Abstract
Phthalates are ubiquitous environmental exposures that may be implicated in inflammatory processes, as demonstrated by previous in vivo and in vitro studies. Few human studies have substantiated these observations. This study sought to examine whether maternal phthalate exposures impact inflammatory processes, as measured by circulating inflammatory biomarkers, in the PROTECT cohort in northern Puerto Rico. Inflammatory biomarkers included matrix metalloproteinases 1, 2, and 9 (MMPs), C-reactive protein (CRP), vascular cell adhesion molecule-1 (VCAM), and intercellular cell adhesion molecule-1 (ICAM). Biomarkers were measured in maternal serum samples collected during pregnancy. 19 phthalate metabolites were assessed in urinary samples collected at three study visits across pregnancy. Phthalates with <50% of measurements above the limit of detection were excluded from analysis. We utilized linear mixed effect models to estimate associations between interquartile range increases in phthalate metabolite concentrations and percent changes in inflammatory biomarkers. Our results revealed significant associations between mono-n-butyl phthalate (MBP) and higher MMP1 by 7.86% (95% CI: 0.49, 15.76) and between mono oxononyl phthalate (MONP) and higher MMP2 by 8.30% (95% CI: 2.22, 14.75). We observed negative or null associations between phthalate metabolites and MMP2, MMP9, ICAM, VCAM, and CRP. Many results were significantly modified by fetal sex, particularly those between di-2-ethylhexyl phthalate (DEHP) metabolites and MMP1 (p-interaction: MEHHP=0.01, MEOHP= 0.04, MECPP= 0.01) and MMP2 (p-interaction: MEHHP=0.03, MEOHP=0.01, MECPP=0.01), for which associations were positive among only women carrying female fetuses. MMPs have been previously associated with preeclampsia and hypertensive pregnancy disorders as mediators of artery remodeling. Hence, our findings suggest a potential role for phthalates in mediating the maternal inflammatory response, as well as significant sexual dimorphism in these relationships, which has implications for several adverse pregnancy outcomes.
Keywords: phthalates, inflammation, matrix metalloproteinase, PROTECT, pregnancy
Graphical Abstract
1. Introduction
Phthalates are a class of man-made chemicals ubiquitously found in the modern environment through their uses as plasticizers and solvents. The range of possible phthalate exposure sources is extensive, ranging from food packaging to personal care products (Kelley et al., 2012). High molecular weight (HMW) phthalates including di-2-ethylhexyl phthalate (DEHP), di-decyl phthalate (DiDP), and di-nonyl phthalate (DiNP) are prevalent in products such as food and water packaging and PVC-containing products (Marie et al., 2015). Low molecular weight (LMW) phthalates including dibutyl phthalate (DBP), dimethyl phthalate (DMP), and diethyl phthalate (DEP) are commonly used as solvents and adhesives in products such as personal care products (Sathyanarayana, 2008). In addition to their large presence in plastics, phthalates have a substantial environmental presence due to their noncovalent bonding allowing for spontaneous volatilization and leaching into food and water sources (Serrano et al., 2014). Significant phthalate metabolite concentrations were found in pregnant women of the PROTECT cohort, and increased concentrations were associated with women who reported using perfume and cosmetics in addition to other activities and dietary factors (Rodríguez-Carmona et al., 2020). Reduction and control of phthalate exposures are challenging due to these chemical properties and wide range of applications and sources, making research into their human health effects important.
Despite the prevalence of phthalate exposure, the scope of their human health effects is not well established. Current scientific literature supports associations between phthalate exposures and endocrine disruption with significant implications for maternal and fetal health such as preterm birth, long term maternal weight gain, and preeclampsia (Colón et al., 2000; Latini et al., 2003; Meeker & Ferguson, 2014; Philips et al., 2020; Rodríguez-Carmona et al., 2019; Swan, 2008; Y. Zhang et al., 2009). Preeclampsia and preterm birth are leading causes of maternal mortality and morbidity across the globe and are associated with systemic inflammatory responses (Al-Jameil et al., 2014; Lo et al., 2013). Consequently, investigating the role of common environmental exposures, like phthalates, in maternal inflammation is important to advancing our understanding of these complex pregnancy disorders.
Previous in vivo and in vitro studies support an association between phthalates and inflammatory biomarkers, particularly DEHP/MEHP-induced oxidative stress and consequent upregulation of pro-inflammatory biomarkers (Chen, 2012; Duan et al., 2017; Erkekoglu et al., 2010; Manteiga Sara & Lee Kyongbum, 2017; Stermer et al., 2017; Tetz et al., 2013). However, only a few human studies have sought to corroborate these associations (Bedrosian et al., 2018; Ferguson et al., 2011, 2015; Trim et al., 2021). These studies assessed maternal inflammation using either only matrix metalloproteinases or CRP and interleukins. A previous preliminary PROTECT study investigated the associations between maternal phthalate exposures and biomarkers of inflammation and oxidative stress (Ferguson et al., 2014). However, only CRP and interleukins were measured in that study. Accordingly, this study utilized a diverse range of inflammatory biomarkers including CRP, matrix metalloproteinase-1, 2, and 9 (MMP1, MMP2, MMP9), intercellular adhesion molecule-1 (ICAM), and vascular cell adhesion molecule-1 (VCAM). MMPs are key regulators in tissue remodeling, a process that is suspected to trigger an inflammatory response when incomplete (Chen & Khalil, 2017). Hence, MMPs can serve as biomarkers of changes to vascular remodeling contributing to gestational inflammation.
MMP1, MMP2, and MMP9 are most often examined in pregnancy studies (Estrada-Gutierrez et al., 2011; C. Kim et al., 2022; Nikolov & Popovski, 2021; Sakowicz et al., 2018). CRP upregulation is noted in times of infection, disease, and other conditions that stimulate a systemic inflammatory response (Tjoa et al., 2003). Elevated CRP has been associated with hypertensive pregnancy disorders (Rebelo et al., 2013). ICAM and VCAM molecules are involved in the migration and localization of activated leukocytes to sites of inflammation in endothelial cells (Yusuf-Makagiansar et al., 2002). Thus, these proteins are key elements in either general inflammatory responses or secondary processes that can trigger maternal inflammation when rendered dysfunctional.
The present study examines the role of phthalate exposures in the maternal inflammatory response. To do so, we use data from the PROTECT cohort to investigate associations between urinary phthalate metabolites and inflammatory biomarkers, including CRP, MMP1, MMP2, MMP9, ICAM, and VCAM, during pregnancy. PROTECT is a prospective birth cohort study dedicated to researching the contributions of various environmental contaminants to maternal and fetal health in Puerto Rico (Ferguson et al., 2014). Puerto Rico historically has some of the highest rates of preterm birth and other adverse pregnancy outcomes across the globe (Meeker, Cantonwine, Rivera-González, Ferguson, Mukherjee, Calafat, Ye, Anzalota Del Toro, Crespo, et al., 2013). Previous studies revealed elevated urinary phthalate metabolite concentrations within the PROTECT cohort relative to women in the general US population, and that concentrations of some phthalate metabolites were associated with preterm birth (Ferguson et al., 2019). PROTECT is still actively recruiting pregnant women, and currently does not have enough clinically diagnosed cases of preeclampsia to test associations with phthalate exposure. Therefore, in the present study we sought to test the hypothesis that markers of phthalates exposure are associated with increased biomarkers of gestational inflammation, which is relevant for pregnancy outcomes, within the PROTECT cohort. Our secondary objectives were to evaluate effect modification of these associations by fetal sex and study visit.
