Abstract
Objective
We aim to report the outcomes and feasibility of endoscopic spine surgery used to treat symptomatic spinal metastases patients. This is the most extensive series of spinal metastases patients who underwent endoscopic spine surgery.
Methods
A worldwide collaborative network group of endoscopic spine surgeons, named ‘ESSSORG,’ was established. Patients diagnosed with spinal metastases who underwent endoscopic spine surgery from 2012 to 2022 were retrospectively reviewed. All related patient data and clinical outcomes were gathered and analyzed before the surgery and the follow-time period of 2 weeks, 1 month, 3 months, and 6 months.
Results
A total of 29 patients from South Korea, Thailand, Taiwan, Mexico, Brazil, Argentina, Chile, and India, were included. The mean age was 59.59 years, and 11 of them were female. The total number of decompressed levels was 40. The technique was relatively equal (15 uniportal; 14 biportal). The average length of admission was 4.41 days. Of all patients with an American Spinal Injury Association Impairment Scale of D or lower before surgery, 62.06% reported having at least one recovery grade after the surgery. Almost all clinical outcomes parameters statistically significantly improved and maintained from 2 weeks to 6 months after the surgery. Few surgical-related complications (4 cases) were reported.
Conclusion
Endoscopic spine surgery is a valid option for treating spinal metastases patients as it could yield comparable results to other minimally invasive spine surgery techniques. As the aim is to improve the quality of life, this procedure is valuable and holds value in palliative oncologic spine surgery.
Keywords: Endoscopic spine surgery, Spinal metastases, Minimally invasive spine surgery, Palliative surgery, Quality of life
INTRODUCTION
Spinal metastasis is considered one of the significant challenges in spine care worldwide [1]. Although most are asymptomatic, symptomatic spinal metastases can severely affect the patient’s quality of life by causing excruciating pain, neurological deficit, and deteriorating ambulation status [2,3]. The mainstay of treatment consists of palliative radiation and spine surgery in appropriately selected patients [4,5]. However, considering the natural history of cancer disease and the patient’s deteriorating frailty status, higher complication rates and more surgical stress is suspected in this population compared to other spinal disorders [6]. Recently, minimally invasive spine surgery (MISS) has been implemented. It can reduce the collateral damage to the surrounding structure, yield better results in reducing blood loss and the length of stay (LOS), and consequently enhance patient recovery. It also provides a comparable efficacy in reducing pain and recovering neurological functions compared to traditional surgery [7,8].
Endoscopic spine surgery is one of the recently developed MISS techniques [9]. During the last decade, it has proven its efficacy and safety in treating various spinal pathologies, such as disc herniation, spinal degeneration, and spinal canal stenosis [10-12]. However, report for its use in spinal metastases patients is still rare; most are case reports or case series. Therefore, in this study, we aim to report the outcomes and efficacy of endoscopic spine surgery when treating symptomatic spinal metastases patients from our extensive collaborative network worldwide. To our knowledge, this is considered the most large-scale cohort ever to be reported.
MATERIALS AND METHODS
This is a multinational, multicenter, retrospective cohort study. After Institutional Review Board approval (Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea; KC22RISI0968), a collaborative network group of experienced endoscopic spine surgeons, named ‘Endoscopic Spine Surgery for Spinal Oncology Research Group’ or ‘ESSSORG,’ was created. They were assigned to collect related data from their respective institutes. The group included 14 spine surgeons from 12 healthcare centers in 8 countries, including South Korea, Thailand, Taiwan, Mexico, Brazil, Argentina, Chile, and India. All patients older than 18 years diagnosed with spinal metastases and underwent endoscopic spine surgery as a palliative treatment from 2012 to 2022 were included in the study.
The patient’s preoperative demographic data, including gender, age, primary tumor of origin, involved vertebrae, age-adjusted Charlson Comorbidity Index (ACCI), American Society of Anesthesiologists (ASA) physical status classification grade, history of previous systemic therapy, history of prior radiotherapy, the revised Tokuhashi Score [13], the Spinal Instability Neoplastic Score (SINS) [14], and the Metastatic Epidural Spinal Cord Compression Scale (MESCC) [15,16], were all gathered. The MESCC is a 6-point grading system to describe the severity of epidural spinal cord compression by the tumor. Grades 0, 1a, and 1b are defined as low grades, while grades 2 and 3 are defined as high grades of compression. According to the neurologic, oncologic, mechanical, and systemic or NOMS framework [17], the use of low and high grades MESCC could help guide the decision of treatment. However, the role of radiation options or surgery is still controversial in grade 1c [15,17]. Therefore, the collected radiographic images of MESCC were accordingly interpreted into low (grades 0, 1a, or 1b), intermediate (grade 1c), and high grades (grades 2, 3), respectively. At the time of data collection, we also asked all the collaborated surgeons to confirm the dead or alive status of their included patients. One’s status that could not be retrieved was categorized as not known or loss follow-up.
The surgical-related data, including the total number of decompressed levels for each patient, the technique used, the approach used, the use of additional spinal stabilization or augmentation procedure, operative time, and intraoperative blood loss, were also collected. The technique used consists of uniportal (full-endoscopic) surgery or biportal (unilateral biportal endoscopic; UBE) surgery, respectively. The approach used consists of interlaminar and transforaminal techniques. The main surgery goal is to palliatively decompress the spinal canal or the foramen and remove the tumor. So, these approaches would aim to decompress those sites, not discectomy as used to perform in the traditional endoscopic spine surgery procedure. All the techniques and approaches were performed under general anesthesia. To corporate the concept of separation surgery, ventral side decompression was also performed in every case if it was feasible and not limited by anatomical hindrance (e.g., in the cervical spine).
