Abstract
Objective
To evaluate the efficacy and safety of stereotactic body radiotherapy (SBRT) in patients with adrenal gland metastasis (AGM) of oligometastatic lung cancer.
Methods
Between June 2013 and May 2021, 44 patients with oligometastatic lung cancer (51 AGMs) were treated with SBRT. Forty-six (90%) lesions received a biological effective dose (BED10, α/β = 10) of 100 Gy. The primary endpoint was local control (LC). Local control (LC), overall survival (OS), and progression-free survival (PFS) curves were calculated by the Kaplan-Meier method.
Results
The median follow-up was 23 months. The most common histology was non-small cell lung cancer (88.6%). The 1- and 2-year LC rates were both 95% and 91%, respectively. Overall survival was better in patients with solitary AGMs in univariate analysis.
Conclusion
This study demonstrated that SBRT with higher BED is associated with satisfactory LC and low toxicity rates in patients with AGM of oligometastatic lung cancer.
Keywords: Stereotactic body radiotherapy, adrenal gland metastases, oligometastatic lung cancer, biologically effective dose
INTRODUCTION
The adrenal glands, with their plentiful blood supply, are among the most common sites of metastatic involvement [1]. Autopsy studies documented that 27–38% of all fatal malignancies are attributable to lung cancer, which is the leading cause of adrenal gland metastasis (AGM) [2]. Various treatment approaches have been developed to manage AGM, including palliative radiotherapy, chemotherapy, surgery, and local ablation. External beam radiation therapy for AGM was previously used for pain palliation, and the overall response rate (defined as complete or significant pain relief) was 40–80% [3-4]. Several studies reported prolonged survival rates after adrenal metastasectomy in selected patients with isolated AGMs; however, this intervention has the potential to cause perioperative complications and adrenal insufficiency [5]. Image-guided percutaneous ablation (IGPA), which is a safe and well-tolerated modality for unresectable primary or metastatic AGM, remarkably contributes to the confined control of tumors with diameters less than 5 cm in the short term [6].
AGMs are frequently occult. Computed tomography (CT), magnetic resonance imaging, and positron emission tomography (PET) can help differentiate incidental adrenal adenomas from small metastases [7-8]. Recently, advances in technology and a better understanding of oligometastatic disease (defined as ≤5 lesions in ≤3 organ sites), which represents a distinct entity, have led to a paradigm shift in the role of stereotactic body radiotherapy (SBRT) in the treatment of patients with 1–5 distant metastases, resulting in prolonged overall and progression-free survival [9-10].
Studies of SBRT for AGM used a wide range of dose and fractionation schedules with inconsistent results regarding control of the metastases and survival rates [11-22]. In this retrospective analysis, we reported our experience with SBRT for patients with AGM of oligometastatic lung cancer and evaluated local control (LC) and treatment response.
PATIENTS AND METHODS
Data collection and study population
Our Hospital Ethical Committee approved this study with the approval number ASM-EK-20 / 127. The retrospective analysis consisted of patients with AGM of oligometastatic lung cancer treated with SBRT in our institution between June 2013 and May 2021. All patients had pathologically confirmed cancer diagnoses and underwent PET before SBRT. The inclusion criteria for SBRT treatment were as follows: age greater than 18 years, Karnofsky performance score of 70 or higher, oligometastatic disease, lesion diameter less than 6 cm, and the absence of previous radiotherapy or surgery in the respective region. All patients were medically or technically inoperable, or they refused surgical treatment. Written informed consent was obtained from all patients at the time of registration.
Technical approaches for treatment
Simulation and contouring
Each patient was immobilized using a vacuum bed and a wing board. After immobilization, a 10-phase 4D-CT scan was acquired using a CT simulator (GE, Milwaukee, WI, USA) and a respiratory positioning management device (Varian Medical Systems, Palo Alto, CA, USA). In the simulation, IV contrast was given to all patients. No patient was given oral contrast. Average intensity projection (AIP)-CT images were created from 10-phase 4D-CT images. All image sets (10-phase 4D-CT and AIP-CT) were imported into the Eclipse treatment planning system (Varian Medical Systems). A radiation oncologist delineated the gross tumor volume (GTV) on each phase of 4D-CT. All GTVs were accumulated on the AIP-CT image to create the internal target volume (ITV). The planning target volume (PTV) was generated by adding a 5-mm margin to the ITV. All organs at risk were delineated on AIP-CT. Abdominal compression was not administered to any patient.