2. Methods
2.1. Study Population
Data for this analysis was obtained from the PROTECT prospective birth cohort in Puerto Rico. The PROTECT cohort was started in 2011 and recruitment is ongoing. Details on study protocols and recruitment have been described previously (Meeker, Cantonwine, Rivera-González, Ferguson, Mukherjee, Calafat, Ye, Anzalota Del Toro, Crespo-Hernández, et al., 2013). In short, women were recruited at median 14 weeks gestation between 2011 and 2019 and participated in up to three study visits – two clinic visits occurring at median 18 (range 16–20) and 26 (range 24–28) weeks gestation, and one in-home visit occurring at median 22 (range 20–24) weeks gestation. Women were included in the study if they were between the ages of 18 and 40 years, had their first clinical visit before their 20th gestational week, did not take oral contraceptives in the three months prior to becoming pregnant, did not use in vitro fertilization to become pregnant, and had no known preexisting medical or obstetric conditions. Information on demographics and other relevant health information was collected at the first study visit. The demographics of the participants in this analysis were similar to those of the overall PROTECT birth cohort (Cathey et al., 2022; Rodríguez-Carmona et al., 2020).
This study was approved by the research and ethics committees of the University of Michigan School of Public Health, University of Puerto Rico, Northeastern University, and participating hospitals and clinics. All methods reported in this study were performed in accordance with relevant guidelines and regulations imposed by those institutions. All study participants provided full informed consent prior to participation.
2.2. Phthalate Exposure Assessment
Urine samples were collected into polypropylene containers from study participants at all three study visits (16–20, 20–24, and 24–28 weeks gestation). 491 women provided one sample and 327 women provided two samples. Samples across study visits were averaged using geometric means. Samples were then aliquoted at the University of Puerto Rico and shipped overnight at −80°C on dry ice to the Centers for Disease Control and Prevention for analysis. Samples were analyzed using solid phase extraction high-performance liquid chromatography-isotope dilution tandem mass spectrometry, described in more detail elsewhere (CDC, 2013). Each analytical batch included 40 unknown samples, five reagent blanks, and two high- and two low-concentration quality control materials. All quality control materials were characterized by 60 repeated measurements in a 3-week period to define control limits for each phthalate metabolite. Further details on sample analysis and quality control are published elsewhere (Kato et al., 2005; Silva et al., 2007, 2019). Samples obtained in earlier years of the PROTECT study beginning in 2011 were analyzed for 11 phthalate metabolites: mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-3-carboxypropyl phthalate (MCPP), mono carboxyisononyl phthalate (MCNP), mono carboxyisooctyl phthalate (MCOP), monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), and mono-isobutyl phthalate (MiBP). Later batches added in 2013 included the metabolites mono-hydroxyisobutyl phthalate (MHiBP), mono-hydroxybutyl phthalate (MHBP), mono isononyl phthalate (MNP), cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH), and cyclohexane-1,2-dicarboxylic acid monocarboxy isooctyl ester (MCOCH), and finally the metabolites mono oxononyl phthalate (MONP), mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP), and mono-2-ethyl-5-hydrohexyl terephthalate (MEHHTP) were the last to be added to the analytical panel in 2015. Values detected below the LOD were assigned a value of the LOD divided by the square root of two (Hornung & Reed, 1990).
2.3. Inflammatory and MMP biomarker Assessment
Inflammatory biomarkers were quantified from serum samples using customized Luminex assay from Invitrogen following the manufacturer’s recommended protocol, modified to include overnight incubation (with shaking) at 4°C. Most of the targets of interest required dilution prior to assay: C-reactive protein (CRP, Catalog #EPX01A10288901) was diluted 2000-fold; matrix metalloproteinase-2 (MMP2, Catalog #EPX01A12132901) and MMP9 (Catalog #EPX01A12016901) were diluted 50-fold; intracellular adhesion molecule (ICAM, Catalog #EPX01A10201901) and vascular cell adhesion molecule (VCAM, Catalog #EPX01A10232901) were diluted 200-fold; and MMP1 (Catalog #PPX07MX322FV) required no dilution. A Luminex-200 plate reader using xPonent software was used to acquire the raw data, which were compiled using Milliplex analyst (5.1.0.0). All samples were run in duplicate and then duplicate measures were averaged. Luminex assays were performed in the Rogel Cancer Center’s Immune Monitoring Shared Resource Center at the University of Michigan.
Based on the measurements of 8 standard concentrations provided by the manufacturer, a seven parameter standard curve was utilized to convert optical density values into concentrations (pg/mL). Based on percent recovery outside the ideal range of 70–130% (ratio of observed to expected concentration) we eliminated the highest standard concentration from the standard curve. Coefficients of variation (CVs) were then calculated. Sample CVs above 30% (CRP, N=260; MMP1, N=39; MMP2, N=9; MMP9, N=67; ICAM, N=43; VCAM, N=47), which indicate errors in pipetting and sample preparation, were eliminated from all subsequent analyses. After removal of sample CVs above 30%, intra-assay CVs ranged from 3.7% (VCAM) to 14.9% (CRP), and inter-assay CVs ranged from 6.1% (MMP2) to 12.0% (VCAM). All samples measured below the lower limit of detection (LOD) were assigned a value of the LOD divided by the square root of 2, and samples measured above the upper LOD (CRP, N=23) were assigned a value of the upper LOD.
2.4. Statistical Analyses
Initially, our study population consisted of 890 women (providing 1238 samples) for whom we had biomarker data on at least one exposure-outcome pair. We explored various possible covariates among this population of women – maternal age, education level, marital status, employment status, annual household income, smoking status, exposure level to secondhand smoke, alcohol use, parity, pre-pregnancy body mass index (BMI), and fetal sex. Covariates were selected based on a priori knowledge and are consistent with previously published PROTECT analyses. This assessment yielded models which adjusted for categorical maternal age and education level, and continuous pre-pregnancy BMI. Models additionally adjusted for specific gravity, which was measured using a digital handheld refractometer (AtagoCo., Ltd., Tokyo, Japan), to account for differences in urinary dilution. There were some study participants who were missing data on included covariates, resulting in a final sample size of 818 women (providing 1145 samples).
Distributions of exposure and outcome biomarker concentrations were assessed at each study visit. Intraclass correlation coefficients (ICCs) were also calculated to assess between- and within-person variability of biomarker concentrations across study visits. All biomarkers displayed right-skewed distributions and were natural log-transformed for all subsequent analyses.
Linear mixed effects models (LMEs) were used to regress inflammatory biomarkers on phthalates and included random intercepts for study participant. Sensitivity analyses were then employed to explore effect modification by fetal sex and differences between study visits. These analyses were achieved using 95% confidence intervals and interaction terms between fetal sex or study visit indicator variables and phthalate exposure variables. All results can be interpreted as the percent change in inflammatory biomarker concentration associated with an interquartile range (IQR) increase in phthalate metabolite. Significance level was set to alpha=0.05. All analyses were completed using R software and the default setting, corresponding to no within-group correlation.
3. Results
3.1. Demographics
Characteristics of the study population, including demographics and other relevant health information, are shown in Table 1. The majority of participants were under the age of 30 years (68.3%), had attained at least some college education (55.6%), were either married or cohabitating (80.7%), were employed (63.8%), lived in a home earning less than $30,000 per year (64.2%), were never-smokers (86.0%), reported no exposure to secondhand smoke (89.4%), were nulliparous (43.2%), and had a pre-pregnancy BMI ≤ 25 kg/m2 (52.3%). There were more pregnancies with a male fetus (52.1%) than with a female fetus (47.9%).
Table 1.
