Abstract
Chemotherapy, in combination with immune checkpoint blockade (ICB) targeting to programmed death‐1 (PD‐1) or its ligand PD‐L1, is one of the first‐line treatments for patients with advanced non–small‐cell lung cancer (NSCLC). However, a large proportion of patients, especially those with PD‐L1 negative tumors, do not benefit from this treatment. This may be due to the existence of multiple immunosuppressive mechanisms other than the PD‐1/PD‐L1 axis. Human leukocyte antigen‐G (HLA‐G) has been identified as an immune checkpoint protein (ICP) and a neoexpressed tumor‐associated antigen (TAA) in a large proportion of solid tumors. In this study, we evaluated the induction of HLA‐G as well as PD‐L1 using sublethal doses of chemotherapeutics including pemetrexed in different NSCLC cell lines. Except for gefitinib, most of the chemotherapeutic agents enhanced HLA‐G and PD‐L1 expression in a dose‐dependent manner, whereas pemetrexed and carboplatin treatments showed the most consistent upregulation of PD‐L1 and HLA‐G in each cell line. In addition to protein levels, a novel finding of this study is that pemetrexed enhanced the glycosylation of HLA‐G and PD‐L1. Pemetrexed potentiated the cytotoxicity of cytotoxic T lymphocytes (CTLs) to treat NSCLC. Both in vitro and in vivo experiments revealed that CTL‐mediated cytotoxicity was most pronounced when both anti‐PD‐L1 and anti‐HLA‐G ICBs were combined with pemetrexed treatment. In conclusion, anti‐HLA‐G could be an intervention strategy in addition to the anti‐PD‐1/PD‐L1 pathway for NSCLC. Moreover, dual targeting of PD‐L1 and HLA‐G combined with pemetrexed might have a better extent of CTL‐based immunotherapy.
Keywords: CTLs, HLA‐G, NSCLC, PD‐L1, pemetrexed
Pemetrexed might enhance the expression and glycosylation of HLA‐G and PD‐L1, which could provide a beneficial TME for CTL cytotoxicity against NSCLC. Dual targeting of PD‐L1 and HLA‐G combined with pemetrexed might have a better extent of CTL‐based immunotherapy.
Abbreviations
- CAR
Chimeric Antigen Receptor
- HLA‐G
human leukocyte antigen‐G
- ICB
immune checkpoint blockade
- ICP
immune checkpoint protein
- ILT2
immunoglobulin‐like transcript 2
- IVIS
in vivo bioluminescence imaging system
- KIR2DL4
killer cell immunoglobulin‐like receptor 2DL4
- MFI
mean fluorescence intensity
- NSCLC
non–small‐cell lung cancer
- PD‐1
programmed cell death‐1
- PD‐L1
programmed cell death ligand 1
- PNGase F
Peptide‐N‐Glycosidase F
- TAA
tumor‐associated antigen
- TKI
tyrosine kinase inhibitor
- TME
tumor microenvironment
1. INTRODUCTION
Immunotherapy against PD‐L1 or its receptor PD‐1 has been proven to improve the outcomes of patients with NSCLC. 1 However, a significant proportion of patients is unresponsive to this immunotherapy due to antigen deficiency or acquired resistance and the possibility of multiple immunosuppressive mechanisms other than PD‐1/PD‐L1. 2 , 3
HLA‐G, a non‐classical MHC class I antigen, is known to be a potent ICP in addition to PD‐L1, and a neoexpressed TAA in numerous solid tumors. 4 , 5 It has recently been considered an effective target for CAR immune cell therapy. 6 , 7 HLA‐G plays a role in the immune escape of cancers through binding to its receptors such as KIR2DL4, ILT2, ILT4, CD8 and CD160. 8 , 9 , 10 , 11 Accordingly, the possibility of targeting HLA‐G to address the immune evasion in the treatment of solid tumors is implied.
In addition to TKI targeting for treatment of EGFR‐mutant NSCLC, chemotherapy is the most used strategy. Pemetrexed works by inhibiting multiple enzymes involved in folate metabolism and the synthesis of purine and pyrimidine nucleotides, ultimately hindering RNA and DNA synthesis. 12 Carboplatin is a second generation of platinum‐based chemotherapeutic drug, which can crosslink with purine bases on DNA, thus interfering with DNA repair mechanisms, causing DNA damage, and subsequently inducing apoptosis in cancer cells. 13 Chemotherapies have been revealed to moderate the TME and the development of drug resistance through modulating ICP expression. 14 , 15 Conversely, T‐cell‐based therapy is a promising and rapidly evolving field that sheds light on the treatment of cancer. It is currently being evaluated in clinical trials across multiple cancer types, alone or in combination with other treatments. 16
In the present study, we used chemotherapeutics to treat certain NSCLC cell lines and assessed whether different chemotherapeutics affect the performance of ICPs, thereby modulating the cytolytic activity of T cells against NSCLC. Then explored the best combination of chemotherapy and immunotherapy for the treatment of NSCLC. The results indicated that, at sublethal doses, treatment with pemetrexed, gemcitabine, carboplatin and paclitaxel could enhance the expression of both PD‐L1 and HLA‐G in most of the NSCLC cell lines that we evaluated in this study. Among them, pemetrexed and carboplatin had the most significant effects on PD‐L1 and HLA‐G performance. In addition to the protein level, there was a corresponding increase in the glycosylation of PD‐L1 and HLA‐G proteins following pemetrexed treatment. Conversely, the EGFR‐TKI gefitinib had little effect on the expression of either PD‐L1 or HLA‐G in NSCLC cells. Pemetrexed and carboplatin enhanced the cytotoxicity of CD8+ T cells (also known as CTLs) against NSCLC cells in vitro. Furthermore, both in vitro and in vivo experiments revealed that pemetrexed treatment in combination with dual ICBs targeting PD‐L1 and HLA‐G significantly promoted CTL‐mediated cytotoxicity in NSCLC. These marked findings demonstrate the importance of HLA‐G and also suggest that dual targeting of PD‐L1 and HLA‐G combined with pemetrexed and T‐cell therapy might be a new strategy for the clinical treatment of NSCLC.
2. MATERIALS AND METHODS
2.1. Cell culture
NSCLC cell lines A549 (CCL‐185™), H358 (CRL‐5807™), H1650 (CRL‐5883™) and H1975 (CRL‐5908™) were purchased from ATCC, USA. All cell lines were maintained in RPMI medium supplemented with 10% FBS (Gibco™/Thermo Fisher Scientific), 1× MEM non‐essential amino acids solution (NEAA) (Gibco™/Thermo Fisher Scientific), 1 mM sodium pyruvate (Gibco™/Thermo Fisher Scientific) and 2 mM l‐glutamine (Gibco™/Thermo Fisher Scientific) in a humidified atmosphere of 5% CO2 at 37°C.
2.2. Cell viability
NSCLC cell lines were individually treated with the chemotherapeutics pemetrexed (Merck), gemcitabine (Merck), carboplatin (Merck), paclitaxel (Merck) and gefitinib (Merck) at different concentrations for 48 h. Cell viability was determined using Cell Counting Kit‐8 (CCK‐8; TargetMol®) according to the manufacturer's instructions. The absorbance was measured at 450 nm using a molecular devices SpectraMax® M2 multilabel microplate reader (Marshall Scientific).
