Abstract
The effect of body mass index (BMI) on esophageal and gastric carcinogenesis might be heterogeneous, depending on subtype or subsite. However, findings from prospective evaluations of BMI associated with these cancers among Asian populations have been inconsistent and limited, especially for esophageal adenocarcinoma and gastric cardia cancer. We performed a pooled analysis of 10 population‐based cohort studies to examine this association in 394,247 Japanese individuals. We used Cox proportional hazards regression to estimate study‐specific hazard ratios (HRs) and 95% confidence intervals (CIs), then pooled these estimates to calculate summary HRs with a random effects model. During 5,750,107 person‐years of follow‐up, 1569 esophageal cancer (1038 squamous cell carcinoma and 86 adenocarcinoma) and 11,095 gastric (728 cardia and 5620 noncardia) cancer incident cases were identified. An inverse association was observed between BMI and esophageal squamous cell carcinoma (HR per 5‐kg/m2 increase 0.57, 95% CI 0.50–0.65), whereas a positive association was seen in gastric cardia cancer (HR 1.15, 95% CI 1.00–1.32). A nonsignificant and significant positive association for overweight or obese (BMI ≥25 kg/m2) relative to BMI <25 kg/m2 was observed with esophageal adenocarcinoma (HR 1.32, 95% CI 0.80–2.17) and gastric cardia cancer (HR 1.24, 95% CI 1.05–1.46), respectively. No clear association with BMI was found for gastric noncardia cancer. This prospective study—the largest in an Asian country—provides a comprehensive quantitative estimate of the association of BMI with upper gastrointestinal cancer and confirms the subtype‐ or subsite‐specific carcinogenic impact of BMI in a Japanese population.
Keywords: body mass index, esophageal cancer, gastric cancer, large‐scale population‐based cohort studies, pooled analysis
The impact of BMI on upper gastrointestinal cancer by subtype or subsite among Asians is inconclusive. Using data from 10 large‐scale population‐based cohort studies, we evaluated the association between BMI and upper gastrointestinal cancers for 394,247 Japanese individuals. With 1038 esophageal squamous cell carcinoma, 86 esophageal adenocarcinoma, 728 gastric cardia cancer, and 5620 gastric noncardia cancer cases, we confirmed the subtype‐ or subsite‐specific carcinogenic impact of BMI in an Asian population.
Abbreviations
- aHR
adjusted hazard ratio
- AICHI
Three‐Prefecture Cohort Study in Aichi
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- ICD‐O‐3
International Classification of Diseases for Oncology, third edition
- ICD‐O‐3‐M
ICD‐O‐3 morphology
- ICD‐O‐3‐T
ICD‐O‐3 tomography
- JACC
Japan Collaborative Cohort Study
- JPHC‐ I
Japan Public Health Center‐ based Prospective Study I
- JPHC‐ II
Japan Public Health Center‐ based Prospective Study II
- LSS
Life Span Study
- MIYAGI‐I
Miyagi Cohort Study
- MIYAGI‐II
Three‐Prefecture Cohort Study in Miyagi
- OHSAKI
Ohsaki Cohort Study
- OSAKA
Three‐Prefecture Cohort Study in Osaka
- RR
relative risk
- TAKAYAMA
Takayama Study
1. INTRODUCTION
Esophageal and gastric cancers remain common worldwide, affecting more than 604,000 and 1,089,000 people in 2020, respectively. 1 Incidence rates are highest in Asian countries, among which Japan showed the third‐ and second‐largest number of new cases (~26,000 and ~138,000 cases), respectively. 1 The etiologies of these cancers are heterogeneous, depending on subtype or subsite. Of the two most common histological subtypes of esophageal cancer, squamous cell carcinoma is the predominant histological subtype in Asians, and is strongly associated with alcohol drinking and smoking. 2 , 3 The other most common subtype, esophageal adenocarcinoma, is more common in Caucasians, and the major risk factors are obesity and smoking. 2 , 3 For gastric cancer, the most common subtype is adenocarcinoma, but etiology differs between subsites: gastric cardia cancer appears to share a common etiology with esophageal adenocarcinoma and is associated with obesity and smoking, whereas noncardia cancer—the most prevalent subsite in Asia—is associated with Helicobacter pylori infection and smoking. 4 , 5 , 6
A number of epidemiological studies have confirmed a positive association between body mass index (BMI) and esophageal adenocarcinoma and gastric cardia cancer. 7 , 8 , 9 The World Cancer Research Fund and American Institute of Cancer Research accordingly concluded that this association was “convincing” for esophageal adenocarcinoma and “probable” for gastric cardia cancer. 3 , 4 One plausible mechanism for this association is that the development of gastroesophageal reflux disease or inflammation of the esophagus promoted by greater body fatness induces Barrett's esophagus, resulting in increased risk of esophageal adenocarcinoma and gastric cardia cancer. 10 , 11 In contrast, however, epidemiological evidence has shown an inverse association between BMI and esophageal squamous cell carcinoma, 7 and no clear association between BMI and gastric noncardia cancer. 9
Given that Asians generally have greater adiposity with the same BMI 12 and different dietary activities and lifestyles compared with Caucasians, it is crucial to assess whether the magnitude and direction of the associations between BMI and risk of upper gastrointestinal cancer in Asians are comparable to those in Caucasians. Several epidemiological studies have evaluated the association between BMI and esophageal and gastric cancer risk among Asian populations, 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 but the findings were inconsistent and limited, particularly with regard to esophageal adenocarcinoma and gastric cardia cancer. A recent large‐scale pooled analysis in Asian countries with more than 800,000 individuals reported that underweight (BMI <18.5 kg/m2) and extreme obesity (BMI ≥35 kg/m2) were associated with the mortality risk of overall esophageal and esophageal squamous cell carcinoma, but showed no clear association between esophageal adenocarcinoma and BMI. 20 A second pooled analysis including more than 500,000 Asian individuals showed a U‐shaped association between BMI and incidence risk of overall gastric and gastric noncardia cancer, but failed to confirm increased risk of gastric cardia cancer incidence in overweight and obese people. 19 These findings suggest that the association between BMI and these cancers shows different patterns between populations. However, considering that lifestyle and environmental factors are strongly associated with both BMI as well as esophageal and gastric cancer risk, unmeasured confounders due to the pooling of various populations with heterogeneous lifestyle and environmental factors might bias the results.
Here, we conducted a pooled analysis of 10 population‐based prospective cohort studies in Japan comprising approximately 400,000 subjects and evaluated the association of BMI with esophageal and gastric cancers by subtype or subsite with unified BMI categories. This study included general Japanese populations sharing a similar lifestyle and living environment, used incidence rather than mortality as an end point, and adjusted for several important covariates uniformly across cohorts, making it possible to increase the generalizability of the results and identify the risk contribution of BMI directly.
2. METHODS
2.1. Study population
To evaluate the association between lifestyle factors and cancer risk, the Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan has been evaluating the association between lifestyle factors and cancer risk by conducting pooled analyses using original data from large‐scale population‐based cohort studies in Japan that met inclusion criteria described elsewhere. 21 The present study included 10 studies: the Japan Public Health Center‐based Prospective Study (JPHC‐I and ‐II), 22 the Japan Collaborative Cohort Study (JACC), 23 the Miyagi Cohort Study (MIYAGI‐I), 24 the Three‐Prefecture Cohort Study in Miyagi (MIYAGI‐II), 25 the Three‐Prefecture Cohort Study in Aichi (AICHI), 25 the Takayama Study (TAKAYAMA), 26 the Ohsaki Cohort Study (OHSAKI), 27 the Three‐Prefecture Cohort Study in Osaka (OSAKA), 25 and the Life Span Study (LSS) 28 (Table 1). We excluded subjects with a past history of cancer at baseline, subjects with unknown information on BMI, subjects with extreme values of BMI (BMI <14 or >40 kg/m2), and subjects with estimated radiation doses from the atomic bombings of ≥100 mGy (for LSS). All studies were reviewed and approved by their relevant institutional ethics review boards. The JPHC‐I and ‐II, 17 , 18 MIYAGI‐II, 15 and TAKAYAMA 16 studies have already evaluated the association between BMI and esophageal or gastric cancer risk in their respective cohorts. We reanalyzed the association using the updated datasets of these studies.
