Abstract
The cancer stem cell (CSC) theory features typically rare self‐renewing subpopulations that reconstitute the heterogeneous tumor. Identification of molecules that characterize the features of CSCs is a key imperative for further understanding tumor heterogeneity and for the development of novel therapeutic strategies. However, the use of conventional markers of CSCs is still insufficient for the isolation of bona fide CSCs. We investigated organoids that are miniature forms of tumor tissues by reconstructing cellular diversity to identify specific markers to characterize CSCs in heterogeneous tumors. Here, we report that the receptor for hyaluronan‐mediated motility (RHAMM) expresses in a subpopulation of CD44+ conventional human colorectal CSC fraction. Single‐cell transcriptomics of organoids highlighted RHAMM‐positive proliferative cells that revealed distinct characteristics among the various cell types. Prospectively isolated RHAMM+CD44+ cells from the human colorectal cancer tissues showed highly proliferative characteristics with a self‐renewal ability in comparison with the other cancer cells. Furthermore, inhibition of RHAMM strongly suppressed organoid formation in vitro and inhibited tumor growth in vivo. Our findings suggest that RHAMM is a potential therapeutic target because it is a specific marker of the proliferative subpopulation within the conventional CSC fraction.
Keywords: cancer stem cells, colorectal cancer, organoid, CD44, RHAMM
This research highlights RHAMM+ proliferative cells within the conventional CD44+ colorectal cancer stem cell fraction. The identification of cycling cells within cancer stem cells led to a better characterization of tumor heterogeneity and the potential development of a novel therapeutic strategy.
Abbreviations
- CSC
cancer stem cell
- RHAMM
receptor for hyaluronan‐mediated motility
1. INTRODUCTION
Heterogeneous tumor tissues are composed of various cancer cell subpopulations including cancer stem cells (CSCs), the phenotypically distinct subpopulation of cancer cells. 1 , 2 , 3 CSCs possess the self‐renewal ability and multilineage differentiation ability that contribute to tumor heterogeneity. 4 , 5 , 6 Analysis of CSCs in clinical tissues necessitates their prospective isolation based on their specific cell surface markers. 7 , 8 In human colorectal cancer (CRC), cell surface markers such as CD44, 9 , 10 CD133, 11 LGR5, 12 and CD166 9 , 13 are reported as representative CSC markers. However, the use of these markers is still insufficient for the efficient enrichment of cells with CSC properties that show a self‐renewal ability in vivo or in vitro. In xenograft assays, the frequency of tumor‐initiating cells among CD44+ colorectal cancer cells was shown to range from 1 out of 62–223 cells. 14 Regarding in vitro models based on clinical colorectal cancer tissues, the organoid‐formation assay is an ideal experimental tool for phenotypic characterization of the self‐renewal ability with recapitulating clinical specimens. 15 , 16 , 17 In our previous study, only 1.5% of CD44+ cells exhibited a self‐renewal ability in an in vitro organoid model. 10 These findings suggest that conventional CSC markers of colorectal cancer are inadequate for the enrichment of bona fide CSCs. Therefore, the identification of robust surface molecules that specifically mark CSCs is warranted to understand tumor heterogeneity and develop novel therapeutic strategies.
There is a growing interest in the characterization of adult stem cells with proliferative character, 18 , 19 although they have long been regarded as slow‐cycling cells capable of self‐renewal and multilineage differentiation. 20 LGR5+ adult intestinal stem cells, the normal counterpart of the CSCs of the colon and rectum, can change their phenotype plastically from a proliferative state to the quiescent state. 21 , 22 , 23 , 24 Although the phenotypic character of isolated CSCs based on several surface markers has been fully elucidated, further investigations are still required to understand the cell cycle status of CSCs in colorectal cancer.
Accumulated evidence suggests that the molecular function of CSC markers contributes to their specific character. 25 , 26 Transmembrane glycoprotein CD44 is a widely accepted cell surface marker of CSCs in colon 9 , 10 , 14 gastric, 27 prostate, 28 and other solid tumors. 29 CD44 was shown to act as a ligand‐binding receptor by interacting with extracellular matrix (ECM) elements such as hyaluronan; this was shown to enhance the CSC properties by participating in signal transduction through the formation of transmembrane complexes. 30 The receptor for hyaluronan‐mediated motility (RHAMM) is expressed extracellularly and intracellularly in various tumor types; it was shown to form a complex with CD44 upon hyaluronan binding. 31 Extracellular RHAMM–CD44 partnering sustains CD44 surface display and enhances CD44‐mediated signaling through ERK1/2 and SRC, which promote the invasion, cell motility, and proliferation of cancer cells. 31 , 32 , 33 , 34 These data suggest that the co‐existence of RHAMM and CD44 on the cell surface is a major determinant of the properties of cancer cells.
Here, we sought to characterize the proliferative state of colorectal CSCs using transcriptomics of organoids and colorectal cancer tissues. We identified RHAMM as a cell surface marker that allows separation of the conventional CD44+ CSC fraction. The proliferative character of RHAMM+CD44+ cells suggests that these represent a phenotypically distinct subpopulation of the conventional CSCs that facilitates a further understanding of tumor biology and the development of novel therapeutic strategies.
2. MATERIALS AND METHODS
2.1. Flow cytometry and cell sorting
Isolated cells were incubated with PI (BD Bioscience; #556463) to exclude nonviable cells. Appropriate isotype‐matched, control monoclonal antibodies were used to determine the level of background staining. Cells were stained with FITC‐conjugated anti‐EpCAM (Biolegend; clone 9C4, #324204), Brilliant Violet 421‐conjugated anti‐CD44 (BD Bioscience; clone G44‐26, #562890), APC‐conjugated CD168/RHAMM (Bioss; bs‐4736R‐APC), PE‐Vio770‐conjugated CD133 (Miltenyi Biotec; clone AC133, #130–113‐672), PE‐conjugated CD166 (BioLegend; clone 3A6, #343904), and APC‐Cy7‐conjugated CD45 (BioLegend; clone H130, #304014). Cells were analyzed and sorted using the BD FACS Aria 2 and Aria 3 cell‐sorting system (BD Bioscience).
