Abstract
Background
High-power short-duration (HPSD) and cryoballoon ablation (CBA) has been used for pulmonary vein isolation (PVI).
Objective
We aimed to compare the efficacy of PVI between CBA and HPSD ablation in patients with paroxysmal atrial fibrillation (PAF).
Methods
We retrospectively analyzed 251 consecutive PAF patients from January 2018 to July 2020. Of them, 124 patients (mean age 57.2 ± 10.1 year) received HPSD and 127 patients (mean age 59.6 ± 9.4 year) received CBA. In HPSD group, the radiofrequency energy was set as 50 W/10 s at anterior wall and 40 W/10 s at posterior wall. In CBA group, 28 mm s generation cryoballoon was used for PVI according the guidelines.
Results
There was no significant difference in baseline characteristics between these 2 groups. The time to achieve PVI was significantly shorter in cryoballoon ablation group than in HPSD group (20.6 ± 1.7 min vs 51.8 ± 36.3, P = 0.001). The 6-month overall recurrence for atrial tachyarrhythmias was not significantly different between the two groups (HPSD:14.50% vs CBA:11.0%, P = 0.40). There were different types of recurrent atrial tachyarrhythmia between these 2 groups. Recurrence as atrial flutter was significantly more common in CBA group compared to HPSD group (57.1% vs 12.5%, P = 0.04).
Conclusion
In PAF patients, CBA and HPSD had a favourable and comparable outcome. The recurrence pattern was different between CBA and HPSD groups.
Keywords: Atrial fibrillation, Pulmonary vein isolation, High power short duration ablation, Cryoballoon ablation, Recurrence
Abbreviations
- 1. HPSD
High power short duration ablation
- 2. CBA
Cryoballoon ablation
- 3. PVI
Pulmonary vein isolation
- 4. PAF
Paroxysmal atrial fibrillation
- 5. RFCA
Radiofrequency catheter ablation
- 6. PVP
Pulmonary vein potentials
1. Introduction
Paroxysmal atrial fibrillation (PAF) is one of the most common arrhythmia, and associated with increased risk of heart failure, cognitive decline, stroke and mortality [[1], [2], [3]]. Durable pulmonary vein isolation (PVI) is the most imperative step in catheter ablation of AF [4,5]
The high-power short duration (HPSD) radiofrequency (RF) application causes more resistive heating and wider tissue injury characterized by the broader and shallower lesions. In contrast, RF application of lower power and longer duration results in larger conductive heating in deeper tissue and leads to deeper lesion with more risk of collateral damage [6,7]. This has been validated in in-vivo animal studies where the longer duration ablations had led to more steam-pops and complications. In PVI, HPSD approach was introduced to reduce collateral damage during ablation and decrease the procedure time [8]. However, the complexity of radiofrequency catheter ablation (RFCA) procedure and the point by point catheter ablation demands longer learning time. The cryoballoon ablation (CBA) is relatively simple technique for PVI and achieve tissue necrosis by deep freezing. The FIRE and ICE trial demonstrated comparable safety and efficacy of CBA and RFCA for PVI in patients with AF [9]. However, comparison of HPSD and CBA for PVI in patients with paroxysmal AF (PAF) is still unclear. This retrospective analysis was designed to examine the efficacy and outcome of PVI using these modalities.
2. Methods
2.1. Patient population
This study retrospectively analyzed 251 consecutive PAF patients attending Taipei Veterans general hospital for catheter ablation for first time from January 2018 to December 2019. Of them, 124 patients (mean age 57.7 ± 11.9 year) received HPSD ablation protocol and 127 patients (mean age 59.6 ± 9.4 year) received CBA for PVI. Type of modality use for ablation was selected as per patient choice. PAF was defined according to the statement from the 2017 Heart Rhythm Society Expert Consensus as atrial fibrillation that is self-terminating or with intervention within seven days of onset [10].. Patients with structural heart disease, valvular atrial fibrillation, and history of prior atrial fibrillation ablation were excluded. All clinical data were obtained after the approval from the Taipei Veterans General Institutional ethics committee.
