Abstract
Objective
This study aimed to investigate the current status of postoperative management of uterine endometrial cancer (EC) in Korea.
Methods
A mail survey was administered to members of the Korean Gynecologic Oncology Group and Korean Radiation Oncology Group. A total of 38 gynecologic cancer surgeons (GYNs) and 31 radiation oncologists (RO) in 43 institutions was responded. The questionnaire consisted of general questions for clinical decision and clinical case questions. The GYN and RO responses were compared using chi-square statistics.
Results
The 2 expert groups had similar responses for clinical decision based on the results of the Gynecologic Oncology Group (GOG)-249 and Postoperative Radiation Therapy for Endometrial Carcinoma-III trials in the early-stage EC. In contrast, the responses based on GOG-258 results differed, as GYNs most frequently opted for sequential chemotherapy (CTx) and radiotherapy (RT), while ROs preferred concurrent chemoradiotherapy in locally advanced stage (p<0.05). Based on the GOG-258, GYNs preferred CTx alone for adjuvant treatment of serous or clear cell adenocarcinoma histology, whereas ROs advocated for combined CTx and RT (sequential or concurrent). Among the clinical case questions, GYNs were more likely than ROs to choose CTx alone rather than the combination of CTx and RT (sequential or concurrent) as the answers to case questions representing patients with locally advanced stage or unfavorable histology (all p<0.05).
Conclusion
The present study showed several different opinions of GYNs and ROs regarding adjuvant treatment for EC, particularly for adjuvant RT in advanced stage or unfavorable histology.
Keywords: Endometrial Neoplasms, Radiotherapy, Hysterectomy, Drug Therapy
Synopsis
Based on the recent large-scale clinical trials, this study investigated the current status of postoperative management of EC. Gynecologic cancer surgeons and radiation oncologists have different opinions on the administration of adjuvant radiotherapy and the difference was more pronounced in locally advanced and unfavorable histology.
INTRODUCTION
Endometrial cancer (EC) is the seventh most common malignancy in females in Korea, and its incidence rapidly increased from 5.6 per 100,000 in 2010 to 8.8 per 100,000 in 2019 [1]. EC is treated primarily by surgical resection and various adjuvant strategies, such as pelvic radiotherapy (RT) and systemic chemotherapy (CTx). The risk assessment for recurrence of early-stage EC generally considers various clinicopathological factors, such as the histological grade, myometrial invasion, lymphovascular invasion, tumor location, and patient age. In addition to the traditional risk classification, including low, intermediate, and high-risk groups, intermediate risk has been subdivided into high–intermediate risk and low–intermediate risk according to the Gynecologic Oncology Group (GOG)-99 trial [2]. Advanced-stage EC also comprises a heterogeneous group of diseases ranging from direct local extension of the disease to nodal or distant metastasis.
To support evidence-based clinical guidance on adjuvant treatment for EC, a series of large-scale clinical trials has been performed by the GOG and the Postoperative Radiation Therapy for Endometrial Carcinoma (PORTEC) study group. The results of 3 landmark studies have been recently published by the GOG-249 [3], PORTEC-III [4], and GOG-258 [5] trial groups (Table 1). These studies may affect the clinical decisions of gynecologic cancer experts, and there is a need to investigate how this has influenced current patterns of practice. The Korean Gynecologic Oncology Group (KGOG) and the Korean Radiation Oncology Group (KROG) are organizations of gynecologic oncologists and radiation oncologists (ROs) that were initiated in 2002 and 2001, respectively, for clinical multi-institutional collaborative study and research. This study was designed with this infrastructure to identify the current patterns of practice of adjuvant treatment for EC in Korea by distributing a survey to the KGOG and KROG members. Importantly, we compared the patterns of practice between gynecologic cancer surgeons (GYNs) and ROs with an intent to identify the discrepancies between the experts and improve patient care for EC in Korea.
Table 1. Summary of recent clinical trials according to the Participant, Intervention, Comparison, and Outcome assessment form.
| Trials | Patient | Intervention | Comparison | Outcome |
|---|---|---|---|---|
| GOG-249 | Stage I HIR–stage II endometrioid, stage I–II unfavorable histology | VBT + CTx* | EBRT | 1. 5-yr RFS and OS: no difference |
| 2. Vaginal recurrence: no difference | ||||
| 3. Pelvic or para-aortic recurrence: more common in VBT | ||||
| 4. Acute toxicity: more common in VBT | ||||
| 5. Late toxicity: similar | ||||
| PORTEC-III | Stage I HR–stage II–III endometrioid, stage I–III unfavorable histology | CCRT + CTx† | EBRT + CTx† | 1. OS: CCRT better |
| 2. Failure free survival: CCRT better | ||||
| 3. DM: more common in EBRT alone | ||||
| 4. Complication: ≥ grade 2 more common in CCRT, especially neuropathy | ||||
| GOG-258 | Stage III–IVA any histology + stage I–II unfavorable histology | CCRT + CTx† | CTx alone‡ | 1. RFS no difference |
| 2. Vaginal & pelvic and PAN recurrence: CCRT better | ||||
| 3. DM: CTx better | ||||
| 4. More grade 4 or higher toxicity in CTx (Tx related death 2patients) |
CCRT, concurrent chemoradiotherapy; CTx, chemotherapy; DM, distant metastasis; EBRT, external beam radiotherapy; GOG, Gynecologic Oncology Group; HIR, high intermediate risk; HR, high risk; OS, overall survival; PAN, para-aortic lymph node; PORTEC, Postoperative Radiation Therapy for Endometrial Carcinoma; RFS, recurrence-free survival; Tx, treatment; VBT, vaginal brachytherapy.
