Abstract
Background
Gene expression profiling tests can predict the risk of disease recurrence and select patients who are expected to benefit from therapy, while allowing other patients to forgo therapy. For breast cancers, these tests were initially designed to tailor chemotherapy decisions, but recent evidence suggests that they may also guide the use of endocrine therapy. This study evaluated the cost effectiveness of a prognostic test, MammaPrint®, to guide the use of adjuvant endocrine therapy in patients eligible according to Dutch treatment guidelines.
Methods
We constructed a Markov decision model to calculate the lifetime costs (in 2020 Euros) and effects (survival and quality-adjusted life-years) of MammaPrint® testing versus usual care (endocrine therapy for all patients) in a simulated cohort of patients. The population of interest includes patients for whom MammaPrint® testing is currently not indicated, but for whom it may be possible to safely omit endocrine therapy. We applied both a health care perspective and a societal perspective and discounted costs (4%) and effects (1.5%). Model inputs were obtained from published research (including randomized controlled trials), nationwide cancer registry data, cohort data and publicly available data sources. Scenario and sensitivity analyses were conducted to explore the impact of uncertainty around input parameters. Additionally, threshold analyses were performed to identify under which circumstances MammaPrint® testing would be cost effective.
Results
Adjuvant endocrine therapy guided by MammaPrint® resulted in fewer side effects, more (quality-adjusted) life-years (0.10 and 0.07 incremental QALYS and LYs, respectively) and higher costs (€18,323 incremental costs) compared with the usual care strategy in which all patients receive endocrine therapy. While costs for hospital visits, medication costs and productivity costs were somewhat higher in the usual care strategy, these did not outweigh costs of testing in the MammaPrint® strategy. The incremental cost-effectiveness ratio was €185,644 per QALY gained from a healthcare perspective and €180,617 from a societal perspective. Sensitivity and scenario analyses showed that the conclusions remained the same under changed input parameters and assumptions. Our results show that MammaPrint® can become a cost-effective strategy when either the price of the test is reduced (> 50%), or the proportion of patients for which treatment is altered (i.e. those with ultra-low risk) increases to > 26%.
Conclusion
Standard MammaPrint® testing to guide the use of endocrine therapy in our simulated patient population appears not to be a cost-effective strategy compared with usual care. The cost effectiveness of the test can be improved by reducing the price or preselecting a population more likely to benefit from the test.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-023-01277-4.
Key Points for Decision Makers
| Gene expression profiling (GEP) tests are developed to personalize treatment by selecting patients who are expected to benefit from therapy, while allowing other patients to forgo therapy. |
| We found that GEP testing is an effective but not a cost-effective strategy to guide the use of endocrine therapy decisions in patients with early-stage breast cancer. |
| GEP testing to guide endocrine therapy in early-stage breast cancer is currently not cost effective and therefore not recommended to be used in clinical practice. |
Introduction
A paradigm shift is taking place in oncology towards de-escalation of treatment with the aim of improving and personalizing care [1]. Treatment de-escalation includes reducing ineffective care or care that provides patients with no net benefit (i.e. treatments for which the benefits do not counterbalance the harmful effects). Evidence-based de-escalation strategies offer advantages to patients because they can safely forgo therapy without compromising outcome [2]. Additionally, avoiding ineffective treatments may also reduce health care costs [3]. Risk stratification by gene expression profiling (GEP) is an approach to personalize and de-escalate treatment. Patients who are expected to benefit from therapy are distinguished from patients that can forgo therapy.
Breast cancer is the most common cancer in women and the most frequent cause of cancer-related death among women worldwide. Currently, several GEP tests are available for breast cancer and some of these are recommended for use in clinical practice, including MammaPrint®, Oncotype DX®, EndoPredict®, Prosigna® and Breast Cancer Index [2, 4]. Retrospective and prospective studies have shown that these tests can accurately identify patients who have a low risk of disease recurrence and who can safely forgo adjuvant chemotherapy [2]. Despite the fact that GEP tests are quite expensive, they are considered to be cost effective to identify patients who can forgo adjuvant chemotherapy in many countries. The costs of testing can be offset by gains in health-related quality of life (HRQoL) combined with savings in costs for chemotherapy and related adverse events [4, 5].
Recent studies suggest that MammaPrint® is also suitable for another purpose, that is, to guide endocrine therapy (ET) decisions in patients with early-stage breast cancer. This concerns patients who already have an excellent survival rate without chemotherapy. Nevertheless, (inter)national guidelines currently recommend (including for these patients) 5 years of adjuvant endocrine therapy (ET) to reduce the risk of disease recurrence [6, 7]. Although ET is typically inexpensive, it can cause several side effects. While only a small proportion of patients develop severe side effects such as endometrial cancer and thromboembolism, less severe adverse events, such as hot flashes, arthralgia, vaginal dryness, emotional lability and symptoms of depression, are seen frequently [8–10]. The latter are typically not life-threatening, but they often impact patients’ quality of life and social functioning. Additionally, side effects are also associated with increased costs, for instance due to more visits to health care professionals and reduced work productivity [11].
In the Netherlands, MammaPrint® is currently commercially available but not reimbursed from the basic benefit package [12, 13]. Possibly, the new indication of MammaPrint® does qualify for reimbursement. For this purpose, information about the cost effectiveness is valuable but to the best of our knowledge currently lacking. As such, we conducted a cost-effectiveness analysis of MammaPrint® to guide ET decisions in patients in the Netherlands.
Methods
Study Design
We constructed a decision analytic model to estimate the incremental costs per (quality-adjusted) life-year (QALY/LY) of MammaPrint® testing to assign ET in patients with ER+/HER2−, lymph node negative and either grade 1 with a tumor size of 2–3 cm, or grade 2 and tumor size of 1–2 cm. We assumed 100% test accuracy for MammaPrint®. The model simulates the course of events of 1000 patients aged 63 years (i.e. average age of patients with the above-mentioned characteristics in the Netherlands [14]) for two strategies: (i) MammaPrint® testing to guide ET or (ii) not testing and give ET according to current guidelines to all patients. The latter includes 2.5 years’ tamoxifen followed by 2.5 years’ aromatase inhibitors (AI) or 5 years’ AI for postmenopausal patients and 5 years’ tamoxifen combined with ovarian suppression for premenopausal patients [15, 16]. Our analysis followed the Dutch pharmacoeconomic guidelines. As such, we used a societal perspective and included both direct and indirect medical and non-medical consumption costs and productivity costs [17]. We also reported results from the health care perspective and for both perspectives we reported results without indirect costs. Costs are valued in 2020 euros and costs and health outcomes are discounted at a rate of 4% and 1.5%, respectively [17].
