Summary:
Primary culture of rat islets of Langerhans lose glucose responsiveness and eventually die when cultured for a long period of time. In this study we evaluated the effect of matrigel, a basement membrane extract, on (i) islet cell survival, (ii) cell responsiveness following a glucose challenge, and (iii) mRNA levels for insulin, glucagon, and somatostatin. Pancreatic islets were isolated by collagenase digestion and plated in culture dishes either coated or not with a matrigel layer. Using the reverse hemolytic plaque assay, we determined the total number of insulin-secreting cells and the amount of insulin secreted by individual beta cells. After 1 h of exposure to 5 mM glucose, β cells from 6-month-old rat islets cultured for 6 weeks on matrigel showed an equal number of insulin-secreting cells compared to freshly isolated islets cultured for only 3 days in the absence of matrigel (39.5 ± 2.5 vs. 37.1 ± 2.6%). Furthermore, the release of insulin by cells cultured on matrigel for 6 weeks increased in a glucose-dependent manner (p < 0.001) and showed an ED50 of 7 mM. However, the amount of insulin released per single β cell was reduced by 40–60% (p < 0.02) compared to that released from isolated β cells derived from a 3-day culture of islets. Finally, there was a 35–55% increase (p < 0.05) in the levels of insulin, glucagon, and somatostatin mRNAs in cells cultured for 6 weeks on matrigel. These data suggest a trophic effect of matrigel on the maintenance of normal β-cell activity and function and may lead the way to the development of a new model for the study of pancreatic islets in long-term culture.
Keywords: Islets of Langerhans, Matrigel, Insulin, Glucagon, Somatostatin
Development of non-insulin-dependent diabetes mellitus (NIDDM) is characterized by an impairment of pancreatic β-cell function as well as a diminished effectiveness of insulin action at its target tissues (1,2). The understanding of the biological basis of the β-cell defect that occurs in NIDDM (3) as well as in normal aging (4–8) has been greatly limited by the difficulty to develop in vitro models using pancreatic islet cells. Primary culture of islets of Langerhans have been showed to lose glucose-stimulated insulin response after a few days of culture, and they eventually die within a week after plating (9). The difficulty of using isolated β cells for physiological or pharmacological studies that need more than 3–5 days of culture has directed many scientists toward the use of tumor cell lines. A number of pancreatic β-cell tumor lines have been derived from insulinomas developed in transgenic mice expressing the SV40 T antigen gene under control of the insulin promoter (10). However, tumor cell lines derived from pancreatic insulin-secreting cells retain only some features of the normal β cells (11,12), and for many purposes, they are not good substitutes for normal β cells.
The study of islet-cell physiology in vitro requires that the in vivo properties are retained for a prolonged period of time. Therefore, we explored the possibility of culturing normal β-cells for weeks by plating them on dishes coated with extracellular matrix, with the hope that this model would more closely resemble the in vivo physiology. The Englebreth–Holm–Swarm (EHS) carcinoma was used as source of extracellular matrix to culture the pancreatic islets of Langerhans. The tumor has been propagated in C57BL mice for more than 30 years (13) and is a source of biologically active basement membrane proteins (matrigel) (14). Matrigel contains laminin, collagen IV, heparin sulfate proteoglycan, and a variety of associated growth factors (15,16) and exhibits diverse biological activities that include the stimulation of tumor growth (17,18) and the ability to induce or maintain differentiation of cultured cells (19,20).
In the present study, we investigated the effect of matrigel on long-term culture of β cells and on the release of insulin and the mRNA levels for insulin, glucagon, and somatostatin.
MATERIALS AND METHODS
Animals.
Six-month-old male Wistar rats were housed at the Gerontology Research Center, under a 12-h light/12-h dark schedule with lights on at 0700. The animals were fasted overnight prior to removal of the pancreas. All procedures were in accordance with the federal guidelines and were approved by the Animal Resource Section of the National Institute on Aging, National Institutes of Health.
Preparation of matrigel.
The EHS carcinoma was used as a source of extracellular matrix (matrigel) (14) and utilized to plate rat pancreatic islets. Samples of matrigel were kindly donated by Dr. Antonino Passaniti (National Institute on Aging, NIH, Baltimore, MD, U.S.A.). Induction of the tumor in rats as well as its purification and isolation of extracellular matrix was performed as described previously (21). Characterization of matrigel components and its biological activity have been previously reported (13–20).
Isolation of pancreatic islets.
Islets of Langerhans were isolated as previously described (22) with minor modifications. Briefly, the whole pancreas was perfused with Hank’s balanced salt solution (HBSS) containing 1.1% collagenase (Sigma, St. Louis, MO, U.S.A.). The pancreas was then dissected into ~1-mm pieces and digested with 3.4% collagenase at 37°C for 20 min, followed by 0.75% collagenase for 5 min. Pancreatic tissue was removed after each exposure to the enzyme. The digested tissue was washed 10 times with 1% bovine serum albumin (BSA) in HBSS and layered on a 40/60 Percoll gradient. Islets were hand-picked under a dissecting microscope (Bausch & Lomb; Rochester, NY, U.S.A.) (15× magnification) with a Lang–Levi pipette and transferred into HBSS medium containing 1% BSA. Islets were then washed, repicked, and plated into new dishes to obtain a pure islet preparation free of acinar tissue (23). Islets (50 per well) were plated in 12-well dishes (Co-star, Cambridge, MA, U.S.A.) previously coated with matrigel (200 μl/well of matrigel at 8–10 mg/ml), with RPMI-1640 medium and 5% fetal calf serum (FCS; GIBCO Laboratories, Gaithersburg, MD, U.S.A.), and cultured for 6 weeks. Control islets were plated in the absence of matrigel and cultured for 3 days.
All experiments were performed using β cells cultured for 3 days (in the absence of matrigel) or for 6 weeks (in the presence of matrigel). The days of culture were determined considering that, for the control sample, at least 3 days is needed to obtain a culture free of nonislet cells, while 6 weeks was chosen as a reasonable long-term culture, as islets cultured simply in medium (in absence of matrigel) have lost glucose responsiveness as early as 1 week (9).
Dispersion of pancreatic islets into single cells.
