Abstract
Background
Lung transplantation median survival has seen improvements due to recognition of short-term survival factors but continues to trail behind other solid organs due to limited understanding of long-term survivorship. Given the creation of the United Network for Organ Sharing (UNOS) database in 1986, it was difficult to accrue data on long-term survivors until recently. This study characterizes factors impacting lung transplant survival beyond 20 years, conditional to 1-year survival.
Methods
Lung transplant recipients listed in UNOS from 1987 to 2002 who survived to 1 post-transplant year were reviewed. Kaplan-Meier and adjusted Cox regression analyses were performed at 20 and 10 years to identify risk factors associated with long-term outcomes independent of their short-term effects.
Results
A total of 6,172 recipients were analyzed, including 472 (7.6%) recipients who lived 20+ years. Factors associated with increased likelihood of 20-year survival were female-to-female gender match, recipient age 25–44, waitlist time >1 year, human leukocyte antigen (HLA) mismatch level 3, and donor cause of death: head trauma. Factors associated with decreased 20-year survival included recipient age ≥55, chronic obstructive pulmonary disease/emphysema (COPD/E) diagnosis, donor smoking history >20 pack-years, unilateral transplant, blood groups O&AB, recipient glomerular filtration rate (GFR) <10 mL/min, and donor GFR 20–29 mL/min.
Conclusions
This is the first study identifying factors associated with multiple-decade survival following lung transplant in the United States. Despite its challenges, long-term survival is possible and more likely in younger females in good waitlist condition without COPD/E who receive a bilateral allograft from a non-smoking, gender-matched donor of minimal HLA mismatch. Further analysis of the molecular and immunologic implications of these conditions are warranted.
Keywords: Long-term survival, bronchiolitis obliterans, bilateral transplant, gender matching
Highlight box.
Key findings
• Long-term survival following lung transplantation is more likely in young, female recipients without COPD and in healthy waitlist condition who receive a bilateral allograft from a non-smoking, gender-matched donor of minimal HLA mismatch.
What is known and what is new?
• CLAD remains a barrier to long-term survival following lung transplantation, which lags significantly behind other solid organs.
• This manuscript is the first to identify factors associated with long-term survival up to 20 years post-transplant.
What is the implication, and what should change now?
• Further investigation into the roles that HLA antigens, Y chromosome antigens, and cigarette smoke may play in CLAD pathogenesis is recommended.
• Bilateral transplantation should be favored in regions where donor supply allows.
Introduction
The first human lung transplantation (LTx) was performed nearly sixty years ago, but survival beyond one year was not achieved until 1988 (1,2). Since then, improvements in surgical technique, perioperative critical care, waitlist allocation, and immunosuppression have made 1-year survival an expectation rather than an exception. Today, LTx is standard therapy for end-stage pulmonary disease, with over 2,500 procedures performed annually in the United States (US). Even so, the incidence of chronic lung allograft dysfunction (CLAD) leaves 5-year LTx survival at around 60% (3,4), trailing behind 5-year heart and kidney survival at 85% and 80%, respectively (5,6).
Though small strides continue to be made to extend LTx median survival time, improvement is mostly confined to the first post-transplant year due to concentrated efforts to reduce primary graft dysfunction and acute rejection (7), with survival dropping off consistently thereafter. Thus, CLAD remains a constant threat to long-term LTx survival (8). While characteristics associated with short-term outcomes like primary graft dysfunction and 1-year mortality are well established, less is known about factors associated with long-term outcomes. Characterizing multiple-decade survivors may provide clues to conditions that promote sustained long-term graft function. An analysis of this sort requires long-term follow-up of a robust number of patients, which was not possible until recently.
The purpose of this study was to analyze recipient, donor and operative characteristics associated with 20-year survival following LTx using over 6,000 recipients in the United Network for Organ Sharing (UNOS) Database, which required inclusion of patients transplanted between 1987 and 2002. Despite the changes in LTx since 1987—namely, bilateral LTx [1988; controllable variable], bronchial anastomosis [1990], tacrolimus Food and Drug Administration approval [1994; survival comparable to cyclosporine (9)], implementing the Lung Allocation Score (LAS) [2005; slight survival increase (10)], inclusion of donation after cardiac death (DCD) donors [2012; outcomes on par with brain-dead donors (11)], and the recent advent of ex vivo lung perfusion (EVLP)—the factors that characterize those first few hundred to achieve 20 years of post-transplant survival remain relevant to our limited contemporary understanding of CLAD. Moreover, our analysis was restricted to only those patients who successfully survived the first post-transplant year, which isolated those factors specifically associated with long-term survival. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1414/rc).
Methods
Study design
We retrospectively identified all patients listed for LTx using the UNOS 2022 Thoracic Database. This study was exempt from institutional review board review, as analysis included data from a publicly available database with de-identified patient information. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study population
Our population of interest was all adult LTx recipients with at least twenty years of follow-up data from transplantation date. To assess 20-year follow-up, only transplants between October 16, 1987, and July 1, 2002, were included (n=8,871). Exclusion criteria included multi-organ transplant (n=20), previous transplant or subsequent retransplant (n=595), and death within one year following LTx (n=2,084). We chose to assess long-term outcomes conditional to 1-year survival to avoid bias from predictors of short-term mortality. Our final cohort included 6,172 adults who underwent LTx during the study period. Demographic data are included in Table 1. We analyzed outcomes from transplantation date until death (n=5,293), lost to follow-up (n=407), or 20-year survival (n=472). Those lost to follow-up before 20 years post-transplant were censored at date of last known follow-up.
