ABSTRACT
Recent evidence has suggested that changes in maternal gut microbiota in early life may generate neurobiological consequences associated with psychiatric-related abnormalities. However, the number of studies on humans investigating this problem is limited, and preclinical findings sometimes conflict. Therefore, we run a meta-analysis to examine whether maternal microbiota disturbance (MMD) during neurodevelopment might affect the offspring during adulthood. We found thirteen studies, from a set of 459 records selected by strategy registered on PROSPERO (#289224), to target preclinical studies that evaluated the behavioral outcomes of the rodents generated by dams submitted to perinatal enteric microbiota perturbation. The analysis revealed a significant effect size (SMD = −0.51, 95% CI = −0.79 to −0.22, p < .001, T2 = 0.54, I2 = 79.85%), indicating that MMD might provoke behavioral impairments in the adult offspring. The MMD also induces a significant effect size for the reduction of the sociability behavior (SMD = −0.63, 95% CI = −1.18 to −0.07, p = 0.011, T2 = 0.30, I2 = 76.11%) and obsessive-compulsive-like behavior (SMD = −0.68, 95% CI = −0.01 to −1.36, p = 0.009, T2 = 0.25, I2 = 62.82%) parameters. The effect size was not significant or inconclusive for memory and anxiety-like behavior, or inconclusive for schizophrenia-like and depressive-like behavior. Therefore, experimental perinatal MMD is vertically transmitted to the offspring, negatively impacting behavioral parameters related to psychiatric disorders.
KEYWORDS: Maternal microbiome disturbance, neurodevelopment, gut-brain axis, psychiatric disorder, sociability, autism spectrum disorder
GRAPHICAL ABSTRACT
1. Introduction
Although the knowledge of the existence of microorganisms and their involvement in diseases begins at 15th century with the germ theory by Girolamo Fracastoro and Marcus von Plenciz, chinese medicine from the 4th century already used the Yellow Soups as a rudimentary microbiota transplantation method for treating intestinal disease.1 However, with recent advances in techniques for genome sequencing that allowed a deep uncovering of microbe species and variabilities,2 accumulated data reveal the influence of the gut microbiota on several human physiological processes, such as the sensitization and balance of the immune system3, hormone production,4 aging,5 and metabolism.6
Therefore, Sudo and colleagues7 provided evidence for the interplay between enteric microbiota and stress responses coordinated by the hypothalamic–pituitary–adrenal (HPA) axis. They reveal an exacerbated curve of serum glucocorticoids released after stress exposure in animal germ-free (GF) compared with the specific pathogen-free (SPF) group. Interestingly, they also highlighted that those responses of the HPA axis are normalized as GF animals are colonized with a commensal bacteria Bifidobacterium infantis. However, the regenerative effects of gut colonization are only observed as they occur in juvenile animals, before completing the neural maturation. Therefore, these findings already suggest the presence of a mechanism by which the gut microbiota could modulate the HPA stress response during neurodevelopment.8. Since then, the bidirectional interplay between host intestinal commensal bacteria and central nervous system (CNS) superior functions, such as emotion,9 effectiveness,10 sociability,11 and cognition12 have attracted attention. The gut-brain axis (GBA) promotes a complex bidirectional communication between gut commensal bacteria, the immune system, the enteric nervous system, and the neural system, commanding activities responsible for brain development, health, and disease.13,14
The role of GBA in brain development and maturation from gestational phases to adulthood neurological outcomes has been revealed in both clinical15,16 and preclinical studies.17 With different approaches, studies have highlighted the hypothesis that neurodevelopmental dysfunction induced by maternal microbiota disturbance (MMD) may result in psychiatric disorders, such as autism spectrum disorder (ASD),18 multiple sclerosis,19 generalized anxiety (GA),20 and schizophrenia (SZ).21
Although consistent results describe the neural mechanisms involving the microbiome to CNS functions, collectively, the evidence is sometimes conflicting or inconclusive.22 Methodological limitations of long-term follow-up of patient cohorts from pregnancy to adulthood may explain the limited number of studies and unsatisfying results.23 Furthermore, differences in the experimental protocols, such as the microbiota manipulation technique chosen, different behavioral analysis paradigms, and the distinct methods applied for microbiota sampling and analysis add high variability, which can result in a disagreement between preclinical studies.24–26 For instance, several studies using microbiota manipulation, such as diet, chemical agents, probiotics, prebiotics, or symbiotics have shown differences in the impact on bacterial populations, with divergent effects on the host outcomes.27,28 Therefore, we run over the current literature aimed at investigating whether MMD during the embryonic developmental window might impact the behavioral outcomes of offspring during adulthood life. To achieve this goal, we performed a systematic review with meta-analysis to combine the results of behavioral tests of the offspring generated by dams submitted to enteric microbiota perturbation during pregnancy.
2. Methods
2.1. Literature search
This study is registered to PROSPERO (#289224) and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,29 and the recommendations for carrying out meta-analyses.30 A searchable review question was structured using the PICO tool as follows: Population = laboratory rodents; Intervention = treatment of female progenitors during the pregnancy antibiotic, probiotic, prebiotic, symbiotic, microbiota colonization, microbiota transferring, and diet manipulation in any dose, via or time of administration; Control = treatment of female progenitors during the pregnancy with vehicle or saline or non-treated with intervention; Outcome = behavioral outcome of the offspring in the infancy, youth, or adulthood in relevant behavioral tests. The analyzed behaviors were those aiming to investigate depressive and anxiety-like behaviors, social deficits, cognition alterations, schizophrenic, panic, and obsessive-compulsive behaviors. A systematic computerized literature search of PubMed, EMBASE, and Web of Science was conducted to target original articles that investigate the effect of maternal microbiota perturbations on offspring behavioral outcomes using the search strategy design described in (Supplementary Table S1).
2.2. Eligibility criteria and screening
Rodent pre-clinical studies in any language, any date, and any journal were included if they aimed to experimentally manipulate maternal microbiota during pregnancy or until weaning and performed a behavioral analysis on offspring in adulthood. There were no studies with a protocol of MMD before gestation. The MMD window was selected due to previous clinical and preclinical evidence showing that the first offspring microbiome is mainly formed by maternal vertical transmission.31,32 The following inclusion criteria were applied to the screening of the relevant studies: (1) used adult laboratory rodents (rats or mice) of any sex and strain; (2) altered the maternal gestational microbiota through the use of antibiotics, probiotics, prebiotics or symbiotics, microbiota transfer, or dietary manipulations with caloric changes, fiber consumption, or ingestion of environmental contaminants; (3) characterized maternal microbiota profile; (4) the control group did not undergo any manipulation of the microbiota; (5) performed the relevant behavioral tests in the offspring older than 21 days. The following behavioral tests were eligible: Forced swimming test; Tail suspension test; Learned helplessness; Novelty suppressed feeding test; Sucrose spray test; Sucrose preference; Social default test; Elevated plus maze test; Vogel conflict test; Open field test; Light dark box; Elevated zero maze; Three chamber social interaction test; Three chamber social interaction test; Social interaction; Ultrasonic vocalization; Water maze test; Novel object recognition test; T test; Marble burying; Prepulse inhibition; Barnes maze test; and Y maze test.
Studies were excluded if they (1) were reviews, systematic reviews, or meta-analyses; (2) did not manipulate the maternal microbiota during pregnancy; (3) did not characterize the maternal microbiota; (4) performed the maternal microbiota manipulation by using behavioral or environmental stress, maternal immune-stimulating, and immune-suppressors; (5) conduct a co-treatment with two or more intervention factors; (6) performed no relevant behavioral study in the offspring of the experimental dams. Four reviewers (F.F., L.H., G.R., and A.K.) independently screened all titles and abstracts before full-text retrieval. The full texts of potentially eligible articles were assessed independently by at least two reviewers, and discrepancies were resolved through debate and consensus of the four authors for the final decision.
2.3. Assessment of study quality
Two independent reviewers (L.H. and G.R) evaluated the risk of bias using the RoB Syrcle tools33, which assess general aspects of experimental design and more specific features of animal research. In case of disagreement between the authors in some aspects of the evaluation, a third reviewer (F.F.) was consulted to make the final decision (Supplementary Table S2).
2.4. Data extraction, global, and stratified meta-analysis
Qualitative information was extracted by reviewers (F.F., L.H., G.R., and A.K.) using a predefined data extraction sheet (Supplementary Table S3), with the following items: article DOI, year of publication, experimental animal (species, age, and sex), microbiota manipulation technique, behavioral analysis conducted in the offspring, type of outcome measure and statistics [unity, mean, standard error, and sample size (n)]. When sample sizes were reported as intervals, the average between the numbers was used as the sample size (e.g., when 6–12 animals per group were reported, the sample size was considered as 9 animals per group).
