Abstract
Objective:
To evaluate the effectiveness of micro-invasive glaucoma surgery (MIGS) with and without concurrent phacoemulsification.
Design:
Multicenter retrospective cohort study.
Participants:
Patients in the IRIS® Registry (Intelligent Research in Sight) who underwent Xen gel stent (ab interno), endoscopic cyclophotocoagulation (ECP), goniotomy, or canaloplasty from 2013 through 2019.
Methods:
Kaplan-Meier survival analysis was used to assess reoperation rates. We defined reoperation as any subsequent glaucoma surgery occurring 1 month to 3 years after the initial procedure. Multivariable cox proportional hazard models were used to determine factors predictive of reoperation.
Main outcome measures:
reoperation rate, mean intraocular pressure (IOP) and visual acuity (VA), postoperative complications, predictors of reoperation, and reoperation procedure type.
Results:
A total of 79,363 eyes from 57, 561 patients were included with 15,118 eyes (19 %) receiving stand-alone MIGS and 64,245 eyes (81%) receiving MIGS concurrent with phacoemulsification. Overall, patients who underwent MIGS concurrently with phacoemulsification had lower reoperation rates compared to those undergoing stand-alone MIGS. This was most pronounced for the ECP and goniotomy/canaloplasty groups. At two years postoperatively, the cumulative reoperation rate when a procedure was performed alone (without cataract surgery) was 15% for ECP, 24% for Xen, and 24% for goniotomy/canaloplasty compared to 3% for ECP, 19% for Xen, and 6% for goniotomy/canaloplasty performed concurrently with phacoemulsification (p<0.001 for each stand-alone MIGS vs MIGS with phacoemulsification). Black race, older age, coding diagnosis of moderate and severe glaucoma, higher baseline IOP, and glaucoma subtype were associated with higher reoperation risk. While IOP decreased in all groups, those who underwent stand-alone MIGS had a more substantial decrease in mean IOP. MIGS complication rates based on diagnosis codes were low overall: 1% for ECP, 1% for Xen, and 2% for goniotomy/canaloplasty.
Conclusions:
In current US clinical practice, MIGS has substantially lower reoperation rates when performed with phacoemulsification, especially for ECP and goniotomy/canaloplasty. Approximately one-sixth of patients undergoing stand-alone ECP and one quarter of patients undergoing stand-alone Xen or goniotomy/canaloplasty require reoperation by 2 years. Black patients, diagnosis coding of moderate to severe glaucoma, and higher baseline IOP were associated with higher risk of reoperation after MIGS procedures.
Keywords: microinvasive glaucoma surgery, endoscopic cyclophotocoagulation, Xen, goniotomy, canaloplasty, phacoemulsification, reoperation
Introduction
Micro-invasive glaucoma surgeries (MIGS) have been developed as a less traumatic surgical method of treating glaucoma refractory to medical therapy.1These techniques are meant to be less invasive and safer than traditional surgical interventions, namely trabeculectomy and glaucoma drainage devices (GDD).2 MIGS procedures have been proposed to fill the treatment gap between medical/laser therapy and traditional filtration surgeries in mild-moderate glaucoma cases where vision-threatening surgical complication risks may outweigh the benefits of intraocular pressure (IOP) reduction.3
MIGS refers to conjunctival sparing ab interno drainage techniques and includes procedures such as endoscopic cyclo-photocoagulation (ECP), Trabectome, ab-interno canaloplasty, ab-interno trabeculotomy, goniotomy, trabecular bypass devices such as iStent and Hydrus, as well as suprachoroidal shunts (Cypass) and subconjunctival stents like the Xen. Though ECP and goniotomy/trabeculotomy procedures have been in use since the 1990’s and 1930’s respectively, their efficacy has not been fully demonstrated because most of the published work to date are retrospective series without control groups that do not account for the IOP-lowering effect of phacoemulsification.4–9 In recent years, a number of randomized controlled trials have assessed the efficacy of novel MIGS devices such as iStent, Hydrus and Cypass in combination with phacoemulsification.10–13 These studies however have been few to date with relatively limited follow-up. Importantly, the majority have been restricted to subsets of patients that may not be representative of current US population and practice patterns. Additionally, little is known about patient factors which predict positive outcomes following MIGS procedures.
To address these limitations, we analyzed data from the American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight), a large longitudinal dataset of ophthalmic patients representative of the US population. Using IRIS Registry data, we investigated the rate of reoperation, IOP reduction, visual acuity changes, postoperative complications based on diagnosis coding, and patient factors predictive of surgical success for MIGS procedures.
Methods
Data collection
We used data from the American Academy of Ophthalmology’s IRIS Registry from January 1st, 2013, to December 31st, 2019. The data were retrieved on April 10th, 2021. Data stored within the IRIS Registry are de-identified and Health Insurance Portability and Accountability Act (HIPAA) compliant. Institutional review board approval was not required for this study as it does not constitute human subjects research.
Cohort definitions
Glaucoma patients who underwent at least one MIGS procedure (iStent or Hydrus microstent, Cypass, ab interno Xen gel stent, ECP, goniotomy, or canaloplasty) in at least one eye during the study period were included. All cases were identified using Current Procedural Terminology (CPT) codes: iStent and Hydrus microstent (0191T or 0253T, +0376T for each additional device insertion; hereafter referred to as trabecular bypass stent), Cypass (0474T), Xen gel stent (0449T, +0450T for each additional device insertion; internal approach into the subconjunctival space), ECP (66711), goniotomy or ab interno trabeculotomy or Trabectome (65820, 66990; hereafter referred to as goniotomy), and ab interno canaloplasty (66174, 66175). The earliest recorded MIGS procedure per eye was used as the index procedure, and the date of the earliest recorded MIGS procedure was denoted as the index date. Only eyes with more than 3 months of follow-up after the index MIGS procedure were included in analyses. We additionally identified eyes that underwent trabeculectomy (66270, 66172, 66183), and GDDs (66179 without graft, 66180 with graft) occurring at least 3 months after the index MIGS procedure date.
We excluded 121,523 eyes that underwent trabecular bypass stent or Cypass as index procedures from further analysis, because it was not possible to distinguish iStent and Hydrus based on CPT codes and iStent is often performed primarily for medication reduction, information on which is lacking and incomplete in the IRIS Registry (Figure 1). Cypass had limited follow-up time as it was removed from the market in August of 2018. Eyes receiving two or more different glaucoma surgical procedures on the same date in the same eye were excluded from analyses for both index and subsequent operations (17,473 eyes). Duplicate procedures were identified as the same procedure on the same day in the same patient in the same eye. The initial procedure observation was kept, and subsequent entries were excluded. Additionally, duplicate IOP measurements of the same IOP on the same date in the same patient and the same eye were excluded, and the initial IOP observation was kept. Patients with poor data quality (i.e., inadequate exam documentation or missing IOP measurements) or missing eye laterality were excluded (121,523 eyes). Additionally, we excluded the top 0.1% of IOP outliers (>= 50mmHg, 4686 observations). No lower limit was set for IOP.
Figure 1.
