Abstract
In folk medicine, Inula viscosa (Asteraceae) has been traditionally utilized for treating various ailments, including diabetes, bronchitis, diarrhea, rheumatism, and injuries. In this study, we aimed to investigate the chemical composition, antioxidant, antiproliferative, and apoptotic properties of I. viscosa leaf extracts. Extraction was performed using solvents of varying polarities. Antioxidant activity was determined using Ferric reducing antioxidant power (FRAP) and 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays. The results revealed that aqueous ethanol (70%) and aqueous ethyl acetate (70%) extracts contained high levels of phenols (645.58 ± 8.77 mg CE/g) and flavonoids (180.69 ± 1.54 mg QE/g), respectively. Aqueous ethanol (70%) extract exhibited the highest antioxidant activity with IC50 of 572.74 μmol TE/g DW (μmol Trolox equivalent in 1g of dry extract) in the ABTS assay and 76862.06 μM TE/g DW in the FRAP test. All extracts showed a considerable dose-dependent cytotoxic effect on cancerous HepG2 cells (P < 0.05). The aqueous ethanol extract demonstrated the highest inhibitory effect (IC50 = 1.67 mg/ml). Treatment with aqueous ethanol (70%) and pure ethyl acetate extracts significantly increased the number of apoptotic cells to 8 and 6%, respectively, in HepG2 cells (P < 0.05). Additionally, the aqueous ethanol extract significantly elevatedreactive oxygen species (ROS) levels (53%) in HepG2 cells. The molecular docking study identified paxanthone and banaxanthone E as the compounds that exhibited the highest binding affinities with BCL-2. This study demonstrated the potent antioxidant, antiproliferation, and intracellular ROS production of I. viscosa leaf extracts. Further studies should be conducted to identify the active compounds involved.
Keywords: Inula viscosaleaf extracts, antioxidant, cytotoxic effect, HepG2 cells, ROS, molecular docking
Introduction
The prevalence of hepatocellular cancer (HCC) is significant, as it is the fifth most prevalent and widespread cancer in the world (Chidambaranathan-Reghupaty et al., 2021). According to the International Agency for Research on Cancer report in 2020, liver cancer affected 905 700 people worldwide, and 830 200 people died from this disease. Hepatic cell damage caused by oxidative stress and inflammation is associated with hepatocarcinogenesis (Manosroi et al., 2015). Given the high worldwide prevalence of HCC, there is an urgent need to find new treatments to combat this multifactorial syndrome.
Surgical interventions (tumor resection and liver transplantation), percutaneous interventions (ethanol injection and radiofrequency thermal ablation), radiation therapy, drugs,and gene and immune therapies have significantly reduced liver diseases in HCC therapy. However, the lack of efficient in vitro models remains a significant challenge in discovering new therapies for hepatic carcinomas (Kumariet al., 2018). To reduce the morbidity and mortality of HCC, early diagnosis and the development of novel systemic therapies for advanced disease, including drugs, gene, and immune therapies, as well as primary HCC prevention, are crucial (Gosalia et al., 2017). Recent studies have focused on finding safe and effective natural anticancer medicines (Khan et al., 2020). Medicinal plants are the most promising source of novel chemical compounds with anticancer effects due to their successful clinical application.
Polyphenols, which comprise phenolic acids, flavonoids, and tannins, are a fascinating class of plant chemical compounds with anticancer characteristics (Cory et al., 2018; Lee and Lee, 2021). Antioxidants of plant origin have been suggested to possess favorable pharmacological effects such as antitumor, anticarcinogenic, and hepatoprotective effects due to their free radical scavenging properties, as suggested by several studies (Bahmani et al., 2016; Remila et al., 2015; Rozenblat et al., 2008).
Inula viscosa (Dittrichia viscosa) is a plant that belongs to the Asteraceae family and is native to the Mediterranean Sea. This plant has numerous beneficial effects on the body, including antimicrobial, antioxidant, antiulcerogenic, antihelmintic, and antidiabetic effects, as reported in several studies (Alkofahi et al., 1999; Chahmi et al., 2015; Hernández et al., 2007; Kheyar-Kraouche., et al., 2018; Oka et al., 2001; Talib et al., 2012). The leaves of I. viscosa have been used as a decoction in Algeria to treat various ailments, such as bronchitis and diabetes, as well as a cataplasm for injuries and rheumatic pain, according to studies (Baba Aissa, 2000; Haoui et al., 2015).
Phytochemicals were extracted from I. viscosa using solvents with varying polarities. In a previous study, Kheyar-Kraouche et al. (2018) identified 51 compounds in the ethanolic extract of I. viscosa leaves, including 11 phenolic acids, 23 flavonoids, one lignin, and 12 terpenoids. Of these compounds, 26 were described for the first time in I. viscosa. Additionally, several studies have reported on the anticancer effect of different extracts from various parts of this plant. Previous studies have indicated that extracts of I. viscosa and phytochemicals derived from this plant exert cytotoxicity against colon cancer (Bar-Shalom et al., 2019), cervical cancer (Virdi et al., 2020), breast cancer (Messaoudi et al., 2016), and melanoma cancer (Kamer Colak et al., 2021).
Building ontheprevious study, we aimed to investigate the impact of solvent polarity on the extraction efficiency of phenolic contents of I. viscosa leaves, as well as to evaluate the antioxidant activity, antiproliferative, and apoptotic effects on HepG2 liver cancer cells. Additionally, we assessed the affinity of the main phenolic compounds for binding to BCL-2, the antiapoptotic protein, using a molecular docking study.
Materials and methods
Plant material
The leaves of I. viscosa were collected from the Kharrata forest, which is situated at a distance of 58 km from Bejaia city in Algeria (coordinates: N 36°45′00″, E 5°04′00″″). The plant was identified botanically at the Laboratory of Plants Biotechnology and Ethnobotany, University Abderahmane Mira, Bejaia (Algeria). A voucher specimen was deposited there with the reference number IV017. The dried plant material was ground into a fine powder (with a diameter of 63 m) using an electric mill (Kika Labortechnik, Staufen, Germany), and then stored in a tightly closed container at 4°C for later use.
Extraction of phenolic compounds
To extract active compounds from the plant material, several solvents were utilized, including distilled water, ethanol (100%), ethanol (70%), ethyl acetate (100%), ethyl acetate (70%), chloroform (100%), and chloroform (70%). Each solvent was used to macerate 1 g of plant powder in 4 ml of the respective solvent at room temperature for 24 h. After centrifugation at 1500 g/min, the supernatant was retrieved, and the crude extract was obtained by drying. The extraction yield was calculated using the formula: yield% = weight of dried extract/ weight of the plant sample. Seven different extracts were collected and tested for various activities.
Total phenolic compounds, flavonoids, and condensed tannins determination
The total phenolic content was determined using the Folin-Ciocalteu technique (Kähkönen et al., 1999). Total flavonoid content was measured by the aluminum chloride colorimetric assay, as described by Maksimović et al. (2005). To determine the content of tannins, the vanillin technique adapted from Sun et al. (1998) was used.
Antioxidant activity
ABTS assay
The scavenging activity of the different extracts against the 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS)) free radical was evaluated according to the method described by Re et al. (1999). ABTS+ was generated by combining 7 mM ABTS at pH 7.4 with 2.5 mM potassium persulfate (final concentration) and then storing it at room temperature for 16 h before use. The liquid was diluted with ethanol to obtain a solution with an absorbance of 0.70 ± 0.02 at 734 nm. For each sample, a diluted ethanol solution (100 μl) was allowed to react with a new ABTS solution (900 μl) for 6 min before measuring the absorbance. The results were reported as Trolox equivalent.
Ferric-reducing antioxidant power (FRAP)
To determine the antioxidant activity of the samples, the FRAP test was used (Benzie et al., 1996). The FRAP reagent was prepared freshly by mixing 25 ml of acetate buffer (300 mM, pH 3.6), 2.5 ml of tripyridyl-s-triazine solution (10 mM), and 2.5 ml of FeCl3 solution (20 mM). The reagent was warmed to 37°C for 30 min, and then 50 μl of different concentrations of extracts (1000, 500, 250, 125, 62.5, and 31.25 μg/ml) were added to 950 μl of fresh FRAP reagent, and the absorbance was measured after an incubation time of 30 min at 37°C.
