Abstract
Background
Treatment patterns for intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) differ, but limited studies exist comparing them. This study examines differences in molecular profiling rates and treatment patterns in these populations, focusing on use of adjuvant, liver-directed, targeted, and investigational therapies.
Methods
This multicenter collaboration included patients with ICC or ECC treated at 1 of 8 participating institutions. Retrospective data were collected on risk factors, pathology, treatments, and survival. Comparative statistical tests were 2-sided.
Results
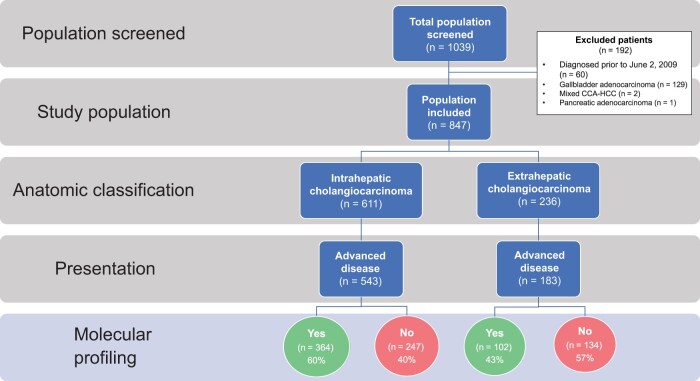
Among 1039 patients screened, 847 patients met eligibility (ICC = 611, ECC = 236). Patients with ECC were more likely than those with ICC to present with early stage disease (53.8% vs 28.0%), undergo surgical resection (55.1% vs 29.8%), and receive adjuvant chemoradiation (36.5% vs 4.2%) (all P < .00001). However, they were less likely to undergo molecular profiling (50.3% vs 64.3%) or receive liver-directed therapy (17.9% vs 35.7%), targeted therapy (4.7% vs 18.9%), and clinical trial therapy (10.6% vs 24.8%) (all P < .001). In patients with recurrent ECC after surgery, the molecular profiling rate was 64.5%. Patients with advanced ECC had a shorter median overall survival than those with advanced ICC (11.8 vs 15.1 months; P < .001).
Conclusions
Patients with advanced ECC have low rates of molecular profiling, possibly in part because of insufficient tissue. They also have low rates of targeted therapy use and clinical trial enrollment. While these rates are higher in advanced ICC, the prognosis for both subtypes of cholangiocarcinoma remains poor, and a pressing need exists for new effective targeted therapies and broader access to clinical trials.
Cholangiocarcinoma (CCA) is a rare, aggressive cancer of the bile ducts with a poor prognosis. It is anatomically subclassified into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC). ECC is further subclassified into perihilar CCA, also known as Klatskin tumors, and distal CCA (1,2). This classification has varied over time, with perihilar tumors being classified as ICC until the International Classification of Diseases (ICD) coding system (ICD-O-3) was adopted in 2001 (3,4). The reclassification of perihilar tumors as ECC poses challenges in characterizing epidemiologic trends and patterns of treatment utilization in the patients with ICC vs ECC.
ICC and ECC have been suspected to be distinct entities as early as Klatskin’s description of the hilar tumor in 1965 (5). This theory has been supported by reports of histologic heterogeneity, including disparate cells of origin (6,7). The recent focus on the molecular characterization of CCA has suggested even more heterogeneity in the pathogenesis of ICC and ECC and has provided insights into therapeutic targets unique to each subtype that may result in more tailored approaches to this disease.
Prior reports comparing the anatomic subtypes of CCA have not yet comprehensively examined differences in treatment utilization patterns in large studies (1,2,6-32). Surgical resection is the cornerstone of management for ICC and ECC, and although liver transplant is uncommonly used in CCA, it is considered more commonly in ECC than ICC. However, only a minority of patients are eligible for these approaches as most patients with CCA present with advanced stage disease. Liver-directed therapy (LDT) is sometimes used for locally advanced unresectable ICC, but it is less commonly used in ECC and usually palliatively in this setting (33-42). Most importantly, details regarding the patterns of use and efficacy of systemic therapies, particularly targeted and investigational therapies, in different subtypes of CCA are limited (43-54).
Single institution retrospective reports often contain comprehensive data but are restricted by small numbers of patients and capture practice patterns individual to the institution. Registry studies, such as those involving the Surveillance, Epidemiology, and End Results Program or National Cancer Database databases, involve large sample sizes but are often constrained by incomplete treatment data and variations in data reporting. We sought to overcome these limitations through a multi-institutional collaboration between high-volume CCA programs and through granular data collection using uniform definitions for data points. We placed a particular focus on patterns of use of systemic therapy in advanced ICC vs ECC, particularly targeted therapy and clinical trial enrollment.
Methods
Data collection
Patients were selected from institutional tumor registries using the ICD codes for CCA (ICD-9-155.1, 156.9, and 156.1 or ICD-10 C22.1, 24.9, 24.8, and 24.0) or from institutional databases of patients with CCA. Eligible patients were aged 18 years or older with a confirmed histologic diagnosis of CCA after June 2, 2009. This date was chosen because the ABC-02 data for gemcitabine and cisplatin were publicly presented and became the standard first-line treatment of advanced CCA on this date. Data were collected on demographics, risk factors, treatments, pathology, tumor genotyping, and survival outcomes through retrospective review of the electronic medical record. Self-reported demographics included age, gender, race (White, Black, Asian, or Other defined as not one of the above or not specific), and ethnicity. Medical oncologists with an expertise in hepatobiliary tumors performed quality control checks on the database. Eight institutions participated in this study: Massachusetts General Cancer Center, University of California San Francisco, MD Anderson Cancer Center, Mayo Clinic, Vanderbilt University, University of Virginia, Beth Israel Deaconess Medical Center, and St. Vincent’s Medical Center. The data were collected in accordance with individual institutional review board–approved protocols and in accordance with the Declaration of Helsinki.
Molecular profiling
Tumor tissue underwent molecular profiling as a routine part of clinical care using FoundationOne (55) or institutional platforms (Supplementary Table 1, available online).
Statistical analysis
A set of prespecified definitions were used across institutions for uniform data collection as per Supplementary Table 2 (available online). Data were extracted by medical trainees or trained research assistants, and attending medical oncologists adjudicated ambiguous cases. Fisher exact, χ2, and Wilcoxon rank sum tests were used for comparisons of independent variables. Statistical analyses were performed using Graph Pad Prism version 9 and Medcalc version 19.7, and multivariate analyses were performed with STATA17. A 2-sided test with a P value less than .05 was considered statistically significant. Recurrence-free survival (RFS), progression-free survival (PFS), and overall survival (OS) were calculated by Kaplan-Meier analysis, and log-rank sum tests were used to calculate P values between the cohorts. Patients who did not meet a particular endpoint at the time of last follow-up were censored for that endpoint.
Results
Baseline characteristics and clinical presentation
Among the 1039 patients screened, 847 were eligible, including 611 (72.1%) patients with ICC and 236 (27.9%) with ECC (Table 1). The median age for patients with ICC vs ECC was similar (63.3 vs 65.4 years, respectively; P = .23). Patients with ICC were more likely to be female (51.1% vs 41.9%; P = .02), and those with ECC were more likely to be male (58.1% vs 48.9%; P = .02). Patients with ICC more commonly had a history of chronic hepatitis B infection than patients with ECC (6.3% vs 0.8%; P = .02). No difference was seen in the frequency of other risk factors between the 2 groups.
Table 1.
