Abstract
Background:
Pediatric patients with cancer infected with COVID-19 may be at higher risk of severe disease and may be unable to mount an adequate response to the virus due to compromised immunity secondary to their cancer therapy.
Procedure:
This study presents immunologic analyses of 20 pediatric patients with cancer, on active chemotherapy or having previously received chemotherapy, and measures their immunoglobulin titers and activation of cellular immunity response to acute SARS-CoV-2 infection and COVID-19 vaccination compared with healthy pediatric controls.
Results:
Forty-three patients were enrolled, of which ten were actively receiving chemotherapy, ten had previously received chemotherapy, and twenty-three were healthy controls. Pediatric patients with cancer had similar immunoglobulin titers, antibody binding capacity, and effector function assay activity after vaccination against COVID-19 compared with healthy controls, though more variability in response was noted in the cohort actively receiving chemotherapy. Compared with acute infection, vaccination against COVID-19 produced superior immunoglobulin responses, particularly IgA1, IgG1, and IgG3, and elicited superior binding capacity and effector function in children with cancer and healthy controls.
Conclusions:
Pediatric patients receiving chemotherapy and those who had previously received chemotherapy had adequate immune activation after both vaccination and acute infection compared to healthy pediatric controls, although there was a demonstrated variability in response for the patients on active chemotherapy. Vaccination against COVID-19 produced superior immune responses compared to acute SARS-CoV-2 infection in pediatric patients with cancer and healthy children, underscoring the importance of vaccination even in previously infected individuals.
Keywords: COVID-19, immunity, vaccination, cancer, coronavirus, immunosuppressed
INTRODUCTION
The novel coronavirus SARS-CoV-2 has caused significant burden of disease and mortality worldwide since its emergence.1 Early data indicated reduced severity of disease among pediatric patients compared with the adult population, which has been repeatedly demonstrated as the pandemic has continued.2–4 Despite this relative protection of younger age, there has been significant concern that the risk of mortality with SARS-CoV-2 infection may be higher for children with underlying illness such as cancer, compared to healthy children.5,6 These concerns stem from data in adult oncology patients that demonstrates increased risk of serious disease and mortality with COVID-19 as well as known worse clinical outcomes in these patients with other viral respiratory illnesses such as influenza H1N1.7–9
Available data regarding severity of COVID-19 disease in immunocompromised pediatric patients remains scarce, and early data did not suggest a more severe disease course compared to immunocompetent children.9–16 However, a large scale multi-center analysis of pediatric patients with cancer demonstrated higher incidence of severe disease due to COVID-19 as measured by rates of hospitalization, ICU admission, and death in these patients compared to healthy controls.17
Responses to mRNA vaccines against SARS-CoV-2 in pediatric patients with cancer have been preliminarily investigated including one study in twenty-one pediatric patients receiving chemotherapy, which demonstrated development of immunity as measured by anti-RBD IgG titers and neutralization titers, albeit lower than in healthy controls. Stronger responses were elicited in children on maintenance therapy or with solid tumors compared to children in intensive phases of chemotherapy or with hematologic malignancies.18 Vaccination for children with cancer has long been an active area of investigation due to concern for immune dysfunction secondary to cancer directed therapy or the primary malignancy. After chemotherapy, children with cancer appear to retain some residual protection from childhood vaccinations however the degree of protection maintained appears to vary by intensity of therapy, type of cancer, and time elapsed since the vaccine was received.19,20 Current CDC recommendations for patients with altered immunocompetence state that children should be fully vaccinated before receiving any immunosuppressive drugs, not receive vaccination during active treatment, and that some children such as HSCT recipients will require re-vaccination after therapy.21
Comprehensive data on immunosuppressed pediatric patients with COVID-19, specifically comparing laboratory analyses of these patients’ immune responses to acute infection with responses to vaccination, remain lacking. We present immunologic serum profiling including quantitative analyses of immunoglobulin response as well as assays measuring antibody-dependent immune activation of twenty pediatric patients with cancer for both acute SARS-CoV-2 infection and COVID-19 vaccination.
METHODS
Biospecimens:
Specimens from the Massachusetts General Hospital (MGH) Pediatric COVID-19 Biorepository, which was established at the outset of the COVID-19 pandemic, collecting biospecimens from children infected with, exposed to, or vaccinated against SARS-CoV-2.22 Participants were enrolled in the IRB-approved MGH Pediatric COVID-19 Biorepository (MGB 2020P000955) or the Pediatric Biorepository (MGB 2016P000949) from 2/01/21 to 1/24/22. Informed consent and assent when appropriate were obtained prior to enrollment and specimen collection and all protocols were reviewed and approved by the Mass General Brigham (MGB) Institutional Review Board for ethical considerations. The biorepository database was used to identify potentially eligible study participants, and inclusion criteria were confirmed by chart review. Demographic data, clinical histories, and laboratory results were obtained by electronic medical record review. Control samples were taken from healthy immunocompetent children with acute SARS-CoV-2 infection or after recent COVID-19 vaccination. The healthy control cohort was a randomly selected from a larger cohort of vaccinated children that had been extensively studied to characterize the pediatric humoral response to the Pfizer mRNA vaccines.23 None of the participants in the healthy vaccinated control cohort reported prior SARS-CoV-2 infection and serological evidence of prior infection with SARS-CoV-2, tested by SARS-CoV-2 Nucleocapsid specific IgG1, was not observed.23 All participants received the Pfizer-BioNTech two dose primary vaccination series. Samples were taken at two time points in the vaccination cohort (V0: before first dose of vaccine and V2: after second dose of vaccine) and at one time point in the acute infection cohort (after COVID-19 positive PCR).
Assay overview:
Details about the assays used for this study have been previously described and are described in more detail in the supplementary materials.24–26 Briefly, serum samples were analyzed for IgA, IgM and IgG (IgG1, IgG2, IgG3 and IgG4) antibodies against several viral antigens including spike of the wild-type SARS-CoV-2 virus. Commercial antibodies were used for IgG assays (Bethyl Laboratories, A80–148B), IgM assays (Thermo Fisher Scientific, MII0401), and IgA assays (Abcam, ab214003). Antibody-dependent effector assays were also performed including antibody-dependent complement deposition (ADCD), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent neutrophil phagocytosis (ADNP). Control samples were included in each sample’s run.
Statistical analysis:
Two-way analysis of variance (ANOVA) Kruskal-Wallis tests with Dunn’s correction for multiple comparisons were used to analyze differences between groups. Mann-Whitney tests were used for comparisons between single groups. An adjusted p-value (using Benjamini-Hochberg correction) of less than 0.05 was considered significant. Data visualization and statistical tests were performed in GraphPad Prism (v9; GraphPad Software) and R Statistical Software (v4.0.3; R Core Team 2021).
