Abstract
Background
Cancer of ovarian, fallopian tube and peritoneal origin, referred to collectively as ovarian cancer, is the eighth most common cancer in women and is often diagnosed at an advanced stage. Women with relapsed epithelial ovarian cancer (EOC) are less well and have a limited life expectancy, therefore maintaining quality of life with effective symptom control is an important aim of treatment. However, the unwanted effects of chemotherapy agents may be severe, and optimal treatment regimens are unclear. Pegylated liposomal doxorubicin (PLD), which contains a cytotoxic drug called doxorubicin hydrochloride, is one of several treatment modalities that may be considered for treatment of relapsed EOCs. This is an update of the original Cochrane Review which was published in Issue 7, 2013.
Objectives
To evaluate the efficacy and safety of PLD, with or without other anti‐cancer drugs, in women with relapsed high grade epithelial ovarian cancer (EOC).
Search methods
We searched CENTRAL, MEDLINE (via Ovid) and Embase (via Ovid) from 1990 to January 2022. We also searched online registers of clinical trials, abstracts of scientific meetings and reference lists of included studies.
Selection criteria
We included randomised controlled trials (RCTs) that evaluated PLD in women diagnosed with relapsed epithelial ovarian cancer.
Data collection and analysis
Two review authors independently extracted data to a pre‐designed data collection form and assessed the risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions guidelines. Where possible, we pooled collected data in meta‐analyses.
Main results
This is an update of a previous review with 12 additional studies, so this updated review includes a total of 26 RCTs with 8277 participants that evaluated the effects of PLD alone or in combination with other drugs in recurrent EOC: seven in platinum‐sensitive disease (2872 participants); 11 in platinum‐resistant disease (3246 participants); and eight that recruited individuals regardless of platinum sensitivity status (2079 participants). The certainty of the evidence was assessed for the three most clinically relevant comparisons out of eight comparisons identified in the included RCTs.
Recurrent platinum‐sensitive EOC
PLD with conventional chemotherapy agent compared to alternative combination chemotherapy likely results in little to no difference in overall survival (OS) (hazard ratio (HR) 0.93, 95% confidence interval (CI) 0.83 to 1.04; 5 studies, 2006 participants; moderate‐certainty evidence) but likely increases progression‐free survival (PFS) (HR 0.81, 95% CI 0.74 to 0.89; 5 studies, 2006 participants; moderate‐certainty evidence). The combination may slightly improve quality of life at three months post‐randomisation, measured using European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (mean difference 4.80, 95% CI 0.92 to 8.68; 1 study, 608 participants; low‐certainty evidence), but this may not represent a clinically meaningful difference.
PLD in combination with another chemotherapy agent compared to alternative combination chemotherapy likely results in little to no difference in the rate of overall severe adverse events (grade ≥ 3) (risk ratio (RR) 1.11, 95% CI 0.95 to 1.30; 2 studies, 834 participants; moderate‐certainty evidence). PLD with chemotherapy likely increases anaemia (grade ≥ 3) (RR 1.37, 95% CI 1.02 to 1.85; 5 studies, 1961 participants; moderate‐certainty evidence). The evidence is very uncertain about the effect of PLD with conventional chemotherapy on hand‐foot syndrome (HFS)(grade ≥ 3) (RR 4.01, 95% CI 1.00 to 16.01; 2 studies, 1028 participants; very low‐certainty evidence) and neurological events (grade ≥ 3) (RR 0.38, 95% CI 0.20 to 0.74; 4 studies, 1900 participants; very low‐certainty evidence).
Recurrent platinum‐resistant EOC
PLD alone compared to another conventional chemotherapy likely results in little to no difference in OS (HR 0.96, 95% CI 0.77 to 1.19; 6 studies, 1995 participants; moderate‐certainty evidence). The evidence is very uncertain about the effect of PLD on PFS (HR 0.94, 95% CI 0.85 to 1.04; 4 studies, 1803 participants; very low‐certainty evidence), overall severe adverse events (grade ≥ 3) (RR ranged from 0.61 to 0.97; 2 studies, 964 participants; very low‐certainty evidence), anaemia (grade ≥ 3) (RR ranged from 0.19 to 0.82; 5 studies, 1968 participants; very low‐certainty evidence), HFS (grade ≥ 3) (RR ranged from 15.19 to 109.15; 6 studies, 2184 participants; very low‐certainty evidence), and the rate of neurological events (grade ≥ 3)(RR ranged from 0.08 to 3.09; 3 studies, 1222 participants; very low‐certainty evidence).
PLD with conventional chemotherapy compared to PLD alone likely results in little to no difference in OS (HR 0.92, 95% CI 0.70 to 1.21; 1 study, 242 participants; moderate‐certainty evidence) and it may result in little to no difference in PFS (HR 0.94, 95% CI 0.73 to 1.22; 2 studies, 353 participants; low‐certainty evidence). The combination likely increases overall severe adverse events (grade ≥ 3) (RR 2.48, 95% CI 1.98 to 3.09; 1 study, 663 participants; moderate‐certainty evidence) and anaemia (grade ≥ 3) (RR 2.38, 95% CI 1.46 to 3.87; 2 studies, 785 participants; moderate‐certainty evidence), but likely results in a large reduction in HFS (grade ≥ 3) (RR 0.24, 95% CI 0.14 to 0.40; 2 studies, 785 participants; moderate‐certainty evidence). It may result in little to no difference in neurological events (grade ≥ 3) (RR 1.40, 95% CI 0.85 to 2.31; 1 study, 663 participants; low‐certainty evidence).
Authors' conclusions
In platinum‐sensitive relapsed EOC, including PLD in a combination chemotherapy regimen probably makes little to no difference in OS compared to other combinations, but likely improves PFS. Choice of chemotherapy will therefore be guided by symptoms from previous chemotherapy and other patient considerations. Single‐agent PLD remains a useful agent for platinum‐resistant relapsed EOC and choice of agent at relapse will depend on patient factors, e.g. degree of bone marrow suppression or neurotoxicity from previous treatments. Adding another agent to PLD likely increases overall grade ≥ 3 adverse events with little to no improvement in survival outcomes. The limited evidence relating to PLD in combination with other agents in platinum‐resistant relapsed EOC does not indicate a benefit, but there is some evidence of increased side effects.
Keywords: Female; Humans; Antineoplastic Agents; Antineoplastic Agents/adverse effects; Carcinoma, Ovarian Epithelial; Carcinoma, Ovarian Epithelial/drug therapy; Ovarian Neoplasms; Ovarian Neoplasms/drug therapy; Randomized Controlled Trials as Topic; Recurrence; Systematic Reviews as Topic
Plain language summary
What are the harms and benefits of using pegylated liposomal doxorubicin in women with recurrent epithelial ovarian cancer, alone or in combination with other drugs?
What is the aim of this review?
The aim of this Cochrane Review update was to summarise benefits and unwanted effects of using a coated form of a chemotherapy drug, pegylated liposomal doxorubicin (PLD), for treatment of women with epithelial ovarian cancer (EOC) that had progressed/returned after initial treatment. The review authors collected and analysed all relevant studies to answer this question and found 26 studies, adding 12 studies to the original version of this review.
Key messages
Women whose EOC returned more than six months after finishing their last treatment who were treated with PLD alongside other chemotherapy survived for a similar length of time to women treated with alternative combinations. It may also take longer for their cancer to re‐grow than with alternative combinations. Quality of life may slightly improve with PLD treatment. Apart from anaemia, which was more common in women taking the PLD treatment, severe side effects were similar to those seen in women on alternative combinations.
In women whose EOC returned within six months of finishing their last platinum treatment, PLD alongside other chemotherapy, versus alterative combination chemotherapy, probably works as well in terms of improving how long they live, but we are uncertain about other unwanted effects and benefits. PLD alongside other chemotherapy versus PLD alone likely makes little difference in how long women survive, and may make little difference in how long it takes for their cancer to re‐grow, but the combination likely increases overall severe unwanted effects and the risk of severe anaemia.
What was studied in this review?
The choice of chemotherapy in women with relapsed EOC is influenced by the duration of platinum‐free interval (length of time from the last platinum‐based chemotherapy to the time of disease progression). This is because a short 'platinum‐free interval' suggests that their disease will no longer respond to platinum‐based chemotherapy. Women who relapse within one month of receiving platinum therapy, or who progress on therapy have 'platinum‐refractory' disease; women who relapse between one and six months after platinum therapy have 'platinum‐resistant' disease; and women who relapse more than six months after platinum therapy have 'platinum‐sensitive' disease.
Doxorubicin hydrochloride is an anti‐cancer drug that works by interfering with cancer cell DNA. However, it can have unwanted effects on the heart. Coating the drug within a protective shell allows it to reach higher concentrations in cancer cells whilst protecting the heart. This coated chemotherapy is called pegylated liposomal doxorubicin (PLD).
We wanted to determine how PLD could be used best in women with EOC that has returned, Most of these women will have a limited life expectancy, so consideration of quality of life is important in making treatment choices. One specific side effect of PLD is called hand‐foot syndrome (HFS). This is reddening, swelling, numbness and skin peeling of the palms of the hands and soles of the feet.
What are the main results of the review?
We added 12 studies to the previous review, so now include 26 studies with a total of 8277 women with recurrent EOC. Seven studies looked at platinum‐sensitive disease (2827 women); 11 platinum‐resistant disease (3246 women); and eight recruited women who had both platinum‐sensitivity and platinum resistant disease (2079 women).
Recurrent platinum‐sensitive EOC
We found five studies for women with platinum‐sensitive disease using PLD in combination with chemotherapy versus alternative combination chemotherapy. The PLD combination likely makes little difference in how long women survive (overall survival, OS), but likely increases the time to further relapse (progression‐free survival, PFS). There may be a slight improvement in quality of life. There may be little to no difference in the overall number of severe unwanted effects, although adding PLD causes more anaemia. We are uncertain about the effect of PLD with chemotherapy on other individual unwanted effects.
Recurrent platinum‐resistant EOC
We found six studies for women with platinum‐resistant disease using PLD alone compared to conventional chemotherapy. PLD alone likely makes little difference in OS. We are very uncertain about the effect on PFS, overall severe unwanted effects (i.e. those that require hospital treatment, e.g. blood transfusion), severe anaemia (grade ≥ 3), HFS, and the rate of severe unwanted effects on the nervous system (e.g. permanent numbness in fingers and toes).
We found two studies that compared PLD alongside other chemotherapy combination with PLD alone. PLD in combination likely makes little difference in OS, and it may make little difference in PFS. The combination likely increases overall severe unwanted effects and anaemia. Combination treatment likely results in a large reduction in HFS, but may result in little difference in unwanted effects on the nervous system.
Several studies compared PLD alone with new targeted agents or immunotherapy, but we are very uncertain about the benefit of adding these to PLD.
How up to date is this review?
We searched electronic databases and other resources for studies of PLD for relapsed EOC, and included 26 studies up to January 2022.
Summary of findings
Summary of findings 1. Summary of findings 1: PLD with chemotherapy compared to alternative combination chemotherapy in recurrent platinum‐sensitive EOC.
|
Patient or population: adult women with recurrent platinum‐sensitive EOC Setting: specialist hospital Intervention: PLD with conventional chemotherapy Comparison: conventional chemotherapy alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comment | |
| Risk with conventional chemotherapy alone | Risk with PLD with conventional chemotherapy | |||||
|
Overall survival (OS) Assessed with: survival status Follow‐up: median range 25.5 to 49 months |
Averagea | HR 0.93 (0.83 to 1.04) | 2006 (5 RCTs) | ⊕⊕⊕⊝ Moderateb | PLD with conventional chemotherapy likely results in little to no difference in OS. | |
| 284 per 1000 | 310 per 1000 (260 to 361) | |||||
|
Progression‐free survival (PFS) Assessed with: progression free status according to RECIST Follow‐up: median range 11.3 to 49 months |
Averagec | HR 0.81 (0.74 to 0.89) | 2006 (5 RCTs) | ⊕⊕⊕⊝ Moderated | PLD with conventional chemotherapy likely increases PFS. | |
| 377 per 1000 | 454 per 1000 (412 to 495) | |||||
|
Quality of life (QoL) Assessed with: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 Follow‐up: 3 months post‐randomisation |
The mean change from baseline in quality of life (Global Health score) was ‐2.2 points. | MD 4.8 points higher (0.92 higher to 8.68 higher) | ‐ | 608 (1 RCT) | ⊕⊕⊝⊝ Lowe | PLD with conventional chemotherapy evidence may slightly improve QoL. |
|
Overall severe adverse events (grade ≥ 3) Assessed with: WHO classification where stated |
222 per 1000 | 245 per 1000 (211 to 289) | RR 1.11 (0.95 to 1.30) | 834 (2 RCTs) | ⊕⊕⊕⊝ Moderated | PLD with conventional chemotherapy likely results in little to no difference in overall severe adverse events (grade ≥3). |
|
Anaemia (grade ≥ 3) Assessed with: WHO classification or CTCAE (v2.0‐4.03) where stated |
69 per 1000 | 95 per 1000 (65 to 140) | RR 1.37 (1.02 to 1.85) | 1961 (5 RCTs) | ⊕⊕⊕⊝ Moderated | PLD with conventional chemotherapy likely increases anaemia (grade ≥ 3). |
| Hand‐foot syndrome (grade ≥ 3) Assessed with: WHO classification or CTCAE (v2.0‐4.03) where stated | 4 per 1000 | 15 per 1000 (4 to 60) | RR 4.01 (1.00 to 16.01)f | 1028 (2 RCTs) | ⊕⊝⊝⊝ Very lowd,g | The evidence is very uncertain about the effect of PLD with conventional chemotherapy on hand‐foot syndrome (grade ≥ 3). |
|
Neurological (grade ≥ 3) Assessed with: WHO classification or CTCAE (v2.0‐4.03) where stated |
33 per 1000 | 19 per 1000 (4 to 100) | RR 0.38 (0.20 to 0.74) | 1900 (4 RCTs) | ⊕⊝⊝⊝ Very lowb,d,h | The evidence is very uncertain about the effect of PLD with conventional chemotherapy on neurological events (grade ≥ 3). |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; EOC: epithelial ovarian cancer; HR: hazard ratio; MD: mean difference; OS: overall survival; PLD: pegylated liposomal doxorubicin; RECIST: Response Evaluation Criteria in Solid Tumors; RCT: randomised control trail; RR: risk ratio; WHO: World Health Organization
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations
aThe control risk is an average number of participants reported alive at 36 months in CALYPSO, Fujiwara 2019 and Fujiwara 2019 trials. bDowngraded by one level due to imprecision (wide confidence interval around the effect estimate crossing a line of no difference). cThe control risk is an average number of participants reported alive at 12 months in CALYPSO, Fujiwara 2019 and Pfisterer 2020 trials. dDowngraded by one level due to risk of bias (open‐label design). eDowngraded by two levels due to imprecision (very wide confidence interval around the effect estimate crossing line of no difference). fNote: 3out of 5 trials contributed to synthesis reported no events of HFS. In the fourth trial, there was only a single event in PLD with conventional chemotherapy arm. gDowngraded by two levels due to imprecision (very wide confidence interval around the effect estimate including the line of no difference). hDowngraded by one level due to inconsistency (notable difference between the direction of the effect between different drugs as conventional chemotherapy, test for subgroup difference P = 0.01).
Summary of findings 2. Summary of findings 2: PLD alone compared to other conventional chemotherapy in recurrent platinum‐resistant EOC.
|
Patient or population: adult women with recurrent platinum‐resistant EOC Setting: specialist hospital Intervention: PLD alone Comparison: other conventional chemotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conventional chemotherapy | Risk with PLD | |||||
|
Overall survival (OS) Assessed with: survival status Follow‐up: median range 10 to 29.2 months |
Averagea | HR 0.96 (0.77 to 1.19) | 1995 (6 RCTs) | ⊕⊕⊕⊝ Moderateb,c,d | PLD likely results in little to no difference in OS. | |
| 24 per 1000 | 28 per 1000 (12 to 57) | |||||
|
Progression‐free survival (PFS) Assessed with: progression‐free status per RECIST 1.1 Follow‐up: median range 10 to 29.2 months |
Averagee | HR 0.94 (0.85 to 1.04) | 1803 (4 RCTs) | ⊕⊝⊝⊝ Very lowd,f,g | The evidence is very uncertain about the effect of PLD on PFS. | |
| 19 per 1000 | 24 per 1000 (16 to 34) | |||||
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported. |
| Overall severe adverse events (grade > 3) Assessed with: unclear method | Risk with gemcitabine | RR ranged from 0.61 to 0.97 | 964 (2 RCTs) | ⊕⊝⊝⊝ Very lowd,f,h | The evidence is very uncertain about the effect of PLD on overall severe adverse events (grade >3). | |
| 60 per 1000 | Ranged from 37 to 58 | |||||
| Risk with patupilone | ||||||
| 600 per 1000 | Ranged from 366 to 582 | |||||
| Anaemia (grade ≥ 3) Assessed with: CTCAE v2.0 & 4.0 where reported | Risk with gemcitabine | RR ranged from 0.19 to 0.82 | 1968 (5 RCTs) | ⊕⊝⊝⊝ Very lowd,f,h | The evidence is very uncertain about the effect of PLD on anaemia (grade ≥ 3). | |
| 50 per 1000 | Ranged from 10 to 41 | |||||
| Risk with topotecan | ||||||
| 280 per 1000 | Ranged from 53 to 230 | |||||
| Hand‐foot syndrome (grade ≥ 3) Assessed with: CTCAE v2.0 & 4.0 where reported | No occurrences of hand‐foot syndrome in the control arms of included studies. | RR ranged from 15.19 to 109.15 | 2184 (6 RCTs) | ⊕⊝⊝⊝ Very lowf,h | The evidence is very uncertain about the effect of PLD on hand‐foot syndrome (grade ≥ 3). | |
| 0 per 1000 | Ranged from 55 to 230 per 1000 | |||||
|
Neurological (grade ≥ 3) Assessed with: CTCAE v2.0 where reported |
Risk with patupilone | RR ranged from 0.08 to 3.09 | 1222 (3 RCTs) | ⊕⊝⊝⊝ Very lowf,i,j | The evidence is very uncertain about the effect of PLD on neurological events (grade ≥ 3). | |
| 62 per 1000 | Ranged from 5 to 192 | |||||
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; EOC: epithelial ovarian cancer; HR: hazard ratio; PFS: progression‐free survival; PLD: pegylated liposomal doxorubicin; RECIST: Response Evaluation Criteria in Solid Tumors; RCT: randomised control trial; RR: risk ratio
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations
aThe control risk is an average number of participants reported alive at 36 months in CORAIL and Colombo 2012 trials. bNote: Despite I2 = 86% we decided not to downgrade the evidence due to inconsistency as the confidence intervals around the effects in the individual trials overlap and all trials show no evidence of an effect of PLD with conventional chemotherapy on overall survival. cNote: 3 studies included participants with recurrent EOC regardless of platinum sensitivity status (Gordon 2001; MITO‐3; NCT00653952, 624 participants). dDowngraded by one level due to imprecision (wide confidence interval around the effect estimate crossing the line of no difference). eThe control risk is an average number of participants reported alive at 12 months in CORAIL and Colombo 2012 trials. fDowngraded by one level due to the risk of bias (open‐label design). gDowngraded by one level due to indirectness (two trials contributing evidence included participants with recurrent EOC regardless of platinum sensitivity status). hDowngraded by four levels due to imprecision (extreme values of effect estimates and confidence intervals). iDifferences depending on the type of conventional chemotherapy (P = 0.04). jDowngraded by two levels due to imprecision (risk ratio estimates in the studies ranging from 0.08 to 3.09).
Summary of findings 3. Summary of findings 3: PLD with chemotherapy compared to PLD alone in recurrent platinum‐resistant epithelial ovarian cancer.
|
Patient or population: adult women with recurrent platinum‐resistant epithelial ovarian cancer Setting: specialist hospital Intervention: PLD with conventional chemotherapy Comparison: PLD alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with PLD alone | Risk with PLD with conventional chemotherapy | |||||
|
Overall survival (OS) Assessed with: survival status Follow‐up: median 17.4 months |
Averagea | HR 0.92 (0.70 to 1.21) | 242 (1 RCT) | ⊕⊕⊕⊝ Moderateb | PLD with conventional chemotherapy likely results in little to no difference in OS. | |
| 12 per 1000 | 17 per 1000 (5 to 45) | |||||
|
Progression‐free survival (PFS) Assessed with: progression‐free status per RECIST 1.1 assessed by BICR Follow‐up: median 17.4 months |
Averagec | HR 0.94 (0.73 to 1.22) | 353 (2 RCTs) | ⊕⊕⊝⊝ Lowb,d | PLD with conventional chemotherapy may result in little to no difference in PFS. | |
| 41 per 1000 | 50 per 1000 (20 to 97) | |||||
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported. |
|
Overall severe adverse events (grade ≥ 3) Assessed with: CTCAE v4.03 treatment‐emergent AEs |
224 per 1000 | 556 per 1000 (444 to 693) | RR 2.48 (1.98 to 3.09) | 663 (1 RCT) | ⊕⊕⊕⊝ Moderated,e | PLD with conventional chemotherapy likely increases overall severe adverse events (grade ≥ 3). |
|
Anaemia (grade ≥ 3) Assessed with: CTCAE v4.03 treatment‐emergent AEs |
54 per 1000 | 129 per 1000 (79 to 210) | RR 2.38 (1.46 to 3.87) | 785 (2 RCTs) | ⊕⊕⊕⊝ Moderated | PLD with conventional chemotherapy likely increases anaemia (grade ≥ 3). |
|
Hand‐foot syndrome (grade ≥ 3) Assessed with: CTCAE v4.03 treatment‐emergent AEs |
186 per 1000 | 45 per 1000 (26 to 74) | RR 0.24 (0.14 to 0.40) | 785 (2 RCTs) | ⊕⊕⊕⊝ Moderated,e | PLD with conventional chemotherapy likely results in a large reduction in hand‐foot syndrome (grade ≥ 3). |
|
Neurological (grade ≥ 3) Assessed with: CTCAE v4.03 treatment‐emergent AEs |
73 per 1000 | 102 per 1000 (62 to 168) | RR 1.40 (0.85 to 2.31) | 663 (1 RCT) | ⊕⊕⊝⊝ Lowb,d | PLD with conventional chemotherapy may result in little to no difference in neurological events (grade ≥ 3). |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
BICR: blinded independent central review; CI: confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; EOC: epithelial ovarian cancer; HR: hazard ratio; OS: overall survival; PFS: progression‐free survival; PLD: Pegylated liposomal doxorubicin; RECIST: Response Evaluation Criteria in Solid Tumors; RR: risk ratio
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations
aThe control risk is an average number of participants reported alive at 36 months in Colombo 2012 and CORAIL trials (arms with PLD alone). bDowngraded by one level due to imprecision (wide confidence interval around the effect estimate crossing a line of no difference). cThe control risk is an average number of participants reported alive at 12 months in PRECEDENT (olaratumab), Colombo 2012 (PLD alone arm), and CORAIL (PLD alone arm) trials. dDowngraded by one level due to risk of bias (open‐label design). eNote: evidence includes data from participants with recurrent EOC regardless of platinum sensitivity status.
Background
Description of the condition
Ovarian cancer is the eighth most common cancer in women worldwide, responsible for approximately 313,959 new cancer cases per annum (GLOBOCAN 2020). In Europe, it is the eighth most common cancer in women, the fifth most common cause of cancer deaths, and the most lethal gynaecological cancer (ECIS 2020). The cumulative risk of being diagnosed with ovarian cancer is approximately 1% in Europe and North America, and 0.6% to 0.7% in the rest of the world (GLOBOCAN 2020); this risk increases with age.
Women with ovarian cancer classically fail to develop symptoms that are recognised as suspicious until the development of advanced disease. This absence of obvious symptoms in early stages results in 60% to 70% of women presenting with the International Federation of Gynecology and Obstetrics (FIGO) stages III to IV disease (Gaitskell 2022), characterised by widespread tumour dissemination within and/or beyond the abdominal cavity (Jemal 2008; see Table 4 for FIGO staging).
1. FIGO staging of ovarian cancer*.
| Stage | Extent of tumour | Substage | Details |
| I | Tumour confined to ovaries or fallopian tube(s) | Ia | Tumour limited to one ovary (capsule intact) or fallopian tube; no tumour on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings. |
| Ib | Tumour limited to both ovaries (capsules intact) or fallopian tubes; no tumour on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings. | ||
| Ic | IC: tumour limited to one or both ovaries or fallopian tubes, with any of the following: IC1: surgical spill; IC2: capsule ruptured before surgery or tumour on ovarian or fallopian tube surface; IC3: malignant cells in the ascites or peritoneal washings. |
||
| II | Tumour involves one or both ovaries or fallopian tubes with pelvic extension (below pelvic brim) or primary peritoneal cancer | IIa | Extension and/or implants on uterus and/or fallopian tubes and/or ovaries |
| IIb | Extension to other pelvic intraperitoneal tissues | ||
| III | Tumour involves one or both ovaries or fallopian tubes, or primary peritoneal cancer, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes | IIIa | IIIA1: positive retroperitoneal lymph nodes only (cytologically or histologically proven): IIIA1(i) Metastasis up to 10 mm in greatest dimension; IIIA1(ii) Metastasis more than 10 mm in greatest dimension; IIIA2: microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes. |
| IIIb | Macroscopic peritoneal metastasis beyond the pelvis up to 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes. | ||
| IIIc | IIIC: macroscopic peritoneal metastasis beyond the pelvis more than 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumour to capsule of liver and spleen without parenchymal involvement of either organ) | ||
| IV | Distant metastasis excluding peritoneal metastases | IVa | Pleural effusion with positive cytology |
| IVb | Parenchymal metastases and metastases to extra‐abdominal organs (including inguinal lymph nodes and lymph nodes outside the abdominal cavity) |
FIGO: International Federation of Gynaecology and Obstetrics. * From FIGO 2014.
Overall, considering the estimated survival of all women and all stages of ovarian cancer, the five‐year survival rate is between 35% and 45% amongst high‐income countries (ICBP 2019). This rate has increased over time, owing to general improvements in healthcare and novel treatments in recent years, such as maintenance poly (ADP‐ribose) polymerase (PARP) inhibitors. When considering survival stratified by diagnostic staging, women with FIGO stage I ovarian cancer in England are estimated to have a five‐year survival rate of around 94%. In contrast, the five‐year survival rate for stage IV disease is less than 20% (Cancer Research UK 2019).
Over 90% of all malignant ovarian tumours are epithelial in origin, termed epithelial ovarian cancer (EOC). Of these, the most common subtype is high‐grade serous carcinoma (HGSC; ESMO 2019). Internationally, current guidelines and treatment paradigms for EOC recommend surgical removal and cytoreduction of the tumour alongside any other visible macroscopic disease, at primary diagnosis if feasible. In most cases, determined by tumour stage and histological subtype, treatment with platinum‐based chemotherapy is also advised in the neo‐adjuvant or adjuvant setting, if performance status permits. This chemotherapy is usually carboplatin and paclitaxel combination therapy, although single‐agent platinum may be considered, determined by a variety of clinical features (ESMO 2019).
HGSC is typically characterised as having marked sensitivity to platinum‐based conventional chemotherapies in the first‐line setting. However, although most tumours (70% to 80%) will initially respond to first‐line chemotherapy, most will subsequently relapse and require further chemotherapy (NICE 2003). At this time, the choice of which chemotherapy women will then be treated with is influenced by the woman's platinum‐free interval (PFI), regarded as the length of time from completion of the last platinum‐based chemotherapy to the time of disease progression. Typically, women who are still classified as platinum‐sensitive (PFI > 12 months) or partially platinum‐sensitive (PFI 6 to 12 months) would be rechallenged with platinum‐based chemotherapy in combination with another agent, including paclitaxel, gemcitabine or pegylated liposomal doxorubicin (PLD; ESMO 2019; NICE 2016; Pfisterer 2006).
Conversely, women regarded as platinum‐resistant (PFI < six months) or platinum‐refractory (PFI < one month or progression during first‐line therapy) should be treated with non‐platinum single agents, including paclitaxel, topotecan, gemcitabine and PLD (ESMO 2019; Pfisterer 2006; Naumann 2011). However, response rates in this group are poor (10% to 15%) and the median overall survival (OS) is approximately 12 months (Naumann 2011). Despite considerable research in this setting in recent years, no improvements in survival have been achieved.
Description of the intervention
For the treatment of women with platinum‐resistant ovarian cancer, PLD had been traditionally recommended as monotherapy at a starting dose of 50 mg/m², given intravenously every four weeks, for up to six cycles. Early termination of the course may occur in the event of unacceptable toxicity, clinical disease progression, or radiological disease progression (EMA 2010). Several recent studies have investigated using lower PLD doses (30 to 45 mg/m²), particularly when combined with other agents, such as carboplatin (in platinum‐sensitive disease) or dexamethasone (CALYPSO; HeCOG 2010; OVA‐301), in an attempt to ameliorate side effects and toxicity. In this setting, a dose of 40 mg/m² given four‐weekly is commonly used in clinical practice.
The most common side effect of PLD is nausea (EMA 2010); however, other frequently associated toxicities include palmar‐plantar erythrodysesthesia (PPE; also known as hand‐foot syndrome (HFS)), stomatitis/mucositis and neutropenia (CAELYX PI; EMA 2010). Hand‐foot syndrome, characterised by palmar‐plantar erythema, desquamation, (redness and skin peeling of palms and soles)and pain, usually occurs after two or three cycles and can be severely disabling; its presence often results in PLD dose reduction or discontinuation. Severe PPE (Common Terminology Criteria for Adverse Events (CTCAE) Grade 3/4) is reported to occur in approximately 20% of women who commence PLD therapy at the 50 mg/m² dose (Lorusso 2007). Numerous approaches to hand‐foot syndrome management have been described; however, there is an absence of high‐quality evidence to support these strategies, and treatment is mostly supportive (von Moos 2008).
How the intervention might work
Available since the 1960s, doxorubicin hydrochloride is an anthracycline cytotoxic chemotherapy drug that belongs to the anthracycline class (EMA 2010). Its main mode of action is through binding with both the enzyme topoisomerase II and DNA, forming a complex resulting in lethal double‐stranded DNA breaks (Zunino 2002). Although anthracyclines are key anti‐tumour agents and shown to be effective, those treated are at risk of cardiotoxicity and other adverse effects (Zunino 2002). Liposomal doxorubicin was subsequently developed, with the aim of reducing cardiotoxicity risk whilst preserving anti‐tumour efficacy, relative to conventional doxorubicin (Theodoulou 2004).
Pegylated liposomal doxorubicin is a formulation of liposomal doxorubicin coated in polyethylene glycol, a hydrophilic coating that protects the liposomes from detection by the body's reticular endothelial system. This coating subsequently reduces the active substance's degradation rate and increases its circulating half‐life, compared to conventional and liposomal doxorubicin (Gabizon 2001). Pegylated liposomes are small enough to extravasate out of leaky tumour vasculature (CAELYX PI), and the absence of functional lymphatic drainage within cancer tissues results in high uptake and retention of PLD by the tumour. In addition, the increased circulating half‐lifetime, conferred by the pegylation, increases the number of passes the drug makes through the tumour microvasculature; this ultimately results in higher intratumoural delivery of cytotoxic agents (Gabizon 2001). The significantly lower risk of cardiotoxicity seen with PLD is thought to be due to the tight capillary junctions in the cardiac muscle, limiting the concentration of the drug able to penetrate cardiac tissues (Theodoulou 2004).
Why it is important to do this review
The European Society of Medical Oncology (ESMO) guidelines recommend PLD as a treatment option for women with recurrent disease, licensed in the UK as monotherapy or in combination with platinum‐based compounds (ESMO 2019;https://www.nice.org.uk/guidance/ta389NICE 2016).
Within the last decade, difficulties in the production of PLD resulted in the disruption of patient care, alongside the suspension of some ongoing trials (INOVATYON; TRINOVA‐2). Since production has been re‐established, the potential for use of this drug as part of routine care has improved once more.
As new agents are being developed continually for the treatment of women with EOC, there remains a continual need to compare existing standards of care, including the role of PLD in both platinum‐sensitive and platinum‐resistant settings. The relative efficacy and toxicity of PLD compared to other new agents is unclear. This review aims to update the previous Cochrane Review (Lawrie 2013), incorporating new research findings in order to evaluate further the efficacy and safety of PLD compared with other chemotherapeutic agents.
Objectives
To evaluate the efficacy and safety of PLD, with or without other anti‐cancer drugs, in women with relapsed high grade epithelial ovarian cancer (EOC).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were eligible for inclusion.
Types of participants
We included women ≥ 18 years of age with relapsed high grade epithelial ovarian cancer of any stage, including women with both platinum‐sensitive and platinum‐resistant disease. We excluded studies of participants with low grade serous carcinoma or non‐epithelial histology.
Types of interventions
PLD in combination with platinum‐based therapy versus platinum‐based therapy with another agent, e.g. PLD plus carboplatin versus paclitaxel (PAC) plus carboplatin.
Other chemotherapy agent(s) versus PLD, e.g. topotecan (TOP) versus PLD.
PLD plus other agent(s) versus PLD alone or with placebo, e.g. trabectedin (TBD) plus PLD versus PLD.
Types of outcome measures
The primary and secondary outcomes of this review are as follows.
Primary outcomes
Overall survival (OS): survival until death from all causes
Progression‐free survival (PFS): survival until disease progression
Secondary outcomes
Severe adverse events, classified according to commonly used toxicity scoring criteria (e.g. CTCAE 2017), including haematological, gastrointestinal, genitourinary, dermatological, neurological, pulmonary, and other severe adverse events
Quality of life, measured by a validated scale, e.g. the European Organisation for Research and Treatment of Cancer (EORTC) Core Quality of Life questionnaire (EORTC QLQ‐C30)
Symptom control, including dose reductions and delays
Search methods for identification of studies
We sought papers in all languages and obtained translations when necessary.
Electronic searches
We searched the following electronic databases on 4 January 2022 (also see Cochrane Gynaecological Cancer Group methods used in reviews):
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 12, 2021), in the Cochrane Library;
MEDLINE via Ovid (1990 to 3 January 2022);
Embase via Ovid (1990 to 2021 week 52).
The CENTRAL, MEDLINE and Embase search strategies, based on terms related to the review topic, are presented in Appendix 1, Appendix 2, and Appendix 3, respectively. As PLD has been recently developed, searches before 1990 would not have been relevant; therefore databases were searched from 1990 until January 2022. We identified all relevant articles on PubMed and, using the 'related articles' feature, we carried out a further search for newly published articles.
Searching other resources
We searched the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com/rct), www.clinicaltrials.gov and the Physicians Data Query (PDQ) (www.cancer.gov/clinicaltrials) for ongoing trials. We also searched the abstracts of the American Society for Clinical Oncology (ASCO) Annual Meetings from 2000 to 2022. Where necessary, we attempted to contact the main investigators of relevant ongoing trials for further information. In addition, we checked the citation lists of included studies to identify other reports/studies.
Data collection and analysis
Selection of studies
For this update of the review, we downloaded all titles and abstracts retrieved by electronic searching to Covidence 2019 and removed duplicates. At least two review authors (a combination of EN, RN, EB, SV and JM) for this update of the review independently examined the remaining references. We excluded those studies that clearly did not meet the inclusion criteria, and obtained copies of the full text of potentially relevant references. At least two review authors (a combination of RN, EN, KE‐S, EB and SV) for this update of the review independently assessed the eligibility of retrieved papers, with appeal to JM where there were disagreements and referral to Dr Miller for expert advice, as required (see Acknowledgements). We documented reasons for exclusion for key excluded papers.
Data extraction and management
For included studies, we extracted the following data where possible.
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
-
Study population
total number enrolled
participant characteristics
age
previous therapy (including platinum sensitivity or resistance)
comorbidities
-
Ovarian cancer details at diagnosis
FIGO stage
histological cell type
tumour grade
performance status
extent of disease
Total number of intervention groups
-
Intervention details
details of PLD including dose, regimen, frequency and the number of cycles
comparison details including type of control and dose, regimen, frequency and number of cycles, if appropriate
Proportion of participants who received all/part/none of the intended treatment
Delays in treatment
Risk of bias in study (see Assessment of risk of bias in included studies)
Duration of follow‐up
-
Outcomes – overall survival, PFS, QoL, symptom control and adverse events
for each outcome: outcome definition (with diagnostic criteria if relevant)
unit of measurement (if relevant)
for scales: upper and lower limits, and whether high or low score is good
results: number of participants allocated to each intervention group
for each outcome of interest: sample size; missing participants
Data extraction of outcome data from each trial
We extracted data on outcomes as follows.
For time‐to‐event data (OS and PFS), we extracted the hazard ratio (HR), log of the hazard ratio (log(HR)) and its standard error (SE) from trial reports where possible. If these were not reported, we attempted to estimate them from other reported statistics using available methods (Tierney 2007).
For dichotomous outcomes (e.g. adverse events), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at an endpoint, in order to estimate a risk ratio (RR).
For continuous outcomes (e.g. QoL measures), we extracted the mean difference (MD) and standard deviation (SD) between the final value of the outcome measure in each treatment arm at the end of follow‐up. If SDs of final values were not available, we used change scores if their SDs were available. If no SDs were available, we omitted these trials from the analyses.
Where possible, we extracted data relevant to an intention‐to‐treat analysis (ITT), in which participants were analysed in groups to which they were assigned. Where time‐to‐event outcomes were assessed by more than one method, e.g. independent radiology review, investigator assessment or independent oncology review, we used the independent radiology review data. We noted the time points at which outcomes were collected and reported. Where data from several time points were reported, we used the data from the last assessment in our meta‐analyses if appropriate. Where a trial evaluated the same drug in two or more different doses, we extracted all the combined data but in the data synthesis used and only the estimated individual data for the most efficacious dose/regimen versus the comparator.
Two review authors (a combination of RN, EN, KE‐S, EB and SV) independently extracted data from the selected trials using piloted data extraction forms specially designed for the review. Where there was disagreement between the two review authors, this was resolved by discussion with JM.
Assessment of risk of bias in included studies
We assessed the risk of bias in included RCTs using Cochrane's RoB 1 tool and the criteria specified in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included assessment of the following domains.
-
Selection bias
random sequence generation
allocation concealment
-
Performance bias
blinding of participants and personnel (participants and treatment providers)
-
Detection bias
blinding of outcome assessment
-
Attrition bias
incomplete outcome data: we recorded the proportion of participants whose outcomes were not reported at the end of the study and considered greater than 20% attrition to be at a high risk of bias
-
Reporting bias
selective reporting of outcomes
Other possible sources of bias
Two authors assessed the risk of bias independently (a combination of RN, EN, KE‐S, EB and SV) and resolved differences by discussion or by appeal to a third review author (JM). Results are presented in a risk of bias summary graph. We interpreted the results of the meta‐analyses in light of the findings with respect to risk of bias.
Measures of treatment effect
We used hazard ratio (HR) for time‐to‐event data, risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with multi‐arm trials
The JAVELIN Ovarian 200 trial had multiple treatment groups (three‐arm trial), and so we divided the control group between the treatment groups and treated comparisons between each treatment group and a split control group as independent comparisons for all adverse event outcomes.
Dealing with missing data
We did not impute missing outcome data.
Assessment of heterogeneity
We assessed heterogeneity between trials by visual inspection of forest plots, by estimation of the percentage heterogeneity (I² statistic) between trials that cannot be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity ‐ Chi² test (Deeks 2001). We regarded statistical heterogeneity as substantial if the I² was greater than 50% and either Tau2 (a measure of between‐study variance) was greater than 0, or the P value of the Chi² test was less than 0.10. If there was evidence of substantial heterogeneity, we investigated the possible reasons for this and reported it.
Assessment of reporting biases
There was an insufficient number of included studies to adequately evaluate the potential for small study effects, such as publication bias, using funnel plots.
Data synthesis
Where we deemed it clinically and methodologically appropriate, we meta‐analysed the trial data. Our main approach was to pool data in a two‐stage, fixed‐effect, inverse‐variance meta‐analysis based on the assumption that all trials included in a given comparison were conducted under sufficiently similar conditions and in similar populations. We applied the random‐effects, inverse variance model in comparisons with platinum‐resistant EOC where we included data from trials that evaluated the effect of treatment options in populations with recurrent EOC regardless of platinum‐sensitivity status. If the outcome was rare (few events), we used the Mantel‐Haenszel models (fixed or random).
Dealing with non‐proportional hazards
If studies identified non‐proportional hazards, we used the reported hazards ratios as a measure of the effect, if reported. However, we indicated the detection of non‐proportionality, reported value of the log‐rank test and alternative measure of the effect (e.g. restricted mean survival times) if reported.
Approach to the synthesis of adverse events
Based on the availability of information on whether the reported adverse events were treatment‐related or not, we synthesised the data as outlined earlier (all adverse events of the same kind). If the trial report did not provide this information, we examined the case‐by‐case suitability of using the data in the analysis.
Subgroup analysis and investigation of heterogeneity
We grouped the RCTs by Types of interventions. Where the types of interventions differed within a comparison, e.g. other drugs versus PLD, we subgrouped data by the comparator drug.
Sensitivity analysis
We performed sensitivity analyses on survival outcomes to explore the influence of our decision to incorporate data from the trials with recurrent EOC regardless of its platinum sensitivity status.
Summary of findings and assessment of the certainty of the evidence
Based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017a), we prepared a summary of findings table to present the results of the following outcomes.
Overall survival (OS)
Progression‐free survival (PFS)
Quality of life (QoL)
Adverse events: overall severe adverse events (Grade ≥ 3)
Adverse events: anaemia (Grade ≥ 3)
Adverse events: hand‐foot syndrome (Grade ≥ 3)
Adverse events: neurological (Grade ≥ 3)
For each assumed risk cited in the tables, we provided a rationale and used the GRADE system to rank the quality of the evidence (Schünemann 2017b). We downgraded evidence by ‐1 or ‐2 if the following limitations were present, according to their seriousness: study design limitations, inconsistency, imprecision, indirectness and publication bias. Where the evidence was based on single studies, or where there was no evidence on a specific outcome, we included the outcome in the summary of findings tables and graded or explained accordingly. We downgraded evidence of a clear effect derived from a single small study, and resolved any differences by discussion. We reported and interpreted results based on the Cochrane Effective Practice and Organisation of Care and interactive GRADEpro summary of findings table guidance (EPOC 2015; Schünemann 2019).
Results
Description of studies
Results of the search
In the original version of the review, 1602 unique references were identified by the database searches, 17 trials by the trial registry searches, and an additional three studies from review of reference lists of included studies (1622 references in total) (Figure 1). Titles and abstracts of 1512 records were excluded and the full text of 110 potentially eligible publications obtained, including the trial registry records. After evaluating these full texts, seven studies were excluded (20 records) (see Characteristics of excluded studies) and the details of the 16 ongoing trials added to the Characteristics of ongoing studies section of the review (18 records). Fourteen completed RCTs (72 records) met the inclusion criteria. One of these was not yet published in full (PRECEDENT); the investigators were contacted and a copy of the unpublished manuscript obtained. Additional unpublished data were also obtained from the investigators of two other studies (Kaye 2012; MITO‐3). Where studies included PLD as part of an arm, attempts were made to extract data separately for those who received PLD. The authors were contacted for data separated by PLD and included where provided.
1.
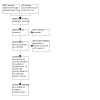
PRISMA flow chart of original version of review (Lawrie 2013) ‐ search to 15 October 2012
For the 2022 update (up to January 2022), we identified 683 unique references by database searches and trial registry searches, and found another six from other sources (Figure 2). After screening titles and abstracts, we identified 96 studies for full‐text review. We excluded 55 studies: 17 studies were clearly irrelevant, as per Cochrane guidelines (Section 4.6.5; Lefebvre 2022), and we have not described these. We have detailed the other 38 records in the Characteristics of excluded studies table. In total, we included 12 further studies, detailed in the Characteristics of included studies table, some of which were identified as ongoing studies in the previous version of the review. In total, we now include 26 studies in this update, with 23 contributing data to the meta‐analysis.
2.
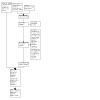
Flow diagram for search for review update from February 2013 to January 2022
We identified a total of 12 Ongoing studies, and 13 studies are Studies awaiting classification pending further information from the authors, which we have requested, largely for separation of data for PLD‐treated participants from a 'chemotherapy of physician's choice' arm.
Included studies
Treatment options for women with recurrent EOC vary depending on whether their disease is likely to respond to platinum‐based chemotherapy. Women within each of these groups have differing prognoses and response rates to treatment compared to those within other treatment groups. We therefore divided our studies between those including women with:
platinum‐sensitive relapsed EOC (relapse > 12 months after last platinum‐base chemotherapy);
platinum‐resistant or refractory relapsed EOC (no response to last platinum‐based chemotherapy (refractory) or relapse within six months of last platinum‐based chemotherapy (resistant); and
regardless of platinum‐resistance (including those with partially‐platinum‐resistant EOC (relapse between six and 12 months of last platinum‐based chemotherapy).
Please see Table 5 for details of included studies by comparison groups and Table 6 for details of median survival times in included studies.
