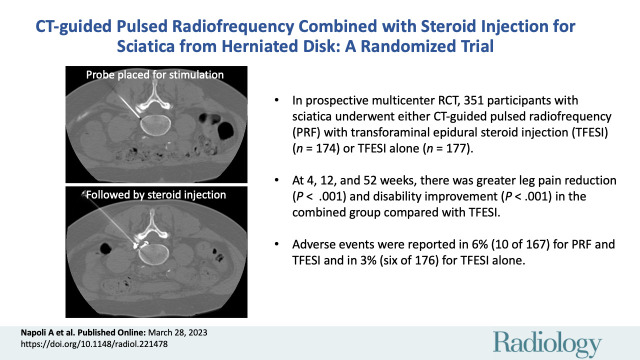
Abstract
Background
Evidence regarding effective nonsurgical management of sciatica remains limited.
Purpose
To determine a difference in effectiveness between combined pulsed radiofrequency (PRF) and transforaminal epidural steroid injection (TFESI) treatment versus TFESI alone for sciatic pain due to lumbar disk herniation.
Materials and Methods
This prospective multicenter double-blind randomized clinical trial was conducted between February 2017 and September 2019 in participants with sciatica due to lumbar disk herniation lasting 12 weeks or longer that was not responsive to conservative treatment. Study participants were randomly assigned to undergo one CT-guided treatment with combined PRF and TFESI (n = 174) or TFESI alone (n = 177). The primary outcome was leg pain severity, as assessed with the numeric rating scale (NRS) (range, 0–10) at weeks 1 and 52 after treatment. Secondary outcomes included Roland-Morris Disability Questionnaire (RMDQ) score (range, 0–24) and Oswestry Disability Index (ODI) score (range, 0–100). Outcomes were analyzed according to the intention-to-treat principle via linear regression.
Results
Mean age of the 351 participants (223 men) was 55 years ± 16 (SD). At baseline, NRS was 8.1 ± 1.1 in the PRF and TFESI group and 7.9 ± 1.1 in the TFESI group. NRS was 3.2 ± 0.2 in the PRF and TFESI group and 5.4 ± 0.2 in the TFESI group (average treatment effect, 2.3; 95% CI: 1.9, 2.8; P < .001) at week 1 and 1.0 ± 0.2 and 3.9 ± 0.2 (average treatment effect, 3.0; 95% CI: 2.4, 3.5; P < .001), respectively, at week 52. At week 52, the average treatment effect was 11.0 (95% CI: 6.4, 15.6; P < .001) for ODI and 2.9 (95% CI: 1.6, 4.3; P < .001) for RMDQ, favoring the combined PRF and TFSEI group. Adverse events were reported in 6% (10 of 167) of participants in the PRF and TFESI group and in 3% (six of 176) of participants in the TFESI group (eight participants did not complete follow-up questionnaires). No severe adverse events occurred.
Conclusion
In the treatment of sciatica caused by lumbar disk herniation, pulsed radiofrequency combined with transforaminal epidural steroid injection is more effective for pain relief and disability improvement than steroid injection alone.
© RSNA, 2023
Supplemental material is available for this article.
See also the editorial by Jennings in this issue.
Summary
In a randomized trial of participants with sciatica, CT-guided pulsed radiofrequency combined with transforaminal epidural steroid injection led to superior pain reduction and disability improvement over 52 weeks versus steroid injection alone.
Key Results
■ In a prospective multicenter randomized trial, 351 participants with sciatica underwent either CT-guided pulsed radiofrequency (PRF) combined with transforaminal epidural steroid injection (TFESI) (n = 174) or TFESI alone (n = 177).
■ At 4, 12, and 52 weeks, there was greater leg pain reduction (P < .001) and greater disability improvement (P < .001) in the combined PRF and TFESI group compared with the TFESI alone group.
■ Adverse events were reported in 6% of participants (10 of 167) for combined PRF and TFESI and in 3% of participants (six of 176) for TFESI alone.
Introduction
Sciatic pain due to lumbar disk herniation can be disabling. Most patients with sciatica will recover spontaneously or with conservative treatment (1–3). If symptoms prove refractory, however, patients are at increased risk for unfavorable outcomes and increased use of health care services. Observational cohorts report that 45% of patients with sciatica do not have a meaningful improvement in their condition after 1 year (4), and 34% report chronic pain beyond 2 years (5). The goal of nonsurgical care is to provide the most effective means of symptom resolution, while still avoiding the need for a surgical procedure. However, in many cases, conventional approaches are ineffective (6–9).
In this context, minimally invasive interventional therapy has become increasingly popular in patients with sciatica refractory to noninvasive care since it can provide pain relief during the timeframe of physiologic resolution of a lumbar disk–mediated process. Among available options, transforaminal epidural steroid injection (TFESI) is the only interventional procedure recommended in clinical guidelines (5,10). However, the duration of benefit is usually short, and additional treatments are often necessary (11,12).