Patient characteristics and presentation in intrahepatic cholangiocarcinoma vs extrahepatic cholangiocarcinoma
| Diagnosis (n = 847a) |
|||
|---|---|---|---|
| Characteristics | Intrahepatic cholangiocarcinoma (n = 611) | Extrahepatic cholangiocarcinoma (n = 236) | P |
| Age | |||
| ≤ 50 years old | 95 (15.5%) | 29 (12.3%) | .23 |
| > 50 years old | 516 (84.5%) | 207 (87.7%) | |
| Median (range) | 63.3 (22.5-92.5) | 65.4 (22.0-93.9) | |
| Gender | |||
| Male | 299 (48.9%) | 137 (58.1%) | .02 |
| Female | 312 (51.1%) | 99 (41.9%) | |
| Ethnicity | |||
| Hispanic or Latino | 19/242 (7.9%) | 13/101 (12.9%) | .15 |
| Non-Hispanic or Latino | 223/242 (92.1%) | 88/101 (87.1%) | |
| Unknown | 369 (60.4%) | 135 (57.2%) | |
| Race | |||
| Asian | 39/493 (7.9%) | 11/184 (6.0%) | |
| Black | 25/493 (5.1%) | 9/184 (4.9%) | |
| Other | 10/493 (2.0%) | 2/184 (1.1%) | |
| White | 419/493 (85.0%) | 162/184 (88.0%) | .18 |
| ECOG PS at Diagnosis | |||
| 0 | 65/175 (37.1%) | 21/52 (40.4%) | .89 |
| 1 | 94/175 (53.7%) | 27/52 (51.9%) | |
| 2 | 16/175 (9.1%) | 4/52 (7.7%) | |
| Risk Factors | |||
| Primary Sclerosing Cholangitis | 24/597 (4.0%) | 8/236 (3.4%) | .67 |
| Diabetes | 66/402 (16.4%) | 35/189 (18.5%) | .53 |
| Cirrhosis | 43/400 (10.8%) | 13/189 (6.9%) | .13 |
| Median BMI, kg/m2 | 27.3 (15.4-59.2) | 26.8 (17.6-65.8) | NE |
| Mean BMI, kg/m2 | 28.21 | 27.64 | .28 |
| BMI ≥ 30, kg/m2 | 159/477 (33.3%) | 50/190 (26.3%) | .08 |
| HBV | 17/272 (6.3%) | 1/121 (0.8%) | .02 |
| HCV | 29/486 (6.0%) | 6/198 (3.0%) | .11 |
| Presentation | |||
| Resected, resectable, or transplanted | 171 (28.0%) | 127 (53.8%) | <.00001 |
| Locally advanced | 139 (22.7%) | 54 (22.9%) | .97 |
| Primary metastatic | 301 (49.3%) | 55 (23.3%) | <.00001 |
| Metastatic Sites (Patients with Primary Metastatic Disease) | |||
| Liver | 205/222 (92.3%) | 24/41 (58.5%) | <.00001 |
| Lymph Node | 115/222 (51.8%) | 11/41 (26.8%) | .003 |
| Lung | 59/222 (26.6%) | 4/41 (9.8%) | .02 |
| Bone | 33/222 (14.9%) | 2/41 (4.9%) | .08 |
| Peritoneum | 27/222 (12.2%) | 13/41 (31.7%) | .001 |
| Other | 12/222 (5.4%) | 4/41 (9.8%) | .28 |
| Bilirubin | |||
| Median (range) | 0.6 (0.0-31.9) | 1.9 (0.2-35.0) | <.00001 |
| Bilirubin > 2 mg/dL | 61/460 (13.3%) | 83/172 (48.3%) | <.00001 |
| CA19-9, U/mL | |||
| Median (range) | 84 (0-364, 190) | 179.5 (0-22, 432) | .11 |
Denominators denote number of patients with known status of variable. BMI = body mass index; ECOG PS = Eastern Cooperative Oncology Group Performance Status; HBV = hepatitis B virus; HCV = hepatitis C virus; NE = not evaluated.
Stage at diagnosis and patterns of metastasis were also statistically significantly different between the ICC and ECC cohorts. Patients with ECC were more likely to present with early stage disease amenable to surgery or transplant (53.8% vs 28.0%; P < .00001), and those with ICC were more likely to present with metastatic disease (49.3% vs 23.3%; P < .00001). Patterns of metastasis also differed between the patients. ICC, when compared with ECC, was more likely to metastasize to the liver (92.3% vs 58.5%; P < .00001), lung (26.6% vs 9.8%; P = .02), and lymph nodes (51.8% vs 26.8%; P = .003) and less likely to metastasize to the peritoneum (31.7% vs 12.2%; P = .001). Multifocal liver involvement of ICC vs liver metastasis could not be distinguished.
Genomics
Genomic profiling data were available for 64.3% (n = 349/543) of patients with advanced ICC and 50.3% (n = 92/183) of patients with advanced ECC (P < .001) (Figure 1). Molecular alterations more commonly seen in ICC included FGFR2 fusions (21.0% vs 0%; P < .00001), any FGFR alteration (20.6% vs 6.0%; P = .002), and IDH1 mutations (20.3% vs 0%; P < .00001). Genomic alterations that were statistically significantly more likely to occur in patients with ECC included mutations in KRAS (37.1% vs 12.1%; P < .00001), TP53 (34.4% vs 20.2%; P = .004), SMAD4 (8.2% vs 2.0%; P = .003), and APC (8.2% vs 1.8%; P = .001) (Table 2).
Figure 1.
Study consort diagram. Of 1039 patients screened, 847 patients with cholangiocarcinoma (CCA) met eligibility for inclusion in the study, including 611 (72.1%) patients with intrahepatic cholangiocarcinoma (ICC) and 236 (27.9%) with extrahepatic cholangiocarcinoma (ECC). Molecular profiling was performed during routine clinical care in 64.3% of patients with advanced ICC and 50.3% of patients with advanced ECC. CCA-HCC = mixed cholangiocarcinoma-hepatocellular carcinoma.
Table 2.
Most common molecular alterations in intrahepatic cholangiocarcinoma vs extrahepatic cholangiocarcinoma
| Prevalence % (alteration present/known status) |
|||
|---|---|---|---|
| Molecular aberrationa | Intrahepatic cholangiocarcinoma | Extrahepatic cholangiocarcinoma | P |
| FGFR2 Fusion | 21.0 (48/229) | 0 (0/55) | <.00001 |
| Any FGFR alteration | 20.6 (64/311) | 6.0 (5/83) | .002 |
| IDH1 | 20.3 (70/344) | 0 (0/96) | <.001 |
| CDKN2A | 7.2 (25/348) | 5.1 (5/99) | .45 |
| HER2 Amplification | 6.8 (17/249) | 1.4 (1/73) | .07 |
| HER2 Mutation | 6.1 (20/329) | 2.2 (2/89) | .15 |
| ATM | 5.7 (13/221) | 6.6 (4/61) | .84 |
| ARID1A | 5.0 (11/222) | 3.2 (2/63) | .55 |
| PIK3CA | 4.9 (17/348) | 2.0 (2/99) | .21 |
| cMET | 4.6 (15/329) | 1.1 (1/88) | .14 |
| MAP2K1 or MAP3K1 | 3.2 (11/342) | 5.3 (5/95) | .35 |
| SMAD4 | 2.0 (7/342) | 8.2 (8/98) | .003 |
| APC | 1.8 (6/342) | 8.2 (8/97) | .001 |
| TP53 | 20.2 (69/342) | 34.4 (33/96) | .004 |
| KRAS | 12.1 (42/346) | 37.1 (36/97) | <.00001 |
Mutations with ≥5% frequency in either ICC or ECC were included for analysis. ICC = intrahepatic cholangiocarcinoma; ECC = extrahepatic cholangiocarcinoma; FGFR = fibroblast growth factor receptor; IDH1 = isocitrate dehydrogenase 1; CDKN2A = cyclin dependent kinase inhibitor 2A; HER2 = human epidermal growth factor receptor 2; ATM = ataxia-telangiectasia mutated; ARID1A = AT-rich interactive domain-containing protein 1A; PIK3CA = phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; cMET = mesenchymal epithelial transition factor; MAPK = mitogen-activated protein kinase kinase; SMAD4 = SMA- and MAD-related protein 4; APC = adenomatous polyposis coli; TP53 = tumor protein 53; KRAS = Kirsten rat sarcoma viral oncogene homolog.