2. Included studies by comparison group.
| Study Name | Alternative name/ trial registry number | 2016 Review | Number of participants | Study design | Experimental treatment | Control treatment | Mechanism of action | Platinum sensitivity | Dose of PLD | Duration of FU | 6‐month PFS rate | 2‐year OS rate | Notes |
| Platinum sensitive | |||||||||||||
| Other conventional chemotherapy | |||||||||||||
| SWOG S0200 | NCT00043082 | Yes | 61 | Phase III multicentre RCT; open‐label | Carboplatin (AUC 5) AND PLD every 4 weeks |
Carboplatin (AUC 5) every 4 weeks | C: alkylating agent. Forms platinum complexes, causing inter‐ and intra‐strand DNA cross‐linkage. Resultant alteration to DNA structure, inhibiting synthesis. | PS | 30 mg/m2 | Median 22.4 months | Not known | Not known | |
| CALYPSO | NCT00538603 | Yes | 976 | Phase III multicentre non‐inferiority RCT; open‐label | Carboplatin (AUC 5) AND PLD every 4 weeks |
Carboplatin (AUC 5) AND Paclitaxel (175mg/m2) every 3 weeks |
C: as above P: impairs cellular division and causes cytotoxicity by inhibiting microtubule formation. |
PS | 30 mg/m2 | Median 49 months (0 to 68 months) | 85.2% PLD+C 79.9% C+T |
61.2% PLD+C 64.2% C+T |
|
| HeCOG 2010 | ACTRN12609000436279 | Yes | 204 (189 eligible) |
Phase II RCT; open‐label | Carboplatin (AUC 5) AND PLD every 4 weeks |
Carboplatin (AUC 5) AND Paclitaxel (175mg/m2) every 3 weeks |
As above | PS | 45 mg/m2 | Median 43.6 months (95% CI 0.1 to 74.8) | NR | NR | |
| Fujiwara 2019 | UMIN 000,005,487 | No | 100 | Phase II RCT; open‐label | Carboplatin (AUC 5) AND PLD every 4 weeks |
Carboplatin (AUC 4) AND Gemcitabine (1000 mg/m2) on days 1 and 8, every 3 weeks |
C: as above G: a nucleoside analogue that interferes with DNA synthesis. S‐phase specific. |
PS | 30 mg/m2 | 24 months | 77.6% PLD+C 80% G+C |
63.3%PLD+C 66% G+C |
|
| Pfisterer 2020 | NCT01837251 | No | 682 | Phase III multicentre RCT; open‐label | Bevacizumab (10 mg/kg) AND Carboplatin (AUC 4) AND PLD every 4 weeks Followed by maintenance bevacizumab (15 mg/kg) every 3 weeks |
Bevacizumab (15 mg/kg) AND Carboplatin (AUC 4) AND Gemcitabine 1000 mg/m2 every 3 weeks Followed by maintenance bevacizumab (15 mg/kg) every 3 weeks |
B: angiogenesis inhibitor; selectively targets VEGF | PS | 30 mg/m2 | 30 months | 84.9% C+PLD+Bev 84.3% C+G+Bev |
53.7% C+PLD+Bev vs 56.5% C+PLD +Bev |
|
| Monk 2020 | NCT01846611 | No | 581 (576 assigned to treatment) | Phase III multi‐centre RCT; open‐label | Trabectedin (1.1 mg/m2) AND PLD every 3 weeks |
PLD every 4 weeks | Complex and not fully understood. Blocks DNA binding and reverses transcription. | PS | 30 mg/m2 in combined arm. 50 mg/m2 in alone arm. |
Median 23.8 months | 40.1% TBD/PLD vs 41.1% PLD | 28.9% TBD/PLD vs 19.9% PLD | |
| Targeted therapy | |||||||||||||
| TRINOVA‐2 | NCT01281254 | No | 223 | Phase III multi‐centre RCT; double‐blind | Trebananib AMG386 (15 mg/kg) every week AND PLD every 4 weeks |
PLD every 4 weeks AND Placebo every week |
Angiogenesis inhibitor; selectively targets angiopoietin‐1/‐2 | PS | 50 mg/m2 | Median 12.4 months | 59.6% PLD/TREB vs 48.6% PLD/Placebo | 10.5% PLD/TREB vs 8.3% PLD/Placebo | |
| Platinum resistant | |||||||||||||
| Other conventional chemotherapy | |||||||||||||
| Mutch 2007 | NCT00191607 | Yes | 195 | Phase III open‐label multicentre RCT | Gemcitabine (1000 mg/m²) day 1 and 8, every 3 weeks | PLD every 4 weeks | As above | PR | 50 mg/m2 | 29.2 months | Data not available | Data not available | Kaplan‐Meier curves shown in paper but without numbers at each time point. Authors contacted for further details. |
| ASSIST‐5 | NCT00350948 | Yes | 125 | Phase III, multicentre RCT, open‐label | Canfosfamide 1000 mg/m² AND PLD every 4 weeks |
PLD every 4 weeks | As above | PR | 50 mg/m2 | The median PFS was 5.6 months for canfosfamide + PLD (n = 65) versus 3.7 months for PLD (n = 60) (hazards ratio, 0.92; P = 0.7243) | Study terminated | Study terminated | |
| ASSIST‐3 | NCT00102973 | Yes | 247 | Phase III multicentre RCT | Canfosfamide (750 mg/m²) AND Carboplatin (AUC 5) every 4 weeks |
PLD every 4 weeks | Glutathione analogue. Activated by glutathione S‐transferase P1‐1, inhibiting cancer cell proliferation and driving apoptosis. | PR | 50 mg/m² | NR | NR | NR | |
| Colombo 2012 | NCT00262990 | Yes | 829 | Phase III open‐label RCT | Patupilone (10 mg/m²) every 3 weeks | PLD every 4 weeks | Impairs cellular division and causes cytotoxicity by inhibiting microtubule formation. | PR | 50 mg/m2 | 27 months | 21.1% PAT vs 18% PLD | 13.8% PAT vs 12.3% PLD | |
| CORAIL | NCT02421588 | No | 442 (10 did not receive study treatment) |
Phase III multicentre, open‐label RCT | Lurbinectedin (3.2 mg/m2) every 3 weeks | PLD every 4 weeks OR Topotecan 1.50 mg/m2 days 1 to 5, every 3 weeks |
Prevents DNA transcription and also influences the tumour microenvironment to prevent cancer growth. | PR | 40mg/m2 | 30 months | Not available for PLD alone (18.6% LUR vs 17.2% PLD/TOP) |
Not available for PLD alone (18.6% LUR vs 17.6% PLD/TOP) |
|
| Targeted therapy | |||||||||||||
| APPROVE | NCT04348032 | No | 152 | Phase II, multicentre RCT; open‐label | Apatinib 250 mg orally once daily AND PLD every 4 weeks |
PLD every 4 weeks | Selectively inhibits VEGFR‐2 | PR | 40 mg/m2 | Median 8.1 months | NR | NR | |
| Banerjee 2018 | NCT01991210 | No | 95 | Phase II multi‐centre RCT; open‐label | Lifastuzumab vedotin (2.4 mg/kg) every 3 weeks. Dose modification if BMI ≥ 35 kg/m2 |
PLD every 4 weeks | Targeted chemotherapy (monomethyl auristatin E); antibody drug conjugate. Inhibits cellular division through prevention of tubulin polymerisation. | PR | 40 mg/m2 | Median 6.6 months | 19.6% PLD vs 14.9% LIFA |
NR | |
| McGuire 2018 | NCT00913835 | No | 125 randomised (123 treated) | Phase II multi‐centre RCT | Olaratumab (20 mg/kg) every 2 weeks AND PLD every 4 weeks |
PLD every 4 weeks | Monoclonal antibody PDGFRα inhibitor, inhibiting cell growth | PR | 40 mg/m² | NR | 35.5% OLA/PLD vs 34.4% PLD | 31.5% OLA/PLD vs 42.9% PLD | |
| PRECEDENT | NCT00722592 | Yes | 149 | Phase II multi‐centre RCT | Vintafolide (2.5 mg IV three times per week during weeks 1 and 3) AND PLD every 4 weeks |
PLD every 4 weeks | Small molecule drug conjugate. Folic acid‐desacetylvinblastine conjugate, binding to the folate receptor. Subsequently, microtubule dysfunction and cellular death occurs. | PR | 50 mg/m2 | 18 months | 44% Vintafolide + PLD v 32.7% PLD alone | NR | |
| PROCEED 2014 | NCT01170650 | No (A.C.) | 321 | Phase III multi‐centre RCT | Vintafolide (2.5 mg IV three times per week during weeks 1 and 3) AND PLD every 4 weeks |
PLD every 4 weeks | Small molecule drug conjugate. Folic acid‐desacetylvinblastine conjugate, binding to the folate receptor. Subsequently, microtubule dysfunction and cellular death occurs. | PR | 50 mg/m2 | 2.8 months | NR | NR | |
| Immunotherapy | |||||||||||||
| JAVELIN Ovarian 200 | NCT02580058 | No | 566 | Phase III, multicentre RCT; open‐label | Arm 1 (188): Avelumab (10 mg/kg) every 2 weeks | Arm 3 (190): PLD every 4 weeks. | PD‐L1 inhibitor, reversing immune‐evasion and inducing T‐cell‐induced cancer cell death. | PR | 40 mg/m2 | 30 months | NR | NR | |
| JAVELIN Ovarian 200 | NCT02580058 | No | 566 | Phase III, multicentre RCT; open‐label | Arm 2 (188): Avelumab 10 mg/kg every 2 weeks AND PLD every 4 weeks |
Arm 3 (190): PLD every 4 weeks. | As above | PR | 40 mg/m2 | 30 months | NR | NR | |
| Platinum resistant and sensitive | |||||||||||||
| Other conventional chemotherapy | |||||||||||||
| MITO‐3 | Yes | 153 | Phase III multicentre RCT | Gemcitabine (1000 mg/m²) days 1, 5, 8, and 15, every 4 weeks | PLD every 4 weeks | As above | PR and PPS | 40 mg/m2 | 39 weeks | NR (reported TTP) | At 24 weeks GEM 81.0% vs PLD 71.4% |
||
| Gordon 2001 | Yes | 481 | Phase III multicentre open‐label RCT | Topotecan (1.5 mg/m²) every 3 weeks | PLD every 4 weeks | Topoisomerase I inhibitor, required for transcription, replication, mitosis. Resultant impaired cell division. | PR and PS | 50 mg/m² | Requested from authors | Requested from authors | Requested from authors | ||
| NCT00653952 | NCT00653952 | No | 216 (220 recruited according to 2002 abstract) | Phase III open‐label RCT | Paclitaxel 175 mg/m2 every 3 weeks | PLD 50 mg/m2 every 4 weeks | As above | PS and PR | 50 mg/m2 | Minimum of 12 months | NR | NR | "The study was closed to new subjects in 1999, because of poor accrual after paclitaxel was approved for use in combination with platinum‐based therapy for the first‐line treatment of ovarian cancer by the European Agency for the Evaluation of Medicinal Products." |
| NCT01840943 | NCT01840943 | No | 32 (planned recruitment 120) | Phase III multicentre RCT (methodology unclear) | Topotecan (1.25 mg/m2) days 1 and 5, every 4 weeks | PLD every 4 weeks | As above | PR and PS | 50 mg/m² | NR | PLD 42.9% vs topotecan 16.7% | Data not available | 8/32 lost to follow up; 11/32 withdrew consent. Data not included in meta‐analysis due to high RoB. |
| OVA‐301 | Yes | 672 | Phase III multi‐centre RCT; open‐label | Trabectedin 1.1 mg/m2 every 3 weeks AND PLD |
PLD every 4 weeks | As above | PR and PS | 30 mg/m2 in combined arm. 50 mg/m2 in alone arm |
Median 17 months | 36.9% TBD/PLD vs 29.3% PLD | NR | ||
| Targeted therapy | |||||||||||||
| Kaye 2012 | NCT00628251 | Yes | 97 | Phase II open‐label multicentre RCT | Olaparib 200 mg twice daily continuously (32 women) OR Olaparib 400 mg twice daily continuously (32 women) |
PLD every 4 weeks | Polyadenosine diphosphate–ribose polymerase (PARP) inhibitor, resulting in impaired DNA damage repair. | PR and PPS | 50 mg/m2 | NR | 46.9% olaparib vs 45.5% PLD | NR | |
| M200 | NCT00635193 | Yes | 127 | Multicentre open‐label RCT | Volociximab M200 (15 mg/kg) every week OR M200 (15 mg/kg) every 2 weeks AND PLD every 4 weeks |
PLD every 4 weeks | Angiogenesis inhibitor; anti‐integrin antibody targeting α5β1. Resultantly induces endothelial cell apoptosis | PR and PS | 40 mg/m² | NR | NR | NR | |
| Immunotherapy | |||||||||||||
| Monk 2017 | NCT01666444 | No | 297 | Phase II multi‐centre RCT; double‐blind | Motolimod (VTX‐2337) AND PLD |
PLD AND placebo |
Motolimod, a TLR 8 inhibitor, reversing immune‐evasion and inducing T‐cell‐induced cancer cell death. | PR and PS | 40 mg/m2 | NR | NR | NR | |
Abbreviations: AUC = area under the curve; BEV = bevacizumab; CAN = canfosfamide; carbo = carboplatin; GEM = gemcitabine; HR = hazard ratio; LIFA = lifastuzumab vedotin; LUR = lurbinectedin; MOT = motolimod (VTX‐2337); NA = not available; NR = not recorded; OLA = olaparib; OMab = olaratumab; OS = overall survival; PAC = paclitaxel; PARP = poly adenosine diphosphate–ribose polymerase; PAT = patupilone; PD‐L1 = programmed cell death ligand 1; PFI = platinum‐free interval; PFS= progression‐free survival; PLD = pegylated liposomal doxorubicin; PPS = partially platinum‐sensitive (recurrence of 7 to 12 months of platinum‐based therapy); PR = platinum‐resistant (recurrence within 6 months of platinum‐based therapy); PRef = platinum‐refractory (recurrence within 1 month of, or during, platinum‐based therapy); PS = platinum‐sensitive (recurrence > 12 months after platinum‐based therapy); RCT = randomised control trial; RR = relative risk; TBD = trabectedin; TLR = toll‐like receptor; TOP = topotecan; TTD = time to death; TTP = time to progression; VEGF = vascular endothelial growth factor; VEGFR‐2 = vascular endothelial growth factor receptor‐2
3. Platinum sensitivity status and median survival times in participants of included studies.
| Platinum‐resistant data (PFI ≤6 months) | |||||||||
| STUDY NAME | Other drug arm | PLD arm | N (other drug) | N (PLD) | Median PFS for other arm in weeks | Median PFS for PLD arm in weeks | Median OS for other arm in weeks | Median OS for PLD arm in weeks | Comment |
| Colombo 2012 | PAT | PLD | 412 | 416 | 16 | 16 | 57 | 54 | 17% of these women had non‐measurable disease. |
| Mutch 2007 | GEM | PLD | 99 | 96 | 15 | 13 | 54 | 58 | 36% of these women had non‐measurable disease. |
| Gordon 2001 | TOP | PLD | 125 | 130 | 14 | 9 | 41 | 36 | It is unclear why survival in the PLD arm of this PR subgroup is so much shorter than that of the other trials. |
| ASSIST‐3 | CAN/carbo | PLD | NA | NA | 15 | 15 | NA | NA | Limited available data. Additional data were requested from Telik but not obtained. |
| Kaye 2012 | OLA | PLD | 16 | 14 | NA | NA | NA | NA | Small study, subgroup data not available. |
| McGuire 2018 | PLD/OMab | PLD | 62 | 61 | 17 | 16 | 66 | 65 | PFS data also provided by PR and platinum‐refractory, but small numbers |
| MITO‐3 | GEM | PLD | 43 | 43 | NA | NA | NA | NA | Subgroup data not available. |
| PRECEDENT | EC145/PLD | PLD | 100 | 49 | 21 | 12 | 60 | 72 | Unpublished OS data. Study was not adequately powered to assess OS. |
| OVA‐301 | TBD/PLD | PLD | 118 | 124 | 17 | 16 | 61 | 53 | Subgroup analysis was pre‐planned for PFS but was exploratory for OS. |
| ASSIST‐5 | CAN/PLD | PLD | 65 | 60 | 24 | 16 | NA | NA | Pre‐planned subgroup analysis favoured the CAN/PLD group for PFS. Final OS results were not published. Additional data were requested from Telik but not obtained. |
| Partially platinum‐sensitive data (PFI 6 to 12 months) | |||||||||
| CALYPSO | PAC/carbo | PLD/carbo | 183 | 161 | 38 | 40 | NA | NA | PFS HR = 0.73 (95% CI 0.58 to 0.90, P = 0.004) from Gladieff 2012; OS HR = 1.01 (0.80 to 1.28) from Wagner 2012. |
| OVA‐301 | TBD/PLD | PLD | 123 | 90 | 32 | 24 | 96 | 71 | TTP data from Poveda 2011 and exploratory TTD data from Monk 2012. PFS HR = 0.65 (95% CI 0.45 to 0.92; P = 0.015); OS HR = 0.64 (95% CI 0.47 to 0.86; P = 0.0027). |
| Platinum‐sensitive data (PFI > 6months) | |||||||||
| Gordon 2001 | TOP | PLD | 111 | 109 | 23 | 29 | 70 | 108 | Exploratory analysis. The greatest effect was seen in the PPS subgroup (N = 112; HR = 1.58, 95% CI 1.07 to 2.34; P = 0.021). |
| OVA‐301 | TBD/PLD | PLD | 215 | 202 | 39 | 32 | 116 | 103 | Subgroup analysis was pre‐planned for PFS but was exploratory for OS. |
| SWOG S0200 | Carbo | PLD/carbo | 30 | 31 | 34 | 51 | 77 | 133 | Small study which closed early. |
| HeCOG 2010 | PAC/carbo | PLD/carbo | 96 | 93 | 46 | 51 | 126 | 106 | ‐ |
| CALYPSO | PAC/carbo | PLD/carbo | 509 | 466 | 40 | 48 | 141 | 132 | ‐ |
| Platinum‐resistant and platinum‐sensitive data combined | |||||||||
| MITO‐3 | GEM | PLD | 76 | 77 | 20 | 16 | 51 | 56 | PR + PPS |
| Kaye 2012 | OLA | PLD | 32 | 33 | 38 | 30 | NA | 76 | PR + PPS. Unpublished TTD data obtained from investigators. Phase II study not powered to assess survival. |
| Monk 2017 | MOT/PLD | PLD | 148 | 149 | 20.6 | 22.3 | 77.6 | 81 | PR + PPS |
| NCT00653952 | PAC | PLD | 108 | 108 | NA | NA | 56.3 | 46.4 | PR + PS |
| Gordon 2001 | TOP | PLD | 235 | 239 | 17 | 16.1 | 60 | 63 | PR + PS |
| OVA‐301 | TBD/PLD | PLD | 337 | 335 | 31 | 25 | 95 | 81 | PR + PS |
Conversions from published data (months to weeks) were performed assuming one month to be 4.3 weeks, and then rounding the answer to the nearest week.
*This is from the comparison CAN versus active control (PLD and TOP data combined). The PLD group had an improved PFS compared with the TOP group, but we were unable to obtain separate data.
Abbreviations: CAN = canfosfamide; carbo = carboplatin; GEM = gemcitabine; HR = hazard ratio; MOT = motolimod (VTX‐2337); NA = not available; OLA = olaparib; OMab = olaratumab; OS = overall survival; PAC = paclitaxel; PAT = patupilone; PFI = platinum‐free interval; PFS= progression‐free survival; PLD = pegylated liposomal doxorubicin; PPS = partially platinum‐sensitive (recurrence of 7 to 12 months of platinum‐based therapy); PR = platinum‐resistant (recurrence within 6 months of platinum‐based therapy); PRef = platinum‐refractory (recurrence within 1 month of, or during, platinum‐based therapy); PS = platinum‐sensitive (recurrence >12 months after platinum‐based therapy); TBD = trabectedin; TOP = topotecan; TTD = time to death; TTP = time to progression
Overall, we included 26 studies with a total of 8277 participants (APPROVE; ASSIST‐3; ASSIST‐5; Banerjee 2018; CALYPSO; Colombo 2012; CORAIL; Fujiwara 2019; Gordon 2001; HeCOG 2010; JAVELIN Ovarian 200; Kaye 2012; M200; McGuire 2018; MITO‐3; Monk 2017; Monk 2020; Mutch 2007; NCT00653952; NCT01840943; OVA‐301; Pfisterer 2020; PRECEDENT; PROCEED 2014; SWOG S0200; TRINOVA‐2).
Several studies were included in the previous version of the review as ongoing studies (McGuire 2018; Monk 2017; PROCEED 2014; TRINOVA‐2), or included studies without data (NCT00653952, formerly O'Byrne 2002 in the previous version of the review), and are now included.
Platinum‐sensitive EOC
We included seven studies (2872 participants; ITT efficacy data reported for 2807) evaluating the effect of PLD in women with platinum‐sensitive recurrent epithelial ovarian cancer. These were grouped by the type of comparison treatment; conventional chemotherapy or targeted therapy (CALYPSO; Fujiwara 2019; HeCOG 2010; Monk 2020; Pfisterer 2020; SWOG S0200; TRINOVA‐2).
PLD with conventional chemotherapy compared to other combination chemotherapy
SWOG S0200 is a phase III multicentre open‐label RCT comparing carboplatin with PLD 30 mg/m2 against PLD alone. It was terminated early due to poor accrual after 61 women were recruited, slowed by the publication of the ICON‐4 study showing the benefit of adding paclitaxel to carboplatin. Unpublished survival data were shared by authors. PFS was significantly improved by the addition of PLD to carboplatin. The final OS was not statistically significantly different between treatment arms.
CALYPSO is a phase III multicentre non‐inferiority RCT comparing carboplatin with PLD 30 mg/m2 against carboplatin with paclitaxel. The study included 976 women. All participants had platinum sensitive disease and had previously received taxane therapy. It was standard for corticosteroids and anti‐emetics to be given as pre‐medication with the addition of clemastine and ranitidine in the carboplatin‐paclitaxel arm. Significantly more women in the carboplatin‐paclitaxel arm discontinued treatment before six cycles had been completed (110/507 versus 70/466), mainly due to toxicity (73/507 women versus 27/466 women; P < 0.001).
HeCOG 2010 is a phase II randomised control trial including 189 women with platinum sensitive disease. Participants were randomised to carboplatin and PLD 45 mg/m2 or carboplatin and paclitaxel. Most participants had received prior taxane therapy (88% in the carboplatin and paclitaxel group; 93% in the carboplatin and pegylated liposomal doxorubicin). Both groups received dexamethasone, dyphenhydramine and ranitidine pre‐medication ‐ this was given via both IV and oral routes to paclitaxel recipients.
Fujiwara 2019 conducted a phase II multicentre open‐label RCT. This recruited 100 women in Japan to either carboplatin and PLD 30mg/m2 or carboplatin and gemcitabine (1000 mg/m2 days 1 and 8). The primary outcome was PFS with ORR, OS, toxicity and dose administration as secondary outcomes. There were no obvious differences in toxicity, but PLD had a more favourable risk‐benefit profile. The PLD arm required fewer dose reductions (relative dose intensity 88.9% versus 53.1%) and had a higher six‐cycle completion rate (63.3% versus 31.4%), the most common reason being neutropenia or thrombocytopenia in the gemcitabine arm.
Pfisterer 2020 was an international open‐label phase III multicentre RCT that recruited 682 participants with platinum‐sensitive recurrent EOC (stratified to recurrence six to 12 months or > 12 months post platinum) to receive bevacizumab and carboplatin with either PLD 30 mg/m2 or gemcitabine. After six cycles of treatment, both arms then received maintenance bevacizumab. Nearly half of participants had previously received anti‐angiogenic treatment (47.7% in the carboplatin/gemcitabine/bevacizumab group versus 47.2% in the carboplatin/PLD/bevacizumab). The primary outcome was PFS by Response Evaluation Criteria in Solid Tumors (RECIST), and secondary outcomes PFS by serum cancer antigen 125 (CA‐125), OS and QoL (EORCT QLQ‐C30 or ovarian cancer‐specific module (QLQ‐OV28)).
PLD with conventional chemotherapy compared to PLD alone
Monk 2020 was an international, multicentre open‐label phase III RCT, in which 576 participants were assigned to either PLD 30 mg/m2 and trabectedin or PLD alone 50 mg/m2. The primary outcome was OS and secondary outcomes were PFS and ORR. Participants were stratified by time from platinum to recurrence (six to 12 months, 12 to 24 months or > 24 months). Eighty per cent of participants had received a previous taxane.
PLD with targeted therapy compared to PLD alone
TRINOVA‐2 was a randomised phase III double‐blind, placebo‐controlled trial that allocated 223 participants to PLD 50 mg/m2 with trebananib or with placebo. Stratification was by platinum free interval (0 to 6 months or 6 to 12 months). Owing to PLD shortages, enrolment was paused and the study then terminated.
Platinum‐resistant EOC
Eleven studies (3246 participants; ITT efficacy data reported for 3234) evaluated the effect of PLD in participants with platinum‐resistant recurrent EOC. These were grouped by the type of comparison treatment; conventional chemotherapy (ASSIST‐3; ASSIST‐5; Colombo 2012; CORAIL; Mutch 2007), targeted therapy (APPROVE; Banerjee 2018; McGuire 2018; PRECEDENT; PROCEED 2014) or immunotherapy (JAVELIN Ovarian 200). JAVELIN Ovarian 200 had two arms, and so was included in the comparison of immunotherapy versus PLD and of PLD with immunotherapy versus PLD alone.
Conventional chemotherapy alone compared to PLD alone
Mutch 2007 was a phase III open‐label multicentre RCT, in which 195 women with platinum‐resistant disease that had reoccurred within six months were assigned to either gemcitabine (n = 99) or PLD 50 mg/m2 (n = 96). If participants experienced disease progression or unacceptable toxicity, or if cumulative PLD dose exceeded 500 mg/m², they could cross over to the alternative drug. Cross‐over therapy was administered to 130 participants, with 66 participants receiving gemcitabine and 64 participants receiving PLD. In the gemcitabine group, 60.6% had received one prior platinum regimen and 39.4% had received two prior regimens; in the PLD group, 67.7% had received one prior platinum regimen and 32.3% had received two prior regimens. Quality of life was assessed by the Functional Assessment of Cancer Therapy–Ovarian (FACT‐O) questionnaire.
ASSIST‐3 was a phase III multicentre RCT. The available conference abstract reported that 247 participants, with disease recurrence within six months, were randomised to either canfosfomide with carboplatin or PLD 50 mg/m2. The methods of randomisation and allocation were not described, and full results were not available.
Colombo 2012 was a phase III open‐label international multicentre RCT that recruited 829 women with platinum resistant disease. They were assigned to patupilone or PLD 50 mg/m2. The primary outcome was OS and secondary outcomes were PFS, overall response rate (ORR) and serious adverse events (SAE). Progression was determined by a blinded, central review of results. Quality of life was measured using the FACT‐O questionnaire.
CORAIL was a randomised phase III study of lurbinectedin versus PLD 50 mg/m2 or topotecan in people with platinum‐resistant disease. 442 participants were stratified by platinum free interval (one to three months, over three months). The primary end point was PFS, determined by independent review committee. Data for PLD alone were included in the supplementary material.
PLD with conventional chemotherapy compared to PLD alone
ASSIST‐5 was a phase III multicentre RCT in which 125 participants with platinum‐resistant disease were allocated to canfosfamide with PLD 50 mg/m2 or PLD 50 mg/m2 alone. The study was paused whilst the results of ASSIST‐1 were reviewed and then terminated, meaning the planned enrolment of 244 participants was not reached. The PFS was performed on interim analysis data, and OS was not reported.
Targeted therapy alone compared to PLD alone
Banerjee 2018 was an international, multicentre, randomised, open‐label, phase II study that randomised 95 participants to lifastuzumab or PLD 40 mg/m2. Lifastuzumab is an antibody–drug conjugate comprising humanised IgG1 anti‐NaPi2b monoclonal antibody and an antimitotic agent which blocks the polymerisation of tubulin. The drug targets the sodium‐dependent phosphate transporter NaPi2b. QoL was measured by the two‐item global health status/quality of life (GHS/QOL) questionnaire.
PLD with targeted therapy compared to PLD alone
APPROVE was a multicentre open‐label phase II trial study evaluating the effect of adding apatinib to PLD 40 mg/m2. Apatinib is a tyrosine kinase inhibitor that selectively inhibits vascular endothelial growth factor (VEGF) receptor 2 administered orally. The trial randomised 152 women with platinum‐resistant disease. All participants had previously received a taxane, and 3.8% of participants in the apatinib arm had received prior antiangiogenic therapy versus 0% in the PLD alone arm.
McGuire 2018 was a multicentre open‐label phase II trial study evaluating the effect of adding olaratumab, an antiplatelet derived growth factor receptor alpha (PDGFR‐alpha) antibody to PLD 40 mg/m2. The trial randomised 123 women with platinum‐resistant recurrent EOC were randomised to receive PLD with or without olaratumab. PDGFRα activity has pro‐angiogenic activity and modulates the tumour or stromal microenvironment to promote metastases, hence blocking this pathway might slow disease progression.
PROCEED 2014 was a randomised double‐blind phase III trial comparing PLD 50 mg/m2 with vintafolide against PLD alone in the treatment of platinum resistant disease. Following ther review of interim data at the first scheduled futility analysis, the study was temporarily halted and then permanently stopped. The trial randomised 321 participants. Overall survival data were not included in the final analysis and the median follow‐up duration was only 2.8 months.
PRECEDENT compared vintafolide and PLD 50 mg/m2 in the treatment of platinum resistant disease. This phase II open‐label multicentre RCT randomised 162 women at a 2:1 ratio to PLD with or without vintafolide. Participants were categorised according to folate receptor status (100% positive, 10% to 90% or 0% folate receptor (FR)‐positive lesions) and a threshold analysis performed based on these results. Progression was assessed by blinded independent review.
Immunotherapy alone compared to PLD alone
JAVELIN Ovarian 200 was a multicentre international phase III open‐label study that randomised 566 participants 1:1:1 to receive avelumab, avelumab and PLD or PLD alone (40 mg/m2). We included the two independent comparisons within this study in separate comparison groups of this review.
PLD with immunotherapy compared to PLD alone
One of the comparison arms of JAVELIN Ovarian 200 compared PLD in combination with avelumab versus PLD alone. Participants were randomised 1:1:1 to receive avelumab (188), avelumab and PLD (188) or PLD alone (190).
Platinum‐resistant and platinum‐sensitive recurrent EOC
We included eight studies with 2079 participants (ITT efficacy data reported for 2075) with both platinum sensitive and resistant disease (Gordon 2001; Kaye 2012; M200; MITO‐3; Monk 2017; NCT00653952; NCT01840943; OVA‐301). Four studies compared the use of PLD alone with a single‐agent conventional chemotherapy (Gordon 2001; MITO‐3; NCT00653952; NCT01840943). One study compared PLD alone with a single targeted therapy (Kaye 2012). OVA‐301 compared PLD in combination with the chemotherapy agent trabectedin with PLD alone, whereas M200 and Monk 2017 compared PLD in combination with volociximab and motolimod, respectively, with PLD alone and PLD with conventional chemotherapy compared to PLD alone in recurrent platinum‐resistant or platinum‐sensitive EOC.
OVA‐301 randomised 572 participants 1:1 to receive either trabectedin with PLD 30 mg/m2 or PLD 50mg/m2 alone. Response was assessed by blinded, independent reviewers. The trial assessed QoL assessment using the e QLQ‐C30 questionnaire. 42% of participants in the trabectedin arm required granulocyte colony‐stimulating factor (G‐CSF) compared with 17% for PLD alone. Women in the trabectedin arm were given an anti‐emetic premedication which was not routinely administered for PLD alone.
Conventional chemotherapy alone compared to PLD alone
Gordon 2001 randomised 481 participants to receive either topotecan or PLD 50 mg/m2. QLQ‐C30 was used to assess quality of life. Those receiving PLD were significantly less likely to experience dose delays.
MITO‐3 was a phase III randomised multicentre trial comparing gemcitabine (n = 77) to PLD 40 mg/m2 (n = 76) Recurrence of EOC had occurred within six months of prior platinum therapy for 43 participants in each arm and between seven and 12 months for 34 and 33 participants in the gemcitabine and PLD arms, respectively. Methyprednisolone IV premedication was given to those receiving PLD. The trial measured QoL using the QoL‐C30 questionnaire. G‐CSF was required in 14% of participants receiving gemcitabine and 5% receiving PLD.
NCT00653952 was a phase III open‐label randomised study comparing PLD and paclitaxel in participants with first relapse after chemotherapy with a platinum‐based regimen, stratified by platinum‐sensitivity. Participants were randomised to receive either PLD 50 mg/m2 every 28 days or paclitaxel 175 mg/m2 every 21 days. Participants were to have been treated for up to one year. The study was halted to new recruits in 1999 due to low accrual after 50% of planned participants were recruited (216 participants), after paclitaxel was approved for use in combination with platinum‐based therapy for the first‐line treatment of ovarian cancer by the European Agency for the Evaluation of Medicinal Products. Reported outcomes were therefore limited to OS and adverse event forms received prior to the database lock on 8 January 2004, and are limited to a clinical study report.
NCT01840943 was a phase III open‐label randomised comparative bridging study. The study aimed to recruit 120 participants with both platinum resistant and sensitive disease to receive either topotecan or PLD 50 mg/m2. The trial recruited 32 participants and of these, 26 were randomised; 25 participants did not complete the study: 11 participants withdrew, eight were lost to follow up and six did not complete due to the physician's decision. The study was terminated due to a medication supply issue. The study has been included in our review but has not been included in the data analysis due to high risk of bias.
Target therapy alone compared to PLD alone
Kaye 2012 was a phase II open‐label multicentre RCT in which 97 participants with BRCA1 or BRCA2 mutations were randomised 1:1:1 to receive olaparib 200 mg bd, 400 mg bd or PLD 50 mg/m2. The primary outcome was reported for the olaparib arms combined and individually, versus the PLD arm. All participants were within 12 months of previous platinum therapy, 56.3%, 50.0% and 42.4%, respectively, had platinum resistant disease.
PLD with target therapy compared to PLD alone
M200 was an open‐label study that recruited participants to receive PLD 40mg/m2 alone, PLD with the anti‐angiogenic integrin inhibitor, volociximab (PLD + V), or volociximab alone (V). The trial randomised 127 participants with platinum‐sensitive or resistant disease to the arms PLD (n = 66), PLD + V (n = 34) and V (n = 27). Only a conference abstract was available.
PLD with immunotherapy compared to PLD alone
Monk 2017 conducted a phase 2, placebo‐controlled trial of PLD (40 mg/m2) with the toll‐like receptor 8 agonist, motolimod, or with placebo. The trial randomly assigned 297 participants 1:1. Participants were stratified by platinum free interval (< 6 months or 6 to 12 months); 51.2% had platinum resistant disease.
Details of study funding and potential conflicts of interest are outlined in Characteristics of included studies and summarised in Other potential sources of bias.
Excluded studies
We excluded 38 studies with reasons, as per Cochrane guidelines (Section 4.6.5; Lefebvre 2022), which are detailed in the Characteristics of excluded studies table.
Review articles: A'hern 1995 review of addition of doxorubicin to chemotherapy regimes; Bookman 2002 review of incorporation of new cytotoxic agents; Graybill 2014 review of vintafolide.
Ineligible study design: not an RCT (Aracil 2013; Basu 2013; Kavanagh 2004; Khokhlova 2012; NCT03639246 2018); prospective study (Basu 2011); exploratory analysis (Colombo 2014a); prediction model (Cherchi 2003; Herzog 2014; Palaia 2006; PiSARRO 2016; Scarfone 2006; Trillsch 2016; Wydra 2014).
Ineligible population as they evaluated PLD for first‐line drug treatment of EOC (GOG0182/ICON 5; MITO‐2 2011) or as maintenance treatment after successful first‐line treatment (AGOG06‐001). MILO ENGOT‐ov11 was a study in low‐grade serous ovarian carcinoma, which we excluded a priori in this update.
Ineligible intervention: Bakrin 2018 intraperitoneal PLD‐based chemotherapy versus intravenous chemotherapy; Bhowmik 2018 branded PLD versus generic PLD; Colombo 2014 PLD in both arms; Lai 2018 PLD and carboplatin versus no treatment; INOVATYON PLD and carboplatin versus PLD and trabectidin; Marme 2019 PLD both arms with atezolizumab or placebo, NCT02891824 2016 PLD or other physician's choice chemotherapy with carboplatin and either atezolizumab or placebo; Monk 2016 and NCT02641639 2015 PLD and bevacizumab both arms with CA4P or placebo; NCT03632798 2018 and NCT03699449 2018 multiple‐arm comparison of durvalumab with other agents; NCT03949283 2019 treatment according to cancer stem cell assay; Shoji 2018 chemotherapy with or without bevacizumab; Yabuno 2019 PLD dose comparison; Lorusso 2014 doxorubicin not PLD; Nagao 2016 low‐dose paclitaxel added to other chemotherapy.
Ineligible comparison: Lindemann 2017 used chemotherapy versus hormonal treatment.
For further details of these excluded studies, see Characteristics of excluded studies.
Studies Awaiting Classification
Thirteen studies are awaiting classification (ASSIST‐1 2009; AURELIA 2012; FORWARD I; HECTOR; MITO‐16 MANGO OV2b; MITO‐23; MITO‐8 2017; MORAb‐003; NCT03690739; Oza 2019; PROVE 2011; SOLO3; Volasertib Trial). For details of these studies see Table 7. Some of these were previously in Ongoing studies and have now had data published in abstract form at least (MITO‐23; MITO‐8 2017; Oza 2019).
4. Studies awaiting classification by comparison group.
| Study Name | Alternative name/ trial registry number | 2016 Review | Number of participants | Study design | Experimental treatment | Control treatment | Mechanism of action | Platinum sensitivity | Dose of PLD | Duration of FU | 6‐month PFS rate | 2‐year OS rate | Notes |
| Platinum sensitive | |||||||||||||
| Other conventional chemotherapy | |||||||||||||
| MITO‐16 MANGO OV2b | NCT01802749 | No | 406 | Phase III open‐label multicentre RCT | Carboplatin AND Paclitaxel OR Gemcitabine OR PLD |
Bevacizumab AND Carboplatin AND Paclitaxel OR Gemcitabine OR PLD |
B: angiogenesis inhibitor; selectively targets VEGF C: alkylating agent. Forms platinum complexes, causing inter‐ and intra‐strand DNA cross‐linkage. Resultant alteration to DNA structure, inhibiting synthesis. P: impairs cellular division and causes cytotoxicity by inhibiting microtubule formation. G: a nucleoside analogue that interferes with DNA synthesis. S‐phase specific. |
PS | 30 mg/m2 | NR (abstract only accessible) | NR (abstract only accessible) | NR (abstract only accessible) | |
| MITO‐8 2017 | NCT657878 | Yes | 215 | Phase III multicentre RCT; open‐label | Carboplatin AND Paclitaxel THEN PLD |
PLD
THEN Carboplatin AND Paclitaxel |
As above | PPS | 40 mg/m2 | NR | NR at 6 months | 41.7% PBC then NPBC versus 31.8% NPBC versus PBC | Amendment made during study to include topotecan, gemcitabine, carboplatin/gemcitabine or any other drug approved in this setting as NPBC. |
| MITO‐23 | NCT02903004 | No | 242 | Phase III multicentre RCT; open‐label | Trabectedin (1.3 mg/m2) | Chemotherapy of physician's choice (PLD or topotecan or gemcitabine or weekly paclitaxel or carboplatin) | T: inhibition of transcription factor‐DNA binding. Also binds and alkylates DNA. | PS | 40 mg/m2 | NR (time frame for study: 4 years) | NR (abstract only reported) | NR (abstract only reported) | National Cancer Institute Common Toxicity Criteria (NCI‐CTC) version 4.0 |
| HECTOR | NCT00170677 | No | 550 | Phase III multicentre RCT | Topotecan (0.75 mg/m2) AND carboplatin every 3 weeks |
Carboplatin AND Paclitaxel OR Gemcitabine OR PLD |
T: topoisomerase I inhibitor, required for transcription, replication, mitosis. Resultant impaired cell division. | PS | 30 mg/m2 | Median 20 months | NR | NR | |
| Targeted therapy | |||||||||||||
| MORAb‐003 | NCT02289950 | No | 211 | Randomised phase II placebo control trial | Farletuzumab (weekly 10 mg/kg first 2 weeks followed by 5 mg/kg) OR placebo |
Carboplatin AND Paclitaxel OR Carboplatin OR PLD |
Humanised monoclonal antibody that minds to the human folate receptor alpha | PS | 30 mg/m2 | NR | NR | NR | |
| PROVE 2011 | NCT01388621 | No | 96 | Open‐label randomised phase II trial | Panitumumab 6 mg/kg day 1 and day 15, every 3 or 4 weeks | Investigators Choice of Carboplatin AND gemcitabine, 3 weekly OR carboplatin (AUC5) AND PLD (40 mg/m²) 4 weekly |
Human monoclonal antibody targeting the epidermal growth factor receptor | PS | 40 mg/m² | NR | NR | NR | |
| Platinum resistant | |||||||||||||
| Other conventional chemotherapy | |||||||||||||
| Oza 2019 | NCT01696032 | No | 103 | Multicentre, randomised, open‐label phase II trial | Guadicitabine (30mg/m2) AND Carboplatin AUC 4 |
Topotecan OR PLD OR Paclitaxel OR Gemcitabine |
Decitabine prodrug, inducing non‐specific genome‐wide hypomethylation, inducing cell cycle S‐phase arrest | PR | 40 to 50 mg/m2 | NR | NR | NR | |
| AURELIA 2012 | NCT00976911 | No | 361 | Phase II, mutlicentre, open‐label, RCT | Bevacizumab (10mg/kg) | Paclitaxel OR PLD OR Topotecan |
As above | PR | 40 mg/m2 | 13.9 months chemo arm/13.0 months in bevacizumab arm | 20.3% in chemo arm versus 49.2% bevacizumab arm | 15.9% in chemo arm versus 41.9% in bevacizumab arm | 2.2% gastrointestinal perforation rate in bevacizumab arm |
| ASSIST‐1 2009 | PMID 19515553 | No | 461 | Phase III active control trial | Canfosfamide (1000mg/m2) 3 weekly | PLD OR Topotecan |
Glutathione analog phosphorodiamidate prodrug, activated by GST P1‐1 in cancer cells | PR | 50 mg/m2 | NR | NR | NR | Allocation to PLD or topotecan dependent on previous therapy received |
| Targeted therapy | |||||||||||||
| FORWARD I | NCT02631876 | No | 366 | Multicentre, randomised phase III trial | Mirvetuximab soravtansine (6mg/kg) | Paclitaxel OR PLD OR Topotecan |
Folate receptor alpha specific antibody‐drug conjugate (maytansinoid payload DM4), an anti‐tubulin agent | PR | 40 mg/m2 | Median 12.5 months | 21.8% in mirvetuximab arm versus 22.9% in physician's chemotherapy arm | 11.7% in mirvetuximab arm versus 9.3% in physician's chemotherapy arm | |
| Volasertib Trial | NCT01121406 | No | 110 | Phase II open‐label multicentre RCT | Volastertib | Paclitaxel OR Gemcitabine OR Topotecan OR PLD |
PLK1 (polo‐like kinase 1) inhibitor, inducing mitotcycle arrest and apoptosis, notably in rapidly dividing cancer cells | PR | 40 mg/m2 | NR | 27.8% volasertib versus 38.2% chemotherapy | NR | PFS reported at 24 weeks rather than 6 months |
| Platinum resistant and sensitive | |||||||||||||
| Targeted therapy | |||||||||||||
| SOLO3 | NCT00628251 | No | 266 | Phase II open‐label multicentre RCT | Olaparib 300 mg twice a day | PLD OR Paclitaxel OR Gemcitabine OR Topotecan |
Poly adenosine diphosphate–ribose polymerase (PARP) inhibitor, resulting in impaired DNA damage repair. | PR or PPS | 50 mg/m2 | Median 13.8 months olaparib and 3.9 months chemotherapy | 70.8% olaparib versus 53.4% chemotherapy | NR | |
Abbreviations: AUC = area under the curve; BEV = bevacizumab; CAN = canfosfamide; carbo = carboplatin; CTC = common toxicity criteria; DNA = deoxyribonucleic acid; GEM = gemcitabine; GST P1‐1 = glutathione S‐transferase P1‐1; HR = hazard ratio; LIFA = Lifastuzumab vedotin; LUR = Lurbinectedin; MOT = motolimod (VTX‐2337); NA = not available; NCI = National Cancer Institute; NPBC = non‐platinum‐based chemotherapy: NR = not recorded; OLA = olaparib; OMab = olaratumab; OS = overall survival; PAC = paclitaxel; PARP = poly adenosine diphosphate–ribose polymerase; PBC =platinum‐based chemotherapy; PAT = patupilone; PD‐L1 = programmed cell death ligand 1; PLK1 = polo‐like kinase 1; PFI = platinum‐free interval; PFS= progression‐free survival; PLD = pegylated liposomal doxorubicin; PPS = partially platinum‐sensitive (recurrence of 7 to 12 months of platinum‐based therapy); PR = platinum‐resistant (recurrence within 6 months of platinum‐based therapy); PRef = platinum‐refractory (recurrence within 1 month of, or during, platinum‐based therapy); PS = platinum‐sensitive (recurrence >12 months after platinum‐based therapy); RCT = randomised control trial; RR = relative risk; TBD = trabectedin; TLR = toll‐like receptor; TOP = topotecan; TTD = time to death; TTP = time to progression; VEGF = vascular endothelial growth factor; VEGFR‐2 = vascular endothelial growth factor receptor‐2
In all of these cases, participants in the arm containing PLD received either PLD or an alternative therapeutic agent; many of these studies compared 'chemotherapy of physician's choice (CPC)' to CPC plus another agent. We contacted the authors of each of these studies to request the data of those who received PLD; however, this extracted data has not yet been made available to us for inclusion in this review. As we were not able to separate PLD from other therapies, these studies remain in the awaiting classification section.
ASSIST‐1 2009 randomised participants to either canfosfomide or active control arm. In the active control arm participants received either PLD 50 mg/m2 or topotecan dependent on their previous failed second line therapy.
AURELIA 2012 randomised 361 participants with platinum resistant disease to receive bevacizumab with one of paclitaxel, PLD (40 mg/m2) or topotecan (n = 179) or chemotherapy alone (n = 182). Investigators selected the chemotherapy agent on an individual participant basis: 126 were given PLD, 115 paclitaxel and 120 topotecan.
FORWARD I randomised 366 people with platinum resistant disease and whose tumours were positive for FRa expression 2:1 to receive either mirvetuximab, soravtansine or investigator's choice chemotherapy (paclitaxel (n = 80), PLD 40 mg/m2 (n = 110) or topotecan n = 58)). The primary outcomes was blinded assessment of PFS. QoL was assessed by QLQ‐OV28.
HECTOR was a phase III multicentre randomised control trial in which topotecan and carboplatin were compared with standard platinum‐based chemotherapy in people with platinum‐sensitive recurrent ovarian cancer. 550 participants were randomised 1:1 to receive carboplatin and topotecan or investigator's choice of paclitaxel (n = 79), gemcitabine (n = 191) or PLD (n = 5) with carboplatin.
In MITO‐16 MANGO OV2b, 406 participants with platinum sensitive recurrent ovarian cancer were randomised to receive investigator's choice chemotherapy (PLD 30 mg/m2, gemcitabine or paclitaxel) and carboplatin, gemcitabine with or without bevacizumab. The PFS for this study was reported by chemotherapy backbone.
MITO‐8 2017 was an open‐label, prospective, randomised, superiority trial in which participants with platinum sensitive disease were randomised to receive either the non‐platinum based chemotherapy (NPBC) followed by PBC or the standard reverse treatment sequence. NPBC agents included PLD 40 mg/m2, topotecan and gemcitabine. PBC included carboplatin with paclitaxel and carboplatin with gemcitabine.
Preliminary data from MITO‐23 have been presented as a meeting abstract. Participants were randomised to receive trabectedin 1.3 mg/m2 every 21 days or CPC (including PLD, carboplatin, gemcitabine, weekly paclitaxel, or topotecan).