For each primary outcome, a standardized mean difference (SMD) was calculated per study. Studies were stratified according to the theoretical paradigm (social behavior, memory, anxiety, depressive, schizophrenic, or obsessive-compulsive behavior) for subgroup meta-analysis independent of the features of the population, intervention, control, or outcomes. When feasible (at least two studies per subgroup), the meta-analyses were performed using a random effect model to estimate the combined effect size (CES, Hedges’g) with 95% confidence interval (95% CI), publication bias (Forest plot, Trim-and-Fill), and Heterogeneity (I2, Q statistic and the associated p-value, alpha = 0.05).
The combined effect size for all measures was calculated normalizing by behavioral impairment or standard behavioral, according to the outcome of the animal task. Therefore, a negative effect size was observed when treatments reduced the probability of exhibition of that particular activity (Mttd = mean of the treated group) compared to the control group (Mctl = mean of the treated group), resulting in a negative value (Mttd – Mctl < 0). For example, it was considered behavioral impairment the reduction of open arm exploration time in the Elevated Plus Maze test (interpreted as anxiety-like behavior), the reduction in the distance moved in the target quadrant in the Morris Water Maze (interpreted as memory impairment), the reduction in sugar consumption in the Sucrose Preference test (interpreted as anhedonia-like behavior), or the reduction in sociability time in the Three Chamber test (interpreted as social deficits). When the behavioral impairment results in an increase in the probability of a particular activity, such as the increase in buried marbles in the Marble Burying test (interpreted as stereotyped behavior), or increased latency to find the target hole in Barnes Maze (interpreted as memory impairments), compared to the control group, the size effect result (Mttd – Mctl) was multiplying by −1, as provided at the raw data (Supplementary table S3).
Calculations and figures were prepared using the Software Meta-essentials by Suurmond, van Rhee, and Hak34 (www.erim.eur.nl/research-support/meta-essentials/downloads). In the case of studies applying two or more behavioral tests to the same cohort of animals, one of them was randomly selected to be analyzed.
The combined effect sizes (CES) Hedges’g were categorized as very small (SMD = up to 0.2), small (SMD = between 0.2 and 0.5), moderate (SMD = 0.5–0.8), and large (SMD higher than 0.8).35 The values of I2 ranging from 0% to 100%, indicate proportion of heterogeneity interpreted as low to up to 25%, moderate in between 25% and 75% or high for above 75%. The 95% confidence interval (95% CI) excluding the null was considered significant or conclusive while 95% CI including the null was considered inconclusive. P-values lower than alpha (< .05) were interpreted as conclusive.
3. Results
3.1. Search result
From a total of 459 records identified in the database-searching strategy, 331 had the title and abstract screened after removing 128 duplicates (Figure 1). Fourteen articles fulfilled the eligibility criteria. After a full text examination, the two articles were excluded as they did not match the inclusion criteria.36,37 One eligible article found by a manual search was included.38 A total of 13 publications and 21 studies, reporting at least 1129 animals, were included in this review: Afroz et al., 2021;39 Bruce-Keller et al., 2017;20 Buffington et al., 2016;40 Champagne-Jorgensen et al., 2020;41 Hill et al., 2021;42 Lebovitz et al., 2019;11 Lyu et al., 2021;28 Sanguinetti et al., 2019;43 Tochitani et al., 2016;27 Vuong et al., 2020;44 Xiao et al., 2020;45 Yu et al., 2020;46 Leclercq et al., 2017.38
Figure 1.
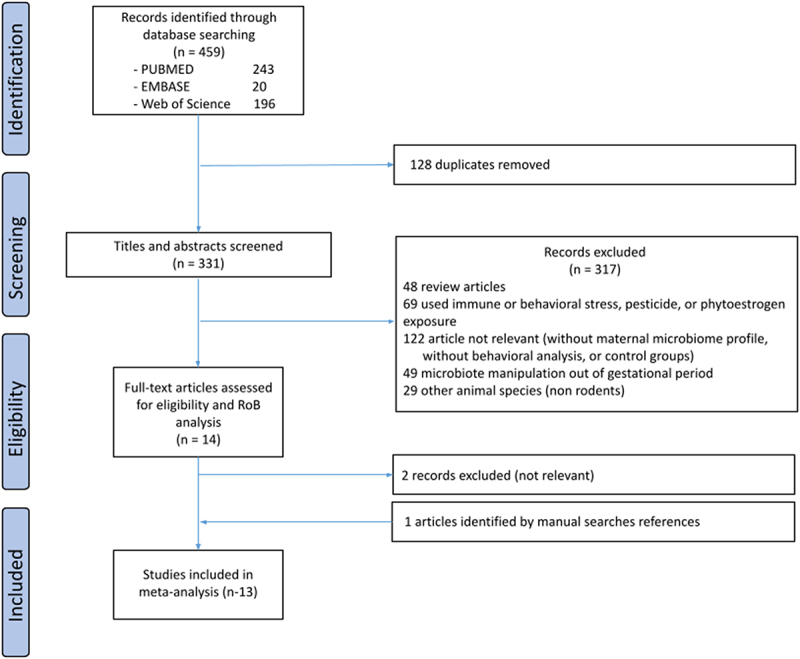
PRISMA flow diagram of the study.
3.2. Description of the eligible articles
The characteristics of the selected articles are presented in Table 1. Most of the articles included in the present work used C57BL/6N (N = 9)11,20,27,28,39,40,42,44,46 mice as animal models. However, other rodent models were used, such as BALB/c (N = 2)38,41 and B6129SF2/J (N = 1) mice43 and Sprague-Dawley rats (N = 1).45
Table 1.
Characteristics of the articles included in the present study.
| Reference (author and year) |
Behavioral paradigm |
Experimental manipulation | Animal | Mean age (weeks) |
Sex | Sample size |
Behavior assessments |
|---|---|---|---|---|---|---|---|
| Lebovitz, 2019 | Sociability | Antibiotic | C57BL/6N | 3 | ND | 86 | Three Chamber |
| Bruce-Keller, 2017 | Fecal Transplantation | C57BL/6N | 10 | M; F | 60 | Ultrasonic vocalization | |
| Tochitani, 2016 | Antibiotic | C57BL/6N | 12 | M | 29 | Social interaction | |
| Buffington., 2016 | High-fat diet | C57BL/6N | 10 | M | 32 | Social Interaction | |
| Jorgensen, 2019 | Antibiotic | BALB/c | 6 | M; F | 59 | Three Chamber | |
| Leclercq, 2017 | Antibiotic | BALB/c | 6 | M; F | 51 | Three Chamber | |
| Afroz, 2021 | High-salt diet | C57BL/6N | 9 | M; F | 52 | Three Chamber | |
| Bruce-Keller, 2017 | OCD | Fecal Transplantation | C57BL/6N | 10 | M; F | 60 | Marble burying |
| Buffington., 2016 | High-fat diet | C57BL/6N | 10 | M | 32 | Marble burying | |
| Afroz, 2021 | High-salt diet | C57BL/6N | 9 | M; F | 52 | Marble burying | |
| Lyu, 2021 | Silver nanoparticle | C57BL/6N | 11 | ND | 23 | Elevated plus maze | |
| Vuong, 2020 | Schizophrenia | Antibiotic | C57BL/6N | 8 | ND | 70 | Prepulse inhibition |
| Bruce-Keller, 2017 | Anxiety | Fecal transplantation | C57BL/6N | 10 | M; F | 60 | Open field |
| Tochitani, 2016 | Antibiotic | C57BL/6N | 12 | M | 79 | Open field | |
| Buffington, 2016 | High-fat diet | C57BL/6N | 10 | M | 32 | Open field | |
| Jorgensen, 2019 | Antibiotic | BALB/c | 6 | M; F | 59 | Elevated plus maze | |
| Yu, 2020 | Low-fiber diet | C57BL/6N | 6 | ND | 34 | Elevated plus maze | |
| Lyu, 2021 | Silver nanoparticle | C57BL/6N | 11 | ND | 23 | Elevated plus maze | |
| Hill, 2021 | Antibiotic | C57BL/6N | 7 | M; F | 20 | Open field | |
| Leclercq, 2017 | Antibiotic | BALB/c | 6 | M; F | 51 | Elevated plus maze | |
| Bruce-Keller, 2017 | Depression | Fecal transplantation | C57BL/6N | 10 | M; F | 60 | Sucrose preference |
| Yu., 2020 | Memory | Low-fiber diet | C57BL/6N | 6 | ND | 34 | Barnes Maze |
| Sanguinetti, 2019 | High-fat diet | B6129SF2/J | 14 | ND | 45 | Y maze | |
| Lyu, 2021 | Silver nanoparticle | C57BL/6N | 11 | ND | 23 | Barnes Maze | |
| Xiao, 2020 | Lead acetate | Sprague-Dawley | 8 | M | 20 | Morris Water Maze |
OCD: obsessive-compulsive disorder; ND: non-defined; M: male; F: female.
Although most studies have split males and females into independent groups,11,20,27,28,38–42,45 some have not differentiated between the sexes.43,46 In this case, the animals were considered to have non-defined sex. As defined in the inclusion criteria, only animals tested from 21 days onwards were included in this meta-analysis. Thus, the animals underwent behavioral tests between an average of 3 and 14 weeks.
