Abstract
Preventing abnormal scar formation and correcting non-aesthetic mature scars are important to prevent physical and psychosocial consequences of abnormal scarring. Evidence-based guidelines for scar management in Asian patients recommend first-line silicone-based products. Dermatix®* Ultra and Dermatix Ultra Kids are topical silicone gels containing a vitamin C ester that helps lighten scar tissue. Herein, we report a case series including patients with hypertrophic and keloid scars treated with Dermatix, showing that Dermatix is effective for scar treatment and prevention, as well as expert consensus supporting the safe and effective use of Dermatix.
Keywords: adults, hypertrophic scars, keloid, paediatric, real-world, scar prevention, scar treatment, topical silicone gel
Introduction
Hypertrophic scars and keloids develop following abnormal fibrous wound healing due to aberrant regulation of tissue repair and regeneration. They are disfiguring and prone to recurrence and, accordingly, represent a major therapeutic problem for physicians.1 Therapeutic options for the management of hypertrophic scars and keloids include intralesional corticosteroid injections with/without cryotherapy, intralesional 5-fluorouracil, laser therapy, and topical silicone-based products.2–4 Combined therapeutic approaches are frequently effective for successful healing.1
Silicone gel sheeting has been used successfully for more than 30 years in hypertrophic and keloid scar management.5–7 Topical silicone gel is also effective for the treatment of hypertrophic and keloid scars and of post-laser exfoliation erythema.8–11 A 90-day comparative prospective study showed that both Dermatix silicone gel and silicone gel sheeting reduced symptoms of itching, irritation, and skin maceration in abnormal scarring, but patients rated Dermatix silicone gel as easier to use.11 A recent systematic review and meta-analysis reported that, in comparison to no treatment or placebo gels, fluid silicone gel was associated with both prophylactic and curative effects on hypertrophic scars.12
Although the mechanism of action of silicone gel products is not completely understood, it is known that they reduce transepidermal water loss and increase stratum corneum hydration. In wound healing, dehydration of an immature stratum corneum can occur, leading to the stimulation of keratinocytes, which release cytokines that in turn activate dermal fibroblasts. These synthesize and release excessive collagen, leading to abnormal scarring.13
Guidelines for scar management in Asian patients indicate that there is strong evidence for the efficacy of silicone-based products, including silicone gel, and recommend silicone-based products as first-line therapy due to their ease of use.14 Vitamin C is a potent antioxidant and topical vitamin C has been shown to be a useful agent for the treatment of photoaging and other dermatological conditions.15 Vitamin C derivatives are used in various skin-care products for skin lightening and protection against ultraviolet A/B light. Dermatix (Registered trademark, Menarini, Singapore) Ultra and Dermatix Ultra Kids are silicone gels that contain vitamin C ester. A 2013 observational study of fine surgical scars in Asian facial skin found that Dermatix Ultra formulation normalized scar pigmentation and flattened scars.16
The case series of Asian patients presented here examines the effectiveness of Dermatix Ultra silicone gel for the treatment of hypertrophic scars, including keloid, in routine clinical practice. Patients were treated at centres in China, Hong Kong, Philippines, Singapore, Taiwan, Thailand and Vietnam. The clinicians who treated these patients are experienced in the management of scars and are experts in plastic/reconstructive surgery, breast surgery, obstetrics and gynaecology, burns, and dermatology. As a separate part of the current manuscript, these clinicians also provide their expert opinion regarding the use of Dermatix Ultra silicone gel for the management of abnormal scars.
Methods
Case series
The case series consisted of 11 patients with visible hypertrophic scars treated with topical Dermatix Ultra silicone gel alone or following appropriate additional therapeutic modalities, including wound debridement and xenograft, surgical excision, or laser therapy.
In November 2022, a panel of nine clinical experts met to review real-world scar management using topical silicone gel, including discussion of individual cases, and to identify areas of consensus based on their clinical expertise. The cases presented in this report were anonymized and discussed at the Experts meeting. No information is reported that could enable patients to be identified; therefore, no patient consent to report these cases was required.
Expert opinion
In addition, the experts who provided the cases completed a separate survey of their opinions regarding the use of Dermatix Ultra silicone gel in routine clinical practice. The questionnaire consisted of 21 statements (13 concerning use in adult patients and 8 in paediatric patients), for each of which the respondents chose from the following response options: “Agree”, “Somewhat agree” or “Disagree”. After completing the survey, the experts met to discuss the findings and identify areas of consensus.
Results
Case series
Case studies included patients with traumatic scars (n=4) or patients undergoing elective surgery (n=4) or caesarean section (n=3). Case study details are summarized in Table 1.
Table 1.
Summary of case studies.