2.2. HPSD ablation protocol
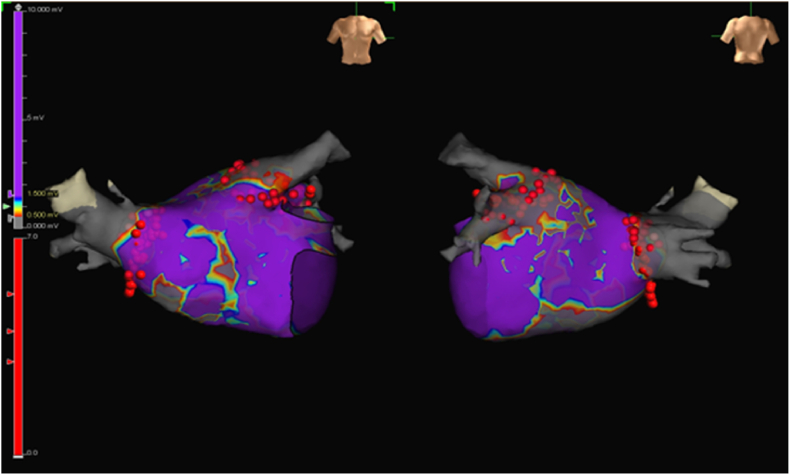
The details of PVI procedure have been described in previous publication [8]. Briefly, after providing written informed consent, each patient underwent an electrophysiological study and ablation. In our HPDS ablation protocol, the electroanatomical map of left atrium was created by Ensite™ Precision (Abbott. St. Jude).(see Fig. 3) An Inquiry AFocus II™ (electrode spacing:3.5-3.5-3.5) spiral catheter or Advisor HD Grid mapping catheter (Abbott. St. Jude) was used to record the PVPs. PVI was performed in antrum circumferential ablation pattern using the TactiCath™ Quartz Contact Force Ablation Catheter (Abbott. St. Jude). The power setting was 50 W for anterior wall and 40 W for posterior wall. Each point ablation time was setting as 10 s. The contact force was at least 10 g (10–30 g) at each point. During ablation, bipolar voltage electrogram (EGM) were observed and recorded.
Fig. 3.
Voltage map and ablation tags of an patient who underwent HPSD ablation.
The endpoint of PVI was elimination of all the PVPs. Superior vena cava and left atrial appendage pacing manoeuvre was used to exclude interference of far-field signals. Exit block was confirmed by pacing from every electrode of spiral catheter or from HD grid catheter within the PV. Recheck for PV reconnection was performed after a waiting period of 30 min. Gaps were defined as the area of acute PV reconnection. If PVPs recurred, additional ablation was applied at the reconnection site.
2.3. Cryoballoon ablation
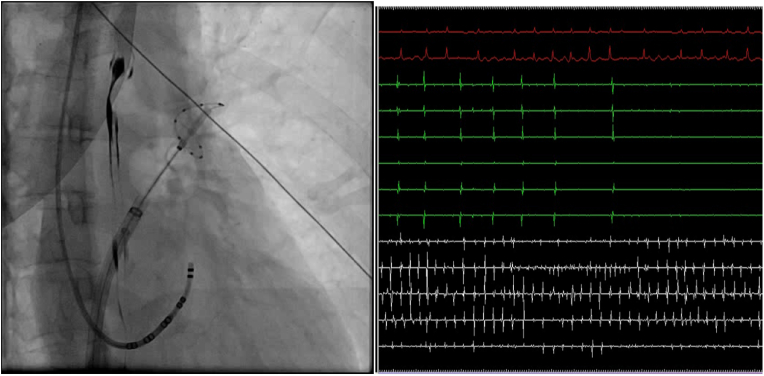
After obtaining trans-septal access, an exchange wire was kept within the left superior PV (LSPV), and a steerable 15-F over-the-wire sheath (FlexCath Advance; Medtronic Inc, Minneapolis, MN) was positioned in the left atrium.(see Fig. 4) A 28-mm second-generation cryoballoon (Arctic Front Advance; Medtronic Inc) was advanced over the 20- mm-diameter inner lumen mapping catheter (Achieve Mapping Catheter; Medtronic Inc) to the left atrium. Ablative lesions were created using intra-catheter temperatures of around −50 °C delivered to each PV. During the CBA procedure, the balloon was placed at the antrum of a PV and advanced toward the PV to obtain occlusion, which was tested with radiopaque contrast agent injection through the balloon's distal tip lumen. Retention of contrast agent within the PV indicated occlusion. Cooling duration of 4 min was used during PVI, therapy was interrupted if PVI was not achieved within 60 s and balloon was repositioned. After PVI a bonus freeze was administered. If PVPs persist after cryoablation then point by point RF ablation was done with a 4 mm tip RF ablation catheter. During all right-sided ablations, phrenic nerve function was monitored by high output (8–12 mA) pacing from the superior vena cava. The LSPV was treated first, followed by the left inferior PV (LIPV), right inferior PV (RIPV), and right superior PV (RSPV). If AF persisted after PVI then, sinus rhythm was restored by Direct current electrical cardioversion. Non-PV triggers were identified and ablated with the methods described in our previous publications [[11], [12], [13]].
