Abstract
Significance.
IQOS is a heated tobacco product that has been widely advertised by Philip Morris International (PMI) as a reduced-exposure product compared to cigarettes. Reduced exposure results from reduced emission of toxicants which could be influenced by product constituents and user behavior. This study aims to assess the influence of user behavior, including device cleaning and puffing parameters, on toxicant emissions from IQOS.
Methods.
IQOS aerosols were generated by a smoking machine using the combination of two cleaning protocols (after one stick vs. 20 sticks) and five puffing regimes (including standard cigarette puffing regimes and IQOS-tailored regimes). The generated aerosols were analyzed by targeted methods for phenol and carbonyl quantification, and by chemical screening for the identification of unknown compounds.
Results.
Puffing parameters significantly affected phenol and carbonyl emissions while device cleaning had no effect. Harsher puffing conditions like more, longer, and larger puffs yielded higher levels for most toxicant emissions. Comparing the obtained data with data reported by PMI on 50 cigarette brands smoked under different puffing regimes showed various trends for phenol and carbonyl emissions, with IQOS emissions sometimes higher than cigarettes. Also, the chemical screening resulted in the tentative identification of ~100 compounds in the IQOS aerosols (most of limited toxicity data).
Conclusion.
This study showed that puffing parameters, but not device cleaning, have significant effects on carbonyl, phenol, and other emissions. Data analysis highlighted the importance of comparing IQOS emissions to an array of commercial cigarettes tested under different puffing regimes before accepting reduced exposure claims.
Keywords: IQOS, puffing parameters, device cleaning, carbonyls, phenols, chemical screening, toxicants
INTRODUCTION
Decades of research have linked cigarette smoking to deleterious health effects such as lung cancer, cardiovascular diseases, and chronic pulmonary diseases.[1–3] Cigarette smokers are usually aware of the health risks of this behavior, yet they fail to quit, due to their dependence on the psychoactive drug nicotine.[4] Recently, smokers have been offered alternative nicotine-delivering products that are supposedly less harmful compared to combustible cigarettes.[5] Examples of alternative tobacco products that are now available in the market include heated tobacco products (HTPs) and electronic nicotine delivery systems (ENDS), among others.[6, 7]
One of the newly introduced HTPs is IQOS, manufactured by Philip Morris International, Inc. (PMI).[8] IQOS releases nicotine-containing aerosol by heating a tobacco stick to 350 °C, lower than the 950 °C of a combustible cigarette.[9] IQOS is leading other HTPs in the global market, having about 17 million users across over 60 countries.[10] In July 2020, the US Food and Drug Administration (FDA) authorized IQOS to be marketed with claims of “reduced exposure” but not “reduced risk” in comparison to combustible cigarettes as part of the FDA modified risk tobacco product (MRTP) regulatory mechanism.[11] Thereafter, IQOS popularity and prevalence in the US population were anticipated to grow.[10] However, IQOS sales in the United States were halted in November 2021, due to possible infringement on patent rights with a rival tobacco company; IQOS is expected to return to the US market in 2023.[12, 13]
The MRTP application relied on data presented by PMI including chemical analysis of IQOS emissions, toxicity assessment, and assessment of the reduction in biomarkers of potential harm in smokers switching to IQOS for a short period.[14] Although the FDA deemed the evidence presented by PMI sufficient for a “reduced exposure” claim authorization, independent researchers examining PMI data or presenting their own data criticized the FDA decision.[6, 15, 16] Moreover, the MRTP application requires the assessment of the influence of use patterns on aerosol composition to ensure that the candidate MRTP consistently lowers user exposure to toxicants.[17, 18] User behavior is important in two aspects: the device cleaning between use sessions and the puffing regime that may alter the heating temperature. While most studies looked at the effect of the puffing regime on toxic emissions from IQOS,[16, 19–23] only one study looked at the effect of device cleaning.[24] According to this report, if the IQOS device is not cleaned properly, residues can accumulate in the heating chamber.[24, 25] We hypothesized that heating these residues may increase the emissions of toxicants or the formation of unexpected chemical compounds; this possibility was also highlighted in a recent review of the chemical analysis of IQOS emissions.[26] The current study was designed to evaluate the effect of user behavior, including crossover conditions of the puffing regime and device cleaning, on the emission of toxicants from IQOS. Specifically, we assessed the effect of residue build-up and puffing intensity on the emission of selected toxicants (phenols and carbonyls) as markers of pyrolysis of IQOS HeatStick constituents (i.e., glycerol and cellulose), and we screened IQOS aerosols for the formation of IQOS-specific toxicants.
MATERIALS AND METHODS
Materials
Gas sampling H Series Cartridges (H30, 6 mL capacity) with high-purity silica coated with 2,4-dinitrophenylhydrazine (DNPH) were procured from Sigma Aldrich. HPLC-grade ethanol was purchased from Fisher Scientific International. HPLC-grade tetrahydrofuran, ethyl acetate, acetonitrile, and ethanol were procured from Sigma-Aldrich. N,O-Bis (trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (25561-30-2) was purchased from Fluka. Quartz Fiber Filter pads (QR-100, 47 mm) were obtained from ADVENTEC. Deuterated standards of p-cresol-d8 and benzene-d6 were procured from Absolute Standards and used as internal standards (IS) for phenol quantification. Hydrochloric acid (HCl; 37%; CAS 7647-01-0), ascorbic acid (CAS 50-81-7), and sodium bicarbonate (CAS 144-55-8) were procured from Sigma-Aldrich and Fluka. IQOS devices (IQOS 2.4), including Marlboro HEETs (aka HeatSticks; Amber tobacco flavor), were bought from the official website in the US in 2021.
Study design
The study was designed to include two cleaning protocols (CP-1 and CP-2) and five puffing regimes (PRs): Health Canada Intense (HCI), International Standardization Organization (ISO), and PR-1, PR-2, and PR-3. The cleaning protocol CP-1 represents the behavior of an extremely cautious user that cleans the device after each use while CP-2 (the recommended cleaning protocol by the manufacturer) represents a user that cleans the device after 20 sticks. The details of the puffing parameters and experimental design are shown in Figure 1. CP-2 was tested with all the puffing regimes, while CP-1 was only tested with HCI and ISO.[24] This procedure allowed an understanding of the effect of device cleaning and puffing parameters on the generated toxicants, mainly phenols and carbonyls. For chemical screening, only ISO, HCI, and PR-3 were tested with CP-2. Puffing regimes included HCI and ISO for direct comparison with combustible cigarette data and PR-1, PR-2, and PR-3 that were arrived at using the limited published literature on IQOS users’ puffing behavior.[27–29] These regimes cover the variations that may be experienced by users in puffing rate and intensity (puffing flow rate, puff duration, and puff volume), and frequency (number of puffs and inter-puff intervals).
Figure 1.
