Abstract
Objective:
To study the association between development of moderate or greater depression during curative-intent therapy and overall survival (OS) in patients with stages II-IV head and neck cancer (HNC).
Methods:
In this secondary analysis of a randomized double-blind placebo-controlled trial, of 148 eligible participants diagnosed with stages II-IV HNC but without baseline depression, 125 were evaluable and were randomly allocated to prophylactic escitalopram oxalate (n=60) or placebo (n=65). Participants were followed for development of moderate or greater depression, using Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR, range 0-27, score ≥11 indicated moderate or greater depression), and were stratified by demographics; cancer site and stage; and primary treatment modality (surgery with or without radiotherapy vs. radiotherapy with or without chemotherapy). Single variable and multivariable Cox proportional-hazard models were used to evaluate differences in OS.
Results:
22 of 125 patients (17.6%) developed clinically significant depression during HNC treatment. The mean follow-up was 5.0 years (SD 2.4). OS was similar for patient groups, when stratified by development of moderate or greater depression (HR 0.54 [CI, 0.21-1.43]) or use of prophylactic antidepressant (HR 0.64 [CI, 0.34-1.21]).
Conclusion:
There was no significant association between OS and development of moderate or greater depression in patients being treated for stages II-IV HNC, or between OS and use of prophylactic antidepressant escitalopram. Prophylactic antidepressants may be considered in patients with HNC for prevention of clinically significant depression and may offer improved quality of life outcomes.
Trial Registration:
clinicaltrials.gov Identifier: NCT00536172
Keywords: Head and Neck Cancer, Survival, Depression, Prophylaxis, Antidepressant
Introduction:
Patients with head and neck cancer (HNC) have an increased risk of developing depression.1,2 Depression has been associated with adverse outcomes including poor quality of life, malnutrition, poor compliance with therapy, and risk of suicide in patients diagnosed with HNC.3–6 Additionally, several small studies suggest association between baseline depression in patients with HNC and decreased survival outcomes.5,7–10
However, many patients who are not depressed at baseline may experience moderate or greater depression during or after the course of curative intent therapy for HNC, which may in turn contribute to significant adverse outcomes and influence poor survivorship experience following treatment of HNC.11–14 Analyses of Surveillance Epidemiology and End Results (SEER)-Medicare data suggest that while approximately 10% of patients diagnosed with HNC have a pre-existing diagnosis of depression, an additional 8% may develop depression after being diagnosed with HNC. However, these data likely represent underestimation of the burden of disease due to under diagnosis, and may not represent the experiences of younger, non-Medicare enrolled patients.12 As such, a true estimate of the burden of de novo depression among patients diagnosed with HNC remains elusive. Further, there is limited information about whether de novo development of clinically significant depression, in patients with HNC receiving definitive treatment, influences survival outcomes.11,12
This study investigates the relationship between overall survival (OS) and development of moderate or greater depression in patients receiving curative intent treatment for stages II-IV HNC, but without baseline depression. Additionally, we investigate if use of prophylactic antidepressant escitalopram oxalate independently influences OS outcomes.
Materials and Methods:
This study represents an ad-hoc, secondary analysis of prospectively collected data, from a randomized double-blind placebo-controlled clinical trial (Clinicaltrials.gov identifier NCT00536172). The detailed methodology of the Prevention of Depression in Patients Being Treated for Head and Neck Cancer Trial (PROTECT) and baseline characteristics, eligibility and exclusion criteria for patient enrollees have been published previously14, but a brief summary and a CONSORT diagram (figure 3) is being provided here. Patients with stages II-IV HNC, but without moderate or greater depression at baseline, who were about to initiate curative intent treatment were enrolled between January 2008 and December 2011 at an academic and a community-based tertiary HNC care center. Patients were screened for psychiatric illness at baseline using the Mini-International Neuropsychiatric Interview. One hundred and forty-eight participants were randomized to receive escitalopram oxalate as prophylactic antidepressant or matched placebo in 1:1 ratio for 16 weeks. Informed written consents were obtained and the study was approved by the institutional review boards at the University of Nebraska Medical Center and Nebraska Methodist Hospital.
Figure 3.
