Abstract
Aim: We aimed to investigate the associations of serum alkaline phosphatase (ALP) levels with incident cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, as well as their subtypes, among men and women in a prospective cohort study.
Methods: A total of 11,408 men and 14,981 women were included to evaluate the associations between ALP levels and incident CVD. Participants were divided into four groups according to the quartiles of serum ALP levels in men and women separately. Cox proportional hazard models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: During an average follow-up of 7.3 years, 7,015 incident CVDs (5,561 CHDs and 1,454 strokes) were documented. After adjustments for age, body mass index, smoking status, drinking status, diabetes, hyperlipidemia, hypertension, physical activity, aspirin usage, anticoagulants usage, menopausal status (women only), family history of CVD, estimated glomerular filtration rate, white blood cell counts, and admission batch and comparing the lowest quartile of ALP, the adjusted HRs (95% CIs) of participants in the highest quartile were 1.22 (1.11–1.34) for CVD, 1.14 (1.02–1.28) for CHD, 1.43 (1.18–1.73) for stroke, 1.31 (1.09–1.57) for acute coronary syndrome (ACS), 1.37 (1.11–1.70) for ischemic stroke, and 1.75 (1.10–2.79) for hemorrhagic stroke in men and 1.12 (1.01–1.23) for CVD, 1.10 (0.99–1.23) for CHD, 1.18 (0.92–1.51) for stroke, 1.23 (1.03–1.47) for ACS, 1.10 (0.83–1.45) for ischemic stroke, and 1.54 (0.90–2.65) for hemorrhagic stroke in women. The ALP–CVD associations remained significant even within the normal ranges of ALP levels (40–150 U/L). Moreover, linear dose–response relationships were found between ALP levels and incident CVD.
Conclusions: Higher ALP levels, even within the normal range, were significantly associated with increased risks of CVD, in a dose-dependent manner. These findings suggested that regular monitoring of ALP levels may help in improving the early identification of the population at higher CVD risk.
Keywords: Alkaline phosphatase, Epidemiology, Prospective cohort study, Cardiovascular disease
Introduction
The liver plays an essential role in lipid, glucose, and protein metabolism 1) . Liver enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase, γ-glutamyl transferase, and alkaline phosphatase (ALP), are commonly used as markers of liver injury in the clinic 2) , which are suggested to be associated with diabetes 3) , cardiovascular morbidity, and mortality in epidemiological studies 4 - 8) . Among these liver enzymes, ALP can catalyze the hydrolysis of inorganic pyrophosphate, a vascular calcification inhibitor 9) . Increased hydrolysis of inorganic pyrophosphate by ALP can act to enhance the mineralization of vessels 9) . In human, ALP can be classified into four isoenzymes mainly according to the specificity of the tissue to be expressed, termed as tissue-nonspecific ALP (expressed in the liver, bone, and kidney), intestinal ALP, placental ALP, and germ cell ALP 10) . Compared with aminotransferases and γ-glutamyl transferase, ALP has received less attention, and its significance for incident cardiovascular disease (CVD) and its subtypes are less certain.
Although accumulating epidemiological studies suggested that elevated circulating ALP levels were associated with higher risks of CVD 5 , 6 , 8 , 11 - 15) and mortality 7 , 14) , many of these studies were conducted among participants at high vascular risk 6) or those with pre-existing disease 13 - 15) , which might limit the generalizability of the findings. With the exception of one study that performed the analyses in Japanese men and women separately 8) , other studies conducted the analyses in men only 6) or in men and women together 5 , 11 - 13 , 15) . Given that menopausal status is one of the known factors influencing ALP levels 16) , it is necessary to investigate the associations of ALP levels with CVD in men and women respectively. In addition, evidence regarding the association of ALP with subtypes of coronary heart disease (CHD) and stroke is limited. Moreover, the dose–response relationships between ALP levels and CVD remained inconsistent 5 , 11) . Furthermore, whether higher ALP levels, even within the normal range, would be associated with an increased risk of CVD is largely unclear.
To fill these knowledge gaps, we aimed to examine the relationships of serum ALP levels with risk of CVD, CHD, and stroke, as well as their subtypes, in a large prospective cohort of Chinese middle-aged and older men and women.
Materials and Methods
Study Design and Population
Data were derived from the Dongfeng–Tongji cohort, an ongoing prospective cohort study in Shiyan, Hubei, China, and the details of the Dongfeng–Tongji cohort have been previously described elsewhere 17) . Briefly, all living retired employees (n=31,000) of Dongfeng Motor Corporation were invited, and a total of 27,009 (87% response rate) retired employees from the Dongfeng Motor Corporation completed questionnaires and clinical measurements between September 2008 and June 2010 in the baseline survey. In 2013, all participants (n=27,009) were invited to a follow-up examination with a follow-up rate of 96.2% (n=25,978). Furthermore, 14,120 participants were newly enrolled in the follow-up survey from April to October 2013. In this study, 14,120 newly-enrolled participants in the follow-up survey (2013) combined with the 27,009 participants in the baseline survey (2008–2010) were included for the association analysis. Hence, we had a total sample size of 41,129 participants with baseline information. After excluding participants with CVD, severely abnormal electrocardiogram (including atrial fibrillation, atrial flutter, pre-excitation syndrome, pacemaker rhythm, and frequent premature ventricular contractions), cancer, liver disease (including self-reported hepatitis, diagnosed hepatitis B virus infection, diagnosed fatty liver disease, and liver cirrhosis), liver enzyme levels greater than two times the upper limit of normal (to minimize the influence of potential liver injury) 18) , missing values of ALP at baseline, and those without follow-up information, we included 26,389 participants (11,408 men and 14,981 women) in this study. The baseline characteristics between individuals included in the present study and those with missing values of ALP are presented in Supplementary Table 1 . Detailed information regarding participant selection is provided in Supplementary Fig.1 .
Supplementary Table 1. A comparison of the baseline characteristics between the included participants and participants with missing value of ALP.
| Characteristics | Included participants | Individuals with missing value of ALP | P value |
|---|---|---|---|
| N | 26389 | 3097 | |
| Age, y | 61.6 (8.0) | 62.3 (8.1) | <0.001 |
| Male, % | 11408 (43.2) | 1291 (41.7) | 0.10 |
| BMI*, kg/m2 | 24.2 (3.3) | 24.0 (2.4) | 0.34 |
| SBP*, mmHg | 130.7 (20.2) | 135.7 (19.6) | <0.001 |
| DBP*, mmHg | 78.3 (11.6) | 81.5 (9.9) | <0.001 |
| Fasting blood glucose*, mmol/L | 5.6 (5.2-6.2) | 5.6 (5.2-6.3) | 0.83 |
| Total cholesterol*, mmol/L | 5.0 (4.4-5.7) | 5.6 (5.1-6.1) | <0.001 |
| Total glyceride*, mmol/L | 1.2 (0.9-1.7) | 1.8 (1.1-2.3) | <0.001 |
| HDL-C*, mmol/L | 1.4 (1.2-1.7) | 1.6 (1.3-2.3) | <0.001 |
| LDL-C*, mmol/L | 2.9 (2.4-3.4) | 3.3 (2.7-3.8) | <0.001 |
| High school or above*, % | 10849 (41.1) | 1359 (43.9) | 0.007 |
| Current smoker*, % | 4912 (18.6) | 582 (18.8) | 0.03 |
| Current drinker*, % | 6404 (24.3) | 650 (21.0) | <0.001 |
| Physical activity, % | 18730 (71.0) | 1971 (63.6) | <0.001 |
| Diabetes, % | 4261 (16.2) | 297 (9.6) | <0.001 |
| Hyperlipidemia, % | 10806 (41.0) | 684 (22.1) | <0.001 |
| Hypertension, % | 13241 (50.2) | 1379 (44.5) | <0.001 |
| Family history of CVD, % | 2999 (11.4) | 312 (10.1) | 0.03 |
| Anticoagulants use, No. (%) | 375 (1.4) | 46 (1.5) | 0.78 |
| Aspirin use, No. (%) | 2500 (9.5) | 278 (9.0) | 0.37 |
| Postmenopausal women, No. (%) | 12431 (47.1) | 1453 (46.9) | 0.74 |
| eGFR*, mL/min/1.73 m2 | 82.6 (70.4-94.6) | 82.7 (82.7-82.7) | 0.14 |
| WBC*, 109/L | 5.6 (4.7-6.7) | 3.9 (3.6-6.1) | <0.001 |
| ALT*, U/L | 19.0 (14.0-26.0) | 19.0 (15.0-28.0) | 0.50 |
Note: Normally distributed variables were presented as mean (SD), non-normally distributed variables were presented as median (IQR) and categorical variables were presented as a number (%).
Abbreviations: ALP, alkaline phosphatase; BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; WBC, white blood cell.
*Data were incomplete for these variables. For totally 26,389 participants included in the analysis, 365 (1.4%), 252 (1.0%), 329 (1.2%), 324 (1.2%), 834 (3.2%), 38 (0.1%), 31 (0.1%), 34 (0.1%), 175 (0.7%), 128 (0.5%), 43 (0.2%), 51 (0.2%), 1867 (7.1%), and 236 (9.0%) of participants had missing data for BMI, fasting blood glucose, SBP, DBP, total cholesterol, triglyceride, HDL-C, LDL-C, education, smoking status, drinking status, eGFR, WBC, and ALT, respectively. The other variables included in the analyses did not have missing data. For the 3097 participants with missing value of ALP, 1490 (48.1%), 2980 (96.2%), 1496 (48.3%), 1499 (48.4%), 2731 (88.2%), 2811 (90.8%), 2834 (91.2%), 2743 (88.3%), 28 (0.9%), 8 (0.3%), 4 (0.1%), 2978 (96.2%), 3019 (97.5%), and 2994 (96.7%) of participants had missing data for BMI, fasting blood glucose, SBP, DBP, total cholesterol, triglyceride, HDL-C, LDL-C, education, smoking status, drinking status, eGFR, WBC, and ALT, respectively.
Supplementary Fig.1. Flow chart of study participants for the association analysis of serum ALP with CVD.
Abbreviations: ACS, acute coronary syndrome; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHD, coronary heart disease; CVD, cardiovascular disease; DFTJ, Dongfeng-Tongji; HBV, hepatitis B virus.
All study protocols were approved by the Ethics and Human Subject Committee of Tongji Medical College. All participants provided written informed consents.
Ascertainment of Outcomes
Participants were followed up longitudinally from the baseline survey to December 31, 2018. All retired employees were covered by Dongfeng Motor Corporation’s healthcare service system (including five company-owned hospitals, one Center of Disease Control and Prevention, and one Social Insurance Center) 17) , and each participant had a unique medical insurance number, which made it available to track the individual’s morbidity and mortality records. For all suspected CVD events that first occurred after enrollment, an expert panel of physicians reviewed hospital records, medical insurance documents, and death certificate to confirm CVD events. The outcomes of the present study were incident CVD, including incident CHD (I20–I25) and stroke (I60–I61, I63–I64, I69.0–I69.1, and I69.3–I69.4), whichever came first. CHD was diagnosed following the World Health Organization criteria using typical clinical symptoms, cardiac enzymes, and electrocardiographic findings 19) or death with CHD as the underlying cause according to the 10th version of the International Classification of Diseases and Related Health Problems (ICD-10). Acute coronary syndrome (ACS) is a severe type of CHD, including acute myocardial infarction (I21.0–I21.4) and unstable angina (I20.0–I20.1 and I20.9) 20 , 21) . Stroke is defined as sudden or rapid onset of a typical neurological deficit of vascular origin that persisted more than 24 h or till death 22) . Stroke subtypes are classified into ischemic stroke and hemorrhagic stroke 23 , 24) .
Serum ALP Measurement and Assessment of Covariates
Blood samples were collected after an overnight fast. Serum ALP levels were measured by ARCHITECT Ci8200 automatic analyzer (Abbott Laboratories, Abbott Park, Chicago, IL, USA) using Abbott Diagnostics reagents following standard experimental procedures from the manufacturer. The normal range of ALP varies between different laboratories, whereas the normal range of serum ALP in the current study was 40–150 U/L.
Data on age, sex, education, smoking status, drinking status, physical activity, family history of CVD, medication usage, and dietary habits were collected with semistructured questionnaires by trained interviewers. Data on height, weight, blood pressure, fasting blood glucose, lipid levels, and other biochemistry indicators were also collected from physical measurements and medical examinations in the health examination center in Sinopharm Dongfeng General Hospital. Further information on assessment of covariates is provided in the Supplementary Methods.
Statistical Analysis
Participants were divided into four groups according to quartiles of serum ALP levels of men and women at baseline. Basic characteristics of the study participants were described as mean (standard deviation, SD) and median (interquartile range) for continuous variables or presented as frequency (percentage) for categorical variables. Differences in baseline characteristics across quartiles were compared by one-way analysis of variance test, Kruskal–Wallis test, or chi-square test as appropriate. Person-years for each participant were calculated from the date of recruitment until the date of the first CVD event, the date of death, or the end of follow-up (December 31, 2018), whichever came first. Variables with missing values (range 0.1%–7.1%) were imputed by multiple imputation method.
We used Cox proportional hazard models (using age as time scale) to evaluate the associations of serum ALP levels with incident CVD, CHD, stroke, and their subtypes. Participants with the first quartile of ALP levels were set as the reference group. In model 1, we adjusted age and admission batch. In model 2, we additionally adjusted established vascular risk factors, including body mass index (BMI), smoking status, drinking status, diabetes, hyperlipidemia, hypertension, physical activity, aspirin usage, anticoagulants usage, menopausal status (women only), family history of CVD, and estimated glomerular filtration rate (eGFR). In model 3, we further adjusted white blood cells (WBCs), the biomarker of inflammation. In addition, we divided participants into four groups according to quartiles of those whose ALP levels were within normal range (40–150 U/L) and examined the association of ALP within normal range with all abovementioned outcomes. General linear regressions were applied to examine the association of ALP levels with major cardiovascular risk factors.