2.3. Flow cytometry
NSCLC cells were seeded into 12‐well plates and treated with chemotherapeutics for 48 h. Cells were then stained with FITC‐conjugated anti‐PD‐L1 or anti‐HLA‐G antibodies. The induction of PD‐L1 and HLA‐G expression was determined using flow cytometry (BD Bioscience). The MFI was analyzed using FlowJo™ software (FlowJo, LLC/BD Bioscience). The phenotypes and activating status of human PBMC‐derived T cells were assessed and analyzed using flow cytometry (SONY SA3800 spectral cell analyzer) after staining with specific antibodies.
2.4. Glycosylation analysis
Peptide‐N‐glycosidase F (PNGase F; New England Biolabs) and tunicamycin (Tu) were individually added to assess the glycosylation status of PD‐L1 and HLA‐G. PNGase F was added to total protein lysate extracted from cancer cells treated with/without chemotherapeutics. Analytical procedures were performed according to the manufacturer's instructions. The detection of glycosylated/de‐glycosylated protein levels was analyzed using western blot (WB) analysis. Tunicamycin was added to the culture medium of NSCLC cells in the presence/absence of chemotherapeutics for 18 h followed by the administration of proteasome inhibitor MG132 for an additional 6 h. After incubation, protein extraction and WB analysis were performed to identify the glycosylated/de‐glycosylated status of PD‐L1 and HLA‐G in NSCLC cells.
2.5. Western blot analysis
Cells were harvested and then lysed with RIPA buffer (Thermo Scientific™) for total protein lysate. For cytosol and membrane protein fractionations, cells were lysed using Mem‐PER™ Plus Membrane Protein Extraction Kit (Thermo Scientific™) according to the manufacturer's instructions. The procedure for WB analysis was performed as described elsewhere 17 using specific antibodies. The blots were then imaged using a ChemiDoc Touch Imaging System (Bio‐Rad).
2.6. Isolation, activation, and expansion of human CTLs
Human PBMCs were purchased from STEMCELL Technologies Inc., Canada. Isolation of lymphocytes from PBMCs was performed using EasySep™ human T‐cell isolation kit (STEMCELL) according to the manufacturer's instructions. The enriched cell suspension (lymphocytes) was harvested for subsequent activation and expansion by incubating with ImmunoCult™ Human CD3/CD28 T Cell Activator (STEMCELL) for up to 3 days. The activated T cells were then expanded and maintained in X‐VIVO™ 15 serum‐free hematopoietic cell medium (Lonza, Swiss) with 5% human AB serum (Sigma‐Aldrich®/Merck Millipore) and 10 ng/mL human recombinant IL‐2 (PeproTech/Thermo Fisher Scientific) in a humidified atmosphere of 5% CO2 at 37°C.
2.7. T‐cell‐mediated tumor cell‐killing assay
Cancer cells were seeded into 12‐well plates and cultured overnight followed by pemetrexed treatment for 48 h. The activated CLTs were stained with CytoTell™ green (Biomol GmbH) according to the manufacturer's instructions, and then co‐cultured with A549 or H1975 cells at different effector‐to‐target cell ratios (E:T ratios). After 24 or 48 h incubation, killing of NSCLC cells was determined using propidium iodide (PI; BD Bioscience) staining and followed by flow cytometry analysis (BD Biosciences). The percentage of cell death was further analyzed using FlowJo™ software (FlowJo, LLC/BD Bioscience).
2.8. Cytokine assay
The concentration of granzyme B in the co‐culture medium was measured using ELISA kits (granzyme B; Abcam) according to the manufacturer's instructions. The optical density of each sample was determined using a microplate reader (molecular devices SpectraMax® M2 multilabel microplate reader; Marshall Scientific) set to 450 nm.
2.9. In vivo xenograft mouse models
Female immunodeficient NOD.Cg‐Prkdc SCID /ll2rg tm1vst /Vst (NPG) mice (5–6 weeks old) were purchased from BioLASCO Taiwan Co., Ltd. All procedures and the care of experimental animals were conducted under ARRIVE guidelines and the experimental protocol (CMUIACUC‐2021‐416) was approved by the Institutional Animal Care and Use Committee (IACUC) of the China Medical University. NPG mice were randomly divided into six groups. A549‐Luc cells (CCL‐185‐LUC2™, ATCC) at a cell concentration of 5 × 105 in 50 μl of RPMI medium were mixed thoroughly with an equal volume of Coring® Matrigel matrix (Corning) and inoculated subcutaneously into the right side of the neck of each mouse. After 7 days (start of pemetrexed treatment, recorded as day 0), pemetrexed at a concentration of 50 mg/kg was injected weekly through the tail vein for 2 weeks. For ICB treatment, anti‐PD‐L1 (Atezolizumab; 2 mg/kg), anti‐HLA‐G (2 mg/kg) or a combination of these two antibodies were administrated by tail vein injection together with 5 × 106 activated T cells once a week for 3 weeks. Tumor volume was monitored weekly using IVIS (PerkinElmer).
2.10. Antibodies
Antibodies against Actin (MA5‐15739), GAPDH (MA5‐15738) for WB and HLA‐G (MA1‐10365) for flow cytometry, and HLA‐G (87G; 14‐9957‐82, eBioscience™) for ICB were purchased from Thermo Fisher Scientific. Antibodies against PD‐L1 (Ab205921), PD‐L1 (NBP1‐76769) for WB and PD‐L1 (FAB1561G‐100) for flow cytometry were purchased from Abcam Inc., Novus Biologicals, LLC. and R&D systems, respectively. Monoclonal antibody Atezolizumab for PD‐L1 ICB was purchased from Roche. Antibodies APC anti‐human CD107a (LAMP‐1, 328620), Alexa Fluor® 647 anti‐human CD107a (LAMP‐1, 328612), PE anti‐human CD178 (Fas‐L, 306407), Pacific Blue™ anti‐human CD3 (300329), FITC anti‐human CD314 (NKG2D, 320820), PE anti‐human CD4 (300539), FITC anti‐human CD8 (344704), and PE/Cyanine7 anti‐human CD8a (300914) were purchased from BioLegend. Anti‐HLA‐G (79769) and anti‐Na+/K+‐ATPase (3010S) for WB were purchased from Cell Signaling Technology.
2.11. Statistical analysis
All data in this study were represented as the mean ± SD and were statistically analyzed using a paired Student's t‐test. Significant differences were shown as: *p < 0.05, **p < 0.01, and ***p < 0.001. All statistical values were generated from at least three replicate experiments with similar patterns.
3. RESULTS
3.1. Endogenous expression of PD‐L1 and HLA‐G varies among NSCLC cell lines
To evaluate the expression of endogenous PD‐L1 and HLA‐G in different NSCLC cell lines, A549, H358, H1650, and H1975 cells were prepared in an appropriate culture system followed by WB and flow cytometry analysis. The results showed that the protein levels of PD‐L1 and HLA‐G were divergent among NSCLC cell lines. Higher expression of PD‐L1 was observed in H1975 and H358 cells compared with other cell lines (Figure 1A). Among these cell lines, the membrane PD‐L1 expression was higher in the H1975 cell line (Figure 1B). Figure 1C shows the divergence of HLA‐G levels in the total cell lysates of individual cell lines. HLA‐G protein levels were higher in H358 and H1975 than in the other two cell lines (Figure 1C). However, membrane HLA‐G was lower in H358 cells and higher in H1975 and H1650 cells (Figure 1D). The expression patterns of PD‐L1 and HLA‐G were identified using siRNA (Figures S1 and S2).
