Abstract
Purpose
The purpose of this study was to perform a systematic review with meta-analysis on the long-term survival rates of zygomatic implants (ZI). ZI success, prostheses survival and success, sinus pathology and patient reported outcomes were also investigated.
Methods
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines were followed. Embase and OvidMedline databases were searched alongside the grey literature. The systematic review was recorded in PROSPERO (CRD42022358024). Studies reporting titanium/titanium alloy ZI survival data, ZI-supported prosthesis data, ZIs directly compared to any other implant therapy including grafted sites, a minimum follow-up time of 3 years and a minimum number of 10 patients were included. All study designs were considered if they met the inclusion criteria. Studies not involving ZIs, ZIs not made from titanium/titanium alloy, a follow-up time of < 3 years or < 10 patients, animal studies and in vitro studies were excluded. Long-term follow-up has not been defined in the literature. A minimum of 3 years follow-up was considered acceptable to capture survival after initial healing, alongside in-function prosthesis data via delayed or immediate load protocols. ZI success, was predominantly defined as ZI survival without biological or neurological complications. Meta-analyses were performed for ZI survival, ZI failure incidence, ZI success, loading protocol, prosthesis survival, and prevalence of sinusitis using random effects models. Descriptive analysis was used for ZI success, prosthesis success and patient reported outcome measures.
Results
Five hundred and seventy-four titles were identified, of which 18 met the inclusion criteria. Eligible studies included 1349 ZIs in 623 patients. Mean follow-up period was 75.4 months (range 36–141.6). The mean survival of ZIs was 96.2% [95% CI: 93.8; 97.7] at 6 years. Mean survival for delayed loading was 95% [95% CI: 91.7; 97.1] and 98.1% [95% CI: 96.2; 99.0] for immediate loading (p = 0.03). Annual incidence rate of ZI failure was 0.7% [95% CI 0.4; 1.0]. Mean ZI success was 95.7% [95% CI 87.8; 98.6]. Mean prosthesis survival was 94% [95% CI 88.6; 96.9]. Sinusitis prevalence was 14.2% [95% CI 8.8; 22.0] at 5 years. Patients’ reported increased satisfaction with ZIs.
Conclusions
ZIs have long-term survival comparable to conventional implants. Immediate loading showed a statistically significant increase in survival over delayed loading. Prosthesis survival was similar to that of prostheses supported by conventional implants, with similar complications. Sinusitis was the most frequently encountered biological complication. Patients reported improved outcome measures with ZI use.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40729-023-00479-x.
Keywords: Zygoma, Implant, Atrophic maxilla, Survival, Rehabilitation, Sinusitis, Patient reported outcome
Introduction
Implant-supported rehabilitation of the maxilla represents a significant surgical challenge when bone volumes are inadequate to allow placement of conventional implants with various treatment modalities explored [1]. Patients face the likelihood of protracted treatment times with significant morbidity related to donor harvest sites, and the additional costs related to the use of biomaterials. Prosthodontic rehabilitation may also be delayed, as two-stage procedures are required if primary stability is not achieved, or when hard tissue reconstruction is required prior to implant placement. These challenges may act as barriers to the provision of care, which may otherwise improve the quality of patients’ lives.
The use of the zygoma as an anchorage site was first explored by Branemark in 1988, using customised, increased-length conventional implants. This technique was first reported in the literature by Aparicio [2], when stabilising a graft in the pre-maxilla using the zygomatic process of the maxilla for anchorage. These implants may present an opportunity to bypass the conventional route of hard tissue regeneration, as well as to reduce treatment time frames [3]. Zygomatic implant (ZI) designs are also evolving to meet increased demand in the face of an expanding range of clinical indications, such as oncologic resections or trauma, with key roles including the retention of obturators or in combination with free flap reconstructions [4].
Treatment modalities include ZIs splinted to conventional implants in the anterior maxilla; or the use of a quad zygoma approach [5] for fixed or removable reconstructions, initial prosthetic rehabilitation followed a two-stage approach, but immediate loading has been identified as a viable treatment modality too [6, 7], with some studies reporting this as the preferred protocol in terms of survival outcomes. The anterior–posterior (AP) spread for prosthetic reconstruction can also improved in the case of significantly pneumatised sinuses extending into the canine region, when options are limited for tilted implants [8]. The ability to deliver implants without the conventional challenges as described above, alongside immediate loading, presented a significant step forwards in terms of patient experience and expediency of care in compromised clinical situations. ZIs appear to show good survival rates in the short to medium term [9], however, complications include sinus pathology and oro-antral communications [10, 11]. Prostheses supported by ZIs also appear to show high survival in the short [12] and medium term [13]. There is little data on the long-term survival and success of the prosthetic reconstructions supported by ZIs, or on patient reported outcomes. Therefore, the aim of this systematic review was to assess long-term ZI survival rates, and to report on biological, prosthetic, mechanical and patient reported outcomes (PROMS) based on previously published clinical studies. The null hypothesis was no difference in the prevalence of ZI survival, or the prevalence and complications related to ZI-supported reconstructions, when compared to conventional implants and their reconstructions in the maxilla.
Methods
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines were followed for this review. The systematic review was recorded in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42022358024.
Eligibility criteria
Inclusion criteria were formulated using the PIO format. The population (P) included adults over the age of 18, who had received titanium/titanium alloy ZIs. The intervention (I) was the use of restored and unrestored ZIs. The outcome (O) was ZI survival with secondary outcomes including ZI success, prosthetic survival and success, prosthetic complications, sinus pathology and patient reported outcomes over a minimum of 3 years follow-up.
Inclusion criteria
Clinical studies which met the following inclusion criteria were included: Studies including ZIs, studies including ZI-supported fixed or removable reconstructions, ZIs made from titanium/titanium alloy, studies directly comparing ZIs to any other conventional implant therapy (included grafted sites), a minimum follow-up time of 3 years and a minimum number of 10 patients. Study designs included were randomised controlled trials, clinical trials, prospective case series and retrospective case series.
Exclusion criteria
The exclusion criteria were clinical studies that did not involve ZIs, ZIs not made from titanium/titanium alloy, a follow-up time of less than 3 years, or with less than 10 patients included. Animal studies and in vitro studies were excluded.
Study identification
Studies were extracted from Embase, dating 1974 to June 21, 2022 and from Ovid MEDLINE dating 1946 to June 21 2022. The full search strategy table is tabulated in Additional file 1: Table S1. The search was not limited by language.
Outcome measures
ZI survival (presence or absent at follow-up) was the primary outcome measure. Secondary outcomes included ZI success, ZI-supported prosthesis survival, success and complications, sinus pathology and patient reported outcomes. Heterogeneity was noted in the reporting of ZI success, which was predominantly defined as ZI survival without biological or neurological complications across the studies.
Data extraction
Data were independently extracted and assessed by two reviewers (MBR and TD). Collection included authors, year of publication, patient data, outcomes reported, loading protocols and follow-up periods. Outcomes recorded were ZI survival, ZI success, ZI-supported prosthesis survival and/or success, sinus data and PROMs. Study authors were contacted in the event of missing data and the report excluded in the event of no reply or inadequate data.
Risk of bias assessment
The papers included were case series reports. Therefore, the critical appraisal checklist for case series, developed by the Joanna Briggs Institute (critical appraisal tools for use in systematic reviews, 2017) was used to assess the risk of bias. This checklist identified the completeness of the report, risk of bias, and the accuracy of reporting. Reports were independently assessed by two reviewers (MBR and AP) with conflicting outcomes resolved by discussion with a third reviewer (TD).
Statistical analysis
Effect measures were generated for ZI survival prevalence (%), ZI failure incidence rate (%/year), ZI success prevalence (%), prosthesis survival prevalence (%), and sinusitis prevalence (%). ZI failure incidence rate was calculated as the total annual failure incidence rate over the complete follow-up (%/year), failure incidence in the first year (%), and annual failure incidence after the first year of follow-up (%). In addition, an a priori specified subgroup analysis comparing the ZI survival (%) between the different loading protocols (immediate versus delayed) was performed. Statistical heterogeneity was assessed as substantial if I2 was > 50% [14]. All meta-analyses were performed in R (v4.2.2, meta-package) using a random effects model with the DerSimonian–Laird estimator. Reporting bias was assessed through funnel plots if > 10 studies were available per endpoint if no clinical or statistical heterogeneity was observed. In all analyses, p < 0.05 (two-tailed) was considered statistically significant.
Results
Selection process
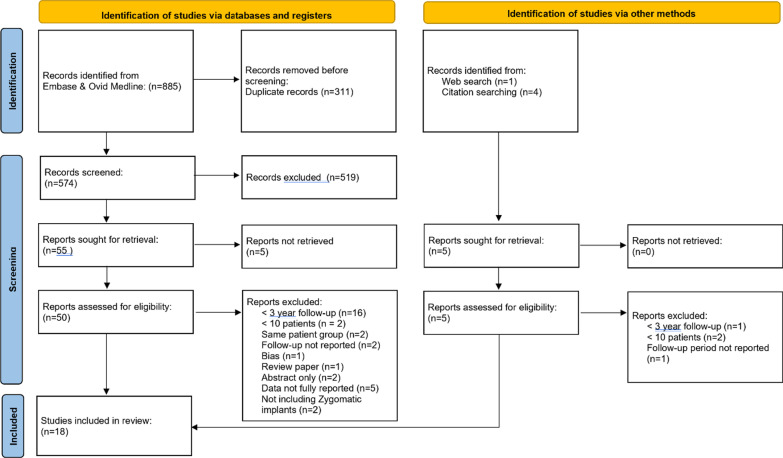
An initial screening of 574 titles and abstracts was carried out by the main author (MBR) (Fig. 1). The grey literature search identified 5 studies. 55 papers were subsequently sought for retrieval. Full-text articles were not retrieved for 5 reports leaving 50 reports to be assessed for eligibility.
Fig. 1.