Chemotherapy regimen: *paclitaxel + carboplatin 3 cycle or †paclitaxel + carboplatin 4 cycle or ‡paclitaxel + carboplatin 6 cycle.
MATERIALS AND METHODS
A mail survey involving an electronic questionnaire consisting of 25 questions regarding the status of adjuvant treatment and case scenarios was distributed to members of the KGOG and KROG. The questionnaire was designed by the principal investigator (K.J.Y.) and was approved by the Disease Committee of Uterine Endometrial Cancer of the KGOG (KGOG-2028) and the gynecologic cancer study branch of the KROG (KROG-2104). In accordance with Declaration of Helsinki, the institutional review boards of National Cancer Center granted an exemption for this study. As a general questionnaire for clinical decision, intended to investigate the up-to-date clinical practice after the recent GOG and PORTEC studies, the 13 questions were composed of 6 demographic and 7 clinical trial questions (Table S1). Because the risk assessment of recurrence of EC includes various clinicopathologic factors, the survey with specific clinical case questions was also asked, consisted of 4 questions regarding stage I/II endometrioid EC, one regarding stage III/IV endometrioid EC, 3 regarding an unfavorable histology, and 2 regarding uterine sarcoma and carcinosarcoma (Table S2). The respondents were reminded of the results of the relevant clinical trial. The adjuvant treatment options given to the respondents included CTx alone, external beam RT (EBRT) alone, sequential use of CTx and EBRT (CTx + EBRT), vaginal brachytherapy (VBT) alone, CTx with VBT, or concurrent chemoradiotherapy (CCRT). As a last, 17 questions specific to GYNs and 16 questions specific to ROs were administered, for more detailed description of their clinical practice.
Initially, we identified 145 members in KGOG and 74 ROs who sub-specialized in gynecology oncology in KROG and mailed the questionnaire to all KGOG and KROG members in May 2021, and the last date for receipt of responses was July 13, 2021. Finally, a total of 38 GYNs and 31 ROs in 43 institutions was participated in the survey. The response rate was 26.2% (38/145) and 41.8% (31/74), respectively. One of the considerations for response rate is that some responders noted he or she responded the survey as a representative of gynecology oncology surgeons in their institution. A standardized computer software package (SPSS version 27.0; IBM Inc., Armonk, NY, USA) was used for the statistical analysis. Chi-square statistics were used to compare data between the GYN and RO groups, and a p-value <0.05 was considered significant.
RESULTS
1. General questionnaire for clinical decision
The respondents’ general information is summarized in Table 2. Overall, the RO respondents were younger and had a shorter history of practice in the field of gynecologic cancer compared with the GYNs. All responders were working in academic (university) institutions and the location of hospital was 69.5% in capital area and 30.5% in non-capital area, respectively.
Table 2. Characteristics of the respondents.
| Characteristics | GYN | RAD | p-value* | |
|---|---|---|---|---|
| No. of physician | 38 | 31 | ||
| No. of institution | 30 | 29 | ||
| Age of physician | 0.070 | |||
| 30–40s | 23 (60.5) | 25 (80.7) | ||
| 50–60s | 15 (39.5) | 6 (19.4) | ||
| Year of practice | <0.001 | |||
| <10 | 10 (26.3) | 22 (71.0) | ||
| ≥10 | 28 (73.7) | 9 (29.0) | ||
| Patient size volume (No. of uterine cancer per year) | 0.518 | |||
| <10 | 1 (2.6) | 4 (12.9) | ||
| 10–20 | 10 (26.3) | 6 (19.4) | ||
| 20–30 | 6 (15.8) | 5 (16.1) | ||
| 30–50 | 6 (15.8) | 4 (12.9) | ||
| 50–100 | 7 (18.4) | 4 (12.9) | ||
| >100 | 8 (21.1) | 8 (25.8) | ||
| Brachytherapy facility | 0.403 | |||
| Yes | 21 (55.3) | 14 (45.2) | ||
| No | 17 (44.7) | 17 (54.8) | ||
GYN, gynecologic cancer surgeon; RO, radiation oncologist.
