Abstract
In Brazil, the use of Eucalyptus is focused on the production of wood or pulp for the paper industry but without any general recovery of waste, with leaves and branches being left on the ground. One possibility is to use these residues as raw materials in the production of industrially relevant and value-added compounds such as essential oil. The aim of the present study was to investigate the chemical composition, yield, anti-inflammatory/antinociceptive activities, and acute toxicity in mice, as well as the antimicrobial effects of essential oils from the leaves of 7 varieties of Eucalyptus and hybrids against Escherichia coli, Staphylococcus aureus, and Candida albicans. The extraction of oils was carried out using hydrodistillation, and they were analyzed by gas chromatography coupled to mass spectrometry. Urocam and Grancam were the plants that obtained the highest oil yield, with yields of 3.32 and 2.30%, respectively. The main chemical components identified in these plants were 1.8 cineole and α-pinene. The antinociceptive effect of the 7 oils (50 mg/kg, p.o.) was initially assessed in the acetic acid-induced writhing test. In this assay, a significant (p < 0.05) antinociceptive/anti-inflammatory effect was observed from 4 tested essential oils (E. benthamii, E. saligna, and the hybrids Urocam and Grancam) when compared to the vehicle-treated group. This effect was then confirmed in the formalin-induced paw licking test. No toxicological effects or alterations were observed in motor coordination after the administration of the studied oils to the animals. In the antimicrobial evaluation, the seven essential oils inhibited the growth of S. aureus, E. coli, and C. albicans at different concentrations. Collectively, these results demonstrate that the essential oil from the leaves and branches of Eucalyptus species and varieties present potential biomedical applications and represent a source of antimicrobial and/or anti-inflammatory compounds.
1. Introduction
Eucalyptus species (Myrtaceae) are native to Australia, New Zealand, and Tasmania [1] and are well known for their economic importance in wood and paper production (forest industry) [2, 3]. Eucalyptus plantations occupy 5.7 million hectares of planted trees in Brazil [4], and the main destination of these plants is the forestry industry, generating economic benefits but, on the other hand, millions of tons of waste, which result in considerable costs for the industry and the environment [3].
In Brazil, the use of Eucalyptus is focused on the production of wood or pulp for the paper industry [5], but without any general recovery of waste, with leaves and branches being left on the ground. One possibility is to use these residues as raw materials in the production of industrially relevant and value-added compounds such as essential oil [6]. In fact, Eucalyptus essential oil is widely used in industry, especially in pharmaceuticals and perfumery. Eucalyptus is a heteroblastic species, and genetic variations have been detected both within and between populations at the juvenile coppice and adult leaf stages, suggesting that the populations may involve quite different ontogenetic trajectories [7].
Some substances of therapeutic interest found in Eucalyptus essential oils are α-pinene, α-phellandrene, cineole, limonene, and terpineol [8, 9]. According to Piccinelli et al. [10], limonene presents antidepressant, antinociceptive effects (in neuropathic pain), and anti-inflammatory potential. Eucalyptol (cineole) inhibits tumor necrosis factor (TNF), interleukin-1, leukotrienes and thromboxane in inflammatory cells, responsible for the activation of the inflammation process [11]. Bayala et al. [12] demonstrated that α-pinene, limonene, and cineole also exhibit satisfactory anti-inflammatory activity.
Currently, nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids are the first-line drugs used to reduce the harmful events associated with inflammation [13]. These drugs exhibit important side effects, ranging from gastric irritation and ulcers to liver toxicity and chronic renal failure [14]. The scarce options for safe drugs for the treatment of chronic inflammatory diseases, such as arthritis, have led to the discovery of new medicinal agents derived from plants [15]. Thus, the disadvantages of using NSAIDs can be minimized by replacing them with safer and more efficient compounds derived from medicinal plants [15].
Essential oils from Eucalyptus species have been reported to have antimicrobial activity against Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria, indicating that the oil can be studied as a possible natural antibiotic for the treatment of various infectious diseases caused by these two bacteria [16]. Such investigations suggest that Eucalyptus major compound, 1,8-cineole, may be responsible for this action, highlighting the importance of the discovery of new substances with antimicrobial potential. Microbial resistance has been growing and rendering treatment of infections difficult with the therapeutic options available; therefore, the research and development of new substances with antimicrobial potential are of primary importance [17].
Literature appraisal on the subject demonstrates the importance of preclinical investigation of the possible anti-inflammatory, antinociceptive, and antimicrobial action of different essential oils from the leaves of Eucalyptus species (Eucalyptus benthamii Maiden & Cambage, Eucalyptus dunnii Maiden, Eucalyptus saligna Smith, and Eucalyptus grandis Hill & Maiden) and hybrids (Urograndis − E. grandis × Eucalyptus urophylla ST Blake; Grancam − E. grandis × Eucalyptus camaldulensis Dehnh; Urocam − E. urophylla × E. camaldulensis), which are abundantly cultivated in Brazil and had never been studied regarding these biological activities [18].
Considering the abovementioned scenario, the exploration of the biological and chemical properties of plant residues from the paper industry could represent a valuable approach for these by-products. Therefore, the aim of this study was to evaluate the antinociceptive, anti-inflammatory, and antimicrobial properties of the essential oils extracted from the leaves of seven varieties of Eucalyptus, as well as to determine their yield and chemical composition.
2. Materials and Methods
2.1. Eucalyptus Sampling, Essential Oil Extraction, and Chemical Analysis
Adult Eucalyptus trees from four species -Eucalyptus benthamii Maiden & Cambage, Eucalyptus dunnii Maiden, Eucalyptus saligna Smith, and Eucalyptus grandis Hill&Maiden - and three hybrids: Urograndis (E. grandis × Eucalyptus urophylla ST Blake), Grancam (E. grandis × Eucalyptus camaldulensis Dehnh), and Urocam (E. urophylla × E. camaldulensis) were collected at the Agricultural Research Company and Rural Extension of Santa Catarina experimental area located in Guantambú, Santa Catarina state, Brazil (27°07055S, 52°44′04″W). The essential oils were obtained by steam distillation using 200 g fresh plant leaves over 120 min in four repetitions [19]. The yield % (wt/wt, based on the fresh weight of the mature leaves) of the extracted oil from each sample was determined by the following equation:
| (1) |
Chemical composition of the essential oil samples was determined by gas chromatography using an Agilent GC/MS (7890B) coupled to a quadripolar mass spectrometer (5977A) (Agilent Technologies, Palo Alto, CA, USA). A HP-SMS 5% Phenyl Methyl Silox capillary column (30 m × 250 μm × 0.25 μm) connected to a flame ionization detector (FID) was used with mobile phase flow (carrier gas: He) adjusted to 1.0 mL·min−1. The GC temperature program was 60°C at 4.0 min, then up to 240°C at a rate of 10°C per min, then to 300°C at a rate of 40°C per min (maintained for 5 min). The injector temperature was 280°C. Oil samples were diluted with methanol.
For GC/MS detection, an electron ionization system was used with ionization energy set at 70 eV and mass range m/z 40–300. The chemical components were detected and identified by comparison of the mass spectra using the NIST 5.01 Mass Spectral Library (Agilent P/N G1033A) and comparing retention times; homologous C8–C30 n-alkane series by determining the linear retention index (LRI) according to Van Den Dool and Kratz [20]. The relative amounts of individual components were performed by integrating the peak in the FID chromatogram and expressed as percent of area.
2.2. Animals and Treatments
Male Swiss (Mus musculus) mice aged 6–8 weeks (25–35 g), bred in the Unochapecó Animal Facility (Chapecó, Brazil), were used. The animals were kept in acrylic cages (28 × 12.5 × 19 cm) in groups of 4 mice per cage, fed with standard laboratory food (Biobase®) and water ad libitum in an air-conditioned room (22–24°C), 12 h light/dark cycle (lights on at 6 a.m.) and air humidity between 40 and 60%. Eight mice were used per experimental group and were set in the experimental room for at least 1 h prior to the experiments. The animals were fasted for 2 h (no water restriction) prior to oral administration. The tests were performed at 23 ± 1°C in the absence of stressful factors such as sounds, odors, and high luminosity. The doses of oils used in this study were chosen based on their 1,8-cineole content. Evidence shows that this compound presents anti-inflammatory activity at 40 mg/kg (p.o.) [21]. Therefore, the oils were administered at 50 mg/kg (p.o.). Only one species of Eucalyptus evaluated in this study does not present cineole in its composition; however, the same dose (50 mg/kg) was used. The solubilization of the oils was performed in saline solution with the aid of 1% polysorbate 80. Indomethacin (10 mg/kg, p.o.) was used as positive control [22, 23]. Animal care was conducted according to the ethical principles of the Directive 2010/63/EU [24] and Brazilian law no 11.794 (2008) [25]. The protocols were approved by the Ethics Committee of the Community University of Chapecó Region (approval number 005/2020).