TABLE 1.
Characteristics of the cohort studies included in the present pooled analysis
| Study | Initial population | Age range at baseline (years) | Follow‐up (start) | Follow‐up (end) | Average follow‐up period (years) | Number of subjects | Number of cases | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | EC (M, F) | GC (M, F) | ESCC | EA | GCC | GNCC | |||||||
| JPHC‐I | Japanese residents of five public health center areas in Japan | 40–59 | 1990 | 2013 | 20.9 | 20,383 | 22,201 | 221, 22 | 991, 394 | 201 | 13 | 76 | 1105 | Subjects of one public health center area were excluded due to lack of incidence data |
| JPHC‐II | Japanese residents of six public health center areas in Japan | 40–69 | 1993 | 2013 | 17.2 | 29,150 | 32,673 | 276, 29 | 1235, 498 | 243 | 8 | 101 | 1121 | |
| JACC | Residents from 45 areas throughout Japan | 40–79 | 1988 | 2009 | 13.2 | 24,755 | 35,659 | 149, 21 | 1091, 614 | 73 | 14 | 79 | 633 | 22 selected areas with cancer incidence follow‐up data were used in this analysis |
| MIYAGI‐I | Residents of 14 municipalities in Miyagi Prefecture, Japan | 40–64 | 1990 | 2014 | 21.3 | 21,094 | 22,657 | 259, 31 | 1432, 567 | 213 | 32 | 191 | 1331 | |
| MIYAGI‐II | Residents of three municipalities in Miyagi Prefecture, Japan | 40+ | 1984 | 1992 | 7.6 | 13,027 | 15,956 | 60, 8 | 332, 140 | 0 | 0 | 67 | 107 | |
| AICHI | Residents of two municipalities in Aichi Prefecture, Japan | 40–103 | 1985 | 2000 | 11.6 | 15,217 | 16,863 | 60, 7 | 405, 187 | 29 | 1 | 27 | 24 | |
| TAKAYAMA | Residents of Takayama city, Gifu Prefecture, Japan | 35–101 | 1992 | 2008 | 13.6 | 13,398 | 15,568 | 57, 10 | 411, 218 | 49 | 3 | 10 | 81 | |
| OHSAKI | Residents of 14 municipalities in Miyagi Prefecture, Japan | 40–79 | 1994 | 2008 | 10.7 | 21,498 | 23,196 | 175, 38 | 954, 375 | 160 | 13 | 131 | 746 | |
| OSAKA | Residents of four municipalities in Osaka Prefecture, Japan | 40–97 | 1983 | 1998–2000 | 11.4 | 15,920 | 17,973 | 80, 10 | 537, 265 | 24 | 2 | 21 | 158 | |
| LSS | Atomic bomb survivors in Hiroshima and Nagasaki, Japan | 46–104 | 1991 | 2003 | 10.8 | 6541 | 10,518 | 45, 11 | 263, 186 | 46 | 0 | 25 | 314 |
LSS originally started in 1950 This analysis included subjects who responded to the 1991 survey |
| Total | 180,983 | 213,264 | 1382, 187 | 7651, 3444 | 1038 | 86 | 728 | 5620 | ||||||
Abbreviations: AICHI, The Three‐Prefecture Cohort Study in Aichi; EA, esophageal adenocarcinoma; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; F, female; GC, gastric cancer; GCC, gastric cardia cancer; GNCC, gastric noncardia cancer; JACC, The Japan Collaborative Cohort Study; JPHC, The Japan Public Health Center‐based Prospective Study; LSS, Life Span Study; M, male; MIYAGI‐I, The Miyagi Cohort Study; MIYAGI‐II, The Three‐Prefecture Cohort Study in Miyagi; OHSAKI, The Ohsaki Cohort Study; Osaka, The Three‐Prefecture Cohort Study in Osaka; TAKAYAMA, The Takayama Study. In ESCC and EA, we excluded MIYAGI‐II, in which no information on histological type was available.
2.2. Assessment of exposure
Each study collected information on height and weight at baseline using a custom‐developed self‐administered questionnaire. BMI was calculated as weight in kilograms divided by height in meters squared. A number of studies conducted validation studies on BMI that had been estimated from self‐reported weight and height. These reported correlation coefficients between BMI estimated from the questionnaire and BMI estimated from actually measured values for weight and height of 0.89 in men and 0.90 in women for JPHC‐I and ‐II, 29 0.91 in the two sexes for MIYAGI‐I, 30 and 0.88 in the two sexes for OHSAKI. 31 Correlation coefficients for self‐reported versus measured height and weight values in both sexes were 0.93 and 0.97 for TAKAYAMA, respectively. 32 In contrast, JACC, MIYAGI‐II, AICHI, OSAKA, and LSS did not provide information on the validation of BMI, but JACC used the same questions on height and weight as MIYAGI‐I while MIYAGI‐II, AICHI, OSAKA, and LSS used similar questions on height and weight to JPHC‐I and ‐II. Important covariates for esophageal and gastric cancers—cumulative smoking exposure, 6 alcohol consumption, 3 , 4 , 33 history of diabetes, 34 , 35 , 36 vegetable and fruit intake, 3 , 37 salt intake, 38 green tea intake, 39 and physical activity 3 —were also collected via self‐administered questionnaire.
2.3. Follow‐up and outcome assessment
As shown in Table 1, participants had been followed from baseline survey to last follow‐up date in all studies. Residential status, survival, date of death, and date of moving out were confirmed using residential registries managed by municipalities in the respective study areas. Incident cancer cases were identified using local cancer registries or via direct access with main regional hospitals. Esophageal cancer was identified by the International Classification of Diseases for Oncology, third edition (ICD‐O‐3) 40 tomography (ICD‐O‐3‐T) codes of C15.0‐C15.9. Esophageal squamous cell carcinoma included ICD‐O‐3 morphology (ICD‐O‐3‐M) 8050–8078 and 8083–8084. 41 Esophageal adenocarcinoma included ICD‐O‐3‐M 8140–8141, 8143–8145, 8190–8231, 8260–8265, 8310, 8401, 8480–8490, 8550–8552, 8570–8574, and 8576. 41 Gastric cancer included ICD‐O‐3‐T C16.0‐C16.9, with cancers of the cardia including ICD‐O‐3‐T C16.0 and those of the noncardia stomach including ICD‐O‐3‐T C16.1–C16.6.