Table 1. Demographics and characteristics.
| Characteristics | Entry completion | 1–20 year survivors (N=5,700) | 20+ year survivors (N=472) | P value |
|---|---|---|---|---|
| Preoperative characteristics | ||||
| Age (years), mean ± SD | 100.0% | 49.6±11.5 | 39.5±10.6 | <0.001 |
| BMI (kg/m2), mean ± SD | 98.9% | 23.5±4.7 | 21.6±4.2 | <0.001 |
| Blood group | ||||
| A | 100.0% | 41.1% | 46.4% | 0.024 |
| B | 10.9% | 15.7% | 0.002 | |
| O | 43.3% | 34.3% | <0.001 | |
| AB | 4.7% | 3.6% | 0.267 | |
| Diagnosis | ||||
| Alpha-1-antitrypsin deficiency | 99.2% | 9.9% | 7.7% | 0.112 |
| COPD/emphysema | 48.6% | 19.2% | <0.001 | |
| Cystic fibrosis | 11.9% | 35.5% | <0.001 | |
| IPF/UIP | 14.4% | 8.7% | <0.001 | |
| Pulmonary hypertension | 4.4% | 8.9% | <0.001 | |
| Male recipient | 100.0% | 48.5% | 46.2% | 0.325 |
| African American recipient | 100.0% | 5.7% | 5.1% | 0.557 |
| Waitlist time (years), mean ± SD | 100.0% | 1.00±0.95 | 1.27±1.04 | <0.001 |
| Donor age (years), mean ± SD | 100.0% | 30.3±12.9 | 28.5±12.3 | 0.003 |
| Male donor | 100.0% | 64.7% | 55.7% | <0.001 |
| African American donor | 100.0% | 13.5% | 9.3% | 0.011 |
| Donor cigarette use | 79.1% | 30.2% | 26.5% | 0.125 |
| Donor creatinine, mean ± SD | 78.8% | 1.21±1.71 | 1.22±2.05 | 0.920 |
| Donor COD | ||||
| Anoxia | 99.4% | 4.1% | 5.8% | 0.081 |
| CVA/stroke | 33.8% | 30.9% | 0.211 | |
| Head trauma | 51.0% | 54.2% | 0.183 | |
| Perioperative characteristics | ||||
| Hospital admission | 99.5% | 4.8% | 4.7% | 0.948 |
| ICU admission | 1.8% | 2.8% | 0.134 | |
| Cold ischemia time (hours), mean ± SD | 90.1% | 4.39±1.73 | 5.20±1.80 | <0.001 |
| Recipient creatinine, mean ± SD | 80.0% | 0.94±1.30 | 0.90±0.91 | 0.490 |
| Life support | 99.5% | 2.8% | 4.1% | 0.104 |
| ABO matching | ||||
| Identical | 100.0% | 91.5% | 92.0% | 0.712 |
| Compatible | 8.5% | 8.1% | 0.741 | |
| Incompatible | 0.1% | 0.0% | 0.618 | |
| Perioperative characteristics | ||||
| HLA mismatch level | ||||
| 0 | 82.0% | 0.0% | 0.0% | 0.678 |
| 1 | 0.5% | 0.8% | 0.497 | |
| 2 | 3.4% | 4.0% | 0.551 | |
| 3 | 12.0% | 13.4% | 0.399 | |
| 4 | 27.6% | 28.4% | 0.733 | |
| 5 | 35.7% | 33.1% | 0.285 | |
| 6 | 20.7% | 20.4% | 0.872 | |
| Gender match (donor to recipient) | ||||
| Male to male | 100.0% | 39.4% | 35.0% | 0.056 |
| Male to female | 25.3% | 20.8% | 0.029 | |
| Female to female | 26.2% | 33.1% | 0.001 | |
| Female to male | 9.1% | 11.2% | 0.129 | |
| Single lung transplant | 100.0% | 63.7% | 23.5% | <0.001 |
| Hospital length of stay (days), mean ± SD | 100.0% | 20.9±26.2 | 22.2±25.9 | 0.584 |
| Post-transplant complications | ||||
| Stroke | 80.2% | 1.6% | 1.5% | 0.884 |
| Dialysis | 80.2% | 1.9% | 3.3% | 0.059 |
| Recipient COD | N=5,282 | N=171 | ||
| Graft failure | 94.1% | 20.8% | 15.2% | 0.074 |
| Pulmonary | 19.2% | 17.5% | 0.593 | |
| Infection | 17.1% | 13.5% | 0.209 | |
| Malignancy | 10.2% | 9.4% | 0.712 | |
| Cardiovascular | 4.9% | 7.0% | 0.201 | |
| Multiple organ failure | 4.2% | 7.0% | 0.069 | |
| Renal failure | 3.3% | 7.0% | 0.007 | |
| Cerebrovascular | 1.6% | 2.3% | 0.444 | |
| Hemorrhage | 1.0% | 0.6% | 0.586 | |
| Non-compliance | 0.4% | 0.0% | 0.409 | |
| Other | 17.4% | 20.5% | 0.295 |
P values <0.05 were considered statistically significant. BMI, body mass index; COPD, chronic obstructive pulmonary disease; IPF/UIP, idiopathic pulmonary fibrosis/usual interstitial pneumonitis; COD, cause of death; CVA, cerebrovascular accident; HLA, human leukocyte antigen.
Statistical analysis
Data were analyzed using Stata 17.0 (Stata Corp, College Station, TX, USA). Continuous variables were summarized using mean ± standard deviation and compared using the Student’s t-test. Categorical variables were compared using the Chi-square test. Results were considered significant at P<0.05. The outcome of interest was patient survival, and Kaplan-Meier survival analysis was used to estimate survival over time.
Univariable Cox regression was performed to determine factors associated with 20-year survival. Factors significant on univariable were included in multivariable Cox regression analysis (Table 2). Significant 20-year factors on multivariable were also evaluated on Kaplan-Meier analysis (Figures 1-4). Univariable and multivariable Cox regression was then repeated for 10-year survival to compare factors significant at 10 and 20 years (Table 3). Cox regression for 5-year survival conditional to 1-year survival is listed in Table S1.
Table 2. Univariable and multivariable cox regression: 20-year survival.