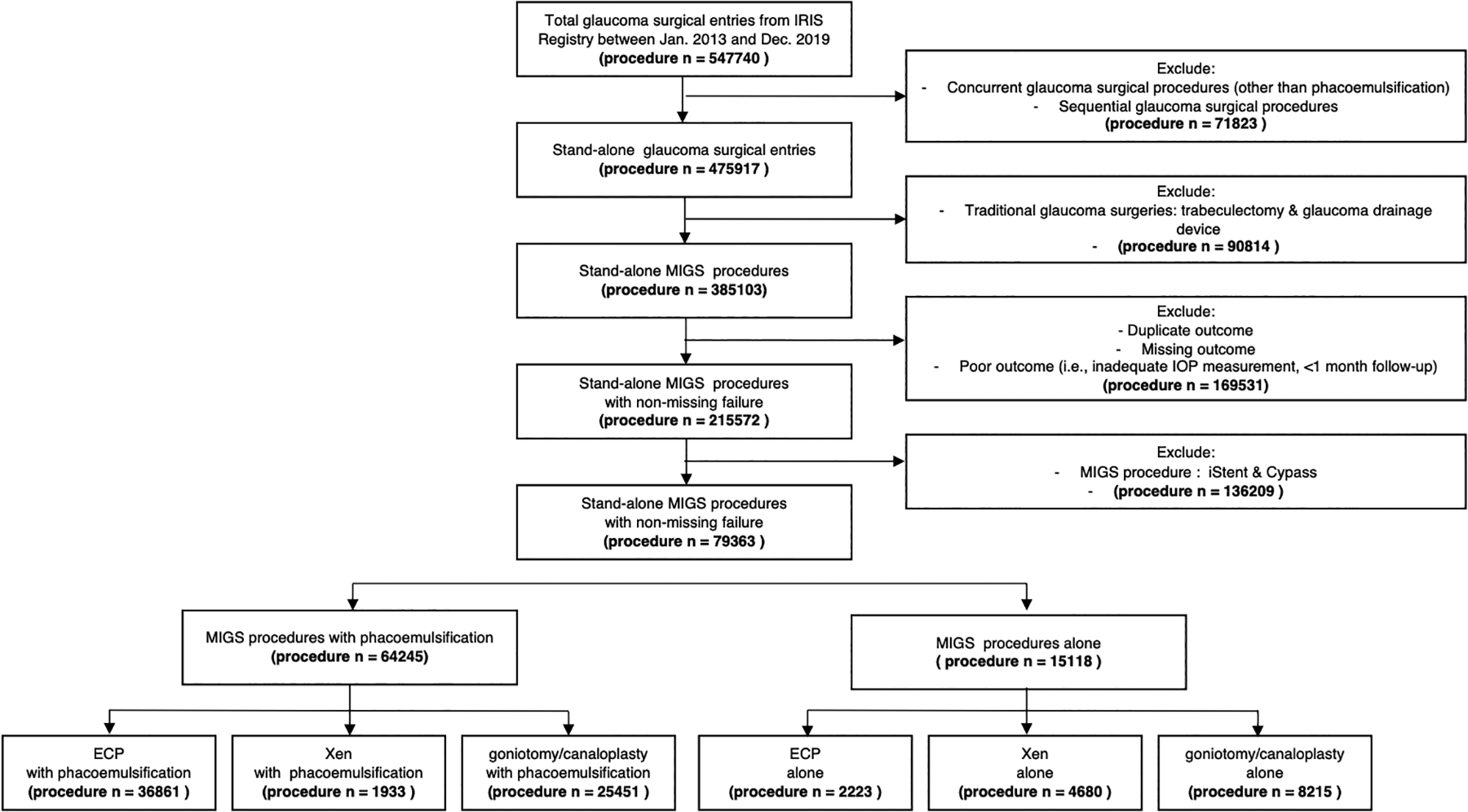
Flowchart of Micro-invasive Glaucoma Surgery procedure identification in IRIS Registry
We differentiated all qualifying MIGS procedures based on whether they were performed concurrently with phacoemulsification (CPT codes: 66982, 66984), defined as procedure and phacoemulsification codes submitted on the same date for the same eye. We divided the cohort into two groups: (a) stand-alone MIGS procedures, (b) MIGS procedures concurrent with phacoemulsification.
Outcome definitions
The primary outcome measure was time to failure by reoperation criteria, which was defined as any subsequent occurrence of MIGS procedures or traditional glaucoma surgeries occurring 1 month to 3 years after the index date. We excluded reoperations occurring in the first month to eliminate observations likely to be duplicates (e.g., index Xen followed by Xen less than one week later).
Secondary outcomes included (1) mean IOP change and (2) mean visual acuity (VA) change (represented using the logarithm of the minimum angle of resolution (logMAR) scale) at 1 month, 3 months, 6 months, 1 year, 2 years, and 3 years postoperatively. Baseline IOP and VA were defined as the mean of the 3 measurements immediately prior to the index MIGS procedure. We used this method to generate a more accurate baseline IOP because IOP often fluctuates (mean number of days between 3 IOP measurements: 61.5 ± 177.2 days). Sensitivity analysis was performed by using the latest measurement prior to the index MIGS procedure and revealed no difference in mean baseline IOP or VA. Visual acuity observations recorded as “counting fingers”, “hand motion”, “light perception”, and “no light perception” were converted to a logMAR of 2.2, 2.3, 2.4, and 2.5, respectively.14 We excluded visual acuity observations recorded in Jaeger notation or listed as “true/false” because Jaeger charts are not standardized and therefore cannot be accurately converted to logMAR. (3) The number of acute postoperative complications within the first 90 days was recorded for each procedure and were defined using International Classification of Diseases (ICD) 10 or 9 codes for corneal edema (H18.2 / 371.2), dislocated intra-ocular lens (H27.1 / 379.34), endophthalmitis (H44.001 / 360.0/360.1), hyphema (H21.0 / 364.41), hypotony (H44.4 / 360.3), iridocyclitis (H20 / 364.0–364.3), macular edema (H59.03/362.07/ 362.83), malignant glaucoma (H40.83 / 365.83), retinal detachment (H33.0 / 361), vitreous hemorrhage (H43.1 / 379.23), and choroidal hemorrhage (H31.3 / 363.61).
Other covariates definitions
To describe baseline features of those undergoing MIGS procedures, we collected demographics (age, gender, race, and ethnicity), baseline IOP, and glaucoma diagnosis type and severity.
Glaucoma specific ICD 9 and 10 codes filed with the patient identification number were used to define six categories of glaucoma diagnosis: (i) suspect glaucoma (365.00 365.01–365.06/H40.01-06) (ii) primary open angle glaucoma (POAG) (365.00 365.01–365.06 365.1 365.10 365.15 365.11/ H40.01-06 H40.1 H40.10 H40.11 H40.15), (iii) primary angle-closure glaucoma (PACG) (365.2 365.20–365.24 / H40.2 H40.20-H40.24), (iv) normal tension glaucoma (NTG) (365.12 / H40.12), (v) pigmentary (365.13/ H40.13) or pseudoexfoliation glaucoma (PEXG) (365.52 / H40.14), and (vi) secondary glaucoma due to drugs (365.3 365.31 365.32 / H40.6), due to eye trauma (365.65/ H40.3), due to eye inflammation (365.62/ H40.4), due to other eye disorders (365.6 365.60 365.61 365.64 / H40.5), other specified glaucoma (365.14 365.4 365.41–365.44 365.5 365.51 365.59 365.63 365.8 365.81 365.82 365.83 365.89 / Q15.0 H40.8 H40.81-H40.83 H40.89), and unspecified glaucoma (365.9 / H40.9). Only diagnoses documented prior to the index procedure were used. If the patient had multiple diagnoses listed prior to their index procedure, the more severe diagnosis was used based on the following severity definition. Severity of glaucoma was identified using ICD-9 codes (365.7/365.70: unspecified, 365.71: mild, 365.72: moderate, 365.73: severe, 365.74: indeterminate) and the 4th decimal place of ICD-10 codes (0: unspecified, 1: mild, 2: moderate, 3: severe, 4: indeterminate).
Statistical analysis
Distribution of demographic and baseline ocular characteristics for each MIGS procedure, stratified as stand-alone versus with concurrent phacoemulsification were reported using descriptive statistics. Continuous variables were expressed as mean and standard deviation (SD), and categorical variables were expressed as frequencies and percentages. ANOVA with Bonferroni correction, Chi-Square test, and Fisher’s test were used to compare each stand-alone MIGS versus MIGS concurrent with phacoemulsification.
Mean IOP and VA for each MIGS procedure, stratified by concurrent phacoemulsification, were graphed to depict change over time. We did not calculate medication use as the accuracy of listed medications in the EMR can be unreliable.15 To account for missing data as well as longitudinal nature of data, change in mean IOP and mean logMAR from baseline over time were both calculated using linear mixed effect models. Percentage change from baseline at 1 year postoperatively was calculated for IOP and compared across procedure groups using student’s t-tests.
Kaplan-Meier survival analysis was performed to assess failure rate by reoperation criteria, and data were expressed as time to reoperation with confidence intervals (CI) for each MIGS procedure. Log-rank tests were used to compare survival curves. Multiple imputation was performed to account for missing data in multivariable analysis using the mice package in R. With this method, we created five iterations of the dataset with predicted values for missing variables based on the distribution of observed data values. Using the aggregated imputed data, multivariable Cox proportional hazard models were then used to determine patient factors and preoperative ocular features predictive of reoperation using the survival package in R. We excluded patients with a diagnosis of glaucoma suspect (3570 eyes (10%)) from the reoperation analysis because patients with suspect glaucoma are typically not candidates for surgical glaucoma treatment and because these patients are not assigned glaucoma severity which was included in our models. Final models were adjusted for age, sex, race, ethnicity, glaucoma diagnosis, glaucoma severity, and baseline IOP by stepwise selection methods.
P-values < 0.05 were considered statistically significant. We performed the multiple imputation analysis in RStudio and R version 4.1.3 and all other statistical analyses using Stata version 16 (StataCorp).
Results
A total of 285,103 eyes from 189,503 glaucoma patients undergoing at least one MIGS were identified from the IRIS Registry during the study period. After applying our exclusion criteria, 79,363 eyes from 57,561 glaucoma patients remained. Of these, 15,118 eyes (19%) underwent stand-alone MIGS procedures, and 64,245 eyes (81%) underwent MIGS procedures concurrent with phacoemulsification (Figure 1).
