Abstract
Background
Knowledge regarding predictors of clinical and radiographic failures of middle meningeal artery (MMA) embolization (MMAE) treatment for chronic subdural hematoma (CSDH) is limited.
Purpose
To identify predictors of MMAE treatment failure for CSDH.
Materials and Methods
In this retrospective study, consecutive patients who underwent MMAE for CSDH from February 2018 to April 2022 at 13 U.S. centers were included. Clinical failure was defined as hematoma reaccumulation and/or neurologic deterioration requiring rescue surgery. Radiographic failure was defined as a maximal hematoma thickness reduction less than 50% at last imaging (minimum 2 weeks of head CT follow-up). Multivariable logistic regression models were constructed to identify independent failure predictors, controlling for age, sex, concurrent surgical evacuation, midline shift, hematoma thickness, and pretreatment baseline antiplatelet and anticoagulation therapy.
Results
Overall, 530 patients (mean age, 71.9 years ± 12.8 [SD]; 386 men; 106 with bilateral lesions) underwent 636 MMAE procedures. At presentation, the median CSDH thickness was 15 mm and 31.3% (166 of 530) and 21.7% (115 of 530) of patients were receiving antiplatelet and anticoagulation medications, respectively. Clinical failure occurred in 36 of 530 patients (6.8%, over a median follow-up of 4.1 months) and radiographic failure occurred in 26.3% (137 of 522) of procedures. At multivariable analysis, independent predictors of clinical failure were pretreatment anticoagulation therapy (odds ratio [OR], 3.23; P = .007) and an MMA diameter less than 1.5 mm (OR, 2.52; P = .027), while liquid embolic agents were associated with nonfailure (OR, 0.32; P = .011). For radiographic failure, female sex (OR, 0.36; P = .001), concurrent surgical evacuation (OR, 0.43; P = .009), and a longer imaging follow-up time were associated with nonfailure. Conversely, MMA diameter less than 1.5 mm (OR, 1.7; P = .044), midline shift (OR, 1.1; P = .02), and superselective MMA catheterization (without targeting the main MMA trunk) (OR, 2; P = .029) were associated with radiographic failure. Sensitivity analyses retained these associations.
Conclusion
Multiple independent predictors of failure of MMAE treatment for chronic subdural hematomas were identified, with small diameter (<1.5 mm) being the only factor independently associated with both clinical and radiographic failures.
© RSNA, 2023
Supplemental material is available for this article.
See also the editorial by Chaudhary and Gemmete in this issue.
Summary
Small diameter (<1.5 mm) was the only factor independently associated with both clinical and radiographic failure of middle meningeal artery embolization for treatment of chronic subdural hematoma.
Key Results
■ In this retrospective study of 530 patients and 636 middle meningeal artery (MMA) embolization procedures, small MMA diameter (<1.5 mm) was the only factor independently associated with both clinical (adjusted odds ratio [OR], 2.52; P = .027) and radiographic (adjusted OR, 1.7; P = .044) failures.
■ Anticoagulation medications were independently associated with clinical failure (adjusted OR, 3.23; P = .007), while liquid embolic materials were associated with nonfailure (adjusted OR, 0.32; P = .011).
■ Midline shift (1-mm increments) (adjusted OR, 1.1; P = .02) and superselective MMA catheterization only (without targeting the main MMA trunk) (adjusted OR, 2; P = .029) were associated with radiographic failure; conversely, concurrent surgical evacuation (adjusted OR, 0.43; P = .009), female sex (adjusted OR, 0.36; P = .001), and a longer imaging follow-up time were associated with nonfailure.
Introduction
Chronic subdural hematomas (CSDHs) are among the most common and challenging pathologies in neurosurgery (1,2), and their incidence is expected to increase as the population ages and comorbidity burden increases (3). Treatment typically involves surgical evacuation using burr holes or craniotomy, but recurrence rates range from 5% to 37% (4,5). Endovascular middle meningeal artery (MMA) embolization (MMAE) has recently emerged as a stand-alone alternative or adjunct modality to conventional surgery (5–10). Little is known regarding potential predictors of MMAE treatment failure and success, in part because of short follow-up times, small sample sizes, relatively low reported rates of treatment failure, and mostly single-center reports. Therefore, we aimed to evaluate clinical and radiographic outcomes of patients with CSDH undergoing MMAE from a large U.S. multicenter database to identify predictors of clinical and radiographic failure.
Materials and Methods
This retrospective and Health Insurance Portability and Accountability Act–compliant study was approved with a waiver of informed consent by the local institutional review board at each institution.
Patient Selection
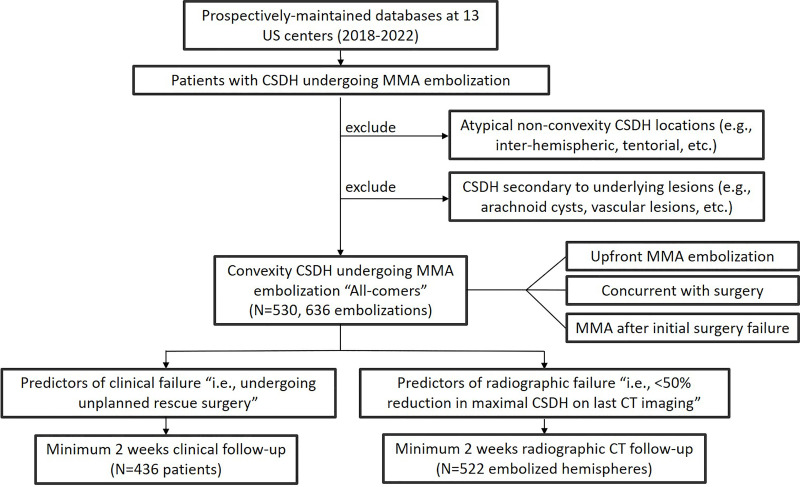
Consecutive patients from 13 high-volume North American cerebrovascular centers who underwent MMAE for CSDH from February 2018 to April 2022 were included. We included patients undergoing MMAE (a) as a stand-alone new treatment (ie, no prior treatment), (b) during the same hospitalization as open surgery (ie, concurrent MMAE), or (c) after failure of prior surgical evacuation or at a planned later time point (ie, different admission from initial surgery). The decision to perform MMAE was at the discretion of the treating neurointerventionalist; however, the procedure was generally used in patients with CSDH causing mild clinical symptoms without altered sensorium or significant motor deficits. Similarly, the embolic materials used, procedural details and techniques, and postprocedural follow-up were chosen according to the treating neurointerventionalist's discretion and institutional protocols. The following exclusion criteria were applied: (a) atypical nonconvexity CSDH locations (eg, interhemispheric or tentorial), (b) CSDH secondary to underlying lesions (eg, arachnoid cysts and vascular lesions), (c) clinical follow-up less than 2 weeks (excluded from the clinical failure analysis if the patient did not undergo rescue treatment during the index admission), and (d) no follow-up CT or an imaging follow-up time less than 2 weeks (excluded from radiographic failure analysis). More details on patient selection for the study are provided in Appendix S1.
Study Outcomes
The main clinical outcome measures were the independent predictors of clinical failure, defined as hematoma reaccumulation or neurologic deterioration requiring unplanned rescue surgical intervention during the initial admission or postprocedural period after the index MMAE (minimum clinical follow-up of 2 weeks). The primary radiographic outcome measures were the independent predictors of radiographic failure, which was defined as a less than 50% reduction in maximal hematoma thickness on the last available follow-up CT images (minimum imaging follow-up of 2 weeks). Secondary end points included functional clinical outcomes at the last available follow-up as assessed using the modified Rankin scale (mRS), with a favorable outcome defined as an mRS score of 0–2.