2.3. Acetic Acid-Induced Abdominal Writhing Test
In this model, abdominal writhing was induced in mice according to the procedure described by Santos et al. [26] and adapted by our research group [27]. Contractions of the abdominal muscles together with the extension of one of the hind legs occur in response to an intraperitoneal (i.p.) injection of 0.6% acetic acid (10 mL/kg). The animals received vehicle, indomethacin (10 mg/kg - positive control [28]), or 50 mg/kg of the oils orally (p.o.) 1 h before the exposure to the injection of acetic acid. Before acetic acid administration, the animals remained in the transparent observation chamber for 20 min for recognition and adaptation to the site. Immediately after administration, abdominal contortions were quantified for 20 min. The oils that presented better effectiveness for the follow-up of the next behavioral trials were selected.
2.4. Formalin-Induced Paw Licking Test
This assay allows evaluating both neurogenic and inflammatory pain: direct stimulation of nociceptive fibers happens at first (1st phase) and the inflammatory reaction (characterized by the release of inflammatory mediators) in a second moment (2nd phase) [29]. The test was performed as previously described by Santos and Calixto [30] with minor modifications [27]: inflammation induction occurred by intraplantar (i.pl.) administration of 1% formalin (20 μL) in the dorsal region of the right hind paw of mice. They were treated orally with vehicle, indomethacin (10 mg/kg - positive control [23]), or the oils under study 1 h before the exposure to formalin. Before formalin administration, the animals remained in the transparent observation chamber for 20 min for recognition and adaptation to the site. Immediately after formalin administration, mice were observed for 30 min: the number of paw elevations was quantified in the first 5 min and in the last 15 min. It was considered as paw elevation behavior any movement not associated with locomotion, ranging from a discrete elevation or contraction of the muscles of the animal's thigh to vigorous movement or licking and biting the paw.
2.5. Evaluation of Locomotor/Exploratory Activity
2.5.1. Open Field Test
This assay was performed as described by Müller et al. [31] in order to verify a possible nonspecific effect of the oils on the spontaneous exploratory and locomotor activities of the animals, which may influence the results regarding the antinociceptive/anti-inflammatory activity. Mice were treated orally with vehicle or the oils that showed positive results in the nociception/inflammation tests. After 60 min, they were placed in the center of a black waterproofed MDF box (40 × 30 × 30 cm) with its bottom divided in 24 equal squares. After 5 min of familiarization, the number of crossings, rearing, and fecal bolus were quantified for 10 min.
2.5.2. Rotarod Test
This model was carried out to verify the effects of the oils on motor coordination of the animals, as described by Neves et al. [32]. The rotarod device consisted of a cylinder (6 cm of diameter) rotating at 6.5 rpm. Initially, mice were trained to balance on the device for 5 min, including falls. Twenty-four hours later, the animals were submitted to a baseline responsiveness determination, when only the ones that presented a minimum continuous permanence time of 90 s were considered able to continue the test. Immediately after this session, the selected animals were treated orally with vehicle or the oils. One hour later, the test was performed and the longer permanence time (seconds) and number of falls within the observation period (5 min) were recorded.
2.5.3. Acute Toxicity
The acute oral toxicity test was carried out following Guideline 423 from the Organization for Economic Cooperation and Development [33]. Nonpregnant female mice were treated with vehicle (n = 3, control group) or the Eucalyptus essential oils that showed significant results in the nociception/inflammation assays, at 2000 mg/kg (n = 6). Their behavior was observed individually with special attention in the first 4 h and 12 h after the treatment with regard to the following occurrence of: piloerection, palpebral ptosis, abdominal contortions, changes in locomotion, hypothermia, increased muscular tonus, shaking, posterior paws stoppage, salivation, bronchial secretion, convulsions, and deaths. The animals' body weight, food intake, and deaths were registered every 2 days. On the 15th day after the treatment, they were euthanized by inhalation of isoflurane (dose of 10–12% in inhaled air) and confirmation by cervical displacement [34]; CONCEA [35] and the organs (liver, kidneys, adrenal glands, spleen, lungs, heart, and brain) relative weight (%) and macroscopic aspect were registered.
2.6. Antimicrobial Strains and Media
According to the method described by Gasparetto et al. [36], the antibacterial activity of the essential oils was evaluated against 1 Gram-positive (Staphylococcus aureus - ATCC 6538) and 1 Gram-negative (Escherichia coli - ATCC 25922) bacteria. The antifungal activity against the pathogenic fungi Candida albicans (ATCC 24433) was determined using the dilution technique. The Mueller–Hinton agar was the culture medium used for bacteria, which were incubated for 18 to 24 h at 35°C. For growing C. albicans, Sabouraud Dextrose 4% agar was used and the incubation conditions used were 24 to 48 h at 30°C. The strains were standard reference of the American Type Culture Collection (ATCC) (Rockville, MD, USA).
2.6.1. Antimicrobial Assay
The minimum inhibitory concentration (MIC) of essential oils was determined by agar dilution assay according to the CLSI [37], with minor modifications [35]. The assay was carried out on slants (1 mL) against bacteria and C. albicans. A series of dilutions was prepared, concentrations ranging from 25,000 ppm-48 ppm. Afterwards, 1 μL of inoculum suspension was added to each slant, except for the sterile control. A drug-free solution was also used as a blank control. Each assay was repeated 3 times. The bacterial strains were incubated at 35°C for 18 to 24 h and the yeast, at 30°C for 24 to 48 h. The MICs were visually recorded after 24 h for bacteria, 48 h for the yeast, and in accordance with control fungus growth for the remaining fungi [38]. For each concentration that did not show microbial growth in the MIC assay, the respective subcultures were performed in a medium without essential oil, followed by incubation and subsequent verification of the minimum microbicidal concentration (MMC) [39].
2.7. Statistical Analysis
Dataset normality was evaluated by the Shapiro−Wilk test. Considering that the results were parametric, the data of the behavioral assays and organs' weight were evaluated by one-way analysis of variance (ANOVA) followed by Bonferroni´s test. Body weight and food intake were evaluated by repeated measures two-way ANOVA (factor 1: day; factor 2: treatment) post-hoc Bonferroni test. The software GraphPad Prism 5.01 (GraphPad Software®, San Diego, California, USA) was used for the analysis of the results. Results were expressed as mean ± standard error of the mean (S.E.M.). P values less than 0.05 were considered significant.
3. Results and Discussion
3.1. Yield and Chemical Analyses of Essential Oils
The yield and compounds identified in the essential oils with their respective concentrations are shown in Table 1. The essential oil yield of the different varieties of Eucalyptus was in the range of 0.77-3.32%, in agreement with other works reported in the literature [40]. Knowledge of the essential oil yield is important for both commercial purposes and scientific research since it can affect the availability and economic viability of Eucalyptus essential oil production [41]. The plants that showed the greatest potential were Urcan and Grancan varieties, with yields of 3.32 and 2.30%, respectively.
Table 1.
