Abstract
Background
Using data collected in the prospective observational study CIRSE Registry for SIR-Spheres Therapy, the present study aimed at identifying predictors of adverse events (AEs) following transarterial radioembolization (TARE) with Yttrium-90 resin microspheres for liver tumours.
Methods
We analysed 1027 patients enrolled between January 2015 and December 2017 and followed up for 24 months. Four hundred and twenty-two patients with hepatocellular carcinoma (HCC), 120 with intrahepatic carcinoma (ICC), 237 with colorectal liver metastases and 248 with liver metastases from other primaries were included. Prognostic factors were calculated with a univariable analysis by using the overall AEs burden score (AEBS).
Results
All-cause AEs were reported in 401/1027 (39.1%) patients, with AEs associated with TARE, such as abdominal pain (16.6%), fatigue (17%), and nausea (11.7%) reported most frequently. Grade 3 or higher AEs were reported in 92/1027 (9%) patients. Reports on grade ≥ 3 gastrointestinal ulcerations (0.4%), gastritis (0.3%), radiation cholecystitis (0.2%) or radioembolization-induced liver disease (0.5%) were uncommon. Univariable analysis showed that in HCC, AEBS increased for Eastern Cooperative Oncology Group (ECOG) 0 (p = 0.0045), 1 tumour nodule (0.0081), > 1 TARE treatment (p = 0.0224), no prophylactic embolization (p = 0.0211), partition model dosimetry (p = 0.0007) and unilobar treatment target (0.0032). For ICC, > 1 TARE treatment was associated with an increase in AEBS (p = 0.0224), and for colorectal liver metastases, ECOG 0 (p = 0.0188), > 2 prior systemic treatments (p = 0.0127), and 1 tumour nodule (p = 0.0155) were associated with an increased AEBS.
Conclusion
Our study confirms that TARE is a safe treatment with low toxicity and a minimal impact on quality of life.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00270-023-03391-4.
Introduction
Transarterial radioembolization (TARE) is a treatment modality for cancer patients with liver-dominant disease not suitable for surgical or ablative therapies, or who experienced no response, significant side effects or intolerance when treated with systemic therapies [1–9]. Depending on the origin of the tumour—be it primary such as hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC) or metastatic liver disease—TARE is included as a palliative treatment option in several treatment guidelines, due to its favourable toxicity profile and ability to facilitate local tumour control [1–10].
Previous reports on the safety and toxicity after TARE follow a trend of focusing on grade ≥ 3 adverse events (AEs) [11–15]. While insightful, those reports ignore the more commonly occurring grade 1 and 2 AEs. Despite their mildness, frequently occurring grade 1 and 2 AEs can put a burden on patients’ health and should, therefore, be included in the safety evaluation of a treatment. To that end, La-Rademacher et al. (2020) developed a single measure that reflects the overall AE burden by including all AEs graded and reported during a trial into a single numeric value [16]. This adverse event burden score (AEBS) allows for inter- and intra-study comparisons between groups and treatments and can be applied to find risk factors for adverse events or associations between adverse events and other numeric factors, such as quality-of-life data.
Using the data from the prospective real-world observational study, the CIRSE Registry for SIR-Spheres Therapy (CIRT), we aimed to provide a comprehensive overview of adverse events observed after TARE and to find factors in the patient baseline data and treatment modalities that can predict an increased AEBS or reduced global health score (GHS). This evaluation was performed for the whole cohort, with an emphasis on HCC, ICC and colorectal liver metastases (mCRC).
Methods
Study Design
The prospective multicentre observational study CIRT (NCT02305459) was conducted by the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) to evaluate the real-world clinical application and outcomes of TARE with Y90 resin microspheres (SIR-Spheres® Y-90 resin microspheres, Sirtex Medical Pty Limited; St. Leonards, NSW, Australia). Sites were invited to participate if they performed at least 40 TARE cases in total and ten cases in the twelve months prior to invitation. The 27 participating sites were identified and enrolled between April 2014 and April 2017.
The CIRT methodology was previously published [17]. Data were collected using a customised electronic data capturing system and electronic case report form that was developed by ConexSys Inc (Lincoln, RI, USA) and hosted on a local secure server in Vienna, Austria, maintained by ITEA (Vienna, Austria). Statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC, USA) and RStudio under R4.0.0 (R Foundation, Vienna, Austria).
Patient Selection and Data Collection
Patients included in the analysis were adults scheduled to receive TARE with Y90 resin microspheres for primary or metastatic liver cancer. There were no specific inclusion or exclusion criteria. The indication for TARE, the treatment design, the methods used for dose calculation and the follow-up regime were made according to the centres’ internal standards. All included patients signed an informed consent form. This research project was performed in accordance with the ethical standards of the applicable institutional and/or national ethics committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
At the time of the first treatment, baseline data, demographics and treatment-related data were collected. Information concerning safety, toxicities and quality of life were gathered at every follow-up up to 24 months. Safety outcomes are described as severe day-of-treatment complications and occurrences of any adverse events after treatment, according to the Common Terminology Criteria for Adverse Events, version 4.03. Previous studies have associated adverse events such as abdominal pain, fatigue, fever, nausea, vomiting, gastrointestinal ulceration, gastritis, radiation cholecystitis, radiation pancreatitis, radiation pneumonitis and radioembolization-induced liver disease (REILD) with the application of TARE and were, thus, specifically included in the case report form [18–20]. An open text field allowed for collecting details on occurrences of other adverse events. Quality-of-life data were collected on a voluntary basis for the patient using the questionnaire QLQ-C30 from the European Organisation for the Research and Treatment of Cancer (EORTC). The questionnaire was presented before the TARE treatment and at every follow-up. Remote monitoring was performed, but no onsite monitoring or source document verification was done.
Definitions and Outcome Measures
To describe and compare safety data, the proportion of patients with at least 1 AE, as well as the AE burden score (AEBS), were used. To summarise, the AEBS represents the weighted sum of all AEs and their respective weighted grades [16]. Since all AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), which reflects comparable severity between AE types, the weight of an AE type was defined as the CTCAE grade. Therefore, the AEBS was calculated by taking the sum of all AE grades per patient (see Supplement 1 for an extended explanation of the AEBS, including the weight of grade 5 AEs, and AEs excluded from the AEBS calculation).
Deterioration of quality-of-life (QOL) data was evaluated using the EORTC Scoring Manual 3.0 [21]. A 10-point change in the score from baseline (first TARE treatment) was considered to be clinically relevant. If there was a 10-point decrease in the function domain or a 10-point increase in the symptom score from baseline, the patient’s QOL in that domain or for that symptom was considered to be deteriorated. This evaluation could be done only for patients who completed the QOL questionnaire at baseline and during, at least, one follow-up visit. Here, we report only on GHS.
Statistics
Data are presented as mean ± standard deviation or median (interquartile range [IQR]) for continuous variables and number (%) for categorical variables. Adverse events are reported as occurrences per patient. When reported per time interval, adverse events are reported per occurrence within the time intervals < 1 month, 1–4 months, and > 4 months. Data are presented for the whole cohort and cancer types with > 100 patients (HCC, ICC and mCRC).
Univariable analyses were performed with the Wilcoxon rank-sum test if the number of groups was 2, or with the Kruskal–Wallis test (nonparametric alternative to the ANOVA) if the number of groups exceeded 2, due to the non-normal distribution of the AEBS data. The statistical significance level was set to p < 0.05 (two-sided). Due to the large number of patients for which no AE was reported (AEBS = 0), it was not possible to perform a multivariable analysis.
Results
We analysed 1197 TARE treatments from 1027 patients included in the CIRT study. Data on baseline and treatment application have been previously reported, which also included, as supplementary information, AE data outlined in Table 1 [22]. Safety data in patients with mCRC and HCC have been previously reported as supplementary information [23, 24].
Table 1.
