Abstract
Silica based nanoparticles have been demonstrated to have intrinsic biologic activity towards the skeleton and to function by promoting the differentiation of bone forming osteoblasts while inhibiting the differentiation of bone resorbing osteoclasts. The excitement surrounding nanomedicine in part revolves around the almost unlimited possibilities for varying the physicochemical properties including size, composition, and surface charge. To date few studies have attempted to manipulate these characteristics in concert to optimize a complex biologic outcome. Towards this end, spherical silica nanoparticles of various sizes (50–450 nm), of different surface properties (OH, CO2H, NR4+, mNH2), and of different composition (silica, gold, and polystyrene) were synthesized and evaluated for biological activity toward skeletal cells. Osteoblast activity was most influenced by composition and size variables, whereas osteoclasts were most affected by surface property variation. The study also establishes nanoparticle mediated suppression of Nfatc1, a key transcriptional regulator for osteoclast differentiation, identifying a novel mechanism of action. Collectively, the study highlights how during the design of bioactive nanoparticles, it is vital to consider not only the myriad of physical properties that can be manipulated, but also that the characteristics of the target cell plays an equally integral role in determining biological outcome.
Keywords: silica nanoparticles, surface charge, size, composition, bone cells
1. Introduction:
The emergence of nanotechnology has generated new opportunities to manipulate nanomaterials based on composition and size which can be further modified or functionalized through changes in charge or addition of biologic or chemical compounds. However, the specific physical properties of nanoparticles that impart these important bio-activities are not well defined. Although the general effects of composition, size, and surface on cell-material interactions are beginning to be elucidated [1–4], the potential contribution or effect of these parameters on a therapeutic basis represents an area of biomedicine that has only begun to be explored [5]. A more complete understanding of how the physicochemical properties of nanomaterials interface with biological systems will allow enhanced optimization of nanomaterials. This, in turn, will allow for highly targeted regulation of cell bioactivity that will lead to greater therapeutic benefit as well as provide insight into the basic biology cell-material interaction.
Silica has a number of properties related to synthesis and biocompatibility that makes it a suitable material for varied biomedical applications [6]. Silica’s adaptable physical characteristics such as shape, surface charge, size distribution, post-synthesis surface modifications, and incorporation of other compounds (composites) make it unique compared to other nanomaterial composition. These properties are also known to vary significantly depending on the synthesis method. The sol-gel method (Stöber method) [7] provides biological advantages of precise control over size, shape, and surface charge and produces nanoparticles that are highly dispersible in most medium including alcohol, water, and PBS in a wide range of pHs [6, 8]. Highly dispersible products, defined as a uniform distribution in solution (mono-dispersed), are an important factor for both biocompatibility and reproducibility. Dietary silica and synthetic amorphous silica is considered a GRAS agent (generally recognized as safe) and has been approved by US-FDA, independent of both thermal (pyrolysis) and wet synthesis methods (precipitation and gel). In regards to the safety of silica based nanoparticles, it has been suggested that a more detailed analysis of physicochemical properties is required to fully identify any potential toxicity [9].
One particular biomedical application of silica has been in the general field of bone biology. Silica and derivatives such as bioactive glass have been studied for a half a century for bone repair and dental applications because of the mechanical and aesthetic properties, and biocompatibility [6, 10]. The skeleton is critical to human health as it represents the main structural component of the body required for locomotion, organ protection, blood cell production, among many other functions. A decrease in the quality or density of bone, commonly referred to as osteoporosis, can have a significant and negative impact on health through increased risk of fracture [11]. Osteoporosis, and bone disease in general, is the result of an imbalance between bone forming osteoblasts and bone resorbing osteoclasts [12]. Silica nanoparticles have recently been demonstrated to improve bone mass in both young and old mice and in culture to modulate promote differentiation of bone forming cells (osteoblasts) while inhibiting differentiation of bone resorbing cells (osteoclasts) suggesting an inherent bioactivity [13–15].
Here we investigated the physical qualities of silica nanoparticles including size, surface properties, and composition that impart the inherent bioactive properties towards osteoblasts and osteoclasts with the ultimate goal of optimizing these desired bioactive responses. Towards this end, spherical silica nanoparticles of 50, 100, 200 and 450 nm were synthesized by the Stӧber method [16, 17] to assess the optimal size for the biological effect. To assess the contribution of surface properties we assessed two negatively charged particles; the as-prepared base 50 nm silica particles (OH- surface) and surface modified (CO2H) as well as two positively charged particles modified by addition of quaternary ammonium salt (NR4+) and multi-amines (mNH2). Finally, composition was assessed using 50 nm spherical polystyrene and gold particles. A bone marrow stromal cell (BMSC) culture was used as a model of osteoblast differentiation while the monocyte/macrophage pre-osteoclast cell line RAW264.7 was used as a model of osteoclast differentiation. Size, charge, and composition were found to affect osteoblast and osteoclasts differently. The study not only provides additional insight into the inherent bioactive nature of silica nanoparticles, but also highlights the flexibility of nanotechnology to finely tune materials for therapeutic purposes.
2. Materials and Methods
2.1. Synthesis of silica nanoparticles
Chemicals for the synthesis of nanoparticles were obtained from Sigma-Aldrich (St. Louis, MO) and Gelest (Morrisville, PA). The 50, 100, 200, and 450 nm silica nanoparticles (SiO2) were synthesized by the Stöber method as described previously [17]. Briefly, tetraethyl orthosilicate was added in ethanol anhydrous and stirred for 5 minutes. Ammonia or diluted ammonia solution [17] was added with stirring. After 16 to 24 hours, silica nanoparticles were purified by centrifugation at 15,000 rpm for 20 min and re-dispersed followed by three additional wash-centrifugation steps. Finally, nanoparticles were centrifuged at 3,000 rpm for 5 minutes to remove any aggregated particles, and the purified silica nanoparticles were centrifuged and re-suspended in sterile water, PBS, or medium. Gold nanoparticles (50 nm) (GNP) with citrate were purchased from Nanopartz (Loveland, CO). 50 nm polystyrene particles (PS) (Spherical Polybead Carboxylate Microspheres) with carboxyl groups were obtained from Polysciences, Inc. (Warrington, PA). Gold nanoparticles and polystyrene particles were used without further purification.
2.2. Surface modifications of silica nanoparticles
Surface modifications of silica nanoparticles were performed as the follows; N-trimethoxysilylpropyl-N,N,N-trimethylammonium chloride (NR4+:PTMA) for positive charge, (3-trimethoxysilylpropyl)diethylenetriamine (mNH2:DETA) for positive charge and amine groups, and N-(trimethoxysilylpropyl)ethylenediamine triacetate, trisodium salt (CO2H) for negative charge and carboxylate groups were reacted with the as-prepared 50 nm silica nanoparticles (OH). All chemicals for the surface modifications were obtained from Gelest (Morrisville, PA). For the NR4+ modification, 50 mg of the as-prepared silica nanoparticles were dispersed in 10 ml of ethanol anhydrous followed by addition of 500 mg of the PTMA and then pH was adjusted to pH10 with ammonia. For the modification from OH to mNH2 and CO2H, 500 mg (0.51 ml) of DETA or 500 mg (1.1 ml) of COOH was added similar to PTMA (10–15 times excess of the as-prepared by weight). After 16 hours nanoparticles were purified as the 50 nm silica nanoparticles above and the modified particles were dispersed in water, PBS, or culture media.
2.3. Characterization of silica nanoparticles
The size and shape of all nanoparticles was characterized by transmission electron microscope (TEM) (JEOL JEM-1400, Peabody, MA). Polystyrene nanoparticles were stained with 1% uranyl acetate prior to TEM. For the measurement of the size by Dynamic Light Scattering (DLS) and surface charge by zeta potential nanoparticles were dispersed in; sterile water (Thermo Fisher Scientific, Waltham, MA), 1x DPBS (without calcium and magnesium) (Cellgro, Manassas, VA), or α-Modified Eagle’s Medium (α-MEM; Thermo Scientific). All samples were measured six times for size and ten times for the surface charge by NanoComposix (San Diego, CA). Gold, polystyrene, and silica (450 nm) nanoparticles were dispersed as the particles above and zeta potential and DLS measured using Zetasizer Nano ZS90 (Malvern, UK) by IMGT (Seungnam, Korea).
2.4. Cell culture and reagents
The murine macrophage/monocyte (pre-osteoclast) cell line RAW264.7 and the murine pre-osteoblast cell line MC3T3-E1[18] were obtained from ATCC (Manassas, VA). The bone marrow stromal cells (BMSC) were originally isolated from murine bone marrow by centrifugation [19]. All cells were used within 10 passages. Pre-osteoblasts (BMSC and MC3T3-E1) were cultured in growth medium; α-Modified Eagle’s Medium (α-MEM; Thermo Scientific) supplemented with 50 U/ml penicillin, 50mg/ml streptomycin, 2mM L-glutamine (Thermo Scientific) and 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA). Pre-osteoclasts (RAW264.7 cells) were cultured in growth medium; Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Scientific) supplemented with 50 U/ml penicillin, 50mg/ml streptomycin, 2mM L-glutamine, and 10% FBS. All cells were cultured at 37°C in 5% CO2.
2.5. Osteoblast differentiation and mineralization
Osteoblast differentiation was assessed using a culture of murine BMSCs previously demonstrated capable of differentiating to osteoblasts and producing mineral [19, 20] and the murine pre-osteoblast cell line MC3T3-E1 [18, 21]. Stock cells (passage 5) were cultured in growth medium (αMEM and 10% FBS) and subcultured every three to 4 days for up to 10 passages. For differentiation assays, cells were originally seeded in 24-well plates at approximately 5×104 in growth medium and switched to osteoblast differentiation after 72 hours. Osteoblast differentiation medium consisted of growth medium supplemented with filtered sterilized 50 μg L-ascorbate and 10 mM β-glycerophosphate (Sigma-Aldrich) [21]. Nanoparticles, at 10 μg/ml unless otherwise noted, were added with differentiation medium changes twice per week and after 7 to 14 days cells were stained with 40mM Alizarin Red S (Sigma-Aldrich) for 15 minutes to visualize mineralization. Excess stain was removed by washing with distilled water. Mineralization was quantified using ImageJ [22]. Graphs represent the average of 4 to 6 independent experiments which consisted of the average of 2 to 4 wells per experiment. Samples for RNA analyses were generated from cells differentiated on 24-well plates ± nanoparticles, as above, and harvested after 7 days.
2.6. Osteoclast differentiation and TRAP assays
Stock cells (< passage 3) were cultured in growth medium (DMEM and 10% FBS) and subcultured every 2 to 3 days for up to 5 passages. To induce osteoclast formation RAW264.7 cells were plated in 48-well plates (3000xcm2) and 24 hours later culture medium switched to differentiation medium; α-MEM + 10% FBS supplemented with 15 ng/mL RANKL (R&D Systems, Minneapolis, MN). Nanoparticles were added with the change to differentiation medium at 25 μg/ml unless otherwise noted. Upon formation of osteoclasts (visualized by light microscopy), usually between days 3 and 4, cells were stained for tartrate-resistant alkaline phosphatase (TRAP) (Sigma-Aldrich) and TRAP+ multinucleated cells (≥ 3 nuclei) were quantitated under light microscopy [23]. Samples used for RNA studies were generated from RAW264.7 cells plated on 10cm plates and treated under differentiation conditions as above ± nanoparticles and harvested in TRIzol (Invitrogen) after 16 hours.
