Abstract
Objective
To evaluate the relationship between Parkinson’s disease with dementia and cortical proteinopathies in a large population of pathologically confirmed Parkinson’s patients.
Methods
We reviewed clinical data from all patients with autopsy data seen in the Movement Disorders Center at Washington University, St. Louis between 1996 and 2019. All patients with parkinsonism and autopsy-confirmed Lewy body pathology were included. We used logistic regression and MANCOVA to investigate the relationship between neuropathology and dementia.
Results
A total of 165 Parkinson’s patients met inclusion criteria. Among these, 128 had clinical dementia. Those with dementia had greater mean ages of motor onset and death but equivalent mean disease duration. The delay between motor symptom onset and dementia was one year or less in 14 individuals, meeting research diagnostic criteria for possible or probable dementia with Lewy bodies (DLB). Braak Lewy body stage was associated with diagnosis of dementia, whereas severities of Alzheimer disease neuropathologic change (ADNC) and small vessel pathology did not. Pathology of individuals diagnosed with DLB did not differ significantly from that of other Parkinson’s patients with dementia. 6% of individuals with Parkinson’s and dementia did not have neocortical Lewy bodies; 68% with Parkinson's but without dementia did have neocortical Lewy bodies.
Interpretation
Neocortical Lewy bodies almost always accompany dementia in Parkinson's; however, they also appear in most Parkinson’s patients without dementia. In some cases, dementia may occur in Parkinson’s patients without neocortical Lewy bodies, ADNC, or small vessel disease. Thus, other factors not directly related to these classic neuropathologic features may contribute to Parkinson's dementia.
Keywords: Parkinson disease, dementia, synucleinopathy
INTRODUCTION
Parkinson’s disease (PD) is a progressive disorder characterized primarily by the clinical features of bradykinesia with rest tremor, rigidity, or both. The underlying disease mechanism is based on neuronal loss affecting the dopaminergic nigrostriatal pathway. Lewy bodies (LB) and Lewy neurites, eosinophilic intraneuronal cytoplasmic inclusions consisting primarily of aggregated α-synuclein, typically occur in conjunction with neuronal loss in catecholaminergic brainstem nuclei including the substantia nigra and locus coeruleus. Other subcortical populations including serotonergic neurons in the raphe nuclei and cholinergic neurons in the nucleus basalis of Meynert, are also affected in early stages.1,2 Limbic and neocortical Lewy bodies and neurites often become evident with disease progression.
Although intellectual function remains relatively spared in most people with early PD, cognitive impairment and dementia often develop in advanced disease. The spectrum of cognitive impairment ranges from subjective cognitive decline and mild cognitive impairment (PD-MCI) to frank dementia (PDD). 3,4 The prevalence of PDD increases with age, with 80% or more of PD patients developing dementia within 20 years after diagnosis.5 Dementia with Lewy bodies (DLB) is a disorder closely related to PDD and with similar neuropathology. DLB is characterized clinically by progressive disabling cognitive impairment leading to dementia, occurring before or concurrently with motor parkinsonism.6 In research studies in which distinction needs to be made between DLB and PDD, a 1-year rule between the onset of dementia and parkinsonism is recommended.
The neuropathological basis for dementia in PDD and DLB is controversial. A recent systematic review indicates that limbic and/or neocortical α-synuclein pathology occurs in virtually all cases of PDD, although coexisting Alzheimer pathology (inclusive of β -amyloid and characteristic neurofibrillary tangle [NFT] pathology) may independently contribute to dementia in PD in some patients.7 Cortical NFT pathology may relate more strongly to cognitive status in PD than β-amyloid.7 Multiple studies investigating the role of cerebrovascular pathology including cerebral amyloid angiopathy (CAA) have not shown an association with dementia in PD.7
The objective of our study was to evaluate the relationship between PD and dementia with cortical proteinopathies in a large population of pathologically confirmed PD patients treated in the Movement Disorders Center at Washington University School of Medicine in St. Louis. We hypothesized that dementia would be associated more strongly with neocortical LBs8 than Alzheimer disease neuropathologic change (ADNC) or cerebral small vessel pathology. We also sought to determine whether patients with PDD vs DLB showed significant differences in cortical LBs, ADNC, or small vessel pathology.
METHODS
We reviewed clinical data maintained in an electronic medical record from all patients treated in the Movement Disorders Center at Washington University in St. Louis (WUSTL) between 1996 and 2019 (n = 19,947). All patients at this center are routinely invited to participate in the brain donation program, regardless of clinical diagnosis. All those with a clinical diagnosis of parkinsonism and autopsy-confirmed Lewy body pathology underwent further analysis (n = 211). Each patient had been examined by a subspecialty-trained movement disorders neurologist who recorded the clinical status, including both the movement disorder and cognitive function, in the electronic medical record at each visit. For this study, a clinical diagnosis of dementia was based upon the historical narrative indicating progressive cognitive impairment sufficient to interfere with activities of daily living. When available, scores for the Mini-Mental State Examination (MMSE)9, Montreal Cognitive Assessment (MoCA)10, or both provided supportive evidence for presence or absence of dementia. Age at onset was defined as the patient’s age at the first motor symptom of PD. Disease duration was defined as the interval between motor symptom onset and death. The WUSTL Human Research Protection Office approved this study. All participants or their next of kin provided written informed consent.
Neuropathologic examinations were performed at WUSTL as described previously.11 However, immunohistochemistry (IHC) for α-synuclein using the LB509 monoclonal antibody was introduced in 1998 and was supplanted by IHC using phosphorylation-specific anti-α-synuclein antibodies in 2009. IHC for phosphorylated tau (using PHF1 antibody, courtesy of Dr. P. Davies, Feinstein Institute for Medical Research, Manhasset, NY) and for β-amyloid (using 10D5 [Eli Lilly] or 4G8 antibodies) was performed routinely. Individuals with neuropathologic examinations completed before the routine use of these IHC techniques were excluded. Neuropathologic examinations after mid-2008 routinely also included IHC for phosphorylated TAR DNA-binding protein of 43 kDa (pTDP-43, Cosmo Bio USA); in this cohort, 29 cases without dementia and 108 cases with dementia had been evaluated with pTDP-43 IHC.