Chemical composition and yield (%) of essential oil from different Eucalyptus varieties.
| RT | RI-N | RI-E | E. benthamii | Grancam | E. dunnii | E. grandis | E. saligna | Urocam | Urograndis | |
|---|---|---|---|---|---|---|---|---|---|---|
| α-pinene | 4.24 | 937 | 937 | 17.39 | 35.92 | 8.55 | 30.54 | 33.32 | 2.90 | 26.44 |
| Camphene | 4.48 | 943 | 953 | 0.87 | 3.00 | 0.77 | 0.95 | |||
| β-pinene | 5.10 | 943 | 993 | 2.75 | ||||||
| p-cymene | 5.71 | 1036 | 1033 | 41.20 | 0.79 | 1.93 | 3.60 | 0.85 | 28.87 | |
| β-terpinyl acetate | 5.78 | 1348 | 1032 | 3.70 | ||||||
| D-limonene | 5.78 | 1018 | 1032 | 10.56 | 3.52 | 3.74 | 5.17 | 3.23 | ||
| 1.8-cineole | 5.86 | 1023 | 1036 | 38.43 | 63.01 | 8.20 | 40.04 | 82.15 | 11.74 | |
| γ-terpinene | 6.31 | 998 | 961 | 4.19 | 5.67 | |||||
| Fenchol | 7.42 | 1125 | 1121 | 0.96 | 3.68 | 0.83 | ||||
| α-Campholenal | 7.61 | 1128 | 1131 | 0.44 | 2.41 | 2.23 | 0.60 | |||
| L-pinocarveol | 7.97 | 1135 | 1150 | 2.46 | 2.93 | 1.63 | 0.97 | |||
| Pinocarvone | 8.35 | 1164 | 1170 | 1.10 | 0.63 | 0.38 | ||||
| (−)-borneol | 8.43 | 1173 | 1175 | 1.39 | 9.73 | |||||
| Camphol | 8.44 | 1148 | 1175 | 0.69 | 2.21 | 2.02 | ||||
| 4-terpinenol | 8.63 | 1137 | 1185 | 2.51 | 0.94 | |||||
| α-terpineol | 8.91 | 1172 | 1200 | 2.62 | 8.42 | 9.38 | 3.76 | 1.36 | 3.44 | |
| α-terpineol acetate | 11.87 | 1333 | 1356 | 6.45 | 3.60 | |||||
| Caryophyllene | 13.27 | 1494 | 1430 | 1.30 | ||||||
| Aromandendrene | 13.59 | 1439 | 1466 | 11.45 | 3.04 | |||||
| Cadina-1(10).4-diene | 15.03 | 1537 | 1532 | 1.24 | ||||||
| Epiglobulol | 15.72 | 1530 | 1472 | 4.17 | 1.91 | |||||
| Spathulenol | 16.03 | 1536 | 1490 | 1.29 | 4.40 | 2.33 | ||||
| Globulol | 16.15 | 1578 | 1497 | 18.01 | 7.40 | 2.40 | 1.39 | |||
| Himbaccol | 16.29 | 1530 | 1507 | 0.77 | ||||||
| Viridiflorol | 16.30 | 1554 | 1507 | 1.48 | ||||||
| Total | 98.92 | 97.50 | 98.12 | 73.33 | 98.69 | 98.88 | 96.71 | |||
|
| ||||||||||
| Oil yield (%) | 1.72 (±0.19) | 2.30 (±0.20) | 1.56 (±0.15) | 0.77 (±0.04) | 0.85 (±0.09) | 3.32 (±0.21) | 1.35 (±0.14) | |||
LR-N-bookstore linear retention index national institute of standards and technology, RT-retention time, and RI-E-experimental retention index.
It can also be seen from Table 1 that 1,8-cineole (eucalyptol) is present in all species studied except for E. benthamii. Note that the hybrid Urocam presented the highest concentration of this compound (82.18%), which makes this plant a potential source for this compound. Besides being a biologically active compound, it has a high therapeutic index and antiseptic properties [42]. According to Caceres et al. [43], eucalyptol inhibits the arachidonic acid pathway, thus inhibiting inflammatory mediators, and also 1,8-cineole and glucocorticoids have a mechanism of inhibiting inflammatory mediators in common.
Except for Grancam, α-pinene was also abundant in all Eucalyptus varieties studied. Such a compound has been considered an anti-inflammatory agent, mainly in osteoarthritis, besides presenting antioxidant and antiallergic activities and antimicrobial effectiveness against some strains of S. aureus and methicillin-resistant Staphylococcus aureus, among others [44, 45].
Another pinene that is part of the chemical composition of Eucalyptus essential oils is β-pinene, which presents biological activities such as anti-inflammatory, antidepressant, anxiolytic, hypotensive, antimicrobial, and myorelaxant, among others [46]. A high concentration of p-cymene in Eucalyptus essential oil was reported by Almeida et al. [47], with possible antiseptic activity that inhibited the growth of S. aureus and E. coli.
γ-Terpinene has been reported to present significant antimicrobial activity [48] and bacteriostatic activity against some microorganisms has been attributed to terpineol [49]. The Benthamii plant presented globulol as one of the major components (18.01%); this sesquiterpene has antifungal properties against a variety of fungal species as well as bacteria [50] and antimicrobial and antioxidant activities [51].
D-Limonene, a monoterpene found in several essential oils of aromatic species, was observed in 5 of the 7 Eucalyptus varieties studied, with concentrations ranging from 3.23 to 10.56%. D-limonene is a multifunctional compound with a potent therapeutic effect, widely used in the food and pharmaceutical industries [52, 53].
Overall, this study provides valuable information about the chemical composition of essential oils from the seven varieties of Eucalyptus cultivated in Brazil, which may explain the results of anti-inflammatory and antimicrobial activity obtained in this work and may have important applications in several industries, including pharmaceutical, cosmetic, and food.
3.2. Antinociceptive and Anti-Inflammatory Activities
3.2.1. Acetic Acid-Induced Abdominal Writhing Test
The acetic acid-induced abdominal writhing test showed that the positive control group (indomethacin at 10 mg/kg) and the groups treated with Urocam, Grancam, E. benthamii, and E. saligna (50 mg/kg, p.o.) presented a significant lower number of abdominal writhing when compared to the group treated with vehicle (F (8, 46) = 15.12, P < 0.0001) (Figure 1) (F (8, 63) = 15.12, P < 0.001).
Figure 1.
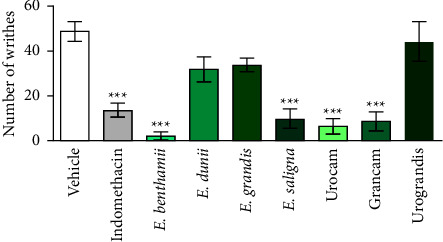
Effect of essential oils on leaves of Eucalyptus species obtained by supercritical CO2 on acetic acid-induced abdominal writhing test. Mice were treated with vehicle (0.9% NaCl plus 1% of tween 80, 10 ml/kg, p.o., negative control group), or indomethacin (10 mg/kg p.o., positive control group), or the essential oils of E. benthamii, E. dunnii, E. grandis, E. saligna, Urocam, Grancam, or Urograndis (50 mg/kg, p.o.), 1 h before i.p. acetic acid (0.6%) administration (n = 8). One-way ANOVA followed by Bonferroni´s test: ∗∗∗P < 0.001 compared to the vehicle-treated (negative control) group. Results expressed as mean ± S.E.M.
It is known that 1,8-cineole presents anti-inflammatory and antinociceptive properties, while α-pinene shows anti-inflammatory and antiallergic activities [45, 54]. Results reported in this work agree with other studies that demonstrated that Eucalyptus essential oil reduced the number of abdominal writhing, similarly to the positive control used (indomethacin) [55], which may be related to the presence of 1,8-cineole and α-pinene in the essential oils.
Based on the chemical composition of the oils investigated, the following may be pointed out as the most efficient with regard to pharmacological effect: Urocam (82.15% of 1,8-cineole and 2.90% of α-pinene), Grancam (38.43% of 1,8-cineole and 35.92% of α-pinene), E. benthamii (satisfactory result with 41.20 of p-cymene and 17.39% of α-pinene only), and E. saligna (40.04% of 1,8-cineole and 33.32% of α-pinene). Considering the results obtained in the acetic-acid induced writhing test, these 4 plants - Urocam, Grancam, E. benthamii, and E. saligna - were selected to be used in the following assays.
3.2.2. Formalin-Induced Paw Licking Test
In order to confirm the antinociceptive and/or anti-inflammatory activities of Eucalyptus essential oils, the ones that presented the best results on acetic acid-induced abdominal writhing assays were also tested in the formalin-induced paw licking test. This assay consists of two phases: the first one (0–5 min) corresponds to neurogenic pain, and the second one (15–30 min) is related to the inflammatory response. Neurogenic pain occurs in response to the direct stimulation of nociceptors by formalin, which results in the release of other mediators that perform local responses, causing vasodilation, activation of synaptic fibers, and leukocyte chemotaxis. In the inflammatory response induced by formalin, there is the release of inflammatory mediators that end up activating the inflammatory cascade and sensitizing nociceptive pathways [56]. Both phases can be inhibited by central-acting drugs, such as opioids, while NSAIDs are more effective in preventing inflammatory pain [57].
Figure 2 represents the effects of E. benthamii, E. saligna, Urocam, and Grancam, tested in the formalin test. In the first phase (Figure 2(a)), the time that the animals spent licking the paw was significantly (F (5, 42) = 32.60, P < 0.0001) reduced by the essential oils of the four species when compared to the vehicle-treated group, as well as the animals treated with indomethacin.