Indication and the decision-making to perform the surgery in spinal metastases patients are based on many factors, such as the patient’s performance status, the systemic burden of disease, life expectancy, the controlling status of systemic disease, and the systemic treatment options available, according to an integrated multidisciplinary algorithm from the report of International Spine Oncology Consortium [18], which should be made in a case-by-case basis. Then, the definite options to include for the surgery will be decided based on the NOMS decision framework [17], or the mechanical, neurological, oncological, preferred treatment, or the MNOP algorithm [18]. Posterior instrumentation, especially via the percutaneous technique, can be added if indicated (e.g., mechanical back pain or SINS of equal or more than 7 points, according to the aforementioned algorithm). However, because metastatic cancer disease cannot be cured and has a short life expectancy, the aggressiveness and costly definite surgical options should be balanced. Therefore, sometimes, not all the indicated procedures can be performed for the patients. All these decision-making processes should also be consensus from a multidisciplinary care team and also aligned with the expectation of patients and their respective families [18,19]. Nevertheless, according to the above, endoscopic spine surgery would be performed as an alternative to the traditional open, mini-open, or tubular surgery, aiming to decompress the spine as indicated in spinal metastases patients.
Several parameters and patient-reported outcomes were used to evaluate the procedure’s results, including the Eastern Cooperative Oncology Group (ECOG) status, the American Spinal Injury Association (ASIA) Impairment Scale, the pain Numeric Rating Scale (NRS), Oswestry Disability Index (ODI), Neck Disability Index (NDI; in cases with cervical region involvement), and the EuroQoL 5-Dimension 5-Levels Utility (EQ5D5L-U) and visual analogue scale (EQ5D5L-VAS), were all collected at the preoperative period and postoperative period of 2 weeks, 1 month, 3 months, and 6 months, respectively. The total LOS in the hospital, follow-up time, and the latest known patient status (whether the patient is alive or has already passed away) were also gathered. Surgical-related complications that occurred during the perioperative or postoperative period, such as neurological progression, wound infection or dehiscence, cerebrospinal fluid leakage, instrumentation failure, local recurrence, the incidence of revision surgery, and intraoperative mortality, were reported together with the occurred time and its related treatment.
In order to compare the preoperative and postoperative data at different time points (months), we employed 2 statistical tests depending on the distribution of the data: the paired t-test and the paired samples Wilcoxon test. The choice of the statistical test was based on the normality of the data distribution. Before conducting the tests, we checked the normality of the data using the Shapiro-Wilk test. If the data met the assumptions of normality, we performed a paired t-test. In cases where the data violated the assumption of normality, we used the non-parametric paired samples Wilcoxon test as an alternative. For each test, we calculated the test statistic and corresponding p-value. We considered a p-value of less than 0.05 as statistically significant. The Stata 17 (Stata Crop., College Station, TX, USA) software was used for all statistical analyses.
RESULTS
A total of 29 patients were included, and 11 (37.93%) were female. The mean age was 59.59±12.61 years. The total number of metastatic involved vertebrae from the patients was 47 levels; most came from the lumbar region (23 levels, 48.9%), followed by the thoracic (17 levels, 36.2%), cervical (4 levels, 8.5%), and sacral region (3 levels, 6.4%), respectively. The mean ACCI was 9.29±2.32. Most patients had a high MESCC grade before the surgery (25 patients, 53.2%). SINS were interpreted as stable and potentially unstable for 6 (20.7%) and 14 (48.3%), respectively. The revised Tokuhashi Score was also low (< 6 months) in most of the patients (21 patients, 72.4%), while the mean follow-up time was 8.52±8.1 months. All the details of the demographic data of the included patients are shown in Table 1.
Table 1.
Demographic data of the patients included (n=29)
| Parameter | Value |
|---|---|
| Age (yr) | 59.59 ± 12.61 (33–89) |
| Sex | |
| Male | 18 (62.1) |
| Female | 11 (37.9) |
| No. of involved vertebrae | 47 (100) |
| Cervical | 4 (8.5) |
| Thoracic | 17 (36.2) |
| Lumbar | 23 (48.9) |
| Sacral | 3 (6.4) |
| Primary tumors of origin | |
| Lung | 8 (27.6) |
| Prostate | 4 (13.8) |
| Myeloma | 4 (13.8) |
| Lymphoma | 2 (6.9) |
| Breast | 2 (6.9) |
| Colon | 2 (6.9) |
| Others* | 7 (24.1) |
| MESCC grade | |
| Low (0, 1a, 1b) | 17 (36.2) |
| Intermediate (1c) | 5 (10.6) |
| High (2, 3) | 25 (53.2) |
| SINS | |
| Stable (0–6) | 6 (20.7) |
| Potentially unstable (7–12) | 14 (48.3) |
| Unstable (13–18) | 9 (31.0) |
| ACCI | 9.29 ± 2.32 (4–12) |
| Previous systematic therapy | |
| Yes | 13 (44.8) |
| No | 15 (51.7) |
| Previous radiotherapy | |
| Yes | 7 (24.1) |
| No | 21 (72.4) |
| Types of radiotherapy received | |
| SRS | 2 (6.7) |
| cEBRT | 19 (63.3) |
| Revised Tokuhashi score | |
| < 6 Months | 21 (72.4) |
| 6–12 Months | 3 (10.3) |
| > 12 Months | 4 (13.8) |
| Follow-up time (mo) | 8.52 ± 8.10 (0.7–30) |
| Confirmed dead | 14 (48.3) |
| Confirmed alive | 8 (27.6) |
| Not know (loss follow-up) | 7 (24.1) |
Values are presented as mean±standard deviation (range) or number (%).
ACCI, age-adjusted Charlson Comorbidity Index; cEBRT, conventional external beam radiation; MESCC, metastatic epidural spinal cord compression; SINS, Spinal Instability Neoplastic Score; SRS, stereotactic radiosurgery.
Others consist of each one of the primary tumors of origin as the followings: liver, thyroid, rectum, kidney, bladder, nasopharynx, and leukemia.