The restricted cubic splines with 3 knots (5th, 50th, and 95th) 25) were constructed to flexibly display the linear associations between ALP levels and CVD, CHD, and stroke after full adjustment, using min value of ALP levels as reference, and ALP levels <1th percentile or >99th percentile were excluded to avoid influence from extreme values. In addition, we conducted stratified analyses by baseline characteristics, including age (<60 or ≥ 60 years), BMI (<24 or ≥ 24 kg/m2) 26) , ever smoking (yes or no), ever drinking (yes or no), diabetes (yes or no), hyperlipidemia (yes or no), hypertension (yes or no), eGFR (<90 or ≥ 90 mL/min/1.73 m2), and menopausal status (women only), to explore whether the associations of ALP levels with cardiovascular outcomes varied in different subgroups.
For sensitivity analyses, ALT levels were further adjusted to examine whether the association of interest was independent of other liver enzymes. In the 26,389 participants included in the present study, only 8,617 participants who newly enrolled in the cohort in 2013 were measured with bone mineral density, among which a total of 3,115 participants were diagnosed with osteoporosis. Considering the relatively small sample size of participants with information of osteoporosis, we did not perform the analysis with adjustment of osteoporosis, but adjusting self-reported arthritis instead to control the confounding brought by bone metabolism. Dietary habits were also included in models to reduce the confounding of diet. Moreover, participants with chronic kidney disease (CKD) (defined as eGFR <60 mL/min/1.73 m2 only or either eGFR <60 mL/min/1.73 m2 or urinary protein with one or more “+”) were further excluded to control for the confounding by kidney dysfunction. Moreover, participants with liver disease were further included to control for the confounding by liver disease.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A p value below 0.05 (two sides) was considered as statistically significant.
Results
Baseline Characteristics of the Study Population
General characteristics of study participants by quartiles of baseline serum ALP levels are shown in Table 1 . The mean (SD) age was 65.2 (6.5) years old among men (43.2%) and 58.9 (8.0) years old among women (56.8%). Compared with the lower ALP group, both men and women in the higher groups tended to be current smokers or noncurrent drinkers and to have lower eGFR and higher WBC levels and exhibited a higher proportion of participants with diabetes, hyperlipidemia, and hypertension and a lower proportion of those with family history of CVD. In addition, women in the higher group were likely to have a higher proportion of postmenopause. The distribution of ALP levels is presented in Supplementary Fig.2 .
Table 1. Basic characteristics of study participants according to serum ALP levels in men and women.
| Variables | Quartiles of ALP levels*, U/L | p value | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Men (n= 11,408) | <69 | 69-84 | 84-100 | >100 | |
| No. (%) | 2676 (23.5) | 2901 (25.4) | 3050 (26.7) | 2781 (24.4) | |
| Age, y | 65.2 (6.5) | 65.4 (6.5) | 65.2 (6.3) | 65.1 (6.8) | 0.40 |
| BMI†, kg/m2 | 24.4 (3.2) | 24.3 (3.1) | 24.3 (3.1) | 24.1 (3.1) | <0.001 |
| High school or above†, No. (%) | 1131 (42.3) | 1191 (41.1) | 1228 (40.3) | 1051 (37.8) | 0.005 |
| SBP†, mmHg | 132.1 (19.6) | 133.1 (20.2) | 133.9 (20.1) | 134.5 (20.5) | <0.001 |
| DBP†, mmHg | 79.1 (11.4) | 79.4 (11.9) | 79.9 (11.8) | 80.4 (12.2) | <0.001 |
| Fasting blood glucose†, mmol/L | 5.7 (5.2-6.3) | 5.7 (5.2-6.2) | 5.7 (5.2-6.3) | 5.7 (5.2-6.3) | 0.29 |
| Total cholesterol†, mmol/L | 4.9 (4.3-5.5) | 4.8 (4.3-5.5) | 4.8 (4.3-5.5) | 4.8 (4.2-5.5) | 0.13 |
| Total glyceride†, mmol/L | 1.1 (0.8-1.6) | 1.2 (0.8-1.6) | 1.2 (0.9-1.7) | 1.3 (0.9-1.8) | <0.001 |
| HDL-C†, mmol/L | 1.4 (1.2-1.6) | 1.3 (1.2-1.6) | 1.3 (1.1-1.6) | 1.3 (1.1-1.6) | <0.001 |
| LDL-C†, mmol/L | 2.8 (2.4-3.3) | 2.8 (2.4-3.3) | 2.8 (2.3-3.4) | 2.8 (2.3-3.4) | 0.40 |
| Current smoker†, No. (%) | 966 (36.1) | 1091 (37.6) | 1257 (41.2) | 1300 (46.8) | <0.001 |
| Current drinker†, No. (%) | 1297 (48.5) | 1324 (45.6) | 1314 (43.1) | 1117 (40.2) | <0.001 |
| Physical activity, No. (%) | 1939 (72.5) | 2139 (73.7) | 2257 (74.0) | 2006 (72.1) | 0.29 |
| Diabetes, No. (%) | 487 (18.2) | 475 (16.4) | 567 (18.6) | 561 (20.2) | 0.003 |
| Hyperlipidemia, No. (%) | 1047 (39.1) | 1205 (41.5) | 1340 (43.9) | 1299 (46.7) | <0.001 |
| Hypertension, No. (%) | 1432 (53.5) | 1600 (55.2) | 1720 (56.4) | 1642 (59.0) | <0.001 |
| Family history of CVD, No. (%) | 261 (9.8) | 214 (7.4) | 227 (7.4) | 240 (8.6) | 0.003 |
| Anticoagulants use, No. (%) | 40 (1.5) | 44 (1.5) | 45 (1.5) | 40 (1.4) | 1.00 |
| Aspirin use, No. (%) | 300 (11.2) | 303 (10.4) | 322 (10.6) | 259 (9.3) | 0.14 |
| eGFR†, mL/min/1.73 m2 | 82.6 (70.5-93.8) | 81.6 (70.0-92.9) | 80.8 (69.2-93.0) | 80.2 (68.6-91.5) | <0.001 |
| WBC†, 109/L | 5.6 (4.8-6.7) | 5.9 (4.9-7.0) | 6.0 (5.1-7.0) | 6.2 (5.2-7.3) | <0.001 |
| ALT†, U/L | 19.0 (15.0-26.0) | 19.0 (15.0-26.0) | 20.0 (15.0-27.0) | 21.0 (16.0-29.0) | <0.001 |
| ALP, U/L | 61.0 (54.0-65.0) | 76.0 (73.0-80.0) | 91.0 (87.0-96.0) | 115.0 (106.0-127.0) | <0.001 |
| Women (n= 14,981) | <71 | 71-87 | 87-105 | >105 | |
| No. (%) | 3720 (24.8) | 3661 (24.4) | 3861 (25.8) | 3739 (25.0) | |
| Age, y | 56.5 (8.0) | 58.6 (7.9) | 59.8 (7.9) | 60.6 (7.7) | <0.001 |
| BMI†, kg/m2 | 23.8 (3.3) | 24.0 (3.3) | 24.1 (3.4) | 24.4 (3.4) | <0.001 |
| High school or above†, No. (%) | 1757 (47.2) | 1610 (44.0) | 1575 (40.8) | 1306 (34.9) | <0.001 |
| SBP†, mmHg | 125.6 (19.7) | 127.4 (19.5) | 129.4 (19.7) | 132.0 (20.9) | <0.001 |
| DBP†, mmHg | 76.4 (11.1) | 76.7 (11.2) | 77.3 (10.9) | 78.4 (11.7) | <0.001 |
| Fasting blood glucose†, mmol/L | 5.4 (5.1-5.9) | 5.5 (5.1-6.0) | 5.6 (5.1-6.1) | 5.6 (5.2-6.2) | <0.001 |
| Total cholesterol†, mmol/L | 5.0 (4.4-5.7) | 5.2 (4.6-5.8) | 5.2 (4.6-5.9) | 5.3 (4.6-5.9) | <0.001 |
| Total glyceride†, mmol/L | 1.1 (0.8-1.6) | 1.2 (0.9-1.7) | 1.3 (0.9-1.8) | 1.4 (1.0-1.9) | <0.001 |
| HDL-C†, mmol/L | 1.5 (1.3-1.7) | 1.5 (1.3-1.7) | 1.5 (1.2-1.7) | 1.4 (1.2-1.7) | <0.001 |
| LDL-C†, mmol/L | 2.8 (2.4-3.3) | 3.0 (2.5-3.5) | 3.0 (2.5-3.6) | 3.0 (2.5-3.6) | <0.001 |
| Current smoker†, No. (%) | 47 (1.3) | 70 (1.9) | 81 (2.1) | 100 (2.7) | <0.001 |
| Current drinker†, No. (%) | 420 (11.3) | 341 (9.3) | 328 (8.5) | 263 (7.0) | <0.001 |
| Physical activity, No. (%) | 2523 (67.8) | 2541 (69.4) | 2696 (69.8) | 2629 (70.3) | <0.001 |
| Diabetes, No. (%) | 435 (11.7) | 485 (13.3) | 569 (14.7) | 682 (18.2) | <0.001 |
| Hyperlipidemia, No. (%) | 1213 (32.6) | 1434 (39.2) | 1621 (42.0) | 1647 (44.1) | <0.001 |
| Hypertension, No. (%) | 1448 (38.9) | 1594 (43.5) | 1815 (47.0) | 1990 (53.2) | <0.001 |
| Family history of CVD, No. (%) | 576 (15.5) | 514 (14.0) | 528 (13.7) | 439 (11.7) | <0.001 |
| Anticoagulants use, No. (%) | 45 (1.2) | 52 (1.4) | 56 (1.5) | 53 (1.4) | 0.80 |
| Aspirin use, No. (%) | 308 (8.3) | 314 (8.6) | 351 (9.1) | 343 (9.2) | 0.47 |
| Postmenopausal women, No. (%) | 2584 (69.5) | 3011 (82.3) | 3452 (89.4) | 3467 (92.7) | <0.001 |
| WBC†, 109/L | 5.2 (4.3-6.2) | 5.3 (4.5-6.3) | 5.5 (4.6-6.6) | 5.7 (4.8-6.7) | <0.001 |
| eGFR†, mL/min/1.73 m2 | 88.1 (74.5-99.5) | 85.3 (72.2-97.2) | 82.3 (70.1-94.6) | 80.5 (68.8-92.9) | <0.001 |
| ALT†, U/L | 17.0 (13.0-23.0) | 18.0 (14.0-24.0) | 19.0 (14.0-26.0) | 20.0 (15.0-28.0) | <0.001 |
| ALP, U/L | 61.0 (54.0-67.0) | 79.0 (75.0-83.0) | 95.0 (91.0-100.0) | 121.0 (112.0-135.0) | <0.001 |
Note: Normally distributed variables were presented as mean (SD), non-normally distributed variables were presented as median (IQR) and categorical variables were presented as a number (%). Abbreviations: ALP, alkaline phosphatase; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; CVD, cardiovascular disease; WBC, white blood cell; eGFR, estimated glomerular filtration rate; ALT, alanine aminotransferase.
*The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-100 U/L), and Q4 (>100 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-87 U/L), Q3 (87-105 U/L), and Q4 (>105 U/L), respectively.
†Data were incomplete for these variables. 159 (1.4%), 65 (0.6%), 106 (0.9%), 143 (1.3%), 139 (1.2%), 369 (3.2%), 15 (0.1%), 15 (0.1%), 14 (0.1%), 31 (0.3%), 18 (0.2%), 22 (0.2%), 809 (7.1%), and 100 (0.9%) of men had missing data for BMI, education, fasting blood glucose, SBP, DBP, total cholesterol, triglyceride, HDL-C, LDL-C, smoking status, drinking status, eGFR, WBC, and ALT, respectively. 206 (1.4%), 110 (0.7%), 146 (1.0%), 186 (1.2%), 185 (1.2%), 465 (3.1%), 23 (0.2%), 16 (0.1%), 20 (0.1%), 97 (0.6%), 25 (0.2%), 29 (0.2%), 1058 (7.1%), and 136 (0.9%) of women had missing data for BMI, education, fasting blood glucose, SBP, DBP, total cholesterol, triglyceride, HDL-C, LDL-C, smoking status, drinking status, eGFR, WBC, and ALT, respectively.
Supplementary Fig.2. Distributions of baseline serum ALP levels in men and women.
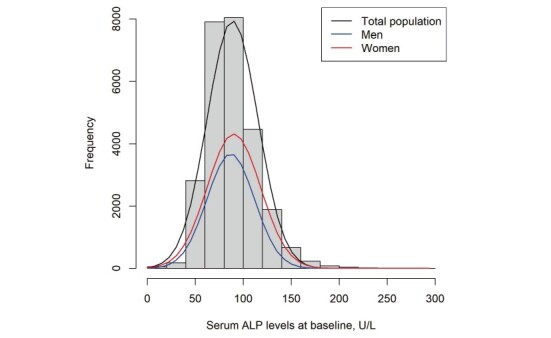
Abbreviations: ALP, alkaline phosphatase.
