Highlights
-
•
Cardiac arrhythmias are a leading cause of morbidity and mortality, at least in part because of gaps in the knowledge of the fundamental mechanisms that negatively affect clinical management.
-
•
Inflammatory activation is increasingly recognized as a nonconventional risk factor for arrhythmias: this review will discuss the basic mechanisms underlying arrhythmogenic effects of proinflammatory cytokines and their translational affect in the clinical setting.
-
•
Based on the strong background provided by the basic and clinical studies here reviewed, large randomized clinical trials are warranted to verify whether anticytokine-targeted therapies can represent a new important avenue for the treatment and prevention of cardiac arrhythmias.
Key Words: basic mechanisms, cardiac arrhythmias, clinical studies, inflammatory cytokines
Summary
Inflammatory activation is increasingly recognized as a nonconventional risk factor for arrhythmias, and experimental studies provided robust evidence that this association is mediated by direct arrhythmogenic effects of proinflammatory cytokines on cardiac cells. Additionally, inflammatory cytokines can favor arrhythmias indirectly through multiple systemic effects. Accumulating data confirm the clinical relevance of these mechanisms; the largest evidence being available for atrial fibrillation, acquired long-QT syndrome, and ventricular arrhythmias. However, clinical management of arrhythmias largely neglects inflammatory cytokines. This review integrates basic science and clinical research to present an updated overview of the topic and provides future directions for patient’s management.
Central Illustration
Cardiac arrhythmias are associated with high morbidity and mortality worldwide.1 This is due, at least in part, to gaps in the knowledge of the basic mechanisms that may have negative impacts on the effectiveness of therapeutic interventions.
Accumulating data from experimental and clinical studies strongly indicate that inflammatory activation can promote a wide spectrum of cardiac arrhythmias, because of specific activities of proinflammatory cytokines on arrhythmogenesis. Despite this evidence, inflammation is still largely neglected in the diagnostic and therapeutic management of arrhythmias. In fact, inflammatory markers, particularly circulating cytokines, are not routinely measured in patients with arrhythmic events, and no agents targeting the inflammatory system are used in clinical practice to prevent or treat arrhythmias at this time.
The purpose of this review is integrating basic science and clinical research to present an updated overview of the topic, also providing future directions in patient’s management. Given that the very large majority of the available data is about tumor necrosis factor-alpha (TNF), interleukin (IL)-1, IL-6, and to a lesser extent, IL-17, these proinflammatory cytokines will represent the focus of the review.
Basic Mechanisms: Inflammatory Cytokines and Arrhythmogenesis
Large experimental evidence indicates that inflammatory cytokines affect arrhythmogenesis via multiple mechanisms, including direct cardiac effects and indirect activities mediated by cytokine-associated systemic changes.
Direct cardiac effects
A wide number of experimental studies provides robust evidence that inflammatory cytokines can significantly enhance the risk of arrhythmic events, including tachyarrhythmias and conduction disturbances/bradyarrhythmias, by directly promoting electrical and structural cardiac remodeling via effects on cardiac myocytes and fibroblasts. The mechanisms demonstrated comprise the following: 1) prolongation of the ventricular action potential duration (APD) caused by membrane ion channels modulation; 2) impairment of intracellular Ca++-handling proteins with spontaneous diastolic Ca++ release from the sarcoplasmic reticulum (SR); 3) gap junction dysfunction, via changes in connexins; and 4) promotion of cardiac fibrosis. Whereas electrical changes occur early (hours, days) and are reversible, structural remodeling requires a longer time to develop (weeks, months).
APD prolongation
The duration of the action potential (AP) in cardiomyocytes is determined by the sequential activation of inward depolarizing currents, primarily conducted by Na+ and Ca++ channels, and outward repolarizing currents, mostly through K+ channels.2,3 It is well established that QT interval on the surface electrocardiogram (ECG) reflects the APD in ventricles.2 Whenever a dysfunction of 1 or more of these channels produces an inward shift in the overall balance of currents, APD prolongs and, therefore, the QT interval does too.2,3 APD prolongation in turn facilitates inward L-type calcium current (ICaL) reactivation, thereby the occurrence of early afterdepolarizations (EADs) leading to triggered tachyarrhythmias caused by abnormal ectopic firing.4 Accordingly, heart rate-corrected QT interval (QTc) prolongation is recognized in the clinical setting as an important risk factor for life-threatening ventricular arrhythmias (VAs), particularly torsades de pointes (TdP), and sudden cardiac death (SCD).3
Extensive experimental evidence demonstrates that proinflammatory cytokines can prolong APD and QTc by directly interfering with the function of several ion channels of the ventricular myocyte (inflammatory cardiac channelopathies) (Table 1, Figure 1).5, 6, 7, 8
Table 1.
APD/QT-Prolonging Effects of Proinflammatory Cytokines and Arrhythmogenesis: Data From Experimental Studies
| Cytokine | Effects on Ion Currents | Effects on Ion Channels/Molecular Mechanisms | Effect on APD | Effect on QT/QTc Interval | Effect on EAD/Arrhythmia Susceptibility |
|---|---|---|---|---|---|
| TNF | Ito decrease15, 16, 17, 18 | Reduced expression of Kv4.2/Kv4.3 potassium channels,15,17,18 mediated by iNOS induction,18 ROS generation,18 and KChIP-2 inhibition17 | Prolongation12,18,19 | Prolongation10,11,14 | Enhanced12,13 |
| IKr decrease19 | Functional impairment of hERG, mediated by TNF-R I engagement and ROS production19 | ||||
| IKs decrease20 | Mediated by sphingosine-1-phosphate generation and cyclic AMP decrease20 | ||||
| IKur decrease15,16 | Reduced expression of Kv1.5 potassium channel15 | ||||
| INa increase21 | NA | ||||
| IL-1 | Ito decrease24 | NA | Prolongation23, 24, 25, 26 | Prolongation24,26 | Enhanced24,26 |
| ICaL increase23 | Mediated by activation of cyclo-oxygenase and lipoxygenase pathways23 | ||||
| NA | Reduced expression of the KCNJ2 gene, encoding the Kir2.1 channel that conducts the inward rectifier potassium current IK128 | ||||
| IL-6 | ICaL increase31 | Enhancement of Cav1.2 calcium channel function,29,31 dependent on a SHP2/ERK-mediated phosphorylation of the serine residue at position 1,82930 | Prolongation25,31, 32, 33, 34, 35 | Prolongation33,34 | Enhanced35 |
| IKr decrease32, 33, 34, 35 | Reduced expression and function of hERG, mediated by IL-6-receptor engagement and Janus-kinase pathway activation32 | ||||
| IKs decrease35 | Impaired mitochondrial ATP production, required for IKs activation caused by PKA-dependent phosphorylation of Kv7.1 potassium subunits35 | ||||
| IL-17 | Ito decrease39 | Mediated by NF-κB–dependent down-regulation of KChIP-239 | Prolongation37, 38, 39 | NA | Enhanced37, 38, 39 |
AMP = adenosine monophosphate; APD = action potential duration; ATP = adenosine triphosphate; EADs = early afterdepolarizations; hERG = human ether-a-go-go-related gene K+-channel; ICaL = L-type calcium channel current; IKr = rapidly activating component of the delayed outward-rectifying current; IKs = slowly activating component of the delayed outward-rectifying current; IKur = ultra-rapidly activating component of the delayed outward-rectifying current; IL = interleukin; INa = sodium current; iNOS = inducible nitric oxide synthase; Ito = transient K+-outward current; KChIP-2 = K(+) channel-interacting protein; Kv = potassium channel proteins; NA = not available; NF-κB = nuclear factor κ light chain enhancer of activated B cells; PKA = protein-kinase A; ROS = reactive oxygen species; SHP/ERK = Src homology 2 domain-containing phosphatase/extracellular signal-regulated kinase; TNF = tumor necrosis factor-alpha; TNF-R1 = tumor necrosis factor receptor-1.
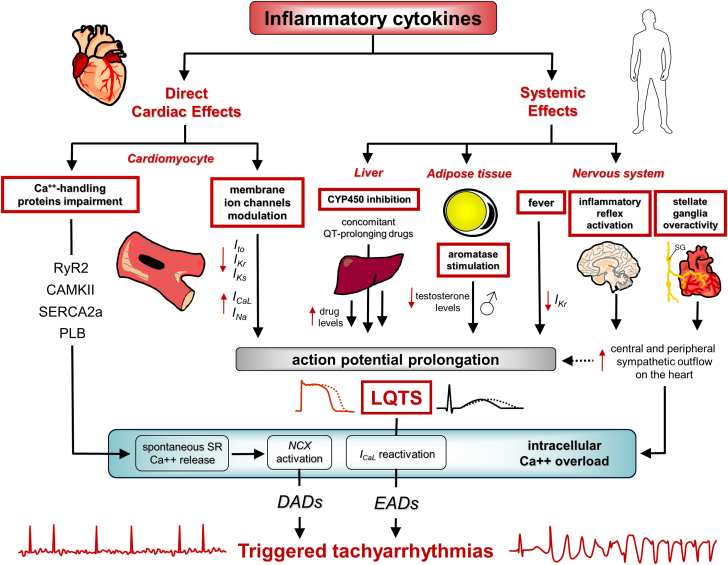
Figure 1.
Arrhythmogenic Effects of Inflammatory Cytokines: Triggered Tachyarrhythmias
Inflammatory cytokines can favor the development of triggered activity-induced tachyarrhythmias through multiple direct and indirect mechanisms. Direct cardiac effects on the cardiomyocyte include the following: 1) modulation of expression/function of several ion channels on the membrane, that is, inhibition of the outward repolarizing currents—transient outward potassium current (Ito), slow component of the delayed-rectifier potassium current (IKs), and rapid component of the delayed-rectifier potassium current (IKr)—and enhancement of the inward depolarizing currents—L-type calcium current (ICaL) and sodium current (INa)—leading to ventricular action potential prolongation; 2) impairment of key intracellular Ca++-handling proteins, that is, ryanodine receptor-2 (RyR2), calmodulin-dependent protein kinase II (CAMKII), sarco-endoplasmic reticulum Ca++ adenosine triphosphatase, and phospholamban (PLB) promoting spontaneous diastolic Ca++ leakage from the sarcoplasmic reticulum. Indirect cardiac effects are the result of cytokine-induced systemic changes, including the following: 1) cardiac sympathetic nervous system overactivation, through both central (inflammatory reflex) and peripheral (left stellate ganglia) mechanisms, which further aggravates cardiomyocyte action potential prolongation and Ca++ overload; 2) induction of fever leading to temperature-mediated cardiac ion channel biophysical changes (ie, IKr decrease); 3) reduction of testosterone levels in male subjects, caused by enhanced aromatase activity and androgen-to-estrogen conversion in adipose tissue, in turn prolonging action potential/QTc duration; 4) inhibition of liver cytochrome p450 (CYP450)-dependent drug metabolism, favoring blood accumulation of concomitant QT-prolonging drugs. Collectively, all these mechanisms can elicit triggered tachyarrhythmias, as a result of enhanced ectopic firing caused by intracellular Ca++ overload-induced afterdepolarizations, either early afterdepolarization (EAD) and delayed afterdepolarization (DAD), in turn resulting from action potential prolongation, which promotes inward ICaL reactivation, and dysfunction of Ca++-handling proteins with spontaneous Ca++ release in diastole, respectively. Red arrows pointing up indicate increase; red arrows pointing down indicate decrease. LQTS = long QT-syndrome; NCX = Na+/Ca++ exchanger; SERCA2a = sarcoplasmic reticulum calcium–adenosine triphosphatase–2a; SR = sarcoplasmic reticulum.
The inhibitory effects of TNF on outward potassium currents are the best documented.9 Several studies have demonstrated that transgenic mice overexpressing TNF show evident APD and QT-interval prolongation, as well as increased vulnerability to VAs and premature mortality.10, 11, 12, 13, 14 In left ventricular myocytes obtained from these animals, a significant lower density of the transient outward potassium current (Ito) and the rapidly activating, slowly inactivating delayed rectifier currents was observed, with a concomitant reduction of the related potassium channel proteins (Kv4.2, Kv4.3, and Kv1.5).12,15 Consistent results were found in mouse ventricular myocytes from animals chronically injected with TNF16 or incubated in vitro with TNF.17,18 These changes are mediated by an intracellular pathway leading to nitric oxide synthase overexpression, reactive oxygen species generation, and inhibition of the K+ voltage-gated channel interacting protein-2, the regulatory subunit of Ito.17,18 TNF can also prolong APD by inhibiting the rapid (IKr), and the slow (IKs) components of the delayed-rectifier potassium current and augmenting the sodium current (INa). Wang et al19 reported that the incubation of HEK293 cells or canine cardiomyocytes with TNF reduced IKr and prolonged APD because of a reactive oxygen species–mediated ether-a-go-go-related gene K+ channel (hERG) loss of function. Moreover, Hatada et al20 showed that isoprotenerol-activated IKs was inhibited in TNF-cultured guinea pig ventricular myocytes, possibly via sphingosine-1-phospate production and cyclic adenosine monophosphate decrease. Finally, an acute increase of INa amplitude was observed by Lin et al21 after incubation of single rat ventricular myocytes with TNF. The evidence that this effect was more pronounced in cells obtained from the M (mid-myocardial) region when compared to other layers of the myocardium, suggests that TNF can also increase transmural dispersion of ventricular repolarization,21 a key additional electrophysiological abnormality required for TdP development in the setting of APD/QTc prolongation.22
Other data point to a similar impact for IL-1 and IL-6 on ventricular APD, as a result of multiple activities of these cytokines on both outward K+ and inward Ca++ currents (Table 1).
In a pioneering study, Li et al23 demonstrated that incubation of guinea pig papillary myocytes with IL-1β induced significant APD prolongation, in association with ICaL increase. More recently, consistent IL-1–induced APD prolongation was reported by Monnerat et al24 and Sattayaprasert et al25 in rat ventricular myocytes and human induced pluripotent stem cell–derived cardiomyocytes (hiPS-CMs), along with Ito inhibition.24 Moreover, in an experimental mouse model of diabetes mellitus, elevated cardiac levels of IL-1β were associated with QT-interval prolongation and an increased ventricular tachycardia (VT) inducibility.26 Optical mapping experiments in these hearts showed that short-term in vitro perfusion with IL-1β prolonged APD and promoted EAD.26 Conversely, blockade of IL-1 signaling by administering the IL-1-receptor (IL-1R) antagonist anakinra or genetically deleting the IL-1R, prevented QTc prolongation and VAs in the same animal model,24,26 as well as in mice with autoimmune myocarditis.27 Finally, our own recent study28 suggests an additional inhibitory effect of IL-1 on the expression of the ventricular Kir2.1 channel, which is encoded by the KCNJ2 gene and conducts the inward rectifier potassium current IK1 (Table 1).
Several studies are also available supporting the ability of IL-6 to prolong ventricular APD by directly inducing complex changes in cardiac electrophysiology. The group of Ogawa29, 30, 31 was the first to provide evidence that in rat ventricular myocytes IL-6 can enhance ICaL,29, 30, 31 via a phosphorylation-mediated potentiation of the Cav1.2 subunit function,30 and prolong APD.31 These findings were more recently confirmed and expanded by our research group who demonstrated the electrophysiological basis for IL-6–induced ventricular APD prolongation also critically involving IKr inhibition,32, 33, 34 an effect resulting from combined depressive activities on protein expression and activation kinetics of the hERG-K+ channel in cardiac cells.32 In addition, we showed that the direct administration of IL-6 to guinea pigs acutely prolongs QTc,33,34 which was prevented by the IL-6R blocker tocilizumab.33 A substantial APD-prolonging effect for IL-6 was also reported by Sattayaprasert et al25 in hiPS-CMs, and by Chowdhury et al35 in adult guinea pig ventricular myocytes where an additional contribution caused by IL-6–dependent depression of IKs density was also demonstrated. Importantly, the last study also provided direct evidence that IL-6 can promotes arrhythmogenesis, manifested as triggered EADs and spontaneous beats (Table 1).