The islets were dispersed into individual cells (23–25) with a specially constructed, blunt-ended Pasteur pipette. The dispersed cells were maintained in petri dishes (Fisher Scientific Co., Columbia, MD, U.S.A.) overnight in RPMI-1640 medium containing 10% FCS with 400 U/ml penicillin and 200 μg/ml streptomycin. A total cell count was performed prior to their utilization for the plaque assay, and the suspension was adjusted to 2 × 105 cells/ml. Viability of the cells was determined by exclusion of trypan blue. Only islet preparations showing at least 95% viable cells were used for subsequent experiments.
The reverse hemolytic plaque assay (RHPA).
The RHPA was used to identify individual cells that secreted insulin (23,26,27). The assay was performed as described previously (23,27) with minor modifications. One milliliter of the dispersed islet cells (~180,000 cells/ml) was mixed with 1 ml of 18% protein A (Sigma)-coated erythrocytes (Colorado Serum Co., Denver, CO, U.S.A.). This mixture was infused into Cunningham chambers formed on a glass microscope slide previously coated with poly-l-lysine (0.25 mg/ml for 20 min; Sigma) (26). Cell attachment occurred during a 45-min incubation at 37°C. After 1 h of incubation, chambers were rinsed with glucose-free medium to remove any nonadhering cells. Cells were then exposed to 2.5, 5, 11, or 20 mM glucose in the presence of insulin antiserum (Arnel Laboratories, New York, NY, U.S.A.) at a dilution of 1:150. One hour later, insulin antibody was removed by washing the cells once with RPMI-1640 medium. Thereafter, 100 μl of a 1:25 dilution of guinea pig complement (Colorado Serum Co.) was added for 20 min to cause lysis of red cells bound to insulin–antiinsulin complexes. A ring of lysed red cells (or “plaque”) surrounded β cells that released insulin. Cells were then fixed with 1% glutaraldehyde, stained with methyl green pyronine, and permanently mounted.
The percentage of plaque-forming cells was determined by systematically scanning each slide at a final magnification of 160. A plaque was defined as a concentric zone of erythrocyte hemolysis with a diameter larger than the pancreatic cell that it surrounded. On average, 350–400 pancreatic cells per slide were counted. The number of β cells that released insulin was expressed as a percentage of the total islet cells counted in the chamber. Plaque area was quantified by image analysis (23). The sizes of ~50 plaque/samples were measured using a Nikon Optiphot-2 microscope (Nikon, Melville, NY, U.S.A.) to collect images on a CCD camera and associated image processor (Universal Imaging, West Chester, PA, U.S.A.).
Medium collection and insulin measurement.
Pancreatic islets cells were cultured from normal 6-month-old Wistar rats as described previously (23). Prior to any experiment, the islets on matrigel were maintained in culture medium (RPMI-1640) in the presence of 6 mM glucose, 1 mM glutamine (GIBCO), 100 U/ml penicillin and 100 μg/ml streptomycin (GIBCO), and 5% FCS. The percentage of FCS was increased up to 10% in the 2 days preceding any experiments.
The day of the experiment, the culture medium was changed and replaced with a buffer containing 3.3 mM glucose, 130 mM NaCl, 5 mM KCl, 1 mM NaH2PO4, 1 mM MgSO4, 1.2 mM CaCl2, 25 mM HEPES (pH 7.4), and 0.1% BSA (radioimmunoassay grade; Sigma) in a 37°C humidified incubator for 60 min. This buffer was then discarded and replaced with 1 ml of fresh buffer with various glucose concentrations for 60 min. An aliquot of the medium was removed and centrifuged and the supernatant assayed for insulin release by radioimmunoassay using a rabbit anti-human insulin antibody (Peninsula Laboratory, Inc., Belmont, CA, U.S.A.) with 15,000–20,000 cpm (3.5–4.5 pmol) of 125I-insulin (Amersham Corp., Arlington Heights, IL, U.S.A.) and rat insulin as standard.
Oligonucleotide primers and probes, cDNAs, and polymerase chain reaction (PCR).
Oligonucleotides were synthesized on an Applied Biosystem DNA synthesizer, cleaved from the resin with concentrated ammonium hydroxide at room temperature, and deprotected by heating overnight at 55°C. After purification with Nensorb Prep columns (New England Nuclear, Boston, MA. U.S.A.), oligonucleotides were used for reverse transcription–PCR (RT-PCR) to generate cDNA probes. PCR primers were designed according to the published rat cDNA sequences (28,29). To generate rat somatostatin cDNA probe we used upstream primer (U) 5′-ATGCTGTCCTGCCGTCTCCAG-3′ and down-stream primer (D) 5′-CCAGAAGTTGAAGTTCTTGCAGCC-3′; for glucagon cDNA, U was 5′-CATTCACAGGGCACATTCACC-3′ and D was 5′-CATCAACCACTGCACAAAATCC-3′. RT-PCR was performed as described previously (30,31).
Insulin mRNA levels were detected by using the full-length rat insulin II cDNA probe of 440 bp, kindly donated by Dr. S. J. Giddings (Washington University, St Louis, MO, U.S.A.) (32). An oligonucleotide homologous to the poly(A) tail of messenger RNA was also synthesized (5′ GATGGATCCTGCAGAAGCTTTTTTTTTTTTTTTTT 3′) and used to quantify total cellular mRNA. cDNAs and oligonucleotide probes were radiolabeled using [α32P]dCTP or [γ32P]ATP (Amersham Corp.), respectively.
RNA isolation and slot-blot analysis.