Figure 2.
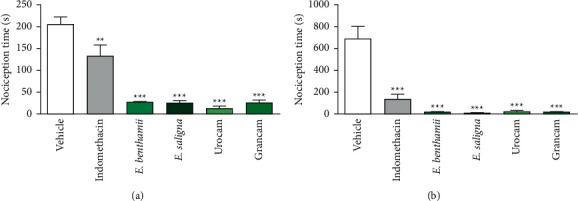
Effect of essential oils of leaves of E. benthamii, E saligna, Urocam, and Grancam, on formalin test: 1st phase (a) and 2nd phase (b). Mice were treated with vehicle (0.9% NaCl plus 1% of tween 80, 10 ml/kg, p.o., negative control group), or indomethacin (10 mg/kg p.o., positive control group), or the essential oils of E. benthamii, E. saligna, Urocam, and Grancam, (50 mg/kg, p.o.), 1 h before formalin (1%, i.pl.) administration (n = 8). One-way ANOVA followed by the Bonferroni´s test: ∗∗P < 0.01 and ∗∗∗P < 0.001 compared to the vehicle-treated (negative control) group. Results expressed as mean ± S.E.M.
The short nociception time in the first phase may indicate that the antinociceptive effect had the involvement of the opioid pathway, since it is known that some compounds of the oils, such as β-pinene and p-cymene, present an antinociceptive effect acting as a partial agonist of μ-opioid receptors [58, 59].
In the second phase of the test (Figure 2(b)), when the anti-inflammatory response of Eucalyptus essential oils tested were evaluated, and there was also a significant (F (5, 42) = 24.12, P < 000.1) reduction in paw licking time for all oils tested as well as indomethacin when compared to the vehicle-treated group.
The results of this test indicate that the essential oils of the leaves of the species E. benthamii, E. saligna, Urocam, and Grancam present antinociceptive action with central and peripheral effects by responding to neurogenic and inflammatory pain caused by formalin injection. This is probably related to the major compounds of the essential oils studied, 1,8- cineole, p-cymene, and α-pinene, which present anti-inflammatory and antinociceptive activities [43, 59].
The compound 1,8-cineole inhibits cyclooxygenase enzymes, resulting in the decrease on the production of TNF-α, leukotriene B4, and thromboxane A2, and it is also a potent inhibitor of cytokines [43]. The study of Santos and Rao [60] corroborates such facts: in the carrageenan test, which induces paw edema, 1,8-cineole at 400 mg/kg (p.o.) inhibited the edema, and, according to the authors, it indicates a decrease in the production of inflammatory mediators. In addition, there was also a reduction in the nociception time in both phases of the formalin test at the same dose of 1,8-cineole.
α-Pinene, when investigated through the carrageenan-induced paw edema test, inhibited the edema and also decreased leukocyte migration at 200 and 400 mg/kg [45]. In the study by Lee et al. [55], Eucalyptus essential oil at 45 mg/kg p.o. significantly reduced the nociception time only in the 2nd phase of the formalin test.
3.3. Evaluation of Locomotor/Exploratory Activity
3.3.1. Open Field Test
The open field test was carried out to exclude the possibility that the results of the behavioral assays of nociception and inflammation were related to a nonspecific effect of the essential oils of E. benthamii, E. saligna, Urocam, and Grancam on spontaneous exploratory and locomotor activities of the animals.
Figure 3 illustrates the number of crossings of the animals treated with the essential oils. There was a significant reduction in the number of crossings (Figure 3(a)) of animals treated with all oils studied (F (5, 42) = 18.03, P < 000.1). Also, according to Figure 3(b), mice treated with E. benthamii, Urocam, and Grancam (F (5, 42) = 6.53, P = 0.0004) showed a significant reduction in the number of rearing compared to the vehicle-treated group. On the one hand, substances that increase the number of crossings and rearing are considered as stimulants [61]. On the other hand, a reduction in these parameters, as caused by the oils under study, is indicative of central depressant effects [61]. In line with our observations, Santos and Rao [60] demonstrated that 1,8-cineole caused a reduction in the locomotion of the animals in the open field test, hence suggesting a sedative effect. Furthermore, it is known that the number of crossings can be affected by central-acting drugs or peripheral muscle relaxers [62]; the number of rearing assesses the degree of sedation and anxiety and can be altered by drugs with anxiolytic/anxiogenic activity; and the number of fecal bolus can be altered by anxiolytic, anxiogenic, spasmolytic, or spasmogenic drugs [63].
Figure 3.
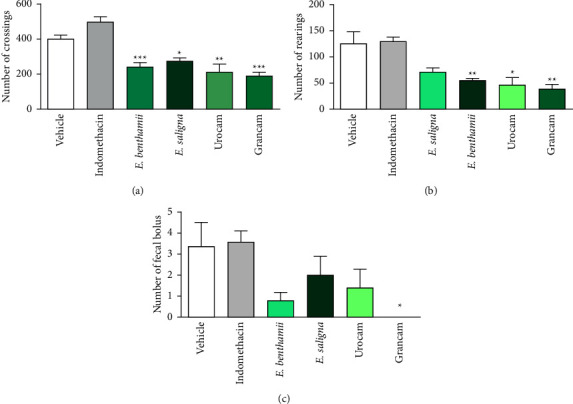
Effect of essential oils on leaves of E. benthamii, E saligna, Urocam, and Grancam on the open field test: number of crossings (a), rearing (b) and fecal bolus (c) expelled during the session. Mice were treated with vehicle (0.9% NaCl plus 1% of tween 80, 10 ml/kg, p.o., negative control group), or indomethacin (10 mg/kg p.o., positive control group) or the essential oils of E. benthamii, E. saligna, Urocam, or Grancam (50 mg/kg, p.o.), 1 h before the exposure to the apparatus (n = 8). One-way ANOVA followed by Bonferroni´s test: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 compared to the vehicle-treated (negative control) group. Results expressed as mean ± S.E.M.
In Figure 3(c), the number of fecal bolus is depicted: the animals treated with Grancam showed a significant (F (5, 42) = 2.767, P = 0.038) reduction in the number of fecal bolus expelled compared to the group treated with vehicle. The reduction in the number of fecal bolus may be related to the involvement of the opioid receptors in the mechanism of action of this oil since opioid drugs present regularly reported peripheral side effects such as constipation, urinary retention, and pruritus [64].
The satisfactory antinociceptive activity as well as the constipation and sedative effects are qualities of opioid drugs [65]. Indeed, the chemical composition of the oils tested demonstrated the presence of some compounds that act as opioid agonists, such as β-pinene [58]. Therefore, it can be suggested that the oils tested are very efficient in the pain management process, perhaps with theinvolvement of the opioid pathway. However, their mechanism of antinociceptive action must be elucidated.
3.3.2. Rotarod Test
Considering that a sedative effect of the oils was detected in the open field test, the rotarod test was performed to verify the effect of the oils on the integrity of the motor coordination of mice.
Table 2 shows the results of the permanence time and number of falls of the animals treated with the oils in the rotarod test. There was no significant difference between the parameters evaluated between the groups. Thus, these results indicate that the essential oils of E. benthamii, E. saligna, Urocam, and Grancam did not alter the integrity of the motor coordination of the animals. Sobreira et al. [66] also noted that 1,8-cineole, the major component of Eucalyptus essential oil, did not cause changes in the motor coordination of the animals in the rotarod test, therefore in agreement with the results of this study.
Table 2.
Effects of Eucalyptus essential oils on the motor coordination of mice, evaluated by the rotarod test. Mice were treated with vehicle (0.9% NaCl plus 1% of tween 80, 10 ml/kg, p.o., negative control group) or indomethacin (10 mg/kg p.o., positive control group) or the essential oils of E. benthamii, E. saligna, Urocam, or Grancam (50 mg/kg, p.o.) (n = 8).
| Treatment | Permanence time (s) | Number of falls |
|---|---|---|
| Vehicle | 299.3 ± 0.75 | 0.12 ± 0.12 |
| Indomethacin | 299.3 ± 0.49 | 0.25 ± 0.16 |
| E. benthamii | 273.7 ± 1.58 | 0.12 ± 0.54 |
| E. saligna | 255.7 ± 2.70 | 0.37 ± 0.79 |
| Urocam | 300.0 | 0.0 |
| Grancam | 300.0 | 0.0 |
One-way ANOVA. Results expressed as mean ± S.E.M.
