Abstract
Introduction
Stereotactic body radiation therapy (SBRT) is increasingly utilized for patients with recurrent and metastatic sarcoma. SBRT affords the potential to overcome the relative radioresistance of sarcomas through delivery of a focused high biological effective dose (BED) as an alternative to invasive surgery. We report local control outcomes after metastatic sarcoma SBRT based on radiation dose and histology.
Methods
From our IRB-approved single-institution registry, all patients treated with SBRT for metastatic sarcoma between 2014 and 2020 were identified. Kaplan-Meier analysis was used to estimate local control and overall survival at 1 and 2 years. A receiver operating characteristic (ROC) curve was generated to determine optimal BED using an α/β ratio of 3. Local control was compared by SBRT dose using the BED cut point and evaluated by histology.
Results
Forty-two patients with a total of 138 lesions met inclusion criteria. Median imaging follow up was 7.73 months (range 0.5–35.0). Patients were heavily pre-treated with systemic therapy. Median SBRT prescription was 116.70 Gy BED (range 66.70–419.30). Desmoplastic small round cell tumor, Ewing sarcoma, rhabdomyosarcoma, and small round blue cell sarcomas were classified as radiosensitive (n = 63), and all other histologies were classified as radioresistant (n = 75). Local control for all lesions was 66.7% (95% CI, 56.6-78.5) at 1 year and 50.2% (95% CI, 38.2–66.1) at 2 years. Stratifying by histology, 1- and 2-year local control rates were 65.3% and 55.0%, respectively, for radiosensitive, and 68.6% and 44.5%, respectively, for radioresistant histologies (p = 0.49). The ROC cut point for BED was 95 Gy. Local control rates at 1- and 2-years were 75% and 61.6%, respectively, for lesions receiving >95 Gy BED, and 46.2% and 0%, respectively, for lesions receiving <95 Gy BED (p = 0.01). On subgroup analysis, local control by BED > 95 Gy was significant for radiosensitive histologies (p = 0.013), and trended toward significance for radioresistant histologies (p = 0.25).
Conclusion
There is a significant local control benefit for sarcoma SBRT when a BED > 95 Gy is used. Further investigation into the dose–response relationship is warranted to maximize the therapeutic index.
Keywords: Sarcoma, SBRT, dose, local control, biological effective dose
BACKGROUND
Sarcomas represent approximately 1% of all malignancies and 10% of pediatric malignancies.1 While sarcoma encompasses an extremely heterogeneous group of histologies, these tumors are generally known to have aggressive growth patterns and a predisposition for distant metastasis. While prognosis for localized disease is driven primarily by tumor size and histologic grade, patients with metastatic disease have a poor prognosis with 20-30% event free survival.2 Therapy for metastatic disease is typically multimodal involving surgical resection, systemic chemotherapy, and radiotherapy. Historical data demonstrates that aggressive local control of sarcoma metastases can extend both overall and progression free survival.3,4
Stereotactic body radiation therapy (SBRT) uses advanced techniques of radiation intensity modulation, image guidance, patient immobilization, and real-time target visualization to precisely deliver high fractional doses of radiation in fewer fractions resulting in local tumor ablation.5 While the indications for SBRT are rapidly expanding, contemporary use commonly includes medically inoperable early stage non-small cell lung cancer, localized prostate cancer, hepatocellular carcinoma, cholangiocarcinoma, locally advanced pancreatic cancer, and oligometastatic cancers.6 Sarcomas are generally considered to be radioresistant, however important exceptions include rhabdomyosarcomas, Ewing’s sarcoma, and myxoid liposarcoma.7,8 The high fractional doses with SBRT increase the biological effective dose (BED)9 which theoretically provides opportunity to overcome the radioresistance of sarcomas to conventional radiotherapy. Available data have shown SBRT to be a promising therapeutic modality for select patients with metastatic sarcoma.10-12 Initial retrospective series using a range of dose and fractionations demonstrate local control ranging from 83 to 96% for sarcoma metastases treated with SBRT.13-15 Early data from a multi-institution phase II trial using 40 Gy delivered over 5 fractions (corresponding to 146.67 Gy BED) demonstrated local control of 95%.10 SBRT for bony metastases has been incorporated into prospective clinical trials for metastatic Ewings sarcoma through the Children’s Oncology Group (NCT02306161) and on the Phase II trial for metastatic non-rhabdomyosarcoma tumors (NCT01763970). These trials incorporate a SBRT dose regimen that stems from the median dose used from the initial Mayo clinic retrospective series.13 Although this early data is promising, questions remain regarding the optimal metastatic SBRT dose regimen and whether there are differences in response based on histology.
