Summary
The gas exchange surface of the lungs is lined by an epithelium consisting of alveolar type (AT) I and ATII cells. ATII cells function to produce surfactant, play a role in host defense and fluid and ion transport, and serve as progenitors. ATI cells are important for gas exchange and fluid and ion transport. Our understanding of the biology of these cells depends on investigation of isolated cells. Here, we present methods for the isolation of mouse and rat ATII cells.
Keywords: Alveolar epithelium, lung digestion, ATII
1. Introduction
The alveolar epithelium consists of ATI and ATII cells. ATI cells cover 95-98% of the alveolar surface and play a role in gas exchange and ion transport. ATII cells are critical for surfactant production and host defense and function as epithelial progenitors during homeostasis and repair. Abnormal ATII cell function has been implicated in a variety of diseases, including pulmonary fibrosis, emphysema, the acute respiratory distress syndrome, and lung cancer. Much of our knowledge regarding the function of ATII cells derives from studies in which cells have been isolated and cultured (1). Previously, ATII cells have been isolated using a variety of methods. Here, we present a protocol for the isolation and purification of mouse ATII cells using magnetic bead separation. Purification is accomplished in two stages: first, CD45+ cells are depleted using negative selection, then EpCAM+ cells are purified using positive selection (2). We also include an established protocol for the isolation of rat ATII cells using discontinuous density centrifugation (3–6).
2. Materials
2.1. Mouse ATII Cell Isolation
C57BL/6 mice (see Note 1)
Pentobarbital sodium (Fatal Plus 260 mg/ml stock diluted 1:20 in saline)
20 gauge Luer stub adapter
Perfusion device: 10 ml disposable syringe attached to a 27 gauge needle
Suture: 2-0 silk suture
Surgical scissors
Media: 500 ml DMEM/F12 supplemented with 200 mM L-Glutamine (final concentration), 10 ml Pen-Strep (10,000 U/ml penicillin, 10,000 U/ml streptomycin), 10 ml MEM non-essential amino acids, 10 ml 1M HEPES. Filter to sterilize.
Ca2+ Mg2+-free phosphate-buffered saline (PBS)
1% low melting point agar in Ca2+ Mg2+-free PBS. Warm to 45°C.
Media w/ DNAse: 0.01% DNAse in media
Buffer #1: Ca2+ Mg2+-free PBS containing 2 mM EDTA and 0.5% BSA. Filter to sterilize. De-gas and keep at 4°C prior to use.
Buffer #2: Ca2+ Mg2+-free PBS containing 0.5% BSA. Filter to sterilize. De-gas and keep at 4°C prior to use.
Dispase: diluted 1:10 in Ca2+ Mg2+-free Hank’s balanced salt solution (HBSS). Equilibrate to room temperature prior to use.
Petri dish
Razor blade
Filters: 100 μM, 40 μM, 20 μM
Biotinylated anti-mouse CD326/EpCAM antibody (Miltenyi Biotech)
FcR Blocking Reagent (Miltenyi)
Anti-biotin MicroBeads (Miltenyi)
CD45 MicroBeads (Miltenyi)
LS Columns (Miltenyi)
Pre-Separation Filters (Miltenyi)
MACS multi-stand w/QuadroMACS or MidiMACS separator (Miltenyi)
14 ml round bottom tubes
15 ml conical tubes
50 ml conical tubes
2.2. Rat ATII Cell Isolation
Sprague-Dawley rat
Fatal Plus/Heparin: Mix 0.15 ml FatalPlus (260 mg/ml) and 0.85 ml Heparin (1000 U/ml)
Plastic Beakers: Three 100 ml beakers, two 250 ml beakers, and one 400 ml beaker (see Note 2)
Plastic Erlenmeyer Flask: 250 ml (see Note 2)
Suture: 2-0 silk suture
Surgical scissors
Luer stub adapter: 15 gauge
Perfusion syringe: 20 ml disposable syringe attached to a 14 gauge catheter.
BSS-A: 15.9 g NaCl, 0.8 g KCl, 0.1 g NaH2PO4 (monobasic), 0.64 g Na2HPO4 (dibasic), 5.06 g HEPES, 2 g D-Glucose (Dextrose), adjust to pH 7.4, and adjust volume to 2 liters with deionized water. Sterile filter. Warm to 37°C.
BSS-A w/EGTA: Mix 37.5 ml of BSS-A and 750 μl 0.1 M EGTA. Adjust to pH 7.4. Sterile filter.
Saline: Warm to 37°C.
Board at angle to allow lavage to drain by gravity.
BSS-B: 15.52 g NaCl, 0.772 g KCl, 0.1 g NaH2PO4 (monobasic), 0.618 g Na2HPO4 (dibasic), 4.93 g HEPES, 2 g D-Glucose (dextrose), 0.558 g Calcium chloride dihydrate, 0.638 g magnesium sulfate 7-hydrate, adjust to pH 7.4, and adjust volume to 2 liters with deionized water. Sterile filter and place at 4°C.