Antiproliferative activity
Cell culture
The adherent HepG2 human hepatoma cells (ATCC: HB-8065) were obtained from the American Type Culture Collection (ATCC, https://www.atcc.org/). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal calf serum and 0.1% (v/v) streptomycin. The cell culture was maintained at 37°C in a humidified incubator with 5% CO2 saturation.
Cell viability assay
To determine cell growth, the MTT test was used, which is based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) by intact cells' mitochondrial dehydrogenase into an insoluble purple formazan product (Stockert et al., 2012). HepG2 cells were incubated in 96-well plates at a concentration of 5×103 cells per well. After a 24-h incubation period at 37°C, cells were treated for 24, 48, or 72 h with plant extracts at different concentrations (0.05–2.5 mg/ml). Control cells were incubated with serum-free medium (DMEM) or with DMSO (0.5 v/v%) at 37°C. Treated cells were incubated with 30 μl of MTT reagent for 2 h. A scanning multiwell spectrophotometer (Thermo Scientific Multiskan EX, Monza, Italy) set to 550 nm was used to measure the amount of formazan dye produced. The results were expressed as a percentage of cell viability relative to control (%). Experiments were performed in triplicate using at least two independent assays. The half-maximal inhibitory concentration (IC50) was also calculated from dose–response curves using Graph Pad Prism software (Graph Pad Software, Inc., La Jolla, CA, USA).
Tali TMapoptosis kit-annexin V Alexa Fluor 488 and propidium iodide
An early stage of apoptosis was determined using annexin V, as reported by Ota et al. (2006). An Alexa Fluor 488 annexin V/dead cell apoptosis kit (Molecular Probes, CA, USA) was used to evaluate apoptosis, which was defined as the translocation of phosphatidylserine to the cell surface. HepG2 cells (5×105 cells/well) were seeded in 12-well plates and treated with the MTT IC50 value of each extract for 24 h. The cells were then washed twice with PBS buffer, and stained with annexin V-FITC and propidium iodide (PI), and the results were assessed using flow cytometry (FACS Calibur, Becton Dickinson, USA).
Measurement of ROS production
The generation of intracellular reactive oxygen species (ROS) was evaluated using 2,7′-dichlorofluorescein diacetate (DCFH-DA) (Sigma Chemical Company, St. Louis, MO, USA) in both treated and control cells, as described by Chang et al. (2001). Cells were seeded at a density of 2×105 cells per well and treated with I. viscosa extracts at their respective IC50 values for 24 h. After incubation, cells were detached with trypsin-EDTA and washed with PBS. Control and treated cells were resuspended in 0.5 ml of PBS containing 10 M DCFH-DA and incubated with 4 mM H2O2 (as an inducer of ROS generation) for 30 min at 37°C. ROS production was measured using a luminescence spectrophotometer (Perkin-Elmer, MA).
Statistical analysis
The results were expressed as the mean ± standard deviation of three independent measurements. One-way analysis of variance, followed by Tukey's test, was performed using Graph Pad Prism 5.0 software (Graph Pad, San Diego, CA, USA). The data were considered statistically significant at P < 0.05.
In silico molecular docking study
The phenolic compounds of I. viscosa leaves identified by Kheyar-Kraouche et al. (2018) using liquid chromatography linked to photodiode array detection and electrospray ionization mass spectrometry (LC-DAD-ESIMS/MS) were utilized to determine the most active compounds that inhibit BCL-2 antiapoptotic protein. A total of 26 compounds, including 4-caffeoylquinic acid, chlorogenic acid, caffeic acid, caffeoylquinic acid, catechin, coumaroyl, delphinidin, dicaffeoylquinic acid, galloylquinic acid, (Epi), gallocatechin-gallate, genkwanin, naringenin, proanthocyanidin dimer, protocatechuic acid, quercetin rhamnoside, quercetin, banaxanthone e, rosmarinic acid, padmatin, cirsiliol, mangostin, spinacetin, rosmanol, paxanthone, caffeic acid phenethyl ester, medioresinol, hispidulin, and rhamnetin, were selected as ligands for the docking analysis. Venetoclax and Obatoclax, the first selective BCL-2 inhibitors for routine clinical use, were used as control ligands.
The 3D molecular structures of the ligands were obtained from PubChem and created using AutoDock Tools (Morris et al., 2009) by minimizing energy and adding hydrogen atoms and charges, as well as adjusting the number of active torsions. The results were saved in pdbqt format.
The crystal structure of human BCL-2 (PDB ID: 6QGK) was downloaded in pdb format from the RCSB Protein Data Bank (https://www.rcsb.org/). Using BIOVIA Discovery Studio v. 2021, water molecules, heteroatoms, and nonstandard ligands were removed from the macromolecules and saved in pdbqt format. AutoDock Vina software (Morris et al., 2009) was used to perform docking calculations. The target structures were docked with ligands, and the values of the grid boxes were determined through a series of trials and errors. For this investigation, the chosen grid box was (x 40% –0.698, y 40% –4.034, z 40% 20.268 at 0.375 Å spacing centered).
Multiple conformations for the ligand were generated in the docking procedure, and the final energy refinement of the ligand posture was performed. The docking score of the optimum pose into the target proteins was computed for all of the examined bioactive compounds.
Results
Extraction yield and phenolic compound contents
Table 1 shows the extraction yield, total phenols, flavonoids, and condensed tannins of I. viscosa leaves using different extraction solvents. The yields of bioactive compounds varied significantly depending on the solvent used. The highest extraction yields were obtained from the aqueous ethanol (70%) extract (33.98%), followed by water and aqueous ethyl acetate (70%) extracts (18.54 and 15.84%, respectively).
Table 1.
Determination of extraction yield and phenolic compound contents in the I. viscosa plant extracts
| Extracts | Extraction of yield [%] | Total phenols [mg Eq catechin/g extract] | Flavonoids [mg Eq quercetin/g extract] | Tannins [mg Eq tannic acid/g extract] |
|---|---|---|---|---|
| Water | 18.54 | 308.41 ± 11.93 c | 115.60 ± 2.25 c | 21.14 ± 5.09 a |
| Ethanol (100%) | 9.92 | 403.28 ± 6.78 b | 72.46 ± 4.61 d | 24.69 ± 13.02 a |
| Aqueous ethanol (70%) | 33.98 | 645.58 ± 8.77 a | 150.50 ± 2.22 b | 7.13 ± 9.14 b |
| Ethyl acetate (100%) | 5.95 | 170.46 ± 9.82 e | 76.05 ± 2.33 d | 37.84 ± 0.43 a |
| Aqueous ethyl acetate (70%) | 15.84 | 410.71 ± 4.56 b | 180.69 ± 5.64 a | 1.60 ± 1.76 b |
| Chloroform (100%) | 5.14 | 122.02 ± 12.02 f | 18.34 ± 5.45 e | 25.03 ± 4.23 a |
| Aqueous chloroform (70%) | 10.07 | 242.76 ± 9.01 d | 23.50 ± 1.23 e | 4.61 ± 3.42 b |
Each value in the table is the mean ± standard deviation (n = 3); data were analyzed using a one-way ANOVA (analysis of variance); different letter(s) indicate that the values are significantly different (P < 0.05)
The use of pure solvents resulted in the lowest levels of extraction yield (<10%). Moreover, the concentration of phenolic compounds was strongly affected by the extraction solvents used. Aqueous ethanol (70%) extract showed the highest concentration of total phenols (645.58 ± 8.77 mg CE/g). Similarly, this extract also exhibited a high level of flavonoids (150.50 ± 2.22 mg QE/g), although the highest level of flavonoids was obtained from the ethyl acetate (70%) extract (180.69 ± 1.54mg QE/g). Notably, pure chloroform extract contained a considerable level of condensed tannins (37.48 ± 0.67 mg TAE/g).
