ABSTRACT
The development of multicellular complex organisms relies on coordinated signaling from the microenvironment, including both biochemical and mechanical interactions. To better understand developmental biology, increasingly sophisticated in vitro systems are needed to mimic these complex extracellular features. In this Primer, we explore how engineered hydrogels can serve as in vitro culture platforms to present such signals in a controlled manner and include examples of how they have been used to advance our understanding of developmental biology.
Keywords: Hydrogels, Extracellular matrix, Mechanobiology, In vitro culture
Summary: This Primer introduces hydrogel technologies and discusses how engineered hydrogels can advance our understanding of developmental biology.
Introduction
Cells within developing tissues encounter a plethora of extracellular cues that influence their fate, from the transmission of signals through receptor-ligand interactions and soluble factors to those physically provided by the extracellular matrix (ECM) (Abdal Dayem et al., 2018; Clause et al., 2010). Extracellular cues begin after fertilization, where the early cell-rich embryo relies predominantly on cell-cell interactions to determine which cells are internalized to form the inner cell mass to give rise to the blastocyst (Rouault and Hakim, 2012). Eventually, gastrulation separates the embryo into multiple layers, and this is the first embryonic time point at which ECM is detected in zebrafish (Latimer and Jessen, 2010). The cues offered by this matrix in turn influence the collective cell migration of each germ layer. As the organism continues to develop, resident stem cells rely on mechanical (via direct biophysical forces) or biochemical cues from the ECM, as well as genetic and epigenetic cues from other cells, to give rise to tissue patterning (Crowder et al., 2016; Walma and Yamada, 2020). The ECM in developing embryos is also heterogeneous (Loganathan et al., 2012), with the timing and cascade of ECM components depending on the species and tissue of interest, often beginning with the deposition of adhesive proteins such as fibronectin (Hayes et al., 2001).
Much of what we have learned about development has been through animal models; however, the complexity of these organisms motivates the development of in vitro models that can both recapitulate and decouple aspects of this dynamic microenvironment to probe important questions related to developmental biology. Traditional cell culture platforms have relied on rigid substrates (e.g. plastic, glass) that exhibit mechanical properties beyond that of native tissue and present static, uniform signals to cells. However, biomaterials, and particularly hydrogels, are playing an important role to address these limitations through the development of in vitro models that recapitulate some of the functions and properties of the native ECM. Hydrogels are water-swollen crosslinked polymer networks that exhibit numerous biochemical and biophysical properties that can be tailored for specific applications (Fig. 1). In this Primer, we first introduce the basic properties of hydrogels and offer an overview of how hydrogels are fabricated. We then discuss how hydrogels can be engineered to create controlled and complex cell culture platforms. Finally, we describe how hydrogels can inform (and are informing) our understanding of development and differentiation. Overall, these new platforms and technologies will expand the tools available for future exploration of developmental processes.
Fig. 1.
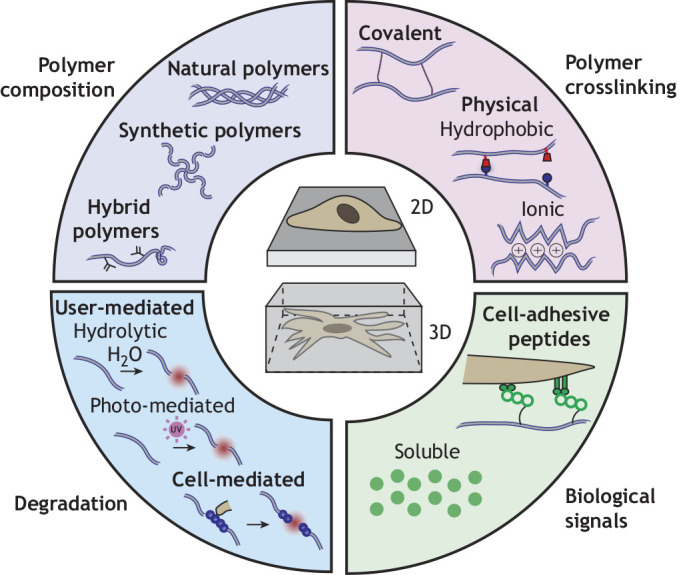
Engineering hydrogels to present extracellular signals to cells. Hydrogels consist of polymer networks in which polymer composition (natural, synthetic or hybrid polymers), polymer crosslinking (covalent or physical) and network degradation (user-mediated and cell-mediated) can be tuned. In addition, biological signals may be introduced as ligands to promote cell adhesion or as soluble factors. Cells interface with hydrogels either through seeding atop (2D) or being encapsulated within (3D).
Hydrogel properties
The chemical and mechanical properties of hydrogels can be customized through changes in polymer chemistry, crosslink type, degradation, geometry and the introduction of specific signals and peptides (Fig. 1). Here, we briefly introduce some key features of hydrogels. For a more exhaustive and detailed overview of engineered hydrogel components, we refer readers to Muir and Burdick (2021).
Polymer chemistry and composition
Hydrogels may be composed of natural ECM derivatives, such as collagen, gelatin or Matrigel, which contain native cell adhesion molecules (e.g. integrin binding sites) and ECM degradation sites that mimic those found in vivo (Nicolas et al., 2020) (Table 1). Furthermore, decellularized ECM has also been used to form natural hydrogels, whereby all cellular components are removed, leaving behind key extracellular frameworks and biological signals. The main advantage of natural hydrogels is their increased ability to mimic the complex ECM found in vivo and the ease of obtaining and using these materials. However, given that these hydrogels are derived from native tissue, batch-to-batch variability is high, and the composition is often poorly defined.
Table 1.
Benefits and drawbacks of commonly used hydrogels
To overcome concerns of batch-to-batch variability and mechanical integrity of naturally derived hydrogels (Haut, 1983), engineered synthetic hydrogels are also being explored for cell culture. When trying to probe specific questions related to how extracellular cues (e.g. adhesion, stiffness, degradation) may influence cell processes, engineered hydrogels may be advantageous over natural hydrogels as these features may be decoupled from one another. Synthetic polymers, such as poly(ethylene glycol) (PEG), offer excellent modification potential to exert control over physical properties of the hydrogels and cellular interactions, such as through the controlled introduction of cell adhesion peptides and matrix degradation sites (Salinas and Anseth, 2008). However, these materials often require complex and time-consuming synthesis processes, although they are increasingly becoming available commercially.
Finally, hybrid polymers, including chemically modified natural biopolymers such as gelatin methacryloyl or methacrylated hyaluronic acid, exhibit features of native ECM owing to their biological origin, but can also be modified to control certain properties, such as mechanics (Zhu et al., 2019) (Fig. 1). Thus, hybrid materials draw on the strengths of both natural and synthetic polymers to mimic how cells interact with ECM in vivo, while also allowing the user increased control over hydrogel crosslinking or cell-material interactions. Notably, the introduction of these chemical modifications must be done carefully, so as to not interfere with native sequences required for cell adhesion and degradation.
Polymer crosslinking
Hydrogels are formed through the crosslinking of polymers to form a network. This crosslinking can occur through physical crosslinking, covalent crosslinking or combinations of the two (Hennink and van Nostrum, 2002) (Fig. 1). Physical crosslinks are generally weak, due to ionic charge or shared hydrophobicity. These crosslinks are usually dynamic and reversible, which allow cells to remodel and exert tractions on their extracellular environments (Yang et al., 2021). In contrast, covalent crosslinks involve a chemical reaction that is often initiated with a stimulus (e.g. light, heat, pH, etc.). These crosslinks are stronger and thus enhance the stability and mechanical properties of hydrogels. Because these crosslinks are usually permanent, degradation must be inherent or engineered into the hydrogel for cellular remodeling.