BSS-B w/BSA: 15 ml BSS-B containing 1% BSA (final concentration) in a small plastic beaker. Adjust to pH 7.2-7.4 and warm to 37°C.
BSS-B w/elastase: Stir 172 U of elastase into 42 ml of BSS-B. Cover with saran wrap and place in a shaking water bath at 37°C. Make fresh.
Fluorocarbon-albumin emulsion: Mix and sonicate approximately 5 ml of BSS-B w/BSA with 1.5 ml of 0.2 uM filtered Fluorocarbon FC-40. Sonicate until completely mixed and emulsion turns white. Add sonicated FC-40 to remaining 10 ml of BSS-B with BSA. Swirl to mix.
0.1 M sodium phosphate: Slowly add 0.1 M monobasic sodium phosphate to 0.1 M dibasic sodium phosphate until the pH is 7.4 at 25°C.
Krebs/HEPES buffer preparation: Mix 1,257.6 ml H2O, 10.8 g NaCl (0.9% final concentration), 36 ml 0.1 M sodium phosphate, 48 ml 0.15 M KCl, and 14.4 ml 1M HEPES.
Optiprep with HEPES: Add 2.5 ml of 1M HEPES to a 250 ml bottle of Optiprep (Accurate Chemical).
Lightweight Optiprep: Add 8 ml of fetal bovine serum (FBS) to 45 ml of Optiprep with HEPES. Add a pinch of Phenol Red until solution is cherry red in color and bring total volume of mixture to 400 ml with Krebs/HEPES Buffer. Stir until completely mixed (see Note 3). The hydrometer reading should be between 1.038 and 1.045 (see Note 4).
Heavyweight Optiprep: Add 8 ml of FBS to 110 ml of Optiprep with HEPES. Bring mixture to 400 ml with Krebs/HEPES Buffer. Stir until completely mixed (see Note 3). Hydrometer reading should be between 1.088 and 1.092 (see Note 4).
Optiprep Gradient: Add 10 ml of lightweight Optiprep (red) to a 50 ml conical tube. Then slowly inject 10 ml of heavyweight Optiprep to the bottom of the conical tube (below the lightweight Optiprep); avoid mixing of layers. Store in the dark at 4°C until use.
Mincing scissors and plastic mincing beakers (see Note 2)
Fetal bovine serum (FBS)
DNase I: 4 mg/ml in BSS-B
Filtering device: 60 ml syringe with needle adaptor cut off
Gauze pads
Rubber bands
3. Methods
3.1. Mouse ATII Cell Isolation
3.1.1. Lung Digestion
Anesthetize mouse with intraperitoneal (i.p.) injection of 200 mg/kg Fatal-Plus in 400 μl saline (see Note 1). Once the mouse is fully anesthetized, perform the following steps at a rapid pace. Speed of the entire protocol is key for cell yield, purity, and viability.
Open the peritoneal cavity and cut the descending aorta/inferior vena cava. Open thoracic cavity, cut the diaphragm away from the walls, pin back the walls of the thoracic cavity, and remove the thymus.
Perfuse the pulmonary vasculature with 20 ml of Ca2+ Mg2+-free PBS by inserting a 20 ml syringe attached to a 27-gauge needle into the right ventricle (see Note 5). Do not cut the left atrium.
After perfusing, remove the skin and muscle covering the trachea. Push under the trachea with forceps and pull two separate sutures through. Cut a slit in the ventral surface of the trachea making sure to avoid cutting all the way through. Insert a 20 gauge Luer stub adapter into the trachea and securely tie it in place with one of the sutures. Leave the other suture untied.
Instill 1 ml of Ca2+ Mg2+-free PBS through the Luer stub adapter, keeping the syringe attached. Next, withdraw the PBS back into the syringe to lavage the lung: approximately 800 μl will be returned.
Instill 3 ml of room temperature dispase diluted 1:10 in Ca2+ Mg2+-free HBSS into lungs. Immediately following dispase, instill 0.3 ml of 45°C 1% low melting agarose in Ca2+ Mg2+-free PBS into the lungs leaving the syringe attached to the Luer stub adapter (7).
With the 1 ml syringe and Luer stub adapter still attached, securely tie the trachea off distal to the Luer stub adapter using the untied, remaining suture. This knot will prevent the dispase and agarose from leaking out once the syringe and Luer stub adapter are removed.
Remove the syringe and Luer stub adapter from the trachea. Remove the lungs from the thorax by holding the suture and cutting behind the trachea. Be careful not to cut the lungs. The heart can remain attached to the lungs at this point.
Place the lungs into 3 ml of dispase diluted 1:10 in Ca2+ Mg2+-free HBSS in a 14 ml round bottom tube and incubate for 30 min at room temperature.
After incubation, place lungs in a petri dish. Remove heart. Dissect away the trachea and the bronchial tree.
Chop the lung with a razor blade back and forth from different directions until the tissue has the consistency of a thick soup/puree (Fig. 1). This requires about 3 min of continuous chopping.
