Abstract
Background
Poly (ADP-ribose) polymerase inhibitors (PARPi) have revolutionized the treatment of ovarian cancer; however, real-world data on kidney function among patients treated with PARPi are lacking.
Methods
We identified adults treated with olaparib or niraparib between 2015 and 2021 at a major cancer center in Boston, MA, USA. We determined the incidence of any acute kidney injury (AKI), defined as at least a 1.5-fold rise in serum creatinine from baseline in the first 12 months following PARPi initiation. We calculated the percentage of patients with any AKI and sustained AKI and adjudicated the etiologies by manual chart review. We compared trajectories in estimated glomerular filtration rate (eGFR) among PARPi-treated and carboplatin and paclitaxel-treated patients with ovarian cancer, matched by baseline eGFR.
Results
Of 269 patients, 60 (22.3%) developed AKI, including 43 of 194 (22.1%) olaparib-treated patients and 17 of 75 (22.7%) niraparib-treated patients. Only 9 of 269 (3.3%) had AKI attributable to the PARPi. Of the 60 patients with AKI, 21 (35%) had sustained AKI, of whom 6 had AKI attributable to the PARPi (2.2% of the whole cohort). eGFR declined within 30 days post-PARPi initiation by 9.61 (SD = 11.017) mL/min per 1.73 m2 but recovered by 8.39 (SD = 14.05) mL/min per 1.73 m2 within 90 days after therapy cessation. There was no difference in eGFR at 12 months post-therapy initiation in patients receiving PARPi or controls receiving carboplatin and paclitaxel (P = .29).
Conclusions
AKI is common following PARPi initiation as is a transient decline in eGFR; however, sustained AKI directly attributable to the PARPi and long-term eGFR decline are uncommon.
Ovarian cancer historically had a uniformly poor prognosis due to the relatively advanced stage by the time it is detected. Poly (ADP-ribose) polymerase inhibitors (PARPi) are a major breakthrough in the maintenance treatment of ovarian cancer, leading to improved progression-free survival in both new and relapsed settings (1-3). These drugs target PARP-1 and PARP-2, enzymes responsible for repairing breaks in single strands of DNA (4). As a result, inhibition of these enzymes allows for single-strand breaks to progress to double-strand breaks, destabilizing DNA. Three PARPi—olaparib, rucaparib, and niraparib—have been approved for the treatment of advanced ovarian cancer, and olaparib and talazoparib have also been approved for HER2-negative, BRCA-mutated breast cancer.
Animal models suggest that PARP-1 is upregulated in the setting of acute kidney injury (AKI) and tubular injury and may help mediate resistance to ischemic reperfusion injury (5). Thus, preclinical data suggest that PARPi may in fact protect against or attenuate AKI, though this has not been tested in human studies. In vitro studies also suggest that PARPi may inhibit creatinine secretion without changing glomerular filtration rate (GFR) (6). One small study (n = 20) showed that nearly one-third of patients with ovarian cancer treated with niraparib had a decline in estimated GFR (eGFR) within weeks of starting treatment (7). Most patients had stabilization of eGFR, although 3 patients had persistent AKI for unclear reasons. To date, no studies have examined eGFR decline in a large cohort of patients receiving PARPi or with a longer duration of follow-up. We therefore aimed to examine kidney function trajectories of patients with ovarian cancer treated with PARPi.
Methods
We identified all female patients aged 18 years and older who were treated with maintenance niraparib or olaparib for ovarian cancer between January 2015 and January 2021 at Brigham and Women’s Hospital and Dana-Farber Cancer Institute using the Oncology Data Retrieval System. Baseline kidney function and eGFR were determined using the most proximal serum creatinine (SCr) value before initiating therapy. Baseline kidney function was defined by creatinine-based eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration 2021 race-free equation (8). Patients were followed-up for up to 12 months after therapy initiation and 12 months post therapy cessation.
Patients without a baseline SCr in the 6 months preceding PARPi initiation, or without at least 1 follow-up SCr within 30 days of starting therapy, were excluded. Additionally, patients were excluded if they had a history of end-stage kidney disease, if they were initiated on a PARPi before 2015, or if they were missing a start and/or an end date for PARPi treatment.
Patient demographics, laboratory studies, medications, and comorbidities were collected using the Research Patient Data Registry, the data warehouse of the Mass General Brigham health-care network. The start and stop date of the PARPi was confirmed using manual electronic medical record review, and patients without clearly documented therapy start dates were excluded. Comorbidities were defined using diagnosis and medication codes. Previous chemotherapy (eg, carboplatin or cisplatin, bevacizumab) was defined by receiving at least 1 dose in the year before PARPi initiation.