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | ||
| Recipient factors | |||||
| Age (years) | |||||
| 18–24 | 0.94 | 0.352 | |||
| 25–34 | 0.64 | <0.001 | 0.77 | <0.001 | |
| 35–44 | 0.74 | <0.001 | 0.83 | <0.001 | |
| 45–54 | 0.96 | 0.117 | |||
| 55–64 | 1.47 | <0.001 | 1.24 | <0.001 | |
| ≥65 | 1.65 | <0.001 | 1.51 | <0.001 | |
| Blood group | |||||
| A | 0.94 | 0.016 | 1.04 | 0.497 | |
| B | 0.94 | 0.117 | |||
| O | 1.07 | 0.011 | 1.11 | 0.048 | |
| AB | 1.15 | 0.030 | 1.23 | 0.015 | |
| BMI | |||||
| <18.5 | 0.86 | <0.001 | 0.92 | 0.524 | |
| 18.5–24.9 | 0.93 | 0.008 | 0.87 | 0.287 | |
| 25.0–29.9 | 1.17 | <0.001 | 1.00 | 0.971 | |
| 30.0–34.9 | 1.13 | 0.014 | 0.97 | 0.828 | |
| 35.0–39.9 | 1.02 | 0.847 | |||
| ≥40.0 | 1.56 | 0.183 | |||
| Diagnosis | |||||
| Alpha-1-antitrypsin deficiency | 0.94 | 0.181 | |||
| COPD/emphysema | 1.39 | <0.001 | 1.10 | 0.036 | |
| Cystic fibrosis | 0.65 | <0.001 | 1.08 | 0.222 | |
| IPF/UIP | 1.16 | <0.001 | 1.02 | 0.733 | |
| Pulmonary hypertension | 0.77 | <0.001 | 0.99 | 0.941 | |
| GFR (mL/min) | |||||
| <10 | 1.86 | 0.004 | 1.87 | 0.021 | |
| 10–19 | 1.00 | 0.989 | |||
| 20–29 | 1.29 | 0.238 | |||
| 30–39 | 0.97 | 0.801 | |||
| 40–49 | 0.94 | 0.462 | |||
| ≥50 | 0.99 | 0.813 | |||
| Life support before transplant | 0.82 | 0.012 | 0.91 | 0.278 | |
| Waitlist time | |||||
| <6 months | 1.15 | <0.001 | 0.96 | 0.389 | |
| 6 months to 1 year | 1.13 | <0.001 | Reference | ||
| 1–2 years | 0.91 | 0.002 | 0.90 | 0.016 | |
| >2 years | 0.75 | <0.001 | 0.79 | <0.001 | |
| Donor/transplant factors | |||||
| Donor age (years) | |||||
| <15 | 0.91 | 0.134 | |||
| 15–24 | 0.96 | 0.092 | |||
| 25–34 | 0.95 | 0.169 | |||
| 35–44 | 1.07 | 0.047 | 1.04 | 0.366 | |
| 45–54 | 1.07 | 0.072 | |||
| ≥55 | 1.14 | 0.052 | |||
| Donor cause of death | |||||
| CVA | 1.07 | 0.019 | Reference | ||
| Anoxia | 0.84 | 0.010 | 0.87 | 0.058 | |
| Head trauma | 0.94 | 0.020 | 0.92 | 0.009 | |
| Donor cigarette use | 1.09 | 0.011 | 1.08 | 0.025 | |
| Donor ethnicity: African American | 1.07 | 0.001 | 1.04 | 0.082 | |
| Donor GFR (mL/min) | |||||
| <10 | 0.92 | 0.489 | |||
| 10–19 | 1.12 | 0.367 | |||
| 20–29 | 1.26 | 0.012 | 1.27 | 0.023 | |
| 30–39 | 1.01 | 0.931 | |||
| 40–49 | 1.01 | 0.924 | |||
| ≥50 | 0.96 | 0.261 | |||
| Gender match | |||||
| Male to male | 1.06 | 0.035 | 0.95 | 0.144 | |
| Male to female | 1.08 | 0.080 | |||
| Female to male | 0.97 | 0.458 | |||
| Female to female | 0.88 | <0.001 | 0.89 | 0.003 | |
| ABO incompatible | 1.02 | 0.968 | |||
| HLA mismatch level | |||||
| 0 | 0.70 | 0.614 | |||
| 1 | 0.85 | 0.364 | |||
| 2 | 0.91 | 0.182 | |||
| 3 | 0.92 | 0.029 | 0.89 | 0.010 | |
| 4 | 1.00 | 0.931 | |||
| 5 | 1.02 | 0.522 | |||
| 6 | 1.06 | 0.067 | |||
| Ischemic time (hours) | |||||
| <1.0 | 1.06 | 0.735 | |||
| 1.0–1.9 | 1.34 | <0.001 | 1.20 | 0.013 | |
| 2.0–2.9 | 1.20 | <0.001 | 1.05 | 0.342 | |
| 3.0–3.9 | 1.19 | <0.001 | 1.05 | 0.232 | |
| 4.0–4.9 | 0.99 | 0.758 | |||
| ≥5.0 | 0.75 | <0.001 | 1.00 | 0.924 | |
| Size match: undersized donor | 1.00 | 0.956 | |||
| Size match: oversized donor | 1.03 | 0.429 | |||
| Unilateral lung transplant | 1.68 | <0.001 | 1.30 | <0.001 | |
P<0.05 is significant on multivariable analysis. BMI, body mass index; COPD, chronic obstructive pulmonary disease; IPF/UIP, idiopathic pulmonary fibrosis/usual interstitial pneumonitis; GFR, glomerular filtration rate; CVA, cerebrovascular accident; HLA, human leukocyte antigen.
Figure 1.
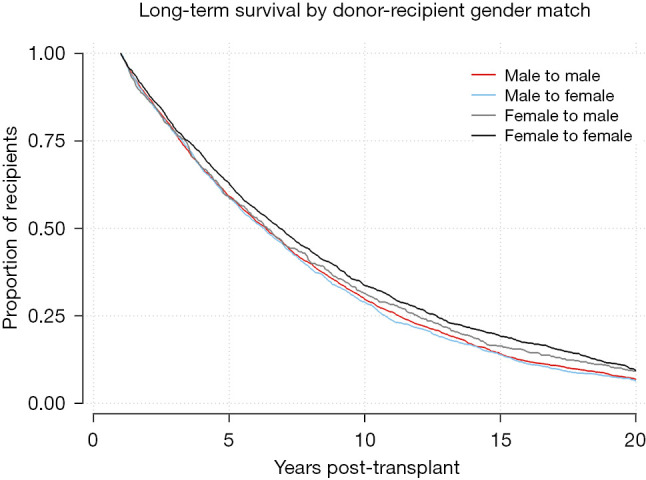
Kaplan-Meier survival function for 1-year survivors by gender-matched cohort. Log-Rank test P=0.0001.