FIGURE 1.
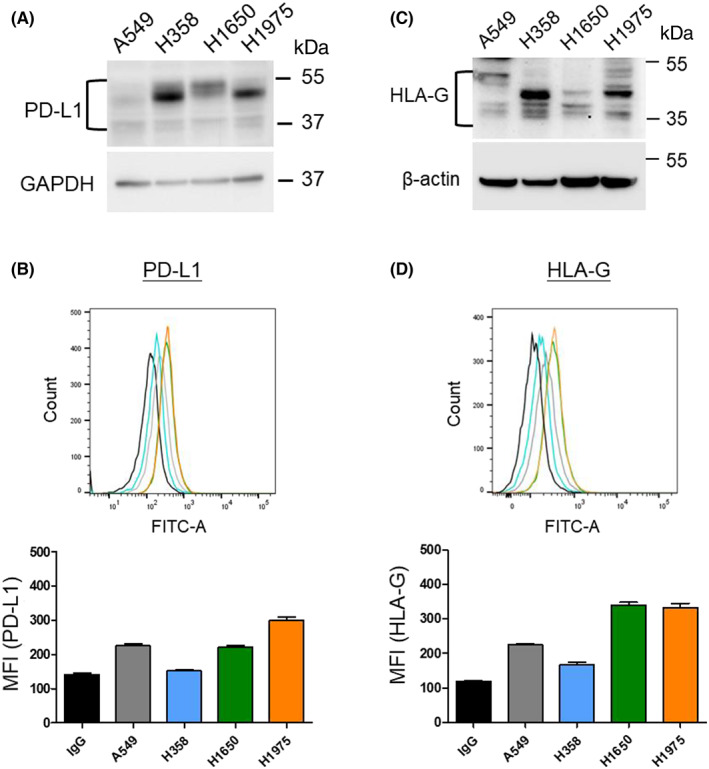
Endogenous expression of PD‐L1 and HLA‐G in NSCLC cell lines. (A) WB analysis of PD‐L1 expression and (B) flow cytometry analysis for membrane PD‐L1 in NSCLC cell lines. (C) WB analysis of endogenous HLA‐G level and (D) flow cytometry analysis for membrane HLA‐G expression in NSCLC cell lines.
3.2. Chemotherapeutics induce the expression and glycosylation of PD‐L1 in NSCLC cell lines
The regulation of PD‐L1 expression was assessed following sublethal doses of chemotherapeutics including pemetrexed, gemcitabine, carboplatin, paclitaxel, and gefitinib (Iressa). The anti‐cancer activity of each chemotherapeutic agent against individual NSCLC cell lines is shown in Figure 2A. Sublethal doses below IC50 of each agent were determined to treat individual cell lines and followed by flow cytometry analysis to evaluate the effects of these chemotherapeutics on membrane PD‐L1 regulation (Figure 2B). The quantitative results in Figure 2B indicated that, under sublethal dosage, pemetrexed, gemcitabine and carboplatin could induce membrane PD‐L1 upregulation in all cell lines. Membrane PD‐L1 in H1650 cells was unaffected after paclitaxel treatment, whereas it was increased in A549, H358 and H1975 cell lines. In particular, gefitinib did not induce an increase in membrane PD‐L1 expression in most of the NSCLC cell lines. Evaluation of PD‐L1 expression in both total cell lysates and membrane fractions of A549 and H1975 cells after pemetrexed (PEM) or carboplatin (CAR) treatment also revealed elevated levels of PD‐L1, whereas it was not significantly increased after gefitinib (GEF) treatment (Figure 2C,D). Glycosylation is known to stabilize PD‐L1 and thereby enhances its activity. We further evaluated the effect of sublethal doses of pemetrexed, carboplatin and gefitinib treatment on PD‐L1 glycosylation. Tunicamycin (Tu), which inhibits protein N‐linked glycosylation, was added to the culture medium to evaluate the effect of chemotherapeutics on PD‐L1 glycosylation. The results showed that pemetrexed (PEM), as well as carboplatin (CAR), induced the expression and glycosylation of PD‐L1 in A549 and H1975 cells (Figure 2E), whereas gefitinib (GEF) had no effect on PD‐L1 glycosylation in both cell lines (Figure S3). The fold changes of glycosylated/de‐glycosylated PD‐L1 are indicated in red. In addition to tunicamycin, recombinant glycosidase PNGase F was used to confirm the effect of pemetrexed on PD‐L1 glycosylation. The results indicate that pemetrexed‐enhanced PD‐L1 levels are, at least partially, due to the increase in PD‐L1 glycosylation (Figure 2F).
FIGURE 2.
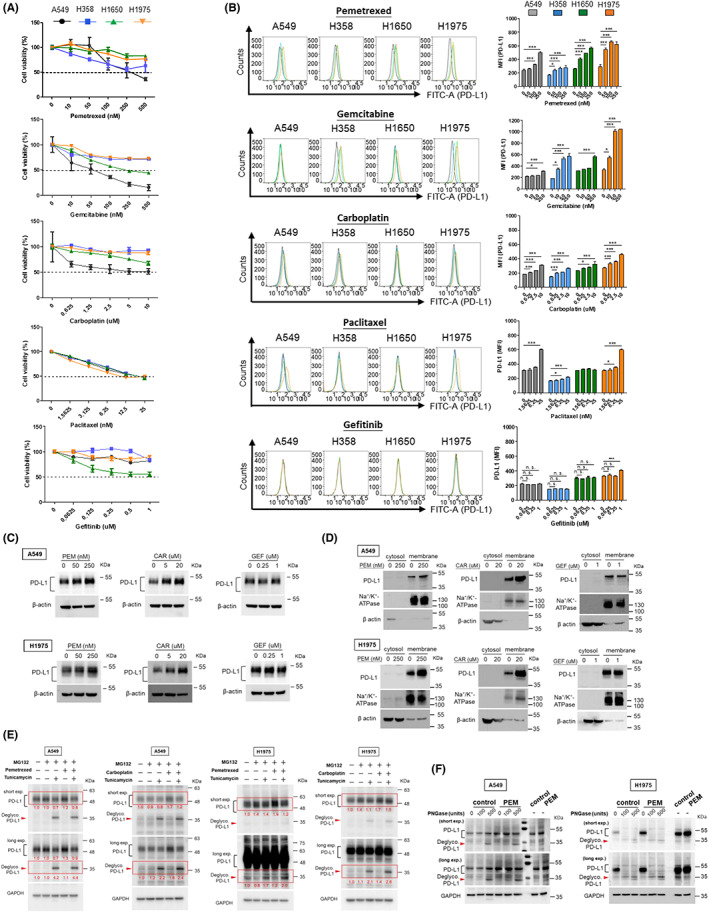
Induction and modulation of PD‐L1 by chemotherapeutics in NSCLC cell lines. (A) NSCLC cells were treated with chemotherapeutics (pemetrexed, gemcitabine, carboplatin, paclitaxel and gefitinib, respectively) at indicated series of increasing concentrations in complete RPMI medium. After 48 h, cell viability was measured using CCK8 assay and absorbance was determined using a cytometer. (B) NSCLC cells were treated with chemotherapeutics as indicated. After 48 h, the cell membrane PD‐L1 expression was analyzed using flow cytometry. The mean fluorescence intensity (MFI) was displayed to identify the expression of membrane PD‐L1. Quantitative results were statistically analyzed from three independent replicates and the significant differences were expressed as *p < 0.05, **p < 0.01 and ***p < 0.001. A549 and H1975 cells were subjected to chemotherapeutics at indicated concentrations for 48 h (CAR, carboplatin; GEF, gefitinib; PEM, pemetrexed). PD‐L1 protein level in (C) total cell lysate, (D) cytosol and membrane fractionations was determined using western blot. Na+/K+ ATPase served as an internal control for the membrane fraction and β‐actin for the cytosolic fraction. (E) A549 and H1975 cells were treated with pemetrexed (250 nM for A549 and 50 nM for H1975) and carboplatin (40 μM), respectively, for 48 h. During treatment, 5 μg/mL tunicamycin and 10 μM MG132 were added into the medium for 24 h and 6 h, respectively, before protein extraction. Glycosylated and de‐glycosylated PD‐L1 were detected by WB analysis. (F) A549 and H1975 cells were treated with pemetrexed (PEM) for 48 h prior to protein extraction. Cell lysates were then incubated with PNGase F to digest the glycosylated proteins and followed by WB analysis to assess the glycosylation/de‐glycosylation pattern of PD‐L1 in cells. Red arrowheads indicate de‐glycosylated PD‐L1.