Pulsed radiofrequency (PRF) relies on a neuromodulatory effect through the intermittent administration of energy stimulating the dorsal root ganglion, which is thought to be the relay of neurologic symptoms in sciatica (13). In a pilot retrospective study, the combination of PRF and TFESI provided superior clinical pain relief in patients with acute sciatica when compared with PRF or TFESI alone (14). However, higher quality evidence in support of the utility of combined treatment is presently lacking, and we hypothesized that combined PRF and TFESI would be more effective than TFESI alone.
The purpose of this study was to determine a difference in effectiveness between combined PRF and TFESI treatment versus TFESI alone for sciatic pain due to lumbar disk herniation.
Materials and Methods
Study Design
We conducted a prospective phase III randomized double-blind controlled clinical trial comparing TFESI alone with combined PRF and TFESI in participants with sciatica due to lumbar disk herniation lasting 12 weeks or more. Participants were recruited at two tertiary university hospitals (Policlinico Umberto I–Sapienza University of Rome; IRCCS Istituto Ortopedico Rizzoli) and one spine clinic (Centro SaNa Servizi Sanitari Privati) in Italy. The medical ethics committee at each participating center approved the study protocol. Written informed consent was obtained from all participants.
The protocol of this study has been previously published (15); this trial was registered with ClinicalTrials.gov (NCT04209322).
Study Participants
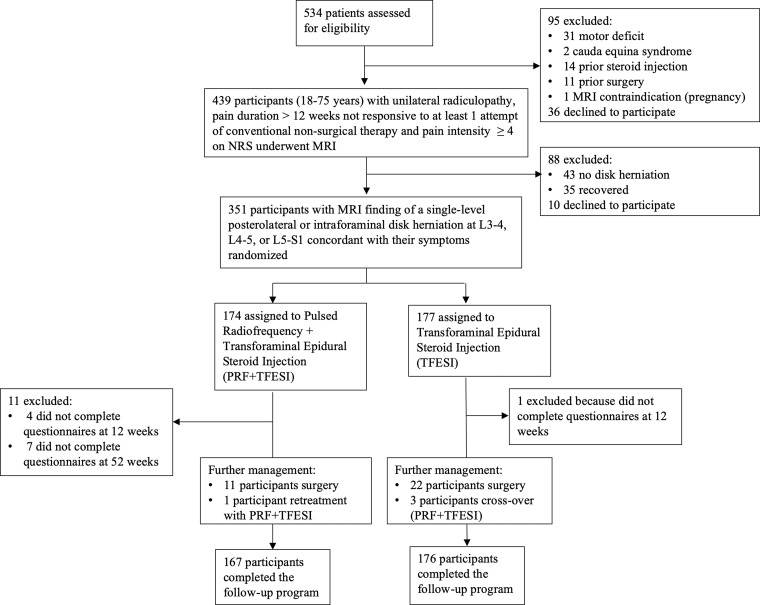
Participants were consecutively recruited from individuals with sciatica identified by a group of general practitioners or referred to study institutions between February 2017 and September 2019. Trial coordinators performed an initial telephone screening of referred individuals who were subsequently assessed by a trial spine specialist (orthopedic surgeon, neurosurgeon, or interventional neuroradiologist; A.N., G.F., U.A., P.S., P.G.N., J.L., R.T.) to ensure inclusion criteria were met based on a clinical visit and image review (Fig 1, Table S1).
Figure 1:
Flowchart shows enrollment and randomization criteria. NRS = numeric rating scale.
Randomization and Masking
A computer-generated permuted-block scheme was used for randomization, with unstratified variable-block sizes of 2 and 4. The order of blocks was also randomized, with participants assigned (1:1 ratio) to the PRF and TFESI (treatment) group or the TFESI alone (control) group. One hour before randomization, participants were evaluated again, and those who no longer showed symptoms were excluded. The principal investigator at each site (G.A., R.T., A.B.), who assessed the outcomes and participants, was blinded to randomization and treatment; the treating physician (A.N., P.G.N., P.S., U.A., G.F.) could not be blinded. An independent statistician generated the randomization sequence, and every time would conceal allocations in sealed opaque envelopes for the team designated to the participant’s care. To preserve masking, all participants received preoperative instruction regarding the possibility of tingling and electric pain sensations (due to sensitive stimulation or therapeutic delivery of PRF) during the procedure.
Procedures
All participants underwent preprocedural unenhanced low-dose CT (Aquilion, Toshiba Medical Systems; Somatom Edge Plus, Siemens Healthineers; Brilliance, Philips Medical Systems) in the prone position. A 22-gauge needle electrode with a 10-mm active tip (Boston Scientific) was introduced and advanced, and a single oblique axial unenhanced CT scan (section thickness, 3 mm; 120 kV; variable tube current) was repeated to show that the needle tip was proximate to the target dorsal root ganglion with the lateral foraminal portal of entry (16). Sensitive stimulation (50 Hz) PRF current with a threshold of 0.2 V or less was used in all participants to confirm proper positioning by evoking tingling or electric pain in the dermatome that had to match the target dorsal root ganglion.
