Abstract
Background:
Host autoimmune activity in myocarditis has been proposed to play a role in development of cardiac disease, but evidence of autoimmunity and relationship to outcomes have not been evaluated in pediatric myocarditis.
Methods:
We performed a multi-institutional study of children with clinical myocarditis. Newly diagnosed patients were followed for up to 12 months and previously diagnosed patients at a single follow-up for serum levels of autoantibodies to human cardiac myosin, beta-adrenergic receptors 1 and 2, muscarinic-2 receptors, and antibody-mediated protein kinase A (PKA) activation in heart cells in culture. Results were compared with those of healthy control children.
Results:
Both previously diagnosed patient at follow-up (P = .0061) and newly diagnosed patients at presentation (P = .0127) had elevated cardiac myosin antibodies compared with control subjects. Antibody levels were not associated with recovery status at follow-up in either group. PKA activation was higher at presentation in the newly diagnosed patients who did not recovery normal function (P = .042).
Conclusions:
Children with myocarditis have evidence of autoantibodies against human cardiac myosin at diagnosis and follow-up compared with control subjects. Differences in antibody-mediated cell signaling may contribute to differences in patient outcomes, as suggested by elevated antibody-mediated PKA activation in heart cells by the serum from nonrecovered patients.
Keywords: Myocarditis, autoantibody, autoimmunity
Myocarditis leads to substantial morbidity and mortality in children, with almost 25% of patients experiencing death or heart transplantation by 3 years after diagnosis, and only 60% attaining echocardiographic normalization during the same period.1 Proposed mechanisms of tissue injury and cardiac dysfunction include direct injury from infectious agents as well as activation of the host immune system, including production of autoantibodies against cardiac tissue.2 Auto-antibodies against human cardiac myosin have been shown to directly target myosin and cross-react with beta-adrenergic receptor on cardiac tissue, with downstream effects on cyclic adenosine monophosphate (cAMP)–dependent activation of protein kinase A in heart cells in culture.3 Increased protein kinase A activation has been associated with cellular apoptosis after passive transfer of antibody, suggesting a possible mechanism of antibody-mediated cellular damage.4,5 Muscarinic receptor antibodies have been reported to induce a dilated cardiomyopathy phenotype in murine models6 and are associated with arrhythmias in other autoimmune diseases.7
Earlier reports evaluating the role of autoimmunity in the development of cardiac disease have found elevated levels of antibodies to cardiac proteins, such as cardiac myosin and beta-receptors, in adults with myocarditis and dilated cardiomyopathy,8,9 and adults with persistent anti-myosin antibodies had an associated lower rate of cardiac recovery at follow-up.10,11 Anti–muscarinic receptor antibodies have also been detected at high levels in adults with dilated cardiomyopathy as well as patients with atrial fibrillation.7,12 However, the presence of cardiac autoantibodies in clinical myocarditis and their relationship to clinical outcomes have not been evaluated in children. We hypothesized that children with clinical myocarditis would have evidence of autoimmunity at diagnosis, and that persistence of cardiac autoantibodies would be associated with cardiac dysfunction at follow-up.
Methods
Evaluation of Children Newly Diagnosed With Clinical Myocarditis
At total of 6 regional institutions participated in patient enrollment during the study period with Saint Louis Children’s Hospital acting as the coordinating institution. This study was approved by the Institutional Review Board at each of the participating study institutions. Informed consent was given by the subject’s guardian for all subjects, and informed assents were obtained from all subjects age ≥7 years if able.
Patients were classified as clinical myocarditis if their presentation was consistent with one of the following clinical myocarditis phenotypes: a) history of recent heart failure symptoms and echocardiographic evidence of significant left ventricular dysfunction without identifiable etiology; b) clinical diagnosis of myocarditis with myocardial biopsy consistent with the “Dallas criteria” (histopathologic findings of myocardial necrosis and infiltration of inflammatory cells)13; or c) clinical history of acute chest pain with ST-segment changes according to electrocardiography and/or elevated troponin levels without evidence of coronary artery abnormalities according to coronary angiography, with or without cardiac magnetic resonance imaging consistent with an “acute coronary syndrome” phenotype.14,15 Significant left ventricular dysfunction was defined as age-adjusted left ventricle shortening fraction z-score <2 standard deviations according to echocardiography. Heart failure symptoms included tachypnea, dyspnea, edema, weight loss, arrhythmia, and cardiogenic shock.
Patients were enrolled in 1 of 2 study groups based on timing of diagnosis of myocarditis. The 1st group consisted of newly diagnosed patients who were enrolled as part of a multicenter prospective longitudinal analysis to evaluate serial changes in markers of autoimmunity and cardiac function over time. Patients were eligible for enrollment in the newly diagnosed group if they were <18 years old at enrollment, presented at one of the participating study institutions for initial evaluation within 6 months of onset of clinical symptoms, and met the study definition of myocarditis and/or dilated cardiomyopathy. Newly diagnosed patients were followed for up to 12 months after diagnosis in conjunction with routine clinical follow-up. For patients who withdrew from the study or were lost to follow-up during the study period, data from the last evaluation were used for analysis. Patients who died or underwent heart transplantation during the 12-month study follow-up period were considered as non-recovered in the data analysis. Echocardiography was performed at the institution where each patient was enrolled, but was reviewed by an experienced pediatric cardiologist experienced in advanced imaging and blinded to patient status at the coordinating institution.