Fig. 4.
Cryoballoon in LSPV showing entrance block after cryoballoon ablation.
Time to PVI was calculated by time to achieve bidirectional block in all four pulmonary veins after placing ablation and mapping catheters in the left atrium.
2.4. Follow up after procedure
Patients come for follow up at 3,6 and 9 weeks after the procedure and then every 6 weeks at outpatient clinic after discharge. All patients underwent ECGs at every opd visit. The post ablation follow-up included 24-hour Holter monitoring and/or cardiac event recording with a duration of 1 week was performed 3 months and 6 months after ablation, or whenever patients reported clinical symptoms. Long-term efficacy was assessed on the basis of a resting surface 12-lead electrocardiogram, 24-hour Holter monitoring, and/or 1-week recordings of a cardiac event recorder. The recurrent atrial tachyarrhythmia (including AF, atrial flutter, and focal atrial tachycardia) was defined as an episode of atrial tachyarrhythmia lasting more than 30 s after blanking period (3 months after ablation). Atrial flutter was diagnosed based on P wave analysis from electrocardiogram.
2.5. Statistical analysis
All continuous variables were reported as mean ± standard deviation (SD), while categorical variables were reported as proportions. Between-groups comparisons of continuous variables and proportional variables were performed using Student's t-test and chi-square test respectively. A two-sided P < 0.05 was considered significant. All statistical analyses were performed using SPSS software version 20.0 (SPSS Inc, Chicago).
3. Result
3.1. Patient characteristics
The baseline characteristics and demographics of the patients are shown in Table 1. There was no significant difference in baseline characteristics between the 2 groups.
Table 1.
Baseline characteristics.
| CBA group (n = 127) | HPSD group (n = 124) | P value | |
|---|---|---|---|
| Age, y/o | 57.7 ± 11.9 | 57.2 ± 10.1 | 0.953 |
| Males (%) | 92 (73%) | 77 (62%) | 0.218 |
| BMI, kg/m2 | 24.9 + 3.4 | 22.9 + 5.6 | 0.812 |
| LAD, mm | 37.8 ± 5.3 | 37.7 ± 5.0 | 0.845 |
| LVEF, % | 60.1 ± 5.1 | 60.0 ± 5.37 | 0.783 |
| DM (%) | 17 (13%) | 16 (12.9%) | 0.909 |
| HTN (%) | 57 (45%) | 44 (35%) | 0.129 |
| CAD (%) | 28 (17%) | 33 (27%) | 0.399 |
| Smoking (%) | 26 (20%) | 29 (23%) | 0.576 |
3.2. Ablation procedure
The parameters of ablation procedure were shown in Table 2. Time to achieve PVI was significantly shorter in cryoballoon ablation group (20.6 ± 1.7 min vs 51.8 ± 36.3 min, P = 0.001). As there is a learning curve with HPSD procedure, time to achieve pulmonary vein isolation become progressively become shorter as the experience with the procedure increases. 8/127 patients (6.2%) received touch-up radiofrequency ablation in CBA group for PV gaps after balloon application. 9/124 (7.2%) patients required additional PV ablation for PV gaps after initial ablation in the HPSD group. There were no major procedure related complications in any of the group.