Baseline Characteristics:
Demographic and ocular characteristics of each MIGS procedure stratified by concurrent phacoemulsification are summarized in Table 1. Of all MIGS procedures, ECP (94%) and goniotomy/canaloplasty (76%) were more likely to be performed with phacoemulsification than ab intero Xen (29%). Regardless of concurrent phacoemulsification, all MIGS procedures were performed more frequently in females, Non-Hispanic White patients, and patients with POAG. Compared with MIGS concurrent with phacoemulsification, those receiving stand-alone MIGS tended to be younger (66.9 vs 70.1 for ECP and 65.4 vs 71.1 for goniotomy/canaloplasty; p<0.001) and have a higher baseline IOP (20.2 vs 17.7 for ECP, 21.0 vs 18.5 for Xen, 21.2 vs 17.3 for goniotomy/canaloplasty; all p<0.001). Additionally, eyes receiving stand-alone ECP and goniotomy/canaloplasty were less likely to have a diagnosis of POAG (53% vs 62% for ECP and 67% vs 75% for goniotomy/canaloplasty) but more likely to have a diagnosis of secondary glaucoma (22% vs 3% for ECP and 12% vs 3% for goniotomy/canaloplasty) and severe glaucoma (49% vs 28% for ECP and 50% vs 31% for goniotomy/canaloplasty, p<0.001 for all) than ECP and goniotomy/canaloplasty concurrent with phacoemulsification. There was no significant difference in glaucoma severity among ab interno Xen procedures performed with versus without concurrent phacoemulsification (p=0.195).
Table 1.
Baseline demographics
| MIGS procedures | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ECP (n=39,084) |
XEN (n=6,613) |
Goniotomy/Canaloplastyc (n=33,666) |
|||||||
| Stand-alone (n=2,223) |
Phacoemulsification (n=36,861) |
P value | Stand-alone (n=4,680) |
Phacoemulsification (n=1,933) |
P value | Stand-alone (n=8,215) | Phacoemulsification (n=25,451) |
P value | |
| Age (mean years, SD) | 66.9(13.9) | 70.1(7.8) | P<0.001 | 72.8(9.6) | 72.7(7.5) | P=0.835 | 65.4(16.8) | 71.1(8.3) | P<0.001 |
| Male (n, %) | 959(43.3) | 15,557 (42.3) | P=0.369 | 2,142(46.0) | 990(51.3) | P<0.001 | 3,795(46.4) | 11,415(45.0) | P=0.026 |
| - Other race a | 81(4.3) | 1,192(3.8) | 104(3.6) | 70(4.9) | 403(5.6) | 1,190(5.7) | |||
| - Hispanic | 260(13.9) | 2,234(7.1) | 324(7.7) | 159(9.3) | 644(8.8) | 2,081(9.6) | |||
| - Secondary glaucoma b | 166(22.0) | 308(3.4) | 223(6.3) | 64(4.5) | 511(11.9) | 478(2.9) | |||
| - Severe | 417(49.3) | 2,950(27.7) | 2,415(64.9) | 918(64.2) | 2,351(50.3) | 4,964(31.3) | |||
| Baseline IOP (mean mmHg, SD) | 20.2(6.9) | 17.7(4.6) | P<0.001 | 21.0(6.4) | 18.5(5.7) | P<0.001 | 21.2(6.5) | 17.3(4.5) | P<0.001 |
| Baseline VA (mean logMAR, SD) | 0.78(0.71) | 0.49(0.41) | P<0.001 | 0.39(0.44) | 0.52(0.49) | P<0.001 | 0.38(0.45) | 0.49(0.42) | P<0.001 |
Abbreviation: MIGS, Microinvasive Glaucoma Surgery; ECP, Endocyclophotocoagulation; IOP, intra-ocular pressure; VA, visual acuity.
Keynote: % = column percentage
Other race = non-Hispanic/Latino Asian, or other
Secondary glaucoma = glaucoma secondary to medications, trauma, inflammation, unspecified
Either Goniotomy, ab interno trabeculotomy, or Trabectome (same CPT code)
Number of missing data: age-12.5%; gender-0.4%; race-15.0%; ethnicity-14.3%; diagnosis-54.7%; severity-53.1%;
Mean intraocular pressure over time
There was a statistically greater percentage reduction in mean IOP from baseline to postoperative year one in each stand-alone MIGS versus MIGS concurrent with phacoemulsification (Figure 2A). Patients in the MIGS with phacoemulsification groups had lower baseline IOP compared to those in the stand-alone MIGS groups. (Baseline IOP- stand-alone MIGS vs MIGS concurrent with phacoemulsification: 20.1 mmHg vs 17.7 mmHg for ECP; 21.2 mmHg vs 17.4 mmHg for goniotomy/canaloplasty; 21.1 mmHg vs 18.5 mmHg for Xen). The absolute IOP (% change from baseline) at postoperative year one for stand-alone MIGS vs MIGS concurrent with phacoemulsification was 16.6 mmHg (−17%) vs 15.7 mmHg (−11%) for ECP, 15.6 mmHg (−26%) vs 15.1 mmHg (−13%) for goniotomy/canaloplasty, and 14.3 mmHg (−32%) vs 14.4 mmHg (−22%) for Xen, p<0.001 for all (Figure 2A).
Figure 2. Mean Intra-Ocular Pressure and Visual Acuity over course of follow-up.
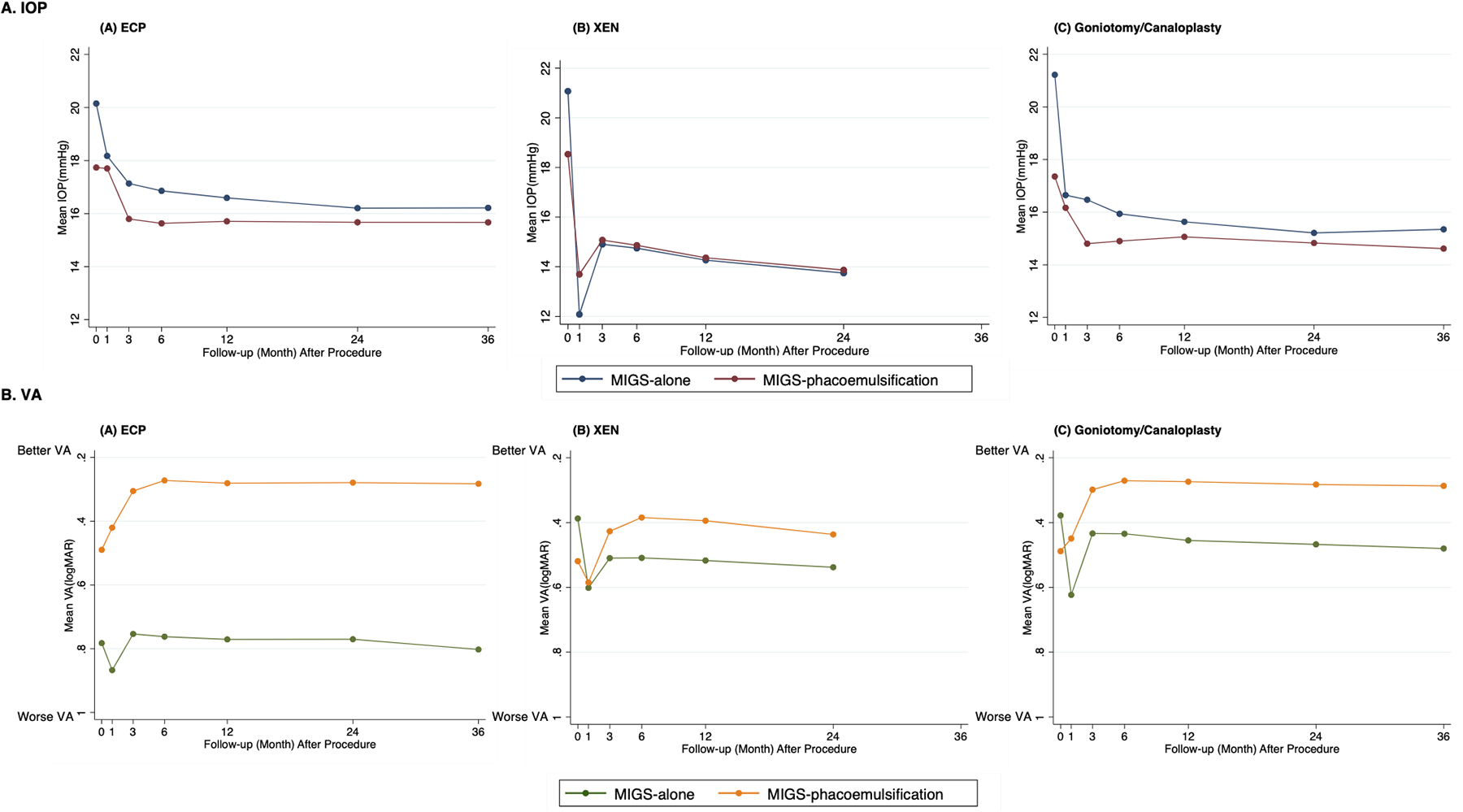
A. mean IOP over time: X axis: Follow-up (month) after procedure. Y axis: Mean IOP (mmHg). Color (Blue: MIGS procedure alone.; Red: MIGS procedure with phacoemulsification.). B. mean VA over time: X axis: Follow-up (month) after procedure. Y axis: Mean VA (logMAR). Color (Green: MIGS procedure alone; Yellow: MIGS procedure with phacoemulsification). Abbreviation: MIGS, micro-invasive glaucoma surgery.