Degradation
For studies in which cells are encapsulated within hydrogels in 3D, polymers or crosslinks may have inherent or engineered degradation sites (either within the polymer or within crosslinks) to allow cells to interact with their extracellular environment as they would in vivo, including to migrate or remodel their pericellular environments. Ultimately, these sites break with stimulus (either user-mediated or cell-mediated), thus weakening the hydrogel (Fig. 1). User-mediated degradation may be engineered through different stimuli, including hydrolytic degradation (Lin and Lin, 2021), which is often dependent on temperature and pH of the environment, or through photo-mediated degradation, in which chemical moieties become unstable upon light stimulus (Kloxin et al., 2009). This allows the user to dictate the extent to which cells may interact with their extracellular environments, facilitating studies of cell behavior in response to dynamic and fully defined changes in their environment. In addition, cell-mediated degradation can be introduced through matrix metalloproteinase (MMP) degradable crosslinkers (Wade et al., 2015), where an inflammatory environment (where MMPs are found in higher concentrations) may be leveraged for faster hydrogel degradation. This approach also enables cells to interact with their environment in a manner more like that observed in vivo.
Degradation may also be useful as a mechanism to control the release of embedded soluble cues. In this case, the weakening of hydrogels enables factors that may have been previously trapped to be released from the hydrogel, thus impacting cells in the vicinity. Strategies for designing hydrogels for controlled release have been studied extensively in the context of drug delivery (Li and Mooney, 2016).
Geometry
Hydrogels can be designed as 2D substrates, where a cell is cultured atop a hydrogel that supports cell adhesion. This approach is similar to traditional culture on plastic or glass but harnesses material tunability with respect to chemical and physical properties, presented either as static and uniform or with dynamic spatiotemporal properties (Fig. 1). 2D approaches are often simpler to engineer because hydrogels can be fabricated before seeding cells, thus negating the constraint of considering whether hydrogel precursors are cytotoxic.
Cells can also be fully encapsulated within hydrogels for 3D culture, where cells are suspended within the hydrogel precursor components and then the hydrogel is formed around them (Caliari and Burdick, 2016) (Fig. 1). These systems are often more physiologically relevant and can elicit signaling pathways that are akin to those found in vivo. In addition, hydrogels can be further processed into increasingly complex 3D structures (e.g. 3D printing; see Box 1) for use as in vitro models (Vanderburgh et al., 2017). However, 3D systems are often more challenging to fabricate given that cell viability must be maintained throughout fabrication and all components (e.g. polymer, crosslinker) must be cytocompatible. This sometimes presents a challenge, given that many synthetic hydrogels rely on crosslinkers or reaction-induction agents that may be cytotoxic. However, these challenges can be mitigated through introducing reactive groups directly onto polymers to eliminate the need for crosslinkers or choosing alternate crosslinking strategies. Other limitations to 3D cell culture include the possibility that 3D networks may also have reduced diffusivity, where bulky molecules such as antibodies may be limited in their ability to reach encapsulated cells. In addition, although imaging approaches are rapidly advancing, hydrogels must often be imaged using 3D microscopy methods, such as confocal imaging, or must be sectioned for use with conventional 2D imaging techniques.
Box 1. Combining hydrogels with other bioengineering technologies.
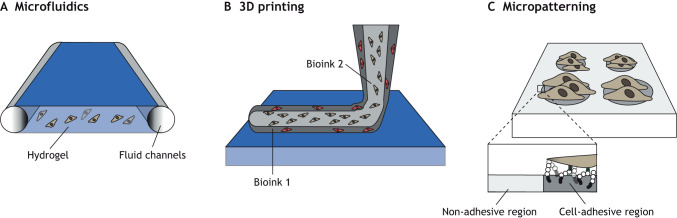
Other tools can be used in conjunction with hydrogels to further control their presentation to cells. For example, hydrogels are often used to support cells within microfluidic devices, where fluid flow occurs through defined geometries and exposes cells to physiologically relevant shear forces (A) (Bonner et al., 2022). These tools are often used to create miniature models (organ-on-a-chip) of various tissues, particularly with the inclusion of vascular structures.
In 3D printing, hydrogels are used as bioinks, where materials are deposited into desired structures either with cells already encapsulated or for seeding with cells (Moroni et al., 2018). This technique enables precise spatial control of complex architectures akin to those found in native tissues through using desired inks and cell populations (B). This method is especially useful when exploring questions of cell proximity or effects of distance on co-culture (Piard et al., 2019). Examples of 3D printing include depositing hydrogels coaxially (simultaneously concentric) with distinct bioinks and populations of cells to spatially pattern them or printing cells initially in a sacrificial ink, where the ink is later removed to enable cell-cell interactions (Jeon et al., 2020; Taymour et al., 2021).
In a method similar to 3D printing, other studies have used acoustophoresis, in which acoustic waves propagate in convective flows to collect cells in predefined areas (Ma et al., 2020). Hydrogels are then used to stabilize the organized cells for further culture. Magnetic fields can also be used to organize cells before encapsulation within hydrogels (Zlotnick et al., 2020). Undoubtedly, these advanced methods and approaches will allow for further processing of hydrogels into desirable configurations for cell culture.
Micropatterning is yet another technique where the spatial presentation of adhesive signals to cells is used to isolate cells to specific areas, typically using photolithography techniques (reviewed by Blin, 2021). These patterns can be large enough to confine a monolayer of cells, be the size of a single cell or be used to pattern multiple adhesive areas within a cell (Yoney et al., 2018; Muncie et al., 2020; Yang et al., 2016) (C).
Peptides and signals
The incorporation of peptides, proteins or other signals into hydrogels can further alter biological interactions, such as cell adhesion (Yang et al., 2005). These peptides can be covalently attached to the hydrogel or be embedded within the hydrogel and released upon degradation or diffusion. For example, the delivery of soluble factors (e.g. growth factors, cytokines, etc.) can be controlled in time and in space to modulate cellular behaviors (Nurkesh et al., 2020) (Fig. 1). We discuss the applications and insights from these approaches in later sections.
Hydrogel preparation
Once the design criteria, hydrogel constituents and desired geometry are identified, hydrogel preparation is often simple. Cells can either be placed atop or encapsulated within hydrogels for 2D and 3D cell studies, respectively. In 2D studies, the user mixes the polymers, the crosslinkers and any desired additional biological signals. This prepolymer (the solution created from the mixture of the previous components) is then placed within a desired geometry (usually via a previously fabricated mold) and crosslinked (with or without stimulus). Cells, cell pellets, organoids or tissue explants are then seeded or placed on top of the hydrogels. Media is then added on top for cell culture atop the hydrogel. For 3D studies, the prepolymer is combined with the cell source and then placed within the mold. The hydrogel is then crosslinked and media is added. The addition of various components can be completed simultaneously or sequentially, depending on the method of incorporation or desired outcomes. This straight-forward fabrication process suggests that labs not accustomed to using hydrogels for in vitro studies can incorporate them into their studies with ease (Caliari and Burdick, 2016).
Hydrogels to increase the complexity and control of in vitro systems
Hydrogels enable the user to control detailed aspects of the extracellular environment across different length scales, widening the breadth of ECM-based inquiries a developmental biologist can pursue in vitro. In this section, we provide examples where in vitro environments can be engineered to study biological behavior.