We determined the frequency of any AKI in the first 12 months following PARPi initiation, which was defined as at least a 1.5-fold rise in SCr from baseline. AKI severity was defined using modified Kidney Disease Improving Global Outcomes (KDIGO) criteria, where stage I was a 1.5-fold to 1.9-fold increase in SCr from baseline; stage II was a 2-fold to 2.9-fold rise from baseline; and stage III was at least a 3-fold increase in SCr from baseline or initiation of kidney replacement therapy (9). We calculated the percentage of patients who had sustained AKI, defined as a persistent elevation of SCr of at least 1.5-fold from baseline for 2 or more consecutive days within 12 months after therapy initiation. Severity of any AKI was also graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (10).
The underlying etiologies of any AKI and sustained AKI were adjudicated based on manual chart review by 2 nephrologists, and a third nephrologist resolved disagreements. The cause of sustained AKI was divided into 4 categories: potentially PARPi-related AKI, hemodynamic AKI or acute tubular necrosis (ATN), urinary tract obstruction, and other (11). PARPi-related AKI was defined as AKI attributed to PARPi based on subspecialist evaluation or unexplained sustained AKI correlating with PARPi initiation. These patients did not have evidence for an alternative cause for AKI, such as hemodynamic AKI or ATN or obstruction. Hemodynamic AKI or ATN included AKI that occurred in the context of dehydration (poor oral intake, diarrhea, vomiting), tumor lysis syndrome, septic or ischemic ATN, or nephrotoxin exposure. Obstructive AKI included all causes of confirmed bilateral ureteral or urinary outlet obstruction. Other causes of AKI included etiologies related to other drugs (eg, bevacizumab-induced thrombotic microangiopathy).
We compared eGFR trajectories among PARPi-treated patients and controls with ovarian cancer who received carboplatin and paclitaxel chemotherapy without PARPi. Patients treated with carboplatin and paclitaxel served as comparators because these standard-of-care drugs allow us to interrogate the natural history of eGFR decline expected in patients with ovarian cancer. For this analysis, control patients were excluded if they received PARPi, were missing a baseline SCr in the 6 months before chemotherapy initiation, or if they were missing at least 1 follow-up SCr in the 30 days following treatment initiation. Controls and PARPi-treated patients were matched 1:1 by baseline eGFR to within 5 mL/min per 1.73 m2 but were not matched on other baseline characteristics.
Statistical analysis
Baseline characteristics were summarized using χ2 or Fisher exact test for categorical data and Student t test or Wilcoxon rank sum test for normally distributed and skewed data, respectively. A logistic regression model was used to examine risk factors for AKI among patients receiving PARPi. Variables were selected based on biological plausibility and statistical significance to generate the final multivariable model. Finally, in both PARPi-treated patients and controls receiving carboplatin and paclitaxel therapy, we plotted the monthly mean eGFR calculated using the average of all eGFRs measured within the preceding month for the first 12 months of therapy. We then plotted the mean monthly eGFR in the 12 months following cessation of therapies. We calculated the proportion of patients with a sustained decline in eGFR, which was defined as at least a 30% decline sustained for 90 consecutive days or more. Patients were censored at their death date or loss to follow-up, defined as their last available SCr value.
All analyses were performed using R 4.1.1 and SAS 9.4. The Mass General Brigham Institutional Review Board approved this study and waived the need for informed consent.
