Abstract
Purpose:
Metastatic melanoma is a tumor amenable to immunotherapy in part due to the presence of antigen-specific tumor-infiltrating lymphocytes (TIL). These T cells can be activated and expanded for adoptive cell transfer (ACT), which has resulted in relatively high rates of clinical responses. Similarly, immune checkpoint inhibitors, specifically PD-1 blocking antibodies, augment antitumor immunity and increase the influx of T cells into tumors. Thus, we hypothesized that addition of PD-1 inhibition may improve the outcomes for patients undergoing ACT with TIL.
Patients and Methods:
Stage III/IV metastatic melanoma patients with unresectable disease who were anti-PD-1 treatment-naïve were enrolled. TIL were generated in the presence of anti-4–1BB antibody in vitro and expanded for ACT. Patients in Cohort 1 received TIL infusion followed by nivolumab. Patients in Cohort 2 also received nivolumab prior to surgical harvest and during TIL production.
Results:
A total of 11 patients were enrolled, all of whom were evaluated for response, and nine completed ACT. Predominantly CD8+ TIL were successfully expanded from all ACT-treated patients and were tumor-reactive in vitro. The trial met its safety endpoint, as there were no protocol-defined dose-limiting toxicity events. The objective response rate was 36% and median progression-free survival was 5 months. Two non-responders who developed new metastatic lesions were analyzed to determine potential mechanisms of therapeutic resistance, which included clonal divergence and intrinsic TIL dysfunction.
Conclusions:
Combination therapy with TIL and nivolumab was safe and feasible for metastatic melanoma patients and provides important insights for future therapeutic developments in ACT with TIL.
INTRODUCTION
Over the past 15 years, immunotherapy has become a frontline treatment option for metastatic melanoma, largely due to its relatively high immunogenicity. T cells with the ability to specifically recognize melanoma antigens are critical to this immune-mediated response. Adoptive cell therapy (ACT) with tumor-infiltrating lymphocytes (TIL) exploits an in situ population of enriched, tumor-specific T cells, removes them from a suppressive microenvironment, and induces their expansion ex vivo in favorable conditions to high numbers for reinfusion. TIL are able to traffic to tumors if administered after lymphodepleting chemotherapy and with interleukin-2 (IL-2) resulting in objective response rates (ORR) of 30–50% across various institutional series.1–11 Despite these impressive results, improvements in treatment outcomes are potentially achievable through the incorporation of in vitro and in vivo agents that could reduce TIL generation time, enhance tumor reactivity and TIL persistence. Our previous work with the inclusion of 4–1BB agonism (anti-CD137 agonistic antibody) to IL-2 during in vitro TIL generation has consistently demonstrated enhanced kinetics of proliferation and increased tumor-specific activity of TIL in both mouse and human preclinical models.3, 12 Therefore, 4–1BB agonism was included in the TIL generation for this trial.
Programmed cell death protein-1 (PD-1 or CD279) is an immune checkpoint receptor expressed by activated T cells. Extensive work has demonstrated that T cells expressing PD-1 include a subset of tumor-reactive lymphocytes.13–16 However, interaction between PD-1 and its ligands programmed cell death ligand-1 and −2 (PD-L1 and PD-L2) in peripheral tissues such as tumors and stromal cells leads to the suppression of these tumor-reactive T cells.17–19 In murine models, blockade of PD-1/PD-L1 interactions demonstrated enhanced T cell proliferation, persistence, and antitumor activity.17–20 In a pivotal phase 3 clinical trial of anti-PD-1 antibody for patients with metastatic melanoma, there were statistically significant improvements in progression-free survival (hazard ratio 0.58) and overall survival (OS) at one year (hazard ratio 0.69) when compared to ipilimumab, leading to FDA approval of anti-PD-1 for the frontline treatment of metastatic melanoma.21 The replacement of exhausted clones within the tumor microenvironment with a renewed infiltrate of tumor-specific clones is essential to the therapeutic efficacy of PD-1 blockade.22
The combination of anti-PD-1 and TIL has the potential to harness a potent pool of tumor-reactive T cells and overcome their inhibition in vivo. With this in mind, we combined the anti-PD-1 blocking antibody, nivolumab, with TIL therapy in anti-PD-1 and TIL treatment-naïve patients with metastatic melanoma. Each of two cohorts in this trial included anti-PD-1 dosing following ACT with TIL, while the second cohort also received anti-PD-1 prior to surgical tumor harvest and during the TIL generation period. We reasoned that dual therapy may be beneficial in terms of TIL persistence upon infusion, and that pretreatment with anti-PD-1 may increase T cell infiltration as well as tumor specificity. In a prior trial at our institution, combination ipilimumab with TIL ACT maintained treatment efficacy similar to previously reported trials, while minimizing patient drop-outs during TIL generation.23 Our institution has also recently reported the successful combination of anti-PD-1 and TIL transfer in anti-PD-1 refractory metastatic non-small cell lung cancer patients with an initial reduction in tumor burden for 85% of patients and a median change in target lesion size of −35.5%.24
Herein, we report a Phase I clinical trial in which nivolumab was administered in combination with TIL ACT. The safety and feasibility of this approach were the primary endpoints, with the expectation that these results would inform the recommended phase 2 schedule for combination treatment. We further hypothesized that utilizing nivolumab prior to surgical tumor harvest and during the interval required for TIL generation would increase T cell trafficking to the tumor and potentially improve the quality of the TIL generated, while mitigating against tumor progression in the time interval between the harvest and infusion of the TIL. Initial TIL generation was supplemented with anti-4–1BB (CD137) agonistic antibody to improve the rate of successful cultures, growth kinetics, and CD8+ T cell composition.3 The trial also assessed the potential for nivolumab to impact the persistence of TIL after transfer, a characteristic that is critical for achieving durable clinical responses.
Materials and Methods
Patients and Treatments
Patients with unresectable stage III/IV metastatic melanoma, an ECOG status of 0–1, and no prior anti-PD-1 or TIL therapy were eligible for enrollment. Other prior treatment for metastatic melanoma was allowed if completed at least three weeks before enrollment. Patients were assessed to determine their ability to tolerate ACT plus lymphodepletion and IL-2 and were required to have measurable residual disease after harvest of at least one lesion for TIL generation. In addition, patients were required to be 18 years or older with adequate organ function and a positive EBV antibody titer. Patients were excluded if they were pregnant or breast-feeding or had an active systemic infection, serologic evidence of syphilis, Hepatitis B or C, HTLV-I or II, or HIV. The trial was carried out in compliance with ethical guidelines laid out in the Declaration of Helsinki, the International Conference on Harmonized Tripartite Guidelines for Good Clinical Practice and the US Common Rule. All eligible patients signed a written informed consent to the IRB-approved clinical trial protocol (NCT02652455).
Dose-limiting toxicity (DLT) was defined separately for each attributable element in the protocol regimen. DLT related to ACT was defined as any non-hematologic grade ≥4 adverse events which occurred upon or up to six weeks after ACT, consistent with our previous TIL trials.23, 25, 26 DLT related to nivolumab was defined as any grade ≥3 immune-related adverse events definitely attributable to nivolumab occurring within the same DLT window of up to six weeks after ACT, with the exception of rash that was required to be grade ≥4.