Planning and treatment
All plans were generated using the volumetric modulation arc therapy (VMAT) technique. Generally, two partial arcs and 6FFF energy were chosen for planning purposes. The progressive resolution optimizer and anisotropic analytical algorithms were used for optimization and final dose calculation, respectively (Varian Medical Systems). During the planning, more than 95% of PTV was aimed to receive the prescription dose, but in case PTV intersects with some critical organs (stomach, bowel, etc.), the coverage of PTV was reduced by giving priority to critical organs, and they received dose below tolerance limits. In these patients, ITV was provided to be covered with the prescribed dose. For 5 fractions, the dosimetric constraints were as follows: (1) liver: D700cc < 15 Gy; (2) spinal cord: Dmax < 20 Gy, D0,35cc < 18Gy; (3) Colon: Dmax < 38 Gy, D20cc < 25Gy; (4) duodenum: Dmax < 32 Gy, D5cc < 18 Gy; (5) Stomach: Dmax < 32Gy, D10cc < 18Gy (6) Kidney: total kidneys, D200cc < 17.5 Gy; ipsilateral kidney, V15Gy < 35%. All patients were treated using the Trubeam STX (Varian Medical Systems) machine with high-definition MLC systems and six dimensions (6D) couch once VMAT plans were loaded. Cone beam computed tomography (CBCT) was mandatory for all patients. General bone matching was done with kV-kV image pair. After that first CBCT was taken for the tumor matching and controlling the situations of OARs, and the required shifts were applied in 6D. These shifts were controlled with a second CBCT, and irradiation was initiated. Real-time Position Management (RPM; Varian Medical Systems, Palo Alto, CA) was used to monitor.
Follow-up and response evaluation
Four to six weeks after the completion of SBRT, all patients underwent assessments, including a comprehensive medical history review, physical examination, and serum electrolyte level measurement. Additionally, patients were re-evaluated every three months after SBRT using contrast-enhanced CT or PET/CT. The treatment response was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST, v1.1) or via 18F-fluorodeoxyglucose (FDG)-PET using the PET Response Evaluation Criteria In Solid Tumors v1.0 [23]. The response to adrenal SBRT was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Early and late toxicities were scored according to the Common Terminology Criteria for Adverse Events (CTCAE, v4.3). Overall survival (OS), progression-free survival (PFS), LC, and the time to local failure were calculated starting from the end date of SBRT.
Statistical analysis
LC, OS, and PFS curves were calculated by the Kaplan–Meier method using SPSS v25.0 (IBM, USA). Prognostic factors were evaluated using univariate log-rank and Cox proportional hazards models. Multivariate analysis was not performed because of the limited number of events. Two-sided P values less than 0.05 were considered statistically significant.
RESULTS
Patient characteristics
Patient characteristics are outlined in Table 1. Forty-four patients with 51 AGMs were treated with SBRT between June 2013 and May 2021. The median patient age was 61.3 years (range 42–84), and the majority (82.6%) of patients were male. Only three patients were diagnosed with lung cancer, and both primary and adrenal metastases were treated locally. Although all other patients received adrenal SBRT, their primary tumors were under control. Twenty-one patients (48%) had the synchronous oligometastatic disease (≤6 months from the first diagnosis), and 23 patients (52%) metachronous oligometastatic disease (>6 months from the first diagnosis). At the time of SBRT, 18 patients (41%) had isolated adrenal metastasis. Fourteen of these patients (78%) had non-small-cell lung cancer (NSCLC). Four of the six patients with bilateral adrenal disease underwent bilateral SBRT for the concurrent disease. A single lesion was irradiated in two patients who subsequently developed contralateral AGM during the follow-up period. AGM was present at the time of diagnosis in 15 patients (34%). The most common histology was adenocarcinoma (68%), followed by squamous cell carcinoma (21%) and small-cell carcinoma (11%). In total, 42 patients (95%) had a previous history of chemotherapy, whereas six patients (14%) had previously received checkpoint inhibitor immunotherapy and four patients had target therapy. No patient received systemic therapy concurrent with SBRT. After the adrenal gland, SBRT total of sixteen patients received immunotherapy. Three of these patients were small-cell lung cancer, and in two NSCLC cancer patients, immunotherapy was suspended due to lung toxicity after 2 and 3 months. Two patients progressed after 6 and 8 months. There are a total of six metastatic NSCLC patients who started first-line immunotherapy. A total of 33 patients were examined for PD-1 and PD-L1, and it was negative (0%) in 22 patients. All metastases were detected using FDG-PET/CT, and the median FDG SUVmax was 8.9 (range, 3.3–42.7).