Data Collection
The relevant demographic data, CSDH characteristics, procedural details, and outcome variables were collected for eligible patients. More information on the collected data is provided in Appendix S1.
Statistical Analysis
Basic descriptive statistics for categorical variables are reported as proportions and were compared using the χ2 test. Continuous variables are reported as means and SDs or medians and IQRs and were compared using the t test or Mann-Whitney U test, as appropriate, based on data normality. A multivariable logistic regression model was constructed to evaluate independent predictors of clinical MMAE failure by including factors with P ≤ .2 at univariable comparison and potentially important variables identified from the literature. Another multivariable logistic regression model was constructed to identify independent predictors of radiographic MMAE failure. The confounders controlled in the regression models were age, sex, concurrent surgical evacuation, midline shift, hematoma thickness, MMA main trunk diameter (ie, intracranial anterior division of the MMA distal to the petrous branch origin bifurcation before termination into frontal and parietal branches), history of prior surgical evacuation, pretreatment baseline antiplatelet and anticoagulation therapy, admission platelet count, type of embolic material used (particle or liquid), MMA catheterization technique (nonselective, selective, combination), and imaging follow-up times (for radiographic failure). Follow-up was categorized into four time intervals as follows: follow-up A, 2–4 weeks; follow-up B, greater than 4 weeks to 6 weeks; follow-up C, greater than 6 weeks to 90 days; and follow-up D, greater than 90 days. To assess temporal changes in radiographic hematoma thickness during imaging follow-up, preprocedural maximal CSDH thickness measurements were categorized into four groups as follows: group 1, less than 10 mm; group 2, 10 mm to less than 15 mm; group 3, 15 mm to less than 20 mm; and group 4, greater than or equal to 20 mm. The preprocedural CSDH categories and imaging follow-up intervals were included in the model, both as categorical variables, with less than 10-mm thickness and 2–4 weeks of follow-up used as the reference categories. The rates of radiographic success were plotted over time and stratified according to CSDH thickness group to delineate the temporal trend of changes in CSDH maximal thickness in response to MMAE. Sensitivity analyses were performed to identify predictors of clinical and radiographic failure in (a) patients undergoing MMAE as stand-alone treatment; (b) the stand-alone MMAE group, with greater than or equal to 10-mm maximal hematoma thickness, to assess “true CSDHs” that might otherwise warrant surgical intervention; and (c) patients with greater than or equal to 90 days of imaging follow-up. All statistical analyses were performed with STATA version 15.0 (StataCorp), with two-sided P < .05 considered indicative of a statistically significant difference.
Results
Baseline Patient Characteristics
Overall, 530 patients (mean age, 71.9 years ± 12.8 [SD]; 386 men) with 636 subdural hematoma lesions who underwent 636 MMAE procedures were included in this analysis (Fig 1). The details of baseline characteristics are summarized in Table 1 and presented in Appendix S1.
Figure 1:
Flowchart shows patient inclusion and exclusion. CSDH = chronic subdural hematoma, MMA = middle meningeal artery.
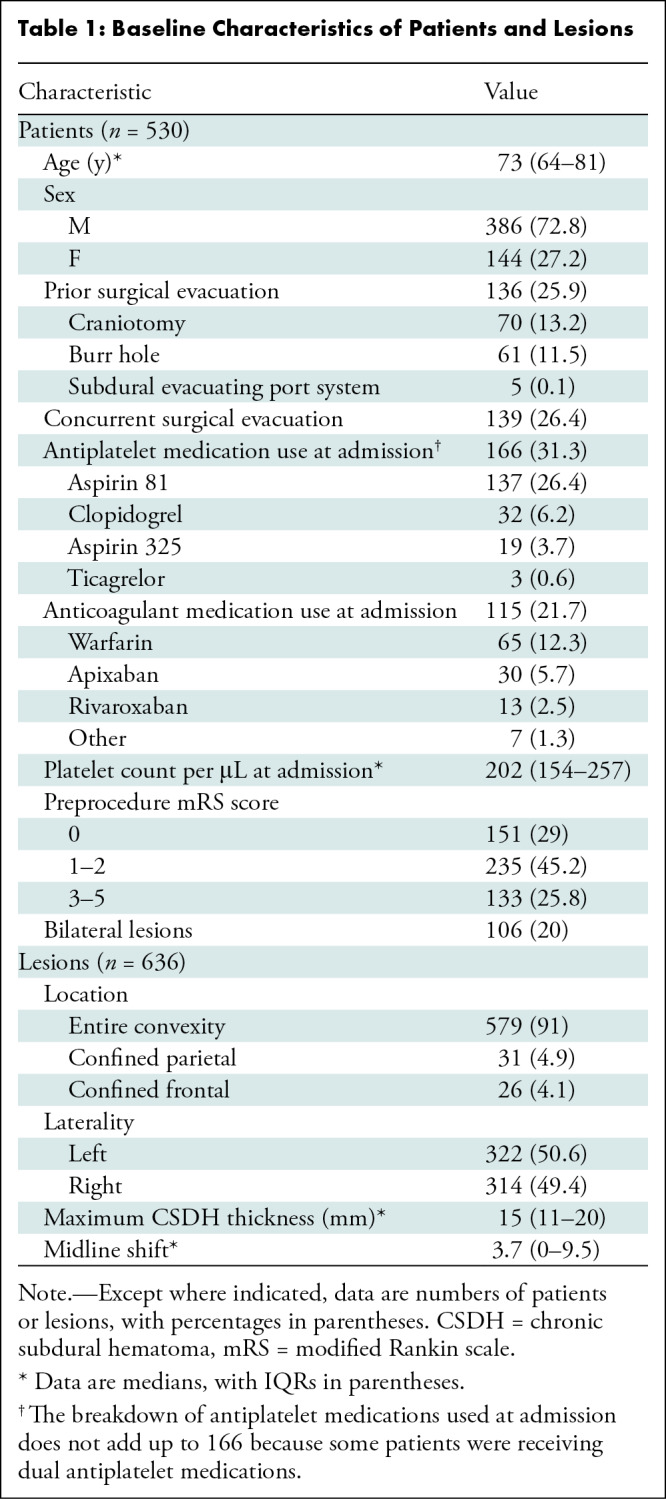
Table 1:
Baseline Characteristics of Patients and Lesions
Procedural Details
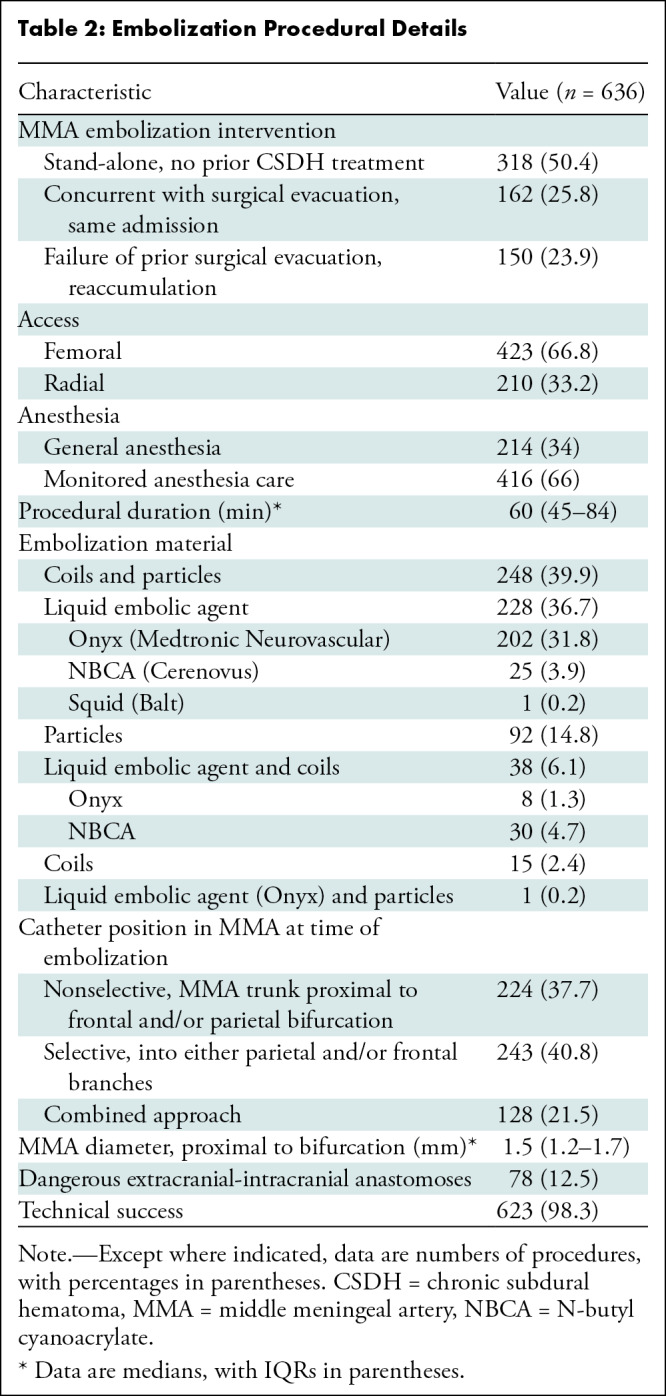
Approximately half of the MMAE procedures (318 of 636; 50.4%) for CSDH were stand-alone treatments with no prior treatments, while 25.8% (162 of 636) were performed concurrently with surgery and 23.9% (150 of 636) were performed for prior surgical failure. The procedural details are summarized in Table 2 and Appendix S1.