3.4. Acute Toxicity
In the acute toxicity assay, the selected essential oils were administered at a single dose of 2000 mg/kg (p.o.). Figures 4(a) and 4(b) present the relative body weight and food intake of the animals, respectively.
Figure 4.
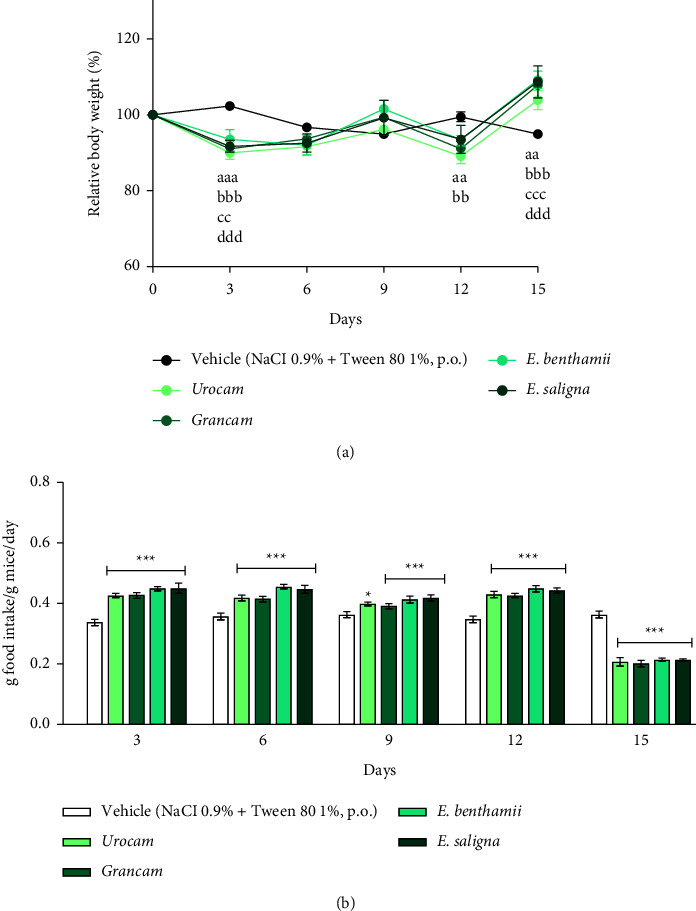
Acute oral toxicity (2000 mg/kg, p.o.) on relative body weight (%) (a) on female mice food intake (g food intake/g mice/day) and (b). Mice were treated with vehicle (0.9% NaCl plus 1% of tween 80, 10 ml/kg, p.o., negative control group, n = 3) or the essential oils of Urocam, Grancam, E. benthamii, or E. saligna (2000 mg/kg, p.o., n = 6). Repeated measures two-way ANOVA followed by the Bonferroni test: (a) compared to the vehicle-treated (negative control) group, Urocam: aaP < 0.01, aaaP < 0.001; Grancam: bbP < 0.01, bbbP < 0.001; E. benthamii: ccP < 0.01, cccP < 0.001; E. saligna: dddP < 0.001. (b) ∗P < 0.05, and ∗∗∗P < 0.001 compared to the vehicle-treated (negative control) group at the same day. Results expressed as mean ± S.E.M.
A two-way RM ANOVA revealed a significant effect of day (F (5, 125) = 56.77 , P < 0.0001])and a significant interaction between factors (day x treatment) (F (20, 125) = 7.943, P < 0.0001) on the relative body weight of animals. No effect of treatment was detected on mice relative body weight (F (4, 25) = 0.7158, P = 0.589).
Regarding food intake, a two-way RM ANOVA revealed significant effect of day (F (4, 100) = 656.8 , P < 0.0001), treatment (F (4, 25) = 4.94, P = 0.0045) and a significant interaction between factors (day × treatment) (F (16, 100) = 49.20, P < 0.0001). Posthoc analysis revealed that on the 3rd day of the experiment, a significant decrease in body weight (P < 0.01 for E. benthamii and P < 0.001 for the other ones) and a significant increase in food intake (P < 0.001) were observed in the groups treated with Eucalyptus species when compared to the vehicle-treated group. At the last experimental day, the animals treated with Eucalyptus ingested significantly (P < 0.001) less food and their body weight significantly increased (P < 0.01 for Urocam and P < 0.001 for the other ones) in comparison with the vehicle-treated group.
These data suggest that Eucalyptus essential oils present a metabolic side effect. On the 15th day of observation, when the animals treated with the oils ingested less food and recovered their body weight, it was likely that the effect of Eucalyptus essential oils on metabolism had stopped [66, 67]. Some tests on the toxicity of Eucalyptus essential oils in rats showed that their median lethal dose (LD50) was 4,440 mg/kg, and their major oil compound, 1,8-cineole, had a LD50 of 2,480 mg/kg. Tests on mammals such as koalas demonstrated a certain toxicity of the Eucalyptus oils tested [68]. In addition, there was no difference in both the body weight of animals and organs compared to the vehicle-treated group when isolated 1,8-cineole was tested [69].
The effect of essential oils on the weight of animals' organs is shown in Figure 5. The treatments with all studied essential oils significantly reduced the relative weight of spleen (F (4, 25) = 7.024, P = 0.0006) (Figure 5(a)) and heart (F (4, 25) = 6.205, P = 0.0013) (Figure 5(b)). There were no effects of the essential oils on the relative weight of mice's adrenals (F (4, 25) = 1.347, P = 0.280) (Figure 5(c)), brain (F (4, 25) = 0.466, P = 0.760) (Figure 5(d)), kidneys (F (4, 25) = 1.397, P = 0.234) (Figure 5(e)), liver (F (4, 25) = 2.202, P = 0.097) (Figure 5(f)), and thymus (F (4, 25) = 0.867, P = 0.497) (Figure 5(g)).
Figure 5.
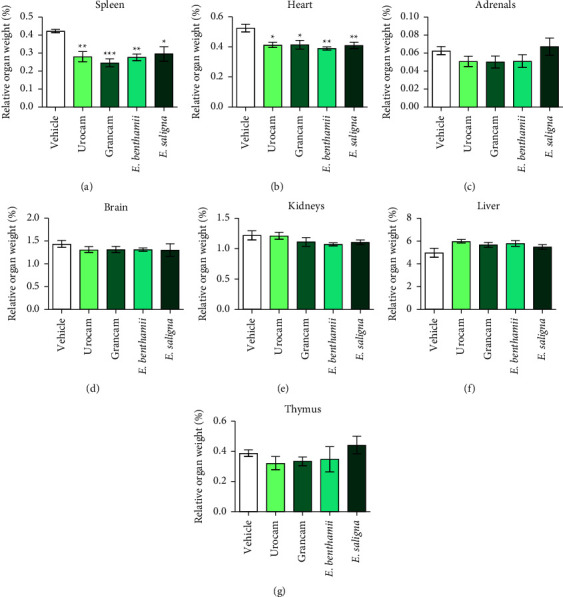
Acute oral toxicity (2000 mg/kg, p.o.) on relative organs weight (%): spleen (a), heart (b), adrenals (c), brain (d), kidneys (e), liver (f), and thymus (g). Mice were treated with vehicle (0.9% NaCl plus 1% of tween 80, 10 ml/kg, p.o., negative control group, n = 3) or the essential oils of Urocam, Grancam, E. benthamii, or E. saligna (2000 mg/kg, p.o., n = 6). One-way ANOVA followed by Bonferroni test: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 compared to the vehicle-treated (negative control) group at the same day. Results expressed as mean ± S.E.M.
Unlike the results found here, there are no reports of splenic and cardiac toxicity related to Eucalyptus essential oils in the literature. Furthermore, the reduction in the proportion of cardiac weight has also not been described for Eucalyptus species and; therefore, there is no conclusive cause of the reason why this effect was observed.
The results of acute toxicity need to be confirmed in repeated dose toxicity studies, with the use of extrapolated working doses, as recommended by the OECD [33]. Anyway, the treatment with the Eucalyptus oils studied did not cause any toxicity sign or death during the study period. Thus, according to Guideline 423 [33], they can be classified in category 5 of the Globally Harmonized System (LD50 is > 2000 mg/kg-5000 mg/kg for nonvulnerable populations).