Manipulating cell interactions and adhesion
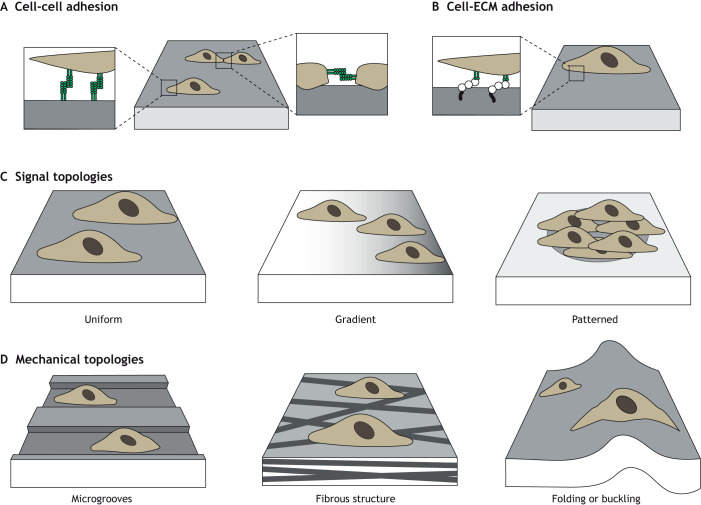
Cell-cell interactions can be controlled through the overall density of cells cultured with hydrogels, through the spatial micropatterning of adhesion onto hydrogels to control the area of cellular interactions (Box 1) or via hydrogel chemical modification to present moieties from specific receptors that mediate cell-cell binding (Zhang et al., 2021). For example, N-Cadherin (Fig. 2A), a transmembrane protein crucial to cell-cell adhesion, can be chemically conjugated onto hydrogels either as a full protein or peptide sequence (e.g. HAVDI) to simulate direct cell-cell contacts, enabling the user to probe how these cues influence cell differentiation (Cosgrove et al., 2016) (Table 2).
Fig. 2.
Increasing complexity of extracellular environments through hydrogels. Hydrogels can be engineered to recapitulate aspects of extracellular environments. For example, they can be designed to display cell-cell (A) and cell-ECM (B) moieties. In addition, hydrogels may present various 2D uniform (left), gradient (middle) or patterned (right) molecular cues to guide cellular behaviors (C) or pseudo 3D topologies such as microgrooves (left), fibrous structures (middle) or through folding or buckling of 2D substrates (right panel) (D).
Table 2.
Peptide sequences on hydrogels to promote cell-material interactions
Although cell-cell adhesion is featured prominently in early development, many tissues reach a crucial transition during which the production of ECM increases and cells are more likely to experience ECM-binding than cell-cell binding. To that end, full-length adhesion proteins or a wide range of peptides that mimic these cell-matrix adhesions have been incorporated into hydrogels and can render inert hydrogels adhesive (Fig. 2B; Table 2). Numerous integrin complexes exist, each with preferential adhesion to different sites on various ECM proteins, such as fibronectin, collagen and laminin. The most widely used adhesive domain to mimic ECM is arginylglycylaspartic acid (RGD), a peptide sequence in fibronectin; when ligated to non-adhesive hydrogels, cells can readily attach to these modified materials (Pierschbacher and Ruoslahti, 1984). Additional peptide sequences in fibronectin, such as FN9*10 and Fn4G, can also be conjugated onto hydrogels (Hui et al., 2021). GFOGER is a peptide sequence that mimics adhesion to collagen I (Clark et al., 2020; Knight et al., 1998). Sequences from laminin, such as IKVAV or YIGSR, have also been introduced to modulate developmental signaling (Kim et al., 2018; Tashiro et al., 1989). Because of the increased control over cell-matrix adhesion sites, hydrogels can be designed to alter not only the type but also the number of sites presented to cells, enabling exploration of the role of cell adhesion on cell behavior and differentiation (Yang et al., 2005). Although these peptides have reduced affinity compared with full native proteins, users can also combine different ratios of adhesion peptides to more closely control and mimic the complex adhesion-initiated signaling that may occur in vivo (Li et al., 2017; Cosgrove et al., 2016).
Creating complex signaling environments
Hydrogels can be used in conjunction with higher-order tools (Box 1) to control soluble factor presentation. For example, 3D printing enables a user to macroscopically build more complex factor release patterns through varying bioinks and spatiotemporal deposition (Miller et al., 2009; Kuzucu et al., 2021). Furthermore, microfluidic channels have been explored to present signaling gradients using different solutions in two parallel microfluidic channels to create gradients within an intermediary hydrogel (O'Grady et al., 2019).
Hydrogels can also be used as substrates to probe the biophysical interactions between cells and their extracellular environments. For example, cells exert tractions as they spread and migrate (Saez et al., 2007). Increasingly complex methods of tracking cell-exerted traction forces have been developed, but almost all rely on viewing deformation of an embedded fiduciary marker and making assumptions on the properties of the surrounding hydrogel to extrapolate mechanical forces (Legant et al., 2010; Mohagheghian et al., 2018).
To increase the spatial complexity of these signals, stimulus can be selectively applied to regions of the hydrogel to create gradients or through micropatterning (Box 1). For example, spatial stiffness gradients can be introduced using such techniques (Hadden et al., 2017), including using a gradient photomask (Rape et al., 2015). Similarly, photo-patterning (where photo-stimulus is applied to specific regions via a photomask) can create spatial patterns in crosslinking to differentially introduce mechanical or biological features (Vega et al., 2018) (Fig. 2C). Hydrogels have been used to explore a range of other topographic cues, through the introduction of nanocraters (small pits), microgrooves (small grooves) or even fibrous (rope-like) topologies (Jeon et al., 2015; Abagnale et al., 2015; Davidson et al., 2019). These materials offer proof-of-concept platforms to explore the role of topographic features inherent in developmental processes (Fig. 2D).
In addition, all tissues may temporally vary in mechanical properties, whether through prescribed changes during developmental transitions or through inherent viscoelasticity, meaning that the mechanical properties of the tissue vary depending on the time scale at which it is probed. Hydrogels can be used to mimic the dynamic transitions in extracellular properties that occur throughout development. For example, hydrogels have been designed to change in stiffness in response to user-mediated cues, such as photo-crosslinking or the addition of crosslinkers (Guvendiren and Burdick, 2012; Arkenberg et al., 2020). Hydrogels can also be engineered to tune the viscoelasticity of complex 2D and 3D environments (Lee et al., 2017).
Improving organoid reproducibility and complexity
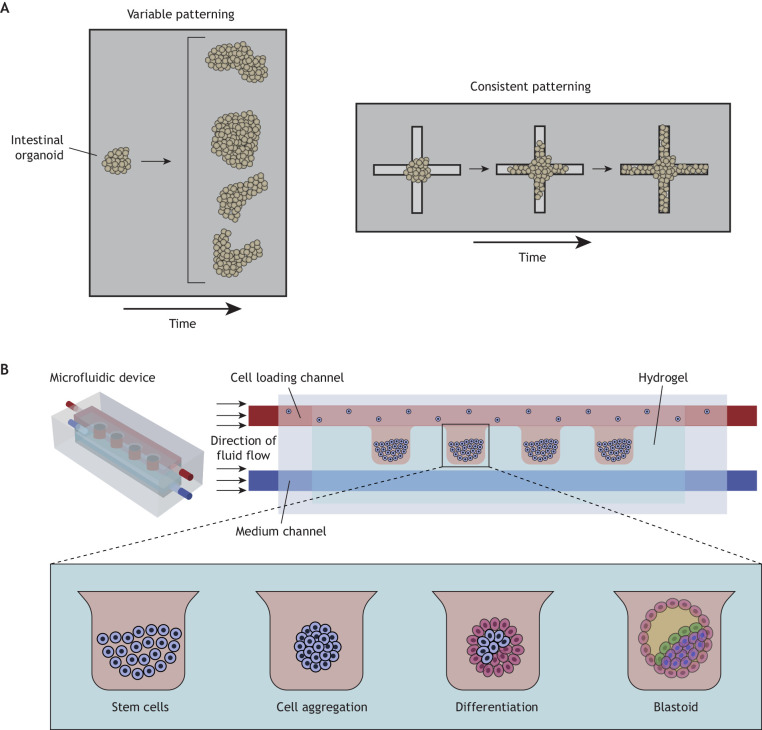
Organoids are 3D multicellular structures that are derived from stem cells that differentiate to exhibit key features of their respective organs (Hofer and Lutolf, 2021). Classically, organoid cultures have been developed with Matrigel matrices, both for fabrication and culture (Viola et al., 2020; Zheng et al., 2019). However, due to the lack of reproducibility between these animal-derived matrices and the random presentation of cues to the organoid, organoids are often inconsistently patterned with heterogeneous structures (Fig. 3A) (Shankaran et al., 2021). To overcome this limitation, rationally designed hydrogels can be used to culture organoids (Gjorevski et al., 2016), where the programmable nature of these engineered environments enables greater reproducibility (Cruz-Acuña et al., 2017). In addition, fully defined hydrogels can support the culture and maturation of organoids from various tissues that would otherwise rely on poorly defined substrates to develop in vitro, including the bone marrow, pancreas and lungs (Vallmajo-Martin et al., 2020; Candiello et al., 2018; Loebel et al., 2022). Due to the sensitivity of organoids in culture, attention must be paid to how the hydrogel is introduced, such as the potential toxicity of crosslinkers within synthetic hydrogels to encapsulate organoids.