Antioxidant capacities of I. viscosa extracts
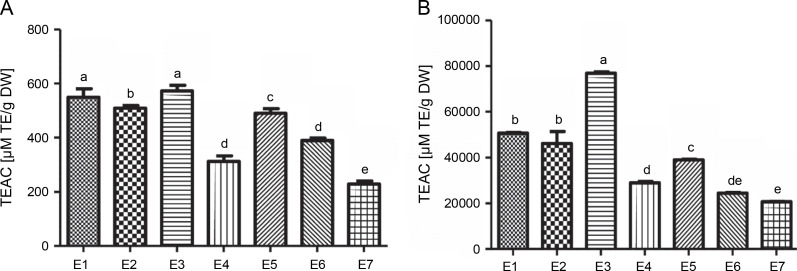
The antioxidant activity of I.viscosa leaf extracts was evaluated using ABTS radical scavenging activity and ferric reducing antioxidant power (FRAP) (Fig.1). The results showed that the antioxidant activity varied significantly depending on the extraction solvent used. Aqueous ethanol (70%)and water extracts had the best potential to scavenge ABTS, with values of 572.74 and 549.53 μmol TE/g extract, respectively. Notably, there was no significant difference (P < 0.05) between these two extracts. The lowest ABTS scavenging capacity was observed in the aqueous chloroform (70%) extract, with a value of 229.22 μmol TE/g extract.
Fig. 1.
The trolox equivalent antioxidant capacities of various extractsof Inula viscosa using ABTS(A) and FRAP (B) methods; E1 – water, E2 – ethanol (100%), E3 – aqueous ethanol (70%), E4 – ethyl acetate (100%), E5 – aqueous ethyl acetate (70%), E6 – chlorofom (100%) and E7 – aqueous chloroform (70%)
In contrast, the aqueous ethanol (70%) extract exhibited the highest reducing capacity with a value of 76 862.06 μM TE/g DW for the FRAP test, followed by water, ethanol (100%), aqueous ethyl acetate (70%), ethyl acetate (100%), chloroform (100%), and aqueous chloroform (70%)extracts, with values ranging between 20 729.88 and 50 660.92 μM TE/g DW.
Cell viability
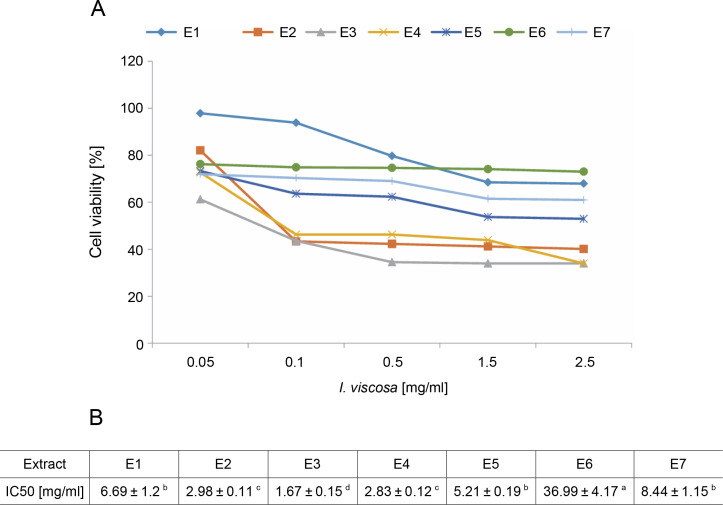
To evaluate the cytotoxicity of I.viscosa leaf extracts on the proliferation of human liver cancer cell lines, HepG2 cells were treated with various concentrations of different extracts for 24, 48, or 72 h, and cellular viability was measured using the MTT assay to determine the IC50 values of I.viscosa extracts (Fig. 2). After 24 h of incubation, all extracts showed a significant (P < 0.05) cytotoxic effect against HepG2 cancer cells in a time-dependent and dose-dependent manner (Fig. 2A). As shown in Figure 2B, the aqueous ethanol (70%) extract exhibited the highest inhibitory effects, with an IC50 value of 1.67 mg/ml, followed by pure ethyl acetate and ethanol extracts. Pure chloroform (IC50 = 36.99 mg/ml) showed the lowest reduction in cell viability.
Fig. 2.
Dose-dependent inhibition of human liver cancer cell (Hep G2) by I. viscosa extracts: (A) – cells were treated with DMSO vehicle or the indicated concentrations of I. viscosa extracts for 24 h; cell viability was determined using MTT assayand expressed as means ± SD of three independent experiments (n = 3), columns with different superscript letters are significantly different (P < 0.05); (B) – IC50 values of I.viscosa extracts were determined based on the dose–response curves; E1 – water, E2 – ethanol (100%), E3 – aqueous ethanol (70%), E4 – ethyl acetate (100%), E5 – aqueous ethyl acetate (70%), E6 – chloroform (100%) and E7 – aqueous chloroform (70%)
I. viscosa extracts induced apoptosis in HepG2 cells
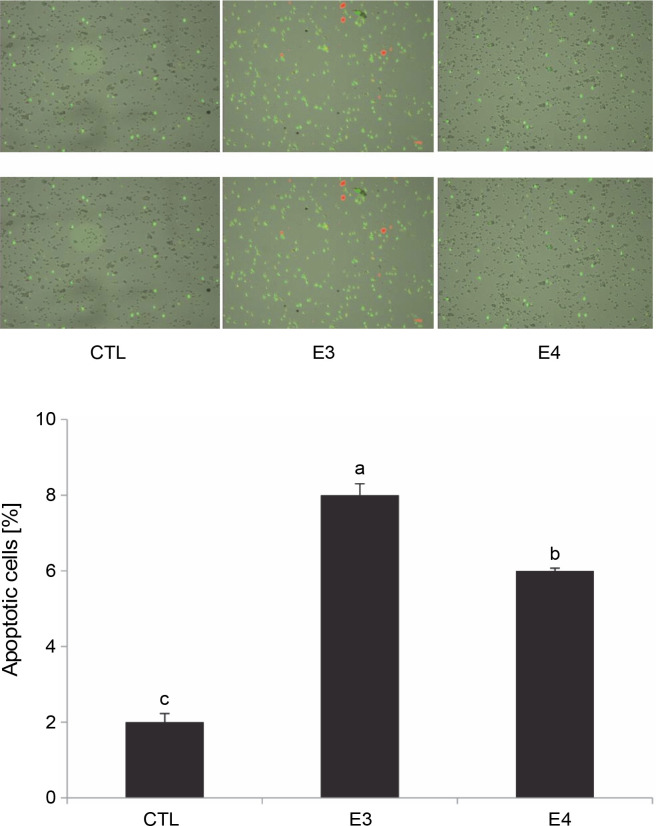
Based on the MTT results, the aqueous ethanol (70%) and pure ethyl acetate extracts were chosen for further experiments to understand the mechanism of I. viscosa cytotoxicity. After 24 h of treatment, HepG2 cells were collected, and flow cytometry was utilized to quantify apoptosis using annexin-V Alexa fluor 488/PI staining (Fig. 3). The number of apoptotic cells significantly increased (P < 0.05) after extract treatment. The percentage of apoptotic cells in HepG2 cells treated with the aqueous ethanol and pure ethyl acetate extracts increased to 8 and 6%, respectively, in comparison to the control group (2% cells) without the extract (Fig. 3).
Fig. 3.
I. viscosa extracts induced apoptotic cell death in human liver cancer cells (Hep G2) after treatment with IC50 for 24 h; values are expressed as mean ± SD of three independent experiments (n = 3); columns with different superscript letters are significantlydifferent (P < 0.05); CTL – untreated cells, E3 – aqueous ethanol, E4 – pure ethyl acetate
Effects of I. viscosa on ROS production in HepG2 cells
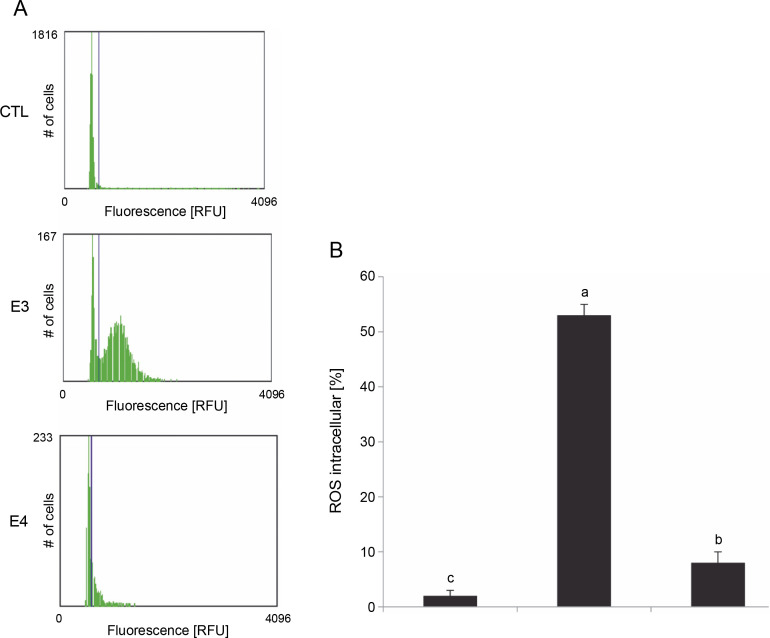
The effect of I. viscosa extract on intracellular ROS generation was studied by measuring changes in the fluorescence intensity of Cell-ROX Orange (Fig. 4).