Mean visual acuity over time
In contrast to ab interno Xen and goniotomy/canaloplasty, those who underwent stand-alone ECP had a significantly worse baseline VA than those who underwent ECP concurrent with phacoemulsification (Figure 2B). VA significantly worsened from baseline in stand-alone Xen and goniotomy/canaloplasty at all time points, while no difference in VA change was found in stand-alone ECP at postoperative year one. All MIGS concurrent with phacoemulsification resulted in an improvement in VA after postoperative month three (Figure 2B). At postoperative year one, the percentage improvement in logMAR VA from baseline for stand-alone ECP vs ECP concurrent with phacoemulsification was (1% vs 43%, p<0.001). Goniotomy/canaloplasty with phacoemulsification improved VA by 44% while stand-alone goniotomy/canaloplasty worsened visual acuity by 20%, p<0.001. Xen with phacoemulsification improved visual acuity by 24% while stand-alone Xen worsened VA by 33% (p<0.001).
Failure by re-operation criteria over time
Kaplan-Meier probability plots of reoperation for those undergoing MIGS procedures with and without phacoemulsification are shown in Figure 3. Overall, patients who underwent MIGS concurrent with phacoemulsification had reoperation less often versus those undergoing stand-alone MIGS. This was most pronounced for ECP and goniotomy/canaloplasty (Figure 3). For stand-alone MIGS, 24% receiving goniotomy/canaloplasty, 24% receiving Xen, and 15% of eyes receiving ECP required additional intervention by two years postoperatively (Table 2). For MIGS procedures with phacoemulsification, ECP and goniotomy/canaloplasty groups had lower reoperation rates of 3% and 6% two years postoperatively, respectively. On the other hand, similar reoperation rates were found for ab interno Xen regardless of whether it was performed concurrently with or without phacoemulsification although the reoperation rate for Xen with phacoemulsification was statistically lower (24.% and 19% reoperation rates at postoperative year two respectively, p<0.001).
Figure 3. Kaplan-Meier analysis of cumulative reoperation rates.
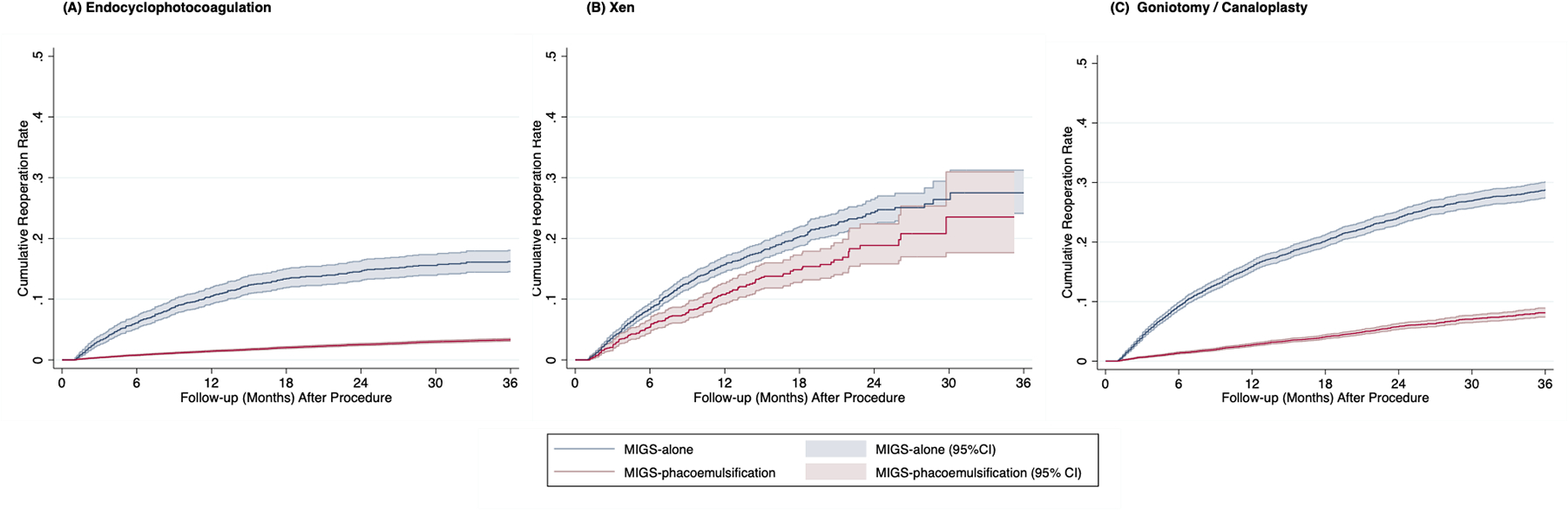
X axis: Follow-up (month) after procedure. Y axis: Cumulative reoperation rate. Color (Blue: MIGS procedure alone.; Red: MIGS procedure with phacoemulsification.) Abbreviation: MIGS, micro-invasive glaucoma surgery.
Table 2.
MIGS procedures – Reoperation at time intervals
| ECP | XEN | Goniotomy + Canaloplasty | ||||
|---|---|---|---|---|---|---|
| Year | Stand-alone (n=2,223) |
phacoemulsification (n=36,861) |
Stand-alone (n=4,680) |
phacoemulsification (n=1,933) |
Stand-alone (n=8,215) |
phacoemulsification (n=25,451) |
| Failure (% (95% CI)) by reoperation failure criteria | ||||||
| 0.25 | 3.1 (2.5 – 3.9) | 0.4 (0.3 – 0.5) | 3.6 (3.1 – 4.2) | 2.1 (1.5 – 2.8) | 4.0 (3.6 – 4.5) | 0.6 (0.5 – 0.7) |
| 0.5 | 6.1 (5.2 – 7.2) | 0.8 (0.7 – 0.9) | 8.3 (7.5 – 9.2) | 5.4 (4.4 – 6.6) | 9.2 (8.6 – 9.9) | 1.4 (1.2 – 1.5) |
| 1 | 10.5 (9.2 – 11.9) | 1.4 (1.3 – 1.6) | 15.7 (14.5 – 17.0) | 10.8 (9.2 – 12.6) | 15.9 (15.0 – 16.8) | 2.7 (2.5 – 3.0) |
| 2 | 14.6 (13.0 – 16.3) | 2.5 (2.4 – 2.7) | 24.3 (22.2 – 26.4) | 18.9 (15.8 – 22.4) | 24.0 (22.9 – 25.2) | 5.8 (5.3 – 6.3) |
| 3 | 16.2 (14.5 – 18.1) | 3.3 (3.1 – 3.5) | 27.5 (24.1 – 31.3) | - | 28.7 (27.4 – 30.1) | 8.1 (7.4 – 8.9) |
| p-value a | P<0.001 | P<0.001 | P<0.001 | |||
Abbreviation: ECP, Endocyclophotocoagulation.
Keynote:
pairwise comparison (each MIGS procedures alone vs MIGS procedures concurrent with phacoemulsification)
Multivariable Cox proportional hazard models: risk of re-operation failure
Multivariable Cox proportional hazard models for failure by re-operation criteria are shown in Table 3 for each MIGs procedure. Non-Hispanic Black race (compared with non-Hispanic White race) was associated with an increased risk of reoperation in all MIGS procedures regardless of concurrent phacoemulsification, except for stand-alone ECP (Table 3A/3B/3C). Additionally, older age was associated with a small decreased risk of reoperation after goniotomy/canaloplasty concurrent with phacoemulsification (aHR=0.98; 95%CI [0.97–0.98]; p<0.001). When compared to POAG, PACG was a risk factor for failure after ECP when performed with concurrent phacoemulsification that associated with a 70% increased risk of reoperation (PACG: aHR=1.70; 95%CI [1.39–2.08]; p<0.001). PEXG was associated with an increased risk of reoperation after both stand-alone Xen (aHR=1.53; 95%CI [1.16–2.02]; p=0.003) and ECP concurrent with phacoemulsification (aHR=1.89; 95%CI [1.46–2.45]; p<0.001). Secondary glaucoma was associated with a two times higher risk of reoperation after ECP with phacoemulsification (aHR=2.06; 95%CI [1.57–2.69]; p<0.001).
