Abstract
Large-scale deployment of proton exchange membrane (PEM) water electrolyzers has to overcome a cost barrier resulting from the exclusive adoption of platinum group metal (PGM) catalysts. Ideally, carbon-supported platinum used at cathode should be replaced with PGM-free catalysts, but they often undergo insufficient activity and stability subjecting to corrosive acidic conditions. Inspired by marcasite existed under acidic environments in nature, we report a sulfur doping–driven structural transformation from pyrite-type cobalt diselenide to pure marcasite counterpart. The resultant catalyst drives hydrogen evolution reaction with low overpotential of 67 millivolts at 10 milliamperes per square centimeter and exhibits no degradation after 1000 hours of testing in acid. Moreover, a PEM electrolyzer with this catalyst as cathode runs stably over 410 hours at 1 ampere per square centimeter and 60°C. The marked properties arise from sulfur doping that not only triggers formation of acid-resistant marcasite structure but also tailors electronic states (e.g., work function) for improved hydrogen diffusion and electrocatalysis.
A marcasite-type cobalt diselenide exhibits promise as an efficient cathode in proton exchange membrane water electrolyzers.
INTRODUCTION
Electrochemical splitting of water into green hydrogen (H2) fuels, especially driven by renewable electricity, offers an elegant path toward carbon-neutral energy society (1, 2). Since proposed in 1789, alkaline water electrolysis has been progressively developed as a matured technology for industrial H2 production, but its limited current density (high ohmic resistance), low partial load range, and low operating pressure are often drawbacks (3, 4). Proton exchange membrane (PEM) water electrolysis that relies on proton transfers can effectively surmount these issues in alkali, but the corrosive acidic environments require the use of expensive platinum group metal (PGM) catalysts, raising the stack cost (4–6). In present-day PEM electrolyzers, carbon-supported platinum (Pt/C) remains the catalyst of choice for the cathodic hydrogen evolution reaction (HER). Thanks to the fast HER kinetics in acid, the Pt loading at the cathode is typical 0.5 to 1.0 mgPt cm−2 per PEM electrolyzer (5), whose cost is lower than that of IrOx catalyst (~2 mgIr cm−2) used at the anode (4); thus, less previous research efforts have been made on the cost reduction at the cathode. While the Pt/C catalyst contributes small to the system cost nowadays, future large-scale H2 production at terawatt range would demand a substantial amount of Pt, which is not sustainable and hampers commercialization of industrial-grade PEM electrolyzers (6, 7).
To enable widespread prevalence of PEM electrolyzers, the Pt loading at the cathode should be substantially reduced or ideally the Pt catalyst should be replaced with PGM-free materials (6, 7). Over the past decade, PGM-free catalysts for the acidic HER have been well explored (6, 8–21), exemplified by the molybdenum disulfide (MoS2) catalyst inspired by nitrogenase enzyme (8–10). Subsequently, numerous transition metal dichalcogenides (11–15) and phosphides (16–20) were designed and synthesized to display decent activities in rotating disk electrode (RDE) measurements. Recently, King et al. (17) have demonstrated a very promising PEM electrolyzer using cobalt phosphide (CoP) as the HER catalyst, illustrating the practical relevance of PGM-free cathode (17). Despite big advances, under acidic environments, PGM-free materials generally show high propensity to chemical and structural changes, and even component dissolution, leading to decreased catalytic performances (4–6). To date, the design of PGM-free HER catalysts with exceptional activity and stability, particularly under realistic PEM electrolysis condition, remains a daunting challenge.
Besides MoS2, transition metals and chalcogens can form many other important groups of minerals in nature, for example, pyrite (11, 12, 22, 23) and marcasite (24, 25). We (22) and others (11, 23) have reported pyrite-type cobalt diselenide (CoSe2) to be promising catalyst for a variety of reactions with notable performances. Furthermore, we recently found that partial transformation of pyrite CoSe2 into marcasite structure enables enhanced HER stability in acidic electrolyte (13). Unfortunately, this pyrite-marcasite mixed CoSe2 catalyst still falls short of the requirement of practical PEM electrolysis. Yet, in nature, marcasite minerals often form under very acidic environments (26); moreover, the dissolution rate was experimentally observed to be more than 10-fold lower for marcasite than for pyrite (27). We therefore reason that marcasite-type CoSe2 might show the potential as cathode for PEM electrolyzers, given its corrosion-resistant feature in acid.
Here, we report a complete structural transformation from pyrite- to marcasite-type CoSe2 driven by sulfur doping. Not only creating marcasite structure with improved acid stability, but also the doping effect caused by partial substitution of Se with S also tailors electronic structures of the catalyst for more favorable hydrogen diffusion in the electrical double layer (EDL) and adsorption on the catalytic surface. The resultant sulfur-doped marcasite CoSe2 (M-CoSe2) as cathode was demonstrated to be active and stable in a practical PEM electrolyzer, highlighting potential of PGM-free catalysts for commercial-scale water electrolysis.
RESULTS
S doping–driven pyrite-to-marcasite transformation in CoSe2
Structurally, both pyrite and marcasite CoSe2 (P-CoSe2 and M-CoSe2, respectively) have Co atoms occurring in octahedral coordination and contain characteristic Se─Se pairs (28). In comparison to pyrite whose metal octahedra do not share edges with each other, the Co coordination octahedra in marcasite share two edges (29). P-CoSe2 in principle can be transformed into marcasite by rotating the Se─Se pairs through 90° (Fig. 1A) (29, 30). We thus sought to obtain pure marcasite structure by using our previously reported P-CoSe2 nanobelts (31), whereas only thermal annealing cannot overcome the high-energy barrier of the structural transformation (fig. S1). Heteroatom doping (32, 33) has been demonstrated as an effective means of triggering structural transformation and is also expected to tune the electronic structure. Moreover, given that the substitution of more polarization Se with S in CoSe2 has been described to result in greater covalency (34), we therefore chose S for doping to induce pyrite-to-marcasite transformation. Density functional theory (DFT) calculations predicted that the energy barrier for pyrite-to-marcasite transformation can be notably reduced when S doping was considered (Fig. 1B and see Materials and Methods).
Fig. 1. Pyrite-to-marcasite structural transformation.
(A) Atomic arrangements of P-CoSe2 (001) (left) and M-CoSe2 (101) (right). Arrows indicate the direction of inclination of Se─Se pairs. (B) Free energy diagram for the energetics of pyrite-to-marcasite transformation in CoSe2. Insets present the models of initial states (IS; P-CoSe2), transition states (TS), and final states (FS; M-CoSe2) without S (top) and with S (down) dopant. (C and E) HRTEM images of P-CoSe2 (C) along the [001] orientation and M-CoSe1.28S0.72 (E) along the [101] orientation. The inset in each image shows corresponding fast Fourier transform pattern. Scale bars 1 nm. (D and F) High-magnification HRTEM image of P-CoSe2 (D) and M-CoSe2 (F) from the marked area in (C) and (E), respectively. The inset in each image shows corresponding atomic model viewed along the same direction. (G and H) X-ray powder diffraction patterns (G) and Raman spectra (H) of P-CoSe2 and M-CoSe1.28S0.72. a.u., arbitrary unit. JCPDS, Joint Committee on Powder Diffraction Standards.
The S doping–driven pyrite-to-marcasite transformation in CoSe2 was realized by annealing freshly synthesized P-CoSe2 nanobelts with sublimed sulfur powder under an argon atmosphere (see Materials and Methods and fig. S2). Scanning electron microscopy (SEM) image shows that the belt-like morphology was well preserved after reaction (fig. S3); moreover, energy-dispersive x-ray spectrum elemental mapping exhibits uniform doping of S across the whole structure (figs. S4 and S5). The atomic lattice patterns of P-CoSe2 and M-CoSe2 were imaged using high-resolution transmission electron microscopy (HRTEM) along the [001] and [101] orientations, revealing cubic (Fig. 1C) and orthorhombic (Fig. 1E) structures, respectively. Corresponding Fourier transforms shown as insets of Fig. 1 (C and E) exhibit clear differences between the symmetries of the P-CoSe2 and M-CoSe2. The magnified images at atomic-resolution show continuous lattice without distortion and defects after structural transformation (Fig. 1, D and F). X-ray diffraction data in Fig. 1G confirm the structural change from cubic P-CoSe2 to orthorhombic M-CoSe2. After transformation, the typical Raman peak of P-CoSe2 at 189 cm−1 from Se─Se stretching mode was shifted to 167 cm−1, further verifying the formation of M-CoSe2 (Fig. 1H) (35). We note that this method enables tunable doping level of S in CoSe2 (figs. S6 to S12 and table S1), which potentially permits a controllable tailoring of the electronic structure. Furthermore, this S doping–driven structural transformation was observed to proceed very fast (within 1 min; fig. S11), indicative of low kinetic barriers of transformation caused by S doping.