Certain histologic subtypes are known to be more radioresistant than others. In vitro radiation studies have found significant differences in radiosensitivity among different histologic subtypes of soft tissue sarcoma.16 Various genetic markers have been associated with radiosensitivity, and gene signatures have been developed to predict radiosensitivity in sarcoma.17 Dose prediction models have also been developed using genomic data to predict radiosensitivity and optimize radiation dose.18,19 Despite these recent developments in the pre-clinical space, there is a paucity of clinical data describing the relationships between radiation dose, relative histologic radiosensitivity, and local control in metastatic sarcoma.
In this study, we sought to determine whether local control rates would vary by sarcoma histologic subtype as divided by radiosensitive versus radioresistant. In addition, we sought to calculate an optimal BED cutoff associated with greater local control rates after SBRT.
METHODS
Cohort
Patients diagnosed with metastatic sarcoma and treated with SBRT between 2014 and 2020 were included as part of this IRB approved single institution registry. We included lesions encoded as clear cell sarcoma, desmoplastic small round cell tumor, Ewing sarcoma, osteosarcoma, paraganglioma, rhabdomyosarcoma, small round blue cell tumor, and synovial sarcoma based on the pathology report. Patients had to also have at least one metastatic lesion treated with SBRT. Nearly all patients had received multiple lines of systemic therapy prior to SBRT.
Treatment
The majority of patients were treated with a regimen of 35 Gy over 5 fractions corresponding to 116.67 Gy BED. The full dose range was 66.70 to 419.30 Gy BED. All regimens were delivered in five or fewer fractions, with a range of 1 to 5. Clinical decisions regarding SBRT dose regimen incorporated prior radiotherapy therapy, proximity of normal tissues, and concurrent therapies. Technical details for SBRT technique have been described elsewhere.11,20
Each lesion was classified as radiosensitive or radioresistant based on histology. Desmoplastic small round cell tumor, Ewing sarcoma, rhabdomyosarcoma, myxoid liposarcoma, and small round blue cell tumors were classified as more radiosensitive, and all other histologies were classified as radioresistant. We used this classification based on multiple basic science and clinical studies comparing the relative radioresistance of sarcoma histologies.19,21,22 For each lesion and treatment session, total dose and fractionation was recorded and converted to biological effective dose (BED) using an α/β ratio of 3.23 Patients received routine post-treatment radiological imaging (CT, PET/CT, or MRI) to monitor for local control. Local failure was defined by enlarged lesions on sequential imaging studies or interpretation of recurrence by a radiologist and/or tumor board.
A smoothed time-dependent receiver operating characteristic curve method was used to determine an optimal BED cut point to stratify high dose and low dose treatment groups based on local control.24 The dose value that maximized the Youden index, defined as the sum of sensitivity and specificity minus 1, was selected. The Kaplan-Meier method was used to compare local control rates first by radiosensitivity and then by dose treatment groups. A stratified Kaplan-Meier analysis then analyzed the interaction of radiosensitivity and dose on local control. Log-rank statistics were used to compare the groups. All data were stored in a secure RedCap registry and analyses were performed in R (v3.6.3).25,26
RESULTS
Forty-two patients with one hundred thirty-eight lesions were included in this study as described in Table 1. Median age of this cohort was 21 [range: 4 to 47]. Median clinical follow up time was 24 months [range: 6 to 35]. Median imaging follow up was 7.73 [range: 0.46 to 35.01] months, and 34 (28.8%) lesions had confirmed local recurrence.
Table 1.
Patient characteristics
| Number of Patients | 42 |
| Age | 21.46 [4.10, 47.70] |
| Male Sex (%) | 24 (57.1) |
| Average Pretreatment KPS (%) | |
| ≤70 | 6 (15.0) |
| 70–80 | 13 (32.5) |
| 80–90 | 17 (42.5) |
| 90–100 | 4 (10.0) |
| Follow up Time (months) | 23.84 [6.00, 35.01] |
Data is presented as either median [min to max] or as number in category (percentage of whole group).
Characteristics of the 138 lesions are detailed in Table 2. Sixty two lesions (44.9%) were considered radiosensitive based on primary lesion histology. Each lesion received a median total dose of 30 Gy [range: 15 to 60 Gy] in a median of 4 fractions [range: 1 to 5 fractions]. The median BED was 116.70 Gy [range: 66.70 to 419.30]. This correlated with a 5 fraction regimen of at least 35 Gy delivered with 7 Gy per fraction. Table 3 depicts every dose regimen used with 35 Gy delivered in 5 fractions (n = 17), 40 Gy delivered in 5 fractions (n = 24), and 21 Gy delivered in 3 fractions (n = 22) being the most common regimens.
Table 2.