2.7. RNA extraction, cDNA synthesis, and qRT-PCR
RNA was extracted using TRIzol reagent following the manufacturer’s protocol (Invitrogen) and cDNA was synthesized using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) or Geneamp RNAPCR kit (Applied Biosystems), and qRT-PCR was performed using EvaGreen qPCR master mix (Biotium, Hayward CA) on an Applied Biosystems-StepOnePlus or BioRAD Icycler Primers were designed using qPrimerDepot software (http://mouseprimerdepot.nci.nih.gov/) as described previously [20] and fold change was calculated using the 2(-ΔΔCt) method [24]. Primer sequences as follows: 18S (control) 5’-TTGACGGAAGGGCACCACCAG-3’ and 5’-GCACCACCACCCACGGAATCG-3, Alp (alpl) 5’-ACAGACCCTCCCCACGAGT-3’ and 5’-TGTACCCTGAGATTCGTCCC-3’, OSC (bglap) 5’- AAGCAGGAGGGCAATAAGGT-3’ and 5’- CAAGCAGGGTTAAGCTCACA −3’, Nfatc1 (nfatc1) 5’-AGTCTCTTTCCCCGACATCA-3’ and 5’-GATCCGAAGCTCGTATGGAC-3’, RANK (tnfrsf11a) 5’- CTGATGAGAAGGGAGCCTCA −3’ and 5’- CCGCTAGAGATGAACGTGGA −3’.
2.8. Cell viability assays
Cells were plated at ~5×103 cells per well (~50–60% confluent) in 96 well plates in 100 μl growth medium. After 24 hours, nanoparticles were added as indicated. Cell viability was measured after 72 hours by adding 20 μl of XTT reagent (3- (4,5-dimethylthiazol-2-yl)-5-(3-carboxylmethoxyphenyl)-2-(4-sulfonyl)- 2H-tetrazolium) according to the manufacturer’s protocol (Promega, Madison, WI) and quantitated by spectrophotometer (VersaMax microplate reader; Molecular Devices, San Jose CA).
2.9. Statistical analysis
Data are presented as the mean ± standard deviation (Stdev). Statistical analyses were performed using excel (Microsoft). Comparisons of nanoparticle treated cells were against the positive control (differentiated osteoblasts (+AA/BGP) or osteoclasts (+RANKL) using Student’s t test. *P< 0.05, **P< 0.01, **P< 0.005.
3. Results
3.1. Synthesis and characterization of varied sized silica nanoparticles
To determine the effects of size on the biologic activity of spherical silica nanoparticles various sized particles were synthesized by the Stöber method [16] similar to previous descriptions [17]. The particles were characterized for shape, size, and charge. TEM revealed spherical nanoparticles for all synthesized sizes (Fig. 1A). Size was evaluated by Dynamic Light Scattering (DLS) (Fig. 1B) and was consistent with the predicted size as well as TEM data. Measurement of zeta potential revealed all particles had a relatively negative charge (~ −25 to −60 mV) (Fig. 1C) as expected with the as-prepared particles possessing OH surface groups. Analysis of cell viability after 72 hours of treatment did not identify substantial cytotoxicity for any of the sized particles in either BMSCs (Fig. 1D) or RAW264.7 cells (Fig. 1E).
Fig. 1.
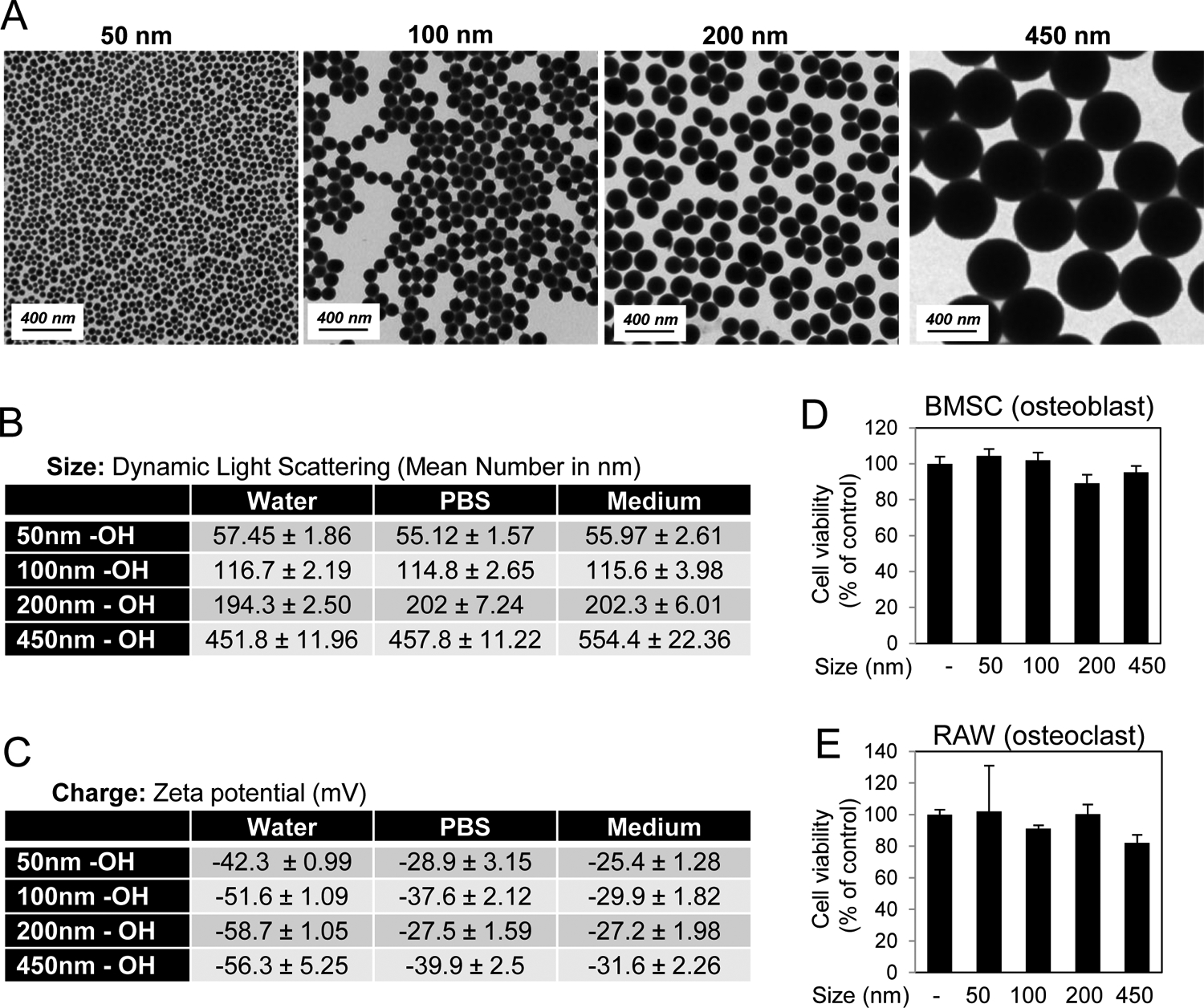
Characterization of varying sized silica nanoparticles. A) Shape was assessed by Transmission Electron Microscopy. B) Size of nanoparticles was characterized by Dynamic Light Scattering in water, phosphate buffered saline (PBS), and medium (Avg. of 3 readings). C) Zeta Potential of nanoparticles was measured in water, DPBS, and cell culture medium (Avg. of 3 readings). Cell viability of D) BMSCs in response to 72 hr treatment with 10 μg/ml nanoparticles and E) RAW264.7 cells in response to 72 hr treatment with 25 μg/ml nanoparticles as indicated (N=3–6). Avg.±Stdev.
3.2. Defining the optimal size of silica nanoparticles for enhancement of osteoblastogenesis
Bone forming osteoblasts, including bone marrow stromal cells (BMSCs) derive from the mesenchyme lineage and can be differentiated in culture to produce a hydroxyapatite embedded collagen matrix. Two key marker genes unique to the osteoblast differentiation process are the membrane bound enzyme alkaline phosphatase (ALP; gene name Alpl) and the secreted matrix protein osteocalcin (OCN: gene name Ocn or Bglap) [25, 26]. BMSCs were treated with the varying size silica nanoparticles and cultures stained for mineralization. Expression of the osteoblast marker genes ALP and OCN were also quantified. A concentration of 10 μg/ml was used as this was a dose (~EC90) previously determined to positively regulate osteoblast [23]. The results from multiple experiments and wells were averaged and revealed that only the 50 nm nanoparticles consistently and significantly enhanced differentiation and mineralization (Fig. 2A,B) and gene expression (Fig. 2C,D). The 100 nm particles had a generally positive effect although not to the extent of the 50 nm and the 200 nm had no substantial difference from control. Somewhat surprisingly, the 450 demonstrated decreased mineralization and osteoblast marker gene expression (Fig. 2), which was not altered with increased concentrations (Supplemental Fig.S1). To confirm that the difference was not due to internalization, 500 nm fluorescent silica nanoparticles were used and images demonstrated that they were effectively taken up by the BMSCs (Supplemental Fig.S2A,B). Taken together, the results identify 50 nm as the optimal size for spherical silica nanoparticles to enhance osteoblast differentiation and mineralization.
Fig. 2. Silica nanoparticle size and enhancement of osteoblast differentiation.
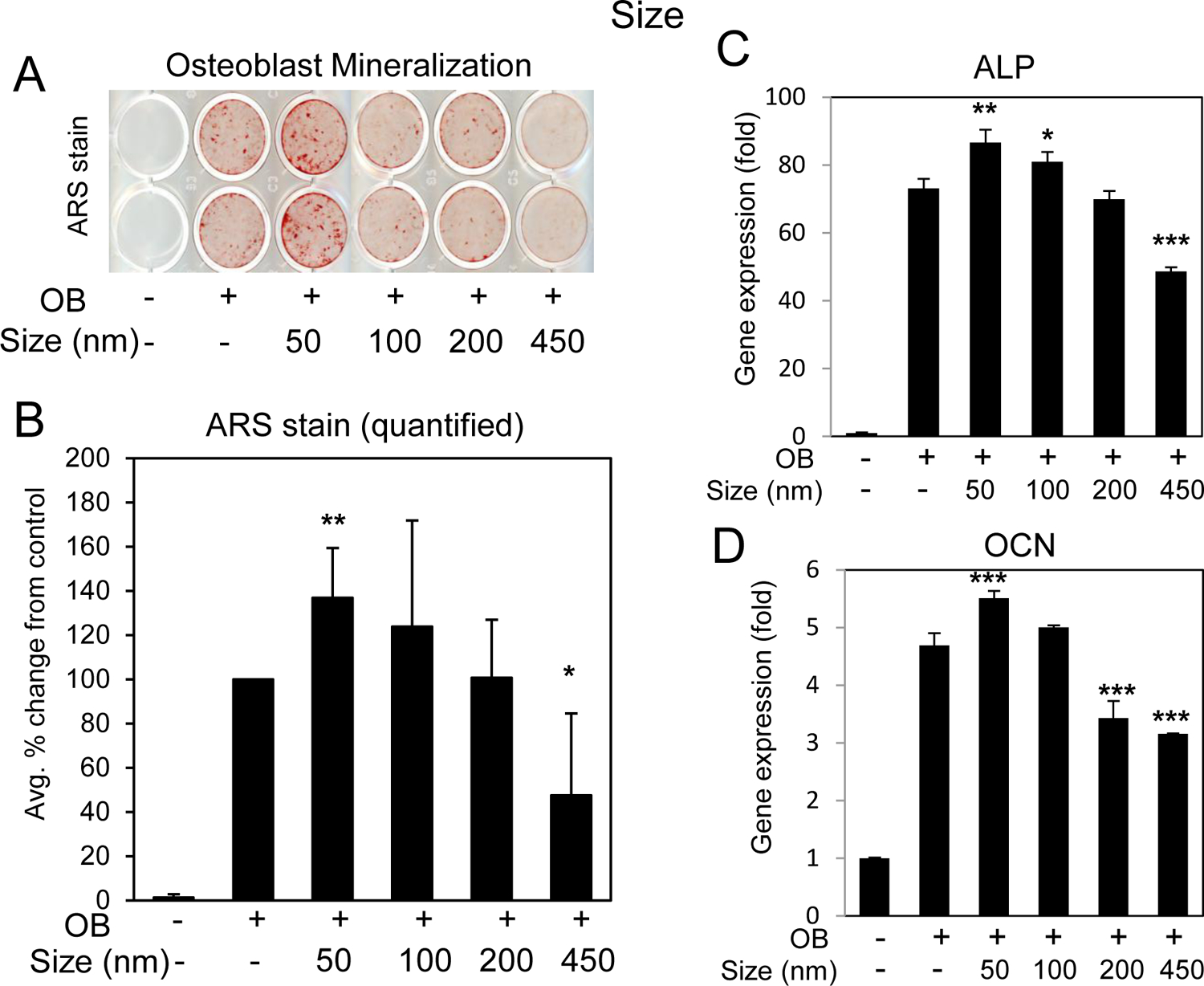
BMSCs were cultured in growth medium or medium supplemented with ascorbic acid and beta glycerophosphate (OB) and treated with 10 μg/ml silica nanoparticles of varying size as indicated. Cells were stained after 11–14 days for A) mineralization with Alizarin Red S staining and B) the average of six independent experiments quantified by image J. Data are expressed and percent change from the positive control (+OB) ±Stdev. Gene expression of C) alkaline phosphatase (ALP) and D) osteocalcin (OCN) are expressed as fold change (±Stdev) relative to untreated. Statistical analysis compares nanoparticle treated relative to positive control (+OB) by Student’s t test. *P< 0.05, **P< 0.01, **P< 0.005.