Spectroscopic analysis
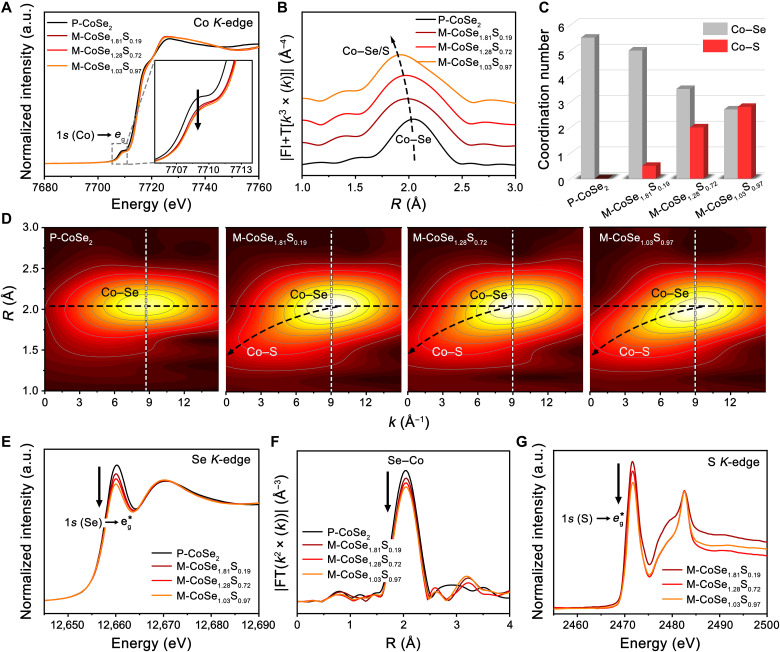
We studied the impact of S doping on the electronic and coordination structures of Co using x-ray absorption near-edge spectroscopy (XANES) at the Co K-edge. As Fig. 2A shows, both P-CoSe2 and M-CoSe2 with different levels of S doping exhibit a pre-edge peak at ~7709.2 eV originated from the Co intra-atomic 1s → eg transition (36). The existence of pre-edge feature after structural transformation indicates that Co cations remain in octahedral environments (36). However, the intensity of this peak decreases greatly after incorporating S into CoSe2 (inset in Fig. 2A), pointing to increased eg filling of Co cations (36). Extended x-ray absorption fine structure (EXAFS) reveals an obvious decrease in Co─Se bond distance, from 2.04 to 1.91 Å, with increasing S doping levels (Fig. 2B), which was caused by the formation of Co─S bonds whose distance is shorter than that of Co─Se bonds. By fitting the Co K-edge EXAFS (fig. S13 and table S2), we disclose a decrease in the first shell coordination number (CN) for Co─Se(from 5.5 to 2.7) and a monotonic increase in the CN for Co─S (from 0 to 2.8), as the S doping amount in the structure increases (Fig. 2C), suggesting highly tailored local coordination environments. Wavelet-transformed EXAFS shows that, compared with that in P-CoSe2, the Co─Se bonding in S-doped M-CoSe2 is extended to high-k direction (from 8.7 to 9 Å−1) (Fig. 2D), indicative of enhanced Co─Se covalency resulted from the doping of more electronegative S atoms (37). The wavelet transform analyses also show a gradually emerged Co─S scattering as the S doping level increases (Fig. 2D).
Fig. 2. Spectroscopic studies.
(A and B) Co K-edge XANES spectra (A) and corresponding Fourier transforms of k3-weighted EXAFS spectra (B) for P-CoSe2 and different S-doped M-CoSe2. FT, Fourier transform. (C) Coordination numbers in the first coordination shell of Co atoms for P-CoSe2 and different S-doped M-CoSe2 by fitting the Co K-edge EXAFS. (D) Wavelet transforms of k3-weighted EXAFS spectra of the Co K-edge for P-CoSe2 and different S-doped M-CoSe2. (E and F) Se K-edge XANES spectra (E) and corresponding Fourier transforms of k2-weighted EXAFS spectra (F) for P-CoSe2 and different S-doped M-CoSe2. (G) S K-edge XANES spectra of P-CoSe2 and different S-doped M-CoSe2.
The Se K-edge XANES spectra for P-CoSe2 and different S-doped M-CoSe2 all exhibit a sharp peak at ~12,660.1 eV (1s → eg* transition), whose intensity gradually decreased as the doping amount increases (Fig. 2E), suggesting low spatial overlap (hybridization) of Se 4p and Co 3d states (38). Se K-edge EXAFS data in Fig. 2F reveals that the bond intensity of Se─Co reduces as the S doping level increases, indicating decreased Se─Co coordination number. For the S K-edge, the intensity of S intra-atomic 1s → eg* transition peak (~2471.7 eV) was also observed to decrease as S doping increases (Fig. 2G), implying gradually lowered overlap of S 3p states and Co 3d orbitals (39). Overall, these spectroscopic studies reveal that S doping enables a controllable tailoring of the electronic structure and local coordination environment of CoSe2.
HER performance in acidic media
We assessed the HER performances of P-CoSe2 and different S-doped M-CoSe2 in Ar-saturated 0.5 M H2SO4 solution (pH 0.32) in a three-electrode setup, with a reference measurement of commercial Pt/C (20 wt % Pt on Vulcan XC72R carbon) for comparison (see Materials and Methods). Our series of measurements unveil that M-CoSe1.28S0.72 obtained at 400°C for 5 min shows the optimal property (figs. S14 to S16). By optimization, the catalyst loading on glassy carbon RDE was fixed at 1.0 mg cm−2 (fig. S17). Figure 3A shows that P-CoSe2 demands an overpotential of 268 mV at 10 mA cm−2 (normalized on the basis of geometrical surface area), which decreased to 67 mV for M-CoSe1.28S0.72, approaching to 28 mV over Pt/C catalyst. Tafel analysis in Fig. 3B yields a slope of 59, 50, and 32 mV dec−1 for P-CoSe2, M-CoSe1.28S0.72, and Pt/C catalysts, respectively. Moreover, electrochemical impedance spectroscopy (Fig. 3C and fig. S18) measured at 200-mV overpotential gives a charge transfer resistances (Rct) of 1.5 ohms for M-CoSe1.28S0.72, which is greatly smaller than that of 145.6 ohms for P-CoSe2, correlating to improved electron transfer from the M-CoSe1.28S0.72 surface to H+ ions (discharge process) (40). We further used gas chromatography to detect and quantify the H2 gas evolved from the M-CoSe1.28S0.72–docerated carbon paper electrode at 10, 100, and 500 mA cm−2. The measured H2 product at the three current densities all matches well with the theoretical values, corresponding to Faradaic efficiency of ~100% (fig. S19).
Fig. 3. HER performance.
(A and B) HER polarization curves (A) and Tafel plots (B) of P-CoSe2, M-CoSe1.28S0.72 and commercial Pt/C. Catalyst loading: ~1.00 mg cm−2. Sweep rate: 2 mV s−1. Rotation rate: 1600 rpm. (C) Electrochemical impedance spectra of P-CoSe2 and M-CoSe1.28S0.72. (D) pH-dependent HER polarization curves at a rotation rate of 1600 rpm and a sweep rate of 2 mV s−1 for M-CoSe1.28S0.72. (E) Free energy diagrams for hydrogen adsorption at different sites on the P-CoSe2, M-CoSe1.28S0.72, and M-CoSe1.03S0.97. (F) Chronopotentiometry (E ~ t) recorded on P-CoSe2 and M-CoSe1.28S0.72 catalysts at a constant current density of 10 mA cm−2. (G) Comparison of overpotential at 10 mA cm−2 and operating lifetime of M-CoSe1.28S0.72 with that of reported catalysts in acidic electrolytes. NDs, nanodots. NF, nanofoam. (H) Polarization curves of the PEM electrolysers with P-CoSe2, M-CoSe1.28S0.72, and commercial Pt/C as cathode catalysts. (I) Chronopotentiometry curve of the PEM electrolyser with M-CoSe1.28S0.72 as cathode and commercial IrO2 as anode operated at 1A cm−2 and 60°C. Insets show photographs of the PEM electrolyser device.
