Abstract
Concurrent exercise and metformin administration may reduce the acute and chronic effects of exercise on glucose metabolism in the patients with type 2 diabetes (T2D). However, several studies suggest that combing metformin and exercise treatment may have neither additive effect nor even cause adverse effects in T2D patients. This case report aimed to highlight the challenges associated with prescribing exercise to type 2 diabetes patients undergoing metformin treatment. A 67-years old woman was followed-up for five months, including assessment of the acute and chronic glucose and lactate metabolism induced by concomitant exercise and metformin. The findings were four-fold: 1) During a high-intensity interval training bout, blood glucose systematically decreased, while blood lactate concentrations fluctuated randomly; 2) Basal blood lactate levels were well above 2 mmol/L on days with medication only; 3) Combined exercise and metformin administration induced additive effects on the normalization of glucose and 4) high levels of physical activity had a positive impact on the continuous glucose fluctuations, while decreased levels of physical activity induced a large fluctuation of glucose due to home confinement of an infectious disease caused by the SARS-CoV-2 virus. Our findings showed that when combined with exercise and metformin treatment for T2D patients, exercise may contribute to improving glycemic control while metformin may elevate lactate levels in the long term. The observed results underline the need to prescribe exercise and monitor lactate levels for reducing possible risks associated with metformin treatment and reinforce the importance of tailoring exercise therapy.
Keywords: Exercise medicine, Hyperlactatemia, Blood glucose, Exercise intervention, Case report
Abbreviation
- T2D
type 2 diabetes
- COVID-19
Coronavirus disease
- HOMA-IR
homeostatic model assessment of insulin resistance
- HIIT
high intensity interval training
- NoMed/Ex
neither medication nor exercise
- Med
only medication
- Med/Ex
medication + exercise
- CGM
continuous glucose monitoring system
- TIR
time in range
- TBR
time below range
- TAR
time above range
- PA
physical activity
Introduction
The treatment of type 2 diabetes (T2D) is multivariate, commonly including medical therapies as well as lifestyle interventions, such as physical exercise and dietary modifications.1 However, concurrent exercise and metformin administration may blunt the acute2,3 and chronic effects of exercise on glucose metabolism.4,5 Furthermore, due to the impaired aerobic-oxidative capacity and lactate transport, some T2D patients exhibit increased lactate levels, which are related to the manifestation and progression of insulin resistance.6 Although the risk of developing lactic acidosis is rare, but it is associated with mortality in severely ill diabetes patients admitted to intensive care units.7, 8, 9 In addition, metformin may further increase basal blood lactate concentrations, while exercise may contribute to reducing lactate production and improving lactate transport and clearance, but few studies have shown reduced basal lactate levels in T2DM patients following exercise training.6 Consequently, prescribing exercise to patients undergoing metformin treatment requires careful consideration of benefits but also associated risks. Especially the latter has not yet received thorough scientific attention. Thus, this case report aims to encourage scientific discourse and evoke future studies in T2D patients treated with metformin focusing on the following key elements: 1) Assessment of the acute effects of exercise on glucose and lactate in patients treated with metformin; 2) Assessment of changes in basal glucose and lactate concentrations in patients with and without medication and exercise; 3) Long-term changes of continuous glucose concentrations alongside with changes in physical activity levels during home confinement due to an infectious disease caused by the SARS-CoV-2 virus (COVID-19).
Materials and methods
The patient was a 67-years old woman. She was diagnosed with type 2 diabetes in 2009. Her medication was prescribed by her physician and did not change during the past 5 years. The prescribed daily dose of metformin was 1 500 mg/day (Metforem, Orion Oyj Orion Pharma, Finland; each tablet contains 750 mg of metformin hydrochloride), ingested in two daily doses of 750 mg/day concomitantly with breakfast and dinner, respectively. In addition, she was prescribed 100 mg sitagliptin (Januvia-sitagliptin, Mrck Sharp & Dohme Corp., Finland), that was administered in the morning with breakfast and she received statins in order to reduce blood lipids (10 mg, administered in the evening; Atorvastatin, Orion, Sun Pharmaceutical Industries Europe B.V., Finland). Body weight was maintained at 51 kg and the body mass index was 20.4 kg⋅m−2 throughout the study period. On the basis of self-estimates and smart band records (the patient's heart rate ranged from 52 to 135 times/min monthly from 2020 to 2022), the physical activity level was considered stable and characterized as moderate. The average daily steps ranged from 6 000 to 8 000 steps per day. The participant did not perform any form of HIIT before the start of the study.