Table 1.
Patient characteristics
| Characteristic | Number (%) or median (range) |
|---|---|
| No. of patients | 44 |
| Age (years) | 61.3 (42–84) |
| Gender (female/male) | 9/35 |
| Karnofsky performance score | 90 (70–100) |
| Histology of the primary tumour | |
| Adenocarcinoma | 30 (68 %) |
| Squamous cell lung cancer | 9 (21 %) |
| Small-cell lung cancer | 5 (11%) |
| Type of metastasis | |
| Solitary adrenal metastasis | 18 (41%) |
| Other oligometastasis | 26 (59%) |
| Timing of adrenal metastases | |
| Synchronous | 21 (48%) |
| Metachronous | 23 (52%) |
| Laterality | |
| Left | 25 (57%) |
| Right | 19 (43%) |
| Bilateral | 4 (9%) |
Treatment characteristics
SBRT was delivered in five fractions at a median dose of 50 Gy (range, 45–50). Forty-six lesions (90%) received a biologically effective amount (BED10) of 100 Gy. The median BED10 (α/β = 10) was calculated as 100 Gy (range, 85.5–100), and the median duration of treatment was eight days (range, 5– 10). The median (range) tumor diameter, median GTV, and median PTV were 2 cm (1–5.5), 22.02 cm3 (5.2–91.9), and 71.17 cm3 (22–249.5), respectively. The mean PTV coverage by prescribed dose (V100%) was 92.3 % (84% - 97%) (Table 2).
Table 2.
Treatment characteristics
| Characteristic | Number (%) or median (range) |
|---|---|
| No. of fractions | 5 |
| Total dose, Gy | 50 (45–50) |
| Total BED10, Gy | 100 (85.5–100) |
| Gross tumour volume, cm3 | 22.02 (5.2–91.9) |
| Planning target volume, cm3 | 71.17(22–249.5) |
| Mean dose, Gy | 55.86(49.93–63.17) |
| Maximum dose, Gy | 60.96 (53.77–67.57) |
| Minimum dose, Gy | 42.75 (24.86–53.35) |
| The mean PTV coverage by prescribed dose (V100%) |
92.3 % (84% - 97%) |
| Conformity index | 0.9 (0.77–1.01) |
| Gradient index | 3.58 (2.99–5.79) |
BED: biological effective dose
Survival outcomes
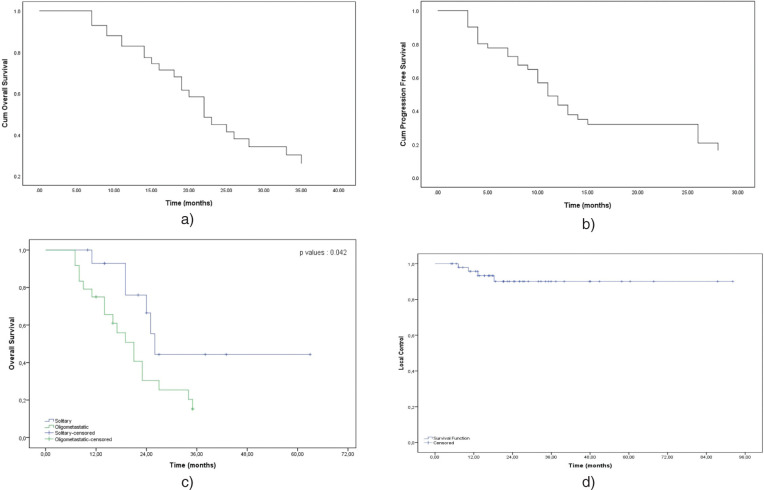
The median follow-up after the initial diagnosis was 36 months (range, 6–86), and that after adrenal SBRT was 23 months (range, 6–63). The median time from the primary diagnosis to AGM was nine months (range, 0–44). The median time from the primary tumor diagnosis to SBRT was 11 months (range, 3–44), and the median time from adrenal metastasis to SBRT was one month (range, 0–23). In total, 15 patients (29.5 %) were still alive at the end of the follow-up. Survival curves are presented in Fig. 1. The median OS after SBRT was 35 months (95% confidence interval [CI] = 23.59–46.40), and the 1- and 2-year OS rates were 85.2 and 57.3%, respectively. İn univariate analysis OS was better in patients with solitary adrenal metastasis than in patients with other metastasis (26 months vs. 20 months, p = 0.042). There was no significant difference in OS values between NSCLC patients who received immunotherapy and did not (40 months vs. 27 months, p = 0.073). No distant metastasis occurred in nine patients (23%) during follow-up. The median PFS after SBRT was 13 months (95% CI = 9.54–16.45), and the 1- and 2-year PFS rates were 45 and 30%, respectively. There was no difference in PFS after SBRT between patients