Figure 2.
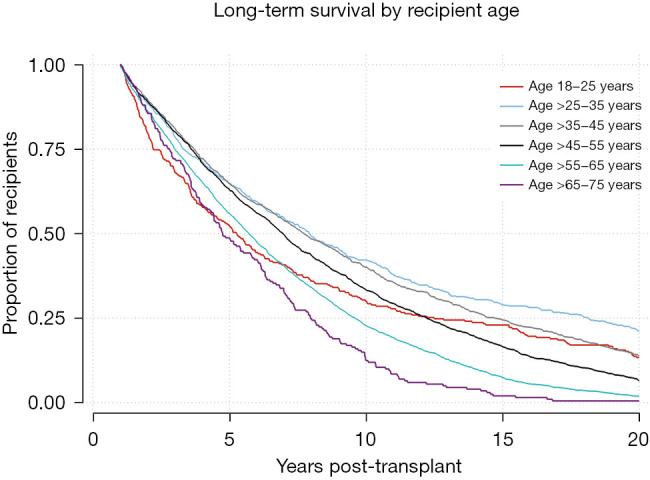
Kaplan-Meier survival function for 1-year survivors by recipient age. Log-Rank test P<0.0001.
Figure 3.
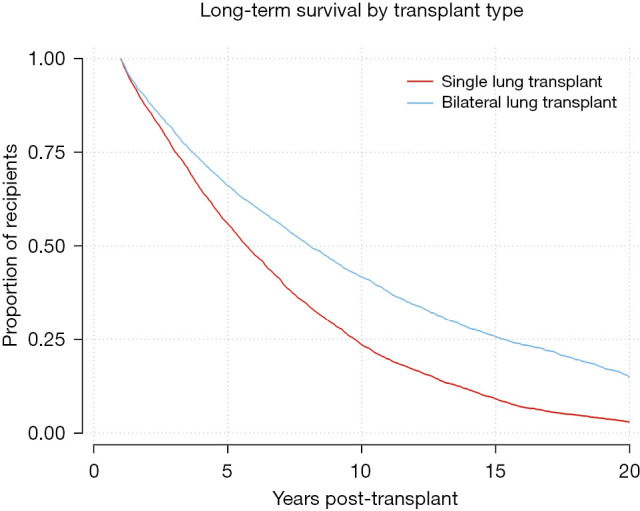
Kaplan-Meier survival function for 1-year survivors by single versus bilateral transplant. Log-Rank test P<0.0001.
Figure 4.
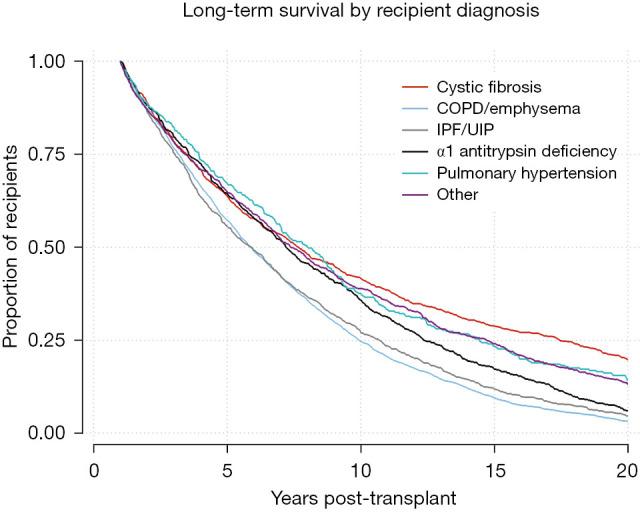
Kaplan-Meier survival function for 1-year survivors by recipient diagnosis. Log-Rank test P<0.0001. COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; UIP, usual interstitial pneumonitis.
Table 3. Univariable and multivariable cox regression: 10-year survival.
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | ||
| Recipient factors | |||||
| Age (years) | |||||
| 18–24 | 1.13 | 0.092 | |||
| 25–34 | 0.74 | <0.001 | 0.60 | <0.001 | |
| 35–44 | 0.76 | <0.001 | 0.56 | <0.001 | |
| 45–54 | 0.89 | 0.001 | 0.57 | <0.001 | |
| 55–64 | 1.35 | <0.001 | 0.71 | .004 | |
| ≥65 | 1.57 | <0.001 | 0.85 | 0.263 | |
| Blood group | |||||
| A | 0.94 | 0.046 | 0.96 | 0.313 | |
| B | 0.98 | 0.679 | |||
| O | 1.04 | 0.176 | |||
| AB | 1.17 | 0.025 | 1.18 | 0.044 | |
| BMI | |||||
| <18.5 | 0.90 | 0.019 | 0.95 | 0.543 | |
| 18.5–24.9 | 0.93 | 0.013 | 0.92 | 0.182 | |
| 25.0–29.9 | 1.15 | <0.001 | 1.04 | 0.532 | |
| 30.0–34.9 | 1.08 | 0.171 | |||
| 35.0–39.9 | 0.93 | 0.622 | |||
| ≥40.0 | 1.54 | 0.223 | |||
| Diagnosis | |||||
| Alpha-1-antitrypsin deficiency | 0.85 | 0.003 | Reference | ||
| COPD/emphysema | 1.29 | <0.001 | 1.11 | 0.088 | |
| Cystic fibrosis | 0.75 | <0.001 | 1.00 | 0.957 | |
| IPF/UIP | 1.13 | 0.004 | 1.02 | 0.744 | |
| Pulmonary hypertension | 0.80 | 0.004 | 1.00 | 0.967 | |
| GFR (mL/min) | |||||
| <10 | 2.01 | 0.001 | 2.02 | 0.012 | |
| 10–19 | 0.67 | 0.329 | |||
| 20–29 | 1.22 | 0.436 | |||
| 30–39 | 1.04 | 0.788 | |||
| 40–49 | 1.01 | 0.955 | |||
| ≥50 | 0.94 | 0.347 | |||
| Life support before transplant | 0.78 | 0.012 | 0.84 | 0.096 | |
| Waitlist time | |||||
| <6 months | 1.15 | <0.001 | 0.99 | 0.772 | |
| 6 months to 1 year | 1.12 | 0.001 | Reference | ||
| 1–2 years | 0.91 | 0.007 | 0.93 | 0.143 | |
| >2 years | 0.75 | <0.001 | 0.83 | 0.001 | |
| Donor/transplant factors | |||||
| Donor age (years) | |||||
| <15 | 0.96 | 0.575 | |||
| 15–24 | 0.95 | 0.081 | |||
| 25–34 | 0.94 | 0.095 | |||
| 35–44 | 1.11 | 0.009 | 1.08 | 0.097 | |
| 45–54 | 1.06 | 0.178 | |||
| ≥55 | 1.08 | 0.341 | |||
| Donor cause of death | |||||
| CVA | 1.08 | 0.014 | Reference | ||
| Anoxia | 0.81 | 0.009 | 0.80 | 0.012 | |
| Head trauma | 0.93 | 0.026 | 0.90 | 0.009 | |
| Donor cigarette use | 1.09 | 0.022 | 1.07 | 0.062 | |
| Donor ethnicity: African American | 1.06 | 0.007 | 1.03 | 0.317 | |
| Donor GFR (mL/min) | |||||
| <10 | 0.91 | 0.509 | |||
| 10–19 | 1.08 | 0.599 | |||
| 20–29 | 1.28 | 0.016 | 1.34 | 0.011 | |
| 30–39 | 1.03 | 0.736 | |||
| 40–49 | 1.03 | 0.664 | |||
| ≥50 | 0.94 | 0.169 | |||
| Gender match | |||||
| Male to male | 1.04 | 0.168 | |||
| Male to female | 1.07 | 0.071 | |||
| Female to male | 0.99 | 0.891 | |||
| Female to female | 0.90 | 0.002 | 0.90 | 0.017 | |
| ABO incompatible | 1.06 | 0.936 | |||
| HLA mismatch level | |||||
| 0 | 1.36 | 0.661 | |||
| 1 | 0.78 | 0.265 | |||
| 2 | 0.85 | 0.069 | |||
| 3 | 0.88 | 0.006 | 0.87 | 0.012 | |
| 4 | 1.01 | 0.677 | |||
| 5 | 0.99 | 0.818 | |||
| 6 | 1.13 | 0.001 | 1.08 | 0.079 | |