In the PRF and TFESI group, therapeutic PRF was then administered in one 10-minute session with E-dose control, that allowed for automatic control of pulse settings (voltage or pulse width) to optimize nerve exposure to the electric field (17), avoiding temperature increases over a specific threshold. A G4 RF Generator (Boston Scientific) was used for all treatments. Once the appropriate spread of contrast material was confirmed using CT fluoroscopy, a local anesthetic mixed with steroid solution (1 mL lidocaine [20 mg per milliliter] and 2 mL dexamethasone [10 mg per milliliter] or 2 mL triamcinolone acetonide [40 mg per milliliter]) was administered immediately after the PRF administration, without altering the needle position (Fig 2). Details on the CT fluoroscopy technique are available in Appendix S1.
Figure 2:
![CT-guided pulsed radiofrequency (PRF) with transforaminal epidural steroid injection. A 62-year-old woman underwent PRF followed by transforaminal epidural steroid injection for sciatica due to left contained intraforaminal disk herniation at the L4-5 level. (A) A 22-gauge needle electrode with a 10-mm active tip was introduced and advanced using one 3-mm oblique axial unenhanced CT scan, which revealed that the needle tip was proximate to the target dorsal root ganglion with the lateral foraminal portal of entry. (B) Sensitive stimulation (50 Hz) PRF current with a threshold of no more than 0.2 V was used to confirm proper positioning by evoking tingling or electric pain in the dermatome that had to match the target dorsal root ganglion. Therapeutic PRF was then performed in one 10-minute session with E-dose functionality, maintaining temperature below the threshold for neural damage (42°C) and a constant voltage (45 V) (G4 RF Generator; Boston Scientific). Immediately after PRF administration, epidural spread of 0.3 mL of contrast material (350 mg of iodine per milliliter, Iomeron; Bracco) was confirmed using intermittent intraprocedural CT fluoroscopic imaging with no intravascular contrast material flow demonstration. A combination of steroid and anesthetic (1 mL lidocaine [ 20 mg/mL] and 2 mL dexamethasone [10 mg/mL]) was then injected without altering needle position to conclude the procedure.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/d13b/10323291/795965fa94e3/radiol.221478.fig2.jpg)
CT-guided pulsed radiofrequency (PRF) with transforaminal epidural steroid injection. A 62-year-old woman underwent PRF followed by transforaminal epidural steroid injection for sciatica due to left contained intraforaminal disk herniation at the L4-5 level. (A) A 22-gauge needle electrode with a 10-mm active tip was introduced and advanced using one 3-mm oblique axial unenhanced CT scan, which revealed that the needle tip was proximate to the target dorsal root ganglion with the lateral foraminal portal of entry. (B) Sensitive stimulation (50 Hz) PRF current with a threshold of no more than 0.2 V was used to confirm proper positioning by evoking tingling or electric pain in the dermatome that had to match the target dorsal root ganglion. Therapeutic PRF was then performed in one 10-minute session with E-dose functionality, maintaining temperature below the threshold for neural damage (42°C) and a constant voltage (45 V) (G4 RF Generator; Boston Scientific). Immediately after PRF administration, epidural spread of 0.3 mL of contrast material (350 mg of iodine per milliliter, Iomeron; Bracco) was confirmed using intermittent intraprocedural CT fluoroscopic imaging with no intravascular contrast material flow demonstration. A combination of steroid and anesthetic (1 mL lidocaine [ 20 mg/mL] and 2 mL dexamethasone [10 mg/mL]) was then injected without altering needle position to conclude the procedure.
In participants undergoing TFESI alone, once the appropriate spread of contrast material was confirmed using CT fluoroscopy, a mixed local anesthetic and steroid solution identical to that used in the PRF and TFESI group was administered within 10 minutes to avoid temporal bias. All procedures were performed in an outpatient clinic, without general anesthesia. Participants were followed for 52 weeks, with intermediate visits at 1, 4, and 12 weeks after treatment.
Outcomes
The primary outcome measurement was leg pain intensity, as reported by participants on a numeric rating scale (NRS) (range, 0–10; with 0 indicating no pain and 10 indicating the worst possible pain) as a mean of the perceived pain over the previous 7 days; minimal clinical important difference from baseline to follow-up was 2 points (18). Pain recovery was defined as complete or nearly complete resolution of symptoms (NRS, 0–1). NRS was assessed 1 week after treatment (primary end point) and 4, 12, and 52 weeks after treatment (secondary end points).
Secondary outcome measures included the extent of disability assessed with the modified Roland-Morris Disability Questionnaire (RMDQ) for sciatica (range, 0–24 with higher scores indicating greater disability; minimal clinical important difference, 4 points) (19) and the Oswestry Disability Index (ODI) (version 2.0, MODEMS) (system based on a 0%–100% scale, with higher scores indicating greater disability; minimal clinical important difference, 10%) (20) evaluated at 4, 12, and 52 weeks.
