Abstract
Background
Real-life spectrum and survival implications of immune-related adverse events (irAEs) in patients treated with extended interval dosing (ED) immune checkpoint inhibitors (ICIs) are unknown.
Methods
Characteristics of 812 consecutive solid cancer patients who received at least 1 cycle of ED monotherapy (pembrolizumab 400 mg Q6W or nivolumab 480 mg Q4W) after switching from canonical interval dosing (CD; pembrolizumab 200 mg Q3W or nivolumab 240 mg Q2W) or treated upfront with ED were retrieved. The primary objective was to compare irAEs patterns within the same population (before and after switch to ED). irAEs spectrum in patients treated upfront with ED and association between irAEs and overall survival were also described.
Results
A total of 550 (68%) patients started ICIs with CD and switched to ED. During CD, 225 (41%) patients developed any grade and 17 (3%) G3 or G4 irAEs; after switching to ED, any grade and G3 or G4 irAEs were experienced by 155 (36%) and 20 (5%) patients. Switching to ED was associated with a lower probability of any grade irAEs (adjusted odds ratio [aOR] = 0.83, 95% confidence interval [CI] = 0.64 to 0.99; P = .047), whereas no difference for G3 or G4 events was noted (aOR = 1.55, 95% CI = 0.81 to 2.94; P = .18). Among patients who started upfront with ED (n = 232, 32%), 107 (41%) developed any grade and 14 (5%) G3 or G4 irAEs during ED. Patients with irAEs during ED had improved overall survival (adjusted hazard ratio [aHR] = 0.53, 95% CI = 0.34 to 0.82; P = .004 after switching; aHR = 0.57, 95% CI = 0.35 to 0.93; P = .025 upfront).
Conclusions
Switching ICI treatment from CD and ED did not increase the incidence of irAEs and represents a safe option also outside clinical trials.
Immune checkpoint inhibitors (ICIs) have deeply changed clinical practice in the field of medical oncology. Despite their first introduction as traditional body weight–based dosing regimens, simulation pharmacokinetics studies demonstrated that weight provides only a marginal contribution to ICIs physiological distribution; therefore ICI flat doses became the standard (1-3).
Recently, long life expectancy of patients treated with ICIs, high health-care costs, and the need to reduce avoidable hospital admissions during COVID-19 crises led to an increasing interest in alternative longer dosing schedules. According to clinical trials data, adoption of extended interval dosing (ED) ICIs (pembrolizumab 400 mg Q6W and nivolumab 480 mg Q4W) offers similar outcomes and safety compared with canonical interval dosing (CD) schedules (200 mg Q3W and 240 mg Q2W, respectively) (4-7). This makes the pair with economic and logistic advantages provided by ED ICIs, which seem to be unquestionable. Although in the real-life setting, an increasingly wide percentage of patients has been shifted to (or treated upfront with) ED ICIs, incidence, clinical patterns, and survival implications for patients who develop immune-related adverse events (irAEs) during ED ICIs are unknown. In a recent study involving 45 patients with advanced non-small cell lung cancer (NSCLC), the switching of pembrolizumab from CD to ED resulted in the manifestation of different and worsening irAEs (8). In this multicenter cohort study, we aim to provide further insights on this topic by 1) investigating the safety of switching the ICI interval dosing from CD to ED across multiple cancer types and different indications, 2) characterizing the spectrum of irAEs in cancer patients treated upfront with ED ICIs, and (3) describing the association between irAEs and overall survival (OS) in ED-treated patients.
Methods
Study design and population
To investigate the primary objective of our study, which was to characterize incidence and spectrum of irAEs in patients switched to ED ICIs and compare them with those before switching (during CD ICI treatment), we designed the multicenter Extended interval Dosing in patients receiving Immune Checkpoint Inhibitors study. Patients with a diagnosis of malignancy undergoing treatment with ICIs as monotherapy (viz., pembrolizumab and nivolumab) for an approved oncological indication between April 2015 and December 2021 were retrospectively identified from electronic medical records at 30 European oncological departments (Supplementary Table 1, available online) and entered into a prospectively maintained database. Patients were included if they were aged 18 years and older and if they were switched from the CD (pembrolizumab 2 mg/kg or 200 mg Q3W and nivolumab 3 mg/kg or 240 mg Q2W) to the ED (pembrolizumab 400 mg Q6W and nivolumab 480 mg Q4W) of the same ICI (first switch reported in May 2018) or if they had started upfront with ED (first upfront ED treatment reported in May 2018). This allowed us to compare irAEs patterns within the same population (before and after switch to ED) but also to describe the irAEs spectrum in cancer patients treated upfront with ED ICIs.
irAEs were evaluated according to Common Terminology Criteria for Adverse Events (version 5) and further defined according to the organ or system involved as follows based on previous retrospective studies (9,10) and Society for Immunotherapy of Cancer guidelines (11): thyroiditis, diarrhea or colitis, endocrine (excluding thyroid disorders), hepatitis, neurologic, arthralgia, asthenia (or fatigue), dermatitis, pneumonitis, others (cardiac, pyrexia, anorexia, renal, hematologic, rheumatic other than arthralgia or arthritis, pulmonary other than pneumonitis, gastrointestinal other than diarrhea or colitis). Investigators assigned the respective irAE to the patient after excluding other alternative diagnosis, based on multidisciplinary evaluation, clinical benefit after ICI discontinuation and/or immunosuppressive treatment, or pathologic evidence of irAE. Multisystem irAEs were defined as irAEs involving more than 1 organ system. irAEs data were collected until death or date of last contact if patients were still alive or lost at follow-up. The data cutoff period was March 2022.
The following clinicopathological and treatment characteristics were also collected at start of upfront CD or ED: age, gender, weight, height, smoking status, past medical and family history, Eastern Cooperative Oncology Group Performance Score, concomitant medications, tumor type, driver mutations, treatment setting, number and site of metastasis, and previous local and systemic treatments.
To switch from CD to ED, a patient must have survived until that point, and no events (deaths) can be expected before. Therefore, OS was calculated as time from ED ICI start (after switching for patients who received upfront CD) until death from any cause; patients still alive at the time of data cutoff (March 2022) were censored at the date of last contact. Ethical approval to conduct this study was obtained by the respective local ethical committees on human experimentation of each participating center, after previous approval by the coordinating center (Comitato Etico Regionale delle Marche, Reference Number 2021 389). All study-related procedures and data collection were conducted in accordance with the Declaration of Helsinki and in accordance with Good Clinical Practice.