Results
Baseline characteristics
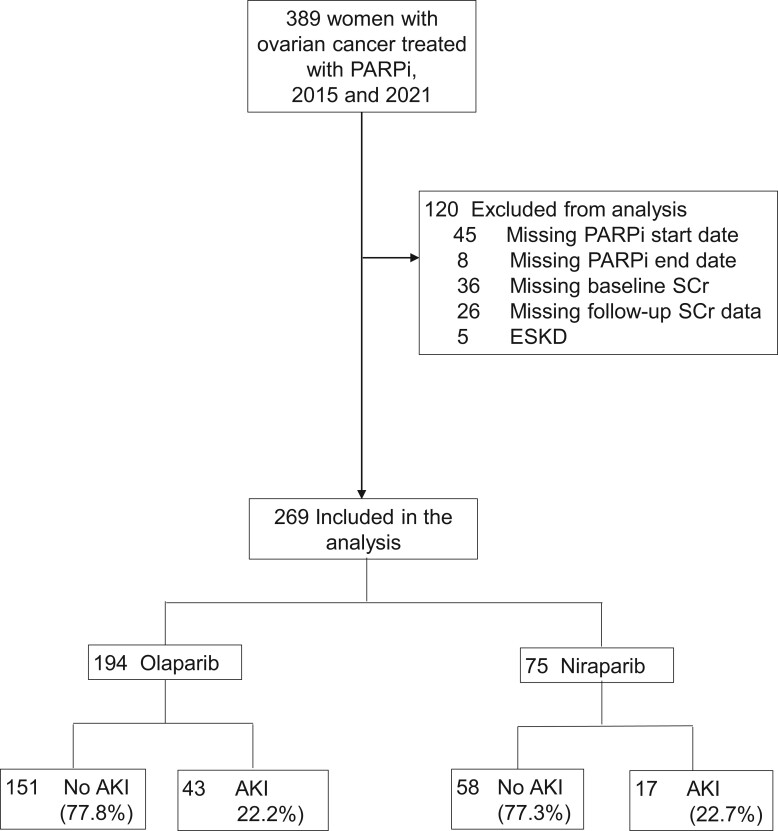
Of 389 patients treated with PARPi, 269 were included in the analysis after the exclusions shown in Figure 1. Of these, 194 (72.1%) were treated with olaparib and 75 (27.9%) with niraparib. The median age was similar in the olaparib (67 years, interquartile range [IQR] = 59-72 years) and niraparib-treated patients (65 years, IQR = 58-72 years) (Table 1). A numerically greater proportion of niraparib patients had diabetes mellitus (16% vs 11.3%) and hypertension (68% vs 60.8%) and received prior treatment with carboplatin or cisplatin (82.7% vs 71.6%) in the year preceding PARPi initiation.
Figure 1.
Flowchart of inclusion for poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi)-treated patients. Acute kidney injury (AKI) was defined as at least a 1.5-fold rise in serum creatinine (SCr) from baseline in the 12 months following PARPi initiation. ESKD = end-stage kidney disease.
Table 1.
Baseline characteristics of poly (ADP-ribose) polymerase inhibitor (PARPi)-treated patients and controls
| PARPi type |
Matched controls | ||
|---|---|---|---|
| Variablea,b | Olaparib (n = 194) | Niraparib (n = 75) | (n = 213)a |
| Age at initiation, y (IQR) | 67 (59-72) | 65 (58-72) | 65 (57-73) |
| Race, no. (%) | |||
| White | 171 (88.1) | 70 (93.3) | 175 (82.2) |
| Non-White | 23 (11.9) | 5 (6.7) | 38 (17.8) |
| Comorbidities, no. (%) | |||
| Hypertension | 118 (60.8) | 51 (68.0) | 134 (62.9) |
| Diabetes mellitus | 22 (11.3) | 12 (16.0) | 54 (25.4) |
| Cirrhosis | 3 (1.6) | 1 (1.3) | 1 (0.5) |
| Laboratory data | |||
| Baseline eGFR,c mL/min per 1.73 m2 (IQR) | 82 (69-97) | 86 (74-96) | 85 (71-97) |
| eGFR categories, no. (%) | |||
| <60 | 22 (11.3) | 6 (8.0) | 23 (10.8) |
| ≥60 | 172 (88.7) | 69 (92.0) | 190 (89.2) |
| Albumin, g/dL (IQR) | 4.2 (3.9-4.4) | 4.1 (3.9-4.4) | 4.1 (3.8-4.4) |
| Magnesium, mg/dL (IQR) | 1.8 (1.6-1.9) | 1.8 (1.6-1.9) | 1.8 (1.7-2.0) |
| Hemoglobin, g/dL (IQR) | 11.6 (10.5-12.7) | 12.4 (10.8-12.4) | 11.3 (10.2-12.6) |
| Platelets, ×109/L (IQR) | 216 (171-261) | 206 (171-283) | 255 (105-328) |
| White blood cell count, x109/l (IQR) | 5.0 (4.0-6.4) | 5.2 (4.1-6.6) | 6.4 (4.8-8.3) |
| Prior platin-based chemotherapy,d no. (%) | 139 (71.6) | 62 (82.7) | — |
| Concurrent bevacizumab, no. (%) | 17 (8.8) | 8 (10.7) | 30 (14.1) |
All patients were female and had ovarian cancer. Controls received carboplatin and paclitaxel. A total of 213 control patients were matched to 213 of the 269 PARPi-treated patients by baseline eGFR (±5 mL/min).
Missing data: 6 (2.2%) were missing albumin, 31 (11.5%) were missing magnesium, 4 (1.5%) were missing white blood cell count, 4 (1.5%) were missing hemoglobin, and 4 (1.5%) were missing platelets.
Baseline estimated glomerular filtration rate (eGFR) was calculated based on Chronic Kidney Disease-Epidemiology Collaboration equation without race.