3.3. Significance of size for silica nanoparticle inhibition of osteoclast differentiation
Osteoclasts are the counterpart to osteoblasts in bone metabolism and function as the cells responsible for bone resorption. Osteoclasts derive from the monocyte/macrophage lineage and can be generated in vitro through the addition of the ligand (RANKL) which binds to the cell surface receptor RANK (Receptor Activator of Nuclear factor Kappa-B) [27] initiating cell signaling cascades to activate the NF-κB (nuclear factor kappa-B) pathway [28–30] as well as stimulate transcription of osteoclast specific genes such as Nfatc1 (Nuclear factor of activated T cells cytoplasmic 1) and RANK (encoded by the gene Tnfrsf11α) [31–34]. To determine if size had the same effects on pre-osteoclasts, RAW264.7 cells were differentiated by the addition of RANKL to the culture for 3–4 days in the presence or absence of 25 μg/ml (~IC90) [23] of silica nanoparticles of varied size. Staining and quantification of osteoclast formation, defined as TRAP+ and containing three or more nuclei, suggested that all sizes of the nanoparticles were capable of significantly inhibiting osteoclastogenesis (Fig. 3A,B). Based on the results of a pilot time course (Supplemental Fig. S3) the effect of size on differentiation marker genes was investigated 16 hours after the addition of RANKL. Analysis of osteoclastic gene expression identified a significant stimulation of Nfatc1 and RANK in response to RANKL relative to time 0 control (untreated) as expected. Treatment of the cells two hours prior to addition of RANKL with nanoparticles resulted in inhibition of gene expression relative to RANKL alone which strongly correlated with inhibition of osteoclast differentiation (Fig. 3C,D). Whereas the size of the particles had a differential effect on osteoblasts, size does not seem to play a role in the activity of spherical silica nanoparticles towards osteoclasts.
Fig. 3. Silica nanoparticle size and inhibition of osteoclast differentiation.
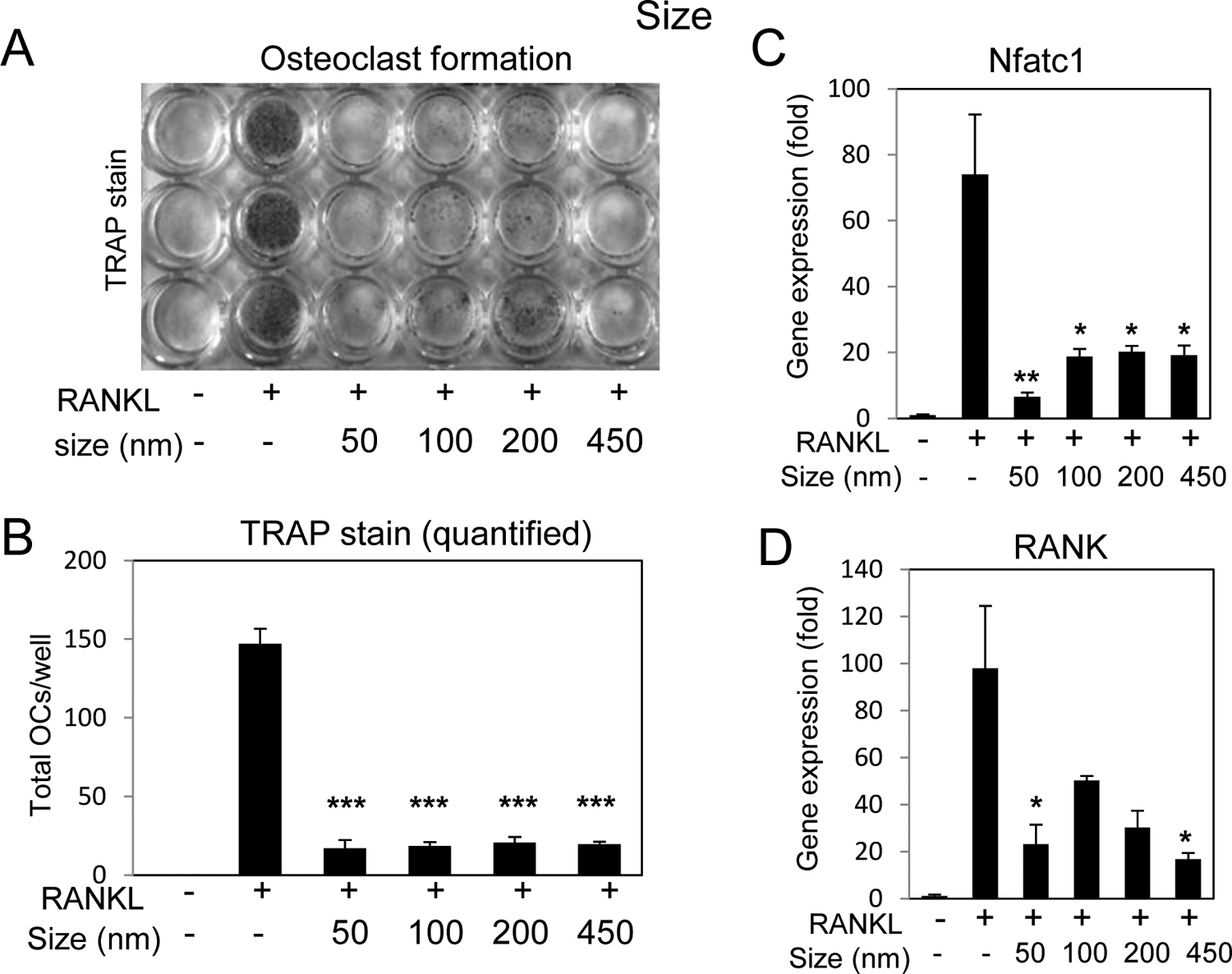
A) RAW264.7 cells were cultured in growth medium or medium supplemented with RANKL (15 ng/ml) and treated with silica nanoparticles of varying size as indicated and after 3–4 days the cultures were stained for tartrate resistant alkaline phosphatase (TRAP). B) Multinucleated (≥3 nuclei) TRAP positive cells were quantified were quantified from plates in (A); average of 3 wells ±Stdev and representative of 3 independent experiments. Cells were treated with RANKL (15 ng/ml) for 16 hours and harvested for RNA analysis of C) Nfatc1 or D) RANK by qRT-PCR expressed as fold change relative to untreated control Avg.±Stdev. *P< 0.05, **P< 0.01, and ***P< 0.005 relative to RANKL treated Students t test (n=3).
3.4. Modification and characterization of silica nanoparticles with varied surface properties
An advantage of silica based nanomaterials is the ability to relatively easily modify the surface. Altering the surface of nanomaterials has been demonstrated to effect the interaction with cell membranes as well as cell entry and toxicity [3]. The sol-gel method results in terminal OH groups (Si-OH, silanol group, essentially a hydroxyl group) on the surface that can be relatively easily coupled with silane-containing compounds by condensation under acidic or basic condition. To test the significance of the surface properties for silica nanoparticles influencing osteoblast and osteoclast differentiation, the surface of the 50 nm base particles were modified by simple hydrolysis-condensation reaction. In addition to the base particle (OH surface and negative charge) three other surfaces were chemically modified to create; NR4+ (PTMA) - positively charged, mNH2 (DETA) - positively charged with amine groups, and CO2H (Carboxylic acid) - negatively charged. The NR4+ has a quaternary ammonium salt, independent of pH, while the mNH2 has both primary and secondary amine groups and the CO2H has a carboxylic acid. The charge of the OH and NR4+ is not altered under the physiological pH change, while the CO2H and mNH2 can be altered from CO2− to CO2H and NH2 to NH3+ under acidic conditions. The surface modified particles originated from the same aliquot of base particles and the expected consistency of size and shape was confirmed by TEM (Fig. 4A). Results identified that the change in surface charge of the NR4+ and mNH2 modifications led to an increase in hydrodynamic size in culture medium to approximately 2000 nm as detected by DLS (Fig. 4B). The OH terminated and CO2H particles were negative in charge and again the NR4+ and mNH2 surface modifications resulted in a more neutral charge although still slightly negative (Fig. 4C). Analysis of cell viability suggested no substantial differences between the particles in the BMSC or RAW264.7 cells (Fig. 4D,E).
Fig. 4. Characterization of nanoparticles with varied surface properties.