Safety outcomes after TARE
| Variables | Categories | HCC | ICC | mCRC | Other mets | All |
|---|---|---|---|---|---|---|
| Length of hospital stay | n | 496 (100) | 144 (100) | 264 (100) | 293 (100) | 1197 (100) |
| < 24 h | 116 (23.4) | 27 (18.8) | 57 (21.6) | 85 (29.0) | 285 (23.8) | |
| 24 to < 36 h | 64 (12.9) | 12 (8.3) | 41 (15.5) | 37 (12.6) | 154 (12.9) | |
| 36 to < 48 h | 52 (10.5) | 12 (8.3) | 39 (14.8) | 44 (15.0) | 147 (12.3) | |
| 48 to < 72 h | 221 (44.6) | 74 (51.4) | 79 (29.9) | 91 (31.0) | 465 (38.8) | |
| ≥ 72 h | 43 (8.7) | 19 (13.2) | 48 (18.2) | 36 (12.3) | 146 (12.2) | |
| Deceased | n | 422 (100) | 120 (100) | 237 (100) | 248 (100) | 1027 (100) |
| Within 30 days* | 3 (0.7) | 1 (0.8) | 4 (1.7) | 2 (0.8) | 10 (1.0) | |
| Severe periprocedural complications | n | 11 (2.2) | 6 (4.2) | 9 (3.4) | 19 (6.5) | 44 (3.7) |
| Abdominal pain | 3 (0.6) | 3 (2.1) | 8 (3.0) | 12 (4.8) | 26 (2.3) | |
| Vomiting | 2 (0.4) | 1 (0.7) | 3 (1.1) | 2 (0.7) | 8 (0.7) | |
| Other | 6 (1.2) | 2 (1.4) | 1 (0.4) | 3 (1) | 10 (0.8) | |
| Patients with at least one adverse event | 155 (36.7) | 49 (40.8) | 96 (40.5) | 102 (41.1) | 402 (39.1) | |
| Patients with at least one adverse events (all) | Abdominal Pain | 60 (14.2) | 25 (20.8) | 34 (14.3) | 51 (20.5) | 170 (16.6) |
| Fatigue | 65 (15.4) | 23 (19.2) | 33 (13.9) | 54 (21.7) | 175 (17.0) | |
| Fever | 25 (5.9) | 7 (5.8) | 14 (5.9) | 9 (3.6) | 55 (5.4) | |
| Nausea | 37 (8.8) | 14 (11.7) | 28 (11.8) | 41 (16.5) | 120 (11.7) | |
| Vomiting | 22 (5.2) | 9 (7.5) | 15 (6.3) | 20 (8.1) | 66 (6.4) | |
| Gastrointestinal Ulceration | 3 (0.7) | 5 (4.2) | 3 (1.3) | 2 (0.8) | 13 (1.3) | |
| Gastritis | 3 (0.7) | 2 (1.7) | 6 (2.5) | 3 (1.2) | 14 (1.4) | |
| Radiation Cholecystitis | 0 (0.0) | 1 (0.8) | 1 (0.4) | 2 (0.8) | 4 (0.4) | |
| Radiation Pancreatitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.1) | |
| Radiation pneumonitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Radioembolization-Induced Liver Disease | 6 (1.4) | 3 (2.5) | 1 (0.4) | 3 (1.2) | 13 (1.3) | |
| Other | 90 (21.3) | 25 (20.8) | 63 (26.6) | 48 (22.6) | 234 (22.8) | |
| Patients with at least one grade ≥ 3 adverse event | 30 (7.1) | 15 (12.5) | 25 (10.5) | 22 (8.9) | 92 (9.0) | |
| Patients with at least one ≥ grade 3 adverse events | Abdominal Pain | 9 (2.1) | 4 (3.3) | 4 (1.7) | 8 (3.2) | 25 (2.4) |
| Fatigue | 6 (1.4) | 2 (1.7) | 0 (0.0) | 6 (2.4) | 14 (1.4) | |
| Fever | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) | |
| Nausea | 3 (0.7) | 0 (0.0) | 1 (0.4) | 1 (0.4) | 5 (0.5) | |
| Vomiting | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) | |
| Gastrointestinal Ulceration | 1 (0.2) | 1 (0.8) | 2 (0.8) | 0 (0.0) | 4 (0.4) | |
| Gastritis | 0 (0.0) | 1 (0.8) | 2 (0.8) | 0 (0.0) | 3 (0.3) | |
| Radiation Cholecystitis | 0 (0.0) | 1 (0.8) | 1 (0.4) | 0 (0.0) | 2 (0.2) | |
| Radiation Pancreatitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Radiation pneumonitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Radioembolization-Induced Liver Disease | 3 (0.7)** | 2 (1.7) | 0 (0.0) | 0 (0.0) | 5 (0.5) | |
| Other | 15 (3.6) | 8 (6.7) | 18 (7.6) | 10 (4.0) | 51 (5.0) | |
Data are presented as n (%)
ICC intrahepatic cholangiocarcinoma, HCC hepatocellular carcinoma, mCRC metastatic colorectal cancer, TARE transarterial radioembolization
The study included 1027 patients with a total of 1197 treatments
*7/10 patients died from intra-hepatic or extra-hepatic disease progression; 1 patient died from pleural effusion and ascites; for 2 patients, the cause of death is unrelated to the treatment or the disease
**2/3 (66.7%) cases of Radioembolization-Induced Liver Disease in HCC patients were grade 5
Safety
Across indications, the number of patients who experienced at least one severe periprocedural complication after a treatment was low (44/1197, 3.7%), primarily abdominal pain (26/1197, 2.3%, Table 1). All-cause AEs were reported in 402/1027 (39.1%) patients, and grade 3 or higher AEs were reported in 92/1027 (9%) patients. Patients with other grade ≥ 3 AEs (51/1027, 5.0%) are reported in Table 2. Within 1 month after treatment (Table 3), the largest groups of reported AEs were abdominal pain (126/909, 13.9%), fatigue (104/909, 11.4%) and nausea (69/909, 7.6%). Between 1 and 4 months, occurrences of these AEs decreased, while cases of radiation cholecystitis (3/726, 0.4%), radiation pancreatitis (1/726, 0.1%), REILD (10/726, 1.4%) and other AEs (62/726, 8.5%%) increased. AEs in the category “Other” occurred mostly after 4 months (156/639, 24.4%). Supplement 2 provides the occurrences of AEs per time interval for HCC, ICC and mCRC. The thirty-day mortality rate was 10/1027 (1.0%, Table 1). Abnormal laboratory values (Table 4) were reported in 792/1027 (77.1%) patients, with 245/1027 (23.9%) patients showing at least 1 value with grade 3 or higher.
Table 2.
Other serious adverse events
| Category* | Adverse event | Grade 3 (n = 72) | Grade 4 (n = 10) | Grade 5 (n = 3) | Total (n = 84) |
|---|---|---|---|---|---|
| Analytical | Anaemia | 3 | 3 | ||
| Thrombocytopenia | 1 | 1 | |||
| Bleeding | Oesophagus | 4 | 1 | 4 | |
| Gastrointestinal | 1 | 2 | 1 | 4 | |
| Haemorrhage at ileostomy | 1 | 1 | |||
| Fundal variceal haemorrhage | 1 | 1 | |||
| Bleeding from gastric ulcer | 1 | 1 | |||
| Left liver lobe | 1 | 1 | |||
| Rectum | 1 | 1 | |||
| Cardio-pulmonary | Heart failure | 1 | 1 | ||
| Respiratory failure | 1 | 1 | |||
| Pleural effusion | 1 | 1 | 2 | ||
| Circulatory insufficiency | 1 | 1 | |||
| Central embolism of the pulmonal artery | 1 | 1 | |||
| Dyspnoea | 1 | 1 | |||
| Digestive | Sub-occlusion | 1 | 1 | 2 | |
| Diarrhoea | 3 | 1 | 4 | ||
| Mouth ulceration | 1 | 1 | |||
| General | Depression | 2 | 2 | ||
| Somnolence | 4 | 4 | |||
| Anorexia | 3 | 3 | |||
| Weight loss | 1 | 1 | |||
| Sleep disorder | 1 | 1 | |||
| Infectious | Cholangio-sepsis | 1 | 1 | ||
| Streptococci infection | 1 | 1 | |||
| Sepsis for staphylococcus aureus with endocarditis | 1 | 1 | |||
| Bilateral lower limb gangrene | 1 | 1 | |||
| Infection of necrotic tumour areas in liver | 1 | 1 | |||
| Infection with E. coli | 1 | 1 | |||
| Infected biloma | 1 | 1 | |||
| Spontaneous bacterial peritonitis | 1 | 1 | |||
| Liver and portal system | Cholangitis | 2 | 1 | 3 | |
| Ascites | 7 | 1 | 8 | ||
| Jaundice | 3 | 1 | 4 | ||
| Cholestasis | 1 | 1 | |||
| Intrahepatic thrombosis of the portal vein | 1 | 1 | |||
| Infarction of ventral part of the spleen | 1 | 1 | |||
| Hepatic encephalopathy | 2 | 2 | |||
| Biloma | 1 | 1 | |||
| Deterioration of the liver function | 1 | 1 | |||
| Neurological, pain, and other sensitive disorders | Hand-foot syndrome | 3 | 3 | ||
| Bone pain | 2 | 2 | |||
| Epileptic seizure | 1 | 1 | |||
| Back pain | 1 | 1 | |||
| Renal and fluid balance | Acute renal failure | 2 | 2 | ||
| Other | Wound rupture after liver surgery | 1 | 1 | ||
| Teeth destroyed | 1 | 1 | |||
| Rash of the skin due to antibiotics | 1 | 1 |
*51 patients experienced 84 severe adverse events in the “other” category
Table 3.