Prior chemotherapy defined as chemotherapy received in the 1 year preceding PARPi initiation. In the treatment group, 137 olaparib recipients (70.6%) had carboplatin vs 61 niraparib recipients (81.3%). Among olaparib recipients, 5 patients (2.6%) had previously received cisplatin vs 3 patients (4%) among niraparib recipients.
Characteristics and risk factors for AKI
The median time to closest available SCr before PARPi initiation (eg, baseline SCr) was 0 days (IQR = 0-6 days). Of 269 patients treated with PARPi, 60 (22.3%) developed AKI, including 43 of 194 (22.2%) olaparib-treated patients and 17 of 75 (22.7%) niraparib-treated patients (Figure 1). The underlying etiologies of any AKI are shown in Supplementary Figure 1 (available online). Most patients had hemodynamic-mediated AKI, whereas only 7 (16.2%) of the olaparib-treated patients and 2 (11.8%) of the niraparib-treated patients had AKI attributable to the PARPi (and therefore 3.3% of the whole cohort had PARPi-related AKI). Of the 43 patients with AKI on olaparib, 40 were stage I, 1 was stage II, and 2 were stage III (Supplementary Figure 2, A, available online). Of the 17 niraparib patients with AKI, 15 were stage I, 2 were stage II, and 0 were stage III (Supplementary Figure 2, B, available online). Based on CTCAE criteria, 70 patients had AKI (vs 60 based on KDIGO staging); of these 70 patients, 51 (72.8%) had grade 1, 16 (22.9%) had grade 2, and 3 (4.3%) had grade 3 AKI (Figure 2, A). The breakdown of AKI by CTCAE criteria among olaparib and niraparib patients is shown in Figure 2, B. No patients receiving either PARPi were treated with kidney replacement therapy in the 12 months following drug initiation.
Figure 2.
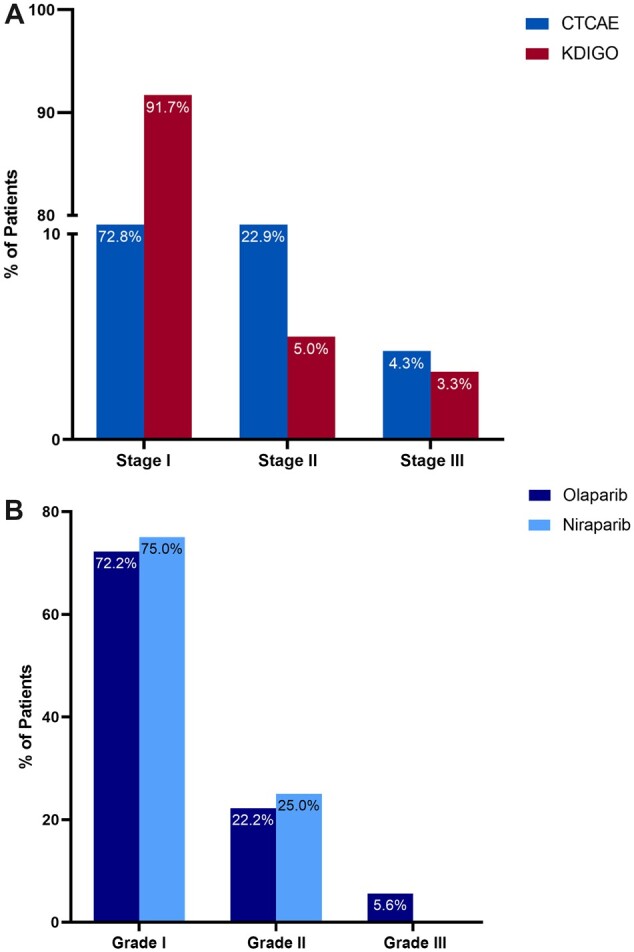
A) Incidence of any acute kidney injury (AKI) based on Common Terminology Criteria for Adverse Events (CTCAE) vs Kidney Disease Improving Global Outcomes (KDIGO) criteria. B) Incidence of any AKI based on CTCAE among olaparib-treated and niraparib-treated patients. Based on CTCAE criteria (version 5.0), 70 patients had any AKI, of whom 51 (72.8%) had grade 1, 16 (22.9%) had grade 2, and 4 (4.3%) had grade 3. Based on KDIGO criteria, 60 patients had AKI, of whom 55 (91.7%) had stage I, 3 (5.0%) had stage II, and 2 (3.3%) had stage III AKI.