Additional data collected at 52 weeks included the global perceived effect scale, an outcome measure for musculoskeletal conditions (current symptoms as compared with baseline on a scale from −5 [vastly worse] to +5 [completely recovered]), satisfaction with the current status (on a scale from 0 to 100, a higher score indicates better quality of life), and adherence to the study (expressed in terms of the number of participants exiting the study for other therapeutic options).
An assessment of adverse events was conducted over the follow-up period through clinical surveillance and formally at 52 weeks after treatment according to the Cardiovascular and Interventional Radiological Society of Europe, or CIRSE, classification system (21).
All questionnaires were completed at hospital visits at each time point. Participants were allowed to cross over and undergo the nonallocated treatment if primary treatment was ineffective from week 4 after treatment.
Statistical Analyses
We determined a minimum sample of 328 participants (164 per group) was necessary to achieve a 95% power in detecting a between-group difference of at least one NRS point at 1 week, with α = 5% and a SD of 2.5. We sought to enroll 350 participants to account for attrition due to anticipated loss to follow-up.
Analysis was performed following the intention-to-treat principle by two independent statisticians with identical involvement in the protocol and access to data (A.D.M., G.F.; 5 and 10 years of experience, respectively). Two-sided P ≤ .05 indicated a significant difference. Between-group differences for primary and secondary outcomes were analyzed at each timepoint with linear regression; pretreatment values and treatment centers as a random effect were included in the analysis model to adjust for any baseline differences. Categorical outcomes derived from definitions of pain improvement and recovery were analyzed with the χ2 test. Follow-up scores for NRS, modified RMDQ for sciatica, and ODI were analyzed with a repeated measures mixed-effects model (Appendix S1). Global perceived effect scale and levels of satisfaction with the current status were analyzed with the Mann-Whitney U test. To quantify the overall effect of treatment over the follow-up period, the Student t test was used to compare between-group scores of the area under the curve (AUC) of each outcome measure. Finally, Cox proportional hazards regression was used to compare rates of pain improvement. Subgroups were preplanned according to baseline demographics (sex, body mass index, age), leg pain, and functional severity. Predefined values of significance were 0.10 for testing the interaction term between each identified subgroup and the randomization variable for treatment to account for the lower power of the interaction test. Analyses were performed using SPSS, version 26 (IBM) (2).
Results
Characteristics of the Study Participants
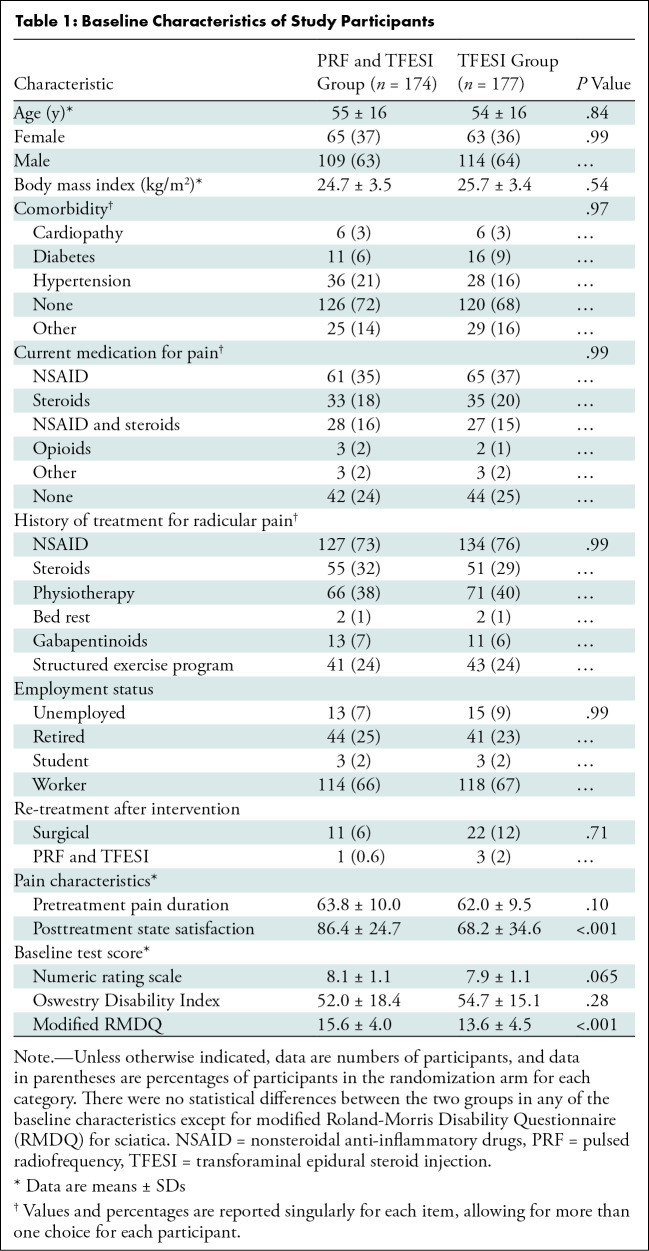
Among 534 participants with sciatica due to lumbar disk herniation identified between February 1, 2017, and November 30, 2019, 439 participants were referred for neurologic examination and MRI (see Appendix S1 for the imaging protocol), after initial telephone screening. Ultimately, 351 participants underwent randomization: 174 were assigned to the PRF and TFESI group (mean age, 55 years ± 16 [SD], 109 male) and 177 were assigned to the TFESI group (mean age, 54 years ± 16; 114 male) (Fig 2). The trial stopped when all participants had completed follow-up. Baseline characteristics are shown in Table 1. At the time of treatment, mean and SD duration of sciatic symptom was 15 weeks ± 2.0. All randomized participants underwent assigned treatment.