After initial assessments, the patient was followed up over a period of 4 months. The case study design is summarized in Fig. 1. A fasting blood sample was collected at the beginning and the end of the study to assess biomarkers that characterize the patient. These markers included lipid and glucose metabolism-related variables, liver enzymes as well as blood counts by conventional methods (Supplemental Digital Content [Table S1]). The intra- and inter-assay coefficients of variation were 3.4% and 2.0% for glucose, 11% and 3.4% for insulin, and 7.4% and 8.4% for non-esterified fatty acid, respectively. The HOMA-IR index (homeostatic model assessment of insulin resistance) was calculated as (fasting glucose × fasting insulin/22.5). The food intake was well controlled by food diaries (range from 1 178–1 236 kCal/day, Supplemental Digital Content [Table S1]).
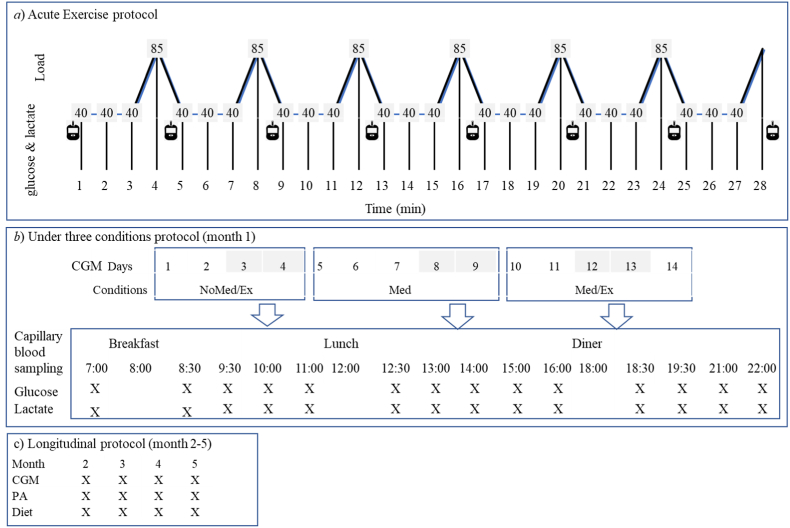
Fig. 1.
Study design of the data collection. a) Acute exercise protocol: A single bout of high intensity interval training was performend. b) Time-series protocol: Glucose and lactate concentrations were assessed in three conditions: NoMed/Ex: no medication and no exercise; Med: medication (Metformin + Januvia); Med/Ex: medication (Metformin + Januvia) + exercise (high intensity interval training). c) Longitudinal protocol: blood glucose concentrations, physical activity and diet were assessed. CGM: Continuous Glucose Monitoring; PA: Physical activity.
The data collection included three parts.
-
1)
For the purpose of assessing the acute effects of a single bout of exercise with medication on blood glucose and lactate concentrations (Fig. 1a), initially a maximal exercise test was performed to define the maximum load on a cycle ergometer (Ergomedic 839E, Monark Exercise AB, Varberg, Sweden). Forty-eight hours later, the patient performed a single high-intensity interval training (HIIT) session at about 45 min post-lunch. The protocol consisted of six 1-min bouts of high-intensity cycling at 85% of the peak power, separated by 3-min bouts at 40%, leading to a total exercise duration of 27 min. This protocol was used in a previous study and was shown to be safe in T2D patients treated with metformin.10 Blood glucose and lactate levels were assessed from capillary blood samples drawn from the finger after each high-intensity bout as well as immediately after completion of the exercise.