3.5. Antimicrobial Assay
The seven oils demonstrated activity against S. aureus (Gram-positive bacteria) and E. coli (Gram-negative bacteria) (Table 3). Besides, they also presented activity against the fungus C. albicans, as shown in Table 4. Estanislau et al. [70] evaluated the antimicrobial activity of 5 Eucalyptus species and also obtained promising results against S. aureus and E. coli for all oils tested. However, the technique used by them was the disk diffusion method; therefore. it is not possible to advance in this discussion.
Table 3.
Antibacterial activity of Eucalyptus essential oils against S. aureus and E. coli.
| Minimum inhibitory concentration (MIC)/minimum microbicidal concentration (MMC) | ||
|---|---|---|
| Essential oil | Gram-positive bacteria | Gram-negative bacteria |
| S. aureus (%) | E. coli (%) | |
| E. benthamii | 0.625/1.25 | 1.25/2.5 |
| E. dunii | 0.312/0.625 | 0.312/0.625 |
| E. grandis | 0.625/1.25 | 1.25/1.25 |
| E. saligna | 0.625/1.25 | 1.25/2.5 |
| Urocam | 1.25/2.5 | 1.25/1.25 |
| Grancam | 0.312/0.625 | 0.625/1.25 |
| Urograndis | 0.625/1.25 | 1.25/1.25 |
Table 4.
Antibacterial activity of Eucalyptus essential oils against C. albicans.
| Minimum inhibitory concentration (MIC)/minimum microbicidal concentration (MMC) | |
|---|---|
| Essential oil | C. albicans (%) |
| E. benthamii | 0.156/0.312 |
| E. dunii | 0.156/0.312 |
| E. grandis | 0.625/0.625 |
| E. saligna | 0.312/0.312 |
| Urocam | 1.250/1.250 |
| Grancam | 0.625/1.250 |
| Urograndis | 0.312/0.625 |
Additionally, E. dunnii and Grancam had the best performance in MIC and MMC assays for the two bacteria under study. The 2 species have different concentrations of active substances: E. dunnii presents 8.55% α-pinene and 63.01% of 1,8-cineole, while Grancam presents 35.92% of α-pinene and 38.43% of 1,8-cineole. These compounds are responsible for the main antimicrobial effectiveness against some strains of S. aureus and have high therapeutic indexes [42, 44]. E. dunnii and Grancam present 8.42% and 2.62% of terpineol, respectively, which is responsible for bacteriostatic action [49].
The compound D-limonene is present in E. dunnii (3.52%) and in Grancam (10.52%) and possesses antimicrobial activity [71]. β-pinene also presents antimicrobial activity and is part of Grancam (2.75%) essential oils [46].
Furthermore, the essential oil of the studied varieties has other components in lower concentrations in its composition. According to Chorianopoulos et al. [72], antimicrobial activity occurs not only through the presence of major compounds but also by the influence of other components at lower concentrations that can cause synergistic, additive, or antagonistic interactions.
The antifungal activity of the oils was slightly more promising in view of the results of MIC and MMC. Cimanga et al. [73] compared the antimicrobial activity of the essential oils of some Eucalyptus species. The major compounds (such as 1,8-cineole and α-pinene) were also tested isolated and had less activity than the essential oil, stating that the combination of compounds is important for the antimicrobial activity of Eucalyptus oil [73], thus showing that it is not possible to compare the compounds of the oils isolated.
The mechanism of inhibitory/microbicidal action of the essential oils may be related to their characteristics, such as hydrophobicity, which allows their interaction with cellular lipid structures, promoting increased permeability and consequent extensive efflux of electrolytes, which play an essential role in cell homeostasis [74].
According to Burt [75], the difficulty in comparing the results obtained by several authors is often mentioned as a problem faced in the study of the antimicrobial activity of essential oils and plant-derived products, since there are variations in the methods used.
Results reported in this work reinforce the information that Eucalyptus essential oils have antimicrobial activity, especially antifungal, with the advantage of being a natural product. According to Rahman and Kang [76], the risk that pathogenic microorganisms will develop resistance to essential oils is very low, since they contain a mixture of antimicrobial substances that act through various mechanisms. These characteristics represent an advantage of essential oils as antimicrobial drugs, which can be promising for applications in different areas.
4. Conclusion
In this study, the great chemical variability that exists between the essential oils of the various Eucalyptus species was demonstrated, highlighting Urocam, Grancam, and E. dunii, which obtained the highest oil yield with high concentrations of 1.8 cineole and a-pinene. In addition, the essential oils of Urocam, Grancam, E. benthamii, and E. saligna have antinociceptive and anti-inflammatory activities and are devoid of acute toxicity. Also, the seven varieties of Eucalyptus studied are active against S. aureus, E. coli, and C. albicans. Finally, the Eucalyptus essential oils represent an important source of antinociceptive, anti-inflammatory, and antimicrobial compounds, such as 1,8-cineole and α-pinene. Future studies on the elucidation of the mechanism of the antinociceptive action of the oils are required.
Acknowledgments
This study was supported by FAPESC (Chamada Pública, grant number 04/2019 TO No 2020TR735), Programa de Bolsas Universitárias de Santa Catarina-Uniedu Art. 170 and 171 CE numbers 013/2019 and 023/2019, respectively.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Grattapaglia D., Vaillancourt R. E., Shepherd M., et al. Progress in Myrtaceae genetics and genomics: Eucalyptus as the pivotal genus. Tree Genetics & Genomes . 2012;8(3):463–508. doi: 10.1007/s11295-012-0491-x. [DOI] [Google Scholar]
- 2.Seth M. K. Trees and their economic importance. The Botanical Review . 2003;69(4):321–376. doi: 10.1663/0006-8101(2004)069[0321:tatei]2.0.co;2. [DOI] [Google Scholar]
- 3.Sembiring N., Napitupulu H. L., Sembiring M. T., Ishak A., Gunawan H. A. Fulfilling Eucalyptus raw materials for pulp and paper production plants. IOP Conference Series: Earth and Environmental Science . 2021;912 doi: 10.1088/1755-1315/912/1/012008.012008 [DOI] [Google Scholar]
- 4.Amorim V. D. S. S. D., Monteiro K. M. S., Sousa G. O., Damascena J. F., Pereira J. A., Moraes W. D. S. Os benefícios ambientais do plantio de eucalipto: revisão de literatura. Research Society and Development . 2021;10(11) doi: 10.33448/rsd-v10i11.19604. [DOI] [Google Scholar]
- 5.Silva A. P. S., Coelho S. T. Biomass residues from sustainable forest management in Brazil. Proceedings of the European Biomass Conference and Exhibition Proceedings; December 2020; Brasília, Brazil. [Google Scholar]
- 6.Dhakad A. K., Pandey V. V., Beg S., Rawat J. M., Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. Journal of the Science of Food and Agriculture . 2018;98(3):833–848. doi: 10.1002/jsfa.8600. [DOI] [PubMed] [Google Scholar]
- 7.Wallis I. R., Keszei A., Henery M. L., et al. A chemical perspective on the evolution of variation in Eucalyptus globulus. Perspectives in Plant Ecology, Evolution and Systematics . 2011;13(4):305–318. doi: 10.1016/j.ppees.2011.05.005. [DOI] [Google Scholar]
- 8.Batish D. R., Singh H. P., Kohli R. K., Kaur S. Eucalyptus essential oil as a natural pesticide. Forest Ecology and Management . 2008;256(12):2166–2174. doi: 10.1016/j.foreco.2008.08.008. [DOI] [Google Scholar]
- 9.Cheng S.-S., Huang C.-G., Chen Y.-J., Yu J.-J., Chen W.-J., Chang S.-T. Chemical compositions and larvicidal activities of leaf essential oils from two Eucalyptus species. Bioresource Technology . 2009;100(1):452–456. doi: 10.1016/j.biortech.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Piccinelli A. C., Morato P. N., dos Santos Barbosa M., et al. Limonene reduces hyperalgesia induced by gp120 and cytokines by modulation of IL-1 β and protein expression in spinal cord of mice. Life Sciences . 2017;174:28–34. doi: 10.1016/j.lfs.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Juergens U. R., Stöber M., Schmidt-Schilling L., Kleuver T., Vetter H. Antiinflammatory effects of euclyptol (1.8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. European Journal of Medical Research . 1998;3(9):407–412. [PubMed] [Google Scholar]