Flow diagram of study identification process
These reports were independently reviewed according to the inclusion and exclusion criteria by two reviewers (MBR and TD). The studies included, along with the extracted data, are presented in Table 1, whilst patient demographics are presented in Table 2.
Table 1.
Datasets extracted from the included studies (n = 18)
| Study | Year of publication | Number of patients | Mean follow-up (months) | Outcomes reported | Loading protocol | Study type | Zygomatic implant number | Female | Mean age | Age range | Smokers |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kahnberg et al. 2007 | 2007 | 76 | 36 |
Survival Prosthesis success Sinus data PROM |
Delayed | Prospective Case Series | 145 | 57 | 58 | 35–77 | 15 |
| Pellegrino et al. 2020 | 2020 | 20 | 39.9 |
Survival Success Prosthesis survival Prosthesis success PROM |
Immediate | Prospective Case Series | 73 | NR | 56.7 | ± 12.6 | 9 |
| Aparicio et al. 2014 | 2014 | 80 | 55.44 |
Survival Sinus data PROM |
Delayed | Retrospective Case Series | 157 | 55 | 3.81 | 42.78–64.78 | 24 |
| Coppede et al. 2017 | 2017 | 42 | 60 | Survival | Immediate and delayed | Prospective Case Series | 94 | 32 | 58 | 37–79 | 8 |
| Davo and Pons 2015 | 2015 | 14 | 60 |
Survival Success Prosthesis survival Sinus data PROM |
Immediate | Prospective Case Series | 68 | 10 | 57.7 | 41–78 | NR |
| Malo et al. 2014 | 2014 | 39 | 60 |
Survival Success Prosthesis survival Sinus data PROM |
Immediate | Retrospective Case Series | 92 | 30 | 53.5 | 32–77 | 4 |
| Davo, Malevez and Pons 2013 | 2013 | 36 | 60 |
Survival Success Prosthesis success Sinus data |
Immediate | Prospective Case Series | 69 | 23 | 57.5 | 34–79 | NR |
| Davo 2009 | 2009 | 21 | 60 |
Survival Prosthesis success Sinus data |
Delayed | Retrospective Case Series | 39 | 16 | 51.4 | 36–72 | NR |
| Branemark et al. 2004 | 2004 | 28 | 60 |
Survival Prosthesis survival Sinus data |
Delayed | Prospective Case Series | 52 | 16 | 58.3 | 39–79 | NR |
| Yates et al. 2013 | 2013 | 23 | 72 |
Survival Success |
Delayed | Retrospective Case Series | 43 | 13 | NR | NR | 6 |
| Bedrossian E. 2010 | 2010 | 36 | 84 | Survival | Immediate | Prospective Case Series | 74 | 22 | NR | NR | NR |
| Agliardi et al. 2017 | 2017 | 15 | 85.04 |
Survival Success Prosthesis success Sinus data PROM |
Immediate | Prospective Case Series | 42 | 13 | 62 | 46–70 | NR |
| Chana et al. 2019 | 2019 | 45 | 90 |
Survival Prosthesis survival Sinus data |
Immediate and delayed | Retrospective Case Series | 88 | 23 | 56.27 | ± 13 | 14 |
| Miglioranca et al. 2012 | 2012 | 21 | 96 |
Survival Prosthesis survival |
Immediate | Prospective Case Series | 40 | 13 | 55 | ± 6.66 | 14 |
| Fortin 2017 | 2017 | 58 | 100.8 |
Survival Success |
Immediate and delayed | Retrospective Case Series | 107 | 40 | 65.3 | ± 8 | NR |
| Bothur et al. 2015 | 2015 | 14 | 111.6 |
Survival Prosthesis survival |
Delayed | Prospective Case Series | 58 | 9 | 60 | 51–78 | 3 |
| Aparicio et al. 2014 | 2014 | 22 | 120 |
Survival Prosthesis survival Sinus data PROM |
Delayed | Prospective Case Series | 41 | 14 | 63.10 | 48–80 | 5 |
| Di Cosola et al. 2021 | 2021 | 33 | 141.6 |
Survival Success Sinus data |
Immediate and delayed | Retrospective case series | 67 | 17 | 59.1 | NR | 7 |
Table 2.
Included studies and characteristics (n = 18)
| Study | Study type | Zygomatic implant number | Female | Mean age | Age range | Smokers |
|---|---|---|---|---|---|---|
| Kahnberg et al. 2007 | Prospective case series | 145 | 57 | 58 | 35–77 | 15 |
| Pellegrino et al. 2020 | Prospective case series | 73 | NR | 56.7 | ± 12.55 | 9 |
| Aparicio et al. 2014 | Retrospective case series | 157 | 55 | 3.81 | 42.78–64.78 | 24 |
| Coppede et al. 2017 | Prospective case series | 94 | 32 | 58 | 37–79 | 8 |
| Davo and Pons 2015 | Prospective case series | 68 | 10 | 57.7 | 41–78 | NR |
| Malo et al. 2014 | Retrospective case series | 92 | 30 | 53.5 | 32–77 | 4 |
| Davo, Malevez and Pons 2013 | Prospective case series | 69 | 23 | 57.5 | 34–79 | NR |
| Davo 2009 | Retrospective case series | 39 | 16 | 51.4 | 36–72 | NR |
| Branemark et al. 2004 | Prospective case series | 52 | 16 | 58.3 | 39–79 | NR |
| Yates et al. 2013 | Retrospective case series | 43 | 13 | NR | NR | 6 |
| Bedrossian 2010 | Prospective case series | 74 | 22 | NR | NR | NR |
| Agliardi et al. 2017 | Prospective case series | 42 | 13 | 62 | 46–70 | NR |
| Chana et al. 2019 | Retrospective case series | 88 | 23 | 56.27 | ± 12.95 | 14 |
| Miglioranca et al. 2012 | Prospective case series | 40 | 13 | 55 | ± 6.66 | 14 |
| Fortin 2017 | Retrospective case series | 107 | 40 | 65.3 | ± 8 | NR |
| Bothur et al. 2015 | Prospective case series | 58 | 9 | 60 | 51–78 | 3 |
| Aparicio et al. 2014 | Prospective case series | 41 | 14 | 63.10 | 48–80 | 5 |
| Di Cosola et al. 2021 | Retrospective case series | 67 | 17 | 59.1 | NR | 7 |
NR not reported
Excluded studies and the reasons for exclusion are presented in Additional file 1: Table S2.
Zygomatic implant survival
Survival description across studies
Eligible studies included a total of 1349 ZIs placed in 623 patients. Survival was determined by the presence or absence of a ZI at completion of the study. The mean follow-up period across all studies was 75.4 months (6.3 years) with a follow-up range of 36 to 141.6 months (3 years–11.8 years). Table 3 reports the ZI survival dataset.
Table 3.
Zygomatic implant survival dataset
| Study | Patient number | Zygomatic implant number | Number of failed implants | Number of patients failure in | Survival | Follow-up Mean (months) |
Follow-up range (months) |
|---|---|---|---|---|---|---|---|
| Kahnberg et al. 2007 | 76 | 145 | 5 (3.45%) | 4 (5.26%) | 96.5 | 36 | N/A |
| Pellegrino et al. 2020 | 20 | 73 | 2 (2.75%) | 2 (10%) | 97.3 | 39.9 | ± 19.5 |
| Aparicio et al. 2014 | 80 | 157 | 5 (3.18%) | 2 (2.5%) | 96.8 | 55.4 | ± 234.7 |
| Coppede et al. 2017 | 42 | 94 | 1 (1.06%) | 1 (2.38%) | 98.9 | 60 | N/A |
| Davo and Pons 2015 | 14 | 68 | 0 (0.00%) | 0 (0.00%) | 100 | 60 | N/A |
| Malo et al. 2014 | 39 | 92 | 1 (1.09%) | 1 (2.56%) | 98.9 | 60 | N/A |
| Davo, Malevez and Pons 2013 | 36 | 69 | 1 (1.45%) | 1 (2.78%) | 98.6 | 60 | N/A |
| Davo 2009 | 21 | 39 | 1 (2.56%) | 1 (4.76%) | 97.4 | 60 | N/A |
| Branemark et al. 2004 | 28 | 52 | 3 (5.77%) | 3 (10.71%) | 94.2 | 60 | 60–120 |
| Yates et al. 2013 | 23 | 43 | 6 (13.95%) | 6 (26.09%) | 86.1 | 72 | 48–72 |
| Bedrossian 2010 | 36 | 74 | 2 (2.70%) | 2 (5.56%) | 97.3 | 84 | − 84 |
| Agliardi et al. 2017 | 15 | 42 | 0 (0.00%) | 0 (0.00%) | 100 | 85 | 73–91 |
| Chana et al. 2019 | 45 | 88 | 5 (5.69%) | 3 (6.66%) | 94.3 | 90 | Not reported |
| Miglioranca et al. 2012 | 21 | 40 | 1 (2.50%) | 1 (4.76%) | 97.5 | 96 | Not reported |
| Fortin 2017 | 58 | 107 | 0 (0.00%) | 0 (0.00%) | 100 | 101 | 60–156 |
| Bothur et al. 2015 | 14 | 58 | 2 (3.45%) | 1 (7.14%) | 96.6 | 112 | 69.6–144 |
| Aparicio et al. 2014 | 22 | 41 | 2 (4.88%) | 1 (4.55%) | 95.1 | 120 | ± 154.1 |
| Di Cosola et al. 2021 | 33 | 67 | 16 (23.88%) | 8 (24.24%) | 76.1 | 142 | 109–198 |
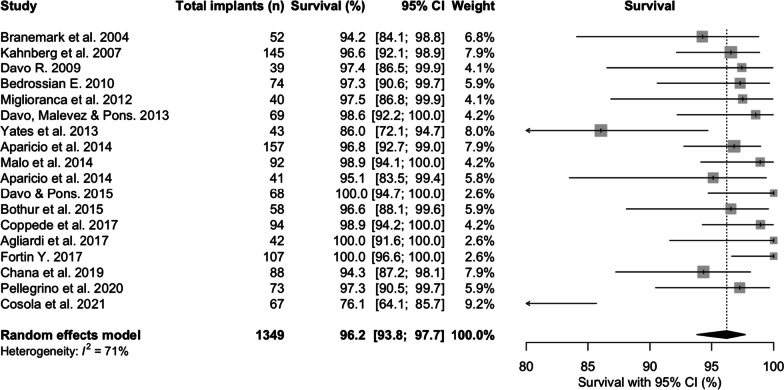
The mean survival at 75.4 months was 96.2% [95% CI 93.8; 97.7] (Fig. 2). The lowest survival was 76.1% [95% CI 64.1; 85.7] survival at 141.6 months [30], whilst 3 studies reported 100% survival at 60, 85, and 101 months, respectively [13, 18, 27].
