Abstract
This study was designed to examine the feasibility of analyzing heart rate variability (HRV) data from repeat-flier astronauts at matching days on two separate missions to assess any effect of repeated missions on brain plasticity and psychological resilience, as conjectured by Demertzi. As an example, on the second mission of a healthy astronaut studied about 20 days after launch, sleep duration lengthened, sleep quality improved, and spectral power (ms2) co-varying with activity of the salience network (SN) increased at night. HF-component (0.15–0.50 Hz) increased by 61.55%, and HF-band (0.30–0.40 Hz) by 92.60%. Spectral power of HRV indices during daytime, which correlate negatively with psychological resilience, decreased, HF-component by 22.18% and HF-band by 37.26%. LF-component and LF-band, reflecting activity of the default mode network, did not change significantly. During the second mission, 24-h acrophases of HRV endpoints did not change but the 12-h acrophase of TF-HRV did (P < 0.0001), perhaps consolidating the circadian system to help adapt to space by taking advantage of brain plasticity at night and psychological resilience during daytime. While this N-of-1 study prevents drawing definitive conclusions, the methodology used herein to monitor markers of brain plasticity could pave the way for further studies that could add to the present results.
Subject terms: Neuroscience, Physiology, Psychology, Environmental sciences, Planetary science, Cardiology, Health care, Astronomy and planetary science, Engineering
Introduction
Herein, we illustrate our method for investigating brain plasticity of an astronaut by assessing how sleep performance and heart rate variability (HRV), gauging activity of intrinsic networks of the brain, particularly the default mode network (DMN) and salience network (SN), changed from first-time to second-time long-duration spaceflight 4 years later.
Magnetic resonance imaging (MRI) studies showed narrowing of the central sulcus, upward shift of the brain, and narrowing of cerebrospinal fluid spaces at the vertex in most astronauts examined1–9. Impaired cerebrovascular circulation in microgravity may induce cortical reorganization. Understanding the effects of spaceflight on the human central nervous system is pivotal for the development of adequate countermeasures. Maximizing crew performance and health is crucial for the success and safety of future prolonged space missions, including missions to the moon or Mars10–12.
The central nervous system seems capable of adaptation to microgravity by the process of neuroplasticity, as previously shown in animals13. Yet, little is known about the effects of microgravity and gravity transitions on the human brain14. After exposure to microgravity, significant differences in resting-state functional connectivity between motor cortex and cerebellum, and changes within the DMN have been reported2,14. Changes in brain function could account for the fact that second-time flyers are less prone to some microgravity-related problems than first-time flyers, given the process of neural adaptation, as conjectured by some14–16. It is thus important to learn how long physiological adaptation processes last. Research investigating space travelers at different intervals post-flight could answer this question.
The intimate brain–heart connection enunciated by Claude Bernard can be studied by analyzing HRV17. HRV may reflect the activity of the coordinating system18–21, notably brain functional connectivity, including the DMN and the SN, which integrates the brainstem nuclei that directly regulate the heart. The heart and brain are connected bi-directionally, and HRV varies in concert with changes in brain functional connectivity. As we reported earlier19,20, HRV may serve as a proxy for ‘vertical integration’ of the brain in association with DMN and SN functions. HRV may provide information on how the brain coordinates with the periphery, and may thus inform about the extent of adaptive adjustment and brain plasticity17. Accordingly, herein, we analyze HRV in the course of space missions to gain knowledge about brain plasticity and adaptation to space19–22.
Many investigations recently showed how psychological resilience can be assessed using imaging modalities within the brain, such as low-frequency fluctuations in the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), posterior cingulate cortex (PCC) and thalamus, largely comprising the brain functional networks of the DMN and SN23,24. Astronauts aboard the International Space Station (ISS) are well motivated and become even more so with subsequent missions.
Subject and methods
Subject
A healthy astronaut participated in the ISS Japan Aerospace Exploration Agency (JAXA) investigation named “Biological Rhythms 24 Hrs & 48 Hrs”. Stays in space lasted approximately 4.5 and 6 months on the first and second flights, respectively. The astronaut had passed class III physical examinations from the National Aeronautics and Space Administration (NASA). The study was approved by the Institutional Review Boards of NASA, ESA (European Space Agency), Pro0406 (MODCR940)—Amd-10, and JAXA, JX-IRBA-20-084 Amd-10. Informed consent was obtained from the astronaut. A detailed explanation of the study protocol was given to the astronaut before obtaining written, informed consent, according to the Declaration of Helsinki Principles. All methods were performed in accordance with the JAXA/ESA/NASA guidelines and regulations.
Experimental protocols
Ambulatory around-the-clock ECG records were obtained over 24 h on the first flight and over 48 h on the second flight by a two-channel Holter recorder (FM-180; Fukuda Denshi, Tokyo, Japan). Measurements were taken four times during each mission: once before flight, twice during flight on the ISS (ISS01 and ISS02), and once after the mission (Post) (Mission 1/2: Pre: 189/50 days before launch; ISS01 and ISS02: day 18/21 and day 67/181 after launch, respectively; Post: 138/188 days after return to Earth).
For assessing microgravity-induced brain plasticity, we focused only on ISS01 because measurements were obtained at about the same time after launch in both missions, on days 18 and 21 on the ISS, respectively. Because the second session on the ISS took place much later on the second than on the first spaceflight (on day 181 versus day 67), data collected during ISS02 were not suitable for analysis.
Analysis of HRV
Data collection and measurement procedures were conducted as previously reported19,20,25–28. Briefly, for HRV measurements, the RR intervals between normal QRS waveforms were extracted as normal-to-normal (NN) intervals, which were A/D converted (125-Hz) with 8-ms time resolution. The authors first confirmed that all artifacts were actually removed and that the data excluded supraventricular or ventricular arrhythmia. First, time-domain measures (CVRR, r-MSSD and pNN50), Lorenz plot (Length, Width and Length/Width ratio), and conventional frequency-domain measures (TF-, ULF-, VLF-, LF- and HF-HRV and LF/HF ratio)29 and β, reflecting the intrinsic cardiovascular regulatory system, were obtained with the Maximum Entropy Method (MEM) software (MemCalc/CHIRAM, Suwa Trust GMS, Tokyo, Japan)30. Time series of NN intervals covering 5-min intervals were analyzed by the MEM to compute the spectral power in different frequency regions. Next, HRV measures reflecting dynamics of brain functional connectivity17–19,23,24,31–36 were assessed, as defined in the lower part of Table 1. Frequency regions examined were 0.05–0.15 Hz (LF-component) and 0.15–0.50 Hz (HF-component), according to Chang et al.18; 0.01–0.05 Hz (LF-band), 0.05–0.10 Hz (MF1-band), 0.10–0.15 Hz (MF2-band), and 0.15–0.20 Hz (HF01-band), according to Baria et al.37; 0.20–0.30 Hz (HF02-band), 0.30–0.40 Hz (HF03-band), and 0.40–0.50 Hz (HF04-band), according to Chen and Glover38.
Table 1.
Frequency-domain measures of heart rate variability (HRV).
| Frequency-domain measures (units, ms2) | Frequency range (Hz) | Description | Related references | Brief physiologic correlation | |
|---|---|---|---|---|---|
| Conventional frequency-domain measures of HRV | TF-HRV | 0.0001–0.50 | Variance of all N–N intervals over 3-h interval | 29 | Index suggestive of anti-aging or longevity |
| ULF-HRV | 0.0001–0.003 | Power in ULF range. Fluctuations in N–N intervals with underlying cycle length [> 5 min − ≤ 3 h] | Predominantly 3-h rhythm, but other influences, including activity and neuroendocrine rhythms, contribute to ULF. Circadian rhythms, core body temperature, metabolism, hormones, and intrinsic rhythms generated by the heart all contribute to ULF | ||
| VLF-HRV | 0.003–0.04 | Power in VLF range, Infra-slow oscillation (ISO) | VLF is the most predictive of adverse outcomes, including all-cause motality. Historically, VLF may reflect both vagal control of heart rate and also the effect of the renin-angiotensin system. Recently, VLF rhythm appears to be produced by the heart itself and may be an intrinsic rhythm that is fundamental to health and well-being | ||
| LF-HRV | 0.04–0.15 | Power in LF range | LF primarily reflects baroreflex activity while at rest | ||
| HF-HRV | 0.15–0.40 | Power in HF range | Relative vagal modulation of heart rate in response to respiration. Higher values reflect higher parasympathetic (vagal) influence or greater degree of erractic rhythm | ||
| HRV measures reflecting dynamics of brain functional connectivity | LF-component | 0.05–0.15 | Power in LF-component range | 18,19 | Index reflecting the Default Mode Network (DMN) circuit, acting via the Temporo-Parietal Junction networks. Increase in LF-component suggests DMN’s role in adaptation to novel environment and in coordinating functional connections with other brain networks, including the Salience Network |
| HF-component | 0.15–0.50 | Power in HF-component range | Index reflecting the Salience Network circuit, acting via the lateral orbitofrontal cortex loop, involved in the adaptation process. Increases in HF-component are accompanied by increases in functional connectivity between the dorsal anterior cingulate cortex and regions including the basal ganglia, thalamus, midbrain and brainstem, and between the amygdala and regions including the basal ganglia, anterior insula and dorsolateral prefrontal cortex | ||
| LF-band | 0.01–0.05 | Power in LF-band, Infra-slow oscillation (ISO) | 19,37 | Index primarily related to brain’s DMN activity, in medial prefrontal and precuneus/posterior cingulate cortex parts | |
| MF1-band | 0.05–0.10 | Power in MF1-band range, ISO | Index primarily related to brain’s DMN activity, mainly in the thalamus and basal ganglia | ||
| MF2-band | 0.10–0.15 | Power in MF2-band range | Index primarily related to brain’s DMN activity, mainly in the orbitofrontal, insular and temporal cortex parts | ||
| HF01-band | 0.15–0.20 | Power in HF1-band range | Index primarily related to medial orbitofrontal cortex (mOFC)/medial prefrontal cortex (mPFC)-guided core integration system | ||
| HF02-band | 0.20–0.30 | Power in HF2-band range | 17,19,31–34,38 | Index reflecting relative vagal modulation of heart rate in response to respiration | |
| HF03-band | 0.30–0.40 | Power in HF3-band range | Index reflecting psychological resilience related to brain’s Salience Network activity, primarily in anterior cingulate cortex on subjective well-being | ||
| HF04-band | 0.40–0.50 | Power in HF4-band range | Index reflecting psychological resilience related to brain’s Salience Network activity, primarily in anterior cingulate cortex on subjective well-being | ||
| Infraslow oscillation (ISO) at night | 0.01–0.10 | Power in frequency range of 0.01–0.10 Hz | 20,23,24,35,36 | HRV index coordinating brain dynamics via thalamic astrocytes plays key role in adaptation to novel environments; 0.02 Hz or 1/min fluctuations underly unconscious processing of information among resting state networks | |
| LFFs (low-frequency fluctuations) during daytime | Recently, LFFs are noted HRV indices reflecting psychological resilience related to brain’s DMN activity, primarily in orbitofrontal cortex on subjective well-being | ||||
A positive response in these bands is thought to indicate how astronauts adapt to the space environment. Increases in the LF- and MF1-bands reflect an activation of the DMN’s medial prefrontal cortex (mPFC), posterior parietal cortex, posterior portion of precuneus and posterior cingulate cortex. Changes in the MF2- and HF01-, HF02-, HF03-, and HF04-bands show dynamic interactions among the DMN and SN, i.e., the alerted DMN involved in the adaptation to a novel environment18,19,37,38.