Finally, recent data suggest that IL-17 is a new and potentially important player for APD prolongation and associated arrhythmogenesis.36 Chang et al37 found that acute IL-17 infusion in rabbits with ischemic heart failure (HF) significantly prolonged APD and induced VAs in vivo and in vitro in a dose-dependent manner.37 These findings, which were reproduced by Tsai et al38 in isolated healthy rabbit hearts perfused with IL-17, were prevented/reversed by anti-IL-17–neutralizing antibodies.37,38 Moreover, in a murine model of diabetes mellitus showing APD prolongation associated to Ito depression, knockout of IL-17A–encoding gene restored these electrophysiological changes by attenuating the nuclear factor κ light chain enhancer of activated B cells–dependent down-regulation of K+ voltage-gated channel interacting protein-2 (Table 1).39
It is important to note that in vivo, during inflammatory activation, all these cytokines are concomitantly released, and for this reason it is likely that their overall electrophysiological effects may be cumulative. In agreement, a recent simulation study incorporated part of the above-mentioned experimental data on TNF, IL-1, and IL-6 into a human cell model and confirmed that each cytokine leads to a significant prolongation of APD and QT interval, in the presence of increased transmural and regional repolarization heterogeneities. These changes were significantly enhanced when the effects of the 3 cytokines were analyzed in combination (Figure 1).40
Spontaneous diastolic SR Ca++ release
Robust experimental evidence provides support for a significant impact of inflammatory cytokines on SR Ca++ release and uptake in cardiac myocytes. Spontaneous diastolic Ca++ leak from the SR is known to be proarrhythmogenic because it activates the electrogenic Na+/Ca++ exchanger (NCX). The resulting transient inward Na+ current can generate delayed afterdepolarizations (DADs), trigger new premature APs, and initiate tachyarrhythmias, including atrial fibrillation (AF) and VT.41
Several reports demonstrated that TNF, IL-1β, and IL-6 can impair the expression and the function of key intracellular Ca++-handling proteins in different cell models (atrial HL-1 cells, mice ventricular cardiomyocytes, hiPS-CMs), including ryanodine receptor-2 (RyR2), the SR channels through which Ca++ is released, SR Ca++–adenosine triphosphatase–2a (SERCA2a), which transports Ca++ inside the SR lumen during cardiac myocyte relaxation, and phospholamban, a physiologic SERCA2a inhibitor (Table 2).42, 43, 44, 45, 46, 47, 48, 49 The arrhythmogenicity of these changes is supported by the evidence that in transgenic mice overexpressing TNF, periods of rapid pacing induced abnormal increase of diastolic Ca++ and elicited atrial and ventricular tachyarrhythmias.10,12 Moreover, Duncan et al50 demonstrated that coexposure of rat ventricular myocytes to IL-1β and TNF induced asynchronous Ca++ release during electrical stimulation and increased the frequency of localized Ca++ release events and spontaneous Ca++ waves; all of these changes are suggestive of an enhanced Ca++ leak from the SR. Consistent findings were obtained by Zu et al51,52 with TNF and IL-6, and by Monnerat et al24 with IL-1β, who additionally reported how cytokine-induced spontaneous sparks in isolated atrial and ventricular myocytes were associated with oxidation and phosphorylation of the calmodulin-dependent protein kinase II, a master activator of important Ca++ handling proteins (including RyR2).24,51 An increased frequency of spontaneous Ca++ sparks in myocytes isolated from diabetic mouse hearts overexpressing IL-1β was also demonstrated in a study by Liu et al,26 where evidence of increased inducibility of VAs and RyR2 hyperactivation caused by oxidative changes was concomitantly provided. Moreover, TNF-treated cardiomyocytes showed increased NCX activity, enhanced transient inward Na+ current, and larger amplitude of DADs than control cells (Table 2).53
Table 2.
Effects of Proinflammatory Cytokines on Cardiac Ca++-handling Proteins and Associated Arrhythmogenesis: Data From Experimental Studies
| Cytokine | Effects on Intracellular Ca++-Handling Proteins Activity |
Effects on Spontaneous Ca++ Release/NCX Activity | Effects on DAD/Arrhythmia Susceptibility | |||
|---|---|---|---|---|---|---|
| RyR2 | CAMKII | SERCA2a | PLB | |||
| TNF | Increased (↑phosphorylation)51 | Increased (↑oxidation)51 | Reduced (↓expression and function)46,53,230,231 | Increased (↓phosphorylation)232 | Enhanced10,12,50,51,53 | Enhanced10,12,53 |
| IL-1 | Increased (↑oxidation)26 | Increased (↑oxidation)24 | Reduced (↓expression)42, 43, 44, 45,55 | NA | Enhanced24,50,55,56 | Enhanced24,26,55,56 |
| IL-6 | Increased (↑expression)57,233 | Increased234,235 | Reduced (↓expression)46,47 | Increased (↓phosphorylation)48,236 | Enhanced25,52 | Enhanced57 |
| IL-17 | Increased (↑expression)38 | NA | Reduced (↓expression)38,237 | Increased (↑expression)38 | Enhanced38 | Enhanced38 |
CAMKII = calmodulin-dependent protein kinase II; DADs = delayed afterdepolarizations; NCX = Na+/Ca++ exchanger; PLB = phospholamban; RyR2 = ryanodine receptor-2; SERCA2a = sarcoplasmic reticulum calcium—adenosine triphosphatase–2a; other abbreviations as in Table 1.
The pathogenic link connecting cytokine-induced Ca++ handling abnormalities and tachyarrhythmias was further strengthened by studies exploring the antiarrhythmic potential of targeted cytokine blockade. Stamm et al54 demonstrated that the intracellular Ca++ handling abnormalities induced in isolated rat hearts following lipopolysaccharide stimulation were effectively reverted by the administration of a neutralizing anti-TNF antibody. In a post–myocardial infarction (MI) mouse model, a 4-day course of anakinra treatment leading to decreased Ca++ alternans magnitude and increased expression of SERCA2a, was associated with a reduction of triggered VAs.55 Three other studies conducted in different murine models of IL-1–driven diseases (diabetes mellitus or renal ischemia/reperfusion injury) obtained consistent results, specifically demonstrating that IL-1R antagonist administration caused substantial reduction of VT inducibility,24,26,56 along with decreased RyR2 oxidation26 and a close to normal duration and amplitude of Ca++ transient.56 Moreover, pretreatment with anti-IL-6 monoclonal antibody reversed the impaired expression of RyR2 and phosphorylated phospholamban, the prolongation and regional heterogeneity of Ca++ transient duration, as well as the increased vulnerability to AF development observed in a rat model of sterile pericarditis, independent of cardiac fibrosis (Table 2).57
Finally, a very recent study performed on isolated perfused rabbit hearts demonstrated that IL-17 enhances intracellular Ca++ transient duration and VA susceptibility, along with up-regulation of NCX, RyR2, and phospholamban and inhibition of SERCA2a, and that all these changes were reversed in the presence of anti-IL-17 antibody (Table 2).38
Gap junction impairment
Gap junctions are intercellular channels that play a key role in the propagation of cardiac impulse by mediating electrical coupling between cardiomyocytes. Each adjacent cell contributes to the junction with a hemichannel or connexon, formed by 6 pore-forming subunits or connexins (Cxs).2,58 Cardiac Cxs, that is, Cx40, Cx43, and Cx45, are differently expressed/coexpressed in chamber- and myocyte-specific manners.2,58 Particularly, whereas the ventricle is characterized by Cx43-containing gap-junctions, both Cx43 and Cx40 construct these channels in the atria. All 3 Cxs contribute to formation of gap junctions in the conduction system, although specific contribution varies in nodal cells and different levels of the His-Purkinje system.2,58 Moreover, recent data demonstrated that gap junctions containing Cx43 also electrically couple cardiomyocytes with macrophages, and that these cells have a high physiological relevance in facilitating electrical conduction in the distal part of the atrioventricular (AV) node.59
Accumulating evidence indicates that inflammatory cytokines can induce gap-junction dysfunction in cardiac myocytes via impaired expression, distribution, and function of Cxs (specifically Cx40 and Cx43). These changes can promote re-entrant atrial and ventricular tachyarrhythmias and conduction disturbances/bradyarrhythmias by favoring a slowed and heterogeneous impulse propagation throughout the heart. Whereas TNF and IL-1 were the first and most intensively investigated, recent data point to similar arrhythmogenic effects for IL-6 and IL-17 (Table 3).
Table 3.
Effects of Proinflammatory Cytokines on Cardiac Cxs and Associated Arrhythmogenesis: Data From Experimental Studies
| Cytokine | Effects on Cxs |
Effect on Gap-Junction Function | Effect on Conduction Velocity | Effect on Arrhythmia Susceptibility | |
|---|---|---|---|---|---|
| Cx40 | Cx43 | ||||
| TNF | Reduced expression11,49,60, 61, 62 | Reduced expression and impaired distribution11,49,60 | Impaired coupling49 | Reduced11 | Enhanced11 |
| IL-1 | NA | Reduced expression and impaired distribution/function55,63, 64, 65, 66 | Impaired coupling63,65,66 | Reduced55,66 | Enhanced55 |
| IL-6 | Reduced expression69 | Reduced expression69,70 | NA | Reduced33,70 | Enhanced33 |
| IL-17 | NA | Reduced expression71 | NA | Reduced37 | Enhanced37,71 |
Cxs = connexins; other abbreviations as in Table 1.
In transgenic mice with cardiac-restricted overexpression of TNF, Cx40 and Cx43 were found to be down-regulated and/or irregularly dispersed throughout the sarcolemma in atria, bundle branches, and ventricles. These changes were associated with a slower and abnormal conduction velocity during the electrical mapping, as well as with an increased incidence of atrial tachyarrhythmias and conduction abnormalities on the ECG (wider P-wave and QRS duration) when compared to wild-type animals.11 In agreement, Liew et al60 reported a reduced Cx40 and Cx43 expression in the atria of mice injected with TNF, and several in vitro investigations reproduced consistent inhibitory effects in different cell models following TNF incubation.49,61,62 In particular, a recent study performed in hiPS-CMs demonstrated that TNF treatment not only decreased Cx40 expression and Cx43 localization in the plasma membrane, but it also impaired intercellular coupling with reduced Ca++ propagation (Table 3).49 Unlike TNF, all the available studies involving IL-1 were specifically focused on Cx43, so that the impact of this cytokine on Cx40 and other Cxs is yet unknown. Coppen et al63 were the first to report that 24-48 hours of incubation of rat neonatal ventricular myocytes with IL-1β caused an evident decrease of Cx43 expression, along with a reduction of functional intercellular communication via gap junctions between cardiomyocytes. Confirmatory results on the same cell model were provided by Baum et al,64,65 who also provided evidence for increased internalization and abnormal distribution of the protein into the myocyte. In addition, Zhong et al66 demonstrated that IL-1β also increases Cx43 phosphorylation at Ser368 (pS368Cx43) in rat embryonic heart cells and that this biochemical modification impairs cell-to-cell communication. The significant impact of these changes on ventricular arrhythmic risk was substantiated by animal studies. In a mouse model of MI, where an ∼3-fold increase in cardiac IL-1β expression was found, Cx43 expression markedly decreased, in the presence of Cx43 internalization and lateralization.67 In these hearts, a significant slowing of the conduction velocity was demonstrated along with an increased propensity to re-entrant VAs.67 Animal treatment with a 4-day course of anakinra improved conduction velocity and reduced spontaneous and inducible VAs, along with an increased Cx43 cardiac expression.55 Furthermore, in a rat model of autoimmune myocarditis characterized by enhanced tissue expression of IL-1,68 slowed intraventricular conduction (as reflected by QRS prolongation on the surface ECG) matched with elevated cardiac levels of pS368Cx43.66 Accordingly, IL-1β perfusion of isolated hearts of normal rats, not only increased pS368Cx43 but also impaired cell-to-cell communication and prolonged QRS duration.66
More recently, our group was the first to demonstrate that IL-6 potently inhibited Cx40 and Cx43 in cultured cardiomyocytes and macrophages,69,70 and that this effect was rescued on preincubation of cells with a monoclonal anti-IL-6 antibody.69 Moreover, in guinea pigs, acute IL-6 injection was associated with a slowing of AV conduction (PR-interval and PR-segment prolongation)33,70 and markedly enhanced the propensity to drug-associated severe bradyarrhythmias (complete AV dissociation and asystole).33
Regarding IL-17, it has been demonstrated that the acute administration of this cytokine in a Langendorff rabbit heart model decreased conduction velocity and promoted VAs, whereas perfusion with an anti-IL-17–neutralizing antibody prevented both effects.37 In the same study, the investigators also provided evidence for enhanced VA inducibility in rabbits with HF on chronic IL-17 administration.37 Coherently, decreasing IL-17 myocardial expression in infarcted rats was associated with increased Cx43 expression and reduced propensity to induced VAs.71
Cardiac fibrosis
It is well established that cardiac fibrosis can significantly disturb electrical impulse propagation and generate re-entry circuits, thus contributing to the occurrence of both conduction defects and re-entrant VAs.72 A large number of experimental studies have implicated inflammatory cytokines, specifically TNF, IL-1, and IL-6 in the pathogenesis of cardiac fibrosis. As recently reviewed in an excellent paper by Frangogiannis,73 multiple mechanisms are involved, including direct activation of myofibroblast-driven extracellular matrix synthesis, and indirect effects mediated by macrophage recruitment, transforming growth factor-β up-regulation, and production of matrix-regulator molecules.
Several animal model studies provided evidence that cytokine-induced fibrogenic changes in the heart are proarrhythmic. Saba et al10 reported that, when compared with control animals, mice with cardiac-specific TNF overexpression showed increased atrial collagen deposition associated with higher incidence of atrial tachyarrhythmias and conduction disturbances (PR-interval prolongation and longer AV-nodal Wenckebach periodicity). Accordingly, in perfused hearts isolated from these animals but not from control animals, programmed stimulation with single extra beats elicited re-entrant atrial arrhythmias. Later, Dai et al74 demonstrated that in a rat model of rheumatoid arthritis (RA), characterized by high circulating inflammatory cytokines, AF inducibility was significantly enhanced compared with in control rats, the duration of the arrhythmia being directly correlated with TNF and IL-6 levels. In these animals, a prolonged atrial conduction time and significant atrial fibrosis were also concomitantly observed. Moreover, rabbits with ischemic HF chronically treated with IL-17 showed increased left ventricle collagen production and fibrosis along with higher susceptibility to induced VAs.37 Further support of these findings is provided by the observation that down-regulation of IL-17 ventricular expression led to a significant decrease of myocardial fibrosis and VA susceptibility during programmed electrical stimulation.75 Moreover, pharmacological inhibition of IL-1–induced IL-6 release with colchicine was associated with lower propensity to atrial fibrosis and AF induction in the rat sterile pericarditis model, and these effects are abolished by IL-6 administration.76 In the same model, other investigators demonstrated how anti-IL-17 antibody treatment alleviated atrial collagen expression and fibrosis and concomitantly suppressed AF development.77
Indirect systemic effects
Besides the direct impact on cardiac electrophysiology, inflammatory cytokines can induce important systemic effects that might further favor arrhythmia development in an indirect manner. These mechanisms include the following: 1) fever, responsible for temperature-mediated modifications of cardiac ion channel biophysics;78,79 2) activation of the cardiac sympathetic nervous system; 3) inhibition of cytochrome p450 in the liver, increasing bioavailability of several medications, including QT-prolonging drugs; and 4) stimulation of aromatase activity in adipose tissue with enhanced androgen-to-estrogen conversion, leading to reduced testosterone levels and increased long-QT syndrome (LQTS)/TdP risk in male patients (Figure 1).