To evaluate how autoimmunity may be related to long-term outcomes in children with myocarditis, children who had been diagnosed with myocarditis in the past and had continued cardiology follow-up at one of the participating study institutions were enrolled in the 2nd group. Patients were eligible for enrollment in the previously diagnosed group if they were <18 years old at enrollment, presented for follow-up >6 months but <6 years after initial diagnosis, and had a diagnosis consistent with one of the clinical phenotypes of myocarditis as described above. Previously diagnosed patients were evaluated at a single time point in conjunction with a routine clinical follow-up appointment along with echocardiography and laboratory studies. Complete information from time of diagnosis for 1 patient in the previously diagnosed group was unavailable and not included in the analysis of characteristics, although the patient was included in the analysis of recovery and autoimmunity.
Control Group
To determine normal baseline levels for comparison of autoimmunity testing in children, control patients were enrolled for a 1-time blood sample at the time of routine intravenous line placement or blood drawing during an outpatient procedure for same-day surgery at the coordinating institution. Control subjects had to meet the same exclusion criteria as study subjects, have a negative history for recent illness or fever, and have no recent history of systemic steroid use or other immunomodulator therapy. A total of 26 children were enrolled in the control group.
Laboratory Evaluation and Technique
Specific anticardiac autoantibodies were evaluated based on both availability of assays and previously reported interactions of antibodies and the development of myocarditis, dilated cardiomyopathy, and/or arrhythmia in both nonhuman animals and humans, as previously described.3,8,16,17 Study and control patients had blood samples collected for measurement of human cardiac myosin antibodies, beta-1 adrenergic receptor antibodies, beta-2 adrenergic receptor antibodies and muscarinic receptor 2 antibodies, and antibody-mediated protein kinase A activation as previously described.3,8,16 All measurements of antibody levels and protein kinase A activation in heart cells were performed at a core research laboratory with previously published experience with the specific antibody assays and protein kinase A analysis (Cunningham Laboratory at the University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma).3,8 The core research laboratory is part of a similar ongoing National Institutes of Health–funded study of autoimmunity in adults with dilated cardiomyopathy (grant R01HL56267).
Statistical Analysis
Study data were analyzed with the use of SPSS version 20 and Graphpad Prism version 4. Categoric data were compared by means of the Fisher exact test. Continuous data are reported as mean ± SD for normally distributed data and median (interquartile range) for nonparametric data. Continuous data were compared with the use of Student t test or Mann-Whitney U test when appropriate. A P value of <.05 was considered to be statistically significant.
Correlation with antibody titers or protein kinase A activation and other variables was performed by means of the Pearson correlation coefficient. Comparison of antibody titers or protein kinase A activation between study and control patients was performed with the use of an analysis of covariance to adjust for any difference in age between the groups.
Results
Children With Myocarditis and/or Dilated Cardiomyopathy
During the study enrollment period fromApril 2011 to December 2012, 17 patients were enrolled in the newly diagnosed group and 12 in the previously diagnosed group. Group characteristics are described in Table 1.All patients were diagnosed within a month of symptoms onset. Both groups had similarly depressed cardiac systolic function and dilation by left ventricle shortening fraction z-score (−9.1 vs −10.9; P = .46) and left ventricle dimension z-scores according to echocardiography (left ventricle end diastolic dimension 5 vs 2.6 cm [P = .22]; left ventricle end systolic dimension 7.9 vs 6.1 cm [P = .57]) at presentation. A total of 3 of the 17 newly diagnosed patients had a normal measured shortening fraction at diagnosis, but clinical presentation was consistent with the “acute coronary syndrome” phenotype.
Table 1.
Patient Characteristics at Diagnosis
| Characteristic | Newly Diagnosed (n = 17) | Previously Diagnosed (n = 11; 11/12 With Complete Data) | P Value |
|---|---|---|---|
| Age at diagnosis (y) | 1.33 (0.63–12) | 0.83 (0.12–4.1) | .16 |
| Time from symptoms to diagnosis (d) | 9.7 ± 8.9 | 6.7 ± 5.8 | .54 |
| Symptoms before diagnosis Respiratory or gastrointestinal prodrome |
13 (76%) | 10 (91%) | .301 |
| Chest pain | 5 (29%) | 0 | .069 |
| Cadiogenic shock | 1 (6%) | 3 (27%) | .141 |
| Inotropic support | 10 (59%) | 11 (100%) | .025* |
| Ventilator support | 6 (35%) | 9 (82%) | .028* |
| Mechanical circulatory support | 4 (24%) | 6 (55%) | .008* |
| Treatment with IVIG | 6 (35%) | 8 (73%) | .076 |
| Treatment with steroids | 1 (6%) | 6 (55%) | .008* |
| Echocardiogram at diagnosis Initial LV SF z-score |
−9.1 (−15.5 to −2.6) | −10.9 (−14.2 to −5) | .46 |
| LVEDd (z-score) | 5 (0.88–9.3) | 2.6 (−0.48–5.1) | .22 |
| LVESd (z-score) | 7.9 (1.7–14.6) | 6.1 (0.62–9.9) | .57 |
Values are presented as median (interquartile range), mean ± SD, or n (%). IVIG, intravenous immunoglobulin; LVEDd, left ventricular end-diastolic dimension; LVESd, left ventricular end-systolic dimension.
P < .05.
