Abstract
Medicinal plants are promising sources of natural substances with biological functions and several drugs have been developed from traditional medicine. This study aimed to determine the chemical components of a hydromethanolic extract from Foeniculum vulgare seeds. Total phenolic, flavonoid, and flavonol contents were assessed, and gas chromatography-mass spectrometry (GC-MS) analysis was performed. To investigate the anti-inflammatory activity of F. vulgare seed hydromethanolic extract, its effects on protein denaturation, protease activity, membrane stabilization, and heat-induced hemolysis in red blood cells were evaluated in vitro. F. vulgare seed extract showed significant inhibition of protein denaturation (35.68±0.4%), protease activity (58.09±0.1%), and heat-induced hemolysis in red blood cells (9.67±0.3%) at concentrations of 200, 250, and 200 μg/mL, respectively, compared to the reference drug indomethacin (P<0.001). This remarkable anti-inflammatory activity may be attributable to the abundance of flavonoids in the F. vulgare seed extract. GC-MS confirmed the presence of linalool and fatty acids (palmitic and oleic acids), which have potential anti-inflammatory activities. Therefore, the hydromethanolic extract of F. vulgare seeds may be a valuable anti-inflammatory candidate in the years ahead.
Keywords: flavonoids, flavonols, hemolysis, protease inhibitors, protein denaturation
INTRODUCTION
Inflammation is the bodily response to mechanical, chemical, or physical injury, as well as microbial invasion. Interactions between cellular and humoral factors in inflammation are complex and poorly understood (Wu et al., 2014). The primary purpose of inflammation is to rid the body of foreign matter or damaged cells and initiate wound healing. Paradoxically, inflammation is involved in the etiology of several diseases, including rheumatoid arthritis, arteriosclerosis, inflammatory bowel disease, myocarditis, infections, metabolic disorders, and cancer. Anti-inflammatory medications can affect the pathophysiology of inflammation by reducing tissue damage and enhancing a patients’ quality of life (Nunes et al., 2020). Steroidal and nonsteroidal medicines are the main groups of anti-inflammatory agents. However, anti-inflammatory drug use is linked to a number of complications, including adrenal atrophy (Phalitakul et al., 2011), osteoporosis, and a decrease in the body’s ability to respond to injury or illness (Craig and Stitzel, 2004). Conversely, owing to the inhibition of both physiological and inflammatory prostaglandins and concomitant leukotriene synthesis, nonsteroidal anti-inflammatory drugs may result in peptic ulcers and bronchospasm (Abdulkhaleq et al., 2018).
The increase in the incidence of multiple-drug resistance and the negative side effects of anti-inflammatory drugs have issued the necessity of the development of new anti-inflammatory drugs from alternative sources. Owing to the variety of their chemical structures and effects, molecules from medicinal plants exhibiting anti-inflammatory properties have the potential of satisfying this demand (Fabricant and Farnworth, 2001). Foeniculum vulgare, often known as fennel, has been used by conventional healers for various illnesses affecting the reproductive, endocrine, digestive, and respiratory systems. It is one of the oldest herbs native to the Mediterranean region and belongs to the Apiaceae family (Hornok, 1992). F. vulgare extracts and included compounds have been observed to be endowed with a number of properties, including anti-aging, anticolitic, antiallergic, antihirsutism, antimicrobial and antiviral, antinociceptive, antipyretic, antimutagenic, antithrombotic, antispasmodic, apoptotic, anxiolytic, cardiovascular, antitumor, diuretic, estrogenic, expectorant, galactogenic, hypoglycemic, hypolipidemic, and hepatoprotective effects. Furthermore, it has gastrointestinal, memory-enhancing, and oculohypotensive activities (Pradhan et al., 2008; Rasul et al., 2012; Koppula and Kumar, 2013; Rahimi and Ardekani, 2013; Tripathi et al., 2013). F. vulgare essential oil has valuable antioxidant, anticancer, antifungal, and antibacterial activities (Moura et al., 2005; El-Awadi and Hassan, 2010; Altameme et al., 2015). The phenolic naturally occurring substances in F. vulgare are believed to be linked to the management of oxidative stress-induced diseases, including cancer, inflammation, and heart conditions (Badgujar et al., 2014). This study aimed to investigate the anti-inflammatory effect of a hydromethanolic extract of F. vulgare seeds in vitro by evaluating its capacity to inhibit the protein denaturation, stabilize the red blood cell (RBC) membrane in heat-induced hemolysis case, and inhibit the protease activity. Phytochemical screening was performed to identify the main bioactive chemical groups via gas chromatography-mass spectrometry (GC-MS) analysis and quantify the different phenolic compounds enclosed in the extract by colorimetric reactions and spectrophotometric assays.
MATERIALS AND METHODS
Plant material
F. vulgare seeds were purchased at a small grocery store in Jijel, Algeria. They were dried in the dark at ambient temperature before being ground to make a dry powder.
F. vulgare extract preparation
Phenolic compound extraction from the seed powder of the plant was performed using a solid-liquid method according to the procedure of Owen and Johns (1999), with slight modifications. The plant powder was macerated at room temperature for 72 h in 80/20 (v/v) water-methanol at a solid/liquid ratio of 1/10 (w/v) under constant stirring using a magnetic stirrer. The hydromethanolic macerate was filtered using a Whatman paper; subsequently, the filtrate was concentrated at 40°C in a rotary evaporator (Laborota 4003, Heidolph Instruments). After evaporation, the extract was introduced into a Memmert-type oven (Model, Memmert) at 40°C until its weight was stabilized to provide a crude extract. The following formula was used to determine the extraction yield:
where W is the weight of the extract following evaporation of the solvent (g) and W0 is the weight of the test portion (g).