with solitary adrenal metastasis and those with other oligometastases (14 months vs. ten months, p = 0.15). Similarly, there was no significant difference in PFS values between NSCLC patients who received and did not receive immunotherapy (13 months vs. ten months, p = 0.22). Other clinical characteristics (age, tumor laterality, synchronous vs. metachronous disease, FDG SUVmax before SBRT, response to SBRT, tumor size) were analyzed as prognostic factors for OS and PFS. No significant prognostic difference was found. The Cox proportional hazards analysis results are presented in Table 3. Four lesions developed local recurrence in only three patients, resulting in a 1- and 2-year LC rate of 95% and 91%. All these patients were treated with BED10 of 100 Gy. In addition, all patients with local recurrence had systemic progressions with local failure.According to RECIST, the local overall response rate was 77.1% (CR = 33%, PR = 44.1%). Meanwhile, three lesions (20.7%) exhibited SD, and PD was noted for one lesion (2.2%).
Figure 1.
Kaplan Meier survival curves for: a) progression-free survival in all patients; b) overall survival in all patients; c) overall survival of patients with solitary versus other oligometastatic diseases (p=0.042); d) local control for patients with adrenal gland metastases treated with SBRT
Table 3.
Univariate Cox proportional hazards analysis of prognostic factors
| Factors | All patients | Solitary | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Overall survival | ||||||||
| Age (≤65 years vs. >65 years) | 1.187 | 0.524–2.685 | 0.680 | 1.014 | 0.904–1.138 | 0.806 | ||
| Tumour laterality (bilateral vs. unilateral;a left vs. rightb) |
0.837 | 0.205–3.415 | 0.804 | 0.711 | 0.129–3.911 | 0.695 | ||
| Synchronous vs. metachronous | 0.681 | 0.302–1.532 | 0.353 | 0.420 | 0.089–1.989 | 0.271 | ||
| İmminothreapy vs no | 1.27 | 0.25 – 0.38 | 0.072 | — | — | — | ||
| Solitary vs. other oligometastasis | 2.497 | 0.988–6.320 | 0.045 | — | — | — | ||
| SUVmax of AGMc | 1.042 | 0.957–1.135 | 0.344 | 1.085 | 0.925–1.262 | 0.328 | ||
| Complete response vs. others | 0.510 | 0.138–1.879 | 0.312 | 0.479 | 0.085–2.697 | 0.404 | ||
| Tumour size (≤3 cm vs. >3 cm) | 1.012 | 0.982–1.043 | 0.426 | 1.008 | 0.957–1.062 | 0.764 | ||
| Progression-free survival | ||||||||
| Age (≤65 years vs. >65 years) | 1.105 | 0.760–1.606 | 0.602 | 0.970 | 0.879–1.071 | 0.547 | ||
| Tumour laterality (bilateral vs. unilateral;a left vs. rightb) |
0.496 | 0.128–1.921 | 0.310 | 0.675 | 0.167–2.724 | 0.581 | ||
| Metachronous vs. synchronous | 1.018 | 0.44–2.201 | 0.964 | 1.085 | 0.269–4.368 | 0.909 | ||
| Solitary vs. other oligometastasis | 1.708 | 0.78–3.743 | 0.181 | — | — | — | ||
| İmminotherapy vs no | 1.47 | 0.12- 0.25 | 0.224 | — | — | — | ||
| SUVmax of AGMc | 1.014 | 0.936–1.100 | 0.730 | 1.016 | 0.893–1.160 | 0.812 | ||
| Complete response vs. others | 1.134 | 0.349–3.684 | 0.834 | 1.194 | 0.246–2.892 | 0.826 | ||
| Tumour size (≤3 cm vs. >3 cm) | 1.002 | 0.974–1.032 | 0.870 | 0.907 | 0.546–1.504 | 0.704 | ||
aAll patients.