Patients with at least 1 adverse event per time interval: < 1 month, 1–4 months, > 4 months
| < 1 month | 1–4 months | > 4 months | |
|---|---|---|---|
| Patients with at least 1 follow-up per time interval | 909 | 726 | 639 |
| Adverse events (all grades, n) | 392 | 302 | 472 |
| Abdominal pain | 126 (13.9) | 65 (9) | 94 (14.7) |
| Fatigue | 104 (11.4) | 75 (10.3) | 128 (20) |
| Fever | 11 (1.2) | 19 (2.6) | 20 (3.1) |
| Nausea | 69 (7.6) | 44 (6.1) | 50 (7.8) |
| Vomiting | 48 (5.4) | 13 (1.8) | 16 (2.5) |
| Gastrointestinal ulceration | 3 (0.3) | 6 (0.8) | 5 (0.8) |
| Gastritis | 8 (0.9) | 5 (0.7) | 1 (0.2) |
| Radiation cholecystitis | 0 (0) | 3 (0.4) | 1 (0.2) |
| Radiation pancreatitis | 0 (0) | 1 (0.1) | 0 (0) |
| Radiation pneumonitis | 0 (0) | 0 (0) | 0 (0) |
| Radioembolization-induced liver disease | 2 (0.2) | 10 (1.4) | 1 (0.2) |
| Other | 20 (2.2) | 62 (8.5) | 156 (24.4) |
| Adverse events (grade 3–5, n) | 52 | 40 | 67 |
| Abdominal pain | 36 (4) | 6 (0.8) | 11 (1.7) |
| Fatigue | 3 (0.3) | 5 (0.7) | 7 (1.1) |
| Fever | 0 (0) | 1 (0.1) | 1 (0.2) |
| Nausea | 0 (0) | 4 (0.6) | 1 (0.2) |
| Vomiting | 8 (0.9) | 1 (0.1) | 1 (0.2) |
| Gastrointestinal ulceration | 0 (0) | 3 (0.4) | 2 (0.3) |
| Gastritis | 2 (0.2) | 1 (0.1) | 0 (0) |
| Radiation cholecystitis | 0 (0) | 0 (0) | 0 (0) |
| Radiation pancreatitis | 0 (0) | 0 (0) | 0 (0) |
| Radiation pneumonitis | 0 (0) | 0 (0) | 0 (0) |
| Radioembolization-induced liver disease | 1 (0.1) | 4 (0.6) | 0 (0) |
| Other | 2 (0.2) | 15 (2.1) | 44 (6.9) |
Data are presented as n (%). The percentage is taken over the number of patients with at least 1 follow-up per time interval
The number of adverse events per time intervals for hepatocellular carcinoma, intrahepatic cholangiocarcinoma and colorectal cancer liver metastases are included in Supplement 2
Table 4.
Abnormal laboratory values: patients with at least one occurrence per grade
| Category | HCC | ICC | mCRC | Other mets (248) | Total (1027) |
|---|---|---|---|---|---|
| Patients with at least 1 abnormal laboratory value | 359 (85.1) | 96 (80) | 158 (66.7) | 179 (72.2) | 792 (77.1) |
| Patients with at least 1 grade ≥ 3 abnormal laboratory value | 129 (30.6) | 27 (22.5) | 42 (17.7) | 47 (19) | 245 (23.9) |
| Albumin decreased—grade | |||||
| n | 422 | 120 | 237 | 248 | 1027 |
| 1 | 167 (39.6) | 26 (21.7) | 36 (15.2) | 47 (19) | 276 (26.9) |
| 2 | 117 (27.7) | 25 (20.8) | 29 (12.2) | 38 (15.3) | 209 (20.4) |
| 3 | 19 (4.5) | 4 (3.3) | 9 (3.8) | 2 (0.8) | 34 (3.3) |
| ALT increased—grade | |||||
| n | 422 | 120 | 237 | 248 | 1027 |
| 1 | 197 (46.7) | 49 (40.8) | 80 (33.8) | 106 (42.7) | 432 (42.1) |
| 2 | 31 (7.3) | 2 (1.7) | 15 (6.3) | 20 (8.1) | 68 (6.6) |
| 3 | 13 (3.1) | 0 (0) | 9 (3.8) | 6 (2.4) | 28 (2.7) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.1) |
| AST increased—grade | |||||
| n | 422 | 120 | 237 | 248 | 1027 |
| 1 | 258 (61.1) | 55 (45.8) | 108 (45.6) | 118 (47.6) | 539 (52.5) |
| 2 | 66 (15.6) | 12 (10) | 24 (10.1) | 23 (9.3) | 125 (12.2) |
| 3 | 25 (5.9) | 3 (2.5) | 13 (5.5) | 14 (5.6) | 55 (5.4) |
| 4 | 3 (0.7) | 0 (0) | 0 (0) | 1 (0.4) | 4 (0.4) |
| Bilirubin increased—grade | |||||
| n | 422 | 120 | 237 | 248 | 1027 |
| 1 | 154 (36.5) | 24 (20) | 57 (24.1) | 44 (17.7) | 279 (27.2) |
| 2 | 141 (33.4) | 22 (18.3) | 35 (14.8) | 34 (13.7) | 232 (22.6) |
| 3 | 63 (14.9) | 15 (12.5) | 19 (8) | 20 (8.1) | 117 (11.4) |
| 4 | 18 (4.3) | 3 (2.5) | 6 (2.5) | 7 (2.8) | 34 (3.3) |
| INR increased | |||||
| n | 422 | 120 | 237 | 248 | 1027 |
| 1 | 133 (31.5) | 17 (14.2) | 38 (16) | 26 (10.5) | 214 (20.8) |
| 2 | 57 (13.5) | 10 (8.3) | 16 (6.8) | 14 (5.6) | 97 (9.4) |
| 3 | 14 (3.3) | 1 (0.8) | 1 (0.4) | 3 (1.2) | 19 (1.9) |
| Neutrophil count decreased—grade | |||||
| n | 422 | 120 | 237 | 248 | 1027 |
| 1 | 36 (8.5) | 14 (11.7) | 8 (3.4) | 16 (6.5) | 74 (7.2) |
| 2 | 22 (5.2) | 5 (4.2) | 7 (3) | 8 (3.2) | 42 (4.1) |
| 3 | 6 (1.4) | 1 (0.8) | 5 (2.1) | 11 (4.4) | 23 (2.2) |
| 4 | 0 (0) | 1 (0.8) | 1 (0.4) | 1 (0.4) | 3 (0.3) |
| Platelet count decreased—grade | |||||
| n | 422 | 120 | 237 | 248 | 1027 |
| 1 | 216 (51.2) | 55 (45.8) | 58 (24.5) | 78 (31.5) | 407 (39.6) |
| 2 | 73 (17.3) | 17 (14.2) | 10 (4.2) | 10 (4) | 110 (10.7) |
| 3 | 27 (6.4) | 5 (4.2) | 1 (0.4) | 5 (2) | 38 (3.7) |
| 4 | 5 (1.2) | 1 (0.8) | 2 (0.8) | 1 (0.4) | 9 (0.9) |
Data are presented as n (%)
ALT Alanine transaminase, AST Aspartate aminotransferase, ICC intrahepatic cholangiocarcinoma, INR International Normalised Ratio, HCC hepatocellular carcinoma, mCRC metastatic colorectal cancer, TARE transarterial radioembolization
Prognostic Factors
For the calculation of the overall AEBS, 620 patients had no AEs (AEBS = 0), 31 patients with ungraded AEs and 118 patients AEs only in the “Other” category were excluded, leaving a total of 258 patients with an AEBS > 0. The mean and standard deviation for the total overall AEBS were 1.4 ± 3.8 for all AEs and 0.2 ± 1.2 for serious AEs. Univariable analysis (Table 5) showed that the variables > 1 TARE treatments (mean ± SD 2.6 ± 5.2, p < 0.0001), no prophylactic embolization (2.0 ± 4.9, p = 0.0222), partition model (2.1 ± 4.9, p = 0.022), > 2 prior systemic treatments (2.1 ± 5.0, p < 0.0001) and unilobar treatments (2.2 ± 4.6 for left lobe treatments and 1.7 ± 4.2 for right lobe treatments, p = 0.0448) were predictors of an increased overall AEBS, in addition to Eastern Cooperative Oncology Group (ECOG) 0 (1.9 ± 4.5, p < 0.0001), 1 tumour nodule (2.5 ± 5.4, p = 0.0002) and the percentage of tumour invasion in the left liver lobe of > 20% (3.5 ± 5.5, p = 0.0283). Furthermore, cancer type was associated with a difference in AEBS (p = 0.0422).