Table 3A.
ECP – Cox Proportional Hazard Model for risk of reoperation
| ECP | ||||
|---|---|---|---|---|
| Stand-alone ECP | ECP with Phacoemulsification | |||
| Multivariable Model | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 0.99 (0.98 – 1.00) | P=0.046 | 0.99 (0.99 – 1.00) | P=0.074 |
| Male a | 1.04 (0.83 – 1.31) | P=0.730 | 1.06 (0.96 – 1.18) | P=0.247 |
| - Other e | 0.98 (0.51 – 1.87) | P=0.940 | 1.61 (1.23 – 2.10) | P<0.001 |
| - Secondary glaucoma f | 1.44 (0.79 – 2.60) | P=0.277 | 2.06 (1.57 – 2.69) | P<0.001 |
| - Severe | 2.35 (0.93 – 5.96) | P=0.122 | 8.61 (6.43 – 11.54) | P<0.001 |
| Baseline IOP | 1.03 (1.01– 1.05) | P=0.010 | 1.07 (1.06 – 1.08) | P<0.001 |
Note:
Reference for Multivariate Model: age, gender ((a) reference: female), race ((b) reference: Non-Hispanic White), glaucoma type ((c) reference: Primary open angle glaucoma), severity ((d) reference: mild glaucoma)), and baseline IOP
Other race = non-Hispanic/Latino Asian, or other
Secondary glaucoma = glaucoma secondary to medications, trauma, inflammation, unspecified
Table 3B.
XEN – Cox Proportional Hazard Model for risk of reoperation
| XEN | ||||
|---|---|---|---|---|
| Stand-alone XEN | XEN with Phacoemulsification | |||
| Multivariable Model | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 0.99 (0.98 – 1.00) | P=0.094 | 0.99 (0.97 – 1.01) | P=0.476 |
| Male a | 0.88 (0.75 – 1.03) | P=0.113 | 1.00 (0.74 – 1.33) | P=0.974 |
| - Other e | 1.59 (1.10 – 2.28) | P=0.013 | 0.97 (0.43 – 2.18) | P=0.936 |
| - Secondary glaucoma f | 0.72 (0.48 – 1.09) | P=0.124 | 0.48 (0.19 – 1.18) | P=0.112 |
| - Severe | 1.32 (0.90 – 1.92) | P=0.159 | 1.76 (0.83 – 3.72) | P=0.152 |
| Baseline IOP | 1.03 (1.02 – 1.05) | P<0.001 | 1.08 (1.06 – 1.11) | P<0.001 |
Note:
Reference for Multivariate Model: age, gender ((a) reference: female), race ((b) reference: Non-Hispanic White), glaucoma type ((c) reference: Primary open angle glaucoma), severity ((d) reference: mild glaucoma)), and baseline IOP
Other race = non-Hispanic/Latino Asian, or other
Secondary glaucoma = glaucoma secondary to medications, trauma, inflammation, unspecified
Table 3C.
Ab Interno Goniotomy/Canaloplasty – Cox Proportional Hazard Model for risk of reoperation
| Goniotomy + Canaloplasty | ||||
|---|---|---|---|---|
| Stand-alone Goniotomy + Canaloplasty | Goniotomy + Canaloplasty with Phacoemulsification | |||
| Multivariable Model | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 1.01 (1.00 – 1.01) | P=0.002 | 0.98 (0.97 – 0.98) | P<0.001 |
| Male a | 0.89 (0.81 – 0.98) | P=0.022 | 1.21 (1.05 – 1.39) | P=0.010 |
| - Othere | 1.13 (0.88 – 1.45) | P=0.329 | 1.26 (0.92 – 1.72) | P=0.150 |
| - Secondary glaucomaf | 0.82 (0.66 – 1.02) | P=0.099 | 1.34 (0.93 – 1.94) | P=0.135 |
| - Severe | 1.46 (1.22 – 1.74) | P<0.001 | 3.39 (2.62 – 4.39) | P<0.001 |
| - Baseline IOP | 1.05 (1.04 – 1.06) | P<0.001 | 1.10 (1.08 – 1.11) | P<0.001 |
Note:
Reference for Multivariate Model: age, gender ((a) reference: female), race ((b) reference: Non-Hispanic White), glaucoma type ((c) reference: Primary open angle glaucoma), severity ((d) reference: mild glaucoma)), and baseline IOP
Other race = non-Hispanic/Latino Asian, or other
Secondary glaucoma = glaucoma secondary to medications, trauma, inflammation, unspecified
A higher risk of reoperation was seen in those with diagnosis of moderate and severe glaucoma when compared to mild glaucoma among all MIGS procedures except for stand-alone ECP and Xen with and without phacoemulsification. Higher baseline IOP was associated with increased failure risk for all MIGS procedures (Table 3A/3B/3C).
Type of Reoperation Procedure
Regardless of concurrent phacoemulsification, trabeculectomy and GDD were two most common subsequent surgeries for those undergoing ab interno Xen and goniotomy/canaloplasty, followed by subsequent MIGS procedures. 75% of initial stand-alone Xen and 81% of initial Xen with phacoemulsification underwent traditional glaucoma surgeries when reoperation was required and 69% of initial stand-alone goniotomy/canaloplasty and 71% of initial goniotomy/canaloplasty with phacoemulsification underwent traditional glaucoma surgeries when reoperation was required. However, different from ab interno Xen and goniotomy/canaloplasty, subsequent GDD was the most common reoperation type, followed by subsequent ECP being the second most common reoperation type for those undergoing initial stand-alone ECP (31% of stand-alone ECP underwent repeat ECP when subsequent surgery was required). (Figure 4).
Figure 4. Secondary procedures after initial Micro-Invasive Glaucoma Surgery procedure.
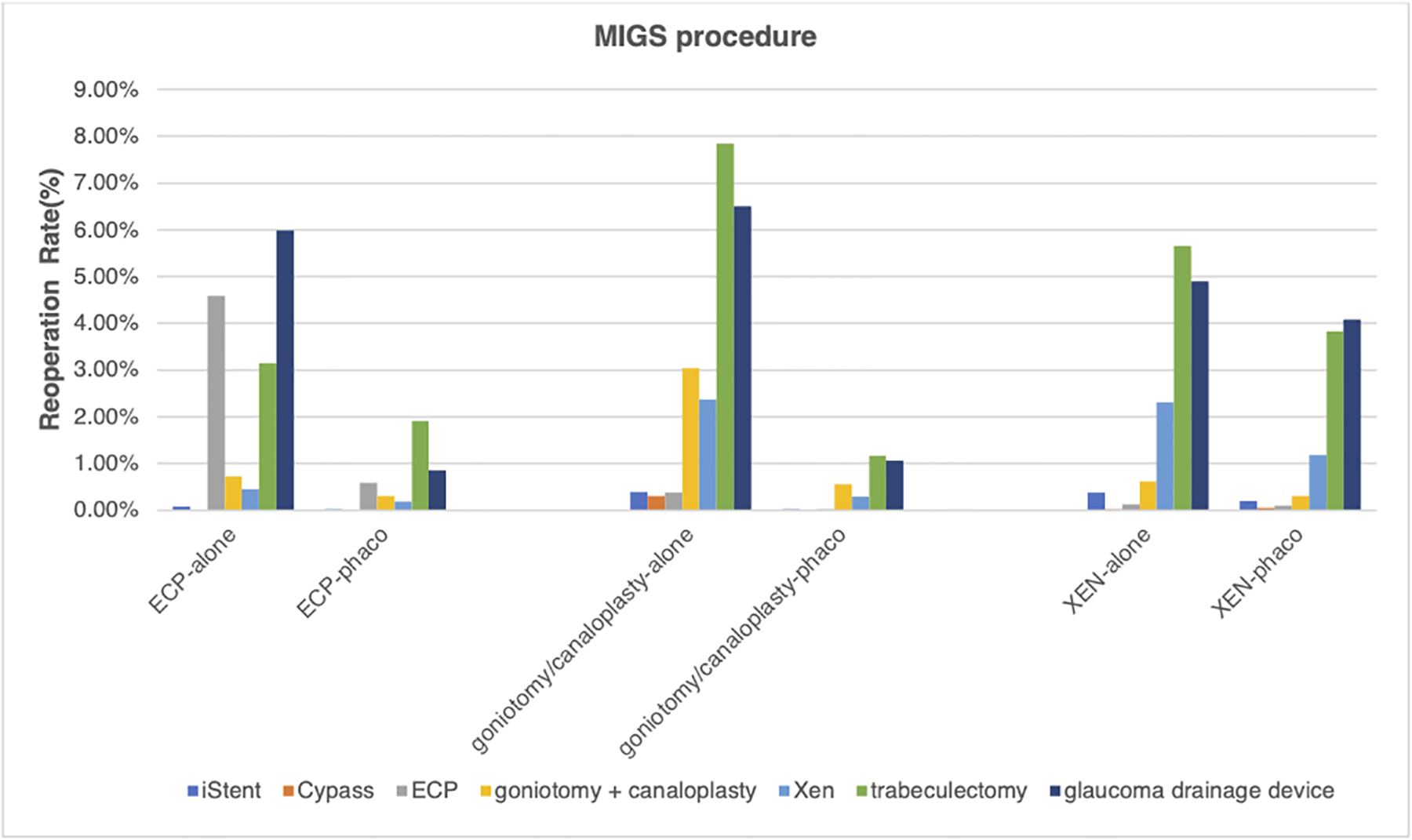
Y axis: percentage of secondary glaucoma surgical procedures (%). X axis: MIGS procedure type, with or without phacoemulsification. Color (Blue: iStent; Orange: Cypass; Grey: ECP; Yellow: Xen; light blue: goniotomy; green: canaloplasty; Navy blue: trabeculectomy; Brown: glaucoma drainage device). Abbreviation: phaco, phacoemulsification; MIGS, micro-invasive glaucoma surgery.