Model Description
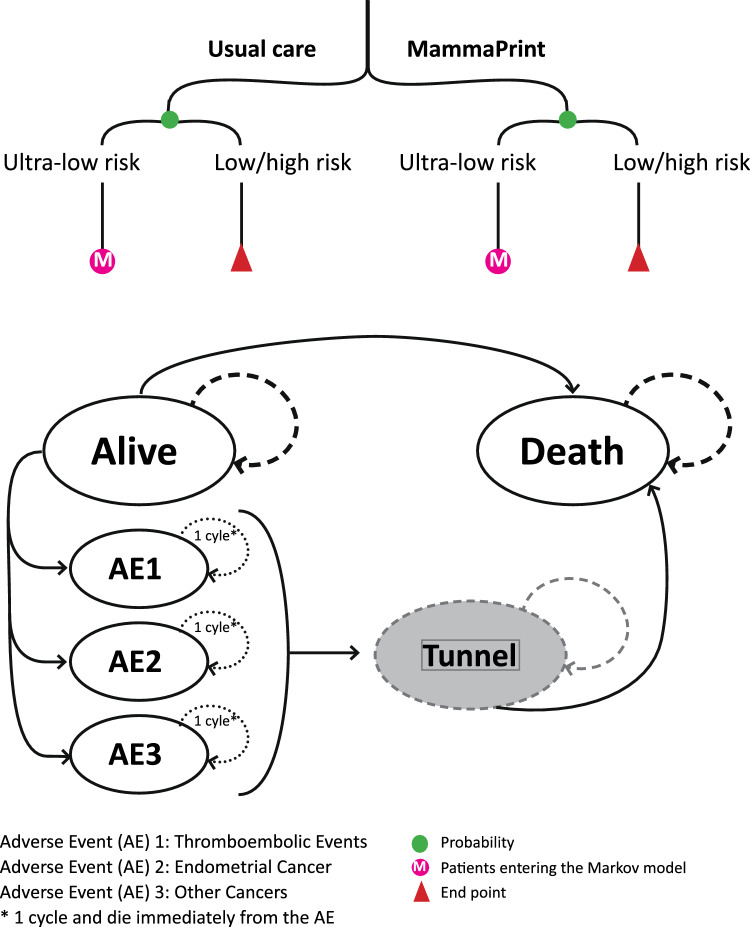
A decision tree was combined with a semi-Markov model to simulate a cohort of patients with early breast cancer (eBC) with the above-mentioned characteristics. The decision tree included the two alternative strategies: (i) test patients with MammaPrint® and guide their adjuvant ET accordingly and (ii) treat all patients with adjuvant ET (Fig. 1), and was used to calculate the proportion of patients with ultra-low risk. In each of the branches of the tree, the population was distributed among the clinical risk of recurrence scores as defined by MammaPrint®: ultra-low (13%, n = 131), low or high (50% low + 37% high = 87%, n = 869) risk [7]. Note: we assumed the same proportion of ultra-low–risk patients in the usual care group.
Fig. 1.
Schematic overview of the model
The semi-Markov model simulated the patients with an ultra-low risk over different health states, until death, using transition probabilities and a cycle length of 3 months. We included the MammaPrint® test costs for all patients that needed to be tested (N = 1000). Treatment costs and outcomes were not included for the patients with a low and high risk (N = 869) because these are not altered with the test. The health states in the Markov model included (i) ‘alive’ which are patients diagnosed with eBC (and the characteristics mentioned above), (ii) ‘endometrial cancer’, ‘other malignancies’, ‘thromboembolic event’, which are three health states representing major adverse events (AEs) related to ET, and (iii) ‘death’. We did not include a breast cancer recurrence health state in our model because we assumed that the probability of recurrence would be the same in ultra-low–risk patients in the MammaPrint® and usual care strategies [6, 7]. All ultra-low–risk patients, regardless of whether they received ET or not, initially entered the ‘alive’ state. From this health state they could die of any cause or develop a major AE and potentially die. Patients could only be in one health state at a time and experience one major AE in their lifetime. Patients who were cured from a major AE entered a new tunnel health state and were assumed to have the same survival as patients in the alive state.
Costs and health-related quality-of-life values (also known as utilities) were attributed to the health states of the model. The total costs and effects of the two strategies were calculated by summing up the health state costs and effects of all cycles (for n = 131 ultra-low–risk patients). Test costs of the entire cohort (n = 1000) were added for patients in the MammaPrint® strategy. Average costs and effects were calculated for the ultra-low–risk patients (thus dividing the total costs and effects by 131). The incremental cost-effectiveness ratio (ICER) was calculated as follows: (average costs MammaPrint® strategy − average costs usual care strategy)/(average effects MammaPrint® strategy − average effects usual care strategy). To determine whether the MammaPrint® strategy was cost effective compared with usual care, we compared the ICERs with the different willingness-to-pay (WTP) thresholds valid in the Netherlands (i.e. 20,000, 50,000 or 80,000 per QALY gained) [18].
Input Parameters
Model inputs used to estimate costs and effects were identified using targeted literature searches and obtained from published research (including randomized controlled trials [RCTs]), nationwide cancer registry data, cohort data and publicly available data sources. Studies were selected based on their relevance to our model and the level of evidence according to the evidence-based medicines criteria [19]. For costs and utility parameters we preferred Dutch studies but if these were not available we used studies from other developed countries. A distribution around the input parameter was defined. All input parameters together with their uncertainty distributions are presented in Tables 1, 2, 3, 4, 5 and 6.
Table 1.
Model input parameters for estimation of costs and effects related to MammaPrint® and endocrine therapy: Information about the characteristics of the patient population simulated in this study
| Parameter | Value | SE | Alpha | Beta | Source |
|---|---|---|---|---|---|
| Mean age (years) | 63.3 | n.v. | n.a. | n.a. | Netherlands Cancer Registry (national data) [14] |
| Premenopausal (%) | 18 | 0.003 | 5257 | 23,351 | Netherlands Cancer Registry (proportions based on national data) [14] |
| Postmenopausal (%) | 82 | 23,351 | 5257 | ||
| Adjuvant endocrine treatment | [24] | ||||
| 5 years of AI (%) | 74 | 0.012 | 380 | 1071 | |
| 2.5 years of tamoxifen followed by 2.5 years AI (%) | 26 | 1071 | 380 | ||
| Employed (%) | 53 | 0.001 | 300,289 | 270,602 | Statistics Netherlands [42] |
AI aromatase inhibitor, n.a. not applicable, n.v. not varied, SE standard error
Table 2.
Model input parameters for estimation of costs and effects related to MammaPrint® and endocrine therapy: Transition probabilities used to populate the decision tree and Markov model
| Value | SE | Alpha | Beta | Source | |
|---|---|---|---|---|---|
| Decision tree—probabilities | |||||
| Ultra-low risk of recurrence based on MammaPrint® (%) | 13 | 0.012 | 98 | 652 | [7] |
| Low or high risk of recurrence based on MammaPrint® (%) | 87 | 652 | 98 | ||
| Markov model—probabilities | |||||
| Probability of death | |||||
| Women with ultra-low risk of recurrence based on MammaPrint® | Based on life tablesa (Supplemental file 10) | [20] | |||
| Probability to adhere to ET | |||||
| Year 1 (%) | 87 | n.v. | n.v. | n.v. | [24] |
| Year 2 (%) | 78 | ||||
| Year 3 (%) | 69 | ||||
| Year 4 (%) | 63 | ||||
| Year 5 (%) | 49 | ||||
ET endocrine therapy, n.v. not varied, PSA probabilistic sensitivity analyses, SE standard error
aAnnual probabilities are reported by Statistics Netherlands, we calculated probabilities for each cycle. These probabilities are not varied in the PSA
Table 3.
Model input parameters for estimation of costs and effects related to MammaPrint® and endocrine therapy: Probabilities of major adverse events in premenopausal and postmenopausal patients treated with and without ET and probabilities of death from major adverse events
| Probabilities of major adverse events—probabilities of thromboembolic events are applied for 5 years and of endometrium cancer and other cancers for 10 years | ||||
|---|---|---|---|---|
| Post-menopausal women | Per cycle (SE—Alpha/Beta) | Sources | ||
| AI [21] | Tamoxifen followed by AI [21] | No ET—RR [8] | ||
| Thromboembolic events (%) | 0.0484 (0.0003–2.4/4850) | 0.1038 (0.0005–5/4809) | 0.031 (0.001–7/2241/0.00–11/2229a) | [8, 21] |
| Endometrium cancer (%) | 0.0057 (0.0004–0.2/3075) | 0.0189 (0.0004–0.6/3044) | 0.0091 (0.001–8/2240/0.00–5/2235a) | |
| Other cancers (%) | 0.1933 (0.0008 –5.9/3069) | 0.1732 (0.0008–5.3/3040) | 0.1419b (0.002–28/2220/0.003–38/2205a) | |
| Pre-menopausal women | Per cycle (SE—Alpha/Beta) | Sources | ||
|---|---|---|---|---|
| Tamoxifen + ovarian suppression—[9, 43] | No ET—[9] | |||
| Thromboembolic events (%) | 0.063 (0.0005–1/2325) | 0.039 (0.0007–0.5/1005) | [9, 43] | |
| Endometrium cancer (%) | 0.015 (0.0004–1.7/3577) | 0.010 (0.0004–1.7/3573) | ||
| Other cancers (%) | 0.122 (0.0004–1.7/3577) | 0.109 (0.0004–1.7/3573) | ||
| Death from: | Per cycle (SE) | Sources | ||
|---|---|---|---|---|
| Thromboembolic events (%) | 7.7 (0.006–91/1111b) | [9, 22] | ||
| Endometrium cancer (%) | 21 (0.021–78/296b) | [44] | ||
| Other cancers (%) | 27 (0.027–72/197b) | Assumptions |
Values in italics indicate SE—Alpha/Beta
AI aromatase inhibitor, ET endocrine therapy, RR relative risk, SE standard error
aProbabilistic RRs were calculated by dividing two probabilities and using the SE of both. Probabilistic RRs were applied to the probabilistic
bSE assumed to be 10% of mean values of exemestane
Table 4.
