Abstract
Objective
To examine the effectiveness of progestin re-treatment for recurrent endometrial intraepithelial neoplasia (EIN), atypical endometrial hyperplasia (AH) and endometrial cancer (EC) following initial fertility-sparing treatment.
Methods
A comprehensive systematic review and meta-analysis were conducted by an Expert Panel of the Japan Society of Gynecologic Oncology Endometrial Cancer Committee. Multiple search engines, including PubMed/MEDLINE and the Cochrane Database, were searched in December 2021 using the keywords “Endometrial neoplasms,” “Endometrial hyperplasia,” “Endometrial intraepithelial neoplasia,” “Fertility preservation,” “Progestins,” AND “Recurrence.” Cases describing progestin re-treatment for recurrent EIN, AH and EC were compared with cases that underwent conventional hysterectomy. The primary outcomes were survival and disease recurrence, and the secondary outcome was pregnancy.
Results
After screening 238 studies, 32 with results for recurrent treatment were identified. These studies included 365 patients (270 received progestin re-treatment and 95 underwent hysterectomy). Most progestin re-treatment involved medroxyprogesterone acetate or megestrol acetate (94.5%). Complete remission (CR) following progestin re-treatment was achieved in 219 (81.1%) cases, with 3-, 6- and 9-month cumulative CR rates of 22.8%, 51.7% and 82.6%, respectively. Progestin re-treatment was associated with higher risk of disease recurrence than conventional hysterectomy was (odds ratio [OR]=6.78; 95% confidence interval [CI]=1.99–23.10), and one patient (0.4%) died of disease. Fifty-one (14.0%) women became pregnant after recurrence, and progestin re-treatment demonstrated a possibility of pregnancy (OR=2.48; 95% CI=0.94–6.58).
Conclusion
This meta-analysis suggests that repeat progestin therapy is an effective option for women with recurrent EIN, AH and EC, who wish to retain their fertility.
Keywords: Endometrial Neoplasm, Endometrial Hyperplasia, Fertility Preservation, Progestins, Recurrence
Synopsis
Although conservative treatment was effective for recurrent disease after complete remission (CR), re-recurrence rates were high. Long-term follow-up and close surveillance are required after achieving CR. Conservative treatment may be an option in women with recurrence who desire fertility preservation.
Graphical Abstract
INTRODUCTION
Endometrial cancer (EC) is the most commonly diagnosed gynecological malignancy in developed countries, with an increasing incidence in recent decades and an estimated incidence in Japan of 17,089 in 2018 [1,2,3]. Although EC is typically diagnosed in postmenopausal women, 3%–14% of cases occur in women younger than 40 years. The overall incidence of EC has recently increased by 2.9% per year in Japan; most rapidly in the under-40s, many of whom are nulliparous and thus have a strong desire to retain their fertility. Among these cases, 86.4% were classified as stage I, with 92.1% 5-year overall survival in Japan [3]. Considering the recent rise in the incidence of EC in women who desire to preserve their fertility and who have a relatively good prognosis, it is imperative to provide effective fertility-sparing treatment options.
Several studies have reported promising results for progestin-based conservative treatment instead of conventional hysterectomy in patients with early-stage grade 1 EC (ECG1) or atypical endometrial hyperplasia (AH) [4]. Progestin-based conservative treatments, such as medroxyprogesterone acetate (MPA), megestrol acetate (MA), gonadotropin-releasing hormone (GnRH), or more recently, a levonorgestrel-releasing intrauterine system (LNG-IUS), are widely accepted as the main fertility-sparing treatments. Although progestin-based conservative treatments can achieve a high response rate of 76%–81%, they carry inherent risks, including a lack of efficacy, risk of relapse, and missed diagnosis of ovarian or lymph node involvement. Most patients who experience recurrence after progestin-based conservative treatment undergo definitive surgical management, including hysterectomy. However, given that patients may still have a strong desire for fertility preservation despite recurrence, repeat progestin-based conservative treatment may be needed.
Few comprehensive meta-analyses have investigated the use of progestin therapy in patients with EC who wish to preserve their fertility, especially among patients with persistent or recurrent disease following initial fertility-sparing treatment. We therefore conducted a systematic review and meta-analysis to evaluate the suitability of repeat progestin-based conservative treatment in women with recurrent endometrial intraepithelial neoplasia (EIN), AH or EC after achieving complete remission (CR).
MATERIALS AND METHODS
1. Source and study selection
We carried out a literature search of the PubMed/MEDLINE and Cochrane databases on 1 December 2021, according to the MOOSE guidelines for systematic reviews, using the entry keywords “Endometrial neoplasms,” “Endometrial Hyperplasia,” “endometrial intraepithelial neoplasia,” “Fertility-preservation,” “Progestins,” and “Recurrence.” The eligibility criteria were patients with initial fertility-sparing treatment for EIN, AH and ECG1 who experienced recurrent disease after achieving CR.
The eligibility criteria were articles published in English, with adequate descriptions of patient demographics, types of initial treatment and re-treatment, treatment responses, survival outcomes, and follow-up. Articles were first extracted from meta-analyses and randomized controlled trials with high-quality evidence; however, few articles had high-quality evidence according to conventional criteria, and retrospective interventional studies and observational studies with large numbers of cases were therefore selected. The references in each article were then reviewed and articles considered to be clinically important that met the inclusion criteria were also added. Finally, any article that met the inclusion criteria was assessed. Articles with a lack of information on re-treatment outcomes, patients with non-recurrent EIN, AH and ECG1, and basic research were excluded.
2. Clinical information
The following variables were abstracted from the case descriptions for eligible articles: year and country of publication, patient demographics at the time of cancer diagnosis, including age at diagnosis, parity, body mass index (BMI), personal history of other malignancy, initial disease (EIN, AH or ECG1), and initial treatment type (MA, MPA, LNG-IUS, or other), time from CR to recurrence, recurrent disease (EIN, AH, ECG1, or progressive disease status), fertility-sparing retreatment (MA/MPA, or other), response to re-treatment (CR or persistent disease [PD]), pregnancy followed by hysterectomy or after fertility-sparing retreatment, and final follow-up duration after the completion of re-treatment and disease status.