bSolitary adrenal metastasis.
cBefore SBRT.
HR: hazard ratio; CI: confidence interval, AGM: adrenal gland metastasis
Toxicity
The treatment was well tolerated. No grade ≥3 early or late adverse effects were observed after SBRT, according to CTCAE. Six patients (13.6%) complained of grade 1 fatigue, nausea, and early-term pain. Grade 2 diarrhea was observed in three patients (6.8%) with left-sided adrenal metastasis and tumor sizes of 4.5, 5, and 5.5 cm, respectively (Table 4).
Table 4.
Toxicity according to common terminology criteria for adverse events
| Grade 1, n (%) | Grade 2, n (%) | |
|---|---|---|
| Acute toxicity | 6 (13.6) | 3 (6.8) |
| Fatigue | 2 (33.3) | 0 |
| Gastrointestinal | 3 (50) | 3 (100) |
| Abdominal pain | 1 (16.6) | 0 |
| Chronic toxicity | 1 (2.2) | 0 |
| Abdominal pain | 1 (100) | 0 |
DISCUSSION
Several previous studies have evaluated the use of SBRT for treating AGM’s (Table 5). Only one randomized comparative trial has examined local treatment in the management of AGM [24]. Currently, adrenalectomy is the most common treatment modality in patients who are candidates for surgery. Gunjur et al. compared the results of surgery and local ablation therapy in patients with AGM of oligometastatic cancer. They reported 2-year OS and LC rates of 63 and 19% in the surgery group versus 84 and 46% in the SBRT group. Factors that influenced the survival rates were that patients in the SBRT group were not candidates for surgery because of the presence of comorbidities, their performance status had declined, and this group included a high number of patients with extra-adrenal diseases (52% vs. 25%). In this review, the reason for the lower LC rate was that the reported results were 2-year LC rates from only six studies, and the 1- and 2-year LC rates using a median BED10 < 60 Gy were 44–66 and 27–44%, respectively, in three of the studies [25]. Other minimally invasive ablation procedures such as radiofrequency ablation (RFA) and microwave ablation (MWA). A retrospective study identified a local failure in 23 of 71 non-small cell lung cancer patients one year after treatment, and the LC rate was 67.6% who received image-guided RFA and MWA [6].
In a study by Chance et al., the 1-year LC rate was 74%, and no local failure was observed in patients treated with BED10 > 100 Gy [13]. In a recent publication, the 1- and 3-year LC rates were 93.4 and 80.8%, respectively [17]. In this study, the comparative survival rates between patients with small and large tumors using a cut-off of 2.9 cm were significantly different (median OS: 54 months vs. 11 months, p = 0.01); however, no such association was found with LC. Finally, in a study by Zhao et al., the 1- and 2-year LC rates in the BED10 ≥ 85.5 Gy group were both 100%, compared with 92.3 and 23.1%, respectively, in the BED10 < 85 Gy group (p = 0.007). Univariate analysis in their studies revealed a GTV of less than 30 ml, which was significantly correlated with LC (p = 0.003) [16]. In a pooled meta-analysis and systematic review by Chen WC et al., in patients with AGM who underwent SBRT were BED10 of 60Gy, 80 Gy, and 100 Gy predicted 1-year LC of 70.5%, 84.8%, and 92.9% and 2-year LC of 47.8%, 70.1%, and 85.6%, respectively [26]. In another review, a one-year LC of over 95% was obtained at an approximately biological equivalent dose with α/β=10 Gy (BED10) of 116.4 Gy [20]. These data are consistent with studies that reported BED10 ≥ 100 Gy in patients with primary NSCLC and pulmonary metastasis increased the LC rate [27].