Fig. 3.
Examples of higher-order hydrogel fabrication for increasingly complex architectures. (A) Intestinal organoids in Matrigel typically form buds in a variable manner (left panel). However, hydrogels can be used to support organoid culture and guide morphogenesis in a fully defined and reproducible manner whereby organoids consistently form crypt-like buds along photo-softened areas (right panel). Based on studies from Gjorevski et al. (2022). (B) Hydrogels can also be used within microfluidics to direct spatial embryonic development into 3D structures within hydrogel compartments that modulate environmental signals. Adapted with permission from Bonner et al. (2022), which was re-drawn from Zheng et al. (2019).
Organoid cultures often require a substrate that can be remodeled to support tissue expansion. As synthetic chemistries have increased in complexity, hydrogels are better able to support this tissue expansion through a variety of means. Through degradation, hydrogels have been used to guide intestinal organoid crypt formation, where user-defined photo-softening of adjacent hydrogel dictated areas of local crypt-like bud development, thus controlling their spatial patterning (Fig. 3A) (Gjorevski et al., 2022). Another recent study tuned the stress relaxation behavior of organoid extracellular environments and, because of the ability of the organoid to remodel its dynamic environments, observed enhanced differentiation of intestinal organoids compared with those maintained in static environments (Chrisnandy et al., 2022). Undoubtedly, the ability to engineer hydrogels has already offered insights into organoid culture and patterning and represents a method to investigate how extracellular cues may influence organ development.
Employing hydrogels to understand development and differentiation
Hydrogels can be engineered to present complex chemical and mechanical features, which can influence morphogenesis and cell differentiation, as well as cell migration (Elosegui-Artola et al., 2022), proliferation (Cameron et al., 2011), and tissue deposition (Lee et al., 2017). Here, we discuss recent progress in which hydrogels support the study of key developmental processes in vitro.
Implantation
Given the versatility of hydrogels in modeling interactions between cells and extracellular environments, they may be used to model the intricate implantation process, whereby the fetal blastocyst must interact with the maternal endometrium. To study the endometrium itself, endometrial epithelial and stromal cells have been co-cultured in hydrogels, where cell adhesion to fibronectin and basement membrane domains enhances robust epithelial layer formation through promoting ECM deposition. These models are also hormone responsive, where decidualization cues promote the release of prolactin and IGFBP1 levels and demonstrate the use of this system as a proof-of-principle in vitro model (Cook et al., 2017).
Hydrogels have also been used to study the dynamics of blastocyst implantation. Implantation requires that embryos undergo hatching, in which blastocysts break out of the zona pellucida. Mammalian embryos have been confined in polyacrylamide hydrogels and the quantification of hydrogel deformation has been used to determine embryonic pressure, revealing that reduced pressure results in delayed hatching (Leonavicius et al., 2018).
In addition, fetal-maternal interactions can be modeled with hydrogels. For example, hydrogels have been fabricated to match the extracellular features of the endometrium, such as stiffness and adhesion sites, to introduce a synthetic in vitro endometrium model that promotes angiogenesis from the implanting trophoblast (Govindasamy et al., 2021). Hydrogels composed of decellularized matrices have also been used to demonstrate that a proliferating endometrium is more supportive of embryonic development when compared with non-proliferative endometrium (Campo et al., 2019). Additionally, hydrogels have been precisely tuned to explore the physiologically relevant mechanical properties and biological factors that influence trophoblast implantation and invasion, which has revealed that cortisol has a minimal effect on endometrial proliferation and proper receptor expression is crucial for normal implantation (Zambuto et al., 2019; Zambuto et al., 2020; Stern-Tal et al., 2020).
Cell differentiation
Totipotent cells in the embryo rely on external cues to direct differentiation. Hydrogels can be used to provide control over these cues to understand their effect on patterning. For example, Matrigel has been used to investigate the influence of cell density on human embryonic stem cell (hESC) lineage decisions, revealing that lower cell densities lead to the development of amniotic-like tissues, whereas higher cell densities lead to the development of mesodermal-like tissues before gastrulation (Chen et al., 2021). This study has informed parameters of embryonic cultures for in vitro hESC models, supporting future in vitro studies on differentiation.
The ability to regulate mechanics by controlling the stiffness of hydrogels has been instrumental in revealing how cells integrate biophysical cues to direct differentiation. For example, hydrogels have been engineered with controlled substrate stiffness for hESC culture, revealing that compliant substrates induce expression of mesoderm-like signals, whereas stiffer substrates do not induce similar expression (Przybyla et al., 2016). Hydrogels have also been designed to spatially present signals to cells. For example, human mesenchymal stromal cells (hMSCs) have been placed atop hydrogels containing micropatterned, small domains of both stiff and soft regions, such that cues are sensed differentially across a single cell (Yang et al., 2016). Interestingly, substrates with stiff and soft regions randomly micropatterned across the cell and entirely soft hydrogels inhibit osteogenic lineages, whereas regular patterning of stiff and soft regions and entirely stiff hydrogels promote osteogenic lineages. These findings are helpful to understand differentiation of cells in normal tissues versus within pathological tissue, in which the local microenvironment may become disorganized. Furthermore, temporal changes in hydrogel rigidity that may occur throughout development influence cell differentiation. Dynamically stiffening hydrogels have demonstrated that, in hMSCs, the amount of time the cell resides on soft substrates before stiffening influences the extent of differentiation: immediate stiffening universally promotes osteogenic phenotypes, whereas delayed stiffening (some time on soft substrates) leads to a subset of adipogenic phenotypes (Guvendiren and Burdick, 2012). In dynamically softening hydrogels, the timeline for mechanical changes also dictates the extent of fibroblast to myofibroblast phenotype changes, where short exposure times to stiff substrates allows myofibroblasts to return to a quiescent phenotype, but longer exposures increases the persistence of the activated myofibroblast (Walker et al., 2021). Both examples underscore how changes in extracellular biophysical properties in response to development or disease can influence phenotype.
Although 2D substrates can constrain cell monolayers and be controlled for topography, many developmental processes occur in 3D and hydrogels must also recapitulate this dimensionality in vitro. Hydrogels have been used to provide a variety of cell scaffolding environments to hESCs to observe amnion development (Shao et al., 2017). When exposed to variable environments of biophysical properties and dimensionality, including stiff 2D culture, soft 2D culture, stiff 3D culture and soft 3D culture, the last of which most closely mimics the tissue niche available for implantation, only hESCs exposed to the soft 3D culture develop a squamous epithelial morphology. This suggests that these hESCs differentiate into a tissue more akin to the developing amnion than the cells in the non-native environments. This study underscores how early embryonic development relies on environment stiffness and dimensionality, two key features of the extracellular environment that can be precisely controlled with hydrogels.
