Abstract
Since its development in the 1970s, X-ray CT has emerged as a landmark diagnostic imaging modality of modern medicine. Technological advances have been crucial to the success of CT imaging, as they have increasingly enabled improvements in image quality and diagnostic value at increasing radiation dose efficiency. With recent advances in engineering and physics, a novel technology has emerged with the potential to surpass several shortcomings and limitations of current CT systems. Photon-counting detector (PCD)-CT might substantially improve and expand the applicability of CT imaging by offering intrinsic spectral capabilities, increased spatial resolution, reduced electronic noise and improved image contrast. In this review we sought to summarize the first clinical experience of PCD-CT. We focused on most recent prototype and first clinically approved PCD-CT systems thereby reviewing initial publications and presenting corresponding clinical cases.
Introduction
X-ray CT has emerged as a landmark diagnostic imaging modality of modern medicine since its development in the 1970’s. The benefits and clinical demand for CT imaging continue to rise due to continued technical advancements facilitating improvements in image quality and diagnostic value at constantly higher radiation dose efficiency, together with broadening of the clinical indications. 1–4
The X-ray detector constitutes a decisive component of a CT system that critically influences image quality and dose efficiency. Accordingly, the detector design has undergone radical transformations and improvements. Currently, most available CT systems use solid-state energy-integrating detectors (EIDs) with third-generation rotate–rotate designs to convert X-rays to electrical signals. The indirect conversion technology of EIDs is based on a scintillator layer converting X-ray photons to visible light, which is then detected by a photodiode layer and converted into an electric output signal that is proportional to the total energy deposited during a measurement interval. 4–8 As the detector element integrates the energy from all photons, the output electrical signal does not convey any information on the energy of individual photons. Ultimately, in a substrate layer the generated signal is transmitted to analog electronics for amplification. 6,9
Despite its continuous merits, EIDs exhibit some inherent limitations. First, the detector design and more importantly the detector element size of current CT systems limits its maximum achievable spatial resolution. The maximum achievable spatial resolution of a CT system depends on both the size of the focal spot of the X-ray tube and the size of the detector elements—both must be matched and approximately equal. The spatial resolution as a function of the spatial frequency (in line pairs per cm) is described by the so-called modulation transfer function (MTF) and shows the relative contrast with which small periodic structures are represented in the image. The MTF can be modified by choosing different convolution kernels, but in doing so, the resolution limit of the measurement system cannot be exceeded. The ultimate resolution limit is reached at a spatial frequency equal to 1/(2*pixel size) (Nyquist theorem)—so detectors must be made smaller to improve spatial resolution. This is a problem for EID detectors, because the individual elements have to be separated by thin septa to avoid optical cross-talk. With smaller detector elements, more area is occupied by the optically isolating septa, which absorb X-rays without contributing to the detector signal, resulting in lower dose efficiency. 5,7,10 Second, electronic readout noise stemming from the analog electronic circuits remains a problem of EIDs. In case of high photon flux on the detector, the effect of electronic readout noise on image quality is negligible. At low to very low radiation doses, however, the number of detectable photons is low and electronic readout noise may become apparent thus degrading image quality. 6 Third, each photon contributes to the total input detector signal with an amount that is proportional to its own energy. Thus, the relative contribution of high-energy photons to the total signal is higher than that of low-energy photons. This underweighting of low-energy photons can be suboptimal as low contrast differences are most prominent at low X-ray energies. Contrast-enhanced CT scans in particular show suboptimal iodine contrast. 5,6 Fourth, most current high-end CT systems offer dual-energy (DE) applications. DECT enables functional imaging by exploiting material-specific differences in X-ray attenuation at different X-ray energies. 11,12 DECT data can be generated by means of various technologies including dual-source (DS), rapid kV switching, and dual-layer (DL) detector technology. However, each of these technologies has its specific set of inherent limitations such as imperfect spatial or temporal registration of data sets, field of view (FOV) restrictions and limitations in terms of tube voltage selection and the use of tube current modulation. Furthermore, for most of these technologies, DECT data are only available if a specific workflow has been selected prior to image acquisition. 13
With recent advances in engineering and physics, a novel technology has emerged with the potential to surpass many of the shortcomings and limitations of current CT systems. Photon-counting detector (PCD-) CT is a promising technology that might substantially improve and expand the applicability of CT imaging.
In this review, we want to summarize the basic technical features and the initial experience with PCD-CT by reviewing first publications with prototype and clinical systems and try to illustrate with case examples how this emerging technology may translate into improved clinical diagnostics.
Photon-counting detector CT
Detailed reviews about the technical principles of PCD-CT systems have been published elsewhere. 5–7,10 Therefore, we will not provide too much detail on these aspects but will rather focus on basic specifications and the first experience with the new technology.
Basic physical principles of the detector
PCDs differ considerably from EIDs. In contrast to EIDs, which require a separate scintillator layer to convert X-rays to light, PCDs use a single layer of a semi-conductor made of cadmium telluride (CdTe), cadmium zinc telluride (CZT), or silicon. A large bias voltage is applied between a cathode on top of and pixelated anodes at the bottom of the semi-conductor. Each incident X-ray photon produces a cloud of positive and negative charges which are separated in the strong electric field and pulled away from each other rapidly. The electrons move towards the anodes to generate an electric signal that is registered by an attached electronic readout circuit. Thus, with PCDs X-ray photons are directly converted into an electrical signal and each photon leads to an electrical pulse whose amplitude is directly proportional to the energy of the photon. The PCD then counts the number of pulses to quantify the number of incident X-ray photons and compares the amplitude of each pulse to several pre-set threshold levels that are set by means of multiple electronic comparators and counters. Specifically, an initial threshold is set at a level that is higher than the electronic noise level but lower than the pulses of incident photons (e.g. at 25 keV). Furthermore, as all pulses are additionally compared to further threshold levels, photons can be assigned to energy bins depending on their energy. 5–7,10
The detector design of PCDs relying on a direct conversion technology overcomes the above-mentioned issues of EIDs. First, by eliminating the scintillator material and consequently the optically isolating septa, the detector elements of PCDs can be made much smaller, thus offering improved spatial resolution. Second, by thresholding incoming photons according to their energies, electronic noise can be eliminated and spectral (i.e. dual- or multienergy) imaging becomes inherently available. Third, as the detective quantum efficiency (DQE) of a photon counting detector is approximately constant as a function of X-ray energy, 5 there is no underweighting of low-energy X-ray photons as with EIDs, and image contrasts can be improved. 5,6 In a practical detector design, the thickness of the semi-conductor layer of a PCD has to be chosen large enough to provide a total DQE similar to EIDs. Thin layers of about 1.4–2 mm are sufficient for CdTe or CZT because of their high atomic number. Si with its low atomic number and low absorption efficiency in the X-ray energy range relevant to medical CT requires thick layers >30 mm. 5,6 Still, the exact total DQE strongly depends on the detector design.