We explored whether tissue availability explained the low rate of genomic profiling seen in patients with ECC. Molecular testing in patients with ECC is often complicated by nondiagnostic biopsies and inadequate tissue specimens because of limitations of brushings and fine needle aspirates (5). Thus, we evaluated patients who had tumor recurrence after resection where tissue specimens were expected to be of sufficient quantity and quality. Of the 76 patients with ECC with tumor recurrence after surgical resection, 49 (64.5%) had molecular profiling, similar to the overall profiling rate in patients with ICC. Data on attempted but failed genotyping efforts from patients with advanced ECC were not available.
Treatment patterns
Differences in presentation and genomics also led to notable differences in treatment patterns. Patients with ECC more commonly underwent surgical resection or transplant than those with ICC (55.1% vs 29.8%; P < .00001) (Table 3). Resected ICCs were statistically significantly larger than ECCs (tumor size ≥ 5 cm in 58.0% vs 5.3%; P < .00001), but no difference was seen between the groups in rates of margin or node positivity or lymphovascular invasion. Despite this, patients with ECC were statistically significantly more likely to receive some form of adjuvant therapy (58.3% vs 29.7%; P < .00001).
Table 3.
Treatment differences in early stage intrahepatic cholangiocarcinoma vs extrahepatic cholangiocarcinoma
| Diagnosis (n = 847a) |
|||
|---|---|---|---|
| Characteristics | Intrahepatic cholangiocarcinoma (n = 611) | Extrahepatic cholangiocarcinoma (n = 236) | P |
| Underwent surgery or transplant, yes | 182 (29.8%) | 130 (55.1%) | <.00001 |
| Of those who underwent surgery | |||
| Tumor Resection Pathology | |||
| Median tumor size of resected patients, cm | 5.5 (0.7-20.0) | 2.5 (0.1-7.2) | NE |
| Tumor size ≥ 5 cm | 91/157 (58.0%) | 6/114 (5.3%) | <.00001 |
| Node status N0 | 36/60 (60.0%) | 52/105 (49.5%) | .19 |
| Node status N1 | 24/60 (40.0%) | 51/105 (48.6%) | .29 |
| Node status N2 | 0/60 (0%) | 2/105 (1.9%) | .53 |
| Margin status R0 | 136/158 (86.1%) | 100/122 (82.0%) | .35 |
| Margin status R1 | 17/158 (10.8%) | 20/122 (16.4%) | .17 |
| Margin status R2 | 5/158 (3.2%) | 2/122 (1.6%) | .42 |
| LVI present | 61/104 (58.7%) | 69/101 (68.3%) | .15 |
| Adjuvant Therapy | |||
| Adjuvant therapy status known | 165/182 (90.7%) | 115/130 (88.5%) | NE |
| Treated with adjuvant therapy | 49/165 (29.7%) | 67/115 (58.3%) | <.00001 |
| Adjuvant chemotherapy only | 44/165 (26.7%) | 59/115 (51.3%) | <.00001 |
| Adjuvant radiation only | 12/165 (7.3%) | 50/115 (43.5%) | <.00001 |
| Adjuvant chemoradiation | 7/165 (4.2%) | 42/115 (36.5%) | <.00001 |
Denominators denote number of patients with known status of variable. NE = not evaluated; LVI = lymphovascular invasion.
Differences between the ICC and ECC cohorts were also seen in utilization of locoregional therapy and systemic therapy for unresectable and metastatic disease. Patients with advanced ICC more commonly received LDT (35.7% vs 17.9%; P = .00001), particularly radioembolization (22.2% vs 0%; P < .001), though patients with ECC were more likely to receive radiation to the liver (90.3% vs 56.8%; P = .0004) (Table 4). This may reflect the preponderance of data for intra-arterial therapies in ICC (34,38), whereas LDT data in ECC are mainly limited to scant reports on external beam radiation therapy and ablation for the management of malignant biliary tract obstruction (33,40,42,56). Similarly, patients with ICC more commonly received 3 or more lines of palliative chemotherapy (24.2% vs 14.7%; P = .03) and triplet first-line chemotherapy (9.0% vs 2.6%; P = .02), whereas patients with ECC were more likely to receive singlet first-line chemotherapy (21.6% vs 10.6%; P = .002). Patients with ECC were also more likely to receive 5-fluorouracil–based first-line palliative chemotherapy (24.1% vs 9.3%; P = .00003), whereas patients with ICC were more likely to receive gemcitabine-based first-line therapy (86.1% vs 69.8%; P = .00006).
Table 4.
Treatment differences in patients with unresectable or metastatic intrahepatic cholangiocarcinoma vs extrahepatic cholangiocarcinoma
| Diagnosis (n = 847a) |
|||
|---|---|---|---|
| Characteristics | Intrahepatic cholangiocarcinoma (n = 611) | Extrahepatic cholangiocarcinoma (n = 236) | P |
| Total No. of patients with advanced disease | |||
| Locally advanced, primary metastatic, recurrent metastatic | 543 (88.9%) | 183 (77.5%) | .00002 |
| Liver-directed therapy (LDT) in patients with advanced disease | |||
| Known status of LDT | 493/543 (90.8%) | 173/183 (94.5%) | .11 |
| Received LDT | 176/493 (35.7%) | 31/173 (17.9%) | .00001 |
| % of those receiving LDT | |||
| Liver radiation | 100 (56.8%) | 28 (90.3%) | .0004 |
| Extrahepatic radiation | 58 (33.0%) | 12 (38.7%) | .05 |
| Ablation | 19 (10.8%) | 4 (12.9%) | .73 |
| Radioembolization | 39 (22.2%) | 0 (0%) | .002 |
| Chemoembolization | 22 (12.5%) | 1 (3.2%) | .13 |
| Bland embolization | 4 (2.3%) | 1 (3.2%) | .75 |
| Irreversible electroporation | 8 (4.6%) | 0 (0%) | .61 |
| Palliative Systemic Chemotherapy in Patients with Advanced Disease | |||
| Known status of palliative systemic chemotherapy | 589/611 (96.4%) | 219/236 (92.8%) | .0249 |
| Treated with palliative systemic chemotherapy | 388/589 (65.9%) | 116/219 (53.0%) | .0008 |
| Median No. of lines of systemic therapy | 1 (0-7) | 1 (0-5) | NE |
| Mean No. of lines of systemic therapy | 1.6 | 1.1 | <.0001 |
| 1 line of systemic therapy | 154 (39.7%) | 41 (35.3%) | .40 |
| 2 lines of systemic therapy | 97 (25.0%) | 23 (19.8%) | .25 |
| ≥3 lines of systemic therapy | 94 (24.2%) | 17 (14.7%) | .03 |
| Singlet first line palliative therapy | 41 (10.6%) | 25 (21.6%) | .002 |
| Doublet first line palliative therapy | 310 (79.9%) | 84 (72.4%) | .09 |
| Triplet first line palliative therapy | 35 (9.0%) | 3 (2.6%) | .02 |
| Fluoropyrimidine-based first line palliative therapy | 36 (9.3%) | 28 (24.1%) | .00003 |
| Gemcitabine-based first line palliative therapy | 334 (86.1%) | 81 (69.8%) | .00006 |
| Fluoropyrimidine and Gemcitabine-based first line palliative therapy | 5 (1.3%) | 1 (0.9%) | .71 |
| Other | 11 (2.8%) | 5 (4.3%) | .43 |
| Targeted Therapy in Patients with Advanced Disease | |||
| Known status of targeted therapy | 520/543 (95.8%) | 170/183 (92.9%) | NE |
| Received targeted therapy | 98 (18.9%) | 8 (4.7%) | <.00001 |
| Clinical Trial in Patients with Advanced Disease | |||
| Known status of clinical trial | 520/543 (95.8%) | 170/183 (92.9%) | |
| Enrolled in a clinical trial | 129 (24.8%) | 18 (10.6%) | .00009 |
| Recurrence Free Survival, mo | |||
| All resected patients | 13.9 | 15.6 | .31 |
| First Progression Free Survival, mo | |||
| All patients who received first line Gemcitabine + Cisplatin | 4.53 | 6.0 | .38 |
| Second Progression Free Survival, mo | |||
| All patients who received second line mFOLFOX | 2.0 | 3.3 | .26 |
| Median Overall Survival, mo | |||
| All patients | 21.4 | 18.5 | .80 |
| From diagnosis of advanced disease | 15.1 | 11.8 | <.001 |
Denominators denote number of patients with known status of variable. NE = not evaluated.