MORAb‐003 randomised participants with platinum sensitive first relapse and a low CA125 to farletuzumab or placebo in addition to either carboplatin and paclitaxel or carboplatin and PLD.
PROVE 2011 randomised 96 participants to receive panitumumab in addition to carboplatin and gemcitabine and carboplatin and PLD 40 mg/m2. Only a conference abstract is available.
In Oza 2019, participants with platinum resistant disease received either guadecitabine and carboplatin or treatment of choice (topotecan, PLD (40 to 50 mg/m2), paclitaxel, or gemcitabine). Participants were randomised 1:1 to either arm. Results were not available for PLD alone.
SOLO3 was a phase II study of olaparib versus physician's choice single‐agent non‐platinum chemotherapy (PLD 50 mg/m2 n = 47, paclitaxel n = 20, gemcitabine n = 13, or topotecan n = 8) in participants with platinum sensitive disease and a germline BRCA mutation. Objective response rate was reported by individual chemotherapy agent, but no other PLD‐alone data were available.
The Volasertib Trial was a phase II randomised trial of volasertib versus investigator's choice chemotherapy. There were no restrictions on the chemotherapy agent but PLD, topotecan, paclitaxel, and gemcitabine were recommended.
As this review includes studies over a significant time‐frame, different classification systems for adverse events were used. These are detailed in Table 8.
5. Adverse events.
| Study Name | Alternative name/ trial registry number | AE code (CTCAE, MedDRA) | AEs listed or AE number only | Results listed as number of participants with at least one AE, or total number of AEs | Type of AE: are AEs in the TRAEs or all AEs/TEAEs? |
| Platinum sensitive | |||||
| Other conventional chemotherapy | |||||
| SWOG S0200 | NCT00043082 | CTCAE 2.0 | G3 and G4 AEs are split in table | Number of participants experiencing any particular AE, listing the highest grade they experienced (may have had the same AE twice) | Not stated but looks like all AEs |
| CALYPSO | NCT00538603 | CTCAE (version not stated) | G3 and G4 AEs are split in table | Number of participants experiencing any particular AE, listing the highest grade they experienced (may have had the same AE twice) | Not stated but looks like all AEs |
| HeCOG 2010 | ACTRN12609000436279 | Toxicity as per WHO classification | G3 and G4 AEs are split in table | Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time | Not stated but looks like all AEs |
| Fujiwara 2019 | UMIN 000,005,487 | Not stated | G3 and G4 AEs are split in table | Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | Not stated but looks like all AEs |
| Pfisterer 2020 | NCT01837251 | CTCAE 4.03 | Table lists Grade 1 to 2 in > 10% of total participants, and any grade ≥ 3 G3 and G4 AEs split in table |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | All AEs |
| Monk 2020 | NCT01846611 | CTCAE 4.0 | G3 or G4 if occurring in ≥ 5% total participants G3 and G4 AEs split in table |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TEAEs |
| Targeted therapy | |||||
| TRINOVA‐2 | NCT01281254 | CTCAE 3.0 | Table lists treatment‐AEs if occurring in 10% of total participants Table lists cumulative values (i.e. G3 or higher) |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TEAEs |
| Platinum resistant | |||||
| Other conventional chemotherapy | |||||
| Mutch 2007 | Not listed | CTCAE 2.0 | G3 and G4 AEs split in table | Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | All |
| ASSIST‐5 | NCT00350948 | Not stated | Not reported | Not reported | Not reported |
| ASSIST‐3 | NCT00102973 | Not stated | Only information on AE: "Dose reductions for HFS and stomatitis were 15% and 4% respectively, in the intervention arm compared with 42% and 25% respectively in the PLD arm" |
Not reported | Not reported |
| Colombo 2012 | NCT00262990 | Not stated | Table shows AEs occurring in >10% participants, regardless of relationship to drug G3 and G4 combined in the table. |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TEAEs |
| CORAIL | NCT02421588 | CTCAE 4.0 | Table shows TRAEs occurring in ≥ 10% of participants in any of the treatment arms. G3 and G4 combined in the table. |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TRAEs |
| Targeted therapy | |||||
| APPROVE | NCT04348032 | CTCAE 4.0 | Table: TEAEs occurring in > 10% of participants in either group. G3 and G4 AEs split in table. |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | Treatment‐emergent |
| Banerjee 2018 | NCT01991210 | Not stated | All AEs listed if occurring in ≥ 20% of participants in either arm | Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | All AEs |
| McGuire 2018 | NCT00913835 | CTCAE 3.0 | TEAEs occurring in ≥ 10% of participants and with a ≥ 5% between‐arm difference | Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TEAEs |
| PRECEDENT | NCT00722592 | CTCAE 3.0 | Grade 3/4 AEs listed together. | Not clear but looks like number of participants experiencing any particular AE | TEAEs |
| Immunotherapy | |||||
| JAVELIN Ovarian 200 | NCT02580058 | CTCAE 4.03 | TRAEs of G1–2 occurring in ≥ 10% of participants and G3–5 occurring in ≥2% of participants are shown G3/G4/G5 are all shown separately on table. |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TRAEs |
| JAVELIN Ovarian 200 | NCT02580058 | CTCAE 4.03 | TRAEs of G1 to 2 occurring in ≥ 10% of participants and G3 to 5 occurring in ≥2% of participants are shown G3/G4/G5 are all shown separately on table. |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TRAEs |
| Monk 2017 | NCT01666444 | CTCAE 4.0 | TEAEs with ≥ 5% difference incidence between arms. | Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TEAEs |
| Platinum resistant and sensitive | |||||
| Other conventional chemotherapy | |||||
| NCT00653952 (Formerly O'Byrne 2002 in the previous, 2013 review) | NCT00653952 | Not stated | Only information on AE: "The overall number of adverse events was equivalent in either arm. Nausea and vomiting, stomatitis and plantar‐palmar erythrodysesthesia were seen more frequently with PLD whereas alopecia, myalgia, arthralgia and paraesthesiae occurred more commonly with paclitaxel" |
Not reported | Not reported |
| MITO‐3 | Not listed | Not stated | SAE and PPE numbers only | Not clear but looks like number of participants experiencing any particular AE | Not stated but looks like all AEs |
| Gordon 2001 | Not listed | Not stated | Not reported | Not reported | Not reported |
| NCT01840943 | NCT01840943 | MedDRA 15.0 | G3/4 AEs listed together | Not stated | Not stated |
| OVA‐301 | PMID 20516432 | CTCAE 3.0 | Table: TEAEs occurring in > 5% of participants in either group. G3 and G4 AEs split in table. |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | TRAEs |
| Targeted therapy | |||||
| Kaye 2012 | NCT00628251 | CTCAE 3.0 | Table: AEs (any grade) occurring in > 30% of participants in either group. Split into G1&2 or G3&4. |
Number of participants experiencing any particular AE ‐ does not state if this is the highest grade they experienced, if they had the same AE > 1 time. | All AEs |
| M200 | NCT00635193 | Not stated | Only information regarding AE: "The incidence of AEs was balanced across treatment groups. The most common Grade 3/4 AEs (≥ 5% in any group) were abdominal pain, intestinal obstruction, ascites, fatigue, hypoalbuminemia, and cytopenias" |
Not reported | Not stated |
Abbreviations: AE ‐ Adverse Events; CTCAE ‐ Common Terminology Criteria for Adverse Events; G1 ‐ Grade 1; G2 ‐ Grade 2; G3 ‐ Grade 3; HFS ‐ Hand‐Foot Syndrome; NCT ‐ National Clinical Trial; PLD ‐ Pegylated Liposomal Doxorubicin; PPE ‐ Palmar Plantar Erythrodysesthesia; SAE ‐ Serious Adverse Event; TEAE ‐ Treatment‐Emergent Adverse Events; TRAE ‐ Treatment‐Related Adverse Events; WHO ‐ World Health Organisation
Risk of bias in included studies
Figure 3 lists all included studies alongside the authors' judgements about risk of bias for each.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Most included studies were multicentre studies, with central randomisation and treatment allocation after registration with the organising centre. Therefore, the risk of selection bias was low. The methods of randomisation were not described in six studies (ASSIST‐3; Banerjee 2018; M200; Monk 2017; NCT00653952; NCT01840943)
Methods of allocation concealment were unclear in eleven studies (APPROVE; ASSIST‐3; Banerjee 2018; Gordon 2001; M200; Monk 2017; NCT00653952; NCT01840943; OVA‐301; PROCEED 2014; TRINOVA‐2).
Blinding
Almost all the included studies were open‐label, i.e. the participants and attending healthcare professionals were aware of the associated group allocation; therefore, all studies were at a high risk of performance bias. As an exception to this, three studies were double‐blinded (Monk 2017; PROCEED 2014; TRINOVA‐2). Method of blinding was not documented for one full‐text publication, with trial no protocol available (HeCOG 2010), and unclear in another (ASSIST‐3), as this was not described.
All but one included study (NCT00653952 ‐ as described above) assessed disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) and/or Gynecologic Cancer Intergroup (GCIG) criteria (CA‐125) (Therasse 2000; Rustin 1996); however, in most studies, it was unclear what methods, if any, were used to minimise detection bias ‐ only seven studies reported assessor blinding or independent radiologist or oncologist review (CALYPSO; Colombo 2012; Gordon 2001; JAVELIN Ovarian 200; MITO‐3; OVA‐301; PRECEDENT).
Incomplete outcome data
Attrition rates were high in ASSIST‐3 for primary outcomes, and these data were not included. A high risk of bias was assigned to NCT01840943 as 11/26 participants withdrew and 8/26 were lost to follow up, and to PROCEED 2014 as only interim data were available. Three other studies did not clearly state the total numbers of participants evaluated per outcome (i.e. denominators were missing) (M200; Mutch 2007; NCT00653952). APPROVE was assigned an unclear risk of bias as 57/74 and 53/72 did not complete six cycles of PLD.
Two trials that reported QoL data had low attrition rates(JAVELIN Ovarian 200; Pfisterer 2020). However, attrition rates for QoL outcomes were high (> 20%) in seven of the nine studies that reported this outcome (CALYPSO; Colombo 2012; Gordon 2001; Kaye 2012; MITO‐3; Mutch 2007; OVA‐301). As attrition rates for other outcomes in these studies were low, overall we adjudged the attrition bias to be low for these studies. We retained the 'unclear' rating for Mutch 2007 due to the unclear denominator.
Selective reporting
Most included studies reported their prespecified outcomes. NCT00653952 was closed early due to accrual problems, after 50% of participants were recruited. Only OS and adverse event data were presented, via an online clinical study report, after agreement with the sponsor company's Oncology Division. NCT01840943 reporting was incomplete when compared to the planned protocol.
Two studies reported only limited data in the abstracts of conference proceedings that could not be adequately evaluated for reporting bias (ASSIST‐3; M200); to our knowledge, these studies have not been published in full. ASSIST‐5 was temporarily put on hold in June 2007 to review the results of the single‐agent trial (ASSIST‐1 2009). The clinical hold was released in October 2007, but the sponsor decided not to enrol any additional patients and closed the trial early (planned enrolment = 244, actual enrolment = 125). Overall survival data for ASSIST‐5 have not been published and, to our knowledge, neither have the review findings, despite closing over a decade ago. Similarly, preliminary data from ASSIST‐3 were presented in 2007, but further data have not been published.
Mutch 2007 was judged at high risk of bias as they did not describe hazard ratios, number of events, and censoring for the primary outcome (PFS) or OS and only limited (non‐comparative) QoL data were reported. PROCEED 2014 was judged as high risk of bias due to high censoring rates and short follow‐up period.
Finally, in the TRINOVA‐2 trial we noted a discrepancy in the reported secondary outcomes; we did not have access to the study protocol, but secondary outcomes are published differently to those listed on clinicaltrials.gov (OS, ORR and duration of response, vs OS only). This may be attributed to reporting bias.
Other potential sources of bias
We judged four studies to be at high risk of other potential risks of bias (ASSIST‐3; M200; NCT00653952; NCT01840943). The results of ASSIST‐3, M200 and NCT00653952 have not been published in full, there is a potentially high risk of bias associated with the non‐publication of these studies. NCT00653952 enrolled women with relapsed EOC (platinum‐sensitive or platinum‐resistant) to PLD or PAC. As previous therapy with PLD or PAC was an exclusion criterion, once PAC/carboplatin became a first‐line chemotherapy combination option for EOC (NICE 2003), accrual was slow and the study became largely irrelevant. However, 220 women (out of a target of 438) were randomised and started on treatment and, ideally, the results of this terminated study should have been published. We were unsuccessful in our attempts to obtain these data or further information. Similarly, we were unable to obtain missing data for ASSIST‐3, despite previous attempts to contact the investigators and Telik.
SWOG S0200 (PLD/carboplatin versus carboplatin alone for platinum‐sensitive relapsed EOC) was another study that closed early due to slow accrual following the release of the initial ICON‐4 results, which showed the combination of PAC/carboplatin to be superior to carboplatin alone for women with platinum‐sensitive relapsed EOC, and for other reasons. SWOG S0200 is therefore limited by a small sample size (61 evaluable participants). However, unlike NCT00653952, the investigators of SWOG S0200 published their final results in full. We therefore judged this study to be at unclear risk of other bias.
In OVA‐301, bias may have occurred as a result of discrepancies in the premedications given to the intervention arm (notably dexamethasone), or the lower PLD dose in the intervention arm, which may have influenced outcomes. We judged this study to be at unclear risk of bias.
NCT01840943 has been included in the review, but not in the meta‐analysis, due to overall high risk of bias in the study. This study was closed early and only included 32 participants. Reporting of the methodology was extremely scanty or unclear.
All but two studies (HeCOG 2010 and MITO‐3) were funded by drug manufacturers with a commercial interest in either PLD or the comparator drugs. We therefore judged the remaining 18 studies to be at unclear risk of bias (APPROVE; ASSIST‐5; Banerjee 2018; CALYPSO; Colombo 2012; CORAIL; Fujiwara 2019; Gordon 2001; JAVELIN Ovarian 200; Kaye 2012; McGuire 2018; Monk 2017; Monk 2020; Mutch 2007; OVA‐301; Pfisterer 2020; PRECEDENT; PROCEED 2014; SWOG S0200; TRINOVA‐2), unless they were at high risk for other reasons, as described above.
Effects of interventions
See: Table 1; Table 2; Table 3
Twenty‐six included studies evaluated the effect of PLD in recurrent EOC: six in women with recurrent platinum‐sensitive disease (2604 participants), and 11 in women with recurrent platinum‐resistant disease (3153 participants). In the remaining nine studies participants were included regardless of platinum‐sensitivity status (2520 participants). We graded the certainty of the evidence of the three most clinically relevant comparisons. As per the methods section (Data synthesis), we combined data from trials that evaluated the effect of treatment options in populations with recurrent EOC regardless of platinum‐sensitivity status with those with platinum‐resistant recurrence in meta‐analyses, and performed sensitivity analysis to determine the effect of excluding studies including the mixed population.
Recurrent platinum‐sensitive EOC
PLD with conventional chemotherapy compared to other combination chemotherapy
See Table 1.
Overall survival (OS)
PLD with chemotherapy likely results in little to no difference in OS (HR 0.93, 95% CI 0.83 to 1.04; P = 0.18, I2 = 23%; 5 studies, 2006 participants; moderate‐certainty evidence; Analysis 1.1) compared to chemotherapy alone.
1.1. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 1: Overall survival
Progression‐free survival (PFS)
PLD with chemotherapy likely increases PFS (HR 0.81, 95% CI 0.74 to 0.89; P < 0.0001, I2 = 16%; 5 studies, 2006 participants; moderate‐certainty evidence; Analysis 1.2) compared to chemotherapy alone.
1.2. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 2: Progression‐free survival
Quality of life (QoL)
PLD with chemotherapy compared to chemotherapy alone may slightly improve QoL at three months post‐randomisation measured using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (MD 4.80, 95% CI 0.92 to 8.68; 1 study, 608 participants; low‐certainty evidence; Analysis 1.3), but this may not represent a clinically meaningful difference.
1.3. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 3: Quality of life
Adverse events
Trials contributing to this comparison did not report the following safety outcomes: diarrhoea (grade ≥ 3), and dose reductions or dose delays.
PLD with chemotherapy compared to chemotherapy alone likely results in little to no difference in overall severe adverse events (grade ≥ 3) (RR 1.11, 95% CI 0.95 to 1.30; P = 0.17, I2 = 0%; 2 studies, 834 participants; moderate‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 4: Overall Severe Adverse Events (grade ≥ 3)
PLD with chemotherapy likely increases anaemia (grade ≥ 3) (RR 1.37, 95% CI 1.02 to 1.85; P = 0.04, I2 = 22%; 5 studies, 1961 participants; moderate‐certainty evidence; Analysis 1.5) and neutropenia (grade ≥ 3) (RR 0.78, 95% CI 0.70 to 0.88; P < 0.0001; I2 = 75%; 5 studies, 1961 participants; Analysis 1.8).
1.5. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 5: SevAE: Anaemia (grade ≥ 3)
1.8. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 8: SevAE: Neutropenia (grade ≥ 3)
PLD with conventional chemotherapy likely results in a large decrease in hypersensitivity reactions (grade ≥ 3) (RR 0.27, 95% CI 0.15 to 0.48; P < 0.0001, I2 = 0%; 5 studies, 1961 participants; Analysis 1.14) and may result in a large increase in stomatitis (grade ≥ 3) (RR 2.52, 95% CI 1.01 to 6.28; P = 0.05, I2 = 0%; 3 studies, 1801 participants; Analysis 1.10) and arthralgia (grade ≥ 3) RR 0.19, 95% CI 0.04 to 0.85; P = 0.03, I2 = 0%; 4 studies, 1862 participants; Analysis 1.13).
1.14. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 14: SevAE: Hypersensitivity reactions (grade ≥ 3)
1.10. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 10: SevAE: Stomatitis (grade ≥ 3)
1.13. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 13: SevAE: Arthralgia/myalgia (grade ≥ 3)
PLD with conventional chemotherapy may result in little to no difference in vomiting (grade ≥ 3) (RR 1.26, 95% CI 0.78 to 2.02; P = 0.34, I2 = 0%; 4 studies, 1862 participants; Analysis 1.11), fatigue (grade ≥ 3) (RR 1.00, 95% CI 0.67 to 1.50; P = 0.99, I2 = 0%; 4 studies, 1862 participants; Analysis 1.12), discontinuation of treatment due to toxicity (RR 0.93, 95% CI 0.75 to 1.15; P = 0.50, I2 = 93%; 4 studies, 1862 participants; Analysis 1.16) and need for G‐CSF (RR 1.14, 95% CI 0.85 to 1.54; P = 0.38, I2 = 0%; 2 studies, 838 participants; Analysis 1.18).
1.11. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 11: SevAE: Vomiting (grade ≥ 3)
1.12. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 12: SevAE: Fatigue (grade ≥ 3)
1.16. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 16: Discontinuation due to toxicity
1.18. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 18: Granulocyte colony stimulating factor (G‐CSF) required
The evidence is very uncertain about the effect of PLD with conventional chemotherapy on hand‐foot syndrome (grade ≥ 3) (RR 4.01, 95% CI 1.00 to 16.01; P = 0.05, I2 = 0%; 2 studies, 1028 participants; very low‐certainty evidence; Analysis 1.6), neurological events (grade ≥ 3) RR 0.38, 95% CI 0.20 to 0.74; P = 0.004, I2 = 67%; 4 studies, 1900 participants; very low‐certainty evidence; Analysis 1.7), thrombocytopenia (grade ≥ 3) (RR 1.14, 95% CI 0.90 to 1.44; P = 0.28, I2 = 90%; 5 studies, 1961 participants; Analysis 1.9), treatment‐related death due to serious adverse events (RR 1.44, 95% CI 0.29 to 7.15; P = 0.66, I2 = 33%; 3 studies, 1801 participants; Analysis 1.15), and need for antibiotics (RR 1.12, 95% CI 0.57 to 2.21; P = 0.73, I2 = 37%; 2 studies, 1144 participants; Analysis 1.17).
1.6. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 6: SevAE: Hand‐foot syndrome (grade ≥ 3)
1.7. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 7: SevAE: Neurological (grade ≥ 3)
1.9. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 9: SevAE: Thrombocytopenia (grade ≥ 3)
1.15. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 15: Serious AE: Treatment‐related death
1.17. Analysis.

Comparison 1: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy, Outcome 17: Antibiotics required
PLD with conventional chemotherapy compared to PLD alone
The evidence in this comparison is limited to a combination of PLD with trabectedin (Monk 2020; OVA‐301).
Overall survival (OS)
PLD with trabectedin likely results in little to no difference in OS compared to PLD alone (HR 0.87, 95% CI 0.74 to 1.02; P = 0.09, I2 = 0%; 2 studies, 1006 participants; Analysis 2.1).
2.1. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 1: Overall survival
Progression‐free survival (PFS)
PLD with trabectedin likely increases PFS slightly compared to PLD alone (HR 0.85, 95% CI 0.72 to 1.00; P = 0.05, I2 = 53%; 2 studies, 1006 participants; Analysis 2.2).
2.2. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 2: Progression‐free survival
Quality of life (QoL)
Outcome not reported.
Adverse events
Trials contributing to this comparison did not report the following safety outcomes: neurological events (grade ≥ 3), diarrhoea (grade ≥ 3), arthralgia/myalgia (grade ≥ 3), hypersensitivity reactions (grade ≥ 3), treatment‐related death due to serious adverse events, discontinuation of treatment due to toxicity, dose reductions or delays, and need for antibiotics or G‐CSF.
PLD with trabectedin compared to PLD alone likely results in a large reduction in hand‐foot syndrome (grade ≥ 3) (RR 0.30, 95% CI 0.15 to 0.59; 1 study, 568 participants; Analysis 2.5) and likely increases overall severe adverse events (grade ≥ 3) (RR 1.33, 95% CI 1.20 to 1.47; 1 study, 568 participants; Analysis 2.3) compared to PLD alone.
2.5. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 5: SevAE: Hand‐foot syndrome (grade ≥ 3)
2.3. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 3: Overall Severe Adverse Events (grade ≥3)
PLD with trabectedin compared to PLD alone may result in a large increase in anaemia (grade ≥ 3) (RR 3.01, 95% CI 1.87 to 4.85; 1 study, 568 participants; Analysis 2.4), neutropenia (grade ≥ 3) (RR 2.07, 95% CI 1.59 to 2.70; 1 study, 568 participants; Analysis 2.6) and fatigue (grade ≥ 3) (RR 4.37, 95% CI 1.96 to 9.75; 1 study, 568 participants; Analysis 2.10), although risk of stomatitis (grade ≥ 3) was decreased by the combination of PLD and trabectedin compared with PLD alone (RR 0.21, 95% CI 0.08 to 0.56; 1 study, 568 participants; Analysis 2.8).
2.4. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 4: SevAE: Anaemia (grade ≥3)
2.6. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 6: SevAE: Neutropenia (grade ≥ 3)
2.10. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 10: SevAE: Fatigue (grade ≥ 3)
2.8. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 8: SevAE: Stomatitis (grade ≥ 3)
PLD with trabectedin compared to PLD alone likely results in an increase in vomiting (grade ≥ 3) (RR 3.55 95% CI 1.34 to 9.43; 1 study, 568 participants; Analysis 2.9) compared to PLD alone.
2.9. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 9: SevAE: Vomiting (grade ≥ 3)
The evidence is very uncertain about the effect of PLD with trabectedin on thrombocytopenia (grade ≥ 3) (RR 14.13, 95% CI 4.44 to 45.03; 1 study, 568 participants; Analysis 2.7).
2.7. Analysis.

Comparison 2: Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 7: SevAE: Thrombocytopenia (grade ≥ 3)
Recurrent platinum‐resistant EOC
PLD alone compared to conventional chemotherapy
See Table 2.
Overall survival (OS)
PLD likely results in little to no difference in OS (HR 0.96, 95% CI 0.77 to 1.19; P = 0.70, I2 = 84%; 6 studies, 1995 participants; moderate‐certainty evidence; Analysis 3.1) compared to chemotherapy alone. Findings of sensitivity analysis using studies that recruited only individuals with platinum‐resistant EOC were consistent with the main analysis (HR 1.08, 95% CI 0.96 to 1.23 ; 3 studies, 1369 participants).
3.1. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 1: Overall survival
Progression‐free survival (PFS)
The evidence is very uncertain about the effect of PLD on PFS (HR 0.94, 95% CI 0.85 to 1.04; P = 0.23, I2 = 0%; 4 studies, 1803 participants; very low‐certainty evidence; Analysis 3.2) compared to chemotherapy alone. Findings of sensitivity analysis using studies that recruited only individuals with platinum‐resistant EOC were consistent with the main analysis (HR 0.95, 95% CI 0.83 to 1.09; 2 studies, 1176 participants).
3.2. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 2: Progression‐free survival
Quality of life (QoL)
Outcome not reported.
Adverse events
Trials contributing to this comparison did not report the following safety outcomes: arthralgia/myalgia (grade ≥ 3), treatment‐related death due to serious adverse events, treatment discontinuation due to toxicity, and a need for antibiotics or G‐CSF.
The evidence is very uncertain about the effect of PLD compared to chemotherapy alone on overall severe adverse events (grade >3) (RR ranged from 0.61 to 0.97; 2 studies, 964 participants; very low‐certainty evidence; Analysis 3.3), anaemia (grade ≥ 3) (RR ranged from 0.19 to 0.82; 5 studies, 1968 participants; very low‐certainty evidence; Analysis 3.4), hand‐foot syndrome (grade ≥ 3) (RR ranged from 15.19 to 109.15; 6 studies, 2184 participants; very low‐certainty evidence; Analysis 3.5), neurological events (grade ≥ 3)(RR ranged from 0.08 to 3.09; 3 studies, 1222 participants; very low‐certainty evidence; Analysis 3.6), neutropenia (grade ≥ 3)(RR ranged from 0.16 to 3.36; 5 studies, 2055 participants; Analysis 3.7), thrombocytopenia (grade ≥ 3) (RR ranged from 0.04 to 0.53; 4 studies, 1157 participants; Analysis 3.8), stomatitis (grade ≥ 3) (RR ranged from 2.53 to 20.15; 6 studies, 2184 participants; Analysis 3.9), vomiting (grade ≥ 3) (RR ranged from 0.14 to 0.74; 4 studies, 1494 participants; Analysis 3.10), diarrhoea (grade ≥ 3) (RR ranged from 0.09 to 0.60; 4 studies, 1494 participants; Analysis 3.11), fatigue (grade ≥ 3) (RR ranged from 0.40 to 0.80; 5 studies, 1556 participants; Analysis 3.12), rate of hypersensitivity reactions (grade ≥ 3) (RR 2.96, 95% CI 0.32 to 27.77; 1 study, 143 participants; Analysis 3.13), rate of dose reductions (RR 1.04, 95% CI 0.54 to 2.01; P = 0.91, I2 = 91%; 4 studies, 1773 participants; Analysis 3.14), and dose delays (RR 0.97, 95% CI 0.55 to 1.69; P = 0.90, I2 = 80%; 5 studies, 962 participants; Analysis 3.15).
3.3. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 3: Overall Severe Adverse Events (grade ≥ 3)
3.4. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 4: SevAE: Anaemia (grade ≥ 3)
3.5. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 5: SevAE: Hand‐foot syndrome (grade ≥ 3)
3.6. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 6: SevAE: Neurological (grade ≥ 3)
3.7. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 7: SevAE: Neutropenia (grade ≥ 3)
3.8. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 8: SevAE: Thrombocytopenia (grade ≥ 3)
3.9. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 9: SevAE: Stomatitis (grade ≥ 3)
3.10. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 10: SevAE: Vomiting (grade ≥ 3)
3.11. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 11: SevAE: Diarrhoea (grade ≥ 3)
3.12. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 12: SevAE: Fatigue (grade ≥ 3)
3.13. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 13: SevAE: Hypersensitivity reactions (grade ≥ 3)
3.14. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 14: Dose reductions
3.15. Analysis.

Comparison 3: Platinum‐resistant recurrent EOC: PLD versus other chemotherapy, Outcome 15: Dose delays
PLD with conventional chemotherapy compared to PLD alone
See Table 3.
Overall survival (OS)
PLD with conventional chemotherapy likely results in little to no difference in OS (HR 0.92, 95% CI 0.70 to 1.21; 1 study, 242 participants, moderate‐certainty evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 1: Overall survival
Progression‐free survival (PFS)
PLD with conventional chemotherapy may result in little to no difference in PFS (HR 0.94, 95% CI 0.73 to 1.22; P = 0.64, I2 = 0%; 2 studies, 353 participants; low‐certainty evidence; Analysis 4.2).
4.2. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 2: Progression‐free survival
Quality of life (QoL)
Outcome not reported.
Adverse events
Trials contributing to this comparison did not report the following safety outcomes: diarrhoea (grade ≥ 3), arthralgia (grade ≥ 3), hypersensitivity reactions (grade ≥ 3), treatment discontinuation due to toxicity and a need for antibiotics or G‐CSF.
PLD with conventional chemotherapy likely increases overall severe adverse events (grade ≥ 3) (RR 2.48, 95% CI 1.98 to 3.09; 1 study, 663 participants; moderate‐certainty evidence; Analysis 4.3), anaemia (grade ≥ 3) (RR 2.38, 95% CI 1.46 to 3.87; P = 0.0005, I2 = 0%; 2 studies, 785 participants; moderate‐certainty evidence; Analysis 4.4), and neutropenia (grade ≥ 3) (RR 2.75, 95% CI 2.23 to 3.38; P < 0.00001, I2 = 0%; 2 studies, 785 participants; Analysis 4.7) compared to PLD alone.
4.3. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 3: Overall Severe Adverse Events (grade ≥ 3)
4.4. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 4: SevAE: Anaemia (grade ≥ 3)
4.7. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 7: SevAE: Neutropenia (grade ≥ 3)
PLD with conventional chemotherapy likely results in a large reduction in hand‐foot syndrome (grade ≥ 3) (RR 0.24, 95% CI 0.14 to 0.40; P < 0.00001, I2 = 48%; 2 studies, 785 participants; moderate‐certainty evidence; Analysis 4.5) compared to PLD alone.
4.5. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 5: SevAE: Hand‐foot syndrome (grade ≥ 3)
PLD with conventional chemotherapy may result in a large increase in thrombocytopenia (grade ≥ 3) (RR 7.70, 95% CI 3.90 to 15.19; P < 0.00001, I2 = 0%; 2 studies, 785 participants; Analysis 4.8), vomiting (grade ≥ 3) (RR 3.77, 95% CI 1.91 to 7.41; P = 0.0001, I2 = 54%; 2 studies, 785 participants; Analysis 4.10) and fatigue (grade ≥ 3) (RR 2.20, 95% CI 1.02 to 4.76; 1 study, 663 participants; Analysis 4.11); and may result in a large reduction in stomatitis (grade ≥ 3) (RR 0.30, 95% CI 0.13 to 0.70; P = 0.005, I2 = 60%; 2 studies, 785 participants; Analysis 4.9).
4.8. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 8: SevAE: Thrombocytopenia (grade ≥ 3)
4.10. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 10: SevAE: Vomiting (grade ≥ 3)
4.11. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 11: SevAE: Fatigue (grade ≥ 3)
4.9. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 9: SevAE: Stomatitis (grade ≥ 3)
PLD with conventional chemotherapy may increase the rate of dose delays (RR 1.50, 95% CI 1.00 to 2.26; 1 study, 535 participants; Analysis 4.13), and may result in little to no difference in neurological events (grade ≥ 3) (RR 1.40, 95% CI 0.85 to 2.31; 1 study, 663 participants; low‐certainty evidence; Analysis 4.6) and rate of dose reductions (RR 1.07, 95% CI 0.53 to 2.14; 1 study, 535 participants; Analysis 4.14).
4.13. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 13: Dose delays
4.6. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 6: SevAE: Neurological (grade ≥ 3)
4.14. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 14: Dose reductions
The evidence is very uncertain about the effect of PLD with conventional chemotherapy on treatment‐related death due to serious adverse events (RR 2.48, 95% CI 0.48 to 12.68; 1 study, 663 participants; Analysis 4.12).
4.12. Analysis.

Comparison 4: Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone, Outcome 12: Serious AE: Treatment‐related death
PLD compared to targeted therapy alone
Overall survival (OS)
PLD likely results in little to no difference in OS (HR 0.99, 95% CI 0.44 to 2.25; 1 study, 65 participants; Analysis 5.1) compared to targeted therapy.
5.1. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 1: Overall survival
Progression‐free survival (PFS)
PLD likely results in little to no difference in PFS (HR 1.23, 95% CI 0.82 to 1.84; P = 0.31, I2 = 0%; 2 studies, 160 participants; Analysis 5.2) compared to targeted therapy.
5.2. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 2: Progression‐free survival
Quality of life (QoL)
Outcome not reported.
Adverse events
Trials contributing to this comparison did not report the following safety outcomes: thrombocytopenia (grade ≥ 3), arthralgia (grade ≥ 3), hypersensitivity reactions (grade ≥ 3), treatment‐related death due to serious adverse events, treatment discontinuation due to toxicity, dose delays and need for antibiotics or G‐CSF.
PLD may result in a large reduction in anaemia (grade ≥ 3) (RR 0.12, 95% CI 0.02 to 0.97; P = 0.05, I2 = 0%; 2 studies, 157 participants; Analysis 5.4) compared to targeted therapy.
5.4. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 4: SevAE: Anaemia (grade ≥ 3)
PLD may result in little to no difference in overall severe adverse events (grade ≥ 3) (RR 1.12, 95% CI 0.73 to 1.71; 1 study, 93 participants; Analysis 5.3) and the rate of dose reductions (RR 0.90, 95% CI 0.42 to 1.92; 1 study, 64 participants; Analysis 5.12) compared to targeted therapy.
5.3. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 3: Overall Severe Adverse Events (grade ≥ 3)
5.12. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 12: Dose reductions
The evidence is very uncertain about the effect of PLD compared to targeted therapy on hand‐foot syndrome (grade ≥ 3) (RR 25.00, 95% CI 1.54 to 405.08; P = 0.02, I2 = 0%; 2 studies, 157 participants; Analysis 5.5), neurological events (grade ≥ 3) (RR 0.98, 95% CI 0.06 to 15.19; 1 study, 93 participants; Analysis 5.6), neutropenia (grade ≥ 3) (RR 0.33, 95% CI 0.07 to 1.53; 1 study, 93 participants; Analysis 5.7), stomatitis (grade ≥ 3) (RR 5.87, 95% CI 0.72 to 47.84; P = 0.10; 2 studies (1 of which had 0 events), 157 participants; Analysis 5.8), vomiting (grade ≥ 3) (RR 1.31, 95% CI 0.30 to 5.70; P = 0.72, I2 = 0%; 2 studies, 157 participants; Analysis 5.9), diarrhoea (grade ≥ 3) (RR 2.06, 95% CI 0.27 to 15.56; P = 0.48, I2 = 0%; 2 studies, 157 participants; Analysis 5.10), and fatigue (grade ≥ 3) (RR 1.18, 95% CI 0.38 to 3.72; P = 0.78, I2 = 0%; 2 studies, 157 participants; Analysis 5.11).
5.5. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 5: SevAE: Hand‐foot syndrome (grade ≥ 3)
5.6. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 6: SevAE: Neurological (grade ≥ 3)
5.7. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 7: SevAE: Neutropenia (grade ≥ 3)
5.8. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 8: SevAE: Stomatitis (grade ≥ 3)
5.9. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 9: SevAE: Vomiting (grade ≥ 3)
5.10. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 10: SevAE: Diarrhoea (grade ≥ 3)
5.11. Analysis.

Comparison 5: Platinum‐resistant recurrent EOC: PLD versus targeted therapy, Outcome 11: SevAE: Fatigue (grade ≥ 3)
PLD with targeted therapy compared to PLD alone
Overall survival (OS)
PLD with targeted therapy likely results in little to no difference in OS (HR 0.93, 95% CI 0.75 to 1.15; P = 0.53, I2 = 0%; 4 studies, 647 participants; Analysis 6.1) compared to PLD alone. Findings of sensitivity analysis using studies that recruited only individuals with platinum‐resistant EOC were consistent with the main analysis (HR 0.84, 95% CI 0.56 to 1.27; 2 studies, 301 participants).
6.1. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 1: Overall survival
Progression‐free survival (PFS)
PLD with targeted therapy may result in little to no difference in PFS (HR 0.78, 95% CI 0.58 to 1.04; P = 0.09, I2 = 63%; 5 studies, 877 participants; Analysis 6.2) compared to PLD alone. Sensitivity analysis using studies that recruited only individuals with platinum‐resistant EOC showed some evidence of beneficial effects from combined treatment compared to PLD alone (HR 0.65, 95% CI 0.42 to 1.00; 3 studies, 531 participants).
6.2. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 2: Progression‐free survival
Quality of life (QoL)
Outcome not reported.
Adverse events
Trials contributing to this comparison did not report the following safety outcomes: arthralgia/myalgia (grade ≥ 3), hypersensitivity reactions (grade ≥ 3), treatment discontinuation due to toxicity, dose delays, and need for antibiotics or G‐CSF. Reported adverse events are mainly treatment‐emergent.
PLD with targeted therapy may increase hand‐foot syndrome (grade ≥ 3) (RR 1.75, 95% CI 1.07 to 2.88; P = 0.03, I2 = 0%; 4 studies, 647 participants; Analysis 6.5) compared to PLD alone.
6.5. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 5: SevAE: Hand‐foot syndrome (grade ≥ 3)
PLD with targeted therapy may result in little to no difference in anaemia (grade ≥ 3) (RR 0.63, 95% CI 0.32 to 1.26; P = 0.19, I2 = 0%; 4 studies, 647 participants; Analysis 6.4) and neutropenia (grade ≥ 3) (RR 1.09, 95% CI 0.69 to 1.74; P = 0.70, I2 = 16%; 4 studies, 647 participants; Analysis 6.7) compared to PLD alone.
6.4. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 4: SevAE: Anaemia (grade ≥ 3)
6.7. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 7: SevAE: Neutropenia (grade ≥ 3)
The evidence is very uncertain about the effect of PLD with targeted therapy on overall severe adverse events (grade ≥ 3) (RR 1.15, 95% CI 0.90 to 1.48; P = 0.27, I2 = 69%; 4 studies, 794 participants; Analysis 6.3); neurological events (grade ≥ 3) (RR 4.25, 95% CI 0.23 to 77.45; P = 0.33; 2 studies, 378 participants; Analysis 6.6); thrombocytopenia (grade ≥ 3) (RR 1.03, 95% CI 0.11 to 9.49; P = 0.98, I2 = 51%; 2 studies, 303 participants; Analysis 6.8); stomatitis (grade ≥ 3) (RR 0.97, 95% CI 0.43 to 2.19; P = 0.93, I2 = 12%; 4 studies, 647 participants; Analysis 6.9); vomiting (grade ≥ 3) (RR 0.81, 95% CI 0.35 to 1.87; P = 0.63, I2 = 0%; 3 studies, 490 participants; Analysis 6.10); diarrhoea (grade ≥ 3) (RR 0.64, 95% CI 0.18 to 2.24; P = 0.49, I2 = 0%; 2 studies, 344 participants; Analysis 6.11); fatigue (grade ≥ 3) (RR 2.08, 95% CI 0.30 to 14.52; P = 0.46, I2 = 67%; 3 studies, 490 participants; Analysis 6.12); rate of treatment‐related death due to serious adverse events (RR 1.02, 95% CI 0.33 to 3.20; P = 0.97, I2 = 0%; 5 studies, 956 participants; Analysis 6.13) and rate of dose reductions (RR 14.00, 95% CI 1.89 to 103.76; 1 study, 148 participants; Analysis 6.14).
6.3. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 3: Overall Severe Adverse Events (grade ≥3)
6.6. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 6: SevAE: Neurological (grade ≥ 3)
6.8. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 8: SevAE: Thrombocytopenia (grade ≥ 3)
6.9. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 9: SevAE: Stomatitis (grade ≥ 3)
6.10. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 10: SevAE: Vomiting (grade ≥ 3)
6.11. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 11: SevAE: Diarrhoea (grade ≥ 3)
6.12. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 12: SevAE: Fatigue (grade ≥ 3)
6.13. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 13: Serious AE: Treatment‐related death
6.14. Analysis.

Comparison 6: Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone, Outcome 14: Dose reductions
PLD compared to immunotherapy
Overall survival (OS)
PLD likely results in little to no difference in OS (HR 0.88, 95% CI 0.68 to 1.13; 1 study, 378 participants; Analysis 7.1) compared to immunotherapy.
7.1. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 1: Overall survival
Progression‐free survival (PFS)
PLD likely increases PFS (HR 0.60, 95% CI 0.42 to 0.84; 1 study, 378 participants; Analysis 7.2) compared to immunotherapy.
7.2. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 2: Progression‐free survival
Quality of life (QoL)
Outcome not reported.
Adverse events
Trials contributing to this comparison did not report the following safety outcomes: arthralgia/myalgia (grade ≥ 3), hypersensitivity reactions (grade ≥ 3), treatment‐related death due to serious adverse events, treatment discontinuation due to toxicity, dose delays and need for antibiotics or G‐CSF. The rate of neurological events (grade ≥ 3) was noted in JAVELIN Ovarian 200, but no events of this grade occurred during the course of the trial. Reported adverse events are mainly treatment‐emergent.
PLD likely results in a large increase in overall severe adverse events (grade ≥ 3) (RR 1.97, 95% CI 1.33 to 2.92; 1 study, 364 participants; Analysis 7.3) compared to immunotherapy.
7.3. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 3: Overall Severe Adverse Events (grade ≥ 3)
The evidence is very uncertain about the effect of PLD on anaemia (grade ≥ 3) (RR 3.17, 95% CI 0.87 to 11.52; 1 study, 364 participants; Analysis 7.4); hand‐foot syndrome (grade ≥ 3) (RR 20.07, 95% CI 1.18 to 342.24; 1 study, 364 participants; Analysis 7.5); neutropenia (grade ≥ 3) (RR 20.07, 95% CI 1.18 to 342.24; 1 study, 364 participants; Analysis 7.6); thrombocytopenia (grade ≥ 3) (RR 3.17, 95% CI 0.13 to 77.27; 1 study, 364 participants; Analysis 7.7); stomatitis (grade ≥ 3) (RR 8.45, 95% CI 1.07 to 66.89; 1 study, 364 participants; Analysis 7.8); vomiting (grade ≥ 3) (RR 3.17, 95% CI 0.33 to 30.19; 1 study, 364 participants; Analysis 7.9); diarrhoea (grade ≥ 3) (RR 0.10, 95% CI 0.01 to 1.72; 1 study, 364 participants; Analysis 7.10); fatigue (grade ≥ 3) (RR 7.39, 95% CI 0.38 to 142.12; 1 study, 364 participants; Analysis 7.11); and the rate of dose reductions (RR 5.07, 95% CI 1.98 to 13.00; 1 study, 364 participants; Analysis 7.12).
7.4. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 4: SevAE: Anaemia (grade ≥ 3)
7.5. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 5: SevAE: Hand‐foot syndrome (grade ≥ 3)
7.6. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 6: SevAE: Neutropenia (grade ≥ 3)
7.7. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 7: SevAE: Thrombocytopenia (grade ≥ 3)
7.8. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 8: SevAE: Stomatitis (grade ≥ 3)
7.9. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 9: SevAE: Vomiting (grade ≥ 3)
7.10. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 10: SevAE: Diarrhoea (grade ≥ 3)
7.11. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 11: SevAE: Fatigue (grade ≥ 3)
7.12. Analysis.

Comparison 7: Platinum‐resistant recurrent EOC: PLD versus immunotherapy, Outcome 12: Dose reductions
PLD with immunotherapy compared to PLD alone
Overall survival (OS)
PLD with immunotherapy likely results in little to no difference in OS (HR 0.89, 95% CI 0.69 to 1.15; P = 0.38, I2 = 0%; 2 studies, 675 participants; Analysis 8.1) compared to PLD alone. Findings of sensitivity analysis using studies that recruited only individuals with platinum‐resistant EOC were consistent with the main analysis (HR 0.89, 95% CI 0.69 to 1.15; 1 study, 378 participants).
8.1. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 1: Overall survival
Progression‐free survival (PFS)
PLD with immunotherapy may result in little to no difference in PFS (HR 0.78, 95% CI 0.54 to 1.14; P = 0.20, I2 = 0%; 2 studies, 675 participants; Analysis 8.2) compared to PLD alone. Findings of sensitivity analysis using studies that recruited only individuals with platinum‐resistant EOC were consistent with the main analysis (HR 0.78, 95% CI 0.54 to 1.14; 1 study, 378 participants).
8.2. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 2: Progression‐free survival
Quality of life (QoL)
Outcome not reported.
Adverse events
Trials contributing to this comparison did not report the following safety outcomes: arthralgia/myalgia (grade ≥ 3), hypersensitivity reactions (grade ≥ 3), treatment discontinuation due to toxicity, dose delays and a need for antibiotics or G‐CSF.
PLD with immunotherapy may increase the rate of dose reductions (RR 1.90, 95% CI 1.22 to 2.98; 1 study, 359 participants; Analysis 8.14).
8.14. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 14: Dose reductions
PLD with immunotherapy may result in little to no difference in overall severe adverse events (grade ≥ 3) (RR 1.12, 95% CI 0.96 to 1.30; P = 0.14, I2 = 62%; 2 studies, 653 participants; Analysis 8.3); anaemia (grade ≥ 3) (RR 0.65, 95% CI 0.27 to 1.57; P = 0.34, I2 = 0%; 2 studies, 653 participants; Analysis 8.4); hand‐foot syndrome (grade ≥ 3) (RR 1.07, 95% CI 0.81 to 1.42; P = 0.62, I2 = 62%; 2 studies, 653 participants; Analysis 8.5); neurological events (grade ≥ 3) (RR 0.98, 95% CI 0.70 to 1.37; P = 0.90, I2 = 0%; 2 studies, 653 participants; Analysis 8.6); neutropenia (grade ≥ 3) (RR 1.07, 95% CI 0.47 to 2.48; P = 0.87, I2 = 0%; 2 studies, 653 participants; Analysis 8.7); stomatitis (grade ≥ 3) (RR 1.39, 95% CI 0.62 to 3.11; P = 0.43, I2 = 0%; 2 studies, 653 participants; Analysis 8.9), and vomiting (grade ≥ 3) (RR 0.99, 95% CI 0.38 to 2.60; P = 0.99, I2 = 23%; 2 studies, 653 participants, Analysis 8.10).