3. Definitions
Treatment outcome was classified as follows: CR, no residual disease in post-treatment biopsy; PD, residual, stable, or PD in post-treatment biopsy; and progressive disease, defined as worsening historical grade or increased FIGO stage. Retreatment failure was defined as residual disease or progressive disease. Disease progression at the time of recurrence was defined as grade 2 EC (ECG2) and EC with more than FIGO stage IB disease. Treatment outcome was determined at the completion of re-treatment. The duration of progestin therapy was abstracted from the records and defined as the treatment duration. Information on the presence or absence of disease recurrence was abstracted for cases with a follow-up duration.
4. Statistical analysis
The primary aim of the analysis was to compare the mortality and recurrence rates between patients re-treated with fertility-sparing surgery and conventional hysterectomy for recurrent AH and ECG1. The secondary aims were to examine the cumulative response rate of fertility-sparing re-treatment and the pregnancy rate.
Continuous variables were assessed for normality by the Kolmogorov–Smirnov test and expressed as mean (±standard deviation) or median (interquartile range [IQR]). The statistical significance of continuous variables was determined by Student’s t-test or Mann–Whitney U test, as appropriate. Categorical or ordinal variables were assessed by χ2 or Fisher’s exact test, as appropriate, and expressed as odds ratio (OR) with 95% confidence interval (CI). Survival analysis with log-rank test for univariate analysis was performed, and the statistical significance was expressed with the hazard ratio (HR) and 95% CI. Cumulative response rates after fertility-sparing re-treatment were estimated at 3, 6 and 9 months after treatment, respectively. All statistical tests were two-tailed, and a p value <0.05 was considered statistically significant. Analyses were carried out using the Statistical Package for the Social Sciences, version 28.0 (SPSS Inc., Chicago, IL, USA).
5. Data extraction and management
Data were entered into a reference database and extracted independently by three reviewers who were blinded to each other’s reviews (I.M., H.M., and personnel from the Japan Medical Library Association). The quality of the studies was assessed independently by two reviewers (I.M. and H.M.), and any disagreements were resolved via discussion with a third reviewer from the Expert Panel of the Japan Society of Gynecologic Oncology Endometrial Cancer Committee. If any data were missing or the methods were unclear, further information was obtained from other published literature on the same trial or by direct inquiry to the authors. Data that were still missing were excluded from the analysis. For each study, we recorded the detailed methods, study population and sample size, inclusion and exclusion criteria, interventions and comparisons, patient demographics, and pregnancy and survival outcome.
6. Assessment of risk of bias
Because there were no randomized controlled trials evaluating survival outcomes between fertility-sparing re-treatment and conventional hysterectomy for recurrent AH and ECG1, we analyzed interventional or observational studies that evaluated initial fertility-sparing treatments and which described disease recurrence and its re-treatment, as surrogate measures. Using the Risk of Bias in Non-randomized Studies for Interventions (ROBINS-I) tool [5], two authors (I.M. and H.M) assessed the risk of bias independently for each study, including selection, preference, detection, attribution, reporting, and other possible types of bias (Table S1). Because it was not possible to blind either the participants or physicians to the assigned treatment, blinding (performance and detection bias) was only assessed for outcomes. Publication bias was assessed by funnel plot analysis.
7. Assessment of heterogeneity
OR was used as a measure of association across studies and a Mantel–Haenszel random-effects model was used to calculate the summary estimates [6]. The heterogeneity of each study was assessed by visual inspection of forest plots and by statistical evaluation using Cochran’s Q test and the I2 test. Data from the studies were synthesized to obtain overall estimates of treatment effects using RevMan software (version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The level of confidence in the summary data was examined using the Grading of Recommendations Assessment Development and Evaluation (GARDE) for studies of interventions and diagnostic test accuracy.
RESULTS
The literature search identified 238 articles published during the study period (Fig. 1). Among these, 208 articles were excluded because they were reports of ongoing trials without survival outcomes, lacked recurrence or re-treatment details, reported non-target diseases, or were non-English articles. The remaining 30 articles met the criteria for further assessment, and a full-content review of these articles was performed. A further 12 articles were added from the reference lists. Thirty-two articles, comprising 371 patients with recurrent AH and ECG1, finally met the inclusion criteria for this review [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Among these, six cases were excluded because of grade 2–3 histology. Thirty-two articles reporting data for a total of 365 women, including 270 (74.0%) who received fertility-sparing retreatment and 95 (26.0%) who received conventional hysterectomy, were thus included in the final analysis (Table 1).
Fig. 1. Flow diagram of study selection for systematic review.
Table 1. Characteristics of eligible studies.