We demonstrate that the HER activity of M-CoSe1.28S0.72 is largely dependent of the H+ ion concentration. The pH-dependent experiments reveals that HER rate decreases with increasing the pH (Fig. 3D). For pH 2.5 to 4, diffusion-limiting currents are seen, which indicate that the HER on M-CoSe1.28S0.72 surface is controlled by the mass transport of H+ ions, unveiling the critical role of efficient proton transfer in the interfacial EDL (41). To probe mechanistic insights into the observed HER performance, we calculated the hydrogen adsorption free energy (ΔGH), showing that M-CoSe1.28S0.72 at Co sites has the smallest ΔGH value of 0.05 eV, close to the thermoneutral ΔGH ≈ 0 (Fig. 3E and figs. S20 to S22). We thus regard the enhanced HER over M-CoSe1.28S0.72 to the Co sites, whose coordination environment and electronic structure were optimized by S dopants.
We further examined the long-term stability—an even more formidable challenge for PGM-free catalysts in acidic electrolytes. We evaluated the electrochemical stability of M-CoSe1.28S0.72 on carbon paper at 10 mA cm−2 in 0.5 M H2SO4 solution. As Fig. 3F shows, the M-CoSe1.28S0.72 catalyst performs stably, exhibiting no sign of improvement in overpotential after 1000 hours of continuous operation. By stark contrast, the P-CoSe2 undergoes a rapid degradation owing to the dissolution of Co atoms into the acidic electrolyte (fig. S23). The composition and electronic states of M-CoSe1.28S0.72 after the stability test were well retained (figs. S24 to S26). We also compared the performance metrics of our M-CoSe1.28S0.72 with those previously reported in terms of overpotential at 10 mA cm−2 and operating lifetime. Under similar testing conditions, the results obtained over M-CoSe1.28S0.72 greatly surpass prior PGM-free catalysts achieved by other approaches (Fig. 3G and table S3).
The high HER activity and stability motivated us to explore the performance of M-CoSe1.28S0.72 in realistic PEM electrolyzers. We thus incorporated the M-CoSe1.28S0.72 catalyst in the cathode and commercial IrO2 in the anode of a 4-cm2 PEM electrolyzer using a Nafion 115 PEM (see Materials and Methods for details). We fed pure water instead of aqueous H2SO4 from the viewpoint of practical use despite its larger ohmic loss. The polarization curves obtained at 60°C show that M-CoSe1.28S0.72 requires a cell voltage of 1.79 V at 1 A cm−2, far exceeding that of P-CoSe2 catalyst (Fig. 3H). Intriguingly, this voltage is mere 110 mV higher than that of using Pt/C cathode. By assuming that all metal atoms are HER active, we estimated the turnover frequency (TOF) of M-CoSe1.28S0.72 to be 0.95 H2 s−1 (fig. S27). If only the surface atoms (~2.5%) are considered, then the TOF of M-CoSe1.28S0.72 is estimated as 38.4 H2 s−1 (fig. S27). Despite these metrics still compare inferior to commercial Pt/C, our results indeed suggest great application potential of the M-CoSe1.28S0.72 cathode. The application potential of M-CoSe1.28S0.72 catalyst can be further demonstrated by the notable stability of the PEM electrolyzer, showing no appreciable voltage increase over 410 hours of continuous testing at 1 A cm−2 (Fig. 3I). In addition, the catalyst loss after long-term operation is mere ~2% by the weight (fig. S28). To our best knowledge, this represents one of the most promising PGM-free cathode catalyst that has shown both high activity and stability in a realistic PEM electrolyzer (table S4).
Mechanistic investigation
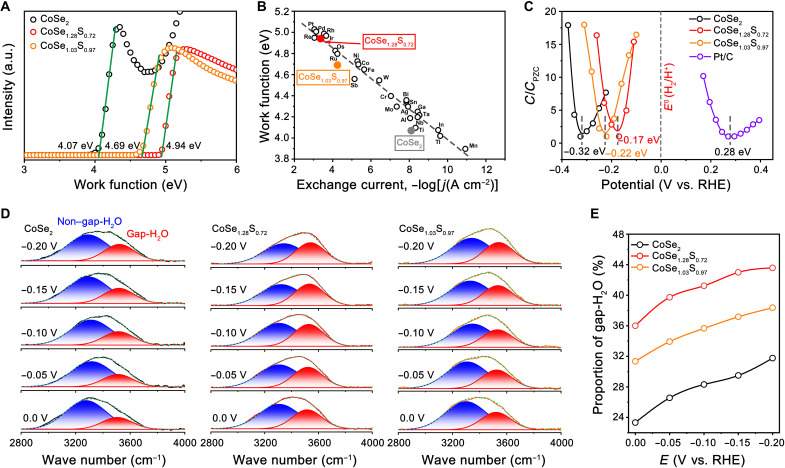
To obtain mechanistic insights into the enhanced acidic HER performance of M-CoSe1.28S0.72, work function (WF) and potential of zero charge (PZC) of three selected catalysts (i.e., P-CoSe2, M-CoSe1.28S0.72, and M-CoSe1.03S0.97) were measured and analyzed. We studied catalysts’ WFs because this electronic property has been reported to show a linear correlation with HER reactivity in acidic electrolytes (42, 43). Moreover, catalysts’ WFs were also found to be positively correlated to the PZC in acid (44), which could lead to variation in interfacial environment and consequently altered HER kinetics (45, 46). We measured the WFs using ultraviolet photoelectron spectroscopy (UPS) and observed an increase in WF from 4.07 eV for P-CoSe2 to 4.94 eV for M-CoSe1.28S0.72 (Fig. 4A). Further increasing S doping level to M-CoSe1.03S0.97, however, results in a reduced WF value of 4.69 eV. We plotted the measured WFs as a function of exchange current densities for different catalysts and find that HER intrinsic activity increases linearly with WF, in agreement with the observation reported by Trasatti for metals (42) (Fig. 4B). The PZC, which was measured by Gouy-Chapman capacitance method (47), increases from −0.32 V for P-CoSe2 to −0.17 V for M-CoSe1.28S0.72, which decreases to −0.22 V by further raising S doping amount to M-CoSe1.03S0.97 (Fig. 4C), showing similar trend observed in WF measurements. By comparison, PZC was measured to be 0.28 V for Pt/C catalyst, reasonably agreeing with the literature data (45).
Fig. 4. Electronic property.
(A) UPS spectra of the P-CoSe2, M-CoSe1.28S0.72, and M-CoSe1.03S0.97. (B) Linear relationship between the WFs and the exchange current densities of different catalysts. The original data of different metals are taken from the report of Trasatti (42). (C) PZC values measured for P-CoSe, M-CoSe1.28S0.72, M-CoSe1.03S0.97, and the Pt/C catalyst. (D) In situ SEIRAS spectra recorded at potentials from 0 to −0.2 V on P-CoSe2 (left), M-CoSe1.28S0.72 (middle), and M-CoSe1.03S0.97 (right) in Ar-saturated 0.5 M H2SO4. (E) Corresponding proportion of gap-H2O molecules in the EDL of the P-CoSe2, M-CoSe1.28S0.72, and M-CoSe1.03S0.97 catalysts.
Generally, PZC has a direct impact on the interfacial electrical field, which, in turn, affects the interfacial water configuration and consequently the proton diffusion in the EDL (45, 46). Very recently, by combining ab initio molecular dynamic simulation and in situ surface-enhanced infrared adsorption spectroscopy (SEIRAS), Li et al. (46) described that water distribution and the connectivity of H-bond networks in the EDL mostly determine the reactivity of hydrogen electrocatalysis. To characterize interfacial water configuration in the EDL, we performed in situ SEIRAS spectra on the studied catalysts at different biases in Ar-saturated 0.5 M H2SO4 electrolyte (see Materials and Methods and fig. S29). As shown in Fig. 4D, the SEIRAS spectra of all catalysts exhibit asymmetric peaks spanned a broad frequency range from ~2900 to ~3800 cm−1, corresponding to the O─H stretching band of interfacial water (46, 48). Using Gaussian fitting, the O─H stretching peaks could be consistently deconvoluted into two components representing water molecules in gap region (gap-H2O at ~3540 cm−1) and other interfacial water molecules (non–gap-H2O at ~3357 cm−1), agreeing with prior report (46). Figure 4E reveals that, for the M-CoSe1.28S0.72 electrode, the proportion of gap-H2O molecules exceeds that of P-CoSe2 and M-CoSe1.03S0.97 electrodes at all potentials examined. This observation could be the result of its PZC closer to the equilibrium potential (Fig. 4C), which barely influenced the interfacial electrical field and resulted in negligible perturbation of the H-bond networks in the EDL, thus facilitating hydrogen diffusion for HER (45, 46).