- 12.Bayala B., Bassole I. H. N., Gnoula C., et al. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina faso. PLoS One . 2014;9(3) doi: 10.1371/journal.pone.0092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batlouni M. Anti-inflamatórios não esteroides: efeitos cardiovasculares, cérebro-vasculares e renais. Arquivos Brasileiros de Cardiologia . 2010;94(4):556–563. doi: 10.1590/s0066-782x2010000400019. [DOI] [PubMed] [Google Scholar]
- 14.Kunanusorn P., Teekachunhatean S., Sangdee C., Panthong A. Antinociceptive and anti-inflammatory activities of a Chinese herbal recipe (DJW) in animal models. International Journal of Applied Research in Natural Products . 2009;2(1):1–8. [Google Scholar]
- 15.Khan J., Alexander A., Ajazuddin A., Saraf S., Saraf S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. Journal of Controlled Release . 2013;168(1):50–60. doi: 10.1016/j.jconrel.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Bachir R. G., Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pacific Journal of Tropical Biomedicine . 2012;2(9):739–742. doi: 10.1016/s2221-1691(12)60220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loureiro R. J., Roque F., Teixeira Rodrigues A., Herdeiro M. T., Ramalheira E. Use of antibiotics and bacterial resistances: brief notes on its evolution. Revista Portuguesa de Saude Publica . 2016;34(1):77–84. doi: 10.1016/j.rpsp.2015.11.003. [DOI] [Google Scholar]
- 18.Santos P. E. T. D., Paludzyszyn Filho E., Silva L. T. D. M. D., Vandresen P. B. Genetic variation for growth and selection in adult plants of Eucalyptus badjensis. Genetics and Molecular Biology . 2015;38(4):457–464. doi: 10.1590/s1415-475738420150041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaral W., Deschamps C., Bizzo H. R., Pinto M. A. S., Biasi L. A., Da Silva L. E. Essential oil yield and composition of native tree species from Atlantic Forest, South of Brazil. Journal of Essential Oil-Bearing Plants . 2017;20(6):1525–1535. doi: 10.1080/0972060x.2017.1346484. [DOI] [Google Scholar]
- 20.van Den Dool H., Kratz P. D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. Journal of Chromatography A . 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 21.Martins A. O. B. P. B., Rodrigues L. B., Cesário F. R. A. S., et al. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1,8-cineole (eucalyptol) Biomedicine & Pharmacotherapy . 2017;96:384–395. doi: 10.1016/j.biopha.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S., Chattopadhyay D., Mandal A., Kaity S., Samanta A. Bioactivity guided isolation of antiinflammatory, analgesic, and antipyretic constituents from the leaves of Pedilanthus tithymaloides (L.) Medicinal Chemistry Research . 2013;22(9):4347–4359. doi: 10.1007/s00044-012-0449-4. [DOI] [Google Scholar]
- 23.Trevisan G., Rossato M. F., Hoffmeister C., et al. Antinociceptive and antiedematogenic effect of pecan (Carya illinoensis) nut shell extract in mice: a possible beneficial use for a by-product of the nut industry. Journal of Basic and Clinical Physiology and Pharmacology . 2014;25(4):401–410. doi: 10.1515/jbcpp-2013-0137. [DOI] [PubMed] [Google Scholar]
- 24.Eur-lex. Directive 2010/63/Eu of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. 2010. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 .
- 25. Lei No 11.794, De 8 De Outubro De 2008: Estabelece Procedimentos Para O Uso Científico De Animais . Brasília, Brazil: Brasil: Congresso Nacional; 2008. [Google Scholar]
- 26.Santos A. R. S., Miguel O. G., Yunes R. A., Calixto J. B. Antinociceptive properties of the new alkaloid, Cis-8, 10-Di-N- propyllobelidiol hydrochloride dihydrate isolated from Siphocampylus verticillatus: evidence for the mechanism of action. Journal of Pharmacology and Experimental Therapeutics . 1999;289(1):417–426. [PubMed] [Google Scholar]
- 27.Morgan L. V., Petry F., Scatolin M., et al. Investigation of the anti-inflammatory effects of stigmasterol in mice: insight into its mechanism of action. Behavioural Pharmacology . 2021;32(8):640–651. doi: 10.1097/fbp.0000000000000658. [DOI] [PubMed] [Google Scholar]
- 28.Scapinello J., Müller L. G., Schindler M. S. Z., et al. Antinociceptive and anti-inflammatory activities of Philodendron bipinnatifidum schott ex endl (araceae) Journal of Ethnopharmacology . 2019;236:21–30. doi: 10.1016/j.jep.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 29.Hunskaar S., Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain . 1987;30(1):103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 30.Santos A. R. S., Calixto J. B. Ruthenium red and capsazepine antinociceptive effect in formalin and capsaicin models of pain in mice. Neuroscience Letters . 1997;235(1–2):73–76. doi: 10.1016/s0304-3940(97)00722-2. [DOI] [PubMed] [Google Scholar]
- 31.Müller L. G., Salles L. A., Stein A. C., et al. Antidepressant-like effect of Valeriana glechomifolia meyer (valerianaceae) in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry . 2012;36(1):101–109. doi: 10.1016/j.pnpbp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Neves G., Menegatti R., Antonio C. B., et al. Searching for multi-target antipsychotics: discovery of orally active heterocyclic N-phenylpiperazine ligands of D2-like and 5-HT1A receptors. Bioorganic & Medicinal Chemistry . 2010;18(5):1925–1935. doi: 10.1016/j.bmc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 33.OECD. Guideline 423. Acute Oral Toxicity – Acute Toxic Class Method . Paris, France: OECD, Publishing; 2001. [Google Scholar]
- 34.Viana F. A. B. Guia Terapêutico Veterinário . 3rd. São Paulo, Brazil: Livraria Lmc; 2014. [Google Scholar]
- 35.Conselho Nacional de Controle de Experimentação Animal. Diretriz da prática de eutanásia do CONCEA . Brasília, Brazil: Federal University of Espírito Santo; 2018. [Google Scholar]
- 36.Gasparetto A., Bella Cruz A., Wagner T. M., Bonomini T. J., Correa R., Malheiros A. Seasonal variation in the chemical composition, antimicrobial and mutagenic potential of essential oils from Piper cernuum. Industrial Crops and Products . 2017;95:256–263. doi: 10.1016/j.indcrop.2016.10.030. [DOI] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard M07-A8 . Detroit, MI, USA: Wayne; 2009. [Google Scholar]
- 38.Zacchino A. S., Gupta M. P. Manual de técnicas in vitro para la detección de compuestos antifúngicos. Corpus Editorial y Distribuidora . 2007;85:p. 176. [Google Scholar]
- 39.Rahalison L., Hamburger M., Monod M., Frenk E., Hostettmann K. Antifungal tests in phytochemical investigations: comparison of bioautographic methods using phytopathogenic and human pathogenic fungi. Planta Medica . 1994;60(1):41–44. doi: 10.1055/s-2006-959405. [DOI] [PubMed] [Google Scholar]
- 40.Barbosa L. C. A., Filomeno C. A., Teixeira R. R. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules . 2016;21(12):1671–1704. doi: 10.3390/molecules21121671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silveira A. C., Lazzarotto M. Óleos essenciais de espécies de Eucaliptos . Brasília, Brazil: Embrapa; 2021. [Google Scholar]
- 42.Ferrari F., Cogo P. M. Planejamento Fatorial do Processo de Obtenção do Óleo Essencial de Folhas e Galhos Residual do Eucalyptus viminalis por hidrodestilação . Pato Branco, Brazil: Universidade Tecnológica Federal do Paraná; 2013. [Google Scholar]
- 43.Caceres A. I., Liu B., Jabba S. V., Achanta S., Morris J. B., Jordt S. E. Transient receptor potential cation channel subfamily M member 8 channels mediate the anti-inflammatory effects of eucalyptol. British Journal of Pharmacology . 2017;174(9):867–879. doi: 10.1111/bph.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa D. F. N. Potencial Imunomodulador e Antimicrobiano Do (+)-α-Pineno e (+)-β-Pineno . João Pessoa, Brazil: Universidade Federal Da Paraíba; 2017. [Google Scholar]