Fig. 2.
Zygomatic implant survival prevalences at latest follow-up (%)
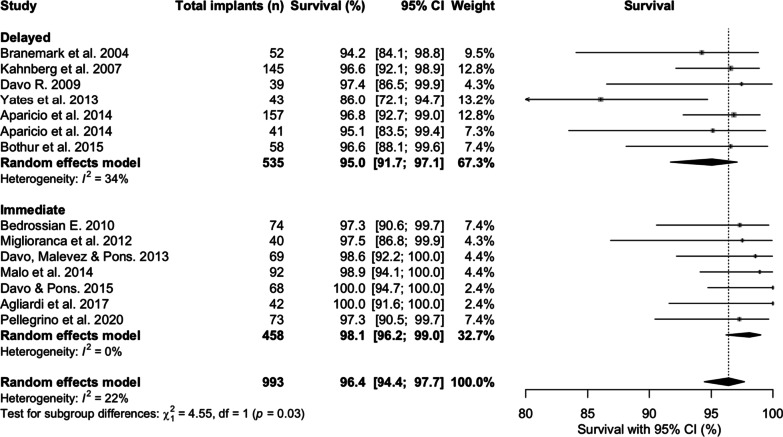
When comparing studies with immediate loading to delayed (Fig. 3), mean survival prevalences for delayed load protocols were 95% [95% CI 91.7; 97.1] over a mean of 69.3 months follow-up, and 98.1% [95% CI 96.2; 99.0] over a mean of 73.6 months follow-up for immediate loading protocols (p = 0.03).
Fig. 3.
Subgroup analysis comparing the zygomatic implant survival prevalences at latest follow-up between delayed versus immediate loading protocols
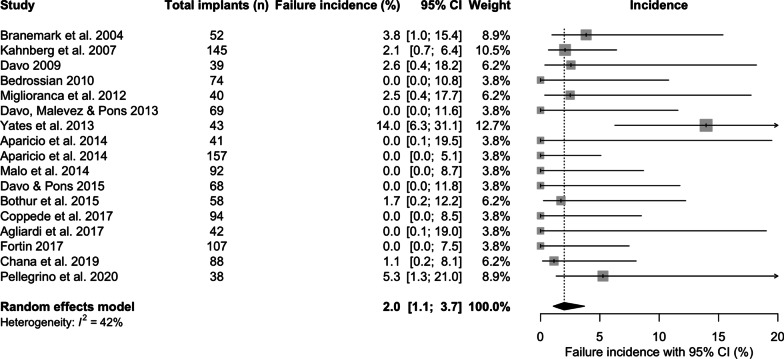
Incidence of zygomatic implant failure
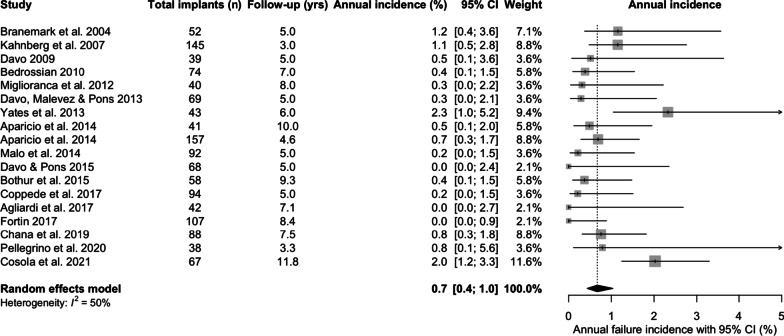
Zygomatic implant failure was defined as the antagonist of ZI survival. The total annual incidence of ZI failure across the studies was 0.7%/year [95% CI 0.4; 1.0] (Fig. 4).
Fig. 4.
Total annual incidence (%/year) of failure of zygomatic implants
The incidence of ZI failure within the first year was 2% [95% CI 1.1; 3.7] following placement (Fig. 5), whilst the incidence of ZI failure after the first year following placement was 0.5%/year [95% CI 0.3; 0.7] (Fig. 6)
Fig. 5.
Incidence of zygomatic implant failure within the first year (%)
Fig. 6.
Incidence of zygomatic implant failure after the first year (%)
A significant relationship between implant failure and gender was reported by one study, Di Cosola et al. [30] (males compared with females (p < 0.05). No other evaluated conditions (age, smoking, hypertension, or diabetes) were correlated with failure. No statistically significant association between implant failure and sex, surface finish, implant length, or position (p > 0.05) [25]. No other studies identified relationships between implant failure and patient demographic.
The classification of ZI placement across the studies was split between the classical Branemark approach (intrasinus), the extra-sinus classification as reported by Miglioranca [26], and the zygomatic anatomy guided approach (ZAGA) developed by Carlos Aparicio [31]. Within this systematic review, no relationship was identified between approaches and survival rates.
Table 4 documents reasons for ZI loss where reported.
Table 4.
ZI loss reported across studies
| Reason attributed to ZI loss | Total number of lost ZIs | Total number of ZIs combined in studies | Study |
|---|---|---|---|
| Sinusitis | 19 (12.25%) | 155 | Di Cosola et al., Chana et al. |
| Oro-antral communication | 1 (1.09%) | 92 | Malo et al. |
| Failure of osseointegration | 5 (2.34%) | 214 | Chana et al., Bedrossian et al., Brannemark et al. |
| Loss of osseointegration | 11 (2.39%) | 461 | Coppede et al., Bothur et al., Yates et al., Davo, Malevez and Pons, kahnberg et al., Branemark et al., Aparicio et al. |
| Infection or peri-implantitis | 4 (3.10%) | 129 | Yates et al., Aparicio et al. |
| Pain | 3 (2.36%) | 127 | Chana et al., Davo |
| Incorrect position | 1 (2.32%) | 43 | Yates et al. |
| Zygomatic implant fracture | 1 (0.64%) | 157 | Aparicio et al. |
| Unreported reasons for failure | 8 (3.37%) | 237 | Fortin, Pellegrino et al., Davo and Pons, Agliardi et al. |
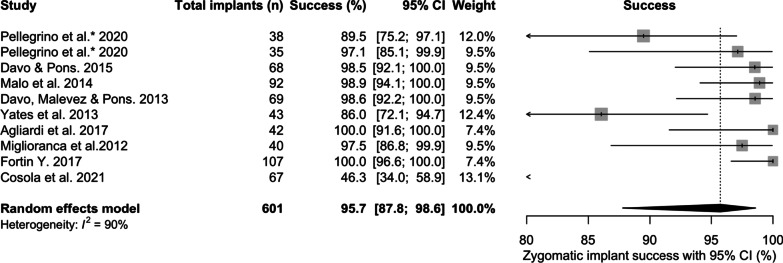
Zygomatic implant success
The mean ZI success was 95.7% [95% CI 87.8; 98.6] (Fig. 7) over a mean follow-up of 71.5 months. ZI success was predominantly defined as ZI survival without biological or neurological complications. Success ranged from 46.3% [95% CI 34.0; 58.9] [30] to 100% [95% CI 91.6; 100] [13]. Failure to meet success criteria included unfavourable positioning [18], peri-implant mucositis [16], bleeding on probing and increased pocket depths [19, 25], recession and extra-oral Infective processes [16, 27].
Fig. 7.
Zygomatic implant success prevalence over the follow-up period
Table 5 reports ZI success data and reasons for failure to meet success criteria across the studies.
Table 5.
Zygomatic implant success report
| Study | Zygomatic implant number | Success | Failure to meet success | Patient number | Follow-up | Follow-up range |
|---|---|---|---|---|---|---|
|
Pellegrino et al.a 2020 Atrophic Oncologic |
38 35 |
89.8% (CI: 60.4–97.7%) (n = 34) 96.7% (CI:79.2–99.5%) (n = 34) |
Mucositis: 13.1% (3.7–41%) Mucositis: 39.7% (9.7–91.7%) Extra-oral swelling (n = 1) |
10 10 |
39.9 | ± 19.5 |
| Davo and Pons 2015 | 68 | 98.5% (n = 67) | Unfavourable position (n = 1) | 14 | 60 | N/A |
| Malo et al. 2014 | 92 | 98.8% (n = 91) | PPD > 4 mm (n = 23) | 39 | 60 | N/A |
| Davo, Malevez and Pons. 2013 | 69 | 98.55% (n = 68) | NR | 42 | 60 | N/A |
| Yates et al. 2013 | 43 | 86.05% (n = 37) | Recession of 2–4 threads (n = 6) | 25 | 72 | 48–72 |
| Agliardi et al. 2017 | 42 | 100% (n = 42) | NR | 15 | 85 | 73–91 |
| Miglioranca et al. 2012 | 40 | 97.5% (n = 39) | NR | 21 | 96 | Not reported |
| Fortin 2017 | 107 | 100% (n = 107) | Successful treatment of infection by implant apicoectomy. (not included in success data by study) | 58 | 100.8 | 60–156 |
| Cosola et al. 2021 | 67 | 46.3% (n = 36) | 28 ZI (41.8%) experienced infective complications defined as sinusitis, oro-antral fistula or soft tissue infection. Early neurologic pain following treatment in 8 ZI (11.9%) in 5 patients | 33 | 141.6 | 109–198 |
NR not reported
aReported as two groups in the same study
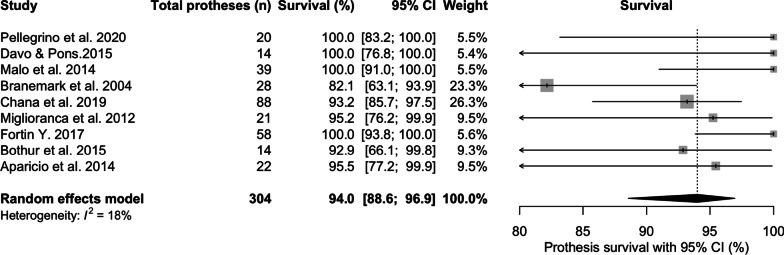
Zygomatic implant-supported prostheses: survival and success
The mean prosthesis survival was 94% [95% CI 88.6; 96.9] at 76 months of mean follow-up (Fig. 8). Seven studies conducted an immediate load protocol, with an interim prosthesis placed immediately after surgery, and was replaced after 3–6 months with the permanent prosthesis. Four conducted both immediate and delayed protocols and 7 conducted delayed protocols.