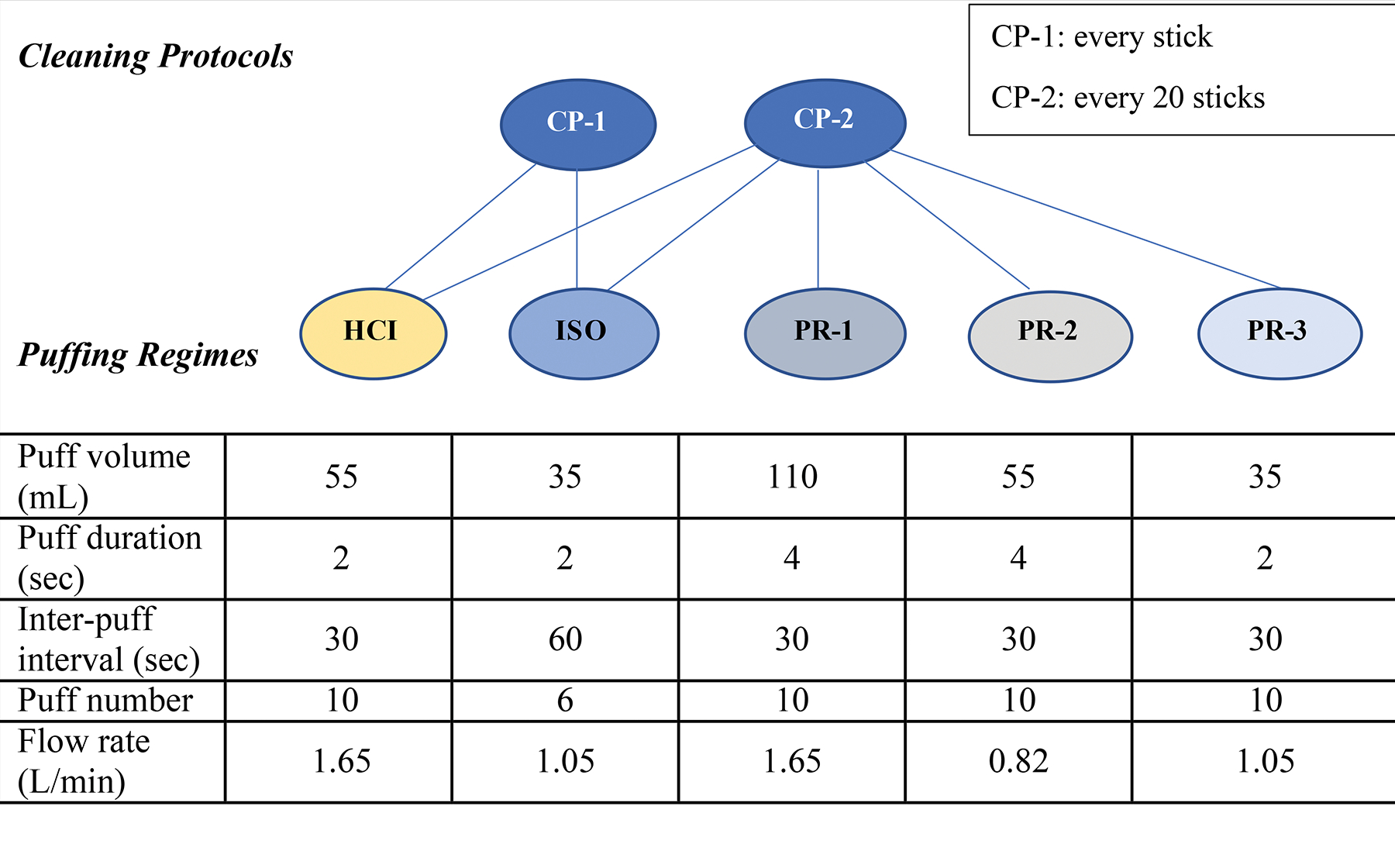
Study design with details of cleaning protocols (CP) and puffing regimes (PR).
Aerosol generation and sampling
The American University of Beirut Aerosol Research Lab Vaping Instrument (ALVIN), was used to generate IQOS aerosols.[30] The generated aerosols were sampled and analyzed by targeted and chemical screening methods, under different smoking parameters, for a comprehensive analysis of IQOS emissions. Figure 2 shows the experimental setup that was used to generate IQOS aerosols in two independent sampling sets (S1 and S2) conducted on different days. For S1, the particulate phase of aerosol was collected on filter pads (Pall Type A/E, 47 mm) for the quantification of phenols and post hoc chemical screening, and the gaseous phase from S1 was trapped using a DNPH cartridge for the quantification of carbonyls. For S2, the particulate phase was trapped on filters and the gaseous phase was cryogenically trapped at −50 °C using two methanol-containing impingers connected in series and placed after the filter. The filter was extracted with ethyl acetate, and aliquots of these extracts and the impingers were screened on gas chromatography-tandem mass spectrometry (GC-MS).
Figure 2.
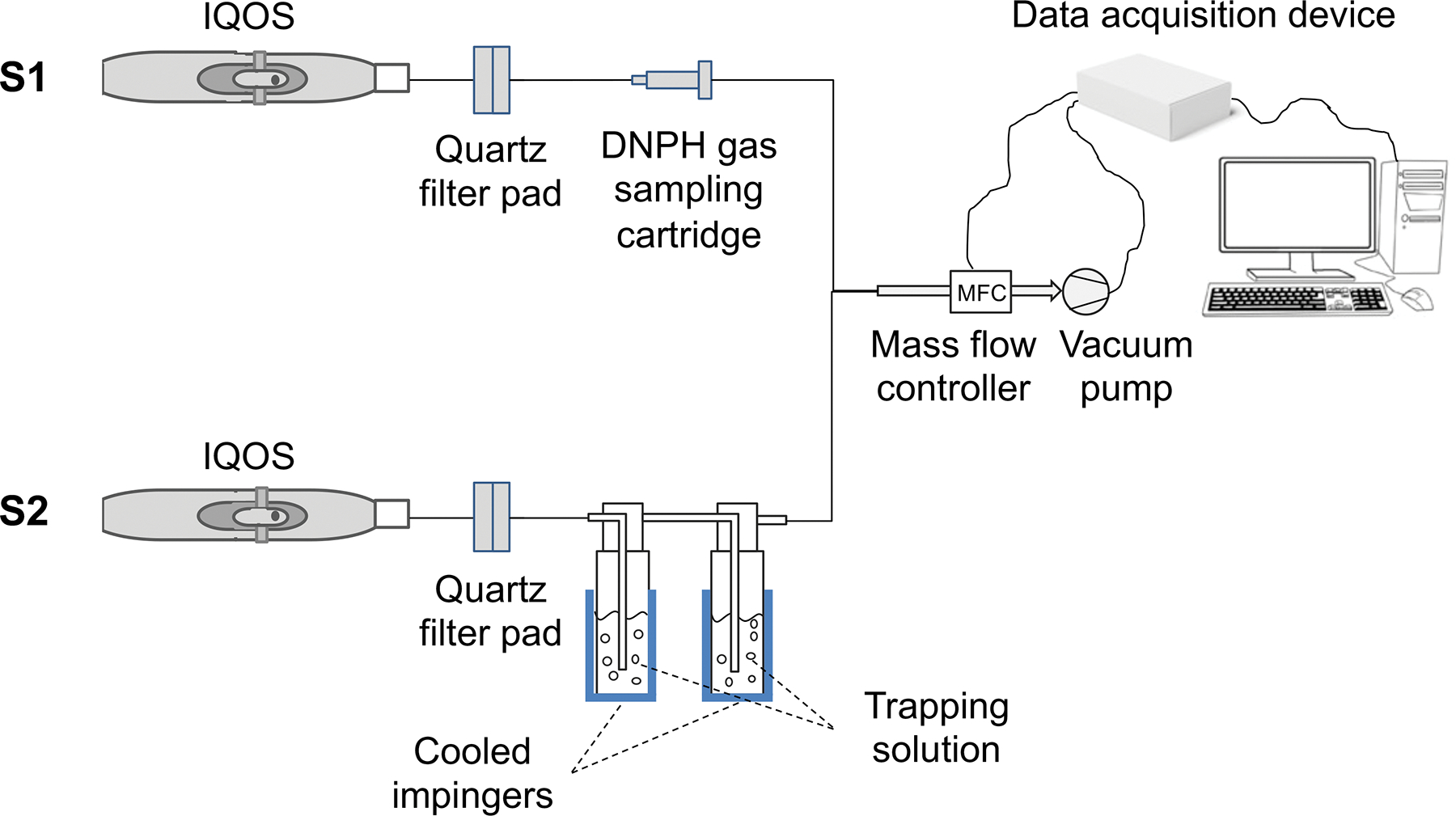
Schematic of the experimental setup that was used to assess IQOS emissions. The two sampling sets, S1 and S2, were conducted separately for the quantification of carbonyls and phenols, and the screening for IQOS-specific toxicants, respectively. S1 and S2 were schematically combined for simplicity.