Neuropathologic diagnoses were based on standard criteria.2,12, 13 The diagnosis of PD was based on the presence of LBs within and the loss of pigmented neurons from the substantia nigra. Limbic and neocortical LBs, although not required for the diagnosis of PD, were present in many of these patients. The distribution of Lewy body pathology was staged from 0 – 6 as described previously.12 We defined neocortical involvement as Braak LB stage 5 or 6. β-amyloid plaque distribution, NFT stage, and neuritic plaque score were rated according to National Institute on Aging–Alzheimer’s Association (NIA-AA) guidelines.13 These scores were specified in the neuropathological record for cases autopsied after 2012. In autopsies from 2012 and earlier, the scores were determined from the narrative record describing these neuropathological features. The degree of AD neuropathologic change (ADNC) for each patient, defined as “none, low, intermediate or high” was determined according to these guidelines. Small vessel diseases CAA and arteriolosclerosis were each graded as “none, mild, moderate, or severe.”
Statistical analyses were performed using a combination of R (version 4.2)14 and IBM SPSS 28 (IBM Corp., Armonk, N.Y., USA). Data were missing for approximately 20% of education scores; multiple imputation was employed to fill missing data (R package: mice).15 Logistic regression models were fit using presence of dementia as the dependent variable to test the relationship between dementia and Braak LB stage, ADNC severity, and severity of small vessel pathology with and without covariates of sex, race, level of education, age at disease onset, and age at death. In the subgroup of participants with a dementia diagnosis, logistic regression also was fit with PDD vs DLB group as a dependent variable, with significant relationships between pathology and group assessed with and without the above covariates. We also ran similar models replacing ADNC severity with β-amyloid plaque distribution, NFT stage, and neuritic plaque score. Comparisons of means between pathological and diagnostic groups were performed using multivariate analysis of covariance (MANCOVA). For both logistic regression and MANCOVA analyses, all appropriate assumptions of linearity and normality were tested for, and models were fit with and without outliers to assess the impact on significant results. For MANCOVA, where assumptions of homogeneity of variance and covariance or multivariate normality were not met, Pillai’s trace (V) was substituted for Wilk’s lambda (λ) as the test statistic. Unadjusted comparisons of individual continuous and categorical measures were performed using Student’s T-tests and χ2 tests, respectively. Where necessary, multiple comparisons were controlled using the Bonferroni method.
RESULTS
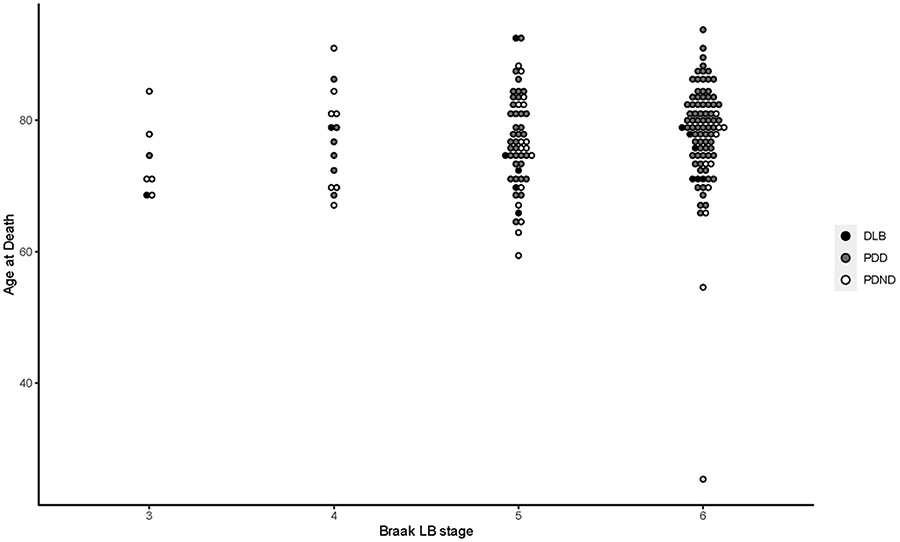
Records of a total of 211 patients, all with neuropathologically confirmed Lewy body pathology and a clinical diagnosis of parkinsonism, were reviewed. From this total, 165 patients had sufficiently detailed clinical and neuropathological records including IHC to be analyzed further. In this group, 128 patients had a clinical diagnosis of dementia. Mean age at motor onset and age at death were both slightly greater among those with dementia (combined PDD and DLB) vs patients with PD but no dementia (PDND), although these differences were not significant after correction for multiple comparisons (p < 0.05), while disease duration did not differ significantly between these two groups (Table 1). Mean ages at motor onset, dementia, and death, total disease duration, and interval between motor onset and dementia did not differ significantly between Braak LB stages when adjusted for sex, race and education using one-way MANCOVA (F[12, 310] = 0.691, λ = 0.932, p = 0.760). The categorization of patients by dementia status and Braak stage with corresponding temporal features of their disease courses is summarized in Table 1. Figure 1 displays the relationship between Braak stage and age of death for DLB, PDD and PDND patients.
Table 1.