The majority of included articles used antibiotics11,27,38,41,44,46 or diet manipulation39,40,43,46 as the experimental approach for maternal microbiota perturbation, followed by environmental contaminant by silver28 and lead45 and fecal transplantation20 (Figure 2a). Manipulations in the different selected articles that occurred during pregnancy and cold also included the breastfeeding period, encompassing the perinatal period. The 16s V4 DNA sequencing method for microbiome characterization was used for 11 articles,11,20,27,28,34–44 one used shotgun metagenomic,28 and one used bacterial culture11 (Figure 2b).
Figure 2.
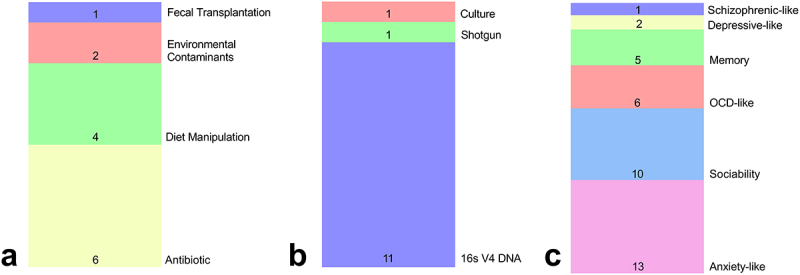
Descriptive analysis of the articles included in the present study. (a) The methodology used to manipulate the maternal gestational microbiota. (b) The method used to characterize the maternal gestational microbiota. (c) Behavioral paradigm analyzed.
The eligible articles used the following outcome measures of behavioral analyses: (1) Open field: inner zone locomotor activity, time spent in the inner zone, or the number of entries in the inner zone; (2) Elevated plus maze (EPM): open arm exploration time (OAE), head dipping; (3) Three Chamber: sociability (score), time spent with social novelty, time in social chamber; (4) Ultrasonic vocalization (UV): vocalizing time; (5) Reciprocal social interaction (RSI): interaction frequency; (6) Barnes Maze (BM): latency to find the target; (7) Y maze (YM): alternation triplets; (8) Morris water maze (MWM): distance traveled in the target quadrant; (9) Marble burying (MB): number of buried marbles; (10) Prepulse inhibition test (PPI): prepulse inhibition; (11) Sucrose preference test (SP): volume consumption.
Thirteen independent groups of animals from the selected articles evaluated anxiety-related behavior, through Open Field20,27,40,42 or Elevated Plus Maze tests;28,38,41,46 ten accessed social deficits behaviors using Three Chamber,11,38,39,41 Ultrasonic Vocalization.20 or Reciprocal Social Interaction tests;27,40 six evaluated obsessive-compulsive behavior using Marble Burying test20,39,40 or Stereotyped Self-grooming;28 five surveyed memory through Barnes Maze.28,46 Y Maze,43 or Morris Water Maze;45 two assessed Depressive-like behavior applying Sucrose Preference test;20 and one investigated Schizophrenia using prepulse Inhibition test44 (Figure 2c).
3.3. Assessment of study quality
The Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) risk of bias (RoB) tool was used to assess the risk of bias in the included animal studies. The risk of bias was low for most of the studies, as described in Supplementary Table S2.
3.4. Global meta-analysis: CESs of maternal enteric microbiome disruption on offspring behavioral outcomes
The analysis combined of all thirty-five behavior outcomes revealed a significant effect size (SMD = −0.51, 95% CI = −0.79 to −0.22, p < .001) indicating that MMD provokes behavioral impairments in the adult offspring (Figure 3). According to Hedges’g criteria, the effect size for the preset collection of data was moderate and associated with high heterogeneity (T2 = 0.54, I2 = 79.85%).
Figure 3.
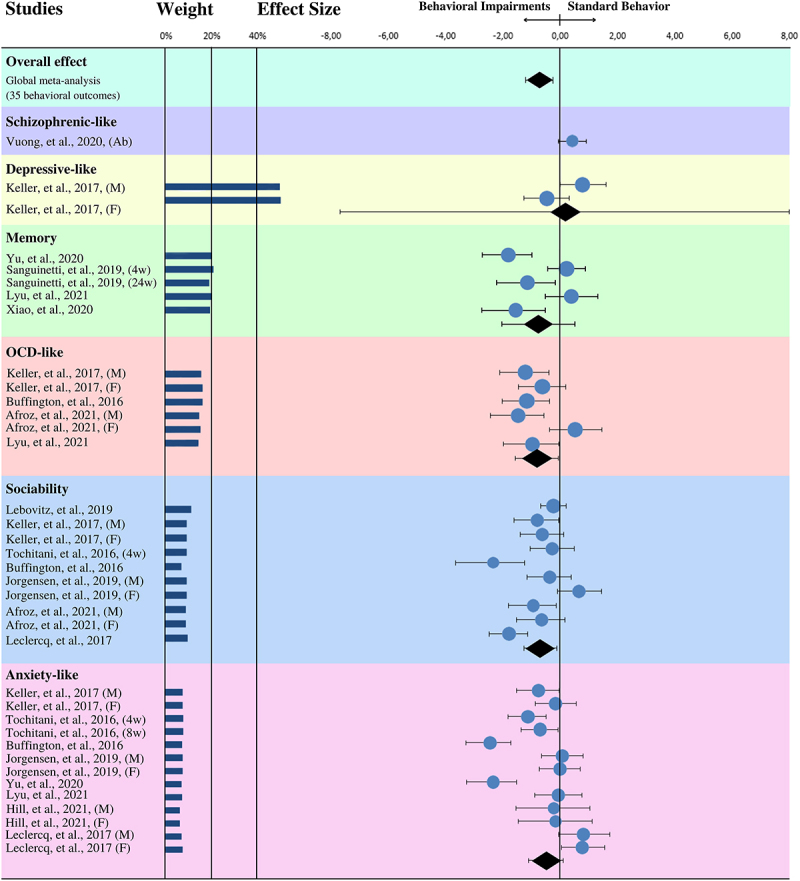
Forest plot graph of the effects of MMD on offspring behavior. M: males; F: females; Ab: Antibiotic; Gf: Germ-free. Only independent groups from the same study were used.
3.5. Stratified meta-analysis: CESs of maternal enteric microbiome disruption on offspring memory, and social, anxiety-like, depressive-like, schizophrenic-type, and obsessive-compulsive behaviors
The estimated CES in the meta-analysis stratified by each outcome reveals that MMD is significatively associated with reduction of sociability behavior (SMD = −0.63, 95% CI = −1.18 to − 0.07, p = 0.011, T2 = 0.30, I2 = 76.11%), and obsessive-compulsive-like behavior (SMD = −0.68, 95% CI = −0.01 to −1.36, p = 0.009, T2 = 0.25, I2 = 62.82%). According to Hedges’g criteria, the effect size for all stratified behaviors was moderate and associated with moderate heterogeneity (Figure 3).
The CESs were moderate and not significant statistically in the studies investigating anxiety-like behavior (SMD = −0.45, 95% CI = −1.07 to 0.17, p = 0.113, T2 = 0.83, I2 = 84.42%) and memory (SMD = −0.67, 95% CI = −1.85 to 0.50, p = 0.111, T2 = 0.79, I2 = 83.41%), and very small in depressive-like behaviors, also not significant statistically (SMD = 0.16, 95% CI = −7.16 to 7.46, p = 0.784, T2 = 0.53, I2 = 80.09%), both with high heterogeneity. As only one experimental group for schizophrenia-like behavior was found in the literature that fulfilled our inclusion criteria, it was not possible to perform a meta-analysis for this model (Figure 3).
4. Discussion
In the last decade, the maternal microbiota has received remarkable attention due to its interference with brain development, resulting in long-lasting effects on the offspring.17,45 According to the developmental origins of health and disease hypothesis.46 during the perinatal period, the development and maturation of several body regulatory systems, such as immune, endocrine, and neural systems, occurs with particular susceptibility to environmental factors.47–49 However, there is still a limited number of clinical studies investigating the role of the maternal enteric microbiome in infant neurodevelopment. Moreover, so far as we know, no study has directly investigated the effects of maternal dysbiosis on offspring’s behavior. Most protocols have used the GF animals or antibiotic, pre-, or probiotic treatments for microbiota manipulation. The present meta-analysis investigated whether the disturbance of maternal enteric microbiota would be associated with behavioral abnormalities manifested by the offspring during adult life. We access this hypothesis by reviewing thirteen eligible, from a set of 459 rodent preclinical studies, identified using a search strategy to determine offspring’s behavioral outcomes generated in MMD protocol.