Phenol Quantification
Four phenols (phenol, catechol, hydroquinone, and resorcinol) were extracted from filter pads using a liquid-liquid extraction method developed by our lab.[31] In brief, this method includes immersing the filter pad in an acidic solution to remove nicotine, then, ethyl acetate was added to extract the phenolic compounds. The extracted phenolic compounds were derivatized using BSTFA before analysis on GC-MS. The total particulate matter (TPM) collected on the filter pad was measured gravimetrically.
Carbonyl Quantification
Carbonyls were extracted from the 2,4-DNPH-coated silica cartridges by a mixture of acetonitrile and ethanol (10/90 v/v) and run through high-performance liquid chromatography (HPLC) for analysis as described before.[32] The analyzed carbonyls include formaldehyde, acetaldehyde, acetone, acrolein, crotonaldehyde, propionaldehyde, and butyraldehyde.
Chemical Screening
For the chemical screening of the S1 particle phase, the collected GC-MS spectra were screened post-hoc against the NIST library.
For S2, both particle and gas phase aliquots were tested for chemical screening. The collected gases using the impingers were injected directly while a two-step extraction was done on the particle phase that was captured on quartz filters for matrix reduction. Benzene-d6 was used as IS for semi-quantification. For the first step, the filter was extracted with ethyl acetate and an aliquot of the extract was injected into GC-MS for chemical screening. The remaining organic phase was further extracted with acidified water. This step removed nicotine, propylene glycol, and glycerol from the sample, which are the main contributors to the particulate matter. An aliquot of the organic phase was then injected into GC-MS. Both extracts were spiked with benzene-d6 as IS. For all chemical screening experiments, peak deconvolution was done manually, and the resulting peaks were compared against the NIST library. A peak that has a match and a reverse match factor of >750 was considered acceptable. Semi-quantification was done using the IS peak areas and concentration.
GC-MS method for chemical screening
The GC-MS analysis was performed on a Thermo Trace GC ITQ-900 instrument equipped with a thermostatically controlled AI 3000 autosampler and a TG-5MS Thermo Scientific fused silica capillary GC column (30 m × 0.25 mm × 0.25 μm). The mass spectrometer ionization mode was electron ionization (EI) at 70 eV. The carrier gas was helium delivered at a flow rate of 1 mL/min. The injector temperature was 250 °C, with a splitless injection of 1 μL using a single tapper gooseneck, deactivated, glass wool-free liner. The oven temperature program was as follows: hold at 70 °C for 1 min, ramp up 10 °C/min to 200 °C, ramp up 40 °C/min to 250 °C, and hold for 1 min. The total run time was 45min, and the solvent delay time was 3 min and 4.5min for the methanol and ethyl acetate samples, respectively.
Statistical analysis
The association between IQOS emissions with device cleanness and puffing parameters including puffing duration, number of puffs, and flow rate was analyzed using a general linear model statistical analysis (GLM). Since puff volume is the result of flow rate multiplied by puff duration, it was not included in the analysis. Statistical analyses were performed using SPSS version 25.0 (significance at p < 0.05).
RESULTS
Table 1 shows the summary of the quantification of carbonyl and phenol emissions in IQOS aerosols generated using a smoking machine. The data showed that regardless of cleaning and puffing conditions, IQOS emitted an average of 25.14 ± 8.38 mg/stick of total particulate matter (TPM) with relative standard deviations (RSD) within each condition ranging from 0.7–33.7%. The carbonyls and phenols could vary slightly (e.g., acetaldehyde and propionaldehyde) or by orders of magnitude (e.g., acrolein) depending on the IQOS use conditions (i.e., CP and PR). Globally, HCI puffing conditions yielded higher levels of emissions compared to ISO, comparable to PR2 and PR3, and lower than PR1. There was no trend of variation in toxicant emissions correlated with device cleanness, i.e., toxicants from the twentieth stick with residue buildup (Figure S1) were comparable to those from the first stick for CP2, and similarly, emissions before and after cleaning the device in CP1 showed no significant difference.
Table 1.
Summary of data on carbonyl and phenol emissions (μg/stick) from IQOS under two cleaning protocols and five puffing regimes.