Demographics and other relevant health information for 890 women in PROTECT.
| N | % | |
|---|---|---|
| Maternal Age | ||
| 18–24 | 321 | 36.1% |
| 25–29 | 287 | 32.2% |
| 30–34 | 187 | 21.0% |
| 35–41 | 95 | 10.7% |
| Missing | 0 | |
| Maternal Education | ||
| GED or less | 184 | 21.2% |
| Some college | 299 | 34.4% |
| Bachelors or higher | 386 | 44.4% |
| Missing | 21 | |
| Marital Status | ||
| Single | 168 | 19.3% |
| Married | 470 | 54.0% |
| Cohabitating | 232 | 26.7% |
| Missing | 20 | |
| Currently Employed | ||
| No | 314 | 36.2% |
| Yes | 554 | 63.8% |
| Missing | 22 | |
| Annual Household Income | ||
| <10k | 236 | 30.6% |
| 10k – <30k | 259 | 33.6% |
| 30k – <50k | 182 | 23.6% |
| ≥50k | 93 | 12.1% |
| Missing | 120 | 15.6% |
| Smoking Status | ||
| Never | 765 | 86.0% |
| Ever | 111 | 12.5% |
| Current | 14 | 1.6% |
| Missing | 0 | |
| ETS | ||
| Never | 725 | 89.4% |
| 1 hour | 40 | 4.9% |
| > 1 hour | 46 | 5.7% |
| Missing | 79 | |
| Alcohol Use | ||
| Never | 437 | 50.3% |
| Yes, before pregnancy | 376 | 43.3% |
| Yes, currently | 56 | 6.4% |
| Missing | 21 | |
| Number of Children | ||
| 0 | 378 | 43.2% |
| 1 | 307 | 35.1% |
| 2–5 | 189 | 21.6% |
| Missing | 16 | |
| BMI | ||
| (0, 25] | 433 | 52.3% |
| (25, 29.9] | 235 | 28.4% |
| (29.9, 51] | 160 | 19.3% |
| Missing | 62 | |
| Fetal Sex | ||
| Female | 355 | 47.9% |
| Male | 386 | 52.1% |
| Missing | 149 |
3.2. Biomarker Distributions
Inflammatory biomarkers included in this study were CRP, MMP1, MMP2, MMP9, ICAM, and VCAM, distributions for which are shown in Table 2. All measurements were above the limit of detection (LOD). ICC characterizes the within-subject variability relative to total (within-subject plus between-subject) variability across visits with values between 0–1. ICC values closer to 1 indicate low temporal variability within subjects whereas ICC values closer to 0 indicate high temporal variability. MMP1, MMP2, and ICAM marker measurements displayed low temporal variability within subjects (ICC =0.79, 0.77, 0.86). Meanwhile, MMP9 and VCAM marker measurements exhibited moderate within-subject temporal variability (ICC =0.49, 0.47).
Table 2.
Distributions of inflammatory biomarkers, by study visit, among 818 women in PROTECT.
| Visit | N | Min | p25 | Med | p75 | p90 | Max | GM | SD | IQR | ICC (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | 1 | 623 | 86675 | 1920120 | 3816094 | 6611702 | 11294355 | 32013346 | 3349068 | 4625260 | 4691582 | 0.68 (0.62, 0.74) |
| 3 | 411 | 132096 | 1667929 | 3588576 | 6913178 | 12282266 | 53806064 | 3219248 | 5370313 | 5245249 | ||
| MMP1 | 1 | 731 | 1.58 | 156 | 312 | 558 | 1020 | 4301 | 295 | 504 | 402 | 0.79 (0.74, 0.82) |
| 3 | 479 | 7.84 | 190 | 380 | 665 | 1261 | 6006 | 356 | 595 | 475 | ||
| MMP2 | 1 | 743 | 698 | 7935 | 9446 | 11430 | 26805 | 79722 | 10740 | 12644 | 3495 | 0.77 (0.73, 0.81) |
| 3 | 487 | 4690 | 7838 | 9261 | 10868 | 14488 | 71698 | 9907 | 8974 | 3030 | ||
| MMP9 | 1 | 716 | 978 | 23439 | 33419 | 46046 | 57445 | 159501 | 31810 | 17693 | 22607 | 0.49 (0.40, 0.56) |
| 3 | 468 | 1700 | 22399 | 32707 | 45025 | 55720 | 119523 | 31106 | 17059 | 22626 | ||
| ICAM | 1 | 698 | 85337 | 506320 | 609455 | 781580 | 1051434 | 8172562 | 646066 | 506357 | 275261 | 0.86 (0.83, 0.88) |
| 3 | 450 | 218407 | 500036 | 607207 | 791400 | 1188143 | 9983930 | 662515 | 641760 | 291364 | ||
| VCAM | 1 | 686 | 37339 | 206742 | 253639 | 305048 | 367289 | 788578 | 243715 | 86844 | 98307 | 0.47 (0.38, 0.55) |
| 3 | 455 | 57195 | 215569 | 263873 | 319075 | 377158 | 1440323 | 260460 | 106129 | 103506 |
All biomarkers were measured above the limit of detection (LOD) in 100% of samples. All concentrations are presented in pg/mL.
GM: geometric mean; SD: standard deviation; IQR: interquartile range; ICC: intraclass correlation coefficient; CI: confidence interval.
3.3. Exposure Descriptives
Distributions of phthalate metabolites are shown in Table S1. For most of the metabolites analyzed, >90% of measurements were above the LOD. MCPP, MEHP, and MHBP measurements were the exceptions with %>LOD values ranging from 81–88%, 80–84%, and 77–86% respectively. MCOCH, MHINCH, and MNP measurement data were excluded from the present analysis due to %>LOD values <50%. Most of the analyzed phthalate metabolites additionally exhibited high within-subject variability with ICC values ranging from 0.10 to 0.46.
3.4. Main Effects
Overall, most of the phthalate metabolites examined were not significantly associated with inflammatory biomarkers in adjusted linear mixed effects models as shown in Table 3. However, all three MMPs were significantly associated with at least one phthalate metabolite. MBP was associated with a 7.86% increase (95% CI: 0.49, 15.76) in MMP1. MONP and MCNP were associated with an 8.30% increase (95% CI: 2.22, 14.75) and 4.51% decrease (95% CI: 1.40, 7.53) in MMP2, respectively. MECPTP was associated with a 6.62% decrease (95% CI: 1.05, 11.9) in MMP9. MEHP was associated with a 3.55% decrease (95% CI: 0.59, 6.42) in ICAM. Of note, these associations were largely not significantly modified by study visit.
Table 3.
Effect estimates and 95% confidence intervals from linear mixed effects models for the associations between phthalate metabolites and inflammatory biomarkers among 818 women in PROTECT. Estimates refer to the percent change in inflammatory biomarker with an interquartile range increase in phthalate metabolite.