RESULTS
Study Population
Forty-three patients were enrolled, including 10 who were actively receiving chemotherapy (‘active treatment’), 10 who had previously received chemotherapy (‘prior treatment’), and 23 healthy controls. One patient in the active treatment cohort and two patients in the prior treatment cohort provided samples both after acute infection and during vaccination series. The active treatment cohort demonstrated an average age of 7.3 years (SD ± 2.3 years) and 80% were male. Varied malignancy diagnoses were represented including B-cell ALL, Wilms tumor, APML, astrocytoma, high grade glioma, Hodgkin lymphoma, and Langerhans cell histiocytosis. Their samples were obtained on average 3.1 weeks after their positive COVID test (n=3) or 4.5 weeks after their second vaccine dose (n=8). [Table 1, extended clinicodemographic data presented in Supplemental Table 1]
TABLE 1.
Demographic, clinical, and time course data for the active treatment, off treatment, and control cohorts.
| Active Treatment (n= 10) | Off Treatment (n= 10) | Control Cohort (n= 23) | |
|---|---|---|---|
| Age at enrollment, mean years (SD) | 7.3 (2.3) | 9.4 (5.6) | 9.14 (3.9) |
| Male, n (% of group) | 8 (80) | 6 (60) | 12 (52.2) |
| Hispanic, n (% of group) | 3 (30) | 6 (60) | 15 (65.2) |
| Race, n (% of group) | |||
| Black | 1 (10) | 0 (0) | 2 (8.7) |
| White | 8 (80) | 5 (50) | 12(52.2) |
| Asian | 0 (0) | 1 (10) | 3(13) |
| Other | 1 (10) | 4 (40) | 3(13) |
| Cancer diagnosis, n (% of group) | |||
| Hematological | 8 (80) | 8 (80) | - |
| Solid | 2 (20) | 2 (20) | - |
| Time between acute infection or initiation of vaccine series and last chemotherapy, average weeks (SD) | - | 126 (268) | - |
| Acute SARS-CoV-2 infection, n (% of group) | 3 (30) | 5 (50) | 8 (35) |
| Time from infection to blood draw, weeks, avg (SD) | 3.1 (1.5) | 6.5 (3.0) | 5.50 (2.1) |
| Vaccination, n (% of group) | 8 (80) | 7 (70) | 15 (65) |
| Time from second vaccination to blood draw, weeks, avg (SD) | 4.5 (1.3) | 5.3 (2.2) | 3.1 (1.3) |
The prior treatment cohort demonstrated an average age of 9.4 years (SD ± 5.6 years) and 60% were male. Varied malignancy diagnoses were again represented including B-cell ALL, Ewing sarcoma, and Wilms tumor. The average time elapsed between enrollment and last chemotherapy was 126 weeks (SD: 268). Their samples were obtained on average 6.5 weeks after their positive COVID test (n=5) or 5.3 weeks after their second vaccine dose (n=7). [Table 1]
Twenty-three healthy controls were selected as comparators who were 52% male and had an average age of 9.1 years. Their samples were obtained on average 5.5 weeks after their positive COVID test or 3.1 weeks after their second vaccine dose. [Table 1]
Patients with cancer have similar but more variable immunoglobulin titers after vaccination compared with healthy controls
Patients in all three groups demonstrated similar pre-vaccination antibody titers against WT-Spike. [Figure 1A–D] Patients in the healthy and active treatment cohorts demonstrated higher post-vaccination anti-WT-Spike IgM (p=0.01 and p<0.0001) compared to pre-vaccine baseline levels, while patients in the prior treatment cohort demonstrated similar IgM levels (p=0.37). [Figure 1A] Patients in the healthy and prior treatment cohorts also demonstrated higher post-vaccination anti-WT-Spike IgA1 (p=0.02 and p<0.0001) compared to pre-vaccine baseline levels, while patients in the active treatment cohort demonstrated a similar pattern though it was not statistically significant (p=0.07). [Figure 1B] Patients in all three groups demonstrated higher post-vaccination anti-WT-Spike IgG1 (p=0.03, p=0.001, p<0.0001) and higher post-vaccination anti-WT-Spike IgG3 (p=0.0006, p=0.0023, p<0.0001) compared to pre-vaccine baseline levels. [Figure 1C–D]