Association between Serum ALP and Incident CVD
After an average follow-up of 7.3 years, among 11,408 men and 14,981 women with serum ALP measurements, a total of 7,015 participants developed incident CVD (3,520 men [incidence rate: 44.0 per 1,000 person-years] and 3,495 women [incidence rate: 30.9 per 1,000 person-years]), including 5,561 CHD (2,607 men [incidence rate: 31.5 per 1,000 person-years] and 2,954 women [incidence rate: 25.7 per 1,000 person-years]) and 1,454 stroke (913 men [incidence rate: 10.3 per 1,000 person-years] and 541 women [incidence rate: 4.4 per 1,000 person-years]) cases ( Supplementary Fig.1 ) . In model 3 ( Table 2 ) , after adjustment for potential covariates, the ALP levels were positively associated with incident CVD (hazard ratio [HR] 1.26, 95% confidence interval [CI] 1.12–1.42), CHD (HR 1.13, 95% CI 0.99–1.29), and stroke (HR 1.68, 95% CI 1.33–2.13) per unit increased natural log-transformed ALP levels in men ( Table 2 ) . Compared to the lowest quartile of serum ALP, the participants in the highest quartile had HRs (95% CIs) of 1.22 (1.11–1.34) for CVD, 1.14 (1.02–1.28) for CHD, and 1.43 (1.18–1.73) for stroke. Specially, comparing the extreme quartiles, the HRs (95% CIs) were 1.31 (1.09–1.57) for incident ACS, 1.37 (1.11–1.70) for ischemic stroke, and 1.75 (1.10–2.79) for hemorrhagic stroke in men ( Table 2 ) . In contrast, in women, compared to the lowest quartile of serum ALP, the participants in the highest quartile had a 1.12 (1.01–1.23) higher risk for CVD, 1.10 (0.99–1.23) higher risk for CHD, and 1.23 (1.03–1.47) higher risk for ACS. No statistical significance was observed between serum ALP with incident stroke, in which the HRs (95% CIs) were 1.18 (0.92–1.51) for incident stroke, 1.10 (0.83–1.45) for ischemic stroke, and 1.54 (0.90–2.65) for hemorrhagic stroke when comparing the extreme quartiles. When restricting the analysis to participants within the normal range (40–150 U/L) of ALP levels, similar results were observed ( Supplementary Table 2 ) .
Table 2. Adjusted hazard ratios for incident CVD, CHD, stroke, and their subtypes according to serum ALP levels in men and women.
| Outcomes† | Quartiles of ALP levels*, U/L | p for trend§ | Natural log- transformed continuous | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Men | ||||||
| CVD | ||||||
| No. of cases/person years | 746/19,226 | 857/20,390 | 997/21,269 | 920/19,110 | ||
| Model 1 | 1.00 (Ref) | 1.08 (0.98-1.19) | 1.22 (1.11-1.34) | 1.27 (1.15-1.40) | <0.001 | 1.34 (1.20-1.51) |
| Model 2 | 1.00 (Ref) | 1.09 (0.98-1.20) | 1.21 (1.10-1.33) | 1.23 (1.11-1.35) | <0.001 | 1.27 (1.13-1.43) |
| Model 3 | 1.00 (Ref) | 1.08 (0.98-1.19) | 1.20 (1.09-1.32) | 1.22 (1.11-1.34) | <0.001 | 1.26 (1.12-1.42) |
| CHD | ||||||
| No. of cases/person years | 567/19,877 | 638/21,138 | 751/21,921 | 651/19,908 | ||
| Model 1 | 1.00 (Ref) | 1.05 (0.94-1.18) | 1.22 (1.09-1.36) | 1.18 (1.05-1.32) | <0.001 | 1.19 (1.04-1.36) |
| Model 2 | 1.00 (Ref) | 1.06 (0.95-1.19) | 1.20 (1.08-1.34) | 1.15 (1.02-1.29) | 0.007 | 1.14 (1.00-1.30) |
| Model 3 | 1.00 (Ref) | 1.06 (0.94-1.18) | 1.20 (1.07-1.34) | 1.14 (1.02-1.28) | 0.01 | 1.13 (0.99-1.29) |
| ACS | ||||||
| No. of cases/person years | 203/18,085 | 290/19,418 | 348/19,976 | 274/17,993 | ||
| Model 1 | 1.00 (Ref) | 1.33 (1.11-1.59) | 1.58 (1.33-1.88) | 1.39 (1.16-1.66) | <0.001 | 1.43 (1.15-1.76) |
| Model 2 | 1.00 (Ref) | 1.33 (1.11-1.59) | 1.54 (1.29-1.83) | 1.33 (1.11-1.59) | 0.004 | 1.33 (1.08-1.64) |
| Model 3 | 1.00 (Ref) | 1.32 (1.10-1.58) | 1.53 (1.28-1.82) | 1.31 (1.09-1.57) | 0.007 | 1.31 (1.06-1.62) |
| Stroke | ||||||
| No. of cases/person years | 179/21,130 | 219/22,498 | 246/23,692 | 269/21,221 | ||
| Model 1 | 1.00 (Ref) | 1.15 (0.94-1.40) | 1.24 (1.02-1.50) | 1.53 (1.26-1.84) | <0.001 | 1.84 (1.45-2.32) |
| Model 2 | 1.00 (Ref) | 1.15 (0.94-1.40) | 1.21 (0.99-1.46) | 1.44 (1.19-1.75) | <0.001 | 1.70 (1.34-2.15) |
| Model 3 | 1.00 (Ref) | 1.14 (0.94-1.39) | 1.20 (0.99-1.45) | 1.43 (1.18-1.73) | <0.001 | 1.68 (1.33-2.13) |
| Ischemic stroke | ||||||
| No. of cases/person years | 151/20,990 | 173/22,303 | 197/23,449 | 217/21,011 | ||
| Model 1 | 1.00 (Ref) | 1.08 (0.86-1.34) | 1.18 (0.96-1.46) | 1.46 (1.19-1.80) | <0.001 | 1.75 (1.35-2.28) |
| Model 2 | 1.00 (Ref) | 1.09 (0.87-1.35) | 1.15 (0.93-1.43) | 1.39 (1.12-1.71) | 0.001 | 1.62 (1.25-2.11) |
| Model 3 | 1.00 (Ref) | 1.08 (0.87-1.34) | 1.15 (0.93-1.42) | 1.37 (1.11-1.70) | 0.002 | 1.61 (1.24-2.09) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 28/20,359 | 46/21,700 | 49/22,755 | 52/20,248 | ||
| Model 1 | 1.00 (Ref) | 1.54 (0.96-2.46) | 1.58 (0.99-2.52) | 1.91 (1.21-3.02) | 0.009 | 2.27 (1.33-3.90) |
| Model 2 | 1.00 (Ref) | 1.50 (0.94-2.41) | 1.52 (0.95-2.42) | 1.77 (1.11-2.81) | 0.03 | 2.09 (1.22-3.58) |
| Model 3 | 1.00 (Ref) | 1.49 (0.93-2.39) | 1.51 (0.94-2.40) | 1.75 (1.10-2.79) | 0.03 | 2.06 (1.20-3.55) |
| Women | ||||||
| CVD | ||||||
| No. of cases/person years | 694/27,881 | 772/27,868 | 974/28,805 | 1055/28,401 | ||
| Model 1 | 1.00 (Ref) | 0.97 (0.88-1.08) | 1.12 (1.01-1.24) | 1.18 (1.07-1.30) | <0.001 | 1.19 (1.06-1.32) |
| Model 2 | 1.00 (Ref) | 0.96 (0.87-1.07) | 1.11 (1.00-1.22) | 1.12 (1.01-1.23) | 0.003 | 1.11 (1.00-1.24) |
| Model 3 | 1.00 (Ref) | 0.96 (0.87-1.07) | 1.11 (1.00-1.22) | 1.12 (1.01-1.23) | 0.003 | 1.11 (1.00-1.24) |
| CHD | ||||||
| No. of cases/person years | 592/28,243 | 661/28,245 | 821/29,308 | 880/28,951 | ||
| Model 1 | 1.00 (Ref) | 0.98 (0.88-1.10) | 1.11 (1.00-1.23) | 1.16 (1.04-1.29) | <0.001 | 1.15 (1.02-1.29) |
| Model 2 | 1.00 (Ref) | 0.97 (0.87-1.08) | 1.10 (0.99-1.22) | 1.10 (0.99-1.23) | 0.01 | 1.09 (0.97-1.22) |
| Model 3 | 1.00 (Ref) | 0.97 (0.87-1.08) | 1.10 (0.99-1.22) | 1.10 (0.99-1.23) | 0.02 | 1.08 (0.96-1.22) |
| ACS | ||||||
| No. of cases/person years | 197/26,220 | 250/25,953 | 292/26,413 | 344/25,925 | ||
| Model 1 | 1.00 (Ref) | 1.09 (0.90-1.31) | 1.17 (0.97-1.40) | 1.32 (1.11-1.58) | 0.001 | 1.28 (1.05-1.57) |
| Model 2 | 1.00 (Ref) | 1.06 (0.88-1.28) | 1.15 (0.95-1.37) | 1.24 (1.04-1.48) | 0.01 | 1.19 (0.98-1.45) |
| Model 3 | 1.00 (Ref) | 1.06 (0.88-1.28) | 1.14 (0.95-1.37) | 1.23 (1.03-1.47) | 0.01 | 1.18 (0.96-1.44) |
| Stroke | ||||||
| No. of cases/person years | 102/29,877 | 111/30,069 | 153/31,807 | 175/31,371 | ||
| Model 1 | 1.00 (Ref) | 0.95 (0.72-1.24) | 1.15 (0.89-1.48) | 1.27 (0.99-1.63) | 0.02 | 1.41 (1.06-1.88) |
| Model 2 | 1.00 (Ref) | 0.94 (0.72-1.23) | 1.13 (0.88-1.46) | 1.18 (0.92-1.51) | 0.07 | 1.29 (0.98-1.71) |
| Model 3 | 1.00 (Ref) | 0.94 (0.72-1.23) | 1.13 (0.88-1.45) | 1.18 (0.92-1.51) | 0.08 | 1.29 (0.97-1.71) |
| Ischemic stroke | ||||||
| No. of cases/person years | 82/29,788 | 82/29,929 | 123/31,639 | 133/31,178 | ||
| Model 1 | 1.00 (Ref) | 0.86 (0.63-1.17) | 1.13 (0.85-1.49) | 1.17 (0.89-1.55) | 0.08 | 1.34 (0.97-1.85) |
| Model 2 | 1.00 (Ref) | 0.85 (0.63-1.16) | 1.11 (0.84-1.48) | 1.10 (0.83-1.45) | 0.21 | 1.24 (0.91-1.71) |
| Model 3 | 1.00 (Ref) | 0.85 (0.63-1.16) | 1.11 (0.84-1.47) | 1.10 (0.83-1.45) | 0.22 | 1.24 (0.90-1.70) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 20/29,485 | 29/29,650 | 30/31,247 | 42/30,721 | ||
| Model 1 | 1.00 (Ref) | 1.33 (0.75-2.36) | 1.24 (0.70-2.20) | 1.70 (0.99-2.93) | 0.06 | 1.71 (0.93-3.16) |
| Model 2 | 1.00 (Ref) | 1.32 (0.74-2.34) | 1.20 (0.68-2.12) | 1.54 (0.89-2.64) | 0.15 | 1.50 (0.82-2.74) |
| Model 3 | 1.00 (Ref) | 1.32 (0.74-2.33) | 1.20 (0.68-2.13) | 1.54 (0.90-2.65) | 0.14 | 1.51 (0.82-2.76) |
*The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-100 U/L), and Q4 (>100 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-87 U/L), Q3 (87-105 U/L), and Q4 (>105 U/L), respectively.
†Model 1 were adjusted for age and admission batch. Model 2 were additionally adjusted for BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), family history of CVD, and eGFR. Model 3 were further adjusted for WBC.
§p for trend was obtained by assigning the median value to each group and used this as a continuous variable in Cox regression models.
Abbreviations: ACS, acute coronary syndrome; ALP, alkaline phosphatase; BMI, body mass index; CHD, coronary heart disease; CI, coefficient interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; WBC, white blood cell.
Supplementary Table 2. Adjusted hazard ratios for incident CVD, CHD, stroke, and their subtypes according to serum ALP levels within normal range (40-150 U/L) in men and women (n = 25,545) .
