Abstract
The European Commission requested the EFSA Panel on Plant Health to prepare and deliver risk assessments for commodities listed in Commission Implementing Regulation (EU) 2018/2019 as ‘High risk plants, plant products and other objects’. This Scientific Opinion covers plant health risks posed by plants of Acer pseudoplatanus imported from the United Kingdom (UK) as: (a) 1‐ to 7‐year‐old bare root plants for planting, (b) 1‐ to 7‐year‐old plants in pots and (c) bundles of 1‐ to 2‐year‐old whips and seedlings, taking into account the available scientific information, including the technical information provided by the UK. All pests associated with the commodity were evaluated against specific criteria for their relevance for this opinion. Six EU quarantine pests and four pests not regulated in the EU fulfilled all relevant criteria and were selected for further evaluation. For these pests, the risk mitigation measures implemented in the technical dossier from the UK were evaluated taking into account the possible limiting factors. For the selected pests, an expert judgement is given on the likelihood of pest freedom taking into consideration the risk mitigation measures acting on the pest, including uncertainties associated with the assessment. The degree of pest freedom varies among the pests evaluated, with Meloidogyne mali or M. fallax being the pest most frequently expected on the imported plants. The Expert Knowledge Elicitation indicated with 95% certainty that 9,792 or more plants in pots per 10,000 will be free from Meloidogyne mali or M. fallax.
Keywords: maple, European Union, commodity risk assessment, plant health, plant pest
1. Introduction
1.1. Background and terms of reference as provided by European Commission
1.1.1. Background
The Plant Health Regulation (EU) 2016/2031 1 , on the protective measures against pests of plants, has been applied from December 2019. Provisions within the above Regulation are in place for the listing of ‘high risk plants, plant products and other objects’ (Article 42) on the basis of a preliminary assessment, and to be followed by a commodity risk assessment. A list of ‘high risk plants, plant products and other objects’ has been published in Regulation (EU) 2018/2019 2 . Scientific opinions are therefore needed to support the European Commission and the Member States in the work connected to Article 42 of Regulation (EU) 2016/2031, as stipulated in the terms of reference.
1.1.2. Terms of reference
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002 3 , the Commission asks EFSA to provide scientific opinions in the field of plant health.
In particular, EFSA is expected to prepare and deliver risk assessments for commodities listed in the relevant Implementing Act as ‘High risk plants, plant products and other objects’. Article 42, paragraphs 4 and 5, establishes that a risk assessment is needed as a follow‐up to evaluate whether the commodities will remain prohibited, removed from the list and additional measures will be applied or removed from the list without any additional measures. This task is expected to be on‐going, with a regular flow of dossiers being sent by the applicant required for the risk assessment.
Therefore, to facilitate the correct handling of the dossiers and the acquisition of the required data for the commodity risk assessment, a format for the submission of the required data for each dossier is needed.
Furthermore, a standard methodology for the performance of ‘commodity risk assessment’ based on the work already done by Member States and other international organisations needs to be set.
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002, the Commission asks EFSA to provide scientific opinion in the field of plant health for Acer pseudoplatanus from the UK taking into account the available scientific information, including the technical dossier provided by the UK.
1.2. Interpretation of the terms of reference
The EFSA Panel on Plant Health (hereafter referred to as ‘the Panel') was requested to conduct a commodity risk assessment of Acer pseudoplatanus from the UK following the Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019). Taking into account the available scientific information, including the technical information provided by the UK.
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Protocol on Ireland/Northern Ireland in conjunction with Annex 2 to that protocol, for the purposes of this opinion, references to the United Kingdom do not include Northern Ireland.
The EU quarantine pests that are regulated as a group in the Commission Implementing Regulation (EU) 2019/2072 4 were considered and evaluated separately at species level.
Annex II of Implementing Regulation (EU) 2019/2072 lists certain pests as non‐European populations or isolates or species. These pests are regulated quarantine pests. Consequently, the respective European populations, or isolates, or species are non‐regulated pests.
Annex VII of the same Regulation, in certain cases (e.g. point 32), makes reference to the following countries that are excluded from the obligation to comply with specific import requirements for those non‐European populations, or isolates, or species: Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (SeveroZapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug), San Marino, Serbia, Switzerland, Türkiye, Ukraine and the United Kingdom (except Northern Ireland 5 ).
Consequently, for those countries,
any pests identified, which are listed as non‐European species in Annex II of Implementing Regulation (EU) 2019/2072 should be investigated as any other non‐regulated pest.
any pest found in a European country that belongs to the same denomination as the pests listed as non‐European populations or isolates in Annex II of Implementing Regulation (EU) 2019/2072, should be considered as European populations or isolates and should not be considered in the assessment of those countries.
Pests listed as ‘Regulated Non‐Quarantine Pest' (RNQP) in Annex IV of the Commission Implementing Regulation (EU) 2019/2072 and deregulated pests (i.e. pest which were listed as quarantine pests in the Council Directive 2000/29/EC and were deregulated by Commission Implementing Regulation (EU) 2019/2072) were not considered for further evaluation. In case a pest is at the same time regulated as an RNQP and as a protected zone quarantine pest, in this Opinion, it should be evaluated as quarantine pest.
In its evaluation, the Panel:
Checked whether the provided information in the technical dossier (hereafter referred to as ‘the Dossier’) provided by the applicant (United Kingdom, Department for Environment Food and Rural Affairs – hereafter referred to as ‘DEFRA’) was sufficient to conduct a commodity risk assessment. When necessary, additional information was requested to the applicant.
Selected the relevant Union quarantine pests and protected zone quarantine pests (as specified in Commission Implementing Regulation (EU) 2019/2072, hereafter referred to as ‘EU quarantine pests’) and other relevant pests present in the UK and associated with the commodity.
Did not assess the effectiveness of measures for Union quarantine pests for which specific measures are in place for the import of the commodity from the UK in Commission Implementing Regulation (EU) 2019/2072 and/or in the relevant legislative texts for emergency measures and if the specific country is in the scope of those emergency measures. The assessment was restricted to whether or not the applicant country implements those measures.
Assessed the effectiveness of the measures described in the Dossier for those Union quarantine pests for which no specific measures are in place for the importation of the commodity from the UK and other relevant pests present in the UK and associated with the commodity.
Risk management decisions are not within EFSA's remit. Therefore, the Panel provided a rating based on expert judgement regarding the likelihood of pest freedom for each relevant pest given the risk mitigation measures proposed by DEFRA of the UK.
2. Data and methodologies
2.1. Data provided by DEFRA of the UK
The Panel considered all the data and information (hereafter called ‘the Dossier’) provided by DEFRA of the UK in May 2022 including the additional information provided by DEFRA of the UK in January 2023, after EFSA's request. The Dossier is managed by EFSA.
The structure and overview of the Dossier is shown in Table 1. The number of the relevant section is indicated in the Opinion when referring to a specific part of the Dossier.
Table 1.
Structure and overview of the Dossier
| Dossier Section | Overview of contents | Filename |
|---|---|---|
| 1.0 | Technical dossier | Acer pseudoplatanus commodity information FINAL draft |
| 2.0 | Pest list | Acer_pest_list_final_checked |
| 3.0 | Additional information: answers | Acer pseudoplatanus additional information 3 Nov 2022 |
| 4.0 | Additional information: distribution of Acer pseudoplatanus plants | Acer_pseudoplatanus_distribution (1) |
| 5.0 | Additional information: pest details | Acer_pseudoplatanus‐EFSA_pest_detail_request_Jan23 |
| 6.0 | Additional information: producers sample product list | A.pseudoplatanus_producers_sample_product_list |
The data and supporting information provided by DEFRA of the UK formed the basis of the commodity risk assessment. Table 2 shows the main data sources used by DEFRA of the UK to compile the Dossier (Dossier Sections 1.0 and 2.0).
Table 2.
Databases used in the literature searches by DEFRA of the UK
2.2. Literature searches performed by EFSA
Literature searches in different databases were undertaken by EFSA to complete a list of pests potentially associated with Acer pseudoplatanus. The following searches were combined: (i) a general search to identify pests reported on Acer pseudoplatanus and Acer species reported as Acer sp. and Acer spp. in the databases, (ii) a search to identify any EU quarantine pest reported on Acer as genus and subsequently (iii) a tailored search to identify whether the above pests are present or not in the UK. The searches were run between July and August 2022. No language, date or document type restrictions were applied in the search strategy.
The Panel used the databases indicated in Table 3 to compile the list of pests associated with the tree species listed above. As for Web of Science, the literature search was performed using a specific, ad hoc established search string (see Appendix B). The string was run in ‘All Databases’ with no range limits for time or language filters. This is further explained in Section 2.3.2.
Table 3.
Databases used by EFSA for the compilation of the pest list associated with Acer pseudoplatanus
Additional searches, limited to retrieve documents, were run when developing the Opinion. The available scientific information, including previous EFSA opinions on the relevant pests and diseases (see pest data sheets in Appendix A) and the relevant literature and legislation (e.g. Regulation (EU) 2016/2031; Commission Implementing Regulations (EU) 2018/2019; (EU) 2018/2018 and (EU) 2019/2072), were taken into account.
2.3. Methodology
When developing the Opinion, the Panel followed the EFSA Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019).
In the first step, pests potentially associated with the commodity in the country of origin (EU‐quarantine pests and other pests) that may require risk mitigation measures are identified. The EU non‐quarantine pests not known to occur in the EU were selected based on evidence of their potential impact in the EU. After the first step, all the relevant pests that may need risk mitigation measures were identified.
In the second step, the implemented risk mitigation measures for each relevant pest were evaluated.
A conclusion on the pest freedom status of the commodity for each of the relevant pests was determined and uncertainties identified using expert judgements.
Pest freedom was assessed by estimating the number of infested/infected units out of 10,000 exported units. Further details on the methodology used to estimate the likelihood of pest freedom are provided in Section 2.3.4.
2.3.1. Commodity data
Based on the information provided by DEFRA of the UK, the characteristics of the commodity were summarised.
2.3.2. Identification of pests potentially associated with the commodity
To evaluate the pest risk associated with the importation of the commodity from the UK, a pest list was compiled. The pest list is a compilation of all identified plant pests reported as associated with Acer pseudoplatanus, Acer sp., Acer spp. and all EU quarantine pests reported as associated with Acer as a genus based on information provided in the Dossier Sections 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 and on searches performed by the Panel. The search strategy and search syntax were adapted to each of the databases listed in Table 3, according to the options and functionalities of the different databases and CABI keyword thesaurus.
The scientific names of the host plants (i.e. Acer, Acer sp., Acer spp., A. pseudoplatanus) were used when searching in the EPPO Global database and CABI Crop Protection Compendium. The same strategy was applied to the other databases excluding EUROPHYT and Web of Science.
EUROPHYT was investigated by searching for the interceptions associated with A. pseudoplatanus imported from the whole world from 1995 to May 2020 and TRACES‐NT from May 2020 to 22 December 2022, respectively. For the pests selected for further evaluation, a search in the EUROPHYT and/or TRACES‐NT was performed for the years between 1995 and December 2022 for the interceptions from the whole world, at species level.
The search strategy used for Web of Science Databases was designed combining English common names for pests and diseases, terms describing symptoms of plant diseases and the scientific and English common names of the commodity and excluding pests which were identified using searches in other databases. The established search strings are detailed in Appendix B and they were run on 29 June and 14 July 2022.
The titles and abstracts of the scientific papers retrieved were screened and the pests associated with Acer sp., Acer spp. and A. pseudoplatanus were included in the pest list. The pest list was eventually further compiled with other relevant information (e.g. EPPO code per pest, taxonomic information, categorisation, distribution) useful for the selection of the pests relevant for the purposes of this Opinion.
The compiled pest list (see Microsoft Excel® in Appendix F) includes all identified pests that use as hosts Acer sp., Acer spp. and A. pseudoplatanus.
The evaluation of the compiled pest list was done in two steps: First, the relevance of the EU‐quarantine pests was evaluated (Section 4.1); second, the relevance of any other plant pest was evaluated (Section 4.2).
Pests for which limited information was available on one or more criteria used to identify them as relevant for this Opinion, e.g. on potential impact, are listed in Appendix E (List of pests that can potentially cause an effect not further assessed).
2.3.3. Listing and evaluation of risk mitigation measures
All implemented risk mitigation measures were listed and evaluated. When evaluating the likelihood of pest freedom of the commodity, the following types of potential infection/infestation sources for Acer pseudoplatanus in export nursery were considered (see also Figure 1):
pest entry from surrounding areas,
pest entry with new plants/seeds,
pest spread within the nursery.
Figure 1.
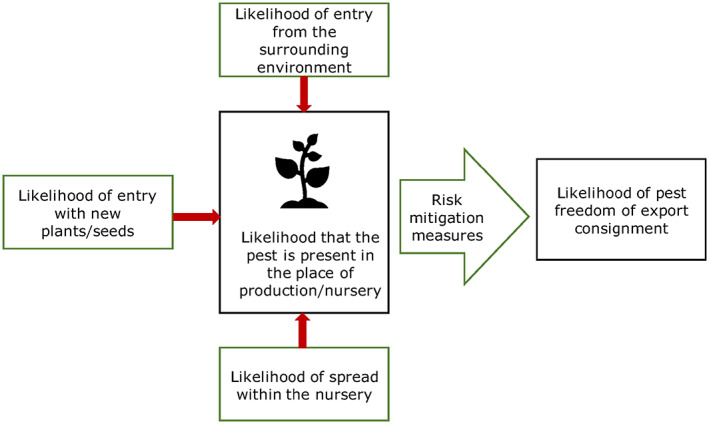
Conceptual framework to assess likelihood that plants are exported free from relevant pests (Source: EFSA PLH Panel, 2019)
The risk mitigation measures proposed by DEFRA of the UK were evaluated with Expert Knowledge Elicitation (EKE) according to the Guidance on uncertainty analysis in scientific assessment (EFSA Scientific Committee, 2018).
Information on the biology, likelihood of entry of the pest to the export nursery, of its spread inside the nursery and the effect of measures on the specific pests were summarised in data sheets of pests selected for further evaluation (see Appendix A).
2.3.4. Expert knowledge elicitation
To estimate the pest freedom of the commodity, an EKE was performed following EFSA guidance (Annex B.8 of EFSA Scientific Committee, 2018). The specific question for EKE was: ‘Taking into account (i) the risk mitigation measures in place in the nurseries, and (ii) other relevant information, how many of 10,000 commodity units, either single plants or bundles of plants will be infested with the relevant pest when arriving in the EU?’
The risk assessment considers bundles of 5–15 bare root whips and 1‐ to 2‐year‐old seedlings, 1‐ to 7‐year‐old bare root single plants and 1‐ to 7‐year‐old single plants in pots.
The following reasoning is given for considering bundles of whips and seedlings:
There is no quantitative information available regarding clustering of plants during production.
Plants are grouped in bundles of 5, 10 or 15 after sorting.
For the pests under consideration, a cross‐contamination during transport is possible.
The following reasoning is given for considering single plants (bare root or plants in pots):
The inspections before export are targeted on individual plants.
It is assumed that the product will be distributed in the EU as individual plants to the consumer.
The EKE question was common to all pests for which the pest freedom of the commodity was estimated.
The uncertainties associated with the EKE were taken into account and quantified in the probability distribution applying the semi‐formal method described in section 3.5.2 of the EFSA‐PLH Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Finally, the results were reported in terms of the likelihood of pest freedom. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
3. Commodity data
3.1. Description of the commodity
The commodities of Acer pseudoplatanus (common name: sycamore; family: Sapindaceae) to be imported from the UK to EU are whips, bare root plants and rooted plants grown in containers (cells, pots, tubs) (Dossier Sections 1.0 and 3.0). According to the Dossier Section 3.0, none of the nurseries expected to export to the EU are using grafting in the production of A. pseudoplatanus.
The commodities are as follows:
-
–
Whips: The age of plants is between 1 and 2 years (Dossier Section 1.0). The diameter is between 4 and 10 mm. Whips are slender, unbranched trees. Whips can be bare root or containerised. Bare root whips may have some leaves at the time of export, particularly when exported in November (Dossier Section 3.0).
-
–
Bare root plants: The age of plants is between 1 and 7 years (Dossier Section 1.0). The diameter is between 30 and 40 mm for 7‐year‐old plants. Bare root plants may have some leaves at the time of export, particularly when exported in November (Dossier Section 3.0).
-
–
Rooted plants in pots: The age of plants is between 1 and 7 years (Dossier Section 1.0). The diameter is between 4 and 88 mm. The plants in pots may be exported with leaves, depending on the timing of the export (Dossier Section 3.0).
Small plants can be considered as seedlings.
The growing media are virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) (Dossier Sections 1.0 and 3.0) complying with the requirements for growing media as specified in the Annex VII of the Commission Implementing Regulation 2019/2072.
The commodities can be classified as ‘bare root plants’ and ‘rooted plants in pots’ in line with ISPM 36 (FAO, 2019).
According to Dossier Section 1.0, the trade volume is up to 18,000 bare root plants and 9,000 rooted plants in pots per year. The trade of these plants will mainly be to Northern Ireland and the Republic of Ireland.
Concerning the intended use of the commodities (Dossier Section 1.0), plants are supplied direct to professional operators and traders. Uses may include propagation, growing on, onward trading or direct sales to final consumers but will generally fall into two categories:
-
–
Tree production and further growing on by professional operators, or
-
–
Direct sales to final users as ornamental plants.
3.2. Description of the production areas
There are four known nurseries in the UK that are producing A. pseudoplatanus plants for the export to the EU (Dossier Section 3.0). The nurseries are shown in Figure 2.
Figure 2.
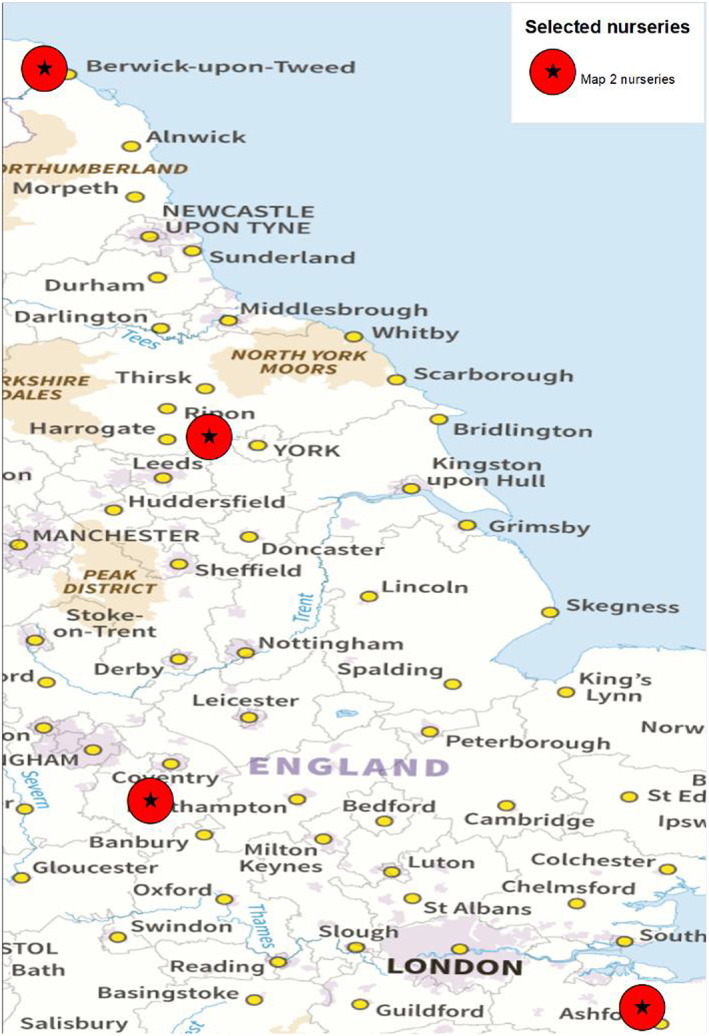
Nurseries in the UK of Acer pseudoplatanus plants for the export to the EU (Source: Dossier Section 3.0)
Acer species are grown in Great Britain in line with the Plant Health (Amendment etc.) (EU Exit) Regulations 2020 8 and the Plant Health (Phytosanitary Conditions) (Amendment) (EU Exit) Regulations 2020 7 . These regulations are broadly similar to EU phytosanitary regulation (Dossier Section 1.0). Producers do not set aside separate areas for export production. All plants within UK nurseries are grown under the same phytosanitary measures, meeting the requirements of the UK Plant Passporting regime (Dossier Section 1.0).
The size of nurseries is between 8 and 150 ha.
The nurseries also grow other plant species as shown in Appendix C. The minimum and maximum proportion of A. pseudoplatanus compared to the other plant species grown in the nurseries is between 0.4% and 2%. Most of the nurseries intending to export to the EU also produce plants for the local market, and there is no distancing between production areas for the export and the local market (Dossier Section 3.0).
Based on the global Köppen–Geiger climate zone classification (Kottek et al., 2006), the climate of the production areas of Acer pseudoplatanus in the UK is classified as Cfb, i.e. main climate (C): warm temperate; precipitation (f): fully humid; temperature (b): warm summer.
The nurseries are kept clear of non‐cultivated herbaceous plants. In access areas, non‐cultivated herbaceous plants are kept to a minimum and only exist at nursery boundaries. Non‐cultivated herbaceous plants grow on less than 1% of the nursery area. The predominant species is rye grass (Lolium sp.). Other identified species include dandelions (Taraxacum officinale), hairy bittercress (Cardamine hirsuta), common daisy (Bellis perennis), creeping cinquefoil (Potentilla reptans) and bluebells (Hyacinthoides non‐scripta). These are all extremely low in number (Dossier Section 3.0).
There are hedges surrounding the export nurseries made up of a range of species including hazel (Corylus avellana), yew (Taxus baccata), holly (Ilex), ivy (Hedera), alder (Alnus glutinosa), cherry laurel (Prunus laurocerasus), hawthorn (Crataegus), blackthorn (Prunus spinosa) and leylandii (Cupressus x leylandii) (Dossier Section 3.0).
The closest Acer plants grown in the surroundings are 10 m away from the nurseries (Dossier Section 3.0).
Nurseries are predominately situated in the rural areas. The surrounding land would tend to be arable farmland with some pasture for animals and small areas of woodland. Hedges are often used to define field boundaries and grown along roadsides (Dossier Section 3.0).
Arable crops within a radius of 2 km from the nurseries are rotated in line with good farming practice and could include oilseed rape (Brassica napus), wheat (Triticum), barley (Hordeum vulgare), turnips (Brassica rapa subsp. rapa), potatoes (Solanum tuberosum) and maize (Zea mays) (Dossier Section 3.0).
Pastures are present within a radius of 2 km from the nurseries and are predominantly ryegrass (Lolium sp.) (Dossier Section 3.0).
Woodland is present within a radius of 2 km from the nurseries. Woodlands tend to be a standard UK mixed woodland, with a range of UK native trees such as oak (Quercus robur), pine (Pinus), poplar (Populus), ash (Fraxinus), sycamore (Acer pseudoplatanus), holly (Ilex), Norway maple (Acer platanoides) and field maple (Acer campestre). The nearest woodland in one of the nurseries borders the boundary fence (Dossier Section 3.0).
It is not possible to identify what plant species are growing within the gardens of private dwellings within a radius of 2 km from the nurseries (Dossier Section 3.0).
Other plants likely to be present in the surroundings of the nurseries (within 2 km radius) are Abies spp., Aesculus spp., Allium porrum, Alnus spp., Beta vulgaris, Betula spp., Camellia spp., Capsicum annuum, Castanea spp., Daucus carota, Fagus spp., Hordeum vugare, Juglans regia, Lolium multiflorum, Magnolia spp., Malus spp., Morus spp., Picea spp., Pinus spp., Populus spp., Prunus spp., Quercus spp., Rhododendron spp., Rosa spp., Salix spp., Sambucus spp., Solanum lycopersicum, Solanum tuberosum, Sorbus spp., Syringa spp., Ulmus spp., Viburnum spp. and Wisteria spp. (Dossier Section 3.0).
Based on the global Köppen–Geiger climate zone classification (Kottek et al., 2006), the climate of the production areas of A. platanoides in the UK is classified as Cfb, i.e. main climate (C): warm temperate; precipitation (f): fully humid; temperature (b): warm summer.
3.3. Production and handling processes
3.3.1. Source of planting material
The starting material of the commodities is a mix of seeds and seedlings depending on the nursery (Dossier Section 3.0).
Seeds purchased in the UK are certified under The Forest Reproductive Material (Great Britain) Regulations 2002. Seedlings sourced in the UK are certified with UK Plant Passports. Seedlings from the EU and New Zealand are certified with phytosanitary certificates. Many plants are obtained from EU (mostly the Netherlands) and New Zealand. These are the only two sources of the plants obtained from abroad (Dossier Section 3.0).
None of the nurseries expected to export to the EU produce plants from grafting, they use only seed and seedlings; therefore, there are no mother plants of A. pseudoplatanus present in the nurseries (Dossier Section 3.0).
3.3.2. Production cycle
Plants are either grown in containers (cells, pots, tubes, etc.) or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). Only certain growth stages are maintained under protection, such as initial seed germination. Plants grown under protection are maintained in plastic polytunnels, or in glasshouses which typically consist of a metal or wood frame construction and glass panels (Dossier Section 3.0). The panel assumes that potted plants could be cultivated for the whole period in pots or grown in the field and then transplanted in pots at a later stage. In this last case it is assumed that the roots will be washed before potting and soil removed as required by the legislation for a commodity to be exported to the EU.
According to the Dossier Section 1.0, planting could take place any time during the year. Bare root plants are harvested in winter (November–April) to be able to lift plants from the field and because this is the best time to move dormant plants. Rooted plants in pots can be moved at any point in the year to fulfil consumer demand. These will likely be destined for garden centre trade rather than nurseries.
The growing media are virgin peat or peat‐free compost. This compost is heat treated by commercial suppliers during production to eliminate pests and diseases. It is supplied in sealed bulk bags or shrink‐wrapped bales and stored off the ground on pallets, these are free from contamination. Where delivered in bulk, compost is kept in a dedicated bunker, either indoors, or covered by tarpaulin outdoors, and with no risk of contamination with soil or other material (Dossier Section 1.0).
The irrigation is done on the need basis and could be overhead, subirrigation or drip irrigation. Water used for irrigation can be drawn from several sources, the mains supply, bore holes or from rainwater collection or watercourses (Dossier Section 3.0). Additional information on water used for irrigation is provided in Appendix D. Regardless of the source of the water used to irrigate, none of the nurseries have experienced the introduction of a pest/disease as a result of contamination of the water supply (Dossier Section 3.0).
Growers are required to assess water sources, irrigation and drainage systems used in the plant production for the potential to harbour and transmit plant pests. Water is routinely sampled and sent for analysis. No quarantine pests have been found (Dossier Section 1.0).
Growers must assess weeds and volunteer plants for the potential to host and transmit plant pests and have an appropriate programme of weed management in place on the nursery (Dossier Section 1.0).
General hygiene measures are undertaken as part of routine nursery production, including disinfection of tools and equipment between batches/lots and different plant species (Dossier Sections 1.0 and 3.0). The tools are dipped and wiped with a clean cloth between trees to reduce the risk of virus and bacterial transfer between subjects. There are various disinfectants available, with Virkon S (active substance: potassium peroxymonosulfate and sodium chloride) being a common example (Dossier Section 3.0).
Growers keep records to allow traceability for all plant material handled. These records must allow a consignment or consignment in transit to be traced back to the original source, as well as forward to identify all trade customers to which those plants have been supplied (Dossier Section 1.0).
3.3.3. Pest monitoring during production
All producers are registered as professional operators with the UK Competent Authority via the Animal and Plant Health Agency (APHA) for England and Wales, or with the Science and Advice for Scottish Agriculture (SASA) for Scotland, and are authorised to issue the UK plant passports, verifying they meet the required national sanitary standards. The Competent Authority inspect crops at least once a year to check they meet the standards set out in the guides. Assessments are normally made based on visual examinations, but samples may be taken for laboratory analysis to get a definitive diagnosis (Dossier Section 1.0).
The Plant Health and Seeds Inspectorate (PHSI), part of APHA, executes plant health policy, except forestry matters, in England and Wales under a Memorandum of Understanding with DEFRA and with the Welsh Government. In Scotland, this role is carried out by inspectors in the Rural Payments and Inspections Division and the Horticulture and Marketing Unit, in SASA. PHSI and Scottish inspectors carry out import, export, monitoring and survey inspections, issue phytosanitary certificates and oversee import controls, issuing of plant passports and eradication campaigns (Dossier Section 1.0).
The sanitary status of production areas is controlled by the producers as part of these schemes, as well as via official inspections by APHA Plant Health and Seeds Inspectors (PHSI) or with SASA (Scotland) Dossier Section 1.0).
All producers are subject to regular inspections by plant health inspectors as part of either Plant Passporting audits or a programme of general surveillance of all registered producers (Dossier Section 1.0).
The UK plant health inspectors monitor for pests and diseases during crop certification and passporting inspections. In addition, the PHSI (in England and Wales) carry out a programme of Quarantine Surveillance in registered premises, inspecting plants grown and moving within the UK market. Similar arrangements operate in Scotland (Dossier Section 1.0).
According to the Dossier Section 1.0, the objective of the quarantine surveillance is to ensure that:
-
–
The plant passport regime is being operated effectively.
-
–
Quarantine organisms are not spread on plants and plant produce which are not subject to plant passporting.
-
–
The UK plant health authorities have early warning of any new threat from a previously unknown pest or disease which has become established within the UK.
-
–
Plant health authorities can take informed decisions on the scope and operation of the plant passport regime.
According to the Dossier Section 1.0, the quarantine surveillance programme centres on a risk‐based selection of premises to visit, based on size, types of plants grown, source of plants and the producer's track record of pest and disease issues. Guidance on visit frequency is given to inspectors to ensure that those sites which present the greatest risk are visited more frequently than those of lower risk. The risk category assigned to a premise determines the frequency of visit:
-
–
very high risk (multiple visits per year);
-
–
high risk (two/three visits per year);
-
–
medium risk (annual visit);
-
–
low risk (once every 3 years).
Inspections are targeted both at the plants or products which present the greatest risk, and also a wider range of plants and plant products which are monitored for more general risks, including those highly polyphagous pests whose range may be unknown or still increasing. The UK inspectors receive comprehensive training on the full range of symptoms caused by pests and diseases, to allow them to detect any new and emerging risks, and during a visit to a nursery, they are free to inspect any plants on that nursery. Samples of pests and plants showing any suspicious symptoms are routinely sent to the laboratory for testing (Dossier Section 1.0).
In the last 3 years, there has been a substantial level of inspection of registered Acer producers, both in support of the Plant Passporting scheme (checks are consistent with EU legislation, with a minimum of once a year for authorised operators) and as part of the Quarantine Surveillance programme (Great Britain uses the same framework for its surveillance programme as the EU) (Dossier Section 1.0).
Plant material is regularly monitored for plant health issues. Pest monitoring is carried out by trained nursery staff via crop walking and records kept of this monitoring. Qualified agronomists also undertake crop walks to verify the producer's assessments. Curative or preventative actions are implemented together with an assessment of phytosanitary risk. Unless a pest can be immediately and definitively identified as non‐quarantine, growers are required to treat it as a suspect quarantine pest and notify the competent authority (Dossier Section 1.0).
The crops are inspected visually on a regular basis by competent nursery staff as part of the growing process. All plants are also carefully inspected by nurseries on arrival and dispatch for any plant health issues (Dossier Section 3.0).
It is a legal requirement under the UK Plant Health law for any person in charge of a premise to notify the Competent Authority of the presence, or suspected presence, of a plant pest. The requirement is not limited to those organisms listed in the UK legislation, but is also required for any organism not normally present in the UK which is likely to be injurious to plants (Dossier Section 1.0).
The nurseries follow the Plant Health Management Standard issued by the Plant Healthy Certification Scheme of which DEFRA, the Royal Horticultural Society and others contribute to via The Plant Health Alliance Steering Group (Dossier Section 3.0).
The UK surveillance is based on visual inspection with samples taken from symptomatic material, and where appropriate, samples taken from asymptomatic material (e.g. plants, tubers, soil, watercourses). According to the Dossier Section 3.0, for sites with the likelihood of multiple pest and host combinations (e.g. ornamental and retail sites), standard methods are used for site selection and visit frequency, whereby clients are assessed taking into account business activity, size of business and source material, so e.g. a large propagator using third country material receives 10 visits per year whilst a small retailer selling locally sourced material is visited once every second year. Where pest‐specific guidelines are absent, inspectors select sufficient plants to give a 95% probability of detecting symptoms randomly distributed on 1.5% of plants in a batch/consignment. For inspections of single hosts, possibly with multiple pests, survey site selection is often directed to specific locations identified by survey planners, e.g. 0.5% of ware potato production land is annually sampled for potato cyst nematode (PCN) with farms randomly selected and sampled at a rate of 50 cores per hectare (Dossier Section 3.0).
During production, in addition to the general health monitoring of the plants by the nurseries, official growing season inspections are undertaken by the UK Plant Health Service at an appropriate time, taking into consideration factors such as the likelihood of pest presence and growth stage of the crop. Where appropriate this could include sampling and laboratory analysis. Official sampling and analysis could also be undertaken nearer to the point of export depending on the type of analysis and the import requirements of the country being exported to. Samples are generally taken on a representative sample of plants, in some cases, however, where the consignment size is quite small, all plants are sampled. Magnification equipment is provided to all inspectors as part of their standard equipment and is used during inspections when appropriate (Dossier Section 3.0).
All residues or waste materials shall be assessed for the potential to host, harbour and transmit pests (Dossier Section 1.0).
Incoming plant material and other goods such as packaging material and growing media, that have the potential to be infected or harbour pests, are checked on arrival. Growers have procedures in place to quarantine any suspect plant material and to report findings to the authorities (Dossier Section 1.0).
3.3.4. Pest management during production
Crop protection is achieved using a combination of measures including approved plant protection products, biological control or physical measures. Plant protection products are only used when necessary and records of all plant protection treatments are kept (Dossier Section 1.0).
Pest and disease pressure vary from season to season. Product application takes place only when required and depends on situation (disease pressure, growth stage, etc. and environmental factors) at that time. Subject to this variation in pest pressure, in some seasons few, if any, pesticides are applied; in others, it is sometimes necessary to apply preventative and/or control applications of pesticides. In many circumstances also, biological control is used to control outbreaks, rather than using chemical treatments.
Examples of typical treatments used against mildew, Botrytis, spider mites, aphids and thrips are detailed in the Dossier Section 3. These would be applied at the manufacturers recommended rate and intervals (Dossier Section 3.0).
There are no specific measures/treatments against the soil pests. However, containerised plants are grown in trays on top of protective plastic membranes to prevent contact with soil. Membranes are regularly refreshed when needed. Alternatively, plants may be grown on raised galvanised steel benches stood on gravel as a barrier between the soil and bench feet and/or concreted surfaces (Dossier Section 3.0).
Post‐harvest and through the autumn and winter, nursery management is centred on pest and disease prevention and maintaining good levels of nursery hygiene. Leaves, pruning residues and weeds are all removed from the nursery to reduce the number of overwintering sites for pests and diseases (Dossier Section 1.0).
3.3.5. Inspections before export
The UK NPPO carries out inspections and testing where required by the country of destination's plant health legislation, to ensure all requirements are fulfilled and a valid phytosanitary certificate with the correct additional declarations is issued (Dossier Section 1.0).
Separate to any official inspection, plant material is checked by growers for plant health issues prior to dispatch (Dossier Section 1.0).
A final pre‐export inspection is undertaken as part of the process of issuing a phytosanitary certificate. These inspections are generally undertaken as near to the time of export as possible, usually within 1–2 days, and not more than 2 weeks before export. Phytosanitary certificates are only issued if the commodity meets the required plant health standards after inspection and/or testing according to appropriate official procedures (Dossier Section 3.0).
The protocol for plants infested by pests during inspections before export is to treat the plants, if they are on site for a sufficient period of time, or to destroy any plants infested by pests otherwise. All other host plants in the nursery would be treated. The phytosanitary certificate for export will not be issued until the UK Plant Health inspectors confirm that the plants are free from pests (Dossier Section 3.0).
3.3.6. Export procedure
Bare root plants are lifted, washed free from soil with a low‐pressure washer in the outdoors nursery area away from packing/cold store area (Dosser Section 3.0).
The maximum time from the harvesting of bare root plants to the export is up to 5 months. Plants are stored in cold store or heeled into soil (but before export they would be washed to ensure freedom from soil). Most plants for export would be kept in cold store (Dossier Section 3.0).
The preparation of the commodities for export is carried out inside the nurseries in a closed environment, e.g. packing shed (Dosser Section 3.0).
Plants are transported by lorry (size dependant on load quantity). Sensitive plants are occasionally transported by temperature‐controlled lorry if weather conditions during transit are likely to be very cold. Bare root plants are exported from November and April, while rooted plants in pots are mainly exported between September and May, although these can be moved at any point in the year to fulfil consumer demand (Dossier Section 1.0).
According to the Dossier Section 3.0, the commodities are dispatched as single bare root trees or in bundles as follows:
-
–
25 or 50 for seedlings or transplants;
-
–
5, 10 or 15 for whips.
Bare root plants are placed in bundles, wrapped in polythene and packed and distributed on ISPM 15 certified wooden pallets, or metal pallets. Alternatively, they may be placed in pallets which are then wrapped in polythene. Small volume orders may be packed in waxed cardboard cartons or polythene bags and dispatched via courier (Dossier Sections 1.0 and 3.0).
Rooted plants in pots are transported on Danish trolleys for smaller containers, or certified pallets, or individually in pots for larger containers (Dossier Section 1.0).
4. Identification of pests potentially associated with the commodity
The search for potential pests associated with the commodity rendered 1924 species (see Microsoft Excel® file in Appendix F).
4.1. Selection of relevant EU‐quarantine pests associated with the commodity
The EU listing of union quarantine pests and protected zone quarantine pests (Commission Implementing Regulation (EU) 2019/2072) is based on assessments concluding that the pests can enter, establish, spread and have potential impact in the EU.
Thirty‐six EU‐quarantine species that are reported to use commodity as a host plant were evaluated (Table 4) for their relevance of being included in this Opinion.
Table 4.
Overview of the evaluation of the 36 EU‐quarantine pest species known to use Acer spp. as a host plant for their relevance for this opinion
| No. | Pest name according to EU legislation (a) | EPPO code | Group | Pest present in the UK | Acer confirmed as a host (reference) | Pest can be associated with the commodity | Pest relevant for the Opinion |
|---|---|---|---|---|---|---|---|
| 1 | Anisandrus maiche as Scolytinae spp. (non‐European) | ANIDMA | Insects | No | Acer barbinerve, A. mandshuricum, A. pictum var. mono (EPPO, online) | Not assessed | No |
| 2 | Anoplophora chinensis | ANOLCN | Insects | No | Acer campestre, A. palmatum, A. platanoides, A. pseudoplatanus (EPPO, online) | Not assessed | No |
| 3 | Anoplophora glabripennis | ANOLGL | Insects | No | Acer platanoides, A. pseudoplatanus (EPPO, online) | Not assessed | No |
| 4a | Bemisia tabaci (non‐European populations) | BEMITA | Insects | No | Acer palmatum (CABI, online) | Not assessed | No |
| 4b | Bemisia tabaci (European populations) | BEMITA | Insects | Yes | Acer palmatum (CABI, online) | Yes | Yes |
| 5 | Candidatus Phytoplasma fragariae related strains (YN‐169, YN‐10G) | PHYPFG | Phytoplasmas | No | Acer (EPPO, online) | Not assessed | No |
| 6 | Choristoneura conflictana | ARCHCO | Insects | No | Acer negundo (Robinson et al., online) | Not assessed | No |
| 7 | Choristoneura parallela | CHONPA | Insects | No | Acer rubrum (Robinson et al., online) | Not assessed | No |
| 8 | Choristoneura rosaceana | CHONRO | Insects | No | Acer palmatum (EPPO, online) | Not assessed | No |
| 9 | Cnestus mutilatus as Scolytinae spp. (non‐European) | XYLSMU | Insects | No | Acer palmatum (EPPO, online) | Not assessed | No |
| 10 | Corthylus punctatissimus as Scolytinae spp. (non‐European) | CORHPU | Insects | No | Acer platanoides (CABI, online) | Not assessed | No |
| 11 | Cryphonectria parasitica | ENDOPA | Fungi | Yes | Acer palmatum (Farr and Rossman, online) | Yes | Yes |
| 12 | Davidsoniella virescens | CERAVI | Fungi | No | Acer campestre (CABI, online) | Not assessed | No |
| 13 | Diabrotica undecimpunctata undecimpunctata | DIABUN | Insects | No | Acer (EPPO, online) | Not assessed | No |
| 14 | Entoleuca mammata | HYPOMA | Fungi | Yes | Acer (Hawksworth, 1972) | Yes | Yes |
| 15 | Euwallacea fornicatus sensu lato (including: Euwallacea fornicatus sensu stricto, Euwallacea fornicatior, Euwallacea kuroshio and Euwallacea perbrevis) |
XYLBFO EUWAWH EUWAFO EUWAKU EUWAPE |
Insects | No | Acer palmatum (EPPO, online) | Not assessed | No |
| 16 | Euwallacea interjectus as Scolytinae spp. (non‐European) | XYLBIN | Insects | No | Acer negundo (EPPO, 2020) | Not assessed | No |
| 17 | Euwallacea validus as Scolytinae spp. (non‐European) | XYLBVA | Insects | No | Acer pensylvanicum (EPPO, 2020) | Not assessed | No |
| 18 | Longidorus diadecturus | LONGDI | Nematodes | No | Acer (Xu and Zhao, 2019) | Not assessed | No |
| 19 | Lopholeucaspis japonica | LOPLJA | Insects | No | Acer palmatum (CABI, online; García Morales et al., online) | Not assessed | No |
| 20 | Lycorma delicatula | LYCMDE | Insects | No | Acer palmatum, A. platanoides, A. pseudoplatanus (EPPO, online) | Not assessed | No |
| 21 | Meloidogyne chitwoodi | MELGCH | Nematodes | No | Acer palmatum (Ferris, online) | Not assessed | No |
| 22 | Meloidogyne fallax | MELGFA | Nematodes | Yes | Acer palmatum (Ferris, online) | Yes | Yes |
| 23 | Monarthrum mali as Scolytinae spp. (non‐European) | MNTHMA | Insects | No | Acer rubrum (EPPO, 2020) | Not assessed | No |
| 24 | Neocosmospora ambrosia | FUSAAM | Fungi | No | Uncertain | Not assessed | No |
| 25 | Neocosmospora euwallaceae | FUSAEW | Fungi | No | Acer palmatum (EPPO, online) | Not assessed | No |
| 26 | Oemona hirta | OEMOHI | Insects | No | Acer palmatum (EPPO, online) | Not assessed | No |
| 27 | Phymatotrichopsis omnivora | PHMPOM | Fungi | No | Acer negundo, A. saccharinum (Farr and Rossman, online) | Not assessed | No |
| 28a | Phytophthora ramorum (EU isolates) | PHYTRA | Oomycetes | No | Acer pseudoplatanus (EPPO, online) | Not assessed | No |
| 28b | Phytophthora ramorum (non‐EU isolates) | PHYTRA | Oomycetes | Yes | Acer pseudoplatanus (EPPO, online) | Yes | Yes |
| 29 | Popillia japonica | POPIJA | Insects | No | Acer palmatum, A. platanoides (EPPO, online) | Not assessed | No |
| 30 | Scirtothrips dorsalis | SCITDO | Insects | Yes | Acer palmatum (CABI, online) | Yes | Yes |
| 31 | Stenoscelis hylastoides as Scolytinae spp. (non‐European) | STEWHY | Insects | No | Acer (Plant Pest Information Network) | Not assessed | No |
| 32 | Trirachys sartus | AELSSA | Insects | No | Acer (EPPO, online) | Not assessed | No |
| 33 | Xiphinema americanum sensu stricto | XIPHAA | Nematodes | No | Acer negundo, A. saccharum (Xu and Zhao, 2019) | Not assessed | No |
| 34 | Xiphinema rivesi (non‐EU populations) | XIPHRI | Nematodes | No | Acer palmatum (Xu and Zhao, 2019) | Not assessed | No |
| 35 | Xylella fastidiosa | XYLEFA | Bacteria | No | Acer platanoides (CABI, online), A. pseudoplatanus | Not assessed | No |
| 36 | Xylosandrus compactus as Scolytinae spp. (non‐European) | XYLSCO | Insects | No | Acer pseudoplatanus (Francardi et al., 2017) | Not assessed | No |
Commission Implementing Regulation (EU) 2019/2072.
The relevance of an EU‐quarantine pest for this opinion was based on evidence that:
the pest is present in the UK;
the commodity is host of the pest;
one or more life stages of the pest can be associated with the specified commodity.
Pests that fulfilled all criteria were selected for further evaluation.
Table 4 presents an overview of the evaluation of the 36 EU‐quarantine pest species that are reported as associated with the commodity.
Of these 36 EU‐quarantine pest species evaluated, six species are present in the UK and all six species (Bemisia tabaci (European populations), Cryphonectria parasitica, Entoleuca mammata, Meloidogyne fallax, Phytophthora ramorum and Scirtothrips dorsalis) can be associated with the commodity and hence were selected for further evaluation.
4.2. Selection of other relevant pests (non‐regulated in the EU) associated with the commodity
The information provided by the UK, integrated with the search performed by EFSA, was evaluated to assess whether there are other potentially relevant pests potentially associated with the commodity species present in the country of export. For these potential pests that are non‐regulated in the EU, pest risk assessment information on the probability of entry, establishment, spread and impact is usually lacking. Therefore, these pests were also evaluated to determine their relevance for this Opinion based on evidence that:
the pest is present in the UK;
the pest is (i) absent or (ii) has a limited distribution in the EU;
the commodity is a host of the pest;
one or more life stages of the pest can be associated with the specified commodity;
the pest may have an impact in the EU.
For non‐regulated species with a limited distribution (i.e. present in one or a few EU MSs) and fulfilling the other criteria (i.e. c, d and e), either one of the following conditions should be additionally fulfilled for the pest to be further evaluated:
Official phytosanitary measures have been adopted in at least one EU MS;
Any other reason justified by the working group (e.g. recent evidence of presence).
Pests that fulfilled the above‐listed criteria were selected for further evaluation.
Based on the information collected, 1,882 potential pests known to be associated with the species commodity were evaluated for their relevance to this Opinion. Species were excluded from further evaluation when at least one of the conditions listed above (a‐e) was not met. Details can be found in Appendix F (Microsoft Excel® file). Of the evaluated EU non‐quarantine pests, four pests (Coniella castaneicola, Eulecanium excrescens, Meloidogyne mali and Takahashia japonica) were selected for further evaluation because they met all of the selection criteria. More information on these four pests can be found in the pest datasheets (Appendix A).
4.3. Overview of interceptions
Data on the interception of harmful organisms on plants of A. pseudoplatanus can provide information on some of the organisms that can be present on A. pseudoplatanus despite the current measures taken. According to EUROPHYT, online (accessed on 22 December 2022) and TRACES‐NT, online (accessed on 22 December 2022), there were no interceptions of plants for planting of A. pseudoplatanus from the UK destined to the EU Member States due to the presence of harmful organisms between the years 1995 and 22 December 2022.
4.4. List of potential pests not further assessed
From the list of pests not selected for further evaluation, the Panel highlighted 12 species (see Appendix E) for which currently available evidence provides no reason to select these species for further evaluation in this Opinion. A specific justification of the inclusion in this list is provided for each species in Appendix E.
4.5. Summary of pests selected for further evaluation
The 10 pests satisfying all the relevant criteria listed above in Sections 4.1 and 4.2 are included in Table 5. The effectiveness of the risk mitigation measures applied to the commodity was evaluated for these selected pests.
Table 5.
List of relevant pests selected for further evaluation
| Number | Current scientific name | EPPO code | Name used in the EU legislation | Taxonomic information | Group | Regulatory status |
|---|---|---|---|---|---|---|
| 1 | Bemisia tabaci | BEMITA | Bemisia tabaci Genn. (European populations) |
Hemiptera Aleyrodidae |
Insects | EU protected zone quarantine pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 2 | Coniella castaneicola | – | – |
Diaporthales Schizoparmaceae |
Fungi | Not regulated in the EU |
| 3 | Cryphonectria parasitica | ENDOPA | Cryphonectria parasitica (Murrill) Barr |
Diaporthales Cryphonectriaceae |
Fungi | EU protected zone quarantine pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 4 | Entoleuca mammata | HYPOMA | Entoleuca mammata (Wahlenb.) Rogers and Ju |
Xylariales Xylariaceae |
Fungi | EU protected zone quarantine pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 5 | Eulecanium excrescens | – | – |
Hemiptera Coccidae |
Insects | Not regulated in the EU |
| 6 | Meloidogyne fallax | MELGFA | Meloidogyne fallax Karssen |
Rhabditida Meloidogynidae |
Nematodes | EU quarantine pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 7 | Meloidogyne mali | MELGMA | – |
Rhabditida Meloidogynidae |
Nematodes | Not regulated in the EU |
| 8 | Phytophthora ramorum | PHYTRA | Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in ‘t Veld |
Peronosporales Peronosporaceae |
Oomycetes | EU quarantine pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 9 | Scirtothrips dorsalis | SCITDO | Scirtothrips dorsalis Hood |
Thysanoptera Thripidae |
Insects | EU quarantine pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 10 | Takahashia japonica | TAKAJA | – |
Hemiptera Coccidae |
Insects | Not regulated in the EU |
5. Risk mitigation measures
For the selected pests (Table 5), the Panel evaluated the likelihood that it could be present in the A. pseudoplatanus nurseries by evaluating the possibility that the commodity in the export nurseries is infested either by:
introduction of the pest from the environment surrounding the nursery;
introduction of the pest with new plants/seeds;
spread of the pest within the nursery.
The information used in the evaluation of the effectiveness of the risk mitigation measures is summarised in pest data sheets (see Appendix A).
5.1. Risk mitigation measures applied in the UK
With the information provided by the UK (Dossier Sections 1.0, 2.0, 3.0 and 4.0), the Panel summarised the risk mitigation measures (see Table 6) that are implemented in the production nursery.
Table 6.
Overview of implemented risk mitigation measures for Acer pseudoplatanus plants designated for export to the EU from the UK
| Number | Risk mitigation measure | Implementation in the UK |
|---|---|---|
| 1 | Registration of production sites | All producers are registered as professional operators with the UK Competent Authority via APHA for England and Wales, or SASA for Scotland, and are authorised to issue the UK plant passports, verifying they meet the required national sanitary standards (Dossier Section 1.0). |
| 2 | Physical separation | Producers do not set aside separate areas for export production. All plants within the UK nurseries are grown under the same phytosanitary measures, meeting the requirements of the UK Plant Passporting regime (Dossier Section 1.0). |
| 3 | Certified plant material | Seeds purchased in the UK are certified under The Forest Reproductive Material (Great Britain) Regulations 2002. Seedlings sourced in the UK are certified with UK Plant Passports. Seedlings from the EU countries are certified with phytosanitary certificates. Many plants are obtained from EU (mostly the Netherlands) and New Zealand. These are the only two sources of the plants obtained from abroad (Dossier Section 3.0). |
| 4 | Growing media | The growing media are virgin peat or peat‐free compost. This compost is heat treated by commercial suppliers during production to eliminate pests and diseases. It is supplied in sealed bulk bags or shrink‐wrapped bales and stored off the ground on pallets, these are free from contamination. Where delivered in bulk, compost is kept in a dedicated bunker, either indoors, or covered by tarpaulin outdoors, and with no risk of contamination with soil or other material (Dossier Section 1.0). |
| 5 | Surveillance, monitoring and sampling | For additional information, see Section 3.3.3 Pest monitoring during production. |
| 6 | Hygiene measures |
Growers must assess weeds and volunteer plants for the potential to host and transmit plant pests and have an appropriate programme of weed management in place on the nursery (Dossier Section 1.0). General hygiene measures are undertaken as part of routine nursery production, including disinfection of tools and equipment between batches/lots (Dossier Section 1.0).and different plant species (Dossier Sections 1.0 and 3.0). The tools are dipped and wiped with a clean cloth between trees to reduce the risk of virus and bacterial transfer between subjects. There are various disinfectants available, with Virkon S being a common example (Dossier Section 3.0). |
| 7 | Removal of infested plant material | Post‐harvest and through the autumn and winter, nursery management is centred on pest and disease prevention and maintaining good levels of nursery hygiene. Leaves, pruning and weeds are all removed from the nursery to reduce the number of over wintering sites for pests and diseases (Dossier Section 1.0). |
| 8 | Irrigation water | Water for irrigation is routinely sampled and sent for analysis (Dossier Section 1.0). |
| 9 | Application of pest control products |
Crop protection is achieved using a combination of measures including approved plant protection products, biological control or physical measures. Plant protection products are only used when necessary and records of all plant protection treatments are kept (Dossier Section 1.0). Examples of typical treatments used against mildew, Botrytis, spider mites, aphids and thrips are detailed in the Dossier Section 3. These would be applied at the manufacturers recommended rate and intervals (Dossier Section 3.0). |
| 10 | Measures against soil pests | There are no specific measures/treatments against the soil pests. However, containerised plants are grown in trays on top of protective plastic membranes to prevent contact with soil. Membranes are regularly refreshed when needed. Alternatively, plants may be grown on raised galvanised steel benches stood on gravel as a barrier between the soil and bench feet and/or concreted surfaces (Dossier Section 3.0). |
| 11 | Inspections and management of plants before export |
The UK NPPO carries out inspections and testing where required by the country of destination's plant health legislation, to ensure all requirements are fulfilled and a valid phytosanitary certificate with the correct additional declarations is issued (Dossier Section 1.0). Separate to any official inspection, plant material is checked by growers for plant health issues prior to dispatch (Dossier Section 1.0). A final pre‐export inspection is undertaken as part of the process of issuing a phytosanitary certificate. These inspections are generally undertaken as near to the time of export as possible, usually within 1–2 days, and not more than 2 weeks before export. Phytosanitary certificates are only issued if the commodity meets the required plant health standards after inspection and/or testing according to appropriate official procedures (Dossier Section 3.0). The protocol for plants infested by pests during inspections before export is to treat the plants, if they are on site for a sufficient period of time, or to destroy any plants infested by pests otherwise. All other host plants in the nursery would be treated. The phytosanitary certificate for export will not be issued until the UK Plant Health inspectors confirm that the plants are free from pests (Dossier Section 3.0). |
| 12 | Separation during transport to the destination |
According to the Dossier Section 3.0, the commodities are dispatched as single bare root trees or in bundles as follows:
Bare root plants are placed in bundles, wrapped in polythene, and packed and distributed on ISPM 15 certified wooden pallets, or metal pallets. Alternatively, they may be placed in pallets which are then wrapped in polythene. Small volume orders may be packed in waxed cardboard cartons or polythene bags and dispatched via courier (Dossier Sections 1.0 and 3.0). Rooted plants in pots are transported on Danish trolleys for smaller containers, or certified pallets, or individually in pots for larger containers (Dossier Section 1.0). |
5.2. Evaluation of the current measures for the selected relevant pests including uncertainties
For each evaluated pest, the relevant risk mitigation measures acting on the pest were identified. Any limiting factors on the effectiveness of the measures were documented.
All the relevant information including the related uncertainties deriving from the limiting factors used in the evaluation are summarised in a pest data sheet provided in Appendix A. Based on this information, for each selected relevant pest, an expert judgement is given for the likelihood of pest freedom taking into consideration the risk mitigation measures and their combination acting on the pest.
An overview of the evaluation of each relevant pest is given in the sections below (Sections 5.2.1–5.2.9). The outcome of the EKE regarding pest freedom after the evaluation of the currently proposed risk mitigation measures is summarised in Section 5.2.10.
5.2.1. Overview of the evaluation of Bemisia tabaci (European populations) (Hemiptera; Aleyrodidae)
| Overview of the evaluation of Bemisia tabaci (European populations) for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,967 out of 10,000 bundles | 9,981 out of 10,000 bundles | 9,989 out of 10,000 bundles | 9,995 out of 10,000 bundles | 9,999.2 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested bundles | 0.8 out of 10,000 bundles | 5 out of 10,000 bundles | 11 out of 10,000 bundles | 19 out of 10,000 bundles | 33 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK, with few occurrences but continuously intercepted. The UK outbreaks of B. tabaci have been restricted to greenhouses. The pest is extremely polyphagous. Other traded plants present in the surroundings of the nursery could be a source of the pest. Polytunnels and glasshouses in the nurseries could act as a reservoir of the pest. The pest could go undetected during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of B. tabaci between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). There were four interceptions of B. tabaci from the UK in 2007 and 2015 on other plants already planted likely produced under protected conditions (EUROPHYT, online) Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
| Overview of the evaluation of Bemisia tabaci (European populations) for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,951 out of 10,000 plants | 9,971 out of 10,000 plants | 9,984 out of 10,000 plants | 9,993 out of 10,000 plants | 9,999 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 1 out of 10,000 plants | 7 out of 10,000 plants | 16 out of 10,000 plants | 29 out of 10,000 plants | 49 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK, with few occurrences but continuously intercepted. The UK outbreaks of B. tabaci have been restricted to greenhouses. The pest is extremely polyphagous. Other traded plants present in the surroundings of the nursery could be a source of the pest. Polytunnels and glasshouses in the nurseries could act as a reservoir of the pest. The pest could go undetected during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of B. tabaci between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). There were four interceptions of B. tabaci from the UK in 2007 and 2015 on other plants already planted likely produced under protected conditions (EUROPHYT, online). Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
| Overview of the evaluation of Bemisia tabaci (European populations) for plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,934 out of 10,000 plants | 9,961 out of 10,000 plants | 9,979 out of 10,000 plants | 9,991 out of 10,000 plants | 9,998.4 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 1.6 out of 10,000 plants | 9 out of 10,000 plants | 21 out of 10,000 plants | 39 out of 10,000 plants | 66 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK, with few occurrences but continuously intercepted. UK outbreaks of B. tabaci have been restricted to greenhouses. The pest is extremely polyphagous. Other traded plants present in the surroundings of the nursery could be a source of the pest. Polytunnels and glasshouses in the nurseries could act as a reservoir of the pest. The pest could go undetected during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of B. tabaci between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). There were four interceptions of B. tabaci from the UK in 2007 and 2015 on other plants already planted likely produced under protected conditions (EUROPHYT, online). Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Bemisia tabaci (European populations) (Section A.1 in Appendix A).
5.2.2. Overview of the evaluation of Coniella castaneicola (Diaporthales; Schizoparmaceae)
| Overview of the evaluation of Coniella castaneicola for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,847 out of 10,000 bundles | 9,920 out of 10,000 bundles | 9,955 out of 10,000 bundles | 9,977 out of 10,000 bundles | 9,994 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 6 out of 10,000 bundles | 23 out of 10,000 bundles | 45 out of 10,000 bundles | 80 out of 10,000 bundles | 153 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Coniella castaneicola is present in the UK, although reports are still scattered. Despite there is uncertainty on the host status of A. pseudoplatanus, Acer sp. is reported as a host of the pathogen. Infection may occur by means of conidia through wounds. Infection courts represented by wounds and injuries of biotic and abiotic origin are expected to be present. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the treatment of the growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) the removal of infected plant material; and (e) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of C. castaneicola between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of Coniella castaneicola for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,841 out of 10,000 plants | 9,922 out of 10,000 plants | 9,958 out of 10,000 plants | 9,981 out of 10,000 plants | 9,996 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 4 out of 10,000 plants | 19 out of 10,000 plants | 42 out of 10,000 plants | 78 out of 10,000 plants | 159 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Coniella castaneicola is present in the UK, although reports are still scattered. Despite there is uncertainty on the host status of A. pseudoplatanus, Acer sp. is reported as a host of the pathogen. Infection may occur by means of conidia through wounds. Infection courts represented by wounds and injuries of biotic and abiotic origin are expected to be frequent. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the treatment of the growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) the removal of infected plant material; and (e) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of C. castaneicola between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of Coniella castaneicola for plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,813 out of 10,000 plants | 9,894 out of 10,000 plants | 9,935 out of 10,000 plants | 9,963 out of 10,000 plants | 9,987 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 13 out of 10,000 plants | 37 out of 10,000 plants | 65 out of 10,000 plants | 106 out of 10,000 plants | 187 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Coniella castaneicola is present in the UK, although reports are still scattered. Despite there is uncertainty on the host status of A. pseudoplatanus, Acer sp. is reported as a host of the pathogen. Infection may occur by means of conidia through wounds. Infection courts represented by wounds and injuries of biotic and abiotic origin are expected to be frequent. Plants can be exported during the vegetation period (with leaves). The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the treatment of the growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) the removal of infected plant material; and (e) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of C. castaneicola between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Coniella castaneicola (Section A.2 in Appendix A).
5.2.3. Overview of the evaluation of Cryphonectria parasitica (Diaporthales; Cryphonectriaceae)
| Overview of the evaluation of Cryphonectria parasitica for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,963 out of 10,000 bundles | 9,981 out of 10,000 bundles | 9,989 out of 10,000 bundles | 9,995 out of 10,000 bundles | 9,999 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 1 out of 10,000 bundles | 5 out of 10,000 bundles | 11 out of 10,000 bundles | 19 out of 10,000 bundles | 37 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Cryphonectria parasitica is present in the UK, although not widely distributed, while its main host, i.e. Castanea spp., has scattered distribution in the UK. Despite there is high uncertainty on the level of susceptibility of Acer spp. to the pathogen, infection courts (e.g. pruning and grafting wounds, accidental breaking of twigs before export) are expected to be present. The main hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity, although unlikely, may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) hygiene measures with particular reference to the disinfection of tools and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of C. parasitica between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of Cryphonectria parasitica for bare root plants/trees and plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,954 out of 10,000 plants | 9,975 out of 10,000 plants | 9,985 out of 10,000 plants | 9,992 out of 10,000 plants | 9,997 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 3 out of 10,000 plants | 8 out of 10,000 plants | 15 out of 10,000 plants | 25 out of 10,000 plants | 46 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Cryphonectria parasitica is present in the UK, although not widely distributed, while its main host, i.e. Castanea spp., has scattered distribution in the UK. Despite there is high uncertainty on the level of susceptibility of Acer spp. to the pathogen, infection courts (e.g. pruning and grafting wounds, accidental breaking of twigs before export) are expected to be present. The main hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity, although unlikely, may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) hygiene measures with particular reference to the disinfection of tools; and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of C. parasitica between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Cryphonectria parasitica (Section A.3 in Appendix A).