Statistical analysis
Clinicopathological characteristics were presented using count and percentage for categorical variables, median, and range for continuous variables. McNemar test was used to compare irAEs onset before and after switch to ED. To adjust for exposure time (represented by number of cycles) that may affect the chance of irAEs onset, nested logistic regressions with intraclass correlation correction between different ICI interval dosing on the same patient were used. More precisely, because different treatment schedules of the same patients become part of the model, a nested model has been implemented to avoid the risks associated with nonindependence. In other words, this approach avoids the bias in direct comparisons of coefficients across models related to the scale changes that accompany changes in the set of explanatory variables. A sensitivity analysis was also performed stratifying patients by tumor type.
OS curves were plotted using the Kaplan-Meier method, and differences in probability of surviving between the strata were evaluated by log-rank (Mantel-Cox) test. As the incidence of irAEs is time dependent (12,13), those patients quickly interrupting ICI treatment were exposed to the potential triggering effect for a shorter time and had a lower risk of experiencing irAEs. For minimizing the immortal time bias, a landmark method was then used, and all patients who died before 3 months were excluded from the OS analysis. The cutoff point of 3 months was chosen to evaluate the impact of early and late onset irAEs, as median time to onset of irAEs usually ranges between 2 and 16 weeks from ICIs start. Among patients who switched to ED, 39 were excluded from the 12-week landmark analysis because of death before the 3-month cutoff; 39 patients among those who started upfront with ED were also excluded from the 12-week landmark analysis. To evaluate the association of irAEs onset with OS independent of other clinicopathological factors, a multivariable proportional hazard regression model was built.
Data for this study were collected in a REDCap (Research Electronic Data Capture) database, and analyses were conducted using R (version 4.0.3; R Foundation). All P values are 2-sided, and confidence intervals (CIs) are at the 95% level, with statistical significance defined as a P value of no more than .05.
Results
Patient characteristics
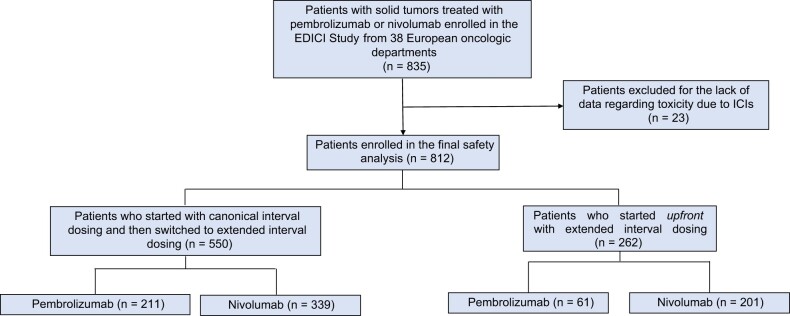
A total of 835 patients were enrolled in the Extended interval Dosing in patients receiving Immune Checkpoint Inhibitors study. Among these, 812 were included in the final safety analysis (Figure 1). ICI treatment was represented by nivolumab in 540 (66.5%) patients and pembrolizumab in 272 (33.5%). The most common tumor types were melanoma (n = 456, 56.2%) and NSCLC (n = 204, 25.1%), with 663 (81.6%) patients being treated in the advanced or metastatic setting.
Figure 1.
STROBE diagram of the EDICI study. EDICI = Extended interval Dosing in patients receiving Immune Checkpoint Inhibitors; ICIs = immune checkpoint inhibitors.
Among the enrolled patients, 550 (67.7%) started ICIs with CD and subsequentially switched to ED. The exposure time was similar, with a median number of 13 CD cycles and 7 ED cycles (1 cycle of ED corresponding to 2 cycles of CD in terms of exposure time). The main reason for switching to ED was physicians’ choice (n = 465, 84.6%); 73 (13.2%) patients requested to switch. The remaining (n = 262, 32.3%) patients started upfront with ED and were exposed to the drug for a median of 7 cycles.
At a median follow-up of 24.8 (95% CI = 23.0 to 26.4) months, median OS was 67.2 (95% CI = 56.2 to not reached [NR]) months in the whole cohort. Among the 812 patients, 368 (45.3%) experienced 1 or more irAEs regardless of the treatment schedule, including 52 (6.4%) G3-G4 irAEs.
The clinical baseline characteristics of the whole cohort, stratified by tumor type, treatment initiation (upfront CD vs upfront ED), and irAEs onset are outlined in Table 1 and Supplementary Tables 2 and 3 (available online).
Table 1.