Total RNA from primary culture of pancreatic islets was isolated from 6-month-old rat islets. Two groups of islets were used, both exposed to 5 mM glucose prior to any RNA extraction. The first group consisted of freshly isolated islets that were cultured only for 3 days in the absence of matrigel, while the second group of islets was cultured on a layer of matrigel for 6 weeks. RNA extraction was performed using a modification of the guanidinium thiocyanatephenol–chloroform method (33). Fifteen micrograms of total RNA from pancreatic islets cultured in the presence (6-week culture) or absence (3-day culture) of a matrigel layer was diluted in sterile TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.4) to a final volume of 50 μl. Twenty microliters of 37% formaldehyde and 10 μl of 20× SSC (3 M NaCl, 0.3 M Na3 citrate–2H2O, up to 1 liter H2O, pH 7), were added, and the solution was placed at 6°C for 15 min. The samples were then diluted with 1 ml of cold 10× SSC, and aliquots of 300 μl of diluted RNA were applied in triplicate to a nytran membrane (Schleicher and Schuell, Keene, NH, U.S.A.) in a slot-blot minifold. After absorption of RNA through the membrane, the wells were washed three times with 400 μl cold 1× SSC and the membrane was baked for 2 h at 80°C in a vacuum oven. The nytran was prehybridized at 37°C in the presence of 5× SSPE(3M NaCl, 0.2 M NaHPO4–H2O, 0.02 M EDTA–Na2, up to 1 liter H2O, pH 7.4), 5× Denhardt’s solution, 1× sodium dodecyl sulfate (SDS), and 200 mg/ml salmon sperm DNA. The membranes were then hybridized with 0.5–1 × 106 cpm of 32P-radiolabeled oligonucleotide-dT20/ml of hybridization solution in the absence of salmon sperm DNA. After an overnight incubation at 37°C, the blots were washed twice in 2× SSPE, 0.05% SDS for 15 min at 45°C, and autoradiography was performed. Hybridization with 32P-radiolabeled rat insulin, glucagon, and somatostatin was performed on the same blots and carried out at 42°C overnight. The hybridization solution for these three cDNA probes contained the same reagents described above, with the addition of 50% formamide. The blots were washed at 55°C under the same conditions used for oligonucleotide-dT20 and exposed to Kodak XR-Omat film overnight at −70°C with intensifying screens.
Quantification of the autoradiograms.
The relative levels of mRNA for insulin, glucagon, and somatostatin on nitrocellulose membranes were quantified using a Betascope 603 Blot analyzer (Betagen, Waltham, MA, U.S.A.). The relative intensity of the bands is expressed as counts per minute. After quantification, the blots were washed twice for 20 min with 0.1× SSPE and 0.01% SDS at 80°C to remove the radiolabeled probe. The nitrocellulose was then subjected to a new hybridization with another pancreatic cDNA probe and autoradiographed, and the level of hybridization quantified by β scanning. Each nitrocellulose was rehybridized three times and the efficiency of the stripping of the radiolabeled probes was verified by exposing each membrane to x-ray film overnight at −70°C. To correct for possible differences in loading of total RNA, data were normalized by rehybridizing the same membranes with oligonucleotide-dT20 probe. The mRNA levels are expressed as a percentage of the value obtained with the 3-day islet culture.
Statistical analysis.
The data are expressed as the mean ± SEM. Significance of the data was evaluated by nonpaired Student’s t test (p < 0.05 was considered significant). One-way analysis of variance (ANOVA) was used to evaluate statistical significance when more than two datum points were analyzed.
RESULTS
Isolation of pancreatic islets of Langerhans.
Pancreatic islets of Langerhans were isolated and hand-picked as described above. Endocrine cells were identified according to their morphological features and by microscopic recognition of secretory granules in the cytoplasm (Leitz microscope; 750× magnification). Approximately 355 ± 70 islets (mean ± SEM) were isolated from each 6-month-old rat pancreas. The islet diameter had an average of 150–350 μm and showed a regular spherical shape with well-delimited smooth borders by day 3 after imbedding into the matrigel (Fig. 1). There was no change in islet diameter following culture of islets for 6 weeks.
FIG. 1.
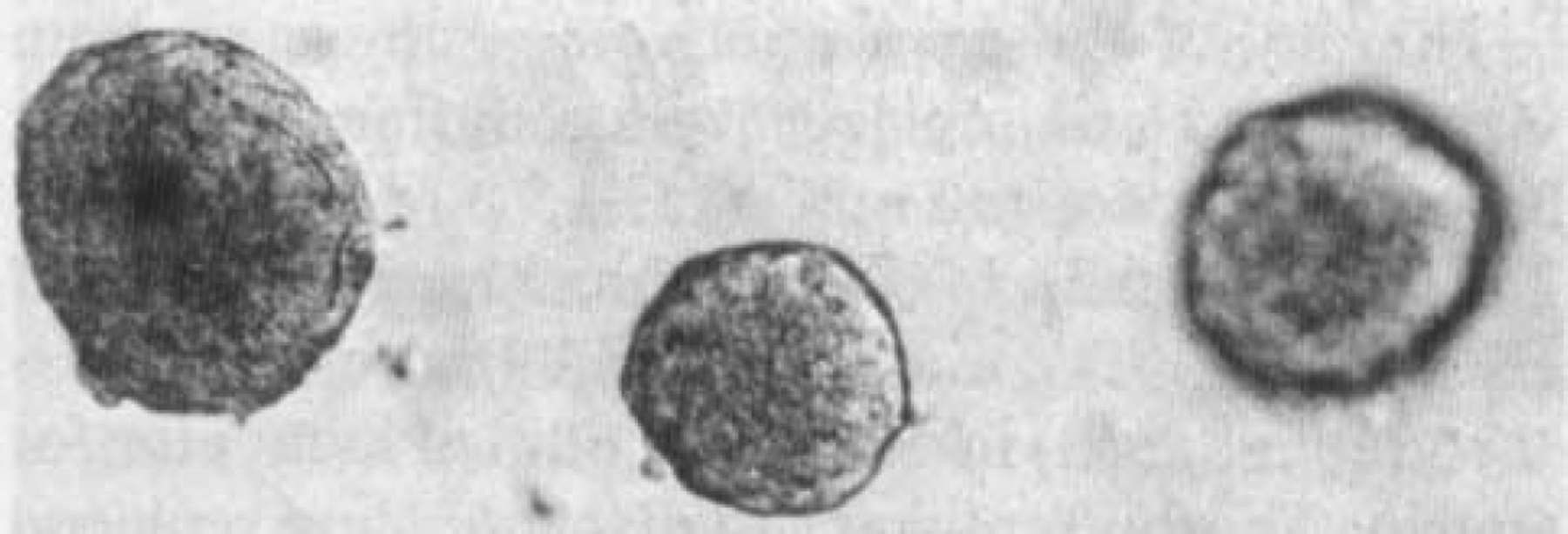
Pancreatic islets of Langerhans. Rat pancreatic islets were grown for 6 weeks on a layer of matrigel in the presence of RPMI-1640 medium containing 6 mM glucose and 5% FCS. The islets embedded in the extracellular matrix showed a smooth three-dimensional structure; by day 3, no contaminating fibroblasts could be seen on the surrounding extracellular matrix. The picture was taken under 100× magnification using a phase-contrast microscope.