| Author, year, initial No. patients | Study type | Country | Study population | ||||
|---|---|---|---|---|---|---|---|
| Recurrent No. patients | Hysterectomy | Progestin re-treatment | Progestin treatment type | Follow-up (mo) | |||
| Song et al., 2022 (n=37) [7] | Retrospective | China | 10 | 1 | 9 | MPA 250–500, MA 160–320 | 51 (5–168) |
| He et al., 2020 (n=110) [8] | Retrospective | China | 25 | 0 | 25 | MPA 250 | 61 (3–104) |
| Giampaolino et al., 2019 (n=69) [9] | Retrospective | Italy | 4 | 1 | 3 | LNG-IUS | na |
| Wang et al., 2019 (n=161) [38] | Retrospective | China | 8 | 14 | 23 | MPA/MA/GnRH+LNG-IUS or Letrozole | 33 (5–160) |
| Yamagami et al., 2018 (n=162) [10] | Retrospective | Japan | 82 | 0 | 82 | MAP 400–600 | 71 (5–209) |
| Laurelli et al., 2016 (n=21) [11] | Prospective | Italy | 2 | 1 | 1 | HR+LNG-IUS | 40 (13–79) |
| Mitsuhashi et al., 2016 (n=36) [12] | Prospective | Japan | 8 | 2 | 6 | MPA 400 | 38 (9–66) |
| Baek et al., 2016 (n=31) [13] | Retrospective | Korea | 6 | 2 | 4 | MPA 40–120, MA 80–160 | 12 (3–29) |
| Ohyagi-Hara et al., 2015 (n=27) [14] | Retrospective | Japan | 9 | 6 | 3 | MPA 400–600 | 39 (3–154) |
| De Marzi et al., 2015 (n=23) [15] | Retrospective | Italy | 1 | 0 | 1 | MA 160 | 25 (8–37) |
| Wang et al., 2014 (n=37) [16] | Retrospective | Taiwan | 15 | 7 | 8 | MA 160 | 79 (19–253) |
| Shan et al., 2013 (n=21) [17] | Prospective | China | 6 | 3 | 3 | MA 160 | 32 (15–66) |
| Kim et al., 2013 (n=16) [18] | Prospective | Korea | 2 | 2 | 0 | 31 (16–50) | |
| Park et al., 2013 (n=141) [19] | Retrospective | Korea | 45 | 12 | 33 | MPA 80–500, MA 80–160 | 52 (24–160) |
| Park et al., 2012 (n=14) [20] | Retrospective | Korea | 3 | 0 | 3 | 36 (18–135) | |
| Koskas et al., 2012 (n=22) [21] | Retrospective | France | 3 | 3 | 0 | MPA 10, MA 160 | 39 (14–86) |
| Dursun et al., 2012 (n=43) [22] | Retrospective | Turkey | 2 | 2 | 0 | 49 (5–156) | |
| Fujiwara et al., 2012 (n=59) [23] | Retrospective | Japan | 22 | 2 | 20 | MPA 400–600 | 66 (11–251) |
| Laurelli et al., 2011 (n=14) [24] | Prospective | Italy | 1 | 1 | 0 | 40 (13–79) | |
| Minig et al., 2011 (n=34) [25] | Prospective | Spain | 6 | 5 | 1 | HR+LNG-IUS | 29 (4–102) |
| Perri et al., 2011 (n=27) [26] | Retrospective | Canada | 15 | 4 | 11 | MPA 600, MA 160–320 | 5 (1–17) |
| Eftekhar et al., 2009 (n=21) [27] | Prospective | Iran | 3 | 0 | 3 | MA 160 | 48 (18–84) |
| Hahn et al., 2009 (n=35) [28] | Retrospective | Korea | 9 | 5 | 4 | MPA 250–1,000 mg, MA 160 mg | 39 (5–108) |
| Ushijima et al., 2007 (n=45) [29] | Prospective | Japan | 14 | 5 | 9 | MPA 600 | 48 (25–73) |
| Yamazawa et al., 2007 (n=9) [30] | Prospective | Japan | 2 | 2 | 0 | 36 (24–69) | |
| Minaguchi et al., 2007 (n=30) [31] | Retrospective | Japan | 9 | 4 | 5 | MPA 600 | 41 (2–109) |
| Niwa et al., 2005 (n=12) [32] | Prospective | Japan | 8 | 4 | 4 | MPA | 49 (33–63) |
| Ota et al., 2005 (n=12) [33] | Retrospective | Japan | 2 | 1 | 1 | MPA 600 | 44 (13–127) |
| Yang et al., 2005 (n=6) [34] | Retrospective | Taiwan | 2 | 2 | 0 | 38 (14–132) | |
| Gotlieb et al., 2003 (n=13) [35] | Retrospective | Israel | 5 | 2 | 3 | MA 160 | 82 (6–358) |
| Wang et al., 2002 (n=9) [36] | Prospective | Taiwan | 3 | 1 | 2 | MA 160 | 69 (25–113) |
| Kaku et al., 2001 (n=39) [37] | Retrospective | Japan | 4 | 1 | 3 | MPA 400–600 | 32 (10–133) |
Doses given as mg/day, unless otherwise indicated.
MA, megestrol acetate; MPA, medroxyprogesterone acetate; GnRH, gonadotropin-releasing hormone; HR, hysteroscopic resection; LNG-IUS, levonorgestrel-releasing intrauterine system.
The demographic profiles of the articles and patients are shown in Table 2 and Table S2. Most articles were published in 2011 or later (83.3%) (p=0.011 in atypical hyperplasia/EIN, p<0.006 in endometrial adenocarcinoma, respectively) and were carried out in Asia (88.5%, p=0.002 in endometrial adenocarcinoma). The median patient age was 33.0 (IQR: 29.0–37.0) years and approximately three-quarters of patients had ECG1 disease at initial diagnosis (80.3%). Most patients were nulliparous (45.8%, p<0.001 in endometrial adenocarcinoma) and had recurrent ECG1 disease (68.8%). The main initial progestin-based conservative treatment was MPA (n=234, 62.4%), followed by MA (n=62, 18.0%), MPA or MA (n=49, 14.1%), and LNG-IUS (n=15, 4.1%). The median time from initial CR to recurrence was 13.0 (IQR: 7.5–23.0) months (p=0.183 in atypical hyperplasia/EIN, p<0.003 in endometrial adenocarcinoma, respectively). There was a significant difference in recurrence-free period between primary progestin treatment and recurrence in re-treatment/hysterectomy groups (median 12.2 versus 20.0 months, p<0.001). The main repeated progestin-based conservative treatment was progestin therapy (93.7%), including MPA (n=193, 76.3%), MA (n=24, 9.5%), and MPA or MA (n=26, 10.4%). There was no significant difference in prognosis depending on the re-treatment type (MA, MPA or LNG-IUS) in the recurrent setting (p=0.12). A total of 208 patients (77.0%) underwent primary and recurrent treatment with similar progestin-based conservative treatment regimens.