-
2)
For the purpose of assessing the changes in blood glucose and lactate concentrations both with and without medication and/or exercise, the patient followed a time series protocol (Fig. 1b) during the first month of the study. This protocol consisted of 4 days with neither medication nor exercise (NoMed/Ex), 5 days with medication only (Med), and another 5 days of medication + exercise (Med/Ex). To ensure the reliability of data, the first 2–3 days of each measurement period were excluded from the analysis. The sampling included blood glucose (OneTouch Ultra Vue®, Johnson & Johnson (China) Medical Devices Co., LTD) and lactate (Lactate Pro 2, Arkray Inc., Kyoto, Japan) concentrations of capillary blood from fingertips during a day for multiple times (see Fig. 1b). According to the manufacturer, the precision error for the lactate assessment was < 4.3%. In addition, the patient was provided with a Continuous Glucose Monitoring System (CGM, FreeStyle Libre™; Abbott Diabetes Care, Witney, UK), which is factory-calibrated and measures glucose concentrations from the interstitial fluid and automatically stores the data every 15 min continuously for 14 days. The sensor was placed on the belly of the m. triceps surae according to the manufacturer's instruction and removed after 14 days to upload the data. HIIT was performed once a day on a cycle ergometer for the medication + exercise condition (Fig. 1b). The protocol was the same as performed in the acute exercise test. Blood lactate and glucose concentrations were determined after each high-intensity bout as well as immediately post-exercise.
-
3)
For the purpose of assessing long-term changes in blood glucose concentrations associated with changes in physical activity patterns due to COVID-19 home confinement, the CGM was used to measure glucose concentrations in the interstitial fluid (Fig. 1c). The data included in this case report were collected in four periods of 14 days each during a period of 4 months (i.e. once per month). CGM results are presented as mean and two cutoffs were used for a time in range (TIR: % of readings 4.4–7.8 mmol/L); Time below range (TBR: % of readings and < 4.4 mmol/L); Time above range (TAR: % of readings and > 7.8 mmol/L). To ensure valid readings, only data from day 3–13 of each period were used for the statistical analysis.
A wearable tracker (Huawei B5, worn at least 12 h a day) was used to assess the level of physical activity (PA) indicated by the daily average steps displayed on the screen. Furthermore, dietary information was collected by food records with random 3–5 days monthly during the 4-month period (Fig. 1c).
Results
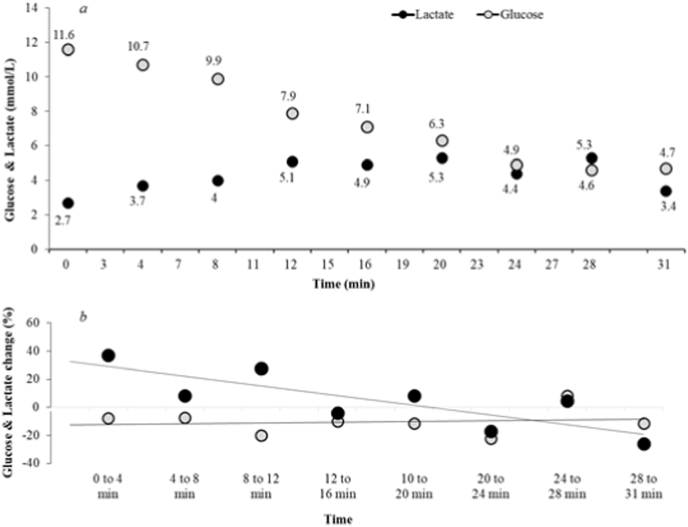
During acute HIIT, blood glucose decreased, while lactate increased gradually until 20 min (Fig. 2a). Thereafter, the blood lactate was maintained above 3 mmol/L, while blood glucose plateaued at ∼5.6 mmol/L. The variations of decrease in glucose between each incremental load were relatively similar (with approximately 1 mmol/L), while the changes in blood lactate fluctuated randomly throughout HIIT (Fig. 2b).
Fig. 2.
a) Concentrations of blood glucose and blood lactate assessed from capillary blood during the initial high-intensity exercise, which was performed at about 45 min post-lunch. b) Percentage changes in blood glucose and blood lactate between two consecutive points during high-intensity exercise.