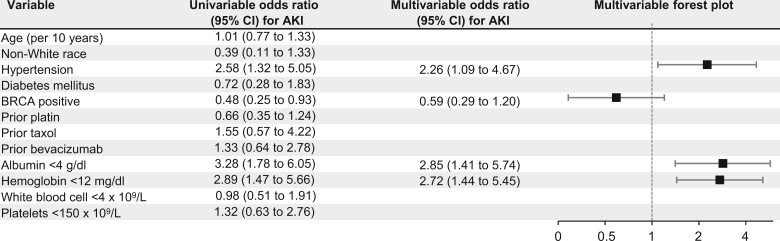
Neither age, race, hypertension nor prior treatment with platin, paclitaxel, or bevacizumab was associated with AKI in univariable-adjusted or multivariable-adjusted analyses (Figure 3). Hypertension (odds ratio [OR] = 2.26, 95% confidence interval [CI] = 1.09 to 4.67), baseline albumin less than 4 g/dL vs at least 4 g/dL (OR = 2.85, 95% CI = 1.41 to 5.74), and baseline hemoglobin less than 12 g/dL vs at least 12 g/dL (OR = 2.72, 95% CI = 1.44 to 5.45) were each independently associated with AKI in multivariable models.
Figure 3.
Risk factors for acute kidney injury (AKI) in poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi)-treated patients. Of 269 patients treated with olaparib or niraparib, 60 (22.3%) developed AKI. CI = confidence interval.
Sustained vs transient AKI
Of the 60 patients with AKI, 21 (35%) had sustained AKI, of whom 17 (81%) had stage I, 3 (14.3%) had stage II, and 1 (4.7% had stage III AKI (Figure 4, A). Fifteen of the 21 patients were on olaparib, and 6 were on niraparib (Supplementary Figure 3, A and B, available online). In reviewing the underlying etiologies for sustained AKI, 7 (33.3%) had hemodynamic-mediated AKI, 6 had AKI from obstruction (28.6%), 6 had AKI that was attributable to the PARPi (28.6%; 2.2% of the whole cohort), and 1 patient (4.8%) had AKI secondary to bevacizumab-associated thrombotic microangiopathy (clinically diagnosed) (Figure 4, B; Supplementary Table 1, available online). None of the patients underwent kidney biopsy. Urinalysis findings before and after sustained AKI are depicted in Supplementary Table 1 (available online).
Figure 4.
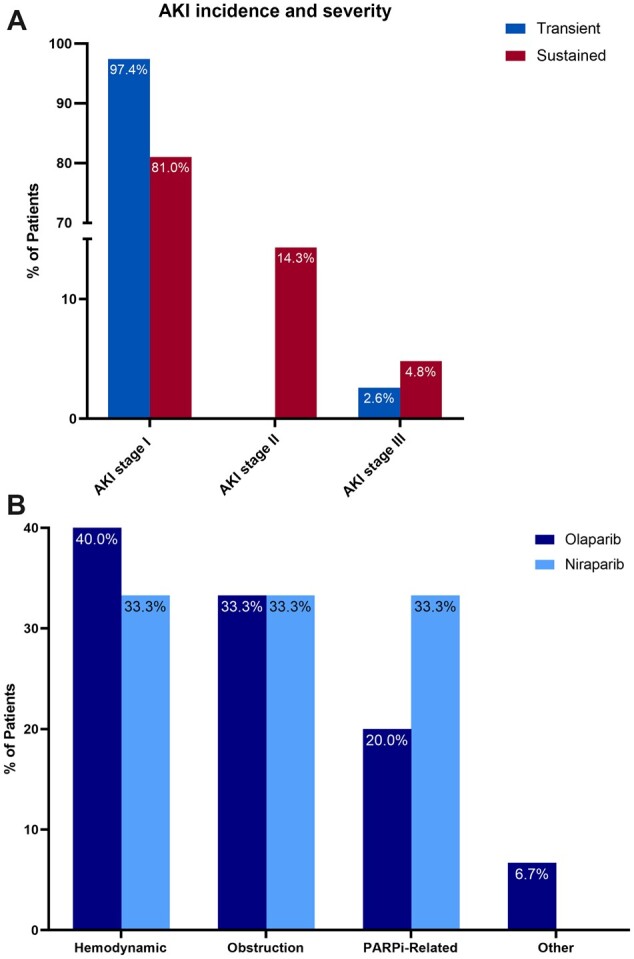
A) Incidence of acute kidney injury (AKI) by stage among patients with sustained and transient AKI. B) Etiology of sustained AKI among olaparib-treated and niraparib-treated patients. Sustained AKI was defined as a persistent elevation of serum creatinine of at least 1.5-fold from baseline for 2 or more consecutive days within the first year after therapy initiation. PARPi = poly (ADP-ribose) polymerase inhibitor.