Baseline clinical characteristics by tumor type
| Characteristic | NSCLC (%) | Melanoma (%) | Renal (%) | Other (%) |
|---|---|---|---|---|
| No., n = 812 | 204 (25.1) | 456 (56.2) | 141 (17.4) | 11 (1.3) |
| Age, median (range), y | 68 (43-85) | 67 (26-94) | 67 (43-86) | 68 (61-81) |
| Gender | ||||
| Female | 73 (35.8) | 184 (40.4) | 31 (22) | 1 (9.1) |
| Male | 131 (64.2) | 272 (59.6) | 110 (78) | 10 (90.9) |
| ECOG-PS | ||||
| 0-1 | 181 (88.7) | 439 (96.3) | 134 (95) | 11 (100) |
| ≥2 | 22 (10.8) | 16 (3.5) | 7 (5) | 0 |
| Unknown | 1 (0.5) | 1 (0.2) | 0 | 0 |
| Smoking status | ||||
| Current | 61 (29.9) | 59 (12.9) | 15 (10.6) | 3 (27.3) |
| Former | 128 (62.7) | 119 (26.1) | 72 (51.1) | 6 (54.5) |
| Never | 15 (7.4) | 278 (61) | 54 (38.3) | 2 (18.2) |
| Treatment setting | ||||
| First line | 138 (67.6) | 232 (50.9) | 8 (5.7) | 2 (18.2) |
| ≥ Second line | 66 (32.4) | 75 (16.4) | 133 (94.3) | 9 (81.8) |
| Adjuvant | 0 | 149 (32.7) | 0 | 0 |
| No. of metastatic sitesa | ||||
| <2 | 61 (29.9) | 86 (28) | 23 (16.3) | 1 (9.1) |
| ≥2 | 119 (58.3) | 180 (58.6) | 111 (78.7) | 9 (81.8) |
| Unknown | 24 (11.8) | 41 (13.4) | 7 (5) | 1 (9.1) |
| Surgeryb | ||||
| Yes | 34 (16.7) | 393 (86.2) | 121 (85.8) | 7 (63.6) |
| No | 170 (83.3) | 63 (13.8) | 20 (14.2) | 4 (36.4) |
| Concomitant radiotherapyc | ||||
| Yes | 37 (18.1) | 85 (18.6) | 33 (23.4) | 1 (9.1) |
| No | 166 (81.4) | 370 (81.1) | 107 (75.9) | 10 (90.9) |
| Unknown | 1 (0.5) | 1 (0.3) | 1 (0.7) | 0 |
| Type of ICI | ||||
| Pembrolizumab | 169 (82.8) | 87 (19.1) | 5 (3.5) | 11 (100) |
| Upfront CD | 138 (67.6) | 62 (13.6) | 3 (2.1) | 8 (72.7) |
| Upfront ED | 31 (15.2) | 25 (5.5) | 2 (1.4) | 3 (27.3) |
| Nivolumab | 35 (17.2) | 369 (80.9) | 136 (96.5) | 0 |
| Upfront CD | 34 (16.7) | 208 (45.6) | 97 (68.8) | 0 |
| Upfront ED | 1 (0.5) | 161 (35.3) | 39 (27.7) | 0 |
| irAEs onset | ||||
| Yes | 95 (46.6) | 252 (55.3) | 60 (42.6) | 4 (36.4) |
| No | 109 (53.4) | 204 (44.7) | 81 (57.4) | 7 (63.6) |
Percentage calculated on the number of patients with metastatic cancer. CD = canonical interval dosing; ECOG-PS = Eastern Operative Oncology Group Performance Score; ED = extended interval dosing; ICI = immune checkpoint inhibitor; irAEs = immune-related adverse events; NSCLC = non-small cell lung cancer.
Surgery refers to resection of primitive tumor or metastatic site or both.
Radiotherapy concomitant to ICIs refers to primitive tumor, metastatic site, or both.
Spectrum and comparison of irAEs in patients who switched from CD to ED
Among patients who started with CD ICI and subsequentially switched to ED (n = 550), 225 (40.9%) patients developed irAEs of any grade, and 17 (3.1%) patients had G3 or G4 events during CD; once they switched to ED-ICI, irAEs of any grade and G3 or G4 events were experienced by 179 (37.1%) and 23 (4.8%) patients, respectively (P = .09 for any grade irAEs and P = .11 for G3 or G4 irAEs). After adjusting for exposure time in a multivariable nested logistic regression model, ED treatment was associated with a lower probability of irAEs of any grade (adjusted odds ratio [aOR] = 0.83, 95% CI = 0.64 to 0.99; P = .047), and no difference in the likelihood of experiencing G3 or G4 events was noted (aOR = 1.55, 95% CI = 0.81 to 2.94; P = .18). Sensitivity analysis stratified by tumor type showed that melanoma patients had lower risk of any grade irAEs after switching to ED (aOR = 0.59, 95% CI = 0.41 to 0.85; P = .005) and similar risk of G3 or G4 irAEs (aOR = 1.06, 95% CI = 0.43 to 2.60; P = .89). No difference between CD and ED was noted in NSCLC patients, either in terms of any grade (aOR = 1.31, 95% CI = 0.80 to 2.12; P = .27) or G3 or G4 irAEs (aOR = 2.97, 95% CI = 0.73 to 11.98; P = .12).
Noteworthy, 78 (44.6%) of 179 cases of any grade irAEs and 12 (52.2%) of G3 or G4 irAEs during ED represented de novo toxicity, meaning that patients had not experienced any irAEs during CD. In a subgroup of patients, any grade (77 of 179; 43%) or G3 or G4 (7 of 23; 30.4%) irAEs had arisen after only 1 ED administration. Thirty-four (6.2%) patients switched back to CD, and the main reason for returning to CD was toxicity (n = 15, 44.1%).
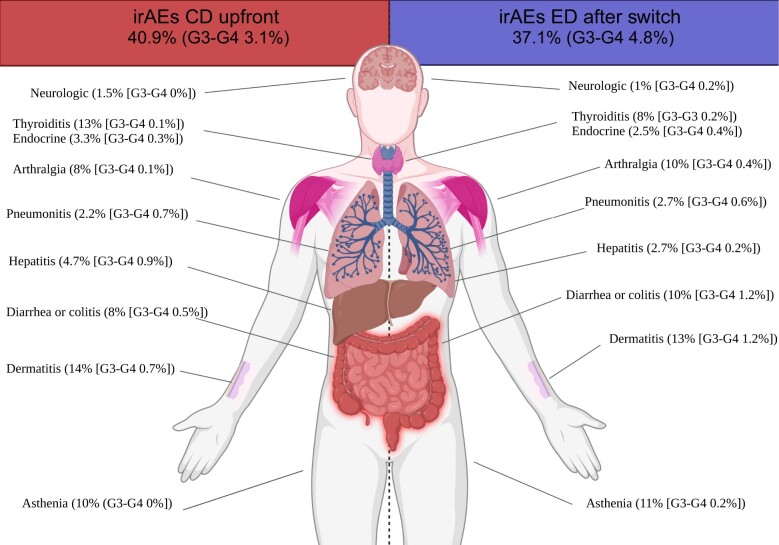
The most common irAEs (any grade) in patients during CD ICI were dermatitis (n = 77, 14%), thyroiditis (n = 69, 12.6%), and asthenia (n = 57, 10.4%) (Figure 2; Supplementary Table 4, available online for stratification by tumor type); the spectrum of irAEs did not change after switching to ED, either at time of first switching (after first ED administration; Supplementary Table 5, available online, for stratification by tumor type) or long-term, with dermatitis (n = 62, 12.8%; P = .24), asthenia (n = 53, 10.9%; P = .71), and diarrhea or colitis (n = 46, 9.5%; P = .77) being the most common irAEs (any grade) (Figure 2; Supplementary Table 6, available online, for stratification by tumor type); also looking at more worrisome toxicities such as pneumonitis (P = .45) and hepatitis (P = .12), no statistically significant differences were noted after switching; 104 (18.9%) patients developed multisystem irAEs during CD and 79 (16.4%) after switching to ED (P = .21), with the difference being statistically significant after adjusting for the number of cycles administered (ED vs CD: aOR = 0.80, 95% CI = 0.58 to 0.99; P = .049).