Postoperative complications
Postoperative ocular complications that were included in encounter diagnoses within the first 90 days after the index MIGS procedure are listed in Table 4. Overall, MIGS procedures had low total documented complication rates: 1% for ECP, 1% for Xen, and 2% for goniotomy/canaloplasty (p<0.001). Hyphema was the most common complication diagnosed after ab interno Xen and goniotomy/canaloplasty, and it was significantly more common than after ECP (38% for Xen and 67% for goniotomy/canaloplasty vs. 5% for ECP, p<0.001). Corneal edema and iridocyclitis were more likely to be diagnosed after ECP and goniotomy/canaloplasty than after ab interno Xen (34% and 27% for ECP, 33% and 37% for goniotomy/canaloplasty vs 13% and 7% for Xen; each pairwise comparison: p<0.001). Vitreous hemorrhage was diagnosed more commonly after ab interno Xen and goniotomy/canaloplasty compared to ECP (17% for Xen, 20% for goniotomy/canaloplasty vs 7% for ECP; each pairwise comparison: p<0.001). Diagnosis of choroidal hemorrhage, endophthalmitis, and retinal detachment were very low in all MIGS procedures, especially for the ECP and goniotomy/canaloplasty groups (Table 4).
Table 4.
Ocular complications within 90 days of the index procedure
| Microinvasive Glaucoma surgery | |||
|---|---|---|---|
| Complication (n=1,018) | ECP (n=39,098) |
XEN (n=6,618) |
goniotomy + canaloplasty (n=33,746) |
| corneal edema | 132 (0.34%) | 13 (0.20%) | 113 (0.33%) |
| dislocated intra-ocular lens | 3 (0.01%) | 0 (0.00%) | 2 (0.01%) |
| endophthalmitis | 12 (0.03%) | 6 (0.09%) | 8 (0.02%) |
| hyphema | 19 (0.05%) | 25 (0.38%) | 225 (0.67%) |
| hypotony | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| iridocyclitis | 107 (0.27%) | 7 (0.11%) | 124 (0.37%) |
| macular edema | 54 (0.14%) | 7 (0.11%) | 11 (0.03%) |
| malignant glaucoma | 31 (0.08%) | 0 (0.00%) | 5 (0.01%) |
| retinal detachment | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| vitreous haemorrhage | 27 (0.07%) | 11 (0.17%) | 69 (0.20%) |
| choroidal haemorrhage | 2 (0.01%) | 4 (0.06%) | 1 (0.00%) |
| TOTAL | 387 (0.99%) | 73 (1.10%) | 558 (1.66%) |
Legend: an ocular complication that occurred either intraoperatively or within 90 postoperative days
Abbreviation: MIGS, Microinvasive Glaucoma Surgery; ECP, Endo cyclophotocoagulation.
Keynote: % = column percentage
Discussion
Summary of findings
In this study of MIGS effectiveness using the IRIS Registry, we found significant IOP reduction in all groups but that those who underwent MIGS concurrent with phacoemulsification had a significantly lower rate of reoperation than those undergoing stand-alone MIGS. Roughly 1 in 7 eyes that underwent stand-alone ECP required re-operation at 2 years while nearly 1 in 4 eyes that underwent stand-alone goniotomy/canaloplasty or ab interno Xen (with or without phacoemulsification) required another operation. We identified several patient and ocular factors associated with higher and lower hazard for reoperation, namely race, glaucoma severity, baseline IOP, and type of glaucoma. The two most common choices of subsequent operation were trabeculectomy and GDD for those with index ab interno Xen and goniotomy/canaloplasty regardless of concurrent phacoemulsification, while subsequent ECP was the second most common reoperation type after initial stand-alone ECP. Importantly, coded MIGS complication rates were low overall, with corneal edema, iridocyclitis, and hyphema being the three most common complications.
Reoperation rate
There are several possible reasons for lower reoperation rates seen after MIGS concurrent with phacoemulsification. First, eyes with visually significant cataract but less severe glaucoma may receive surgery primarily to treat their cataract and only undergo a concurrent MIGS procedure for better IOP control or to reduce medication burden. Therefore, these eyes may have better glaucoma control at baseline, lower odds of disease progression and less need for additional IOP lowering procedures. Our finding of lower baseline IOP in eyes undergoing MIGS concurrent with phacoemulsification supports this explanation. Additionally, older age in patients undergoing concurrent cataract surgery may influence the reoperation decision. Clinicians may be more reluctant to perform additional procedures in older patients with shorter life expectancy and less time for glaucoma disease progression.
In contrast to ECP and goniotomy/canaloplasty, we found similar reoperation rates for ab interno Xen with and without concurrent phacoemulsification. In accordance with our findings, a previous prospective multicenter cohort study of 202 eyes16 and a prospective single center study of 149 eyes17 reported similar success rates with similar reduction in IOP percentage between Xen-alone and Xen-phacoemulsification at 1 and 2 years, respectively. Although these studies defined the success rate by IOP reduction criteria as opposed to our criteria of reoperation, their findings support that the long-term effectiveness and IOP-lowering effect of the Xen gel stent is likely similar between Xen with and without phacoemulsification. However, considering that patients in this study undergoing MIGS with concurrent phacoemulsification have lower IOP at baseline, we would expect lower reoperation rates in patients undergoing Xen with phacoemulsification compared to Xen alone. Our finding of similar reoperation rates between ab interno Xen with and without phacoemulsification suggests Xen with phacoemulsification may be failing at a higher rate than expected or that surgeons are choosing Xen for eyes that need lower IOP. Xen is FDA approved for patients with refractory glaucoma, and the IOP lowering effect of phacoemulsification may be limited in this more severe form of glaucoma. It is also possible that the pro-inflammatory effect of phacoemulsification at the time of Xen implantation causes scarring and damages bleb function, leading to higher reoperation rates. It is important to note that patients in the stand-alone Xen group may be pseudophakic since the IRIS Registry does not have information on lens status at the time of entrance into the database. A retrospective study from Widder et al.18 on 261 eyes undergoing Xen reported that pseudophakic eyes had the most favorable success rate compared to phakic eyes and combination surgery with phacoemulsification. Thus, it is possible our reoperation rate for stand-alone Xen is lower than what it would be for phakic patients undergoing stand-alone Xen. Future studies investigating the effectiveness of stand-alone Xen stratified by lens status are warranted.