5.2.4. Overview of the evaluation of Entoleuca mammata (Xylariales; Xylariaceae)
| Overview of the evaluation of Entoleuca mammata for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,942 out of 10,000 bundles | 9,967 out of 10,000 bundles | 9,983 out of 10,000 bundles | 9,993 out of 10,000 bundles | 9,999.1 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 0.9 out of 10,000 bundles | 7 out of 10,000 bundles | 17 out of 10,000 bundles | 33 out of 10,000 bundles | 58 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Entoleuca mammata is present in the UK, although not widely distributed. Despite there is uncertainty on the host status of A. pseudoplatanus, other Acer spp. are reported as hosts of the pathogen. Mechanical wounds including pruning wounds are expected to be present and may represent infection courts. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) the removal of infected plant material; and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of E. mammata between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of Entoleuca mammata for bare root plants/trees and plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,927 out of 10,000 plants | 9,961 out of 10,000 plants | 9,979 out of 10,000 plants | 9,991 out of 10,000 plants | 9,998 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 2 out of 10,000 plants | 9 out of 10,000 plants | 21 out of 10,000 plants | 39 out of 10,000 plants | 73 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Entoleuca mammata is present in the UK, although not widely distributed. Despite there is uncertainty on the host status of A. pseudoplatanus, other Acer spp. are reported as hosts of the pathogen. Mechanical wounds including pruning wounds are expected to be present and may represent infection courts. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) the removal of infected plant material; and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of E. mammata between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Entoleuca mammata (Section A.4 in Appendix A).
5.2.5. Overview of the evaluation of Eulecanium excrescens (Hemiptera; Coccidae)
| Overview of the evaluation of Eulecanium excrescens for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,897 out of 10,000 bundles | 9,943 out of 10,000 bundles | 9,972 out of 10,000 bundles | 9,989 out of 10,000 bundles | 9,997.3 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested bundles | 2.7 out of 10,000 bundles | 11 out of 10,000 bundles | 28 out of 10,000 bundles | 57 out of 10,000 bundles | 103 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK, and specifically in the location where nurseries are sited. First instar (crawlers) can be easily spread with the wind from the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected or confused with nymphs of other species during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of E. excrescens between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
| Overview of the evaluation of Eulecanium excrescens for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,922 out of 10,000 plants | 9,955 out of 10,000 plants | 9,975 out of 10,000 plants | 9,988 out of 10,000 plants | 9,996 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 4 out of 10,000 plants | 12 out of 10,000 plants | 25 out of 10,000 plants | 45 out of 10,000 plants | 78 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK, and specifically in the location where nurseries are sited. First instar (crawlers) can be easily spread with the wind from the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected or confused with nymphs of other species during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of E. excrescens between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
| Overview of the evaluation of Eulecanium excrescens for plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,896 out of 10,000 plants | 9,942 out of 10,000 plants | 9,968 out of 10,000 plants | 9,986 out of 10,000 plants | 9,995.6 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 4.4 out of 10,000 plants | 14 out of 10,000 plants | 32 out of 10,000 plants | 58 out of 10,000 plants | 104 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK, and specifically in the location where nurseries are sited. First instar (crawlers) can be easily spread with the wind from the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected or confused with nymphs of other species during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products; and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of E. excrescens between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Eulecanium excrescens (Section A.5 in Appendix A).
5.2.6. Overview of the evaluation of Meloidogyne fallax and M. mali (Rhabditida; Meloidogynidae)
| Overview of the evaluation of Meloidogyne fallax and M. mali for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,901 out of 10,000 bundles | 9,940 out of 10,000 bundles | 9,960 out of 10,000 bundles | 9,975 out of 10,000 bundles | 9,989 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 11 out of 10,000 bundles | 25 out of 10,000 bundles | 40 out of 10,000 bundles | 60 out of 10,000 bundles | 99 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Meloidogyne fallax and M. mali are present in the UK with restricted distribution. Suitable hosts are present both in the nurseries and in the surroundings. Acer palmatum is a host of M. fallax. Acer palmatum and A. pseudoplatanus are hosts of M. mali. Given that both nematodes are highly polyphagous, it is likely that also other Acer species could be used as host plants. The pest can enter into the nurseries and spread within the nurseries with infected plant material and movement of soil attached to machinery and shoes. The plants could become infected during the growth in the soil in the fields. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the nematodes. These measures include (a) the use of certified plant material; (b) the use of heat‐treated growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; and (d) hygiene measures. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of M. fallax between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of M. mali between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures Low‐pressure water is used for washing roots before export. This washing may not be as effective as using high‐pressure water in removing the soil, thereby making symptoms less visible.
Main uncertainties
|
||||
| Overview of the evaluation of Meloidogyne fallax and M. mali for bare root plants/trees and plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,792 out of 10,000 plants | 9,873 out of 10,000 plants | 9,927 out of 10,000 plants | 9,967 out of 10,000 plants | 9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 6 out of 10,000 plants | 33 out of 10,000 plants | 73 out of 10,000 plants | 127 out of 10,000 plants | 208 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Meloidogyne fallax and M. mali are present in the UK with restricted distribution. Suitable hosts are present both in the nurseries and in the surroundings. Acer palmatum is a host of M. fallax. Acer palmatum and A. pseudoplatanus are hosts of M. mali. Given that both nematodes are highly polyphagous, it is likely that also other Acer species could be used as host plants. The pest can enter into the nurseries and spread within the nurseries with infected plant material and movement of soil attached to machinery and shoes. The plants could become infected during the growth in the soil in the fields. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the nematodes. These measures include (a) the use of certified plant material; (b) the use of heat‐treated growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) hygiene measures; and (e) separation of the pots from soil. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of M. fallax between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of M. mali between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures Low‐pressure water is used for washing roots before export for bare root plants. This washing may not be as effective as using high‐pressure water in removing the soil, thereby making symptoms less visible.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Meloidogyne fallax (Section A.6 in Appendix A) and M. mali (Section A.7 in Appendix A).
5.2.7. Overview of the evaluation of Phytophthora ramorum (Peronosporales; Peronosporaceae)
| Overview of the evaluation of Phytophthora ramorum for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,872 out of 10,000 bundles | 9,922 out of 10,000 bundles | 9,957 out of 10,000 bundles | 9,981 out of 10,000 bundles | 9,995 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 5 out of 10,000 bundles | 19 out of 10,000 bundles | 43 out of 10,000 bundles | 78 out of 10,000 bundles | 128 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including A. pseudoplatanus and other Acer species. The main hosts (e.g. Rhododendron spp., Larix spp., etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy Phytophthora ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of Phytophthora ramorum for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,886 out of 10,000 plants | 9,936 out of 10,000 plants | 9,964 out of 10,000 plants | 9,983 out of 10,000 plants | 9,995 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 5 out of 10,000 plants | 17 out of 10,000 plants | 36 out of 10,000 plants | 64 out of 10,000 plants | 114 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including A. pseudoplatanus and other Acer species. The main hosts (e.g. Rhododendron spp., Larix spp. etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy Phytophthora ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of Phytophthora ramorum for plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,808 out of 10,000 plants | 9,891 out of 10,000 plants | 9,939 out of 10,000 plants | 9,971 out of 10,000 plants | 9,992 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 8 out of 10,000 plants | 29 out of 10,000 plants | 61 out of 10,000 plants | 109 out of 10,000 plants | 192 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including A. pseudoplatanus and other Acer species. The main hosts (e.g. Rhododendron spp., Larix spp. etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy Phytophthora ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Phytophthora ramorum (Section A.8 in Appendix A).
5.2.8. Overview of the evaluation of Scirtothrips dorsalis (Thysanoptera; Thripidae)
| Overview of the evaluation of Scirtothrips dorsalis for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,987 out of 10,000 bundles | 9,994 out of 10,000 bundles | 9,997 out of 10,000 bundles | 9,999 out of 10,000 bundles | 9,999.87 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 0.13 out of 10,000 bundles | 1 out of 10,000 bundles | 3 out of 10,000 bundles | 6 out of 10,000 bundles | 13 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The presence of the pest is doubtful in the UK, although not declared as eradicated. The adults fly and can be spread by the wind from the greenhouse where it was detected to the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected because symptoms are generic. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products; and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of S. dorsalis between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures Detection can be difficult and require expert identification.
Main uncertainties
|
||||
| Overview of the evaluation of Scirtothrips dorsalis for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,986 out of 10,000 plants | 9,993 out of 10,000 plants | 9,996 out of 10,000 plants | 9,998 out of 10,000 plants | 9,999.4 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 0.6 out of 10,000 plants | 2 out of 10,000 plants | 4 out of 10,000 plants | 7 out of 10,000 plants | 14 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The presence of the pest is doubtful in the UK, although not declared as eradicated. The adults fly and can be spread by the wind from the greenhouse where it was detected to the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected because symptoms are generic. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products; and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of S. dorsalis between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures Detection can be difficult and require expert identification.
Main uncertainties
|
||||
| Overview of the evaluation of Scirtothrips dorsalis for plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with few exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,981 out of 10,000 plants | 9,990 out of 10,000 plants | 9,994 out of 10,000 plants | 9,996.9 out of 10,000 plants | 9,999.1 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 0.9 out of 10,000 plants | 3.1 out of 10,000 plants | 6 out of 10,000 plants | 10 out of 10,000 plants | 19 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The presence of the pest is doubtful in the UK, although not declared as eradicated. The adults fly and can be spread by the wind from the greenhouse where it was detected to the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected because symptoms are generic and because the species is difficult to detect when overwintering in the soil. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products; (d) removal of infested plant material; (e) using clean substrate. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of S. dorsalis between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures Detection can be difficult especially in the soil and require expert identification.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Scirtothrips dorsalis (Section A.9 in Appendix A).
5.2.9. Overview of the evaluation of Takahashia japonica (Hemiptera; Coccidae)
| Overview of the evaluation of Takahashia japonica for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the median). | ||||
| Percentile of the distribution | 5% | 25% | median | 75% | 95% |
| Proportion of pest‐free bundles | 9,903 out of 10,000 bundles | 9,947 out of 10,000 bundles | 9,972 out of 10,000 bundles | 9,988 out of 10,000 bundles | 9,997.6 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested bundles | 2.4 out of 10,000 bundles | 12 out of 10,000 bundles | 28 out of 10,000 bundles | 53 out of 10,000 bundles | 97 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK. First instar (crawlers) can be easily spread with the wind from the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected or confused with nymphs of other species during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products; and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of T. japonica between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
| Overview of the evaluation of Takahashia japonica for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,916 out of 10,000 plants | 9,953 out of 10,000 plants | 9,978 out of 10,000 plants | 9,992 out of 10,000 plants | 9,998.6 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 1.4 out of 10,000 plants | 8 out of 10,000 plants | 22 out of 10,000 plants | 47 out of 10,000 plants | 84 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK. First instar (crawlers) can be easily spread with the wind from the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected or confused with nymphs of other species during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products; and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of T. japonica between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
| Overview of the evaluation of Takahashia japonica for plants in pots up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | median | 75% | 95% |
| Proportion of pest‐free plants | 9,899 out of 10,000 plants | 9,943 out of 10,000 plants | 9,972 out of 10,000 plants | 9,990 out of 10,000 plants | 9,997.3 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 2.7 out of 10,000 plants | 10 out of 10,000 plants | 28 out of 10,000 plants | 57 out of 10,000 plants | 101 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK. First instar (crawlers) can be easily spread with the wind from the surroundings of the nurseries. The pest is extremely polyphagous. There are host species in the surroundings of the nurseries. An initial infestation of the pest could go undetected or confused with nymphs of other species during inspections. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pest. These measures include (a) inspections, surveillance, monitoring, sampling and laboratory testing; (b) hygiene measures; (c) application of pest control products; and (d) removal of infested plant material. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of T. japonica between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures None.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Takahashia japonica (Section A.10 in Appendix A).
5.2.10. Outcome of expert knowledge elicitation
Table 7 and Figures 3, 4 and 5 show the outcome of the EKE regarding pest freedom after the evaluation of the implemented risk mitigation measures for all the evaluated pests.
Table 7.
Assessment of the likelihood of pest freedom following evaluation of current risk mitigation measures against pests and pathogens on Acer pseudoplatanus plants designated for export to the EU. In panel A, the median value for the assessed level of pest freedom for each pest is indicated by ‘M', the 5% percentile is indicated by ‘L' and the 95% percentile is indicated by ‘U'. The percentiles together span the 90% uncertainty range regarding pest freedom. The pest freedom categories are defined in panel B of the table
| Number | Group | Pest species | Sometimes pest free | More often than not pest free | Frequently pest free | Very frequently pest free | Extremely frequently pest free | Pest free with some exceptional cases | Pest free with few exceptional cases | Almost always pest free |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Insects | Bemisia tabaci/whips | LM | U | ||||||
| 2 | Fungi | Coniella castaneicola/whips | L | M | U | |||||
| 3 | Fungi | Cryphonectria parasitica/whips | LM | U | ||||||
| 4 | Fungi | Entoleuca mammata/whips | L | M | U | |||||
| 5 | Insects | Eulecanium excrescens/whips | L | M | U | |||||
| 6 | Nematodes | Meloidogyne fallax and M. mali/whips | L | MU | ||||||
| 7 | Oomycetes | Phytophthora ramorum/whips | L | M | U | |||||
| 8 | Insects | Scirtothrips dorsalis/whips | L | MU | ||||||
| 9 | Insects | Takahashia japonica/whips | L | M | U | |||||
| 10 | Insects | Bemisia tabaci/bare root plants | LM | U | ||||||
| 11 | Fungi | Coniella castaneicola/bare root plants | L | M | U | |||||
| 12 | Fungi | Cryphonectria parasitica/bare root plants | LM | U | ||||||
| 13 | Fungi | Entoleuca mammata/bare root plants | L | M | U | |||||
| 14 | Insects | Eulecanium excrescens/bare root plants | L | M | U | |||||
| 15 | Nematodes | Meloidogyne fallax and M. mali/bare root plants | L | M | U | |||||
| 16 | Oomycetes | Phytophthora ramorum/bare root plants | L | M | U | |||||
| 17 | Insects | Scirtothrips dorsalis/bare root plants | L | MU | ||||||
| 18 | Insects | Takahashia japonica/bare root plants | L | M | U | |||||
| 19 | Insects | Bemisia tabaci / plants in pots | L | M | U | |||||
| 20 | Fungi | Coniella castaneicola / plants in pots | L | M | U | |||||
| 21 | Fungi | Cryphonectria parasitica/plants in pots | LM | U | ||||||
| 22 | Fungi | Entoleuca mammata/plants in pots | L | M | U | |||||
| 23 | Insects | Eulecanium excrescens/plants in pots | L | M | U | |||||
| 24 | Nematodes | Meloidogyne fallax and M. mali/plants in pots | L | M | U | |||||
| 25 | Oomycetes | Phytophthora ramorum/plants in pots | L | M | U | |||||
| 26 | Insects | Scirtothrips dorsalis/plant in pots | L | M | U | |||||
| 27 | Insects | Takahashia japonica/plants in pots | L | M | U |
Figure 3.
Elicited certainty (y‐axis) of the number of pest‐free Acer pseudoplatanus whips and seedlings (x‐axis; log‐scaled) out of 10,000 bundles designated for export to the EU from the UK for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
Figure 4.
Elicited certainty (y‐axis) of the number of pest‐free Acer pseudoplatanus bare root plants up to 7 years old (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU from the UK for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
Figure 5.
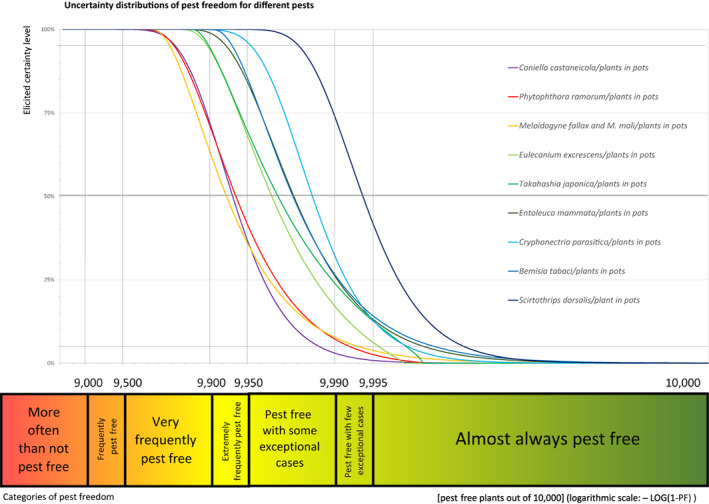
Elicited certainty (y‐axis) of the number of pest‐free Acer pseudoplatanus plants in pots up to 7 years old (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU from the UK for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
Figure 6 provides an explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the implemented risk mitigation measures for bundles of Acer pseudoplatanus whips designated for export to the EU for Coniella castaneicola.
Figure 6.
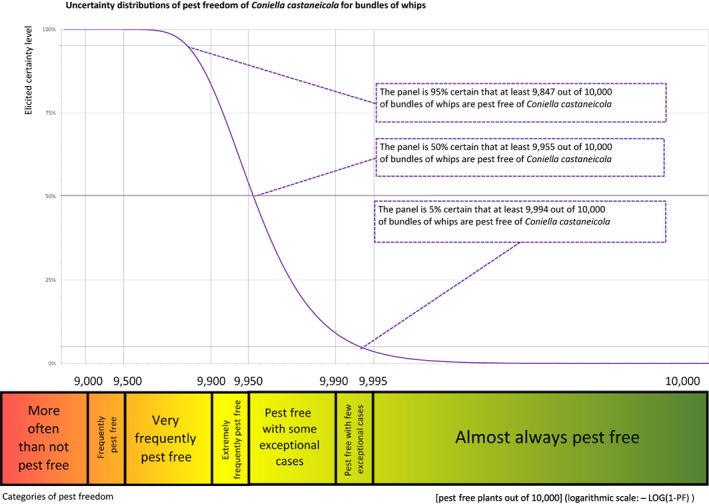
Explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the implemented risk mitigation measures for plants designated for export to the EU based on the example of Coniella castaneicola on Acer pseudoplatanus bundles of whips and seedlings
PANEL A
| Pest freedom category | Pest‐free plants out of 10,000 | |
|---|---|---|
| Sometimespest free | ≤ 5,000 | |
| More often than notpest free | 5,000– ≤ 9,000 | |
| Frequentlypest free | 9,000– ≤ 9,500 | |
| Very frequently pest free | 9,500– ≤ 9,900 | |
| Extremely frequently pest free | 9,900– ≤ 9,950 | |
| Pest free with some exceptional cases | 9,950– ≤ 9,990 | |
| Pest free with few exceptional cases | 9,990– ≤ 9,995 | |
| Almost always pest free | 9,995– ≤ 10,000 |
PANEL B
| Legend of pest freedom categories | |
|---|---|
| L | Pest freedom category includes the elicitedlower bound of the 90% uncertainty range |
| M | Pest freedom category includesthe elicited median |
| U | Pest freedom category includes the elicitedupper bound of the 90% uncertainty range |
6. Conclusions
There are 10 pests identified to be present in the UK and considered to be potentially associated with the commodities imported from the UK and relevant for the EU.
These pests are Bemisia tabaci, Coniella castaneicola, Cryphonectria parasitica, Entoleuca mammata, Eulecanium excrescens, Meloidogyne fallax, Meloidogyne mali, Phytophthora ramorum, Scirtothrips dorsalis and Takahashia japonica. The likelihood of the pest freedom after the evaluation of the implemented risk mitigation measures for the commodities designated for export to the EU was estimated. In the assessment of risk, the age of the plants was considered, reasoning that older trees are more likely to be infested mainly due to longer exposure time and larger size.
For Bemisia tabaci, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,967 and 10,000 bundles of whips and seedlings per 10,000 will be free from B. tabaci. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,951 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from B. tabaci. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘Almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,934 and 10,000 plants in pots up to 7 years old per 10,000 will be free from B. tabaci.
For Coniella castaneicola, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,847 and 10,000 bundles of whips and seedlings per 10,000 will be free from C. castaneicola. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,841 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from C. castaneicola. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,813 and 10,000 plants in pots up to 7 years old per 10,000 will be free from C. castaneicola.
For Cryphonectria parasitica, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,963 and 10,000 bundles of whips and seedlings per 10,000 will be free from C. parasitica. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,954 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from C. parasitica. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,954 and 10,000 plants in pots up to 7 years old per 10,000 will be free from C. parasitica.
For Entoleuca mammata, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from pest free with some exceptional cases' to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,942 and 10,000 bundles of whips and seedlings per 10,000 will be free from E. mammata. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,927 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from E. mammata. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,927 and 10,000 plants in pots up to 7 years old per 10,000 will be free from E. mammata.
For Eulecanium excrescens, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,897 and 10,000 bundles of whips and seedlings per 10,000 will be free from E. excrescens. The likelihood of pest freedom for bare root plants/ up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,922 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from E. excrescens. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,896 and 10,000 plants in pots up to 7 years old per 10,000 will be free from E. excrescens.
For Meloidogyne fallax, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,901 and 10,000 bundles of whips and seedlings per 10,000 will be free from M. fallax. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,792 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from M. fallax. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,792 and 10,000 plants in pots up to 7 years old per 10,000 will be free from M. fallax.
For Meloidogyne mali, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,901 and 10,000 bundles of whips and seedlings per 10,000 will be free from M. mali. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,792 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from M. mali. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,792 and 10,000 plants in pots up to 7 years old per 10,000 will be free from M. mali.
For Phytophthora ramorum, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free´. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,872 and 10,000 bundles of whips and seedlings per 10,000 will be free from P. ramorum. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases' with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,886 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from P. ramorum. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases'. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,808 and 10,000 plants in pots up to 7 years old per 10,000 will be free from P. ramorum.
For Scirtothrips dorsalis, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with few exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,987 and 10,000 bundles of whips and seedlings per 10,000 will be free from S. dorsalis. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,986 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from S. dorsalis. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘pest free with few exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,981 and 10,000 plants in pots up to 7 years old per 10,000 will be free from S. dorsalis.
For Takahashia japonica, the likelihood of pest freedom for bundles of whips and seedlings following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,903 and 10,000 bundles of whips and seedlings per 10,000 will be free from T. japonica. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,916 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from T. japonica. The likelihood of pest freedom for plants in pots up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,899 and 10,000 plants in pots up to 7 years old per 10,000 will be free from T. japonica.
Abbreviations
- APHA
Animal and Plant Health Agency
- CABI
Centre for Agriculture and Bioscience International
- DEFRA
Department for Environment Food and Rural Affairs
- EKE
Expert Knowledge Elicitation
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- ISPM
International Standards for Phytosanitary Measures
- NPPO
National Plant Protection Organisation
- PHSI
Plant Health and Seeds Inspectorate
- PLH
Plant Health
- PRA
Pest Risk Assessment
- RNQPs
Regulated Non‐Quarantine Pests
- SASA
Science and Advice for Scottish Agriculture
Glossary
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017).
- Measures
Control (of a pest) is defined in ISPM 5 (FAO, 2017) as ‘Suppression, containment or eradication of a pest population’ (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk mitigation measures that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017).
- Protected zone
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017).
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017).
- Risk mitigation measure
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A risk mitigation measure may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017).
Appendix A – Data sheets of pests selected for further evaluation
A.1. Bemisia tabaci (European populations)
A.1.1. Organism information
| Taxonomic information |
Current valid scientific name: Bemisia tabaci Synonyms: Aleurodes inconspicua, Aleurodes tabaci, Bemisia achyranthes, Bemisia bahiana, Bemisia costa‐limai, Bemisia emiliae, Bemisia goldingi, Bemisia gossypiperda, Bemisia gossypiperda mosaicivectura, Bemisia hibisci, Bemisia inconspicua, Bemisia longispina, Bemisia lonicerae, Bemisia manihotis, Bemisia minima, Bemisia minuscula, Bemisia nigeriensis, Bemisia rhodesiaensis, Bemisia signata, Bemisia vayssieri Name used in the EU legislation: Bemisia tabaci Genn. (European populations) Order: Hemiptera Family: Aleyrodidae Common name: cassava whitefly, cotton whitefly, silver‐leaf whitefly, sweet‐potato whitefly, tobacco whitefly Name used in the Dossier: – |
|
| Group | Insects | |
| EPPO code | BEMITA | |
| Regulated status |
The pest is listed in Annex III as protected zone quarantine pest Bemisia tabaci Genn. (European populations) for Ireland and Sweden. Bemisia tabaci is included in the EPPO A2 list (EPPO, online_a). The species is a quarantine pest in Belarus, Moldova, Norway and New Zealand. It is on A1 list of Azerbaijan, Chile, Georgia, Kazakhstan, Ukraine and the UK. It is on A2 list of Bahrain, East Africa, Southern Africa, Russia, Türkiye and EAEU (= Eurasian Economic Union – Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) (EPPO, online_b). |
|
| Pest status in the UK |
Bemisia tabaci (European populations) is present in the UK, with few occurrences (CABI, online; EPPO, online_c) and it is continuously intercepted to the UK. The intercepted populations were identified as B biotype Middle East‐Asia Minor 1 (=MEAM1) and Q biotype Mediterranean (=MED) (Cuthbertson, 2013). From 1998 to 2015, there were between 7 and 35 outbreaks per year of B. tabaci in the UK and all the findings were subject to eradication. The UK outbreaks of B. tabaci have been restricted to greenhouses and there are no records of the whitefly establishing outdoors during summer (Cuthbertson and Vänninen, 2015; Bradshaw et al., 2019). According to the Dossier Section 5.0, B. tabaci is present: not widely distributed and under official control, restricted to four outbreak sites in 2022–2023 in contained environments (glasshouses). Many interceptions and outbreaks (356 in total in 2021), but all outbreaks subject to eradication measures. Not known outdoors (i.e. not under protection) and not thought to be able to establish outdoors. |
|
| Pest status in the EU |
Bemisia tabaci (European populations) is widespread in the EU – Austria, Belgium, Bulgaria, Croatia, the Republic of Cyprus, Czechia, Finland, France, Germany, Greece, Hungary, Italy, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovenia and Spain (CABI, online; EPPO, online_c). It is absent from Denmark, Estonia, Ireland, Latvia, Lithuania, Slovakia and Sweden (CABI, online; EPPO, online_c). In the EU, B. tabaci is mainly present in the greenhouses, with exception of Mediterranean coastal region (Cyprus, Greece, Malta, Italy, south of France, certain parts of Spain and Portugal), where the whitefly occurs also outdoors (EFSA PLH Panel, 2013). |
|
| Host status on Acer |
Acer palmatum is reported host of Bemisia tabaci in China (Li et al., 2011; CABI, online). Other reported hosts are A. buergerianum (Li et al., 2011) and A. macrophyllum (Yassin and Bendixen, 1982). There is no information on whether B. tabaci can also attack Acer pseudoplatanus. |
|
| PRA information | Available Pest Risk Assessments:
|
|
| Other relevant information for the assessment | ||
| Biology |
Bemisia tabaci is a cosmopolitan whitefly present on all continents except for Antarctica (CABI, online; EPPO, online_c). In the literature, it is reported as either native to Africa, Asia, India, North America or South America (De Barro et al., 2011). However, based on mtCO1 (mitochondrial cytochrome oxidase 1) sequence, its origin is most likely to be sub‐Saharan Africa (De Barro, 2012). Bemisia tabaci is a complex of at least 40 cryptic species that are morphologically identical but distinguishable at molecular level (Khatun et al., 2018). The species differ from each other in host association, spread capacity, transmission of viruses and resistance to insecticides (De Barro et al., 2011). Bemisia tabaci develops through three life stages: egg, nymph (four instars) and adult (Walker et al., 2010). Nymphs of Bemisia tabaci mainly feed on phloem in minor veins of the underside leaf surface (Cohen et al., 1996). Adults feed on both phloem and xylem of leaves (Walker et al., 2010, citing others). Honeydew is produced by both nymphs and adults (Davidson et al., 1994). Bemisia tabaci is multivoltine with up to 15 generations per year (Ren et al., 2001). The life cycle from egg to adult requires from 2.5 weeks up to 2 months depending on the temperature (Norman et al., 1995) and the host plant (Coudriet et al., 1985). In the southern California desert on field‐grown lettuce (from 27 October 1983 to 4 January 1984), B. tabaci completed at least one generation (Coudriet et al., 1985). In Israel, the reproduction of B. tabaci was much reduced in winter months, but adults emerging in December survived and started ovipositing at the end of the cold season (Avidov, 1956). The most cold‐tolerant stage are eggs (at temperatures of −2°, −6°, −10°C) and the least tolerant are large nymphs. Short periods of exposure in 0° to −6°C have little effect on mortality. As the temperature lowers to −10°C, the duration of time required to cause significant mortality shortens dramatically (Simmons and Elsey, 1995). Females can lay more than 300 eggs (Gerling et al., 1986), which can be found mainly on the underside of the leaves (CABI, online). Females develop from fertilised and males from unfertilised eggs (Gerling et al., 1986). Eggs are yellowish white and with age turn golden brown. Their size is about 0.19–0.20 mm long and 0.10–0.12 mm wide. First‐instar nymph (=crawler) is scale‐like, elliptical, darker yellow in colour and about 0.26 mm long and 0.15 mm wide. Crawlers have legs and crawl actively on leaves before they settle down and moult through second‐ (0.38 mm long and 0.24 mm wide), third‐ (0.55 mm long and 0.35 mm wide) |
|
|
and fourth‐instar nymph (0.86 mm long and 0.63 mm wide) (Hill, 1969). Fourth‐instar nymph (=pupa) stops feeding and moults into an adult (Walker et al., 2010, citing others). Adult emerges through a ‘T'‐shaped rupture in the pupal case (El‐Helaly et al., 1971). Adults are pale yellow and have two pairs of white wings dusted with a white waxy powder (Hill, 1969). Female is approximately 1 mm long. Males are smaller about 0.8 mm long (EFSA PLH Panel, 2013). Out of all life stages, only first‐instar nymph (= crawler) and adults are mobile. Movement of crawlers by walking is very limited, usually within the leaf where they hatched (Price and Taborsky, 1992) or to more suitable neighbouring leaves. The average distance was estimated within 10–70 mm (Summers et al., 1996). For these reasons, they are not considered to be good colonisers. On a contrary, adults can fly reaching quite long distances in a search of a permanent host. According to a study done by Cohen et al. (1988), some of the marked individuals were trapped 7 km away from the initial place after 6 days. Long‐distance passive dispersal by wind is also possible (Byrne, 1999). Bemisia tabaci is an important agricultural pest that is able to transmit more than 121 viruses (belonging to genera Begomovirus, Crinivirus, Ipomovirus, Carlavirus and Torradovirus) and cause significant damage to food crops such as tomatoes, cucurbits, beans and ornamental plants (EFSA PLH Panel, 2013). However, these viruses are not reported to infect Acer species. Possible pathways of entry for B. tabaci are plants for planting including cuttings and rooted ornamental plants; cut flowers and branches with foliage; fruits and vegetables; human‐assisted spread; natural spread such as wind (EFSA PLH Panel, 2013). |
||
| Symptoms | Main type of symptoms | Main symptoms of B. tabaci on plants are chlorotic spotting, decrease of plant growth, deformation of fruits, deformation of leaves, intervein yellowing, leaf yellowing, leaf curling, leaf crumpling, leaf vein thickening, leaf enations, leaf cupping, leaf loss, necrotic lesions on stems, plant stunting, reduced flowering, reduced fruit development, silvering of leaves, stem twisting, vein yellowing, wilting, yellow blotching of leaves, yellow mosaic of leaves, presence of honeydew and sooty mould. These symptoms are plant responses to the feeding of the whitefly and to the presence of transmitted viruses (EPPO, 2004; EFSA PLH Panel, 2013; CABI, online). |
| Presence of asymptomatic plants | Symptoms of B. tabaci being present on the plants are usually visible. However, B. tabaci is a vector of several viruses and their infection could be asymptomatic. | |
| Confusion with other pests |
Bemisia tabaci can be easily confused with other whitefly species such as B. afer, Trialeurodes lauri, T. packardi, T. ricini, T. vaporariorum and T. variabilis. A microscopic slide is needed for morphological identification (EPPO, 2004). Different species of B. tabaci complex can be distinguished using molecular methods (De Barro et al., 2011). |
|
| Host plant range |
Bemisia tabaci has a wide host range, including more than 1,000 different plant species (Abd‐Rabou and Simmons, 2010). Some of the many hosts of Bemisia tabaci are Abelmoschus esculentus, Amaranthus blitoides, Amaranthus retroflexus, Arachis hypogaea, Atriplex semibaccata, Bellis perennis, Borago officinalis, Brassica oleracea var. botrytis, B. oleracea var. gemmifera, B. oleracea var. italica, Bryonia dioica, Cajanus cajan, Capsella bursa‐pastoris, Capsicum annuum, Citrus spp., Crataegus spp., Cucumis sativus, Cucurbita pepo, Erigeron canadensis, Euphorbia pulcherrima, Gerbera jamesonii, Glycine max, Gossypium spp., G. hirsutum, Hedera helix, Ipomoea batatas, Lactuca sativa, L. serriola, Lavandula coronopifolia, Ligustrum lucidum, L. quihoui, L. vicaryiis, Manihot esculenta, Melissa officinalis, Nicotiana tabacum, Ocimum basilicum, Origanum majorana, Oxalis pes‐caprae, Phaseolus spp., Phaseolus vulgaris, Piper nigrum, Potentilla spp., Prunus spp., Rosa spp., |
|
|
Rubus fruticosus, Salvia officinalis, S. rosmarinus, Senecio vulgaris, Sinningia speciosa, Solanum lycopersicum, S. melongena, S. nigrum, S. tuberosum, Sonchus oleraceus, Stellaria media, Tagetes erecta, Taraxacum officinale, Thymus serpyllum, Urtica urens, Vitis vinifera and many more (Li et al., 2011; EFSA PLH Panel, 2013; CABI, online; EPPO, online_d). Acer palmatum and A. buergerianumare are reported hosts in China (Li et al., 2011; CABI, online). For a full host list, refer to Li et al. (2011), EFSA PLH Panel (2013); CABI (online) and EPPO (online_d). |
||
| Reported evidence of impact | Bemisia tabaci (European populations) is EU protected zone quarantine pest. | |
| Evidence that the commodity is a pathway | Bemisia tabaci is continuously intercepted in the EU on different commodities including plants for planting (EUROPHYT/TRACES‐NT, online). Therefore, the commodity is a pathway for B. tabaci. Plants can carry leaves at the time of export which can host all life stages of the pest. | |
| Surveillance information |
Bemisia tabaci is regulated quarantine pest in the UK. As such, the policy for any outbreak is to eradicate the population. The UK makes many interceptions of B. tabaci and experiences a few outbreaks each year (356 interceptions and outbreaks in 2021), but all outbreaks are under protection and subject to eradication measures. This pest has never established outdoors in the UK (Dossier Section 3.0). As part of an annual survey at ornamental retail and production sites (frequency of visits determined by a decision matrix), B. tabaci is inspected for on common host plants. In addition, all tomato and pepper production sites subject to annual inspection (Dossier Sections 3.0 and 5.0). |
|
A.1.2. Possibility of pest presence in the nursery
A.1.2.1. Possibility of entry from the surrounding environment
Bemisia tabaci (European populations) is present in the UK with few occurrences (location not specified) (CABI, online; EPPO, online_c) and is continuously intercepted in the UK. The UK outbreaks of B. tabaci have been restricted to glasshouses and there are no records of B. tabaci establishing outdoors during summer (Cuthbertson and Vänninen, 2015; Bradshaw et al., 2019). Bradshaw et al. (2019) indicate that theoretically B. tabaci (in summertime) could complete one generation across most of Scotland, and one to three generations over England and Wales. However, the temperatures experienced during the cold days and nights during summer may be low enough to cause chilling injury to B. tabaci, thereby inhibiting development and preventing establishment in the UK. It is unlikely, therefore, that this pest will establish outdoors in the UK under current climate conditions.
The possible entry of B. tabaci from surrounding environment to the nurseries may occur through adult dispersal and passively on wind currents (Cohen et al., 1988; Byrne, 1999; EFSA PLH Panel, 2013).
Bemisia tabaci is a polyphagous species that can infest number of different plants. Suitable hosts of B. tabaci like Acer spp., Beta vulgaris, Camellia spp., Capsicum annuum, Crataegus spp., Daucus carota, Hedera spp., Ilex spp., Magnolia spp., Malus spp., Morus spp., Prunus spp., Rhododendron spp., Rosa spp., Salix spp., Solanum lycopersicum, Solanum tuberosum, Ulmus spp., Viburnum spp. and Wisteria spp. are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
Exact locations where the whitefly is present.
-
–
Possibility of spread beyond the infested greenhouses.
-
–
Possibility of the whitefly to survive the UK summer in outdoor conditions.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries from surrounding environment, even though it is only reported to be present in greenhouses. In the surrounding area, suitable hosts are present and the pest can spread by wind and adult flight.
A.1.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0). Seeds are not a pathway for the whitefly.
In addition to Acer plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the whitefly (such as Acacia spp., Ajuga spp., Allium spp., Arbutus spp., Artemisia spp., Aster spp., Aucuba spp., Berberis spp., Buxus spp., etc.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the whitefly could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0), which is not a pathway for the whitefly.
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries with new seedlings of Acer and new plants of other species used for plant production in the area. The entry of the pest with seeds and the growing media the Panel considers as not possible.
A.1.2.3. Possibility of spread within the nursery
Acer plants are either grown in containers (cells, pots, tubes, etc.) outdoors/in the open air or in field. Cell grown trees may be grown in greenhouses; however, most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The whitefly can attack other suitable plants (such as Acacia spp., Ajuga spp., Allium spp., etc.), non‐cultivated herbaceous plants (Bellis perennis, Potentilla spp., Taraxacum officinale) present within the nurseries and hedges surrounding the nurseries (Crataegus spp., Hedera spp., Ilex spp. and Prunus spp.) (Dossier Sections 3.0 and 6.0).
The whitefly within the nurseries can spread by adult flight and wind. Spread within the nurseries through equipment and clothing is less relevant as the distance walked is very limited and of a short duration.
Uncertainties:
-
–
Possibility of the whitefly to survive the UK summer in outdoor conditions.
-
–
Possibility that greenhouses are heated which allows the pest to overwinter.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nurseries is possible either by wind and active flight.
A.1.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Bemisia tabaci between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.1.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Bemisia tabaci (European populations) is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
As the plant passport is very similar to the EU one, the plants shall be free from quarantine pests and RNQPs.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. Physical separation is not a barrier for B. tabaci because the adults can fly. |
| 3 | Certified plant material | Yes | Only applicable to seedlings with leaves. |
| 4 | Growing media | No | Not relevant. |
| 5 | Surveillance, monitoring and sampling | Yes |
Although the plants are thoroughly checked during the production, later infestation by B. tabaci can go undetected.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
Weeding can have some effect on the reduction of Bemisia populations. The other measures are not relevant.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
Removal of infested plant material can have some effect on the reduction of Bemisia populations.
Uncertainties:
|
| 8 | Irrigation water | No | Not relevant. |
| 9 | Application of pest control products | Yes |
Chemical measures may have some effect on the pest.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
Although the plants are thoroughly checked 2 weeks before the export, infestation by B. tabaci can go undetected.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. Plants are not individually separated during transportation. The pest can infest other plants. |
A.1.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.1.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bundles of whips and seedlings
This scenario assumes a low pressure of the pest in the nurseries and the surrounding. In this scenario, it is assumed that leaves are not present when the plants are exported. Pesticide treatments are effective.
A.1.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bundles of whips and seedlings
This scenario assumes a high pressure of the pest in the nurseries and the surrounding. Some leaves may be present and eggs laid underneath the leaves, crawlers can move and hide and may be overlooked. Low‐density populations can also be overlooked. The pesticides used may not be effective against B. tabaci.
A.1.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bundles of whips and seedlings (Median)
The uncertainties which were identified are equal in both directions and can lead equally to over‐ or underestimate the number of infested bundles.
A.1.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a medium uncertainty in the first quartile, and a medium uncertainty above the median because of restricted distribution of the pest. It is very unlikely to be present outdoors and maple is not a major host. It is a quarantine pest in the UK and therefore more likely to be detected and that measures are taken.
A.1.5.5. Elicitation outcomes of the assessment of the pest freedom for Bemisia tabaci (European populations) on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infestation (Table A.1) and pest freedom (Table A.2).
Table A.1.
Elicited and fitted values of the uncertainty distribution of pest infestation by Bemisia tabaci (European populations) per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 5 | 10 | 20 | 40 | ||||||||||
| EKE | 0.141 | 0.374 | 0.785 | 1.66 | 2.92 | 4.61 | 6.45 | 10.7 | 16.1 | 19.4 | 23.5 | 28.0 | 32.8 | 36.5 | 40.0 |
The EKE results are the BetaGeneral (0.94432, 2.5871, 0, 48.5) distribution fitted with @Risk version 7.6.
Table A.2.
The uncertainty distribution of bundles free of Bemisia tabaci (European populations) per 10,000 bundles calculated by Table A.1
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,960 | 9,980 | 9,990 | 9,995 | 10,000 | ||||||||||
| EKE results | 9,960 | 9,964 | 9,967 | 9,972 | 9,976 | 9,981 | 9,984 | 9,989 | 9,994 | 9,995 | 9,997 | 9,998 | 9,999.2 | 9,999.6 | 9,999.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infested bundles, the pest freedom was calculated (i.e. = 10,000 – number of infested bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.2.
Figure A.1.
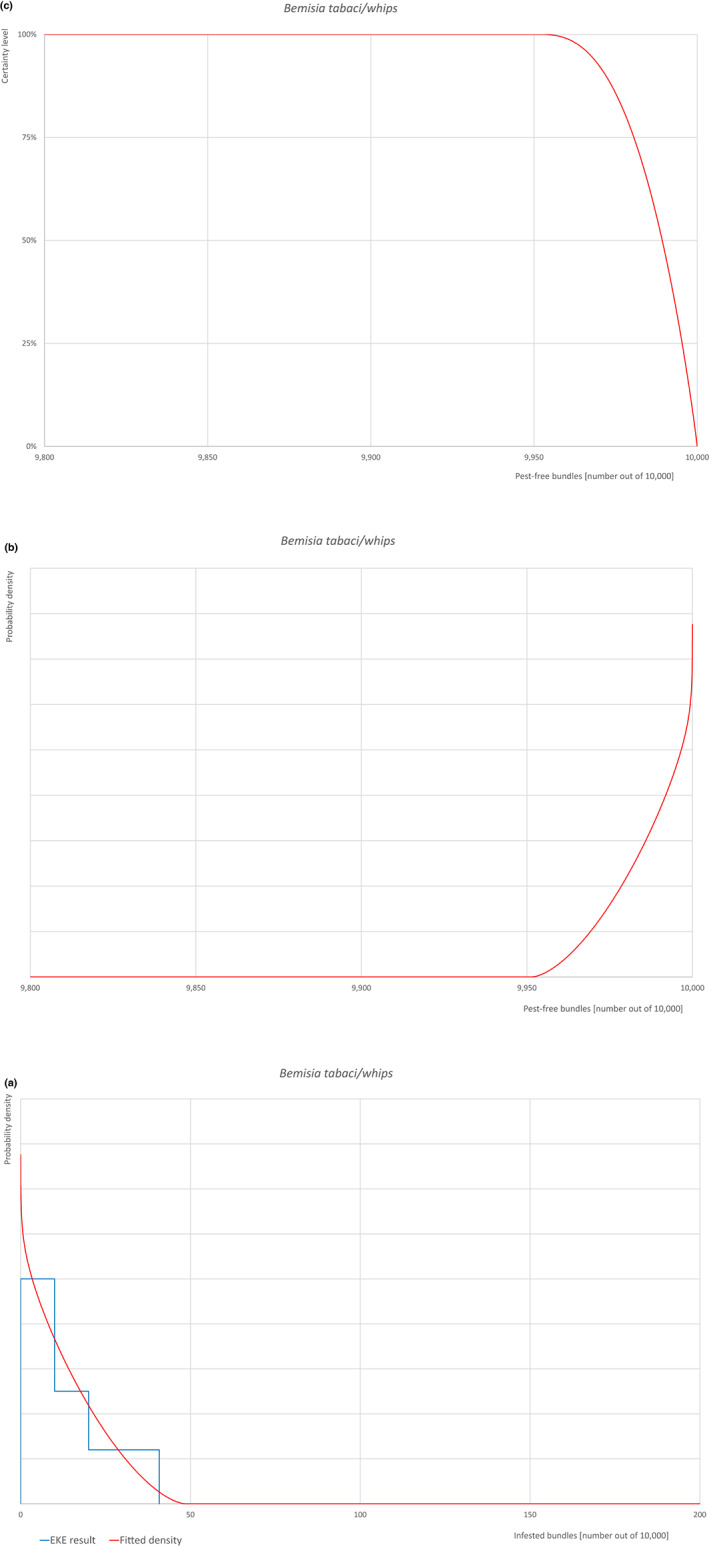
(a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
Figure A.2.
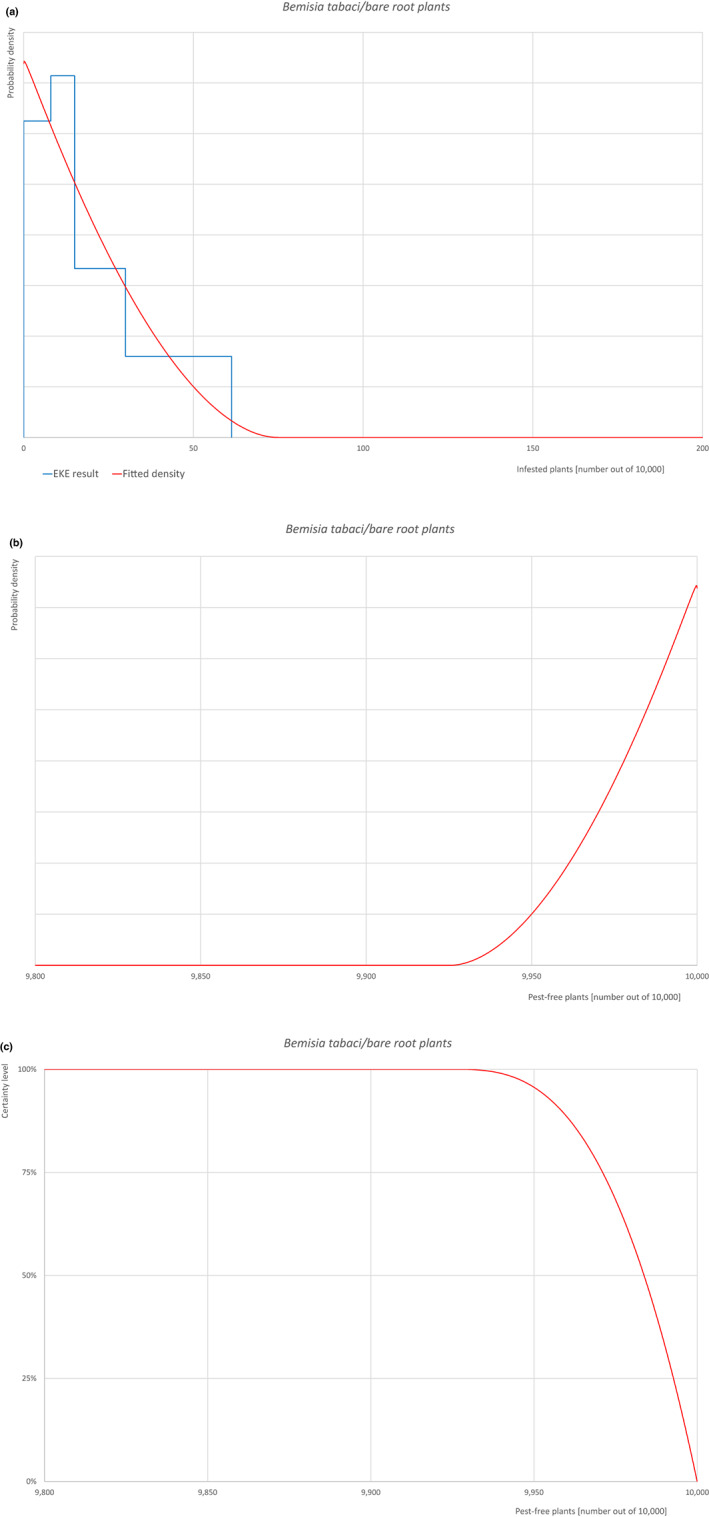
(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
Figure A.3.
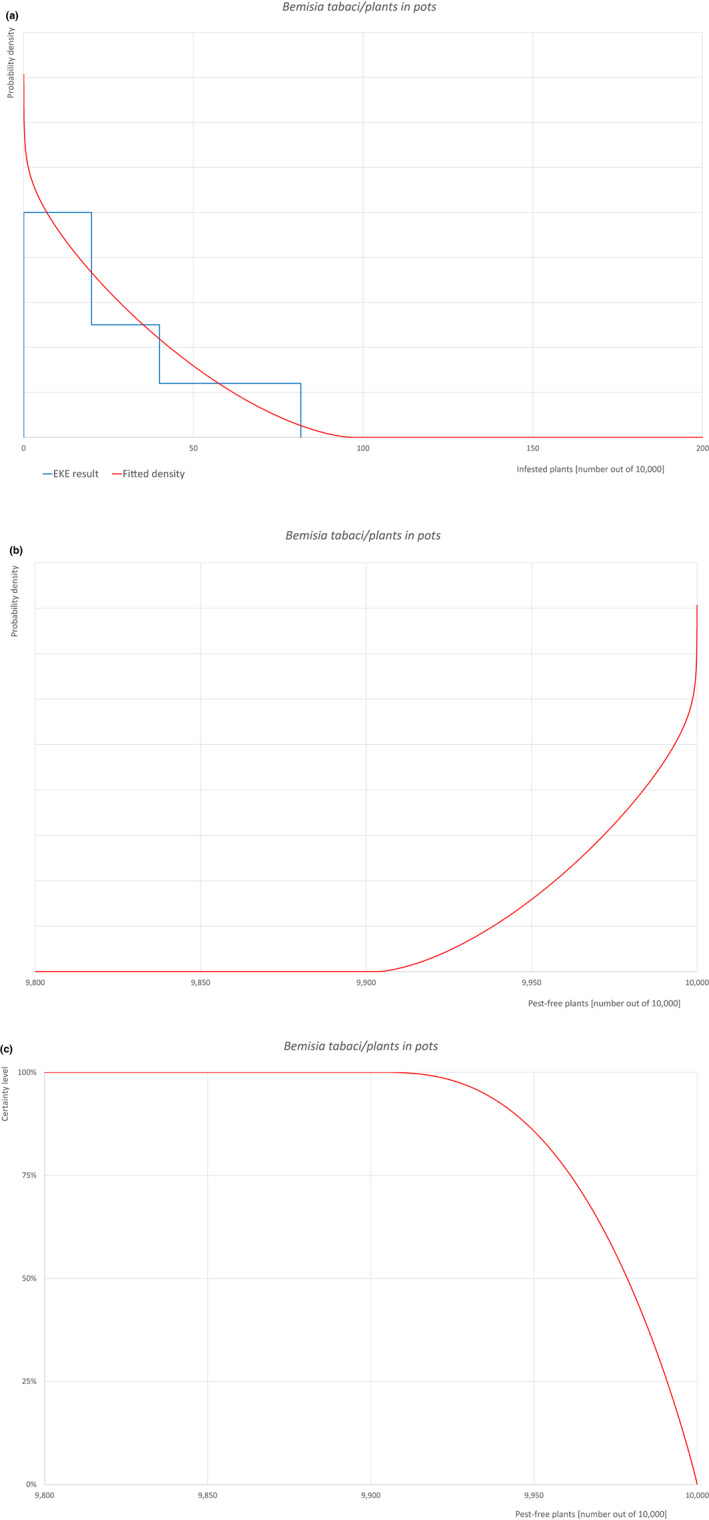
(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
Figure A.4.
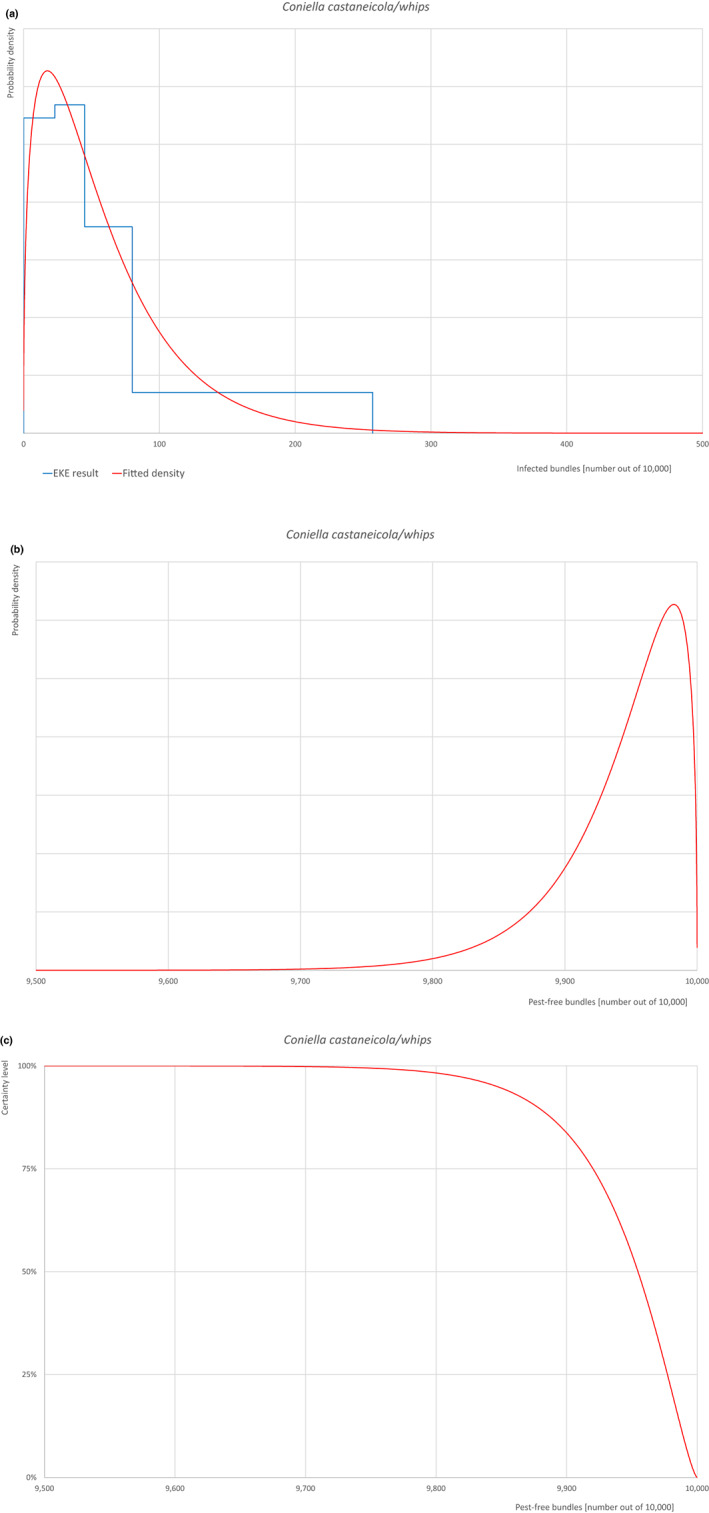
(a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
Figure A.5.
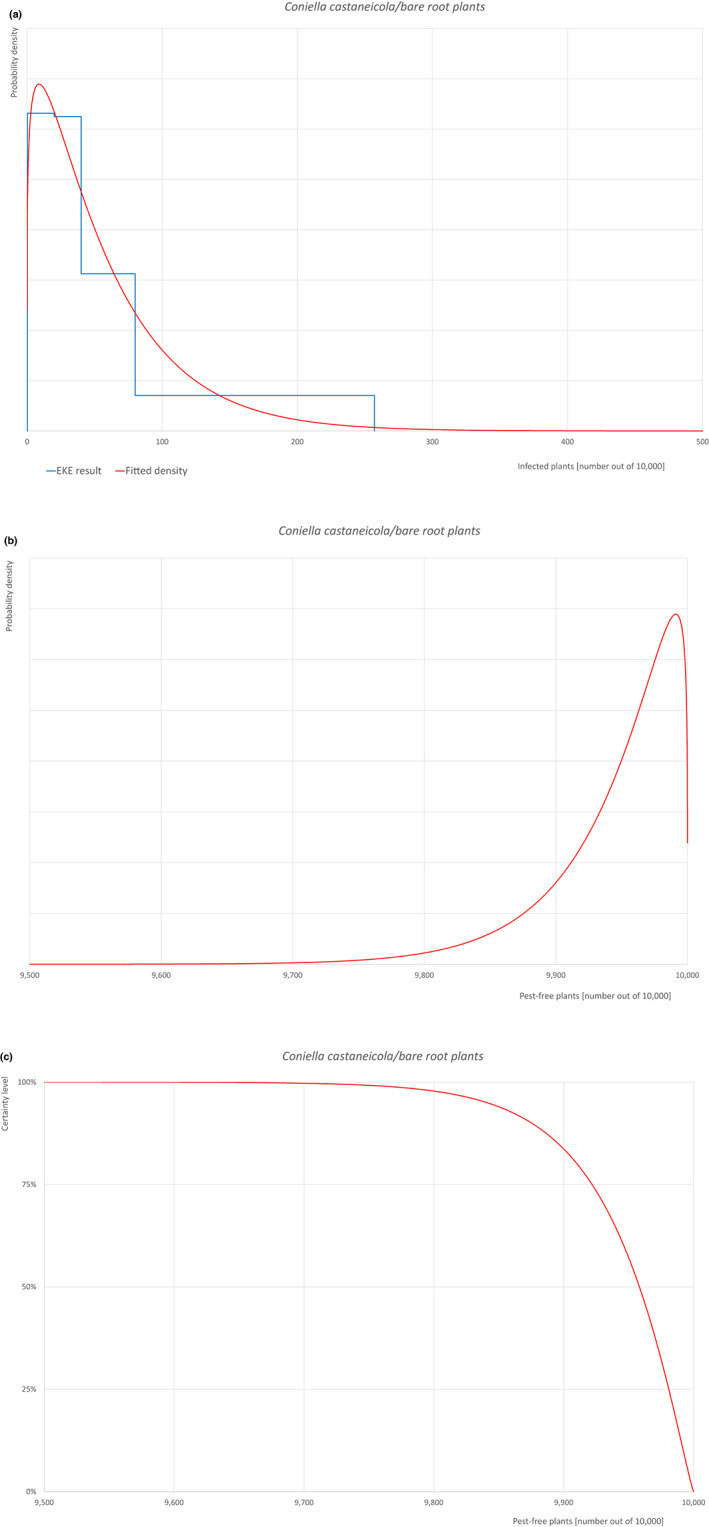
(a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
Figure A.6.
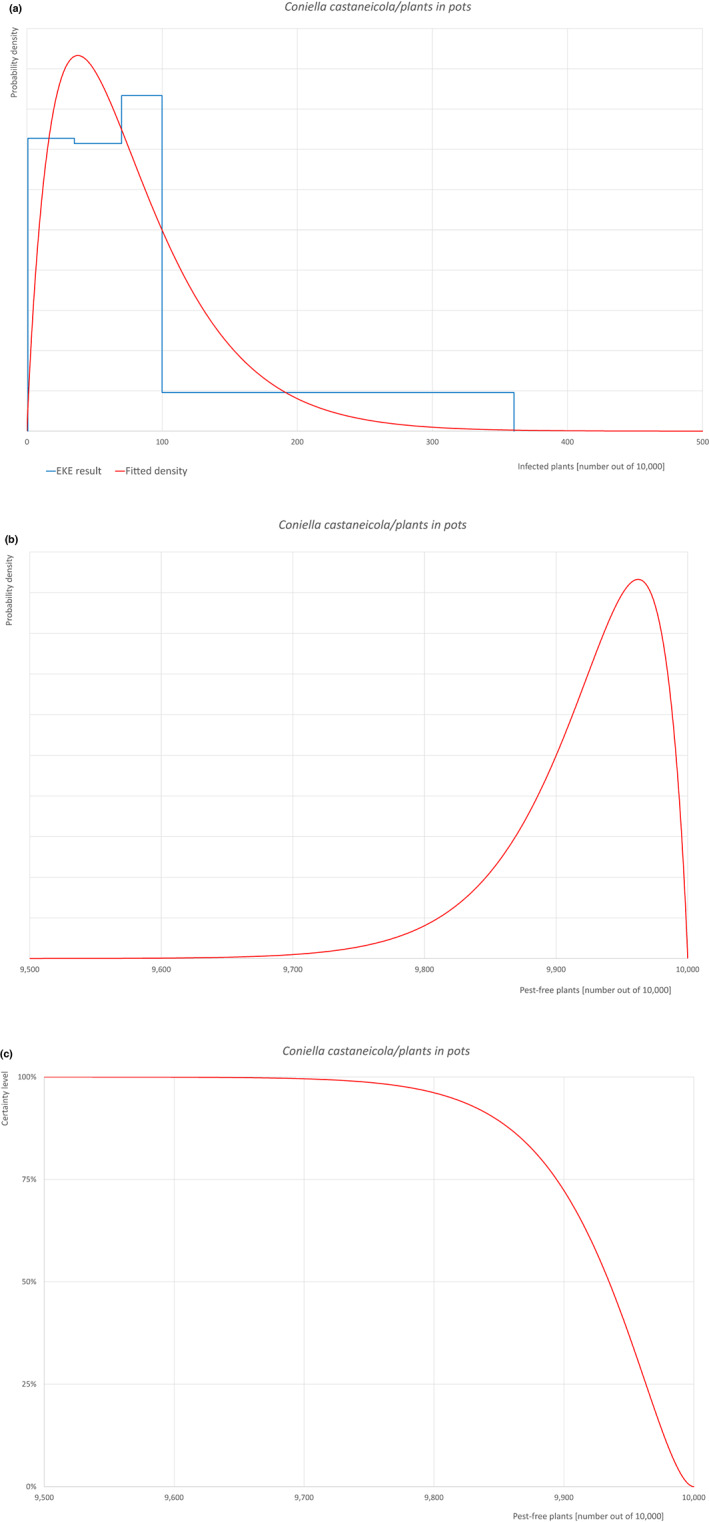
(a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
Figure A.7.