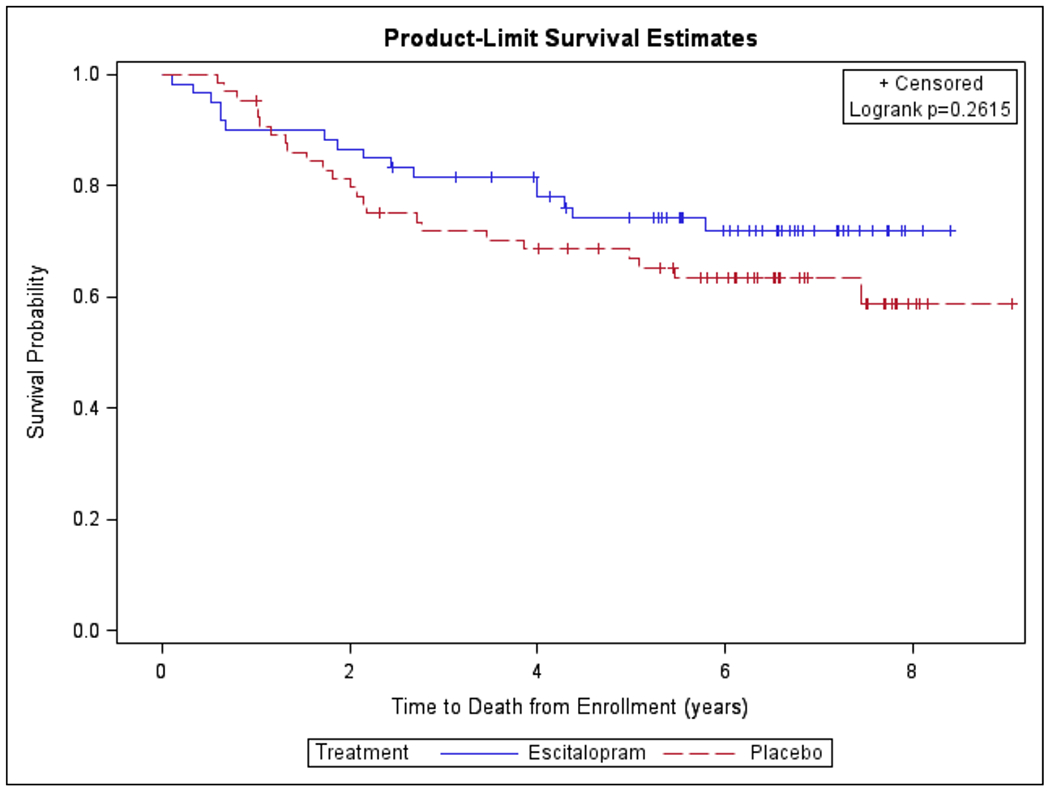
Kaplan-Meier Survival Curve Modeled for Time to Death from Enrollment stratified by Prophylactic Use of Antidepressant Escitalopram Oxalate
We stratified participants by age, sex, cancer site and stage (early [stage II] vs. advanced [stages III and IV]), and primary modality of treatment (surgery with or without radiotherapy vs. radiotherapy with or without chemotherapy), and randomized them to escitalopram oxalate or placebo arms (74 patients each). Participants were administered a clinician rated and a self-reported screening tool in order to identify depressive symptomatology at each rating interval. The Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) is a previously validated tool which was chosen as the primary measurement tool to reduce burden of the study on patients who are often overwhelmed by their disease and treatment-related factors. Participants who scored 11 or greater on the QIDS-SR (permissible range 0-27) were identified to have met the primary endpoint of moderate or greater depression. In order to maximize patient safety, participants were allowed to exit the study in order to receive appropriate medical care and psychiatric counseling when they met the primary endpoint. All participants received standard education and counseling from physicians and nurses and were offered the opportunity to join an optional monthly survivor support group, but did not receive formal psychotherapy.
Of the 148 participants who underwent randomization, 125 could be evaluated for primary endpoints and survival outcomes. In this group of patients who were diagnosed with stages II-IV HNC but without baseline depression, 60 were randomly allocated to receive prophylactic escitalopram oxalate and 65 others received matched placebo. Moderate or greater depression that developed during treatment (de novo depression) was considered as the primary endpoint in this study and participants who experienced this endpoint were identified in each arm. Per study design, 6 patients who developed depression in the intervention cohort and 16 others in the placebo cohort were requested to discontinue the pills prescribed to them at study initiation, and were referred for mental health consultation and medical management of depression. Thirty patients in the intervention arm and 28 in the placebo arm continued with use of pills allocated to them for the intended 16 week duration.