The dried extract was stored in a dry sterile flask in the dark at 4°C for pharmacological tests.
Total phenolic compound assessment
The quantity of phenolics in the extract was measured using the Folin-Ciocalteu reagent according to the method of Othman et al. (2007), with minor modifications. A 0.2-mL aliquot of the extract or standard solution was mixed with 1.5 mL of Folin-Ciocalteu reagent (1/10). After 5 min in the dark at room temperature, 1.5 mL of a 7.5% sodium carbonate (Na2CO3) solution was added to the mixture, which was subsequently incubated in the dark at room temperature for 90 min. Absorbance against a blank was read at 750 nm. The average total phenolic compound content is expressed as mg equivalent of gallic acid (EGA) per gram of crude extract (mg EGA/g). The trial was performed in triplicate and the average value was determined.
Total flavonoid assessment
The flavonoid content was spectrophotometrically estimated according to the method of Djeridane et al. (2006), with slight modifications. Here 1.5 mL of the extract or standard solution was added to 1.5 mL of 2% aluminum chloride (AlCl3). The absorbance against a blank was read at 430 nm after 30 min using the SPECORDⓇ 50 PLUS Spectrophotometer (Analytik Jena). The results were presented in mg equivalent of quercetin (EQ) per gram of crude extract (mg EQ/g). The trial was performed in triplicate and the average value was calculated.
Flavonol assessment
The flavonol content was determined using the colorimetric method described by Shehata et al. (2009), with slight modifications. A 1-mL aliquot of the extract was mixed with 1 mL of 2% AlCl3; subsequently, 3 mL of a 5% sodium acetate solution was added. The mixture was incubated for 30 min in the dark at room temperature. The absorbance was read at 440 nm. The results were presented in mg EQ/g of crude extract.
GC-MS analysis
The phytochemical investigation of the extract was performed on a Shimadzu QP 2010 GC-MS equipment (Shimadzu), with (70 eV) electron impact ionization. An SE 30 capillary column (0.25×25 mm, CS-Chromatographie Service GmbH) coated with 5% diphenyl and 95% dimethylpolysiloxane was used. An oven was set to increase the temperature from 50°C to 250°C at a rate of 50°C/min. The transfer line temperature was 250°C. Helium was used as the carrier gas, flowing at a rate of 1.5 mL/min with a 20:0 split ratio. The mass range and scan time were 40∼350 m/Z and 0.50 s, respectively. The results were analyzed using the National Institute of Standards and Technology mass spectral library of the GC-MS data system. The determination of the percentage of each component was based on peak area.
Evaluation of the anti-inflammatory activity of F. vulgare extract
Protein denaturation assay: The test of inhibition of protein denaturation was performed following the method of Kar et al. (2012). The following three solutions were prepared: the negative control solution (0.5 mL), which contains 0.45 mL 5% bovine serum albumin (BSA) and 0.05 mL distilled water; the standard solution (0.5 mL), which contains 0.45 mL 5% BSA and 0.05 mL indomethacin at different concentrations (50∼250 μg/mL); and the test solution (0.5 mL), which contains 0.45 mL 5% BSA and 0.05 mL of the extract at different concentrations (50∼250 μg/mL). The pH of all the aforementioned solutions was brought to 6.3 with 1 N hydrochloric acid (HCl). The samples were kept for 20 min at 37°C; subsequently, the temperature was increased to maintain the sample at 57°C for 3 min. Next, 2.5 mL of phosphate buffer solution (pH 6.3) was added after cooling. The absorbance was recorded using an ultraviolet-visible spectrophotometer at 416 nm. The protein denaturation inhibition percentage was determined using the following equation:
The control represents 100% of the protein denaturation. The results were compared with those of the standard solution of indomethacin.
Cell membrane stabilization assay: The principle of this assay is to evaluate the capacity of the extract to preserve the RBC membrane from heat-induced hemolysis. This test was performed following the method described by Kar et al. (2012), with minor modifications. First, a suspension of RBCs was prepared by combining equal portions of blood and a sterilized Alsever’s solution. The suspension was centrifuged at 1,107 g to recuperate the pellet (containing packed cells) which was washed with iso-saline water and subsequently added to normal saline to prepare a 10% (v/v) suspension. This suspension was conserved at 4°C before use.
Different concentrations of F. vulgare extract and indomethacin were prepared (50∼1,000 μg/mL) in normal saline. They were separately mixed with 1 mL phosphate buffer, 2 mL hypo-saline, and 0.5 mL of the 10% RBC suspension. All test mixtures were heated at 56°C for 30 min and centrifuged at 769 g for 10 min. Physiological saline was used as a negative control and the absorbance was read at 560 nm. The percentage of inhibition of hemolysis of RBCs was calculated using the following formula:
Protease inhibition assay: The Sakat et al. (2010) technique was used to perform this test. Here, 0.06 mg of trypsin, 1 mL of 20 mM Tris-HCl buffer (pH 7.4), and 1 mL of the extract at various concentrations (50∼250 g/mL) were incorporated into the reaction mixture (2 mL). Distilled water and indomethacin were used as the negative control and the standard, respectively. After 5 min of incubation at 37°C, 1 mL of the buffer solution containing 0.8% (w/v) casein was added to the reaction mixture. After another 20 min of inhibition, 2 mL acetic acid was added to the mixture to terminate the reaction. After centrifuging the suspension, the absorbance of the supernatant was measured at 210 nm against the buffer solution blank. The experiment was performed three times and the following formula was used to determine the protease inhibitory activity:
Statistical analysis
Each analysis was performed in triplicate and all the results were expressed as mean±standard deviation. Statistical analysis were performed using the analysis of variance test coupled with Sidak’s multiple comparisons tests using GraphPad Prism version 7.00 for windows (GraphPad). The results were compared with those of a reference drug (indomethacin), and the differences between the means were considered not significant when P>0.05, significant when 0.05>P>0.01, highly significant when 0.01>P>0.001, and very highly significant when P<0.001.