eGFR decline among PARPi-treated patients and controls
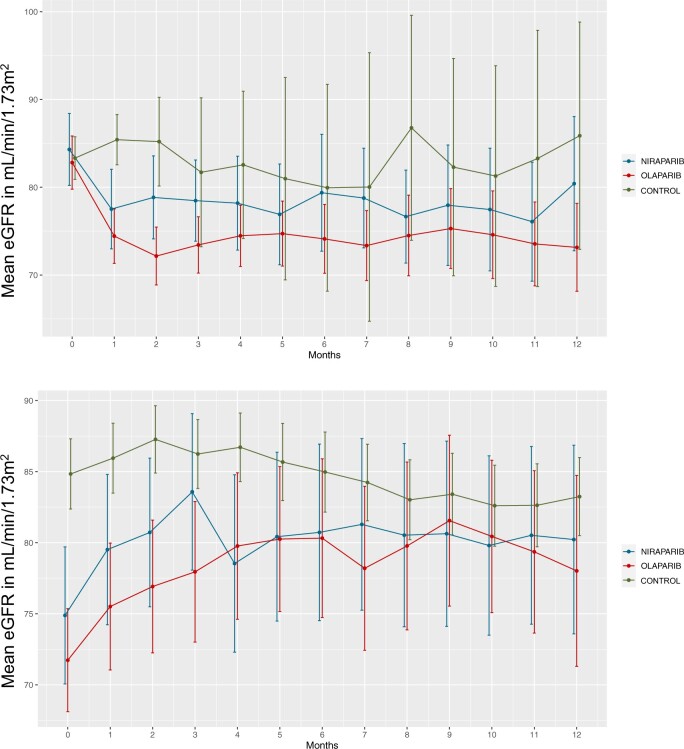
After implementing exclusion criteria, there were 213 control patients treated with carboplatin and paclitaxel matched 1:1 with 213 PARPi-treated patients (Supplementary Figure 4, available online). Baseline characteristics of PARPi-treated patients vs controls were largely similar, except that controls treated with carboplatin and paclitaxel were more likely to have diabetes mellitus and had higher baseline platelet and white blood cell counts (Table 1). PARPi recipients experienced an initial 9.61 (SD = 11.017) mL/min per 1.73 m2 decline in their eGFR by 30 days after initiation of therapy compared with controls (−1.86 [SD = 11.869] mL/min per 1.73 m2) (Figure 5, A). eGFR subsequently stabilized for the remainder of the 12-month follow-up period. There was no statistically significant difference between mean eGFR at 12 months after therapy initiation between PARPi recipients and controls (P = .29). Within the first 12 months after therapy initiation, there were 11 patients who had a sustained decline of at least 30% in eGFR, of whom 2 (18.2%) received niraparib, 4 (36.4%) received olaparib, and 5 (45.5%) received carboplatin and paclitaxel (Supplementary Figure 5, available online). Following therapy cessation, survivors experienced a modest eGFR recovery (8.39 [SD = 4.05] mL/min per 1.73 m2) within 90 days compared with controls (−1.197 [SD = 11.71] mL/min per 1.73 m2) (Figure 5, B). eGFR remained stable for the remainder of the posttherapy cessation follow-up period.
Figure 5.
A) Estimated glomerular filtration rate (eGFR) trajectories among poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi)-treated and matched chemotherapy-treated controls for 12 months following therapy initiation. B) eGFR trajectories among PARPi-treated and matched chemotherapy-treated controls for 12 months following therapy cessation. A total of 213 controls receiving carboplatin and paclitaxel therapy for ovarian cancer were matched by baseline eGFR to 213 PARPi-treated patients and not based on other baseline characteristics. For Panel A, we followed the mean eGFR trend from therapy initiation until 12 months. Patients were dropped at their end of therapy or loss of follow-up. For panel B, we followed the mean eGFR from therapy cessation until 12 months. Patients were dropped at loss of follow-up or death. Error bars = confidence intervals.
Discussion
PARPi have transformed the landscape of therapy for ovarian cancer and are now approved as maintenance therapy following platinum-based chemotherapy in both the upfront and recurrent disease settings in patients with and without BRCA mutations. However, data on AKI in the real-world setting are lacking. In this cohort study of more than 250 PARPi-treated patients, we found that AKI was common in the 6 months following initiation. However, the majority of AKI was mild (92% stage I AKI), and AKI was rarely sustained 2 days and more. Furthermore, few patients (only 2.2% of overall cohort) had an episode of sustained AKI directly attributable to the PARPi. Although a subset had a steep decline in eGFR in the first 30 days following PARPi initiation that was more pronounced than in controls receiving carboplatin and paclitaxel for ovarian cancer, reassuringly, PARPi-treated patients had stabilization or improvement of their eGFR over an extended follow-up, suggesting that these patients can likely continue PARPi therapy safely.