Figure 2.
Spectrum of irAEs for cancer patients before (during upfront CD) and after switch to ED, overall and per organ or system involved. CD = canonical interval dosing; ED = extended interval dosing; irAEs = immune-related adverse events.
Spectrum of irAEs in patients who started upfront with ED
Among patients who started upfront with ED (262), 107 (40.8%) developed irAEs of any grade and 14 (5.3%) G3 or G4 irAEs during ED. Only 18 (6.8%) patients switched to CD, mainly because of toxicity (n = 7, 38.8%).
Patients who started upfront with ED experienced dermatitis (n = 32, 12.2%), diarrhea or colitis (n = 32, 12.2%), and thyroiditis (n = 26, 9.9%) as most common irAEs (any grade) (Supplementary Table 7, available online, for stratification by tumor type). Any grade pneumonitis and hepatitis were observed in 16 (6.1%) and 12 (4.6%) patients during upfront ED, and multisystem irAEs were registered in 30 (11.4%) patients.
Association between irAEs onset and survival
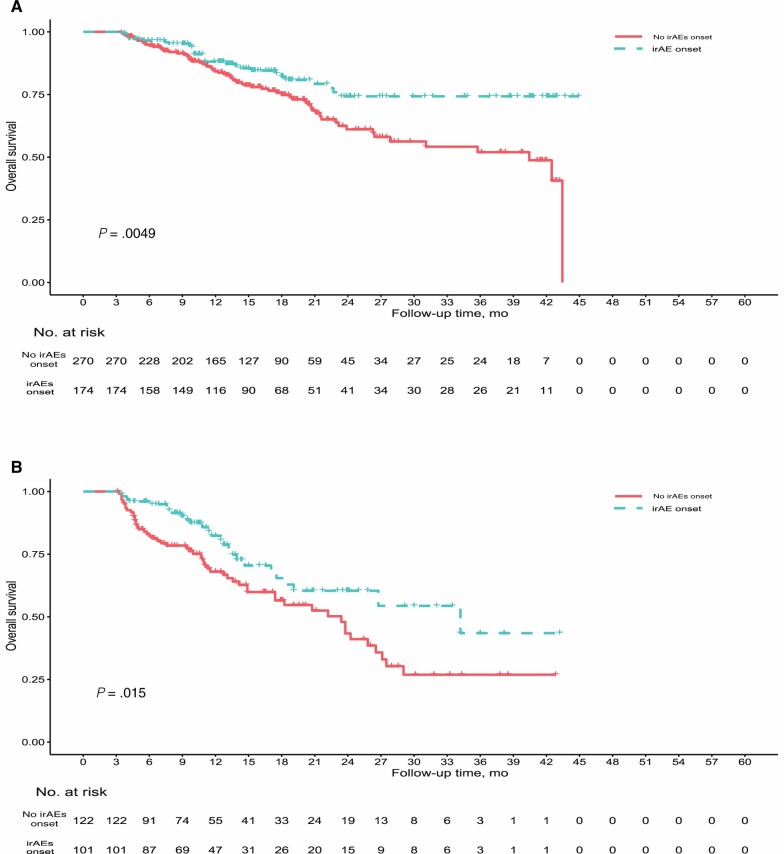
Patients who developed irAEs during ED also had longer OS compared with the no irAEs group. Among patients who switched to ED and were included in the landmark analysis (n = 444), median OS was NR (95% CI = NR to NR) in the irAEs group vs 40.4 (95% CI = 26.4 to NR) months in the no irAEs group (P = .005). Among patients who started upfront with ED and were included in the landmark analysis (n = 223), median OS was 34.2 (95% CI = 19.1 to NR) months in the irAEs group vs 23.4 (95% CI = 17.4 to 27.1) months in the no irAEs group (P = .01) (Figure 3). This association between irAEs onset and OS was confirmed in a multivariable model, which included tumor type, treatment setting, and Eastern Cooperative Oncology Group Performance Score as other variables (adjusted hazard ratio [aHR] = 0.53, 95% CI = 0.34 to 0.82; P = .004 and aHR = 0.57, 95% CI = 0.35 to 0.93; P = .02, respectively; Tables 2 and 3).
Figure 3.
Overall survival stratified by irAEs onset in patients who switched from CD to ED (A) and in patients treated upfront with ED ICIs (B). Overall survival was calculated since start of ED treatment. Landmark method was used to correct for immortal time bias (all patients who died before 3 months were excluded from the analysis). CD = canonical interval dosing; ED = extended interval dosing; ICI = immune checkpoint inhibitors; irAEs = immune-related adverse events.
Table 2.
Multivariable Cox regression model of overall survival in patients who switched from CD to ED ICIsa
| Characteristic | Hazard ratio (95% CI) | P |
|---|---|---|
| irAEs onset | ||
| No | Referent | |
| Yes | 0.52 (0.33 to 0.81) | .004 |
| Tumor type | ||
| NSCLC | Referent | |
| Melanoma | 1.24 (0.71 to 2.18) | .43 |
| Renal | 0.99 (0.55 to 1.77) | .97 |
| Other | 1.49 (0.34 to 6.45) | .58 |
| Treatment setting | ||
| Adjuvant | Referent | |
| Advanced or metastatic | 1.03 (0.50 to 2.11) | .92 |
| ECOG PS | ||
| 0 | Referent | |
| ≥1 | 3.29 (2.14 to 5.06) | <.001 |
Overall survival was calculated since start of ED treatment. Landmark method was used to correct for immortal time bias (all patients who died before 3 months were excluded from the analysis). CD = canonical interval dosing; CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group Performance Score; ED = extended interval dosing; ICIs = immune checkpoint inhibitors; irAEs = immune-related adverse events; NSCLC = non-small cell lung cancer.
Table 3.