Table 2:
Embolization Procedural Details
Radiologic and Clinical Outcomes
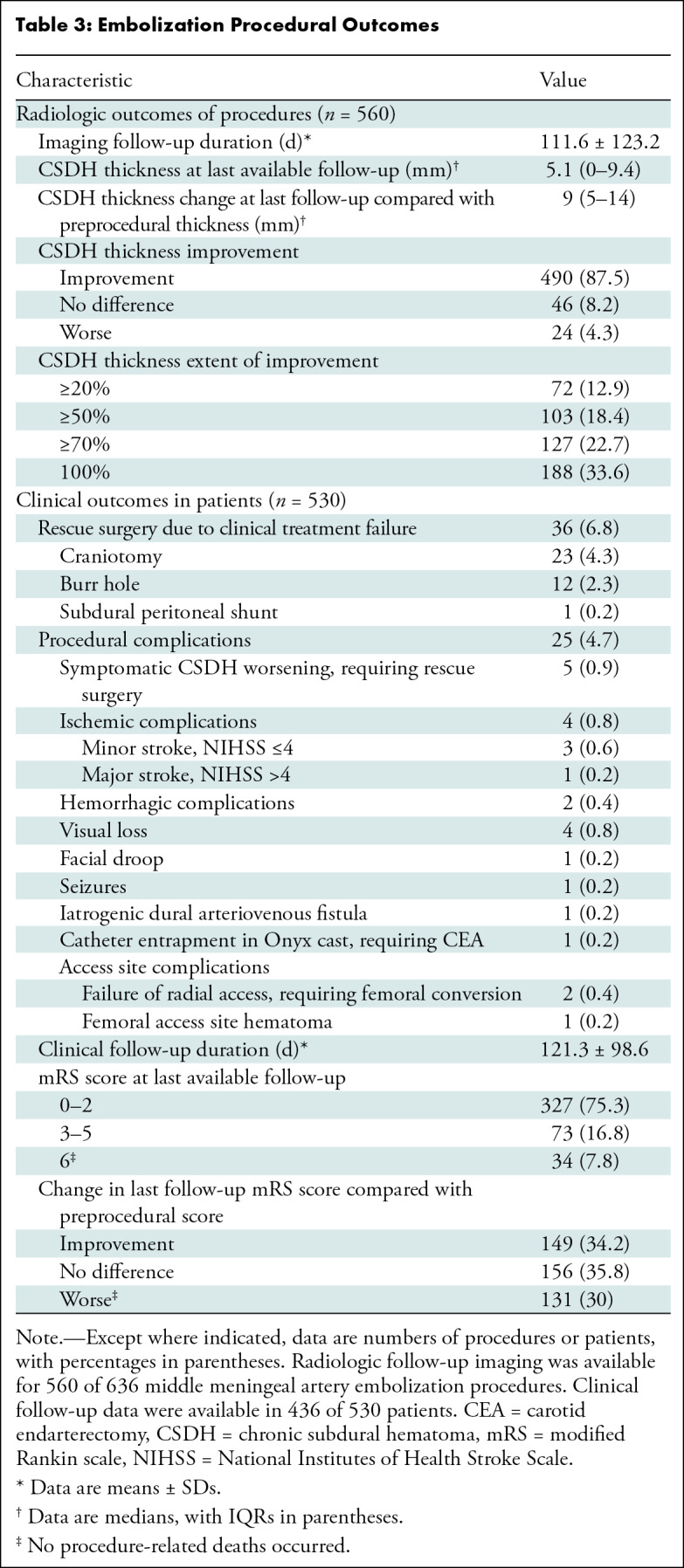
Radiologic follow-up imaging was available for 560 of 636 MMAE procedures (88.1%) with a mean follow-up of 111.6 days ± 123.2. Radiologic improvement at the last follow-up was noted in 87.5% (490 of 560) of MMAE procedures, with a median 5.1-mm CSDH thickness on the last follow-up images (and a median 9-mm change in maximal thickness compared with the thickness before MMAE). The primary radiologic end point (≥50% reduction in maximal thickness with a minimum of 2 weeks imaging follow-up) was achieved in 73.8% (385 of 522) of MMAE procedures. Unchanged CSDH thickness on the last follow-up images occurred in 8.2% (46 of 560) and CSDH worsening in 4.3% (24 of 560) of procedures. Clinical treatment failure requiring unplanned rescue surgery occurred in 6.8% (36 of 530) of patients at a mean clinical follow-up of 121.3 days, with craniotomy being the most frequently performed rescue surgery (23 of 36 patients; 63.9%). The overall procedural complications rate was 4.7% (25 of 530 patients), including symptomatic CSDH worsening that required rescue surgery in 0.9% (five of 530) of patients. Other procedural complications included ischemic complications (0.8%, four of 530 patients), including one major stroke (0.2%, one of 530 patients), hemorrhagic complications (0.4%, two of 530 patients), and visual loss (0.8%, four of 530 patients). Clinical follow-up data were available in 436 of 530 patients (82.3%), with a favorable mRS score in 75% (327 of 436) of patients at the last follow-up. Compared with scores before the procedure, mRS scores at the last follow-up were improved in 34.2% (149 of 436), unchanged in 35.8% (156 of 436), and worse in 30% (131 of 436) of patients. The all-cause mortality rate was 7.8% (34 of 436 patients), with no procedure-related mortality (Table 3).