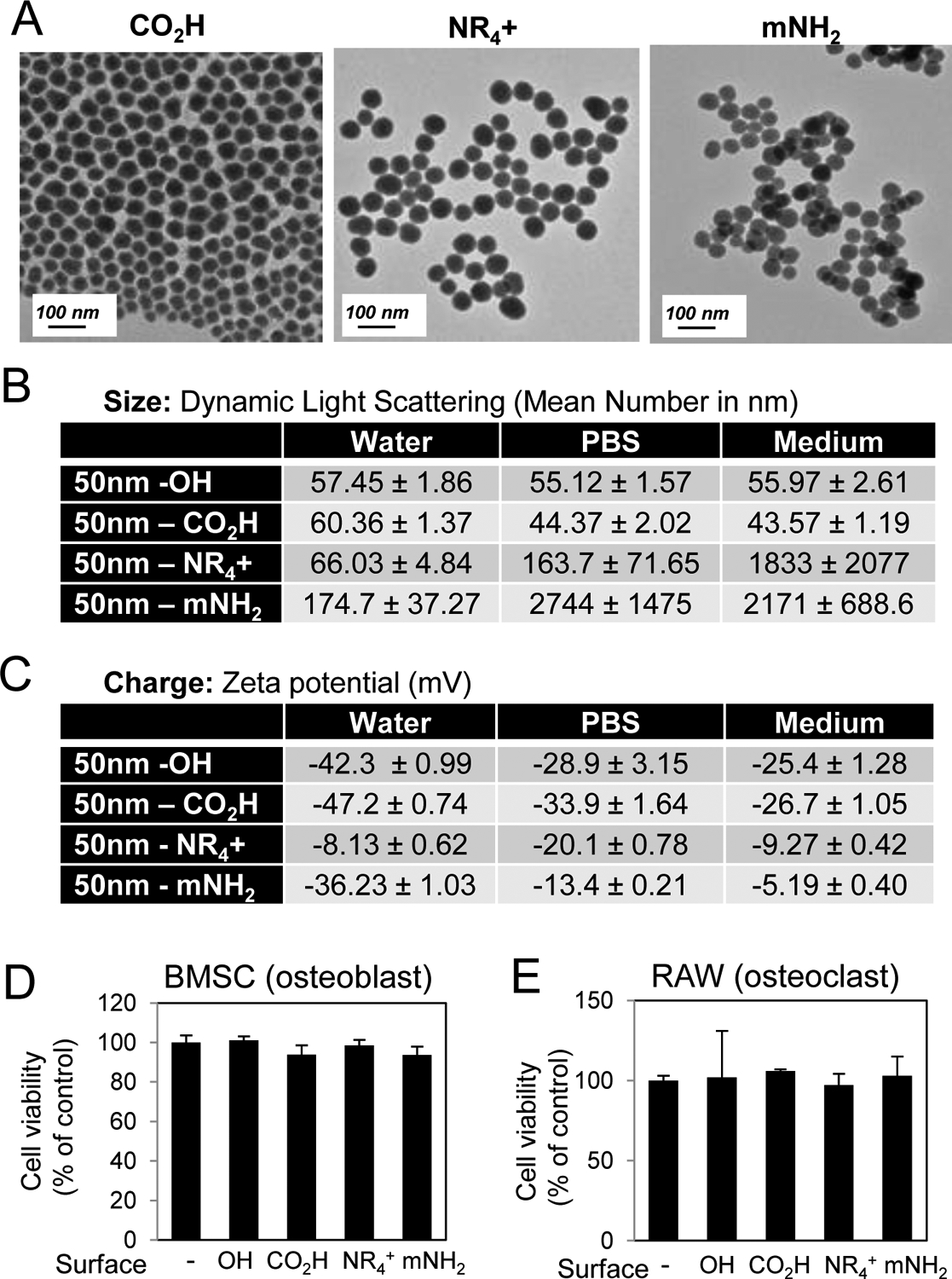
A) The shape of nanoparticles with varied surfaces was characterized by TEM and B) size was assessed by Dynamic Light Scattering in water, PBS, and medium (Avg. of 3 readings). C) Zeta Potential of nanoparticles was measured in water, DPBS, and cell culture medium (Avg. of 3 readings). Cell viability of D) BMSCs in response to 72 hr treatment with 10 μg/ml nanoparticles and E) RAW264.7 cells in response to 72 hr treatment with 25 μg/ml nanoparticles as indicated (N=3–6). Avg.±Stdev.
3.5. Analysis of surface property for the biological activity of silica nanoparticles in osteoblasts
BMSCs and pre-osteoblasts were differentiated in the presence of the four different surface modified 50 nm silica nanoparticles; OH, NR4+, mNH2, and CO2H. Analysis of mineralization suggested that all particles enhanced osteoblastogenesis with CO2H being the most potent (Fig. 5A,B). Gene expression results generally correlated with the mineralization suggesting that all nanoparticles enhanced differentiation beyond control and again, CO2H being the most potent (Fig. 5C,D). The NR4+ and mNH2 particles showed enhanced ALP and slightly repressed OCN which might be due to the transient nature of the expression of these marker genes however the functional output of mineralization suggested enhancement by all surface modified particles relative to control. Taken together, the results suggest that surface properties are not a strong determinant of the biological effects of silica nanoparticles towards osteoblast differentiation.
Fig. 5. Nanoparticle surface properties and enhancement of osteoblast differentiation.
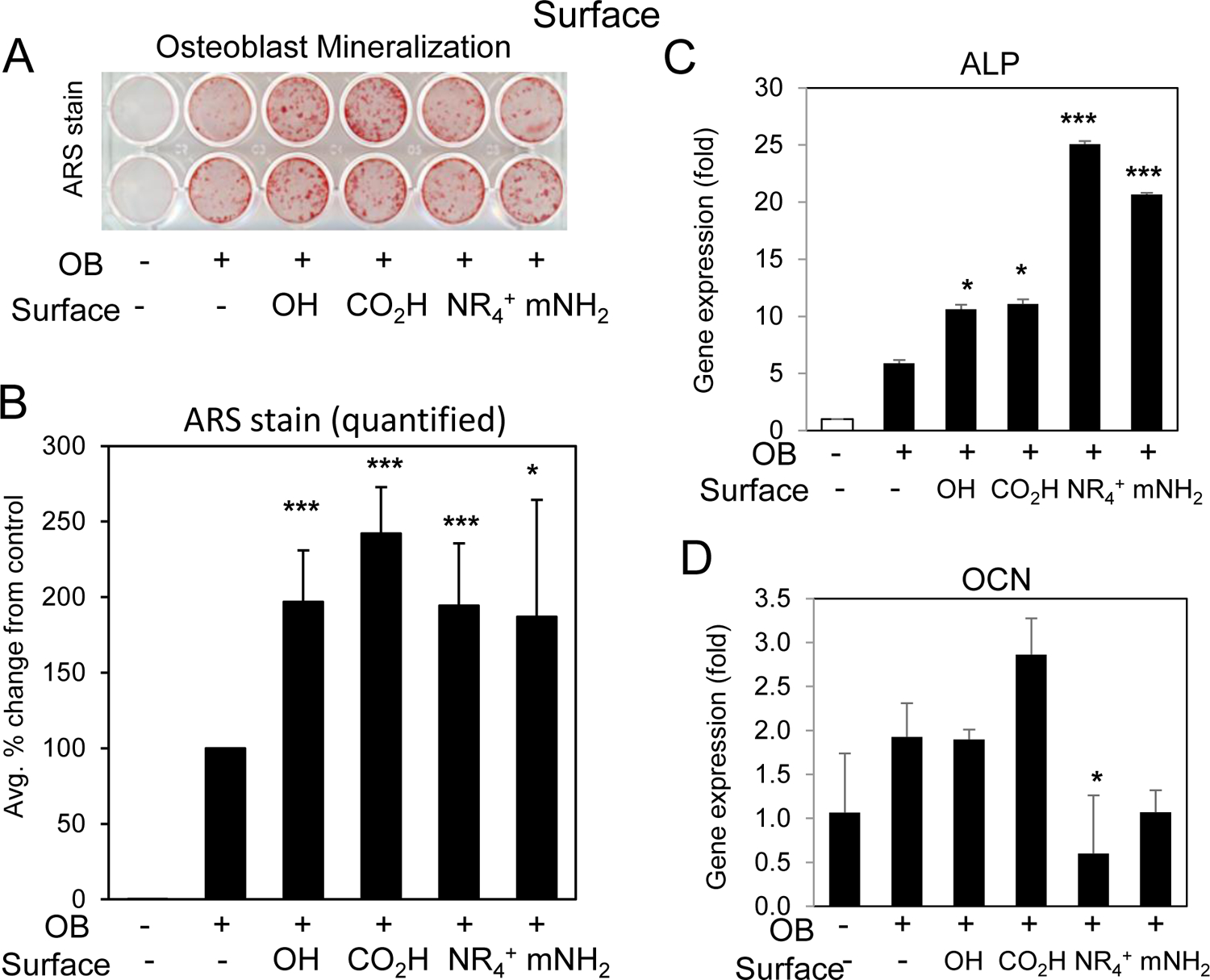
BMSCs or MC3T3-E1 cells were cultured in growth medium or medium supplemented with ascorbic acid and beta glycerophosphate (OB) and treated with 10 μg/ml silica nanoparticles of varying surface properties as indicated. Cells were stained after 11–14 days for A) mineralization with Alizarin Red S staining and B) the average of six independent experiments quantified by image J. Data are expressed and percent change from the positive control (+OB) ±Stdev. Gene expression of C) alkaline phosphatase (ALP) and D) osteocalcin (OCN) are expressed as fold change (±Stdev) relative to untreated. Statistical analysis compares nanoparticle treated relative to positive control (+OB) by Student’s t test. *P< 0.05, **P< 0.01, **P< 0.005.
3.6. Nanoparticle surface properties alter the effects on osteoclasts
To determine if surface properties altered the inhibition of osteoclastogenesis by 50 nm silica nanoparticles RAW264.7 were differentiated in the presence of nanoparticles with varying surface modification and stained for TRAP. As expected, the OH terminated nanoparticles completely inhibited osteoclast differentiation and the CO2H was also strongly inhibitory (Fig. 6A,B). However, the NR4+ and mNH2 nanoparticles had a more limited inhibitory effect on the differentiating osteoclasts. Gene expression confirmed the inhibitory effects of OH and CO2H particles on Nfatc1 and RANK expression and in agreement with the TRAP+ staining results, the NR4+ and mNH2 particles had much less impact (Fig. 6C,D). To confirm that the least inhibitory nanoparticles were internalized by the RAW264.7 cells a fluorescent variant of NR4+ [17] was used and the results demonstrated extensive uptake (Supplemental Fig.S2C,D) in agreement with a previous report [35]. Collectively, the results suggest the surface properties of 50 nm silica nanoparticles are critical for the biological effect on osteoclast differentiation with more negatively charged surfaces providing a greater inhibitor influence on osteoclastogenesis.
Fig. 6. Silica nanoparticle surface properties and enhancement of osteoclast differentiation.