| Cleaning Protocol | Puffing Regime | Stick Number* | TPM (mg) | FA | AA | Ac | ACr | PA | CA | BA | Phenol | Catechol | Resorcinol | HQ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP1 | HCI | 1 | 28.30 ± 8.25 | 1.42 ± 0.30 | 176.94 ± 3.58 | 43.52 ± 1.72 | 0.29 ± 0.06 | 15.75 ± 0.39 | 5.94 ± 0.28 | 22.29 ± 5.19 | 0.70 ± 0.20 | 12.82 ± 2.41 | 0.14 ± 0.00 | 6.59 ± 2.91 |
| 2 | 32.70 ± 1.13 | 2.20 ± 0.37 | 176.97 ± 7.84 | 43.66 ± 2.05 | 0.26 ± 0.03 | 16.75 ± 0.39 | 6.10 ± 0.60 | 19.34 ± 0.65 | 0.71 ± 0.23 | 9.84 ± 3.47 | 0.13 ± 0.00 | 2.69 ± 3.45 | ||
| ISO | 1 | 15.60 ± 2.00 | 1.05 ± 0.18 | 141.13 ± 6.14 | 34.77 ± 1.11 | 0.08 ± 0.14 | 10.95 ± 1.39 | 4.17 ± 0.77 | 14.96 ± 0.39 | 0.37 ± 0.03 | 5.54 ± 0.54 | 0.12 ± 0.00 | 0.67 ± 0.58 | |
| 2 | 16.37 ± 0.12 | 0.95 ± 0.15 | 148.77 ± 4.07 | 35.33 ± 0.93 | 0.24 ± 0.21 | 11.00 ± 2.39 | 4.00 ± 0.17 | 14.49 ± 0.36 | 0.31 ± 0.01 | 5.71 ± 1.10 | 0.12 ± 0.00 | 3.27 ± 0.72 | ||
| CP2 | HCI | 1 | 28.87 ± 9.62 | 1.31 ± 0.66 | 179.43 ± 4.69 | 44.06 ± 1.42 | 4.64 ± 0.79 | 10.07 ± 7.39 | 5.97 ± 0.39 | 22.58 ± 0.86 | 0.62 ± 0.15 | 13.08 ± 1.04 | 0.13 ± 0.00 | 3.33 ± 2.97 |
| 5 | 29.07 ± 4.40 | 1.98 ± 0.34 | 170.59 ± 17.27 | 43.31 ± 2.15 | 4.94 ± 0.58 | 9.44 ± 5.39 | 5.92 ± 0.13 | 21.72 ± 1.83 | 0.72 ± 0.06 | 12.37 ± 2.95 | 0.13 ± 0.00 | 3.33 ± 2.72 | ||
| 10 | 27.77 ± 3.41 | 2.30 ± 0.23 | 166.07 ± 11.32 | 42.58 ± 2.07 | 5.39 ± 0.24 | 9.07 ± 2.39 | 5.84 ± 0.46 | 21.65 ± 0.78 | 0.73 ± 0.26 | 10.78 ± 3.65 | 0.12 ± 0.01 | 3.77 ± 2.61 | ||
| 20 | 30.07 ± 0.40 | 2.69 ± 1.29 | 176.35 ± 16.23 | 44.39 ± 1.05 | 5.75 ± 1.05 | 9.73 ± 0.39 | 6.34 ± 0.56 | 22.61 ± 1.38 | 0.71 ± 0.04 | 13.31 ± 2.60 | 0.13 ± 1.00 | 5.07 ± 2.53 | ||
| 21 | 26.30 ± 1.85 | 2.45 ± 0.36 | 172.50 ± 0.45 | 40.71 ± 4.77 | 5.37 ± 0.20 | 9.59 ± 2.39 | 5.94 ± 0.53 | 21.96 ± 0.92 | 0.69 ± 0.03 | 12.61 ± 1.67 | 0.13 ± 0.00 | 5.05 ± 0.65 | ||
| ISO | 1 | 10.43 ± 1.17 | 0.41 ± 0.25 | 125.64 ± 18.22 | 33.97 ± 2.09 | 0.89 ± 0.50 | 7.07 ± 5.39 | 3.55 ± 0.73 | 15.00 ± 1.15 | 0.11 ± 0.04 | 4.15 ± 2.43 | 0.12 ± 0.01 | 0.88 ± 0.62 | |
| 5 | 11.57 ± 3.90 | 0.40 ± 0.14 | 131.33 ± 6.76 | 34.52 ± 2.30 | 1.03 ± 0.14 | 7.64 ± 1.39 | 3.32 ± 0.35 | 16.06 ± 1.55 | 0.14 ± 0.13 | 4.15 ± 0.70 | 0.12 ± 0.00 | 0.81 ± 0.43 | ||
| 10 | 13.47 ± 4.35 | 0.66 ± 0.14 | 126.82 ± 12.24 | 24.12 ± 0.81 | 1.26 ± 0.19 | 7.44 ± 1.39 | 3.32 ± 0.12 | 15.53 ± 2.20 | 0.30 ± 0.38 | 6.53 ± 0.59 | 0.12 ± 0.00 | 1.66 ± 1.37 | ||
| 20 | 12.01 ± 2.16 | 0.48 ± 0.20 | 130.10 ± 12.96 | 35.15 ± 3.53 | 0.80 ± 0.50 | 7.51 ± 5.39 | 3.21 ± 0.50 | 16.02 ± 1.91 | 0.41 ± 0.47 | 4.88 ± 0.54 | 0.12 ± 0.01 | 1.33 ± 0.66 | ||
| 21 | 12.87 ± 2.86 | 0.78 ± 0.54 | 131.54 ± 9.06 | 36.22 ± 1.32 | 1.13 ± 0.32 | 7.46 ± 3.39 | 3.37 ± 0.32 | 16.08 ± 0.83 | 0.18 ± 0.19 | 6.48 ± 1.76 | 0.12 ± 0.00 | 2.65 ± 1.60 | ||
| PR1 | 1 | 35.07 ± 4.33 | 3.59 ± 1.11 | 172.77 ± 0.99 | 46.46 ± 0.42 | 9.51 ± 1.60 | 9.84 ± 6.39 | 8.73 ± 0.41 | 22.58 ± 1.07 | 2.02 ± 0.62 | 14.12 ± 0.68 | 0.13 ± 1.00 | 4.65 ± 2.59 | |
| 5 | 35.53 ± 7.05 | 4.44 ± 0.91 | 180.82 ± 7.16 | 49.81 ± 2.17 | 9.98 ± 0.30 | 10.40 ± 3.39 | 9.26 ± 0.56 | 23.81 ± 0.64 | 2.04 ± 0.39 | 23.67 ± 14.11 | 0.20 ± 0.12 | 22.98 ± 31.81 | ||
| 10 | 31.57 ± 2.23 | 5.20 ± 0.27 | 189.15 ± 10.58 | 50.50 ± 0.59 | 11.45 ± 0.15 | 10.89 ± 1.39 | 9.79 ± 0.41 | 24.46 ± 1.44 | 2.64 ± 0145 | 15.97 ± 0.46 | 0.14 ± 0.00 | 4.63 ± 0.45 | ||
| 20 | 35.53 ± 0.65 | 4.69 ± 0.94 | 187.62 ± 8.63 | 51.57 ± 0.94 | 11.14 ± 0.58 | 11.07 ± 5.39 | 9.92 ± 1.54 | 24.32 ± 1.13 | 2.81 ± 0.04 | 19.39 ± 2.26 | 0.14 ± 0.00 | 5.72 ± 1.21 | ||
| 21 | 34.37 ± 6.05 | 5.85 ± 1.20 | 195.68 ± 13.48 | 50.70 ± 3.45 | 11.52 ± 1.14 | 11.35 ± 1.39 | 9.50 ± 2.26 | 23.69 ± 2.90 | 2.51 ± 0.72 | 16.75 ± 6.37 | 0.13 ± 1.01 | 4.66 ± 2.98 | ||
| PR2 | 1 | 27.30 ± 4.42 | 1.00 ± 0.55 | 190.70 ± 13.78 | 48.73 ± 1.56 | 3.53 ± 0.90 | 10.50 ± 9.39 | 5.28 ± 0.18 | 23.60 ± 1.63 | 0.40 ± 0.15 | 15.08 ± 0.41 | 0.14 ± 0.00 | 3.98 ± 1.56 | |
| 5 | 29.63 ± 0.95 | 1.27 ± 0.67 | 191.50 ± 11.99 | 49.80 ± 1.93 | 3.98 ± 0.01 | 10.52 ± 0.39 | 5.44 ± 0.14 | 24.08 ± 2.29 | 0.42 ± 0.55 | 16.55 ± 0.79 | 0.14 ± 0.00 | 6.30 ± 2.11 | ||
| 10 | 28.37 ± 1.46 | 1.69 ± 0.54 | 198.59 ± 3.29 | 52.17 ± 1.68 | 4.09 ± 0.59 | 10.97 ± 5.39 | 5.61 ± 0.41 | 24.75 ± 3.30 | 0.52 ± 0.11 | 16.68 ± 2.10 | 0.14 ± 0.00 | 4.82 ± 2.38 | ||