Fig. 8.
Prosthesis survival prevalence at latest follow-up period (%)
Table 6 reports prosthesis survival and success data. Survival was determined by the presence or absence of the permanent reconstruction at time of study completion. Success criteria varied across the reports. The main subgroups for failure to meet success included prosthetic tooth loss, chipping or fracture of the veneering material, abutment or screw fracture and abutment or screw loosening.
Table 6.
Prosthesis survival and success data
| Study | Prosthesis number | Prosthesis survival | Prosthesis success | Success criteria | Prosthesis fracture | Prosthetic tooth loss | Screw/abutment loosening/fracture | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Kahnberg et al. 2007 | 60 | 83.34% (n = 50) | Clinically stable prosthesis that not been removed for two weeks or more during the study period | 2 cases requiring laboratory repair | 1 patient with loosening of ball attachments | 36 | ||
| Pellegrino et al. 2020 | 20 | 100% (n = 20) |
90% (n = 9) (95% CI: 47.3–98.5%) Atrophic 58.3% (n = 6) (95% CI: 22.9–82%) Oncologic difference between the groups was not statistically significant (p = 0.77) |
Support of all placed implants, extended to the first molar region, not need to be reduced from the day of delivery to the final follow-up | Prosthetic screw loosening and abutment fractures. All unsuccessful prosthetic events occurred in the first year, with the majority experienced by the oncologic group. 5-year follow-up complication probability values were 10.7% [95% CI 4.1–26%] for the atrophic group, and 10.7% [95% CI 3.5–29.6%] for the oncologic group | 39.9 | ||
| Aparicio et al. 2014 | 80 | NR | NR | NR | 65 events involving fracture of the prosthetic acrylic coating material occurred. 2 fractures of the porcelain coating occurred. No metal framework fractures | NR | 16 screws and abutments loosened within the ZAGA group, and 7 screws fractured | 55.44 |
| Davo and Pons 2015 | 14 | 100% (n = 14) | Clinically stable prosthesis that not been removed for two weeks or more during the study period | 1 incidence of acrylic fracture from the underlying substructure at 36 months | 1 incident involving loss of an anterior tooth from an acrylic prothesis | Fracture of 1 abutment at 12 months | 60 | |
| Coppede et al. 2017 | 42 | NR | NR | NR | NR | Fractures or detachments in 5 fixed implant-supported restorations (14.7%) | NR | 60 |
| Malo et al. 2014 | 39 | 100% (n = 39) | NR | NR | 3 prostheses fracturing in 3 patients. 2 acrylic prostheses fractured at 48 and 58 months of follow-up, whilst a metal ceramic prosthesis fractured at 49 months | NR | Abutment screw loosening occurred in 1 patient at 54 months and prosthetic screw loosening in 2 patients at 47 and 55 months | 60 |
| Davo, Malevez and Pons 2013 | 36 | NR | 97.2% (n = 35 | Clinically stable prosthesis that not been removed for two weeks or more during the study period | NR | Replacement of resin teeth in 4 definitive acrylic fixed prostheses after 4 years of function due to extreme tooth wear | NR | 60 |
| Branemark et al. 2004 | 28 | 82% (n = 23) | NR | NR | NR | NR | 60 | |
| Davo 2009 | 21 | NR | 95.8% (n = 20) | Clinically stable prosthesis that not been removed for two weeks or more during the study period | NR | NR | NR | 60 |
| Agliardi et al. 2017 | 83 | NR | 100% (n = 83) | In function without mobility or pain, even if one or more of the implants were lost | NR | NR | NR | 85.1 |
| Chana et al. 2019 | 88 | 93.98% (n = 82) | NR | NR | Failure only in the fixed prosthetic group, when compared to the removable prosthetic group (n = 5/56 and n = 0/27, respectively). Failure was related to 1 fixed acrylic bar and 4 metal acrylic fixed prostheses | NR | Reported loosening in 7 abutments. 1 patient experienced abutment loosening twice within a 12-month period | 90 |
| Miglioranca et al. 2012 | 21 | 95.2% (n = 20) | NR | NR | Fracture of the metal bar supporting a prosthesis in 1 patient | NR | NR | 96 |
| Fortin Y. 2017 | 58 | 100% (n = 58) | NR | NR | NR | NR | NR | 100.8 |
| Bothur et al. 2015 | 14 | 92.9% (n = 13) | NR | NR | NR | NR | Reported loosening of 2 angled abutments and a third with a damaged thread | 111.6 |
| Aparicio et al. 2014 | 22 | 95.5% (n = 21) | NR | 4 incidents of fracture of the acrylic coating material, 25 fractures of the porcelain coating material, and fracture of 2 metal frameworks | Reported 6 episodes of screw fractures and 9 episodes of screw or abutment loosening | 120 |
Sinus pathology
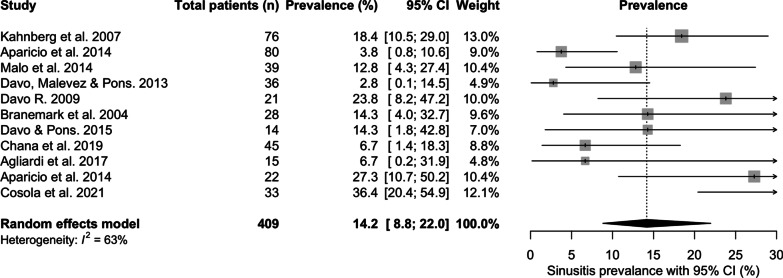
Sinusitis
Sinusitis was reported by 11 studies with a total prevalence of 14.2% [95% CI 8.8; 22.0] over a mean of 65.4 months follow-up (Fig. 9). The prevalence of sinusitis ranged from 2.8% [95% CI 0.1;14.5] at 60 months mean follow-up [20], to 36.4% [95% CI 20.4; 54.9] at 141.6 months of mean follow-up [30]. Disease was diagnosed clinically, radiographically, using patient reported questionnaires, or combined methods [11]. Sinusitis was the most commonly reported factor related to implant loss.
Fig. 9.
Prevalence of sinusitis (%)
Table 7 reports the data involving the sinus within the studies.
Table 7.
Reported sinus pathology
| Study | Mean follow-up | % of patients experiencing sinusitis during follow-up | Individual patient level timeframe data | Presence of oro-antral communication/fistula during follow-up | Intra or extra-sinus zygomatic implant path | Zygomatic implant loss related to sinusitis | Management | Previous history of sinusitis |
|---|---|---|---|---|---|---|---|---|
| Kahnberg et al. 2007 | 36 | 18.4% (n = 14) | Not reported | 3.9% (n = 3) | Path involving the sinus | Not reported | Not reported | Not reported |
| Aparicio et al. 2014 | 55.24 | 3.7% (n = 3) | Not reported | 2.5% (n = 2) | Path involving the sinus | Not reported | Not reported | Not reported |
| Malo et al. 2014 | 60 | 13% (n = 5) | Sinusitis diagnosed in 2 patients at 2 months then individual patients at 6, 12 and 24 months, respectively | 1.08% (n = 1) | Extra-maxillary approach | 1.08% (n = 1) in 2.6% (n = 1) patient | Antibiotics for 2 patients and surgical management for 2 successful for 4 of 5 patients | 13% (n = 5) patients |
| Davo, Malevez and Pons 2013 | 60 | 1.7% (n = 1) | Not reported |
Sinusitis (n = 1) Pain and swelling at zygomatic level (n = 1) |
Intra and extra-maxillary paths | Not reported | Antibiotics and meatotomy successful | Not reported |
| Davo 2009 | 60 | 23.8% (n = 5) | Not reported | Not reported | Path involving the sinus | No failures due to sinusitis | 2 patients Antibiotics, 2 patients meatotomy or 1 patient Caldwell Luc. All successful treatments | Not reported |
| Branemark et al. 2004 | 60 | 14.2% (n = 4) | Not reported | Not reported | Path involving the sinus | Not reported | Meatotomy on 4 patients | 3.5% (n = 1) patient |
| Davo and Pons 2015 | 60 | 14% (n = 2) | Sinus infection diagnosed at 24 and 30 months, respectively | Fistula at 1 implant | Path involving the sinus | Not reported | Antimicrobial therapy successful in managing disease | Not reported |
| Chana et al. 2019 | 90 | 6.6% (n = 3) | Not reported | Not reported | Intra and extra-maxillary paths | 3.4% (n = 3) in 6.6% (n = 3) patients | Not reported | Not reported |
| Agliardi et al. 2017 | 85.04 | 6.7% (n = 1) | 5 months after placement | 6.7% (n = 1) | Path involving the sinus | 0% (n = 0) | Antimicrobial therapy successful in managing disease | Not reported |
| Aparicio et al. 2014 | 120 | 27.3% (n = 6) | Post placement, 1–2 years, 2–3 years, 7–8, 8–9, 10–11 | 3.75% (n = 80) | Path involving the sinus | 1.5% (n = 2) in 4.5% (n = 1) patient | 5 patients successfully managed with antimicrobials | Not reported |
| Cosola et al. 2021 | 141.6 | 32.8% (n = 12) | Not reported | Not reported | 70.2% (n = 47) completely intrasinusal. 29.85% (n = 20) not completely intrasinusal | 23.9% (n = 16) in 24.24% (n = 8) patients | Not reported | Not reported |
Oral health impact of zygomatic implant therapy
Patient reported outcomes using the Oral Health Impact 14 score (OHIP14) were reported by Pellegrino et al. [16] and Davo and Pons [18]. Pellegrino et al. [16] reported OHIP scores of 30.4 (± 9.5) pre-operative and 6.3 (± 3.7) after 60 months follow-up. Davo and Pons [18] reported total mean OHIP14 scores of 3.4, 2.5, 3.8 at 1, 2 and 5 year follow-up recalls, respectively. No pre-operative OHIP14 questionnaires were carried out.