2.4. Statistical analysis
A Cox proportional hazards regression model was used to estimate study‐specific hazard ratios (HRs) and their two‐sided 95% confidence intervals (CIs) for the incidence of esophageal or gastric cancer per 5‐kg/m2 increase in BMI. We calculated person‐years of follow‐up from the date of the baseline survey to the first occurrence of the date of diagnosis, date of death, date of loss to follow‐up (migration from the study area), or date of termination of follow‐up. All studies estimated two types of HR: model 1 adjusted for sex, age at baseline, and area (for multicentric studies, i.e., JPHC‐I, JPHC‐II, JACC, and LSS), and model 2 adjusted for covariates in model 1 as well as cumulative smoking exposure (pack‐years: 0, 0< and ≤20, >20), alcohol consumption (nondrinker, occasional drinker [<1 day/week], and current drinker [1 to 4 days/week, ≥5 days/week and <23 ethanol g/day, ≥5 days/week and ≥23 ethanol g/day]), and history of diabetes (no, yes). Pack‐years were established as the product of the number of packs smoked per day by the number of years of smoking.
Among the six studies with available information on total energy intake, vegetable intake, fruit intake, salt intake, consumption of green tea, and physical activity (JPHC‐I, JPHC‐II, JACC, MIYAGI‐I, TAKAYAMA, and OHSAKI), we also estimated HRs by making the same adjustments as in model 2, but also with adjustment for total energy intake (quartiles), vegetable intake (quartiles), fruit intake (quartiles), salt intake (quartiles), consumption of green tea (<1 cup/day, 1–2 cups/day, 3–4 cups/day, ≥5 cups/day), and physical activity (seldom, sometimes, frequently) (model 3). Consumption of vegetables, fruit, and salt was adjusted for total energy intake by the residual method 42 and classified into quartiles using sex‐ and cohort‐specific cutoff points. Because the questionnaires were not homogeneous across the studies, we created a variable for physical activity using broad exposure categories as follows: seldom (JPHC‐I, JPHC‐II, JACC, MIYAGI‐I, and OHSAKI: seldom; TAKAYAMA: never), sometimes (JPHC‐I and JPHC‐II: <5 days/week; JACC, MIYAGI‐I, TAKAYAMA, and OHSAKI: <5 h/day), frequently (JPHC‐I and JPHC‐II: almost every day; JACC, MIYAGI‐I, TAKAYAMA, and OHSAKI: ≥5 h/day).
HRs with exclusion of cases diagnosed in the first 3 years of follow‐up were estimated to examine possible reverse causation. To investigate whether the effect of BMI was homogeneous within strata of smoking status and sex, we performed stratified analyses according to smoking status (never, ever) and sex. Furthermore, with the same covariate adjustment, we evaluated the impact of overweight (25 to <30 kg/m2) or obese (≥30 kg/m2) on esophageal and gastric cancer risks relative to <25 kg/m2. Subjects were additionally classified into the following six categories (<18.5, 18.5 to <21, 21 to <23, 23 to <25, 25 to <30, and ≥30 kg/m2) and HR was estimated for each category relative to a reference category of 21 to <23 kg/m2. Among covariates, the ratio of missing data was 9.3% for cumulative smoking exposure (n = 36,772), 7.8% for alcohol consumption (n = 30,633), 9.4% for history of diabetes (n = 37,126), 6.9% for energy intake (n = 19,352), 2.2% for vegetable intake (n = 6072), 2.2% for fruit intake (n = 6281), 5.5% for consumption of green tea (n = 15,474), and 5.9% for physical activity (n = 16,606). Missing data for each covariate were coded as indicator terms.
Additionally, we pooled study‐specific results using a random effects model. 43 The degree of between‐study heterogeneity was analyzed with Cochran's Q‐statistic and I 2‐statistic. 44 The proportional hazards assumption was evaluated graphically with log‐negative‐log plots, which revealed no major violations of the proportional hazards assumption. All analyses were performed using SAS version 9.4 (SAS Institute, Inc.) or STATA version 17.0 (Stata Corporation). Two‐sided p values of <0.05 were considered statistically significant.
3. RESULTS
This study included 394,247 subjects (180,983 males and 213,264 females) with 5,750,107 person‐years of follow‐up (average follow‐up: 13.8 years). In total, we identified 1569 incident esophageal cancer cases (1382 males and 187 females), including 1038 esophageal squamous cell carcinoma and 86 esophageal adenocarcinoma cases, and 11,095 incident gastric cancer cases (7651 males and 3444 females), including 728 gastric cardia cancer and 5620 gastric noncardia cancer cases (Table 1).
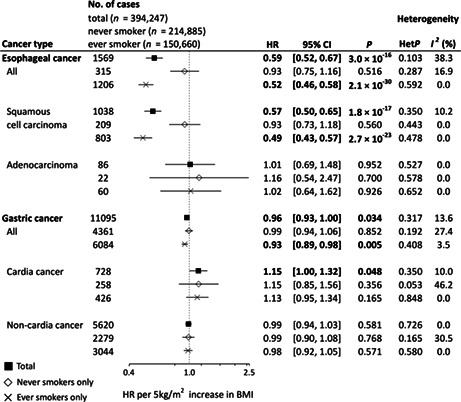
Figure 1 shows adjusted (model 2) HRs (aHRs) per 5‐kg/m2 increase in BMI. A significant inverse association was observed between BMI and risk of overall esophageal cancer (aHR 0.59, 95% CI 0.52–0.67). Stratification by subtype demonstrated that the inverse association was restricted to squamous cell carcinoma (aHR 0.57, 95% CI 0.50–0.65), with no association seen in adenocarcinoma (aHR 1.01, 95% CI 0.69–1.48). Furthermore, these inverse associations observed in overall esophageal cancer and esophageal squamous cell carcinoma were stronger in ever smokers (esophageal cancer: aHR 0.52, 95% CI 0.46–0.58; esophageal squamous cell carcinoma: aHR 0.49, 95% CI 0.43–0.57). For gastric cancer, although we observed a significant inverse association of BMI with overall gastric cancer (aHR 0.96, 95% CI 0.93–1.00), stratification by subsite demonstrated a positive association in gastric cardia cancer (aHR 1.15, 95% CI 1.00–1.32) and no association in gastric noncardia cancer (aHR 0.99, 95% CI 0.94–1.03). The inverse association in overall gastric cancer became slightly stronger in ever smokers (aHR 0.93, 95% CI 0.89–0.98) and unclear in never smokers (aHR 0.99, 95% CI 0.94–1.06), but consistent results across each stratum of smoking status were observed for both gastric cardia and noncardia cancers.
FIGURE 1.
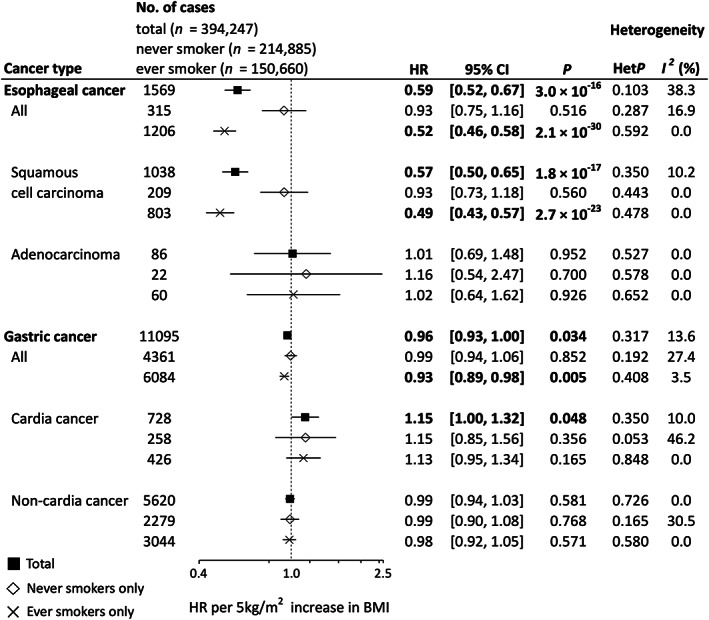
Forest plot of hazard ratios (HRs) and 95% confidence intervals (CIs) for each cancer per 5‐kg/m2 increase in body mass index (BMI). HRs were calculated by a random effects model by pooling study‐specific HR adjusted for sex, age, area (for multicentric studies, namely JPHC‐I, JPHC‐II, JACC, and LSS), pack‐years (0, 0< and ≤20, >20), alcohol consumption (nondrinker, occasional drinker [<1 day/week], and current drinker [1–4 days/week, ≥5 days/week and <23 ethanol g/day, ≥5 days/week and ≥23 ethanol g/day]), and history of diabetes. Between‐study heterogeneity for the risk estimate by trend analysis was evaluated using the Q‐statistic and the I 2‐statistic. The Q‐statistic was considered statistically significant when p < 0.10 and 0% of the I 2‐statistic represented no heterogeneity. HR values in bold show statistical significance (p < 0.05). For esophageal squamous cell carcinoma and esophageal adenocarcinoma, we excluded MIYAGI‐II, in which no information on histological type was available. JACC, Japan Collaborative Cohort Study; JPHC, Japan Public Health Center‐based Prospective Study; TAKAYAMA, Takayama Study; LSS, Life Span Study.