The total number of levels decompressed is 40. For a single operation, 1-, 2-, and 3-level of decompression were performed in 21 cases (70%), 8 cases (26.67%), and 1 case (3.33%), respectively. Because 1 patient received 2 surgeries at different locations of pathology during a single admission, the total number of surgeries performed is 30. Although the technique used was equal regarding the uniportal (full-endoscopic) or biportal (UBE) endoscopy (15 surgeries, 50% each), the approach used mainly was interlaminar (22 surgeries, 73.33%) followed by the transforaminal (8 surgeries, 26.67%). Spinal instrumentation was included in 8 surgeries (26.7%), and cement augmentation was included in 4 surgeries (13.3%). The mean operative time was 150.86±64.45 minutes (range, 40–315 minutes), the estimated blood loss was 94.63±85.11 mL (range, 5–300 mL), and the length of admission was 4.41±3.38 days (range, 1–18 days). All the details of the surgical-related data of the included patients are shown in Table 2.
Table 2.
Surgical-related data of the included patients
| Parameter | Value |
|---|---|
| No. of surgeries performed* | 30 |
| Technique used | |
| Uniportal endoscopy | 15 (50.0) |
| Biportal endoscopy | 15 (50.0) |
| Total number of decompressed level | 40 |
| No. of level decompressed | |
| 1 Level | 21 (70.0) |
| 2 Level | 8 (26.7) |
| 3 Level | 1 (3.3) |
| Approach used | |
| Interlaminar | 30 (75.0) |
| Transforaminal | 10 (25.0) |
| The use of spinal instrumentation | |
| Yes | 8 (26.7) |
| No | 22 (73.3) |
| The use of cement augmentation | |
| Yes | 4 (13.3) |
| No | 26 (86.7) |
| Operative time (min) | 150.86 ± 94.45 (40–315) |
| Estimated blood loss (mL) | 94.63 ± 85.11 (5–300) |
| Length of admission (day) | 4.41 ± 3.38 (1–18) |
Values are presented as number (%) or mean±standard deviation (range).
One patient received 2 surgeries at different locations of pathology during a single admission.
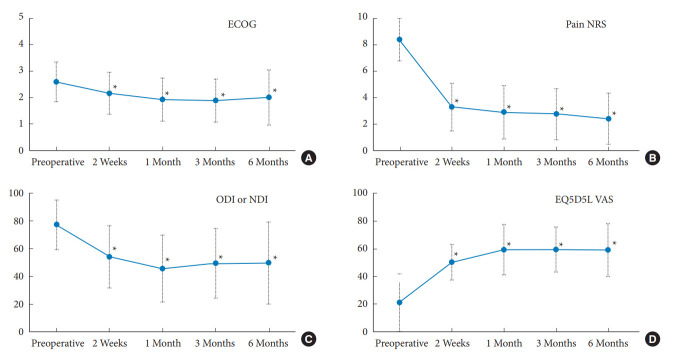
The number of patients stratified by their preoperative and postoperative neurological status according to the ASIA Impairment Scale is shown in Table 3. Of these, 27 patients (93.1%) had an ASIA Impairment Scale of D or lowered before the surgery, and up to 18 patients (66.67%) had at least one recovery grade after the surgery. Before the surgery, the pain NRS (8.41±1.8) and the ODI or NDI (77.27±17.96) decreased significantly at 2 weeks (pain NRS=3.3±1.81, p < 0.001; ODI or NDI=54.13±22.46, p < 0.0001), and able to maintain throughout the follow-up period of 1 month (pain NRS=2.91±2.02, p < 0.0001; ODI or NDI=45.62±24.13, p < 0.0001), 3 months (pain NRS=2.77±1.93, p < 0.0001; ODI or NDI=49.7±29.67, p < 0.0001), and 6 months (pain NRS=2.42±1.93, p < 0.0001; ODI or NDI= 49.7±29.67, p < 0.005). Other parameters, such as ECOG, EQ5D5L-U, and EQ5D5L-VAS, followed the same trend with the improvement achieved rapidly 2 weeks after the surgery and could be maintained up to at least 3 months after the surgery; all statistically significant differences from before the surgery (Fig. 1). All the details of the patient’s clinical outcomes before and after the surgery at each follow-up time are shown in Table 4. The survival analysis revealed an overall mean survival of 9.15 months. The Kaplan-Meier graph is displayed in Fig. 2.
Table 3.
The number of patients stratified by their neurological status according to the American Spinal Injury Association (ASIA) Impairment Scale compared before and after the endoscopic decompression spine surgery
| Before surgery | After surgery |
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| A | 1 | 1 | - | - | - |
| B | - | 1 | 1 | 1 | 1 |
| C | - | 1 | - | 7 | 1 |
| D | - | - | - | 7 | 5 |
| E | - | - | - | - | 2 |
Fig. 1.
Charts representing the improvement of clinical outcome parameters compared between preoperative and each postoperative periods. (A) The Eastern Cooperative Oncology Group (ECOG). (B) The pain Numeric Rating Scale (NRS). (C) The Oswestry Disability Index (ODI) or the Neck Disability Index (NDI). (D) The EuroQoL 5-Dimension 5-Levels visual analogue scale (EQ5D5L VAS). *p < 0.05, statistically significant differences.
Table 4.