RESULTS
Extraction yield
The F. vulgare extraction yield was 13.33%.
Phenolic content of F. vulgare extract
The results obtained were expressed in mg EGA/g of raw extract, using the linear regression equation of the calibration curve of gallic acid (y=0.0069x+0.0272, R2=0.9987). The total phenolic constituents of the extract were observed to constitute 38.2±2.3 mg EGA/g of raw extract. The total flavonoid content was determined using the equation of the linear regression of the quercetin calibration curve (y=0.0022x+0.0094, R2=0.9956). The content was estimated at 122.1±1.4 mg EQ/g of raw extract. The flavonol content was determined using the equation of the linear regression of the quercetin calibration curve (y=0.0004x+0.0077, R2=0.9844). The content was estimated at 205.2±2.3 mg EQ/g of raw extract. These significant total flavonoid and flavonol levels indicate that F. vulgare is rich in flavonoids and flavonols. The total phenolic compound, flavonoid, and flavonol contents are demonstrated in Table 1.
Table 1.
Total phenol, flavonoid, and flavonol contents of the hydromethanolic extract of Foeniculum vulgare seeds
| Total phenols (mg EGA/g) | Flavonoids (mg EQ/g) | Flavonols (mg EQ/g) |
|---|---|---|
| 38.2±2.3 | 122.1±1.4 | 205.2±2.3 |
EGA, equivalent of gallic acid; EQ, equivalent of quercetin.
GC-MS results
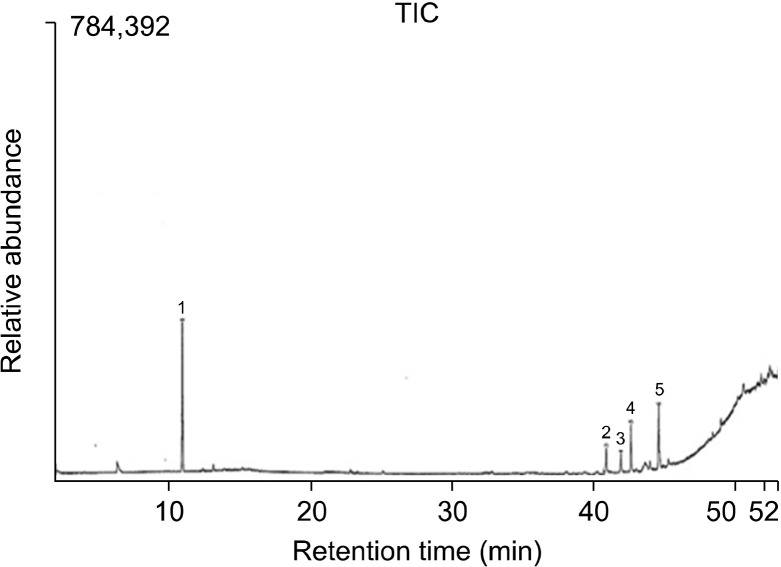
The qualitative and quantitative profiles of F. vulgare extract are presented in Fig. 1. Compounds are listed according to their mass/charge ratio (m/z) in Table 2. Results showed that the F. vulgare sample contained linalool and oleic acid as major constituents (34.47% and 14.28%, respectively).
Fig. 1.
Gas chromatography-mass spectrometry profile of Foeniculum vulgare. 1, linalool (major component); 2, palmitic acid; 3, diisobutyl phthalate; 4, eriostoic acid; and 5, oleic acid (major component). TIC, total ion chromatogram.
Table 2.
Qualitative and quantitative compositions of the hydromethanolic extract of Foeniculum vulgare seeds according to gas chromatography-mass spectrometry analysis
| Peak | IUPAC name | Chemical name | Height (%) | M/Z |
|---|---|---|---|---|
| 1 | 1,6-Octadien-3-ol, 3,7-dimethyl | Linalool | 34.47 | 93.10 |
| 2 | Hexadecanoic acid, methyl ester | Palmitic acid | 5.48 | 74.00 |
| 3 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl)ester | Diisobutyl phthalate | 3.68 | 149.05 |
| 4 | 2H,8H-Benzol[1,2-b:5,4-b’]dipyran-10-propanoic acid 5-methoxy-2,2,8,8-tetramethyl,methyl ester | Eriostoic acid | 10.58 | 343.05 |
| 5 | 9-Octadecenoic acid(Z)-, methyl ester | Oleic acid | 14.28 | 55.00 |
IUPAC, International Union of Pure and Applied Chemistry; M/Z, mass/charge ratio.