Distribution of synucleinopathy by Braak Lewy body stage and basic demographics
| Lewy body stage* (no. of patients) |
Age at onset of motor symptoms (yr) mean±SD |
Age at onset of dementia (yr) mean±SD |
Duration of motor symptoms (yr) mean±SD |
Age at death (yr) mean±SD |
|---|---|---|---|---|
| PDND (37) | 56.6 ± 14.6† | - | 17.0 ± 9.7 | 73.4 ± 11.5† |
| Braak 3 (5) | 62.5 ± 11.1 | - | 12.1 ± 5.8 | 74.6 ± 6.7 |
| Braak 4 (7) | 63.9 ± 13.9 | - | 13.7 ± 9.8 | 77.6 ± 9.0 |
| Braak 5 (15) | 56.1 ± 12.9 | - | 19.0 ± 10.6 | 75.1 ± 8.8 |
| Braak 6 (10) | 49.4 ± 17.1 | - | 18.7 ± 9.7 | 68.0 ± 16.9 |
| PDD (114) | 62.4 ± 9.6 | 74.1 ± 6.8‡ | 16.4 ± 6.5‡ | 78.8 ± 6.0‡ |
| Braak 3 (1) | 56 | 73 | 19 | 75 |
| Braak 4 (6) | 60.1 ± 1.6 | 72.5 ± 7.0 | 16.1 ± 7.3 | 76.2 ± 6.1 |
| Braak 5 (34) | 62.0 ± 11.0 | 73.1 ± 7.7 | 16.1 ± 7.3 | 77.9 ± 6.1 |
| Braak 6 (73) | 63.0 ± 9.4 | 74.7 ± 6.4 | 16.5 ± 6.4 | 79.5 ± 5.9 |
| DLB (14) | 64.1 ± 8.5 | 64.9 ± 8.3 | 10.1 ± 4.1 | 74.2 ± 6.5 |
| Braak 3 (1) | 64 | 64 | 4 | 68 |
| Braak 4 (1) | 68 | 68 | 11 | 79 |
| Braak 5 (6) | 61.9 ± 11.8 | 63.8 ± 11.7 | 12.4 ± 3.9 | 74.3 ± 9.3 |
| Braak 6 (6) | 65.6 ± 6.0 | 65.7 ± 6.3 | 8.6 ± 3.6 | 74.2 ± 3.7 |
Lewy body stage according to Braak H, Del Tredici K, Rub U et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24:197–211
indicates significant difference (prior to correction for multiple comparisons) between PDND and combined dementia group, p < 0.05
indicates significant difference between PDD and DLB, p < 0.05
Figure 1.
Raw data for age at death grouped by Braak Lewy body stage. DLB, PDD, and PDND diagnoses are indicated for each individual.
We performed logistic regression to ascertain the association of pathology with diagnosis of dementia. A model was constructed to predict diagnosis of dementia using Braak LB stage, ADNC stage, and cerebral small vessel pathology (arteriolosclerosis and CAA) as independent variables, with additional covariates of sex, race, education, age at motor onset, and age at death. This model was statistically significant, (df = 24, χ2 = 53.38, p < 0.001) and correctly classified 84.8% of cases. Braak LB stage significantly related to dementia (df = 3, Wald χ2 = 15.09, p = 0.002), with Braak LB stage 6 independently predicting dementia diagnosis (p = 0.004, OR 37.6, 95% CI 3.9-362.2), although Braak LB stage 5 did not (p = 0.078, OR 7.46, 95% CI 0.80-69.48). ADNC stage did not significantly relate to dementia diagnosis (df = 3, Wald χ2 = 0.930, p = 0.818); nor did arteriolosclerosis (df = 3, Wald χ2 = 2.73, p = 0.436) or CAA (df = 4, Wald χ2 = 3.85, p = 0.427) (Table 2). Similar results were observed when each AD pathology type was considered independently of the others, with and without accounting for clinical covariates. Only 9 patients in this sample had ADNC score of “high.” All of these had a diagnosis of dementia and neocortical Lewy bodies (8 Braak stage 6, 1 Braak stage 5). When β-amyloid plaque score, NFT score, and neuritic plaque score were substituted for the composite ADNC severity score, none significantly related to dementia diagnosis (β-amyloid: df = 3, Wald χ2 = 2.257, p = 0.521; NFT: df = 3, Wald χ2 = 0.852, p = 0.837; neuritic plaque: df = 3, Wald χ2 = 1.711, p = 0.634) (Table 2). NIA-AA scores are listed in Tables 3 and 4. When Braak LB stage was dichotomized into stages 1-4 (no neocortical LB) versus stages 5-6 (neocortical LB), and ADNC was dichotomized into “none/low” versus “intermediate/high” within a similar logistic regression model, neocortical LB significantly predicted dementia (p = 0.001, OR = 9.0, 95% CI = 2.4-34.2), while ADNC did not (p = 0.232, OR = 2.3, 95% CI = 0.6 – 9.1).
Table 2.
A. Logistic regression model predicting diagnosis of dementia. For each pathological predictor, omnibus significance (via Wald χ2) is followed by odds ratios and significance for each predictor level. B. Supplemental model analyzing NIA-AA pathological categories comprising ADNC score as predictors of dementia.