Table 2. Patient demographics in fertility-sparing retreatment and conventional hysterectomy groups for recurrent disease.
| Variables | Total | Recurrent treatment | p-value | |||
|---|---|---|---|---|---|---|
| Hysterectomy | Progestin Tx | |||||
| Total | 365 (100) | 95 (26.0) | 270 (74.0) | |||
| Year of publication | <0.001 | |||||
| ≤2010 | 61 (16.7) | 27 (28.4) | 34 (12.6) | |||
| >2011 | 304 (83.3) | 68 (71.6) | 236 (87.4) | |||
| Region of publication | <0.001 | |||||
| Asia | 323 (88.5) | 76 (80.0) | 247 (91.5) | |||
| Europe | 17 (4.7) | 11 (11.6) | 6 (2.2) | |||
| Other | 25 (6.8) | 8 (8.4) | 17 (6.3) | |||
| Initial disease | 0.043 | |||||
| Atypical hyperplasia/EIN | 72 (19.7) | 12 (12.6) | 60 (22.2) | |||
| Endometrial adenocarcinoma G1 | 293 (80.3) | 83 (87.4) | 210 (77.8) | |||
| Age (yr)* | 33.0 (29.0–37.0) | 35.0 (32.5–37.5) | 32.0 (29.0–36.0) | 0.007 | ||
| <30 | 55 (15.1) | 5 (5.3) | 50 (18.5) | |||
| 30–39 | 145 (39.7) | 36 (37.9) | 109 (40.4) | |||
| ≥40 | 13 (3.6) | 4 (4.2) | 9 (3.3) | |||
| Missing | 152 (41.6) | 50 (52.6) | 102 (37.8) | |||
| Parity* | <0.001 | |||||
| Nullipara | 167 (45.8) | 20 (21.1) | 147 (54.4) | |||
| Multipara | 6 (1.4) | 1 (1.1) | 5 (1.9) | |||
| Missing | 192 (52.6) | 74 (77.9) | 118 (43.7) | |||
| BMI* | 22.7 (19.5–23.8) | 24.0 (19.5–26.0) | 22.7 (19.4–26.7) | <0.001 | ||
| <25 | 99 (27.1) | 11 (11.6) | 88 (32.6) | |||
| 25–29 (overweight) | 33 (9.0) | 5 (6.3) | 28 (10.4) | |||
| ≥30 (obese) | 24 (6.6) | 3 (3.2) | 21 (7.8) | |||
| Missing | 209 (57.3) | 76 (80.0) | 133 (49.3) | |||
| Initial progestin treatment | 0.010 | |||||
| MPA/MA | 345 (94.5) | 84 (88.4) | 261(96.7) | |||
| LNG-IUS | 15 (4.1) | 8 (8.4) | 7 (2.6) | |||
| Other | 5 (1.4) | 3 (3.2) | 2 (0.7) | |||
| Time to recurrence from CR (mo) | 13.0 (7.5–23.0) | 20 (10.0–39.0) | 12.2 (6.9–21.2) | <0.001 | ||
| Recurrent disease | <0.001 | |||||
| Atypical hyperplasia/EIN | 114 (31.2) | 13 (13.7) | 101 (37.4) | |||
| Endometrial adenocarcinoma | 251 (68.8) | 82 (86.3) | 169 (62.6) | |||
| Disease progress at the time of recurrent† | 24 (6.6) | 13 (13.7) | 11 (4.1) | 0.001 | ||
| Progestin re-treatment | ||||||
| MPA/MA | - | 253 (93.7) | ||||
| Other | - | 17 (6.3) | ||||
| Regimen change‡ | - | 62 (23.0) | ||||
| Treatment outcome | <0.001 | |||||
| Complete remission | 301 (82.5) | 82 (98.8) | 219 (81.1) | |||
| Residual disease | 40 (11.0) | 1 (1.2) | 39 (14.4) | |||
| Unknown | 24 (6.6) | 12 (12.6) | 12 (4.4) | |||
| Final disease status | <0.001 | |||||
| Disease death | 1 (0.3) | 0 | 1 (0.4) | |||
| Disease recurrence | 100 (27.4) | 4 (4.2) | 96 (35.6) | |||
| Progressed/stable disease | 34 (9.3) | 0 | 34 (12.6) | |||
| Pregnancy | 51 (14.0) | 2 (2.1) | 49 (18.1) | <0.001 | ||
Data are shown as median (range) or number (%).
CR, complete remission; EIN, endometrioid intraepithelial neoplasia; MA, megestrol acetate; MPA, medroxyprogesterone acetate; LNG-IUS, levonorgestrel-releasing intrauterine system.
*At initial treatment; †Disease progress at the time of recurrent including endometrial adenocarcinoma G2 and endometrial cancer with more than stage IB disease; ‡Regimen change including the alteration of progestin type (MPA, MA, IUS, and other progesterone) or dose change.
Compared with the conventional hysterectomy group, patients who received repeated progestin-based conservative treatment were reported significantly more recently (87.4% versus 71.6%), were more likely to be reported from Asia (91.5% versus 80.0%), and were more likely to be younger at diagnosis (32.0 versus 35.0 years), nulliparous (54.4% versus 21.1%), have an appropriate BMI (32.6% versus 11.6%), have received initial MPA or MA treatment (96.7% versus 88.4%), and to have AH recurrence (37.4% versus 13.7%), respectively (all p<0.05). The rates of initial disease were similar in the repeated progestin-based conservative treatment and conventional hysterectomy groups (ECG1; 75.5% versus 85.2%, respectively; p=0.07), whereas disease at recurrence related to progressed disease was associated with the likelihood of undergoing conventional hysterectomy (13.7% versus 4.1%, p<0.001). The rate of disease progression at the time of recurrence was higher in women with EC compared with those with AH/EIN. CR rate was lower in women with recurrent EC treated with repeated progestin-based conservative treatment, compared with women with recurrent AH/EIN.