One of the eligibility criteria requests the confirmation of disturbance of maternal enteric microbiota by at least one validated method. Only one article used growth culture media characterize the microbiota profile, while the other twelve used culture-independent methods. Among them, one study used the shotgun method, and eleven characterized the maternal microbiota by using the 16s metagenomics method.50 The metagenomic methods allow the characterization of a greater number of microorganisms and the processing of significant amounts of data than culture-dependent methods.51 The cost decrease observed in recent decades, especially to 16s metagenomics, explains the exponential number of studies in the field. Although the search strategy included other species as non-human primates, only studies with rodents matched all the criteria. Nine in thirteen studies used mice of the C57BL/6N strain as rodent models, three used other mouse strains (BALB/c and B6129SF2/J), and only one used rats (Sprague-Dawley).
This variability in species/strain may have contributed to the heterogeneity of the meta-analysis. Even though most studies have used GF compared to SPF animals, or protocols for microbiota recolonization of GF animals, the present meta-analysis did not include studies with GF protocols because this condition has been associated with several behavioral and neurodevelopmental abnormalities, and they have not been considered clinically relevant models because its weakness in causality.52
In the present meta-analysis, the combined effects for behavioral outcomes to animal models of major depression disorder, memory, obsessive-compulsive disorder, sociability impairments, and generalized anxiety reveal that MMD might negatively impact the expression of offspring’s behavior during adult life. The meta-analysis stratified for sociability outcomes confirmed a significant impact of MMD on the offspring’s behavior. This finding corroborates other studies showing that maternal microbiota might influence the social behavior of the offspring, being associated with increasing the risk of neurodevelopmental disorders including ASD.18,53,54 For instance, Bruce-Keller et al. demonstrated in 2017 that microbiota transplantation from animals submitted to the High Fat Diet (HFD) to pregnant females decreased the sociability of offspring in both males and females.20 Although this study did not investigate the neural mechanism, it suggested that a healthy maternal microbiota is important for the offspring`s social behavioral development. Lastly, Afroz et al.39 also observed that a high-salt diet during pregnancy reduced the maternal Lactobacillus population, which was related to lower sociability in the offspring, both in males and females.
Social impairments were also observed in studies that used small doses of antibiotics as models of MMD, an approach close to what is observed clinically. Jorgensen and colleagues demonstrated that even low levels of penicillin exposure can lead to social impairments only in male rodents. These behavioral alterations were correlated with brain AVPR1A, AVPR1B, and OXTR altered expression, and decreased balance of splenic FOXP3+ regulatory T cells.38,41 Prenatal penicillin exposure also led to distinct microbiota compositions clustered differently by sex.41 His team continued Leclercq et al.'s work, which in 2017 had already shown that small doses of penicillin during pregnancy and the perinatal period caused deficits in the animal's sociability.38 Regardless of gender, and an increased AVPR1b expression in the prefrontal cortex of both sexes, but not in the hippocampus. This gene encodes a receptor for vasopressin, important for arginine-vasopressin signaling, and is related to the aggressive behavior of offspring.55 The group also observed a systemic increase in pro-inflammatory cytokines, such as interleukin (IL)-6, IL-10, and CXCL15, in the animals’ prefrontal cortex, but without systemic changes.
After that, different studies analyzed brain impairments that could explain the effects of MMD, causing social disabilities in offspring. As observed by Lebovitz et al., one of the mechanisms by which a model of antibiotic-based bacterial depletion in maternal microbiota impairs the proper offspring neurobehavioral development is due to an accentuated prefrontal cortex microglial expression of Cx3cr1, a chemokine receptor for neuron-derived Cx3cl1 (fractalkine). This signaling pathway involves premature senescence of microglia and dysfunctional remodeling of synapses, leading to deficits in the social preference in males and females during the three-chamber test, which can be rescued by Lactobacillus murinus HU-1 or Cx3cr1 Knockout11.
Buffington and colleagues also explored the role of the Limosilactobacillus genus on mechanisms of social deficits induced by dysbiosis40. The group observed that pups of females that submitted to MMD showed social deficits related to lower long-term potentiation (LTP) in the ventral tegmental area (VTA) in dopaminergic neurons during social stimuli, and has a reduction in hypothalamic oxytocinergic neurons when compared to the pups generated in female control. Treating these pups with L. reuteri restores the number of hypothalamic oxytocinergic neurons, which reestablish LTP in the VTA and, consequently, improves the sociability of the animals.
Finally, the study conducted by Tochitani et al. did not observe social impairments in male mice.27 In that work, behavioral analysis was performed on the offspring of females submitted to MMD during pregnancy at four and eight weeks of life. However, the authors report that animals exhibit locomotor impairments at week four, which could be a confounding factor for the sociability test. Also, social behavior was not evaluated at week eight, when the animal no longer showed locomotor impairments. Taken together, these studies accumulate evidence that MMD modulates social behavior in the offspring and highlight the role of the Lactobacillus, Lacticaseibacillus, and Limosilactobacillus genus in sustaining a healthy microbiota for sociability in adult life.11,20,27,38–41
The CES stratified analysis also demonstrated that MMD increases the stereotypical behavior of the offspring. Stereotypical behaviors are symptoms of different disorders such as OCD, ASD56 and SZ,57,characterized by repetitive, functionless motor behavior.57 Stereotyped behavior seems to be related mainly to excessive dopaminergic activity in the basal ganglia,58 which is influenced by the gut microbiota. One of the gold standard models for studying this behavior in mice, with great validity and easy implementation, is the MB test.59 All three studies performed the test on males found that MMD elicits stereotyped behaviors in the offspring.20,39,40 Of these, only two also performed in females,20,39 which did not show significant differences from the control group. In addition to the MB test, another way to analyze stereotyped behaviors is through the quantification of unnecessary and exacerbated compulsive behaviors in mice, such as excessive grooming,28 increased chewing of non-nutritive kaolin clay,60 and excessive head-dipping behaviors.60 In the study by Lyu and collaborators, mice that underwent MMD through exposure to AgnNP spent more time engaged in stereotypic head-dipping behaviors compared to controls.28 Together these results suggest that MMD might have a sex-dependent effect for offspring`s stereotyped behaviors, corroborating the neurodevelopmental hypothesis for ASD.61
Regarding the other behaviors analyzed, it has already been demonstrated that colonization of healthy mice with fecal microbiota provided by donor mice subjected to stress protocols is sufficient to reproduce the stress-related behavior, including anxiety and depressive-like behaviors, in the recipient mice.62 In addition, Schmidt et al. have shown that FMT from healthy adult rats prevented both gut dysbiosis and anxiety-like behaviors development in those animals suffering spinal cord injury.63 Moreover, previous studies also have shown that adult GF animals exhibit a constitutive reduction of anxiety-related behaviors compared to SPF animals.64 This anxiolytic-like response of GF animals also occurs when they are exposed to stress,65 and it is reversed when mice receive microbiota of healthy SPF animals via FMT.65 Although all these pieces of evidence suggest that gut microbiota participants in anxiety-like responses, we found a marginal combined size effect of the MMD on offspring anxiety-like behavior in the present data set. However, we apply a meta-analysis for adult mice undergoing maternal enteric dysbiosis, a protocol different from the results above. Moreover, several animal models that explore anxiety-related behavior are susceptible to other environmental stress.66 Thus, studies aimed to assess the effects of MDD on offspring behavior could have been contaminated by confounding factors.
The same occurs with the CES for memory-related behaviors. D’Amato and coworkers have shown that transplantation of the microbiota from aged mice to adult mice affects spatial learning and memory via modulation of hippocampal synaptic plasticity.67 A sharp decrease in bacteria associated with the production of short-chain fatty acids (SCFAs) (Lachnospiraceae, Faecalibaculum, and Ruminococcaceae) was observed in the transplanted mice. Also, using transplantation techniques, the microbiota of a rodent model of Alzheimer’s disease for C57BL/6 mice decreased neurogenesis in the adult hippocampus and Brain-Derived Neurotrophic Factor (BDNF) expression, and increased p21 expression, leading to memory impairment.68 However, the results of stratified CES in the present study showed no effect of MMD on offspring memory, suggesting that these changes observed in adult animals, at least for the present collection of data, may not be related to MMD. Regarding behaviors related to depression and schizophrenia, the number of results obtained in the present data collection does not permit conclusions so far, and further studies are needed.
GBA is one of the most complex communication systems evolving immunological, endocrine, metabolic, and neural pathways.69–75 However, the mechanism whereby environmental factors during the perinatal-phase compromise the GBA and cause negative consequences to neural development remains to be elucidated.76–78
Dietary habits have been shown a consistent and reproducible factor in shaping the composition of the gut microbiome,79 leading to impairments in memory, exploration, and social behavior in adulthood in rodents.40,43 Different diets provide different nutrients that can select the composition of the microbiota, as shown in clinical80 and preclinical81 studies. In addition, various components of the maternal diet play beneficial roles in mental health depending on the gut microbiome.82 For example, inulin is a soluble fiber that cannot be digested by the body,83 but is metabolized by enteric microbiota, leading to the production of SCFAs.84 Several studies have reported that diet-induced MMD, e.g., inadequate dietary fiber or excessive consumption of high-fat or high-salt foods, disrupt the gut microbiota of the offspring85 and also modify the production of maternal gut metabolites that can cross the placenta and the embryo blood–brain barrier (BBB).86 Both have been associated with neurodegenerative diseases and behavioral disorders.87 In addition, transplantation of the HFD gut microbiota into female mice before breeding can disrupt social behavior in males, but not in females and offspring.20 This study adds to evidence that behavioral impairments induced by HFD may be vertically transmitted to the offspring through the microbiota in a sex-specific manner.