2.2. Xenotransplantation into immunodeficient mice
NOD/SCID/IL2Rγnull (NSG) mice 35 were purchased from Charles River Laboratories Japan. C57BL/6. Rag2nullIL2Rγnull mice with NOD‐type SIRPA (BRGS) mice were developed in our laboratory. 36 Both types of mice were bred and housed at Kyushu University. BRGS or NSG mice aged 4–20 weeks old were administered subcutaneous injection of cells resuspended in 50 μL of PBS and 50 μL of Matrigel. Tumor growth was followed up to 6 months. Estimated tumor volume was calculated using the formula:
tumor volume = 1/2 × length × width × width.
2.3. Tissue processing and organoid culture
We obtained surgically resected colorectal cancer tissues from 48 patients. Table S1 summarizes the clinical characteristics of these patients. The specimens were washed in PBS, minced with a razor blade, and incubated with Collagenase Type 3 (Worthington; catalog # LS004182) and DNAase1 (Wako; catalog # 043–26,773) for 60 min at 37°C using a gentleMACS dissociator. After the enzymatic digestion, the samples were sequentially filtered through 100‐ and 40‐μm cell strainers (Corning; catalog # 352340 and 352,360), and the cells were inoculated with BD Pharm Lyse (BD Biosciences; catalog # 555899) for 5 min to lyse the red blood cells. For sorting experiments, the isolated cells were sorted using surface markers and cultured from a single cell. Isolated cells were embedded in Matrigel (Corning; catalog # 356237) on ice and seeded in 24‐well or 96‐well plates depending on the cell number. Cells formed an organoid in several days, were passaged weekly by dissociating the cells with trypsin, and were replated under the conditions described previously. 10
2.4. Limiting dilution assay
For the limiting dilution assay, the cells were dissociated into single cells, and the sorted cells were diluted to the desired cell number. Cells were injected subcutaneously into the flanks of immunodeficient mice, and the number of tumors formed out of the number of sites injected was scored to determine the frequency of cancer‐initiating cells using ELDA software (http://bioinf.wehi.edu.au/software/elda/).
2.5. Viral transduction of organoids
Cells were dissociated and filtered through a cell strainer to eliminate cell clumps. Cells were diluted in the medium mentioned above in 250‐μL volumes and incubated with viral particles in a 24‐well dish. After 24 h of transfection, cells were washed and resuspended in culture medium for organoid culture or injected into immunodeficient mice as described above. The vectors showed expression of GFP, which made it possible to determine the proportion of successfully transduced cells. Transduction efficiency of more than 95% was observed by fluorescence‐activated cell sorting (FACS), excluding dead cells. Transduction efficiency was determined after culture and after the sacrifice of mice.
2.6. Plasmids and reagents
HMMR‐specific shRNA lentiviral vector was purchased from ORIGENE (TL312389). Lentiviruses containing shHMMR (RHAMM) were generated by transient transduction of the lentiviral vector together with the packaging of HEK293T cells with PAX2 and VSVG helper plasmids, as described. 10 The medium for the 293T cells was changed at 18 h after transfection, and the viral supernatant was collected, filtered, and stored at −80°C.
2.7. RNA isolation, RT‐PCR assay, and microarray analysis
Total RNA was extracted using TRIzol (Thermo Fisher Scientific; catalog # 15596026), in accordance with the manufacturer's instructions, and cDNA was generated using reverse transcriptase SuperScript Vilo (Thermo Fisher Scientific; catalog # 11755050). Quantitative real‐time PCR (qPCR) was carried out using the StepOnePlus Real‐time PCR System. Reactions were run in triplicate in three independent experiments. The geometric mean of the housekeeping gene GAPDH was used as an internal control. TaqMan gene expression assay IDs were: HMMR, Hs00234864_m1; GAPDH, Hs02758991_g1.
All reagents and instruments including probes, were obtained from Applied Biosystems, unless specified otherwise.
For microarray analysis, target populations ranging from 5000 to 10,000 cells were sorted directly into TRIzol. Total RNA was extracted and purified using the RNeasy Micro Kit (Qiagen; catalog # 74004). cRNA (1.5 μg) from each sample was hybridized to the Illumina BeadChip. Gene expression data were imported and estimated with quantile normalization using GeneSpring GX software (Agilent Technologies). The microarray data were deposited in the Gene Expression Omnibus (GEO) with the codes GSE100433 10 and GSE137919.
2.8. Single‐cell RNA‐sequencing of organoids
Organoids were grown from a single cell in a 24‐well plate. The isolated cells were washed and resuspended at a concentration of 500 cells/μL. Cell suspensions were mixed with Fluidigm C1 Suspension Reagents (Fluidigm 100–5315), added with LIVE/DEAD staining solution (Life Technologies), and loaded onto a C1 chip (Fluidigm 100–5760). Bright‐field images of captured cells were acquired using BZ‐X710 (Keyence). Single‐cell RNA isolation and amplification were performed using Fluidigm C1 Single Cell Auto Prep IFC, according to the manufacturer's protocol. The SMARTer Ultra Low RNA Kit (Clontech) was used for cDNA synthesis from single cells. Illumina library was constructed using the Nextera XT DNA Library Preparation Kit (Illumina), according to the manufacturer's protocol. cDNA library preparation was performed according to the manufacturer's protocol (PN100‐7168 G1). Quantification of cDNA libraries was performed using the Qubit dsDNA HS Assay Kit (Life Technologies) and high‐sensitivity DNA chips (Agilent). Sequencing was performed on an Illumina HiSeq 2500 system (Illumina) to achieve an average of 8.5 million reads per cell. Raw sequence data were converted to FASTQ format. Reads were mapped to the reference genome (GRCh37/hg19). Fragments per kilobase of transcript per million mapped reads (count) values were quantified and concatenated into a resulting gene expression matrix for each library. Gene expression data were imported and analyzed using GeneSpring GX software (Agilent Technologies) and SeqGeq (BD Bioscience).
2.9. Gene Set Enrichment Analysis (GSEA)
The Broad Institute provides a Java implementation of the GSEA method on its website. The gene sets were provided by MSigDB (http://software.broadinstitute.org/gsea/msigdb/index.jsp). GSEA was performed to analyze the enrichment of the gene sets following the developer's protocol. 37
Enrichment scores of pathways were calculated in GSEA and were listed in bar plots.
2.10. Statistical analysis
Unless mentioned otherwise, the results are representative of replicated experiments and are presented as mean ± standard deviation from triplicate samples or randomly chosen cells within a field. All statistical analyses were performed using the JMP software (version 11.0; SAS institute). Unpaired two‐tailed Student's t‐test was used to compare two groups and one‐way ANOVA was used to compare three or more groups. p‐values <0.05 were considered indicative of statistical significance.