Temporal evolution
Computational power
Until recently, the use of PCDs has mainly been limited to nuclear imaging as the X-ray photon-count rate in CT imaging is much higher than in nuclear medicine. 14 Specifically, the PCD has to register each incoming X-ray photon before the next one arrives. If the PCD does not manage this, a pile-up occurs where the photons can no longer be separately registered and the count rate is no longer proportional to the X-ray flux, leading to image quality degradation. A further challenge concerns the cross-talk between detector elements. X-ray photons hitting the detector close to the border of a detector element produce charge clouds that spread out and may be wrongfully registered by more than one detector element. This results in a loss of spatial resolution and spectral separation. By implementing faster readout electronics, smaller pixel sizes, subdivided detector elements, and optimized electronic circuits that can detect coincidental registration of photons, these hurdles were recently overcome, making industrial production of PCD-CT possible. 5,6,10
PCD-CT systems
A series of prototype PCD-CT systems enabled pre-clinical research but were all limited in one way or another. One prototype system was a whole-body research PCD-CT system built (SOMATOM Count, Siemens Healthineers, Forchheim, Germany) using a modified DS CT platform (SOMATOM Definition Flash, Siemens Healthineers), with the B-subsystem equipped with a CdTe PCD array. 2 × 2 subpixels of the photon counting detector can be binned to a “sharp pixel” or “ultra-high resolution (UHR) pixel” with a pixel size of 0.45 × 0.45 mm2 (0.25 × 0.25 mm2 at the isocenter), 4 × 4 subpixels can be binned to a “MACRO pixel” with a size of 0.9 × 0.9 mm2 (0.5 × 0.2 mm2 at the isocenter) comparable to today’s medical CT systems. A detailed description of this system can be found elsewhere. 5,15–20 The main limitations of this scanner included a detector z-coverage of 8–16 mm depending on the acquisition mode, an in-plane FOV of the PCD array of 27.5 cm at the isocenter, and a lack of angular tube current modulation (for comparison: a conventional SOMATOM Definition Flash scanner has a detector z-coverage of 38.4 mm and a maximum in-plane FOV of 50 cm 21 ). In addition, due to the hybrid design, DS PCD scanning at high temporal resolution was not possible. Later, a further investigational whole-body full FOV single-source PCD-CT system (SOMATOM Count Plus, Siemens Healthineers, Forchheim, Germany) was developed thereby overcoming most of the key limitations of the hybrid DS scanner by offering a 50 cm scan FOV, 57.6 mm longitudinal detector coverage as well as automatic exposure control in both angular and longitudinal directions. 22 Recently, another prototype single-source CT scanner with a full FOV silicon-based PCD (modification of a commercial Lightspeed VCT scanner, GE Healthcare, Chicago, Illinois, USA) was presented with a phantom study. 23 Another, further advanced PCD-CT prototype system based on a CZT detector (modified Brilliance iCT scanner, Philips Healthcare) and capable of human imaging was recently developed. 24,25 This system enables cardiac imaging on a level and beyond the technical performance of a comparable EID-CT system. 24,26 This prototype relies on a single-layer of energy-sensitive PCDs of 2 mm thick CZT with five adaptable energy thresholds set at 30, 51, 62, 72 and 81 keV and a detector element size of 0.27 × 0.27 mm2 at the isocenter. An in-plane FOV of 50 cm and a z-coverage of 1.76 cm (64 × 0.275 mm at the isocenter) is offered. Tube voltage can be set at 80, 100, 120 or 140 kV, and tube current can be modulated between 10 and 500 mAs. The system has a focal spot of 0.6 × 0.7 mm and a gantry rotation time of 0.33–1 s for 2400 projections per rotation.
Importantly, however, a PCD-CT system has recently been cleared for clinical use (NAEOTOM Alpha; Siemens Healthineers/ FDA approval September 30, 2021). A detailed description of this CT system has been published recently. 8 In brief, this scanner exhibits a DS geometry with a minimum gantry rotation time of 0.25 s offering a temporal resolution of 66 ms. The system uses two dedicated PCDs with 1.6 mm thick CdTe. Each detector element has a size of 0.151 × 0.176 mm2 projected to the isocenter. The detector pixels can be read out either independently, thus allowing for UHR imaging with a z-coverage of 24 mm (120 × 0.2 mm at the isocenter) or can be binned into 2 × 2 groups (resulting pixel size 0.302 × 0.352 mm2 at the isocenter) for standard imaging with a z-coverage of 57.6 mm (144 × 0.4 mm at the isocenter). Scans acquired in the spectral mode use fixed energy bin thresholds of 20/35/55/70 keV and offer full spectral image information. The system is equipped with two Vectron tubes each with 120 kW power output. To match the spatial resolution of the detector, the Vectron tubes offer several focal spots with dimensions down to 0.4 mm x 0.4 mm2 (0.181 × 0.181 mm2 at the isocenter) for UHR scanning.
Images can be reconstructed with a range of slice thicknesses as low as 0.2 mm depending on the protocol and with various matrix sizes (512 × 512, 768 × 768, 1024 × 1024). 8,22,27 A novel iterative reconstruction (IR) algorithm named Quantum Iterative Reconstruction (QIR) that is suitable for the reconstruction of spectral imaging data 28 has been introduced. As with previous IR algorithms, higher strength levels lead to greater noise reductions. 27,28 A representative image example illustrating the performance of the novel IR algorithm is provided in Figure 1.
Figure 1.
63-year-old male patient (body weight 77 kg) with multifocal hepatocellular carcinoma. Contrast-enhanced abdominal portal venous phase scans were acquired on a clinical PCD-CT system (NAEOTOM Alpha, Siemens Healthineers) in the spectral imaging mode at 120 kV with a tube current of 86 mAs. The CTDIvol was 6.7 mGy. Virtual monoenergetic images at 60 keV were reconstructed with a 2 mm slice thickness without Quantum Iterative Reconstruction and with all strength levels of QIR (QIR 1–4). CTDIvol, volume of CT dose index; PCD, Photon-counting detector; QIR, Quantum Iterative Reconstruction
Translating the benefits of PCD-CT into clinical routine
Improved spatial resolution without dose penalty
The lack of a scintillator layer and the use of subdivided detectors in a PCD enables dose-efficient UHR imaging. A range of technological approaches have previously enabled UHR imaging for EID-CT including the use of very small detector element sizes or the implementation of comb filters to reduce detector aperture in the z-axis and/or in-plane. All these approaches, however, were associated with manufacturing difficulties or reduced dose efficiency of up to 50%. 27,29,30
The detector design of the current PCD-CT systems bypass these difficulties, by exhibiting a 125 µm limiting (4.00 lp/mm) (NAEOTOM Alpha, Siemens Healthineers) 8 to 178 µm limiting (2.81 lp/mm) (Philips Healthcare prototype) in-plane spatial resolution. 24,25 The limiting spatial resolution is defined as the spatial frequency at 0% MTF for the sharpest convolution kernel available at the CT system. For comparison, the limiting spatial resolution of a comparable EID system (SOMATOM Force, Siemens Healthineers) is 240 µm (2.08 lp/mm).