Figure 2 shows aHRs for being overweight or obese (BMI ≥25 kg/m2) relative to BMI <25 kg/m2. Being overweight or obese was inversely associated with the risk of overall esophageal cancer (aHR 0.64, 95% CI 0.56–0.73) and esophageal squamous cell carcinoma (aHR 0.56, 95% CI 0.47–0.67). In contrast, we observed a nonsignificant and significant positive association of overweight or obesity with esophageal adenocarcinoma (aHR 1.32, 95% CI 0.80–2.17) and gastric cardia cancer (aHR 1.24, 95% CI 1.05–1.46), respectively.
FIGURE 2.
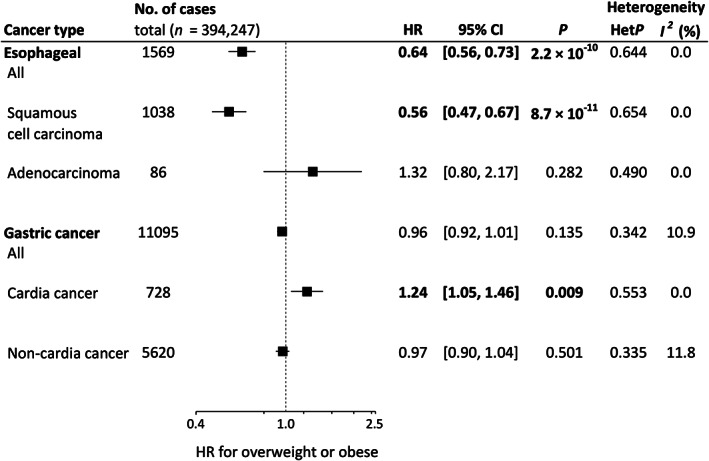
Forest plot of hazard ratios (HRs) and 95% confidence intervals (CIs) for overweight or obese (body mas index [BMI] ≥25 kg/m2) relative to BMI <25. HRs were calculated by a random effects model by pooling study‐specific HR adjusted for sex, age, area (for multicentric studies, namely JPHC‐I, JPHC‐II, JACC, and LSS), pack‐years (0, 0 < and ≤20, >20), alcohol consumption (nondrinker, occasional drinker [<1 day/week], and current drinker [1–4 days/week, ≥5 days/week and <23 ethanol g/day, ≥5 days/week and ≥23 ethanol g/day]), and history of diabetes. Between‐study heterogeneity for the risk estimate by trend analysis was evaluated using the Q‐statistic and the I 2‐statistic. The Q‐statistic was considered statistically significant when p < 0.10 and 0% of the I 2‐statistic represented no heterogeneity. HRs values in bold show statistical significance (p < 0.05). For esophageal squamous cell carcinoma and esophageal adenocarcinoma, we excluded MIYAGI‐II, in which no information on histological type was available. JACC, Japan Collaborative Cohort Study; JPHC, Japan Public Health Center‐based Prospective Study; TAKAYAMA, Takayama Study; LSS, Life Span Study.
Risks of esophageal and gastric cancers by six BMI categories are shown in Table 2. A nonlinear association was suggested for gastric cardia cancer due to a nonsignificant positive association in underweight (BMI <18.5 kg/m2, aHR 1.16, 95% CI 0.79–1.70) and overweight (25 to <30 kg/m2, aHR 1.12, 95% CI 0.92–1.37) or obese (≥30 kg/m2, aHR 1.04, 95% CI 0.57–1.87). On stratification by smoking status (Table 2), results indicated U‐shaped associations in overall esophageal cancer, esophageal squamous cell carcinoma, overall gastric cancer, and gastric cardia cancer among never smokers, although some estimates which included only a small number of cases were unstable. No between‐study heterogeneity was observed in most estimates (Figures 1 and 2, and Table 2).
TABLE 2.
Risks of esophageal and gastric cancers by BMI category
| Esophageal cancer | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Squamous cell carcinoma | Adenocarcinoma | ||||||||||||
| BMI kg/m2 | Subjects (n) | Person‐ years | Ca (n) | HR | 95% CI | HetP, I 2 | Ca (n) | HR | 95% CI | HetP, I 2 | Ca (n) | HR | 95% CI | HetP, I 2 |
| All subjects | ||||||||||||||
| <18.5 | 21,466 | 254,959 | 129 | 1.48 | 1.14–1.91 | 0.3, 20 | 51 | 1.31 | 0.90–1.90 | 0.2, 28 | 0 | ND | – | |
| 18.5 to <21 | 79,688 | 1,108,234 | 394 | 1.31 | 1.15–1.50 | 0.9, 0 | 284 | 1.30 | 1.10–1.53 | 0.9, 0 | 19 | 0.91 | 0.48–1.72 | 0.9, 0 |
| 21 to <23 | 104,714 | 1,531,131 | 471 | 1.00 | Reference | – | 320 | 1.00 | Reference | 27 | 1.00 | Reference | – | |
| 23 to <25 | 95,164 | 1,431,439 | 326 | 0.75 | 0.61–0.91 | 0.1, 40 | 231 | 0.76 | 0.62–0.93 | 0.3, 19 | 16 | 0.91 | 0.46–1.82 | 0.7, 0 |
| 25 to <30 | 85,053 | 1,300,950 | 235 | 0.64 | 0.55–0.76 | 0.4, 0 | 143 | 0.56 | 0.46–0.68 | 0.5, 0 | 23 | 0.95 | 0.52–1.71 | 0.6, 0 |
| ≥30 | 8162 | 123,394 | 14 | 0.64 | 0.37–1.10 | 0.8, 0 | 9 | 0.54 | 0.28–1.05 | 0.9, 0 | 1 | 1.19 | 0.15–9.22 | – |
| Never smokers | ||||||||||||||
| <18.5 | 11,174 | 139,846 | 12 | 1.69 | 0.82–3.50 | 0.6, 0 | 6 | 1.60 | 0.62–4.14 | 0.9, 0 | 0 | ND | – | |
| 18.5 to <21 | 41,595 | 601,085 | 55 | 1.02 | 0.71–1.45 | 0.5, 0 | 34 | 0.92 | 0.59–1.45 | 0.5, 0 | 4 | 0.84 | 0.21–3.37 | 0.6, 0 |
| 21 to <23 | 56,251 | 852,112 | 85 | 1.00 | Reference | – | 61 | 1.00 | Reference | 7 | 1.00 | Reference | – | |
| 23 to <25 | 51,834 | 803,621 | 83 | 0.93 | 0.68–1.27 | 0.4, 0 | 57 | 0.90 | 0.62–1.30 | 0.8, 0 | 2 | 1.18 | 0.11–13.05 | – |
| 25 to <30 | 48,999 | 770,799 | 75 | 0.83 | 0.60–1.14 | 0.5, 0 | 46 | 0.72 | 0.49–1.07 | 0.8, 0 | 9 | 0.65 | 0.20–2.14 | 0.8, 0 |