| A. | ||||
|---|---|---|---|---|
| Pathology | Odds Ratio (95% CI) | Wald χ2 | Significance | |
| Lewy Body (Braak) | 0.002* | |||
| Braak 4a | 1.68 (0.16 – 18.08) | 0.670 | ||
| Braak 5a | 7.46 (0.80 – 69.49) | 0.078 | ||
| Braak 6a | 35.18 (3.21 – 385.64) | 0.004* | ||
| ADNC | 0.818 | |||
| Lowb | 1.33 (0.46 – 3.89) | 0.601 | ||
| Intermediateb | 2.34 (0.42 – 13.11) | 0.335 | ||
| Highb | † | 0.999 | ||
| CAA | 0.427 | |||
| Minimalb | 0.35 (0.02 – 6.48) | 0.478 | ||
| Mildb | 0.51 (0.15 – 1.72) | 0.280 | ||
| Moderateb | 0.11 (0.01 – 1.10) | 0.061 | ||
| Severeb | 0.47 (0.03 – 7.32) | 0.590 | ||
| Arteriolosclerosis | 0.436 | |||
| Mildb | 2.67 (0.14 – 52.01) | 0.518 | ||
| Moderateb | 1.83 (0.09 – 36.77) | 0.694 | ||
| Severeb | 13.11 (0.30 – 575.3) | 0.182 | ||
| B. | ||||
| Pathology | Odds Ratio (95% CI) | Wald χ2 | Significance | |
| β-amyloid | 2.257 | 0.521 | ||
| Score 1c | 2.64 (0.731 – 9.51) | 2.196 | 0.138 | |
| Score 2c | 1.37 (0.33 – 5.67) | 0.192 | 0.662 | |
| Score 3c | 1.53 (0.13 – 17.44) | 0.116 | 0.733 | |
| NFT | 0.852 | 0.837 | ||
| Score 1c | 0.39 (0.045 – 3.34) | 0.746 | 0.388 | |
| Score 2c | 0.56 (0.043 – 7.36) | 0.194 | 0.659 | |
| Score 3c | † | 3.0 x 10−6 | 0.999 | |
| Neuritic plaques | 1.711 | 0.634 | ||
| Score 1c | 6.80 (0.39 – 120.1) | 1.711 | 0.191 | |
| Score 2c | † | 4.0 x 10−6 | 0.998 | |
| Score 3c | † | 1.65 x 10−10 | 0.999 | |
ADNC = Alzheimer disease neuropathologic change, CAA = Cerebral amyloid angiopathy, NFT = neurofibrillary tangles
denotes significance at p = 0.05.
Odds ratio with 95% CI could not be calculated.
Contrast with Braak stage 3.
Contrast with “none”.
Contrast with score 0.
Table 3.
Alzheimer Disease Neuropathologic Scores
| AD Neuropathologic Change “ABC” Scores* | |||
|---|---|---|---|
| AD Neuropathologic Change Severity Scale |
“A” β-amyloid |
“B” Neurofibrillary tangles |
“C” Neuritic plaques |
| PDND (37) | |||
| 0 | 13 | 4 | 36 |
| 1 | 14 | 28 | 1 |
| 2 | 7 | 5 | 0 |
| 3 | 3 | 0 | 0 |
| PDD (114) | |||
| 0 | 24 | 3 | 77 |
| 1 | 26 | 72 | 24 |
| 2 | 31 | 28 | 10 |
| 3 | 33 | 11 | 3 |
| DLB (14) | |||
| 0 | 2 | 1 | 9 |
| 1 | 5 | 8 | 2 |
| 2 | 3 | 4 | 3 |
| 3 | 4 | 1 | 0 |
Neuropathology scores according to Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012; 123:1–11
Table 4.
Lewy body stage and level of Alzheimer disease neuropathologic change
| Lewy body stage (no. of patients) |
Level of Alzheimer disease neuropathologic change* | |||
|---|---|---|---|---|
| Not | Low | Intermediate | High | |
| PDND (37) | ||||
| Braak 3 (5) | 2 | 3 | 0 | 0 |
| Braak 4 (7) | 1 | 6 | 0 | 0 |
| Braak 5 (15) | 5 | 7 | 3 | 0 |
| Braak 6 (10) | 5 | 4 | 1 | 0 |
| PDD (114) | ||||
| Braak 3 (1) | 1 | 0 | 0 | 0 |
| Braak 4 (6) | 4 | 2 | 0 | 0 |
| Braak 5 (34) | 10 | 18 | 5 | 1 |
| Braak 6 (73) | 10 | 39 | 17 | 7 |
| DLB (14) | ||||
| Braak 3 (1) | 0 | 1 | 0 | 0 |
| Braak 4 (1) | 0 | 1 | 0 | 0 |
| Braak 5 (6) | 2 | 4 | 0 | 0 |
| Braak 6 (6) | 1 | 1 | 3 | 1 |
Level of AD neuropathological change according to Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012; 123:1-11
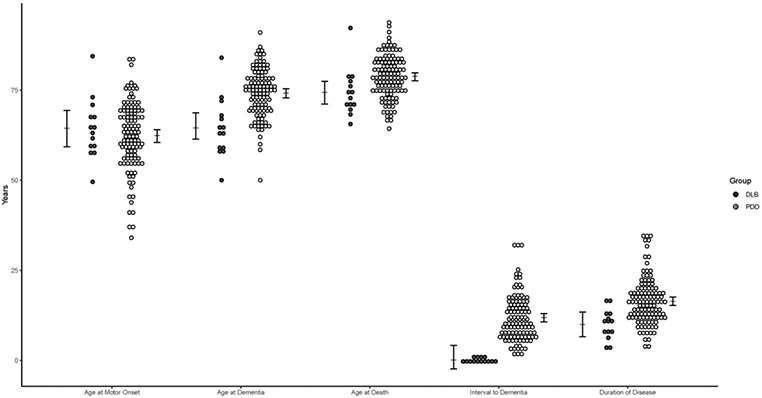
The interval between motor onset and dementia onset ranged from −0.6 to 32 years (mean 10.6 ± 7.0 years). This interval was one year or less in 14 individuals; thus, they were assigned to the DLB group per current research diagnostic criteria.6 We conducted one-way MANCOVA to evaluate mean ages of motor onset, dementia and death, time to dementia, and total disease duration by diagnosis of PDD versus DLB while adjusting for sex, race, and level of education. There was an overall effect of diagnostic group on temporal variables (F[4,119] = 21.621, V = 0.421, p < 0.001). PDD and DLB groups significantly differed by age at death (F[1,122] = 6.75, p = 0.011, difference DLB −4.4 years), age at dementia onset (F[1,122] = 21.54, p < 0.001, difference DLB −9.0 years), time to dementia (F[1,122] = 38.52, p < 0.001, difference DLB −10.94 years) and total disease duration (F[1,122] = 12.34, p < 0.001, difference DLB −6.4 years), as displayed in Figure 2.