Fig. 4.
Effect of I.viscosa extracts on ROS intracellular production of Hep G2 after treatment with IC50 for 24 h; values are expressed as mean ± SD of three independent experiments (n =3); columns with different superscript letters are significantly different (P < 0.05); CTL – untreated cells, E3 – aqueous ethanol and E4 – pure ethyl acetate
Based on the results presented in Figure 4, it can be observed that I. viscosa extracts induced a significant increase in intracellular ROS production in HepG2 cells in a solvent-dependent manner (P < 0.05). Notably, cells treated with the aqueous ethanol extract (IC50, 24h) exhibited the highest level of ROS production (53%) when compared to the control cells (2%) without extract (Fig. 4). Additionally, the level of ROS production was higher in cells treated with aqueous ethanol extract than in cells pretreated with pure ethyl acetate (8%).
Molecular docking studies
Overall, 26 phenolic compounds from I. viscosa and the standards (venetoclax and obatoclax) were dockedto the active site of BCL-2 (PDB ID: 6QGK). The ligands were classified according to their binding energy (kcal/mol) with the protein (Table 2). The interactions between antiapoptotic protein BCL-2 (PDB ID: 6QGK) and chemicals as well as the binding energies of the complexes were used to analyze the data.
Table 2.
Phenolic compound docking results
| Compounds name | Binding energy [Kcal/mol] |
|---|---|
| 4Caffeoylquinic acid | –5.9 |
| Chlorogenic acid | –6.5 |
| Cafeic acid | –5.7 |
| Caffeoylquinic acid | –5.8 |
| Catechin | –6.5 |
| Coumaroyl | –6.2 |
| Delphinidin | –6.9 |
| DiCaffeoylquinic acid | –6.9 |
| Galloylquinic acid | –5.3 |
| (Epi)-gallocatechin-gallate | –7.1 |
| Genkwanin | –6.9 |
| Naringenin | –7.3 |
| Proanthocyanidin dimer | –7.1 |
| Protocatechuic acid | –5.3 |
| Quercetin rhamnoside | –7.2 |
| Quercetin | –7.0 |
| Banaxanthone E | –7.6 |
| Rosmarinic acid | –6.2 |
| Padmatin | –6.4 |
| Cirsiliol | –5.9 |
| Mangostin | –6.6 |
| Spinacetin | –5.8 |
| Rosmanol | –7.0 |
| Paxanthone | –7.6 |
| Caffeic acid phenethyl ester | –6.4 |
| Medioresinol | –6.9 |
| Hispidulin | –7.1 |
| Rhamnetin | –6.9 |
| Venetoclax | –7.8 |
| Obatoclax | –7.7 |
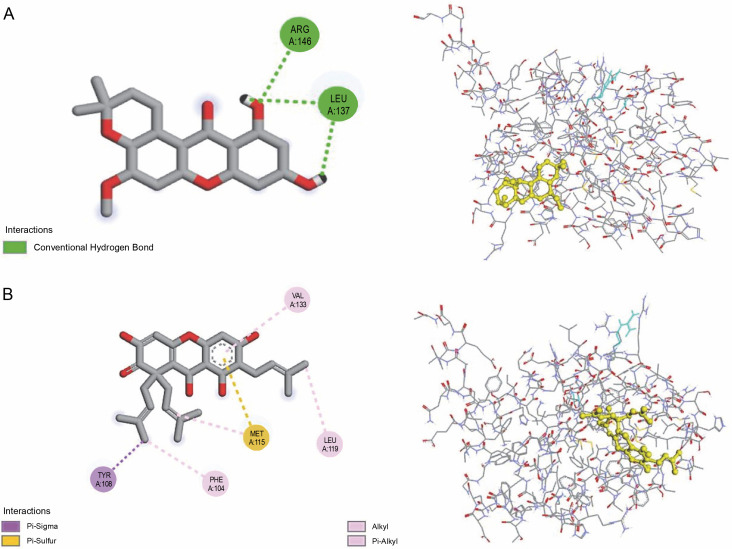
All investigated ligands were compared with the standard molecules, obatoclax, and venetoclax, based on the interaction energy criteria (interaction energy: –7.7 and –7.8 kcal/mol, respectively). Among the 26 docked compounds (Table 2), Banaxanthone E (7.6 kcal/mol) and paxanthone (7.6 kcal/mol) exhibited good binding interaction with the antiapoptotic protein BCL-2. Therefore, these compounds were predicted to have the most stable molecular binding with BCL-2. Figure 5 illustrates the molecular interaction of Banaxanthone E and paxanthone in the active site of BCL-2. These compounds interact with the antiapoptotic protein BCL-2 through hydrogen and hydrophobic interactions. Paxanthone binds to the active site of BCL-2 through three hydrogen bonding interactions of free hydroxyl groups with Arg146 and Leu137 residues. Banaxanthone E, on the other hand, interacts with Val133, Leu119, Met115, Phe104, and Tyr108 residues through pi sigma, pi-alkyl, alkyl, and pi-sulfur interactions (Table 3).
Fig. 5.
Binding interactions of paxanthone (A) and banaxanthone E (B) in BCL-2
Table 3.
Interacting residues of BCL-2 with phenolic compounds from I. viscosa and the number of hydrophobic and hydrogen bonds
| Compounds name | Interacting residues | Number of hydrophobic interaction | Number of hydrogen bond |
|---|---|---|---|
| 4Caffeoylquinic acid | PHE112 TYR108 PHE104 MET115 LEU137 GLU136 |
1 1 1 – – – |
– – – 1 1 1 |
| Chlorogenic acid | PHE104 | 1 | – |
| Caffeoylquinic acid | TYR108 | 1 | – |
| Catechin | LEU137 PHE104 TYR108 PHE112 |
1 1 1 1 |
– – – – |
| Coumaroyl | MET115 PHE112 TYR108 PHE104 ARG146 |
2 1 1 2 1 |
– – – – – |
| Delphinidin | GLU136 | – | 1 |
| DiCaffeoylquinic acid | PHE112 PHE104 TYR108 MET115 LEU137 GLU136 |
1 1 1 – 1 |
– – – 1 – 1 |
| (Epi)-gallocatechin-gallate | MET115 PHE112 PHE104 ARG146 LEU137 |
1 – 1 – – |
– 1 – 1 1 |
| Genkwanin | PHE104 PHE112 LEU137 ARG146 |
1 1 2 2 |
– – – 1 |
| Naringenin | MET115 PHE104 TYR108 |
– 2 1 |
1 – – |
| Proanthocyanidin dimer | MET115 VAL133 LEU137 |
1 – 1 |
– 1 – |
| Protocatechuic acid | ARG109 ALA113 PHE112 VAL156 SER105 |
1 – 1 1 – |
– 1 – – 1 |
| Quercetin rhamnoside | LEU137 | 1 | – |
| Quercetin | ARG146 PHE104 |
1 1 |
– – |
| Banaxanthone E | PHE104 TYR108 MET115 LEU119 VAL133 MET115 |
1 1 1 1 1 Pi-Sulfur |
– – – – – – |
| Rosmarinic acid | MET115 LEU137 PHE112 GLU136 |
1 – 1 1 |
1 1 – 1 |
| Padmatin | PHE112 | – | 1 |
| Cirsiliol | PHE104 PHE112 |
1 1 |
– – |
| Mangostin | MET115 PHE112 |
1 1 |
– – |
| Spinacetin | MET115 | – | 1 |
| Caffeic acid phenethyl ester | ARG146 LEU137 PHE112 MET115 |
– – 1 1 |
1 1 – – |
| Medioresinol | MET115 VAL133 ARG146 |
1 – – |
– 1 2 |
| Nepetin | PHE104 TYR108 PHE112 ASP111 |
1 1 1 – |
– – – 1 |
| Vénétoclax | PHE112 MET115 VAL133 ARG139 |
1 1 – – |
– – 1 1 |
| Obatoclax | PHE104 PHE112 MET115 VAL133 LEU137 |
1 1 1 1 – |
– – – 1 1 |
Discussion
In the last few decades, cancer treatment has primarily relied on cytotoxic chemotherapy and radiation therapy. However, the efficacy of these treatments has been hindered by their negative side effects and the development of resistance (Schirrmacher, 2019). To overcome these challenges, there is a need for new effective cancer therapies that are selective and have fewer side effects. Natural products have emerged as a promising source of anticancer medications, with many being more effective and less hazardous than traditional therapies (Rayan et al., 2017). Approximately 60% of cancer medications in use today are derived from natural compounds, many of which originate from plants (Newman and Cragg, 2020). Phenolic compounds, in particular, have demonstrated remarkable therapeutic and preventive properties against various forms of human cancer. They contain functional groups that enable them to exert multiple anticancer mechanisms, such as inducing apoptosis, autophagy, cell cycle arrest at different stages, and inhibiting telomerase (Islam et al., 2021; Rauf et al., 2022).
