Abstract
Background
Durvalumab and atezolizumab have recently been approved in extensive small cell lung cancer (SCLC) with moderate median overall survival (OS) improvements. However, only limited data exist regarding the impact of immunotherapy in real-world SCLC patients. This study sought to assess the efficacy and safety of atezolizumab plus chemotherapy and durvalumab plus chemotherapy in the treatment of SCLC in a real-world setting.
Methods
A retrospective cohort study of all patients treated for SCLC with chemotherapy with PD-L1 inhibitor, at 3 centers in China between February 1, 2020 and April 30, 2022. Patient characteristics, adverse-events and survival analyses were conducted.
Results
A total of 143 patients were enrolled in this study, 100 were treated with durvalumab and the remainder with atezolizumab. The baseline characteristics of the two groups were fundamentally balanced before using PD-L1 inhibitors (P>0.05). The median OS (mOS) of the patients who received durvalumab or atezolizumab as the first-line treatment were 22.0 and 10.0 months, respectively (P=0.03). Survival analysis of patients with brain metastasis (BM) revealed that the median progression-free survival (mPFS) of patients without BM treated with durvalumab plus chemotherapy (5.5 months) was longer than that of those with BM (4.0 months) (P=0.03). In contrast, in the atezolizumab plus chemotherapy regimen, BM did not affect survival. In addition, the addition of radiotherapy to treatment with PD-L1 inhibitors in combination with chemotherapy has a tendency to improve long-term survival. As for safety analysis, there was no significant difference in the incidence of immune-related adverse events (IRAEs) during PD-L1 inhibitor therapy between the 2 groups (P>0.05). And during treatment with immunochemotherapy, radiotherapy was not associated with the development of IRAE (P=0.42) but increased the risk of immune-related pneumonitis (P=0.026).
Conclusions
The implication of this study for clinical practice is a preference for durvalumab in first-line immunotherapy for SCLC. In addition, appropriate radiotherapy during treatment with PD-L1 inhibitors in combination with chemotherapy may prolong long-term survival, but the occurrence of immune-related pneumonitis should be vigilant. Data from this study are limited and the baseline characteristics of the two populations still need to be more finely classified.
Keywords: Atezolizumab, durvalumab, immune-related adverse events, progression-free survival, overall survival
Highlight box.
Key findings
• In the first-line treatment of SCLC, the efficacy of durvalumab plus chemotherapy was better than that of atezolizumab plus chemotherapy.
• The IRAEs reported for these 2 immune checkpoint inhibitors (ICIs) were similar to those reported in previous clinical studies, but the most common IRAEs differed.
• Appropriate addition of radiotherapy during immunotherapy prolonged long-term survival time, but increased the risk of immune-related pneumonitis.
What is known and what is new?
• IMPOWER 133 and CASPIAN have showed that both atezolizumab and durvalumab significantly improved the survival of first-line extensive-SCLC.
• This real-world study showed that durvalumab was superior to atezolizumab in terms of long-term survival as a first-line treatment.
What is the implication, and what should change now?
• Our findings suggested that clinicians should pay attention to potential IRAEs before initiating immunotherapy. The addition of radiotherapy (RT) during immunotherapy significantly improved the long-term survival, but the occurrence of immune-associated pneumonitis should be monitored.
Introduction
Lung cancer is still the leading cause of cancer death worldwide (1). Significant progress has been made in the use of immunotherapy and targeted therapy for non-small cell lung cancer (NSCLC), which has led to improvements in the response and survival rates of patients (2,3). However, small cell lung cancer (SCLC), is an aggressive malignancy that accounts for 14% of all lung cancer cases (4), and advances in its treatment are relatively backward (5). SCLC is characterized by abnormal respiratory symptoms, early metastasis, and a poor prognosis (6,7). The main metastatic sites of SCLC are the lymph nodes, brain, bone, liver, pleural effusion, and adrenal gland, and the 5-year survival rate of SCLC is about 7% (8,9).
Platinum-based chemotherapy is the standard treatment regimen for both limited disease (LD) and extensive disease (ED), for which platinum plus etoposide combination is the preferred treatment regimen (10). In the absence of any breakthrough in treatment strategies and any improvements in patient prognosis for >20 years, the introduction of immune checkpoint inhibitors (ICIs) has been a welcome relief. ICIs have shifted the treatment methods and improved the survival of ED-SCLC patients. At present, the most common targets of ICIs are programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) (11-14).