TIL Production
Surgically harvested tumor specimens were minced into 1–3 mm3 fragments for TIL generation under sterile conditions. Tumor fragments (36–48 per patient) were placed into 24-well plates with media containing 6000 IU/mL IL-2 (aldesleukin, Prometheus Laboratories, Inc., San Diego, CA, USA), 10% human AB serum (Access Biologicals LLC, Vista, CA, USA), and 10 ug/mL anti-4–1BB monoclonal agonistic antibody (BMS-663513; Bristol Myers Squibb, New York, NY, USA). TIL were expanded under these conditions for up to five weeks, keeping individual fragments separate. Each well was assessed for confluency 2–3 times per week, expanded to new wells at >80% confluency, and supplemented with the aforementioned media containing 1 ug/mL anti-4–1BB every 3–4 days.
Tumor Digest
Excess tumor material that was not used to generate TIL was physically and enzymatically digested to prepare a single cell suspension of target tumor cells. After mincing, tumor material was gently agitated in media containing collagenase II, collagenase IV, hyaluronidase, and DNase (Fisher Scientific, Pittsburgh, PA, USA). The digested material was then filtered, red blood cells lysed, and viable cells counted and cryopreserved until utilization in functional assays.
TIL Selection
Expanded TIL were co-cultured with tumor cells to determine antitumor reactivity. Autologous tumor cells from the tumor digest and HLA-mismatched control tumor cells were utilized as targets. TIL and tumor cells were plated at a 1:1 ratio in 96-well plates (1×105 cells/well each) overnight. Where indicated, MHC Class I interaction was impeded via the HLA-ABC blocking antibody clone W6/32 (BioLegend, Inc., San Diego, CA, USA) at 10 ug/mL. Co-culture supernatants were collected and assayed for interferon-gamma (IFN-γ) release by ELISA (R&D Systems, Minneapolis, MN, USA and BioLegend, Inc., San Diego, CA, USA). TIL that produced >200 pg/mL IFN-γ and at least a two-fold increase over the HLA-mismatched control were designated as tumor-reactive.
Rapid Expansion Protocol
TIL from tumor-reactive fragments were collected and enumerated. A maximum of 12 GREX 100M flasks (Wilson Wolf, Saint Paul, MN, USA) were seeded with 5.0×106 TIL each for a total of 6.0×107 TIL at the initiation of the rapid expansion protocol (REP). REP media consisted of 200 mL AIM-V (Invitrogen, Waltham, MA, USA) and 200 mL RPMI containing 10% human AB serum, 1% HEPES, and 0.1% 2-mercaptoethanol. TIL were cultured at a 1:200 ratio with irradiated feeder cells pooled from three healthy donors. REP media was supplemented with 30 ng/mL OKT3 (Ortho Biotech, Bridgewater, NJ, USA) and 3000 IU/mL IL-2. On Day 4, an additional 200 mL of the above REP media was added to each flask and supplemented with 3000 IU/mL IL-2. On day 7, TIL were resuspended, counted, and expanded into a maximum of 36 GREX 100M flasks. Each flask was seeded with 1.0–7.5×108 TIL and filled to 1 L total volume of AIM V and 3000 IU/mL IL-2 and cultured for an additional 7 days. No anti-4–1BB was included in the rapid expansion steps.
TIL Infusion & Nivolumab Treatment
After the REP, TIL were harvested, washed, and concentrated to between 100–500 mL for infusion. Viable cells were quantified after staining with acridine orange and propidium iodine on the Cellometer Auto 2000 (Nexcelom Bioscience, Lawrence, MA, USA). Infusion product TIL were labeled with 7-AAD, CD45, CD3, CD4, and CD8 antibodies (see Supplementary Methods Table) followed by acquisition on a BD FACSCanto (BD Biosciences, San Jose, CA, USA) and analysis by FlowJo software (RRID:SCR_008520; TreeStar, Inc., Ashland, OR, USA). Prior to infusion, patients were lymphodepleted with cyclophosphamide (Cy) (60 mg/kg/day) on day −7 and −6, followed by fludarabine (Flu) (25 mg/m2) on days −5, −4, −3, −2, and −1. After sterility testing, the TIL product was infused intravenously (IV) by gravity drip into the patient at 300 mL/hour. For cohort 1, nivolumab (BMS-936558; Bristol Myers Squibb, New York, NY USA) was administered IV (3 mg/kg) beginning two weeks after the TIL transfer, continuing every 14 days for six months, then every 90 days for 18 months unless unacceptable toxicity or tumor progression supervened. For cohort 2, nivolumab was administered IV (3 mg/kg) every two weeks for two doses prior to TIL harvest then was continued every two weeks during TIL propagation. Nivolumab was stopped two weeks prior to the TIL transfer then resumed at the identical dosing schedule to cohort 1 after the TIL transfer.
PBMC Collection
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (GE Healthcare Biosciences, Pittsburgh, PA, USA) gradient separation and cryopreserved from blood draws at indicated weekly time points. PBMC collection was continued in this manner every three months while patients remained on study. Apheresis was performed at the six-week time point and processed as above.
Flow Cytometry
An aliquot of the infusion product was cryopreserved at the time the product was prepared for infusion. Upon recovery, aliquoted TIL were washed with PBS, counted, and stained for flow cytometric analysis. Viability staining was completed according to the manufacturer’s protocol (see table). Antibody surface staining occurred at 4°C for 30 minutes, protected from light, in PBS containing 5% FBS, 1mM EDTA, and 0.1% sodium azide. Where indicated, intracellular staining for FOXP3 was performed following the manufacturer’s protocol (BD Biosciences #560098 or ThermoFisher #552300). Cells were stained at concentrations between 0.25–1.0×106 TIL/sample and fixed in 2% paraformaldehyde until acquisition on a BD LSRII or BD Celesta (BD Biosciences, San Jose, CA, USA) instrument. FlowJo software was utilized for FACS analysis and gates were drawn according to fluorescence minus one (FMO) staining on a per patient basis. See Supplementary Methods Table for panel information.
TCRβ Sequencing
Cryopreserved TIL and PBMC samples were thawed into pre-warmed media and counted. Where indicated, CD4+ and CD8+ TIL populations were isolated by magnetic bead separation (Miltenyi Biotec, Bergisch Gladbach, Germany) on MACS columns from bulk TIL products. DNA was extracted using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the kit’s protocol. DNA was quantified via Nanodrop and deep level TCRβ sequencing was performed by the Moffitt Cancer Center (MCC) Molecular Genomics Core using the ImmunoSEQ TCRβ Kit v3 (Adaptive Biotechnologies, Seattle, WA, USA). Data were uploaded to the Adaptive Biotechnologies server for analysis on the Adaptive ImmunoSEQ Analyzer 3.0.
Murine ACT Model
A patient-derived xenograft (PDX) tumor line was established in NSG mice from subcutaneously injected tumor digest from Patient 7. PDX tumors were harvested, physically and enzymatically digested, and injected subcutaneously into NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(CMV-IL2)4–2Jic/JicTac (hIL-2 NOG; RRID:IMSR_TAC:13440) mice (Taconic Biosciences, Rensselaer, NY, USA). When tumors reached ~20–30 mm2, TIL were adoptively transferred via IV tail vein injection. Tumor area was monitored by calipers. All animal studies were approved by the institutional IACUC review board (R IS00006131 and R IS00010199).
TIL Production for Research Use
Excess tumor tissue from resected specimens were collected under separate IRB-approved non-therapeutic research protocols (MCC50148, MCC50193, MCC50232, and MCC50326) after informed written consent and processed and cultured as above. These non-therapeutic studies were conducted under the ethical principles laid out by the Declaration of Helsinki and in accordance with the US Common Rule.