Multivariable Cox regression model of overall survival in patients treated upfront with ED ICIsa
| Characteristic | Hazard ratio (95% CI) | P |
|---|---|---|
| irAEs onset | ||
| No | Referent | |
| Yes | 0.57 (0.35 to 0.93) | .02 |
| Tumor type | ||
| NSCLC | Referent | |
| Melanoma | 1.16 (0.58 to 2.33) | .66 |
| Renal | 0.51 (0.22 to 1.14) | .10 |
| Treatment setting | ||
| Adjuvant | Referent | |
| Advanced or metastatic | 7.66 (1.84 to 31.89) | .005 |
| ECOG PS | ||
| 0 | Referent | |
| ≥1 | 1.74 (1.08 to 2.79) | .02 |
Landmark method was used to correct for immortal time bias (all patients who died before 3 months were excluded from the analysis). CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group Performance Score; ED = extended interval dosing; ICIs = immune checkpoint inhibitors; irAEs = immune-related adverse events; NSCLC = non-small cell lung cancer.
Discussion
The findings of this international multicenter pan-cancer cohort study suggest that switching from CD to ED during ICI treatment did not worsen the safety profile. After switching to ED, any grade and G3 or G4 irAEs occurred in 37.1% and 4.8% of patients, respectively, and only 6.2% of patients returned to CD. Dermatitis, asthenia, and diarrhea or colitis were the most common irAEs after switching to ED treatment. The progressive reduction of toxicities observed after switching to ED in our study, with an incidence even lower than that reported in clinical trials, somehow corroborates previous observations (particularly in the real-life context) indicating that prolonged ICI treatment does not lead to an increased cumulative incidence of irAEs (14-16).
Nevertheless, some irAEs cases after switch to ED represented de novo toxicity, revealing that the pathobiology of immune-related toxicity might differ between the 2 schedules; moreover, 43% of any grade and 30.4% of G3 or G4 irAEs occurred after only 1 ED administration. This phenomenon was already observed in a recently published retrospective study limited to NSCLC patients (8); on one hand, this might reflect the increase in peak concentrations (Cmax) observed with ED compared with CD and the peak proliferative response of CD8+ T cells occurring in the first weeks after switching (4,17,18); on the other hand, it suggests that surveillance should be more intensive during the first ED cycles and that biomarkers of toxicity should be found to support the decision making (19).
To this extent, investigating how the 2 schedules differently affect the abundance of specific circulating immune cell types and/or T-cell receptor diversity might help predict irAEs onset and improve clinical management (20).
The study also investigated a separate cohort of pan-cancer patients treated upfront with ED, showing a real-life incidence and a spectrum of irAEs in line with those observed in historical cohort of patients treated with CD and with those reported by clinical trials with ED ICIs (7,10,13,15). The lack of a control cohort treated with upfront CD with similar baseline characteristics and follow-up time prevented us from making comparisons to avoid selection bias; in fact, the cohort included in our study was skewed toward long survival and good tolerability as, to transition from CD to ED, a patient must have survived and tolerated CD ICI well enough. However, a recent study that has shown no differences in time-to-treatment discontinuation (a measure of real-world effectiveness) between upfront CD and upfront ED also tried to infer irAEs incidence using incident levothyroxine and prednisone prescription and found no discrepancies between the 2 groups (21).
Finally, this analysis revealed that irAEs onset during ED was associated with improved OS. Although results in melanoma and NSCLC patients treated with CD ICIs are contradictory, due in part to methodological limits (10,13,22,23), our results suggest that irAEs might be considered a surrogate of clinical activity in the setting of ED ICIs. External validation among more homogeneous patient populations will be needed to confirm this observation.
Besides the retrospective nature that may have led to underreporting of irAEs (in particular grades 1-2), another limitation of this study is represented by missing data about treatment discontinuation. Nevertheless, considering the occurrence of de novo toxicity that may develop in a subset of patients, these findings demonstrate that switching ICI treatment from CD and ED did not increase the incidence of irAEs across different indications. Because the need of remodulating patients’ accesses to oncology departments is increasing, this treatment schedule represents an important alternative for treating physicians. Further prospective studies with proper comparison should look at the safety of this approach when used upfront, investigate ED efficacy data outside of clinical trials, and deepen the potential economic impact of this strategy.
Supplementary Material
Acknowledgements
LC was supported by the European Society of Medical Oncology (ESMO) with an ESMO Translational Research Fellowship. ESMO had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Luca Cantini, Clinical Oncology, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria delle Marche, Ancona, Italy; Department of Pulmonary Medicine, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands; Labcorp Drug Development Inc, Princeton, NJ, USA.
Francesco Paoloni, Clinical Oncology, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria delle Marche, Ancona, Italy.
Federica Pecci, Clinical Oncology, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria delle Marche, Ancona, Italy.
Francesco Spagnolo, Medical Oncology Unit 2, Istituto di ricovero e cura a carattere scientific (IRCCS) Ospedale Policlinico San Martino, Genova, Italy.
Carlo Genova, Academic Medical Oncology Unit, Istituto di ricovero e cura a carattere scientific (IRCCS) Ospedale Policlinico San Martino, Genoa, Italy; Department of Internal Medicine and Medical Specialties, School of Medicine, University of Genoa, Italy.
Enrica Teresa Tanda, Medical Oncology Unit 2, Istituto di ricovero e cura a carattere scientific (IRCCS) Ospedale Policlinico San Martino, Genova, Italy.
Sophie Aerts, Department of Pulmonary Medicine, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Sara Elena Rebuzzi, Department of Internal Medicine and Medical Specialties, School of Medicine, University of Genoa, Italy; Medical Oncology Unit, Ospedale San Paolo, Savona, Italy.
Giuseppe Fornarini, Medical Oncology Unit 1, Istituto di ricovero e cura a carattere scientific (IRCCS) Ospedale Policlinico San Martino, Genoa, Italy.
Federica Zoratto, UOC Oncologia, Ospedale Santa Maria Goretti, Latina, Italy.
Sara Fancelli, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Clinical Oncology Unit, Careggi University Hospital, Florence, Italy.
Alessio Lupi, Clinical Oncology, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria delle Marche, Ancona, Italy.
Carminia Maria Della Corte, Department of Precision Medicine, University of Campania, Italy.