Fever-induced changes in cardiac electrophysiology
Inflammatory cytokines are the key molecules responsible for fever development, via direct activation of the thermoregulatory neurons of the preoptic hypothalamic region.80 Several investigators provided evidence that fever can promote LQTS and related life-threatening VAs,81,82 particularly in the presence of concomitant genetic or acquired (drugs, hypokalemia) IKr defects,79,81, 82, 83 by interfering with the temperature-sensitive biophysical properties of the hERG-K+ channel.79,83 Specifically, neonatal rat ventricular myocytes exposed to febrile temperature (40 °C) showed IKr decrease, hERG-K+–channel down-regulation, and APD prolongation through altered K+ dependence.79 In agreement, fever and hypokalemia demonstrated synergistic QT-prolonging effects in an in vivo rabbit model.79 Brugada syndrome (BrS) is an arrhythmogenic channelopathy associated with a high incidence of SCD, in most cases caused by a loss-of-function of the Na+ channel Nav1.5 resulting in electrophysiological transmural changes in the right ventricle outflow tract.84 Fever represents a well-known acquired factor unmasking BrS in predisposed subjects,85 in other words, carriers of latent Na+ channels dysfunction.86,87 Accordingly, evidence indicates that biophysical properties of the Nav1.5 channel are substantially altered by high temperature, resulting in a less efficient kinetics with INa decrease.88,89 Thus, although quite different pathophysiological bases underlie LQTS and BrS, cytokine-induced fever may significantly enhance, through different mechanisms, the arrhythmogenic potential associated with both conditions.
Enhanced cardiac sympathetic system activation
Autonomic nervous system activation, representing an established triggering factor for malignant tachyarrhythmias,90 is a well-recognized systemic change associated to the inflammatory response.91 It has been known for many years that it results from direct effects of cytokines, particularly IL-1, IL-6, and TNF, on the autonomic centers of the brain, principally the hypothalamus, leading to an enhanced sympathetic outflow throughout the body.91, 92, 93 These systemic changes, primarily aimed to control cytokine production by targeting the inhibitory β2-adrenoceptors in circulating lympho-monocytes (a component of the inflammatory reflex, a self-modulating loop to prevent disproportionate immuno-inflammatory activation),92 can also markedly increase ventricular electric instability. Accordingly, cardiac autonomic dysfunction with increased sympathetic nervous system activity is commonly found in several systemic immuno-mediated inflammatory diseases, including inflammatory arthritis and connective tissue diseases, as well as in heart inflammatory cardiac diseases, such as viral myocarditis and acute rheumatic fever.7,8,94, 95, 96, 97, 98 Moreover, anticytokine-targeted treatment with the anti-TNF monoclonal antibody infliximab acutely dampened the increased sympathetic tone in patients with chronic inflammatory arthritis.99 Animal studies further support the relevance of these mechanisms. In a mouse model of RA showing high circulating levels of IL-1 and TNF, sympathovagal index and plasma level of catecholamines were increased, along with APD prolongation and enhanced VA vulnerability.100 Furthermore, in rats with MI presenting elevated norepinephrine concentrations associated to high VA propensity, injection of IL-1β antagonist gevokizumab in the paraventricular nucleus of the hypothalamus improved these changes by attenuating sympathetic hyperactivity.101
In addition, several recent studies demonstrated that inflammatory cytokines could enhance cardiac sympathetic system activation also via peripheral effects on the left stellate ganglia (LSG). Specifically, injection of IL-1 into the canine LSG, promoted VAs by increasing the sympathetic outflow on the heart,102 whereas in an animal model of Kawasaki disease, IL-1 blockade by anakinra reversed cardiac ganglionic inflammation along with the associated increase in heart rate and QTc.27 Furthermore, IL-1β is elevated in LSG of rat with HF, but IL-1β dampening by macrophage depletion attenuates neuronal excitability and cardiac sympathetic overactivation, as well as QTc prolongation and VT/ventricular fibrillation (VF) occurrence.103 Finally, IL-17 microinjection into canine LSG led to unstable ventricular electrophysiology in normal structural hearts, reversed by anti-IL-17 monoclonal antibody administration.104
It is anticipated that cytokine-induced cardiac sympathetic overactivation can promote triggered tachyarrhythmias by favoring, via several mechanisms, intracellular Ca++ overload and afterdepolarizations (EADs and DADs).105 In fact, it has been demonstrated that catecholamines promote ICaL reactivation (a key electrophysiological mechanism responsible for EADs)4 directly, via phosphorylation of the L-type Ca++ channel,106 and indirectly by promoting APD prolongation, as a result of complex changes in ICaL, IKs, and IKr.107,108 Moreover, catecholamines also facilitate spontaneous SR Ca++ release and enhance the activity of the electrogenic NCX, leading to transient inward Na+ current increase with higher propensity for DADs (Figure 1).105,109,110
Increased bioavailability of QT-prolonging drugs
Inflammatory cytokines can reduce liver metabolism by directly inhibiting cytochrome p450 (CYP), thereby increasing bioavailability of a number of medications (Figure 1). In vitro studies have demonstrated that IL-1, IL-6, and TNF strongly decrease messenger RNA and/or protein expression of major CYP isoenzymes in human hepatocytes and hepatoma cell lines, most importantly CYP3A4,111, 112, 113, 114 and that these effects were significantly restored by specific anticytokine agents (IL-1Ra, anti-IL-6 monoclonal antibodies).113,114 Accordingly, Wollmann et al115 demonstrated that IL-6 levels inversely correlated with CYP3A4 activity in patients with RA. Accumulating evidence indicates that these changes significantly suppress patient’s ability to metabolize CYP3A4 substrate drugs,116, 117, 118 including several QT-prolonging medications such as antimicrobials (macrolides and azole antifungals) and psychoactive drugs.116 Thus, in the case that 1 or more of these (frequently used) drugs are concomitantly administered, it is expected that cytokine elevation may indirectly enhance the associated risk of LQTS and related VAs by abnormally increasing their bioavailability in the patient.
Reduction of testosterone levels in male patients
Specifically in male patients, inflammatory cytokines could also promote VAs by inducing hypogonadism (Figure 1), which is increasingly recognized as an important risk factor for LQTS/TdP,119, 120, 121, 122 by removing the physiological APD shortening effect of testosterone on the ventricular cardiomyocyte.123 In fact, testosterone can decrease QTc by both increasing the repolarizing K+ currents IKr and IKs and decreasing the depolarizing current ICaL.124
Many studies provided evidence for a direct interplay between inflammation and depressed gonad function in male patients.125 Testosterone levels are frequently reduced in men with chronic inflammatory diseases,126 and Rivier et al127 demonstrated how hypogonadism could be induced by injecting IL-1β in rats. Moreover, a randomized clinical trial provided evidence that anakinra administration can increase testosterone levels in obese men with testosterone deficiency.128 Several mechanisms may account for these changes, both central on gonadotropin secretion, and peripheral on androgen-to-estrogen conversion.126 In this regard, a recent study conducted in our institution demonstrated that in male patients with active inflammatory diseases, testosterone levels were significantly reduced, but promptly normalized in association with the decrease in IL-6 levels. Reduction of testosterone levels, which also inversely correlated with 17-β estradiol over time, significantly contributed to inflammation-induced QTc prolongation.129 Moreover, in men with TdP, both active systemic inflammation and hypogonadism were frequently present (∼70%-80% of cases), with significant correlations among IL-6, testosterone, and 17-β estradiol levels.129
Collectively, all these direct and indirect effects support an important role for inflammatory cytokines in promoting both tachyarrhythmias, re-entry-driven and triggered activity-induced, and conduction disturbances/bradyarrhythmias. Gap-junction dysfunction and cardiac fibrosis can lead to a slowed and heterogenous intracardiac conduction, favoring re-entry circuits formation and conduction defects development (Figure 2). At the same time, ectopic firing leading to triggered tachyarrhythmias is enhanced, because of an increased propensity to EADs and DADs, caused by intracellular Ca++ overload. In turn, this alteration can be favored by cytokines by promoting both APD prolongation (caused by direct modulation of ion channels and indirect effects on liver, adipose tissue, and nervous system), which facilitates inward ICaL reactivation, and Ca++-handling proteins dysfunction, resulting in spontaneous SR Ca++ release, each of these mechanisms being further enhanced by cytokine-induced sympathetic overactivation (Figure 1).
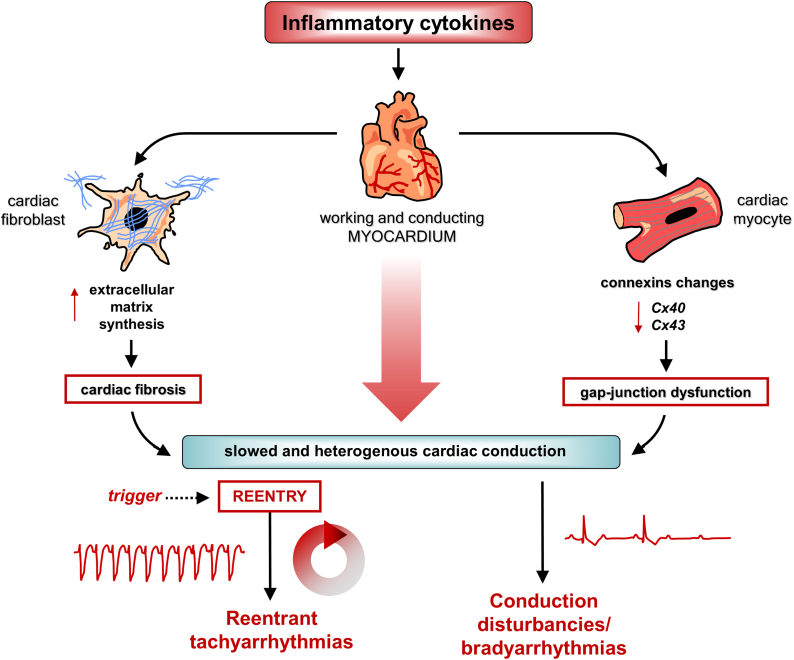
Figure 2.
Arrhythmogenic Effects of Inflammatory Cytokines: Re-entrant Tachyarrhythmias and Conduction Disturbances/Bradyarrhythmias
Inflammatory cytokines increase the propensity to re-entrant tachyarrhythmias and conduction disturbances/bradyarrhythmias via direct effects on myocardial cells. Specifically, these molecules can induce: 1) gap-junction dysfunction, caused by connexin40 (Cx40) and Cx43 changes in cardiac myocytes (and beyond); and 2) cardiac fibrosis, by activating cardiac fibroblasts and promoting extracellular matrix synthesis. These alterations are responsible for a slowed and inhomogeneous conduction of the cardiac electric impulse both in the working myocardium and in the conduction system, where the formation of re-entry circuits and the development of bradyarrhythmias/conduction disturbances are promoted, respectively. Red arrows pointing up indicate increase; red arrows pointing down indicate decrease.
Clinical Evidence
Besides the large body of experimental studies discussed, accumulating clinical data strongly support the hypothesis that systemic inflammation, via cytokines elevation, is a key player for the development of a wide spectrum of cardiac arrhythmias in humans. Although most of the currently available evidence is regarding AF and LQTS/VAs, recent data suggest that inflammatory cytokines may be also significantly involved in AV blocks and arrhythmogenic cardiomyopathy (ACM), as well as in COVID-19–associated arrhythmias.
Atrial fibrillation
AF represents the most common type of sustained tachyarrhythmia worldwide, with an estimated prevalence in the general population of ∼0.5%-1%.130 From an electrophysiological point of view, it is well established that AF development implicates both rapid ectopic firing, predominantly induced by DAD-induced triggered activity, and a vulnerable re-entrant substrate resulting from a slowed and inhomogeneous conduction throughout the atria.131
Robust clinical data support the association between systemic inflammation and increased risk of AF. Specifically, several meta-analyses of observational studies provided evidence that levels of inflammatory cytokines, particularly IL-6, strongly and independently predict AF occurrence and recurrence both in patients with overt cardiac diseases and otherwise healthy subjects (Table 4). By cumulatively including over 4,000 patients from 31 studies, Wu et al132 found that circulating IL-6 and TNF concentration were associated with AF risk in the general population, because the levels of both cytokines were significantly higher in AF cases than in control cases. Moreover, baseline IL-6 was greater in patients who developed postoperative AF than in those who remained in sinus rhythm, and baseline IL-6 was also associated with AF recurrence after catheter ablation.132 These findings were supported and refined by 3 more recent and focused meta-analyses. Jiang et al133 (9 studies) and Boyalla et al134 (8 studies) confirmed that in patients showing postablation AF recurrence, IL-6 levels before the procedure were significantly higher when compared to those in whom AF did not recur. Furthermore, pooling the data from 8 studies on postoperative AF, Weymann et al135 provided evidence that not only baseline but also postoperative levels of IL-6 strongly associated with the development of this type of AF.
Table 4.
Meta-Analyses of Clinical Studies Showing an Association Between Cytokine Levels and AF Occurrence/Recurrence
| First Author, Year | Cytokine | Pooled Studies (n) | Pooled Patients (n) |
Main Findings | |
|---|---|---|---|---|---|
| AF | Control Subjects | ||||
| Wu et al,132 2013 | TNF | 9 | 718 | 1,489 | In the general population, circulating TNF and IL-6 levels are higher in AF cases than control cases (TNF pooled SMD: 2.20; 95% CI: 1.17-3.23; P < 0.001; IL-6 pooled SMD: 0.89; 95% CI: 0.64-1.15; P < 0.001). In patients undergoing cardiac surgery (CABG), baseline IL-6 levels are higher in AF cases than in patients who remained in sinus rhythm (pooled SMD: 1.03; 95% CI: 0.03-2.04; P = 0.04). In patients with AF undergoing catheter ablation, baseline IL-6 levels are higher in recurrent AF cases than in patients who remained in sinus rhythm (pooled SMD: 0.55; 95% CI: 0.25-0.85; P < 0.001). |
| IL-6 | 30 | 1,992 | 2,264 | ||
| Jiang et al,133 2017 | IL-6 | 9 | 749 | — | In patients with AF undergoing catheter ablation, baseline IL-6 levels are higher in recurrent AF cases than in patients who remained in sinus rhythm (pooled SMD: 0.37; 95% CI: 0.21-0.52; P < 0.001). |
| Weymann et al,135 2018 | IL-6 | 8 | 315 | 581 | In patients undergoing cardiac surgery (CABG and/or valvular surgery, or both), both baseline and postoperative IL-6 levels are higher in AF cases than in patients who remained in sinus rhythm (pooled SMD baseline IL-6: 0.40; 95% CI: 0.23-0.57; P < 0.001; pooled SMD postoperative IL-6: 1.66; 95% CI: 1.43-1.90; P < 0.001). |
| Boyalla et al,134 2022 | IL-6 | 6 | 327 | — | In patients with AF undergoing catheter ablation, baseline IL-6 levels are higher in recurrent AF cases than in patients who remained in sinus rhythm (pooled OR: 1.83; 95% CI: 1.18-2.84; P < 0.001). |
AF = atrial fibrillation; CABG = coronary artery bypass graft; OR = odds ratio; SMD = standardized mean difference; other abbreviations as in Table 1.