Cosine curve fitting for estimating amplitude and phase by cosinor
The MEM software (MemCalc/Win, Suwa Trust GMS, Tokyo, Japan)30 was used to fit a single 24-h or 12-h cosine curve individually to each of the HRV measures by cosinor39–41. The 24-h and 12-h amplitudes and acrophases together with the MESOR (Midline Estimating Statistic Of Rhythm, a rhythm-adjusted mean) were thereby estimated. Changes in biological rhythm amplitude and acrophase assessed the response in rhythmicity of each biological rhythmic component to the space environment.
Sleep duration and sleep quality
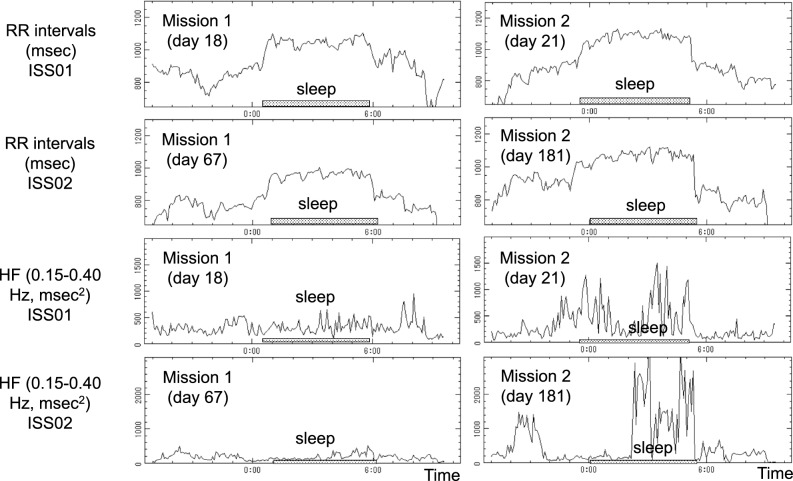
Sleep duration at night was estimated by using circadian profiles of RR-intervals and 5-min HF endpoints of HRV25,28,29,42. Sleep duration on the second spaceflight was estimated as the average of the two consecutive sleep spans of the 48-h ECG record. Sleep quality was determined based on whether a sleep-related increase in RR-interval and in HF of HRV could be observed or not, as shown in Fig. 1. For reference, results during ISS02 are also depicted.
Figure 1.
Estimation of sleep span and assessment of sleep quality. RR-intervals (first two rows) and HF-HRV (last two rows) of the first (left) and second (right) spaceflight assess sleep duration and sleep quality during ISS01 and ISS02, respectively. HF-HRV spectral power, reflecting sleep quality, is clearly larger on the second than on the first spaceflight during both ISS01 and ISS02. Sleep-related increase in RR-intervals also appears to be larger on the second than on the first spaceflight.
Statistical analyses
Data shown in Table 2 are expressed as mean ± standard error (SE). The ECG recording was started at 13:25 during the 1st space-flight mission, and at 17:50 during the 2nd space-flight mission. For comparison of HRV indices, statistical analyses were applied on hourly averages of the 5-min estimates in order to minimize serial correlation. Paired hourly HRV indices were compared between the two spaceflights, focusing on ISS01 (days 18 and 21 after launch, respectively), using the paired t-test. Cohen’s distance was determined to assess effect size. The Stat Flex (Ver. 6) software (Artec Co., Ltd., Osaka, Japan) was used. P-values less than 0.05 were considered to indicate statistical significance.
Table 2.
Comparison of heart rate variability between the two spaceflights suggests role of brain functional network for faster adaptation to microgravity on second spaceflight.
| Unit, or frequency range (Hz) | Day time (awake) | Night time (asleep) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mission 1 (day 18 after launch) | Mission 2 (day 21 after launch) | Paired t-test | n | Mission 1 (day 18 after launch) | Mission 2 (day 21 after launch) | Paired t-test | ||||||||
| Mean | SE | Mean | SE | t-value | p-value | Mean | SE | Mean | SE | t-value | p-value | ||||
| HR | bpm | 18 | 75.9 | 2.7 | 71.4 | 1.7 | − 1.415 | 0.1752 | 6 | 58.3 | 1.0 | 56.5 | 1.1 | − 3.277 | 0.0220 |
| NN | ms | 18 | 813.3 | 21.8 | 849.9 | 17.5 | 1.330 | 0.2012 | 6 | 1032.1 | 16.5 | 1065.3 | 19.7 | 3.100 | 0.0269 |
| β | 0.0001–0.01 | 17 | − 1.2065 | 0.0875 | − 0.9793 | 0.0465 | 1.799 | 0.0953 | 6 | − 0.7533 | 0.0732 | − 0.5400 | 0.0991 | 3.719 | 0.0137 |
| CVRR | ms | 18 | 8.08 | 0.30 | 7.40 | 0.44 | − 1.178 | 0.2549 | 6 | 5.67 | 0.30 | 5.79 | 0.28 | 0.521 | 0.6246 |
| r-MSSD | ms | 18 | 41.8 | 2.2 | 32.2 | 1.9 | − 4.697 | 0.0002 | 6 | 45.7 | 1.2 | 52.0 | 5.2 | 1.191 | 0.2869 |
| pNN50 | % | 18 | 17.7 | 1.4 | 9.2 | 1.4 | − 5.178 | 0.0001 | 6 | 20.4 | 1.0 | 15.4 | 3.4 | − 1.481 | 0.1986 |
| Length | ms | 18 | 300.2 | 12.1 | 263.6 | 8.9 | − 2.252 | 0.0378 | 6 | 302.1 | 18.6 | 305.2 | 11.9 | 0.151 | 0.8857 |
| Width | ms | 18 | 138.2 | 18.8 | 81.9 | 5.7 | − 2.965 | 0.0087 | 6 | 119.3 | 3.5 | 159.3 | 17.6 | 2.629 | 0.0466 |
| Len/Wid | – | 18 | 2.62 | 0.09 | 3.69 | 0.25 | 4.416 | 0.0004 | 6 | 2.55 | 0.09 | 2.42 | 0.31 | − 0.390 | 0.7129 |
| TF | 0.0001–0.50 | 17 | 8857.4 | 1400.4 | 5351.3 | 693.0 | − 1.871 | 0.0840 | 6 | 4238.2 | 460.1 | 4498.2 | 348.7 | 0.656 | 0.5408 |
| ULF | 0.0001–0.003 | 17 | 6227.8 | 1479.7 | 2672.4 | 583.3 | − 1.846 | 0.0878 | 6 | 1168.7 | 223.1 | 879.4 | 160.7 | − 1.961 | 0.1071 |
| VLF | 0.003–0.04 | 18 | 1566.0 | 112.6 | 1642.1 | 129.6 | 0.482 | 0.6360 | 6 | 1724.7 | 172.8 | 2083.1 | 284.0 | 1.327 | 0.2419 |
| LF | 0.04–0.15 | 18 | 920.2 | 61.1 | 872.4 | 48.5 | − 0.703 | 0.4919 | 6 | 999.7 | 142.6 | 978.2 | 108.8 | − 0.210 | 0.8422 |
| HF | 0.15–0.40 | 18 | 283.1 | 29.9 | 227.7 | 26.4 | − 2.108 | 0.0501 | 6 | 317.4 | 20.5 | 510.7 | 88.9 | 2.064 | 0.0939 |
| LF/HF | – | 18 | 3.63 | 0.20 | 4.85 | 0.29 | 4.266 | 0.0005 | 6 | 3.44 | 0.51 | 2.62 | 0.30 | − 1.236 | 0.2714 |
| LF-component | 0.05–0.15 | 18 | 783.2 | 50.9 | 747.0 | 42.3 | − 0.617 | 0.5451 | 6 | 819.3 | 113.1 | 809.2 | 104.1 | − 0.151 | 0.8859 |
| HF-component | 0.15–0.50 | 18 | 321.0 | 34.1 | 249.8 | 29.1 | − 2.479 | 0.0240 | 6 | 345.1 | 20.3 | 557.5 | 97.1 | 2.095 | 0.0903 |
| LF-band | 0.01–0.05 | 18 | 1035.6 | 86.7 | 1052.0 | 60.6 | 0.198 | 0.8453 | 6 | 1448.7 | 152.3 | 1531.0 | 187.8 | 0.402 | 0.7040 |
| MF1-band | 0.05–0.10 | 18 | 522.1 | 34.1 | 462.0 | 23.0 | − 1.623 | 0.1230 | 6 | 643.9 | 101.3 | 635.4 | 86.3 | − 0.183 | 0.8619 |
| MF2-band | 0.10–0.15 | 18 | 261.1 | 18.7 | 285.1 | 21.6 | 0.938 | 0.3612 | 6 | 175.4 | 15.5 | 173.8 | 26.7 | − 0.060 | 0.9548 |
| HF01-band | 0.15–0.20 | 18 | 111.1 | 10.9 | 95.4 | 9.0 | − 1.418 | 0.1743 | 6 | 102.5 | 10.2 | 138.3 | 17.5 | 2.256 | 0.0737 |
| HF02-band | 0.20–0.30 | 18 | 113.5 | 13.1 | 95.6 | 14.5 | − 1.415 | 0.1750 | 6 | 162.2 | 13.5 | 270.9 | 55.1 | 1.757 | 0.1392 |
| HF03-band | 0.30–0.40 | 18 | 58.5 | 6.9 | 36.7 | 5.2 | − 4.791 | 0.0002 | 6 | 52.7 | 2.1 | 101.5 | 20.8 | 2.338 | 0.0665 |
| HF04-band | 0.40–0.50 | 18 | 37.9 | 4.5 | 22.1 | 3.1 | − 5.531 | 0.0003 | 6 | 27.7 | 0.9 | 46.8 | 9.0 | 2.203 | 0.0788 |
Tests applied on hourly averages of 5-min intervals in order to eliminate or at least reduce serial correlation.