*The p-value by χ2 test.
Regarding postoperative management in the early-stage disease, the questionnaire was conducted based on the results of PORTEC-II, GOG 249, and PORTEC-III trials (Table 3). For the high-intermediate risk early-stage EC patients, in which PORTEC-II trial compared the efficacy of VBT vs. EBRT after hysterectomy [6], the distribution of responses was similar between GYNs and ROs. Performing VBT alone was most common answer in both groups. For the high-intermediate to high risk early-stage EC patients, in which GOG-249 compared the efficacy of CTx + VBT vs. EBRT after hysterectomy [3], the responses were also similar between GYNs and ROs. Performing adjuvant EBRT alone was most common answer in both groups. For high-risk stage I and stage II–III EC patients, in which PORTEC-III compared the efficacy of EBRT and CCRT [4], the most frequent responses of GYNs and ROs were sequential CTx + EBRT and CCRT, respectively.
Table 3. Comparison of distribution of responses between GYN and RO on preferences of adjuvant management based on the result of recent clinical trials.
| No. | Characteristics | Preferred adjuvant management | Relevant trial | ||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||||
| 1 | Stage I grade 1–2 with >1/2 myometrial invasion or stage I grade 3 with < 1/2 myometrial invasion | GYN | VBT only (47.3%) | EBRT (23.7%) | EBRT due to no VBT facility (18.4%) | PORTEC-II | |
| RO | VBT only (54.8%) | EBRT (32.3%) | EBRT due to no VBT facility (9.7%) | ||||
| 2 | Stage I, II high-intermediate risk | GYN | EBRT alone (50.0%) | EBRT + CTx (18.4%) | VBT only (13.1%) | CCRT (10.5%) | GOG-249 |
| RO | EBRT alone (74.2%) | CCRT (16.1%) | |||||
| 3 | High-risk stage I and stage II–III | GYN | EBRT + CTx sequential (42.1%) | CCRT + adj. CTx (15.8%) | CCRT (15.8%) | EBRT alone (13.2%) | PORTEC-III |
| RO | EBRT + CTx sequential (29.0%) | CCRT + adj. CTx (25.8%) | CCRT (22.6%) | ||||
| 4 | Stage III–IV endometrioid | GYN | EBRT + CTx sequential (57.9%) | CTx only (28.9%) | CCRT (13.2%) | GOG-258 | |
| RO | CCRT (41.9%) | EBRT + CTx sequential (35.4%) | CTx only (12.9%) | ||||
| 5 | Endometrial cancer with serous or clear cell adenocarcinoma histology | GYN | CTx only (47.3%) | EBRT + CTx sequential (34.2%) | CCRT (18.4%) | GOG-258 | |
| RO | CCRT (51.6%) | EBRT + CTx sequential (29.0%) | |||||
| 6 | Stage I, II serous or clear cell adenocarcinoma | GYN | CTx only (60.5%) | EBRT + CTx sequential (21.1%) | |||
| RO | EBRT + CTx sequential (32.2%) | CCRT + adj. CTx (22.5%) | CCRT (16.1%) | ||||
| 7 | Stage III, IV serous or clear cell adenocarcinoma | GYN | CTx only (57.9%) | EBRT + CTx sequential (34.2%) | |||
| RO | CCRT + adj. CTx (35.4%) | EBRT + CTx sequential (25.8%) | CCRT (22.5%) | ||||
adj., adjuvant; CCRT, concurrent chemoradiotherapy; CTx, chemotherapy; EBRT, external beam radiotherapy; GOG, Gynecologic Oncology Group; GYN, gynecologic cancer surgeon; PORTEC, Postoperative Radiation Therapy for Endometrial Carcinoma; RO, radiation oncologist; VBT, vaginal brachytherapy.
Regarding postoperative management in the locally advanced disease (Table 3), the questionnaire was conducted based on the results of GOG-258 trial [5]. For the stage III–IV EC patients, in which GOG-258 compared the efficacy of CCRT and CTx alone, the distribution of the responses revealed differences between GYNs and ROs. Sequential CTx + EBRT and CCRT were the most common treatments ordered by GYNs and ROs, respectively.
Regarding postoperative management in EC with unfavorable histology, the questionnaire was conducted based on the result of recent clinical GOG-258 trial [5]. For stage I–IV EC with serous or clear cell adenocarcinoma histology, in which GOG-258 compared the efficacy of CCRT and CTx alone, the distribution of the responses revealed differences between GYNs and ROs. Postoperative CTx alone was the most common treatment provided by GYNs, whereas CCRT was the most common by ROs. Although there were no relevant specific clinical trials to unfavorable histology group, those patients were divided into stage I, II and stage III, IV and then the clinical practice preferences were asked. As shown in Table 3, GYNs consistently chose CTx alone to treat stage I/II and III/IV patients, whereas ROs preferred sequential CTx + EBRT for stage I/II and CCRT + CTx for stage III/IV patients, respectively.