Descriptive data for all lesions
| Characteristics of lesions | N = 138 |
| Radiosensitive lesions | 63 (45.6) |
| Histologic subtypes (%) | |
| Clear cell carcinoma | 3 (2.2) |
| Desmoplastic small round cell tumor | 22 (16.0) |
| Ewing sarcoma | 30 (21.9) |
| Osteosarcoma | 54 (39.4) |
| Paraganglioma | 7 (5.1) |
| Rhabdomyosarcoma | 7 (5.1) |
| Small round blue cell tumor | 4 (2.9) |
| Synovial sarcoma | 11 (8.0) |
| Received concurrent systemic therapy (%) | 79 (57.2) |
| Location of metastases (%) | |
| Bone | 104 (75.4) |
| Soft tissue | 16 (11.6) |
| Brain | 1 (0.7) |
| Lung | 16 (11.6) |
| Liver | 1 (0.7) |
| Radiation Dosimetry | |
| Mean biological effective dose | 116.70 [66.70, 419.30] |
| Planning target volume (cc) | 40.00 [0.18, 806.00] |
| Planning target volume for radioresistant lesions |
33.00 [0.18, 623.00] |
| Planning target volume for radiosensitive lesions |
77.00 [4.70, 806.00] |
| Lesions receiving a low dose (BED < 95 Gy) |
45 (32.6) |
| Dose per fraction (Gy) | 8.0 [5.0, 34.0] |
| Fractions | 4 [1, 5] |
| Total dose (Gy) | 30.0 [15.0, 60.0] |
Desmoplastic small round cell tumor, Ewing sarcoma, rhabdomyosarcoma, myxoid liposarcoma, and small round blue cell tumors were classified as radiosensitive, and all other histologies were classified as radioresistant. Concurrent therapy includes any chemotherapy or immunotherapy received at time of treatment. Recurrence was determined by CT or PET imaging surveillance. Data is presented as either median [min to max] or as number in category (percentage of whole group).
Table 3.
List of all dose regimens in study arranged by increasing BED
| Total Dose (Gy) | Fractions | BED (Gy) | Lesions |
|---|---|---|---|
| 25 | 5 | 66.67 | 2 |
| 21 | 3 | 70.00 | 22 |
| 24 | 3 | 88.00 | 7 |
| 15 | 1 | 90.00 | 2 |
| 30 | 5 | 90.00 | 12 |
| 16 | 1 | 101.33 | 2 |
| 30 | 4 | 105.00 | 7 |
| 27 | 3 | 108.00 | 1 |
| 35 | 5 | 116.67 | 17 |
| 18 | 1 | 126.00 | 13 |
| 30 | 3 | 130.00 | 10 |
| 37.5 | 5 | 131.25 | 2 |
| 40 | 5 | 146.67 | 24 |
| 24 | 1 | 216.00 | 3 |
| 50 | 5 | 216.67 | 9 |
| 48 | 4 | 240.00 | 1 |
| 60 | 5 | 300.00 | 1 |
| 34 | 1 | 419.33 | 3 |
One- and two-year local control rates for all treated lesions were 66.7% (95% CI: 56.6% to 78.5%) and 50.2% (95% CI: 38.2% to 66.1%), respectively. One- and two-year local control rates for radioresistant lesions were 68.6% (95% CI: 55.8% to 84.4%) and 44.5% (95% CI: 28.4% to 69.8%), respectively. For radiosensitive lesions, the one-year and two-year local control rates were 65.3% (95% CI: 50.8% to 84.0%) and 55.0% (95% CI: 38.5% to 78.7%) respectively. Local control outcomes for treated lesions can be found in Table 4.
Table 4.
Local control rates by radiosensitivity and dose
| 1 Year Local Control (95% CI) | 2 Year Local Control (95% CI) | p value | |
|---|---|---|---|
| All Lesions | 66.7% (56.6% to 78.5%) | 50.2% (38.2% to 66.1%) | |
| Radioresistant Lesions | 68.6% (55.8% to 84.4%) | 44.5% (28.4% to 69.8%) | p = 0.49 |
| Radiosensitive Lesions | 65.3% (50.8% to 84.0%) | 55.0% (38.5% to 78.7%) | |
| Lesions treated ≥95 Gy BED | 75.0% (64.3% to 87.6%) | 61.6% (47.8% to 79.4%) | p = 0.0099 |
| Lesions treated <95 Gy BED | 46.2% (28.2% to 75.6%) | NAa |
Local control rates are given as rate (95% CI).
aNo lesion treated with low radiation dose (<95 Gy BED) was controlled for 2 years.