| 20 | 28.47 ± 3.06 | 1.44 ± 0.87 | 193.92 ± 12.24 | 50.65 ± 2.60 | 4.64 ± 0.09 | 10.79 ± 0.39 | 5.72 ± 0.57 | 25.11 ± 1.19 | 0.43 ± 0.21 | 13.68 ± 1.37 | 0.13 ± 0.01 | 4.40 ± 0.66 | ||
| 21 | 26.83 ± 1.63 | 1.91 ± 1.09 | 184.81 ± 17.79 | 48.82 ± 3.35 | 4.98 ± 1.07 | 10.15 ± 0.39 | 5.78 ± 0.71 | 23.82 ± 0.55 | 0.53 ± 1.22 | 15.04 ± 1.96 | 0.13 ± 1.01 | 6.56 ± 2.54 | ||
| PR3 | 1 | 29.50 ± 2.09 | 0.48 ± 0.25 | 174.45 ± 24.91 | 42.28 ± 4.27 | 2.21 ± 0.60 | 10.59 ± 6.39 | 3.91 ± 0.33 | 19.62 ± 1.14 | 0.19 ± 0.15 | 26.07 ± 15.83 | 0.17 ± 0.05 | 19.44 ± 19.87 | |
| 5 | 22.33 ± 2.72 | 0.65 ± 0.13 | 163.50 ± 6.68 | 40.88 ± 1.47 | 2.09 ± 0.12 | 8.98 ± 1.39 | 3.67 ± 0.33 | 19.55 ± 2.76 | 0.22 ± 0.06 | 29.06 ± 15.47 | 0.17 ± 0.05 | 17.94 ± 16.80 | ||
| 10 | 25.27 ± 7.18 | 1.04 ± 0.49 | 174.34 ± 21.74 | 44.36 ± 7.83 | 2.98 ± 0.41 | 9.53 ± 4.39 | 4.34 ± 1.27 | 20.46 ± 3.97 | 0.53 ± 0.58 | 14.35 ± 4.92 | 0.13 ± 0.01 | 6.12 ± 2.94 | ||
| 20 | 19.53 ± 1.74 | 0.55 ± 0.26 | 156.67 ± 11.04 | 39.31 ± 1.65 | 1.73 ± 0.41 | 8.39 ± 4.39 | 3.36 ± 0.34 | 18.77 ± 0.77 | 0.17 ± 0.01 | 28.70 ± 19.56 | 0.15 ± 0.03 | 11.69 ± 8.18 | ||
| 21 | 24.30 ± 1.57 | 0.95 ± 0.43 | 173.71 ± 5.83 | 43.54 ± 3.71 | 2.68 ± 0.52 | 9.30 ± 5.39 | 4.23 ± 0.57 | 20.92 ± 0.46 | 0.24 ± 0.01 | 12.02 ± 1.27 | 0.13 ± 0.00 | 6.01 ± 1.08 |
TPM: total particulate matter. FA: Formaldehyde, AA: Acetaldehyde, Ac: Acetone, ACr: Acrolein, PA: Propionaldehyde, CA: crotonaldehyde, BA: Butyraldehyde, HQ: Hydroquinone.
Sticks were numbered and sampled before and after cleaning for CP-1 and at different time points for CP-2; sticks whose numbers are not shown in the table were not sampled.
Impact of User Behavior on Toxicants
For device cleaning, the data were organized and coded as new (stick 1 for CP1 and CP2), cleaned (stick 2 for CP1 and 21 for CP2), and aged (stick 5th, 10th, and 20th for CP2) before running the GLM. Our statistical analysis of the carbonyl and phenol data across the different conditions showed that puff duration, number of puffs, and flow rate, but not cleaning condition, have significant effects on IQOS emissions (Table 2). Head-to-head comparison between the 20th stick emissions for two puffing regimes that differ only in puff duration (i.e., HCI and PR-1) showed that longer puff duration yielded higher emissions of all toxicants. The same comparison between PR-1 and PR-2 (flow rate is different) showed that a higher flow rate yielded higher emissions of all toxicants except for AA and BA. Also, a higher number of puffs (PR-3 versus ISO) yielded higher emissions across the board.
Table 2.
Statistical analysis of the impact of user behavior on IQOS emissions.
| Cleaning Protocol | Puff duration (sec) | Number of puffs | Flow rate (L/min) | |
|---|---|---|---|---|
|
| ||||
| TPM | *** | *** | *** | |
| Formaldehyde | *** | *** | ||
| Acetaldehyde | *** | *** | ||
| Acetone | *** | *** | ||
| Acrolein | *** | *** | ||
| Propionaldehyde | ** | |||
| Crotonaldehyde | *** | *** | ||
| Methacrolein | *** | *** | ** | |
| Butyraldehyde | *** | *** | ||
| Glyoxal | *** | |||
| Methylglyoxal | *** | |||
|
| ||||
| Total Carbonyls | *** | *** | *** | |
|
| ||||
| Phenol | * | *** | ** | *** |
| Catechol | *** | |||
| Resorcinol | * | |||
| Hydroquinone | ** | |||
|
| ||||
| Total Phenols | *** | |||
P < 0.05
P < 0.01
P < 0.001.
Chemical Screening
The chemical screening on phenol filters from S1 resulted in the identification of a total number of 56 compounds, as shown in Supplementary Material (Table S1). However, in the chemical screening conducted on the gaseous and particulate phases from S2, 98 compounds were detected across the different puffing regimes. Interestingly, different numbers of chemicals and profiles were detected according to puffing conditions. For example, for the ISO puffing regime, 43 compounds were identified, with 8 compounds detected in the gas phase trap while 35 compounds were detected in the particulate phase trapped on a filter pad. However, for HCI, 56 compounds were detected in total, with 9 compounds in the gas phase and 47 compounds in the particulate phase. When PR-1, PR-2, and PR-3 were used, a total number of 52, 36, and 42 compounds were detected, respectively. The data for the semi-quantification of these emissions are included in the Supplementary Material (Table S2).