Chart review was performed to identify date of last clinical contact or documented date of patient death. Initial survival estimates between patients by stratification based on development of moderate or greater depression, and by receipt of prophylactic antidepressant escitalopram oxalate were illustrated using Kaplan-Meier survival curves. Single variable and multivariable Cox proportional-hazard models were used to further evaluate potential differences in OS between groups. Multivariable analyses controlled for demographics; cancer site and stage; and primary modality of treatment (surgery with or without radiotherapy vs. radiotherapy with or without chemotherapy). Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina). Statistical significance was assigned at p<0.05 and hazard ratios are presented with 95% CIs.
Results:
A total of 125 participants could be evaluated. The average age at enrollment was 59.8 (SD 16.4) years and 82% (102 of 125 participants) were men. Early (stage II) disease was experienced by 28 participants (22%) and 97 others (78%) had advanced (stage III or IV) disease. The primary modality of therapy was surgery with or without adjuvant radiotherapy in 65 patients (52%). Another 60 patients (48%) received radiotherapy with or without chemotherapy as the primary modality of treatment. (Table 1)
Table 1.
Selected baseline characteristics of 125 evaluable patients
| n | % | |
|---|---|---|
| Age at enrollment, mean (SD), years | 59.8 (16.4) | |
| Male gender | 102 | 82.0% |
| Study site | ||
| UNMC* | 59 | 47.0% |
| NMH† | 66 | 53.0% |
| Prognostic stage (clinical) | ||
| II | 28 | 22.0% |
| III or IV | 97 | 78.0% |
| Initial treatment | ||
| Surgery (Not biopsy) | 65 | 52.0% |
| Radiation with/ without chemotherapy | 60 | 48.0% |
| Intervention | ||
| Escitalopram | 60 | 48.0% |
| Placebo | 65 | 52.0% |