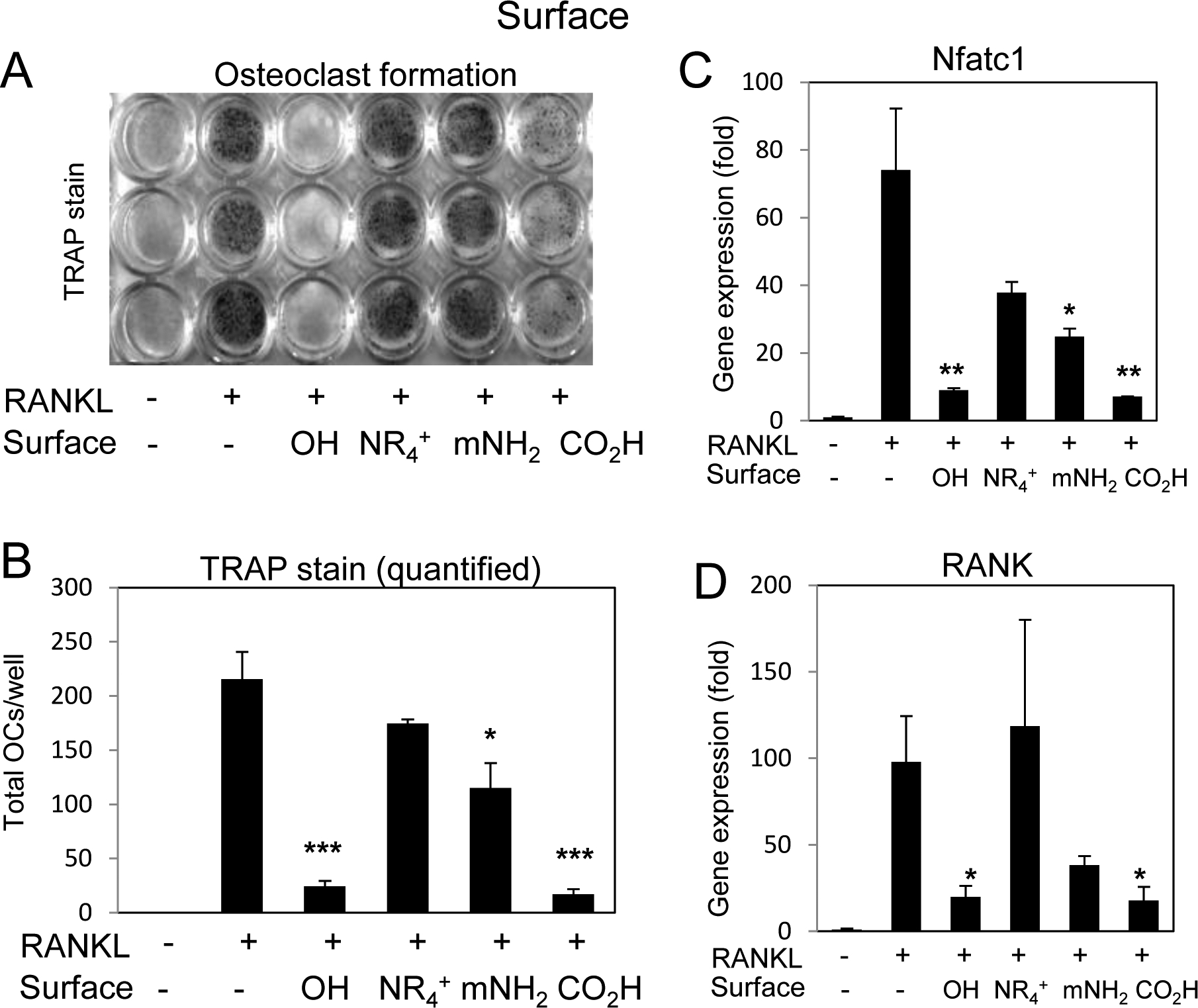
A) RAW264.7 cells were cultured in growth medium or medium supplemented with RANKL (15 ng/ml) and treated with silica nanoparticles with varying surfaces as indicated and after 3–4 days the cultures were stained for tartrate resistant alkaline phosphatase (TRAP). B) Multinucleated (≥3 nuclei) TRAP positive cells were quantified were quantified from plates in (A); average of 3 wells ±Stdev and representative of 3 independent experiments. Cells were treated with RANKL (15 ng/ml) for 16 hours and harvested for RNA analysis of C) Nfatc1 or D) RANK by qRT-PCR expressed as fold change relative to untreated control Avg.±Stdev. *P< 0.05, **P< 0.01, and ***P< 0.005 relative to RANKL treated Students t test (n=3).
3.7. Analysis of nanoparticle composition for bio-active effects
To determine the effect of composition on the ability of 50 nm nanoparticles to influence osteoblasts and osteoclasts we utilized 50 nm silica (SiO2), gold (GNP), and polystyrene (PS) nanoparticles. Silica is one of the most common elements on earth and is found in the food supply, whereas gold is a metal and polystyrene represents a synthetic compound. Furthermore, these compositions were primarily used due to their ease of manipulation to control for precise size and shape as confirmed by TEM (Fig. 7A). The nanoparticles were additionally characterized by DLS and zeta potential (Fig. 7B,C) and exhibited a negative charge similar to the SiO2 particles. Analysis of viability suggested that all three spherical particles (silica, gold, and polystyrene) did not negatively impact osteoblast viability however GNPs decreased RAW264.7 cell viability by approximately 50% (Fig. 7D,E). Because the larger silica particles did not impact RAW264.7 cell viability (Fig.1D,E) and the charge was within the range of other particles used in the study, the results suggest the possibility that the macrophage/pre-osteoclast cells can discriminate particles based on composition.
Fig. 7. Characterization of spherical nanoparticles of varying composition.
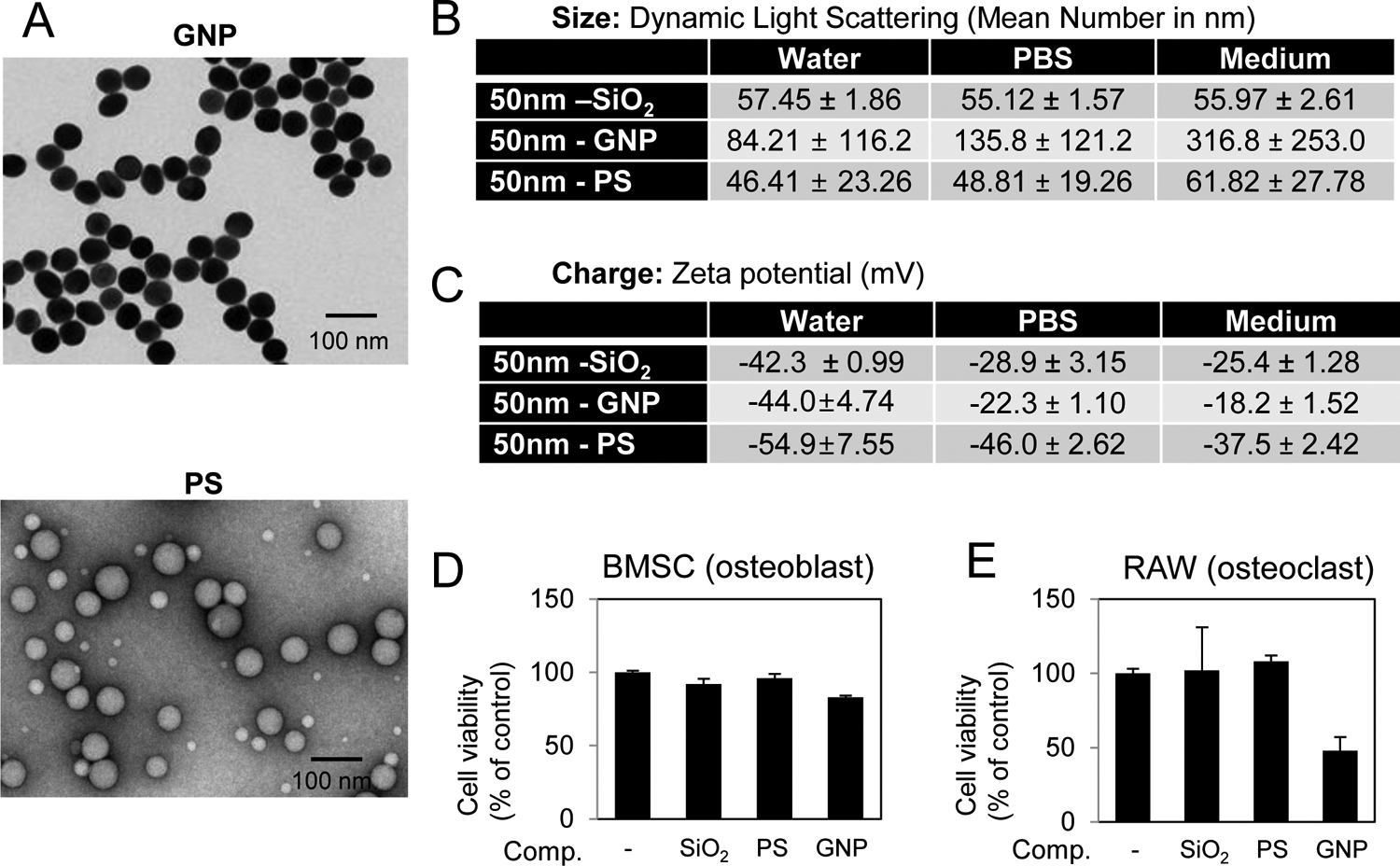
A) The shape of the gold (GNP) and polystyrene (PS) nanoparticles was characterized by TEM and B) size by Dynamic Light Scattering in water, PBS, and medium (Avg. of 3 readings). C) Zeta Potential of nanoparticles was measured in water, DPBS, and cell culture medium (Avg. of 3 readings). Cell viability of D) BMSCs in response to 72 hr treatment with 10 μg/ml nanoparticles and E) RAW264.7 cells in response to 72 hr treatment with 25 μg/ml nanoparticles as indicated (N=3–6). Avg.±Stdev.
3.8. Silica is optimal for the enhancement of osteoblastogenesis
To determine the effect of nanoparticle composition on the enhancement of osteoblast differentiation BMSCs were treated with the three spherical nanoparticles of various compositions for 7–14 days. Cells were stained for mineralization or harvested for RNA analysis. The results revealed that SiO2 particles are significantly better than either GNPs or PS particles for the enhancement of osteoblast differentiation and mineralization even though the shape and charge are very similar (Fig. 8A,B). Interestingly, although GNP did not enhance mineralization, osteoblast marker gene expression was increased (Fig. 8C,D) suggesting the possibility that the GNP might interfere with matrix maturation and/or calcium-phosphate deposition. Taken together, the results suggest that the chemical composition of nanoparticles plays a role in the biological effects on osteoblasts suggesting the possibility that osteoblasts have a mechanism to discriminate composition.
Fig. 8. Nanoparticle composition and enhancement of osteoblast differentiation.