3. RESULTS
3.1. Characterization of proliferative CD44+ human colorectal cancer stem cells
Cancer stem cells are phenotypically characterized by high tumor‐initiating capacity and a high potential for organoid formation. 9 , 10 , 14 , 38 To further investigate the properties of CSCs, we evaluated our previously reported transcriptomic data pertaining to human colorectal CD44+ CSCs. 10 Gene expression of CD44+ CSCs and CD44− non‐CSCs from primary colorectal cancer tissues (n = 4) and organoids (n = 4) purified by FACS were analyzed. We performed GSEA to identify the biological characteristics and key molecules that define CSCs. Compared with CD44− cells, gene sets associated with cell proliferation were significantly enriched in CD44+ CSCs of primary tissues and organoids (Figure 1A). To further identify the transcriptomic character of CSCs that across the systems, we focused on differentially expressed genes (DEGs) that were universally expressed in CD44+ CSCs from both primary tissues and organoids with a fold change (FC) cut‐off ≥2.0. In particular, the representative cell proliferation marker (MKI67), proliferative cell nuclear antigen (PCNA), 39 DNA topoisomerase II alpha (TOP2A), a baculoviral inhibitor of apoptosis repeat‐containing protein 5 (BIRC5/Survivin), 14 master regulator of cell proliferation, forkhead box M1 (FOXM1), 40 E2F family, 41 and cyclin families (CDK4 and CDK6) 42 were highly expressed in CD44+ CSCs of primary tissues and organoids (Figure 1B). These results showed that CD44+ CSCs represented the proliferative cell population in human colorectal cancer.
FIGURE 1.
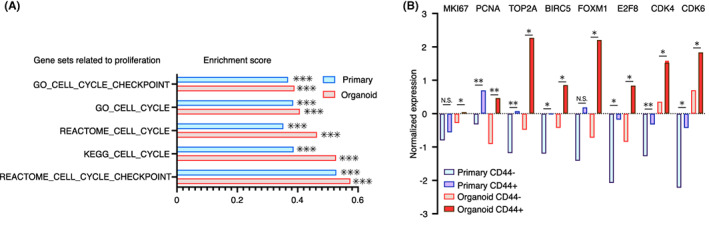
Characterization of proliferative CD44+ human colorectal cancer stem cells. (A) Results of Gene Set Enrichment Analysis (GSEA) showing significantly enriched cell cycle‐related gene sets in CD44+ CSCs of primary tissues (n = 4) and organoids (n = 4). Enrichment scores and nominal p‐values are shown. (B) Bar graph showing the normalized intensity values of expressions of representative proliferative genes in CD44− and CD44+ cells of primary (n = 4) and organoids (n = 4). Data are based on the microarray of four biological replicates for FACS‐purified CD44− and CD44+ cells of primary tissues (n = 4) and organoids (n = 4). Two‐tailed Student's t‐test was used for statistical analysis. *p < 0.05; **p < 0.01; ***p < 0.001; N.S, not significant. Statistics of GSEA was performed according to the developer's protocol.
3.2. Single‐cell RNA‐sequencing identified RHAMM+ proliferative cell population in organoid
To elucidate the proliferative nature of CD44+ CSCs, we used organoid as a model in which intratumoral heterogeneity is preserved including CSCs. 43 , 44 , 45 We isolated individual cells from patient‐derived organoids and generated a single‐cell full‐length transcriptome using SMART‐seq. We profiled transcriptome of 568 cells from four patients (Figure 2A). Firstly, we evaluated cellular heterogeneity in colon cancer organoids based on transcriptomic data by applying principal component analysis (PCA) to identify proliferative subpopulation within an organoid (n = 4). We found that principal components (PC) separated cells into two distinct subpopulations in each of the four samples (Figure 2B). PC1 high PC2 low cells were proliferative subpopulations indicated by higher expression of MKI67 (Figure 2C). We compared the PC1 high PC2 low proliferative subpopulation and the PC1 low PC2 high nonproliferative subpopulations, and extracted the top 100 DEG of each subpopulation (Table S2). Hierarchical clustering of all single cells using the top 100 genes of each subpopulation highlighted the proliferative subpopulation with a higher expression of proliferative markers (MKI67, PCNA, TOP2A, BIRC5) and critical cell proliferation regulators (MYBL2, FOXM1, CCNA2, CCNB1, CDK1) with statistical significance (Figure S1A). Interestingly, the nonproliferative subpopulation showed a higher expression of differentiation marker of the colon (KRT19, CDX2) suggesting that the organoid contains subpopulations of CSCs and differentiated cells (Figure S1A). We particularly focused on the cell surface molecule HMMR that encoded the cell surface and intracellular protein RHAMM within a proliferative subpopulation that has been shown to regulate cell proliferation. 31 , 32 , 33 , 34 We performed a correlation analysis of the top 100 genes of each subpopulation, including HMMR. HMMR showed a fairly high correlation with proliferative genes (MKI67, AURKA, FOXM1) that indicated that HMMR+ cells were proliferative (Figure S1B). Notably, the mapping of HMMR‐positive cells in a PCA plot overlapped with the proliferative subpopulation we identified (Figure 2D). In contrast, CD44‐positive cells were broadly expressed in the entire population (Figure 2E), suggesting that conventional CD44‐positive cell population could be further characterized into a proliferative subpopulation by the expression of HMMR (RHAMM). We next characterized the gene expression of HMMR (RHAMM)+CD44+ cells compared with HMMR (RHAMM)−CD44+ cells. Higher expression of MKI67, PCNA, TOP2A, BIRC5, FOXM1 and lower expression of KRT19, KRT20, CDX2 were observed in HMMR (RHAMM)+CD44+ cells (Figure 2F). Taken together, we identified an HMMR (RHAMM)+CD44+ proliferative subpopulation using organoids that recapitulated the intratumoral heterogeneity of human colorectal cancer.
FIGURE 2.