Table 2.
The parameters of ablation procedure in CBA and HPSD groups.
| CBA group (n = 127) | HPSD group (n = 124) | P value | |
|---|---|---|---|
| PVI Time, min | 20.6 ± 1.7 | 51.8 ± 36.3 | 0.001 |
| PV Gaps, n (%) | 8 (6.3%) | 9 (7.2%) | NS |
| Complications, n (%) | 0 | 0 | NS |
3.3. Recurrence
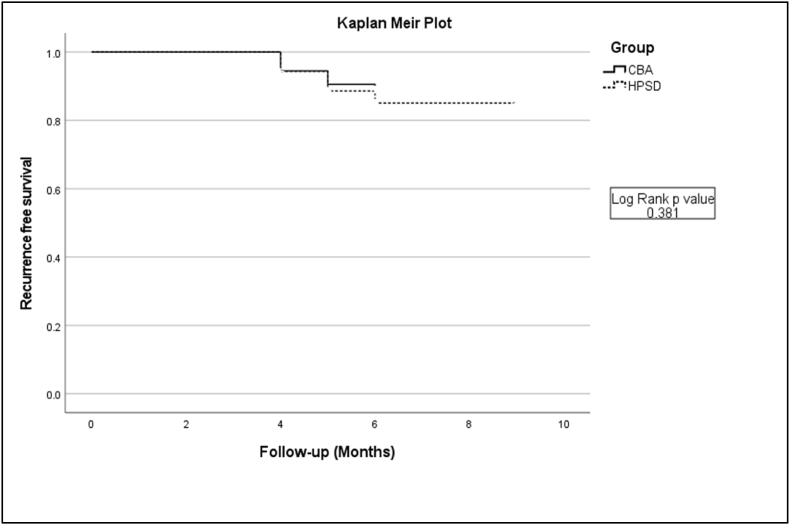
After 3 months of blanking period, 18/124 (14.5%) patients in the HPSD group and 14/127 (11.0%) patients in the CBA group had recurrent atrial arrhythmias (P = 0.40) during 6-month's follow-up. There was no difference in the number of antiarrhythmic drugs used after ablation between both groups. Residual PVP ablation by touch-up RFA was not associated with recurrence in CBA group (recurrence: 0/8 vs no recurrence: 14/119, P = 0.59). The Kaplan-Meier curve did not show significant difference in recurrence between the two groups 6-month's follow-up (Log rank P = 0.318). (Fig. 1).
Fig. 1.
A, Kaplan-Meier curve of recurrent atrial tachyarrhythmia after the index procedure during follow-up between the two groups (High power short duration group (HPSD) and Cryoballoon ablation group (CBA)) (Log rank p value 0.381).
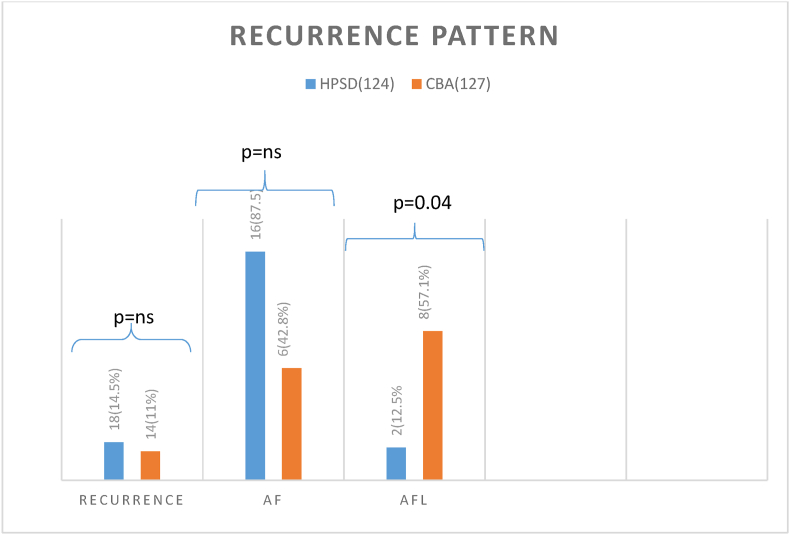
There were different recurrent patterns between 2 groups (Fig. 2). A significantly higher incidence of recurrent atrial flutter in CBA group was found compared to HPSD group (57.1% vs 11.1%, P = 0.04).