Based on the UHR mode of a prototype PCD-CT system with 150 µm limiting spatial resolution (3.33 lp/mm) (SOMATOM Count) , Leng et al were able to show that the UHR mode with a pixel size of 0.25 × 0.25 mm2 at the isocenter exhibited a 87% improvement in spatial resolution (10% MTF) using the sharpest available convolution kernel as compared to the so-called MACRO scan mode of the same system with 0.5 × 0.5 mm2 pixel size (i.e. standard-resolution PCD-CT) comparable to today’s medical CT systems. Alternatively, a 15% reduction in image noise could be achieved at the same in-plane spatial resolution (sharpest kernel available in the MACRO mode) for scans acquired with the UHR scan mode as compared to the MACRO scan mode. 6,15 Klein et al and Pourmorteza et al confirmed that the UHR mode achieves lower noise levels than the standard MACRO mode at comparable spatial resolution because detector cell binning is avoided. This further highlights the high dose efficiency of the PCD-CT UHR mode. 18,31
Importantly, using dedicated phantom experiments, cadaveric and patient scans, both Leng et al 15,32 and Zhou et al 33 provided evidence that the UHR PCD-CT mode outperforms the comb-filter UHR EID-CT mode when using comparable imaging and reconstruction parameters. The latter study even demonstrated 40% less noise for PCD-CT UHR-based temporal bone imaging as compared to comb-filter UHR EID-CT imaging at matching radiation doses and reconstruction settings. 33
A variety of further studies have been published on HR and UHR PCD-CT imaging highlighting its value for lung assessment, 15,25,27,34–39 cardiac imaging 20,24,40–44 and musculoskeletal applications. 19,33,45–48
UHR imaging is of particular interest for these anatomical areas because the clear depiction of fine details may effectively enhance diagnostic accuracy. In case of lung imaging, fine parenchymal changes may be better detected, delineated and characterized. 15,36–38 Inoue et al scanned 30 patients with suspicion of interstitial lung disease on a research PCD-CT system in the UHR mode with optimized reconstruction settings and on standard-of-care EID-CT systems. PCD-CT improved reader’s confidence for the presence of imaging findings of reticulation, ground-glass opacities, and mosaic pattern as determined by three thoracic radiologists. Furthermore, reader confidence in the probability of usual interstitial pneumonia increased for one of the three thoracic radiologists. Lastly, overall image quality and sharpness of PCD-CT images was deemed improved despite the slightly lower radiation dose (median CTDIvol of 6.49 mGy for PCD-CT vs 7.88 mGy for EID-CT). 38 A further clinical study with 80 systemic sclerosis patients showed that the UHR mode of the first clinical PCD-CT system maintains image quality and diagnostic accuracy for the assessment of interstitial lung disease at only 33% of the dose of comparable EID-CT scans performed on a third generation (i.e. latest generation) DS EID-CT scanner. 39
In regard to coronary artery imaging, plaque visualization and stent imaging can be improved. 24,40 Two recent studies assessing the UHR scan mode of clinical PCD-CT in patients referred for coronary CT angiography have shown that coronary arteries can be visualized in excellent quality with improved visualization of non-calcified plaque components and with reduced blooming of calcified plaques. 43,44 For bone imaging, fine bone details and previously occult hairline fractures may become apparent. 33,46,49,50
In a clinical feasibility study including 32 patients who underwent both UHR PCD-CT imaging on a prototype CT system with optimized reconstruction settings and standard-of-care EID-CT imaging, Baffour et al reported improved visualization of osseous structures of the pelvis and shoulder for UHR PCD-CT imaging at a 31–47% lower radiation dose. A further study on 29 multiple myeloma patients showed that the visualization of lytic bone lesions, medullary lesions and fat attenuation in myeloma lesions could significantly be improved with a clinical PCD-CT in the UHR mode as compared to standard-of-care EID-CT systems with matching reconstruction parameters and radiation dose. 51 Further clinical studies will demonstrate whether or not the improvements in anatomic display will also impact on therapeutic management and ultimately, on patient outcome. 52
Representative PCD-CT cases scanned with the UHR mode illustrating the benefits for coronary CT angiography and upper ankle joint imaging can be found in Figures 2–3.
Figure 2.
65-year-old male patient (body weight 94 kg) with calcified coronary plaques in the left main and left anterior descending coronary artery with an Agatston Score of 245. Coronary CTA images were acquired in the UHR mode (z-coverage of 24 mm) on a clinical dual source PCD-CT system (NAEOTOM Alpha, Siemens Healthineers) at 120 kV with a tube current of 61 mAs. The gantry rotation time was 0.25 s, with a temporal resolution of 66 ms. The CTDIvol was 46.3 mGy. Images at different slice thicknesses and kernels were reconstructed. Note the improved sharpness of anatomical structures, vessels, and calcified coronary plaques on UHR images reconstructed with the Bv64 kernel and 0.2 mm section thickness. CTA, CT angiography; CTDIvol, volume of CT dose index; PCD, Photon-counting detector; UHR, ultra-high resolution.
Figure 3.
32-year-old male patient (body weight 64 kg) presenting with a depression fracture of the talus. The fracture gap extends to the lateral part of the talus. Images were acquired in the UHR mode (z-coverage of 24 mm) on a clinical PCD-CT system (NAEOTOM Alpha, Siemens Healthineers) at 120 kV and with a tube current of 40 mAs. Radiation dose was 3.23 mGy CTDIvol. Images were reconstructed with a Br56 kernel, 2 mm section thickness and a 512 × 512 matrix size, with a Qr68 kernel, 1 mm section thickness and a 512 × 512 matrix size and with a sharp Qr72 kernel, 0.2 mm section thickness (UHR image) and 1024 × 1024 matrix size. The left image represents the default setting for bone imaging, while the right image leverages the potential of PCD-CT UHR imaging. Note the excellent visualization of bone features and trabeculae and the exquisite delineation of the small fracture gap. CTDIvol, volume of CT dose index; PCD, Photon-counting detector; UHR, ultra-high resolution.
Improved CNR and noise properties
Improved contrast-to-noise ratio (CNR) and noise performance stemming from factors such as lack of electronic noise or equal weighting of all X-ray photons (no underweighting of low energy photons) are a key feature of PCD-CT systems enabling radiation and contrast media dose reduction for routine clinical imaging. 53
Using the first clinical PCD-CT system, Liu et al quantified the ability of PCD-CT to eliminate electronic background noise, with it achieving mean percent noise reductions of up to 74% at a radiation dose level of 0.4 mGy CTDIvol compared to a third generation (i.e. latest generation) DS EID-CT system. 54 Rajagopal et al investigated the technical performance of a prototype PCD-CT system for low dose abdominal CT imaging in a phantom 55 and found that both for PCD-CT and EID-CT spatial resolution as a function of noise and contrast remained unaffected by dose while PCD-CT achieved a 22–24% improvement in noise across four radiation dose levels ranging from 1.7 to 6 mGy CTDIvol. Consequently, this improved noise performance could be translated to a 29–41% improvement in CNR and a 20–36% improvement in detectability index. Using the first clinical PCD-CT, Racine et al confirmed that PCD-CT outperforms third generation (i.e. latest generation) DS EID-CT for the detection of hypo- and hyperattenuating focal liver lesions across a wide range of radiation dose levels. 56 Gutjahr et al showed that iodine CNR was improved by 11–38% for a prototype PCD-CT system relative to EID-CT at matching scan and tube voltage settings of 80–140 kV. 21 This was further confirmed by Sawall et al who showed that with a prototype PCD-CT, dose-normalized iodine CNR could be improved by up to 37% relative to EID-CT, thus potentially enabling a radiation dose reduction of up to 46%. 57 More recently, Booij et al investigated the iodine CNR benefits of the first clinical PCD-CT on VMI relative to a third generation (i.e. latest generation) DS EID-CT system run in DE mode with largely matching imaging and reconstruction settings. The authors confirmed that the CNR benefits of PCD-CT were also applicable for VMI, with low keV images below 60 keV exhibiting a 55–75% higher CNR than their EID-CT based counterparts depending on the tube voltage settings. 58
Further experiments on sinus and temporal bone imaging with a prototype PCD-CT have shown significant radiation dose reductions between 56 and 85% relative to EID-CT depending on the exact imaging protocol without compromising image contrast and image noise. 19 Specifically, experiments were performed using an UHR PCD-CT mode with an additional tin (Sn) filter at 100 kV tube voltage. Besides the use of the tin filter, the authors also cite the advantages of the UHR PCD-CT mode with better intrinsic detector resolution and the detector design of PC detectors that eliminates the need for comb/grid filters for UHR imaging as reasons for the improved performance of the PCD-CT system. More recent studies performed on the first clinical PCD-CT system support these previous results, with two experimental studies showing that the clinical PCD-CT outperforms third generation (i.e. latest generation) DS EID-CT systems across a variety of widely matching protocol parameters, reconstruction settings and radiation dose levels in terms of objective and subjective image quality. 59,60
The improved performance of PCD-CT was also confirmed in-vivo in a variety of clinical studies. Exemplarily, Symons et al showed that a prototype PCD-CT exhibited between 15 and 17% lower noise than EID-CT for 120 kV and 100 kV dose-matched chest CT scans. 61 Importantly, low dose PCD-CT imaging of the lungs was also associated with improved HU stability relative to EID-CT. 62 Specifically, across tube voltage settings of 80, 100 and 120 kV, attenuation values of lung equivalent foams of a dedicated phantom as measured on PCD-CT remained stable while the attenuation values of EID-CT decreased by up to approximately 5 HU when decreasing the dose level from 3 to 0.75 mGy CTDIvol.