In a few studies, the study groups entirely consisted of patients with AGM secondary to lung carcinoma. In a retrospective study, Holy et al. analyzed the outcomes of adrenal SBRT in 18 patients with adrenal metastasis secondary to NSCLC and reported 1- and 2-year LC rates of 89 and 83%, respectively. In their study, the median PFS and OS for patients with solitary AGM were 12 and 23 months, respectively [11]. In a study by Gamsiz et al., SBRT was performed in 15 patients with AGM secondary to NSCLC with 17 lesions. The SBRT dosage prescribed in their study was 30 Gy in three fractions, and the median follow-up and median PFS were 16 and 10 months, respectively. The LC and OS rates were 86.7 and 33%, respectively [12]. In a study by Celik et al. involving 15 patients with oligometastatic AGM of NSCLC, the median OS was 17.3 months, and 1- and 2-year OS rates were 93.3 and 66.6%, respectively. In addition, the median PFS was 10.5 months, and the 1- and 2-year PFS rates were 60 and 46.6%, respectively. The prescribed treatment dosage was 42 Gy in six fractions. The median LC was 11 months, and the 1- and 2-year LC rates were 60 and 46.6%, respectively [15]. The results in the current study are consistent with these previous reports.
SBRT, which is a non-invasive treatment modality, has few side effects. In the literature, CTCAE grade 3 and higher toxicity rate is 1.5% and 0.2% [26]. Grade 4 toxicity was reported in only two patients in this analysis (gastrointestinal bleeding from a duodenal ulcer and a perforated pyloric ulcer 14 months after SBRT). In our practice, AAPM Task Group 101 protocols are followed [28]. The most commonly prescribed treatment dosage was 50 Gy delivered in five fractions in our study, and no grade 3-5 gastrointestinal toxicity was observed. When we investigated the gastric and intestinal dosage of three patients who developed grade 2 diarrhea, Dmax was lower than 32 Gy. These events were resolved in these patients after 3–5 days of anti-diarrhoeal therapy.
The tumor itself, as well as local therapy, may cause adrenal insufficiency. The rate of symptomatic adrenal insufficiency in patients with adrenal malignancies is 1% [29]. There are no reports of adrenal insufficiency greater than grade 3 after SBRT in the literature [26]. In one study, three patients with bilateral adrenal metastasis developed grade 1–2 adrenal insufficiency after six weeks, four months, and seven months, respectively [14]. Toesca et al. recorded no early or late side effects in four patients with bilateral adrenal metastasis who underwent SBRT [17], in line with our findings. However, in an analysis of postoperative complication risks in 7829 patients who underwent adrenalectomy, the rates of complications after bilateral and unilateral adrenalectomy were 23.4 and 15%, respectively (p < 0.001), and the rates of complications of adrenalectomy for malignant and benign lesions were 23 and 13%, respectively (p < 0.0001) [30].
This study clarifies the efficacy and safety of high-dose SBRT in patients with AGM secondary to lung cancer using homogeneous radiation doses and fractions.However, the limitations of this study include its retrospective nature, single-center design, a small number of patients, the prior receipt of a variety of systemic treatments among the patients, and short follow-up duration. Thus, prospective randomized comparative studies are required to determine the most effective treatment modality with minimum side effects.
This study demonstrated that SBRT with higher BED is associated with satisfactory LC and low toxicity rates in patients with AGM of oligometastatic lung cancer. SBRT can be considered a first-line treatment and an alternative to surgery in such patients.
ACKNOWLEDGMENTS
We want to thank Enago (enago.com) for the English language editing.
Funding: None.