8.3. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 3: Overall Severe Adverse Events (grade ≥ 3)
8.4. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 4: SevAE: Anaemia (grade ≥ 3)
8.5. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 5: SevAE: Hand‐foot syndrome (grade ≥ 3)
8.6. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 6: SevAE: Neurological (grade ≥ 3)
8.7. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 7: SevAE: Neutropenia (grade ≥ 3)
8.9. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 9: SevAE: Stomatitis (grade ≥ 3)
8.10. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 10: SevAE: Vomiting (grade ≥ 3)
The evidence is very uncertain about the effect of PLD with immunotherapy on thrombocytopenia (grade ≥ 3) (RR 0.32, 95% CI 0.01 to 7.91;1 study, 359 participants; Analysis 8.8); diarrhoea (grade ≥ 3) (RR 3.64, 95% CI 0.60 to 21.92; P = 0.16, I2 = 0%; 2 studies, 653 participants; Analysis 8.11); fatigue (grade ≥ 3) (RR 2.75, 95% CI 1.00 to 7.55; P = 0.05, I2 = 0%; 2 studies, 653 participants; Analysis 8.12); and the rate of treatment‐related death due to serious adverse events (RR 3.00, 95% CI 0.12 to 73.05; 1 study, 294 participants; Analysis 8.13).
8.8. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 8: SevAE: Thrombocytopenia (grade ≥ 3)
8.11. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 11: SevAE: Diarrhoea (grade ≥ 3)
8.12. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 12: SevAE: Fatigue (grade ≥ 3)
8.13. Analysis.

Comparison 8: Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone, Outcome 13: Serious AE: Treatment‐related death
Discussion
Summary of main results
In this update to the review, we include 26 RCTs with a total of 8277 participants recruited that evaluated the effects of PLD alone or in combination with other drugs in recurrent EOC (ITT efficacy data reported for 8116).
Seven in platinum‐sensitive disease (2872 participants; ITT efficacy data reported for 2807)
Eleven in platinum‐resistant disease (3246 participants; ITT efficacy data reported for 3234)
Eight that recruited individuals regardless of platinum sensitivity status (2079 participants; ITT efficacy data reported for 2075)
We assessed the certainty of the evidence for the three most clinically relevant comparisons, out of eight comparisons identified in the included RCTs.
Recurrent platinum‐sensitive EOC
PLD with conventional chemotherapy compared to alternative conventional chemotherapy likely results in little to no difference in OS, but likely increases progression‐free survival (PFS). The combination of PLD and chemotherapy may slightly improve the quality of life at three months post‐randomisation, measured using European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30, but this may not represent a clinically meaningful difference.
PLD added to chemotherapy compared to chemotherapy alone likely results in little to no difference in the rate of overall severe adverse events (grade ≥ 3). PLD with chemotherapy likely increases anaemia (grade ≥ 3). The evidence is very uncertain about the effect of PLD with conventional chemotherapy on hand‐foot syndrome (HFS) (grade ≥ 3) and neurological events (grade ≥ 3).
Recurrent platinum‐resistant EOC
PLD alone compared to conventional chemotherapy likely results in little to no difference in OS. The evidence is very uncertain about the effect of PLD on PFS, overall severe adverse events (grade ≥3), anaemia (grade ≥3), HFS (grade ≥3), and the rate of neurological events (grade ≥3). However, there were no cases of HFS in the non‐PLD arm and between 55 and 104 cases per 1,000 in the PLD arm.
PLD with conventional chemotherapy compared to PLD alone likely results in little to no difference in OS, and it may result in little to no difference in PFS. The combination likely increases overall severe adverse events (grade ≥3) and anaemia (grade ≥ 3), but likely results in a large reduction in HFS (grade ≥ 3). It may result in little to no difference in neurological events (grade ≥ 3).
Several studies included PLD either as a comparison to, or with or without, targeted agents or immunotherapy. This is because novel agents are often first tested in participants with relapsed disease, and PLD is commonly used in these settings, so is a frequent 'physician's choice' chemotherapy. Due to the heterogeneity of treatments and their targets, we have not formally included them within our summary of findings, as the certainty of these treatment combinations is low or very low. The data for individual comparisons is presented in the results and analyses sections.
Overall completeness and applicability of evidence
We included 26 studies where data were available for the use of PLD in women with relapsed EOC. A number of other studies used PLD as part of treatment, either as monotherapy or in combination, which examined the effect of adding another agent to the physicians choice of chemotherapy treatment. We were not able to obtain data from these studies for those treated with PLD in the intervention or comparison arm, so a review of individual participant data, including data from these studies, may alter our findings.
We are relatively confident that we have captured the majority of studies assessing PLD in relapsed EOC, having identified ongoing studies in a previous review and compared included studies to recent systematic reviews. In addition, this review has been performed alongside other reviews of targeted agents in ovarian cancer. Studies have been shared between review teams where there was found to be overlap, so the search net has effectively been wider than the search strategy of each individual review. However, treatment of relapsed EOC is a fast‐moving field, so studies may have been missed.
The studies included in the review are applicable to many women with relapsed EOC, as they include those with platinum‐sensitive and resistant disease. However, the age of women in studies is younger than those outside of clinical trials, which should be noted when making decisions about individual patient care, as this may affect the benefit and risks of treatment.
Data for disease response, including survival outcomes, is reported more consistently than harms and quality of life outcomes. This is common across oncology studies and remains disappointing, limiting the ability of women to make truly informed decisions about their treatment options.
In summary, PLD may have similar levels of benefit to other chemotherapy options, either alone or in combination with other chemotherapy and targeted treatments, but has a differing side effect profile. Optimal choice of treatment for women with relapsed EOC will therefore depend on patient factors, comorbidity, residual side effects to other treatments and individual preference.
Quality of the evidence
This is a comprehensive review of literature on the effect of PLD in the treatment of relapsed EOC. Nevertheless, the certainty of evidence for survival outcomes was moderate to very low, with the certainty of harms and quality of life outcomes mainly very low. The main difficulty in the assessment of study limitations was suboptimal or inadequate reporting of important features of trial design, such as randomisation sequence generation (in 6 of 26 studies this domain was unclear) or allocation concealment (unclear in 11 of 26 studies).
Only three studies clearly blinded participants and personnel (Monk 2017; PROCEED 2014; TRINOVA‐2), with another two at unclear risk for this domain (ASSIST‐3; HeCOG 2010). The rest of the studies were open‐label in design, which put the studies at high risk of bias for blinding for all outcomes except OS (Figure 3). Overall, no study was deemed at low risk of bias in all evaluated domains and only one study in six domains (MITO‐3).
Reporting of adverse outcomes was inconsistent, impeding combination of data in meta‐analyses, which reduces the certainty of our findings.
Potential biases in the review process
We attempted to prevent bias in the review by including grey literature and making every effort to obtain missing data from the investigators. However, as discussed above, we were unable to obtain data for PLD‐treated participants in several studies, which remain Studies awaiting classification. PLD is a commonly used treatment in recurrent EOC, so we may not have identified all studies, despite a systematic approach, and welcome further data from other studies not included in this review.
The majority of the authors, including the senior author, have no links to drug companies, any financial interest in the prescription of chemotherapeutic agents, nor were they involved in the conduct of the included studies.
Agreements and disagreements with other studies or reviews
When considering this review in the context of the published literature studying the benefits of PLD in EOC, existing systematic reviews generally agree with our outcomes (where comparison was possible), but there are also some disagreements, as highlighted below.
PLD with conventional chemotherapy compared to conventional chemotherapy alone in recurrent platinum‐sensitive EOC
Four published systematic reviews compare the combination of PLD and platinum‐based chemotherapy (primarily carboplatin) with other platinum‐based combinations (Edwards 2015 (16 studies (28 publications), including 5368 participants);Li 2021 (described as including 10 studies with 3747 participants); Shi 2020 (7 studies, including 3676 participants); Staropoli 2014 (14 studies, including 5760 participants)). Three of these investigated the value of PLD in both the platinum‐sensitive and platinum‐resistant setting, whereas Shi 2020 only focussed on the role of PLD in the platinum‐sensitive setting, combined with carboplatin.
Considering combination platinum/PLD chemotherapy, studies by Li 2021 and Shi 2020 agree that PLD‐containing regimens likely produce no difference in OS, when compared to carboplatin/paclitaxel (HR 1.00, and HR 0.98, respectively). Staropoli 2014 drew similar conclusions (HR 0.96, 95% CI 0.82 to 1.12). However, their analysis also included a study comparing carboplatin/PLD to platinum monotherapy, alongside studies using combination platinum/paclitaxel chemotherapy. In addition, their analysis included studies treating women in the first‐line setting, so was not limited to the relapsed, platinum‐sensitive patient cohort. Edwards 2015 noted an overall survival advantage of platinum/PLD combination chemotherapy, but this was when compared to platinum monotherapy alone (HR 1.267, favouring PLD, 95% CI 1.03 to 1.55).
Regarding PFS in this population, all reviews agreed with our findings, suggesting likely improvement in PFS with PLD. Edwards 2015, Li 2021 and Shi 2020 estimated preference for PLD/carboplatin over PLD/paclitaxel combination chemotherapy, with HR 0.82, HR 0.85, and HR 0.87, respectively; no 95% confidence intervals crossed 1.0. In addition, Li 2021 noted that no difference in PFS was observed when PLD was dosed at 30 mg/m2, in combination with carboplatin, compared to 45 mg/m2. Six out of the seven included studies in Shi 2020 also used the 30 mg/m2 dose, possibly attributing to the non‐inferiority of the lower dose in this setting. Staropoli 2014 observed an increased PFS with PLD/carboplatin combined chemotherapy (HR 0.83, 95% CI 0.74 to 0.94); this comparison was confined to studies using either carboplatin monotherapy or carboplatin/paclitaxel.
Considering adverse events and treatment toxicities in this population, Shi 2020 concluded that anaemia grade ≥ 3 was more likely with platinum/PLD, compared to platinum/paclitaxel (RR 0.52, 95% CI 0.38 to 0.70), in agreement with our findings. Grade ≥ 3 neutropenia was not significantly different with platinum/PLD (RR 1.03, 95% 0.78‐1.35). In contrast to our uncertainty around grade ≥ 3 thrombocytopenia, Shi 2020 reported RR 0.30 favouring platinum/paclitaxel (95% CI 0.19 to 0.47). They found that G3/4 allergy was also more common with platinum/PLD (RR 1.86, 95% CI 1.06 to 3.24).
Li 2021 included a comprehensive analysis of treatment toxicities using combination chemotherapies in relapsed, platinum‐sensitive EOC. They also agree that platinum/PLD is more likely to result in grade ≥ 3 allergy (RR 0.38 favouring platinum/paclitaxel, 95% CI 0.19 to 0.78) and grade ≥ 3 anaemia (RR 1.86, 95% CI 1.22 to 2.71). In contrast with our results, G3/4 neutropenia was less likely when using carboplatin/PLD (over carboplatin/paclitaxel; RR 0.76, 95% CI 0.67 to 0.86). Interestingly, they also reported no significant difference in the incidence of G3/4 anaemia and G3/4 thrombocytopenia at PLD 30 mg/m2 versus 45 mg/m2. In agreement with Shi 2020, G3/4 thrombocytopenia was also more common with PLD/platinum, over paclitaxel/platinum (HR 2.67, 95% CI 1.94 to 3.67).
Regarding HFS and mucositis, Li 2021 assessed these toxicities considering CTCAE grade 2 and above. They noted HFS was higher in carboplatin/PLD than carboplatin/paclitaxel (RR 6.12, 95% CI 3.84 to 9.76), as was G2 and above mucositis (RR 2.12, 95% CI 1.53 to 2.93). Interestingly, grade 3/4 HFS rates were comparable between carboplatin/PLD and carboplatin/paclitaxel (RR 2.76, 95% CI 0.50 to 15.60).
Edwards 2015, a health technology assessment for the National Institute for Healthcare Research, also included a network meta‐analysis (NMA) and cost‐effectiveness analysis of treatment for recurrent EOC. The cost‐effectiveness analysis included 21 economic evaluations. They concluded that in platinum‐sensitive disease treated with non‐platinum‐based therapies, it was unclear whether PLD would be considered cost‐effective compared with paclitaxel at a threshold of GBP 30,000 per additional Quality Adjusted Life Year (QALY), and that the addition of trabectedin to PLD was unlikely to be considered cost‐effective. For women with platinum‐resistant recurrence, it was unlikely that topotecan would be considered cost‐effective compared with PLD.
PLD with conventional chemotherapy compared to PLD alone in recurrent platinum‐sensitive EOC and platinum‐resistant EOC
No previously published systematic reviews or meta‐analyses exist comparing PLD monotherapy with PLD plus another conventional chemotherapy. This is the case for both platinum‐sensitive and platinum‐resistant settings.
PLD compared to conventional chemotherapy alone in recurrent platinum‐resistant EOC, and PLD compared to targeted therapy alone in recurrent platinum‐resistant EOC
Our review suggests that PLD likely results in no change in overall survival, alongside reporting uncertainty regarding PFS and the benefit of PLD monotherapy, when compared to other conventional chemotherapy monotherapies.
Comparison with existing literature and previously published reviews is limited in this setting. Staropoli 2014 compared PLD to chemotherapy monotherapy, but only included one study in this PFS subgroup analysis (Colombo 2012). When comparing OS, they included two studies and predicted PLD had little influence (HR 1.06. 95% CI 0.92 to 1.23). Li 2021 grouped all monotherapies together under the one analysis (PLD versus conventional chemotherapy monotherapy, and PLD versus targeted treatment monotherapy). For this subgroup, though they identified no difference in PFS (HR 1.02, 95% CI 0.90 to 1.16) or OS (HR 0.88, 95% CI 0.77 to 1.01), their data cannot be directly compared with our own results, which split chemotherapy and targeted therapy monotherapy subgroups, and analysed them separately. Finally, Edwards 2015 concluded that in the platinum‐resistant/refractory setting, PLD monotherapy resulted in no statistically significant differences in PFS or OS, compared to comparators.
When considering adverse events, it is once again not possible to directly compare our results to existing reviews and meta‐analyses. For adverse event analysis, Staropoli 2014 grouped together platinum‐resistant trials with two studies that used non‐conventional chemotherapies, and these trials also used a cohort including both platinum‐resistant and partial platinum‐sensitive patients. They did note, in this grouped analysis, that PLD was not superior even when considering toxicity profiles, but further comparison is limited.
Li 2021 also analysed treatment toxicities for PLD versus other monotherapy, but as all other monotherapies were grouped together (including PARP inhibitors and antibody‐drug conjugates) comparison with our data is limited. In their grouped analysis, they did report worse grade ≥ 3 mucositis/stomatitis with PLD (RR 0.10 favouring other agents, 95% CI 0.04 to 0.23) and HFS (RR 0.03 favouring other agents, 95% CI 0.01 to 0.09), alongside noting no difference in grade 3/4 anaemia, grade 3/4 thrombocytopenia, and grade 3/4 neutropenia. Further subgroup analysis of toxicities in the context of PLD versus other monotherapy also concluded that there was a dose‐dependent relationship for mucositis and PPE severity, considering the two different PLD doses of 30 mg/m2 and 50 mg/m2.
PLD with targeted therapy compared to PLD alone in recurrent platinum‐resistant EOC
No previously published systematic reviews or meta‐analyses exist comparing PLD monotherapy with PLD plus a targeted therapy. This is the case for both platinum‐sensitive and platinum‐resistant settings.
PLD compared to immunotherapy in recurrent platinum‐resistant EOC, and PLD with immunotherapy compared to PLD alone in recurrent platinum‐resistant EOC
No previously published systematic reviews or meta‐analyses investigating PLD in EOC have included immunotherapy trials. This is the case for both platinum‐sensitive and platinum‐resistant settings.
Authors' conclusions
Implications for practice.
In platinum‐sensitive recurrence, pegylated liposomal doxorubicin (PLD), in combination with a platinum agent, has similar efficacy to other combinations, in terms of overall survival (OS), although likely improves progression‐free survival (PFS). The side effect profiles differ and this, in conjunction with consideration of patient‐factors (residual toxicities of previous treatment, disease‐related symptoms, other comorbidities), is likely to determine best options for treatment.
In platinum‐resistant relapse, PLD alone appears to have similar efficacy to other chemotherapy regimens and addition of another chemotherapy agent to PLD has minimal benefit over PLD alone, but likely increases severe adverse events. Given those with relapsed epithelial ovarian cancer (EOC) have life‐limiting disease, treatment options that minimise toxicities and improve quality of life are preferable.
Effectiveness and harms of targeted therapy and immunotherapy remain uncertain and require further examination in clinical trials.
Most importantly, women with relapsed ovarian cancer have a life‐limiting illness. Evidence shows that doctors tend to be overly optimistic about prognosis, both with themselves and even more so with patients, and may be reluctant to have conversations about end of life (e.g. Barclay 2010; Lamont 2001; Stone 2007). The median OS and PFS times in the studies, outlined in Table 6, are of women fit enough to enter clinical studies, who have generally better performance status and fewer comorbidities than the general ovarian cancer patient population. Therefore, the survival of the average woman with relapsed ovarian cancer is likely to be shorter. Involvement of palliative care, alongside active treatment, may be appropriate, as discussed recently by Temkin 2022. However, nearly half (47.1%) of participants with advanced ovarian cancer, who had no further routine treatment options and were taking part in phase 1 studies, had no specialist palliative care alongside their experimental treatment (Moroney 2019).
Implications for research.
Treatment of advanced and recurrent cancers requires a careful balance of risks and benefits, the details of which need to be understood by both patient and clinician in order to make decisions about the best treatment for individuals. Without good evidence for both efficacy and harms, these decisions will be made with insufficient information, which may not be in the patient's best interest. It is therefore disappointing that toxicity and quality of life data continue to be so poorly reported, with over‐reliance on surrogate outcomes, rather than those more meaningful to patients, as previously discussed (Tattersall 2022). Agreement of minimum reporting standards and core outcomes, especially those important to patients, with study protocols published in advance, should be a requirement for all future clinical studies. Early involvement of patient advocacy groups in clinical trial design is critical to this, and should be mandated. Furthermore, standardised reporting of harms data would help to inform patients and facilitate analysis in secondary research, as outlined by the CoRe Outcomes in Women’s and Newborn health (CROWN) initiative.
As PLD is a recognised treatment in recurrent EOC, studies are likely to continue to include PLD in their arms, either in combination with, or in comparison to, another treatment. This is often as part of a 'physician choice' chemotherapy, which may mean that study arms contain a variety of chemotherapy treatments within each arm. Open sharing of data, broken down by specific treatment, should be a requirement of clinical studies. We are disappointed that further study data could not be included in this review, despite contacting authors for data limited to those treated with PLD. We hope that publication of this review will encourage more open sharing in the future, so these data will be able to inform patient care in the future.
There is now a bewildering array of options for treatment of EOC in second‐ and third‐line settings, and those beyond. Studies using genomic sequencing and liquid‐based biomarkers to predict which chemotherapy agent to use are ongoing (as reviewed by Khan 2021). Ideally, these studies should be brought together, with the aid of meta‐analysis and machine‐learning, in order to produce decision‐aids, to help clinicians and their patients to make informed choices, based on evidence of effectiveness and harms.
What's new
| Date | Event | Description |
|---|---|---|
| 3 July 2023 | New search has been performed | New search undertaken on 4 January 2022. |
| 3 July 2023 | New citation required but conclusions have not changed | Twelve new studies added. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 7, 2013
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
| 1 April 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 15 October 2012 | Amended | New search performed. |
| 24 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful to the following people for their generous assistance.
Dr Rowan Miller for her expert medical oncology clinical advice throughout the conduct of this review and for her helpful comments and challenge.
Authors of the protocol and original version of the review: Tess Lawrie selected and classified studies, abstracted and entered data and wrote the first draft of the review. Andy Bryant abstracted and checked data, provided statistical and methodological support and reviewed the first draft. Alison Cameron and Emma Gray co‐wrote the protocol and reviewed the first draft. Julia Dawson, who assisted with the sifting of the database searches.
The Managing Editors, Clare Jess and Gail Quinn, and the rest of the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Team (GNOC) for their invaluable administrative and editorial support.
Jo Platt for designing and running the searches.
Copy Edit Support and Joey Kwong, from the Cochrane Central Editorial Unit, for support with response to copy edit queries and aligning past and current versions of this review.
Cate Newell and her team (including Florence Gregory, Natalie Parsley and Dan Wolfe) at Somerset NHS Foundation Trust Library, UK, for obtaining copies of the many full texts.
Nicoletta Colombo for supplying Qol data for Colombo 2012.
Gabriella Ferrandina for supplying raw PFS data from MITO‐3.
Wendel Naumann for allowing us access to the unpublished manuscript of PRECEDENT and for providing additional unpublished data.
Eric Pujade‐Lauraine and Nicholas Gane for additional data on side‐effects from CALYPSO.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
The authors and GNOC, are grateful to the following peer reviewers for their time and comments: Christine Ang, Ruth Payne, Andrew Bryant and Hanna Dahlstrand.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL
MeSH descriptor Ovarian Neoplasms explode all trees
ovar* near/5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)
(#1 OR #2)
MeSH descriptor Doxorubicin explode all trees
doxorubicin
caelyx
doxil
(#4 OR #5 OR #6 OR #7)
(#3 AND #8)
Appendix 2. MEDLINE search strategy
Medline Ovid
exp Ovarian Neoplasms/
(ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)).mp.
1 or 2
exp Doxorubicin/
doxorubicin.mp.
caelyx.mp.
doxil.mp.
4 or 5 or 6 or 7
3 and 8
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
10 or 11 or 12 or 13 or 14 or 15 or 16
9 and 17
exp animals/ not humans.sh.
18 not 19
Key:
mp = protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier pt = publication type ab = abstract ti = title sh = subject heading
Appendix 3. EMBASE search strategy
EMBASE Ovid
exp ovary tumor/
(ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)).mp.
1 or 2
exp doxorubicin/
doxorubicin.mp.
caelyx.mp.
doxil.mp.
4 or 5 or 6 or 7
3 and 8
crossover procedure/
randomized controlled trial/
single blind procedure/
random*.mp.
factorial*.mp.
(crossover* or cross over* or cross‐over).mp.
placebo*.mp.
(doubl* adj blind*).mp.
(singl* adj blind*).mp.
assign*.mp.
allocat*.mp.
volunteer*.mp.
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
9 and 22
Key:
mp = protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier pt = publication type ab = abstract ti = title sh = subject heading
Data and analyses
Comparison 1. Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus alternative combination chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 5 | 2006 | Hazard Ratio (IV, Fixed, 95% CI) | 0.93 [0.83, 1.04] |
| 1.1.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.40, 1.21] |
| 1.1.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1164 | Hazard Ratio (IV, Fixed, 95% CI) | 1.01 [0.88, 1.17] |
| 1.1.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 99 | Hazard Ratio (IV, Fixed, 95% CI) | 1.04 [0.61, 1.78] |
| 1.1.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 682 | Hazard Ratio (IV, Fixed, 95% CI) | 0.81 [0.67, 0.98] |
| 1.2 Progression‐free survival | 5 | 2006 | Hazard Ratio (IV, Fixed, 95% CI) | 0.81 [0.74, 0.89] |
| 1.2.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Hazard Ratio (IV, Fixed, 95% CI) | 0.52 [0.31, 0.88] |
| 1.2.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1164 | Hazard Ratio (IV, Fixed, 95% CI) | 0.84 [0.75, 0.95] |
| 1.2.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 99 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.45, 1.05] |
| 1.2.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 682 | Hazard Ratio (IV, Fixed, 95% CI) | 0.81 [0.68, 0.96] |
| 1.3 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 PLD with carboplatin versus paclitaxel with carboplatin | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Overall Severe Adverse Events (grade ≥ 3) | 2 | 834 | Risk Ratio (IV, Fixed, 95% CI) | 1.11 [0.95, 1.30] |
| 1.4.1 PLD with carboplatin versus paclitaxel with carboplatin | 1 | 173 | Risk Ratio (IV, Fixed, 95% CI) | 1.11 [0.94, 1.31] |
| 1.4.2 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.72, 1.89] |
| 1.5 SevAE: Anaemia (grade ≥ 3) | 5 | 1961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.02, 1.85] |
| 1.5.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.66 [0.61, 184.70] |
| 1.5.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.03, 2.52] |
| 1.5.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.60, 3.52] |
| 1.5.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.62, 1.58] |
| 1.6 SevAE: Hand‐foot syndrome (grade ≥ 3) | 5 | 1961 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.01 [1.00, 16.01] |
| 1.6.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 68.66] |
| 1.6.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.92, 20.15] |
| 1.6.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.6.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7 SevAE: Neurological (grade ≥ 3) | 4 | 1900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.20, 0.74] |
| 1.7.1 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.08, 0.48] |
| 1.7.2 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.13, 73.34] |
| 1.7.3 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.48, 12.68] |
| 1.8 SevAE: Neutropenia (grade ≥ 3) | 5 | 1961 | Risk Ratio (IV, Fixed, 95% CI) | 0.78 [0.70, 0.88] |
| 1.8.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Risk Ratio (IV, Fixed, 95% CI) | 14.52 [2.04, 103.16] |
| 1.8.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (IV, Fixed, 95% CI) | 0.81 [0.70, 0.94] |
| 1.8.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 99 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.64, 1.01] |
| 1.8.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (IV, Fixed, 95% CI) | 0.54 [0.38, 0.77] |
| 1.9 SevAE: Thrombocytopenia (grade ≥ 3) | 5 | 1961 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [0.90, 1.44] |
| 1.9.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Risk Ratio (IV, Fixed, 95% CI) | 3.87 [1.21, 12.36] |
| 1.9.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (IV, Fixed, 95% CI) | 2.69 [1.83, 3.96] |
| 1.9.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 99 | Risk Ratio (IV, Fixed, 95% CI) | 0.75 [0.46, 1.23] |
| 1.9.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (IV, Fixed, 95% CI) | 0.53 [0.36, 0.78] |
| 1.10 SevAE: Stomatitis (grade ≥ 3) | 4 | 1900 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.01, 6.28] |
| 1.10.1 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.90, 6.61] |
| 1.10.2 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.10.3 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.31, 28.43] |
| 1.11 SevAE: Vomiting (grade ≥ 3) | 5 | 1961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.78, 2.02] |
| 1.11.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 68.66] |
| 1.11.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.68, 2.00] |
| 1.11.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.11.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.54, 4.13] |
| 1.12 SevAE: Fatigue (grade ≥ 3) | 5 | 1961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.50] |
| 1.12.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.26, 8.09] |
| 1.12.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.63, 1.62] |
| 1.12.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.12.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.39, 2.09] |
| 1.13 SevAE: Arthralgia/myalgia (grade ≥ 3) | 4 | 1862 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.85] |
| 1.13.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.63] |
| 1.13.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.48] |
| 1.13.3 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.78] |
| 1.14 SevAE: Hypersensitivity reactions (grade ≥ 3) | 5 | 1961 | Risk Ratio (IV, Fixed, 95% CI) | 0.27 [0.15, 0.48] |
| 1.14.1 PLD with carboplatin versus carboplatin alone | 1 | 61 | Risk Ratio (IV, Fixed, 95% CI) | 0.09 [0.01, 1.53] |
| 1.14.2 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (IV, Fixed, 95% CI) | 0.29 [0.15, 0.54] |
| 1.14.3 PLD with carboplatin versus gemcitabine with carboplatin | 1 | 99 | Risk Ratio (IV, Fixed, 95% CI) | 0.20 [0.01, 4.14] |
| 1.14.4 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (IV, Fixed, 95% CI) | 0.20 [0.01, 4.11] |
| 1.15 Serious AE: Treatment‐related death | 3 | 1801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.29, 7.15] |
| 1.15.1 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.37 [0.26, 111.66] |
| 1.15.2 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.44] |
| 1.16 Discontinuation due to toxicity | 3 | 1811 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.75, 1.15] |
| 1.16.1 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1150 | Risk Ratio (IV, Fixed, 95% CI) | 0.38 [0.26, 0.57] |
| 1.16.2 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [1.03, 1.70] |
| 1.17 Antibiotics required | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.17.1 PLD with carboplatin versus paclitaxel with carboplatin | 2 | 1144 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.57, 2.21] |
| 1.18 Granulocyte colony stimulating factor (G‐CSF) required | 2 | 838 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [0.85, 1.54] |
| 1.18.1 PLD with carboplatin versus paclitaxel with carboplatin | 1 | 177 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [0.84, 1.54] |
| 1.18.2 PLD with carboplatin and bevacizumab versus gemcitabine with carboplatin and bevacizumab | 1 | 661 | Risk Ratio (IV, Fixed, 95% CI) | 1.49 [0.25, 8.84] |
Comparison 2. Platinum‐sensitive recurrent EOC: PLD with chemotherapy versus PLD alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Overall survival | 2 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 PLD with trabectedin | 2 | 1006 | Hazard Ratio (IV, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 2.2 Progression‐free survival | 2 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 PLD with trabectedin | 2 | 732 | Hazard Ratio (IV, Fixed, 95% CI) | 0.85 [0.72, 1.00] |
| 2.3 Overall Severe Adverse Events (grade ≥3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 PLD with trabectedin | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 SevAE: Anaemia (grade ≥3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.1 PLD with trabectedin | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 SevAE: Hand‐foot syndrome (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 PLD with trabectedin | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6 SevAE: Neutropenia (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.1 PLD with trabectedin | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.7 SevAE: Thrombocytopenia (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7.1 PLD with trabectedin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.8 SevAE: Stomatitis (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.8.1 PLD with trabectedin | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.9 SevAE: Vomiting (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9.1 PLD with trabectedin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.10 SevAE: Fatigue (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.10.1 PLD with trabectedin | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Platinum‐resistant recurrent EOC: PLD versus other chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Overall survival | 6 | 1995 | Hazard Ratio (IV, Random, 95% CI) | 0.96 [0.77, 1.19] |
| 3.1.1 PLD versus gemcitabine | 2 | 348 | Hazard Ratio (IV, Random, 95% CI) | 0.80 [0.52, 1.21] |
| 3.1.2 PLD versus lurbinectedin | 1 | 348 | Hazard Ratio (IV, Random, 95% CI) | 1.13 [0.88, 1.44] |
| 3.1.3 PLD versus patupilone | 1 | 828 | Hazard Ratio (IV, Random, 95% CI) | 1.08 [0.92, 1.26] |
| 3.1.4 PLD with topotecan | 1 | 255 | Hazard Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 3.1.5 PLD versus paclitaxel | 1 | 216 | Hazard Ratio (IV, Random, 95% CI) | 1.07 [0.81, 1.42] |
| 3.2 Progression‐free survival | 4 | 1803 | Hazard Ratio (IV, Random, 95% CI) | 0.94 [0.85, 1.04] |
| 3.2.1 PLD versus gemcitabine | 1 | 153 | Hazard Ratio (IV, Random, 95% CI) | 1.15 [0.78, 1.70] |
| 3.2.2 PLD versus lurbinectedin | 1 | 348 | Hazard Ratio (IV, Random, 95% CI) | 0.94 [0.73, 1.21] |
| 3.2.3 PLD versus patupilone | 1 | 828 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.81, 1.12] |
| 3.2.4 PLD versus topotecan | 1 | 474 | Hazard Ratio (IV, Random, 95% CI) | 0.89 [0.75, 1.06] |
| 3.3 Overall Severe Adverse Events (grade ≥ 3) | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.1 PLD versus gemcitabine | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.2 PLD versus patupilone | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4 SevAE: Anaemia (grade ≥ 3) | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.4.1 PLD versus gemcitabine | 2 | 338 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.27, 2.11] |
| 3.4.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.40, 1.16] |
| 3.4.3 PLD versus patupilone | 1 | 811 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.42, 1.60] |
| 3.4.4 PLD versus topotecan | 1 | 474 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.11, 0.34] |
| 3.5 SevAE: Hand‐foot syndrome (grade ≥ 3) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 PLD versus gemcitabine | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.19 [2.04, 113.27] |
| 3.5.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 53.70 [3.24, 889.90] |
| 3.5.3 PLD versus patupilone | 1 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 109.10 [6.76, 1760.19] |
| 3.5.4 PLD versus topotecan | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 109.15 [6.78, 1756.69] |
| 3.5.5 PLD versus paclitaxel | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 35.00 [2.13, 574.74] |
| 3.6 SevAE: Neurological (grade ≥ 3) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.1 PLD versus gemcitabine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.2 PLD versus patupilone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.3 PLD versus paclitaxel | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7 SevAE: Neutropenia (grade ≥ 3) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.7.1 PLD versus gemcitabine | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.67] |
| 3.7.2 PLD versus lurbinectedin | 1 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.38, 1.80] |
| 3.7.3 PLD versus patupilone | 1 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [1.79, 6.30] |
| 3.7.4 PLD versus topotecan | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.11, 0.22] |
| 3.8 SevAE: Thrombocytopenia (grade ≥ 3) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.8.1 PLD versus gemcitabine | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.46] |
| 3.8.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.13] |
| 3.8.3 PLD versus topotecan | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.12] |
| 3.9 SevAE: Stomatitis (grade ≥ 3) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.9.1 PLD versus gemcitabine | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.50, 12.82] |
| 3.9.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.85 [2.94, 32.95] |
| 3.9.3 PLD versus patupilone | 1 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 20.15 [4.91, 82.75] |
| 3.9.4 PLD versus topotecan | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.67 [2.66, 145.35] |
| 3.9.5 PLD versus paclitaxel | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.00 [1.45, 83.73] |
| 3.10 SevAE: Vomiting (grade ≥ 3) | 4 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.10.1 PLD versus gemcitabine | 2 | 338 | Risk Ratio (IV, Fixed, 95% CI) | 0.57 [0.21, 1.50] |
| 3.10.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (IV, Fixed, 95% CI) | 0.14 [0.02, 1.10] |
| 3.10.3 PLD verusus patupilone | 1 | 811 | Risk Ratio (IV, Fixed, 95% CI) | 0.74 [0.44, 1.23] |
| 3.11 SevAE: Diarrhoea (grade ≥ 3) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.11.1 PLD versus gemcitabine | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.08, 4.55] |
| 3.11.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 7.16] |
| 3.11.3 PLD versus patupilone | 1 | 812 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.04, 0.17] |
| 3.12 SevAE: Fatigue (grade ≥ 3) | 5 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.12.1 PLD versus gemcitabine | 2 | 338 | Risk Ratio (IV, Fixed, 95% CI) | 0.40 [0.14, 1.14] |
| 3.12.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (IV, Fixed, 95% CI) | 0.54 [0.20, 1.45] |
| 3.12.3 PLD versus patupilone | 1 | 811 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 3.12.4 PLD versus paclitaxel | 1 | 216 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.22, 2.90] |
| 3.13 SevAE: Hypersensitivity reactions (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.1 PLD versus gemcitabine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.14 Dose reductions | 4 | 1773 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.54, 2.01] |
| 3.14.1 PLD versus gemcitabine | 1 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.30, 2.39] |
| 3.14.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (IV, Random, 95% CI) | 2.96 [1.70, 5.18] |
| 3.14.3 PLD versus patupilone | 1 | 811 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.74, 1.23] |
| 3.14.4 PLD versus topotecan | 1 | 474 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.41, 0.67] |
| 3.15 Dose delays | 3 | 962 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.55, 1.69] |
| 3.15.1 PLD versus gemcitabine | 1 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.31, 1.18] |
| 3.15.2 PLD versus lurbinectedin | 1 | 345 | Risk Ratio (IV, Random, 95% CI) | 1.81 [1.09, 2.99] |
| 3.15.3 PLD versus topotecan | 1 | 474 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.69, 0.94] |
Comparison 4. Platinum‐resistant recurrent EOC: PLD with chemotherapy versus PLD alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Overall survival | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 PLD with trabectedin | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Progression‐free survival | 2 | 353 | Hazard Ratio (IV, Fixed, 95% CI) | 0.94 [0.73, 1.22] |
| 4.2.1 PLD with trabectedin | 1 | 228 | Hazard Ratio (IV, Fixed, 95% CI) | 0.95 [0.70, 1.29] |
| 4.2.2 PLD with canfosfamide | 1 | 125 | Hazard Ratio (IV, Fixed, 95% CI) | 0.92 [0.58, 1.46] |
| 4.3 Overall Severe Adverse Events (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3.1 PLD with trabectedin | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4 SevAE: Anaemia (grade ≥ 3) | 2 | 785 | Risk Ratio (IV, Fixed, 95% CI) | 2.38 [1.46, 3.87] |
| 4.4.1 PLD with trabectedin | 1 | 663 | Risk Ratio (IV, Fixed, 95% CI) | 2.54 [1.45, 4.43] |
| 4.4.2 PLD with canfosfamide | 1 | 122 | Risk Ratio (IV, Fixed, 95% CI) | 1.93 [0.71, 5.22] |
| 4.5 SevAE: Hand‐foot syndrome (grade ≥ 3) | 2 | 785 | Risk Ratio (IV, Fixed, 95% CI) | 0.24 [0.14, 0.40] |
| 4.5.1 PLD with trabectedin | 1 | 663 | Risk Ratio (IV, Fixed, 95% CI) | 0.20 [0.11, 0.35] |
| 4.5.2 PLD with canfosfamide | 1 | 122 | Risk Ratio (IV, Fixed, 95% CI) | 0.50 [0.15, 1.62] |
| 4.6 SevAE: Neurological (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.6.1 PLD with trabectedin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.7 SevAE: Neutropenia (grade ≥ 3) | 2 | 785 | Risk Ratio (IV, Fixed, 95% CI) | 2.75 [2.23, 3.38] |
| 4.7.1 PLD with trabectedin | 1 | 663 | Risk Ratio (IV, Fixed, 95% CI) | 2.80 [2.25, 3.48] |
| 4.7.2 PLD with canfosfamide | 1 | 122 | Risk Ratio (IV, Fixed, 95% CI) | 2.19 [1.05, 4.59] |
| 4.8 SevAE: Thrombocytopenia (grade ≥ 3) | 2 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.70 [3.90, 15.19] |
| 4.8.1 PLD with trabectedin | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.56 [3.67, 15.54] |
| 4.8.2 PLD with canfosfamide | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.77 [1.16, 66.41] |
| 4.9 SevAE: Stomatitis (grade ≥ 3) | 2 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.13, 0.70] |
| 4.9.1 PLD with trabectedin | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.59] |
| 4.9.2 PLD with canfosfamide | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.20, 2.49] |
| 4.10 SevAE: Vomiting (grade ≥ 3) | 2 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.77 [1.91, 7.41] |
| 4.10.1 PLD with trabectedin | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.81 [2.16, 10.70] |
| 4.10.2 PLD with canfosfamide | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.37, 5.85] |
| 4.11 SevAE: Fatigue (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.11.1 PLD with trabectedin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.12 Serious AE: Treatment‐related death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.12.1 trabectedin with PLD vs PLD alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.13 Dose delays | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.13.1 canfosfamide with PLD vs PLD alone | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.14 Dose reductions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.14.1 canfosfamide with PLD vs PLD alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Platinum‐resistant recurrent EOC: PLD versus targeted therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Overall survival | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.1 PLD versus olaparib 400 mg | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2 Progression‐free survival | 2 | 160 | Hazard Ratio (IV, Random, 95% CI) | 1.23 [0.82, 1.84] |
| 5.2.1 PLD versus lifastuzumab | 1 | 95 | Hazard Ratio (IV, Random, 95% CI) | 1.28 [0.76, 2.16] |
| 5.2.2 PLD versus olaparib 400mg | 1 | 65 | Hazard Ratio (IV, Random, 95% CI) | 1.16 [0.62, 2.17] |
| 5.3 Overall Severe Adverse Events (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.1 PLD versus lifastuzumab | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.4 SevAE: Anaemia (grade ≥ 3) | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.97] |
| 5.4.1 PLD versus lifastuzumab | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.63] |
| 5.4.2 PLD versus olaparib 400mg | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.98] |
| 5.5 SevAE: Hand‐foot syndrome (grade ≥ 3) | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 25.00 [1.54, 405.08] |
| 5.5.1 PLD versus lifastuzumab | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.5.2 PLD versus olaparib 400mg | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 25.00 [1.54, 405.08] |
| 5.6 SevAE: Neurological (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6.1 PLD versus lifastuzumab | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.7 SevAE: Neutropenia (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.7.1 PLD versus lifastuzumab | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.8 SevAE: Stomatitis (grade ≥ 3) | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 5.87 [0.72, 47.84] |
| 5.8.1 PLD versus lifastuzumab | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 6.85 [0.36, 129.10] |
| 5.8.2 PLD versus olaparib 400mg | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.25, 100.20] |
| 5.9 SevAE: Vomiting (grade ≥ 3) | 2 | 157 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.30, 5.70] |
| 5.9.1 PLD versus lifastuzumab | 1 | 93 | Risk Ratio (IV, Random, 95% CI) | 1.47 [0.26, 8.38] |
| 5.9.2 PLD versus olaparib 400mg | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.07, 15.30] |
| 5.10 SevAE: Diarrhoea (grade ≥ 3) | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.27, 15.56] |
| 5.10.1 PLD versus lifastuzumab | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.19] |
| 5.10.2 PLD versus olaparib 400mg | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.25, 100.20] |
| 5.11 SevAE: Fatigue (grade ≥ 3) | 2 | 157 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.38, 3.72] |
| 5.11.1 PLD versus lifastuzumab | 1 | 93 | Risk Ratio (IV, Random, 95% CI) | 1.47 [0.26, 8.38] |
| 5.11.2 PLD versus olaparib 400mg | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.22, 4.59] |
| 5.12 Dose reductions | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.12.1 PLD versus olaparib 400 mg | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Platinum‐resistant recurrent EOC: PLD with targeted therapy versus PLD alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Overall survival | 4 | 647 | Hazard Ratio (IV, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 6.1.1 PLD with apatinib | 1 | 152 | Hazard Ratio (IV, Random, 95% CI) | 0.66 [0.40, 1.09] |
| 6.1.2 PLD with trebaninib | 1 | 223 | Hazard Ratio (IV, Random, 95% CI) | 0.94 [0.64, 1.39] |
| 6.1.3 PLD with vintafolide | 1 | 149 | Hazard Ratio (IV, Random, 95% CI) | 1.01 [0.68, 1.50] |
| 6.1.4 PLD with olaratumab | 1 | 123 | Hazard Ratio (IV, Random, 95% CI) | 1.10 [0.71, 1.71] |
| 6.2 Progression‐free survival | 5 | 877 | Hazard Ratio (IV, Random, 95% CI) | 0.78 [0.58, 1.04] |
| 6.2.1 PLD with apatinib | 1 | 152 | Hazard Ratio (IV, Random, 95% CI) | 0.44 [0.28, 0.70] |
| 6.2.2 PLD with trebaninib | 1 | 223 | Hazard Ratio (IV, Random, 95% CI) | 0.92 [0.68, 1.24] |
| 6.2.3 PLD with vintafolide | 2 | 379 | Hazard Ratio (IV, Random, 95% CI) | 0.78 [0.52, 1.16] |
| 6.2.4 PLD with olaratumab | 1 | 123 | Hazard Ratio (IV, Random, 95% CI) | 1.04 [0.70, 1.56] |
| 6.3 Overall Severe Adverse Events (grade ≥3) | 4 | 794 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.90, 1.48] |
| 6.3.1 PLD with apatinib | 1 | 146 | Risk Ratio (IV, Random, 95% CI) | 2.22 [1.30, 3.81] |
| 6.3.2 PLD with trebaninib | 1 | 216 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.88, 1.18] |
| 6.3.3 PLD with vintafolide | 1 | 309 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.91, 1.65] |
| 6.3.4 PLD with Olaratumab | 1 | 123 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.69, 1.20] |
| 6.4 SevAE: Anaemia (grade ≥ 3) | 4 | 647 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.32, 1.26] |
| 6.4.1 PLD with apatinib | 1 | 146 | Risk Ratio (IV, Random, 95% CI) | 0.32 [0.03, 3.05] |
| 6.4.2 PLD with trebananib | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.16, 3.13] |
| 6.4.3 PLD with vintafolide | 1 | 157 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.22, 1.28] |
| 6.4.4 PLD with Olaratumab | 1 | 123 | Risk Ratio (IV, Random, 95% CI) | 2.95 [0.32, 27.60] |
| 6.5 SevAE: Hand‐foot syndrome (grade ≥ 3) | 4 | 647 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.07, 2.88] |
| 6.5.1 PLD with apatinib | 1 | 146 | Risk Ratio (IV, Random, 95% CI) | 1.95 [0.37, 10.30] |
| 6.5.2 PLD with trebaninib | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 1.62 [0.86, 3.05] |
| 6.5.3 PLD with vintafolide | 1 | 157 | Risk Ratio (IV, Random, 95% CI) | 2.57 [0.59, 11.16] |
| 6.5.4 PLD with Olaratumab | 1 | 123 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.53, 5.58] |
| 6.6 SevAE: Neurological (grade ≥ 3) | 2 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 4.25 [0.23, 77.45] |
| 6.6.1 PLD with trebaninib | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.6.2 PLD with vintafolide | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 4.25 [0.23, 77.45] |
| 6.7 SevAE: Neutropenia (grade ≥ 3) | 4 | 647 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.69, 1.74] |
| 6.7.1 PLD with apatinib | 1 | 146 | Risk Ratio (IV, Random, 95% CI) | 1.78 [0.70, 4.57] |
| 6.7.2 PLD with trebaninib | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.25, 1.36] |
| 6.7.3 PLD with vintafolide | 1 | 157 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.55, 2.08] |
| 6.7.4 PLD with olaratumab | 1 | 123 | Risk Ratio (IV, Random, 95% CI) | 1.57 [0.55, 4.54] |
| 6.8 SevAE: Thrombocytopenia (grade ≥ 3) | 2 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.11, 9.49] |
| 6.8.1 PLD with apatinib | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 4.87 [0.24, 99.65] |
| 6.8.2 PLD with vintafolide | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.12, 1.79] |
| 6.9 SevAE: Stomatitis (grade ≥ 3) | 4 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.43, 2.19] |
| 6.9.1 PLD with apatinib | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 4.87 [0.24, 99.65] |
| 6.9.2 PLD with trebaninib | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.39, 3.21] |
| 6.9.3 PLD with vintafolide | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.30, 2.96] |
| 6.9.4 PLD with Olaratumab | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.99] |
| 6.10 SevAE: Vomiting (grade ≥ 3) | 3 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.35, 1.87] |
| 6.10.1 PLD with apatinib | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.10.2 PLD with trebaninib | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.39, 3.21] |
| 6.10.3 PLD with Olaratumab | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.13, 1.88] |
| 6.11 SevAE: Diarrhoea (grade ≥ 3) | 2 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.18, 2.24] |
| 6.11.1 PLD with trebaninib | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.14, 2.34] |
| 6.11.2 PLD with olaratumab | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.38] |
| 6.12 SevAE: Fatigue (grade ≥ 3) | 3 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.30, 14.52] |
| 6.12.1 PLD with apatinib | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.12.2 PLD with trebaninib | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.37, 2.46] |
| 6.12.3 PLD with Olaratumab | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 6.89 [0.87, 54.32] |
| 6.13 Serious AE: Treatment‐related death | 5 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.33, 3.20] |
| 6.13.1 PLD with apatinib | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.13.2 PLD with trebaninib | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.14, 6.67] |
| 6.13.3 PLD with vintafolide | 2 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.26, 4.35] |
| 6.13.4 PLD with olaratumab | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.14 Dose reductions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.14.1 PLD with apatinib | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Platinum‐resistant recurrent EOC: PLD versus immunotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Overall survival | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1.1 PLD versus avelumab | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2 Progression‐free survival | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2.1 PLD versus avelumab | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.3 Overall Severe Adverse Events (grade ≥ 3) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.3.1 PLD versus avelumab | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.4 SevAE: Anaemia (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5 SevAE: Hand‐foot syndrome (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.6 SevAE: Neutropenia (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.6.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.7 SevAE: Thrombocytopenia (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.7.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.8 SevAE: Stomatitis (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.8.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.9 SevAE: Vomiting (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.9.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.10 SevAE: Diarrhoea (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.10.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.11 SevAE: Fatigue (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.11.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.12 Dose reductions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.12.1 PLD versus avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Platinum‐resistant recurrent EOC: PLD with immunotherapy versus PLD alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Overall survival | 2 | 675 | Hazard Ratio (IV, Fixed, 95% CI) | 0.89 [0.69, 1.15] |
| 8.1.1 PLD with avelumab | 1 | 378 | Hazard Ratio (IV, Fixed, 95% CI) | 0.89 [0.69, 1.15] |
| 8.1.2 PLD with motolimod | 1 | 297 | Hazard Ratio (IV, Fixed, 95% CI) | 1.22 [0.02, 68.79] |
| 8.2 Progression‐free survival | 2 | 675 | Hazard Ratio (IV, Fixed, 95% CI) | 0.78 [0.54, 1.14] |
| 8.2.1 PLD vs avelumab | 1 | 378 | Hazard Ratio (IV, Fixed, 95% CI) | 0.78 [0.54, 1.14] |
| 8.2.2 PLD with motolimod | 1 | 297 | Hazard Ratio (IV, Fixed, 95% CI) | 1.21 [0.01, 224.96] |
| 8.3 Overall Severe Adverse Events (grade ≥ 3) | 2 | 653 | Risk Ratio (IV, Fixed, 95% CI) | 1.12 [0.96, 1.30] |
| 8.3.1 PLD with avelumab | 1 | 359 | Risk Ratio (IV, Fixed, 95% CI) | 1.35 [1.03, 1.78] |
| 8.3.2 PLD with motolimod | 1 | 294 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [0.87, 1.23] |
| 8.4 SevAE: Anaemia (grade ≥ 3) | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.27, 1.57] |
| 8.4.1 PLD with avelumab | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.24, 1.78] |
| 8.4.2 PLD with motolimod | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.93] |
| 8.5 SevAE: Hand‐foot syndrome (grade ≥ 3) | 2 | 653 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.81, 1.42] |
| 8.5.1 PLD with avelumab | 1 | 359 | Risk Ratio (IV, Fixed, 95% CI) | 1.95 [0.90, 4.21] |
| 8.5.2 PLD with motolimod | 1 | 294 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.73, 1.32] |
| 8.6 SevAE: Neurological (grade ≥ 3) | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.37] |
| 8.6.1 PLD with avelumab | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 71.15] |
| 8.6.2 PLD with motolimod | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.68, 1.34] |
| 8.7 SevAE: Neutropenia (grade ≥ 3) | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.47, 2.48] |
| 8.7.1 PLD with avelumab | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.40, 2.39] |
| 8.7.2 PLD with motolimod | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 21.82] |
| 8.8 SevAE: Thrombocytopenia (grade ≥ 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.8.1 PLD with avelumab | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.9 SevAE: Stomatitis (grade ≥ 3) | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.62, 3.11] |
| 8.9.1 PLD with avelumab | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.67, 3.72] |
| 8.9.2 PLD with motolimod | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 8.10 SevAE: Vomiting (grade ≥ 3) | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.38, 2.60] |
| 8.10.1 PLD with avelumab | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.09] |
| 8.10.2 PLD with motolimod | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.45, 4.31] |
| 8.11 SevAE: Diarrhoea (grade ≥ 3) | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [0.60, 21.92] |
| 8.11.1 PLD with avelumab | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 71.15] |
| 8.11.2 PLD with motolimod | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.45, 35.36] |
| 8.12 SevAE: Fatigue (grade ≥ 3) | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.00, 7.55] |
| 8.12.1 PLD versus avelumab | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.91, 11.58] |
| 8.12.2 PLD with motolimod | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.37, 10.75] |
| 8.13 Serious AE: Treatment‐related death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.13.1 PLD with motolimod | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.14 Dose reductions | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 8.14.1 PLD with avelumab | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
APPROVE.
| Study characteristics | ||
| Methods | Multicentre, randomised, controlled, open‐label, phase II trial | |
| Participants | 152 PR |
|
| Interventions | Intervention: apatinib 250 mg orally once daily and PLD (40 mg/m2) IV every 4 weeks Control: PLD (40 mg/m2) IV every 4 weeks |
|
| Outcomes |
Primary Outcome PFS Secondary outcomes OS Objective response rate (ORR) Disease control rate (DCR) Safety |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (1:1) via an interactive web response system. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | RECIST criteria used but unclear if assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 57/74 and 53/72 did not complete 6 cycles of PLD. |
| Selective reporting (reporting bias) | Unclear risk | OS data immature. |
| Other bias | Unclear risk | 57/74 and 53/72 did not complete 6 cycles of PLD Drs She, Guan, and Hou reported personal fees from Jiangsu Hengrui Pharmaceutical Co outside the submitted work. Dr Wu reported grants from National Key R&D Program of China during the conduct of the study as well as nonfinancial support from Jiangsu Hengrui Pharmaceuticals Co Ltd and the CSPC Pharmaceutical Group Co Ltd outside the submitted work. No other disclosures were reported. |
ASSIST‐3.
| Study characteristics | ||
| Methods | Phase III multicentre RCT (ID not found on trial registries); abstract only; no further methodological details. | |
| Participants | 247 women with PR ROC (resistant and refractory) with measurable disease (RECIST), who had progressed on 2 platinum regimens. | |
| Interventions |
Intervention CAN (750 mg/m²) and carboplatin (AUC 5) (carbo) Control PLD (50 mg/m²) IV every 4 weeks until progression |
|
| Outcomes | ORR PFS Safety QoL |
|
| Notes | Published results included the following statements with little supporting data.