Model input parameters for estimation of costs and effects related to MammaPrint® and endocrine therapy: Probabilities of minor adverse events in premenopausal and postmenopausal patients treated with and without ET
| Probabilities of minor adverse events in different subgroups – applied for 5 years (during ET) | ||||
|---|---|---|---|---|
| Post-menopausal women | Per cycle—for 5 years (SE—Alpha/Beta) | Sources | ||
| AI – [21]a | Tamoxifen followed by AI – [21]a | No ET—RR from—[8]b | ||
| Vasomotor (%) | 35.1 (0.007–1703/3149) | 40.4 (0.007–1945/2869) | 27.9 (0.010 718/1530/0.010–900/1340d) | [8] |
| Vulvovaginal (%) | 6.6 (0.004–320/4532) | 8.4 (0.004–403/4411) | 6.4 (0.008 343/1905/0.008–352/1888d) | |
| Mood (%) | 13.5 (0.005–654/4198) | 10.5 (0.004–504/4310) | 13.4 (0.006–235/2013/0.006–236/2004d) | |
| Cognitive (%) | 10.5c (0.006–236/2004) | 10.5c (0.006–236/2004) | 10.5 (0.009–465/1783/0.009–532/1717d) | |
| Fractures (%) | 0.26 (0.001–13/4839) | 0.17 (0.001–8/4844) | 0.25 (0.005–143/2105/0.005–149/2091d) | |
| Any minor AEf (%) | 35.1 (n.v.) | 40.4 (n.v.) | 27.9 (n.v.) | |
| Pre-menopausal women | Per cycle—for 5 years (SE—Alpha/Beta) | Sources | |
|---|---|---|---|
| Tamoxifen + ovarian suppression–[43] | No ET–RR from [8, 9, 45]e | ||
| Vasomotor (%) | 93.5 (0.005–2175/151) | 63.9 (0.012–988/838/0.011–1233/577) | [8, 43, 45] |
| Vulvovaginal (%) | 49.2 (0.010–1144/1182) | 36.2 (0.011–565/1261/0.011–669/1141) | |
| Mood (%) | 51.4 (0.010–1195/1131) | 47.0 (0.006–235/2013 /0.006–236/2004) | |
| Cognitive (%) | 59.5 (0.010–465/1783) | 41.5 (0.009–465/1783/0.009–523/1717) | |
| Fractures (%) | 0.19 (0.001–2/2321) | 0.17 (0.004–235/3340/0.004–240/3339) | |
| Any minor AEf (%) | 93.5 (n.v.) | 63.9 (n.v.) | |
Values in italics indicate SE—Alpha/Beta
ET endocrine therapy, IBIS1 International Breast Cancer Intervention Study, MAP3 Mammary Prevention.3, n.v. not varied using the SE but using the max of the probabilistic values of all categories, RR relative risk, SE standard error, SOFT Suppression of Ovarian Function Trial, TEAM Tamoxifen Exemestane Adjuvant Multinational
aCrude probabilities from TEAM trial (took the highest % reported if multiple similar AEs were reported)
bApplied RRs to probabilities of exemestane. Probabilistic RRs were calculated by dividing two probabilities and using the SE of both
cObtained from MAP3, because not reported in TEAM
dProbabilistic RRs were calculated by dividing two probabilities and using the SE of both
eMAP3. Was also used because not all events were reported for pre- and post-menopausal women separately in the IBIS1
fMax of the 5 overarching groups using the SE but using the max of the probabilistic values of all categories
Table 5.
Model input parameters for estimation of costs and effects related to MammaPrint® and endocrine therapy: Utility values applied in the model
| Per cycle | SE | Alpha | Beta | Source | |
|---|---|---|---|---|---|
| Utility values | |||||
| First years after primary BC diagnosis | 0.696 | 0.007 | 2719 | 1188 | [25] |
| Subsequent years (utility of the general population of women aged 55–65 years) | 0.89 | 0.089 | 10 | 1 | [26] |
| AE 1: Thromboembolic events—fatal | − 0.056 | 0.006a | 93 | 1581 | [17] (applied for 1 cycle) |
| AE 1: Thromboembolic events—chronic | − 0.004 | 0.0002a | 99 | 46,979 | [17, 53] (see Supplemental file 4) |
| AE 2: Endometrial cancer | − 0.036 | 0.0036a | 95 | 2581 | [27] (applied for 1 cycle) |
| AE 3: Other cancers | − 0.036 | 0.0036a | 95 | 2581 | Assumed to be the same as endometrium cancer (applied for 1 cycle) |
| Dis-utilities of minor adverse events values | |||||
| Dis-utilities of adverse events due to minor AEs—first 3 months | − 0.083 | 0.002 | 2064 | 22,816 | TOTAM study see Supplemental file 5, EQ5D-5L Dutch Tarif |
| Dis-utilities of adverse events due to minor AEs—first 6–24 months | − 0.074 | 0.002 | 1288 | 16,130 | |
| Dis-utilities of adverse events due to minor AEs—24–60 months | − 0.067 | 0.002 | 1782 | 24,830 | |
AEs adverse events, AI aromatase inhibitor, BC breast cancer, EQ5D-5L EuroQol-5 Dimensions – 5 levels, SE standard error
aSE assumed to be 10% of mean
Table 6.