Identification of insulin-secreting cells.
Islets were isolated from the whole pancreas by collagenase treatment, separated into single cells by mechanical trituration, and subjected to the reverse hemolytic plaque assay (23,27). With this technique, a similar number of insulin-secreting β-cells was identified both in 3-day culture and in islet cells maintained on matrigel for 6 weeks (Fig. 2). One hour after exposure to 2.5 mM glucose, 13 ± 2.5% of the islet cells cultured for 3 days were responsive and secreted insulin, compared to 23 ± 3.1% of the islets cultured for 6 weeks on matrigel. The increase in glucose concentration led to a progressive recruitment of new cells secreting insulin, in both short- and long-term cultures (Fig. 2). At the glucose concentration of 11 mM, 58.2 ± 1.3% of the islet cells cultured for 3 days formed hemolytic plaques, versus 56.7 ± 2.0% of cells from long-term islet culture.
FIG. 2.
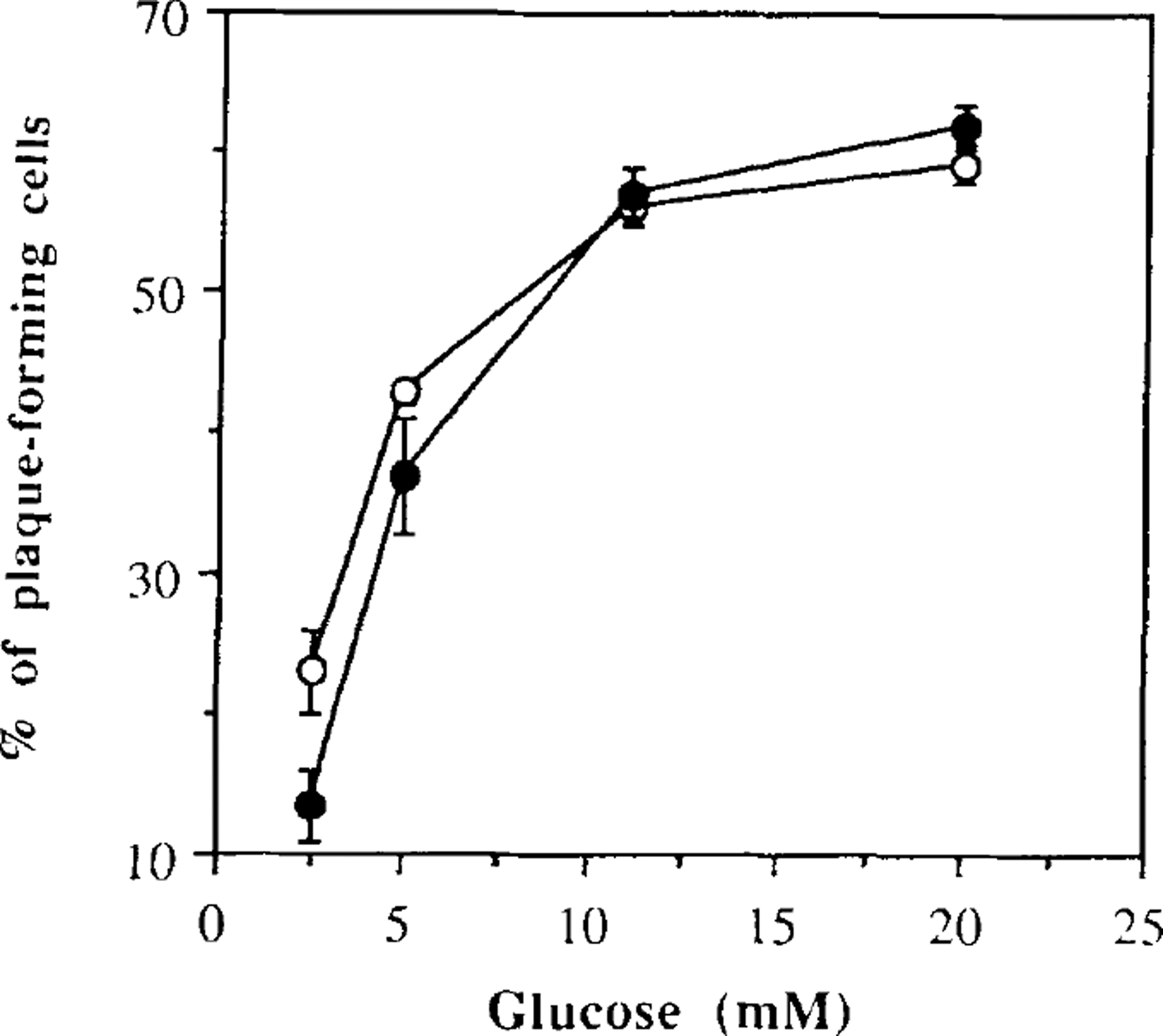
Percentage of plaque-forming cells at various glucose concentrations. Dispersed islet cells from short-term (filled circles) and long-term (open circles) cultures were exposed to different glucose concentrations in the presence of insulin antiserum for 1 h. Datum points express the number of cells secreting insulin over the total number of isolated islet cells. The values shown represent the mean ± SEM from three experiments. Each experiment was performed using a pool of islets obtained from four rats.
Detection of the amount of insulin secreted per single β cell.
Using the reverse hemolytic plaque assay, we were able to quantify the amount of insulin secreted per single β cell by measuring the area of hemolysis surrounding each secreting cell (23). We determined that the amount of insulin released per single β cell increased as a function of the glucose concentration present in the medium both in 3-day islet cultures and in islets maintained for 6 weeks on matrigel (p < 0.001, by ANOVA). However, the amount of insulin released per single β cell from the long-term islet cultures was consistently lower than that from the short-term islet cultures (Fig. 3). At 2.5 and 5 mM glucose, β cells cultured for 6 weeks on matrigel secreted 38 and 63% less insulin than β cells cultured for only 3 days in the absence of matrigel (p < 0.02 and p < 0.005, by Student t test). With higher glucose concentrations, the long-term cultures of β cells were capable of maintaining their glucose-dependent insulin responsiveness, but the plaque area was consistently smaller than in control 3-day cultures (Fig. 3).