The leaves of I. viscosa are commonly used in traditional Algerian medicine to treat various ailments, including anti-helminthic, anti-inflammatory, antiseptic, antipyretic, anticancer, and diabetic therapy (Baba Aissa, 2000; Haoui et al., 2015; Ouahchia et al., 2020; Taïbi et al., 2020).
The extraction of active components is a crucial step in the production of herbal products, as it influences both the quality and quantity of the extracted components. Various factors can affect the extraction yield, such as the extraction method, solvent polarity, temperature, extraction time, sample particle size, pH, chemical nature of phytochemicals, and possible interferences from nonantioxidant compounds (Do et al., 2014; Pérez-Jiménez, et al., 2008). The extraction solvent, in particular, has a significant effect on the yield of extraction and antioxidant activity due to the existence of numerous antioxidant molecules with different chemical properties and polarity (Do et al., 2014; Metrouh-Amir et al., 2015; Sultana et al., 2009). It has been observed that the addition of water to organic solvents can increase the solubility of phenolic compounds by modulating the polarity of the organic solvent (Mohammedi et al., 2011).
In this study, the combination of organic solvents with water resulted in the highest extraction rate and phenolic compound content. The aqueous extract contained higher levels of total phenols and flavonoids, while the extracts obtained with pure solvents showed the highest amount of tannins. These findings are consistent with previous studies that have investigated the effect of solvents on extraction (Koofi et al., 2010; Metrouh-Amir et al., 2015). Metrouh-Amir et al. (2015) reported lower contents of total phenols, flavonoids, and condensed tannins using seven different solvents for the extraction of Matricaria pubescens compared to the results of the present study.
I. viscosa is a known source of various bioactive compounds, including phenolic acids, flavonoids, tannins, guaianolides, sesquiterpenes, sesquiterpene acids, triterpenoids, lactones, costic acid, and essential oils (Brahmi-Chendouh et al., 2019; Kheyar-Kraouche et al., 2018; Ozkan et al., 2019). Due to its high phenolic content, we investigated the antioxidant potential of I. viscosa extracts using two complementary assays (ABTS and FRAP). Our results showed that the aqueous extracts had significantly higher free radical scavenging activity than the pure solvent extracts. According to Babbar et al. (2014) and Temesgen et al. (2022), the differences in antioxidant capacity may be due to the quantity and quality of phenolic compounds present in each solvent.
Phenolic compounds exhibit antioxidant activity by donating hydrogen atoms to free radicals and scavenging other reactive species, such as OH•, NO2 •, N2O3, ONOOH, and HOCl. Additionally, some phenolics, especially diphenolsand polyphenols, can bind to transition metal ions, such as iron and copper, and react with oxygen, leading to forms that are ineffective at generating free radical reactions (Zin et al., 2004). The polarity of the solvent can also affect the antioxidant activity of the extract. Wakeel et al. (2019) and Abarca-Vargas et al. (2016) reported that solvent polarity can influence the extraction of a specific group of antioxidants, thereby affecting the antioxidant activity. Zhang et al. (2018) and Autor et al. (2022) demonstrated that highly polar organic solvents, such as water and alcohols (e.g., ethanol and methanol), are essential in improving phenolic compound extraction yield. Highly hydroxylated aglycone forms of phenolic compounds are soluble in high polar solvents, and the hydroxy groups in phenolic compounds contribute to their antioxidant activity (Kaczorová et al., 2021).
The second objective of this study was to assess the cytotoxicity of I. viscosa leaf extracts on the proliferation of human liver cancer cell lines (HepG2) and explore their cytotoxic mechanism. Previous studies have demonstrated the cytotoxic and anticancer activities of I. viscosa in various cancer cell lines. For example, Kheyar et al. (2022) found that the ethanolic extract of I. viscosa did not exhibit cytotoxicity on nondifferentiated Caco-2 cell lines, but showed growth inhibitory activity against human colon carcinoma HT29 cells. They concluded that I. viscosa extracts caused a significant decrease in cell viability in a dose-dependent manner.
In this study, we found that the aqueous ethanol (70%) and pure ethyl acetate extracts caused a significant reduction in cancer cell viability, which may be due to the presence of compounds with cytotoxic properties. Our extract’s phytochemical analysis (Kheyar-Kraouche et al., 2018) revealed the presence of various phenolic compounds, including chlorogenic acid derivatives, flavonoids, and sesquiterpenes. These compounds have been the focus of many studies, all of which have produced promising results and demonstrated significant inhibition of the proliferation of human cells from various types of cancer (Ayouaz et al., 2021; Huang et al., 2020; Macrì et al., 2020).
Previous studies have established the cytotoxicity potential of I. viscosa extracts on various cancer cell lines, with varying degrees of growth inhibition (Bar-Shalom et al., 2019; Belayachi et al., 2013; Merghoub et al., 2016; Sevgi et al., 2021; Talib et al., 2010; Virdis et al., 2020). For instance, Belayachi et al. (2013) observed a decrease in the proliferation of PC-3 prostate cancer cells in vitro after exposure to I. viscosa's dichloromethane extract. Additionally, Bar-Shalom et al. (2019) demonstrated the antiproliferative effect of I. viscosa’s hexanic extract on colorectal carcinoma cell lines in vitro. Recent study showed the effectiveness of I. viscosa ethanolic extract in reducing the proliferation of a human colorectal adenocarcinoma cell line (HT29) with an IC50 value of 62.39 ± 0.34 μg/ml (Kheyar et al., 2022).
Apoptosis is a crucial physiological mechanism that eliminates damaged or unwanted cells and is considered the preferred way to eliminate cancer cells (Kim et al., 2013; Xie et al., 2014). Defects in apoptosis enable cancer cells to become tumors and make them resistant to therapy (Hassan et al., 2014; Yu and Zhang, 2004). In this study, we examined the effects of I. viscosa extracts on apoptosis in the HepG2 cell line in vitro and intracellular ROS production to explore the mechanisms underlying I. viscosa's anticancer activity. Our Annexin V/PI flow cytometric assay showed that after 24 h of exposure to aqueous ethanol (70%) and pure ethyl acetate extracts, there was a significant increase in apoptosis to 8 and 6%, respectively. These results are consistent with previous studies that have demonstrated the anticancer properties of I. viscosa extracts. For example, Benbacer et al. (2012) reported that I. viscosa extracts had cytotoxic effects on cervical cancer cell lines by reducing proliferation and inducing apoptosis. Merghoub et al. (2016) showed that I. viscosa extracts promoted apoptosis in cervical cancer cells, and the active compounds overcame treatment resistance in neoplastic cells. Additionally, Bar-Shalom et al. (2019) found a proapoptotic effect of I. viscosa extracts on colorectal cancer in vitro and in vivo, with no adverse effects in animal models.