| Outcomes† | Quartiles of ALP levels*, U/L | p for trend§ | Natural log- transformed continuous | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Men | ||||||
| CVD | ||||||
| No. of cases/person years | 732/18,763 | 857/20,390 | 946/20,118 | 899/18,826 | ||
| Model 1 | 1.00 (Ref) | 1.08 (0.97-1.19) | 1.23 (1.11-1.35) | 1.25 (1.14-1.38) | <0.001 | 1.37 (1.20-1.56) |
| Model 2 | 1.00 (Ref) | 1.08 (0.98-1.19) | 1.20 (1.09-1.32) | 1.21 (1.10-1.34) | <0.001 | 1.29 (1.13-1.47) |
| Model 3 | 1.00 (Ref) | 1.08 (0.98-1.19) | 1.20 (1.09-1.32) | 1.20 (1.09-1.33) | <0.001 | 1.28 (1.12-1.46) |
| CHD | ||||||
| No. of cases/person years | 556/19,406 | 638/21,138 | 714/20,733 | 641/19,586 | ||
| Model 1 | 1.00 (Ref) | 1.05 (0.94-1.18) | 1.23 (1.10-1.37) | 1.17 (1.05-1.31) | 0.001 | 1.23 (1.05-1.43) |
| Model 2 | 1.00 (Ref) | 1.06 (0.94-1.18) | 1.21 (1.08-1.35) | 1.14 (1.02-1.28) | 0.007 | 1.16 (1.00-1.36) |
| Model 3 | 1.00 (Ref) | 1.05 (0.94-1.18) | 1.20 (1.08-1.34) | 1.13 (1.01-1.27) | 0.01 | 1.15 (0.99-1.35) |
| ACS | ||||||
| No. of cases/person years | 201/17,646 | 290/19,418 | 330/18,898 | 276/17,726 | ||
| Model 1 | 1.00 (Ref) | 1.31 (1.10-1.57) | 1.58 (1.33-1.88) | 1.41 (1.17-1.69) | <0.001 | 1.52 (1.20-1.92) |
| Model 2 | 1.00 (Ref) | 1.32 (1.10-1.58) | 1.52 (1.28-1.82) | 1.34 (1.12-1.61) | 0.002 | 1.39 (1.10-1.76) |
| Model 3 | 1.00 (Ref) | 1.31 (1.09-1.57) | 1.51 (1.27-1.81) | 1.32 (1.10-1.59) | 0.003 | 1.37 (1.08-1.73) |
| Stroke | ||||||
| No. of cases/person years | 176/20,626 | 219/22,498 | 232/22,419 | 258/20,955 | ||
| Model 1 | 1.00 (Ref) | 1.14 (0.94-1.40) | 1.23 (1.01-1.50) | 1.48 (1.22-1.79) | <0.001 | 1.81 (1.39-2.35) |
| Model 2 | 1.00 (Ref) | 1.14 (0.94-1.39) | 1.19 (0.98-1.45) | 1.40 (1.16-1.70) | <0.001 | 1.67 (1.29-2.18) |
| Model 3 | 1.00 (Ref) | 1.14 (0.93-1.39) | 1.19 (0.98-1.45) | 1.39 (1.14-1.69) | <0.001 | 1.65 (1.27-2.15) |
| Ischemic stroke | ||||||
| No. of cases/person years | 148/20,486 | 173/22,303 | 186/22,188 | 208/20,740 | ||
| Model 1 | 1.00 (Ref) | 1.08 (0.86-1.34) | 1.18 (0.95-1.46) | 1.42 (1.15-1.76) | <0.001 | 1.75 (1.31-2.34) |
| Model 2 | 1.00 (Ref) | 1.08 (0.87-1.35) | 1.15 (0.93-1.43) | 1.35 (1.09-1.67) | 0.004 | 1.62 (1.21-2.16) |
| Model 3 | 1.00 (Ref) | 1.08 (0.87-1.34) | 1.14 (0.92-1.42) | 1.34 (1.08-1.66) | 0.005 | 1.60 (1.19-2.14) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 28/19,863 | 46/21,700 | 46/21,539 | 50/20,001 | ||
| Model 1 | 1.00 (Ref) | 1.50 (0.94-2.40) | 1.53 (0.96-2.45) | 1.82 (1.15-2.89) | 0.02 | 2.16 (1.18-3.94) |
| Model 2 | 1.00 (Ref) | 1.47 (0.92-2.35) | 1.46 (0.91-2.33) | 1.70 (1.07-2.71) | 0.04 | 1.97 (1.08-3.61) |
| Model 3 | 1.00 (Ref) | 1.46 (0.91-2.33) | 1.44 (0.90-2.32) | 1.68 (1.05-2.68) | 0.049 | 1.94 (1.06-3.56) |
| Women | ||||||
| CVD | ||||||
| No. of cases/person years | 677/26,998 | 716/26,008 | 997/29,329 | 970/26,437 | ||
| Model 1 | 1.00 (Ref) | 0.96 (0.86-1.06) | 1.12 (1.02-1.24) | 1.17 (1.06-1.29) | <0.001 | 1.29 (1.13-1.48) |
| Model 2 | 1.00 (Ref) | 0.95 (0.85-1.05) | 1.12 (1.01-1.23) | 1.12 (1.01-1.23) | 0.002 | 1.22 (1.07-1.39) |
| Model 3 | 1.00 (Ref) | 0.95 (0.85-1.05) | 1.12 (1.01-1.23) | 1.11 (1.01-1.23) | 0.002 | 1.22 (1.07-1.39) |
| CHD | ||||||
| No. of cases/person years | 577/27,360 | 614/26,354 | 840/29,838 | 814/26,939 | ||
| Model 1 | 1.00 (Ref) | 0.97 (0.86-1.08) | 1.11 (1.00-1.24) | 1.15 (1.04-1.29) | <0.001 | 1.26 (1.09-1.45) |
| Model 2 | 1.00 (Ref) | 0.96 (0.85-1.07) | 1.11 (1.00-1.24) | 1.11 (1.00-1.24) | 0.009 | 1.20 (1.04-1.38) |
| Model 3 | 1.00 (Ref) | 0.96 (0.85-1.07) | 1.11 (1.00-1.23) | 1.11 (0.99-1.23) | 0.009 | 1.08 (0.96-1.22) |
| ACS | ||||||
| No. of cases/person years | 192/25,382 | 231/24,250 | 299/26,840 | 317/24,168 | ||
| Model 1 | 1.00 (Ref) | 1.07 (0.88-1.30) | 1.18 (0.98-1.42) | 1.31 (1.09-1.57) | 0.001 | 1.39 (1.10-1.77) |
| Model 2 | 1.00 (Ref) | 1.04 (0.86-1.26) | 1.17 (0.97-1.40) | 1.24 (1.04-1.49) | 0.008 | 1.31 (1.03-1.66) |
| Model 3 | 1.00 (Ref) | 1.04 (0.86-1.26) | 1.16 (0.97-1.40) | 1.23 (1.03-1.48) | 0.01 | 1.29 (1.01-1.63) |
| Stroke | ||||||
| No. of cases/person years | 100/28,946 | 102/28,064 | 157/32,377 | 156/29,220 | ||
| Model 1 | 1.00 (Ref) | 0.93 (0.70-1.23) | 1.16 (0.90-1.50) | 1.23 (0.95-1.58) | 0.03 | 1.47 (1.04-2.06) |
| Model 2 | 1.00 (Ref) | 0.92 (0.69-1.21) | 1.13 (0.88-1.46) | 1.14 (0.89-1.47) | 0.13 | 1.33 (0.95-1.86) |
| Model 3 | 1.00 (Ref) | 0.92 (0.69-1.21) | 1.13 (0.88-1.46) | 1.14 (0.88-1.47) | 0.13 | 1.32 (0.94-1.85) |
| Ischemic stroke | ||||||
| No. of cases/person years | 80/28,857 | 75/27,936 | 125/32,197 | 121/29,062 | ||
| Model 1 | 1.00 (Ref) | 0.84 (0.61-1.15) | 1.13 (0.85-1.50) | 1.16 (0.87-1.54) | 0.09 | 1.44 (0.98-2.12) |
| Model 2 | 1.00 (Ref) | 0.83 (0.61-1.14) | 1.12 (0.84-1.48) | 1.09 (0.82-1.45) | 0.21 | 1.33 (0.90-1.94) |
| Model 3 | 1.00 (Ref) | 0.83 (0.61-1.14) | 1.11 (0.84-1.48) | 1.09 (0.82-1.45) | 0.23 | 1.32 (0.90-1.93) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 20/28,563 | 27/27,684 | 32/31,801 | 35/28,626 | ||
| Model 1 | 1.00 (Ref) | 1.32 (0.73-2.38) | 1.30 (0.73-2.30) | 1.53 (0.87-2.69) | 0.17 | 1.58 (0.76-3.25) |
| Model 2 | 1.00 (Ref) | 1.28 (0.71-2.29) | 1.22 (0.69-2.14) | 1.35 (0.77-2.37) | 0.36 | 1.33 (0.65-2.71) |
| Model 3 | 1.00 (Ref) | 1.28 (0.71-2.28) | 1.22 (0.69-2.15) | 1.36 (0.78-2.38) | 0.35 | 1.34 (0.65-2.74) |
*The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-99 U/L), and Q4 (>99 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-86 U/L), Q3 (86-104 U/L), and Q4 (>104 U/L), respectively.
†Model 1 were adjusted for age and admission batch. Model 2 were additionally adjusted for BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), family history of CVD, and eGFR. Model 3 were further adjusted for WBC.
§p for trend was obtained by assigning the median value to each group and used this as a continuous variable in Cox regression models.
Abbreviations: ACS, acute coronary syndrome; ALP, alkaline phosphatase; BMI, body mass index; CHD, coronary heart disease; CI, coefficient interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation, WBC, white blood cell.
The relationship between ALP levels and major cardiovascular risk factors is summarized in Supplementary Table 3 . In both men and women, current smoking and higher levels of systolic blood pressure, diastolic blood pressure, glucose, triglyceride, low-density lipoprotein cholesterol, WBC, and ALT were significantly associated with higher ALP levels, whereas current drinking was inversely associated with ALP levels in both men and women (p<0.05). Advanced age, higher levels of BMI, and postmenopausal status were also associated with higher ALP in women, whereas higher levels of BMI were associated with lower ALP in men ( Supplementary Table 3 ) .
Supplementary Table 3. Associations between serum ALP levels and major cardiovascular risk factors in men and women.
| Variables | Serum ALP levels (U/L) | Serum ALP levels in the normal range (U/L) | ||||
|---|---|---|---|---|---|---|
| β* | SE | p value | β* | SE | p value | |
| Men | ||||||
| Age, years | -0.001 | 0.037 | 0.99 | 0.005 | 0.033 | 0.87 |
| BMI, kg/m2 | -0.224 | 0.074 | 0.003 | -0.111 | 0.066 | 0.09 |
| SBP†, mmHg | 0.476 | 0.167 | 0.004 | 0.261 | 0.148 | 0.08 |
| DBP†, mmHg | 0.634 | 0.282 | 0.02 | 0.757 | 0.249 | 0.002 |
| Fasting blood glucose§, mmol/L | 10.288 | 1.136 | <0.001 | 7.292 | 1.018 | <0.001 |
| Total cholesterol§, mmol/L | -9.234 | 1.710 | <0.001 | -7.754 | 1.531 | <0.001 |
| Total glyceride§, mmol/L | 5.680 | 0.503 | <0.001 | 4.615 | 0.447 | <0.001 |
| HDL-C§, mmol/L | -2.335 | 1.021 | 0.02 | -2.430 | 0.906 | 0.007 |
| LDL-C§, mmol/L | 3.375 | 1.022 | 0.001 | 2.690 | 0.914 | 0.003 |
| Current smoking | 5.738 | 0.554 | <0.001 | 4.974 | 0.491 | <0.001 |
| Current drinking | -4.498 | 0.510 | <0.001 | -4.089 | 0.451 | <0.001 |
| Physical activity | 0.202 | 0.526 | 0.70 | 0.134 | 0.465 | 0.77 |
| Family history of CVD, % | -0.551 | 0.846 | 0.51 | -0.049 | 0.750 | 0.95 |
| Anticoagulants use, No. (%) | -0.324 | 1.968 | 0.87 | 0.165 | 1.726 | 0.92 |
| Aspirin use, No. (%) | -0.893 | 0.780 | 0.25 | -0.671 | 0.688 | 0.33 |
| eGFR†, mL/min×1.73 m2 | 0.082 | 0.079 | 0.30 | 0.179 | 0.070 | 0.01 |
| WBC, 109/L | 1.596 | 0.148 | <0.001 | 1.442 | 1.301 | 0.01 |
| ALT, U/L | 0.275 | 0.022 | <0.001 | 0.209 | 0.019 | <0.001 |
| Women | ||||||
| Age, years | 0.551 | 0.030 | <0.001 | 0.462 | 0.026 | <0.001 |
| BMI, kg/m2 | 0.355 | 0.067 | <0.001 | 0.331 | 0.057 | <0.001 |
| SBP†, mmHg | 0.750 | 0.163 | <0.001 | 0.574 | 0.140 | <0.001 |
| DBP†, mmHg | 0.633 | 0.280 | 0.02 | 0.670 | 0.241 | 0.006 |
| Fasting blood glucose§, mmol/L | 15.370 | 1.261 | <0.001 | 11.769 | 1.097 | <0.001 |
| Total cholesterol§, mmol/L | -8.376 | 1.706 | <0.001 | -7.731 | 1.488 | <0.001 |
| Total glyceride§, mmol/L | 7.167 | 0.499 | <0.001 | 6.108 | 0.431 | <0.001 |
| HDL-C§, mmol/L | 2.225 | 0.998 | 0.03 | 2.119 | 0.862 | 0.01 |
| LDL-C§, mmol/L | 6.058 | 1.080 | <0.001 | 6.348 | 0.938 | <0.001 |
| Current smoking | 4.238 | 1.619 | 0.009 | 4.396 | 1.389 | 0.002 |
| Current drinking | -3.352 | 0.790 | <0.001 | -2.947 | 0.676 | <0.001 |
| Physical activity | 0.442 | 0.486 | 0.36 | 0.606 | 0.417 | 0.15 |
| Family history of CVD, % | -1.180 | 0.660 | 0.07 | -0.579 | 0.564 | 0.30 |
| Anticoagulants use, No. (%) | -0.379 | 1.950 | 0.85 | -1.220 | 1.671 | 0.47 |
| Aspirin use, No. (%) | -1.514 | 0.809 | 0.06 | -1.005 | 0.695 | 0.15 |
| Post-menopausal status, No. (%) | 13.208 | 0.653 | <0.001 | 11.948 | 0.558 | <0.001 |
| eGFR†, mL/min×1.73 m2 | -0.041 | 0.011 | <0.001 | -0.317 | 0.093 | <0.001 |
| WBC, 109/L | 1.374 | 0.156 | <0.001 | 1.061 | 0.133 | <0.001 |
| ALT, U/L | 0.376 | 0.021 | <0.001 | 0.280 | 0.018 | <0.001 |
*The analysis was conducted in participants without missing value. General linear regression was performed with serum ALP levels as dependent variables and adjusted for age, BMI, smoking status, drinking status, physical activity and eGFR. Anti-hypertensive medication, antidiabetic medication and lipid-lowering medication were further adjusted for the association between ALP levels and blood pressure, glucose, and lipids, respectively.
†Per 10 mmHg higher SBP and DBP.
§Glucose, TG, HDL-C, and LDL-C were natural log-transformed before analysis.
Abbreviations: ALP, alkaline phosphatase; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure; SE, standard error; TG, triglyceride.