n number of hourly averages (of 5-min intervals), SE standard error.
P-values not adjusted for multiple testing; P < 0.05 after adjusting for mutiple testing highlighted in bold.
Results
Sleep performance
While sleep duration around day 20 after launch during ISS01 cannot be statistically compared between the two missions without knowing its day-to-day variation for this astronaut, it was more than one hour longer on the second than on the first mission (374 vs. 300 min during ISS01 and 365 vs. 295 min during ISS02). Such large differences between the two missions are not seen before launch (297 vs. 289 min) and after return to Earth (330 vs. 360 min). Sleep quality may also have been improved on the second compared to the first spaceflight, as suggested by a clear increase in spectral power of the HF-HRV, Fig. 1.
Dynamic response of the autonomic nervous system
As shown in Table 2 (right), nighttime HR was lower by about 2 bpm on the second than on the first mission during ISS01 (56.5 vs. 58.3 bpm). A larger change occurred in the intrinsic cardiovascular regulatory function |β| (0.5400 vs. 0.7533). Parasympathetic activity was increased on the second mission, as shown by the Width of Lorenz plot (159.3 vs. 119.3) and by HF-HRV (510.7 vs. 317.4 msec2). Such changes, well exceeding 25% are of sufficient magnitude to serve as biomarkers in future studies.
During daytime (Table 2, left), parasympathetic activity was lower on the second than on the first mission during ISS01, gauged by r-MSSD (32.2 vs. 41.8), pNN50 (9.2 vs. 17.7), Length of Lorenz plot (263.6 vs. 300.2), Width of Lorenz plot (81.9 vs. 138.2), and HF-HRV (227.7 vs. 283.1). Sympathetic activity increased during daytime, gauged by the LF/HF ratio (4.85 vs. 3.63) and Lorenz plot’s Length/Width (3.69 vs. 2.62). Again, these changes are non-negligible, extending from over 10% to about 50%.
Brain functional networks estimated by heart rate variability during spaceflight
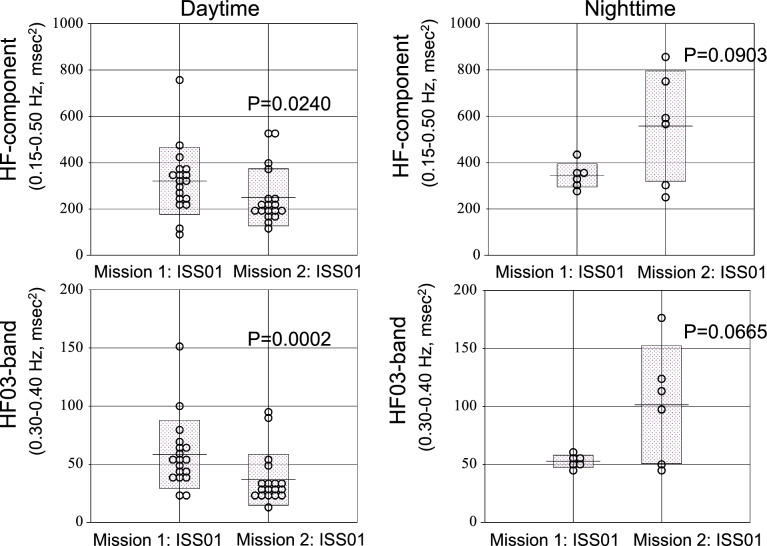
Activity of brain functional networks is reflected in several indices of HRV17–19,31–35. Although not statistically significant, HRV indices reflecting SN activity tended to increase on the second compared to the first spaceflight during nighttime (Table 2, right)17,32,37,43. The HF-component increased by 62% (from 345.1 to 557.5 ms2) (Fig. 2, top right), HF01-band by 35% (from 102.5 to 138.3 ms2), HF02-band by 67% (from 162.2 to 270.9 ms2), HF03-band by 92% (from 52.7 to 101.5 ms2) (Fig. 2, bottom right), and HF04-band by 69% (from 27.7 to 46.8 ms2). These HRV indices changed statistically significant during daytime, (Table 2, left). The HF-component decreased by 22% (Fig. 2, top left), HF03-band and HF04-band decreased by 37% (from 58.5 to 36.7) (Fig. 2, bottom left) and by 42% (from 37.9 to 22.1), respectively. Corresponding effect sizes are medium or large based on Cohen’s distance. It should be possible to detect changes of this magnitude in future studies.
Figure 2.
Brain plasticity at night and psychological resilience during daytime took place on the second mission. HRV indices reflecting SN activity, HF-component and HF03-band, decreased during daytime (left), but increased during nighttime (right). These changes are in agreement with previous investigations showing that brain plasticity takes place at night while psychological resilience takes place during the daytime (see text).
Assessment of circadian and circasemidian components of HRV endpoints
Circadian and circasemidian amplitudes of HRV endpoints are shown in Table 3. On the first spaceflight, the circadian amplitude of HR increased more than two-fold both during ISS01 (255%) and ISS02 (271%) compared to pre-flight, as observed previously28. This was not the case on the second spaceflight. On the first mission, the circadian amplitude of the intrinsic cardiovascular regulatory system function (β) also increased during both ISS01 (303%) and ISS02 (233%), compared to pre-flight. The adaptation behavior of the 12-h component of HRV endpoints was remarkably larger than that of the 24-h rhythm, particularly for TF-HRV, seen in both amplitude and phase. The 12-h amplitude of TF-HRV increased up to 574% and 473% during ISS01 and ISS02, respectively, on the first spaceflight, although similar changes were not clear on the second spaceflight.
Table 3.
Changes of circadian and circasemidian amplitude of heart rate and heart rate variability along with long-duration spaceflight missions.