Fig. 2.
Recurrent patterns of the 2 groups
(Recurrence as Atrial fibrillation (AF) was not significantly different between two groups but recurrence as atypical atrial flutter (AFL) was significantly more common in CBA group (p = 0.04).
4. Discussion
4.1. Main finding
The main findings of this study were [1] The time to achieve PVI was significantly shorter in the CBA group [2], there was no significant difference in incidence of recurrent atrial tachyarrhythmias between CBA and HPSD ablation of PAF [3], there were different recurrent patterns between CBA and HPSD groups [4]. Both the techniques have equal safety profile.
4.2. Ablation procedure
A recent randomized controlled trial reconfirmed our findings except that we find more recurrence as organized atrial tachycardias in the CBA group [14] Recent randomized controlled trials showed equal 1 year arrhythmia free survival with RF ablation by contact force sensing vs cryoballoon catheter [15].(13) [16] In terms of the efficacy of PVI, our result showed that time to achieve PVI was significantly shorter for CBA group than HPSD group. A recent study also showed time to PVI was significantly shorter in CBA group as compared to the HPSD group (19.6+- 5.2 vs 43.3+-14.9 min) [14]. As second generation cryoballoon has large surface area and more efficient cooling, it might contribute to the shorter procedure time in the CBA [17].
No major procedure related complication was noted in either group, suggesting that PVI by HPSD or CBA is safe in agreement with previous data [8,18]. These clinical and animal studies showed that in HPSD, RF applications produce the lesions mainly by resistive tissue heating that occur during early part of RF application and thus avoid conductive heating of distant and deep tissues that is mainly responsible for complications of ablation procedures. In contrast CBA achieve irreversible tissue damage by intracellular freezing below −40′C. As compared with RF lesions, cryoablation results in preservation of tissue architecture with lesser risk of thrombus formation. Histologically CBA results in the creation of well-demarcated homogeneous lesions [19].
4.3. Mechanism of recurrent arrhythmias
In agreement with previous studies [[20], [21], [22]], comparing conventional RFA with CBA, our study also showed rate of recurrence of atrial arrhythmias was not significantly different between these 2 techniques of PVI. A recent study using DE-MRI also showed that PV reconnection rates and recurrence rate were similar in patients who underwent RFA or CBA [23].
Previous studies have shown that PV reconnection is the main cause of recurrence in both RFA and CBA groups [[24], [25], [26], [27]]. Our study showed that the type of recurrence is different between HPSD and CBA groups. Recurrence as atrial flutter was significantly more common in CBA group as compared to HPSD group. Mechanism of atrial flutters after CBA may be linked to more generous LA substrate debulking produced by CBA. The larger area of ablation may cause a potential isthmus critical for development of macroreentrant tachycardias. Furthermore, CBA may produce non-transmural lesion owing to complex anatomy of LA-PV antrum, which could lead to the formation of slow conduction zone. Both factors paved the way to uneven distribution of scar tissue between the cryoballoon-ablated area and anatomical obstacles, resulting in slow conduction, which could serve as the critical isthmus to sustain the organized atrial arrhythmia.
It has been showed that the 28-mm cryoballoon resulted in more antral and generous posterior LA debulking during PV isolation [28]. Our previous study also showed that in comparison with the RFA group, the CBA group had a lower mean LA voltage which might contribute to the formation of LA flutters [25]. Attention should be paid in patients with CBA to identify the potential conduction gap or isthmus which might prevent the recurrence of tachyarrhythmia.