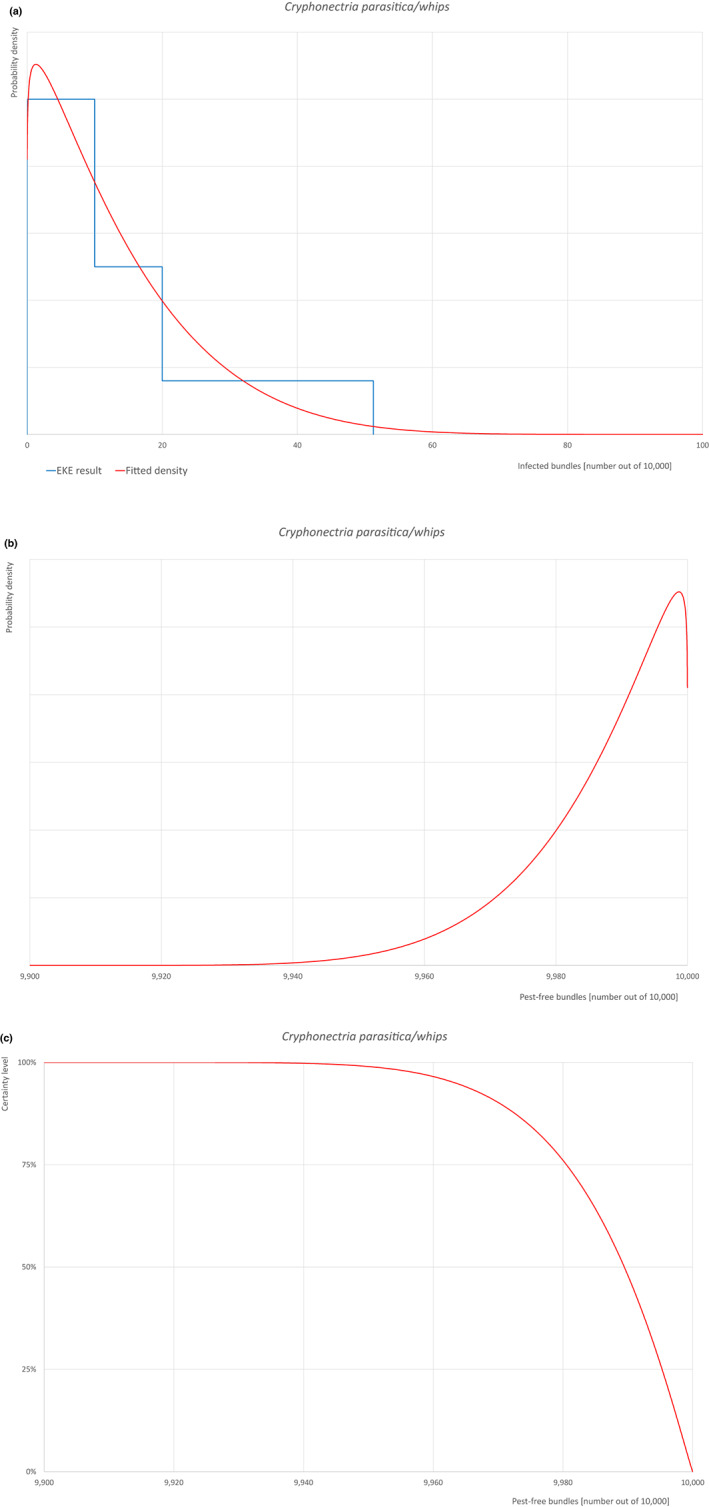
(a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
Figure A.8.
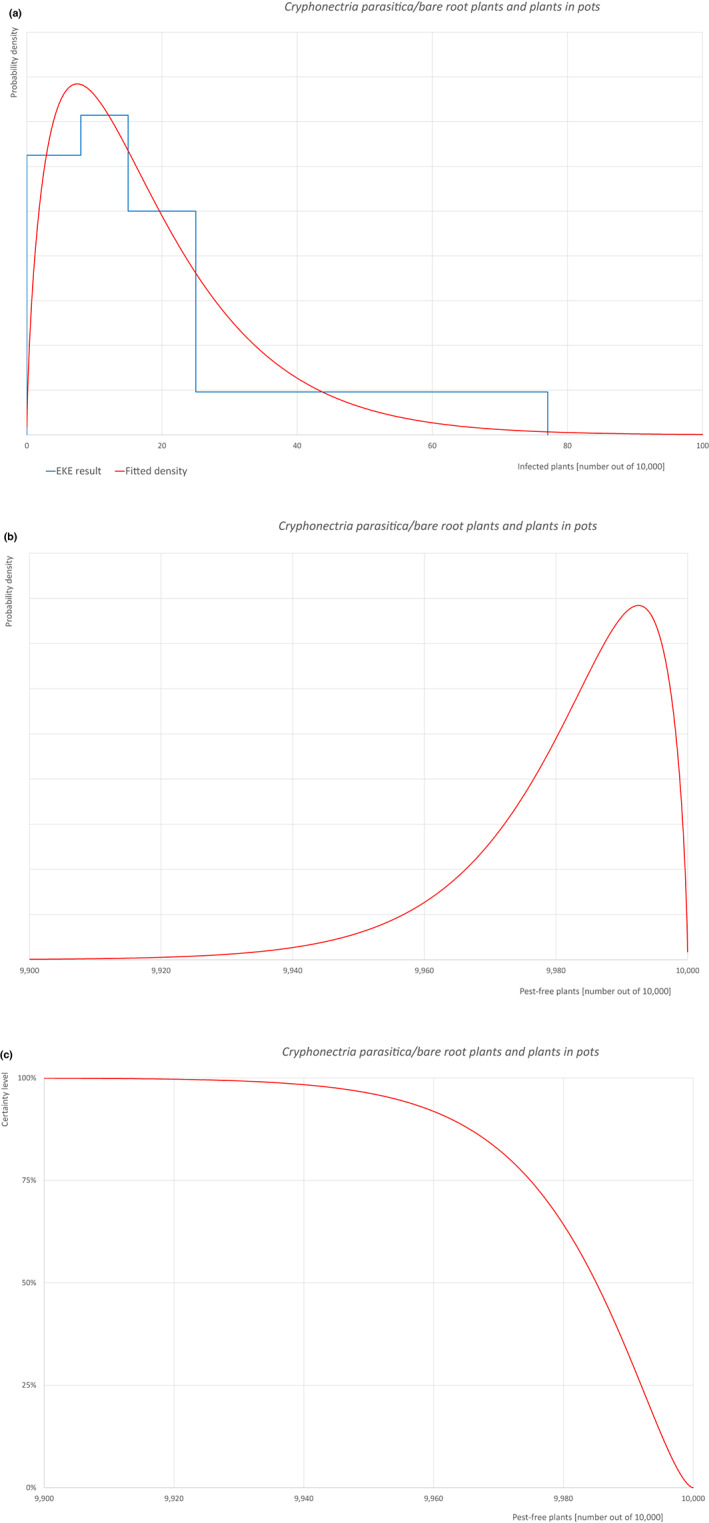
(a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
Figure A.9.
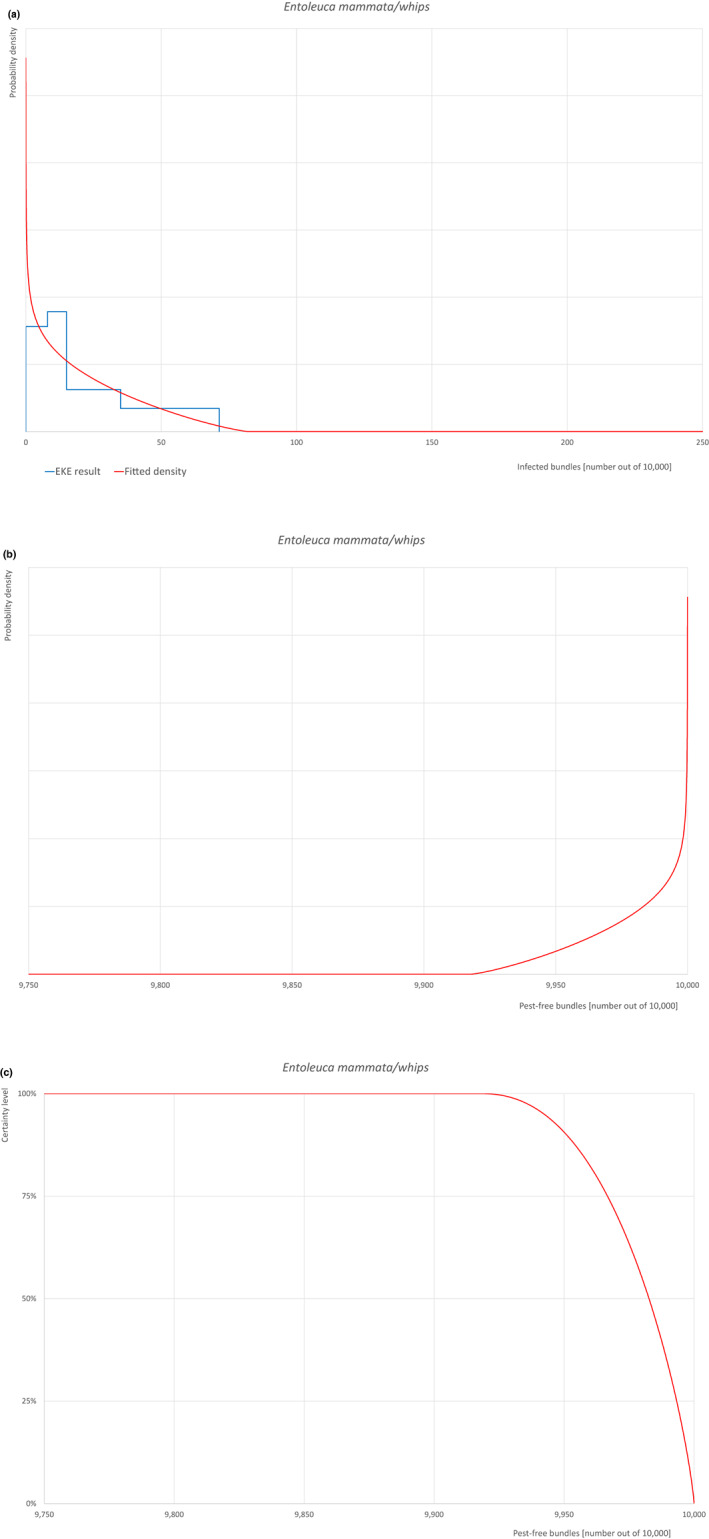
(a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
Figure A.10.
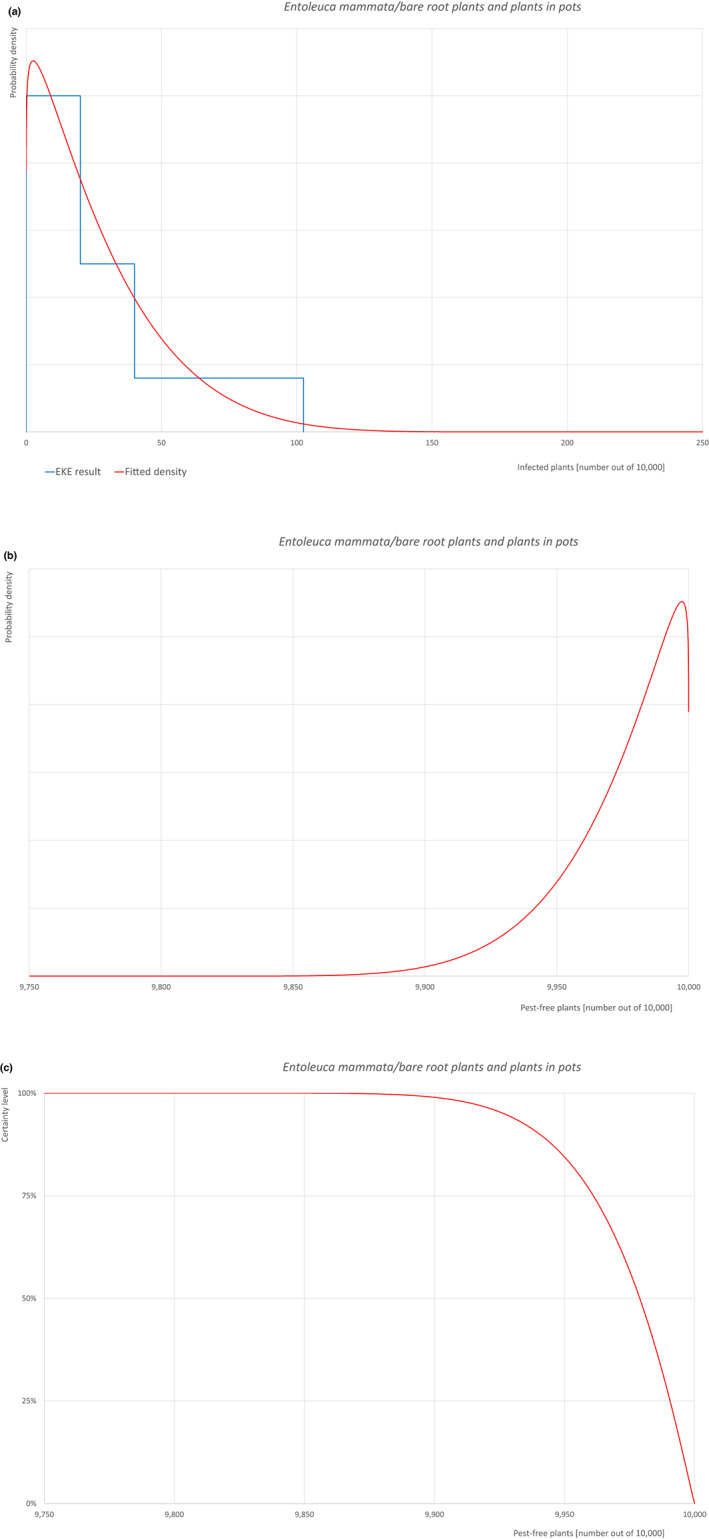
(a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
Figure A.11.
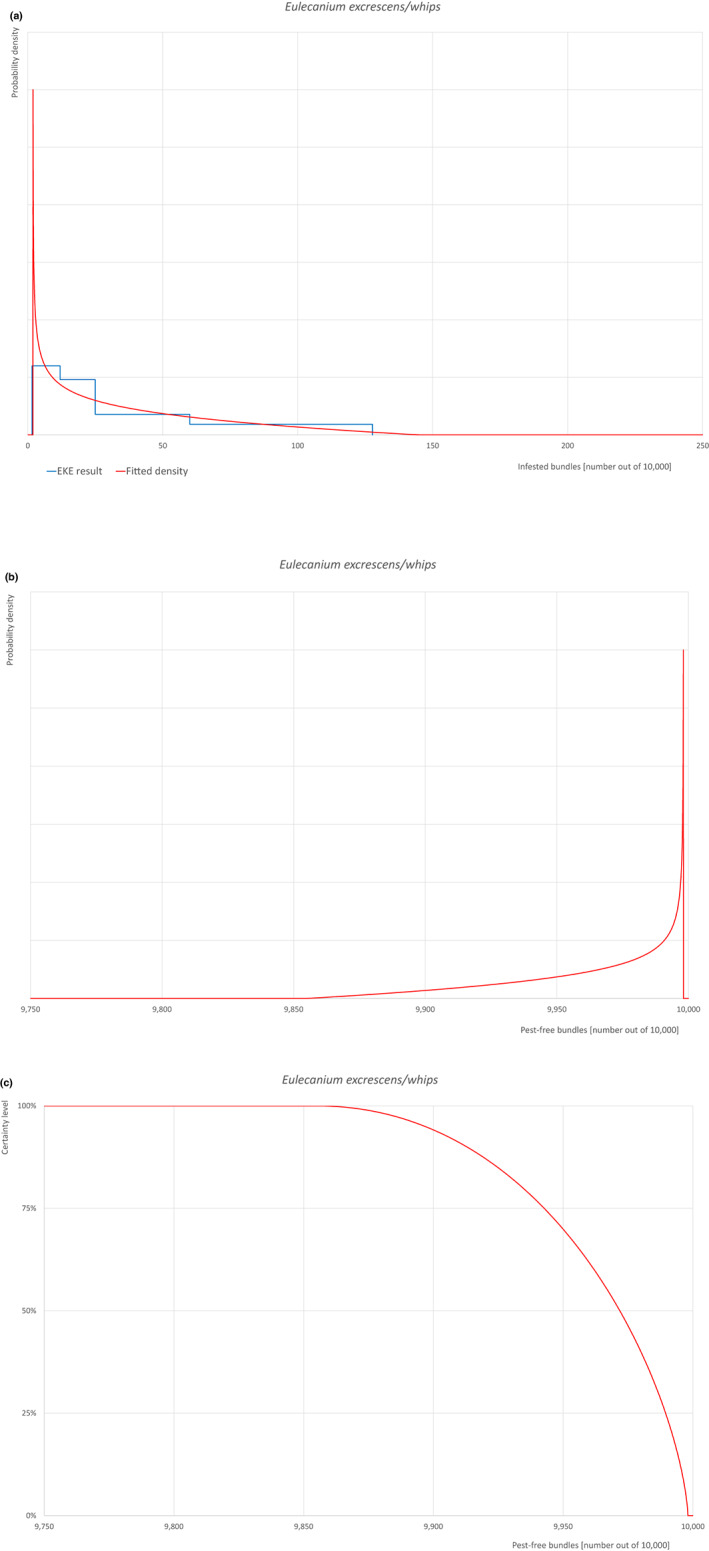
(a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
Figure A.12.
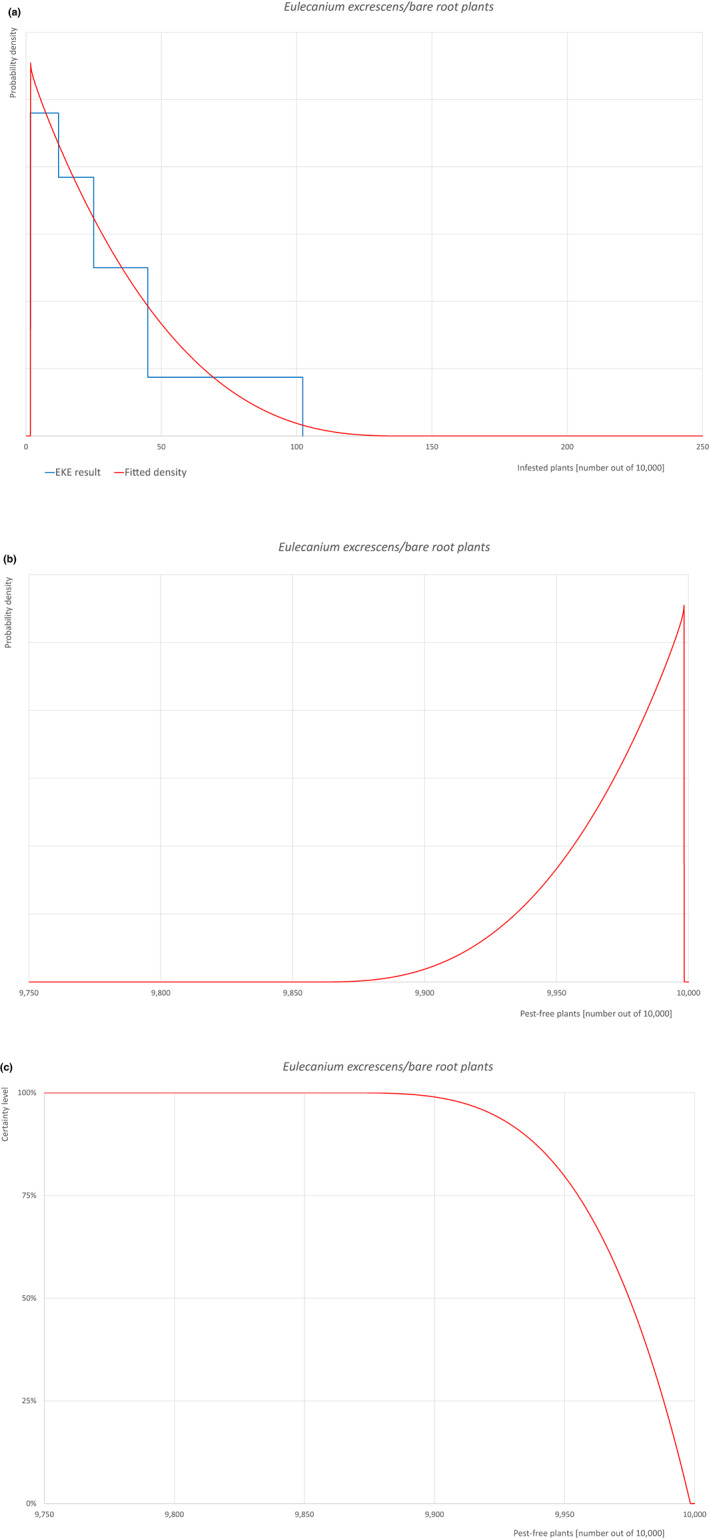
(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
Figure A.13.
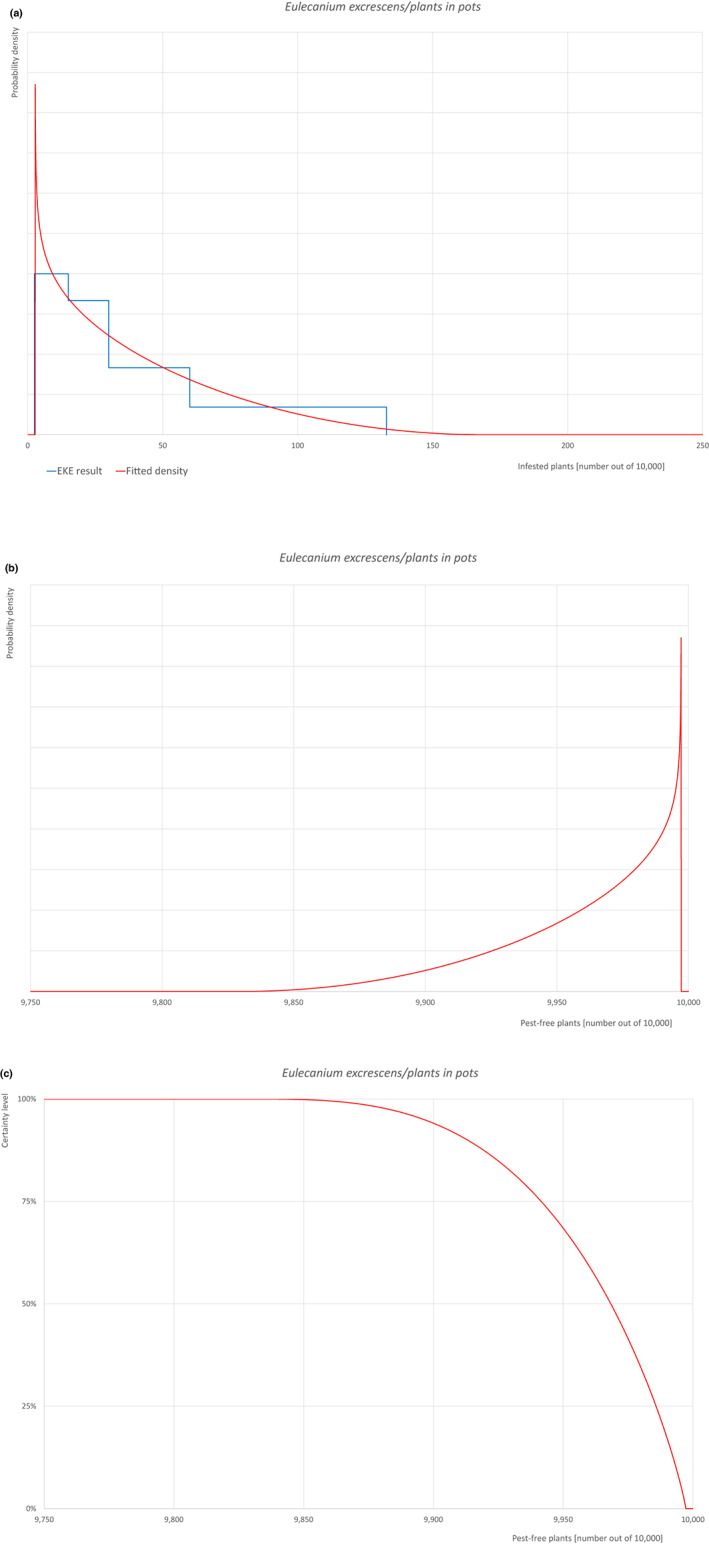
(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
Figure A.14.
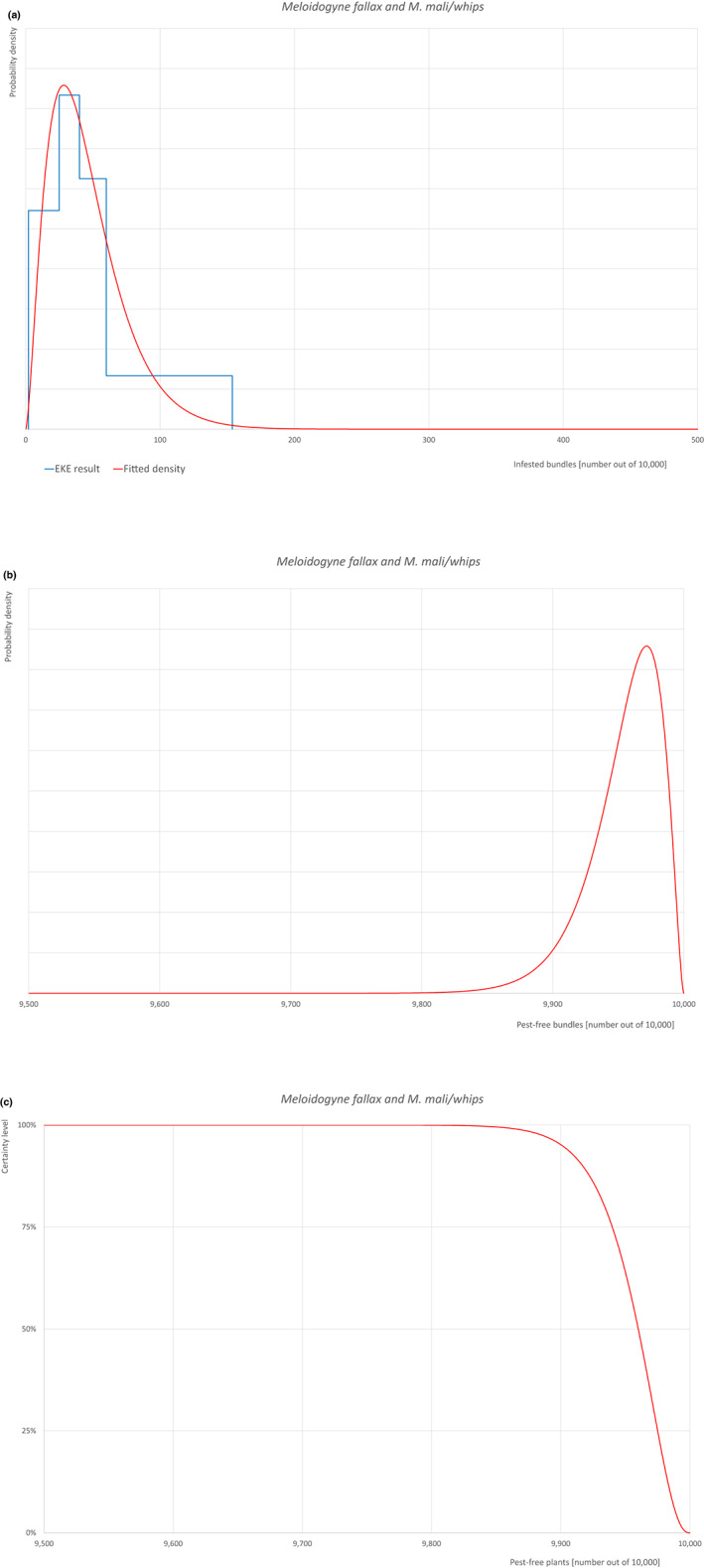
(a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
Figure A.15.
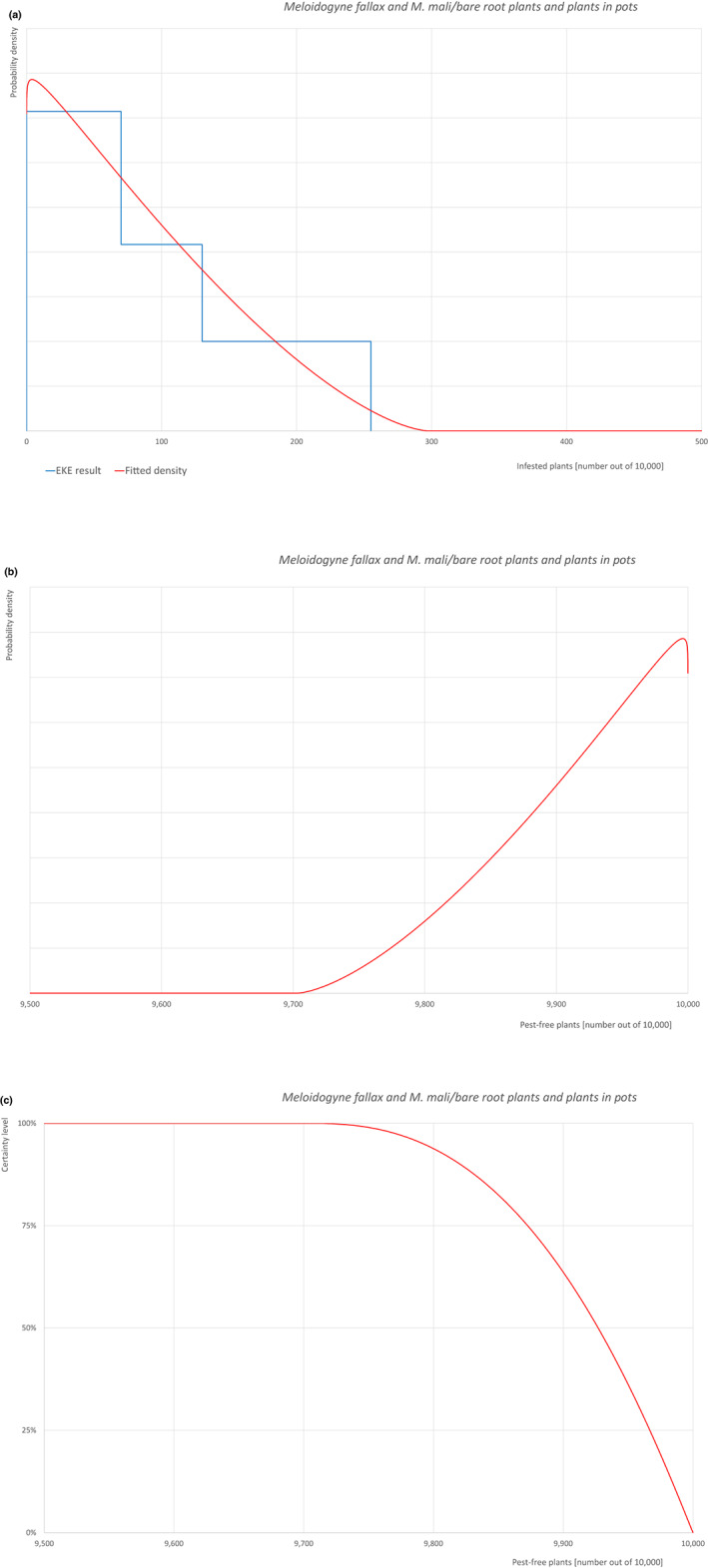
(a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
Figure A.16.
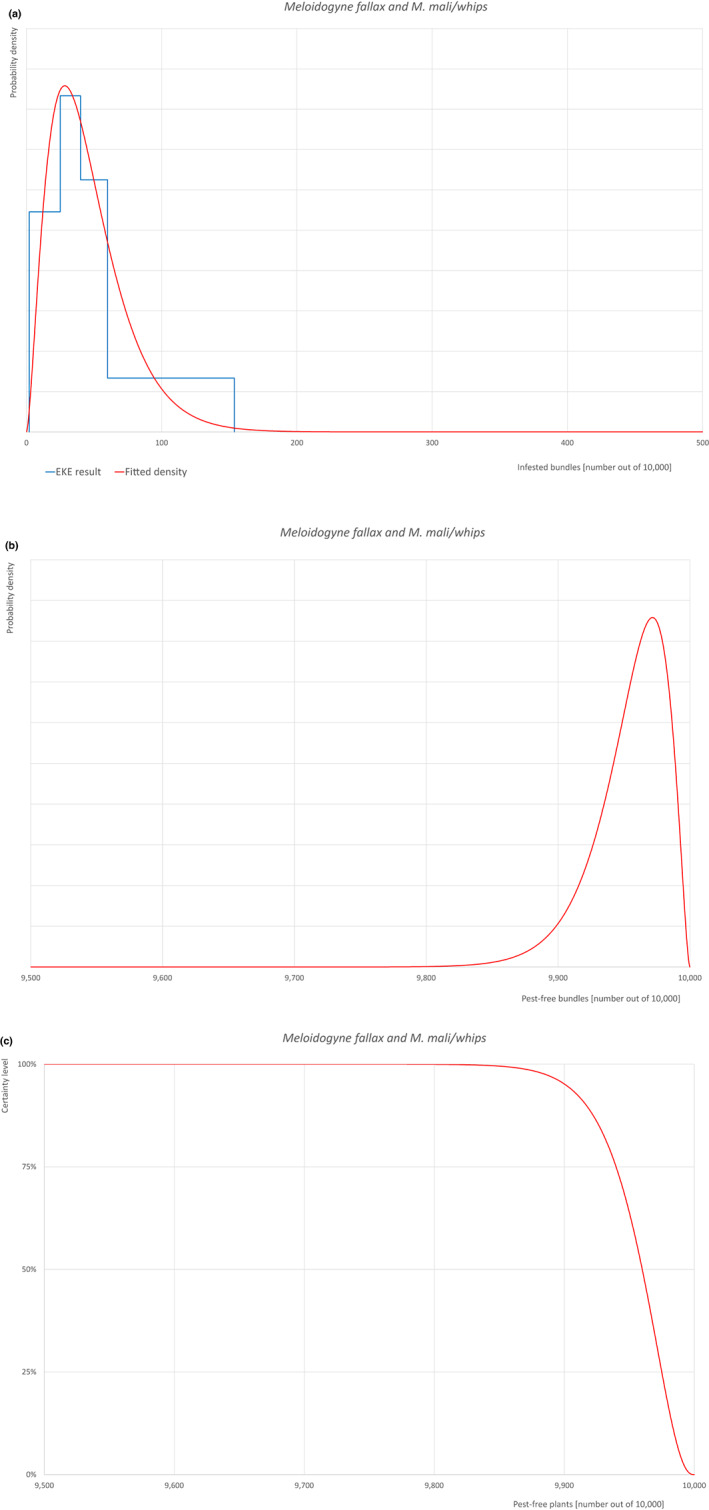
(a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
Figure A.17.
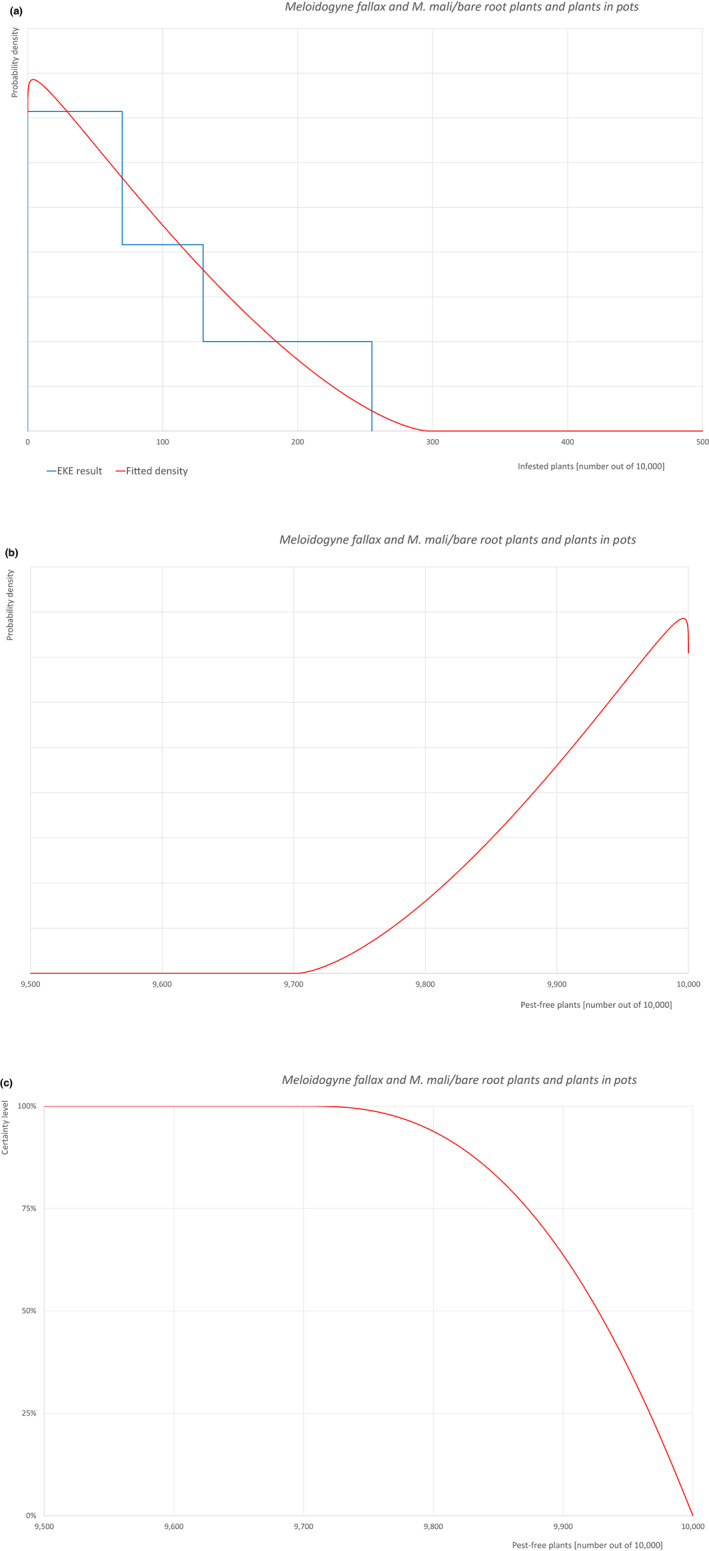
(a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
Figure A.18.
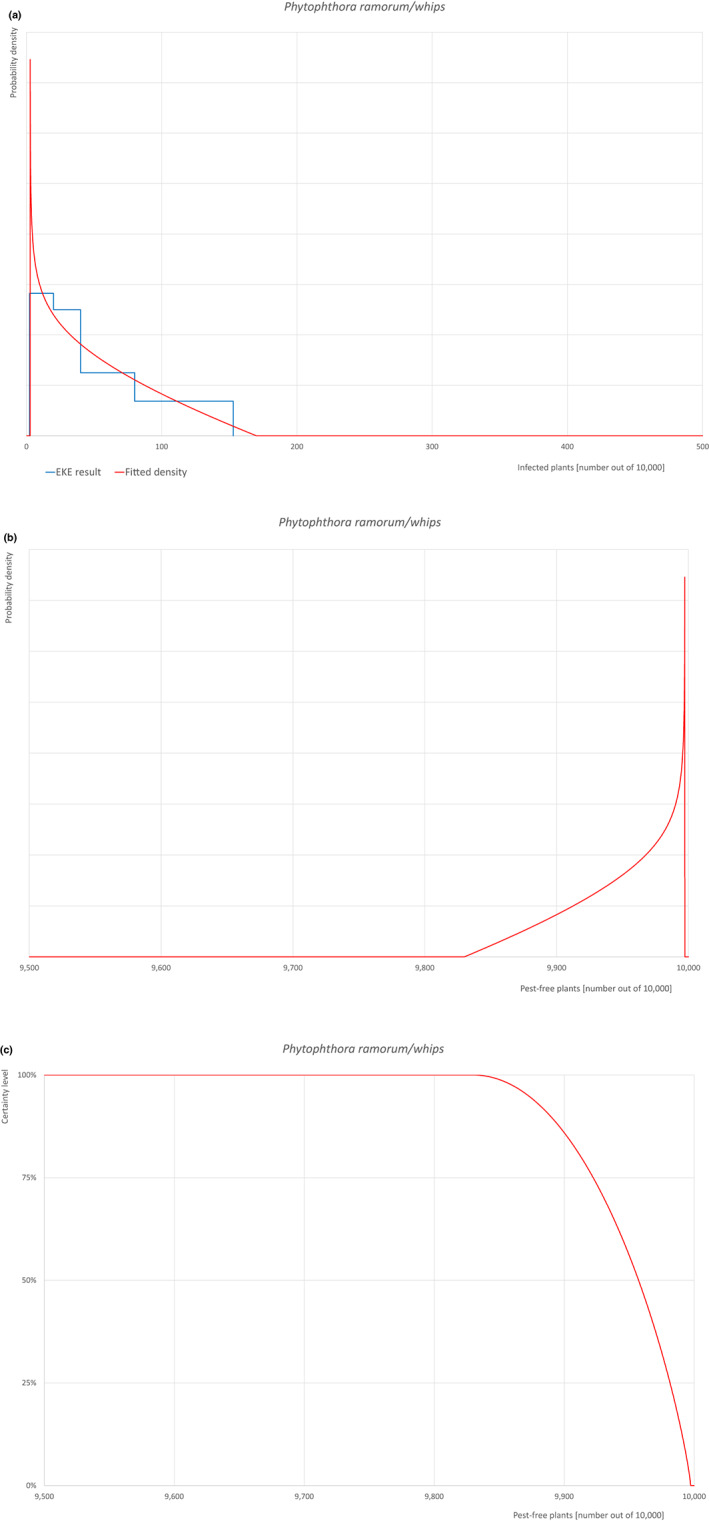
(a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
Figure A.19.
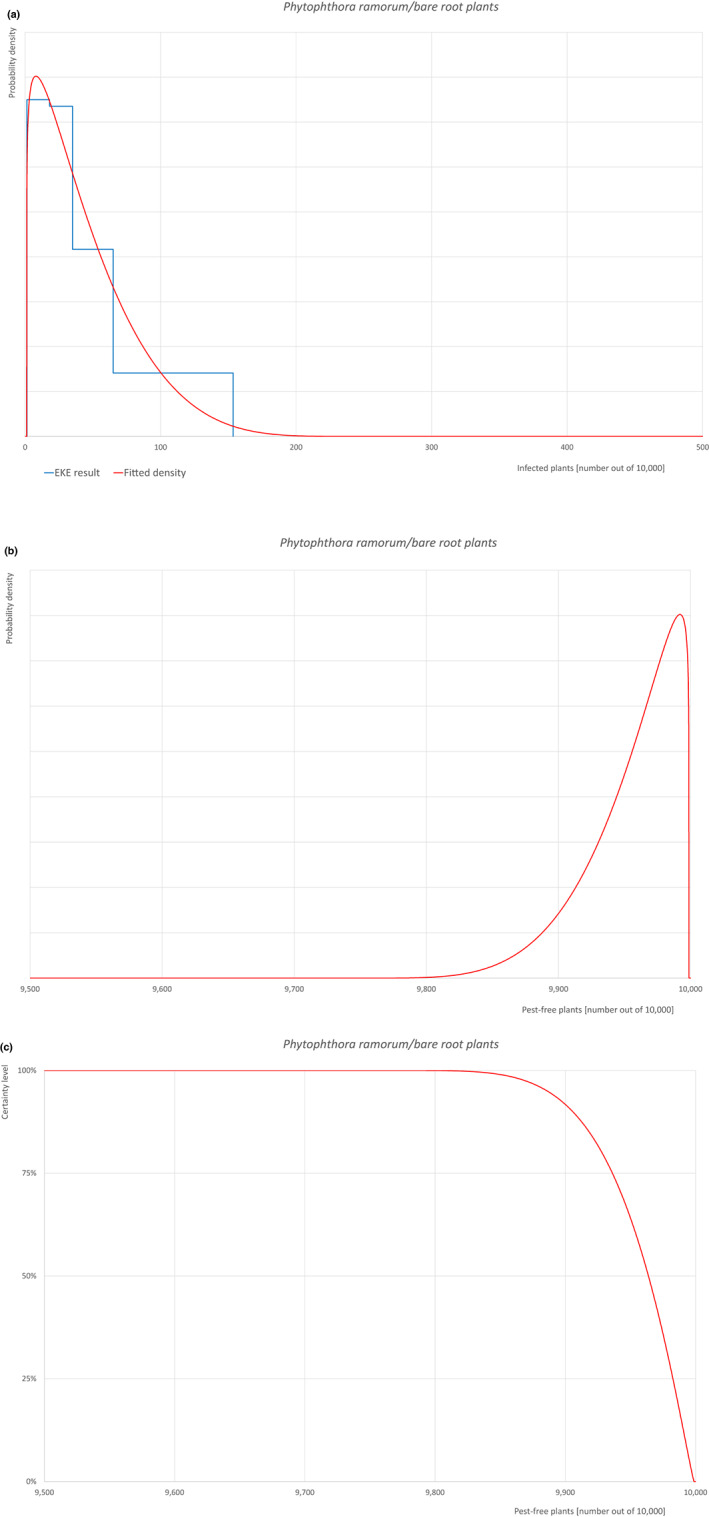
(a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
Figure A.20.
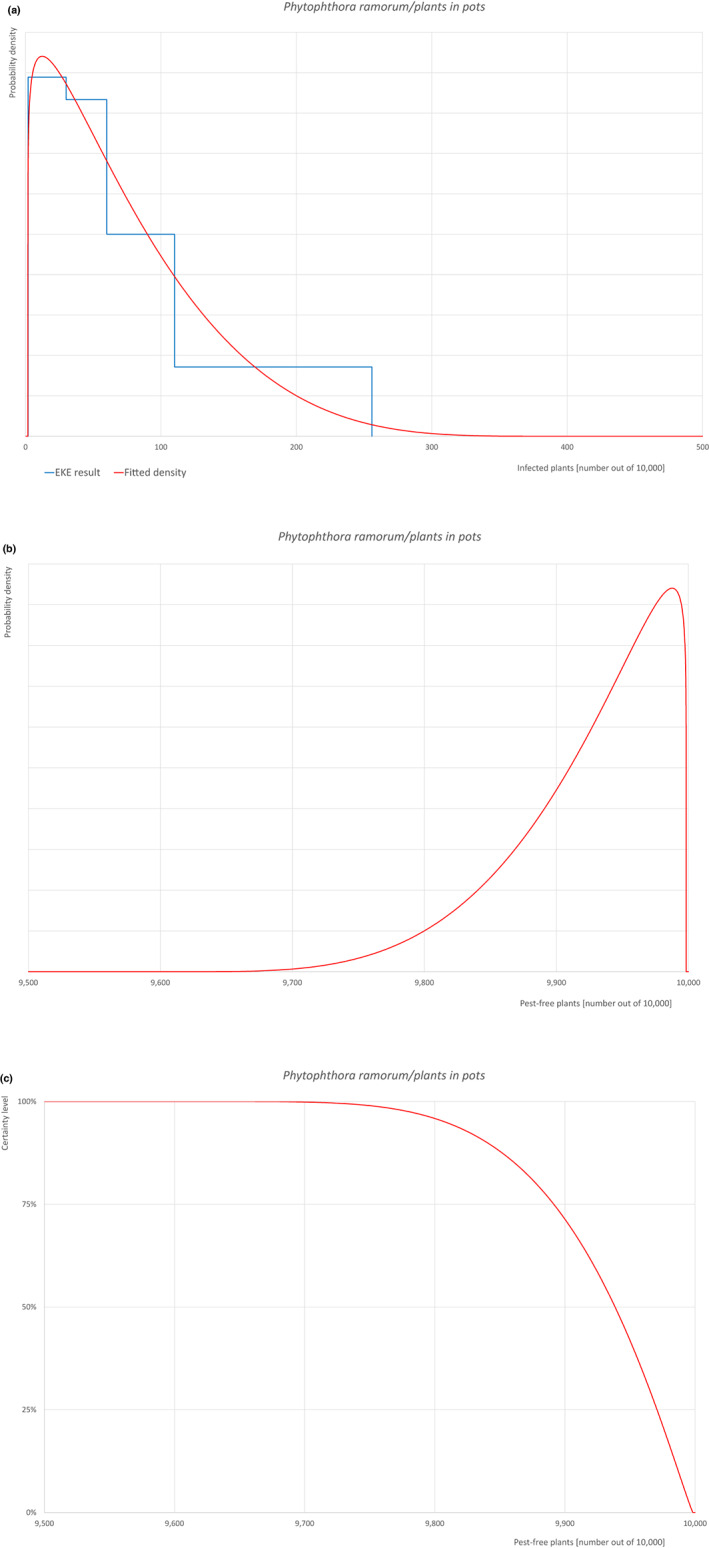
(a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
Figure A.21.
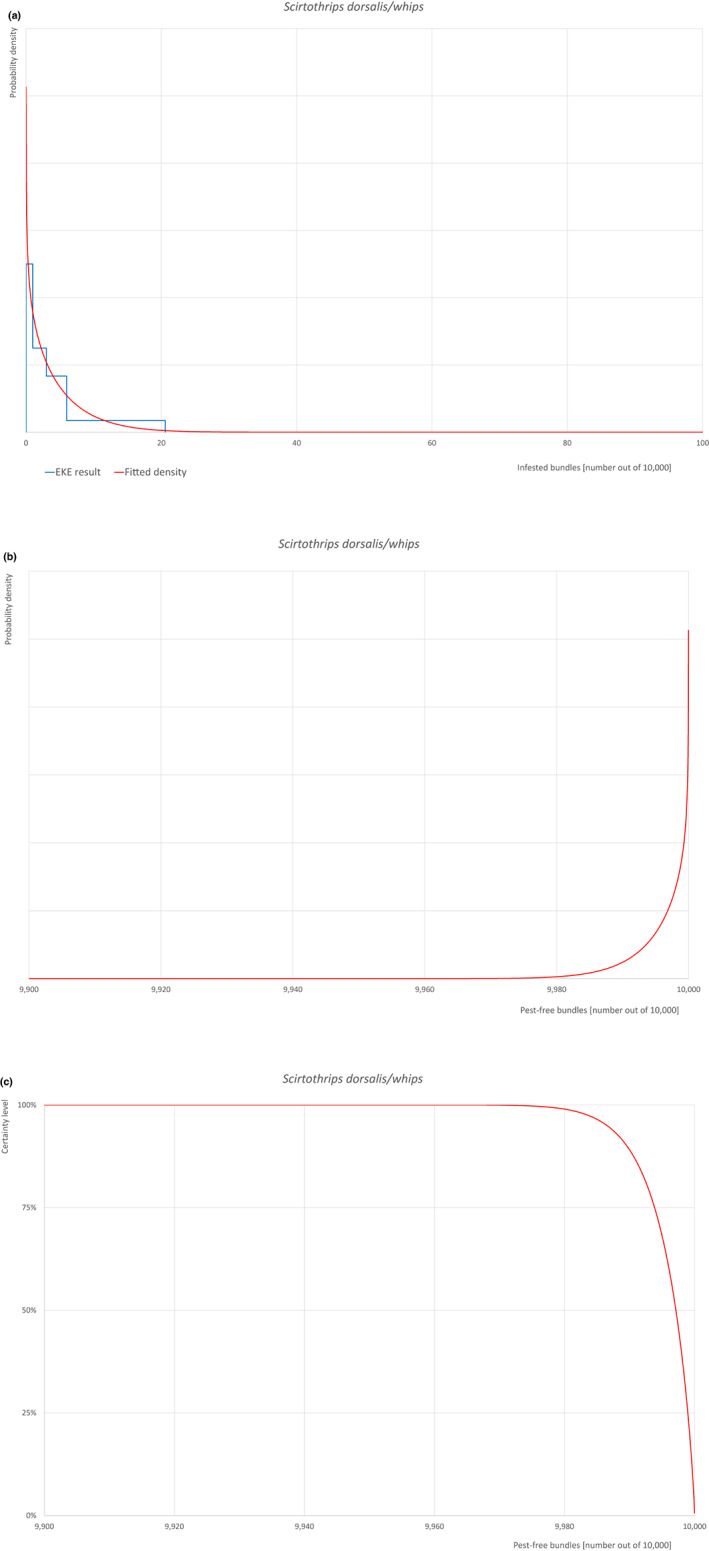
(a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
Figure A.22.
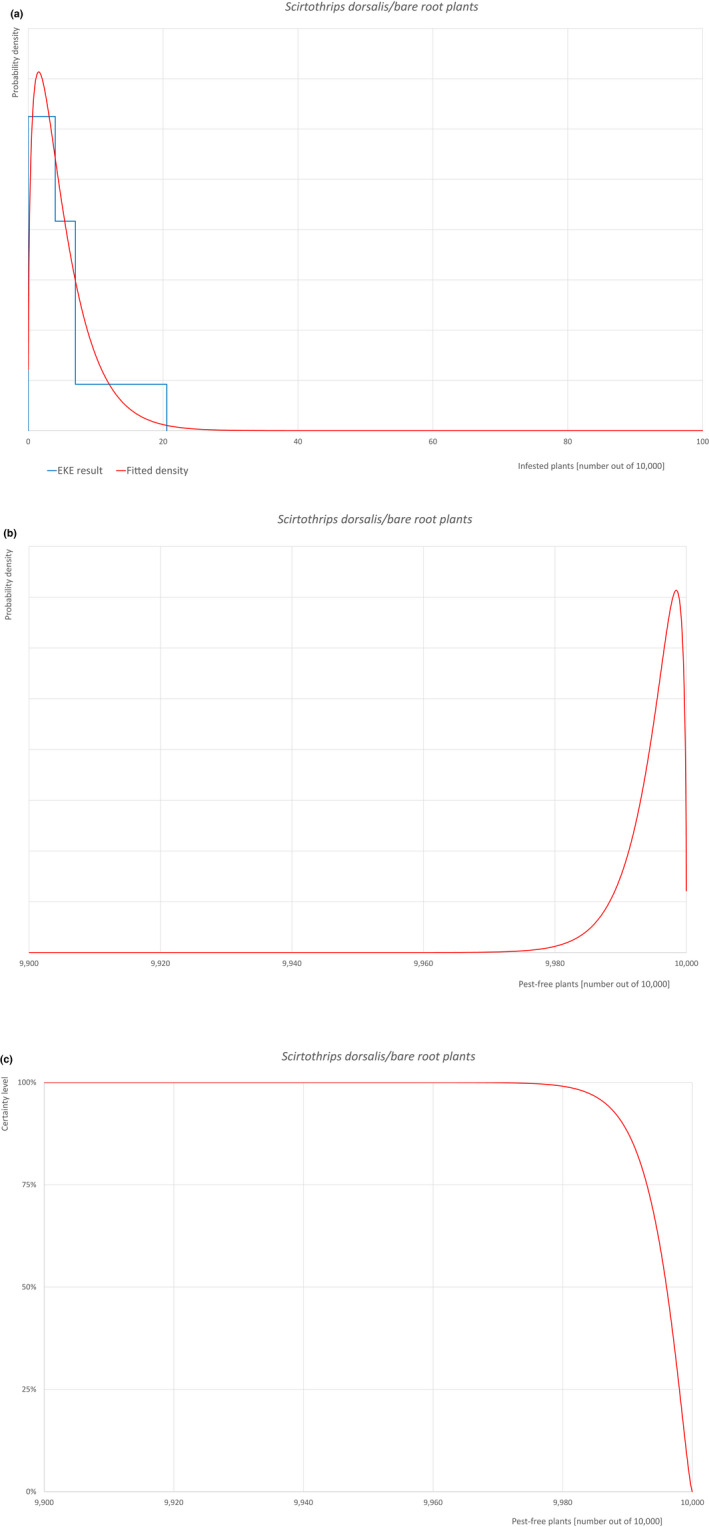
(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
Figure A.23.
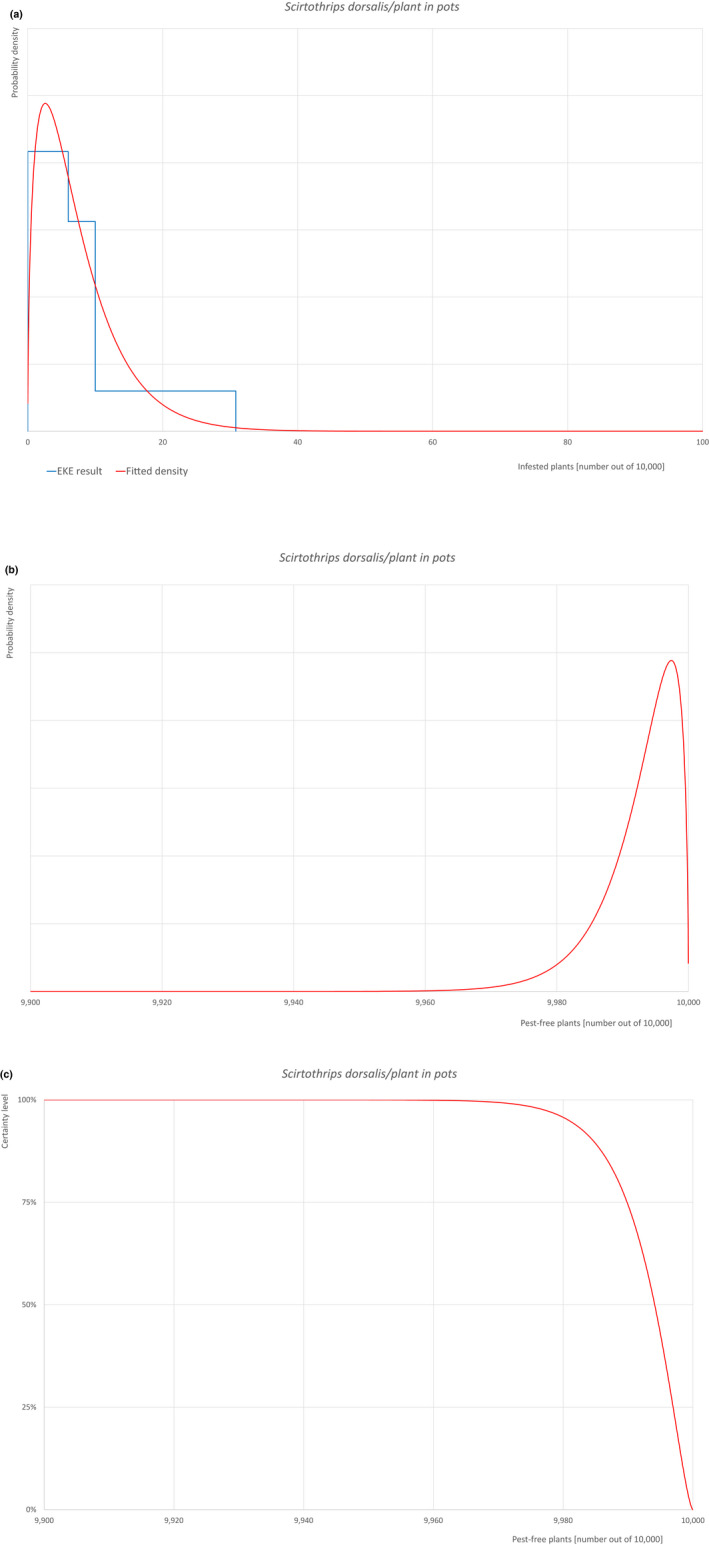
(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
Figure A.24.
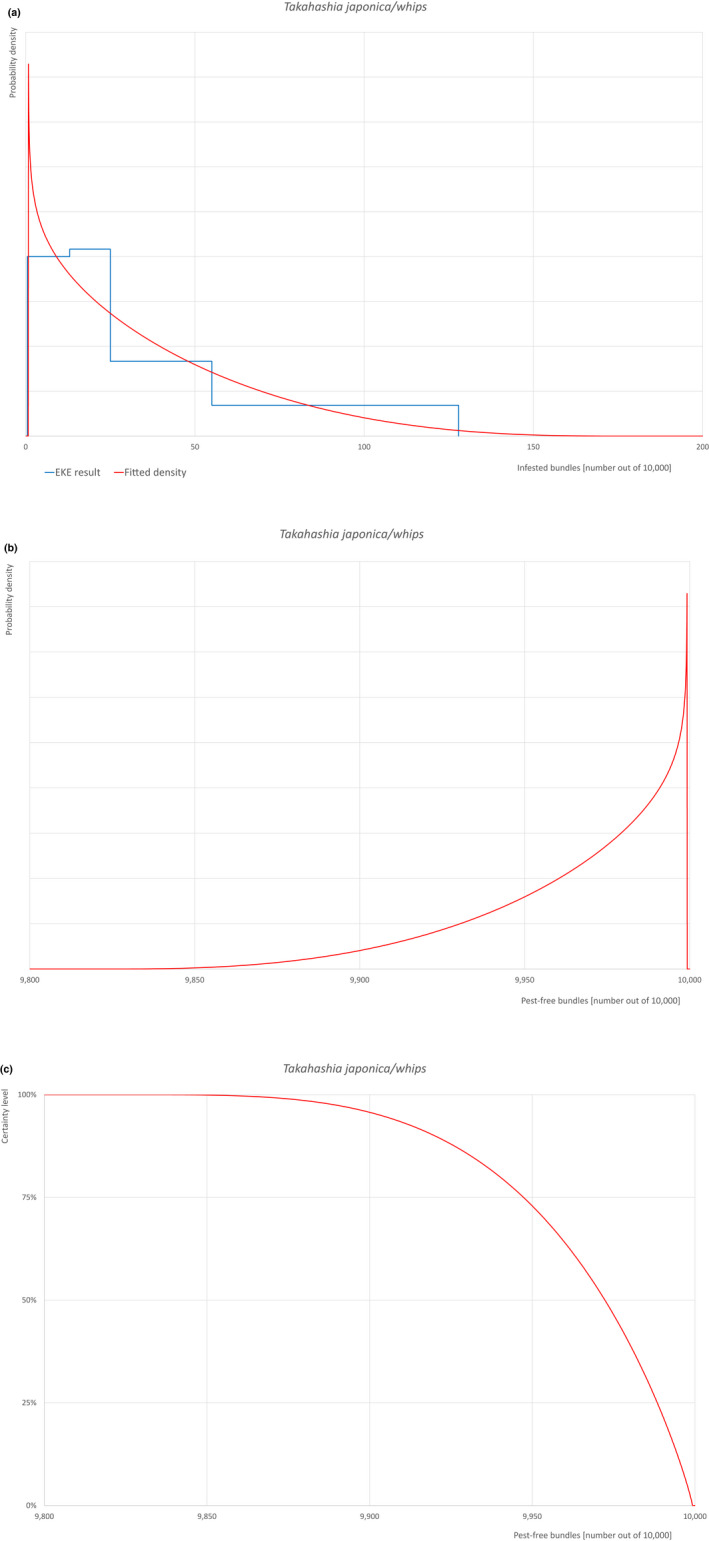
(a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
Figure A.25.