Add 3 ml of media with 0.01% DNase to minced tissue and pipet up and down with a 5 ml pipet.
Filter the lung tissue sequentially through 100, 40, and 20 μm filters pre-wetted with media with 0.01% DNase. To aid with 100 μm filtering, stir solution around with a 5 ml pipet on top of the filter, pushing the tissue through very gently (Fig. 2). Let the solution drain by gravity through the 40 and 20 μm filters. Centrifuge the filtrate at 300 x g for 5 min at 10°C. Set acceleration and deceleration to medium.
Remove the supernatant and resuspend cells in 10 ml of media w/ 0.01% DNase. Count cells using a hemocytometer.
Transfer cells to a 15 ml conical tube and centrifuge at 300 x g for 5 min at 10°C.
Fig. 1.
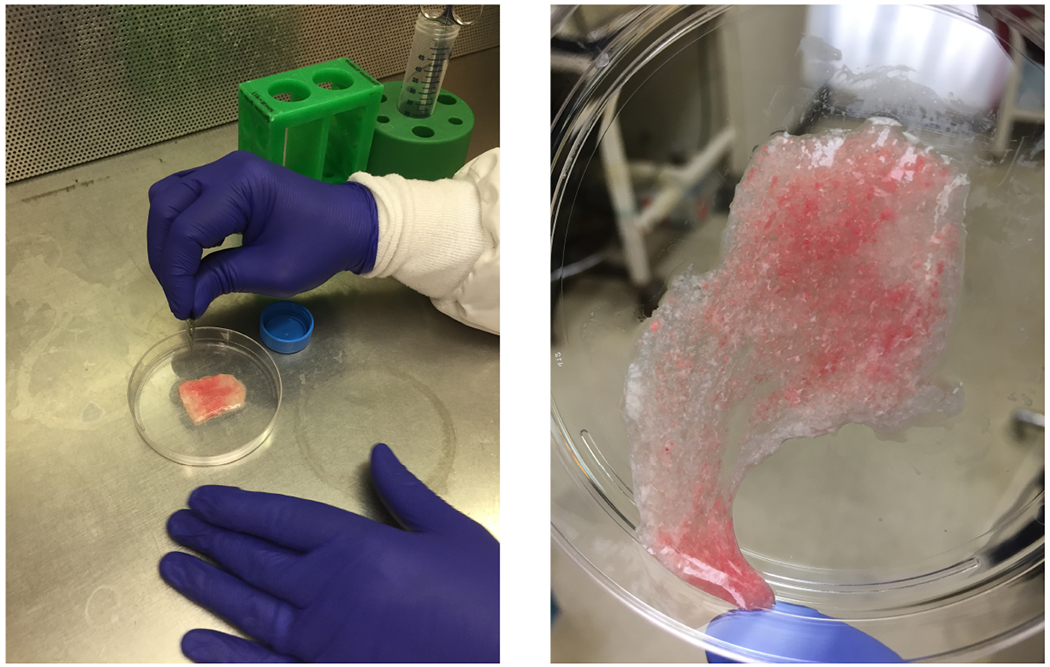
The mouse lung after chopping with the razor blade. The lung should have the consistency of a thick soup/puree.
Fig. 2.
Filtering mouse lung digest through the 100 μm filter. Notice the remains of the tissue that did not pass through the filter.
3.2.2. Isolation of ATII Cells by Magnetic Sorting
Resuspend cells in 500 μl/mouse of Buffer #1.
Add 40 μl/mouse anti-CD45 MicroBeads to deplete leukocytes. Pipet up and down vigorously 5-10 times and then incubate for 15 min at 4°C.
Add 10 ml Buffer #1.
Centrifuge at 300 x g for 5 min at 10°C.
During the 5-minute centrifugation, place LS column into a MidiMACS or QuadroMACS separator. Place pre-separation filter into LS column. Place a 15 ml conical tube under the column.
Rinse pre-separation filter/LS column with 3 ml of Buffer #1.
Place a new 15 ml conical tube under the column.
Resuspend centrifuged cells in 500 μl of Buffer #1.
Add cell suspension to pre-separation filter/LS column.
Wash three times with 3 ml Buffer #1.
Collect flow through, which contains CD45 negative cells. (Optional: count cells here.) Centrifuge flow through at 300 x g for 5 min at 10°C.
Resuspend cells in 100 μl/mouse of Buffer #1 in a 15 ml conical tube. Pipet up and down vigorously 10-20 times with a P1000 Pipetman.
Add 10 μl/mouse biotinylated anti-mouse CD326 (EpCAM). Mix and incubate for 10 min at 4°C.
Add 2 ml/mouse of Buffer #1. Mix and centrifuge at 300 x g for 5 min at 10°C.
Remove the supernatant and resuspend cells in 100 μl/mouse of Buffer #1.
Add 20 μl/mouse anti-biotin MicroBeads. Pipet up and down vigorously 5-10 times and incubate for 15 min at 4°C.