Comparisons of patient’s clinical outcomes before and after surgery at each interested follow-up period
| Parameter | Before surgery (n = 29) | PO 2 weeks (n = 26) | p-value | PO 1 month (n=23) | p-value | PO 3 months (n = 21) | p-value | PO 6 months (n = 12) | p-value |
|---|---|---|---|---|---|---|---|---|---|
| ECOG | 2.59 ± 0.75 | 2.16 ± 0.8 | < 0.05* | 1.92 ± 0.81 | < 0.05* | 1.89 ± 0.81 | < 0.05* | 2 ± 1.04 | < 0.05* |
| Pain NRS | 8.41 ± 1.8 | 3.3 ± 1.81 | < 0.05* | 2.91 ± 2.02 | < 0.05* | 2.77 ± 1.93 | < 0.05* | 2.42 ± 1.93 | < 0.05* |
| ODI or NDI | 77.27 ± 17.96 | 54.13 ± 22.46 | < 0.05* | 45.62 ± 24.13 | < 0.05* | 49.39 ± 25.13 | < 0.05* | 49.7 ± 29.67 | < 0.05* |
| EQ5D5L-U | 0.12 ± 0.42 | 0.38 ± 0.36 | < 0.05* | 0.52 ± 0.33 | < 0.05* | 0.4 ± 0.44 | < 0.05* | 0.34 ± 0.48 | 0.3833 |
| EQ5D5L VAS | 21.27 ± 20.78 | 50.46 ± 12.76 | < 0.05* | 59.58 ± 18.15 | < 0.05* | 59.62 ± 16.39 | < 0.05* | 59.25 ± 19.23 | < 0.05* |
Values are presented as mean±standard deviation.
PO, postoperative; ECOG, Eastern Cooperative Oncology Group; NRS, Numeric Rating Scale; ODI, Oswestry Disability Index; NDI, Neck Disability Index; EQ5D5L, EuroQoL 5-Dimension 5-Levels; EQ5D5L-U, EQ5D5L Utility; VAS, visual analogue scale.
Statistically significant (p<0.05).
Fig. 2.
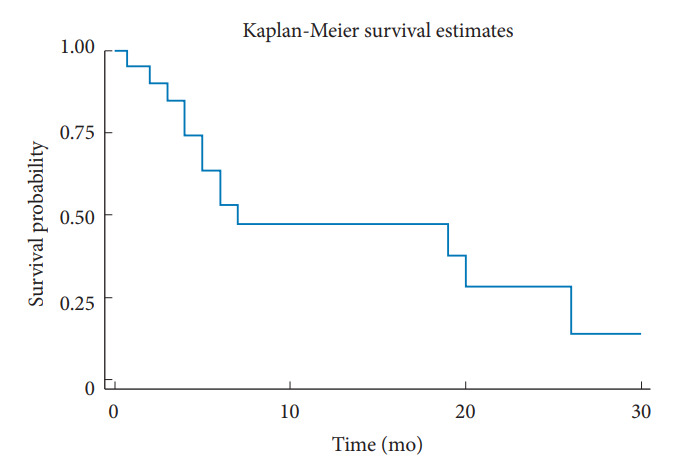
The Kaplan-Meier estimates graph according to the survival analysis
From our cohort, complications occurred in 4 patients (13.79%). One patient had a superficial wound problem 1 week after the surgery, which could resolve with regular wound dressing and empirical oral antibiotics. No organism was found. One patient had a postoperative hematoma with severe progressive back and leg pain symptoms 2 weeks after the surgery. Revision surgery for exploration and hematoma removal was carried out, and the patient’s symptoms were resolved without further complications. Another patient had neurological progression immediately after the surgery, and urgent revision surgery for wide decompression was carried out. The final patient had a local tumor recurrence 4 months after the surgery. He had a new onset of back pain, and the computed tomography scans revealed a progressive pathologic fracture of the previously involved and operating level of the vertebrae. Percutaneous vertebroplasty was carried out, and the patient’s symptoms improved without further complications. A summary of surgical-related complications is displayed in Supplementary Table 1.
DISCUSSION
Treatment in spinal metastases requires multidisciplinary approaches and primarily consists of palliative surgery followed by subsequent conventional external beam radiation or stereotactic radiosurgery (SRS), depending on the tumor type of origin radioresistance [18]. With the patient’s frailty combined with multiple comorbidities, traditional open surgery is usually associated with higher blood loss, prolonged LOS, and delayed recovery time [6,19]. This could also cause the postponement of other subsequent treatment processes, such as radiotherapy or other systemic therapy. Thus, MISS has recently been preferable in treating symptomatic spinal metastases patients. It can limit the unnecessary surrounding soft tissue damage, provide less blood loss, less LOS, and enhance the recovery time with comparable outcomes and rate of complications to the traditional open surgery [7,8,20,21].
Endoscopic spine surgery is considered one of the recent advancements of MISS, especially in degenerative spine disease [9,22]. Using the recent AOSpine Nomenclature in endoscopic spine surgery [23], we summarized these advancements and their expanding indications in Table 5. With the larger working cannula and specialized armamentarium [9], it has been able to expand the surgical indications from simple decompression reaching up to lumbar interbody fusion [24,25], and more complicated spine surgery (e.g., infection) [26], with noninferiority to better results compared to the traditional MISS procedures [10-12,27]. However, because the support of these technical evolutions is very recent, report of its use in spinal metastases is still scarce. To the best of our knowledge, there are only 11 cases from 7 studies in previous literature [28-34]. Although all have revealed good results of rapid pain relief, neurological recovery, less blood loss, and the reduction of LOS, the level of evidence is considered low as they are all case reports or series with an insufficient number of patients (Supplementary Table 2). This could also be from the lower incidence of symptomatic spinal metastases undergone surgery compared to other general spinal diseases [35,36]. Therefore, less number of cases and fewer adoption of endoscopic spine surgery used in spinal metastases is expected. To overcome this, a worldwide collaboration of spine surgeons is established by our senior author (KJS). As a result of the last 10 years from 14 surgeons in 8 countries, there was a total of 29 cases enrolled in our study, which is considered the most extensive cohort to date.
Table 5.