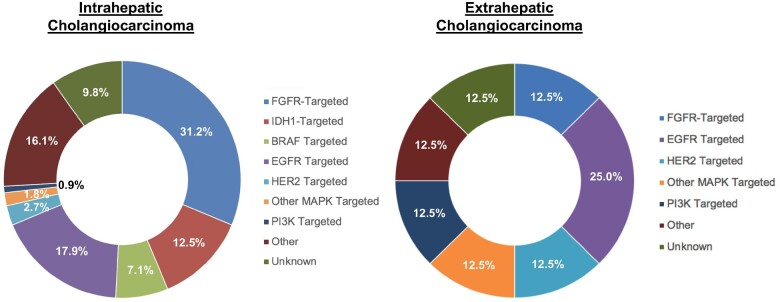
The most clinically significant differences between the ICC and ECC cohorts were in utilization patterns for clinical trials and targeted therapies. Patients with advanced ICC were statistically significantly more likely to receive targeted therapy (18.9% vs 4.7%; P < .00001) and/or participate in a clinical trial (24.8% vs 10.6%; P = .00009) (Table 4). Of the trials where the investigational agent was known, the majority was targeted therapy–based in both groups (64.6% in patients with ICC, 53.9% ECC), followed by immunotherapy-based (21.2% ICC, 38.5% ECC) (Supplementary Figure 1, available online). Patients with ICC who received targeted therapy most commonly received fibroblast growth factor receptor (FGFR)-, isocitrate dehydrogenase 1 (IDH1)-, and epidermal growth factor receptor (EGFR)–targeted therapy (31.2%, 12.5%, and 17.9%, respectively), whereas patients with ECC most commonly received EGFR–targeted (25.0%), followed by human epidermal growth factor receptor 2 (HER2)-, FGFR-, phosphatidylinositol-3 kinase (PI3K)-, and other mitogen-activated protein kinase (MAPK) alteration-targeted agents in 12.5% of patients each for each target (Figure 2). To investigate this further, we again studied the 76 patients with ECC whose disease recurred after surgical resection, of which only 7 (9.2%) enrolled on a clinical trial and only 2 (2.6%) received targeted therapy on or off trial. Of the patients who underwent genomic sequencing but did not receive targeted therapy, a majority (87.0%) of patients did not have actionable targets. Importantly, 35.0% of the patients with advanced ECC who did not receive targeted therapy or enroll into a clinical trial did receive second-line therapy or beyond, suggesting viable treatment options were needed.
Figure 2.
Profile of targeted therapy received in intrahepatic cholangiocarcinoma vs extrahepatic cholangiocarcinoma. Spectrum of targeted therapies received by patients with intrahepatic cholangiocarcinoma (ICC) and patients with extrahepatic cholangiocarcinoma (ECC) during the course of their clinical care. Patients with ICC primarily received FGFR-, EGFR- and IDH1-targeted therapies, and patients with ECC predominantly received EGFR-targeted therapy, followed equally by FGFR-, HER2-, PI3K-, IDH1-, BRAF-, and MAPK-targeted therapies. BRAF = v-raf murine sarcoma viral oncogene homolog B1; EGFR = epidermal growth factor receptor; FGFR = fibroblast growth factor receptor; IDH1 = isocitrate dehydrogenase 1; MAPK = mitogen-activated protein kinase; PI3K = phosphatidylinositol-3 kinase.
Outcomes on systemic therapy and prognosis
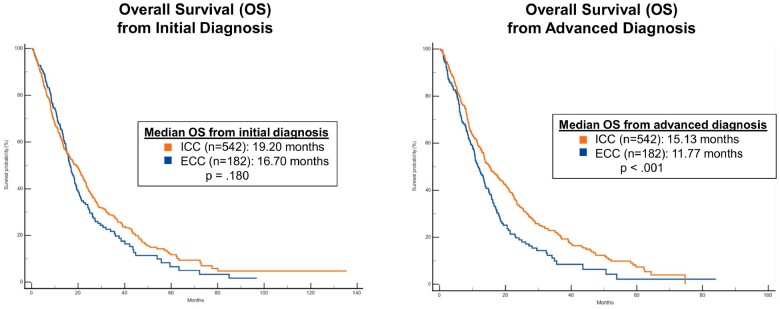
Direct comparisons of ICC and ECC with regard to clinical outcomes are limited (14,57). We compared outcomes on systemic therapy between the cohorts and found median OS from the time of diagnosis of advanced disease was longer in patients with ICC compared with ECC (15.1 vs 11.8 months; P < .001) (Figure 3). This difference was maintained when adjusting for several demographic, clinical, and molecular parameters (Supplementary Table 3, available online). No statistically significant difference in RFS after surgery was seen between the groups.
Figure 3.
Overall survival in intrahepatic cholangiocarcinoma vs extrahepatic cholangiocarcinoma. Overall survival (OS) in patients with intrahepatic cholangiocarcinoma (ICC) compared with patients with extrahepatic cholangiocarcinoma (ECC) from the time of diagnosis and from the time of diagnosis of advanced disease. Patients with ICC lived longer with advanced disease than patients with ECC.
Discussion
The advent of molecular profiling has renewed interest in understanding ICC and ECC as distinct entities beyond genomic signatures. This study is the first multicenter collaboration to examine the differences in treatment patterns between patients with ICC and ECC in a large, modern cohort of patients in granular detail. We identified several key findings: 1) patients with resected or transplanted ECC were more likely to receive adjuvant therapy, 2) patients with advanced ECC were less likely to receive palliative systemic chemotherapy, 3) patients with advanced ECC less commonly enrolled in a clinical trial or received targeted therapy, and 4) patients with advanced ECC had statistically significantly shorter survival than those with advanced ICC.