| ≥30 | 5032 | 78,149 | 5 | 1.28 | 0.49–3.33 | 0.5, 0 | 5 | 1.76 | 0.65–4.75 | 0.4, 0 | 0 | ND | – | |
| Ever smokers | ||||||||||||||
| <18.5 | 8772 | 99,452 | 83 | 1.60 | 1.22–2.09 | 0.3, 14 | 44 | 1.44 | 0.96–2.15 | 0.2, 28 | 0 | ND | – | |
| 18.5 to <21 | 32,780 | 442,798 | 363 | 1.36 | 1.17–1.57 | 1, 0 | 245 | 1.37 | 1.15–1.64 | 1, 0 | 14 | 0.90 | 0.43–1.91 | 0.9, 0 |
| 21 to <23 | 41,232 | 586,281 | 366 | 1.00 | Reference | – | 254 | 1.00 | Reference | 19 | 1.00 | Reference | – | |
| 23 to <25 | 36,736 | 537,031 | 228 | 0.69 | 0.57–0.85 | 0.2, 23 | 166 | 0.71 | 0.57–0.89 | 0.3, 13 | 13 | 1.23 | 0.54–2.77 | 0.9, 0 |
| 25 to <30 | 28,814 | 433,142 | 157 | 0.58 | 0.48–0.71 | 0.6, 0 | 90 | 0.51 | 0.40–0.65 | 0.7, 0 | 13 | 0.99 | 0.46–2.11 | 0.7, 0 |
| ≥30 | 2326 | 34,802 | 9 | 0.86 | 0.33–2.21 | 0.2, 42 | 4 | 0.71 | 0.26–1.92 | 0.8, 0 | 1 | 2.88 | 0.34–24.24 | – |
| Gastric cancer | ||||||||||||||
| All | Cardia cancer | Noncardia cancer | ||||||||||||
| BMI kg/m2 | Subjects (n) | Person‐ years | Ca (n) | HR | 95% CI | HetP, I 2 | Ca (n) | HR | 95% CI | HetP, I 2 | Ca (n) | HR | 95% CI | HetP, I 2 |
| All subjects | ||||||||||||||
| <18.5 | 21,466 | 254,959 | 597 | 1.07 | 0.96–1.20 | 0.2, 32 | 32 | 1.16 | 0.79–1.70 | 0.9, 0 | 203 | 0.97 | 0.84–1.13 | 0.5, 0 |
| 18.5 to <21 | 79,688 | 1,108,234 | 2293 | 1.05 | 0.99–1.12 | 0.3, 19 | 106 | 0.78 | 0.61–0.99 | 0.5, 0 | 1058 | 1.04 | 0.96–1.13 | 0.5, 0 |
| 21 to <23 | 104,714 | 1,531,131 | 2975 | 1.00 | Reference | 206 | 1.00 | Reference | – | 1517 | 1.00 | Reference | – | |
| 23 to <25 | 95,164 | 1,431,439 | 2715 | 0.98 | 0.91–1.05 | 0.1, 45 | 176 | 0.89 | 0.69–1.14 | 0.2, 24 | 1446 | 0.98 | 0.91–1.05 | 0.5, 0 |
| 25 to <30 | 85,053 | 1,300,950 | 2304 | 0.96 | 0.90–1.03 | 0.2, 30 | 196 | 1.12 | 0.92–1.37 | 0.5, 0 | 1275 | 0.96 | 0.89–1.03 | 0.6, 0 |
| ≥30 | 8162 | 123,394 | 211 | 1.05 | 0.88–1.25 | 0.2, 22 | 12 | 1.04 | 0.57–1.87 | 0.5, 0 | 121 | 1.04 | 0.76–1.43 | 0.1, 48 |
| Never smokers | ||||||||||||||
| <18.5 | 11,174 | 139,846 | 238 | 1.22 | 1.02–1.45 | 0.2, 26 | 12 | 1.90 | 0.96–3.76 | 0.9, 0 | 81 | 1.02 | 0.75–1.41 | 0.1, 37 |
| 18.5 to <21 | 41,595 | 601,085 | 788 | 1.07 | 0.94–1.23 | 0.04, 48 | 33 | 0.92 | 0.59–1.41 | 0.9, 0 | 389 | 1.08 | 0.92–1.27 | 0.2, 25 |
| 21 to <23 | 56,251 | 852,112 | 1066 | 1.00 | Reference | 61 | 1.00 | Reference | – | 577 | 1.00 | Reference | – | |
| 23 to <25 | 51,834 | 803,621 | 1085 | 1.04 | 0.94–1.15 | 0.2, 21 | 68 | 1.08 | 0.76–1.54 | 0.7, 0 | 574 | 0.97 | 0.86–1.09 | 0.6, 0 |
| 25 to <30 | 48,999 | 770,799 | 1074 | 1.05 | 0.96–1.15 | 0.4, 5 | 76 | 1.19 | 0.83–1.68 | 0.6, 0 | 595 | 0.99 | 0.88–1.11 | 0.6, 0 |
| ≥30 | 5032 | 78,149 | 110 | 1.14 | 0.91–1.43 | 0.3, 12 | 8 | 1.94 | 0.85–4.43 | 0.6, 0 | 63 | 1.19 | 0.85–1.66 | 0.2, 27 |
| Ever smokers | ||||||||||||||
| <18.5 | 8772 | 99,452 | 326 | 1.00 | 0.87–1.15 | 0.2, 23 | 19 | 1.23 | 0.74–2.06 | 0.8, 0 | 111 | 0.93 | 0.72–1.21 | 0.2, 30 |
| 18.5 to <21 | 32,780 | 442,798 | 1388 | 1.04 | 0.97–1.12 | 0.7, 0 | 67 | 0.72 | 0.53–0.97 | 0.6, 0 | 625 | 1.02 | 0.92–1.14 | 0.4, 7 |
| 21 to <23 | 41,232 | 586,281 | 1739 | 1.00 | Reference | 136 | 1.00 | Reference | – | 866 | 1.00 | Reference | – | |
| 23 to <25 | 36,736 | 537,031 | 1483 | 0.96 | 0.86–1.06 | 0.01, 50 | 95 | 0.83 | 0.63–1.08 | 0.5, 0 | 797 | 0.99 | 0.89–1.11 | 0.3, 15 |
| 25 to <30 | 28,814 | 433,142 | 1066 | 0.90 | 0.79–1.02 | 0.03, 58 | 108 | 1.14 | 0.88–1.47 | 0.7, 0 | 597 | 0.94 | 0.85–1.05 | 0.6, 0 |
| ≥30 | 2326 | 34,802 | 82 | 1.05 | 0.84–1.32 | 0.8, 0 | 1 | 0.77 | 0.10–5.71 | – | 48 | 1.24 | 0.92–1.67 | 0.8, 0 |
Note: HRs were calculated by a random effects model by pooling study‐specific HR adjusted for sex, age, area (for multicentric studies, namely JPHC‐I, JPHC‐II, JACC, and LSS), pack‐years (0, 0< and ≤20, >20), alcohol consumption (nondrinker, occasional drinker [<1 day/week], and current drinker [1–4 days/week, ≥5 days/week and <23 ethanol g/day, ≥5 days/week and ≥23 ethanol g/day]), and history of diabetes. HRs values in bold show statistical significance (p < 0.05). In esophageal squamous cell carcinoma and esophageal adenocarcinoma, we excluded MIYAGI‐II, in which no information on histological type was available.