Statistical Analysis
Up to six patients were accrued per cohort. The primary endpoints of the trial were to determine the safety and feasibility of combination nivolumab with ACT using TIL. Safety and feasibility were defined as the ability to successfully treat at least 67% of the patients in each cohort without reaching DLT. The secondary endpoints consisted of the objective response rate confirmed at 12 weeks after TIL transfer and progression-free and overall survival. Responses were determined by RECIST 1.1 criteria by comparing the baseline radiographic and clinical assessments to those at 12 weeks following ACT with TIL. All statistical analyses were completed using GraphPad Prism v9 (RRID:SCR_002798; GraphPad Software, La Jolla, CA, USA).
Data Availability
The data in this report are available within this manuscript and its supplement or available upon reasonable request from the corresponding author.
Results
Eleven patients with unresectable stage III/IV melanoma were enrolled between March 2016 and May 2018 (Table 1) at a single institution to receive combination nivolumab and ACT with TIL expanded in the presence of anti-4–1BB agonism (NCT02652455). The first six patients were enrolled in Cohort 1 (C1) and were to receive TIL infusion followed by nivolumab treatment beginning two weeks after ACT. Five subsequent patients entered Cohort 2 (C2) and were planned for pre-treatment with nivolumab prior to tumor harvest and during TIL generation, in addition to the C1 regimen of nivolumab after ACT (Figure 1A). Of these eleven patients, five were females and six were males, with a median age of 52 (range 37–68 years). The majority had no prior therapy, while four had previous adjuvant therapy and two each had received either interferon, ipilimumab, and/or combination BRAF/MEK targeted therapy (Table 1). All patients were included in the intent-to-treat analysis of response, however three patients were excluded from correlative analyses due to lack of specimen availability. Patient 2 was not treated with TIL due to progression with new brain metastases prior to lymphodepletion. Of note, this patient also had liver metastases and markedly elevated lactate dehydrogenase (LDH). Patient 5 was removed from all subsequent correlative analyses for non-compliance due to a lack of follow-up prior to first post TIL imaging assessment. Patient 9 elected to forego surgical tumor harvest for TIL propagation due to a response to nivolumab prior to harvest.
Table 1.
Patient Characteristics and Response
| Patient | Age | Sex | M Stage | LDH Level (>ULN) | Prior Therapy | ECOG | Tissue Type | No. IL-2 Doses | No. Nivo Doses | Response at 12 weeks after ACT | Response at 1 year after ACT | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 | ||||||||||||
| 1 | 67 | M | M1c | 1.0X | IFN, Adj | 0 | SQ | 1 | 18 | PR | PR | |
| 2 | 46 | F | M1c | 8.4X | IFN, Adj, D/T | 1 | SQ | NT | NA | PD | PD | Progressed prior to ACT |
| 3 | 54 | M | M1b | <1X | None | 0 | LN | 6 | 2 | PD | PD | |
| 4 | 37 | F | M1c | <1X | None | 0 | SQ | 5 | 5 | PD | PD | |
| 5 | 44 | M | M1c | <1X | Adj, Ipi | 1 | LN | 1 | 1 | PD | PD | Removed: Noncompliance |
| 6 | 55 | F | M1c | <1X | Adj, Ipi | 0 | SQ | 6 | 11 | SD | PD | |
| Cohort 2 | ||||||||||||
| 7 | 52 | F | M1c | 2.4X | None | 1 | LN & Breast | 6 | 8 | SD | PD | |
| 8 | 50 | M | M1a | <1X | D/T, V/C | 0 | LN | 6 | 8 | SD | PD | |
| 9 | 45 | M | M1a | <1X | None | 1 | NA | NT | 2 | PR | PR | Response to Nivolumab |
| 10 | 68 | F | M1c | 1.0X | None | 0 | SQ & LN | 6 | 19 | PR | PR | |
| 11 | 54 | M | M1c | 1.1X | None | 0 | LN | 4 | 21 | PR | PR | |
Nivo= Nivolumab; NA= Not Applicable; NT= Not Treated; SQ= subcutaneous disease, LN= nodal disease; LDH Level= fold above upper limit of normal (>ULN) IFN = Interferon; Adj = Adjuvant; D/T=Dabrafenib/Trametinib; V/C= Vemurafenib/Cobimetinib
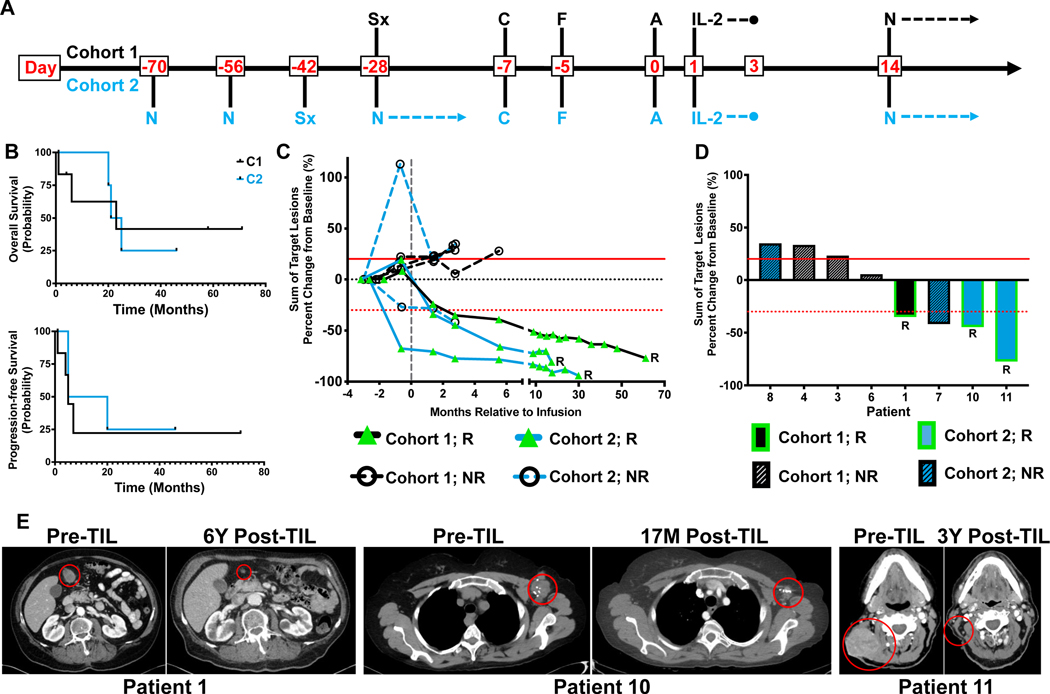
Figure 1. Patient treatment schema and overall trial results.
A. Patient treatment schema for Cohort 1 and 2. N=Nivolumab; Sx=Surgery; C=Cyclophosphamide; F=Fludarabine; A= ACT with TIL. B. Overall survival (OS) and progression-free survival (PFS) in months stratified by cohort (C1 = Black; C2 = Blue). C. Longitudinal tracking of target lesion measurements relative to baseline for individual patients while on study. RECIST criteria is denoted by horizontal dotted red line (response, −30%) and solid red line (progression, +20%). R=Responder. D. Percent change of individual target lesions at 12 weeks. RECIST criteria is denoted by horizontal dotted red line (response, −30%) and solid red line (progression, +20%). R=Responder. E. Computed tomography (CT) images from responders at designated time points. M=months, Y=years.