| Before launch | ISS01 | ISS02 | Before launch | ISS01 | ISS02 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mission 1 | Mission 2 | Mission 1 | Mission 2 | Mission 1 | Mission 2 | Mission 1 | Mission 2 | Mission 1 | Mission 2 | Mission 1 | Mission 2 | ||||
| HR (b/min) | MESOR | 72.86 | 66.61 | 71.65 | 68.18 | 79.42 | 70.03 | β (0.0001–0.01 Hz) | MESOR | − 1.173 | 1.072 | − 1.147 | 0.942 | − 1.070 | 0.867 |
| Circadian amplitude | 4.40 | 9.71 | 11.22 (255%) | 8.38 | 11.93 (271%) | 12.11 (125%) | Circadian amplitude | 0.172 | 0.287 | 0.521 (303%) | 0.356 | 0.401 (233%) | 0.253 | ||
| Circasemidian amplitude | 13.2 | 5.3 | 6.8 | 5.5 | 8.2 | 7.4 | Circasemidian amplitude | 0.194 | 0.127 | 0.094 | 0.144 | 0.329 | 0.294 | ||
| NN-interval (ms) | MESOR | 853.15 | 930.80 | 866.30 | 897.60 | 778.60 | 882.90 | LF-component (ms2) (0.05–0.15 Hz) | MESOR | 1205.8 | 1097.6 | 791.6 | 740.4 | 655.9 | 801.0 |
| Circadian amplitude | 48.14 | 129.80 | 128.70 (267%) | 114.20 | 119.40 (248%) | 148.50 (114%) | Circadian amplitude | 441.8 | 375.6 | 20.1 | 33.7 | 102.9 | 254.2 | ||
| Circasemidian amplitude | 138.3 | 82.1 | 83.3 | 75.9 | 81.2 | 89.5 | Circasemidian amplitude | 352.7 | 295.0 | 104.6 | 36.2 | 61.1 | 139.4 | ||
| r-MSSD (ms) | MESOR | 43.45 | 58.94 | 42.45 | 34.68 | 27.16 | 43.92 | HF-component (ms2) (0.15–0.50 Hz) | MESOR | 443.5 | 728.8 | 319.9 | 284.5 | 168.0 | 485.1 |
| Circadian amplitude | 4.21 | 29.70 | 4.17 | 7.90 | 1.14 | 14.37 | Circadian amplitude | 56.0 | 715.1 | 37.6 | 115.4 | 18.1 | 402.5 | ||
| Circasemidian amplitude | 17.32 | 24.01 | 1.41 | 4.24 | 0.73 | 11.60 | Circasemidian amplitude | 287.8 | 607.0 | 26.1 | 63.4 | 12.6 | 307.4 | ||
| pNN50 ( % ) | MESOR | 16.86 | 21.19 | 18.34 | 9.52 | 6.25 | 12.92 | LF-band (ms2) (0.01–0.05 Hz) | MESOR | 1612.5 | 2156.2 | 1117.0 | 1087.6 | 827.5 | 1331.9 |
| Circadian amplitude | 5.40 | 8.25 | 3.36 | 2.99 | 0.26 | 2.50 | Circadian amplitude | 612.2 | 785.3 | 295.2 | 217.0 | 230.0 | 605.4 | ||
| Circasemidian amplitude | 8.27 | 7.17 | 1.01 | 2.92 | 0.39 | 4.05 | Circasemidian amplitude | 930.1 | 605.9 | 107.1 | 216.1 | 234.6 | 640.1 | ||
| LF/HF (–) | MESOR | 4.42 | 3.31 | 3.60 | 4.36 | 5.61 | 4.01 | MF1-band (ms2) (0.05–0.10 Hz) | MESOR | 763.2 | 803.1 | 549.2 | 498.4 | 434.1 | 550.5 |
| Circadian amplitude | 0.58 | 1.12 | 0.39 | 0.85 | 0.36 | 0.52 | Circadian amplitude | 207.4 | 228.4 | 56.1 | 88.4 | 123.4 | 171.5 | ||
| Circasemidian amplitude | 1.26 | 0.76 | 0.16 | 0.85 | 0.86 | 1.23 | Circasemidian amplitude | 217.2 | 232.0 | 94.8 | 39.1 | 54.9 | 167.9 | ||
| TF (ms2) (0.0001–0.50 Hz) | MESOR | 8012.5 | 9851.4 | 8563.9 | 5578.0 | 4717.9 | 6756.6 | MF2-band (ms2) (0.10–0.15 Hz) | MESOR | 442.6 | 437.9 | 242.4 | 245.1 | 221.8 | 290.9 |
| Circadian amplitude | 3927.2 | 3408.7 | 6385.6 (163%) | 2827.0 | 2152.4 | 2721.3 | Circadian amplitude | 234.9 | 88.9 | 49.9 | 66.1 | 76.1 | 73.7 | ||
| Circasemidian amplitude | 311.3 | 3434.1 | 1785.5 (574%) | 1710.7 | 1473.0 (473%) | 1167.1 | Circasemidian amplitude | 140.2 | 110.4 | 29.5 | 42.5 | 52.8 | 42.7 | ||
| ULF (ms2) (0.0001–0.003 Hz) | MESOR | 4059.4 | 5071.0 | 5874.3 | 2924.9 | 2717.5 | 3449.9 | HF-band (ms2) (0.15–0.20 Hz) | MESOR | 181.9 | 444.2 | 107.6 | 102.0 | 63.8 | 154.5 |
| Circadian amplitude | 2721.4 | 3021.9 | 6757.7 (248%) | 3060.2 | 2255.6 | 3422.8 | Circadian amplitude | 33.4 | 529.3 | 6.1 | 16.3 | 9.7 | 90.6 | ||
| Circasemidian amplitude | 1712.6 | 1776.2 | 1692.6 | 1683.5 | 1449.8 | 2469.7 | Circasemidian Amplitude | 123.2 | 460.1 | 1.9 | 5.5 | 5.8 | 101.8 | ||
| VLF (ms2) (0.003–0.04 Hz) | MESOR | 2606.3 | 3455.9 | 1582.1 | 1538.2 | 1140.9 | 1856.1 | HF02-band (ms2) (0.20–0.30 Hz) | MESOR | 157.4 | 300.2 | 122.6 | 116.4 | 63.6 | 187.0 |
| Circadian amplitude | 1112.3 | 954.2 | 304.4 | 87.7 | 351.5 | 756.2 | Circadian amplitude | 16.8 | 271.7 | 37.1 (221%) | 69.1 | 17.8 | 169.8 | ||
| Circasemidian amplitude | 1520.8 | 1207.1 | 114.3 | 327.7 | 335.2 | 929.7 | Circasemidian amplitude | 99.0 | 271.7 | 19.4 | 40.4 | 5.7 | 117.8 | ||
| LF (ms2) (0.04–0.15 Hz) | MESOR | 1395.8 | 1487.1 | 937.0 | 874.7 | 772.1 | 982.7 | HF03-band (ms2) (0.30–0.40 Hz) | MESOR | 69.6 | 140.6 | 55.6 | 43.6 | 25.4 | 92.8 |
| Circadian amplitude | 483.1 | 382.0 | 25.2 | 52.9 | 122.1 | 162.1 | Circadian amplitude | 10.5 | 142.6 | 2.6 | 21.9 | 1.8 | 68.4 | ||
| Circasemidian amplitude | 392.2 | 390.8 | 125.5 | 15.1 | 84.7 | 220.3 | Circasemidian amplitude | 43.5 | 112.3 | 6.7 | 12.5 | 3.8 | 54.4 | ||
| HF (ms2) (0.15–0.40 Hz) | MESOR | 408.89 | 909.60 | 285.70 | 262.40 | 152.75 | 443.30 | HF04-band (ms2) (0.40–0.50 Hz) | MESOR | 34.6 | 60.7 | 34.2 | 23.0 | 15.2 | 47.3 |
| Circadian amplitude | 56.82 | 988.70 | 40.46 | 104.60 | 19.29 | 286.70 | Circadian amplitude | 1.0 | 60.8 | 3.1 (317%) | 9.8 | 1.6 | 30.1 | ||
| Circasemidian amplitude | 265.3 | 821.4 | 23.9 | 55.6 | 12.3 | 260.5 | Circasemidian amplitude | 24.2 | 46.6 | 5.7 | 6.4 | 1.8 | 19.9 | ||
Mission 2 took place about 4 years after Mission 1; ISS01 and ISS02 took place on days 18/21 and 67/181 after launch during mission 1/2, respectively.
Results highlighted in bold indicate changes larger than 200%.
An apparent phase shift of the 24-h and 12-h components of HRV endpoints in response to spaceflight was observed after fitting a single 24-h or 12-h cosine curve separately to the 20 HRV measures by cosinor. Results are summarized in Table 4, where misaligned circadian phases occurring at unusual times (such as day-night reversals), are shown in bold. On the first but not on the second spaceflight, quite a few HRV endpoints show circadian misalignment pre-flight (Table 4, left), suggesting that circadian desynchrony due to social jetlag was larger on the first than on the second mission.
Table 4.
Circadian and circasemidian phase changes in heart rate variability indices in response to space flight differ between the two long-term missions.
| Mission 1 | Mission 2 | ||||||
|---|---|---|---|---|---|---|---|
| Circadians | Before | ISS01 | ISS02 | Circadians | Before | ISS01 | ISS02 |
| HR (b/min) | 16:55 | 14:18 | 15:10 | HR (b/min) | 15:26 | 15:01 | 12:18 |
| NN-interval (ms) | 6:22 | 2:45 | 3:15 | NN-interval (ms) | 3:08 | 2:52 | 0:46 |
| r-MSSD (ms) | 14:08 | 2:59 | 2:48 | r-MSSD (ms) | 2:13 | 1:46 | 1:32 |
| pNN50 ( % ) | 15:34 | 2:07 | 17:56 | pNN50 ( % ) | 3:29 | 0:28 | 1:10 |
| LF/HF ratio (–) | 16:17 | 16:35 | 05:57 | LF/HF ratio (–) | 13:49 | 13:13 | 16:58 |
| LF-component (ms2) (0.05–0.15 Hz) | 16:32 | 21:10 | 7:35 | LF-component (ms2) (0.05–0.15 Hz) | 6:27 | 23:04 | 2:30 |
| HF-component (ms2) (0.15–0.50 Hz) | 14:41 | 4:08 | 4:08 | HF-component (ms2) (0.15–0.50 Hz) | 3:32 | 2:03 | 2:43 |
| TF (ms2) (0.0001–0.50 Hz) | 13:00 | 12:23 | 12:09 | TF (ms2) (0.0001–0.50 Hz) | 10:21 | 17:40 | 09:17 |
| ULF (ms2) (0.0001–0.003 Hz) | 13:30 | 12:37 | 13:01 | ULF (ms2) (0.0001–0.003 Hz) | 11:11 | 17:25 | 10:36 |
| VLF (ms2) (0.003–0.04 Hz) | 14:03 | 05:59 | 07:08 | VLF (ms2) (0.003–0.04 Hz) | 06:13 | 03:47 | 01:58 |
| LF (ms2) (0.04–0.15 Hz) | 16:15 | 00:36 | 07:06 | LF (ms2) (0.04–0.15 Hz) | 04:09 | 23:47 | 00:49 |
| HF (ms2) (0.15–0.40 Hz) | 14:39 | 04:02 | 04:21 | HF (ms2) (0.15–0.40 Hz) | 01:56 | 01:52 | 01:54 |
| LF-band (ms2) (0.01–0.05 Hz) | 13:52 | 04:25 | 05:27 | LF-band (ms2) (0.01–0.05 Hz) | 04:45 | 03:54 | 02:08 |
| MF1-band (ms2) (0.05–0.10 Hz) | 16:44 | 01:17 | 05:03 | MF1-band (ms2) (0.05–0.10 Hz) | 03:55 | 00:58 | 01:07 |
| MF2-band (ms2) (0.10–0.15 Hz) | 16:21 | 14:40 | 13:18 | MF2-band (ms2) (0.10–0.15 Hz) | 05:20 | 14:24 | 14:17 |
| HF-band (ms2) (0.15–0.20 Hz) | 15:51 | 09:19 | 09:07 | HF-band (ms2) (0.15–0.20 Hz) | 01:53 | 03:40 | 02:37 |