Few studies have explored kidney-related side effects in patients treated with PARPi. Phase III trials suggest that a grade 1 or 2 increase in SCr occurred in 11% of olaparib-treated patients (12) and 15% of rucaparib-treated patients (13) and was not reported in a large niraparib trial (14). PARPi, particularly olaparib, have been shown to inhibit multidrug and toxin extruder 1 and 2 and organic cation transporters 1 and 2 (15). There may be class differences between drugs, because niraparib does not interact with proximal tubular transporters responsible for PARPi secretion (15). Interestingly, we found a similar initial eGFR decline within 30 days and near-equivalent rates of any AKI and sustained AKI in the olaparib-treated and niraparib-treated patients. One study of 20 patients with advanced ovarian cancer treated with niraparib, with a baseline eGFR of 52-102 mL/min per 1.73 m2 before starting therapy, observed that eGFR declined to 35-90 mL/min per 1.73 m2 after receiving carboplatin and then further declined by an additional 28% after starting niraparib treatment before stabilizing after several weeks (7). Patients did not have any proteinuria, hematuria, or leukocytes on urinalysis, suggesting that there was no considerable intrinsic kidney damage. In our cohort, patients had a transient decline in eGFR within 30 to 60 days followed by stabilization of eGFR, which could similarly indicate that PARPi therapy leads to transient inhibition of tubular creatinine secretion that occurs without intrinsic AKI (“pseudo- AKI”). Additionally, after manually reviewing the underlying etiologies for sustained AKI, only a small proportion of patients (eg, <1 in 3 of those with sustained AKI and approximately 2% of the entire cohort) had AKI directly attributable to the PARPi.
Animal studies suggest that PARPi may play a role in mediating resistance to AKI. In experimental studies of ischemic reperfusion injury, rats treated with PARPi had improved SCr and increased eGFR (16,17). Histologic evaluation of rat kidneys demonstrated preserved tubular architecture in the PARPi-treated rats (16). Given these findings from preclinical studies, the potential etiologies for AKI in patients receiving PARPi are unclear. It is possible that the duration of exposure to these drugs matters whereby transient exposure is protective, yet prolonged exposure may lead to AKI.
Though this is the largest real-world study, to our knowledge, to characterize AKI rates and eGFR trajectories in patients treated with PARPi, there are limitations. First, we do not have cystatin C or measured GFR in any patients, and we therefore cannot differentiate true AKI from “pseudo-AKI.” Second, we do not have data from kidney biopsies in the patients with sustained AKI attributable to the PARPi. Third, our multivariable model may be susceptible to unmeasured confounding. Fourth, we cannot generalize our findings to other PARPi, including rucaparib and talazoparib, which were excluded from our analysis. Fifth, we matched control patients who received carboplatin and paclitaxel therapy with PARPi-treated patients by baseline eGFR because this is the strongest determinant of eGFR decline; however, we did not match other characteristics due to relatively small numbers of patients and the fact that patient characteristics were largely similar between groups (Table 1).
In summary, PARPi may cause a transient decline in eGFR and AKI in 1 in 5 patients; however, sustained, clinically significant AKI is rare, with only 2.2% of the whole cohort having sustained AKI directly attributable to the PARPi. These findings should reassure oncologists and nephrologists who frequently comanage patients receiving PARPi therapy and must weigh the pros and cons of continuing therapy. Discovery of biomarkers differentiating “pseudo-AKI” from true AKI, as well as more regular measurement of cystatin C levels, may help inform the management of patients treated with PARPi.
Supplementary Material
Acknowledgements
The funders did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Contributor Information
Shruti Gupta, Division of Renal Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA; Medical Oncology, Adult Survivorship Program, Dana-Farber Cancer Institute, Boston, MA, USA.
Paul E Hanna, Department of Medicine, Division of Nephrology, Massachusetts General Hospital, Boston, MA, USA.
Tianqi Ouyang, Department of Medicine, Division of Nephrology, Massachusetts General Hospital, Boston, MA, USA.
Karla Sofia Yamada, Division of Renal Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Rani Sawtell, Department of Medicine, Division of Nephrology, Massachusetts General Hospital, Boston, MA, USA.
Qiyu Wang, Department of Medicine, Division of Nephrology, Massachusetts General Hospital, Boston, MA, USA.
Nurit Katz-Agranov, Department of Medicine, Division of Nephrology, Massachusetts General Hospital, Boston, MA, USA.
Lea Feghali, Division of Renal Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Carolyn N Krasner, Division of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Sara Bouberhan, Department of Medicine, Division of Hematology/Oncology, Massachusetts General Hospital, Boston, MA, USA.
