and unstaged NC women exist, but were obscured in our sample. However, data from Smith et al. (2019) suggest this is unlikely. In their study, DA release (as measured via [18F]fallypride paired with D-amphetamine) did not differ between women using hormonal contraception and NC women staged within the first 10 days of their menstrual cycle.
Another consideration is that FMT signal increases over the adult lifespan. Braskie et al. (2008) observed greater striatal FMT Ki values in older participants (mean age = 67) relative to younger participants (mean age = 23). In young adults, higher FMT Ki values in caudate are associated with increased working memory capacity (Cools et al. 2008). In contrast, in older adults greater striatal FMT signal may reflect potential compensation for deficits elsewhere in the DA system (e.g. PFC). In a recent study of DA synthesis and working memory capacity in cognitively normal older adults (Ciampa et al. 2021), we observed that adults with the highest FMT Ki values also display the greatest atrophy in posterior parietal cortex, raising the possibility of a compensatory response with aging. In the present study of younger adults, HC users were slightly older than NC participants (2 years on average), but the age range of our sample was limited (18–28 years) and results remained significant after controlling for age. Thus, it is unlikely that the group differences we observed are attributable to general effects of aging. Furthermore, our results do not support the idea that higher FMT Ki values reflect suboptimal DA functioning, given that HC users had higher FMT Ki values and greater cognitive flexibility. Higher FMT Ki values in young adults have consistently been associated with better cognitive flexibility (Berry et al. 2016, 2018b) as well as with working memory capacity (Cools et al. 2008).
Consistent effects across hormonal contraceptive regimens
The women in our HC group were on different forms of hormonal contraception, including the combined oral contraceptive pill, vaginal ring, subdermal implant, injection, and hormonal IUD. Exploratory analyses suggest that the relationship between HC use and potentiated DA synthesis capacity is independent of route of administration (Supplementary Fig. 1). Similarly, in their population-level study of hormonal contraception use and mood disorders, Skovlund et al. (2016) report an increased risk with hormonal contraceptive use, regardless of method and formulation. Hormonal contraception can alter endogenous hormone concentrations to varying extents depending on the formulation and method of delivery. Oral contraceptives and the depot medroxyprogesterone injection exert powerful and sustained suppression of endogenous sex hormone production (Gaspard et al. 1983; Croxatto and Mäkäräinen 1998; Rivera et al. 1999), whereas hormonal IUDs and implants generally exert less pronounced suppression of endogenous hormone levels (Barbosa et al. 1990; Luukkainen et al. 1990; Xiao et al. 1995; Croxatto and Mäkäräinen 1998; Coelingh Bennink 2000). Therefore, the impact of HC on DA may occur in part by altering endogenous hormone levels, but the observed effects are unlikely to be solely attributable to endogenous hormone modulation, per se.
The synthetic hormones introduced by the HC regimen, not the alteration of endogenous hormones alone, may be driving changes within the DA system. In one of the few studies of synthetic hormones’ effects on striatal DA, Jori and Dolfini (1976) report increased turnover of striatal DA in intact female rats after acute and chronic oral administration of steroid contraceptive drug combinations (mestranol with either lynestrenol, norethindrone, or norethynodrel). While endogenous estrogen levels are suppressed in users of OC, the exogenous estrogen (typically ethinylestradiol) is considered to be significantly more potent than its endogenous analog (Helgason et al. 1982; Jeyakumar et al. 2011). If synthetic hormones’ potency confers an enhanced estrogenic effect (see Hampson 2023, for discussion), this could be contributing to the augmented DA synthesis we see in HC users relative to nonusers. However, this account could not fully explain the pattern of results observed here, since users of progestin-only IUDs were similarly affected (n.b. Skovlund et al. 2016).