| Case number | Patient | History | Treatment | Outcome |
|---|---|---|---|---|
| Traumatic scars | ||||
| Case 1 (Figure 1) | 26-year-old man with burns on the face, neck, and anterior upper trunk from gasoline leakage TBSA 8%, third-degree burns |
After STSG treatment, the hypertrophic scar from the wound felt itchy and reddish | Tracheostomy Early wound debridement and tangential excision; wound xenograft for 7 days; STSG on neck Dermatix Ultra BID applied to burn area for 90 days |
Improvement in scarring following 90 days of Dermatix Ultra application |
| Case 2 (Figure 2) | 5-year-old boy, with facial scald injury; TBSA 3%, second-degree grade burns | 3 weeks later from injury, the wound was healed and silicone gel was applied to prevent hypertrophic scar | Wound debridement; xenograft for 2 weeks Dermatix Ultra silicone gel BID applied for 7 weeks |
Scarring was not noticeable at 60 days of follow-up |
| Case 3 (Figure 3) | 7-year-old boy with an erythematous and hypertrophic scar on his forehead following a 3-week-old injury | The scar remained painful and itchy | Pulsed dye laser treatment every 4 weeks plus topical Dermatix Ultra silicone gel | Marked improvement after three courses of treatment |
| Case 4 (Figure 4) | 45-year-old man with facial injury and scarring | Fell from motorcycle and sustained facial injury Residual post-inflammatory erythema and indentation 3 months after injury without signs of improvement |
Fractional pico-second laser and Dermatix Ultra silicone treatment (1 tube for 3 months) | Marked improvement observed following two sessions of laser therapy and 3 months of Dermatix Ultra silicone gel treatment |
| Surgical/procedural scars | ||||
| Case 5 (Figure 5) | 25-year-old woman undergoing lower eyelid surgery for lower eyelid defect | Infantile haemangioma | Lower eyelid reconstruction Scar on lower eyelid treated with Dermatix Ultra silicone gel BID for >6 months |
Improvement in scarring during 11 months of post-operative monitoring |
| Case 6 (Figure 6) | 35-year-old woman receiving revisional breast surgery and abdominal surgery | The patient underwent revisional breast surgery 2 years ago a plastic surgeon and, 1 year later, she had elective abdominal surgery (at her local hospital) due to hepatobiliary disease | Dermatix Ultra silicone gel was initiated since day 7 post-breast surgery and applied twice-daily per protocol for at least 6 months; nonetheless, the abdominal scar was neither treated nor advised for any scar prevention protocol | No hypertrophic or keloid scar formation at 1 and 2 years post-revisional breast surgery; in contrast, hypertrophic scar formation was evident on the non-treated abdominal scar after 6 months |
| Case 7 (Figure 7) | 3-month-old child born with bilateral complete cleft lip and palate | No family history | Planned intervention: presurgical nasoalveolar molding; bilateral cheiloplasty aged 3 months (Noordhoff bilateral cheiloplasty) Post-operative botulinum toxin injection, 3M surgical taping and 6-month Dermatix Ultra silicone gel treatment |
Scarring was not noticeable at 6 and 9 months of follow-up |
| Case 8 (Figure 8) | 45-year-old man with nasal hypopigmentation and an atrophic and hypertrophic scar | Skin necrosis due to rhinoplasty 2 months previously | Fractional ablative CO2 laser surgery every 4 weeks plus application of topical Dermatix Ultra silicone gel | Improvement in scarring following four rounds of treatment |
| Surgical scars – caesarean section | ||||
| Case 9 (Figure 9) | 30-year-old woman, 38 weeks pregnant (by gestational age); scheduled caesarean section due to breech presentation | G1P0 No history of operation; no major disease or drug history BMI: 25 kg/m2 |
Initial treatment after surgery with Dermatix Ultra silicone gel applied BID from 2 weeks post-surgery for 6 months | No adverse effects Patient overall satisfaction score with Dermatix Ultra was 4.5 out of 5: “very satisfied” (easy to apply, user-friendly, excellent wound healing) |
| Case 10 (No figure) | 29-year-old pregnant woman undergoing caesarean section | G1P0; no GDM or PMH. Pregnancy-induced seafood allergic reaction during second to third trimester (eczema rash) over hands BMI: 20 kg/m2 |
Post-caesarean section: skin closure with Dermabond (no sutures used); Cerederm dressing for 14 days Application of Dermatix Ultra silicone gel OD for 2 months |
At 12 months post-caesarean section, scar had lightened and faded (almost invisible) No wound infection or itch noted Patient satisfied with Dermatix Ultra application |
| Case 11 (Figure 10a) | 28-year-old pregnant woman with abdominal keloid scar undergoing caesarean section | G2P1 (1011) Right salpingectomy (2009; aged 26 years), after a raised 6 × 0.5 cm midline lower abdominal keloid scar formed, which was sometimes itchy, with intermittent shooting pain; during the ninth month of pregnancy, the keloid scar was 10 × 1 cm, brown coloured and raised; caesarean section performed in 2010 (patient aged 28 years) |
Keloid was excised and sutured Application of Dermatix Ultra silicone gel from 2 weeks post-caesarean section for 6 months |
No keloid scar formation after 6 months of treatment with Dermatix Ultra silicone gel and the patient (currently aged 42 years) has a nice, flat one-line scar |
No pre-treatment photo is available.
BID, twice daily; G, gravida; GDM, gestational diabetes mellitus; OD, once daily; P, parity; PMH, progressive macular hypomelanosis; STSG, split-thickness skin graft; TBSA, total body surface area.
Patients suffering from third-degree or second-degree burns (cases 1 and 2) showed marked improvement in scarring following wound debridement, xenograft and Dermatix Ultra silicone gel treatment (Figures 1 and 2). Despite extensive facial scarring in a 5-year-old boy (case 2), scarring was not noticeable at the 60-day follow-up (Figure 2). Following an injury 3 weeks previously, an erythematous and hypertrophic scar on the forehead of a 7-year-old boy (case 3) was treated with pulsed dye laser every 4 weeks plus topical Dermatix Ultra silicone gel. The scar was barely visible after three courses of pulsed dye laser treatment (Figure 3). A 45-year-old man who had residual post-inflammatory erythema and indentation 3 months after a facial injury without signs of improvement (case 4) showed improvement following two sessions of laser therapy and 3 months of Dermatix Ultra silicone gel treatment (Figure 4).