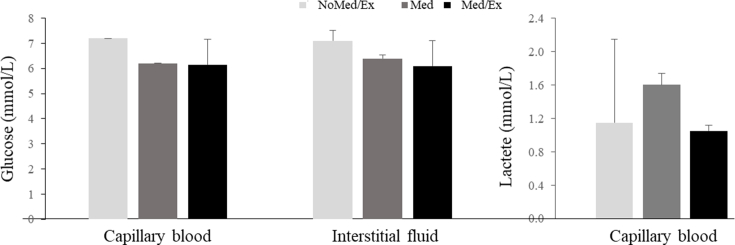
NoMed/Ex showed an approximately 1 mmol/L higher glucose concentration compared to Med and Med/Ex (Supplemental Digital Content [Fig. S1]). The lactate concentration was below 2 mmol/L in all conditions. However, Med led to an approximately 0.5 mmol/L higher concentration.
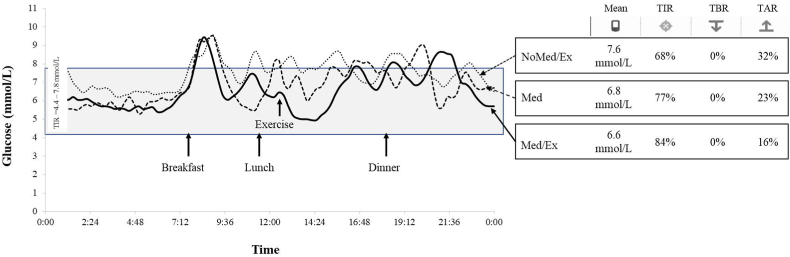
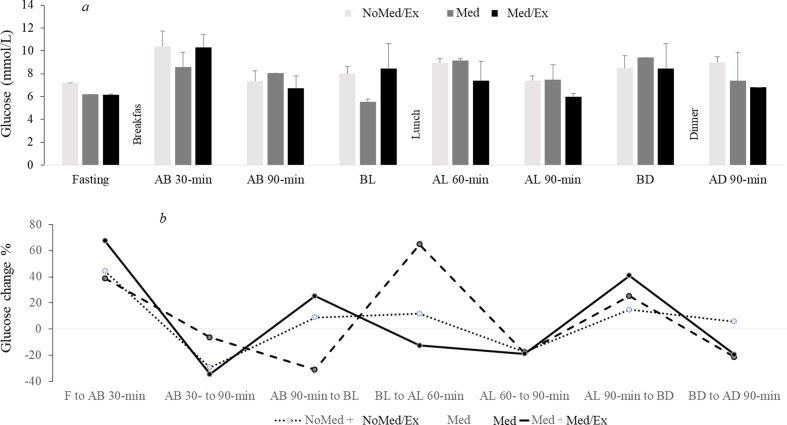
In NoMed/Ex, the glucose level was on average 7.6 mmol/L, with 68% of the time in range (TIR: 4.4–7.8 mmol/L) and 32% of the time above range (TAR: > 7.8 mmol/L; Fig. 3). In Med, the mean was 6.8 mmol/L with 77% TIR and 23% TAR, while in Med/Ex, the mean was 6.6 mmol/L with 84% TIR and 16% TAR. Following exercise performed at 45 min post-lunch, the glucose level decreased until 90 min post-lunch and exercise but then increased again until before dinner (Supplemental Digital Content [Fig. S2]).
Fig. 3.
Continuous glucose concentrations assessed from interstitial fluid under the three conditions. NoMed/Ex: no medication and no exercise; Med: only medication; Med/Ex: medication and exercise; TIR: time in range % of readings 4.4–7.8 mmol/L; TBR: time below range % of readings < 4.4 mmol/L; and TAR: TAR: time above range % of readings > 7.8 mmol/L.