Anti-inflammatory activity of F. vulgare
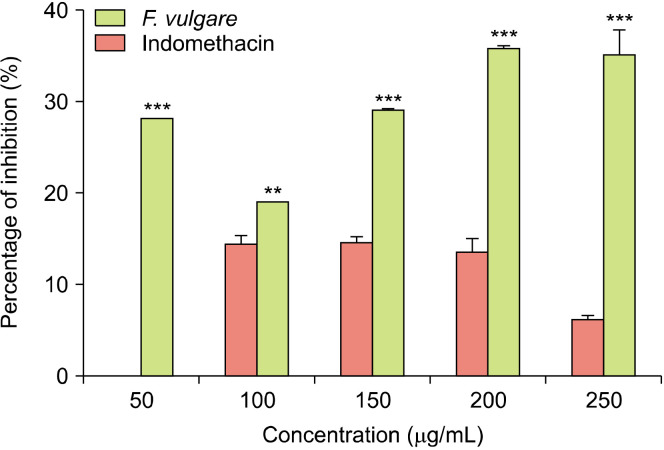
The effect of F. vulgare extract on protein denaturation: According to the results presented in Fig. 2, the hydromethanolic extract of F. vulgare seeds provoked an increase in protein denaturation inhibition, which was highly significant at 100 μg/mL concentration (P<0.01) and very highly significant at 50, 150, 200, and 250 μg/mL concentrations (P<0.001). F. vulgare exhibited the maximum percentage of protein denaturation inhibition of 35.68±0.40% at 200 μg/mL. Conversely, indomethacin exhibited a maximal inhibition of 14.55±0.70% at 150 μg/mL concentration at which F. vulgare extract reached a percentage of inhibition of 29.08±0.10%. These results show that the hydromethanolic extract of F. vulgare has anti-inflammatory activity by inhibiting protein denaturation and this activity is more significant than that shown by indomethacin.
Fig. 2.
The percentage of inhibition of protein denaturation by the hydromethanolic extract of Foeniculum vulgare and indomethacin at different concentrations. Results are expressed as mean±standard deviation (n=3 replicates). Comparison was performed between F. vulgare extract and indomethacin at each concentration using analysis of variance (ANOVA) followed by Sidak’s multiple comparisons test at **P<0.01 and ***P<0.001.
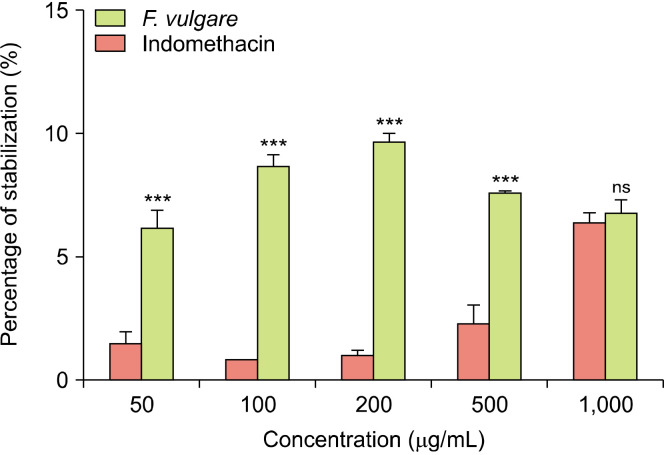
The effect of F. vulgare extract on RBC membrane stabilization: The results demonstrated in Fig. 3 show a reduction in RBC hemolysis when treated with the hydromethanolic extract of F. vulgare at concentrations ranging from 50 to 1,000 μg/mL. This reduction was more significant than that observed with indomethacin, and the difference in the activity was very highly significant (P<0.001) at all concentrations except for the 1,000 μg/mL concentration at which the difference in the effect was not significant (P=0.8910).
Fig. 3.
The percentage of stabilization of heat-induced hemolysis by the hydromethanolic extract of Foeniculum vulgare and indomethacin at different concentrations. Results are expressed as mean±standard deviation (n=3 replicates). Comparison was performed between F. vulgare extract and indomethacin at each concentration using analysis of variance (ANOVA) followed by Sidak’s multiple comparisons test at ***P<0.001. ns, not significant.
The maximum inhibition was 9.67±0.30% with the extract of F. vulgare at the 200 μg/mL concentration, whereas indomethacin had a maximum inhibition of 6.30±0.30% at the 1,000 μg/mL concentration. These findings suggest that the hydromethanolic extract of F. vulgare seeds may have a significant competence to stabilize the cell membrane.
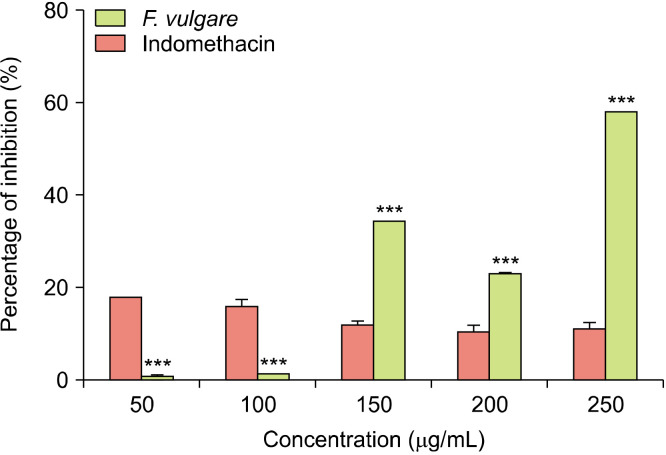
The effect of F. vulgare extract on protease activity: The percentage of inhibition of trypsin (a protease) by the hydromethanolic extract of F. vulgare and indomethacin is illustrated in Fig. 4. The results indicate that at 150, 200, and 250 μg/mL concentrations, F. vulgare extract had a very significantly higher protease inhibitory effect than indomethacin (P<0.001), with values of 34.38±0.00, 23.00±0.37, and 58.09±0.10%, respectively, for F. vulgare extract, and 11.82±0.95, 10.42±1.63, and 10.97±1.63%, respectively, for indomethacin. Conversely, at 50 and 100 μg/mL concentrations, indomethacin had a significantly higher protease inhibitory effect of 17.81±0.10% and 15.80±1.54%, respectively, whereas that of F. vulgare extract was 0.93±0.20% and 1.24±0.14%, respectively. From these results, it can be presumed that F. vulgare has anti-inflammatory activity by inhibiting the action of proteases, which is more significant than that shown by indomethacin at concentrations higher than 100 μg/mL.