The receiver operating characteristic curve showing the optimal BED cutoff associated with durable local control is depicted in Figure 1. The dose point at which the curve had the greatest vertical distance from the diagonal was 98.014 Gy and thus was selected as the cut off. This point also corresponds to the dose cut off with the best Youden index (sensitivity + specificity – 1). The ideal cut off of 98.014 Gy corresponded to a Youden index of 0.324. We used the cleaner number of 95 Gy to partition the cohort into high and low dose groups as it partitioned identically to 98.014 Gy. Forty-five lesions (32.6%) were treated with an SBRT prescription with a BED < 95 Gy. Lesions treated with a BED > 95 Gy experienced 1- and 2-year local control rates of 75.0% (95% CI: 64.3% to 87.6%) and 61.6% (95% CI: 47.8% to 79.4%), respectively. Lesions treated with a BED < 95 Gy experienced a 1-year local control rate of 46.2% (95% CI: 28.2% to 75.6%). No lesions treated with a BED < 95 Gy were observed to be locally controlled at 2 years.
Figure 1.
Survival receiver operator curve to determine optimal BED cutpoint
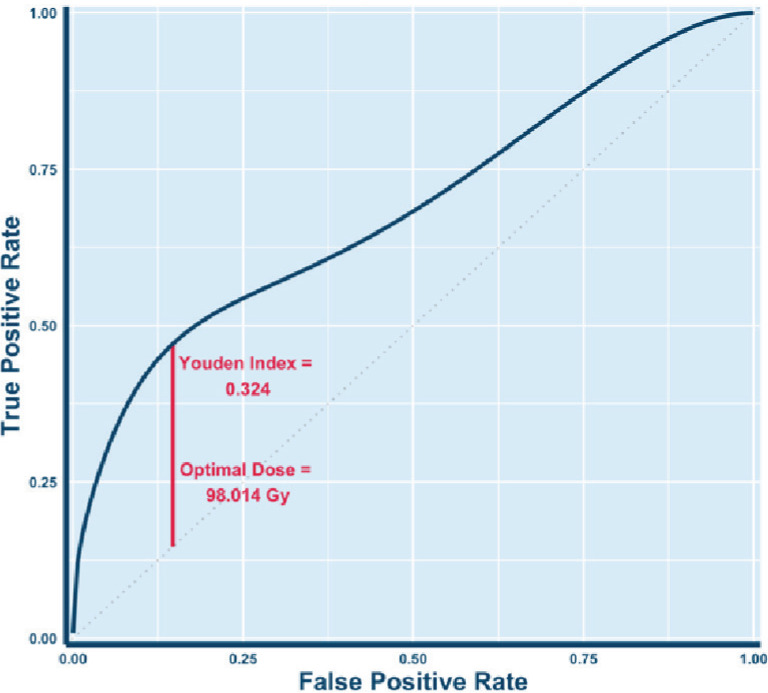
Note – The ROC was calibrated for 22 months of local control. The optimal cut point of BED = 98.014 Gy was determined by selecting the dose at which the Youden index (sensitivity + specificity – 1) was at a maximum (i.e. point on curve with furthest vertical distance from diagonal). The Youden index of this cut point was 0.324.
Figure 2 depicts the Kaplan-Meier analysis of local control comparing the two dose groups in the radioresistant lesion subset. Within this cohort, the 1-year local control rates were 52.5% (95% CI: 26.2% to 100%) for those treated with a BED < 95 Gy and 73.7% (95% CI: 60.7% to 89.7%) for those treated with a BED > 95 Gy. This numerical difference between the cohorts was not statistically significantly (p = 0.25), but only a small percentage (N = 17, 23%) received a BED < 95 Gy in this group. However, among the subset of radiosensitive lesions, the 1-year local control rates were 46.2% (95% CI: 25.3% to 84.6%) for those treated with a BED < 95 Gy and 79.3% (95% CI: 63.1% to 99.7%) for those treated with a BED > 95 Gy (Figure 3). This difference was statistically significant, p = 0.013. Table 5 lists the local control rates of the groups in Figures 2 and 3.
Figure 2.
Kaplan-Meier curve comparing local control by dose in radioresistant lesions
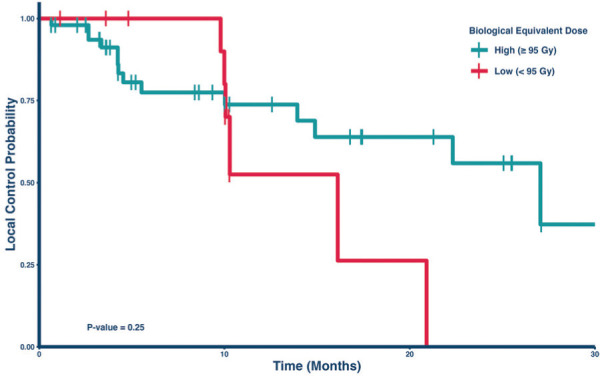
Note – There was no significant difference in local control rates by dose amount (p = 0.25).
Figure 3.