Besides the most investigated IL-6 and TNF, other inflammatory cytokines have been associated in clinical studies with an increased risk of AF. A case-control study involving 53 patients with nonvalvular AF found that subjects with persistent AF had significantly increased serum levels of microvesicle-bound IL-1β when compared to control subjects.136 In another study performed in patients who underwent open-heart surgery, IL-1β messenger RNA level in epicardial adipose tissue was higher in those with persistent AF than in the control group and represented an independent risk factor for the presence of this arrhythmia.137 A larger case-control study (336 patients with AF vs 336 patients without AF) showed that elevated plasma IL-17 was independently associated with AF risk in a multivariate conditional logistic regression analysis.138
Moreover, other investigators provided evidence that the proarrhythmic effects of cytokines documented by basic investigations are relevant for AF development in the clinical setting, particularly those promoting re-entry circuits such as atrial fibrosis and Cx expression down-regulation. Stanciu et al139 reported that serum levels of both IL-6 and extracellular matrix remodeling markers (metalloproteinase-9/tissue inhibitor of metalloproteinase-1) were significantly higher in patients with AF than in control subjects. Consistently, 2 studies conducted on specimens from the right atrium of patients with valvular disease found that those with AF showed an increased tissue expression of inflammatory cytokines (TNF, IL-6, IL-1β) along with a more pronounced atrial fibrosis when compared to patients with valvular disease who are on sinus rhythm.140,141 In addition, our group demonstrated that P-wave dispersion, an ECG marker of inhomogeneous propagation of sinus impulses in the atrial myocardium, which has proven to be a sensitive and specific predictor of AF in different clinical settings,142 was increased in patients with active inflammatory diseases of different origin, but promptly normalized when IL-6 levels declined. In these patients, Cx40 and Cx43 expression in peripheral blood mononuclear cells (PBMCs), which strongly correlated with that in atrial tissue, inversely associated to P-wave dispersion and IL-6 changes.69
QTc prolongation and VAs/SCD
Accumulating studies in patients with different inflammatory diseases, as well as with noninflammatory heart diseases and general population subjects, provided evidence that high blood concentration of inflammatory cytokines is associated with an increased risk of acquired LQTS and VAs/SCD (Table 5).
Table 5.
Clinical Studies Showing an Association Between Cytokine Levels and QTc Prolongation/VAs/SCD
| First Author | Study Population | Subjects, n | Controls, n | Key Findings |
|---|---|---|---|---|
| Adlan et al144 | RA | 112 | — | QTc duration positively correlated with circulating levels of TNF, IL-1β and IL-6 |
| Lazzerini et al145 | RA | 17 | — | Anti-IL-6 therapy (TCZ) was associated with a rapid (12-week) QTc shortening that correlated with the TNF level decrease |
| Kobayashi et al146 | RA | 94 | 42 | Anti-IL-6 therapy (TCZ) was associated with a rapid (24-week) QTc shortening |
| Pisoni et al148 | CTD | 73 | — | QTc prolongation was independently predicted by elevated IL-1β levels |
| Lazzerini et al147 | Acute infections | 41 | 25 | QTc prolongation was common (39%) and QTc duration correlated over time with IL-6 and IL-1 levels; in these patients, Kir2.1 channel mRNA expression in PBMCs (which associated with that measured in the ventricular tissue), inversely correlated to IL-1 blood changes |
| Wu et al149 | HIV infection | 774 | 652 | Independent association between increasing tertiles of IL-6 and longer QTc duration |
| Wu et al150 | HIV infection | 774 | 652 | QT-interval subcomponents (To-p, Tp-e) were higher in patients with HIV than in control subjects; the highest tertile of IL-6 was associated with a 7.3 ms longer To-p. |
| Heravi et al151 | HIV infection | 589 | 534 | QTV was increased in patients with HIV than in control subjects and associated with ventricular arrhythmia burden; higher levels of IL-6 and sTNF-R2 were associated with higher QTV |
| Lazzerini et al152 | Various inflammatory diseases | 46 | — | QTc prolongation in 26% of patients; CRP reduction was associated with significant QTc shortening, which correlated over time with IL-6 level decrease |
| Lazzerini. et al152 | TdP | 40 | 20 | In patients with TdP, circulating IL-6 was ∼15–20× higher than in control subjects and comparable to patients with active RA |
| Elmas et al153 | CAD | 50 | — | In patients with AMI who developed VF, IL-6 levels were higher than in patients with AMI who were not complicated with VF |
| Safranow et al154 | CAD | 167 | — | IL-6 plasma concentration was an independent predictor for severe VAs, including sustained VT or VF |
| Fisman et al155 | CAD | 3,090 | — | Patients with CAD who presented with SCD had higher IL-6 levels than those who did not; each 1 pg/mL IL-6 increase was associated with a 1.70 increased relative odd of subsequent SCD |
| Streitner et al156 | Recipients of ICDs | 47 | — | IL-6 serum levels were prospectively associated with an increased risk of spontaneous VT/VF events |
| Streitner et al157 | Recipients of ICDs | 86 | — | Gradual IL-6 increase when subjects without ICD intervention were compared to those with a single VT/VF event and those developing electrical storm |
| Cheng et al159 | Recipients of ICDs | 1,189 | — | Higher IL-6 levels independently increased the risk of shocks for VT/VF and mortality |
| Wu et al160 | Recipients of ICDs | 382 | — | Circulating IL-6 was a top predictor for appropriate ICD discharge and SCD |
| Medenwald et al161 | General population | 1,716 | — | sTNF-R1 levels independently correlated with QTc duration in women |
| Empana et al162 | General population | 9,771 | — | IL-6 was an independent predictor of SCD in asymptomatic men |
| Hussein et al163 | General population | 5,382 | — | IL-6 was an independent predictor of SCD, beyond traditional risk factors |
AMI = acute myocardial infraction; CAD = coronary artery disease; CRP = C-reactive protein; CTD = connective tissue disease; ICD = implantable cardioverter-defibrillator; mRNA = messenger RNA; PBMCs = peripheral blood mononuclear cells; QTV = QT-interval variability; RA = rheumatoid arthritis; SCD = sudden cardiac death; sTNF-R1/2 = soluble TNF-receptor-1/2; TCZ = tocilizumab; TdP = torsades de pointes; To-p = interval from the onset to the peak of the T-wave; Tp-e = interval from the peak to the end of the T-wave; VAs = ventricular arrhythmias; VF = ventricular fibrillation; VT = ventricular tachycardia; other abbreviations as in Table 1.
In RA, a chronic inflammatory disease characterized by increase prevalence of QTc prolongation along with a significantly higher risk of SCD/cardiac arrest when compared to the general population,8,143 circulating levels of TNF, IL-1β, and IL-6 positively correlated with QTc duration.144 In these patients, IL-6R blockade with tocilizumab rapidly normalized QTc,145,146 an effect that strongly correlated with TNF level decrease.145,146 Accordingly, cases of marked QTc prolongation complicated by TdP have been reported in patients with RA and elevated TNF, IL-1β, and/or IL-6 levels.147 In connective tissue diseases, another group of autoimmune inflammatory diseases frequently associated with acquired LQTS, Pisoni et al148 provided evidence that serum IL-1β was an independent predictor for the presence of QTc prolongation. Moreover, we showed that during acute infections, QTc was commonly and significantly prolonged, and that infection recovery led to a rapid and significant QTc shortening, in turn correlating with IL-6 and IL-1 level reduction.28 Notably, in these patients Kir2.1 channel messenger RNA expression in PBMCs (which correlated with that measured in the ventricular tissue) inversely associated with IL-1 blood changes.28 A significant association between elevated IL-6 levels and increased QT-interval parameters was also demonstrated by several recent studies performed in subjects living with HIV infection,149, 150, 151 as well as in a cohort of patients with high C-reactive protein levels caused by different inflammatory diseases.152 As a further support of these findings, in a cohort of 40 patients with TdP consecutively collected from the general population regardless of concomitant diseases and ongoing therapies, most subjects presented with high circulating IL-6 levels when the arrhythmia occurred (on average ∼15-20× higher than for control subjects), frequently as a result of a definite inflammatory disease (acute infections, active immuno-inflammatory diseases, other).152
The ventricular proarrhythmic potential of inflammatory cytokines is clinically relevant not only during active inflammatory diseases, but also in the presence of a low-grade chronic inflammatory state as observed in patients with structural heart diseases, such as coronary artery disease (CAD) and HF, as well as in otherwise healthy individuals of the general population. Elmas et al153 found that patients who developed VF during acute MI showed higher IL-6 serum concentration than those who did not experience this arrhythmic complication. In a larger cohort of 167 patients with CAD, IL-6 plasma concentration was reported to be a strong independent predictor for severe VAs, including sustained VT/VF.154 Moreover, among 3,090 patients with CAD followed for a mean of 6.3 years, subjects who presented SCD showed greater IL-6 levels when compared with those who did not, each 1 pg/mL IL-6 increase leading to a 1.70 increased relative odd of subsequent SCD.155
Further support derives from populations who have received implantable cardioverter-defibrillators (ICDs). In 47 consecutive patients with ICDs who have CAD or HF caused by idiopathic dilated cardiomyopathy, IL-6 serum levels at baseline and at 9-month follow-up were prospectively associated with an increased risk of spontaneous VT/VF events.156 Moreover, in a following study performed in the same but expanded cohort (86 patients with ICDs), the investigators reported a gradual progressive IL-6 increase when blood samples from subjects without ICD intervention were compared to those with a single VT/VF event (∼2× higher) and those developing electrical storm (>3×) during 9-month follow-up. Notably, in patients with electrical storm, IL-6 levels measured within 60 minutes after events were significantly higher (doubled) with respect to event-free determinations.157 Moreover, in patients with electrical storm, the neutrophil-to-lymphocyte ratio, a surrogate for IL-1 activity, predicted recurrence of in-hospital arrhythmias.158 Consistent data were obtained by 2 larger cohort studies. In the PROSe-ICD (Prospective Observational Study of Implantable Cardioverter Defibrillators), which enrolled 1,189 patients with systolic HF who underwent ICD implantation for primary prevention of SCD, higher IL-6 levels independently increased the risk of ICD shocks for adjudicated VT/VF and mortality.159 Furthermore, among 382 patients with ICDs who have cardiomyopathy who were analyzed by a random forest statistical method, circulating IL-6 resulted to be a top predictor for appropriate ICD discharge and SCD together with HF hospital admissions and abnormal cardiac magnetic resonance.160
Focusing on the general population, the CARLA (CARdiovascular diseases, Living and Ageing in Halle) study found that in a cohort of 1,716 subjects soluble TNF-R1 (a circulating stabile marker of TNF system activation) was independently associated with QTc duration in women.161 Moreover, 2 large prospective population studies (PRIME [Etude Prospective de l’Infarctus du Myocarde] on 9,971 men followed over 10 years; Cardiovascular Health Study on 5,282 older adults followed over 17 years) provide strong evidence that IL-6 is an independent predictor of SCD in asymptomatic individuals, beyond traditional risk factors.162,163
Other cardiac rhythm disorders
Besides AF and VAs, preliminary evidence from recent studies suggests that inflammatory cytokines may play a clinically relevant role in AV blocks, as well as in inherited arrhythmogenic disorders, particularly ACM.
In a cohort of patients with active inflammatory diseases of different origin, our group demonstrated that AV conduction indices (PR interval, PR segment) were transiently but significantly increased, and correlated over time with IL-6 levels in the blood. Specifically, in subjects who presented with AV block during active disease, IL-6 levels were 2× higher when compared to those who did not. Moreover, Cx43 expression in PBMCs, which was correlative of that measured in the cardiac tissue, inversely associated with circulating IL-6.70 The potentially relevant role of inflammatory cytokines in delaying AV conduction is further and intriguingly supported by the results of a genome-wide association study involving 16,468 individuals of European ancestry. In this population, single-nucleotide-variation (formerly single-nucleotide-polymorphism) in the gene encoding IL-17D, a member of the IL-17 cytokine family, was strongly associated with PR-segment duration, which specifically represents the period during which electrical signals from the atria are delayed at the AV node.164 The significance of this finding was strengthened by quantitative polymerase chain reaction experiments that showed that IL17D gene was expressed significantly higher in the AV node compared with in the left ventricle.164
ACM is a genetically determined disorder caused by mutations in proteins constituting desmosomes, which are intercellular adhesion plaques placed within intercalated disks that connect cardiomyocytes. In these patients, where remodeling of gap junction with loss of Cx43 appears to be a consistent feature, the risk of life-threatening VAs is markedly enhanced.165 Accumulating recent evidence robustly suggests that inflammation is deeply involved in the pathogenesis of ACM, including some human studies specifically demonstrating the contribution of inflammatory cytokines.165 Campian et al166 found that both regional myocardial inflammation, as assessed by gallium-67 scintigraphy, and plasma cytokines concentration (IL-1β, IL-6, TNF) were significantly increased in patients with ACM when compared with control subjects. Consistently, other investigators167,168 provided evidence that in ACM also circulating levels of cytokine receptors (TNF-R1/R2, IL6-R, IL1-R1) are elevated and associated with VA risk even after adjustment for ventricular function.168 Moreover, in a pathological study on 9 patients with ACM, Asimaki et al167 reported that myocardial expression of IL-17 and/or TNF was substantially enhanced in all cases.
BrS and congenital LQTS are 2 other inherited arrhythmogenic disorders in which the potential role of inflammation as a driving force for clinical expression and severity has been progressively emerging over the past couple decades.7,169, 170, 171, 172, 173 The critical impact of inflammatory cytokines in promoting, directly and indirectly, APD/QTc prolongation has been extensively reviewed herein. Moreover, evidence for a pathogenic involvement of Cx43 abnormalities in BrS is ever more reported.84,174 Despite such intriguing premises, to our knowledge no studies exploring the potential arrhythmogenic role of cytokines in these 2 conditions are currently available. The same applies to catecholaminergic polymorphic ventricular tachycardia a rare inherited channelopathy characterized by adrenergic-induced bidirectional or polymorphic VT or VF caused by mutations in genes encoding RyR2 and RyR2-regulatory proteins leading to spontaneous Ca++ diastolic release from SR, particularly following intense adrenergic activation.175 Again, although several investigators176, 177, 178, 179 reported bidirectional VT in the setting of inflammatory heart diseases, and many studies described herein demonstrated that inflammatory cytokines markedly increase both SR Ca++ leakage in diastole and sympathetic cardiac activation, to date there is no information regarding the potential impact of these molecules, and more generally of inflammation, on this arrhythmogenic disorder.
Overall, even though the body of clinical data linking inflammatory cytokines and arrhythmias is consistent, most of the available studies are correlative. Thus, further investigations providing causative evidence are warranted to confirm or refute a connection between the specific cytokines discussed and the associated arrhythmias.
COVID-19, Inflammatory Cytokines, and Cardiac Arrhythmias
The potential clinical relevance of inflammatory cytokines in boosting arrhythmic risk recently has been raising a deal of great general attention due the current pandemic of COVID-19, an acute inflammatory disorder characterized by high-level circulating cytokines along with an unexpected high prevalence of arrhythmic events.180, 181, 182 Large studies reported an overall prevalence of arrhythmias in COVID-19 ranging from 10% to 20%, an incidence which greatly increases in patients who are severely ill. Supraventricular tachyarrhythmias, especially AF, are the most reported and encompass >60% of all arrhythmias. The other forms include VAs and bradyarrhythmias/conduction defects, significantly contributing to disease mortality.183,184
Early in the pandemic period, it was believed that COVID-19–associated arrhythmias were mostly the consequence of direct virus-induced cardiac injury and electrophysiological interference of repurposed drugs, such as antimalarials, azithromycin, and protease inhibitors.185 However, as the knowledge of the disease increased, it became increasingly clear that only rarely does the virus invade the heart.186 Moreover, arrhythmic risk in patients with COVID-19 persisted to be elevated despite off-label medications that were ever less used because they were not effective. Thus, it became evident that other proarrhythmic factors, less specifically associated with COVID-19, but more generally present in all severe pneumonia regardless of the specific etiology, such as hypoxia caused by respiratory impairment and high-grade systemic inflammation, were most commonly and importantly involved.185 In this scenario, the discrete impact of inflammatory cytokines on arrhythmogenesis seems to be particularly relevant,181 also because it is increasingly recognized that a number of patients with COVID-19 (and subjects with non–COVID-19 pneumonia)187 can develop arrhythmic events, which are also life-threatening, despite no severe respiratory impairment, provided that a significant systemic inflammatory activation was present.188, 189, 190 Altogether, these findings point to inflammatory cytokines as important drivers of COVID-19–associated arrhythmias, a view supported by several recent clinical studies.34,191, 192, 193, 194, 195 Guan et al191 and Bagnato et al192 found that among patients hospitalized with COVID-19, subjects presenting atrial arrhythmias and AF, respectively, had increased IL-6 levels compared to those who did not. These findings were confirmed in a larger retrospective analysis involving 3,970 patients during the hospital stay with COVID-19 in New York City, reported that new-onset AF/atrial flutter occurrence was associated with inflammatory markers, including IL-6, independent of the characteristics at baseline.193 In another large study involving 965 patients admitted with COVID-19 where an independent association between infection status and QTc prolongation was demonstrated, Rubin et al194 provided evidence for a direct correlation between IL-6 levels and QTc maximum. Accordingly, our group demonstrated that in a cohort of patients with active severe COVID-19 and elevated IL-6 blood concentration, QTc was significantly prolonged independent of myocardial injury/strain and QT-prolonging risk factors, but promptly normalized in correlation with IL-6 decrease.34 Moreover, Hu et al195 showed that cytokine storm with rapidly elevated IL-6 associated with SCD among patients with critical COVID-19. Finally, it is well known that obesity is associated with a chronic low-grade inflammatory state, caused by a dysregulated production of IL-6, TNF, and other cytokines by the adipose tissue,196 along with a higher risk of cardiac arrhythmias and SCD.197,198 Cumulative data indicate that in patients with COVID-19 obesity correlates with a pronounced aberrant innate immune reaction and predicts mortality.199,200 Thus, it is intriguing to speculate that an exaggerated inflammatory response to the virus could make these subjects particularly susceptible to cytokine-driven arrhythmic events, at least in part accounting for the worst prognosis observed.