Table 1:
Baseline Characteristics of Study Participants
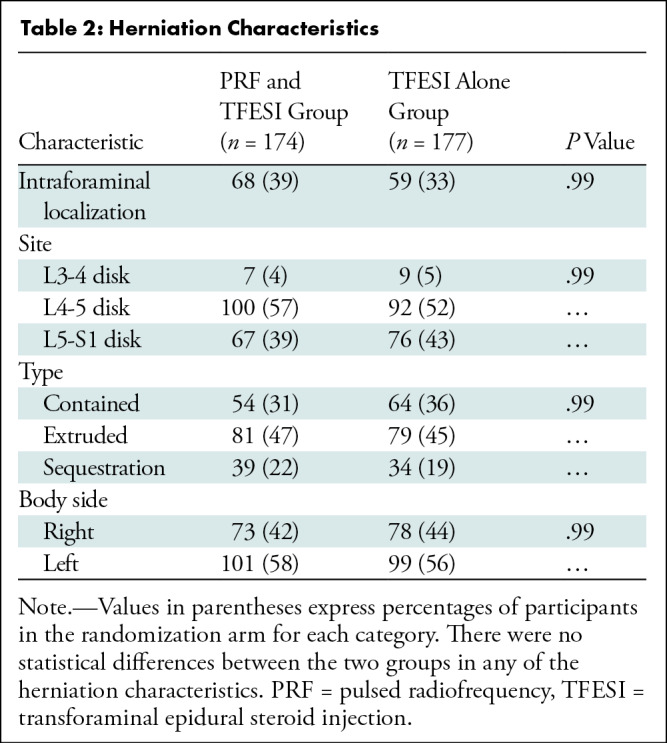
During the follow-up period, 7% (12 of 174) of participants who underwent PRF and TFESI and 14% (25 of 177) of participants who underwent TFESI had intractable residual symptoms requiring further management, as indicated by a mean NRS of 8.3 ± 1.5 and 8.3 ± 1.0, respectively. Among these, 11 of 12 participants in the PRF and TFESI group and 22 of 25 participants in the TFESI group underwent surgery; mean time from the first intervention to surgery was 7.0 weeks ± 2.0 in the PRF and TFESI group and 6.0 weeks ± 1.5 in the TFESI group. One of 11 participants in the PRF and TFESI group underwent re-treatment at week 7 after the first treatment session, whereas three of 25 participants in the TFESI group crossed over a mean of 8.0 weeks ± 1.5 after the first intervention. In the PRF and TFESI group, four and seven of 174 participants did not complete questionnaires at 12 and 52 weeks, respectively; in the TFESI group, one of 176 participants did not complete the questionnaire at 12 weeks. Thus, 167 of 174 participants in the PRF and TFESI group and 176 of 177 participants in the TFESI group completed the study at 52 weeks (Table 2).
Table 2:
Herniation Characteristics
Pain Assessment
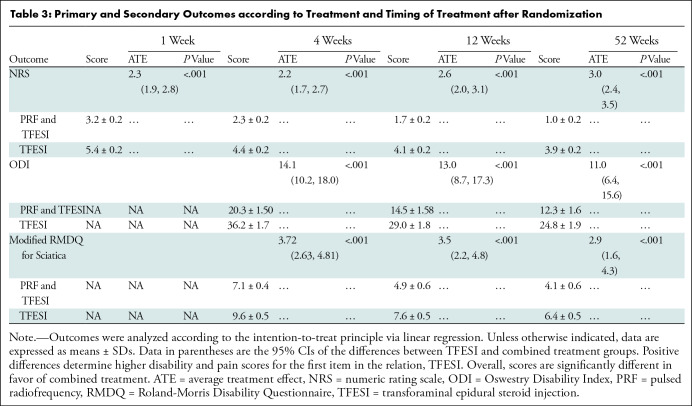
The average treatment effect, as measured with NRS, after adjustment for pretreatment score values and treatment centers between groups at week 1 was 2.3 (95% CI: 1.9, 2.8; P < .001) (mean score, 3.2 ± 0.2 in the PRF and TFESI group and 5.4 ± 0.2 in the TFESI group) (Table 3); at week 52, it was 3.0 (95% CI: 2.4, 3.5; P < .001) (mean score, 1.0 ± 0.16 in the PRF and TFESI group and 3.9 ± 0. 2 in the TFESI group). At final follow-up, 96% (161 of 167) of participants in the PRF and TFESI group compared with 69% (121 of 176) of participants in the TFESI group experienced an improvement in pain (P < .001); complete pain recovery was greater in the PRF and TFESI group at 68% (114 of 167) compared with 13% (22 of 176) in the TFESI group (P < .001).