Safety, Feasibility, and Toxicity
The primary endpoints of safety and feasibility were met, since at least four patients (≥67%) per cohort were successfully treated with ACT without reaching DLT. Patients received a median of eight doses of nivolumab and six doses of IL-2. Non-hematologic adverse events were primarily grade 3 and were largely attributed to either the Cy/Flu lymphodepleting regimen or the IL-2 and TIL transfer (Supplementary Table 1). One patient presented with grade 4 elevated lipase which was attributed to the Cy/Flu preconditioning and did not alter the course of treatment. Two grade 3 toxicities definitely attributable to nivolumab occurred in a single patient. These events comprised grade 3 colitis that occurred outside of the six-week DLT window, and grade 3 rash that did not reach the grade ≥4 severity to be deemed a DLT.
Clinical Response and Outcome
Patient responses at six weeks after TIL infusion were confirmed at 12 weeks and constituted the secondary endpoint for the trial. The ORR was 36% with four patients achieving a partial response. Median OS was 23 months (both cohorts 23 months), while PFS was 5 months (C1: 5 months, C2: 12.5 months) (Figure 1B). Each of the three responding patients who received TIL had responses lasting more than 18 months, with two responses ongoing (46+ and 71+ months) at the time of publication (Figure 1C). In total, 69% of the monitored target lesions decreased in size from baseline measurements after ACT, including three target lesions from a non-responder (Figure 1D). (Figure 1E).
TIL Infusion
There were no manufacturing failures, as TIL were successfully expanded from all patient samples utilizing media containing IL-2 and anti-4–1BB. Aggregating all specimen samples, 59% of plated tumor fragments successfully generated TIL (Figure 2A). After initial “pre-REP” outgrowth, TIL underwent REP and produced an average of 1435-fold expansion, with no observed difference between cohorts (Figure 2B). An average of 8.5×1010 TIL were infused per patient (range: 5.3×1010−1.3 ×1011) (Figure 2C). TIL generation with combination anti-4–1BB and IL-2 was compared to IL-2 alone utilizing a separate set of fragments from trial-derived excess surgical specimens not utilized for treatment. Similar efficiency was observed between these two groups, although the majority of paired samples (5 of 6) attained an increase in the fraction of fragments that yielded TIL in the presence of anti-4–1BB compared to IL-2 alone (Figure 2D).
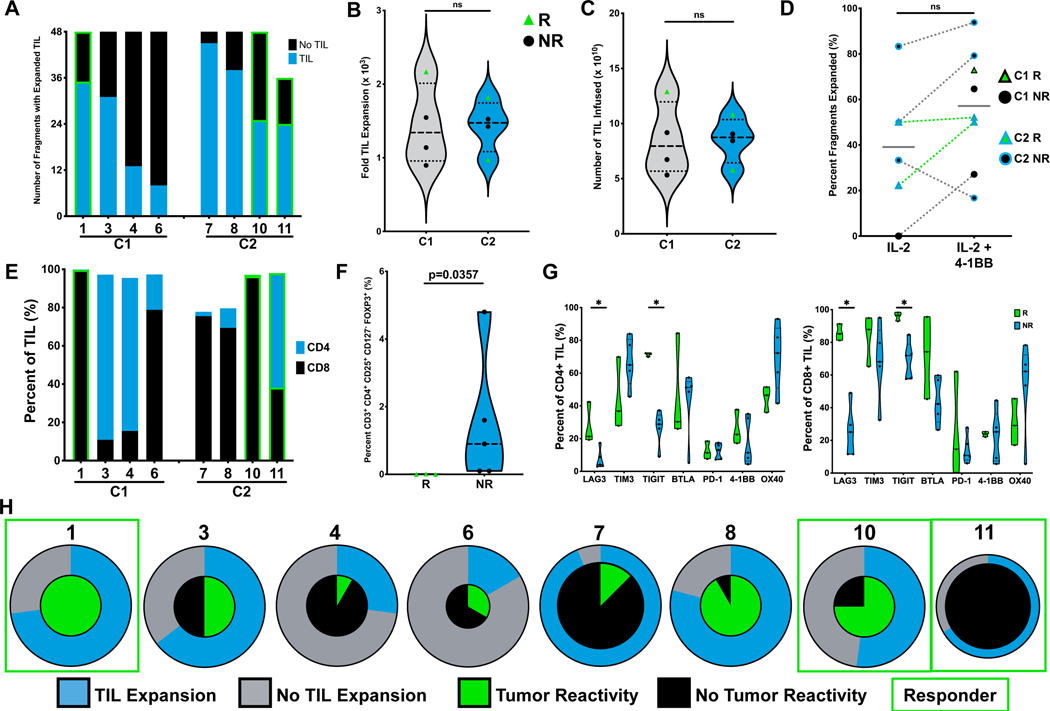
Figure 2. Characterization of infused TIL.
A. Number of tumor fragments which produced at least six wells of expanded TIL as a proportion of total fragments initially plated. Responders highlighted with green border. B. Fold expansion of TIL during the REP phase of production. Mann-Whitney test. C. Absolute number of TIL infused per patient. Mann-Whitney test. D. Analysis of percent of tumor fragments which expanded TIL in media containing IL-2 +/− anti-4–1BB agonistic antibody. Mann-Whitney test. E. Frequency of single positive CD4 and CD8 T cells for infused TIL products. F. Flow cytometry analysis of TIL for FOXP3 expression on CD3+ CD4+ CD127- T cells. Mann-Whitney test. p=0.0357. G. Surface expression of co-stimulatory and co-inhibitory markers on patient TIL. Mann-Whitney test. *p<0.05. H. Percent of tumor fragments with TIL expanded represented in outer ring for each patient. Center circle represents percent of fragments which generated TIL reactive to AT. Each pie size is proportional to the number of tumor fragments present in each condition.
TIL products were composed primarily of CD8+ T cells at infusion (Figure 2E). Of the CD4+ TIL, only a small fraction were CD25+ CD127- FOXP3+ T cells, which were found to be statistically significantly enriched in the TIL product of non-responders, as the three responders all had lower values than did the five non-responders (p = 0.0357; Figure 2F). When stratified by response, further phenotypic evaluation of the infusion product indicated that both CD8+ and CD4+ TIL from responders displayed increased LAG3 (CD223) and TIGIT surface expression (Figure 2G). Infused TIL were predominantly effector memory (TEM) or terminal effector (TE) T cells, and we observed that responders were enriched for central memory T cells (TCM) when compared to non-responders (Supplementary Figure 1). Tumor reactivity was detected from at least one tested fragment from each patient, except for Patient 11 who still achieved a partial response (Figure 2H). Collectively, a mean of 39% of all tested fragments produced tumor-reactive TIL (C1: 50% vs C2: 32%). Furthermore, TIL produced IFNg in response to HLA-matched tumors, indicating that T cell clones recognizing shared tumor antigens may be present within the infusion product.