Kaplan-Meier Curve comparing local control by dose in radiosensitive lesions
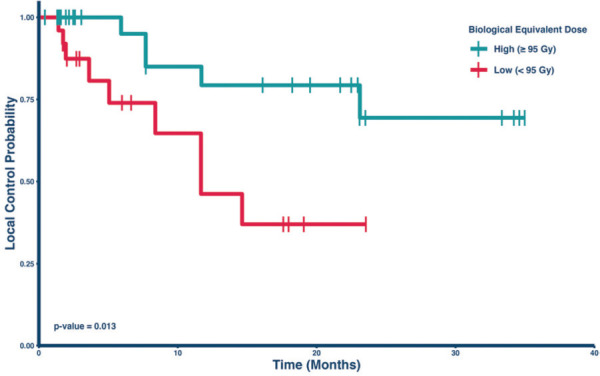
Note – Lesions treated with a high dose of radiation had significantly longer local control rates compared to lesions treated with a low dose (p = 0.013).
Table 5.
Local control rates after portioning by radiation dose and radiation sensitivity
| Radioresistant lesions | |||
|---|---|---|---|
| Low BED Dose (n = 17) | High BED Dose (n = 58) | p value | |
| 1 year local control | 52.5% (26.2% to 100%) | 73.7% (60.7% to 89.7%) | p = 0.25 |
| 2 year local control | NAa | 55.9% (38.2% to 82.0%) | |
| Radiosensitive lesions | |||
| BED < 95 Gy (n = 29) | BED ≥ 95 Gy (n = 34) | p value | |
| 1 year local control | 46.2% (25.3% to 84.6%) | 79.3% (63.1% to 99.7%) | p = 0.013 |
| 2 year local control | NAa | 69.4% (49.0% to 98.3%) | |
Local control rates are given as rate (95% CI).
aNo lesion in this group reached 2 year local control.
aNo lesions treated with low radiation dose (< 95 Gy BED) were controlled at 2 years.
Table 6 lists all acute and late toxicities experienced by the cohort. Almost all toxicities were grade 1 or 2 with skin changes and GI symptoms being the most common. One 1 grade 3 toxicity occurred as a late toxicity, which was in a patient who experienced enteritis and required 1 month in the ICU for sepsis. This patient had SBRT for retreatment of local progression and had concurrent and adjuvant high dose chemotherapy. No grade 4 or 5 toxicities occurred.
Table 6.
List of all toxicities experienced by cohort
| Acute toxicities | |
|---|---|
| Toxicity | Frequency |
| Grade I Fatigue | 12 |
| Grade I Constipation | 3 |
| Grade I Dermatitis | 1 |
| Grade I Alopecia | 1 |
| Grade I Fatigue | 2 |
| Grade I Chest pain | 2 |
| Grade I Nausea | 3 |
| Grade I Diarrhea | 2 |
| Grade I Pneumonitis | 1 |
| Grade I Other skin changes | 1 |
| Grade I Headache | 2 |
| Grade I Esophagitis | 1 |
| Grade I Mucositis | 1 |
| Grade I Dysphagia | 1 |
| Grade I Pain | 1 |
| Grade I Anorexia | 1 |
| Grade II Myositis | 1 |
| Late toxicities | |
| Toxicity | Frequency |
| Grade 2 Skin changes | 5 |
| Grade 2 Cough | 1 |
| Grade 2 Pain | 1 |
| Grade 2 Nausea | 2 |
| Grade 2 Pneumonitis | 1 |
| Grade 3 Enteritis | 1 |
DISCUSSION
We report a large series investigating the efficacy of SBRT in providing local control for metastatic sarcoma patients, specifically exploring the interactions between BED of the SBRT treatment and the relative radioresistance of heterogeneous sarcoma histologies. Although the local control at one year of 66.7% is lower than other published series, our inclusion of a heterogeneously treated group allowed us to ask this important SBRT dose question. Our institutional SBRT strategy has changed over time from being more conservative with SBRT dose at the beginning and now reflecting the SBRT dose regimens used on Children’s Oncology Group trials.Among the 138 lesion treated, we observed a dose-response relationship with local control of metastatic sarcoma. Intrinsic histologic radiosensitivity of a lesion by itself was not observed to be associated with local control. After calculating an optimal cut point to stratify lesions into high and low dose groups, an association between improved local control and patients who received a BED of SBRT >95 was observed.
Several different radiation dose and fractionation schemes have been used to treat metastases from sarcoma. The Mayo Clinic published an initial series of 14 Ewings or osteosarcoma patients treated with SBRT using a range of SBRT doses, with a median dose of 40 Gy over 5 fractions (147.67 Gy BED) for targets treated for a definitive intent, resulting in a local control rate of 85%.13 A retrospective review of 15 patients with metastatic sarcoma treated with palliative radiotherapy found recurrence in three patients using 39 Gy delivered in 13 fractions (78 Gy BED).27 Our earlier review of metastatic sarcoma in pediatric and AYA patients found local control to be 83% at one year among 88 lesions treated with a median dose of 30 Gy in 5 fractions (90 Gy BED).11 Although in general sarcomas are considered radioresistant, they include a heterogeneous group of histologies which may have various dose response relationships.