Alessandro Parisi, Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy.
Chiara Bennati, S Maria delle Croci Hospital, AUSL della Romagna, Ravenna, Italy.
Cinzia Ortega, Oncology, Asl Cn2, Ospedale Michele e Pietro Ferrero, Verduno, Italy.
Francesco Atzori, Medical Oncology Unit, University Hospital and University of Cagliari, Cagliari, Italy.
Pier Luigi Piovano, Oncology Unit, Azienda Ospedaliera “SS. Antonio e Biagio e C. Arrigo”, Alessandria, Italy.
Corrado Orciuolo, Istituto di ricovero e cura a carattere scientific (IRCCS), National Cancer Institute Regina Elena, Rome, Italy.
Michele De Tursi, Department of Innovative Technologies in Medicine and Dentistry, University G. D’Annunzio, Chieti-Pescara, Italy.
Michele Ghidini, Oncology Unit, Fondazione Istituto di ricovero e cura a carattere scientific (IRCCS) Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy.
Andrea Botticelli, Medical Oncology Unit A, Policlinico Umberto I, Radiological, Oncological, Pathological Sciences Department, Sapienza University of Rome, Italy.
Simone Scagnoli, Medical Oncology Unit B, Policlinico Umberto I, Rome, Italy.
Lorenzo Belluomini, Section of Oncology, Department of Medicine, University of Verona School of Medicine and Verona University Hospital Trust, Verona, Italy.
Rita Leporati, Medical Oncology, Fondazione Istituto di ricovero e cura a carattere scientific (IRCCS) Istituto Nazionale dei Tumori, Milano, Italy.
Antonello Veccia, Medical Oncology, Santa Chiara Hospital, Largo Medaglie d’Oro 1, Trento, Italy.
Anna Maria Di Giacomo, Center for Immuno-Oncology, University of Siena, University Hospital of Siena, Siena, Italy.
Lucia Festino, Melanoma Unit, Cancer Immunotherapy and Development Therapeutics Unit, Istituto Nazionale Tumori Istituto di ricovero e cura a carattere scientific (IRCCS) Fondazione “G. Pascale”, Naples, Italy.
Diego Cortinovis, SC Oncologia Medica ASST H S Gerardo, Monza, Italy.
Mirko Acquati, SC Oncologia Medica ASST H S Gerardo, Monza, Italy.
Marco Filetti, Phase 1 Unit, Fondazione Policlinico Universitario Agostino Gemelli, Istituto di ricovero e cura a carattere scientific (IRCCS), Rome, Italy.
Raffaele Giusti, Medical Oncology Unit, Sant’Andrea Hospital of Rome, Italy.
Marco Tucci, Medical Oncology Unit, Department of Interdisciplinary Medicine, University of Bari Aldo Moro, Italy.
Maria Chiara Sergi, Medical Oncology Unit, Department of Interdisciplinary Medicine, University of Bari Aldo Moro, Italy.
Mattia Garutti, CRO Aviano, National Cancer Institute, Istituto di ricovero e cura a carattere scientific (IRCCS), Aviano, Italy.
Fabio Puglisi, CRO Aviano, National Cancer Institute, Istituto di ricovero e cura a carattere scientific (IRCCS), Aviano, Italy; Department of Medicine (DAME), University of Udine, Udine, Italy.
Sara Manglaviti, Thoracic Unit, Medical Oncology Department 1, Fondazione Istituto di ricovero e cura a carattere scientific (IRCCS) Istituto Nazionale dei Tumori di Milano, Italy.
Fabrizio Citarella, Department of Medical Oncology, Campus Bio-Medico University, Rome, Italy.
Matteo Santoni, Oncology Unit, Macerata Hospital, Macerata, Italy.
Erika Rijavec, Medical Oncology Unit, Ospedale di Circolo e Fondazione Macchi, asst Settelaghi, Varese, Italy.
Giuseppe Lo Russo, Thoracic Unit, Medical Oncology Department 1, Fondazione Istituto di ricovero e cura a carattere scientific (IRCCS) Istituto Nazionale dei Tumori di Milano, Italy.
Daniele Santini, UOC Oncologia Medica Territoriale, Sapienza Università, Polo Pontino, Rome, Italy.
Alfredo Addeo, Oncology Department, University Hospital Geneva, Geneva, Switzerland.
Lorenzo Antonuzzo, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Clinical Oncology Unit, and Medical Oncology Unit, Careggi University Hospital, Florence, Italy.
Alice Indini, Medical Oncology Unit, Ospedale di Circolo e Fondazione Macchi, asst Settelaghi, Varese, Italy.
Marco Bruno Luigi Rocchi, Biomolecular Sciences Department, University of Urbino, Urbino, Italy.
Alessio Cortellini, Division of Cancer, Department of Surgery and Cancer, Imperial College London, Hammersmith Hospital, London, UK.
Francesco Grossi, Medical Oncology Unit, Ospedale di Circolo e Fondazione Macchi, asst Settelaghi, University of insubria, Varese, Italy.
Paolo Antonio Ascierto, Melanoma Unit, Cancer Immunotherapy and Development Therapeutics Unit, Istituto Nazionale Tumori Istituto di ricovero e cura a carattere scientific (IRCCS) Fondazione “G. Pascale”, Naples, Italy.