| CRP | ICAM | VCAM | MMP1 | MMP2 | MMP9 | |
|---|---|---|---|---|---|---|
| MBP | 4.91 (−3.82, 14.42) | −0.53 (−3.23, 2.26) | 0.38 (−2.84, 3.71) | 7.86 (0.49, 15.76) | 2.06 (−1.94, 6.23) | −0.69 (−5.31, 4.15) |
| MHBP | 4.50 (−8.04, 18.75) | −0.75 (−4.64, 3.30) | 0.50 (−4.16, 5.38) | 0.00 (−9.44, 10.42) | 5.49 (−0.79, 12.17) | −6.24 (−12.29, 0.22) |
| MIBP | 1.48 (−7.51, 11.35) | −1.60 (−4.63, 1.54) | −1.79 (−5.08, 1.62) | 3.15 (−4.59, 11.53) | 0.03 (−4.22, 4.47) | 0.82 (−4.03, 5.92) |
| MHIBP | 7.85 (−5.47, 23.05) | −3.26 (−7.47, 1.15) | −2.69 (−7.20, 2.04) | −1.57 (−11.45, 9.41) | −3.59 (−9.74, 2.99) | −0.70 (−7.13, 6.16) |
| MEP | 0.43 (−8.08, 9.72) | −2.71 (−5.63, 0.31) | −1.60 (−4.78, 1.68) | 3.38 (−4.04, 11.37) | −1.80 (−5.82, 2.39) | 0.42 (−4.21, 5.27) |
| MEHP | −2.50 (−10.89, 6.69) | −3.55 (−6.42, −0.59) | −0.81 (−4.12, 2.61) | −1.50 (−8.59, 6.14) | 0.15 (−3.98, 4.45) | 2.12 (−2.78, 7.28) |
| MEHHP | 5.19 (−3.13, 14.23) | −1.40 (−3.98, 1.24) | −1.35 (−4.35, 1.75) | 1.21 (−5.33, 8.19) | −0.49 (−4.16, 3.32) | −0.23 (−4.61, 4.35) |
| MEOHP | 3.63 (−4.58, 12.54) | −1.63 (−4.20, 1.02) | −1.06 (−4.09, 2.07) | 2.81 (−3.86, 9.95) | −0.52 (−4.20, 3.31) | −0.71 (−5.11, 3.89) |
| MECPP | 7.00 (−2.15, 17.01) | −1.05 (−3.84, 1.82) | −0.53 (−3.75, 2.79) | 3.38 (−3.78, 11.08) | −1.83 (−5.73, 2.23) | −1.63 (−6.23, 3.18) |
| MCOP | 2.13 (−5.03, 9.83) | −0.29 (−2.59, 2.07) | −0.15 (−2.82, 2.60) | 1.94 (−3.92, 8.16) | −2.31 (−5.50, 0.98) | −3.50 (−7.18, 0.32) |
| MONP | −5.20 (−15.64, 6.53) | −1.32 (−4.31, 1.76) | −3.47 (−7.67, 0.93) | 5.11 (−2.70, 13.54) | 8.30 (2.22, 14.75) | −1.55 (−7.40, 4.68) |
| MCNP | 2.33 (−4.73, 9.91) | 0.39 (−1.82, 2.65) | 1.43 (−1.29, 4.23) | 0.81 (−4.81, 6.76) | −4.51 (−7.53, −1.40) | −1.92 (−5.71, 2.02) |
| MCPP | 2.64 (−5.55, 11.53) | 0.22 (−2.41, 2.92) | −0.11 (−3.11, 2.98) | 1.05 (−5.45, 8.01) | 0.25 (−3.42, 4.05) | −1.58 (−5.81, 2.84) |
| MBZP | −0.61 (−9.07, 8.65) | −1.07 (−4.00, 1.95) | 1.44 (−1.88, 4.88) | 5.48 (−2.15, 13.71) | 0.51 (−3.65, 4.85) | 0.19 (−4.53, 5.14) |
| MECPTP | 7.08 (−3.39, 18.69) | 2.45 (−0.61, 5.61) | −0.18 (−4.17, 3.99) | 3.62 (−3.90, 11.73) | 1.15 (−4.56, 7.20) | −6.62 (−11.87, −1.05) |
| MEHHTP | 11.83 (−1.45, 26.90) | 3.29 (−0.45, 7.18) | 0.52 (−4.39, 5.67) | 1.84 (−7.10, 11.65) | 0.30 (−6.55, 7.64) | −6.53 (−12.88, 0.28) |
All models adjust for categorical maternal education, continuous maternal age and pre-pregnancy BMI, and specific gravity.
3.5. Fetal Sex Effects
Effect modification by fetal sex on the relationship between phthalate metabolites and inflammatory biomarkers was observed for several associations (Figure 1 and Table S2). Significant effect modification by fetal sex was noted for the associations between MMP1 and the following phthalates: MBP with a 15.2% increase (95% CI: 4.55, 27.0; p-int=0.030), MIBP with a 12.8% increase (95% CI: 0.89, 26.0; p-int=0.048), MHIBP with a 16.8% increase (95% CI: 0.44, 35.9; p-int=0.007), MEHHP with a 11.0% increase (95% CI: 0.92, 22.1; p-int=0.011), MEOHP with a 12.5% increase (95% CI: 2.28, 23.7; p-int=0.014), and MECPP with a 14.7% increase (95% CI: 3.63, 26.8; p-int=0.012). Of these MMP1-phthalate associations, all were significantly positive among female fetuses, but none reached statistical significance among male fetuses. Effect modification by fetal sex was additionally observed for the associations between MMP2 and MEHHP with a 3.96% increase (95% CI: 0.02, 8.06; p-int=0.028), MEOHP with a 3.98% increase (95% CI: 0.03, 8.08; p-int=0.009), and MECPP with a 4.27% increase (95% CI: 0.10, 8.62; p-int=0.014), and these associations were also significant among only female fetuses. Effect modification by fetal sex was noted for the association between MMP9 and MCOP with a 6.06% decrease (95% CI: −11.0, −0.84; p-int=0.019), which was only significant among male fetuses.
Figure 1.
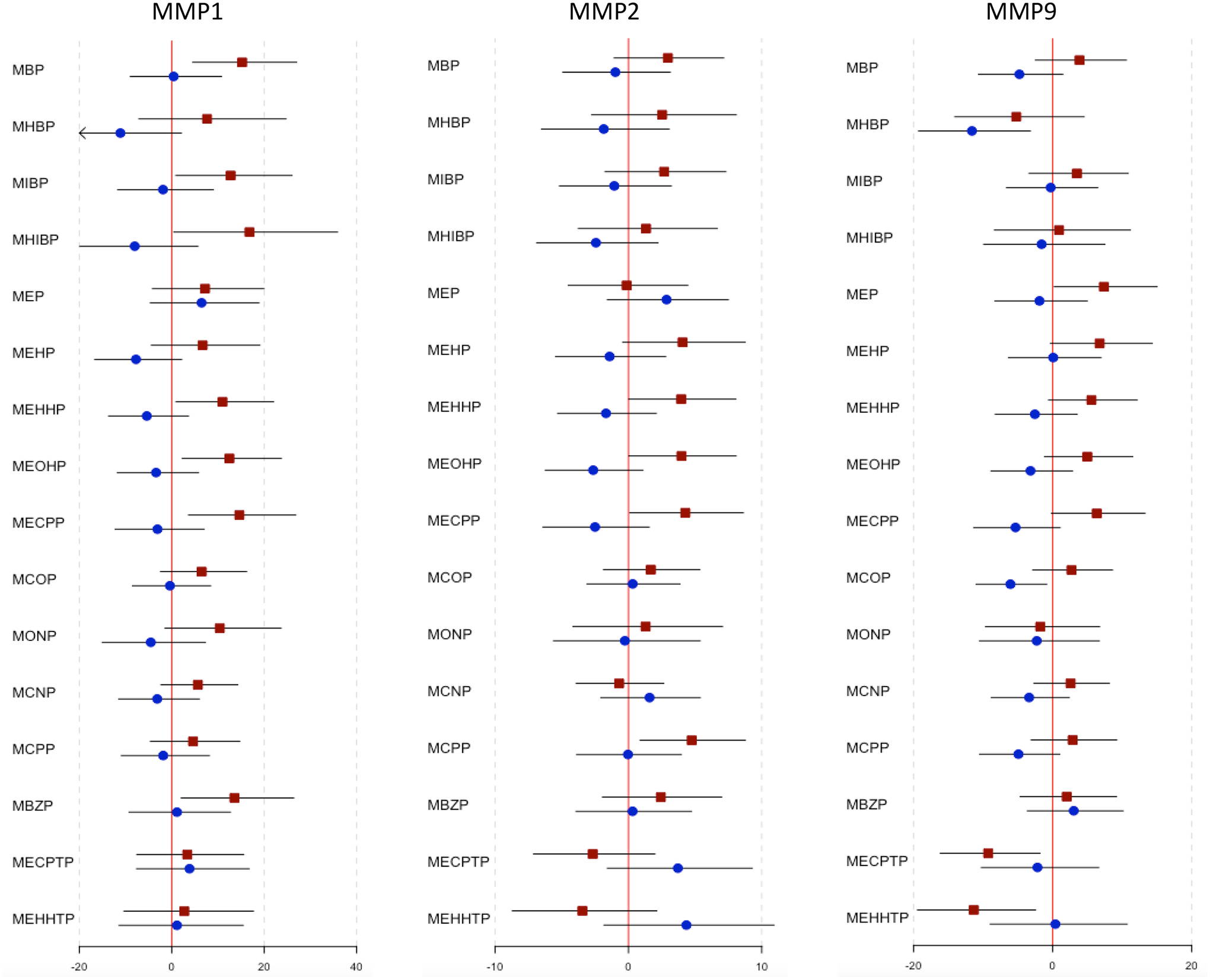
Effect estimates and 95% confidence intervals from linear mixed effects models for the associations between phthalate metabolites and inflammatory biomarkers among 818 women in PROTECT, by fetal sex.