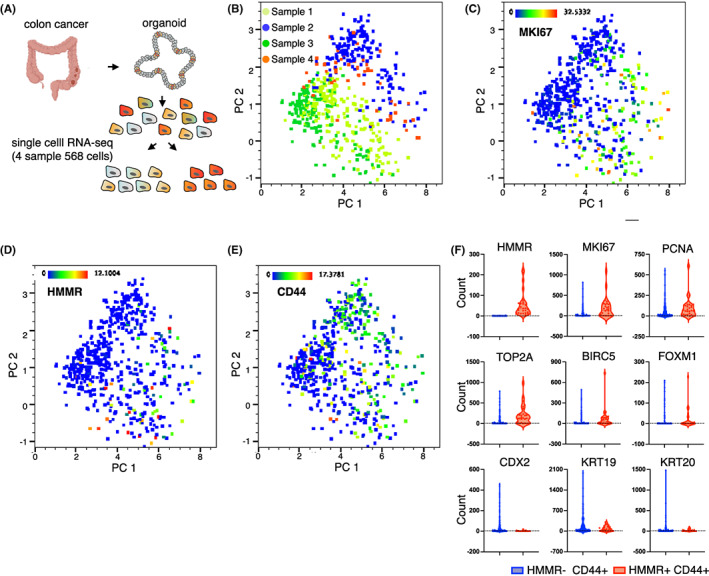
Single‐cell RNA‐sequencing identifies RHAMM+ proliferative cell population in organoids. (A) Workflow showing scheme of the experiment. Four colon cancer patient‐derived organoids were isolated into single cells and 568 cells were subjected to single‐cell RNA‐sequencing. (B) Principal component analysis (PCA) of location‐averaged transcriptome for 568 single cells colored by patient (n = 4). (C) PCA map showing PC1 high PC2 low cells reveals higher MKI67 expressions among four patients. (D, E) Distribution of (D) HMMR‐positive cells and (E) CD44‐positive cells in PCA map. (F) Violin plots showing the mean and variance of gene expressions difference between HMMR (RHAMM)−/+ population within CD44+ cells.
3.3. RHAMM+CD44+ cells represent proliferative character of CSCs
First, the cell surface expression of RHAMM was examined by FACS in 24 patients with surgically resected colorectal cancer tissues (Figure 3A). The expression of RHAMM was detected in all cancer tissue specimens; the median frequency in these specimens (25.7%) was significantly greater than that in normal tissues (10.3%) (Figure 3B). Interestingly, we were able to divide the CD44+ CSC fraction into CD44+RHAMM+ and CD44+RHAMM− fractions; the median frequency of these fractions was 7.1% (CD44+RHAMM+) and 10.3% (CD44+RHAMM−), respectively (Figure 3C). To characterize the gene expression patterns of RHAMM+CD44+ CSCs, we purified 5000 numbers of RHAMM+CD44+ and RHAMM−CD44+ cells by FACS and subjected these to microarray analysis. GSEA revealed that the enrichment of cell cycle‐related genes in the RHAMM+CD44+ fraction compared with the RHAMM−CD44+ fraction (Figure 3D). The clear enrichment of the top 100 proliferative genes (Table S2) was also observed in RHAMM+CD44+ cells with high expression of proliferative genes including MKI67, PCNA, TOP2A, BIRC5, AURKA, and master regulators of proliferation (FOXM1, MYBL2) (Figure 3E). Furthermore, the RHAMM+CD44+ fraction exhibited enrichment of the MAPK signaling pathway and SRC signaling pathway, which are known to be activated by hyaluronan‐mediated binding of CD44 and RHAMM 31 , 32 , 33 , 34 (Figure 3F). These results suggested that RHAMM+CD44+ cells represented the proliferative characteristics of CSCs.
FIGURE 3.
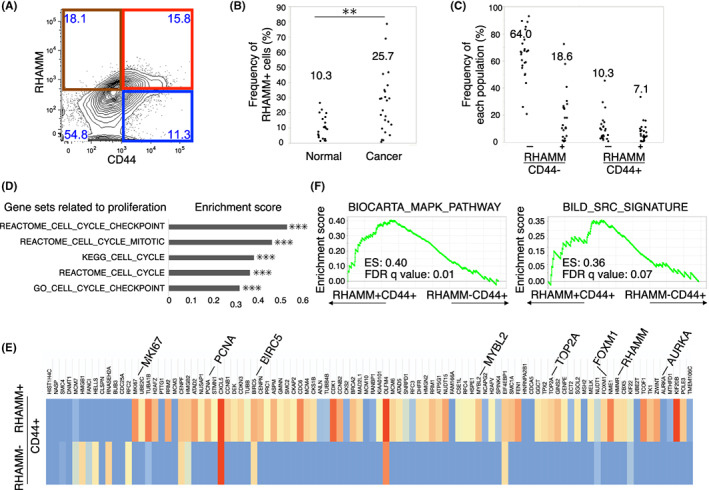
RHAMM+CD44+ CSCs retained CSC signature as well as proliferative gene expression patterns. (A) Representative FACS plot of primary human colorectal cancer tissues. Each subpopulation is segmented by red (RHAMM+CD44+), blue (RHAMM−CD44+), brown (RHAMM+CD44−), and no‐color (RHAMM−CD44−). (B) Dot plot showing RHAMM‐positive cells among human colonic normal (n = 20) and cancer tissues (n = 24) analyzed by FACS. Median frequencies of biological replicates are indicated. Two‐tailed Student's t‐test was used for statistical analysis. **p < 0.01. (C) Dot plot showing the frequency of each population. Median frequencies of biological replicates (n = 24) are indicated. (D) Bar graph of enrichment scores in GSEA regarding cell cycle‐related gene sets upregulated in RHAMM+CD44+ cells (n = 3) compared with RHAMM−CD44+ cells (n = 3). Nominal p‐values are shown. ***p < 0.001. (E) Heatmap showing the expression of proliferative related genes in RHAMM+CD44+ cells (n = 3) compared with RHAMM−CD44+ cells (n = 3). Gene expression of biologically replicated three samples was normalized. Expression levels of HMMR (RHAMM), representative proliferative genes (MKI67, PCNA TOP2A, BIRC5, AURKA), master regulators of cell cycle (FOXM1, MYBL2) are highlighted. (F) GSEA tables of MAPK signaling and SRC signaling that were significantly enriched in RHAMM+CD44+ cells (n = 3). Enrichment score and false discover rate (FDR) q values are shown. Data are based on the microarray data of biological replicates for FACS‐purified RHAMM+CD44+ cells compared with RHAMM−CD44+ cells of primary tissues (n = 3). Statistics of GSEA was performed according to the developer's protocol.