Here, DA synthesis capacity was similarly elevated in users of OC (“the pill,” which is primarily a combination of ethinyl estradiol and progestin) and users of other forms of hormonal contraception (including implants, injection, and hormonal IUDs) that primarily contain progestin. This suggests that the progestin component, alone or in concert with endogenous or exogenous estrogen, could be influencing the observed effects. Skovlund et al. (2016) proposed that their findings of increased risk of depression across all types of HC reflected the influence of progestins. A general consensus from animal and human research is that endogenous estradiol augments DA function (reviewed in Barth et al. 2015), whereas the influence of progesterone has not been fully characterized (Hidalgo and Pletzer 2017). Still, progesterone receptor expression in embryonic DA neurons suggests a potentially powerful role of progesterone in modulating DA signaling. In a study of mouse embryonic stem cells, Díaz et al. (2007) studied the expression of steroid hormone receptors in differentiated DA neurons. They report that 92% of DA neurons expressed progesterone receptors and only 19% of these neurons co-expressed TH and ER-α. Other studies report effects of progesterone, independent of estrogens, on DA release (Dluzen and Ramirez 1990; Petitclerc et al. 1995). Future investigations delineating the influence of synthetic progestins alone and in combination with ethinyl estradiol on dopaminergic function will provide mechanistic insight into the results reported here.
Hormonal modulation of dorsal caudate vs striatum broadly
We observed a significant difference in DA synthesis capacity between HC and NC groups across the striatum, and post hoc tests revealed the strongest effect to be in dorsal caudate (Fig. 1). Thus, hormonal contraception may broadly alter striatal DA synthesis capacity, or do so more selectively within dorsal caudate. In a case study of oral contraceptive-induced hemichorea using 18FDG-PET, investigators observed striatal hypermetabolism, with increased glucose metabolism in the body of the left caudate nucleus (contralateral to the dyskinesia) (Vela et al. 2004), suggesting augmented effects of OC in the caudate.
Individual differences in DA synthesis capacity are tied to cognitive flexibility
Hormonal contraceptive users differed from NC women on switch cost but not on distractor cost in this task-switching paradigm, suggesting a specific effect on cognitive flexibility. This reduced switch cost (i.e. greater cognitive flexibility) in hormone users is consistent with our observation of greater striatal DA synthesis capacity in hormone users relative to NC women, and aligns with models of cognitive control, which posit unique roles of prefrontal and striatal DA on cognitive stability (i.e. distractor cost) and flexibility (switch cost), respectively (Cools and D’Esposito 2011). Previous studies have reported an association between task-switching performance and DA synthesis capacity, specifically in the dorsal caudate (Klanker et al. 2013; Berry et al. 2016, 2018b). Our results suggest an influence of hormonal contraceptive use on the corticostriatal circuitry underlying executive functioning. Future studies should consider whether other measures of executive functioning are influenced, and, by extension, whether dopaminergic medications used to treat disorders of executive function (e.g. ADHD) exert unique effects with or without concomitant use of hormonal contraception.
Strengths and limitations
Together, this study provided a unique opportunity to examine differences in basal DA receptor occupancy, stimulated DA release, and DA synthesis capacity in a single cohort, based on women’s hormonal contraceptive status. However, a number of limitations should be considered. First, these data were collected as part of a parent study that was not designed with a woman’s health lens in mind. For this reason, our sample is representative of contraceptive use by the general pool of women enrolled in the parent neuroimaging study. The route of administration and formulation of the hormonal contraceptive regimen varied (e.g. patch, pill, IUD, and implant) among HC participants. Detailed information on participants’ age of initiation, duration of hormone use, and schedule would further enhance our understanding of the time course with which hormonal contraceptives impacts the DA system. Second, NC participants were not staged according to menstrual cycle phase. Thus, the robust differences we observed may reflect stable characteristics of the DA system that differ between groups as opposed to state-dependent effects sensitive to hormonal variation across the cycle. It remains a possibility that there are differences in DA signaling within NC women over time or between contraceptive users and NC women at specific cycle stages (as opposed to NC women generally). Finally, though we determined that a number of non-hormonal factors (e.g. working memory span, impulsivity, and smoking status) that could influence DA neurotransmission were likely not contributing to our findings, there are other factors (e.g. IQ) that future studies should also take into consideration.