Model input parameters for estimation of costs and effects related to MammaPrint® and endocrine therapy: Costs input parameters (costs in Euros)
| Direct health care costs | |||||
|---|---|---|---|---|---|
| Once | SE | Alpha | Beta | Source | |
| MammaPrint® costs | €2674 | fixed | n.a. | n.a. | [32] |
| Endocrine therapy | Per cycle | SE | |||
| Average of AIs | €31.03 | 3.10a | 100 | 0.31 | [33] |
| Tamoxifen (generic) | €19.11 | 1.91a | 100 | 0.19 | |
| Ovarian suppression—first cycle 3 injections per cycle | €908.91 | 90.9a | 100 | 9.09 | |
| Ovarian suppression—subsequent cycles 1 injection per cycle | €302.97 | 30.3a | 100 | 3.03 | |
| Minor adverse event costs | |||||
|---|---|---|---|---|---|
| Per cycle | SE | Alpha | Beta | Source | |
| Vasomotor | €22.17 | 0.22a | 100 | 0.22 | [39] + assumptions |
| Vulvovaginal | €22.17 | 0.22a | 100 | 0.22 | |
| Mood | €22.17 | 0.22a | 100 | 0.22 | |
| Cognitive | €22.17 | 0.22a | 100 | 0.22 | |
| Once | SE | Alpha | Beta | Source | |
| Fractures | €5486 | €1321b | 16 | 343 | [46] (once) |
| Major adverse event health care costs | |||||
|---|---|---|---|---|---|
| Once | SE | Alpha | Beta | Source | |
| Thromboembolic events | €4459 | €1130b | 16 | 279 | [47] |
| Endometrium cancer | €15,292 | €562 | 739 | 21 | [48] |
| Other malignancies | €29,897 | €7474b | 16 | 1869 | [49–51] |
| Indirect health care costs | |||||
| Per cycle | SE | Alpha | Beta | Source | |
| Indirect medical costs | Based on PAID 3.0 | n.v. | n.a. | n.a. | [34] |
| End-of-life costs | n.v. | n.a. | n.a. | ||
| Patient and family costs | |||||
|---|---|---|---|---|---|
| Per cycle | SE | Alpha | Beta | Source | |
| Travel costs | |||||
| Patients with minor AEs | €4.64 | €0.46a | 100 | 0.05 | [17] |
| Informal care costs | |||||
| Patients who died from major AEs | €2680 | €670b | 16 | 168 | [52] |
| Costs made in other sectors | |||||
|---|---|---|---|---|---|
| Per cycle | SE | Alpha | Beta | Source | |
| Costs due to productivity losses for patients treated with ET with related AEs —first 3 months | €3094 | €656 | 22 | 139 | TOTAM study (see Supplemental file 7) [28, 29] |
| Costs due to productivity losses for patients treated with ET-related AEs —next 3 months | €907 | €348 | 7 | 133 | |
| Costs due to productivity losses for patients treated with ET-related AEs —subsequent months | €846 | €342 | 6 | 138 | |
| Costs due to productivity losses for patients without ET-related AEs —first 3 months | €320 | €320 | 1 | 320 | |
| Costs due to productivity losses for patients without ET-related AEs — 3–6 months | €346 | €163 | 5 | 77 | |
| Costs due to productivity losses for patients without ET-related AEs—subsequent months | 0 | €0.5 | 1 | 250 | |
| Cost due to productivity losses for patients who died from major adverse events | €3501 | €875 | 16 | 219 | (costs of 1 friction costs period = 102 days) [17] |
| Non-medical consumption costs in life years gained | |||||
| Non-medical consumption costs for each age ≥63 y | Based on PAID 3.0 | n.v. | n.a. | n.a. | [34] |
AEs adverse events, AIs aromatase inhibitors (anastrozole, letrozole, exemestane), ET endocrine therapy, n.a. not applicable, n.v. not varied, PAID Practical Application to Include Disease Costs
aSE assumed to be 10% of mean
bSE assumed to be 25% of mean
Probabilities
Mortality from any cause was assumed to be equal to that of the general female population aged 63 years. We assumed no difference in all-cause mortality between the ultra-low–risk group treated with or without ET. This assumption was supported by the excellent metastatic free and overall survival of ultra-low–risk patients, both with and without treatment [6, 7]. Dutch life tables were used to calculate death probabilities per cycle [20].
Probabilities of major adverse events (per cycle) were calculated for four groups: premenopausal and postmenopausal women not treated with ET, postmenopausal women treated with an AI for 5 years, postmenopausal women treated with 2.5 years of tamoxifen followed by 2.5 years of AI and premenopausal women treated with tamoxifen for 5 years combined with ovarian suppression. Probabilities were obtained from the literature [8, 21]. Supplemental file 1 provides more information about the RCTs and exact calculations (see electronic supplementary material [ESM]).
Probabilities of death from major adverse events were calculated based on different publications. For the thromboembolic events, we weighted the proportion of patients with deep vein thrombosis (DVT) and pulmonary embolism (PE) [9, 22]. For endometrium cancer and other cancers, we assumed that patients would only die from metastatic disease. The occurrence of metastatic disease was estimated for 21% of patients who would develop endometrium cancer and for 27% of patients who would develop other malignancies (Supplement file 2, see ESM). Probabilities of death were applied to all patients who entered the major AE health state for the first time. By using this approach, we assumed that patients would either die from the AE within 3 months of diagnosis or would survive the AE and die from any other cause. We also assumed that patients who experienced a major adverse event would cease treatment and thus not experience a reduction in quality of life and costs due to further minor and major AEs beyond 3 months.
Minor adverse events related to ET were clustered in four overarching groups based on the publication of Kadakia et al. (2016) [23], which was an appropriate categorization according to a clinical oncologist: (i) vasomotor symptoms, which include hot flashes and night sweating; (ii) vulvovaginal symptoms, which include symptoms such as vaginal dryness and bleeding; (iii) mood symptoms, which mainly include depressive symptoms; (iv) cognitive symptoms including tiredness and forgetfulness etc.; (v) musculoskeletal symptoms for which we assumed that mainly fractures would result in costs and reductions in quality of life [23]. Probabilities of these minor AEs for patients treated with and without ET were recorded for the same groups as the major adverse events, using the same data sources. We calculated the probability of having any minor AE by taking the highest proportion of the AE groups. We assumed that minor AEs would occur directly from the start of treatment and would remain for the full 5 years of treatment (except for fractures) (Supplemental file 3, see ESM).
It is well known that many breast cancer patients discontinue ET early and therefore assuming full adherence would overestimate costs. Since the proportions of adherence were not reported in detail in the RCTs, the discontinuation rates were obtained from a Dutch publication [24]. This percentage was used to reduce ET medication costs.
Health Effects—Quality of Life
For each health state, survival was weighted by utility values to estimate QALYs. Utility values for the first year in the ‘alive’ health state were based on the study of Lidgren et al. (2007), who report utility values based on the EuroQol 5-dimensions 3-levels (EQ5D-3L) of breast cancer patients during the first year after diagnosis [25]. For the remaining years, utility values were based on the female population from the Netherlands aged 55–65 years obtained with EQ5D-3L and valued with the Dutch tariff [26]. The utility value of patients in the death state was assumed to be zero.
Dis-utilities for thromboembolic events (chronic and fatal) and endometrium cancer were obtained from the literature (Supplemental file 4, see ESM) [27]. We assumed dis-utilities for other cancers similar to disutility for endometrium cancer. The dis-utilities for acute thromboembolic events, endometrium cancer and other cancers were applied for one cycle whereas the chronic thromboembolic dis-utilities were applied for a lifelong duration.
Dis-utilities were also applied for patients who experienced any minor AE. These dis-utilities were calculated based on patient-level data from the Therapeutic Drug Monitoring Of TAMoxifen (TOTAM) study (Dutch Trial Registry; NL6918) (Supplemental file 5, see ESM) [28, 29]. This study evaluated therapeutic drug monitoring-guided dosing of adjuvant tamoxifen and collected data on adverse events, quality of life (EQ5D-5L) and productivity losses (Institute for Medical Technology Assessment [iMTA] Productivity Cost Questionnaire [PCQ]) 3, 6 and 24 months post-initiation of tamoxifen [30]. The EQ5D-5L data were valued using the Dutch tariff [31].
Costs
The costs of MammaPrint® (€2765) were based on the commercial tariff reported in previous publications [32]. Costs of ET and ovarian suppression (gonadotropin-releasing hormone agonists) were based on national tariffs [33]. Costs of ET-related minor AEs included those of visits to health care professionals (including procedures for fractures) (Supplemental file 6, see ESM), travel costs and costs of productivity losses. Travel costs were calculated by multiplying the average distance to the health care facility with the average travel costs per kilometer (Dutch costing manual) [17]. Productivity losses related to experiencing minor AEs were calculated based on patient-level data of patients in the TOTAM study (see Supplemental file 7 in the ESM for more information) [28, 29]. Costs of productivity losses were applied for 15 cycles because the average age of women in our model was 63.3 years and the retirement age in the Netherlands is 67 years in 2024 [31].
Health care costs of ET-related major AEs were based on published literature (Supplement file 8, see ESM). These costs were applied to all patients entering the major AE health state. Productivity costs (of one friction period) of major AEs and costs of informal care (of 59 hours per month) were applied only to patients dying from the events [17].
End-of-life costs, unrelated health care costs and non-medical consumption costs in life-years gained were obtained from the Practical Application to Include Disease Costs (PAID 3.0) [34].
Accounting for Uncertainty
We conducted univariate sensitivity analyses to describe the impact of uncertainty in the input parameters in our model. Input parameters were varied with ±30% and we reported the most influential parameters (i.e. deviations from the base case ICER of >€10,000). In addition to the univariate sensitivity analyses, we also performed a probabilistic sensitivity analysis using Monte Carlo simulations (1000 iterations). Beta distributions were assigned to probabilities and utility parameters, and gamma distributions to cost parameters.