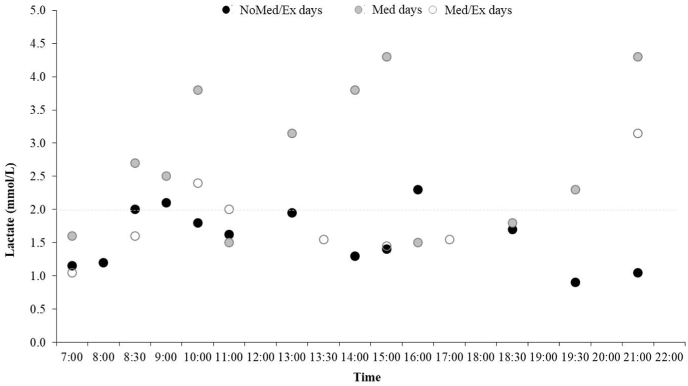
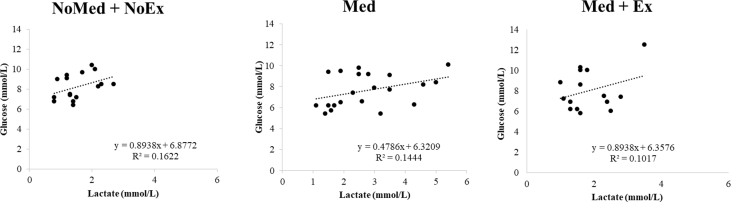
In NoMed/Ex and Med/Ex, the capillary blood lactate concentration was 23% and 37.5% higher than 2 mmol/L, respectively (Fig. 4), while in Med the lactate concentration was 66.7% higher (Fig. 4). The glucose concentrations correlated positively with lactate (Supplemental Digital Content [Fig. S3]).
Fig. 4.
Changes of capillary blood lactate concentrations throughout a day in three conditions. NoMed/Ex: no medication and no exercise; Med: only medication; Med/Ex: medication and exercise.
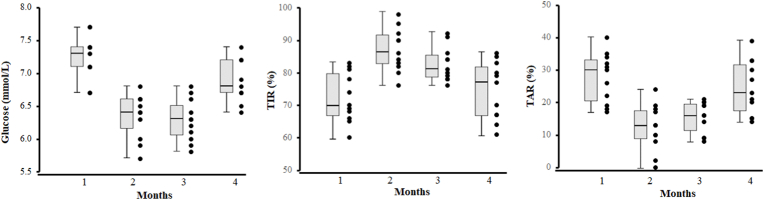
Body weight was maintained at 51 kg and the body mass index was 20.4 kg·m−2 throughout the study period. The average daily steps during months 1–4 were 3 102, 8 518, 8 227, and 2 116, respectively. The food intake was well controlled by food diaries (range from 1 178–1 236 kCal/day). Clinical biomarkers collected before and after the study are provided in Supplemental Digital Content (Table S1). In months including 3 weeks of home confinement and lower levels of PA, the mean glucose levels were significantly higher than that of months with free living and higher PA levels (p < 0.001 for all, Fig. 5). Months with higher PA levels also showed a higher TIR and lower TAR (p = 0.004, p < 0.001).
Fig. 5.
CGM results during the 4-month period for 11 days in each month. Left: Average glucose concentrations; Middle: TIR: time in range % of readings 4.4–7.8 mmol/L; Right: TAR: time above range % of readings > 7.8 mmol/L.
Discussion
In this case report, we made several interesting observations: 1) During an acute HIIT bout, reductions in glucose between each incremental load were relatively similar, while the changes in blood lactate fluctuated randomly throughout the session. 2) The medication alone days led to an approximately 0.5 mmol/L higher fasting blood lactate concentration compared to the other two conditions and lactate levels correlated positively with glucose in all three conditions. 3) We found an additive effect of combined exercise and metformin assessed by CGM in the short term. 4) In the long term, high amounts of PA had a positive impact on glucose fluctuation, while decreased levels of PA clearly induced a large fluctuation of glucose.
On the one hand, our data clearly underline the beneficial effects of combined exercise and metformin treatment for T2D patients. While metformin treatment reduces glucose concentrations, the normalization of glucose is further augmented acutely with a single bout of exercise and chronically through increased levels of PA. This finding is in line with previous data reporting that acute and chronic exercise can decrease the mean 24-h glucose concentrations and the amount of time in hyperglycemia and glycemic variability measured by CGM in adults with T2D.11
However, on the other hand, our data also highlighted potential risks associated with metformin treatment that has previously not been stressed. The fluctuations of blood lactate concentrations were much higher, reaching concentrations of up to ∼4.5 mmol/L, on days with metformin alone as compared to no medication days. Hyperlactatemia has been reported in T2D patients12 with conceivable negative consequences (i.e. progression of insulin resistance and reduced physical performance).13,14 Indeed, these days with medication and no exercise were also accompanied by an increase in anecdotally reported muscle cramps, causing a considerable amount of discomfort. However, when combined with exercise, the blood lactate concentrations decreased. These findings expand upon a previous study by de Sousaa et al., who observed fasting lactate concentrations to decrease after 12 weeks of exercise plus a caloric-restricted diet in T2D, most of who were treated with metformin15 and is likely a result of improved lactate clearance as has been shown in other studies.16, 17, 18 However, the absence of a group that performed exercise without a caloric-restricted diet complicated this study result interpretation, leaving the question of the independent effect of exercise on fasting lactate levels unanswered.