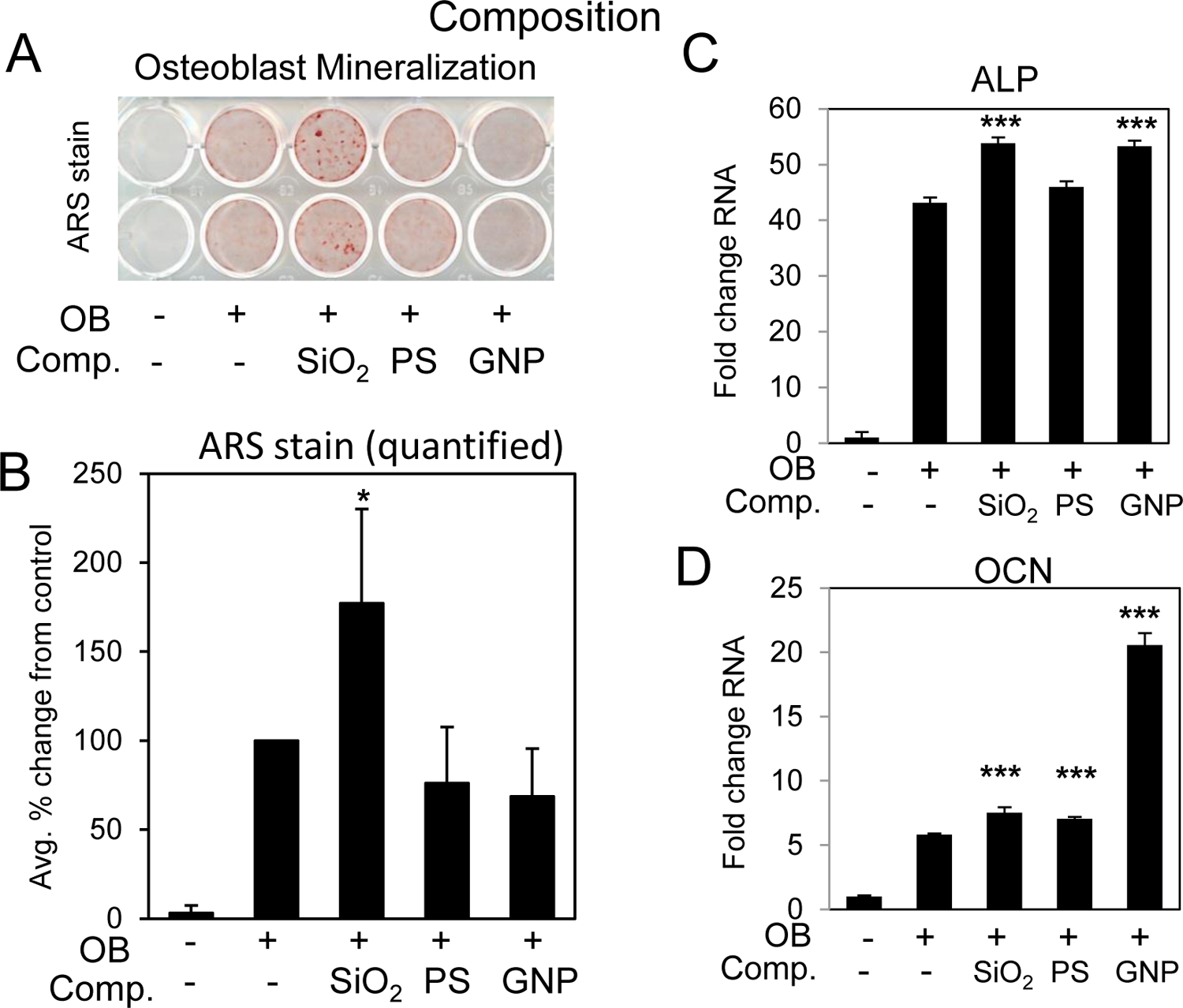
BMSCs were cultured in growth medium or medium supplemented with ascorbic acid and beta glycerophosphate (OB) and treated with 10 μg/ml of 50 nm nanoparticles composed of SiO2:silica nanoparticles, GNP:gold nanoparticles and PS:polystyrene. Cells were stained after 11–14 days for A) mineralization with Alizarin Red S staining and B) the average of four independent experiments quantified by image J. Data are expressed and percent change from the positive control (+OB) ±Stdev. Gene expression of C) alkaline phosphatase (ALP) and D) osteocalcin (OCN) are expressed as fold change (±Stdev) relative to untreated. Statistical analysis compares nanoparticle treated relative to positive control (+OB) by Student’s t test. *P< 0.05, **P< 0.01, **P< 0.005.
3.9. Nanoparticle composition is less critical for osteoclast inhibition
To assess the effects of composition on osteoclastogenesis RAW264.7 cells were differentiated to osteoclasts with RANKL in the presence of our panel of nanoparticles. Results suggest that all nanoparticles regardless of composition negatively impacted osteoclast differentiation (Fig. 9A,B). The decrease in osteoclast number was accompanied by a decrease in Nfatc1 and RANK gene expression (Fig. 9C,D). Because of the toxicity associated with the GNPs it is difficult at this time to determine the overall effects on the osteoclast differentiation process however the similarities between the PS and SiO2 in shape, size, and charge (negative) suggest that composition does not play a substantial role in the inhibition of differentiation.
Fig. 9. Nanoparticle composition and inhibition of osteoclast differentiation.
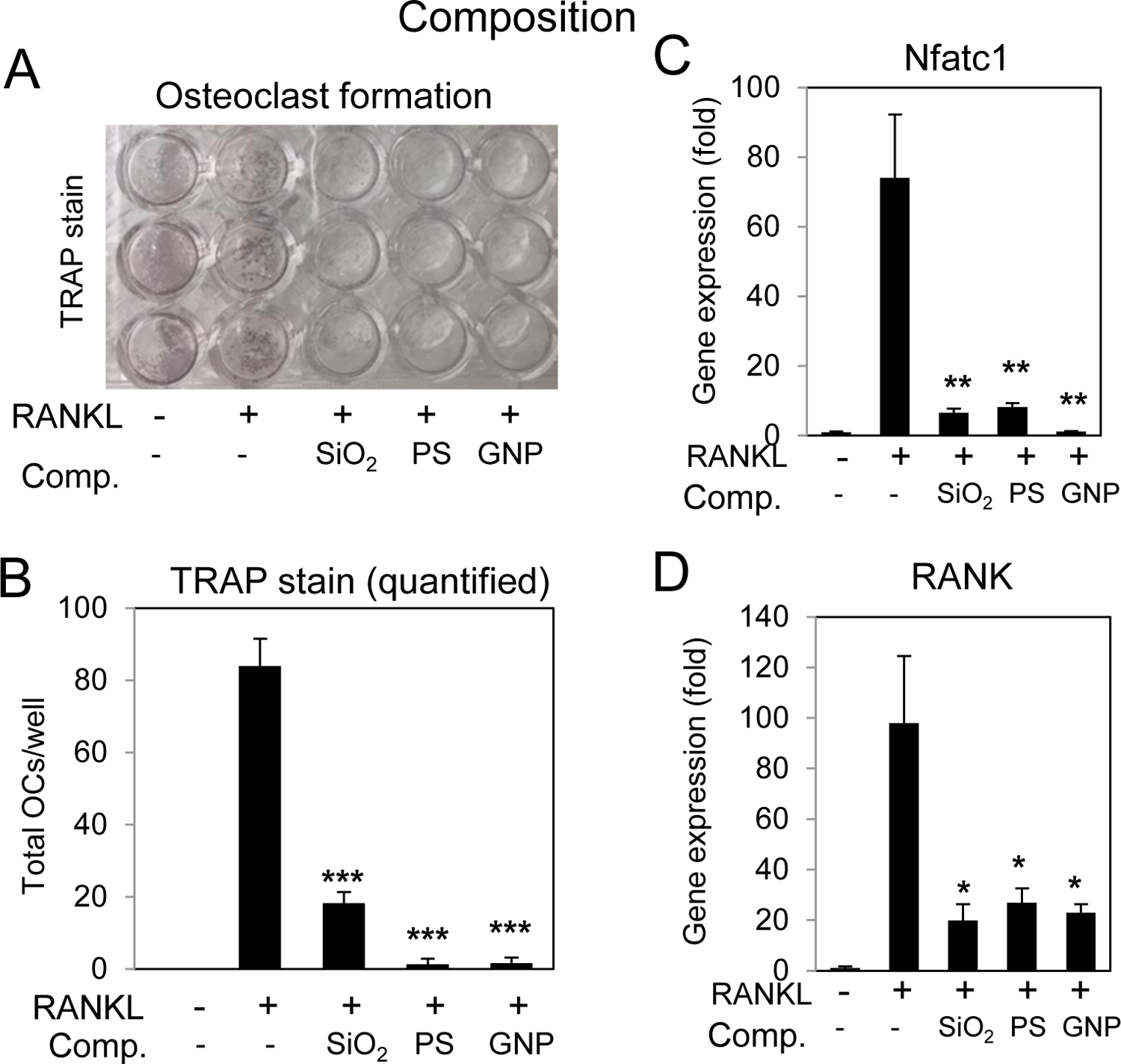
A) RAW264.7 cells were cultured in growth medium or medium supplemented RANKL (15 ng/ml) and treated with spherical 50nm nanoparticles of varying compositions; SiO2:silica nanoparticles, GNP:gold nanoparticles and PS:polystyrene as indicated and after 3–4 days the cultures were stained for tartrate resistant alkaline phosphatase (TRAP). B) Multinucleated (≥3 nuclei) TRAP positive cells were quantified were quantified from plates in (A); average of 3 wells ±Stdev and representative of 3 independent experiments. Cells were treated with RANKL (15 ng/ml) for 16 hours and harvested for RNA analysis of C) Nfatc1 or D) RANK by qRT-PCR expressed as fold change relative to untreated control Avg.±Stdev. *P< 0.05, **P< 0.01, and ***P< 0.005 relative to RANKL treated Students t test (n=3).
4. Discussion:
Nanotechnology has the ability to create materials with uniquely varied size, surface properties, and composition. To take full advantage of this technology the effects of these physical traits on cell function must first be understood beyond simple toxicity. Previous studies have identified a positive effect of silica nanoparticles on osteoblast differentiation and mineralization while inhibiting osteoclast differentiation in agreement with previous studies [13, 14, 23, 36, 37]. Studies related to bone metabolism have suggested a beneficial effect of dietary silica on skeletal development in rats and chicks [38–42], while clinical studies have reported strong positive associations between dietary silica intake and BMD in human (reviewed in [43–45]). Silica is also being investigated for use in combination with hydroxyapatite/bioceramic artificial bone scaffolds, where it is reported to enhance osteoconductivity [46–49]. Here we investigated the physical properties of silica nanoparticles that impart bioactivity towards bone regulating cells; osteoblasts and osteoclasts.
To characterize the effects of size, surface charge, and composition of our particles on the interaction with ions, minerals, and amino acids which the nanoparticles might be exposed in biological applications we measured size by DLS and charge by zeta potential in water, PBS, and medium. The different sized particle all contain OH surface groups and as expected all demonstrated a negative overall charge and the different mediums did not substantially alter size (Fig. 1B,C). However, results identified that the change in surface charge of the NR4+ and mNH2 modifications led to an increase in hydrodynamic diameter in culture medium to approximately 2000 nm as detected by DLS (Fig. 4B). DLS measurements also determined that an increase in size of GNPs size when suspended in PBS relative to water which increased further when suspended in medium (Fig. 7B). The increase in size is likely due to the interaction of GNPs with salts in PBS or salts and amino acids in medium whereas SiO2 and PS maintained a relative constant size in all solutions. Overall, the charge moved towards neutral in the more complex solutions (medium) but still maintained a relative negative charge for all particles (Fig. 7C). Although the hydrodynamic size changed for a number of the particles in response to modification, TEM confirmed the expected size of the solid phase.
Although osteoclast differentiation was inhibited by all silica nanoparticles regardless of size, the osteoblast response was substantially influenced by size. Nanoparticle size, of varied compositions, has been demonstrated to influence the mechanism and rate of internalization, cellular localization, and cytotoxicity [1] and how they are recognized and processed in vivo [5, 50, 51]. However, few studies have investigated the effects of size on bioactivity. Here, we determined that SiO2 nanoparticle size was important for the positive effects on osteoblast differentiation with smaller particles (50–100 nm) being superior to 450 nm particles for the stimulation of differentiation and mineralization. The effect of size on osteoblasts is in general agreement with two other studies. Xu et al., compared ~100, ~300, and ~600 nm silica particles in MC3T3-E1 pre-osteoblasts and found that although all particles enhanced osteoblast differentiation to a degree the 100 nm particles provided the greatest positive response [36]. Yang et al., using human mesenchymal stem cells treated with irradiated silica nanoparticles of ~50 ~200 and ~400 nm found that 200 nm provided the best increase in mineralization at 100 μg/ml while the benefits of 50 and 200 nm were similar at 10 μg/ml [37]. Unlike our study which found an inhibitory effect of 450 nm particles, this study did not find a negative impact of 400 nm particles and at certain concentrations found some positive benefit to osteoblast differentiation. There are a number of differences between the studies including post-synthesis treatment of the nanoparticles which included drying and irradiation as well as cell type used and differentiation protocol, single treatment verse multiple in our study which could explain the difference. Another interesting finding regarding size is that although the mNH2 particle was measured as larger by DLS (174 nm, likely due to hydrogen bonding) the particle still promoted mineralization in contrast with the true 200 nm particle assessed in Figure 2. The result suggests that osteoblasts are more responsive to the size of the solid phase of silica particle as opposed to hydrodynamic size due to surface interactions.