FIGURE 1.
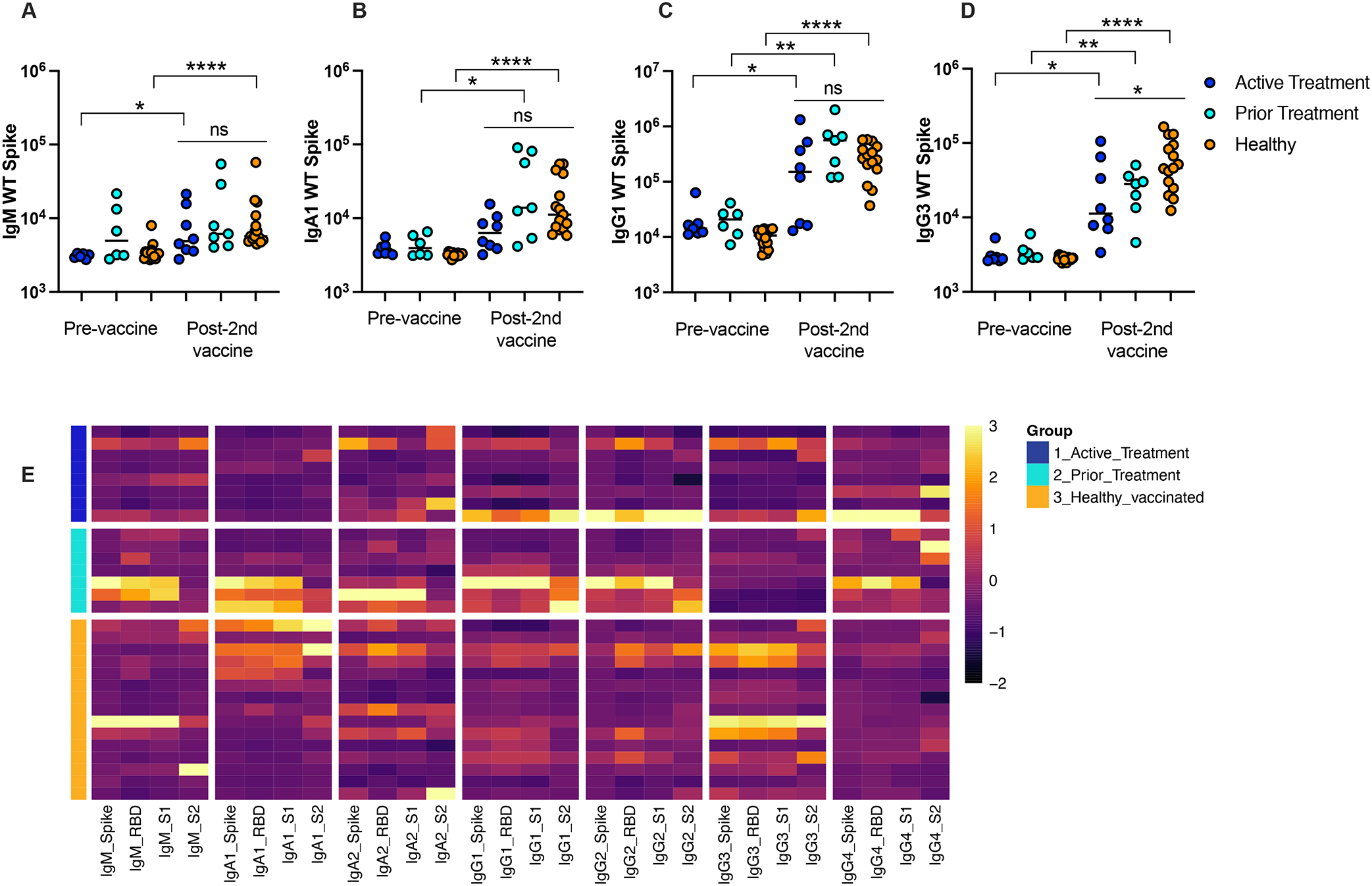
Immunoglobulin titers against SARS-CoV-2 WT-Spike antigen at the pre-vaccine and post- second vaccine timepoints for active treatment, prior treatment, and healthy cohorts including (A) IgM, (B) IgA1, (C) IgG1 and (D) IgG3. (E) Heatmap illustrates relative levels of IgM, IgA1, IgA2, IgG1, IgG3 and IgG4 against Spike, RBD, S1 and S2 proteins for the WT strain of SARS-CoV-2. Antibody levels are shown for Active Treatment, Prior Treatment and Healthy vaccinated groups. MFI data are z-scored across columns, with each row representing a different individual.
Compared with healthy controls, post-vaccination anti-WT-Spike IgM, IgA1, and IgG1 titers did not differ significantly in the active treatment or prior treatment cohorts. However, anti-WT-Spike IgG3 titers were lower in the active treatment cohort compared to healthy controls (p=0.03) with no difference detected between the prior treatment cohort and healthy controls. [Figure 1A–D]
To further study the impact of cancer on SARS-CoV-2-specific humoral immunity, antibody levels against other antigen targets including RBD, S1 and S2 were measured in the plasma of vaccinated patients with cancer compared to vaccinated healthy patients. Figure 1E displays these findings as a heatmap with relative levels of IgM, IgA1, IgA2, IgG1, IgG3 and IgG4 against Spike, RBD, S1 and S2 proteins for the WT strain of SARS-CoV-2 for each enrolled individual. Median fluorescence intensity values were z-scored across each column. As represented on the heatmap, antibody response against RBD, S1 and S2 showed similar patterns to antibodies against Spike for all three groups of children. Indeed, similar titers were observed between children with cancer and healthy controls for IgM, IgA1, IgA2, IgG1, and IgG4, and only IgG3 showed higher levels in the healthy group compared to both active and prior treatment groups. [Figure 1E] Of note, in the active treatment group, three patients persistently lagged behind their cohort in immunoglobulin titer levels: no apparent pattern was identified separating these patients from their cohort with respect to age, diagnosis, timing of chemotherapy relative to COVID vaccination, or chemotherapy regimen, raising concern about a possible non-responder subgroup. [Supplemental Table 1]
Patients with cancer have similar binding capacity and effector function after vaccination as healthy controls
To evaluate functional differences between groups, antibody binding to FcR was measured, in addition to antibody-dependent complement deposition (ADCD), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent neutrophil phagocytosis (ADNP). Patients in all three groups demonstrated similar pre-vaccination binding capacity against SARS-CoV-2 WT-Spike. Patients in all three groups (active treatment, prior treatment, and healthy controls) demonstrated higher post-vaccination binding capacity against SARS-CoV-2 WT-Spike antigen as measured by FcgR2a (p=0.23, p=0.0012, p<0.0001), FcgR2b (p=0.15, p=0.0012, p<0.0001), FcgR3a (p=0.05, p=0.0012, p<0.0001), and FcgR3b (p=0.04, p=0.0012, p<0.0001) compared to pre-vaccine baseline levels; these differences were statistically significant for all cohorts in FcgR3b and for the prior treatment and healthy cohorts for all binding capacity assays. No statistically significant differences were found between the cohorts at the post-vaccine time point. [Figure 2A]
FIGURE 2.