Notably, there is a discrepancy in our study between lower reoperation rates yet less substantial IOP reduction for MIGS concurrent with phacoemulsification compared to stand-alone MIGS. It has been noted in previous literature reviews19–21 that eyes with a higher baseline IOP experience a larger pressure reduction, whether expressed in mmHg or as percentages. Therefore, this discrepancy may be related to a higher baseline IOP in the stand-alone MIGS group, leading to greater IOP reduction compared to MIGS with concurrent phacoemulsification. Even though there is more IOP reduction in stand-alone MIGS, the absolute IOPs are quite similar between all stand-alone and phacoemulsification-combined procedures by one year postoperatively. Despite this, it is possible that patients undergoing stand-alone MIGS had lower target IOP compared to patients undergoing MIGS with concurrent phacoemulsification who may have better controlled glaucoma at baseline. Thus, patients in the stand-alone MIGS group may undergo more procedures for more aggressive IOP control. Similarly, our finding of improved VA in all MIGS procedures concurrent with phacoemulsification groups but declined or un-changed VA in stand-alone MIGS procedures groups at one year post-operatively despite similar baseline VA may point to more advanced and uncontrolled glaucoma in stand-alone group. The only exception to this trend was seen among those who underwent stand-alone ECP had significantly worse baseline VA compared to the combined ECP and phacoemulsification group. This may be because ECP is often reserved for patients with refractory glaucoma who have failed previous glaucoma surgical interventions.22,23 Additionally, potential complications of intractable vision loss caused by macular edema and hypotony due to aqueous shutdown may have contributed to worse visual outcomes in this group.4
Predictors of reoperation
We identified several risk factors for reoperation in our analyses. Non-Hispanic Black patients who underwent MIGS procedures were at higher risk of reoperation compared to non-Hispanic White patients. This supports the findings of the Advanced Glaucoma Intervention Study (AGIS) trial24 which showed that Black patients have a higher failure rate following initial standard glaucoma interventions compared to White patients.25 With glaucoma filtering surgeries being the only surgical treatment option available at the time (without any MIGS procedure on the market yet), the AGIS trial suggests that either Black patients respond poorly to surgery or have more aggressive glaucoma progression.23 In addition, disparity in access and utilization of eye care leading to more severe and higher glaucoma progression may also in part explain the higher reoperation rate in Black patients.26 Among baseline ocular factors, severe glaucoma and higher baseline IOP were associated with a higher reoperation rate in all MIGS procedures. A lower IOP target is often set in severe glaucoma to protect what is left of the nerve fiber layer.27,28 Therefore, patients with severe glaucoma may require more interventions to reach a lower target IOP.
Our study found that different types of glaucoma were associated with risk of reoperation failure. Eyes with PACG that underwent ECP concurrent with phacoemulsification were more likely to receive an additional reoperation than those with POAG, while this effect was not seen in those eyes receiving stand-alone ECP. Previous studies29–31 have reported that IOP reduction after primary lens extraction is consistently greater in eyes with PACG than in eyes with POAG. Because ECP is typically performed as a stand-alone procedure only in pseudophakic eyes, our findings of no increased reoperation risk for PACG patients in the stand-alone ECP group suggests that lens removal prior to ECP may be superior to lens removal at the time of ECP. It is also possible that eyes undergoing ECP with concurrent phacoemulsification have more severe PACG and require acute lens removal whereas those undergoing stand-alone ECP have better disease control given previous lens removal. The higher reoperation risk in secondary glaucoma eyes receiving ECP with phacoemulsification compared to POAG eyes reflect a higher baseline IOP and subsequent need for increased interventions in this glaucoma subtype.
Interestingly, patients with pigmentary/PEXG were more likely than POAG patients to require another operation after receiving an initial stand-alone Xen. Because the underlying mechanism of these glaucoma subtypes is due to accumulated extracellular material and pigment within trabecular meshwork, the lumen of the Xen gel stent might be at risk for internal obstruction by pseudoexfoliation material. However, a recent study17 investigating 107 eyes showed contrasting results that the Xen gel implant as a stand-alone or combined procedure demonstrated similar complete success rates (IOP<=16mmHg) in PEXG and POAG eyes (63% vs 42%, p=0.06) at 1 year and that PEXG diagnosis was a potential predictive factor for surgical success. Future RCTs are needed to shed light on the role of MIGS in PEXG.
Reoperation
Patients who underwent index MIGS procedures most often underwent reoperation with traditional glaucoma surgery while patients who underwent initial ECP were also more likely to receive another ECP for reoperation. ECP is a potentially titratable and repeatable procedure used to lower IOP in a wide variety of glaucoma types and severities. Through different approaches (anterior vs posterior)23 and ablation of varying degrees and lengths of the ciliary processes (one vs two corneal incisions),32 the aggressiveness of the procedure can be titrated to potentially reach a lower target IOP. Our results show that repeat ECP is often used when an initial ECP is unsuccessful whereas traditional glaucoma surgery is used for reoperation in the setting of failed goniotomy/canaloplasty or ab interno Xen.
Postoperative complications
Overall, the documented complication rates for MIGS in the IRIS Registry database were very low, substantially lower than in previous studies.33,34 Postoperative complications are almost certainly underestimated here because ICD coding of postoperative complications may not be common in clinical practice. However, our findings regarding types of complications seen after MIGS and the rare rate of visually threatening complications are similar to what has been documented.33 Among angle-based MIGS procedures like OMNI and GATT, complications other than hyphema tend to be similar to those from phacoemulsification alone. Additionally, most of the complications seen are transient and do not require additional surgical intervention.35
Using the IRIS Registry, we present the outcomes of MIGS procedures in current US clinical practice. In contrast to previous prospective studies and RCTs which compare MIGS procedures concurrent with phacoemulsification to phacoemulsification alone, here we compare MIGS procedures performed with concurrent phacoemulsification to stand-alone MIGS.
Several limitations of this analysis should be considered. First, given the retrospective and nonrandomized nature of our study, we are unable to determine the exact indications for each procedure, and there are almost certainly underlying differences between patients in the various procedure groups that bias our results. Additionally, after applying exclusion criteria, we excluded approximately 75% of the MIGS procedures initially identified. While we feel that strict exclusion criteria are necessary for this large database study, it is possible that limiting the cohort may bias the results. Because of missing data on medications in the IRIS Registry, we were not able to include IOP in our failure criteria. Some patients may receive MIGS procedures for reducing medication burden rather than IOP reduction and should not be counted as a failure if IOP remains the same, yet their medication burden is reduced. Second, we were unable to determine patients’ lens status prior to the entry into the IRIS Registry. This is particularly true for those who underwent stand-alone goniotomy or canaloplasty as they may have been phakic or pseudophakic. This may partially bias our outcomes when comparing the effectiveness of stand-alone MIGS to MIGS combined with phacoemulsification. Additionally, patients in both the stand-alone MIGS and MIGS with phacoemulsification groups may have undergone glaucoma surgery prior to entering the IRIS Registry, although we would expect this to be evenly distributed across the groups. Procedures in the IRIS Registry are based on billing codes extracted from the electronic health records. This limited our ability to evaluate more specifically which MIGS procedure was performed because some CPT codes include a group of similar procedures (e.g., CPT code 65820 indicates either ab interno goniotomy, ab interno trabeculotomy, or Trabectome). Additionally, while ab interno Xen and ab externo Xen were differentiated by CPT codes (CPT code : 0449T for ab interno Xen; 66183 for ab externo Xen) in our study, it may not always be possible to make this distinction accurately. Also, Xen implants placed ab externo are coded under CPT code 66183 which also includes procedures like the ExPRESS shunt as part of a trabeculectomy. Although we chose the codes most specific for each procedure, minor overlaps may influence the results. Additionally, we did not have information on ophthalmologist type (comprehensive vs subspecialist ophthalmologist). Bias may arise due to the surgeons’ proficiency and access to MIGS. Last, the IRIS Registry captures a greater proportion of private ophthalmic practices vs tertiary academic centers, which potentially limits the generalizability of our results.
Conclusion
We found that in the current US clinical practice in the IRIS Registry, MIGS has substantially lower reoperation rates when performed with phacoemulsification. Importantly, nearly one quarter of patients undergoing stand-alone ab interno Xen and goniotomy/canaloplasty required reoperation by 2 years. Black patients, eyes with moderate to severe glaucoma, and high baseline IOP had higher risk of reoperation after MIGS procedures.
Financial Support:
This work was supported by the UNC Chapel Hill Scott Neil Schwirck fellowship (EC), NIH grant numbers R01EY030575 (TE), P30EY003790 (TE), K23EY032634 (NZ), Research to Prevent Blindness Career Development Award (NZ), and the Massachusetts Eye and Ear Clinical Data Science Fund. The funding organizations had no role in the design or conduct of this research.