5. Limitations
Limitations of this study should be appreciated. First since it is a single Centre a retrospective study it is difficult to compare these ablation modalities head to head, Second as continuous rhythm monitoring was not done so the recurrence may be underreported, Third the use of propensity score matching could have been useful, Fourth the follow up time is only 6 months so long term results may be different, Fifth not all the patients with recurrence underwent repeat invasive study so type of recurrence was assessed by only surface ECG and rhythm monitoring, Sixth the definition of HPSD ablation is arbitrary and optimal power and duration settings likely to very depending on the left atrial wall thickness at a constant contact force.
6. Conclusion
CBA ablation has a similar efficacy and safety as HPSD in PAF patients with shorter procedure time. However, the recurrent patterns were different between these 2 groups. Prospective study is warrant to identify the possible mechanism.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Declaration of competing interest
The Authors declares that they have no conflict of interest.
Acknowledgement
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Schnabel R.B., Yin X., Larson M.G., Magnani J.W., Ellinor P.T., Philip A. Fifty-year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the community renate. Lancet. 2015;386(9989):154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Wolf P.A., D'Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998 Sep 8;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Dietzel J, Haeusler KG, Endres M. Does atrial fibrillation cause cognitive decline and dementia? Europace. 2018 Mar 1;20(3):408–419. doi: 10.1093/europace/eux031. [DOI] [PubMed] [Google Scholar]
- 4.Haïssaguerre M., Jaïs P., Shah D.C., Takahashi A., Hocini M., Quiniou G., et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998 Sep 3;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 5.Chen S.A., Hsieh M.H., Tai C.T., Tsai C.F., Prakash V.S., Yu W.C., et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999 Nov 2;100(18):1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 6.Bourier F., Duchateau J., Vlachos K., Lam A., Martin C.A., Takigawa M., et al. High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electrophysiol. 2018 Nov 1;29(11):1570–1575. doi: 10.1111/jce.13724. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson B., Chen X., Pehrson S., Svendsen J.H. The effectiveness of a high output/short duration radiofrequency current application technique in segmental pulmonary vein isolation for atrial fibrillation. Europace. 2006 Nov;8(11):962–965. doi: 10.1093/europace/eul100. [DOI] [PubMed] [Google Scholar]
- 8.Winkle R.A., Mohanty S., Patrawala R.A., Mead R.H., Kong M.H., Engel G., et al. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm. 2019 Feb 1;16(2):165–169. doi: 10.1016/j.hrthm.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Kuck K.-H., Brugada J., Fürnkranz A., Metzner A., Ouyang F., Chun K.R.J., et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016 Jun 9;374(23):2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 10.Calkins H., Hindricks G., Cappato R., Kim Y.H., Saad E.B., Aguinaga L., et al. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–e444. doi: 10.1016/j.hrthm.2017.05.012. 2017 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin W.S., Tai C.T., Hsieh M.H., Tsai C.F., Lin Y.K., Tsao H.M., et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003 Jul 1;107(25):3176–3183. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 12.Tsai C.F., Tai C.T., Hsieh M.H., Lin W.S., Yu W.C., Ueng K.C., et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000 Jul 4;102(1):67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Fürnkranz A., Bordignon S., Dugo D., Perotta L., Gunawardene M., Schulte-Hahn B., et al. Improved 1-year clinical success rate of pulmonary vein isolation with the second-generation cryoballoon in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25(8):840–844. doi: 10.1111/jce.12417. [DOI] [PubMed] [Google Scholar]
- 14.Pak H.N., Park J.W., Yang S.Y., Kim T.H., Uhm J.S., Joung B., et al. Cryoballoon versus high-power, short-duration radiofrequency ablation for pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a single-center, prospective, randomized study. Circ Arrhythm Electrophysiol. 2021;14(9):886–896. doi: 10.1161/CIRCEP.121.010040. [DOI] [PubMed] [Google Scholar]