Analyses defining an ideal target SBRT dose for metastatic sarcoma lesions have not been sufficiently explored. A retrospective review of patients with metastatic sarcoma treated with SBRT found a positive correlation between local control and dose to clinical target volume.15 In our retrospective study, we did not have enough lesions to properly draw a dose response curve for continuous analysis. We thus opted for a discrete analysis by finding an optical dose cut off that is associated with greater local control. There is a difference in local control across our cut point for radiosensitive and radioresistant lesions. Also, recent SBRT data demonstrates improved local control and radiographic response for bone targets as compared to soft tissue.12 Our analysis did not evaluate lesion type, but the majority of targets treated were osseous.
In theory, a sufficiently high dose of radiation can eliminate any lesion; however, ideally the minimum dose to achieve local control is sought in order to minimize radiation toxicity. A high dose per fraction of SBRT also carries with it a risk of late skin and soft tissue toxicity and unknown risks on growth and development in a younger population. Patient selection and thoughtful consideration of concurrent therapies is also critical. Brown et al., described three late grade 3 toxicities including a sacral plexopathy in a patient treated with 60 Gy over 10 fractions for reirradiation after prior 59.4 Gy conventional radiotherapy and a myonecrosis in a patient who received 50 Gy over 5 fractions with concurrent gemcitabine.13 Our earlier retrospective analysis of SBRT for 31 patients with metastatic sarcoma revealed only one patient developed a late grade 3 radiation related toxicity (intestinal obstruction). This patient had previously received 18 Gy in a single fraction to a large local recurrence treated with conventional radiotherapy 18 months prior. This patient’s spine radiosurgery was given with concurrent chemotherapy, followed by gemcitabine and docetaxel which certainly may have contributed to the patient’s grade 3 toxicity.11 Similarly, in our current cohort, one patient experienced a grade 3 complication of enteritis.
Factors associated with increased radioresistance in lesions include larger tumor volumes, hypoxic conditions, and enhanced DNA repair mechanisms.28 Complicating the picture is that radiotherapy, similar to chemotherapy, can select for tumor cell subpopulations that are more resistant, leading to a recurrence that is more radioresistant than the original lesion.29 This is often the setting when SBRT is used in a highly pretreated population.
Proposed strategies against radioresistance include use of synergistic systemic agents. If radiation therapy does not induce sufficient DNA damage to kill a tumor cell, chemotherapy agents such as 5FU and cisplatin can cause additional damage that reach the required threshold.30 A drug that could inhibit DNA damage repair pathways would also have a synergistic effect with radiation therapy lowering the required dose to kill a tumor. Additionally, radiation itself may synergize with immunotherapy by promoting the production of neoantigens and stimulating an immune response.31
Additional work that seeks to optimize ideal SBRT dose to treat sarcoma should consider factors such as synergistic therapy, intrinsic tumor biology, and target type (ie. bone versus soft tissue). In our analysis, we show that for metastatic sarcoma lesions, a BED of at least 95 Gy is needed for reliable local control. We believe the next step is designing prospective studies that compare how SBRT dose regimens interact with these other factors that may impact control.
Limitations
This study has several limitations. First, this study is subject to the standard confounding that accompanies retrospective analyses. Further, because metastatic sarcoma is relatively rare and SBRT has limited indications in this clinical scenario, the relatively small sample size limited the power of our analyses. However, to our knowledge, this is the largest cohort of patients with metastatic sarcoma from a single institution analyzed for SBRT local control and dose response. While there are no standardized protocols for radiation dose prescription, certain dose regimens were more represented than others in this analysis. As such, constructing a continuous dose-responsive curve to precisely describe the relationship between dose and local control was not possible. Therefore, it remains unknown how much each incremental adjustment of SBRT radiation dose affects local control. Lastly, median surveillance follow up time was limited so this study cannot comment on whether local control rates are stable in the patients treated with high dose SBRT beyond 2 years. However, there is evidence that local failure, if it were to occur, happens mostly within the first 6 months in patients with metastatic sarcoma with one study finding a median time to recurrence of 159 days.32,33
CONCLUSION
For patients undergoing stereotactic body radiotherapy for metastatic sarcoma, BED of at least 95 Gy was associated with better local control. These findings have implications for treatment planning and prospective study design. Further study is needed to optimize local control by exploring relationships between SBRT dose regimens, specific tumor histology, target type, and concurrent therapies.