Many prior attempts at comparing management approaches in ICC and ECC have focused on the perioperative setting (2,28,47,58,59), however, the earlier stage at presentation for many patients with ECC introduces inherent bias (2,5). We confirmed this pattern of earlier stage presentation in ECC and also uncovered statistically significantly different rates of adjuvant therapy use in ECC and ICC. Patients with ECC were more likely to receive adjuvant therapy, although there were no statistically significant differences between the groups in pathology-based prognostic factors other than tumor size, which was greater in the ICC group. Historically, much of the data in support of adjuvant therapy in CCA was in patients with ECC (44,47,60-65), particularly findings from the Southwest Oncology Group (SWOG) S0809 study, which supported adjuvant chemoradiotherapy in patients with ECC or gallbladder carcinoma (66). In 2019, the phase III BILCAP trial also demonstrated a trend toward improved OS with adjuvant capecitabine in patients with ICC (hazard ratio [HR] = 0.65, 95% confidence interval [CI] = 0.35 to 1.18; P = .47) (60). Thus, rates of adjuvant therapy use in patients with ICC and with ECC may become more comparable over time. Notably, in spite of, or perhaps because of, the aforementioned differences in receipt of adjuvant treatment, there was no statistically significant difference in median RFS between the groups. Further investigation into novel perioperative approaches, including biomarkers predictive of benefit, holds the potential to improve outcomes for patients with CCA and save some patients unnecessary treatment.
Most patients with CCA present with advanced disease (2,22,28,29,51), and thus we performed a focused analysis on the use of systemic therapies. Patients with ECC were statistically significantly less likely than those with ICC to receive any palliative chemotherapy and less likely to receive multiple lines of therapy. Several prior studies have reported similar observations (67-69). A study by the Dutch Pancreatic Cancer Group demonstrated that although the use of palliative chemotherapy in patients with distal CCA improved over time, only 32.9% of patients received at least 1 line of palliative chemotherapy by 2016 (67). The limited use of systemic therapy in patients with ECC may in part be related to 1) worse nutritional and performance status due to patterns of metastasis (ie, high rates of multi-organ and peritoneal metastasis) (69-71) and 2) local tumor-related complications such as biliary obstruction leading to elevated liver function tests and recurrent cholangitis (2,5,35,70,72,73).
The rate of incorporation of targeted therapies and clinical trials into treatment was encouraging in our population, but both were lowest for the patients with ECC. This higher trial accrual rate in ICC vs ECC appears to be a widespread problem. In the European MOSCATO-01 trial of molecularly matched targeted therapies where 4.0% of the 1035-patient study population had biliary tract cancer (BTC), a majority (67.0%) of them had ICC (54). Further, in a single-institution study, of the 40 patients with BTC enrolled on a phase I trial, only 4 (10.0%) had ECC as compared with 30 (75.0%) patients with ICC (49). Low rates of trial enrollment among patients with ECC may be related to the aforementioned barriers to receiving systemic therapy and also to lower rates of molecular profiling, a lack of actionable genomic alterations, and poor access to suitable trials for rare targets or biomarker-agnostic trials.
Several potential avenues exist to directly address these problems. More than half of the patients with advanced ECC did not have molecular profiling performed, limiting the potential for employing targeted therapy and clinical trials. Encouragingly, patients who had tumor recurrence after surgery had rates of profiling on par with those seen in ICC, suggesting adequate quantity and quality of tissue is a relevant challenge. Cholangioscopy-guided forceps biopsy can offer more adequate tissue acquisition than brushings alone (74,75) in those without surgical tissue. Further, the use of circulating tumor DNA for molecular profiling has shown similar capture rates for actionable alterations as tissue genotyping in BTC (76-79), providing a rapid, noninvasive approach to identifying patients for molecularly matched therapy. Additionally, tissue-agnostic basket trials such as the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) and American Society of Clinical Oncology (ASCO) Targeting Agent and Profiling Utilization Registry (TAPUR) trials may offer access to trial therapies for uncommon cancers with uncommon targets. Given most CCAs do not harbor targets that are currently actionable, clinical trials of biomarker-agnostic therapies are also key to improving outcomes for these patients. Finally, patients with CCA can benefit from advances in other cancers with overlapping molecular profiles. For example, 37.0% of patients with ECC in our study had KRAS alterations. Recent advances in targeting KRAS G12C in lung and colorectal cancer (80-82), and ongoing trials targeting other KRAS variants, open the door for CCA-specific investigations.
Patients with advanced ECC had a worse survival than those with ICC in our study, and the contribution of the treatment pattern differences noted herein requires further study. Notably, the difference in survival was not accounted for by controlling for known positive and negative prognostic mutations, such as FGFR2 fusions found at higher frequencies in ICC and associated with improved survival (16,52,83) and TP53 or KRAS mutations found at higher frequencies in ECC and associated with worse survival (16,84). This disparity persisted on multivariate analysis when controlling for differences in utilization of potentially life-prolonging palliative therapy, however, unknown factors such as recurrent jaundice and cholangitic sepsis due to biliary obstruction may have contributed as main causes of morbidity and mortality in patients with ECC (85-89).
A limitation of our study is the patient population was limited to those evaluated primarily by medical oncologists at academic cancer centers, potentially limiting the generalizability of our findings to the broader CCA population. Although these patients may have been more likely to seek out clinical trials or undergo molecular profiling, this should not appreciably impact the differences we noted between patients with different anatomic subtypes of CCA. Additionally, given the retrospective data collection, selection bias and incomplete data availability may have impacted the findings. We aimed to minimize their impact by performing a large study across multiple institutions and limiting our analyses to variables with enough known values to achieve sufficient power to calculate statistically significant differences.
Taken together, our findings suggest a critical need for profiling approaches that perform well in the setting of scant tissue obtained in CCA, education of clinicians and patients regarding the potential of molecular profiling to open up therapeutic avenues, and development of drugs for currently actionable and nonactionable genomic alterations and that are biomarker and molecularly agnostic. Lastly, further investigation into the factors contributing to the difference in survival between patients with ICC and ECC has the potential to inform treatment decision making. Ultimately, our study suggests nuanced differences between ICC and ECC beyond anatomic location that warrant treatment of them as distinct entities.
Supplementary Material
Acknowledgements
We would like to acknowledge support from the Cholangiocarcinoma Foundation (CCF), the International Cholangiocarcinoma Research Network (ICRN), Jacqui Lewis and the Rare Initiative, and Joe and Katie Comeau.
Contributor Information
Kristen Spencer, Department of Medicine, New York University Langone Health Perlmutter Cancer Center, New York University School of Medicine, New York, NY, USA.
Leontios Pappas, Department of Medicine, Mass General Cancer Center, Harvard Medical School, Boston, MA, USA.
Islam Baiev, Department of Medicine, Mass General Cancer Center, Harvard Medical School, Boston, MA, USA.
Jordan Maurer, Department of Medicine, Mass General Cancer Center, Harvard Medical School, Boston, MA, USA.
Andrea Grace Bocobo, Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, CA, USA.
Karen Zhang, Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, CA, USA.
Apurva Jain, Division of Cancer Medicine, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Anaemy Danner De Armas, Division of Cancer Medicine, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Stephanie Reyes, Duke University School of Medicine, Durham, NC, USA.
Tri Minh Le, Department of Medicine, University of Virginia Comprehensive Cancer Center, Charlottesville, VA, USA.
Osama E Rahma, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Jennifer Stanton, Department of Medicine, Mass General Cancer Center, Harvard Medical School, Boston, MA, USA.
Thomas T DeLeon, Division of Hematology/Oncology, Mayo Clinic, Scottsdale, AZ, USA.