Add 2 ml/mouse of Buffer #1. Mix and centrifuge at 300 x g for 5 min at 10°C.
Rinse new pre-separation filter and LS Column with 3 ml of Buffer #1 during centrifugation time. Leave the flow-through in the 15 ml conical collection tube.
Remove the supernatant and resuspend cells in 500 μl of Buffer #1. Pipet up and down vigorously 10 times.
Add cells to Pre-Separation Filter on LS Column. Do not apply pressure to the column.
Wash filter one time with 3 ml of Buffer #1. Then wash twice with 3 ml of Buffer #2.
Remove column from magnet, place into a new 15 ml conical, and add 5 ml of Buffer #2 to LS Column. Using the plunger, strongly push out EpCAM+ cells with plunger. This should be fast, taking only 1 sec for the whole solution to come through.
Dilute an aliquot of cells 1:10 in trypan blue and count live and dead cells.
Fig. 3.
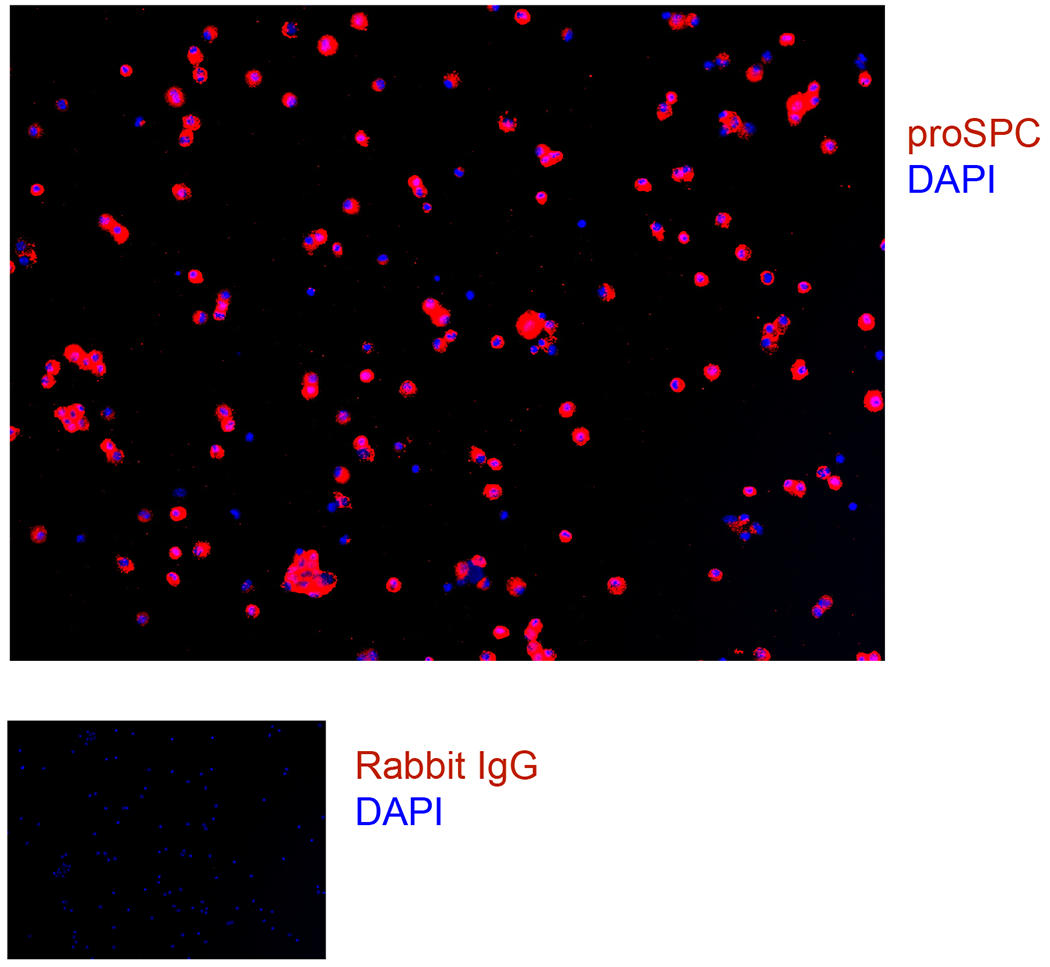
Mouse cell isolation cytospins were fixed and stained for proSPC and DAPI.
3.2. Rat ATII Cell Isolation1
Anesthetize a rat with a 1 ml intraperitoneal injection of Fatal Plus/Heparin solution prepared as described above. Once rat is fully anesthetized, perform the following steps at a rapid pace.
Open the peritoneal cavity and cut the descending aorta/inferior vena cava. Remove the skin and muscle covering the trachea. Push under the trachea with forceps and pull a suture through. Cut a slit in the ventral surface of the trachea making sure to avoid cutting all the way. Insert a 15-gauge Luer stub adapter into the trachea and securely tie it in place with a 2-0 silk suture in a double knot. It is important that this knot be tight since lungs will be held by this suture in later steps.