Table 3:
Embolization Procedural Outcomes
Changes in CSDH Thickness over Time
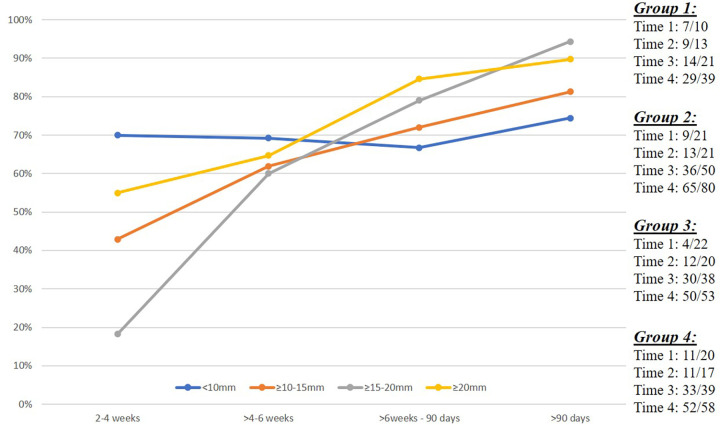
In patients with a baseline CSDH thickness less than 10 mm (group 1), radiographic success was achieved in 70% (seven of 10) at 2–4 weeks and in 74.4% (29 of 39) at greater than 90 days. In contrast, radiographic success was achieved in only 18.2% (four of 22) of patients at 2–4 weeks in group 3 (maximal CSDH thickness 15 mm to <20 mm), but that percentage increased to 60% (12 of 20), 78.9% (30 of 38), and 94.2% (49 of 52) at greater than 4 weeks to 6 weeks, greater than 6 weeks to 90 days, and greater than 90 days, respectively. In patients with a baseline CSDH thickness greater than or equal to 20 mm (group 4), radiographic success was achieved in 55% (11 of 20) at 2–4 weeks, which increased to 64.7% (11 of 17), 84.6% (33 of 39), and 89.7% (52 of 58) at greater than 4 weeks to 6 weeks, greater than 6 weeks to 90 days, and greater than 90 days, respectively. For patients in group 2 (maximal CSDH thickness 10 mm to <15 mm), radiographic success rates continuously increased from 42.9% (nine of 21) at 2–4 weeks to 61.9% (13 of 21), 72% (36 of 50), and 81.3% (65 of 80) at greater than 4 weeks to 6 weeks, greater than 6 weeks to 90 days, and greater than 90 days, respectively (Fig 2). Case examples from the four CSDH groups are provided in Figure 3.
Figure 2:
Temporal depiction of chronic subdural hematoma (CSDH) response after middle meningeal artery (MMA) embolization. Line graph shows the percentage of CSDHs that achieved radiographic success (≥50% reduction in maximal hematoma thickness), stratified according to preprocedural CSDH maximal thickness (group 1, <10 mm; group 2, 10 mm to <15 mm; group 3, 15 mm to <20 mm, and group 4, ≥20 mm) over prespecified imaging follow-up intervals, including 2–4 weeks (14–28 days), greater than 4 weeks to 6 weeks (29–42 days), 6 weeks to 90 days (43–90 days), and greater than 90 days. The data on the right indicate the number of cases achieving the end point (numerator) from the total cases (denominator) for each CSDH group over each time interval.
Figure 3:
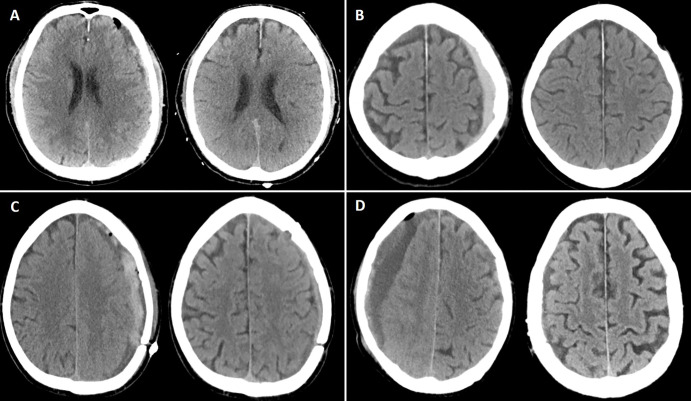
Illustrative cases of each preprocedural CSDH maximal thickness group. (A) Noncontrast head CT image (left) shows a maximal hematoma thickness of 9 mm (group 1, <10 mm) in a 77-year-old man who underwent middle meningeal artery embolization (MMAE) concurrently with left-sided craniotomy for hematoma evacuation. Follow-up noncontrast head CT image (right) at 4 months shows complete resolution of the hematoma. (B) Noncontrast head CT image (left) shows a maximal hematoma thickness of 11.6 mm (group 2, 10 mm to <15 mm) in an 80-year-old man who underwent MMAE following recurrence after a prior left-sided burr hole procedure (3 months earlier). Follow-up noncontrast head CT image (right) at 9 weeks shows complete resolution of the CSDH. (C) Noncontrast head CT image (left) shows a maximal hematoma thickness of 16 mm (group 3, 15 mm to <20 mm) in a 68-year-old man who underwent MMAE following recurrence after prior left-sided craniotomy. Follow-up noncontrast head CT image (right) at 6 weeks shows a minimal hematoma thickness of 5 mm and resolution of symptoms. (D) Noncontrast head CT image (left) shows a maximal hematoma thickness of 25 mm (group 4, ≥20 mm) in a 94-year-old man who underwent MMAE following recurrence after prior left-sided craniotomy (2 months earlier). Follow-up noncontrast head CT image (right) at 8 weeks shows near resolution of the right-sided hematoma.
Predictors of Clinical Failure
Univariable analysis.—Patients in the clinical failure group were older (P = .006) and more likely to be receiving anticoagulation medication (P = .02), with a smaller median MMA diameter (P = .027) and longer hospital stay (P < .001). The results of univariable comparisons between the clinical failure and nonfailure groups are detailed in Appendix S1 and Table S1.
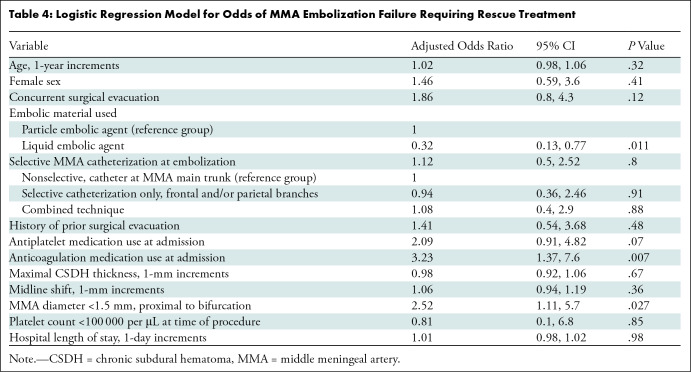
Multivariable analysis.—When controlling for potential confounders in a multivariable regression model, anticoagulation medication use at admission (adjusted odds ratio [OR], 3.23; P = .007) and an MMA diameter less than 1.5 mm (adjusted OR, 2.52; P = .027) were independent predictors of clinical treatment failure. The use of liquid embolic agents was independently associated with nonfailure compared with the reference group of particle embolic agents (adjusted OR, 0.32; P = .011), while there was no evidence of an association between antiplatelet medication use at admission and clinical failure (adjusted OR, 2.09; P = .07) (Table 4).
Table 4:
Logistic Regression Model for Odds of MMA Embolization Failure Requiring Rescue Treatment
Subgroup sensitivity analyses.—When patients with concurrent surgical evacuation with MMAE were excluded (Table S2), anticoagulation medication use (adjusted OR, 4.57; P = .005) and an MMA diameter less than 1.5 mm (adjusted OR, 3.43; P = .029) remained independently associated with clinical failure. Subsequently, patients who underwent concurrent surgical evacuation and those who underwent MMAE as a stand-alone treatment, with a maximal hematoma thickness less than 10 mm, were excluded (Table S3), whereby anticoagulation medication use (adjusted OR, 3.64; P = .025) and an MMA diameter less than 1.5 mm (adjusted OR, 3.39; P = .048) retained independent associations with clinical failure.