Marc Roth, Department of Medical Oncology, St. Luke’s Cancer Institute, Kansas City, MO, USA.
Mary Linton B Peters, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Andrew X Zhu, Jiahui International Cancer Center, Jiahui Health, Shanghai, China.
Jochen K Lennerz, Center for Integrated Diagnostics, Department of Pathology, Massachusetts General Hospital/Harvard Medical School, Boston, MA, USA.
A John Iafrate, Center for Integrated Diagnostics, Department of Pathology, Massachusetts General Hospital/Harvard Medical School, Boston, MA, USA.
Kylie Boyhen, Yale University, New Haven, CT, USA.
Christine VanCott, Department of Medicine, HCA Florida South Tampa Hospital, Tampa, FL, USA.
Lewis R Roberts, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Stacie Lindsey, Cholangiocarcinoma Foundation, Herriman, UT, USA.
Nora Horick, Department of Medicine, Mass General Cancer Center, Harvard Medical School, Boston, MA, USA.
Laura Williams Goff, Department of Medicine, Vanderbilt-Ingram Cancer Center, Nashville, TN, USA.
Kabir Mody, Division of Hematology/Oncology, Mayo Clinic, Jacksonville, FL, USA.
Mitesh J Borad, Division of Hematology/Oncology, Mayo Clinic, Scottsdale, AZ, USA.
Rachna T Shroff, University of Arizona Cancer Center, University of Arizona, Tucson, AZ, USA.
Robin Kate Kelley, Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, CA, USA.
Milind M Javle, Division of Cancer Medicine, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Lipika Goyal, Department of Medicine, Mass General Cancer Center, Harvard Medical School, Boston, MA, USA.
Data availability
Individual participant data cannot be shared publicly due to our institutions’ ethical regulations, aiming to protect the privacy of individuals that participated in the study. All source data is available and will be archived at Massachusetts General Cancer Center. The data sharing of individual patient data from each participating institution will be subject to the policy and procedures of each institution. Data requests will be honored pending approval of the institutional review board of each institution in accordance with appropriate human subjects protections.
Author contributions
Kristen Spencer, DO, MPH (Conceptualization; Methodology; Project administration; Writing—original draft; Writing—review & editing); Robin Kate Kelley, MD (Writing—review & editing); Rachna T. Shroff, MD (Writing—review & editing); Mitesh J. Borad, MD (Writing—review & editing); Kabir Mody, MD (Writing—review & editing) Laura Williams Goff, MD, MSCI, MMHC (Writing—review & editing); Nora Horick, MS (Formal analysis; Writing—review & editing); Stacie Lindsey, BS (Writing—review & editing); Lewis R. Roberts, MB ChB, PhD (Writing—review & editing); Christine VanCott, MD, FACS, CPE (Writing—review & editing); Kylie Boyhen, CCRP (Data curation; Writing—review & editing); A. John Iafrate, MD, PhD (Writing—review & editing); Jochen K. Lennerz, MD, PhD (Writing—review & editing); Andrew X. Zhu, MD, PhD (Writing—review & editing); Mary Linton B. Peters, MD (Writing—review & editing); Marc Roth, MD (Writing—review & editing); Thomas T. DeLeon, MD (Writing—review & editing); Jennifer Stanton, BS (Data curation; Writing—review & editing); Osama E. Rahma, MD (Writing—review & editing); Tri Minh Le, MD (Data curation; Writing—review & editing); Stephanie Reyes, MD (Data curation; Writing—review & editing); Anaemy Danner De Armas, MPH (Data curation; Writing—review & editing); Apurva Jain, BS (Data curation; Writing—review & editing); Karen Zhang, BS (Data curation; Writing—review & editing); Andrea Grace Bocobo, BS (Data curation; Writing—review & editing); Jordan Maurer, BS, MS (Data curation; Formal analysis; Writing—review & editing); Islam Baiev, BS (Data curation; Formal analysis; Writing—review & editing); Leontios Pappas, MD, PhD (Data curation; Formal analysis; Methodology; Writing—review & editing); Milind M. Javle, MD (Writing—review & editing); and Lipika Goyal, MD, MPhil (Conceptualization; Methodology; Writing—review & editing).
Funding
No funding was provided for this project.
Conflicts of interest
KS reports advisory/consultancy: QED Therapeutics, Helsinn, the Lynx Group, and Caris Life Sciences.
LP no disclosures. IB no disclosures. JM no disclosures. AGB no disclosures. KZ no disclosures. AJ no disclosures. ADD no disclosures. SR no disclosures. TML no disclosures.
OER reports research support from Merck. Speaker for activities supported by educational grants from BMS and Merck. Consultant for Merck, Celgene, Five Prime, GSK, Bayer, Roche/Genentech, Puretech, Imvax, Sobi, Boehringer Ingelheim. In addition, Dr Rahma has patent “Methods of using pembrolizumab and trebananib” pending.
JS no disclosures. TTD no disclosures. MR no disclosures.
MLBP institutional funding from Taiho, AstraZeneca, Exelixis, BeiGene, Berg, Merck, Bayer, Nucana, Lilly, and Helsinn Therapeutics; personal consulting fees from Agios. MLBP reports National Cancer Institute (K08CA248473).
AXZ reports advisory/consultancy: Sanofi-aventis, Lilly, Merck, Eisai, Sirtex. Employment: I-MAB Biopharma.
JKL no disclosures.
JKL reports National Institutes of Health (NIH) (R37 CA225655).
AJI no disclosures. KB no disclosures. CV no disclosures.
LRR reports research funding (to their institution) from Bayer, Boston Scientific, Exact Sciences, Fujifilm Medical Sciences, Gilead Sciences, Glycotest Inc, RedHill Biopharma, TARGET PharmaSolutions, and QED Therapeutics; and service on scientific advisory committees or as a consultant for AstraZeneca, Bayer, Eisai, Exact Sciences, Gilead Sciences, Global Life Science Consulting, GRAIL Inc, Hepion, MedEd Design LLC, Medscape, Novartis Venture Fund, Pontifax, Roche, and The Lynx Group.
SL no disclosures. NH no disclosures.
LWG reports advisory/consultancy: QED, Genentech, Merck, AstraZeneca, Exelixis, Boehringer Ingelheim. Research Funding: BMS, Agios, ASLAN, BeiGene, Basilea, Merck.
KM reports Stock and Other Ownership Interests: CytoDyn; Consulting or Advisory Role: Celgene, Genentech/Roche, AstraZeneca, Ipsen, Boston Scientific, Incyte (Inst), QED Therapeutics; Research Funding: FibroGen (Inst), Senhwa Biosciences (Inst), MedImmune (Inst), Agios (Inst), ArQule (Inst), Taiho Pharmaceutical (Inst), Gritstone Bio (Inst), Incyte (Inst), Merck (Inst), Vyriad (Inst), Turnstone Bio (Inst), AstraZeneca (Inst), Basilea (Inst).
MB no relevant disclosures.
RTS reports funding from Bayer, Bristol-Myers Squibb, Exelixis, IMV Inc, Loxo, Novocure, NUCANA, Pieris, QED Therapeutics, Rafael Pharmaceuticals, Seagen, and Taiho.
honoraria for serving on scientific advisory committees or as a consultant from AstraZeneca, Boehringer Ingelheim Pharma, CAMI, Clovis, Genentech, Incyte, Merck, QED Therapeutics, Servier, Taiho, Zymeworks, and Syros.