To understand how S doping tunes the electronic structure (i.e., WF and consequent PZC) of CoSe2, we further performed Co L-edge x-ray absorption spectroscopy (XAS). From Fig. 5A, we observed two main peaks that were attributed to the transitions of Co 2p3/2 (L3 edge) and 2p1/2 core (L2 edge) electrons to unoccupied eg orbital (49), complementary to above Co K-edge results. In comparison to P-CoSe2, the intensity of L3 edge decreases gradually as the S doping level increases. Because no oxidation state change of Co was detected by x-ray photoelectron spectroscopy (XPS) (fig. S30), the intensity reduction should be ascribed to the spin-state transition of Co ions from low-spin (LS) to high-spin (HS) states after S incorporation (50). To investigate this, we carried out temperature-dependent magnetizations with a magnetic field of H = 1 kOe under field-cooling procedures, which enables us to probe the spin structures of Co ions. As Fig. 5B shows, the susceptibility, χ, derived from the magnetizations follows Curie-Weiss behavior above 250 K. By fitting the linear regions, we obtained effective magnetic moment μeff and the consequent unpaired electron for each sample (see Materials and Methods for details, fig. S31, and table S5). We find that the number of unpaired electron increases with increasing S doping amount (Fig. 5B, inset). For P-CoSe2, the number was calculated to be 1.03, reasonably matching with its LS state (t2g6eg1) reported in literature (51). The number increases to 2.03 for M-CoSe1.28S0.72 and 2.98 for M-CoSe1.03S0.97, which indicates a gradual transition from LS to HS (t2g5eg2), consistent with the XAS results. We further find that the HER activities of studied catalysts exhibit a volcano relationship as a function of the unpaired electron number, where the activities are compared in terms of the overpotential at 10 mA cm−2 (Fig. 5C). Among these catalysts, M-CoSe1.28S0.72 with unpaired electron number of 2.03 sits at the top of the volcano.
Fig. 5. Enhancement mechanism.
(A) Co L-edge XAS of P-CoSe2 and different S-doped M-CoSe2. (B) Temperature-dependent susceptibilities for P-CoSe2 and different S-doped M-CoSe2. Inset shows the corresponding unpaired d electron number. (C) Volcano relationship between the HER activity and the unpaired d-electron number of different catalysts. (D and E) Crystal structures (D) and schematic energy band diagrams (E) of the P-CoSe2 (left), M-CoSe1.28S0.72 (middle), and M-CoSe1.03S0.97 (right) catalysts. (F) Schematic illustration of the electronic coupling between H and Co in P-CoSe2 (left), M-CoSe1.28S0.72 (middle), and M-CoSe1.03S0.97 (right) during Volmer reaction.
The above studies reveal that S doping enables fine modulation of the electron filling between eg and t2g orbitals, thus tailoring the electronic structure for enhanced HER. In P-CoSe2 structure, the Co ions bind with six Se atoms in an octahedral CoSe6 coordination via hybridization of Co 3d and Se 4p orbitals (Fig. 5D) (29, 30). As analyzed by Co K-edge EXAFS above, the addition of S monotonously increased the Co─S coordination number (CNCo─S = 0 to 2.8) as the doping amount increased, yielding altered octahedral coordination environment (Fig. 5D). Because the bandwidth of S 3p is narrower than that of Se 4p states (52) (fig. S32) and partially replacing Se with S leads to increased Se─Se/S bond distance and consequently a decrease in the Se─Se/S bonding-antibonding splitting (53). This will caused an attenuated orbital overlap between Co d and Se/S p orbitals, which makes the eg orbital moves closer to the Fermi energy EF (Fig. 5E) (34). As a result, t2g electron becomes readily to transfer into eg orbital, therefore mediating the spin states.
Furthermore, the downshift of eg orbital toward the Fermi level after S doping can also result in enhanced electron-nucleus interaction, which gives rise to increased WF (42, 54), consistent with our WF measurement in Fig. 4A. We emphasize that much higher S doping (e.g., M-CoSe1.03S0.97), however, leads to decreased WF because of forming HS state caused by further reduced ligand-field splitting energy (55), as schematically shown in Fig. 5E. Owing to the positive correlation between WF and PZC in acid (44), the above results together explain why M-CoSe1.28S0.72 has the largest WF and PZC as measured experimentally.
On the basis of above results, we interpret the optimal HER performance over M-CoSe1.28S0.72 as the improved WF and PZC. In acidic electrolytes, the superior WF of M-CoSe1.28S0.72 relative to P-CoSe2 and M-CoSe1.03S0.97 reflects its strongly bound nature of surface electrons, which attracts a high local H+ ion concentration near the electrode and thus an enhanced HER kinetics. This finding is consistent with the work of Trasatti (42, 44), which exhibited that greater WF can lead to higher exchange current for HER on metals. Meanwhile, the large PZC of M-CoSe1.28S0.72 that approaches the equilibrium potential suggests less disturbed interfacial electrical field and thereby a better connectivity of H-bond networks, benefiting H+ ion diffusion toward the catalyst surface (45, 46). When arriving at the reaction surface, H+ first demands a d-electron pair to adhere. After that, it experiences a discharge process and then the resultant adatom requires an empty semi-d orbital to form σ-bonding *H (Volmer reaction) (56). Compared with P-CoSe2, the M-CoSe1.28S0.72 catalyst contains more exposed d-electron pairs and empty semi-d–orbital sites, thereby giving superior HER rate (Fig. 5F). Regarding M-CoSe1.03S0.97, the substantial downshift of eg orbital toward the Fermi level, however, causes too strong *H adsorption and therefore limited HER kinetics (Fig. 5F and fig. S33).
DISCUSSION
In summary, we have demonstrated a complete pyrite-to-marcasite structural transformation in CoSe2 induced by sulfur doping. The obtained M-CoSe1.28S0.72 catalyst shows outstanding HER activity and stability in acidic electrolyte. Moreover, a PEM electrolyzer assembled with this catalyst as cathode can be stably operated at 1 A cm−2 for over 410 hours, suggesting potential practical use. The high operating stability of our catalyst can be explained by a greater covalency of Co─Se(S) bonds in marcasite caused by sulfur doping. In addition, this doping also suitably tailors electronic structures (i.e., WF and consequent PZC) of the catalyst, leading to an improved proton transfer in EDL, and a more favorable hydrogen adsorption for enhanced HER. While our PEM performance still compares inferior to the platinum-based counterpart, this work opens up the possibility of developing low-cost PEM electrolyzers that make use of PGM-free materials as electrode catalysts. Future effort should focus on further improving the activity and stability of the PGM-free catalysts, aiming to make future terawatt-scale PEM electrolysis a reality.
MATERIALS AND METHODS
Material synthesis
All chemicals were used as received without further purification. The M-CoSe1.28S0.72 was prepared through a two-step method. First, P-CoSe2 nanobelts were synthesized as described in our previous work (31). Then, 50 mg of fresh P-CoSe2 nanobelts and 0.5 g of sublimated sulfur powder were placed at two ceramic boats, respectively, while sublimated sulfur powder is at the upstream side. The samples were heated at 400°C for 5 min with a heating rate of 2°C min−1 in Ar atmosphere. The synthetic temperature, time and the mass of sublimated sulfur powder, as well as the obtained products were summarized in table S1. All the obtained samples were carefully washed and dried before use.
Material characterizations
X-ray powder diffraction (XRD) was obtained from a Japan Rigaku DMax-γA rotation anode x-ray diffractometer equipped with graphite monochromatized Cu-K radiation. The morphology of the samples was achieved by SEM (Zersss Supra 40) and TEM (Hitachi H7700). HRTEM measurements and energy-dispersive spectroscopy (EDS) mappings were carried out using FEI Talos F200X, equipped with Super X-EDS system (four systematically arranged windowless silicon drift detectors) at 200 kV. Raman spectra analysis carried out with a LABRAM-HR confocal laser micro-Raman spectrometer with a wavelength of 532 nm. XPS data were gathered using an ESCALAB-MKII x-ray photoelectron spectrometer with Mg Kα radiation as an exciting source (Mg Kα = 1253.6 eV). UPS was obtained at the BL11U beamline of National Synchrotron Radiation Laboratory in Hefei (China). The x-ray absorption spectra of Co L-edges were taken on the BL10B beamline of National Synchrotron Radiation Laboratory in Hefei (China). The x-ray absorption spectra of Co and Se K-edges were carried out at the beamline 1W1B station of Beijing Synchrotron Radiation Facility (China), and the S K-edges were performed at the beamline 4B7A station of Beijing Synchrotron Radiation Facility (China). Inductively coupled plasma atomic emission spectroscopy data were investigated by an Optima 7300 DV instrument.