Abbreviations: BMI, body mass index; Ca, cases; CI, confidence interval; HetP, P value from test of heterogeneity; HR, hazard ratio; JACC, Japan Collaborative Cohort Study; JPHC, Japan Public Health Center‐based Prospective Study; LSS, Life Span Study; ND, no cases in this stratum.
Results remained largely unchanged after additional adjustment for total energy intake, vegetable intake, fruit intake, salt intake, consumption of green tea, and physical activity (model 3) and the exclusion of cases diagnosed early within 3 years after enrollment (Tables S1–S3). Further analyses stratified by sex are shown in Table S4. We did not perform analyses by subtype or subsite in females due to the small number of female cases. The results were mostly consistent between sexes in overall esophageal and gastric cancers, but we observed a greater decrease in HR per 5‐kg/m2 for overall esophageal cancer in males (aHR 0.56, 95% CI 0.49–0.64) than in females (aHR 0.74, 95% CI 0.59–0.94).
4. DISCUSSION
Using data from 10 population‐based cohort studies comprising a total of 394,247 Japanese subjects, we evaluated the association between BMI and risk of upper gastrointestinal cancer incidence by subtype and subsite in an Asian population, where evidence to date has been inconsistent. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 This is one of the largest prospective analyses in an Asian country, with 1569 incident esophageal cancer cases (1038 esophageal squamous cell carcinoma and 86 esophageal adenocarcinoma cases) and 11,095 incident gastric cancer cases (728 gastric cardia and 5620 gastric noncardia cases). The results enabled us to confirm the heterogeneous impact of BMI on upper gastrointestinal cancer according to subtype or subsite 3 , 4 in an Asian population, as is also seen in Caucasians. Specifically, a significant inverse association was observed between BMI and risk of esophageal squamous cell carcinoma (HR per 5‐kg/m2 increase in BMI 0.57, 95% CI 0.50–0.65), whereas a significant positive association was seen in gastric cardia cancer (HR 1.15, 95% CI 1.00–1.32). The magnitude of the effect of BMI on these cancers was equivalent to that observed in the previous meta‐analyses conducted mainly in Caucasians (relative risk [RR] of 5‐kg/m2 increase in BMI 0.64, 95% CI 0.56–0.73 for esophageal squamous cell carcinoma; RR 1.23, 95% CI 1.07–1.40 for gastric cardia cancer). 3 , 11 Being overweight or obese (BMI ≥25 kg/m2) relative to BMI <25 kg/m2 was inversely associated with the risk of esophageal squamous cell carcinoma (HR 0.56, 95% CI 0.47–0.67). In contrast, we observed a nonsignificant and significant positive association of overweight or obesity with esophageal adenocarcinoma (HR 1.32, 95% CI 0.80–2.17) and gastric cardia cancer (HR 1.24, 95% CI 1.05–1.46), respectively. In addition, we found no clear association of gastric noncardia cancer with BMI, consistent with a previous meta‐analysis. 9
This study included the largest number of incident cases of esophageal squamous cell carcinoma to date and provides the strongest evidence yet that leanness is a risk factor for esophageal squamous cell carcinoma. Increased risks among lean subjects have been consistently observed in other smoking‐associated cancer sites, 45 including lung 46 , 47 and head and neck, 48 making this relationship more convincing. Given that smoking decreases appetite 49 and that smokers accordingly tend to be leaner than nonsmokers, confounding by smoking has been one of the primary explanations for this association. To disentangle the effects of leanness and smoking on risk of esophageal squamous cell carcinoma epidemiologically, five studies 20 , 50 , 51 , 52 , 53 evaluated consistency in the inverse association with BMI by smoking status. Three reported a consistent inverse association with BMI in never and ever smokers. 50 , 51 , 52 Consistent with these, the present study suggests that risk is elevated in those with low BMI within both strata of smoking status (Table 2). With respect to other sites, one large prospective study showed the association of higher BMI with reduced risk of lung cancer only in ever smokers, 47 whereas large collaborative analyses involving multiple cohort studies and case–control studies showed the association of leanness with lung cancer 46 and head and neck cancer, 48 respectively, regardless of smoking status. Taken together, the effect of low BMI on never smokers is also likely to be carcinogenic, although this study could not provide conclusive evidence on never smokers despite having the largest number of esophageal squamous cell carcinoma cases.
It is important to note that, judging from the significantly higher inverse effect among ever smokers than never smokers (Figure 1), significant effect modification between BMI and smoking on the risk of esophageal squamous cell carcinoma is likely present. Studies evaluating biomarkers showed increased levels of DNA adducts 54 and an oxidative stress marker 55 among lean smokers compared with nonlean smokers, suggesting a biological connection between esophageal squamous cell carcinoma and leanness among smokers. Furthermore, the recent Mendelian randomization analysis demonstrated a complex bidirectional relation between obesity and smoking. 56 We therefore consider that our results highlight the importance of jointly considering both smoking and BMI in reducing the risk of esophageal squamous cell carcinoma. Still, although we included pack‐years as a covariate in the stratified analysis by smoking status to obviate concerns about residual confounding, we cannot completely deny the possibility that the impact of residual confounding remains. The underlying mechanisms of this association may be heterogeneous by smoking status, 57 and further biological studies are needed.
One of the considerable strengths of this study was that it included a very large number of general Japanese participants and a substantial number of incident cases. This allowed us to perform a comprehensive evaluation of BMI effects on upper gastrointestinal cancers, including stratified analyses by subtype or subsite, investigation of nonlinearity, and effect modification. In addition, we unified the categories of BMI and multiple important covariates across cohorts. Together, these various factors allow us to ignore any potential heterogeneity that can occur in meta‐analyses of published studies.
Several limitations of this study also warrant mention. First, we did not perform analyses for abdominal obesity, such as waist circumference and waist‐to‐hip ratio, because our study is an aggregation of cohort studies and not all studies had such information. Although findings for the association between abdominal obesity and esophageal or gastric cancer risk were inconsistent, 50 , 53 , 58 , 59 , 60 the European Prospective Investigation into Cancer and Nutrition, a multicenter prospective cohort study, suggested positive associations for both waist circumference and waist‐to‐hip ratio on adjustment of BMI, even in esophageal squamous cell carcinoma. 50 , 53 Abdominal obesity might therefore be an important risk factor for upper gastrointestinal cancer independent of BMI. Second, we conducted our analysis using only the baseline questionnaire and were therefore unable to account for changes in BMI or other covariate exposure status that occurred after enrollment. Third, we did not observe any significant HRs for esophageal adenocarcinoma. The number of esophageal adenocarcinoma cases was only 86, and the estimates for adenocarcinoma were accordingly unstable. Therefore, the nonsignificant results and smaller magnitude of the dose‐dependent effect of BMI in this study (HR 5‐kg/m2 increase in BMI 1.01, 95% CI 0.69–1.48) than in the previous meta‐analysis (RR 5‐kg/m2 increase in BMI 1.48, 95% CI 1.35–1.62) 11 on esophageal adenocarcinoma can be considered partially due to the small sample size. Further studies to evaluate the impact of BMI on esophageal adenocarcinoma among Asians with a larger sample size are needed. Lastly, although we took account of important potential confounders, the possibility of residual confounders remains.