Cesar M Castro, Department of Medicine, Division of Hematology/Oncology, Massachusetts General Hospital, Boston, MA, USA.
Meghan E Sise, Department of Medicine, Division of Nephrology, Massachusetts General Hospital, Boston, MA, USA.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Author contributions
Shruti Gupta, MD, MPH (Conceptualization; Supervision; Writing – review & editing); Paul E. Hanna, MD, MSc (Data curation; Formal analysis; Visualization); Tianqi Ouyang, MPH (Formal analysis); Karla Sofia Yamada, MD (Visualization); Rani Sawtell, HS (Data curation); Qiyu Wang, MD (Data curation); Nurit Katz-Agranov, MD (Data curation); Lea Feghali, MD (Data curation); Carolyn N. Krasner, MD (Conceptualization; Supervision; Writing – review & editing); Sara Bouberhan, MD (Conceptualization; Supervision; Writing – review & editing); Cesar M. Castro, MD (Conceptualization; Supervision; Writing – review & editing); Meghan E. Sise, MD, MS (Conceptualization; Supervision; Writing – review & editing).
Funding
This work was funded by NIH NIDDK K23DK125672 (to S. Gupta) and R01DK140839 (to M. Sise).
Conflicts of interest
S. Gupta reports research support from the National Institutes of Health, NIDDK K23DK125672. She also reports research funding from BTG International and GE Healthcare outside the submitted work. She is a member of GlaxoSmithKline’s (GSK) Global Anemia Council, a consultant for Secretome and Proletariat Therapeutics, and founder of the American Society of Onconephrology. M. Sise reports research support from the NIH, NIDDK R01DK140839. She also reports research funding from Gilead, Abbvie, EMD-Serono, and Angion outside of the submitted work. She has served on a scientific advisory board for Travere, Novartis, and Mallinckrodt. C. Castro reports research funding from NIH, NCI R01CA264363; U01CA233360; and P50CA240243. He also reports research funding from Canon USA, Inc outside the submitted work. He is a consultant for Advanced Medical, Qiagen, Aikili Bio, and InfiniteMD.
References
- 1. González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391-2402. doi: 10.1056/nejmoa1910962 [DOI] [PubMed] [Google Scholar]
- 2. Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416-2428. doi: 10.1056/nejmoa1911361 [DOI] [PubMed] [Google Scholar]
- 3. Penson RT, Valencia RV, Cibula D, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38(11):1164-1174. doi: 10.1200/J.Clin.Oncol.19.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Murcia G, Schreiber V, Molinete M, et al. Structure and function of poly(ADP-ribose) polymerase. Mol Cell Biochem. 1994;138(1-2):15-24. doi: 10.1007/BF00928438. [DOI] [PubMed] [Google Scholar]
- 5. Zheng J, Devalaraja-Narashimha K, Singaravelu K, Padanilam BJ.. Poly(ADP-ribose) polymerase-1 gene ablation protects mice from ischemic renal injury. Am J Physiol - Ren Physiol. 2005;288(2):F387-F398. doi: 10.1152/ajprenal.00436.2003. [DOI] [PubMed] [Google Scholar]
- 6. Zibetti Dal Molin G, Westin SN, Msaouel P, Gomes LM, Dickens A, Coleman RL.. Discrepancy in calculated and measured glomerular filtration rates in patients treated with PARP inhibitors. Int J Gynecol Cancer. 2020;30(1):89-93. doi: 10.1136/ijgc-2019-000714. [DOI] [PubMed] [Google Scholar]
- 7. Lazareth H, Delanoy N, Cohen R, et al. Nephrotoxicity associated with niraparib. Am J Kidney Dis. 2020;76(6):898-900. doi: 10.1053/j.ajkd.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 8. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi: 10.1056/nejmoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kellum JA, Lameire N, Aspelin P, et al. ; Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(suppl 1):1-141. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 10. National Institute of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.; 2017. doi: 10.1080/00140139.2010.489653. [DOI]
- 11. Seethapathy H, Zhao S, Chute D, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14(12):1692-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274-1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 13. Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949-1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164. doi: 10.1056/nejmoa1611310. [DOI] [PubMed] [Google Scholar]
- 15. LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL.. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15-e28. doi: 10.1016/S1470-2045(18)30786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin DR, Lewington AJP, Hammerman MR, Padanilam BJ.. Inhibition of poly(ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):R1834-R1840. doi: 10.1152/ajpregu.2000.279.5.r1834. [DOI] [PubMed] [Google Scholar]
- 17. Shen Y, Aoyagi-Scharber M, Wang B.. Trapping poly(ADP-Ribose) polymerase. J Pharmacol Exp Ther. 2015;353(3):446-457. doi: 10.1124/jpet.114.222448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.