RKK reports research funding (to institution) from Agios, Astra Zeneca, Bayer, BMS, Eli Lilly, EMD Serono, Exelixis, Genentech/Roche, Loxo Oncology, Merck, Novartis, Partner Therapeutics, QED, Relay Therapeutics, Surface Oncology, Taiho; honoraria for serving on scientific advisory committees or as a consultant from Agios, Astra Zeneca, Exelixis, Ipsen, Merck (to institution) and from Exact Sciences, Gilead, and Kinnate (to self).
MMJ reports research funding (to institution) from Merck, EMD Serono, Novartis, Eli Lilly, Astra Zeneca, Genentech, Transthera, Meclun, BMS, Incyte, QED, Taiho, Servier, Oncosil, Basilea, Nucana and to self or as advisory board/DSMB member from Incyte, Zymeworks, Mundi Pharma, Nucana, MORE health and Origimed.
LG reports research funding (to their institution) from Adaptimmune, Bayer, Merck, Macrogenics, Genentech, Novartis, Incyte, Loxo Oncology, Relay Therapeutics, QED, Taiho Oncology, Leap Therapeutics, Bristol Myers Squibb, Nucana, and Servier; honoraria (to self) for serving on scientific advisory committees or as a consultant from Alentis Therapeutics AG, Black Diamond, Basilea, Genentech, Exelixis, Kinnate, H3Biomedicine, Incyte Corporation, QED Therapeutics, Sirtex Medical Ltd, The Servier Group, SIRTEX, Taiho Oncology, TranstheraBio, and participation on data safety monitoring boards for AstraZeneca. LG receives funding from the American Cancer Society Clinical Scientist Development Grant 134013‐CSDG‐19‐163‐01‐TBG, the NIH/NCI Gastrointestinal Cancer SPORE P50 CA127003, V Foundation for Cancer Research Translational Grant, and the Cholangiocarcinoma Foundation Andrea Marie Fuquay Research Fellowship.
References
- 1. Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472-477. [DOI] [PubMed] [Google Scholar]
- 2. Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463-473. discussion 473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56(4):848-854. [DOI] [PubMed] [Google Scholar]
- 4. Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98(12):873-875. [DOI] [PubMed] [Google Scholar]
- 5. Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38(2):241-256. [DOI] [PubMed] [Google Scholar]
- 6. Bragazzi MC, Ridola L, Safarikia S, et al. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann Gastroenterol. 2018;31(1):42-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cardinale V, Carpino G, Reid L, et al. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J Gastrointest Oncol. 2012;4(5):94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahn DH, Bekaii-Saab T.. Biliary cancer: intrahepatic cholangiocarcinoma vs. extrahepatic cholangiocarcinoma vs. gallbladder cancers: classification and therapeutic implications. J Gastrointest Oncol. 2017;8(2):293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142(4):1021-1031 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antwi SO, Mousa OY, Patel T.. Racial, ethnic, and age disparities in incidence and survival of intrahepatic cholangiocarcinoma in the United States; 1995-2014. Ann Hepatol. 2018;17(4):604-614. [DOI] [PubMed] [Google Scholar]
- 11. Bertuccio P, Bosetti C, Levi F, et al. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24(6):1667-1674. [DOI] [PubMed] [Google Scholar]
- 12. Burke EC, Jarnagin WR, Hochwald SN, et al. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228(3):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cardinale V, Semeraro R, Torrice A, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: new insight into epidemiology and risk factors. World J Gastrointest Oncol. 2010;2(11):407-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gatto M, Alvaro D.. Cholangiocarcinoma: risk factors and clinical presentation. Eur Rev Med Pharmacol Sci. 2010;14(4):363-367. [PubMed] [Google Scholar]
- 16. Javle M, Bekaii-Saab T, Jain A, et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer. 2016;122(24):3838-3847. [DOI] [PubMed] [Google Scholar]
- 17. Khan SA, Tavolari S, Brandi G.. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39(suppl 1):19-31. [DOI] [PubMed] [Google Scholar]
- 18. Khan SA, Taylor-Robinson SD, Toledano MB, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37(6):806-813. [DOI] [PubMed] [Google Scholar]
- 19. Kirstein MM, Vogel A.. Epidemiology and risk factors of cholangiocarcinoma. Visc Med. 2016;32(6):395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mosadeghi S, Liu B, Bhuket T, et al. Sex-specific and race/ethnicity-specific disparities in cholangiocarcinoma incidence and prevalence in the USA: an updated analysis of the 2000-2011 Surveillance, Epidemiology and End Results registry. Hepatol Res. 2016;46(7):669-677. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003-1010. [DOI] [PubMed] [Google Scholar]
- 22. Patel N, Benipal B.. Incidence of cholangiocarcinoma in the USA from 2001 to 2015: a US cancer statistics analysis of 50 states. Cureus. 2019;11(1):e3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saha SK, Zhu AX, Fuchs CS, et al. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaib Y, El-Serag HB.. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115-125. [DOI] [PubMed] [Google Scholar]
- 26. Shaib YH, El-Serag HB, Davila JA, et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128(3):620-626. [DOI] [PubMed] [Google Scholar]
- 27. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. [DOI] [PubMed] [Google Scholar]
- 28. Singal AG, Rakoski MO, Salgia R, et al. The clinical presentation and prognostic factors for intrahepatic and extrahepatic cholangiocarcinoma in a tertiary care centre. Aliment Pharmacol Ther. 2010;31(6):625-633. [DOI] [PubMed] [Google Scholar]
- 29. Surveillance, Epidemiology, and End Results (SEER) Program Database. Cancer stat facts: liver and intrahepatic bile duct cancer. 2018. https://seer.cancer.gov/statfacts/html/livibd.html. Accessed December 12, 2019.