3.4. RHAMM expression enriched the functional CSCs within CD44+ CRC cells
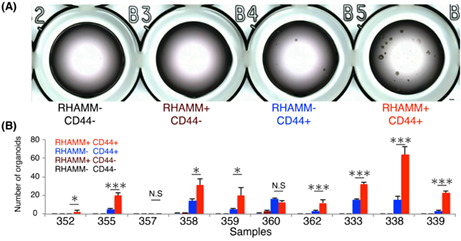
In our previous study, ~1%–2% of CD44+ cells among freshly isolated human colorectal cancer tissues were found to form organoids 10 ; this suggests that only a small fraction of CD44+ cells possessed the properties of CSCs. To investigate the organoid‐forming ability of RHAMM‐positive cells, we purified 1000 cells of each fraction (CD44−RHAMM−, CD44−RHAMM+, CD44+RHAMM−, CD44+RHAMM+), and evaluated the efficiency of organoid formation in 96‐well culture plate. CD44+ cells exhibited distinctly greater organoid‐forming ability compared with CD44− cells (CD44+: 1.25% vs CD44−: 0%) (Figure 4A), which was consistent with our previous report. 10 Of note, RHAMM+CD44+ fraction showed a significantly greater efficiency for organoid formation within the CD44+ fraction (RHAMM+CD44+: 2.0% vs. RHAMM−CD44+: 0.5%) in eight out of 10 samples (one sample did not show organoid formation in any fraction) (Figure 4B). To determine the frequency of tumor‐initiating cells in RHAMM+CD44+ cells in vivo, we injected different cell numbers of purified RHAMM+CD44+ and RHAMM−CD44+ cells (100, 500, 2500 cells) into immunodeficient mice. The frequency of tumor‐initiating cells was calculated using the estimation method for limiting dilution assay. We calculated the tumor‐initiating cells of 1 in 279 cells (sample #369), 453 cells (sample #297), and 772 cells (sample #364) for RHAMM+CD44+ cells, and 1 in 7985 cells (sample #369), 4125 cells (sample #297), and 5963 cells (sample #364) for RHAMM−CD44+ cells (Figure 4C; Table S3). Furthermore, representative CSC surface markers, such as CD133 and CD166, were highly enriched in RHAMM+CD44+ cells compared to the other fractions including RHAMM−CD44+ cells (Figure 4D). These results collectively suggest that RHAMM is a marker that can enrich the functional CSCs among the CD44+ conventional CSC population.
FIGURE 4.
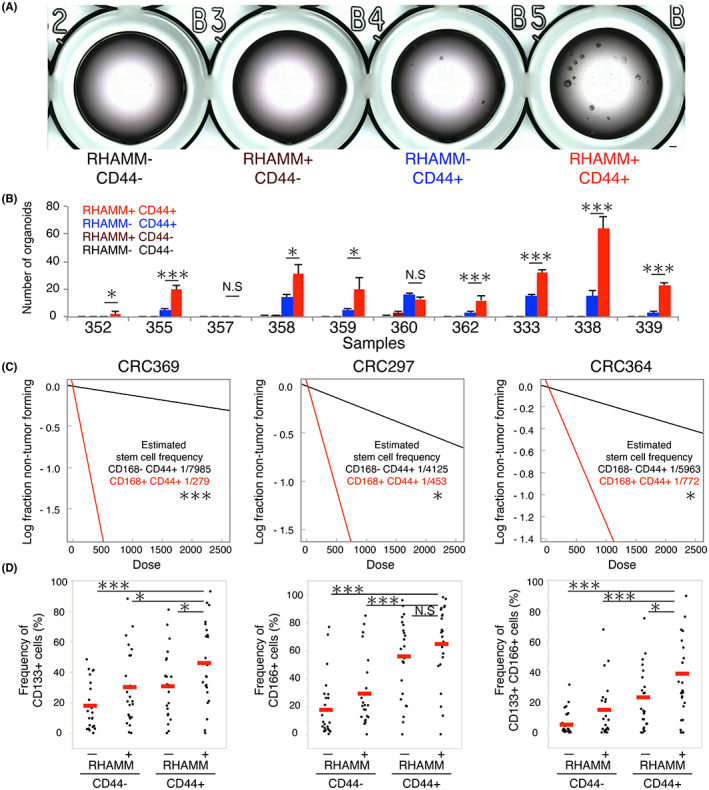
RHAMM expression enriched the functional CSCs within CD44+ CRC cells. (A) Bright‐field images of organoid‐cultured FACS‐purified 1000 cells of each fraction in 96‐well plates. Bar indicates 500 μm. (B) Bar graph showing the number of organoids cultured FACS‐purified cells in 96 well plates (n = 10). Mean values from three independent experiments are shown. Two‐tailed Student's t‐test was used for statistical analysis. (C) Limiting dilution analysis of FACS‐purified RHAMM+CD44+ and RHAMM−CD44+ cells in xenograft assay using three samples (n = 3). Data are plotted on a log scale. Stem cell frequencies and p‐values were estimated according to the developers' protocol. (D) Dot plots showing the frequencies of CD133+, CD166+, and CD133+CD166+ cells in each population analyzed by FACS. Median values are indicated by red lines (n = 24). RHAMM+CD44+ cells are compared with other populations. Two‐tailed Student's t‐test was used for statistical analysis. *p < 0.05; **p < 0.01; ***p < 0.001; N.S, not significant.
3.5. RHAMM is a potential therapeutic target in colorectal cancer
We next tested whether RHAMM is a functional molecule in CSCs. To this end, we transduced two lentiviral short hairpin RNAs (shRNA) into organoids. We confirmed the sufficient reduction of HMMR expression (Figure S2). Subsequently, we evaluated the effects of HMMR silencing on organoid formation. Significant inhibition of organoid‐forming ability was observed in seven clinical colorectal cancer samples, which suggests a close involvement of RHAMM in maintaining the organoid‐forming ability of CSCs, at least in vitro (Figure 5A,B). RHAMM inhibition does not affect CD44 expression (Figure S3A,B). Next, we transplanted the 5000 cells derived from organoids after 24 h transfection of shRNA and scrambled shRNA into immunodeficient mice. Knockdown of HMMR significantly reduced the tumor formation, which suggests that RHAMM inhibition impaired the tumor‐initiating potential of CSCs in vivo (Figure 5C). To evaluate the chemosensitivity of RHAMM+CD44+ cells, we evaluated the Oxaliplatin sensitivity of RHAMM knocked‐down CD44‐positive colon cancer cell line. Oxaliplatin sensitivity was reduced in the HMMR knocked‐down cell line (Figure S4), suggesting that cytotoxic drugs can target the proliferative characteristics of RHAMM‐positive cells. These results collectively suggest that RHAMM is a functional cell surface molecule required for maintaining the proliferative character of CSCs, and that RHAMM is a potential therapeutic target in human colorectal cancer.