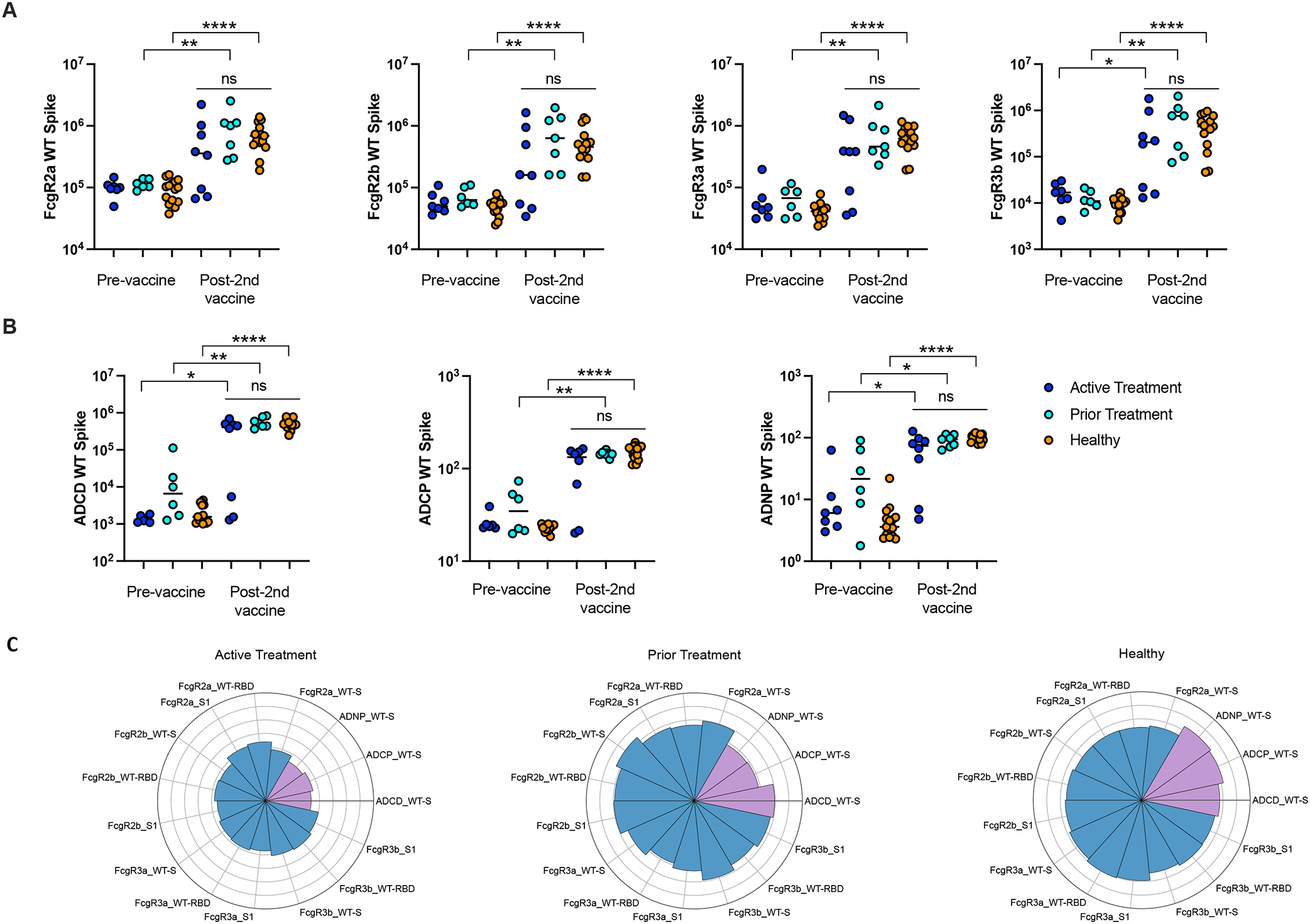
Binding capacity and functional activity against SARS-CoV-2 WT-Spike antigen at the pre-vaccine and post- second vaccine timepoints for active treatment, prior treatment, and healthy cohorts including (A) binding capacity of FcgR2a, FcgR2b, FcgR3a, FcgR3b, (B) functional activity of ADCD, ADCP and ADNP, (C) The Nightingale rose plots show the average of the z-scored value for antibody functions (in purple) and binding to FcgR (blue) in the Active Treatment, Prior Treatment and Healthy groups. Each wedge corresponds to a SARS-CoV-2 antibody feature, and the length of the wedge represents the magnitude of the response.
Patients in all three groups demonstrated similar pre-vaccination effector function against SARS-CoV-2 WT-Spike. Patients in all three groups (active treatment, prior treatment, and healthy controls) demonstrated higher post-vaccination effector function against SARS-CoV-2 WT-Spike antigen as measured by ADCD (p=0.013, p=0.0012, p<0.0001), ADCP (p=0.142, p=0.0012, p<0.0001), and ADNP activity (p=0.014, p=0.014, p<0.0001) compared to pre-vaccine baseline levels. These differences were statistically significant for all cohorts for the ADCD and ADNP assays and for the prior treatment and healthy cohorts for the ADCP assay. No statistically significant differences were found between the cohorts at the post-vaccine time point. [Figure 2B]
Functional analyses were also performed for antibodies against RBD and S1. As shown on the Nightingale rose plots, similar profiles were observed against Spike, RBD and S1 after SARS-CoV-2 vaccination. More precisely, results showed a strong vaccine-induced humoral activation in all three groups, including active treatment, prior treatment, and healthy groups. However, the healthy and prior treatment groups demonstrated slightly higher response to vaccination compared to a diminished response in the active treatment group, which might be due to the variable immune activation observed in that active treatment group, with some individuals having low antibody functionality. [Figure 2C] Of note, in this active treatment group, the same three patients persistently lagged behind their cohort in binding capacity and effector activity as previously seen in immunoglobulin titers, potentially representing non-responders.
Vaccination produces superior protective immunoglobulin titers compared to natural infection
Patients in all three groups (active treatment, prior treatment, and healthy controls) demonstrated a pattern of higher post-acute infection anti-WT-Spike IgM (p=0.20, p=0.19, p=0.017) compared with post-vaccination titers in healthy controls, although the difference was statistically significant in the healthy cohort group. [Figure 3A] Patients in all three groups (active treatment, prior treatment, and healthy controls) demonstrated inferior post-acute infection anti-WT-Spike IgA1 (p=0.04, p=0.02, p=0.0037), IgG1 (p=0.005, p=0.14, p=0.0165), and IgG3 (p=0.009, p=0.035, p=0.0043) compared with post-vaccination titers in healthy controls. This pattern was statistically significant in all cohorts for IgA1 and IgG3, and for the active treatment and healthy cohorts in IgG3. [Figure 3B–D]
FIGURE 3.