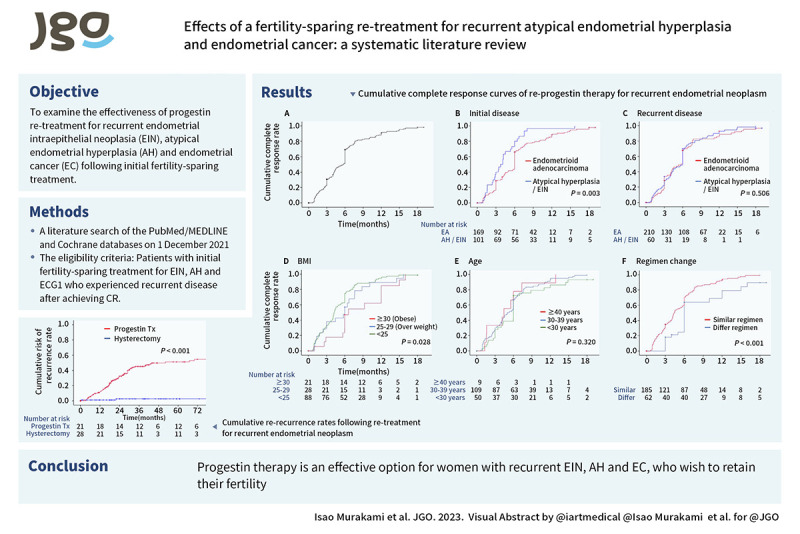
Time-dependent analysis of progestin re-treatment and cumulative complete response curves for patients in the repeated progestin-based conservative treatment group (n=253) are shown in Fig. 2A–F. The median re-treatment duration was 6.0 (IQR: 4.3–7.9) months. CR was achieved in 219 (85.3%) cases with progestin re-treatment, with 3-, 6-, and 9-month cumulative CR rates of 22.8%, 51.7%, and 82.6%, respectively. CR rate of initial histology at EC was significantly lower than that of initial histology at AH/EIN at progestin re-treatment (p=0.003). The cumulative CR rate did not differ significantly in relation to recurrent disease (6-month CR rates AH, ECG1, and PD: 50.9%, 53.7%, and 46.2%, respectively, p=0.506) or age (<30, 30–39, and ≥40 years: 42.0%, 52.7%, and 62.5%, respectively, p=0.320). Obesity at initial treatment (BMI ≥30) was associated with a significantly lower CR rate than the other groups (BMI <25, 25–29, and ≥30: 58.6%, 42.9%, and 18.5%, respectively, p=0.028). Regimen change at repeated progestin-based conservative treatment was also associated with a significantly lower CR rate (different versus same regimen: 45.4% versus 78.3%, respectively, p<0.001) but recurrent disease was similar among those groups (ECG1: 59.6% versus 53.2%, PD: 7.7% versus 10.5%, respectively, p=0.506). Among the 270 cases that received repeated progestin-based conservative treatment, initial histology of endometrial adenocarcinoma, BMI ≥ 30, and regimen change for treatment of recurrence were significantly associated with lower remission rate after progestin re-treatment (CR rates at 6 months: initial endometrial adenocarcinoma 33.4%; BMI ≥30, 29.0%; and regimen change, 28.2%; p<0.05). On multivariable analysis, regimen change remained a risk factor for progestin re-treatment failure (HR=4.22; 95% CI=1.59–11.19; p=0.004) (Table S3).
Fig. 2. Cumulative complete response curves of re-progestin therapy for recurrent endometrial neoplasm. Log-rank test for p-values. Cumulative complete remission curves for progestin retreatment were constructed for: whole cohort (panel A), initial disease (panel B), recurrent disease (panel C), BMI (panel D), Age (panel E), and regimen change at recurrent treatment (panel F).
AH, atypical endometrial hyperplasia; BMI, body mass index; EA, endometrial adenocarcinoma; EIN, endometrial intraepithelial neoplasia.
Ninety-six of 270 women who received repeated progestin-based conservative treatment had re-recurrence. Eighty-seven (91%) women had intrauterine recurrence and nine (9%) women had ovarian recurrence. Four of 270 women in the conventional hysterectomy group had re-recurrence. All four women had recurrence in ovary. The cumulative re-recurrence rates following re-treatment for recurrent AH and ECG1 are shown in Fig. 3 and Fig. S1. The median time to re-recurrence was 18.8 (IQR: 12–29.0) months after CR following the initial treatment. One hundred (27.4%) women had subsequent re-recurrence during the study period and 34 (9.3%) women had stable disease or progressive disease following re-treatment at recurrence. Univariate analysis showed that the recurrence rate was higher in women treated with repeated progestin-based conservative treatment compared with those treated with conventional hysterectomy (HR=18.6; 95% CI=4.56–75.76; 5-year rate: 54.6% versus 1.9%, respectively). The re-recurrence rate was higher in women with recurrent AH/EIN and EC treated with repeated progestin-based conservative treatment compared with those treated with conventional hysterectomy. The results of meta-analysis of the data from seven articles are shown in Fig. 4. Patients in the repeated progestin-based conservative treatment group were significantly more likely to experience recurrence compared with the conventional hysterectomy group (OR=6.78; 95% CI=1.99–23.10; p<0.001).
Fig. 3. Cumulative re-recurrence rates following re-treatment for recurrent endometrial neoplasm. Endometrial neoplasm including atypical hyperplasia/endometrioid intraepithelial neoplasia or endometrial adenocarcinoma.
Fig. 4. Forest plot comparing re-recurrence rates between re-progestin therapy and conventional hysterectomy groups with recurrent atypical endometrial hyperplasia or endometrial cancer.
CI, confidence interval.
We also analyzed the re-treatment and pregnancy rates during the study period in nine articles. Two women in the conventional hysterectomy group underwent hysterectomy after delivery because they were aged >40 years [31]. The frequency of pregnancy following re-treatment was significantly higher in the fertility-sparing re-treatment group compared with the conventional hysterectomy group (pregnancy rate: 19.1% versus 2.5%, respectively), but there was no significant difference in pregnancy rates between the two groups with recurrent AH or EC (OR=2.61; 95% CI=0.97–7.01; p=0.062) (Fig. S2).
DISCUSSION
1. Principal findings
The present systematic review and meta-analysis investigated the efficacy and safety of repeated progestin-based conservative treatment for women with recurrence of AH or EC who wish to preserve their fertility. The results showed that 85.3% of women who received repeated progestin-based conservative treatment achieved CR, with a median remission time of 6.0 months. Repeated progestin-based conservative treatment is thought to be effective for treating recurrent disease after achieving CR, similar to first-round fertility-sparing treatment. However, the incidence of repeat recurrence was approximately 20-fold higher in the fertility-sparing re-treatment compared with the conventional hysterectomy group.
2. Results and clinical implications
Repeated progestin-based conservative treatment may be acceptable for recurrent disease. However, this option is associated with potential problems, including disease progression during treatment, synchronous ovarian cancer, and a high rate of re-recurrence. Moreover, the number of patients with early-stage AH or EC treated with initial or repeated progestin-based conservative treatment is expected to increase. Long-term follow-up and close surveillance are warranted to investigate the effects of this regimen.