2. Specific clinical case questions
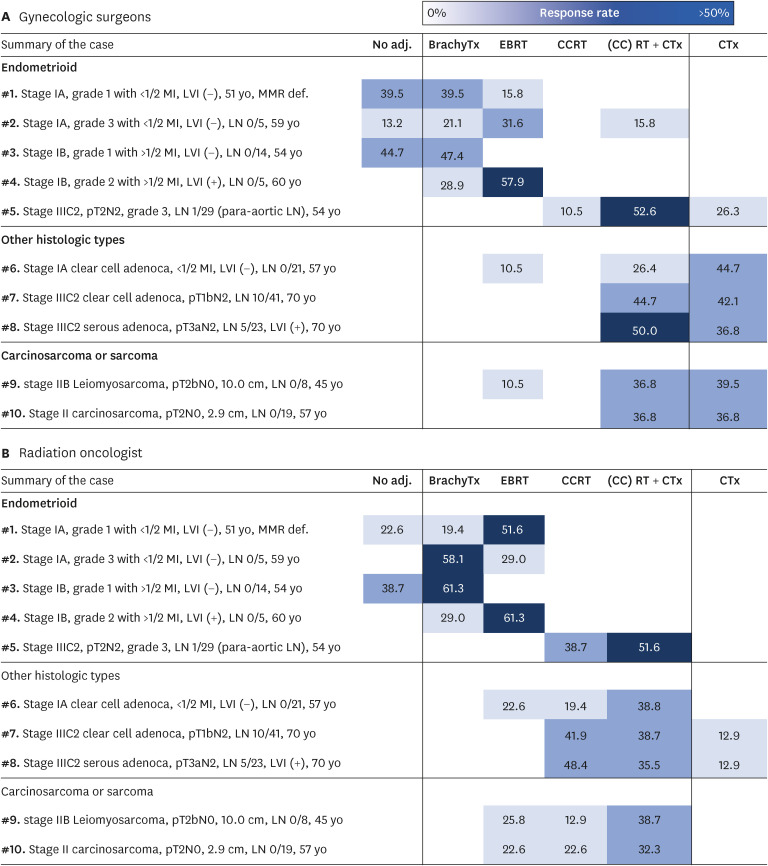
Summary of clinical case scenarios and distributions of responses from GYN and RO are visualized in Fig. 1. For the first clinical case with a DNA mismatch repair deficiency, GYNs preferred close observation or administering postoperative VBT, whereas ROs were inclined to administer EBRT (p<0.05). For the second case with early-stage high grade EC, GYNs and ROs the most commonly chose EBRT and VBT, respectively (p<0.05). For the third case having low grade EC with deep myometrial invasion, almost half of the clinicians (38.7%–44.7%) preferred close observation and the remaining half wanted to add VBT (47.4%–61.3%). For the fourth case having grade 2 EC, deep myometrial invasion, and positive lymphovascular invasion, EBRT was the first choice (57.9%–61.3%) for both expert groups. For the fifth case with locally advanced grade 3 EC, sequential CTx + EBRT was the most frequently selected in both groups. For the sixth case with stage IA uterine clear cell adenocarcinoma, GYNs preferred to administer CTx alone, whereas ROs preferred to administer EBRT sequentially or concurrently with CTx (p<0.05). For the seventh and eighth case with locally advanced uterine clear cell adenocarcinoma and serous adenocarcinoma, respectively, approximately 50% of GYNs chose CTx alone. In contrast, ROs chose EBRT sequentially or concurrently with CTx rather than CTx alone (p<0.05). For the ninth and tenth case with uterine leiomyosarcoma and carcinosarcoma, respectively, most GYNs preferred CTx alone, whereas most ROs thought that RT was still necessary for postoperative management (p<0.05). Overall, responses of GYN are more distributed in the column of CTx alone compared to that of RO.
Fig. 1. Summary of clinical cases and distributions of responses between (A) gynecologic surgeon and (B) radiation oncologist.
adj., adjuvant; BrachyTx, brachytreatment; CCRT, concurrent chemoradiotherapy; CTx, chemotherapy; EBRT, external beam radiotherapy; LN, lymph node; RT, radiotherapy.