Summary of the recent evolution in lumbar endoscopic spine surgery according to the targeted pathology
| Procedure | Targeted | AOSpine Nomenclature [21] | Level of skills |
|---|---|---|---|
| Discectomy | Herniated disc | TELD | Basic |
| Discectomy | Herniated disc | IELD | Basic |
| Foraminoplasty | Herniated disc | TELD with foraminoplasty | Basic |
| Foraminotomy | SAP | TELF | Intermediate |
| Foraminotomy | Contralateral SAP | ICELF | Intermediate |
| Decompression | Central canal+lateral recess | LE-ULBD | Intermediate |
| Ventral facetectomy | SAP+lateral recess | TELF with TE-LRD | Master |
| Fusion | Instability | Endoscopic TLIF | Master |
TELD, transforaminal endoscopic lumbar discectomy; IELD, interlaminar endoscopic lumbar discectomy; TELF, transforaminal endoscopic lumbar foraminotomy; ICELF, interlaminar contralateral endoscopic lumbar foraminotomy; LE-ULBD, lumbar endoscopic unilateral laminotomy for bilateral decompression; TE-LRD, transforaminal endoscopic lateral recess decompression; SAP, superior articular process; TLIF, transforaminal lumbar interbody fusion.
From our study, good to excellent outcomes were achieved as all clinical outcomes related to the patient’s quality of life were improved significantly and could be maintained up to 6 months after the surgery. Among 27 patients with an ASIA Impairment Scale of D or lower, up to 66.67% achieved at least one recovery grade. Moreover, the mean length of admission from our study was also low (4.41±3.38 days). These results were comparable or even better to previous reports of spinal metastases patients treated with MISS. Miscusi et al. [20] compared the outcomes of MISS with traditional open surgery for thoracic spinal metastases patients with acute myelopathy. Although no apparent significant differences regarding neurological status improvement were found, significant improvements in decreasing blood loss (mean, 240 mL), operative time (mean, 2.2 hours), and LOS (mean, 7.2 days) were achieved from the MISS group. This can also indirectly indicate that this enhanced recovery time, as revealed by the shortened LOS, could also allow patients to receive other adjunct cancer-related treatment faster than traditional procedures. Thus, it can potentially enhance the overall survival of metastatic cancer patients. Complication rates were also comparable to previous studies. Vargas et al. [37] retrospectively reviewed the records of 205 patients regarding wound complications in spinal metastases patients and reported rates of wound complications between 10.8%–14.3%. Another review by Igoumenou et al. [38] has also demonstrated relatively comparable rates of various complication types to our study, including wound infection and dehiscence (1.5% to 30%), hemorrhage and hematomas (5.9% to 12%), and neurological complications (0.6% to 14.5%).
One of the potential limitations of the ESS for patients with spinal metastases lies in the learning curve itself. Adopting this technique for this specific type of patient would be more challenging than disc herniation or degenerative diseases. Initially, almost all techniques used in the previous study were full-endoscopic with a transforaminal approach (Supplementary Table 2). This means that the endoscopic spine surgery from those studies was able to do the role of the local decompression around the intervertebral foramen, primarily only for pain reduction. This differs from our study, as 30 out of 40 operated levels (75%) used the interlaminar approach. Because this approach is as feasible for central and bilateral decompression as traditional microsurgery, it allowed surgeons to achieve better spinal canal decompression. Another reason could be that the adoption of endoscopic spine surgery is relatively new in spinal metastases treatment. The interlaminar approach is more familiar to many surgeons as it shares the same features as the traditional posterior approach and could also shorten the overall learning curve process.
The number of full-endoscopic and UBE surgeries that surgeons used in our cohort is relatively the same. The UBE technique uses the 2 independent portals to work and has a more flexible working angle. This could ease the learning curve and be adopted more readily by many surgeons familiar with arthroscopy or tubular spine surgery [9,39]. Nevertheless, both techniques provided better intraoperative visualization than tubular-based microsurgery. The concept of separation surgery is to allow the safe delivery of the SRS after surgery to achieve better local tumor control by adequately separating the neural element from the tumor to a certain margin (usually 2–5 mm) [18,21]. Therefore, using an interlaminar approach with either full-endoscopic or UBE, the optimal visualization provided by both techniques is another potential advantage, especially when doing the ventral side decompression into the vertebral body, to ultimately achieve the goal of separation surgery (Figs. 3, 4).
Fig. 3.
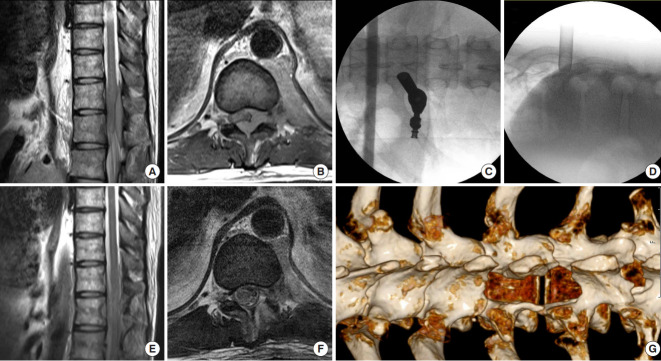
A patient with acute onset of both sides of paraplegia underwent full-endoscopic decompression surgery. (A, B) Preoperative magnetic resonance imaging (MRI) images reveal T10–12 epidural metastases and severe spinal cord compression. (C, D) Intraoperative fluoroscopic images show the optimal position for the interlaminar approach used in this case. (E, F) Postoperative MRI images demonstrate the tumor has been removed and the neural element was free. (G) Postoperative computed tomography scan showed the postlaminectomy site, where wide decompression was achieved.
Fig. 4.