Since in our case study, blood lactate measured at any time point was well above 1 mmol/L, this might be one of the reasons for the discomfort experienced by the patient, indicating the importance of monitoring lactate concentrations for T2D patients. But no regular lactate tests were conducted prior to inclusion in the study for this patient. However, the role of lactate in patients treated with metformin remains somewhat equivocal. Despite the potential negative effects of high lactate concentrations on insulin resistance,13,14 it has been speculated that high lactate concentrations and consequently high rates of cell-to-cell shuttles may also aid the actions of metformin.19 Thus, future studies should elucidate the associations between blood lactate accumulation and progression in T2D patients.
Another important observation of this case is the change in glucose concentrations alongside changes in PA due to COVID-19 home confinement. When PA levels were low at the follow-up month 2 and 5, the mean glucose concentrations were significantly higher and less TIR of glucose was found as compared to that obtained in months 3 and 4, characterized by free-living and consequently higher levels of PA. These findings indicate the importance for T2D patients to maintain PA even during times of home confinement, in order to preserve glucose management. In the present case, about 8 000 steps per day showed a sufficient effect of PA on glycemic control.
Conclusion
Our findings showed that when combined with exercise and metformin treatment for T2D patients, exercise may contribute to improving glycemic control while metformin may elevate lactate levels in the long term. This supports assumptions based on which exercise should also be considered as a means to reduce possible risks associated with metformin treatment. However, considering the large heterogeneity in the observed exercise-induced changes in glucose metabolism, our results also underline the importance of tailoring exercise therapy to the metabolic needs of the individual T2D patient to help optimize treatment outcomes, and further research is warranted to shed light on the interactive mechanisms of exercise and metformin on lactate concentrations. Such approaches are already used within the context of precision medicine20 and voices have also been raised to translate these approaches into exercise medicine.21 The findings of our case, therefore, report reinforcing these attempts for patients with T2D. It is obvious that in patients similar to the case described here, exercise may contribute to improving glycemic control in the long term and alleviate the elevating effect of metformin on lactate in short term. However, we clearly encourage full-scale studies in order to expand on these findings by also including the timing of exercise in relation to the administration of medication.
Ethical approval statement
The study was approved by the Ethic Committee at Shanghai Jiao Tong University (No. B2020023I).
Authors’ contributions
WY designed the study. SL, SML, WY and SC collected data. SL, MS and SC performed data interpretation. SL and SML edited the figure and tables. SL and WY wrote the first draft of the manuscript. SML, MS, WY and SC critically reviewed the manuscript and made revisions. All authors contributed to the article and approved the submitted version.
Consent for publication
The patient provided her written informed consent to participate in this study.
Submission statement
The manuscript has not been published and is not under consideration for publication elsewhere.
Conflict of interest
The authors declare that they have no known competing financial interests that could have appeared to influence the work reported in this paper. Shulin Cheng is an editorial board member for Sports Medicine and Health Science and was not involved in the editorial review or the decision to publish this article.
Acknowledgments
We thank Hui Zuo and Tao Zhang for their help in managing the patient. This work was supported by the China Postdoctoral Science Foundation (2021M692090), Guided Scientific Research Project of Shiyan Science and Technology Bureau (21Y17) and Shanghai Philosophy and Social Science Planning Project (2022ZTY003).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.smhs.2023.02.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Fig. S1.
Fig. S2.
Fig. S3.