In summary, this study—the largest prospective study conducted in an Asian country to date—obtained a comprehensive quantitative estimate of the association between BMI and upper gastrointestinal cancer incidence. Our results confirm the subtype‐ or subsite‐specific effect of BMI, and therefore carry important implications for primary prevention strategies against upper gastrointestinal cancer incidence.
FUNDING INFORMATION
The National Cancer Center Research and Development Fund, Grant/Award Number 2021‐A‐16, 30‐A‐15, and 27‐A‐4, 24‐A‐3; Health and Labour Sciences Research Grants for the Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare, Japan, Grant/Award Number H21‐3jigan‐ippan‐003, H18‐3jigan‐ippan‐001, and H16‐3jigan‐010; Japan and the US government: the Radiation Effects Research Foundation (RERF) Research Protocol A2‐15.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
ETHICS STATEMENT
All studies obtained written informed consent from all participants and were approved by their respective institutional review boards.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Tables S1–S4
ACKNOWLEDGMENTS
This study was supported by the National Cancer Center Research and Development Fund, Grant/Award Number 2021‐A‐16, 30‐A‐15, 27‐A‐4, 24‐A‐3, and Health and Labour Sciences Research Grants for the Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare, Japan, Grant/Award Number H21‐3jigan‐ippan‐003, H18‐3jigan‐ippan‐001, and H16‐3jigan‐010. The Radiation Effects Research Foundation (RERF) is funded by Japan and the US government (RERF Research Protocol A2‐15).
APPENDIX 1.
1.1.
Research group member (as of April 2023)
Manami Inoue (Principal investigator); Sarah Krull Abe, Norie Sawada (National Cancer Center); Takashi Kimura (Hokkaido University); Yumi Sugawara (Tohoku University); Shuhei Nomura (The University of Tokyo); Hidemi Takimoto (National Institutes of Biomedical Innovation, Health and Nutrition); Hidemi Ito; Isao Oze (Aichi Cancer Center); Yingsong Lin (Aichi Medical University); Keiko Wada (Gifu University); Tetsuhisa Kitamura (Osaka University); Mai Utada (Radiation Effects Research Foundation).
Past members:
Akihisa Hidaka, Mayo Hirabayashi, Motoki Iwasaki, Yuri Kitamura, Keitaro Matsuo, Tetsuya Mizoue, Nagisa Mori, Michihiro Muto, Chisato Nagata, Mariko Naito, Tomio Nakayama, Yoshikazu Nishino, Atsuko Sadakane, Eiko Saito, Ritsu Sakata, Shizuka Sasazuki, Taichi Shimazu, Hiroyuki Shimizu, Kemmyo Sugiyama, Hidekazu Suzuki, Akiko Tamakoshi, Keitaro Tanaka, Shiori Tanaka, Yoshitaka Tsubono, Ichiro Tsuji, Shoichiro Tsugane, Kenji Wakai, Yoko Yamagiwa, Taiki Yamaji
Koyanagi YN, Matsuo K, Ito H, et al. Body mass index and esophageal and gastric cancer: A pooled analysis of 10 population‐based cohort studies in Japan. Cancer Sci. 2023;114:2961‐2972. doi: 10.1111/cas.15805
Keitaro Matsuo, Norie Sawada, Chisato Nagata, and Manami Inoue are editorial board members of Cancer Science (as of November 2022).
Contributor Information
Keitaro Matsuo, Email: kmatsuo@aichi-cc.jp.
Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan:
Manami Inoue, Sarah Krull Abe, Norie Sawada, Takashi Kimura, Yumi Sugawara, Shuhei Nomura, Hidemi Takimoto, Hidemi Ito, Isao Oze, Yingsong Lin, Keiko Wada, Tetsuhisa Kitamura, Mai Utada, Akihisa Hidaka, Mayo Hirabayashi, Motoki Iwasaki, Yuri Kitamura, Keitaro Matsuo, Tetsuya Mizoue, Nagisa Mori, Michihiro Muto, Chisato Nagata, Mariko Naito, Tomio Nakayama, Yoshikazu Nishino, Atsuko Sadakane, Eiko Saito, Ritsu Sakata, Shizuka Sasazuki, Taichi Shimazu, Hiroyuki Shimizu, Kemmyo Sugiyama, Hidekazu Suzuki, Akiko Tamakoshi, Keitaro Tanaka, Shiori Tanaka, Yoshitaka Tsubono, Ichiro Tsuji, Shoichiro Tsugane, Kenji Wakai, Yoko Yamagiwa, and Taiki Yamaji
REFERENCES
- 1. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer. 2020. Accessed October 12, 2022. https://gco.iarc.fr/today
- 2. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400‐412. [DOI] [PubMed] [Google Scholar]
- 3. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report . Diet, nutrition, physical activity and oesophageal cancer. 2018. Accessed March 9, 2023. https://www.wcrf.org/wp‐content/uploads/2021/02/oesophageal‐cancer‐report.pdf
- 4. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report . Diet, nutrition, physical activity and stomach cancer. 2016. Accessed March 9, 2023. https://www.wcrf.org/sites/default/files/Stomach‐cancer‐report.pdf
- 5. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827‐1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian J, Zuo C, Liu G, et al. Cumulative evidence for the relationship between body mass index and the risk of esophageal cancer: an updated meta‐analysis with evidence from 25 observational studies. J Gastroenterol Hepatol. 2020;35:730‐743. [DOI] [PubMed] [Google Scholar]
- 8. Turati F, Tramacere I, La Vecchia C, Negri E. A meta‐analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol. 2013;24:609‐617. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Liu L, Wang X, et al. Body mass index and risk of gastric cancer: a meta‐analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev. 2013;22:1395‐1408. [DOI] [PubMed] [Google Scholar]
- 10. Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8:340‐347. [DOI] [PubMed] [Google Scholar]
- 11. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report . Body fatness and weight gain and the risk of cancer. 2018. Accessed March 9, 2023. https://www.wcrf.org/wp‐content/uploads/2021/01/Body‐fatness‐and‐weight‐gain_0.pdf
- 12. WHO expert consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157‐163. [DOI] [PubMed] [Google Scholar]
- 13. Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456‐463. [DOI] [PubMed] [Google Scholar]
- 14. Smith M, Zhou M, Whitlock G, et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta‐analysis of published studies. Int J Cancer. 2008;122:1604‐1610. [DOI] [PubMed] [Google Scholar]
- 15. Kuriyama S, Tsubono Y, Hozawa A, et al. Obesity and risk of cancer in Japan. Int J Cancer. 2005;113:148‐157. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka T, Nagata C, Oba S, Takatsuka N, Shimizu H. Prospective cohort study of body mass index in adolescence and death from stomach cancer in Japan. Cancer Sci. 2007;98:1785‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song H, Saito E, Sawada N, et al. Body mass index change during adulthood and risk of oesophageal squamous‐cell carcinoma in a Japanese population: the Japan public health (JPHC)‐based prospective study. Br J Cancer. 2017;117:1715‐1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirabayashi M, Inoue M, Sawada N, et al. Effect of body‐mass index on the risk of gastric cancer: a population‐based cohort study in a Japanese population. Cancer Epidemiol. 2019;63:101622. [DOI] [PubMed] [Google Scholar]
- 19. Jang J, Lee S, Ko KP, et al. Association between body mass index and risk of gastric cancer by anatomic and histologic subtypes in over 500,000 east and southeast Asian cohort participants. Cancer Epidemiol Biomarkers Prev. 2022;31:1727‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S, Jang J, Abe SK, et al. Association between body mass index and oesophageal cancer mortality: a pooled analysis of prospective cohort studies with >800,000 individuals in the Asia cohort consortium. Int J Epidemiol. 2022;51:1190‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saito E, Inoue M, Tsugane S, et al. Smoking cessation and subsequent risk of cancer: a pooled analysis of eight population‐based cohort studies in Japan. Cancer Epidemiol. 2017;51:98‐108. [DOI] [PubMed] [Google Scholar]