This study leveraged an existing multimodal molecular PET imaging cohort, which gave us the rare opportunity to identify broad differences in DA neurotransmission between NC women and those using hormonal contraception. This analysis provides researchers with a strong rationale for conducting additional studies designed explicitly to test the breadth and depth with which hormonal contraceptives influence major neuromodulatory systems. Strikingly little has been done to investigate the impact of chronic ovarian hormone suppression and synthetic hormone regimens on brain regions that are densely populated with sex hormone receptors and modulated by sex hormones (Taylor et al. 2021; Petersen et al. 2023). This multi-tracer molecular PET imaging study, which allows a comprehensive assessment of DA receptor occupancy, DA release, and DA synthesis in a single cohort, represents a critical step toward that goal.
Conclusions
This PET imaging study revealed differences in DA synthesis capacity between hormonal contraceptive users and naturally cycling women. Measures of DA binding potential and stimulated DA release were similar between groups. Hormonal contraception (in the form of pill, shot, implant, ring, or IUD) is used by ~400 million women worldwide (United Nations 2019), yet few studies have examined whether hormonal manipulations impact basic properties of the DA system. Findings from this study begin to address this critical gap in women’s health. Moving forward, it is important to consider hormone use as a factor in studies of DA function. More broadly, our findings motivate consideration of the clinical implications of concomitant use of commonly used DA-based medications and hormonal contraceptives.
Funding
The National Institutes of Health AG044292 (WJ); the Daryl and Marguerite Errett Discovery Award (CMT); NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (EGJ); the Ann S. Bowers Women’s Brain Health Initiative (EGJ, MTD).
Conflict of interest statement: The authors report no conflicts of interest.
Author contributions
The parent project was conceived by MTD and WJJ, with study aims conceived by CMT and EGJ; WJJ, DJF, RLW and A.S.B. and performed the experiments; data analysis was conducted by CMT and DJF; CMT and EGJ wrote the manuscript; MTD, ASB, DJF, WJJ, RLW, CMT, and EGJ edited the manuscript.
Caitlin Taylor (Conceptualization, Formal analysis, Visualization, Writing—original draft, Writing—review & editing), Daniella L. Furman (Formal analysis, Investigation, Methodology, Visualization, Writing—review & editing), Anne Berry (Investigation, Writing—review & editing), Robert L. White III (Investigation, Writing—review & editing), William Jagust (Conceptualization, Investigation, Writing—review & editing), Mark D'Esposito (Conceptualization, Writing—review & editing), and Emily Jacobs (Conceptualization, Writing—original draft, Writing—review & editing)
Supplementary Material
Contributor Information
Caitlin M Taylor, Department of Psychological & Brain Sciences, University of California, Santa Barbara, CA 93106, United States.
Daniella J Furman, Department of Neurology, University of California San Francisco, San Francisco, CA 94143, United States.
Anne S Berry, Department of Psychology, Brandeis University, Waltham, MA 02453, United States.
Robert L White, III, Department of Neurology, Washington University School of Medicine, St. Louis, MO 63112, United States.
William J Jagust, Helen Wills Neuroscience Institute, University of California Berkeley, Berkeley, CA 94720, United States; Lawrence Berkeley National Laboratory, Berkeley, CA 94720, United States.
Mark D’Esposito, Helen Wills Neuroscience Institute, University of California Berkeley, Berkeley, CA 94720, United States; Department of Psychology, University of California Berkeley, Berkeley, CA 94720, United States.
Emily G Jacobs, Department of Psychological & Brain Sciences, University of California, Santa Barbara, CA 93106, United States; Neuroscience Research Institute, University of California Santa Barbara, Santa Barbara, CA 93106, United States.