- 30. Taylor-Robinson SD, Toledano MB, Arora S, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48(6):816-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5(10):1221-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. West J, Wood H, Logan RF, et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94(11):1751-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alis H, Sengoz C, Gonenc M, et al. Endobiliary radiofrequency ablation for malignant biliary obstruction. Hepatobiliary Pancreat Dis Int. 2013;12(4):423-427. [DOI] [PubMed] [Google Scholar]
- 34. Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111(2):213-220. [DOI] [PubMed] [Google Scholar]
- 35. Boulay BR, Birg A.. Malignant biliary obstruction: from palliation to treatment. World J Gastrointest Oncol. 2016;8(6):498-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brunner TB, Blanck O, Lewitzki V, et al. Stereotactic body radiotherapy dose and its impact on local control and overall survival of patients for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. Radiother Oncol. 2019;132:42-47. [DOI] [PubMed] [Google Scholar]
- 37. Gkika E, Hallauer L, Kirste S, et al. Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer. 2017;17(1):781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20(12):3779-3786. [DOI] [PubMed] [Google Scholar]
- 39. Jung DH, Kim MS, Cho CK, et al. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat Oncol J. 2014;32(3):163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohnishi H, Asada M, Shichijo Y, et al. External radiotherapy for biliary decompression of hilar cholangiocarcinoma. Hepatogastroenterology. 1995;42(3):265-268. [PubMed] [Google Scholar]
- 41. Shinohara ET, Mitra N, Guo M, et al. Radiotherapy is associated with improved survival in adjuvant and palliative treatment of extrahepatic cholangiocarcinomas. Int J Radiat Oncol Biol Phys. 2009;74(4):1191-1198. [DOI] [PubMed] [Google Scholar]
- 42. Steel AW, Postgate AJ, Khorsandi S, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73(1):149-153. [DOI] [PubMed] [Google Scholar]
- 43. Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doherty MK, Knox JJ.. Adjuvant therapy for resected biliary tract cancer: a review. Chin Clin Oncol. 2016;5(5):64. [DOI] [PubMed] [Google Scholar]
- 45. Doherty B, Nambudiri VE, Palmer WC.. Update on the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19(1):2. [DOI] [PubMed] [Google Scholar]
- 46. Anderson C, Kim R.. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer Treat Rev. 2009;35(4):322-327. [DOI] [PubMed] [Google Scholar]
- 47. Heron DE, Stein DE, Eschelman DJ, et al. Cholangiocarcinoma: the impact of tumor location and treatment strategy on outcome. Am J Clin Oncol. 2003;26(4):422-428. [DOI] [PubMed] [Google Scholar]
- 48. Shirabe K, Shimada M, Harimoto N, et al. Intrahepatic cholangiocarcinoma: its mode of spreading and therapeutic modalities. Surgery. 2002;131(suppl 1):S159-64. [DOI] [PubMed] [Google Scholar]
- 49. Subbiah IM, Subbiah V, Tsimberidou AM, et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: eliciting early response signals from phase 1 trials. Oncotarget. 2013;4(1):156-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sulpice L, Rayar M, Boucher E, et al. Treatment of recurrent intrahepatic cholangiocarcinoma. Br J Surg. 2012;99(12):1711-1717. [DOI] [PubMed] [Google Scholar]
- 51. Valle JW. Advances in the treatment of metastatic or unresectable biliary tract cancer. Ann Oncol. 2010;21(suppl 7):vii345-vii348. [DOI] [PubMed] [Google Scholar]
- 52. Valle JW, Lamarca A, Goyal L, et al. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7(9):943-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vauthey JN, Blumgart LH.. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14(2):109-114. [DOI] [PubMed] [Google Scholar]
- 54. Verlingue L, Malka D, Allorant A, et al. Precision medicine for patients with advanced biliary tract cancers: an effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer. 2017;87:122-130. [DOI] [PubMed] [Google Scholar]
- 55. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuvshinoff BW, Armstrong JG, Fong Y, et al. Palliation of irresectable hilar cholangiocarcinoma with biliary drainage and radiotherapy. Br J Surg. 1995;82(11):1522-1525. [DOI] [PubMed] [Google Scholar]
- 57. Waseem D, Tushar P.. Intrahepatic, perihilar and distal cholangiocarcinoma: management and outcomes. Ann Hepatol. 2017;16(1):133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baton O, Azoulay D, Adam DV, et al. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg. 2007;204(2):250-260. [DOI] [PubMed] [Google Scholar]
- 59. Hanazaki K, Kajikawa S, Shimozawa N, et al. Prognostic factors of intrahepatic cholangiocarcinoma after hepatic resection: Univariate and multivariate analysis. Hepatogastroenterology. 2002;49(44):311-316. [PubMed] [Google Scholar]
- 60. Primrose JN, Fox RP, Palmer DH, et al. ; for the BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663-673. [DOI] [PubMed] [Google Scholar]
- 61. Hughes MA, Frassica DA, Yeo CJ, et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys. 2007;68(1):178-182. [DOI] [PubMed] [Google Scholar]
- 62. Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934-1940. [DOI] [PubMed] [Google Scholar]
- 63. Kim S, Kim SW, Bang YJ, et al. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2002;54(2):414-419. [DOI] [PubMed] [Google Scholar]
- 64. Kim TH, Han SS, Park SJ, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):e853-e859. [DOI] [PubMed] [Google Scholar]
- 65. Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565-574. [DOI] [PubMed] [Google Scholar]
- 66. Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: a phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol. 2015;33(24):2617-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Strijker M, Belkouz A, van der Geest LG, et al. ; for the Dutch Pancreatic Cancer Group. Treatment and survival of resected and unresected distal cholangiocarcinoma: a nationwide study. Acta Oncol. 2019;58(7):1048-1055. [DOI] [PubMed] [Google Scholar]
- 68. Roos E, Strijker M, Franken LC, et al. Comparison of short- and long-term outcomes between anatomical subtypes of resected biliary tract cancer in a Western high-volume center. HPB (Oxford). 2020;22(3):405-414. [DOI] [PubMed] [Google Scholar]
- 69. Wang J, Bo X, Nan L, et al. Landscape of distant metastasis mode and current chemotherapy efficacy of the advanced biliary tract cancer in the United States, 2010-2016. Cancer Med. 2020;9(4):1335-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: Implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689-1700. [DOI] [PubMed] [Google Scholar]
- 71. Sallinen V, Siren J, Makisalo H, et al. Differences in prognostic factors and recurrence patterns after curative-intent resection of perihilar and distal cholangiocarcinomas. Scand J Surg. 2020;109(3):219-227. [DOI] [PubMed] [Google Scholar]
- 72. Ercolani G, Dazzi A, Giovinazzo F, et al. Intrahepatic, peri-hilar and distal cholangiocarcinoma: three different locations of the same tumor or three different tumors? Eur J Surg Oncol. 2015;41(9):1162-1169. [DOI] [PubMed] [Google Scholar]
- 73. Yang LC, Shi HY, Huang JW, et al. Biliary stenting for unresectable cholangiocarcinoma: a population-based study of long-term outcomes and hospital costs in Taiwan. Kaohsiung J Med Sci. 2015;31(7):370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015;82(4):608-614 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012;75(2):347-353. [DOI] [PubMed] [Google Scholar]
- 76. Pascual J, Attard G, Bidard FC, et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2022;33(8):750-768. [DOI] [PubMed] [Google Scholar]
- 77. Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med. 2020;26(12):1859-1864. [DOI] [PubMed] [Google Scholar]
- 78. Berchuck JE, Facchinetti F, DiToro DF, et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann Oncol. 2022;33(12):1269-1283. [DOI] [PubMed] [Google Scholar]
- 79. Mody K, Kasi PM, Yang J, et al. Circulating tumor DNA profiling of advanced biliary tract cancers. J Clin Oncol Precis Oncol. 2019;3:1-9. [DOI] [PubMed] [Google Scholar]
- 80. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fakih MG, Kopetz S, Kuboki Y, et al. Sotorasib for previously treated colorectal cancers with KRAS(G12C) mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23(1):115-124. [DOI] [PubMed] [Google Scholar]
- 82. Janne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non-small-cell lung cancer harboring a KRAS(G12C) mutation. N Engl J Med. 2022;387(2):120-131. [DOI] [PubMed] [Google Scholar]
- 83. Mertens JC, Rizvi S, Gores GJ.. Targeting cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 pt B):1454-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Montal R, Sia D, Montironi C, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73(2):315-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mosconi S, Beretta GD, Labianca R, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69(3):259-270. [DOI] [PubMed] [Google Scholar]
- 86. Patel T. Cholangiocarcinoma–controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8(4):189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lamarca A, Benafif S, Ross P, et al. Cisplatin and gemcitabine in patients with advanced biliary tract cancer (ABC) and persistent jaundice despite optimal stenting: effective intervention in patients with luminal disease. Eur J Cancer. 2015;51(13):1694-1703. [DOI] [PubMed] [Google Scholar]
- 89. Elganainy D, Holliday EB, Taniguchi CM, et al. Dose escalation of radiotherapy in unresectable extrahepatic cholangiocarcinoma. Cancer Med. 2018;7(10):4880-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data cannot be shared publicly due to our institutions’ ethical regulations, aiming to protect the privacy of individuals that participated in the study. All source data is available and will be archived at Massachusetts General Cancer Center. The data sharing of individual patient data from each participating institution will be subject to the policy and procedures of each institution. Data requests will be honored pending approval of the institutional review board of each institution in accordance with appropriate human subjects protections.