Fig. 4.
The percentage of inhibition of trypsin by the hydromethanolic extract of Foeniculum vulgare and indomethacin at different concentrations. Comparison was performed between F. vulgare extract and indomethacin at each concentration using analysis of variance (ANOVA) followed by Sidak’s multiple comparisons test at ***P<0.001.
DISCUSSION
Recently, the search for phytochemicals with anti-inflammatory effects has been increasing owing to their potential use in the prevention and management of numerous chronic and infectious diseases. In the present study, we have noted strong anti-inflammatory activities for the methanolic extract of F. vulgare. Chemically, Foeniculum species are recognized by the presence of essential oils (Özbek et al., 2003), sterols (Ivanov, 1979), coumarins (Kwon et al., 2002), and flavonoids (Parejo et al., 2004). Certain Foeniculum species have been linked to specific bioactivities, including antioxidant and antibacterial properties for F. vulgare aerial parts (Ruberto et al., 2000) and analgesic and anti-inflammatory properties for the plant’s fruits (Choi and Hwang, 2004). Methanol was used as an extraction solvent since it is already evident that methanol is better for phenol and flavonoid extraction owing to its high polar propriety (Kothari et al., 2012). F. vulgare hydromethanolic extract seems to include phenolic groups, expressed in EGA. The phenolic content was observed to be 38.2±2.3 mg EGA/g, which is greater than that observed in other medicinal plants already studied for their anti-inflammatory activity, including the hydromethanolic extract of Spergularia marina that contained 17 mg EGA/g (Kong, 2014). Furthermore, the flavonoid content noted in F. vulgare was 122.1±1.4 mg EGA/g, which is higher than that reported by Cherbal et al. (2012) in Pistachia lentiscus L. (38.7±0.02 mg EQ/g) and Amezouar et al. (2013) in Erica arborea L. (54.08±0.031 mg EQ/g), and even much higher than the total flavonoid content of the hydro-alcoholic extracts of F. vulgare (12.3±0.18 mg EQ/g) studied by Badgujar et al. (2014). Our present study indicates that F. vulgare involves significant flavonoid content, which may be responsible for the activity of the extract, as it is repeatedly proven by several studies that a multitude of flavonoids have anti-inflammatory properties. In fact, the anti-inflammatory effects of morin, rutin, and quercetin (flavonols) were explored in animal models, and several mechanisms elucidating the anti-inflammatory action of flavonoids have been illustrated, including radical scavenging and anti-oxidative activities, control of cellular activities of inflammation-associated cells, regulation of arachidonic acid metabolism enzymes [cyclooxygenase (COX), phospholipase A2, and lipoxygenase] and nitric oxide synthase (NOS), and regulation of gene expression of pro-inflammatory molecules (García-Lafuente et al., 2009; Ham et al., 2021).
Protein denaturation is a well-recognized cause of inflammation. The production of autoantigens in inflammatory diseases, including rheumatoid arthritis, may be caused by in vivo denaturation of proteins. As a part of the investigation of the anti-inflammatory mechanisms, the capacity of F. vulgare seed extract to prevent protein denaturation was investigated. Protein denaturation is a phenomenon wherein proteins lose their secondary and tertiary configurations owing to external stress, such as heat, or compounds, such as strong acids or bases, or inorganic solvents, thereby causing alteration of function since most biological proteins lose their activity when denaturized (Marliyah and Ananthi, 2015). One mechanism contributing to the anti-inflammatory activity of F. vulgare may be its capacity to reduce protein heat denaturation. Our results showed that F. vulgare seed extract produced a significant protein denaturation inhibition at different concentrations, with a maximum inhibitory activity of 35.68±0.40% shown at 200 μg/mL concentration. However, we have observed that the percentage of inhibition was greater at 50 μg/mL than that shown at 100 μg/mL. It is common that a particular pharmacological effect is non-concentration-related. In some cases, unexpected relationships between concentration and outcomes occur. One of the reasons that resolve the paradox of apparently non-concentration-related effect is the imprecision or inaccuracy in the measurement of either the concentration or the effect. Moreover, concentration-response relationships generally depend on the exposure time. Accordingly, the nonlinear concentration-response relationship observed may be because of the expanded exposure time at 50 μg/mL concentration.