Table 5.
Univariable analysis of statistically significant predictors of increased overall adverse events burden score
| Variable | Categories | N | Mean AEBS ± SD | P value |
|---|---|---|---|---|
| Complete cohort (all cancer types, n = 1027) | ||||
| Cancer type | Hepatocellular carcinoma | 422 | 1.2 ± 3.8 | 0.0422 |
| Intrahepatic cholangiocarcinoma | 120 | 2.0 ± 4.7 | ||
| Colorectal cancer liver mets | 237 | 1.2 ± 3.1 | ||
| Neuroendocrine tumour liver mets | 58 | 2.3 ± 5.5 | ||
| Breast cancer liver mets | 47 | 2.2 ± 4.7 | ||
| Pancreatic cancer liver mets | 32 | 1.1 ± 1.5 | ||
| Melanoma liver mets | 32 | 1.2 ± 1.8 | ||
| ECOG | 0 | 625 | 1.9 ± 4.5 | < 0.0001 |
| 1 | 316 | 0.7 ± 1.8 | ||
| ≥ 2 | 75 | 0.4 ± 1.4 | ||
| Number of prior treatments | 0 | 521 | 1.3 ± 3.9 | < 0.0001 |
| 1 | 191 | 0.5 ± 1.4 | ||
| 2 | 98 | 0.8 ± 2.0 | ||
| > 2 | 217 | 2.6 ± 5.0 | ||
| Number of tumour nodules | 1 | 230 | 2.5 ± 5.4 | 0.0002 |
| 2–5 | 253 | 1.2 ± 3.4 | ||
| > 5 | 245 | 1.3 ± 3.3 | ||
| Uncountable | 299 | 0.8 ± 2.5 | ||
| 10–20% | 99 | 2.7 ± 6.1 | ||
| > 20% | 103 | 2.2 ± 4.4 | ||
| Percentage tumour invasion in the liver (left lobe) | < 10% | 224 | 2.1 ± 4.9 | 0.0283 |
| 10–20% | 73 | 2.2 ± 4.9 | ||
| > 20% | 85 | 3.5 ± 5.5 | ||
| Number of TARE treatments | 1 | 869 | 1.2 ± 3.4 | < .0001 |
| ≥ 2 | 158 | 2.6 ± 5.2 | ||
| Prophylactic embolization | Yes | 412 | 0.8 ± 2.1 | 0.0222 |
| No | 477 | 2.0 ± 4.9 | ||
| Dose methodology | BSA/mBSA | 736 | 1.1 ± 3.2 | 0.0022 |
| Partition | 279 | 2.1 ± 4.9 | ||
| TARE Target | Left lobe | 124 | 2.2 ± 4.6 | 0.0448 |
| Right lobe | 337 | 1.7 ± 4.2 | ||
| Whole liver (segmental) | 98 | 0.9 ± 3.3 | ||
| Whole liver (sequential) | 136 | 1.0 ± 2.4 | ||
| Whole liver (single catheter) | 121 | 1.1 ± 3.7 | ||
| Whole liver (split administration) | 171 | 1.1 ± 2.9 | ||
| Hepatocellular carcinoma (n = 422) | ||||
| ECOG | 0 | 260 | 1.7 ± 4.7 | 0.0045 |
| 1 | 131 | 0.6 ± 1.5 | ||
| ≥ 2 | 31 | 0.0 ± 0.1 | ||
| Number of prior treatments | 0 | 381 | 1.3 ± 3.9 | 0.0107 |
| 1 | 35 | 0.6 ± 2.2 | ||
| 2 | 4 | 1.0 ± 2.0 | ||
| > 2 | 2 | 8.5 ± 0.7 | ||
| Number of tumour nodules | 1 | 136 | 2.2 ± 5.0 | 0.0081 |
| 2–5 | 138 | 1.0 ± 3.5 | ||
| > 5 | 72 | 1.0 ± 3.4 | ||
| Uncountable | 76 | 0.3 ± 1.1 | ||
| Number of TARE treatments | 1 | 353 | 1.2 ± 3.8 | 0.0224 |
| ≥ 2 | 69 | 1.6 ± 3.9 | ||
| Prophylactic embolization | No | 217 | 1.8 ± 4.9 | 0.0211 |
| Yes | 138 | 0.7 ± 2.2 | ||
| Dose methodology | BSA/mBSA | 245 | 0.7 ± 2.8 | 0.0211 |
| Partition | 173 | 2.0 ± 4.8 | ||
| TARE Target | Left lobe | 56 | 1.8 ± 4.6 | 0.0032 |
| Right lobe | 177 | 1.6 ± 4.2 | ||
| Whole liver (segmental) | 66 | 0.2 ± 0.9 | ||
| Whole liver (sequential) | 33 | 1.1 ± 2.8 | ||
| Whole liver (single catheter) | 53 | 1.4 ± 5.1 | ||
| Whole liver (split administration) | 37 | 0.2 ± 0.9 | ||
| Intrahepatic cholangiocarcinoma (n = 120) | ||||
| Number of TARE treatments | 1 | 97 | 1.6 ± 3.5 | 0.0224 |
| ≥ 2 | 23 | 3.7 ± 7.8 | ||
| Colorectal liver metastases (n = 237) | ||||
| ECOG | 0 | 143 | 1.8 ± 3.8 | 0.0188 |
| 1 | 72 | 0.5 ± 1.5 | ||
| ≥ 2 | 18 | 0.5 ± 1.1 | ||
| Number of prior treatments | 0 | 21 | 1.1 ± 2.6 | 0.0127 |
| 1 | 69 | 0.3 ± 1.0 | ||
| 2 | 41 | 0.7 ± 2.1 | ||
| > 2 | 106 | 2.1 ± 4.1 | ||
| Number of tumour nodules | 1 | 27 | 2.5 ± 4.8 | 0.0155 |
| 2–5 | 50 | 1.7 ± 3.5 | ||
| > 5 | 79 | 1.4 ± 3.2 | ||
| Uncountable | 81 | 0.4 ± 1.4 | ||
Other, non-significant variables for the whole cohort, hepatocellular carcinoma, intrahepatic cholangiocarcinoma and colorectal liver metastases included in the univariable analysis: Gender; Presence of extra-hepatic disease; prior systemic therapy; prior hepatic procedures; percentage of tumour invasion in the whole liver; percentage of tumour invasion in the right liver lobe; Unilobar treatment, left vs right; Prescribed activity, < 1 GBq, 1.1–1.15 GBq, 1.5–1.82 GBq, > 1.82 GBq. The complete tables can be found in Supplement 3
Analyses were performed using the Wilcoxon rank-sum test if the number of groups is 2 or with the Kruskal–Wallis test (nonparametric alternative to the ANOVA) if the number of groups exceed 2. Multivariable analysis could not be performed due to the high number of patients with no adverse events
BSA body surface area, ECOG Eastern Cooperative Oncology Group, GBq giga-becquerel, mBSA modified body surface area, SD standard deviation, TARE transarterial radioembolization
Variables associated with a difference in AEBS for HCC were ECOG 0 (p = 0.0045), number of prior systemic treatments (p = 0.0107), 1 tumour nodule (0.0081), number of TARE treatments (p = 0.0224), no prophylactic embolization (p = 0.0211) partition model dosimetry (p = 0.0007) and unilobar treatment target (0.0032). For ICC, only the number of TARE treatments was associated with a change in AEBS (p = 0.0224), and for colorectal liver metastases, ECOG 0 (p = 0.0188), > 2 prior systemic treatments (p = 0.0127), and 1 tumour nodule (p = 0.0155) were associated with a change in AEBS (Table 5). Supplements 3 A-D describe the outcomes of all variables included in the univariable analysis.