Joachim G J V Aerts, Department of Pulmonary Medicine, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Rossana Berardi, Clinical Oncology, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria delle Marche, Ancona, Italy.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Author contributions
Luca Cantini, MD (Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Writing – original draft; Writing – review & Editing); Lucia Festino, MD (Investigation; Writing – original draft; Writing – review & Editing); Diego Cortinovis, MD (Investigation; Writing – original draft; Writing – review & Editing); Mirko Acquati, MD (Investigation; Writing – original draft; Writing – review & Editing); Marco Filetti, MD (Investigation; Writing – original draft; Writing – review & Editing); Raffaele Giusti, MD (Investigation; Writing – original draft; Writing – review & Editing); Marco Tucci, MD, PhD, Prof (Investigation; Writing – original draft; Writing – review & Editing); Maria Chiara Sergi, MD (Investigation; Writing – original draft; Writing – review & Editing); Mattia Garutti, MD (Investigation; Writing – original draft; Writing – review & Editing); Fabio Puglisi, MD, PhD, Prof (Investigation; Writing – original draft; Writing – review & Editing); Sara Manglaviti, MD (Investigation; Writing – original draft; Writing – review & Editing); Anna Maria Di Giacomo, MD, Prof (Investigation; Writing – original draft; Writing – review & Editing); Fabrizio Citarella, MD (Investigation; Writing – original draft; Writing – review & Editing); Erika Rijavec, MD (Investigation; Writing – original draft; Writing – review & Editing); Giuseppe Lo Russo, MD, PhD (Investigation; Writing – original draft; Writing – review & Editing); Daniele Santini, MD, PhD (Investigation; Writing – original draft; Writing – review & Editing); Alfredo Addeo, MD (Investigation; Writing – original draft; Writing – review & Editing); Lorenzo Antonuzzo, MD, PhD, Prof (Investigation; Writing – original draft; Writing – review & Editing); Alice Indini, MD (Investigation; Writing – original draft; Writing – review & Editing); Marco Bruno Luigi Rocchi, MD, Prof (Data curation; Methodology; Supervision; Writing – original draft; Writing – review & Editing); Alessio Cortellini, MD, PhD (Investigation; Methodology; Writing – original draft; Writing – review & Editing); Francesco Grossi, MD, Prof (Investigation; Writing – original draft; Writing – review & Editing); Paolo Antonio Ascierto, MD, Prof (Investigation; Writing – original draft; Writing – review & Editing); Matteo Santoni, MD, PhD (Investigation; Writing – original draft; Writing – review & Editing); Joachim GJV. Aerts, MD, PhD, Prof (Conceptualization; Data curation; Formal analysis; Supervision; Validation; Writing – original draft; Writing – review & Editing); Antonello Veccia, MD (Investigation; Writing – original draft; Writing – review & Editing); Lorenzo Belluomini, MD (Investigation; Writing – original draft; Writing – review & Editing); Francesco Paoloni, MD (Conceptualization; Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & Editing); Federica Pecci, MD (Conceptualization; Data curation; Formal analysis; Methodology; Visualization; Writing – original draft; Writing – review & Editing); Francesco Spagnolo, MD (Data curation; Validation; Writing – original draft; Writing – review & Editing); Carlo Genova, MD, PhD (Data curation; Writing – original draft; Writing – review & Editing); Enrica Teresa Tanda, MD (Data curation; Investigation; Writing – original draft; Writing – review & Editing); Sophie Aerts, MD (Investigation; Writing – original draft; Writing – review & Editing); Sara Elena Rebuzzi, MD (Investigation; Writing – original draft; Writing – review & Editing); Giuseppe Fornarini, MD (Investigation; Writing – original draft; Writing – review & Editing); Federica Zoratto, MD (Investigation; Writing – original draft; Writing – review & Editing); Sara Fancelli, MD (Investigation; Writing – original draft; Writing – review & Editing); Rita Leporati, MD (Investigation; Writing – original draft; Writing – review & Editing); Alessio Lupi, MD (Investigation; Writing – original draft; Writing – review & Editing); Alessandro Parisi, MD (Investigation; Writing – original draft; Writing – review & Editing); Chiara Bennati, MD (Investigation; Writing – original draft; Writing – review & Editing); Cinzia Ortega, MD (Investigation; Writing – original draft; Writing – review & Editing); Francesco Atzori, MD (Investigation; Writing – original draft; Writing – review & Editing); Pier Luigi Piovano, MD (Investigation; Writing – original draft; Writing – review & Editing); Corrado Orciuolo, MD (Investigation; Writing – original draft; Writing – review & Editing); Michele De Tursi, MD, Prof (Investigation; Writing – original draft; Writing – review & Editing); Michele Ghidini, MD (Investigation; Writing – original draft; Writing – review & Editing); Andrea Botticelli, MD, PhD (Investigation; Writing – original draft; Writing – review & Editing); Simone Scagnoli, MD (Investigation; Writing – original draft; Writing – review & Editing); Carminia Maria Della Corte, MD (Investigation; Writing – original draft; Writing – review & Editing); Rossana Berardi, MD (Conceptualization; Data curation; Formal analysis; Supervision; Validation; Writing – original draft; Writing – review & Editing).
Funding
This work did not receive funding.
Conflicts of interest
LC reported stock and/or other ownership interests in Labcorp Drug Development. FS reported receiving personal fees from Bristol-Myers Squibb, Novartis, Merck, Sun Pharma, Sanofi, and Pierre-Fabre outside the submitted work and serving on the advisory boards of Merck, Pierre-Fabre, Sun Pharma, and Philogen. CG reported receiving personal fees from Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Roche, Sanofi, Takeda, and ThermoFisher outside the submitted work. ETT reported receiving personal fees from Merck, and Bristol-Myers Squibb. CB reported serving as advisory board member for Bristol-Myers Squibb, Merck, Boheringer Ingelheim, and AstraZeneca; and receiving personal fees from AstraZeneca, Bristol-Myers Squibb, Novartis outside the submitted work. CO reported receiving personal fees from Merck, Bristol-Myers Squibb, Janssen, Merck, and Astellas outside the submitted work. MG reported serving as advisory board member and receiving personal fees from Merck, Servier, Eli Lilly, Amgen, and Italfarmaco outside the submitted work. AB reported serving as advisory board member and receiving personal fees from Merck, Bristol-Myers Squibb, Incyte, Pierre-Fabre, Novartis, Roche, Eli Lilly, Pfizer, Daichii Sankyo, Seagen, and Gilead outside the submitted work. SS reported serving as advisory board member and receiving personal fees from Novartis, Roche, Pierre-Fabre, Eli Lilly, Pfizer, and Daichii Sankyo outside the submitted work. LB reported receiving personal fees from AstraZeneca, Roche, and Merck outside the submitted work. AMDG reported serving as advisory board member for