Scenario and Threshold Analyses
In scenarios, we explored the impact of different assumptions for the incidence of ‘other cancers’, the share of ultra-low–risk patients identified, the amount of productivity costs, the utility values of minor adverse events and the share of patients who would receive MammaPrint®. In addition, threshold analyses were performed to evaluate at what price and incidence rate (i.e. proportion of ultra-low–risk patients identified with MammaPrint®) testing would be cost effective (Supplemental file 9, see ESM).
Model Validation
Our cost-effectiveness model was validated using the Assessment of the Validation Status of Health Economic decision models tool (AdViSHE) [35]. The conceptual model of our study followed the same structure as that of many previously conducted health economic models to evaluate interventions for early-stage breast cancer. The model was constructed according to the “Principles of good practice for decision analytic modeling in health-care evaluation: Report of the ISPOR task force on good research practices—Modeling studies” with close collaboration between health economic and clinical experts. Extreme value testing was performed for several parameters and validity checks were built into the model (i.e. constant number of patients in each cycle of the model). The model results were extensively discussed in the team. Finally, several scenario analyses were performed to assess the robustness of the results and back-of-the-envelope calculations were performed to assess whether the results were as expected (see Supplemental file 11 in the ESM).
The analyses were performed in Microsoft Excel and the list of important assumptions can be found in Supplemental file 12 (see ESM).
Results
The results of the base-case analysis are presented in Tables 2, 3 and 4.
Effectiveness
The MammaPrint® strategy yielded more QALYs and LYs and fewer AEs than the usual care strategy (Table 7). The model estimated an average of 16.73 QALYs and 19.20 LYs per patient in the MammaPrint® strategy and 16.63 QALYs and 19.14 LYs in the usual care strategy (discounted results). Moreover, in the usual care strategy, more patients experienced a major AE. Specifically, in the MammaPrint strategy, 0.82 patients were estimated to experience a thromboembolic event (within 5 years from the start of treatment), 0.45 patients would develop endometrial cancer (within 10 years) and 6.64 patients another cancer (within 10 years). In the usual care strategy, thromboembolic events, endometrial cancer and other cancers were estimated to occur in 1.59, 0.49 and 8.49 patients, respectively.
Table 7.
Model results: average discounted life-years, quality-adjusted life-years per patient and total number of patients with major adverse events
| Intervention | QALYs first 5 y | QALYs all years |
LY first 5 y | LY all years | Patients with thromboembolic events | Patients with endometrial cancer | Patients with other cancers |
|---|---|---|---|---|---|---|---|
| First 5 y | First 10 y | First 10 y | |||||
| MammaPrint® | 4.05 | 16.73 | 4.96 | 19.20 | 0.82 | 0.45 | 6.64 |
| Usual care | 4.00 | 16.63 | 4.95 | 19.14 | 1.59 | 0.49 | 8.49 |
| Increments | 0.05 | 0.10 | 0.01 | 0.07 | − 0.77 | − 0.04 | − 1.86 |
LY life-years, QALYs quality-adjusted life-years, y years
Costs
The average costs (over the 131 patients with ultra-low risk) from the health care perspective were €22,366 per patient for MammaPrint® and €4029 for the usual care strategy, respectively. The most important cost driver was the MammaPrint® costs, which were €20,472 per patient in the MammaPrint strategy and zero in the usual care strategy. The average costs from the health care perspective including costs in life-years gained were €185,770 and €166,874 for MammaPrint® and usual care, respectively. In addition to the costs of MammaPrint®, important cost drivers were the indirect medical costs (total MammaPrint®: €138,590, usual care: €138,038), followed by the end-of-life costs (total MammaPrint®: €24,814, usual care: €24,807).
From the societal perspective (excluding costs in life-years gained), the average costs were €165,062 versus €147,298 per patient in the MammaPrint® and usual care strategies, respectively. When including the costs in life-years gained, the costs were €328,466 (MammaPrint®) and €310,143 (usual care). Major cost drivers were the costs of MammaPrint®, drug acquisition costs and the costs of productivity losses (MammaPrint®: €2645, usual care: €3641). When we included the costs in life-years gained, these also contributed significantly (Table 8).
Table 8.
Model results: average discounted costs per patient and increments (costs in Euros)
| Perspective | Intervention | Direct medical costs | Indirect medical costs | Costs in other sectors | Patient and family costs | Total costs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test costs | Drug acquisition costsa | Minor adverse events (HC) | Major adverse events (HC) | End-of-life costs | Indirect medical costs | Productivity costs | Indirect consumption costs | Informal care costs | Travel costs | |||
| Health care—only direct HCC | MammaPrint® | 20,472 | n.a. | 569 | 1326 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 22,366 |
| Usual care | n.a. | 1683 | 640 | 1706 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4029 | |
| Increments | 20,472 | − 1683 | − 72 | − 380 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 18,337 | |
| Health care | MammaPrint® | 20,472 | n.a. | 569 | 1326 | 24,814 | 138,590 | n.a. | n.a. | n.a. | n.a. | 185,770 |
| Usual care | n.a. | 1683 | 640 | 1706 | 24,807 | 138,038 | n.a. | n.a. | n.a. | n.a. | 166,874 | |
| Increments | 20,472 | − 1683 | − 72 | − 380 | 7 | 552 | n.a. | n.a. | n.a. | n.a. | 18,896 | |
| Societal—excl. costs in life-years gained | MammaPrint® | 20,472 | n.a. | 569 | 1326 | n.a. | n.a. | 2645 | 139,989 | 33 | 29 | 165,062 |
| Usual care | n.a. | 1683 | 640 | 1706 | n.a. | n.a. | 3641 | 139,545 | 43 | 40 | 147,298 | |
| Increments | 20,472 | − 1683 | − 72 | − 380 | n.a. | n.a. | − 997 | 444 | − 10 | − 10 | 17,764 | |
| Societal | MammaPrint® | 20,472 | n.a. | 569 | 1326 | 24,814 | 138,590 | 2645 | 139,989 | 33 | 29 | 328,466 |
| Usual care | n.a. | 1683 | 640 | 1706 | 24,807 | 138,038 | 3641 | 139,545 | 43 | 40 | 310,143 | |
| Increments | 20,472 | − 1683 | − 72 | − 380 | 7 | 552 | − 997 | 444 | − 10 | − 10 | 18,323 | |
ET endocrine therapy, excl. excluding, HC health care, HCC health care costs, n.a. not applicable
a Includes costs of bone density measures once every 5 years for patients treated with ET
Incremental Cost-Effectiveness Ratio
Depending on the perspective and inclusion of costs in LY gained; ICERs ranged from €175,107 to €185,644 per QALY gained (Table 9). Incremental differences in QALYs were mainly driven by differences in survival time due to the occurrence of major AEs and differences in costs by the price of MammaPrint®.
Table 9.
Discounted incremental cost-effectiveness ratios (costs in Euros)
| Perspective | ||||
|---|---|---|---|---|
| Health care—only direct HCC | Health care | Societal—excl. costs in LY gained | Societal | |
| Incremental costs (EUR) per LY gained | 276,928 | 285,370 | 268,281 | 276,723 |
| Incremental costs (EUR) per QALY gained | 180,152 | 185,644 | 175,107 | 180,617 |
HCC health care costs, EUR Euros, LY life-years, QALY quality-adjusted life-year
Univariate Sensitivity Analysis
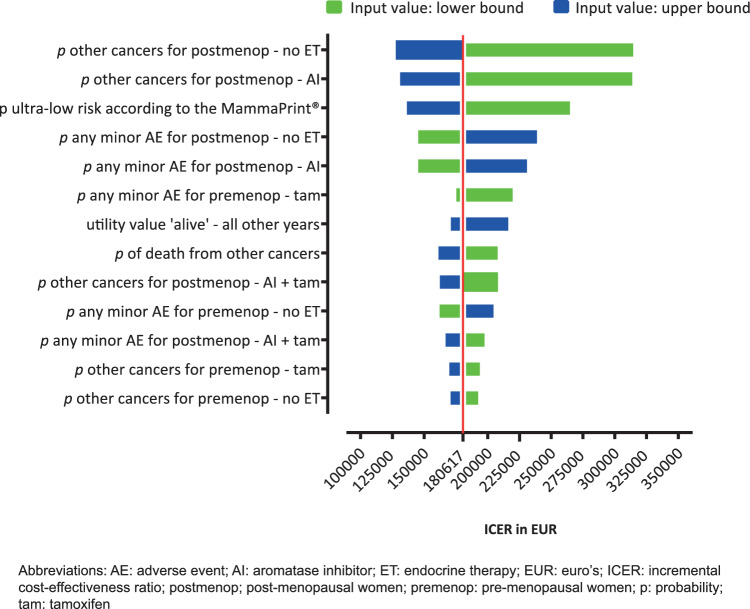
The 13 most influential parameters in our model are shown in Fig. 2. Changing the probability of getting another cancer (post-menopausal women) by +30% resulted in the highest ICER of €316,905 per QALY gained. Other influential parameters were the probability of patients with ultra-low risk as compared with the population tested with MammaPrint®, the probabilities of having any adverse events (in different subgroups) and the probability of other cancers for other subgroups.