To further investigate the results of the univariable analysis, a comparison of reported AEs per time interval (< 1 month, 1–4 months, and > 4 months) was performed (Supplement 4A–E). This comparison revealed that the reporting of symptomatic adverse events (abdominal pain, fatigue, nausea, vomiting and fever) was different between patients with ECOG 0 compared to ECOG > 0, 1 tumour nodule compared to > 1 tumour nodule, unilobar treatments versus bilobar treatments, activity calculations with partition model compared to standard body surface area, and patients that had no prophylactic embolization compared to those that had prophylactic embolization.
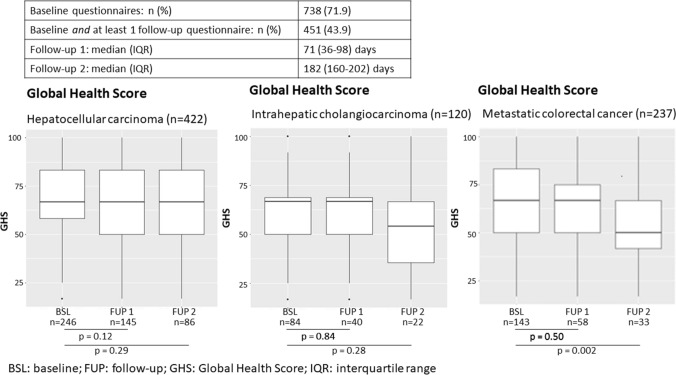
Global Health Score
In total, 451 patients were eligible for quality-of-life deterioration evaluation, having completed a questionnaire both at baseline and during at least one follow-up visit. Deterioration of GHS with 10 points was found in 241 patients (53.4%). However, in the HCC and ICC cohorts, GHS was largely maintained during the first 2 follow-up periods (Fig. 1). The mCRC cohort showed similar results at the first follow-up period, but GHS was significantly reduced at the second follow-up period (p = 0.002). Supplement 5 shows a strong correlation between the deterioration of QOL over different dimensions, including GHS, and an increase in overall AEBS (p values ranging from < 0.001 to 0.0199). Univariable analysis (Table 6) shows that ECOG 0, > 1 TARE treatment and partition model were associated with ≥ 10 points deterioration of QOL (p = 0.0027, p = 0.0014 and p = 0.0041, respectively).
Fig. 1.
Change in Global Health Score from baseline to follow-up 1 and follow-up 2 for HCC, ICC and mCRC
Table 6.
Predictors for a deterioration of quality of life
| Variable | Type | < 10 points deterioration | ≥ 10 points deterioration | All | P value |
|---|---|---|---|---|---|
| Gender | Female | 67 (34.0%) | 81 (33.6%) | 148 (33.8%) | 0.4399 |
| Male | 123 (62.4%) | 156 (64.7%) | 279 (63.7%) | ||
| Unknown | 7 (3.6%) | 4 (1.7%) | 11 (2.5%) | ||
| ECOG | 0 | 122 (62.6%) | 183 (76.3%) | 305 (70.1%) | 0.0027 |
| 1 | 60 (30.8%) | 52 (21.7%) | 112 (25.7%) | ||
| ≥ 2 | 13 (6.7%) | 5 (2.1%) | 18 (4.1%) | ||
| Prior chemotherapy | No | 113 (57.4%) | 133 (55.2%) | 246 (56.2%) | 0.6483 |
| Yes | 84 (42.6%) | 108 (44.8%) | 192 (43.8%) | ||
| Prior locoregional treatments | No | 118 (59.9%) | 144 (59.8%) | 262 (59.8%) | 0.975 |
| Yes | 79 (40.1%) | 97 (40.2%) | 176 (40.2%) | ||
| Number of TARE treatments | 1 TARE | 168 (85.3%) | 175 (72.6%) | 343 (78.3%) | 0.0014 |
| 2 or more TARE | 29 (14.7%) | 66 (27.4%) | 95 (21.7%) | ||
| Location of liver tumours | Left | 23 (25.0%) | 29 (21.5%) | 52 (22.9%) | 0.5357 |
| Right | 69 (75.0%) | 106 (78.5%) | 175 (77.1%) | ||
| Prophylactic embolization | No | 96 (65.3%) | 140 (70.7%) | 236 (68.4%) | 0.2859 |
| Yes | 51 (34.7%) | 58 (29.3%) | 109 (31.6%) | ||
| Dose methodology | Partition Model | 75 (38.7%) | 125 (52.5%) | 200 (46.3%) | 0.0041 |
| BSA/mBSA | 119 (61.3%) | 113 (47.5%) | 232 (53.7%) |
Analyses were performed using the Wilcoxon rank-sum test if the number of groups is 2 or with the Kruskal–Wallis test (non-parametric alternative to the ANOVA) if the number of groups exceed 2
BSA body surface area, ECOG Eastern Cooperative Oncology Group, mBSA modified body surface area, TARE transarterial radioembolization
Discussion
This large prospective multicentre study describes how TARE treatments with Y90 resin microspheres are performed in patients with primary and metastatic liver disease and shows that regardless of indication, experienced interventional radiologists can apply the treatment safely with a low occurrence of severe AEs and low AEBS. We identified several predictors for an increased AEBS regardless of cancer type, described the predictors of an increased AEBS for HCC, ICC and colorectal liver metastases, specifically, and determined predictors for a deterioration of GHS. We furthermore established that a strong correlation between the AEBS and GHS exists.
Application-Related Adverse Events
Severe AEs associated with the technical application of TARE are gastritis, gastrointestinal ulcerations, radiation cholecystitis, radiation pancreatitis, radiation pneumonitis and REILD [20]. In our cohort, we found that each of these non-target radiation AEs occurred in less than 2% of the patients. We reported that 13/1027 patients (1.3%) experienced REILD, of which half were grade 3 or higher. This occurrence of REILD is on the lower end of the range reported in a systematic review, which identified that the incidence of symptomatic REILD varied between 0 and 31%, although, in most reports, the incidence was 0–8% [25]. Furthermore, our cohort reported no occurrences of radiation pneumonitis, which is consistent with the generally low median lung shunt findings of 5–7% [26]. Supplement 6 discusses the severe AEs in more detail.
Prognostic Factors for Adverse Events
Prior studies that identified prognostic factors for AEs were based on small retrospective cohorts [27–29]. The exception was the RESIN study, which included 614 patients with various indications and reported on predictors for AEs within 6 months after treatment [30].
In HCC, we found that ECOG 0, number of prior systemic treatments, 1 tumour nodule, number of TARE treatments, partition model dosimetry, and unilobar treatment target were associated with an increase in AEBS. Prior studies evaluating prognostic factors for safety and toxicity reported on severe AEs and commonly ignored the burden of symptomatic post-embolization AEs [27–29]. While the randomised trials SIRveNIB and SARAH reported adverse events of all grades [31, 32], comparing the complete safety profiles of TARE and sorafenib is complicated without the use of a numeric metric such as the AEBS. Furthermore, it should be noted that important differences in safety outcomes after TARE with glass or resin in HCC have been described [33].
In mCRC, our findings that > 2 lines of prior chemotherapy before TARE increased the AEBS reflect the current understanding regarding hepatotoxicities following oxaliplatin and irinotecan [34, 35]. Also here, we found ECOG 0 and 1 tumour nodule to be predictive of an increased AEBS, whereby the impact of low tumour volume on toxicity outcomes has been described in the RESIN study as well [30].
Interestingly, our cohort-wide findings suggest that partition model dosimetry predicts an increase in AEBS. This finding is not completely understood. Our data show that patients treated with partition model dosimetry had fewer cases of gastritis, gastrointestinal ulcerations, REILD, radiation pancreatitis or radiation cholecystitis, but presented more symptomatic adverse events than patients treated with body surface area (BSA) activity calculation methods. While partition model has demonstrated to increase the tumour-absorbed dose compared to standard activity calculations [36], several studies showed that an increase in tumour-absorbed dose was not associated with an increase in toxicity and that instead the level of toxicity was associated with an increase in parenchyma-absorbed dose [27, 37, 38]. Furthermore, it has been demonstrated that partition model dosimetry is associated with an increased survival [39], which means that patients may receive further treatments that cause additional AEs.