References
- Algeri S, Ponzio F, Dolfini E, Jori A. Biochemical effects of treatment with oral contraceptive steroids on the dopaminergic system of the rat. Neuroendocrinology. 1976:22(4):343–351. [DOI] [PubMed] [Google Scholar]
- Allen GFG, Neergheen V, Oppenheim M, Fitzgerald JC, Footitt E, Hyland K, Clayton PT, Land JM, Heales SJR. Pyridoxal 5′-phosphate deficiency causes a loss of aromatic l-amino acid decarboxylase in patients and human neuroblastoma cells, implications for aromatic l-amino acid decarboxylase and vitamin B6 deficiency states. J Neurochem. 2010:114(1):87–96. [DOI] [PubMed] [Google Scholar]
- Armbruster DJN, Ueltzhöffer K, Basten U, Fiebach CJ. Prefrontal cortical mechanisms underlying individual differences in cognitive flexibility and stability. J Cogn Neurosci. 2012:24(12):2385–2399. [DOI] [PubMed] [Google Scholar]
- Asghari R, Lung MSY, Pilowsky PM, Connor M. Sex differences in the expression of serotonin-synthesizing enzymes in mouse trigeminal ganglia. Neuroscience. 2011:199:429–437. [DOI] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Howes OD. Tobacco smoking and dopaminergic function in humans: a meta-analysis of molecular imaging studies. Psychopharmacology. 2019:236(4):1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JM, Bond SW, Handley SL. Effects of long-term treatment with contraceptive steroids on plasma and brain tryptophan, brain 5-hydroxytryptamine, and locomotor activity in female mice [proceedings]. Br J Pharmacol. 1977:59(3):531P–532P. [PMC free article] [PubMed] [Google Scholar]
- Barbosa I, Bakos O, Olsson S-E, Odlind V, Johansson EDB. Ovarian function during use of a levonorgestrel-releasing IUD. Contraception. 1990:42:51–66. [DOI] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015:9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999:64:803–812. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha J-H. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989:35:117–125. [DOI] [PubMed] [Google Scholar]
- Bennink HJTC, Schreurs WHP. Disturbance of tryptophan metabolism and its correction during hormonal contraception. Contraception. 1974:9:347–356. [DOI] [PubMed] [Google Scholar]
- Berry AS, Shah VD, Baker SL, Vogel JW, O’Neil JP, Janabi M, Schwimmer HD, Marks SM, Jagust WJ. Aging affects dopaminergic neural mechanisms of cognitive flexibility. J Neurosci. 2016:36:12559–12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Shah VD, Furman DJ, White Iii RL, Baker SL, O’Neil JP, Janabi M, D’Esposito M, Jagust WJ. Dopamine synthesis capacity is associated with D2/3 receptor binding but not dopamine release. Neuropsychopharmacology. 2018a:43:1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Shah VD, Jagust WJ. The influence of dopamine on cognitive flexibility is mediated by functional connectivity in young but not older adults. J Cogn Neurosci. 2018b:30:1330–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, White RL, Furman DJ, Naskolnakorn JR, Shah VD, D’Esposito M, Jagust WJ. Dopaminergic mechanisms underlying normal variation in trait anxiety. J Neurosci. 2019:39:2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007:30:194–202. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Wilcox CE, Landau SM, O’Neil JP, Baker SL, Madison CM, Kluth JT, Jagust WJ. Relationship of striatal dopamine synthesis capacity to age and cognition. J Neurosci. 2008:28:14320–14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Cortés R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience. 1989:28:275–290. [DOI] [PubMed] [Google Scholar]
- Ciampa CJ, Parent JH, Lapoint MR, Swinnerton KN, Taylor MM, Tennant VR, Whitman AJ, Jagust WJ, Berry AS. Elevated dopamine synthesis as a mechanism of cognitive resilience in aging. Cereb Cortex. 2021:32(3):2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh Bennink HJT. The pharmacokinetics and pharmacodynamics of Implanon®, a single-rod etonogestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000:5:12–20. [PubMed] [Google Scholar]
- Cools R, Arnsten AFT. Neuromodulation of prefrontal cortex cognitive function in primates: the powerful roles of monoamines and acetylcholine. Neuropsychopharmacology. 2022:47:309–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011:69:e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008:28:1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto HB, Mäkäräinen L. The pharmacodynamics and efficacy of Implanon®11Norplant® is a registered trademark of the Population Council, New York.: an overview of the data. Contraception. 1998:58:91S–97S. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009:34:548–554. [DOI] [PubMed] [Google Scholar]