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: Rashad Rzazade, Ngoc T.Pham, Hale Basak Caglar
Data collection: Rashad Rzazade, Menekse Turna, Mehmet Dogu Canoglu
Data analysis and interpretation: Rashad Rzazade, Ngoc T.Pham
Manuscript writing: Rashad Rzazade, Esra Kucukmorkoc
Final approval of manuscript: Hale Basak Caglar, Rashad Rzazade
REFERENCES
- 1.Kung AW, Pun KK, Lam K, Wang C, Leung CY. Addisonian crisis as presenting feature in malignancies. Cancer 1990. Jan65(1):177-9. [DOI] [PubMed] [Google Scholar]
- 2.Lo CY, van Heerden JA, Soreide JA, Grant CS, Thompson GB, Lloyd RV, Harmsen WS. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996. Apr83(4):528-31. [DOI] [PubMed] [Google Scholar]
- 3.Soffen EM, Solin LJ, Rubenstein JH, Hanks GE. Palliative radiotherapy for symptomatic adrenal metastases. Cancer 1990. Mar65(6):1318-20. [DOI] [PubMed] [Google Scholar]
- 4.Short S, Chaturvedi A, Leslie MD. Palliation of symptomatic adrenal gland metastases by radiotherapy. Clin Oncol (R Coll Radiol). 1996;8(6):387-9. [DOI] [PubMed] [Google Scholar]
- 5.Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor R, Coit DG, Bepler G. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: A systematic review and pooled analysis. J Clin Oncol. 2008. Mar26(7):1142– 7 [DOI] [PubMed] [Google Scholar]
- 6.Botsa EI, Thanou IL, Papatheodoropoulou AT, Thanos LI. Thermal ablation in the management of adrenal metastasis originating from non-small cell lung cancer: A 5-year single-center experience. Chin Med J (Engl). 2017;130(17):2027-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpi S, Ali JM, Tasker A, Peryt A, Aresu G, Coonar AS. The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Ann Transl Med. 2018;6(5):95-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vito d’Amuri F, Maestroni U, Pagnini F, Russo U, Melani E, Ziglioli F, Negrini G, Cella S, Cappabianca S, Reginelli A, Barile A, de Filippo M. Magnetic resonance imaging of adrenal gland: State of the art. Gland Surg. 2019;8(Suppl 3):S223-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez DR, Tang C, Zhang J, Blumenschein GR, Hernandez M, Jack Lee J, Ye R, Palma DA, Louie A V., Ross Camidge D, Doebele RC, Skoulidis F, Gaspar LE, Welsh JW, Gibbons DL, Karam JA, Kavanagh BD, Tsao AS, Sepesi B, Swisher SG, Heymach J V. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palma DA, Olson R, Harrow S, Gaede S, Louie A v., Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Bauman GS, Warner A, Senan S. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet [Internet]. 2019;393(10185):2051-8. Available from: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 11.Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187:245-51. 10.1007/s00066-011-2192-z [DOI] [PubMed] [Google Scholar]
- 12.Gamsiz H, Beyzadeoglu M, Sager O, Demiral S, Dincoglan F, Uysal B, Onal E, Dirican B. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori. 2015;101(1):98-103. [DOI] [PubMed] [Google Scholar]
- 13.Chance WW, Nguyen QN, Mehran R, Welsh JW, Gomez DR, Balter P, Komaki R, Liao Z, Chang JY. Stereotactic ablative radiotherapy for adrenal gland metastases: Factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract Radiat Oncol [Internet]. 2017;7(3):e195-203. Available from: 10.1016/j.prro.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 14.Franzese C, Franceschini D, Cozzi L, Agostino G, Comito T, De Rose F, Navarria P, Mancosu P, Tomatis S, Fogliata A, Scorsetti M. Minimally ınvasive stereotactical radio-ablation of adrenal metastases as an alternative to surgery. Cancer Res Treat. 2017;49(1):20-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celik E, Semrau R, Baues C, Trommer-Nestler M, Baus W, Marnitz S. Robot-assisted extracranial stereotactic radiotherapy of adrenal metastases in oligometastatic non-small cell lung cancer. Anticancer Res. 2017. Sep37(9):5285-91. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Zhu X, Fei J, Ren H, Cao Y, Ju X, Yuan Z, Zhang H. Short-term outcomes and clinical efficacy of stereotactic body radiation therapy (SBRT) in treatment of adrenal gland metastases from lung cancer. Radiat Oncol. 