FIGURE 5.
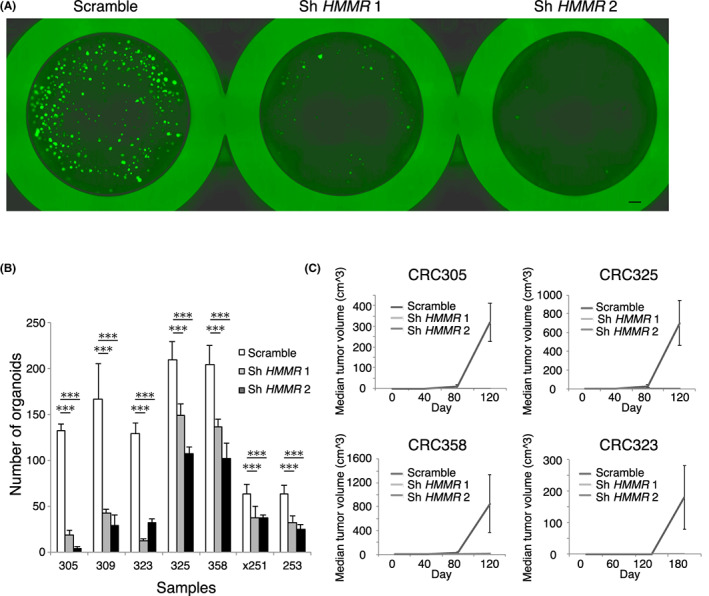
RHAMM is a potential therapeutic target in colorectal cancer. (A) Fluorescent images of GFP‐expressing vector transduced organoids. Organoid formation was inhibited by knockdown of HMMR using two shRNA vectors. Bar indicates 500 μm. (B) Bar graph showing the number of organoids after seeding 5000 transfected cells. Organoid formation was significantly inhibited by knockdown of HMMR (n = 7). Mean values from three independent experiments are shown. (C) Line graphs showing tumor volumes of transfected cells in xenograft assay. Tumor‐initiating ability was inhibited by knockdown of HMMR (n = 4). Mean values from three independent experiments are shown. Two‐tailed Student's t‐test was used for statistical analysis. ***p < 0.001.
4. DISCUSSION
Identification of markers to characterize CSCs is a key to understanding tumor heterogeneity, and identifying potential therapeutic targets. Here, we report a cell surface marker RHAMM identifies the proliferative subpopulation among the conventional CD44+ CSC population. Results of our transcriptomic analysis of RHAMM+CD44+ cells are consistent with previous studies; several studies have shown that proliferative signal transduction is mediated via extracellular binding between RHAMM and CD44, which activates ERK and SRC signaling. 31 , 32 , 33 , 34 In general, adult stem cells are maintained in a state of quiescence under conditions of homeostasis; however, these retain the ability for rapid expansion. 21 , 22 , 23 , 24 Nonetheless, there is no clear consensus whether CSCs reside in a proliferative or quiescent state. Active signal transduction that regulates the properties of CSCs may provide insights into the cell cycle status of CSCs. For example, active Wnt signaling in human colorectal CSCs 12 , 46 was shown to indicate the proliferative status of CSCs, a phenomenon similar to that observed in some adult stem cells such as intestinal, 15 , 18 , 21 , 23 , 24 hair follicle, 19 and liver 22 stem cells. In this study, we clarified that CD44+ cells were in a proliferative state compared with CD44− cells; furthermore RHAMM+CD44+ cells represented the most proliferative subpopulation among these by transcriptomic analysis based on the prospective isolation of CSCs from primary tissues and organoids. We showed the proliferative character of RHAMM+ and CD44+ cells which were isolated by antibody‐based detection of cell surface protein expression. Single‐cell transcriptomic data for organoids showed a high correlation of HMMR but not a perfect correlation of CD44 with proliferative genes (Figure S1B). The discrepancy between mRNA and cell surface expression of CD44 for marking proliferative cell population is observed in the study, it might be due to complex causes including post‐translational modifications of CD44 47 and the existence of the CD44 variant. Cell surface RHAMM was identified as a mitogenic factor required for cell motility, and evidence of its role in G2M progression has already been demonstrated. 31 Furthermore, RHAMM has been reported to promote the localization of CD44 on the cell surface shown by co‐immunoprecipitation of RHAMM and CD44 in a breast cancer cell line, 32 leading to the formation of RHAMM–CD44–ERK1/2 complexes and activation of MAPK signaling. 31 , 32 , 33 , 34 These data suggest that RHAMM+CD44+ cells should reveal active MAPK signaling based on the RHAMM–CD44–ERK1/2 complex on the cell surface and potentially possess a proliferative capacity. The enrichment of MAPK and ERK signaling‐related genes in GSEA of RHAMM+CD44+ cells is also consistent with this theoretical background. As CD44 variant (v) isoforms are known CSC markers and reported to transduce MAPK signaling 48 through binding with hyaluronic acid as in CD44 standard isoforms, RHAMM potentially contributes to CD44v‐positive CSC biology. Because co‐expression of RHAMM and CD44v has been reported in B cell lymphomas, 49 further investigation on the correlation between RHAMM and CD44v in broad cancer types is imperative. TGF‐β signaling is another pathway that potentially attenuates CSC properties, however we did not observe any effect on RHAMM expression (Figure S5). Given that intestinal stem cells dynamically change their phenotype between the proliferative state and the quiescent state, 21 , 22 , 23 , 24 further studies should investigate the mechanism by which the RHAMM+CD44+ subpopulation transitions from a proliferative state to a dormant state and phenotypically change into another population.