The above-mentioned data on the relationship between inflammatory cytokines and cardiac arrhythmias in COVID-19 are mostly correlative and might lose significance in the long term as the impact of the disease progressively subsides, nevertheless COVID-19 has provided us with the unique opportunity to realize how much, in the clinical practice, systemic cytokine release can increase arrhythmic risk. This is a lesson to take with us into the post–COVID-19 era.181
Clinical Trials
Despite the wealth of preclinical and observational data, however, there are very limited data regarding anti-inflammatory therapies in patients with cardiac arrhythmias.201 Indirect evidence of a benefit may be inferred from nonspecific anti-inflammatory treatments, such as statins, which suggest, although do not prove, a benefit of inhibiting inflammation. A study evaluating the effect of statins in patients with ICD found that those who had higher statin usage had a significantly reduced risk of SCD or VAs compared with those who had lower statin use.202 Similarly, in a population-based study in Taiwan of 1 million patients with HF, statin therapy lowered the risk of VAs and SCD.203 A subanalysis of the JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) trial showed that rosuvastatin treatment was associated with a lower relative risk of developing AF when compared with placebo.204 Moreover, 2 recent meta-analyses of randomized clinical trials found a significant association between statin therapy and decreased occurrence of postoperative AF,205,206 although other large studies reported conflicting results.207,208
Specifically, whereas an accurate review of the published reports searching for targeted anticytokine drugs substantially revealed no clinical trials in patients with or at risk for arrhythmic events, several considerations support feasibility and effectiveness of this approach. Anticytokine-targeted treatments, including anti-TNF, anti-IL-6, anti-IL-1, and anti-IL-17 medications, are already widely and safely used in clinical practice for the treatment of several autoimmune inflammatory diseases,209 and some of them (anti-IL-6 [tocilizumab], anti-TNF [infliximab], anti-IL-1 [anakinra]) have demonstrated that they significantly dampen the increased arrhythmic risk observed in these patients.145,146,210,211 Several randomized, placebo-controlled clinical trials evaluated the impact of anticytokine therapies on cardiovascular disease, including a very large clinical trial of canakinumab (an anti-IL-1β monoclonal antibody) in patients with prior MI212 and many small clinical trials of anakinra in patients with acute MI, HF, pericarditis, myocarditis, and sarcoidosis.213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226 Two other small trials involving single-injection tocilizumab in patients with acute MI have been completed,227,228 and a large phase III clinical trial with ziltivekimab, IL-6 antibody for secondary cardiovascular prevention is ongoing (ZEUS [A Research Study to Look at How Ziltivekimab Works Compared to Placebo in People With Cardiovascular Disease, Chronic Kidney Disease, and Inflammation]; NCT05021835). Although none of these studies focused on arrhythmic risk, inflammatory activation was significantly attenuated, along with improvement of cardiovascular outcomes and a favorable safety profile.215, 216, 217,219, 220, 221, 222,224,228 Finally, a small pilot randomized trial recently demonstrated that a single subcutaneous injection of canakinumab administered 60 minutes after effective electrical cardioversion in a small cohort of 11 patients with persistent AF and elevated high-sensitivity C-reactive protein levels (vs 13 with placebo) was associated to a near-to-significance ∼60% reduced risk of AF recurrence at 6-month follow-up (HR: 0.36; 95% CI: 0.11-1.15; P = 0.09), without safety concerns, including infections.229 Notably, in none of the above-mentioned trials were cytokine levels measured, although these data are expected to be crucial for guiding a truly high-precision targeted anticytokine therapy and achieving its maximum effectiveness.
Thus, defining the potential impact of anti-inflammatory cytokine-targeted therapies on cardiac arrhythmias continues to represent a knowledge gap and an unmet clinical need.
Conclusions
The large body of data reviewed strongly supports the conclusion that inflammatory cytokines (TNF, IL-1, IL-6, and IL-17) play a crucial role for the development of many forms of cardiac arrhythmias, and this in turn is well consistent with the multifaceted spectrum of proarrhythmic effects they exert (Central Illustration). The arrhythmogenic potential of cytokines seems to be correlated with circulating levels and is substantial in very different clinical settings, including acute and chronic inflammatory diseases independent of specific etiology, noninflammatory structural heart disorders, as well as unselected individuals from the general population. This evidence, further strengthening their role of fundamental downstream mediators of proarrhythmic changes occurring during inflammatory activation, points to these molecules as precision targets for novel antiarrhythmic therapies, ideally a personalized treatment focused on that specific cytokine(s) elevated in the blood of that specific patient with cardiac arrhythmias. Even though these data, in connection with the strong background provided by the basic and clinical studies reviewed here, are very attracting and promising, large randomized clinical trials are warranted to verify whether anticytokine–targeted therapies can actually represent a new important avenue for the treatment and prevention of cardiac arrhythmias.
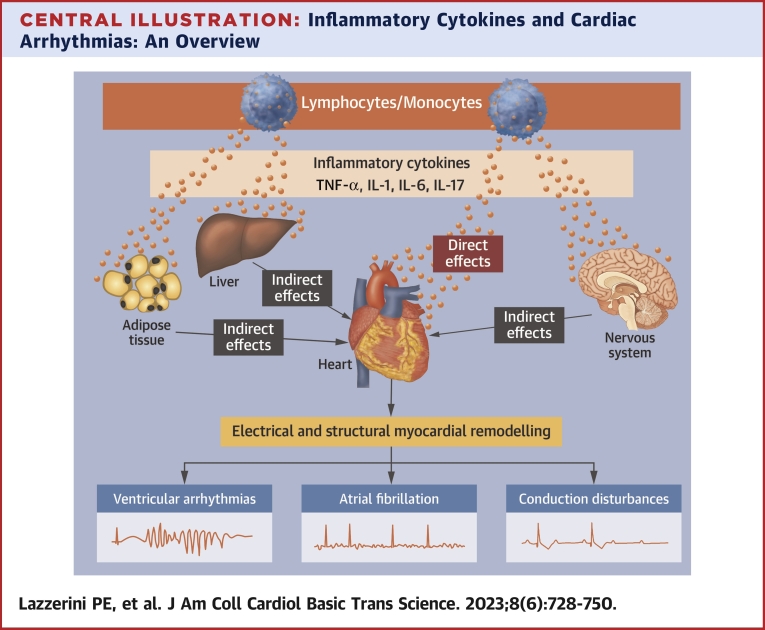
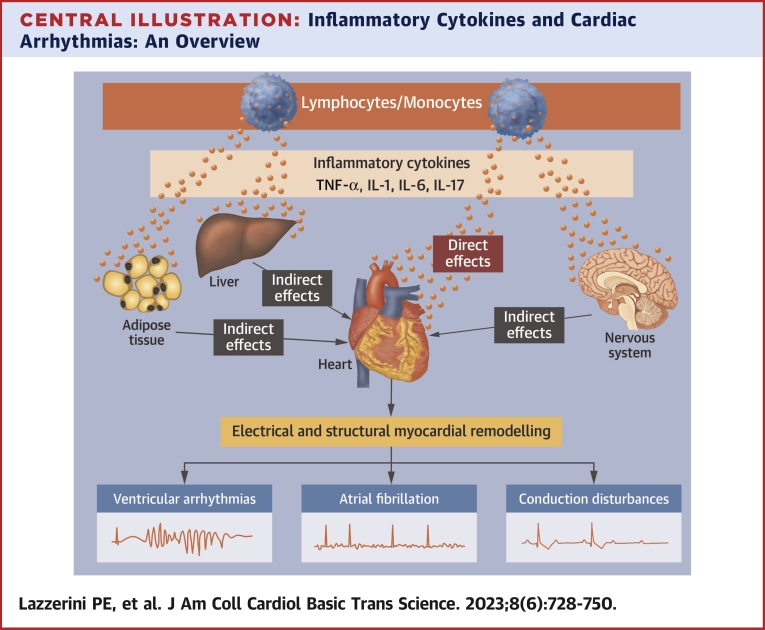
Central Illustration.
Inflammatory Cytokines and Cardiac Arrhythmias: An Overview
Proinflammatory cytokines tumor necrosis factor (TNF)-alpha, interleukin (IL)-1, IL-6, and IL-17 deeply affect arrhythmogenesis via both direct activities on cardiac cells and indirect systemic effects on nervous system, liver, and adipose tissue. The resulting electrical and structural remodeling of the myocardium can promote a wide spectrum of cardiac arrhythmias, including atrial fibrillation, ventricular arrhythmias, and conduction disturbances.
Funding Support and Author Disclosures
This work was supported by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), Progetti di Rilevante Interesse Nazionale (PRIN), and Bando 2017, protocollo 2017XZMBYX (to Drs Lazzerini and Capecchi); Bando Ricerca COVID-19 Toscana–2021, Progetto PRECARVID (to Drs Lazzerini and Capecchi); Biomedical Laboratory Research and Development Service of Veterans Affairs Office of Research and Development, Merit Review grant I01 BX002137 (to Dr Boutjdir); National Heart, Lung, and Blood Institute 1R01HL164415-01 (to Dr Boutjdir); and US Department of Defense Award Number W81XWH-21-1-0424 (to Dr Boutjdir). Dr Lazzerini received a grant from Roche Italia SpA outside the submitted work, in 2018. Dr Abbate has served as a consultant for Cardiol, Implicit Biosciences, Kiniksa, Novartis, Novo-Nordisk, Olatec, R-Pharm, Sanofi, and Serpin Pharma, unrelated to the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 2.Grant A.O. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 3.Drew B.J., Ackerman M.J., Funk M., et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–947. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wit A.L. Afterdepolarizations and triggered activity as a mechanism for clinical arrhythmias. Pacing Clin Electrophysiol. 2018;41(8):883–896. doi: 10.1111/pace.13419. [DOI] [PubMed] [Google Scholar]
- 5.Lazzerini P.E., Capecchi P.L., El-Sherif N., Laghi-Pasini F., Boutjdir M. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazzerini P.E., Laghi-Pasini F., Boutjdir M., Capecchi P.L. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19:63–64. doi: 10.1038/s41577-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 7.Lazzerini P.E., Capecchi P.L., Laghi-Pasini F. Long QT syndrome: an emerging role for inflammation and immunity. Front Cardiovasc Med. 2015;2:26. doi: 10.3389/fcvm.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzerini P.E., Capecchi P.L., Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38:1717–1727. doi: 10.1093/eurheartj/ehw208. [DOI] [PubMed] [Google Scholar]
- 9.Capecchi P.L., Laghi-Pasini F., El-Sherif N., Qu Y., Boutjdir M., Lazzerini P.E. Autoimmune and inflammatory K. Heart Rhythm. 2019;16:1273–1280. doi: 10.1016/j.hrthm.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Saba S., Janczewski A.M., Baker L.C., et al. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-{alpha. Am J Physiol Heart Circ Physiol. 2005;289:H1456–H1467. doi: 10.1152/ajpheart.00733.2004. [DOI] [PubMed] [Google Scholar]
- 11.Sawaya S.E., Rajawat Y.S., Rami T.G., et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am J Physiol Heart Circ Physiol. 2007;292:H1561–H1567. doi: 10.1152/ajpheart.00285.2006. [DOI] [PubMed] [Google Scholar]
- 12.London B., Baker L.C., Lee J.S., et al. Calcium-dependent arrhythmias in transgenic mice with heart failure. Am J Physiol Heart Circ Physiol. 2003;284:H431–H441. doi: 10.1152/ajpheart.00431.2002. [DOI] [PubMed] [Google Scholar]
- 13.Ntari L., Sakkou M., Chouvardas P., et al. Comorbid TNF-mediated heart valve disease and chronic polyarthritis share common mesenchymal cell-mediated aetiopathogenesis. Ann Rheum Dis. 2018;77:926–934. doi: 10.1136/annrheumdis-2017-212597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakkou M., Chouvardas P., Ntari L., et al. Mesenchymal TNFR2 promotes the development of polyarthritis and comorbid heart valve stenosis. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petkova-Kirova P.S., Gursoy E., Mehdi H., McTiernan C.F., London B., Salama G. Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H2098–H2107. doi: 10.1152/ajpheart.00097.2005. [DOI] [PubMed] [Google Scholar]
- 16.Grandy S.A., Fiset C. Ventricular K+ currents are reduced in mice with elevated levels of serum TNFalpha. J Mol Cell Cardiol. 2009;47:238–246. doi: 10.1016/j.yjmcc.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Kawada H., Niwano S., Niwano H., et al. Tumor necrosis factor-alpha downregulates the voltage gated outward K+ current in cultured neonatal rat cardiomyocytes: a possible cause of electrical remodeling in diseased hearts. Circ J. 2006;70:605–609. doi: 10.1253/circj.70.605. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Velasco M., Ruiz-Hurtado G., Hurtado O., Moro M.A., Delgado C. TNF-alpha downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am J Physiol Heart Circ Physiol. 2007;293:H238–H245. doi: 10.1152/ajpheart.01122.2006. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Wang H., Zhang Y., Gao H., Nattel S., Wang Z. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J Biol Chem. 2004;279:13289–13292. doi: 10.1074/jbc.C400025200. [DOI] [PubMed] [Google Scholar]
- 20.Hatada K., Washizuka T., Horie M., et al. Tumor necrosis factor-alpha inhibits the cardiac delayed rectifier K current via the asphingomyelin pathway. Biochem Biophys Res Commun. 2006;344:189–193. doi: 10.1016/j.bbrc.2006.03.115. [DOI] [PubMed] [Google Scholar]
- 21.Lin X., Liu N., Lu J., et al. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm. 2011;8:1923–1930. doi: 10.1016/j.hrthm.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Sherif N., Turitto G., Boutjdir M. Acquired long QT syndrome and electrophysiology of torsade de pointes. Arrhythm Electrophysiol Rev. 2019;8:122–130. doi: 10.15420/aer.2019.8.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y.H., Rozanski G.J. Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovasc Res. 1993;27:525–530. doi: 10.1093/cvr/27.3.525. [DOI] [PubMed] [Google Scholar]
- 24.Monnerat G., Alarcón M.L., Vasconcellos L.R., et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun. 2016;7 doi: 10.1038/ncomms13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sattayaprasert P., Vasireddi S.K., Bektik E., et al. Human cardiac mesenchymal stem cells remodel in disease and can regulate arrhythmia substrates. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H., Zhao Y., Xie A., et al. Interleukin-1β, oxidative stress, and abnormal calcium handling mediate diabetic arrhythmic risk. J Am Coll Cardiol Basic Trans Science. 2021;6:42–52. doi: 10.1016/j.jacbts.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe M., Rastelli D.D., Gomez A.C., et al. IL-1-dependent electrophysiological changes and cardiac neural remodeling in a mouse model of Kawasaki disease vasculitis. Clin Exp Immunol. 2020;199:303–313. doi: 10.1111/cei.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazzerini P.E., Acampa M., Laghi-Pasini F., et al. Cardiac arrest risk during acute infections: systemic inflammation directly prolongs QTc interval via cytokine-mediated effects on potassium channel expression. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008627. [DOI] [PubMed] [Google Scholar]
- 29.Murata M., Fukuda K., Ishida H., et al. Leukemia inhibitory factor, a potent cardiac hypertrophic cytokine, enhances L-type Ca2+ current and [Ca2+]i transient in cardiomyocytes. J Mol Cell Cardiol. 1999;31:237–245. doi: 10.1006/jmcc.1998.0866. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi E., Fukuda K., Miyoshi S., et al. Leukemia inhibitory factor activates cardiac L-Type Ca2+ channels via phosphorylation of serine 1829 in the rabbit Cav1.2 subunit. Circ Res. 2004;94:1242–1248. doi: 10.1161/01.RES.0000126405.38858.BC. [DOI] [PubMed] [Google Scholar]
- 31.Hagiwara Y., Miyoshi S., Fukuda K., et al. SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent I CaL, [Ca2+]i transient, and APD increase in cardiomyocytes. J Mol Cell Cardiol. 2007;43:710–716. doi: 10.1016/j.yjmcc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Aromolaran A.S., Srivastava U., Alí A., et al. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X., Wang Y., Xiao Y., et al. Arrhythmogenic mechanisms of interleukin-6 combination with hydroxychloroquine and azithromycin in inflammatory diseases. Sci Rep. 2022;12:1075. doi: 10.1038/s41598-022-04852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazzerini P.E., Accioli R., Acampa M., et al. Interleukin-6 elevation is a key pathogenic factor underlying COVID-19-associated heart rate-corrected QT interval prolongation. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.893681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhury M.K.H., Martinez-Mateu L., Do J., Aromolaran K.A., Saiz J., Aromolaran A.S. Macrophage-dependent interleukin-6-production and inhibition of IK contributes to acquired QT prolongation in lipotoxic guinea pig heart. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222011249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazzerini P.E., Laghi-Pasini F., Boutjdir M., Capecchi P.L. Commentary: systemic effects of IL-17 in inflammatory arthritis. Front Cardiovasc Med. 2019;6:183. doi: 10.3389/fcvm.2019.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang S.L., Hsiao Y.W., Tsai Y.N., et al. Interleukin-17 enhances cardiac ventricular remodeling via activating MAPK pathway in ischemic heart failure. J Mol Cell Cardiol. 2018;122:69–79. doi: 10.1016/j.yjmcc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Tsai Y.N., Hsiao Y.W., Lin S.F., et al. Proinflammatory cytokine modulates intracellular calcium handling and enhances ventricular arrhythmia susceptibility. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.623510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D.S., Xue G.L., Yang J.M., et al. Knockout of interleukin-17A diminishes ventricular arrhythmia susceptibility in diabetic mice via inhibiting NF-κB-mediated electrical remodeling. Acta Pharmacol Sin. 2022;43:307–315. doi: 10.1038/s41401-021-00659-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bi X., Zhang S., Jiang H., et al. Mechanistic insights into inflammation-induced arrhythmias: a simulation study. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.843292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlotthauer K., Bers D.M. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization: underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 42.Thaik C.M., Calderone A., Takahashi N., Colucci W.S. Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes. J Clin Invest. 1995;96:1093–1099. doi: 10.1172/JCI118095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Combes A., Frye C.S., Lemster B.H., et al. Chronic exposure to interleukin 1beta induces a delayed and reversible alteration in excitation-contraction coupling of cultured cardiomyocytes. Pflugers Arch. 2002;445:246–256. doi: 10.1007/s00424-002-0921-y. [DOI] [PubMed] [Google Scholar]