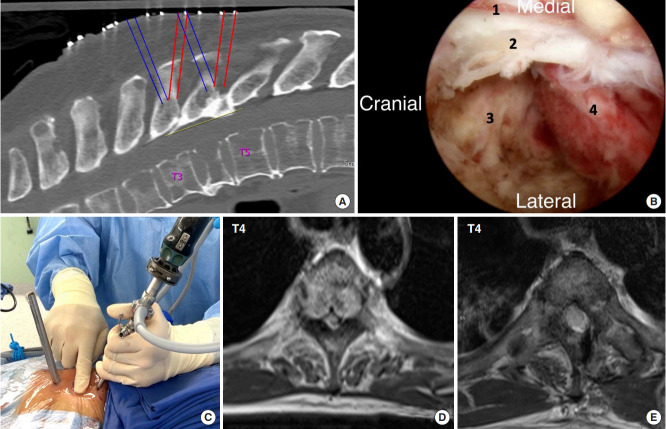
A patient with T4 metastases and acute incomplete spinal cord compression underwent unilateral biportal endoscopic surgery. (A) Annotations in the preoperative computed tomography scan revealed 3 incisions were planned for portals insertion. (B) Intraoperative view during T3–4 decompression demonstrating an optimal visualization, which could facilitate the separation surgery. While the endoscope was approaching from the right side, No. 1 is the lateral border of the dural sac. No. 2 is the posterior longitudinal ligament. No. 3 is the T3–4 intervertebral disc space, and No. 4 is the tumor tissue involving the T4 vertebral body. (C) The photograph demonstrating irrigation flow control using the planned incision portals can help with the bleeding control and provide better visualization. (D, E) Axial preoperative and postoperative magnetic resonance imaging images of the T4 level reveal that wide decompression was achieved.
Intraoperative hemostasis is one of the major concerns during surgery in spinal metastases patients. More excessive bleeding may negatively affect the visualization during endoscopic spinal procedures. Similar to other surgeries, several options are available for us to use for achieving better hemostasis control during endoscopic spinal surgery, including radiofrequency ablation probes or various types of hemostatic agents, such as bone wax, gelatin foam, or human gelatine-thrombin matrix sealant [40]. The manual control pressure of the saline irrigation for a brief period is also an advantage in full-endoscopic spine surgery. Furthermore, UBE could also provide some unique benefits for hemostasis. Optimizing the irrigation flow control during the UBE procedure or creating a new channel to allow more irrigation flow can provide better visualization (Fig. 4). As to our studies, the mean estimated blood loss is 94.63 mL (range, 5–300 mL), which is considered comparable or less bleeding to other MISS in the literature [8,20,21]. Lastly, although we do not have the data regarding the preoperative endovascular embolization used in our series, it is also one of the good options for spinal metastases patients to enhance bleeding control, as proven in previous literature [41]. However, controversy regarding its effectiveness still exists, especially in patients with nonhypervascular tumors of origin [42].
Theoretically, saline irrigation during endoscopic spine surgery in spinal metastases patients might have a negative impact as the tumor cell could be spread, causing more contamination. However, there is still no clear evidence that endoscopic spine surgery will cause more contamination than traditional or MISS when treating spinal metastases. Moreover, our study found only 1 case (3.45%) of local tumor recurrence. Park and Jeon [43] conducted a retrospective study of 102 symptomatic spinal metastases patients who underwent spinal surgery. Primary tumors mainly consisted of gastrointestinal, breast, and lung in origin, and they reported a symptomatic tumor recurrence rate of 2.94%. Another prospective nonrandomized MISS study by Tancioni et al. [44] also found a symptomatic local recurrence rate of 8% among the spinal metastases patients in their cohort. Further studies focusing on the extent of tumor cell contamination or the recurrence rate after the surgery in these populations that underwent endoscopic spine surgery with a larger population will be beneficial to confirm this finding.
Patient selection is also challenging in surgically treated spinal metastases patients. Following the algorithms, all have recommended many systemic factors to consider, including the patient’s performance status, the systemic burden of disease, life expectancy, and the controlling status of systemic disease, to name a few [18]. However, with the nature of the ultraminimally invasiveness of ESS, it is possible that more frailty of patients with those systemic burdens could benefit from the decompression surgery, ultimately enhancing their quality of life throughout their remaining survival time. This was also demonstrated well in our study. Although from our series, the preoperative mean ACCI was high (9.29±2.32), the patients still benefited from the surgery by having a better quality of life, and the complication rates were also comparable with previous studies, as previously mentioned.
Several limitations exist in our studies. With the nature of the retrospective study, it will unavoidably be subject to bias. In the future, a control group, such as open surgery or other MISS techniques, to compare with the ESS technique will be very helpful in demonstrating its efficacy in these patients. Endoscopic spine surgery is a relatively new technique, mainly when used to treat spinal metastases patients. Therefore, various factors regarding differences in practice, equipment settings, or learning curves between surgeons could not be fully controlled and might affect the overall results. Moreover, with the complexity of the procedure, it is possible that we may not cover some patients with multiple levels of involvement of spinal metastases that need to be decompressed, and this could lead to selective bias. Lastly, with the nature of the oncologic study, the poor prognosis of these patients could impact our study's drop-out rate. However, from our research, the overall mean survival time was 9.15 months which is similar to the spinal metastases studies from previous literature.
CONCLUSION
As a result of the most extensive number of cases to date from the collaboration of 12 healthcare centers in 8 countries worldwide, endoscopic spinal surgery is a valid option in the palliative treatment of spinal metastases. It can achieve the same goal of decompression while providing comparable to excellent outcomes to the other MISS technique. The enhanced recovery time could also allow patients to go on other cancer-related treatments more rapidly.
Footnotes
Conflict of Interest
The corresponding author (JSK) is a consultant of Richard Wolf, GmbH, and Elliquence, LLC. The other authors have no conflicts of interest to declare.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: JSK; Data curation: SS, YJK, WT, AA, JQO, VK, HC, CCV, MVS, FVI, LHL, CMC, PL, SMP, JSK; Formal analysis: SS, YL; Methodology: YL, JSK; Project administration: JSK; Visualization: SS, YJK, YL, WT, KJ, KKP, JSK; Writing - original draft: SS, YJK, YL, KJ, KKP, JHK, AM; Writing - review & editing: SS, YJK, YL, WT, AA, JQO, VK, CCV, MVS, FVI, LHL, CMC, PL, SMP, KJ, KKP, JHK, AM, PL, SW, JSK.