Several studies have shown that inducing intracellular ROS in cancer cells through bioactive compound therapy is a promising alternative in developing anticancer drugs. Increasing ROS levels through natural product therapy not only regulates the cell cycle butalso triggers apoptotic cell death by altering intracellular signaling molecules such as the Bcl-2 family and caspases (Kim et al., 2016). Our results demonstrate that I. viscosa extracts stimulate intracellular ROS generation and influence apoptosis in cancer cells. Talib et al. (2012) reported the ability of I. viscosa's phenolic compounds to induce apoptosis in MCF-7 cells, as evidenced by the presence of DNA fragmentation, nuclear condensation, and formation of apoptotic bodies in treated cancer cells. Indeed, the main phenolic components found in I. viscosa extracts were flavonoids, sesquiterpenes, chlorogenic acid, and its derivatives (Brahmi-Chendouh et al., 2019; Danino et al., 2009; Gökbulut, 2016; Kheyar-Kraouche et al., 2018; Sevgi et al., 2021). These molecules were found to have cytotoxicity and induce apoptosis or necrosis in various cancer cell lines, including human melanoma cell lines (Rozenblat et al., 2008), cultured oral KB epithelial cells (Chang et al., 2002), and osteosarcoma cells (Salzillo et al., 2021).
BCL-2 is a target for cancer therapy as it prevents programmed cell death induced by various stimuli, including hypoxia and oxidative stress (Frenzel et al., 2009). Therefore, developing novel Bcl-2 inhibitors from phytochemicals is of high interest. In this study, molecular docking was performed using AutoDock Vina software to examine the interaction between 26 phenolic compounds identified in I. viscosa’s ethanolic extract, as described by Kheyar-Kraouche et al. (2018), and BCL-2 (PDB ID: 6QGK). Through this study, we were able to define the amino acid residues involved in the binding interactions as well as the protein–ligand binding ener-
gies. BCL-2 is a well-known target in cancer research as it prevents programmed cell death induced by hypoxia and oxidative stress (Frenzel et al., 2009). Therefore, developing BCL-2 inhibitors from phytochemicals is of great interest. In this study, we performed molecular docking of 26 phenolic compounds identified in I. viscosa's ethanolic extract toward BCL-2 (PDB ID: 6QGK) using AutoDock Vina software. We were able to define the amino acid residues involved in the binding interactions and determine the protein–ligand binding energies. The study found that two xanthones, paxanthone and banaxanthone E, previously reported in I. viscosa extracts by Kheyar-Kraouche et al. (2018), hada good affinity for the examined antiapoptotic protein BCL-2, similar to obatoclax and venetoclax. Previous studies have reported that banaxanthone E showed cytotoxicity (Auranwiwat et al., 2016; Thanh et al., 2021) and exhibited antiproliferative activity by suppressing cell-cycle progression at the G2/M phase and activating the caspase pathway in human lung cancer cell lines (Thanh et al., 2021) and breast cancer cells (Nguyen et al., 2021). An interesting feature of this compound is that it binds through hydrophobic interactions with BCL-2's binding pocket. Several studies have shown that binding inhibitors with the hydrophobic groove of BCL-2 activate the apoptotic process (Souers et al., 2013; Verma et al., 2017; Vogler, 2014; Voss et al., 2010). Furthermore, Verma et al. (2017) demonstrated that hydrophobic interactions are critical for stable complex formation. Hydrogen bonds play a significant role in the binding modes and docking mechanism, and the key interactions between BCL-2's groove amino acids and paxanthone are hydrogen bonds. We used BIOVIA Discovery Studio v. 2021 to superimpose the redocked complex on BCL-2 (PDB ID: 6QGK), and our results showed that both this protein and banaxanthone E had a low root mean square deviation (RMSD) of 0.8 Å, comparable to the findings of Ismail et al. (2022a, 2022b), which suggested that docking accuracy increases with decreasing RMSD.
Conclusion
In summary, the results of this study suggest that I. viscosa has promising bioactive compounds with the potential as natural sources of antioxidants and anticancer agents. The choice of extraction solvent significantly influenced the extraction efficiency of phenolic compounds, which contributed to the antioxidant activity of the extracts. The aqueous ethanol (70%) and pure ethyl acetate extracts showed the most significant antiproliferative and apoptotic effects on HepG2 cells. Additionally, the molecular docking results indicated that paxanthone and banaxanthone E havea high affinity towards the antiapoptotic protein BCL-2 mainly through hydrophobic interactions. Further research on I. viscosa's potential anticancer properties should involve more cancer cell lines and animal studies. Furthermore, the isolation and characterization of bioactive compounds from I. viscosa should be the focus of future research to identify potential lead compounds for the development of natural anticancer agents.
Acknowledgments
The authors gratefully acknowledgethe Algerian Minister of Higher Education and Scientific Research for their support. The authors would also like to express their appreciation to Prof. Maurizio Battino for generously providing laboratory facilities at Dipartimento di Scienze Cliniche Specialistiche ed Odontostomatologiche (DISCO)-Sez. Biochimica, Facoltà di Medicina, Università Politecnica delle Marche, Ancona 60131, Italy, and to Dr. Francesca Giampieri and Dr. Tamara Forbes-Hernandez for their invaluable guidance during the experimental work.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- Abarca-Vargas R., Peña Malacara C.F., Petricevich V.L. (2016) Characterization of chemical compounds with antioxidant and cytotoxic activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) extracts. Antioxidants (Basel). 5: 45. 10.3390/antiox5040045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkofahi A., Atta A.H. (1999) Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J. Ethnopharmacol. 67: 341–345. 10.1016/s0378-8741(98)00126-3 [DOI] [PubMed] [Google Scholar]
- Auranwiwat C., Laphookhieo S., Rattanajak R., Kamchonwongpaisan S., Pyne S.G., Ritthiwigrom T. (2016) Antimalarial polyoxygenated and prenylated xanthones from the leaves and branches of Garcinia mckeaniana. Tetrahedron 72(43): 6837–6842. 10.1016/j.tet.2016.09.018 [DOI] [Google Scholar]
- Autor E., Cornejo A., Bimbela F., Maisterra M., Gandía L.M., Martínez-Merino V. (2022) Extraction of phenolic compounds from Populus salicaceae Bark. Biomolecules 12(4): 539. 10.3390/biom12040539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayouaz S., Oliveira-Alves S.C., Serra A.T., Lefsih K., Samah M., Bento da Silva A Madani K., Bronze M.R. (2021) LC-DAD-ESI-MS/MS analysis and cytotoxic and antiproliferative effects of chlorogenic acid derivative rich extract from Nerium oleander L. pink flowers. Food Funct. 12(8): 3624–3634. 10.1039/d0fo02640a [DOI] [PubMed] [Google Scholar]
- Baba Aissa F. (2000) Encyclopédie des plantes utiles : flore d'Algérie et du Maghreb. Substances végétales d'Afrique d'Orient et d'Occident, Édas. Ed. Librairie moderne . Rouiba, Algérie: 252–253. [Google Scholar]
- Babbar N., Oberoi H.S., Sandhu S.K., Bhargav V.K. (2014) Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J. Food Sci. Technol. 51(10): 2568–2575. 10.1007/s13197-012-0754-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani M., Shirzad H., Mirhosseini M., Mesripour A., Rafieian-Kopaei M. (2016) A review on ethnobotanical and therapeutic uses of Fenugreek (Trigonella foenum-graceum L). J. Evid. Based. Complement. Altern. Med. 21: 53–62. 10.1177/2156587215583405 [DOI] [PubMed] [Google Scholar]
- Bar-Shalom R., Bergman M., Grossman S., Azzam N., Sharvit L., Fares F. (2019) Inula viscosa extract inhibits growth of colorectal cancer cells in vitro and in vivo through induction of apoptosis. Front. Oncol. 9: 227. 10.3389/fonc.2019.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayachi L., Aceves-Luquero C., Merghoub N., Bakri Y., Fernández de Mattos S., Amzazi Set al. (2013) Screening of North African medicinal plant extracts for cytotoxic activity against tumor cell lines. Eur. J. Med. Plants 3: 310–332. 10.9734/EJMP/2013/3403 [DOI] [Google Scholar]