DISCUSSION
We found that puff duration, flow rate, and the number of puffs had significant effects on toxicant emissions from IQOS while device cleaning did not. For puffing parameters, longer puff duration, higher puffing flow rate, and more puffs yielded higher emissions (e.g., formaldehyde, acetaldehyde, and catechol). However, a recent systematic review combining data from studies on IQOS emissions showed no significant effects of puffing parameters on carbonyl emissions.[33] There is a need for industry-independent studies that systematically assess the impact of puffing parameters on IQOS emissions.[19, 34, 35] Moreover, the findings in our current study call for the development of specific puffing regimes obtained from human puffing recording studies for the accurate assessment of IQOS emissions. For example, experienced IQOS users might be brought into a laboratory setting to use IQOS ad libitum while their puff topography is recorded, and the resulting puff topography records could be used to program a smoking machine that replicates IQOS-experienced participants’ behavior.[36] Otherwise, studies that compare IQOS to cigarettes using “standard” regimes (i.e., ISO and HCI) could be misleading.
Most studies on IQOS are largely focused on specific compounds of toxicological interest, such as the FDA list of harmful and potentially harmful constituents (HPHCs),[19, 20, 37, 38] with only two non-targeted analyses or chemical screening that were reported by the manufacturer.[39, 40] Toxicant characterization for IQOS aerosols to date has been guided by the list of HPHCs known to be present in combustible cigarette smoke.[39, 41, 42] This list includes nicotine, humectants, tobacco-specific nitrosamines (TSNAs), gases (carbon monoxide, ammonia, and hydrogen cyanide), radicals, carbonyls, VOCs, phenols, aromatic amines (AAs), and polycyclic aromatic hydrocarbons (PAHs).[33] Most reports showed equivalent delivery of nicotine from IQOS compared to cigarettes and a significant decrease in the levels of toxicants like VOCs, AAs, small gases, radicals, phenols, and PAHs, and partial reduction in carbonyls and TSNAs.[19, 20, 37, 38] This significant reduction in the levels of the majority of the analyzed HPHCs is attributed to the low temperature reached in IQOS preventing high-temperature pyrolysis and pyrosynthesis usually encountered in cigarette combustion.[20] However, most of these comparisons are made with one cigarette comparator (e.g., 3R4F) with IQOS aerosols and cigarette smoke generated mostly under HCI conditions.[33] For a more comprehensive approach, we computed the relative reduction of carbonyl and phenols in IQOS aerosol in our data in comparison to emissions in the smoke generated from combustible cigarettes under HCI, ISO, and Massachusetts Department of Public Health (MDPH) smoking regimes as reported by PMI in Counts et al. 2005 (Figure 3).[43] The cigarette data are reported as range limits of all cigarettes under a given puffing regime (e.g., Min cig-ISO is the lowest yield for cigarette smoke generated under ISO conditions; Min cig-ISO is not necessarily the same cigarette as Min cig-HCI or Min cig-MDPH)
Figure 3.
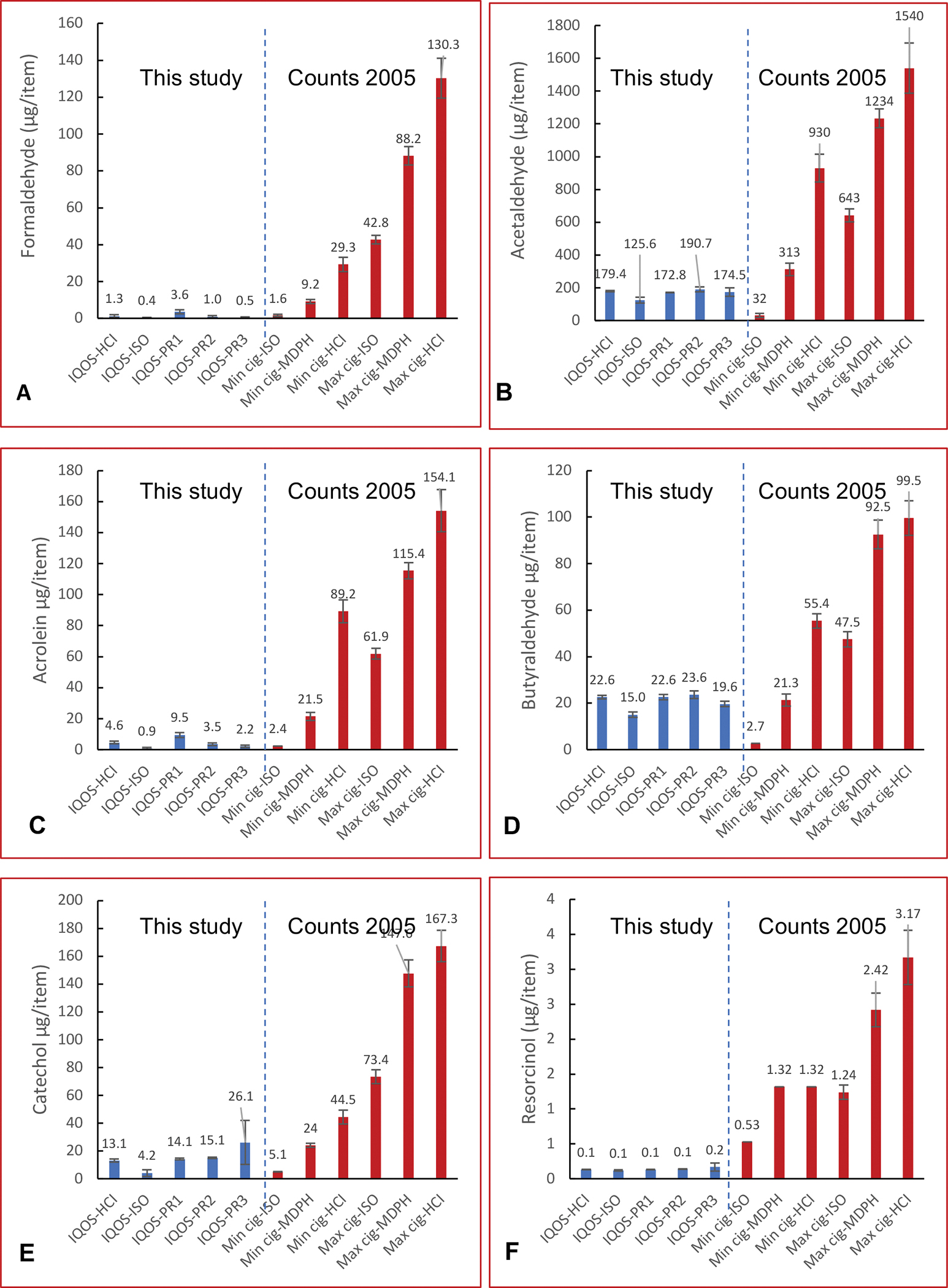
Comparison of phenol and carbonyl IQOS emissions from this work to combustible cigarette emissions under different puffing conditions from Counts et al. 2005. Data on cigarettes are shown as the minimum (min) and maximum (max) for each puffing regime.