Abbreviations:
- MIGS
microinvasive glaucoma surgery
- GDD
glaucoma drainage device
- ECP
endoscopic cyclophotocoagulation
- IRIS® Registry
Intelligent Research in Sight
- IOP
intraocular pressure
- CPT
Current Procedural Terminology
- VA
visual acuity
- logMAR
logarithm of the minimum angle of resolution
- ICD
International Classification of Diseases
- POAG
primary open angle glaucoma
- PACG
primary angle-closure glaucoma
- NTG
normal tension glaucoma
- PEXG
pseudoexfoliation glaucoma
- ANOVA
analysis of variance
- SD
standard deviation
- CI
confidence interval
- FDA
US Food and Drug Administration
- aHR
adjusted hazard ratio
- GATT
gonioscopy-assisted transluminal trabeculotomy
- TM
trabecular meshwork
- RCT
randomized controlled trial
Footnotes
Paper presentation at the American Glaucoma Society Annual Meeting, 2022
Conflict of Interest:
No conflicting relationship exists for any author
Reference
- 1.Francis BA, Singh K, Lin SC, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118(7):1466–1480. doi: 10.1016/j.ophtha.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 2.Coleman AL. Advances in glaucoma treatment and management: surgery. Invest Ophthalmol Vis Sci. 2012;53(5):2491–2494. doi: 10.1167/iovs.12-9483l [DOI] [PubMed] [Google Scholar]
- 3.SooHoo JR, Seibold LK, Radcliffe NM, Kahook MY. Minimally invasive glaucoma surgery: current implants and future innovations. Can J Ophthalmol J Can Ophtalmol. 2014;49(6):528–533. doi: 10.1016/j.jcjo.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol. 1997;124(6):787–796. doi: 10.1016/s0002-9394(14)71696-4 [DOI] [PubMed] [Google Scholar]
- 5.Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40(8):1313–1321. doi: 10.1016/j.jcrs.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 6.Morales J, Al Qahtani M, Khandekar R, et al. Intraocular Pressure Following Phacoemulsification and Endoscopic Cyclophotocoagulation for Advanced Glaucoma: 1-Year Outcomes. J Glaucoma. 2015;24(6):e157–162. doi: 10.1097/IJG.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 7.Salinas L, Chaudhary A, Berdahl JP, et al. Goniotomy Using the Kahook Dual Blade in Severe and Refractory Glaucoma: 6-Month Outcomes. J Glaucoma. 2018;27(10):849–855. doi: 10.1097/IJG.0000000000001019 [DOI] [PubMed] [Google Scholar]
- 8.Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: Short-term results. J Cataract Refract Surg. 2017;43(9):1197–1201. doi: 10.1016/j.jcrs.2017.06.046 [DOI] [PubMed] [Google Scholar]
- 9.Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-Month Outcomes of Goniotomy Performed Using the Kahook Dual Blade Combined with Cataract Surgery in Eyes with Medically Treated Glaucoma. Adv Ther. 2018;35(9):1460–1469. doi: 10.1007/s12325-018-0755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE, US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi: 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 11.Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, Randomized, Controlled Pivotal Trial of an Ab Interno Implanted Trabecular Micro-Bypass in Primary Open-Angle Glaucoma and Cataract: Two-Year Results. Ophthalmology. 2019;126(6):811–821. doi: 10.1016/j.ophtha.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Vold S, Ahmed IIK, Craven ER, et al. Two-Year COMPASS Trial Results: Supraciliary Microstenting with Phacoemulsification in Patients with Open-Angle Glaucoma and Cataracts. Ophthalmology. 2016;123(10):2103–2112. doi: 10.1016/j.ophtha.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 13.García-Feijoo J, Rau M, Grisanti S, et al. Supraciliary Micro-stent Implantation for Open-Angle Glaucoma Failing Topical Therapy: 1-Year Results of a Multicenter Study. Am J Ophthalmol. 2015;159(6):1075–1081.e1. doi: 10.1016/j.ajo.2015.02.018 [DOI] [PubMed] [Google Scholar]
- 14.Pistilli M, Joffe MM, Gangaputra SS, et al. Visual Acuity Outcome over Time in Non-Infectious Uveitis. Ocul Immunol Inflamm. 2021;29(6):1064–1071. doi: 10.1080/09273948.2019.1687733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monte AA, Anderson P, Hoppe JA, Weinshilboum RM, Vasiliou V, Heard KJ. The Accuracy of Electronic Medical Record Medication Reconciliation in Emergency Department Patients. J Emerg Med. 2015;49(1):78–84. doi: 10.1016/j.jemermed.2014.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reitsamer H, Sng C, Vera V, et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2019;257(5):983–996. doi: 10.1007/s00417-019-04251-z [DOI] [PubMed] [Google Scholar]
- 17.Mansouri K, Guidotti J, Rao HL, et al. Prospective Evaluation of Standalone XEN Gel Implant and Combined Phacoemulsification-XEN Gel Implant Surgery: 1-Year Results. J Glaucoma. 2018;27(2):140–147. doi: 10.1097/IJG.0000000000000858 [DOI] [PubMed] [Google Scholar]
- 18.Widder RA, Dietlein TS, Dinslage S, Kühnrich P, Rennings C, Rössler G. The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2018;256(4):765–771. doi: 10.1007/s00417-018-3899-7 [DOI] [PubMed] [Google Scholar]
- 19.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol Chic Ill 1960. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268 [DOI] [PubMed] [Google Scholar]
- 20.Wang SY, Azad AD, Lin SC, Hernandez-Boussard T, Pershing S. Intraocular Pressure Changes after Cataract Surgery in Patients with and without Glaucoma: An Informatics-Based Approach. Ophthalmol Glaucoma. 2020;3(5):343–349. doi: 10.1016/j.ogla.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindén C, Heijl A, Jóhannesson G, Aspberg J, Andersson Geimer S, Bengtsson B. Initial intraocular pressure reduction by mono‐ versus multi‐therapy in patients with open‐angle glaucoma: results from the Glaucoma Intensive Treatment Study. Acta Ophthalmol (Copenh). 2018;96(6):567–572. doi: 10.1111/aos.13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima FE, Magacho L, Carvalho DM, Susanna RJ, Avila MP. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma. 2004;13(3):233–237. doi: 10.1097/00061198-200406000-00011 [DOI] [PubMed] [Google Scholar]
- 23.Tan JCH, Francis BA, Noecker R, Uram M, Dustin L, Chopra V. Endoscopic Cyclophotocoagulation and Pars Plana Ablation (ECP-plus) to Treat Refractory Glaucoma. J Glaucoma. 2016;25(3):e117–122. doi: 10.1097/IJG.0000000000000278 [DOI] [PubMed] [Google Scholar]
- 24.Ederer F, Gaasterland DE, Sullivan EK, AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials. 1994;15(4):299–325. doi: 10.1016/0197-2456(94)90046-9 [DOI] [PubMed] [Google Scholar]
- 25.Williams AM, Huang W, Muir KW, Stinnett SS, Stone JS, Rosdahl JA. Identifying risk factors for blindness from primary open-angle glaucoma by race: a case–control study. Clin Ophthalmol Auckl NZ. 2018;12:377–383. doi: 10.2147/OPTH.S143417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and Ethnic Disparities in Health Care Access and Utilization Under the Affordable Care Act. Med Care. 2016;54(2):140–146. doi: 10.1097/MLR.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK, CIGTS Study Investigators. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology. 2009;116(2):200–207. doi: 10.1016/j.ophtha.2008.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The advanced glaucoma intervention study (AGIS): 7. the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/S0002-9394(00)00538-9 [DOI] [PubMed] [Google Scholar]
- 29.Wishart PK, Atkinson PL. Extracapsular cataract extraction and posterior chamber lens implantation in patients with primary chronic angle-closure glaucoma: effect on intraocular pressure control. Eye Lond Engl. 1989;3 (Pt 6):706–712. doi: 10.1038/eye.1989.109 [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Hayashi H, Nakao F, Hayashi F. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg. 2001;27(11):1779–1786. doi: 10.1016/s0886-3350(01)01036-7 [DOI] [PubMed] [Google Scholar]
- 31.Mierzejewski A, Eliks I, Kałuzny B, Zygulska M, Harasimowicz B, Kałuzny JJ. Cataract phacoemulsification and intraocular pressure in glaucoma patients. Klin Oczna. 2008;110(1–3):11–17. [PubMed] [Google Scholar]
- 32.Kahook MY, Lathrop KL, Noecker RJ. One-site Versus Two-site Endoscopic Cyclophotocoagulation. J Glaucoma. 2007;16(6):527–530. doi: 10.1097/IJG.0b013e3180575215 [DOI] [PubMed] [Google Scholar]
- 33.Yook E, Vinod K, Panarelli JF. Complications of micro-invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29(2):147–154. doi: 10.1097/ICU.0000000000000457 [DOI] [PubMed] [Google Scholar]
- 34.Vinod K, Gedde SJ. Safety profile of minimally invasive glaucoma surgery. Curr Opin Ophthalmol. 2021;32(2):160–168. doi: 10.1097/ICU.0000000000000731 [DOI] [PubMed] [Google Scholar]
- 35.Chen X zhu, Liang Z qiao, Yang K yi, et al. The Outcomes of XEN Gel Stent Implantation: A Systematic Review and Meta-Analysis. Front Med. 2022;9. Accessed March 20, 2022. https://www.frontiersin.org/article/10.3389/fmed.2022.804847 [DOI] [PMC free article] [PubMed] [Google Scholar]