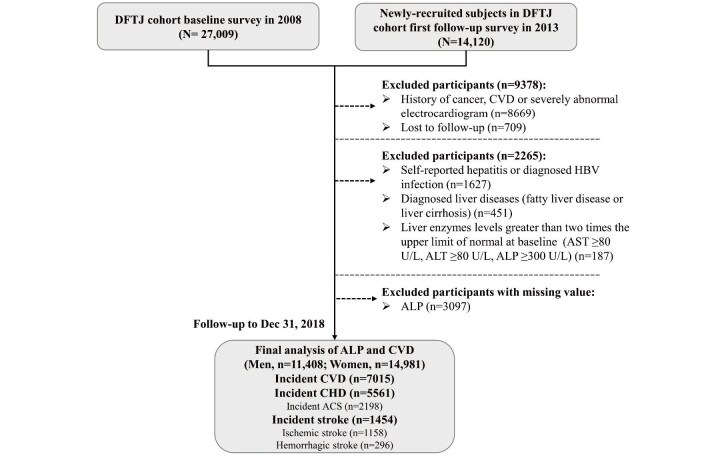
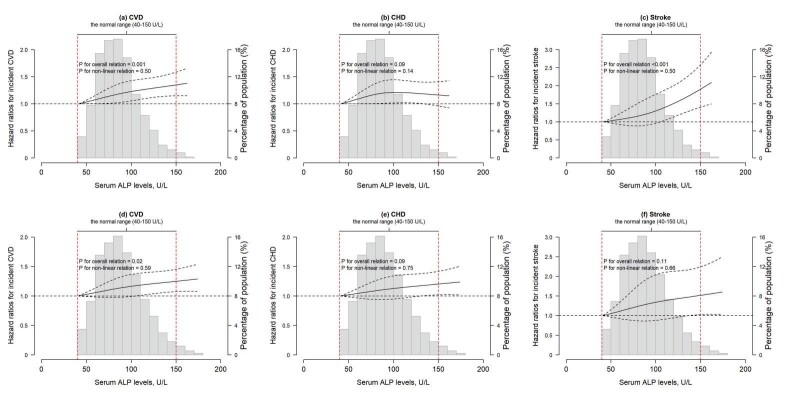
Restricted cubic spline plots revealed positive linear relationships of serum ALP with incident CVD in men and women ( Fig.1 ) (both plinear <0.05). In addition, a positive linear relationship of serum ALP with incident stroke was observed in men ( plinear <0.05). Results of subgroup analyses are presented in Fig.2 . All subgroup analyses generated consistent results (p>0.05 for all interaction).
Fig.1. The restricted cubic splines for associations of serum ALP levels with incident CVD, CHD, and stroke in men and women.
The associations of serum ALP levels with incident CVD, CHD, and stroke among men (a, b, and c) and women (d, e, and f), with min value of ALP levels in each group as the reference. HRs were calculated by adjusting for age, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), family history of CVD, eGFR, WBC, and admission batch. In each figure, the black solid line represents the HRs, the gray dotted lines depict the 95% CIs, and the vertical dotted lines show the normal range of serum ALP levels (40–150 U/L).
Fig.2. Associations between serum ALP levels and incident CVD, CHD, and stroke in subgroups in men and women.
Stratified analysis for the association between serum ALP levels and incident CVD, CHD, and stroke among men (a, b, and c) and women (d, e, and f) with comparison of the extreme quartiles. All models were adjusted for age, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), family history of CVD, eGFR, WBC, and admission batch (except for the corresponding stratified variables). Interactions were tested by including a multiplicative interaction term in the model. Ever-smokers included current and former smokers, and ever-drinkers included current and former drinkers. The total number and the number of events for each stratification characteristic were slightly different because of missing values for BMI, smoking status, drinking status, and eGFR. The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69–84 U/L), Q3 (84–100 U/L), and Q4 (>100 U/L), whereas the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71–87 U/L), Q3 (87–105 U/L), and Q4 (>105 U/L), respectively.
For sensitivity analyses, the results did not change significantly when further adjusting ALT ( Supplementary Table 4 ) , self-reported arthritis ( Supplementary Table 5 ) , and dietary habits ( Supplementary Table 6 ) ; excluding participants with CKD ( Supplementary Tables 7 – 8 ) ; or including participants with liver disease ( Supplementary Table 9 ) .
Supplementary Table 4. Adjusted hazard ratios for incident CVD, CHD, stroke, and their subtypes according to serum ALP levels with further adjustment of ALT in men and women.
| Outcomes | Quartiles of ALP levels*, U/L | p for trend§ | Natural log- transformed continuous | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Men | ||||||
| CVD | ||||||
| No. of cases/person years | 746/19,226 | 857/20,390 | 997/21,269 | 920/19,110 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.08 (0.98-1.19) | 1.20 (1.09-1.32) | 1.22 (1.11-1.35) | <0.001 | 1.27 (1.13-1.43) |
| CHD | ||||||
| No. of cases/person years | 567/19,877 | 638/21,138 | 751/21,921 | 651/19,908 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.06 (0.94-1.18) | 1.20 (1.08-1.34) | 1.14 (1.02-1.28) | 0.009 | 1.13 (0.99-1.30) |
| ACS | ||||||
| No. of cases/person years | 203/18,085 | 290/19,418 | 348/19,976 | 274/17,993 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.32 (1.10-1.58) | 1.53 (1.28-1.82) | 1.31 (1.09-1.57) | 0.007 | 1.31 (1.06-1.62) |
| Stroke | ||||||
| No. of cases/person years | 179/21,130 | 219/22,498 | 246/23,692 | 269/21,221 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.14 (0.94-1.39) | 1.20 (0.99-1.46) | 1.44 (1.19-1.75) | <0.001 | 1.71 (1.34-2.17) |
| Ischemic stroke | ||||||
| No. of cases/person years | 151/20,990 | 173/22,303 | 197/23,449 | 217/21,011 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.08 (0.87-1.34) | 1.15 (0.93-1.42) | 1.37 (1.11-1.70) | 0.002 | 1.61 (1.23-2.09) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 28/20,359 | 46/21,700 | 49/22,755 | 52/20,248 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.51 (0.94-2.42) | 1.54 (0.97-2.46) | 1.85 (1.16-2.96) | 0.02 | 2.26 (1.30-3.93) |
| Women | ||||||
| CVD | ||||||
| No. of cases/person years | 694/27,881 | 772/27,868 | 974/28,805 | 1055/28,401 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.96 (0.87-1.07) | 1.11 (1.00-1.22) | 1.12 (1.02-1.24) | 0.002 | 1.12 (1.00-1.25) |
| CHD | ||||||
| No. of cases/person years | 592/28,243 | 661/28,245 | 821/29,308 | 880/28,951 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.97 (0.87-1.08) | 1.10 (0.99-1.22) | 1.11 (0.99-1.23) | 0.01 | 1.09 (0.97-1.23) |
| ACS | ||||||
| No. of cases/person years | 197/26,220 | 250/25,953 | 292/26,413 | 344/25,925 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.06 (0.88-1.28) | 1.13 (0.95-1.36) | 1.22 (1.02-1.46) | 0.02 | 1.16 (0.95-1.42) |
| Stroke | ||||||
| No. of cases/person years | 102/29,877 | 111/30,069 | 153/31,807 | 175/31,371 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.94 (0.72-1.23) | 1.13 (0.88-1.46) | 1.19 (0.92-1.52) | 0.07 | 1.30 (0.98-1.72) |
| Ischemic stroke | ||||||
| No. of cases/person years | 82/29,788 | 82/29,929 | 123/31,639 | 133/31,178 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.85 (0.63-1.16) | 1.11 (0.84-1.47) | 1.10 (0.83-1.45) | 0.23 | 1.23 (0.90-1.70) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 20/29,485 | 29/29,650 | 30/31,247 | 42/30,721 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.33 (0.75-2.35) | 1.22 (0.69-2.16) | 1.59 (0.92-2.74) | 0.12 | 1.57 (0.86-2.89) |
*The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-100 U/L), and Q4 (>100 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-87 U/L), Q3 (87-105 U/L), and Q4 (>105 U/L), respectively.
†Models were adjusted for age, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), eGFR, family history of CVD, WBC, admission batch, and ALT.
§p for trend was obtained by assigning the median value to each group and used this as a continuous variable in Cox regression models.
Abbreviations: ACS, acute coronary syndrome; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BMI, body mass index; CHD, coronary heart disease; CI, coefficient interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; WBC, white blood cell.
Supplementary Table 5. Adjusted hazard ratios for incident CVD, CHD, stroke, and their subtypes according to serum ALP levels with further adjustment of self-reported arthritis in men and women.
| Outcomes | Quartiles of ALP levels*, U/L | p for trend§ | Natural log- transformed continuous | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Men | ||||||
| CVD | ||||||
| No. of cases/person years | 746/19,226 | 857/20,390 | 997/21,269 | 920/19,110 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.08 (0.98-1.20) | 1.20 (1.09-1.32) | 1.22 (1.11-1.35) | <0.001 | 1.27 (1.12-1.42) |
| CHD | ||||||
| No. of cases/person years | 567/19,877 | 638/21,138 | 751/21,921 | 651/19,908 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.06 (0.94-1.18) | 1.20 (1.07-1.34) | 1.14 (1.02-1.28) | 0.01 | 1.13 (0.99-1.29) |
| ACS | ||||||
| No. of cases/person years | 203/18,085 | 290/19,418 | 348/19,976 | 274/17,993 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.32 (1.11-1.58) | 1.52 (1.28-1.81) | 1.31 (1.09-1.58) | 0.007 | 1.31 (1.06-1.62) |
| Stroke | ||||||
| No. of cases/person years | 179/21,130 | 219/22,498 | 246/23,692 | 269/21,221 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.14 (0.94-1.39) | 1.20 (0.99-1.45) | 1.43 (1.18-1.73) | <0.001 | 1.68 (1.33-2.13) |
| Ischemic stroke | ||||||
| No. of cases/person years | 151/20,990 | 173/22,303 | 197/23,449 | 217/21,011 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.08 (0.87-1.34) | 1.15 (0.93-1.42) | 1.37 (1.11-1.70) | 0.002 | 1.61 (1.24-2.09) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 28/20,359 | 46/21,700 | 49/22,755 | 52/20,248 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.49 (0.93-2.39) | 1.51 (0.95-2.41) | 1.75 (1.10-2.79) | 0.03 | 2.06 (1.20-3.55) |
| Women | ||||||
| CVD | ||||||
| No. of cases/person years | 694/27,881 | 772/27,868 | 974/28,805 | 1055/28,401 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.96 (0.87-1.07) | 1.10 (1.00-1.22) | 1.12 (1.01-1.23) | 0.003 | 1.11 (1.00-1.24) |
| CHD | ||||||
| No. of cases/person years | 592/28,243 | 661/28,245 | 821/29,308 | 880/28,951 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.97 (0.87-1.08) | 1.09 (0.98-1.22) | 1.10 (0.99-1.23) | 0.02 | 1.08 (0.96-1.22) |
| ACS | ||||||
| No. of cases/person years | 197/26,220 | 250/25,953 | 292/26,413 | 344/25,925 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.07 (0.88-1.29) | 1.14 (0.95-1.36) | 1.23 (1.03-1.47) | 0.02 | 1.17 (0.96-1.43) |
| Stroke | ||||||
| No. of cases/person years | 102/29,877 | 111/30,069 | 153/31,807 | 175/31,371 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.94 (0.72-1.23) | 1.12 (0.87-1.45) | 1.18 (0.92-1.51) | 0.08 | 1.29 (0.97-1.71) |
| Ischemic stroke | ||||||
| No. of cases/person years | 82/29,788 | 82/29,929 | 123/31,639 | 133/31,178 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.85 (0.63-1.16) | 1.11 (0.83-1.47) | 1.10 (0.83-1.45) | 0.22 | 1.23 (0.90-1.70) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 20/29,485 | 29/29,650 | 30/31,247 | 42/30,721 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.32 (0.74-2.34) | 1.19 (0.67-2.12) | 1.54 (0.90-2.65) | 0.14 | 1.50 (0.82-2.75) |
*The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-100 U/L), and Q4 (>100 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-87 U/L), Q3 (87-105 U/L), and Q4 (>105 U/L), respectively.
†Models were adjusted for age, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), eGFR, family history of CVD, WBC, admission batch, and self-reported arthritis.
§p for trend was obtained by assigning the median value to each group and used this as a continuous variable in Cox regression models.
Abbreviations: ACS, acute coronary syndrome; ALP, alkaline phosphatase; BMI, body mass index; CHD, coronary heart disease; CI, coefficient interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; WBC, white blood cell.