Open the thoracic cavity, cut the diaphragm carefully so as not to nick the lungs, pin back the walls of the thoracic cavity, and remove the thymus. Make an incision in the right ventricle. Cannulate the pulmonary artery with a 14 gauge catheter attached to a 20 ml syringe and perfuse the lungs with 20 ml of BSS-A. Cut the left atrium to allow exit of the perfusate after the atrium distends and the lungs blanch (see Note 5).
Remove the lungs from the thorax by holding the sutures and cutting behind the trachea. Be careful not to cut the lung tissue. Do not remove the Luer stub adapter.
Place cannulated lung into a plastic beaker with 200 ml of 37°C saline while performing surgery on subsequent animals. Remove the lung from saline and lay it on an angled surface with heart-side facing up and the cannula pointing down (Fig. 4).
Lavage lung five times with BSS-A at 37°C using a 20 ml syringe. Instill solution until lungs fill completely, using approximately 8-12 ml. Remove syringe and let lavage fluid drain by gravity between each lavage. The objective of this step is to remove some of the alveolar macrophages from the airspaces.
Pump sonicated fluorocarbon-albumin emulsion through syringe a few times to mix.
Hold lung upright by cannula and slowly instill ~15 ml of sonicated fluorocarbon-albumin emulsion into lung with a 20 ml syringe over a beaker of saline at 37°C. Stop adding emulsion if lung starts leaking.
Leaving the syringe attached and the lung distended, submerge the lungs into beaker of saline and incubate the lung for 20 min in a 37°C water bath.
After incubation, lavage the lung twice with 8-12 ml of BSS-A and let it drain by gravity, as described above (Fig. 4).
Lavage the lung three more times with 8-12 ml of BSS-A with EGTA.
Load a 60 ml syringe with 40 ml of BSS-B w/ elastase. Lavage the lung one time with 8-10 ml. Let the lavage fluid drain by gravity.
Return the lung to the beaker of saline at 37°C and instill ~10 ml of BSS-B with elastase. Incubate at 37°C for 15 min. Leave the syringe attached to the cannula. Every 5 min, instill an additional 10 ml of BSS-B with elastase.
Remove lung from the saline bath and place it in a plastic cup. Holding the cannula in one hand and the scissors vertically in the other hand, cut straight down to remove the heart, trachea, and mediastinal structures from the lung.
Transfer lung to mincing beaker and add 100 μl of DNase.
Mince for 200 strokes (30 sec) with one pair of large, very sharp scissors until pieces are ~1 mm3 in size (see Note 7). Do not over-mince.
Transfer minced lung into a 250 ml plastic Erlenmeyer flask (see Note 2) containing 5 ml of FBS to quench the elastase. Rinse mincing beaker and scissors well with BSS-B and add additional tissue to minced lung mixture.
Shake mixture vigorously in a water bath for 5 min at 37°C.
Use a 60 ml syringe with the needle adaptor cut off (Fig. 5). Then use a rubber band to attach gauze or Nitex filter to the cut off end of the syringe. Before filtering, wet each filter with BSS-B. Do not let the filter touch the filtrate in the cup. Pour half of the minced lung into one set of filters and the other half into the other. Filter sequentially through: (1) 2 layers of gauze, (2) 4 layers of gauze, and (3) 20 μm Nitex nylon filtration fabric.
Make sure to rinse each cup w/BSS-B after draining filtrate from syringe. Apply pressure to the open end of the syringe with the palm of your hand to aid in pushing the cells through the filter (Fig. 6).
With a 10 ml pipette, add filtrate to the top layer on the Optiprep gradient with the tip right above the lightweight (red) optiprep layer. Slowly expel filtrate onto the gradient so that the layers do not mix (Fig. 7). Balance gradients with BSS-B and centrifuge for 20 min at 1,000 RPM at 4°C. Set acceleration and deceleration to low.
From this point on, handle cells in a sterile manner. Aspirate using a circular motion to ensure even aspiration down to the 15 ml level and discard. With a 10 ml pipette, remove the gradient contents between the 15 and 5 ml marks; this contains the ATII cells. Transfer to a sterile 50 ml conical tube.
Add sterile BSS-B at 4°C to bring the volume up to 40 ml. Centrifuge 10 min at 1,000 RPM at 4°C.
Aspirate the supernatant and resuspend the cells in 10 ml of sterile BSS-B. Bring the volume up to 40 ml and centrifuge at 850-1,000 RPM for 10 min at 4°C.
Aspirate the supernatant and immediately resuspend cells in 10 ml of 37°C media. Any delay in resuspending the cells will cause them to clump.
Dilute an aliquot of cells 1:10 in trypan blue and count live and dead cells.
Fig. 4.