The clinical activity of immunotherapies in patients with refractory or metastatic SCLC has been observed (15-18). Anti-PD-1 drugs have been shown to have promising therapeutic effects as a later-line treatment for ED-SCLC patients (15,19). However, the objective response rate (ORR) of PD-1 inhibitor monotherapy is only 10–20% (15,19). In the Keynote-604 study (NCT03066778), pembrolizumab or placebo was added to the standard chemotherapy of ED-SCLC. Compared with placebo, pembrolizumab did not show improvement in overall survival (OS) with ED-SCLC.
To date, 2 mature large-scale phase-III clinical trials have been published, which have led to a long-awaited shift in the treatment paradigm and improved disease response and ED-SCLC patient prognosis (20,21). IMpower133 and CASPIAN have provided substantial evidence of the benefits of adding atezolizumab and durvalumab, respectively, to chemotherapy, which led to the approval of these ICIs in the front-line treatment of ED-SCLC (20,21).
Although data from clinical trials indicate comparable overall survival for atezolizumab and durvalumab (20,22), clinical trials only recruit well-defined patients and do not reflect the heterogeneity of patients and diseases. Meanwhile, given the diverse efficacy and absence of head-to-head researches conducted to evaluate the efficacy among them, it might bring with confusion on selection in clinical practice. Real-world data are therefore needed to validate which immune checkpoint inhibitor is recommended in clinical practice for the treatment of patients with SCLC. This study was conducted in large tertiary care general hospitals in provincial capitals. Clinical records related to SCLC patients diagnosed at these three large medical centers in the last 2 years were collected, collated and analyzed. A review of the literature was conducted to compare the similarities and differences in the efficacy and safety of PD-L1 inhibitors between clinical studies and real-world treatment settings; and to directly compare the efficacy and safety of these two PD-L1 inhibitors and explore the optimal treatment strategies for SCLC patients in a real-world setting. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-588/rc).
Methods
Study population
A total of 143 SCLC patients who met the inclusion criteria were collected from February 1, 2020 to April 30, 2022 in three provincial general hospitals (The First Affiliated Hospital of USTC, The First Affiliated Hospital of Anhui Medical University, The Anhui Chest Hospital) in the capital of Anhui Province as outpatients or inpatients.
Inclusion criteria:
Patients with histologically or cytologically confirmed SCLC as defined by the Veterans Administration Lung Study Group staging system.
Age ≥18 years.
Eastern Cooperative Oncology Group (ECOG) score between 0 and 2.
Receiving treatment with durvalumab or atezolizumab.
All conditions must be met to be included in this study.
Exclusion criteria:
Patients with significant deficiencies in relevant medical records.
Previous autoimmune disease or interstitial lung disease.
Patients with previous use of PD-1 inhibitors.
Data collection
Data included patients’ demographics and baseline characteristics (sex, age, smoking status, background diseases, ECOG); disease characteristics (metastatic sites at diagnosis, stage at diagnosis) and follow up indicators (follow-up time, progression-free survival (PFS), OS, immune-related adverse events (IRAEs), interventions after the occurrence of IRAEs).
Observed indicators
Clinical efficacy
Patients receiving durvalumab or atezolizumab in combination with chemotherapy were evaluated for efficacy every 2 courses of treatment. Clinical outcomes were assessed according to RECIST version 1.1 and were classified into the following states: Complete response (CR), Partial response (PR), Stable disease (SD) and Progressive disease (PD). We sought to assess the OS (the time from the initiation of immunotherapy to the time of death from any cause) and PFS (the time from the initiation of immunotherapy to disease progression according to the RECIST or death from any cause) in the targeted population.
Immune-related adverse events
To investigate the safety of PD-L1 inhibitors in combination with chemotherapy regimens, we screened for all drug-related adverse events by reviewing all clinical records and laboratory tests during the use of durvalumab or atezolizumab. IRAEs were screened by reviewing clinical records, radiology reports and pathology during treatment with PD-L1 inhibitors and were based on the National Cancer Institute common terminology criteria for adverse events. IRAEs were graded on a scale of severity from 1–5, with grades 1–2 being considered low grade IRAEs and grades 3–5 being considered high grade IRAEs.
Statistical analysis
This was a descriptive study for which no theoretical calculation of the number of patients to be included was made. Clinical characteristics, safety, and survival outcomes were compared using the Fisher’s exact (descriptive analysis) and log-rank (Kaplan-Meier) tests. IBM SPSS Statistics version 26.0 (IBM SPSS Inc., Chicago, USA) was used for the statistical analysis. A P value <0.05 was set as the significance level.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics boards of The First Affiliated Hospital of USTC (2023KY Ethics Review No. 003). The First Affiliated Hospital of Anhui Medical University and The Anhui Chest Hospital were informed and agreed with the study. Individual consent for this retrospective analysis was waived.