ACKNOWLEDGMENTS
IRB approval status: Reviewed and approved by the Cleveland Clinic Institutional Review Board
Authors’ declaration of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: Erin Murphy, Timothy Smile, Shireen Parsai, James Broughman, Ahmed Halima
Data collection: Eashwar Somasundaram, Timothy Smile, Shireen Parsai, Ahmed Halima, Jacob G Scott, Timothy Chan, Shauna Campbell, Lilyana Angelov, Peter M Anderson, Stacy Zahler, Matteo Trucco, Stefanie M Thomas, Shavaughn Johnson, Peng Qi, Anthony Magnelli, Chirag Shah, Lukas Nystrom, Dale Shepard, George Thomas Budd, Nathan Mesko
Data analysis and interpretation: Eashwar Somasundaram, Chandana Reddy, Timothy Smile, Ahmed Halima
Manuscript writing: Eashwar Somasundaram, Timothy Smile, Ahmed Halima
Final approval of manuscript: All authors
REFERENCES
- 1.Williams RF, Fernandez-Pineda I, Gosain A. Pediatric sarcomas. Surg Clin North Am 201696(5):1107-1125. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez Tejada FN, Zamudio A, Marques-Piubelli ML, Cuglievan B, Harrison D. Advances in the management of pediatric sarcomas. Curr Oncol Rep 202023(1):3. [DOI] [PubMed] [Google Scholar]
- 3.Pastorino U. History of the surgical management of pulmonary metastases and development of the International Registry. Semin Thorac Cardiovasc Surg 200214(1):18-28. [DOI] [PubMed] [Google Scholar]
- 4.Haeusler J, Ranft A, Boelling T, Gosheger G, Braun-Munzinger G, Vieth V, Burdach S, van den Berg H, Juergens H, Dirksen U. The value of local treatment in patients with primary, disseminated, multifocal Ewing sarcoma (PDMES) Cancer 2010116(2):443-450. [DOI] [PubMed] [Google Scholar]
- 5.Chang BK, Timmerman RD. Stereotactic body radiation therapy: A comprehensive review Am J Clin Oncol 200730(6):637-644. [DOI] [PubMed] [Google Scholar]
- 6.Chang BK, Timmerman RD. Stereotactic body radiation therapy: A comprehensive review Am J Clin Oncol 200730(6):637-644. [DOI] [PubMed] [Google Scholar]
- 7.Lansu J, Bovée J, Braam P, van Boven H, Flucke U, Bonenkamp JJ, Miah AB, Zaidi SH, Thway K, Bruland Ø S, Baldini EH, Jebsen NL, Scholten AN, van den Ende PLA, Krol ADG, Ubbels JF, van der Hage JA, van Werkhoven E, Klomp HM, van der Graaf WTA, van Coevorden F, Schrage Y, van Houdt WJ, Haas RL. Dose reduction of preoperative radiotherapy in myxoid liposarcoma: A nonrandomized controlled trial JAMA Oncol 20217(1):e205865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhry V, Goldberg S, DeLaney TF, Cote GM, Chebib I, Kim J, Lozano-Calderón SA, De Amorim Bernstein K. Myxoid liposarcoma: Treatment outcomes from chemotherapy and radiation therapy Sarcoma. 20182018:8029157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall EJ GA. Radiobiology for the radiologist, 8th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2018. [Google Scholar]
- 10.Elledge CR, Krasin MJ, Ladra MM, Alcorn SR, Han P, Gibbs IC, Hiniker SM, Laack NN, Terezakis SA. A multi-institutional phase 2 trial of stereotactic body radiotherapy in the treatment of bone metastases in pediatric and young adult patients with sarcoma Cancer 2021127(5):739-747. [DOI] [PubMed] [Google Scholar]
- 11.Parsai S, Sedor G, Smile TD, Scott J, Ochocki A, Vassil N, Zahler S, Angelov L, Chao ST, Qi P, Anderson P, Murphy ES. Multiple site SBRT in pediatric, adolescent, and young adult patients with recurrent and/or metastatic sarcoma Am J Clin Oncol 202144(3):126-130. [DOI] [PubMed] [Google Scholar]
- 12.Tinkle CL, Singh C, Lloyd S, Guo Y, Li Y, Pappo AS, DuBois SG, Lucas JT, Haas-Kogan DA, Terezakis SA, Braunstein SE, Krasin MJ. Stereotactic body radiation therapy for metastatic and recurrent solid tumors in children and young adults Int J Radiat Oncol Biol Phys 2021109(5):1396-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown LC, Lester RA, Grams MP, Haddock MG, Olivier KR, Arndt CA, Rose PS, Laack NN. Stereotactic body radiotherapy for metastatic and recurrent ewing sarcoma and osteosarcoma Sarcoma 20142014:418270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E, Jeans E, Shinohara ET, Stavas MJ. Stereotactic body radiotherapy (SBRT) for metastatic and recurrent soft tissue and bone sarcomas Int J Radiat Oncol Biol Phys. 201799(2, Supplement):E754. [Google Scholar]