Signaling and patterning
Morphogen gradients also play important roles in tissue development, such as in patterning vertebral axes (Akieda et al., 2019). Morphogen gradient studies performed in vitro often rely on plated cells stimulated with induction factors through changing media composition (Yoney et al., 2018). However, the limitations to this approach include the fact that the extent and differential gradient presented to these cells remains unknown and most gradients presented to the cells in vitro will equilibrate over time, which does not mimic in vivo conditions. Here, hydrogels offer an opportunity to better recapitulate true morphogen gradients in vitro. For example, proof-of-principle hydrogel systems have been designed with a source/sink mechanism by which diffusion of molecules from a source to a sink establishes a persistent soluble factor gradient (Regier et al., 2019). Reservoirs can be replenished to maintain consistent gradients and the profile of factors within the gradient can be tailored by device design. In addition, other hydrogel systems have been introduced into microfluidic systems that introduce soluble factor gradients using adjacent microfluidic channels containing unique induction ports. This design led to graded cell differentiation throughout the hydrogel (O'Grady et al., 2019).
In another recent study (Fig. 3B), hESCs were clustered into hydrogel pockets using microfluidics where induction signals were used to guide cell behaviors in an environment in which luminogenesis and other key embryonic development events post-implantation could be monitored. In this study, a hydrogel precursor flowed into a microfluidic channel and contracted with gelation, forming a hydrogel pocket (Zheng et al., 2019). Two parallel border channels were loaded either with hESCs that were deposited into clusters within the hydrogel pockets or with induction signals, introducing patterning within the cell clusters. Here, microfluidics enabled the recapitulation of developmental events in a reproducible manner and revealed, for the first time, the spatial progression of embryonic development post-implantation.
Morphogenesis and regeneration
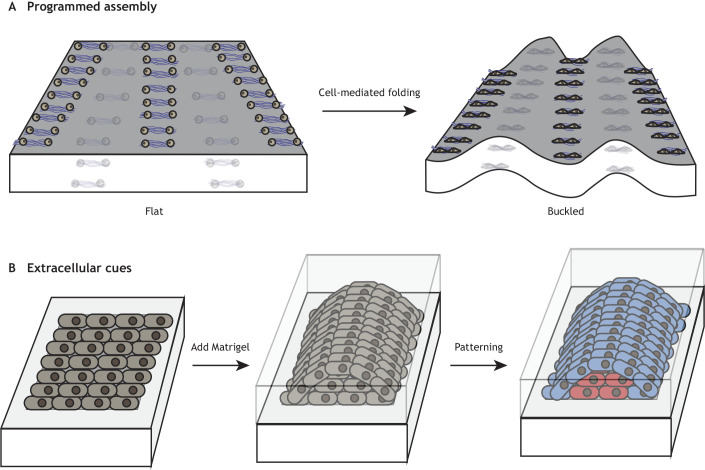
In many cases of organ morphogenesis, such as in the brain, gut and lung, a smooth monolayer of cells will undergo buckling and folding, introducing 3D cues to cells spread on a 2D surface (Nelson, 2016). To recapitulate this in vitro, cells can be strategically organized via DNA-patterned assembly under collagen I embedded in Matrigel. These cells reliably contract to induce folding into relevant in vitro geometries (Fig. 4A) (Hughes et al., 2018). These hydrogels have been designed to mimic the curvature that develops in mesenchymal condensates during gut villus development. Future studies could use such hydrogel-based systems to further study curvature development and its consequences on cellular behavior.
Fig. 4.
Modeling morphogenesis using hydrogels. (A) DNA-programmed assembly of cells allows the precise engineering of hydrogel folding to study this crucial developmental morphogenetic process. Based on studies from Hughes et al. (2018). (B) The temporal addition of other extracellular cues and morphogen patterns can also promote patterning. Based on studies from Karzbrun et al. (2021).
In neural-tube morphogenesis, another study has controlled 2D epithelium monolayer development and observed subsequent self-patterning of a 3D tube (Fig. 4B) (Karzbrun et al., 2021). The authors confined cells within micropatterned regions, which increased the control over initial cell density and behavior of the 2D stem-cell cultures. Afterwards, the material transitioned into a neural tube with the addition of Matrigel. This study also demonstrated that proper neural tube formation relies on optimal micropattern dimensions.
The extracellular basis of vascular morphogenesis has also been widely studied using hydrogels, given the importance of the vasculature in development and regeneration of tissues. Hydrogels have been used to determine the factors that promote tubulogenesis, revealing that an optimal concentration of the adhesive peptide RGD is required and that increased RGD does not promote further lumen formation (Hanjaya-Putra et al., 2011). Further studies have demonstrated that the introduction of dynamic hydrogels increases integrin clustering to promote further formation of vasculature (Wei et al., 2020). Related to vascular morphogenesis, many studies have used hydrogels as engineered scaffolds to regenerate tissue in vivo. If the reader is interested in exploring how hydrogels have been used as translational tissue engineered constructs, we refer them to the following reviews by Lee and Mooney (2001) and Hunt et al. (2014).
Summary and future perspectives
The developing embryo relies on a myriad of biochemical and physical cues to guide development. Hydrogels offer an opportunity to engineer user-defined precision into the extracellular environment, enabling investigators to systematically decouple and explore the role of these cues on developmental processes in vitro. These technologies may prove useful for developmental processes that are still not understood and difficult to study in vivo. For example, the transition of developing tissues from fluid-like to solid-like, where cells may be caged and packed tightly, is well known in development (Kang et al., 2021). How much of this transition is due to cell behavior versus ECM deposition is not yet known and, although hydrogels have not been used to study these transitions, they have been used to impart confinement on different cellular systems (Dudaryeva et al., 2021). Here, hydrogels may offer a valuable tool with which to continue probing packing phenomena that are crucial to tissue development. Undoubtedly, as hydrogels evolve to become increasingly complex, developmental biologists can probe increasingly advanced questions on the extracellular regulators of cell differentiation and tissue patterning.
Footnotes
Funding
The authors are supported by funding from the National Institutes of Health (F30AG074508) and the National Science Foundation (CMMI-1548571). Deposited in PMC for release after 12 months.