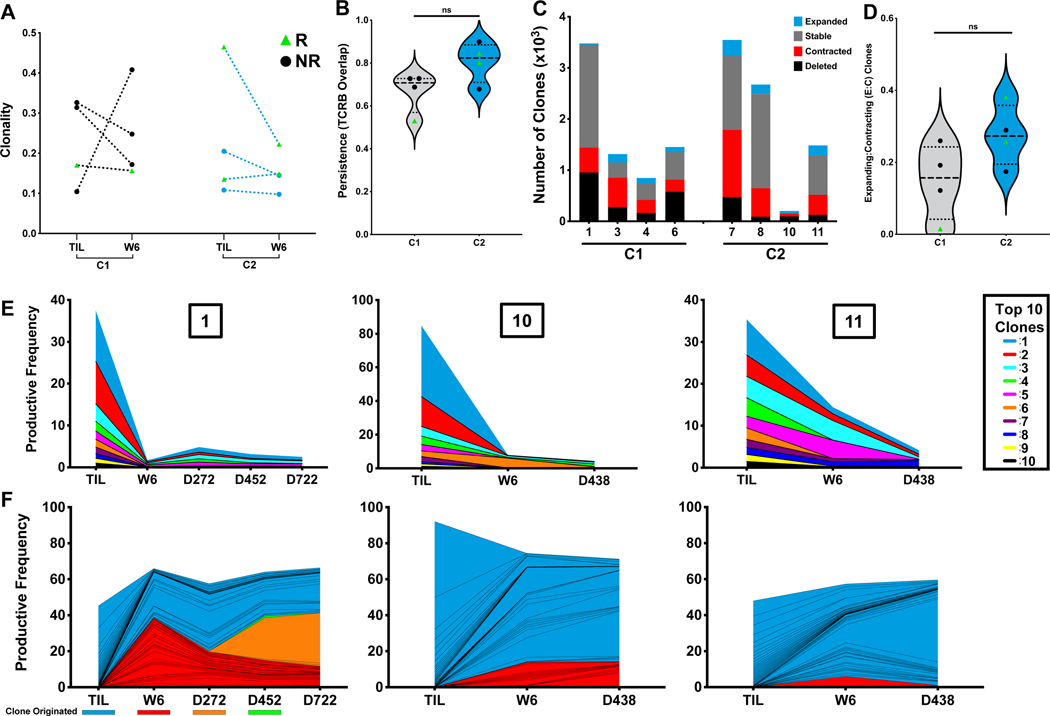
Persistence and Clonality
To further characterize the infused TIL, TCRβ sequencing was performed. TIL products were similar in clonality between cohorts and between responders and non-responders (Figure 3A). Persistence was relatively high across all patients and trended toward increased values in C2 (Figure 3B) with a greater ratio of expanded to contracted clones than their C1 counterparts (Figure 3C and 3D). For each of the three long-term responders who received ACT, additional sequencing was performed from PBMCs isolated throughout the duration of response. The top ten most prevalent clones accounted for between 35% (Patient 11) and 85% (Patient 10) of the infusion product in these patients. Over one year after infusion (day 438 or day 452), these TIL clones were still detectable at substantial levels measuring 3–4% of the entire peripheral blood repertoire. When this analysis was expanded to include the top 25 clones from each sample, greater than 50% of the TCRβ sequences originated in the TIL infusion product for the two patients in C2. The C1 long-term responder (Patient 1) maintained 25% of clones in these same parameters two years following infusion (Figure 3E & 3F).
Figure 3. T cell clonal analysis.
A. T cell clonality determined by TCRβ sequencing in TIL and six-week apheresis samples. B. Persistence in vivo measured by Overlap metric in TCRβ sequences between TIL and six-week apheresis sample. C. Absolute number of clones categorized by relative frequency between TIL infusion and six-week apheresis. D. Ratio of T cell clones with increased frequency (expanding) to decreased frequency (contracting) from infused TIL product to six-week time point. Mann-Whitney test. E. Clonal tracking by TCRβ sequence of the top ten infused TIL clones in peripheral blood samples from long-term responders. F. Stacked productive frequency of the top twenty-five shared T cell clones across long-term responders in peripheral blood. Colors indicate time point of clonal origin as indicated.
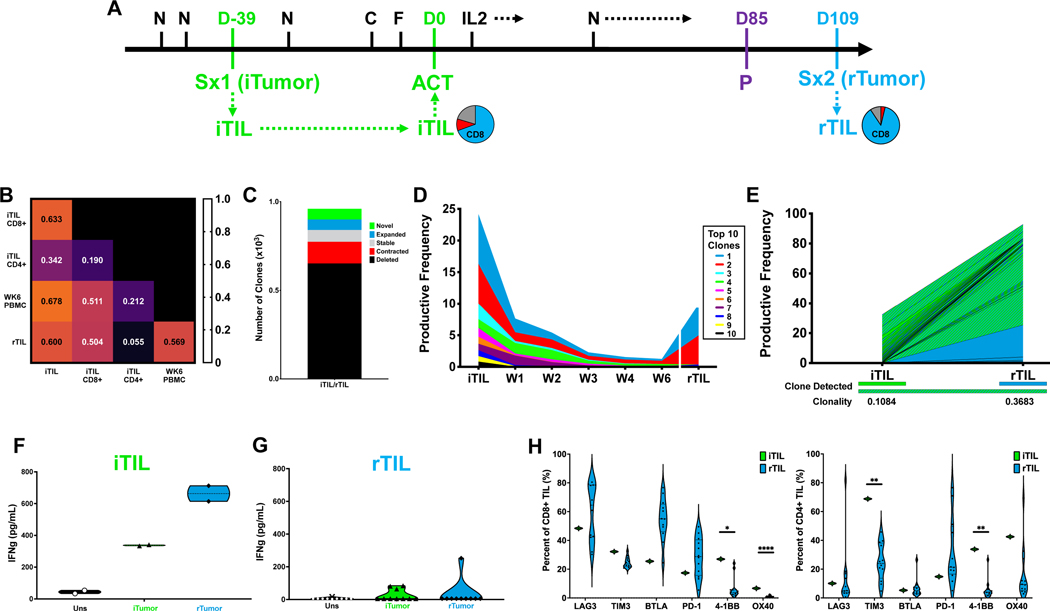
Patient 7 Case Study
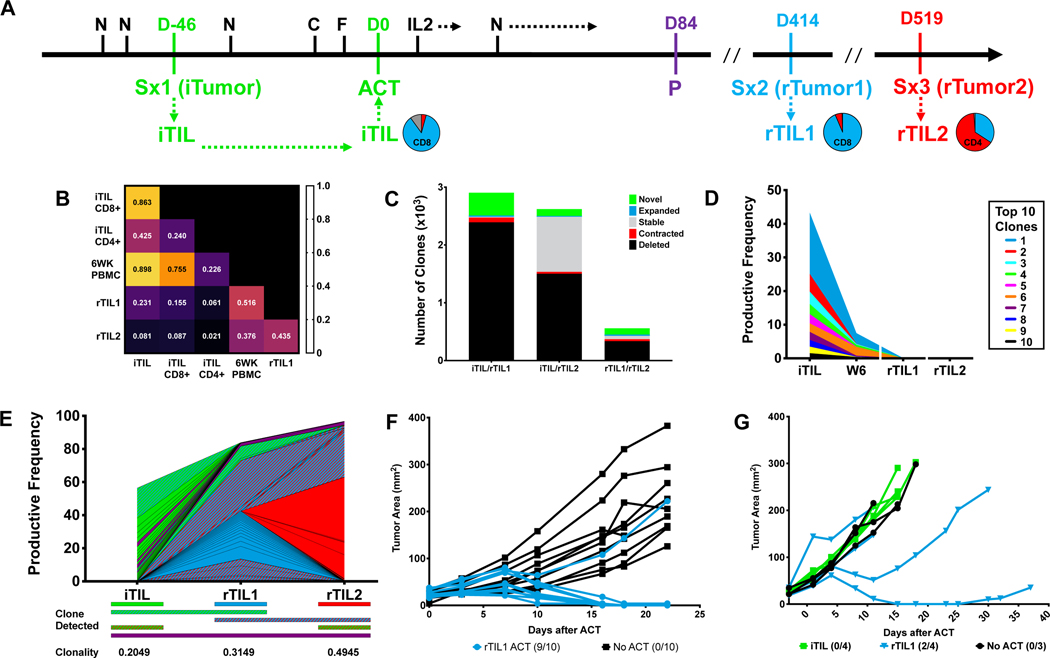
We further investigated Patient 7 (C2) as a case study as the clinical course of this patient included multiple recurrent lesions following ACT (Figure 4A). The initial tumor (iTumor) of the patient generated an initial TIL (iTIL) product from fragments at a relatively high rate (Figure 2H), and seven of these fragments were selected for REP based on IFN-γ production in response to autologous tumor. The iTIL infusion product was predominantly CD8+ T cells (85.8%) (Figure 4A). After ACT, the patient received five additional doses of nivolumab and achieved a status of stable disease at 12 weeks. Each of the three target lesions regressed by greater than 30% from baseline (Figure 1D). Despite this, the patient ultimately progressed after five months, manifested by two breakthrough brain metastases. These recurrences (rTumor1 and rTumor2) were acquired by surgical extirpation and TIL (rTIL1 and rTIL2, respectively) were expanded for non-therapeutic research purposes (Figure 4A).