Supplementary Material
Supplementary Tables 1 and 2 can be found via https://doi.org/10.14245/ns.2346274.137.
Summary of surgical-related complications and its detail
Summary of the previous reports of endoscopic surgery in spinal metastases patients
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Fomchenko EI, Bayley JC, Alvarez-Breckenridge C, et al. Spinal metastases and the evolving role of molecular targeted therapy, chemotherapy, and immunotherapy. Neurospine. 2022;19:978–93. doi: 10.14245/ns.2244290.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Versteeg AL, Elkaim LM, Sahgal A, et al. Steroids in the management of preoperative neurological deficits in metastatic spine disease: results from the EPOSO study. Neurospine. 2022;19:43–50. doi: 10.14245/ns.2142768.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barzilai O, McLaughlin L, Lis E, et al. Outcome analysis of surgery for symptomatic spinal metastases in long-term cancer survivors. J Neurosurg Spine. Apr Apr 26;:1–6. doi: 10.3171/2019.2.SPINE181306. doi: . [Epub] [DOI] [PubMed] [Google Scholar]
- 5.Taechalertpaisarn P, Wilartratsami S, Phisalprapa P, et al. Cost-utility analysis compared between radiotherapy alone and combined surgery and radiotherapy for symptomatic spinal metastases in Thailand. Neurospine. 2022;19:334–47. doi: 10.14245/ns.2142948.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hersh AM, Pennington Z, Hung B, et al. Comparison of frailty metrics and the Charlson Comorbidity Index for predicting adverse outcomes in patients undergoing surgery for spine metastases. J Neurosurg Spine. 2021;36:849–57. doi: 10.3171/2021.8.SPINE21559. [DOI] [PubMed] [Google Scholar]
- 7.Barzilai O, Bilsky MH, Laufer I. The role of minimal access surgery in the treatment of spinal metastatic tumors. Global Spine J. 2020;10(2 Suppl):79S–87S. doi: 10.1177/2192568219895265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuckerman SL, Laufer I, Sahgal A, et al. When less is more: the indications for MIS techniques and separation surgery in metastatic spine disease. Spine (Phila Pa 1976) 2016;41 Suppl 20(Suppl 20):S246–53. doi: 10.1097/BRS.0000000000001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jitpakdee K, Liu Y, Heo DH, et al. Minimally invasive endoscopy in spine surgery: where are we now? Eur Spine J. 2023 Mar 1; doi: 10.1007/s00586-023-07622-7. doi: . [Epub] [DOI] [PubMed] [Google Scholar]
- 10.Li WS, Yan Q, Cong L. Comparison of endoscopic discectomy versus non-endoscopic discectomy for symptomatic lumbar disc herniation: a systematic review and meta-analysis. Global Spine J. 2022;12:1012–26. doi: 10.1177/21925682211020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Kim YJ, Ryu KS, et al. Comparison of the clinical and radiological outcomes of full-endoscopic laminotomy and conventional subtotal laminectomy for lumbar spinal stenosis: a randomized controlled trial. Global Spine J. 2023 Feb 9;:21925682231155846. doi: 10.1177/21925682231155846. doi: . [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson JNA, Subramanian AS, Scott CEH. A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur Spine J. 2017;26:847–56. doi: 10.1007/s00586-016-4885-6. [DOI] [PubMed] [Google Scholar]
- 13.Gruenberg M, Mereles ME, Willhuber GOC, et al. Usefulness of Tokuhashi score in survival prediction of patients operated for vertebral metastatic disease. Global Spine J. 2017;7:260–5. doi: 10.1177/2192568217699186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Versteeg AL, Sahgal A, Laufer I, et al. Correlation between the Spinal Instability Neoplastic Score (SINS) and patient reported outcomes. Global Spine J. 2021 Jul 26;:21925682211033591. doi: 10.1177/21925682211033591. doi: . [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13:324–8. doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 16.Di Perna G, Cofano F, Mantovani C, et al. Separation surgery for metastatic epidural spinal cord compression: a qualitative review. J Bone Oncol. 2020;25:100320. doi: 10.1016/j.jbo.2020.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18:744–51. doi: 10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spratt DE, Beeler WH, de Moraes FY, et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. Lancet Oncol. 2017;18:e720–30. doi: 10.1016/S1470-2045(17)30612-5. [DOI] [PubMed] [Google Scholar]
- 19.Hussain I, Hartley BR, McLaughlin L, et al. Surgery for metastatic spinal disease in octogenarians and above: analysis of 78 patients. Global Spine J. 2021 Oct 20;:21925682211037936. doi: 10.1177/21925682211037936. doi: . [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miscusi M, Polli FM, Forcato S, et al. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: surgical technique and early clinical results. J Neurosurg Spine. 2015;22:518–25. doi: 10.3171/2014.10.SPINE131201. [DOI] [PubMed] [Google Scholar]
- 21.Kumar N, Tan JH, Thomas AC, et al. The utility of ‘minimal access and separation surgery’ in the management of metastatic spine disease. Global Spine J. 2022 Feb 28;:21925682211049803. doi: 10.1177/21925682211049803. doi: . [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Kotheeranurak V, Quillo-Olvera J, et al. A 30-year worldwide research productivity of scientific publication in full-endoscopic decompression spine surgery: quantitative and qualitative analysis. Neurospine. 2023;20:374–89. doi: 10.14245/ns.2245042.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofstetter CP, Ahn Y, Choi G, et al. AOSpine consensus paper on nomenclature for working-channel endoscopic spinal procedures. Global Spine J. 2020;10(2 Suppl):111S–121S. doi: 10.1177/2192568219887364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quillo-Olvera J, Quillo-Resendiz J, Quillo-Olvera D, et al. Ten-step biportal endoscopic transforaminal lumbar interbody fusion under computed tomography-based intraoperative navigation: technical report and preliminary outcomes in Mexico. Oper Neurosurg (Hagerstown) 2020;19:608–18. doi: 10.1093/ons/opaa226. [DOI] [PubMed] [Google Scholar]