- 45.Kummer R. Efeitos do p-cimeno e do α-pineno sobre a resposta inflamatória aguda . Maringá, Brazil: Universidade Estadual De Maringá; 2015. [Google Scholar]
- 46.Moreira I. J. A., Serafini M. R., Lucca Junior W., et al. Prospecção tecnológica da utilização do β-pineno. Revista Gestão, Inovação e Tecnologias . 2013;3(2):186–194. doi: 10.7198/s2237-0722201300020015. [DOI] [Google Scholar]
- 47.Almeida A. C., Morão R. P., Martins E. R., et al. Atividade antisséptica do óleo essencial de Lippia origanoides Cham. (Alecrim-pimenta) na presença de leite bovino. Pesquisa Veterinária Brasileira . 2016;36(9):905–911. doi: 10.1590/s0100-736x2016000900018. [DOI] [Google Scholar]
- 48.Cosentino S., Tuberoso C. I. G., Pisano B., et al. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Letters in Applied Microbiology . 1999;29(2):130–135. doi: 10.1046/j.1472-765x.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 49.Barel S., Segal R., Yashphe J. The antimicrobial activity of the essential oil from Achillea fragrantissima. Journal of Ethnopharmacology . 1991;33(1–2):187–191. doi: 10.1016/0378-8741(91)90177-f. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira J. D., Alves C. C. F., Miranda M. L. D., et al. Rendimento, composição química e atividades antimicrobiana e antioxidante do óleo essencial de folhas de Campomanesia adamantium submetidas a diferentes métodos de secagem. Revista Brasileira de Plantas Medicinais . 2016;18(2):502–510. doi: 10.1590/1983-084x/15_206. [DOI] [Google Scholar]
- 51.Ghazghazi H., Essghaier B., Riguene H., et al. Phytochemical analysis, antioxidant and antimicrobial activities of Eucalyptus essential oil: a comparative study between Eucalyptus marginata L. and Eucalyptus paucilora L. Revue Roumaine de Chimie . 2020;64(12):1055–1062. doi: 10.33224/rrch/2019.64.12.05. [DOI] [Google Scholar]
- 52.Anandakumar P., Kamaraj S., Vanitha M. K. D-limonene: a multifunctional compound with potent therapeutic effects. Journal of Food Biochemistry . 2021;45(1) doi: 10.1111/jfbc.13566. [DOI] [PubMed] [Google Scholar]
- 53.Hafeez A., Männer K., Schieder C., Zentek J. Effect of supplementation of phytogenic feed additives (powdered vs. encapsulated) on performance and nutrient digestibility in broiler chickens. Poultry Science . 2016;95(3):622–629. doi: 10.3382/ps/pev368. [DOI] [PubMed] [Google Scholar]
- 54.Li Y., Lai Y., Wang Y., Liu N., Zhang F., Xu P. 1, 8-cineol protect against influenza-virus-induced pneumonia in mice. Inflammation . 2016;39(4):1582–1593. doi: 10.1007/s10753-016-0394-3. [DOI] [PubMed] [Google Scholar]
- 55.Lee G., Park J., Kim M. S., Seol G. H., Min S. S. Analgesic effects of Eucalyptus essential oil in mice. The Korean Journal of Pain . 2019;32(2):79–86. doi: 10.3344/kjp.2019.32.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batista E. K. F., Trindade H. I., Lira S. R. S., Muller J., Silva L., Batista M. Atividades antinociceptiva e antiinflamatória do extrato etanólico de Luehea divaricata. Revista Brasileira de Plantas Medicinais . 2016;18(2):433–441. doi: 10.1590/1983-084x/15_140. [DOI] [Google Scholar]
- 57.Rosland J. H., Tjølsen A., Mæhle B., Hole K. The formalin test in mice: effect of formalin concentration. Pain . 1990;42(2):235–242. doi: 10.1016/0304-3959(90)91167-h. [DOI] [PubMed] [Google Scholar]
- 58.Liapi C., Anifantis G., Chinou I., Kourounakis A. P., Theodosopoulos S., Galanopoulou P. Antinociceptive properties of 1,8-cineole and β- pinene, from the essential oil of Eucalyptus camaldu lensis leaves, in rodents. Planta Medica . 2007;73(12):1247–1254. doi: 10.1055/s-2007-990224. [DOI] [PubMed] [Google Scholar]
- 59.Hajhashemi V., Ghannadi A., Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytotherapy Research . 2004;18(3):195–199. doi: 10.1002/ptr.1390. [DOI] [PubMed] [Google Scholar]
- 60.Santos F. A., Rao V. Efeitos antiinflamatórios e antinociceptivos do 1,8-cineol, um óxido terpenóide presente em muitos óleos essenciais de plantas . Ceará, Brazil: Universidade Federal do Ceará; 1999. [Google Scholar]
- 61.de Lacerda G. F. M. L. Ansiedade em modelos animais: efeito de drogas nas dimensões extraídas da análise fatorial . Ceará, Brazil: Universidade Federal do Paraná; 2006. [Google Scholar]
- 62.Carlini E. A., Mendes F. R. Protocolos Em Psicofarmacologia Comportamental . 1st. São Paulo, Brazil: Editora Unifesp; 2011. [Google Scholar]
- 63.Sturman O., Germain P. L., Bohacek J. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress: The International Journal on the Biology of Stress . 2018;21(5):443–452. doi: 10.1080/10253890.2018.1438405. [DOI] [PubMed] [Google Scholar]
- 64.Al-Hasani R., Bruchas M. R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology . 2011;115(6):1363–1381. doi: 10.1097/aln.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunton L. L., Hilal-Dandan R., Knollmann B. C. As Bases Farmacológicas da Terapêutica de Goodman e Gilman . 13th. Porto Alegre, Brazil: AMGH; 2018. [Google Scholar]
- 66.Sobreira D. N. F. R., Monteiro A. B., Alencar de Menezes I. R., et al. Effects of the Hyptis martiusii Benth. leaf essential oil and 1,8-cineole (eucalyptol) on the central nervous system of mice. Food and Chemical Toxicology . 2019;133 doi: 10.1016/j.fct.2019.110802.110802 [DOI] [PubMed] [Google Scholar]
- 67.Betti A. H., Stein A. C., Dallegrave E., et al. Acute and repeated-doses (28 days) toxicity study of Hypericum polyanthemum Klotzsch ex Reichardt (Guttiferare) in mice. Food and Chemical Toxicology . 2012;50(7):2349–2355. doi: 10.1016/j.fct.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 68.Vilela G. R. Efeito do óleo essencial de Eucalyptus globulus sobre espécies produtoras de aflatoxinas . Piracicaba, Brazil: Universidade de São Paulo; 2007. [Google Scholar]
- 69.Xu J., Hu Z., Wang C., et al. Acute and subacute toxicity study of 1,8-cineole in mice. International Journal of Clinical and Experimental Pathology . 2014;7(4):1495–1501. [PMC free article] [PubMed] [Google Scholar]
- 70.Estanislau A. A., Barros F. A. S., Peña A. P., Santos S., Ferri P., Paula J. Composição química e atividade antibacteriana dos óleos essenciais de cinco espécies de Eucalyptus cuItivadas em Goiás. Revista Brasileira de Farmacognosia . 2001;11(2):95–100. doi: 10.1590/s0102-695x2001000200005. [DOI] [Google Scholar]
- 71.Falcão D. Q., Menezes F. S. Revisão etnofarmacológica, farmacológica e química do gênero Hyptis. Revista Brasileira de Farmacognosia . 2003;84(3):69–74. [Google Scholar]
- 72.Chorianopoulos N., Kalpoutzakis E., Aligiannis N., Mitaku S., Nychas G. J., Haroutounian S. A. Essential oils of Satureja, Origanum, and Thymus species: chemical composition and antibacterial activities against foodborne pathogens. Journal of Agricultural and Food Chemistry . 2004;52(26):8261–8267. doi: 10.1021/jf049113i. [DOI] [PubMed] [Google Scholar]
- 73.Cimanga K., Kambu K., Tona L., et al. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. Journal of Ethnopharmacology . 2002;79(2):213–220. doi: 10.1016/s0378-8741(01)00384-1. [DOI] [PubMed] [Google Scholar]
- 74.Nascimento P. F. C., Nascimento A. C., Rodrigues C. S., et al. Atividade antimicrobiana dos óleos essenciais: uma abordagem multifatorial dos métodos. Revista Brasileira de Farmacognosia . 2007;17(1):108–113. doi: 10.1590/s0102-695x2007000100020. [DOI] [Google Scholar]
- 75.Burt S. Essential oils: their antibacterial properties and potential applications in foods-a review. International Journal of Food Microbiology . 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 76.Rahman A., Kang S. C. Inhibition of foodborne pathogens and spoiling bacteria by essential oil and extracts of Erigeron ramosus (WALT.) B.S.P. Journal of Food Safety . 2009;29(2):176–189. doi: 10.1111/j.1745-4565.2009.00149.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