Predictors of Radiographic Failure
Univariable analysis.—For this analysis, 431 of 530 patients (81.3%) who underwent MMAE for 522 CSDHs had greater than or equal to 2 weeks of imaging follow-up. Patients who experienced radiographic failure were older (P = .003) and more likely to be men (P < .001), with a lower platelet count (P = .014) and smaller median MMA diameter (P = .04). Higher rates of favorable clinical outcomes were noted in the radiographic nonfailure group (81% vs 69%, P = .01). The results of univariable comparisons between the radiographic failure and nonfailure groups are detailed in Appendix S1 and Table S4.
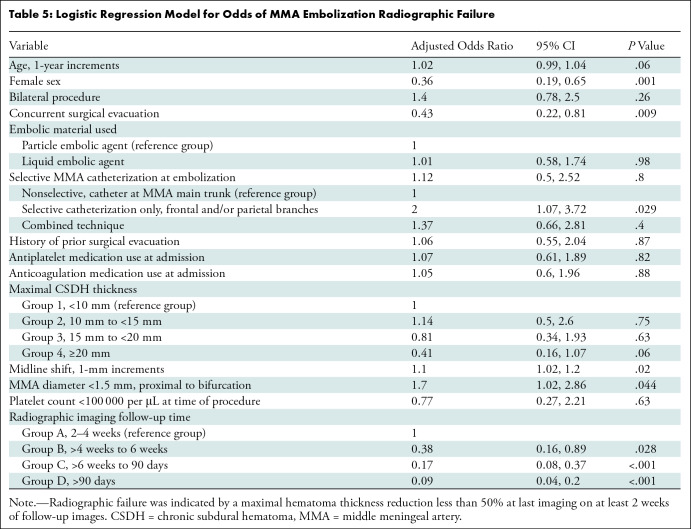
Multivariable analysis.—When controlling for potential confounders in a multivariable logistic regression model, female sex (adjusted OR, 0.36; P = .001) and concurrent surgical evacuation (adjusted OR, 0.43; P = .009) were independently associated with radiographic nonfailure. Later follow-up time categories were independent predictors against radiographic failure when compared with group A (2–4 weeks) as the reference, including group B (>4 weeks to 6 weeks) (adjusted OR, 0.38; P = .028), group C (>6 weeks to 90 days) (adjusted OR, 0.17; P < .001), and group D (>90 days) (adjusted OR, 0.09; P < .001). In contrast, superselective MMA catheterization only (adjusted OR, 2; P = .029), midline shift (adjusted OR, 1.1; P = .02), and an MMA diameter less than 1.5 mm (adjusted OR, 1.7; P = .044) were independently associated with radiographic failure (Table 5).
Table 5:
Logistic Regression Model for Odds of MMA Embolization Radiographic Failure
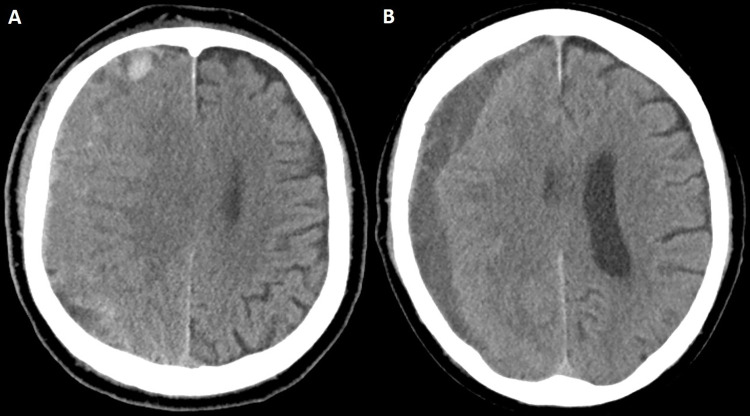
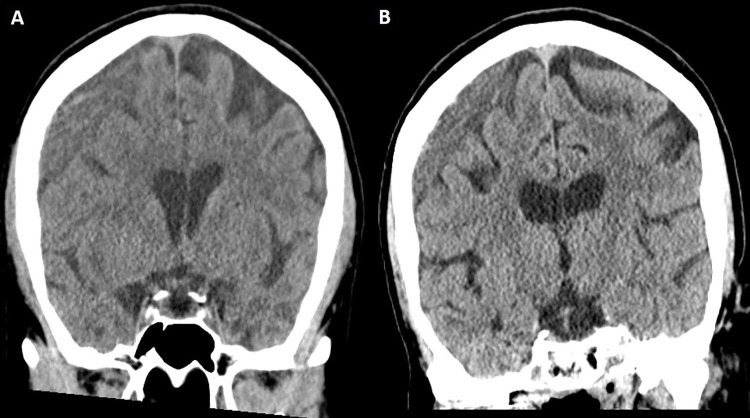
Subgroup sensitivity analyses.—When patients who underwent concurrent surgical evacuation with MMAE were excluded (Table S5), female sex (adjusted OR, 0.3; P = .002) and a longer follow-up time (group C adjusted OR, 0.13; P < .001; group D adjusted OR, 0.06; P < .001) were independently associated with radiographic nonfailure. Selective MMA catheterization only (adjusted OR, 5.01; P < .001), midline shift (adjusted OR, 1.19; P = .002), and an MMA diameter less than 1.5 mm (adjusted OR, 2.3; P = .012) were independently associated with radiographic failure. Subsequently, when patients who underwent concurrent surgical evacuation and those who underwent MMAE as a stand-alone treatment, with maximal hematoma thickness less than 10 mm, were excluded (Table S6), female sex (adjusted OR, 0.27; P = .004), CSDH group 3 (≥20 mm) (adjusted OR, 0.25; P = .009), and a longer follow-up time, including groups C (adjusted OR, 0.07; P < .001) and D (adjusted OR, 0.03; P < .001), were independently associated with nonfailure. Selective MMA catheterization only (adjusted OR, 4.81; P < .001), midline shift (adjusted OR, 1.22; P = .003), and an MMA diameter less than 1.5 mm (adjusted OR, 2.8; P = .007) were independently associated with radiographic failure. When follow-up groups A–C were excluded in the final sensitivity analysis, female sex (adjusted OR, 0.11; P = .011) and CSDH group 4 (≥20 mm) (adjusted OR, 0.1; P = .012) were independently associated with nonfailure, while age (adjusted OR, 1.05; P = .027), bilateral procedures (adjusted OR, 3.81; P = .019), selective MMA catheterization only (adjusted OR, 3.3; P = .044), anticoagulation medication use (adjusted OR, 3.61; P = .034), midline shift (adjusted OR, 1.26; P = .007), and an MMA diameter less than 1.5 mm (adjusted OR, 2.7; P = .043) were all independent predictors of radiographic failure (Table S7). Imaging examples for clinical and radiographic failures are provided in Figures 4 and 5, respectively.
Figure 4:
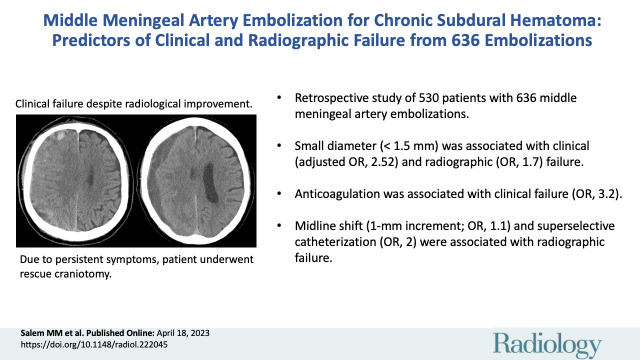
Case illustration of clinical failure. (A) Preprocedural head CT image in a 74-year-old man with an extensive cardiovascular history (receiving aspirin and clopidogrel) and stage IV lung cancer shows a right-sided isodense subacute subdural hematoma. The patient was initially managed conservatively and eventually offered middle meningeal artery embolization because of persistent headaches, which was uneventful. The patient subsequently returned from rehabilitation after 4 weeks. (B) Head CT image in the same patient shows changes in hematoma density and decreased size. Despite these radiologic changes, the patient underwent subsequent rescue craniotomy because of progression of headaches.