2018. Oct 22;13(1):205 doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toesca DAS, Koong AJ, Eyben R Von, Koong AC, Chang DT. Stereotactic body radiation therapy for adrenal gland metastases: Outcomes and toxicity. Adv Radiat Oncol. 2018;3(4):621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figura NB, Oliver DE, Mohammadi H, Martinez K, Grass GD, Hoffe SE, Johnstone PAS, Frakes JM. Novel dose escalation approaches for stereotactic body radiotherapy to adrenal oligometastases: A single-ınstitution experience. Am J Clin Oncol: Cancer Clin Trials. 2020;43(2):107-14. [DOI] [PubMed] [Google Scholar]
- 19.König L, Häfner MF, Katayama S, Koerber SA, Tonndorf-Martini E, Bernhardt D, von Nettelbladt B, Weykamp F, Hoegen P, Klüter S, Susko MS, Debus J, Hörner-Rieber J. Stereotactic body radiotherapy (SBRT) for adrenal metastases of oligometastatic or oligoprogressive tumor patients. Radiat Oncol 2020;15(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stumpf PK, Yorke ED, El Naqa I, Cuneo KC, Grimm J, Goodman KA. Modeling of tumor control probability in stereotactic body radiation therapy for adrenal tumors. Int J Radiat Oncol Biol Phys. 2021;110(1);217-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehret F, Kaul D, Kufeld M, Endt C vom, Budach V, Senger C, Fürweger C, Haidenberger A, Muacevic A. Robotic stereotactic body radiotherapy for the management of adrenal gland metastases: A bi-institutional analysis. J Cancer Res Clin Oncol 2023. Mar 49(3):1095-1101. 10.1007/s00432-022-03943-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baydoun A, Chen H, Poon I, Badellino S, Dagan R, Erler D, Foote MC, Louie AV, Redmond KJ, Ricard U, Sahgal A, Biswas T. Outcomes and toxicities in oligometastatic patients treated with stereotactic body radiotherapy for adrenal gland metastases: A multi-institutional retrospective study. Clin Transl Radiat Oncol 2022;33:159-164. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-47. [DOI] [PubMed] [Google Scholar]
- 24.Franzese C, Nicosia L, Facondo G, lo Faro L, Cuccia F, Vullo G, Osti MF, Alongi F, Scorsetti M. Stereotactic body radiation therapy for adrenal gland metastases: Outcome and predictive factors from a multicenter analysis. Clin Exp Metastasis. 2021. Dec 1;38(6):511-8. 10.1007/s00432-021-03807-z [DOI] [PubMed] [Google Scholar]
- 25.Gunjur A, Duong C, Ball D, Siva S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’ — A systematic review. Cancer Treat Rev. 2014; 40(7), 838-846. DOI: [DOI] [PubMed] [Google Scholar]
- 26.Chen WC, Baal JD, Baal U, Pai J, Gottschalk A, Boreta L, Braunstein SE, Raleigh DR. Stereotactic body radiation therapy of adrenal metastases: A pooled meta-analysis and systematic review of 39 studies with 1006 patients. Int J Radiat Oncol Biol Phys. 2020. May 1;107(1):48-61. 10.1016/j.ijrobp.2020.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieber J, Streblow J, Uhlmann L, Flentje M, Duma M, Ernst I, Blanck O, Wittig A, Boda-heggemann J, Krempien R, Lohaus F, Desirée N, Eble MJ, Imhoff D, Kahl H, Petersen C, Gerum S, Henkenberens C, Adebahr S, Hass P, Schrade E, Wendt TG, Hildebrandt G, Andratschke N, Sterzing F, Guckenberger M. Lung cancer stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases — A pooled analysis of the German working group “stereotactic radiotherapy.” Lung Cancer. 2016;97:51-8. 10.1016/j.lungcan.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 28.Benedict SH, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Purdie T, Schell MC, Salter B, Solberg T, Song DY, Timmerman R, Verellen D, Wang L. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys 2010. Aug37(8):4078-101. 10.1118/1.3438081 [DOI] [PubMed] [Google Scholar]
- 29.Lo KY, Lo CY. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002. Jan56(1):95-101. doi: [DOI] [PubMed] [Google Scholar]
- 30.Hauch A, Al-Qurayshi Z, Kandil E. Factors associated with higher risk of complications after adrenal surgery. Ann Surg Oncol 2015;22(1):103-10. 10.1245/s10434-014-3750-2 [DOI] [PubMed] [Google Scholar]