Overall median survival had not been reached at the time of the 2007 ASCO proceedings where these results were reported. Subgroup analyses of women with time from last carboplatin dose (TFP) = 6 months 'reported large differences in ORR and QoL and statistical significance in PFS and survival' in favour of the experimental group (CAN/carboplatin), but this subgroup consisted of 19 women in each group and 58% (11/19) of the CAN/carboplatin arm were censored (compared with 3/19 in the PLD arm). We emailed Dr Rose and Telik, Inc in November 2012 for further information and final survival and safety data but received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | ORR results differed between clinician and independent radiological assessments, however it is not stated which assessment was used in the analyses. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Insufficient data. The abstract states "25% of patients discontinued treatment without documented progression." Final results not reported. Censoring imbalance. |
| Selective reporting (reporting bias) | High risk | Preliminary results were reported at ASCO 2007 with scant useful data. Overall survival was not reported. |
| Other bias | High risk | Publication bias. We were unable to obtain any useful data despite several attempts to contact the first author and Telik. We assessed the overall risk of bias of this study as high. This study was sponsored by Telik. Authors reported to stocks in Telik and received honararia. Telik is the company that developed the study drug (TLK286). |
ASSIST‐5.
| Study characteristics | ||
| Methods | Phase III multicentre RCT (US, Brazil, Belgium, UK). Accrual from September 2006 to June 2007. Followed up every 8 weeks. (ID: NCT00350948) | |
| Participants | 125 women with PR ROC. Included if: ≥ 18 years old; 1 or 2 previous platinum‐based chemotherapy regimens given; measurable disease defined by RECIST; ECOG PS 0,1 or 2; and adequate bone marrow reserves and cardiac, renal and hepatic function were required. Bulky disease was defined as tumour mass ≥ 5cm. | |
| Interventions |
Intervention Canfosfomide (CAN) (1000 mg/m²) IVI for 30 min followed by PLD (50 mg/m²) on day 1 every 28 days Control PLD (50 mg/m²) IVI for 60 min on day 1 every 28 days |
|
| Outcomes |
Primary outcome PFS Secondary outcome ORR, SAE (NCI‐CTCAE v3.0) |
|
| Notes | This study was temporarily put on hold in June 2007 to review the results of the single‐agent trial (ASSIST‐1) in PR ROC. The clinical hold was released in October 2007, but the sponsor decided not to enrol any additional patients. Patients requiring dose reductions for HFS and stomatitis were 15% and 4%, respectively, in the intervention arm compared with 42% and 25%, respectively, in the PLD arm; i.e. CAN appeared to decrease the rate of HFS and stomatitis when combined with PLD. Premedication (ondansetron and IV corticosteroids) was the same in both arms. For the exploratory subgroup of PR ROC women with platinum‐refractory or primary platinum resistance (i.e. excluding secondary platinum resistance), the difference in PFS was significantly in favour of arm 1 (HR = 0.55; P value 0.0425). Also in this subgroup, median survival for arm 1 was 11.8 months versus 7.8 months in arm 2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation with stratification for ECOG PS, prior best response to platinum‐based chemotherapy and bulky disease. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether assessors were blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/60 women in the PLD arm did not receive any study drug and so were not included in the SAE analyses. |
| Selective reporting (reporting bias) | Unclear risk | OS was not reported as there was an insufficient number of death events at the time of reporting. We requested final OS data from Telik Inc but have as yet received no reply to our queries. Although immature OS data are not necessarily a high risk of selective reporting, this study completed over a decade ago, so this is judged to be at high risk of bias. |
| Other bias | Unclear risk | This trial closed early. Planned enrolment n = 244, actual enrolment n = 125. See notes above. As a result of the clinical hold, 35 patients (21 in combination arm and 14 in PLD arm) were not able to complete their assigned therapy as per protocol. |
Banerjee 2018.
| Study characteristics | ||
| Methods | International, multicentre, randomised, open‐label, phase II study | |
| Participants | 95 participants with PR ROC. No more than 1 previous CT for ROC Inclusion criteria ≥ 18 years of age Eastern Cooperative Oncology Group (ECOG) performance status 0‐1 Received no more than one line of chemotherapy for platinum resistant disease and up to three lines total Primary platinum‐refractory disease defined as disease progression during or within 2 months of a first‐line, platinum‐containing chemotherapy regimen Suitable to start treatment with PLD Adequate organ function measured within 10 days of randomisation Highly effective form of contraception through the course of study treatment and for 6 months after the last dose of study treatment Willing and able to perform a patient‐reported outcome survey Exclusion criteria Other malignancy within the last 5 years, except adequately treated squamous carcinoma of the skin, limited basal cell skin cancer, carcinoma in situ of the cervix or synchronous primary endometrial cancer or prior primary endometrial cancer Antitumor therapy, including chemotherapy, biologic, experimental, or hormonal therapy, within 4 weeks. Palliative radiation within 2 weeks. Major surgical procedure within 4 weeks. Prior anthracycline therapy, including prior treatment with PLD in any setting. Prior treatment with NaPi2b or SCL34A2 (solute carrier family 34 member 2 gene) targeted therapy. History of severe allergic or anaphylactic reactions to monoclonal antibody therapy (or recombinant antibody‐related fusion proteins) Grade >1 toxicity (except alopecia and anorexia) from prior therapy or Grade >1 neuropathy from any cause Left ventricular ejection fraction below the lower limit of normal. Significant cardiovascular disease or pulmonary disease Untreated or active CNS metastases (progressing or requiring anticonvulsants or corticosteroids for symptomatic control) Known active infection, or any major episode of infection requiring treatment with IV antibiotics or hospitalisation within 4 weeks Clinically significant liver disease, including viral or other hepatitis, current alcohol abuse, or cirrhosis. HIV seropositive status, hepatitis B or C Pregnant or breastfeeding |
|
| Interventions |
Intervention Lifastuzumab 2.4 mg/kg IV every 3 weeks. Control PLD 40 mg/m2 IV every 4 weeks. |
|
| Outcomes |
Primary outcome PFS Secondary outcomes ORR (RECIST) Duration of OR, OS, SAE Area under the concentration time curve lifastuzumab Maximum concentration of lifastuzumab Clearance of lifastuzumab Elimination half‐time of lifastuzumab Volume of distribution at steady state of lifastuzumab Percentage of participants with anti‐therapeutic antibodies against lifastuzumab |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Equal assignment to each of the two arms (1:1). Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95 patients randomised, 93 patients received at least one dose of study drug. At data cutoff there were 64 PFS events, 23 OS events, and 22 patients remained on study. |
| Selective reporting (reporting bias) | Low risk | Same outcome measures reported in study protocol and final results. |
| Other bias | Unclear risk | Funding by Genentech, Inc., South San Francisco, CA, USA (no grant number applies). Authorwise: PT, KL, ES, AV, YC, JCM, DJM, VL, YW, and EWH are employees of Genentech, Inc., and shareholders of Roche. There may be some bias that the study was funded by Genentech, done by Genentech, and produced by Roche. |
CALYPSO.
| Study characteristics | ||
| Methods | Phase III open‐label multicentre non‐inferiority RCT. Accrual from April 2005 to September 2007. (ID: NCT00538603) | |
| Participants | 976 women with PS ROC (recurrence > 6 months after first or second line platinum‐based chemotherapy and had received a taxane). Included if ECOG ≤ 2; previous taxane therapy; measurable or assessable disease; life‐expectancy of at least 12 weeks; and adequate bone marrow, renal and hepatic function. Patients with pre‐existing peripheral neuropathy grade > 1 were excluded. | |
| Interventions |
Intervention (509 women): carboplatin (AUC 5) + PAC (175 mg/m²) every 3 weeks Control (466 women): carboplatin (AUC 5) + PLD (30 mg/m²) every 4 weeks Premedication of antiemetics (5HT agonist) and dexamethasone was to given to all women; those in the carboplatin /PAC arm also received clemastine and ranitidine. |
|
| Outcomes |
Primary outcome PFS Secondary outcomes OS SAE QoL (QLQ C30 and OV 28) assessed at baseline, 3,6, 9 and 12 months |
|
| Notes | Overall, severe non‐haematological toxicity occurred in 36.8% of the PAC/carboplatin arm compared with 28.4% of the PLD/carboplatin arm (P < 0.01). Significantly fewer severe allergic reactions (grade 3 to 4) were observed in the PLD/carboplatin arm than in the PAC/carboplatin arm: 2.4% versus 8.8%, respectively (P < 0.001) (in reference Joly et al. Gynecologic Oncology 2011;122(2):226‐32). Significantly more women in the PAC/carboplatin arm discontinued treatment before six cycles had been completed (110/507 versus 70/466), mainly due to toxicity (73/507 women versus 27/466 women; P < 0.001). In total, 90% of women received post‐progression treatment, 69% received two or more lines. The proportion of women in the PAC/carboplatin arm who received PLD as post‐study therapy (68%) was significantly higher than the proportion of women in the PLD/carboplatin arm who received PAC (43%; P < 0.001); this may have influenced OS HRs in the direction of the PAC/carboplatin arm. We obtained unpublished data on non‐haematological adverse effects (grade 3 to 4) from the investigators. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised. Randomisation was in permuted blocks of 6, with stratification by measurable disease, treatment‐free interval (6 to 12 versus > 12 months) and centre. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluation assessments were independently reviewed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition for survival and toxicity outcomes. Regarding QoL data, 79% of women in the carbo/PAC arm and 84% of women in the carbo/PLD arm had QoL data at baseline and one other point in the study. The most complete data set (< 20% missing data) was available at 3 months post‐randomisation, therefore we used these data. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Unclear risk | Baseline characteristics were similar and arms were well balanced for stratification factors. Imbalance in treatment allocation (509 versus 467) was consistent with chance. Industry funded "Supported by a research grant from Schering‐Plough" and COI statement as follows: "Employment or Leadership Position: None Consultant or Advisory Role:Jalid Sehouli, Ovarian Cancer (C); Gunnar Kristensen, Boehringer Ingelheim (C), AstraZeneca (C), Roche (C); Christian Jackisch, Essex Pharma Germany (C), Schering‐Plough (C) Stock Ownership: None Honoraria: Eric Pujade‐Lauraine, Schering‐Plough; Uwe Wagner, Essex Pharma GmbH; Mark Heywood, Schering‐Plough Canada; Sandro Pignata, Schering‐Plough, Roche; Jalid Sehouli, GlaxoSmithKline, Essex Pharma; Gunnar Kristensen, Schering‐Plough; Andreas du Bois, Schering‐Plough Research Funding: Sandro Pignata, ATRC; Jalid Sehouli, Ovarian Cancer; Andreas du Bois, Schering‐Plough, GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Eli Lilly Expert Testimony: None Other Remuneration: Uwe Wagner, Essex Pharma GmbH" |
Colombo 2012.
| Study characteristics | ||
| Methods | Phase III open‐label RCT conducted in 22 countries; accrual between November 2005 and March 2009 ID: NCT00262990 | |
| Participants | 829 women with PR ROC following ≤ 3 platinum‐taxane based regimens. Measurable and non‐measurable disease (but CA125 elevated at baseline); ovarian, fallopian and primary peritoneal cancer included. Excluded if peripheral neuropathy, unresolved bowel obstruction or diarrhoea within 7 days of start of treatment. | |
| Interventions |
Intervention PAT (10 mg/m²) IVI q3wk Control PLD (50 mg/m²) IVI q4wk No routine premedication was given to either arm. |
|
| Outcomes |
Primary outcome OS Secondary outcomes PFS ORR SAE |
|
| Notes | Women were assessed 8‐weekly; median follow‐up was 27 months. Arms received a median of 4.5 and 3 cycles for PAT and PLD respectively. Median TTP was 15.9 weeks for both arms. Median time to death was 56.6 weeks versus 54.4 weeks in favour of PAT. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Allocation via an interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded central review of results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few women lost to follow‐up and low attrition (< 20%) in most analyses. As with other studies, QoL data suffered from high attrition rates, and therefore we could not use it in the meta‐analyses. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk | Baseline characteristics were similar. Supported by Novartis Pharmaceuticals (clinical studies and medical editorial assistance). Conflicts of interst statements "Employment or Leadership Position: Dirk Weber, Novartis Pharmaceuticals (C); Mona El‐Hashimy, Novartis Pharmaceuticals (C); Farida Souami, Novartis Pharmaceuticals (C); Patricia Wing, Novartis Pharmaceuticals (C) Consultant or Advisory Role: Aristotelis Bamias, Novartis Pharmaceuticals (C) Stock Ownership: Dirk Weber, Novartis Pharmaceuticals; Mona El‐Hashimy, Novartis Pharmaceuticals; Jingjin Li, Novartis Pharmaceuticals; Patricia Wing, Novartis Pharmaceuticals Honoraria: Aristotelis Bamias, Novartis Pharmaceuticals Research Funding: None Expert Testimony: None Other Remuneration: None". |
CORAIL.
| Study characteristics | ||
| Methods | Randomised international multicentre phase III study | |
| Participants | 442 participants with PR ROC Inclusion criteria Histologically or cytologically confirmed unresectable epithelial ovarian, primary peritoneal or fallopian tube cancer. ≥ 18 years of age Measurable disease by RECIST criteria Eastern Cooperative Oncology Group (ECOG) performance status 0‐2 Received no more than three lines of chemotherapy Platinum refractory or platinum sensitive disease (PFI < 1 or > 6 m) Adequate organ function Not pregnant and on medically acceptable form of contraception Voluntary, written informed consent Exclusion criteria Other malignancy within the last 3 years, except curatively treated basal cell carcinoma or squamous cell carcinoma of the skin, carcinoma in situ of the breast or cervix Therapy within 3 weeks Prior therapy with PM01183, trabectedin, or with both PLD and topotecan Grade ≤ 1 from any previous treatment (excluding grade ≤ 2 alopecia or peripheral neuropathy) History of cardiac disease Bowel obstruction Brain or leptomeningeal metastases Active uncontrolled infection Patients with any immunodeficiency, HIV, hepatitis or cirrhosis, hepatitis B or C Pregnant or breastfeeding |
|
| Interventions |
Intervention Lurbinectedin given 3.2 mg/m2 IV as a 1‐hour infusion on Day 1 every 3 weeks (3 weeks = one treatment cycle) Control PLD given if previous treatment with topotecan, or topotecan given if previous treatment with PLD. PLD (50 mg/m2, every 4 weeks) or topotecan (1.5 mg/m2, every 3 weeks) |
|
| Outcomes |
Primary outcome PFS Secondary outcomes PFS OS ORR DoR Best response according to Ca 125 AE & SAE All‐cause mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation method using Medidata Balance, 1:1. |
| Allocation concealment (selection bias) | Low risk | Randomised allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported on planned outcomes. |
| Selective reporting (reporting bias) | Low risk | Planned outcome data complete. |
| Other bias | Unclear risk | Study sponsored by PharmaMar, manufacturers of Lurbinectedin. |
Fujiwara 2019.
| Study characteristics | ||
| Methods | A phase II multicentre, open‐label, randomised controlled study in Japan | |
| Participants | 100 women with PS ROC, > 19 years, PS ≤ 2, life expectancy at least 4 months, and adequate bone marrow, renal, and hepatic function. Inclusion criteria Ovarian, primary peritoneal or fallopian tube cancer ≥ 20 years of age Measurable disease or assessable lesions by RECIST criteria Eastern Cooperative Oncology Group (ECOG) performance status 0‐2 Adequate bone marrow and organ function Life expectancy of 16 weeks or more Exclusion criteria Elevated CA125 without measurable disease or assessable lesions |
|
| Interventions |
Intervention PLD 30 mg/m2 plus carboplatin AUC 5 mg/ mL/min on day 1, every 4 weeks Control GEM 1000 mg/m2 on days 1 and 8 plus carboplatin AUC 4 mg/mL/min on day 1, every 3 weeks for at least 6 cycles |
|
| Outcomes |
Primary outcome PFS 6 month/1 year survival rate Secondary outcomes ORR OS SAE Dose administration |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned. |
| Allocation concealment (selection bias) | Low risk | Centrally randomised. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for response efficacy 42/49 and 39/50. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported. |
| Other bias | Unclear risk | Janssen supported study and is producer of PLD. |
Gordon 2001.
| Study characteristics | ||
| Methods | Phase III multicentre open‐label RCT with 104 sites in the USA and Europe that recruited participants between May 1997 and March 1999. | |
| Participants | 481 women with ROC (PS or PR) who had recurred or failed first‐line platinum‐based chemotherapy; with measurable disease, or measurable and assessable disease; adequate bone marrow, renal, hepatic and cardiac function; Karnofsky performance status ≥ 60%; expected to live > 3 months | |
| Interventions |
Intervention PLD 50 mg/m² IV over 1 hour, every 4 weeks Control TOP 1.5 mg/m²/d IV over 30 min x 5d, every 3 weeks |
|
| Outcomes |
Primary outcome PFS Secondary outcome ORR, OS, SAE, QoL (QLQ‐C30) |
|
| Notes | Seven women received no treatment after randomisation and were excluded from most analyses. G‐CSF was given to women who experienced febrile neutropenia, prophylactically in the following cycles; 29.1 % TOP versus 4.6% PLD received G‐CSF. The Investigators concluded that PLD was the treatment of choice among non‐platinum agents for women with ROC, especially platinum‐sensitive disease. 72% and 74% of women in the TOP and PLD groups, respectively, received prior taxane therapy. Median TTP was 17 weeks versus 16.1 weeks in favour of the TOP arm. Median time to death was 59.7 weeks versus 62.7 weeks in favour of the PLD arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation; stratified by platinum sensitivity and bulky disease. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent radiological review used for primary outcome (PFS). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition low for primary outcomes (high for QoL data). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. Censoring = 13%. |
| Other bias | Unclear risk | Funded by drug manufacturer. |
HeCOG 2010.
| Study characteristics | ||
| Methods | Phase II RCT of the Hellenic Cooperative Oncology Group. Accrual from October 1999 to December 2005. | |
| Participants | 189 women with PS ROC (≥ 6 months after platinum‐based chemotherapy). Included if ECOG 0‐2; life expectancy ≥ 3 months; and adequate bone marrow, renal, hepatic function. Patients with residual neurotoxicity from previous platinum and/or taxane chemotherapy and those with other cancers were excluded. | |
| Interventions |
Intervention Carboplatin (AUC 5) + PAC 175 mg/m² over 3 hours, every 3 weeks Control Carboplatin (AUC 5) + PLD 45 mg/m², every 4 weeks Standard premedication included dexamethasone, dyphenhydramine and ranitidine for both groups, although the PAC group received both an oral (12 hours prior) and an IV dose (30 min prior to PAC administration). Six cycles intended. |
|
| Outcomes |
Primary outcome ORR (WHO criteria or CA‐125 Rustin's criteria) and toxicity Secondary outcomes TTP OS |
|
| Notes | 204 women were randomised but 15 were subsequently considered to be ineligible and excluded. Median follow‐up 43.6 months (95% CI 0.1 to 74.8). 88% and 93%, respectively, received previous taxane‐containing therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Central randomisation/allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (< 20%). Fifteen post‐randomisation exclusions due to non‐eligibility including other cancers, non‐measurable disease without CA‐125 elevations. Eleven lost medical records, (5 in CP arm and 6 in CLD arm); 8 and 5 women lost to follow‐up respectively. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | None noted. Baseline characteristics were similar. |
JAVELIN Ovarian 200.
| Study characteristics | ||
| Methods | Phase 3, randomised, open‐label study, multicentre, international (USA, Australia, Austria, Belgium, Canada, Czechia, Denmark, France, Greece, Honk Kong, Hungary, Ireland, Israel, Italy, Japan, Korea, Netherlands, Norway, Poland, Russia, Singapore, Spain, Switzerland, Taiwan, United Kingdom) | |
| Participants | 566 women with PR ROC Inclusion criteria Histologically confirmed epithelial ovarian, primary peritoneal or fallopian tube cancer, including malignant mixed Müllerian tumors with high grade serous component. ≥ 18 years of age Measurable disease by RECIST criteria that has not previously been irradiated Received up to three lines of chemotherapy for platinum sensitive disease, most recently platinum containing, and no prior systemic therapy for platinum resistant disease Exclusion criteria Non epithelial tumor or ovarian tumors with low malignant potential (ie, borderline tumors). Other malignancy within the last 5 years, except for adequately treated basal cell or squamous cell skin cancer, or carcinoma in situ of the breast or of the cervix. Prior therapy with an anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2, anti CD137, or anti‐cytotoxic T lymphocyte associated antigen 4 antibody Severe gastrointestinal conditions such as bowel obstruction, or uncontrolled diarrhoea within 4 weeks or history of inflammatory bowel disease. Symptomatic brain metastases requiring steroids Active autoimmune disease that might deteriorate when receiving an immunostimulatory agents |
|
| Interventions | Randomisation 1:1:1 Arm 1 n = 188 Avelumab 10 mg/kg monotherapy as a 1‐h IV infusion once every 2 weeks Arm 2 n = 188 Avelumab 10 mg/kg every 2 weeks plus PLD 40 mg/m2 every 4 weeks, each as 1‐h IV infusions Arm 3 n = 190 PLD 40 mg/m2 alone as a 1‐h IV infusion every 4 weeks. |
|
| Outcomes |
Primary outcomes OS PFS Secondary outcomes ORR (BICR and investigator) PFD (RECIST) DoR (BICR) Disease control SAE TEAE QoL (EORTC QLQ‐C30) Time to deterioration in abdominal/GI Symptom (subscale of EORTC QLQ‐OV28) Change from baseline in EQ‐VAS score at end of treatment |
|
| Notes |
Main result The checkpoint inhibitor, avelumab, alone or with PLD, did not significantly improve PFS or OS versus PLD. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment by Interactive Response Technology (IRT) system in 1:1:1 ratio to avelumab alone (Arm A), avelumab plus PLD, (Arm B) or PLD alone (Arm C). |
| Allocation concealment (selection bias) | Low risk | Central randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessed by blinded independent central review (BICR). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow table provided, with all patients included in full analysis set. |
| Selective reporting (reporting bias) | Low risk | Outcomes on study protocol and outcomes on published results match. |
| Other bias | Unclear risk | The study was sponsored by Pfizer. |
Kaye 2012.
| Study characteristics | ||
| Methods | Phase II open‐label multicentre RCT; 1:1:1 ratio (ID: NCT00628251) | |
| Participants | 97 women with ROC within 12 months of receiving platinum‐based chemotherapy with confirmed BRCA1/2 germline mutations; one or more measurable lesion; ECOG PS 0‐2; estimated life expectancy ≥ 16 weeks; adequate bone marrow, hepatic and renal function. Excluded if previous PARP inhibitors or anthracyclines; brain metastases; other malignant disease; persistent toxic effects of treatment; LVEF < 50% | |
| Interventions |
Arm 1 OLA 200 mg twice daily continuously (32 women) Arm 2 OLA 400 mg twice daily continuously (32 women) Arm 3 PLD 50 mg/m² IVI every 4 weeks (33 women) |
|
| Outcomes |
Primary outcome PFS (RECIST‐assessed) Secondary outcomes ORR Duration of treatment response Tumour size OS SAE QoL (FACT‐O) |
|
| Notes | PARP nuclear enzymes facilitate DNA repair. Olaparib is a PARP inhibitor selective for homologous‐recombination‐deficient cells, such as those with BRCA1/2 deficiency. The primary outcome was reported for the olaparib arms combined and individually, versus the PLD arm. We used the results from the OLA 400 mg arm versus PLD. Median time to progression was 38 weeks versus 30 weeks in favour of OLA. Median time to death was not calculable for the OLA group and was 76 weeks for the PLD group (unpublished data). Corticosteroids and serotonin antagonists were given to 22/33 (67%) and 14/33 (42%) of the women in the PLD group respectively versus 12.5 % and 12.5% of the OLA group respectively, but it was not possible to determine whether they were given as premedication or at another time (unpublished information). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block randomisation, stratified according to BRCA status and platinum sensitivity (≤ 6 months and > 6 months). |
| Allocation concealment (selection bias) | Low risk | Allocation via an Interactive Voice Response System. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 'Centrally reviewed tumour assessments' were used for analyses; investigator‐assessed primary outcome; assessor blinding/independence not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the PLD arm, 5/33 discontinued treatment for unknown reasons versus 1/64 in the olaparib arm. Otherwise, attrition rates seem low. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. Results are not reported for platinum‐sensitive subgroups; these data were requested from the lead investigator on the 6 December 2012. |
| Other bias | Unclear risk | Baseline characteristics were similar except that more women in Arm 2 had received > 2 prior chemotherapy regimens. The study was supported by AstraZeneca, the manufacturer of Olaparib. Employment or Leadership Position: Mark Wickens, AstraZeneca (C); Elizabeth S. Lowe, AstraZeneca (C); James Carmichael, AstraZeneca (C) Consultant or Advisory Role: Stan B. Kaye, AstraZeneca (C); Charlie Gourley, GlaxoSmithKline (C), Roche (C), Schering‐Plough (C); Michael Friedlander, AstraZeneca Advisory Board (C); Bella Kaufman, AstraZeneca (U) Stock Ownership: Mark Wickens, AstraZeneca; Elizabeth S. Lowe, AstraZeneca; James Carmichael, AstraZeneca Honoraria: Stan B. Kaye, AstraZeneca Advisory Board; Charlie Gourley, Chugai Pharmaceutical, GlaxoSmithKline, PharmaMar, Roche, Schering‐Plough Research Funding: Ursula Matulonis, AstraZeneca; Charlie Gourley, AstraZeneca; Beth Y. Karlan, AstraZeneca; Michael Friedlander, AstraZeneca Expert Testimony: None Other Remuneration: Charlie Gourley, MSD (Schering‐Plough), PharmaMar |
M200.
| Study characteristics | ||
| Methods | Multicentre open‐label RCT; enrolment in the USA from July 2007 to Oct 2008. (ID: NCT00635193) | |
| Participants | 127 women with stage III/IV PS or PR ROC. Maximum of 2 prior chemotherapy treatments (at least one of which was platinum/taxane based); at least one measurable lesion to assess response by RECIST. | |
| Interventions | Volociximab (M200) is an anti‐angiogenic integrin inhibitor/monoclonal antibody. Two dosage regimes were tested combined with PLD versus PLD alone: Arm 1 PLD 40 mg/m² every 4 weeks (66 women) Arm 2 M200 15 mg/kg weekly + PLD 40 mg/m² every 4 weeks (34 women) Arm 3 M200 15 mg/kg every 2 weeks + PLD 40 mg/m² every 4 weeks (27 women) |
|
| Outcomes | Efficacy, safety and tolerability | |
| Notes | No useable data. Results were reported as follows: "The most common Grade 3 to 4 AEs (≥ 5% in any group) were abdominal pain, intestinal obstruction, ascites, fatigue, hypoalbuminemia, and cytopenias. The incidence of AEs was balanced across treatment groups. There were no CRs; PRs were 16%, 18%, and 19%....Preliminary analysis of PFS suggested that there was a low probability of detecting a statistically significant difference in favor of V [ Volociximab] +PLD, so the study was closed to enrollment." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Efficacy and safety were not clearly detailed in the ASCO 2009 abstract which is the only publication for this study. |
| Selective reporting (reporting bias) | High risk | Baseline data were not reported. Study outcomes not reported, except in abstract. |
| Other bias | High risk | Funding: Biogen Idec and Protein Design Labs, Inc. Limited information was available and results have not been published in full. Dr Obrocea of Abbott Laboratories was emailed on 28 November 2012 for final study data. |
McGuire 2018.
| Study characteristics | ||
| Methods | Randomised multicentre open‐label phase II study | |
| Participants | 123 participants with PR ROC from the USA, UK and Spain. They were recruited between June 2009 and February 2014. Inclusion criteria Histologically or cytologically confirmed epithelial ovarian, primary peritoneal, fallopian tube cancer, or ovarian clear cell carcinoma Measurable disease by RECIST Eastern Cooperative Oncology Group (ECOG) performance status 0‐1 Adequate bone marrow, organ function and echocardiogram Informed consent Effective form of contraception Exclusion criteria Increased CA125 in the absence of concomitant clinical or radiographic progression Other malignancy within the last 3 years except curatively resected non‐melanomatous skin cancer, curatively treated cervical carcinoma in‐situ or other primary solid tumour treated with curative intent Major surgery, open biopsy or significant traumatic injury within the last 4 weeks. Participation in clinical trials of experimental agents within 4 weeks Prior treatment with more than 1 biologic and/or more than one hormonal therapy Prior treatment with other agents targeting PDGF or PDGF receptor. Received an anthracycline for any indication in the past. Radiotherapy, chemotherapy, or biologic therapy directed at the malignant tumour within 3 weeks prior to randomisation, or hormonal therapy directed at the malignant tumour within 1 week. Known allergies to compounds of chemical or biologic composition similar to that of IMC‐3G3 Current Grade > 1 toxicity or ≥ Grade 2 side effects due to agents administered more than 28 days prior to randomisation. Unstable angina pectoris, angioplasty, cardiac stenting, or myocardial infarction 6 months prior to randomisation. Uncontrolled symptomatic congestive heart failure, uncontrolled hypertension, clinically significant arrhythmia Suspected impending bowel obstruction Brain metastases of leptomeningeal disease Uncontrolled intercurrent illness including, serious or nonhealing active wound, ulcer, or bone fracture. HIV seropositive status History of uncontrolled hereditary or acquired bleeding or thrombotic disorders Psychiatric illness/social situations that would limit compliance with study requirements Pregnant or breastfeeding |
|
| Interventions |
Intervention Olaratumab (20 mg/kg IV every 2 weeks and PLD (40 mg/m² every 4 weeks) Control PLD (40 mg/m² every 4 weeks) |
|
| Outcomes |
Primary outcome PFS Secondary outcomes OS ORR DoR Safety |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study site personnel randomised patients using either a call‐in Interactive Voice Response System (IVRS) or Interactive Web Response System (IWRS). The IVRS/ IWRS assigned a unique identification number to each patient. |
| Allocation concealment (selection bias) | Low risk | Study site personnel randomized patients using either a call‐in Interactive Voice Response System (IVRS) or Interactive Web Response System (IWRS). The IVRS/ IWRS assigned a unique identification number to each patient. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Patients underwent radiographic disease assessment approximately every 8 weeks. Not described how this was assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was one patient lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Study registered at clinicaltrials.gov. Outcomes reported in study protocol match outcome reported in results paper. |
| Other bias | Unclear risk | This work was sponsored by ImClone System LLC, a wholly owned subsidiary of Eli Lilly and Company. The study was designed by the funder, ImClone /Eli Lilly and Company, with input from ovarian cancer experts. The data were analysed and interpreted by ImClone /Eli Lilly and Company in collaboration with the academic authors. All authors had access to all the data and vouch for the accuracy and completeness of the data and analyses reported and for the fidelity of the study to the study protocol. All authors had final responsibility for the decision to submit for publication. All authors participated in the drafting of the manuscript and/or critical revisions of subsequent drafts. Writing and editorial assistance was provided by Syneos Health on behalf of ImClone /Eli Lilly and Company. |
MITO‐3.
| Study characteristics | ||
| Methods | Phase III multicentre RCT; accrual from January 2003 to January 2007. | |
| Participants | 153 women with ROC that had relapsed within 12 months (PPS and PR ROC) of receiving one platinum/paclitaxel regimen. Women had measurable or assessable disease (RECIST), adequate hepatic, renal, cardiac and bone marrow function, no prior malignancies, and were expected to live > 3 months. | |
| Interventions |
Intervention GEM (1000 mg/m²) days 1, 5, 8, 15, every 4 weeks Control PLD (40 mg/m²) IVI, every 4 weeks Methylprednisolone 20 mg was given as premedication to the PLD arm. |
|
| Outcomes |
Primary outcome Time to progression Secondary outcomes OS ORR SAE QoL (QLQ‐C30) |
|
| Notes | Trial used a lower (40 mg/m²) dose of PLD to minimise SAEs. Post‐progression treatment was only documented in 36 participants so OS data difficult to interpret. Median TTP was 20 weeks versus 16 weeks in favour of GEM. Median time to death was 51 weeks versus 56 weeks in favour of PLD. HR for OS and PFS not given but requested from Dr Ferrandina on 3 December 2012. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Random assignment by central telephone service. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and physicians not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary outcome was TTP. PFS/OS were not reported clearly with HRs, but we were able to obtain these from the investigators in January 2013. 79% of participants completed the QoL questionnaire. |
| Other bias | Low risk | Treatment groups were well‐balanced for baseline characteristics. |
Monk 2017.
| Study characteristics | ||
| Methods | Phase II, randomised controlled trial, double‐blinded, multicentre in the USA. | |
| Participants | 297 participants (148 in experimental Arm; PLD + VTX‐2337, 149 in control Arm; PLD + placebo) Inclusion criteria Recurrent or persistent epithelial ovarian, primary peritoneal or fallopian tube carcinoma. Serous adenocarcinoma, endometrioid adenocarcinoma, mucinous adenocarcinoma, undifferentiated carcinoma, clear cell adenocarcinoma, mixed epithelial adenocarcinoma, transitional cell carcinoma, malignant Brenner's tumour or adenocarcinoma not otherwise specified. Measurable disease by RECIST Eastern Cooperative Oncology Group (ECOG) performance status 0‐1 No more than one cytotoxic regimen for management of recurrent or persistent disease or one biologic/targeted therapy Received a platinum‐based chemotherapeutic regimen for management of primary disease containing carboplatin, cisplatin or another organoplatinum compound Adequate bone marrow and organ function Recovered from effects of recent surgery, radiotherapy or chemotherapy Free of active infection Informed consent Effective form of contraception Any prior radiation therapy must be completed at least four weeks prior to registration Exclusion criteria Other malignancy within the last 3 years, except for non‐melanoma skin cancer Radiotherapy or chemotherapy within the last 3 years other than for ovarian, fallopian tube or primary peritoneal cancer Chemotherapy, biologic/targeted agents and immunologic agents, within 3 weeks Hormonal therapy within the last 1 week Investigational agent within the last 4 weeks Prior treatment with VTX‐2337, doxorubicin, PLD, or any other anthracycline. Clinically significant cardiovascular disease Gastrointestinal obstruction Requiring parenteral hydration or nutrition Brain metastases or primary brain tumour, seizures not controlled with standard medical therapy, cerebrovascular accident, transient ischaemic attack or subarachnoid haemorrhage within six months Current or use in last 2 weeks of corticosteroids or requiring systemic immunosuppressive therapy Active autoimmune disease Pregnant or breastfeeding |
|
| Interventions | Randomisation 1:1
Intervention PLD 40 mg/m2 plus VTX‐2337 ‐ PLD on Day 1 plus VTX‐2337 on Day 3, Day 10, and Day 17 for the first 4 cycles. Starting with cycle 5, the dose regimen will be PLD on Day 1 plus VTX‐2337 on Day 3 only, without additional doses of VTX‐2337 on Days 10 and Day 17. Control PLD 40 mg/m2 plus placebo. The starting dose schedule is PLD on Day 1 plus placebo on Day 3, Day 10, and Day 17 for the first 4 cycles. Starting with cycle 5, the dose regimen will be PLD on Day 1 plus placebo on Day 3 only. |
|
| Outcomes |
Primary outcome OS Secondary outcomes PFS AE‐ frequency and severity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not specified how allocation was done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of both participants and personnel, use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant flow data provided in clinicaltrials.gov does not suggest high risk of bias. |
| Selective reporting (reporting bias) | Low risk | Outcomes on study protocol on clinicaltrials.gov similar to those reported in the results paper. |
| Other bias | Unclear risk | Study sponsored by VentiRx Pharmaceuticals. Principal Investigators are not employed by the organisation sponsoring the study. There is not an agreement between Principal Investigators and the Sponsor (or its agents) that restricts the PI's rights to discuss or publish trial results after the trial is completed. |
Monk 2020.
| Study characteristics | ||
| Methods | Open‐label, randomised, active‐controlled phase 3 study International, multicentre |
|
| Participants | n = 576 Inclusion criteria Histologically confirmed epithelial ovarian cancer, primary peritoneal or fallopian tube cancer ≥ 18 years of age Measurable disease by RECIST Eastern Cooperative Oncology Group (ECOG) performance status 0‐1 Received no more than 2 lines of systemic therapy Second‐line treatment with a platinum‐based regimen, with progression of disease after attaining a complete or partial response Progression of disease based on imaging after the second‐line platinum‐based regimen Adequate bone marrow, organ function and echocardiogram Side effects of prior treatment resolved to at least Grade 1 Effective form of contraception and negative pregnancy test at screening Exclusion criteria Mucinous histology Other malignancy within the last 3 years, except for non‐metastatic basal cell or squamous cell skin cancer, or non‐invasive malignancy requiring ongoing therapy Radiation therapy, experimental therapy, hormonal therapy, prior chemotherapy or biological therapy within the last 3 weeks Prior treatment with more than 2 lines of systemic therapy Prior treatment with doxorubicin or other anthracycline at cumulative doses greater than 300 mg/m2 Currently enrolled in an investigational study Known allergies, hypersensitivity, or intolerance to PLD, dexamethasone, or their excipients Myocardial infarction within 6 months, unstable angina, NYHA class II or greater heart failure, severe uncontrolled ventricular arrhythmias, clinically significant pericardial disease, ECG evidence of acute ischaemic or conductive system abnormalities Uncontrolled seizures Active systemic infection likely to interfere with study Significant chronic liver disease Uncontrolled diabetes Newly diagnosed DVT Psychiatric disorder, including dementia, that prevents compliance with protocol Pregnant or breastfeeding |
|
| Interventions |
Intervention PLD 30 mg/m2 for 1.5 hrs followed by trabectedin 1.1 mg/ m2 for 3 hrs every 3 weeks Control PLD 50 mg/m2 for 1.5 hrs every 4 weeks |
|
| Outcomes |
Primary outcome OS Secondary outcomes PFS ORR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned in 1:1 ratio to treatment groups based on a computer‐generated randomisation schedule prepared before the study by or under the supervision of the sponsor. |
| Allocation concealment (selection bias) | Low risk | The interactive voice response system (IVRS) and/or interactive web response system (IWRS) assigned a unique treatment code, which dictated the treatment assignment and matching study drug kit for the subject. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study ‐ the requirement of a central venous catheter, limited to the combination trabectedin+DOXIL arm, precludes blinded treatment in this study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not possible due to drug delivery method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14% attrition due to loss to follow‐up/withdrawal from study 289 enrolled in experimental arm, and 286 received treatment. 287 enrolled in standard arm, and 282 received treatment. |
| Selective reporting (reporting bias) | Low risk | Study protocol outcomes and results paper outcome match. |
| Other bias | Unclear risk | Study sponsored by Janssen, manufacturers of Caelyx |
Mutch 2007.
| Study characteristics | ||
| Methods | Phase III open‐label multicentre RCT; accrual from July 2002 to May 2004 at 44 sites in the USA. | |
| Participants | 195 women with PR ROC who had received 1 to 2 prior platinum‐based chemotherapy regimens with measurable (RECIST) or assessable disease (Zubrod performance status of 0 to 2 and adequate bone marrow, hepatic and neurological function. | |
| Interventions |
Intervention GEM (1000 mg/m²) IV day 1 and day 8, every 3 weeks Control PLD (50 mg/m²) IV every 4 weeks |
|
| Outcomes |
Primary outcome PFS Secondary outcomes OS SAE (NCI‐CTCAE v 2.0) QoL (FACT‐O) |
|
| Notes | If participants experienced disease progression, unacceptable toxicity or if cumulative PLD dose exceeded 500 mg/m², they crossed over to the alternative drug. Median follow‐up was 29.2 months. 99% of women had received prior taxane. Median TTP was 15.4 weeks versus 13.3 weeks in favour of the GEM arm. Median time to death was 54.4 versus 57.9 weeks in favour of the PLD arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Independent assessment/blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of events/(total number evaluated) and censoring was not described for the primary outcome (PFS) or OS. Attrition for QoL outcomes not reported. Additional data requested from authors 4 December 2012. |
| Selective reporting (reporting bias) | High risk | HRs, number of events, and censoring was not described for the primary outcome (PFS) or OS. Limited (non‐comparative) QoL data reported. Additional data requested from authors 4 December 2012. |
| Other bias | Unclear risk | Study funded by drug manufacturers |
NCT00653952.