Figure 4. Patient 7 Case Study.
A. Patient treatment schema annotated with CD4 and CD8 ratios of expanded CD3+ TIL from each surgical specimen; blue=CD8+, red=CD4+. N=Nivolumab; D=Day; Sx=Surgery; iTIL=initial TIL; C=Cyclophosphamide; F=Fludarabine; ACT=Adoptive Cell Transfer; rTIL=recurrence-derived TIL; P=Progression. B. TCRβ sequence overlap between indicated TIL and peripheral blood samples. C. Absolute number of clones categorized by relative frequency between TIL infusion and six-week apheresis. D. Clonal tracking by TCRβ sequences of the top ten infused TIL clones in peripheral blood samples from long-term responders. E. The top 25 shared clones were tracked by TCRβ sequencing between TIL products generated from resected samples. Individual clones are denoted by color in which samples each were detected. F-G. PDXs were established from rTumor1 in NOG IL2 mice and treated IV with ACT with indicated TIL (5e6 TIL). Tumor area was measured and the ratio of responding mice to total mice is in parenthesis.
After six weeks, the iTIL were persistent in the periphery (89.8% TCRβ overlap) and similar in composition to the infusion product. However, substantially less clonal overlap was observed between iTIL and TIL generated from either of the brain metastases (Figure 4B). Further analysis confirmed that the majority of iTIL clones were deleted between the iTIL and rTIL1 or rTIL2 (Figure 4C). Analysis of the top ten iTIL clones, which comprised greater than 40% of the infused TIL, confirmed that each of these clones were excluded from the infiltrate in both of the recurrent lesions (Figures 4D), indicating a complete loss of the dominant TIL clones originating from the infusion product. To further support this, we analyzed the top 25 shared clones within each TIL population. The majority of clones within the rTIL1 (73%) and rTIL2 (94%) repertoire originated independently of the infused TIL product, while only 2% of clones were shared across all three surgical specimens (Figure 4E).
Further ex vivo analysis demonstrated that while the iTIL were highly responsive to the patient’s iTumor (Figure 2H), iTIL reactivity towards rTumor1 was substantially impaired both in PDX murine models and the corresponding in vitro co-culture (Figure 4G). In contrast, rTIL1 efficiently responded to rTumor1 in these PDX murine models (Figure 4F and 4G), despite inability to control metastatic outgrowth in the patient. The ability of the iTIL to recognize the iTumor coupled with an inferior response of the iTIL toward the recurrent lesions is consistent with the patient’s clinical trajectory and lack of response to ACT. Altogether, these data support a shift in the clonal distribution of the T cell repertoire between the initial harvested specimen and subsequent recurrences.
Patient 8 Case Study
Patient 8, another member of C2, received iTIL in combination with nivolumab treatment but recurred with a brain lesion after ACT. The brain lesion was resected and analyzed after surgical extirpation (Figure 5A). The iTIL from the patient were efficiently generated from 79% of fragments and 92% of the tested fragments contained tumor-reactive TIL (Figure 2H). This predominantly tumor-reactive infusion product primarily consisted of CD8+ T cells that were highly persistent within the circulation of the patient (Figure 5B). This patient had stable disease at the 12-week time point before ultimately progressing and requiring additional surgical intervention at 15 weeks (rTumor). Again, rTIL were expanded for non-therapeutic research purposes from the rTumor sample and found to be CD8-predominant. Strong overlap (60%) in the TCRβ sequences between iTIL and rTIL suggested persistence and infiltration of the infused product into the recurrent brain metastasis (Figure 5B). While many individual clones were not maintained, the two most prevalent clones in the iTIL population demonstrated a robust presence in the rTIL population, while steadily declining in the periphery (Figure 5C & 5D). Furthermore, 67% of the clonal repertoire present in the rTIL population were shared with the top 25 clones among the infused TIL product (Figure 5E), indicating a high degree of clonal continuity after ACT.
Figure 5. Patient 8 Case Study.
A. Patient treatment schema annotated with CD4 and CD8 ratios for expanded TIL from each surgical specimen; blue=CD8, red=CD4. N=Nivolumab; D=Day; Sx=Surgery; iTIL=initial TIL; C=Cyclophosphamide; F=Fludarabine; ACT=Adoptive Cell Transfer; rTIL=recurrence-derived TIL; P=Progression. B. TCRβ sequence overlap between indicated TIL and peripheral blood samples. C. Absolute number of clones categorized by relative frequency between TIL infusion and six-week apheresis. D. Clonal tracking by TCRβ sequences of the top ten infused TIL clones in peripheral blood samples from long-term responders. E. The top 25 shared clones were tracked by TCRβ sequencing between TIL products generated from resected samples. Individual clones are denoted by color in which samples each were detected. F. TIL and tumor were co-cultured at 1:1 ratio as indicated. IFN-γ concentration was measured by ELISA on cell culture supernatants. Markers represent technical replicates. G. TIL and tumor were co-cultured at 1:1 ratio as indicated. IFN-γ concentration was measured by ELISA on cell culture supernatants. Markers represent rTIL from individual fragments. H. Cell surface expression of checkpoint molecules on TIL by flow cytometry. Markers represent bulk population for iTIL, individual fragments for rTIL. Unpaired T test. p<0.05.
To investigate the function of these TIL, in vitro co-cultures with tumor were performed. The iTIL product demonstrated high IFN-γ production in response to both the iTumor and rTumor, indicating shared antitumor recognition between lesions. Only 30% of rTIL fragments produced low levels of IFN-γ in response to the iTumor, and just 10% of rTIL fragments released IFN-γ in response to rTumor (Figure 5F & 5G), suggesting an intrinsic loss of rTIL function despite the substantial clonal overlap. Flow cytometric profiling of co-stimulatory receptor expression indicated that rTIL expressed diminished levels of 4–1BB on both CD8+ and CD4+ T cells as well as reduced TIM3 and OX40 on CD4+ and CD8+ T cells, respectively. In totality, these data support substantial tumor infiltration and persistence of tumor-reactive iTIL; however, the rTIL were marked by an inferior co-stimulatory profile suggesting these TIL may be lacking critical intrinsic activation receptors.