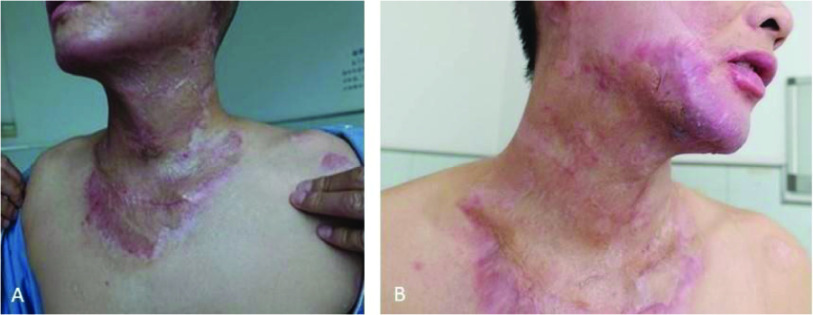
Figure 1.
Improvement in scarring in a 26-year-old man with third-degree burns to his face, neck and anterior upper trunk following 90 days Dermatix Ultra application (case 1).
(A) Pre-treatment; (B) Post-treatment (3 months).
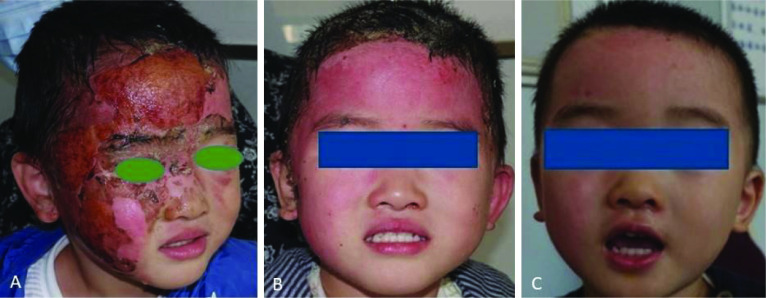
Figure 2.
Improvement in scarring in a 5-year-old boy with second-degree facial scald injury treated with wound debridement, 2-week xenograft, and Dermatix Ultra silicone gel for 7 weeks (case 2).
(A) day 1; (B) day 14; (C) day 60.
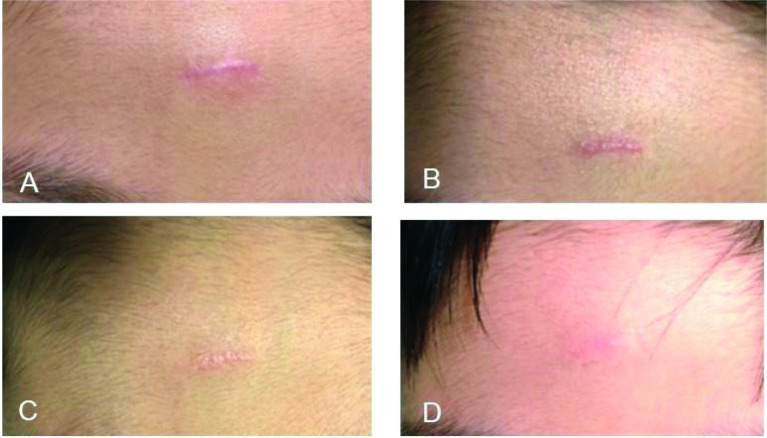
Figure 3.
7-year-old boy with an erythematous and hypertrophic scar treated with laser treatment (pulsed dye laser) every 4 weeks plus topical Dermatix Ultra silicone gel (case 3).
(A) Pre-treatment; (B) After 1 round of treatment; (C) After 2 rounds of treatment; (D) After 3 rounds of treatment.
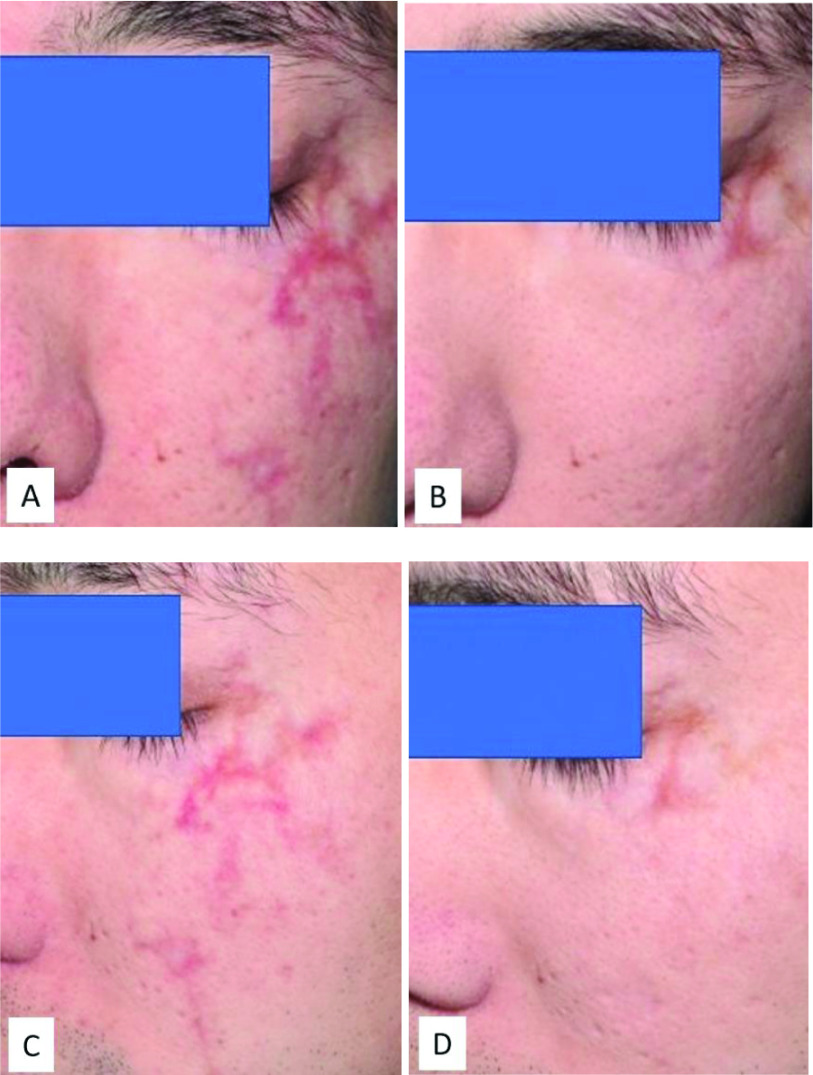
Figure 4.