Red squares denote estimates for female fetuses and blue circles denote estimates for male fetuses. Effect estimates measured as % change in inflammatory biomarker concentration per interquartile (IQR) increase in phthalate metabolite concentration.
4. Discussion
The present study is one of the first to examine associations between maternal phthalate exposure and this set of inflammatory biomarkers, including MMP1, MMP2, MMP9, ICAM, VCAM, and CRP, during pregnancy. We previously examined associations between phthalate metabolites and CRP and biomarkers of oxidative stress in a preliminary subset of this cohort (Ferguson et al., 2014). In our primary analysis we observed several associations between MMPs and maternal phthalate metabolites. ICAM was associated with one phthalate metabolite, whereas no associations were observed for VCAM and CRP. Notably, our results revealed significant effect modification of multiple phthalate and inflammatory biomarker associations by fetal sex. Associations between phthalate metabolites and MMP1 and MMP2 were mostly significant and positive only among mothers carrying female fetuses, while the associations with MMP9 were limited.
Of the inflammatory biomarkers assessed here, the MMPs were most notably associated with maternal phthalate exposure, although the overall effect direction was mixed. MMPs are enzymes that aid in the degradation and remodeling of the extracellular matrix and are essential in vascular remodeling during pregnancy (Chen & Khalil, 2017; Palei et al., 2012). Given these roles, changes in expression of MMPs are associated with multiple adverse pregnancy outcomes, including preeclampsia and spontaneous preterm birth, highlighting the importance of understanding environmental MMP regulators (Lyall et al., 2013; Pandey & Awasthi, 2019; Tency et al., 2012).
Our results showed that maternal MBP concentrations were associated with increased MMP1. An in vitro study using human vascular smooth cells found that MMP1 activated and recruited neutrophils evidenced by increased neutrophil migration in MMP1-treated tissues (Estrada-Gutierrez et al., 2011). Neutrophil migration is associated with an increased inflammatory response. The capacity of MMP1 to recruit neutrophils may also be mediated by the secretion of interleukin-8 (IL-8), a pro-inflammatory cytokine involved in neutrophil recruitment that is associated with maternal phthalate exposure as well (Vetrano et al., 2010). Our present findings suggest a potential role of maternal phthalate exposures in altering maternal MMP1, indicative of an enhanced maternal inflammatory response. This study is one of the first to demonstrate associations between upregulated MMP1 and phthalate exposures, warranting further investigation.
In our present study, we discovered both positive and negative associations between maternal phthalate exposure and MMP2, as well as a negative association with MMP9. Two separate in vitro studies using endometrial cells and breast cancer cells, respectively, found that DEHP exposure upregulated MMP2 and MMP9 (S. H. Kim et al., 2015; S. Zhang et al., 2016). Another in vitro study reported that DBP treatment of endothelial cells altered expression of the MMP2 gene, the direction of regulation depending on the length of exposure (Stanic et al., 2022). Other studies have also investigated associations between MMP2 and MMP9 with maternal inflammation, particularly in the context of preeclampsia (Eleuterio et al., 2015; Montagnana et al., 2009; Myers et al., 2005; Palei et al., 2012). These studies generally found that MMP2 was upregulated in women with preeclampsia, characterized by inflammation, but null differences in MMP9 levels. The lack of conclusive associations between MMP9 and maternal inflammation suggests that circulating MMP9 may be a less robust inflammatory biomarker relative to MMP1 and MMP2, clarifying our unexpected observations regarding MMP9. The mixed associations with MMP2 may be the result of complex interactions between MMP2 and regulatory elements, including tissue inhibitor of metalloproteinase 2 (TIMP2) as examined in Eleuterio et al. However, further research is required to elucidate our findings between maternal phthalate exposure and MMP2 and MMP9.
ICAM and VCAM are endothelial cell adhesion molecules that partake in the systemic inflammatory response when activated by pro-inflammatory cytokines (Videm & Albrigtsen, 2008). Thus, they can be utilized as markers of endothelial activation and systemic inflammatory responses. Here, we did not observe any changes in VCAM but did note a downregulation of ICAM with biomarkers of maternal phthalate exposure. A similar cohort study revealed null findings of VCAM and ICAM with inflammatory biomarkers suggesting the possibility that circulating VCAM and ICAM may not be sensitive markers of maternal inflammation, perhaps due to interactions with regulatory elements such as thromboxane (Lewis et al., 2010).
We did not detect any significant associations between maternal phthalate exposure and CRP, despite its well-documented indication of an inflammatory response. These findings are consistent with our previous study utilizing this biomarker in the PROTECT cohort where CRP was marginally associated with increases in MCPP and MCNP (Ferguson et al., 2014). In a separate analysis of the LIFECODES cohort, CRP was not found to be significantly associated with phthalate metabolites (Ferguson et al., 2015). However, CRP was significantly associated with MBzP and MiBP in an NHANES 1999–2006 sample, which may be attributed to physiological differences between pregnant women and the general US population or other differences between studies and study populations (Ferguson et al., 2011). Another consideration is that CRP was measured in the second trimester, which is characterized by a more anti-inflammatory profile compared to the other trimesters (Mor et al., 2017). Thus, associations between CRP and maternal phthalate exposures may be more appropriately evaluated in another timeframe.
Most of the phthalate metabolites we observed to be significantly associated with inflammatory biomarkers were metabolites of high molecular weight phthalates, apart from MBP which is a derivative of dibutyl phthalate (DBP). In an exploratory subset of the LIFECODES birth cohort, we previously observed suggestive positive associations between MMP1 and DBP metabolites (Bedrosian et al., 2018), which is consistent with our present findings in PROTECT. We noted associations between urinary DEHP metabolites with MMP1 and MMP9 in LIFECODES as well. In this study, however, we only found associations between MEHP, a DEHP metabolite, and ICAM. The prevalence of HMW phthalates in our observed associations supports prioritizing the reduction of HMW phthalate exposures.
A notable observation in the present study was fetal sex effect modification of associations between maternal phthalate exposure and inflammatory biomarkers, particularly by female fetal sex for MMPs. These sex-specific effects may be attributed to sexual dimorphism in the maternal secretion of growth factors and pro-inflammatory cytokines, which aid in the activation and regulation of MMPs, VCAM, and ICAM. Women carrying a male fetus have been linked to higher concentrations of pro-inflammatory cytokines and higher concentrations of angiogenic growth factors (Enninga et al., 2015). A recent study also reported more robust inflammatory cytokine mRNA expression associated with prenatal phthalate exposure in the placentae of male fetuses than female fetuses (Wang et al., 2020). These findings could additionally be explained by sex-specific phthalate-induced endocrine disruption concerning human chorionic gonadotropin (hCG) and its transcription factor, peroxisome proliferator-activated receptor gamma (PPARγ) (Adibi et al., 2015, 2017). Given the critical role of hCG in placental function, its dimorphic expression by fetal sex may increase the risk of placental dysfunction and its accompanying inflammatory response in one sex (Rosenfeld, 2015).