We also reported that patients with ECOG 0, unilobar treatments and 1 tumour nodule have an increased AEBS. As the data in Supplement 4 shows, these healthier patients reported more symptomatic AEs within the first month after treatment. We can hypothesise that patients with low tumour burden and fewer cancer-related symptoms are more aware of changes in their well-being and thus more likely to report mild symptomatic adverse events compared to patients with increased tumour-related symptoms or experience with prior treatments. Moreover, especially in our HCC cohort, 55.2% of the patients did not receive prior hepatic procedures and 89.3% did not receive prior systemic therapy [22], meaning that a substantial number of HCC patients had little experience with cancer treatments. Our results show that these healthier patients do report an increased number of post-TARE symptomatic adverse events. Indeed, some studies have shown that psychological distress and poor communication can lead to an increase in patient-reported symptomatic AEs [40, 41].
Quality of Life
This prospective study shows that TARE has a minimum effect on QOL. Predictors for a deterioration of GHS were ECOG 0, > 1 TARE treatment and partition model dosimetry. Previous studies have shown that patients treated with TARE for HCC maintain a stable quality-of-life outcome [10, 42, 43] and TARE is considered favourable to TACE in that respect [44–46]. This is consistent with our findings, which shows no deterioration of GHS for HCC and ICC, and a minor deterioration of GHS after 6 months in the mCRC cohort. An extended discussion of quality of life after TARE can be found in Supplement 6.
Limitations
The patient population included in this study is heterogeneous in presentation, treatment history and treatment pathway following TARE. Therefore, it should be considered that reported AEs might have been influenced by other treatments, or, vice versa, treatment-related AEs were considered unrelated due to concomitant therapies. In general, AEs are known to be underreported, both in clinical studies and to regulatory bodies [47–49]. Moreover, interventional radiology departments did not always have the appropriate infrastructure to consistently perform follow-ups, which contributed to the increased number of censored patients during follow-up and may have contributed to a potential underreporting of AEs and decreased returns of QOL questionnaires. Furthermore, the commonality of post-embolization syndrome may have caused some investigators to consider it as an expected side effect of TARE, instead of a reportable AE. Finally, the TARE treatments were performed between 2015 and 2017. Changes in practice and that investigator-determined follow-up regimes were not always optimal for detecting early AEs may not be reflected in the data. Despite the large number of patients without any reported AEs and potential missing data, our sample displays a high degree of internal validity as suggested by the strong correlation between an increase of overall AEBS and deterioration of QOL.
When interpreting the predictors for AEBS outcomes, it should be considered that for this analysis we combined all reported AEs in the follow-up period. Despite our findings that AEs in the “other” category were primarily occurring > 4 months after treatment, the exclusion of other AEs from the AEBS analysis could introduce a bias. We, therefore, supplemented this analysis by presenting adverse events outcomes per time interval (< 1 month, 1–4 months, and > 4 months after TARE). However, potential discrepancies in the number of follow-ups per time interval when comparing variables were not evaluated.
Finally, data points that may affect occurrences of REILD or other serious AEs, such as liver reserve, treated liver volume, or liver absorbed dose were not collected during the study, and their confounding impact should be considered when interpreting the results.
Conclusion
This large prospective dataset on the use of TARE with Y90 resin microspheres shows that, in the real-world setting, TARE is very safe with minimal impact on GHS. It was established that the AEBS is a viable way to report on AEs and find prognostic factors for safety-related outcomes and correlates with deterioration of GHS. Predictors for an increased AEBS are cancer type, > 2 prior systemic treatments, multiple TARE treatments, no prophylactic embolization, ECOG 0, low tumour burden and partition model dosimetry. Predictors for a deterioration of GHS were ECOG 0, > 1 TARE treatment and partition model dosimetry. Further studies on intra-arterial treatments are encouraged to use the AEBS to accurately evaluate predictors for safety outcomes and improve patient safety.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors want to thank the patients, the CIRT investigators and site staff involved in the study, in particular the local study nurses that contributed significantly to the quality of the collected data through feedback and comments during the data collection phase. The authors are indebted to Prof. José Ignacio Bilbao, who initiated the study and was chairperson of the CIRT Steering Committee between 2015 and 2018 and is now enjoying his well-deserved retirement. The CIRT study was funded by an independent investigator-initiated research grant from SIRTEX Medical Europe GmbH (Bonn, Germany). The electronic data capturing system was developed and supported by ConexSys Inc (Lincoln, RI, USA), and a customised data management system was designed by Joaquin Padilla Montani (Vienna, Austria). Necessary server infrastructure was developed and maintained by ITEA GmbH (Vienna, Austria). The authors thank the CIRSE Central Office and the staff of the CIRSE Clinical Research department for their support during the design and setup of the study and drafting of the manuscript. On behalf of the CIRT Principal Investigators: Thomas Albrecht, Vivantes Klinikum Neukölln, Department for Radiology and Interventional Therapy, Rudower Str. 48, 12351, Berlin, Germany. Olivier D’Archambeau, University Hospital Antwerp, Department of Radiology, Wilrijkstraat 10, 2650, Antwerp, Belgium. Tugsan Balli, Cukurova University, Radiology Department, Balcalı Hospital, 01330, Adana, Turkey. Sadik Bilgic, Ankara University, Department of Radiology, Medical Faculty, Cebeci, 06590, Ankara, Turkey. Allan Bloom, Hadassah-Hebrew University Medical Center, Department of Radiology, Jerusalem, Israel. Roberto Cianni, Department of Interventional Radiology, S. Camillo Hospitals, Circonvallazione Gianicolense 85, 00149, Rome, Italy. Roberto Cioni, University of Pisa, Diagnostic and Interventional Radiology, Department of Translational Research and New Technologies in Medicine, Via U. Foscolo 5, 50059, Vinci, Pisa, Italy. Roman Fischbach, Asklepios Klinik Altona, Department of Radiology and Neuroradiology, Paul-EhrlichStraße 1, 22763, Hamburg, Germany. Patrick Flamen, Institute Jules Bordet, Université Libre de Bruxelles, Nuclear Medicine Department, 121 Boulevard de Waterloo, 1000, Brussels, Belgium. Laurent Gerard, University Hospital of Liege, Division of Radiology, domaine du Sart-Tilman B35, 4000, Liège, Belgium. Rita Golfieri, Department of Radiology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Italy. Gerd Grözinger, Eberhard Karls University, Department of Diagnostic and Interventional Radiology, Hoppe-Seyler-Str. 3, D-72076, Tübingen, Germany. Thomas Helmberger, Department of Radiology, Neuroradiology and Minimal-Invasive Therapy, Klinikum Bogenhausen, Englschalkinger Str. 77, 81925, Munich, Germany. Marcus Katoh, Helios Hospital Krefeld, Department of Diagnostic and Interventional Radiology, Lutherplatz 40, 47805, Krefeld, Germany. Michael Koehler, University Hospital Muenster, Department of Clinical Radiology, Albert-Schweitzer-Strasse 33, 48129, Muenster, Germany. Jan Robert Kröger, Johannes Wesling Klinik Minden, Universitätsinstitut für Radiologie, Neuroradiologie und Nuklearmedizin der Mühlenkreiskliniken, Hans-Nolte-Straße 1, 32429 Minden, Germany. Christiane Kuhl, University Hospital Aachen, Department of Radiology, Pauwelsstr. 30, 52074, Aachen, Germany. Geert Maleux, Radiology, Univeritair Ziekenhuis Leuven, Herestraat 49, 3000, Leuven, Belgium. Franco Orsi, European Institute of Oncology, Interventional Radiology Division, Via Ripamonti 435, 20100, Milan, Italy. Murat Özgün, St. Franziskus Hospital, Department of Radiology, Hohenzollernring 70, 48145, Muenster, Germany. Maciej Pech, Department of Radiology and Nuclear Medicine, University of Magdeburg, Magdeburg, Germany. Thomas Pfammatter, Institute of Diagnostic and Interventional Radiology, Universitätsspital Zürich, Zürich, Switzerland. Peter Reimer, Academic Teaching Hospital the University of Freiburg, Städtisches Klinikum Karlsruhe, Institute for Diagnostic and Interventional Radiology, Moltkestrasse 90, 76133, Karlsruhe, Germany. Maxime Ronot, APHP, University Hospitals Paris Nord Val de Seine, Department of Radiology, 100 bd general Leclerc, Beaujon, Clichy, Hauts-de-Seine, France. Bruno Sangro, Liver Unit and HPB Oncology Area, Clínica Universidad de Navarra and CIBEREHD, Avda. Pio XII 36, 31008, Pamplona, Spain. Axel Schmid, University Hospital Erlangen, Department of Radiology, Maximiliansplatz 1, 91054, Erlangen, Germany. Alessandro Vit, Azienda Ospedaliero Universitaria, SOC Diagnostica Angiografica e Radiologia Interventistica, via Grazzano 150/C, 33100, Udine, Italy.