Improvement in facial scarring in a 45-year-old man following 2 sessions of laser therapy and 3 months of Dermatix Ultra silicone gel treatment (case 4).
(A) and (C) Pre-treatment; (B) and (D) Post-treatment (3 months).
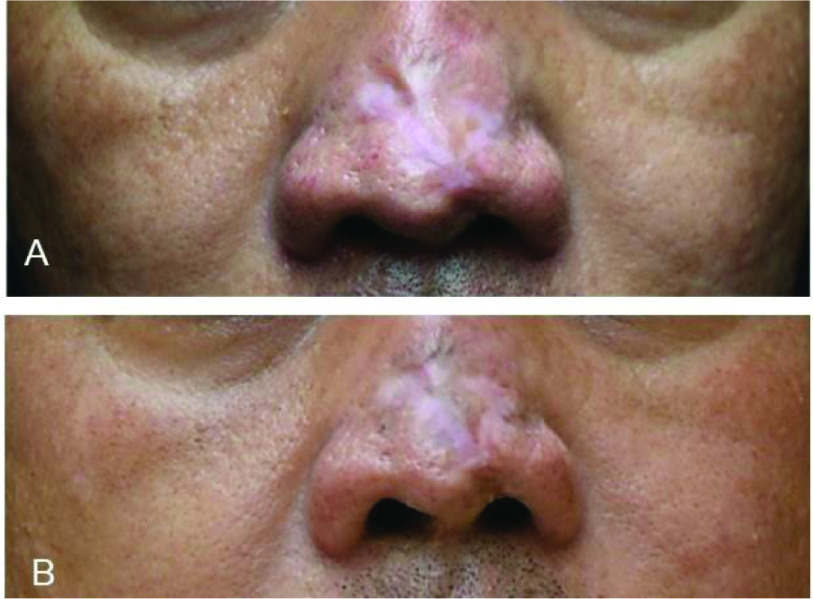
Amongst surgical cases, scarring in a 25-year-old woman (case 5) undergoing lower eyelid surgery and treated with Dermatix Ultra silicone gel gradually improved during 11 months of post-operative monitoring (Figure 5). Treatment of a breast augmentation surgical scar (case 6) with Dermatix Ultra silicone gel prevented visible scarring, comparison with an untreated abdominal scar clearly shows the benefit of treatment (Figure 6). Following planned surgery for bilateral complete cleft lip and palate in a 3-month-old child (case 7) treated with an injection of botulinum toxin and topical Dermatix Ultra silicone gel for 6 months, scarring was not noticeable at 9 months of follow-up (Figure 7). Treatment of a 45-year-old man with a hypopigmented, atrophic and hypertrophic nasal scar (case 8) using four rounds of fractional ablative CO2 laser surgery plus topical Dermatix Ultra silicone gel led to improvement in scarring (Figure 8).
Figure 5.
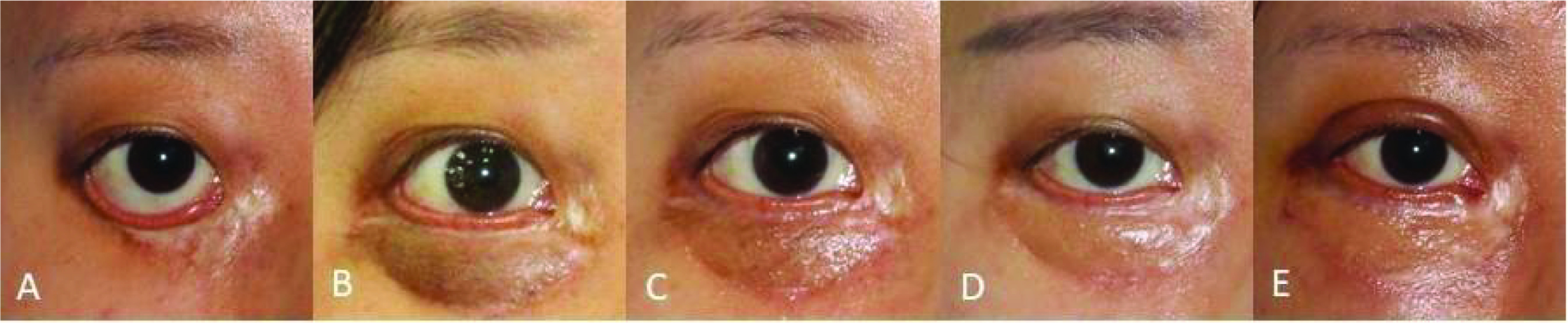
Improvement in scarring in a 25-year-old woman undergoing lower eyelid surgery treated with Dermatix Ultra silicone gel (case 5).