| HF02-band (ms2) (0.20–0.30 Hz) | 12:53 | 03:27 | 02:43 | HF02-band (ms2) (0.20–0.30 Hz) | 02:30 | 01:44 | 02:20 |
| HF03-band (ms2) (0.30–0.40 Hz) | 13:37 | 03:33 | 22:29 | HF03-band (ms2) (0.30–0.40 Hz) | 01:43 | 01:58 | 02:20 |
| HF04-band (ms2) (0.40–0.50 Hz) | 00:37 | 14:51 | 18:55 | HF04-band (ms2) (0.40–0.50 Hz) | 01:38 | 01:11 | 01:40 |
| β | 06:32 | 01:36 | 02:10 | β | 01:36 | 04:38 | 23:11 |
| Mission 1 | Mission 2 | ||||||
|---|---|---|---|---|---|---|---|
| Circasemidians | Before | ISS01 | ISS02 | Circasemidians | Before | ISS01 | ISS02 |
| HR (b/min) | − 355 | − 328 | − 312 | HR (b/min) | − 292 | − 306 | − 305 |
| NN-interval (ms) | − 82 | − 238 | − 225 | NN-interval (ms) | − 207 | − 37 | − 211 |
| r-MSSD (ms) | − 93 | − 20 | − 235 | r-MSSD (ms) | − 207 | − 16 | − 236 |
| pNN50 ( % ) | − 98 | − 359 | − 316 | pNN50 ( % ) | − 212 | − 357 | − 247 |
| LF/HF ratio (–) | − 9 | − 352 | − 6 | LF/HF ratio (–) | − 299 | − 271 | − 342 |
| LF-component (ms2) (0.05–0.15 Hz) | − 98 | − 9 | − 10 | LF-component (ms2) (0.05–0.15 Hz) | − 215 | − 356 | − 229 |
| HF-component (ms2) (0.15–0.50 Hz) | − 93 | − 205 | − 284 | HF-component (ms2) (0.15–0.50 Hz) | − 223 | − 17 | − 242 |
| TF (ms2) (0.0001–0.50 Hz) | − 320 | − 320 | − 302 | TF (ms2) (0.0001–0.50 Hz) | − 246 | − 92 | − 302 |
| ULF (ms2) (0.0001–0.003 Hz) | − 4 | − 323 | − 310 | ULF (ms2) (0.0001–0.003 Hz) | − 255 | − 97 | − 316 |
| VLF (ms2) (0.003–0.04 Hz) | − 93 | − 288 | − 258 | VLF (ms2) (0.003–0.04 Hz) | − 240 | − 62 | − 241 |
| LF (ms2) (0.04–0.15 Hz) | − 281 | − 11 | − 194 | LF (ms2) (0.04–0.15 Hz) | − 209 | − 19 | − 227 |
| HF (ms2) (0.15–0.40 Hz) | − 92 | − 211 | − 280 | HF (ms2) (0.15–0.40 Hz) | − 206 | − 16 | − 233 |
| LF-band (ms2) (0.01–0.05 Hz) | − 92 | − 240 | − 243 | LF-band (ms2) (0.01–0.05 Hz) | − 230 | − 62 | − 236 |
| MF1-band (ms2) (0.05–0.10 Hz) | − 94 | − 17 | − 217 | MF1-band (ms2) (0.05–0.10 Hz) | − 208 | − 29 | − 221 |
| MF2-band (ms2) (0.10–0.15 Hz) | − 284 | − 337 | − 342 | MF2-band (ms2) (0.10–0.15 Hz) | − 214 | − 320 | − 260 |
| HF-band (ms2) (0.15–0.20 Hz) | − 92 | − 8 | − 281 | HF-band (ms2) (0.15–0.20 Hz) | − 206 | − 37 | − 235 |
| HF02-band (ms2) (0.20–0.30 Hz) | − 91 | − 221 | − 262 | HF02-band (ms2) (0.20–0.30 Hz) | − 38 | − 15 | − 238 |
| HF03-band (ms2) (0.30–0.40 Hz) | − 96 | − 6 | − 309 | HF03-band (ms2) (0.30–0.40 Hz) | − 207 | − 19 | − 250 |
| HF04-band (ms2) (0.40–0.50 Hz) | − 284 | − 354 | − 322 | HF04-band (ms2) (0.40–0.50 Hz) | − 204 | − 5 | − 264 |
| β | − 90 | − 32 | − 230 | β | − 206 | − 27 | − 45 |
Bold cells mean circadian phase misalignment induced by astronaut social jetlag on Earth before spaceflight mission.
Circasemidian acrophases expressed in (negative) degrees, with 360° ≡ 12 h, 0° = 00:00.
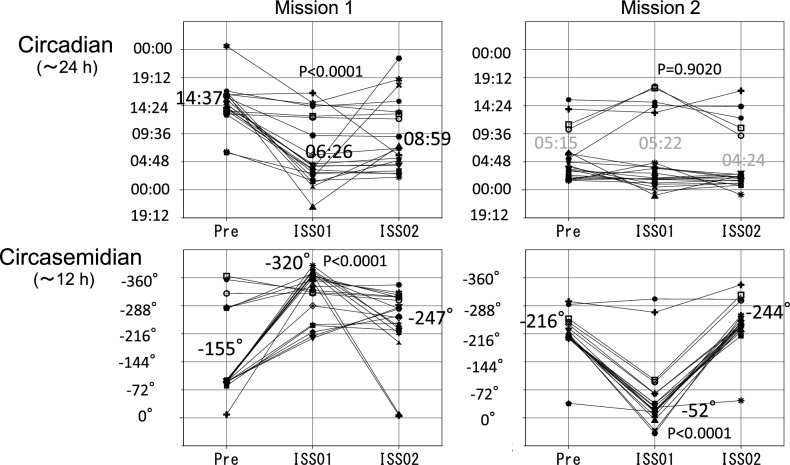
The recovery process of such internal desynchrony of the 20 HRV indices during spaceflight is illustrated in Fig. 3. It depicts the time course of the circadian (Fig. 3, top) and circasemidian (Fig. 3, bottom) acrophases during the first (Fig. 3, left) and second (Fig. 3, right) missions. On the first mission, circadian acrophases advanced on average by about 8 h, from 14:37 (pre-flight) to 6:26 (ISS01). Any misalignment of these circadian acrophases improved during ISS01, maintaining a similar timing of 8:59 during ISS02 (Fig. 3, top left). Circasemidian acrophases showed an average phase-delay of 165° (5.5 h) from − 155° (05:10 and 17:10) to − 320° (10:40 and 22:40) during ISS01, and were maintained at − 247° (08:14 and 20:14) during ISS02 (Fig. 3, bottom left). The 5-time amplified 12-h component may thus help restore internal synchrony to the 24-h clock. Presumably, circadian acrophases may have over-adjusted to 06:26 during ISS01 and were readjusted to 08:59 during ISS02 (Fig. 3, top left).
Figure 3.
Changes in 24-h and 12-h acrophases of HRV endpoints during two space missions. On the first mission (Mission 1, left), circadian acrophases advanced on average by about 8 h from pre-flight to ISS01 (from 14:37 to 06:26, P < 0.0001). Circadian acrophases were mostly restored during ISS01 and ISS02, when they averaged 08:59 (top left). Circasemidian acrophases were delayed on average by 165° (5.5 h) from − 155° (05:10 and 17:10) to − 320° (10:40 and 22:40) (P < 0.0001) between pre-flight and ISS01 (bottom left). On the second spaceflight (Mission 2, right), circadian acrophases showed no significant changes on average from Pre (05:15) to ISS01 (05:22) and ISS02 (04:24). Circasemidian acrophases were phase-advanced on average by164° (about 5.5 h) from − 216° (07:12 and 19:12) to − 52° (01:44 and 13:44) (P < 0.0001), returning to their original acrophase of − 244° (08:08 and 20:08) during ISS02 (bottom right). Acrophases of 12-h component expressed in (negative) degrees, with 360° ≡ 12 h, 0° = 00:00.
During the second mission, circadian acrophases, on average, showed no significant changes from pre-flight (05:15) to ISS01 (05:22) and ISS02 (04:24) (Fig. 3, top right). Circasemidian acrophases, by contrast, advanced on average by 164° (5.5 h) from − 216° (07:12 and 19:12) to − 52° (01:44 and 13:44) (P < 0.0001), returning to their original phase of − 244° (08:08 and 20:08) during ISS02 (Fig. 3, bottom right). As observed previously20, the 12-h component may help restore appropriate circadian timing in the presence of desynchrony. Mapping the circadian and circasemidian amplitude and phase characteristics could thus serve as important markers of adaptation to the space environment.
Discussion
Repeated HRV monitoring over 24 h of a healthy astronaut during two space missions, 4 years apart, served to illustrate methods to assess changes in brain plasticity and psychological resilience. Whereas conclusions cannot be derived from data from a single astronaut monitored for only 24 h at discrete times before, during and after a mission in space, the methodology used herein could be used in future studies to determine whether neural adaptation improves on repeated missions, as observed herein around day 20 after launch. During nighttime, sleep improved, and HRV activity co-varying with brain neural activity in the SN accelerated, while decelerating during daytime. HRV endpoints reflecting DMN activity showed no differences between the two space missions.
Stimulating environment and brain plasticity
Brain plasticity refers to the capacity of neurons and of neural circuits in the brain to change, structurally and functionally, in response to experience. This property is fundamental for the adaptability of behavior, for learning and memory processes, brain development, and brain repair. Exposure to stimulating environments has repeatedly been shown to strongly influence brain plasticity. Thus, it is a crucial underlying component of the enormous challenge of space adaptation for astronauts. Neural plasticity can take place at several levels, from synaptic plasticity at the (sub)cellular level to plasticity at the system and network levels44–46. Brain plasticity can be studied with a number of methods, such as electroencephalography (EEG)/evoked potentials (ERPs), structural and functional MRI and transcranial magnetic stimulation (TMS). In addition to functional brain response47–50 illustrated herein, recent work showed structural changes in the brain after long-duration space flights4–7 resulting from alterations in sleep performance or functional brain networks. Both aspects were estimated by HRV in a healthy astronaut who also took part in repeated space missions20,21,28.