Oral Health Impact Profile Edentulous questionnaires (OHIP EDENT) were reported for 22 patients within a control group (Classical Branemark approach) [29]. 84% reported satisfaction scores above 80%. 31.8% of those patients reporting a maximum satisfaction score of 100%. This control group was compared against a test group (ZAGA approach), of which 76.3% (n = 61) recorded satisfaction rates of between 81 and 100% [11]. The difference between the groups was not statistically significant (P = 0.92).
A Likert scale to investigate satisfaction following a full-arch, immediate restoration supported by ZIs, was reported by Agliardi et al. [13]. Aesthetics and function were reported as excellent or very good by the entire group of 15 patients. Phonetics was considered excellent or very good by 13 of 15 patients.
Subjective satisfaction for oral rehabilitation using ZIs and prosthetic reconstructions were reported by Kahnberg et al. [15]. 73 Patients reported 86% satisfaction with the aesthetics and 71% satisfaction with the functional outcomes at 3 years of follow-up.
Malo et al. [19] assessed aesthetic and functional complaints in a group of 39 patients over a 5-year period. There were no reports of complaints at the final follow-up by any patients.
Assessment of bias
The risk of bias assessment is summarised in Table 8. Consecutive inclusion of participants and complete inclusion of participants presented the most common risk of bias, as this was unclear in several studies. Older studies reported data in a less systematic fashion when compared to contemporary studies. Descriptive analysis was the most common reporting methodology.
Table 8.
Assessment of studies using the Joanna Briggs Institute critical appraisal tools for case series
| Study | Was there clear criteria for inclusion? | Was the condition measured in a standard, reliable way for all participants? | Were valid methods used for identification of the condition for all participants included in the case series | Did the case series have consecutive inclusion of the participants | Did the case series have complete inclusion of participants | Was there clear reporting of the demographics of the participants in the study | Was the clear reporting of clinical information of the participants | With the outcomes or follow-up results of cases clearly reported | Was there clear reporting of the presenting site/clinic demographic information | Was statistical analysis appropriate |
|---|---|---|---|---|---|---|---|---|---|---|
| Kahnberg et al. 2007 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Not applicable |
| Pellegrino et al. 2020 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Unclear | Yes |
| Aparicio et al. 2014 | Unclear | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Not applicable |
| Coppede et al. 2017 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Not applicable |
| Davo & Pons. 2015 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | No | Not applicable |
| Malo et al. 2014 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Not applicable |
| Davo, Malevez and Pons 2013 | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Not applicable |
| Davo 2009 | Yes | Yes | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | Not applicable |
| Branemark et al. 2004 | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Not applicable |
| Yates et al. 2013 | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes |
| Bedrossian 2010 | Yes | Yes | Yes | No | No | No | Unclear | Yes | No | Not applicable |
| Agliardi et al. 2017 | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Not applicable |
| Chana et al. 2019 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Not applicable |
| Miglioranca et al. 2012 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Not applicable |
| Fortin 2017 | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Not applicable |
| Bothur et al. 2015 | Unclear | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Not applicable |
| Aparicio et al. 2014 | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes |
| Cosola et al. 2021 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes |
Discussion
ZIs present a therapeutic opportunity to rehabilitate patients who lack either the desire to undergo extensive augmentation procedures, or lack the anatomical structures required, to deliver conventional implant therapy in the maxilla. Custom, increased-length, end osseous implants anchored in the zygomatic process was first reported by Aparicio et al. in 1993 [2], with subsequent international studies exploring this treatment modality and its outcomes. The present systematic review focused on long-term ZI survival (≥ 3 years). Unfortunately no controlled trials, randomised or otherwise, that met our criteria were identified. Severe maxillary atrophy was the predominant clinical indication, and described patients who were unable to receive conventional implants without additional augmentation procedures. Other clinical indications for ZIs included trauma, cleft, and oncologic patient groups. There was a paucity of studies meeting the inclusion criteria for ZI placement in oncology patients. This could be related to the reduced survival rates of such individuals, alongside the increased risk of osteoradionecrosis (ORN) for those who received adjuvant radiotherapy to the zygomatic region. Only one study within this systematic review [16] compared ZI survival in the atrophic maxilla against ZIs placed after oncologic resections, and identified no difference in survival between the groups.
Zygomatic implant survival
In this review, ZI survival was 96.2% [95% CI 93.8; 97.7] over the mean follow-up of 75.4 months (6.3 years). The results are consistent with two other systematic reviews. The first included 68 studies [32], which reported a cumulative survival rate of 95.2% with no minimum follow-up, to a maximum follow-up of 12 years. The second, Sola Perez et al. [33] reported survival rates of 98.5% within the first year, 97.5% between 1 and 3 years, 96.8% at 3–5 years and 96.1% at more than 5 years. The current study also investigated the overall incidence of ZI failure (failure being defined as the antagonist of ZI survival), incidence of failure within the first year, and incidence of failure in subsequent years. When ZI failure did occur, sinusitis, oro-antral communications, failure of osseointegration, loss of osseointegration, infection or peri-implantitis, pain and zygomatic implant fracture were the related factors in descending order of frequency. A higher incidence of failure was identified within the first year (2%) compared to that of subsequent years (0.5%/year), whilst the overall annual failure incidence was 0.7% with intra-study mean follow-up times ranging from 36 to 142 months, again corroborated by the systematic review from Chrcanovic et al. [34], and Chrcanovic et al. [32]. These findings reflect the possibility that some patients will suffer from early infective, or medically related, post-surgical complications, which may result in ZI loss.
We found the mean ZI survival rate to be higher for immediate Loading protocols over delayed loading protocols. Di Cosola et al. [30] reported that immediate loading resulted in significantly lower risk of infective, neurological and overall complications compared with the two-step rehabilitation. The latter finding was supported by Chrcanovic et al. [32], who hypothesised this to be related to the length of follow-up, as delayed loading protocols were associated with increased time frames allowing greater chance of failure to occur during this period. The slightly higher ZI survival rate could also be due to evolution in the delivery of ZI therapy. Delayed load protocols are associated with the original technical approach, whilst immediate load protocols were introduced alongside refinements to the surgical technique, operator familiarity with the procedure, and ZI/ZI reconstruction design innovations. The difference was statistically significant between the protocols (p = 0.03) but may not be clinically relevant, and so should be interpreted with caution. Both loading protocols result in high ZI survival prevalence.
A common theme across all studies, was the combination of ZI in the posterior maxilla splinted to conventional implants in the anterior maxilla. Moraschini et al. [35], through a systematic review, compared ZI survival to conventional implant survival, which reported survival at 96.5% ± 5.0 for ZIs and 95.8% ± 6.4% for conventional implants at 78 months of mean follow-up. These findings suggest that ZIs have long-term survival rates comparable to conventional implants, with a positive inference for the ability to deliver dual implant modality supported reconstructions.
ZI survival rates also appear to be comparable to alternative techniques in the atrophic maxilla, including short implants, tilted implants, and implants placed in grafted sinuses. Slot et al. [36] reported 96.1% and 100% survival at 10 years in a randomised controlled trial of 6 or 4 conventional implants, respectively, when supporting maxillary overdentures. A meta-analysis and systematic review by Kotsovilis et al. [37] revealed no statistically significant difference in survival between short (≤ 8 mm or < 10 mm) and conventional (≥ 10 mm) rough surface implants placed in partially or totally edentulous individuals. ZIs may confer treatment time benefits to patients due to the immediacy of reconstruction when compared to implants placed in grafted sites. Further benefits are realised through reduced morbidity, due to the absence of a second donor sites, and potential cost savings due to a reduced number of procedures or number of conventional implant required to support full-arch prostheses. However, ZI placement is an advanced surgical procedure, requiring appropriate technical skillsets, and may also require sedation or a general anaesthetic, which in turn, adds additional cost and complexity to the process.
Zygomatic implant success
ZI success was 96%. Reporting ZI success demonstrated challenges because of significant heterogeneity (I2=90%) across the studies. ZIs in situ was a prerequisite, whilst specifying an absence of pain [13, 16, 17, 19, 20, 26] may have reflected reports of pain and neurosensory disturbances in the zygomaticofacial and infraorbital regions reported in other studies [21, 24, 25]. Absence of infection (included sinus disease, oro-antral communications, peri-implantitis and peri-implant mucositis) was included by 3 studies [13, 19, 23]. There are no recognised criteria for ZI success, and criteria for conventional implant success, as reported by Albrektsson and Isidor [38], have been suggested as a reference. Radiographic changes were considered as success criteria for 3 studies [13, 19, 21]. 3-dimensional imaging may provide the most accurate data on bone volume maintenance, but is unsuitable as a tool for monitoring success due to the dose of radiation received by the patient. Plain film, 2-dimensional, radiographic examinations are unsatisfactory for monitoring bone volume around ZIs, due to anatomical challenges and the possible fixture head positions in relation to the alveolar crest [39]. An attempt to standardise these reporting challenges has been suggested by Aparicio et al. [40] with the publication of the ORIS criteria (offset of prosthesis, rhino-sinusitis status, infection in soft tissue, stability of ZI) for documenting ZI success.