- 15.Stragliotto CL, Karlsson K, Lax I, Rutkowska E, Bergh J, Strander H, Blomgren H, Friesland S. A retrospective study of SBRT of metastases in patients with primary sarcoma Med Oncol 201229(5):3431-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas RL, Floot BGJ, Scholten AN, van der Graaf WTA, van Houdt W, Schrage Y, van de Ven M, Bovée JVMG, van Coevorden F, Vens C. Cellular radiosensitivity of soft tissue sarcoma Radiat Res 2021196(1):23-30. [DOI] [PubMed] [Google Scholar]
- 17.Tang Z, Zeng Q, Li Y, Zhang X, Ma J, Suto MJ, Xu B, Yi N. Development of a radiosensitivity gene signature for patients with soft tissue sarcoma Oncotarget 20178(16):27428-27439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott JG, Berglund A, Schell MJ, Mihaylov I, Fulp WJ, Yue B, Welsh E, Caudell JJ, Ahmed K, Strom TS, Mellon E, Venkat P, Johnstone P, Foekens J, Lee J, Moros E, Dalton WS, Eschrich SA, McLeod H, Harrison LB, Torres-Roca JF. A genome-based model for adjusting radiotherapy dose (GARD): A retrospective, cohort-based study Lancet Oncol 201718(2):202-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang G, Yuan Z, Ahmed K, Welsh EA, Fulp WJ, Gonzalez RJ, Mullinax JE, Letson D, Bui M, Harrison LB, Scott JG, Torres-Roca JF, Naghavi AO. Genomic identification of sarcoma radiosensitivity and the clinical implications for radiation dose personalization Translat Oncol 202114(10):101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsai S, Juloori A, Angelov L, Scott JG, Krishnaney AA, Udo-Inyang I, Zhuang T, Qi P, Kolar M, Anderson P, Zahler S, Chao ST, Suh JH, Murphy ES. Spine radiosurgery in adolescents and young adults: Early outcomes and toxicity in patients with metastatic Ewing sarcoma and osteosarcoma J Neurosurg Spine. 2019:1-8. [DOI] [PubMed] [Google Scholar]
- 21.Terezakis SA, Wharam MD. Radiotherapy for rhabdomyosarcoma: indications and outcome Clin Oncol (R Coll Radiol) 201325(1):27-35. [DOI] [PubMed] [Google Scholar]
- 22.Wunder JS, Nielsen TO, Maki RG, O’Sullivan B, Alman BA. Opportunities for improving the therapeutic ratio for patients with sarcoma Lancet Oncol 20078(6):513-524. [DOI] [PubMed] [Google Scholar]
- 23.Haas RL, Floot BGJ, Scholten AN, Graaf WTAvd, Houdt Wv, Schrage Y, Ven Mvd, Bovée JVMG, Coevorden Fv, Vens C. Cellular radiosensitivity of soft tissue sarcoma Radiat Res 2021196(1):23-30. [DOI] [PubMed] [Google Scholar]
- 24.Beyene KM, El Ghouch A. Smoothed time-dependent receiver operating characteristic curve for right censored survival data Stat Med 202039(24):3373-3396. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 26. Team R. RStudio: Integrated Development Environment for R. In. Boston, MA2020.
- 27.Soyfer V, Corn BW, Kollender Y, Tempelhoff H, Meller I, Merimsky O. Radiation therapy for palliation of sarcoma metastases: A unique and uniform hypofractionation experience Sarcoma. 20102010:927972-927972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willers H, Azzoli CG, Santivasi WL, Xia F. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer Cancer J 201319(3):200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rycaj K, Tang DG. Cancer stem cells and radioresistance Int J Radiat Biol 201490(8):615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Citrin DE, Mitchell JB. Altering the response to radiation: sensitizers and protectors Semin Oncol 201441(6):848-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers Int J Mol Sci 201415(1):927-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brett C, Newman NB, Kim E, Jeans E, Shinohara ET. Efficacy of SBRT for local control in recurrent and metastatic sarcoma Int J Radiat Oncol Biol Phys. 2018102(3):e260. [Google Scholar]
- 33.Stachelek GC, Ligon JA, Vogel J, Levin AS, Llosa NJ, Ladle BH, Meyer CF, Terezakis SA, Morris CD, Ladra MM, Pratilas CA. Predictors of recurrence and patterns of initial failure in localized ewing sarcoma: A contemporary 20-year experience Sarcoma. 20212021:6681741. [DOI] [PMC free article] [PubMed] [Google Scholar]