Results
Baseline characteristics of the 143 SCLC patients
We identified 201 patients with SCLC who received durvalumab or atezolizumab at the 3 centers from February 1, 2020 to April 30, 2022. Among these patients, 58 were excluded, as they did not meet the study criteria or for other reasons. Ultimately, 143 patients were included in this analysis, 100 of these patients were treated with durvalumab and 43 with atezolizumab (Figure 1). Regarding the median age, it was 61 years in the durvalumab group [interquartile range (IQR), 23–82] and 64 years in the atezolizumab group (IQR, 43–81); the gender ratio was predominantly male in both groups, with 72 (72%) male patients in the durvalumab group and 29 (67%) male patients in the atezolizumab group; 90 (90%) in the durvalumab group were in the extensive stage at diagnosis compared to 34 (79%) in the atezolizumab group; with regard to metastases at baseline in patients, they were mainly in bone, brain and liver, with 33 (33%), 23 (23%) and 14 (14%) cases in the durvalumab group compared to 8 (19%), 7 (16%) and 5 (12%) cases in the atezolizumab group, respectively. Over the course of treatment, the number of patients treated with radiotherapy was 42 (42%) and 32 (74%) in the durvalumab and atezolizumab groups, respectively; and there was a high proportion of first-line immunotherapy in both groups. Overall, the baseline demographics and disease characteristics were well balanced between the durvalumab plus chemotherapy and atezolizumab plus chemotherapy groups (Table 1).
Figure 1.
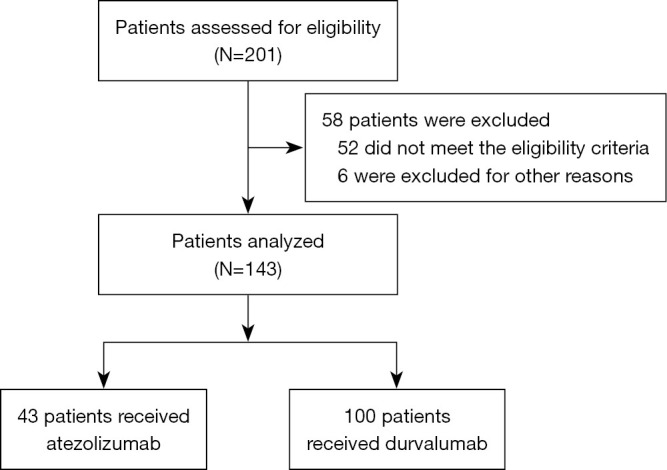
Flow chart of study profile.
Table 1. Baseline characteristics of the patients in the durvalumab plus chemotherapy (durvalumab) versus atezolizumab plus chemotherapy (atezolizumab) groups.
| Characteristics | Durvalumab (N=100) | Atezolizumab (N=43) | P |
|---|---|---|---|
| Median age [range], years | 61 [23–82] | 64 [43–81] | 0.54 |
| Age group, n [%] | 0.9 | ||
| <65 years | 57 [57] | 24 [56] | |
| ≥65 years | 43 [43] | 19 [44] | |
| Sex, n [%] | 0.3 | ||
| Men | 72 [72] | 29 [67] | |
| Women | 28 [28] | 14 [33] | |
| ECOG performance status, n [%] | 0.83 | ||
| ≤1 | 94 [94] | 40 [94] | |
| ≥2 | 6 [6] | 3 [6] | |
| Smoking status, n [%] | 0.27 | ||
| Never | 60 [60] | 27 [63] | |
| Current | 33 [33] | 10 [23] | |
| Former | 7 [7] | 6 [14] | |
| Disease stage at initiation of immunotherapy, n [%] | 0.13 | ||
| Limited stage | 10 [10] | 9 [21] | |
| Extensive stage | 90 [90] | 34 [79] | |
| Brain or central nervous system metastasis, n [%] | 0.37 | ||
| Yes | 23 [23] | 7 [16] | |
| No | 77 [77] | 36 [83] | |
| Liver metastases, n [%] | |||
| Yes | 14 [14] | 5 [12] | 0.7 |
| No | 86 [86] | 38 [88] | |
| Bone metastases, n [%] | 0.08 | ||
| Yes | 33 [33] | 8 [19] | |
| No | 67 [67] | 35 [81] | |
| Radiotherapy, n [%] | 0.06 | ||
| Yes | 42 [42] | 32 [74] | |
| No | 58 [58] | 11 [26] | |
| Brain radiotherapy, n [%] | 0.07 | ||
| Yes | 22 [22] | 4 [9] | |
| No | 78 [78] | 39 [91] | |
| Immunotherapy at the first line, n [%] | 0.14 | ||
| Yes | 71 [71] | 29 [67] | |
| No | 29 [29] | 14 [33] |
ECOG, Eastern Cooperative Oncology Group.