- 22. Tsugane S, Sobue T. Baseline survey of JPHC study—design and participation rate. Japan public health center‐based prospective study on cancer and cardiovascular diseases. J Epidemiol. 2001;11:S24‐S29. [DOI] [PubMed] [Google Scholar]
- 23. Tamakoshi A, Yoshimura T, Inaba Y, et al. Profile of the JACC study. J Epidemiol. 2005;15(Suppl 1):S4‐S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsuji I, Nishino Y, Tsubono Y, et al. Follow‐up and mortality profiles in the Miyagi cohort study. J Epidemiol. 2004;14(Suppl 1):S2‐S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marugame T, Sobue T, Satoh H, et al. Lung cancer death rates by smoking status: comparison of the three‐prefecture cohort study in Japan to the cancer prevention study II in the USA. Cancer Sci. 2005;96:120‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura K, Nagata C, Wada K, et al. Cigarette smoking and other lifestyle factors in relation to the risk of pancreatic cancer death: a prospective cohort study in Japan. Jpn J Clin Oncol. 2011;41:225‐231. [DOI] [PubMed] [Google Scholar]
- 27. Tsuji I, Nishino Y, Ohkubo T, et al. A prospective cohort study on National Health Insurance beneficiaries in Ohsaki, Miyagi prefecture, Japan: study design, profiles of the subjects and medical cost during the first year. J Epidemiol. 1998;8:258‐263. [DOI] [PubMed] [Google Scholar]
- 28. Sakata R, McGale P, Grant EJ, Ozasa K, Peto R, Darby SC. Impact of smoking on mortality and life expectancy in Japanese smokers: a prospective cohort study. BMJ. 2012;345:e7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inoue M, Sobue T, Tsugane S, Group JS . Impact of body mass index on the risk of total cancer incidence and mortality among middle‐aged Japanese: data from a large‐scale population‐based cohort study—the JPHC study. Cancer Causes Control. 2004;15:671‐680. [DOI] [PubMed] [Google Scholar]
- 30. Kuriyama S, Ohmori K, Miura C, et al. Body mass index and mortality in Japan: the Miyagi cohort study. J Epidemiol. 2004;14(Suppl 1):S33‐S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagai M, Kuriyama S, Kakizaki M, et al. Impact of obesity, overweight and underweight on life expectancy and lifetime medical expenditures: the Ohsaki cohort study. BMJ Open. 2012;2(3):e000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shimizu N, Nagata C, Shimizu H, et al. Height, weight, and alcohol consumption in relation to the risk of colorectal cancer in Japan: a prospective study. Br J Cancer. 2003;88:1038‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamura T, Wakai K, Lin Y, et al. Alcohol intake and stomach cancer risk in Japan: a pooled analysis of six cohort studies. Cancer Sci. 2022;113:261‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petrick JL, Li N, Anderson LA, et al. Diabetes in relation to Barrett's esophagus and adenocarcinomas of the esophagus: a pooled study from the international Barrett's and esophageal adenocarcinoma consortium. Cancer. 2019;125:4210‐4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang W, Ren H, Ben Q, Cai Q, Zhu W, Li Z. Risk of esophageal cancer in diabetes mellitus: a meta‐analysis of observational studies. Cancer Causes Control. 2012;23:263‐272. [DOI] [PubMed] [Google Scholar]
- 36. Ge Z, Ben Q, Qian J, Wang Y, Li Y. Diabetes mellitus and risk of gastric cancer: a systematic review and meta‐analysis of observational studies. Eur J Gastroenterol Hepatol. 2011;23:1127‐1135. [DOI] [PubMed] [Google Scholar]
- 37. Shimazu T, Wakai K, Tamakoshi A, et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: a pooled analysis of four cohort studies. Ann Oncol. 2014;25:1228‐1233. [DOI] [PubMed] [Google Scholar]
- 38. Tsugane S, Sasazuki S, Kobayashi M, Sasaki S. Salt and salted food intake and subsequent risk of gastric cancer among middle‐aged Japanese men and women. Br J Cancer. 2004;90:128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inoue M, Sasazuki S, Wakai K, et al. Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut. 2009;58:1323‐1332. [DOI] [PubMed] [Google Scholar]
- 40. International Classification of Diseases for Oncology. 2000. 3rd Edn: Geneva, Switzerland: World Health Organization.
- 41. Bray F, Colombet M, Mery L, et al. Cancer incidence in five continents volume XI: International Agency for Research on Cancer Lyon. France 2021.
- 42. Willett W. Nutritional Epidemiology. 2nd ed. Oxford University Press; 1998:288‐291. [Google Scholar]
- 43. DerSimonian R, Laird N. Meta‐analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 45. Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu D, Zheng W, Johansson M, et al. Overall and central obesity and risk of lung cancer: a pooled analysis. J Natl Cancer Inst. 2018;110:831‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith L, Brinton LA, Spitz MR, et al. Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst. 2012;104:778‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gaudet MM, Olshan AF, Chuang SC, et al. Body mass index and risk of head and neck cancer in a pooled analysis of case‐control studies in the international head and neck cancer epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39:1091‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mineur YS, Abizaid A, Rao Y, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sanikini H, Muller DC, Sophiea M, et al. Anthropometric and reproductive factors and risk of esophageal and gastric cancer by subtype and subsite: results from the European prospective investigation into cancer and nutrition (EPIC) cohort. Int J Cancer. 2020;146:929‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanikini H, Muller DC, Chadeau‐Hyam M, Murphy N, Gunter MJ, Cross AJ. Anthropometry, body fat composition and reproductive factors and risk of oesophageal and gastric cancer by subtype and subsite in the UK biobank cohort. PLoS One. 2020;15:e0240413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lindkvist B, Johansen D, Stocks T, et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the me‐can project. BMC Cancer. 2014;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Steffen A, Schulze MB, Pischon T, et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18:2079‐2089. [DOI] [PubMed] [Google Scholar]
- 54. Godschalk RW, Feldker DE, Borm PJ, Wouters EF, van Schooten FJ. Body mass index modulates aromatic DNA adduct levels and their persistence in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:790‐793. [PubMed] [Google Scholar]
- 55. Mizoue T, Kasai H, Kubo T, Tokunaga S. Leanness, smoking, and enhanced oxidative DNA damage. Cancer Epidemiol Biomarkers Prev. 2006;15:582‐585. [DOI] [PubMed] [Google Scholar]
- 56. Carreras‐Torres R, Johansson M, Haycock PC, et al. Role of obesity in smoking behaviour: mendelian randomisation study in UK biobank. BMJ. 2018;361:k1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers — a different disease. Nat Rev Cancer. 2007;7:778‐790. [DOI] [PubMed] [Google Scholar]
- 58. Steffen A, Huerta JM, Weiderpass E, et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European prospective investigation into cancer and nutrition. Int J Cancer. 2015;137:646‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. MacInnis RJ, English DR, Hopper JL, Giles GG. Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer. 2006;118:2628‐2631. [DOI] [PubMed] [Google Scholar]
- 60. O'Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH‐AARP diet and health study. Gut. 2012;61:1261‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