- Daabees TT, Mohy El-Din MM, Zeitoun R, Makar AB. Injectable and oral contraceptive steroids in relation to some neurotransmitters in the rat brain. Biochem Pharmacol. 1981:30:1581–1585. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994:5(1):27–41. [DOI] [PubMed] [Google Scholar]
- Díaz NF, Guerra-Arraiza C, Díaz-Martínez NE, Salazar P, Molina-Hernández A, Camacho-Arroyo I, Velasco I. Changes in the content of estrogen α and progesterone receptors during differentiation of mouse embryonic stem cells to dopamine neurons. Brain Res Bull. 2007:73:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geana A, Ohm F, Doll BB, Frank MJ. The straw that broke the Camel’s back: natural variations in 17β-Estradiol and COMT-Val158Met genotype interact in the modulation of model-free and model-based control. Front Behav Neurosci. 2021:15:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinology. 1984:39:149–155. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. In vitro progesterone modulation of amphetamine-stimulated dopamine release from the corpus striatum of ovariectomized estrogen-treated female rats: response characteristics. Brain Res. 1990:517:117–122. [DOI] [PubMed] [Google Scholar]
- Ebadi M. Regulation and function of pyridoxal phosphate in CNS. In: Selected topics from neurochemistry. Pergamon: Elsevier; 1985. p. 341–376 [Google Scholar]
- Farde L, Hall H, Ehrin E, Sedvall G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science. 1986:231:258–261. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Parametric Mapping: The Analysis of Functional Brain Images. Academic: Amsterdam, 2007. [Google Scholar]
- Furman DJ, White RL III, Naskolnakorn J, Ye J, Kayser A, D’Esposito M. Effects of dopaminergic drugs on cognitive control processes vary by genotype. J Cogn Neurosci. 2020:32:804–821. [DOI] [PubMed] [Google Scholar]
- Furman DJ, Pappas I, White RL, Kayser AS, D’Esposito M. Enhancing dopamine tone modulates global and local cortical perfusion as a function of COMT val158met genotype. NeuroImage. 2021:242:118472. [DOI] [PubMed] [Google Scholar]
- Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey-Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983:27:577–590. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997:6:279–287. [DOI] [PubMed] [Google Scholar]
- Hahn A, Reed MB, Pichler V, Michenthaler P, Rischka L, Godbersen GM, Wadsak W, Hacker M, Lanzenberger R. Functional dynamics of dopamine synthesis during monetary reward and punishment processing. J Cereb Blood Flow Metab. 2021:41:2973–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994:11:245–256. [DOI] [PubMed] [Google Scholar]
- Hampson E. Oral contraceptives in the central nervous system: basic pharmacology, methodological considerations, and current state of the field. Front Neuroendocrinol. 2023:68:101040. [DOI] [PubMed] [Google Scholar]
- Hampson E, Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci. 1988:102:456–459. [DOI] [PubMed] [Google Scholar]
- Hartvig P, Lindner KJ, Bjurling P, Långström B, Tedroff J. Pyridoxine effect on synthesis rate of serotonin in the monkey brain measured with positron emission tomography. J Neural Transm. 1995:102:91–97. [DOI] [PubMed] [Google Scholar]
- Helgason S, Damber M-G, Von Schoultz B, Stigbrand T. Estrogenic potency of oral replacement therapy estimated by the induction of pregnancy zone protein. Acta Obstet Gynecol Scand. 1982:61:75–79. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Lopez E, Pletzer B. Interactive effects of dopamine baseline levels and cycle phase on executive functions: the role of progesterone. Front Neurosci. 2017:11:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang S-C, Ichise M, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007:27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Ito H, Ota M, Ikoma Y, Seki C, Yasuno F, Takano A, Maeda J, Nakao R, Suzuki K, Suhara T. Quantitative analysis of dopamine synthesis in human brain using positron emission tomography with L-[β-11C]DOPA. Nucl Med Commun. 2006:27:723–731. [DOI] [PubMed] [Google Scholar]
- Ito H, Shidahara M, Takano H, Takahashi H, Nozaki S, Suhara T. Mapping of central dopamine synthesis in man, using positron emission tomography with l-[β-11C]DOPA. Ann Nucl Med. 2007:21:355–360. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007:30:188–193. [DOI] [PubMed] [Google Scholar]