Merck Sharpe & Dohme, Bristol-Myers Squibb, Incyte, Pierre-Fabre, Sanofi, GlaxoSmithKline, and Novartis; and receiving personal fees from Merck Sharpe & Dohme, Roche, Bristol-Myers Squibb, Sanofi, Pierre-Fabre, GlaxoSmithKline, and Vyvamed outside the submitted work. DC reported receiving personal fees from Merck, Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Roche, Novartis, Sanofi, Amgen outside the submitted work. MaG reported serving as advisory board member for Novartis, Eli Lilly, Pierre-Fabre, and Roche; and receiving personal fees from Daichii Sankyo outside the submitted work. FaP reported receiving grants from AstraZeneca, Eisai, and Roche; and receiving personal fees from Amgen, AstraZeneca, Daichii Sankyo, Celgene, Eisai, Eli Lilly, Gilead, GlaxoSmithKline, Ipsen, Merck, Novartis, Pierre-Fabre, Pfizer, Roche, Seagen, Takeda, and Viatris outside the submitted work. SM reported serving on the advisory board of Italfarmaco. GLR reported serving as a consultant/advisory board member for Merck, Bristol-Myers Squibb, Roche, AstraZeneca, Novartis, Italfarmaco, Pfizer, Sanofi. AA reported serving as a consultant/advisory board member for Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novartis, Pfizer, PharmaMar, Roche, Sanofi, Aventis, and Takeda; and receiving personal fees from AstraZeneca, Amgen, Novartis, Eli Lilly outside the submitted work. AC reported receiving grants from Merck, AstraZeneca, IQVIA, and Oncoc4; and receiving personal fees from EISAI and AstraZeneca outside the submitted work. PAA reported serving as consultant/advisory board member for Bristol-Myers Squibb, Roche, Merck, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-Al Health, ValoTX, Replimmune; and receiving grants from Bristol-Myers Squibb, Roche, Pfizer, Sanofi outside the submitted work. JGJVA reported receiving grants from Amphera, Eli Lilly, and Roche, holding ownership interest (including patents) in Amphera, and serving as a consultant/advisory board member for Amphera, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck, Takeda, Bayer, AstraZeneca, and Roche outside of the submitted work. RB reported serving as a consultant/advisory board member for AstraZeneca, BMS, Boehringer Ingelheim, EISAI, Novartis, MSD, Otsuka, Eli Lilly, Pierre Fabre, GSk, Italfarmaco, Incyte, Seagen, and Roche, outside the submitted work. All remaining authors have no disclosures to report.
References
- 1. Sheng J, Srivastava S, Sanghavi K, et al. Clinical pharmacology considerations for the development of immune checkpoint inhibitors. J Clin Pharmacol. 2017;57(suppl 10):S26-S42. doi: 10.1002/JCPH.990. [DOI] [PubMed] [Google Scholar]
- 2. Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5(1):1-9. doi: 10.1186/S40425-017-0242-5/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28(8):2002-2008. doi: 10.1093/ANNONC/MDX235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao X, Shen J, Ivaturi V, et al. Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types. Ann Oncol. 2020;31(2):302-309. doi: 10.1016/J.ANNONC.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 5. Lala M, Li TR, de Alwis DP, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. 2020;131:68-75. doi: 10.1016/J.EJCA.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 6. Long GV, Tykodi SS, Schneider JG, et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol. 2018;29(11):2208-2213. doi: 10.1093/ANNONC/MDY408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lala M, Akala O, Chartash E, et al. Abstract CT042: pembrolizumab 400 mg Q6W dosing: first clinical outcomes data from Keynote-555 cohort B in metastatic melanoma patients. Cancer Res. 2020;80(suppl 16):CT042-CT042. doi: 10.1158/1538-7445.AM2020-CT042. [DOI] [Google Scholar]
- 8. Higashiyama RI, Yoshida T, Yagishita S, et al. Safety implications of switching pembrolizumab dosage from 200 mg every 3 weeks to 400 mg every 6 weeks in patients with advanced NSCLC. J Thorac Oncol. 2022;17(10):1227-1232. doi: 10.1016/J.JTHO.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 9. Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20(4):237-247.e1. doi: 10.1016/J.CLLC.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 10. Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non–small cell lung cancer. JAMA Oncol. 2020;6(12):1952-1956. doi: 10.1001/JAMAONCOL.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6):e002435. doi: 10.1136/JITC-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559-574. doi: 10.1093/ANNONC/MDV623. [DOI] [PubMed] [Google Scholar]
- 13. Cortellini A, Friedlaender A, Banna GL, et al. Immune-related adverse events of pembrolizumab in a large real-world cohort of patients with NSCLC with a PD-L1 expression≥ 50% and their relationship with clinical outcomes. Clin Lung Cancer. 2020;21(6):498-508.e2. doi: 10.1016/J.CLLC.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 14. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. Published online, doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 15. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-1030. doi: 10.1200/J.Clin.Oncol.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schouten RD, Muller M, de Gooijer CJ, Baas P, van den Heuvel M. Real life experience with nivolumab for the treatment of non-small cell lung carcinoma: data from the expanded access program and routine clinical care in a tertiary cancer centre–The Netherlands Cancer Institute. Lung Cancer. 2018;126:210-216. doi: 10.1016/J.LUNGCAN.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 17. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 18. Kim KH, Cho J, Ku BM, et al. The first-week proliferative response of peripheral blood PD-1þCD8þ T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019;25(7):2144-2154. doi: 10.1158/1078-0432.CCR-18-1449. [DOI] [PubMed] [Google Scholar]
- 19. Xu Y, Fu Y, Zhu B, Wang J, Zhang B. Predictive biomarkers of immune checkpoint inhibitors-related toxicities. Front Immunol. 2020;11:2023. doi: 10.3389/FIMMU.2020.02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lozano AX, Chaudhuri AA, Nene A, et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med. 2022;28(2):353-362. doi: 10.1038/s41591-021-01623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strohbehn GW, Holleman R, Burns J, et al. Adoption of extended-interval dosing of single-agent pembrolizumab and comparative effectiveness vs standard dosing in time-to-treatment discontinuation. JAMA Oncol. 2022;8(11):1663. doi: 10.1001/JAMAONCOL.2022.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kfoury M, Najean M, Lappara A, et al. Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, considering immortal-time bias. Cancer Treat Rev. 2022;110:102452. doi: 10.1016/J.CTRV.2022.102452. [DOI] [PubMed] [Google Scholar]
- 23. Villacampa G, Hernando-Calvo A, Berché R, et al. Response to “Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias.” Cancer Treat Rev. 2022;111:102465. doi: 10.1016/J.CTRV.2022.102465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.