Since the erythrocyte membrane is homologous to the lysosomal membrane (Shenoy et al., 2010), RBC membrane preservation has been adopted as a tool for examining the in vitro anti-inflammatory action. This suggests that if the extract can preserve the RBC membrane, it may also be capable of protecting the lysosomal membrane. Lysosomal stability is crucial in regulating the inflammatory response by blocking the extracellular release of lysosomal components of activated neutrophils, including bactericidal enzymes and proteases, which further exacerbate tissue inflammation and damage. Nonsteroidal medications act by either suppressing lysosomal enzymes or maintaining lysosomal membranes (Hu and Xu, 2011). RBC membrane stabilization was evaluated to establish the mechanism of the anti-inflammatory action of F. vulgare extract. The extract efficiently inhibited the heat-induced hemolysis. This provides evidence for the membrane stabilization effect of F. vulgare extract as another mechanism for its anti-inflammatory effect.
Proteolytic enzymes are essential regulators and modulators of the inflammatory response (Reshma et al., 2014). It was previously established that the lysosomal proteinase of activated neutrophils has a significant impact on tissue damage expansion during inflammatory responses (Leelaprakash and Dass, 2011). Consequently, protease inhibitors have received increasing attention as powerful tools for inactivating proteases in the pathogenic process of inflammation (Ruiz-Ruiz et al., 2017). The hydromethanolic extract of F. vulgare exhibited a significant anti-proteinase activity, with a maximum inhibition of 58.09±0.10% at 250 μg/mL, whereas indomethacin showed a maximum proteinase inhibitory action of 17.81±0.10% at 50 mg/mL. Since flavonoids have been reported to inhibit enzymes with various catalytic activities, their high concentration in the F. vulgare extract may be responsible for the significant activity it exhibits.
We further investigated the chemical composition of F. vulgare using GC-MS analysis and identified five different components, among which three have anti-inflammatory activity, namely linalool (C10H18O, the major constituent of F. vulgare extract), palmitic, and oleic acids (fatty acids). Linalool, also called monoterpenol (Ilc et al., 2016) or 3,7-dimethyl-1,6-octadien-3-ol (El Asbahani et al., 2015), is an acyclic monoterpene tertiary alcohol observed in the essential oils of several plant species (Aprotosoaie et al., 2014; Raguso, 2016). It is non-toxic and has been shown in various in vitro and in vivo studies to have various bioactivities that can be exploited for pharmaceutical purposes, such as anticancer, anti-inflammatory, antimicrobial, anti-hyperlipidemic, antidepressant, antioxidant, analgesic, and neuroprotective activities (Pereira et al., 2018). Linalool’s anti-inflammatory activity has been explored in vivo and in vitro and has been observed to attenuate the lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 production, block the nuclear factor-kappa B (NF-κB) pathway and mitogen-activated protein kinases pathway activation, and inhibit inflammatory cell infiltration and IL-1β, IL-8, and monocyte chemoattractant protein-1 production (Ma et al., 2015). This study has concluded that cytokine production suppression is related to linalool’s inhibitory impact on the NF-κB cellular pathway and COX-2, indicating that linalool can be used to reduce the inflammatory responses induced by conditions, including diabetes and cardiovascular diseases (Chang and Shen, 2014). Furthermore, linalool may prevent the synthesis of nitric oxide (NO), which is involved in the generation of edema, vasodilation, and the attraction of the immunological cells to the site of infection (Pereira et al., 2018).
Several studies have reported that fatty acid anti-inflammatory properties are related to lower TNF-α, IL-1α, IL-1β, and IL-6 levels (James et al., 2000). It is confirmed that palmitic acid, a saturated fatty acid, can influence immunological responses by specifically affecting T cells or inhibiting phospholipase A2, which is responsible for arachidonic acid and lysophospholipid release, which is the first step in the production of powerful inflammatory mediators, including leukotriene and prostaglandins (Aparna et al., 2012). Oleic acid, another F. vulgare constituent, is an indispensable fatty acid also known as omega 9. It can suppress endothelial cell stimulation and decrease inflammatory molecule expression (Carluccio et al., 1999). Moreover, oleic acid inhibits the release of pro-NO and prostaglandin E2 mediators as well as the production of NOS and COX-2 in LPS-stimulated microglial cells. This anti-inflammatory activity of oleic acid is linked to the inhibition of reactive oxygen species (Oh et al., 2009).
In conclusion, protein denaturation, membrane stabilization, and protease inhibition assays showed that F. vulgare seed extract had anti-inflammatory potential. Phytochemical screening revealed that F. vulgare seed extract contains a substantial flavonoid content that could be implicated in its anti-inflammatory activity. GC-MS analysis revealed that this extract contains essential oils and fatty acids, including linalool, palmitic acid, and oleic acid, which may be implicated in the anti-inflammatory activity observed in vitro. These findings provide scientific evidence supporting the traditional use of F. vulgare and the potential of its seeds for use in developing anti-inflammatory drugs.
Footnotes
FUNDING
None.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: AC, SH. Analysis and interpretation: all authors. Data collection: MB (Mouna Bouabdallah), MB (Mouna Benhalla). Writing the article: AC, MB (Mouna Bouabdallah), MB (Mouna Benhalla). Critical revision of the article: AC. Final approval of the article: all authors. Statistical analysis: AC, MB (Mouna Bouabdallah), MB (Mouna Benhalla). Overall responsibility: AC.