Figure 2.
Raw data for time course of disease grouped by PDD vs DLB diagnosis with estimated marginal means for time course of disease compared between PDD and DLB groups, derived from MANCOVA analysis adjusting for sex, race, and level of education. Error bars indicate 95% confidence intervals.
A logistic regression model was employed to evaluate the effect of pathology (Braak stage, ADNC, cerebral small vessel pathologies) on diagnosis of PDD vs DLB, covarying for sex, race, and education level. When only pathology was considered, the model was not statistically significant (df = 13, χ2 = 12.136, p = 0.517). While accounting for demographic covariates, none of the pathological variables significantly related to diagnosis of PDD vs DLB, including Braak LB stage (df = 3, Wald χ2 = 0.2.902, p = 0.407), ADNC stage (df = 3, Wald χ2 = 2.230, p = 0.526), CAA severity (df = 4, Wald χ2 = 1.996, p = 0.737), and arteriolosclerosis (df = 3, Wald χ2 = 2.269, p = 0.518). When substituted for composite ADNC score, neither β-amyloid plaque score, NFT score, nor neuritic plaque score significantly related to PDD versus DLB diagnosis (β-amyloid: df = 3, Wald χ2 = 1.571, p = 0.666; NFT: df = 3, Wald χ2 = 1.469, p = 0.689; neuritic plaque: df = 3, Wald χ2 = 3.515, p = 0.319).
Other neuropathological findings included TDP-43 immunoreactive neuronal cytoplasmic inclusions (consistent with limbic-predominant age-related TDP-43 encephalopathy – neuropathologic change [LATE-NC])16 in 1/29 (3.4%) of patients without dementia and 21/108 (19.4%) patients with dementia (χ2 = 4.3396, p = 0.037). TDP-43 positivity did not differ between PDD (19/97) and DLB (2/11) groups (χ2 = 0.0125, p = 0.911). TDP-43 positivity was recorded as sparse or mild in all but 3 participants, 2 of whom were designated stage II, and 1 as stage IV (on a I - VI scale).17 Hippocampal sclerosis was present in 3 patients, all of whom had dementia and were very mildly TDP-43 positive. Argyrophilic grain disease (AGD) was present in 3/37 (8.1%) patients without dementia (stage I in 2, stage II in 1) and 9/128 (7.0%) patients with dementia (stage I in 6, stage II in 3).18 AGD was not reported in any of the DLB individuals. In individuals who died in 2015 or later, aging-related tau astrogliopathy (ARTAG)19 was recorded in 2/15 (13.3%) PDND patients and 21/48 (43.8%) patients with dementia, one with DLB (χ2 = 4.562, p = 0. 033). These observations and their association with Lewy body stage are summarized in Table 5.
Table 5.
TDP-43 positivity, ARTAG and AGD in Parkinson patients categorized by dementia status and stratified by Braak Lewy body stage*
| Lewy body stage | TDP-43 positivity No. of cases (total assessed) |
ARTAG No. of cases (total assessed) |
AGD No. of cases (total assessed) |
|---|---|---|---|
| PDND | |||
| Braak 3 | 0 (4) | 0 (1) | 0 (5) |
| Braak 4 | 0 (4) | 0 (3) | 0 (7) |
| Braak 5 | 0 (13) | 1 (7) | 1 (15) |
| Braak 6 | 1 (8) | 1 (4) | 2 (10) |
| PDD | |||
| Braak 3 | 0 (1) | 0 (1) | 0 (1) |
| Braak 4 | 0 (6) | 3 (5) | 1 (6) |
| Braak 5 | 2 (32) | 10 (19) | 1 (34) |
| Braak 6 | 17 (58) | 7 (18) | 7 (73) |
| DLB | |||
| Braak 3 | 0 (1) | 0 (1) | 0 (1) |
| Braak 4 | 0 (1) | 0 (1) | 0 (1) |
| Braak 5 | 1 (4) | 0 (1) | 0 (6) |
| Braak 6 | 1 (5) | 1 (2) | 0 (6) |
Lewy body stage according to Braak H, Del Tredici K, Rub U et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24:197–211
TDP-43: TAR DNA-binding protein immunoreactive neuronal cytoplasmic inclusions
ARTAG: age-related tau astrogliopathy
AGD: argyrophilic grain disease
PDND: Parkinson disease without dementia
PDD: Parkinson disease with dementia
DLB: dementia with Lewy bodies
DISCUSSION
This study provides evidence that the development of dementia in Lewy body diseases closely relates to the presence of neocortical LBs. This agrees with previous work reporting a correspondence between cognitive status and Braak LB stage in PD, although that study did not report specifically on dementia.20 Also consistent is a systematic review of 44 autopsy studies involving 2002 cases with PD, 57.2% of whom developed dementia, which reported that limbic and neocortical α-synuclein pathology had the strongest association with dementia.7 Two important caveats to this, however, are that neocortical synucleinopathy frequently occurred in patients without clinical dementia (25/37 patients in our PDND group) and that dementia may occur in the absence of neocortical synucleinopathy (7/114 patients in our PDD group). Two of these 7 patients had severe small vessel disease raising the possibility of a vascular cause for their dementia. Therefore, neocortical LBs are neither sufficient nor necessary for dementia in PD, although most patients with PD and dementia did exhibit neocortical LBs.