Efficacy
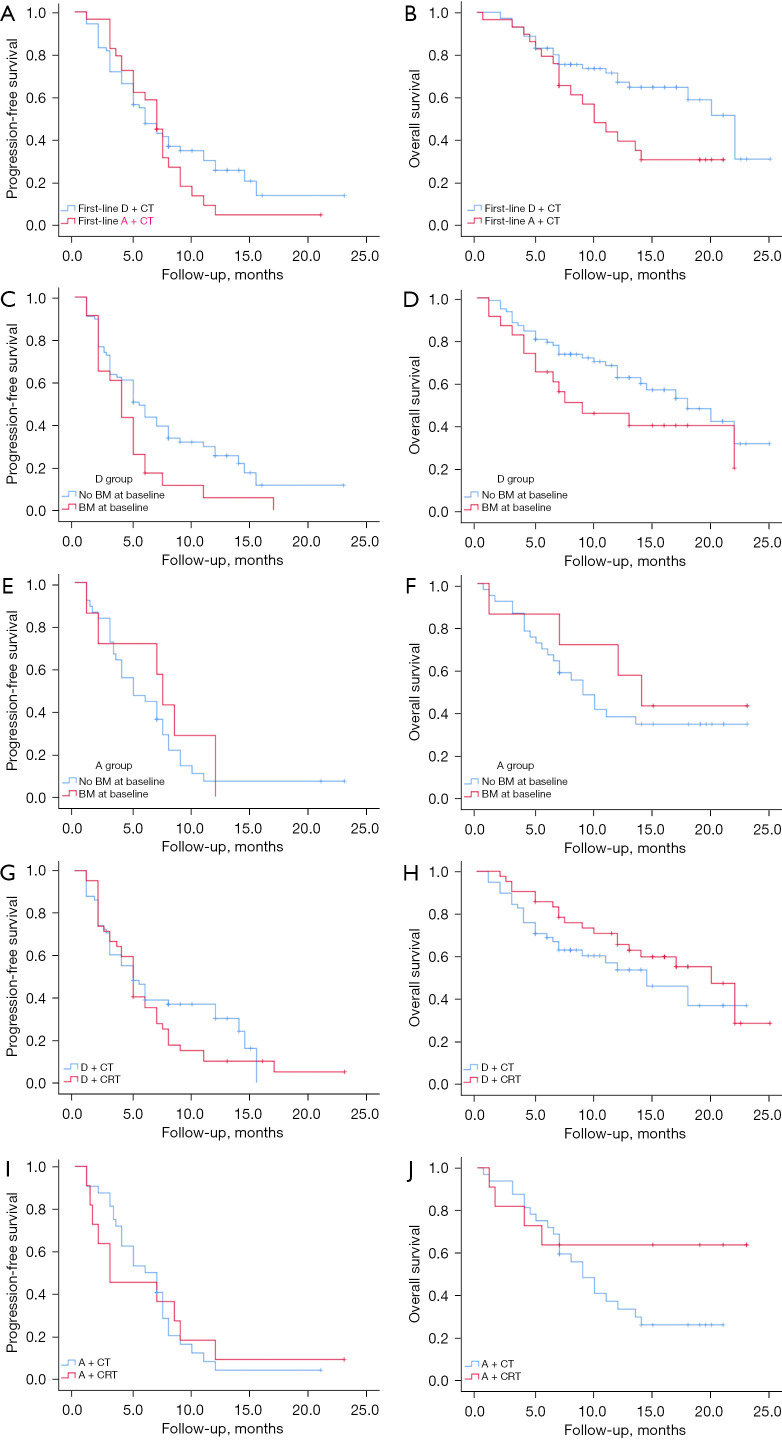
In the target cohort, 100 patients were treated with durvalumab, of which 71 received it as a first-line treatment and 29 as a second- or later-line treatment, while 43 patients were treated with atezolizumab, of which 29 received it as a first-line treatment and 14 as a second- or later-line treatment (Table 1). At the data cut-off time, a total of 26 (37%) patients who received durvalumab as a first-line treatment and 18 (62%) patients who received atezolizumab as a first-line treatment had died. When the PFS of both was analyzed, the mPFS in the durvalumab and atezolizumab groups were 6.0 and 7.0 months respectively, with no statistically significant difference (P=0.41; Figure 2A). The mOS of the durvalumab treatment group [22.0 months; 95% confidence interval (CI): 18.4–25.6] was significantly longer than that of the atezolizumab treatment group (10.0 months; 95% CI: 7.0–13.0). The stratified hazard ratio (HR) for death was 2.03 (95% CI: 1.11–3.73; P=0.029; Figure 2B).