Our results generally indicate that female fetal sex, not male fetal sex, enhanced the associations between maternal phthalate exposures and circulating inflammatory biomarkers. The contrast between our findings and the literature could be the result of using circulating inflammatory biomarkers versus placental or MMPs versus inflammatory cytokines to assess gestational inflammation. Despite these differences, our findings introduce an intriguing avenue for future research examining the role of fetal sex in differential inflammatory responses to prenatal phthalate exposures.
This study has several limitations. Our inflammatory biomarker measurements were based on circulating levels as opposed to tissue sampling, which was not feasible for our study. Previous studies have reported changes in TIMP concentrations associated with maternal inflammation; thus, measuring MMP/TIMP ratios, which we did not analyze, may contextualize changes in total MMP concentrations (Chen & Khalil, 2017; Deng et al., 2015; Estrada-Gutierrez et al., 2011; Palei et al., 2012). We also noted high variability within subjects in phthalate metabolite measurements across pregnancy as evidenced by low to moderate ICC values. This variability has similarly been noted in other studies measuring urinary phthalate metabolites in pregnant women, which may be improved by increasing the number of samples per subject (Casas et al., 2018; Fisher et al., 2015; Philippat et al., 2021; Shin et al., 2019). Additionally, our use of a significance level of 0.05 leaves room for Type I error in our results and the potential for overestimation of some associations.
Despite the aforementioned weaknesses, our study offers many strengths. We utilized a large sample size with repeated measurements taken throughout pregnancy, allowing us to better assess phthalate exposure and the outcomes. Furthermore, we analyzed a diverse and novel set of inflammatory biomarkers. This is the first human study, to our knowledge, to examine the role of phthalate exposure on the maternal inflammatory response collectively using CRP, MMPs, ICAM, and VCAM biomarkers. Our observed associations between MMPs, key regulators of gestational vascular remodeling, and maternal phthalate exposures provide evidence that phthalates may impact the vascular remodeling process during pregnancy promoting maternal inflammation. Increased maternal inflammation and deficits in vascular remodeling may contribute to gestational pathologies, including preeclampsia, preterm birth, and fetal growth restriction (Cotechini et al., 2014; Ulrich et al., 2019). Thus, these inflammatory biomarkers can be versatilely used as indicators of several major adverse pregnancy outcomes. Additionally, our findings of fetal sex effect modification provide intriguing insight into the sexual dimorphism of inflammatory responses and potentially its related pathologies. Further research is needed to understand the balance of MMPs with other regulatory elements in maternal inflammatory responses as well as the impacts of timing and placental versus plasma sampling for sex-specific effect modifications. Recruitment is ongoing in the PROTECT cohort; as our sample size increases, we plan to assess the impact of phthalate exposure on gestational inflammation, and the role that MMPs play in mediating those relationships.
Supplementary Material
Highlights.
Phthalates are ubiquitous exposures found in many plastics and consumer products
Matrix metalloproteinases are associated with maternal inflammation
Phthalate metabolites were associated with changes in matrix metalloproteinases
Phthalate exposure may impact the development of inflammatory maternal disease
Fetal sex moderates these associations
Acknowledgments
We would like to extend our gratitude to all PROTECT study participants and their families. We also thank the nurses and research staff who participated in cohort recruitment and follow up, as well as the Federally Qualified Health Centers (FQHC) and clinics in Puerto Rico who facilitated participant recruitment, including Morovis Community Health Center (FQHC), Prymed: Ciales Community Health Center (FQHC), Camuy Health Services, Inc. (FQHC), and the Delta OBGyn (Prenatal Clinic).
Funding
This study was supported by the Superfund Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; grant number P42ES017198). Additional support was provided from NIEHS grant numbers R01ES032203, R01ES031591, P30ES017885, T32ES007062, and the Environmental influences on Child Health Outcomes (ECHO) program grant number UH3OD023251. ECHO is a nationwide research program supported by the NIH, Office of the Director to enhance child health.
This study was approved by the research and ethics committees of the University of Michigan School of Public Health, University of Puerto Rico, Northeastern University, and participating hospitals and clinics. The patients/participants provided their written informed consent to participate in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Credit authorship contributions statement
C.W.L.: Investigation; Writing – original draft. A.L.C.: Formal analysis; Methodology; Writing – review & editing. D.J.W: Conceptualization; Writing – review & editing; Funding acquisition. Z.Y.R.: Data curation; Project administration. C.M.V.: Data curation; Project administration. A.N.A.: Conceptualization; Funding acquisition. J.F.C.: Conceptualization; Funding acquisition. J.D.M.: Conceptualization; Funding acquisition; Supervision; Writing - review & editing.
References
- Adibi JJ, Lee MK, Naimi AI, Barrett E, Nguyen RH, Sathyanarayana S, Zhao Y, Thiet M-P, Redmon JB, & Swan SH (2015). Human Chorionic Gonadotropin Partially Mediates Phthalate Association With Male and Female Anogenital Distance. The Journal of Clinical Endocrinology and Metabolism, 100(9), E1216–E1224. 10.1210/jc.2015-2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Zhao Y, Zhan LV, Kapidzic M, Larocque N, Koistinen H, Huhtaniemi IT, & Stenman U-H (2017). An Investigation of the Single and Combined Phthalate Metabolite Effects on Human Chorionic Gonadotropin Expression in Placental Cells. Environmental Health Perspectives, 125(10), 107010. 10.1289/EHP1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jameil N, Aziz Khan F, Fareed Khan M, & Tabassum H (2014). A Brief Overview of Preeclampsia. Journal of Clinical Medicine Research, 6(1), 1–7. 10.4021/jocmr1682w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian LD, Ferguson KK, Cantonwine DE, McElrath TF, & Meeker JD (2018). Urinary phthalate metabolite concentrations in relation to levels of circulating matrix metalloproteinases in pregnant women. Science of The Total Environment, 613–614, 1349–1352. 10.1016/j.scitotenv.2017.09.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, Basagaña X, Sakhi AK, Haug LS, Philippat C, Granum B, Manzano-Salgado CB, Brochot C, Zeman F, de Bont J, Andrusaityte S, Chatzi L, Donaire-Gonzalez D, Giorgis-Allemand L, Gonzalez JR, Gracia-Lavedan E, Grazuleviciene R, Kampouri M, Lyon-Caen S, … Vrijheid M (2018). Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environment International, 121, 561–573. 10.1016/j.envint.2018.09.046 [DOI] [PubMed] [Google Scholar]
- Cathey AL, Watkins DJ, Rosario ZY, Vélez C, Mukherjee B, Alshawabkeh AN, Cordero JF, & Meeker JD (2022). Biomarkers of Exposure to Phthalate Mixtures and Adverse Birth Outcomes in a Puerto Rico Birth Cohort. Environmental Health Perspectives, 130(3), 037009. 10.1289/EHP8990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2013). Metabolites of phthalates and phthalate alternatives. 42.