Another way to trigger MMD applied in the studies included in the present meta-analysis was the consumption of environmental contaminants, such as silver and lead. These substances are widely used by the food and pharmaceutical industries because of their beneficial antimicrobial and antifungal properties.88 However, chronic exposure to environmental pollutants has been linked to the development of neurodegenerative diseases, as these substances can accumulate in various organs, such as the brain and gut, with harmful effects. Moreover, due to their antimicrobial activity and accumulation in the gut, these materials can alter the microbiota when ingested, depending on the dose and format of the nanoparticles.28 In two papers presented here,28,45 environmental contaminants were found in association with MMD. The contaminant induced the reduction in resident hippocampal microglial cells and stereotyped behavior in the offspring.28 Memory impairment was also reported to be associated with morphological abnormalities in hippocampal dendritic spines,45 which could be reversed with a multispecies probiotic containing genus Bifidobacterium, Lactobacillus, Lacticaseibacillus, Limosilactobacillus, and Streptococcus.45 Together, these findings reinforce the role of environmental contaminants in MMD and its consequences to neurodevelopmental and mental health.
Antibiotic administration, in turn, has been pointed out as the most useful method for MMD induction generating a GF-like phenotype. These drugs may influence neurodevelopment by (1) altering the maternal gestational microbiota;89 (2) changing the profile of microorganisms passed to the offspring;90 (3) changing the absorption of nutrients by the mother, which may affect the microbiota and milk composition;91 (4) causing hyperactivation of the HPA axis by altering the maternal microbiota and impairing maternal nurturing behavioral in the postnatal period, which is a stress factor for the offspring.27 Yet, a common criticism regarding the use of antibiotics to deplete the microbiota in animal models is the lack of similarity with what actually occurs in clinics, which use significantly lower doses.38
Furthermore, different combinations and concentrations of antibiotics are used in the literature and, despite generally impacting the most abundant phyla such as Firmicutes and Bacteroidetes, this diversity causes different changes at lower taxonomic levels.92 In addition, clinical evidence has already shown that the individual’s microbiota before the start of antibiotic treatment is one of the main determining factors for the disturbances that will be caused in the microorganisms populations.93 Together, the combination of antibiotics used and the profile of the healthy microbiota of the animals used in each study might contribute to the heterogeneity observed between the different studies.
In the context of the neuro-immune system, a body of evidence suggests that inflammatory unbalance during pregnancy might facilitate neurodevelopmental psychiatric illness. Elevated levels of gestational maternal pro-inflammatory markers including C reactive protein (CRP) and pro-inflammatory cytokines such as IL-6, IL-1, and IFN-gamma have been associated with ASD,94 SZ,95 Parkinson96 and Alzheimer disease.97–102 Inflammatory environments seem to be related to a brain region-specific microglial activation, such as prefrontal cortex, hippocampus, and amygdala, with elevated expression of microglial senescence genes (such as Trp53 and I1β),11 inflammatory mediators (e.g. Cx3cr1),11 and reduced oxytocin signaling,40 which are correlated with dysfunctional modeling of synapses and behavior. Finally, evidence from our group and others103 has shown a remarkable effect on hippocampal biomarkers of synaptic plasticity when the dysbiosis is provoked during the weaning period (data not published). Therefore, the immune system has been considered a crucial for maintaining hippocampal neurogenesis, social behavior, and cognitive functions.104,105 In particular, for neurodevelopment, the gut maternal enteric microbiota has emerged as a crucial player in the immune system balance and maturation. For instance, in a model of reversible colonization of germ-free mice during gestation, the microbial shapes the neonatal immune system even before birth through molecular signals derived from the microbiota of the mother.3 Otherwise, maternal microbiota was important to mature intestinal innate immune cells and to alter intestinal gene expression profiles in the offspring.3,106 A clinical study with a cohort of 1074 newborn infants revealed that maternal gut microbiota might conditionate the composition of child immune cells.107 Infants clustered by Dialister, Escherichia, and Ruminococcus present lower proportion of granulocytes, and higher proportion of both central naïve CD4 T cells (CD4+/CD45RA+/CD31−) and naïve regulatory T cells (Treg) (CD4+/CD45RA+/FoxP3low). However, the meaning of this last finding for neurodevelopment remains to be elucidated. Therefore, the effect of MMD may have multiple pathways facilitate this bidirectional communication between the maternal gut microbiota and the offspring brain, including direct and humoral interactions of microbes or their metabolites with intrinsic and extrinsic neurons,108–110 lymphoid organs-derived soluble inflammatory mediators,111 and/or translocation of gut microbial products, such as tryptophan metabolites or SCFAs, into the brain circulation.112–114
Preclinical evidence is further consolidated regarding the neural pathways of enteric dysbiosis on behavior. For example, Sgritta et al.4 demonstrated that L. reuteri treatment reverses social deficits in a model of autism, including in GF animals, via activation of afferent fibers from the vagus nerve, an important nerve involved in the interaction between the periphery and CNS, for more details see.115 This group observed that L. reuteri treatment induces vagal signals to the hypothalamus . paraventricular nuclei (PVN), stimulating oxytocin production via neurons projecting to the VTA, restoring mechanisms of synaptic potentiation in this region.4 These findings link the adverse effects of maternal diet- and antibiotic-induced disruption of gut dysbiosis and neurobehavioral impairment of offspring.20
In addition, gut-brain communication also involves the production of hormones, neurotransmitters, and metabolites by enteric microbiota.116 These signaling molecules have local117 and systemic118 effects, and some of them can cross the BBB and act directly on the central nervous system. Regarding the impact of the maternal gestational microbiota on offspring neurodevelopment, preclinical103 and clinical53 studies in recent decades have focussed primarily on the production of maternal microbial metabolites capable of crossing the placenta and the embryo BBB,119 such as SCFAs. These small organic monocarboxylic acid molecules are the main products of microbial anaerobic fermentation of indigestible polysaccharides, such as dietary fiber, e.g., inulin, and resistant starch in the colon.120 Locally, the effects of SCFAs range from maintaining intestinal barrier integrity, mucus production, and protection against inflammation to reducing the risk of colorectal cancer.106 However, a fraction of these molecules also reach the systemic circulation, and they are capable of crossing both the placenta119 and the BBB,119 which is rich in SCFA transporters.108 In the brain, SCFAs regulate BBB integrity and function,121 promote microglial maturation,122 reduce neuroinflammation in models of LPS administration123 and ischemic stroke124, and affect neuronal function by modulating levels of neurotransmitters and neurotrophic factors.108 It is therefore speculated that SCFAs play a central role in both microbiota-gut-brain crosstalk108 and communication between the maternal microbiota and the embryo,125 consequently influencing neurodevelopment46 and behavior126 in the offspring.
Finally, it has been shown that some of the physiological impairments caused by altered gut microbiota may be antagonized, especially if treatment is given in the early stages of neurodevelopment, by (1) oral supplementation with specific probiotics (L. murinus HU-1 and L. reuteri);11,40 (2) co-housing with animals with a healthy microbiota;40 (3) treatment with metabolites from the maternal microbiota (e.g., butyrate, trimethylamine N-oxide, and imidazole propionate)44,46 and hormones (e.g., oxytocin);40 (4) or even targeting inflammatory components in the brain (e.g., Cx3cr1).11 Such manipulations may serve as future therapeutic approaches in an area that needs more research. The available meta-analysis suggests that maternal gut dysbiosis influences offspring behavior, which in turn is related to individual mental health and susceptibility to psychiatric disorders.
The principal limitation of the studies included in this meta-analysis is the lack of consistency in the standardization of protocols utilized among the different laboratories leading to low reproducibility. Many variables result in conflicting outcomes, including sexual dimorphism, behavioral tests, time and type of maternal dysbiosis utilized, assays to determine the microbiota, species, strain, and age of rodents, dose, and type of antibiotic that the animals were exposed to generate gut dysbiosis. Among these factors, the behavioral differences observed in sexual dimorphism were considered relevant given that, although many studies were performed utilizing male and female rodents,11,20,28,38,39,41,42,44 those that investigated males and females individually observed that the male was more sensitive to maternal microbiota-induced behavioral alteration than the females.20,39,41,42 Sexual dimorphism has already been reported for the social behavior parading in a study that used female offspring in the social interaction test.127 Even in humans, sexual dimorphism may influence the behavior or neurological outcomes linked to enteric microbiota. For instance, a study that evaluated the alpha diversity in six-week-old babies revealed that the higher diversity may be associated with internalizing (anxiety and depression) behavior only in boys, but not among girls, evaluated at age of three years old.128 Therefore, despite the high heterogeneity, the present study shows by the current scientific literature the importance of the maternal microbiota in the social behavior of the offspring. Further meta-analyses discriminating behavior outcomes for male and female animals might better clarify the sexual dimorphism associated with MMD.