Figure 2.
Kaplan-Meier graphs of the survival outcomes in the target cohort. (A,B) Durvalumab plus chemotherapy versus atezolizumab plus chemotherapy as a first-line treatment; patients without BM versus those with BM in the durvalumab (C,D) or atezolizumab (E,F) group; RT versus no RT receipt in the durvalumab (G,H) or atezolizumab (I,J) groups. D + CT, durvalumab + chemotherapy; A + CT, atezolizumab + chemotherapy; D + CRT, durvalumab + chemoradiotherapy; A + CRT, atezolizumab + chemoradiotherapy; BM, brain metastasis; RT, radiotherapy.
The CASPIAN and Impower 133 studies did not address the management of patients with brain metastasis (BM), but patients with SCLC are extremely vulnerable to BM (22). In this study, we found that the PFS of the patients without BM who received a combination regimen of chemotherapy plus durvalumab (median: 5.5 months) was longer than that of patients with BM (median: 4.0 months; P=0.03) (Figure 2C); Regarding the long-term survival analysis, mOS was 18.0 months and 9.0 months in BM-free versus BM, respectively (P=0.12; Figure 2D). In contrast, in the treatment regimen of atezolizumab combined with chemotherapy, no effect of BM on survival was revealed (Figure 2E,2F).
We further analysed the difference in the curative effect between ICI combined with chemoradiotherapy (CRT) and ICI combined with chemotherapy. Among the target cohort analyzed, the mPFS was 5.0 months in the durvalumab plus chemoradiotherapy (D + CRT) group and 5.0 months in the durvalumab plus chemotherapy (D + CT) group (P=0.29; Figure 2G); in terms of long-term survival, the radiotherapy group benefited over the no-radiation group, but the difference was not statistically significant (P=0.21; Figure 2H). In the atezolizumab combination chemotherapy regimen, the addition of radiotherapy had no effect on mPFS. For long-term survival, the mOS endpoint was not reached in the atezolizumab + chemoraditherapy (A + CRT) group, but the addition of radiotherapy tended to improve long-term survival (Figure 2I,2J).
Safety
The cohort that could be evaluated for safety included the patients who received at least 1 dose of durvalumab or atezolizumab. The median dose of durvalumab was 6.0 doses (range, 1–23), and that of atezolizumab was also 5.0 doses (range, 1–31). The IRAEs are listed in Table 2. In this multicenter, real-world study, the main IRAEs were pneumonitis, chest tightness, hepatitis, rash, and endocrine toxicity. There were 23 patients (23%) in the durvalumab treatment group who had at least an IRAE of any grade, and 7 (16%) in the atezolizumab treatment group. And the most common IRAE in durvalumab group was pneumonitis (6%) compared to endocrine system disease (7%) in atezolizumab group. In addition, no deaths due to IRAE were seen in either group.
Table 2. Summary of the immune-related adverse events and drug exposure in the durvalumab and atezolizumab groups.