Pourmorteza et al demonstrated improved image quality of a prototype PCD-CT for brain imaging relative to EID-CT exhibiting 12.8–20.6% less image noise and 15.7–33.3% improved soft-tissue CNR. 63 Lastly, in a combined phantom and in-vivo study Symons et al demonstrated improved coronary artery calcium scoring (CACS) accuracy at low radiation doses for PCD-CT relative to EID-CT owing to the improved noise and CNR properties of PCD-CT. 64 The improved detection and quantification accuracy of CACS at low radiation doses was later confirmed by van der Werf et al using a different PCD-CT concept. 65
Recently, two patient studies have been published on the performance of clinical PCD-CT operated at 120 kV compared to EID-CT scans performed on a third generation (i.e. latest generation) DS EID-CT scanner with automated tube voltage selection. These were intraindividual comparison studies in whom the same patients underwent scans both with PCD-CT and EID-CT within a relatively short time period. For abdominal CT, 50 keV VMI reconstructions of PCD-CT exhibited higher vascular and parenchymal CNR than polychromatic EID-CT reconstructions at similar subjective image quality. 66 For high-pitch CT angiography of the aorta, 45 keV VMI reconstructions of PCD-CT showed improved vascular CNR and similar subjective image quality as compared to polychromatic EID-CT reconstructions. 67
In a recent study, Higashigaito et al could show that CT angiography of the aorta using PCD-CT and administering 20% reduced contrast media volume provides non-inferior image quality compared to CT angiography of the aorta using EID-CT in the same patients and at matched radiation dose. 53
Representative images of patient studies illustrating the improved noise and CNR characteristics of PCD-CT are shown in Figures 4–6.
Figure 4.
47-year-old male patient (body weight 85 kg) with COVID-19-associated pneumonia presenting with ground-glass opacities and mild reticular abnormalities. Non-enhanced chest CT was acquired on a clinical PCD-CT system (NAEOTOM Alpha, Siemens Healthineers) in the UHR mode at 120 kV; the CTDIvol was 0.55 mGy. CTDIvol, volume of CT dose index; PCD, Photon-counting detector; UHR, ultra-high resolution.
Figure 5.
82-year-old male patient (body weight 81 kg) undergoing CTA for follow-up after endovascular treatment of an abdominal aortic aneurysm. Images were acquired on a third generation dual-source EID-CT system (SOMATOM Force, Siemens Healthineers) with automated tube voltage selection (80 kV) and CTA with a clinical dual-source PCD-CT system (NAEOTOM Alpha, Siemens Healthineers) (120 kV) at matched radiation dose (CTDIvol 6.1 mGy) and using the same contrast media protocol. Note the reduced noise and improved contrast on PCD-CT images. CTA, CT angiography; CTDIvol, volume of CT dose index; PCD, Photon-counting detector; EID, energy-integrating detector; PCD, Photon-counting detector.
Figure 6.
71-year-old female patient (body weight 75 kg) with a cyst in liver segment VII. Contrast-enhanced abdominal portal venous phase images were acquired on a third generation dual-source EID-CT system (SOMATOM Force, Siemens Healthineers) with automatic tube voltage selection (120 kV) and with a clinical PCD-CT system (NAEOTOM Alpha, Siemens Healthineers) in the spectral imaging mode at 120 kV at matched radiation dose (CTDIvol 7.24 mGy) and using the same contrast media protocol. Note the improved iodine contrast and lesion conspicuity on PCD-CT images. CTDIvol, volume of CT dose index; EID, energy-integrating detector; PCD, Photon-counting detector.
Intrinsic spectral capabilities
A key feature of PCD-CT is its ability to provide spectral information from every scan due to the detector being able to count and characterize individual photons according to their energy. In contrast, most EID-CT systems require the user to choose between single-energy or DE scan modes prior to image acquisition. The intrinsic spectral capabilities of the first clinical DS PCD-CT system with 0.25 s rotation time allows for multienergy imaging at 66 ms temporal resolution and high pitch multienergy imaging with helical pitch values up to 3.2. When implemented as an ECG-triggered high-pitch scan mode for cardiac imaging, the latter may enable radiation dose reductions of up to a factor of 2 as compared to other DSCT scan techniques such as ECG-triggered sequential step-and-shoot and ECG-gated spiral with X-ray pulsing. 8,67,68
Currently, ECG-gated UHR imaging represents an exception to this rule: due to the vast amount of image information that has to be processed, spectral image information is not yet available for this imaging mode on the first clinical DS PCD-CT system. However, additional updates to the scanner’s soft- and hardware should be able to fix the problem in the near future.
The spectral capabilities of PCD-CT allow the user to reconstruct iodine maps, virtual non-contrast (VNC) and virtual monoenergetic images (VMIs) from every acquisition. The use of VMI for routine clinical PCD-CT imaging has exemplarily been shown for abdominal imaging, 28,66 cardiac CT 69–71 and coronary calcium quantification, 72 high-pitch CT angiography of the aorta, 67 lung 35,73 and brain imaging. 74 The benefits of low keV VMI include better visualization of small low-contrast structures and increased iodine signal. For CT angiography examinations, the latter may be particularly promising as high pitch CTA together with low keV VMI may set new standards in terms of radiation and contrast media dose reductions. 8,53
Concerning VNC images and iodine maps, Rajendran et al 8 and Sartoretti et al 75 have demonstrated the feasibility of reconstructing these images from routine clinical PCD-CT scans. Quantitative accuracy of reconstructions was high with VNC showing mean absolute errors of 4 HU 75 and iodine maps exhibiting a root mean squared error of 0.5 mg/cm3 for iodine concentration. 8 For VNC imaging, the high quantitative accuracy was later further confirmed in a larger clinical study encompassing 100 patients who underwent a triphasic examination on the first clinical PCD-CT system. Attenuation errors of VNC images were less than 5 HU in 76% and less than 10 HU in 95% of measurements compared with true non-contrast images across a variety of abdominal organs and regions. Furthermore, diagnostic image quality of VNC images as determined by two independent readers was achieved in 99 and 100% of cases, respectively. 76
The clinical benefits of the routine availability of VNC images and iodine maps from PCD-CT include, among others, emphysema quantification from VNC images, 77 assessment of adrenal adenomas from VNC images, 78 anemia detection and quantification from VNC images 79 and myocardial extracellular volume quantification based on iodine maps from a single cardiac late enhancement scan. 70
Beyond the opportunities discussed above, PCD-CT harbors further potential in terms of improved and novel spectral imaging applications. The spectral data allow for the reconstruction of further spectral images such as calcium-only or virtual non-calcium (VNCa) images. In this regard, a novel vascular calcium removal algorithm has been recently introduced that aims to surpass the performance of previous similar algorithms designed for DECT capable EID-CT systems. 80 With this novel algorithm, high quality VNCa images can be reconstructed thus counteracting the problem of blooming artifacts from heavily calcified plaques on standard monoenergetic or polychromatic images. 81 A representative image example illustrating the performance of the novel algorithm for high quality VNCa imaging is provided in Figure 7.
Figure 7.
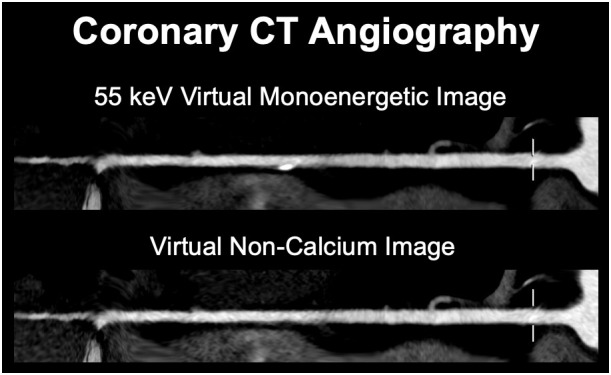
67-year-old male patient (body weight 71 kg) with atypical chest pain. Coronary CTA was performed on a clinical dual source PCD-CT system (NAEOTOM Alpha, Siemens Healthineers) at 120 kV (CTDIvol 10.1 mGy). Virtual monoenergetic images at 55 keV and VNCa images using a novel vascular calcium removal algorithm (PureLumen) were generated. A calcified plaque can be seen in the distal right coronary artery, which is subtracted on a dual-energy basis in the VNCa (PureLumen) images. CTA, CT angiography; CTDIvol, volume of CT dose index; PCD, Photon-counting detector; VNCa, virtual non-calcium.