FIG. 3.
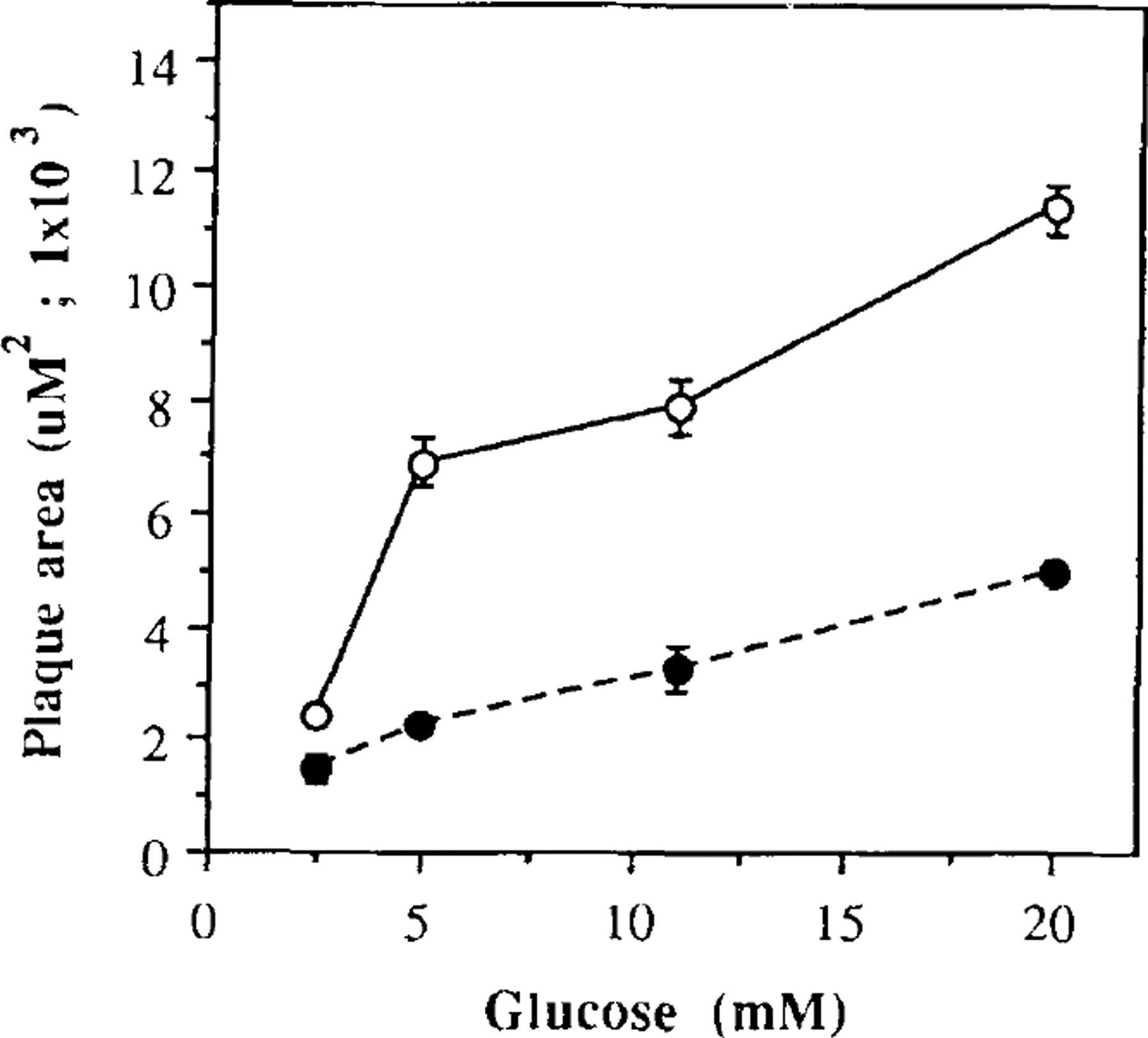
Mean plaque areas at various glucose concentrations. Dispersed islet cells from short-term (open circles) and long-term (filled circles) cultures were exposed to different glucose concentrations in the presence of insulin antiserum for 1 h. Datum points express the mean plaque area, which corresponds to the extension of the area of erythrocyte hemolysis. The values shown represent the mean ± SEM from three experiments. Each experiment was performed using pool of islets obtained from four rats.
Insulin accumulation into the culture medium.
Islet β cells cultured for 6 weeks on matrigel released insulin into the culture medium in a glucose-dependent fashion (p < 0.001, by ANOVA) (Fig. 4), with an ED50 of 7 mM. The islets responded acutely to 2.5, 5, 11, and 20 mM glucose with a 1.35 ± 0.17-, 1.47 ± 0.21-, 2.39 ± 0.07-, and 4.03 ± 0.08-fold increase in insulin secretion over the levels seen without glucose, respectively. Therefore, it appears that maintaining islets for 6 weeks on a layer of extracellular matrix does not reduce the ability of β cells to release insulin following a glucose challenge.
FIG. 4.