- Benbacer L., Merghoub N., El Btaouri H., Gmouh S., Attaleb M., Morjani H., Amzazi S., El Mzibri M. (2012) Antiproliferative effect and induction of apoptosis by Inula viscosa L. and retamamonosperma L. extracts in human cervical cancer cells. Top Cervical Cancer Advoc. Prev. 16: 267–284. 10.5772/30025 [DOI] [Google Scholar]
- Benzie I., Strain J. (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power: the FRAP assay”. Anal. Biochem. 239: 70–76. 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- Brahmi-Chendouh N., Piccolella S., Crescente G., Pacifico F., Boulekbache L., Hamri-Zeghichi S. et al. (2019) A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J. Food Drug. Anal. 27: 692–702. 10.1016/j.jfda.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. (2018) The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 5: 87. 10.3389/fnut.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahmi N., Anissi J., Jennan S., Farah A., Sendide K., El Hassoun M. (2015) Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pac. J. Trop. Biomed. 5: 228–233. 10.1016/S2221-1691(15)30010-1 [DOI] [Google Scholar]
- Chang M.C., Ho Y.S., Lee P.H., Chan C.P., Lee J.J., Hahn L.J., Wang Y.J., Jeng J.H. (2001) Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis 22: 1527–1535. 10.1093/carcin/22.9.1527 [DOI] [PubMed] [Google Scholar]
- Chidambaranathan-Reghupaty S., Fisher P.B., Sarkar D. (2021) Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 149: 1–61. 10.1016/bs.acr.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danino O., Hugo E., Gottlieb S.G., Bergman M. (2009) Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 42: 1273–1280. 10.1016/j.foodres.2009.03.023 [DOI] [Google Scholar]
- Do Q.D., Angkawijaya E.A., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y-H. (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic. J. Food Drug. Anal. 22: 296–302. 10.1016/j.jfda.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel A., Grespi F., Chmelewskij W., Villunger A. (2009) Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis 14(4): 584–596. 10.1007/s10495-008-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosalia A.J., Martin P., Jones P.D. (2017) Advances and future directions in the treatment of hepatocellular carcinoma. Gastroenterol. Hepatol. (NY) 13(7): 398–410. [PMC free article] [PubMed] [Google Scholar]
- Gökbulut A. (2016) Determination of hispidulin in the flowers of Inula viscosa (L.) aiton using HPLC and HPTLC methods. Turk. J. Pharm. Sci. 13: 159–166. 10.5505/tjps.2016.47955 [DOI] [Google Scholar]
- Haoui I.E., Derriche R., Madani L, Oukali Z. (2015) Analysis of the chemical composition of essential oil from Algerian Inula viscosa (L.) Aiton. Arab. J. Chem. 8: 587–590. 10.1016/j.arabjc.2011.05.005 [DOI] [Google Scholar]
- Hassan M., Watari H., AbuAlmaaty A., Ohba Y., Sakuragi N. (2014) Apoptosis and molecular targeting therapy in cancer. BioMed. Res. Int. 2014: 150845. 10.1155/2014/150845 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hernández V., Recio M.C., Manez S., Giner R.M., Riou J.F. (2007) Effects of naturally occurring dihydro flavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 81: 480–488. 10.1016/j.lfs.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Huang X.B., Yuan L.W., Shao J., Yang Y., Liu Y., Lu J.J., Chen L. (2020) Cytotoxic effects of flavonoids from root of Sophora flavescens in cancer cells. Nat. Prod. Res. 11: 1–6. 10.1080/14786419.2020.1712382 [DOI] [PubMed] [Google Scholar]
- Islam B.U., Suhail M., Khan M.K., Zughaibi T.A., Alserihi R.F., Zaidi S.K., Tabrez S. (2021) Polyphenols as anticancer agents: toxicological concern to healthy cells. Phytother. Res. 35: 6063–6079. [DOI] [PubMed] [Google Scholar]
- Ismail N.Z., Adebayo I.A., Mohamad Zain N.N., Arsad H. (2022a) Molecular docking of compounds from Clinacanthus nutans extract detected by GC-MS analysis with the SARS-CoV-2 main protease and ACE2 protein. Nat. Prod. Res. 36(11): 2848–2852. [DOI] [PubMed] [Google Scholar]
- Ismail N.Z., Mohamed W.A.S., Ab. Rahim N., Hashim N.M., Adebayo I.A., Mohamad Zain N.N., Arsad H. (2022b) Molecular docking and molecular dynamic simulations of apoptosis proteins with potential anticancer compounds present in Clinacanthus nutans extract using gas chromatography–mass spectrometry. J. Biomol. Struct. Dyn.: 1–17. [DOI] [PubMed] [Google Scholar]
- Kähkönen M.P., Hopia A.I., Vuorela H.J, Rauha J.P., Pihlaja K., Kujala T.S. (1999) Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 47: 3954–3962. 10.1021/jf990146l [DOI] [PubMed] [Google Scholar]
- Kamer Colak K., Egeli U., Eryilmaz I.E., Aybastier O., Malyer H., Cecener G., Tunca B. (2021) The anticancer effect of Inula viscosa methanol extract by miRNAs' re-regulation: an in vitro study on human malignant melanoma cells. Nutr. Cancer 11: 1–14. 10.1080/01635581.2020.1869791 [DOI] [PubMed] [Google Scholar]
- Kaczorová D., Karalija E., Dahija S., Bešta-Gajević R., Parić A., Ćavar Zeljković S. (2021) Influence of extraction solvent on the phenolic profile and bioactivity of two Achillea species. Molecules 26(6): 1601. 10.3390/molecules26061601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T., Ali M., Khan A., Nisar P., Jan S.A., Afridi S., Shinwari Z.K. (2020) Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 10: 47. 10.3390/biom10010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheyar-Kraouche N., Bento da Silva A., Serra A.T., Bedjou F., Bronze M.R. (2018) Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J. Pharm. Biomed. Anal. 156: 297–306. 10.1016/j.jpba.2018.04.047 [DOI] [PubMed] [Google Scholar]
- Kheyar N., Bellik Y., Serra A.T., Kheyar F., Bedjou F. (2022) Inula viscosa phenolic extract suppresses colon cancer cell proliferation and ulcerative colitis by modulating oxidative stress biomarkers. BioTechnologia 103(3): 269–281. 10.5114/bta.2022.118670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.T., Lee S.H., Kim Y.M. (2016) Torilis japonica extractgenerated intracellular ROS induces apoptosis by reducing the mitochondrial membrane potential via regulation of the AMPK-p38 MAPK signaling pathway in HCT116 colon cancer. Int. J. Oncol. 49: 1088–1098. 10.3892/ijo.2016.3578 [DOI] [PubMed] [Google Scholar]
- Kim H., Moon J.Y., Ahn K.S., Cho S.K. (2013) Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 596496. 10.1155/2013/596496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffi E., Sea T., Dodehe Y., Soro S. (2010) Effect of solvent type on extraction of polyphenols from twenty-three Ivorian plants. J. Anim. Plant Sci. 5: 550–558. [Google Scholar]
- Kumari R., Sahu M.K., Tripathy A., Uthansingh K., Behera M. (2018) Hepatocellular carcinoma treatment: hurdles, advances and prospects. Hepat. Oncol. 5(2): HEP08. 10.2217/hep-2018-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Lee Y.J. (2021) Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 15: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacrX R., Musolino V., Gliozzi M., Carresi C., Maiuolo J., Nucera S., Scicchitano M., Bosco F., Scarano F., Ruga S. et al. (2020) Ferula L. plant extracts and dose-dependent activity of natural sesquiterpene ferutinin: from antioxidant potential to cytotoxic effects. Molecules 25: 5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimović Z., Malencié ND., Kovacevié N. (2005) Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 96: 873–877. 10.1016/j.biortech.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Manosroi A., Akazawa H., Kitdamrongtham W., Akihisa T., Manosroi W., Manosroi J. (2015) Potent antiproliferative effect on liver cancer of medicinal plants selected from the Thai/Lanna Medicinal. Plant Recipe Database ‘‘MANOSROI III’’: 11. 10.1155/2015/397181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merghoub N., Btaouri H., Benbacer L., Gmouh S., Trentesaux C., Brassart B. et al. (2016) Inula viscosa extracts induces telomere shortening and apoptosis in cancer cells and overcome drug resistance. Nutr. Cancer 68: 131–143. 10.1080/01635581.2016.1115105 [DOI] [PubMed] [Google Scholar]