However, the duration of re-treatment following CR and specific progestin-based re-treatment regimen have varied among studies to date, and the conclusions thus remain unclear. Various regimens have been proposed as alternative options for recurrent disease, including MA, MPA, LNG-IUS, metformin, and GnRH agonists [38,39,40,41,42]. Moreover, several studies have reported improved therapeutic effects following prolonged treatment [43]. Interestingly, the present systematic review and meta-analysis found that using the same regimen for re-treatment as for the initial progestin treatment could be more effective than changing to a different regimen. Furthermore, 94 (28.7%) patients who received repeated progestin-based conservative treatment re-relapsed, with a median remission time of 18.8 months. A previous study reported that the recurrence rate following repeated progestin-based conservative treatment was significantly higher than that following initial progestin-based conservative treatment (54% and 76%, respectively) [44]. These results were probably related to the efficacy of progestin to the individual tumor. Minaguchi et al. have reported that presence of PTEN-null cells and weak phosphor-Akt expression in pre-treatment specimens were associated with no response to progestin-based treatment or relapse after progestin-based treatment, could represent the consequence of biological sensitivity of tumor to the progestin-based treatment [31]. A stricter follow-up regimen, such as pelvic magnetic resonance imaging, chest–pelvic computed tomography (or positron emission tomography–computed tomography), or total endometrial curettage, should therefore be mandatory after achieving CR. Further investigations with different regimens and additional treatment and follow-up periods are warranted.
Repeated progestin treatment may be associated with disease progression during treatment. Extrauterine disease, such as pelvic lymph node involvement, has been reported after progestin-based conservative treatment [37,45]. Moreover, synchronous ovarian cancer is also a concern in young women treated with repeated progestin-based conservative treatment. A prior study reported that 11% of patients aged ≤45 years had synchronous ovarian cancer, compared with only 2% of those aged >45 years [46]. However, another study including a large multicenter analysis by the Korean Society of Gynecologic Oncology found that, although 4.5% of young patients with EC had synchronous ovarian cancer, it was not identified in patients with low-risk EC [47]. These reports suggest that repeated progestin-based conservative treatment could increase the risks of progression and synchronous ovarian cancer, and careful diagnosis and counselling should thus be discussed with the patient when re-starting treatment. A laparoscopic procedure to rule out extrauterine disease, including adnexal exploration, might be helpful for pretreatment evaluation [48].
Consistent with past findings, a BMI ≥30 kg/m2 was significantly associated with a higher risk of failure to achieve CR in the present study [44,49,50]. Park et al. [51] proposed that being overweight was an important predictor of a poor response and failure to achieve CR. Weight reduction should therefore be recommended in obese women, such as those with a BMI ≥30 kg/m2, in terms of both their general health and fertility-preserving outcomes.
3. Strengths and limitations
The main strength of this study was its design: as a systematic review and meta-analysis, the data obtained and analyzed were more reliable than those obtained from any individual investigation. To the best of our knowledge, this is the first systematic review and meta-analysis of the repeated use of progestin-based conservative treatment. However, the study also had a few limitations. First, there were no phase III trials comparing repeated progestin-based conservative treatment with standard treatment as a control arm. Therefore, this study surrogated and analyzed the individual-level data from each study. There were no data regarding the side effects of progestin re-treatment because the data were retrospective. Second, in several cases, it was not possible to collect all data that we analyze, as we recruited several studies. In addition, despite their methodological quality, the recruited studies differed in their data collection, design, methodology, and included populations. Finally, these data were based on mixed population. Women in our study received different regimens and dose of progestin even initial or recurrent treatment. Those may influence the frequency of the recurrence of disease.
In conclusion, repeated progestin-based conservative treatment may be effective in patients with recurrent AH and EC after achieving CR, but re-recurrence rates are high post-treatment. However, repeated progestin-based conservative treatment may thus be an option for women who wish to preserve their fertility after recurrence.
ACKNOWLEDGEMENTS
This project was assisted by an Expert Panel of the Japan Society of Gynecologic Oncology, via the Medical Information Network Distribution Service, which received financial support from the Ministry of Health, Labour and Welfare of Japan as a consignment project. Additionally, the authors thank Sho Sasaki and Toshio Morizane at Minds, the Japan Council for Quality Health Care for guidance and assistance, Shinichi Abe at Jikei University for the literature survey, and Wataru Yamagami MD, PhD, and Mikihiko Ushijima MD, PhD, for providing additional information on their study.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: M.I., M.H., H.Y., K.Y., B.T., N.S.
- Data curation: M.I., M.H.
- Formal analysis: M.I., M.H.
- Methodology: M.I., M.H.
- Project administration: K.Y., B.T., N.S.
- Supervision: M.M., H.Y., K.Y., B.T., N.S.
- Writing - original draft: M.I., M.H.
- Writing - review & editing: M.T., T.Y., T.T., M.M., H.Y., K.Y., B.T., N.S.
SUPPLEMENTARY MATERIALS
Summary of risks of bias for each study
Patient demographics in fertility-sparing retreatment and conventional hysterectomy groups for recurrent disease
Multivariate analysis for risk factors of progestin retreatment failure (n=270)
Cumulative re-recurrence rates following re-treatment for recurrent endometrial neoplasm according to recurrent diseases.