(A) Pre-surgery; (B) 2 months post-surgery; (C) 3 months post-surgery; (D) 6 months post-surgery; (E) 11 months post-surgery.
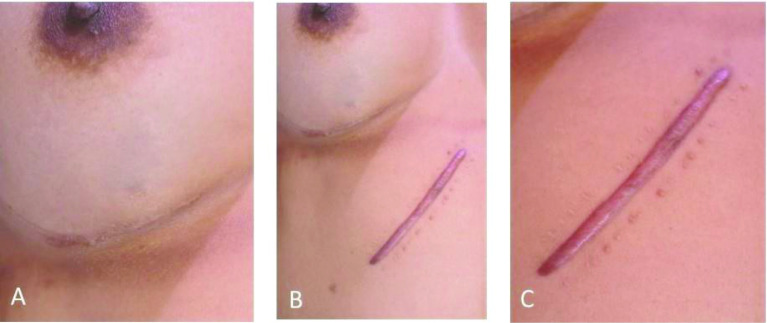
Figure 6.
Improvement of scarring in a 35-year-old woman post-revisional breast surgery treated with Dermatix Ultra silicone gel (case 6).
(A) 2-year post-revisional breast surgery scar treated with Dermatix Ultra silicone gel; (B) Dermatix Ultra-treated 2-year post-revisional breast surgery scar compared with untreated 6-month post-surgical abdominal scar; (C) Untreated 3-month post-surgical abdominal scar showing hypertrophic scar formation (untreated site).
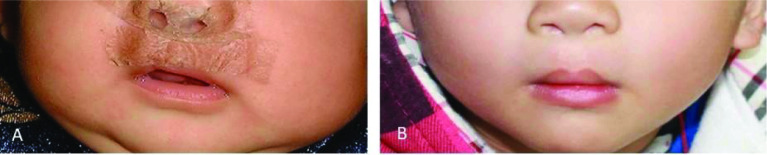
Figure 7.
Newborn child with scarring following surgery for bilateral complete cleft lip and palate treated with botulinum toxin injection and 6-month Dermatix Ultra Kids silicone gel (case 7).
(A) Post-surgical scarring; (B) 9-month follow-up.
Figure 8.
Improvement in scarring in a 45-year-old man with hypopigmented, atrophic, and hypertrophic scar on his nose treated with four rounds of fractional ablative CO2 laser surgery plus topical Dermatix Ultra silicone gel (case 8).
(A) Pre-treatment; (B) Post-treatment (4 months).
Application of Dermatix Ultra silicone gel for 6 months to a caesarean section scar in a 30-year-old woman (case 9) showed gradual improvement in the appearance of the scar (Figure 9). The patient was “very satisfied” with the treatment, which was easy to apply, user-friendly and provided excellent wound healing. In a second patient who underwent caesarean section (case 10) and had application of Dermatix Ultra silicone gel for 2 months, the scar was well healed at 12 months, appearing lighter and faded, and with no keloid developing (figure not available). In case 11, a raised midline lower abdominal keloid scar (following salpingectomy at the age of 26 years) was excised at the time of a caesarean section (aged 28 years). There was no keloid scar formation after 6 months of treatment with Dermatix Ultra silicone gel (Figure 10) and the patient (currently aged 42 years) has a nice, flat one-line scar.
Figure 9.
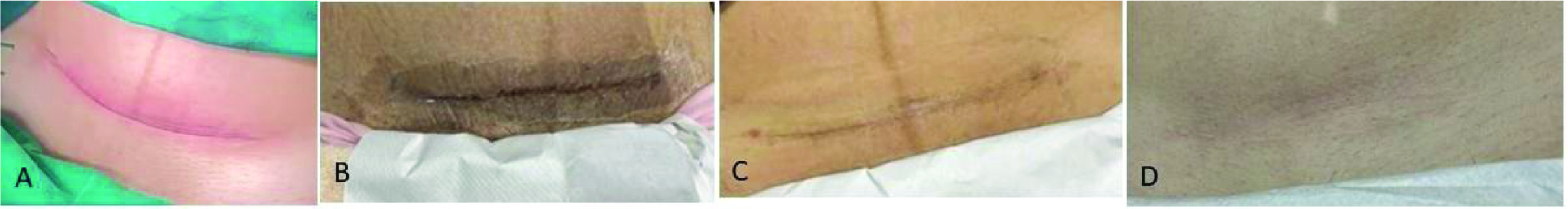
Improvement in caesarean section scar in a 30-year-old woman following application of Dermatix Ultra silicone gel from 2 weeks post-surgery for 6 months (case 9).
(A) Pre-treatment; (B) 10 days post-surgery; (C) 1-month post-surgery; (D) 6 months post-surgery.
Figure 10.