| Follow-up Duration, mean, years | 5.0 (2.4) | |
| Time to death for patients who died during follow up, mean, years | 2.4 (1.8) | |
University of Nebraska Medical Center
Nebraska Methodist Hospital
%, percentage value
SD, standard deviation
Twenty-two patients (17.6%) in the entire cohort developed the primary endpoint of moderate or greater depression, including 6 patients (10% of 60) in the prophylactic antidepressant arm and 16 (25% of 65) in the placebo arm. The mean follow up duration was 5.0 (SD 2.4) years and the mean time to death for patients who died during follow up was 2.4 (SD 1.8) years. (Table 1)
The Kaplan-Meier curves denoting probability of survival and time to death from enrollment in years are presented in figures 1 and 2, and the Cox proportional-hazard models for time to death from enrollment are presented in tables 2 and 3. In single variable analyses, the hazard ratio for time to death between patients who developed moderate or greater depression during the course of HNC treatment was similar to those who did not develop this outcome (HR 0.61 [95% CI, 0.24-1.56]). When controlling for age, sex, cancer stage, and choice of therapy, the lack of association between the primary endpoint and OS persisted (HR 0.54 [95% CI, 0.21-1.43]). (Table 2)
Figure 1:
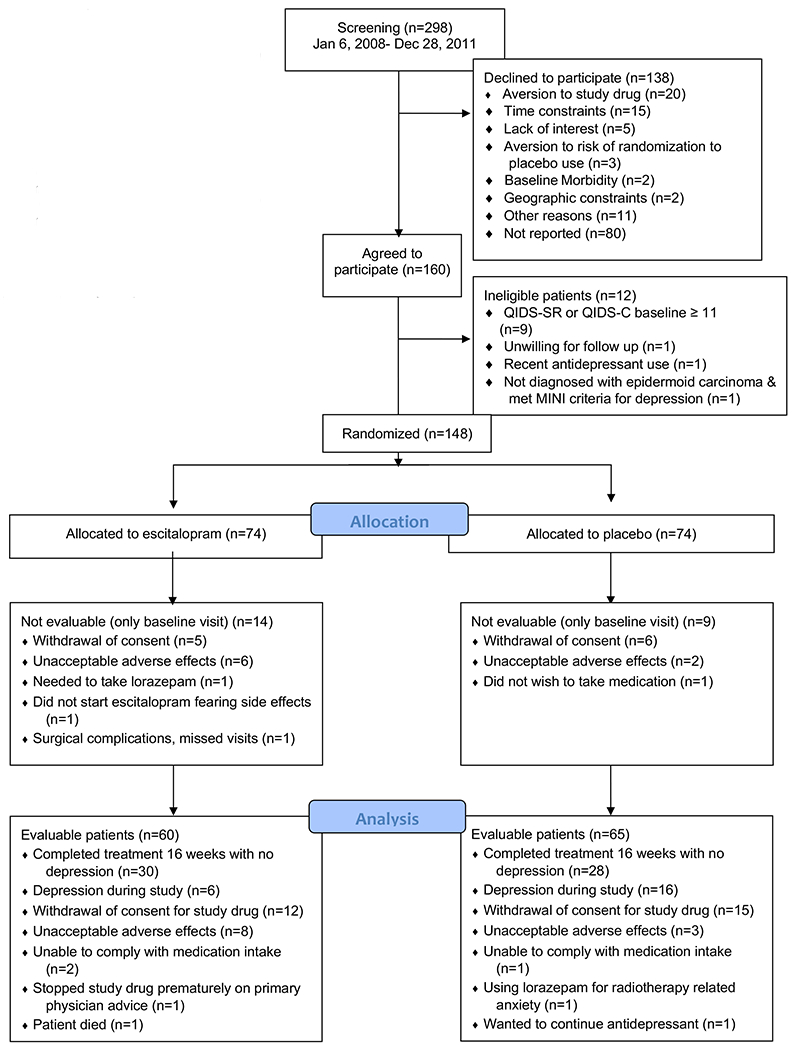
CONSORT Diagram Identifying Study Flow and Enrollment of Study Participants14
Trial Registration: clinicaltrials.gov Identifier: NCT00536172
MINI, Mini-International Neuropsychiatric Interview
QIDS-SR, Quick Inventory of Depressive Symptomatology- Self-Rated
QIDS-C, Quick Inventory of Depressive Symptomatology- Clinical
Figure 2.
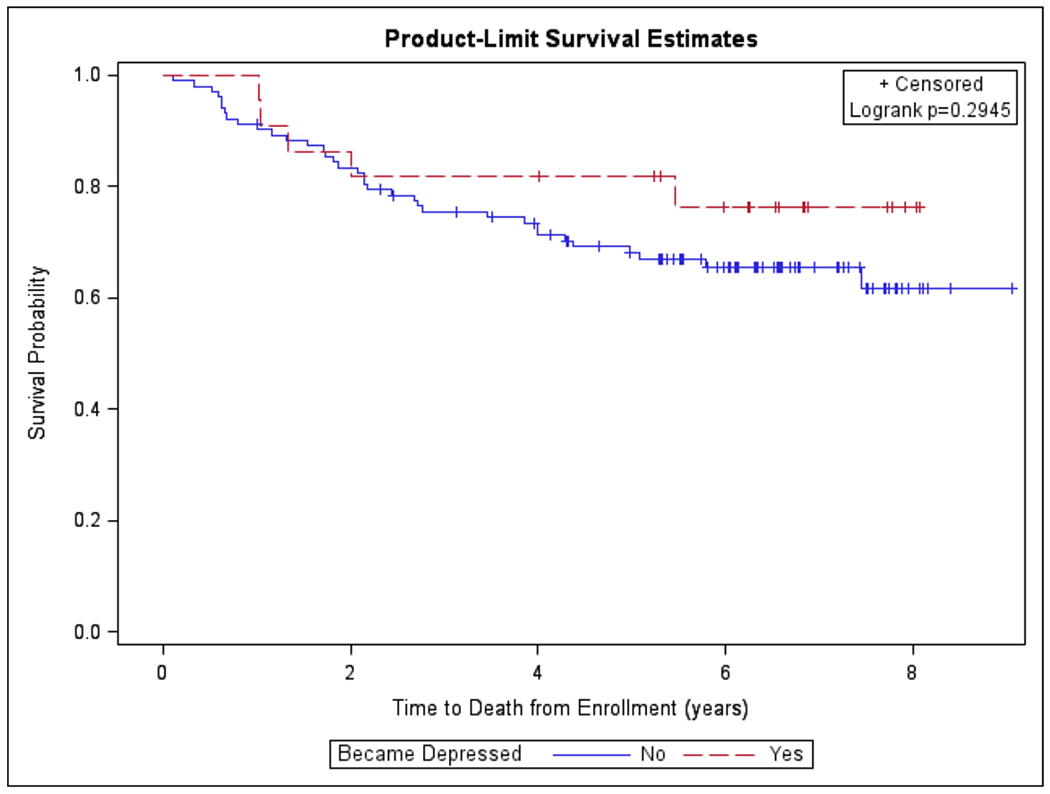
Kaplan-Meier Survival Curve Modeled for Time to Death from Enrollment stratified by Primary Outcome of Moderate or Greater Depression
Table 2.