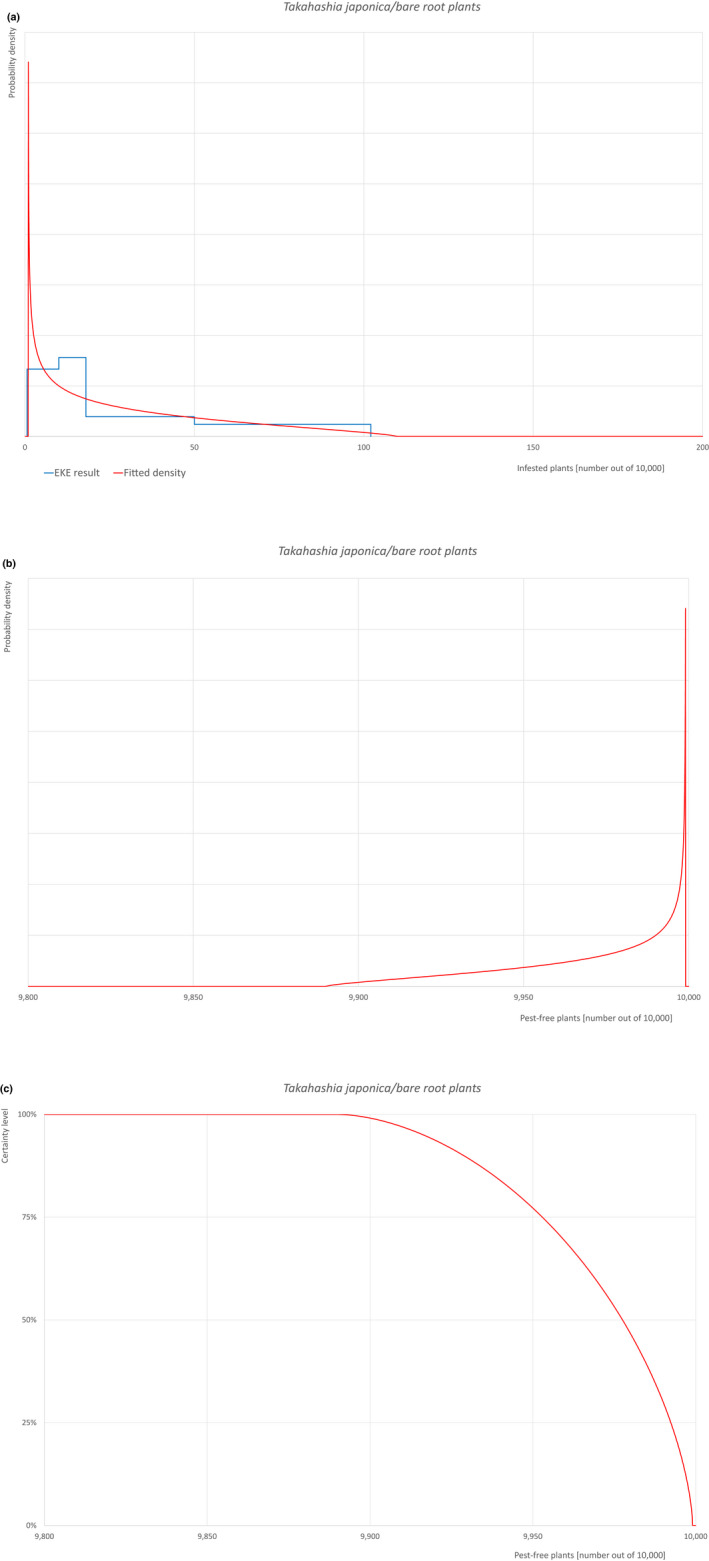
(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
Figure A.26.
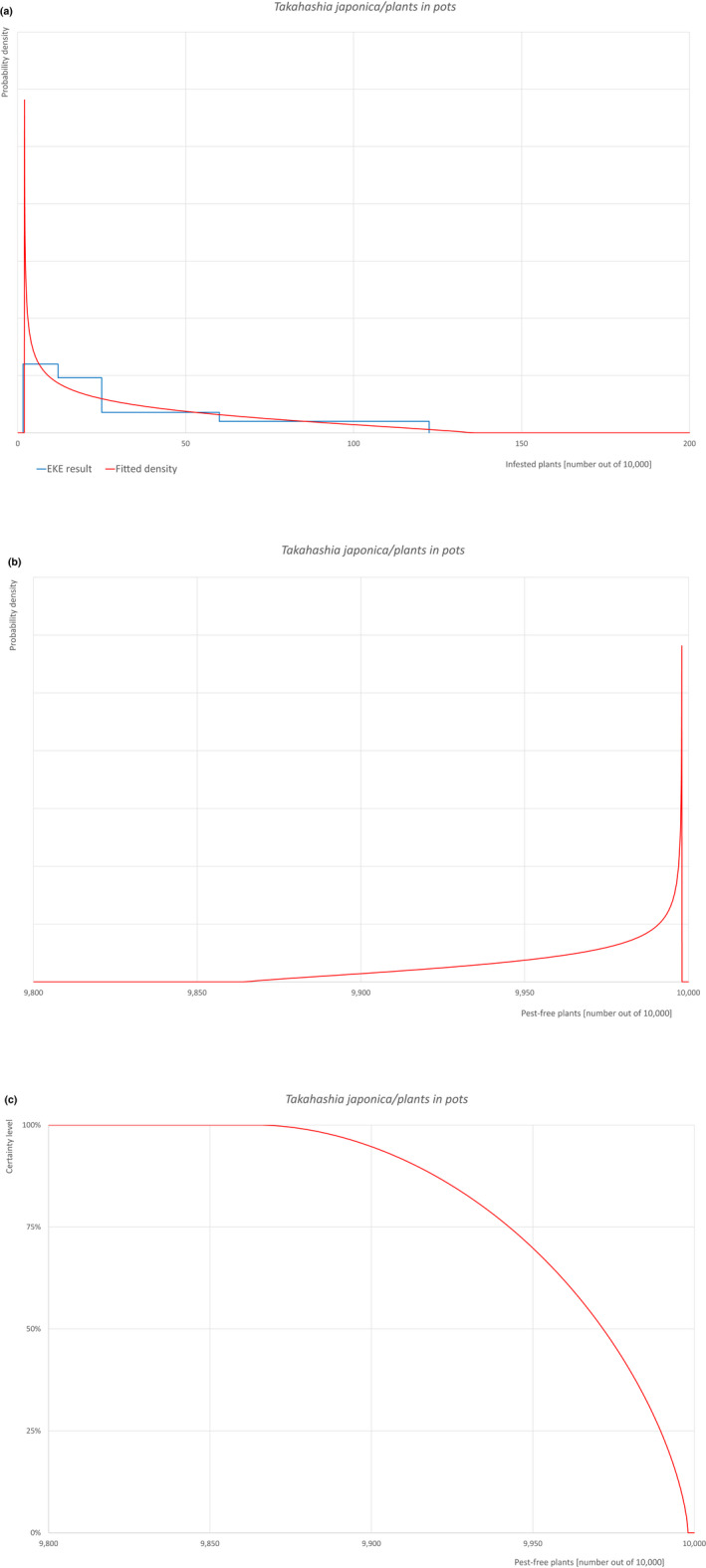
(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.1.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.1.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants/trees up to 7 years old
This scenario assumes a low pressure of the pest in the nurseries and the surrounding. In this scenario it is assumed that leaves are not present when the plants are exported. Pesticide treatments are effective.
A.1.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants/trees up to 7 years old
This scenario assumes a high pressure of the pest in the nurseries and the surrounding. Leaves may be present and eggs laid underneath the leaves, crawlers can move and hide and may be overlooked. Low‐density populations can also be overlooked. The pesticides used may not be effective against B. tabaci. Seven‐year‐old plants have more leaves compared to younger plants and hence more possibilities for the pest to hide and being overlooked.
A.1.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants/trees up to 7 years old (Median)
The uncertainties which were identified are equal in both directions and can lead equally to over‐ or underestimate the number of infested plants.
A.1.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a medium uncertainty in the first quartile, and a medium uncertainty above the median because of restricted distribution of the pest. It is very unlikely to be present outdoors and maple is not a major host. It is a quarantine pest in the UK and therefore more likely to be detected and that measures are taken.
A.1.6.5. Elicitation outcomes of the assessment of the pest freedom for Bemisia tabaci (European populations) on bare root plants/trees up to 7 years old
The following tables show the elicited and fitted values for pest infestation (Table A.3) and pest freedom (Table A.4).
Table A.3.
Elicited and fitted values of the uncertainty distribution of pest infestation by Bemisia tabaci (European populations) per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 8 | 15 | 30 | 60 | ||||||||||
| EKE | 0.269 | 0.675 | 1.36 | 2.76 | 4.71 | 7.26 | 10.0 | 16.3 | 24.1 | 29.0 | 35.0 | 41.6 | 48.8 | 54.4 | 59.9 |
The EKE results are the BetaGeneral (1.0037, 2.8237, 0, 74.5) distribution fitted with @Risk version 7.6.
Table A.4.
The uncertainty distribution of plants free of Bemisia tabaci (European populations) per 10,000 plants calculated by Table A.3
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,940 | 9,970 | 9,985 | 9,992 | 10,000 | ||||||||||
| EKE results | 9,940 | 9,946 | 9,951 | 9,958 | 9,965 | 9,971 | 9,976 | 9,984 | 9,990 | 9,993 | 9,995 | 9,997 | 9,999 | 9,999.3 | 9,999.7 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.4.
A.1.7. Overall likelihood of pest freedom for plants in pots up to 7 years old
A.1.7.1. Reasoning for a scenario which would lead to a reasonably low number of infested plants in pots up to 7 years old
This scenario assumes a low pressure of the pest in the nurseries and the surrounding. Symptoms are developed and visible, chlorotic spotting well visible, honeydew and ants are present. Pesticide treatments are effective. It is assumed that the commodity is mainly 1‐year‐old plants which have less leaves and hence less possibilities of the pest to hide and to be overlooked.
A.1.7.2. Reasoning for a scenario which would lead to a reasonably high number of infested plants in pots up to 7 years old
This scenario assumes a high pressure of the pest in the nurseries and the surrounding. Eggs laid underneath the leaves, crawlers can move and hide and may be overlooked. Low‐density populations can also be overlooked. The pesticides used may not be effective against B. tabaci. Seven‐year‐old plants have more leaves compared to younger plants and hence more possibilities for the pest to hide and being overlooked.
A.1.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested plants in pots up to 7 years old (Median)
The uncertainties which were identified are equal in both directions, and can lead equally to over‐ or underestimate the number of infested individual plants
A.1.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a medium uncertainty in the first quartile, and a medium uncertainty above the median because of restricted distribution of the pest. It is very unlikely to be present outdoors and maple is not a major host. It is a quarantine pest in the UK and therefore more likely to be detected and that measures are taken.
A.1.7.5. Elicitation outcomes of the assessment of the pest freedom for Bemisia tabaci (European populations) on plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infestation (Table A.5) and pest freedom (Table A.6).
Table A.5.
Elicited and fitted values of the uncertainty distribution of pest infestation by Bemisia tabaci (European populations) per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 10 | 20 | 40 | 80 | ||||||||||
| EKE | 0.283 | 0.749 | 1.57 | 3.32 | 5.84 | 9.22 | 12.9 | 21.4 | 32.1 | 38.8 | 47.1 | 56.0 | 65.5 | 72.9 | 80.1 |
The EKE results are the BetaGeneral (1.0037, 2.8237, 0, 74.5) distribution fitted with @Risk version 7.6.
Table A.6.
The uncertainty distribution of plants free of Bemisia tabaci (European populations) per 10,000 plants calculated by Table A.5
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,920 | 9,960 | 9,980 | 9,990 | 10,000 | ||||||||||
| EKE results | 9,920 | 9,927 | 9,934 | 9,944 | 9,953 | 9,961 | 9,968 | 9,979 | 9,987 | 9,991 | 9,994 | 9,997 | 9,998.4 | 9,999.3 | 9,999.7 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.6.
A.1.8. Reference list
Abd‐Rabou S and Simmons AM, 2010. Survey of reproductive host plants of Bemisia tabaci (Hemiptera: Aleyrodidae) in Egypt, including new host records. Entomological News, 121, 456–465. https://doi.org/10.3157/021.121.0507
Avidov Z, 1956. Bionomics of the tobacco whitefly (Bemisia tabaci Cennad.) in Israel. Ktavin, 7, 25–41.
Bradshaw CD, Hemming D, Baker R, Everatt M, Eyre D and Korycinska A, 2019. A novel approach for exploring climatic factors limiting current pest distributions: a case study of Bemisia tabaci in north‐west Europe and assessment of potential future establishment in the United Kingdom under climate change. PLoS One, 14, e0221057, 18 pp. https://doi.org/10.1371/journal.pone.0221057
Byrne DN, 1999. Migration and dispersal by the sweet potato whitefly, Bemisia tabaci. Agricultural and Forest Meteorology, 97, 309–316. https://doi.org/10.1016/s0168-1923(99)00074-x
CABI (Centre for Agriculture and Bioscience International), online. Bemisia tabaci (tobacco whitefly). Available online: https://www.cabi.org/cpc/datasheet/8927#F8A36FF8-D287-4CBD-A0C8-B380F2CFB753 [Accessed: 28 November 2022].
Cohen S, Kern J, Harpaz I and Ben‐Joseph R, 1988. Epidemiological studies of the tomato yellow leaf curl virus (TYLCV) in the Jordan Valley, Israel. Phytoparasitica, 16, 259. https://doi.org/10.1007/bf02979527
Cohen AC, Henneberry TJ and Chu CC, 1996. Geometric relationships between whitefly feeding behavior and vascular bundle arrangements. Entomologia Experimentalis et Applicata, 78, 135–142. https://doi.org/10.1111/j.1570-7458.1996.tb00774.x
Coudriet DL, Prabhaker N, Kishaba AN and Meyerdirk DE, 1985. Variation in developmental rate on different host and overwintering of the sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae). Environmental Entomology, 14, 516–519. https://doi.org/10.1093/ee/14.4.516
Cuthbertson AG, 2013. Update on the status of Bemisia tabaci in the UK and the use of entomopathogenic fungi within eradication programmes. Insects, 4, 198–205. https://doi.org/10.3390/insects4020198
Cuthbertson AG and Vänninen I, 2015. The importance of maintaining Protected Zone status against Bemisia tabaci. Insects, 6, 432–441. https://doi.org/10.3390/insects6020432
Davidson EW, Segura BJ, Steele T and Hendrix DL, 1994. Microorganisms influence the composition of honeydew produced by the silverleaf whitefly, Bemisia argentifolii. Journal of Insect Physiology, 40, 1069–1076. https://doi.org/10.1016/0022-1910(94)90060-4
De Barro PJ, 2012. The Bemisia tabaci species complex: questions to guide future research. Journal of Integrative Agriculture, 11, 187–196. https://doi.org/10.1016/s2095-3119(12)60003-3
De Barro PJ, Liu SS, Boykin LM and Dinsdale AB, 2011. Bemisia tabaci: a statement of species status. Annual Review of Entomology, 56, 1–19. https://doi.org/10.1146/annurev-ento-112408-085504
DEFRA (Department for Environment, Food and Rural Affairs), online_a. UK Risk Register Details for Bemisia tabaci non‐European populations. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=13756&riskId=13756 [Accessed: 28 November 2022].
DEFRA (Department for Environment, Food and Rural Affairs), online_b. UK Risk Register Details for Bemisia tabaci European populations. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=13756&riskId=27242 [Accessed: 28 November 2022].
EFSA PLH Panel (EFSA Panel on Plant Health), 2013. Scientific Opinion on the risks to plant health posed by Bemisia tabaci species complex and viruses it transmits for the EU territory. EFSA Journal 2013;11(4):3162, 45 pp. https://doi.org/10.2903/j.efsa.2013.3162
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Zappalà L, Gómez P, Lucchi A, Urek G, Tramontini S, Mosbach‐Schulz O, de la Peña E and Yuen J, 2021. Scientific Opinion on the commodity risk assessment of Persea americana from Israel. EFSA Journal 2021;19(2):6354, 195 pp. https://doi.org/10.2903/j.efsa.2021.6354
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Chatzivassiliou E, Di Serio F, dos Santos Baptista PC, Gonthier P, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Debode J, Manceau C, Gardi C, Mosbach‐Schulz O and Potting R, 2022a. Scientific report on the commodity risk assessment of specified species of Lonicera potted plants from Turkey. EFSA Journal 2022;20(1):7014, 56 pp. https://doi.org/10.2903/j.efsa.2022.7014
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Chatzivassiliou E, Di Serio F, Baptista P, Gonthier P, Jaques Miret JA, Fejer Justesen A, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Debode J, Manceau C, Gardi C, Mosbach‐Schulz O and Potting R, 2022b. Scientific Opinion on the commodity risk assessment of Jasminum polyanthum unrooted cuttings from Uganda. EFSA Journal 2022;20(5):7300, 83 pp. https://doi.org/10.2903/j.efsa.2022.7300
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Debode J, Manceau C, Gardi C, Schulz OM, Akrivou A, Antonatos S, Beris D, Kritikos C, Kormpi M, Papachristos D, Reppa C and Potting R, 2022c. Scientific Opinion on the commodity risk assessment of Berberis thunbergii potted plants from Turkey. EFSA Journal 2022;20(6):7392, 43 pp. https://doi.org/10.2903/j.efsa.2022.7392
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stancanelli G, Stergulc F and Gonthier P, 2022d. Scientific Opinion on the commodity risk assessment of Ligustrum delavayanum topiary plants grafted on Ligustrum japonicum from the UK. EFSA Journal 2022;20(11):7593, 88 pp. https://doi.org/10.2903/j.efsa.2022.7593
El‐Helaly MS, El‐Shazli AY and El‐Gayar FH, 1971. Biological Studies on Bemisia tabaci Genn. (Homopt., Aleyrodidae) in Egypt 1. Zeitschrift für angewandte Entomologie, 69, 48–55. https://doi.org/10.1111/j.1439-0418.1971.tb03181.x
EPPO (European and Mediterranean Plant Protection Organisation), 2004. Diagnostic protocols for regulated pests Bemisia tabaci, PM 7/35(1). OEPP/EPPO Bulletin, 34, 281–288.
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2022–09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Bemisia tabaci (BEMITA), Categorization. Available online: https://gd.eppo.int/taxon/BEMITA/categorization [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Bemisia tabaci (BEMITA), Distribution. Available online: https://gd.eppo.int/taxon/BEMITA/distribution [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Bemisia tabaci (BEMITA), Host plants. Available online: https://gd.eppo.int/taxon/BEMITA/hosts [Accessed: 28 November 2022].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
Gerling D, Horowitz AR and Baumgaertner J, 1986. Autecology of Bemisia tabaci. Agriculture, Ecosystems and Environment, 17, 5–19. https://doi.org/10.1016/0167-8809(86)90022-8
Hill BG, 1969. A morphological comparison between two species of whitefly, Trialeurodes vaporariorum (Westw.) and Bemisia tabaci (Genn.) (Homoptera: Aleurodidae) which occur on tobacco in the Transvaal. Phytophylactica, 1, 127–146.
Khatun MF, Jahan SH, Lee S and Lee KY, 2018. Genetic diversity and geographic distribution of the Bemisia tabaci species complex in Bangladesh. Acta Tropica, 187, 28–36. https://doi.org/10.1016/j.actatropica.2018.07.021
Li S‐J, Xue X, Ahmed MZ, Ren S‐X, Du Y‐Z, Wu J‐H, Cuthbertson AGS and Qiu B‐L, 2011. Host plants and natural enemies of Bemisia tabaci (Hemiptera: Aleyrodidae) in China. Insect Science 18, 101–120. https://doi.org/10.1111/j.1744-7917.2010.01395.x
Norman JW, Stansty DG, Ellsworth PA and Toscano NCPC, 1995. Management of silverleaf whitefly: a comprehensive manual on the biology, economic impact and control tactics. USDA/CSREES Grant Pub. 93‐EPIX‐1‐0102. 13 pp.
Price JF and Taborsky D, 1992. Movement of immature Bemisia tabaci (Homoptera: Aleyrodidae) on poinsettia leaves. The Florida Entomologist, 75, 151–153. https://doi.org/10.2307/3495495
Ren S‐X, Wang Z‐Z, Qiu B‐L and Xiao Y, 2001. The pest status of Bemisia tabaci in China and non‐chemical control strategies. Insect Science, 8, 279–288. https://doi.org/10.1111/j.1744-7917.2001.tb00453.x
Simmons AM and Elsey KD, 1995. Overwintering and cold tolerance of Bemisia argentifolii (Homoptera: Aleyrodidae) in coastal South Carolina. Journal of Entomological Science, 30, 497–506. https://doi.org/10.18474/0749-8004-30.4.497
Summers CG, Newton Jr AS and Estrada D, 1996. Intraplant and interplant movement of Bemisia argentifolii (Homoptera: Aleyrodidae) crawlers. Environmental Entomology, 25, 1360–1364. https://doi.org/10.1093/ee/25.6.1360
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Walker GP, Perring TM and Freeman TP, 2010. Life history, functional anatomy, feeding and mating behavior. In Stansly PA and Naranjo SE (Eds.), Bemisia: bionomics and management of a global pest, Springer, Dordrecht, 109–160. https://doi.org/10.1007/978-90-481-2460-2_4
Yassin MA and Bendixen LE, 1982. Weed hosts of the cotton whitefly (Bemisia tabaci (Genn.)) Homoptera Aleyrodidae. The Ohio State University, Research Bulletin 1144, June 1982, 10 pp.
A.2. Coniella castaneicola
A.2.1. Organism information
| Taxonomic information |
Current valid scientific name: Coniella castaneicola Synonyms: Anthasthoopa simba, Asteromella castaneicola, Coniella simba, Dothidella castaneicola, Embolidium eucalypti, Gloeosporium castaneicola, Phyllosticta castanicola, Pilidiella castaneicola (according to Index Fungorum) Name used in the EU legislation: – Order: Diaportales Family: Schizoparmaceae Common name: white rot, Coniella leaf blight Name used in the Dossier: Coniella castaneicola |
|
| Group | Fungi | |
| EPPO code | – | |
| Regulated status |
Coniella castaneicola is neither regulated in the EU, nor listed by EPPO. Coniella castaneicola is quarantine pathogen for New Zealand (MAF Biosecurity New Zealand, 2009), Western Australia (Australian Department of Agriculture, 2014) and Korea (Korea Government, 2013). |
|
| Pest status in the UK |
Coniella castaneicola is present in the UK, where it is found in the London area (Elmbridge, Wandsworth) and in south England (New Forest) (NBN Atlas, online; Dossier Section 5.0). The pathogen was recorded from England in 1991 (South Hampshire), 1997 (Surrey), 2001 (Surrey) and from Scotland in 2006 (Dawyck Botanic Garden) (NBN atlas, online). In 2015 it was found on cupules of Castanea sativa from Studland, Dorset, England (Dorset nature, online). |
|
| Pest status in the EU | Coniella castaneicola is present in the EU in Germany (Kehr and Wulf, 1993) and Latvia, where it was found on few strawberry plantations in Kurzeme, in 2007 and 2008 (Laugale et al., 2009). | |
| Host status on Acer | The only available record for Acer sp. being a host of Coniella castaneicola is from Canada (Farr and Rossman, online, citing Nag Raj, 1993). | |
| PRA information | Available Pest Risk Assessment:
|
|
| Other relevant information for the assessment | ||
| Biology |
Coniella castaneicola is an ascomycete fungus causing rot of fruits and leaf spots on a number of hosts throughout the world, frequently found on living, decaying and dead leaves (Farr and Rossman, online). It is present in Africa (South Africa, Nigeria) (Van Niekerk et al., 2004; Australian department of Agriculture, 2014); Asia (China, Korea, India, Indonesia, Pakistan, Japan, Taiwan) (Farr and Rossman, online; Australian department of Agriculture, 2014; Wang and Lin, 2004); Australia (Australian department of Agriculture, 2014); North America and Caribbean (Canada, the US, Cuba) (Farr and Rossman, online); South America (Brazil) (Barreto et al., 2022). |
|
|
Coniella castaneicola is also present in Europe in Germany (Kehr and Wulf, 1993), Latvia (Laugale et al., 2009), Switzerland (Bissegger and Sieber, 1994), Russia (Melkumov, 2014) and the UK (GBIF, online; NBN Atlas, online). There is poor information on the biology and life cycle of C. castaneicola; however, its biology is considered very similar to that of Pilidiella diplodiella, so that the two species have been assessed together in Australia on grapevine (Australian Department of Agriculture, 2014). Coniella castaneicola is mostly known as a pathogen of grapevine, affecting peduncle, rachis, pedicel and berries; secondarily it is found on foliage of deciduous trees. Infections are frequently associated with hailstorms causing wounds on grapes and foliage. Heavy rain, sun scorch and wounding caused by insects can also facilitate infection to a lesser extent (Australian Department of Agriculture, 2014). The pathogen reproduces sexually and asexually, producing ascospores and conidia, respectively, both able to cause infection and dispersed by air or water. Conidia are also able to survive in the environment for long time. Infection rapidly develops at temperatures of 24–27°C, slowly at temperatures below 15°C and only slightly above 34°C. Incubation period varies from 3 to 8 days, depending on temperature, relative humidity, means of penetration and the tissue infected (Australian Department of Agriculture, 2014). Pycnidia and conidia of the pathogen overwinter on dead leaves and survive in the soil for long time (up to 15 years in case of P. diplodiella); conidia may germinate under favourable conditions and establish infection on suitable hosts. Conidia are dispersed over short distances by water splash from infected plant material as well as contaminated soil. On medium‐long distances, both ascospores and conidia may be dispersed by air currents. The movement of infected material or nursery stock and contaminated soil may also contribute to spreading of the pathogen (Australian Department of Agriculture, 2014). |
||
| Symptoms | Main type of symptoms |
Typical symptom on grapevine is white rot of peduncle, rachis, pedicel and berries. The infection begins as small, pale brown, elongated depressions, which may rapidly spread in favourable conditions, causing drying and falling of berries (Australian Department of Agriculture, 2014). According to Kaneko (1981), on Castanea and Quercus species in Japan the first symptom on leaves in summer is sparse small spots pale brown, becoming greyish white in colour. The spots increase in size and form irregular‐shaped lesions causing marked leaf blight. Pycnidia are produced in the lesions on both leaf surfaces as minute black points. Usually, the disease seems not causing premature defoliation. No information about the symptoms on Acer leaves was found. |
| Presence of asymptomatic plants | In Switzerland, C. castaneicola was isolated from young healthy shoots of Castanea sativa (Bissegger and Sieber, 1994). | |
| Confusion with other pests |
On grapevine, Coniella castaneicola and Pilidiella diplodiella cause very similar symptoms, hardly distinguishable. On deciduous trees, the symptoms of C. castaneicola may possibly be confused with those of foliage diseases caused by other ascomycete fungi, also depending on the host plant. Identification of the pathogen cannot be done on a symptomatic basis and requires microscopic examination of isolates in cultures or infected plant material with fruiting structures by specialists. A good description of sexual morph of the pathogen on Castanea is provided by Jiang et al. (2021). |
|
| Host plant range |
Coniella castaneicola has a variety of hosts including Acer sp., Carya sp., Castanea sativa, C. crenata, C. mollissima, C. dentata, Castanea spp., Castanopsis sempervirens, Eucalyptus grandis, Eucalyptus spp., Fragaria spp., Liquidambar styraciflua, Mangifera indica, Quercus alba, Q. rubra, Quercus sp., Rhus copallina, Rhus spp., Rosa rugosa‐prostrata, Syzygium aromaticum, Vaccinium virgatum, Vitis cordifolia and V. vinifera (Crous and Van der Linde, 1993; Farr and Rossman, online). Other host plants recognised in Europe are Aesculus hippocastanum (Melkumov, 2014) and Quercus robur (Kehr and Wulf, 1993). |
|
| Reported evidence of impact |
Coniella castaneicola and Pilidiella diplodiella are mostly known as causing damage to grapevine berries, leading to crop losses and reduced marketability. In regions where hailstorms are frequent, white rot caused by C. castaneicola and P. diplodiella can lead to crop losses of 20–80% (Australian Department of Agriculture, 2014). Coniella castaneicola is also known to cause leaf and fruit diseases of strawberry in the US but no information on the economic significance was found (Australian Department of Agriculture, 2014). In Latvia the pathogen has only a little economic significance in strawberry plantations (Laugale et al., 2009). Coniella castaneicola is commonly found on leaves of Eucalyptus species, in plantations and nurseries in South Africa, Brazil and Australia, but is considered of minor importance as a pathogen causing leaf spot (Van Niekerk et al., 2004; Australian Department of Agriculture, 2014). In September 2020, C. castaneicola was observed on blueberries (Vaccinium virgatum) in Nanchang, China. The pathogen caused damage to the leaves (blight, curling, falling off), dieback and even shoot blight. Subsequently the pathogen lowered yield potential (floral buds' development was affected when the leaves fell off) (Lai et al., 2022). |
|
| Evidence that the commodity is a pathway | Although C. castaneicola has never been intercepted on plants for planting, the pathogen can move both via infected leaves on plants and contaminated soil in potted plants, therefore Acer plants for planting may be a pathway. | |
| Surveillance information | Coniella castaneicola is not under official control in the UK (Dossier Section 5.0). | |
A.2.2. Possibility of pest presence in the nursery
A.2.2.1. Possibility of entry from the surrounding environment
Coniella castaneicola is present in the UK in the London area and southern England (South Hampshire, Surrey, Dorset) and Scotland (Dawyck Botanic Garden; NBN atlas, online; Dorset nature, online; Dossier Section 5.0).
The pathogen can naturally spread with ascospores and conidia dispersed by air currents also over long distance, as well as with conidia transported with rain and water splash on short distances.
Coniella castaneicola can infect Acer spp., Castanea spp. (mostly C. sativa), which are present within 2 km from the nurseries, together with other suitable hosts like Quercus robur and Quercus spp., Aesculus spp. and Rosa spp. (Dossier Section 3.0).
Uncertainties:
-
–
The presence of the pathogen on host plants in the surrounding area.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries from surrounding environment via conidia and ascospores transported by wind and air currents.
A.2.2.2. Possibility of entry with new plants/seed
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0).
In addition to Acer plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are suitable hosts for the pathogen such as Aesculus hippocastanum, Castanea spp. and Quercus spp. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0).
Pycnidia and conidia of Coniella and Pilidiella species can survive in the soil for long time (up to 15 years in case of P. diplodiella) (Australian Department of Agriculture, 2014), and therefore could potentially enter by this pathway. However, the growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries via new seedlings of Acer and plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.2.2.3. Possibility of spread within the nursery
Acer plants are either grown in containers (cells, pots, tubes, etc.) outdoors, in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pathogen can infect other suitable plants, including Aesculus spp., Acer spp., Quercus spp. present within the nurseries (Dossier Sections 3.0 and 6.0).
Coniella castaneicola can naturally spread within the nurseries by rain, water splash, air currents and movement of soil. It can also be spread with tools/machinery/containers with contaminated soil and/or infected debris of host plants.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible by air currents, rain and water splash tools/machinery/containers with contaminated soil and/or infected debris of host plants.
A.2.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Coniella castaneicola between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.2.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Coniella castaneicola is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
Although the pathogen is not regulated, the risk mitigation measure could have some effects in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
The risk mitigation measure could have some effects in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 4 | Growing media | Yes |
As the pathogen can survive in the soil for long time, this measure, in particular using heat‐treated growing media, could be effective in reducing the likelihood of introduction of the pathogen into the nurseries.
Uncertainties:
|
| 5 | Surveillance, monitoring and sampling | Yes |
Although the pathogen is not regulated, the risk mitigation measure could have some effects in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 6 | Hygiene measures | No |
Not relevant.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This measure could have some effect.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Overhead irrigation could favour foliar infections and spread of the pathogen by water splash.
Uncertainties:
|
| 9 | Application of pest control products | Yes |
Some fungicides could reduce the likelihood of the infection by the pathogen.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
Although the pathogen is not regulated, the risk mitigation measure could have some effects in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.2.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.2.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Acer spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections, and that infected leaves are removed from the ground thereby reducing the inoculum pressure.
A.2.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Acer spp. to be hosts for the pathogen. The scenario also assumes that wounds (e.g. pruning wounds) representing infection courts may be present, that infected leaves are not completely removed from the ground and that symptoms of the disease are not easily recognisable during inspections.
A.2.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection. Acer spp. are considered minor hosts.
A.2.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.2.5.5. Elicitation outcomes of the assessment of the pest freedom for Coniella castaneicola on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infection (Table A.7) and pest freedom (Table A.8).
Table A.7.
Elicited and fitted values of the uncertainty distribution of pest infection by Coniella castaneicola per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 23 | 45 | 80 | 250 | ||||||||||
| EKE | 1.95 | 3.78 | 6.30 | 10.7 | 16.1 | 22.8 | 29.7 | 45.3 | 65.9 | 79.8 | 98.8 | 122 | 153 | 184 | 223 |
The EKE results are BetaGeneral (1.4281, 244.51, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.8.
The uncertainty distribution of bundles free of Coniella castaneicola per 10,000 bundles calculated by Table A.7
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,920 | 9,955 | 9,977 | 9,999 | ||||||||||
| EKE results | 9,777 | 9,816 | 9,847 | 9,878 | 9,901 | 9,920 | 9,934 | 9,955 | 9,970 | 9,977 | 9,984 | 9,989 | 9,994 | 9,996 | 9,998 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles, the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.8.
A.2.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.2.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants/trees up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Acer spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections, and that infected leaves are removed from the ground thereby reducing the inoculum pressure.
A.2.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants/trees up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Acer spp. to be hosts for the pathogen. The scenario also assumes that wounds (e.g. pruning wounds) representing infection courts are frequent, that infected leaves are not completely removed from the ground, and that symptoms of the disease are not easily recognisable during inspections.
A.2.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants/trees up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection. Acer spp. are considered minor hosts.
A.2.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.2.6.5. Elicitation outcomes of the assessment of the pest freedom for Coniella castaneicola on bare root plants/trees up to 7 years old
The following tables show the elicited and fitted values for pest infection (Table A.9) and pest freedom (Table A.10).
Table A.9.
Elicited and fitted values of the uncertainty distribution of pest infection by Coniella castaneicola per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 20 | 40 | 80 | 250 | ||||||||||
| EKE | 1.07 | 2.34 | 4.28 | 7.96 | 12.8 | 19.1 | 25.8 | 41.7 | 63.3 | 78.2 | 98.8 | 125 | 159 | 193 | 238 |
The EKE results are the BetaGeneral (1.1831, 208.25, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.10.
The uncertainty distribution of plants free of Coniella castaneicola per 10,000 plants calculated by Table A.9
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,920 | 9,960 | 9,980 | 9,999 | ||||||||||
| EKE results | 9,762 | 9,807 | 9,841 | 9,875 | 9,901 | 9,922 | 9,937 | 9,958 | 9,974 | 9,981 | 9,987 | 9,992 | 9,996 | 9,998 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.10.
A.2.7. Overall likelihood of pest freedom for plants in pots up to 7 years old
A.2.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Acer spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections, and that infected leaves are removed from the ground thereby reducing the inoculum pressure during production and preventing the pathogen to be exported in plant material dropped on to the substrate present in pots.
A.2.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Acer spp. to be hosts for the pathogen. The scenario also assumes that several consignments are traded during the vegetation period (with leaves), that wounds representing infection courts are frequent, that infected leaves are not completely removed from the ground, and that symptoms of the disease are not easily recognisable during inspections.
A.2.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection. Acer spp. are considered minor hosts.
A.2.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.2.7.5. Elicitation outcomes of the assessment of the pest freedom for Coniella castaneicola on plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infection (Table A.11) and pest freedom (Table A.12).
Table A.11.
Elicited and fitted values of the uncertainty distribution of pest infection by Coniella castaneicola per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 35 | 70 | 100 | 350 | ||||||||||
| EKE | 5.34 | 8.87 | 13.2 | 20.0 | 27.8 | 36.9 | 45.8 | 65.3 | 89.6 | 106 | 127 | 153 | 187 | 220 | 263 |
The EKE results are the BetaGeneral (1.9134, 242.75, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.12.
The uncertainty distribution of plants free of Coniella castaneicola per 10,000 plants calculated by Table A.11
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,650 | 9,900 | 9,930 | 9,965 | 9,998 | ||||||||||
| EKE results | 9,737 | 9,780 | 9,813 | 9,847 | 9,873 | 9,894 | 9,910 | 9,935 | 9,954 | 9,963 | 9,972 | 9,980 | 9,987 | 9,991 | 9,995 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.12.
A.2.8. Reference list
Australian Department of Agriculture, 2014. Draft report for the non‐regulated analysis of existing policy for table grapes from Japan. Department of Agriculture, 392 pp.
Barreto GG, Gusmão LFP and Dianese JC, 2022. Checklist of ascomycetes recorded on Eucalyptus in Brazil (1976–2022). Asian Journal of Mycology, 5, 107–129.
Bissegger M and Sieber TN, 1994. Assemblages of endophytic fungi in coppice shoots of Castanea sativa. Mycologia, 86, 648–655. https://doi.org/10.2307/3760535
Crous PW and Van der Linde EJ, 1993. New and interesting records of South African fungi. XI. Eucalyptus leaf fungi. South African Journal of Botany, 59, 300–304.
Dorset nature, online. Coniella castaneicola. Available online: http://www.dorsetnature.co.uk/pages-fungi/f-124.html [Accessed: 4 November 2022].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
Farr DF and Rossman AY. Fungal Databases, U.S. National Fungus Collections, ARS, USDA, online. Coniella castaneicola. Available online: https://nt.ars-grin.gov/fungaldatabases. [Accessed: 18 February 2023].
GBIF (Global Biodiversity Information Facility) Secretariat, online. GBIF BackBone Taxonomy. Available online: https://www.gbif.org/ [Accessed: 18 February 2023].
Jiang N, Fan X and Tian C, 2021. Identification and characterization of leaf‐inhabiting fungi from Castanea plantations in China. Journal of Fungi, 7, 1–59 https://doi.org/10.3390/jof7010064
Kaneko S, 1981. Fungi inhabiting Fagaceous trees III Coniella leaf blight of Quercus and Castanea caused by Coniella castaneicola. Japanese Journal of Phytopathology, 47, 80–83.
Kehr RD and Wulf A, 1993. Fungi associated with above‐ground portions of declining oaks (Quercus robur) in Germany. European Journal Forest Pathology, 23, 18–27.
Korea Government, 2013. List of plant quarantine fungi in Korea newly revised in 2013. Research in Plant Disease, 19, 237–241.
Lai J, Xiong G, Liu B, Kuang W and Song S, 2022. First report of Coniella castaneicola causing leaf blight on blueberry (Vaccinium virgatum) in China. Plant Disease, 106, 1298.
Laugale V, Lepse L, Vilka L, and Rancāne R, 2009. Incidence of fruit rot on strawberries in Latvia, resistance of cultivars and impact of cultural systems. Sodininkystė ir daržininkystė, 28, 3, 125–134.
MAF Biosecurity New Zealand 2009. Import health standard commodity sub‐class: fresh fruit/vegetables mango, Mangifera indica from Australia. Ministry of Agriculture and Forestry, 19 pp.
Melkumov GM, 2014. Substrate specialization of causative agents of diseases of the tree component of the park areas of the city of Voronezh. Bulletin of the Voronezh State Agrarian University, (1–2), 57–62. (in Russian).
Nag Raj TR, 1993. Coelomycetous anamorphs with appendage‐bearing conidia. Mycologue Publications, Waterloo, Ontario, 1101 pp.
NBN Atlas (The National Biodiversity Network), online. Available online: https://nbnatlas.org/about-nbn-atlas/ [Accessed: 4 November 2022].
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Van Niekerk JA, Groenewald JZ, Verkley GJM, Fourie PH, Wingfield MJ and Crous PW, 2004. Systematic reappraisal of Coniella and Pilidiella, with specific reference to species occurring on Eucalyptus and Vitis in South Africa. Mycological Research, 108, 283–303. https://doi.org/10.1017/s0953756204009268
Wang CL and Lin CC, 2004. Five new records of ascomycetes in Taiwan. Fungal Science, 19, 21–29.
A.3. Cryphonectria parasitica
A.3.1. Organism information
| Taxonomic information |
Current valid scientific name: Cryphonectria parasitica Synonyms: Diaporthe parasitica, Endothia gyrosa var. parasitica, Endothia parasitica, Valsonectria parasitica (according to Index Fungorum) Name used in the EU legislation: Cryphonectria parasitica (Murrill) Barr [ENDOPA] Order: Diaporthales Family: Cryphonectriaceae Common name: chestnut blight, blight of chestnut, canker of chestnut, blight of oak Name used in the Dossier: Cryphonectria parasitica |
|
| Group | Fungi | |
| EPPO code | ENDOPA | |
| Regulated status |
The pathogen is listed in Annex III and in Annex VI of Commission Implementing Regulation (EU) 2019/2072 as Cryphonectria parasitica (Murrill) Barr. [ENDOPA]. It is EU protected zone quarantine pests of Czechia, Ireland, Sweden and the UK (Northern Ireland) and also RNQP (Regulated non‐quarantine pest) for plants for planting other than seeds of Castanea. Cryphonectria parasitica is a quarantine pest in Israel, Morocco, Norway and the US (EPPO, online_a). Cryphonectria parasitica is included in the EPPO A2 and in the A2 list of Jordan, Türkiye and COSAVE (Comite de Sanidad Vegetal del Cono Sur – Argentina, Brazil, Chile, Paraguay, Peru and Uruguay). It is also reported on A1 list of Argentina, Azerbaijan, Chile, the UK and IAPSC (Inter‐African Phytosanitary Council) (EPPO, online_a). |
|
| Pest status in the UK |
Cryphonectria parasitica is present in the UK (CABI, online; Farr and Rossman, online). The pathogen was apparently eradicated after the first findings in 2011, then newly recorded in 2016; it was suggested that C. parasitica has been introduced to the UK multiple times over at least two decades through international plant trade (Perez‐Sierra et al., 2019). According to EPPO (online_b), the pathogen is present in the UK with restricted distribution. During surveys held in 2017–2018 and 2019–2020, Cryphonectria parasitica was detected in Berkshire, Buckinghamshire, Cornwall, Derbyshire, Devon, Dorset, London, West Sussex, Jersey and Guernsey (Perez‐Sierra et al., 2019; Romon‐Ochoa et al., 2022; EPPO, online_c; Forestry Commission, online). According to the Dossier Section 5.0, Cryphonectria parasitica is present, not widely distributed and under official control in Great Britain. It is present in central and southern England. In North Ireland, the pathogen is not recorded. |
|
| Pest status in the EU |
Cryphonectria parasitica is present in the EU. It is widespread in Croatia, Italy and Portugal. It has restricted distribution in Austria, Belgium, Bulgaria, France, Germany, Greece, Hungary, Romania, Slovakia, Slovenia and Spain. The pathogen is present with few occurrences in Czechia and the Netherlands. In Poland, the pathogen was eradicated (EPPO online_b). Different areas in the EU have different strains of C. parasitica, the ability of new strains to spread in areas already infested by other strains seems to be very limited (EFSA PLH Panel, 2016). |
|
| Host status on Acer |
Cryphonectria parasitica may infect Acer palmatum (Spaulding, 1961; Farr and Rossman, online) and Acer rubrum (Anderson and Babcock, 1913; Shear et al., 1917). There is no information on whether C. parasitica can also attack Acer pseudoplatanus. Acer spp. are reported as minor incidental hosts by Rigling and Prospero (2018). |
|
| PRA information | Available pest risk assessment:
|
|
| Other relevant information for the assessment | ||
| Biology |
Cryphonectria parasitica is a pathogen in the family Cryphonectriaceae, native to East Asia (EPPO, online_b). It is present in Africa (Tunisia), Asia (China, India, Iran, Japan, North and South Korea, Taiwan), Europe, North America (Canada, the US) and Oceania (Australia) (EPPO, online_b). The biology section is based on the studies on chestnut, one of the major hosts. Cryphonectria parasitica is a bark pathogen that infects the tissue through wounds or growth cracks in the bark. The pathogen can also infect abandoned galls of the gall wasp Dryocosmus kuriphilus (Meyer et al., 2015). Hail wounds have been documented as important infection courts (Lione et al., 2020). The infection is caused by asexual and sexual spores. The infection develops in a lesion and a canker, which eventually kills the plant part distal to the infection. The pathogen can saprophytically colonise recently (1 year) dead stems or branches (Hepting, 1974; Prospero et al., 2006). Then stromata develop. Stromata can contain sexual fruiting bodies (perithecia), asexual ones (pycnidia) or both. Pycnidia produce conidia that are released in tendrils in moist condition and splash dispersed by rain in a few metres range. Conidia can also be dispersed by birds, insects and windborne dust over long distances (Wendt et al., 1983; Russin et al., 1984). Once in the ground conidia can survive for a long time (Heald and Studhalter, 1914). Perithecia produce ascospores that can be dispersed by wind over hundreds of metres and are relatively short‐lived. Ascospores are discharged from spring to autumn during warm rains (Heald and Gardner, 1914; Guérin et al., 2001). Sexual reproduction can be by both, outcrossing and self‐fertilisation (Marra et al., 2004). In northern Italy, it has been reported that C. parasitica can release propagules all over the year, though with significant seasonal peaks in the spring and fall. Large propagule loads were significantly correlated with an increasing number of rainy days of the week (days providing 1 ‐10 mm/day of water) (Lione et al., 2022). In newly established populations, asexual reproduction via conidia is often the predominant spreading mechanism (Rigling and Prospero, 2018). The canker growth can be as fast as 1 mm per day when the average daily temperature is 20°C, with a peak at 27°C and slowed down below 20°C (Bazzigher, 1981). The optimal germination temperature of conidia is 25–26°C, the ascospores' one is 21°C (Fulton, 1912). Humidity promotes spore release (Griffin, 1986), but drought stress can increase incidence and mortality of the pathogen (Roane et al., 1986; Waldboth and Oberhuber, 2009). The pathogen's ability to infect a new host is dependent on the age of the wound: on European chestnut C. parasitica cannot establish itself in wounds of four or more days (Bazzigher and Schmid, 1962). Cryphonectria parasitica can also show an endophytic behaviour, it has been found in symptomless stems 3 months after inoculation (Guérin and Robin, 2003) or developed its symptoms after 16 months of quarantine in Australia (Cunnington and Pascoe, 2003). On chestnut fruits, the fungus is associated with only the nutshell (Jaynes and Depalma, 1984). |
|
|
In newly colonised territories, the population usually consists of one or few genotypes, limiting sexual reproduction and long‐range dispersal via ascospores. In most populations in Europe, random mating has been ruled out and, even then, ascospores are not likely to be the primary inoculum (Milgroom and Cortesi, 1999). The main mycovirus acting as biological control agent for C. parasitica, reducing its virulence, in Europe is Cryphonectria hypovirus 1 (CHV‐1), one of the four known species of the genus Hypovirus (Turina and Rostagno, 2007). CHV‐1 can spread via hyphal anastomosis from one individual to another or via conidia, but not via ascospores. Fungi‐feeding mites can be important for the spread of CHV‐1 (Bouneb et al., 2016). Cryphonectria parasitica, like many fungi has a vegetative incompatibility (vic) mechanism. This mechanism usually hinders the transmission of mycoviruses including CHV1. Up to date, there are 64 genetically defined vic genotypes (Short et al., 2015). According to EFSA PLH Panel (2016), the main pathways of entry for C. parasitica are plants for planting (including seedlings, scions, rootstocks, ornamental plants), wood with bark (including chips, wood for tannin production, hoops for barrels), fruit (nuts), soil and growing media (including isolated chestnut bark), natural spread of airborne inoculum, biological agents able to mechanically transfer the fungus (e.g. birds, mammals, insects, mites, etc.) and machinery (construction, terracing, etc.) and pruning/cutting tools. According to EUROPHYT (online), Cryphonectria parasitica was intercepted 14 times on wood and bark of Castanea sp. or Castanea sativa. Once it was intercepted on Castanea sativa plants intended for planting: not yet planted. Cryphonectria parasitica is singlehandedly responsible for the removal from the forest dominant plane of Castanea dentata in North America. Impact of the pathogen is strongly dependent on host availability, host susceptibility and virulence of the Cryphonectria parasitica strain. An in‐depth analysis of the impact of introduction of new strains of the pathogen in EU countries where C. parasitica is already established and in countries where it is absent is available in the EFSA Pest Risk Assessment for Cryphonectria parasitica (EFSA PLH Panel, 2016). |
||
| Symptoms | Main type of symptoms |
Cryphonectria parasitica only attacks the aboveground tree parts. Symptoms vary depending on the age of the host tree, its species, and the virulence of the particular pathogen strain (Heiniger and Rigling, 1994; Prospero and Rigling, 2013). Virulent strains on susceptible trees produce in few months cankers that can kill branches or twigs (Diller, 1965). On susceptible Castanea species, one of the first symptoms is branch wilting with wilted leaves hanging on the branches. Cankers typically appear as sunken, reddish‐brown bark lesions. Below the cankers, trees can produce epicormic shoots. At the canker border and under the bark, the fungus develops pale brown mycelial fans. On more resistant tree species (Asian chestnut species, oaks), cankers typically have a swollen appearance and are superficial or callused. There is no information on the symptoms caused by C. parasitica on Acer plants. |
| Presence of asymptomatic plants | Cryphonectria parasitica can show an endophytic behaviour, imported chestnut plants have developed symptoms after 16 months of quarantine (Cunnington and Pascoe, 2003). | |
| Confusion with other pests |
Cryphonectria parasitica symptoms can be confused with other cankers in the first stages, but the presence of mycelial fans and appearance of the fruiting bodies makes the identification clear. Isolated on potato dextrose agar can identify also hypovirus‐infected fungi, and molecular methods have been developed for identification (EFSA PLH Panel, 2014). Some confusion can occur with cancers caused by Gnomonopsis castaneae (Lione et al., 2019). |
|
| Host plant range |
Main host of Cryphonectria parasitica are Castanea dentata and C. sativa. Other hosts in the Castanea genus are C. crenata, C. henryi, C. mollissima, C. ozarkensis, C. pumila and C. seguinii. Among oaks the known hosts are Quercus alba, Q. coccinea, Q. frainetto, Q. ilex, Q. montana, Q. petraea, Q. prinus, Q. pubescens, Q. stellata, Q. suber, Q. velutina and Q. virginiana. Cryphonectria parasitica was also reported on Aesculus hippocastanum, Carya ovata, Carpinus betulus, Eucalyptus camaldulensis, E. haemastoma, E. microcorys, E. punctata, E. robusta, Rhus typhina and Fagus sylvatica (EPPO, online_d; Farr and Rossman, online). The reports for Fagus sylvatica are only taken from artificial inoculation (Dennert et al., 2020). Acer palmatum is a known host for C. parasitica (EPPO, online_d; Farr and Rossman, online). Cryphonectria parasitica has also been reported on Acer rubrum in North America (Anderson and Babcock, 1913; Shear et al., 1917). Inoculation experiments indicated that bark of Acer rubrum is much less susceptible than the bark of Quercus sp. (Baird, 1991). |
|
| Reported evidence of impact | Cryphonectria parasitica is EU protected zone quarantine pest. | |
| Evidence that the commodity is a pathway | Host plants for planting, excluding seeds, but including dormant plants, have been identified as pathways by EFSA PLH Panel (2014), and have been historically pathways even after quarantine (Cunnington and Pascoe, 2003). | |
| Surveillance information | Cryphonectria parasitica is a regulated quarantine pest for Great Britain subject to eradication measures, unless in the wider environment where a containment policy may be taken dependent on the site. As part of an annual survey at ornamental retail and production sites (frequency of visits determined by a decision matrix), Cryphonectria parasitica is inspected for on common hosts plants (Dossier Section 3.0 and 5.0). | |
A.3.2. Possibility of pest presence in the nursery
A.3.2.1. Possibility of entry from the surrounding environment
Cryphonectria parasitica is present in the UK with restricted distribution mostly in central and southern England (Dossier Section 5.0; Forestry Commission, online).
The pathogen can naturally spread with ascospores dispersed by air currents over hundreds of metres, as well as with conidia transported with rain splash over short distances. However, conidia can also be dispersed by birds, insects and wind over long distances (Wendt et al., 1983; Russin et al., 1984).
Cryphonectria parasitica principally infects Castanea species mostly C. sativa, which is present within 2 km radius from the nurseries, together with other suitable hosts like Quercus spp. and other plants that the pathogen was reported on like Aesculus hippocastanum and Fagus spp. (Dossier Section 3.0).
Uncertainties:
-
–
The susceptibility of Acer to the pathogen.
-
–
The dispersal range of animals carrying C. parasitica inoculum (e.g. birds, insects and mites).
-
–
The role of animals in C. parasitica dispersal.
-
–
No information available on the distance of the nurseries to sources of pathogen in the surrounding environment.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for Cryphonectria parasitica to enter the nurseries from surrounding environment via conidia and ascospores transported by air currents, birds and insects.
A.3.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0).
In addition to Acer, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are suitable hosts for the pathogen such as Castanea spp. and Quercus spp. and other plants that the pathogen was reported on like Aesculus hippocastanum, Carpinus betulus and Fagus sylvatica. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). Although soil and growing media are considered pathways of minor importance (EFSA, 2016), the conidia of Cryphonectria parasitica can survive in the soil for long time (Heald and Studhalter, 1914) and therefore could potentially enter by this way. However, the growing media are certified and heat treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
The susceptibility of other plants in the nursery to the pathogen.
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries via new seedlings of Acer and plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.3.2.3. Possibility of spread within the nursery
Acer plants are either grown in containers (cells, pots, tubes, etc.) outdoors, in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pathogen can infect other plants, such as Aesculus spp., Acer spp., Castanea spp., Fagus spp., Quercus spp., etc., present within the nurseries (Dossier Sections 3.0 and 6.0).
If sporulating infections occur in the nurseries, Cryphonectria parasitica can naturally spread within the nurseries by rain/water splash, air currents, transported by insects, mites and birds. Human assisted spread could be mostly via contaminated equipment, but tools used in the nurseries are disinfected before being used on different plants (Dossier Section 3.0).
Uncertainties:
-
–
The host suitability of Acer to Cryphonectria parasitica.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible by rain/water splash, air currents and transport of insects, mites and birds.
A.3.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Cryphonectria parasitica between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.3.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Cryphonectria parasitica is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 4 | Growing media | No | Not relevant. |
| 5 | Surveillance, monitoring and sampling | Yes |
This measure could have some effect.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
The desinfection of tools with appropriate product can prevent the spread of the pathogen within the nurseries.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This measure could have some effect.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Overhead irrigation can increase the likelihood of spread of the pathogen by water splash.
Uncertainties:
|
| 9 | Application of pest control products | Yes |
Although C. parasitica is generally not a target of the pesticide treatments in the nurseries, some fungicides could reduce the likelihood of the infection by the pathogen.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
This measure could have some effect.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.3.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.3.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The plants are exposed to the pathogen for only short period of time. The scenario assumes Acer spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes Acer spp. to be hosts for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings. Acer spp. are considered minor hosts. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.5.5. Elicitation outcomes of the assessment of the pest freedom for Cryphonectria parasitica on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infection (Table A.13) and pest freedom (Table A.14).
Table A.13.
Elicited and fitted values of the uncertainty distribution of pest infection by Cryphonectria parasitica per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 5 | 10 | 20 | 50 | ||||||||||
| EKE | 0.209 | 0.494 | 0.952 | 1.86 | 3.10 | 4.72 | 6.46 | 10.5 | 15.9 | 19.47 | 24.2 | 29.8 | 36.6 | 42.8 | 50.0 |
The EKE results are the BetaGeneral (1.0764, 6.8505, 0, 100) distribution fitted with @Risk version 7.6.
Table A.14.
The uncertainty distribution of bundles free of Cryphonectria parasitica per 10,000 bundles calculated by Table A.13
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,950 | 9,980 | 9,990 | 9,995 | 10,000 | ||||||||||
| EKE results | 9,950 | 9,957 | 9,963 | 9,970 | 9,976 | 9,981 | 9,984 | 9,989 | 9,994 | 9,995 | 9,997 | 9,998 | 9,999 | 10,000 | 10,000 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles, the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.14.
A.3.6. Overall likelihood of pest freedom for bare root plants/trees and plants in pots up to 7 years old
A.3.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees and plants in pots up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Acer spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees and plants in pots up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Acer spp. to be hosts for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees and plants in pots up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause some infection. Acer spp. are considered minor hosts. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.6.5. Elicitation outcomes of the assessment of the pest freedom for Cryphonectria parasitica on bare root plants/trees and plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infection (Table A.15) and pest freedom (Table A.16).
Table A.15.
Elicited and fitted values of the uncertainty distribution of pest infection by Cryphonectria parasitica per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 8 | 15 | 25 | 75 | ||||||||||
| EKE | 0.936 | 1.65 | 2.58 | 4.10 | 5.90 | 8.02 | 10.2 | 14.9 | 21.0 | 25.1 | 30.6 | 37.4 | 46.2 | 54.9 | 66.1 |
The EKE results are the BetaGeneral (1.6776, 908.36, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.16.
The uncertainty distribution of plants free of Cryphonectria parasitica per 10,000 plants calculated by Table A.15
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,925 | 9,975 | 9,985 | 9,992 | 10,000 | ||||||||||
| EKE results | 9,934 | 9,945 | 9,954 | 9,963 | 9,969 | 9,975 | 9,979 | 9,985 | 9,990 | 9,992 | 9,994 | 9,996 | 9,997 | 9,998 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.16.
A.3.7. Reference list
Anderson PJ and Babcock DC, 1913. Field studies on the dissemination and growth of the chestnut blight fungus. Pennsylvania Chestnut Tree Blight Commission, 3, 46.
Anderson A, Baker R, Parkinson N, Reed P and Woodward S, 2013. Rapid pest risk analysis for Cryphonectria parasitica. The Food and Environment Research Agency, 23 pp.
Baird RE, 1991. Growth and stromata production of hypovirulent and virulent strains of Cryphonectria parasitica on dead Quercus rubra and Acer rubrum. Mycologia, 83, 158–162. https://doi.org/10.2307/3759931
Bazzigher G, 1981. Selection of blight‐resistant chestnut trees in Switzerland. Forest Pathology, 11, 199–207. https://doi.org/10.1111/j.1439-0329.1981.tb00088.x
Bazzigher G and Schmid P, 1962. Methodik zur Prüfung der Endothia‐Resistenz bei Kastanien. Journal of Phytopathology, 45, 169–189.
Biosecurity Australia, 2006. Technical justification for Australia's requirement for wood packaging material to be bark free. Biosecurity Australia, Canberra, Australia. 123 pp.
Bouneb M, Turchetti T, Nannelli R, Roversi PF, Paoli F, Danti R and Simoni S, 2016. Occurrence and transmission of mycovirus Cryphonectria hypovirus 1 from dejecta of Thyreophagus corticalis (Acari, Acaridae). Fungal Biology, 120, 351–357. https://doi.org/10.1016/j.funbio.2015.11.004
CABI, online. Datasheet for Cryphonectria parasitica (blight of chestnut). Available online: https://www.cabi.org/isc/datasheet/21108 [Accessed: 28 November 2022].
Cunnington JH and Pascoe IG, 2003. Post entry quarantine interception of chestnut blight in Victoria. Australasian Plant Pathology, 32, 569. https://doi.org/10.1071/AP03067
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Cryphonectria parasitica. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=11469 [Accessed: 28 November 2022].
Dennert F, Rigling D, Meyer JB, Schefer C, Augustiny E and Prospero S, 2020. Testing the pathogenic potential of Cryphonectria parasitica and related species on three common European Fagaceae. Frontiers in Forests and Global Change, 52, 8 pp. https://doi.org/10.3389/ffgc.2020.00052
Diller JD, 1965. Chestnut Blight. Forest Pest Leaflet 94. U.S. Department of Agriculture Forest Service, Washington, DC. 7 pp.
EFSA PLH Panel (EFSA Panel on Plant Health), 2014. Scientific Opinion on the pest categorisation of Cryphonectria parasitica (Murrill) Barr. EFSA Journal 2014;12(10):3859, 42 pp. https://doi.org/10.2903/j.efsa.2014.3859
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Maresi G, Prospero S, Vettraino AM, Vloutoglou I, Pautasso M and Rossi V, 2016. Risk assessment and reduction options for Cryphonectria parasitica in the EU. EFSA Journal 2016;14(12):4641, 54 pp. https://doi.org/10.2903/j.efsa.2016.4641
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F and Gonthier P, 2022. Scientific Opinion on the commodity risk assessment of Acer palmatum plants grafted on Acer davidii from China. EFSA Journal 2022;20(5):7298, 262 pp. https://doi.org/10.2903/j.efsa.2022.7298
EPPO (European and Mediterranean Plant Protection Organization), online_a. Cryphonectria parasitica (ENDOPA), Categorization. Available online: https://gd.eppo.int/taxon/ENDOPA/categorization [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Cryphonectria parasitica (ENDOPA), Distribution. Available online: https://gd.eppo.int/taxon/ENDOPA/distribution [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Cryphonectria parasitica (ENDOPA), Distribution details in United Kingdom. Available online: https://gd.eppo.int/taxon/ENDOPA/distribution/GB [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Cryphonectria parasitica (ENDOPA), Hots plants. Available online: https://gd.eppo.int/taxon/ENDOPA/hosts [Accessed: 28 November 2022].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
Farr DF and Rossman AY. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases. [Accessed: 28 November 2022].
Fulton HR, 1912. Recent notes on the chestnut bark disease. Pennsylvania Chestnut Blight Conference Report, Harrisburg, PA, the US, 48–56.
Griffin GJ, 1986. Chestnut blight and its control. Horticultural Reviews, 8, 291–335.
Guérin L, Froidefond G and Xu X‐M, 2001. Seasonal patterns of dispersal of ascospores of Cryphonectria parasitica (chestnut blight): Dispersal of C. parasitica ascospores. Plant Pathology, 50, 717–724. https://doi.org/10.1046/j.1365-3059.2001.00600.x
Guérin L and Robin C, 2003. Seasonal effect on infection and development of lesions caused by Cryphonectria parasitica in Castanea sativa. Forest Pathology, 33, 223–235. https://doi.org/10.1046/j.1439-0329.2003.00329.x
Heald FD and Gardner MW, 1914. Longevity of pycnospores of the chestnut blight fungus in soil. Journal of Agricultural Research, 2, 67–75.
Heald FD and Studhalter RA, 1914. Birds as carriers of the chestnut blight fungus. Journal of Agricultural Research, 2, 405–422.