Anti–Human Cardiac Myosin Autoantibodies Are Elevated in Children With Both Newly and Previously Diagnosed Myocarditis Compared with Control Children
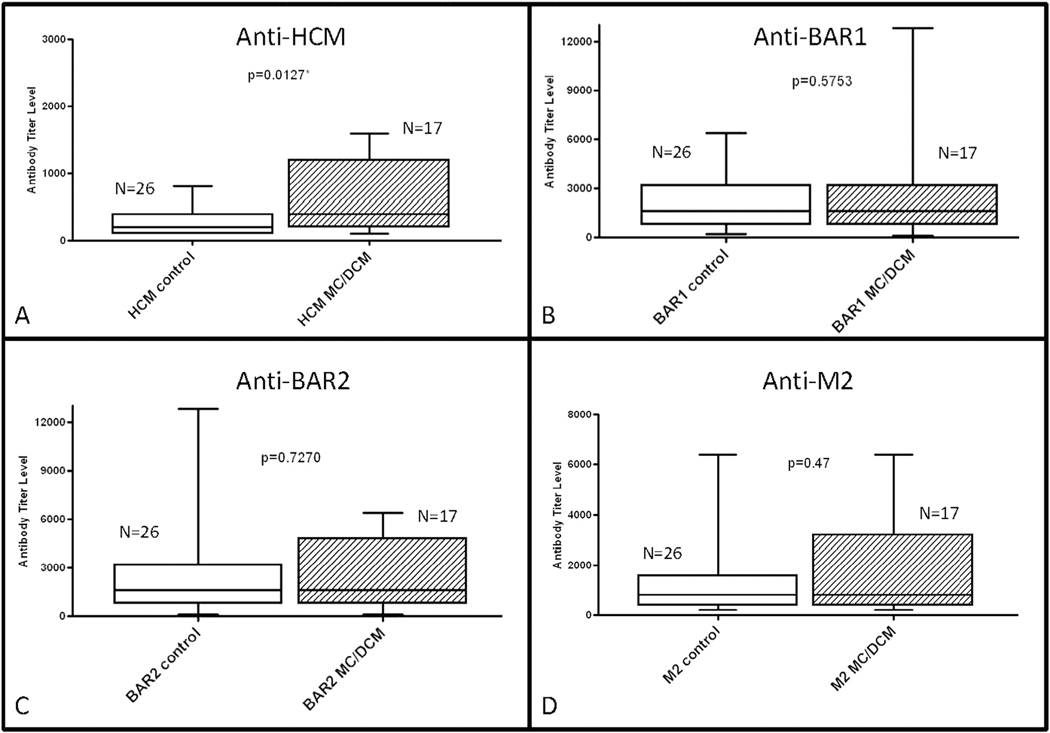
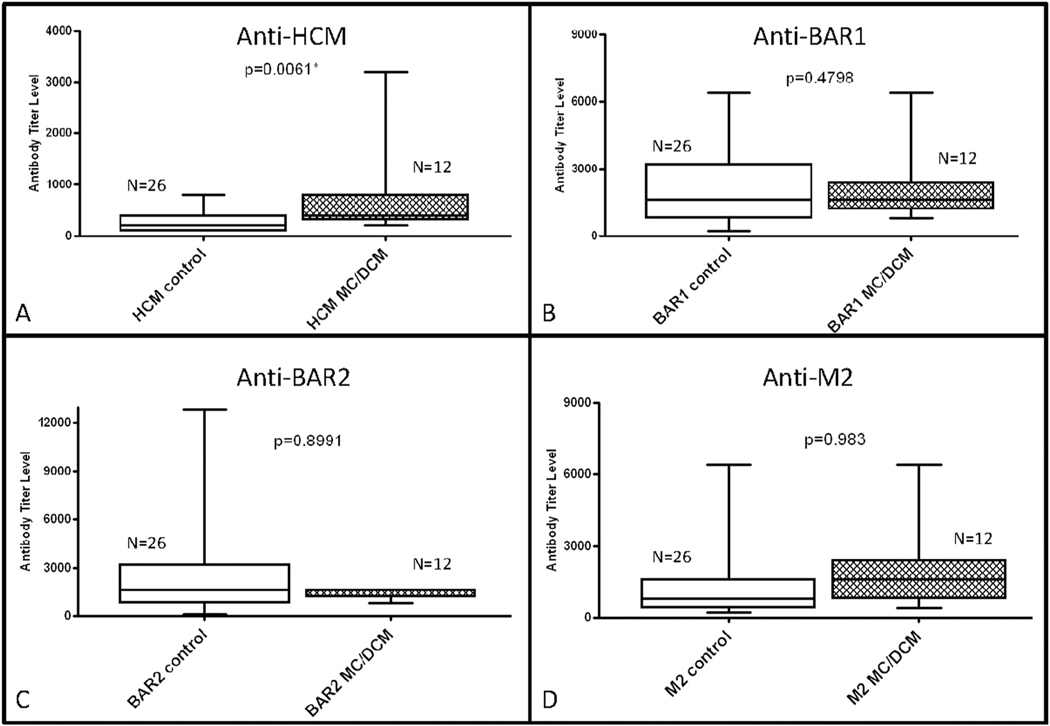
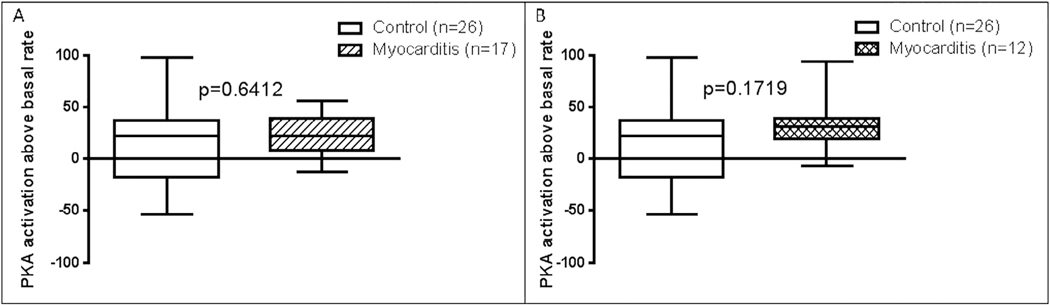
The newly diagnosed group had significantly higher human cardiac myosin antibody levels at time of diagnosis compared with the control group (n = 26; P = .0127; Fig. 1). The previously diagnosed group also had higher human cardiac myosin antibody levels at follow-up compared with the control group (P = .0061; Fig. 2). There was no significant difference in levels of beta-adrenergic receptor 1 (P = .58), beta-adrenergic receptor 2 (P = .73), or muscarinic receptor 2 (P = .47) antibody levels (Fig. 1) or of antibody-mediated protein kinase A activation in heart cells (P = .64; Fig. 3) at diagnosis in the newly diagnosed group compared with the control group. Comparison of the control group with the previously diagnosed group also revealed no significant difference in beta-adrenergic receptor 1 (P = .48), beta-adrenergic receptor 2 (P = .89), muscarinic receptor 2 (P = .98; Fig. 2), or in antibody-mediated protein kinase A activation in heart cells (P = .17; Fig. 3).