| Ischemic time (hours) | |||||
| <1.0 | 0.92 | 0.694 | |||
| 1.0–1.9 | 1.30 | <0.001 | 1.22 | 0.017 | |
| 2.0–2.9 | 1.18 | <0.001 | 1.06 | 0.325 | |
| 3.0–3.9 | 1.18 | <0.001 | 1.09 | 0.101 | |
| 4.0–4.9 | 0.98 | 0.611 | |||
| ≥5.0 | 0.75 | <0.001 | 0.99 | 0.858 | |
| Size match: undersized donor | 0.98 | 0.734 | |||
| Size match: oversized donor | 0.99 | 0.827 | |||
| Unilateral lung transplant | 1.58 | <0.001 | 1.31 | <0.001 | |
P<0.05 is significant on multivariable analysis. BMI, body mass index; COPD, chronic obstructive pulmonary disease; IPF/UIP, idiopathic pulmonary fibrosis/usual interstitial pneumonitis; GFR, glomerular filtration rate; CVA, Cerebrovascular accident; HLA, human leukocyte antigen.
Potential recipient and donor risk factors were evaluated for entry completion and statistical significance. Missing variables were imputed using predictive mean matching multiple imputation. Continuous variables were stratified into categorical variables for Cox regression analysis using standard ranges [body mass index (BMI)], groups of ten [age, glomerular filtration rate (GFR)], by hour (ischemic time), or at the authors’ discretion (waitlist time). Size matching was based on donor height as a percentage of recipient height, with undersized and oversized ranges corresponding to the bottom and top 10th percentiles, respectively. Reference ranges were used for pretransplant diagnosis, waitlist time, and donor cause of death to avoid multivariable collinearity.
Additional analyses
The supplementary materials include additional and subgroup analyses. Tables S2,S3 contains propensity score matching for further evaluation of our findings on unilateral transplantation, ischemia time, and female-to-female gender matching. First, the 1–20 and 20+ year survivor groups were matched by proportion of unilateral transplants alone to evaluate the extent to which the significant cold ischemic time difference between groups was merely due to bilateral transplants reporting ischemic time as the average of both allografts. Separately, female recipients with male versus female donors were matched using significant factors from Tables 1-3, along with donor-to-recipient height ratio to account for organ size. Subsequently, our 20-year multivariable Cox regression model—with donor: recipient height ratio included as a covariate—was run on the matched cohort to evaluate the remaining protective effect of female recipients with female donors compared to those with male donors. Tables S4,S5 represent our multivariable model applied to graft survival instead of patient survival and a subgroup analysis by graft versus non-graft recipient cause of death, respectively.
Results
Study population
Our patient cohort included 6,172 patients, 48.4% of whom were male, with a mean age of 48.8±11.8 years. Mean donor age was 30.2±12.9 years, with 3,739 single lung transplants (60.6%) and 2,433 bilateral lung transplants (39.4%). Recipient diagnoses included chronic obstructive pulmonary disease/emphysema (COPD/E) (n=2,852, 46.2%), idiopathic pulmonary fibrosis/usual interstitial pneumonia (IPF/UIP) (n=871, 14.1%), cystic fibrosis (CF) (n=843, 13.7%), alpha-1-antitrypsin deficiency (n=487, 7.9%) and pulmonary hypertension (PH) (4.8%, n=295), with 824 patients (13.4%) of other miscellaneous diagnoses.