3. GYN-specific questionnaire
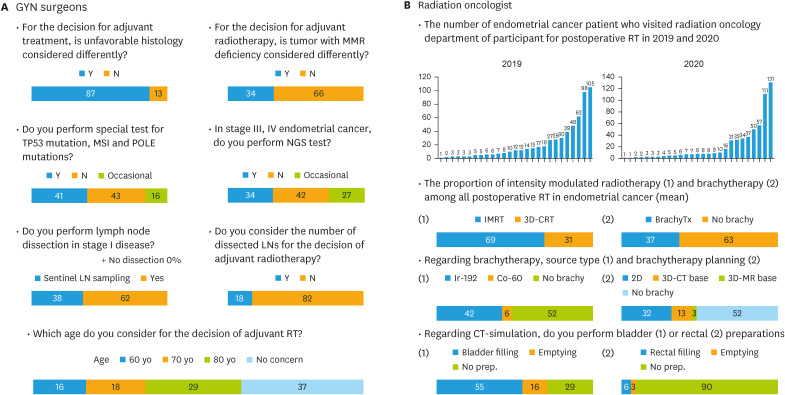
For more detailed description of clinical practice in GYN, seventeen questions were posed specifically to GYNs. The most severe postoperative complication was considered lymphedema (69%), followed by wounds (16%). The most severe radiation-associated complication was considered small/large intestinal toxicity (80%), followed by bladder toxicity (17%). Questions were asked regarding various determinants of the adjuvant treatment choice, including the molecular subtype, lymph node (LN) dissection, and age, and the results are summarized in Fig. 2A. The results of the GOG-122 trial [7], which compared the efficacy of whole abdominal RT and CTx for EC, affected the preference of GYNs, as it made them hesitate to administer postoperative RT rather than CTx (71%).
Fig. 2. The distribution of responses for specific questionnaire for (A) gynecologic surgeon and (B) radiation oncologist.
CT, computed tomography; GYN, gynecologic cancer surgeon; MMR, mismatch repair; MSI, microsatellite instable; NGS, next-generation sequencing; POLE, polymerase epsilon; RT, radiotherapy.
4. RO-specific questionnaire
For more detailed description of clinical practice in RO, sixteen questions were specifically posed to ROs, and the results are summarized in Fig. 2B. Intensity-modulated RT (IMRT) was the main RT modality (mean 69% of all institutions) used in 2019–2020, which may be closely associated with the approval of IMRT for gynecological cancer by government health insurance in July 2015. The vaginal cylinder type of brachytherapy applicator was more commonly used (67%) than the ovoid pair type. The frequency of brachytherapy ranged from 2 to 5 times per week, but twice per week was most common (73%). The most common brachytherapy fraction size and total dose were 500 cGy (47%) and 3,000 cGy (47%), respectively. After postoperative RT, 81% of the participants conducted a regular follow-up in both GYN and RO clinic.
DISCUSSION
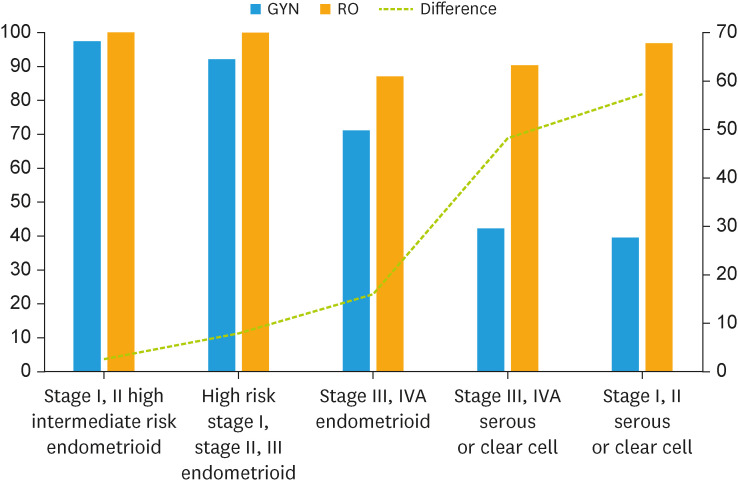
In this study, we observed different opinions between GYNs and ROs regarding postoperative management of EC. Overall, the interpretations and pattern of practice for early-stage EC with reference to the results of the PORTEC-II, GOG-249, and PORTEC-III trials were similar between the 2 expert groups; however, the main areas of disagreement were the interpretation of the GOG-258 results and managing stage III/IVA endometrioid EC, unfavorable histology, and sarcomas (Fig. 3). For such cases, GYNs showed a preference for administering adjuvant CTx alone, whereas ROs advocated for RT with or without combined CTx.
Fig. 3. Comparison of responses between gynecologic surgeon and radiation oncologist regarding the need of adjuvant radiotherapy for each risk groups and histologies of endometrial cancer.
GYN, gynecologic cancer surgeon; RO, radiation oncologist.