Results of Cox Proportional Hazards Models for Time to Death from Enrollment stratified by Primary Outcome of Moderate or Greater Depression (n=125)
| Univariable Model HR (95% CI) | Multivariable Model HR (95% CI) | |
|---|---|---|
| Moderate or Greater Depression During Study: Yes vs. No | 0.61 (0.24, 1.56) | 0.54 (0.21, 1.43) |
| Age (Years) | - | 1.04 (1.01, 1.07) |
| Site: NMH† vs. UNMC* | - | 0.79 (0.42, 1.52) |
| Sex: Female vs. Male | - | 1.12 (0.49, 2.59) |
| Stage: II vs. III or IV | - | 0.46 (0.19, 1.13) |
| Initial Treatment: Radiotherapy vs. Surgery | - | 1.21 (0.63, 2.35) |
University of Nebraska Medical Center
Nebraska Methodist Hospital
Table 3.
Results of Cox Proportional Hazards Models for Time to Death from Enrollment stratified by Prophylactic Use of Antidepressant Escitalopram Oxalate (n=125)
| Univariable Model HR (95% CI) | Multivariable Model HR (95% CI) | |
|---|---|---|
| Intervention: Escitalopram vs. Placebo | 0.70 (0.37, 1.31) | 0.64 (0.34, 1.21) |
| Age (Years) | - | 1.04 (1.02, 1.07) |
| Site: NMH† vs. UNMC* | - | 0.73 (0.39, 1.40) |
| Sex: Female vs. Male | - | 1.12 (0.48, 2.59) |
| Stage: II vs. III or IV | - | 0.47 (0.19, 1.15) |
| Initial Treatment: Radiotherapy vs. Surgery | - | 1.10 (0.58, 2.12) |
University of Nebraska Medical Center
Nebraska Methodist Hospital
When patients were stratified by intervention group to assess the independent association of prophylactic antidepressant use with OS, the unadjusted hazard ratio for patients receiving escitalopram oxalate vs. placebo was 0.70 (95% CI, 0.37-1.31). In the multivariable model, prophylactic escitalopram oxalate use did not demonstrate association with adverse OS (HR 0.64 [95% CI, 0.34-1.21]). (Table 3)
Discussion:
Development of depression in patients undergoing treatment of HNC is a substantial source of morbidity and adverse outcomes.3–5, 7–10 Patients with HNC who suffer depression experience overall diminished health-related quality of life outcomes and decreased compliance with therapy, which may in turn affect survival outcomes. Despite profound implications on survivorship experience, development of depression in such patients is often underdiagnosed creating significant gaps in healthcare delivery and outcomes related to suboptimal care.
Our previously published research suggested that development of clinically significant depression in patients diagnosed with stages II-IV HNC but without baseline depression could be reduced by greater than 50% by using a strategy of prophylactic antidepressant. Furthermore, the sustained improvement in quality of life metrics for up to 12 weeks after cessation of therapy provided additional basis in support of this approach using prophylactic escitalopram oxalate in patients with HNC as part of their multidisciplinary care.14
However, whether the primary outcome of development of moderate or greater depression during HNC therapy or prophylactic intervention with escitalopram oxalate influences survival outcomes in any meaningful manner remained unclear. Separately, several studies suggest that in a different population of patients with baseline depression and HNC, depression may be associated with decreased survival. To investigate the association between survival outcomes and de novo development of depression in patients undergoing curative intent treatment for HNC, our group performed a smaller study of 34 patients which suggested that emergent depression during the course of treatment was associated with increased mortality.11 However, this study was limited in its scope by the size of patient cohort. A more recent study of administrative data from the SEER-Medicare program registry, evaluating 3466 patients with HNC suggested that 18.5% of patients were diagnosed with depression. Compared to patients who were not depressed, patients who were diagnosed with depression after cancer diagnosis were more likely to die of cancer (HR 1.38 [95% CI, 1.16-1.65]) and all-cause mortality (HR 1.40 [95% CI, 1.21-1.62]).12 However, due to the nature and limitations of a large administrative data set, the temporality of depression with relationship to curative intent therapy or interventions related to management of depression cannot be reliably ascertained.