Zygomatic prosthesis survival and success
Prosthesis survival ranged from 82 to 100% with the mean at 94% at 76 months of follow-up. The high prosthesis survival mirrored that of ZI survival, although there was a potential for confounding as the definitive prosthesis was placed between 3 and 6 months after ZI installation. Early ZI failures could have influenced survival of the provisional prosthesis, but not the definitive reconstruction. Provisional prosthesis success was not reported in the literature, but there was little mention of complication rates as a counter argument. The mechanism of catastrophic failure was either related to loss of the supporting ZI or conventional implants, or fracture of the reconstruction [11, 18, 19, 25, 26]. Fracture of the metal substructure or fracture of the ceramic / acrylic attached to the metal substructure were the reported modes of prosthesis failure. Although not reported in this study, reconstructions supported by conventional implants alone showed no statistically significance difference between the construction materials used for full-arch screw-retained prostheses [41]. Mean ZI prosthesis survival appears comparable to full-arch screw-retained fixed prostheses supported by conventional implants. Sailer et al. [42] reported a 5-year cumulative survival of 95.8% for full-arch, screw-retained prostheses, whilst Wittneben et al. [41] reported a 5-year cumulative survival of 96.7% in comparison to this review, with 94% survival at 6 years for combined full-arch and partial ZI-supported reconstruction data.
Significant heterogeneity was found when reporting prosthesis success. Success criteria, such as allowing a prosthesis to be out of the mouth for up to 2 weeks, potentially affected the results and allowed for a higher prosthesis survival rate. In addition, Kahnberg et al. [15] and Branemark et al. [22], when faced with ZI loss, reported modification rather than replacement of the definitive prosthesis. The use of acrylic/acrylic teeth across the majority of studies potentially aided survival rates, as the prosthesis was repairable rather than lost. Chipping of the veneering resin was reported to be the second most common complication after loss of retention [41]. Technical complications for full-arch ZI screw-retained reconstructions appear to reflect those seen in conventional full-arch screw-retained implant reconstructions. Screw and abutment loosening events were frequently reported [11, 15, 16, 18, 19, 25, 28]. These complications have been reported in the implant literature [42], but adverse incidence rates for ZI reconstructions could be influenced by increased movement of the prosthesis due to bending moments of the ZIs [18, 20, 28]. These technical complications occurred regardless of whether the ZIs were splinted to conventional implants, or whether the prosthesis was supported by Quad ZIs. It is also possible that this bending phenomenon contributed to veneering material fractures away from the metal substructure. If a similarity between ZI-supported and conventional implant-supported prostheses is considered, Sailer et al.’s [42] report suggests that patient education and consent is essential in managing patients’ expectations when a 54.1% technical complication rate over 5 years exists for screw-retained, full-arch, conventional implant-supported prostheses.
Sinus pathology
The overall prevalence of sinusitis was 14.2% with a follow-up of 65.4 months. There was significant heterogeneity in the methodology employed to diagnose sinusitis across the studies, which included clinical, radiographic, patient reported questionnaires, or combined methods [11]. The 95% CI ranged from 8.8 to 22%, with an increased prevalence over longer follow-up periods [20, 30]. This finding might suggest a relationship between the two, although a reported confounding factor is the background population prevalence of chronic rhinosinusitis. This has been reported at > 10% in a western population, when measured by objective criteria [43], but when using guideline-based diagnostic criteria, the true prevalence of chronic rhinosinusitis is reportedly less than 5%.
The classical approach, or Branemark protocol [22], for ZI placement is via an intra-sinus route, with a window raised in the lateral sinus wall to allow direct visualisation for ZI placement. In contrast, the extra-sinus approach, first reported by Miglioranca [51] does not breach the sinus cavity during placement although the extra-maxillary approach may do so at the at the most apical extent before entering the Zygoma. Extra-maxillary refers to the coronal portion of the implant at intra-oral exit site. The biological consequences of the presence or absence of a ZI within the sinus cavity are therefore worth consideration. Sinusitis was the most commonly reported factor related to ZI loss within this review. Aparicio et al. [11] reported a statistically significant difference in Lund–MacKay sinus staging score (radiographic examination) between classical and ZAGA groups (p = 0.04). Dual Lanza–Kennedy scores (patient reported) and Lund–MacKay scores, again reported a statistically significant difference between the treatment protocols. Sinus pneumatisation was found to be statistically related to overall complications and implant loss by Di Cosola et al. [30] However, these findings may relate to the fact that larger sinuses require an intra-sinus path for ZI placement, rather than presenting the surgical option for an extra-maxillary approach. It would appear logical that ZIs not breaching the sinus wall would not be likely to cause sinusitis. Ultimately, the patient’s individual anatomy and the desired prosthetic envelope, dictate the ZIs trajectory towards an intra- or extra-sinus approach.
When sinusitis was diagnosed, successful treatment with antibiotics and/or via a surgical meatotomy was reported with no further consequences. ZI failure due to loss of osseointegration, was infrequent when presented on a background of sinusitis, with surgical removal being the more common mode of ZI loss to treat un-resolving sinusitis. This suggests sinusitis may not always be a catastrophic event, although a concern of bacterial colonisation onto an exposed ZI surface within the sinus, with subsequent inflammation or infection might be prudent. Petruson [44] conducted sinuscopies on patients whose ZIs had been in function after at least 1 year. Total or partial mucosal coverage had occurred with no signs of infection or increased secretion. However, there is potential evidence to show that inflammatory bone changes may occur, as Bothur et al. [28] reported signs of osteitis when examining sinus walls (not directly surgically altered) after ZI placement. New bone development, measured radiographically, was seen in all patients within at least one of their sinuses. Di Cosola et al. [30] reported that a sinus mucosa thickness of > 3 mm was related to an increased odds ratio (1:2.8) of infective complications (sinusitis, oro-antral fistula, infection of the soft tissues). Obstruction of the osteum was not associated with implant failure or infective complications. Davo et al. reported no clinical consequences in patients who exhibited radiological thickening of the sinus mucosa associated with zygomatic implants [45]. It appears that ZIs within the sinus are not always a cause of clinically diagnosed or reported sinusitis, and that antibiotic therapy or surgery can successfully treat acute cases of sinusitis. Some cases may persist however, with a need to remove the ZI in order to resolve the situation.
Oro-antral communication was also linked to ZI loss [11, 13, 15, 19, 20]. Preventing oro-antral communications is reliant on hard or soft tissue attachment to the coronal aspect of the implant. It has been suggested that 2 stage procedures, with repeat surgery or abutment changes at this level, could have a negative effect [7]. Early infective processes may also jeopardise this seal, as potentially might bending movements when in function. Patient education, compliance in oral hygiene measures, and prosthesis cleansability are undoubtedly important in maintaining peri-ZI tissue health and for the long-term prevention of peri-ZI mucositis.
Patient reported outcomes with zygomatic implants
Across the studies, there was significant heterogeneity in recording patient reported outcomes. Few studies investigated PROMs with the same tool, which made comparison between studies impossible. PROMs where investigated via the OHIP 14 system [16, 18] developed by Slade et al. [46], the OHIP EDENT [11] or via Likert-style questionnaires [13]. Improvements were reported in all studies comparing qualify of life (QoL) start points to end points. When considering conventional implant literature and associated reconstructions, edentulous patients report improvements in satisfaction when provided with implant retained or supported prostheses, regardless of fixed or removable design. Removable was found to be preferential for performing hygiene related procedures [47]. In addition, either immediate or delayed reconstructions were found to be acceptable. Heydecke et al. [48], in a crossover study, found implant retained removable prostheses to be preferred over fixed reconstructions for phonetics. This was either due to prior patient experience with palatal coverage, or that not enough time was given for adaptation. An extended period for normalisation might therefore be recommended. Conversely, Brennan et al. [49], reported patients with fixed reconstructions were more satisfied than counterparts with removable reconstructions. Edentulism is recognised as having a detractive effect on the emotional status of patients [50]. Any implant-supported or retained reconstruction might reasonably be expected to improve QoL if individuals previously managed with conventional removable prostheses, or had undergone partial or total maxillary resection for trauma or oncology. Factors influencing satisfaction were comfort, aesthetics and phonetics. These are more challenging in ZI therapy, which may have a more palatal emergence profile of the implant platform related to the local anatomy and level of alveolar bone atrophy. This may lead to prostheses that encroach upon the palate, and might challenge patients’ adaptive capabilities. This relies on surgical skill coupled with prosthetically driven planning to reduce the chance of prosthetic complications. The use of PROMs and QoL assessments within ZI research is an essential component of patient-centred research, but standardised criteria and recording by investigators is recommended to construct a more detailed picture.
Limitations of the evidence
Limitations centred around the quality of reporting. Although the inclusion criteria of primary studies were clear, it was often unclear as to whether there had been consecutive and/or complete inclusion of the participants. As such, inclusion bias may have played a part in the selection of participants selected for studies. Older studies were less systematic in their approach to reporting. Patient demographics were generally well reported. Narrative results were sufficient to extract datasets that allowed the systematic review to be conducted along with the meta-analysis. Heterogeneity in reporting ZI success and PROMs was notable across the studies, which challenged comparisons. However, we tried to account for several factors that led to heterogeneity, e.g., the duration of follow-up (i.e. by calculating annual incidence rates) and loading protocol (i.e. by performing subgroup analysis).
Limitations of the review
This systematic review followed the PRISMA guidelines for reporting, which created a framework for the structure, assessment, introspection, and reporting process in order to conduct a review and analysis of the relevant literature. The main limitations were related to the study quality, as no clinical trials or randomised controlled trials were included. In addition, we were unable to analyse ZI success for all studies, or carry out further subgroup analyses of complications due to heterogeneity in reporting. Caution should be used when interpreting the results due to the lack of high-quality evidence.