The comparison depicted in Figure 3 reveals different trends for different toxicants. For some toxicants, there was a robust reduction of up to 99% with respect to all comparators under all puffing conditions (e.g., resorcinol). However, other toxicants showed significant reduction under all conditions except when compared to Min Cig smoke generated under ISO conditions (e.g., formaldehyde and acrolein). In contrast, for certain toxicants, moderate to significant reduction but sometimes significant increase (up to 736%) when IQOS emissions were compared to Min Cig smoke generated under ISO or MDPH puffing conditions (e.g., acetaldehyde, butyraldehyde, and catechol). The comparison of IQOS with a wider range of cigarettes gives a better insight that under certain puffing conditions not all emissions are reduced in IQOS aerosol.[33] Similarly, a report examined PMI data to show significant increases in emissions from IQOS compared to reference cigarettes.[15]
The post-hoc chemical screening conducted on S1 resulted in the identification of 56 compounds. The chemical classes included alkanes, carboxylic acids, ketones, aromatic acids, esters, and substituted hydrocarbons. The toxicity data of most of these compounds remain unknown. However, some of them have been reported to have detrimental effects on human health. For instance, the International Agency for Research on Cancer (IARC) has classified both catechol and 3-chloro-1,2-propanediol as Group 2B, possible human carcinogens.[44, 45]. The chemical screening conducted on S2 resulted in the identification of more compounds (n=96) because of the two-step extraction method that was used to reduce interference from dominant compounds such as humectants. Our chemical screening data were compared with the data reported by PMI-affiliates (Supplementary Material, Table S2),[39] the only available comprehensive chemical characterization of IQOS emissions. The comparison showed that only 28% of our compounds matched PMI’s list, although some of the compounds showed close structural similarity with those identified by PMI. This disparity might be due to variations in the library matching software, the sample preparation, or the less sophisticated analytical instrument used in our work. It should be noted that our screening was done in triplicates in contrast to PMI’s data.
This work has some limitations. Our targeted analysis only focused on carbonyls and phenols, for which reheating of residue buildup (i.e., device cleanliness) did not affect the emissions. However, we recommend further assessment of other emissions like PAHs and solid carbon to determine how reheating of the residue can affect these emissions.[46] Also, because of the low sensitivity of the one-dimensional GC that was used in the chemical screening, and due to the complexity of the analyte matrix leading to the co-elution of peaks, the number of identified compounds was low. In addition, due to the lack of workflow software that uses algorithms for peak deconvolution and library searching, this work was done manually, hence lowering the accuracy of peak identification.
Conclusion
The effect of user behavior which includes puffing regimes and device cleaning on the emission of toxicants showed that puffing parameters such as puff duration, flow rate, and the number of puffs can affect the composition of IQOS aerosol. When compared to an array of cigarettes, IQOS did not have consistently reduced toxicant emissions.
Supplementary Material
What is already known on this topic:
IQOS is a heated tobacco product that is gaining prominence worldwide. IQOS produces aerosol at a significantly lower temperature compared to cigarettes leading to reduced emission of some toxicants compared to selected cigarettes (e.g., 3R4F reference cigarette).
What this study adds:
This study showed that user behavior, mainly the puffing regime, has a significant effect on IQOS emissions. It highlights the importance of generating IQOS aerosol under a range of puffing conditions, including those derived from studies of IQOS users, and comparing emissions to an array of commercially available cigarettes to assess any potential real-life reduction in users’ exposure to toxicants.
How this study might affect research, practice, or policy-
The effect of user behavior and the cigarette comparator should be considered carefully during new tobacco product evaluation and regulation.
Funding.
This research was supported by a Rapid Response Project award under grant number of U54DA036105 from the National Institute on Drug Abuse of the National Institutes of Health (NIH) and the Center for Tobacco Products (CTP) of the U.S. Food and Drug Administration (FDA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Footnotes
Conflict of Interest. TE and AS are paid consultants in litigation against the tobacco and e-cigarette industry and are named on one patent for a device that measures the puffing behavior of e-cigarette users and a patent application for a smoking cessation intervention. TE is also named on a patent application for a smartphone app that determines e-cigarette device and liquid characteristics. All other authors declare no conflict of interest.
References
- 1.Brawley OW, Glynn TJ, Khuri FR, et al. The first surgeon general’s report on smoking and health: The 50th anniversary. CA: A Cancer Journal for Clinicians. 2014;64(1):5–8. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder SA, Koh HK. Tobacco Control 50 Years After the 1964 Surgeon General’s Report. JAMA. 2014;311(2):141–3. [DOI] [PubMed] [Google Scholar]
- 3.Smith CJ, Perfetti TA, Garg R, et al. IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem Toxicol. 2003;41(6):807–17. [DOI] [PubMed] [Google Scholar]
- 4.Murray JB. Nicotine as a Psychoactive Drug. The Journal of Psychology. 1991;125(1):5–25. [DOI] [PubMed] [Google Scholar]
- 5.Hatsukami DK, Carroll DM. Tobacco harm reduction: Past history, current controversies and a proposed approach for the future. Prev Med. 2020;140:106099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glantz SA. Heated tobacco products: the example of IQOS. Tobacco Control. 2018;27:S1–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breland A, Soule E, Lopez A, et al. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci. 2017;1394(1):5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MR, Clark B, Lüdicke F, et al. Evaluation of the Tobacco Heating System 2.2. Part 1: Description of the system and the scientific assessment program. Regulatory Toxicology and Pharmacology. 2016;81:S17–S26. [DOI] [PubMed] [Google Scholar]
- 9.Kopa PN, Pawliczak R. IQOS ? a heat-not-burn (HnB) tobacco product ? chemical composition and possible impact on oxidative stress and inflammatory response. A systematic review. Toxicology Mechanisms and Methods. 2020;30(2):81–7. [DOI] [PubMed] [Google Scholar]
- 10.Abroms L, Levine H, Romm K, et al. Anticipating IQOS market expansion in the United States. Tob Prev Cessat. 2022;8:04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA. FDA Authorizes Marketing of IQOS Tobacco Heating System with ‘Reduced Exposure’ Information 2020. [Available from: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-iqos-tobacco-heating-system-reduced-exposure-information.
- 12.Lucas A Altria can’t sell Iqos in the U.S. as Biden administration opts to not intervene in patent dispute 2021. [Available from: https://www.cnbc.com/2021/11/30/altria-cant-sell-iqos-in-us-as-biden-wont-intervene-in-patent-dispute.html.
- 13.TobaccoReporter. PMI to Make IQOS in the U.S. After Import Ban 2022. [Available from: https://tobaccoreporter.com/2022/02/11/pmi-to-manufacture-iqos-in-the-u-s-following-import-ban/#:~:text=In%20July%202020%2C%20the%20FDA,the%20first%20half%20of%202023.