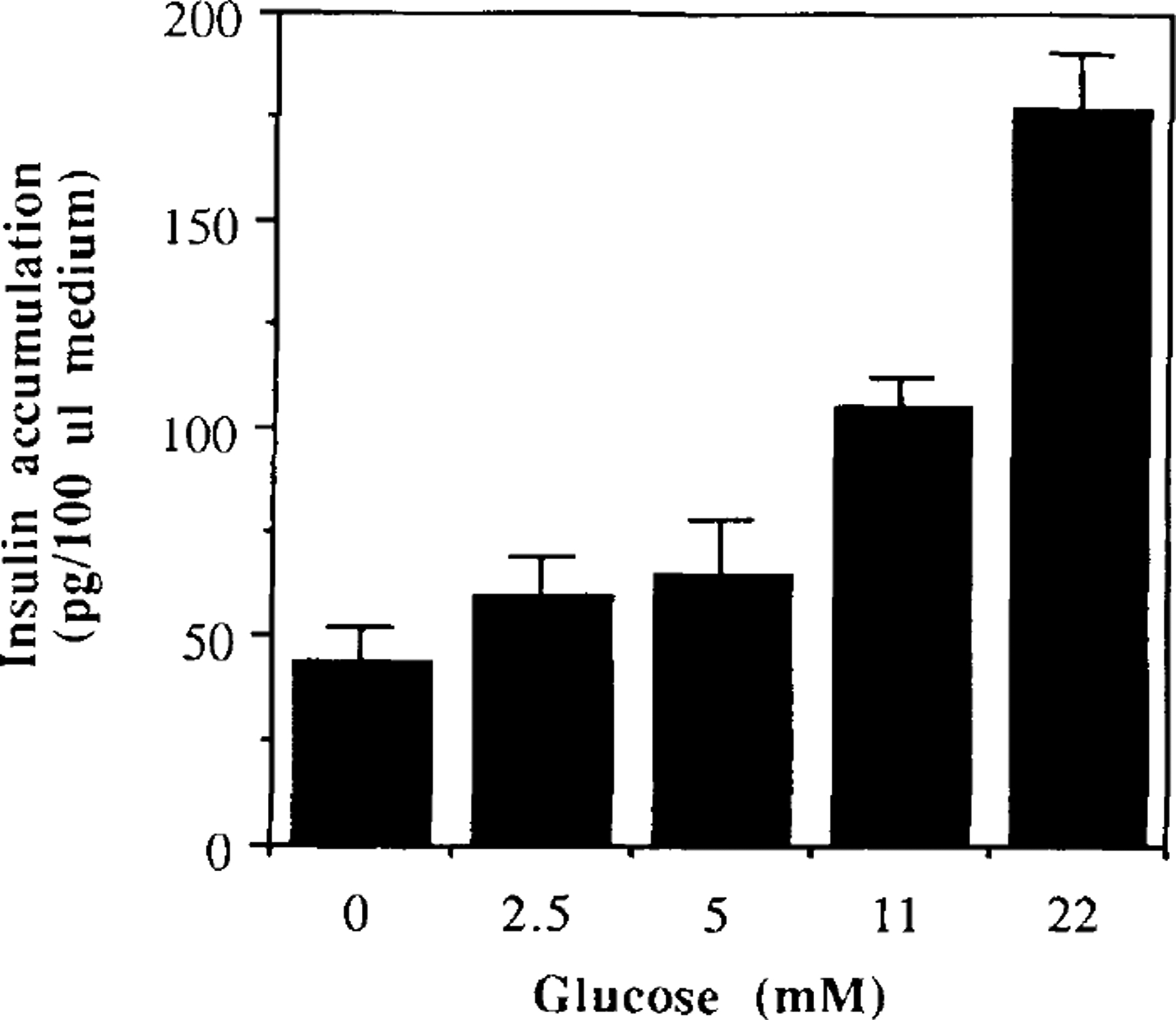
Insulin accumulation into the culture medium. Culture medium obtained from long-term culture of 50 islets previously cultured for 6 weeks on matrigel was assayed for total insulin release by RIA. The values shown represent the mean ± SEM from three experiments.
Detection of insulin, glucagon, and somatostatin mRNA levels in isolated islets.
Prior to RNA extraction, islets cultured for 3 days in the absence of matrigel and islets cultured for 6 weeks on a layer of matrigel were incubated in RPMI-1640 medium in the presence of 5 mM glucose. Messenger RNA levels for insulin, glucagon, and somatostatin were measured by slot-blot analysis. Islets from six rats were pooled for each group, and two different preparations were assayed in two independent experiments. Insulin mRNA levels in long-term culture increased up to 35.2 ± 9.1% over that detected in 3-day islet cultures (p < 0.05 by Student t test) (Fig. 5). Similarly, glucagon and somatostatin mRNAs showed a parallel increase of 45.4 ± 14.2 and 55.5 ± 9.8%, respectively (p < 0.05) (Fig. 5).
FIG. 5.
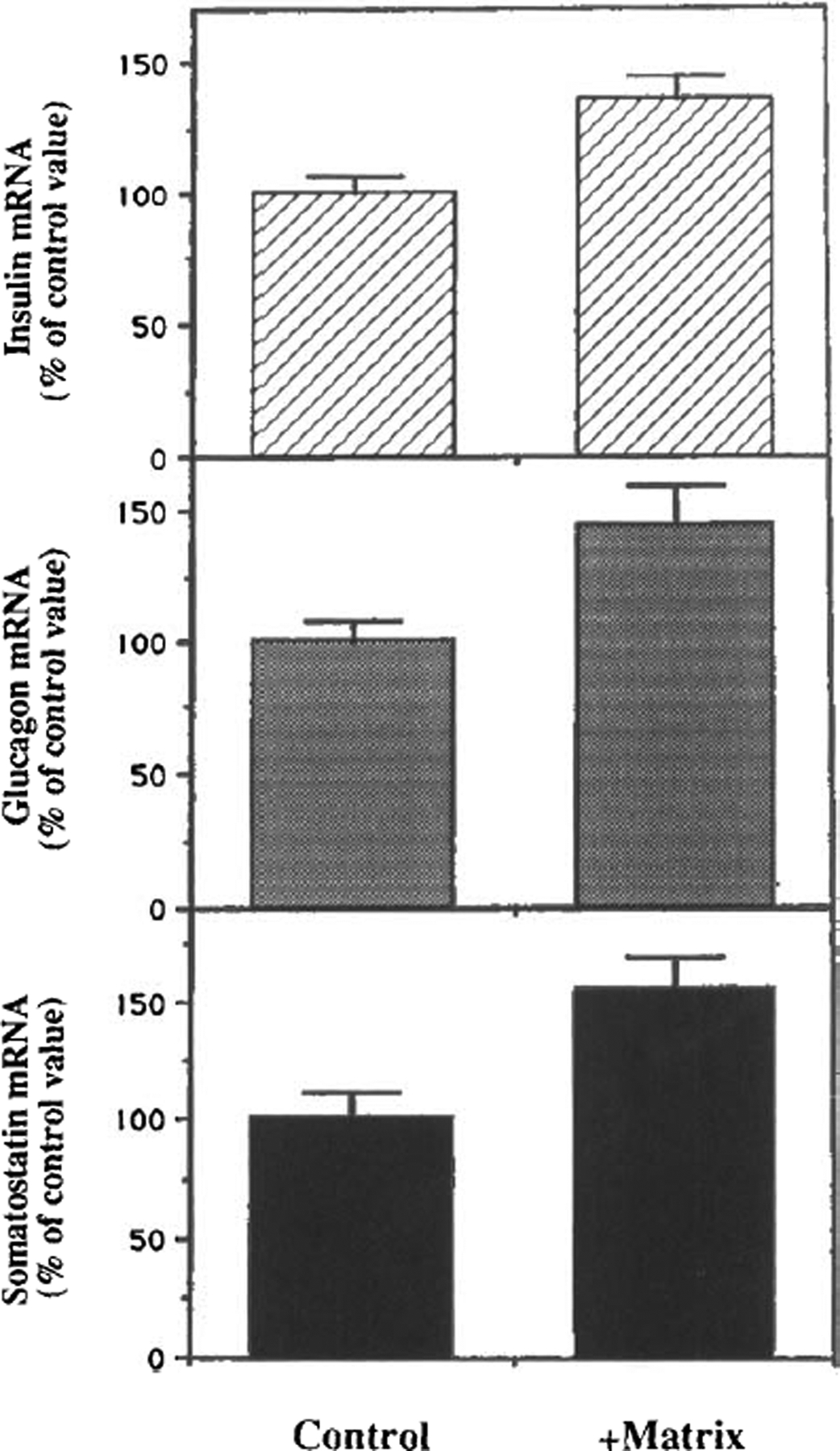
Detection of insulin, glucagon, and somatostatin mRNA levels. Total RNA from islet cultured for 3 days in the absence of matrigel (control; left) or from islets maintained in culture for 6 weeks on dishes coated with matrigel (right) was collected and combined in pools corresponding to each different group. Five micrograms of RNA was subjected to slot-blot analysis and hybridized with the cDNA probe for rat insulin-II (top), glucagon (center), or somatostatin (bottom). The data were normalized by hybridizing the same membrane with oligo-dT20 and are expressed as a percentage of the value observed in the control sample. The figure shows the mean of two independent experiments performed with two independent pools of pancreatic islets.
DISCUSSION
β-cell destruction and dysfunction are etiological factors responsible for the onset of type I and type II diabetes (1). The availability of in vitro models to study the pathophysiology of the β cells would provide a powerful tool for the design of therapeutical interventions capable of interfering with the autoimmune destruction of the β cells in type I diabetes or improving their function in type II diabetes. Unfortunately, in vitro studies have failed in culturing pancreatic islets of Langerhans for a long time. When kept in culture for more than a week, islet cells first lose their glucose responsiveness and eventually die (9). The availability of cell lines derived from insulinoma tumors has partially solved the problem of islet-cell survival in culture (10). Insulinoma cell lines have allowed long-term studies on cell metabolism and provide biological material of an easy accessibility and a low cost. However, their suitability for designing therapeutic agents is debatable. For example, in many insulinoma cell lines, the secretion of insulin is completely independent of the glucose concentration present in the culture medium (9), while in others, the glucose responsiveness is preserved only at very low glucose concentrations (12). Indeed, these cellular responses are in sharp contrast with those observed in vivo (9,10), thereby limiting the use of these tumor cell lines in studies of β-cell physiology and pharmacology.