Fig. 2.
Tornodo diagram (societal perspective, discounted)
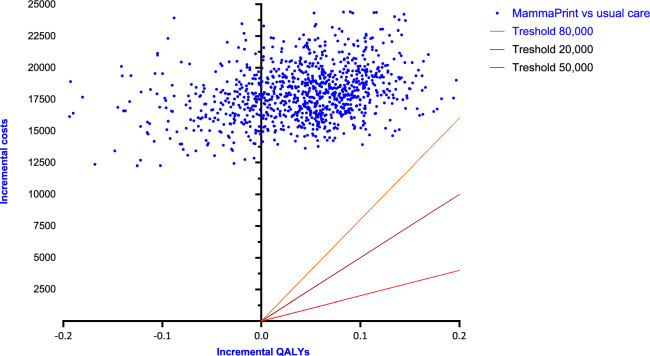
Probabilistic Sensitivity Analysis
The results of the probabilistic sensitivity analyses are shown in the cost-effectiveness plane (Fig. 3) for the societal perspective (discounted). None of the iteration fell below the WTP threshold of €80,000 per QALY, which is the highest WTP threshold used in the Netherlands.
Fig. 3.
Incremental cost-effectiveness (QALYs)
Scenario and Threshold Analyses
The results of the scenario analyses are reported in Table 10. Scenario 1, in which we assumed that patients treated with and without ET had the same chance of having another cancer in the coming 10 years instead of having a higher chance in the ET group (base case), had a major impact on the results. Changing this assumption resulted in significantly fewer QALYs and LYs gained as compared with the base case (1 LY and 5 QALYs vs 10 LY and 13 QALYs). Because the costs and incremental costs barely changed, the ICER significantly increased.
Table 10.
Scenario and threshold analyses (costs in Euros)
| Scenario | Average life-years gained | Average QALYs gained | Average costs (societal perspective) | ICER | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MammaPrint® | Usual care | Increment | MammaPrint® | Usual care | Increment | MammaPrint® | Usual care | Increment | Per LY | Per QALY | |
| Base case | 19.20 | 19.14 | 0.07 | 16.73 | 16.63 | 0.10 | 328,466 | 310,143 | 18,323 | 276,723 | 180,617 |
| Same probability of ‘other cancers’ for patients in MammaPrint® and usual care group | 19.41 | 19.40 | 0.01 | 16.91 | 16.86 | 0.05 | 330,273 | 312,485 | 17,788 | 2,043,152 | 346,434 |
| Smaller difference in productivity costs between MammaPrint® and usual care group | 19.20 | 19.14 | 0.07 | 16.73 | 16.63 | 0.10 | 332,026 | 313,399 | 18,627 | 281,306 | 183.609 |
| Reduced disutility values of minor adverse events | 19.20 | 19.14 | 0.07 | 16.79 | 16.71 | 0.08 | 328,466 | 310,143 | 18,323 | 276,723 | 227.989 |
| Costs of MammaPrint® are attributed to 60% of patients | 19.20 | 19.14 | 0.07 | 16.73 | 16.63 | 0.10 | 320,277 | 310,143 | 10,135 | 153,054 | 99,899 |
| Costs of MammaPrint® only applied to those who experience AEs | 19.20 | 19.14 | 0.07 | 16.61 | 16.47 | 0.14 | 331,388 | 313,985 | 17,403 | 262,818 | 126,074 |
| WTP threshold | Max price and reductions required to be cost effective | ||||||||||
| Varying the price | |||||||||||
| Threshold analysis 1 | 80,000 | Max price 1341 Euro, price reductions of 50% | |||||||||
| Threshold analysis 2 | 50,000 | Max price 944 Euro, price reductions of 65% | |||||||||
| Threshold analysis 3 | 20,000 | Max price 546 Euro, price reductions of 80% | |||||||||
| Varying the proportion of patients with ultra-low risk identified with MammaPrint®—proportion (n) | |||||||||||
| Base case | 13% (7.65) | ||||||||||
| Threshold analysis 1 | 80,000 | 26% (3.83) | |||||||||
| Threshold analysis 2 | 50,000 | 37% (2.70) | |||||||||
| Threshold analysis 3 | 20,000 | 64% (1.56) | |||||||||
AEs adverse events, ICER incremental cost-effectiveness ratio, LY life-years, max maximum, QALYs quality-adjusted life-years, WTP willingness to pay
The threshold analyses revealed that the price of MammaPrint® should be reduced by 50%, 65% or 80% for the MammaPrint® strategy to be cost effective depending on the WTP threshold. Another way for MammaPrint® to become cost effective would be if the proportion of patients for which treatment could be altered (i.e. those with ultra-low risk) increased to at least 26%, 37% or 64% instead of the 13% in the base case (20,000, 50,000, 80,000, respectively).
Discussion
Our study showed that MammaPrint®-guided adjuvant ET yields more QALYs and LYs but substantially higher costs in Dutch early breast cancer patients who are eligible for ET only. The costs were mostly driven by the relatively high number of patients needing to be tested to identify one patient who can safely forgo ET, and the price of MammaPrint®. Depending on the perspective, the ICERs ranged from €174,450 to €185,644. With the willingness-to-pay thresholds used in the Netherlands, the MammaPrint® strategy will not be a cost-effective strategy for guiding adjuvant ET for the population in our study. The proportion of patients for which treatment would be altered needs to be at least 26%, or the price of MammaPrint® needs to be reduced by >50% to make MammaPrint® a cost-effective strategy for use in our study.
To our knowledge, this study is the first to evaluate the cost effectiveness of MammaPrint® to guide the use of adjuvant ET only. Based on the cost-effectiveness results, we would not recommend reimbursing MammaPrint® from the basic benefit package to guide ET treatment decisions. The value for money of the test can possibly be improved by pre-selecting patients eligible for the test. In our comparison, many patients need to be tested with MammaPrint®, to identify one patient that can be classified as being at ultra-low risk of recurrence and can safely forgo ET (i.e. 13%) [6, 7]. This means that the total costs of testing (i.e. population size times the price of the test) are relatively high compared with the share of patients who benefit. Clearly, if fewer patients needed to be tested, the ICER will improve. Previous studies have suggested that the frequency of ultra-low–risk breast cancers is higher in screen-detected cancers and in patients with more favorable characteristics [6]. Additionally, one of our scenario analyses showed that the ICER improves if the test is only used in patients who develop minor symptoms. Further research identifying which patients benefit most from the test may be relevant to optimize the use of MammaPrint®.
Despite the results of our study, we believe that de-escalating ET is relevant for patients with early breast cancer. Not only did the patients in the MammaPrint® strategy experience fewer side effects, but they also had a slightly better quality of life and survival rate. Moreover, health care costs and productivity losses were lower. Since adjuvant ET is frequently used, de-escalation strategies have the potential to reduce the treatment burden in many patients and also save costs to society [36]. Unfortunately, de-escalation studies in this area are scarce, possibly because the consequences of ET-related side effects on patients and society are understudied and underestimated and treatment costs are relatively low [37]. In fact, we did not find a single European study that evaluated the effect of ET and adverse events on productivity losses and few studies that assessed the impact on quality of life and health care costs [38, 39]. This observation is especially notable given the substantial amount of literature on ET. Ultimately, to truly improve patient outcomes and the economics of health care, researchers should include broader outcomes (e.g. quality of life and costs) in their studies.