Figure 5:
Case illustration of radiographic failure. (A) Head CT image in a 63-year-old woman shows a large right-sided chronic subdural hematoma (maximal thickness of 19 mm), which was discovered after the patient fell. Because of progressive headaches, the patient underwent a middle meningeal artery embolization procedure, which was uneventful. (B) Head CT image obtained at the 10-week follow-up (last available) shows minimal reduction of hematoma thickness, and the patient reported little improvement of headaches.
Discussion
Little is currently known about predictors of clinical and radiographic failure of middle meningeal artery (MMA) embolization (MMAE) treatment for chronic subdural hematoma (CSDH). In this large U.S.-based multicenter study examining potential predictors of treatment failure, an MMA diameter less than 1.5 mm was the only independent factor associated with both clinical and radiographic failures. Anticoagulation medication use was associated with clinical failure, whereas use of liquid embolic agents was associated with clinical nonfailure. Midline shift and superselective MMA catheterization alone (without targeting the main trunk) were associated with radiographic failure, whereas female sex, concurrent surgery, and longer follow-up times were significantly associated with radiographic nonfailure. Identification of predictors of MMAE treatment failure is critical because it would allow individualized treatment selection and counseling of patients, as well as identification of end points for future trials. Despite the theoretical advantage of MMAE in poor surgical candidates, we found that anticoagulation medication use was independently predictive of clinical failure, with a similar trend with the use of antiplatelet medication. These results align with those from prior reports of surgically treated CSDH (11–13).
Interestingly, the MMA diameter was the only significant predictor of treatment failure both clinically and radiographically. This may be explained by the suspected pathophysiologic mechanisms of MMA involvement in CSDH; at the beginning, when border cells between the dura mater and the arachnoid break down after incitement (eg, trauma, inflammation, intracranial hypotension), blood and cerebrospinal fluid leak into the subdural space (14), activating an inflammatory cascade (15). This results in formation of CSDH inner and outer membranes with neovascularization of these neomembranes originating from distal MMA branches (16), with repeated microbleeding from these immature MMA neovessels implicated in hematoma enlargement (17). Therefore, the delivery of embolic material through the MMA is likely critical to interrupt the cascade of microbleeding and formation of neomembranes, and a smaller diameter may hinder adequate delivery of embolic material, leading to suboptimal devascularization and eventually treatment failure. Takizawa et al (16) showed that patients with CSDH have a larger MMA diameter compared with controls without CSDH and also compared with themselves intraindividually prior to CSDH development (16). Therefore, it is interesting that some patients in our cohort of failed MMAEs had smaller MMA diameters despite having a CSDH. It is possible that these patients have different pathophysiologic characteristics than patients with a larger MMA diameter, making them nonresponsive to MMAE. A more likely explanation might be additional arterial supply to the CSDH membrane, such as from the occipital artery or accessory MMA and contralateral dural arterial supply (18). Without this information in this cohort, we cannot make a final assessment and future studies are warranted. However, our findings suggest that in patients with CSDH and a small MMA diameter, additional arterial supply to the dura mater and CSDH membrane should be assessed during diagnostic angiograms to potentially identify less common blood supply patterns with potential alternative routes for MMAE to avoid treatment failure (6), particularly with the numerous known anatomic variations of the MMA (19–21).
In our study, liquid embolic agents were protective against clinical failure even after controlling for confounders, although neither embolic material (particle or liquid) had a significant association with radiographic failure. Multiple opinions exist regarding the choice of embolic material (7,22,23); however, the limited head-to-head comparisons of efficacy to date have suggested equivalent radiographic and clinical efficacy (24). It will be important to further investigate the potential benefit of liquid embolic agents in the clinical outcomes seen in our study by using matched analyses. Moreover, we found that selective catheterization of MMA branches without targeting the main trunk was an independent predictor of radiographic failure when controlling for other confounders, including the embolic materials used; however, using a combination of nonselective and selective catheterization was not associated with radiographic failure. At first glance, this appears contrary to our finding on liquid embolic agents and clinical failure because selective embolization is needed to employ liquid embolic agents. However, these results merely suggest that occluding the main trunk (regardless of the embolic material used), whenever safe and feasible, might be an important prerequisite for adequate devascularization of the convexity neomembranes and, therefore, radiographic success (25). Nonetheless, the position of the microcatheter has to be carefully weighed against nontarget embolization risks, especially when dangerous extracranial-intracranial collaterals are suspected (7,8).
Our data corroborate the findings of Onyinzo et al (26) that a higher rate of radiographic resolution occurred in the concurrent embolization and surgery group compared with the stand-alone surgery and embolization groups. Concurrent surgical evacuation was significantly associated with radiographic nonfailure at multivariable analysis (P = .009). The underlying reasons remain unknown, but immediate evacuation of the CSDH concurrently with occlusion of the vascular supply of the neomembranes via the MMA may have a synergistic effect (27). The statistical significance of midline shift in association with radiographic failure in our study further supports this, as we found that each 1-mm incremental increase carried a 10% higher likelihood of radiographic failure when controlling for confounders (P = .044). In contrast to the rapid immediate decompression after surgical evacuation, the course of radiologic improvement after MMAE has not been well described, although it is thought to be slower and with delayed effect (10,24). We used the follow-up time interval of 2–4 weeks as a reference group, in line with Gomez-Paz et al (28), who found a significant reduction in hematoma thickness and midline shift at this time interval, which is further confirmed in our data that demonstrate a greater than 50% radiographic success rate in both CSDH groups 1 and 4 (maximal CSDH thickness <10 mm and ≥20 mm, respectively) at this time point. Paradoxically, there was no evidence that larger CSDHs (particularly group 4) were associated with nonfailure (adjusted OR, 0.41; P = .06; Table 5) in the multivariable analysis, but there was an association in subsequent sensitivity analyses (Tables S5–S7). Furthermore, when excluding CSDHs with less than 90 days of imaging follow-up, the same associations persisted in the multivariable analysis. This suggests that CSDHs with larger diameters are more likely to respond to MMAE than those with smaller diameters. The reasons for this are unknown, and prospective studies with fixed time points for the different subgroups, in addition to volumetric hematoma assessment, should provide more data.
In contrast to clinical nonfailure after conventional surgery, clinical nonfailure after MMAE might be harder to delineate. The reasons for association between clinical nonfailure and radiographic failure remain unknown, but some patients who experience radiographic failure may not need to undergo rescue surgery because of the difference in definition criteria for each end point and general differences in indications, as patients with significant midline shift or clinical deficits are likely to undergo prompt surgical evacuation. In contrast, all patients who experienced the clinical failure end point had evidence of radiographic failure before undergoing rescue surgery, reinforcing the notion that clinical rescue intervention in patients undergoing MMAE is generally radiographically guided, although clinical nuances might vary on a case-by-case basis. Moreover, the criteria for pursuing rescue surgical intervention are not well defined, given the lack of universal indications for MMAE failure and success; thus, some of these MMAE failures might have been the result of imprudent case selection (only five of 36 [13.9%] retreated patients had hematoma enlargement). The difference in definition criteria for the clinical and radiographic failure end points likely contributed to the difference in predicting factors of clinical and radiographic failures, although the underlying reasons cannot be discerned with certainty. There were no conflicts in the reported factors predicting clinical and radiographic failures (eg, one factor predicting clinical failure while also predicting radiographic nonfailure). However, given that clinical failure occurred in only 6.8% of patients and radiographic failure occurred in 26.3% of procedures, with different defining criteria for each end point (as detailed in the Materials and Methods section), there is a higher probability of identifying more factors of radiographic than clinical failure, which was indeed the case in our cohort.