- Jacobs EG, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci. 2011:31:5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Weiss BK, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM. Impact of sex and menopausal status on episodic memory circuitry in early midlife. J Neurosci. 2016:36:10163–10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Weiss B, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM. Reorganization of functional networks in verbal working memory circuitry in early midlife: the impact of sex and menopausal status. Cereb Cortex. 2017:27:2857–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar M, Carlson KE, Gunther JR, Katzenellenbogen JA. Exploration of dimensions of estrogen potency: parsing ligand binding and coactivator binding affinities. J Biol Chem. 2011:286:12971–12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori A, Dolfini E. Modifications of striatal dopamine levels by steroid contraceptive drugs in mice and rats. Neuroendocrinology. 1976:21:74–78. [DOI] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013:7:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998:395:1–17. [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Jorgen B, Haaparanta M, Solin O, Syvälahti E, Salokangas RKR, Hietala J. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002:52:759–763. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996:4:153–158. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O’Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009:19:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalaye J, Booij J, Reneman L, Habraken JBA, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000:27:867–869. [DOI] [PubMed] [Google Scholar]
- Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci. 2000:20:8604–8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque D, Gagnon S, Di Paolo T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci Lett. 1989:98:345–350. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci. 1993:90:8861–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhby AL, Brin M, Gordon M, Davis P, Murphy M, Spiegel H. Vitamin B6 metabolism in users of oral contraceptive agents. I. Abnormal urinary xanthurenic acid excretion and its correction by pyridoxine. Am J Clin Nutr. 1971:24:684–693. [DOI] [PubMed] [Google Scholar]
- Luukkainen T, Lähteenmäki P, Toivonen J. Levonorgestrel-releasing intrauterine device. Ann Med. 1990:22:85–90. [DOI] [PubMed] [Google Scholar]
- Manza P, Shokri-Kojori E, Wiers CE, Kroll D, Feldman D, McPherson K, Biesecker E, Dennis E, Johnson A, Kelleher A, et al. Sex differences in methylphenidate-induced dopamine increases in ventral striatum. Mol Psychiatry. 2022:27:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D-R, Huang Y, Simpson N, Ngo K, Van Heertum R, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. accuracy and precision of D 2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001:21:1034–1057. [DOI] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001:158:1492–1499. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006:59:966–974. [DOI] [PubMed] [Google Scholar]
- Nordström A-L, Olsson H, Halldin C. A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res Neuroimaging. 1998:83:1–6. [DOI] [PubMed] [Google Scholar]
- Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of Estradiol on striatal dopamine synthesis. J Neurochem. 1995:65:1651–1657. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1985:5:584–590. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995:51:768–774. [DOI] [PubMed] [Google Scholar]
- Petersen N, Rapkin AJ, Okita K, Kinney KR, Mizuno T, Mandelkern MA, London ED. Striatal dopamine D 2 -type receptor availability and peripheral 17 β-estradiol. Mol Psychiatry. 2021:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Beltz AM, Casto KV, Taylor CM, Jacobs EG, Sundström-Poromaa I, Pletzer B. Towards a more comprehensive neuroscience of hormonal contraceptives. Nat Neurosci. 2023:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitclerc M, Bédard PJ, Di Paolo T. Progesterone releases dopamine in male and female rat striatum: a behavioral and microdialysis study. Prog Neuro-Psychopharmacol Biol Psychiatry. 1995:19:491–497. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K, SyvÄlahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry. 1998:155:768–773. [DOI] [PubMed] [Google Scholar]
- Porcu P, Serra M, Concas A. The brain as a target of hormonal contraceptives: evidence from animal studies. Front Neuroendocrinol. 2019:55:100799. [DOI] [PubMed] [Google Scholar]