Implications for practice and future research
These results indicate that ZIs are a predictable treatment modality for use in the atrophic maxilla, and represent a reconstructive therapy to consider against techniques including sinus augmentation with conventional implant rehabilitation. Five-year ZI survival rates appear comparable to conventional implants, although mean longer term comparable survival and complication data are still lacking. The reported survival rates are reassuring, as ZI therapy presents the opportunity for immediate reconstruction at the time of implant placement, and reduces both morbidity from donor site procedures and reduces overall treatment times. The study indicates that ZI-supported prosthetic reconstruction survival is satisfactory, with complications comparable to that experienced by reconstructions supported by conventional implants. Further investigation into ZI-supported prosthesis performance, material choices and prosthetic complications are required. Sinusitis appears to be a complication affecting around 14% of individuals with ZIs, but can respond to antibiotic or surgical treatment with total resolution. PROMs indicate that ZIs improve quality of life for those treated in this fashion.
Future research should be focused on the creation of uniform research core datasets to ensure standardised reporting within the field of ZIs, in order to capture and compare study data. There is also a need to identify guidelines for diagnosis and management of sinus pathology related to ZIs. Finally, standardised PROMs should be included when measuring clinical outcomes.
Conclusions
Zygomatic implants may represent a predictable treatment modality for management of the atrophic or resected maxilla, with comparable survival rates to conventional implants over similar time frames. Immediate loading showed a statistically significant increase in survival rates over delayed loading, but this difference may not be clinically significant. Prosthesis survival was satisfactory and similar to that of prostheses supported by conventional implants, with similar complications. Sinusitis was the most frequently encountered biological complication. Patient reported outcomes show an increase in satisfaction when rehabilitated with ZIs.
Supplementary Information
Additional file 1: Table S1: Search strategy. Table S2: Excluded studies sorted according to the reason of exclusion after full-text screening.
Acknowledgements
Evidence search carried out by Jo Hooper from the University Hospitals Bristol & Weston Library & Knowledge Services. Bristol:UK. Statistical analysis was conducted by Gareb Barzi. Advice on Risk of Bias tools was provided by Alex Gormley, PhD candidate, Restorative Department, Bristol Dental Hospital. This systematic review was initiated and steered by the Scientific Committee of the International Team for Implantology (ITI).
Abbreviations
- ZI
Zygomatic implant
- PROM
Patient reported outcome measures
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analyses
- PROSPERO
Prospective Register of Systematic Reviews
- PIO
Patient, intervention, outcome
- OHIP
Oral health impact profile
- OHIP EDENT
Oral health impact profile edentulous
- QoL
Quality of life
- CI
Confidence interval
- ZAGA
Zygoma anatomy guided approach
- ORIS
Offset, rhino-sinus, infection, stability
- NR
Not reported
- N
Number
Author contributions
MBR: data collection, bias analysis, data analysis, drafted document. TD: data collection and review of document. AP: bias analysis and review of document. BG: statistical analysis and revision of document. AV, CM, TB, LB, VK, YW, RJ: project conception and design, review of document. All authors read and approved the final manuscript.
Funding
This review was solely supported by the authors’ institutions. No funding was involved.
Availability of data and materials
All data generated or analysed during the study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Matthew Brennand Roper declares that he has no conflict of interests. He has a grant from BioHorizons outside and unrelated to the submitted work. The authors declare they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Matthew Brennand Roper, Email: mbr@brennandroper.com, Email: matthew.brennand-roper@uhbw.nhs.uk.
Arjan Vissink, Email: a.vissink@umcg.nl.
Tom Dudding, Email: tom.dudding@uhbw.nhs.uk.
Alex Pollard, Email: alex.pollard@uhbw.nhs.uk.
Barzi Gareb, Email: b.gareb@umcg.nl.
Chantal Malevez, Email: c.malevez@delocht.be.
Thomas Balshi, Email: thomas.balshi@pidentalcenter.com.
Lawrence Brecht, Email: lebrecht@nycpros.com.
Vinay Kumar, Email: veezo@rediffmail.com.
Yiqun Wu, Email: yiqunwu@hotmail.com.
Ronald Jung, Email: ronald.jung@zzm.uzh.ch.
References
- 1.Jensen J, Sindet-Pedersen S, Oliver AJ. Varying treatment strategies for reconstruction of maxillary atrophy with implants: results in 98 patients. J Oral Maxillofac Surg. 1994;52(3):210–216. doi: 10.1016/0278-2391(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio C, Branemark PI, Keller EE, Olive J. Reconstruction of the premaxilla with autogenous iliac bone in combination with osseointegrated implants. Int J Oral Maxillofac Implants. 1993;8:61–67. [Google Scholar]
- 3.Bedrossian E, Rangert B, Stumpel L, Indresano T. Immediate function with the zygomatic implant: a graftless solution for the patient with mild to advanced atrophy of the maxilla. Int J Oral Maxillofac Implants. 2006;21(6):937–942. [PubMed] [Google Scholar]
- 4.Butterworth CJ, Lowe D, Rogers SN. The Zygomatic Implant Perforated (ZIP) flap reconstructive technique for the management of low-level maxillary malignancy—clinical & patient related outcomes on 35 consecutively treated patients. Head Neck. 2022;44(2):345–358. doi: 10.1002/hed.26933. [DOI] [PubMed] [Google Scholar]
- 5.Duarte LR, Filho HN, Francischone CE, Peredo LG, Brånemark PI. The establishment of a protocol for the total rehabilitation of atrophic maxillae employing four zygomatic fixtures in an immediate loading system—a 30-month clinical and radiographic follow-up. Clin Implant Dent Relat Res. 2007;9(4):186–196. doi: 10.1111/j.1708-8208.2007.00046.x. [DOI] [PubMed] [Google Scholar]
- 6.Chow J, Hui E, Lee PK, Li W. Zygomatic implants–protocol for immediate occlusal loading: a preliminary report. J Oral Maxillofac Surg. 2006;64(5):804–811. doi: 10.1016/j.joms.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Davó R, Malevez C, Rojas J, Rodríguez J, Regolf J. Clinical outcome of 42 patients treated with 81 immediately loaded zygomatic implants: a 12- to 42-month retrospective study. Eur J Oral Implantol. 2008;9(Suppl 1):141–150. [PubMed] [Google Scholar]
- 8.Krekmanov L, Kahn M, Rangert B, Lindström H. Tilting of posterior mandibular and maxillary implants for improved prosthesis support. Int J Oral Maxillofac Implants. 2000;15(3):405–414. [PubMed] [Google Scholar]
- 9.Aparicio C, Ouazzani W, Aparicio A, Fortes V, Muela R, Pascual A, Codesal M, Barluenga N, Franch M. Immediate/early loading of zygomatic implants: clinical experiences after 2 to 5 years of follow-up. Clin Implant Dent Relat Res. 2010;12(Suppl1):e77–82. doi: 10.1111/j.1708-8208.2008.00134.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahlgren F, Størksen K, Tornes K. A study of 25 zygomatic dental implants with 11 to 49 months follow-up after loading. Int J Oral Maxillofac Implants. 2006;21(3):421–425. [PubMed] [Google Scholar]
- 11.Aparicio C, Manresa C, Francisco K, Aparicio A, Nunes J, Claros P, Potau J. Zygomatic implants placed using the zygomatic anatomy guided approach versus the classical technique: a proposed system to report rhinosinusitis diagnosis. Clin Implant Dent Relat Res. 2014;16:627–642. doi: 10.1111/cid.12047. [DOI] [PubMed] [Google Scholar]
- 12.Maló P, Nobre Mde A, Lopes I. A new approach to rehabilitate the severely atrophic maxilla using extramaxillary anchored implants in immediate function: a pilot study. J Prosthet Dent. 2008;100(5):354–366. doi: 10.1016/S0022-3913(08)60237-1. [DOI] [PubMed] [Google Scholar]
- 13.Agliardi E, Romeo D, Panigatti S, de Araujo Nobre M, Malo P. Immediate full- arch rehabilitation of the severely atrophic maxilla supported by zygomatic implants: a prospective clinical study with minimum follow-up of 6 years. Int J Oral Maxillofac Surg. 2017;46:1592–1599. doi: 10.1016/j.ijom.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022.