Our observation of improved sleep agrees with results from a previous study28 where we assessed sleep quality based on sleep-related changes in RR-intervals and HRV-HF, which we found to be improved in space, and to be associated with increased parasympathetic activity, contrary to previous investigations51–54. Sleep quality was assessed as changes in sleep performance and HRV behavior in specific frequency regions for interpretation in terms of functional brain networks, as done in previous studies20,25,26,28,42. Previous investigations reported shorter sleep duration and inadequate sleep quality of astronauts during spaceflight aboard the ISS. These results were attributed to environmental factors, including exposure to microgravity, the 90-min light–dark cycle from the skylight weightlessness itself, excitement, and workload scheduled by operational demands53,54.
Effect of nighttime HRV changes on brain plasticity in space
Despite increased interest in the effect of spaceflight on the human central nervous system (CNS)15,55, not much is known thus far about the functional and morphological effects of microgravity on the human CNS. Previous studies have shown that CNS changes occur during and after spaceflight in the form of neuro-vestibular problems, alterations in cognitive function and sensory perception, problems with motor function, cephalic fluid shift, and psychological disturbances56,57. In the past few years, advances in structural and functional neuroimaging techniques have shown spaceflight-induced neuroplasticity in humans in several brain regions, including the insular cortex, the temporo-parietal junction, and the thalamus, in relation to short- and long-duration spaceflight1,2,14.
HRV indices that co-vary with SN activity are of particular interest since the SN is linked to the autonomic nervous system function and is sensitive to environmental challenges. The SN is mainly centered on the dorsal anterior cingulate, extending into the perigenual anterior cingulate cortex, and orbital fronto-insular cortices, but it also encompasses the limbic and brainstem areas. Relevance to HF-HRV is suggested by the inclusion of known autonomic nervous system control areas in the SN, and by this vagal marker’s putative role in switching between rest and activity and between internal and external focus of attention.
Further investigations are needed to examine whether acceleration of SN activity starts with nighttime sleep, as observed herein. It would suggest that brain plasticity may be initiated at night. The sensitivity of vagally-induced heart rate reactions to event salience might further suggest relationships between the SN and HF-HRV, as might the apparent overlap between nodes of the SN and areas related to autonomic control.
Identified as related to HF-HRV, the mPFC is important both as a node in the DMN and in the SN58. Anatomically, the mPFC is known to connect to pre-autonomic cell groups in the hypothalamus, periaqueductal gray, and brainstem59,60. If diffuse attention is a major aspect of the functionality of the DMN, then the overlapping membership of the mPFC in the two networks would provide an anatomical site for shifting from DMN activation to SN activation. Some evidence supports the view that DMN activation is switched to SN activation when an interoceptive or environmental stimulus is encoded as significant61.
Daytime HRV fluctuations associated with brain resilience in space
Because HRV may be associated with neural structures that are involved in the appraisal of threat and safety, HRV can be considered a potential marker of stress. HRV reflects the status of one’s ongoing adjustment to constantly changing environmental demands. Previously, under stressful environments, such as performing tasks during a spaceflight mission, HRV was found to be decreased17. Increased HF-HRV is considered to be associated with a positive mood, absence of negative affect, and an alert readiness to engage with the physical and social environment62,63.
Much recent research has found that psychological resilience is mediated by spontaneous brain activity measured with resting-state functional MRI. Although Waugh et al.64 found that when faced with a threat, participants had prolonged changed activity in the insula in response to aversive stimuli, psychological resilience is a complex construct that likely involves different brain functions. Other studies provided evidence that brain resilience is related not only to the insula, but also to the mPFC, OFC, PCC, ACC, and thalamus65–71. In the extant literature, the most consistent brain area related to psychological resilience is the ACC, perhaps because the ACC is associated with many important emotional functions, including motivation, emotion regulation, and attention or adaptation to a novel environment, such as space72–75. Previous investigations on resilience speculated that local activity in the ACC (such as fractional amplitude of low-frequency fluctuations measured by fMRI) would be negatively associated with psychological resilience23,75,76.
The bi-directional connections between heart and brain enunciated by Claude Bernard can be studied by analyzing HRV17,77. Over the past several years, many neuroimaging studies examined the association of HRV endpoints with fluctuations in brain functional connectivity18,19,32–35,59,60,78. They confirmed the existence of intimate connections between the different brain regions and HRV endpoints. They also posited that any changes in brain functional networks, which dynamically adjust the structure of their global and local network connectivity, should affect and change HRV activities in their respective frequency bands. “HRV is like a mirror reflecting the strength of activities of humans’ brain and mind”17,19,77.
Astronauts’ motivation aboard the ISS is also expected to reflect changed activities in the respective HRV frequency bands. Several investigations reported a relation between levels of psychological well-being and HRV35,79, which confirmed a statistically significant negative correlation between life satisfaction and HF-HRV activities35. Should our observation of decreased spectral power of HF-HRV, HF-component, and the series of the HF-band groups, and of lowered r-MSSD, pNN50 and Lorenz plot’s measures (Table 2, left and Fig. 2, left) be confirmed in future studies, it would suggest psychological resilience on repeated space missions.
Role of biological rhythms in the adaptation to the space environment
Whereas the circadian system plays a key role in the adaptation to a novel environment, such as microgravity in space19,20,25–28, ultradian components provided an evolutionary advantage for almost all life forms, from bacteria to humans80–84.
These ultradian rhythms can be expected to be important for the rapid adaptation to microgravity in space. The 12-h (circasemidian) component in particular may be involved85–90. It may reflect the function of two stress response pathways reacting to unfolded protein in the endogenous endoplasmic reticulum (ER) and mitochondria. A 12-h (circasemidian) component characterizes the ER- and mitochondria-associated “unfolded protein response (UPR) cycle”88–93. Several potential roles of the circasemidian clock in coordinating human health have been proposed, such as maintaining metabolic homeostasis87, coordinating sleep quality of slow wave sleep94,95, and mediating aging, especially in the prevention of aging-related metabolic decline87,88,96,97.
Based on our observations herein, the following hypothesis comes to mind. First, when faced with a new environment in space, the 12-h response appears faster and is larger than the circadian response (Table 3). Second, strong 12-h clock regulation might help repair circadian desynchrony (Table 4 and Fig. 3). The more severe internal desynchrony is (Table 4, Flight 1), the larger is the activation of the 12-h component (Table 3 and Fig. 3, Flight 1). Third, a milder circasemidian response during the second than during the first mission suggests that spaceflight-induced neuroplasticity may be present in the astronaut’s brain during the second mission.
Harmonic oscillations of 24 and 12 h likely provide evolutionarily adaptive advantages. The 12-h (circasemidian) component may contribute to consolidating a strong circadian system in space, and may contribute to a better adaptation in space by taking advantage of brain plasticity at night and psychological resilience during daytime.
Limitations
This investigation has several limitations. First, the study is limited to a single astronaut, and results were only compared between missions on a single day (ISS01). Factors other than adaptation to space environment (such as exercise, nutrition, mission tasks, and interpersonal stress) likely contributed in part to the results. As such, results herein do not provide inferential information about the effect of repeated missions of many days flown by a “population” of astronauts. Future studies should be designed to also estimate the uncertainty due to variation between astronauts and between mission days for each astronaut.
Despite the medium to large effect size of changes observed in this illustrative case, serial correlation, reduced by considering hourly averages instead of the original 5-min HRV endpoints, remains an issue preventing the derivation of generalizable inferences. As similar data from other astronauts become available, individual estimates can be used as imputations that no longer depend on the sampling interval.
In view of the importance of the circadian rhythm, there is merit in recording ECG around the clock. Demanding schedules and inconvenience of implementing the monitoring have been limiting factors to obtaining more data or data covering spans longer than 24 or 48 h. As technology advances, ECG monitors may become easier to use for longer spans, and as space exploration expands, more space travelers may participate in similar studies in the future.
Space adaptation of human neural cardiovascular coordination remains a challenge, as mechanisms are diverse and complex. Second, brain oscillatory activity data are lacking. Several studies, however, showed that HRV is associated with structures and functions of the neural network, and HRV is a biomarker reflecting activities of the brain integration system. These associations are extremely complex, however, and have not yet been fully confirmed. Future investigations are needed to directly assess the brain’s oscillatory activity in space. The methodology used in this investigation may help address these complex issues in future studies.
Conclusion
We examined the hypothesis proposed by Demertzi et al.14 that second-time flyers adapt more quickly and are less prone to microgravity-induced problems14,16,21. This demonstration is a simple illustration of methodology aimed to assess changes in brain plasticity and psychological resilience in a single astronaut, limited to comparing HRV endpoints between missions on a single day. Results nevertheless confirm earlier findings that sleep duration lengthened and sleep quality improved in space. The methodology used herein outlines how HRV behavior, which estimates the process of neural adaptation17,20,21,28, can serve to interpret changes in terms of brain functional networks. In the case examined herein, we find that brain plasticity during nighttime and psychological resilience during daytime may help with the adaptation to space’s environment. The 12-h component may have played a role in the adaptation process since it underwent larger changes than the 24-h component in response to the space environment, as assessed around day 20 on the ISS. HRV in the HF spectral region may be critical to assess microgravity-induced brain plasticity and psychological resilience, because HF-HRV reflects the adaptation process. Further studies are needed to examine how adaptation to the microgravity environment in space occurs. The role of functionally integrating the SN, consisting of neural centers (ACC, OFC, Amygdala and Insula), which involves and responds in a task-dependent manner to interceptive-autonomic and reward processes in a task-independent manner to emotional and homeostatic stimuli of personal salience23,35,71,72,75,98–100 may be particularly important, as our data suggest.
Acknowledgements
The authors thank I. Tayama, S. Ishida, N. Inoue, K. Murakami and S. Yamada from the Space Biomedical Research Group, Japan Aerospace Exploration Agency (JAXA), for cooperation in our study. The authors also acknowledge the cooperation of the astronauts, the engineers, staff and managers of JAXA and NASA. The help of Larry A. Beaty to improve the English language for greater clarity and readability is greatly appreciated. JAXA Chronobiology Project was supported by the Japan Aerospace Exploration Agency (K.O., K.M., T.A., S.F., H.O., C.M.) and Halberg Chronobiology Fund (G.C.).