References
- Abagnale, G., Steger, M., Nguyen, V. H., Hersch, N., Sechi, A., Joussen, S., Denecke, B., Merkel, R., Hoffmann, B., Dreser, A.et al. (2015). Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 61, 316-326. 10.1016/j.biomaterials.2015.05.030 [DOI] [PubMed] [Google Scholar]
- Abdal Dayem, A., Lee, S., Y. Choi, H. and Cho, S.-G. (2018). The impact of adhesion molecules on the in vitro culture and differentiation of stem cells. Biotechnol. J. 13, 1700575. 10.1002/biot.201700575 [DOI] [PubMed] [Google Scholar]
- Akieda, Y., Ogamino, S., Furuie, H., Ishitani, S., Akiyoshi, R., Nogami, J., Masuda, T., Shimizu, N., Ohkawa, Y. and Ishitani, T. (2019). Cell competition corrects noisy Wnt morphogen gradients to achieve robust patterning in the zebrafish embryo. Nat. Commun. 10, 4710. 10.1038/s41467-019-12609-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkenberg, M. R., Dimmitt, N. H., Johnson, H. C., Koehler, K. R. and Lin, C.-C. (2020). Dynamic click hydrogels for xeno-free culture of induced pluripotent stem cells. Adv. Biosyst. 4, 2000129. 10.1002/adbi.202000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin, G. (2021). Quantitative developmental biology in vitro using micropatterning. Development 148, dev186387. 10.1242/dev.186387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner, M. G., Gudapati, H., Mou, X. and Musah, S. (2022). Microfluidic systems for modeling human development. Development 149, dev199463. 10.1242/dev.199463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari, S. R. and Burdick, J. A. (2016). A practical guide to hydrogels for cell culture. Nat. Methods 13, 405-414. 10.1038/nmeth.3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, A. R., Frith, J. E. and Cooper-White, J. J. (2011). The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979-5993. 10.1016/j.biomaterials.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Campo, H., García-Domínguez, X., López-Martínez, S., Faus, A., Vicente Antón, J. S., Marco-Jiménez, F. and Cervelló, I. (2019). Tissue-specific decellularized endometrial substratum mimicking different physiological conditions influences in vitro embryo development in a rabbit model. Acta Biomater. 89, 126-138. 10.1016/j.actbio.2019.03.004 [DOI] [PubMed] [Google Scholar]
- Candiello, J., Grandhi, T. S. P., Goh, S. K., Vaidya, V., Lemmon-Kishi, M., Eliato, K. R., Ros, R., Kumta, P. N., Rege, K. and Banerjee, I. (2018). 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials 177, 27-39. 10.1016/j.biomaterials.2018.05.031 [DOI] [PubMed] [Google Scholar]
- Chen, K., Zheng, Y., Xue, X., Liu, Y., Resto Irizarry, A. M., Tang, H. and Fu, J. (2021). Branching development of early post-implantation human embryonic-like tissues in 3D stem cell culture. Biomaterials 275, 120898. 10.1016/j.biomaterials.2021.120898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisnandy, A., Blondel, D., Rezakhani, S., Broguiere, N. and Lutolf, M. P. (2022). Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat. Mater. 21, 479-487. 10.1038/s41563-021-01136-7 [DOI] [PubMed] [Google Scholar]
- Clark, A. Y., Martin, K. E., García, J. R., Johnson, C. T., Theriault, H. S., Han, W. M., Zhou, D. W., Botchwey, E. A. and García, A. J. (2020). Integrin-specific hydrogels modulate transplanted human bone marrow-derived mesenchymal stem cell survival, engraftment, and reparative activities. Nat. Commun. 11, 114. 10.1038/s41467-019-14000-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clause, K. C., Liu, L. J. and Tobita, K. (2010). Directed stem cell differentiation: the role of physical forces. Cell Commun. Adhes. 17, 48-54. 10.3109/15419061.2010.492535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, C. D., Hill, A. S., Guo, M., Stockdale, L., Papps, J. P., Isaacson, K. B., Lauffenburger, D. A. and Griffith, L. G. (2017). Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr. Biol. 9, 271-289. 10.1039/c6ib00245e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove, B. D., Mui, K. L., Driscoll, T. P., Caliari, S. R., Mehta, K. D., Assoian, R. K., Burdick, J. A. and Mauck, R. L. (2016). N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 15, 1297-1306. 10.1038/nmat4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder, S. W., Leonardo, V., Whittaker, T., Papathanasiou, P. and Stevens, M. M. (2016). Material cues as potent regulators of epigenetics and stem cell function. Cell Stem Cell 18, 39-52. 10.1016/j.stem.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Acuña, R., Quirós, M., Farkas, A. E., Dedhia, P. H., Huang, S., Siuda, D., García-Hernández, V., Miller, A. J., Spence, J. R., Nusrat, A.et al. (2017). Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19, 1326-1335. 10.1038/ncb3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, M. D., Song, K. H., Lee, M.-H., Llewellyn, J., Du, Y., Baker, B. M., Wells, R. G. and Burdick, J. A. (2019). Engineered fibrous networks to investigate the influence of fiber mechanics on myofibroblast differentiation. ACS Biomaterials Science & Engineering 5, 3899-3908. 10.1021/acsbiomaterials.8b01276 [DOI] [PubMed] [Google Scholar]
- Dudaryeva, O. Y., Bucciarelli, A., Bovone, G., Huwyler, F., Jaydev, S., Broguiere, N., Al-Bayati, M., Lütolf, M. and Tibbitt, M. W. (2021). 3D confinement regulates cell life and death. Adv. Funct. Mater. 31, 2104098. 10.1002/adfm.202104098 [DOI] [Google Scholar]
- Elosegui-Artola, A., Gupta, A., Najibi, A. J., Seo, B. R., Garry, R., Tringides, C. M., De Lázaro, I., Darnell, M., Gu, W., Zhou, Q.et al. (2022). Matrix viscoelasticity controls spatiotemporal tissue organization. Nat. Mater. 22, 117-127. 10.1038/s41563-022-01400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski, N., Nikolaev, M., Brown, T. E., Mitrofanova, O., Brandenberg, N., Delrio, F. W., Yavitt, F. M., Liberali, P., Anseth, K. S. and Lutolf, M. P. (2022). Tissue geometry drives deterministic organoid patterning. Science 375, eaaw9021. 10.1126/science.aaw9021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski, N., Sachs, N., Manfrin, A., Giger, S., Bragina, M. E., Ordóñez-Morán, P., Clevers, H. and Lutolf, M. P. (2016). Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560-564. 10.1038/nature20168 [DOI] [PubMed] [Google Scholar]
- Govindasamy, N., Long, H., Jeong, H.-W., Raman, R., Özcifci, B., Probst, S., Arnold, S. J., Riehemann, K., Ranga, A., Adams, R. H.et al. (2021). 3D biomimetic platform reveals the first interactions of the embryo and the maternal blood vessels. Dev. Cell 56, 3276-3287.e8. 10.1016/j.devcel.2021.10.014 [DOI] [PubMed] [Google Scholar]
- Guvendiren, M. and Burdick, J. A. (2012). Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 3, 792. 10.1038/ncomms1792 [DOI] [PubMed] [Google Scholar]
- Hadden, W. J., Young, J. L., Holle, A. W., Mcfetridge, M. L., Kim, D. Y., Wijesinghe, P., Taylor-Weiner, H., Wen, J. H., Lee, A. R., Bieback, K.et al. (2017). Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl Acad. Sci. USA 114, 5647-5652. 10.1073/pnas.1618239114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanjaya-Putra, D., Bose, V., Shen, Y.-I., Yee, J., Khetan, S., Fox-Talbot, K., Steenbergen, C., Burdick, J. A. and Gerecht, S. (2011). Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood 118, 804-815. 10.1182/blood-2010-12-327338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut, R. C. (1983). Age-dependent influence of strain rate on the tensile failure of rat-tail tendon. J. Biomech. Eng. 105, 296-299. 10.1115/1.3138422 [DOI] [PubMed] [Google Scholar]
- Hayes, A. J., Benjamin, M. and Ralphs, J. R. (2001). Extracellular matrix in development of the intervertebral disc. Matrix Biol. 20, 107-121. 10.1016/S0945-053X(01)00125-1 [DOI] [PubMed] [Google Scholar]
- Hennink, W. E. and Van Nostrum, C. F. (2002). Novel crosslinking methods to design hydrogels. Adv. Drug Delivery. Rev. 54, 13-36. 10.1016/S0169-409X(01)00240-X [DOI] [PubMed] [Google Scholar]