With PCD-CT, improved material decomposition can potentially be achieved, as the selection of energy thresholds can be tailored towards the spectral behavior of the materials that are to be separated. For example, by selecting optimized energy thresholds high quality VNCa and contrast media maps could be computed from coronary and carotid CTA images acquired with a range of contrast media including iodine and experimental contrast media such as bismuth, tungsten, holmium or hafnium. 82,83 Furthermore, the energy threshold capabilities of PCDs can theoretically also be leveraged for improved simultaneous dual-contrast agent imaging as shown in experimental studies 84–87 or to further reduce metal artifact burden. 88,89
Although the latter applications are still in the preclinical phase of testing, PCD-CT opens up a range of new opportunities that may inspire a new momentum for clinical CT imaging.
Representative image examples highlighting the applications discussed above are provided in Figures 8–9.
Figure 8.
54-year-old male patient (body weight 76 kg) with hepatocellular carcinoma and vascular invasion. Late arterial scans were performed with a clinical PCD-CT (NAEOTOM Alpha, Siemens Healthineers) in the spectral mode with reconstruction of virtual monoenergetic images at 70 keV, virtual non-contrast images, and iodine maps from a single acquisition (CTDIvol3.6 mGy). Although tumor enhancement is seen also on the monoenergetic images, the iodine maps allow for a better appreciation of the carcinoma along with the possibility of quantification of iodine uptake. CTDIvol, volume of CT dose index; PCD, Photon-counting detector.
Figure 9.
59-year-old male patient (body weight 81 kg) with chronic occlusion of the right pulmonary artery and partial occlusion of left pulmonary artery branches resulting in severely reduced perfusion of the right and, to a lesser extent, of the left lung. Axial and coronal thick maximum intensity projection images and coronal PBV map computed from a routine contrast-enhanced chest CT scan acquired on a clinical PCD-CT (NAEOTOM Alpha, Siemens Healthineers) at 120 kV (CTDIvol 2.37 mGy) illustrate both the anatomical and functional situation in the lungs. CTDIvol, volume of CT dose index; PBV, perfused blood volume; PCD, Photon-counting detector.
Conclusion
In this review, we aimed to summarize basic technical principles and potential advantages of PCD-CT. Potential clinical benefits as evidenced by recent publications with this technology are outlined and representative clinical cases from our experience were added to illustrate the added value of the technique.
While the current possibilities of PCD-CT already considerably enhance our diagnostic capabilities, we foresee developments with spectrally optimized contrast media in combination with adaptable energy threshold imaging to enter clinical CT imaging in the near future.
Footnotes
Funding: JW: Institutional grants via Clinical Trial Center Maastricht: Bard, Bayer, Boston, Brainlab, GE, Philips, Siemens; Speaker’s bureau via Maastricht UMC+: Bayer, Siemens HA: Institutional grants: Bayer, Guerbet, Siemens, Canon; Speaker´s bureau: Siemens TF: Employee of Siemens Healthineers, Forchheim, Germany. Open access funding provided by Universitat Zurich.
Author Contributions: T. S. and H. A. reviewed the literature. T. S., J. W., T. F. and H. A. wrote the paper. All coauthors contributed constructively to the manuscript.
Contributor Information
Thomas Sartoretti, Email: thomas.sartoretti@usz.ch.
Joachim E Wildberger, Email: j.wildberger@mumc.nl.
Thomas Flohr, Email: thomas.flohr@siemens-healthineers.com.
Hatem Alkadhi, Email: hatem.alkadhi@usz.ch.
REFERENCES
- 1. Smith-Bindman R, Kwan ML, Marlow EC, Theis MK, Bolch W, Cheng SY, et al. Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000-2016. JAMA 2019; 322: 843–56. doi: 10.1001/jama.2019.11456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alkadhi H, Euler A. The future of computed tomography: personalized, functional, and precise. Invest Radiol 2020; 55: 545–55. doi: 10.1097/RLI.0000000000000668 [DOI] [PubMed] [Google Scholar]
- 3. Wildberger JE, Prokop M. Hounsfield’s legacy. Invest Radiol 2020; 55: 556–58. doi: 10.1097/RLI.0000000000000680 [DOI] [PubMed] [Google Scholar]
- 4. Lell MM, Wildberger JE, Alkadhi H, Damilakis J, Kachelriess M. Evolution in computed tomography: the battle for speed and dose. Invest Radiol 2015; 50: 629–44. doi: 10.1097/RLI.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 5. Flohr T, Petersilka M, Henning A, Ulzheimer S, Ferda J, Schmidt B. Photon-Counting CT review. Phys Med 2020; 79: 126–36: S1120-1797(20)30273-8. doi: 10.1016/j.ejmp.2020.10.030 [DOI] [PubMed] [Google Scholar]
- 6. Leng S, Bruesewitz M, Tao S, Rajendran K, Halaweish AF, Campeau NG, et al. Photon-Counting detector CT: system design and clinical applications of an emerging technology. Radiographics 2019; 39: 729–43. doi: 10.1148/rg.2019180115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willemink MJ, Persson M, Pourmorteza A, Pelc NJ, Fleischmann D. Photon-Counting CT: technical principles and clinical prospects. Radiology 2018; 289: 293–312. doi: 10.1148/radiol.2018172656 [DOI] [PubMed] [Google Scholar]
- 8. Rajendran K, Petersilka M, Henning A, Shanblatt ER, Schmidt B, Flohr TG, et al. First clinical photon-counting detector CT system: technical evaluation. Radiology 2022; 303: 130–38. doi: 10.1148/radiol.212579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shefer E, Altman A, Behling R, Goshen R, Gregorian L, Roterman Y, et al. State of the art of CT detectors and sources: a literature review. Curr Radiol Rep 2013; 1: 76–91. doi: 10.1007/s40134-012-0006-4 [DOI] [Google Scholar]
- 10. Sandfort V, Persson M, Pourmorteza A, Noël PB, Fleischmann D, Willemink MJ. Spectral photon-counting CT in cardiovascular imaging. J Cardiovasc Comput Tomogr 2021; 15: 218–25: S1934-5925(20)30503-7. doi: 10.1016/j.jcct.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 11. Postma AA, Das M, Stadler AAR, Wildberger JE. Dual-Energy CT: what the neuroradiologist should know. Curr Radiol Rep 2015; 3: 16: 16. doi: 10.1007/s40134-015-0097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roele ED, Timmer VCML, Vaassen LAA, van Kroonenburgh AMJL, Postma AA. Dual-Energy CT in head and neck imaging. Curr Radiol Rep 2017; 5: 19: 19. doi: 10.1007/s40134-017-0213-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rajiah P, Parakh A, Kay F, Baruah D, Kambadakone AR, Leng S. Update on multienergy CT: physics, principles, and applications. Radiographics 2020; 40: 1284–1308. doi: 10.1148/rg.2020200038 [DOI] [PubMed] [Google Scholar]