DFT calculations
The DFT calculations were performed by Vienna ab initio simulation package (57) program with projector augmented wave method, and the kinetic energy cutoff was set to be 500 eV. The convergence criterion for the electronic self-consistent iteration was set to be 10−7 eV. The atomic positions were fully relaxed until the force on each atom is less than 0.02 eV Å−1. The Perdew-Burke-Ernzerhof (58)–generalized gradient approximation exchange-correlation functional was used throughout. The slab model of P-CoSe2 (001) surface and M-CoSe2 (101) surface were constructed on the basis of the optimized crystal structure, and the S atoms were located at M-CoSe2 (101) surface to consider the effect of the doped S. The vacuum layer was set to be 15 Å to ensure the separation between slabs. To study the rotation of the Se─Se bond, its energy barriers were determined by using the climbing image nudged elastic band method. The Gibbs free energy is calculated by
where EDFT is the total energy from the DFT calculation. EZPE is the zero-point energy, S is the entropy, and T is the temperature.
Electrochemical measurements
All the electrochemical measurements were performed in a standard three-electrode cell at ambient temperature connected to a VSP-300 Potentiostat (BioLogic, France). Ag/AgCl (3.5 M KCl) electrode and graphite rod were used as the reference and counter electrodes, respectively. The potentials reported in this work were normalized versus the RHE through a standard RHE calibration (E = EAg/AgCl + 0.20 V). An RDE with glassy carbon (Pine Research Instrumentation, 5.00 mm in diameter and disk area of 0.196 cm2) was used as the working electrode.
To make the working electrodes, 5 mg of catalyst powder was dispersed in 1 ml of 1:3 (v/v) isopropanol/deionzed water (DIW) mixture with 20 μl of Nafion solution (5 wt %), which was ultrasonicated to yield a homogeneous ink. Then, 40 μl of catalyst ink was pipetted onto the glassy carbon disk to ensure the catalyst loading of ~1.0 mg cm−2. The fresh electrolytes (0.5 M H2SO4) were bubbled with pure argon for 30 min before measurements. The HER polarization curves were recorded at a sweep rate of 2 mV s−1 and 1600 rpm (to remove the H2 bubbles formed in situ) at ambient temperature. The electrochemical impedance spectroscopy measurement was performed in the same configuration at 200-mV overpotential over a frequency range from 100 KHz to 100 mHz at the amplitude of the sinusoidal voltage of 5 mV. The polarization curves were replotted as overpotential (η) versus log current (log j) to get Tafel plots to assess the HER kinetics of investigated catalysts. The Tafel slope (b) can be obtained by fitting the linear portion of the Tafel plots to the Tafel equation [η = b log (j) + a]. The exchange current density (j0) were calculated from Tafel curves using extrapolation method.
The M-CoSe1.28S0.72 modified carbon paper (catalyst loading: ~1.0 mg cm−2) was used as working electrode to conduct chronopotentiometry experiments at a constant current density of 10 mA cm−2. To estimate the double-layer capacitance, cyclic voltammograms were measured at different sweep rates in the potential region of 0 to 0.1 V versus RHE at ambient temperature. All the polarization curves were corrected with iR compensation that resulted from the solution resistance.
PEM water electrolyzer measurements
To prepare the catalyst ink, the catalyst and the Nafion solution (5 wt %) was dispersed in ethanol, followed by ultrasonicated to yield a homogeneous ink. To prepare the catalyst-coated membrane (CCM), the anode (IrO2) and cathode (M-CoSe1.28S0.72) catalysts are sprayed onto sheets of polytetrafluoroethylene (PTFE), respectively. After that, the PTFE-supported cathode catalysts, Nafion 115, and PTFE-supported anode catalysts are hot pressed together at 135°C for 3 min under a pressure of 2 T. After cooling, the PTFE on the surface were carefully peeled off to get the CCM with an electrode area of 4 cm−2. The final catalyst loading was 3.0 mg cm−2 for the anode and 1.0 mg cm−2 on the cathode. The CCM prepared was preserved in distilled water for further measurements.
To construct the PEM electrolyzer for performance evaluation, a titanium felt with Pt coating was used as the porous transport layers (PTLs) in the anode. For the cathode, a titanium felt without Pt coating is used as the PTL. The PEM electrolyzers were operated at 60°C. Deionized water was used as reactant for the constructed PEM electrolyzer, which was supplied through peristaltic pump circulation.
In situ SEIRAS experiments
The Au film was firstly prepared for SEIRAS. Briefly, the silicon prism was washed by aqua regia solution. Then, the silicon prism was polished with a 0.05-μm Al2O3 slurry, followed by ultrasound in acetone and DIW, respectively. After that, the silicon prism was dried, soaking 120 s in the NH4F solution. Last, the silicon prism was immersed in a mixture of gold-plated solution and 2 wt % HF aqueous solution under 55°C water bath for 7 min to chemically deposit gold film. After deposition, the gold film on silicon prism was washed by DIW. Next, the working electrodes were prepared via dropping ink onto the obtained gold film. Briefly, 10 mg of catalyst powder was dispersed in 1 ml of isopropanol, which was ultrasonicated to yield a homogeneous ink. Then, 200 μl of catalyst ink was uniformly pipetted onto the Au film. Last, the obtained working electrode was dried naturally for use.
In situ SEIRAS tests were carried in a spectroelectrochemical cell with a three-electrode configuration. A graphite rod was used as the counter electrode and an Ag/AgCl (3.5 M KCl) electrode as the reference electrode. The cell was integrated into a NICOLET iS50 Fourier transform infrared spectrometer equipped with a liquid nitrogen–cooled mercury cadmium telluride (MCT) detector. During the in situ SEIRAS tests, Ar was kept bubbling into the electrolyte. In addition, each spectrum consisted of 32 single beams with a resolution of 4 cm−1.
Temperature-dependent magnetization measurements
The temperature-dependent magnetization (M) measurements were carried out with a Magnetic Property Measurement System Superconducting Quantum Interference Device (MPMS SQUID) magnetometer and under magnetic field strength (H) of 1 kOe for all the samples. The effective magnetic moments (μeff) for all the samples were obtained via μeff = (8C)1/2 × μB, wherein μB is Bohr magneton and C is Curie constant and is obtained by fitting the susceptibility (χ−1 = M/H) above 250 K via Curie-Weiss law. The fractions of Co ions in HS and LS states are calculated from the relationship: μeff = g × μB × [SHS × (SHS + 1) × VHS + SLS × (SLS + 1) × VLS]1/2, where SHS = 3 and SLS = 1. VHS and VLS (= 1 − VHS) represent to the fractions for Co2+ with HS and LS states, respectively. Consequently, the number of unpaired electron (x) is calculated via x = SHS × VHS + SLS × VLS.
Acknowledgments
Funding: This work was supported by the National Basic Research Program of China (grant 2018YFA0702001), the National Natural Science Foundation of China (grants 22225901, 21975237, and 22175162), the Anhui Provincial Research and Development Program (grant 202004a05020073), the Fundamental Research Funds for the Central Universities (grant WK2340000101), the USTC Research Funds of the Double First-Class Initiative (grant YD2340002007), and the Open Funds of the State Key Laboratory of Rare Earth Resource Utilization (grant RERU2022007).