Therefore, the present work demonstrates the importance of standardizing experimental paradigms in this new area of study so that the large amount of data generated can be analyzed more clearly. It confirms, from the current collection of references, which different environmental disturbances in the maternal enteric microbiota during the gestational period, such as antibiotic administration,11,41 diet,40 and environmental contaminants,20,28,45 can affect the behavior in the offspring during adult life.
Conclusions
Our meta-analysis shows that maternal gut microbiota alterations during pregnancy generate changes in sociability and stereotypic behaviors related to psychiatric disorders in the offspring. The potential long-lasting behavioral disturbances of MMD in memory and mood disorders remain to be better investigated.
Supplementary Material
Acknowledgments
The authors are thankful to Andreia Vieira, Carla Saudanha, and André Sales for their kind office and technical support. CLO received funds from the Alexander von Humboldt Foundation to keep the website CAMARADES BR (https://camaradesbrasil.bio.br/).
Funding Statement
The work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [88887.630534/2021-00]; Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro [E-26/203.383/2021].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2226282
References
- 1.de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M.. Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes. 2017;8:253–19. doi: 10.1080/19490976.2017.1293224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JC, Im S-H. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med. 2020;52(9):1383–1396. doi: 10.1038/s12276-020-0473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 4.Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, Costa-Mattioli M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron [Internet]. 2019;101(2):246–259.e6. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker A, Romano S, Ansorge R, Aboelnour A, Le Gall G, Savva GM, Pontifex MG, Telatin A, Baker D, Jones E, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 2022;10(1):68. doi: 10.1186/s40168-022-01243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jašarević E, Howard CD, Morrison K, Misic A, Weinkopff T, Scott P, Hunter C, Beiting D, Bale TL. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat Neurosci. 2018;21(8):1061–1071. doi: 10.1038/s41593-018-0182-5. [DOI] [PubMed] [Google Scholar]
- 7.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X-N, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Y, Zeng B, Zeng L, Du X, Li B, Huo R, Liu L, Wang H, Dong M, Pan J, et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry. 2018;8(1):187. doi: 10.1038/s41398-018-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S-H, Yoon S-H, Jung Y, Kim N, Min U, Chun J, Choi I. Emotional well-being and gut microbiome profiles by enterotype. Sci Rep. 2020;10(1):20736. doi: 10.1038/s41598-020-77673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YM, Mu A, Wallace M, Gengatharan JM, Furst AJ, Bode L, Metallo CM, Ayres JS. Microbiota control of maternal behavior regulates early postnatal growth of offspring. Sci Adv. 2021;7(5). doi: 10.1126/sciadv.abe6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebovitz Y, Kowalski EA, Wang X, Kelly C, Lee M, McDonald V, Ward R, Creasey M, Mills W, Gudenschwager Basso EK, et al. Lactobacillus rescues postnatal neurobehavioral and microglial dysfunction in a model of maternal microbiome dysbiosis. Brain Behav Immun. 2019;81:617–629. doi: 10.1016/j.bbi.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Meyer K, Lulla A, Debroy K, Shikany JM, Yaffe K, Meirelles O, Launer LJ. Association of the gut microbiota with cognitive function in midlife. JAMA Netw Open. 2022;5(2):e2143941. doi: 10.1001/jamanetworkopen.2021.43941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safadi JM, Quinton AMG, Lennox BR, Burnet PWJ, Minichino A. Gut dysbiosis in severe mental illness and chronic fatigue: a novel trans-diagnostic construct? A systematic review and meta-analysis. Mol Psychiatry. 2022;27(1):141–153. doi: 10.1038/s41380-021-01032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46(1):77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Drell T, Štšepetova J, Simm J, Rull K, Aleksejeva A, Antson A, Tillmann V, Metsis M, Sepp E, Salumets A, et al. The influence of different maternal microbial communities on the development of infant gut and oral microbiota. Sci Rep. 2017;7(1):9940. doi: 10.1038/s41598-017-09278-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson SL, O’Hely M, Jacka FN, Ponsonby A-L, Symeonides C, Loughman A, Collier F, Moreno-Betancur M, Sly P, Burgner D, et al. Maternal prenatal gut microbiota composition predicts child behaviour. EBioMedicine. 2021;68:103400. doi: 10.1016/j.ebiom.2021.103400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luck B, Engevik MA, Ganesh BP, Lackey EP, Lin T, Balderas M, Major A, Runge J, Luna RA, Sillitoe RV, et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci Rep. 2020;10(1):7737. doi: 10.1038/s41598-020-64173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Yang J, Zhang J, Liang C, Wang Y, Chen B, Zhao C, Wang J, Zhang G, Zhao D, et al. Correlation of gut microbiome between ASD children and mothers and potential biomarkers for risk assessment. Genom Proteom Bioinform. 2019;17(1):26–38. doi: 10.1016/j.gpb.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiuolo J, Musolino V, Gliozzi M, Carresi C, Scarano F, Nucera S, Scicchitano M, Oppedisano F, Bosco F, Macri R, et al. Involvement of the intestinal microbiota in the appearance of multiple sclerosis: aloe vera and citrus bergamia as potential candidates for intestinal health. Nutrients. 2022;14(13):14. doi: 10.3390/nu14132711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce-Keller AJ, Fernandez-Kim S-O, Townsend RL, Kruger C, Carmouche R, Newman S, Salbaum JM, Berthoud H-R, Rosenfeld CS. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. Plos One. 2017;12(4):e0175577. doi: 10.1371/journal.pone.0175577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y, Cheng K, Zhou C, Wang H, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 23.van Beurden YH, de Groot PF, van Nood E, Nieuwdorp M, Keller JJ, Goorhuis A, Beurden YH, Groot PF. Complications, effectiveness, and long term follow-up of fecal microbiota transfer by nasoduodenal tube for treatment of recurrent Clostridium difficile infection. United Eur Gastroenterol J. 2017;5(6):868–879. doi: 10.1177/2050640616678099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karp NA. Reproducible preclinical research—Is embracing variability the answer? Plos Biol. 2018;16(3):e2005413. doi: 10.1371/journal.pbio.2005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eberl G. Addressing the experimental variability associated with the microbiota. Mucosal Immunol. 2015;8(3):487–490. doi: 10.1038/mi.2015.26. [DOI] [PubMed] [Google Scholar]
- 26.Moore RJ, Stanley D. Experimental design considerations in microbiota/inflammation studies. Clin Trans Immunol. 2016;5(7):e92. doi: 10.1038/cti.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tochitani S, Ikeno T, Ito T, Sakurai A, Yamauchi T, Matsuzaki H, Shankar K. Administration of non-absorbable antibiotics to pregnant mice to perturb the maternal gut microbiota is associated with alterations in offspring behavior. Plos One. 2016;11(1):e0138293. doi: 10.1371/journal.pone.0138293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyu Z, Ghoshdastidar S, Rekha KR, Suresh D, Mao J, Bivens N, Kannan R, Joshi T, Rosenfeld CS, Upendran A. Developmental exposure to silver nanoparticles leads to long term gut dysbiosis and neurobehavioral alterations. Sci Rep. 2021;11(1):6558. doi: 10.1038/s41598-021-85919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Forero DA, Lopez-Leon S, González-Giraldo Y, Bagos PG, Markel S. Ten Simple rules for carrying out and writing meta-analyses. Plos Comput Biol. 2019;15(5):e1006922. doi: 10.1371/journal.pcbi.1006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yassour M, Jason E, Hogstrom LJ, Arthur TD, Tripathi S, Siljander H, Selvenius J, Oikarinen S, Hyöty H, Virtanen SM, et al. Strain-level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host & Microbe. 2018;24(1):146–154.e4. doi: 10.1016/j.chom.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue C, Xie Q, Zhang C, Hu Y, Song X, Jia Y, Shi X, Chen Y, Liu Y, Zhao L, et al. Vertical transmission of the gut microbiota influences glucose metabolism in offspring of mice with hyperglycaemia in pregnancy. Microbiome. 2022;10(1):122. doi: 10.1186/s40168-022-01318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW, de Vries RB. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol [Internet]. 2014;14(1):43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: a free and simple tool for meta-analysis. Res Synth Methods. 2017;8(4):537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4(3):279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall BL, Liu Y, Farrington MJ, Mao J, Helferich WG, Schenk AK, Bivens NJ, Sarma SJ, Lei Z, Sumner LW, et al. Early genistein exposure of California mice and effects on the gut microbiota–brain axis. J Endocrinol. 2019;242(2):139–157. doi: 10.1530/JOE-19-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry [Internet]. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 38.Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, Koren O, Forsythe P, Bienenstock J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8(1):15062. doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afroz KF, Reyes N, Young K, Parikh K, Misra V, Alviña K. Altered gut microbiome and autism like behavior are associated with parental high salt diet in male mice. Sci Rep. 2021;11(1):8364. doi: 10.1038/s41598-021-87678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Champagne-Jorgensen K, Mian MF, Kay S, Hanani H, Ziv O, McVey NReufeld K-A, Koren O, Bienenstock J. Prenatal low-dose penicillin results in long-term sex-specific changes to murine behaviour, immune regulation, and gut microbiota. Brain Behav Immun. 2020;84:154–163. doi: 10.1016/j.bbi.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Hill EM, Howard CD, Bale TL, Jašarević E. Perinatal exposure to tetracycline contributes to lasting developmental effects on offspring. Anim Microbiome. 2021;3(1):37. doi: 10.1186/s42523-021-00099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanguinetti E, Guzzardi MA, Tripodi M, Panetta D, Selma-Royo M, Zega A, Telleschi M, Collado MC, Iozzo P. Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Sci Rep. 2019;9(1):12609. doi: 10.1038/s41598-019-48090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586(7828):281–286. doi: 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao J, Wang T, Xu Y, Gu X, Li D, Niu K, Wang T, Zhao J, Zhou R, Wang H-L. Long-term probiotic intervention mitigates memory dysfunction through a novel H3K27me3-based mechanism in lead-exposed rats. Transl Psychiatry. 2020;10(1):25. doi: 10.1038/s41398-020-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu L, Zhong X, He Y, Shi Y. Butyrate, but not propionate, reverses maternal diet-induced neurocognitive deficits in offspring. Pharmacol Res. 2020;160:105082. doi: 10.1016/j.phrs.2020.105082. [DOI] [PubMed] [Google Scholar]
- 47.Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Monk C, Fernández CR. Neuroscience advances and the developmental origins of health and disease research. JAMA Netw Open [Internet]. 2022;5(4):e229251–e229251. doi: 10.1001/jamanetworkopen.2022.9251. [DOI] [PubMed] [Google Scholar]
- 49.Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009;27(5):391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson D, Bonham KS, Rowland S, Pattanayak CW, Consortium RESONANCE, Klepac-Ceraj V, Deoni SCL, D’Sa V, Bruchhage M, Volpe A, et al. Comparative analysis of 16S rRNA gene and metagenome sequencing in pediatric gut microbiomes. Front Microbiol [Internet]. 2021;12. doi: 10.3389/fmicb.2021.670336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galloway-Peña J, Hanson B. Tools for analysis of the microbiome. Dig Dis Sci. 2020;65(3):674–685. doi: 10.1007/s10620-020-06091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uzbay T. Germ-free animal experiments in the gut microbiota studies. Curr Opin Pharmacol. 2019;49:6–10. doi: 10.1016/j.coph.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Urbonaite G, Knyzeliene A, Bunn FS, Smalskys A, Neniskyte U. The impact of maternal high-fat diet on offspring neurodevelopment. Front Neurosci. 2022;16:909762. doi: 10.3389/fnins.2022.909762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta L, Hoffman KW. Exploring the intersection of the microbiome and the developing brain: impacts on schizophrenia risk. Schizophr Res. 2022;247:92–100. doi: 10.1016/j.schres.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Wersinger SR, Ginns EI, O’Carroll A-M, Lolait SJ, Young WS III. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry [Internet]. 2002;7(9):975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham AB, Schreibman L. Stereotypy in autism: the importance of function. Research in Autism Spectrum Disorders [Internet]. 2008;2(3):469–479. https://www.sciencedirect.com/science/article/pii/S1750946707000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrens M, Hulstijn W, Lewi PJ, De Hert M, Sabbe BGC. Stereotypy in schizophrenia. Schizophr Res. 2006;84(2–3):397–404. doi: 10.1016/j.schres.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Ridley RM. The psychology of perseverative and stereotyped behaviour. Progress in Neurobiology [Internet]. 1994;44(2):221–231. https://www.sciencedirect.com/science/article/pii/0301008294900396 [DOI] [PubMed] [Google Scholar]
- 59.Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp. 2013;82:50978. doi: 10.3791/50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robbins TW, Vaghi MM, Banca P. Obsessive-compulsive disorder: puzzles and prospects. Neuron. 2019;102:27–47. doi: 10.1016/j.neuron.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 61.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11(7):490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, Li X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress [Internet]. 2019;22(5):592–602. doi: 10.1080/10253890.2019.1617267. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt EKA, Torres-Espin A, Raposo PJF, Madsen KL, Kigerl KA, Popovich PG, Fenrich KK, Fouad K, Di Giovanni S. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. Plos One. 2020;15(1):e0226128. doi: 10.1371/journal.pone.0226128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu T-W, Park Y-M, Holscher HD, Padilla J, Scroggins RJ, Welly R, Britton SL, Koch LG, Vieira-Potter VJ, Swanson KS, et al. Physical activity differentially affects the cecal microbiota of ovariectomized female rats selectively bred for high and low aerobic capacity. Plos One. 2015;10(8):e0136150. doi: 10.1371/journal.pone.0136150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, Wang H, Zhou C, Fang L, Li W, et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. 2017;7:489. doi: 10.3389/fcimb.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohl F. Animal models of anxiety. Handb Exp Pharmacol. 2005;169:35–69. [DOI] [PubMed] [Google Scholar]
- 67.D’Amato A, Di Cesare Mannelli L, Lucarini E, Man AL, Le Gall G, Branca JV, Ghelardini C, Amedei A, Bertelli E, Regoli M, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome [Internet]. 2020;8(1):140. doi: 10.1186/s40168-020-00914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim N, Jeon SH, Ju IG, Gee MS, Do J, Oh MS, Lee JK. Transplantation of gut microbiota derived from Alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav Immun. 2021;98:357–365. doi: 10.1016/j.bbi.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82(7):472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 70.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59(12):1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jašarević E, Rodgers AB, Bale TL. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol Stress. 2015;1:81–88. doi: 10.1016/j.ynstr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lima-Ojeda JM, Rupprecht R, Baghai TC. “I am i and my bacterial circumstances”: linking gut microbiome, neurodevelopment, and depression. Front Psychiatry [Internet]. 2017;8:153. doi: 10.3389/fpsyt.2017.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winter G, Hart RA, Charlesworth RPG, Sharpley CF. Gut microbiome and depression: what we know and what we need to know. Rev Neurosci. 2018;29(6):629–643. doi: 10.1515/revneuro-2017-0072. [DOI] [PubMed] [Google Scholar]
- 74.O’Mahony SM, Clarke G, Dinan TG, Cryan JF. Early-life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience. 2017;342:37–54. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 75.Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh S, Sharma P, Pal N, Kumawat M, Shubham S, Sarma DK, Tiwari RR, Kumar M, Nagpal R. Impact of environmental pollutants on gut microbiome and mental health via the gut–brain axis. Microorganisms. 2022;10(7):10. doi: 10.3390/microorganisms10071457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, Ducarmon QR, Keller JJ, Kuijper EJ, Contarino MF. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. doi: 10.3389/fcimb.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10:572912. doi: 10.3389/fcimb.2020.572912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Besson AA, Lagisz M, Senior AM, Hector KL, Nakagawa S. Effect of maternal diet on offspring coping styles in rodents: a systematic review and meta-analysis. Biol Rev Camb Philos Soc. 2016;91(4):1065–1080. doi: 10.1111/brv.12210. [DOI] [PubMed] [Google Scholar]
- 80.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beam A, Clinger E, Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13(8):2795. doi: 10.3390/nu13082795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, Jacka F, Dinan TG, Cryan JF. Diet and the microbiota–gut–brain axis: sowing the seeds of good mental health. Adv Nutr. 2021;12(4):1239–1285. doi: 10.1093/advances/nmaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ellegård L, Andersson H, Bosaeus I. Inulin and oligofructose do not influence the absorption of cholesterol, or the excretion of cholesterol, Ca, Mg, Zn, Fe, or bile acids but increases energy excretion in ileostomy subjects. Eur J Clin Nutr. 1997;51(1):1–5. doi: 10.1038/sj.ejcn.1600320. [DOI] [PubMed] [Google Scholar]
- 84.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9 [DOI] [PubMed] [Google Scholar]