- Chen. (2012). Mono-2-ethylhexyl phthalate induced loss of mitochondrial membrane potential and activation of Caspase3 in HepG2 cells. Environmental Toxicology and Pharmacology, 33(3), 421–430. 10.1016/j.etap.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Chen J, & Khalil RA (2017). Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. Progress in Molecular Biology and Translational Science, 148, 87–165. 10.1016/bs.pmbts.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón I, Caro D, Bourdony CJ, & Rosario O (2000). Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environmental Health Perspectives, 108(9), 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, & Graham CH (2014). Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. Journal of Experimental Medicine, 211(1), 165–179. 10.1084/jem.20130295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C-L, Ling S-T, Liu X-Q, Zhao Y-J, & Lv Y-F (2015). Decreased expression of matrix metalloproteinase-1 in the maternal umbilical serum, trophoblasts and decidua leads to preeclampsia. Experimental and Therapeutic Medicine, 9(3), 992–998. 10.3892/etm.2015.2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Wang L, Han L, Wang B, Sun H, Chen L, Zhu L, & Luo Y (2017). Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environment International, 109, 53–63. 10.1016/j.envint.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Eleuterio NM, Palei ACT, Rangel Machado JS, Tanus-Santos JE, Cavalli RC, & Sandrim VC (2015). Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health, 5(2), 205–208. 10.1016/j.preghy.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Enninga EAL, Nevala WK, Creedon DJ, Markovic SN, & Holtan SG (2015). Fetal Sex-Based Differences in Maternal Hormones, Angiogenic Factors, and Immune Mediators During Pregnancy and the Postpartum Period. American Journal of Reproductive Immunology, 73(3), 251–262. 10.1111/aji.12303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkekoglu P, Rachidi W, Yuzugullu OG, Giray B, Favier A, Ozturk M, & Hincal F (2010). Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicology and Applied Pharmacology, 248(1), 52–62. 10.1016/j.taap.2010.07.016 [DOI] [PubMed] [Google Scholar]
- Estrada-Gutierrez G, Cappello RE, Mishra N, Romero R, Strauss JF, & Walsh SW (2011). Increased Expression of Matrix Metalloproteinase-1 in Systemic Vessels of Preeclamptic Women. The American Journal of Pathology, 178(1), 451–460. 10.1016/j.ajpath.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, Rivera-González LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, Jiménez-Vélez B, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, & Meeker JD (2014). Urinary Phthalate Metabolite Associations with Biomarkers of Inflammation and Oxidative Stress Across Pregnancy in Puerto Rico. Environmental Science & Technology, 48(12), 7018–7025. 10.1021/es502076j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Loch-Caruso R, & Meeker JD (2011). Urinary Phthalate Metabolites in Relation to Biomarkers of Inflammation and Oxidative Stress: NHANES 1999–2006. Environmental Research, 111(5), 718–726. 10.1016/j.envres.2011.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Mukherjee B, Loch-Caruso R, & Meeker JD (2015). Associations between Maternal Biomarkers of Phthalate Exposure and Inflammation Using Repeated Measurements across Pregnancy. PLOS ONE, 10(8), e0135601. 10.1371/journal.pone.0135601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, Vélez Vega C, Cordero JF, Alshawabkeh A, & Meeker JD (2019). Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environment International, 132, 105099. 10.1016/j.envint.2019.105099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Mallick R, LeBlanc A, Hauser R, Feeley M, Koniecki D, Ramsay T, Provencher G, Bérubé R, & Walker M (2015). Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of Exposure Science & Environmental Epidemiology, 25(3), 231–239. 10.1038/jes.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene, 5(1), 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Kato K, Silva MJ, Needham LL, & Calafat AM (2005). Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Analytical Chemistry, 77(9), 2985–2991. 10.1021/ac0481248 [DOI] [PubMed] [Google Scholar]
- Kelley KE, Hernández-Díaz S, Chaplin EL, Hauser R, & Mitchell AA (2012). Identification of Phthalates in Medications and Dietary Supplement Formulations in the United States and Canada. Environmental Health Perspectives, 120(3), 379–384. 10.1289/ehp.1103998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Cathey AL, Watkins DJ, Mukherjee B, Rosario-Pabón ZY, Vélez-Vega CM, Alshawabkeh AN, Cordero JF, & Meeker JD (2022). Maternal blood metal concentrations are associated with matrix metalloproteinases (MMPs) among pregnant women in Puerto Rico. Environmental Research, 209, 112874. 10.1016/j.envres.2022.112874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Cho S, Ihm HJ, Oh YS, Heo S-H, Chun S, Im H, Chae HD, Kim C-H, & Kang BM (2015). Possible Role of Phthalate in the Pathogenesis of Endometriosis: In Vitro, Animal, and Human Data. The Journal of Clinical Endocrinology & Metabolism, 100(12), E1502–E1511. 10.1210/jc.2015-2478 [DOI] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, & Mazzeo P (2003). In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environmental Health Perspectives, 111(14), 1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DF, Canzoneri BJ, Gu Y, Zhao S, & Wang Y (2010). Maternal Levels of Prostacyclin, Thromboxane, ICAM, and VCAM in Normal and Preeclamptic Pregnancies. American Journal of Reproductive Immunology, 64(6), 376–383. 10.1111/j.1600-0897.2010.00861.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JO, Mission JF, & Caughey AB (2013). Hypertensive disease of pregnancy and maternal mortality. Current Opinion in Obstetrics and Gynecology, 25(2), 124–132. 10.1097/GCO.0b013e32835e0ef5 [DOI] [PubMed] [Google Scholar]
- Lyall F, Robson SC, & Bulmer JN (2013). Spiral Artery Remodeling and Trophoblast Invasion in Preeclampsia and Fetal Growth Restriction. Hypertension, 62(6), 1046–1054. 10.1161/HYPERTENSIONAHA.113.01892 [DOI] [PubMed] [Google Scholar]
- Manteiga Sara & Lee Kyongbum. (2017). Monoethylhexyl Phthalate Elicits an Inflammatory Response in Adipocytes Characterized by Alterations in Lipid and Cytokine Pathways. Environmental Health Perspectives, 125(4), 615–622. 10.1289/EHP464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Vendittelli F, & Sauvant-Rochat M-P (2015). Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environment International, 83, 116–136. 10.1016/j.envint.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, Ye X, Anzalota Del Toro LV, Crespo N, Jiménez-Vélez B, Alshawabkeh AN, & Cordero JF (2013). Distribution, variability and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental Science & Technology, 47(7), 3439–3447. 10.1021/es400510g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, Ye X, Anzalota Del Toro LV, Crespo-Hernández N, Jiménez-Vélez B, Alshawabkeh AN, & Cordero JF (2013). Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental Science & Technology, 47(7), 3439–3447. 10.1021/es400510g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, & Ferguson KK (2014). Urinary Phthalate Metabolites Are Associated With Decreased Serum Testosterone in Men, Women, and Children From NHANES 2011–2012. The Journal of Clinical Endocrinology and Metabolism, 99(11), 4346–4352. 10.1210/jc.2014-2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnana M, Lippi G, Albiero A, Scevarolli S, Salvagno GL, Franchi M, & Guidi GC (2009). Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and preeclamptic pregnancy. Journal of Clinical Laboratory Analysis, 23(2), 88–92. 10.1002/jcla.20295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Aldo P, & Alvero AB (2017). The unique immunological and microbial aspects of pregnancy. Nature Reviews Immunology, 17(8), 469–482. 10.1038/nri.2017.64 [DOI] [PubMed] [Google Scholar]
- Myers JE, Merchant SJ, Macleod M, Mires GJ, Baker PN, & Davidge ST (2005). MMP-2 Levels are Elevated in the Plasma of Women Who Subsequently Develop Preeclampsia. Hypertension in Pregnancy, 24(2), 103–115. 10.1081/PRG-200059836 [DOI] [PubMed] [Google Scholar]