In order to overcome limitations posed by a small patient cohort, limited follow up, and constraints of administrative datasets, we performed an ad-hoc secondary analysis of the data from the PROTECT trial. Our findings suggest that after controlling for demographic variables, cancer stage, and primary modality of treatment, OS is similar between patients diagnosed with stages II-IV HNC without baseline depression who subsequently develop moderate or greater depression during the course of HNC treatment versus others who do not develop this endpoint. These findings are in contradistinction to reports from Lazure et al11 and Rieke et al12, and serve to inform clinicians and patients when discussing role of de novo depression during or after therapy. Our findings may be reflective of a longer follow up duration, the very controlled study environment of a randomized clinical trial, and treatment of patients at two high volume tertiary HNC care centers. These results may provide basis for additional research into the role of depression diagnosed during survivorship for patients affected by HNC and its relationship to survival outcomes.
Several studies have previously investigated depression and its association with adverse outcomes in the HNC population. However, most rely on baseline depression in order to measure outcomes. For example, Zimmaro and colleagues suggest that greater depressive symptoms at baseline were associated with shorter survival (HR 0.868 [95% CI, 0.819-0.921]). They, however, temper their findings by suggesting that depression-survival relationship may be affected by concurrent observations of poorer treatment response in this patient population.5 In a mixed cohort with variety of cancer diagnoses, Suthahar et al suggest that baseline depression is an independent prognostic factor associated with decreased survival. However, the contribution of patients with HNC to the overall cohort was relatively modest at 11% of the study population, limiting its generalizability to a larger HNC population.7 Another study of 241 patients with HNC and 3-year follow up suggested that baseline depression was experienced by 26.9% of patients and associated with decreased OS compared to patients who were not depressed at baseline (70.8% vs. 82.7%, respectively [p=0.045]).8 Separately, Chen and colleagues examined patients with HNC who were about to initiate definitive radiotherapy in a cohort of 133 patients, baseline depression was identified in 23% and the 2-year survival for patients who were identified as “extremely” or “somewhat” depressed was 71% compared to 86% for those patients who did not report such baseline characteristics (p=0.026).9
In contrast, our findings related to survival and its relationship to de novo depression that develops after the diagnosis and through the treatment of HNC are particularly important since it has been shown that depression related events peak at 2-3 months after diagnosis.15, 16 As a result, focusing exclusively on baseline depression and its association with adverse outcomes under-recognizes the scope of the problem and does disservice to a large number of patients affected by HNC who may develop depression after initial diagnosis and during their treatment course. Our findings are disparate from those of Jansen et al who investigated depressive symptoms in patients with HNC, the course of such symptoms, and their relationship to OS. They find that 20% of their patient cohort experienced persistent or recurrent or late depressive symptoms as identified on the Hospital Anxiety and Depression Scale. They find that OS was poorer for patients in this group compared to those who never experienced such symptoms (HR 1.66 [95% CI, 1.09-2.53]), although patients in the late depression group had a higher preponderance of patients with severe comorbidities.13
As a distinct advantage over previously published reports, the trial design in the current study allowed us to further investigate whether administration of prophylactic escitalopram oxalate influenced OS. We found no significant difference in OS between patients who received prophylactic antidepressant versus those who received a matched placebo.
While this finding is novel, it should not serve to dissuade clinicians and patients from considering and incorporating prophylactic antidepressant use as part of multidisciplinary HNC care at the time of diagnosis given the established benefit of prophylactic antidepressants in significant reduction of risk for subsequent moderate or greater depression.14 In addition to prevention of clinically significant depression, participants in the PROTECT trial who received prophylactic antidepressant experienced superior quality of life outcomes in a sustained fashion compared to those receiving placebo. For context, the proportion of study participants reporting good, very good, or outstanding health related quality of life outcome (QOL) and overall QOL measures at 6 months from baseline assessment was higher for patients receiving prophylactic escitalopram (96% and 100%, respectively) compared to those receiving placebo (78.6% and 85.7%, respectively).14
We previously reported findings from the PROTECT trial that suggest low incidence of adverse events related to the use of prophylactic antidepressant escitalopram. Among the trial participants, the most common side effects that were encountered included insomnia, ejaculatory delay, nausea, sweating, fatigue, or somnolence. More than 9 in 10 patients in each group reported that they experienced adverse effects less than 25% of the time. Additionally, more than 8 in 10 patients in the study group reported adverse effects related impairment to be mild. There were no statistically significant differences between groups in terms of adverse effects, although more patients dropped out of the treatment group than the placebo group.14
The calculated number needed to treat of 6.8 in order to prevent clinically significant depression using prophylactic antidepressant escitalopram remains modest compared to many commonly accepted preventative interventions in the field of medicine.14 Consequently, despite lack of survival benefit, we feel that prophylactic antidepressants should be considered when discussing multidisciplinary care as part of shared decision making for patients newly diagnosed with HNC.