Fig. 1.
Comparison of median antibody titers between healthy pediatric control subjects and newly diagnosed myocarditis patients at initial diagnosis. (A) Serum titers of anti–human cardiac myosin (HCM), (B) anti–beta-adrenergic receptor 1 (BAR1), (C) anti–beta-adrenergic receptor 2 (BAR2), and (D) anti–muscarinic receptor 2 (M2) were compared between groups. Only anti-HCM titers were significantly higher in myocarditis patients compared with control subjects. P < .05 was considered to be statistically significant.
Fig. 2.
Comparison of median antibody titers between healthy pediatric control subjects and previously diagnosed myocarditis patients at follow-up. (A) Serum titers of anti–human cardiac myosin (HCM), (B) anti–beta-adrenergic receptor-1 (BAR1), (C) anti–beta-adrenergic receptor-2 (BAR2), and (D) anti–muscarinic receptor (M2) were compared between groups. Only anti-HCM titers were significantly higher in myocarditis patients compared with controls. P < .05 considered to be statistically significant.
Fig. 3.
Comparison of median level of antibody-mediated protein kinase A (PKA) activation above basal rate in healthy pediatric control subjects (A) compared with newly diagnosed myocarditis patients at time of initial diagnosis and (B) compared with previously diagnosed myocarditis patients at follow-up. P < .05 was considered to be statistically significant.
Taking into account possible differences in the maturity of the immune system with age, antibody levels and protein kinase A activation were analyzed for correlation with patient age. Median age for the control group was 4.2 years (interquartile range [IQR] 0.96–9.8) compared with median age of the new diagnosed group of 1.3 years (IQR 0.6–12; P = .46). The median age of the control group was significantly higher than the previously diagnosed group by 0.83 years (IQR 0.12–4.1; P = .02). Looking at specific antibody levels compared with age, only beta-adrenergic receptor 1 (Pearson coefficient 0.639; P = .006), beta-adrenergic receptor 2 (Pearson coefficient 0.552; P = .022) and muscarinic receptor 2 (Pearson coefficient 0.567; P = .340) antibody levels were found to correlate with age, with higher titers observed in older patients. In recognition of age differences among the control and study groups, we adjusted for patient age with the use of an analysis of covariance for comparison of study patients and control subjects, including antibodies and antibody-mediated protein kinase A activation. The analysis of covariance revealed that anti–human cardiac myosin antibody levels remained significantly different between the control patients and either the newly diagnosed (P = .0016) or the previously diagnosed (P = .0126) group.
Antibody-Mediated Protein Kinase A Activation in Heart Cells in Newly Diagnosed Myocarditis Group Correlated With Nonrecovery
In the newly diagnosed group, 59% of patients (10/17) had normal systolic function according to echocardiography at the last follow-up during the study period. Of those patients who did not recover, 2 (11%) died, 4 (23%) underwent heart transplantation, and 1 (5%) had persistent dilated cardiomyopathy at 12-month follow-up. All episodes or death or transplantation occurred ≤3months after diagnosis. Neither antibody titers nor protein kinase A activation correlated with initial left ventricle shortening fraction z-score at time of diagnosis. Of the 12 patients in the previously diagnosed group, 9 (75%) had recovery of normal systolic function according to echocardiography at clinical follow-up 26 ± 16 months after diagnosis.