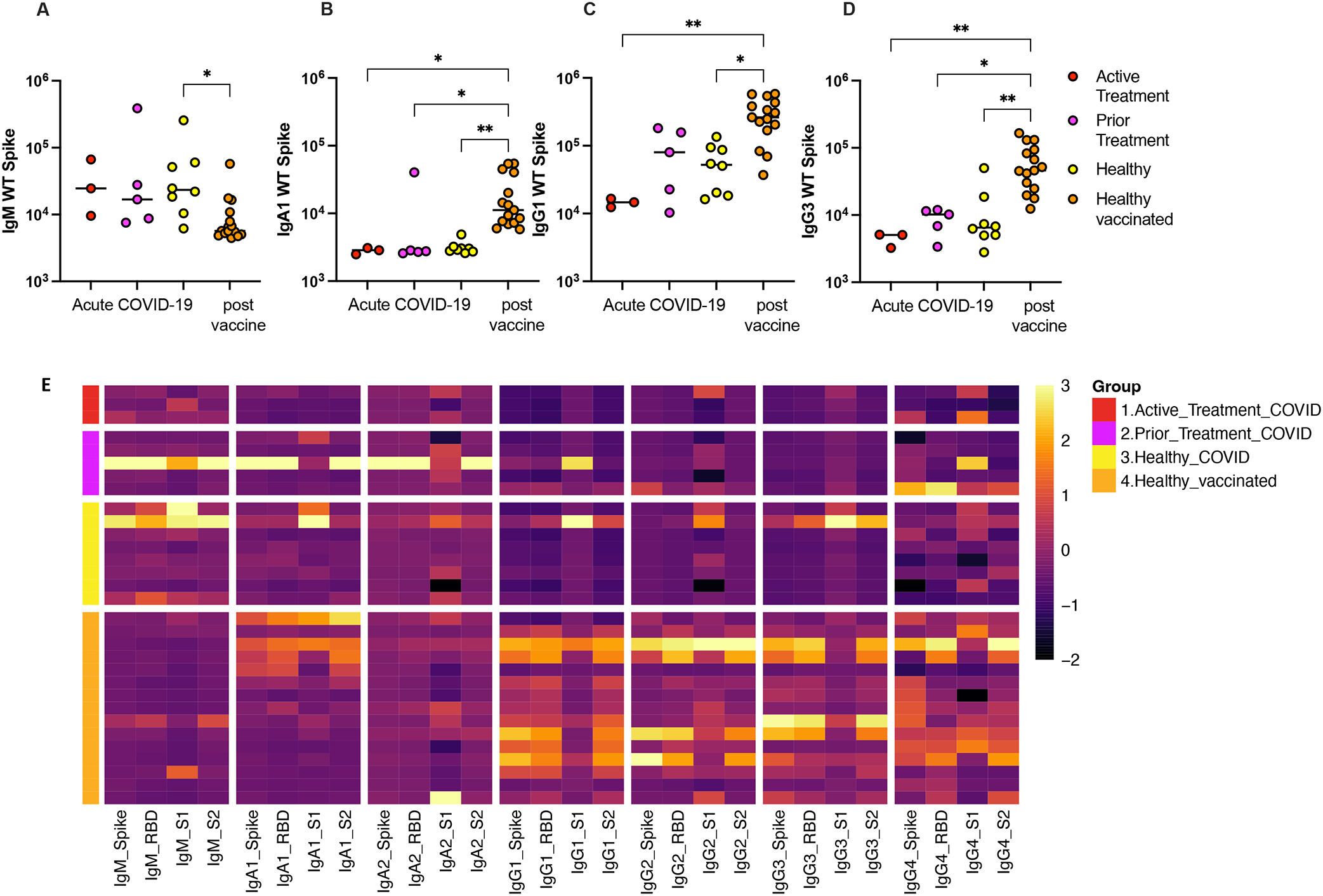
Immunoglobulin titers against SARS-CoV-2 WT-Spike antigen after COVID-19 infection in active treatment, prior treatment, and health cohorts compared with response to vaccination in healthy children including (A) IgM, (B) IgA1, (C) IgG1 and (D) IgG3. (E) Heatmap illustrates relative levels of IgM, IgA1, IgA2, IgG1, IgG3 and IgG4 against Spike, RBD, S1 and S2 proteins for the WT strain of SARS-CoV-2. Antibody levels are shown for Active Treatment, Prior Treatment and Healthy groups, all infected with SARS-CoV-2, in addition to Healthy vaccinated group. MFI data are z-scored across columns, with each row representing a different individual.
Similar findings were demonstrated against other WT antigens including RBD, S1, and S2 for all cohorts, with healthy vaccinated children mounting the highest IgG and IgA titers compared to acute infection in all cohorts. [Figure 3E]
Vaccination produces superior binding capacity and effector activity compared to natural infection
For all three cohorts (active treatment, prior treatment, and healthy controls), vaccination produced a pattern of higher FcR binding against SARS-CoV-2 WT-Spike antigen as measured by FcgR2a (p=0.016, p=0.066, p=0.0017), FcgR2b (p=0.0089, p=0.061, p=0.0033), FcgR3a (p=0.0018, p=0.061, p=0.0038), and FcgR3b (p=0.0053, p=0.059, p=0.0073) compared to acute SARS-CoV-2 infection. These differences were statistically significant in the healthy cohort and the active treatment cohort. [Figure 4A]
FIGURE 4.
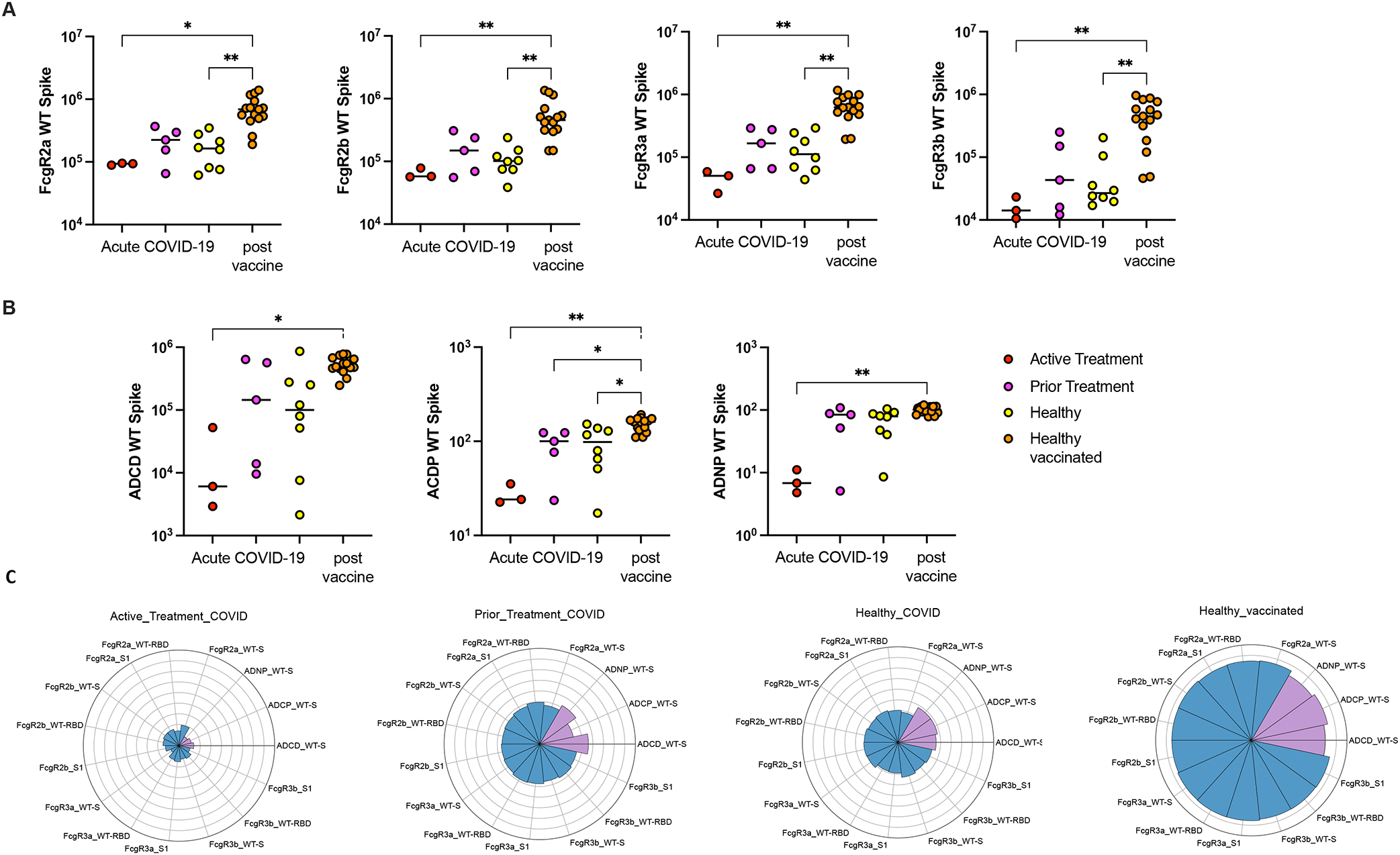
Binding capacity and functional activity against SARS-CoV-2 WT-Spike antigen after acute SARS-CoV-2 infection in active treatment, prior treatment, and health cohorts compared with response to vaccination in healthy children including (A) binding capacity of FcgR2a, FcgR2b, FcgR3a, FcgR3b, (B) functional activity of ADCD, ADCP and ADNP. (C) The Nightingale rose plots illustrate the average of the z-scored value for antibody functions (in purple) and binding to FcgR (blue) in the Active Treatment, Prior Treatment and Healthy groups infected with SARS-CoV-2, as well as the Healthy vaccinated group. Each wedge corresponds to a SARS-CoV-2 antibody feature, and the length of the wedge represents the magnitude of the response.