Table 3:
Primary and Secondary Outcomes according to Treatment and Timing of Treatment after Randomization
NRS scores were analyzed using a mixed-effects model. There was an estimated between-group mean difference across all time points of 2.2 in favor of PRF and TFESI (95% CI: 1.1, 2.8; P = .003). No interaction term was found to be significant. The model B (Appendix S1), adjusting for additional covariates, reported an effect estimate difference of 1.6 points in favor of PRF and TFESI (95% CI: 1.0, 2.0; P < .001). Further differences regarding herniation characteristics (anatomic site, intra- or extraforaminal position, disk containment, body side, type of steroid) were not significant. Details on mixed-effect model analysis are provided in Appendix S1.
Analysis of AUC showed a between-group difference in terms of overall outcome for pain in favor of PRF and TFESI; for the NRS, mean AUC was 687.5 ± 71.4 and 1090.4 ± 89.2, respectively, in the PRF and TFESI combined and TFESI alone groups (P < .001) (Fig 3A).
Figure 3:
(A) Numeric rating scale (NRS) for leg pain, (B) Roland-Morris Disability Questionnaire (RMDQ), and (C) Oswestry Disability Index (ODI). Panels show curves of means with 95% CIs for the 52-week observation period. Follow-up scores are denoted at 1 week (for NRS only) and at 4, 12, and 52 weeks. Values on the y-axis are expressed as different outcome measures in each panel. Blue indicates the pulsed radiofrequency (PRF) and transforaminal epidural steroid injection (TFESI) treatment group, while red indicates the TFESI group. Mean and mean differences are indicated over the panels for the area under the curve analysis with the respective 95% CIs, as analyzed with the Student t test. Results show statistical significance in favor of the combined treatment protocol at all time points for the NRS and RMDQ and ODI scales.
The hazard ratio for pain improvement on NRS as the end point was 1.62 (95% CI: 1.3, 2.5) in favor of PRF and TFESI for all participants. Analysis of treatment groups according to predefined baseline characteristics showed that combined treatment was beneficial in all subgroups except the underweight population, which accounted for 1.4% (five of 343) of participants. No categorical interaction term was found to be significant (Fig S1).
Disability
The modified RMDQ for sciatica scores showed average treatment effect of 3.0 points (95% CI: 1.6, 4.3) at week 52; the mixed-effect models A and B estimated a between-group mean difference of 1.6 (95% CI: 0.8, 2.6) and 3.8 (95% CI: 0.0, 7.7), respectively. ODI scores reported average treatment effect of 10.0 (95% CI: 5.4, 14.6) at week 52; estimated mean differences at mixed-effect models A and B were 0.9 (95% CI: 0.8, 1.0) and 0.8 (95% CI: 0.0, 1.7). Details on mixed-effect model analysis are provided in Appendix S1.
Analysis of the AUC showed a between-group difference in terms of overall outcome for disability scores in favor of PRF and TFESI. For the modified RMDQ for sciatica, mean AUC was 1700.3 ± 150.0 in the PRF and TFESI group and 2379.3 ± 185.5 in the TFESI group (P = .001). For the ODI, mean AUC was 4929.2 ± 481.8 in the PRF and TFESI group and 9295.4 ± 728.4 in the TFESI group (P < .001) (Fig 3B, 3C).
At final follow-up, the mean global perceived effect scale was 3.7 ± 0.3 in the PRF and TFESI group and 1.8 ± 0.2 in the TFESI group (P < .001), while the satisfaction rate was 86.4 ± 24.7 and 68.0 ± 34.6, respectively (P < .001).
Adverse Events
No procedure-related complications occurred. No participants died during the study period. No serious adverse events were reported. There were 25 adverse events: 14 occurred in 10 participants who had PRF and TFESI, and 11 occurred in six participants who had TFESI (Appendix S1).
Discussion
In our trial, both transforaminal epidural steroid injection (TFESI) alone and combined pulsed radiofrequency (PRF) and TFESI led to pain improvement; 161 of 167 (96%) participants in the PRF and TFESI group and 121 of 176 (69%) participants in the TFESI group experienced decreased pain at final follow-up (P < .001). PRF added to TFESI demonstrated more prominent reductions in leg pain even over the 1st week after treatment: for the leg pain intensity score, a between-group difference in favor of the PRF and TFESI group was encountered at week 1 and persisted at week 52.