Our observation that Braak LB stage 4 disease (corresponding with basal prosencephalic and/or mesocortical LBs) more commonly occurs in the PDND group than in the PDD/DLB groups, supports the concept that PD pathology progresses in a caudal-to-rostral fashion from the brainstem through the limbic system to the neocortex. The degree of neocortical spread appears to influence dementia, with Braak LB stage significantly relating to dementia diagnosis. In particular, Braak LB stage 6 (involving further spread into sensorimotor and temporal cortical regions12) independently predicts dementia diagnosis in our cohort, distinguishing it from stages 4 and 5, although Braak stage 5 may have been predictive of dementia in a larger sample. PDD patients with neocortical involvement typically have clinically more advanced disease than those PDND patients with pathology limited to stage 4. This observation also indicates that limbic Lewy body pathology alone with sparing of the neocortex is an uncommon feature in PDD. This contrasts with guidelines for the assessment of neuropathologic findings in DLB that suggest patients with limbic Lewy-related pathology and no significant ADNC have a high likelihood of having a typical DLB clinical syndrome.6
Cortical α-synuclein deposition in the form of Lewy bodies and Lewy neurites is not the only proteinopathy evident in these patients. β-amyloid accumulation was evident in the majority of patients, regardless of the presence of dementia (Table 3). NFT pathology was also commonly seen in all groups (Table 3). This agrees with observations of Smith et al.,7 although severity of tau pathology did not significantly relate with PDD vs DLB diagnosis or with presence of dementia. TDP-43 positivity was evident in a minority of patients and more common in the presence of dementia, generally consistent with the report of Nakashima-Yasuda et al.21 Of note, however, is that very few individuals in our study had more than mild TDP-43 positivity. In any aging population, coincident ADNC is an important consideration. In our cohort, a total of 34/128 patients had “intermediate” or “high” AD pathology meeting ADNC criteria sufficient to account for dementia, all of whom had neocortical LBs (Braak LB stage 5 or 6), a higher rate than we reported previously.8 The presence of these criteria in 4 of our patients without dementia demonstrates their non-specificity. Given the co-incidence of pathology in these patients, it is not possible to determine which protein(s) accounted for the cognitive changes in individual cases, although LB disease significantly related to dementia in our models whereas AD pathology did not. Quantitative measures indicate that neocortical α-synuclein correlates with neocortical β-amyloid, further confounding interpretation of how these individual proteinopathies contribute to cognitive impairment (in the absence of significant ADNC changes).22 Extant literature contains conflicting reports regarding the importance of coincident ADNC in PDD, with some authors suggesting that it is uncommon23,24 and others suggesting a close relationship between the two pathologies. Jellinger et al. reported 40 cases with a high probability of AD from 66 PDD patients using NIA-Reagan diagnostic criteria.25 Our data falls midway between these reports.
DLB is poorly represented in our patient population. DLB is characterized clinically by progressive disabling cognitive impairment leading to dementia occurring before or concurrently with motor parkinsonism.6 These individuals are more likely to seek evaluation at a dementia center rather than a movement disorders center. Thus, our brain donation cohort reflects the bias of those patients referred to a movement disorder center. DLB does not have a disease-specific pathological marker.26 Substantial pathological overlap exists between DLB and PDD; in fact, these conditions are pathologically indistinguishable. They both likely represent the same synucleinopathy, with clinicopathologic diagnosis assigned according to the timing of onset of the dementia with respect to onset of motor parkinsonism.27 Consistent with this concept, recent criteria have de-emphasized the “one-year rule” to distinguish PDD from DLB.6,28 These comments notwithstanding, in our patient group the mean age of motor onset did not differ significantly between DLB and PDD groups. After controlling for covariates, age of dementia onset in DLB was approximately 9 years earlier than in PDD. The DLB patients died about 4 years earlier than PDD patients, with a shorter total disease duration about 6 years less. The degree of LB disease, β-amyloid deposition, and tau pathology was essentially the same in the 2 groups. Some authors have suggested that striatal β-amyloid deposition distinguishes PDD from DLB.7,29 This corresponds to a NIA-AA β-amyloid score ≥ 2.13,30 In our patients, 62% of DLB patients met this condition vs 55% in the PDD group, arguing against the value of striatal β-amyloid deposition in differential diagnosis, consistent with the observations of Kalaitzakis et al.31 We did not apply the DLB neuropathologic criteria of McKeith et al. because these criteria assume a clinical diagnosis of DLB.6 Our patients had dementia and parkinsonism but did not necessarily have other core clinical features required for the diagnosis.
Of particular interest is the presence of discordant cases, i.e., those in which a cortical proteinopathy was present in the absence of clinical dementia and those in which dementia was present in the absence of cortical proteinopathy. Not surprisingly, because α-synuclein is a major component of LBs, by definition some degree of synucleinopathy had to be present, at least in the brainstem, in all patients. Neocortical LBs were evident in all patient groups although a small number of individuals in both the PDD and DLB groups had only Braak stage 3 or 4 disease, indicating absent or only very rare neocortical LBs. This suggests that other features in addition to neocortical synucleinopathy contribute to development of dementia in the setting of PD. In general, the patients with dementia who lacked neocortical synucleinopathy also had a minor degree of cortical tauopathy and/or β-amyloid accumulation, implicating factors other than cortical LB disease, β-amyloidopathy, or NFT formation. Similarly, the presence of cortical tauopathy or β-amyloidopathy in the absence of synuclein accumulation does not appear to relate to dementia (in the absence of frank AD).
Other neuropathological findings in our patients included ARTAG, a recently described neuropathological entity characterized by astroglial tau pathology in the aged brain.19 The clinical significance is uncertain. The significance of our observation of a higher incidence of ARTAG in patients with dementia vs those without (in the subset of patients who died in 2015 or later) is unclear. Argyrophilic grain disease (AGD) is an age-associated tauopathy staged from I - III.18 More advanced stages of AGD may contribute to cognitive decline in the elderly. AGD was uncommon in our study participants. None had stage III AGD while 4 (plus 1 individual without dementia) had stage II disease. The potential roles of ARTAG and AGD will be an important consideration in future studies of dementia in PD.