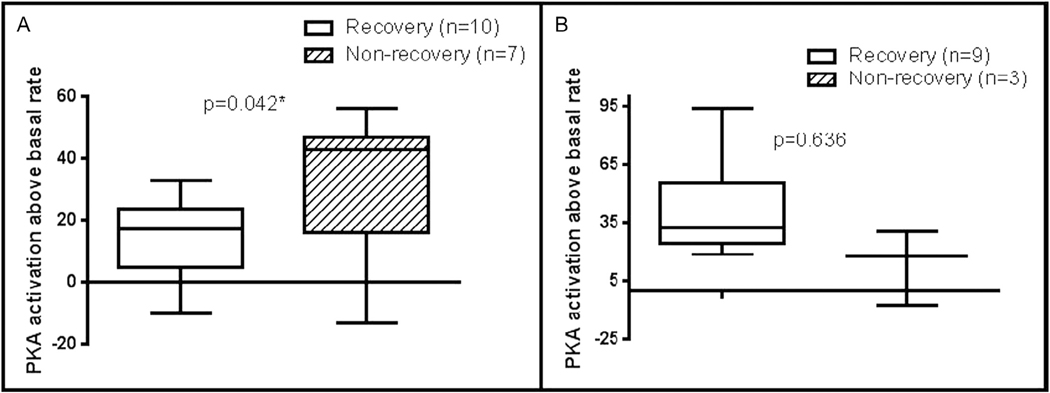
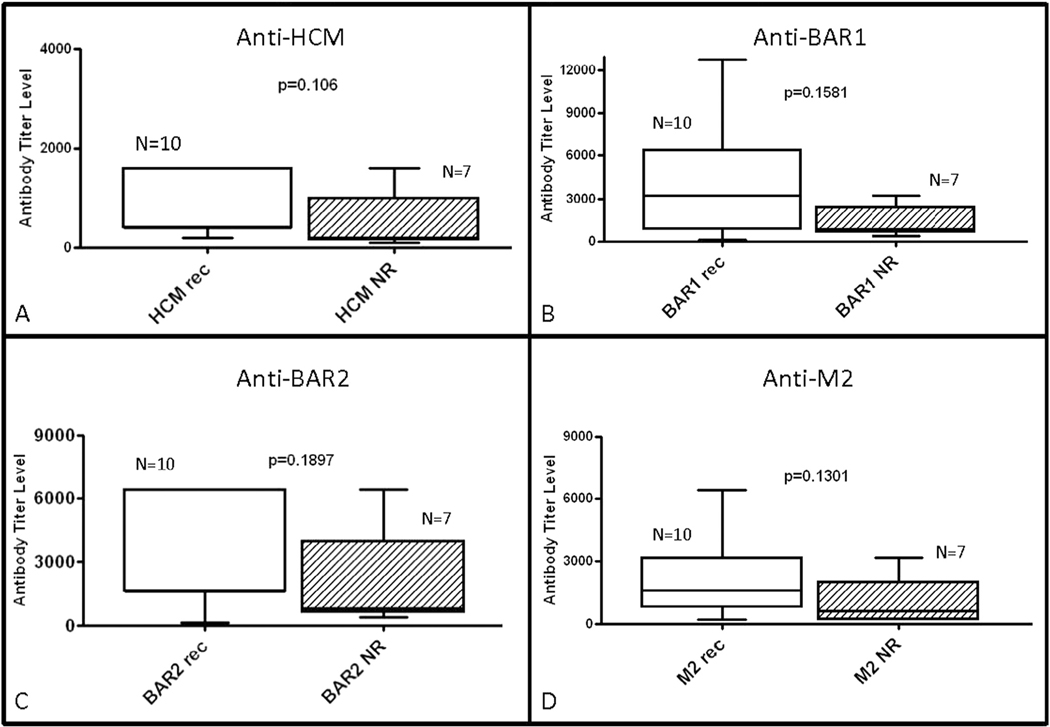
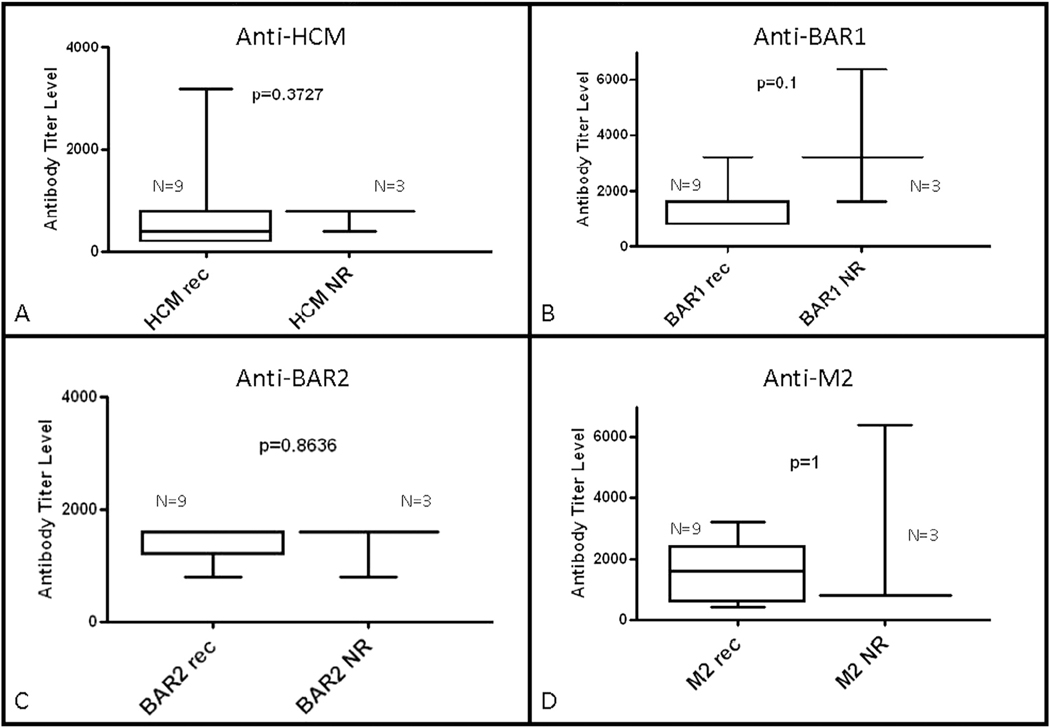
Analysis of results from time of diagnosis revealed that antibody-mediated signaling of protein kinase A activation in heart cells was higher at diagnosis in patients who did not recover normal function (43 vs 17.5; P = .042; Fig. 4). However, autoantibody titers at the time of diagnosis were not significantly different based on recovery status at last follow-up in the newly diagnosed group (Fig. 5). Antibody levels and antibody-mediated protein kinase A activation in heart cells were also not significantly different based on recovery at follow-up in the previously diagnosed group (Figs. 4 and 6). Although not reaching statistical significance, there was a trend toward higher anti–beta-adrenergic receptor 1 antibody compared with the other antibody titers in the nonrecovered previously diagnosed group.