Author contributions: M.-R.G. supervised the project. X.-L.Z. performed the experiments and collected and analyzed the data. X.-Z.S. performed the XAFS measurements. S.-J.H. carried out the DFT calculations. L.S. performed the HRTEM measurements. P.-C.Y. and Y.-H.W. performed the SEM measurements. P.-P.Y., F.-Y.G., Z.-Z.W., L.-P.C., and Y.-R.Z. helped with electrochemical data collection and analysis. M.-R.G. and X.-L.Z. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S33
Tables S1 to S5
References
REFERENCES AND NOTES
- 1.S. Chu, A. Majumdar, Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012). [DOI] [PubMed] [Google Scholar]
- 2.E. Taibi, R. Miranda, W. Vanhoudt, T. Winkel, J.-C Lanoix, F. Barth, Hydrogen from renewable power: Technology outlook for the energy transition (International Renewable Energy Agency, 2018). [Google Scholar]
- 3.A. Buttler, H. Spliethoff, Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 82, 2440–2454 (2018). [Google Scholar]
- 4.M. Carmo, D. L. Fritz, J. Mergel, D. Stolten, A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 38, 4901–4934 (2013). [Google Scholar]
- 5.M. Bernt, A. Hartig-Weiß, M. F. Tovini, H. A. el-Sayed, C. Schramm, J. Schröter, C. Gebauer, H. A. Gasteiger, Current challenges in catalyst development for PEM water electrolyzers. Chem. Ing. Tech. 92, 31–39 (2020). [Google Scholar]
- 6.X. Sun, K. Xu, C. Fleischer, X. Liu, M. Grandcolas, R. Strandbakke, T. Bjørheim, T. Norby, A. Chatzitakis, Earth-abundant electrocatalysts in proton exchange membrane electrolyzers. Catalysts 8, 657 (2018). [Google Scholar]
- 7.L. Bertuccioli, A. Chan, D. Hart, F. Lehner, B. Madden, E. Standen, Development of water electrolysis in the European Union (Fuel Cells and Hydrogen Joint Undertaking, 2014).
- 8.H. I. Karunadasa, E. Montalvo, Y. Sun, M. Majda, J. R. Long, C. J. Chang, A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 335, 698–702 (2012). [DOI] [PubMed] [Google Scholar]
- 9.T. F. Jaramillo, K. P. Jørgensen, J. Bonde, J. H. Nielsen, S. Horch, I. Chorkendorff, Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007). [DOI] [PubMed] [Google Scholar]
- 10.H. Wang, Z. Lu, S. Xu, D. Kong, J. J. Cha, G. Zheng, P.-C. Hsu, K. Yan, D. Bradshaw, F. B. Prinz, Y. Cui, Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. U.S.A. 110, 19701–19706 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D. Kong, H. Wang, Z. Lu, Y. Cui, CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 136, 4897–4900 (2014). [DOI] [PubMed] [Google Scholar]
- 12.D.-Y. Wang, M. Gong, H.-L. Chou, C.-J. Pan, H.-A. Chen, Y. Wu, M.-C. Lin, M. Guan, J. Yang, C.-W. Chen, Y.-L. Wang, B.-J. Hwang, C.-C. Chen, H. Dai, Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets–carbon nanotubes for hydrogen evolution reaction. J. Am. Chem. Soc. 137, 1587–1592 (2015). [DOI] [PubMed] [Google Scholar]
- 13.X.-L. Zhang, S. J. Hu, Y. R. Zheng, R. Wu, F. Y. Gao, P. P. Yang, Z. Z. Niu, C. Gu, X. Yu, X. S. Zheng, C. Ma, X. Zheng, J. F. Zhu, M. R. Gao, S. H. Yu, Polymorphic cobalt diselenide as extremely stable electrocatalyst in acidic media via a phase-mixing strategy. Nat. Commun. 10, 5338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.M. A. Lukowski, A. S. Daniel, C. R. English, F. Meng, A. Forticaux, R. J. Hamers, S. Jin, Highly active hydrogen evolution catalysis from metallic WS2 nanosheets. Energ. Environ. Sci. 7, 2608–2613 (2014). [Google Scholar]
- 15.J. Yang, A. R. Mohmad, Y. Wang, R. Fullon, X. Song, F. Zhao, I. Bozkurt, M. Augustin, E. J. G. Santos, H. S. Shin, W. Zhang, D. Voiry, H. Y. Jeong, M. Chhowalla, Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution. Nat. Mater. 18, 1309–1314 (2019). [DOI] [PubMed] [Google Scholar]
- 16.M. Cabán-Acevedo, M. L. Stone, J. R. Schmidt, J. G. Thomas, Q. Ding, H. C. Chang, M. L. Tsai, J. H. He, S. Jin, Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 14, 1245–1251 (2015). [DOI] [PubMed] [Google Scholar]
- 17.L. A. King, M. K. A. Hubert, C. Capuano, J. Manco, N. Danilovic, E. Valle, T. R. Hellstern, K. Ayers, T. F. Jaramillo, A non-precious metal hydrogen catalyst in a commercial polymer electrolyte membrane electrolyser. Nat. Nanotechnol. 14, 1071–1074 (2019). [DOI] [PubMed] [Google Scholar]
- 18.E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis, R. E. Schaak, Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 135, 9267–9270 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Y. Xu, R. Wu, J. Zhang, Y. Shi, B. Zhang, Anion-exchange synthesis of nanoporous FeP nanosheets as electrocatalysts for hydrogen evolution reaction. Chem. Commun. 49, 6656–6658 (2013). [DOI] [PubMed] [Google Scholar]
- 20.J. Cai, Y. Song, Y. Zang, S. Niu, Y. Wu, Y. Xie, X. Zheng, Y. Liu, Y. Lin, X. Liu, G. Wang, Y. Qian, N-induced lattice contraction generally boosts the hydrogen evolution catalysis of P-rich metal phosphides. Sci. Adv. 6, eaaw8113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.M. Chatenet, B. G. Pollet, D. R. Dekel, F. Dionigi, J. Deseure, P. Millet, R. D. Braatz, M. Z. Bazant, M. Eikerling, I. Staffell, P. Balcombe, Y. Shao-Horn, H. Schäfer, Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 51, 4583–4762 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.M.-R. Gao, Y.-R. Zheng, J. Jiang, S.-H. Yu, Pyrite-type nanomaterials for advanced electrocatalysis. Acc. Chem. Res. 50, 2194–2204 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Y. Dou, C. T. He, L. Zhang, H. Yin, M. Al-Mamun, J. Ma, H. Zhao, Approaching the activity limit of CoSe2 for oxygen evolution via Fe doping and Co vacancy. Nat. Commun. 11, 1664 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.L. Liang, H. Cheng, F. Lei, J. Han, S. Gao, C. Wang, Y. Sun, S. Qamar, S. Wei, Y. Xie, Metallic single-unit-cell orthorhombic cobalt diselenide atomic layers: Robust water-electrolysis catalysts. Angew. Chem. Int. Ed. 54, 12004–12008 (2015). [DOI] [PubMed] [Google Scholar]
- 25.J. A. Tossell, D. J. Vaughan, J. K. Burdett, Pyrite, marcasite, and arsenopyrite type minerals - crystal chemical and structural principles. Phys. Chem. Miner. 7, 177–184 (1981). [Google Scholar]
- 26.J. R. Craig, F. M. Vokes, The metamorphism of pyrite and pyritic ores: An overview. Mineral. Mag. 57, 3–18 (1993). [Google Scholar]
- 27.M. P. C. Asta, P. Acero, Dissolution kinetics of marcasite at acidic pH. Eur. J. Mineral. 22, 49–61 (2010). [Google Scholar]
- 28.R. Sun, M. K. Y. Chan, G. Ceder, First-principles electronic structure and relative stability of pyrite and marcasite: Implications for photovoltaic performance. Phys. Rev. B 83, 235311 (2011). [Google Scholar]
- 29.G. Brostigen, A. Kjekshus, On the relationships between the structure types pyrite, marcasite, and arsenopyrite. Acta Chem. Scand. 24, 2983–2992 (1970). [Google Scholar]
- 30.I. Dodony, M. Pósfai, A. R. Buseck, Structural relationship between pyrite and marcasite. Am. Mineral. 81, 119–125 (1996). [Google Scholar]
- 31.M.-R. Gao, W.-T. Yao, H.-B. Yao, S.-H. Yu, Synthesis of unique ultrathin lamellar mesostructured CoSe2−amine (protonated) nanobelts in a binary solution. J. Am. Chem. Soc. 131, 7486–7487 (2009). [DOI] [PubMed] [Google Scholar]