Our findings in relation to the proliferative status of colorectal CSCs have therapeutic implications with respect to the targeting of this trait. Expression of RHAMM was shown to correlate with worse prognosis in the context of breast cancer 50 and colon cancer. 51 RHAMM expression was shown to be a marker of aggressive subpopulation associated with invasive characteristics; in addition, RHAMM expression was an independent predictor of tumor–node–metastasis (TNM) classification. 52 , 53 In our study, inhibition of HMMR (RHAMM) resulted in the loss of CSC properties, as reflected in decreased organoid formation and tumorigenicity. These data are consistent with previous studies in which silencing of HMMR (RHAMM) was shown to suppress stemness in glioblastoma, 54 and inhibit proliferation of breast cancer, 32 lung cancer, 34 and colon cancer 53 cells. Although it is preferable to investigate RHAMM expression depending on molecular subtype, RHAAM is known to be expressed in the cell membrane and cytoplasm in most of the proficient/deficient mismatch repair (MMR) colorectal cancer patients (>90%) compared with normal mucosa,52 suggesting that RHAMM seems to be one of the therapeutic targets in colorectal cancer patients. At the same time, the limitations of targeting only the RHAMM+ subpopulation have to be considered. In our study, RHAMM+ cells accounted for only 25.7% of the entire population of human colorectal cancer cells, although these were the most proliferative cells with stemness. Taking into consideration tumor heterogeneity and the hypothesized plasticity between the RHAMM+CD44+ subpopulation and other populations, targeting only RHAMM is insufficient but a multitarget strategy may be required to eradicate all cancer cells.
In summary, we identified a novel subpopulation among human colorectal cancer cells. RHAMM+CD44+ proliferative cells with stem cell activity represent a subpopulation within the conventional CD44+ fraction. The proliferative characteristics of CSCs represented by the RHAMM+CD44+ subpopulation were identified by combining multiple transcriptomics. Our findings facilitate better characterization of tumor heterogeneity and the potential development of novel therapeutic strategies.
AUTHOR CONTRIBUTIONS
M.N. and Y.K. conceived and designed the study. M.N. and K.M. performed and analyzed the transcriptomics. T.R. and T.I. performed single‐cell transcriptome analysis. S.M. constructed the shRNA. T.I., T.Y, and K.Y. developed the methodology of experiments. M Nakamura contributed to providing clinical materials. M.N. and Y.K. wrote reviewed, and/or revised the manuscript. N.T., F.H. contributed to the acquisition of data and technical support. Y.K., H.A., H.K., T.M., C.K., E.B., K.A. provided study supervision.
FUNDING INFORMATION
This study was supported in part by WAT‐NeW (to M.N. Takarabio and Fluidigm Single Cell Analysis Award), the Shin‐Nihon Foundation of Advanced Medical Research (to M.N., Y.K., H.A.), a Grant‐in‐Aid for Early‐Career Scientists (to M.N. No.19 K16852), a Grant‐in‐Aid for Young Scientists (A) (to Y.K. No.16748470), a Grant‐in‐Aid for Scientific Research (S) (to K.A. No.16747244), a Grant‐in‐Aid for Scientific Research (C) (to E.B., #15 K08970), a Grant‐in‐Aid for Scientific Research (B) (to Y.K. No.19109659), a Grant‐in‐Aid for Scientific Research (C) (to H.A., #18 K07202) from Ministry of Education, Culture, Sports, Science, and Technology of Japan. This study was also supported in part by a Grant‐in‐Aid for Japan Agency for Medical Research and Development (to Y.K. 16,768,249 and to K.A. 16,770,576).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICAL APPROVAL
All human studies were performed in accordance with the principles of the Declaration of Helsinki principles and the study was approved by the Research Ethics Committee on Human Experimentation of Kyushu University.
Approval of the research protocol by an Institutional Reviewer Board.
Informed Consent: Written informed consent was obtained from all subjects prior to their enrolment.
Registry and Registration No. of the study: #28–95 (Research Ethics Committee on Human Experimentation of Kyushu University). #25–106 (Animal Care Committee of Kyushu University).
Animal Studies: All animal experiments were performed in accordance with the institutional guidelines approved by the Animal Care Committee of Kyushu University.
Supporting information
Appendix S1.
Table S2A.
Table S3A.
ACKNOWLEDGMENTS
The authors especially thank N. Torada, T. Ueki, and T. Manabe, S. Nagai from the Department of Surgery and Oncology, Kyushu University, for providing clinical colorectal cancer tissues. They also thank N. Hosono from Fluidigm for performing single‐cell RNA‐sequencing, and T. Sugio and T. Jiroumaru for performing microarray analysis. Co‐workers at our laboratories, K. Sagara, Y. Okumura, M. Ito, and K. Tsuchihashi, supported this work.
Nakano M, Taguchi R, Kikushige Y, et al. RHAMM marks proliferative subpopulation of human colorectal cancer stem cells. Cancer Sci. 2023;114:2895‐2906. doi: 10.1111/cas.15795
Contributor Information
Yoshikane Kikushige, Email: kikushige.yoshikane.726@m.kyushu-u.ac.jp.
Eishi Baba, Email: baba.eishi.889@m.kyushu-u.ac.jp.
DATA AVAILABILITY STATEMENT
The datasets generated during the current study are available from the GEO with numbers GSE100433, GSE137919, and GSE140897.