3.3. Chemotherapeutics induce HLA‐G expression and glycosylation in NSCLC cell lines
The effects of sublethal doses of chemotherapeutics on membrane HLA‐G regulation in individual NSCLC cell lines were assessed using flow cytometry analysis (Figure 3A). The quantitative results in Figure 3A demonstrate that pemetrexed, carboplatin and paclitaxel induced membrane HLA‐G upregulation in all cell lines in a dose‐dependent manner. Gemcitabine enhanced membrane HLA‐G expression in H358 and H1975 cells, but not A549 and H1650 cell lines. Consistent with membrane PD‐L1 expression, gefitinib did not affect membrane HLA‐G expression in any of the cell lines we tested. We further assessed HLA‐G protein levels in total cell lysates and membrane fractions of A549 and H1975 cells following pemetrexed, carboplatin or gefitinib treatment. The increase in HLA‐G protein in total cell lysate (Figure 3B) and membrane fraction (Figure 3C) after pemetrexed (PEM) or carboplatin (CAR) treatment showed the same trend in both A549 and H1975 cell lines, whereas gefitinib (GEF) had little effect on HLA‐G expression in both cell lines. We further evaluated the glycosylation of HLA‐G after pemetrexed treatment using PNGase F. The results in Figure 3D demonstrated that pemetrexed enhanced the glycosylation of HLA‐G protein in both A549 and H1975 cells.
FIGURE 3.
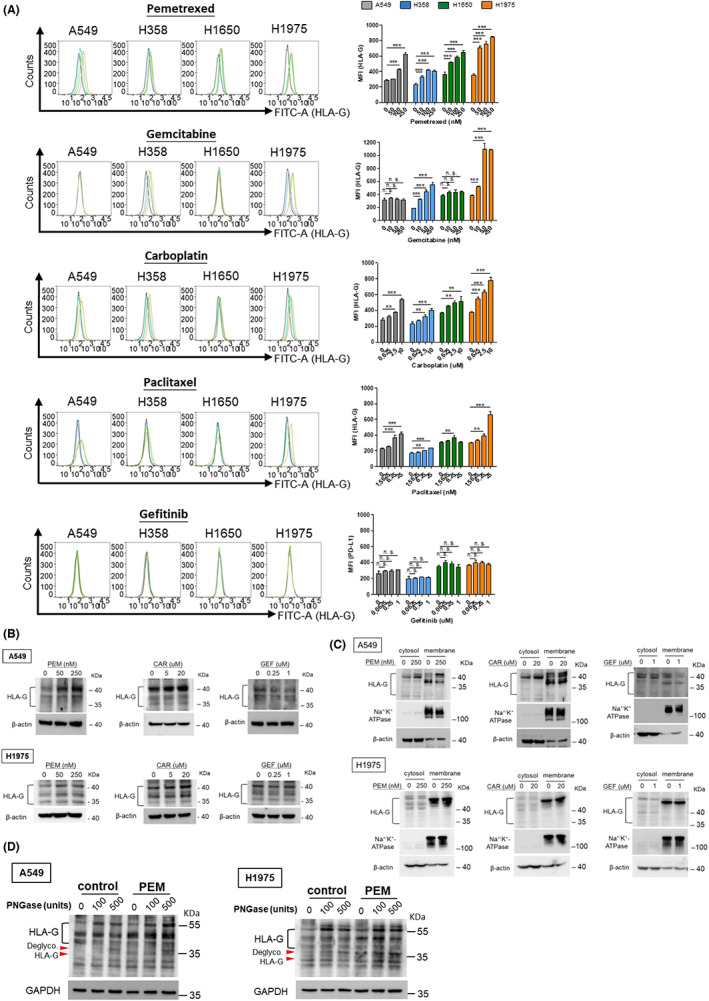
HLA‐G induction by chemotherapeutics in NSCLC cell lines (A) NSCLC cells were treated with chemotherapeutics at indicated concentrations for 48 h. After that, membrane HLA‐G was analyzed by flow cytometry and the MFI was displayed to identify the expression of membrane HLA‐G (right part of panel A). The quantitative results were statistically analyzed from three independent replicates and the significant differences were expressed as *p < 0.05, **p < 0.01 and ***p < 0.001. NSCLC cells were subjected to pemetrexed (PEM), carboplatin (CAR) or gefitinib (GEF) treatment for 48 h, HLA‐G protein levels in total lysate (B) and cytosol/membrane fractionations (C) were determined using western blot (WB) analysis. (D) A549 and H1975 cells were treated with pemetrexed (PEM; 250 nM and 50 nM, respectively) for 48 h followed by protein extraction. Cell lysates were then incubated with PNGase F to digest the glycosylated proteins followed by WB analysis to assess the glycosylation/de‐glycosylation pattern of HLA‐G. Red arrowheads indicate de‐glycosylated HLA‐G.