- Messaoudi M., Chahmi N., El Mzibri M., Gmouh S., Amzazi S., Benbacer L., El Hassouni M. (2016) Cytotoxic effect and chemical composition of Inula viscosa from three different regions of Morocco. Eur. J. Med. Plants 16: 1–9. 10.9734/EJMP/2016/28340 [DOI] [Google Scholar]
- Metrouh-Amir H., Duarte C.M.M., Maiza F. (2015) Solvent effect on total phenolic contents, antioxidant, and antibacterial activities of Matricaria pubescens. Ind. Crops Prod. 67: 249–256, 10.1016/j.indcrop.2015.01.049 [DOI] [Google Scholar]
- Mohammedi Z., Atik F. (2011) Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) Karst. Int. J. Pharma. Bio. Sci. 2: 609–615. [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30(16): 2785–2791. 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83: 770–803. [DOI] [PubMed] [Google Scholar]
- Nguyen T.T.H., Qu Z., Nguyen V.T., Nguyen T.T., Le T.T.A., Chen S., Ninh S.T. (2021) Natural prenylated xanthones as potential inhibitors of PI3k/Akt/mTOR pathway in triple negative breast cancer cells. Planta Med. 10.1055/a-1728-5166 [DOI] [PubMed] [Google Scholar]
- Oka Y., Ben-Daniel B., Cohen Y. (2001) Nematicidal activity of powder and extracts of Inula viscosa. Nematology 3: 735–742. 10.1163/156854101753625245 [DOI] [Google Scholar]
- Ota E., Nagashima Y., Shiomi K., Sakurai K., Kojima C., Waalkes P.M., Himeno S. (2006) Caspase-independent apoptosis induced in rat liver cells by plancitoxin I, the major lethal factor from the crown-of-thorns starfish Acanthaster planci venom. Toxicon 48: 1002–1010. 10.1016/j.toxicon.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Ouahchia C., Hamaidi-Chergui F., Cherif H.-S., Hemma R., Negab I., Azine K., Saidi F. (2020) Total phenolic content, anti-inflammatory, analgesic, and antipyretic activities of some extracts of Inula viscosa (L.) from Algeria. Phytothérapie 18: 81–91. [Google Scholar]
- Ozkan E., Pehlivan Karakas F., Birinci Yildirim A.B., Tas I., Eker I., Zeynep Yavuz M., Ucar Turker A. (2019) Promising medicinal plant Inula viscosa L. antiproliferative, antioxidant, antibacterial and phenolic profiles. Progr. Nutr. 21(3): 652–661. [Google Scholar]
- Pérez-Jiménez J., Arranz S., Tabernero M., Díaz-Rubio M.E., Serrano J., Goñi I. et al. (2008) Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res. Int. 41: 272–285. 10.1016/j.foodres.2007.12.004 [DOI] [Google Scholar]
- Rauf A., Shariati M.A., Imran M., Bashir K., Khan S.A., Mitra S., Emran T.B., Badalova K., Uddin M., Mubarak M.S. (2022) Comprehensive review on naringenin and naringin polyphenols as a potent anticancer agent. Environ. Sci. Pollut. Res. 29: 1–17. [DOI] [PubMed] [Google Scholar]
- Rayan A., Raiyn J., Falah M. (2017) Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS One 12(11): e0187925. 10.1371/journal.pone.0187925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 26: 1231–1237. 10.1016/S0891-5849(98)00315-3 [DOI] [PubMed] [Google Scholar]
- Remila S., Atmani-Kilani D., Delemasure S., Connat J.L., Azib L., Richard T., Atmani D. (2015) Antioxidant, cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts. Eur. J. Integr. Med. 7: 274–286. 10.1016/j.eujim.2015.03.009 [DOI] [Google Scholar]
- Rozenblat S., Grossman S., Bergman M., Gottlieb H., Cohen Y., Dovrat S. (2008) Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem. Pharmacol. 75: 369–382. 10.1016/j.bcp.2007.08.024 [DOI] [PubMed] [Google Scholar]
- Salzillo A., Ragone A., Spina A., Naviglio S., Sapio L. (2021) Chlorogenic acid enhances doxorubicin-mediated cytotoxic effect in Osteosarcoma cells. Int. J. Mol. Sci. 22: 8586. 10.3390/ijms22168586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirrmacher V. (2019) From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 54(2): 407–419. 10.3892/ijo.2018.4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevgi E., Dag A., K2z2larslan-Hançer Ç., Atasoy S., ZenginKurt B., Aksakal Ö. (2021) Evaluation of cytotoxic and antioxidant potential of Dittrichia viscosa (L.) Greuter used in traditional medicine. J. Ethnopharmacol 276: 114211. 10.1016/j.jep.2021.114211 [DOI] [PubMed] [Google Scholar]
- Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L, Catron N.D, Chen J., Dayton B.D. et al. (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19(2): 202–208. 10.1038/nm.3048 [DOI] [PubMed] [Google Scholar]
- Stockert Ota J.C., Blázquez-Castro A., Cañete M., Horobin R.W., Villanueva A. (2012) MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 114(8): 785–796. 10.1016/j.acthis.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Sultana B., Anwar F., Ashraf M. (2009) Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 14: 2167–2180. 10.3390/molecules14062167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Richardo-da-Silvia J.M., Spranger I. (1998) Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 46: 4267–4274. 10.1021/jf980366j [DOI] [Google Scholar]
- Taïbi K., Ait Abderrahim L., Ferhat K., Betta S., Taïbi F., Bouraada F., Boussaid M. (2020) Ethnopharmacological study of natural products used for traditional cancer therapy in Algeria. Saudi Pharm. J. 28(11): 1451–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talib W.H., Mahasneh A.M. (2010) Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci. Pharm. 78: 33–45. 10.3797/scipharm.0912-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talib W.H., Musa H., Zarga A., Mahasneh A.M. (2012) Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 17: 3291–3303. 10.3390/molecules17033291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temesgen Shibiru J.M., Sasikumar Meseret C.E. (2022) Effect of extraction solvents on total polyphenolic content and antioxidant capacity of Syzygium aromaticum L. Flower bud from Ethiopia. BioMed Res. Intern. 2022. 10.1155/2022/4568944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh N.H., Thu Ha NT., Tra NT. et al. (2021) Bannaxanthone E induced cell-cycle arrest and apoptosis in human lung cancer cell line. Nat. Prod. Commun. 16(11): 1–6. 10.1177/1934578X211059010 [DOI] [Google Scholar]
- Verma S., Singh A., Kumari A., Tyagi C., Goyal S., Jamal S., Grover A. (2017) Natural polyphenolic inhibitors against the antiapoptotic BCL-2. J. Recept. Signal Transduct. Res. 37(4): 391–400. 10.1080/10799893.2017.1298129 [DOI] [PubMed] [Google Scholar]
- Virdis P., Migheli R., Galleri G., Fancello S., Cadoni M.P.L., Pintore G. et al. (2020) Antiproliferative and proapoptotic effects of Inula viscosa extract on Burkitt lymphoma cell line. Tumor Biol. 1: 9. 10.1177/1010428319901061 [DOI] [PubMed] [Google Scholar]
- Vogler M. (2014) Targeting BCL2-proteins for the treatment of solid tumours. Adv. Med. 2014: 943648. 10.1155/2014/943648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss V., Senft C., Lang V., Ronellenfitsch M.W., Steinbach J.P., Seifert V., Kogel D. (2010) The pan-BCL-2 inhibitor (–)-gossypol triggers autophagic cell death in malignant glioma. Mol. Cancer Res. 8: 1002–1016. 10.1158/1541-7786.MCR-09-0562 [DOI] [PubMed] [Google Scholar]
- Wakeel A., Jan S.A., Ullah I., Shinwari Z.K., Xu M. (2019) Solvent polarity mediates phytochemical yield and antioxidant capacity of Isatis tinctoria. Peer J. 7: e7857. 10.7717/peerj.7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Yi X., Wang R., Wang L., He G., Zhu M. et al. (2014) 14-Thienyl methylene matrine (YYJ18), the derivative from matrine, induces apoptosis of human nasopharyngeal carcinoma cells by targeting MAPK and PI3K/Akt pathways in vitro. Cell Physiol. Biochem. 33: 1475–1483. 10.1159/000358712 [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang L. (2004) Apoptosis in human cancer cells. Curr. Opin. Oncol. 16: 19–24, 10.1097/00001622-200401000-00005 [DOI] [PubMed] [Google Scholar]
- Zhang Q.W., Lin L.G., Ye W.C. (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 13: 20. 10.1186/s13020-018-0177-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zin Z.M., Hamid A.A., Osman A., Saari N. (2006) Antioxidative activities of chromatographic fractions obtained from root, fruit and leaf of Mengkudu (Morinda citrifolia L.). Food Chem. 94: 169–178. 10.1016/j.foodchem.2004.08.048 [DOI] [Google Scholar]