- 14. Taguchi K, Iwanczyk JS.. Vision 20/20: Single photon counting x-ray detectors in medical imaging: Vision 20/20: Photon counting detectors. Med Phys 2013;40:100901. doi: 10.1118/1.4820371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leng S, Rajendran K, Gong H, Zhou W, Halaweish AF, Henning A, et al. 150-μm spatial resolution using photon-counting detector computed tomography technology: technical performance and first patient images. Invest Radiol 2018; 53: 655–62. doi: 10.1097/RLI.0000000000000488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu Z, Leng S, Jorgensen SM, Li Z, Gutjahr R, Chen B, et al. Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array. Phys Med Biol 2016; 61: 1572–95. doi: 10.1088/0031-9155/61/4/1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pourmorteza A, Symons R, Sandfort V, Mallek M, Fuld MK, Henderson G, et al. Abdominal imaging with contrast-enhanced photon-counting CT: first human experience. Radiology 2016; 279: 239–45. doi: 10.1148/radiol.2016152601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klein L, Dorn S, Amato C, Heinze S, Uhrig M, Schlemmer H-P, et al. Effects of detector sampling on noise reduction in clinical photon-counting whole-body computed tomography. Invest Radiol 2020; 55: 111–19. doi: 10.1097/RLI.0000000000000616 [DOI] [PubMed] [Google Scholar]
- 19. Rajendran K, Voss BA, Zhou W, Tao S, DeLone DR, Lane JI, et al. Dose reduction for sinus and temporal bone imaging using photon-counting detector CT with an additional tin filter. Invest Radiol 2020; 55: 91–100. doi: 10.1097/RLI.0000000000000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mannil M, Hickethier T, von Spiczak J, Baer M, Henning A, Hertel M, et al. Photon-Counting CT: high-resolution imaging of coronary stents. Invest Radiol 2018; 53: 143–49. doi: 10.1097/RLI.0000000000000420 [DOI] [PubMed] [Google Scholar]
- 21. Gutjahr R, Halaweish AF, Yu Z, et al. Human Imaging With Photon Counting–Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Invest Radiol 2016;51:421–9. doi: 10.1097/RLI.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajendran K, Petersilka M, Henning A, et al. Full field-of-view, high-resolution, photon-counting detector CT: technical assessment and initial patient experience. Phys Med Biol 2021;66:205019. doi: 10.1088/1361-6560/ac155e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silva J Grönberg F, Cederström B, et al. Resolution characterization of a silicon-based, photon-counting computed tomography prototype capable of patient scanning. J Med Imag 2019; 6: 1. doi: 10.1117/1.JMI.6.4.043502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Si-Mohamed SA, Boccalini S. Lacombe H, et al.coronary CT angiography with photon-counting CT: first-in-human results. Radiology 2022. doi: 10.1148/radiol.211780 [DOI] [PubMed] [Google Scholar]
- 25. Si-Mohamed S, Boccalini S, Rodesch P-A, et al. Feasibility of lung imaging with a large field-of-view spectral photon-counting CT system. Diagnostic and Interventional Imaging 2021;102:305–12. doi: 10.1016/j.diii.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 26. Boccalini S, Si-Mohamed SA, Lacombe H, Diaw A, Varasteh M, Rodesch P-A, et al. First in-human results of computed tomography angiography for coronary stent assessment with a spectral photon counting computed tomography. Invest Radiol 2022; 57: 212–21. doi: 10.1097/RLI.0000000000000835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sartoretti T, Racine D, Mergen V, Jungblut L, Monnin P, Flohr TG, et al. Quantum iterative reconstruction for low-dose ultra-high-resolution photon-counting detector CT of the lung. Diagnostics (Basel) 2022; 12(): 522. doi: 10.3390/diagnostics12020522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sartoretti T, Landsmann A, Nakhostin D, Eberhard M, Roeren C, Mergen V, et al. Quantum iterative reconstruction for abdominal photon-counting detector CT improves image quality. Radiology 2022; 303: 339–48. doi: 10.1148/radiol.211931 [DOI] [PubMed] [Google Scholar]
- 29. Meyer M, Haubenreisser H, Raupach R, Schmidt B, Lietzmann F, Leidecker C, et al. Initial results of a new generation dual source CT system using only an in-plane comb filter for ultra-high resolution temporal bone imaging. Eur Radiol 2015; 25: 178–85. doi: 10.1007/s00330-014-3406-4 [DOI] [PubMed] [Google Scholar]
- 30. Oostveen LJ, Boedeker KL, Brink M, Prokop M, de Lange F, Sechopoulos I. Physical evaluation of an ultra-high-resolution CT scanner. Eur Radiol 2020; 30: 2552–60. doi: 10.1007/s00330-019-06635-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pourmorteza A, Symons R, Henning A, Ulzheimer S, Bluemke DA. Dose efficiency of quarter-millimeter photon-counting computed tomography: first-in-human results. Invest Radiol 2018; 53: 365–72. doi: 10.1097/RLI.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 32. Leng S, Yu Z, Halaweish A, Kappler S, Hahn K, Henning A, et al. Dose-efficient ultrahigh-resolution scan mode using a photon counting detector computed tomography system. J Med Imaging (Bellingham) 2016; 3: 043504: 043504. doi: 10.1117/1.JMI.3.4.043504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou W, Lane JI, Carlson ML, Bruesewitz MR, Witte RJ, Koeller KK, et al. Comparison of a photon-counting-detector CT with an energy-integrating-detector CT for temporal bone imaging: a cadaveric study. AJNR Am J Neuroradiol 2018; 39: 1733–38. doi: 10.3174/ajnr.A5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartlett DJ, Koo CW, Bartholmai BJ, Rajendran K, Weaver JM, Halaweish AF, et al. High-Resolution chest computed tomography imaging of the lungs: impact of 1024 matrix reconstruction and photon-counting detector computed tomography. Invest Radiol 2019; 54: 129–37. doi: 10.1097/RLI.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jungblut L, Blüthgen C, Polacin M, Messerli M, Schmidt B, Euler A, et al. First performance evaluation of an artificial intelligence-based computer-aided detection system for pulmonary nodule evaluation in dual-source photon-counting detector CT at different low-dose levels. Invest Radiol 2022; 57: 108–14. doi: 10.1097/RLI.0000000000000814 [DOI] [PubMed] [Google Scholar]
- 36. Ferda J, Vendiš T, Flohr T, Schmidt B, Henning A, Ulzheimer S, et al. Computed tomography with a full FOV photon-counting detector in a clinical setting, the first experience. Eur J Radiol 2021; 137: 109614: S0720-048X(21)00094-2. doi: 10.1016/j.ejrad.2021.109614 [DOI] [PubMed] [Google Scholar]
- 37. Zhou W, Montoya J, Gutjahr R, Ferrero A, Halaweish A, Kappler S, et al. Lung nodule volume quantification and shape differentiation with an ultra-high resolution technique on a photon counting detector CT system. Proc SPIE Int Soc Opt Eng 2017; 10132: 101323Q. doi: 10.1117/12.2255736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inoue A, Johnson TF, White D, Cox CW, Hartman TE, Thorne JE, et al. Estimating the clinical impact of photon-counting-detector CT in diagnosing usual interstitial pneumonia. Invest Radiol 2022; 57: 734–41. doi: 10.1097/RLI.0000000000000888 [DOI] [PubMed] [Google Scholar]
- 39. Jungblut L, Euler A, von Spiczak J, Sartoretti T, Mergen V, Englmaier V, et al. Potential of photon-counting detector CT for radiation dose reduction for the assessment of interstitial lung disease in patients with systemic sclerosis. Invest Radiol 2022; 57: 773–79. doi: 10.1097/RLI.0000000000000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rajagopal JR, Farhadi F, Richards T, Nikpanah M, Sahbaee P, Shanbhag SM, et al. Evaluation of coronary plaques and stents with conventional and photon-counting CT: benefits of high-resolution photon-counting CT. Radiol Cardiothorac Imaging 2021; 3(): e210102. doi: 10.1148/ryct.2021210102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petritsch B, Petri N, Weng AM, Petersilka M, Allmendinger T, Bley TA, et al. Photon-Counting computed tomography for coronary stent imaging: in vitro evaluation of 28 coronary stents. Invest Radiol 2021; 56: 653–60. doi: 10.1097/RLI.0000000000000787 [DOI] [PubMed] [Google Scholar]