Effector functions against SARS-CoV-2 WT-Spike were also compared between the cohorts (active treatment, prior treatment, and healthy controls). Vaccination produced higher ADCD (p=0.0167, p=0.71, p= 0.054), higher ADCP (p=0.0055, p=0.04, p=0.035), and ADNP (p=0.0062, p=0.35, p=0.066) compared to acute SARS-CoV-2 infection. These differences were statistically significant for all groups for ADCP and the active treatment cohort for all assays. [Figure 4B]
As represented on the Nightingale rose plots, similar findings were demonstrated against RBD and S1 for all cohorts [Figure 4C]. For each functional feature, z-scored values showed that healthy vaccinated individuals demonstrated the most robust response to vaccination compared to a slightly diminished response in the prior treatment group and markedly decreased response in the active treatment group.
Lastly, comparisons of WT-Spike-specific differences in median immunoglobulin titers, FcR binding, and functional assays are represented as heatmaps between the active chemotherapy group and the healthy vaccinated group, with the healthy vaccinated group demonstrating the most robust response and the infected, active treatment group displaying the least robust response. [Figure 5A–B] Tile color indicates the groups with highest humoral response (red showing higher response in active chemotherapy group with COVID-19 infection and active chemotherapy group after vaccination, blue representing enrichment in healthy vaccinated group). The upper panel demonstrates comparable but a trend towards inferior responses for children on active chemotherapy after vaccination compared to healthy children after vaccination with no statistical significance detected between the groups. [Figure 5A] The lower panel demonstrates significantly inferior humoral responses among children on active treatment following SARS-CoV-2 infection compared to healthy vaccinated children, except for IgM production and FcαR binding which were non-significantly higher following natural infection compared with vaccination. [Figure 5B]
FIGURE 5.
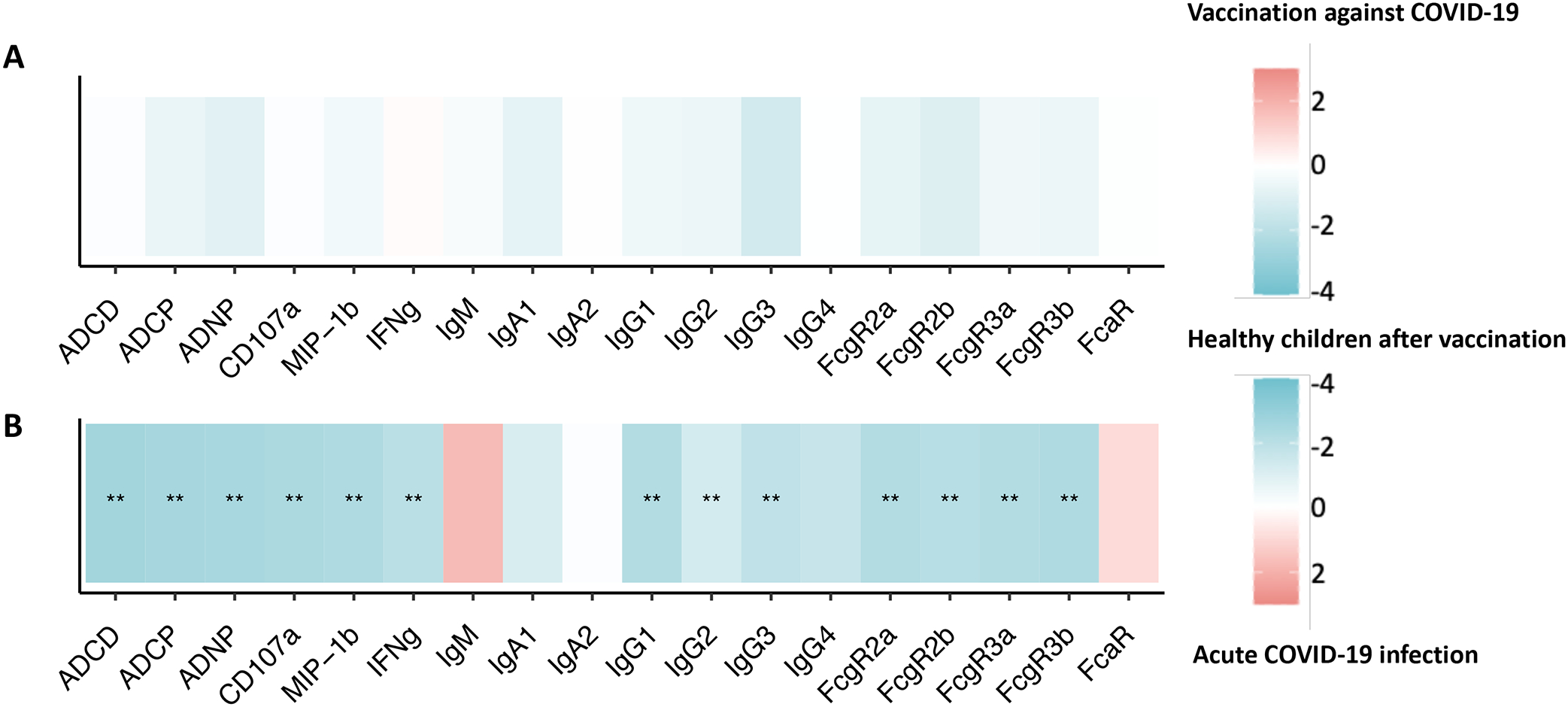
Heatmaps showing WT-Spike-specific differences between the (A) active chemotherapy group with SARS-CoV-2 infection and the healthy vaccinated group and (B) active chemotherapy group after COVID-19 vaccination and healthy vaccinated group. The color of the tiles shows the group in which antibody response was higher: red for active chemotherapy group with SARS-CoV-2 infection and active chemotherapy group after vaccination, blue for healthy vaccinated group. Statistical significance was calculated with a Wilcoxon-signed rank test, followed by Benjamini-Hochberg correction for multiple testing. **p < 0.01.