- 44.McTiernan C.F., Lemster B.H., Frye C., Brooks S., Combes A., Feldman A.M. Interleukin-1 beta inhibits phospholamban gene expression in cultured cardiomyocytes. Circ Res. 1997;81:493–503. doi: 10.1161/01.res.81.4.493. [DOI] [PubMed] [Google Scholar]
- 45.Patten M., Hartogensis W.E., Long C.S. Interleukin-1beta is a negative transcriptional regulator of alpha1-adrenergic induced gene expression in cultured cardiac myocytes. J Biol Chem. 1996;271:21134–21141. doi: 10.1074/jbc.271.35.21134. [DOI] [PubMed] [Google Scholar]
- 46.Wu C.K., Lee J.K., Chiang F.T., et al. Plasma levels of tumor necrosis factor-α and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med. 2011;39:984–992. doi: 10.1097/CCM.0b013e31820a91b9. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka T., Kanda T., Takahashi T., Saegusa S., Moriya J., Kurabayashi M. Interleukin-6-induced reciprocal expression of SERCA and natriuretic peptides mRNA in cultured rat ventricular myocytes. J Int Med Res. 2004;32:57–61. doi: 10.1177/147323000403200109. [DOI] [PubMed] [Google Scholar]
- 48.Yu X.W., Chen Q., Kennedy R.H., Liu S.J. Inhibition of sarcoplasmic reticular function by chronic interleukin-6 exposure via iNOS in adult ventricular myocytes. J Physiol. 2005;566:327–340. doi: 10.1113/jphysiol.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saraf A., Rampoldi A., Chao M., et al. Functional and molecular effects of TNF-α on human iPSC-derived cardiomyocytes. Stem Cell Res. 2021;52 doi: 10.1016/j.scr.2021.102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duncan D.J., Yang Z., Hopkins P.M., Steele D.S., Harrison S.M. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium. 2010;47:378–386. doi: 10.1016/j.ceca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo S., Li L.L., Ruan Y.F., et al. Acute administration of tumour necrosis factor-α induces spontaneous calcium release via the reactive oxygen species pathway in atrial myocytes. Europace. 2018;20:1367–1374. doi: 10.1093/europace/eux271. [DOI] [PubMed] [Google Scholar]
- 52.Zuo S., Li L., Jiang L., et al. Pravastatin alleviates intracellular calcium dysregulation induced by Interleukin-6 via the mitochondrial ROS pathway in adult ventricular myocytes. J Pharmacol Sci. 2020;143:141–147. doi: 10.1016/j.jphs.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Lee S.H., Chen Y.C., Chen Y.J., et al. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80:1806–1815. doi: 10.1016/j.lfs.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 54.Stamm C., Cowan D.B., Friehs I., Noria S., Del Nido P.J., McGowan F.X. Rapid endotoxin-induced alterations in myocardial calcium handling: obligatory role of cardiac TNF-alpha. Anesthesiology. 2001;95:1396–1405. doi: 10.1097/00000542-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 55.De Jesus N.M., Wang L., Lai J., et al. Antiarrhythmic effects of interleukin 1 inhibition after myocardial infarction. Heart Rhythm. 2017;14:727–736. doi: 10.1016/j.hrthm.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alarcon M.M.L., Trentin-Sonoda M., Panico K., et al. Cardiac arrhythmias after renal I/R depend on IL-1β. J Mol Cell Cardiol. 2019;131:101–111. doi: 10.1016/j.yjmcc.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Liao J., Zhang S., Yang S., et al. Interleukin-6-mediated-Ca2+ handling abnormalities contributes to atrial fibrillation in sterile pericarditis rats. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.758157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Severs N.J., Bruce A.F., Dupont E., Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hulsmans M., Clauss S., Xiao L., et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017;169:510–522e20. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liew R., Khairunnisa K., Gu Y., et al. Role of tumor necrosis factor-α in the pathogenesis of atrial fibrosis and development of an arrhythmogenic substrate. Circ J. 2013;77:1171–1179. doi: 10.1253/circj.cj-12-1155. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Cobo M., Gingalewski C., Drujan D., De Maio A. Downregulation of connexin 43 gene expression in rat heart during inflammation: the role of tumour necrosis factor. Cytokine. 1999;11:216–224. doi: 10.1006/cyto.1998.0422. [DOI] [PubMed] [Google Scholar]
- 62.Sun Z., Zhou D., Xie X., et al. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res Cardiol. 2016;111:63. doi: 10.1007/s00395-016-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coppen S.R., Fukushima S., Shintani Y., et al. A factor underlying late-phase arrhythmogenicity after cell therapy to the heart: global downregulation of connexin43 in the host myocardium after skeletal myoblast transplantation. Circulation. 2008;118:S138–S144. doi: 10.1161/CIRCULATIONAHA.107.779629. [DOI] [PubMed] [Google Scholar]
- 64.Baum J.R., Long B., Cabo C., Duffy H.S. Myofibroblasts cause heterogeneous Cx43 reduction and are unlikely to be coupled to myocytes in the healing canine infarct. Am J Physiol Heart Circ Physiol. 2012;302:H790–H800. doi: 10.1152/ajpheart.00498.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baum J.R., Dolmatova E., Tan A., Duffy H.S. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated connexin43 regulation in the heart. Front Physiol. 2012;3:272. doi: 10.3389/fphys.2012.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong C., Chang H., Wu Y., et al. Up-regulated Cx43 phosphorylation at Ser368 prolongs QRS duration in myocarditis. J Cell Mol Med. 2018;22:3537–3547. doi: 10.1111/jcmm.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Jesus N.M., Wang L., Herren A.W., et al. Atherosclerosis exacerbates arrhythmia following myocardial infarction: role of myocardial inflammation. Heart Rhythm. 2015;12:169–178. doi: 10.1016/j.hrthm.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H., Li W., Gu W., Kong Y., Yang N., Chen L. Immunoregulatory effects of carvedilol on rat experimental autoimmune myocarditis. Scand J Immunol. 2010;71:38–44. doi: 10.1111/j.1365-3083.2009.02347.x. [DOI] [PubMed] [Google Scholar]
- 69.Lazzerini P.E., Laghi-Pasini F., Acampa M., et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6–mediated changes in connexin expression. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazzerini P.E., Acampa M., Cupelli M., et al. Unravelling atrioventricular block risk in inflammatory diseases: systemic inflammation acutely delays atrioventricular conduction via a cytokine-mediated inhibition of connexin43 expression. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang H.Y., Li X., Tian Y. Telmisartan reduces arrhythmias through increasing cardiac connexin43 by inhibiting IL-17 after myocardial infarction in rats. Eur Rev Med Pharmacol Sci. 2017;21:5283–5289. doi: 10.26355/eurrev_201711_13853. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen M.N., Kiriazis H., Gao X.M., Du X.J. Cardiac fibrosis and arrhythmogenesis. Compr Physiol. 2017;7:1009–1049. doi: 10.1002/cphy.c160046. [DOI] [PubMed] [Google Scholar]
- 73.Frangogiannis N.G. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dai H., Wang X., Yin S., et al. Atrial Fibrillation Promotion in a Rat Model of Rheumatoid Arthritis. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsiao Y.W., Tsai Y.N., Huang Y.T., et al. Rhodiola crenulata reduces ventricular arrhythmia through mitigating the activation of IL-17 and inhibiting the MAPK signaling pathway. Cardiovasc Drugs Ther. 2021;35:889–900. doi: 10.1007/s10557-020-07072-z. [DOI] [PubMed] [Google Scholar]
- 76.Wu Q., Liu H., Liao J., et al. Colchicine prevents atrial fibrillation promotion by inhibiting IL-1β-induced IL-6 release and atrial fibrosis in the rat sterile pericarditis model. Biomed Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110384. [DOI] [PubMed] [Google Scholar]
- 77.Fu X.X., Zhao N., Dong Q., et al. Interleukin-17A contributes to the development of post-operative atrial fibrillation by regulating inflammation and fibrosis in rats with sterile pericarditis. Int J Mol Med. 2015;36:83–92. doi: 10.3892/ijmm.2015.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim S.M., Pak H.N., Lee M.H., Kim S.S., Joung B. Fever-induced QTc prolongation and ventricular fibrillation in a healthy young man. Yonsei Med J. 2011;52:1025–1027. doi: 10.3349/ymj.2011.52.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Y., Wang T., Guo J., et al. Febrile temperature facilitates hERG/IKr degradation through an altered K(+) dependence. Heart Rhythm. 2016;13:2004–2011. doi: 10.1016/j.hrthm.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 80.Evans S.S., Repasky E.A., Fisher D.T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amin A.S., Herfst L.J., Delisle B.P., et al. Fever-induced QTc prolongation and ventricular arrhythmias in individuals with type 2 congenital long QT syndrome. J Clin Invest. 2008;118:2552–2561. doi: 10.1172/JCI35337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burashnikov A., Shimizu W., Antzelevitch C. Fever accentuates transmural dispersion of repolarization and facilitates development of early afterdepolarizations and torsade de pointes under long-QT conditions. Circ Arrhythm Electrophysiol. 2008;1:202–208. doi: 10.1161/CIRCEP.107.691931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo J., Zhan S., Lees-Miller J.P., Teng G., Duff H.J. Exaggerated block of hERG (KCNH2) and prolongation of action potential duration by erythromycin at temperatures between 37 degrees C and 42 degrees C. Heart Rhythm. 2005;2:860–866. doi: 10.1016/j.hrthm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 84.Sieira J., Dendramis G., Brugada P. Pathogenesis and management of Brugada syndrome. Nat Rev Cardiol. 2016;13:744–756. doi: 10.1038/nrcardio.2016.143. [DOI] [PubMed] [Google Scholar]
- 85.Antzelevitch C., Yan G.X., Ackerman M.J., et al. J-wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Europace. 2017;19:665–694. doi: 10.1093/europace/euw235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adler A., Topaz G., Heller K., et al. Fever-induced Brugada pattern: how common is it and what does it mean? Heart Rhythm. 2013;10:1375–1382. doi: 10.1016/j.hrthm.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mizusawa Y., Morita H., Adler A., et al. Prognostic significance of fever-induced Brugada syndrome. Heart Rhythm. 2016;13:1515–1520. doi: 10.1016/j.hrthm.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 88.Dumaine R., Towbin J.A., Brugada P., et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 89.Keller D.I., Rougier J.S., Kucera J.P., et al. Brugada syndrome and fever: genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res. 2005;67:510–519. doi: 10.1016/j.cardiores.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 90.Schwartz P.J. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat Rev Cardiol. 2014;11:346–353. doi: 10.1038/nrcardio.2014.19. [DOI] [PubMed] [Google Scholar]
- 91.Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve--an integrative interface between 2 supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 92.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 93.Martelli D., Yao S.T., McKinley M.J., McAllen R.M. Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol. 2014;592:1677–1686. doi: 10.1113/jphysiol.2013.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stojanovich L. Autonomic dysfunction in autoimmune rheumatic disease. Autoimmun Rev. 2009;8:569–572. doi: 10.1016/j.autrev.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 95.Adlan A.M., Lip G.Y., Paton J.F., Kitas G.D., Fisher J.P. Autonomic function and rheumatoid arthritis: a systematic review. Semin Arthritis Rheum. 2014;44:283–304. doi: 10.1016/j.semarthrit.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 96.Gao X., Peng L., Zeng Q., Wu Z.K. Autonomic nervous function and arrhythmias in patients with acute viral myocarditis during a 6-month follow-up period. Cardiology. 2009;113:66–71. doi: 10.1159/000167794. [DOI] [PubMed] [Google Scholar]
- 97.Cheng Z., Li-Sha G., Yue-Chun L. Autonomic nervous system in viral myocarditis: pathophysiology and therapy. Curr Pharm Des. 2016;22:485–498. doi: 10.2174/1381612822666151222160810. [DOI] [PubMed] [Google Scholar]
- 98.Karacan M., Ceviz N., Olgun H. Heart rate variability in children with acute rheumatic fever. Cardiol Young. 2012;22:285–292. doi: 10.1017/S1047951111001429. [DOI] [PubMed] [Google Scholar]
- 99.Lazzerini P.E., Acampa M., Hammoud M., et al. Arrhythmic risk during acute infusion of infliximab: a prospective, single-blind, placebo-controlled, crossover study in patients with chronic arthritis. J Rheumatol. 2008;35:1958–1965. [PubMed] [Google Scholar]
- 100.Lin T.T., Sung Y.L., Syu J.Y., et al. Anti-inflammatory and antiarrhythmic effects of beta blocker in a rat model of rheumatoid arthritis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y., Yin J., Wang C., et al. Microglial Mincle receptor in the PVN contributes to sympathetic hyperactivity in acute myocardial infarction rat. J Cell Mol Med. 2019;23:112–125. doi: 10.1111/jcmm.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang M., Li S., Zhou X., et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286–293. doi: 10.1016/j.ijcard.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 103.Zhang D., Hu W., Tu H., et al. Macrophage depletion in stellate ganglia alleviates cardiac sympathetic overactivation and ventricular arrhythmogenesis by attenuating neuroinflammation in heart failure. Basic Res Cardiol. 2021;116:28. doi: 10.1007/s00395-021-00871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deng J., Zhou X., Wang M., et al. The effects of interleukin 17A on left stellate ganglion remodeling are mediated by neuroimmune communication in normal structural hearts. Int J Cardiol. 2019;279:64–71. doi: 10.1016/j.ijcard.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 105.ter Bekke R.M., Volders P.G. Arrhythmogenic mechano-electric heterogeneity in the long-QT syndrome. Prog Biophys Mol Biol. 2012;110:347–358. doi: 10.1016/j.pbiomolbio.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 106.Kamp T.J., Hell J.W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 107.Xie X., Visweswaran R., Guzman P.A., Smith R.M., Osborn J.W., Tolkacheva E.G. The effect of cardiac sympathetic denervation through bilateral stellate ganglionectomy on electrical properties of the heart. Am J Physiol Heart Circ Physiol. 2011;301:H192–H199. doi: 10.1152/ajpheart.01149.2010. [DOI] [PubMed] [Google Scholar]