Numerous other factors potentially contribute to dementia that do not directly relate to cortical proteinopathy but were not investigated in the present study. Dysfunction of resting state functional connectivity networks could be one such factor. We previously reported longitudinal reductions in functional connectivity networks in PD participants without dementia, predicted in part by baseline CSF α-synuclein levels but not by β-amyloid levels.32,33 Disruption of dorsal attention and frontoparietal resting state networks relate to cognitive dysfunction in PD. 34 A meta-analysis of 72 publications supported the concept that network dysfunction is tightly linked to motor, cognitive and psychiatric symptoms.35
While the scoring system for cortical proteinopathy in our study provides limited regional information, metabolic imaging studies suggested that hypometabolism in visual association and posterior cingulate cortices occurs early in the course of declining cognition in PD with more widespread cortical changes occurring with disease progression.36 Studies correlating regional cortical proteinopathy and metabolism have not been published.
All patients with PD have a significant loss of dopaminergic neurons in nigrostriatal projections. Those with PDD also have a substantial loss of neurons in the lateral dopaminergic system to frontal, parietal and temporal cortical regions.37 Other neurotransmitter systems are also potentially involved. Basal forebrain neurons, often involved in Braak LB stage 4 (and greater), are the major source of cholinergic innervation to the neocortex, hippocampus, and amygdala. We observed regional deficits in serotonergic projections to cortical and limbic regions and widespread deficits in noradrenergic projections in our previous postmortem study in people with PD and dementia.38 Decreased grey matter volume and increased mean diffusivity in the nucleus basalis of Meynert has been reported as predictive for developing cognitive impairment in cognitively intact patients with PD,39 consistent with a previous report of significant reductions of cortical choline acetyltransferase activity in PDD.40 The relationship, if any, between neurotransmitter abnormalities, cortical proteinopathies, and cognition has not yet been addressed.
Neuroinflammation is another potential contributing factor. A previous report identified an inverse association between PD and inflammatory bowel disease,41 a condition treated with anti-inflammatory or immunosuppressant agents as well as, in a population-based case-control study, a lower risk of PD in individuals receiving immunosuppressants.42 Inflammation could be secondary to ongoing neuronal cell death in PD, but it has also been suggested that misfolded α-synuclein itself might play a direct role.43 Significant cortical microglial activation has been reported with [C-11]PK11195/PET studies in both PD and PDD.44 That study also showed an inverse correlation between microglial activation and MMSE scores, suggesting a role for neuroinflammation in declining cognition. Zhang et al reported that extracellular aggregated α-synuclein activates microglia and that microglial activation enhanced dopaminergic neurodegeneration.45 Lastly, other conditions such as LATE-NC, argyrophilic grain disease, hippocampal sclerosis, and cerebrovascular disease may contribute in individual patients. Further study is necessary to determine the relative importance of these other potential contributing factors and their relationship to cortical proteinopathies.
The strengths of this study are the longitudinal follow up by movement disorders specialists of all cases prior to autopsy, the relatively large number of autopsy cases and the careful postmortem examinations including immunohistochemistry in all cases. We also provide strong evidence that neocortical synucleinopathy or other proteinopathy including beta-amyloid or tau is neither sufficient nor necessary for development of dementia in people with PD. This strongly suggests that other factors play a role in dementia in these cases, and these other factors also may be critical for those with neocortical synucleinopathy.
A limitation of this and previous clinicopathological correlative studies is their dependence on direct observations of the deposition of aggregated protein species with IHC. Quantitation of the neocortical proteinopathy is approximate at best, typically based on semiquantitative scoring systems with limited regional data.13 We previously reported methods to address these limitations by quantifying the burden of insoluble protein accumulation in frozen tissue samples from individual brain regions but these methods have not yet been applied to most of the cases in this report.22 As a retrospective chart review, this study depends on the accuracy of clinical information available within the medical record. Not all patients had standard MMSE or MoCA assessments but subspecialty-trained movement disorders neurologists were responsible for all assessments, and the narrative record provided a clear indication of the presence of declining cognition. In future studies, implementation of comprehensive cognitive testing would allow stronger assessment of the relationship between quality/severity of dementia and pathology. Other factors to consider include the potential contribution of "cognitive reserve" in addition to potential other detrimental or protective factors in lifestyle, environment, etc. The cumulative effects of both protective and harmful factors throughout a person's lifetime are important in consideration of dementia risk. We have not included PD-MCI because the narrative record did not always provide sufficient information to diagnose this clinical entity. Selection bias of autopsy cases is unlikely to be a major issue. All patients in our center are routinely invited to participate in our brain donation program early in the course of their clinic involvement, regardless of clinical diagnosis. The greatest limitation is the relatively few number of DLB cases compared to PDD, likely reflecting the referral bias through our movement disorders center.