| Study characteristics | ||
| Methods | Phase 3, randomised, open‐label study | |
| Participants | The study was halted to new recruits in 1999 due to low accrual after 50% of planned participants were recruited (216 participants). Inclusion criteria
Exclusion criteria
|
|
| Interventions | PLD (50 mg/m2) every 28 days for up to 1 year versus Paclitaxel (175 mg/m2) Day 1 every 3 weeks |
|
| Outcomes |
Primary outcome Time to progression (TTP) Secondary outcomes Response rates Time to response Duration of response Quality of life assessment Survival |
|
| Notes | Terminated in 2010 due to poor accrual. Results released online. https://yoda.yale.edu/sites/default/files/nct00653952.pdf https://clinicaltrials.gov/ct2/show/NCT00653952?term=nct00653952&draw=2&rank=1 Previous abstract (O'Byrne 2002 in previous review) stated "This study is listed as 'Terminated' on the NCT registry after enrolling 220 women." The only published report is an ASCO 2002 abstract which had no data that could be included in our meta‐analyses. Results were reported as follows: "A preliminary analysis indicates that the overall progression‐free survival rates are similar between the two arms (PLD: 21.7 versus paclitaxel: 22.4 weeks; P = 0.15). The overall response rates for PLD and paclitaxel are 17.8% and 22.4%, respectively (P = 0.34). Median overall survival times are 45.7 weeks for PLD and 56.1 weeks for paclitaxel (P = 0.44). No significant difference was seen in median progression‐free or overall survival for platinum sensitive or refractory patients in either treatment arm. The overall number of adverse events was equivalent in either arm. Nausea and vomiting, stomatitis and plantar‐palmar erythrodysesthesia were seen more frequently with PLD whereas alopecia, myalgia, arthralgia and paraesthesiae occurred more commonly with paclitaxel. These findings clearly indicate that PLD has comparable efficacy to paclitaxel in taxane naive patients with ROC. PLD may be particularly suitable for those patients with musculoskeletal disorders or for whom the prospect of alopecia has a significant adverse psychological effect." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not provided. |
| Allocation concealment (selection bias) | Unclear risk | Details not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | AE data reported for all 216, although initial abstract stated 220 recruited. |
| Selective reporting (reporting bias) | High risk | The study was terminated due to poor accrual after approximately 50% of the planned subjects had been entered. Therefore, efficacy analyses were limited to overall survival only, pursuant to an agreement with the Oncology Division. |
| Other bias | High risk | Study terminated early due to poor accrual. Data available online but not published in peer‐reviewed journal, as far as we have been able to ascertain. Study sponsored by Johnson & Johnson Pharmaceutical Research & Development, L.L.C. |
NCT01840943.
| Study characteristics | ||
| Methods | Phase III randomised control trial Multicentre ‐ China |
|
| Participants | n = 32 Inclusion criteria Histologically confirmed epithelial ovarian cancer Measurable disease Received no more than one platinum based chemotherapy Demonstrate adequate organ function and echocardiogram Effective form of contraception Other invasive malignancy within the past 5 years except curatively treated basal cell or squamous cell carcinoma of the skin or carcinoma in situ of the cervix Exclusion criteria Chemotherapy within 4 weeks Prior therapy with PLD or topotecan Myocardial infarct within 6 months, class II or greater heart failure, uncontrolled angina, severe uncontrolled ventricular arrhythmias, clinically significant pericardial disease Uncontrolled systemic infection that requires systemic treatment Pregnant or breastfeeding or planning to become pregnant while enrolled in this study or within 1 year after the last dose of study medication |
|
| Interventions |
Intervention IV PLD 50 mg/m2 Day 1 of each cycle as: 60 to 90‐minute infusion to the participants not undergoing pharmacokinetic (PK) evaluation and 90‐minute infusion to the participants undergoing PK evaluation Outcome IV topotecan (TOP) 1.25 mg/m2 per day administered for 30 minutes duration, on Day 1 to Day 5 of each cycle. |
|
| Outcomes |
Primary outcome PFS Secondary outcomes OS Duration of PFS Number of participants With response Time to response Duration of response Health‐related quality of life assessment (HQL) Maximum plasma concentration of PLD Time to reach the maximum plasma concentration of PLD Area under the plasma concentration of PLD Apparent terminal elimination half‐life of plasma concentration of PLD Apparent terminal elimination rate constant of plasma concentration of PLD Systemic clearance of plasma concentration of PLD Apparent volume of distribution of plasma concentration of PLD Number of participants with adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation methods not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | PLD 14: withdrawal 5; physician decision 4; lost to follow‐up 4. Topotecan 12: withdrawal 6; physician decision 2; lost to follow‐up 4. |
| Selective reporting (reporting bias) | High risk | Extremely low numbers and incomplete compared to planned protocol. |
| Other bias | High risk | Study overall at extremely high risk of bias. Study funded by Xian‐Janssen Pharmaceutical Ltd. |
OVA‐301.
| Study characteristics | ||
| Methods | Phase III multicentre RCT (21 countries); recruited from April 2005 to May 2007. Participants were followed up every 8 weeks. | |
| Participants | 672 women with PR ROC (PFI < 6 months) and women with PS ROC (PFI ≥ 6 months), excluding platinum refractory patients. Planned enrolment was 650 women. Included if measurable disease was present (defined by RECIST); only 1 prior platinum‐based regimen received; ECOG PS 0,1 or 2; PFI based on radiological evaluation; no other major medical conditions. |
|
| Interventions |
Intervention PLD (30 mg/m²) IVI for 90 min + trabectedin (TBD) (1.1 mg/m²) IVI for 3‐hours, every 21 days Control PLD (50 mg/m²) IVI for 90 min, every 28 days |
|
| Outcomes |
Primary outcome PFS Secondary outcomes OS ORR Duration of response SAE (NCI‐CTCAE v3.0) Tertiary outcome QoL |
|
| Notes | Growth factor was necessary in 42% arm 1 versus 17% arm 2 to treat neutropenia (precise figures were not given). There were more withdrawals in the TBD arm than the PLD alone arm due to patient choice or adverse events (126 versus 89 participants). Dexamethasone was given to the TBD group only to reduce hepatic toxicity (personal communication). When results were subgrouped by platinum sensitivity, only women in the PS ROC group experienced significantly longer PFS with arm 1; i.e. TBD + PLD offered no significant additional benefit over PLD alone for women with PR ROC. Similarly, for OS, only the PPS ROC subgroup of arm 1 had a statistically significantly longer OS than the arm 2 subgroup (HR 0.59; 95% CI 0.42 to 0.82; P value 0.0015). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central block randomisation (1:1) with stratification by platinum sensitivity and ECOG PS. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent radiological assessment and oncologist review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All expected outcomes were reported, although missing data was > 20% for QoL outcomes. |
| Selective reporting (reporting bias) | Unclear risk | The reduced rate of PLD toxicity reported in the combination arm could have been due to dexamethasone premedication, or the lower dose of PLD used. |
| Other bias | Unclear risk | Women in arm 2 had a significantly longer PFI than arm 1 (P value 0.009) which may have biased the survival data in the direction of PLD alone. When the investigators adjusted OS results for the PFI and other prognostic factors in ad hoc exploratory analyses, the adjusted OS produced a statistically significant result in favour of arm 1 (HR = 0.82; 95% CI 0.69 to 0.98; P value 0.0285). Conflict of interest: Employment or Leadership Position: Stanley B. Kaye, PharmaMar Board of Directors (C); Youn Choi Park, Johnson & Johnson Pharmaceutical Research & Development (C); Claudia A. Lebedinsky, PharmaMar (C) Consultant or Advisory Role: Thomas J. Herzog, Genentech (C), GlaxoSmithKline (C), Johnson & Johnson/OrthoBiotech (C); Carolyn N. Krasner, Johnson & Johnson (U); Jan B. Vermorken, Johnson & Johnson (C), PharmaMar (C); Franco M. Muggia, Johnson & Johnson (C); Andrés M. Poveda, PharmaMar (C) Stock Ownership: Youn Choi Park, Johnson & Johnson; Claudia A. Lebedinsky, Grupo Zeltia Honoraria: Thomas J. Herzog, GlaxoSmithKline, Johnson & Johnson/OrthoBiotech, PTI, Eli Lilly; Carolyn N. Krasner, Johnson & Johnson; Jan B. Vermorken, PharmaMar; Franco M. Muggia, Ortho Biotech; Eric Pujade‐Lauraine, Schering Plough, Pharmamar Research Funding: Bradley J. Monk, Johnson & Johnson; Hextan Yuen‐Sheung Ngan, Johnson & Johnson Pharmaceutical Research & Development Expert Testimony:Bradley J. Monk, Johnson & Johnson–Oncologic Drugs Advisory Committee (C) Other Remuneration: None |
Pfisterer 2020.
| Study characteristics | ||
| Methods | International multicentre open‐label, randomised, phase 3 trial | |
| Participants | n = 682 Inclusion criteria Histologically confirmed epithelial ovarian, primary peritoneal or fallopian tube cancer ≥ 18 years of age Measurable or non‐measurable disease by RECIST or CA 125 assessable disease (GCIG criteria) or histological proven diagnosis of relapse Eastern Cooperative Oncology Group (ECOG) performance status 0‐2 Adequate bone marrow and organ function. Normal blood pressure or adequately treated and controlled hypertension (either systolic BP ≤ 140 mmHg and/or diastolic BP ≤ 90 mmHg) Patients commencing cytotoxic chemo‐therapy after cytoreductive surgery must be able to do so within 8 weeks Effective form of contraception Exclusion criteria Ovarian tumours of low malignant potential Other malignancy within the last 5 years Administration of other simultaneous chemotherapy drugs, any other anticancer therapy or antineoplastic hormonal therapy, or simultaneous radiotherapy during the trial treatment period Prior radiotherapy to the abdomen or pelvis Surgery (including open biopsy) within 4 weeks prior to anticipated first dose of bevacizumab Known hypersensitivity to trial chemotherapeutic agents, bevacizumab, Chinese hamster ovary cell products or other recombinant human or humanised antibodies Significant heart disease, e.g. congestive heart failure Grade 3 or 4, myocardial infarction or unstable angina within ≤ 6 months, poorly controlled cardiac arrhythmia despite medication; peripheral vascular disease grade ≤ 3. Prior history of hypertensive crisis or hypertensive encephalopathy Current, clinically relevant bowel obstruction, including sub‐occlusive disease, related to underlying disease. Patients with evidence of abdominal free air not explained by paracentesis or recent surgical procedure. History of VEGF therapy related abdominal fistula or gastrointestinal perforation. Brain metastases or spinal cord compression. Previous cerebrovascular accident, transient ischaemic attack or subarachnoid haemorrhage Non‐healing wound, active ulcer or bone fracture Current or recent chronic use of aspirin > 325 mg/day History of thrombotic or haemorrhagic disorders within 6 months. Evidence of bleeding diathesis or significant coagulopathy or requirement of therapeutic anticoagulation using marcumar, warfarin or PTT‐prolonging heparin Significant traumatic injury during 4 weeks prior to randomisation Pregnant or breastfeeding |
|
| Interventions |
Intervention n = 345: bevacizumab (BEV) 10mg/kg day 1 and 15, plus PLD 30mg/m2 day1, plus carboplatin AUC4 day 1; every 4 weeks up to 6 cycles, followed by BEV 15mg/kg every 3 weeks until progression/toxicity Control n= 337: BEV 15 mg/kg day 1 plus gemcitabine 1000 mg/m2 day 1 + 8 plus carboplatin AUC4 day 1; every 3 weeks up to 6 cycles, followed by BEV 15 mg/kg every 3 weeks until progression/toxicity |
|
| Outcomes |
Primary outcomes PFS by RECIST Median progression‐free survival was 13·3 months (95% CI 11·7 to 14·2) in the PLD/carbo/BEV group versus 11·6 months (11·0 to 12·7) in the GEM/Carbo/BEV group (HR 0·81, 95% CI 0·68 to 0·96; P = 0·012). Secondary outcomes PFS by CA125 Health related quality of life Overall survival |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned centrally 1:1. |
| Allocation concealment (selection bias) | Low risk | Randomly assigned centrally by authorised personnel from the Coordinating Center for Clinical Trials at Philipps‐University of Marburg, Germany. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label, no blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participant data presented on flow diagram. |
| Selective reporting (reporting bias) | Low risk | Same outcomes on study protocol and clinical trials.gov and results paper. |
| Other bias | Unclear risk | Funding: Hoffmann‐La Roche. The funder of the study had no role in study design, data collection, data analysis, or data interpretation. |
PRECEDENT.
| Study characteristics | ||
| Methods | Phase II open‐label multicentre RCT; randomisation ratio EC145 (Vintafolide) + PLD to PLD was 2:1; recruitment between September 2008 and June 2010 in the USA, Canada and Poland. | |
| Participants | 162 women with PR ROC (149 had measurable disease); ≥ 18 years; ECOG performance status of 0‐2; measurable disease; ≤ 2 prior systemic cytotoxic regimens and adequate organ function. Excluded if prior exposure to PLD, folate‐receptor (FR) targeted therapy or vinca‐containing compounds; recent surgery; serious comorbidities; concurrent malignancy. | |
| Interventions |
Intervention (100 women): EC145 (2.5 mg IV days 1,3 and 5, weeks 1 and 3, every 4 weeks) + PLD (50 mg/m²), every 4 weeks Control (49 women): PLD (50 mg/m²) IV, every 4 weeks EC145 is a folate‐linked vinca alkaloid. Premedication was optional, but considered not necessary for EC145 administration. |
|
| Outcomes |
Primary outcome PFS assessed within 12 months following completion of accrual using RECIST and clinical findings. Secondary outcomes OS assessed within 18 months after PFS analysis; ORR; safety and tolerability; correlation between therapeutic response and 99mTc‐EC20 levels. |
|
| Notes | We contacted the investigators, who gave us access to their unpublished manuscript and provided us with additional unpublished data. The independent radiologic committee (IRC) assessment in women with more than one CT scan correlation was 74%. PFS was not significantly different between the treatment groups for the IRC assessment except for the subgroup of folate‐receptor positive women. One woman in each group required growth factor support (unpublished data). Median OS was unusually long in the PLD only arm (16.8 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation 2:1 EC145/PLD:PLD. Stratified according to primary or secondary platinum resistance, treatment centre, and baseline CA‐125 (< 200 versus ≥ 200 U/ml). |
| Allocation concealment (selection bias) | Low risk | Central randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was based upon investigator assessment using RECIST criteria; however, blinded assessment was performed by an IRC to check for investigator bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Censoring due to clinical progression was 12% and 10% for treatment arms respectively. Eight women in the EC145 arm were withdrawn from EC145 due to treatment related AEs (7.5%) but were included in ITT analyses. Women with non‐measurable disease (13) were included in the safety analyses but excluded from the survival analyses. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. Sensitivity analysis performed for primary outcome. |
| Other bias | Unclear risk | Baseline characteristics were similar between the arms except for the number of tumour lesions, which was greater in the EC145 arm, however this was not a prognostic factor for shorter PFS. Conflict of interest: Supported by Endocyte who developed the study drug. Employment or Leadership Position: Chandra D. Lovejoy, Endocyte (C); Christopher P. Leamon, Endocyte (C) Consultant or Advisory Role: Robert L. Coleman, Endocyte (C); Robert A. Burger, Endocyte (C); Richard T. Penson, Endocyte (C); James T. Symanowski, Endocyte (C); Chandra D. Lovejoy, Endocyte (C) Stock Ownership: Chandra D. Lovejoy, Endocyte; Christopher P. Leamon, Endocyte; David E. Morgenstern, Endocyte Honoraria: Robert L. Coleman, Endocyte; Richard T. Penson, Endocyte Research Funding: R. Wendel Naumann, Endocyte; Robert L. Coleman, Endocyte; Robert A. Burger, Endocyte; Edward A. Sausville, Endocyte; Sharad A. Ghamande, Endocyte; Nashat Y. Gabrail, Endocyte; Stephen E. DePasquale, Endocyte; Lucy Gilbert, Endocyte; Michael G. Teneriello, Endocyte; Wael A. Harb, Endocyte; Panagiotis A. Konstantinopoulos, Endocyte; Richard T. Penson, Endocyte Expert Testimony: None Patents: None Other Remuneration: None |
PROCEED 2014.
| Study characteristics | ||
| Methods | Multicentre international randomised double‐blind phase III trial The primary analysis was conducted in FR (100%) patients as determined by 99mTc‐etarfolatide scan |
|
| Participants | 182 participants ≥ 18 years old PR Pathology confirmed epithelial ovarian, fallopian tube, or primary peritoneal carcinoma ECOG 0‐2 Measurable disease by RECIST Adequate bone marrow, hepatic, and renal function |
|
| Interventions | Vintafolide 2.5 mg IV injection three times a week, weeks 1 and 3 of a 4‐week cycle AND PLD 50 mg/m2 every 4 weeks versus Placebo AND PLD 50 mg/m2 every 4 weeks |
|
| Outcomes |
Primary outcome PFS Secondary outcomes OS Disease control rate Duration of disease control ORR Duration of response QoL CA125 response rate CA125 PFS Pharmacokinetics Archived tumour specimen biomarker analysis AE SAE Death |
|
| Notes | Initial recruitment delayed due to PLD availability. At interim futility analysis the Data and Safety Monitoring Board (DSMB) recommended the trial stop because it did not meet the efficacy hurdle specified in the statistical analysis plan. Interim analysis data only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | RECIST criteria, unclear if blinded assessors . |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Interim analysis data only. |
| Selective reporting (reporting bias) | High risk | Short duration of follow‐up and high censoring rates. |
| Other bias | Unclear risk | At interim futility analysis the DSMB recommended the trial stop because it did not meet the efficacy hurdle specified in the statistical analysis plan. 321 of planned 350 patients randomised. Sponsored by Endocyte, who developed the study drug. |
SWOG S0200.
| Study characteristics | ||
| Methods | Phase III multicentre RCT. Accrual from August 2002 to December 2004. (ID:NCT00043082) | |
| Participants | 61 women with PS ROC or peritoneal cancer; a progression‐free and platinum‐free interval of 6 to 24 months according to RECIST or GCIG CA‐125 criteria; progression following first‐line platinum‐based chemotherapy and up to 12 courses of non‐platinum containing consolidation treatment; Zubrod performance status 0‐1. | |
| Interventions |
Intervention PLD (30 mg/m²) IV plus carbo IV (AUC = 5 mg/mL/min), every 4 weeks Control Carboplatin IV (AUC = 5 mg/mL/min), every 4 weeks Patients could receive a premed of intravenous dexamethasone (20 mg) plus IV granisetron before carboplatin dose, and further dexamethasone on days 2,3, and 4. G‐CSF was allowed to treat G3 to 4 neutropenia when it occurred, and then subsequently to prevent it. |
|
| Outcomes |
Primary OS Secondary PFS, ORR, toxicity |
|
| Notes | The accrual goal was 900, but study was discontinued due to slow accrual. Unpublished final survival data related to the 2010 publication was received from investigators on 13 December 2012. PFS was significantly improved by the addition of PLD to carboplatin. The final OS was not statistically significantly different between treatment arms, in contrast to the earlier report of 2008 where OS was significantly longer in the PLD/carboplatin arm. Despite using a lower dose of PLD, this trial had a relatively high rate of haematological SAEs (G3 to 4) in the PLD/carboplatin arm compared with the carboplatin alone arm (neutropenia 48% versus 3%; anaemia 16% versus 0%; thrombocytopenia 39% versus 10%). Eight women in the carboplatin arm had allergic reactions (any grade) compared with 0 in the PLD/carbo arm. The HFS rate was 3/31 (10%) in the PLD/carbo arm. The proportion of women in each group who received a dexamethasone premed was not described. Investigators concluded that PLD/carboplatin dosing interval was more convenient than the PAC/carboplatin and GEM/carboplatin alternatives; that PLD was well tolerated with no significant HFS problems; and that "administering PLD with carboplatin appears to substantially reduce the incidence of platinum‐associated hypersensitivity reactions." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. |
| Selective reporting (reporting bias) | Low risk | Final HRs for survival were not published; however, the investigators provided us with these unpublished data. |
| Other bias | Unclear risk | This study closed early due to insufficient accrual and the final sample size was not powered to detect a survival difference. Conflict of interest: this investigation was supported in part by Ortho Biotech Products, L.P. |
TRINOVA‐2.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled phase 3 study from April 2011 to October 2013 in 69 sites in 16 countries (USA, Australia, Austria, Belgium, Canada, Denmark, France, Germany, Hong Kong, Hungary, Italy, New Zealand, Poland, Singapore, Slovakia, Switzerland, Taiwan, UK) | |
| Participants | 223 women with PR ROC Inclusion criteria Histologically or cytologically confirmed epithelial ovarian, primary peritoneal, or fallopian tube cancer ≥ 18 years of age One prior platinum‐based chemotherapeutic regimen for primary disease containing carboplatin, cisplatin, or another organoplatinum compound Radiographically documented disease progression Adequate bone marrow and organ function Exclusion criteria Platinum‐free interval > 12 months from their last platinum based therapy Major surgery within the last 4 weeks or still recovering from prior surgery Prior treatment with more than 3 regimens of anti‐cancer therapy for epithelial ovarian, primary peritoneal or fallopian tube cancer Prior treatment with PLD or any anthracycline‐based or mitoxantrone‐based chemotherapy CNS metastases |
|
| Interventions |
Intervention IV PLD 50 mg/m2 once every 4 weeks plus weekly IV trebananib 15 mg/kg Control IV PLD 50 mg/m2 once every 4 weeks plus weekly IV placebo |
|
| Outcomes |
Primary outcome PFS Secondary outcomes OS ORR DoR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised, however method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment for mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. Outcomes were assessed using the RECIST criteria. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient flow chart included and all patients accounted for. |
| Selective reporting (reporting bias) | High risk | No study protocol available. Clinical trials.gov has only overall survival (OS) as secondary outcome. Overall response rate (ORR) and duration of response (DOR) are added as outcomes in results. |
| Other bias | Unclear risk | Sponsored by Amgen, the maker of the study drug |
AE: adverse event; ALT: alanine amino transferase; ASCO: American Society of Clinical Oncology; AST: aspartate amino transferase; AUC: area under the curve; bd: twice daily; BEV: bevacizumab; BRCA: breast cancer antgen; CA125: cancer antigen 125; CAN: canfosfomide; CRs: complete responses; DCR: disease control rate; DoR: duration of response; DSMB: Data and Safety Monitoring Board; EC145: vintafolide; ECG: electrocardiogram; ECOG: Eastern Cooperative Oncology Group; FR: folate receptor; GCIG: Gynecologic Cancer Intergroup; G‐CSF: granulocyte colony stimulating factor; GEM: gemcitabine; HQL: Health‐related quality of life assessment; HR: hazard ratio; IV: intravenous; IVI:intravenous infusion; M200: volociximab; NCI‐CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; NYHA: New York Heart Association; OLA: olaparib; ORR: objective (or overall) response rate; OS; overall survival; PAC: paclitaxel; PARP: Poly(ADP‐ribose) polymerase; PD: programmed death receptor; PDGF: platelet‐derived growth factor; PDGF‐R; platelet‐derived growth factor receptor; PD‐L: programmed death ligand; PFI: platinum‐free interval; PFS: progression‐free survival; PK: pharmacokinetic; PLD: peglyated liposomal doxorubicin; PPS; partially platinum‐sensitive; PR: platinum resistant (or refractory); PRs: partial responses; PS: platinum sensitive; PTT: prothrombin time; QoL: quality of life; RCT: randomised control trial; RECIST: Response Evaluation Criteria in Solid Tumours; ROC: recurrent/relpased ovarian cancer; SAE: serious adverse events; TBD: trabectedin; Tc: technetium; TOP: topotecan; TTP: time‐to‐progression; VTX‐2337: motolimod.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| A'hern 1995 | Ineligible intervention ‐ review of previous studies focusing on addtion of doxorubicin to chemotherapy regimes. |
| AGOG06‐001 | Phase III RCT of maintenance pegylated liposomal doxorubicin (PLD)/carboplatin versus without in patients with advanced ovarian cancer after first‐line treatmetn. Patients therefore did not have relpasoed EOC. (ANZCTR reg. ID: ACTRN12607000329460) |
| Aracil 2013 | Ineligible study design ‐ not an RCT. |
| Bakrin 2018 | Study protocol of a Phase III trial comparing progression‐free survival between cisplatin + PLD as Pressurized Intraperitoneal Chemotherapy (PIPAC) and chemotherapy alone. Results not available at time of review update. |
| Basu 2011 | Ineligible study design ‐ prospective study, not an RCT. |
| Basu 2013 | Ineligible study design, not an RCT. |
| Bhowmik 2018 | Ineligible intervention ‐ compares generic with branded PLD. |
| Bookman 2002 | Ineligible study design ‐ review of developmental chemotherapy in advanced OC and description of Phase III trial protocol. |
| Cherchi 2003 | Ineligible study design ‐ not an RCT. |
| Colombo 2014 | Ineligible intervention. |
| Colombo 2014a | Ineligible study design ‐ exploratory analysis of results. |
| GOG0182/ICON 5 | Ineligible study group ‐ use of PLD as first line agent, not in ROC. |
| Graybill 2014 | Ineligible study design ‐ abstract describing other studies only. |
| Herzog 2014 | Prediction model taking data from other study already included in review. |
| INOVATYON | Ineligible comparison as PLD in both arms, combined with different chemo agents in each arm, therefore not PLD randomisation. Phase III international, randomised study of trabectedin plus pegylated liposomal doxorubicin (PLD) versus carboplatin plus PLD in patients with ovarian cancer progressing within 6 to 12 months of last platinum (ID: NCT01379989). |
| Kavanagh 2004 | Ineligible study design ‐ not an RCT. |
| Khokhlova 2012 | Ineligible study design ‐ cis+trabectedin versus PLD+trabectadin |
| Lai 2018 | Ineligible intervention ‐ compares chemotherapy to observation only. |
| Lindemann 2017 | Ineligible comparison ‐ chemotherapy versus hormonal treatment |
| Lorusso 2014 | Ineligible intervention ‐ doxorubicin not PLD |
| Marme 2019 | Ineligible intervention ‐ studies efficacy and safety of atezolizumab v placebo. |
| MILO ENGOT‐ov11 | Participants had low grade serous ovarian cancer. |
| MITO‐2 2011 | Ineligible study cohort ‐ use of PLD as first line treatment, not for patients with ROC. |
| Monk 2016 | Ineligible intervention ‐ comparing use of bevacizumab with CA4p v bevacizumab with placebo. |
| Nagao 2016 | Ineligible intervention‐ pilot study of addition of low‐dose paclitaxel to carboplatin‐based combination chemotherapy |
| NCT02641639 2015 | Ineligible intervention ‐ does not study use of PLD. |
| NCT02891824 2016 | Ineligible intervention ‐ does not study use of PLD. |
| NCT03632798 2018 | Ineligible intervention ‐ PCC v ChemoID‐guided treatment. |
| NCT03639246 2018 | Ineligible study design ‐ phase 1b/2 ‐ no randomisation. |
| NCT03699449 2018 | Ineligible intervention ‐ compares durvalumab with other agents. Wrong primary outcome ‐ ORR. |
| NCT03949283 2019 | Ineligible intervention ‐ PCC v ChemoID‐guided treatment. Ineligible outcomes ‐ primary outcome ORR. |
| Palaia 2006 | Ineligible study design ‐ not an RCT. |
| PiSARRO 2016 | Not an RCT ‐ phase 1b study of APR‐246 with carboplatin and PLD. |
| Scarfone 2006 | Not an RCT. |
| Shoji 2018 | Ineligible intervention ‐ compares chemo +/‐ bevacizumab. |
| Trillsch 2016 | Subanalysis of aurelia ‐ compares PPR v SPR. Does not meet intervention. |
| Wydra 2014 | Ineligible intervention ‐ patients who discontinued PLD and continued on vintafolide only. |
| Yabuno 2019 | Does not meet intervention criteria ‐ comparing dosage regimens. |
Abbreviations: AE = adverse effect; EOC: epithelial ovarian cancer; GEM = gemcitabine; HRQoL = health related quality of life; OC = ovarian cancer; ORR = objective response rate; PCC = physician choice chemotherapy; PLD = pegylated liposomal doxorubicin; PR ROC = platinum refractory relapsed ovarian cancer; RCT = randomised controlled trial; ROC = relapsed ovarian cancer; TOP = topotecan;
Characteristics of studies awaiting classification [ordered by study ID]
ASSIST‐1 2009.
| Methods | International, multicentre randomised active control trial The choice of PLD or TOPO for patients randomised to the active control arm was based on the prior failed second‐line therapy |
| Participants | 461 participants PR Inclusion criteria ≥ 18 years of age Eastern Cooperative Oncology Group (ECOG) performance status 0‐2 Histologically or cytologically proven advanced epithelial ovarian, fallopian tube or peritoneal carcinoma Failed one second line therapy with TOPO or PLD Adequate haematopoietic, hepatic and renal function Exclusion criteria Bone marrow transplant Prior malignancy except curative treatment of cervical carcinoma in situ, BCC or SCC skin Significant cardiac disease Hypercalcaemia Systemic infection |
| Interventions | Canfosfamide 1000mg/m2 3 weekly versus PLD 50mg/m2 4 weekly or TOPO at 1.5mg/m2 3 weekly |
| Outcomes | Primary Outcome OS Secondary Outcomes Safety PFS ORR |
| Notes |
AURELIA 2012.
| Methods | Multicentre, open‐label, randomised, two‐arm phase III trial |
| Participants | 361 |
| Interventions | Intervention Chemotherapy of choice plus bevacizumab 10mg/kg every 2 weeks Control Chemotherapy of choice alone (paclitaxel 80mg/m2 days 1, 8, 15, 22 every 4 weeks; PLD 40mg/m2 day 1 every 4 weeks; topotecan 4mg/m2 days 1, 8, 15 every 4 weeks or 1.25mg/m2 on days 1 to 5 every 3 weeks) |
| Outcomes |
Primary outcome
Percentage of participants with disease progression or death, progression free survival. Secondary outcomes Percentage of participants with best overall confirmed objective response of complete response (CR) or partial response (PR) per modified RECIST Duration of objective response Percentage of participatns who died Overall survival Quality of life (EORTC OV 28). |
| Notes | Median PFS was 3.4 months (95% CI 2.2 to 3.7) for the chemotherapy alone arm and 6.7 months (95% CI 5.7 to 7.9) in the chemotherapy with bevacizumab arm. There was no statistically significant difference in overall survival. The results are presented as chemotherapy of choice and have not been separated into individual results (PLD/paclitaxel/topotecan). We have emailed the researchers for individual response. Until then, we have moved this study into awaiting classification. |
FORWARD I.
| Methods | Open‐label phase 3 randomised control trial; international multicentre (131 study locations in the USA, Belgium, Bosnia, Canada, Czech Republic, France, Ireland, Italy, Russia, Serbia, Spain, Switzerland, UK). Randomised 2:1 intervention versus control. |
| Participants | 366 patients with relapsed platinum‐resistant tubo‐ovarian/primary peritoneal cancer. At least 1 and no more than 3 previous lines of chemotherapy. Females >18 years of age. Folate receptor‐alpha receptor positive tumour expression. Clear cell and low grade histology types excluded. |
| Interventions | Mirvetuximab soravtansine 6 mg/kg IV on D1 of 3‐weekly cycles versus chemotherapy of physician's choice (paclitaxel; topotecan; PLD). Study drug continued until progressive disease as per RESIST criteria or unacceptable toxicity or withdrawal of consent or termination of study. |
| Outcomes | OS PFS ORR |
| Notes | Unable to extract data specific for PLD group. Data not able to be included in meta‐analysis. |
HECTOR.
| Methods | Phase III multicentre RCT |
| Participants | 550 women with PS ROC. |
| Interventions | Arm 1:TC Arm 2: GC or PC or PLDC |
| Outcomes | Primary: PFS Secondary: toxicity |
| Notes | Interim data of the first 200 women was presented at ASCO 2012. |
MITO‐16 MANGO OV2b.
| Methods | Open‐label, multicentre, phase III, randomised trial; NCT01802749 |
| Participants | 406 participants with recurrent EOC |
| Interventions | Combination chemotherapy with ONE of the following regimens:
versus Combination chemotherapy AND bevacizumab with ONE of the following regimens:
|
| Outcomes |
Primary outcome Progression free survival (12 months) assessed by local Investigator Secondary outcomes Overall survival (12 months) Number of complete or partial responses (6 months) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 Worst grade toxicity per patient (evaluated every 3 weeks up to 12 months) according to Common Toxicity Criteria for Adverse Events v. 4.03 Number of patients taking oral antidiabetic therapy (at baseline) Number of patients taking antithrombotic therapy (at baseline) Progression‐free survival (12 months) as measured by independent central review Other outcomes Predictive clinical factors for efficacy of bevacizumab (12 months) Correlation of baseline plasma biomarker expression and clinical outcome (12 months) |
| Notes | For carboplatin with PLD, median PFS was 9.0 months (7.8 to 10.0) without bevacizumab and 12.5 months (10.9 to 15.2) with bevacizumab. Contact authors for PLD‐only data from each arm. |
MITO‐23.
| Methods | Open‐label, prospective, multicentre, randomised phase III. Patients will be randomly assigned in a 1:1 ratio to treatment arms. |
| Participants | 242 participants Inclusion Criteria Female of age 18 years or older Histologically or cytologically documented invasive epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer Platinum resistant or sensitive patients with either:
Adequate organ functions:
Exclusion Criteria
Other serious illnesses, such as:
|
| Interventions | Trabectedin 1.3 mg/m2 on day 1 3‐weekly versus Chemotherapy of physician's choice (PLD 40 mg/m2 every 28 days or topotecan 4 mg/m2 days 1, 8, 15 every 28 days or gemcitabine 1000 mg/m2 days 1, 8, 15 every 28 days, or weekly paclitaxel 80 mg/m2 days 1, 8, 15 every 28 days or carboplatin AUC 5‐6 3‐weekly or 4‐weekly) |
| Outcomes |
Primary outcome Overall survival (OS) (4 years) Secondary Outcomes Progression‐free survival (PFS) (4 years) assessed by radiological criteria; CA 125 increases alone (GCIG criteria of progression) will not be considered as progression of disease without a radiological confirmation of progression. Duration of Response (4 years) Adverse events (4 years) Incidence of adverse events, according to the National Cancer Institute Common Toxicity Criteria (NCI‐CTC) version 4.0. |
| Notes | Estimated 4‐year follow up https://clinicaltrials.gov/ct2/show/NCT02903004 November 2022: Abstracts for conferences published but full data not yet published. Will request data for PLD‐only once further data available. |
MITO‐8 2017.
| Methods | International, multicentre, randomised, open‐label, superiority trial (Belgium, Germany, Italy) |
| Participants | 215 participants with PS ROC with disease recurrence between 6 and 12 months after a first‐line platinum based therapy, but no more than 2 previous lines of previous therapy and a life expectancy of more than 3 months. |
| Interventions |
Intervention n = 107 PLD 40 mg/m2 day 1 every 28 days OR topotecan as per institutional guidelines OR gemcitabine 1000 mg/m2 on days 1, 8, 15 every 28 days Control n = 108 carboplatin AUC 5 day 1 every 28 days plus either paclitaxel 175 mg/m2 day 1 every 21 days OR gemcitabine 1000 mg/m2 on days 1, 8, 15 every 28 days |
| Outcomes |
Primary outcome OS Secondary outcome PFS QOL OR Worst grade toxicity for each patient |
| Notes |
Inclusion criteria Histological or cytologically confirmed ovarian cancer Received no more than two lines of chemotherapy Life expectancy ≥ 12 weeks Informed consent Exclusion criteria Other malignancy, previous or current, except adequately treated basal cell carcinoma or squamous cell carcinoma of the skin or carcinoma in situ of the cervix Prior therapy with stealth liposomal doxorubicin Grade ≤ 3 residual peripheral neuropathy Heart disease (congestive heart failure, myocardial infarction within 6 months from study entry, atrioventricular block of any grade, severe arrhythmias) Present or suspected hemorrhagic syndromes |
MORAb‐003.
| Methods | Randomised phase II placebo control trial |
| Participants | 211 participants Low CA125 Platinum sensitive first relapse |
| Interventions | Carboplatin with paclitaxel or carboplatin with PLD AND Farletuzumab (weekly 10 mg/kg first 2 weeks followed by 5 mg/kg) OR placebo |
| Outcomes |
Primary outcome PFS Secondary outcomes OS Best Overall Response Time to Tumour Response Duration of Response Percentage of Participants Achieving Each Second Platinum‐Free Interval Stratified by First Platinum‐Free Interval |
| Notes |
NCT03690739.
| Methods | Open‐label, prospective, randomised, controlled, parallel group, multicentre phase III |
| Participants | 330 patients randomised in a 1:1 ratio. Inclusion criteria
Exclusion criteria
|
| Interventions | Arm A ‐ platinum‐based chemotherapy according to investigator's discretion Arm B ‐ pegylated liposomal doxorubicin 30 mg/m² + trabectedin 1.1 mg/m² every 3 weeks |
| Outcomes |
Primary outcome Symptom benefit rate Secondary outcomes Deterioration in quality of life outcomes Progression‐free survival Response rate (RECIST v1.1) Quality of life (EORTC QLQ‐C30 and EORTC QLQ‐OV 28) Overall survival |
| Notes | AGO‐OVAR 2.32 Closed early due to lack of recruitment. |
Oza 2019.
| Methods | Multicentre, randomised, open‐label phase II trial conducted at 20 centres in the USA, UK, and Canada |
| Participants | 103 participants (51 in experimental arm, 49 in chemotherapy of choice arm) |
| Interventions | Intervention: guadicitabine 30mg/m2 s.c. OD on days 1‐5, + carboplatin IV AUC 4 on day 8 (51 patients) Control: treatment choice of tocotecan IV 3.5 to 4.0mg/m2/week on day 1/8/15 (20 patients), PLD IV 40 to 50mg/m2 on day 1 (15 patients), paclitaxel IV 60 to 80mg/m2/week on day 1/8/15/22 (11 patients) or gemcitabine IV 800 to 100mg/m2 on day 1/8/15 (3 patients) |
| Outcomes |
Primary outcome Progression free survival (PFS) Secondary outcomes Objective response rate (ORR), defined as complete response (CR) and partial response (PR) based on both measurable and evaluable disease PFS at 6 months Clinical benefit rate (CBR: defined as CR+PR+SD for at least 3 months) Proportion of patients with CA‐ 125 reduction of at least 50%, duration of response (DOR) Overall survival (OS); in participants crossing over from the TC to the G+C arm, ORR was measured. Response was assessed using RECIST v1.1 for patients with measurable disease (20), and modified Rustin criteria for patients with detectable disease according to CA‐125 criteria (21, 22). Tumour measurements were obtained by CT or MRI at screening, after every two cycles for the first six cycles, and every 3 months until progression. |
| Notes | E‐mailed author for separated results ‐ awaiting response. Until then study included in the awaiting classification studies. |
PROVE 2011.
| Methods | Open‐label randomised phase II Trial |
| Participants | Platinum sensitive 102 randomised, 96 participants Inclusion criteria Epithelial ovarian/ fallopian/ peritoneal cancer No more than 2 prior treatment lines Measurable disease or elevated CA125 KRAS wild type |
| Interventions | Investigators choice of gemcitabine 1000 mg/m² days 1/8 and carboplatin (AUC 4) 3 weekly or PLD 30 mg/m² 3 weekly and carboplatin (AUC 5) 4 weekly with or without panitumumab 6 mg/kg days 1/15 4 weekly |
| Outcomes |
Primary outcome PFS Secondary outcomes Duration of tumour response PFS OS Toxicity Tumour response rate |
| Notes |
SOLO3.
| Methods | Randomised, controlled, open‐label, phase III trial conducted in 13 countries |
| Participants | 266 participants underwent random assignment. 178 participants were randomly assigned to olaparib and 88 to chemotherapy (PLD, n = 47; paclitaxel, n = 20; gemcitabine, n = 13; topotecan, n = 8; intent‐to‐treat population). |
| Interventions | Participants were randomly assigned 2:1 to olaparib tablets 300 mg twice a day or to physician’s choice of single‐agent chemotherapy: PLD 50 mg/m2 on day 1 every 4 weeks; paclitaxel 80 mg/m2 on days 1, 8, 15, and 22 every 4 weeks; gemcitabine 1000 mg/m2 on days 1, 8, and 15 every 4 weeks; or topotecan 4 mg/m2 on days 1, 8, and 15 every 4 weeks. The investigator made his/her chemotherapy choice before random assignment. |
| Outcomes |
Primary outcome
ORR as assessed by blinded independent central review (BICR) in the measurable disease analysis set (MDAS) using RECIST version 1.1. Secondary outcomes PFS as assessed by BICR Investigator‐assessed PFS Time from random assignment to second progression or death (PFS2) Overall survival (OS) Time from random assignment to first subsequent therapy or death (TFST) Time to earliest progression by RECIST or cancer antigen‐125 (CA‐125) or death Time from random assignment to study treatment discontinuation or death (TDT). |
| Notes | We emailed the authors in November 2020, but they were not able to provide us with separated data for PLD only. Therefore, this study has been placed in th awaiting classification group. |
Volasertib Trial.
| Methods | International, open‐label, controlled, randomised, phase II trial conducted at 31 centres in five countries (Belgium, France, Slovakia, Spain, and Sweden) |
| Participants | A total of 122 patients were screened between 23 April 2010, and 21 April 2011, and 110 participants (volasertib, n = 55; chemotherapy, n = 55) were randomly assigned. Of these participants, 109 (volasertib, n = 54; chemotherapy, n = 55) were treated and included in the analysis. |
| Interventions | Participants were randomly assigned 1:1 to receive either volasertib 300 mg by intravenous infusion every 3 weeks or an investigator’s choice of single‐agent nonplatinum cytotoxic chemotherapy. The investigator was free to choose the most appropriate nonplatinum cytotoxic single agent according to patient status (previous chemotherapy, cumulative toxic effects, performance status, and nutritional status), the summary of product characteristics, and the local standard of care. There were no restrictions but the following single agents were recommended: pegylated liposomal doxorubicin, topotecan, paclitaxel, or gemcitabine. |
| Outcomes |
Primary outcome
24‐week disease control rate (CR, PR or SD; according to RECIST vl.l). Secondary outcomes Best overall response (according to RECIST vl.l) OS PFS QoL and symptom control safety Pharmacokinetics of volasertib Biomarker analysis. |
| Notes |
C = carboplatin; GEM = gemcitabine; PAC = paclitaxel; PLD = pegylated liposomal doxorubicin
Characteristics of ongoing studies [ordered by study ID]
ABT‐888/NCT01113957.
| Study name | A trial of ABT‐888 in combination with temozolomide versus pegylated liposomal doxorubicin alone in ovarian cancer |
| Methods | Phase II open‐label multicentre RCT |
| Participants | 150 women with recurrent high grade serous OC; must be PR or unable to tolerate platinum‐based therapy |
| Interventions | ABT‐888 + temozolomide versus PLD |
| Outcomes |
Primary outcome
ORR based on tumour measurements and CA125 levels (assessed every 3 months for 3 years) Secondary outcomes PFS, OS, 12‐month survival rate, 6‐month PFS rate, duration of response, safety and tolerability, QoL |
| Starting date | March 2010 |
| Contact information | Yan Luo (Abbott): yan.luo@abbott.com |
| Notes | End date: Mar 2013. No results available, checked 23 November 2022 |
ATI0918/NCT01715168.
| Study name | A cross‐over bioequivalence study of intravenously administered ATI0918 and DOXIL/CAELYX in patients with ovarian cancer |
| Methods | Phase I single‐blind RCT |
| Participants | 40 women with ROC |
| Interventions | PLD (50 mg/m²) versus ATI‐0918 |
| Outcomes | Pharmaco‐equivalence outcomes |
| Starting date | Oct 2012 |
| Contact information | Karen Kuhn: kkuhn@ockham.com |
| Notes | May 2013 Nov 2022 ‐ looks to be completed, no results |
EPIK‐O/ENGOT‐OV61.
| Study name | EPIK‐O/ENGOT‐OV61: a phase 3, randomised study of alpelisib + olaparib in patients with no germline BRCA mutation detected, platinum‐resistant or‐refractory, high‐grade serous ovarian cancer |
| Methods | Phase 3, randomised, open‐label, controlled trial |
| Participants | 358 PR |
| Interventions |
Intervention: alpelisib and olaparib Control: paclitaxel or PLD |
| Outcomes |
Primary outcome
PFS Secondary outcomes OS Overall response rate Clinical benefit rate Safety Quality of life |
| Starting date | |
| Contact information | |
| Notes |
MIRASOL.
| Study name | MIRASOL |
| Methods | Phase III randomised open‐label trial |
| Participants | Estimated enrolment 430 PR |
| Interventions |
Intervention: mirvetuximab soravtansine Control: paclitaxel or PLD or topotecan |
| Outcomes |
Primary outcome
PFS Secondary outcomes Safety and tolerability ORR OS Patient‐reported outcomes (QLQ‐OV28) Duration of response CA‐125 response |
| Starting date | 31 December 2019 |
| Contact information | ImmunoGen, Inc. 781‐895‐0600 medicalaffairs@immunogen.com |
| Notes |
MIROVA.
| Study name | Mirvetuximab Soravtansine (IMGN853), in Folate Receptor Alpha (FRα) High Recurrent Ovarian Cancer (MIROVA) NCT04274426 |
| Methods | Phase II randomised open‐label |
| Participants | Estimated enrolment 136 participants |
| Interventions | Intervention: carboplatin and mirvetuximab soravtansine Control: carboplatin and PLD or gemcitabine or paclitaxel |
| Outcomes |
Primary outcome
PFS Secondary outcomes OS Objective response rate PFS OS ORR Time to serological progressive disease Time to first subsequent treatment Time to second subsequent treatment Patient‐reported outcomes Safety and tolerability |
| Starting date | 13 October 2021 |
| Contact information | Michaela Fredrich +49 611 880467 ext 42 mfredrich@ago‐ovar.de |
| Notes |
MITO‐27.