| All-cause IRAEs, treatment-related AEs and AESIs | Durvalumab group (N=100) | Atezolizumab group (N=43) | |||||
|---|---|---|---|---|---|---|---|
| Any grade | ≥3 grade | P | Any grade | ≥3 grade | P | ||
| Any IRAE, n (%) | 23 (23.0) | 10 (10.0) | 0.37 | 7 (16.3) | 1 (2.3) | 0.22 | |
| Im pneumonitis, n (%) | 6 (6.0) | 2 (2.0) | 0.61 | 1 (2.3) | 0 | 1.00 | |
| Im chest distress, n (%) | 1 (1.0) | 0 | 1.00 | 0 | 0 | 1.00 | |
| Im diseases of endocrine system, n (%) | 3 (3.0) | 2 (2.0) | 0.53 | 3 (7.0) | 0 | 0.16 | |
| Im hepatitis, n (%) | 5 (5.0) | 4 (4.0) | 0.31 | 0 | 0 | 0.52 | |
| Im rash, n (%) | 4 (4.0) | 1 (1.0) | 1.00 | 2 (4.7) | 0 | 1.00 | |
| Im esophagitis, n (%) | 1 (1.0) | 0 | 1.00 | 0 | 0 | 0.22 | |
| Im peritonitis, n (%) | 1 (1.0) | 0 | 1.00 | 0 | 0 | 0.22 | |
| Im enteritis, n (%) | 2 (2.0) | 0 | 1.00 | 1 (2.3) | 0 | 1.00 | |
| Im toxicity, n (%) | 2 (2.0) | 0 | 1.00 | 0 | 0 | 1.00 | |
| Im damage, n (%) | 2 (2.0) | 0 | 1.00 | 1 (2.3) | 1 (2.3) | 0.36 | |
| Im cardiotoxicity, n (%) | 4 (4.0) | 2 (2.0) | 0.36 | 0 | 0 | 1.00 | |
| Im fatigue, n (%) | 2 (2.0) | 1 (1.0) | 1.00 | 0 | 0 | 1.00 | |
| Number of patients with IRAEs, n (%) | 23 (23.0) | 11 (11.0) | 7 (16.3) | 1 (2.3) | 0.37 | ||
| Number of doses received, median [range] | 6.0 [1–23] | 5.0 [1–31] | 0.91 | ||||
| Treated with steroids therapy, n (%) | 11 (47.8) | 0 | 0.06 | ||||
| Any permanent cessation due to IRAEs, n (%) | 10 (10.0) | 2 (4.7) | 0.79 | ||||
| Any IRAE leading to death | 0 | 0 | 1 | ||||
IRAE, immune-related adverse event; AE, adverse event; Im, immune-mediated; AESI, adverse event of special interest.
The relevance of the radiotherapy (including chest radiotherapy, prophylactic cranial irradiation and bone radiotherapy) to the development of IRAE and immune-related pneumonitis was also analyzed in this study. Due to the limited number of cases, the population using durvalumab and atezolizumab were pooled together for analysis, with a total of 53 in the radiotherapy group and 90 in the non-radiotherapy group, and a univariate analysis found that radiotherapy had no correlation with the occurrence of IRAE (P=0.42) (Table 3). However, radiotherapy increased the risk of immune-related pneumonitis (P=0.026) (Table 3).
Table 3. Correlation of radiotherapy with the development of IRAE and immune-related pneumonitis.
| IRAE and pneumonitis | Non-radiotherapy group [n=90], n [%] | Radiotherapy group [n=53], n [%] | P |
|---|---|---|---|
| Without IRAE | 73 [81] | 40 [76] | 0.42 |
| With IRAE | 17 [19] | 13 [24] | |
| Without ir-pneumonitis | 89 [98.9] | 48 [90.6] | 0.026 |
| Pneumonitis | 1 [1.1] | 5 [9.4] |
IRAE, immune-related adverse event; ir, immune-related.
Discussion
SCLC is a tumor with a high degree of malignancy and a poor prognosis (23). Although platinum-based first-line regimens have high objective remission rates in the short term, once the disease has progressed, the effectiveness of subsequent treatment is often limited and the desired outcome is difficult to achieve. In recent years, with the rapid development of oncology treatment, immunotherapy has become another important therapy after chemotherapy and targeted therapy in the treatment of various tumors. Trials by IMPOWER133 (20) and CASPIAN (21) have established the place of PD-L1 inhibitors in combination with chemotherapy in the first-line treatment of SCLC, thus enabling a 30-year of changing the treatment paradigm for SCLC.
There are more studies on comparative efficacy analysis between PD-1 inhibitors, PD-L1 inhibitors and CTLA-4 inhibitors, while there is a lack of data on comparative efficacy between several PD-L1 inhibitors. According to the guideline of Chinese Society of Clinical Oncology (CSCO), atezolizumab + chemotherapy and durvalumab + chemotherapy had been recommended for treating ES-SCLC. The network meta-analysis by Chen et al. revealed that there was no statistical difference observed in the indirect comparison of PFS or OS among agents of atezolizumab and durvalumab as first-line treatment in patients with extensive-stage SCLC. Besides, durvalumab was shown superiority on ORR when compared with atezolizumab, however, with a significantly higher risk of immune-related AEs when compared with atezolizumab (24). Our study was a direct comparison between atezolizumab and durvalumab in first-line treatment of SCLC and revealed that the mOS of the durvalumab plus chemotherapy front-line patients was longer than that of the atezolizumab plus chemotherapy front-line patients (22.0 vs. 10.0 months; HR =2.03; 95% CI: 1.11–3.73). Notably, the mOS of the patients who received the first-line treatment of durvalumab plus chemotherapy in our study was 22.0 months, which was much higher than that of those who received CASPIAN, which has been reported to be 13.0 months (22). There may be various reasons for this finding. Firstly, radiotherapy (RT) can prolong the survival of SCLC patients treated with ICIs. And 33.8% (24/71) of the patients in first-line durvalumab plus chemotherapy group received radiotherapy in the real-world study. Whereas, patients in the experimental group in the CASPIAN study did not undergo radiotherapy (2). In addition, 10% (7/71) of patients with SCLC were in the LD, while all patients in CASPIAN study were in the ED.