REFERENCES
- Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM. The crucial roles of inflammatory mediators in inflammation: A review. Vet World. 2018;11:627–635. doi: 10.14202/vetworld.2018.627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altameme HJ, Hameed IH, Kareem MA. Analysis of alkaloid phytochemical compounds in the ethanolic extract of Datura stramonium and evaluation of antimicrobial activity. Afr J Biotechnol. 2015;14:1668–1674. [Google Scholar]
- Amezouar F, Badri W, Hsaine M, Bourhim N, Fougrach H. Antioxidant and anti-inflammatory activities of Moroccan Erica arborea L. Pathol Biol. 2013;61:254–258. doi: 10.1016/j.patbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Des. 2012;80:434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- Aprotosoaie AC, Hăncianu M, Costache II, Miron A. Linalool: a review on a key odorant molecule with valuable biological properties. Flavour Fragr J. 2014;29:193–219. doi: 10.1002/ffj.3197. [DOI] [Google Scholar]
- Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014:842674. doi: 10.1155/2014/842674. https://doi.org/10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carluccio MA, Massaro M, Bonfrate C, Siculella L, Maffia M, Nicolardi G, et al. Oleic acid inhibits endothelial activation: A direct vascular antiatherogenic mechanism of a nutritional component in the mediterranean diet. Arterioscler Thromb Vasc Biol. 1999;19:220–228. doi: 10.1161/01.ATV.19.2.220. [DOI] [PubMed] [Google Scholar]
- Chang MY, Shen YL. Linalool exhibits cytotoxic effects by activating antitumor immunity. Molecules. 2014;19:6694–6706. doi: 10.3390/molecules19056694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbal A, Kebieche M, Madani K, El-Adawi H. Extraction and valorization of phenolic compounds of leaves of Algerian Pistacia lentiscus. Asian J Plant Sci. 2012;11:131–136. doi: 10.3923/ajps.2012.131.136. [DOI] [Google Scholar]
- Choi EM, Hwang JK. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia. 2004;75:557–565. doi: 10.1016/j.fitote.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Craig CR, Stitzel RE. Modern pharmacology with clinical applications. Lippincott Williams & Wilkins; 2004. pp. 486–498. [Google Scholar]
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- El-Awadi ME, Hassan EA. Physiological responses of fennel (Foeniculum vulgare Mill) plants to some growth substances. The effect of certain amino acids and a pyrimidine derivative. J Am Sci. 2010;6:120–125. [Google Scholar]
- El Asbahani A, Miladi K, Badri W, Sala M, Aït Addi EH, Casabianca H, et al. Essential oils: from extraction to encapsulation. Int J Pharm. 2015;483:220–243. doi: 10.1016/j.ijpharm.2014.12.069. [DOI] [PubMed] [Google Scholar]
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- Ham JR, Yun KW, Lee MK. Anti-inflammatory and antioxidant in vitro activities of Magnoliae flos ethanol extract. Prev Nutr Food Sci. 2021;26:485–491. doi: 10.3746/pnf.2021.26.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornok L. The cultivation of medicinal plants. In: Hornok L, editor. Cultivation and Processing of Medicinal Plants. John Wiley & Sons; 1992. pp. 187–196. [Google Scholar]
- Hu QP, Xu JG. Profiles of carotenoids, anthocyanins, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. J Agric Food Chem. 2011;59:2026–2033. doi: 10.1021/jf104149q. [DOI] [PubMed] [Google Scholar]
- Ilc T, Parage C, Boachon B, Navrot N, Werck-Reichhart D. Monoterpenol oxidative metabolism: role in plant adaptation and potential applications. Front Plant Sci. 2016;7:509. doi: 10.3389/fpls.2016.00509. https://doi.org/10.3389/fpls.2016.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov SA. Natural antioxidants 4: Antioxidants in the fatty oil of Foeniculum vulgare Miller. Fette Seifen Anstrichm. 1979;81:105–107. doi: 10.1002/lipi.19790810302. [DOI] [Google Scholar]
- James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343S. [DOI] [PubMed] [Google Scholar]
- Kar B, Kumar RBS, Karmakar I, Dola N, Bala A, Mazumder UK, et al. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac J Trop Biomed. 2012;2:S976–S980. doi: 10.1016/S2221-1691(12)60346-3. [DOI] [Google Scholar]
- Kong CS. Anti-inflammatory activity of the solvent-partitioned fractions from Spergularia marina in LPS-stimulated RAW 264.7 cells. Prev Nutr Food Sci. 2014;19:261–267. doi: 10.3746/pnf.2014.19.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula S, Kumar H. Foeniculum vulgare Mill (Umbelliferae) attenuates stress and improves memory in Wister rats. Trop J Pharm Res. 2013;12:553–558. doi: 10.4314/tjpr.v12i4.17. [DOI] [Google Scholar]
- Kothari V, Gupta A, Naraniwal M. Comparative study of various methods for extraction of antioxidant and antibacterial compounds from plant seeds. J Nat Remedies. 2012;12:162–173. [Google Scholar]
- Kwon YS, Choi WG, Kim WJ, Kim WK, Kim MJ, Kang WH, et al. Antimicrobial constituents of Foeniculum vulgare. Arch Pharm Res. 2002;25:154–157. doi: 10.1007/BF02976556. [DOI] [PubMed] [Google Scholar]
- Leelaprakash G, Dass SM. In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int J Drug Dev Res. 2011;3:189–196. [Google Scholar]