Characteristics for 1–20-year survivors and 20+ year survivors are illustrated in Table 1. In total, 472 recipients (7.6%) lived 20 or more years following transplantation. 20+ year survivors demonstrated lower recipient age (39.5 vs. 49.6 years, P<0.001), donor age (28.5 vs. 30.3 years, P=0.003), and recipient BMI (21.6 vs. 23.5 kg/m2, P<0.001). They also spent roughly three more months on the waitlist (1.27 vs. 1.00 years, P<0.001). 20+ year survivors were more likely to have pretransplant diagnosis of CF (35.5% vs. 11.9%, P<0.001) or PH (8.9% vs. 4.4%, P<0.001), while less likely to have IPF/UIP (8.7% vs. 14.4%, P<0.001) or COPD/E (19.2% vs. 48.6%, P<0.001). While there was no significant difference with regards to recipient gender, 20+ year survivors were more likely to have a female donor (44.3% vs. 35.3%, P<0.001) and less likely to have an African American donor (9.3% vs. 13.5%, P=0.011).
Additionally, single lung transplants accounted for less than one-quarter of 20+ year survivors but nearly two-thirds of 1–20-year survivors (23.5% vs. 63.7%, P<0.001). There were also significantly more female-to-female recipient-donor gender pairs in the 20+ year survival cohort (33.1% vs. 26.2%, P=0.001) and fewer male-to-female matches (20.8% vs. 25.3%, P=0.029). 20+ year survivors also experienced longer cold ischemic times (5.20 vs. 4.39 hours, P<0.001). There were no significant differences between cohorts regarding recipient gender or race, donor or recipient creatinine, life support status, hospital admission status, donor cigarette use, donor cause of death, ABO matching, or human leukocyte antigen (HLA) mismatch level. Importantly, these similarities between cohorts largely extended to post-transplant outcomes, with no significant differences in hospital length of stay, post-transplant complications, or recipient cause of death (most common causes: graft failure, pulmonary cause, infection, and malignancy). However, 20+ year survivors exhibiting slightly more renal failure as a cause of death (7.0% vs. 3.3%, P=0.007) (Table 1).
Multivariable analysis
Univariable and multivariable Cox regression for 20-year survival is reflected in Table 2. Risk factors associated with death prior to 20 years after transplantation include recipient age ≥55 years [hazard ratio (HR) =1.24–1.51], recipient pretransplant diagnosis of COPD/E (HR =1.10), donor cigarette use (HR =1.08), and single lung transplant (HR =1.30), along with decreased donor and recipient GFR and short ischemic time. Protective factors for 20+ year survival include recipient age 25–45 (HR =0.77–0.83), female-to-female gender matching (HR =0.89), HLA mismatch level 3 (HR =0.89), and waitlist time >1 year (HR =0.79–0.90).
Univariable and multivariable Cox regression for 10-year survival is reflected in Table 3. The recipient age range associated with greater likelihood of 10+ year survival was broader (25–64 years) than it was in the 20+ year analysis, while longer waitlist time was only protective at >2 years. COPD/E diagnosis and donor smoking history were no longer risk factors for reduced likelihood of 10+ year survival as they had been in the 20+ year survival analysis. Otherwise, most of the 20-year factors remained significant at 10 years; namely: single lung transplant, recipient GFR <10 mL/min, and donor GFR 20–29 mL/min were risk factors, while female-to-female gender matching and HLA mismatch level 3 were protective at 10 years. At 5 years, recipient aged 18–24 bore out as a new significant risk factor (HR =2.02) (Table S1).
Kaplan-Meier analysis
Kaplan-Meier analysis was performed to visualize various trends in long-term outcomes conditional to successful 1-year survival over the 20-year period following LTx. Figure 1 shows long-term survivorship by gender-matched cohort. Figure 2 illustrates survival trends to 20 years by recipient age. Figure 3 demonstrates difference in long-term survival between unilateral and bilateral lung transplants. Long-term survival by recipient diagnosis is available in Figure 4. Log-rank tests for each Kaplan-Meier analysis were significant at P≤0.0001.
Propensity score matching
Propensity score matching is available in the supplemental materials as Tables S2,S3. Matching the 1–20 and 20+ year survivor groups for proportion of unilateral transplants alone resulted in a 97.3% reduction in the bias of cold ischemia time between groups, resulting in a non-significant difference in ischemia time (P=0.858) (Table S2). Female recipients with male versus female donors were matched using significant factors from Tables 1-3 and donor-to-recipient height ratio to account for organ size. Female-to-female gender matching compared to male-to-female remained significant (HR =0.85, P=0.001) using our 20-year multivariable Cox regression model even after matching and adjusting for donor: recipient height (Table S3).
Discussion
This study determined the factors influencing LTx survival up to and exceeding twenty years post-transplant via multivariable analysis. While several studies have investigated factors associated with long-term outcomes (7,12,13), none have explored multiple decades. Significant changes in LTX have occurred since 1987 and must be considered when utilizing the UNOS database. Relevant changes include bilateral LTx [1988], the technique of bronchial anastomosis [1990], treatment with tacrolimus [1994], allocation using the LAS [2005], utilization of DCD donors [2012], and use of EVLP. The most significant change that limits the generalizability of our results to a contemporary cohort is the LAS, which initiated allocation based on estimated urgency and utility rather than waitlist time (10). Specifically, the LAS favored transplantation of more IPF/UIP recipients than our cohort, which has drastically reduced waitlist mortality. These improvements mainly bolstered 1-year survival; while long-term survival is improving, it continues to lag significantly behind other solid organs (8). Understanding of this discrepancy is limited to the notion that immunosuppressed and nonsterile lungs yield infection, innate immune activation, and ultimately CLAD (14); however, because this phenomenon remains poorly understood, a characterization of long-term survivors remains essential novel information.
In comparing demographics between 1–20-year and 20+ year survivors, factors associated with 20+ year survival included lower recipient and donor age (Table 1). Recipients living 20+ years were 10 years younger at time of transplant and received lungs from donors 2 years younger. Importantly, advanced donor age was not associated with reduced likelihood of 10+ or 20+ year survival after controlling for other factors (Tables 2,3). This is consistent with a previous study showing lack of effect of donor age on 5-year survival (15). By contrast, smoking bore significant risk. Both donor cigarette use >20 pack-years and recipient diagnosis of COPD/E remained moderate long-term risk factors independent their short-term effects. There were no meaningful differences in postoperative complications such as stroke or dialysis or cause of death, with graft failure, pulmonary cause, infection, and malignancy representing the most common causes for both cohorts (Table 1). This indicates that a constellation of protective factors allowed long-term survivors to delay the advent of similar outcomes.