- 108.Winter J., Tipton M.J., Shattock M.J. Autonomic conflict exacerbates long QT associated ventricular arrhythmias. J Mol Cell Cardiol. 2018;116:145–154. doi: 10.1016/j.yjmcc.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kashimura T., Briston S.J., Trafford A.W., et al. In the RyR2(R4496C) mouse model of CPVT, β-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res. 2010;107:1483–1489. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 110.Viatchenko-Karpinski S., Györke S. Modulation of the Ca(2+)-induced Ca(2+) release cascade by beta-adrenergic stimulation in rat ventricular myocytes. J Physiol. 2001;533:837–848. doi: 10.1111/j.1469-7793.2001.t01-1-00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aitken A.E., Morgan E.T. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–1693. doi: 10.1124/dmd.107.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abdel-Razzak Z., Loyer P., Fautrel A., et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993;44:707–715. [PubMed] [Google Scholar]
- 113.Mimura H., Kobayashi K., Xu L., et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105–110. doi: 10.1016/j.dmpk.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 114.Dickmann L.J., Patel S.K., Rock D.A., Wienkers L.C., Slatter J.G. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–1422. doi: 10.1124/dmd.111.038679. [DOI] [PubMed] [Google Scholar]
- 115.Wollmann B.M., Syversen S.W., Vistnes M., Lie E., Mehus L.L., Molden E. Associations between cytokine levels and CYP3A4 phenotype in patients with rheumatoid arthritis. Drug Metab Dispos. 2018;46:1384–1389. doi: 10.1124/dmd.118.082065. [DOI] [PubMed] [Google Scholar]
- 116.White C.M. Inflammation suppresses patients' ability to metabolize cytochrome P450 substrate drugs. Ann Pharmacother. 2022;56:809–819. doi: 10.1177/10600280211047864. [DOI] [PubMed] [Google Scholar]
- 117.Schmitt C., Kuhn B., Zhang X., Kivitz A.J., Grange S. Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89:735–740. doi: 10.1038/clpt.2011.35. [DOI] [PubMed] [Google Scholar]
- 118.Zhuang Y., de Vries D.E., Xu Z., et al. Evaluation of disease-mediated therapeutic protein-drug interactions between an anti-interleukin-6 monoclonal antibody (sirukumab) and cytochrome P450 activities in a phase 1 study in patients with rheumatoid arthritis using a cocktail approach. J Clin Pharmacol. 2015;55:1386–13894. doi: 10.1002/jcph.561. [DOI] [PubMed] [Google Scholar]
- 119.Salem J.E., Waintraub X., Courtillot C., et al. Hypogonadism as a reversible cause of torsades de pointes in men. Circulation. 2018;138:110–113. doi: 10.1161/CIRCULATIONAHA.118.034282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salem J.E., Yang T., Moslehi J.J., et al. Androgenic effects on ventricular repolarization: a translational study from the International Pharmacovigilance Database to iPSC-Cardiomyocytes. Circulation. 2019;140:1070–1080. doi: 10.1161/CIRCULATIONAHA.119.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lazzerini P.E., Bertolozzi I., Acampa M., et al. Androgen deprivation therapy for prostatic cancer in patients with torsades de pointes. Front Pharmacol. 2020;11:684. doi: 10.3389/fphar.2020.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasegawa K., Ito H., Kaseno K., et al. Impact of medical castration on malignant arrhythmias in patients with prostate cancer. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gutierrez G., Wamboldt R., Baranchuk A. The impact of testosterone on the QT interval: a systematic review. Curr Probl Cardiol. 2022;47 doi: 10.1016/j.cpcardiol.2021.100882. [DOI] [PubMed] [Google Scholar]
- 124.Salem J.E., Alexandre J., Bachelot A., Funck-Brentano C. Influence of steroid hormones on ventricular repolarization. Pharmacol Ther. 2016;167:38–47. doi: 10.1016/j.pharmthera.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 125.Mohamad N.V., Wong S.K., Wan Hasan W.N., et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22:129–140. doi: 10.1080/13685538.2018.1482487. [DOI] [PubMed] [Google Scholar]
- 126.Cutolo M., Straub R.H. Sex steroids and autoimmune rheumatic diseases: state of the art. Nat Rev Rheumatol. 2020;16:628–644. doi: 10.1038/s41584-020-0503-4. [DOI] [PubMed] [Google Scholar]
- 127.Rivier C., Vale W. In the rat, interleukin-1 alpha acts at the level of the brain and the gonads to interfere with gonadotropin and sex steroid secretion. Endocrinology. 1989;124:2105–2109. doi: 10.1210/endo-124-5-2105. [DOI] [PubMed] [Google Scholar]
- 128.Ebrahimi F., Urwyler S.A., Straumann S., et al. IL-1 Antagonism in men with metabolic syndrome and low testosterone: a randomized clinical trial. J. Clin Endocrinol Metab. 2018;103:3466–3476. doi: 10.1210/jc.2018-00739. [DOI] [PubMed] [Google Scholar]
- 129.Lazzerini P.E., Cantara S., Bertolozzi I., et al. Transient hypogonadism is associated with heart rate-corrected QT prolongation and torsades de pointes risk during active systemic inflammation in men. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.023371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fuster V., Rydén L.E., Cannom D.S., et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 131.Nattel S., Burstein B., Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 132.Wu N., Xu B., Xiang Y., et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol. 2013;169:62–72. doi: 10.1016/j.ijcard.2013.08.078. [DOI] [PubMed] [Google Scholar]
- 133.Jiang H., Wang W., Wang C., Xie X., Hou Y. Association of pre-ablation level of potential blood markers with atrial fibrillation recurrence after catheter ablation: a meta-analysis. Europace. 2017;19:392–400. doi: 10.1093/europace/euw088. [DOI] [PubMed] [Google Scholar]
- 134.Boyalla V., Harling L., Snell A., et al. Biomarkers as predictors of recurrence of atrial fibrillation post ablation: an updated and expanded systematic review and meta-analysis. Clin Res Cardiol. 2022;111:680–691. doi: 10.1007/s00392-021-01978-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weymann A., Popov A.F., Sabashnikov A., et al. Baseline and postoperative levels of C-reactive protein and interleukins as inflammatory predictors of atrial fibrillation following cardiac surgery: a systematic review and meta-analysis. Kardiol Pol. 2018;76:440–451. doi: 10.5603/KP.a2017.0242. [DOI] [PubMed] [Google Scholar]
- 136.Wang H., Yan H.M., Tang M.X., et al. Increased serum levels of microvesicles in nonvalvular atrial fibrillation determined by ELISA using a specific monoclonal antibody AD-1. Clin Chim Acta. 2010;411:1700–1704. doi: 10.1016/j.cca.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 137.Liu Q., Zhang F., Yang M., Zhong J. Increasing level of interleukin-1β in epicardial adipose tissue is associated with persistent atrial fibrillation. J Interferon Cytokine Res. 2020;40:64–69. doi: 10.1089/jir.2019.0098. [DOI] [PubMed] [Google Scholar]
- 138.Wu N., Xu B., Liu Y., et al. Elevated plasma levels of Th17-related cytokines are associated with increased risk of atrial fibrillation. Sci Rep. 2016;6 doi: 10.1038/srep26543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stanciu A.E., Vatasescu R.G., Stanciu M.M., Serdarevic N., Dorobantu M. The role of pro-fibrotic biomarkers in paroxysmal and persistent atrial fibrillation. Cytokine. 2018;103:63–68. doi: 10.1016/j.cyto.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 140.Wang C.H., Hu D.Y., Tang C.Z., et al. [Changes of interleukin-1beta and tumor necrosis factor-alpha of right atrial appendages in patients with rheumatic valvular disease complicated with chronic atrial fibrillation] Zhonghua Xin Xue Guan Bing Za Zhi. 2005;33:522–525. [PubMed] [Google Scholar]
- 141.Qu Y.C., Du Y.M., Wu S.L., Chen Q.X., Wu H.L., Zhou S.F. Activated nuclear factor-kappaB and increased tumor necrosis factor-alpha in atrial tissue of atrial fibrillation. Scand Cardiovasc J. 2009;43:292–297. doi: 10.1080/14017430802651803. [DOI] [PubMed] [Google Scholar]
- 142.Chen L.Y., Ribeiro A.L.P., Platonov P.G., et al. P wave parameters and indices: a critical appraisal of clinical utility, challenges, and future research: a consensus document endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardiology. Circ Arrhythm Electrophysiol. 2022;15 doi: 10.1161/CIRCEP.121.010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hegazy H., Folke F., Coronel R., Torp-Pedersen C., Gislason G.H., Eroglu T.E. Risk of out-of-hospital cardiac arrest in patients with rheumatoid arthritis: a nationwide study. Open Heart. 2022;9 doi: 10.1136/openhrt-2022-001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adlan A.M., Panoulas V.F., Smith J.P., Fisher J.P., Kitas G.D. Association between corrected QT interval and inflammatory cytokines in rheumatoid arthritis. J Rheumatol. 2015;42:421–428. doi: 10.3899/jrheum.140861. [DOI] [PubMed] [Google Scholar]
- 145.Lazzerini P.E., Acampa M., Capecchi P.L., et al. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res (Hoboken) 2015;67:332–339. doi: 10.1002/acr.22455. [DOI] [PubMed] [Google Scholar]
- 146.Kobayashi H., Kobayashi Y., Yokoe I., et al. Heart rate-corrected QT interval duration in rheumatoid arthritis and its reduction with treatment with the interleukin 6 inhibitor tocilizumab. J Rheumatol. 2018;45:1620–1627. doi: 10.3899/jrheum.180065. [DOI] [PubMed] [Google Scholar]
- 147.Lazzerini P.E., Capecchi P.L., Bertolozzi I., et al. Marked QTc prolongation and torsades de pointes in patients with chronic inflammatory arthritis. Front Cardiovasc Med. 2016;3:31. doi: 10.3389/fcvm.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pisoni C.N., Reina S., Arakaki D., Eimon A., Carrizo C., Borda E. Elevated IL-1β levels in anti-Ro/SSA connective tissue diseases patients with prolonged corrected QTc interval. Clin Exp Rheumatol. 2015;33:715–720. [PubMed] [Google Scholar]
- 149.Wu K.C., Zhang L., Haberlen S.A., et al. Predictors of electrocardiographic QT interval prolongation in men with HIV. Heart. 2019;105:559–565. doi: 10.1136/heartjnl-2018-313667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu K.C., Bhondoekhan F., Haberlen S.A., et al. Associations between QT interval subcomponents, HIV serostatus, and inflammation. Ann Noninvasive Electrocardiol. 2020;25 doi: 10.1111/anec.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heravi A.S., Etzkorn L.H., Urbanek J.K., et al. HIV infection is associated with variability in ventricular repolarization: the Multicenter AIDS Cohort Study (MACS) Circulation. 2020;141:176–187. doi: 10.1161/CIRCULATIONAHA.119.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lazzerini P.E., Laghi-Pasini F., Bertolozzi I., et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart. 2017;103:1821–1829. doi: 10.1136/heartjnl-2016-311079. [DOI] [PubMed] [Google Scholar]
- 153.Elmas E., Hölzer L., Lang S., et al. Enhanced proinflammatory response of mononuclear cells to in vitro LPS-challenge in patients with ventricular fibrillation in the setting of acute myocardial infarction. Cytokine. 2008;43:138–142. doi: 10.1016/j.cyto.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 154.Safranow K., Dziedziejko V., Rzeuski R., et al. Inflammation markers are associated with metabolic syndrome and ventricular arrhythmia in patients with coronary artery disease. Postepy Hig Med Dosw (Online) 2016;70:56–66. doi: 10.5604/17322693.1194612. [DOI] [PubMed] [Google Scholar]
- 155.Fisman E.Z., Benderly M., Esper R.J., et al. Interleukin-6 and the risk of future cardiovascular events in patients with angina pectoris and/or healed myocardial infarction. Am J Cardiol. 2006;98:14–18. doi: 10.1016/j.amjcard.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 156.Streitner F., Kuschyk J., Veltmann C., et al. Prospective study of interleukin-6 and the risk of malignant ventricular tachyarrhythmia in ICD-recipients--a pilot study. Cytokine. 2007;40:30–34. doi: 10.1016/j.cyto.2007.07.187. [DOI] [PubMed] [Google Scholar]
- 157.Streitner F., Kuschyk J., Veltmann C., et al. Role of proinflammatory markers and NT-proBNP in patients with an implantable cardioverter-defibrillator and an electrical storm. Cytokine. 2009;47:166–172. doi: 10.1016/j.cyto.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 158.Damonte J.I., Del Buono M.G., Thomas G.K., et al. Arrhythmic recurrence and outcomes in patients hospitalized with first episode of electrical storm. Am J Cardiol. 2022;172:40–47. doi: 10.1016/j.amjcard.2022.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cheng A., Zhang Y., Blasco-Colmenares E., et al. Protein biomarkers identify patients unlikely to benefit from primary prevention implantable cardioverter-defibrillators: findings from the Prospective Observational Study of Implantable Cardioverter Defibrillators (PROSE-ICD) Circ Arrhythm Electrophysiol. 2014;7:1084–1091. doi: 10.1161/CIRCEP.113.001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu K.C., Wongvibulsin S., Tao S., et al. Baseline and dynamic risk predictors of appropriate implantable cardioverter defibrillator therapy. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Medenwald D., Kors J.A., Loppnow H., et al. Inflammation and prolonged QT time: results from the Cardiovascular Disease, Living and Ageing in Halle (CARLA) study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Empana J.P., Jouven X., Canouï-Poitrine F., et al. C-reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: the PRIME study. Arterioscler Thromb Vasc Biol. 2010;30:2047–2052. doi: 10.1161/ATVBAHA.110.208785. [DOI] [PubMed] [Google Scholar]
- 163.Hussein A.A., Gottdiener J.S., Bartz T.M., et al. Inflammation and sudden cardiac death in a community-based population of older adults: the Cardiovascular Health Study. Heart Rhythm. 2013;10:1425–1432. doi: 10.1016/j.hrthm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 164.Verweij N., Mateo Leach I., van den Boogaard M., et al. Genetic determinants of P wave duration and PR segment. Circ Cardiovasc Genet. 2014;7:475–481. doi: 10.1161/CIRCGENETICS.113.000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Asatryan B., Asimaki A., Landstrom A.P., et al. Inflammation and immune response in arrhythmogenic cardiomyopathy: state-of-the-art review. Circulation. 2021;144:1646–1655. doi: 10.1161/CIRCULATIONAHA.121.055890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Campian M.E., Verberne H.J., Hardziyenka M., et al. Assessment of inflammation in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Nucl Med Mol Imaging. 2010;37:2079–2085. doi: 10.1007/s00259-010-1525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Asimaki A., Tandri H., Duffy E.R., et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:743–752. doi: 10.1161/CIRCEP.111.964890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Broch K., Leren I.S., Saberniak J., et al. Soluble ST2 is associated with disease severity in arrhythmogenic right ventricular cardiomyopathy. Biomarkers. 2017;22:367–371. doi: 10.1080/1354750X.2016.1278266. [DOI] [PubMed] [Google Scholar]