- Ma J, Xu H, Wu J, Qu C, Sun F, Xu S. Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int Immunopharmacol. 2015;29:708–713. doi: 10.1016/j.intimp.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Marliyah M, Ananthi T. In vitro anti-inflammatory activity of seed extract of Zea mays (L.) J Glob Biosci. 2015;4:2168–2173. [Google Scholar]
- Moura LS, Carvalho RN, Jr, Stefanini MB, Ming LC, Meireles MAA. Supercritical fluid extraction from fennel (Foeniculum vulgare): global yield, composition and kinetic data. J Supercrit Fluids. 2005;35:212–219. doi: 10.1016/j.supflu.2005.01.006. [DOI] [Google Scholar]
- Nunes CDR, Barreto Arantes M, Menezes de Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de Moraes L, et al. Plants as sources of anti-inflammatory agents. Molecules. 2020;25:3726. doi: 10.3390/molecules25163726. https://doi.org/10.3390/molecules25163726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YT, Lee JY, Lee J, Kim H, Yoon KS, Choe W, et al. Oleic acid reduces lipopolysaccharide-induced expression of iNOS and COX-2 in BV2 murine microglial cells: possible involvement of reactive oxygen species, p38 MAPK, and IKK/NF-κB signaling pathways. Neurosci Lett. 2009;464:93–97. doi: 10.1016/j.neulet.2009.08.040. [DOI] [PubMed] [Google Scholar]
- Othman A, Ismail A, Ghani NA, Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100:1523–1530. doi: 10.1016/j.foodchem.2005.12.021. [DOI] [Google Scholar]
- Owen PL, Johns T. Xanthine oxidase inhibitory activity of northeastern North American plant remedies used for gout. J Ethnopharmacol. 1999;64:149–160. doi: 10.1016/S0378-8741(98)00119-6. [DOI] [PubMed] [Google Scholar]
- Özbek H, Uğraş S, Dülger H, Bayram I, Tuncer I, Oztürk G, et al. Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia. 2003;74:317–319. doi: 10.1016/S0367-326X(03)00028-5. [DOI] [PubMed] [Google Scholar]
- Parejo I, Jauregui O, Sánchez-Rabaneda F, Viladomat F, Bastida J, Codina C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography-negative electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2004;52:3679–3687. doi: 10.1021/jf030813h. [DOI] [PubMed] [Google Scholar]
- Pereira I, Severino P, Santos AC, Silva AM, Souto EB. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf B Biointerfaces. 2018;171:566–578. doi: 10.1016/j.colsurfb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Phalitakul S, Okada M, Hara Y, Yamawaki H. Vaspin prevents TNF-α-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-κB and PKCθ activation in cultured rat vascular smooth muscle cells. Pharmacol Res. 2011;64:493–500. doi: 10.1016/j.phrs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Pradhan M, Sribhuwaneswari S, Karthikeyan D, Minz S, Sure P, Chandu Atul N, et al. In-vitro cytoprotection activity of Foeniculum vulgare and Helicteres isora in cultured human blood lymphocytes and antitumour activity against B16F10 melanoma cell line. Res J Pharm Technol. 2008;1:450–452. [Google Scholar]
- Raguso RA. More lessons from linalool: insights gained from a ubiquitous floral volatile. Curr Opin Plant Biol. 2016;32:31–36. doi: 10.1016/j.pbi.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Rahimi R, Ardekani MR. Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chin J Integr Med. 2013;19:73–79. doi: 10.1007/s11655-013-1327-0. [DOI] [PubMed] [Google Scholar]
- Rasul A, Akhtar N, Khan BA, Mahmood T, Uz Zaman S, Khan HM. Formulation development of a cream containing fennel extract: in vivo evaluation for anti-aging effects. Pharmazie. 2012;67:54–58. [PubMed] [Google Scholar]
- Reshma, Arun KP, Brindha P. In vitro anti-inflammatory, antioxidant and nephroprotective studies on leaves of Aegle marmelos and Ocimum sanctum. Asian J Pharm Clin Res. 2014;7:121–129. [Google Scholar]
- Ruberto G, Baratta MT, Deans SG, Dorman HJ. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000;66:687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz JC, Matus-Basto AJ, Acereto-Escoffié P, Segura-Campos MR. Antioxidant and anti-inflammatory activities of phenolic compounds isolated from Melipona beecheii honey. Food Agric Immunol. 2017;28:1424–1437. doi: 10.1080/09540105.2017.1347148. [DOI] [Google Scholar]
- Sakat SS, Juvekar AR, Gambhire MN. In-vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci. 2010;2:146–155. [Google Scholar]
- Shehata F, Fathy A, Abdelhameed M, Moustafa SF. Preparation and properties of Al2O3 nanoparticle reinforced copper matrix composites by in situ processing. Mater Des. 2009;30:2756–2762. doi: 10.1016/j.matdes.2008.10.005. [DOI] [Google Scholar]
- Shenoy S, Shwetha K, Prabhu K, Maradi R, Bairy KL, Shanbhag T. Evaluation of antiinflammatory activity of Tephrosia purpurea in rats. Asian Pac J Trop Med. 2010;3:193–195. doi: 10.1016/S1995-7645(10)60007-7. [DOI] [Google Scholar]
- Tripathi P, Tripathi R, Patel RK, Pancholi SS. Investigation of antimutagenic potential of Foeniculum vulgare essential oil on cyclophosphamide induced genotoxicity and oxidative stress in mice. Drug Chem Toxicol. 2013;36:35–41. doi: 10.3109/01480545.2011.648328. [DOI] [PubMed] [Google Scholar]
- Wu Q, Yu L, Qiu J, Shen B, Wang D, Soromou LW, et al. Linalool attenuates lung inflammation induced by Pasteurella multocida via activating Nrf-2 signaling pathway. Int Immunopharmacol. 2014;21:456–463. doi: 10.1016/j.intimp.2014.05.030. [DOI] [PubMed] [Google Scholar]