Our results on single versus bilateral LTx align with the most recent, statistically rigorous studies of large datasets. For instance, Weiss et al. demonstrated a doubled 10-year survival rate among younger patients receiving bilateral LTx compared to unilateral (16). A 2020 multicenter study even found that bilateral LTx specifically reduced acute rejection rates (17). Perhaps most relevant was the demonstration that bilateral recipients exhibit both milder symptoms and decreased incidence of bronchiolitis obliterans syndrome (BOS)—a prevalent subset of CLAD and a predominant cause of death in the late post-transplant period (18,19). Our analysis likewise demonstrates that a double lung yields superior individual longevity (Figure 3), though not necessarily overall life-years gained. Universal bilateral LTx would reduce the number of available organs for waitlisted patients and may only be feasible in regions where donor supply adequately satisfies demand (20).
Our analysis produced some unexpected results. Size matching was not associated with 10+ or 20+ year survival despite being a focus theme for the 2019 International Society for Heart and LTx report (3). Paradoxically, shorter cold ischemia time may appear to be a long-term risk factor; however ischemic time for double lungs reflects the mean value for both lungs, an important consideration for future UNOS studies (21). After propensity score matching for unilateral transplant alone, the association between longer ischemic time and survival disappeared entirely. Thus, our findings on ischemic time are likely only a reflection of an increased proportion of bilateral LTx in the 20+ year survival cohort, for whom the mean ischemic time is necessarily higher. Another surprising finding was that increased waitlist time was protective for both 10- and 20-year survival. Organ allocation during the study period was based purely on waitlist time, so this finding is likely influenced by survivorship bias. Patients with lower acuity of illness or comorbidity burden would be able to live longer without LTx, resulting in the cohort with longer waitlist times demonstrating increased post-LTx survival longer by comparison. Still, this finding underscores the importance of listing patients in the best overall condition possible and preserving vital organ function like GFR through the perioperative period to maximize longevity. This concept should be tempered by the fact that there remain major challenges to LTx; generally, recipients should be listed when their expected chance of surviving 1–2 years without transplant is less than 50%.
Our findings raised several immunological considerations. Reduced HLA mismatch was a long-term protective factor. This is unsurprising given that HLA mismatch has been shown to bring about CLAD via acute cellular rejection (22). While our findings on recipient gender were consistent with prior observations favoring female recipients (23), the advantage for female-to-female donor-recipient matching was novel. Gender matching for LTx has been evaluated in the past with varying conclusions: a smaller study found no significant correlation (24), with a slightly larger study noting increased 5-year survival, indicating female-to-male mismatch as the strongest predictor of reduced survival (25). By contrast, our propensity matched, adjusted Cox regression (Table S3) revealed female donors to be significantly protective compared to male donors when the recipient is female. It is possible that the favorability of female-to-female matching over male-to-female is due to an immunological advantage of chromosomal matching. Transplanting an XX female donor into an XX female recipient eliminates the possibility of developing an immune response to Y chromosome antigens (26). Still, 69% of our 20+ year survivors were not female-to-female, and given the donor shortage, this level of matching may not always be feasible.
A final consideration is how our findings relate to the contemporary state of LTx and predicted 20-year survival. We compared the characteristics of our cohort with a study on recent trends in LTx (27). Since our 1987–2002 cohort, recipients have become older, more male, and of higher BMI. Donors are also older on average, with less cigarette use. Donor cause of death has seen more anoxia and less head trauma recently, both of which are protective compared to cerebrovascular accident, which has remained constant. Pretransplant diagnosis has perhaps seen the most significant change. As medical therapies continue to improve, the contemporary cohort is comprised of fewer patients with CF, PH, or COPD/E, in exchange for increased IPF/UIP. Perhaps most significant is the drastic increase in proportion of bilateral LTx from about 40% to over 70%, which is likely to have the most optimistic impact on 20-year survival of today’s recipients (27).
The demand for LTx continues to climb in the US (28), where cost per life-year gained is significantly higher than other solid organ transplants (29). Our findings suggest areas for future investigation on the immunological roles that histocompatibility antigens, Y chromosome antigens, and carbon black from smoking may play in CLAD pathogenesis. They also support bilateral LTx and provide a unique and optimistic perspective on life-years gained in LTx given a favorable balance of protective and risk factors.
Limitations
A limitation inherent to our research question was that our cohort was transplanted twenty years ago. Alterations in immunosuppressive regimens, procurement technique, preservation methods (i.e., Perfadex), and transplantation protocols limit generalizability to the contemporary era. UNOS collects data on US transplants, so our results may not be generalizable internationally. Though data entry is mandatory in all US transplant centers, patient registries suffer from entry variability. Cause of death, among other variables, can be subject to provider interpretation. Though our primary outcome was quantifiable survival, the UNOS dataset cannot portray what quality of life may have accompanied these additional years of life. A final limitation is a lack of consideration of events following LTx, but we censored patients that did not survive one year to reduce confounding effects of early postoperative events.
Conclusions
Our investigation of 6,172 LTx recipients living one year after LTx used multivariable analysis to identify factors associated with 20+ year survival. This analysis is important for both patient and provider education, granting insight into conditions that predispose recipients to sustained long-term graft function and providing optimism for longevity in patients surviving to one year. Protective factors included younger recipient age, bilateral LTx, and female-to-female gender matching. Our results substantiate the long-term benefits of minimal donor and recipient smoking history, irrespective of their associated protective effects in the short-term, while also favoring increased utilization of donors of advanced age. Additionally, management of recipient factors like renal function during the perioperative period contributed to patient longevity. Finally, our findings on female-to-female gender matching, HLA matching, and donor smoking history denote long-term immunologic considerations that warrant further investigation into the molecular mechanisms underlying long-term graft dysfunction.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1414/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1414/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1414/coif). GL serves as a consultant and scientific board advisor for TransMedics and Abiomed Breethe, and he receives research grant support from TransMedics, Abiomed Breethe, AtriCure, and the JLH Foundation. The other authors have no conflicts of interest to declare.