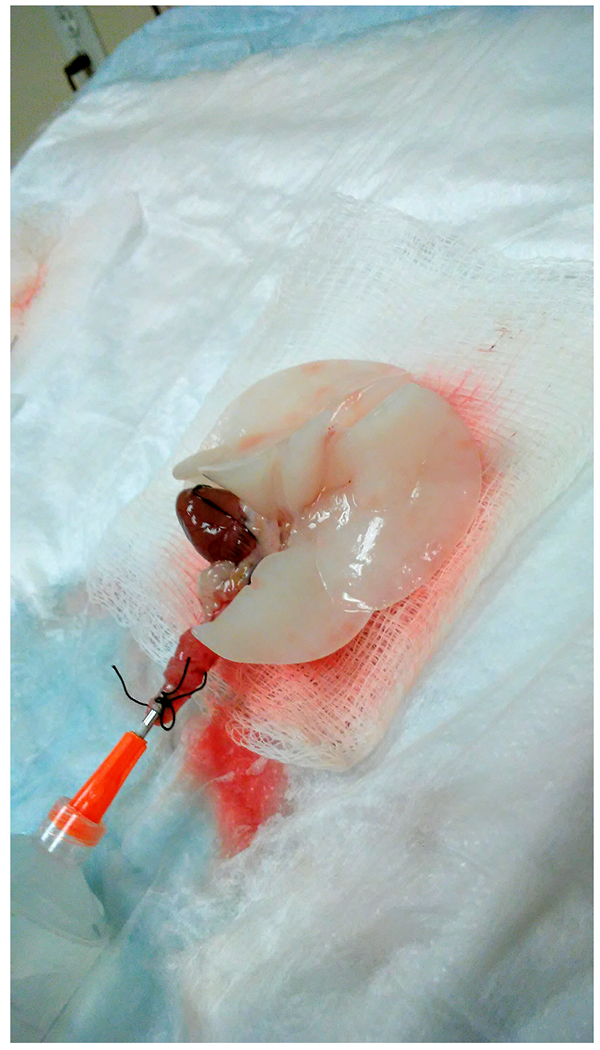
Lungs on board angled to allow lavage to drain by gravity. The trachea is pointing down, and the heart is facing up.
Fig. 5.

Filtering device made from a 60 ml syringe with the needle adaptor cut off. The filter, made of layered gauze, is attached using a rubber band.
Fig. 6.
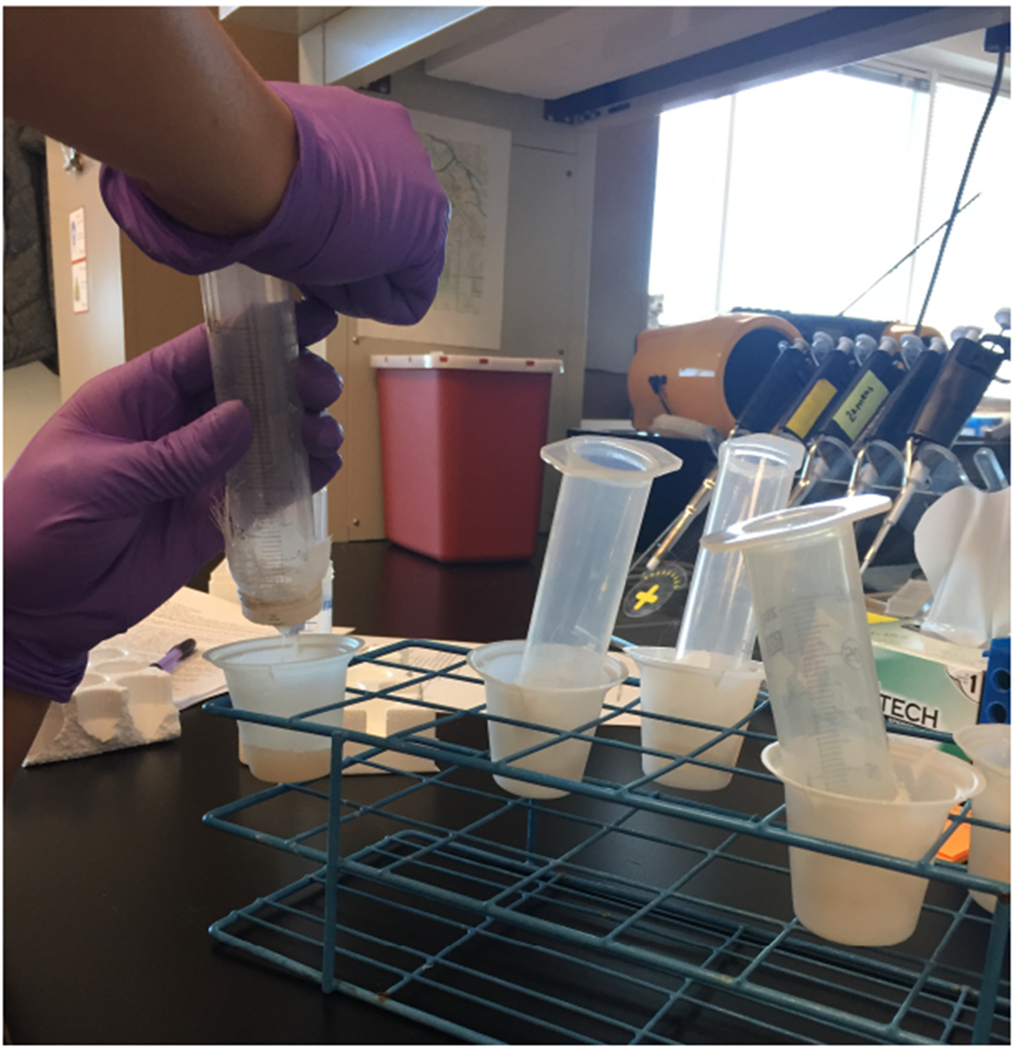
To aid in filtering, apply pressure to the open end of the syringe with the palm of your hand.