- 15.Kahnberg K-E, Henry P, Hirsch J-M, Ohrnell L-O, Andreasson L, Branemarl P-I, Chiapasco M, Gynther G, Finne K, Higuchi K, Isaksson S, Malevez C, Neukam F, Sevetz E, Urgell J, Widmark G, Bolind P. Clinical evaluation of the zygoma implant: 3-year follow-up at 16 clinics. J Oral Maillofac Surg. 2007;65:2033–2038. doi: 10.1016/j.joms.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrino G, Francesco Basile F, Relics D, Ferri A, Grande F, Tarsitano A, Marchetti C. Computer-aided rehabilitation supported by zygomatic implants: a cohort study comparing atrophic with oncologic patients after five years of follow-up. J Clin Med. 2020;9:3254. doi: 10.3390/jcm9103254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppede A, de Mayo T, de sa Zamper-lini M, Amorin R, DePadua APAT, Shibli JA. Three-year clinical prospective follow-up of extrasinus zygomatic implants for the rehabilitation of the atrophic maxilla. Clin Implant Dent Relat Res. 2017;19:926–934. doi: 10.1111/cid.12517. [DOI] [PubMed] [Google Scholar]
- 18.Davo R, Pons O. 5-year outcome of cross-arch prostheses supported by four immediately loaded zygomatic implants: a prospective case series. Eur J Oral Implantol. 2015;8:169–174. [PubMed] [Google Scholar]
- 19.Malo P, de Araujo NM, Lopes A, Ferro A, Moss S. Five-year outcome of a retrospective cohort study on the rehabilitation of completely edentulous atrophic maxillae with immediate loaded zygomatic implants placed extra-maxillary. Eur J Oral Implantol. 2014;7:267–281. [PubMed] [Google Scholar]
- 20.Davo R, Malevez C, Pons O. Immediately loaded zygomatic implants: a five year prospective study. Eur J Oral Implantol. 2013;6:39–47. [PubMed] [Google Scholar]
- 21.Davo R. Zygomatic implants placed with a two-stage procedure: a 5-year retrospective study. Eur J Oral Implantol. 2009;2:115–124. [PubMed] [Google Scholar]
- 22.Brånemark PI, Gröndahl K, Ohrnell LO, Nilsson P, Petruson B, Svensson B, Engstrand P, Nannmark U. Zygoma fixture in the management of advanced atrophy of the maxilla: technique and long-term results. Scand J Plast Reconstr Surg Hand Surg. 2004;38(2):70–85. doi: 10.1080/02844310310023918. [DOI] [PubMed] [Google Scholar]
- 23.Yates J, Brook I, Patel R, Wragg P, Atkins S, El-Awa A, Bakri I, Bolt R. Treatment of the edentulous atrophic maxilla using zygomatic implants: evaluation of survival rates over 5–10 years. Int J Oral Maxillofac Surg. 2014;43:237–242. doi: 10.1016/j.ijom.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Bedrossian E. Rehabilitation of the edentulous maxilla with the zygoma concept: a 7-year prospective study. Int J Oral Maxillofac Implants. 2010;25:1213–1221. [PubMed] [Google Scholar]
- 25.Chana H, Smith G, Bansal H, Zahra D. A retrospective cohort study of the survival rate of 88 zygomatic implants placed over an 18 year period. Int J Oral Maxillofac Implants. 2019;34:461–470. doi: 10.11607/jomi.6790. [DOI] [PubMed] [Google Scholar]
- 26.Miglioranca R, Sotto-Maior B, Senna P, Francischone C, Del Bel CA. Immediate occlusal loading of extrasinus zygomatic implants: a prospective cohort study with a follow-up period of 8 years. Int J Oral Maxillofac Surg. 2012;41:1072–1076. doi: 10.1016/j.ijom.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Fortin Y. Placement of zygomatic implants into the male prominence of the maxillary bone for apical fixation: a clinical report 5 to 13 years. Int J Oral Maxillofac Implants. 2017;32:633–641. doi: 10.11607/jomi.5230. [DOI] [PubMed] [Google Scholar]
- 28.Bothur S, Kullendorff B, Olsson-Sandin G. Asymptomatic chronic rhinosinusitis and osteitis in patients treated with multiple zygomatic implants: a long-term radiographic follow-up. Int J Oral Maxillofac Implants. 2015;30:161–168. doi: 10.11607/jomi.3581. [DOI] [PubMed] [Google Scholar]
- 29.Aparicio C, Manresa C, Francisco K, Ouazzani W, Claros P, Potau J, Aparicio A. The long-term use of zygomatic implants: a 10-year clinical and radiographic report. Clin Implant Dent Relat Res. 2014;16(3):447–459. doi: 10.1111/cid.12007. [DOI] [PubMed] [Google Scholar]
- 30.Di Cosola M, Ballini A, Zhurakivska K, Ceccarello A, Nocini R, Malcangi A, Mori G, Lo Muzio L, Cantore S, Olivo A. Retrospective analysis of clinical and radiologic data regarding zygomatic implant rehabilitation with a long-term follow-up. Int J Environ Res Public Health. 2021;18:12963. doi: 10.3390/ijerph182412963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aparicio C. A proposed classification for zygomatic implants based on the zygoma anatomy guided approach (ZAGA). A cross-sectional survey. Eur J Oral Implantol. 2011;4:269–275. [PubMed] [Google Scholar]
- 32.Chrcanovic BR, Albrektsson T, Wennerberg A. Survival and complications of zygomatic implants: an updated systematic review. J Oral Maxillofac Surg. 2016;74:1949–1964. doi: 10.1016/j.joms.2016.06.166. [DOI] [PubMed] [Google Scholar]
- 33.Solà Pérez A, Pastorino D, Aparicio C, Pegueroles Neyra M, Khan RS, Wright S, Ucer C. Success rates of zygomatic implants for the rehabilitation of severely atrophic maxilla: a systematic review. Dent J. 2022;10:151. doi: 10.3390/dj10080151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrcanovic BR, Abreu MH. Survival and complications of zygomatic implants: a systematic review. Oral Maxillofac Surg. 2003;17:81. doi: 10.1007/s10006-012-0331-z. [DOI] [PubMed] [Google Scholar]
- 35.Moraschini V, de Queiroz TR, Sartoretto SC, de Almeida DCF, Calasans-Maia MD, Louro RS. Survival and complications of zygomatic implants compared to conventional implants reported in longitudinal studies with a follow-up period of at least 5 years: a systematic review and meta-analysis. Clin Implant Dent Relat Res. 2022;1–13. [DOI] [PubMed]
- 36.Slot W, Raghoebar GM, Cune MS, Vissink A, Meijer HJA. Maxillary overdentures supported by four or six implants in the anterior region: 10-year randomized controlled trial results. J Clin Periodontol. 2023;50(1):36–44. doi: 10.1111/jcpe.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotsovilis S, Fourmousis I, Karroussis IK, Bamia C. A systematic review and meta-analysis on the effect of implant length on the survival of rough surface dental implants. J Periodontol. 2009;80:1700–1718. doi: 10.1902/jop.2009.090107. [DOI] [PubMed] [Google Scholar]
- 38.Albrektsson T, Isador F. Consensus report of Session IV. In: Lang NP, Karring T, editors. Proceedings of the 1st European Workshop on Periodontology. London: Quintessence. 1994;365–369.
- 39.Malevez C, Abarca M, Durdu F, Daelemans P. Clinical outcome of 103 consecutive zygomatic implants: a 6–48 months follow-up study. Clin Oral Implants Res. 2004;15:18–22. doi: 10.1046/j.1600-0501.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 40.Aparicio C, Lopez-Piriz R, Albrektsson T. ORIS criteria of success for the zygoma-related rehabilitation: the (revisited) zygoma success code. Int J Oral Maxillofac Implants. 2020;35:366–378. doi: 10.11607/jomi.7488. [DOI] [PubMed] [Google Scholar]
- 41.Wittneben JG, Millen C, Brägger U. Clinical performance of screw- versus cement-retained fixed implant-supported reconstructions—a systematic review. Int J Oral Maxillofac Implants. 2014;29(Suppl):84–98. doi: 10.11607/jomi.2014suppl.g2.1. [DOI] [PubMed] [Google Scholar]
- 42.Sailer I, Muhlemann S, Zwahlen M, Hammerle CHF, Schneider D. Cemented and screw-retained implant reconstructions: a systematic review of the survival and complication rates. Clin Oral Implants Res. 2012;23(Suppl. 6):163–201. doi: 10.1111/j.1600-0501.2012.02538.x. [DOI] [PubMed] [Google Scholar]
- 43.Sedaghat AR, Kuan EC, Scadding GK. Epidemiology of chronic rhinosinusitis: prevalence and risk factors. J Allergy Clin Immunol Pract. 2022;10(6):1395–1403. doi: 10.1016/j.jaip.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Petruson B. Sinuscopy in patients with titanium implants in the nose and sinuses. Scand J Plast Reconstr Surg Hand Surg. 2004;38(2):86–93. doi: 10.1080/02844310310023909. [DOI] [PubMed] [Google Scholar]
- 45.Davo R, Malevez C, Lopez-Orellana C, Pastor-Bevia F, Rojas J. Sinus reactions to immediately loaded zygomatic implants: clinical and radiological study. Eur J Oral Implantol. 2008;1:53–60. [PubMed] [Google Scholar]
- 46.Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. 1997;25(4):284–290. doi: 10.1111/j.1600-0528.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 47.Feine J, Abou-Ayash S, Al Mardini M, de Santana RB, Bjelke-Holtermann T, Bornstein MM, Braegger U, Cao O, Cordaro L, Eycken D, Fillion M, Gebran G, Huynh-Ba G, Joda T, Levine R, Mattheos N, Oates TW, Abd-Ul-Salam H, Santosa R, Shahdad S, Storelli S, Sykaras N, Treviño Santos A, Stephanie Webersberger U, Williams MAH, Wilson TG, Jr, Wismeijer D, Wittneben JG, Yao CJ, Zubiria JPV. Group 3 ITI Consensus Report: patient-reported outcome measures associated with implant dentistry. Clin Oral Implants Res. 2018;29(Suppl16):270–275. doi: 10.1111/clr.13299. [DOI] [PubMed] [Google Scholar]
- 48.Heydecke G, Boudrias P, Awad MA, De Albuquerque RF, Lund JP, Feine JS. Within-subject comparisons of maxillary fixed and removable implant prostheses: patient satisfaction and choice of prosthesis. Clin Oral Implants Res. 2003;14(1):125–130. doi: 10.1034/j.1600-0501.2003.140117.x. [DOI] [PubMed] [Google Scholar]
- 49.Brennan M, Houston F, O'Sullivan M, O'Connell B. Patient satisfaction and oral health-related quality of life outcomes of implant overdentures and fixed complete dentures. Int J Oral Maxillofac Implants. 2010;25(4):791–800. [PubMed] [Google Scholar]
- 50.Fiske J, Davis DM, Frances C, Gelbier S. The emotional effects of tooth loss in edentulous people. Br Dent J. 1998;184(2):90–93. doi: 10.1038/sj.bdj.4809551. [DOI] [PubMed] [Google Scholar]
- 51.Migliorança RM, Coppedê A, Dias Rezende RC, de Mayo T. Restoration of the edentulous maxilla using extrasinus zygomatic implants combined with anterior conventional implants: a retrospective study. Int J Oral Maxillofac Implants. 2011;26(3):665–672. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1: Search strategy. Table S2: Excluded studies sorted according to the reason of exclusion after full-text screening.
Data Availability Statement
All data generated or analysed during the study are included in this published article.