Author contributions
K.O. and G.C. wrote the first draft of the manuscript and prepared the figures. K.O., H.O. and C.M. designed the study, and K.M., T.A., S.F. and H.O. contributed to the acquisition of data. K.O., G.C., Y.K. and K.S. analyzed the data, and K.O., G.C., Y.K., K.S., K.M., T.A., S.F., H.O. and C.M. contributed to the writing and editing of the manuscript. All authors read and contributed to the final version of the manuscript.
Data availability
Restrictions from Japan’s Aerospace Exploration Agency (JAXA) apply to the availability of the data supporting the findings of this study. The data were used under license for the current study. Although data are not publicly available, they are available to collaborating parties under ethical approval from JAXA.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roberts DR, et al. Effects of spaceflight on astronaut brain structure as indicated on MRI. N. Engl. J. Med. 2017;377:1746–1753. doi: 10.1056/NEJMoa1705129. [DOI] [PubMed] [Google Scholar]
- 2.Van Ombergen, A. et al. Intrinsic functional connectivity reduces after first-time exposure to short-term gravitational alterations induced by parabolic flight. Sci. Rep. 7, 3061. 10.1038/s41598-017-03170-5 (2017). [DOI] [PMC free article] [PubMed]
- 3.Hupfeld, K.E. et al. The impact of 6 and 12 months in space on human brain structure and intracranial fluid shifts. Cereb. Cortex Commun. 1, tgaa023. 10.1093/texcom/tgaa023 (2020). [DOI] [PMC free article] [PubMed]
- 4.Jillings, S. et al. Macro- and microstructural changes in cosmonauts' brains after long-duration spaceflight. Sci. Adv. 6, eaaz9488. 10.1126/sciadv.aaz9488 (2020). [DOI] [PMC free article] [PubMed]
- 5.Barisano, G. et al. The effect of prolonged spaceflight on cerebrospinal fluid and perivascular spaces of astronauts and cosmonauts. Proc. Natl. Acad. Sci. U S A. 119, e2120439119. 10.1073/pnas.2120439119 (2022). [DOI] [PMC free article] [PubMed]
- 6.Doroshin, A. et al. Brain connectometry changes in space travelers after long-duration spaceflight. Front. Neural. Circ. 16, 815838. 10.3389/fncir.2022.815838 (2022). [DOI] [PMC free article] [PubMed]
- 7.Hupfeld KE, et al. Longitudinal MRI-visible perivascular space (PVS) changes with long-duration spaceflight. Sci. Rep. 2022;12:7238. doi: 10.1038/s41598-022-11593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wostyn P, Mader TH, Gibson CR, Nedergaard M. Does long-duration exposure to microgravity lead to dysregulation of the brain and ocular glymphatic systems? Eye Brain. 2022;14:49–58. doi: 10.2147/EB.S354710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wostyn, P., Mader, T.H., Gibson, C.R. & Nedergaard, M. The effect of long-duration spaceflight on perivascular spaces within the brain. Proc. Natl. Acad. Sci. USA. 119, e2207724119. 10.1073/pnas.2207724119 (2022). [DOI] [PMC free article] [PubMed]
- 10.Pavez Loriè, E. et al. The future of personalized medicine in space: from observations to countermeasures. Front. Bioeng. Biotechnol. 9, 739747. 10.3389/fbioe.2021.739747 (2021). [DOI] [PMC free article] [PubMed]
- 11.Scott JM, Stoudemire J, Dolan L, Downs M. Leveraging spaceflight to advance cardiovascular research on earth. Circ. Res. 2022;130:942–957. doi: 10.1161/CIRCRESAHA.121.319843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidler, R.D., Stern, C., Basner, M., Stahn, A.C., Wuyts, F.L. & Zu Eulenburg, P. Future research directions to identify risks and mitigation strategies for neurostructural, ocular, and behavioral changes induced by human spaceflight: A NASA-ESA expert group consensus report. Front. Neural. Circ. 16, 876789. 10.3389/fncir.2022.876789 (2022). [DOI] [PMC free article] [PubMed]
- 13.Ross MD. A spaceflight study of synaptic plasticity in adult rat vestibular maculas. Acta. Otolaryngol. 1994;516:1–14. [PubMed] [Google Scholar]
- 14.Demertzi, A., et al. Cortical reorganization in an astronaut's brain after long-duration spaceflight. Brain Struct. Funct.221, 2873–2876. Erratum in: Brain Struct. Funct. 221, 2877 (2016). [DOI] [PMC free article] [PubMed]
- 15.Clément G, Reschke M, Wood S. Neurovestibular and sensorimotor studies in space and Earth benefits. Curr. Pharm. Biotechnol. 2005;6:267–283. doi: 10.2174/1389201054553716. [DOI] [PubMed] [Google Scholar]
- 16.Pechenkova E, et al. Alterations of functional brain connectivity after long-duration spaceflight as revealed by fMRI. Front. Physiol. 2019;10:761. doi: 10.3389/fphys.2019.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Chang C, et al. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otsuka K, et al. Circadian challenge of astronauts’ unconscious mind adapting to microgravity in space, estimated by heart rate variability. Sci. Rep. 2018;8:10381. doi: 10.1038/s41598-018-28740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuka K, et al. Unconscious mind activates central cardiovascular network and promotes adaptation to microgravity possibly anti-aging during 1-year-long spaceflight. Sci. Rep. 2022;12:11862. doi: 10.1038/s41598-022-14858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rusanov, V.B., Fomina, E.V. & Orlov, O.I. Does heart rate variability reflect brain plasticity as a likely mechanism of adaptation to space mission? Front. Space Technol.3, 998610. 10.3389/frspt.2022.998610 (2022).
- 22.Baevsky RM, Chernikova AG, Funtova II, Tank J. Assessment of individual adaptation to microgravity during long term& space flight based on stepwise discriminant analysis of heart rate variability parameters. Acta Astronaut. 2011;69:1148–1152. doi: 10.1016/j.actaastro.2011.07.011. [DOI] [Google Scholar]
- 23.Kong F, et al. Neural correlates of psychological resilience and their relation to life satisfaction in a sample of healthy young adults. Neuroimage. 2015;123:165–172. doi: 10.1016/j.neuroimage.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Kong F, et al. The resilient brain: psychological resilience mediates the effect of amplitude of low-frequency fluctuations in orbitofrontal cortex on subjective well-being in young healthy adults. Soc. Cogn. Affect. Neurosci. 2018;13:755–763. doi: 10.1093/scan/nsy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuka, K. et al. Intrinsic cardiovascular autonomic regulatory system of astronauts exposed long-term to microgravity in space: Observational study. NPJ Microgravity. 1, 15018. 10.1038/npjmgrav.2016.31 (2015). Erratum in: NPJ Microgravity.8, 12 (2016). [DOI] [PMC free article] [PubMed]
- 26.Otsuka, K., et al. Long-term exposure to space’s microgravity alters the time structure of heart rate variability of astronauts. Heliyon. 2, e00211. 10.1016/j.heliyon.2016.e00211 (2016). [DOI] [PMC free article] [PubMed]
- 27.Otsuka, K. et al. Anti-aging effects of long-term space missions, estimated by heart rate variability. Sci. Rep. 9, 8995. 10.1038/s41598-019-45387-6 (2019) [DOI] [PMC free article] [PubMed]
- 28.Otsuka, K. et al. Astronauts well-being and possibly anti-aging improved during long-duration spaceflight. Sci. Rep. 11, 14907. 10.1038/s41598-021-94478-w (2021). [DOI] [PMC free article] [PubMed]
- 29.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 93, 1043–1065 (1996). [PubMed]
- 30.Saito, K., Koyama, A., Yoneyama, K., Sawada, Y., & Ohtomo, N. A recent advances in time series analysis by maximum entropy method. Hokkaido University Press (Sapporo) (1994).