3.4. Chemotherapeutics enhance the cytotoxicity of CTLs against NSCLC cell lines
According to the above results, pemetrexed and carboplatin upregulated PD‐L1 and HLA‐G proteins in all the NSCLC cell lines used in this study, while gefitinib did not affect either PD‐L1 or HLA‐G (Figures 2 and 3). We were therefore concerned whether pemetrexed and carboplatin could moderate the expression of ICPs thereby altering the TME and further influencing the anti‐cancer effects of immune cells. Consequently, we further investigated the effects of pemetrexed, carboplatin and gefitinib on the cytotoxic activity of CTLs against NSCLC. After isolation, expansion, and activation from PBMCs, the phenotypic characterization of the CD8+ T cells was identified through flow cytometry analysis (Figure 4A). A549 and H1975 cells were treated with sublethal doses of pemetrexed (250 nM for A549, and 50 nM for H1975 cells), carboplatin (10 μM) and gefitinib (1 μM) for 48 h, and then co‐cultured with T cells for 24 h. The results for the cytolytic cancer cells showed that pemetrexed and carboplatin enhanced the cytotoxicity of CTLs against A549 and H1975 cells, while gefitinib increased the cytotoxicity of CTLs against A549 but not H1975 cells (Figure 4B,C). The release of granzyme B and the expression of CD107a on the cell surface of CD8+ T cells after co‐cultured with A549 and H1975 cells were assessed in the presence/absence of pemetrexed to identify the antitumor activity of CTLs. The results in Figure 4D show that an increase in granzyme B caused by pemetrexed treatment was detected after co‐culture of T cells with A549 and H1975 cells, while the expression of CD107a on the surface of CD8+ T cells was enhanced (Figure 4E).
FIGURE 4.
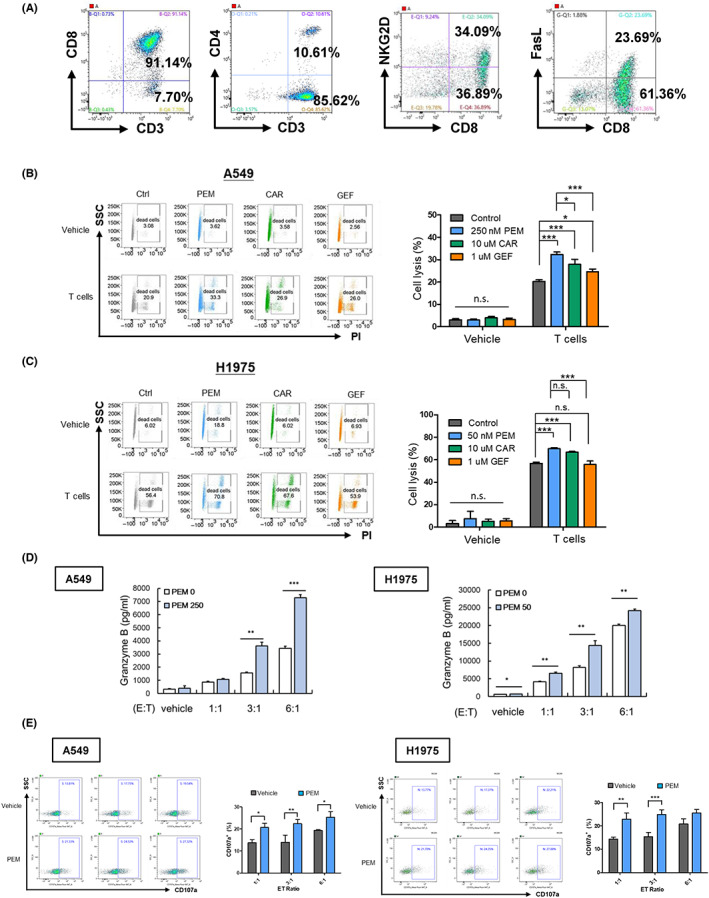
Pemetrexed enhances in vitro cytotoxicity of CTLs against NSCLC cells. (A) The phenotypes and activating status of human PBMC‐derived T cells were assessed through flow cytometry. (B) A549 cells were treated with or without pemetrexed (PEM; 250 nM), carboplatin (CAR; 10 μM) or gefitinib (GEF; 1 μM) for 48 h, then cocultured with CTLs for an additional 48 h at ET ratio of 3:1 or 6:1, respectively. Cell lysis were analyzed using PI staining using flow cytometry. (C) H1975 cells were treated with or without pemetrexed (PEM; 50 nM), carboplatin (CAR; 10 μM) or gefitinib (GEF; 1 μM) for 48 h, then cocultured with activated CTLs at an ET ratio of 3:1 or 6:1, respectively, for an additional 24 h. Cell lysis was analyzed by PI staining using flow cytometry. A549 and H1975 cells were treated with pemetrexed (PEM; 250 nM and 50 nM, respectively), for 48 h. Cells were then co‐cultured with CTLs for an additional 24 h followed by (D) ELISA assay for the detection of secreted granzyme B, and (E) flow cytometry analysis for the expression of CD107a on CD8+ T cells. Statistical analysis was performed on the quantitative results of three independent replicates, and significant differences were expressed as *p < 0.05, **p < 0.01 and ***p < 0.001.
3.5. Pemetrexed enhances cytotoxicity of combined ICB and CTLs therapy in NSCLC cell lines
ICB therapy has been clinically shown to favor T‐cell recognition and the elimination of cancer cells. As pemetrexed could induce the expression and glycosylation of both PD‐L1 and HLA‐G (Figures 2 and 3), and conversely enhance the cytotoxicity of CTLs against NSCLC cells (Figure 4). We further evaluated whether pemetrexed affected the combination of ICB and CTLs in NSCLC. The in vitro co‐culture experiments showed that, in the absence of pemetrexed, anti‐PD‐L1 (α‐PD‐L1) had no effect on CTL cytotoxicity against A549, but anti‐HLA‐G (α‐HLA‐G) with or without α‐PD‐L1 statistically increased CTL‐caused cytolysis of A549 cells (Figure 5A), whereas neither α‐PD‐L1 nor α‐HLA‐G alone had any effect on the cytotoxicity of CTLs against H1975 cells (Figure 5B). In contrast, both in A549 and H1975 cell lines, a sublethal dose of pemetrexed significantly enhanced the cytotoxic effect of ICB (α‐PD‐L1 and/or α‐HLA‐G) and CTL combination therapy (Figure 5A,B). The results also suggested a synergistic effect of α‐PD‐L1 and α‐HLA‐G on CTL cytotoxicity in the presence of pemetrexed. In addition, pemetrexed increased granzyme B release (Figure 5C) and CD107a expression (Figure 5D) in A549 and H1975 cells in the combination of ICB (α‐PD‐L1 and/or α‐HLA‐G) and CTLs.
FIGURE 5.