TIL Generation in the Context of anti-PD-1 Therapy
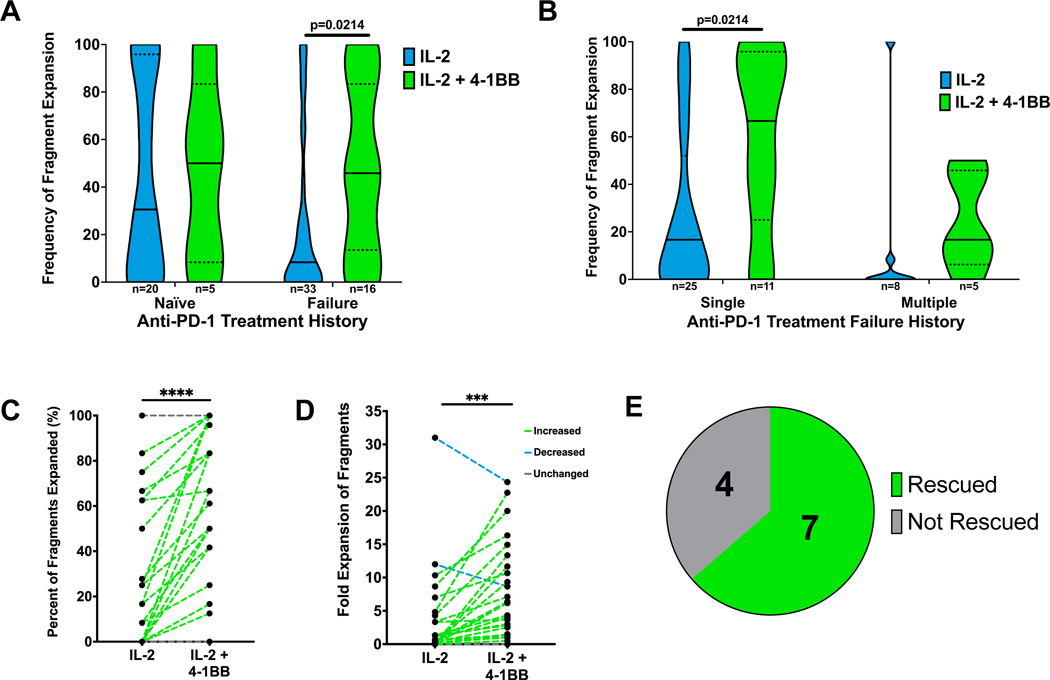
With these observed deficiencies in TIL isolated from recurrent metastases following treatment in patients 7 and 8, we sought to further investigate TIL quality and strategies to improve TIL production in the context of disease progression after prior anti-PD-1 therapy. In Figure 2D, we found that the addition of anti-4–1BB to TIL cultures from anti-PD-1 naïve patients on the clinical trial resulted in a trend toward improved TIL generation, consistent with our previous work.3 From a separate group of metastatic melanoma patients outside of the clinical trial, we cultured tumor fragments in media containing IL-2 with or without supplemental anti-4–1BB. Prior progression on anti-PD-1 therapy had a clear negative effect on ex vivo TIL expansion with IL-2 alone. The addition of agonistic anti-4–1BB plus IL-2 to TIL cultures from patients who had relapsed on prior PD-1 blockade significantly improved TIL generation (p < 0.05; Figure 6A). When patients in this category were stratified, the addition of agonistic anti-4–1BB resulted in a statistically significantly greater frequency of fragment expansion for patients who had failed a single course of anti-PD-1, while only a trend in this direction was observed for failure of multiple courses of anti-PD-1 therapy (Figure 6B).
Figure 6. Agonistic anti-4–1BB antibody improves TIL generation.
A-B. Percent of fragments with expanded TIL in IL-2 and IL-2 and anti-4–1BB supplemented media, stratified by patient therapy history. Patient samples were from a separate group of metastatic melanoma patients, independent of the clinical trial. Single= one prior anti-PD-1 therapy. Multiple= more than one prior course of anti-PD-1 therapies. Number of patient samples indicated (n). Mann-Whitney Test. *p<0.05. C. Tumor fragments were plated for TIL generation in the indicated conditions and the percent of fragments with expanded TIL was reported. Each paired data point represents an individual patient tumor sample. Paired t test. p=0.0009. D. Tumor fragments were plated for TIL generation in the indicated conditions and fold change of the number of wells with expanded TIL was reported. Each paired data point represents an individual patient tumor sample. Paired t test. p<0.0001. E. Isolated subset of samples which did not expand TIL in single agent IL-2. Samples which demonstrated TIL generation with the addition of anti-4–1BB to paired TIL cultures were determined to be rescued. n=11.
With IL-2 alone, only 27% of tumor fragments from specimens previously treated with anti-PD-1 successfully generated TIL. In paired melanoma specimens, the addition of agonistic anti-4–1BB plus IL-2 increased TIL culture success rate on a per fragment basis and improved TIL fold expansion (Figure 6C and 6D). Impressively, of the specimens where TIL were not generated with single-agent IL-2, the addition of anti-4–1BB rescued TIL production for 64% of these tumors (Figure 6E). These data suggest that the addition of anti-4–1BB may be critical for future clinical TIL production as patients are treated with checkpoint blockade as frontline therapy with consideration of TIL therapy at the time of progression after checkpoint blockade.
Discussion
We completed a Phase I clinical trial for anti-PD-1 naïve metastatic melanoma patients investigating the combination of anti-PD-1 and ACT with TIL generated in the presence of agonistic anti-4–1BB in vitro. The primary endpoint of this study was met as the treatment was determined to be both safe and feasible. Adverse events were primarily related to the lymphodepletion regimen and IL-2 administered immediately following ACT. The current study enrolled 11 patients who achieved an ORR of 36% and observed a decrease in 69% of all target lesions.1, 23–27 Three of these patients achieved a partial response lasting longer than one year following combination ACT and nivolumab therapy, while one additional patient responded to nivolumab preceding TIL treatment that was ultimately not administered.
Current timelines of TIL production can include up to eight weeks between tumor harvest and TIL administration, a substantial gap during which patients suffering from already advanced disease are susceptible to further tumor progression and clinical deterioration preventing scheduled ACT. Anti-PD-1 monotherapy is effective at reducing tumor burden in metastatic melanoma patients, which may render a patient’s tumor burden more amenable to TIL therapy.28 Based on prior studies, we hypothesized that pretreatment with nivolumab would curb patient progression during TIL generation and allow more patients to complete treatment with ACT.23 To this end, all five patients enrolled in C2 received TIL transfer, except Patient 9 who achieved a response on nivolumab monotherapy and elected not to complete ACT, while one of six patients in C1 progressed prior to ACT. The degree to which anti-PD-1 treatment alone contributed to patient responses in this trial was not specifically investigated, however anti-PD-1 pretreatment provided patients the potential opportunity to respond prior to ACT in C2, as with Patient 9. Furthermore, we observed a decreased tumor burden in two additional patients in C2 with anti-PD-1 pretreatment between baseline and ACT. Responses were evaluated at 12 weeks following ACT by intent-to-treat analysis. The response rate in this study is similar to previously reported response rates involving TIL, including PD-1 refractory patients.23, 25, 26, 29 While the relatively small number of patients is a limitation of this study, these individual cases support the notion that anti-PD-1 pretreatment alleviated patient attrition when combined with ACT, and the intervening use of lymphodepleting chemotherapy following anti-PD-1 pretreatment did not appear to have a deleterious effect on subsequent tumor responses.