- 25.Lin GX, Chen CM, Rui G, et al. A pilot study of endoscopeassisted MITLIF with fluoroscopy-guided technique: intraoperative objective and subjective evaluation of disc space preparation. BMC Surg. 2022;22:109. doi: 10.1186/s12893-022-01559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin GX, Kim JS, Sharma S, et al. Full endoscopic discectomy, debridement, and drainage for high-risk patients with spondylodiscitis. World Neurosurg. 2019;127:e202–11. doi: 10.1016/j.wneu.2019.02.206. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Wang H, Li W, et al. Comparative effects and safety of full-endoscopic versus microscopic spinal decompression for lumbar spinal stenosis: a meta-analysis and statistical power analysis of 6 randomized controlled trials. Neurospine. 2022;19:996–1005. doi: 10.14245/ns.2244600.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joo YC, Ok WK, Baik SH, et al. Removal of a vertebral metastatic tumor compressing the spinal nerve roots via a single-port, transforaminal, endoscopic approach under monitored anesthesia care. Pain Physician. 2012;15:297–302. [PubMed] [Google Scholar]
- 29.Tsai SH, Wu HH, Cheng CY, et al. Full endoscopic interlaminar approach for nerve root decompression of sacral metastatic tumor. World Neurosurg. 2018;112:57–63. doi: 10.1016/j.wneu.2018.01.075. [DOI] [PubMed] [Google Scholar]
- 30.Gao Z, Wu Z, Lin Y, et al. Percutaneous transforaminal endoscopic decompression in the treatment of spinal metastases: a case report. Medicine (Baltimore) 2019;98:e14819. doi: 10.1097/MD.0000000000014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senturk S, Unsal UU. Percutaneous endoscopic interlaminar decompression of hypervascular spinal metastases. World Neurosurg. 2020;134:182–6. doi: 10.1016/j.wneu.2019.10.175. [DOI] [PubMed] [Google Scholar]
- 32.Henderson F, Jr, Hubbard ZS, Jones S, et al. Endoscopic decompression of epidural spinal metastasis causing lumbar radiculopathy through a transforaminal approach: report of two cases. AME Case Rep. 2020;4:2. doi: 10.21037/acr.2019.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telfeian AE, Oyelese A, Fridley J, et al. Endoscopic surgical treatment for symptomatic spinal metastases in long-term cancer survivors. J Spine Surg. 2020;6:372–82. doi: 10.21037/jss.2019.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotheeranurak V, Jitpakdee K, Pornmeechai Y, et al. Posterior endoscopic cervical decompression in metastatic cervical spine tumors: an alternative to palliative surgery. J Am Acad Orthop Surg Glob Res Rev. 2022;6:e22.00201. doi: 10.5435/JAAOSGlobal-D-22-00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshihara H, Yoneoka D. Trends in the surgical treatment for spinal metastasis and the in-hospital patient outcomes in the United States from 2000 to 2009. Spine J. 2014;14:1844–9. doi: 10.1016/j.spinee.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 36.Luksanapruksa P, Santipas B, Ruangchainikom M, et al. Epidemiologic study of operative treatment for spinal metastasis in Thailand: a review of National Healthcare Data from 2005 to 2014. J Korean Neurosurg Soc. 2021;65:57–63. doi: 10.3340/jkns.2020.0330. [DOI] [PubMed] [Google Scholar]
- 37.Vargas E, Mummaneni PV, Rivera J, et al. Wound complications in metastatic spine tumor patients with and without preoperative radiation. J Neurosurg Spine. 2023;38:265–70. doi: 10.3171/2022.8.SPINE22757. [DOI] [PubMed] [Google Scholar]
- 38.Igoumenou VG, Mavrogenis AF, Angelini A, et al. Complications of spine surgery for metastasis. Eur J Orthop Surg Traumatol. 2020;30:37–56. doi: 10.1007/s00590-019-02541-0. [DOI] [PubMed] [Google Scholar]
- 39.Gadjradj PS, Vreeling A, Depauw PR, et al. Surgeons learning curve of transforaminal endoscopic discectomy for sciatica. Neurospine. 2022;19:594–602. doi: 10.14245/ns.2244342.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Huec JC, AlEissa S, Bowey AJ, et al. Hemostats in spine surgery: literature review and expert panel recommendations. Neurospine. 2022;19:1–12. doi: 10.14245/ns.2143196.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luksanapruksa P, Buchowski JM, Tongsai S, et al. Systematic review and meta-analysis of effectiveness of preoperative embolization in surgery for metastatic spine disease. J Neurointerv Surg. 2018;10:596–601. doi: 10.1136/neurintsurg-2017-013350. [DOI] [PubMed] [Google Scholar]
- 42.Groot OQ, van Steijn NJ, Ogink PT, et al. Preoperative embolization in surgical treatment of spinal metastases originating from non-hypervascular primary tumors: a propensity score matched study using 495 patients. Spine J. 2022;22:1334–44. doi: 10.1016/j.spinee.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Park JH, Jeon SR. Pre- and postoperative lower extremity motor power and ambulatory status of patients with spinal cord compression due to a metastatic spinal tumor. Spine (Phila Pa 1976) 2013;38:E798–802. doi: 10.1097/BRS.0b013e3182927559. [DOI] [PubMed] [Google Scholar]
- 44.Tancioni F, Navarria P, Pessina F, et al. Early surgical experience with minimally invasive percutaneous approach for patients with metastatic epidural spinal cord compression (MESCC) to poor prognoses. Ann Surg Oncol. 2012;19:294–300. doi: 10.1245/s10434-011-1894-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of surgical-related complications and its detail
Summary of the previous reports of endoscopic surgery in spinal metastases patients