BM is common in patients with SCLC, and is associated with poor clinical outcomes (25,26). Consistent with previous findings, among patients who received durvalumab plus chemotherapy, patients without BM had a longer PFS (P=0.03), patients without BM were also found to benefit from long-term survival, but this did not reach statistical significance. Conversely, no significant difference was found in the survival time (PFS and OS) of patients with and without BM who received atezolizumab plus chemotherapy. This may have been due to the small number of patients in the atezolizumab treatment group, and the smaller number of patients with BM. Further research needs to be conducted on the role of immunotherapy in SCLC patients with BM.
The efficacy of ICIs has been shown to be promising, however, the IRAEs caused by ICIs requires attention. IRAEs are toxic reactions caused by the abnormal activation of the systematic immune system, which usually affect multiple organs. Studies have shown that the incidence of immune-related toxic reactions caused by ICI drugs might be about 30%, and that <20% of individuals suffer from high-level IRAEs (27-29). Clinicians should be aware of the potential IRAEs, including pneumonitis, hyperthyroidism, hypothyroidism, colitis, and cytopenia. The results of this study showed that the spectrum of IRAEs for durvalumab and atezolizumab was similar to that of the existing clinical studies, but in the atezolizumab group, some IRAEs were not explored due to the limited number of patients. Statistical results from this study indicated that the most common IRAEs for durvalumab and atezolizumab were not the same. Therefore, clinicians should assess patients’ general condition, underlying diseases and relevant laboratory test results prior to initiating immunotherapy, to fully inform patients of potential IRAEs that may occur during immunotherapy, and pay attention to the high likelihood of immune-related events and make timely adjustments to the treatment regimen. In addition, we found that the survival of patients treated with PD-L1 inhibitors combined with chemotherapy and RT was longer than that of those who did not receive RT, and the addition of RT did not increase the risk of IRAEs. However, clinicians need to be alert to the occurrence of immune-related pneumonitis and IRAEs in clinical practice.
Recent breakthroughs in “immunotherapy in combination with chemotherapy” have changed the previous standard of care for SCLC and have shown signs of improving outcomes for SCLC patients (20,22), as further validated by our real-world clinical data. In 2022, with the publication of data from clinical studies of our self-developed serplulimab and adebrelimab, which break through the previous magnitude of benefit of immune checkpoint inhibitors, a new record of OS for first-line treatment of ES-SCLC was set, providing more drug options for first-line treatment of SCLC (30,31). SCLC must be firmly targeting precision therapy and immunotherapy. Although immune drugs have improved survival in SCLC patients to some extent, there is still huge room for improvement in their long-term survival rates. With the exploration and deeper understanding of the molecular typing and immune microenvironment of SCLC, the more rational use and combination of existing drugs and the development of more effective new drugs will drive the precision treatment of SCLC and improve patient survival faster.
The design of this retrospective observational study had some limitations. First, the number of patients in the durvalumab-treated and atezolizumab-treated groups was not consistent Second, the lesion assessments and IRAE gradings may have differed depending on the evaluation criteria applied by different clinicians. Third, many patients did not receive the standard dose of PD-L1 inhibitors due to economic or other factors.
In summary, this was the first real-world research to compare the efficacy and safety of a combination regimen of chemotherapy with 2 different PD-L1 inhibitors in SCLC. This real-world research study showed that durvalumab combined with chemotherapy improved the OS of patients more than atezolizumab combined with chemotherapy in the first-line treatment of SCLC patients. This finding has not been reported in previous clinical studies. Notably, this study also found that in both the durvalumab or atezolizumab combined with chemotherapy treatment groups, the OS of the patients who received RT was significantly longer than that of the patients who did not receive RT, and there was no significant difference in the incidence of IRAEs between the patients who did and did not receive RT. However, clinicians need to be alert to the occurrence of immune-related pneumonitis.