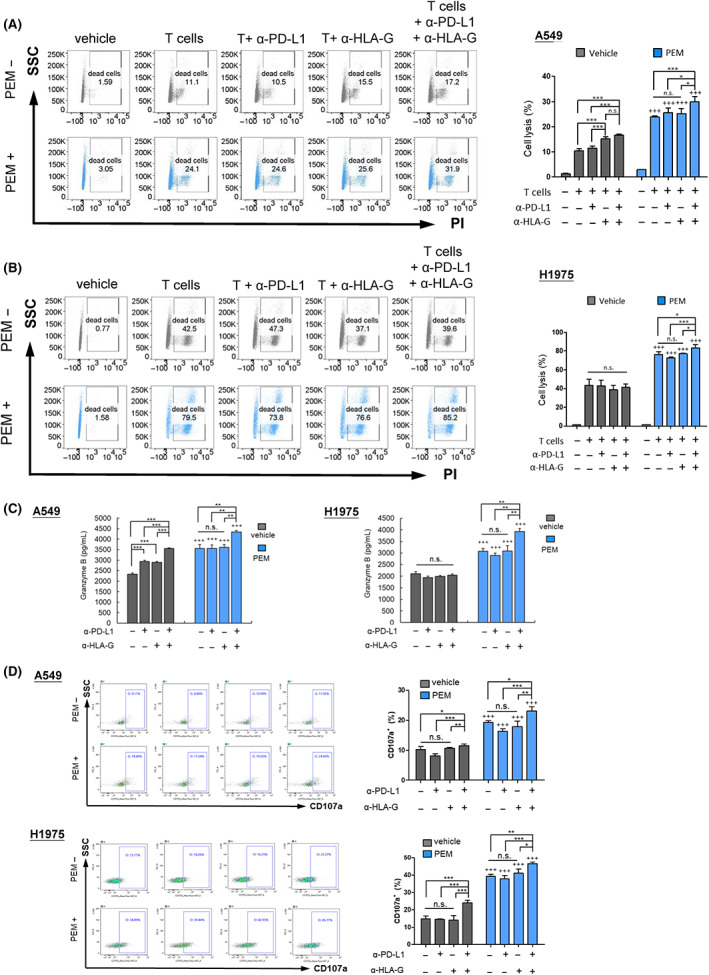
Pemetrexed enhances the anti‐cancer effect of CTLs combined with anti‐PD‐L1 plus anti‐HLA‐G antibodies in the treatment of NSCLC cells. A549 (A) and H1975 (B) cells were treated with or without pemetrexed (PEM) for 48 h, then co‐cultured with CD8+ T cells at an ET ratio of 6:1 plus α‐PD‐L1 (10 μg/mL), α‐HLA‐G (10 μg/mL) or a combination of both α‐PD‐L1 and α‐HLA‐G treatment for 24 h (H1975) or 48 h (A549). Cell lysis was analyzed using PI staining and flow cytometry. (C) NSCLC cells were treated with or without pemetrexed (PEM) for 48 h, and then co‐cultured with CD8+ T cells in the presence of α‐PD‐L1, α‐HLA‐G or the combination of both for an additional 16 h. The secreted granzyme B in culture medium was detected through ELISA, and (D) the expression of CD107a on activated CD8+ T cells was measured using flow cytometry. Statistical analysis was performed on the results of three independent replicates, and significant differences were expressed as *p < 0.05, **p < 0.01 and ***p < 0.001.
3.6. The in vivo effect of pemetrexed on the combination therapy of ICB and CTLs for NSCLC
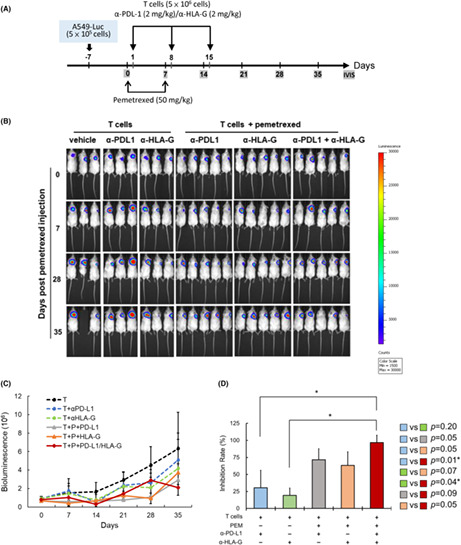
To assess the in vivo effect of pemetrexed on the combination therapy of ICB and CTLs against NSCLC, a xenograft immunodeficient NPG mouse model was performed. The experimental timeline is shown in Figure 6A. Pemetrexed, CD8+ T cells, α‐PD‐L1 and/or α‐HLA‐G antibodies were injected via the tail vein as described in Materials and Methods. Images of mice monitoring the bioluminescence signal of inoculated tumors using IVIS spectroscopy are shown in Figure 6B. Quantification of the bioluminescence signal and tumor inhibition rate showed that α‐PD‐L1 plus α‐HLA‐G combined with CD8+ T cells showed the best tumor inhibition in the presence of pemetrexed (Figure 6C,D).
FIGURE 6.
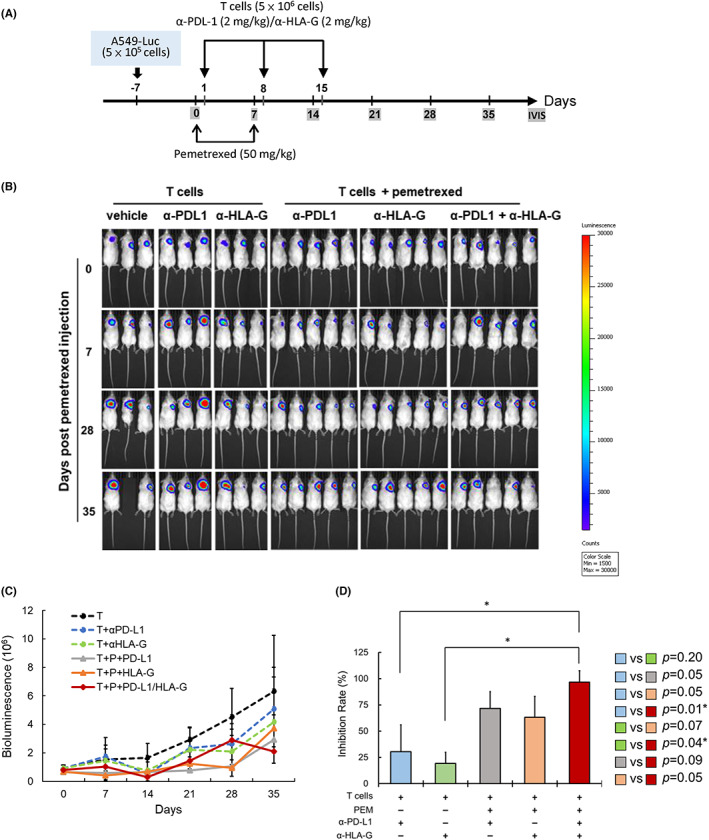
The effect of pemetrexed on the combination therapy of ICB and CTLs for the treatment of NSCLC in the xenograft animal model. (A) The experimental design and protocol. A549‐Luc cells (5 × 105 cells/mice) were subcutaneously injected into right back side of the mice. On day 0, mice were injected with pemetrexed (50 mg/kg) through the tail vein once a week for 2 weeks. On day 1 and for the following 2 weeks, mice were weekly injected with CD8+ T cells (5 × 106 cells/mice) with ICB (α‐PDL‐1, 2 mg/kg and/or α‐HLA‐G, 2 mg/kg) via the tail vein. Images were taken every week. (B) IVIS images of A549‐Luc tumors in mice treated with CD8+ T and α‐PDL‐1/α‐HLA‐G in the presence or absence of pemetrexed, and following the procedure described in (A). (C) Mice were injected with d‐luciferin (120 mg/kg), and then the tumor size was detected and quantified using bioluminescence photometry. (D) The inhibition rate of tumor compared with the group for CD8+ T cells (*p < 0.05).