Indeed, the importance of recognizing depression in patients affected by HNC, not only at baseline, but also through their survivorship is highlighted by the elevated risk of suicide in this population. In this study, no patient exhibited suicidality during the course of prophylactic antidepressant or placebo use and during the follow up period for behavioral health and quality of life related outcomes. However, the subsequent long term data collected for secondary assessment of overall survival, relying on review of medical charts and other sources of information on vital statistics may not reliably capture information about suicide attempts in the long-term. As a result, we cannot offer insights regarding long-term incidence of suicidality in the study cohort. Other data suggest that patients with HNC have more than three times the incidence of suicide compared to the general population in the United States.6 Therefore, early recognition, continued surveillance, prevention and treatment of depression in patients with HNC should be an important goal for clinicians and caregivers as part of comprehensive cancer care. Use of prophylactic antidepressants should be considered as one of the many tools, along with others such as behavioral healthcare consultation, counseling, and psychotherapy, which clinicians should incorporate when discussing management options with patients diagnosed with HNC.
Our study has several limitations. First, the clinical trial sample size calculation was not powered to detect survival differences. This limitation of the ad-hoc secondary analyses is offset by the unique nature of an interventional trial focused exclusively on patients with HNC. Second, the patient population in this study comprised of a mixed cohort with various head and neck sites of primary malignancy that were staged using the seventh edition of American Joint Committee on Cancer Staging System. As a result of recent revision in staging system, some patients that were staged in a particular prognostic group may now be assigned a different group. Clinicians may need to exercise caution when drawing inferences from the stratification used in the current study. Third, the results of this study reflect outcomes in a closely monitored patient cohort that participated in a randomized clinical trial at two tertiary HNC care centers. Inferences from data provided here may be influenced by other factors related to treatment site and non-clinical trial environment in a real-world setting.
Conclusion:
In this study of patients who were diagnosed with stages II-IV head and neck cancer, but without baseline depression, development of moderate or greater depression during curative intent treatment course was not associated with reduced overall survival. Although prophylactic use of antidepressant escitalopram oxalate was not associated with any survival benefit, the importance of this endpoint pales in comparison to the detriment of depression on the well-being and survivorship experience of patients with head and neck cancer.4,6 Based on previously published evidence14, clinicians may consider prophylactic antidepressant use as part of good clinical practice in comprehensive head and neck cancer care, in order to substantially reduce risk of de novo development of clinically significant depression and to provide improved quality of life in patients undergoing therapy for head and neck cancer.
Acknowledgements:
This manuscript is presented on behalf of the PROTECT study team: Mary Morris, BSN, James C. Lynch, PhD, Jonathan D. Beck, PharmD, Daniel D. Lydiatt, MD, Oleg N. Militsakh, MD, Alan T. Richards, MD, Russell B. Smith, MD, Aaron M. Wieland, MD, Katerina Goldman, PA-C, Jennelea R. Montanez, PA-C, Lora L. Dosen, BSN, Nikie Herrera, RN, Jane Hill, LPN, Sheryl Jungbluth, BSN, Danya O’Brien, RN, Nicole R. Strohman, BSN, Matthew Egbert, MD, Christopher J. Kratochvil, MD, Ashish Sharma, MD, Jane Theobald, MD, Steven P. Wengel, MD, Paula Danekas, PharmD, Randy Rasmussen, PharmD, Barbara L. Bayer, MSN, Micki T. Bethea, BS, Deborah S. Heimes, BS, Delores A. McArthur-Miller, MA, and Rosella Squires, BS. James Anderson, PhD, chaired the data safety monitoring committee with Charles A. Enke, MD, Mark H. Fleisher, MD, Donald Leopold, MD, Fredrick Petty, PhD, Toby L. Schonfeld, PhD, and Weining Zhen, MD. Special thanks to Kendra Schmid, PhD and Diane Bessette, PA, for their assistance with the conduct of the study.