Heiniger U and Rigling D, 1994. Biological control of chestnut blight in Europe. Annual Review of Phytopathology, 32, 581–599. https://doi.org/10.1146/annurev.py.32.090194.003053
Hepting GH, 1974. Death of the American chestnut. Journal of Forest History, 18, 60–67. https://doi.org/10.2307/3983346
Jaynes RA and DePalma NK, 1984. Natural infection of nuts of Castanea dentata by Endothia parasitica. Phytopathology, 74, 296. https://doi.org/10.1094/Phyto-74-296
Lione G, Danti R, Fernandez‐Conradi P, Ferreira‐Cardoso JV, Lefort F, Marques G, Meyer JB, Prospero S, Radócz L, Robin C, Turchetti T, Vettraino AM and Gonthier P, 2019. The emerging pathogen of chestnut Gnomoniopsis castaneae: the challenge posed by a versatile fungus. European Journal of Plant Pathology, 153, 671–685. https://doi.org/10.1007/s10658-018-1597-2
Lione G, Giordano L, Turina M and Gonthier P, 2020. Hail‐induced infections of the chestnut blight pathogen Cryphonectria parasitica depend on wound size and may lead to severe diebacks. Phytopathology, 110, 1280–1293. https://doi.org/10.1094/PHYTO‐01‐20‐0006‐R
Lione G, Brescia F, Giordano L and Gonthier P, 2022. Effects of seasonality and climate on the propagule deposition patterns of the chestnut blight pathogen Cryphonectria parasitica in orchards of the Alpine district of NorthWestern Italy. Agriculture 12, 644. https://doi.org/10.3390/agriculture12050644
Marra RE, Cortesi P, Bissegger M and Milgroom MG, 2004. Mixed mating in natural populations of the chestnut blight fungus, Cryphonectria parasitica. Heredity, 93, 189–195. https://doi.org/10.1038/sj.hdy.6800492
Meyer JB, Gallien L and Prospero S, 2015. Interaction between two invasive organisms on the European chestnut: does the chestnut blight fungus benefit from the presence of the gall wasp? FEMS Microbiology Ecology, 91, fiv122. https://doi.org/10.1093/femsec/fiv122
Milgroom MG and Cortesi P, 1999. Analysis of population structure of the chestnut blight fungus based on vegetative incompatibility genotypes. Proceedings of the National Academy of Sciences, 96, 10518–10523. https://doi.org/10.1073/pnas.96.18.10518
Perez‐Sierra A, Romon‐Ochoa P, Gorton C, Lewis A, Rees H, Van Der Linde S and Webber J, 2019. High vegetative compatibility diversity of Cryphonectria parasitica infecting sweet chestnut (Castanea sativa) in Britain indicates multiple pathogen introductions. Plant Pathology, 68, 727–737. https://doi.org/10.1111/ppa.12981
Prospero S, Conedera M, Heiniger U and Rigling D, 2006. Saprophytic activity and sporulation of Cryphonectria parasitica on dead chestnut wood in forests with naturally established hypovirulence. Phytopathology, 96, 1337–1344. https://doi.org/10.1094/PHYTO-96-1337
Prospero S and Rigling D, 2013. Chestnut blight. Infectious forest diseases. In: Gonthier P, Nicolotti G. (Eds.), Infectious forest diseases. CAB International, Wallingford, UK, 318–339.
Rigling D and Prospero S, 2018. Cryphonectria parasitica, the causal agent of chestnut blight: invasion history, population biology and disease control: Cryphonectria parasitica. Molecular Plant Pathology, 19, 7–20. https://doi.org/10.1111/mpp.12542
Roane MK, Griffin GJ and Elkins JR, 1986. Chestnut blight, other Endothia diseases, and the genus Endothia. APS Press, American Phytopathological Society, St. Paul, MN, the US, vii + 53 pp.
Romon‐Ochoa P, Kranjec Orlović J, Gorton C, Lewis A, van der Linde S and Pérez‐Sierra A, 2022. New detections of chestnut blight in Great Britain during 2019–2020 reveal high Cryphonectria parasitica diversity and limited spread of the disease. Plant Pathology, 71, 793–804. https://doi.org/10.1111/ppa.13523
Russin JS, Shain L and Nordin GL, 1984. Insects as carriers of virulent and cytoplasmic hypovirulent isolates of the chestnut blight fungus. Journal of Economic Entomology, 77, 838–846.
Shear CL, Stevens NE and Tiller RJ, 1917. Endothia parasitica and related species. Bulletin of the United States Department of Agriculture, 380, 1–82. https://doi.org/10.5962/bhl.title.64538
Short DPG, Double M, Nuss DL, Stauder CM, MacDonald W and Kasson MT, 2015. Multilocus PCR assays elucidate vegetative incompatibility gene profiles of Cryphonectria parasitica in the United States. Applied and Environmental Microbiology, 81, 5736–5742. https://doi.org/10.1128/AEM.00926-15
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Turina M and Rostagno L, 2007. Virus‐induced hypovirulence in Cryphonectria parasitica: still an unresolved conundrum. Journal of Plant Pathology, 14.
Waldboth M and Oberhuber W, 2009. Synergistic effect of drought and chestnut blight (Cryphonectria parasitica) on growth decline of European chestnut (Castanea sativa). Forest Pathology, 39, 43–55. https://doi.org/10.1111/j.1439-0329.2008.00562.x
Wendt R, 1983. Association of Endothia parasitica with mites isolated from cankers on American chestnut trees. Plant Disease, 67, 757. https://doi.org/10.1094/PD-67-757
A.4. Entoleuca mammata
A.4.1. Organism information
| Taxonomic information |
Current valid scientific name: Entoleuca mammata Synonyms: Anthostoma blakei, Anthostoma morsei, Fuckelia morsei, Hypoxylon blakei, Hypoxylon holwayi, Hypoxylon mammatum, Hypoxylon morsei, Hypoxylon pauperatum, Hypoxylon pruinatum, Nemania mammata, Rosellinia pruinata, Sphaeria mammata, Sphaeria pruinata (according to Index Fungorum) Name used in the EU legislation: Entoleuca mammata (Wahlenb.) Rogers and Ju Order: Xylariales Family: Xylariaceae Common name: hypoxylon canker of poplar, canker of aspen Name used in the Dossier: Entoleuca mammata, Hypoxylon mammatum |
|
| Group | Fungi | |
| EPPO code | HYPOMA | |
| Regulated status |
Entoleuca mammata is listed in Annex III of Commission Implementing Regulation (EU) 2019/2072 as protected zone quarantine pest for Ireland. The pathogen is quarantine pest in China and Israel. It is on the A1 list of Türkiye (EPPO, online_a). |
|
| Pest status in the UK |
Entoleuca mammata is present in the UK, with few occurrences in England, Wales, Channel Islands and Scotland (CABI, online; EPPO, online_b). According to Dossier Section 5.0 the pathogen is present: not widely distributed and not under official control. |
|
| Pest status in the EU | Entoleuca mammata is currently present in the EU in 19 MS: Austria, Belgium, Croatia, Czechia, Finland, France, Germany, Greece, Italy, Lithuania, the Netherlands, Slovakia, Slovenia, Sweden (EFSA PLH Panel, 2017); Denmark (GBIF, online); Estonia (Lutter et al., 2019) Latvia (Zeps et al., 2016); Poland and Spain (Farr and Rossman, online). | |
| Host status on Acer |
Entoleuca mammata was reported on Acer ginnala, A. rubrum, A. saccharum, A. saccarophorum and Acer sp. (Manion and Griffin, 1986; Farr and Rossman, online). There is no information on whether Entoleuca mammata can also infect Acer pseudoplatanus. |
|
| PRA information | Pest risk assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
Entoleuca mammata is an ascomycete fungus known as an important agent of canker disease in Populus species, mostly Populus tremuloides and P. tremula; other hardwood species like Salix spp. can also be infected (EFSA PLH Panel, 2017). |
|
|
The pathogen is native to North America and was introduced into Europe several centuries ago (Kasanen et al., 2004); it is now largely spread in the temperate zones of the northern hemisphere. Entoleuca mammata is present in Canada and in some northern states of the US (Alaska, Iowa, Michigan, Minnesota, Montana, New Hampshire, New York, Wisconsin). In Europe, in addition to the 19 mentioned EU MS (see above), it is reported in Andorra, Bosnia and Herzegovina, Montenegro, North Macedonia, Russia (Southern Russia and Western Siberia) Serbia, Switzerland, Ukraine and the UK (CABI, online; EPPO, online_b) and Norway (NBIC, online). The presence of E. mammata in Australia should be considered doubtful, as it is limited to a few specimens in herbarium without other records (EPPO, online_b). The ascospores of E. mammata can infect the living wood of the hosts penetrating in the periderm and invading tissues under healthy bark and through mechanical wounds, as well as through injuries caused by woodpeckers and insects, in particular the North American cerambycid beetles (mostly Saperda inornata and Oberea spp.) (Anderson et al., 1979a) and the cicada Magicicada septendedecim (Ostry and Anderson, 1983) not occurring in Europe; water stress can increase host susceptibility (EFSA PLH Panel, 2017). Entoleuca mammata is mostly found on trees 15–40 years old, but all ages can be infected (EFSA PLH Panel, 2017; EPPO online_c). Infection usually starts from branches and twigs and then can spread to the main stem. The cankers expand very rapidly (7–8 cm per month) in summer and more slowly during winter; branches and stems can be girdled causing drying and breakage. The pathogen mostly develops in the range from 8 to 32°C, the optimum temperature is 28°C; toxins host‐specific produced by the fungus are involved in pathogenesis (Stermer et al., 1984; EFSA PLH Panel, 2017; EPPO, online_c). Entoleuca mammata overwinters in host tissues as both mycelium and spores. 5 to 14 months after infection conidia are produced, but their role in the disease transmission is considered not relevant (EFSA PLH Panel, 2017). The pathogen spreads over long distances via windborne ascospores, which are produced only 2–3 years after infection; cankers on felled trees on the ground can continue to produce ascospores for 23 months. Ascospores are dispersed with a temperature above −4°C and wet weather; a minimum of 16°C is required for starting germination, which became rapid at 28–32°C (EFSA PLH Panel, 2017). Infected wood, mostly with bark, may be a pathway for passive spread of E. mammata in international trade; however, also young plants may carry ascospores or mycelium of the fungus, which can exist as a latent infection on living material inadvertently moved (EFSA PLH Panel, 2017; EPPO online_c). |
||
| Symptoms | Main type of symptoms |
The symptoms are observed on Populus trees. Early symptoms of cankers on the bark appear as slightly sunken, yellowish‐orange areas with an irregular border. Young cankers can be easily identified by removing the bark to expose the white mycelium in the cambial zone. The outer bark in older cankers is then lifted into blister‐like patches and break away, exposing blackened areas prominently visible on green branches and trunks. Callus formation only occasionally develops because cankers spread very quickly (Anderson et al., 1979b; EPPO, online_c). Wilting of leaves may be observed when the trees are girdled, as well as sprouting of new shoots on stem and branches. Infected trees can be secondarily colonised by other fungi, accelerating the host decline (EPPO, online_c). There is no information on the symptoms caused to Acer plants. |
| Presence of asymptomatic plants | The disease caused by E. mammata has a latent period and symptoms can appear only 2 years after the ascospore infection; therefore, asymptomatic plants can be found (Ostry and Anderson, 2009). | |
| Confusion with other pests |
Some Hypoxylon species present in Europe on deciduous trees (H. confluens and H. udum) show symptoms similar to those of E. mammata but can be easily distinguished in laboratory by the ascospore characteris tics (EFSA PLH Panel, 2017). |
|
| Host plant range |
In North America, Entoleuca mammata mainly infects quacking aspen (Populus tremuloides); minor damage is recorded on P. grandidentata, P. balsamifera and various Populus hybrids. Other secondary hosts in North America are Acer, Alnus, Betula, Carpinus, Fagus, Picea, Pyrus, Salix, Sorbus and Ulmus (Manion and Griffin, 1986). In Europe, the main host is Populus tremula; other hosts are Populus alba, P. nigra, P. trichocarpa and the hybrid P. tremula x P. tremuloides (Ostry, 2013). |
|
| Reported evidence of impact |
Entoleuca mammata is an important pathogen of poplars in the US and Canada, causing economic losses of millions of dollars a year (Anderson et al., 1979b; Ostry, 2013; EFSA PLH Panel, 2017). In Europe Entoleuca mammata is known as a pest of low importance, although damage on Populus tremula has been reported in France (Pinon, 1976), Italy and Sweden (EFSA PLH Panel, 2017). Data on the incidence and impact of E. mammata on other woody species, Acer included, is not available and may be considered negligible. |
|
| Evidence that the commodity is a pathway | Plants for planting may carry ascospores and mycelium of E. mammata also as asymptomatic plants (EFSA PLH Panel; EPPO online_c); therefore, the commodity is a pathway. | |
| Surveillance information | Entoleuca mammata is not a regulated pest for Great Britain and it is not under official control and surveillance in the UK (Dossier Sections 3.0 and 5.0). | |
A.4.2. Possibility of pest presence in the nursery
A.4.2.1. Possibility of entry from the surrounding environment
Entoleuca mammata is present in the UK in England, Wales, Channel Islands and Scotland (EPPO, online_b; CABI, online; Dossier Section 5.0).
The pathogen can naturally spread with ascospores dispersed by air currents also over long distance.
Entoleuca mammata can infect Acer spp., Alnus spp., Betula spp., Fagus spp., Picea spp., Populus spp., Salix spp., Sorbus spp. and Ulmus spp. which are present within 2 km from the nurseries.
Uncertainties:
-
–
The presence of the pathogen on host plants in the surrounding area.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for Entoleuca mammata to enter the nurseries from surrounding environment via ascospores transported by wind and air currents.
A.4.2.2 Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0).
In addition to Acer plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are suitable hosts for the pathogen such as Alnus spp., Betula spp., Carpinus spp., Fagus spp., Picea spp., Populus tremula, Populus spp., Pyrus spp., Salix spp., Sorbus spp. and Ulmus spp. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). The growing media are certified and heat treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0). There is no evidence that soil or growing media may be a pathway for Entoleuca mammata.
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries via new seedlings of Acer and plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.4.2.3. Possibility of spread within the nursery
Acer plants are either grown in containers (cells, pots, tubes, etc.) outdoors, in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pathogen can infect other suitable plants, such as Acer spp., Alnus spp., Betula spp. etc. present within the nurseries (Dossier Sections 3.0 and 6.0).
Once entered, ascospores of Entoleuca mammata could be produced on infected plants and naturally spread within the nurseries by air currents.
Uncertainties:
-
–
Whether ascospores are produced on infected nursery plants.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible by air currents.
A.4.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Entoleuca mammata between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.4.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Entoleuca mammata is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of the presence of the pathogen on the commodity.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of the presence of the pathogen on the commodity.
Uncertainties:
|
| 4 | Growing media | No | Not relevant. |
| 5 | Surveillance, monitoring and sampling | Yes |
This measure could have some effect.
Uncertainties:
|
| 6 | Hygiene measures | No |
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This measure could have some effect.
Uncertainties:
|
| 8 | Irrigation water | No | Not relevant. |
| 9 | Application of pest control products | Yes |
Although E. mammata is generally not a target of the pesticide treatments in the nurseries, some fungicides could reduce the likelihood of the infection by the pathogen.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
This measure could have some effect.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.4.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.4.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Acer spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.4.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Acer spp. to be hosts for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.4.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection through mechanical wounds. Acer spp. are considered minor hosts.
A.4.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.4.5.5 Elicitation outcomes of the assessment of the pest freedom for Entoleuca mammata on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infection (Table A.17) and pest freedom (Table A.18).
Table A.17.
Elicited and fitted values of the uncertainty distribution of pest infection by Entoleuca mammata per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 8 | 15 | 35 | 70 | ||||||||||
| EKE | 0.117 | 0.365 | 0.865 | 2.06 | 3.96 | 6.68 | 9.79 | 17.3 | 27.1 | 33.3 | 41.0 | 49.1 | 57.7 | 64.1 | 70.1 |
The EKE results are the BetaGeneral (0.80639, 2.2251, 0, 82) distribution fitted with @Risk version 7.6.
Table A.18.
The uncertainty distribution of bundles free of Entoleuca mammata per 10,000 bundles calculated by Table A.17
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,930 | 9,965 | 9,985 | 9,992 | 10,000 | ||||||||||
| EKE results | 9,930 | 9,936 | 9,942 | 9,951 | 9,959 | 9,967 | 9,973 | 9,983 | 9,990 | 9,993 | 9,996 | 9,998 | 9,999.1 | 9,999.6 | 9,999.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.18.
A.4.6. Overall likelihood of pest freedom for bare root plants/trees and plants in pots up to 7 years old
A.4.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees and plants in pots up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Acer spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.4.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees and plants in pots up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Acer spp. to be hosts for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.4.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees and plants in pots up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection through mechanical wounds. Acer spp. are considered minor hosts.
A.4.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.4.6.5. Elicitation outcomes of the assessment of the pest freedom for Entoleuca mammata on bare root plants/trees and plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infection (Table A.19) and pest freedom (Table A.20).
Table A.19.
Elicited and fitted values of the uncertainty distribution of pest infection by Entoleuca mammata per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 10 | 20 | 40 | 100 | ||||||||||
| EKE | 0.418 | 0.987 | 1.90 | 3.72 | 6.20 | 9.44 | 12.9 | 21.1 | 31.8 | 38.9 | 48.4 | 59.5 | 73.3 | 85.6 | 100 |
The EKE results are the BetaGeneral (1.0764, 6.8505, 0, 200) distribution fitted with @Risk version 7.6.
Table A.20.
The uncertainty distribution of plants free of Entoleuca mammata per 10,000 plants calculated by Table A.19
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,900 | 9,960 | 9,980 | 9,990 | 10,000 | ||||||||||
| EKE results | 9,900 | 9,914 | 9,927 | 9,940 | 9,952 | 9,961 | 9,968 | 9,979 | 9,987 | 9,991 | 9,994 | 9,996 | 9,998 | 9,999.0 | 9,999.6 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.20.
A.4.7. Reference list
Anderson NA, Ostry ME and Anderson GW, 1979a. Insect wounds as infection sites for Hypoxylon mammatum on trembling aspen. Phytopathology 69, 476–479. https://doi.org/10.1094/phyto-69-476
Anderson RL, Anderson GW and Schipper AL Jr, 1979b. Hypoxylon canker of aspen. USDA Forest Insect and Disease Leaflet, 6, 6 pp.
CABI (Centre for Agriculture and Bioscience International), online. Hypoxylon mammatum (poplar canker). Available online: https://www.cabi.org/cpc/datasheet/28323 [Accessed: 12 October 2022].
DEFRA (Department for Environment, Food and Rural Affairs), online. UK risk register details for Entoleuca mammata. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=11840 [Accessed: 26 February 2023].
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Boberg J, Gonthier P and Pautasso M, 2017. Scientific Opinion on the pest categorisation of Entoleuca mammata. EFSA Journal 2017;15(7):4925, 25 pp. https://doi.org/10.2903/j.efsa.2017.4925
EPPO (European and Mediterranean Plant Protection Organization), online_a. Entoleuca mammata (HYPOMA), Categorization. Available online: https://gd.eppo.int/taxon/HYPOMA/categorization [Accessed: 26 February 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Entoleuca mammata (HYPOMA), Distribution. Available online: https://gd.eppo.int/taxon/HYPOMA/distribution [Accessed: 26 February 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Datasheets on Quarantine Pests: Hypoxylon mammatum (HYPOMA), Documents. Available online: https://gd.eppo.int/taxon/HYPOMA/documents [Accessed: 26 February 2023].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
Farr DF and Rossman AY. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases [Accessed: 12 October 2022].
GBIF (Global Biodiversity Information Facility) Secretariat, online. GBIF BackBone Taxonomy. Available online: https://www.gbif.org/ [Accessed: 24 February 2023].
Kasanen R, Hantula J, Ostry ME, Pinon J and Kurkela T, 2004. North American populations of Entoleuca mammata are genetically more variable than populations in Europe. Mycological Research, 108, 766–774. https://doi.org/10.1017/s0953756204000334
Klejdysz T, Kubasik W, Strażyński P, Gawlak M, Pruciak A, Rzepecka D, Kałuski T, online. Express pest risk analysis for Hypoxylon mammatum. Available online: https://www.plantquarantine.pl//user_storage/36/pliki/pra_133/Entoleuca%20mammata.pdf [Accessed: 2 March 2023].
Lutter R, Drenkhan R, Tullus A, Jürimaa K, Tullus T and Tullus H, 2019. First record of Entoleuca mammata in hybrid aspen plantations in hemiboreal Estonia and stand‐environmental factors affecting its prevalence. European Journal of Forest Research, 138, 263–274. https://doi.org/10.1007/s10342-019-01165-7
Manion PD and Griffin DH, 1986. Sixty‐five years of research on Hypoxylon canker of aspen. Plant Disease, 70, 803–808. https://doi.org/10.1094/pd-70-803
NBIC (Norwegian Biodiversity Information Center) online. Entoleuca mammata. Available online: https://artsdatabanken.no/Taxon/Entoleuca%20mammata/82864 [Accessed: 27 February 2023].
Ostry ME, 2013. Hypoxylon canker. In: Gonthier P and Nicolotti G (Eds.), Infectious forest diseases. CABI International, Wallingford, 407–419.
Ostry ME and Anderson NA, 1983. Infection of trembling aspen by Hypoxylon mammatum through cicada oviposition wounds. Phytopathology, 73, 1092–1096. https://doi.org/10.1094/phyto-73-1092
Ostry ME and Anderson NA, 2009. Genetics and ecology of the Entoleuca mammata–Populus pathosystem: implications for aspen improvement and management. Forest Ecology and Management, 257, 390–400. https://doi.org/10.1016/j.foreco.2008.09.053
Pinon J, 1976. A serious threat to the Alpine aspen: Hypoxylon mammatum canker. Revue Forestière Française, 28, 30–34.
Stermer BA, Scheffer RP and Hart JH, 1984. Isolation of toxins from Hypoxylon mammatum and demonstration of some toxin effects on selected clones of Populus tremuloides. Phytopathology, 74, 654–658. https://doi.org/10.1094/phyto-74-654
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Zeps M, Adamovics A, Smilga J and Sisenis L, 2016. Productivity and quality of hybrid aspen at the age of 18 years. Research for Rural Development, 2, 55–61.
A.5. Eulecanium excrescens
A.5.1. Organism information
| Taxonomic information |
Current valid scientific name: Eulecanium excrescens Synonyms: Lecanium excrescens Name used in the EU legislation: – Order: Hemiptera Family: Coccidae Common name: excrescent scale, wisteria scale Name used in the Dossier: Eulecanium excrescens |
|
| Group | Insects | |
| EPPO code | – | |
| Regulated status |
The pest is neither regulated in the EU nor listed by EPPO. Eulecanium excrescens is listed in the UK Plant Health Risk Register but archived in 2020 as considered to pose a low risk to the UK (DEFRA, online). |
|
| Pest status in the UK |
Eulecanium excrescens is present in the UK as introduced species since at least 2000 (MacLeod and Matthews, 2005 Dossier Section 5.0) with restricted distribution to the Greater London Area (e.g. Bishops Strotford, Hornchurch, London, Maidenhead, Roystone, Wickford) where it is considered an ‘established exotic pest' (MacLeod and Matthews, 2005). Outside this area, E. excrescens has been reported in a few localities of the neighbouring county of Hertfordshire (Salisbury et al., 2010) and more recently (2021) in Gloucester (GBIF, online). According to the Dossier Section 5.0, the pest is present in the UK: not widely distributed and not under official control. |
|
| Pest status in the EU | Eulecanium excrescens is absent from the EU (García Morales et al., online). | |
| Host status on Acer | Acer pseudoplatanus is a host for E. excrescens (Malumphy, 2005). | |
| PRA information | Pest Risk Assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
Eulecanium excrescens is a polyphagous soft scale native to Asia (China) and introduced in the US, where it is present in California, Connecticut, New York, Oregon and Pennsylvania (MacLeod and Matthews, 2005; Malumphy, 2005); in Europe it is present only in the UK (Malumphy, 2005; GBIF, online). It is absent from Africa, Central and South America and Oceania (García Morales et al., online). There is no scales have specific information about the number of life stages of E. excrescens. However, soft three development stages: eggs, nymphs (two or three instars) and adult (Camacho and Chong, 2015). The nymphs of E. excrescens are orange or pale brown with rectangular whitish encrustations on their surface; the first‐instar nymphs are mobile (crawlers) and disperse actively by crawling away from their mothers or passively by wind or phoresis. Both nymphs and adults of soft scales (except adult males when present) feed on twigs and leaves sucking phloem and often producing large amount of honeydew (Camacho and Chong, 2015). In the UK, E. excrescens is a parthenogenetic species and has one generation per year; the nymphs overwinter and reach maturity in April. The large globular adult females (10–13 mm) are dark brown, covered by grey powdery wax. They lay eggs in May, each female lays about 2,000 eggs. The crawlers emerge in May–June and settle to the underside of leaves; in autumn, before the leaf fall, they move from the leaves to the woody parts to overwinter (Malumphy, 2005). |
|
|
Eulecanium excrescens can spread naturally by crawling of first‐instar nymphs on very short distances, and passively via air currents and wind of the same development stage. No specific information on the natural dispersal of E. excrescens is available, which is considered ‘likely slow’ (MacLeod and Matthews, 2005). However, it is known that some soft scale crawlers may be dispersed by wind to 55 m to > 4 km (Camacho and Chong, 2015). Passive transport of nymphs on medium–long distance is also possible by phoresis mostly with birds and ants, and human‐assisted spread may occur via infested plants. |
||
| Symptoms | Main type of symptoms |
Only a non‐specific leaf loss and slow dieback may be sometimes observed as a symptom on plants strongly attacked by E. excrescens, as a result of large amount of sap removed by the scale. The main evidence of the pest attack is the presence of colonies of adults and nymphs producing honeydew on the stem and branches. Adults of E. excrescens are large size (10–13 mm in length/diameter), and this make the pest easy to detect, since no other soft scale species raises that size in the UK. |
| Presence of asymptomatic plants | There is no information on the presence of asymptomatic plants, although initial infestations caused by a few insects may go unnoticed. | |
| Confusion with other pests | In the UK, other six species of Eulecanium are found (Malumphy, 2005) but none raises the size of adult females of E. excrescens, therefore no confusion with other species is possible at that stage. The nymphs may be confused with those of other Eulecanium, as well as with other soft scale species and need to be examined in laboratory for identification with the support of diagnostic keys. | |
| Host plant range |
Eulecanium excrescens feeds on a wide host plant range including Acer pseudoplatanus, Juglans regia, Malus spp., Prunus dulcis, P. armeniaca, P. persica, Prunus spp., Pyrus communis, Ulmus spp. and Zelkova serrata. In the UK, the scale also attacks some vines species as Ceanothus sp., Podranea ricasoliana, Parthenocyssus quinquefolia, P. tricuspidata and mostly Wisteria spp. The latter appears to be the preferred host genus as it is in the US (Malumphy, 2005; Salisbury et al., 2010). |
|
| Reported evidence of impact |
Eulecanium excrescens is a sap sucker able to damage host plants by removing large quantities of sap, so causing weakening, leaf loss and dieback; large amount of honeydew is also produced, reducing photosynthesis and disfiguring ornamental plants in parks and gardens (MacLeod and Matthews, 2005). In China, E. excrescens is regarded as a pest damaging fruit orchards of Malus, Prunus and Pyrus (MacLeod and Matthews, 2005). In the US, the scale is included in the list of pests harmful to hazelnut (Corylus avellana) production in Oregon (Murray and Jepson, 2018); not regarded as a pest of economic importance in California (MacLeod and Matthews, 2005). Impact on ornamental Wisteria may be relevant in case of heavy infestation, as these vines are often cultivated in gardens and for covering walls and buildings in the UK. No information on damage on Acer was found. |
|
| Evidence that the commodity is a pathway | The pest occurs on bark. Although the source of infestations in the UK is unknown (MacLeod and Matthews, 2005) and no interception is recorded (EUROPHYT/TRACES, online), plants for planting (except seeds bulbs and tubers) are pathways for E. excrescens according to DEFRA (online). The commodities including dormant plants are pathways for the pest. | |
| Surveillance information | Eulecanium excrescens is not under official control and surveillance in the UK, as does not meet criteria of quarantine pest for Great Britain (Dossier Section 5.0). | |
A.5.2. Possibility of pest presence in the nursery
A.5.2.1. Possibility of entry from the surrounding environment
Eulecanium excrescens is present in the UK, with restricted distribution to the Great London area and some findings in Hertfordshire and Gloucester (MacLeod and Matthews, 2005; Salisbury et al., 2010; GBIF, online; Dossier Section 5.0).
Eulecanium excrescens has limited ability of active spreading, but the first‐instar nymphs of the pest (crawlers) may be naturally dispersed by phoresis and wind over distances of some kilometres.
Eulecanium excrescens can feed on Acer pseudoplatanus, Juglans regia, Prunus spp., Ulmus spp. and Wisteria spp. which are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
The presence of the pest on host plants in the surrounding area.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries from surrounding environment via first‐instar nymphs (crawlers) transported by wind and birds.
A.5.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0).
In addition to Acer plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are suitable hosts for the pathogen such as Juglans regia, Malus spp., Prunus, Pyrus, Ulmus and Wisteria sinensis. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pest could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). The growing media are certified and heat treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0). There is no evidence that soil or growing media may be a pathway for E. excrescens.
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries via new seedlings of Acer and plants of other species used for plant production in the area. The entry of the pest with seeds and the growing media the Panel considers as not possible.
A.5.2.3. Possibility of spread within the nursery
Acer plants are either grown in containers (cells, pots, tubes, etc.) outdoors, in the open air or in field. Cell grown trees may be grown in greenhouses; however, most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pest can attack other suitable plants, such as Juglans regia, Malus, Prunus, Pyrus, Ulmus and Wisteria present within the nurseries (Dossier Sections 3.0 and 6.0).
Once entered, the pest can naturally spread within the nurseries both by crawling of the nymphs plant to plant, and by air currents and phoresis with birds and ants; nymphs could also move with equipment/clothes of the nursery staff.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nurseries is possible by crawling, air currents and phoresis.
A.5.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Eulecanium excrescens between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.5.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Eulecanium excrescens is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
Although E. excrescens is not a quarantine pest, it has been listed the UK Plant Health Risk Register until 2020 and it is easy to spot. The risk mitigation measure could have some effects in reducing the likelihood of the presence of the pest on the commodity.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. Physical separation is not a barrier for E. excrescens because crawlers can be easily spread by wind and air currents. |
| 3 | Certified plant material | Yes |
The risk mitigation measure could have some effects in reducing the likelihood of the presence of the pest on the commodity.
Uncertainties:
|
| 4 | Growing media | No | Not relevant. Eulecanium excrescens is not present in soil. |
| 5 | Surveillance, monitoring and sampling | Yes |
Incoming plant material is thoroughly checked, E. excrescens is easy to spot. The risk mitigation measure could have some effects in reducing the likelihood of the presence of the pest on the commodity.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
Nymphs could move with equipment/clothes of the nursery staff. The described measures could have very little effect in reducing the likelihood of the presence of the pest on the commodity. Eulecanium excrescens is not present on weeds.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
Removal of generically damaged plants could reduce the probability of carrying E. excrescens.
Uncertainties:
|
| 8 | Irrigation water | No | Not relevant. |
| 9 | Application of pest control products | Yes |
Chemical control measures adopted when the pest is found may have a substantial effect of pest suppression.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
Inspection should detect E. excrescens as signs are clear (honeydew).
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. Acer plants are not individually separated during transportation. |
A.5.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.5.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bundles of whips and seedlings
This scenario assumes a low pressure of the pest in the nurseries and the surrounding. In this scenario it is also assumed that plants are very small and therefore symptoms and signs are easy to spot, as small plants can be more accurate inspected. Honeydew is present and adult females are settled and big, so they are easy to be spotted during inspections, and measures against the pest can be taken.
A.5.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bundles of whips and seedlings
This scenario assumes a high pressure of the pest in the nurseries and the surrounding. It also assumes that the pest is spreading. No measures are being taken by the UK against this pest. Leaves can mask the presence of the pest on the twigs. Crawlers can move and hide and may be overlooked. The scenario assumes that low‐density populations can be overlooked during inspections, and that mode of action of the pesticides used may not be effective against E. excrescens. Finally, the scenario assumes that pest is coming from the surroundings and a bundle effect is expected to increase the number of infestations.
A.5.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bundles of whips and seedlings (Median)
The Panel assumes a high efficacy of the surveillance and inspections conducted in the nursery due to the small size of the plants and the big size of adult females. The Panel also assumes that symptoms as honeydew are easily visible and, therefore, measures against the pest can be taken. These considerations lead Panel to indicate low value of the median.
A.5.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile and a medium uncertainty above the median because symptoms because of the ease of detection of signs and symptoms of the pest, and because clustering of infestation is unknown or unclear.
A.5.5.5. Elicitation outcomes of the assessment of the pest freedom for Eulecanium excrescens on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infestation (Table A.21) and pest freedom (Table A.22).
Table A.21.
Elicited and fitted values of the uncertainty distribution of pest infestation by Eulecanium excrescens per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 12 | 25 | 60 | 125 | ||||||||||
| EKE | 2.00 | 2.19 | 2.67 | 4.04 | 6.54 | 10.5 | 15.4 | 28.2 | 45.8 | 57.3 | 71.7 | 87.1 | 103 | 115 | 126 |
The EKE results are the BetaGeneral (0.66731, 2.0139, 1.93, 145) distribution fitted with @Risk version 7.6.
Table A.22.
The uncertainty distribution of bundles free of Eulecanium excrescens per 10,000 bundles calculated by Table A.21
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,875 | 9,940 | 9,975 | 9,988 | 9,998 | ||||||||||
| EKE results | 9,874 | 9,885 | 9,897 | 9,913 | 9,928 | 9,943 | 9,954 | 9,972 | 9,985 | 9,989 | 9,993 | 9,996 | 9,997.3 | 9,997.8 | 9,998.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested bundles, the pest freedom was calculated (i.e. = 10,000 – number of infested bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.22.
A.5.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.5.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants/trees up to 7 years old
This scenario assumes a low pressure of the pest in the nurseries and the surrounding. In this scenario, it is also assumed that symptoms are easy to spot as honeydew is present and adult females are settled and big. Therefore, signs and symptoms are easy to be spotted during inspections, and measures against the pest can be taken.
A.5.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants/trees up to 7 years old
This scenario assumes a high pressure of the pest in the nurseries and the surrounding. It also assumes that the pest is spreading and that no measures are being taken by the UK against this pest. The scenario assumes that low‐density populations can be overlooked during inspections, that leaves can mask the presence of the pest on the twigs, and that mode of action of the pesticides used may not be effective against E. excrescens.
A.5.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants/trees up to 7 years old (Median)
The Panel assumes a high efficacy of the surveillance and inspections conducted in the nursery due to big size of adult females. The Panel also assumes that symptoms as honeydew are easily visible and, therefore, measures against the pest can be taken. These considerations lead Panel to indicate low value of the median.
A.5.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because of the ease of detection of signs and symptoms of the pest and because of the effectiveness of measures conducted to control the pest in the nursery.
A.5.6.5. Elicitation outcomes of the assessment of the pest freedom for Eulecanium excrescens on bare root plants/trees up to 7 years old
The following tables show the elicited and fitted values for pest infestation (Table A.23) and pest freedom (Table A.24).
Table A.23.
Elicited and fitted values of the uncertainty distribution of pest infestation by Eulecanium excrescens per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 12 | 25 | 45 | 100 | ||||||||||
| EKE | 2.01 | 2.57 | 3.52 | 5.48 | 8.24 | 11.9 | 15.9 | 25.2 | 37.1 | 44.8 | 54.7 | 65.7 | 78.5 | 89.0 | 100 |
The EKE results are the BetaGeneral (0.99307, 3.6847, 1.65, 140) distribution fitted with @Risk version 7.6.
Table A.24.
The uncertainty distribution of plants free of Eulecanium excrescens per 10,000 plants calculated by Table A.23
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,900 | 9,955 | 9,975 | 9,988 | 9,998 | ||||||||||
| EKE results | 9,900 | 9,911 | 9,922 | 9,934 | 9,945 | 9,955 | 9,963 | 9,975 | 9,984 | 9,988 | 9,992 | 9,995 | 9,996 | 9,997 | 9,998 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.24.
A.5.7. Overall likelihood of pest freedom for plants in pots up to 7 years old
A.5.7.1. Reasoning for a scenario which would lead to a reasonably low number of infested plants in pots up to 7 years old
This scenario assumes a low pressure of the pest in the nurseries and the surrounding. In this scenario, it is also assumed that symptoms and signs are easy to be spotted, as honeydew is present and adult females are settled and big. Therefore, inspections can be effective and measures against the pest can be taken consequently. Finally, the scenario assumes that the commodity is rarely exported with leaves, period when active crawlers and recent infestations could lead to undetected infestations.
A.5.7.2. Reasoning for a scenario which would lead to a reasonably high number of infested plants in pots up to 7 years old
This scenario assumes a high pressure of the pest in the nurseries and the surrounding. It also assumes that the pest is spreading and that no measures are being taken by the UK against this specific pest. The scenario assumes that low‐density populations can be overlooked during inspections, that leaves can mask the presence of the pest on the twigs, and that mode of action of the pesticides used may not be effective against E. excrescens. The scenario assumes that the commodity can be exported any time of the year and with leaves, so period when crawlers are active and recent infestations could lead to undetected infestations. Finally, the scenario assumes that the pest can be hidden and not detected in the bigger trees with large canopy, compared to the previous commodities.
A.5.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested plants in pots up to 7 years old (Median)
The Panel assumes a high efficacy of the surveillance and inspections conducted in the nursery due to big size of adult females. The Panel also assumes that symptoms as honeydew are easily visible and, therefore, measures against the pest can be taken. These considerations lead Panel to indicate low value of the median.
A.5.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because of the ease of detection of signs and symptoms of the pest, and because of the effectiveness of measures conducted to control the pest in the nursery.
A.5.7.5. Elicitation outcomes of the assessment of the pest freedom for Eulecanium excrescens on plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infestation (Table A.25) and pest freedom (Table A.26).
Table A.25.
Elicited and fitted values of the uncertainty distribution of pest infestation by Eulecanium excrescens per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 3 | 15 | 30 | 60 | 130 | ||||||||||
| EKE | 3.00 | 3.48 | 4.39 | 6.48 | 9.69 | 14.2 | 19.3 | 31.6 | 47.9 | 58.5 | 71.9 | 86.9 | 104 | 117 | 131 |
The EKE results are the BetaGeneral (0.85608, 2.9928, 2.75, 170) distribution fitted with @Risk version 7.6.
Table A.26.
The uncertainty distribution of plants free of Eulecanium excrescens per 10,000 plants calculated by Table A.25
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,870 | 9,940 | 9,970 | 9,985 | 9,997 | ||||||||||
| EKE results | 9,869 | 9,883 | 9,896 | 9,913 | 9,928 | 9,942 | 9,952 | 9,968 | 9,981 | 9,986 | 9,990 | 9,994 | 9,995.6 | 9,996.5 | 9,997.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.26.
A.5.8. Reference list
Camacho ER and Chong JH, 2015. General biology and current management approaches of soft scale pests (Hemiptera: Coccidae). Journal of Integrated Pest Management, 6, 1–22. https://doi.org/10.1093/jipm/pmv016
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Eulecanium excrescens. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=23301 [Accessed: 28 February 2023].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Gonthier P, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Zappalà L, Lucchi A, Gómez P, Urek G, Bernardo U, Bubici G, Carluccio AV, Chiumenti M, Di Serio F, Fanelli E, Marzachì C, Kaczmarek A, Mosbach‐Schulz O and Yuen J, 2023. Scientific Opinion on the commodity risk assessment of Malus domestica plants from United Kingdom. EFSA Journal 2023;21(5):8002, 146 pp. https://doi.org/10.2903/j.efsa.2023.8002
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: A literature‐based model of scale insect biology and systematics, Eulecanium excrescens. Available online: http://scalenet.info/catalogue/eulecanium%20excrescens/ [Accessed 28 February 2023].
GBIF (Global Biodiversity Information Facility) Secretariat, online. GBIF BackBone Taxonomy. Available online: https://www.gbif.org/ [Accessed: 28 February 2023].
MacLeod A and Matthews L, 2005. Pest risk analysis for Eulecanium excrescens. CSL, Central Science Laboratory, UK. 7 pp.
Malumphy CP, 2005. Eulecanium excrescens (Ferris) (Hemiptera: Coccidae), an Asian pest of woody ornamentals and fruit trees, new to Britain. British Journal of Entomology and Natural History, 18, 45–49.
Murray K and Jepson P, 2018. An integrated pest management strategic plan for hazelnuts in Oregon and Washington. Oregon State University, 57 pp.
Salisbury A, Halstead A and Malumphy C, 2010. Wisteria scale, Eulecanium excrescens (Hemiptera: Coccidae) spreading in South East England. British Journal of Entomology and Natural History, 23, 225–228.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
A.6. Meloidogyne fallax
A.6.1. Organism information
| Taxonomic information |
Current valid scientific name: Meloidogyne fallax Synonyms: Meloidogyne chitwoodi (Baexem) B‐type Name used in the EU legislation: Meloidogyne fallax Karssen [MELGFA] Order: Rhabditida Family: Meloidogynidae Common name: false Columbia root‐knot nematode, root gall nematode Name used in the Dossier: Meloidogyne fallax |
|
| Group | Nematodes | |
| EPPO code | MELGMA | |
| Regulated status |
The pest is listed in Annex II of Regulation (EU) 2019/2072 as Meloidogyne fallax Karssen [MELGFA]. The pest is included in the EPPO A2 list (EPPO, online_a). Meloidogyne fallax is quarantine in Morocco, Moldova and Norway. It is on A1 list of Argentina, Bahrain, Brazil, Egypt, Georgia, Kazakhstan, Russia, Ukraine and EAEU (=Eurasian Economic Union – Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia). It is on A2 list of COSAVE (=Comite de Sanidad Vegetal del Cono Sur – Argentina, Brazil, Chile, Paraguay, Peru and Uruguay) (EPPO, online_b). M. fallax is also quarantine pest in the US (Kantor et al., 2022). In the UK, M. fallax is regulated non‐quarantine pest in Great Britain on potato only, as this is considered to be the main host at risk (EPPO, online_b; DEFRA, online), and it is regulated quarantine pest in Northern Ireland (DEFRA, online; Dossier Section 5.0). |
|
| Pest status in the UK | Meloidogyne fallax is present in the UK (CABI, online; EPPO, online_c) with a restricted distribution in England and Wales and no findings associated to trees (Dossier Sections 2.0, 3.0 and 5.0). M. fallax was also detected in Northern Ireland in 2011 (EPPO, 2015); however, its presence there is currently not confirmed (Dossier Section 5.0). | |
| Pest status in the EU |
Meloidogyne fallax is present in Belgium, France, the Netherlands and Sweden; in the latter country, it is under eradication (DEFRA, online; EPPO, online_c,d). It is transient and under eradication in Germany (CABI, online; EPPO, online_c). M. fallax was present in Ireland in the past century (1965) (Topalović et al., 2017), but it has not been reported since. |
|
| Host status on Acer |
Acer palmatum is reported as a host plant for Meloidogyne fallax in field experiments (den Nijs et al., 2004). There is no information on whether M. fallax can also infest Acer pseudoplatanus. However given the polyphagous nature of M. fallax the panel cannot exclude that A. pseudoplatanus could be a hostplant for the pathogen |
|
| PRA information | Available Pest Risk Assessments:
|
|
| Other relevant information for the assessment | ||
| Biology |
Meloidogyne fallax is a highly polyphagous root‐gall nematode firstly described from the Netherlands and mostly distributed in temperate regions of the world. It is present in Africa (South Africa), Asia (Indonesia), Europe (Belgium, France, Germany, the Netherlands, Switzerland, Sweden, UK), Oceania (Australia, New Zealand), South America (Chile) (CABI, online; EPPO, online_c) According to MacLeod et al. (2012), M. fallax may be more widespread because it is frequently confused with similar species as M. hapla and M. chitwoodi, and not causing clear external symptoms on host plants. Meloidogyne fallax has three development stages: eggs, juveniles (four stages) and adults. The nematode mainly reproduces parthenogenetically, and sexual reproduction can possibly occur under adverse conditions; like other Meloidogyne species, M. fallax has 1–3 generations per year depending on temperature and host availability (MacLeod et al., 2012; EFSA, 2019). Females lay up to 800–1,000 eggs in gelatinous masses on the root surface, in galls and tubers. Hatching can occur at temperatures below 10°C, so that M. fallax is considered cryophilic (MacLeod et al., 2012; EFSA PLH Panel, 2020). The second‐stage juveniles move in the soil and penetrate host roots, start feeding on cortical tissues inducing the formation of root galls; they become sedentary and develop to successive stages by quick moults. The nematode can stay infective in the soil for long time, being also able to survive for more than 300 days at temperatures of 5 and 10°C, and 140 days at higher temperatures (15–25°C). Survival and infectivity may be also related to high soil humidity (100% survival with 98% RH) although in moderate dry soil conditions, M. fallax may survive for more than 9 weeks (MacLeod et al., 2012). As other nematode species living in the soil, Meloidogyne fallax has only little spread capacity, the juvenile stages moving 1–2 m maximum per year depending on type of soil, water availability and other parameters (EFSA, 2019). Water could also disperse the nematode (mainly eggs and juveniles) at short distances. The human‐assisted spread on medium–long distance is very frequent and effective by passive transport. Possible pathways are mainly plants for planting with infected roots; tubers and bulbs; soil and growing media; contaminated tools, machinery, shoes and packaging material (EFSA, 2019). |
|
| Symptoms | Main type of symptoms |
Meloidogyne fallax is a root‐knot nematode. Heavily infested plants show stunting and yellowing on above‐ground parts and galling on roots (Moens et al., 2009; MacLeod et al., 2012; EFSA, 2019). Symptoms of root‐knot nematodes on hardwood trees may show as slow growth, sparse foliage, chlorotic leaves and crown dieback (Riffle, 1963). Symptoms on roots vary with species but should be visible as galls in advanced infections. On potato tubers, M. fallax cause numerous small pimple‐like areas on the surface (EPPO, 2019). No specific information about symptoms on Acer sp. was found. |
| Presence of asymptomatic plants | At the early stages of infection, plants may not show any apparent symptoms on the above ground parts and do not show galls on the roots. In some cases, plants are wilted and lack vigour. The main impact of the pest is on root growth, and on the quality and growth of the plant (Moens et al., 2009; MacLeod et al., 2012; EFSA, 2019). | |
| Confusion with other pests |
Meloidogyne fallax is morphologically very similar to M. chitwoodi and may also be easily confused with other species as M. hapla and M. minor, often found in the same habitat. M. fallax cannot be identified on the basis of sole galls, since other soil nematode cause similar damage and some insects and bacteria can induce comparable galls on roots as well (EFSA, 2019). The nematode can be identified by laboratory tests on morphometric characters, electrophoresis or sequencing /DNA barcoding are needed (EPPO, 2016). |
|
| Host plant range |
Meloidogyne fallax is a polyphagous nematode with a wide host range, including several major horticultural and agricultural crops and a few species of trees, shrubs and herbaceous plants. Main horticultural/agricultural hosts are: Apium graveolens, Allium porrum, Asparagus officinalis, Avena strigosa, Beta vulgaris, Cicorium endivia, Cynara scolymus, Daucus carota, Foeniculum vulgare, Fragaria ananassa, Hordeum vulgare, Lactuca sativa, Lycopersicum esculentum, Medicago sativa, Phaseolus vulgaris, Secale cereale, Solanum nigrum, S. tuberosum, Solanum spp., Triticum aestivum and Zea mays (MacLeod et al., 2012; CABI, online; EPPO, online_e). Woody hosts of M. fallax are Acer palmatum, Betula pendula, Cornus sanguinea, Laburnum anagyroides, Lonicera xylosteum (MacLeod et al., 2012; Ferris, online). For a more exhaustive list of hosts, see de Nijs et al. (2004); MacLeod et al. (2012); CABI (online); EPPO (online_e) and Ferris (online). |
|
| Reported evidence of impact |
Meloidogyne fallax is EU quarantine pest. Meloidogyne fallax is known as species of economic concern on some horticultural crops as potato and carrot, mostly in the Netherlands, but no information is available on yield losses. The main damage observed is the reduction of merchantability in potato tubers (MacLeod et al., 2012). Similarly, no significant damage was observed on strawberries (van der Sommen et al., 2005). Few reports demonstrate the impact of M. fallax on yield or quality of plant products. The nematode species has been reported to be of economic importance on potato and carrot in the Netherlands, but no data were given on yield losses in practice (MacLeod et al., 2012). Damage caused by M. fallax in sports turf were reported in North‐western England in 2015 (EPPO, 2015; DEFRA, 2017). No specific data about damage on Acer sp. was found. |
|
| Evidence that the commodity is a pathway | Meloidogyne nematodes, although rarely identified at species level, are frequently intercepted on plants for planting, including bonsais of Acer palmatum (EUROPHYT/TRACES, online); therefore, the commodity is a possible pathway of entry for M. fallax. | |
| Surveillance information | According to the Dossier Section 3.0 and 5.0, Meloidogyne fallax is not under official surveillance in the UK, as does not meet criteria of quarantine pest for Great Britain. | |
A.6.2. Possibility of pest presence in the nursery
A.6.2.1. Possibility of entry from the surrounding environment
Meloidogyne fallax is present in the UK territory with restricted distribution in England and Wales in agricultural lands and sports turf (Dossier Section 3.0 and 5.0).
The nematode has very limited capacity of movement in the soil (1–2 m) and can only spread by passive transport human assisted with plants for planting with infected roots, infected soil and growing media, and possibly via contaminated tools and machinery. No other possibility of entry in the nurseries is known.
Meloidogyne fallax can infect Allium porrum, Beta vulgaris, Betula, Daucus carota, Hordeum vulgare, Solanum lycopersicum and S. tuberosum which are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
Pest presence and pressure in the surrounding
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible for Meloidogyne fallax to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present, but the nematode cannot enter by other way than human assisted spread.
A.6.2.2. Possibility of entry with new plants/seed
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0). Seeds are not a pathway for the nematode.
In addition to Acer, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are some suitable hosts for the nematode (such as Betula pendula, Cornus sanguinea, Laburnum anagyroides and Lonicera xylosteum). However, there is no information on how and where the plants are produced. Besides, M. fallax may also spread on soil adhering to the roots of non‐host plants (MacLeod et al., 2012). Therefore, if the plants are first produced in another nursery, the nematode could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). Meloidogyne fallax is able to survive in the soil for long time and therefore could potentially enter with infested soil/growing media. However, the growing media are certified and heat treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the nematode to enter the nurseries via infected roots of new seedlings of Acer and plants of other species used for plant production in the area. The entry of the nematode with seeds and the growing media the Panel considers as not possible.
A.6.2.3. Possibility of spread within the nursery
Acer plants are either grown in containers (cells, pots, tubes, etc.) outdoors in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The nematode can infect other suitable plants such as Betula pendula, Cornus sanguinea, Laburnum anagyroides and Lonicera xylosteum, present within the nurseries (Dossier Sections 3.0 and 6.0).
Meloidogyne fallax can spread within the nurseries by movement of soil, water, infested plant material and infected tools, contaminated shoes and machinery. Tools used in the nurseries are disinfected after operation on a stock and before being used on a different plant species (Dossier Section 3.0); however, no information is available on the measures to reduce the risk of contamination of machinery, shoes or other material (i.e. package, bags, etc.).
Uncertainties:
-
–
Possibility that the pest can spread via contaminated soil adhering to shoes, machinery or other material.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the nematode within the nurseries is possible either by movement of infested soil (also via machinery, shoes and other material) water and plant material.
A.6.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Meloidogyne fallax between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.6.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Meloidogyne fallax is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
As the plant passport is very similar to the EU one, the plants shall be free from quarantine pests and RNQPs.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
Seedlings could be a pathway for the nematode.
Uncertainties:
|
| 4 | Growing media | Yes |
Heat treatment and protection of the treated growing media is effective against the nematode.
Uncertainties:
|
| 5 | Surveillance, monitoring and sampling | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Uncertainties:
|
| 9 | Application of pest control products | No | Not relevant. No nematicides are used in the nurseries. |
| 10 | Measures against soil pests | Yes |
Separation of the pots from soil is effective against the nematode.
Uncertainties:
|
| 11 | Inspections and management of plants before export | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. The nematode cannot spread between the roots of the plants when transported to the EU. |
A.6.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.6.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pest in the nurseries and in the surroundings. The plants are exposed to the nematode for only short period of time. The scenario also assumes that root galls are visible while inspecting plants before export and that the second juvenile stage are washed away during the root washing.
A.6.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pest in the nurseries and in the surroundings as many potential hosts are present. The scenario also assumes that root galls are not easily recognisable while inspecting plants before export and that the low‐pressure washing is not effective in removing the second juvenile stage before export.
A.6.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pest in the nurseries and the surroundings and that the plants are exposed to the nematode for only short period of time. The movement of soil from the surrounding into the nurseries is not expected to be significant.
A.6.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pests in the UK including the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.6.5.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne mali and M. fallax on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infection (Table A.27) and pest freedom (Table A.28).
Table A.27.
Elicited and fitted values of the uncertainty distribution of pest infection by Meloidogyne mali and M. fallax per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 3 | 25 | 40 | 60 | 150 | ||||||||||
| EKE | 5.507 | 8.116 | 11.04 | 15.31 | 19.91 | 24.97 | 29.82 | 39.98 | 52.2 | 60.1 | 70.5 | 83.0 | 99.1 | 114.4 | 133.9 |
The EKE results are the BetaGeneral (2.6372, 576.47, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.28.
The uncertainty distribution of bundles free of Meloidogyne mali and M. fallax per 10,000 bundles calculated by Table A.27
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,940 | 9,960 | 9,975 | 9,997 | ||||||||||
| EKE results | 9,866 | 9,886 | 9,901 | 9,917 | 9,929 | 9,940 | 9,948 | 9,960 | 9,970 | 9,975 | 9,980 | 9,985 | 9,989 | 9,992 | 9,994 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles, the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.28.
A.6.6. Overall likelihood of pest freedom for bare root plants/trees and plants in pots up to 7 years old
A.6.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees and plants in pots up to 7 years old
The scenario assumes a low pressure of the pest in the nurseries and in the surroundings. Younger plants are exposed to the nematode for only short period of time. The scenario also assumes that root galls are visible while inspecting plants before export and that the second juvenile stage are washed away during the root washing of bare root plants. The scenario assumes that the root systems of plants have undergone washing and inspection before being transplanted in pots.
A.6.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees and plants in pots up to 7 years old
The scenario assumes a high pressure of the pest in the nurseries and in the surroundings as many potential hosts are present. Older plants are exposed to the nematode for longer period of time. The scenario also assumes that root galls are not easily recognisable while inspecting plants before export and that the low‐pressure washing is not effective in removing the second juvenile stage before export. The scenario assumes that the root systems of plants did not undergone washing and inspection before being transplanted in pots.
A.6.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees and plants in pots up to 7 years old (Median)
The scenario assumes a limited presence of the pest in the nurseries and the surroundings and that the plants are exposed to the nematode for a sufficient period of time for infection to occur. The movement of soil from the surrounding into the nurseries is not expected to be significant.
A.6.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pests in the UK including the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.6.6.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne mali and M. fallax on bare root plants/trees and plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infection (Table A.29) and pest freedom (Table A.30).
Table A.29.
Elicited and fitted values of the uncertainty distribution of pest infection by Meloidogyne mali and M. fallax per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 35 | 70 | 130 | 250 | ||||||||||
| EKE | 1.31 | 3.22 | 6.41 | 12.8 | 21.7 | 33.2 | 45.5 | 73.1 | 107 | 127 | 153 | 179 | 208 | 229 | 250 |
The EKE results are the BetaGeneral (1.0205, 2.5146, 0, 297) distribution fitted with @Risk version 7.6.
Table A.30.
The uncertainty distribution of plants free of Meloidogyne mali and M. fallax per 10,000 plants calculated by Table A.29
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,870 | 9,930 | 9,965 | 9,999 | ||||||||||
| EKE results | 9,750 | 9,771 | 9,792 | 9,821 | 9,847 | 9,873 | 9,893 | 9,927 | 9,955 | 9,967 | 9,978 | 9,987 | 9,994 | 9,997 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.30.
A.6.7. Reference list
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Meloidogyne fallax (false Columbia root‐knot nematode). Available online: https://www.cabi.org/cpc/datasheet/33241 [Accessed: 20 February 2023].
DEFRA (Department for Environment, Food and Rural Affairs), online. UK risk register details for Meloidogyne fallax. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register//viewPestRisks.cfm?cslref=16540 [Accessed: 23 February 2023].
DEFRA (Department for Environment Food and Rural Affairs), 2017. The nematode Meloidogyne fallax in sports turf: symptoms, biosecurity, guidance and control. Plant Pest Factsheet, 5 pp. Available online: https://www.cabdirect.org/cabdirect/abstract/20207800404
den Nijs LJMF, Brinkman H and van der Sommen ATC, 2004. A Dutch contribution to knowledge on phytosanitary risk and host status of various crops for Meloidogyne chitwoodi Golden et al., 1980 and M. fallax Karssen, 1996: an overview. Nematology, 6, 303–312. https://doi.org/10.1163/1568541042360492
EFSA (European Food Safety Authority), den Nijs L, Camilleri M, Diakaki M, Schenk M and Vos S, 2019. Pest survey card on Meloidogyne chitwoodi and Meloidogyne fallax. EFSA supporting publication 2019;EN‐1572, 20 pp. https://doi.org/10.2903/sp.efsa.2019.en-1572
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐ Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Mosbach‐Schulz O and Gonthier P, 2020. Scientific Opinion on the commodity risk assessment of Acer spp. plants from New Zealand. EFSA Journal 2020;18(5):6105, 87 pp. https://doi.org/10.2903/j.efsa.2020.6105
EPPO (European and Mediterranean Plant Protection Organization), 2015. Meloidogyne fallax detected in sports turf in United Kingdom. EPPO Reporting Service No. 10–2015. Available online: https://gd.eppo.int/reporting/article-5137 [Accessed: 20 February 2023].
EPPO (European and Mediterranean Plant Protection Organization), 2016. Diagnostics. PM 7/41 (3). Meloidogyne chitwoodi and M. fallax. EPPO Bulletin, 46, 171–189.
EPPO (European and Mediterranean Plant Protection Organization), 2019. Phytosanitary procedures. PM 3/69 (2) Meloidogyne chitwoodi and M. fallax sampling potato tubers for detection. EPPO Bulletin, 49, 486–487.
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2022–09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 20 February 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Meloidogyne fallax (MELGFA), Categorization. https://gd.eppo.int/taxon/MELGFA/categorization [Accessed: 20 February 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Meloidogyne fallax (MELGFA), Distribution. Available online: https://gd.eppo.int/taxon/MELGFA/distribution [Accessed: 20 February 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Meloidogyne fallax (MELGFA), Reporting. Available online: https://gd.eppo.int/taxon/MELGFA/reporting [Accessed: 20 February 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Meloidogyne fallax (MELGFA), Hosts. Available online: https://gd.eppo.int/taxon/MELGFA/hosts [Accessed: 20 February 2023].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
Ferris H, online. Nemaplex (The Nematode‐Plant Expert Information System). Available online: http://nemaplex.ucdavis.edu/ [Accessed: 4 December 2022].
Kantor M, Handoo Z, Kantor C and Carta L, 2022. Top ten most important U.S.‐regulated and emerging plant‐parasitic nematodes. Horticulturae, 8, 208, 1–26. https://doi.org/10.3390/horticulturae8030208
MacLeod A, Anderson H, Follak S, van der Gaag DJ, Potting R, Pruvost O, Smith J, Steffek R, Vloutoglou I, Holt J, Karadjova O, Kehlenbeck H, Labonne G, Reynaud P, Viaene N, Anthoine G, Holeva M, Hostachy B, Ilieva Z, Karssen G, Krumov V, Limon P, Meffert J, Niere B, Petrova E, Peyre J, Pfeilstetter E, Roelofs W, Rothlisberger F, Sauvion N, Schenck N, Schrader G, Schroeder T, Steinmöller S, Tjou‐Tam‐Sin L, Ventsislavov V, Verhoeven K, and Wesemael W, 2012. Pest risk assessment for the European Community plant health: a comparative approach with case studies. Cases: Meloidogyne chitwoodi and M. fallax. Supporting publications 2012;EN‐319, 1053 pp. Available online: www.efsa.europa.eu/publications
Moens M, Perry RN and Starr JL, 2009. Meloidogyne species–a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL (eds.). Root‐Knot Nematodes. CABI, California, the US, 1–17. https://doi.org/10.1079/9781845934927.0001
Riffle JW, 1963. Meloidogyne ovalis (Nematoda: Heteroderidae), A new species of root‐knot nematode. Helminthological Society of Washington, 30, 287–292.