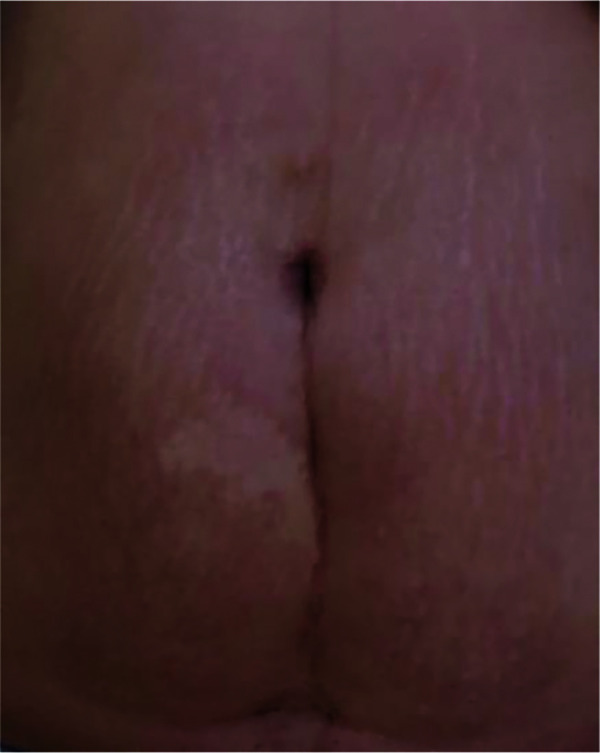
No keloid scar formation after 6 months of treatment with Dermatix Ultra silicone gel; the patient (currently aged 42 years) has a nice, flat 1-line scar (pre-treatment photo not available) (case 11).
Expert opinion
Survey responses are summarized in Table 2. With respect to adult patients, the nine experts were unanimous in agreeing that Dermatix Ultra silicone gel was the preferred first-line treatment for scar management, that it had been clinically proven to be effective on scars, and that the vitamin C ester lipids contained in the gel provided strong lightening effects. There was also unanimous agreement that it should be prescribed early (once the wound closes or stitches are removed) to prevent scar formation and that early use mitigated the formation of keloid scars. Further discussion confirmed that following surgery, treatment with Dermatix Ultra gel should be started after suture removal. If absorbable stitches or tissue glue are used, the gel was usually initiated after approximately 2 weeks. If wounds were in a good condition, treatment could be started as soon as the wound could safely come into contact with water (7–10 days).
Table 2.
Survey of experts’ opinions on the use of Dermatix Ultra gel in routine clinical practice.
| Agree | Somewhat agree | Disagree | |
|---|---|---|---|
| Statements about adult patients | n =9 | ||
| Dermatix Ultra is the preferred first-line treatment for scar management | 9 | 0 | 0 |
| I will consider Dermatix Ultra for my patients as it is clinically proven to be effective on scars | 9 | 0 | 0 |
| Dermatix Ultra should be prescribed early (once the wound closes/stitches are removed) to prevent scar formation | 9 | 0 | 0 |
| Dermatix Ultra’s vitamin C ester lipids provide stronger lightening effects | 9 | 0 | 0 |
| Early use of Dermatix Ultra mitigates keloid scar formation | 9 | 0 | 0 |
| Dermatix Ultra’s formulation provides better lightening and recovery for burn scars | 8 | 1 | 0 |
| Dermatix Ultra is recommended by plastic surgeons, obstetricians and gynaecologists around the world to effectively manage caesarean section scars | 8 | 1 | 0 |
| Dermatix Ultra is safe in pregnant and breastfeeding women | 8 | 1 | 0 |
| Dermatix Ultra’s medical-grade formulation is superior to onion extract formulations | 7 | 2 | 0 |
| CPX (cyclopentasiloxane) technology in Dermatix Ultra provides better outcomes than other silicone formulas | 7 | 2 | 0 |
| Dermatix Ultra has superior efficacy compared with silicone sheets for the management of scars with its ease of application and fast-drying properties | 7 | 2 | 0 |
| To achieve the best outcomes for surgical scars, the optimal duration of use for Dermatix Ultra should be between 3 and 4 months | 7 | 2 | 0 |
| By applying Dermatix Ultra twice daily, patients can see visible results on their scars from day 7 onwards | 4 | 4 | 1 |
| Statements about paediatric patients | n =7 | ||
| Early usage of Dermatix Ultra Kids when wound closes, helps prevent scar formation and produces visible results from day 7 after continuous twice-daily usage | 7 | 0 | 0 |
| Dermatix Ultra Kids is safe in children aged ≥3 months for the treatment of their scars | 6 | 1 | 0 |
| Dermatix Ultra Kids is clinically proven to be effective on keloid scars in children | 6 | 1 | 0 |
| For serious surgical scars, Dermatix Ultra Kids should be used continuously for at least 3 months, preferably up to 6 months | 6 | 1 | 0 |
| Dermatix Ultra Kids is recommended over onion extract formulations for the treatment of children’s scars due to its advanced medical-grade formulation | 6 | 1 | 0 |
| Dermatix Ultra Kids is gentle on children’s sensitive and delicate skin | 6 | 1 | 0 |
| Dermatix Ultra Kids reduces itchiness and is gentle on scars caused by eczema | 5 | 1 | 1 |
| Dermatix Ultra Kids reduces itchiness and prevents scars caused by insect bites | 4 | 3 | 0 |
Almost all experts (8/9) agreed with the statements that Dermatix Ultra gel was recommended for the effective management of caesarean section scars and that it was safe to use in pregnant and breastfeeding women. Most (7/9) agreed that the optimal duration of use for surgical scars was 3–4 months. During the discussion, several noted that they sometimes recommended treatment continuation for up to 6 months, particularly after caesarean sections.
Almost all (8/9) agreed that Dermatix Ultra formulation provided better lightening and recovery for burn scars. A specialist in burns and plastic surgery recommended starting treatment as early as possible in patients with burn wounds, within 7 days after the injury, and continuation for 3 months.