Minimally invasive interventional therapy for sciatica has become increasingly popular, especially in patients with symptoms refractory to noninvasive care, since it can provide pain relief while allowing for the physiologic resolution of symptoms related to lumbar disk herniation. Our results in the TFESI group are in line with those of the recent Nerve Root Block Versus Surgery (NERVES) study (22); this trial showed that use of TFESI as an initial treatment is similarly effective to surgical microdiscectomy at reducing pain and disability from sciatica lasting 6 weeks to 12 months. Given the safety of TFESI, along with the unlikely cost-effectiveness of surgery as a first invasive treatment (22), NERVES results indicate that treating physicians should consider interventional pain management as a stepwise treatment for sciatica without neurologic deficit of up to 12 months duration.
The role of PRF in the management of sciatica has been investigated in limited prior work. One prospective study by Lee et al (23) showed that PRF and TFESI had similar benefits in terms of pain relief and function improvement. However, patient selection was narrowed to those without any improvement from prior TFESI, only a limited portion of participants completed the study, and the follow-up period was 3 months. It remains difficult to compare long-term efficacy demonstrated by our present study (12 months) with data in the literature since available evidence derives from studies with a follow-up period of 6 months or less. Moreover, this is the first prospective randomized trial comparing PRF and TFESI and TFESI alone in the treatment of sciatica due to lumbar disk herniation; thus, it is less affected by temporal biases than previous evidence. The results of our trial show that a combined PRF and TFESI leads to better outcomes at 1 year after a single 10-minute procedure than TFESI alone.
We acknowledge several limitations of our study. First, the efficacy of PRF combined with TFESI could have been evaluated more effectively via use of a sham or placebo group. Second, we used a 22-gauge needle (not a 25-gauge needle, as is often used for TFESI) since the electrode could not fit through the canula of a smaller needle. However, in the matching group, we also used the same gauge to avoid bias (however, a 22-gauge needle is not unusual for lumbar spine injections).
In conclusion, we found that participants who underwent PRF combined with TFESI for persistent sciatica caused by lumbar disk herniation experienced better clinical outcomes in terms of pain relief and functional recovery over the 1st year after treatment as compared with those who underwent TFESI alone. We believe that the randomized double-blind study design, combined with the magnitude of the treatment effect for PRF and TFESI, mean that our results are applicable to the treatment of sciatica resulting from lumbar disk herniation not otherwise responding to conservative therapy.
Further studies should investigate the long-term efficacy of pulsed radiofrequency combined with transforaminal epidural steroid injection as compared with minimally invasive surgery for management of radicular pain secondary to lumbar disk herniation, extend inclusion criteria to individuals with a motor deficit, and possibly perform relative cost-effectiveness analysis.
Disclosures of conflicts of interest: A.N. No relevant relationships. G.A. No relevant relationships. A.D.M. No relevant relationships. E.P. No relevant relationships. R.S. No relevant relationships. G.F. No relevant relationships. U.A. No relevant relationships. P.S. No relevant relationships. P.G.N. No relevant relationships. R.T. No relevant relationships. J.L. No relevant relationships. P.G. Grant from InSightec; consulting fees from HistoSonics; participation on the DataSafety Monitoring Board or Advisory Board of InSightec, Profound, and SonALAsense; stock or stock options in SonALASense. A.B. No relevant relationships. S.P. No relevant relationships. A.J.S. Institution received grants from NIH-NIAMS, the Department of Defense, and OREF; royalties or licenses from Wolters Kluwer and Springer Nature; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from the University of Michigan; leadership or fiduciary role in the North American Spine Society Spine. C.C. No relevant relationships.
Abbreviations:
- AUC
- area under the curve
- NRS
- numeric rating scale
- ODI
- Oswestry Disability Index
- PRF
- pulsed radiofrequency
- RMDQ
- Roland-Morris Disability Questionnaire
- TFESI
- transforaminal epidural steroid injection
References
- 1. Schoenfeld AJ , Kang JD . Decision Making for Treatment of Persistent Sciatica . N Engl J Med 2020. ; 382 ( 12 ): 1161 – 1162 . [DOI] [PubMed] [Google Scholar]
- 2. Mathieson S , Maher CG , McLachlan AJ , et al . Trial of Pregabalin for Acute and Chronic Sciatica . N Engl J Med 2017. ; 376 ( 12 ): 1111 – 1120 . [DOI] [PubMed] [Google Scholar]
- 3. Bailey CS , Rasoulinejad P , Taylor D , et al . Surgery versus Conservative Care for Persistent Sciatica Lasting 4 to 12 Months . N Engl J Med 2020. ; 382 ( 12 ): 1093 – 1102 . [DOI] [PubMed] [Google Scholar]
- 4. Weinstein JN , Lurie JD , Tosteson TD , et al . Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort . JAMA 2006. ; 296 ( 20 ): 2451 – 2459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence . Low back pain and sciatica in over 16s: assessment and management . (NICE QS No. 155). https://www.nice.org.uk/guidance/qs155. Published 2016. Accessed October 10, 2020 . [PubMed]
- 6. Enke O , New HA , New CH , et al . Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis . CMAJ 2018. ; 190 ( 26 ): E786 – E793 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinto RZ , Verwoerd AJH , Koes BW . Which pain medications are effective for sciatica (radicular leg pain)? BMJ 2017. ; 359 : j4248 . [DOI] [PubMed] [Google Scholar]
- 8. Goldberg H , Firtch W , Tyburski M , et al . Oral steroids for acute radiculopathy due to a herniated lumbar disk: a randomized clinical trial . JAMA 2015. ; 313 ( 19 ): 1915 – 1923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gastaldi R , Durand M , Roustit M , et al . Short-term Efficiency and Tolerance of Ketoprofen and Methylprednisolone in Acute Sciatica: A Randomized Trial . Pain Med 2019. ; 20 ( 7 ): 1294 – 1299 . [DOI] [PubMed] [Google Scholar]
- 10. North American Spine Society . Clinical Guidelines for Diagnosis and Treatment of Lumbar Disc Herniation with Radiculopathy . https://www.spine.org/Portals/0/Assets/Downloads/ResearchClinicalCare/Guidelines/LumbarDiscHerniation.pdf. Published 2012. Accessed October 10, 2020 .