| Study name | MITO 27; NCT03539328 |
| Methods | Multicentre, randomised, open‐label trial, |
| Participants | 138 participants Inclusion Criteria Platinum resistant (platinum free interval 1 to 6 months from last platinum dose) ovarian, Fallopian tube or primary peritoneal cancer ≥ 18 years of age Measurable disease or evaluable based on RECIST 1.1 (patients with only CA 125 increase without evidence of disease are not included) Willing to provide tissue from a newly obtained core or excisional biopsy of a tumour lesion Performance status of 0 or 1 on the ECOG Performance Scale Adequate organ function Negative pregnancy test/on contraception, if required Exclusion criteria Received > 2 previous lines of chemotherapy Pregnant or breastfeeding Immunodeficiency, including HIV 1/2 Active infection |
| Interventions | Physician's choice chemotherapy (PLD 40 mg/m2 IV every 4 weeks, or weekly paclitaxel 80 mg/m2 days 1, 8, 15 every 4 weeks, or gemcitabine 1000 mg/m2 days 1 and 8 every 3 weeks) versus Physician's choice chemotherapy (PLD 40 mg/m2 IV every 4 weeks, or weekly paclitaxel 80 mg/m2 days 1, 8, 15 every 4 weeks, or gemcitabine 1000 mg/m2 days 1 and 8 every 3 weeks) plus pembrolizumab 200 mg d1 every 3 weeks via IV infusion. Participants will receive treatments until disease progression, unacceptable toxicity or patient choice to withdraw. At least 6 to 8 cycles of chemotherapy at physician's discretion. In the experimental arm patients who stop chemotherapy for toxicity reasons and whose disease is at least in stabilisation, may continue treatment with pembrolizumab as single agent. |
| Outcomes |
Primary outcome Overall survival (OS) (from randomisation to the date of death, assessed up to 44 months) Secondary outcomes Progression free survival (PFS) (from randomisation to the date of radiological/clinical progression of disease or death, assessed up to 44 months) Response rate (44 months) Adverse events (44 months) Incidence of adverse events, according to the National Cancer Institute Common Toxicity Criteria (NCI‐CTC) version 5.0 Quality of life (44 months) Physical subscale of the National Comprehensive Cancer Network‐Functional Assessment of Cancer Therapy FACT‐Ovarian Symptom Index 18 (FOSI‐18) Changes. The time to an event in PRO (patient‐reported outcome) of worsening of disease symptoms will be defined as the time from randomisation to a 4‐point reduction in the FACT‐O questionnaire to assess PRO of patients receiving chemotherapy plus pembrolizumab with respect to patients receiving chemotherapy alone using Euro‐Quality of Life 5D tool, indicating which statements best describe the patient health state regarding: mobility, self‐care, usual activities, pain/discomfort, anxiety/depression. |
| Starting date | June 2018 |
| Contact information | domenica.lorusso@istitutotumori.mi.it |
| Notes | https://clinicaltrials.gov/ct2/show/NCT03539328?term=NCT03539328&draw=2&rank=1 |
MITO‐29/NCT03467178.
| Study name | MITO29/NCT03467178 |
| Methods | Open‐label, prospective, multicentre, randomised phase II |
| Participants | 119 participants Age ≥ 18 years Inclusion criteria Cytologic/histologic diagnosis of FIGO stage 1 to 4 epithelial, fallopian tube and primary peritoneal cancer (carcinosarcomas are included) 1‐2 prior lines of treatment Relapsed within 6 months after platinum containing regimen Disease measurable or evaluable by RECIST version 1.1 or Ca 125 GCIG criteria (Gynaecologic Cancer Intergroup). No residual peripheral neurotoxicity > Grade 1 from previous chemotherapy treatment Performance Status (PS) 0 to 1 Life expectancy of at least 3 months Adequate organ functions Exclusion criteria Pregnant or breast‐feeding. Serious heart disease. Active infection requiring antibiotics. History of cerebrovascular accident, pulmonary embolism or untreated deep venous thrombosis (DVT) within the past 6 months History of human immunodeficiency virus (HIV) infection or chronic hepatitis B or C. Patients with evidence of interstitial lung disease. |
| Interventions | Carboplatin AUC (Area Under Curve) 5 day 8 every 4 weeks plus decitabine 10 mg/m2 IV days 1 to 5 every 4 weeks versus PLD 40 mg/m2 every 4 weeks or gemcitabine 1000 mg/m2 days 1, 8, 15 every 4 weeks or weekly paclitaxel 80 mg/m2 days 1, 8, 15 every 4 weeks |
| Outcomes |
Primary outcome PFS (from randomisation to the date of radiological/clinical progression of disease or death, assessed up to 3 years) Secondary outcomes Overall survival (OS) (from randomisation to the date of death, assessed up to 3 years) Radiological response rate (in patients with measurable disease) Duration of response Cancer‐Antigen 125 (CA‐125) response rate Toxicity profile Patient Reported Outcome: physical well‐being (3 years) Patient Reported Outcome: social/family well‐being (3 years) Patient Reported Outcome: functional well‐being (3 years) Patient Reported Outcome: emotional well‐being (3 years) Functional Assessment of Cancer Therapy (FACT‐O) and FACT‐O Ovarian Cancer‐specific Subscale (OCS) questionnaire |
| Starting date | 30 July 2018 |
| Contact information | domenica.lorusso@istitutotumori.mi.it |
| Notes | https://clinicaltrials.gov/ct2/show/NCT03467178?term=NCT03467178&draw=2&rank=1 |
NCT01100372.
| Study name | NCT01100372 |
| Methods | Randomised phase II trial |
| Participants | 154 participants Disease characteristics Histologically confirmed ovarian epithelial, fallopian tube, or primary peritoneal cavity cancer Recurrent platinum‐refractory disease OR platinum‐resistant disease Meets ≥ 1 of the following criteria:
Tumour marker progression (CA‐125) according to Rustin criteria, meeting 1 of the following criteria:
Participant characteristics Karnofsky performance status 70 to 100% Life expectancy ≥ 3 months Platelet count ≥ 100,000/mm³ Haemoglobin ≥ 10 g/dL Neutrophil count ≥ 1.5 x 10³/mm³ Serum creatinine < 1.5 times ULN Bilirubin < 1.5 times ULN (< 2.5 times ULN if liver metastases are present) AST/ALT < 2.5 times ULN (unless caused by parenchymal liver metastases) No childbearing capacity LVEF ≥ 50% by ECHO or MUGA scan No significant comorbidity (e.g. uncontrolled infection, clinical signs of cardiac insufficiency, history of myocardial infarction, or cardiac rhythmic disorders (NYHA class III‐IV disease)) No known hypersensitivity to study drugs No active secondary malignant tumour within the past 5 years (e.g. metastases from primary breast cancer) No condition (medical, social, or psychological), that would prevent adequate follow‐up Prior concurrent therapy No prior chemotherapy with PLD, other anthracyclines, or gemcitabine hydrochloride No other concurrent tumour‐specific therapy for ovarian cancer |
| Interventions | PLD on day 1 and gemcitabine on days 1 and 8 every 3 weeks for 6 courses. versus PLD on day 1 every 3 weeks for 6 courses. |
| Outcomes |
Primary outcome
Remission rates (complete response and partial response) Secondary outcomes Quality of life as measured by EORTC‐QLQ30 and QLQ‐OV28 questionnaires Progression‐free survival Overall survival Toxicity |
| Starting date | 8 April 2010 |
| Contact information | Alain Zeimet, Medical University Innsbruck Alain.zeimet@i‐med.ac.at |
| Notes | https://clinicaltrials.gov/ct2/show/NCT01100372?cond=nct01100372&draw=2&rank=1 |
NCT03353831.
| Study name | NTC03353831 |
| Methods | Randomised phase III trial |
| Participants | Platinum resistant Inclusion criteria Epithelial ovarian, fallopian tube, or primary peritoneal cancer Up to three prior therapies Biopsy for PDL1 status |
| Interventions | Paclitaxel or PLD AND Bevacizumab AND Atezolizumab or placebo |
| Outcomes |
Primary outcomes OS PFS |
| Starting date | September 2018 |
| Contact information | AGO Research GmbH |
| Notes |
NCT04739800.
| Study name | Comparison of Standard of Care Treatment With a Triplet Combination of Targeted Immunotherapeutic Agents |
| Methods | Randomised Phase II Trial |
| Participants | Estimated enrolment 164 participants PR |
| Interventions |
Intervention MEDI4736 (durvalumab) and olaparib and cediranib or Durvalumab and cediranib or Olaparib and cediranib Control Standard of care chemotherapy |
| Outcomes |
Primary outcome
PFS Secondary outcomes Objective response rate Duration of response Overall survival Incidence of adverse events |
| Starting date | 28 April 2021 |
| Contact information | |
| Notes |
NCT05092360.
| Study name | Phase 3 Study of Nemvaleukin Alfa in Combination With Pembrolizumab (ARTISTRY‐7) |
| Methods | Phase 3, multicentre, open‐label, randomised study |
| Participants | Estimated enrolment 376 participants PR |
| Interventions |
Intervention: nemvaleukin and pembrolizumab combination Control: PLD or topotecan or paclitaxel or gemcitabine |
| Outcomes |
Primary outcome
PFS Secondary outcomes Objective response rate Overall Survival Rate Disease Control Rate Duration of Response Time to Response CA‐125 Treatment‐emergent adverse events |
| Starting date | 10 February 2022 |
| Contact information | |
| Notes |
PROVE/NCT01388621.
| Study name | |
| Methods | Phase II open‐label RCT |
| Participants | 140 women with ROC |
| Interventions | Panitumumab + carbo + PLD or GEM versus carbo + PLD or GEM (physician's choice) |
| Outcomes |
Primary outcome
PFS Secondary outcomes OS Duration of response SAE Translational research |
| Starting date | |
| Contact information | |
| Notes | End Date: July 2015; once available, we will request data by those participants treated with PLD, and will be transferred to 'awaiting classification' until these are made available by the author (requested). |
Abbreviations: CR = complete response; DCR = disease control rate; DDC = duration of disease control; GEM = gemcitabine; IV: intravenous; OC = ovarian cancer; ORR = objective response rate; OS = overall survival; PFS = progression‐free survival; PLD = pegylated liposomal doxorubicin; PR = partial response; PR ROC = platinum refractory relapsed ovarian cancer; QoL = quality of life; ROC = relapsed ovarian cancer; RCT = randomised controlled trial; SAE = serious adverse event; TOP = topotecan
Differences between protocol and review
In Types of interventions we have included 'PLD in combination with other agent/s versus PLD alone or with placebo', whereas this comparison was not included in the original protocol. In addition, we have removed the comparison 'PLD versus best supportive care', which was included in the protocol. These changes were as per the previous version of the review and were therefore a priori decisions for this update.
An a priori decision was made to include only participants with high‐grade EOC in this update, since low‐grade serous ovarian cancer (LGSOC) is now considered to be a different disease with different aetiology, genetic mutations and biology.
As explained in the text, we changed the comparison groups in the review to align with clinical scenarios of platinum‐sensitive or platinum‐resistant/refractory relapse, in line with other reviews (Gaitskell 2023, Tattersall 2022). This was a decision for this update to be more in line with information required for clinical decision‐making, rather than by intervention.
In comparison to the previous version of the review, our main approach to meta‐analysis was by applying a fixed‐effect model. Our decision was based on the assumption that the evaluated drugs within the individual comparisons are estimating a common treatment effect. The random‐effects model was only applied in comparisons where we incorporated trials with individuals with recurrent EOC regardless of platinum‐sensitivity status. In case of non‐proportionality of hazards (reported or visible on Kaplan Meier curve) we decided to use a hazard ratio estimate as a measure of effect, if available, but acknowledge its limitations.
Contributions of authors
RN, EN and KE‐S contributed equally to the review and are joint first authors.
For this latest update EN, RN, EB, SV and JM sifted the results of the searches, with appeal to JM, where there were disagreements.
RN, EN, KE‐S, EB and SV performed full text screening. Disagreements were resolved by discussion with JM and referral to Dr Miller for expert advice, as required.
RN, EN, KE‐S, EB and SV contributed to data extraction and risk of bias assessment. Disagreements were resolved by discussion with JM.
ER, RN and JM performed the data analysis, with the GRADE assessment performed by ER and JM.
RN, EN, KE‐S, EB and SV contacted authors and pharmaceutical companies for additional information.
JM, RN, KE‐S, EN, ER, and KE‐S wrote the final review.
All current authors approved the final version of the review.
Sources of support
Internal sources
-
National Institute of Health and Care Research (NIHR( Cochrane Review Group (CRG) Infrastructure award, UK
CRG infrasturucture award supported information specialist and managing editorial support for this review update.
External sources
-
Department of Health, UK
NHS Cochrane Collaboration Programme Grant Scheme CPG‐10/4001/12 for original version of review
Declarations of interest
RN ‐ attended GSK‐sponsored educational event but no direct funding received EN ‐ declares no conflict of interest KE‐S ‐ attended industry‐sponsored educational events but no direct funding received. Declares no other conflicts of interests and no funding received. ER ‐ declares no conflict of interest EB ‐ delares no conflict of interest SV ‐ declares no conflict of interest JM ‐ declares no conflict of interest
These authors should be considered joint first author
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
APPROVE {published data only}
- Wang T, Li N, Tang J, Yang HY, Yin R, Jingru Zhang J, et al. Apatinib combined with pegylated liposomal doxorubicin (PLD) versus PLD for platinum-resistant recurrent ovarian cancer (APPROVE): a multicenter, randomized, controlled, open-label, phase II trial. Gynecologic Oncology August 2021;162:Suppl 1. [Google Scholar]
ASSIST‐3 {published data only}
- NCT00102973. TLK286 (telcyta) in combination with carboplatin (paraplatin) versus doxil in platinum refractory or resistant ovarian cancer. clinicalTrails.gov 2005.
- Rose P, Edwards R, Finkler N, Seiden M, Duska L, Krasner C, et al. Phase 3 Study: Canfosfamide (C, TLK286) plus carboplatin (P) vs liposomal doxorubicin (D) as 2nd line therapy of platinum (P) resistant ovarian cancer (OC). Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I 2007;25 Suppl 18:5529. [Google Scholar]
ASSIST‐5 {published data only}
- Vergote I, Finkler N, Hall J, Melnyk O, Edwards R, Jones M, et al. Randomised phase III study of canfosfamide (TLK 286) plus pegylated liposomal doxorubicin (PLD) vs PLD as second-line therapy in platinum (P) refractory or resistant ovarian cancer (OC). Journal of Clinical Oncology 2009;27 Suppl 15:5552. [Google Scholar]
- Vergote I, Finkler NJ, Hall JB, Melnyk O, Edwards RP, Jones M, et al. Randomized phase III study of canfosfamide in combination with pegylated liposomal doxorubicin compared with pegylated liposomal doxorubicin alone in platinum-resistant ovarian cancer. International Journal of Gynecological Cancer 2010;20(5):772-80. [DOI] [PubMed] [Google Scholar]
Banerjee 2018 {published data only}
- Banerjee S, Oza AM, Birrer MJ, Hamilton EP, Hasan J, Leary A, et al. A randomized, open-label, phase II study of anti-NaPi2b antibody-drug conjugate (ADC) lifastuzumab (Lifa) vedotin (DNIB0600A) compared to pegylated liposomal doxorubicin (PLD) in patients (pts) with platinum-resistant ovarian cancer (PROC). Journal of Clinical Oncology 2016;34(15 Suppl):5569. [Google Scholar]
- Banerjee S, Oza AM, Birrer MJ, Hamilton EP, Hasan J, Leary A, et al. Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Annals of Oncology 2018;29(4):917-23. [DOI] [PubMed] [Google Scholar]
- NCT01991210. A Study of DNIB0600A in ocmparison with pegylated liposomal doxorubicin (PLD) in participants with platinum-resistant ovarian cancer (PROC). https://clinicaltrials.gov/ct2/show/NCT01991210 2013.
CALYPSO {published and unpublished data}
- Alexandre J, Brown C, Coeffic D, Raban N, Pfisterer J, Mäenpää J, et al. CA-125 can be part of the tumour evaluation criteria in ovarian cancer trials: experience of the GCIG CALYPSO trial. British Journal of Cancer 2012;106(4):633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre J, Brown C, Priou F, De Rauglaudre G, Pfisterer J, Maenpaa J, et al. Should CA 125 still be part of tumour evaluation criteria in ovarian cancer trials? Experience of the GCIG calypso trial. Annals of Oncology 2010;21(Suppl 8):307-308. [Google Scholar]
- Baumann KH, Pujade-Lauraine E, Jackisch C, Lubbe D, du Bois A, Brown C, et al. Therapy expectations of patients with recurrent platinum-sensitive ovarian cancer-a German substudy of the calypso/ago-ovar 2.9 trial. In: International Journal of Gynecological Cancer, 17th International Meeting of ESGO. 2011.
- Baumann KH, Sehouli J, Du Bois A, Lubbe D, Wimberger P, Beckmann MW, et al. Quality of life and therapy expectations of patients with recurrent platinum-sensitive ovarian cancer. A German substudy of the Calypso/AGO-Ovar 2.9 trial. Cochrane Database of Systematic Reviews (Online) 2012, Issue 5. [DOI: 10.1002/central/CN-01065059/full] [DOI] [Google Scholar]
- Brundage M, Gropp M, Mefti F, Mann K, Lund B, Gebski V, et al. Health-related quality of life in recurrent platinum-sensitive ovarian cancer - results from the CALYPSO trial. Annals of Oncology 2012;23(8):2020-2027. [DOI] [PubMed] [Google Scholar]
- Gladieff L, Ferrero A, De Rauglaudre G, Brown C, Vasey P, Reinthaller A, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Annals of Oncology 2012;23(5):1185-9. [DOI] [PubMed] [Google Scholar]
- Joly F, Ray-Coquard I, Fabbro M, Donoghoe M, Boman K, Sugimoto A, et al. Decreased hypersensitivity reactions with carboplatin-pegylated liposomal doxorubicin compared to carboplatin-paclitaxel combination: analysis from the GCIG CALYPSO relapsing ovarian cancer trial. Gynecologic Oncology 2011;122(2):226-32. [DOI] [PubMed] [Google Scholar]
- Kurtz J, Hilpert F, Dorum A, Veillard A, Elit L, Buck M, et al. Can elderly patients with recurrent ovarian cancer (ROC) be treated with a platinum-based doublet? Results from the CALYPSO trial. Journal of Clinical Oncology 2010;28(15 Suppl):5031. [Google Scholar]
- Kurtz J, Kaminsky M, Floquet A, Veillard A, Kimmig R, Dorum A, et al. Ovarian cancer in elderly patients: carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in late relapse: a Gynecologic Cancer Intergroup (GCIG) CALYPSO sub-study. Annals of Oncology 2011;22(11):2417-23. [DOI] [PubMed] [Google Scholar]
- Kurzeder C Meier W Jackisch C Staehle A Burges A Pfisterer J et al. Pegylated liposomal doxorubicin and carboplatin (C-PLD) versus paclitaxel and carboplatin (C-P) in platinum-sensitive ovarian cancer (OC) patients (pts): Treatment at recurrence and overall survival (OS) final analysis from CALYPSO phase III GCIG trial. Journal of Clinical Oncology 2012;138:10. [Google Scholar]
- Lee C, Friedlander M, Brown C, Gebski V, Georgoulopoulos A, Vergote I, et al. Early decline in cancer antigen 125 as a surrogate for progression-free survival in recurrent ovarian cancer. Journal of the National Cancer Institute 2011;103(17):1338-42. [DOI] [PubMed] [Google Scholar]
- Lee C, Gurney H, Brown C, Sorio R, Donadello N, Tulunay G, et al. Carboplatin-paclitaxel-induced leukopenia and neuropathy predict progression-free survival in recurrent ovarian cancer. British Journal of Cancer 2011;105(3):360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Simes R, Brown C, Lord S, Wagner U, Plante M, et al. Prognostic nomogram to predict progression-free survival in patients with platinum-sensitive recurrent ovarian cancer. British Journal of Cancer 2011;105(8):1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK. Ovar 2.9: A Phase III study compare PLD-doxorubicine-carboplatin (CD) with carboplatin-paclitaxel (CP) in recurrent platin-sensible ovarian cancer. A GCIG study. In: Archives of Gynecology and Obstetrics, Proceedings of the 58th Congress of the German Society for Gynecology and Obstetrics (DGGG). 2011.
- Mahner S, Meier W, Du Bois A, Brown C, Lorusso D, Ferrero A, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in very platinum-sensitive ovarian cancer patients: Results from a subset analysis of the CALYPSO phase III GCIG trial. Journal of Clinical Oncology 2011;29(15 Suppl):5059. [Google Scholar]
- Mahner S, Meier W, du Bois A, Brown C, Lorusso D, Dell'Anna T, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in very platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. European Journal of Cancer 2015;51(3):352-8. [DOI] [PubMed] [Google Scholar]
- Marth C, Alexandre J, Hanker L, Brown C, Kaern J, Heywood M, et al. Pegylated liposomal doxorubicin and carboplatin (C-PLD) versus paclitaxel and carboplatin (C-P) in platinum-sensitive ovarian cancer (OC) patients (pts): Treatment at recurrence and overall survival (OS) final analysis from CALYPSO phase III GCIG trial. Journal of Clinical Oncology 2011;29(15 Suppl):5052. [Google Scholar]
- NCT00189553. Caelyx plus carboplatin versus paclitaxel plus carboplatin in patients with epithelial ovarian cancer in late relapse. https://clinicaltrials.gov/ct2/show/NCT00189553 2005.
- Pujade-Lauraine E, Mahner S, Kaern J, Gebski V, Heywood M, Vasey P, et al. A randomized, phase III study of carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in relapsed platinum-sensitive ovarian cancer (OC): CALYPSO study of the Gynecologic Cancer Intergroup (GCIG). Journal of Clinical Oncology 2009;27(18 Suppl):LBA5509. [Google Scholar]
- Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. Journal of Clinical Oncology 2010;28(20):3323-3329. [DOI] [PubMed] [Google Scholar]
- Simes R, Lee C, Mirza M, Sauthier P, Georgopoulos A, Vergote I, et al. The value of early decrease in CA125 levels as a prognostic or surrogate marker for disease progression in patients with recurrent ovarian cancer. Results from the calypso study. Journal of Clinical Oncology 2010;28(15 Suppl):5080. [Google Scholar]
- Vasey P. A GCIG randomized phase III study of carboplatin (C) & pegylated liposomal doxorubicin (PLD) (C-D) vs carboplatin (C) & paclitaxel (P) (C-P): CALYPSO results in partially platinum-sensitive ovarian cancer (OC) patients. European Journal of Cancer 2009;3(7):11. [Google Scholar]
- Wagner U, Marth C, Largillier R, Kaern J, Brown C, Heywood M, et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. British Journal of Cancer 2012;107(4):588-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åvall-Lundqvist E, Wimberger P, Gladieff L, Gebski V, Huober JB, Floquet A, et al. Pegylated liposomal doxorubicin (PLD)-carboplatin (C) (C-D) vs paclitaxel-carboplatin (C-P) in relapsing sensitive ovarian cancer (OC): A 500-patient interim safety analysis of the CALYPSO GCIG Intergroup phase III study. Journal of Clinical Oncology 2008;26(15 Suppl):5565. [Google Scholar]
Colombo 2012 {published data only}
- Colombo N, Kutarska E, Dimopoulos M, Bae DS, Rzepka-Gorska I, Bidzinski M, et al. Randomized, open-Label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. Journal of Clinical Oncology 2012;30(31):3841-7. [DOI] [PubMed] [Google Scholar]
CORAIL {published data only}
- Gaillard S, Ghamande S, Pardo B, Lorusso D, Vergote I, Papai Z, et al. CORAIL trial: Randomized phase III study of lurbinectedin (PM01183) versus pegylated liposomal doxorubicin (PLD) or topotecan (T) in patients with platinum-resistant ovarian cancer. Gynecologic Cancer 2016;34(15 Suppl):TPS5597. [Google Scholar]
Fujiwara 2019 {published data only}
- Fujiwara H, Ushijima K, Nagao S, Takei Y, Shimada M, Takano M, et al. A phase II randomized controlled study of pegylated liposomal doxorubicin and carboplatin vs. gemcitabine and carboplatin for platinum-sensitive recurrent ovarian cancer (GOTIC003/intergroup study). International Journal of Clinical Oncology 2019;24:1284-91. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ushijima K, Nagao S, Takei Y, Shimada M, Takano M, et al. Phase 2, randomized controlled study of pegylated liposomal doxorubicin and carboplatin versus gemcitabine and carboplatin in platinum-sensitive recurrent ovarian cancer (gotic003/intergroup study). International Journal of Gynecological Cancer 2017;27:1493. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ushijima K, Nagao S, Takei Y, Shimada M, Takano M, et al. Phase 2, randomized controlled study of pegylated liposomal doxorubicin and carboplatin versus gemcitabine and carboplatin in platinum-sensitive recurrent ovarian cancer (gotic003/intergroup study). International Journal of Gynecological Cancer 2018;28:48. [DOI] [PubMed] [Google Scholar]
Gordon 2001 {published data only}
- Capri S, Cattaneo G. Cost-minimization analysis of pegylated liposomal doxorubicin versus topotecan for the treatment of ovarian cancer in Italy. Clinical Therapeutics 2003;25(6):1826-45. [DOI] [PubMed] [Google Scholar]
- Coleman RL, Gordon A, Barter J, Sun S, Rackoff W, Herzog TJ. Early changes in CA125 after treatment with pegylated liposomal doxorubicin or topotecan do not always reflect best response in recurrent ovarian cancer patients. The Oncologist 2007;12(1):72. [DOI] [PubMed] [Google Scholar]
- Gordon A, Sun S, Rackoff W. Incidence of adverse events in women (</=65 or >65 years) with recurrent ovarian cancer receiving pegylated liposomal doxorubicin or topotecan [abstract]. Gynecologic Oncology 2006;101(Suppl 1):60. [Google Scholar]
- Gordon A, Teitelbaum A. Overall survival advantage for pegylated liposomal doxorubicin compared to topotecan in recurrent epithelial ovarian cancer [abstract]. European Journal of Cancer Supplements 2003;5(1):S51. [Google Scholar]
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. Journal of Clinical Oncology 2001;19(14):3312-22. [DOI] [PubMed] [Google Scholar]
- Gordon AN, Tonda M, Sun S, Rackoff W, on behalf of the Doxil Study 30–49 investigators. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecologic Oncology 2004;95(1):1-8. [DOI] [PubMed] [Google Scholar]
HeCOG 2010 {published and unpublished data}ACTRN12609000436279
- Bafaloukos D, Linardou H, Aravantinos G, Papadimitriou G, Bamias A, Fountzilas G, et al. A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study. BMC Medicine 2010;8(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
JAVELIN Ovarian 200 {published data only}
- NCT02580058 - A study of avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in patients with platinum resistant/refractory ovarian cancer (JAVELIN Ovarian 200) NCT02580058. https://clinicaltrials.gov/ct2/show/NCT02580058.
- Pujade-Lauraine E, Fujiwara K, Dychter SS, Devgan G, Monk BJ. Avelumab (anti-PD-L1) in platinum-resistant/refractory ovarian cancer: JAVELIN Ovarian 200 Phase III study design. Future Oncology 2018;14(21):2103-13. [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncology 2021;22(7):1034-1046. [DOI: 10.1016/S1470-2045(21)00216-3] [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit RS, Ray-Coquard IL, et al. Avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant or refractory epithelial ovarian cancer: primary and biomarker analysis of the phase III JAVELIN Ovarian 200 trial. Gynecologic Oncology 2019;154(Supplement 1):21-2. [Google Scholar]
- Pujade-Lourraine E, Colombo N, Disis ML, Fujiwara K, Ledermann JA, Mirza MR, et al. Avelumab (MSB0010718C; anti-PD-LI) +/- pegylated liposomal doxorubicin vs pegylated liposomal doxorubicin alone in patients with platinum-resistant/refractory ovarian cancer: the phase III JAVELIN Ovarian 200 trial. Journal of Clinical Oncology 2016;34(15 Suppl):TPS5600. [Google Scholar]
- Yonemori K, Matsumoto T, Nagao S, Katsumata N, Oda K, Watari H, et al. Avelumab in platinum-resistant/refractory ovarian cancer (PRROC): phase 3 results from JAVELIN Ovarian 200. Annals of Oncology 2019;30(Suppl 6):vi89. [Google Scholar]
Kaye 2012 {published data only}
- Kaye S, Kaufman B, Lubinski J, Matulonis U, Gourley C, Karlan B, et al. Phase II study of the oral PARP inhibitor olaparib (AZD2281) versus liposomal doxorubicin in ovarian cancer patients with BRCA1 and/or BRCA2 mutations. Annals of Oncology 2010;21:304. [Google Scholar]
- Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. Journal of Clinical Oncology 2012;30(4):372-9. [DOI] [PubMed] [Google Scholar]
M200 {published data only}
- Vergote IB, Colombo N, Kutarska E, Del Campo J, Pippitt C, Casado A, et al. Phase II study comparing volociximab (an antiangiogenic antibody) and pegylated liposomal doxorubicin (PLD) with PLD alone in recurrent ovarian or primary peritoneal cancer. Journal of Clinical Oncology 2009;27(15 Suppl):5560. [Google Scholar]
McGuire 2018 {published data only}
- McGuire W, Penson R, Gore M, Herraez A, Peterson P, Shahir A and Ilaria Jr R, . Randomized phase II study of the PDGFRα antibody olaratumab plus liposomal doxorubicin versus liposomal doxorubicin alone in patients with platinum-refractory or platinum-resistant advanced ovarian cancer. BMC Cancer 2018;18:1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire WP, Shah GD, Loizos N, Youssoufian H, Rowinsky EK, Gore ME. Randomized phase II trial of pegylated liposomal doxorubicin (PLD) with or without anti-platelet-derived growth factor receptor-alpha (PDGFR-alpha) monoclonal antibody IMC-3G3 in platinum-refractory/resistant advanced ovarian cancer. In: Journal of Clinical Oncology. Vol. 28. 2010:Suppl 15.
MITO‐3 {published and unpublished data}
- Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. Journal of Clinical Oncology 2008;26(6):890-6. [DOI] [PubMed] [Google Scholar]
Monk 2017 {published data only}
- Coukos G, Brady M, Lankes H, Aghajanian C, Gottardo R, Swisher E, et al. GOG-3003: identifying biomarkers predictive of immune and clinical response in a phase 2 RCT of chemo-immunotherapy in recurrent ovarian cancer. International Journal of Gynecological Cancer 2016;26:26-7. [Google Scholar]
- Monk BJ, Brady MF, Aghajanian C, Lankes H, Rizack T, Leach JW, et al. A phase II, randomized, double-blind, placebo-controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a gynecologic oncology group partners study. Gynecologic Oncology 2017;145(Suppl 1):220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk BJ, Brady MF, Aghajanian C, Lankes HA, Rizack T, Leach J, et al. A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a Gynecologic Oncology Group partners study. Annals of Oncology 2017;28(5):996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monk 2020 {published data only}
- Bradley J Monk, Thomas J Herzog, George Wang, Spyros Triantos, Scott Maul, Roland Knoblauch, Tracy McGowan, Waleed S W Shalaby, Robert L Coleman. A phase 3 randomized, open-label, multicenter trial for safety and efficacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecologic Oncology March 2020;156 (3):535-44. [DOI: 10.1016/j.ygyno.2019.12.043] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Coleman R, Monk B, Knoblauch R, Parekh T, Triantos S, Maul R, et al. A phase III study of trabectedin (T) plus pegylated liposomal doxorubicin (PLD) versus PLD for treatment of advanced relapsed epithelial ovarian, primary peritoneal, or fallopian tube cancer. Journal of Clinical Oncology 2015;33(15 Suppl):TPS5606. [Google Scholar]
- Jones RL, Herzog TJ, Patel SR, Mehren M, Schuetze SM, Tine BA, et al. Cardiac safety of trabectedin monotherapy or in combination with pegylated liposomal doxorubicin in patients with sarcomas and ovarian cancer. Cancer Medician June 2021;10(11):3565-74. [DOI: 10.1002/cam4.3903] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia F, et al. Final survival results of the randomized phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer. Journal of Clinical Oncology 2011;29(15 Suppl):5046. [Google Scholar]
- Monk BJ, Herzog TJ, Triantos S, Maul S, Wang G, Pontes Valero MJ, et al. Impact of prior pegylated liposomal doxorubicin (PLD) treatment in recurrent ovarian cancer (ROC): sub-group analysis from a randomized, open-label study comparing trabectedin (T) and PLD versus PLD alone in ROC (ET743-OVC-3006). Annals of Oncology 2019;Conference: 44th Congress of European Society for Medical Oncology, ESMO 2019. Spain. 30(Supplement 5):v420-1. [Google Scholar]
- Monk BJ, Herzog TJ, Triantos S, Wang G, Shalaby WSW, McGowan T, et al. A randomized, open-label study comparing trabectedin and pegylated liposomal doxorubicin with pegylated liposomal doxorubicin alone for the treatment of advanced-relapsed epithelial ovarian, primary peritoneal, or fallopian tube cancer (ET743-OVC-3006). Gyynecologic Oncology 2019;154(Supplement 1):12-3. [Google Scholar]
Mutch 2007 {published data only}
- Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. Journal of Clinical Oncology 2007;25(19):2811-8. [DOI] [PubMed] [Google Scholar]
- Mutch DG, Orlando M, Goss T, Wang Y, Lilly E, Teneriello MG, et al. Randomized phase III trial of gemcitabine versus pegylated liposomal doxorubicin for patients with platinum-resistent taxane-pretreated ovarian cancer as second or third line therapy [abstract]. Gynecologic Oncology 2006;101(Suppl 1):S14. [Google Scholar]
- NCT00191607. A randomized trial for patients with platinum resistant ovarian, fallopian or primary peritoneal cancer. https://clinicaltrials.gov/ct2/show/NCT00191607 2005.
NCT00653952 {published and unpublished data}
- Doxil: Clinical Study Report. https://yoda.yale.edu/sites/default/files/nct00653952.pdf 15 March 2004. [CSR: https://yoda.yale.edu/sites/default/files/nct00653952.pdf]
- NCT00653952. CAELYX versus paclitaxel HCl in patients with epithelial ovarian carcinoma following failure of first-line, platinum-based chemotherapy. https://clinicaltrials.gov/ct2/show/NCT00653952 2008.
- O'Byrne KJ. A phase III study of Doxil/Caelyx versus paclitaxel in platinum-treated, taxane-naïve relapsed ovarian cancer. In: Proceedings of the American Society of Clinical Oncologists. Vol. 21. 2002:203a.
NCT01840943 {published data only}
- NCT01840943. A study to compare CAELYX with topotecan HCL in patients with recurrent epithelial ovarian carcinoma following failure of first-line, platinum-based chemotherapy. https://clinicaltrials.gov/ct2/show/NCT01840943 2013.
OVA‐301 {published data only}
- Bidzinski M, Poveda A, Vermorken J, Kaye S, Makhson A, Jagiello-Gruszfeld A, et al. Influence of an independent review on PFS and response assessments in a phase III clinical trial in relapsed ovarian cancer. European Journal of Cancer Supplements 2009;2(7):468. [Google Scholar]
- Boman K, Colombo N, Runnebaum IB, Vergote I, Gore M, Oaknin A, et al. Tolerability of trabectedin (TR) plus pegylated liposomal doxorubicin (PLD) in platinum sensitive (p-s) vs. platinum resistant (P-R) patients (PTS) with relapsed ovarian cancer. In: Annals of Oncology; 35th ESMO Congress, Milan, Italy. Vol. 21. Oxford: Oxford University Press, 2010:306.
- Brundage M, Gropp M, Mefti F, Mann K, Lund B, Gebski V, et al. Health-related quality of life in recurrent platinum-sensitive ovarian cancer--results from the CALYPSO trial. Annals of Oncology 2012;23(8):2020-7. [DOI] [PubMed] [Google Scholar]
- Colombo N, Casado A, Fernandez C, Vergote I. Trabectedin plus pegylated liposomal doxorubicin (PLD) prior to subsequent platinum chemotherapy in patients with platinum-resistant (PR) recurrent ovarian cancer (ROC): results from OVA-301 follow-up. Journal of Clinical Oncology 2014;32(15 Suppl):5551. [Google Scholar]
- Colombo N. Efficacy of trabectedin in platinum-sensitive-relapsed ovarian cancer: new data from the randomized OVA-301 study. International Journal of Gynecological Cancer 2011;21(Suppl 1):S12-6. [DOI] [PubMed] [Google Scholar]
- Diebolder H, Runnebaum I. Extending platinum-free interval (PFI) in partially platinum-sensitive (PPS) patients (pts) with recurrent ovarian cancer (ROC) treated with trabectedin (Yondelis) plus pegylated liposomal doxorubicin (Caelyx [PLD]) combination versus PLD alone: Results from a PPS cohort of the OVA-301 phase III study. In: Archives of Gynecology and Obstetrics; 58th Congress of the German Society for Gynaecology and Obstetrics (DGGG). 2010.
- Fisher M, Gore M. Cost-effectiveness of trabectedin plus pegylated liposomal doxorubicin for the treatment of women with relapsed platinum-sensitive ovarian cancer in the UK: analysis based on the final survival data of the OVA-301 trial. Value in Health 2013;16(4):507-516. [DOI] [PubMed] [Google Scholar]
- Gore M, Vergote I, Vasanthan S, Chan S, Arranz JA, Colombo N, et al. Cost-effectiveness of trabectedin in combination with pegylated liposomal doxorubicin for the treatment of women with relapsed platinum-sensitive ovarian cancer in the UK: Analysis based on the final survival data. European Journal of Cancer; 2011 ECCO Multidisciplinary Cancer Congress, Stockholm, Sweden 2011;var. pagings:72138-4. [Google Scholar]
- Herzog TJ, Vermorken JB, Pujade-Lauraine E, Li J, Bayever E, et al. Correlation of CA-125 and RECIST evaluation in recurrent ovarian cancer (ROC): results from a randomized phase III study of trabectedin (T) with pegylated liposomal doxorubicin (PLD) versus PLD alone [abstract]. Journal of Clinical Oncology 2009;27(15 Suppl):5550. [Google Scholar]
- Herzog TJ, Vermorken JB, Pujade-Lauraine E, Provencher DM, Jagiello-Gruszfeld A, Kong B, et al. Correlation between CA-125 serum level and response by RECIST in a phase III recurrent ovarian cancer study. Gynecologic Oncology 2011;122(2):350-355. [DOI] [PubMed] [Google Scholar]
- Kaye SB, Colombo N, Monk BJ, Tjulandin S, Kong B, Roy M, et al. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer delays third-line chemotherapy and prolongs the platinum-free interval. Annals of Oncology 2011;22(1):49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B, Biakhov M, Kelley JL, Nunez J, Lebedinsky C, Parekh TV, et al. Influence of tumor control on tumor-related events (TRE) in relapsed ovarian cancer (ROC): results from OVA-301, a randomized phase III study of trabectedin (Tr) with pegylated liposomal doxorubicin (PLD) versus PLD alone [abstract]. Journal of Clinical Oncology 2010;28(15 Suppl):e15529. [Google Scholar]
- Krasner CN, Poveda A, Herzog T, Vermorken J, Monk B, Zintl P, et al. Health-related quality of life/patient-reported outcomes in relapsed ovarian cancer: results from a randomized phase III study of trabectedin witj pegylated doxorubicin (PLD) versus PLD alone [abstract]. Journal of Clinical Oncology 2009;27(15 Suppl):5526. [Google Scholar]
- Krasner CN, Poveda A, Herzog TJ, Vermorken JB, Kaye SB, Nieto A, et al. Patient-reported outcomes in relapsed ovarian cancer: results from a randomized Phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD alone. Gynecologic Oncology 2012;127(1):161-7. [DOI] [PubMed] [Google Scholar]
- Kurtz JE, Kaminsky MC, Floquet A, Veillard AS, Kimmig R, Dorum, A et al. Ovarian cancer in elderly patients: carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in late relapse: a Gynecologic Cancer Intergroup (GCIG) CALYPSO sub-study. Annals of Oncology 2011;22(11):2417-23. [DOI] [PubMed] [Google Scholar]
- Meerpohl H. Tolerability of long-term use of trabectedin in combination with pegylated liposomal doxorubicin (PLD) in patients (pts) with relapsed ovarian cancer (ROC). In: Archives of Gynecology and Obstetrics; 58th Congress of the German Society for Gynaecology and Obstetrics (DGGG). 2010.
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia F, et al. Final survival results of the randomized phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer [abstract]. Journal of Clinical Oncology 2011;29(15 Suppl):5046. [Google Scholar]
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. Journal of Clinical Oncology 2010;28(19):3107-14. [DOI] [PubMed] [Google Scholar]
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: Overall survival analysis. European Journal of Cancer 2012;48(15):2361-8. [DOI] [PubMed] [Google Scholar]
- Monk BJ. A randomized phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD in relapsed, recurrent ovarian cancer (OC). Current Treatment Options in Oncology 2009;9(1):30. [Google Scholar]
- Poveda A, Kaye SB, McCormack RT, Wang S, Ricci D, Broderick E, et al. Circulating tumor cells (CTC) in a study of relapsed/recurrent advanced ovarian cancer: An exploratory analysis in the ova-301 phase III study of pegylated liposomal doxorubicin (PLD) compared with trabectedin and PLD. Journal of Clinical Oncology 2009;27(15 Suppl):5551. [Google Scholar]
- Poveda A, Monk BJ, Kaye SB, Vermorken JB, Nieto A, Gomez J, et al. Prediction of overall survival (OS) adjusted by continuous platinum-free interval (PFI) at fixed timepoints in patients with recurrent ovarian cancer (ROC): results from OVA-301. European Journal of Cancer 2011;47 Suppl:S536-7. [Google Scholar]
- Poveda A, Monk BJ, Kaye SB, Vermorken JB, Vergote IB, Runnebaum IB, et al. Prediction of progression-free survival (PFS) adjusted by continuous platinum-free interval (PFI) at fixed timepoints in patients with recurrent ovarian cancer (ROC): Results from OVA-301. Journal of Clinical Oncology 2011;29(15 suppl):5067. [Google Scholar]
- Poveda A, Tjulandin S, Kong B, Roy M, Chan S, Filipczyk-Cisarz E, et al. Extending platinum-free interval (PFI) in partially platinum-sensitive (PPS) patients (pts) with recurrent ovarian cancer (ROC) treated with trabectedin (Tr) plus pegylated liposomal doxorubicin (Tr+PLD) versus PLD alone: results from a PPS cohort of a phase III study. Journal of Clinical Oncology 2010;28(15 Suppl):5012. [Google Scholar]
- Poveda A, Vergote I, Tjulandin S, Kong B, Roy M, Chan S, et al. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum-sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial. Annals of Oncology 2011;22(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero I, Colombo N, Kaye SB, Arranz J, Roszak A, Provencher DM, et al. Tolerability of long-term use of trabectedin (Tr) in combination with pegylated liposomal doxorubicin (PLD) in patients (pts) with relapsed ovarian cancer (ROC). Journal of Clinical Oncology 2010;28(15 Suppl):5121. [Google Scholar]
- Romero I, Pujade-Lauraine E, Tanovic A, Gomez Garcia J, Poveda AM. Conservative sensitivity analyses of progression-free survival (PFS) of trabectedin plus pegylated liposomal doxorubicin (PLD) vs. PLD alone in patients with relapsed ovarian cancer: OVA-301 study. Annals of Oncology 2012;23(Suppl 9):ix324-ix325. [Google Scholar]
- Runnebaum IB. Extending platinum-free interval (PFI) in partially platinum-sensitive (PPS) patients (pts) with recurrent ovarian cancer (ROC) treated with trabectedin (Tr) plus pegylated liposomal doxorubicin (Tr + PLD) versus PLD alone: Results from a PPS cohort of a phase III study. In: Archives of Gynecology and Obstetrics; 58th Congress of the German Society for Gynaecology and Obstetrics (DGGG). 2010.
- Vergote I, Bidzinski M, Kelley J, Vasanthan S, Runnebaum I, Vermorken J, et al. Health-Related Quality of Life (HRQoL) / Patient Reported Outcomes (PRO) of patients with partially platinum sensitive (PPS) recurrent ovarian cancer (ROC) treated in a randomized phase III trial of trabectedin and PLD vs PLD alone (OVA-301): An Exploratory Analysis. Gynecological Cancer 2011;47(Suppl 1):S536. [Google Scholar]
- Vergote I, Meerpohl HG, Boman K, Nieto A, Tanovic A, Vermorken JB. Exploratory study of platinum-sensitive (PS) patients who did not achieve a complete response after first-line platinum-based treatment before ova-301 trial. International Journal Gynecological Cancer 2012;22(Suppl 3):225. [Google Scholar]
- Vergote I, Vermorken J, Pujade-Lauraine E, Monk B, Lisyanskaya A, Rolski S, et al. Safety analysis of trabectedin in combination with pegylated liposomal doxorubicin (PLD) vs PLD alone in ovarian cancer patients 65 years of age and older. In: European Journal of Cancer. 2009:var pagings.
Pfisterer 2020 {published data only}
- Pfisterer J, Dean AP, Baumann K, Rau J, Harter P, Joly F et al. Carboplatin/pegylated liposomal doxorubicin/bevacizumab (CD-BEV) vs. carboplatin/gemcitabine/bevacizumab (CG-BEV) in patients with recurrent ovarian cancer: a prospective randomized phase III ENGOT/ GCIG-Intergroup study (AGO study group, AGO-Austria, ANZGOG, GINECO, SGCTG). In: Oncology Research and Treatment. Vol. Conference: 43rd Congress of European Society for Medical Oncology, ESMO 2018. Germany. 29. 2018:viii332-3.
- Pfisterer J, Shannon CM, Baumann K, Rau J, Harter P, Joly F, et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. The Lancet Oncology 2020;21(5):699-709. [DOI] [PubMed] [Google Scholar]
PRECEDENT {published data only}
- Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, et al. PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. Journal of Clinical Oncology 2013 Dec 10;31(35):4400-6. [DOI: 10.1200/JCO.2013.49.7685] [PMID: ] [DOI] [PubMed] [Google Scholar]
PROCEED 2014 {published data only}
- Armour A. A randomised double-blind plase 3 trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD/DOXIL/Caelyx) in combination versus PLD with platinum ressitant ovarian cancer [EC-FV-06 Clinical Study Report]. Clinical Trials Register 15 February 2017.
- NCT01170650. Study for Women With Platinum Resistant Ovarian Cancer Evaluating EC145 in Combination With Doxil® (PROCEED). clinicaltrials.gov 2010.