Quantifying the impact of relatively mild adverse events, such as those related to ET, is complex. As in most cost-effectiveness analyses, we expressed the benefits of omitting ET in QALYs, based on utility measures derived from a generic quality-of-life instrument (i.e. the EQ5D) [40]. Generic instruments cover universal health aspects (e.g. self-care, mobility, pain), which makes them relevant for patients with all types of diseases. The disadvantage is that these instruments are usually not very responsive to specific health problems, such as the menopausal symptoms in patients treated with ET. As such, we may not have fully captured the beneficial effects of omitting ET. Possibly, the value-based health care (VBHC) framework would have been more sensitive to capturing effects related to reductions in minor AEs because this framework is focused on outcomes that are most relevant to patients [41]. Nevertheless, even in this framework, finding the appropriate measure to value the outcomes would be a challenge.
There are a couple of limitations of our study. First, we assumed 100% accuracy of MammaPrint® to stratify patients into the different risk groups and it is unlikely that this assumption holds. However, even with this optimistic assumption, the ICER is already far above the WTP threshold used in the Netherlands. A lower test accuracy would reduce the effects and increase the costs of the MammaPrint® strategy, hence increase the ICER. Second, given the currently available evidence, we could not assess the ICER in different age groups. Further research in potential subgroups is recommended. For example, the share of patients with ultra-low versus low/high risk has a major impact on the ICER and could therefore lead to different conclusions regarding cost effectiveness. Third, transition probabilities of minor AEs related to ET were based on different RCTs which used various definitions and described different levels of detail. For instance, one trial reported vasomotor symptoms without further specifying these, whereas another reported hot flashes, sweating and fatigue separately, even by grade [8, 10]. Fourth, major AE probabilities were based on studies that were not powered to report differences in these outcomes. The reported differences may thus have occurred due to chance. Finally, we assumed that all patients in the usual care strategy were treated with ET (in line with guidelines) and that all pre-menopausal women were treated with ovarian suppression. These assumptions are likely not entirely true. In fact, in daily practice only about 70% of the patients with the characteristics of those in our study actually initiate ET [39]. Moreover, probably not all young women are willing to be treated with ovarian suppression. This suggests that ICERs would be even less favorable. Despite these limitations, we think that our study convincingly showed that the use of MammaPrint as described in this study is effective but not yet a cost-effective strategy.
Conclusion
This study suggests that MammaPrint® testing to guide adjuvant ET in patients only eligible for ET is an effective but not a cost-effective strategy compared with usual care. De-escalating ET appears to offer gains in survival time and quality of life and results in lower direct medical costs and productivity losses. Likely, the cost effectiveness of the test can be improved if it would be possible to pre-select patients who can best benefit from the test.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No funding was acquired for this study.
Competing Interests
M. Luyendijk, S.M. Buijs, S. Siesling have no conflicts of interest to declare. A. Jager reports an unrestricted grant received from Agendia® (MammaPrint®) to set up a database with genomic data (obtained with MammaPrint®) with the objective to discover new prognostic molecular markers within early stage breast cancer patients. Note: no restrictions were imposed on the use of the grant paid to the institute. Dr Blommestein reports a fee for participation in an advisory board from Pfizer, paid to the institute. C.A. Uyl-de Groot reports grants from Boehringer Ingelheim, Astellas, Celgene, Sanofi, Jansen-Cilag, Bayer, Amgen, Genzyme, Merck, Gilead, Novartis, AstraZeneca, Roche, all payments to the institute.
Ethics Approval
The TOTAM study, from which we used patient-level data, was approved by the Local Ethics Committee (Erasmus MC, Rotterdam; MEC 17-548) and was registered at the Dutch Trial Registry (NL6918).
Consent to Participate
All patients participating in the TOTAM study gave their written informed consent for the study and also to use their data for other (related) research.
Consent for Publication
Not applicable.
Availability of Data and Material
The model is available on request.
Authors Contributions
HB, AJ, ML contributed to the development of the study design. HB, AJ, ML, SB contributed to the data collection and data analyses. All authors contributed to the critical revision of the manuscript. All authors read and agreed with the final version of the manuscript.
References
- 1.Piccart MJ, Hilbers FS, Bliss JM, Caballero C, Frank ES, Renault P, et al. Road map to safe and well-designed de-escalation trials of systemic adjuvant therapy for solid tumors. J Clin Oncol. 2020;38:4120–4129. doi: 10.1200/JCO.20.01382. [DOI] [PubMed] [Google Scholar]
- 2.Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700–1712. doi: 10.1093/annonc/mdy537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes JH, Ollendorf DA, Pearson SD, Barry MJ, Kantoff PW, Lee PA, et al. Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158:853–860. doi: 10.7326/0003-4819-158-12-201306180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ontario Health (Quality) Gene expression profiling tests for early-stage invasive breast cancer: A health technology assessment. Ont Health Technol Assess Ser. 2020;20:1–234. [PMC free article] [PubMed] [Google Scholar]
- 5.Retèl VP, Byng D, Linn SC, Jóźwiak K, Koffijberg H, Rutgers EJ, et al. Cost-effectiveness analysis of the 70-gene signature compared with clinical assessment in breast cancer based on a randomised controlled trial. Eur J Cancer. 2020;137:193–203. doi: 10.1016/j.ejca.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Lopes Cardozo JM, Drukker CA, Rutgers EJ, Schmidt MK, Glas AM, Witteveen A, et al. Outcome of patients with an ultralow-risk 70-gene signature in the MINDACT trial. J Clin Oncol. 2022;40:1335–1345. doi: 10.1200/JCO.21.02019. [DOI] [PubMed] [Google Scholar]
- 7.Esserman LJ, Yau C, Thompson CK, Van't Veer LJ, Borowsky AD, Hoadley KA, et al. Use of molecular tools to identify patients with indolent breast cancers with ultralow risk over 2 decades. JAMA Oncol. 2017;3:1503–1510. doi: 10.1001/jamaoncol.2017.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;23:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. doi: 10.1016/S1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, et al. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman NL, Rohrbacker NJ, Bushmakin AG, Whiteley J, Lynch WD, Shah SN. Direct and indirect costs of women diagnosed with menopause symptoms. J Occup Environ Med. 2013;55:465–470. doi: 10.1097/JOM.0b013e3182820515. [DOI] [PubMed] [Google Scholar]
- 12.Federation of Medical Specialists. Dutch Breast Cancer Guidelines – Geneexpression profiling [in Dutch: Borstkanker - Genexpressie profielen]. https://richtlijnendatabase.nl/richtlijn/borstkanker/risicoprofilering/genexpressie_profielen.html. Accessed 20 Jan 2023.
- 13.National Health Care Institute the Netherlands (ZIN). Standpunt MammaPrint® bij vrouwen met vroeg stadium borstkanker (herbeoordeling). 2018. https://www.zorginstituutnederland.nl/publicaties/standpunten/2018/09/27/standpunt-mammaprint-bij-vrouwen-met-vroeg-stadium-borstkanker-herbeoordeling. Accessed 20 Jan 2023.
- 14.Netherlands Comprehensive Cancer Organization (IKNL). 2022. Netherlands Cancer Registry: data available on request.
- 15.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer: version 2.2022 –– December 20, 2021. https://www.nccn.org/guidelines/recently-published-guidelines. Accessed 04 April 2022.
- 16.Federation of Medical Specialists. Dutch Breast Cancer Guidelines. 2020. https://www.oncoline.nl/borstkanker. Accessed 4 Apr 2020.
- 17.National Health Care Institute the Netherlands (ZIN). Dutch Guidelines for Economic Evaluations in Healthcare. 2016. https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg. Accessed 15 Oct 2021.