Abbreviations
- 99mTC MAA
Technetium 99m macroaggregated albumin
- AE
Adverse event
- AEBS
Adverse event burden score
- ALT
Alanine transaminase
- AST
Aspartate aminotransferase
- BSA
Body surface area
- CIRSE
Cardiovascular and Interventional Radiological Society of Europe
- CIRT
CIRSE registry for SIR-spheres therapy
- CTCAE
Common terminology criteria for AEs
- ECOG
Eastern Cooperative Oncology Group
- EORTC
European Organisation for the Research and Treatment of Cancer
- GBq
Giga-becquerel
- GHS
Global Health Score
- HAI
Hepatic arterial infusion
- HCC
Hepatocellular carcinoma
- ICC
Intrahepatic cholangiocarcinoma
- INR
International normalised ratio
- IQR
Interquartile range
- mBSA
Modified body surface area
- mCRC
Metastatic colorectal cancer
- QOL
Quality of life
- RCT
Randomised controlled trial
- REILD
Radioembolization-induced liver disease
- SD
Standard deviation
- SIRT
Selective internal radiation therapy
- TACE
Transarterial chemoembolization
- TARE
Transarterial radioembolization
- Y90
Yttrium-90
Author Contributions
GMa, NS, DA, BP, BS, FK, RC, GMu, TH and NdJ contributed to the study concept, set-up, and design. GMa, TA, IB, RC, BS and TH acquired patient data. GMa, TA, IB, HP, BZ and NdJ analysed and interpreted the data. GMa, TA, IB, HP, BZ and NdJ drafted the manuscript. TH supervised the study. GMa supervised the manuscript drafting and data interpretation. All authors contributed to critical revisions and approved the final version of the manuscript.
Funding
The CIRT study was funded by an independent investigator-initiated research grant from SIRTEX Medical Europe GmbH (Bonn, Germany). CIRSE, the Cardiovascular and Interventional Radiological Society of Europe, is responsible for the independent execution of the CIRT study and has sole ownership of the data.
Declarations
Conflict of interest
Thomas Albrecht received speaker’s honoraria from Sirtex Medical and Pharmacept, he consulted for Siemens Healthineers and operated as a proctor for Sirtex. Dirk Arnold received consulting fees and honoraria for presentations and lectures and travel support from Boston Scientific and Terumo, MSD, BMS, AstraZeneca, Roche, Servier, Sanofi and Merck Serono. He is on the guidelines committee of the European Society for Medical Oncology and supported oncopolicy manuscripts for the European Cancer Organisation. Irene Bargellini received: Institutional research grant from Boston Scientific; consulting fees from AstraZeneca, EISAI, Ge Healthcare, Guerbet, Merck, Sirtex, Terumo; honoraria for lectures from AstraZeneca, Bayer, Boston Scientific, Eisai, Guerbet, Merck, Sirtex, Sobi, Terumo; participation on Independent Data Safety Monitoring Board for AstraZeneca. Frank Kolligs participated on a data safety monitoring or advisory board of Bayer, MSD, and Roche. Geert Maleux received honoraria for speaker’s bureau from Sirtex Medical and operated as a proctor for Sirtex Medical. Bruno Sangro received grants or contracts from Sirtex and BMS, consulting fees from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, Eisai, Eli Lilly, Incyte, Ipsen, Roche, Sirtex Medical, Terumo; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astra Zeneca, Bayer, BMS, Eisai, Incyte, Ipsen, Roche, Sirtex Medical; Participation on a Data Safety Monitoring Board or Advisory Board from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, Eisai, Eli Lilly, Incyte, Ipsen, Roche, Sirtex Medical, Terumo, and has a leadership or fiduciary role in the International Liver Cancer Association. José I. Bilbao operated as proctor for Sirtex Medical. Niklaus Schaefer, Graham Munneke, Roberto Cianni, Bora Peynircioglu, Helena Pereira, Bleranda Zeka, Niels de Jong and Thomas Helmberger had nothing to declare.
Ethics Approval and Consent to Participate
All included patients signed an informed consent form. This research project was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Niels de Jong, Email: dejong@cirse.org.
the CIRT Principal Investigators:
Thomas Albrecht, Olivier D’Archambeau, Tugsan Balli, Sadik Bilgic, Allan Bloom, Roberto Cioni, Roman Fischbach, Patrick Flamen, Laurent Gerard, Rita Golfieri, Gerd Grözinger, Marcus Katoh, Michael Koehler, Jan Robert Kröger, Christiane Kuhl, Franco Orsi, Murat Özgün, Peter Reimer, Maxime Ronot, Axel Schmid, Alessandro Vit, Geert Maleux, Bruno Sangro, Maciej Pech, Thomas Helmberger, Roberto Cianni, and Thomas Pfammatter
References
- 1.Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 22021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329–359. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. [DOI] [PubMed]
- 4.European Association for the Study of the Liver Electronic address EEE, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Giammarile F, Bodei L, Chiesa C, Flux G, Forrer F, Kraeber-Bodere F, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2011;38(7):1393–1406. doi: 10.1007/s00259-011-1812-2. [DOI] [PubMed] [Google Scholar]
- 6.Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 8.Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):871–873. doi: 10.1093/annonc/mdy510. [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 10.Loffroy R, Ronot M, Greget M, Bouvier A, Mastier C, Sengel C, et al. Short-term Safety and quality of life outcomes following radioembolization in primary and secondary liver tumours: a multi-centre analysis of 200 patients in France. Cardiovasc Intervent Radiol. 2021;44(1):36–49. doi: 10.1007/s00270-020-02643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS ONE. 2017;12(9):e0184597. doi: 10.1371/journal.pone.0184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 13.Emmons EC, Bishay S, Du L, Krebs H, Gandhi RT, Collins ZS, et al. Survival and toxicities after (90)Y transarterial radioembolization of metastatic colorectal cancer in the RESIN registry. Radiology. 2022;305(1):228–236. doi: 10.1148/radiol.220387. [DOI] [PubMed] [Google Scholar]
- 14.Frantz S, Matsuoka L, Vaheesan K, Petroziello M, Golzarian J, Wang E, et al. Multicenter evaluation of survival and toxicities of hepatocellular carcinoma following radioembolization: analysis of the RESiN registry. J Vasc Interv Radiol. 2021;32(6):845–852. doi: 10.1016/j.jvir.2021.03.535. [DOI] [PubMed] [Google Scholar]
- 15.Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18(9):1159–1171. doi: 10.1016/S1470-2045(17)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le-Rademacher JG, Hillman S, Storrick E, Mahoney MR, Thall PF, Jatoi A, et al. Adverse event burden score-A versatile summary measure for cancer clinical trials. Cancers. 2020;12(11):3251. doi: 10.3390/cancers12113251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helmberger T, Arnold D, Bilbao JI, de Jong N, Maleux G, Nordlund A, et al. Clinical application of radioembolization in hepatic malignancies: protocol for a prospective multicenter observational study. JMIR Res Protoc. 2020;9(4):e16296. doi: 10.2196/16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilbao JI, Garrastachu P, Herraiz MJ, Rodriguez M, Inarrairaegui M, Rodriguez J, et al. Safety and efficacy assessment of flow redistribution by occlusion of intrahepatic vessels prior to radioembolization in the treatment of liver tumors. Cardiovasc Intervent Radiol. 2010;33(3):523–531. doi: 10.1007/s00270-009-9717-1. [DOI] [PubMed] [Google Scholar]
- 19.Boas FE, Bodei L, Sofocleous CT. Radioembolization of colorectal liver metastases: indications, technique, and outcomes. J Nucl Med. 2017;58(Suppl 2):104s–s111. doi: 10.2967/jnumed.116.187229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahnken AH. Current status of transarterial radioembolization. World J Radiol. 2016;8(5):449–459. doi: 10.4329/wjr.v8.i5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fayers PM AN, Bjordal K, Groenvold M, Curran D, Bottomley A, on, Group. botEQoL. The EORTC QLQ-C30 Scoring Manual, 3rd edition. European Organisation for Research and Treatment of Cancer. 2001.