- 14.Seidenberg AB, Popova L, Ashley DL, et al. Inferences beyond a claim: a typology of potential halo effects related to modified risk tobacco product claims. Tobacco Control. 2020:tobaccocontrol-2019–055560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St.Helen G, Jacob III P, Nardone N, et al. IQOS: examination of Philip Morris International’s claim of reduced exposure. Tobacco Control. 2018;27(Suppl 1):s30–s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popova L, Lempert LK, Glantz SA. Light and mild redux: heated tobacco products’ reduced exposure claims are likely to be misunderstood as reduced risk claims. Tobacco Control. 2018;27:S87–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MR, Clark B, Lüdicke F, et al. Evaluation of the Tobacco Heating System 2.2. Part 1: Description of the system and the scientific assessment program. Regul Toxicol Pharmacol. 2016;81 Suppl 2:S17–s26. [DOI] [PubMed] [Google Scholar]
- 18.FDA. Modified Risk Tobacco Products 2022. [Available from: https://www.fda.gov/tobacco-products/advertising-and-promotion/modified-risk-tobacco-products.
- 19.Schaller JP, Keller D, Poget L, et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul Toxicol Pharmacol. 2016;81 Suppl 2:S27–s47. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Luo Y, Jiang X, et al. Chemical Analysis and Simulated Pyrolysis of Tobacco Heating System 2.2 Compared to Conventional Cigarettes. Nicotine Tob Res. 2019;21(1):111–8. [DOI] [PubMed] [Google Scholar]
- 21.Salman R, Talih S, El-Hage R, et al. Free-Base and Total Nicotine, Reactive Oxygen Species, and Carbonyl Emissions From IQOS, a Heated Tobacco Product. Nicotine & Tobacco Research. 2019;21(9):1285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchiyama S, Noguchi M, Takagi N, et al. Simple Determination of Gaseous and Particulate Compounds Generated from Heated Tobacco Products. Chemical Research in Toxicology. 2018;31(7):585–93. [DOI] [PubMed] [Google Scholar]
- 23.Glantz SA. PMI’s own in vivo clinical data on biomarkers of potential harm in Americans show that IQOS is not detectably different from conventional cigarettes. Tobacco Control. 2018;27:S9–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis B, Williams M, Talbot P. iQOS: evidence of pyrolysis and release of a toxicant from plastic. Tobacco Control. 2019;28(1):34–41. [DOI] [PubMed] [Google Scholar]
- 25.Jankowski M, Brożek GM, Lawson J, et al. New ideas, old problems? Heated tobacco products - a systematic review. Int J Occup Med Environ Health. 2019;32(5):595–634. [DOI] [PubMed] [Google Scholar]
- 26.Uguna CN, Snape CE. Should IQOS Emissions Be Considered as Smoke and Harmful to Health? A Review of the Chemical Evidence. ACS Omega. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poget L, Campelos P, Jeannet C, et al. Development of Models for the Estimation of Mouth Level Exposure to Aerosol Constituents from a Heat-Not-Burn Tobacco Product Using Mouthpiece Analysis. Beitrage zur Tabakforschung International/ Contributions to Tobacco Research. 2017;27(5):42–64. [Google Scholar]
- 28.Haziza C, de la Bourdonnaye G, Merlet S, et al. Assessment of the reduction in levels of exposure to harmful and potentially harmful constituents in Japanese subjects using a novel tobacco heating system compared with conventional cigarettes and smoking abstinence: A randomized controlled study in confinement. Regulatory Toxicology and Pharmacology. 2016;81:489–99. [DOI] [PubMed] [Google Scholar]
- 29.Jones J, Slayford S, Gray A, et al. A cross-category puffing topography, mouth level exposure and consumption study among Italian users of tobacco and nicotine products. Int J Environ Res Public Health. 2020;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shihadeh A, Azar S. A closed-loop control “playback” smoking machine for generating mainstream smoke aerosols. J Aerosol Med. 2006;19(2):137–47. [DOI] [PubMed] [Google Scholar]
- 31.El-Hage R, El-Hellani A, Salman R, et al. Vaped Humectants in E-Cigarettes Are a Source of Phenols. Chemical Research in Toxicology. 2020;33(9):2374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Hellani A, Salman R, El-Hage R, et al. Nicotine and Carbonyl Emissions From Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. Nicotine Tob Res. 2018;20(2):215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Kaassamani M, Yen M, Talih S, et al. Analysis of mainstream emissions, secondhand emissions and the environmental impact of IQOS waste: a systematic review on IQOS that accounts for data source. Tob Control. 2022. [DOI] [PubMed] [Google Scholar]
- 34.McAdam K, Davis P, Ashmore L, et al. Influence of machine-based puffing parameters on aerosol and smoke emissions from next generation nicotine inhalation products. Regul Toxicol Pharmacol. 2019;101:156–65. [DOI] [PubMed] [Google Scholar]
- 35.Goujon C, Kleinhans S, Maeder S, et al. Robustness of HPHC reduction for THS 2.2 aerosol compared with 3R4f reference cigarette smoke under high intensity puffing conditions. Beitrage zur Tabakforschung International/ Contributions to Tobacco Research. 2020;29(2):66–83. [Google Scholar]
- 36.Shihadeh AL, Eissenberg TE. Significance of smoking machine toxicant yields to blood-level exposure in water pipe tobacco smokers. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cancelada L, Sleiman M, Tang X, et al. Heated Tobacco Products: Volatile Emissions and Their Predicted Impact on Indoor Air Quality. Environ Sci Technol. 2019;53(13):7866–76. [DOI] [PubMed] [Google Scholar]
- 38.Mallock N, Boss L, Burk R, et al. Levels of selected analytes in the emissions of “heat not burn” tobacco products that are relevant to assess human health risks. Archives of Toxicology. 2018;92(6):2145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bentley MC, Almstetter M, Arndt D, et al. Comprehensive chemical characterization of the aerosol generated by a heated tobacco product by untargeted screening. Analytical and Bioanalytical Chemistry. 2020;412(11):2675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savareear B, Lizak R, Brokl M, et al. Headspace solid-phase microextraction coupled to comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the analysis of aerosol from tobacco heating product. J Chromatogr A. 2017;1520:135–42. [DOI] [PubMed] [Google Scholar]
- 41.Shein M, Jeschke G. Comparison of Free Radical Levels in the Aerosol from Conventional Cigarettes, Electronic Cigarettes, and Heat-Not-Burn Tobacco Products. Chemical Research in Toxicology. 2019;32(6):1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bitzer ZT, Goel R, Trushin N, et al. Free Radical Production and Characterization of Heat-Not-Burn Cigarettes in Comparison to Conventional and Electronic Cigarettes. Chemical Research in Toxicology. 2020;33(7):1882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Counts ME, Morton MJ, Laffoon SW, et al. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41(3):185–227. [DOI] [PubMed] [Google Scholar]
- 44.Pubchem: 3-Chloro-1,2-propanediol; https://pubchem.ncbi.nlm.nih.gov/compound/3-Chloro-1_2-propanediol; Accessed date: 5/24/2022.
- 45.Catechol (Pyrocatechol); https://www.epa.gov/sites/production/files/2016-09/documents/catechol-pyrocatechol.pdf; Accessed date: 5/24/2022.
- 46.Uguna CN, Snape CE. Should IQOS Emissions Be Considered as Smoke and Harmful to Health? A Review of the Chemical Evidence. ACS Omega. 2022;7(26):22111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