Most experts (7/9) agreed that the medical-grade formulation of Dermatix Ultra was superior to onion extract formulations, that the cyclopentasiloxane technology used in Dermatix Ultra provided better outcomes than other silicone formulations, and that the ease of application and fast-drying nature of the Dermatix Ultra gel contributed to superior efficacy compared with silicone sheets. Four of nine experts agreed that visible results could be seen after 7 days with twice-daily application, four respondents somewhat agreed with the statement, and one was of the opinion that results became apparent after 2–3 weeks of use.
With respect to Dermatix Ultra Kids, the seven experts who responded to this section of the survey unanimously agreed that early use (when the wound had closed) helped prevent scar formation and produced visible results from day 7 after continuous twice-daily usage. Almost all experts (6/7) agreed that it was safe for the treatment of scars in children aged ≥3 months, that it had been clinically proven to be effective on keloid scars in children, that it was gentle on children’s skin, and that it was recommended over onion extract formulations for the treatment of paediatric scars because of its medical-grade formulation. In addition, almost all (6/7) agreed that it should be used continuously for at least 3 months (and preferably 6 months) for serious surgical scars in children. Overall, 5/7 experts agreed that the paediatric preparation reduced itchiness and was gentle on scars caused by eczema, and more than half (57%) agreed that it reduced itchiness and prevented scars caused by insect bites (4/7).
Discussion
Case series
This case series relating to the real-world management of hypertrophic and keloid scars using topical Dermatix Ultra and Dermatix Ultra Kids in routine clinical practice, demonstrates that it is effective for both scar treatment and scar prevention. As scarring may have physical and psychosocial consequences, it is important to minimize scar formation after surgery and to correct non-aesthetic mature scars.3 Cases included patients with traumatic scars, including those from burn or scald injuries, as well as those undergoing a range of surgical procedures, including caesarean section.
The two cases with extensive burn scarring presented here support results from randomized controlled trials demonstrating that silicone gel was an effective treatment for hypertrophic burn scars.17,18 In the case of a 5-year-old child with extensive facial burns treated with wound debridement, xenograft, and Dermatix Ultra Kids silicone gel, scarring was unnoticeable at 60 days of follow-up.
In elective surgical cases, Dermatix Ultra silicone gel markedly improved scarring and, in some cases, scars were barely visible. For example, treatment of the upper lip of a 3-month-old child following surgery for bilateral cleft lip and palate with Dermatix Ultra Kids silicone gel showed the absence of noticeable scarring at 6 and 9 months of follow-up. This case supports results from an observational study of children with unilateral cleft lip surgery that found that silicone gel was non-inferior to silicone sheeting for post-operative care of upper lip scars at 6-month follow-up.19 Topical silicone gel is safe, effective and easy to apply.9–11,20,21 In contrast, topical silicone sheeting can be difficult to apply to scars in some areas, such as joints, and patient compliance can be reduced for visible scars. Silicone sheeting may also lead to skin irritation, especially in hot climates, leading to treatment discontinuation.10 In addition, silicone gel has a potential safety advantage over silicone sheeting as it cannot be swallowed whole by babies, with the consequent risk of gastrointestinal obstruction or respiratory compromise.19
Interestingly, case 6 demonstrates the effect of treated (using Dermatix Ultra) and non-treated surgical scars in the same patient. The treated scar showed a sustained, long-term improvement after 18 months.
Expert opinion
Although the survey involved only a small number of clinicians, their expert opinion provides some additional insight into the use of Dermatix Ultra silicone gel in routine clinical practice. There was unanimous agreement that Dermatix Ultra gel is the preferred first-line treatment for scar management in adult patients and has been proven to be clinically effective. Topical silicone products (sheeting or gel) have been shown to be effective for scar management for over 30 years21,22 and guidelines recommend silicone gel as the first-line prophylactic and treatment option for hypertrophic scars and keloid.3,14 All experts agreed that the vitamin C ester lipids in the formulation improve its lightening effect and that prescribing the gel early (once sutures are removed) helps prevent scar formation, including keloid. There was also unanimous agreement that early use of Dermatix Ultra Kids helps prevent scar formation in children, with regular twice-daily use producing visible results after 1 week.
In order to optimize outcomes, it is important to ensure that clinicians understand the correct usage of Dermatix Ultra gel and that patients are educated about the importance of complying with treatment.
Study limitations
Limitations of this study include the small number of cases (n=11) and expert clinicians surveyed (n=9) and the absence of objective effectiveness outcomes. In addition, as is often the situation with real-world cases, our case series is limited by the relatively poor quality of some of the photos, with inconsistent lighting and views. Nevertheless, all patients reviewed showed visual improvement in scarring following treatment with Dermatix Ultra or Dermatix Ultra Kids silicone gel.
Conclusions
First, this case series of the real-world management of hypertrophic and keloid scars resulting from trauma, general surgery and caesarean section demonstrates that topical Dermatix Ultra gel and Dermatix Ultra Kids silicone gel, for adult and paediatric patients, respectively, is effective for both scar treatment and scar prevention. Second, expert opinion, generated from a survey of clinicians experienced in the management of scars, supports its use in the management of scars and emphasises the importance of starting treatment early for the prevention of abnormal scarring.
Acknowledgements
Under the direction of the authors, medical writing, and editorial support was provided by Robert A Furlong PhD and David P Figgitt PhD, ISMPP CMPP, Content Ed Net, with funding from Menarini Singapore.