DISCUSSION
Vaccines against COVID-19 appear to produce an appropriate humoral response in this small cohort of pediatric patients actively receiving chemotherapy, those who have previously received chemotherapy, and healthy controls. Importantly, greater variability is noted among the patients actively receiving chemotherapy compared to other groups and there is a potential sub-group of non-responders, though our sample size is too small to discern the precise nature of this gap. Antibodies produced as a response to COVID-19 vaccination demonstrate comparable functionality in pediatric patients with cancer compared to healthy controls, though again some patients actively receiving chemotherapy have a reduced response.
Furthermore, COVID-19 vaccinations appear to produce a superior humoral response with improved functionality compared to natural infection in all children included in this study, including children actively receiving chemotherapy, children who had previously received chemotherapy, and healthy controls although the sample size is small and further large-scale investigations will be required to confirm this finding. Nonetheless, these findings underscore the importance of all children receiving COVID-19 vaccination, even if they have been previously infected with SARS-CoV-2 or are receiving chemotherapy.
Despite demonstrated efficacy and safety, COVID-19 vaccine uptake among children nationally remains low, with only 32% of 5–11-year-old children and 58% of 12–17-year-old adolescents having received their full primary vaccination series as of February 2023. Only 12% of children aged 6 months to 4 years have received their first dose, however the vaccine has only been FDA approved in this age group since June 2022 compared with May 2021 and November 2021 for the older age groups.27 Vaccines play a critical role in keeping children safe from serious illness due to COVID-19, and children who are immunosuppressed due to their cancer-directed therapy are at greater risk for severe disease. Emerging vaccine hesitancy data demonstrate moderate vaccine hesitancy for parents of children with cancer, as well as considerable hesitancy in adolescents and young adults with cancer.28,29 Our data underscore the critical importance of encouraging and facilitating COVID-19 vaccination within oncology clinics or the primary care setting for children with cancer, as these patients appear to respond to vaccination appropriately and are notably better protected by vaccination than acute infection.
Of note, our data demonstrated a significant over-representation of Hispanic ethnicity in COVID19+ patients (89%). As has been previously demonstrated, COVID-19 has disproportionately infected and affected Hispanic and non-Hispanic black children in the United States with higher rates of asymptomatic carriage, symptomatic disease, and severe disease.5,30 Reasons for this inequity are multiple but likely include increased exposure due to over-representation in high-risk front-line occupations, greater household size, decreased vaccine and health care access, higher rates of comorbid conditions, and innumerable other effects of structural racism.31–34
This study should be considered within the context of its limitations. Although it is a small, single center cohort study, it includes an in-depth investigation not previously performed in this population. The data were collected prior to vaccine availability for children less than 5 years old; although vaccine efficacy seems comparable for this younger group, this requires further evaluation specifically in younger patients with cancer who may not respond as expected.35 Further, not all types of cancer or chemotherapy regimens were represented in this study, though it did include a wide range of hematologic, solid, and CNS malignancies. The cohorts were also predominantly male, which may limit their generalizability. Lastly, the samples for vaccination were drawn on average slightly closer to the antigenic exposure than the samples for acute infection, which could contribute to the superior response seen, although the difference is on average only 1.6 weeks.
These results demonstrate that for children with cancer as well as healthy children, natural infection with the virus does not confer sufficient protection against COVID-19 compared with vaccination. This suggests that children who have been previously infected should still receive a full vaccination series, regardless of immunocompromised state. Furthermore, children with cancer mount a comparable immunologic response after vaccination compared with healthy children, although their response is variable amongst those actively receiving chemotherapy. Given their unique vulnerability, these children and their families should be strongly encouraged to receive COVID vaccination and recommended booster doses to best protect them against serious outcomes and secondary effects of COVID-19.
Supplementary Material
ACKNOWLEDGEMENTS
The authors graciously acknowledge the patients and families who participated in this study by providing samples for the Biorepository. The authors also wish to thank the clinic staff and providers at MassGeneral for Children (MGfC) Pediatric Hematology-Oncology clinic, without whom this work would not have been possible.
FUNDING SOURCES
We acknowledge support from Massachusetts General for Children, the Ragon Institute of MGH, MIT, and Harvard, Gates Foundation Global Health Vaccine Accelerator Platform funding (OPP1146996 and INV-001650 to G.A.) and the NIH (3R37AI080289-11S1 to G.A., R01AI146785 to G.A., U19AI42790-01 to G.A., U19AI135995-02 to G.A., U19AI42790-01 to G.A., 1U01CA260476-01 to G.A., CIVIC75N93019C00052 to G.A., 5K08HL143183 to L.M.Y.).
ABBREVIATION KEY:
- MGB
Mass General Brigham
- ADCD
antibody-dependent complement deposition
- ADCP
antibody-dependent cellular phagocytosis
- ADNP
antibody-dependent neutrophil phagocytosis
- ANOVA
Analysis of variance
- ALL
Acute lymphoblastic leukemia
- APML
Acute promyelocytic leukemia
- WT
Wild type
- RBD
Receptor-binding domain
- NTD
N-terminal domain
- S1
Spike 1 subunit
- S2
Spike 2 subunit
Footnotes
CONFLICT OF INTEREST STATEMENT
Conflicts of interest are as follows: G.A. is currently an employee of Moderna but was employed by MGH when this data was collected and analyzed. G.A. is a founder of Seromyx Systems, a company developing a platform technology that describes the antibody immune response. G.A.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. All other authors have declared that no conflicts of interest exist.