Our study had limitations. First, this is a retrospective analysis limited by selection bias and the inherent weaknesses of these types of studies, including the absence of a control group. Second, there was heterogeneity in management protocols among participating institutions. Third, radiologic analysis was limited to CSDH maximal thickness because volumetric data were not available. Fourth, the natural history of CSDH, especially with MMAE, remains poorly defined without standardized indications for intervention, and thus an element of overzealous treatment cannot be excluded. Fifth, information regarding placement of subdural drains in patients who received surgery was not available, which might be an uncontrolled confounder of recurrence (29). Sixth, important procedural details, such as the positioning of the microcatheter in relation to important anatomic landmarks (eg, meningolacrimal branch), were not available. Seventh, the different combinations of embolic materials (including different sizes and types of embolization particles [Table S8]) were used according to the operator’s preference, limiting the yield of their comparisons. Eighth, information regarding the duration and timing of anticoagulation and antiplatelet medication use was not available. Finally, there was a relatively short duration of follow-up.
In conclusion, we identified multiple independent predictors of clinical and radiographic middle meningeal artery (MMA) embolization (MMAE) failure in a large multicenter cohort. Anticoagulation medication use and an MMA diameter less than 1.5 mm were independently associated with clinical failure, whereas liquid embolic materials were associated with nonfailure. MMA diameter less than 1.5 mm, midline shift, and superselective MMA catheterization only (without targeting the main MMA trunk) were associated with radiographic failure, whereas concurrent surgical evacuation, female sex, and a longer imaging follow-up time were associated with nonfailure. Controlled comparative investigations of the efficacy and safety of different materials are warranted, and studies with longer imaging and clinical follow-up durations could provide additional data regarding chronic subdural hematoma response to MMAE. These factors might help guide individualized treatment selection and counseling in patients undergoing this procedure.
Acknowledgments
Acknowledgment
We thank our medical editor Kristin Kraus, MSc, for assistance with editing the manuscript.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: M.M.S. No relevant relationships. O.K. No relevant relationships. A.N.H. No relevant relationships. A.A.B. No relevant relationships. M.K. No relevant relationships. C.B. No relevant relationships. J.C.H. No relevant relationships. A.A.M. No relevant relationships. G.C. No relevant relationships. J.M.D. Institutional grants or contracts from National Institutes of Health (NIH), NSF SBIR, University at Buffalo Center for Advanced Technology, Buffalo Translational Consortium, Cummings Foundation, NVIDIA, and Google; royalties from RIST Neurovascular; lecture payment from Medtronic; advisory board for NIH Strokenet; leadership role, Cerebrovascular section of the Congress of Neurological Surgeons; stockholder, QAS.ai, RIST Neurovascular, Cerebrotech, Synchron, and Hyperion. S.N. Lecture payment from Cerenovus; board of directors, Society of NeuroInterventional Surgery. C.M.C. No relevant relationships. H.A.R. No relevant relationships. J.M.M. No relevant relationships. A.M.S. Grants or contracts from Medtronic, Stryker, Penumbra, Avail, and RapidAI; consulting fees from Stryker, Penumbra, RapidAI, and Terumo; medical advisory board, Brain Aneurysm Foundation; stockholder, Avail Med. A.A.K. Consulting fees from Medtronic; payment for expert testimony from Procopio US Attorney; advisory board, Route 92 and Medtronic; leadership role, Congress of Neurological Surgeons and American College of Surgeons; stockholder, Ospitek, Synaptive, and Proximie. B.M.H. No relevant relationships. R.H. Grants or contracts from NIH, Interline Endowment, Microvention, Stryker, CNX, and Balt; consulting fees from Medtronic, Balt, Stryker, Q’Apel Medical, Codman Neuro (J&J), Cerenovus, Microvention, Imperative Care, Phenox, and Rapid Medical; advisory board, MiVI, eLum, Three Rivers, Shape Medical, and Corindus; associate editor of the endovascular section for Neurosurgery Journal; stockholder, InNeuroCo, Cerebrotech, eLum, Endostream, Three Rivers Medical, Scientia, RisT, Blink TBI, and Corindus. O.T. No relevant relationships. E.I.L. Consulting fees from Clarion, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, StimMed, Misionix, and Mosiac; lecture payments from Clarion, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, StimMed, Misionix, and Mosiac; expert testimony payment from Renders Medical; patents planned, issued, or pending for Ultrasonic surgical blade; advisory board for Stryker, NeXtGen Biologics, MEDX, Cognition Medical, Endostream Medical, and IRRAS; stockholder, NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care, Rebound Therapeutics, StimMed, and Three Rivers Medical; chief medical officer for Haniva Technology. R.G. Consulting fees from Medtronic Neurovascular, Balt Neurovascular, and Cerenovus. M.J.L No relevant relationships. A.H.S. Grant from NIH; consulting fees from Amnis Therapeutics, Apellis Pharmaceuticals, Boston Scientific, Canon Medical Systems, Cardinal Health 200, Cerebrotech Medical Systems, Cerenovus, Cerevatech Medical, Cordis, Corindus, Endostream Medical, Imperative Care, InspireMD, Integra, IRRAS AB, Medtronic, MicroVention, Minnetronix Neuro, Peijia Medical, Penumbra, Piraeus Medical, Q’Apel Medical, Rapid Medical, Serenity Medical, Silk Road Medical, StimMed, Stryker Neurovascular, Three Rivers Medical, VasSol, and Viz.ai; stockholder, Adona Medical, Amnis Therapeutics, Bend IT Technologies, BlinkTBI, Borvo Medical, Cerebrotech Medical Systems, Cerevatech Medical, Cognition Medical, Collavidence, CVAID, E8, Endostream Medical, Galaxy Therapeutics, Imperative Care, InspireMD, Instylla, International Medical Distribution Partners, Launch NY, Neurolutions, NeuroRadial Technologies, NeuroTechnology Investors, Neurovascular Diagnostics, Peijia Medical, PerFlow Medical, Piraeus Medical, Q’Apel Medical, QAS.ai, Radical Catheter Technologies, Rebound Therapeutics, RIST Neurovascular, Sense Diagnostics, Serenity Medical, Silk Road Medical, Sim & Cure, SongBird Therapy, Spinnaker Medical, StimMed, Synchron, Three Rivers Medical, Truvic Medical, Tulavi Therapeutics, Vastrax, VICIS, Viseon, and Whisper Medical; payments related to Cerenovus EXCELLENT and ARISE II Trial; Medtronic SWIFT PRIME, VANTAGE, EMBOLISE, and SWIFT DIRECT Trials; MicroVention FRED Trial & CONFIDENCE Study; MUSC POSITIVE Trial; Penumbra 3D Separator Trial, COMPASS Trial, INVEST Trial, MIVI Neuroscience EVAQ Trial; Rapid Medical SUCCESS Trial; and InspireMD C-GUARDIANS IDE Pivotal Trial. P.K. Grants from NIH (1U18EB029353-01), Medtronic (ERP-2019-12070), Siemens (CON30434), and Joe Niekro Foundation (CON30914); consulting fees from Stryker Neurovascular, Imperative Care, Cerenovus, and Microvention; editorial board, Journal of Neurointerventional Surgery. C.S.O. No relevant relationships. B.A.G. Consulting fees from Medtronic and Microvention. A.J.T. Consulting fees from Stryker, Medtronic, and Cerevasc. B.T.J. No relevant relationships. J.K.B. Consulting fees from Q`Apel Medical, Stryker, Medtronic, Cerenovous, and Microvention.