- 15.Andrade J.G., Champagne J., Dubuc M., Deyell M.W., Verma A., Macle L., et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140(22):1779–1788. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 16.Rubesch-Kütemeyer V., Molatta S., Vogt J., Gutleben K.-J., Horstkotte D., Nölker G. Reduction of radiation exposure in cryoballoon ablation procedures: a single-centre study applying intracardiac echocardiography and other radioprotective measures. Europace. 2017 Jun 1;19(6):947–953. doi: 10.1093/europace/euw139. [DOI] [PubMed] [Google Scholar]
- 17.Ciconte G., De Asmundis C., Sieira J., Conte G., Di Giovanni G., Mugnai G., et al. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015;12(4) doi: 10.1016/j.hrthm.2014.12.026. 673-80. [DOI] [PubMed] [Google Scholar]
- 18.Bhaskaran A., Chik W., Pouliopoulos J., Nalliah C., Qian P., Barry T., et al. Five seconds of 50-60 W radio frequency atrial ablations were transmural and safe: an in vitro mechanistic assessment and force-controlled in vivo validation. Europace. 2017 May 1;19(5):874–880. doi: 10.1093/europace/euw077. [DOI] [PubMed] [Google Scholar]
- 19.Hirao T., Nitta J., Adachi A., Takahashi Y., Goya M., Hirao K. First confirmation of histologic changes in the human heart after cryoballoon ablation. Hear Case Reports. 2019;5(2):93–96. doi: 10.1016/j.hrcr.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buiatti A., von Olshausen G., Barthel P., Schneider S., Luik A., Kaess B., et al. Cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: an updated meta-analysis of randomized and observational studies. Europace. 2017 Mar 1;19(3):378–384. doi: 10.1093/europace/euw262. [DOI] [PubMed] [Google Scholar]
- 21.Luik A., Radzewitz A., Kieser M., Walter M., Bramlage P., Hörmann P., et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation. Circulation. 2015 Oct 6;132(14):1311–1319. doi: 10.1161/CIRCULATIONAHA.115.016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasserlauf J., Pelchovitz D.J., Rhyner J., Verma N., Bohn M., Li Z., et al. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. PACE - Pacing Clin Electrophysiol. 2015 Apr 1;38(4):483–489. doi: 10.1111/pace.12582. [DOI] [PubMed] [Google Scholar]
- 23.Alarcón F., Cabanelas N., Izquierdo M., Benito E., Figueras Ventura R.I., Guasch E., et al. Cryoballoon vs. radiofrequency lesions as detected by late-enhancement cardiac magnetic resonance after ablation of paroxysmal atrial fibrillation: a case-control study. Europace. 2020 Mar 1;22(3):382–387. doi: 10.1093/europace/euz309. [DOI] [PubMed] [Google Scholar]
- 24.Higa S., Tai C.T., Chen S.A. Catheter ablation of atrial fibrillation originating from extrapulmonary vein areas: Taipei approach. Heart Rhythm. 2006 Nov;3(11):1386–1390. doi: 10.1016/j.hrthm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Chang T.Y., Lo L.W., Te A.L.D., Lin Y.J., Chang S.L., Hu Y.F., et al. The importance of extrapulmonary vein triggers and atypical atrial flutter in atrial fibrillation recurrence after cryoablation: insights from repeat ablation procedures. J Cardiovasc Electrophysiol. 2019 Jan 1;30(1):16–24. doi: 10.1111/jce.13741. [DOI] [PubMed] [Google Scholar]
- 26.Fichtner S., Czudnochowsky U., Hessling G., Reents T., Estner H., Wu J., et al. Very late relapse of atrial fibrillation after pulmonary vein isolation: incidence and results of repeat ablation. PACE - Pacing Clin Electrophysiol. 2010 Oct;33(10):1258–1263. doi: 10.1111/j.1540-8159.2010.02808.x. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson B., Chen X., Pehrson S., Køber L., Hilden J., Svendsen J.H. Recurrence of pulmonary vein conduction and atrial fibrillation after pulmonary vein isolation for atrial fibrillation: a randomized trial of the ostial versus the extraostial ablation strategy. Am Heart J. 2006;152(3):537.e1–537.e8. doi: 10.1016/j.ahj.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Usui E., Miyazaki S., Taniguchi H., Ichihara N., Kanaji Y., Takagi T., et al. Recurrence after “long-term success” in catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2015 May 1;12(5):893–898. doi: 10.1016/j.hrthm.2015.01.043. [DOI] [PubMed] [Google Scholar]