Fig. 4.
Comparison of median level of antibody-mediated protein kinase A (PKA) activation above basal rate according to recovery status at follow-up (A) in newly diagnosed myocarditis patients at time of initial diagnosis and (B) in previously diagnosed myocarditis patients at follow-up. P < .05 was considered to be statistically significant.
Fig. 5.
Comparison of median antibody titers between newly diagnosed myocarditis patients at initial diagnosis according to recovery status at follow-up (Rec, recovery of normal function; NR, nonrecovery of normal function). Serum titers of (A) anti–human cardiac myosin (HCM), (B) anti–beta-adrenergic receptor 1 (BAR1), (C) anti–beta-adrenergic receptor 2 (BAR2), and (D) anti–muscarinic receptor 2 (M2) were compared between groups. There was no significant difference between groups based on recovery status. P < .05 was considered to be statistically significant.
Fig. 6.
Comparison of median antibody titers between previously diagnosed myocarditis patients at follow-up according to recovery status at follow-up (Rec, recovery of normal function; NR, nonrecovery of normal function). Serum titers of (A) anti–human cardiac myosin (HCM), (B) anti–beta-adrenergic receptor 1 (BAR1), (C) anti–beta-adrenergic receptor 2 (BAR2), and (D) anti–muscarinic receptor 2 (M2) were compared between groups. There was no significant difference between groups based on recovery status. P < .05 was considered to be statistically significant.
Discussion
This is the 1st study to demonstrate evidence of autoimmunity in children with clinically diagnosed myocarditis, where anti–human cardiac myosin antibodies were significantly elevated at both diagnosis and follow-up. Our findings are similar to previously reported elevated anti–human cardiac myosin levels in pediatric Kawasaki patients with associated myocarditis compared with febrile pediatric control subjects18 and adults with myocarditis or dilated cardiomyopathy compared with healthy control subjects.8 In contrast to earlier reports of elevated beta-adrenergic receptor and muscarinic receptor 2 autoantibodies in adults with myocarditis and/or dilated cardiomyopathy,8,19,20 we did not find elevated levels of these antibodies compared with control subjects. Several of the studies in adults did not detail the timing of sampling in relation to onset of cardiac disease, and many of the study populations were mainly nonischemic dilated cardiomyopathy, rather than specifically suspected myocarditis. Our pediatric study population, therefore, may not be easily comparable to the available adult studies. Some of our control patients may have also had unrecognized factors that affected auto antibody levels, as shown by 1 control patient who demonstrated both elevated anti–beta-adrenergic receptor 1 and anti–beta-adrenergic receptor 2 antibody levels compared with other control patients.
Although antibody levels in the newly diagnosed group at diagnosis and in the previously diagnosed group at follow-up were not associated with recovery in our study population, our study demonstrated that non recovered patients had higher antibody-mediated protein kinase A activation at time of diagnosis compared with patients who recovered normal systolic function. It may not be completely unexpected that antibody titers as measured with the use of enzyme-linked immunosorbent assay(ELISA) do not correlate with the protein kinase Aassay results. Antibodies of both low and high avidities are measured by means of ELISA, whereas the protein kinase A functional antibody assay measures higher-avidity antibodies, which are expected to signal the beta-adrenergic receptor leading to a potentially poor outcome.
Antibodies to beta-adrenergic receptor 1 and human cardiac myosin have been shown to stimulate beta-adrenergic receptors and lead to increased protein kinase A activation in murine models.3 Previous studies have shown that prolonged and increased beta-adrenergic receptor1 stimulation leads to increased cardiac myocyte apoptosis and decreased cardiac function.4,5 Increased beta-adrenergic receptor stimulation results in increased cAMP-dependent protein kinase A activation and subsequent changes in calcium regulation in the sarcoplasmic reticulum via alterations in L-type calcium channels and phosphorylation of sarcoplasmic proteins (phospholamban and RyR2).21,22 This effect appears to be robust with beta-adrenergic receptor 1– versus beta-adrenergic receptor 2–specific stimulation.22 Chronic direct protein kinase A activation in transgenic mice was associated with the development of adilated cardiomyopathy phenotype, decreased cardiac function, arrhythmias, and increased sudden death.21 The risk of sudden death correlated with the amount of protein kinase A activation, with increased death in the highest level of activation. Moreover, protein kinase A inhibition in mice exposed to increased beta-adrenergic stimulation prevented the development of cardiac dysfunction, fibrosis, and hypertrophy seen in wildtype mice.22 Evaluation of cardiac myocytes revealed that both apoptosis and calcium deregulation were prevented in the presence of protein kinase A inhibition. In children with myocarditis, it is possible that the ability of specific antibodies to stimulate beta-adrenergic receptors and the level of antibody-mediated protein kinase A activation may account for the differences in disease severity among patients.