- 42. Symons R, De Bruecker Y, Roosen J, Van Camp L, Cork TE, Kappler S, et al. Quarter-millimeter spectral coronary stent imaging with photon-counting CT: initial experience. J Cardiovasc Comput Tomogr 2018; 12: 509–15: S1934-5925(18)30426-X. doi: 10.1016/j.jcct.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 43. Mergen V, Eberhard M, Manka R, Euler A, Alkadhi H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front Cardiovasc Med 2022; 9: 981012: 981012. doi: 10.3389/fcvm.2022.981012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mergen V, Sartoretti T, Baer-Beck M, Schmidt B, Petersilka M, Wildberger JE, et al. Ultra-high-resolution coronary CT angiography with photon-counting detector CT: feasibility and image characterization. Invest Radiol 2022; 57: 780–88. doi: 10.1097/RLI.0000000000000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wehrse E, Sawall S, Klein L, Glemser P, Delorme S, Schlemmer H-P, et al. Potential of ultra-high-resolution photon-counting CT of bone metastases: initial experiences in breast cancer patients. NPJ Breast Cancer 2021; 7: 3: 3. doi: 10.1038/s41523-020-00207-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grunz J-P, Huflage H, Heidenreich JF, Ergün S, Petersilka M, Allmendinger T, et al. Image quality assessment for clinical cadmium telluride-based photon-counting computed tomography detector in cadaveric wrist imaging. Invest Radiol 2021; 56: 785–90. doi: 10.1097/RLI.0000000000000789 [DOI] [PubMed] [Google Scholar]
- 47. Bette SJ, Braun FM, Haerting M, Decker JA, Luitjens JH, Scheurig-Muenkler C, et al. Visualization of bone details in a novel photon-counting dual-source CT scanner-comparison with energy-integrating CT. Eur Radiol 2022; 32: 2930–36. doi: 10.1007/s00330-021-08441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baffour FI, Rajendran K, Glazebrook KN, Thorne JE, Larson NB, Leng S, et al. Ultra-High-Resolution imaging of the shoulder and pelvis using photon-counting-detector CT: a feasibility study in patients. Eur Radiol 2022; 32: 7079–86. doi: 10.1007/s00330-022-08925-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rajendran K, Baffour F, Powell G, Glazebrook K, Thorne J, Larson N, et al. Improved visualization of the wrist at lower radiation dose with photon-counting-detector CT. Skeletal Radiol 2023; 52: 23–29. doi: 10.1007/s00256-022-04117-2 [DOI] [PubMed] [Google Scholar]
- 50. Kämmerling N, Sandstedt M, Farnebo S, Persson A, Tesselaar E. Assessment of image quality in photon-counting detector computed tomography of the wrist-an ex vivo study. Eur J Radiol 2022; 154: 110442: S0720-048X(22)00292-3. doi: 10.1016/j.ejrad.2022.110442 [DOI] [PubMed] [Google Scholar]
- 51. Baffour FI, Huber NR, Ferrero A, Rajendran K, Glazebrook KN, Larson NB, et al. Photon-Counting detector CT with deep learning noise reduction to detect multiple myeloma. Radiology 2023; 306: 229–36. doi: 10.1148/radiol.220311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making 1991; 11: 88–94. doi: 10.1177/0272989X9101100203 [DOI] [PubMed] [Google Scholar]
- 53. Higashigaito K, Mergen V, Eberhard M, Jungblut L, Hebeisen M, Rätzer S, et al. CT angiography of the aorta using photon-counting detector CT with reduced contrast media volume. Radiology: Cardiothoracic Imaging 2023; 5(. doi: 10.1148/ryct.220140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu LP, Shapira N, Chen AA, Shinohara RT, Sahbaee P, Schnall M, et al. First-Generation clinical dual-source photon-counting CT: ultra-low-dose quantitative spectral imaging. Eur Radiol 2022; 32: 8579–87. doi: 10.1007/s00330-022-08933-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rajagopal JR, Farhadi F, Solomon J, Sahbaee P, Saboury B, Pritchard WF, et al. Comparison of low dose performance of photon-counting and energy integrating CT. Acad Radiol 2021; 28: 1754–60: S1076-6332(20)30456-6. doi: 10.1016/j.acra.2020.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Racine D, Mergen V, Viry A, Eberhard M, Becce F, Rotzinger DC, et al. Photon-counting detector CT with quantum iterative reconstruction: impact on liver lesion detection and radiation dose reduction. Invest Radiol 2022. doi: 10.1097/RLI.0000000000000925 [DOI] [PubMed] [Google Scholar]
- 57. Sawall S, Klein L, Amato C, Wehrse E, Dorn S, Maier J, et al. Iodine contrast-to-noise ratio improvement at unit dose and contrast media volume reduction in whole-body photon-counting CT. Eur J Radiol 2020; 126: 108909: S0720-048X(20)30098-X. doi: 10.1016/j.ejrad.2020.108909 [DOI] [PubMed] [Google Scholar]
- 58. Booij R, van der Werf NR, Dijkshoorn ML, van der Lugt A, van Straten M. Assessment of iodine contrast-to-noise ratio in virtual monoenergetic images reconstructed from dual-source energy-integrating CT and photon-counting CT data. Diagnostics (Basel) 2022; 12(): 1467. doi: 10.3390/diagnostics12061467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grunz J-P, Petritsch B, Luetkens KS, Kunz AS, Lennartz S, Ergün S, et al. Ultra-low-dose photon-counting CT imaging of the paranasal sinus with tin prefiltration: how low can we go? Invest Radiol 2022; 57: 728–33. doi: 10.1097/RLI.0000000000000887 [DOI] [PubMed] [Google Scholar]
- 60. Grunz J-P, Heidenreich JF, Lennartz S, Weighardt JP, Bley TA, Ergün S, et al. Spectral shaping via tin prefiltration in ultra-high-resolution photon-counting and energy-integrating detector CT of the temporal bone. Invest Radiol 2022; 57: 819–25. doi: 10.1097/RLI.0000000000000901 [DOI] [PubMed] [Google Scholar]
- 61. Symons R, Pourmorteza A, Sandfort V, Ahlman MA, Cropper T, Mallek M, et al. Feasibility of dose-reduced chest CT with photon-counting detectors: initial results in humans. Radiology 2017; 285: 980–89. doi: 10.1148/radiol.2017162587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Symons R, Cork TE, Sahbaee P, Fuld MK, Kappler S, Folio LR, et al. Low-Dose lung cancer screening with photon-counting CT: a feasibility study. Phys Med Biol 2017; 62: 202–13. doi: 10.1088/1361-6560/62/1/202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pourmorteza A, Symons R, Reich DS, Bagheri M, Cork TE, Kappler S, et al. Photon-Counting CT of the brain: in vivo human results and image-quality assessment. AJNR Am J Neuroradiol 2017; 38: 2257–63. doi: 10.3174/ajnr.A5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Symons R, Sandfort V, Mallek M, Ulzheimer S, Pourmorteza A. Coronary artery calcium scoring with photon-counting CT: first in vivo human experience. Int J Cardiovasc Imaging 2019; 35: 733–39. doi: 10.1007/s10554-018-1499-6 [DOI] [PubMed] [Google Scholar]
- 65. van der Werf NR, Rodesch PA, Si-Mohamed S, van Hamersvelt RW, Greuter MJW, Leiner T, et al. Improved coronary calcium detection and quantification with low-dose full field-of-view photon-counting CT: a phantom study. Eur Radiol 2022; 32: 3447–57. doi: 10.1007/s00330-021-08421-8 [DOI] [PubMed] [Google Scholar]
- 66. Higashigaito K, Euler A, Eberhard M, Flohr TG, Schmidt B, Alkadhi H. Contrast-enhanced abdominal CT with clinical photon-counting detector CT: assessment of image quality and comparison with energy-integrating detector CT. Acad Radiol 2022; 29: 689–97. doi: 10.1016/j.acra.2021.06.018 [DOI] [PubMed] [Google Scholar]