Supplementary Table 6. Adjusted hazard ratios for incident CVD, CHD, stroke, and their subtypes according to serum ALP levels with further adjustment of dietary habits in men and women.
| Outcomes | Quartiles of ALP levels*, U/L | p for trend§ | Natural log- transformed continuous | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Men | ||||||
| CVD | ||||||
| No. of cases/person years | 746/19,226 | 857/20,390 | 997/21,269 | 920/19,110 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.08 (0.98-1.19) | 1.19 (1.09-1.31) | 1.22 (1.10-1.34) | <0.001 | 1.26 (1.12-1.42) |
| CHD | ||||||
| No. of cases/person years | 567/19,877 | 638/21,138 | 751/21,921 | 651/19,908 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.06 (0.94-1.18) | 1.20 (1.07-1.34) | 1.14 (1.01-1.28) | 0.01 | 1.13 (0.99-1.29) |
| ACS | ||||||
| No. of cases/person years | 203/18,085 | 290/19,418 | 348/19,976 | 274/17,993 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.32 (1.10-1.58) | 1.52 (1.28-1.81) | 1.30 (1.08-1.57) | 0.009 | 1.30 (1.05-1.61) |
| Stroke | ||||||
| No. of cases/person years | 179/21,130 | 219/22,498 | 246/23,692 | 269/21,221 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.14 (0.94-1.39) | 1.20 (0.99-1.45) | 1.43 (1.18-1.74) | <0.001 | 1.68 (1.33-2.14) |
| Ischemic stroke | ||||||
| No. of cases/person years | 151/20,990 | 173/22,303 | 197/23,449 | 217/21,011 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.08 (0.86-1.34) | 1.14 (0.92-1.41) | 1.36 (1.10-1.68) | 0.002 | 1.59 (1.22-2.06) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 28/20,359 | 46/21,700 | 49/22,755 | 52/20,248 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.51 (0.94-2.42) | 1.54 (0.97-2.46) | 1.85 (1.16-2.95) | 0.02 | 2.24 (1.29-3.88) |
| Women | ||||||
| CVD | ||||||
| No. of cases/person years | 694/27,881 | 772/27,868 | 974/28,805 | 1055/28,401 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.96 (0.87-1.07) | 1.11 (1.00-1.22) | 1.12 (1.02-1.24) | 0.002 | 1.12 (1.00-1.25) |
| CHD | ||||||
| No. of cases/person years | 592/28,243 | 661/28,245 | 821/29,308 | 880/28,951 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.97 (0.87-1.09) | 1.10 (0.99-1.23) | 1.11 (1.00-1.23) | 0.01 | 1.09 (0.97-1.23) |
| ACS | ||||||
| No. of cases/person years | 197/26,220 | 250/25,953 | 292/26,413 | 344/25,925 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.06 (0.88-1.28) | 1.14 (0.95-1.36) | 1.22 (1.02-1.46) | 0.02 | 1.16 (0.95-1.42) |
| Stroke | ||||||
| No. of cases/person years | 102/29,877 | 111/30,069 | 153/31,807 | 175/31,371 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.94 (0.71-1.23) | 1.13 (0.87-1.45) | 1.18 (0.92-1.52) | 0.07 | 1.30 (0.98-1.72) |
| Ischemic stroke | ||||||
| No. of cases/person years | 82/29,788 | 82/29,929 | 123/31,639 | 133/31,178 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.85 (0.63-1.16) | 1.11 (0.84-1.47) | 1.10 (0.83-1.45) | 0.22 | 1.24 (0.90-1.70) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 20/29,485 | 29/29,650 | 30/31,247 | 42/30,721 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.31 (0.74-2.32) | 1.19 (0.67-2.12) | 1.55 (0.90-2.67) | 0.14 | 1.52 (0.83-2.76) |
*The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-100 U/L), and Q4 (>100 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-87 U/L), Q3 (87-105 U/L), and Q4 (>105 U/L), respectively.
†Models were adjusted for age, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), eGFR, family history of CVD, WBC, admission batch, and dietary habit (yes or no) of meat, fish or seafood, milk or dairy products, beans or soy foods, and fruits or vegetables. If the participant reported eating the food ≥ 5 times per week, the response would be “yes”.
§p for trend was obtained by assigning the median value to each group and used this as a continuous variable in Cox regression models.
Abbreviations: ACS, acute coronary syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BMI, body mass index; CHD, coronary heart disease; CI, coefficient interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; WBC, white blood cell.
Supplementary Table 7. Adjusted hazard ratios for incident CVD, CHD, stroke, and their subtypes according to serum ALP levels after excluding participants with CKD (eGFR <60 mL/min/1.73 m2) in men and women (n = 23,728) .
| Outcomes | Quartiles of ALP levels*, U/L | p for trend§ | Natural log- transformed continuous | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Men | ||||||
| CVD | ||||||
| No. of cases/person years | 641/17,598 | 744/18,371 | 863/19,300 | 794/17,106 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.11 (1.00-1.23) | 1.21 (1.10-1.35) | 1.24 (1.12-1.38) | <0.001 | 1.32 (1.16-1.50) |
| CHD | ||||||
| No. of cases/person years | 490/18,170 | 556/19,036 | 665/19,833 | 560/17,841 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.08 (0.95-1.22) | 1.24 (1.10-1.39) | 1.14 (1.01-1.29) | 0.01 | 1.18 (1.02-1.37) |
| ACS | ||||||
| No. of cases/person years | 172/16,572 | 259/17,519 | 305/18,088 | 234/16,141 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.41 (1.16-1.71) | 1.58 (1.31-1.91) | 1.32 (1.08-1.61) | 0.02 | 1.34 (1.06-1.68) |
| Stroke | ||||||
| No. of cases/person years | 151/19,208 | 188/20,175 | 198/21,465 | 234/18,896 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.18 (0.95-1.46) | 1.15 (0.93-1.42) | 1.51 (1.23-1.86) | <0.001 | 1.76 (1.36-2.29) |
| Ischemic stroke | ||||||
| No. of cases/person years | 128/19,099 | 150/20,002 | 159/21,272 | 196/18,730 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.12 (0.88-1.42) | 1.09 (0.86-1.38) | 1.49 (1.19-1.86) | <0.001 | 1.71 (1.28-2.28) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 23/18,529 | 38/19,479 | 39/20,706 | 38/18,024 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.55 (0.92-2.60) | 1.49 (0.89-2.50) | 1.67 (0.99-2.83) | 0.10 | 2.10 (1.13-3.92) |
| Women | ||||||
| CVD | ||||||
| No. of cases/person years | 605/25,959 | 663/25,513 | 833/25,905 | 899/25,558 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.96 (0.86-1.07) | 1.12 (1.00-1.24) | 1.12 (1.01-1.25) | 0.004 | 1.15 (1.02-1.30) |
| CHD | ||||||
| No. of cases/person years | 514/26,277 | 563/25,861 | 709/26,319 | 746/26,030 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.96 (0.85-1.09) | 1.13 (1.00-1.26) | 1.11 (0.99-1.25) | 0.01 | 1.12 (0.98-1.28) |
| ACS | ||||||
| No. of cases/person years | 167/24,508 | 206/23,852 | 241/23,706 | 293/23,408 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.05 (0.85-1.29) | 1.15 (0.94-1.41) | 1.26 (1.04-1.53) | 0.008 | 1.22 (0.98-1.52) |
| Stroke | ||||||
| No. of cases/person years | 91/27,684 | 100/27,400 | 124/28,462 | 153/28,074 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.96 (0.72-1.27) | 1.04 (0.79-1.37) | 1.16 (0.89-1.52) | 0.15 | 1.34 (0.99-1.83) |
| Ischemic stroke | ||||||
| No. of cases/person years | 73/27,601 | 74/27,276 | 98/28,314 | 118/27,909 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.87 (0.63-1.20) | 1.01 (0.74-1.37) | 1.09 (0.81-1.47) | 0.31 | 1.34 (0.94-1.89) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 18/27,333 | 26/27,025 | 26/27,995 | 35/27,485 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.32 (0.72-2.41) | 1.18 (0.64-2.18) | 1.46 (0.82-2.62) | 0.25 | 1.37 (0.71-2.63) |
*The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-100 U/L), and Q4 (>100 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-87 U/L), Q3 (87-105 U/L), and Q4 (>105 U/L), respectively.
†Models were adjusted for age, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), eGFR, family history of CVD, WBC, and admission batch.
§p for trend was obtained by assigning the median value to each group and used this as a continuous variable in Cox regression models.
Abbreviations: ACS, acute coronary syndrome; ALP, alkaline phosphatase; BMI, body mass index; CHD, coronary heart disease; CI, coefficient interval; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; WBC, white blood cell.
Supplementary Table 8. Adjusted hazard ratios for incident CVD, CHD, stroke, and their subtypes according to serum ALP levels after excluding participants with CKD (eGFR <60 mL/min/1.73 m2 or proteinuria) in men and women (n = 16,676)* .
| Outcomes | Quartiles of ALP levels, U/L† | p for trend‡ | Natural log- transformed continuous | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Men | ||||||
| CVD | ||||||
| No. of cases/person years | 386/11,381 | 421/11,563 | 520/12,287 | 469/10,313 | ||
| HR (95% CI)§ | 1.00 (Ref) | 1.08 (0.94-1.24) | 1.27 (1.11-1.45) | 1.35 (1.18-1.55) | <0.001 | 1.44 (1.21-1.71) |
| CHD | ||||||
| No. of cases/person years | 290/11,799 | 325/11,958 | 405/12,609 | 316/10,817 | ||
| HR (95% CI)§ | 1.00 (Ref) | 1.11 (0.94-1.30) | 1.32 (1.14-1.54) | 1.18 (1.00-1.38) | 0.02 | 1.20 (0.98-1.46) |
| ACS | ||||||
| No. of cases/person years | 112/10,655 | 167/10,949 | 211/11,442 | 154/9800 | ||
| HR (95% CI)§ | 1.00 (Ref) | 1.45 (1.14-1.84) | 1.74 (1.38-2.19) | 1.44 (1.13-1.85) | 0.006 | 1.48 (1.11-1.97) |
| Stroke | ||||||
| No. of cases/person years | 96/12,412 | 96/12,748 | 115/13,697 | 153/11,456 | ||
| HR (95% CI)§ | 1.00 (Ref) | 0.99 (0.75-1.32) | 1.12 (0.86-1.48) | 1.79 (1.38-2.32) | <0.001 | 2.29 (1.62-3.23) |
| Ischemic stroke | ||||||
| No. of cases/person years | 86/12,351 | 81/12,658 | 93/13,572 | 128/11,328 | ||
| HR (95% CI)§ | 1.00 (Ref) | 0.94 (0.70-1.28) | 1.02 (0.76-1.36) | 1.68 (1.27-2.22) | <0.001 | 2.05 (1.41-2.99) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 10/11,925 | 15/12,314 | 22/13,195 | 25/10,838 | ||
| HR (95% CI)§ | 1.00 (Ref) | 1.45 (0.65-3.23) | 2.09 (0.98-4.43) | 2.91 (1.38-6.11) | 0.002 | 4.36 (1.82-10.42) |
| Women | ||||||
| CVD | ||||||
| No. of cases/person years | 367/16,299 | 451/17,003 | 582/17,850 | 638/18,105 | ||
| HR (95% CI)§ | 1.00 (Ref) | 1.02 (0.88-1.17) | 1.20 (1.05-1.37) | 1.21 (1.06-1.37) | <0.001 | 1.24 (1.07-1.45) |
| CHD | ||||||
| No. of cases/person years | 302/16,539 | 377/17,276 | 491/18,141 | 525/18,484 | ||
| HR (95% CI)§ | 1.00 (Ref) | 1.03 (0.89-1.20) | 1.24 (1.07-1.43) | 1.21 (1.05-1.40) | 0.002 | 1.23 (1.04-1.45) |
| ACS | ||||||
| No. of cases/person years | 113/15,307 | 150/15,764 | 181/16,120 | 224/16,407 | ||
| HR (95% CI)§ | 1.00 (Ref) | 1.07 (0.84-1.37) | 1.24 (0.97-1.57) | 1.35 (1.07-1.70) | 0.004 | 1.35 (1.03-1.75) |
| Stroke | ||||||
| No. of cases/person years | 65/17,406 | 74/18,377 | 91/19,742 | 113/19,938 | ||
| HR (95% CI)§ | 1.00 (Ref) | 0.95 (0.68-1.33) | 1.03 (0.75-1.42) | 1.15 (0.84-1.57) | 0.25 | 1.30 (0.89-1.88) |
| Ischemic stroke | ||||||
| No. of cases/person years | 52/17,340 | 54/18,273 | 74/19,623 | 88/19,817 | ||
| HR (95% CI)§ | 1.00 (Ref) | 0.85 (0.58-1.25) | 1.03 (0.72-1.47) | 1.09 (0.77-1.54) | 0.37 | 1.30 (0.86-1.98) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 13/17,133 | 20/18,088 | 17/19,360 | 25/19,470 | ||
| HR (95% CI)§ | 1.00 (Ref) | 1.37 (0.68-2.76) | 1.05 (0.50-2.19) | 1.43 (0.72-2.84) | 0.42 | 1.31 (0.59-2.93) |
*After exclusion, a total of 16,676 participants in the baseline survey (2008–2010) with the information of proteinuria from spot urine test were remained for the association analysis. Proteinuria is defined as urinary protein with one or more “+”.
†The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-100 U/L), and Q4 (>100 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-87 U/L), Q3 (87-105 U/L), and Q4 (>105 U/L), respectively.
§Models were adjusted for age, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, aspirin usage, anticoagulants usage, menopausal status (women only), eGFR, family history of CVD, WBC, and admission batch.
‡p for trend was obtained by assigning the median value to each group and used this as a continuous variable in Cox regression models. Abbreviations: ACS, acute coronary syndrome; ALP, alkaline phosphatase; BMI, body mass index; CHD, coronary heart disease; CI, coefficient interval; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; WBC, white blood cell.