References
- 1.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metabol. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Viskochil R, Malin SK, Blankenship JM, Braun B. Exercise training and metformin, but not exercise training alone, decreases insulin production and increases insulin clearance in adults with prediabetes. J Appl Physiol (1985) 2017;123(1):243–248. doi: 10.1152/japplphysiol.00790.2016. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharoff CG, Hagobian TA, Malin SK, et al. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab. 2010;298(4):E815–E823. doi: 10.1152/ajpendo.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35(1):131–136. doi: 10.2337/dc11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malin SK, Nightingale J, Choi SE, Chipkin SR, Braun B. Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adults. Obesity. 2013;21(1):93–100. doi: 10.1002/oby.20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann C, Brixius K. Hyperlactatemia in type 2 diabetes: can physical training help? J Diabet Complicat. 2015;29(7):965–969. doi: 10.1016/j.jdiacomp.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med. 1998;338(4):265–266. doi: 10.1056/NEJM199801223380415. [DOI] [PubMed] [Google Scholar]
- 8.Wiholm BE, Myrhed M. Metformin-associated lactic acidosis in Sweden 1977-1991. Eur J Clin Pharmacol. 1993;44(6):589–591. doi: 10.1007/BF02440866. [DOI] [PubMed] [Google Scholar]
- 9.Bennis Y, Bodeau S, Batteux B, et al. A study of associations between plasma metformin concentration, lactic acidosis, and mortality in an emergency hospitalization context. Crit Care Med. 2020;48(12):e1194–e1202. doi: 10.1097/CCM.0000000000004589. [DOI] [PubMed] [Google Scholar]
- 10.Huang T, Lu C, Schumann M, et al. Timing of exercise affects glycemic control in type 2 diabetes patients treated with metformin. J Diabetes Res. 2018;2018 doi: 10.1155/2018/2483273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munan M, Oliveira CLP, Marcotte-Chénard A, et al. Acute and chronic effects of exercise on continuous glucose monitoring outcomes in type 2 diabetes: a meta-analysis. Front Endocrinol. 2020;11:495. doi: 10.3389/fendo.2020.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YD, Varasteh BB, Reaven GM. Plasma lactate concentration in obesity and type 2 diabetes. Diabetes Metab. 1993;19(4):348–354. [PubMed] [Google Scholar]
- 13.Choi CS, Kim YB, Lee FN, Zabolotny JM, Kahn BB, Youn JH. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol Endocrinol Metab. 2002;283(2):E233–E240. doi: 10.1152/ajpendo.00557.2001. [DOI] [PubMed] [Google Scholar]
- 14.Lombardi AM, Fabris R, Bassetto F, et al. Hyperlactatemia reduces muscle glucose uptake and GLUT-4 mRNA while increasing (E1alpha)PDH gene expression in rat. Am J Physiol. 1999;276(5):E922–E929. doi: 10.1152/ajpendo.1999.276.5.E922. [DOI] [PubMed] [Google Scholar]
- 15.de Sousa MV, Fukui R, Dagogo-Jack S, Krustrup P, Zouhal H, da Silva MER. Biomarkers of insulin action during single soccer sessions before and after a 12-week training period in type 2 diabetes patients on a caloric-restricted diet. Physiol Behav. 2019;209 doi: 10.1016/j.physbeh.2019.112618. [DOI] [PubMed] [Google Scholar]
- 16.Depocas F, Minaire Y, Chatonnet J. Rates of formation and oxidation of lactic acid in dogs at rest and during moderate exercise. Can J Physiol Pharmacol. 1969;47(7):603–610. doi: 10.1139/y69-106. [DOI] [PubMed] [Google Scholar]
- 17.Eldridge FL. Relationship between turnover rate and blood concentration of lactate in exercising dogs. J Appl Physiol. 1975;39(2):231–234. doi: 10.1152/jappl.1975.39.2.231. [DOI] [PubMed] [Google Scholar]
- 18.Issekutz B, Jr., Shaw WA, Issekutz AC. Lactate metabolism in resting and exercising dogs. J Appl Physiol. 1976;40(3):312–319. doi: 10.1152/jappl.1976.40.3.312. [DOI] [PubMed] [Google Scholar]
- 19.Giaccari A, Solini A, Frontoni S, Del Prato S. Metformin benefits: another example for alternative energy substrate mechanism? Diabetes Care. 2021;44(3):647–654. doi: 10.2337/dc20-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramírez-Vélez R, Lobelo F, Izquierdo M. Exercise for disease prevention and management: a precision medicine approach. J Am Med Dir Assoc. 2017;18(7):633–634. doi: 10.1016/j.jamda.2017.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.