There may be several reasons for the differences in the opinions of GYNs and ROs. The GOG-258 trial demonstrated that CCRT and CTx improved locoregional control and reduced distant metastasis, respectively. ROs may base their opinions on the improved loco-regional control with RT, although it may not improve survival. GYNs may base their opinions on the overall survival rate and recommend systemic treatment that can be directly administered in their clinics. There is a fundamental gap in the understanding of GYNs and ROs regarding the impact of loco-regional recurrence. GYNs suggest that the reduced loco-regional recurrence with RT is less important than the reduced incidence of metastasis with CTx, because distant metastasis determines survival in a large proportion of patients. In contrast, ROs suggest that loco-regional recurrences cause severe symptoms with the potential to substantially compromise the patient’s quality of life. Furthermore, loco-regional recurrences have limited treatment options and may serve as sources for distant seeding of cancer cells.
Gynecological cancer specialists must be cautious when interpreting the results of large-scale clinical trials and translating them into clinical practice. When interpreting the results of PORTEC-II, GOG-249, and PORTEC-III trials, it is important to consider that the methods used for risk stratification and evaluation of LNs were different from the methods used in previous GOG studies. For example, lympho-vascular space invasion is the most important prognostic factor regarding regional recurrence [8,9,10,11]. Even when LNs were evaluated for risk factor and staging, it was based on LN examination varied from removal of suspicious nodes, nodal sampling to partial or complete nodal dissection. Accordingly, risk groups in previous studies had heterogeneous prognostic features that failed to distinguish between loco-regional and systemic relapses. Consistent with this heterogeneity, analysis of the National Cancer Database (NCDB) regarding patients with uterine cancer who were treated between 2004 and 2017 revealed wide variation in survival outcomes according to the tumor characteristics of patients who met the pathological criteria for enrollment in GOG-249, PORTEC-3, and GOG-258 trials (i.e., survival rates of 59.9%–81.7%, 40.2%–81.8%, and 17.5%–75%, respectively) [12]. These findings suggest that the 3 recently published clinical trials included patient cohorts with heterogeneous tumor characteristics. Specifically, among patients who met the criteria for enrollment in the GOG-258 trial, the 5-year overall survival of the 7,012 patients who received CTx alone was significantly lower than the 5-year overall survival of the 8,926 women who received chemoradiotherapy (57.8% and 72.7%, respectively). The NCDB real-world data suggest that the large-scale trials were underpowered and did not represent patients with important tumor characteristics; therefore, the results of the GOG-258 trial should be interpreted with caution.
Considering this background, there is a need to understand how the results of previous clinical trials have been applied to clinical practice. After the PORTEC-1 and GOG-99 trials were published in the early 2000s, Ko et al. [13] assessed changes in the pattern of practice regarding adjuvant RT for EC in the U.S. using the Surveillance, Epidemiology, and End Results database. They compared the use of RT pre- vs. post-publication of 2 sentinel studies. The authors concluded that the utility of adjuvant RT for early-stage EC had not changed despite the proven risk reduction by adjuvant RT that was revealed in the PORTEC-1 and GOG-99 trials [13]. Although the overall use of adjuvant RT has not changed, the use of VBT has gradually increased. Naumann et al. [14] reported a markedly decreased use of pelvic RT and increased use of VBT for early-stage EC between 1999 and 2005. This trend continued until recently, when Modh et al. [15] reported a significant overall increase in the use of VBT of 17.1% during 1995–2000 to 57.1% during 2007–2012 for early-stage EC, along with a proportional decrease in the use of EBRT from 54.0% to 25.5%. In 2019, the GOG-249 trial revealed opposite results; VBT did not demonstrate superior efficacy over EBRT in high–intermediate- to high-risk EC, even with greater pelvic and para-aortic recurrence rates. Therefore, patient selection for VBT in early-stage EC would be an important issue.
In the GOG-122 randomized trial, systemic CTx was advantageous over whole-abdomen RT for locally advanced disease, and thus it became part of standard adjuvant management [7]. As revealed in the present study, the majority of GYNs have been hesitant to proceed with adjuvant RT since the GOG-122 trial. However, many GYNs do not understand that a 2,000 cGy dose of whole abdominopelvic RT represents a suboptimal eradiating dose, as well as a low therapeutic ratio, resulting in high toxicity and low tumor control [16]. IMRT decreases radiation exposure to normal organs and significantly reduces the incidence of treatment-related toxicity compared with the conventional technique [17]. As shown in this study, IMRT has become a mainstay RT technique for EC patients in Korea. In addition, if pelvic RT is omitted, CTx has a limited effect in preventing locoregional recurrence, and the incidence of recurrence approaches 20% [7], resulting in distant progression. Similar results were reported by the GOG-258 trial [5]. Compared with pelvic RT, CTx alone consistently shows a higher incidence of severe toxicity, including mortality [5,7]. Therefore, the adjuvant treatment choice should be determined by understanding the different roles of RT and CTx and by considering treatment-related toxicity, which greatly affects patient quality of life. Notably, the role of adjuvant RT for serous and clear cell adenocarcinoma needs to be discussed between GYNs and ROs because the divergence in opinion between them was noticeable. A subgroup analysis of the PORTEC-3 trial showed that the local relapse rate in patients with serous carcinoma was as high as that in those with endometrioid adenocarcinoma [4]. A collaborative multi-institutional study is warranted to assess the efficacy of RT and CTx in these patient populations.