- 11. Benny B , Azari P . The efficacy of lumbosacral transforaminal epidural steroid injections: a comprehensive literature review . J Back Musculoskeletal Rehabil 2011. ; 24 ( 2 ): 67 – 76 . [DOI] [PubMed] [Google Scholar]
- 12. Burgher AH , Hoelzer BC , Schroeder DR , Wilson GA , Huntoon MA . Transforaminal epidural clonidine versus corticosteroid for acute lumbosacral radiculopathy due to intervertebral disc herniation . Spine 2011. ; 36 ( 5 ): E293 – E300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Napoli A , Alfieri G , Scipione R , Andrani F , Leonardi A , Catalano C . Pulsed radiofrequency for low-back pain and sciatica . Expert Rev Med Devices 2020. ; 17 ( 2 ): 83 – 86 . [DOI] [PubMed] [Google Scholar]
- 14. Ding Y , Li H , Zhu Y , Yao P , Zhao G . Transforaminal epidural steroid injection combined with pulsed radio frequency on spinal nerve root for the treatment of lumbar disc herniation . J Pain Res 2018. ; 11 : 1531 – 1539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scipione R , Alfieri G , De Maio A , et al . STUDY PROTOCOL - pulsed radiofrequency in addition to transforaminal epidural steroid injection in patients with acute and subacute sciatica due to lumbosacral disc herniation: rationale and design of a phase III, multicenter, randomized, controlled trial . Expert Rev Med Devices 2020. ; 17 ( 9 ): 945 – 949 . [DOI] [PubMed] [Google Scholar]
- 16. Yu RK , Lagemann GM , Ghodadra A , Agarwal V . Extraforaminal needle tip position reduces risk of intravascular injection in CT-fluoroscopic lumbar transforaminal epidural steroid injections . J Spine Surg 2016. ; 2 ( 4 ): 246 – 255 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cosman ER Jr , Cosman ER Sr . Electric and thermal field effects in tissue around radiofrequency electrodes . Pain Med 2005. ; 6 ( 6 ): 405 – 424 . [DOI] [PubMed] [Google Scholar]
- 18. Farrar JT , Young JP Jr , LaMoreaux L , Werth JL , Poole MR . Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale . Pain 2001. ; 94 ( 2 ): 149 – 158 . [DOI] [PubMed] [Google Scholar]
- 19. Kim M , Guilfoyle MR , Seeley HM , Laing RJ . A modified Roland-Morris disability scale for the assessment of sciatica . Acta Neurochir (Wien) 2010. ; 152 ( 9 ): 1549 – 1553 ; discussion 1553 . [DOI] [PubMed] [Google Scholar]
- 20. Fairbank JC , Couper J , Davies JB , O’Brien JP . The Oswestry low back pain disability questionnaire . Physiotherapy 1980. ; 66 ( 8 ): 271 – 273 . [PubMed] [Google Scholar]
- 21. Filippiadis DK , Binkert C , Pellerin O , Hoffmann RT , Krajina A , Pereira PL . Cirse quality assurance document and standards for classification of complications: the cirse classification system . Cardiovasc Intervent Radiol 2017. ; 40 ( 8 ): 1141 – 1146 . [DOI] [PubMed] [Google Scholar]
- 22. Wilby MJ , Best A , Wood E , et al . Surgical microdiscectomy versus transforaminal epidural steroid injection in patients with sciatica secondary to herniated lumbar disc (NERVES): a phase 3, multicentre, open-label, randomised controlled trial and economic evaluation . Lancet Rheumatol 2021. ; 3 ( 5 ): e347 – e356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee DG , Ahn SH , Lee J . Comparative Effectivenesses of Pulsed Radiofrequency and Transforaminal Steroid Injection for Radicular Pain due to Disc Herniation: a Prospective Randomized Trial . J Korean Med Sci 2016. ; 31 ( 8 ): 1324 – 1330 . [DOI] [PMC free article] [PubMed] [Google Scholar]