Footnotes
Contributions: All authors contributed equally to the preparation of this article. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: XY, VL, FC, LQ, SS, TT, and YC declare no conflicts of interest. TC declares receiving an honorarium for the expert meeting. CH declares payment or honoraria for lectures, presentations, speakers, bureaus, article writing, or educational events from Biofemme Unilab, Philippine Family Physician, Bayer, Cardinal Santos Medical Center and Quezon City Medical Society, support for attending meetings and/or travel from Cathay Drug Philippines and Biofemme Unilab, and a leadership or fiduciary role at Cardinal Santos Medical Center. DD and DN are employees of A. Menarini Asia-Pacific Pte. Ltd. The International Committee of Medical Journal Editors Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2023/06/dic.2023-4-3-COI.pdf
Funding declaration: Development of the article, questionnaire, and all tasks related to organization of the virtual meeting were funded by A. Menarini Asia-Pacific Pte Ltd.
Correct attribution: Copyright © 2023_YANG X, Lohsiriwat V, Chang FCS, Chye TT, Howard CJ, Qiao L, Shaw SW, Tran TNA, Yung C, Dellosa D, Nagrale D. https://doi.org/10.7573/dic.2023-4-3. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids—a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35(2):171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 2.Gauglitz GG. Management of keloids and hypertrophic scars: current and emerging options. Clin Cosmet Investig Dermatol. 2013;6:103–114. doi: 10.2147/CCID.S35252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg. 2014;67(8):1017–1025. doi: 10.1016/j.bjps.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Poetschke J, Gauglitz GG. Current options for the treatment of pathological scarring. J Dtsch Dermatol Ges. 2016;14(5):467–477. doi: 10.1111/ddg.13027. [DOI] [PubMed] [Google Scholar]
- 5.Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel: a new treatment for hypertrophic scars. Surgery. 1989;106:781–787. [PubMed] [Google Scholar]
- 6.Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel for the prevention and treatment of hypertrophic scar. Arch Surg. 1991;126(4):499–504. doi: 10.1001/archsurg.1991.01410280103016. [DOI] [PubMed] [Google Scholar]
- 7.Fulton JE., Jr Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatol Surg. 1995;21:947–951. doi: 10.1111/j.1524-4725.1995.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 8.Mercer NSG. Silicone gel in the treatment of keloid scars. Br J Plastic Surg. 1989;42:83–87. doi: 10.1016/s0007-1226(89)90119-7. [DOI] [PubMed] [Google Scholar]
- 9.Chan KY, Lau CL, Adeeb SM, et al. A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg. 2005;116:1013–1020. doi: 10.1097/01.prs.0000178397.05852.ce. [DOI] [PubMed] [Google Scholar]
- 10.Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg. 2009;2(2):104–106. doi: 10.4103/0974-2077.58527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chernoff WG, Cramer H, Su-Huang S. The efficacy of topical silicone gel elastomers in the treatment of hypertrophic scars, keloid scars, and post-laser exfoliation erythema. Aesthetic Plast Surg. 2007;31(5):495–500. doi: 10.1007/s00266-006-0218-1. [DOI] [PubMed] [Google Scholar]
- 12.De Decker I, Hoeksema H, Verbelen J, et al. The use of fluid silicone gels in the prevention and treatment of hypertrophic scars: a systematic review and meta-analysis. Burns. 2022;48(3):491–509. doi: 10.1016/j.burns.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesth Plast Surg. 2008;32:82–92. doi: 10.1007/s00266-007-9030-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Choi TH, Liu W, et al. Update on scar management: guidelines for treating Asian patients. Plast Reconstr Surg. 2013;132(6):1580–1589. doi: 10.1097/PRS.0b013e3182a8070c. [DOI] [PubMed] [Google Scholar]
- 15.Farris PK. Topical vitamin C: a useful agent for treating photoaging and other dermatologic conditions. Dermatol Surg. 2005;31(7 Pt 2):814–817. doi: 10.1111/j.1524-4725.2005.31725. discussion 818. [DOI] [PubMed] [Google Scholar]
- 16.Yun IS, Yoo HS, Kim YO, Rah DK. Improved scar appearance with combined use of silicone gel and vitamin C for Asian patients: a comparative case series. Aesthetic Plast Surg. 2013;37(6):1176–1181. doi: 10.1007/s00266-013-0210-5. [DOI] [PubMed] [Google Scholar]
- 17.Momeni M, Hafezi F, Rahbar H, Karimi H. Effects of silicone gel on burn scars. Burns. 2009;35(1):70–74. doi: 10.1016/j.burns.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 18.van der Wal MB, van Zuijlen P, van de Ven P, et al. Topical silicone gel versus placebo in promoting the maturation of burn scars: a randomized controlled trial. Plast Reconstr Surg. 2010;126:524–531. doi: 10.1097/PRS.0b013e3181e09559. [DOI] [PubMed] [Google Scholar]
- 19.Chang CS, Wallace CG, Hsiao YC, et al. Clinical evaluation of silicone gel in the treatment of cleft lip scars. Sci Rep. 2018;8(1):7422. doi: 10.1038/s41598-018-25697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medhi B, Sewal RK, Kaman L, et al. Efficacy and safety of an advanced formula silicone gel for prevention of post-operative scars. Dermatol Ther. 2013;3(2):157–167. doi: 10.1007/s13555-013-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110(2):560–571. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 22.Bleasdale B, Finnegan S, Murray K, et al. The use of silicone adhesives for scar reduction. Adv Wound Care. 2015;4(7):422–430. doi: 10.1089/wound.2015.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]