- 85.Xie R, Sun Y, Wu J, Huang S, Jin G, Guo Z, Zhang Y, Liu T, Liu X, Cao X, et al. Maternal high fat diet alters gut microbiota of offspring and exacerbates DSS-induced colitis in adulthood. Front Immunol. 2018;9:2608. doi: 10.3389/fimmu.2018.02608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellisario V, Panetta P, Balsevich G, Baumann V, Noble J, Raggi C, Nathan O, Berry A, Seckl J, Schmidt M, et al. Maternal high-fat diet acts as a stressor increasing maternal glucocorticoids’ signaling to the fetus and disrupting maternal behavior and brain activation in C57BL/6J mice. Psychoneuroendocrinology. 2015;60:138–150. doi: 10.1016/j.psyneuen.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 87.Winther G, Elfving B, Müller HK, Lund S, Wegener G. Maternal high-fat diet programs offspring emotional behavior in adulthood. Neuroscience. 2018;388:87–101. doi: 10.1016/j.neuroscience.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X-F, Liu Z-G, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C-M, Chou H-C, Y-CSH Y. Maternal antibiotic treatment disrupts the intestinal microbiota and intestinal development in neonatal mice [Internet]. Front Microbiol. 2021;12. doi: 10.3389/fmicb.2021.684233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dierikx TH, Visser DH, Benninga MA, van Kaam AHLC, de Boer NKH, de Vries R, van Limbergen J, de Meij TGJ, van Kaam AHLC, de Boer NKH, et al. The influence of prenatal and intrapartum antibiotics on intestinal microbiota colonisation in infants: a systematic review. J Infect [Internet]. 2020;81(2):190–204. doi: 10.1016/j.jinf.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Demmelmair H, Jiménez E, Collado MC, Salminen S, McGuire MK. Maternal and perinatal factors associated with the human milk microbiome. Curr Dev Nutr. 2020;4(4):nzaa027. doi: 10.1093/cdn/nzaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang L, Bajinka O, Jarju PO, Tan Y, Taal AM, Ozdemir G. The varying effects of antibiotics on gut microbiota. AMB Express. 2021;11(1):116. doi: 10.1186/s13568-021-01274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Nalluri H, Kaiser T, Holtan SG, Khoruts A, Weisdorf DJ, Staley C. Gut microbiota response to antibiotics is personalized and depends on baseline microbiota. Microbiome [Internet]. 2021;9(1):211. doi: 10.1186/s40168-021-01170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chudal R, Brown AS, Gyllenberg D, Hinkka-Yli-Salomäki S, Sucksdorff M, Surcel H-M, Upadhyaya S, Sourander A. Maternal serum C-reactive protein (CRP) and offspring attention deficit hyperactivity disorder (ADHD). Eur Child Adolesc Psychiatry. 2020;29(2):239–247. doi: 10.1007/s00787-019-01372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown AS, Meyer U. Maternal immune activation and neuropsychiatric illness: a translational research perspective. Am J Psychiatry. 2018;175(11):1073–1083. doi: 10.1176/appi.ajp.2018.17121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson’s disease. Front Biosci. 2003;8(6):s826–37. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- 97.Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 2016;12(6):719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 98.Kim E, Paik D, Ramirez RN, Biggs DG, Park Y, Kwon H-K, Choi GB, Huh JR. Maternal gut bacteria drive intestinal inflammation in offspring with neurodevelopmental disorders by altering the chromatin landscape of CD4+ T cells. Immunity. 2022;55(1):145–158.e7. doi: 10.1016/j.immuni.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spini VBMG, Ferreira FR, Gomes AO, Duarte RMF, Oliveira VHS, Costa NB, Ferreira AFF, Dourado MDPB, Ribeiro-Barbosa ER. Maternal immune activation with H1N1 or toxoplasma gondii antigens induces behavioral impairments associated with mood disorders in rodents. Neuropsychobiology. 2020;320. doi: 10.1159/000510791. [DOI] [PubMed] [Google Scholar]
- 100.Ferreira FR, de Moura NSB, Hassib L, Pombo TR, de Moura NSB. Resveratrol ameliorates the effect of maternal immune activation associated with schizophrenia in adulthood offspring. Neurosci Lett [Internet]. 2020;734:135100. doi: 10.1016/j.neulet.2020.135100. [DOI] [PubMed] [Google Scholar]
- 101.Ferreira FR, de Paula GC, de Carvalho RJ V, Ribeiro-Barbosa ER, Spini VBMG, de Paula G, de Carvalho RV. Impact of season of birth on psychiatric disorder susceptibility and drug abuse incidence in a Population from the Köppen Tropical Savanna Region of Brazil. Neuropsychobiology. 2020;79(2):131–140. doi: 10.1159/000503069. [DOI] [PubMed] [Google Scholar]
- 102.Xu Z, Zhang X, Chang H, Kong Y, Ni Y, Liu R, Zhang X, Hu Y, Yang Z, Hou M, et al. Rescue of maternal immune activation-induced behavioral abnormalities in adult mouse offspring by pathogen-activated maternal T(reg) cells. Nat Neurosci. 2021;24(6):818–830. doi: 10.1038/s41593-021-00837-1. [DOI] [PubMed] [Google Scholar]
- 103.Di Gesù CM, Matz LM, Bolding IJ, Fultz R, Hoffman KL, Gammazza AM, Petrosino JF, Buffington SA. Maternal gut microbiota mediate intergenerational effects of high-fat diet on descendant social behavior. Cell Rep. 2022;41:111461. doi: 10.1016/j.celrep.2022.111461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 105.Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535(7612):425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ganal-Vonarburg SC, Fuhrer T, Gomez de Agüero M. Maternal microbiota and antibodies as advocates of neonatal health. Gut Microbes. 2017;8(5):479–485. doi: 10.1080/19490976.2017.1299847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao Y, O’Hely M, Quinn TP, Ponsonby A-L, Harrison LC, Frøkiær H, Tang MLK, Brix S, Kristiansen K, Burgner D, et al. Maternal gut microbiota during pregnancy and the composition of immune cells in infancy. Front Immunol. 2022;13:986340. doi: 10.3389/fimmu.2022.986340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng W, Zhao W, Wu M, Song X, Caro F, Sun X, Gazzaniga F, Stefanetti G, Oh S, Mekalanos JJ, et al. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature [Internet]. 2020;577(7791):543–548.doi: 10.1038/s41586-019-1898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci Off J Soc Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14(3):555–565. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris G, Berk M, Carvalho A, Caso JR, Sanz Y, Walder K, Maes M. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol Neurobiol. 2017;54(6):4432–4451. doi: 10.1007/s12035-016-0004-2. [DOI] [PubMed] [Google Scholar]
- 113.Bosi A, Banfi D, Bistoletti M, Giaroni C, Baj A. Tryptophan metabolites along the microbiota-gut-brain axis: an interkingdom communication system influencing the gut in health and disease. Int J Tryptophan Res. 2020;13:1178646920928984. doi: 10.1177/1178646920928984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoffiz YC, Castillo-Ruiz A, Hall MAL, Hite TA, Gray JM, Cisternas CD, Cortes LR, Jacobs AJ, Forger NG. Birth elicits a conserved neuroendocrine response with implications for perinatal osmoregulation and neuronal cell death. Sci Rep [Internet]. 2021;11(1):2335. doi: 10.1038/s41598-021-81511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840(1):289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 116.Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lai Y, Dhingra R, Zhang Z, Ball LM, Zylka MJ, Lu K. Toward elucidating the human gut microbiota–brain axis: molecules, biochemistry, and implications for health and diseases. Biochem Implications Health Diseases Biochem. 2021;61(24):2806–2821. doi: 10.1021/acs.biochem.1c00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic signaling along the microbiota-gut-brain Axis. Int J Mol Sci. 2019;20(6):20. doi: 10.3390/ijms20061482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ziętek M, Celewicz Z, Szczuko M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients. 2021;13(4):1244. doi: 10.3390/nu13041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol [Internet]. 1996;62(5):1589–1592. doi: 10.1128/aem.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci [Internet]. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, Harada K, Shirawachi S, Asano S, Aizawa H, Yamawaki S, et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res [Internet]. 2018;1680:13–38. https://www.sciencedirect.com/science/article/pii/S0006899317305383 [DOI] [PubMed] [Google Scholar]
- 124.Patnala R, Arumugam TV, Gupta N, Dheen ST. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol Neurobiol [Internet]. 2017;54(8):6391–6411. doi: 10.1007/s12035-016-0149-z. [DOI] [PubMed] [Google Scholar]
- 125.Needell JC, Ir D, Robertson CE, Kroehl ME, Frank DN, Zipris D, Mounier C. Maternal treatment with short-chain fatty acids modulates the intestinal microbiota and immunity and ameliorates type 1 diabetes in the offspring. Plos One. 2017;12(9):e0183786. doi: 10.1371/journal.pone.0183786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Z, Li L, Ma S, Ye J, Zhang H, Li Y, Sair AT, Pan J, Liu X, Li X, et al. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J Agric Food Chem [Internet]. 2020;68(47):13697–13710. doi: 10.1021/acs.jafc.0c04290. [DOI] [PubMed] [Google Scholar]
- 127.Kopachev N, Netser S, Wagner S. Sex-dependent features of social behavior differ between distinct laboratory mouse strains and their mixed offspring. iScience. 2022;25:103735. doi: 10.1016/j.isci.2022.103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Laue HE, Karagas MR, Coker MO, Bellinger DC, Baker ER, Korrick SA, Madan JC. Sex-specific relationships of the infant microbiome and early-childhood behavioral outcomes. Pediatr Res. 2021;92(2):580–591. doi: 10.1038/s41390-021-01785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