Supplementary Table 9. Adjusted hazard ratios for incident CVD, CHD, stroke, and their subtypes according to serum ALP levels after including participants with liver disease in men and women (n = 28,536) .
| Outcomes | Quartiles of ALP levels*, U/L | p for trend§ | Natural log- transformed continuous | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Men | ||||||
| CVD | ||||||
| No. of cases/person years | 819/21,008 | 939/22,307 | 1097/23,060 | 1027/21,321 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.08 (0.98-1.18) | 1.20 (1.10-1.32) | 1.21 (1.11-1.33) | <0.001 | 1.25 (1.11-1.40) |
| CHD | ||||||
| No. of cases/person years | 632/21,690 | 696/23,164 | 820/23,792 | 728/22,213 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.02 (0.92-1.14) | 1.17 (1.06-1.30) | 1.11 (1.00-1.24) | 0.02 | 1.11 (0.98-1.27) |
| ACS | ||||||
| No. of cases/person years | 234/19,772 | 319/21,304 | 374/21,647 | 305/20,117 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.26 (1.06-1.49) | 1.43 (1.21-1.68) | 1.24 (1.04-1.47) | 0.02 | 1.21 (0.99-1.47) |
| Stroke | ||||||
| No. of cases/person years | 187/23,110 | 243/24,619 | 277/25,687 | 299/23,673 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.22 (1.00-1.47) | 1.31 (1.09-1.58) | 1.50 (1.24-1.80) | <0.001 | 1.64 (1.32-2.05) |
| Ischemic stroke | ||||||
| No. of cases/person years | 156/22,959 | 188/24,391 | 216/25,378 | 236/23,404 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.13 (0.92-1.40) | 1.22 (0.99-1.50) | 1.42 (1.15-1.74) | <0.001 | 1.56 (1.21-2.00) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 31/22,323 | 55/23,771 | 61/24,674 | 63/22,610 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.67 (1.07-2.60) | 1.81 (1.17-2.79) | 1.96 (1.27-3.03) | 0.005 | 2.04 (1.29-3.24) |
| Women | ||||||
| CVD | ||||||
| No. of cases/person years | 725/29,176 | 809/29,031 | 1030/30,396 | 1149/30,524 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.97 (0.88-1.08) | 1.12 (1.02-1.24) | 1.14 (1.04-1.25) | <0.001 | 1.14 (1.03-1.27) |
| CHD | ||||||
| No. of cases/person years | 619/29,568 | 692/29,421 | 866/30,928 | 960/31,110 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.98 (0.88-1.09) | 1.11 (1.00-1.23) | 1.13 (1.02-1.25) | 0.003 | 1.12 (1.00-1.26) |
| ACS | ||||||
| No. of cases/person years | 202/27,470 | 265/27,057 | 312/27,926 | 371/27,832 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.12 (0.93-1.34) | 1.19 (1.00-1.43) | 1.27 (1.07-1.51) | 0.006 | 1.22 (1.01-1.47) |
| Stroke | ||||||
| No. of cases/person years | 106/31,270 | 117/31,331 | 164/33,577 | 189/33,757 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.96 (0.74-1.25) | 1.15 (0.90-1.48) | 1.19 (0.93-1.51) | 0.07 | 1.25 (0.96-1.62) |
| Ischemic stroke | ||||||
| No. of cases/person years | 86/31,181 | 87/31,184 | 131/33,397 | 141/33,540 | ||
| HR (95% CI)† | 1.00 (Ref) | 0.87 (0.64-1.17) | 1.12 (0.85-1.48) | 1.08 (0.82-1.42) | 0.28 | 1.16 (0.87-1.56) |
| Hemorrhagic stroke | ||||||
| No. of cases/person years | 20/30,861 | 30/30,897 | 33/32,973 | 48/33,086 | ||
| HR (95% CI)† | 1.00 (Ref) | 1.36 (0.77-2.40) | 1.30 (0.74-2.27) | 1.67 (0.99-2.85) | 0.06 | 1.58 (0.93-2.70) |
*The quartiles of ALP levels in men were Q1 (<69 U/L), Q2 (69-84 U/L), Q3 (84-100 U/L), and Q4 (>100 U/L), while the quartiles of ALP levels in women were Q1 (<71 U/L), Q2 (71-87 U/L), Q3 (87-105 U/L), and Q4 (>105 U/L), respectively.
†Models were adjusted for age, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, diabetes, liver disease, aspirin usage, anticoagulants usage, menopausal status (women only), eGFR, family history of CVD, WBC, and admission batch.
§p for trend was obtained by assigning the median value to each group and used this as a continuous variable in Cox regression models.
Abbreviations: ACS, acute coronary syndrome; ALP, alkaline phosphatase; BMI, body mass index; CHD, coronary heart disease; CI, coefficient interval; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; WBC, white blood cell.
Discussion
In this prospective cohort, we found that higher serum ALP levels, even within the normal range, were significantly, linearly associated with higher risks of CVD in both men and women. Moreover, our analyses showed that per unit increment in natural log-transformed ALP levels was independently associated with 31%, 61%, and 206% greater risks of ACS, ischemic stroke, and hemorrhagic stroke, respectively, in men, whereas a null association was observed among women. Several sensitivity analyses and stratified analyses demonstrated the robustness of our findings.
Our results are consistent with previous studies that suggested that high ALP levels were associated with CVD 5 , 6 , 11 , 12 , 14) , CHD 6 , 11) , stroke 15) , and cardiovascular risk factors, 27 , 28) although many previous studies were conducted among participants at high vascular risk. For instance, involving 3,381 older men (aged 60–79 years), Wannamethee et al. 6) found that per SD increase in log-ALP is associated with 9% and 15% higher risks of CVD and CHD, respectively. Additionally, two hospital-based studies 13 , 14) consisting of participants with CVD at baseline reported consistent findings. Compared with previous studies, our study was performed in a total of 11,408 middle-aged and elderly men and 14,981 middle-aged and elderly women without CVD histories at baseline, and our data provide evidence of the association of ALP with incident CVD in both men and women. In addition, evidence regarding the links between ALP levels and CHD subtypes is sparse. To the best of our knowledge, we demonstrated for the first time a significant positive association of ALP with incident ACS, a severe type of CHD, in men. Moreover, we found that the association of ALP levels with CVD was in a linear dose–response manner. Nonetheless, one prospective study involving 6,974 participants (737 cases) showed a “J-shaped” relationship 11) , whereas a meta-analysis consisting of 33,727 participants (2,097 cases) reported a log–linear relationship between ALP levels and incident CVD 5) . The inconsistency of the dose–response association may be caused by limited data endpoints. Additional observational evidence is needed to better assess the shape of the association.
Findings regarding ALP levels and incident stroke were limited and inconsistent in previous studies 6 , 8 , 11 , 12 , 15) . In contrast to no significant 6 , 11) or marginal 12) associations, we found that high ALP levels were associated with excess risk of stroke in a positive linear association and in a dose-dependent manner in men. However, no significant association was observed in women, potentially because of its relatively less stroke cases (n=541), and the association was likely to be masked by postmenopausal status 29 , 30) (postmenopausal women: n=12,514 [83.5%]) in the present analysis. Of note, we further found the associations of ALP levels with both ischemic stroke and hemorrhagic stroke risks in men and observed the same linear relationships. One study in Japanese populations had investigated the associations of ALP with both total stroke and its subtypes, which found a U-shaped association between ALP levels and total stroke in nondrinkers in both men and women 8) . However, in our study, the significant positive associations between ALP levels and total stroke did not vary according to drinking status in men. The differences in findings may be attributed to differences in statistical power. The relatively low incidence of stroke in previous studies 6 , 8 , 11 , 12) may contribute to their null findings. For example, in the study involving 10,754 Japanese 8) , there were a total of 489 stroke cases, and the limited number of subtype cases in their study precluded robust statistical analysis. There were several factors that may be contributing to the difference in the incidence of stroke between Japanese and participants in our study. Primarily, the mean age of the participants in our study is higher than that of the Japanese study 8) . Given that age is one of the most important risk factors of stroke, it is reasonable that the prevalence of stroke in our study is higher than that of the Japanese study 8) . Furthermore, due to the rapid socioeconomic development, secular trends in stroke incidence in China were observed to be most likely affected by population aging, risk accumulation, and advances in healthcare 31) . In contrast, a population-based stroke registry in Japan observed a significant reduction in incidence rates of stroke from 1990 to 2010 32) . Overall, our study had a relatively larger sample size (11,408 men, 913 stroke cases), which allowed more in-depth analysis on the association of ALP levels with various endpoints in different groups.
ALP commonly includes isoenzymes predominantly derived from the liver and bones and in lesser amounts from the kidneys, intestines, and placenta 2 , 6 , 7 , 9) . The elevation of ALP levels could be caused by liver and nonliver factors. Except for participants with a history of liver disease, we also excluded participant with two times the upper limit of normal liver enzymes and further controlled for ALT levels. Hence, the relationships between ALP levels and CVD in the current study were less likely to be confounded by subclinical liver dysfunction. Nonliver factors that contribute to the elevation of ALP levels include advanced age and female, bone disease, CKD, and inflammation 18) . Serum ALP levels could increase with age, especially for women after menopause 2 , 18) . We found that serum ALP levels were significantly higher among older women in our study. High ALP levels may indicate an increased bone metabolism activity, which could accelerate the development of CVD via cardiovascular calcification 33 , 34) . Given that we did not have information on vascular calcification or bone-specific ALP, this suggestion remains speculative. In line with existing evidence 6) , the significantly positive association persisted between ALP levels and incident CVD after excluding participants with CKD, suggesting that the association of ALP levels with CVD was independent of CKD. Moreover, several studies have shown that ALP levels were associated with inflammation biomarkers such as C-reactive protein 6 , 7 , 11 , 35) . With additional adjustment of WBC, another biomarker of inflammation, the association between ALP and CVD remained significant. Alternatively, blood vitamin D, calcium, and parathyroid hormone levels, associated with serum phosphate levels, were reported to play a critical role in the regulation of bone metabolism 36) and involved in vascular calcification 37) . However, we did not have the available data of vitamin D and parathyroid hormone, although a prior meta-analysis suggested that higher serum phosphate levels, but not serum calcium or parathyroid hormone levels, were associated with CVD risk 38) .
Of note, we found positive associations of serum ALP with both incident ischemic stroke and hemorrhagic stroke. The potential mechanisms underlying the association of ALP with subtypes of stroke are obscure. According to the existing evidence, higher serum ALP might constitute a risk factor for ischemic stroke owing to the progression of vascular calcification 39) . Vascular calcification can accelerate the process of atherosclerosis, which in turn results in vascular aging and involves vessel rupture. In addition, a previous study performed in individuals with hypertension indicated that higher serum ALP was associated with a higher risk of endothelial dysfunction 40) . The endothelium is essential in maintaining vascular homeostasis and is involved in the regulation of blood pressure, promotion of angiogenesis, and control of the coagulation process 41) , which may be involved in the pathogenesis of hemorrhagic stroke 42) .
Interestingly, we found that the associations of serum ALP with incident CVD remained significant within the normal ranges of ALP levels (40–150 U/L). The underlying mechanism through which ALP levels within normal range may contribute to incident CVD remains speculative. Previous studies reported that serum ALP correlated with whole body fat mass and was higher in obese subjects 43) . Furthermore, higher levels of serum ALP were associated with metabolic syndrome 44 , 45) , which is an important risk factor for CVD incidence 46) . In addition, consistent with the study based on the United States National Health and Nutrition Examination Survey 47) , we observed that participants, even within the normal range of ALP levels, who smoked or with higher levels of blood pressure, glucose, or lipids had higher serum ALP levels, which could contribute to the risk of CVD. Even though the associations remain robust after adjustment for the abovementioned factors, residual confounding cannot be fully excluded. Additionally, low ALP levels may indicate poor nutritional intake, although the associations of ALP with CVD remained robust when further adjusting the dietary habits. In contrast, unrecognized liver diseases may contribute to the associations, given that patients with chronic hepatitis can have normal liver enzyme levels 48 , 49) . More investigations are warranted to establish the underlying mechanisms.
Overall, our results revealed the robust dose–response relationships between the serum ALP levels and incident CVD, and participants within the normal range of ALP levels also had a higher risk of CVD. In addition, our study offers evidence for the positive association between ALP and ACS, ischemic stroke, and hemorrhagic stroke in men. Serum ALP measurement involves a routinely available blood test, which is simple, cheap, and standardized, and may be a potential test in screening for CVD risk. Furthermore, given that ALP levels increase with age 2) , our findings highlight the critical issue of the normal limits and target ALP levels for the prevention of CVD, especially for middle-aged and elderly people who are at a higher risk of CVD.
The strengths of our study include the use of prospective cohort study design to evaluate the associations of serum ALP levels with incident CVD, CHD, stroke, and their subtypes in Chinese men and women. To the best of our knowledge, we demonstrated for the first time the significant positive association of ALP levels with incident ACS. We also found the linear relationships of ALP levels within the normal range with incident CVD. Nonetheless, several limitations should be mentioned. Firstly, our study was conducted among middle-aged and elderly participants, which might limit the generalization of the results. Secondly, to minimize the potential confounding of CKD, we controlled for eGFR in the association analysis. The results did not substantially change, when we further excluded participants with CKD in a sensitivity analysis. Thirdly, despite that we have adjusted the admission batch in the analysis to reduce the batch effect, the possibility of batch effect cannot be fully eliminated. Fourthly, medication usage, particularly aspirin and anticoagulation usage, could bring confounding in the association of ALP and CVD risk. Nonetheless, we controlled for aspirin usage and anticoagulation usage within 2 weeks in the analysis, and the association remained significant. Fifthly, despite adjusting several potential confounders, we did not have information of vitamin D, mineral, ABO blood groups, parathyroid hormone, and disease history of hyperthyroidism or hyperparathyroidism; hence, we cannot rule out the possibility of residual confounding. Lastly, we have no measurement of the isoenzymes of ALP that might help in determining the source of the elevation. Further research regarding the association of ALP isoenzymes with CVD is needed.
In conclusion, our study found linear dose–response relationships of serum ALP levels, even within the normal range, with risk of CVD in both men and women and with risk of total stroke, ischemic stroke, and hemorrhagic stroke in men as well. Further research with large sample size is needed to evaluate the normal limits and target levels of ALP for the prevention of CVD, especially for the middle-aged and elderly adults.
Acknowledgements
We thank all staff and participants of the Dongfeng-Tongji cohort study as well as all participants for assisting in collecting the samples and data.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81973128, 81930092], the Fundamental Research Funds for the Central Universities [grant number 2019kfyXMBZ015], and the 111 Project and the Program for Changjiang Scholars and Innovative Research Team in University. The funders of this study had no role in the study design; the collection, analysis, and interpretation of data; writing of the report; or the decision to submit the article for publication.
Competing Interests
The authors declare no competing interests.