- 18.National Health Care Institute the Netherlands (ZIN). Cost-effectiveness in practice [In Dutch: Kosteneffectiviteit in de praktijk]. 2015. https://www.zorginstituutnederland.nl/publicaties/rapport/2015/06/26/kosteneffectiviteit-in-de-praktijk. Accessed 7 June 2022.
- 19.Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A et all. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 2 Jan 2023.
- 20.Statistics Netherlands. Survival probability by gender and age. 2022. https://opendata.cbs.nl/#/CBS/nl/dataset/70701ned/table. Accessed 3 Feb 2022.
- 21.van de Velde JH, Rea D, Seynaeve C, Putter H, Hasenburg A, Vannetzel J. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet Oncol. 2011;377:321–331. doi: 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 22.Næss IA, Christiansen S, Romundstad P, Cannegieter S, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 23.Kadakia KC, Snyder CF, Kidwell KM, Seewald NJ, Flockhart DA, Skaar TC, et al. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist. 2016;21:539–546. doi: 10.1634/theoncologist.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Herk-Sukel MP, van de Poll-Franse LV, Voogd AC, Nieuwenhuijzen GA, Coebergh JWW, Herings R. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat. 2010;122:843–851. doi: 10.1007/s10549-009-0724-3. [DOI] [PubMed] [Google Scholar]
- 25.Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16:1073–1081. doi: 10.1007/s11136-007-9202-8. [DOI] [PubMed] [Google Scholar]
- 26.Janssen M, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König H-H. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019;20:205–216. doi: 10.1007/s10198-018-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snowsill T, Coelho H, Huxley N, Jones-Hughes T, Briscoe S, Frayling IM, et al. Molecular testing for Lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess. 2017;21:1–280. doi: 10.3310/hta21510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braal CL, Jager A, Hoop EO-d, Westenberg JD, Lommen KM, de Bruijn P, et al. Therapeutic drug monitoring of endoxifen for tamoxifen precision dosing: feasible in patients with hormone-sensitive breast cancer. Clin Pharmacokinetics. 2022;61:527–537. doi: 10.1007/s40262-021-01077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braal CL, Kleijburg A, Jager A, Koolen SL, Mathijssen RH, Corro Ramos I, et al. Therapeutic drug monitoring-guided adjuvant tamoxifen dosing in patients with early breast cancer: a cost-effectiveness analysis from the prospective TOTAM TRIAL. Clin Drug Investig. 2022;42:163–175. doi: 10.1007/s40261-021-01114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart-van RL. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health. 2015;18:753–758. doi: 10.1016/j.jval.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Versteegh MM, Vermeulen KM, Evers SM, De Wit GA, Prenger R, Stolk EA. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19:34–52. doi: 10.1016/j.jval.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Retèl VP, Joore MA, Knauer M, Linn SC, Hauptmann M, van Harten WH. Cost-effectiveness of the 70-gene signature versus St. Gallen guidelines and Adjuvant Online for early breast cancer. Eur J Cancer. 2010;46:1382–1391. doi: 10.1016/j.ejca.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 33.National Health Care Institute the Netherlands (ZIN). Pharmacotherapeutic Compass [In Dutch: Farmacotherapeutisch Kompas]. https://www.farmacotherapeutischkompas.nl/. Accessed 27 Aug 2021.
- 34.van Baal PH, Wong A, Slobbe LC, Polder JJ, Brouwer WB, de Wit GA. Standardizing the inclusion of indirect medical costs in economic evaluations. Pharmacoeconomics. 2011;29:175–187. doi: 10.2165/11586130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Vemer P, Corro Ramos I, Van Voorn G, Al M, Feenstra T. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34:349–361. doi: 10.1007/s40273-015-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netherlands Comprehensive Cancer Organization (IKNL). Breast Cancer in the Netherlands: trends 1989-2020 based on data from the Netherlands Cancer Registry [in Dutch: Borstkanker in Nederland trends 1989-2020, gebaseerd op cijfers uit de Nederlandse Kankerregistratie]. https://iknl.nl/getmedia/fe459c3a-c561-40de-b740-fff15997f020/IKNL-Folder-Borstkanker-2020.pdf. Accessed 4 Apr 2022.
- 37.Condorelli R, Vaz-Luis I. Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev Anticancer Ther. 2019;18:1101–1112. doi: 10.1080/14737140.2018.1520096. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira A, Di Meglio A, Pistilli B, Gbenou A, El-Mouhebb M, Dauchy S, et al. Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: a prospective patient-reported outcomes analysis. Ann Oncol. 2019;30:1784–1795. doi: 10.1093/annonc/mdz298. [DOI] [PubMed] [Google Scholar]
- 39.Verbeek JG, Atema V, Mewes JC, van Leeuwen M, Oldenburg HS, van Beurden M, et al. Cost-utility, cost-effectiveness, and budget impact of Internet-based cognitive behavioral therapy for breast cancer survivors with treatment-induced menopausal symptoms. Breast Cancer Res Treat. 2019;178:573–585. doi: 10.1007/s10549-019-05410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4. Oxford: Oxford University Press; 2015. [Google Scholar]
- 41.Walraven J, Jacobs MS, Uyl-de Groot CA. Leveraging the similarities between cost-effectiveness analysis and value-based healthcare. Value Health. 2021;24:1038–1044. doi: 10.1016/j.jval.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Statistics Netherlands. Labor participation. 2022. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/82309NED/table?fromstatweb. Accessed 1 Feb 2022.
- 43.Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Federation of Medical Specialists. Dutch Endometrial Cancer Guidelines. 2011. https://richtlijnendatabase.nl/richtlijn/endometriumcarcinoom/epidemiologie_en_etiologie.html. Accessed 02 June 2022.
- 45.Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 46.Lötters FJ, van den Bergh JP, de Vries F, Rutten-van Mölken MP. Current and future incidence and costs of osteoporosis-related fractures in the Netherlands: combining claims data with BMD measurements. Calcif Tissue Int. 2016;98:235–243. doi: 10.1007/s00223-015-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heisen M, Treur MJ, Heemstra HE, Giesen EB, Postma MJ. Cost-effectiveness analysis of rivaroxaban for treatment and secondary prevention of venous thromboembolism in the Netherlands. J Med Econ. 2017;20:813–824. doi: 10.1080/13696998.2017.1331912. [DOI] [PubMed] [Google Scholar]
- 48.Pennington M, Gentry-Maharaj A, Karpinskyj C, Miners A, Taylor J, Manchanda R, et al. Long-term secondary care costs of endometrial cancer: a prospective cohort study nested within the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) PLoS ONE. 2016;11:e0165539. doi: 10.1371/journal.pone.0165539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vondeling GT, Menezes GL, Dvortsin EP, Jansman FGA, Konings IR, Postma MJ, et al. Burden of early, advanced and metastatic breast cancer in The Netherlands. BMC Cancer. 2018;18:262–273. doi: 10.1186/s12885-018-4158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Linden N, Buter J, Pescott CP, Lalisang RI, De Boer JP, de Graeff A, et al. Treatments and costs for recurrent and/or metastatic squamous cell carcinoma of the head and neck in the Netherlands. Eur Arch Otorhinolaryngol. 2016;273:455–464. doi: 10.1007/s00405-015-3495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noels E, Hollestein L, Luijkx K, Louwman M, de Groot C, Bos R, et al. Increasing costs of skin cancer due to increasing incidence and introduction of pharmaceuticals, 2007–2017. Acta Derm Venereol. 2020 doi: 10.2340/00015555-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gout-Zwart J, Vondeling G, Rozenbaum M, Beby A, Postma M. PCN393-The burden of informal care for breast cancer patients in the Netherlands. Value Health. 2018;21:S81. doi: 10.1016/j.jval.2018.09.183. [DOI] [Google Scholar]
- 53.Lloyd AJ, Dewilde S, Noble S, Reimer E, Lee AY. What impact does venous thromboembolism and bleeding have on cancer patients’ quality of life? Value Health. 2018;21:449–455. doi: 10.1016/j.jval.2017.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.