Fig. 7.
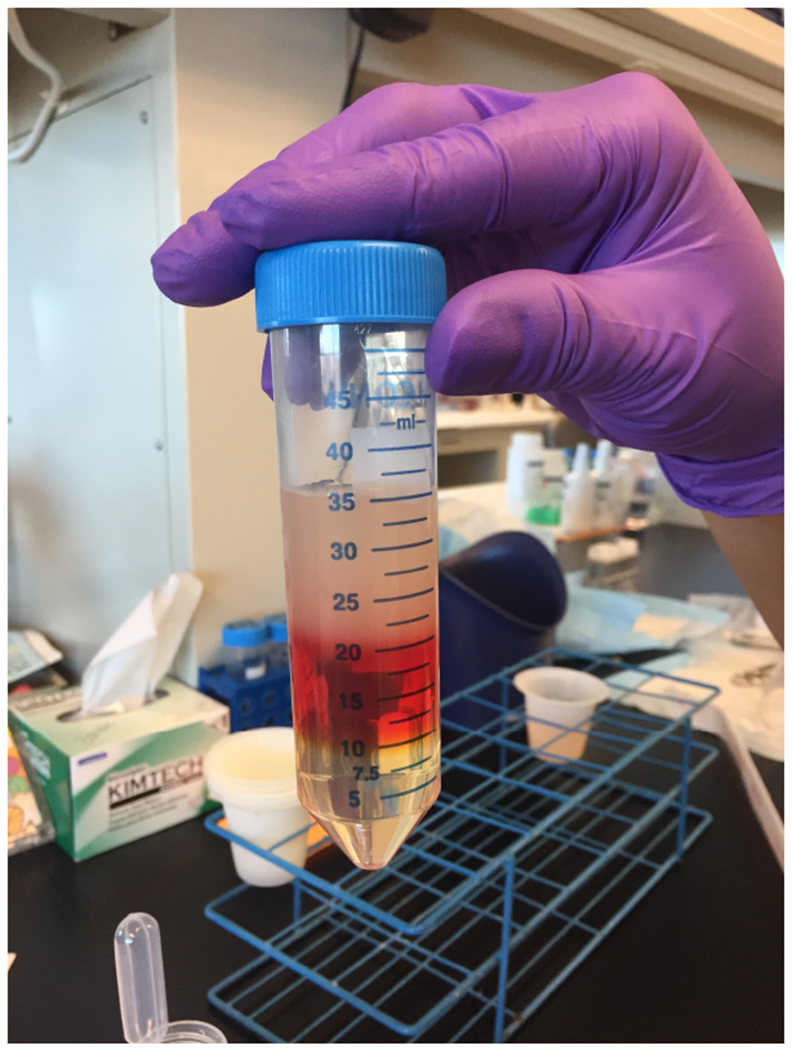
Optiprep gradient showing clear defined layers. Slowly add filtrate to the top layer of the Optiprep gradient so that layers do not mix.
Fig. 8.