4. DISCUSSION
The marked effect of clinical trials has led to the approval of pemetrexed and platinum‐based chemotherapy in combination with α‐PD‐1 as the first‐line treatment for patients with advanced NSCLC. 18 , 19 , 20 In the present study, we assessed the endogenous HLA‐G as well as PD‐L1 expression in a variety of NSCLC cell lines and screened for the induction of these two ICPs by first‐line chemotherapies. The upregulation of ICPs by individual chemotherapies varied among different cell lines. Sublethal doses of pemetrexed and carboplatin enhanced both HLA‐G and PD‐L1 consistently in a dose‐dependent manner in all NSCLC cell lines that we tested in this study. In addition to protein expression, we provide evidence that glycosylation of PD‐L1 and HLA‐G was correspondingly elevated following pemetrexed treatment. This is a novel finding that chemotherapeutics could enhance the glycosylation of PD‐L1, and HLA‐G as well. Furthermore, a sublethal dose of pemetrexed or carboplatin alone enhanced the in vitro cytotoxicity of CTLs to treat NSCLC cells. Pemetrexed potentiated CTL‐mediated cytotoxicity in combination with either α‐PD‐L1 or α‐HLA‐G and, interestingly, the enhancement of CTL‐mediated cytotoxicity was most pronounced when both ICBs were simultaneously combined with pemetrexed. Moreover, the experimental results of the in vivo mouse model corresponded with the in vitro findings, indicating that pemetrexed combined with dual ICBs targeting PD‐L1 and HLA‐G had the best effect on T‐cell immunotherapy for NSCLC.
CTLs play an important role in immune defense and are the principal immune cells for targeting cancer. During cancer progression, CTLs may become dysfunctional or even exhausted within the TME, thereby predisposing cancer to finding adaptive immune resistance. Tumor cells evade immune surveillance and attack by upregulating PD‐L1 and modulating its interaction with the immunosuppressive molecule PD‐1 on T cells, thereby inhibiting their function and leading to exhaustion. 21 Accordingly, reactivation and/or priming CD8+ T cells has become an issue in driving anti‐cancer immunity. ICB targeting the PD‐1/PD‐L1 pathway may alleviate the exhaustion of CD8+ T cells thereby enhancing the effect of T cells to eliminate antigen‐expressing cancer cells. 22 , 23 However, exhausted T cells contain heterogenous populations of cells that respond differently to this immune intervention. 21
The dilemma that antigen‐deficient patients may not benefit from current α‐PD‐L1/α‐PD‐1 treatments suggests an unmet need to evaluate tumor immune escape and combination immunotherapy strategies to improve clinical efficacy. The development of immune cell therapy is currently booming. In addition to genetically modifying the deployment of immune cells, in combination with other therapies, is currently being evaluated in clinical trials. In our study, pemetrexed and carboplatin could significantly improve the cytotoxicity effect of CTLs against NSCLC cells. The release of granzyme B and the expression of CD107a, both reflecting the cytotoxic activity of CTLs in killing cancer cells, were increased according to pemetrexed administration in the co‐culture system of CTLs and NSCLC cells. The potentiation of the cytotoxic activity of CTLs by pemetrexed treatment was improved by either α‐PD‐L1 or α‐HLA‐G, or to a greater extent by both α‐PD‐L1 and α‐HLA‐G at the same time. The results of the in vivo experiments also represent the best therapeutic effect of CD8+ T‐cellular immunotherapy on NSCLC progression under the combination of pemetrexed and dual ICBs.
The approach of reactivating PD‐L1 expression in NSCLC cells, and therefore turning the cold tumor into a “hot” one for ICB and priming a favorable TME to facilitate T‐cell activation, has recently been proposed. 24 , 25 Pemetrexed is known to enhance membrane PD‐L1 expression through multiple pathways including mTOR/P70S6K‐STAT 25 and thymidylate synthase–ROS–NF‐κB axes. 24 In this study, we provide evidence that not only the protein level, but that PD‐L1 glycosylation was also enhanced under pemetrexed treatment. In addition to PD‐L1, HLA‐G expression and glycosylation were increased in the presence of pemetrexed. This indicates a novel finding that pemetrexed may enhance PD‐L1 and HLA‐G persistence and activity in addition to transcriptional and translational means. Moreover, chemotherapies including gemcitabine and carboplatin could enhance membrane PD‐L1 and HLA‐G expression as well. Carboplatin also increased PD‐L1 glycosylation in NSCLC cell lines. Genotoxic stress induces PD‐L1 upregulation in cancers, 26 which enhances the resistance of cancer cells in response to DNA damage. 27 , 28 Both pemetrexed and carboplatin can interrupt DNA and cause genotoxic stress, thus this might be responsible for the upregulation of PD‐L1 and HLA‐G after pemetrexed and carboplatin treatment in this study. In addition, DNA damage in cancer cells may activate the cGAS–STING pathway and, concurrently, the uptake of tumor‐derived DNA leading to the activation of the cGAS–STING pathway in dendritic cells and resulting in cross‐priming of CD8+ T cells for anti‐cancer immunity. 29 Taken together, the participation of the cGAS–STING pathway in pemetrexed or carboplatin‐enhanced cytotoxicity of CTLs in combination with dual ICBs deserves to be further investigated.
The addition of pemetrexed and carboplatin to gefitinib has been reported to significantly improve the clinical outcome of NSCLC patients. 30 From the results of our present study, we speculate that this may be related to the regulation of the tumor microenvironment (TME) by pemetrexed and carboplatin, which is beneficial to the antitumor activity of immune cells, thus complementing the effect of gefitinib. In our study, EGFR inhibitor gefitinib could neither increase the expression nor the glycosylation of PD‐L1 in any of the NSCLC cell lines that we tested in this study. This result is in line with the findings in the published literature that EGF/EGFR signaling upregulates PD‐L1 expression in NSCLC. 31 , 32 This may provide a plausible explanation for the inability of TKI therapy to induce PD‐L1 expression. Moreover, consistent with the effect of gefitinib on PD‐L1 regulation, HLA‐G expression was not affected by gefitinib treatment. Whether the EGFR signaling pathway is involved in HLA‐G modulation deserves further exploration. Our previous study has evaluated the anti‐cancer effect of HLA‐G‐targeting CAR‐engineered NK cells in the treatment of breast, brain, pancreatic and ovarian cancers. 7 In the present study, we revealed that anti‐HLA‐G could be a strategy for immunotherapeutic interventions in addition to anti‐PD‐1/PD‐L1 for NSCLC, and provided clues to develop cancer treatment strategies targeting HLA‐G.
In summary, in addition to PD‐L1, we highlighted that the induction of HLA‐G by pemetrexed, and the administration of dual ICBs α‐HLA‐G and α‐PD‐L1 led to a better extent of T‐cell immunotherapy. The results provide broader possibilities for the clinical application of combination strategies to enhance the efficacy of immune cell therapy in the treatment of NSCLC.
AUTHOR CONTRIBUTIONS
Mei‐Chih Chen, conception and design, manuscript writing. Meng‐Yu Hung, acquisition of data and analysis. Chih‐Ming Pan, instrument and software operation. Shi‐Wei Huang, analysis and interpretation of results. Chia‐Ing Jan, technical support and data analysis. Yu‐Hsuan Li, acquisition of data. Shao‐Chih Chiu, conception, manuscript review and editing. Der‐Yang Cho, supervision, manuscript review and editing.