These observations provide useful information regarding the potential causes of dementia in LB diseases. Although neocortical LB disease appears to play a major role, it is neither necessary nor sufficient for development of dementia in people with PD. Other proteinopathies (tau and/or β-amyloid) may play a role in some patients but there may be other factors that have not yet been fully elucidated at this time.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (NINDS, NIA) (NS075321, NS097799, NS097437, U10NS077384, NINDS K23NS125107), the American Parkinson Disease Association (APDA), the Greater St. Louis Chapter of the APDA, the Jo Oertli Fund, the Barnes Jewish Hospital Foundation (Elliot Stein Family Fund and the Parkinson Disease Research Fund), N Grant Williams Fund, Pohlman Fund, and the Paula and Rodger Riney Fund. Dr. Pietro Mazzoni’s expertise is appreciated. We thank Erin Franklin, Susan Donovan and Michael Baxter, Aime Burns-Harring, other personnel of the Translational Human Neurodegenerative Disease Research (THuNDR) / Betty Martz Laboratory, and the Faculty of the Neuropathology Division at Washington University for their contributions to the Movement Disorder Center neuropathology resource We thank our patients and families without whom this research would not be possible.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
None
REFERENCES
- 1.NI Bohnen, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res 2011;221: 564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]
- 3.Aarsland D, Zaccai J & Brayne C A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord 2005;20:1255–1263 [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7:47. [DOI] [PubMed] [Google Scholar]
- 5.Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844 [DOI] [PubMed] [Google Scholar]
- 6.McKeith IG, Boeve BF, Dickson, et al. Diagnosis and management of dementia with Lewy bodies. Fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C, Malek N, Grosset K, et al. Neuropathology of dementia in patients with Parkinson’s disease: a systematic review of autopsy studies. J Neurol Neurosurg Psychiatry 2019;90:1234–1243 [DOI] [PubMed] [Google Scholar]
- 8.Kotzbauer PT, Cairns NJ, Campbell MC, et al. Pathologic accumulation of α-synuclein and Aβ in Parkinson disease patients with dementia. Arch Neurol 2012;69:1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 11.Franklin EE, Perrin RJ, Vincent B, et al. Alzheimer’s disease neuroimaging initiative. Brain collection, standardized neuropathologic assessment, and comorbidity in Alzheimer’s disease neuroimaging initiative participants. Alzheimers Dement 2015;11:815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 2009;8:1150–1157 [DOI] [PubMed] [Google Scholar]
- 13.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, 2021. https://www.R-project.org/ [Google Scholar]
- 15.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67 [Google Scholar]
- 16.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 2019; 142:1503–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josephs KA, Murray ME, Whitwell JL, et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol 2016; 131: 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito Y, Ruberu NN, Sawabe M, et al. Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 2004;63:911–918 [DOI] [PubMed] [Google Scholar]
- 19.Kovacs GG, Ferrer I, Grinberg LT, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 2016;131:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braak H, Rüb U, Jansen Steur ENH, et al. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 2005;64:1404–1410 [DOI] [PubMed] [Google Scholar]
- 21.Nakashima-Yasuda H, Uryu K, Robinson J et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropath 2007;114:221–229 [DOI] [PubMed] [Google Scholar]
- 22.Miller R, Dhavale D, O’Shea J, et al. Quantifying regional alpha-synuclein, amyloid β and tau accumulation in Lewy body dementia. Ann Clin Transl Neurol 2022;9:106–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aarsland D, Perry R, Brown A, et al. Neuropathology of dementia in Parkinson’s disease: a prospective, community-based study. Ann Neurol 2005;58:773–776 [DOI] [PubMed] [Google Scholar]
- 24.Apaydin H, Ahlskog JE, Parisi JE, et al. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol 2002;59:102–12 [DOI] [PubMed] [Google Scholar]
- 25.Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm 2002;109:329–339 [DOI] [PubMed] [Google Scholar]
- 26.Martin WRW, Miles M, Zhong Q et al. Is levodopa response a valid indicator of Parkinson’s disease? Movement Disorders 2021;36:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Medicine 2018;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders 2015;30:1591–1599 [DOI] [PubMed] [Google Scholar]
- 29.Halliday GM, Song YJC, Harding AJ. Striatal β-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J Neural Transm 2011;118:713–719 [DOI] [PubMed] [Google Scholar]
- 30.Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–1800 [DOI] [PubMed] [Google Scholar]
- 31.Kalaitzakis ME, Walls AJ, Pearce RKB, Gentleman SM. Striatal Aβ peptide deposition mirrors dementia and differentiates DLB and PDD from other Parkinsonian syndromes. Neurobiol Dis 2011;41:377–384 [DOI] [PubMed] [Google Scholar]
- 32.Campbell MC, Koller JM, Snyder AZ, et al. CSF proteins and resting-state functional connectivity in Parkinson disease. Neurology 2015;84:2413–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell MC, Jackson JJ, Koller JM, et al. Proteinopathy and longitudinal changes in functional connectivity networks in Parkinson disease. Neurology 2020;94:e718–e728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baggio HC, Segura B, Sala-Llonch R, et al. Cognitive impairment and resting-state network connectivity in Parkinson’s disease. Hum Brain Mapp 2015;36:199–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ting S, Wang Y, Liu Y, et al. Large-scale network dysfunction in α-synucleinopathy: A meta-analysis of resting-state functional connectivity. eBioMedicine 2022;77:103915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NI Bohnen, Koeppe RA, Minoshima S, et al. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. J Nucl Med 2011;52:848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasikumar S, Strafella AP. Imaging mild cognitive impairment and dementia in Parkinson’s disease. Front Neurol 2020;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buddhala C, Campbell MC, Perlmutter JS, Kotzbauer PT. Correlation between decreased CSF α-synuclein and Aβ1-42 in Parkinson disease. Neurobiol Aging 2015;36:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz J, Pagano G, Bonfante JAF, et al. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain 2018;141:1501–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry EK, Curtis M, Dick DJ, et al. Cholinergic correlates of cognitive impairment in Parkinson' s disease: comparisons with Alzheimer' s disease. J Neurol Neurosurg Psychiatr 1985;48:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camacho-Soto A, Gross A, Searles Nielsen S, et al. Inflammatory bowel disease and risk of Parkinson’s disease in medicare beneficiaries. Parkinsonism Relat Disord 2018;50:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Racette BA, Gross A, Vouri SM, et al. Immunosuppressants and risk of Parkinson disease. Ann Clin Transl Neurol 2018;5:870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pajares M, Rojo AI, Manda G, et al. Inflammation in Parkinson’s disease: mechanisms. Cells 2020;9:1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edison P, Ahmed I, Fan Z, et al. Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacology. 2013;38:938–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Wang T, Pei Z et al. Aggregated α-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 2005;19:533–542 [DOI] [PubMed] [Google Scholar]