- 32.X. Sun, Z. Wang, Z. Li, Y. Q. Fu, Origin of structural transformation in mono- and Bi-layered molybdenum disulfide. Sci. Rep. 6, 26666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.S. Chen, Z. Wang, L. Fan, Y. Chen, H. Ren, H. Ji, D. Natelson, Y. Huang, J. Jiang, C. Zou, Sequential insulator-metal-insulator phase transitions of VO2 triggered by hydrogen doping. Phys. Rev. B 96, 125130 (2017). [Google Scholar]
- 34.V. Johnson, A. Wold, Crystal growth and magnetic properties of compositions in the CoS2:CoSe2 system. J. Solid State Chem. 2, 209–217 (1970). [Google Scholar]
- 35.E. Anastassakis, C. H. Perry, Light scattering and ir measurements in XS2 pryite-type compounds. J. Chem. Phys. 64, 3604–3609 (1976). [Google Scholar]
- 36.M. Sano, XANES study at the Co-K absorption edge in a series of cobalt(III) complexes. Inorg. Chem. 27, 4249–4253 (1988). [Google Scholar]
- 37.S. Kong, X. Lv, X. Wang, Z. Liu, Z. Li, B. Jia, D. Sun, C. Yang, L. Liu, A. Guan, J. Wang, G. Zheng, F. Huang, Delocalization state-induced selective bond breaking for efficient methanol electrosynthesis from CO2. Nat. Catal. 6, 6–15 (2023). [Google Scholar]
- 38.B. Joseph, A. Iadecola, L. Simonelli, Y. Mizuguchi, Y. Takano, T. Mizokawa, N. L. Saini, A study of the electronic structure of FeSe1−xTex chalcogenides by Fe and Se K-edge x-ray absorption near edge structure measurements. J. Phys. Condens. Matter 22, 485702 (2010). [DOI] [PubMed] [Google Scholar]
- 39.C. Sugiura, Sulfur K x-ray absorption spectra of FeS, FeS2, and Fe2S3. J. Chem. Phys. 74, 215–217 (1981). [Google Scholar]
- 40.S. Wang, J. Zhang, O. Gharbi, V. Vivier, M. Gao, M. E. Orazem, Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 1, 41 (2021). [Google Scholar]
- 41.D. Strmcnik, M. Uchimura, C. Wang, R. Subbaraman, N. Danilovic, D. van der Vliet, A. P. Paulikas, V. R. Stamenkovic, N. M. Markovic, Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 5, 300–306 (2013). [DOI] [PubMed] [Google Scholar]
- 42.S. Trasatti, Work function, electronegativity, and electrochemical behaviour of metals. J. Electroanal. Chem. 39, 163–184 (1972). [Google Scholar]
- 43.F. Calle-Vallejo, M. T. Koper, A. S. Bandarenka, Tailoring the catalytic activity of electrodes with monolayer amounts of foreign metals. Chem. Soc. Rev. 42, 5210–5230 (2013). [DOI] [PubMed] [Google Scholar]
- 44.S. Trasatti, Work function, electronegativity, and electrochemical behaviour of metals. J. Electroanal. Chem. 33, 351–378 (1971). [Google Scholar]
- 45.I. Ledezma-Yanez, W. D. Z. Wallace, P. Sebastián-Pascual, V. Climent, J. M. Feliu, M. T. M. Koper, Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2, 17031 (2017). [Google Scholar]
- 46.P. Li, Y. Jiang, Y. Hu, Y. Men, Y. Liu, W. Cai, S. Chen, Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nat. Catal. 5, 900–911 (2022). [Google Scholar]
- 47.A. S. Shatla, M. Landstorfer, H. Baltruschat, On the differential capacitance and potential of zero charge of Au(111) in some aprotic solvents. ChemElectroChem 8, 1817–1835 (2021). [Google Scholar]
- 48.J. Le, Q. Fan, L. Perez-Martinez, A. Cuesta, J. Cheng, Theoretical insight into the vibrational spectra of metal–water interfaces from density functional theory based molecular dynamics. Phys. Chem. Chem. Phys. 20, 11554–11558 (2018). [DOI] [PubMed] [Google Scholar]
- 49.J. Charnock, C. Henderson, J. Mosselmans, R. Pattrick, 3d transition metal L-edge X-ray absorption studies of the dichalcogenides of Fe, Co and Ni. Phys. Chem. Miner. 23, 403–408 (1996). [Google Scholar]
- 50.Y. Tong, Y. Guo, P. Chen, H. Liu, M. Zhang, L. Zhang, W. Yan, W. Chu, C. Wu, Y. Xie, Spin-state regulation of perovskite cobaltite to realize enhanced oxygen evolution activity. Chem 3, 812–821 (2017). [Google Scholar]
- 51.H. Sato, F. Nagasaki, Y. Kani, S. Senba, Y. Ueda, A. Kimura, M. Taniguchi, Electronic structure of CoSe2 studied by photoemission spectroscopy using synchrotron radiation. Solid State Commun. 118, 563–567 (2001). [Google Scholar]
- 52.H. Yamada, K. Terao, M. Aoki, Electronic structure and magnetic properties of CoS2. J. Magn. Magn. Mater. 177-181, 607–608 (1998). [Google Scholar]
- 53.H. Wada, D. Kawasaki, Y. Maekawa, Magnetocaloric effect and magnetoresistance due to itinerant electron metamagnetic transition in Co(S1−xSex)2. IEEE T. Magn. 50, 2501806 (2014). [Google Scholar]
- 54.H. B. Michaelson, The work function of the elements and its periodicity. J. Appl. Phys. 48, 4729–4733 (1977). [Google Scholar]
- 55.T. Oyamada, K. Hongo, Y. Kawazoe, H. Yasuhara, The influence of correlation on the interpretation of Hund's multiplicity rule: A quantum Monte Carlo study. J. Chem. Phys. 125, 014101 (2006). [DOI] [PubMed] [Google Scholar]
- 56.M. Jaksic, Advances in electrocatalysis for hydrogen evolution in the light of the Brewer-Engel valence-bond theory. Int. J. Hydrogen Energy 12, 727–752 (1987). [Google Scholar]
- 57.G. Kresse, J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- 58.J. P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 59.M. Mavrikakis, B. Hammer, J. K. Nørskov, Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 81, 2819–2822 (1998). [Google Scholar]
- 60.Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong, H. Dai, MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011). [DOI] [PubMed] [Google Scholar]
- 61.M.-R. Gao, Z. Y. Lin, T. T. Zhuang, J. Jiang, Y. F. Xu, Y. R. Zheng, S. H. Yu, Mixed-solution synthesis of sea urchin-like NiSe nanofiber assemblies as economical Pt-free catalysts for electrochemical H2 production. J. Mater. Chem. 22, 13662–13668 (2012). [Google Scholar]
- 62.D. Voiry, H. Yamaguchi, J. Li, R. Silva, D. C. B. Alves, T. Fujita, M. Chen, T. Asefa, V. B. Shenoy, G. Eda, M. Chhowalla, Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850–855 (2013). [DOI] [PubMed] [Google Scholar]
- 63.J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. D. Lou, Y. Xie, Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 25, 5807–5813 (2013). [DOI] [PubMed] [Google Scholar]
- 64.J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan, Y. Xie, Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 135, 17881–17888 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Q. Liu, J. Tian, W. Cui, P. Jiang, N. Cheng, A. M. Asiri, X. Sun, Carbon nanotubes decorated with CoP nanocrystals: A highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. Int. Ed. 53, 6710–6714 (2014). [DOI] [PubMed] [Google Scholar]
- 66.X. Chen, D. Wang, Z. Wang, P. Zhou, Z. Wu, F. Jiang, Molybdenum phosphide: A new highly efficient catalyst for the electrochemical hydrogen evolution reaction. Chem. Commun. 50, 11683–11685 (2014). [DOI] [PubMed] [Google Scholar]
- 67.L. Feng, H. Vrubel, M. Bensimon, X. Hu, Easily-prepared dinickel phosphide (Ni2P) nanoparticles as an efficient and robust electrocatalyst for hydrogen evolution. Phys. Chem. Chem. Phys. 16, 5917–5921 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Z. Xing, Q. Liu, A. M. Asiri, X. Sun, High-efficiency electrochemical hydrogen evolution catalyzed by tungsten phosphide submicroparticles. ACS Catal. 5, 145–149 (2015). [Google Scholar]