- Hofer, M. and Lutolf, M. P. (2021). Engineering organoids. Nat. Rev. Mater. 6, 402-420. 10.1038/s41578-021-00279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, A. J., Miyazaki, H., Coyle, M. C., Zhang, J., Laurie, M. T., Chu, D., Vavrušová, Z., Schneider, R. A., Klein, O. D. and Gartner, Z. J. (2018). Engineered tissue folding by mechanical compaction of the mesenchyme. Dev. Cell 44, 165-178.e6. 10.1016/j.devcel.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui, E., Moretti, L., Barker, T. H. and Caliari, S. R. (2021). The combined influence of viscoelastic and adhesive cues on fibroblast spreading and focal adhesion organization. Cell. Mol. Bioeng. 14, 427-440. 10.1007/s12195-021-00672-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, J. A., Chen, R., Van Veen, T. and Bryan, N. (2014). Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2, 5319-5338. 10.1039/C4TB00775A [DOI] [PubMed] [Google Scholar]
- Jeon, H., Koo, S., Reese, W. M., Loskill, P., Grigoropoulos, C. P. and Healy, K. E. (2015). Directing cell migration and organization via nanocrater-patterned cell-repellent interfaces. Nat. Mater. 14, 918-923. 10.1038/nmat4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, S., Heo, J.-H., Kim, M. K., Jeong, W. and Kang, H.-W. (2020). High-precision 3D bio-dot printing to improve paracrine interaction between multiple types of cell spheroids. Adv. Funct. Mater. 30, 2005324. 10.1002/adfm.202005324 [DOI] [Google Scholar]
- Kang, W., Ferruzzi, J., Spatarelu, C.-P., Han, Y. L., Sharma, Y., Koehler, S. A., Mitchel, J. A., Khan, A., Butler, J. P., Roblyer, D.et al. (2021). A novel jamming phase diagram links tumor invasion to non-equilibrium phase separation. iScience 24, 103252-103252. 10.1016/j.isci.2021.103252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzbrun, E., Khankhel, A. H., Megale, H. C., Glasauer, S. M. K., Wyle, Y., Britton, G., Warmflash, A., Kosik, K. S., Siggia, E. D., Shraiman, B. I.et al. (2021). Human neural tube morphogenesis in vitro by geometric constraints. Nature 599, 268-272. 10.1038/s41586-021-04026-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.-Y., Li, H., Song, Y. S., Jeong, H.-S., Yun, H.-Y., Baek, K. J., Kwon, N. S., Shin, Y. K., Park, K.-C. and Kim, D.-S. (2018). Laminin peptide YIGSR enhances epidermal development of skin equivalents. J. Tissue Viability 27, 117-121. 10.1016/j.jtv.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Kloxin, A. M., Kasko, A. M., Salinas, C. N. and Anseth, K. S. (2009). Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59-63. 10.1126/science.1169494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, C. G., Morton, L. F., Onley, D. J., Peachey, A. R., Messent, A. J., Smethurst, P. A., Tuckwell, D. S., Farndale, R. W. and Barnes, M. J. (1998). Identification in collagen type I of an integrin beta1-binding site containing an essential GER sequence. J. Biol. Chem. 273, 33287-33294. 10.1074/jbc.273.50.33287 [DOI] [PubMed] [Google Scholar]
- Kuzucu, M., Vera, G., Beaumont, M., Fischer, S., Wei, P., Shastri, V. P. and Forget, A. (2021). Extrusion-based 3D bioprinting of gradients of stiffness, cell density, and immobilized peptide using thermogelling hydrogels. ACS Biomater. Sci. Eng. 7, 2192-2197. 10.1021/acsbiomaterials.1c00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer, A. and Jessen, J. R. (2010). Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol. 29, 89-96. 10.1016/j.matbio.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Lee, H.-P., Gu, L., Mooney, D. J., Levenston, M. E. and Chaudhuri, O. (2017). Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 16, 1243-1251. 10.1038/nmat4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. Y. and Mooney, D. J. (2001). Hydrogels for tissue engineering. Chem. Rev. 101, 1869-1880. 10.1021/cr000108x [DOI] [PubMed] [Google Scholar]
- Legant, W. R., Miller, J. S., Blakely, B. L., Cohen, D. M., Genin, G. M. and Chen, C. S. (2010). Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Methods 7, 969-971. 10.1038/nmeth.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonavicius, K., Royer, C., Preece, C., Davies, B., Biggins, J. S. and Srinivas, S. (2018). Mechanics of mouse blastocyst hatching revealed by a hydrogel-based microdeformation assay. Proc. Natl Acad. Sci. USA 115, 10375-10380. 10.1073/pnas.1719930115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. and Mooney, D. J. (2016). Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 16071. 10.1038/natrevmats.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Nih, L. R., Bachman, H., Fei, P., Li, Y., Nam, E., Dimatteo, R., Carmichael, S. T., Barker, T. H. and Segura, T. (2017). Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater. 16, 953-961. 10.1038/nmat4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F.-Y. and Lin, C.-C. (2021). Facile synthesis of rapidly degrading PEG-based thiol-norbornene hydrogels. ACS Macro Lett. 10, 341-345. 10.1021/acsmacrolett.1c00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebel, C., Weiner, A. I., Eiken, M. K., Katzen, J. B., Morley, M. P., Bala, V., Cardenas-Diaz, F. L., Davidson, M. D., Shiraishi, K., Basil, M. C.et al. (2022). Microstructured hydrogels to guide self-assembly and function of lung alveolospheres. Adv. Mater. 34, 2202992. 10.1002/adma.202202992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan, R., Potetz, B. R., Rongish, B. J. and Little, C. D. (2012). Spatial anisotropies and temporal fluctuations in extracellular matrix network texture during early embryogenesis. PLoS ONE 7, e38266. 10.1371/journal.pone.0038266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z., Holle, A. W., Melde, K., Qiu, T., Poeppel, K., Kadiri, V. M. and Fischer, P. (2020). Acoustic Holographic Cell Patterning in a Biocompatible Hydrogel. Adv. Mater. 32, 1904181. 10.1002/adma.201904181 [DOI] [PubMed] [Google Scholar]
- Miller, D. E., Phillippi, A. J., Fisher, W. G., Campbell, G. P., Walker, M. L. and Weiss, E. L. (2009). Inkjet printing of growth factor concentration gradients and combinatorial arrays immobilized on biologically-relevant substrates. Comb. Chem. High Throughput Screen. 12, 604-618. 10.2174/138620709788681907 [DOI] [PubMed] [Google Scholar]
- Mohagheghian, E., Luo, J., Chen, J., Chaudhary, G., Chen, J., Sun, J., Ewoldt, R. H. and Wang, N. (2018). Quantifying compressive forces between living cell layers and within tissues using elastic round microgels. Nat. Commun. 9, 1878. 10.1038/s41467-018-04245-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni, L., Burdick, J. A., Highley, C., Lee, S. J., Morimoto, Y., Takeuchi, S. and Yoo, J. J. (2018). Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 3, 21-37. 10.1038/s41578-018-0006-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir, V. G. and Burdick, J. A. (2021). Chemically modified biopolymers for the formation of biomedical hydrogels. Chem. Rev. 121, 10908-10949. 10.1021/acs.chemrev.0c00923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncie, J. M., Ayad, N. M. E., Lakins, J. N., Xue, X., Fu, J. and Weaver, V. M. (2020). Mechanical tension promotes formation of gastrulation-like nodes and patterns mesoderm specification in human embryonic stem cells. Dev. Cell 55, 679-694.e11. 10.1016/j.devcel.2020.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, C. M. (2016). On buckling morphogenesis. J. Biomech. Eng. 138, 021005. 10.1115/1.4032128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas, J., Magli, S., Rabbachin, L., Sampaolesi, S., Nicotra, F. and Russo, L. (2020). 3D Extracellular matrix mimics: fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules 21, 1968-1994. 10.1021/acs.biomac.0c00045 [DOI] [PubMed] [Google Scholar]
- Nurkesh, A., Jaguparov, A., Jimi, S. and Saparov, A. (2020). Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front. Cell Dev. Biol. 8, 638. 10.3389/fcell.2020.00638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'grady, B., Balikov, D. A., Wang, J. X., Neal, E. K., Ou, Y.-C., Bardhan, R., Lippmann, E. S. and Bellan, L. M. (2019). Spatiotemporal control and modeling of morphogen delivery to induce gradient patterning of stem cell differentiation using fluidic channels. Biomater. Sci. 7, 1358-1371. 10.1039/C8BM01199K [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piard, C., Jeyaram, A., Liu, Y., Caccamese, J., Jay, S. M., Chen, Y. and Fisher, J. (2019). 3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance. Biomaterials 222, 119423. 10.1016/j.biomaterials.2019.119423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher, M. D. and Ruoslahti, E. (1984). Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30-33. 10.1038/309030a0 [DOI] [PubMed] [Google Scholar]