Topalović O, Moore JF, Janssen T, Bert W and Karssen G, 2017. An early record of Meloidogyne fallax from Ireland. ZooKeys, 643, 33–52. https://doi.org/10.3897/zookeys.643.11266
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
van der Sommen A, den Nijs L and Karssen G, 2005. The root‐knot nematode Meloidogyne fallax on strawberry in the Netherlands. Plant Disease, 89, 526. https://doi.org/10.1094/pd-89-0526a
A.7. Meloidogyne mali
A.7.1. Organism information
| Taxonomic information |
Current valid scientific name: Meloidogyne mali Synonyms: Meloidogyne ulmi Name used in the EU legislation: – Order: Rhabditia Family: Meloidogynidae Common name: apple root‐knot nematode Name used in the Dossier: Meloidogyne mali |
|
| Group | Nematodes | |
| EPPO code | MELGMA | |
| Regulated status |
Meloidogyne mali is included in the EPPO A2 list (EPPO, online_a) and was recently recommended for regulation as quarantine pest (EPPO, online_b). Meloidogyne mali is quarantine pest in the US and Morocco (EPPO, online_a) and listed as a ‘pest of quarantine interest' in the Dominican Republic (EPPO, 2017); it is also regulated in Colombia, the Republic of Korea, Malaysia and Uruguay (EPPO, 2017). All Meloidogyne species are quarantine pests for Türkiye (EPPO, 2017). |
|
| Pest status in the UK |
Meloidogyne mali is present in the UK in Southern England – two sites in Farnham and Surrey (Dossier Section 3.0) where it was found on elm trees in 2018, as consequence of introduction in the past of infected elms from the Netherlands (Prior et al., 2019). According to the Dossier Section 5.0 the nematode is present in the UK: not widely distributed and not under official control. |
|
| Pest status in the EU |
Meloidogyne mali is currently present in the EU in Austria (de Jong et al., online); it is also present in Belgium (Suwanngam and Wesemael, 2019), Italy (Palmisano and Ambrogioni, 2000) and the Netherlands (Ahmed et al., 2013), in all cases with few occurrences or restricted distribution (EPPO, online_c). M. mali was detected in France (Ile de France) in 2016, but it was eradicated in 2021 (EPPO, online_c). According to Ahmed et al. (2013) and EPPO (2017), M. mali may have a wider distribution in Europe, since elm plants growing in plots infested by the nematode in the Netherlands have been sent to other countries (Belgium, Denmark, France, Germany, Ireland, Italy, Spain, Slovakia, Romania, the UK) to carry out resistance tests against the Dutch Elm Disease (DED). These programmes started from the 80's of the last century (Prior et al., 2019). |
|
| Host status on Acer | Acer palmatum, A. pseudoplatanus, A. x freemanii and Acer saccharinum, are host plants for Meloidogyne mali (EPPO, 2017; Kang et al., 2021). | |
| PRA information | Available pest risk assessments:
|
|
| Other relevant information for the assessment | ||
| Biology |
Meloidogyne mali is a root‐knot nematode inducing root galls on host plants; it is native to Asia (Japan), introduced decades ago to Europe and more recently also to the US (EPPO, 2017; Eisenback et al., 2017) and to the Republic of Korea (Kang et al., 2021). When found in Europe in 2000, the nematode was initially described as a new species, Meloidogyne ulmi (Palmisano and Ambrogioni, 2000) and elms remained long‐time the only known host plants. The synonymy with the well‐known species M. mali was found later, after comparison in the Netherlands with living material from Japan (Ahmed et al., 2013). Meloidogyne mali develops through three stages: eggs, juveniles (four stages) and adults, all living in the root galls. Adult males, second‐stage juveniles and eggs can live also free in the soil (EPPO, 2017). Information on M. mali biology mainly come from Malus sp. in Japan where the nematode and has one generation per year and the life cycle lasts 18–22 weeks. However, it is known that Meloidogyne species can frequently have more generations per year depending on the temperature and the feeding on perennial plants. Only few specific information on the life cycle of M. mali is available. Unlike similar species as M. chitwoodi and M. fallax which are parthenogenetic, Meloidogyne mali reproduces sexually. Like all Meloidogyne root‐knot nematodes it deposits eggs in gelatinous sacs on the surface of galls or within them (EPPO, 2017; EFSA, 2019); in Japan the minimum hatching temperature range of M. mali eggs is 10–15°C (optimal 20–33°C) (EPPO, 2017). As usual in Meloidogyne species, the infective second‐stage juveniles move in the soil and attack the roots penetrating behind the root cap. They start to feed on cortical tissues inducing the formation of giant cells that cause swelling and finally root galls. After moulting, adults develop from the last juvenile stage; females remain into the roots where they lay eggs in a gelatinous matrix, while males leave the galls (EFSA, 2019). It is not clear in what extent the nematode can survive frost conditions during winter. Meloidogyne mali can probably overwinter in the roots of plants growing outdoors, possibly as young females, given that egg‐laying females have been observed in early March (EPPO, 2017). In the US the nematode seems able to survive at minimum winter temperature of −6°C (Pylypenko, 2016). Although Meloidogyne species are known not forming cysts to resist to the absence of host plants for long‐time, M. mali can survive for at least 2 years in root fragments in the soil after removal of infected trees; it is not known, however, if the nematode can also have a diapause period (EPPO, 2017). All Meloidogyne are strictly associated with the roots of plants and are known to be sedentary species, moving in the soil 1–2 m maximum per year, and spread through the roots depending on their size, type of soil, water availability and other parameters (EFSA, 2019). As other species of root‐knot nematodes, the spread on medium‐long distance of Meloidogyne mali is by passive transport, and possible pathways are mainly plants for planting with infected roots, soil and growing media and also contaminated tools and machinery (EPPO, 2017). |
|
| Symptoms | Main type of symptoms |
Plants infected by Meloidogyne mali show root‐knot galls on roots. The galls can be of different size also depending on the hosts and are always visible to the naked eye (0.5–2 cm in diameter) (EPPO, 2018). When a severe root infection occurs, as consequence of the developing of large number of galls the root system can be damaged, reducing uptake of water and minerals and causing symptoms on above‐ground part of plants. Common symptoms are little growth of primary shoots and increase of secondary shoots, leaf fall and general reduction of growth. No specific information about symptoms on Acer was found. |
| Presence of asymptomatic plants | Plants infected by Meloidogyne mali can remain asymptomatic. Damage on above‐ground part of plants goes often unnoticed in early infection stage or when underground attack on roots is light. 30‐year‐old elms gravely infected in the root system were uprooted by wind without any symptom on the crown or foliage (EPPO, 2017). | |
| Confusion with other pests |
Plants infected by Meloidogyne mali appear similar to plants infected by other nematode species or root pathogens living in the soil. The identification of the nematode is not possible on the basis of sole galls. M. mali juveniles and adults are morphologically similar to other Meloidogyne nematodes. For identification to species level, laboratory tests on morphometric characters, electrophoresis or sequencing/DNA barcoding are needed (EPPO, 2018). |
|
| Host plant range |
Meloidogyne mali is a polyphagous nematode feeding on roots of several species of trees, shrubs and herbaceous plants. Some important woody hosts of M. mali are Acer x freemani, A. palmatum. A. pseudoplatanus, Castanea crenata, Euonymus kiautschovicus, E. fortunei, Fagus sylvatica, Lagerstroemia indica, Malus pumila, Morus alba, Prunus serrulata, Quercus robur, Sorbus aucuparia, Taxus baccata, Ulmus glabra, U. parvifolia, Vitis vinifera, Zelkova serrata (EPPO, 2017; DEFRA, online; Ferris, online). Common herbaceous hosts are: Dryopteris filix‐mas, D. carthusiana, Geranium robertianum, Geum coccineum, Impatiens parviflora, Rosa sp., Rubus fruticosus, Taraxacum officinale, Trifolium repens and Urtica dioica (EPPO, 2017; DEFRA, online). For a complete list of hosts see EPPO (2017) and DEFRA (online). |
|
| Reported evidence of impact |
Only poor information on economic impact caused by Meloidogyne mali is available. In Japan, damage on Malus and Morus (15–43% growth reduction) was reported only following inoculation experiments. In Italy slowly declining elms were observed (Palmisano and Ambrogioni, 2000). In the UK, M. mali was only found in elms killed by DED (Prior et al., 2019). Roots damaged by M. mali may be also attacked by secondary pathogen agents. On elm trees in the Netherlands the infection by M. mali caused detriment of stability with uprooting by wind in urban areas (EPPO, 2017). No specific data about damage on Acer was found. |
|
| Evidence that the commodity is a pathway | Meloidogyne mali can travel with plants for planting; therefore, they are possible pathways of entry for the nematode. The pest has been intercepted in China in 2013 and 2020 on Acer palmatum and in 2015 on Lagerstroemia indica, in both cases on plants imported from Japan (EPPO, 2017; GenBank, online). In the period 1995 to December 2022, there have been several interceptions of Meloidogyne species in the EU from Japan, mostly on bonsais, including on hosts of M. mali as Acer palmatum, although in these hosts, the nematode was never identified at species level. | |
| Surveillance information |
According to the Dossier Section 5.0, Meloidogyne mali is not under official surveillance, as does not meet criteria of quarantine pest for Great Britain. A survey was conducted to determine the extent of Meloidogyne mali presence in Surrey; all of the samples outside the two sites where the nematode was found in 2018 were negative, indicating that it has not spread off the sites (Dossier Section 3.0). A containment approach is being implemented in the two sites. No movement of soil from the sites is allowed. No movement of host plants from the sites is allowed. Staff and contractors coming into contact with host plants or soil on sites must remove soil from footwear and equipment before leaving the sites. Only non‐hosts should be planted at the sites (Dossier Section 3.0). |
|
A.7.2. Possibility of pest presence in the nursery
A.7.2.1. Possibility of entry from the surrounding environment
Meloidogyne mali is eradicated from the outbreak locations on Ulmus sp. in the two sites in Southern England (Farnham, Surrey) (Prior et al., 2019; Dossier Sections 3.0 and 5.0). The pest is not regulated in the UK. No presence of the nematode outside the two known sites is reported and a containment approach has been implemented (Dossier Section 3.0).
The nematode can only spread by passive transport with plants for planting with infected roots, infected soil and growing media, and possibly via contaminated tools and machinery. No other possibility of entry in the nurseries is known.
Meloidogyne mali can infect Castanea spp., Fagus spp., Malus spp., Morus spp., Taxus baccata, Quercus robur, Prunus spp., Rosa spp., Solanum lycopersicum, Ulmus spp. which are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
Pest presence and pressure in the surrounding.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for Meloidogyne mali to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present and the nematode could enter by human‐assisted spread.
A.7.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0). Seeds are not a pathway for the nematode.
In addition to Acer, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the nematode (such as Castanea spp., Fagus spp., Malus spp., Prunus spp., Quercus spp., Rosa spp., Sorbus spp., Taxus baccata, Ulmus spp.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the nematode could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). Meloidogyne mali is able to survive both in the soil and in root fragments in the soil for 2 years (EPPO, 2007) and therefore could potentially enter with infested soil/growing media. However, the growing media are certified and heat treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the nematode to enter the nurseries via infected roots of new seedlings of Acer and plants of other species used for plant production in the area. The entry of the nematode with seeds and the growing media the Panel considers as not possible.
A.7.2.3. Possibility of spread within the nursery
Acer plants are either grown in containers (cells, pots, tubes, etc.) outdoors in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The nematode can infect other suitable plants (such as Acer spp., Fagus spp., Quercus spp., Ulmus spp., etc.) present within the nurseries (Dossier Sections 3.0 and 6.0).
Meloidogyne mali can spread within the nurseries by movement of soil, water, infested plant material and infected tools and machinery (EPPO, 2017). However, tools used in the nurseries are disinfected after operation on a stock and before being used on a different plant species (Dossier Section 3.0).
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the nematode within the nurseries is possible either by movement of infested soil, water and plant material.
A.7.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Meloidogyne mali between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.7.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Meloidogyne mali is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
As the plant passport is very similar to the EU one, the plants shall be free from quarantine pests and RNQPs.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material– | Yes |
Seedlings could be a pathway for the nematode. The certification could have an effect on preventing the nematode to enter into the nurseries.
Uncertainties:
|
| 4 | Growing media | Yes |
Heat treatment and protection of the treated growing media is effective against the nematode.
Uncertainties:
|
| 5 | Surveillance, monitoring and sampling | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Uncertainties:
|
| 9 | Application of pest control products | No | Not relevant. No nematicides are used in the nurseries. |
| 10 | Measures against soil pests | Yes |
Separation of the pots from soil is effective against the nematode.
Uncertainties:
|
| 11 | Inspections and management of plants before export | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. The nematode cannot spread between the roots of the plants when transported to the EU. |
A.7.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.7.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pest in the nurseries and in the surroundings. The plants are exposed to the nematode for only short period of time. The scenario also assumes that root galls are visible while inspecting plants before export and that the second juvenile stage are washed away during the root washing.
A.7.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pest in the nurseries and in the surroundings as many potential hosts are present. The scenario also assumes that root galls are not easily recognisable while inspecting plants before export and that the low‐pressure washing is not effective in removing the second juvenile stage before export.
A.7.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pest in the nurseries and the surroundings and that the plants are exposed to the nematode for only short period of time. The movement of soil from the surrounding into the nurseries is not expected to be significant.
A.7.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pests in the UK including the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.7.5.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne mali and M. fallax on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infection (Table A.31) and pest freedom (Table A.32).
Table A.31.
Elicited and fitted values of the uncertainty distribution of pest infection by Meloidogyne mali and M. fallax per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 3 | 25 | 40 | 60 | 150 | ||||||||||
| EKE | 5.507 | 8.116 | 11.04 | 15.31 | 19.91 | 24.97 | 29.82 | 39.98 | 52.2 | 60.1 | 70.5 | 83.0 | 99.1 | 114.4 | 133.9 |
The EKE results are the BetaGeneral (2.6372, 576.47, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.32.
The uncertainty distribution of bundles free of Meloidogyne mali and M. fallax per 10,000 bundles calculated by Table A.31
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,940 | 9,960 | 9,975 | 9,997 | ||||||||||
| EKE results | 9,866 | 9,886 | 9,901 | 9,917 | 9,929 | 9,940 | 9,948 | 9,960 | 9,970 | 9,975 | 9,980 | 9,985 | 9,989 | 9,992 | 9,994 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles, the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.32.
A.7.6. Overall likelihood of pest freedom for bare root plants/trees and plants in pots up to 7 years old
A.7.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees and plants in pots up to 7 years old
The scenario assumes a low pressure of the pest in the nurseries and in the surroundings. Younger plants are exposed to the nematode for only short period of time. The scenario also assumes that root galls are visible while inspecting plants before export and that the second juvenile stage is washed away during the root washing of bare root plants. The scenario assumes that the root systems of plants have undergone washing and inspection before being transplanted in pots.
A.7.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees and plants in pots up to 7 years old
The scenario assumes a high pressure of the pest in the nurseries and in the surroundings as many potential hosts are present. Older plants are exposed to the nematode for longer period of time. The scenario also assumes that root galls are not easily recognisable while inspecting plants before export and that the low‐pressure washing is not effective in removing the second juvenile stage before export. The scenario assumes that the root systems of plants did not undergone washing and inspection before being transplanted in pots.
A.7.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees and plants in pots up to 7 years old (Median)
The scenario assumes a limited presence of the pest in the nurseries and the surroundings and that the plants are exposed to the nematode for a sufficient period of time for infection to occur. The movement of soil from the surrounding into the nurseries is not expected to be significant.
A.7.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pests in the UK including the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.7.6.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne mali and M. fallax on bare root plants/trees and plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infection (Table A.33) and pest freedom (Table A.34).
Table A.33.
Elicited and fitted values of the uncertainty distribution of pest infection by Meloidogyne mali and M. fallax per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 35 | 70 | 130 | 250 | ||||||||||
| EKE | 1.31 | 3.22 | 6.41 | 12.8 | 21.7 | 33.2 | 45.5 | 73.1 | 107 | 127 | 153 | 179 | 208 | 229 | 250 |
The EKE results are the BetaGeneral (1.0205, 2.5146, 0, 297) distribution fitted with @Risk version 7.6.
Table A.34.
The uncertainty distribution of plants free of Meloidogyne mali and M. fallax per 10,000 plants calculated by Table A.33
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,870 | 9,930 | 9,965 | 9,999 | ||||||||||
| EKE results | 9,750 | 9,771 | 9,792 | 9,821 | 9,847 | 9,873 | 9,893 | 9,927 | 9,955 | 9,967 | 9,978 | 9,987 | 9,994 | 9,997 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.34.
A.7.7. Reference list
Ahmed M, van de Vossenberg BTLH, Cornelisse C and Karssen G, 2013. On the species status of the root‐knot nematode Meloidogyne ulmi Palmisano and Ambrogioni, 2000 (Nematoda, Meloidogynidae). ZooKeys, 362, 1–27. https://doi.org/10.3897/zookeys.362.6352
DEFRA (Department for Environment, Food and Rural Affairs), online. UK risk register details for Meloidogyne mali. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=16542 [Accessed: 30 November 2022].
de Jong Y, et al., online. Fauna Europaea ‐ all European animal species on the web. Biodiversity Data Journal. Available online: https://fauna-eu.org/ [Accessed: 3 November 2022].
EFSA (European Food Safety Authority), den Nijs L, Camilleri M, Diakaki M, Schenk M and Vos S, 2019. Pest survey card on Meloidogyne chitwoodi and Meloidogyne fallax. EFSA supporting publication 2019;EN‐1572, 20 pp. https://doi.org/10.2903/sp.efsa.2019.en-1572
EFSA PLH Panel (EFSA Panel on Plant Health), 2015. Scientific opinion on the risks to plant health posed by EU import of soil or growing media. EFSA Journal 2015;13(6):4132, 133 pp. https://doi.org/10.2903/j.efsa.2015.4132
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Gonthier P, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P,Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Zappalà L, Lucchi A, Gómez P, Urek G, Bernardo U, Bubici G, Carluccio AV, Chiumenti M, Di Serio F, Fanelli E, Marzachì C, Kaczmarek A, Mosbach‐Schulz O and Yuen J, 2023. Scientific Opinion on the commodity risk assessment of Malus domestica plants from United Kingdom. EFSA Journal 2023;21(5):8002, 146 pp. https://doi.org/10.2903/j.efsa.2023.8002
Eisenback JD, Graney LS and Vieira P, 2017. First report of the apple root‐knot nematode (Meloidogyne mali) in North America, found parasitizing Euonymus in New York. Plant Disease, 101, 3, 510. https://doi.org/10.1094/pdis-06-16-0894-pdn
EPPO (European and Mediterranean Plant Protection Organization), 2017. Pest risk analysis for Meloidogyne mali, apple root‐knot nematode. EPPO, Paris, 38 pp.
EPPO (European and Mediterranean Plant Protection Organization), 2018. Diagnostics PM 7/136 (1) Meloidogyne mali. Bulletin OEPP/EPPO, 48, 438–445.
EPPO (European and Mediterranean Plant Protection Organization), online_a. Meloidogyne mali (MELGMA), Categorization. Available online: https://gd.eppo.int/taxon/MELGMA/categorization [Accessed: 30 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Meloidogyne mali (MELGMA), Documents. Available online: https://gd.eppo.int/taxon/MELGMA/documents [Accessed: 30 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Meloidogyne mali (MELGMA), Distribution. Available online: https://gd.eppo.int/taxon/MELGMA/distribution [Accessed: 30 November 2022].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
Ferris H, online. Nemaplex (The Nematode‐Plant Expert Information System). Available online: http://nemaplex.ucdavis.edu/ [Accessed: 4 December 2022].
GenBank (National Center for Biotechnology Information), online. Meloidogyne mali. Available online: https://www.ncbi.nlm.nih.gov/nuccore/?term=meloidogyne+mali [Accessed: 8 February 2023].
Kang H, Seo J, Ko HR, Park S, Park NS, Park BY and Choi I, 2021. First report of the apple root‐knot nematode, Meloidogyne mali, on maple trees in the Republic of Korea. Plant Disease. https://doi.org/10.1094/pdis-09-21-2121-pdn
Palmisano A and Ambrogioni L, 2000. Meloidogyne ulmi sp. n., a root‐knot nematode from elm. Nematologia Mediterranea, 28, 279–293.
Prior T, Tozer H, Yale R, Jones EP, Lawson R, Jutson L, Correia M, Stubbs J, Hockland S and Karssen G, 2019. First report of Meloidogyne mali causing root galling to elm trees in the UK. New Disease Reports, 39, 10. https://doi.org/10.5197/j.2044-0588.2019.039.010
Pylypenko LA, 2016. A quickscan pest risk analysis for the Meloidogyne mali. Interdepartmental Thematic Scientific Collection of Plant Protection and Quarantine, 62, 188–200. https://doi.org/10.36495/1606-9773.2016.62.188-200
Suwanngam A and Wesemael WML, 2019. First report of the root‐knot nematode Meloidogyne mali infecting elm trees in Belgium. New Disease Reports, 40, 16. https://doi.org/10.5197/j.2044-0588.2019.040.016
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
A.8. Phytophthora ramorum
A.8.1. Organism information
| Taxonomic information |
Current valid scientific name: Phytophthora ramorum Synonyms: – Name used in the EU legislation: Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in ‘t Veld [PHYTRA] Order: Peronosporales Family: Peronosporaceae Common name: Sudden Oak Death (SOD), ramorum bleeding canker, ramorum blight, ramorum leaf blight, twig and leaf blight Name used in the Dossier: Phytophthora ramorum |
|
| Group | Oomycetes | |
| EPPO code | PHYTRA | |
| Regulated status |
The pathogen is listed in Annex II of Commission Implementing Regulation (EU) 2019/2072 as Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in ‘t Veld [PHYTRA]. The EU isolates of P. ramorum are listed as protected zone quarantine pest The pathogen is included in the EPPO A2 list (EPPO, online_a). Phytophthora ramorum is quarantine in Canada, Israel, Mexico, Morocco and the UK. It is on A1 list of Brazil, Chile, Egypt, Kazakhstan, Türkiye and EAEU (=Eurasian Economic Union: Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) (EPPO, online_b). |
|
| Pest status in the UK |
Phytophthora ramorum is present in the UK (Brown and Brasier, 2007; Dossier Sections 2.0 and 5.0; CABI, online; EPPO, online_c). According to the Dossier Section 5.0, European isolates of Phytophthora ramorum are present in the UK: not widely distributed and under official control. It has been found in most regions of the UK, but it is more often reported in wetter, western regions. |
|
| Pest status in the EU | Phytophthora ramorum is present in the EU and it is currently reported in the following EU Member States: Belgium, Croatia, Denmark, Finland, France, Germany, Ireland, the Netherlands, Poland, Portugal and Slovenia (EPPO, online_c). | |
| Host status on Acer |
Acer pseudoplatanus is a reported host of Phytophthora ramorum (Brown and Brasier, 2007; King et al., 2015; CABI, online; EPPO, online_d; Farr and Rossman, online). Phytophthora ramorum is a pathogen of other Acer species such as Acer circinatum, Acer davidii, Acer laevigatum and Acer macrophyllum (Hayden et al., 2004; Cave et al., 2008; DiLeo et al., 2008; King et al., 2015; CABI, online; EPPO, online_d; Farr and Rossman, online). |
|
| PRA information | Pest risk assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
Phytophthora ramorum is most probably native to East Asia (Poimala and Lilja, 2013; Jung et al., 2021). The pathogen is present in Asia (Japan, Vietnam), Europe (Belgium, Croatia, Denmark, Finland, France, Germany, Guernsey, Ireland, Luxembourg, the Netherlands, Norway, Poland, Portugal, Slovenia, the UK), North America (Canada, the US) and South America (Argentina) (EPPO, online_c). So far there are 12 known lineages of P. ramorum: NA1 and NA2 from North American, EU1 from Europe (including the UK) and North America (Grünwald et al., 2009), EU2 from Northern Ireland and western Scotland (Van Poucke et al., 2012), IC1 to IC5 from Vietnam and NP1 to NP3 from Japan (Jung et al., 2021). Phytophthora ramorum is heterothallic oomycete species belonging to clade 8c (Blair et al., 2008) with two mating types: A1 and A2 (Boutet et al., 2010). Phytophthora species generally reproduce through a) dormant (resting) spores which can be either sexual (oospores) or asexual (chlamydospores); and b) fruiting structures (sporangia) which contain zoospores (Erwin and Ribeiro, 1996). Phytophthora ramorum produces sporangia on the surfaces of infected leaves and twigs of host plants. These sporangia can be splash‐dispersed to other close or carried by wind and rain to longer distances. The sporangia germinate to produce zoospores that penetrate and initiate an infection on new hosts. In infected plant material, the chlamydospores are produced and can serve as resting structures (Davidson et al., 2005; Grünwald et al., 2008). Trunk cankers (e.g. on Quercus) are not known to support sporulation and therefore do not transmit the pathogen (DEFRA, 2008). The pathogen is also able to survive in soil (Shishkoff, 2007). In the west of Scotland, it persisted in soil for at least 2 years after its hosts were removed (Elliot et al., 2013). Oospores were only observed in pairing tests under controlled laboratory conditions (Brasier and Kirk, 2004). Optimal temperatures under laboratory conditions were 16–26°C for growth, 14–26°C for chlamydospore production and 16–22°C for sporangia production (Englander et al., 2006). Phytophthora ramorum is mainly a foliar pathogen; however, it was also reported to infect shoots, stems, and occasionally roots of various host plants (Grünwald et al, 2008, Parke and Lewis, 2007). According to Brown and Brasier (2007), P. ramorum commonly occupies xylem beneath phloem lesions and may spread within xylem and possibly recolonize the phloem from the xylem. Phytophthora ramorum can remain viable within xylem for two or more years after the overlying phloem had been excised. Phytophthora ramorum can disperse by aerial dissemination, water, movement of infested plant material and soil containing propagules on footwear, tires of trucks and mountain bikes, or the feet of animals (Davidson et al., 2002; Brasier, 2008). Infected foliar hosts can be a major source of inoculum, which can lead to secondary infections on nearby host plants. Important foliar hosts in Europe are Rhododendron spp. and Larix kaempferi (Brasier and Webber, 2010, Grünwald et al., 2008). Possible pathways of entry for Phytophthora ramorum are plants for planting (excluding seed and fruit) of known susceptible hosts; plants for planting (excluding seed and fruit) of non‐host plant species accompanied by contaminated attached growing media; soil/growing medium (with organic matter) as a commodity; soil as a contaminant; foliage or cut branches; seed and fruits; susceptible (isolated) bark and susceptible wood (EFSA PLH Panel, 2011). Phytophthora ramorum caused rapid decline of Lithocarpus densiflorus and Quercus agrifolia in forests of California and Oregon (Rizzo et al., 2005) and Larix kaempferi in plantations of southwest England (Brasier and Webber, 2010). |
|
| Symptoms | Main type of symptoms |
Phytophthora ramorum causes different types of symptoms depending on the host species and the plant tissue infected. According to DEFRA (2008), P. ramorum causes three different types of disease:
Symptoms on Quercus species are cankers of red, brown or black colour on trunk, browning of the crown, gradual leaf loss and death of trees (Davidson et al., 2003). Leaf lesions and shoot dieback can be observed on foliar hosts such as Rhododendron, Viburnum, Pieris and Camellia. (Davidson et al., 2003, EPPO, online_e). On Larix kaempferi, P. ramorum causes foliage and bark infection that are visible as wilted shoot tips with blackened needles and stem lesions with resin bleeding (Braiser and Webber, 2010). Symptoms on Lithocarpus densiflorus are lesions on leaves, cankers on trunk, branches and twigs; shoot tip dieback, leaf flagging and formation of a Shepard's crook. The trees can die within 1 year (Davidson et al., 2003). Acer macrophyllum is affected by discoloration of leaves ranging from orange to brown colour, which normally starts from the leaf edges (Davidson et al., 2003). On Acer pseudoplatanus, P. ramorum has been isolated from bleeding bark lesions (Brown and Brasier, 2007). In an inoculation experiment with P. ramorum, low levels of necrosis on leaves were observed on this host species (Denman et al., 2005). |
| Presence of asymptomatic plants |
If roots are infected by P. ramorum, the plants can be without aboveground symptoms for months until developmental or environmental factors trigger disease expression (Roubtsova and Bostock, 2009; Thompson et al., 2021). Application of some fungicides may reduce symptoms and therefore mask infection, making it more difficult to determine whether the plant is pathogen‐free (DEFRA, 2008). |
|
| Confusion with other pests |
Various symptoms caused by P. ramorum can be confused with other pathogens, such as: canker and foliar symptoms caused by other Phytophthora species (P. cinnamomi, P. citricola and P. cactorum); leaf lesions caused by rust in early stages; leafspots caused by sunburn; dieback of twigs and leaves caused by Botryosphaeria dothidea (Davidson et al., 2003). Symptoms in Acer macrophyllum can be confused with drought stress, Xylella leaf scorch and anthracnose (Davidson et al., 2003). Phytophthora ramorum can be easily distinguished from other Phytophthora species based on morphology (Grünwald et al., 2008) and molecular tests. |
|
| Host plant range |
Phytophthora ramorum has a very wide host range, which is expanding. Main host plants include Camellia spp., Larix decidua, L. kaempferi, Pieris spp., Rhododendron spp., Syringa vulgaris, Viburnum spp., and the North American trees species, Lithocarpus densiflorus and Quercus agrifolia (EPPO online_d). Further proven hosts confirmed by Koch's postulates are Abies grandis, A. magnifica, Acer circinatum, A. macrophyllum, A. pseudoplatanus, Adiantum aleuticum, A. jordanii, Aesculus californica, A. hippocastanum, Arbutus menziesii, A. unedo, Arctostaphylos columbiana, A. glauca, A. hooveri, A. manzanita, A. montereyensis, A. morroensis, A. pilosula, A. pumila, A. silvicola, A. viridissima, Calluna vulgaris, Castanea sativa, Ceanothus thyrsiflorus, Chamaecyparis lawsoniana, Chrysolepis chrysophylla, Cinnamomum camphora, Corylus cornuta, Fagus sylvatica, Frangula californica, Frangula purshiana, Fraxinus excelsior, Gaultheria procumbens, G. shallon, Griselinia littoralis, Hamamelis virginiana, Heteromeles arbutifolia, Kalmia spp., Larix × eurolepis, Laurus nobilis,, Lonicera hispidula, Lophostemon confertus, Loropetalum chinense, Magnolia × loebneri, M. oltsopa, M. stellata, Mahonia aquifolium, Maianthemum racemosum, Parrotia persica, Photinia fraseri, Phoradendron serotinum subsp. macrophyllum, Photinia × fraseri, Prunus laurocerasus, Pseudotsuga menziesii var. menziesii, Quercuscerris, Q. chrysolepis, Q. falcata Q. ilex, Q. kelloggii, Q. parvula var. shrevei, Rosa gymnocarpa, Salix caprea, Sequoia sempervirens,, Taxus baccata, Trientalis latifolia, Umbellularia californica, Vaccinium myrtillus, V. ovatum, V. parvifolium and Vinca minor (Cave et al., 2008; APHIS USDA, 2022). |
|
| Reported evidence of impact | Phytophthora ramorum is an EU quarantine pest. | |
| Evidence that the commodity is a pathway | Phytophthora ramorum is continuously intercepted in the EU on different plant species intended for planting (EUROPHYT/TRACES‐NT, online) and according to EFSA PLH Panel (2011), P. ramorum can travel with plants for planting. Therefore, plants for planting are possible pathway of entry for P. ramorum. | |
| Surveillance information |
Phytophthora ramorum at growing sites: infested plants are destroyed and potentially infested plants are ‘held’ (prohibited from moving). The UK has a containment policy in the wider environment with official action taken to remove infected trees (Dossier Section 3.0). As part of an annual survey at ornamental retail and production sites (frequency of visits determined by a decision matrix) Phytophthora ramorum is inspected on common host plants. An additional inspection, during the growing period, is carried out at plant passport production sites. Inspections are carried out at a survey to 300 non‐woodland wider environment sites annually (Dossier Sections 3.0 and 5.0). |
|
A.8.2. Possibility of pest presence in the nursery
A.8.2.1. Possibility of entry from the surrounding environment
Phytophthora ramorum is present in the UK, it has been found in most regions of the UK, but it is more often reported in wetter, western regions (Dossier Section 5.0).
The possible entry of P. ramorum from surrounding environment to the nurseries may occur through aerial dissemination, water and animals (Davidson et al., 2002).
Phytophthora ramorum has wide host range and can infect number of different plants. Suitable hosts of P. ramorum like Abies spp., Acer spp., Aesculus spp., Camellia spp., Castanea spp., Fagus spp., Fraxinus spp., Magnolia spp., Prunus spp., Quercus spp., Rhododendron spp., Rosa spp., Salix spp., Syringa spp. and Viburnum spp. are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
The dispersal range of P. ramorum sporangia.
-
–
No information available on the distance of the nurseries to sources of pathogen in the surrounding environment.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present and the pathogen can spread by wind, rain and infested soil propagules on feet of animals entering the nurseries.
A.8.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0). Seeds are not a pathway for the pathogen.
In addition to Acer plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the pathogen (such as Abies spp., Aesculus spp., Arbutus spp., Calluna spp., Castanea spp., Ceanothus spp., Chamaecyparis spp., Larix spp., etc.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). Phytophthora ramorum is able to survive in soil (Shishkoff, 2007) and therefore could potentially enter with infested soil/growing media. However, the growing media are certified and heat treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries with new seedlings of Acer and new plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.8.2.3. Possibility of spread within the nursery
Acer plants are either grown in containers (cells, pots, tubes, etc.) outdoors/in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pathogen can infect other suitable plants (such as Abies spp., Aesculus spp., Castanea spp., Larix spp., etc.) present within the nurseries and hedges surrounding the nurseries (Prunus spp.) (Dossier Sections 3.0 and 6.0).
Phytophthora ramorum can spread within the nurseries by aerial dissemination, soil, water, movement of infested plant material and animals (Davidson et al., 2002).
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible either by aerial dissemination, animals, movement of infested plant material, soil and water.
A.8.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Phytophthora ramorum between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.8.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Phytophthora ramorum is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
Phytophthora ramorum is a quarantine organism in the UK and targeted by this measure.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
Phytophthora ramorum is a quarantine organism in the UK and targeted by this measure.
Uncertainties:
|
| 4 | Growing media | Yes |
This measure should ensure pest‐free growing media and is expected to prevent the introduction of the pathogen into the nurseries with growing media.
Uncertainties:
|
| 5 | Surveillance, monitoring and sampling | Yes |
This measure has an effect as the pathogen would be detected on nursery‐grown plants, as well as on incoming plant material and growing media, and suspected plant material quarantined.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
General hygiene measures will reduce the likelihood of the pathogen being spread by tools and equipment, although this is not a major pathway for the pest.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This measure could have some effect by removing potentially infested plant material, thus reducing the spread of the pathogen within the nursery.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Testing of irrigation water would detect the pathogen, which can spread by water. Overhead irrigation could favour foliar infections and spread of the pathogen by water splash.
Uncertainties:
|
| 9 | Application of pest control products | Yes |
Some fungicides could reduce the likelihood of foliar infection by the pathogen.
Uncertainties:
|
| 10 | Measures against soil pests | Yes |
This measure could have some effect by preventing root contact with soil where the pathogen may be present.
Uncertainties:
|
| 11 | Inspections and management of plants before export | Yes |
P. ramorum is a quarantine organism in the UK and the EU and this measure is expected to reduce the likelihood of infested plants being exported.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.8.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.8.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Acer spp. to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.8.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.8.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Acer spp. The pathogen is a regulated quarantine pest in the UK and under official control.
A.8.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range).
The limited information on the susceptibility of Acer spp. and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.8.5.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infection (Table A.35) and pest freedom (Table A.36).
Table A.35.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 3 | 20 | 40 | 80 | 150 | ||||||||||
| EKE | 3.00 | 3.62 | 4.85 | 7.74 | 12.2 | 18.6 | 25.7 | 42.8 | 64.4 | 77.7 | 93.9 | 111 | 128 | 140 | 151 |
The EKE results are the BetaGeneral (0.82439, 1.9948, 2.7, 170) distribution fitted with @Risk version 7.6.
Table A.36.
The uncertainty distribution of bundles free of Phytophthora ramorum per 10,000 bundles calculated by Table A.35
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,920 | 9,960 | 9,980 | 9,997 | ||||||||||
| EKE results | 9,849 | 9,860 | 9,872 | 9,889 | 9,906 | 9,922 | 9,936 | 9,957 | 9,974 | 9,981 | 9,988 | 9,992 | 9,995 | 9,996 | 9,997 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles, the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.36.
A.8.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.8.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Acer spp. to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.8.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. Older trees are more likely to become infected due to longer exposure time and larger size. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.8.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Acer spp. The pathogen is a regulated quarantine pest in the UK and under official control.
A.8.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the susceptibility of Acer spp. and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.8.6.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on bare root plants/trees up to 7 years old
The following tables show the elicited and fitted values for pest infection (Table A.37) and pest freedom (Table A.38).
Table A.37.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 18 | 35 | 65 | 150 | ||||||||||
| EKE | 2.01 | 3.04 | 4.67 | 7.81 | 12.0 | 17.4 | 23.1 | 36.2 | 53.0 | 63.9 | 78.0 | 94.4 | 114 | 131 | 150 |
The EKE results are the BetaGeneral (1.1205, 5.2894, 1.2, 250) distribution fitted with @Risk version 7.6.
Table A.38.
The uncertainty distribution of plants free of Phytophthora ramorum per 10,000 plants calculated by Table A.37
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,935 | 9,965 | 9,982 | 9,998 | ||||||||||
| EKE results | 9,850 | 9,869 | 9,886 | 9,906 | 9,922 | 9,936 | 9,947 | 9,964 | 9,977 | 9,983 | 9,988 | 9,992 | 9,995 | 9,997 | 9,998 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.38.
A.8.7. Overall likelihood of pest freedom for plants in pots up to 7 years old
A.8.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Acer spp. to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.8.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. Older trees are more likely to become infected due to longer exposure time and larger size. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.8.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Acer spp. The pathogen is a regulated quarantine pest in the UK and under official control.
A.8.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the susceptibility of Acer spp. and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.8.7.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infection (Table A.39) and pest freedom (Table A.40).
Table A.39.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 3 | 30 | 60 | 110 | 250 | ||||||||||
| EKE | 3.02 | 4.76 | 7.50 | 12.8 | 20.0 | 29.2 | 39.0 | 61.5 | 90.1 | 109 | 132 | 160 | 192 | 219 | 250 |
The EKE results are the BetaGeneral (1.1036, 4.6992, 1.7, 390) distribution fitted with @Risk version 7.6.
Table A.40.
The uncertainty distribution of plants free of Phytophthora ramorum per 10,000 plants calculated by Table A.39
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,890 | 9,940 | 9,970 | 9,997 | ||||||||||
| EKE results | 9,750 | 9,781 | 9,808 | 9,840 | 9,868 | 9,891 | 9,910 | 9,939 | 9,961 | 9,971 | 9,980 | 9,987 | 9,992 | 9,995 | 9,997 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.40.
A.8.8. Reference list
APHIS USDA (Animal and Plant Health Inspection Service U.S. Department of Agriculture), 2022. APHIS lists of proven hosts of and plants associated with Phytophthora ramorum. September 2022. 12 pp. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/pram/downloads/pdf_files/usdaprlist.pdf
Blair JE, Coffey MD, Park SY, Geiser DM and Kang S, 2008. A multi‐locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology, 45, 266–277. https://doi.org/10.1016/j.fgb.2007.10.010
Boutet X, Vercauteren A, Heungens C and Kurt A, 2010. Mating of Phytophthora ramorum: functionality and consequences. In: Frankel SJ, Kliejunas JT; Palmieri KM (eds.). Proceedings of the sudden oak death fourth science symposium. USDA Forest Service, Pacific Southwest Research Station, Albany, CA: US Department of Agriculture, 214, 97–100.
Brasier C and Kirk S, 2004. Production of gametangia by Phytophthora ramorum in vitro. Mycological Research, 108, 823–827. https://doi.org/10.1017/s0953756204000565
Brasier C, 2008. Phytophthora ramorum + P. kernoviae = international biosecurity failure. In: Frankel SJ, Kliejunas JT and Palmieri KM (Eds.), Proceedings of the sudden oak death third science symposium. USDA Forest Service, Pacific Southwest Research Station, Albany, CA: US Department of Agriculture, 214, 133–139.
Brasier C and Webber J, 2010. Sudden larch death. Nature, 466, 824–825. https://doi.org/10.1038/466824a
Brown AV and Brasier CM, 2007. Colonization of tree xylem by Phytophthora ramorum, P. kernoviae and other Phytophthora species. Plant Pathology, 56, 227–241. https://doi.org/10.1111/j.1365-3059.2006.01511.x
CABI (Centre for Agriculture and Bioscience International), online. Phytophthora ramorum (Sudden Oak Death (SOD)). Available online: https://www.cabi.org/cpc/datasheet/40991 [Accessed: 27 September 2022].
Cave GL, Randall‐Schadel B and Redlin SC, 2008. Risk analysis for Phytophthora ramorum Werres, de Cock & Man in't Veld, causal agent of sudden oak death, ramorum leaf blight, and ramorum dieback. US Department of Agriculture, Animal and Plant Health Inspection Service, Raleigh, NC. 88 pp.
Davidson JM, Rizzo DM, Garbelotto M, Tjosvold S and Slaughter GW, 2002. Phytophthora ramorum and sudden oak death in California: II. Transmission and survival. In: Standiford RB, McCreary D and Purcell KL (eds.). Proceedings of the fifth symposium on oak woodlands: Oaks in California's challenging landscape. San Diego, California, US Department of Agriculture, Forest Service, Pacific Southwest Research Station, 184, 741–749.
Davidson JM, Werres S, Garbelotto M, Hansen EM and Rizzo DM, 2003. Sudden oak death and associated diseases caused by Phytophthora ramorum. Plant Health Progress, 4, 12. https://doi.org/10.1094/php-2003-0707-01-dg
Davidson JM, Wickland AC, Patterson HA, Falk KR and Rizzo DM, 2005. Transmission of Phytophthora ramorum in mixed‐evergreen forest in California. Phytopathology, 95, 587–596. https://doi.org/10.1094/phyto-95-0587
DEFRA (Department for Environment, Food and Rural Affairs), 2008. Consultation on future management of risks from Phytophthora ramorum and Phytophthora kernoviae. London, the UK: Department for Environment, Food and Rural Affairs. 22 pp.
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Phytophthora ramorum. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=23022 [Accessed: 12 December 2022].
Denman S, Kirk SA, Brasier CM and Webber JF, 2005. In vitro leaf inoculation studies as an indication of tree foliage susceptibility to Phytophthora ramorum in the UK. Plant Pathology, 54, 512–521. https://doi.org/10.1111/j.1365-3059.2005.01243.x
DiLeo MV, Bienapfl JC and Rizzo DM, 2008. Phytophthora ramorum infects hazelnut, vine maple, blue blossom, and manzanita species in California. Plant Health Progress, (January), 0118–02. https://doi.org/10.1094/php-2008-0118-02-br
EFSA Panel on Plant Health (PLH), 2011. Scientific Opinion on the Pest Risk Analysis on Phytophthora ramorum prepared by the FP6 project RAPRA. EFSA Journal 2011;9(6):2186, 108 pp. https://doi.org/10.2903/j.efsa.2011.2186
Elliot M, Meagher TR, Harris C, Searle K, Purse BV and Schlenzig A, 2013. The epidemiology of Phytophthora ramorum and P. kernoviae at two historic gardens in Scotland. In Frankel SJ, Kliejunas JT, Palmieri KM and Alexander JM (Eds.), Sudden oak death fifth science symposium. Albany, CA, the US: US Department of Agriculture, Forest Service, Pacific Southwest Research Station, 23–32.
Englander L, Browning M and Tooley PW, 2006. Growth and sporulation of Phytophthora ramorum in vitro in response to temperature and light. Mycologia, 98, 365–373. https://doi.org/10.3852/mycologia.98.3.365
EPPO (European and Mediterranean Plant Protection Organization), 2013. Pest risk management for Phytophthora kernoviae and Phytophthora ramorum. EPPO, Paris. Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2021–09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 27 September 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Phytophthora ramorum (PHYTRA), Categorization. Available online: https://gd.eppo.int/taxon/PHYTRA/categorization [Accessed: 27 September 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Phytophthora ramorum (PHYTRA), Distribution. Available online: https://gd.eppo.int/taxon/PHYTRA/distribution [Accessed: 27 September 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Phytophthora ramorum (PHYTRA), Host plants. Available online: https://gd.eppo.int/taxon/PHYTRA/hosts [Accessed: 27 September 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Phytophthora ramorum (PHYTRA), Photos. Available online: https://gd.eppo.int/taxon/PHYTRA/photos [Accessed: 27 September 2022].
Erwin DC and Ribeiro OK, 1996. Phytophthora diseases worldwide. St. Paul, Minnesota: APS Press, American Phytopathological Society, 562 pp.
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ [Accessed: 13 December 2022].
Grünwald NJ, Goss EM and Press CM, 2008. Phytophthora ramorum: a pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Molecular Plant Pathology, 9, 729–740. https://doi.org/10.1111/j.1364-3703.2008.00500.x
Grünwald NJ, Goss EM, Ivors K, Garbelotto M, Martin FN, Prospero S, Hansen E, Bonants PJM, Hamelin RC, Chastagner G, Werres S, Rizzo DM, Abad G, Beales P, Bilodeau GJ, Blomquist CL, Brasier C, Brière SC, Chandelier A, Davidson JM, Denman S, Elliott M, Frankel SJ, Goheen EM, de Gruyter H, Heungens K, James D, Kanaskie A, McWilliams MG, Man in ‘t Veld W, Moralejo E, Osterbauer NK, Palm ME, Parke JL, Perez Sierra AM, Shamoun SF, Shishkoff N, Tooley PW, Vettraino AM, Webber J and Widmer TL, 2009. Standardizing the nomenclature for clonal lineages of the sudden oak death pathogen, Phytophthora ramorum. Phytopathology, 99, 792–795.
Hayden KJ, Rizzo D, Tse J and Garbelotto M, 2004. Detection and quantification of Phytophthora ramorum from California forests using a real‐time polymerase chain reaction assay. Phytopathology, 94, 1075–1083. https://doi.org/10.1094/phyto.2004.94.10.1075
Jung T, Jung MH, Webber JF, Kageyama K, Hieno A, Masuya H, Uematsu S, Pérez‐Sierra A, Harris AR, Forster J, Rees H, Scanu B, Patra S, Kudláček T, Janoušek J, Corcobado T, Milenković I, Nagy Z, Csorba I, Bakonyi J and Brasier CM, 2021. The destructive tree pathogen Phytophthora ramorum originates from the laurosilva forests of East Asia. Journal of Fungi, 7, 226, 32 pp. https://doi.org/10.3390/jof7030226
King KM, Harris AR, Webber JF, 2015. In planta detection used to define the distribution of the European lineages of Phytophthora ramorum on larch (Larix) in the UK. Plant Pathology, 64, 1168–1175. https://doi.org/10.1111/ppa.12345
Parke JL and Lewis C, 2007. Root and stem infection of Rhododendron from potting medium infested with Phytophthora ramorum. Plant Disease, 91, 1265–1270. https://doi.org/10.1094/pdis-91-10-1265
Poimala A and Lilja A, 2013. NOBANIS – Invasive Alien Species Fact Sheet – Phytophthora ramorum. From: Online Database of the European Network on Invasive Alien Species. 14 pp. Available online: https://www.nobanis.org/globalassets/speciesinfo/p/phytophthora-ramorum/phytophthora_ramorum.pdf [Accessed: 12 December 2022].
Rizzo DM, Garbelotto M and Hansen EM, 2005. Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Annual Review of Phytopathology, 43, 13.1–13.27. https://doi.org/10.1146/annurev.phyto.42.040803.140418
Roubtsova TV and Bostock RM, 2009. Episodic abiotic stress as a potential contributing factor to onset and severity of disease caused by Phytophthora ramorum in Rhododendron and Viburnum. Plant Disease, 93, 912–918. https://doi.org/10.1094/pdis-93-9-0912
Sansford CE, Inman AJ, Baker R, Brasier C, Frankel S, de Gruyter J, Husson C, Kehlenbeck H, Kessel G, Moralejo E, Steeghs M, Webber J and Werres S, 2009. Report on the risk of entry, establishment, spread and socio‐economic loss and environmental impact and the appropriate level of management for Phytophthora ramorum for the EU. Deliverable Report 28. EU Sixth Framework Project RAPRA. 310 pp.
Shishkoff N, 2007. Persistence of Phytophthora ramorum in soil mix and roots of nursery ornamentals. Plant Disease, 91, 1245–1249. https://doi.org/10.1094/pdis-91-10-1245
Thompson CH, McCartney MM, Roubtsova TV, Kasuga T, Ebeler SE, Davis CE and Bostock RM, 2021. Analysis of volatile profiles for tracking asymptomatic infections of Phytophthora ramorum and other pathogens in Rhododendron. Phytopathology, 111, 1818–1827. https://doi.org/10.1094/phyto-10-20-0472-r
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Van Poucke K, Franceschini S, Webber J, Vercauteren A, Turner JA, Mccracken AR, Heungens K and Brasier C, 2012. Discovery of a fourth evolutionary lineage of Phytophthora ramorum: EU2. Fungal Biology, 116, 1178–1191. https://doi.org/10.1016/j.funbio.2012.09.003
A.9. Scirtothrips dorsalis
A.9.1. Organism information
| Taxonomic information |
Current valid scientific name: Scirtothrips dorsalis Synonyms: Anaphothrips andreae, Anaphothrips dorsalis, Anaphothrips fragariae, Heliothrips minutissimus, Neophysopus fragariae, Scirtothrips andreae, Scirtothrips dorsalis padmae, Scirtothrips fragariae, Scirtothrips minutissimus, Scirtothrips padmae Name used in the EU legislation: Scirtothrips dorsalis Hood [SCITDO] Order: Thysanoptera Family: Thripidae Common name: Assam thrips, chilli thrips, flower thrips, strawberry thrips, yellow tea thrips, castor thrips Name used in the Dossier: Scirtothrips dorsalis |
|
| Group | Insects | |
| EPPO code | SCITDO | |
| Regulated status |
The pest is listed in Annex II of Commission Implementing Regulation (EU) 2019/2072 as Scirtothrips dorsalis Hood [SCITDO]. Scirtothrips dorsalis is included in the EPPO A2 list (EPPO, online_a). The species is a quarantine pest in Israel, Mexico, Morocco and Tunisia. It is on A1 list of Brazil, Chile, Egypt, Kazakhstan, Russia, Türkiye, Ukraine, the UK and EAEU (Eurasian Economic Union – Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia). It is on A2 list of Bahrain (EPPO, online_b). |
|
| Pest status in the UK |
Scirtothrips dorsalis was found for the first time in the UK in December 2007 in a greenhouse (Palm House) at Royal Botanic Garden Kew in South England (Scott‐Brown et al., 2018). Since 2008 the discovered population has been under official control by the plant health authorities with the objective of achieving complete eradication (Collins, 2010). Eradication measures were applied and since 2019 the pest was no longer found (EPPO, online_c). According to the Dossier Section 5.0, S. dorsalis is present: not widely distributed and under official control. This is a regulated quarantine pest for GB. It has been found in one tropical glasshouse at Kew (Botanic Gardens, Richmond, London), and at no other location. It has been subject to control measures for many years, and there have been no recent records – last official records are from 2012. It is possible that this pest has been eradicated, but the UK is unable to officially confirm this at this time. Therefore, the Panel cannot exclude that the pest is still present in the UK. |
|
| Pest status in the EU |
Scirtothrips dorsalis is present with restricted distribution in Spain and transient in the Netherlands (EPPO, online_c). According to EUROPHYT Oubreaks database (online), there were three outbreaks, which are under eradication:
Scirtothrips dorsalis is continuously intercepted in the EU points‐of‐entry on different commodities: plants for planting; cut flowers and branches with foliage; fruits and vegetables (EUROPHYT/TRACES‐NT, online). |
|
| Host status on Acer |
Acer palmatum is reported host of S. dorsalis (Ohkubo, 1995; CABI, online). Scirtothrips dorsalis is a pest of other Acer species such as A. buergerianum and A. sieboldianum (Ohkubo, 1995; CABI, online). There is no information on whether S. dorsalis can also attack A. pseudoplatanus. |
|
| PRA information | Available pest risk assessments:
|
|
| Other relevant information for the assessment | ||
| Biology |
Scirtothrips dorsalis is a thrips present in Africa (Cote d'Ivoire, Kenya, Uganda), Asia (Bangladesh, Brunei Darussalam, China, India, Indonesia, Iran, Israel, Japan, Malaysia, Myanmar, North Korea, Pakistan, Philippines, South Korea, Sri Lanka, Taiwan, Thailand, Vietnam), Europe (the Netherlands, Spain, Türkiye, the UK), North America (Caribbean, Florida, Georgia, Hawaii, Mexico, Texas), Oceania (Australia, Papua New Guinea, Solomon Islands) and South America (Brazil, Colombia, French Guiana, Suriname, Venezuela) (CABI, online; EPPO, online_c). In the literature, its origin is contradictory, it is reported as either native to Asia, Australasia or South Africa. For more details, refer to Mound and Palmer (1981), Seal et al. (2006), Hoddle et al. (2008), Kumar et al. (2013) and CABI (online). According to Dickey et al. (2015), S. dorsalis is a species complex that includes at least nine cryptic species and two morphologically distinguishable species (S. oligochaetus and S. aff. dorsalis). Scirtothrips dorsalis develops through five life stages: egg, larva (two instars), prepupa, pupa and adult (Dev, 1964; Kumar et al., 2013). They can be found on all the aboveground plant parts (Kumar et al., 2014), and they damage young leaves, buds, tender stems and fruits by sucking tender tissues with their stylets (Kumar et al., 2013). Temperature thresholds for development are 9.7°C and 32°C, with 265 degree‐days required for development from egg to adult (Tatara, 1994). The adult can live up to 13–15 days (Kumar et al., 2013, citing others). Scirtothrips dorsalis can have annually up to 8 generations in Japan (Tatara, 1994). In the US, it was estimated by a degree‐day model that in some of the southern states the thrip can potentially have up to 18 generations per year (Nietschke et al., 2008). Scirtothrips dorsalis can reproduce both sexually and by haplodiploid parthenogenesis, with females developing from fertilised and males from unfertilised eggs (Dev, 1964). Female can lay between 60 and 200 eggs (Seal and Klassen, 2012), which are inserted into soft plant tissues of buds and young leaves near the mid rib or into the veins. But sometimes they are also laid into older leaves (Dev, 1964). The eggs hatch in 6–8 days (Seal and Klassen, 2012). Eggs are glassy white about 0.25 mm long and 0.1 mm wide. First and second‐instar larvae are white, yellow to light orange and their length size ranges between 0.29–0.32 and 0.48–0.59 mm, respectively (Dev, 1964). Prepupa is yellowish and pupa dark yellow (CABI, online) with 0.59–0.63 mm in length (Dev, 1964). Adults are pale yellow to greyish white in colour (Seal and Klassen, 2012). Female is approximately 1.05 mm long and 0.19 mm wide. Males are smaller 0.71 mm long and 0.14 mm wide |
|
|
(Dev, 1964). Larvae and adults tend to gather near the mid‐vein or near the damaged part of leaf tissue. Pupae are found in the leaf litter, on the axils of the leaves, in curled leaves or under the calyx of flowers and fruits (MacLeod and Collins, 2006; Kumar et al., 2013). Prepupa and pupa stages never feed (Tatara, 1994). Adults fly actively for short distances – tens of metres (Masui, 2007a) and passively on wind currents, which enables long‐distance spread (EFSA PLH Panel, 2014). They overwinter as adults (Okada and Kudo, 1982) in bark, litter, soil and protected in plant parts (Shibao, 1991; Holtz, 2006). The thrips cannot survive if the temperature remains below – 4°C for 5 or more days (Nietschke et al., 2008). Scirtothrips dorsalis is a vector of plant viruses including capsicum chlorosis virus (CaCV), chilli leaf curl virus (CLC), melon yellow spot virus (MYSV), peanut chlorotic fan virus (PCFV), peanut necrosis virus (PBNV), peanut yellow spot virus (PYSV), tobacco streak virus (TSV) and watermelon silver mottle virus (WsMoV) (Satyanarayana et al., 1996; Rao et al., 2003; Seal et al., 2010; Kumar et al., 2013). However, these viruses are not reported to infect Acer species. Possible pathways of entry for S. dorsalis are plants for planting, cut flowers, fruits, vegetables, soil and growing media (EFSA PLH Panel, 2014). Scirtothrips dorsalis causes economic loses to chilli (Capsicum annuum) in India with yield loss estimated between 61% and 74% (Kumar et al., 2013, citing others), mango in Malaysia (Aliakbarpour et al., 2010), vegetables in China and the US (Reitz et al., 2011), tea, grapevine and citrus in Japan (Tatara, 1994, citing others; Masui, 2007b). No information is available about damage on Acer species. |
||
| Symptoms | Main type of symptoms |
According to Dev (1964) and Kumar et al. (2013; 2014), main symptoms caused by S. dorsalis are:
When the population is high, thrips may feed on the upper surfaces of leaves and cause defoliation and yield loss (Kumar et al., 2013). There is no information on the symptoms caused to Acer plants. |
| Presence of asymptomatic plants | Plant damage might not be obvious in early infestation or during dormancy (due to absence of leaves). The presence of S. dorsalis on the plants could hardly be observed. | |
| Confusion with other pests |
Plants infested by S. dorsalis appear similar to plants damaged by the feeding of broad mites (Kumar et al., 2013). Due to small size and morphological similarities within the genus, the identification of S. dorsalis, using traditional taxonomic keys, is difficult. The most precise identification of the pest is combination of molecular and morphological methods (Kumar et al., 2013). |
|
| Host plant range |
Scirtothrips dorsalis is a polyphagous pest with more than 100 reported hosts (Kumar et al., 2013). The pest can infest many more plant species, but they are not considered true hosts, since the pest cannot reproduce on all of them (EFSA PLH Panel, 2014). Some of the many hosts of Scirtothrips dorsalis are (alphabetically): Abelmoschus esculentus, Acacia auriculiformis, Acacia brownii, Acer palmatum, A. buergerianum, A. sieboldianum, Actinidia deliciosa, Allium cepa, Allium sativum, Anacardium occidentale, Arachis hypogaea, Asparagus officinalis, Beta vulgaris, Camellia sinensis, Capsicum annuum, Capsicum frutescens, Citrus spp., Citrus aurantiifolia, Citrus sinensis, Cucumis melo, Cucumis sativus, Cucurbita pepo, Dahlia pinnata, Dimocarpus longan, Diospyros kaki, Fagopyrum esculentum, Ficus spp., Ficus carica, Fragaria spp., Fragaria ananassa, Fragaria chiloensis, Glycine max, Gossypium spp., Gossypium hirsutum, Hedera helix, Helianthus annuus, Hevea brasiliensis, Hydrangea spp., Ipomoea batatas, Lablab purpureus, Ligustrum japonicum, Litchi chinensis, Mangifera indica, Melilotus indica, Mimosa spp., Morus spp., Nelumbo spp., Nelumbo lutea, Nelumbo nucifera, Nephelium lappaceum, Nicotiana tabacum, Passiflora edulis, Persea americana, Phaseolus vulgaris, Populus deltoides, Portulaca oleracea, Prunus spp., Prunus persica, Punica granatum, Pyrus spp., Ricinus communis, Rosa spp., Rubus spp., Saraca spp., Solanum spp., Solanum lycopersicum, Solanum melongena, Solanum nigrum, Syzygium samarangense, Tamarindus indica, Viburnum spp., Vigna radiata, Vitis spp., Vitis vinifera, Zea mays subsp. mays and Ziziphus mauritiana (Ohkubo, 1995; Hodges et al., 2005; Kumar et al., 2014; CABI, online). For a full host list refer to Ohkubo (1995), Hodges et al. (2005), Kumar et al. (2014), CABI (online). |
|
| Reported evidence of impact | Scirtothrips dorsalis is an EU quarantine pest. | |
| Evidence that the commodity is a pathway | Scirtothrips dorsalis is continuously intercepted in the EU on different commodities including plants for planting (EUROPHYT/TRACES‐NT, online) and according to EFSA PLH Panel (2014), S. dorsalis can travel with plants for planting. Therefore, plants for planting are possible pathways of entry for S. dorsalis. | |
| Surveillance information | Scirtothrips dorsalis has been found in one tropical glasshouse at Kew (Botanic Gardens, Richmond, London), and at no other location. This pest has been subject to control measures for many years, and there have been no recent records. FERA diagnostics records show that sticky trap surveys at Kew were carried out in November 2007; January 2008; September 2022; October 2022 and November 2022 – all proved negative for the presence of this pest. It is possible that this pest has been eradicated, but the UK is unable to officially confirm this at this time (Dossier Section 3.0). | |
A.9.2. Possibility of pest presence in the nursery
A.9.2.2. Possibility of entry from the surrounding environment
Scirtothrips dorsalis was found in a greenhouse at Kew Gardens in South England in 2007 (Scott‐ Brown et al., 2018) and since then it has been under official control (Dossier Section 5.0), although last official records are from 2012. However, there is no information of the thrips being able to spread beyond the greenhouse.
The possible entry of S. dorsalis from surrounding environment to the nurseries may occur through adult dispersal and passively on wind currents (EFSA PLH Panel, 2014).
Scirtothrips dorsalis is polyphagous species that can infest a number of different plants. Suitable hosts of S. dorsalis like Acer spp., Beta vulgaris, Camellia spp., Capsicum annuum, Hedera spp., Morus spp., Populus spp., Prunus spp., Rhododendron spp., Rosa spp., Solanum lycopersicum, Viburnum spp. and Zea mays are present within 2 km from the nurseries.
Uncertainties:
-
–
Presence of the thrips in the UK.
-
–
Possibility of spread beyond the infested greenhouse.
-
–
Possibility of the thrips to survive the UK winter and summer in outdoor conditions.
Taking into consideration the above evidence and uncertainties, the Panel cannot exclude that the pest is present in the surrounding environment and can enter the nurseries, even though it was found only in one greenhouse. In the surrounding area, suitable hosts are present and the pest can spread by wind and adult flight.
A.9.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0). Seeds are not a pathway for the thrips.
In addition to Acer plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the thrips (such as Euphorbia spp., Gaura spp., Hedera spp., Ligustrum spp., Pittosporum spp., Populus spp., Prunus spp., Rosa spp., etc.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the thrips could possibly travel with them.
According to Shibao (1991) and Holtz (2006), adults overwinter in leaf litter and potting soil. The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries with new seedlings of Acer and new plants of other species used for plant production in the area. The entry of the pest with seeds and the growing media the Panel considers as not possible.
A.9.2.3. Possibility of spread within the nursery
Acer plats are either grown in containers (cells, pots, tubes, etc.) outdoors/ in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The thrips can attack other suitable plants (such as Euphorbia spp., Gaura spp., Hedera spp., etc.), present within the nurseries and hedges surrounding the nurseries (Hedera spp. and Prunus spp.) (Dossier Sections 3.0 and 6.0).
The thrips within the nurseries can spread by adult flight, wind or infested soil. Spread within the nurseries through equipment and tools is not relevant.
Uncertainties:
-
–
Possibility of the thrips to survive the UK winter in outdoor conditions.
-
–
Possibility of different plant host species for trade.
-
–
Possibility that polytunnels are used in a way that allows the pest to overwinter.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nurseries is possible either by wind, active flight or infested soil.
A.9.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Scirtothrips dorsalis between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.9.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Scirtothrips dorsalis is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
As the plant passport is very similar to the EU one, the Acer plants shall be free from quarantine pests.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. Physical separation is not a barrier for S. dorsalis because the adults can fly. |
| 3 | Certified plant material | Yes |
The measure is effective against the pest.
Uncertainties:
|
| 4 | Growing media | No | Not relevant. Scirtothrips dorsalis is not present in soil. |
| 5 | Surveillance, monitoring and sampling | Yes |
Although the incoming plant material is thoroughly checked, later infestation by S. dorsalis can go undetected, because its small size.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
Weeding can have some effect on the reduction of Scirtothrips populations. The other measures are not relevant.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
Removal of generically damaged plants will reduce the probability of carrying S. dorsalis.