REFERENCES
- 1. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105‐111. [DOI] [PubMed] [Google Scholar]
- 2. Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124‐1134. [DOI] [PubMed] [Google Scholar]
- 4. De Sousa EMF, Vermeulen L, Fessler E, Medema JP. Cancer heterogeneity–a multifaceted view. EMBO Rep. 2013;14:686‐695. Cancer heterogeneity—a multifaceted view. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275‐291. [DOI] [PubMed] [Google Scholar]
- 6. Clarke MF. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;380:2237‐2245. [DOI] [PubMed] [Google Scholar]
- 7. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730‐737. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Hajj M, Wicha MS, Benito‐Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983‐3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158‐10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakano M, Kikushige Y, Miyawaki K, et al. Dedifferentiation process driven by TGF‐beta signaling enhances stem cell properties in human colorectal cancer. Oncogene. 2019;38:780‐793. [DOI] [PubMed] [Google Scholar]
- 11. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106‐110. [DOI] [PubMed] [Google Scholar]
- 12. Shimokawa M, Ohta Y, Nishikori S, et al. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature. 2017;545:187‐192. [DOI] [PubMed] [Google Scholar]
- 13. Levin TG, Powell AE, Davies PS, et al. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology. 2010;139(6):2072‐2082.e5. e2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalerba P, Kalisky T, Sahoo D, et al. Single‐cell dissection of transcriptional heterogeneity in human colon tumors. Nat Biotechnol. 2011;29:1120‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato T, Stange DE, Ferrante M, et al. Long‐term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762‐1772. [DOI] [PubMed] [Google Scholar]
- 16. Fujii M, Shimokawa M, Date S, et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18:827‐838. [DOI] [PubMed] [Google Scholar]
- 17. Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586‐1597. [DOI] [PubMed] [Google Scholar]
- 18. Barker N, van Es JH, Jaks V, et al. Very long‐term self‐renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:351‐356. [DOI] [PubMed] [Google Scholar]
- 19. Jaks V, Barker N, Kasper M, et al. Lgr5 marks cycling, yet long‐lived, hair follicle stem cells. Nat Genet. 2008;40:1291‐1299. [DOI] [PubMed] [Google Scholar]
- 20. Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin‐1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149‐161. [DOI] [PubMed] [Google Scholar]
- 21. Yan KS, Chia LA, Li X, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao W, Chen K, Bolkestein M, et al. Dynamics of proliferative and quiescent stem cells in liver homeostasis and injury. Gastroenterology. 2017;153:1133‐1147. [DOI] [PubMed] [Google Scholar]
- 23. Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone‐producing Enteroendocrine cells. Cell Stem Cell. 2017;20:177‐190. [DOI] [PubMed] [Google Scholar]
- 24. Yan KS, Janda CY, Chang J, et al. Non‐equivalence of Wnt and R‐spondin ligands during Lgr5(+) intestinal stem‐cell self‐renewal. Nature. 2017;545:238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19:387‐400. [DOI] [PubMed] [Google Scholar]
- 26. Zoller M. CD44: can a cancer‐initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254‐267. [DOI] [PubMed] [Google Scholar]
- 27. Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patrawala L, Calhoun T, Schneider‐Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696‐1708. [DOI] [PubMed] [Google Scholar]
- 29. Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer‐initiating cells from primary human tumors. Cancer Res. 2008;68:4311‐4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33‐45. [DOI] [PubMed] [Google Scholar]
- 31. Maxwell CA, McCarthy J, Turley E. Cell‐surface and mitotic‐spindle RHAMM: moonlighting or dual oncogenic functions? J Cell Sci. 2008;121:925‐932. [DOI] [PubMed] [Google Scholar]
- 32. Hamilton SR, Fard SF, Paiwand FF, et al. The hyaluronan receptors CD44 and Rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells. J Biol Chem. 2007;282:16667‐16680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tolg C, Hamilton SR, Nakrieko KA, et al. Rhamm−/− fibroblasts are defective in CD44‐mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol. 2006;175:1017‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song JM, Im J, Nho RS, Han YH, Upadhyaya P, Kassie F. Hyaluronan‐CD44/RHAMM interaction‐dependent cell proliferation and survival in lung cancer cells. Mol Carcinog. 2019;58:321‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565‐1573. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamauchi T, Takenaka K, Urata S, et al. Polymorphic Sirpa is the genetic determinant for NOD‐based mouse lines to achieve efficient human cell engraftment. Blood. 2013;121:1316‐1325. [DOI] [PubMed] [Google Scholar]
- 37. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545‐15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vermeulen L, Todaro M, de Sousa MF, et al. Single‐cell cloning of colon cancer stem cells reveals a multi‐lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008;105:13427‐13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kubben FJ, Peeters‐Haesevoets A, Engels LG, et al. Proliferating cell nuclear antigen (PCNA): a new marker to study human colonic cell proliferation. Gut. 1994;35:530‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wierstra I, Alves J. FOXM1, a typical proliferation‐associated transcription factor. Biol Chem. 2007;388:1257‐1274. [DOI] [PubMed] [Google Scholar]
- 41. Weinberg RA. E2F and cell proliferation: a world turned upside down. Cell. 1996;85:457‐459. [DOI] [PubMed] [Google Scholar]
- 42. Hunter T, Pines J. Cyclins and cancer. II: cyclin D and CDK inhibitors come of age. Cell. 1994;79:573‐582. [DOI] [PubMed] [Google Scholar]
- 43. Roerink SF, Sasaki N, Lee‐Six H, et al. Intra‐tumour diversification in colorectal cancer at the single‐cell level. Nature. 2018;556:457‐462. [DOI] [PubMed] [Google Scholar]
- 44. Kopper O, de Witte CJ, Lohmussaar K, et al. An organoid platform for ovarian cancer captures intra‐ and interpatient heterogeneity. Nat Med. 2019;25:838‐849. [DOI] [PubMed] [Google Scholar]
- 45. Patel AP, Tirosh I, Trombetta JJ, et al. Single‐cell RNA‐seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468‐476. [DOI] [PubMed] [Google Scholar]
- 47. Cichy J, Pure E. The liberation of CD44. J Cell Biol. 2003;161:839‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nagel S, Hirschmann P, Dirnhofer S, Gunthert U, Tzankov A. Coexpression of CD44 variant isoforms and receptor for hyaluronic acid‐mediated motility (RHAMM, CD168) is an international prognostic index and C‐MYC gene status‐independent predictor of poor outcome in diffuse large B‐cell lymphomas. Exp Hematol. 2010;38:38‐45. [DOI] [PubMed] [Google Scholar]
- 50. Wang C, Thor AD, Moore DH 2nd, et al. The overexpression of RHAMM, a hyaluronan‐binding protein that regulates ras signaling, correlates with overexpression of mitogen‐activated protein kinase and is a significant parameter in breast cancer progression. Clin Cancer Res. 1998;4:567‐576. [PubMed] [Google Scholar]
- 51. Zlobec I, Terracciano L, Tornillo L, et al. Role of RHAMM within the hierarchy of well‐established prognostic factors in colorectal cancer. Gut. 2008;57:1413‐1419. [DOI] [PubMed] [Google Scholar]
- 52. Lugli A, Zlobec I, Gunthert U, et al. Overexpression of the receptor for hyaluronic acid mediated motility is an independent adverse prognostic factor in colorectal cancer. Mod Pathol. 2006;19:1302‐1309. [DOI] [PubMed] [Google Scholar]
- 53. Mele V, Sokol L, Kolzer VH, et al. The hyaluronan‐mediated motility receptor RHAMM promotes growth, invasiveness and dissemination of colorectal cancer. Oncotarget. 2017;8:70617‐70629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tilghman J, Wu H, Sang Y, et al. HMMR maintains the stemness and tumorigenicity of glioblastoma stem‐like cells. Cancer Res. 2014;74:3168‐3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Table S2A.
Table S3A.
Data Availability Statement
The datasets generated during the current study are available from the GEO with numbers GSE100433, GSE137919, and GSE140897.