REFERENCES
- 1.COVID-19 Data Center. Accessed February 25 2023. https://coronavirus.jhu.edu/
- 2.Castagnoli R, Votto M, Licari A, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. Sep 2020;174(9):882–889. doi: 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 3.Patel NA. Pediatric COVID-19: Systematic review of the literature. Am J Otolaryngol. 2020 Sep - Oct 2020;41(5):102573. doi: 10.1016/j.amjoto.2020.102573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsabouri S, Makis A, Kosmeri C, Siomou E. Risk Factors for Severity in Children with Coronavirus Disease 2019: A Comprehensive Literature Review. Pediatr Clin North Am. February 2021;68(1):321–338. doi: 10.1016/j.pcl.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. Aug 2020;69(32):1081–1088. doi: 10.15585/mmwr.mm6932e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. Sep 2020;174(9):868–873. doi: 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and Cancer: a Comprehensive Review. Curr Oncol Rep. May 2020;22(5):53. doi: 10.1007/s11912-020-00934-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. March 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minotti C, Tirelli F, Barbieri E, Giaquinto C, Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. July 2020;81(1):e61–e66. doi: 10.1016/j.jinf.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari A, Zecca M, Rizzari C, et al. Children with cancer in the time of COVID-19: An 8-week report from the six pediatric onco-hematology centers in Lombardia, Italy. Pediatr Blood Cancer. August 2020;67(8):e28410. doi: 10.1002/pbc.28410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millen GC, Arnold R, Cazier JB, et al. Severity of COVID-19 in children with cancer: Report from the United Kingdom Paediatric Coronavirus Cancer Monitoring Project. Br J Cancer. February 2021;124(4):754–759. doi: 10.1038/s41416-020-01181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhusoodhan PP, Pierro J, Musante J, et al. Characterization of COVID-19 disease in pediatric oncology patients: The New York-New Jersey regional experience. Pediatr Blood Cancer. March 2021;68(3):e28843. doi: 10.1002/pbc.28843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulad F, Kamboj M, Bouvier N, Mauguen A, Kung AL. COVID-19 in Children With Cancer in New York City. JAMA Oncol. 09 January 2020;6(9):1459–1460. doi: 10.1001/jamaoncol.2020.2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marlais M, Wlodkowski T, Vivarelli M, et al. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health. July 2020;4(7):e17–e18. doi: 10.1016/S2352-4642(20)30145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batu ED, Özen S. Implications of COVID-19 in pediatric rheumatology. Rheumatol Int. August 2020;40(8):1193–1213. doi: 10.1007/s00296-020-04612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicastro E, Verdoni L, Bettini LR, et al. COVID-19 in Immunosuppressed Children. Front Pediatr. 2021;9:629240. doi: 10.3389/fped.2021.629240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston EE, Martinez I, Davis ES, et al. SARS-CoV-2 in Childhood Cancer in 2020: A Disease of Disparities. J Clin Oncol. 12 January 2021;39(34):3778–3788. doi: 10.1200/JCO.21.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehrnbecher T, Sack U, Speckmann C, et al. Longitudinal Immune Response to Three Doses of mRNA Vaccine Against COVID-19 in Pediatric Patients Receiving Chemotherapy for Cancer. Clin Infect Dis. Jul 28 2022;doi: 10.1093/cid/ciac570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fioredda F, Cavillo M, Banov L, Plebani A, Timitilli A, Castagnola E. Immunization after the elective end of antineoplastic chemotherapy in children. Pediatr Blood Cancer. Feb 2009;52(2):165–8. doi: 10.1002/pbc.21864 [DOI] [PubMed] [Google Scholar]
- 20.Esposito S, Cecinati V, Brescia L, Principi N. Vaccinations in children with cancer. Vaccine. Apr 26 2010;28(19):3278–84. doi: 10.1016/j.vaccine.2010.02.096 [DOI] [PubMed] [Google Scholar]
- 21.Altered Immunocompetence: General Best Practice Guidelines for Vaccination. ACIP Vaccine Recommendations and Guidelines. Centers for Disease Control and Infection (CDC); 2023. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html [Google Scholar]
- 22.Lima R, Gootkind EF, De la Flor D, et al. Establishment of a pediatric COVID-19 biorepository: unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children. BMC Med Res Methodol. Sep 11 2020;20(1):228. doi: 10.1186/s12874-020-01110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartsch YC, Chen JW, Kang J, et al. BNT162b2 induces robust cross-variant SARS-CoV-2 immunity in children. NPJ Vaccines. Dec 03 2022;7(1):158. doi: 10.1038/s41541-022-00575-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galloway SE, Paul P, MacCannell DR, et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage - United States, December 29, 2020-January 12, 2021. MMWR Morb Mortal Wkly Rep. Jan 22 2021;70(3):95–99. doi: 10.15585/mmwr.mm7003e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norman M, Gilboa T, Ogata AF, et al. Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat Biomed Eng. Dec 2020;4(12):1180–1187. doi: 10.1038/s41551-020-00611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartsch YC, St Denis KJ, Kaplonek P, et al. SARS-CoV-2 mRNA vaccination elicits robust antibody responses in children. Sci Transl Med. Nov 23 2022;14(672):eabn9237. doi: 10.1126/scitranslmed.abn9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Children and COVID-19 Vaccination Trends: AAP Analysis of Data Posted by the Centers for Disease Control and Prevention as of February 15, 2023. 2023. February 15, 2023. Accessed February 26, 2023.
- 28.Skeens MA, Hill K, Olsavsky A, et al. Factors affecting COVID-19 vaccine hesitancy in parents of children with cancer. Pediatr Blood Cancer. June 2022;69(6):e29707. doi: 10.1002/pbc.29707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waters AR, Kepka D, Ramsay JM, et al. COVID-19 Vaccine Hesitancy Among Adolescent and Young Adult Cancer Survivors. JNCI Cancer Spectr. June 2021;5(3):Pkab049. doi: 10.1093/jncics/pkab049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez DA, Hinson JS, Klein EY, et al. SARS-CoV-2 Positivity Rate for Latinos in the Baltimore-Washington, DC Region. JAMA. Jul 28 2020;324(4):392–395. doi: 10.1001/jama.2020.11374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001 Sep-Oct 2001;116(5):404–16. doi: 10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulson M, Geary A, Annesi C, et al. National Disparities in COVID-19 Outcomes between Black and White Americans. J Natl Med Assoc. Apr 2021;113(2):125–132. doi: 10.1016/j.jnma.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In California. Health Aff (Millwood). Jul 2020;39(7):1253–1262. doi: 10.1377/hlthaff.2020.00598 [DOI] [PubMed] [Google Scholar]
- 34.Selden TM, Berdahl TA. COVID-19 And Racial/Ethnic Disparities In Health Risk, Employment, And Household Composition. Health Aff (Millwood). Sep 2020;39(9):1624–1632. doi: 10.1377/hlthaff.2020.00897 [DOI] [PubMed] [Google Scholar]
- 35.Anderson EJ, Creech CB, Berthaud V, et al. Evaluation of mRNA-1273 Vaccine in Children 6 Months to 5 Years of Age. N Engl J Med. Oct 19 2022;doi: 10.1056/NEJMoa2209367 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