- 67. Euler A, Higashigaito K, Mergen V, Sartoretti T, Zanini B, Schmidt B, et al. High-pitch photon-counting detector computed tomography angiography of the aorta: Intraindividual comparison to energy-integrating detector computed tomography at equal radiation dose. Invest Radiol 2022; 57: 115–21. doi: 10.1097/RLI.0000000000000816 [DOI] [PubMed] [Google Scholar]
- 68. Flohr TG, Leng S, Yu L, Aiimendinger T, Bruder H, Petersilka M, et al. Dual-source spiral CT with pitch up to 3.2 and 75 MS temporal resolution: image reconstruction and assessment of image quality. Med Phys 2009; 36: 5641–53. doi: 10.1118/1.3259739 [DOI] [PubMed] [Google Scholar]
- 69. Mergen V, Ried E, Allmendinger T, Sartoretti T, Higashigaito K, Manka R, et al. Epicardial adipose tissue attenuation and fat attenuation index: phantom study and in vivo measurements with photon-counting detector CT. AJR Am J Roentgenol 2022; 218: 822–29. doi: 10.2214/AJR.21.26930 [DOI] [PubMed] [Google Scholar]
- 70. Mergen V, Sartoretti T, Klotz E, Schmidt B, Jungblut L, Higashigaito K, et al. Extracellular volume quantification with cardiac late enhancement scanning using dual-source photon-counting detector CT. Invest Radiol 2022; 57: 406–11. doi: 10.1097/RLI.0000000000000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sartoretti T, McDermott M, Mergen V, Euler A, Schmidt B, Jost G, et al. Photon-Counting detector coronary CT angiography: impact of virtual monoenergetic imaging and iterative reconstruction on image quality. Br J Radiol 2023: 20220466. doi: 10.1259/bjr.20220466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Eberhard M, Mergen V, Higashigaito K, Allmendinger T, Manka R, Flohr T, et al. Coronary calcium scoring with first generation dual-source photon-counting CT-first evidence from phantom and in-vivo scans. Diagnostics (Basel) 2021; 11(): 1708. doi: 10.3390/diagnostics11091708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jungblut L, Kronenberg D, Mergen V, Higashigaito K, Schmidt B, Euler A, et al. Impact of contrast enhancement and virtual monoenergetic image energy levels on emphysema quantification: experience with photon-counting detector computed tomography. Invest Radiol 2022; 57: 359–65. doi: 10.1097/RLI.0000000000000848 [DOI] [PubMed] [Google Scholar]
- 74. Michael AE, Boriesosdick J, Schoenbeck D, Woeltjen MM, Saeed S, Kroeger JR, et al. Image-quality assessment of polyenergetic and virtual monoenergetic reconstructions of unenhanced CT scans of the head: initial experiences with the first photon-counting CT Approved for clinical use. Diagnostics (Basel) 2022; 12(): 265. doi: 10.3390/diagnostics12020265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sartoretti T, Mergen V, Higashigaito K, Eberhard M, Alkadhi H, Euler A. Virtual noncontrast imaging of the liver using photon-counting detector computed tomography: A systematic phantom and patient study. Invest Radiol 2022; 57: 488–93. doi: 10.1097/RLI.0000000000000860 [DOI] [PubMed] [Google Scholar]
- 76. Mergen V, Racine D, Jungblut L, Sartoretti T, Bickel S, Monnin P, et al. Virtual noncontrast abdominal imaging with photon-counting detector CT. Radiology 2022; 305: 107–15. doi: 10.1148/radiol.213260 [DOI] [PubMed] [Google Scholar]
- 77. Jungblut L, Sartoretti T, Kronenberg D, Mergen V, Euler A, Schmidt B, et al. Performance of virtual non-contrast images generated on clinical photon-counting detector CT for emphysema quantification: proof of concept. Br J Radiol 2022; 95(): 20211367. doi: 10.1259/bjr.20211367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lennartz S, Schoenbeck D, Kröger JR, Borggrefe J, Henning Niehoff J. Photon-Counting CT material decomposition: initial experience in assessing adrenal adenoma. Radiology 2023; 306: 202–4. doi: 10.1148/radiol.220919 [DOI] [PubMed] [Google Scholar]
- 79. Decker JA, Huber A, Senel F, Bette S, Braun F, Risch F, et al. Anemia detection by hemoglobin quantification on contrast-enhanced photon-counting CT data sets. Radiology 2022; 305: 650–52. doi: 10.1148/radiol.220063 [DOI] [PubMed] [Google Scholar]
- 80. Mannil M, Ramachandran J, Vittoria de Martini I, Wegener S, Schmidt B, Flohr T, et al. Modified dual-energy algorithm for calcified plaque removal: evaluation in carotid computed tomography angiography and comparison with digital subtraction angiography. Invest Radiol 2017; 52: 680–85. doi: 10.1097/RLI.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 81. Allmendinger T, Nowak T, Flohr T, Klotz E, Hagenauer J, Alkadhi H, et al. Photon-Counting detector CT-based vascular calcium removal algorithm: assessment using a cardiac motion phantom. Invest Radiol 2022; 57: 399–405. doi: 10.1097/RLI.0000000000000853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sartoretti T, Eberhard M, Nowak T, Gutjahr R, Jost G, Pietsch H, et al. Photon-Counting multienergy computed tomography with spectrally optimized contrast media for plaque removal and stenosis assessment. Invest Radiol 2021; 56: 563–70. doi: 10.1097/RLI.0000000000000773 [DOI] [PubMed] [Google Scholar]
- 83. Sartoretti T, Eberhard M, Rüschoff JH, Pietsch H, Jost G, Nowak T, et al. Photon-Counting CT with tungsten as contrast medium: experimental evidence of vessel lumen and plaque visualization. Atherosclerosis 2020; 310: 11–16: S0021-9150(20)30405-6. doi: 10.1016/j.atherosclerosis.2020.07.023 [DOI] [PubMed] [Google Scholar]
- 84. Ren L, Rajendran K, Fletcher JG, McCollough CH, Yu L. Simultaneous dual-contrast imaging of small bowel with iodine and bismuth using photon-counting-detector computed tomography: a feasibility animal study. Invest Radiol 2020; 55: 688–94. doi: 10.1097/RLI.0000000000000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ren L, Huber N, Rajendran K, Fletcher JG, McCollough CH, Yu L. Dual-Contrast biphasic liver imaging with iodine and gadolinium using photon-counting detector computed tomography: an exploratory animal study. Invest Radiol 2022; 57: 122–29. doi: 10.1097/RLI.0000000000000815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Muenzel D, Bar-Ness D, Roessl E, Blevis I, Bartels M, Fingerle AA, et al. Spectral photon-counting CT: initial experience with dual-contrast agent K-edge colonography. Radiology 2017; 283: 723–28. doi: 10.1148/radiol.2016160890 [DOI] [PubMed] [Google Scholar]
- 87. Symons R, Cork TE, Lakshmanan MN, Evers R, Davies-Venn C, Rice KA, et al. Dual-Contrast agent photon-counting computed tomography of the heart: initial experience. Int J Cardiovasc Imaging 2017; 33: 1253–61. doi: 10.1007/s10554-017-1104-4 [DOI] [PubMed] [Google Scholar]
- 88. Do TD, Sawall S, Heinze S, Reiner T, Ziener CH, Stiller W, et al. A semi-automated quantitative comparison of metal artifact reduction in photon-counting computed tomography by energy-selective thresholding. Sci Rep 2020; 10: 21099: 21099. doi: 10.1038/s41598-020-77904-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhou W, Bartlett DJ, Diehn FE, Glazebrook KN, Kotsenas AL, Carter RE, et al. Reduction of metal artifacts and improvement in dose efficiency using photon-counting detector computed tomography and tin filtration. Invest Radiol 2019; 54: 204–11. doi: 10.1097/RLI.0000000000000535 [DOI] [PMC free article] [PubMed] [Google Scholar]