In the present study, we demonstrated that plating of freshly isolated rat islets on a layer of extracellular matrix allows their survival for several weeks; they responded to physiological concentrations of glucose and were capable of modulating the amount of insulin secreted as a function of the concentration of glucose in the medium, in agreement with the findings of Kaiser and Lucks-Cleric et al. (34,35). Of great interest is the fact that the same number of β cells, in both 3-day and 6-week cultures, can still secrete insulin. This means that islets grown on matrigel do not suffer a loss of β cells. Furthermore, a statistically significant increase in the amount of insulin, glucagon, and somatostatin mRNA levels was shown in islets maintained on matrigel for 6 weeks compared to islets cultured for only 3 days in the absence of matrigel. However, despite these beneficial effects, a reduction of the amount of insulin secreted per individual β cell was observed following a glucose challenge. These findings indicate that the extracellular matrix is sufficient to maintain glucose competence, but some other factors (i.e., incretins, growth factors, etc.) or cell–cell interaction within the islet (36) may be necessary for the maximal β-cell response to glucose.
Previous reports demonstrated that the culture of islet cells on a layer of extracellular matrix prolongs their survival (34), possibly by preserving the normal cell–cell interaction within the three-dimensional structure of the islet (35). In this study, we reported that when those islets are separated into single cells, they still maintain their glucose responsiveness, despite the loss of the normal islet structure, and that a similar number of isolated β-cells is in an insulin-secretory mode as in islet cells cultured for only 3 days. This finding demonstrates that cell-to-matrix interaction restores β-cell responsiveness to different concentrations of glucose but it does not seem to be sufficient to maintain the normal magnitude of insulin secretion after a glucose challenge. The latter could be the consequence of the disruption of the islet microanatomy and the loss of islet paracrine interaction (36) induced to obtain single-cell preparations.
In conclusion, pancreatic islets of Langerhans maintained on a layer of extracellular matrix retain their normal morphology and glucose-mediated insulin responsiveness, while having an increase in islet-specific messenger RNAs for insulin, glucagon, and somatostatin. This in vitro system may allow for mid- to long-term experiments required for the testing of novel therapeutic agents designed to improve the activity of islet cells.
Acknowledgment:
We would like to thank Dr. Michel Bernier for reviewing the manuscript and Mr. David Brindley for his editorial support.