Uncertainties:
|
| 8 | Irrigation water | No | Not relevant. |
| 9 | Application of pest control products | Yes |
Chemical control measures adopted when the pest is found may have a substantial effect of pest suppression.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
Inspection should detect S. dorsalis as symptoms are clear.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. Acer plants are not individually separated during transportation. The pest can infest other plants carrying leaves. |
A.9.5.2. Overall likelihood of pest freedom for bundles of whips and seedlings
A.9.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bundles of whips and seedlings
This scenario assumes the absence of the pest in the nurseries and the surrounding as the species was never recorded again after the first detection in the palm house at Kew Garden and it has been never found outside of the greenhouse.
A.9.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bundles of whips and seedlings
This scenario assumes the pest is present in the nurseries and the surrounding although with very low density and low capacity to adapt to the local climatic conditions.
A.9.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bundles of whips and seedlings (Median)
This scenario assumes that the presence of the pest in the nurseries is skewed to the lower values because the conditions for its survival and spread are generally not met. The lack of finding after the first detection indicates a likely absence of the pest and its low capacity to establish on Acer plants in the growing conditions indicated in the Dossier.
A.9.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because symptoms are generic and require an expert identification integrated with molecular methods.
A.9.5.5. Elicitation outcomes of the assessment of the pest freedom for Scirtothrips dorsalis on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infestation (Table A.41) and pest freedom (Table A.42).
Table A.41.
Elicited and fitted values of the uncertainty distribution of pest infestation by Scirtothrips dorsalis per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 1 | 3 | 6 | 20 | ||||||||||
| EKE | 0.0175 | 0.0548 | 0.131 | 0.314 | 0.609 | 1.04 | 1.56 | 2.88 | 4.80 | 6.15 | 8.05 | 10.4 | 13.5 | 16.4 | 20.0 |
The EKE results are the BetaGeneral (0.80489, 10.921, 0, 63) distribution fitted with @Risk version 7.6.
Table A.42.
The uncertainty distribution of bundles free of Scirtothrips dorsalis per 10,000 bundles calculated by Table A.41
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,980 | 9,994 | 9,997 | 9,999 | 10,000 | ||||||||||
| EKE results | 9,980 | 9,984 | 9,987 | 9,990 | 9,992 | 9,994 | 9,995 | 9,997 | 9,998 | 9,999.0 | 9,999.4 | 9,999.7 | 9,999.87 | 9,999.95 | 9,999.98 |
The EKE results are the fitted values.
Based on the numbers of estimated infested bundles, the pest freedom was calculated (i.e. = 10,000 – number of infested bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.42.
A.9.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.9.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants/trees up to 7 years old
This scenario assumes the absence of the pest in the nurseries and the surrounding as the species was never recorded again after the first detection in the palm house at Kew Garden and it has been never found outside of the greenhouse.
A.9.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants/trees up to 7 years old
This scenario assumes the pest is present in the nurseries and the surrounding although with very low density and low capacity to adapt to the local climatic conditions. Older plants could carry a higher number of leaves promoting the establishment of the pest if present in the surroundings.
A.9.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants/trees up to 7 years old (Median)
This scenario assumes that the presence of the pest in the nurseries is skewed to the lower values because the conditions for its survival and spread are generally not met. The lack of finding after the first detection indicates a likely absence of the pest and its low capacity to establish on Acer plants in the growing conditions indicated in the Dossier.
A.9.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because symptoms are generic and require an expert identification integrated with molecular methods.
A.9.6.5. Elicitation outcomes of the assessment of the pest freedom for Scirtothrips dorsalis on bare root plants/trees up to 7 years old
The following tables show the elicited and fitted values for pest infestation (Table A.43) and pest freedom (Table A.44).
Table A.43.
Elicited and fitted values of the uncertainty distribution of pest infestation by Scirtothrips dorsalis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 2 | 4 | 7 | 20 | ||||||||||
| EKE | 0.1741 | 0.3356 | 0.558 | 0.943 | 1.42 | 2.01 | 2.61 | 3.99 | 5.79 | 7.02 | 8.69 | 10.8 | 13.5 | 16.2 | 19.7 |
The EKE results are the BetaGeneral (1.4365, 2,808.7, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.44.
The uncertainty distribution of plants free of Scirtothrips dorsalis per 10,000 plants calculated by Table A.43
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,980 | 9,993 | 9,996 | 9,998 | 10,000 | ||||||||||
| EKE results | 9,980 | 9,984 | 9,986 | 9,989 | 9,991 | 9,993 | 9,994 | 9,996 | 9,997 | 9,998.0 | 9,998.6 | 9,999.1 | 9,999.4 | 9,999.7 | 9,999.8 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.44.
A.9.7. Overall likelihood of pest freedom for plants in pots up to 7 years old
A.9.7.1. Reasoning for a scenario which would lead to a reasonably low number of infested plants in pots up to 7 years old
This scenario assumes absence of the pest in the nurseries and the surrounding as the species was never recorded again after the first detection in the palm house at Kew Garden and it has been never found outside of the greenhouse.
A.9.7.2. Reasoning for a scenario which would lead to a reasonably high number of infested plants in pots up to 7 years old
This scenario assumes the pest is present in the nurseries and the surrounding although with very low density and low capacity to adapt to the local climatic conditions. Older plants could carry a higher number of leaves promoting the establishment of the pest if present in the surroundings. There could be insects overwintering in the soil and these are extremely difficult to detect.
A.9.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested plants in pots up to 7 years old (Median)
This scenario assumes that the presence of the pest in the nurseries is skewed to the lower values because the conditions for its survival and spread are generally not met. The lack of finding after the first detection indicates a likely absence of the pest and its low capacity to establish on Acer plants in the growing conditions indicated in the Dossier.
A.9.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because symptoms are generic and require an expert identification integrated with molecular methods and careful soil inspection with appropriate methods.
A.9.7.5. Elicitation outcomes of the assessment of the pest freedom for Scirtothrips dorsalis on plants in pots up to 7 years old
The following Tables show the elicited and fitted values for pest infestation (Table A.45) and pest freedom (Table A.46).
Table A.45.
Elicited and fitted values of the uncertainty distribution of pest infestation by Scirtothrips dorsalis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 3 | 6 | 10 | 30 | ||||||||||
| EKE | 0.304 | 0.562 | 0.907 | 1.49 | 2.20 | 3.05 | 3.92 | 5.89 | 8.43 | 10.1 | 12.5 | 15.4 | 19.1 | 22.9 | 27.7 |
The EKE results are the BetaGeneral (1.539, 2,074.2, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.46.
The uncertainty distribution of plants free of Scirtothrips dorsalis per 10,000 plants calculated by Table A.45
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,970 | 9,990 | 9,994 | 9,997 | 10,000 | ||||||||||
| EKE results | 9,972 | 9,977 | 9,981 | 9,985 | 9,988 | 9,990 | 9,992 | 9,994 | 9,996 | 9,996.9 | 9,997.8 | 9,998.5 | 9,999.1 | 9,999.4 | 9,999.7 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.46.
A.9.8. Reference list
Aliakbarpour H, Che Salmah MR and Dieng H, 2010. Species composition and population dynamics of thrips (Thysanoptera) in mango orchards of northern peninsular Malaysia. Environmental Entomology, 39, 1409–1419.
CABI (Centre for Agriculture and Bioscience International), online. Scirtothrips dorsalis (chilli thrips). Available online: https://www.cabi.org/cpc/datasheet/49065#REF-DDB-202162 [Accessed: 28 November 2022].
Collins DW, 2010. Thysanoptera of Great Britain: a revised and updated checklist. Zootaxa, 2412, 21–41. doi:10.11646/zootaxa.2412.1.2
DEFRA (Department for Environment, Food and Rural Affairs), online. UK risk register details for Scirtothrips dorsalis. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=21873 [Accessed: 28 November 2022].
Dev HN, 1964. Preliminary studies on the biology of Assam thrips, Scirtothrips dorsalis Hood on tea. Indian Journal of Entomology, 26, 184–194.
Dickey AM, Kumar V, Hoddle MS, Funderburk JE, Morgan JK, Jara‐Cavieres A, Shatters RGJ, Osborne LS and McKenzie CL, 2015. The Scirtothrips dorsalis species complex: endemism and invasion in a global pest. PLoS One, 10, e0123747. 22 pp. 10.1371/journal.pone.0123747
EFSA PLH Panel (EFSA Panel on Plant Health), 2014. Scientific Opinion on the pest categorisation of Scirtothrips dorsalis. EFSA Journal 2014;12(12):3915, 29 pp. https://doi.org/10.2903/j.efsa.2014.3915
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques MA, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Chatzivassiliou E, Debode J, Manceau C, Gardi C, Mosbach‐Schulz O and Potting R, 2020. Scientific Opinion on the commodity risk assessment of Jasminum polyanthum plants from Israel. EFSA Journal 2020;18(8):6225, 78 pp. https://doi.org/10.2903/j.efsa.2020.6225
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Mosbach‐Schulz O and Gonthier P, 2021a. Scientific Opinion on the commodity risk assessment of Ficus carica plants from Israel. EFSA Journal 2021;19(1):6353, 249 pp. https://doi.org/10.2903/j.efsa.2021.6353
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Zappalà L, Gómez P, Lucchi A, Urek G, Tramontini S, Mosbach‐Schulz O, de la Peña E and Yuen J, 2021b. Scientific Opinion on the commodity risk assessment of Persea americana from Israel. EFSA Journal 2021;19(2):6354, 195 pp. https://doi.org/10.2903/j.efsa.2021.6354
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Chatzivassiliou E, Di Serio F, Baptista P, Gonthier P, Jaques Miret JA, Fejer Justesen A, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Debode J, Manceau C, Gardi C, Mosbach‐Schulz O and Potting R, 2022a. Scientific Opinion on the commodity risk assessment of Jasminum polyanthum unrooted cuttings from Uganda. EFSA Journal 2022;20(5):7300, 83 pp. https://doi.org/10.2903/j.efsa.2022.7300
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stancanelli G, Stergulc F and Gonthier P, 2022b. Scientific Opinion on the commodity risk assessment of Ligustrum delavayanum topiary plants grafted on Ligustrum japonicum from the UK. EFSA Journal 2022;20(11):7593, 88 pp. https://doi.org/10.2903/j.efsa.2022.7593
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2022–09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Scirtothrips dorsalis (SCITDO), Categorization. Available online: https://gd.eppo.int/taxon/SCITDO/categorization [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Scirtothrips dorsalis (SCITDO), Distribution. Available online: https://gd.eppo.int/taxon/SCITDO/distribution [Accessed: 28 November 2022].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions – EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
EUROPHYT Oubreaks database, online. European Union Notification System for Plant Health Interceptions – EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
Hodges G, Edwards GB and Dixon W, 2005. Chilli thrips Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) a new pest thrips for Florida. Florida Department of Agriculture and Consumer Service, Department of Primary Industries. Available online: http://www.doacs.state.fl.us/pi/enpp/ento/chillithrips.html
Holtz T, 2006. Scirtothrips dorsalis Hood: Chilli Thrips. New Pest Advisory Group (NPAG) Report. Plant Epidemiology and Risk Analysis Laboratory, Center for Plant Health Science and Technology, USDA‐APPHIS. the US: USDA‐APHIS. Available online: https://mrec.ifas.ufl.edu/lso/DOCUMENTS/Scirtothrips%20dorsalis%20NPAG%20et%20Report%20060310.pdf
Kumar V, Kakkar G, McKenzie CL, Seal DR and Osborne LS, 2013. An overview of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) biology, distribution and management. Weed and pest control‐Conventional and new challenges, 53–77. https://doi.org/10.5772/55045
Kumar V, Seal DR and Kakkar G, 2014. Chilli thrips Scirtothrips dorsalis Hood (Insecta: Thysanoptera: Thripidae). Journal of Entomology and Zoology Studies 2014, 2, 104–106. https://doi.org/10.1007/springerreference_85820
MacLeod A and Collins D, 2006. CSL pest risk analysis for Scirtothrips dorsalis. CSL (Central Science Laboratory), 8 pp.
Masui S, 2007a. Timing and distance of dispersal by flight of adult yellow tea thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae). Japanese Journal of Applied Entomology and Zoology, 51, 137–140. https://doi.org/10.1303/jjaez.2007.137
Masui S, 2007b. Synchronism of immigration of adult yellow tea thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) to citrus orchards with reference to their occurrence on surrounding host plants. Applied Entomology and Zoology, 42, 517–523.
Mound L and Palmer J, 1981 Identification, distribution and host‐plants of the pest species of Scirtothrips (Thysanoptera: Thripidae). Bulletin of Entomological Research, 71, 467–479.
Nietschke BS, Borchert DM, Magarey RD and Ciomperlik MA, 2008. Climatological potential for Scirtothrips dorsalis (Thysanoptera: Thripidae) establishment in the United States. Florida Entomologist, 91, 79–86. https://doi.org/10.1653/0015‐4040(2008)091[0079:cpfsdt]2.0.co;2
Ohkubo N, 1995. Host plants of yellow tea thrips, Scirtothrips dorsalis Hood and annual occurrence on them. Bulletin of the Nagasaki Fruit Tree Experimental Station, 2, 1–16. Available online: https://oa.mg/work/3125044213
Okada T and Kudo I, 1982. Overwintering sites and stages of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in Tea Fields. Japanese Journal of Applied Entomology and Zoology, 26, 177–182.
Rao PRDVJ, Reddy AS, Reddy SV, Thirumala‐Devi K, Chander Rao S, Manoj Kumar V, Subramaniam K, Yellamanda Reddy T, Nigam SN and Reddy DVR, 2003. The host range of Tobacco streak virus in India and transmission by thrips. Annals of Applied Biology, 142, 365–368. https://doi.org/10.1111/j.1744‐7348.2003.tb00262.x
Reitz SR, Yu‐lin G and Zhong‐ren L, 2011. Thrips: Pests of concern to China and the United States. Agricultural Sciences in China, 10, 867–892.
Satyanarayana T, Reddy KL, Ratna AS, Deom CM, Gowda S and Reddy DVR, 1996. Peanut yellow spot virus: a distinct tospovirus species based on serology and nucleic acid hybridization. Annals of Applied Biology, 129, 237–245. https://doi.org/10.1111/j.1744‐7348.1996.tb05748.x
Scott‐Brown AS, Hodgetts J, Hall J, Simmonds MJS and Collins DW, 2018. Potential role of botanic garden collections in predicting hosts at risk globally from invasive pests: a case study using Scirtothrips dorsalis. Journal of Pest Science, 91, 601–611.
Seal DR, Klassen W and Kumar V, 2010. Biological parameters of Scirtothrips dorsalis (Thysanoptera: Thripidae) on selected hosts. Environmental Entomology, 39, 1389–1398. https://doi.org/10.1603/en09236
Seal DR, Ciomperlik M, Richards ML and Klassen W, 2006. Comparative effectiveness of chemical insecticides against the chilli thrips Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), on pepper and their compatibility with natural enemies. Crop Protection, 25, 949–955. https://doi.org/10.1016/j.cropro.2005.12.008
Seal DR and Klassen W, 2012. Chilli thrips (castor thrips, Assam thrips, yellow tea thrips, strawberry thrips), Scirtothrips dorsalis Hood, provisional management guidelines. University of Florida, Gainesville, FL, 4 pp.
Shibao M, 1991. Overwintering sites and stages of the chillie thrip Scirtothrips dorsalis HOOD (Thysanoptera: Thripidae) in Grapevine Fields. Japanese Journal of Applied Entomology and Zoology, 35, 161–163. https://doi.org/10.1303/jjaez.35.161
Tatara A, 1994. Effect of temperature and host plant on the development, fertility and longevity of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae). Applied Entomology and Zoology, 29, 31–37. https://doi.org/10.1303/aez.29.31
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Vierbergen B and van der Gaag DJ, 2009. Pest Risk Assessment Scirtothrips dorsalis. Plant Protection Service, the Netherlands. 9 pp. Available online: https://pra.eppo.int/getfile/ddcf51cf-df6d-40f9-9d28-46f447652ed7
A.10. Takahashia japonica
A.10.1. Organism information
| Taxonomic information |
Current valid scientific name: Takahashia japonica Synonyms: Pulvinaria japonica, Takahashia wuchangensis Name used in the EU legislation: – Order: Hemiptera Family: Coccidae Common name: Asiatic string cottony scale, string cottony scale Name used in the Dossier: Takahashia japonica |
|
| Group | Insects | |
| EPPO code | TAKAJA | |
| Regulated status | Takahashia japonica is neither regulated in the EU, nor anywhere in the world. T. japonica meets the criteria that are within the remit of EFSA to assess for this species to be regarded as a potential Union quarantine pest (EFSA PLH Panel, 2023a). | |
| Pest status in the UK |
Takahashia japonica is present in the UK (Tuffen et al., 2019). The pest was recorded from West Berkshire in 2018 on Magnolia in a private garden (Malumphy et al., 2019; Tuffen et al., 2019). According to the Dossier Section 5.0, T. japonica is present in the UK: not widely distributed and not under official control. |
|
| Pest status in the EU |
Takahashia japonica is present in Croatia and Italy (Limonta and Pellizzari, 2018; Landeka et al., 2021). In Italy, the pest was first reported in 2017 from the Northern provinces of Milano and Varese. High infestations of T. japonica indicated that the pest was most probably introduced some years before its detection (Limonta and Pellizzari, 2018). In Croatia the pest was observed for the first time in 2019 from the city of Pula (Landeka et al., 2021) and eradication measures were applied by cutting down the infested branches and by applying insecticides (EPPO, online). There is no information whether the eradication was successful or not. |
|
| Host status on Acer | Acer pseudoplatanus (Limonta and Pellizzari, 2018), A. buergerianum (Wang et al., 2016), A. negundo and A. pseudosieboldianum (Suh, 2020) are reported to be a host for T. japonica. | |
| PRA information | Available Pest Risk Assessments:
|
|
| Other relevant information for the assessment | ||
| Biology |
Takahashia japonica is a soft scale insect native to Asia (Limonta et al., 2022), where it is reported from China, India, Japan, South Korea and Taiwan. The species has been introduced in Europe (Croatia, Italy and the UK) (Limonta et al., 2022; García Morales et al., online). Takahashia japonica is a parthenogenetic species. The scale has three development stages: egg, nymph (two instars) and adult. In Italy it has one generation annually. Females start laying eggs from late April till early May. The female settles on the twig and in several days creates an ovisac. The ovisac is white string‐like loop that can be up to 7 cm long. Fecundity is very high. It was counted that there are around 1,200 eggs in a 1 cm length of the ovisac. Eggs start to hatch into first‐instar nymphs (crawlers) in early June. The crawlers are small, about 740 μm long and 325 μm wide. The crawlers are the main natural dispersal stage, and they migrate from twigs to underside of leaves. During this migration, the crawlers can be easily carried by the wind, insects or birds to other host plants. Long‐distance dispersal is with infested traded plants. In late August and in September, the population consists of second‐instar nymphs, which are about 1.3 mm long. |
|
|
In September and October, the second‐instar nymphs migrate back from leaves to twigs, to overwinter. Overwintering second‐instar nymphs are brown and covered by transparent wax plates. After overwintering, the nymphs resume activity from March onwards and reach the length of about 1.5 mm and the width of 0.5 mm. The moult to the adult female occurs at the same overwintering site. The first moults occur in early April, and the whole population reaches the adult stage over about 10 days. The adult female's body size increases quickly from about 1.5 to 6–7 mm long and 5 mm wide. In this growing phase, the pre‐ovigerous females feed and produce honeydew droplets (Limonta et al., 2022). Several natural enemies of T. japonica are recorded in the literature. The scale was found to have been parasitised in Italy and the UK (Tuffen et al., 2019; Limonta et al., 2022). Possible pathways of entry for T. japonica are plants for planting (except seeds bulbs and tubers) (DEFRA, online). |
||
| Symptoms | Main type of symptoms |
Heavy infestations of T. japonica on twigs cause dieback and necrosis of buds. It is mainly harmful to young trees (Limonta et al., 2022). The production of honeydew by females is limited (Limonta et al., 2022). From late April onwards (when the females start oviposition), the long white ovisacs hanging from the twigs and branches can be seen. Moreover, the ovisacs persist on the plants long after the eggs have hatched and are still present in winter (Limonta et al., 2022). |
| Presence of asymptomatic plants | Low initial infestations in the absence of ovisacs may be overlooked. | |
| Confusion with other pests | Takahashia japonica can be hardly confused with other scales, due to the characteristic ovisacs hanging from the twigs and branches (Malumphy et al., 2019). | |
| Host plant range | Takahashia japonica is highly polyphagous species with total of 35 known host species in 17 families (Limonta et al., 2022). The hosts are Acer negundo, A. buergerianum, A. pseudoplatanus, A. pseudosieboldianum, Albizia julibrissin, Alnus japonica, Carpinus betulus, Celtis australis, C. sinensis, Citrus sp., Cornus officinalis, Cydonia oblonga, Diospyros kaki, Juglans regia, Lespedeza sp., Lespedeza bicolor, Liquidambar styraciflua, Loropetalum chinense, Magnolia kobus, M. obovate, Malus pumila, Morus sp., M. alba, M. nigra, Parthenocissus tricuspidata, Prunus cerasifera, P. glandulosa, P. salicina, P. tomentosa, Pyrus serotina, Rhododendron schlippenbachii, Robinia pseudoacacia, Salix chaenomeloides, S. glandulosa, Styphnolobium japonicum, Ulmus davidiana and Zelkova serrata (Wang et al., 2016; Limonta and Pellizzari, 2018; Suh, 2020; Limonta et al., 2022; García Morales et al., online). | |
| Reported evidence of impact |
There are no reports of economic or ecological damage induced by T. japonica in Asia (Malumphy et al., 2019). According to Limonta et al. (2022) in Italy its impact on urban trees has mostly involved some honeydew production and the unsightly appearance of infested trees – long white ovisacs hanging from the branches. Takahashia japonica can potentially reduce esthetical value of plants (Malumphy et al., 2019). No data about damage on Acer species is available. |
|
| Evidence that the commodity is a pathway | According to DEFRA (online), T. japonica can travel with plants for planting. Therefore, the commodity is a pathway for T. japonica. | |
| Surveillance information | According to the Dossier Section 5.0, T. japonica is not under official surveillance in the UK, as does not meet criteria of quarantine pest for Great Britain. | |
A.10.2. Possibility of pest presence in the nursery
A.10.2.1 Possibility of entry from the surrounding environment
Takahashia japonica is present in the UK, not widely distributed and not under official control (Dossier Section 5.0). The scale was recorded from West Berkshire in 2018 on Magnolia in a private garden (Malumphy et al., 2019; Tuffen et al., 2019).
The possible entry of T. japonica from surrounding environment to the nurseries may occur through crawler dispersal by wind, infested machinery, with other insects and birds.
Takahashia japonica is polyphagous species that can infest a number of different plants. Suitable hosts of T. japonica like Acer spp., Juglans regia, Magnolia spp., Malus spp., Morus spp., Prunus spp., Salix spp. and Ulmus spp. are present within 2 km from the nurseries.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery. The pest could be present in the surrounding areas because of suitable hosts and the transferring rate could be enhanced by crawler dispersal, via infested machinery, with other insects and birds.
A.10.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are either from the UK, the EU (mostly the Netherlands) or New Zealand (Dossier Section 3.0). Seeds are not a pathway for the scale.
In addition to Acer plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the scale (such as the Netherlands Alnus spp., Carpinus betulus, Celtis australis, Cornus spp., Prunus spp., etc.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the scale could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). The growing media are certified and heat treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0). Soil is not a pathway for the scale.
Uncertainties:
-
–
No information is available on the provenance of plants other than Acer used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries with new seedlings of Acer and new plants of other species used for plant production in the area. The entry of the pest with seeds and the growing media the Panel considers as not possible.
A.10.2.3. Possibility of spread within the nursery
Acer plats are either grown in containers (cells, pots, tubes, etc.) outdoors/ in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The scale can attack other suitable plants (such as Alnus spp., Carpinus betulus, Celtis australis, etc.), present within the nurseries and hedges surrounding the nurseries (Prunus spp.) (Dossier Sections 3.0 and 6.0).
The scale within the nurseries can spread by crawler dispersal (wind), nursery workers and machinery. Spread within the nurseries through equipment and clothing is less relevant as the distance walked is very limited and of a short duration.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nurseries is possible either by wind, nursery workers, machinery, equipment and clothing.
A.10.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Acer plants for planting neither from the UK nor from other countries due to the presence of Takahashia japonica between the years 1995 and December 2022 (EUROPHYT/TRACES‐NT, online).
A.10.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on Takahashia japonica is provided. The description of the risk mitigation measures currently applied in the UK is provided in Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
Although T. japonica is not a quarantine pest, it is easy to spot. The risk mitigation measure could have some effects in reducing the likelihood of the presence of the pest on the commodity.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. Physical separation is not a barrier for T. japonica because crawlers can be easily spread by wind and air currents. |
| 3 | Certified plant material | Yes |
The risk mitigation measure could have some effects in reducing the likelihood of the presence of the pest on the commodity.
Uncertainties:
|
| 4 | Growing media | No | Not relevant. Takahashia japonica is not present in soil. |
| 5 | Surveillance, monitoring and sampling | Yes |
Incoming plant material is thoroughly checked, T. japonica is easy to spot. The risk mitigation measure could have some effects in reducing the likelihood of the presence of the pest on the commodity.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
Nymphs could move with equipment/clothes of the nursery staff. The described measures could have very little effect in reducing the likelihood of the presence of the pest on the commodity. Takahashia japonica is not present on weeds.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
Removal of generically damaged plants could reduce the probability of carrying T. japonica, but dieback and necrosis of buds are only expected in heavy infestations.
Uncertainties:
|
| 8 | Irrigation water | No | Not relevant. |
| 9 | Application of pest control products | Yes |
Chemical control measures adopted when the pest is found may have a substantial effect of pest suppression.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
Inspection should detect T. japonica as signs are clear.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. Acer plants are not individually separated during transportation. |
A.10.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.10.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bundles of whips and seedlings
This scenario assumes a low pressure of the pest in the nurseries and the surrounding: There is only one report from the UK in a private garden in West Berkshire. In this scenario, it is also assumed that signs of the pest are easy to spot as ovisacs are clearly visible and very conspicuous, and because small plants are easier to handle. Therefore, measures against the pest can be taken.
A.10.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bundles of whips and seedlings
This scenario assumes that the pest is present in the nurseries and the surrounding. It also assumes that, although there is only one report from the UK in a private garden in West Berkshire, this report is very recent (2018), so pest may be spreading. The scenario assumes that, in the case of low‐density populations or initial stages of infestation, nymphs can be overlooked during inspections, and mode of action of the pesticides used may not be effective against the pest. Moreover, ovisacs are less frequent in younger plants and therefore not so easily noticed. Finally, the scenario assumes that pest is coming from the surroundings and a bundle effect is expected to increase the number of infestations.
A.10.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bundles of whips and seedlings (Median)
The Panel assumes a high efficacy of the surveillance and inspections conducted in the nursery due to conspicuous aspect of the ovisacs of adult female; therefore, measures against the pest can be taken. The Panel also assumes a very low pressure of the pest from the surroundings. These considerations lead Panel to indicate low value of the median.
A.10.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because of the ease of detection of signs of the pest, because of the low pressure of the pest from the surroundings and because of effectiveness of measures conducted to control the pest in the nursery.
A.10.5.5. Elicitation outcomes of the assessment of the pest freedom for Takahashia japonica on bundles of whips and seedlings
The following tables show the elicited and fitted values for pest infestation (Table A.47) and pest freedom (Table A.48).
Table A.47.
Elicited and fitted values of the uncertainty distribution of pest infestation by Takahashia japonica per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 13 | 25 | 55 | 125 | ||||||||||
| EKE | 1.01 | 1.48 | 2.37 | 4.37 | 7.38 | 11.6 | 16.3 | 27.7 | 42.8 | 52.7 | 65.5 | 80.0 | 96.7 | 111 | 125 |
The EKE results are the BetaGeneral (0.88135, 3.505, 0.75, 175) distribution fitted with @Risk version 7.6.
Table A.48.
The uncertainty distribution of bundles free of Takahashia japonica per 10,000 bundles calculated by Table A.47
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,875 | 9,945 | 9,975 | 9,987 | 9,999 | ||||||||||
| EKE results | 9,875 | 9,889 | 9,903 | 9,920 | 9,934 | 9,947 | 9,957 | 9,972 | 9,984 | 9,988 | 9,993 | 9,996 | 9,997.6 | 9,998.5 | 9,999.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested bundles, the pest freedom was calculated (i.e. = 10,000 – number of infested bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.48.
A.10.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.10.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants/trees up to 7 years old
This scenario assumes a low pressure of the pest in the nurseries and the surrounding: There is only one report from the UK in a private garden in West Berkshire. In this scenario, it is also assumed that signs of the pest are easy to spot as ovisacs are clearly visible and very conspicuous. Therefore, measures against the pest can be taken.
A.10.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants/trees up to 7 years old
This scenario assumes that the pest is present in the nurseries and the surrounding. It also assumes that, although there is only one report from the UK in a private garden in West Berkshire, this report is very recent (2018), so pest may be spreading. The scenario assumes that, in the case of low‐density populations or initial stages of infestation, nymphs can be overlooked during inspections, and mode of action of the pesticides used may not be effective against the pest.
A.10.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants/trees up to 7 years old (Median)
The Panel assumes a high efficacy of the surveillance and inspections conducted in the nursery due to conspicuous aspect of the ovisacs of adult female, therefore, measures against the pest can be taken. The Panel also assumes a very low pressure of the pest from the surroundings. These considerations lead Panel to indicate low value of the median.
A.10.6.4 Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because of the ease of detection of signs of the pest, because of the low pressure of the pest from the surroundings and because of effectiveness of measures conducted to control the pest in the nursery.
A.10.6.5. Elicitation outcomes of the assessment of the pest freedom for Takahashia japonica on bare root plants/trees up to 7 years old
The following tables show the elicited and fitted values for pest infestation (Table A.49) and pest freedom (Table A.50).
Table A.49.
Elicited and fitted values of the uncertainty distribution of pest infestation by Takahashia japonica per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 10 | 18 | 50 | 100 | ||||||||||
| EKE | 0.988 | 1.12 | 1.45 | 2.47 | 4.42 | 7.63 | 11.7 | 22.5 | 37.5 | 47.1 | 59.0 | 71.3 | 83.7 | 92.2 | 99.5 |
The EKE results are the BetaGeneral (0.62668, 1.7132, 0.95, 110) distribution fitted with @Risk version 7.6.
Table A.50.
The uncertainty distribution of plants free of Takahashia japonica per 10,000 plants calculated by Table A.49
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,900 | 9,950 | 9,982 | 9,990 | 9,999 | ||||||||||
| EKE results | 9,901 | 9,908 | 9,916 | 9,929 | 9,941 | 9,953 | 9,963 | 9,978 | 9,988 | 9,992 | 9,996 | 9,998 | 9,998.6 | 9,998.9 | 9,999.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.50.
A.10.7 Overall likelihood of pest freedom for plants in pots up to 7 years old
A.10.7.1. Reasoning for a scenario which would lead to a reasonably low number of infested plants in pots up to 7 years old
This scenario assumes a low pressure of the pest in the nurseries and the surrounding: there is only one report from the UK in a private garden in West Berkshire. In this scenario, it is also assumed that signs of the pest are easy to spot as ovisacs are clearly visible and very conspicuous. Therefore, measures against the pest can be taken.
A.10.7.2. Reasoning for a scenario which would lead to a reasonably high number of infested plants in pots up to 7 years old
This scenario assumes that the pest is present in the nurseries and the surrounding. It also assumes that, although there is only one report from the UK in a private garden in West Berkshire, this report is very recent (2018), so pest may be spreading. Moreover, the scenario assumes that, in the case of low‐density populations or initial stages of infestation, nymphs can be overlooked during inspections, and mode of action of the pesticides used may not be effective against the pest. Finally, this scenario assumes that plants can be exported all year round, and the presence of leaves and the possible presence of undetected nymphs at early stages of infestation could increase the number of infested plants during export.
A.10.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested plants in pots up to 7 years old (Median)
The Panel assumes a high efficacy of the surveillance and inspections conducted in the nursery due to conspicuous aspect of the ovisacs of adult female, therefore, measures against the pest can be taken. The Panel also assumes a very low pressure of the pest from the surroundings. These considerations lead Panel to indicate low value of the median.
A.10.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because of the ease of detection of signs of the pest, because of the low pressure of the pest from the surroundings, and because of effectiveness of measures conducted to control the pest in the nursery.
A.10.7.5. Elicitation outcomes of the assessment of the pest freedom for Takahashia japonica on plants in pots up to 7 years old
The following tables show the elicited and fitted values for pest infestation (Table A.51) and pest freedom (Table A.52).
Table A.51.
Elicited and fitted values of the uncertainty distribution of pest infestation by Takahashia japonica per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 12 | 25 | 60 | 120 | ||||||||||
| EKE | 2.01 | 2.19 | 2.65 | 3.99 | 6.46 | 10.4 | 15.4 | 28.3 | 46.0 | 57.3 | 71.4 | 86.1 | 101 | 112 | 121 |
The EKE results are the BetaGeneral (0.65362, 1.8111, 1.95, 135) distribution fitted with @Risk version 7.6.
Table A.52.
The uncertainty distribution of plants free of Takahashia japonica per 10,000 plants calculated by Table A.51
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,880 | 9,940 | 9,975 | 9,988 | 9,998 | ||||||||||
| EKE results | 9,879 | 9,888 | 9,899 | 9,914 | 9,929 | 9,943 | 9,954 | 9,972 | 9,985 | 9,990 | 9,994 | 9,996 | 9,997.3 | 9,997.8 | 9,998.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.52.
A.10.8. Reference list
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Takahashia japonica. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=27909 [Accessed: 27 February 2023].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P,Chatzivassiliou E, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L,Grégoire J‐C, Malumphy C, Akrivou A, Kertesz V, Maiorano A, Papachristos D and MacLeod A, 2023a. Scientific Opinion on the pest categorisation of Takahashia japonica. EFSA Journal 2023;21(5):8000, 23 pp. https://doi.org/10.2903/j.efsa.2023.8000
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Gonthier P, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P,Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Zappalà L, Lucchi A, Gómez P, Urek G, Bernardo U, Bubici G, Carluccio AV, Chiumenti M, Di Serio F, Fanelli E, Marzachì C, Kaczmarek A, Mosbach‐Schulz O and Yuen J, 2023b. Scientific Opinion on the commodity risk assessment of Malus domestica plants from United Kingdom. EFSA Journal 2023;21(5):8002, 146 pp. https://doi.org/10.2903/j.efsa.2023.8002
EPPO (European and Mediterranean Plant Protection Organization), online. First report of Takahashia japonica in Croatia. Available online: https://gd.eppo.int/reporting/article-7127 [Accessed: 24 October 2022].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 December 2022].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: a literature‐based model of scale insect biology and systematics, Takahashia japonica. Available online: https://scalenet.info/catalogue/Takahashia%20japonica/ [Accessed 24 October 2022].
Landeka N, Uzelac M, Poljuha D and Sladonja B, 2021. The first record of the Asiatic string cottony scale Takahashia japonica in Croatia. Journal of Forestry, 145, 263–267. https://doi.org/10.31298/sl.145.5-6.5
Limonta L and Pellizzari G, 2018. First record of the string cottony scale Takahashia japonica in Europe and its establishment in Northern Italy. Bulletin of Insectology, 71, 159–160.
Limonta L, Porcelli F and Pellizzari G, 2022. An overview of Takahashia japonica: present distribution, host plants, natural enemies and life‐cycle, with observations on its morphology. Bulletin of Insectology, 75, 306–314.
Malumphy C, Tuffen M and Andrew S, 2019. Plant Pest Factsheet: Cotton stringy scale insect: Takahashia japonica. Department for Environment Food and Rural Affairs. 4 pp.
Suh S‐J, 2020. Host plant list of the scale insects (Hemiptera: Coccomorpha) in South Korea. Insecta Mundi, 0757, 1–26.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Tuffen M, Salisbury A and Malumphy CP, 2019. Cotton stringy scale insect, Takahashia japonica (Hemiptera: Coccidae), new to Britain. British Journal of Entomology and Natural History, 32, 1–4.
Wang Y, Zhou Q, Qiao H, Zhang A, Fang Y, Wang X, Zhu C and Zhang Y, 2016. Formal nomenclature and description of cryptic species of the Encyrtus sasakii complex (Hymenoptera Encyrtidae). Scientific Reports, 6, 1–16. https://doi.org/10.1038/srep34372
Appendix B – Web of Science All Databases Search String
1.
In Table B.1, the search string for Acer pseudoplatanus used in Web of Science is reported. Totally, 225 papers were retrieved. Titles and abstracts were screened, and 29 pests were added to the list of pests (see Appendix F).
Table B.1.
String for Acer pseudoplatanus
| Web of Science All databases |
TOPIC: (“Acer pseudoplatanus” OR “common sycamore” OR “great maple” OR “plane maple” OR “sycamore” OR “Acer abchasicum” OR “Acer atropurpureum” OR “Acer bohemicum” OR “Acer dittrichii” OR “Acer erythrocarpum“ OR “Acer euchlorum “ OR “Acer fieberi “ OR “Acer majus” OR “Acer melliodorum” OR “Acer montanum” OR “Acer opizii” OR “Acer opulifolium” OR “Acer procerum” OR “Acer purpureum” OR “Acer quinquelobum” OR “Acer rafinesquianum” OR “Acer villosum” OR “Acer wondracekii” OR “Acer worleei”) AND TOPIC: (pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR host OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR “malaise” OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viroses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR rot OR rots OR rotten OR “damping off” OR “damping‐off” OR blister$ OR “smut” OR mould OR mold OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootknot OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding”) NOT TOPIC: (“winged seeds” OR metabolites OR *tannins OR climate OR “maple syrup” OR syrup OR mycorrhiz* OR “carbon loss” OR pollut* OR weather OR propert* OR probes OR spectr* OR antioxidant$ OR transformation OR RNA OR DNA OR “Secondary plant metabolite$” OR metabol* OR “Phenolic compounds” OR Quality OR Abiotic OR Storage OR Pollen* OR fertil* OR Mulching OR Nutrient* OR Pruning OR drought OR “human virus” OR “animal disease*” OR “plant extracts” OR immunological OR “purified fraction” OR “traditional medicine” OR medicine OR mammal* OR bird* OR “human disease*” OR biomarker$ OR “health education” OR bat$ OR “seedling$ survival” OR “anthropogenic disturbance” OR “cold resistance” OR “salt stress” OR salinity OR “aCER method” OR “adaptive cognitive emotion regulation” OR nitrogen OR hygien* OR “cognitive function$” OR fossil$ OR *toxicity OR Miocene OR postglacial OR “weed control” OR landscape |
In Table B.2, the search string for Acer, Acer sp. and Acer spp. used in Web of Science is reported. Totally, 772 papers were retrieved. Titles and abstracts were screened, and 31 pests were added to the list of pests (see Appendix F).
Table B.2.
String for Acer, Acer sp. and Acer spp.
| Web of Science All databases |
TOPIC: (“Acer” OR “Acer sp.” OR “Acer spp.” OR “maple”) AND TOPIC: (pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR host OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR “malaise” OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viroses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR rot OR rots OR rotten OR “damping off” OR “damping‐off” OR blister$ OR “smut” OR mould OR mold OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootknot OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding”) NOT TOPIC: (“winged seeds” OR metabolites OR *tannins OR climate OR “maple syrup” OR syrup OR mycorrhiz* OR “carbon loss” OR pollut* OR weather OR propert* OR probes OR spectr* OR antioxidant$ OR transformation OR RNA OR DNA OR “Secondary plant metabolite$” OR metabol* OR “Phenolic compounds” OR Quality OR Abiotic OR Storage OR Pollen* OR fertil* OR Mulching OR Nutrient* OR Pruning OR drought OR “human virus” OR “animal disease*” OR “plant extracts” OR immunological OR “purified fraction” OR “traditional medicine” OR medicine OR mammal* OR bird* OR “human disease*” OR biomarker$ OR “health education” OR bat$ OR “seedling$ survival” OR “anthropogenic disturbance” OR “cold resistance” OR “salt stress” OR salinity OR “aCER method” OR “adaptive cognitive emotion regulation” OR nitrogen OR hygien* OR “cognitive function$” OR fossil$ OR *toxicity OR Miocene OR postglacial OR “weed control” OR landscape) NOT TOPIC: (“Acer acuminatum” OR “Acer amamiense” OR “Acer amplum” OR “Acer argutum” OR “Acer barbinerve” OR “Acer brachystephyanum” OR “Acer buergerianum” OR “Acer caesium” OR “Acer calcaratum” OR “Acer caloneurum” OR “Acer campbellii” OR “Acer campestre” OR “Acer capillipes” OR “Acer cappadocicum” OR “Acer carpinifolium” OR “Acer caudatifolium” OR “Acer caudatum” OR “Acer ceriferum” OR “Acer chapaense” OR “Acer chiangdaoense” OR “Acer cinerascentiforme” OR “Acer circinatum” OR “Acer cissifolium” OR “Acer confertifolium” OR “Acer cordatum” OR “Acer coriaceifolium” OR “Acer crassum” OR “Acer crataegifolium” OR “Acer davidii” OR “Acer diabolicum” OR “Acer discolor” OR “Acer distylum” OR “Acer duplicatoserratum” OR “Acer elegantulum” OR “Acer emeiense” OR “Acer erianthum” OR “Acer erythranthum” OR “Acer eucalyptoides” OR “Acer fabri” OR “Acer fengii” OR “Acer fenzelianum” OR “Acer foveolatum” OR “Acer garrettii” OR “Acer glabrum” OR “Acer gracilifolium” OR “Acer granatense” OR “Acer griseum” OR “Acer guanense” OR “Acer guizhouense” OR “Acer hainanense” OR “Acer heldreichii” OR “Acer henryi” OR “Acer huangpingense” OR “Acer hyrcanum” OR “Acer japonicum” OR “Acer jingdongense” OR “Acer laevigatum” OR “Acer laisuense” OR “Acer lanpingense” OR “Acer laurinum” OR “Acer lauyuense” OR “Acer legonsanicum” OR “Acer leipoense” OR “Acer leptophyllum” OR “Acer lichuanense” OR “Acer linganense” OR “Acer longipedicellatum” OR “Acer longipes” OR “Acer lucidum” OR “Acer macrophyllum” OR “Acer mairei” OR “Acer mandshuricum” OR “Acer mapienense” OR “Acer maximowiczianum” OR “Acer mazandaranicum” OR “Acer medogense” OR “Acer miaoshanicum” OR “Acer micranthum” OR “Acer mirabile” OR “Acer miyabei” OR “Acer monspessulanum” OR “Acer morifolium” OR “Acer nayongense” OR “Acer negundo” OR “Acer nipponicum” OR “Acer oblongum” OR “Acer obtusifolium” OR “Acer okamotoi” OR “Acer oligocarpum” OR “Acer olivaceum” OR “Acer oliverianum” OR “Acer opalus” OR “Acer palmatum” OR “Acer pauciflorum” OR “Acer paxii” OR “Acer pectinatum” OR “Acer pehpeiense” OR “Acer pensylvanicum” OR “Acer pentaphyllum” OR “Acer pentapomicum” OR “Acer pictum” OR “Acer pilosum” OR “Acer platanoides” OR “Acer pluridens” OR “Acer poliophyllum” OR “Acer pseudoplatanus” OR “Acer pseudosieboldianum” OR “Acer pseudowilsonii” OR “Acer pubipalmatum” OR “Acer pycnanthum” OR “Acer robustum” OR “Acer rubescens” OR “Acer rubronervium” OR “Acer rubrum” OR “Acer rufinerve” OR “Acer saccharinum” OR “Acer saccharum” OR “Acer salweenense” OR “Acer schneiderianum” OR “Acer sempervirens” OR “Acer shangszeense” OR “Acer shenkanense” OR “Acer shensiense” OR “Acer shihweii” OR “Acer shirasawanum” OR “Acer sichourense” OR “Acer sieboldianum” OR “Acer sikkimense” OR “Acer sino‐oblongum” OR “Acer sinopurpurascens” OR “Acer spicatum” OR “Acer stachyophyllum” OR “Acer sterculiaceum” OR “Acer sutchuenense” OR “Acer sycopseoides” OR “Acer taipuense” OR “Acer tataricum” OR “Acer tegmentosum” OR “Acer tenellum” OR “Acer tibetense” OR “Acer tonkinense” OR “Acer trialatum” OR “Acer tricaudatum” OR “Acer triflorum” OR “Acer truncatum” OR “Acer tschonoskii” OR “Acer turcomanicum” OR “Acer tutcheri” OR “Acer undulatum” OR “Acer velutinum” OR “Acer wangchii” OR “Acer wardii” OR “Acer wuyishanicum” OR “Acer wuyuanense” OR “Acer yangbiense” OR “Acer yaoshanicum” OR “Acer yinkunii” OR “Acer yui” OR “Acer × bornmuelleri” OR “Acer × coriaceum” OR “Acer × freemanii” OR “Acer × martini” OR “Acer × schwerinii” OR “Acer × varbossanium”) NOT TOPIC: (“Anoplophora chinensis” OR “Anoplophora glabripennis” OR “Cacoecimorpha pronubana” OR “Candidatus Phytoplasma fragariae” OR “Ceroplastes ceriferus” OR “Choristoneura conflictana” OR “Choristoneura rosaceana” OR “Comstockaspis perniciosa” OR “Cryphonectria parasitica” OR “Cryptostroma corticale” OR “Diabrotica undecimpunctata undecimpunctata” OR “Euwallacea fornicatus sensu stricto” OR “Halyomorpha halys” OR “Hyphantria cunea” OR “Lopholeucaspis japonica” OR “Malacosoma disstria” OR “Maple mosaic agent” OR “Oemona hirta” OR “Orgyia leucostigma” OR “Ricania speculum” OR “Trichoferus campestris” OR “Trirachys sartus” OR “Xanthomonas acernea” OR “Xylosandrus germanus” OR “Anoplophora glabripennis” OR “Cacoecimorpha pronubana” OR “Cossus cossus” OR “Fomes fomentarius” OR “Hemiberlesia rapax” OR “Inonotus hispidus” OR “Monema flavescens” OR “Operophtera brumata” OR “Phellinus igniarius” OR “Phytophthora cactorum” OR “Popillia japonica” OR “Sawadaea bicornis” OR “Sawadaea tulasnei” OR “Xiphinema rivesi” OR “Xylella fastidiosa” OR “Xylosandrus mutilatus” OR “Anoplophora chinensis” OR “Apiognomonia errabunda” OR “Armillaria mellea” OR “Belonolaimus longicaudatus” OR “Bemisia tabaci” OR “Brevipalpus phoenicis” OR “Chinavia hilaris” OR “Chionaspis acer” OR “Coccus hesperidum” OR “Diaspidiotus perniciosus” OR “Euproctis chrysorrhoea” OR “Ganoderma lucidum” OR “Glomerella cingulata” OR “Lopholeucaspis japonica” OR “Lymantria dispar” OR “Paracolomerus fopingacer” OR “Parthenolecanium corni” OR “Phytoplasma fragariae” OR “Pseudaulacaspis pentagona” OR “Raoiella indica” OR “Rhizobium radiobacter” OR “Rhizobium rhizogenes” OR “Rosellinia necatrix” OR “Tetropium castaneum” OR “Tortrix viridana” OR “Trirachys sartus” OR “Zeuzera pyrina” OR “Ceroplastes rubens” OR “Hyphantria cunea” OR “Peridroma saucia” OR “Pratylenchus penetrans” OR “Saturnia pyri” OR “Sowbane mosaic virus” OR “Taeniothrips inconsequens” OR “Xestia c‐nigrum” OR “Diaspidiotus ostreaeformis” OR “Boisea trivittata” OR “Cryptostroma corticale” OR “Dothiorella iberica” OR “Drepanosiphum platanoidis” OR “Fusarium” OR “Heterarthrus aceris” OR “Heterarthrus leucomelus” OR “Megaplatypus mutatus” OR “Melanaspis tenebricosa” OR “Myrmica rubra” OR “Ossiannilssonola callosa” OR “Pammene fasciana” OR “Periphyllus californiensis” OR “Phomopsis” OR “Phytophthora cambivora” OR “Pratylenchus coffeae” OR “Pterostichus coracinus” OR “Ptilophora plumigera” OR “Pulvinaria regalis” OR “Rhytisma acerinum” OR “Ricania speculum” OR “Synanthedon resplendens” OR “Trichodorus viruliferus” OR “Trichoferus campestris” OR “Valsa sordida” OR “Periphyllus bengalensis” OR “Stomaphis aceris” OR “Yamatocallis obscura” OR “Trichaitophorus acerifolius” OR “Yamatocallis sauteri” OR “Yamatocallis acerisucta” OR “Periphyllus himalayensis” OR “Periphyllus pallidus” OR “Periphyllus tokyoensis” OR “Coptophylla gymnaspis” OR “Eotetranychus carpini” OR “Eotetranychus carpini” OR “Eotetranychus tiliarum” OR “Agrilus viridis” OR “Cerambyx scopolii” OR “Leiopus nebulosus” OR “Anaglyptus mysticus” OR “Saperda scalaris” OR “Orsodacne cerasi” OR “Cryptocephalus pusillus” OR “Crepidodera aurata” OR “Mesites tardii” OR “Rhyncolus gracilis” OR “Barypeithes pellucidus” OR “Phyllobius argentatus” OR “Phyllobius calcaratus” OR “Phyllobius maculicornis” OR “Phyllobius oblongus” OR “Phyllobius roboretanus” OR “Polydrusus cervinus” OR “Polydrusus marginatus” OR “Strophosomus melanogrammus” OR “Hylesinus crenatus” OR “Xyloterus domesticum” OR “Ischnodes sanguinicollis” OR “Melanotus erythropus” OR “Hylecoetus dermestoides” OR “Ischnomera caerulea” OR “Arboridia ribauti” OR “Edwardsiana alnicola” OR “Edwardsiana diversa” OR “Edwardsiana lethierryi” OR “Alebra wahlbergi” OR “Idiocerus vittifrons” OR “Lindbergina aurovittata” OR “Ribautiana debilis” OR “Ribautiana tenerrima” OR “Zygina suavis” OR “Physatocheila harwoodi” OR “Cerostegia japonica” OR “Pulvinaria regalis” OR “Chionaspis salicis” OR “Parlatoria theae” OR “Pseudaulacaspis pentagona” OR “Quadraspidiotus ostreaeformis” OR “Myzus persicae” OR “Drepanosiphum platanoidis” OR “Periphyllus testudinaceus” OR “Tremex columba” OR “Croesus septentrionalis” OR “Zeuzera pyrina” OR “Operophtera brumata” OR “Cyclophora annulata” OR “Actinotia polyodon” OR “Mimas tiliae” OR “Coleophora badiipennella” OR “Incurvaria pectinea” OR “Clepsis rurinana” OR “Aleimma loeflingiana” OR “Pandemis cerasana” OR “Pandemis cinnamomeana” OR “Argyresthia bonnetella” OR “Roeslerstammia erxlebella” OR “Taeniothrips inconsequens” OR “Lymantor coryli” OR “Xyleborus dispar” OR “Xyleborus saxeseni” OR “Acronicta aceris” OR “Ptilodontella cucullina” OR “Meconema thalassinum” OR “Pammene regiana” OR “Acleris sparsana” OR “Apatelodes torrefacta” OR “Halysidota tessellaris” OR “Hyphantria cunea” OR “Lophocampa maculata” OR “Pyrrharctia isabella” OR “Bucculatrix demaryella” OR “Bucculatrix thoracella” OR “Prionoxystus macmurtrei” OR “Prionoxystus robiniae” OR “Zeuzera pyrina” OR “Pseudothyatira cymatophoroides” OR “Dichomeris ustalella” OR “Faristenia acerella” OR “Faristenia geminisignella” OR “Gelechia sestertiella” OR “Alsophila pometaria” OR “Anavitrinella pampinaria” OR “Antepione thisoaria” OR “Ascotis selenaria” OR “Besma endropiaria” OR “Biston betularia” OR “Biston robustus” OR “Campaea perlata” OR “Carsia sororiata” OR “Cepphis armataria” OR “Chloroclysta siterata” OR “Cladara atroliturata” OR “Colotois pennaria” OR “Cyclophora annulata” OR “Ectropis crepuscularia” OR “Endropiodes sp. B” OR “Ennomos magnaria” OR “Ennomos subsignaria” OR “Epirrita dilutata” OR “Erannis defoliaria” OR “Erannis tiliaria” OR “Eubaphe mendica” OR “Euchlaena irraria” OR “Euchlaena serrata” OR “Eupithecia inturbata” OR “Eutrapela clemataria” OR “Heterophleps triguttaria” OR “Hydriomena albifasciata” OR “Inurois fletcheri” OR “Iridopsis larvaria” OR “Itame plumosata” OR “Itame pustularia” OR “Lambdina fervidaria” OR “Lambdina fiscellaria” OR “Lambdina vitraria” OR “Lycia ursaria” OR “Lytrosis unitaria” OR “Melanolophia signataria” OR “Nematocampa limbata” OR “Obrussa sericopeza” OR “Operophtera bruceata” OR “Operophtera brumata” OR “Operophtera occidentalis” OR “Paleacrita vernata” OR “Pero hubneraria” OR “Phigalia titea” OR “Plagodis fervidaria” OR “Plagodis pulveraria” OR “Plagodis serinaria” OR “Probole amicaria” OR “Prochoerodes forficaria” OR “Prochoerodes transversata” OR “Sabulodes caberata” OR “Selenia alciphearia” OR “Selenia kentaria” OR “Xanthorhoe defensaria” OR “Caloptilia aceriella” OR “Caloptilia acerifoliella” OR “Caloptilia bimaculatella” OR “Caloptilia negundella” OR “Caloptilia packardella” OR “Caloptilia sp. A” OR “Caloptilia wakayamensis” OR “Cameraria aceriella” OR “Cameraria saccharella” OR “Phyllonorycter acericola” OR “Phyllonorycter aceripestis” OR “Phyllonorycter clemensella” OR “Phyllonorycter geniculella” OR “Phyllonorycter ginnalae” OR “Phyllonorycter lucidicostella” OR “Phyllonorycter orientalis” OR “Phyllonorycter trinotella” OR “Paraclemensia acerifoliella” OR “Malacosoma americana” OR “Malacosoma disstria” OR “Malacosoma neustria” OR “Phyllodesma americana” OR “Tolype velleda” OR “Acharia stimulea” OR “Heterogenea asella” OR “Lithacodes fasciola” OR “Monema flavescens” OR “Parasa latistriga” OR “Phobetron pithecium” OR “Euproctis celebensis” OR “Euproctis chrysorrhoea” OR “Lymantria dispar” OR “Orgyia antiqua” OR “Orgyia leucostigma” OR “Megalopyge opercularis” OR “Ectoedemia decentella” OR “Ectoedemia sericopeza” OR “Etainia ochrefasciella” OR “Glaucolepis saccharella” OR “Stigmella aceris” OR “Stigmella apicialbella” OR “Achatia distincta” OR “Acronicta aceris” OR “Acronicta alni” OR “Acronicta americana” OR “Acronicta funeralis” OR “Acronicta hastulifera” OR “Acronicta impleta” OR “Acronicta interrupta” OR “Acronicta retardata” OR “Acronicta tristis” OR “Actebia fennica” OR “Amphipyra pyramidoides” OR “Apamea castanea” OR “Aseptis binotata” OR “Brachionycha atossa” OR “Cosmia trapezina” OR “Eucirroedia pampina” OR “Eupsilia transversa” OR “Eupsilia vinulenta” OR “Eurois astricta” OR “Eurois occulta” OR “Euxoa auxiliaris” OR “Homorthodes furfurata” OR “Hypena baltimoralis” OR “Hypena scabra” OR “Lacanobia subjuncta” OR “Lithophane antennata” OR “Lithophane bethunei” OR “Lithophane grotei” OR “Lithophane laticinerea” OR “Lithophane patefacta” OR “Lithophane unimoda” OR “Melanchra adjuncta” OR “Morrisonia confusa” OR “Orthosia pacifica” OR “Orthosia rubescens” OR “Palthis angulalis” OR “Papaipema furcata” OR “Parallelia bistriaris” OR “Peridroma saucia” OR “Polia nimbosa” OR “Polia purpurissata” OR “Psaphida resumens” OR “Pseudohermonassa bicarnea” OR “Spaelotis clandestina” OR “Spiramater lutra” OR “Sunira bicolorago” OR “Sunira decipiens” OR “Xestia c‐nigrum” OR “Xestia dolosa” OR “Zale galbanata” OR “Zale lunata” OR “Zale minerea” OR “Clostera inclusa” OR “Datana ministra” OR “Fusapteryx ladislai” OR “Heterocampa biundata” OR “Heterocampa guttivitta” OR “Heterocampa subrotata” OR “Heterocampa umbrata” OR “Himeropteryx miraculosa” OR “Macrurocampa marthesia” OR “Microphalera grisea” OR “Nadata gibbosa” OR “Oligocentria semirufescens” OR “Ptilodon hoegei” OR “Ptilodon okanoi” OR “Ptilophora jezoensis” OR “Ptilophora nohirae” OR “Schizura concinna” OR “Schizura ipomoeae” OR “Schizura unicornis” OR “Semidonta biloba” OR “Shaka atrovittatus” OR “Stauropus fagi” OR “Symmerista albifrons” OR “Symmerista canicosta” OR “Symmerista leucitys” OR “Tarsolepis japonica” OR “Togepteryx velutina” OR “Neptis philyra” OR “Nymphalis antiopa” OR “Agonopterix pallidior” OR “Antaeotricha leucillana” OR “Cheimophila salicella” OR “Hofmannophila pseudospretella” OR “Machimia tentoriferella” OR “Psilocorsis cryptolechiella” OR “Psilocorsis reflexella” OR “Charadra deridens” OR “Colocasia coryli” OR “Colocasia propinquilinea” OR “Papilio glaucus” OR “Thyridopteryx ephemeraeformis” OR “Glyptocera consobrinella” OR “Oreana unicolorella” OR “Pococera asperatella” OR “Actias artemis” OR “Actias luna” OR “Anisota senatoria” OR “Anisota virginiensis” OR “Antheraea polyphemus” OR “Automeris coresus” OR “Automeris io” OR “Caligula boisduvali” OR “Caligula japonica” OR “Callosamia promethea” OR “Dryocampa rubicunda” OR “Eacles imperialis” OR “Hemileuca eglanterina” OR “Hyalophora cecropia” OR “Hyalophora columbia” OR “Hyalophora euryalus” OR “Hylesia nigricans” OR “Leucanella memusae” OR “Leucanella viridescens” OR “Rhodinia fugax” OR “Rhodinia newara” OR “Saturnia lindia” OR “Carmenta corni” OR “Synanthedon acerni” OR “Synanthedon acerrubri” OR “Synanthedon spuleri” OR “Paonias excaecata” OR “Acleris chalybeana” OR “Acleris forskaleana” OR “Acleris negundana” OR “Acleris semiannula” OR “Acleris sparsana” OR “Aleimma loeflingiana” OR “Ancylis platanana” OR “Apotomis tertiana” OR “Archips argyrospila” OR “Archips fuscocupreanus” OR “Archips ingentana” OR “Archips negundana” OR “Archips rosana” OR “Archips semiferanus” OR “Archips xylosteana” OR “Argyrotaenia mariana” OR “Argyrotaenia velutinana” OR “Choristoneura conflictana” OR “Choristoneura fractivittana” OR “Choristoneura rosaceana” OR “Clepsis persicana” OR “Clepsis rurinana” OR “Cydia candana” OR “Cydia inquinatana” OR “Epinotia rasdolynana” OR “Episimus tyrius” OR “Eucosma rasdolnyana” OR “Eucosma variana” OR “Gypsonoma substitutionis” OR “Homonopsis illotana” OR “Isotrias hybridana” OR “Olethreutes glaciana” OR “Olethreutes nigranum” OR “Pammene regiana” OR “Pandemis cerasana” OR “Pandemis cinnamomeana” OR “Pandemis heparana” OR “Pandemis lamprosana” OR “Parapammene petulantana” OR “Platynota flavedana” OR “Proteoteras aesculana” OR “Proteoteras crescentana” OR “Proteoteras moffatiana” OR “Proteoteras naracana” OR “Proteoteras sp.” OR “Proteoteras willingana” OR “Pseudexentera spoliana” OR “Ptycholoma circumclusana” OR “Ptycholoma lecheana” OR “Sonia canadana” OR “Sparganothis acerivorana” OR “Sparganothis albicaudana” OR “Sparganothis pettitana” OR “Sparganothis reticulatana” OR “Tortrix viridana” OR “Argyresthia subreticulata” OR “Cnephasia incertana” OR “Mesocriconema xenoplax” OR “Ogma octangularis” OR “Paratrichodorus minor” OR “Criconemoides incrassata” OR “Criconemoides parvus” OR “Criconema mutabile” OR “Gracilacus straeleni” OR “Helicotylenchus digonicus” OR “Rotylenchus sp.” OR “Trichodorus beirensis” OR “Helicotylenchus erythrinae” OR “Pratylenchus crenatus” OR “Meloidogyne sp.” OR “Hemicycliophora uniformis” OR “Hemicycliophora zuckermani” OR “Pratylenchus neglectus” OR “Tylenchorhynchus claytoni” OR “Tylenchorhynchus cylindricus” OR “Xiphinema americanum” OR “Xiphinema sp.” OR “Longidorus elongatus” OR “Longidorus paralongicaudatus” OR “Longidorus paravineacola” OR “Pratylenchus vulnus” OR “Merlinius brevidens” OR “Hemicycliophora similis” OR “Tylenchorhynchus maximus” OR “Pratylenchus penetrans” OR “Pratylenchus sp.” OR “Xiphinema bernardi” OR “Xiphinema chambersi” OR “Acanthococcus acericola” OR “Acanthococcus aceris” OR “Acanthococcus azaleae” OR “Acanthococcus macedoniensis” OR “Acanthococcus tokaedae” OR “Acanthomytilus kurdicus” OR “Aonidiella aurantii” OR “Aonidiella orientalis” OR “Aspidiotus hedericola” OR “Aspidiotus mousavii” OR “Aulacaspis aceris” OR “Aulacaspis ligulata” OR “Aulacaspis tubercularis” OR “Cerococcus koebelei” OR “Cerococcus parrotti” OR “Ceroplastes ceriferus” OR “Ceroplastes japonicus” OR “Ceroplastes pseudoceriferus” OR “Ceroplastes rubens” OR “Chionaspis acer” OR “Chionaspis acericola” OR “Chionaspis salicis” OR “Chionaspis sozanica” OR “Chrysomphalus dictyospermi” OR “Clavaspis ulmi” OR “Coccus hesperidum hesperidum” OR “Comstockaspis perniciosa” OR “Crisicoccus matsumotoi” OR “Cryptococcus aceris” OR “Cryptococcus williamsi” OR “Cryptoparlatoreopsis longispina” OR “Diaspidiotus aesculi” OR “Diaspidiotus africanus” OR “Diaspidiotus ancylus” OR “Diaspidiotus forbesi” OR “Diaspidiotus juglansregiae” OR “Diaspidiotus liquidambaris” OR “Diaspidiotus osborni” OR “Diaspidiotus ostreaeformis” OR “Drosicha corpulenta” OR “Drosicha mangiferae” OR “Drosicha stebbingii” OR “Dynaspidiotus abietis” OR “Dysmicoccus wistariae” OR “Epidiaspis leperii” OR “Eulecanium cerasorum” OR “Eulecanium ciliatum” OR “Eulecanium giganteum” OR “Eulecanium nocivum” OR “Eulecanium paucispinosum” OR “Eulecanium tiliae” OR “Ferreroaspis hungarica” OR “Formicococcus acerneus” OR “Heliococcus osborni” OR “Heliococcus stachyos” OR “Icerya purchasi” OR “Kerria communis” OR “Lecanodiaspis prosopidis” OR “Lepidosaphes conchiformis” OR “Lepidosaphes coreana” OR “Lepidosaphes malicola” OR “Lepidosaphes towadensis” OR “Lepidosaphes ulmi” OR “Lepidosaphes yanagicola” OR “Lopholeucaspis japonica” OR “Melanaspis inopinata” OR “Melanaspis louristana” OR “Melanaspis nigropunctata” OR “Melanaspis obscura” OR “Melanaspis tenebricosa” OR “Mesolecanium nigrofasciatum” OR “Mirococcus ostiaplurimus” OR “Morganella cueroensis” OR “Morganella longispina” OR “Neochionaspis kirgisica” OR “Neopinnaspis harperi” OR “Neopulvinaria innumerabilis innumerabilis” OR “Neosteingelia texana” OR “Newsteadia floccosa” OR “Nipponpulvinaria horii” OR “Palaeococcus fuscipennis” OR “Paratachardina pseudolobata” OR “Parlatoreopsis acericola” OR “Parlatoreopsis pyri” OR “Parlatoria camelliae” OR “Parlatoria crotonis” OR “Parlatoria octolobata” OR “Parlatoria oleae” OR “Parlatoria theae” OR “Parthenolecanium cerasifex” OR “Parthenolecanium corni corni” OR “Parthenolecanium glandi” OR “Parthenolecanium persicae” OR “Phenacoccus acericola” OR “Phenacoccus aceris” OR “Phenacoccus grandicarpus” OR “Phenacoccus hortonarum” OR “Phenacoccus insularis” OR “Planococcus angkorensis” OR “Planococcus japonicus” OR “Pseudaonidia duplex” OR “Pseudaulacaspis pentagona” OR “Pseudaulacaspis prunicola prunicola” OR “Pseudococcus comstocki” OR “Pseudococcus maritimus” OR “Pseudococcus sorghiellus” OR “Pseudococcus viburni” OR “Pulvinaria acericola” OR “Pulvinaria brachiungualis” OR “Pulvinaria camelicola” OR “Pulvinaria kuwacola” OR “Pulvinaria nipponica” OR “Pulvinaria nishigaharae” OR “Pulvinaria peregrina” OR “Pulvinaria pulchra” OR “Pulvinaria regalis” OR “Pulvinaria shinjii” OR “Pulvinaria vitis” OR “Rutherfordia major” OR “Spilococcus pacificus” OR “Steingelia gorodetskia” OR “Suturaspis archangelskyae” OR “Takahashia japonica” OR “Takahashiaspis macroporana” OR “Trionymus americanus” OR “Unaspis euonymi” OR “Velataspis dentata” OR “Xylococculus betulae” OR “Amphitetranychus viennensis” OR “Bryobia neoribis” OR “Bryobia praetiosa” OR “Bryobia rubrioculus” OR “Bryobia sarothamni” OR “Eotetranychus aceri” OR “Eotetranychus boreus” OR “Eotetranychus carpini” OR “Eotetranychus coryli” OR “Eotetranychus crossleyi” OR “Eotetranychus dissectus” OR “Eotetranychus pruni” OR “Eotetranychus sexmaculatus” OR “Eotetranychus spectabilis” OR “Eotetranychus tiliarium” OR “Eotetranychus uncatus” OR “Eotetranychus willamettei” OR “Eutetranychus orientalis” OR “Oligonychus aceris” OR “Oligonychus bicolor” OR “Oligonychus endytus” OR “Oligonychus ununguis” OR “Schizotetranychus garmani” OR “Tetranychus canadensis” OR “Tetranychus mcdanieli” OR “Tetranychus turkestani” OR “Tetranychus urticae” OR “Xinella huangshanensis” OR “Abortiporus fractipes” OR “Acanthonitschkea tristis” OR “Acarosporina microspora” OR “Acrothecium apicale” OR “Agaricus primitivus” OR “Agrocybe cylindracea” OR “Aleurocorticium acerinum” OR “Aleurocorticium alliaceum” OR “Aleurocorticium candidum” OR “Aleurocorticium dryinum” OR “Aleurocorticium microsporum” OR “Aleurodiscus acerinus” OR “Aleurodiscus canadensis” OR “Aleurodiscus cerussatus” OR “Allophylaria crystallifera” OR “Alternaria sp.” OR “Ambrosiella brunnea” OR “Ambrosiella hartigii” OR “Amphisphaeria celata” OR “Amylocorticium cebennense” OR “Anthostoma acerinum” OR “Anthostoma saprophilum” OR “Anthostomella eructans” OR “Antrodia malicola” OR “Antrodiella romellii” OR “Antrodiella semisupina” OR “Antrodiella zonata” OR “Apiognomonia errabunda” OR “Apiognomonia hystrix” OR “Apiognomonia sp.” OR “Aporpium caryae” OR “Aquanectria penicillioides” OR “Armillaria gallica” OR “Armillaria mellea” OR “Armillaria ostoyae” OR “Armillaria sinapina” OR “Arthrobotrys arthrobotryoides” OR “Arthrobotryum robustum” OR “Ascochyta sp.” OR “Ascotremella faginea” OR “Ascotremella turbinata” OR “Aspergillus fumigatus” OR “Aspergillus sp.” OR “Asteromella platanoidis” OR “Athelia coprophila” OR “Aureobasidium apocryptum” OR “Auricularia auricula” OR “Auricularia auricula‐judae” OR “Auricularia fuscosuccinea” OR “Auricularia mesenterica” OR “Bactrodesmium abruptum” OR “Bactrodesmium spilomeum” OR “Basidiodendron eyrei” OR “Bertia moriformis” OR “Biscogniauxia capnodes” OR “Biscogniauxia mediterranea” OR “Bispora betulina” OR “Botryobasidium pruinatum” OR “Botryodiplodia acerina” OR “Botryohypochnus isabellinus” OR “Botryosphaeria dothidea” OR “Botryosphaeria obtusa” OR “Botryosphaeria ribis” OR “Botryosphaeria sp.” OR “Botrytis cinerea” OR “Botrytis fuliginosa” OR “Botrytis olivascens” OR “Botrytis pannosa” OR “Botrytis sp.” OR “Brachysporium canadense” OR “Brachysporium nigrum” OR “Brachysporium obovatum” OR “Bulgaria inquinans” OR “Byssochlamys nivea” OR “Byssocorticium atrovirens” OR “Cacumisporium capitulatum” OR “Cacumisporium tenebrosum” OR “Calocera cornea” OR “Calocera palmata” OR “Calonectria morganii” OR “Calonectria pseudopeziza” OR “Caloplaca ulcerosa” OR “Calosphaeria acerina” OR “Calospora platanoidis” OR “Camarophyllus virgineus var. curtipes” OR “Camarops pugillus” OR “Camarosporium acerinum” OR “Candelabrochaete septocystidia” OR “Candelariella xanthostigma” OR “Candida sp.” OR “Cenangium griseum” OR “Centrospora acerina” OR “Cephalosporium sp.” OR “Ceraceomyces americanus” OR “Ceratocystis virescens” OR “Ceratostomella ampullasca” OR “Ceratostomella echinella” OR “Cercophora caudata” OR “Cercospora acericola” OR “Cercospora acerigena” OR “Cercospora acerina” OR “Cercospora aceris” OR “Cercospora sp.” OR “Cerocorticium confluens” OR “Cerrena unicolor” OR “Chaetomella acutiseta var. acutiseta” OR “Chaetomium graminiforme” OR “Chaetosphaerella fusca” OR “Chaetosphaerella fusispora” OR “Chaetosphaerella phaeostroma” OR “Chaetosphaeria myriocarpa” OR “Chaetosphaeria pulviscula” OR “Chlorencoelia torta” OR “Chlorosplenium aeruginascens” OR “Chondrostereum purpureum” OR “Ciboria acericola” OR “Ciboria acerina” OR “Ciboria rufescens” OR “Ciboriopsis simulata” OR “Cirrenalia acericola” OR “Cistella chlorosticta” OR “Cistella dentata” OR “Cladosporium britannicum” OR “Cladosporium cladosporioides” OR “Cladosporium epiphyllum” OR “Cladosporium epiphyllum var. acerinum” OR “Cladosporium sp.” OR “Cladosporium vagans” OR “Clasterosporium caespitulosum” OR “Claussenomyces atrovirens” OR “Claussenomyces prasinula” OR “Clavicorona pyxidata” OR “Climacodon septentrionalis” OR “Clitocybe truncicola” OR “Clypeosphaeria mamillana” OR “Coccomyces coronatus” OR “Coccomyces tumidus” OR “Collema occultatum” OR “Colletotrichum sp.” OR “Collybia lacunosa” OR “Comatricha typhoides” OR “Coniella castaneicola” OR “Coniochaeta ligniaria” OR “Coniophora capnoides” OR “Coniosporium corticale” OR “Coniothecium applanatum” OR “Conoplea globosa” OR “Conoplea olivacea” OR “Conoplea sphaerica” OR “Cordana pauciseptata” OR “Coriolopsis gallica” OR “Coriolopsis rigida” OR “Coriolus pubescens” OR “Coriolus versicolor” OR “Coronophora angustata” OR “Coronophora annexa” OR “Corticium caeruleum” OR “Corticium effuscatum” OR “Corticium galactinum” OR “Corticium rallum” OR “Corticium subgiganteum” OR “Corticium vellereum” OR “Cosmospora arxii” OR “Crepidotus hamulatus” OR “Crepidotus herbarum” OR “Cristella sulphurea” OR “Cristulariella moricola” OR “Crocicreas subhyalinum” OR “Cryphonectria parasitica” OR “Cryptadelphia groenendalensis” OR “Cryptendoxyla hypophloia” OR “Cryptocoryneum condensatum” OR “Cryptodiaporthe acerina” OR “Cryptodiaporthe acerinum” OR “Cryptodiaporthe hystrix” OR “Cryptodiaporthe lebiseyi” OR “Cryptodiaporthe magnispora” OR “Cryptodiscus pallidus” OR “Cryptostroma corticale” OR “Cryptovalsa protracta” OR “Cucurbitaria homalea” OR “Curvularia sp.” OR “Cyathicula subhyalina” OR “Cyathus striatus” OR “Cylindrobasidium evolvens” OR “Cylindrocarpon candidulum” OR “Cylindrocarpon ianthothele var. majus” OR “Cylindrocarpon ianthothele var. rugulosum” OR “Cylindrosporium sp.” OR “Cyptotrama asprata” OR “Cystostereum murrayi” OR “Cytospora chrysosperma” OR “Cytospora leucosperma” OR “Cytospora lutea” OR “Cytospora pseudoplatani” OR “Cytospora pulcherrima” OR “Cytospora sp.” OR “Cytosporella sp.” OR “Dacryopinax elegans” OR “Dactylaria affinis” OR “Dactylaria echinophila” OR “Daedalea ambigua” OR “Daedalea quercina” OR “Daedalea unicolor” OR “Daldinia childiae” OR “Daldinia concentrica” OR “Daldinia loculatoides” OR “Daldinia petriniae” OR “Daldinia sp.” OR “Daldinia vanderguchtiae” OR “Daldinia vernicosa” OR “Dasyscypha soppittii” OR “Dasyscyphus acerinus” OR “Dasyscyphus brevipilus” OR “Dasyscyphus minutissimus” OR “Dasyscyphus patulus” OR “Dasyscyphus pudibundus” OR “Dasyscyphus radotinensis” OR “Dasyscyphus rhytismatis” OR “Datronia mollis” OR “Dematioscypha richonis” OR “Dendrocorticium polygonioides” OR “Dendrophoma sp.” OR “Dendrophora versiformis” OR “Dendrothele acerina” OR “Dendrothele alliacea” OR “Dendrothele candida” OR “Dendrothele dryina” OR “Dendrothele maculata” OR “Dendrothele microspora” OR “Dendrothele strumosa” OR “Dendryphiopsis atra” OR “Dentocorticium sulphurellum” OR “Dermea acerina” OR “Diaporthe acerina” OR “Diaporthe eres” OR “Diaporthe kadsurae” OR “Diaporthe microstroma” OR “Diaporthe ontariensis” OR “Diaporthe pustulata” OR “Diaporthe sp.” OR “Diaporthe subcongrua” OR “Diaporthe varians” OR “Diatrype albopruinosa” OR “Diatrype decorticata” OR “Diatrype flavovirens” OR “Diatrype oregonensis” OR “Diatrype stigma” OR “Diatrypella frostii” OR “Diatrypella quercina” OR “Didymosporina aceris” OR “Dinemasporium acerinum” OR “Dinemasporium decipiens” OR “Diplodia atrata” OR “Diplodia subtecta” OR “Diplodina acerina” OR “Discella pilosula” OR “Discosia artocreas” OR “Doratomyces stemonitis” OR “Dothiorella sp.” OR “Ellisembia adscendens” OR “Elmerina holophaea” OR “Endophragmia bisbyi” OR “Endophragmia boothii” OR “Endophragmiella biseptata” OR “Endophragmiella canadensis” OR “Endophragmiella collapsa” OR “Endophragmiella subolivacea” OR “Endothia sp.” OR “Endoxyla acericola” OR “Entosordaria perfidiosa” OR “Epicoccum sp.” OR “Erysiphe ljubarskii” OR “Eutypa acharii” OR “Eutypa flavovirens” OR “Eutypa lata” OR “Eutypa lata var. aceri” OR “Eutypa leioplaca” OR “Eutypa lejoplaca” OR “Eutypa limaeformis” OR “Eutypa ludibunda” OR “Eutypa maura” OR “Eutypa milliaria” OR “Eutypa spinosa” OR “Eutypa velutina” OR “Eutypella acericola” OR “Eutypella junglandicola” OR “Eutypella leprosa” OR “Eutypella oregonensis” OR “Eutypella paradisiaca” OR “Eutypella parasitica” OR “Eutypella stellulata” OR “Excipularia fusispora” OR “Exidia nucleata” OR “Exidia recisa” OR “Favolus rhipidium” OR “Favolus squamosus” OR “Fibricium subcarneum” OR “Flammulina velutipes” OR “Fomes annosus” OR “Fomes connatus” OR “Fomes fomentarius” OR “Fomes geotropus” OR “Fomes marmoratus” OR “Fomes meliae” OR “Fomes ohiensis” OR “Fomes pini” OR “Fomes populinus” OR “Fomes tenuis” OR “Fomitiporia punctata” OR “Fumago vagans” OR “Fusarium eumartii” OR “Fusarium roseum” OR “Fusarium sp.” OR “Fusicoccum sp.” OR “Ganoderma applanatum” OR “Ganoderma australe” OR “Ganoderma lipsiense” OR “Ganoderma lobatum” OR “Ganoderma lucidum” OR “Ganoderma resinaceum” OR “Ganoderma tsunodae” OR “Geotrichum sp.” OR “Gliocladium viride” OR “Gloeocystidiellum porosum” OR “Gloeophyllum hirsutum” OR “Gloeosporium acericola” OR “Gloeosporium apocryptum” OR “Gloeosporium saccharinum” OR “Glonium stellatum” OR “Gnomonia acerophila” OR “Gnomonia cerastis” OR “Gnomonia setacea” OR “Goniosporium corticale” OR “Gonytrichum caesium var. subglobosum” OR “Grandinia coriaria” OR “Graphium sp.” OR “Graphostroma platystoma” OR “Grifola frondosa” OR “Guepinia elegans” OR “Guepinia spathularia” OR “Gymnopilus spectabilis” OR “Gyromitra infula” OR “Gyrostroma missouriense” OR “Hapalopilus nidulans” OR “Haplographium delicatum” OR “Haploporus odorus” OR “Helicobasidium candidum” OR “Helicoma ambiens” OR “Helicoma perelegans” OR “Helicoma proliferens” OR “Helicoma sp.” OR “Helicomyces roseus” OR “Helicosporium sp.” OR “Helicosporium vegetum” OR “Helminthosporium brachytrichum” OR “Helminthosporium velutinum” OR “Helotium albovirens” OR “Helotium albumineum” OR “Helotium epiphyllum” OR “Helotium fraternum” OR “Helotium naviculisporum” OR “Hendersonia collapsa” OR “Hericium erinaceus” OR “Hericium ramosum” OR “Heterochaetella dubia” OR “Hirschioporus lacteus” OR “Hirschioporus pargamenus” OR “Holwaya leptosperma” OR “Humaria scutellata” OR “Hyalopeziza ciliata” OR “Hyaloscypha lachnobrachya” OR “Hydnum carbonarium” OR “Hymenochaete agglutinans” OR “Hymenochaete badioferruginea” OR “Hymenochaete biformis” OR “Hymenochaete carpatica” OR “Hymenochaete episphaeria” OR “Hymenochaete fuliginosa” OR “Hymenochaete mougeotii” OR “Hymenoscyphus caudatus” OR “Hymenoscyphus epiphyllus” OR “Hymenoscyphus foliicola” OR “Hymenoscyphus phyllogenus” OR “Hymenoscyphus subpallescens” OR “Hyphoderma leoninum” OR “Hyphoderma praetermissum” OR “Hyphoderma rimosum” OR “Hyphoderma setigerum” OR “Hypochnicium subrigescens” OR “Hypocrea gelatinosa” OR “Hypocrea rufa” OR “Hypoderma rufilabrum” OR “Hypoxylon caries” OR “Hypoxylon cohaerens” OR “Hypoxylon dearnessii” OR “Hypoxylon deustum” OR “Hypoxylon fragiforme” OR “Hypoxylon fuscum” OR “Hypoxylon howeanum” OR “Hypoxylon howeianum” OR “Hypoxylon macrocarpum” OR “Hypoxylon mammatum” OR “Hypoxylon mediterraneum” OR “Hypoxylon multiforme var. effusum” OR “Hypoxylon multiforme” OR “Hypoxylon regale” OR “Hypoxylon rubiginosum” OR “Hypoxylon serpens” OR “Hypoxylon serpens var. macrosporum” OR “Hypoxylon viridulosum” OR “Hypsizgus ulmarius” OR “Hypsizygus marmoreus” OR “Hysterium angustatum” OR “Hysterium pulicare” OR “Incrupila melatheja” OR “Incrupila viridipilosa” OR “Inonotus cuticularis” OR “Inonotus dryophilus” OR “Inonotus hispidus” OR “Inonotus radiatus” OR “Irpex griseofuscus” OR “Irpex lacteus” OR “Irpex mollis” OR “Isariopsis sp.” OR “Ischnoderma resinosum” OR “Jattaea echinella” OR “Julella sericea” OR “Junghuhnia separabilima” OR “Kabatiella apocrypta” OR “Kabatiella polyspora” OR “Kalmusia clivensis” OR “Karschia lignyota” OR “Kavinia himantia” OR “Lachnum rhytismatis” OR “Lachnum virgineum” OR “Laestadia pseudoplatani” OR “Laeticorticium roseocarneum” OR “Laetiporus sulphureus var. miniatus” OR “Laetiporus sulphureus” OR “Lagynodella acerina” OR “Lambertella tubulosa” OR “Lampteromyces japonicus” OR “Lanzia luteovirescens” OR “Lanzia rufescens” OR “Lasiosphaeria caudata” OR “Lasiosphaeria glabrata” OR “Lasiosphaeria hirsuta” OR “Lasiosphaeria ovina” OR “Lasiosphaeria pezizula” OR “Lasiosphaeria strigosa” OR “Leiosphaerella falcata” OR “Lentaria byssiseda” OR “Lentomita stylophora” OR “Lentomitella cirrhosa” OR “Lenzites betulina” OR “Lenzites heteromorpha” OR “Lenzites tricolor var. tricolor” OR “Leptosphaeria controversa” OR “Leptosphaeria dioica” OR “Leptosphaeria leucoplaca” OR “Leptosphaeria muelleri” OR “Leptosporomyces ovoideus” OR “Licrostroma subgiganteum” OR “Lopadostoma saprophilum” OR “Lophiostoma microstomum” OR “Lophiostoma tingens” OR “Lophiostoma triseptatum var. pluriseptatum” OR “Lophiostoma viridarium” OR “Lophiotricha viridicoma” OR “Lulworthia grandispora var. apiculata” OR “Lulworthia submersa” OR “Lylea tetracoila” OR “Macrohyporia extensa” OR “Macrophoma haraeana” OR “Macrophoma sp.” OR “Marasmiellus opacus” OR “Massaria inquinans” OR “Massaria lantanae” OR “Massaria vomitoria” OR “Melanconis everhartii” OR “Melanconis sudans” OR “Melanconium crinigerum” OR “Melanoleuca grammopodia var. macrocarpa” OR “Melanomma fuscidulum” OR “Melanomma longicolle” OR “Melanomma medium” OR “Melanomma pulvis‐pyrius” OR “Melasmia acerina” OR “Melasmia aceris‐trifidi” OR “Melogramma aceris” OR “Menispora ciliata” OR “Menispora glauconigra” OR “Menispora tortuosa” OR “Merulius hirtellus” OR “Merulius tremellosus” OR “Moellerodiscus lentus” OR “Mollisia caespiticia” OR “Mollisia cinerea” OR “Mollisia ligni” OR “Mollisina acerina” OR “Monodictys castaneae” OR “Monodictys putredinis” OR “Morchella esculenta” OR “Mycena cyaneobasis” OR “Mycena radicatella” OR “Mycena subcaerulea” OR “Mycocentrospora acerina” OR “Mycoleptodonoides aitchisonii” OR “Mycosphaerella latebrosa” OR “Mycosphaerella maculiformis” OR “Mycosphaerella punctiformis” OR “Myxarium nucleatum” OR “Myxosporium seriatum” OR “Nectria cinnabarina” OR “Nectria coccinea” OR “Nectria coccinea var. faginata” OR “Nectria dealbata” OR “Nectria dematiosa” OR “Nectria episphaeria” OR “Nectria flavoviridis” OR “Nectria fuckeliana” OR “Nectria galligena” OR “Nectria mammoidea” OR “Nectria mammoidea var. rugulosa” OR “Nectria modesta” OR “Nectria nigrescens” OR “Nectria ochroleuca” OR “Nectria pallidula” OR “Nectria pseudotrichia” OR “Nectria purtonii” OR “Nectria ralfsii” OR “Nectria veuillotiana” OR “Nectria viridescens” OR “Nectria vulpina” OR “Nemania atropurpurea” OR “Nemania chestersii” OR “Nemania quadrata” OR “Nemania serpens” OR “Nematoloma sublateritium” OR “Neobulgaria pura” OR “Neonectria ditissima” OR “Neonectria punicea” OR “Niesslia pulchriseta” OR “Nigrospora sp.” OR “Nitschkia brevispina” OR “Nitschkia cupularis” OR “Nodulisporium sp.” OR “Nummularia repanda” OR “Nummularia succenturiata” OR “Odontia acerina” OR “Odontia hydnoides” OR “Odontia subcrinale” OR “Oidium sp.” OR “Oligoporus tephroleucus” OR “Oncopodium aceris” OR “Ophiocordyceps clavulata” OR “Ophiostoma piceae” OR “Ophiostoma piliferum” OR “Ophiostoma roboris” OR “Orbilia inflatula” OR “Orbilia luteorubella” OR “Otthia spiraeae” OR “Oudemansiella mucida” OR “Oxyporus populinus” OR “Pachyella clypeata” OR “Panus laevis” OR “Panus rudis” OR “Papularia arundinis” OR “Papularia sp.” OR “Parmelia glabratula” OR “Parmelia subargentifera” OR “Parmelia subrudecta” OR “Patellaria cylindrospora” OR “Patellariopsis clavispora” OR “Penicillium sp.” OR “Peniophora carnosa” OR “Peniophora cinerea” OR “Peniophora decorticans” OR “Peniophora gracillima” OR “Peniophora heterocystidia” OR “Peniophora hydnoides” OR “Peniophora parasitica” OR “Peniophora velutina” OR “Peniophora violaceolivida” OR “Peniophora viticola” OR “Perenniporia fissiliformis” OR “Periconia pycnospora” OR “Peroneutypa heteracantha” OR “Pestalotia aceris” OR “Pestalotia sp.” OR “Pestalotiopsis aceris” OR “Pestalotiopsis paraguariensis” OR “Pezicula acericola” OR “Pezicula acerina” OR “Pezicula aesculea” OR “Pezicula carnea” OR “Pezicula sp.” OR “Pezicula subcarnea” OR “Peziza cognata” OR “Peziza paulopuncta” OR “Peziza repanda” OR “Peziza simulata” OR “Peziza vinosa” OR “Phaeodiaporthe appendiculata” OR “Phaeoisaria sparsa” OR “Phaeomoniella sp.” OR “Phaeosphaeriopsis sp.” OR “Phaeostalagmus altissimus” OR “Phaeostalagmus cyclosporus” OR “Phanerochaete carnosa” OR “Phanerochaete chrysorhiza” OR “Phanerochaete ericina” OR “Phanerochaete filamentosa” OR “Phanerochaete laevis” OR “Phanerochaete rhodella” OR “Phanerochaete sordida” OR “Phanerochaete velutina” OR “Phellinidium fragrans” OR “Phellinus ferruginosus” OR “Phellinus fragrans” OR “Phellinus igniarius” OR “Phellinus macgregorii” OR “Phellinus melleoporus” OR “Phellinus parmastoi” OR “Phellinus robustus” OR “Phialocephala canadensis” OR “Phialocephala fusca” OR “Phialophora americana” OR “Phialophora sp.” OR “Phialophora verrucosa” OR “Phlebia floridensis” OR “Phlebia radiata” OR “Phlebia setulosa” OR “Pholiota albocrenulata” OR “Pholiota sp.” OR “Phoma consorta” OR “Phoma pezizoides” OR “Phoma sp.” OR “Phomopsis acerina” OR “Phomopsis aceris‐palmatus” OR “Phomopsis lebiseyi” OR “Phragmotrichum rivoclarinum” OR “Phyllactinia guttata” OR “Phyllactinia suffulta” OR “Phyllosticta acericola” OR “Phyllosticta aceris” OR “Phyllosticta capitalensis” OR “Phyllosticta minima” OR “Phyllosticta minutissima” OR “Phyllosticta negundinis” OR “Phyllosticta platanoides” OR “Phyllosticta platanoidis” OR “Phyllosticta sp.” OR “Physalospora malorum” OR “Physcia caesia” OR “Physcia dubia” OR “Phytophthora cactorum” OR “Phytophthora cambivora” OR “Phytophthora cinnamomi” OR “Phytophthora citricola” OR “Phytophthora infestans” OR “Phytophthora sp.” OR “Pilidium acerinum” OR “Plagiostoma barriae” OR “Plagiostoma inclinatum” OR “Plagiostoma petiolophilum” OR “Plagiostoma pseudobavaricum” OR “Plagiostoma sp.” OR “Platystomum compressum var. pseudomacrostomum” OR “Pleomassaria acericola” OR “Pleospora papillata” OR “Pleurophoma pleurospora” OR “Pleurophragmium parvisporum” OR “Pleurotellus hypnophilus” OR “Pleurothecium recurvatum” OR “Pleurotus cornucopiae var. citrinopileatus” OR “Pleurotus ostreatus” OR “Pleurotus salmoneostramineus” OR “Pleurotus serotinus” OR “Pleurotus strigosus” OR “Pleurotus ulmarius” OR “Plicaturopsis crispa” OR “Polyporus admirabilis” OR “Polyporus adustus” OR “Polyporus biformis” OR “Polyporus conchifer” OR “Polyporus cuticularis” OR “Polyporus delectans” OR “Polyporus ectypus” OR “Polyporus floriformis” OR “Polyporus frondosus” OR “Polyporus gilvus” OR “Polyporus glomeratus” OR “Polyporus hispidus” OR “Polyporus lentus” OR “Polyporus litschaueri” OR “Polyporus mori” OR “Polyporus nidulans” OR “Polyporus picipes” OR “Polyporus pinsitus” OR “Polyporus planellus” OR “Polyporus robiniophilus” OR “Polyporus spraguei” OR “Polyporus squamosus” OR “Polyporus tulipiferae” OR “Polyporus umbellatus” OR “Polyporus varius” OR “Polyporus versicolor” OR “Poria ambigua” OR “Poria attenuata” OR “Poria aurea” OR “Poria cinerascens” OR “Poria cocos” OR “Poria elongata” OR “Poria eupora” OR “Poria fimbriatella” OR “Poria fraxinea” OR “Poria isabellina” OR “Poria lurida” OR “Poria mollusca” OR “Poria pulchella” OR “Poria punctata” OR “Poria subacida” OR “Poria tomentocincta” OR “Poria unita” OR “Poria versipora” OR “Propolis lobata” OR “Propolomyces versicolor” OR “Prosthecium innesii” OR “Prosthecium platanoidis” OR “Protoventuria vancouverensis” OR “Pseudobasidiospora caroliniana” OR “Pseudodiplodia corticis” OR “Pseudospiropes longipilus” OR “Pseudospiropes nodosus” OR “Pseudospiropes simplex” OR “Pseudotomentella flavovirens” OR “Pseudotomentella griseopergamacea” OR “Pseudovalsa fusca” OR “Pseudovalsa minima” OR “Ptychogaster cubensis” OR “Pycnoporellus fulgens” OR “Pycnoporus cinnabarinus” OR “Pycnoporus sanguineus” OR “Pyrenopeziza nervicola” OR “Pyrenopeziza petiolaris” OR “Pythium debaryanum” OR “Resinicium bicolor” OR “Rhamphoria bevanii” OR “Rhizoctonia aurantiaca” OR “Rhizoctonia solani” OR “Rhodotorula sp.” OR “Rhytisma acerinum” OR “Rhytisma punctatum” OR “Rigidoporus nigrescens” OR “Rosellinia aquila” OR “Rosellinia cainii” OR “Rosellinia corticium” OR “Rosellinia evansii” OR “Rosellinia medullaris” OR “Rosellinia megalocarpa” OR “Rosellinia necatrix” OR “Russula salmoneolutea” OR “Rutstroemia luteovirescens” OR “Rutstroemia sp.” OR “Rutstroemia sydowiana” OR “Sarcoscypha coccinea” OR “Sarcoscypha occidentalis” OR “Sawadaea bicornis” OR “Sawadaea polyfida var. japonica” OR “Sawadaea sp.” OR “Sawadaea tulasnei” OR “Sawadaia bicornis” OR “Schizophyllum commune” OR “Schizothyrium aceris” OR “Schizoxylon compositum” OR “Schizoxylum cinereum” OR “Scoleconectria atkinsonii” OR “Scopuloides rimosa” OR “Scytinostroma protrusum subsp. septentrionale” OR “Sebacina epigaea” OR “Sebacina incrustans” OR “Sebacina molybdea” OR “Seimatosporium lichenicola” OR “Septobasidium castaneum” OR “Septobasidium pseudopedicellatum” OR “Septomyxa tulasnei” OR “Septonema breviusculum” OR “Septoria acerina” OR “Septoria aceris” OR “Septoria apatela” OR “Septoria epicotylea” OR “Septoria negundinis” OR “Septoria seminalis var. platanoidis” OR “Septoria sp.” OR “Septosporium velutinum” OR “Skeletocutis perennis” OR “Sordaria lutea” OR “Spadicoides atra” OR “Spadicoides bina” OR “Spadicoides obovata” OR “Spegazzinia parkeri” OR “Sphaerella alarum” OR “Sphaeria atriella” OR “Sphaeria caminata” OR “Sphaeria fulvotecta” OR “Sphaeria monilispora” OR “Sphaeria vetusta” OR “Sphaeronaema acerinum” OR “Sphaeropsis grandinea” OR “Sphaeropsis sp.” OR “Sphaerostilbe flammea” OR “Sphaerotheca fuliginea” OR “Splanchnonema pupula” OR “Spongipellis delectans” OR “Spongipellis spumeus” OR “Sporidesmiella brachysporioides” OR “Sporidesmiella hyalosperma var. hyalosperma” OR “Sporidesmium altum” OR “Sporidesmium folliculatum” OR “Sporidesmium hormiscioides” OR “Sporidesmium hysterioideum” OR “Sporidesmium leptosporum” OR “Sporidesmium moriforme” OR “Sporidesmium varians” OR “Sporonema pallidum” OR “Stachybotrys chartarum” OR “Stachycoremium parvulum” OR “Steccherinum ochraceum” OR “Steccherinum peckii” OR “Steccherinum septentrionale” OR “Steccherinum setulosum” OR “Steccherinum tenue” OR “Steganosporium ovatum” OR “Stegonsporium pyriforme” OR “Stereum complicatum” OR “Stereum fuscum” OR “Stereum gausapatum” OR “Stereum hirsutum” OR “Stereum lilacinofuscum” OR “Stereum murrayi” OR “Stereum ostrea” OR “Stictis radiata” OR “Stigmatomassaria pupula” OR “Stigmina robusta” OR “Stilbum giganteum” OR “Streptomyces sp.” OR “Strickeria obducens” OR “Stromatocyphella aceris” OR “Strossmayeria bakeriana” OR “Strossmayeria basitricha” OR “Taeniolella breviuscula” OR “Taphrina sacchari” OR “Taphrina sp.” OR “Teichospora nigrobrunnea” OR “Thanatephorus terrigenus” OR “Thelephora sp.” OR “Thelonectria gongylodes” OR “Thelonectria nodosa” OR “Thyridaria minima” OR “Thyridaria rubronotata” OR “Thyridium stilbostomum” OR “Thyronectria pyrrhochlora” OR “Thyronectria virens” OR “Tilletiopsis lilacina” OR “Tilletiopsis minor var. flava” OR “Tomentella angulospora” OR “Tomentella atrorubra” OR “Tomentella bicolor” OR “Tomentella botryoides” OR “Tomentella bresadolae” OR “Tomentella bryophila” OR “Tomentella calcicola” OR “Tomentella chlorina” OR “Tomentella cinerascens” OR “Tomentella coerulea” OR “Tomentella crinalis” OR “Tomentella epigaea” OR “Tomentella ferruginea” OR “Tomentella fibrosa” OR “Tomentella fuscoferruginosa” OR “Tomentella lateritia” OR “Tomentella microspora” OR “Tomentella neobourdotii” OR “Tomentella ochracea” OR “Tomentella olivascens” OR “Tomentella pilosa” OR “Tomentella punicea” OR “Tomentella ramosissima” OR “Tomentella ruttneri” OR “Tomentella subcinerascens” OR “Tomentella sublilacina” OR “Tomentella subvinosa” OR “Tomentella terrestris” OR “Tomentella umbrinospora” OR “Tomentella violaceofusca” OR “Tomentella viridescens” OR “Tomentella viridis” OR “Tomentella viridula” OR “Trametes cinnabarina var. sanguinea” OR “Trametes elegans” OR “Trametes hirsuta” OR “Trametes malicola” OR “Trametes trogii” OR “Trametes unicolor” OR “Trechispora farinacea” OR “Trematosphaeria pertusa” OR “Tremella foliacea” OR “Tremella lutescens” OR “Triblidium caliciiforme” OR “Trichocladium canadense” OR “Trichocladium sp.” OR “Trichoderma sp.” OR “Trichodiscus virescentulus” OR “Trichothecium sp.” OR “Tripospermum acerinum” OR “Trisulcosporium acerinum” OR “Tubakia dryina” OR “Tubercularia granulata” OR “Tubercularia smaragdina” OR “Tubercularia vulgaris” OR “Tubulicrinis glebulosus” OR “Tulasnella pruinosa” OR “Tulasnella violea” OR “Tympanis acericola” OR “Tympanis acerina” OR “Tympanis bicolor” OR “Tympanis truncatula” OR “Typhula intermedia” OR “Typhula phacorrhiza” OR “Tyromyces balsameus” OR “Tyromyces chioneus” OR “Tyromyces fissilis” OR “Tyromyces fumidiceps” OR “Tyromyces galactinus” OR “Tyromyces immitis” OR “Tyromyces spumeus” OR “Tyromyces stipticus” OR “Tyromyces undosus” OR “Uncinula aceris” OR “Uncinula bicornis” OR “Uncinula circinata” OR “Uncinula nankinensis” OR “Unguiculariopsis ilicincola” OR “Valsa ambiens” OR “Valsa ambiens subsp. ambiens” OR “Valsa ambiens subsp. leucostomoides” OR “Valsa ceratosperma” OR “Valsa magnispora” OR “Valsa myinda” OR “Valsa pauperata” OR “Valsa pseudoplatani” OR “Valsa sordida” OR “Valsaria exasperans” OR “Velutarina rufo‐olivacea” OR “Venturia acerina” OR “Vermicularia ochrochaeta” OR “Verticillium albo‐atrum” OR “Verticillium dahliae” OR “Verticillium nigra” OR “Verticillium sp.” OR “Vesiculomyces citrinus” OR “Vibrissea truncorum” OR “Volvariella bombycina” OR “Vuilleminia comedens” OR “Wardomyces hughesii” OR “Xenasma alboglaucum” OR “Xenasma praeteritum” OR “Xenasma rallum” OR “Xenasma rimicola” OR “Xenasma tulasnelloideum” OR “Xenostigmina zilleri” OR “Xylaria acuta” OR “Xylaria cornu‐damae” OR “Xylaria cubensis” OR “Xylaria longipes” OR “Xylaria polymorpha” OR “Xylaria warburgii” OR “Zygodesmus granulosus” OR “Zygodesmus limonisporus” OR “Biscogniauxia capnodes” OR “Botryosphaeria parva” OR “Pestalotiopsis sp.” OR “Drepanosiphum platanoidis” OR “Periphyllus californiensis” OR “Phloeophagosoma dilutum” OR “Stenoscelis hylastoides” OR “Lopharia spadicea” OR “Stegonsporium pyriforme” OR “Hyphoderma obtusum” OR “Pleurotus ostreatus” OR “Chondrostereum purpureum” OR “Codinaea glauconigra” OR “Costantinella terrestris” OR “Fomes connatus” OR “Ganoderma lucidum” OR “Hypoxylon deustum” OR “Hypoxylon howeanum” OR “Laeticorticium roseocarneum” OR “Menispora glauconigra” OR “Menispora tortuosa” OR “Nectria pithoides” OR “Omphalotus olearius” OR “Ophionectria cerea” OR “Peniophora decorticans” OR “Plectania coccinea” OR “Polyporus brumalis” OR “Polyporus dichrous” OR “Polyporus” OR “Polyporus glomeratus” OR “Spadicoides atra” OR “Stegonsporium pyriforme” OR “Vararia investiens” OR “Massaria pupula” OR “Metasphaeria sepincola” OR “Septogloeum hercynicum” OR “Trichia scabra” OR “Cylindrocladium pacificum” OR “Verticillium nigrescens” OR “Agaricus” OR “Botryosphaeria quercuum sensu” OR “Calonectria kyotensis” OR “Descolea” OR “Eutypa” OR “Gymnopus sp.” OR “Inocybe geophila” OR “Lopharia cinerascens” OR “Verticillium” OR “Dendrothele acerina” OR “Dacrymyces capitatus” OR “Dacrymyces stillatus” OR “Diatrypaceae” OR “Exidia nucleata” OR “Lycoperdon” OR “Phloeospora aceris” OR “Rhytisma acerinum” OR “Suillus grevillei” OR “Pseudomonas syringae pv. aceris” OR “Verticillium intertextum” OR “Aporpium caryae” OR “Cryptodiaporthe myinda” OR “Diatrypella frostii” OR “Helicoma viride” OR “Hypocrea gelatinosa” OR “Polyporus albellus” OR “Polyporus” OR “Polyporus fumidiceps” OR “Polyporus galactinus” OR “Poria elongata” OR “Poria pannocincta” OR “Cecidomyia ocellaris” OR “Dasyneura communis” OR “Rhabdophaga rileyana” OR “Perrisia tympani” OR “Aceria ryderi” OR “Aculops longispinosus” OR “Cnestus mutilatus” OR “Monarthrum mali” OR “Euwallacea fornicatus sensu lato” OR “Hypothenemus eruditus” OR “Megaplatypus mutatus” OR “Platypus koryoensis” OR “Xylosandrus compactus” OR “Longidorus biformis” OR “Longidorus breviannulatus” OR “Longidorus crassus” OR “Longidorus diadecturus” OR “Longidorus paralongicaudatus” OR “Longidorus paravineacola” OR “Xiphinema brevicolle” OR “Xiphinema simile” OR “Nanidorus minor” OR “Paratrichodorus teres”) |
Appendix C – Plant taxa reported to be present in the nurseries of Acer pseudoplatanus
1.
Table C.1.
Plant taxa reported in the Dossier Section 6.0 to be present in the nurseries of Acer pseudoplatanus
| Number | Plant taxa | Number | Plant taxa |
|---|---|---|---|
| 1 | Abelia | 203 | Juniperus |
| 2 | Abies alba | 204 | Juniperus communis |
| 3 | Abies concolor | 205 | Knautia |
| 4 | Abies fraseri | 206 | Kniphofia |
| 5 | Abies grandis | 207 | Laburnum |
| 6 | Abies koreana | 208 | Laburnum anagyroides |
| 7 | Abies nobilis | 209 | Lamium |
| 8 | Abies nordmanniana | 210 | Larix |
| 9 | Abies procera | 211 | Larix decidua |
| 10 | Acacia | 212 | Larix kaempferi |
| 11 | Acanthus | 213 | Larix × eurolepsis |
| 12 | Acer | 214 | Lavandula |
| 13 | Acer campestre | 215 | Lavatera |
| 14 | Acer capillipes | 216 | Leucanthemum |
| 15 | Acer davidii | 217 | Leucothoe |
| 16 | Acer griseum | 218 | Leycesteria |
| 17 | Acer macrocarpa | 219 | Leymus |
| 18 | Acer palmatum | 220 | Liatris |
| 19 | Acer palmatum ‘Atropurpureum' | 221 | Ligularia |
| 20 | Acer pensylvanicum | 222 | Ligustrum |
| 21 | Acer platanoides | 223 | Ligustrum ovalifolium |
| 22 | Acer pseudoplatanus | 224 | Ligustrum ovalifolium ‘Aureum' |
| 23 | Achillea | 225 | Ligustrum vulgare |
| 24 | Acorus | 226 | Liquidambar |
| 25 | Actaea | 227 | Liquidambar styraciflua |
| 26 | Agapanthus | 228 | Liriodendron tulipifera |
| 27 | Agastache | 229 | Liriope |
| 28 | Ajuga | 230 | Lithodora |
| 29 | Akebia | 231 | Lobelia |
| 30 | Alchemilla | 232 | Lonicera |
| 31 | Allium | 233 | Lonicera nitida |
| 32 | Alnus | 234 | Lonicera periclymenum |
| 33 | Alnus cordata | 235 | Lupinus |
| 34 | Alnus glutinosa | 236 | Luzula |
| 35 | Alnus incana | 237 | Lysimachia |
| 36 | Alnus rubra | 238 | Magnolia |
| 37 | Alstroemeria | 239 | Magnolia kobus |
| 38 | Amelanchier | 240 | Mahonia |
| 39 | Amelanchier canadensis | 241 | Malus |
| 40 | Ammonophylla | 242 | Malus sylvestris |
| 41 | Anemanthele | 243 | Matteuccia |
| 42 | Anemone | 244 | Meconopsis |
| 43 | Aquilegia | 245 | Metasequoia glyptostroboides |
| 44 | Araucaria araucana | 246 | Miscanthus |
| 45 | Arbutus | 247 | Molinia |
| 46 | Arbutus unedo | 248 | Monarda |
| 47 | Armeria | 249 | Myrtus |
| 48 | Artemisia | 250 | Nandina |
| 49 | Arum | 251 | Nemesia |
| 50 | Aruncus | 252 | Nepeta |
| 51 | Asplenium | 253 | Nothofagus |
| 52 | Astelia | 254 | Nyssa sylvatica |
| 53 | Aster | 255 | Olearia |
| 54 | Astilbe | 256 | Ophiopogon |
| 55 | Astrantia | 257 | Osmanthus |
| 56 | Athyrium | 258 | Osmunda |
| 57 | Aucuba | 259 | Pachysandra |
| 58 | Baptisia | 260 | Pachystegia |
| 59 | Berberis | 261 | Paeonia |
| 60 | Berberis darwinii | 262 | Panicum |
| 61 | Berberis thunbergii | 263 | Pennisetum |
| 62 | Berberis thunbergii f. atropurpurea | 264 | Penstemon |
| 63 | Bergenia | 265 | Perovskia |
| 64 | Betula | 266 | Persicaria |
| 65 | Betula pendula | 267 | Philadelphus |
| 66 | Betula pubescens | 268 | Phlomis |
| 67 | Betula utilis var. jacquemontii | 269 | Phlox |
| 68 | Blechnum | 270 | Phormium |
| 69 | Brachyglottis | 271 | Photinia |
| 70 | Brunnera | 272 | Photinia × fraseri ‘Red Robin’ |
| 71 | Buddleja | 273 | Phygelius |
| 72 | Buxus | 274 | Physocarpus |
| 73 | Buxus sempervirens | 275 | Physostegia |
| 74 | Calamagrostis | 276 | Picea abies |
| 75 | Calluna | 277 | Picea omorika |
| 76 | Campanula | 278 | Picea orientalis |
| 77 | Carex | 279 | Picea pungens glauca |
| 78 | Carpinus | 280 | Picea sitchensis |
| 79 | Carpinus betulus | 281 | Pinus |
| 80 | Caryopteris | 282 | Pinus nigra var. austriaca |
| 81 | Castanea | 283 | Pinus peuce |
| 82 | Castanea sativa | 284 | Pinus pinaster |
| 83 | Ceanothus | 285 | Pinus pungens glauca |
| 84 | Cedrus atlantica | 286 | Pinus radiata |
| 85 | Cedrus deodara | 287 | Pinus sylvestris |
| 86 | Centaurea | 288 | Pittosporum |
| 87 | Centranthus | 289 | Platanus |
| 88 | Ceratostigma | 290 | Polemonium |
| 89 | Cercidiphyllum japonicum | 291 | Polygonatum |
| 90 | Cercis canadensis | 292 | Polypodium |
| 91 | Chaenomeles | 293 | Polystichum |
| 92 | Chamaecyparis | 294 | Populus |
| 93 | Chamaecyparis lawsoniana | 295 | Populus nigra |
| 94 | Choisya | 296 | Populus tremula |
| 95 | Cistus | 297 | Potentilla |
| 96 | Clematis | 298 | Primula |
| 97 | Convolvulus | 299 | Prunus |
| 98 | Coprosma | 300 | Prunus avium |
| 99 | Coreopsis | 301 | Prunus cera |
| 100 | Cornus | 302 | Prunus cerasifera |
| 101 | Cornus kousa var. chinensis | 303 | Prunus lau.’Rotund’ |
| 102 | Cornus sanguinea | 304 | Prunus laurocerasus |
| 103 | Cortaderia | 305 | Prunus lusitanica |
| 104 | Corydalis | 306 | Prunus padus |
| 105 | Corylus | 307 | Prunus spinosa |
| 106 | Corylus avellana | 308 | Pseudotsuga menziesii |
| 107 | Cosmos | 309 | Pulmonaria |
| 108 | Cotinus | 310 | Pyracantha |
| 109 | Cotoneaster | 311 | Pyrus |
| 110 | Cotoneaster bullatus | 312 | Pyrus communis |
| 111 | Cotoneaster franchettii | 313 | Quercus |
| 112 | Cotoneaster horizontalis | 314 | Quercus ilex |
| 113 | Cotoneaster lacteus | 315 | Quercus palustris |
| 114 | Cotoneaster simonsii | 316 | Quercus petraea |
| 115 | Crataegus | 317 | Quercus robur |
| 116 | Crataegus monogyna | 318 | Quercus rubra |
| 117 | Crocosmia | 319 | Rhamnus |
| 118 | Cryptomeria japonica | 320 | Rhamnus cathartica |
| 119 | Cupressocyparis | 321 | Rhamnus frangula |
| 120 | Cupressocyparis leylandii | 322 | Rhus |
| 121 | Cupressus | 323 | Ribes |
| 122 | Cupressus macrocarpa | 324 | Robinia pseudoacacia |
| 123 | Cynoglossum | 325 | Robinia |
| 124 | Cytisus | 326 | Rosa |
| 125 | Dahlia | 327 | Rosa arvensis |
| 126 | Daphne | 328 | Rosa canina |
| 127 | Davidia involucrata | 329 | Rosa rubiginosa |
| 128 | Delosperma | 330 | Rosa rugosa |
| 129 | Delphinium | 331 | Rosa rugosa ‘Alba’ |
| 130 | Deschampsia | 332 | Rosa rugosa rubra |
| 131 | Deutzia | 333 | Rosa spinosissima |
| 132 | Dicentra | 334 | Rosmarinus |
| 133 | Diervilla | 335 | Rudbeckia |
| 134 | Digitalis | 336 | Salix |
| 135 | Doronicum | 337 | Salix aurita |
| 136 | Dryopteris | 338 | Salix caprea |
| 137 | Echinacea | 339 | Salix cinerea |
| 138 | Echinops | 340 | Salix pentandra |
| 139 | Elaeagnus | 341 | Salix viminalis |
| 140 | Epimedium | 342 | Salvia |
| 141 | Eremurus | 343 | Sambucus |
| 142 | Erigeron | 344 | Sambucus nigra |
| 143 | Eriophorum | 345 | Sanguisorba |
| 144 | Eriostemon | 346 | Santolina |
| 145 | Eryngium | 347 | Sarcococca confusa |
| 146 | Erysimum | 348 | Scabiosa |
| 147 | Escallonia | 349 | Schizostylis |
| 148 | Eucalyptus | 350 | Sedum |
| 149 | Eucalyptus glaucescens | 351 | Senecio |
| 150 | Eucalyptus gunnii | 352 | Sequoia sempervirens |
| 151 | Euonymus | 353 | Sequoiadendron giganteum |
| 152 | Euonymus europaeus | 354 | Sesleria |
| 153 | Euonymus japonicus ‘Bravo’ | 355 | Sorbaria |
| 154 | Euphorbia | 356 | Sorbus |
| 155 | Exochorda | 357 | Sorbus aria |
| 156 | Fagus | 358 | Sorbus aucuparia |
| 157 | Fagus sylvatica | 359 | Sorbus intermedia |
| 158 | Fagus sylvatica ‘Atropurpurea’ | 360 | Sorbus torminalis |
| 159 | Fargesia | 361 | Spiraea |
| 160 | Fatsia | 362 | Stachys |
| 161 | Festuca | 363 | Stachyurus |
| 162 | Filipendula | 364 | Stewartia pseudocamellia |
| 163 | Foeniculum | 365 | Stipa |
| 164 | Forsythia | 366 | Symphiocarpus |
| 165 | Fuchsia | 367 | Symphoricarpos |
| 166 | Galium | 368 | Symphytum |
| 167 | Garrya | 369 | Syringa |
| 168 | Gaultheria procumbens | 370 | Taxodium distichum |
| 169 | Gaultheria shallon | 371 | Taxus |
| 170 | Gaura | 372 | Taxus baccata |
| 171 | Genista | 373 | Tellima |
| 172 | Geranium | 374 | Thalictrum |
| 173 | Geum | 375 | Thuja |
| 174 | Ginkgo biloba | 376 | Thuja plicata |
| 175 | Griselinia | 377 | Thymus |
| 176 | Hakonechloa | 378 | Tiarella |
| 177 | Halesia carolina | 379 | Tilia |
| 178 | Halimium | 380 | Tilia cordata |
| 179 | Hebe | 381 | Tilia platanoides |
| 180 | Hedera | 382 | Tilia platyphyllos |
| 181 | Helenium | 383 | Trachelospermum |
| 182 | Helichrysum | 384 | Trachycarpus fortunei |
| 183 | Helleborus | 385 | Tradescantia |
| 184 | Hemerocallis | 386 | Tricyrtis |
| 185 | Heuchera | 387 | Trollius |
| 186 | Heucherella | 388 | Tsuga heterophylla |
| 187 | Hippophae | 389 | Ulex |
| 188 | Hippophae rhamnoides | 390 | Ulex europaeus |
| 189 | Hosta | 391 | Ulmus |
| 190 | Houttuynia | 392 | Ulmus glabra |
| 191 | Hydrangea | 393 | Uncinia |
| 192 | Hypericum | 394 | Verbena |
| 193 | Iberis | 395 | Veronica |
| 194 | Ilex | 396 | Viburnum |
| 195 | Ilex aquifolium | 397 | Viburnum lantana |
| 196 | ilex crenata | 398 | Viburnum opulus |
| 197 | Ilex x altaclerensis ‘Golden King’ | 399 | Vinca |
| 198 | Imperata | 400 | Weigela |
| 199 | Iris | 401 | Wisteria sinensis |
| 200 | Jasminum | 402 | Cupressocyparis leylandii |
| 201 | Juglans nigra | 403 | Yucca |
| 202 | Juglans regia | 404 | Yucca filamentosa |
Appendix D – Water used for irrigation
1.
All mains water used meets the UK standard Water Supply (Water quality) regulation 2016 and the WHO/EU potable water standards (Drinking water Directive (98/83/EC and the revised Drinking Water Directive 2020/2184) which includes a total freedom from both human and plant pathogens (Article 2–(7)). All mains water conducting pipework fully complies with the UK Water Supply (Water Fittings) regulations of 1999 and the amendments of 2019. Irrigation water used is not stored in any open tanks where airborne contamination could take place and is entirely isolated from any outside exposure (Dossier Section 3.0).
Bore hole water supply: in some cases, where the underlying geology permits, nurseries can draw water directly from bore holes drilled into underground aquafers. The water that fills these aquafers is naturally filtered through the layers of rock (e.g. limestone) over long periods of time, many millennia in some cases. The water from such supplies is generally of such high quality that it is fit for human consumption with little to no further processing and is often bottled and sold as mineral water (Dossier Section 3.0).
Rainwater or freshwater watercourse supply: Some nurseries contributing to this application for both environmental and efficiency reasons use a combination of rain capture systems or abstract directly from available watercourses. All water is passed through a sand filtration system to remove contaminants and is contained in storage tanks prior to use. One nursery that operates this approach is currently in the process of installing additional nanobubble technology to treat the water (Dossier Section 3.0).
Appendix E – List of pests that can potentially cause an effect not further assessed
1.
Table E.1.
List of potential pests not further assessed
| N | Pest name | EPPO code | Group | Present in the UK | Present in the EU | Acer pseudoplatanus/Acer confirmed as a host (reference) | Pest can be associated with the commodity | Impact | Justification for inclusion in this list |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Anthocoptes transitionalis | – | Mites | Yes | Restricted (Hungary) | Acer pseudoplatanus (Dossier Section 2.0) | Yes | No data | Uncertainty about impact. |
| 2 | Aureobasidium apocryptum | – | Fungi | Yes | Restricted (Germany) | Acer (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
| 3 | Aureobasidium pullulans var. pullulans | – | Fungi | Yes | Restricted (Poland, France) | Acer pseudoplatanus (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
| 4 | Cinereomyces lindbladii | CINELI | Fungi | Yes | No | Acer (Farr and Rossman, online) | Uncertain | No data | Uncertainty about impact and about association with the commodities. |
| 5 | Dichomera sauhinetii | – | Fungi | Yes | No data | Acer pseudoplatanus (Bevercombe and Rayner, 1980) | Yes | No data | Uncertainty about impact and the presence in the EU. |
| 6 | Phomopsis lebiseyi | – | Fungi | Yes | Restricted (Portugal) | Acer (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
| 7 | Phyllosticta aceris | – | Fungi | Yes | Restricted (Czechia, Poland, Slovakia) | Acer, A. pseudoplatanus (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
| 8 | Phyllosticta campestris | – | Fungi | Yes | Restricted (Poland) | Acer pseudoplatanus (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
| 9 | Phytophthora obscura | PHYTOB | Oomycetes | Yes | Restricted (Germany, Slovakia) | Acer pseudoplatanus (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
| 10 | Pleuroceras pseudoplatani | – | Fungi | Yes | Uncertain | Acer pseudoplatanus (Farr and Rossman, online) | Yes | Yes | Uncertainty about the distribution in the EU. |
| 11 | Rhizoctonia terrigena | – | Fungi | Yes | Restricted (Denmark) | Acer (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
| 12 | Septomyxa tulasnei | – | Fungi | Yes | Restricted (Poland) | Acer, A. pseudoplatanus (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
Appendix F – Excel file with the pest list of Acer pseudoplatanus
1.
Appendix F can be found in the online version of this output (in the ‘Supporting information section’): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2023.8074#support‐informationsection
Supporting information
Excel file with the pest list of Acer pseudoplatanus
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023. Scientific Opinion on the commodity risk assessment of Acer pseudoplatanus plants from the UK. EFSA Journal 2023;21(7):8074, 271 pp. 10.2903/j.efsa.2023.8074
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00348
Panel members: Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, and Lucia Zappalà.
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements: The Scientific Opinion was prepared in cooperation with the Università degli studi di Padova, Dipartimento Agronomia, Animali, Alimenti, Risorse Naturali e Ambiente (Italy) under the EFSA Art. 36 Framework Partnership Agreement ‘GP/EFSA/ALPHA/2019/01’ – Lot 3 – taxa traded mostly as plants for planting for forestry purposes.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 25 May 2023
Notes
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) 228/2013, (EU) 652/2014 and (EU) 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. OJ L 317, 23.11.2016, pp. 4–104.
Commission Implementing Regulation (EU) 2018/2019 of 18 December 2018 establishing a provisional list of high risk plants, plant products or other objects, within the meaning of Article 42 of Regulation (EU) 2016/2031 and a list of plants for which phytosanitary certificates are not required for introduction into the Union, within the meaning of Article 73 of that Regulation C/2018/8877. OJ L 323, 19.12.2018, pp. 10–15.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019. OJ L 319, 10.12.2019, p. 1–279.
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Protocol on Ireland/Northern Ireland in conjunction with Annex 2 to that Protocol, for the purposes of this Opinion, references to Member States include the United Kingdom in respect of Northern Ireland.
Plant Health (Amendment etc.) (EU Exit) Regulations 2020 of 14 December 2020, No. 1482, 80 pp. Available online: https://www.legislation.gov.uk/uksi/2020/1482/contents/made
Plant Health (Phytosanitary Conditions) (Amendment) (EU Exit) Regulations 2020, No. 1527, 276 pp. Available online: https://www.legislation.gov.uk/uksi/2020/1527/contents/made
References
- Bevercombe GP and Rayner ADM, 1980. Diamond‐bark diseases of sycamore in Britain. New Phytologist, 86, 379–392. 10.1111/j.1469-8137.1980.tb01679.x [DOI] [Google Scholar]
- CABI (Centre for Agriculture and Bioscience International) , online. CABI Crop Protection Compendium. Available online: https://www.cabi.org/cpc/ [Accessed: 1 December 2022]. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2019. Guidance on commodity risk assessment for the evaluation of high risk plants dossiers. EFSA Journal 2019;17(4):5668, 20 pp. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2020. EPPO Technical Document No. 1081, EPPO Study on the risk of bark and ambrosia beetles associated with imported non‐coniferous wood. EPPO Paris. Available online: https://www.eppo.int/RESOURCES/eppo_publications [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://gd.eppo.int/ [Accessed: 1 December 2022].
- EUROPHYT (European Union Notification System for Plant Health Interceptions) , online. Available online: https://ec.europa.eu/food/plants/plant-health-and-biosecurity/European-union-notification-system-plant-health-interceptionsen [Accessed: 22 December 2022].
- FAO (Food and Agriculture Organization of the United Nations) , 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations) , 2017. ISPM (International standards for phytosanitary measures) No. 5. Glossary of phytosanitary terms. FAO, Rome. Available online: https://www.ippc.int/en/publications/622/
- FAO (Food and Agriculture Organization of the United Nations) , 2019. ISPM (International Standards for Phytosanitary Measures) No. 36, Integrated measures for plants for planting. FAO, Rome. Available online: https://www.fao.org/3/k8114e/K8114E.pdf
- Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ [Accessed: 1 December 2022].
- Ferris H, online. Nemaplex (The Nematode‐Plant Expert Information System). Available online: http://nemaplex.ucdavis.edu/ [Accessed: 1 December 2022].
- Francardi V, Noal A, Francescato S, Pinto R, Bruni A, Loffredi L, Bucini D, Guarnieri D, Bellantuono M, Esposito N, Nuccitelli L, Binazzi F, Vitale S, Di Giambattista G, Pf R and Pennacchio F, 2017. Coexistence of Xylosandrus crassiusculus (Motschulsky) and X. compactus (Eichhoff) (Coleoptera Curculionidae Scolytinae) in the National Park of Circeo (Lazio, Italy). Redia, 100, 149–155. 10.19263/redia-100.17.19 [DOI] [Google Scholar]
- García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: a literature‐based model of scale insect biology and systematics. Available online: https://scalenet.info/associates/ [Accessed: 1 December 2022]. [DOI] [PMC free article] [PubMed]
- Hawksworth DL, 1972. Hypoxylon mammatum. [Descriptions of Fungi and Bacteria]. IMI Descriptions of Fungi and Bacteria, 36, 2. [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐ Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Plant Pest Information Network , online. Available online: https://www.mpi.govt.nz/resources-and-forms/registers-and-lists/plant-pest-information-network/ [Accessed: 1 December 2022].
- Robinson GS, Ackery PR, Kitching IJ, Beccaloni GW and Hernández LM, online. HOSTS – a database of the world's Lepidopteran hostplants. Natural History Museum, London. Available online: https://www.nhm.ac.uk/our-science/data/hostplants/search/index.dsml [Accessed: 1 December 2022]. [Google Scholar]
- TRACES‐NT , online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
- Xu YM and Zhao ZQ, 2019. Longidoridae and Trichodoridae (Nematoda: Dorylaimida and Triplonchida). Fauna of New Zealand, 79, 149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel file with the pest list of Acer pseudoplatanus