- Naumann RW Burger RA Sausville EA Kutarska E Ghamande SA DePasquale SE et al. Vintafolide therapy shows greater efficacy in patients with folate receptor positive tumors selected by 99mTc-etarfolatide. European Journal of Cancer 2013;49:S712. [Google Scholar]
- Naumann RW, Gilbert L, Habbe A, Ma H, Ghamande S, Vergote IB. Trial in progress: a randomized double-blind phase 3 trial comparing vintafolide + pegylated liposomal doxorubicin (PLD) versus PLD + placebo in patients with platinum-resistant ovarian cancer (PROCEED). Gynecologic Oncology 2014;133(Suppl 1):177. [Google Scholar]
- Naumann RW, Gilbert L, Miller AM, Ma H, Ghamande SA, Vergote I. A randomized double-blind phase III trial comparing vintafolide plus pegylated liposomal doxorubicin (PLD) versus PLD plus placebo in patients with platinum-resistant ovarian cancer (PROCEED). Journal of Clinical Oncology 2013;31(15 Suppl):TPS5613. [DOI] [PubMed] [Google Scholar]
- Oza AM, Vergote IB, Gilbert LG, Lucy, Ghatage P, Lisyankaya A, et al. A randomized double-blind phase III trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD/Doxil/Caelyx) in combination versus PLD in participants with platinum-resistant ovarian cancer (PROCEED) (NCT01170650). Gynecologic Oncology 2015;137(Suppl 1):5-6. [Google Scholar]
SWOG S0200 {published and unpublished data}
- Alberts DS, Liu PY, Wilczynski S, Clouser M, Lopez A, Lange M, et al. Phase III randomized trial of pegylated liposomal doxorubicin plus carboplatin versus carboplatin in platinum-sensitive patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy: Southwest Oncology Group Protocol S0200. Journal of Clinical Oncology 2007;25(18 Suppl):5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts DS, Liu PY, Wilczynski SP, Clouser MC, Lopez AM, Michelin DP, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200). Gynecologic Oncology 2008;108(1):90-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman M, Moon J, Wilczynski S, Lopez AM, Rowland KM, Michelin DP, et al. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: final survival results of a SWOG (S0200) phase 3 randomized trial. Gynecologic Oncology 2010;116(3):323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
TRINOVA‐2 {published data only}
- Marth C, Vergote I, Colombo N, Kurzeder C, Lorusso D, Clamp A, et al. ENGOT-ov-6/TRINOVA-2: Randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. International Journal of Gynecological Cancer 2015;9:97-9. [DOI] [PubMed] [Google Scholar]
- Marth C, Vergote I, Scambia G, Oberaigner W, Clamp A, Berger R, et al. ENGOT-ov-6/TRINOVA-2: Randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. European Journal of Cancer 2017;70:111-21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
A'hern 1995 {published data only}
- A'hern RP, Gore ME. Impact of doxorubicin on survival in advanced ovarian cancer. Journal of Clinical Oncology 1995;13(3):726-32. [DOI] [PubMed] [Google Scholar]
AGOG06‐001 {published data only}
- Lai C-H. Phase III randomised trial of maintenance pegylated liposomal doxorubicin/carboplatin versus without in patients with advanced ovarian cancer [Ongoing]. Australia and New Zealand Clinical Trials Registry; 2010;ACTRN12607000329460:Accessed on 20/11/12 at http://www.anzctr.org.au/ACTRN12607000329460.aspx.
Aracil 2013 {published data only}
- Aracil M, Nieto A, Tanovic A, Monk BJ, Kaye SB, Poveda A, et al. Low expression of nibrin predicts better prognosis in patients with ovarian cancer treated with trabectedin plus pegylated liposomal doxorubicin (PLD). International Journal of Gynecological Cancer 2013;23(8 SUPPL. 1):133. [Google Scholar]
Bakrin 2018 {published data only}
- Bakrin N, Tempfer C, Scambia G, De Simone M, Gabriel B, Grischke E-M, et al. PIPAC-OV3: a multicenter, open-label, randomized, two-arm phase III trial of the effect on progression-free survival of cisplatin and doxorubicin as pressurized intra-peritoneal aerosol chemotherapy (PIPAC) vs. chemotherapy alone in patients with platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer. Pleura and Peritoneum 2018;3:0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basu 2011 {published data only}
- Basu C. Second line chemotherapy in platinum potentially resistant recurrent epithelial ovarian cancer: Experience from Eastern India. In: International Journal of Gynecological Cancer. Vol. 21. 2011:99.
Basu 2013 {published data only}
- Basu C and Mukhopadhyay A. Second line chemotherapy in epithelial ovarian cancer - issues of quality of life and cost-effective ratio: experience from a cancer institute of eastern India. In: International Journal of Gynecological Cancer. Vol. 23. 2013:852.
Bhowmik 2018 {published data only}
- Bhowmik S, Bhowmik S, Maiiti K, Chakra A, Shahi P, Jain D, et al. Two multicenter Phase I randomized trials to compare the bioequivalence and safety of a generic doxorubicin hydrochloride liposome injection with Doxil® or Caelyx® in advanced ovarian cancer. Cancer Chemotherapy and Pharmacology 2018;82(3):521-32. [DOI] [PubMed] [Google Scholar]
Bookman 2002 {published data only}
Cherchi 2003 {published data only}
- Cherchi PL, Capobianco G, Fattorini F, Canetto AM, Milia L, Cosso M, et al. Second-line therapy with topotecan and pegylated liposomal doxorubicin vs. topotecan slone in patients with recurrent ovarian cancer. In: International Journal of Gynecological Cancer. Vol. 13(Suppl):53. 2003 IGCS Annual Meeting, 2003.
Colombo 2014 {published data only}
- Colombo N, Biagioli E, Copreni E, Floriani I, Fossati R. Trabectedin and pegylated liposomal doxorubicin (PLD) versus carboplatin and PLD in partially platinum-sensitive ovarian cancer patients: Inovatyon study. In: Annals of Oncology. Vol. 25. 2014:iv325.
Colombo 2014a {published data only}
- Colombo N, Tanovic A, Fernandez C, Casado Herraez A. Trabectedin plus pegylated liposomal doxorubicin (PLD) prior to subsequent platinum-based chemotherapy in recurrent ovarian cancer (ROC): results from ova-301 follow-up. In: International Journal of Gynecological Cancer. Vol. 24. 2014:452-3.
GOG0182/ICON 5 {published data only}
- Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. Journal of Clinical Oncology 2009;27(9):1419-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookman MA, Greer BE, Ozols RF. Optimal therapy of advanced ovarian cancer: carboplatin and paclitaxel vs. cisplatin and paclitaxel (GOG 158) and an update on GOG0 182-ICON5. International Journal of Gynecological Cancer 2003;13(6):735-40. [DOI] [PubMed] [Google Scholar]
- Bookman MA, Gynecologic Cancer InterGroup (GCIG). GOG0182-ICON5: 5-arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG-lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced-stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. In: Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I. Vol. 24, 18S (June 20 Supplement). 2006:5002.
- Bookman MA. Erratum. Journal of Clinical Oncology 2009;27(13):2305. [Google Scholar]
- Bookman MA. GOG0182-ICON5: 5-arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG-lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced-stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. [Abstract]. In: Journal of Clinical Oncology. Vol. 24 (Suppl 18): A-5002, 256s. 2006.
- Copeland LJB, Gynecologic Cancer Intergroup (GCIG). Clinical trials of newer regimens for treating ovarian cancer: the rationale for Gynecologic Oncology Group Protocol GOG 182-ICON5. Gynecologic Oncology 2003;90(2 Part 2):S1-7. [DOI] [PubMed] [Google Scholar]
Graybill 2014 {published data only}
Herzog 2014 {published data only}
- Herzog TJ, Anderson K, Wang J, Xu R. Model prediction of mean PFS and OS time from a phase II trial comparing vintafolide plus pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant ovarian cancer patients with 100% folate receptor-positive target. In: Gynecologic Oncology. Vol. 133. 2014:178-9.
INOVATYON {published data only}
- Colombo N, Gadducci A, Sehouli J, Biagioli E, Nyvang GB, Riniker S, et al. LBA30 INOVATYON study: Randomized phase III international study comparing trabectedin/PLD followed by platinum at progression vs carboplatin/PLD in patients with recurrent ovarian cancer progressing within 6-12 months after last platinum line. Annals of Oncology 1 September 2020;31(Supplement 4):S1161. [DOI: ] [Google Scholar]
- Colombo N. INOVATYON STUDY -International, randomized study in patients with ovarian cancer. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01379989.
Kavanagh 2004 {published data only}
- Kavanagh JJ, Garcia A, Choi H, Gershenson DM, Lewis L, Mascavage J, et al. Efficacy of TLK286 (Telcyta) alone and Inparaplatin® or Doxil® (Caelyx®) in platinum resistant ovarian cancer - results from 3 phase 2 studies. In: International Journal of Gynaecological Cancer. Vol. 14 (suppl 1). 2004.
Khokhlova 2012 {published data only}
- Khokhlova SV, Cherkasova MV, Gorbounova VA, Orel NF, Limareva SV. Trabectedin-based combinations in treatment of platinum sensitive recurrence epithelial ovarian cancer (EOC). Pilot study. International Journal of Gynecological Cancer 2012;22(var.pagings):E381. [Google Scholar]
Lai 2018 {published data only}
- Lai CH, Vallikad E, Lin H, Yang LY, Ou YC, Chou HH, et al. Phase III randomized trial of maintenance pegylated liposomal doxorubicin (PLD) / carboplatin versus without in patients with advanced ovarian cancer: an Asian Gynecologic Oncology Group study. In: Journal of Clinical Oncology. Vol. 36. 2018:5584.
Lindemann 2017 {published data only}
- Lindemann K, Gibbs E, Avall-Lundqvist E, Depont Christensen R, Woie K, Kalling M, et al. Chemotherapy vs tamoxifen in platinum-resistant ovarian cancer: a phase III, randomised, multicentre trial (Ovaresist). British Journal of Cancer 2017;116(4):455-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorusso 2014 {published data only (unpublished sought but not used)}
- Lorusso D, Scambia G, Colombo N, Reed N, Pisano C, Lord R, et al. Randomized phase II trial of NGR-hTNF with an anthracycline in platinum-refractory or -resistant ovarian cancer (OC). In: ASCO Meeting Abstracts. Vol. 32. 2014:5523.
Marme 2019 {published data only}
- Marme F, Pautier P, Van Nieuwenhuysen E, Reuss A, Redondo A, Lindemann K, et al. AGO-OVAR 2.29 (ENGOT-ov34): Atezolizumab in combination with bevacizumab and chemotherapy versus bevacizumab and chemotherapy in recurrent ovarian cancer (ROC). In: Journal of Clinical Oncology. Vol. 37. 2019. [DOI] [PubMed]
MILO ENGOT‐ov11 {published data only}
- Monk BJ, Grisham RN, Marth C, Banerjee SN, Hilpert F, Coleman RL, et al. The MEK Inhibitor in Low-Grade Serous Ovarian Cancer (MILO)/ENGOT-ov11 study: a multinational, randomized, open-label phase 3 study of binimetinib (MEK162) versus physician's choice chemotherapy in patients with recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. Journal of Clinical Oncology 2014;32(15 Suppl):TPS5618. [Google Scholar]
- Monk BJ, Grisham RN, Marth C, Banerjee SN, Hilpert F, Coleman RL, et al. The MILO (MEK inhibitor in low-grade serous ovarian cancer)/ENGOT-ov11 study: A multinational, randomized, open-label phase 3 study of binimetinib (MEK162) versus physician's choice chemotherapy in patients with recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. Journal of Clinical Oncology 2015;33(15 Suppl):TPS5610. [Google Scholar]
MITO‐2 2011 {published data only}
- Pignata S, Scambia G, Ferrandina G, Savarese A, Sorio R, Breda E, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized phase III trial. Journal of Clinical Oncology 2011;29(27):3628-35. [DOI] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Scollo P, De Vivo R, et al. Safety of a 3-weekly schedule of carboplatin plus pegylatedliposomal doxorubicin as first line chemotherapy in patients withovarian cancer: preliminary results of the MITO-2 randomized trial. BMC Cancer 2006;6:202:This article is available from: http://www.biomedcentral.com/1471-2407/6/202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Pisano C, et al. Carboplatin and pegylated liposomal doxorubicin for advanced ovarian cancer: preliminary activity results of the MITO-2 phase III trial. Oncology 2009;76(1):49-54. [DOI] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Vernaglia Lombardi A, et al. Carboplatin plus paclitaxel versus carboplatin plus Stealth liposomal doxorubicin in patients with advanced ovarian cancer: preliminary activity results of the MITO-2 randomized multicenter trial [Abstract]. In: Journal of Clinical Oncology; 2007 ASCO Annual meeting. Vol. 25:18S (abstr. 5532). 2007.
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Ferrandina G, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): activity and safety results of the MITO-2 randomized multicenter trial [abstract]. In: Journal of Clinical Oncology; 2009 ASCO Annual Meeting. Vol. 27:15S (suppl; abstr LBA5508). 2009.
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Legge F, et al. Carboplatin (C) plus paclitaxel (P) versus carboplatin plus pegylated liposomal doxorubicin (PLD) in patients with advanced ovarian cancer (AOC): Final analysis of the MITO-2 randomized multicenter trial. In: Journal of Clinical Oncology; 2010 ASCO Annual Meeting. Vol. 28:18s, 2010 (suppl; abstr LBA5033). 2010.
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Legge F, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as firstline treatment for patients with ovarian cancer: The mito-2 (multicentre Italian trials in ovarian cancer) randomized phase III trial. In: Annals of Oncology; 35th ESMO Congress, Milan, Italy. Vol. var. pagings. 2010.
- Pignata S, Scambia G, Savarese A, Zagonel V, Gebbia V, Scollo P, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus Stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): preliminary safety results of the MITO-2 randomized multicenter trial. In: Journal of Clinical Oncology; 2005 ASCO Annual Meeting. Vol. 23:16S (Suppl, Abstr. 5014). 2005.
Monk 2016 {published data only}
- Monk BJ, Herzog T, Alvarez R, Chan J, Chase D, Couchenour R, et al. Focus study: physician's choice chemotherapy (PCC) plus bevacizumab and CA4P versus PCC plus bevacizumab and placebo in platinum-resistant ovarian cancer. International Journal of Gynecological Cancer 2016;26:878-9. [Google Scholar]
Nagao 2016 {published data only}
- Nagao S, Iwasa N, Kurosaki A, Nishikawa T, Hanaoka T, Hasegawa K, et al. The efficacy of low-dose paclitaxel added to combination chemotherapy of carboplatin and gemcitabine or pegylated liposomal doxorubicin. International Journal of Gynaecological Cancer 2016;26(3):443-8. [DOI] [PubMed] [Google Scholar]
NCT02641639 2015 {published data only}
- NCT02641639. FOCUS: PCC + bevacizumab + CA4P versus PCC + bevacizumab + placebo for subjects with platinum resistant ovarian cancer. https://clinicaltrials.gov/ct2/show/NCT02641639 2015.
NCT02891824 2016 {published data only}
- NCT02891824. ATALANTE: atezolizumab vs placebo phase III study in late relapse ovarian cancer treated with chemotherapy + bevacizumab. Https://clinicaltrials.gov/show/NCT02891824 2016;():2016 2016.
NCT03632798 2018 {published data only}
- NCT03632798. Avastin plus chemotherapy vs. avastin plus chemotherapy guided by cancer stem cell test in recurrent ovarian cancer. Https://clinicaltrials.gov/show/nct03632798 2018.
NCT03639246 2018 {published data only}
- NCT03639246. Efficacy and safety study of AVB-S6-500 in patients with platinum-resistant recurrent ovarian cancer. Https://clinicaltrials.gov/show/nct03639246 2018.
NCT03699449 2018 {published data only}
- NCT03699449. An umbrella study of bioomarker-driven targeted therapy in patients with platinum-resistant recurrent ovarian cancer(AMBITION). Https://clinicaltrials.gov/show/nct03699449 2018.
NCT03949283 2019 {published data only}
- NCT03949283. Cancer stem cell assay directed chemotherapy in recurrent platinum resistant ovarian cancer. Https://clinicaltrials.gov/show/nct03949283 2019.
Palaia 2006 {published data only}
- Palaia I, Angioli R, Calcagno M, Zullo MA, Plotti F, Basile S, et al. Liposome-encapsulated doxorubicin citrate in previously treated recurrent/metastatic gynecologic malignancies. International Journal of Gynecological Cancer 2007;17:88-93. [DOI] [PubMed] [Google Scholar]
PiSARRO 2016 {published data only}
- Basu B, Gourley C, Gabra H, Vergote IB, Brenton JD, Abrahmsen L, et al. PiSARRO: a EUTROC phase 1b study of APR-246 with carboplatin (C) and pegylated liposomal doxorubicin (PLD) in relapsed platinum-sensitive high-grade serous ovarian cancer (HGSOC). Annals of Oncology 2016;27:vi123. [Google Scholar]
Scarfone 2006 {published data only}
- Scarfone G, Presti M, Scarabelli C, Polverino GP, Polonio N, Bertoglio S, et al. Pegylated liposomal doxorubicin alone or in combination with platinum compounds in recurrent ovarian cancer after first line chemotherapy containing paclitaxel and carboplatin. In: International Journal of Gynecological Cancer. Vol. 16. 2006:668.
Shoji 2018 {published data only}
- Shoji T, Komiyama S, Kigawa J, Tanabe H, Kato K, Itamochi H, et al. An open-label, randomized, phase II trial evaluating the efficacy and safety of standard of care with or without bevacizumab in platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer patients previously treated with bevacizumab for front-line or platinum-sensitive ovarian cancer: rationale, design, and methods of the Japanese Gynecologic Oncology Group study JGOG3023. BMC Cancer 2018;18(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trillsch 2016 {published data only}
- Trillsch F, Mahner S, Hilpert F, Davies L, Garcia-Martinez E, Kristensen G, et al. Prognostic and predictive effects of primary versus secondary platinum resistance for bevacizumab treatment for platinum-resistant ovarian cancer in the AURELIA trial. Annals of Oncology 2016;27(9):1733-9. [DOI] [PubMed] [Google Scholar]
Wydra 2014 {published data only}
- Wydra D, Ghamande S, Gabrail N, Nowara E, Bidzinski M, De Pasquale S, et al. Vintafolide and its companion imaging agent, etarfolatide-results from a PRECEDENT subanalysis. European Journal of Nuclear Medicine and Molecular Imaging 2014;41:S353-4. [Google Scholar]
Yabuno 2019 {published data only}
- Yabuno A, Tabata T, Michimae H, Oishi T, Nonaka M, Takano M, et al. Randomized phase III trial comparing pegylated liposomal doxorubicin (PLD) 50 mg/m2 and 40 mg/m2 in patients with platinum-resistant Mullerian carcinoma (JGOG3018). In: Journal of Clinical Oncology. Vol. 37. 2019:5550.
References to studies awaiting assessment
ASSIST‐1 2009 {published and unpublished data}
- Vergote I, Finkler N, Campo J, Lohr A, Hunter J, Matei D, et al. Phase 3 randomised study of canfosfamide (Telcyta®, TLK286) versus pegylated liposomal doxorubicin or topotecan as third-line therapy in patients with platinum-refractory or-resistant ovarian cancer. European Journal of Cancer 2009;45(13):2324-32. [DOI] [PubMed] [Google Scholar]
- Vergote I, Finkler N, Campo J, Lohr A, Hunter J, Matei D, et al. Single agent, canfosfamide (C, TLK286) vs pegylated liposomal doxorubicin or topotecan in 3rd-line treatment of platinum refractory or resistant ovarian cancer: phase III study results [Abstract]. In: Journal of Clinical Oncology; 2007 ASCO Annual Meeting. Vol. 25:18S (Suppl, abstr. LBA5528). 2007.
AURELIA 2012 {published data only}
- Hilpert F, Fabbro M, Jesus Rubio M, Reuss A, Rosenberg P, Benedettu Panici P, et al. Symptoms and adverse effects with chemotherapy +/- bevacizumab for platinum-resistant recurrent ovarian cancer: Analysis of the phase III AURELIA trial. Gynecologic Oncology 2013;130(1):e3. [Google Scholar]
- Hoffmann-La Roche. AURELIA: A study of Avastin (Bevacizumab) added to chemotherapy in patients with platinum-resistant ovarian cancer. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT00976911.
- Husain A, Wany Y, Hanker L, Ojeda B, Anttila M, Breda E, et al. Independent radiologic review of AURELIA, a phase 3 trial of bevacizumab plus chemotherapy for platinum-resistant recurrent ovarian cancer. Gynecologic Oncology 2016;142(3):465-470. [DOI] [PubMed] [Google Scholar]
- Kristensen G Berton-Rigaud D Hilpert F Reuss A Bover I Raspagliesi F et al. Effect of bevacizumab (BEV) plus chemotherapy (CT) for platinum-resistant recurrent ovarian cancer (OC) with ascites: analysis of the aurelia trial. ASCO Annual Meeting II, Journal of Clinical Oncology 2012;22(Suppl 3):E103. [Google Scholar]
- Lesnock J, Hamilton C, Darcy K, Havrilesky L, Krivak T, Maxwell G, et al. A cost-effectiveness analysis of the AURELIA trial: bevacizumab and paclitaxel offer a promising clinical and economic combination for platinum-resistant ovarian cancer. Gynecologic Oncology 2016;141:36-37. [Google Scholar]
- Poveda A, Selle F, Hilpert F, Reuss A, Pasic A Savarese A, et al. Weekly paclitaxel (PAC), pegylated liposomal doxorubicin (PLD) or topotecan (TOP) +/- bevacizumab (BEV) in platinum (PT)-resistant recurrent ovarian cancer (OC): analysis by chemotherapy (CT) cohort in the GCIG Aurelia randomised phase III trial. Annals of Oncology 2012;23(Suppl 9):ixe17. [DOI] [PubMed] [Google Scholar]
- Poveda A, Selle F, Hilpert F, Reuss A, Savarese A, Vergote I, et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: Analysis by chemotherapy cohort of the randomized phase III Aurelia trial. Journal of Clinical Oncology 2015;33(32):3836-8. [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. AURELIA: a randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). In: Journal of Clinical Oncology. Vol. Conference: 2012 Annual Meeting of the American Society of Clinical Oncology, ASCO. Chicago, IL United States. Conference Publication: 30. 2012.
- Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. Journal of Clinical Oncology 2014;32(13):1302-8. [DOI] [PubMed] [Google Scholar]
- Sorio R, Roemer-Becuwe C, Hilpert F, Gibbs E, Garcia Y, Kaern J, et al. Safety and efficacy of single-agent bevacizumab-containing therapy in elderly patients with platinum-resistant recurrent ovarian cancer: subgroup analysis of the randomised phase III AURELIA trial. Gynecologic Oncology 2016;144(1):65-71. [DOI] [PubMed] [Google Scholar]
- Sorio R, Roemer-Becuwe C, Hilpert F, Reuss A, Garcia Y, Kaern J, et al. Safety and efficacy of single-agent chemotherapy +/- bevacizumab in elderly patients with platinum-resistant recurrent ovarian cancer: Subgroup analysis of Aurelia. International Journal of Gynecological Cancer 2013;23(8):136-7. [Google Scholar]
- Stockler M, Hilpert F, Friedlander M, Jing M, Wenzel L, Lee C, et al. Health-related quality of life (HRQoL) results from the AURELIA trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum-resistant recurrent ovarian cancer (OC). American Society of Clinical Oncology 2013;31(15 Suppl):5542. [Google Scholar]
- Stockler M, Hilpert F, Friedlander M, King M, Wenzel L, Lee C, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. Journal of Clinical Oncology 2014;32(13):1309-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteveen P, Lortholary A, Fehm T, Poveda A, Reuss A, Havsteen H, et al. Final overall survival (OS) results from AURELIA, an open-label randomised phase III trial of chemotherapy (CT) with or without bevacizumab (BEV) for platinum-resistant recurrent ovarian cancer (OC). European Journal of Cancer 2013;49:S3-4. [Google Scholar]
FORWARD I {published data only}
- Moore K, Oza A, Colombo N, Oaknin A, Scambia G, Lorusso D, et al. FORWARD I (GOG 3011): a phase III study of mirvetuximab soravtansine, a folate receptor alpha (FRalpha)-targeting antibody-drug conjugate (ADC), versus chemotherapy in patients (pts) with platinumresistant ovarian cancer (PROC). Annals of Oncology 2019;30(Suppl 5):v403. [Google Scholar]
- Moore KN, Vergote I, Oaknin A, Colombo N, Banerjee S, Oza A, et al. FORWARD I: a Phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Future Oncology 2018;14(17):1669-78. [DOI] [PubMed] [Google Scholar]
- Moore KN, Vergote I, Oaknin A, Colombo N, Banerjee S, Oza AM, et al. FORWARD I: a phase 3 study to evaluate the safety and efficacy of mirvetuximab soravtansine (IMGN853) versus chemotherapy in adults with folate receptor alpha (FRa)-positive, platinum-resistant epithelial ovarian cancer, primary peritoneal cancer, or primary fallopian tube cancer. Annals of Oncology 2017;28(Supplement 5):353. [Google Scholar]
- Moore KN, Vergote I, Oaknin A, Colombo N, Banerjee SN, Oza AM, et al. FORWARD I (GOG 3011): a randomized phase 3 study to evaluate the safety and efficacy of mirvetuximab soravtansine (IMGN853) versus chemotherapy in adults with folate receptor alpha (FRalpha)-positive, platinum-resistant epithelial ovarian cancer (EOC), primary peritoneal cancer, or primary fallopian tube cancer. Journal of Clinical Oncology 2017;35(15 Suppl):TPS5607. [Google Scholar]
- NCT02631876. PH3 Study of Mirvetuximab Soravtansine vs Investigator's Choice of Chemotherapy in Women With Folate Receptor (FR) Alpha Positive Advanced Epithelial Ovarian Cancer (EOC), Primary Peritoneal or Fallopian Tube Cancer. Cochrane Database of Systematic Reviews 2015.
HECTOR {published data only}
- Mahner S, Chekerov R, Zeimet A, Garcia-Martinez E, Hanker L, Klare P, et al. Topotecan-carboplatin versus standard platinum combinations in patients with platinum-sensitive recurrent ovarian cancer-the gcig intergroup 'HECTOR' phase III trial. In: International Gynecologic Cancer Society. Vol. 22. 2012:E104.
- Meier W, Lichtenegger W, Marth C, Gonzalez-Martin AJ, Harter P, Tome O, et al. Topotecan plus carboplatin versus standard therapy with paclitaxel plus carboplatin (PC) or gemcitabin plus carboplatin (GC) or carboplatin plus pegylated doxorubicin (PLDC): A planed 200-pt interim safety analysis of the NOGGO-AGO-Germany-AGO Austria and GEICO-GCIG Intergroup Study (HECTOR). In: Journal of Clinical Oncology, 2010 ASCO Annual Meeting. Vol. 28:15s, (suppl; abstr 5071). 2010.
- Sehouli J, Chekerov R, Reinthaller A, Richter R, Gonzalez-Martin A, Harter P, et al. Topotecan plus carboplatin versus standard therapy with paclitaxel plus carboplatin (PC) or gemcitabine plus carboplatin (GC) or pegylated liposomal doxorubicin plus carboplatin (PLDC): a randomized phase III trial of the NOGGO-AGO-Study Group-AGO Austria and GEICO-ENGOT-GCIG intergroup study (HECTOR). Annals of Oncology 2016;27(12):2236-41. [DOI] [PubMed] [Google Scholar]
- Sehouli J, Meier W, Wimberger P, Chekerov R, Belau A, Mahner S, et al. Topotecan plus carboplatin versus standard therapy with paclitaxel plus carboplatin (PC) or gemcitabin plus carboplatin (GC) or carboplatin plus pegylated doxorubicin (PLDC): a randomized phase III trial of the NOGGO-AGO-Germany-AGO Austria and GEICO-GCIG intergroup study (HECTOR). Journal of Clinical Oncology 2012;30(15 Suppl):5031. [Google Scholar]
MITO‐16 MANGO OV2b {published data only}
- NCT01802749. Bevacizumab Beyond Progression in Platinum Sensitive Ovarian Cancer. https://clinicaltrials.gov/ct2/show/NCT01802749 2013.
- Pignata S, Lorusso D, Joly F, Gallo C, Colombo N, Sessa C, et al. MITO16b/MANGO–OV2/ENGOT–ov17 Investigators. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: a randomised, phase 3 trial. The Lancet Oncology 2021;22(2):267-76. [DOI] [PubMed] [Google Scholar]
MITO‐23 {published data only}
- NCT02903004. Trial on Trabectedin (ET-743) vs Clinician's Choice Chemotherapy in Recurrent Ovarian, Primary Peritoneal or Fallopian Tube Cancers of BRCA Mutated or BRCAness Phenotype Patients _MITO-23. clinicaltrials.gov 2016.
- Scambia G, Raspagliesi F, Valabrega G, Colombo N, Pisano C, Cassani C, et al. Randomized phase III trial on trabectedin (ET-743) single agent versus clinician’s choice chemotherapy in recurrent ovarian, primary peritoneal, or fallopian tube cancers of BRCA-mutated or BRCAness phenotype patients (MITO23). Abstract for ASCO 2022 meeting 2022.
MITO‐8 2017 {published data only (unpublished sought but not used)}
- NCT00657878. Efficacy Study of Chemotherapy to Treat Ovarian Cancer Recurrence by Prolonging the Platinum Free Interval. https://clinicaltrials.gov/ct2/show/NCT00657878 2008.
- Piccirillo MC, Scambia G, Bologna A, Signoriello S, Vergote I, Baumann K, et al. Quality-of-life analysis of the MITO-8, MaNGO, BGOG-Ov1, AGO-Ovar2.16, ENGOT-Ov1, GCIG study comparing platinum-based versus non-platinum based chemotherapy in patients with partially platinum-sensitive recurrent ovarian cancer. Annals of Oncology 2018;29(5):1189-94. [DOI] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Bologna A, Signoriello S, Vergote IB, Wagner U, et al. Randomized Controlled Trial Testing the Efficacy of Platinum-Free Interval Prolongation in Advanced Ovarian Cancer: the MITO-8, MaNGO, BGOG-Ov1, AGO-Ovar2.16, ENGOT-Ov1, GCIG Study. Journal of Clinical Oncology 2017;35(29):3347-53. [DOI] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Raspagliesi F, Murgia V, Pisano C, Salutari V, et al. The MITO8 phase III international multicenter randomized study testing the effect on survival of prolonging platinum-free interval (PFI) in patients with ovarian cancer (OC) recurring between 6 and 12 months after previous platinum-based chemotherapy: a collaboration of MITO, MANGO, AGO, BGOG, ENGOT, and GCIG. Journal of Clinical Oncology 2016;34(15 Suppl):5505. [Google Scholar]
MORAb‐003 {published data only}
- Herzog TJ, Ghamande SA, Gabra H, Armstrong DK, Fujiwara K, Monk BJ, et al. A randomized, double-blind, placebo-controlled, phase II study to assess the efficacy and safety of farletuzumab (MORAb-003) in combination with carboplatin plus either paclitaxel or pegylated liposomal doxorubicin (PLD) in subjects with low CA125 platinum-sensitive ovarian cancer. In: Journal of Clinical Oncology. Vol. 34. 2016. [NCT02289950] [DOI] [PubMed]
NCT03690739 {published data only}
- NCT03690739. Evaluation of symptom benefit rate of trabectedin/PLD in patients with recurrent ovarian cancer. https://www.clinicaltrials.gov/ct2/show/NCT03690739 2018.
Oza 2019 {published data only}
- Matulonis UA, Oza AM, Secord AA, Roman LD, Blagden SP, Banerjee SN, et al. Epigenetic resensitization to platinum in recurrent, platinum-resistant ovarian cancer (OC) using guadecitabine (SGI-110), a novel hypomethylating agent (HMA): results of a randomized phase II study. Journal of Clinical Oncology 2016;34(15 Suppl):5547. [Google Scholar]
- Oza AM, Matulonis UA, Alvarez Secord A, Nemunaitis J, Roman LD, Blagden SP, et al. A randomized phase 2 trial of epigenetic priming with guadecitabine and carboplatin in platinum-resistant, recurrent ovarian cancer. Clinical Cancer Research 2019;26(5):1009-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
PROVE 2011 {published data only}
- Chekerov R, Klare P, Krabisch P, Potenberg J, Heinrich G, Mueller L, Kurbacher CM, Grischke EM, Braicu EI, Wimberger P, Sehouli J. Panitumumab in platinum-sensitive epithelial ovarian cancer patients with KRAS wild-type: The PROVE-study, a phase II randomized multicenter study of the North-Eastern German Society of Gynaecologic Oncology. In: Journal of Clinical Oncology. Vol. 35. 2017:Suppl 15. [PMID: ]
- NCT01388621. Carboplatin-based chemotherapy with or without panitumumab in platinum-sensitive recurrent ovarian cancer (PROVE). Https://clinicaltrials.gov/show/NCT01388621 2011.
SOLO3 {published data only}
- Penson R, Valencia VR, David C, Colombo N, Leath CA, Bidziński M, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): A randomized phase III trial. Journal of Clinical Oncology February 2020;38(11):1164-74. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA, Bidzihski M, et al. Olaparib monotherapy versus (vs) chemotherapy for germline BRCA-mutated (gBRCAm) platinum-sensitive relapsed ovarian cancer (PSR OC) patients (pts): phase III SOLO3 trial. Journal of Clinical Oncology 2019;37(15 Suppl):5506. [Google Scholar]
Volasertib Trial {published data only}
- Pujade-Lauraine E, Selle F, Weber B, Ray-Coquard IL, Vergote I, Sufliarsky J, et al. Volasertib versus chemotherapy in platinum-resistant or - refractory ovarian cancer: A randomized phase II groupe des investigateurs nationaux pour l'etude des cancers de l'ovaire study. Journal of Clinical Oncology 2016;34(7):706-13. [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E, Weber BE, Ray-Coquard I, Vergote I, Selle F, Del Campo JM, et al. Phase II trial of volasertib (BI 6727) versus chemotherapy (CT) in platinum-resistant/refractory ovarian cancer (OC). Journal of Clinical Oncology 2013;31(15 Suppl):5504. [Google Scholar]
References to ongoing studies
ABT‐888/NCT01113957 {published data only}
- Abbott. A trial of ABT-888 in combination with temozolomide versus pegylated liposomal doxorubicin alone in ovarian cancer [Ongoing]. Accessed on 12/11/12 at http://www.http://clinicaltrials.gov/show/NCT01113957.
ATI0918/NCT01715168 {published data only}
- Kuhn K. A Crossover bioequivalence study of intravenously administered ATI0918 and DOXIL/CAELYX in patients with ovarian cancer. Accessed on 14/11/12 at ttp://www.clinicaltrials.gov/ct2/show/record/NCT01715168.
EPIK‐O/ENGOT‐OV61 {published data only}
- P Konstantinopoulos, A González-Martín, F Cruz, M Friedlander, R Glasspool, D Lorusso, et al. EPIK-O/ENGOT-OV61: a phase 3, randomized study of alpelisib + olaparib in patients with no germline brca mutation detected, platinum-resistant or-refractory, high grade serous ovarian cancer. International Journal of Gynecological Cancer 1/11/2021;31 (Suppl 4):A139‐A140. [DOI: 10.1136/ijgc-2021-IGCS.350] [DOI] [Google Scholar]
MIRASOL {published data only}
- Moore KN, Van Gorp T, Wang J, Esteves B, Zweidler-McKay PA. A study of mirvetuximab soravtansine vs. investigator's choice of chemotherapy in platinum-resistant, advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate receptor-alpha expression (MIRASOL) NCT04209855. Journal of Clinical Oncology 2020;38. [DOI: 10.1200/JCO.2020.38.15_suppl.TPS6103] [NCT: NCT04209855] [DOI]
MIROVA {published data only}
- Mirvetuximab soravtansine (IMGN853), in folate receptor alpha (FRα) high recurrent ovarian cancer. clinicaltrials.gov. [NCT: 04274426]
MITO‐27 {published data only}
- NCT03539328. Study on MK-3475 plus chemotherapy versus chemotherapy alone in recurrent, platinum-resistant ovarian cancer. https://clinicaltrials.gov/ct2/show/NCT03539328 2018.
MITO‐29/NCT03467178 {published data only}
- NCT03467178. Study on decitabine plus carboplatin versus physician's choice chemotherapy in recurrent, platinum-resistant ovarian cancer. https://clinicaltrials.gov/ct2/show/NCT03467178 2018.
NCT01100372 {published data only}
- NCT01100372. Liposome-encapsulated doxorubicin citrate with or without gemcitabine hydrochloride in treating patients with ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cavity cancer. https://clinicaltrials.gov/ct2/show/NCT01100372 2010.
NCT03353831 {published data only}
- Harter P, Pautier P, Nieuwenhuysen EV, Reuss A, Redondo A, Lindemann K, et. Atezolizumab in combination with bevacizumab and chemotherapy versus bevacizumab and chemotherapy in recurrent ovarian cancer - a randomized phase III trial (AGO-OVAR 2.29/ENGOT-ov34). International Journal of Gynecological Cancer Dec 2020;30(12):1997-2001. [DOI] [PubMed] [Google Scholar]
- NCT03353831. Atezolizumab with bevacizumab and chemotherapy vs bevacizumab and chemotherapy in early relapse ovarian cancer. https://clinicaltrials.gov/ct2/show/NCT03353831 2017.
NCT04739800 {published data only}
- Comparison of standard of care treatment with a triplet combination of targeted immunotherapeutic agents. clinicaltrials.gov. [NCT: NCT04739800]
NCT05092360 {published data only}
- Phase 3 study of nemvaleukin alfa in combination with pembrolizumab (ARTISTRY-7). clinicaltrials.gov. [NCT: 05092360]
PROVE/NCT01388621 {published data only}
- Chekerov R, Klare P, Krabisch P, Potenberg J, Heinrich G, Mueller L. Panitumumab in platinum-sensitive epithelial ovarian cancer patients with KRAS wild-type: The PROVE-study, a phase II randomized multicenter study of the North-Eastern German Society of Gynaecologic Oncology. Journal of Clinical Oncology 2017;35:suppl, 5558-5558. [Google Scholar]
- Sehouli J. PROVE A randomized phase II trial of standard carboplatin-based chemotherapy with or without panitumumab in platinum-sensitive recurrent ovarian cancer. Accessed on 14/11/12 at http://clinicaltrials.gov/ct2/show/NCT01388621.
Additional references
Barclay 2010
- Barclay S, Maher J. Having the difficult conversations about the end of life. BMJ 2010/09/16;341:c4862. [DOI] [PubMed] [Google Scholar]
CAELYX PI
- Janssen-Cilag Pty. CAELYX Product Information. www.janssen.com.au/Products/Caelyx Amended 4 January 2011:(Accessed 24 January 2013).
Cancer Research UK 2019
- Ovarian cancer survival statistics. Ovarian cancer survival by stage at diagnosis. Five-year net survival by stage. Last reviewed: 27 November 2019. Available at www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer/survival#heading-Three (accessed 1 September 2022).
Covidence 2019 [Computer program]
- Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2019.
CROWN
- CROWN. Core outcomes in women's and newborn health. [Available from: www.crown-initiative.org/] Accessed 20 Nov 2022.
CTCAE 2017
- CTCAE. Common terminology criteria for adverse events. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf 27 November 2017;v5.0 (CTCAE).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining for heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith, Altman DG, editors(s). Systematic Reviews in Healthcare: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001. [Google Scholar]
ECIS 2020
- ECIS - European Cancer Information System. Estimates of cancer incidence and mortality in 2020, for all cancer sites. Available at ecis.jrc.ec.europa.eu/explorer.php?$0-0$1-AE27$2-All$4-2$3-All$6-0,85$5-2020,2020$7-7$CEstByCancer$X0_8-3$CEstRelativeCanc$X1_8-3$X1_9-AE27$CEstBySexByCancer$X2_8-3$X2_-1-1 (accessed 1 September 2022).
Edwards 2015
- Edwards SJ, Barton S, Thurgar E, Trevor N. Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for advanced recurrent or refractory ovarian cancer: a systematic review and economic evaluation. Health Technology Assessment 2015;19(7):1-480. [DOI: 10.3310/hta19070] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EMA 2010
- European Medicines Agency. EPARs for authorised medicinal products for human use EPARs for authorised medicinal products for human use. www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000089/WC500020173.pdf 2010;accessed 24 January 2013.
EPOC 2015
- Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed 6 July 2016).
ESMO 2019
- Colombo N, Sessa C, du Bois A, Ledermann J, McCLuggage WG, McNeish I, et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Annals of Oncology 2019;30(5):672-705. [DOI] [PubMed] [Google Scholar]
FIGO 2014
- Prat J. FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. Journal of Gynecologic Oncology 2015;26(2):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabizon 2001
- Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clinical Cancer Research 2001;7(2):223-5. [PubMed] [Google Scholar]
Gaitskell 2022
- Gaitskell K, Hermon C, Barnes I, Pirie K, Floud S, Green J, et al. Ovarian cancer survival by stage, histotype, and pre-diagnostic lifestyle factors, in the prospective UK Million Women Study. Cancer Epidemiology 2022;76:102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaitskell 2023
- Gaitskell K, Rogozińska E, Platt S, Chen Y, El Aziz M, Tattersall A, Morrison J. Angiogenesis inhibitors for the treatment of epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2023, Issue 4. Art. No: CD007930. [DOI: 10.1002/14651858.CD007930.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gladieff 2012
- Gladieff L, Ferrero A, De Rauglaudre G, Brown C, Vasey P, Reinthaller A, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Annals of Oncology 2012;23(5):1185-9. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2020
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: Cancer today. IARC Cancer Base 2020;International Agency for Research on Cancer:globocan.iarc.fr.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
ICBP 2019
- Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TML, Myklebust T, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncology November 2019;20(11):1493-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICON‐4
- Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 2003;361:2099–106. [DOI] [PubMed] [Google Scholar]
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA: A Cancer Journal for Clinicians 2008;58:71-96. [DOI] [PubMed] [Google Scholar]
Khan 2021
- Khan MA, Vikramdeo KS, Sudan SK, Singh Se, Wilhite A, Dasgupta S, et al. Platinum-resistant ovarian cancer: From drug resistance mechanisms to liquid biopsy-based biomarkers for disease management. Seminars in Cancer Biology 2021;77:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamont 2001
- Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Annals of Internal Medicine 2001;134:1096-105. [DOI: 10.7326/0003-4819-134-12-200106190-00009] [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Li 2021
- Xi-Ru Li, Yi Zhu, Guo-Nan Zhang, Jian-Ming Huang, Li-Xia Pei. The impact of pegylated liposomal doxorubicin in recurrent ovarian cancer: an updated meta-analysis of randomized clinical trials. Journal of Ovarian Research 2021;14:42:1-12. [DOI: 10.1186/s13048-021-00790-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorusso 2007
- Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin-related palmar-plantar erthrodysesthesia ('hand-foot syndrome'). Annals of Oncology 2007;18(7):1159-64. [DOI] [PubMed] [Google Scholar]
Monk 2012
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: Overall survival analysis. European Journal of Cancer 2012;48(15):2361-8. [DOI] [PubMed] [Google Scholar]
Moroney 2019
- Moroney MR, Hill B, Sheeder J, Robinson Diamond J, Avella-Howell M, Corr B, et al. Integration of specialty palliative care in a phase I ovarian cancer population. Journal of Clinical Oncology 2019;37(31_suppl):75. [DOI: 10.1200/JCO.2019.37.31_suppl.75] [DOI] [Google Scholar]
Naumann 2011
- Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs 2011;71:1397-412. [DOI] [PubMed] [Google Scholar]
NICE 2003
- National Institute for Clinical Excellence. Guidance on the use of paclitaxel in the treatment of ovarian cancer. NICE: Technology Appraisal Guidance- No. 55 2003.
NICE 2016
- National Institute for Health and Care Excellence. Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for treating recurrent ovarian cancer. Technology appraisal guidance [TA389]Published: 27 April 2016. Available at www.nice.org.uk/guidance/ta389 (accessed 1 September 2022).
Pfisterer 2006
- Pfisterer J, Lederman JA. Management of platinum-sensitive recurrent ovarian cancer. Seminars in Oncology 2006;33(2 Suppl 6):S12-6. [DOI] [PubMed] [Google Scholar]
Poveda 2011
- Poveda A, Vergote I, Tjulandin S, Kong B, Roy M, Chan S, et al. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum-sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial. Annals of Oncology 2011;22(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rustin 1996
- Rustin GJ, Nelstrop AE, Tuxen MK, Lambert HE. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Annals of Oncology 1996;7:361-4. [DOI] [PubMed] [Google Scholar]
Schünemann 2017a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2017b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al, on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2019
- Schünemann H J, Santesso N, Brozek J L. Interactive summary of findings tables: the way to present and understand results of systematic reviews. JBI Database Systematic Reviews and Implementation Reports 2019;17(3):259-60. [DOI] [PubMed] [Google Scholar]
Shi 2020
- Shi S-Q, Jiang F-F, Hong T, Zhuang Y, Chen L, Huang X-L. Comparison of pegylated liposomal doxorubicin and paclitaxel plus carboplatin-based chemotherapy as first line treatment for patients with ovarian cancer: a systematic review and meta-analysis of randomized controlled trials. European Review for Medical and Pharmacological Sciences 2020;24(6):2911-27. [DOI: 10.26355/eurrev_202003_20655] [DOI] [PubMed] [Google Scholar]
Staropoli 2014
- Staropoli N, Ciliberto D, Botta C, Fiorillo L, Grimaldi A, Lama S, et al. Pegylated liposomal doxorubicin in the management of ovarian cancer: a systematic review and meta analysis of randomized trials. Cancer Biology & Therapy 2014;15(6):707-20. [DOI: 10.4161/cbt.28557] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2007
- Stone P, Lund S. Predicting prognosis in patients with advanced cancer. Annals of Oncology 2007;18:971-6. [DOI: 10.1093/annonc/mdl343] [DOI] [PubMed] [Google Scholar]
Tattersall 2022
- Tattersall A, Ryan N, Wiggans AJ, Rogozinska E, Morrison J. Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No.: CD007929. Art. No: CD007929. [DOI: 10.1002/14651858.CD007929.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Temkin 2022
- Temkin, SM, Smeltzer MP, Dawkins MD, Boehmer LM, Senter L, Black DR, et al. Improving the quality of care for patients with advanced epithelial ovarian cancer: Program components, implementation barriers, and recommendations. Cancer 2022;128(4):654-64. [DOI: 10.1002/cncr.34023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Theodoulou 2004
- Theodoulou M, Hudis C. Cardiac profiles of liposomal anthracyclines. Cancer 2004;100(10):2052-63. [DOI] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumours: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92:205-16. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:1-16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
von Moos 2008
- Moos R, Thuerlimann BJK, Aapro M, Rayson D, Harrold K, Sehouli J, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. European Journal of Cancer 2008;44:781-90. [DOI] [PubMed] [Google Scholar]
Wagner 2012
- Wagner U, Marth C, Largillier R, Kaern J, Brown C, Heywood M, et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. British Journal of Cancer 2012;107(4):588-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zunino 2002
- Zunino F, Pratesi G. Antitumour Antibodies. In: Souhami RL, Tannock I, Hohenberger P, Horiot J-C, editors(s). Oxford Textbook of Oncology. 2nd edition. Oxford: Oxford University Press, 2002:715-27. [Google Scholar]
References to other published versions of this review
Lawrie 2013
- Lawrie TA, Bryant A, Cameron A, Gray E, Morrison J. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database of Systematic Reviews. Cochrane Database of Systematic Reviews 2013, Issue Issue 7. Art. No.: CD006910. Art. No: CD006910. [DOI: 10.1002/14651858.CD006910.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