References
- 1.Hardy JD, Webb WR, Dalton ML, Jr, et al. Lung homotransplantation in man. JAMA 1963;186:1065-74. 10.1001/jama.1963.63710120001010 [DOI] [PubMed] [Google Scholar]
- 2.Patterson GA, Cooper JD, Goldman B, et al. Technique of successful clinical double-lung transplantation. Ann Thorac Surg 1988;45:626-33. 10.1016/S0003-4975(10)64763-7 [DOI] [PubMed] [Google Scholar]
- 3.Chambers DC, Perch M, Zuckermann A, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant 2021;40:1060-72. 10.1016/j.healun.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2020 Annual Data Report: Lung. Am J Transplant 2022;22 Suppl 2:438-518. 10.1111/ajt.16991 [DOI] [PubMed] [Google Scholar]
- 5.Colvin M, Smith JM, Ahn Y, et al. OPTN/SRTR 2020 Annual Data Report: Heart. Am J Transplant 2022;22 Suppl 2:350-437. 10.1111/ajt.16977 [DOI] [PubMed] [Google Scholar]
- 6.Ghelichi-Ghojogh M, Ghaem H, Mohammadizadeh F, et al. Graft and Patient Survival Rates in Kidney Transplantation, and Their Associated Factors: A Systematic Review and Meta-Analysis. Iran J Public Health 2021;50:1555-63. 10.18502/ijph.v50i8.6801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa J, Benvenuto LJ, Sonett JR. Long-term outcomes and management of lung transplant recipients. Best Pract Res Clin Anaesthesiol 2017;31:285-97. 10.1016/j.bpa.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Panchabhai TS, Chaddha U, McCurry KR, et al. Historical perspectives of lung transplantation: connecting the dots. J Thorac Dis 2018;10:4516-31. 10.21037/jtd.2018.07.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y, Xiao YB, Weng YG. Tacrolimus versus cyclosporine for adult lung transplant recipients: a meta-analysis. Transplant Proc 2009;41:1821-4. 10.1016/j.transproceed.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 10.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016;35:433-9. 10.1016/j.healun.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 11.Bobba CM, Whitson BA, Henn MC, et al. Trends in Donation After Circulatory Death in Lung Transplantation in the United States: Impact Of Era. Transpl Int 2022;35:10172. 10.3389/ti.2022.10172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Divithotawela C, Cypel M, Martinu T, et al. Long-term Outcomes of Lung Transplant With Ex Vivo Lung Perfusion. JAMA Surg 2019;154:1143-50. 10.1001/jamasurg.2019.4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jawitz OK, Raman V, Becerra D, et al. Factors associated with short- versus long-term survival after lung transplant. J Thorac Cardiovasc Surg 2022;163:853-860.e2. 10.1016/j.jtcvs.2020.09.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawashima M, Juvet SC. The role of innate immunity in the long-term outcome of lung transplantation. Ann Transl Med 2020;8:412. 10.21037/atm.2020.03.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holley CT, Kelly RF, Shumway SJ, et al. Clinical implications of donor age: A single-institution analysis spanning 3 decades. J Thorac Cardiovasc Surg 2017;154:2126-2133.e2. 10.1016/j.jtcvs.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 16.Weiss ES, Allen JG, Merlo CA, et al. Factors indicative of long-term survival after lung transplantation: a review of 836 10-year survivors. J Heart Lung Transplant 2010;29:240-6. 10.1016/j.healun.2009.06.027 [DOI] [PubMed] [Google Scholar]
- 17.Todd JL, Neely ML, Kopetskie H, et al. Risk Factors for Acute Rejection in the First Year after Lung Transplant. A Multicenter Study. Am J Respir Crit Care Med 2020;202:576-85. 10.1164/rccm.201910-1915OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni HS, Cherikh WS, Chambers DC, et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J Heart Lung Transplant 2019;38:5-16. 10.1016/j.healun.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bos S, Vos R, Van Raemdonck DE, et al. Survival in adult lung transplantation: where are we in 2020? Curr Opin Organ Transplant 2020;25:268-73. 10.1097/MOT.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 20.Mendogni P, Palleschi A, Tosi D, et al. Lobar Lung Transplantation From Deceased Donor: Monocentric Experience. Transplant Proc 2017;49:682-5. 10.1016/j.transproceed.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 21.Nęcki M, Antończyk R, Pandel A, et al. Impact of Cold Ischemia Time on Frequency of Airway Complications Among Lung Transplant Recipients. Transplant Proc 2020;52:2160-4. 10.1016/j.transproceed.2020.03.047 [DOI] [PubMed] [Google Scholar]
- 22.Rajalingam R. Allele-level HLA matching reduces early rejection in lung transplant recipients. Ann Transl Med 2020;8:275. 10.21037/atm.2020.02.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loor G, Brown R, Kelly RF, et al. Gender differences in long-term survival post-transplant: A single-institution analysis in the lung allocation score era. Clin Transplant 2017. 10.1111/ctr.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fessart D, Dromer C, Thumerel M, et al. Influence of gender donor-recipient combinations on survival after human lung transplantation. Transplant Proc 2011;43:3899-902. 10.1016/j.transproceed.2011.08.101 [DOI] [PubMed] [Google Scholar]
- 25.Demir A, Coosemans W, Decaluwé H, et al. Donor-recipient matching in lung transplantation: which variables are important? Eur J Cardiothorac Surg 2015;47:974-83. 10.1093/ejcts/ezu340 [DOI] [PubMed] [Google Scholar]
- 26.Matthews JC, Aaronson KD. Sex matters, but to what clinical avail? Circ Heart Fail 2009;2:389-92. 10.1161/CIRCHEARTFAILURE.109.902460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miggins JJ, Reul RM, Jr, Loor G, et al. Trends in marginal lung allograft survival: Advanced-age donors improve. Clin Transplant 2022;36:e14777. 10.1111/ctr.14777 [DOI] [PubMed] [Google Scholar]
- 28.Lautner LJ, Freed DH, Nagendran J, et al. Current techniques and the future of lung preservation. Cryobiology 2020;94:1-8. 10.1016/j.cryobiol.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 29.Kaiser LR. Donor lung allocation: who shall live; who shall die? Arch Surg 2011;146:1209-10. 10.1001/archsurg.2011.242 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