Forest plots comparing pregnancy rates between re-progestin therapy and conventional hysterectomy groups with recurrent atypical endometrial hyperplasia or endometrial cancer.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. J Natl Cancer Inst. 2018;110:354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- 3.Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol. 2017;28:e32. doi: 10.3802/jgo.2017.28.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akter S, Goto A, Mizoue T. Smoking and the risk of type 2 diabetes in Japan: a systematic review and meta-analysis. J Epidemiol. 2017;27:553–561. doi: 10.1016/j.je.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z, Liu H, Zhou R, Xiao Z, Wang J, Wang H, et al. The optimal time for the initiation of in vitro fertilization and embryo transfer among women with atypical endometrial hyperplasia and endometrial carcinoma receiving fertility-sparing treatment. Arch Gynecol Obstet. 2022;305:1215–1223. doi: 10.1007/s00404-021-06320-3. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Wang Y, Zhou R, Wang J. Oncologic and obstetrical outcomes after fertility-preserving retreatment in patients with recurrent atypical endometrial hyperplasia and endometrial cancer. Int J Gynecol Cancer. 2020;30:1902–1907. doi: 10.1136/ijgc-2020-001570. [DOI] [PubMed] [Google Scholar]
- 9.Giampaolino P, Di Spiezio Sardo A, Mollo A, Raffone A, Travaglino A, Boccellino A, et al. Hysteroscopic endometrial focal resection followed by levonorgestrel intrauterine device insertion as a fertility-sparing treatment of atypical endometrial hyperplasia and early endometrial cancer: a retrospective study. J Minim Invasive Gynecol. 2019;26:648–656. doi: 10.1016/j.jmig.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Yamagami W, Susumu N, Makabe T, Sakai K, Nomura H, Kataoka F, et al. Is repeated high-dose medroxyprogesterone acetate (MPA) therapy permissible for patients with early stage endometrial cancer or atypical endometrial hyperplasia who desire preserving fertility? J Gynecol Oncol. 2018;29:e21. doi: 10.3802/jgo.2018.29.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurelli G, Falcone F, Gallo MS, Scala F, Losito S, Granata V, et al. Long-term oncologic and reproductive outcomes in young women with early endometrial cancer conservatively treated: a prospective study and literature update. Int J Gynecol Cancer. 2016;26:1650–1657. doi: 10.1097/IGC.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 12.Mitsuhashi A, Sato Y, Kiyokawa T, Koshizaka M, Hanaoka H, Shozu M. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Ann Oncol. 2016;27:262–266. doi: 10.1093/annonc/mdv539. [DOI] [PubMed] [Google Scholar]
- 13.Baek JS, Lee WH, Kang WD, Kim SM. Fertility-preserving treatment in complex atypical hyperplasia and early endometrial cancer in young women with oral progestin: Is it effective? Obstet Gynecol Sci. 2016;59:24–31. doi: 10.5468/ogs.2016.59.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohyagi-Hara C, Sawada K, Aki I, Mabuchi S, Kobayashi E, Ueda Y, et al. Efficacies and pregnant outcomes of fertility-sparing treatment with medroxyprogesterone acetate for endometrioid adenocarcinoma and complex atypical hyperplasia: our experience and a review of the literature. Arch Gynecol Obstet. 2015;291:151–157. doi: 10.1007/s00404-014-3417-z. [DOI] [PubMed] [Google Scholar]
- 15.De Marzi P, Bergamini A, Luchini S, Petrone M, Taccagni GL, Mangili G, et al. Hysteroscopic resection in fertility-sparing surgery for atypical hyperplasia and endometrial cancer: safety and efficacy. J Minim Invasive Gynecol. 2015;22:1178–1182. doi: 10.1016/j.jmig.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang CJ, Chao A, Yang LY, Hsueh S, Huang YT, Chou HH, et al. Fertility-preserving treatment in young women with endometrial adenocarcinoma: a long-term cohort study. Int J Gynecol Cancer. 2014;24:718–728. doi: 10.1097/IGC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 17.Shan BE, Ren YL, Sun JM, Tu XY, Jiang ZX, Ju XZ, et al. A prospective study of fertility-sparing treatment with megestrol acetate following hysteroscopic curettage for well-differentiated endometrioid carcinoma and atypical hyperplasia in young women. Arch Gynecol Obstet. 2013;288:1115–1123. doi: 10.1007/s00404-013-2826-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim MK, Seong SJ, Kim YS, Song T, Kim ML, Yoon BS, et al. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol. 2013;209:358.e1–358.e4. doi: 10.1016/j.ajog.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Park JY, Lee SH, Seong SJ, Kim DY, Kim TJ, Kim JW, et al. Progestin re-treatment in patients with recurrent endometrial adenocarcinoma after successful fertility-sparing management using progestin. Gynecol Oncol. 2013;129:7–11. doi: 10.1016/j.ygyno.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Park H, Seok JM, Yoon BS, Seong SJ, Kim JY, Shim JY, et al. Effectiveness of high-dose progestin and long-term outcomes in young women with early-stage, well-differentiated endometrioid adenocarcinoma of uterine endometrium. Arch Gynecol Obstet. 2012;285:473–478. doi: 10.1007/s00404-011-1959-x. [DOI] [PubMed] [Google Scholar]
- 21.Koskas M, Azria E, Walker F, Luton D, Madelenat P, Yazbeck C. Progestin treatment of atypical hyperplasia and well-differentiated adenocarcinoma of the endometrium to preserve fertility. Anticancer Res. 2012;32:1037–1043. [PubMed] [Google Scholar]
- 22.Dursun P, Erkanli S, Güzel AB, Gultekin M, Tarhan NC, Altundag O, et al. A Turkish Gynecologic Oncology Group study of fertility-sparing treatment for early-stage endometrial cancer. Int J Gynaecol Obstet. 2012;119:270–273. doi: 10.1016/j.ijgo.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara H, Jobo T, Takei Y, Saga Y, Imai M, Arai T, et al. Fertility-sparing treatment using medroxyprogesterone acetate for endometrial carcinoma. Oncol Lett. 2012;3:1002–1006. doi: 10.3892/ol.2012.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurelli G, Di Vagno G, Scaffa C, Losito S, Del Giudice M, Greggi S. Conservative treatment of early endometrial cancer: preliminary results of a pilot study. Gynecol Oncol. 2011;120:43–46. doi: 10.1016/j.ygyno.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, Sideri M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011;22:643–649. doi: 10.1093/annonc/mdq463. [DOI] [PubMed] [Google Scholar]