- 31.Allen B, Jennings JR, Gianaros PJ, Thayer JF, Manuck SB. Resting high-frequency heart rate variability is related to resting brain perfusion. Psychophysiology. 2015;52:277–287. doi: 10.1111/psyp.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jennings JR, Sheu LK, Kuan DC, Manuck SB, Gianaros PJ. Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high-frequency heart rate variability. Psychophysiology. 2016;53:444–454. doi: 10.1111/psyp.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolin S, Boonstra TW, Loo CK, Martin D. Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PLoS ONE. 2017;12:e0181833. doi: 10.1371/journal.pone.0181833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkelmann T, et al. Structural brain correlates of heart rate variability in a healthy young adult population. Brain Struct. Funct. 2017;222:1061–1068. doi: 10.1007/s00429-016-1185-1. [DOI] [PubMed] [Google Scholar]
- 35.Yoshino, K. et al. Relationship between life satisfaction and sympathovagal balance in healthy elderly males at home at night. Health. 4, 1068–1072. 10.4236/health.2012.411163 (2012)
- 36.Luo Y, et al. Resting-state functional connectivity of the default mode network associated with happiness. Soc. Cogn. Affect. Neurosci. 2016;11:516–524. doi: 10.1093/scan/nsv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J. Neurosci. 2011;31:7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen, J.E. & Glover, G.H. BOLD fractional contribution to resting-state functional connectivity above 0.1 Hz. Neuroimage. 107, 207–218 (2015). [DOI] [PMC free article] [PubMed]
- 39.Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 40.Cornelissen G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014;11:16. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otsuka K, Cornelissen G, Halberg F. Chronomics and Continuous Ambulatory Blood Pressure Monitoring—Vascular Chronomics: From 7-Day/24-Hour to Lifelong Monitoring 870 + lxxv. Springer; 2016. [Google Scholar]
- 42.Otsuka K, Ozawa T, Shimada K. New simple method for the analysis of sleep states employing the Holter monitoring system. Auton. Nerv. Syst. 1985;22:252–260. [Google Scholar]
- 43.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slenzka K. Neuroplasticity changes during space flight. Adv Space Res. 2003;31:1595–1604. doi: 10.1016/S0273-1177(03)00011-5. [DOI] [PubMed] [Google Scholar]
- 45.Pearson-Fuhrhop KM, Cramer SC. Genetic influences on neural plasticity. PM R. 2010;2(12 Suppl 2):S227–S240. doi: 10.1016/j.pmrj.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Roy-O’Reilly, M., Mulavara, A. & Williams, T. A review of alterations to the brain during spaceflight and the potential relevance to crew in long-duration space exploration. NPJ Microgravity7, 5. 10.1038/s41526-021-00133-z (2021). [DOI] [PMC free article] [PubMed]
- 47.Takeuchi H, et al. Neural plasticity in amplitude of low frequency fluctuation, cortical hub construction, regional homogeneity resulting from working memory training. Sci. Rep. 2017;7:1470. doi: 10.1038/s41598-017-01460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhat DI, Indira Devi B, Bharti K, Panda R. Cortical plasticity after brachial plexus injury and repair: A resting-state functional MRI study. Neurosurg. Focus. 2017;42:E14. doi: 10.3171/2016.12.FOCUS16430. [DOI] [PubMed] [Google Scholar]
- 49.Xing XX, et al. Brain plasticity after peripheral nerve injury treatment with massage therapy based on resting-state functional magnetic resonance imaging. Neural Regen. Res. 2021;16:388–393. doi: 10.4103/1673-5374.290912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi WQ, et al. Altered spontaneous brain activity patterns in patients with diabetic retinopathy using amplitude of low-frequency fluctuation. World J. Diabetes. 2022;13:97–109. doi: 10.4239/wjd.v13.i2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baevsky RM, et al. Autonomic cardiovascular and respiratory control during prolonged spaceflights aboard the International Space Station. J. Appl. Physiol. 2007;1985(103):156–161. doi: 10.1152/japplphysiol.00137.2007. [DOI] [PubMed] [Google Scholar]
- 52.Baevsky RM, et al. Autonomic regulation of circulation and cardiac contractility during a 14-month space flight. Acta. Astronaut. 1998;42:159–173. doi: 10.1016/S0094-5765(98)00114-3. [DOI] [PubMed] [Google Scholar]
- 53.Dijk DJ, et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1647–1664. doi: 10.1152/ajpregu.2001.281.5.R1647. [DOI] [PubMed] [Google Scholar]
- 54.Flynn-Evans, E.E., Barger, L.K., Kubey, A.A., Sullivan, J.P. & Czeisler, C.A. Circadian misalignment affects sleep and medication use before and during spaceflight. NPJ. Microgravity. 2, 15019. 10.1038/npjmgrav.2015.19 (2016). [DOI] [PMC free article] [PubMed]
- 55.Clément G, Ngo-Anh JT. Space physiology II: adaptation of the central nervous system to space flight–past, current, and future studies. Eur. J. Appl. Physiol. 2013;113:1655–1672. doi: 10.1007/s00421-012-2509-3. [DOI] [PubMed] [Google Scholar]
- 56.Manzey D. Human missions to Mars: New psychological challenges and research issues. Acta. Astronaut. 2004;55:781–790. doi: 10.1016/j.actaastro.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 57.De la Torre GG. Cognitive neuroscience in space. Life (Basel). 2014;4:281–294. doi: 10.3390/life4030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakaki M, et al. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage. 2016;139:44–52. doi: 10.1016/j.neuroimage.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei L, Chen H, Wu G-R. Structural covariance of the prefrontal-amygdala pathways associated with heart rate variability. Front. Hum. Neurosci. 2018;12:2. doi: 10.3389/fnhum.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 63.Porges SW. The polyvagal perspective. Biol. Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Soc. Cogn. Affect. Neurosci. 2008;3:322–332. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: A critical review. Prog. Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 66.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lloyd TJ, Hastings R. Hope as a psychological resilience factor in mothers and fathers of children with intellectual disabilities. J. Intellect. Disabil. Res. 2009;53:957–968. doi: 10.1111/j.1365-2788.2009.01206.x. [DOI] [PubMed] [Google Scholar]
- 68.Reynaud E, et al. Relationship between emotional experience and resilience: An fMRI study in fire-fighters. Neuropsychologia. 2013;51:845–849. doi: 10.1016/j.neuropsychologia.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Sekiguchi A, et al. Resilience after 3/11: Structural brain changes 1 year after the Japanese earthquake. Mol. Psychiatry. 2015;20:553–554. doi: 10.1038/mp.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Satici SA. Psychological vulnerability, resilience, and subjective well-being: The mediating role of hope. Pers. Individ. Differ. 2016;102:68–73. doi: 10.1016/j.paid.2016.06.057. [DOI] [Google Scholar]
- 71.Kong F, et al. Amplitude of low-frequency fluctuations during resting state differentially predicts authentic and hubristic pride. J. Pers. 2018;86:213–219. doi: 10.1111/jopy.12306. [DOI] [PubMed] [Google Scholar]
- 72.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 73.Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 74.Quoidbach J, Berry EV, Hansenne M, Mikolajczak M. Positive emotion regulation and well-being: Comparing the impact of eight savoring and dampening strategies. Pers. Individ. Differ. 2010;49:368–373. doi: 10.1016/j.paid.2010.03.048. [DOI] [Google Scholar]
- 75.King ML. The neural correlates of well-being: A systematic review of the human neuroimaging and neuropsychological literature. Cogn. Affect. Behav. Neurosci. 2019;19:779–796. doi: 10.3758/s13415-019-00720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S, et al. Hope and the brain: Trait hope mediates the protective role of medial orbitofrontal cortex spontaneous activity against anxiety. Neuroimage. 2017;157:439–447. doi: 10.1016/j.neuroimage.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 77.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Mulcahy, J.S., Larsson, D.E.O., Garfinkel, S.N. & Critchley, H.D. Heart rate variability as a biomarker in health and affective disorders: A perspective on neuroimaging studies. Neuroimage. 202, 116072. 10.1016/j.neuroimage.2019.116072 (2019). [DOI] [PubMed]
- 79.Shiga, K. et al. Subjective well-being and month-long LF/HF ratio among deskworkers. PLoS ONE16, e0257062. 10.1371/journal.pone.0257062 (2021). [DOI] [PMC free article] [PubMed]
- 80.Yates, F.E. & Yates, L.B. Ultradian rhytyhms as the dynamic signature of life. (ed. Lloyd, D. & Rossi, E.L.) Ultradian Rhythms from Molecules to Mind. 249–260 (Springer; London, 2008).
- 81.Hughes, M.E. et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 5, e1000442. 10.1371/journal.pgen.1000442 (2009). [DOI] [PMC free article] [PubMed]
- 82.Heijde M, et al. Characterization of two members of the cryptochrome/photolyase family from Ostreococcus tauri provides insights into the origin and evolution of cryptochromes. Plant Cell Environ. 2010;33:1614–1626. doi: 10.1111/j.1365-3040.2010.02168.x. [DOI] [PubMed] [Google Scholar]
- 83.Hancock, A.M. et al. Adaptations to climate-mediated selective pressures in humans. PLoS Genet. 7, e1001375. 10.1371/journal.pgen.1001375 (2011). [DOI] [PMC free article] [PubMed]
- 84.Lopez, L., Fasano, C., Perrella, G. & Facella, P. Cryptochromes and the circadian clock: The story of a very complex relationship in a spinning world. Genes (Basel). 12, 672. 10.3390/genes12050672 (2021). [DOI] [PMC free article] [PubMed]
- 85.Hughes, M. E. et al. Brain-specific rescue of Clock reveals system-driven transcriptional rhythms in peripheral tissue. PLoS. Genet. 8, e1002835. 10.1371/journal.pgen.1002835 (2012). [DOI] [PMC free article] [PubMed]
- 86.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 87.Zhu B, et al. A cell-autonomous mammalian 12 hr clock coordinates metabolic and stress rhythms. Cell Metab. 2017;25:1305–1319.e9. doi: 10.1016/j.cmet.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu B, Dacso CC, O’Malley BW. Unveiling “musica universalis” of the cell: A brief history of biological 12-hour rhythms. J. Endocr. Soc. 2018;2:727–752. doi: 10.1210/js.2018-00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan, Y. et al. 12-h clock regulation of genetic information flow by XBP1s. PLoS Biol. 18, e3000580. 10.1371/journal.pbio.3000580 (2020). [DOI] [PMC free article] [PubMed]
- 90.Balance H, Zhu B. Revealing the hidden reality of the mammalian 12-h ultradian rhythms. Cell Mol. Life Sci. 2021;78:3127–3140. doi: 10.1007/s00018-020-03730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jovaisaite V, Auwerx J. The mitochondrial unfolded protein response—synchronizing genomes. Curr. Opin. Cell Biol. 2015;33:74–81. doi: 10.1016/j.ceb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qureshi MA, Haynes CM, Pellegrino MW. The mitochondrial unfolded protein response: Signaling from the powerhouse. J. Biol. Chem. 2017;292:13500–13506. doi: 10.1074/jbc.R117.791061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopez-Crisosto C, et al. Endoplasmic reticulum-mitochondria coupling increases during doxycycline-induced mitochondrial stress in HeLa cells. Cell Death Dis. 2021;12:657. doi: 10.1038/s41419-021-03945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Koninck GC, Hébert M, Carrier J, Lamarche C, Dufour S. Body temperature and the return of slow wave activity in extended sleep. Electroencephalogr. Clin. Neurophysiol. 1996;98:42–50. doi: 10.1016/0013-4694(95)00215-4. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi M, Morikawa T, Hori T. Circasemidian 12 h cycle of slow wave sleep under constant darkness. Clin. Neurophysiol. 2002;113:1505–1516. doi: 10.1016/S1388-2457(02)00168-2. [DOI] [PubMed] [Google Scholar]
- 96.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 98.Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain A review and meta-analysis. Neurophysiol. Clin. 2000;30:263–288. doi: 10.1016/S0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 99.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 100.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions from Japan’s Aerospace Exploration Agency (JAXA) apply to the availability of the data supporting the findings of this study. The data were used under license for the current study. Although data are not publicly available, they are available to collaborating parties under ethical approval from JAXA.