REFERENCES
- 1.Taylor R, Agius L. The biochemistry of diabetes. Biochem 1988;250:625–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes CJ, Alarcón C. What β-cell defect could lead to hyperproinsulinemia in NIDDM? Diabetes 1994;43:511–7. [DOI] [PubMed] [Google Scholar]
- 3.Leahy JL. Natural history of β-cell dysfunction in NIDDM. Diabetes Care 1990;13:992–1010. [DOI] [PubMed] [Google Scholar]
- 4.Andres R Aging and diabetes. Med Clin North Am 1971;55: 835–45. [DOI] [PubMed] [Google Scholar]
- 5.Andres R, Tobin JD. Aging and the disposition of glucose. Adv Exp Biol Med 1975;61:239–49. [DOI] [PubMed] [Google Scholar]
- 6.Reaven EP, Gold G, Reaven GM. Effect of age on glucose-stimulated insulin release by the β-cell of the rat. J Clin Invest 1979;64:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reaven E, Curry D, Moore J, Reaven G. Effect of age and environmental factors on insulin release from the perfused pancreas of the rat. J Clin Invest 1983;71:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Bergman RN, Pauni G, Porte D Jr. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased β-cell function. J Clin Endocrinol Metab 1985;60:13–70. [DOI] [PubMed] [Google Scholar]
- 9.Kostianovsky M, McDaniel ML, Still MF, Codell RC, Lacy PE. Monolayer cell culture of adult rat islets of Langerhans. Diabetologia 1974;10:337–44. [DOI] [PubMed] [Google Scholar]
- 10.Efrat S, Linde S, Kofod H, et al. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci USA 1988;85:9037–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tal M, Thorens B, Surana M, et al. Glucose transporter isotypes switch in T-antigen-transformed pancreatic β-cell growing in culture and in mice. Mol Cell Biol 1992;12:422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakurada M, Kanatuska A, Saitoch T, et al. Regulation between glucose-stimulated insulin secretion and intracellular calcium accumulation studied with a superinfusion system of a glucose-responsive pancreatic β-cell line MIN 6. Endocrinology 1993;130:2659–65. [DOI] [PubMed] [Google Scholar]
- 13.Swarm RL. Transplantation of a murine chondrosarcoma in mice of different inbred strains. J Natl Cancer Inst 1963;31: 953–74. [PubMed] [Google Scholar]
- 14.Kleinman HK, McGarvey ML, Hassell JR, et al. Basement membrane complexes with biological activity. Biochemistry 1986;25:312–8. [DOI] [PubMed] [Google Scholar]
- 15.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvanson K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 1982;21:6188–93. [DOI] [PubMed] [Google Scholar]
- 16.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res 1992;202:1–8. [DOI] [PubMed] [Google Scholar]
- 17.Friedman R, Kibbey MC, Royce LS, et al. Enhanced tumor growth of both primary and established human and murine tumor cells in athimic mice after coinjection with Matrigel (see comment citation in Medline). J Natl Cancer Inst 1991; 83:769–74 [DOI] [PubMed] [Google Scholar]
- 18.Passaniti A, Isaacs JT, Haney JA, et al. Stimulation of a human prostatic carcinoma tumor growth in athimic mice and control of migration in culture by extracellular matrix. Int J Cancer 1992;51:318–24. [DOI] [PubMed] [Google Scholar]
- 19.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 1988;107: 1589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant DS, Tashiro K, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures. Cell 1989;58:933–43. [DOI] [PubMed] [Google Scholar]
- 21.Pauly RR, Passaniti A, Crow M. et al. Experimental models that mimic the differentiation of vascular cells. Circulation 1992;86:68–73. [PubMed] [Google Scholar]
- 22.Steffes MN, Nielson O, Dyrberg T, Baekkeskov S, Scott J, Lernmark A. Islet transplantation differing in the I and S subregions of the H-2 complex. Transplantation 1981;31: 476–9. [DOI] [PubMed] [Google Scholar]
- 23.Egan JM, Asplin CM, Drumheller MA. et al. , Glucose stimulated insulin release by individual beta cells: potentiation by glyburide. J Exp Med Biol 1991;196:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia 1974;10:431–8. [DOI] [PubMed] [Google Scholar]
- 25.Hiriart M, Matteson DR. Na+ channels and two types of Ca2+ channels in rat pancreatic β-cells identified with the reverse hemolytic plaque assay. J Gen Physiol 1988,91:617–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neill JD, Frawley LS. Detection of hormone release from individual cells in mixed populations using a reverse hemolytic plaque assay. Endocrinology 1983;83:1123–35 [DOI] [PubMed] [Google Scholar]
- 27.Smith PF, Lugue EF, Neill JD. Detection and measurement of secretion from individual neuroendocrine cells using a reverse hemolytic plaque assay. Methods Enzymol 1986;124: 443–65. [DOI] [PubMed] [Google Scholar]
- 28.Fuhrmann G, Heilig R, Kempf J, Ebel A. Nucleotide sequence of the mouse preprosomatostatin gene. Nucleic Acids Res 1990;18:1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetru C, Li KW, Bulet P, Lagueux M, Hoffmann JA. Isolation and structural characterization of an insulin-related molecule, a predominant neuropeptide from Locusta migratoria. Eur J Biochem 1991;201:495–9. [DOI] [PubMed] [Google Scholar]
- 30.Saiki RK, Gelfand DDH, Stoffed S, et al. Primer directed enzymatic amplification of DNA with thermostable DNA polymerase. Science 1988;239:487–91. [DOI] [PubMed] [Google Scholar]
- 31.Shuldiner A, Perfetti R. The polymerase chain reaction. Appl Endocrine Res 1993;23:457–86. [Google Scholar]
- 32.Giddings SJ, Carnaghi LR. The two nonallelic rat insulin mRNAs and pre-mRNAs are regulated coordinately in vivo. J Biol Chem 1988;263:3845–9. [PubMed] [Google Scholar]
- 33.Chomczynski P, Sacchi H. Single step method RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–9. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser N, Corcos AP, Tur-Sinai A, Ariau Y, Cerasi E. Monolayer culture of adult rat pancreatic islets on extracellular matrix: long term maintenance of differentiated β-cell function. Endocrinology 1988;123:834–40. [DOI] [PubMed] [Google Scholar]
- 35.Lucas-Clerc C, Massart C, Campion JP, Launois B, Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol 1993;94:9–20. [DOI] [PubMed] [Google Scholar]
- 36.Hopcroft DW, Mason DR, Scott RS. Structure-function relationships in pancreatic islets: support for intraislet modulation of insulin secretion. Endocrinology 1985;117:2073–80. [DOI] [PubMed] [Google Scholar]