- 169.Frustaci A., Priori S.G., Pieroni M., et al. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation. 2005;112:3680–3687. doi: 10.1161/CIRCULATIONAHA.105.520999. [DOI] [PubMed] [Google Scholar]
- 170.Ohkubo K., Watanabe I., Okumura Y., et al. Right ventricular histological substrate and conduction delay in patients with Brugada syndrome. Int Heart J. 2010;51:17–23. doi: 10.1536/ihj.51.17. [DOI] [PubMed] [Google Scholar]
- 171.Rizzo S., Basso C., Troost D., et al. T-cell-mediated inflammatory activity in the stellate ganglia of patients with ion-channel disease and severe ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:224–229. doi: 10.1161/CIRCEP.113.001184. [DOI] [PubMed] [Google Scholar]
- 172.Pieroni M., Notarstefano P., Oliva A., et al. Electroanatomic and pathologic right ventricular outflow tract abnormalities in patients with Brugada syndrome. J Am Coll Cardiol. 2018;72:2747–2757. doi: 10.1016/j.jacc.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 173.Corrado D., Migliore F., Zorzi A. Brugada syndrome: in search of a cause. J Am Coll Cardiol. 2018;72:2758–2760. doi: 10.1016/j.jacc.2018.08.2199. [DOI] [PubMed] [Google Scholar]
- 174.Nademanee K., Raju H., de Noronha S.V., et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–1986. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Imberti J.F., Underwood K., Mazzanti A., Priori S.G. Clinical challenges in catecholaminergic polymorphic ventricular tachycardia. Heart Lung Circ. 2016;25:777–783. doi: 10.1016/j.hlc.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 176.Berte B., Eyskens B., Meyfroidt G., Willems R. Bidirectional ventricular tachycardia in fulminant myocarditis. Europace. 2008;10:767–768. doi: 10.1093/europace/eun105. [DOI] [PubMed] [Google Scholar]
- 177.Chin A., Nair V., Healey J.S. Bidirectional ventricular tachycardia secondary to subacute myocarditis. Can J Cardiol. 2013;29:254e13–254e14. doi: 10.1016/j.cjca.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 178.Benjamin M.M., Hayes K., Field M.E., Scheinman M.M., Hoffmayer K.S. Bidirectional ventricular tachycardia in cardiac sarcoidosis. J Arrhythm. 2017;33:69–72. doi: 10.1016/j.joa.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Durocher D., El-Hajjaji I., Gilani S.O., Leong-Sit P., Davey R.A., De S.K. Bidirectional ventricular tachycardia in a patient with fulminant myocarditis secondary to cardiac sarcoidosis mimicking giant cell myocarditis. CJC Open. 2021;3:1509–1512. doi: 10.1016/j.cjco.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk, and inflammation: mind the gap. Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 181.Lazzerini P.E., Laghi-Pasini F., Boutjdir M., Capecchi P.L. Inflammatory cytokines and cardiac arrhythmias: the lesson from COVID-19. Nat Rev Immunol. 2022;22:270–272. doi: 10.1038/s41577-022-00714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lazzerini P.E., Laghi-Pasini F., Acampa M., Boutjdir M., Leopoldo Capecchi P. IL-6 (Interleukin 6) blockade and heart rate corrected QT interval prolongation in COVID-19. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Coromilas E.J., Kochav S., Goldenthal I., et al. Worldwide survey of COVID-19 associated arrhythmias. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dherange P., Lang J., Qian P., et al. Arrhythmias and COVID-19: a review. J Am Coll Cardiol EP. 2020;6:1193–1204. doi: 10.1016/j.jacep.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Siripanthong B., Asatryan B., Hanff T.C., et al. The pathogenesis and long-term consequences of COVID-19 cardiac injury. J Am Coll Cardiol Basic Trans Science. 2022;7:294–308. doi: 10.1016/j.jacbts.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Carr G.E., Yuen T.C., McConville J.F., et al. Early cardiac arrest in patients hospitalized with pneumonia: a report from the American Heart Association's Get With The Guidelines-Resuscitation Program. Chest. 2012;141:1528–1536. doi: 10.1378/chest.11-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Elsaid O., McCullough P.A., Tecson K.M., Williams R.S., Yoon A. Ventricular fibrillation storm in Coronavirus 2019. Am J Cardiol. 2020;135:177–180. doi: 10.1016/j.amjcard.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Beer D., Isakadze N., McClellan R., Calkins H., Barth A.S. Acquired long QT and ventricular arrhythmias in the setting of acute inflammation: a case series. J Am Coll Cardiol Case Rep. 2021;3:1103–1107. doi: 10.1016/j.jaccas.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Anupama B.K., Adhikari S., Chaudhuri D. Prolonged QT interval in a patient with coronavirus disease-2019: beyond hydroxychloroquine and azithromycin. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620948407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Guan H., Liu J., Ding J., et al. Arrhythmias in patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: incidences and implications. J Electrocardiol. 2021;65:96–101. doi: 10.1016/j.jelectrocard.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bagnato G., Imbalzano E., Aragona C.O., et al. New-onset atrial fibrillation and early mortality rate in COVID-19 patients: association with IL-6 serum levels and respiratory distress. Medicina (Kaunas) 2022;58:530. doi: 10.3390/medicina58040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Musikantow D.R., Turagam M.K., Sartori S., et al. Atrial fibrillation in patients hospitalized with COVID-19: incidence, predictors, outcomes, and comparison to influenza. J Am Coll Cardiol EP. 2021;7:1120–1130. doi: 10.1016/j.jacep.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rubin G.A., Desai A.D., Chai Z., et al. Cardiac corrected QT interval changes among patients treated for COVID-19 infection during the early phase of the pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hu Z., Li S., Song X. Cytokine storm with rapidly elevated interleukin-6 indicates sudden death in patients with critical COVID-19. Cytokine Growth Factor Rev. 2021;58:30–31. doi: 10.1016/j.cytogfr.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.González-Muniesa P., Mártinez-González M.A., Hu F.B., et al. Obesity. Nat Rev Dis Primers. 2017;3 doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 197.Powell-Wiley T.M., Poirier P., Burke L.E., et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Alí A., Boutjdir M., Aromolaran A.S. Cardiolipotoxicity, inflammation, and arrhythmias: role for interleukin-6 molecular mechanisms. Front Physiol. 2018;9:1866. doi: 10.3389/fphys.2018.01866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zulu M.Z., Sureshchandra S., Pinski A.N., Doratt B., Shen W., Messaoudi I. Obesity correlates with pronounced aberrant innate immune responses in hospitalized aged COVID-19 patients. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.760288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tamara A., Tahapary D.L. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr. 2020;14:655–659. doi: 10.1016/j.dsx.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Armbruster A.L., Campbell K.B., Kahanda M.G., Cuculich P.S. The role of inflammation in the pathogenesis and treatment of arrhythmias. Pharmacotherapy. 2022;42:250–262. doi: 10.1002/phar.2663. [DOI] [PubMed] [Google Scholar]
- 202.Vyas A.K., Guo H., Moss A.J., et al. MADIT-II Research Group Reduction in ventricular tachyarrhythmias with statins in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:769–773. doi: 10.1016/j.jacc.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 203.Liao Y.C., Hsieh Y.C., Hung C.Y., et al. Statin therapy reduces the risk of ventricular arrhythmias, sudden cardiac death, and mortality in heart failure patients: a nationwide population-based cohort study. Int J Cardiol. 2013;168:4805–4807. doi: 10.1016/j.ijcard.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 204.Peña J.M., MacFadyen J., Glynn R.J., Ridker P.M. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2012;33:531–537. doi: 10.1093/eurheartj/ehr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yuan X., Du J., Liu Q., Zhang L. Defining the role of perioperative statin treatment in patients after cardiac surgery: a meta-analysis and systematic review of 20 randomized controlled trials. Int J Cardiol. 2017;228:958–966. doi: 10.1016/j.ijcard.2016.11.116. [DOI] [PubMed] [Google Scholar]
- 206.Elgendy I.Y., Mahmoud A., Huo T., Beaver T.M., Bavry A.A. Meta-analysis of 12 trials evaluating the effects of statins on decreasing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2015;115:1523–1528. doi: 10.1016/j.amjcard.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 207.Zheng Z., Jayaram R., Jiang L., et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med. 2016;374:1744–1753. doi: 10.1056/NEJMoa1507750. [DOI] [PubMed] [Google Scholar]
- 208.Nomani H., Mohammadpour A.H., Reiner Ž., Jamialahmadi T., Sahebkar A. Statin therapy in post-operative atrial fibrillation: focus on the anti-inflammatory effects. J Cardiovasc Dev Dis. 2021;8:24. doi: 10.3390/jcdd8030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schett G., McInnes I.B., Neurath M.F. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. 2021;385:628–639. doi: 10.1056/NEJMra1909094. [DOI] [PubMed] [Google Scholar]
- 210.Senel S., Cobankara V., Taskoylu O., Guclu A., Evrengul H., Kaya M.G. Effect of infliximab treatment on QT intervals in patients with ankylosing spondylitis. J Investig Med. 2011;59:1273–1275. doi: 10.2130/JIM.0b013e3182330720. [DOI] [PubMed] [Google Scholar]
- 211.Bello F., Marchi A., Prisco D., Olivotto I., Emmi G. Antiarrhythmic efficacy of anakinra in a young patient with autoimmune lymphocytic myocarditis. Rheumatology (Oxford) 2020;59:e88–e90. doi: 10.1093/rheumatology/keaa207. [DOI] [PubMed] [Google Scholar]
- 212.Ridker P.M., Everett B.M., Thuren T., et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 213.Abbate A., Toldo S., Marchetti C., Kron J., Van Tassell B.W., Dinarello C.A. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126:1260–1280. doi: 10.1161/CIRCRESAHA.120.315937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Buckley L.F., Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39:2063–2069. doi: 10.1093/eurheartj/ehy128. [DOI] [PubMed] [Google Scholar]
- 215.Abbate A., Wohlford G.F., Del Buono M.G., et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur Heart J Cardiovasc Pharmacother. 2022;8:503–510. doi: 10.1093/ehjcvp/pvab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Abbate A., Trankle C.R., Buckley L.F., et al. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Del Buono M.G., Damonte J.I., Trankle C.R., et al. Effect of interleukin-1 blockade with anakinra on leukocyte count in patients with ST-segment elevation acute myocardial infarction. Sci Rep. 2022;12:1254. doi: 10.1038/s41598-022-05374-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kron J., Crawford T., Mihalick V., et al. Interleukin-1 blockade in cardiac sarcoidosis: study design of the multimodality assessment of granulomas in cardiac sarcoidosis: Anakinra Randomized Trial (MAGiC-ART) J Transl Med. 2021;19:460. doi: 10.1186/s12967-021-03130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Van Tassell B.W., Trankle C.R., Canada J.M., et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Van Tassell B.W., Canada J., Carbone S., et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial) Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Van Tassell B.W., Abouzaki N.A., Oddi Erdle C., et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J Cardiovasc Pharmacol. 2016;67:544–551. doi: 10.1097/FJC.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Van Tassell B.W., Arena R., Biondi-Zoccai G., et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study) Am J Cardiol. 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Abbate A., Van Tassell B.W., Biondi-Zoccai G., et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study] Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Van Tassell B.W., Arena R.A., Toldo S., et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Abbate A., Kontos M.C., Grizzard J.D., et al. VCU-ART Investigators. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–1377e1. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 226.Imazio M., Lazaros G., Gattorno M., et al. Anti-interleukin-1 agents for pericarditis: a primer for cardiologists. Eur Heart J. 2022;43:2946–2957. doi: 10.1093/eurheartj/ehab452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kleveland O., Kunszt G., Bratlie M., et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur Heart J. 2016;37:2406–2413. doi: 10.1093/eurheartj/ehw171. [DOI] [PubMed] [Google Scholar]
- 228.Broch K., Anstensrud A.K., Woxholt S., et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77:1845–1855. doi: 10.1016/j.jacc.2021.02.049. [DOI] [PubMed] [Google Scholar]
- 229.Krisai P., Blum S., Schnabel R.B., et al. Canakinumab after electrical cardioversion in patients with persistent atrial fibrillation: a pilot randomized trial. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.008197. [DOI] [PubMed] [Google Scholar]
- 230.Ntari L., Mantzouratou P., Katsaouni A., Pantos C., Kollias G., Mourouzis I. Changes in thyroid hormone signaling mediate cardiac dysfunction in the Tg197 mouse model of arthritis: potential therapeutic implications. J Clin Med. 2021;10:5512. doi: 10.3390/jcm10235512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Janczewski A.M., Kadokami T., Lemster B., Frye C.S., McTiernan C.F., Feldman A.M. Morphological and functional changes in cardiac myocytes isolated from mice overexpressing TNF-alpha. Am J Physiol Heart Circ Physiol. 2003;284:H960–H969. doi: 10.1152/ajpheart.0718.2001. [DOI] [PubMed] [Google Scholar]
- 232.Yokoyama T., Arai M., Sekiguchi K., et al. Tumor necrosis factor-alpha decreases the phosphorylation levels of phospholamban and troponin I in spontaneously beating rat neonatal cardiac myocytes. J Mol Cell Cardiol. 1999;31:261–273. doi: 10.1006/jmcc.1998.0863. [DOI] [PubMed] [Google Scholar]
- 233.Jiang T., Peng D., Shi W., et al. IL-6/STAT3 signaling promotes cardiac dysfunction by upregulating FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes formation in sepsis mice. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.790612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kumar S., Wang G., Zheng N., et al. HIMF (hypoxia-induced mitogenic factor)-IL (interleukin)-6 signaling mediates cardiomyocyte-fibroblast crosstalk to promote cardiac hypertrophy and fibrosis. Hypertension. 2019;73:1058–1070. doi: 10.1161/HYPERTENSIONAHA.118.12267. [DOI] [PubMed] [Google Scholar]
- 235.Kato T., Sano M., Miyoshi S., et al. Calmodulin kinases II and IV and calcineurin are involved in leukemia inhibitory factor-induced cardiac hypertrophy in rats. Circ Res. 2000;87:937–945. doi: 10.1161/01.res.87.10.937. [DOI] [PubMed] [Google Scholar]
- 236.Gregolin C.S., do Nascimento M., Borges de Souza S.L., et al. Myocardial dysfunction in cirrhotic cardiomyopathy is associated with alterations of phospholamban phosphorylation and IL-6 levels. Arch Med Res. 2021;52:284–293. doi: 10.1016/j.arcmed.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 237.Xue G.L., Li D.S., Wang Z.Y., et al. Interleukin-17 upregulation participates in the pathogenesis of heart failure in mice via NF-κB-dependent suppression of SERCA2a and Cav1.2 expression. Acta Pharmacol Sin. 2021;42:1780–1789. doi: 10.1038/s41401-020-00580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]