Funding/Support:
The original project described was supported by grant R01 MH079420 from the National Institute of Mental Health. Additional support was provided by a research support fund grant from the Nebraska Medical Center and UNMC.
Role of the Sponsor:
Forest Research Institute provided matching placebo and drug. Forest Research Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Other financial disclosures:
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Trial protocol may be accessed at:
Level of Evidence: II
References:
- 1.Duffy SA, Ronis DL, Valenstein M et al. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007. Mar-Apr;48(2):142–8. [DOI] [PubMed] [Google Scholar]
- 2.Frampton M Psychological distress in patients with head and neck cancer: review. Br J Oral Maxillofac Surg. 2001. Feb;39(1):67–70. [DOI] [PubMed] [Google Scholar]
- 3.Chiou WY, Lee MS, Ho HC et al. Prognosticators and the Relationship of Depression and Quality of Life in Head and Neck Cancer. Indian J Cancer. 2013. Jan-Mar;50(1):14–20. [DOI] [PubMed] [Google Scholar]
- 4.Britton B, Clover K, Bateman L et al. Baseline Depression Predicts Malnutrition in Head and Neck Cancer Patients Undergoing Radiotherapy. Support Care Cancer. 2012. Feb;20(2):335–42. [DOI] [PubMed] [Google Scholar]
- 5.Zimmaro LA, Sephton SE, Siwik CJ et al. Depressive Symptoms Predict Head and Neck Cancer Survival: Examining Plausible Behavioral and Biological Pathways. Cancer. 2018. Mar 1;124(5):1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kam D, Salib A, Gorgy G et al. Incidence of Suicide in Patients with Head and Neck Cancer. JAMA Otolaryngol Head Neck Surg. 2015. Dec;141(12):1075–81 [DOI] [PubMed] [Google Scholar]
- 7.Suthahar A, Gurpreet K, Ambigga D et al. Psychological Distress, Quality of Life, and Coping in Cancer Patients: A Prospective Study. Med J Malaysia. 2008. Dec;63(5):362–8. [PubMed] [Google Scholar]
- 8.Kim SA, Roh JL, Lee SA et al. Pretreatment Depression as a Prognostic Indicator of Survival and Nutritional Status in Patients with Head and Neck Cancer. Cancer. 2016. Jan 1;122(1):131–40. [DOI] [PubMed] [Google Scholar]
- 9.Chen AM, Hsu S, Felix C et al. Effect of Psychosocial Distress on Outcome for Head and Neck Cancer Patients Undergoing Radiation. Laryngoscope. 2018. Mar;128(3):641–645. [DOI] [PubMed] [Google Scholar]
- 10.Shinn EH, Valentine A, Jethanandani A et al. Depression and Oropharynx Cancer Outcome. Psychosom Med. 2016. Jan;78(1):38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazure KE, Lydiatt WM, Denman D et al. Association between Depression and Survival or Disease Recurrence in Patients with Head and Neck Cancer Enrolled in a Depression Prevention Trial. Head Neck. 2009. Jul;31(7):888–92. [DOI] [PubMed] [Google Scholar]
- 12.Rieke K, Schmid KK, Lydiatt W et al. Depression and Survival in Head and Neck Cancer Patients. Oral Oncol. 2017. Feb;65:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen F, Verdonck-de Leeuw IM, Cuijpers P et al. Depressive Symptoms in Relation to Overall Survival in People with Head and Neck Cancer: A Longitudinal Cohort Study. Psychooncology. 2018. Jun 21. doi: 10.1002/pon.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lydiatt WM, Bessette D, Schmid KK et al. Prevention of Depression with Escitalopram in Patients undergoing Treatment for Head and Neck Cancer: Randomized, Double-Blind, Placebo-Controlled Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2013. Jul;139(7):678–86. [DOI] [PubMed] [Google Scholar]
- 15.Rieke K, Boilesen E, Lydiatt W et al. Population-Based Retrospective Study to Investigate Preexisting and New Depression Diagnosis Among Head and Neck Cancer Patients. Cancer Epidemiol. 2016. Aug;43:42–8. [DOI] [PubMed] [Google Scholar]
- 16.Lydiatt WM, Moran J, Burke WJ. A Review of Depression in the Head and Neck Cancer Patient. Clin Adv Hematol Oncol. 2009. Jun;7(6):397–403. [PubMed] [Google Scholar]