Supplemental Methods
Covariates Assessment
Education status was indicated as primary school and below, junior high school, senior high school and above. Participants who smoked more than 1 cigarette per day over the past 6 months were defined as current smokers; participants who quitted smoking for more than 6 months were defined as former smokers; otherwise they were defined as never smokers. Current drinkers were defined as participants who drank more than 1 time per week over the past 6 months; former drinkers were those who had stopped drinking for more than 6 months; otherwise they were defined as never drinkers. Physical activity was defined as exercise for at least 30 minutes each time and at least 5 times a week for more than half a year. Medication usage information was collected by face-to-face interview about any medication used in the previous fortnight. In DFTJ cohort, diet was determined via a simplified semi-quantitative food frequency questionnaire. Participants were asked to answer the question “In the past year, how often do you eat the following food”, and the trained interviewer fulfilled the eating frequency of 13 food groups (never and times per day/week/month/year). According to usual intakes of major food groups in the previous 1 year, diet was classified into 5 food groups (meat, fish or seafood, milk or dairy products, beans or soy foods, and fruits or vegetables) for analyses. If the participant reported eating the food ≥ 5 times per week, the response would be “yes”, otherwise the response would be “no”.
Body mass index was calculated as the body mass divided by the square of body height and was expressed in unit of kg/m2. Hypertension was defined as self-reported physician diagnosis, or systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg, or use of anti-hypertensive medications. Diabetes was defined as self-reported physician diagnosis, or fasting glucose ≥ 7.0 mmol/L, or taking anti-diabetic medications (oral hypoglycemic medication or insulin). Hyperlipidemia was defined as self-reported physician diagnosis, or total cholesterol ≥ 6.22 mmol/L, or triglycerides ≥ 2.26 mmol/L, or high-density lipoprotein cholesterol <1.04 mmol/L, or low-density lipoprotein cholesterol ≥ 4.14 mmol/L, or taking anti-hyperlipidemia medications. Estimated glomerular filtration rate in our study was calculated with the Chronic Kidney Disease Epidemiology Collaboration equation1), which was approved to be more accurate than the Modification of Diet in Renal Disease Study equation, especially at a glomerular filtration rate of 60 mL/min/1.73 m2 or greater2, 3). Chronic kidney disease was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2 4).
References
1)Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J and CKD EPI: A new equation to estimate glomerular filtration rate. Ann Intern Med, 2009; 150: 604-612
2)Earley A, Miskulin D, Lamb EJ, Levey AS and Uhlig K: Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med, 2012; 156: 785-795
3)Stevens PE, Levin A and Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members: Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med, 2013; 158: 825-830
4)Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N and Eknoyan G: Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int, 2005; 67: 2089-2100
References
- 1).Rui L: Energy metabolism in the liver. Compr Physiol, 2014; 4: 177-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Pratt DS and Kaplan MM: Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med, 2000; 342: 1266-1271 [DOI] [PubMed] [Google Scholar]
- 3).Xu L, Jiang CQ, Schooling CM, Zhang WS, Cheng KK and Lam TH: Liver enzymes and incident diabetes in China: a prospective analysis of 10 764 participants in the Guangzhou Biobank Cohort Study. J Epidemiol Community Health, 2015; 69: 1040-1044 [DOI] [PubMed] [Google Scholar]
- 4).Targher G and Byrne CD: Circulating markers of liver function and cardiovascular disease risk. Arterioscler Thromb Vasc Biol, 2015; 35: 2290-2296 [DOI] [PubMed] [Google Scholar]
- 5).Kunutsor SK, Apekey TA and Khan H: Liver enzymes and risk of cardiovascular disease in the general population: a meta-analysis of prospective cohort studies. Atherosclerosis, 2014; 236: 7-17 [DOI] [PubMed] [Google Scholar]
- 6).Wannamethee SG, Sattar N, Papcosta O, Lennon L and Whincup PH: Alkaline phosphatase, serum phosphate, and incident cardiovascular disease and total mortality in older men. Arterioscl Throm Vas Biol, 2013; 33: 1070-U1503 [DOI] [PubMed] [Google Scholar]
- 7).Tonelli M, Curhan G, Pfeffer M, Sacks F, Thadhani R, Melamed ML, Wiebe N and Muntner P: Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation, 2009; 120: 1784-1792 [DOI] [PubMed] [Google Scholar]
- 8).Shimizu Y, Imano H, Ohira T, Kitamura A, Kiyama M, Okada T, Ishikawa Y, Shimamoto T, Yamagishi K, Tanigawa T, Iso H and Investigators C: Alkaline phosphatase and risk of stroke among Japanese: the Circulatory Risk in Communities Study (CIRCS). J Stroke Cerebrovasc Dis, 2013; 22: 1046-1055 [DOI] [PubMed] [Google Scholar]
- 9).Schoppet M and Shanahan CM: Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int, 2008; 73: 989-991 [DOI] [PubMed] [Google Scholar]
- 10).Harris H: The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta, 1990; 186: 133-150 [DOI] [PubMed] [Google Scholar]
- 11).Kunutsor SK, Bakker SJ, Kootstra-Ros JE, Gansevoort RT, Gregson J and Dullaart RP: Serum alkaline phosphatase and risk of incident cardiovascular disease: Interrelationship with high sensitivity C-reactive protein. PLoS One, 2015; 10: e0132822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Kabootari M, Raee MR, Akbarpour S, Asgari S, Azizi F and Hadaegh F: Serum alkaline phosphatase and the risk of coronary heart disease, stroke and all-cause mortality: Tehran Lipid and Glucose Study. BMJ Open, 2018; 8: e023735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Park JB, Kang DY, Yang HM, Cho HJ, Park KW, Lee HY, Kang HJ, Koo BK and Kim HS: Serum alkaline phosphatase is a predictor of mortality, myocardial infarction, or stent thrombosis after implantation of coronary drug-eluting stent. Eur Heart J, 2013; 34: 920-931 [DOI] [PubMed] [Google Scholar]
- 14).Abramowitz M, Muntner P, Coco M, Southern W, Lotwin I, Hostetter TH and Melamed ML: Serum alkaline phosphatase and phosphate and risk of mortality and hospitalization. Clin J Am Soc Nephrol, 2010; 5: 1064-1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Kitamura H, Yamada S, Hiyamuta H, Yotsueda R, Taniguchi M, Tokumoto M, Tsuruya K, Nakano T and Kitazono T: Serum alkaline phosphatase levels and increased risk of brain hemorrhage in hemodialysis patients: the Q-Cohort Study. J Atheroscler Thromb, 2022; 29: 923-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y and Kato H: Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res, 2015; 27: 413-418 [DOI] [PubMed] [Google Scholar]
- 17).Wang F, Zhu J, Yao P, Li X, He M, Liu Y, Yuan J, Chen W, Zhou L, Min X, Fang W, Liang Y, Wang Y, Wei S, Liu J, Miao X, Lang M, Jiang X, Zhang P, Li D, Lu C, Wang X, Shi W, Zheng J, Guo H, Zhang X, Yang H, Hu FB and Wu T: Cohort profile: the Dongfeng-Tongji cohort study of retired workers. Int J Epidemiol, 2013; 42: 731-740 [DOI] [PubMed] [Google Scholar]
- 18).Kwo PY, Cohen SM and Lim JK: ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol, 2017; 112: 18-35 [DOI] [PubMed] [Google Scholar]
- 19).Rose GA and Blackburn H: Cardiovascular survey methods. Monogr Ser World Health Organ, 1968; 56: 1-188 [PubMed] [Google Scholar]
- 20).O’Connor RE, Bossaert L, Arntz HR, Brooks SC, Diercks D, Feitosa-Filho G, Nolan JP, Vanden Hoek TL, Walters DL, Wong A, Welsford M, Woolfrey K and Acute Coronary Syndrome Chapter Collaborators: Part 9: Acute coronary syndromes: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation, 2010; 122: S422-465 [DOI] [PubMed] [Google Scholar]
- 21).Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation, 2013; 127: E663-E828 [DOI] [PubMed] [Google Scholar]
- 22).Walker AE, Robins M and Weinfeld FD: The national survey of stroke. Clinical findings. Stroke, 1981; 12: I13-44 [PubMed] [Google Scholar]
- 23).Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL and Marsh EE, 3rd: Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 1993; 24: 35-41 [DOI] [PubMed] [Google Scholar]
- 24).Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MSV, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism: An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2013; 44: 2064-2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Desquilbet L and Mariotti F: Dose-response analyses using restricted cubic spline functions in public health research. Stat Med, 2010; 29: 1037-1057 [DOI] [PubMed] [Google Scholar]
- 26).Zhou BF and Cooperative Meta-Analysis Group of the Working Group on Obesity in C: Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci, 2002; 15: 83-96 [PubMed] [Google Scholar]
- 27).Lee JH, Lee JW and Lee YJ: The Relationship between serum alkaline phosphatase and arterial stiffness in Korean adults. J Atheroscler Thromb, 2019; 26: 1084-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Naito H, Nezu T, Hosomi N, Kuzume D, Aoki S, Morimoto Y, Yoshida T, Kamimura T, Shiga Y, Kinoshita N, Ueno H, Morino H and Maruyama H: Increased serum alkaline phosphatase and functional outcome in patients with acute ischemic stroke presenting a low ankle-brachial index. J Atheroscler Thromb, 2022; 29: 719-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing: Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation, 2020; 142: e506-e532 [DOI] [PubMed] [Google Scholar]
- 30).Rosano GM, Vitale C, Marazzi G and Volterrani M: Menopause and cardiovascular disease: the evidence. Climacteric, 2007; 10 Suppl 1: 19-24 [DOI] [PubMed] [Google Scholar]
- 31).Wang Y, Zhou L, Guo J, Wang Y, Yang Y, Peng Q, Gao Y and Lu W: Secular trends of stroke incidence and mortality in China, 1990 to 2016: The Global Burden of Disease Study 2016. J Stroke Cerebrovasc Dis, 2020; 29: 104959 [DOI] [PubMed] [Google Scholar]
- 32).Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T and Kiyohara Y: Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961-2009). Circulation, 2013; 128: 1198-1205 [DOI] [PubMed] [Google Scholar]
- 33).Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H and Nishizawa Y: Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-α and oncostatin M derived from macrophages. Circ Res, 2002; 91: 9-16 [DOI] [PubMed] [Google Scholar]
- 34).Panh L, Ruidavets JB, Rousseau H, Petermann A, Bongard V, Berard E, Taraszkiewicz D, Lairez O, Galinier M, Carrie D and Ferrieres J: Association between serum alkaline phosphatase and coronary artery calcification in a sample of primary cardiovascular prevention patients. Atherosclerosis, 2017; 260: 81-86 [DOI] [PubMed] [Google Scholar]
- 35).Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, Levy Y, Brook GJ and Aronson D: Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol, 2005; 25: 193-197 [DOI] [PubMed] [Google Scholar]
- 36).Lieben L, Masuyama R, Torrekens S, Van Looveren R, Schrooten J, Baatsen P, Lafage-Proust MH, Dresselaers T, Feng JQ, Bonewald LF, Meyer MB, Pike JW, Bouillon R and Carmeliet G: Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest, 2012; 122: 1803-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Chen NX and Moe SM: Pathophysiology of vascular calcification. Curr Osteoporos Rep, 2015; 13: 372-380 [DOI] [PubMed] [Google Scholar]
- 38).Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ and Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA, 2011; 305: 1119-1127 [DOI] [PubMed] [Google Scholar]
- 39).Lomashvili KA, Garg P, Narisawa S, Millan JL and O’Neill WC: Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int, 2008; 73: 1024-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Perticone F, Perticone M, Maio R, Sciacqua A, Andreucci M, Tripepi G, Corrao S, Mallamaci F, Sesti G and Zoccali C: Serum alkaline phosphatase negatively affects endothelium-dependent vasodilation in naive hypertensive patients. Hypertension, 2015; 66: 874-880 [DOI] [PubMed] [Google Scholar]
- 41).Sierra C, Coca A and Schiffrin EL: Vascular mechanisms in the pathogenesis of stroke. Curr Hypertens Rep, 2011; 13: 200-207 [DOI] [PubMed] [Google Scholar]
- 42).Chrissobolis S, Miller AA, Drummond GR, Kemp-Harper BK and Sobey CG: Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci (Landmark Ed), 2011; 16: 1733-1745 [DOI] [PubMed] [Google Scholar]
- 43).Ali AT, Paiker JE and Crowther NJ: The relationship between anthropometry and serum concentrations of alkaline phosphatase isoenzymes, liver-enzymes, albumin, and bilirubin. Am J Clin Pathol, 2006; 126: 437-442 [DOI] [PubMed] [Google Scholar]
- 44).Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Jr. and Haffner SM: Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes, 2005; 54: 3140-3147 [DOI] [PubMed] [Google Scholar]
- 45).Krishnamurthy VR, Baird BC, Wei G, Greene T, Raphael K and Beddhu S: Associations of serum alkaline phosphatase with metabolic syndrome and mortality. Am J Med, 2011; 124: 566 e561-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Wilson PW, D’Agostino RB, Parise H, Sullivan L and Meigs JB: Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation, 2005; 112: 3066-3072 [DOI] [PubMed] [Google Scholar]
- 47).Cheung BM, Ong KL and Wong LY: Elevated serum alkaline phosphatase and peripheral arterial disease in the United States National Health and Nutrition Examination Survey 1999-2004. Int J Cardiol, 2009; 135: 156-161 [DOI] [PubMed] [Google Scholar]
- 48).Ahmed A and Keeffe EB: Chronic hepatitis C with normal aminotransferase levels. Gastroenterology, 2004; 126: 1409-1415 [DOI] [PubMed] [Google Scholar]
- 49).Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, Chauhan R and Bose S: Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology, 2008; 134: 1376-1384 [DOI] [PubMed] [Google Scholar]