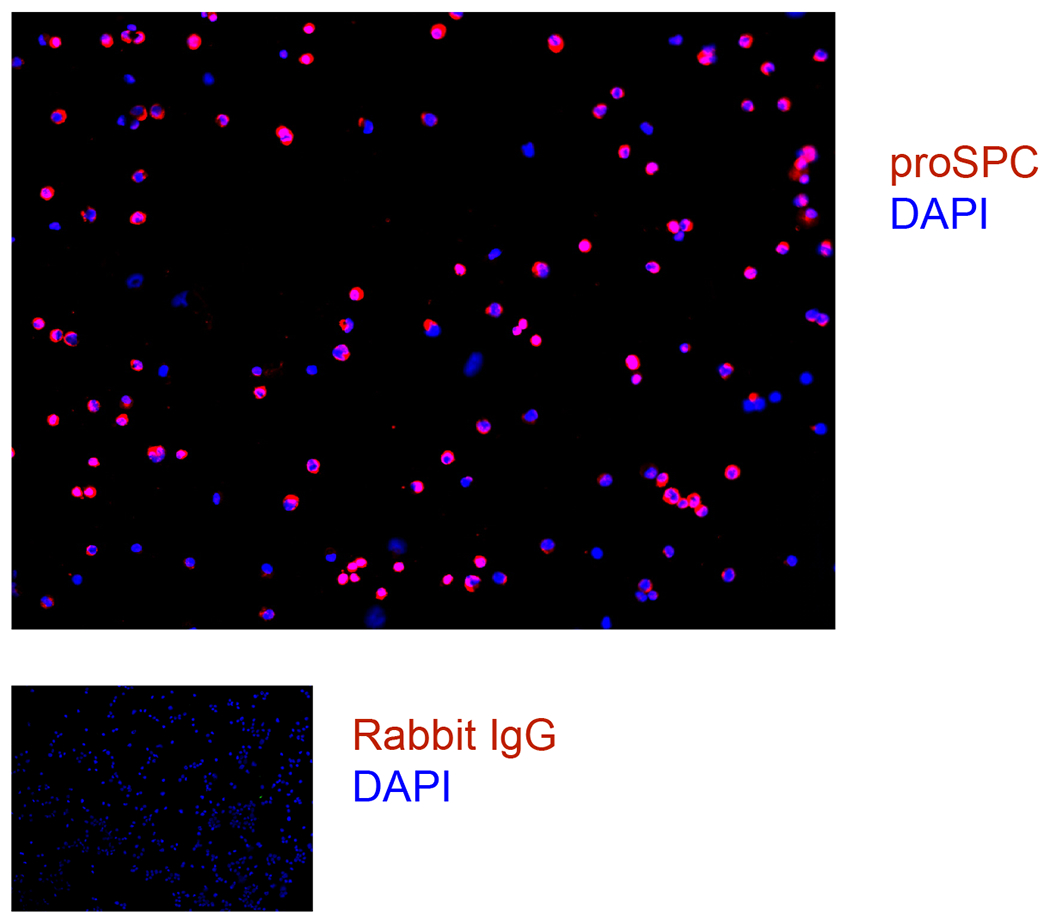
Rat cell isolation cytospins were fixed and stained for proSPC and DAPI.
Acknowledgments
This work was supported by NIH HL131608 (RLZ), the Boettcher Foundation (RLZ), funds from the University of Colorado Denver Department of Medicine Outstanding Early Career Scholars Program (RLZ) and the Hastings Foundation (ZB).
4 Notes
To increase the ATII cell yield, the lungs from 2 mice can be digested together, combining the digests before magnetic sorting.
Do not let cells come into contact with glassware. Cells can adhere to glass surfaces which can cause damage to the cells. Use plastic beakers for cells. If using a glass flask you must siliconize the glass prior to adding cells.
When preparing the lightweight and heavyweight Optiprep gradients, mix with a stir bar until the Optiprep and Krebs buffer are completely mixed so that the two solutions cannot be seen separately at all.
To achieve the proper hydrometer reading, add Optiprep if the hydrometer reading is too low. Add Krebs/HEPES buffer if the hydrometer reading is too high.
A good perfusion is crucial to the ATII cell isolation. Make sure that lungs are white after perfusion.
Use an aliquot of cells to determine purity and viability after every cell isolation. To analyze viability, dilute cells 1:10 in trypan blue and count live and dead cells. To analyze purity, cytospin 100,000 cells in a cytofunnel at 600 RPM for 5 min, fix cells in 4% paraformaldehyde (PFA) for 10 min at room temperature, air dry, and store at −20°C. Immunostain slides with antibodies against proSPC.
To mince, hold the two pairs of scissors together, insert into mincing beaker, and pump scissors rapidly until pieces are ~1 mm in size. Move through this step quickly. This should take only 30 sec.
Conflicts of Interest: None
Not used by Borok lab.
5 References
- 1.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol 1990; 258: L134–147. [DOI] [PubMed] [Google Scholar]
- 2.Messier EM, Mason RJ, Kosmider B. Efficient and rapid isolation and purification of mouse alveolar type II epithelial cells. Exp Lung Res 2012; 38: 363–373. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez RF, Dobbs LG. Isolation and culture of alveolar epithelial Type I and Type II cells from rat lungs. Methods Mol Biol 2013; 945: 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 1986; 134: 141–145. [DOI] [PubMed] [Google Scholar]
- 5.Dobbs LG, Mason RJ. Pulmonary alveolar type II cells isolated from rats. Release of phosphatidylcholine in response to beta-adrenergic stimulation. J Clin Invest 1979; 63: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobbs LG, Geppert EF, Williams MC, Greenleaf RD, Mason RJ. Metabolic properties and ultrastructure of alveolar type II cells isolated with elastase. Biochim Biophys Acta 1980; 618: 510–523. [DOI] [PubMed] [Google Scholar]
- 7.Demaio L, Tseng W, Balverde Z, Alvarez JR, Kim KJ, Kelley DG, Senior RM, Crandall ED, Borok Z. Characterization of mouse alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 2009; 296: L1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]