Furthermore, anti–beta-adrenergic receptor autoantibodies as measured in serum by means of ELISA contain both high and low avidities and may not reflect the disease-producing functional beta-adrenergic receptor and protein kinase A–activating antibody species in the serum.23,24 Thus, not all antibodies measured with the use of ELISA are of high avidity leading to signaling and activation of protein kinase A. Protein kinase A is the most accurate measure of functional autoantibodies that can activate protein kinase A in the heart and presumably thus lead to disease consequences. Therefore, the quantity of antibody may not be as important as the avidity of the antibody and its ability to lead to downstream activation of beta-adrenergic receptor and subsequently increased protein kinase A activation, which has been shown to contribute to cellular damage and heart disease.4 Altered protein kinase A activation has been described in adults with ischemic and nonischemic heart failure compared with control subjects.25
Studies in nonhuman animals and humans have shown that the anti–human cardiac myosin antibodies cross-react with beta-adrenergic receptor and that the activating and signaling anti–human cardiac myosin/anti–beta-adrenergic receptor autoantibodies can be blocked by human cardiac myosin and beta adrenergic receptor, demonstrating that these cross-reactive antibodies are responsible for the signaling.3,8 Passive transfer of the anti–human cardiac myosin/anti-beta adrenergic receptor antibodies was shown to lead to apoptosis.3 Evidence demonstrates that the anti–human cardiac myosin/anti–beta-adrenergic receptor signalling is due to IgG inserum.3,8 The protein kinase A activation of serum was abrogated with the use of anti-IgG beads, indicating that the IgG in serum is responsible for the signalling of the beta-adrenergic receptor. Serum protein kinase A activation can also be blocked by beta-blockers as well as the antigens human cardiac myosin and beta-adrenergic receptor.3,8 Results from nonhuman animal models have also demonstrated the cross-reactivity between the human cardiac myosin and beta-adrenergic receptor in Western blots.3
The use of immunoadsorption in patients with chronic dilated cardiomyopathy suggests that antibodies may play an active role in disease severity and recovery. Adults with dilated cardiomyopathy and antibodies to beta-adrenergic receptor 1 and/or muscarinic receptor 2 who were treated with immunoabsorption had improved ejection fraction, decreased B-type natriuretic peptide, and improved 6-minute walk test if they had negative antibody testing after immunoadsorption therapy compared with nonresponders.26 Another study found that adults with dilated cardiomyopathy treated by means of immunoadsorption followed by intravenous immunoglobulin had improved ejection fraction and exercise parameters at follow-up compared with the untreated dilated cardiomyopathy group.27 The effects of immunoadsorption are most commonly related to antibody removal, but they also may be due to changes in specific T-cell activation or T-cell subsets after antibody removal.28
Study Limitations
Although we were able to demonstrate evidence of functional autoimmunity against the heart in children with clinical myocarditis, our study was limited by small sample size and limited serial follow-up in some study participants. Echocardiography was performed according to institutional protocol, which limited consistent data acquisition for more extensive analysis of functional parameters, including diastolic dysfunction. Also, as part of the observational nature of this study, immunomodulator therapy was used at the discretion of the referring physician. There was no difference in frequency of use of either therapy between patients based on recovery status in our study, but it is unknown how these commonly used therapies affect antibody levels or other specific levels of immune activation in patients with myocarditis.
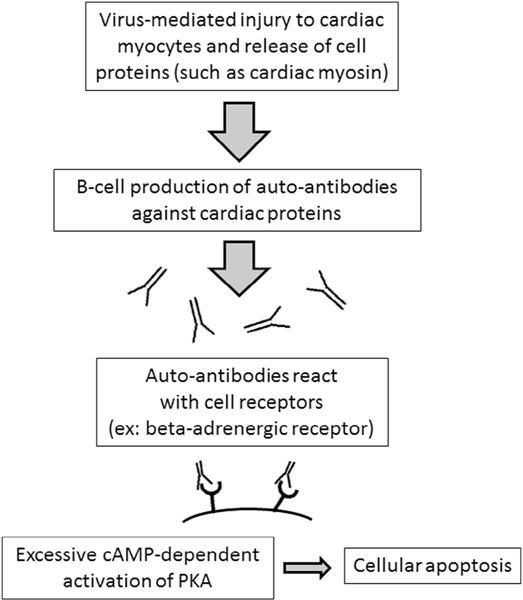
Recovery in pediatric myocarditis is likely multifactorial, with complex interactions between different pathways in the immune systems, including activation of B and T cells, antibody production, cytokine release, and cell signaling. Research to evaluate these relationships is ongoing, and may give more insight into the role of autoantibodies in the future. The effects of immunomodulator therapies, such as intravenous immunoglobulin and steroids, on patient outcomes have been inconsistently reported in the literature, and use varies among centers, as seen in our study population. Understanding the biomarkers of disease severity and mechanism of immune activation in children with myocarditis may help to guide therapy and lead to improved long-term outcomes in the future (Fig. 7).
Fig. 7.
Proposed mechanism for development of autoantibodies to cardiac proteins and downstream cell signaling leading to injury and death of cardiac myocytes in patients with myocarditis.
Conclusion
We present the first evidence of autoimmune activation in children with clinical myocarditis as demonstrated by elevated anti–human cardiac myosin antibodies compared with control children at both diagnosis and follow-up. Most importantly, antibody-mediated protein kinase A activation was associated with nonrecovery, suggesting that the downstream effects of cell signaling by the antibody may be more important than the presence of autoantibodies alone. Further large prospective studies in children with clinical myocarditis are needed to understand the complex relationship of autoimmune activation with patient outcomes and to identify potential targets for therapy.
Funding:
This study was funded in part from a research fellowship training grant from the Myocarditis Foundation and a research grant from the Children’s Discovery Institute of Washington University and Saint Louis Children’s Hospital. MWC was supported by grants R01HL56267 and R01HL35280, has been the recipient of MERIT Award HL35280 from the National Heart, Lung, and Blood Institute, and is supported by a Grant in Aid from the American Heart Association.