- 26.Perri T, Korach J, Gotlieb WH, Beiner M, Meirow D, Friedman E, et al. Prolonged conservative treatment of endometrial cancer patients: more than 1 pregnancy can be achieved. Int J Gynecol Cancer. 2011;21:72–78. doi: 10.1097/IGC.0b013e31820003de. [DOI] [PubMed] [Google Scholar]
- 27.Eftekhar Z, Izadi-Mood N, Yarandi F, Shojaei H, Rezaei Z, Mohagheghi S. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: our experiences with 21 patients. Int J Gynecol Cancer. 2009;19:249–252. doi: 10.1111/IGC.0b013e31819c5372. [DOI] [PubMed] [Google Scholar]
- 28.Hahn HS, Yoon SG, Hong JS, Hong SR, Park SJ, Lim JY, et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer. 2009;19:1068–1073. doi: 10.1111/IGC.0b013e3181aae1fb. [DOI] [PubMed] [Google Scholar]
- 29.Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 30.Yamazawa K, Hirai M, Fujito A, Nishi H, Terauchi F, Ishikura H, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22:1953–1958. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 31.Minaguchi T, Nakagawa S, Takazawa Y, Nei T, Horie K, Fujiwara T, et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer Lett. 2007;248:112–122. doi: 10.1016/j.canlet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Niwa K, Tagami K, Lian Z, Onogi K, Mori H, Tamaya T. Outcome of fertility-preserving treatment in young women with endometrial carcinomas. BJOG. 2005;112:317–320. doi: 10.1111/j.1471-0528.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 33.Ota T, Yoshida M, Kimura M, Kinoshita K. Clinicopathologic study of uterine endometrial carcinoma in young women aged 40 years and younger. Int J Gynecol Cancer. 2005;15:657–662. doi: 10.1111/j.1525-1438.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang YC, Wu CC, Chen CP, Chang CL, Wang KL. Reevaluating the safety of fertility-sparing hormonal therapy for early endometrial cancer. Gynecol Oncol. 2005;99:287–293. doi: 10.1016/j.ygyno.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Gotlieb WH, Beiner ME, Shalmon B, Korach Y, Segal Y, Zmira N, et al. Outcome of fertility-sparing treatment with progestins in young patients with endometrial cancer. Obstet Gynecol. 2003;102:718–725. doi: 10.1016/s0029-7844(03)00667-7. [DOI] [PubMed] [Google Scholar]
- 36.Wang CB, Wang CJ, Huang HJ, Hsueh S, Chou HH, Soong YK, et al. Fertility-preserving treatment in young patients with endometrial adenocarcinoma. Cancer. 2002;94:2192–2198. doi: 10.1002/cncr.10435. [DOI] [PubMed] [Google Scholar]
- 37.Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, Kuwabara Y, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Yu M, Yang JX, Cao DY, Yuan Z, Zhou HM, et al. Prolonged conservative treatment in patients with recurrent endometrial cancer after primary fertility-sparing therapy: 15-year experience. Int J Clin Oncol. 2019;24:712–720. doi: 10.1007/s10147-019-01404-2. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Guo YR, Lin JF, Feng Y, Billig H, Shao R. Combination of diane-35 and metformin to treat early endometrial carcinoma in pcos women with insulin resistance. J Cancer. 2014;5:173–181. doi: 10.7150/jca.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, Shan WW, et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. BJOG. 2020;127:848–857. doi: 10.1111/1471-0528.16108. [DOI] [PubMed] [Google Scholar]
- 41.Hwang JY, Kim DH, Bae HS, Kim ML, Jung YW, Yun BS, et al. Combined oral medroxyprogesterone/levonorgestrel-intrauterine system treatment for women with grade 2 stage IA endometrial cancer. Int J Gynecol Cancer. 2017;27:738–742. doi: 10.1097/IGC.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 42.Zhou H, Cao D, Yang J, Shen K, Lang J. Gonadotropin-releasing hormone agonist combined with a levonorgestrel-releasing intrauterine system or letrozole for fertility-preserving treatment of endometrial carcinoma and complex atypical hyperplasia in young women. Int J Gynecol Cancer. 2017;27:1178–1182. doi: 10.1097/IGC.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Zhou R, Wang H, Liu H, Wang J. Impact of treatment duration in fertility-preserving management of endometrial cancer or atypical endometrial hyperplasia. Int J Gynecol Cancer. 2019;29:699–704. doi: 10.1136/ijgc-2018-000081. [DOI] [PubMed] [Google Scholar]
- 44.Yang B, Xie L, Zhang H, Zhu Q, Du Y, Luo X, et al. Insulin resistance and overweight prolonged fertility-sparing treatment duration in endometrial atypical hyperplasia patients. J Gynecol Oncol. 2018;29:e35. doi: 10.3802/jgo.2018.29.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YB, Holschneider CH, Ghosh K, Nieberg RK, Montz FJ. Progestin alone as primary treatment of endometrial carcinoma in premenopausal women. Report of seven cases and review of the literature. Cancer. 1997;79:320–327. doi: 10.1002/(sici)1097-0142(19970115)79:2<320::aid-cncr15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song T, Seong SJ, Bae DS, Suh DH, Kim DY, Lee KH, et al. Synchronous primary cancers of the endometrium and ovary in young women: a Korean Gynecologic Oncology Group Study. Gynecol Oncol. 2013;131:624–628. doi: 10.1016/j.ygyno.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Morice P, Fourchotte V, Sideris L, Gariel C, Duvillard P, Castaigne D. A need for laparoscopic evaluation of patients with endometrial carcinoma selected for conservative treatment. Gynecol Oncol. 2005;96:245–248. doi: 10.1016/j.ygyno.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 49.Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. 2016;34:4225–4230. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacKintosh ML, Derbyshire AE, McVey RJ, Bolton J, Nickkho-Amiry M, Higgins CL, et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int J Cancer. 2019;144:641–650. doi: 10.1002/ijc.31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JY, Seong SJ, Kim TJ, Kim JW, Bae DS, Nam JH. Significance of body weight change during fertility-sparing progestin therapy in young women with early endometrial cancer. Gynecol Oncol. 2017;146:39–43. doi: 10.1016/j.ygyno.2017.05.002. [stylefix] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of risks of bias for each study
Patient demographics in fertility-sparing retreatment and conventional hysterectomy groups for recurrent disease
Multivariate analysis for risk factors of progestin retreatment failure (n=270)
Cumulative re-recurrence rates following re-treatment for recurrent endometrial neoplasm according to recurrent diseases.
Forest plots comparing pregnancy rates between re-progestin therapy and conventional hysterectomy groups with recurrent atypical endometrial hyperplasia or endometrial cancer.