Conclusions
This study is a comparison of the efficacy and safety of PD-L1 inhibitors in SCLC in a real-word setting. Despite its many limitations, results from this study with respect to guild of selection of PD-L1 inhibitors for the treatment of SCLC and warrant further investigations.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of The First Affiliated Hospital of USTC (2023KY Ethics Review No. 003) and individual consent for this retrospective analysis was waived. The First Affiliated Hospital of Anhui Medical University and The Anhui Chest Hospital were informed and agreed with the study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-588/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-588/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-588/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-588/coif). The authors have no conflicts of interest to declare.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Lahiri A, Maji A, Potdar PD, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer 2023;22:40. 10.1186/s12943-023-01740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu SY, Zhang JT, Zeng KH, et al. Perioperative targeted therapy for oncogene-driven NSCLC. Lung Cancer 2022;172:160-9. 10.1016/j.lungcan.2022.05.007 [DOI] [PubMed] [Google Scholar]
- 4.Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health 2019;85:8. 10.5334/aogh.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Shim HS, Ahn BC, et al. Efficacy and safety of atezolizumab, in combination with etoposide and carboplatin regimen, in the first-line treatment of extensive-stage small-cell lung cancer: a single-center experience. Cancer Immunol Immunother 2022;71:1093-101. 10.1007/s00262-021-03052-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oronsky B, Reid TR, Oronsky A, et al. What's New in SCLC? A Review. Neoplasia 2017;19:842-7. 10.1016/j.neo.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev 2015;29:1447-62. 10.1101/gad.263145.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. 10.1038/s41572-020-00235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko J, Winslow MM, Sage J. Mechanisms of small cell lung cancer metastasis. EMBO Mol Med 2021;13:e13122. 10.15252/emmm.202013122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amarasena IU, Chatterjee S, Walters JA, et al. Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev 2015;2015:CD006849. 10.1002/14651858.CD006849.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Humeau J, Buqué A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 2020;17:725-41. 10.1038/s41571-020-0413-z [DOI] [PubMed] [Google Scholar]
- 12.Qu J, Mei Q, Liu L, et al. The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther Adv Med Oncol 2021;13:1758835921992968. 10.1177/1758835921992968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 14.Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest 2015;125:3377-83. 10.1172/JCI80012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 16.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 2017;35:3823-9. 10.1200/JCO.2017.72.5069 [DOI] [PubMed] [Google Scholar]
- 17.Chiang AC, Sequist LVD, Gilbert J, et al. Clinical Activity and Safety of Atezolizumab in a Phase 1 Study of Patients With Relapsed/Refractory Small-Cell Lung Cancer. Clin Lung Cancer 2020;21:455-463.e4. 10.1016/j.cllc.2020.05.008 [DOI] [PubMed] [Google Scholar]
- 18.Gadgeel SM, Pennell NA, Fidler MJ, et al. Phase II Study of Maintenance Pembrolizumab in Patients with Extensive-Stage Small Cell Lung Cancer (SCLC). J Thorac Oncol 2018;13:1393-9. 10.1016/j.jtho.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol 2020;15:618-27. 10.1016/j.jtho.2019.12.109 [DOI] [PubMed] [Google Scholar]
- 20.Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 21.Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. 10.1016/S1470-2045(20)30539-8 [DOI] [PubMed] [Google Scholar]
- 22.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 23.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Wang J, Xu H. Comparison of atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with extensive-stage small cell lung cancer: A systematic review and network meta-analysis. Medicine (Baltimore) 2021;100:e25180. 10.1097/MD.0000000000025180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rades D, Dziggel L, Segedin B, et al. The first survival score for patients with brain metastases from small cell lung cancer (SCLC). Clin Neurol Neurosurg 2013;115:2029-32. 10.1016/j.clineuro.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 26.Yang F, Markovic SN, Molina JR, et al. Association of Sex, Age, and Eastern Cooperative Oncology Group Performance Status With Survival Benefit of Cancer Immunotherapy in Randomized Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw Open 2020;3:e2012534. 10.1001/jamanetworkopen.2020.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med 2016;4:272. 10.21037/atm.2016.07.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maughan BL, Bailey E, Gill DM, et al. Incidence of Immune-Related Adverse Events with Program Death Receptor-1- and Program Death Receptor-1 Ligand-Directed Therapies in Genitourinary Cancers. Front Oncol 2017;7:56. 10.3389/fonc.2017.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V, Chaudhary N, Garg M, et al. Corrigendum: Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol 2017;8:311. Erratum for: Front Pharmacol 2017;8:49. 10.3389/fphar.2017.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022;328:1223-32. 10.1001/jama.2022.16464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:739-47. 10.1016/S1470-2045(22)00224-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as