Abbreviations:
- CSDH
- chronic subdural hematoma
- MMA
- middle meningeal artery
- MMAE
- MMA embolization
- mRS
- modified Rankin scale
- OR
- odds ratio
References
- 1. Ducruet AF , Grobelny BT , Zacharia BE , et al . The surgical management of chronic subdural hematoma . Neurosurg Rev 2012. ; 35 ( 2 ): 155 – 169 ; discussion 169 . [DOI] [PubMed] [Google Scholar]
- 2. Miranda LB , Braxton E , Hobbs J , Quigley MR . Chronic subdural hematoma in the elderly: not a benign disease . J Neurosurg 2011. ; 114 ( 1 ): 72 – 76 . [DOI] [PubMed] [Google Scholar]
- 3. Ivamoto HS , Lemos HP Jr , Atallah AN . Surgical treatments for chronic subdural hematomas: a comprehensive systematic review . World Neurosurg 2016. ; 86 : 399 – 418 . [DOI] [PubMed] [Google Scholar]
- 4. Liu W , Bakker NA , Groen RJ . Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures . J Neurosurg 2014. ; 121 ( 3 ): 665 – 673 . [DOI] [PubMed] [Google Scholar]
- 5. Ironside N , Nguyen C , Do Q , et al . Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis . J Neurointerv Surg 2021. ; 13 ( 10 ): 951 – 957 . [DOI] [PubMed] [Google Scholar]
- 6. Martínez JL , Domingo RA , Sattur M , et al . The middle meningeal artery: branches, dangerous anastomoses, and implications in neurosurgery and neuroendovascular surgery . Oper Neurosurg (Hagerstown) 2022. ; 22 ( 1 ): 1 – 13 . [DOI] [PubMed] [Google Scholar]
- 7. Shapiro M , Walker M , Carroll KT , et al . Neuroanatomy of cranial dural vessels: implications for subdural hematoma embolization . J Neurointerv Surg 2021. ; 13 ( 5 ): 471 – 477 . [DOI] [PubMed] [Google Scholar]
- 8. Kan P , Maragkos GA , Srivatsan A , et al . Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations . Neurosurgery 2021. ; 88 ( 2 ): 268 – 277 . [DOI] [PubMed] [Google Scholar]
- 9. Ban SP , Hwang G , Byoun HS , et al . Middle meningeal artery embolization for chronic subdural hematoma . Radiology 2018. ; 286 ( 3 ): 992 – 999 . [DOI] [PubMed] [Google Scholar]
- 10. Link TW , Boddu S , Paine SM , Kamel H , Knopman J . Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases . Neurosurgery 2019. ; 85 ( 6 ): 801 – 807 . [DOI] [PubMed] [Google Scholar]
- 11. Motiei-Langroudi R , Stippler M , Shi S , et al . Factors predicting reoperation of chronic subdural hematoma following primary surgical evacuation . J Neurosurg 2018. ; 129 ( 5 ): 1143 – 1150 . [DOI] [PubMed] [Google Scholar]
- 12. Schwarz F , Loos F , Dünisch P , et al . Risk factors for reoperation after initial burr hole trephination in chronic subdural hematomas . Clin Neurol Neurosurg 2015. ; 138 : 66 – 71 . [DOI] [PubMed] [Google Scholar]
- 13. Tahsim-Oglou Y , Beseoglu K , Hänggi D , Stummer W , Steiger HJ . Factors predicting recurrence of chronic subdural haematoma: the influence of intraoperative irrigation and low-molecular-weight heparin thromboprophylaxis . Acta Neurochir (Wien) 2012. ; 154 ( 6 ): 1063 – 1067 ; discussion 1068 . [DOI] [PubMed] [Google Scholar]
- 14. Yamashima T , Yamamoto S , Friede RL . The role of endothelial gap junctions in the enlargement of chronic subdural hematomas . J Neurosurg 1983. ; 59 ( 2 ): 298 – 303 . [DOI] [PubMed] [Google Scholar]
- 15. Yamashima T . The inner membrane of chronic subdural hematomas: pathology and pathophysiology . Neurosurg Clin N Am 2000. ; 11 ( 3 ): 413 – 424 . [PubMed] [Google Scholar]
- 16. Takizawa K , Sorimachi T , Ishizaka H , et al . Enlargement of the middle meningeal artery on MR angiography in chronic subdural hematoma . J Neurosurg 2016. ; 124 ( 6 ): 1679 – 1683 . [DOI] [PubMed] [Google Scholar]
- 17. Gandhoke GS , Kaif M , Choi L , Williamson RW , Nakaji P . Histopathological features of the outer membrane of chronic subdural hematoma and correlation with clinical and radiological features . J Clin Neurosci 2013. ; 20 ( 10 ): 1398 – 1401 . [DOI] [PubMed] [Google Scholar]
- 18. Hubbard ZS , Al Kasab S , Porto GB , Spiotta A . Chronic subdural hematoma recurrence due to contralateral neovascularization following middle meningeal artery embolization . Interv Neuroradiol 2022. ; 28 ( 6 ): 639 – 643 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonasia S , Smajda S , Ciccio G , Robert T . Middle meningeal artery: anatomy and variations . AJNR Am J Neuroradiol 2020. ; 41 ( 10 ): 1777 – 1785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salem MM , Fusco MR , Dolati P , et al . Middle meningeal artery arising from the basilar artery . J Cerebrovasc Endovasc Neurosurg 2014. ; 16 ( 4 ): 364 – 367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuruvilla A , Aguwa AN , Lee AW , Xavier AR . Anomalous origin of the middle meningeal artery from the posterior inferior cerebellar artery . J Neuroimaging 2011. ; 21 ( 3 ): 269 – 272 . [DOI] [PubMed] [Google Scholar]
- 22. Fiorella D , Arthur AS . Middle meningeal artery embolization for the management of chronic subdural hematoma . J Neurointerv Surg 2019. ; 11 ( 9 ): 912 – 915 . [DOI] [PubMed] [Google Scholar]
- 23. Samarage HM , Kim WJ , Zarrin D , et al . The “bright falx” sign-midline embolic penetration is associated with faster resolution of chronic subdural hematoma after middle meningeal artery embolization: a case series . Neurosurgery 2022. ; 91 ( 3 ): 389 – 398 . [DOI] [PubMed] [Google Scholar]
- 24. Scoville JP , Joyce E , Tonetti DA , et al . Radiographic and clinical outcomes with particle or liquid embolic agents for middle meningeal artery embolization of nonacute subdural hematomas . Interv Neuroradiol 2022. . 15910199221104631. Published online June 7, 2022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khorasanizadeh M , Shutran M , Garcia A , et al . Middle meningeal artery embolization with isolated use of coils for treatment of chronic subdural hematomas: a case series . World Neurosurg 2022. ; 165 : e581 – e587 . [DOI] [PubMed] [Google Scholar]
- 26. Onyinzo C , Berlis A , Abel M , Kudernatsch M , Maurer CJ . Efficacy and mid-term outcome of middle meningeal artery embolization with or without burr hole evacuation for chronic subdural hematoma compared with burr hole evacuation alone . J Neurointerv Surg 2022. ; 14 ( 3 ): 297 – 300 . [DOI] [PubMed] [Google Scholar]
- 27. Schwarz J , Carnevale JA , Goldberg JL , Ramos AD , Link TW , Knopman J . Perioperative prophylactic middle meningeal artery embolization for chronic subdural hematoma: a series of 44 cases . J Neurosurg 2021. ; 135 ( 6 ): 1627 – 1635 . [DOI] [PubMed] [Google Scholar]
- 28. Gomez-Paz S , Akamatsu Y , Salem MM , et al . Upfront middle meningeal artery embolization for treatment of chronic subdural hematomas in patients with or without midline shift . Interv Neuroradiol 2021. ; 27 ( 4 ): 571 – 576 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santarius T , Kirkpatrick PJ , Ganesan D , et al . Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial . Lancet 2009. ; 374 ( 9695 ): 1067 – 1073 . [DOI] [PubMed] [Google Scholar]