Although particle size did not appear to play a major role in the osteoclast response, a relatively negative charge (~ −25 to −30 mV) was found to enhance the inhibition of differentiation suggesting surface properties may play a more influential role in the biological response. The surface properties of nanoparticles are known to alter the interaction with cell membranes and internalization kinetics [52, 53] with positively charged particles generally demonstrating more rapid uptake [3, 54]. The surface modifications tested in the current study only minimally affected the osteoblast response based on the result that all surface modified nanoparticles enhanced mineralization, although CO2H was the most beneficial. However, surface property was arguably the most important factor influencing the effects of silica nanoparticles on osteoclast differentiation. The more positive particles NR4+ and mNH2 had a prominent change in size as determined by DLS (Fig. 4B) and exhibited much less potency for inhibition of differentiation, relative to the more negatively charged OH and CO2H. A previous study investigating the effects of nanoparticle charge on uptake by RAW264.7 cells demonstrated that the more positively charged particles are internalized with greater efficiency than negatively charged particles [54]. One potential explanation is that nanoparticles have been demonstrated to create a corona with proteins, amino acids, or inorganic salts in medium or blood [55, 56] and the corona has been demonstrated to differ based on the surface properties (positive, negative, and neutral) of silica nanoparticles in the 20 to 100 nm range [57–61] including RAW264.7 cells [57, 58]. Although numerous proteins have been found to bind differentially to silica nanoparticles with varied surface properties/charges the physiological relevance and biological consequences have yet to be determined [62].
Composition was determined to play an influential role in the biological response of osteoblasts and was associated with decreased viability in osteoclasts. The PS particles with carboxylic acid were similar to silica in shape, charge, and size but had little effect on osteoblast differentiation and no effect on mineralization whereas the 50 nm GNPs seemed to stimulate differentiation but did not increase mineralization. A number of previous studies have suggested either a positive effect or no effect of GNPs on osteoblasts [63–70]. There are a number of potential explanations. The majority of studies that find a positive effect on osteoblast differentiation or mineralization use smaller GNPs with a size of ~20 nm being most effective. Further, these particles are often surfaced modified with for example, chitosan or L-cysteine [68, 69]. In agreement with both the composition and surface property data presented herein, a recent study evaluated various surface modifications of 20nm GNPs on osteoblast differentiation and found either little to no effect or a negative effect in the case of CO2H [63]. When taken with the fact that silica nanoparticles regardless of surface functionalization enhanced osteoblastogenesis the results strongly suggest that composition, at least at the 50 nm size, is a critical factor whereas surface plays only a minor role in the biological response.
The mechanism(s) by which osteoblasts, and possibly osteoclasts, sense and respond to silica remains unknown however, recent studies provide evidence that this in fact does occur. The existence of cellular silica transporters in plants as well as bacteria and lower eukaryotes have been reasonably well defined (reviewed in [71, 72]). Recent studies suggest that silica transporters also exist in mammalian cells. Members of the aquaporin family of transporters have been revealed to transport silica into oocytes and human embryonic kidney cells [73] and therefore might represent a mechanism by which cells could sense silica resulting in phenotypic changes. Furthermore, a 12-mer amino acid sequences have been identified that specifically recognize silica surfaces [74] which suggests that specific intracellular or even extracellular proteins containing this sequence might directly recognize the silica nanoparticle surface and respond accordingly. The demonstrated importance of silica in bone biology points to the potential physiological importance of understanding this phenomenon although this area of investigation has only begun to be explored.
These studies also provide new insight into the mechanism by which silica nanoparticles inhibit osteoclastogenesis. Previous studies on the effects of silica nanoparticles and soluble silica on osteoclasts have identified the inhibition of osteoclastogenesis [14, 75] and this inhibition was associated with suppression of the NF-kB signaling pathway [14]. Analysis of osteoclastic gene expression identified a strong correlation between inhibition of Nfatc1 and RANK and inhibition of osteoclast formation. This correlation was consistent and across nanoparticle size, shape, and composition (Figs. 3, 6, 9). The results point to a functional mechanism by which nanoparticles inhibit osteoclast differentiation. This is in agreement with a recent study that found β-cyclodextrin (CD) surface-functionalized 20–40 nm GNPs inhibited osteoclastogenesis as well as Nfatc1 expression [76]. Additionally, previous studies revealed that 50 nm silica and 150 nm gold nanoparticles are capable of inhibiting NF-κB signaling in pre-osteoclasts [14, 77]. The NF-κB signaling pathway has also been demonstrated as a target of orthosilicic acid and silica nanoparticles in osteoblasts [14, 78] suggesting this may be a general signaling pathway by which cells respond to silica nanomaterials. RANKL is known to stimulate Nfatc1 downstream of the activation of the NF-κB pathway [29, 33] providing a potential cellular mechanism by which nanoparticles inhibit RANKL stimulation of Nfatc1.
5. Conclusions
Previous cell-based and preclinical animal studies have identified silica based nanomaterials as beneficial to bone through increased osteoblast function and decreased osteoclast formation [13–15, 23, 36, 37, 75, 78, 79]. In the current study we assessed the effects of size, surface charge, and composition on the biological effects of silica nanoparticles on the two bone modulating cell types. Interestingly, the hierarchies of the physical properties of nanomaterials that influence osteoblast and osteoclast differentiation differed. Enhancement of osteoblast differentiation was more sensitive to composition and size, with 50 nm silica nanoparticles being optimal. In contrast to the effects on osteoblasts, the surface charge of nanoparticles was the most influential factor for the bioactive effects on osteoclasts with negatively charged particles generating the greatest inhibition. Further, results herein reveal for the first time that silica nanoparticles inhibit the early critical osteoclastogenic transcriptional regulator Nfatc1 and its target gene RANK. In regards to the potential therapeutic use of nanoparticles towards bone metabolism it appears that silica is optimal for enhancement of osteoblast differentiation whereas seemingly any nanoparticle with a relatively negative charge is capable of inhibiting osteoclast differentiation. Clinically, the most commonly used therapeutic for the treatment of osteoporosis is bisphosphonates, a class of drugs that target osteoclasts [80]. However, the options for stimulating osteoblasts remain limited and therefore one goal in the treatment of bone disease is to identify factors capable of promoting bone formation while simultaneously reducing bone resorption. Results generated herein provide additional information as to the design of nanoparticles that could specifically target different aspects of bone metabolism or could even be tweaked to differentially target osteoblasts and osteoclasts in different ratios. The results also highlight the importance of testing the biological effects of a potential therapeutic nanomaterial on different cell types and the need to avoid over-generalizing about the biological effects of the physical properties of nanomaterials.
Supplementary Material
Acknowledgements
This study was supported by a Biomedical Laboratory Research & Development Service Award Number I01BX002363 from the VA Office of Research and Development to G.R Beck. The content of this manuscript is solely the responsibility of the authors and does not represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the United States Government. G.R Beck is also supported by a grant from Emory University Research Committee (00067461). S.W Ha is currently supported by a grant (D171751) from Gyeonggi Technology Development Program funded by Gyeonggi Province, Korea. The authors would like to thank Hong Yi of the Robert P. Apkarian Integrated Electron Microscopy Core at Emory University for expert technical assistance associated with TEM. The core is subsidized by the Emory College of Arts and Sciences and the Emory University School of Medicine and is one of the Emory Integrated Core Facilities. The JEOL JEM-1400 was supported by a National Institutes of Health Grant S10 RR025679.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnology. 12 (2014) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 7 (2012) 5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 6 (2010) 12–21. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Tan J, Thomas A, Ou-Yang D, Muzykantov VR. The shape of things to come: importance of design in nanotechnology for drug delivery. Ther Deliv. 3 (2012) 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Albanese A, Tang PS, Chan WC. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu Rev Biomed Eng. (2012). [DOI] [PubMed] [Google Scholar]
- [6].Ha SW, Weitzmann MN, Beck GR Jr. Dental and Skeletal Applications of Silica-Based Nanomaterials. In: Subramani K, Ahmed W, Hartsfield J, editors. Nanobiomaterials in Clinical Dentistry. Oxford, UK: William Andrew Publishing; 2013. p. 69–91. [Google Scholar]
- [7].Stober W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. Journal of colloid interface science. (1968) 62–69. [Google Scholar]
- [8].Ha SW, Camalier CE, Beck GR Jr., Lee JK. New method to prepare very stable and biocompatible fluorescent silica nanoparticles. Chem Commun (Camb). (2009) 2881–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, Brassinne F, Sebaihi N, Hoet PH. Toxicology of silica nanoparticles: an update. Arch Toxicol. 91 (2017) 2967–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vallet-Regi M, Balas F. Silica materials for medical applications. Open Biomed Eng J. 2 (2008) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, Cummings SR. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2 (2016) 16069. [DOI] [PubMed] [Google Scholar]
- [12].Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 3 Suppl 3 (2008) S131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ha SW, Weitzmann MN, Beck GR Jr., Bioactive silica nanoparticles promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano. 8 (2014) 5898–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Beck GR Jr., Ha SW, Camalier CE, Yamaguchi M, Li Y, Lee JK, Weitzmann MN. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomedicine. 8 (2012) 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Weitzmann MN, Ha SW, Vikulina T, Roser-Page S, Lee JK, Beck GR Jr., Bioactive silica nanoparticles reverse age-associated bone loss in mice. Nanomedicine. 11 (2015) 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stӧber W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci. (1968) 62–69. [Google Scholar]
- [17].Ha SW, Camalier CE, Beck GR Jr., Lee J-K. New method to prepare very stable and biocompatible fluorescent silica nanoparticles. Chem Commun (Camb). (2009) 2881–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 96 (1983) 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Camalier CE, Yi M, Yu LR, Hood BL, Conrads KA, Lee YJ, Lin Y, Garneys LM, Bouloux GF, Young MR, Veenstra TD, Stephens RM, Colburn NH, Conrads TP, Beck GR Jr., An integrated understanding of the physiological response to elevated extracellular phosphate. J Cell Physiol. 228 (2013) 1536–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ha SW, Jang HL, Nam KT, Beck GR Jr., Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials. 65 (2015) 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Beck GR Jr., Sullivan EC, Moran E, Zerler B. Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. J Cell Biochem. 68 (1998) 269–280. [DOI] [PubMed] [Google Scholar]