FUNDING INFORMATION
China Medical University Hospital, Taiwan. (DMR‐CELL‐2107 and DMR‐112‐137 to Mei‐chih Chen).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENTS
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: All procedure and care of experimental animals were conducted under ARRIVE guidelines and the experimental protocol (CMUIACUC‐2021‐416) was approved by the IACUC of the China Medical University.
Supporting information
Figure S1
Figure S2
Figure S3
Appendix S1
ACKNOWLEDGMENTS
The authors thank the Valuable Instrument Center of the Office of Research and Development of China Medical University for supporting the operation of the IVIS.
Chen M‐C, Hung M‐Y, Pan C‐M, et al. Pemetrexed combined with dual immune checkpoint blockade enhances cytotoxic T lymphocytes against lung cancer. Cancer Sci. 2023;114:2761‐2773. doi: 10.1111/cas.15806
Shao‐Chih Chiu and Der‐Yang Cho contributed equally to this study.
Contributor Information
Shao‐Chih Chiu, Email: scchiu@mail.cmu.edu.tw.
Der‐Yang Cho, Email: d5057@mail.cmuh.org.tw.
REFERENCES
- 1. Reslan L, Dalle S, Dumontet C. Understanding and circumventing resistance to anticancer monoclonal antibodies. MAbs. 2009;1:222‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antonia SJ, Vansteenkiste JF, Moon E. Immunotherapy: beyond anti‐PD‐1 and anti‐PD‐L1 therapies. Am Soc Clin Oncol Educ Book. 2016;35:e450‐e458. [DOI] [PubMed] [Google Scholar]
- 3. Venkatraman S, Meller J, Hongeng S, Tohtong R, Chutipongtanate S. Transcriptional regulation of cancer immune checkpoints: emerging strategies for immunotherapy. Vaccines (Basel). 2020;8:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loustau M, Anna F, Drean R, Lecomte M, Langlade‐Demoyen P, Caumartin J. HLA‐G neo‐expression on tumors. Front Immunol. 2020;11:1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curigliano G, Criscitiello C, Gelao L, Goldhirsch A. Molecular pathways: human leukocyte antigen G (HLA‐G). Clin Cancer Res. 2013;19:5564‐5571. [DOI] [PubMed] [Google Scholar]
- 6. Anna F, Bole‐Richard E, LeMaoult J, et al. First immunotherapeutic CAR‐T cells against the immune checkpoint protein HLA‐G. J Immunother Cancer. 2021;9:e001998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jan CI, Huang SW, Canoll P, et al. Targeting human leukocyte antigen G with chimeric antigen receptors of natural killer cells convert immunosuppression to ablate solid tumors. J Immunother Cancer. 2021;9:e003050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yan WH. Human leukocyte antigen‐G in cancer: are they clinically relevant? Cancer Lett. 2011;311:123‐130. [DOI] [PubMed] [Google Scholar]
- 9. Sheu J, Shih IM. HLA‐G and immune evasion in cancer cells. J Formos Med Assoc. 2010;109:248‐257. [DOI] [PubMed] [Google Scholar]
- 10. Pistoia V, Morandi F, Wang X, Ferrone S. Soluble HLA‐G: are they clinically relevant? Semin Cancer Biol. 2007;17:469‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. LeMaoult J, Krawice‐Radanne I, Dausset J, Carosella ED. HLA‐G1‐expressing antigen‐presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA. 2004;101:7064‐7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villela LR, Stanford BL, Shah SR. Pemetrexed, a novel antifolate therapeutic alternative for cancer chemotherapy. Pharmacotherapy. 2006;26:641‐654. [DOI] [PubMed] [Google Scholar]
- 13. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fournel L, Wu Z, Stadler N, et al. Cisplatin increases PD‐L1 expression and optimizes immune check‐point blockade in non‐small cell lung cancer. Cancer Lett. 2019;464:5‐14. [DOI] [PubMed] [Google Scholar]
- 15. Okimoto T, Kotani H, Iida Y, et al. Pemetrexed sensitizes human lung cancer cells to cytotoxic immune cells. Cancer Sci. 2020;111:1910‐1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez C, Gruber I, Arber C. Off‐the‐shelf allogeneic T cell therapies for cancer: opportunities and challenges using naturally occurring “universal” donor T cells. Front Immunol. 2020;11:583716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen MC, Chen KC, Chang GC, et al. RAGE acts as an oncogenic role and promotes the metastasis of human lung cancer. Cell Death Dis. 2020;11:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Y, Wang Z, Fang J, et al. Efficacy and safety of Sintilimab plus Pemetrexed and platinum as first‐line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double‐blind, phase 3 study (oncology pRogram by InnovENT anti‐PD‐1‐11). J Thorac Oncol. 2020;15:1636‐1646. [DOI] [PubMed] [Google Scholar]
- 19. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: a randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol. 2016;17:1497‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first‐line treatment for advanced non‐small‐cell lung cancer (CheckMate 012): results of an open‐label, phase 1, multicohort study. Lancet Oncol. 2017;18:31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurachi M. CD8(+) T cell exhaustion. Semin Immunopathol. 2019;41:327‐337. [DOI] [PubMed] [Google Scholar]
- 22. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234:8509‐8521. [DOI] [PubMed] [Google Scholar]
- 23. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu CS, Lin CW, Chang YH, et al. Antimetabolite pemetrexed primes a favorable tumor microenvironment for immune checkpoint blockade therapy. J Immunother Cancer. 2020;8:e001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cavazzoni A, Digiacomo G, Alfieri R, et al. Pemetrexed enhances membrane PD‐L1 expression and potentiates T cell‐mediated cytotoxicity by anti‐PD‐L1 antibody therapy in non‐small‐cell lung cancer. Cancers (Basel). 2020;12:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sato H, Niimi A, Yasuhara T, et al. DNA double‐strand break repair pathway regulates PD‐L1 expression in cancer cells. Nat Commun. 2017;8:1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheon H, Holvey‐Bates EG, McGrail DJ, Stark GR. PD‐L1 sustains chronic, cancer cell‐intrinsic responses to type I interferon, enhancing resistance to DNA damage. Proc Natl Acad Sci USA. 2021;118:e2112258118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tu X, Qin B, Zhang Y, et al. PD‐L1 (B7‐H1) competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Mol Cell. 2019;74:1215‐1226.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khoo LT, Chen LY. Role of the cGAS‐STING pathway in cancer development and oncotherapeutic approaches. EMBO Rep. 2018;19:e46935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus Gefitinib plus Pemetrexed and carboplatin chemotherapy in EGFR‐mutated lung cancer. J Clin Oncol. 2020;38:124‐136. [DOI] [PubMed] [Google Scholar]
- 31. Hsu PC, Jablons DM, Yang CT, You L. Epidermal growth factor receptor (EGFR) pathway, yes‐associated protein (YAP) and the regulation of programmed death‐ligand 1 (PD‐L1) in non‐small cell lung cancer (NSCLC). Int J Mol Sci. 2019;20:3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen N, Fang W, Zhan J, et al. Upregulation of PD‐L1 by EGFR activation mediates the immune escape in EGFR‐driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10:910‐923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Appendix S1