- 69.M.-R. Gao, M. K. Y. Chan, Y. Sun, Edge-terminated molybdenum disulfide with a 9.4-Å interlayer spacing for electrochemical hydrogen production. Nat. Commun. 6, 7493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.X. Long, G. Li, Z. Wang, H. Y. Zhu, T. Zhang, S. Xiao, W. Guo, S. Yang, Metallic iron–nickel sulfide ultrathin nanosheets as a highly active electrocatalyst for hydrogen evolution reaction in acidic media. J. Am. Chem. Soc. 137, 11900–11903 (2015). [DOI] [PubMed] [Google Scholar]
- 71.W. Chen, J. Gu, Q. Liu, R. Luo, L. Yao, B. Sun, W. Zhang, H. Su, B. Chen, P. Liu, D. Zhang, Quantum dots of 1T phase transitional metal dichalcogenides generated via electrochemical Li intercalation. ACS Nano 12, 308–316 (2018). [DOI] [PubMed] [Google Scholar]
- 72.X. Zhang, Z. Luo, P. Yu, Y. Cai, Y. du, D. Wu, S. Gao, C. Tan, Z. Li, M. Ren, T. Osipowicz, S. Chen, Z. Jiang, J. Li, Y. Huang, J. Yang, Y. Chen, C. Y. Ang, Y. Zhao, P. Wang, L. Song, X. Wu, Z. Liu, A. Borgna, H. Zhang, Lithiation-induced amorphization of Pd3P2S8 for highly efficient hydrogen evolution. Nat. Catal. 1, 460–468 (2018). [Google Scholar]
- 73.Z. Zheng, L. Yu, M. Gao, X. Chen, W. Zhou, C. Ma, L. Wu, J. Zhu, X. Meng, J. Hu, Y. Tu, S. Wu, J. Mao, Z. Tian, D. Deng, Boosting hydrogen evolution on MoS2 via co-confining selenium in surface and cobalt in inner layer. Nat. Commun. 11, 3315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.H. Duan, C. Wang, G. Li, H. Tan, W. Hu, L. Cai, W. Liu, N. Li, Q. Ji, Y. Wang, Y. Lu, W. Yan, F. Hu, W. Zhang, Z. Sun, Z. Qi, L. Song, S. Wei, Single-atom-layer catalysis in a MoS2 monolayer activated by long-range ferromagnetism for the hydrogen evolution reaction: Beyond single-atom catalysis. Angew. Chem. Int. Ed. 60, 7251–7258 (2021). [DOI] [PubMed] [Google Scholar]
- 75.A. Han, X. Zhou, X. Wang, S. Liu, Q. Xiong, Q. Zhang, L. Gu, Z. Zhuang, W. Zhang, F. Li, D. Wang, L. J. Li, Y. Li, One-step synthesis of single-site vanadium substitution in 1T-WS2 monolayers for enhanced hydrogen evolution catalysis. Nat. Commun. 12, 709 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.I. H. Kwak, I. S. Kwon, G. M. Zewdie, T. T. Debela, S. J. Lee, J. Y. Kim, S. J. Yoo, J. G. Kim, J. Park, H. S. Kang, Polytypic phase transition of Nb1–xVxSe2 via colloidal synthesis and their catalytic activity toward hydrogen evolution reaction. ACS Nano 16, 4278–4288 (2022). [DOI] [PubMed] [Google Scholar]
- 77.I. S. Kwon, I. H. Kwak, G. M. Zewdie, S. J. Lee, J. Y. Kim, S. J. Yoo, J. G. Kim, J. Park, H. S. Kang, MoSe2–VSe2–NbSe2 ternary alloy nanosheets to boost electrocatalytic hydrogen evolution reaction. Adv. Mater. 34, 2205524 (2022). [DOI] [PubMed] [Google Scholar]
- 78.J. Xu, G. Shao, X. Tang, F. Lv, H. Xiang, C. Jing, S. Liu, S. Dai, Y. Li, J. Luo, Z. Zhou, Frenkel-defected monolayer MoS2 catalysts for efficient hydrogen evolution. Nat. Commun. 13, 2193 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.T. Corrales-Sánchez, J. Ampurdanés, A. Urakawa, MoS2-based materials as alternative cathode catalyst for PEM electrolysis. Int. J. Hydrogen Energy 39, 20837–20843 (2014). [Google Scholar]
- 80.A. Morozan, H. Johnson, C. Roiron, G. Genay, D. Aldakov, A. Ghedjatti, C. T. Nguyen, P. D. Tran, S. Kinge, V. Artero, Nonprecious bimetallic iron–molybdenum sulfide electrocatalysts for the hydrogen evolution reaction in proton exchange membrane electrolyzers. ACS Catal. 10, 14336–14348 (2020). [Google Scholar]
- 81.H. Kim, H. Park, S. Oh, S.-K. Kim, Facile electrochemical preparation of nonprecious Co-Cu alloy catalysts for hydrogen production in proton exchange membrane water electrolysis. Int. J. Energy Res. 44, 2833–2844 (2020). [Google Scholar]
- 82.H. Kim, E. Hwang, H. Park, B.-S. Lee, J. H. Jang, H.-J. Kim, S. H. Ahn, S.-K. Kim, Non-precious metal electrocatalysts for hydrogen production in proton exchange membrane water electrolyzer. Appl. Catal. B Environ. 206, 608–616 (2017). [Google Scholar]
- 83.K. J. Choi, H. Kim, S.-K. Kim, Multicomponent nonprecious hydrogen evolution catalysts for high performance and durable proton exchange membrane water electrolyzer. J. Power Sources 506, 230200 (2021). [Google Scholar]
- 84.H. Kim, H. Park, D.-K. Kim, I. Choi, S.-K. Kim, Pulse-electrodeposited nickel phosphide for high-performance proton exchange membrane water electrolysis. J. Alloys Compd. 785, 296–304 (2019). [Google Scholar]
- 85.J. W. D. Ng, T. R. Hellstern, J. Kibsgaard, A. C. Hinckley, J. D. Benck, T. F. Jaramillo, Polymer electrolyte membrane electrolyzers utilizing non-precious Mo-based hydrogen evolution catalysts. ChemSusChem 8, 3512–3519 (2015). [DOI] [PubMed] [Google Scholar]
- 86.S. M. Senthil Kumar, K. Selvakumar, R. Thangamuthu, A. Karthigai Selvi, S. Ravichandran, G. Sozhan, K. Rajasekar, N. Navascues, S. Irusta, Hydrothermal assisted morphology designed MoS2 material as alternative cathode catalyst for PEM electrolyser application. Int. J. Hydrog. Energy 41, 13331–13340 (2016). [Google Scholar]
- 87.J. H. Kim, H. Kim, J. Kim, H. J. Lee, J. H. Jang, S. H. Ahn, Electrodeposited molybdenum sulfide as a cathode for proton exchange membrane water electrolyzer. J. Power Sources 392, 69–78 (2018). [Google Scholar]
- 88.C. D. Giovanni, Á. Reyes-Carmona, A. Coursier, S. Nowak, J.−. M. Grenèche, H. Lecoq, L. Mouton, J. Rozière, D. Jones, J. Peron, M. Giraud, C. Tard, Low-cost nanostructured iron sulfide electrocatalysts for PEM water electrolysis. ACS Catal. 6, 2626–2631 (2016). [Google Scholar]
- 89.Z. Xie, S. Yu, X. Ma, K. Li, L. Ding, W. Wang, D. A. Cullen, H. M. Meyer III, H. Yu, J. Tong, Z. Wu, F.-Y. Zhang, MoS2 nanosheet integrated electrodes with engineered 1T-2H- phases and defects for efficient hydrogen production in practical PEM electrolysis. Appl. Catal. B Environ. 313, 121458 (2022). [Google Scholar]
- 90.J. Ampurdanés, M. Chourashiya, A. Urakawa, Cobalt oxide-based materials as non-PGM catalyst for HER in PEM electrolysis and in situ XAS characterization of its functional state. Catal. Today 336, 161–168 (2019). [Google Scholar]
- 91.P. Millet, R. Ngameni, S. A. Grigoriev, N. Mbemba, F. Brisset, A. Ranjbari, C. Etiévant, PEM water electrolyzers: From electrocatalysis to stack development. Int. J. Hydrog. Energy 35, 5043–5052 (2010). [Google Scholar]
- 92.M. T. Dinh Nguyen, A. Ranjbari, L. Catala, F. Brisset, P. Millet, A. Aukauloo, Implementing molecular catalysts for hydrogen production in proton exchange membrane water electrolysers. Coord. Chem. Rev. 256, 2435–2444 (2012). [Google Scholar]
- 93.H. Kim, H. Park, D. K. Kim, S. H. Oh, I. Choi, S. K. Kim, Electrochemically fabricated NiW on a Cu nanowire as a highly porous non-precious-metal cathode catalyst for a proton exchange membrane water electrolyzer. ACS Sustainable Chem. Eng. 7, 8265–8273 (2019). [Google Scholar]
- 94.P. K. R. Holzapfel, M. Bühler, D. Escalera-López, M. Bierling, F. D. Speck, K. J. J. Mayrhofer, S. Cherevko, C. V. Pham, S. Thiele, Fabrication of a robust PEM water electrolyzer based on non-noble metal cathode catalyst: [Mo3S13]2−clusters anchored to N-doped carbon nanotubes. Small 16, 2003161 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S33
Tables S1 to S5
References