- [22].Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9 (2012) 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ha SW, Sikorski JA, Weitzmann MN, Beck GR Jr., Bio-active engineered 50nm silica nanoparticles with bone anabolic activity: Therapeutic index, effective concentration, and cytotoxicity profile in vitro. Toxicol In Vitro. 28 (2014) 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25 (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [25].Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2 (2001) 81–94. [DOI] [PubMed] [Google Scholar]
- [26].Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 4 (1990) 3111–3123. [DOI] [PubMed] [Google Scholar]
- [27].Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 12 (2006) 17–25. [DOI] [PubMed] [Google Scholar]
- [28].Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 40 (2007) 251–264. [DOI] [PubMed] [Google Scholar]
- [29].Abu-Amer Y. NF-kappaB signaling and bone resorption. Osteoporos Int. 24 (2013) 2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Boyce BF, Yao Z, Xing L. Functions of nuclear factor kappaB in bone. Ann N Y Acad Sci. 1192 (2010) 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sitara D, Aliprantis AO. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol Rev. 233 (2010) 286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim JH, Kim N. Regulation of NFATc1 in Osteoclast Differentiation. J Bone Metab. 21 (2014) 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Boyce BF, Yamashita T, Yao Z, Zhang Q, Li F, Xing L. Roles for NF-kappaB and c-Fos in osteoclasts. J Bone Miner Metab. 23 Suppl (2005) 11–15. [DOI] [PubMed] [Google Scholar]
- [34].Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 3 (2002) 889–901. [DOI] [PubMed] [Google Scholar]
- [35].Ha SW, Camalier CE, Weitzmann MN, Beck GR Jr., Lee JK. Long-term monitoring of the physicochemical properties of silica-based nanoparticles on the rate of endocytosis and exocytosis and consequences of cell division. Soft Materials. 11 (2013) 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xu XW, Zhang K, Zhao L, Wang DD, Bu WH, Zheng CY, Sun HC. Characteristics of three sizes of silica nanoparticles in the osteoblastic cell line, MC3T3-E1. Rsc Advances. 4 (2014) 46481–46487. [Google Scholar]
- [37].Yang X, Li Y, Liu X, Huang Q, He W, Zhang R, Feng Q, Benayahu D. The stimulatory effect of silica nanoparticles on osteogenic differentiation of human mesenchymal stem cells. Biomed Mater. 12 (2016) 015001. [DOI] [PubMed] [Google Scholar]
- [38].Schwarz K, Milne DB. Growth-promoting effects of silicon in rats. Nature. 239 (1972) 333–334. [DOI] [PubMed] [Google Scholar]
- [39].Seaborn CD, Nielsen FH. Silicon deprivation decreases collagen formation in wounds and bone, and ornithine transaminase enzyme activity in liver. Biol Trace Elem Res. 89 (2002) 251–261. [DOI] [PubMed] [Google Scholar]
- [40].Seaborn CD, Nielsen FH. Dietary silicon and arginine affect mineral element composition of rat femur and vertebra. Biol Trace Elem Res. 89 (2002) 239–250. [DOI] [PubMed] [Google Scholar]
- [41].Jugdaohsingh R, Calomme MR, Robinson K, Nielsen F, Anderson SH, D’Haese P, Geusens P, Loveridge N, Thompson RP, Powell JJ. Increased longitudinal growth in rats on a silicon-depleted diet. Bone. 43 (2008) 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carlisle EM. Silicon: a requirement in bone formation independent of vitamin D1. Calcif Tissue Int. 33 (1981) 27–34. [DOI] [PubMed] [Google Scholar]
- [43].Jugdaohsingh R. Silicon and bone health. J Nutr Health Aging. 11 (2007) 99–110. [PMC free article] [PubMed] [Google Scholar]
- [44].Nielsen FH. Update on the possible nutritional importance of silicon. J Trace Elem Med Biol. 28 (2014) 379–382. [DOI] [PubMed] [Google Scholar]
- [45].Carlisle EM. Silicon: a possible factor in bone calcification. Science. 167 (1970) 279–280. [DOI] [PubMed] [Google Scholar]
- [46].Gibson IR, Best SM, Bonfield W. Chemical characterization of silicon-substituted hydroxyapatite. J Biomed Mater Res. 44 (1999) 422–428. [DOI] [PubMed] [Google Scholar]
- [47].Keeting PE, Oursler MJ, Wiegand KE, Bonde SK, Spelsberg TC, Riggs BL. Zeolite A increases proliferation, differentiation, and transforming growth factor beta production in normal adult human osteoblast-like cells in vitro. J Bone Miner Res. 7 (1992) 1281–1289. [DOI] [PubMed] [Google Scholar]
- [48].Zou H, Wu S, Shen J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem Rev. 108 (2008) 3893–3957. [DOI] [PubMed] [Google Scholar]
- [49].Muller WE, Boreiko A, Wang X, Krasko A, Geurtsen W, Custodio MR, Winkler T, Lukic-Bilela L, Link T, Schroder HC. Morphogenetic activity of silica and bio-silica on the expression of genes controlling biomineralization using SaOS-2 cells. Calcif Tissue Int. 81 (2007) 382–393. [DOI] [PubMed] [Google Scholar]
- [50].Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 9 (2010) 615–627. [DOI] [PubMed] [Google Scholar]
- [51].Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 25 (2007) 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ha S-W, Camalier CE, Weitzmann MN, Beck GR, Lee J-K. Long-Term Monitoring of the Physicochemical Properties of Silica-Based Nanoparticles on the Rate of Endocytosis and Exocytosis and Consequences of Cell Division. Soft Materials. 11 (2013) 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shahabi S, Treccani L, Dringen R, Rezwan K. Modulation of Silica Nanoparticle Uptake into Human Osteoblast Cells by Variation of the Ratio of Amino and Sulfonate Surface Groups: Effects of Serum. ACS Appl Mater Interfaces. 7 (2015) 13821–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kurtz-Chalot A, Klein JP, Pourchez J, Boudard D, Bin V, Alcantara GB, Martini M, Cottier M, Forest V. Adsorption at cell surface and cellular uptake of silica nanoparticles with different surface chemical functionalizations: impact on cytotoxicity. Journal of Nanoparticle Research. 16 (2014). [Google Scholar]
- [55].Shannahan J. The biocorona: a challenge for the biomedical application of nanoparticles. Nanotechnol Rev. 6 (2017) 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hata K, Higashisaka K, Nagano K, Mukai Y, Kamada H, Tsunoda S, Yoshioka Y, Tsutsumi Y. Evaluation of silica nanoparticle binding to major human blood proteins. Nanoscale Res Lett. 9 (2014) 2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kurtz-Chalot A, Villiers C, Pourchez J, Boudard D, Martini M, Marche PN, Cottier M, Forest V. Impact of silica nanoparticle surface chemistry on protein corona formation and consequential interactions with biological cells. Mater Sci Eng C Mater Biol Appl. 75 (2017) 16–24. [DOI] [PubMed] [Google Scholar]
- [58].Mortensen NP, Hurst GB, Wang W, Foster CM, Nallathamby PD, Retterer ST. Dynamic development of the protein corona on silica nanoparticles: composition and role in toxicity. Nanoscale. 5 (2013) 6372–6380. [DOI] [PubMed] [Google Scholar]
- [59].Vilanova O, Mittag JJ, Kelly PM, Milani S, Dawson KA, Radler JO, Franzese G. Understanding the Kinetics of Protein-Nanoparticle Corona Formation. ACS Nano. 10 (2016) 10842–10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sikora A, Shard AG, Minelli C. Size and zeta-Potential Measurement of Silica Nanoparticles in Serum Using Tunable Resistive Pulse Sensing. Langmuir. 32 (2016) 2216–2224. [DOI] [PubMed] [Google Scholar]
- [61].Bharti B, Meissner J, Klapp SH, Findenegg GH. Bridging interactions of proteins with silica nanoparticles: the influence of pH, ionic strength and protein concentration. Soft Matter. 10 (2014) 718–728. [DOI] [PubMed] [Google Scholar]
- [62].Nguyen VH, Lee BJ. Protein corona: a new approach for nanomedicine design. Int J Nanomedicine. 12 (2017) 3137–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li JJ, Kawazoe N, Chen G. Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials. 54 (2015) 226–236. [DOI] [PubMed] [Google Scholar]
- [64].Yi C, Liu D, Fong CC, Zhang J, Yang M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 4 (2010) 6439–6448. [DOI] [PubMed] [Google Scholar]
- [65].Tsai SW, Liaw JW, Kao YC, Huang MY, Lee CY, Rau LR, Huang CY, Wei KC, Ye TC. Internalized gold nanoparticles do not affect the osteogenesis and apoptosis of MG63 osteoblast-like cells: a quantitative, in vitro study. PLoS One. 8 (2013) e76545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Suh KS, Lee YS, Seo SH, Kim YS, Choi EM. Gold nanoparticles attenuates antimycin A-induced mitochondrial dysfunction in MC3T3-E1 osteoblastic cells. Biol Trace Elem Res. 153 (2013) 428–436. [DOI] [PubMed] [Google Scholar]
- [67].Zhang D, Liu D, Zhang J, Fong C, Yang M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater Sci Eng C Mater Biol Appl. 42 (2014) 70–77. [DOI] [PubMed] [Google Scholar]
- [68].Choi SY, Song MS, Ryu PD, Lam AT, Joo SW, Lee SY. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/beta-catenin signaling pathway. Int J Nanomedicine. 10 (2015) 4383–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang Y, Kong N, Zhang Y, Yang W, Yan F. Size-dependent Effects of Gold Nanoparticles on Osteogenic Differentiation of Human Periodontal Ligament Progenitor Cells. Theranostics. 7 (2017) 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yao Y, Shi X, Chen F. The effect of gold nanoparticles on the proliferation and differentiation of murine osteoblast: a study of MC3T3-E1 cells in vitro. J Nanosci Nanotechnol. 14 (2014) 4851–4857. [DOI] [PubMed] [Google Scholar]
- [71].Ma JF, Yamaji N. A cooperative system of silicon transport in plants. Trends Plant Sci. 20 (2015) 435–442. [DOI] [PubMed] [Google Scholar]
- [72].Marron AO, Ratcliffe S, Wheeler GL, Goldstein RE, King N, Not F, de Vargas C, Richter DJ. The Evolution of Silicon Transport in Eukaryotes. Mol Biol Evol. 33 (2016) 3226–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Garneau AP, Carpentier GA, Marcoux AA, Frenette-Cotton R, Simard CF, Remus-Borel W, Caron L, Jacob-Wagner M, Noel M, Powell JJ, Belanger R, Cote F, Isenring P. Aquaporins Mediate Silicon Transport in Humans. PLoS One. 10 (2015) e0136149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Estephan E, Saab MB, Agarwal V, Cuisinier FJG, Larroque C, Gergely C. Peptides for the Biofunctionalization of Silicon for Use in Optical Sensing with Porous Silicon Microcavities. Advanced Functional Materials. 21 (2011) 2003–2011. [Google Scholar]
- [75].Mladenovic Z, Johansson A, Willman B, Shahabi K, Bjorn E, Ransjo M. Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater. 10 (2014) 406–418. [DOI] [PubMed] [Google Scholar]
- [76].Heo DN, Ko WK, Moon HJ, Kim HJ, Lee SJ, Lee JB, Bae MS, Yi JK, Hwang YS, Bang JB, Kim EC, Do SH, Kwon IK. Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes. ACS Nano. 8 (2014) 12049–12062. [DOI] [PubMed] [Google Scholar]
- [77].Sul OJ, Kim JC, Kyung TW, Kim HJ, Kim YY, Kim SH, Kim JS, Choi HS. Gold nanoparticles inhibited the receptor activator of nuclear factor-kappab ligand (RANKL)-induced osteoclast formation by acting as an antioxidant. Biosci Biotechnol Biochem. 74 (2010) 2209–2213. [DOI] [PubMed] [Google Scholar]
- [78].Zhou X, Moussa FM, Mankoci S, Ustriyana P, Zhang N, Abdelmagid S, Molenda J, Murphy WL, Safadi FF, Sahai N. Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-kappaB activation. Acta Biomater. 39 (2016) 192–202. [DOI] [PubMed] [Google Scholar]
- [79].Mihaila SM, Gaharwar AK, Reis RL, Khademhosseini A, Marques AP, Gomes ME. The osteogenic differentiation of SSEA-4 sub-population of human adipose derived stem cells using silicate nanoplatelets. Biomaterials. 35 (2014) 9087–9099. [DOI] [PubMed] [Google Scholar]
- [80].Teitelbaum SL. Bone resorption by osteoclasts. Science. 289 (2000) 1504–1508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