Footnotes
Disclosures
MWC is chief scientific officer at Moleculera Labs in Oklahoma City, Oklahoma, which performs testing for antineuronal autoantibodies in children. None of the other authors have any potential conflicts of interest.
References
- 1.Foerster SR, Canter CE, Cinar A, Sleeper LA, Webber SA, Pahl E, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the Pediatric Cardiomyopathy Registry. Circ Heart Fail 2010;3:689–97. [DOI] [PubMed] [Google Scholar]
- 2.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol 2008;3:127–55. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol 2006;177:8234–40. [DOI] [PubMed] [Google Scholar]
- 4.Saito S, Hiroi Y, Zou Y, Aikawa R, Toko H, Shibasaki F, et al. Beta-adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J Biol Chem 2000;275:34528–33. [DOI] [PubMed] [Google Scholar]
- 5.Shizukuda Y, Buttrick PM, Geenen DL, Borczuk AC, Kitsis RN, Sonnenblick EH. Beta-adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am J Physiol 1998;275:H961–8. [DOI] [PubMed] [Google Scholar]
- 6.Yoshizawa A, Nagai S, Baba Y, Yamada T, Matsui M, Tanaka H, et al. Autoimmunity against M(2) muscarinic acetylcholine receptor induces myocarditis and leads to a dilated cardiomyopathy–like phenotype. Eur J Immunol 2012;42:1152–63. [DOI] [PubMed] [Google Scholar]
- 7.Stavrakis S, Yu X, Patterson E, Huang S, Hamlett SR, Chalmers L, et al. Activating autoantibodies to the beta-1 adrenergic and M2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J Am Coll Cardiol 2009;54:1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascaro-Blanco A, Alvarez K, Yu X, Lindenfeld J, Olansky L, Lyons T, et al. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity 2008;41:442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail 2002;4:411–7. [DOI] [PubMed] [Google Scholar]
- 10.Lauer B, Schannwell M, Kuhl U, Strauer BE, Schultheiss HP. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol 2000;35:11–8. [DOI] [PubMed] [Google Scholar]
- 11.Warraich RS, Noutsias M, Kazak I, Seeberg B, Dunn MJ, Schultheiss HP, et al. Immunoglobulin G3 cardiac myosin autoantibodies correlate with left ventricular dysfunction in patients with dilated cardiomyopathy: immunoglobulin G3 and clinical correlates. Am Heart J 2002;143:1076–84. [DOI] [PubMed] [Google Scholar]
- 12.Nussinovitch U, Shoenfeld Y. The diagnostic and clinical significance of anti-muscarinic receptor autoantibodies. Clin Rev Allergy Immunol 2012;42:298–308. [DOI] [PubMed] [Google Scholar]
- 13.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1987;1:3–14. [PubMed] [Google Scholar]
- 14.Cooper LT Jr. Myocarditis. N Engl J Med 2009;360:1526–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monney PA, Sekhri N, Burchell T, Knight C, Davies C, Deaner A, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart 2011;97:1312–8. [DOI] [PubMed] [Google Scholar]
- 16.Galvin JE, Hemric ME, Ward K, Cunningham MW. Cytotoxic mAb from rheumatic carditis recognizes heart valves and laminin. J Clin Invest 2000;106:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, et al. Direct evidence for a beta 1-adrenergic receptor–directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest 2004;113:1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham MW, Meissner HC, Heuser JS, Pietra BA, Kurahara DK, Leung DY. Anti–human cardiac myosin autoantibodies in Kawasaki syndrome. J Immunol 1999;163:1060–5. [PubMed] [Google Scholar]
- 19.Baba A, Yoshikawa T, Fukuda Y, Sugiyama T, Shimada M, Akaishi M, et al. Autoantibodies against M2-muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J 2004;25:1108–15. [DOI] [PubMed] [Google Scholar]
- 20.Fu LX, Magnusson Y, Bergh CH, Liljeqvist JA, Waagstein F, Hjalmarson A, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest 1993;91:1964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ Res 2001;89:997–1004. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, et al. Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ Res 2013;112:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mertens NM, Galvin JE, Adderson EE, Cunningham MW. Molecular analysis of cross-reactive anti-myosin/anti-streptococcal mouse monoclonal antibodies. Mol Immunol 2000;37:901–13. [DOI] [PubMed] [Google Scholar]
- 24.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med 2003;9:914–20. [DOI] [PubMed] [Google Scholar]
- 25.Han YS, Arroyo J, Ogut O. Human heart failure is accompanied by altered protein kinase A subunit expression and post-translational state. Arch Biochem Biophys 2013;538:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba A, Akaishi M, Shimada M, Monkawa T, Wakabayashi Y, Takahashi M, et al. Complete elimination of cardiodepressant IgG3 autoantibodies by immunoadsorption in patients with severe heart failure. Circ J 2010;74:1372–8. [DOI] [PubMed] [Google Scholar]
- 27.Herda LR, Trimpert C, Nauke U, Landsberger M, Hummel A, Beug D, et al. Effects of immunoadsorption and subsequent immunoglobulin G substitution on cardiopulmonary exercise capacity in patients with dilated cardiomyopathy. Am Heart J 2010;159:809–16. [DOI] [PubMed] [Google Scholar]
- 28.Bulut D, Scheeler M, Wichmann T, Borgel J, Miebach T, Mugge A. Effect of protein A immunoadsorption on T cell activation in patients with inflammatory dilated cardiomyopathy. Clin Res Cardiol 2010;99:633–8. [DOI] [PubMed] [Google Scholar]