- Przybyla, L., Lakins, J. N. and Weaver, V. M. (2016). Tissue mechanics orchestrate wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell 19, 462-475. 10.1016/j.stem.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape, A. D., Zibinsky, M., Murthy, N. and Kumar, S. (2015). A synthetic hydrogel for the high-throughput study of cell–ECM interactions. Nat. Commun. 6, 8129. 10.1038/ncomms9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier, M. C., Tokar, J. J., Warrick, J. W., Pabon, L., Berthier, E., Beebe, D. J. and Stevens, K. R. (2019). User-defined morphogen patterning for directing human cell fate stratification. Sci. Rep. 9, 6433. 10.1038/s41598-019-42874-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault, H. and Hakim, V. (2012). Different cell fates from cell-cell interactions: core architectures of two-cell bistable networks. Biophys. J. 102, 417-426. 10.1016/j.bpj.2011.11.4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez, A., Ghibaudo, M., Buguin, A., Silberzan, P. and Ladoux, B. (2007). Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl Acad. Sci. USA 104, 8281-8286. 10.1073/pnas.0702259104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas, C. N. and Anseth, K. S. (2008). The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J. Tissue Eng. Regen. Med. 2, 296-304. 10.1002/term.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran, A., Prasad, K., Chaudhari, S., Brand, A. and Satyamoorthy, K. (2021). Advances in development and application of human organoids. 3 Biotech 11, 257. 10.1007/s13205-021-02815-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, Y., Taniguchi, K., Gurdziel, K., Townshend, R. F., Xue, X., Yong, K. M. A., Sang, J., Spence, J. R., Gumucio, D. L. and Fu, J. (2017). Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater. 16, 419-425. 10.1038/nmat4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper, S. D., Facci, L., Williams, G., Williams, E.-J., Walsh, F. S. and Doherty, P. (2004). A dimeric version of the short N-cadherin binding motif HAVDI promotes neuronal cell survival by activating an N-cadherin/fibroblast growth factor receptor signalling cascade. Mol. Cell. Neurosci. 26, 17-23. 10.1016/j.mcn.2003.12.015 [DOI] [PubMed] [Google Scholar]
- Stern-Tal, D., Achache, H., Jacobs Catane, L., Reich, R. and Tavor Re'em, T. (2020). Novel 3D embryo implantation model within macroporous alginate scaffolds. J. Biol. Eng. 14, 18. 10.1186/s13036-020-00240-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro, K., Sephel, G. C., Weeks, B., Sasaki, M., Martin, G. R., Kleinman, H. K. and Yamada, Y. (1989). A synthetic peptide containing the IKVAV sequence from the a chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Chem. 264, 16174-16182. 10.1016/S0021-9258(18)71604-9 [DOI] [PubMed] [Google Scholar]
- Taymour, R., Kilian, D., Ahlfeld, T., Gelinsky, M. and Lode, A. (2021). 3D bioprinting of hepatocytes: core–shell structured co-cultures with fibroblasts for enhanced functionality. Sci. Rep. 11, 5130. 10.1038/s41598-021-84384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallmajo-Martin, Q., Broguiere, N., Millan, C., Zenobi-Wong, M. and Ehrbar, M. (2020). PEG/HA hybrid hydrogels for biologically and mechanically tailorable bone marrow organoids. Adv. Funct. Mater. 30, 1910282. 10.1002/adfm.201910282 [DOI] [Google Scholar]
- Vanderburgh, J., Sterling, J. A. and Guelcher, S. A. (2017). 3D printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening. Ann. Biomed. Eng. 45, 164-179. 10.1007/s10439-016-1640-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega, S. L., Kwon, M. Y., Song, K. H., Wang, C., Mauck, R. L., Han, L. and Burdick, J. A. (2018). Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat. Commun. 9, 614-614. 10.1038/s41467-018-03021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola, J. M., Porter, C. M., Gupta, A., Alibekova, M., Prahl, L. S. and Hughes, A. J. (2020). Guiding cell network assembly using shape-morphing hydrogels. Adv. Mater. 32, 2002195. 10.1002/adma.202002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, R. J., Bassin, E. J., Rodell, C. B. and Burdick, J. A. (2015). Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 6, 6639. 10.1038/ncomms7639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, C. J., Crocini, C., Ramirez, D., Killaars, A. R., Grim, J. C., Aguado, B. A., Clark, K., Allen, M. A., Dowell, R. D., Leinwand, L. A.et al. (2021). Nuclear mechanosensing drives chromatin remodelling in persistently activated fibroblasts. Nat. Biomed. Eng. 5, 1485-1499. 10.1038/s41551-021-00709-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walma, D. A. C. and Yamada, K. M. (2020). The extracellular matrix in development. Development 147, dev175596. 10.1242/dev.175596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z., Schnellmann, R., Pruitt, H. C. and Gerecht, S. (2020). Hydrogel network dynamics regulate vascular morphogenesis. Cell Stem Cell 27, 798-812.e6. 10.1016/j.stem.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B., Wei, K., Loebel, C., Zhang, K., Feng, Q., Li, R., Wong, S. H. D., Xu, X., Lau, C., Chen, X.et al. (2021). Enhanced mechanosensing of cells in synthetic 3D matrix with controlled biophysical dynamics. Nat. Commun. 12, 3514. 10.1038/s41467-021-23120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C., Delrio, F. W., Ma, H., Killaars, A. R., Basta, L. P., Kyburz, K. A. and Anseth, K. S. (2016). Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci. USA 113, E4439-E4445. 10.1073/pnas.1609731113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F., Williams, C. G., Wang, D.-A., Lee, H., Manson, P. N. and Elisseeff, J. (2005). The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 26, 5991-5998. 10.1016/j.biomaterials.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Yoney, A., Etoc, F., Ruzo, A., Carroll, T., Metzger, J. J., Martyn, I., Li, S., Kirst, C., Siggia, E. D. and Brivanlou, A. H. (2018). WNT signaling memory is required for ACTIVIN to function as a morphogen in human gastruloids. eLife 7, e38279. 10.7554/eLife.38279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambuto, S. G., Clancy, K. B. H. and Harley, B. A. C. (2019). A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus 9, 20190016. 10.1098/rsfs.2019.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambuto, S. G., Clancy, K. B. H. and Harley, B. A. C. (2020). Tuning trophoblast motility in a gelatin hydrogel via soluble cues from the maternal–fetal interface. Tissue Eng. Part A 27, 1064-1073. 10.1089/ten.tea.2020.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Zhao, R., Li, B., Farrukh, A., Hoth, M., Qu, B. and Del Campo, A. (2021). Micropatterned soft hydrogels to study the interplay of receptors and forces in T cell activation. Acta Biomater. 119, 234-246. 10.1016/j.actbio.2020.10.028 [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Xue, X., Shao, Y., Wang, S., Esfahani, S. N., Li, Z., Muncie, J. M., Lakins, J. N., Weaver, V. M., Gumucio, D. L.. et al. (2019). Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421-425. 10.1038/s41586-019-1535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, M., Wang, Y., Ferracci, G., Zheng, J., Cho, N.-J. and Lee, B. H. (2019). Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 9, 6863. 10.1038/s41598-019-42186-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnick, H. M., Clark, A. T., Gullbrand, S. E., Carey, J. L., Cheng, X. M. and Mauck, R. L. (2020). Magneto-driven gradients of diamagnetic objects for engineering complex tissues. Adv. Mater. 32, 2005030. 10.1002/adma.202005030 [DOI] [PMC free article] [PubMed] [Google Scholar]