- Rahman MK, Nagatsu T, Sakurai T, Hori S, Abe M, Matsuda M. Effect of pyridoxal phosphate deficiency on aromatic l-amino acid decarboxylase activity with l-dopa and l-5-hydroxytryptophan as substrates in rats. Japn J Pharmacol. 1982:32:803–811. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, Anderson S, Woodward N, Schmidt D, Baldwin R, et al. Sex differences in amphetamine-induced displacement of [18 F]Fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006:163:1639–1641. [DOI] [PubMed] [Google Scholar]
- Rios-Avila L, Coats B, Chi Y-Y, Midttun Ø, Ueland PM, Stacpoole PW, Gregory JF. Metabolite profile analysis reveals association of vitamin B-6 with metabolites related to one-carbon metabolism and tryptophan catabolism but not with biomarkers of inflammation in oral contraceptive users and reveals the effects of oral contraceptives on these processes. J Nutr. 2015:145:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999:181:1263–1269. [DOI] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998:39:904–911. [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991:27:763. [Google Scholar]
- Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry. 2016:73:1154–1162. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 2001:28:595–608. [DOI] [PubMed] [Google Scholar]
- Smith CT, Sierra Y, Oppler SH, Boettiger CA. Ovarian cycle effects on immediate reward selection bias in humans: a role for Estradiol. J Neurosci. 2014:34:5468–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, San Juan MD, Dang LC, Katz DT, Perkins SF, Burgess LL, Cowan RL, Manning HC, Nickels ML, Claassen DO, et al. Ventral striatal dopamine transporter availability is associated with lower trait motor impulsivity in healthy adults. Transl Psychiatry. 2018:8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Dang LC, Burgess LL, Perkins SF, San Juan MD, Smith DK, Cowan RL, Le NT, Kessler RM, Samanez-Larkin GR, et al. Lack of consistent sex differences in d-amphetamine-induced dopamine release measured with [18F]fallypride PET. Psychopharmacology. 2019:236:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossi V, Holden JE, de la Fuente-Fernandez R, Ruth TJ, Stoessl AJ. Effect of dopamine loss and the metabolite 3-O-methyl-[18F]fluoro-dopa on the relation between the 18F-fluorodopa tissue input uptake rate constant Kocc and the [18F]fluorodopa plasma input uptake rate constant Ki. J Cereb Blood Flow Metab. 2003:23:301–309. [DOI] [PubMed] [Google Scholar]
- Sun J, Walker AJ, Dean B, van den Buuse M, Gogos A. Progesterone: the neglected hormone in schizophrenia? A focus on progesterone-dopamine interactions. Psychoneuroendocrinology. 2016:74:126–140. [DOI] [PubMed] [Google Scholar]
- Taxier LR, Gross KS, Frick KM. Oestradiol as a neuromodulator of learning and memory. Nat Rev Neurosci. 2020:21:535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CM, Pritschet L, Jacobs EG. The scientific body of knowledge – whose body does it serve? A spotlight on oral contraceptives and women’s health factors in neuroimaging. Front Neuroendocrinol. 2021:60:100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Rummel J, Floresco SB, Galea LAM. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacology. 2012:37:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations . Contraceptive use by method 2019: data booklet. UN; 2019. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_contraceptiveusebymethod_databooklet.pdf. [Google Scholar]
- Vela L, Sfakianakis GN, Heros D, Koller W, Singer C. Chorea and contraceptives: case report with pet study and review of the literature. Mov Disord. 2004:19:349–352. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Bivins BN, Russell KA, Bailey LB. Oral contraceptive use: impact on folate, vitamin B6, and vitamin B12 status: nutrition reviews©. Nutr Rev. 2011:69:572–583. [DOI] [PubMed] [Google Scholar]
- Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, Links JM, Zacur HA, Harris J, Naidu S, Braestrup C, et al. In vivo measurement of dopamine receptors in human brain by positron emission tomography age and sex differences. Ann N Y Acad Sci. 1988:515:203–214. [DOI] [PubMed] [Google Scholar]
- Xiao B, Zeng T, Wu S, Sun H, Xiao N. Effect of levonorgestrel-releasing intrauterine device on hormonal profile and menstrual pattern after long-term use. Contraception. 1995:51:359–365. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA, Becker JB. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav. 2018:104:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