- 22.Helmberger T, Golfieri R, Pech M, Pfammatter T, Arnold D, Cianni R, et al. Clinical application of trans-arterial radioembolization in hepatic malignancies in Europe: first results from the prospective multicentre observational study CIRSE registry for SIR-spheres therapy (CIRT) Cardiovasc Intervent Radiol. 2021;44(1):21–35. doi: 10.1007/s00270-020-02642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolligs F, Arnold D, Golfieri R, Pech M, Peynircioglu B, Pfammatter T, et al. Factors impacting survival after transarterial radioembolization in patients with hepatocellular carcinoma: results from the prospective CIRT study. JHEP Rep. 2023;5(2):100633. doi: 10.1016/j.jhepr.2022.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaefer N, Grozinger G, Pech M, Pfammatter T, Soydal C, Arnold D, et al. Prognostic factors for effectiveness outcomes after transarterial radioembolization in metastatic colorectal cancer: results from the multicentre observational study CIRT. Clin Colorectal Cancer. 2022. [DOI] [PubMed]
- 25.Braat MN, van Erpecum KJ, Zonnenberg BA, van den Bosch MA, Lam MG. Radioembolization-induced liver disease: a systematic review. Eur J Gastroenterol Hepatol. 2017;29(2):144–152. doi: 10.1097/MEG.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 26.Salem R, Parikh P, Atassi B, Lewandowski RJ, Ryu RK, Sato KT, et al. Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol. 2008;31(5):431–438. doi: 10.1097/COC.0b013e318168ef65. [DOI] [PubMed] [Google Scholar]
- 27.Chiesa C, Mira M, Bhoori S, Bormolini G, Maccauro M, Spreafico C, et al. Radioembolization of hepatocarcinoma with (90)Y glass microspheres: treatment optimization using the dose-toxicity relationship. Eur J Nucl Med Mol Imaging. 2020;47(13):3018–3032. doi: 10.1007/s00259-020-04845-4. [DOI] [PubMed] [Google Scholar]
- 28.Elsayed M, Ermentrout RM, Sethi I, Bercu ZL, Galt JR, Whitmore M, et al. Incidence of radioembolization-induced liver disease and liver toxicity following repeat 90Y-radioembolization: outcomes at a large tertiary care center. Clin Nucl Med. 2020;45(2):100–104. doi: 10.1097/RLU.0000000000002828. [DOI] [PubMed] [Google Scholar]
- 29.Floridi C, Pesapane F, Angileri SA, De Palma D, Fontana F, Caspani F, et al. Yttrium-90 radioembolization treatment for unresectable hepatocellular carcinoma: a single-centre prognostic factors analysis. Med Oncol. 2017;34(10):174. doi: 10.1007/s12032-017-1021-3. [DOI] [PubMed] [Google Scholar]
- 30.Brown D, Krebs H, Brower J, O'Hara R, Wang E, Vaheesan K, et al. Incidence and risk factors for sustained hepatic function toxicity 6 months after radioembolization: analysis of the radiation-emitting sir-spheres in non-resectable liver tumor (RESIN) registry. J Gastrointest Oncol. 2021;12(2):639–657. doi: 10.21037/jgo-20-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 32.Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 33.Kallini JR, Gabr A, Thorlund K, Balijepalli C, Ayres D, Kanters S, et al. Comparison of the adverse event profile of therasphere((R)) with SIR-spheres((R)) for the treatment of unresectable hepatocellular carcinoma: a systematic review. Cardiovasc Intervent Radiol. 2017;40(7):1033–1043. doi: 10.1007/s00270-017-1594-4. [DOI] [PubMed] [Google Scholar]
- 34.Currie BM, Hoteit MA, Ben-Josef E, Nadolski GJ, Soulen MC. Radioembolization-induced chronic hepatotoxicity: a single-center cohort analysis. J Vasc Interv Radiol. 2019;30(12):1915–1923. doi: 10.1016/j.jvir.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Padia SA, Lewandowski RJ, Johnson GE, Sze DY, Ward TJ, Gaba RC, et al. Radioembolization of hepatic malignancies: background, quality improvement guidelines, and future directions. J Vasc Interv Radiol. 2017;28(1):1–15. doi: 10.1016/j.jvir.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(1):17–29. doi: 10.1016/S2468-1253(20)30290-9. [DOI] [PubMed] [Google Scholar]
- 37.van Roekel C, Bastiaannet R, Smits MLJ, Bruijnen RC, Braat A, de Jong H, et al. Dose-effect relationships of (166)Ho radioembolization in colorectal cancer. J Nucl Med. 2021;62(2):272–279. doi: 10.2967/jnumed.120.243832. [DOI] [PubMed] [Google Scholar]
- 38.Alsultan AA, van Roekel C, Barentsz MW, Smits MLJ, Kunnen B, Koopman M, et al. Dose-response and dose-toxicity relationships for glass (90)Y radioembolization in patients with liver metastases from colorectal cancer. J Nucl Med. 2021;62(11):1616–1623. doi: 10.2967/jnumed.120.255745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garin E, Tzelikas L, Guiu B, Chalaye J, Edeline J, De Baere T, Tacher V, Robert C, Assenat E, Terroir-Cassou-Mounat M, Regnault H, Palard X, Laffont S, Campillo-Gimenez B, Rolland Y. Major impact of personalized dosimetry using 90Y loaded glass microspheres SIRT in HCC: Final overall survival analysis of a multicenter randomized phase II study (DOSISPHERE-01). J Clin Oncol. 2020;38(4_suppl):516–516. doi: 10.1200/JCO.2020.38.4_suppl.516. [DOI] [Google Scholar]
- 40.Lang S, Velasco Garrido M, Heintze C. Patients' views of adverse events in primary and ambulatory care: a systematic review to assess methods and the content of what patients consider to be adverse events. BMC Fam Pract. 2016;17:6. doi: 10.1186/s12875-016-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mira JJ, Nebot C, Lorenzo S, Perez-Jover V. Patient report on information given, consultation time and safety in primary care. Qual Saf Health Care. 2010;19(5):e33. doi: 10.1136/qshc.2009.037978. [DOI] [PubMed] [Google Scholar]
- 42.Salem R, Hassan S, Lewandowski RJ, Grace K, Martin RCG, Sichlau MJ, et al. Quality of life after radioembolization for hepatocellular carcinoma using a digital patient-reported outcome tool. J Vasc Interv Radiol. 2020;31(2):311–4. doi: 10.1016/j.jvir.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Xing M, Kokabi N, Camacho JC, Kim HS. Prospective longitudinal quality of life and survival outcomes in patients with advanced infiltrative hepatocellular carcinoma and portal vein thrombosis treated with Yttrium-90 radioembolization. BMC Cancer. 2018;18(1):75. doi: 10.1186/s12885-017-3921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das A, Gabr A, O’Brian DP, Riaz A, Desai K, Thornburg B, Kallini JR, Mouli S, Lewandowski RJ, Salem R. Contemporary systematic review of health-related quality of life outcomes in locoregional therapies for hepatocellular carcinoma. J Vascul Int Radiol. 2019;30(12):1924–1933.e2. doi: 10.1016/j.jvir.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Kirchner T, Marquardt S, Werncke T, Kirstein MM, Brunkhorst T, Wacker F, et al. Comparison of health-related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdom Radiol. 2019;44(4):1554–1561. doi: 10.1007/s00261-018-1802-y. [DOI] [PubMed] [Google Scholar]
- 46.Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11(10):1358–65. doi: 10.1016/j.cgh.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 47.Bakhit M, Jones M, Baker J, Nair R, Yan K, Del Mar C, et al. Reporting of adverse events, conflict of interest and funding in randomised controlled trials of antibiotics: a secondary analysis. BMJ Open. 2021;11(7):e045406. doi: 10.1136/bmjopen-2020-045406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 49.Veitch ZW, Shepshelovich D, Gallagher C, Wang L, Abdul Razak AR, Spreafico A, et al. Underreporting of symptomatic adverse events in phase I clinical trials. J Natl Cancer Inst. 2021;113(8):980–988. doi: 10.1093/jnci/djab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.