The results of phase-III randomized controlled trials can largely affect physicians’ pattern of practice, but these effects often occur gradually. Because no study has assessed the pattern of practice since 3 landmark studies (GOG-249, PORTEC-3, and GOG-258 trials), a survey of the up-to-date pattern of practice regarding adjuvant management for EC is timely and necessary. The first limitation of this study would be its relatively low response rate, particularly among GYNs; however, the response rate should be interpreted with caution. As described in Table 2, the number of institutions was high (i.e., >30 in each group) relative to the number of responders. Generally, more than 2 GYNs were working in an academic hospital; some responders responded to the survey as representatives of GYNs in their institution. Therefore, most responders were active members of KGOG, and the actual response rate would be much higher. The second limitation was that this study was based on a cross-sectional survey; no comparison of the use of RT pre- vs. post-publication of some recent clinical studies could be made. However, this limitation was compensated for by mentioning the relevant clinical trials for each question so that the respondents were aware of the clinical trial in question before responding. This guidance may or may not have influenced the responses; however, it was intended to allow the participants to respond in regard to a future patient. The third limitation was that, considering the outcomes of recent clinical trials, we did not pursue some important factors, including the location of the tumor within the uterus or the type and extent of LN dissection (sentinel LN sampling vs. LN dissection), which may be important factors determining the need for EBRT. Performing LN dissection is another important issue to be investigated [18].
Several points should be considered when interpreting the results of our study. In Korea, the overall utility of RT has continued to rise from 24.3% to 29.1% for all cancers [19]. Of the 90 hospitals operating a facility mainly for EBRT, 31 (34.4%) are equipped for brachytherapy [19]. This low rate of brachytherapy facilities is related to the extremely low medical fee for brachytherapy in Korea [20], whereas the high rate of IMRT since 2015 is related to the inclusion of National Health Insurance coverage in Korea. Changes in the medical and economic environments may influence physicians’ preferences; however, physicians must be conscious of what their counterparts (GYNs vs. ROs) achieve in terms of technical development. Surges in new drug investigations or multi-national clinical trials may also affect daily practice for gynecologic cancers in Korea. Nevertheless, opinions vary among nations. For instance, in a survey conducted among the members of the 4 East Asian GOGs in 2017, CTx was the most preferred treatment for locally advanced diseases in Japan, whereas CCRT was preferred in the other countries [21]. Overall, the pattern of practice results may need to be understood within the medical environment and physicians’ societies, which differ from country to country [22].
In conclusion, there are some discrepancies between GYNs and ROs concerning the interpretations and pattern of practice regarding recent clinical trials associated with postoperative management of EC. Although several large-scale clinical trials have been conducted, they lacked appropriate risk assessment; thus, their conclusions were (at best) provisional and should be adopted on a case-by-case basis, depending on local surgical and radiation oncology expertise. Continuous research efforts for optimal management and active communication among experts are essential in this field.
Footnotes
Funding: This study was supported by a National Cancer Center Grant (NCC 2210580-1) and 2020 cancer research support project from the Korea Foundation for Cancer Research (CB-2020-B-2). The funding source had no role in the study design, data curation, or analysis and interpretation of data.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: L.S.U., K.M.K., K.J.Y.
- Data curation: L.S.U., K.M.K.
- Formal analysis: L.S.U., K.J.Y.
- Investigation: L.S.U., K.M.K., K.Y.J., E.K.Y., W.C.W., K.J.Y.
- Methodology: L.S.U., K.M.K., K.Y.S., K.Y.J., E.K.Y., K.J.Y.
- Project administration: K.M.K.
- Supervision: K.Y.S., E.K.Y., K.J.Y.
- Validation: K.Y.J., E.K.Y., W.C.W.
- Visualization: L.S.U.
- Writing - original draft: L.S.U., K.J.Y.
- Writing - review & editing: K.M.K., K.Y.S., K.Y.J., E.K.Y., W.C.W., K.J.Y.