- Nikolov A, & Popovski N (2021). Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics, 11(3), 480. 10.3390/diagnostics11030480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palei ACT, Sandrim VC, Amaral LM, Machado JSR, Cavalli RC, Duarte G, & Tanus-Santos JE (2012). Association between matrix metalloproteinase (MMP)-2 polymorphisms and MMP-2 levels in hypertensive disorders of pregnancy. Experimental and Molecular Pathology, 92(2), 217–221. 10.1016/j.yexmp.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Pandey M, & Awasthi S (2019). Role of MMP-1, MMP-8 and MMP-9 gene polymorphisms in preterm birth. Journal of Genetics, 99(1), 2. 10.1007/s12041-019-1161-7 [DOI] [PubMed] [Google Scholar]
- Philippat C, Rolland M, Lyon-Caen S, Pin I, Sakhi AK, Sabaredzovic A, Thomsen C, & Slama R (2021). Pre- and early post-natal exposure to phthalates and DINCH in a new type of mother-child cohort relying on within-subject pools of repeated urine samples. Environmental Pollution, 287, 117650. 10.1016/j.envpol.2021.117650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Santos S, Steegers EAP, Asimakopoulos AG, Kannan K, Trasande L, & Jaddoe VWV (2020). Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environment International, 135, 105342. 10.1016/j.envint.2019.105342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo F, Schlüssel MM, Vaz JS, Franco-Sena AB, Pinto TJP, Bastos FI, Adegboye ARA, & Kac G (2013). C-reactive protein and later preeclampsia: Systematic review and meta-analysis taking into account the weight status. Journal of Hypertension, 31(1), 16–26. 10.1097/HJH.0b013e32835b0556 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carmona Y, Ashrap P, Calafat AM, Ye X, Rosario Z, Bedrosian LD, Huerta-Montanez G, Vélez-Vega CM, Alshawabkeh A, Cordero JF, Meeker JD, & Watkins D (2020). Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. Journal of Exposure Science & Environmental Epidemiology, 30(1), 56–69. 10.1038/s41370-019-0168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carmona Y, Cantoral A, Trejo-Valdivia B, Téllez-Rojo MM, Svensson K, Peterson KE, Meeker JD, Schnaas L, Solano M, & Watkins DJ (2019). Phthalate Exposure During Pregnancy and Long-Term Weight Gain in Women. Environmental Research, 169, 26–32. 10.1016/j.envres.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS (2015). Sex-Specific Placental Responses in Fetal Development. Endocrinology, 156(10), 3422–3434. 10.1210/en.2015-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowicz A, Lisowska M, Biesiada L, Rybak-Krzyszkowska M, Gach A, Sakowicz B, Grzesiak M, Huras H, & Pietrucha T (2018). Association of Maternal and Fetal Single-Nucleotide Polymorphisms in Metalloproteinase (MMP1, MMP2, MMP3, and MMP9) Genes with Preeclampsia. Disease Markers, 2018, e1371425. 10.1155/2018/1371425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S (2008). Phthalates and Children’s Health. Current Problems in Pediatric and Adolescent Health Care, 38(2), 34–49. 10.1016/j.cppeds.2007.11.001 [DOI] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, & Sathyanarayana S (2014). Phthalates and diet: A review of the food monitoring and epidemiology data. Environmental Health, 13(1), 43. 10.1186/1476-069X-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H-M, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi D, & Hertz-Picciotto I (2019). Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environment International, 122, 222–230. 10.1016/j.envint.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, & Calafat AM (2007). Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 860(1), 106–112. 10.1016/j.jchromb.2007.10.023 [DOI] [PubMed] [Google Scholar]
- Silva MJ, Wong L-Y, Samandar E, Preau JL, Jia LT, & Calafat AM (2019). Exposure to di-2-ethylhexyl terephthalate in the U.S. general population from the 2015–2016 National Health and Nutrition Examination Survey. Environment International, 123, 141–147. 10.1016/j.envint.2018.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic B, Kokai D, Tesic B, Fa S, Samardzija Nenadov D, Pogrmic-Majkic K, & Andric N (2022). Integration of data from the in vitro long-term exposure study on human endothelial cells and the in silico analysis: A case of dibutyl phthalate-induced vascular dysfunction. Toxicology Letters, 356, 64–74. 10.1016/j.toxlet.2021.12.006 [DOI] [PubMed] [Google Scholar]
- Stermer AR, Murphy CJ, Ghaffari R, Di Bona KR, Voss JJ, & Richburg JH (2017). Mono-(2-ethylhexyl) phthalate-induced Sertoli cell injury stimulates the production of pro-inflammatory cytokines in Fischer 344 rats. Reproductive Toxicology, 69, 150–158. 10.1016/j.reprotox.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH (2008). Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environmental Research, 108(2), 177–184. 10.1016/j.envres.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tency I, Verstraelen H, Kroes I, Holtappels G, Verhasselt B, Vaneechoutte M, Verhelst R, & Temmerman M (2012). Imbalances between Matrix Metalloproteinases (MMPs) and Tissue Inhibitor of Metalloproteinases (TIMPs) in Maternal Serum during Preterm Labor. PLoS ONE, 7(11), e49042. 10.1371/journal.pone.0049042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz LM, Cheng AA, Korte CS, Giese RW, Wang P, Harris C, Meeker JD, & Loch-Caruso R (2013). Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicology and Applied Pharmacology, 268(1), 47–54. 10.1016/j.taap.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjoa ML, van Vugt JMG, Go ATJJ, Blankenstein MA, Oudejans CBM, & van Wijk IJ (2003). Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. Journal of Reproductive Immunology, 59(1), 29–37. 10.1016/S0165-0378(02)00085-2 [DOI] [PubMed] [Google Scholar]
- Trim A, Hankinson SE, Liu S, Shadyab AH, Meliker J, Bao W, Luo J, Liu B, Manson JE, Tinker L, Bigelow C, & Reeves KW (2021). Biomarkers of phthalates and inflammation: Findings from a subgroup of Women’s Health Initiative participants. International Journal of Hygiene and Environmental Health, 234, 113743. 10.1016/j.ijheh.2021.113743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich CC, Arinze V, Wandscheer CB, Copley Salem C, Nabati C, Etezadi-Amoli N, & Burkin HR (2019). Matrix metalloproteinases 2 and 9 are elevated in human preterm laboring uterine myometrium and exacerbate uterine contractility†. Biology of Reproduction, 100(6), 1597–1604. 10.1093/biolre/ioz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrano AM, Laskin DL, Archer F, Syed K, Gray JP, Laskin JD, Nwebube N, & Weinberger B (2010). Inflammatory Effects of Phthalates in Neonatal Neutrophils. Pediatric Research, 68(2), 134–139. 10.1203/PDR.0b013e3181e5c1f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videm V, & Albrigtsen M (2008). Soluble ICAM-1 and VCAM-1 as Markers of Endothelial Activation. Scandinavian Journal of Immunology, 67(5), 523–531. 10.1111/j.1365-3083.2008.02029.x [DOI] [PubMed] [Google Scholar]
- Wang J-Q, Ya-Bin H, Gao H, Jie S, Huang K, Yun-Wei Z, Lei-Jing M, Shan-Shan Z, Xiu-Xiu C, Liang-Jian Z, Su-Fang W, Jia-Hu H, Li-Qi Y, & Fang-Biao T (2020). Sex-specific difference in placental inflammatory transcriptional biomarkers of maternal phthalate exposure: A prospective cohort study. Journal of Exposure Science and Environmental Epidemiology, 30(5), 835–844. 10.1038/s41370-020-0200-z [DOI] [PubMed] [Google Scholar]
- Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, & Siahaan TJ (2002). Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Medicinal Research Reviews, 22(2), 146–167. 10.1002/med.10001 [DOI] [PubMed] [Google Scholar]
- Zhang S, Ma J, Fu Z, Zhang Z, Cao J, Huang L, Li W, Xu P, & Cao X (2016). Promotion of breast cancer cells MDA-MB-231 invasion by di(2-ethylhexyl)phthalate through matrix metalloproteinase-2/-9 overexpression. Environmental Science and Pollution Research, 23(10), 9742–9749. 10.1007/s11356-016-6158-7 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lin L, Cao Y, Chen B, Zheng L, & Ge R-S (2009). Phthalate levels and low birth weight: A nested case-control study of Chinese newborns. The Journal of Pediatrics, 155(4), 500–504. 10.1016/j.jpeds.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