SUPPLEMENTARY MATERIALS
Survey questions distributed to the Korean Gynecologic Oncology Group-Korean Radiation Oncology Group members
Detailed patient information for specific case questions
References
- 1.MacNaughton WK. Review article: new insights into the pathogenesis of radiation-induced intestinal dysfunction. Aliment Pharmacol Ther. 2000;14:523–528. doi: 10.1046/j.1365-2036.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 2.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 3.Randall ME, Filiaci V, McMeekin DS, von Gruenigen V, Huang H, Yashar CM, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810–1818. doi: 10.1200/JCO.18.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273–1285. doi: 10.1016/S1470-2045(19)30395-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317–2326. doi: 10.1056/NEJMoa1813181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wortman BG, Creutzberg CL, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer. 2018;119:1067–1074. doi: 10.1038/s41416-018-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 8.Boothe D, Wolfson A, Christensen M, Francis S, Werner TL, Gaffney DK. Lymphovascular invasion in endometrial cancer: prognostic value and implications on adjuvant radiation therapy use. Am J Clin Oncol. 2019;42:549–554. doi: 10.1097/COC.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 9.Sadozye AH, Harrand RL, Reed NS. Lymphovascular space invasion as a risk factor in early endometrial cancer. Curr Oncol Rep. 2016;18:24. doi: 10.1007/s11912-016-0505-1. [DOI] [PubMed] [Google Scholar]
- 10.Raffone A, Travaglino A, Raimondo D, Neola D, Maletta M, Santoro A, et al. Lymphovascular space invasion in endometrial carcinoma: a prognostic factor independent from molecular signature. Gynecol Oncol. 2022;165:192–197. doi: 10.1016/j.ygyno.2022.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Stålberg K, Bjurberg M, Borgfeldt C, Carlson J, Dahm-Kähler P, Flöter-Rådestad A, et al. Lymphovascular space invasion as a predictive factor for lymph node metastases and survival in endometrioid endometrial cancer - a Swedish Gynecologic Cancer Group (SweGCG) study. Acta Oncol. 2019;58:1628–1633. doi: 10.1080/0284186X.2019.1643036. [DOI] [PubMed] [Google Scholar]
- 12.Boone RM, Praiss AM, Huang Y, Melamed A, Khoury-Collado F, Hou JY, et al. Heterogeneity of outcomes of endometrial cancer patients included in prospective clinical trials. Gynecol Oncol. 2023;169:70–77. doi: 10.1016/j.ygyno.2022.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Ko EM, Funk MJ, Clark LH, Brewster WR. Did GOG99 and PORTEC1 change clinical practice in the United States? Gynecol Oncol. 2013;129:12–17. doi: 10.1016/j.ygyno.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Naumann RW, Coleman RL. The use of adjuvant radiation therapy in early endometrial cancer by members of the Society of Gynecologic Oncologists in 2005. Gynecol Oncol. 2007;105:7–12. doi: 10.1016/j.ygyno.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Modh A, Ghanem AI, Burmeister C, Rasool N, Elshaikh MA. Trends in the utilization of adjuvant vaginal brachytherapy in women with early-stage endometrial carcinoma: results of an updated period analysis of SEER data. Brachytherapy. 2016;15:554–561. doi: 10.1016/j.brachy.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Shah PH, Kudrimoti M, Feddock J, Randall M. Adjuvant treatment for stage IIIC endometrial cancer: options and controversies. Gynecol Oncol. 2011;122:675–683. doi: 10.1016/j.ygyno.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Onal C, Yuce Sari S, Yavas G, Oymak E, Birgi SD, Yigit E, et al. Outcome and safety analysis of endometrial cancer patients treated with postoperative 3D-conformal radiotherapy or intensity modulated radiotherapy. Acta Oncol. 2021;60:1154–1160. doi: 10.1080/0284186X.2021.1926537. [DOI] [PubMed] [Google Scholar]
- 18.Parker JE, Miller DS, Lee J, Carlson M, Lococo S, Lea JS. Current practice patterns in nodal evaluation and adjuvant treatment of advanced stage endometrioid endometrial cancer: an SGO survey. Gynecol Oncol Rep. 2020;34:100620. doi: 10.1016/j.gore.2020.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim E, Jang WI, Kim MS, Paik EK, Kim HJ, Yoo HJ, et al. Clinical utilization of radiation therapy in Korea, 2016. J Radiat Res. 2020;61:249–256. doi: 10.1093/jrr/rrz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JY, Lee KE, Kim K, Lee MA, Yoon WS, Han DS, et al. Choosing wisely: the Korean perspective and launch of the ‘right decision in cancer care’ initiative. Cancer Res Treat. 2020;52:655–660. doi: 10.4143/crt.2020.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Kim JW, Lee TS, Zang R, Chen X, Yang J, et al. Difference in practice patterns in the management of endometrial cancer: a survey of the members of 4 East Asian Gynecologic Oncology Groups. Int J Gynecol Cancer. 2017;27:1888–1894. doi: 10.1097/IGC.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 22.Huh SJ, Nishimura T, Park W, Onishi H, Ahn YC, Nakamura K. Current status and comparison of national health insurance systems for advanced radiation technologies in Korea and Japan. Radiat Oncol J. 2020;38:170–175. doi: 10.3857/roj.2020.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey questions distributed to the Korean Gynecologic Oncology Group-Korean Radiation Oncology Group members
Detailed patient information for specific case questions