PD-1 is expressed by T cells enriched for antigen-specific reactivity and serves to negatively regulate T cell function upon ligand interaction.14–16 We hypothesized that combination PD-1 blockade with ACT might improve treatment efficacy upon TIL infusion by preventing ligand engagement during antitumor responses to sustain T cell responses. TIL from responders expressed elevated LAG3 and TIGIT, negative regulators of T cell function induced after activation, suggesting these may be additional indicators of antigen-specific T cells.30–32 These represent possible targets for additional therapeutic intervention, especially with the recent FDA approval of an anti-LAG3 antibody in combination with nivolumab.33 Additionally, infused TIL had high overall persistence in the peripheral blood of patients across both cohorts at six weeks after transfer and responders were enriched for central memory CD8+ T cells. In long-term responders, these enriched TIL clones were detected over one year after infusion and clones present within the TIL product comprised over 25% of the peripheral repertoire in each of these patients, consistent with previous TIL trials that have correlated persistence with patient response1, 34. Combination checkpoint blockade/ACT in this study led to durable responses, characteristic of ACT responses in other reports.1, 23, 25, 26, 35 The overall TIL persistence indicated that even non-responders may have an altered immune landscape and could potentially be poised to benefit from additional immunotherapeutic options.
We further hypothesized that enriching for tumor-reactive T cell clones would improve the quality of TIL available for infusion. To address this, we supplemented our ex vivo TIL cultures with anti-4–1BB. Previous studies demonstrated that the addition of anti-4–1BB antibody during TIL generation improved TIL yield and skewed the TIL population toward tumor-reactive CD8+ T cells.3, 36, 37 In the current trial, substantially more TIL were infused in comparison to previous TIL ACT trials at our center, likely due to improved growth kinetics with addition of anti-4–1BB antibody.23, 25, 26 A paired comparison of TIL generation with and without supplemental anti-4–1BB demonstrated an increase in productive TIL fragments for five of six patients, including rescue of TIL expansion in one patient who failed to produce TIL with single agent IL-2. Additionally, all eight patient samples produced tumor-reactive TIL upon in vitro co-culture, with the exception of Patient 11. Despite this, Patient 11 achieved a partial response, which is still ongoing at the time of publication, after ACT with predominantly CD4+ TIL. We cannot exclude that this response was driven by nivolumab, however it is possible that antigen-specific TIL were present but not detected in our assays. For example, CD4+ TIL antitumor reactivity is restricted by the ability of the tumor target to efficiently express MHC Class II. This limitation often precludes the ability to accurately assess CD4+ TIL functionality, representing an unmet need in the field of tumor immunology and an active area of research in our laboratory.
We also investigated two patients who progressed with brain metastases following ACT, in order to better understand the mechanisms underlying therapeutic resistance after TIL transfer. We were able to efficiently generate TIL from each resected brain metastasis, indicating T cell infiltration occurs in brain metastasis relapses, and that subsequent ex vivo expansion of TIL from these lesions is feasible. In patient 7, low TCRβ overlap between the iTIL and rTIL suggested inefficient trafficking or persistence of the iTIL at those novel sites, or reduced subsequent ex vivo expansion during rTIL generation. This shift in repertoire coincided with TIL antitumor recognition that was limited to the respective tumor origin of the TIL. These data support the recent report from Yost et al. describing clonal replacement following anti-PD-1 therapy but also may indicate a potential antigenic shift between the original target lesion and subsequent recurrences.22 Due to limited quality of the recovered iTumor specimen, we were unable to accurately compare the neoantigen mutanome between specimens, as well as other potential contributing mechanisms of therapeutic resistance, such as HLA loss and immune receptor ligand expression.
In contrast, analysis of Patient 8 demonstrated substantial overlap in the clonal profile between iTIL and rTIL, suggesting effective iTIL trafficking and infiltration into the relapsed tumor site. Despite successful infiltration, iTIL failed to mediate an antitumor response at this lesion indicating active tumor resistance to T cell-mediated elimination. Additional in vitro analysis demonstrated minimal recognition of autologous tumor by rTIL coupled with a deficient co-stimulatory profile, a known mechanism of immunotherapeutic resistance.38–43 Given that additional resistance mechanisms are possible, future analyses will aim to compare the transcriptome of infused TIL to TIL recovered from those at sites of progression as well as to determine evidence of immunoediting in ACT. These case studies present opportunities to inform future trial designs to investigate additional monitoring and interventional strategies to elucidate the mechanisms and context underlying the efficacy of and resistance to ACT.
As anti-PD-1 therapy has become a widespread frontline treatment, including its use in the adjuvant setting, there are fewer patients eligible for TIL therapy who are anti-PD-1 treatment-naïve. We performed a larger analysis with a separate group of metastatic melanoma patients outside of the clinical trial to determine the effects of disease progression from prior anti-PD-1 on TIL expansion. We found that surgical specimens resected from patients who had previously failed anti-PD-1 therapy generated substantially fewer TIL compared to anti-PD-1 treatment-naïve specimens. Agonistic anti-4–1BB antibody plus IL-2 proved to be an effective strategy to rescue TIL expansion ex vivo following disease progression on anti-PD-1, particularly in samples unable to produce TIL when utilizing single agent IL-2. Going forward, this study underscores the importance of developing improved methods for TIL generation, especially in the context of disease progression after prior immunotherapy, although it has been previously shown that PD-1 refractory patients can be successfully treated with TIL.29
Overall, combination therapy of ACT with TIL expanded ex vivo using anti-4–1BB along with systemically administered anti-PD-1, was found to be a safe and feasible treatment for metastatic melanoma patients. This study represents the first peer reviewed report to our knowledge of TIL infusion in combination with PD-1 blockade in the anti-PD-1-naïve setting. Forthcoming ACT trials for melanoma will predominantly enroll patients who have progressed after anti-PD-1, and thus may necessitate additional combination strategies to enhance TIL generation such as anti-4–1BB used in this report.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
In this Phase I clinical trial, metastatic melanoma patients received adoptive cell therapy (ACT) with tumor-infiltrating lymphocytes (TIL) generated with anti-4–1BB agonistic antibody in vitro in combination with nivolumab infusions. Four out of 11 patients achieved a partial response (36% overall response rate) with median progression-free survival (PFS) of 5 months. Responses to therapy were durable and were characterized by relatively high persistence of infused TIL. Expanded TIL were primarily CD8+ T cells with potent antitumor reactivity detected in vitro, leading to a decrease in tumor burden of 69% of target lesions. Correlative case studies suggested that non-responders manifested varying mechanisms of resistance, which underscore the importance of the function and quality of T cells after anti-PD-1 therapy. Additional research specimens suggested that metastatic melanoma tumors from patients who progressed after anti-PD-1 therapy yielded reduced expansion of TIL, which was rescued by supplemental agonistic anti-4–1BB antibody during TIL production.
ACKNOWLEDGEMENTS
This work was funded by Swim Across America, Iovance Biotechnologies, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. The anti-4-1BB antibody was generously provided by Bristol Meyers Squibb, as well as additional support. S. Pilon-Thomas was supported by an American Cancer Society––Leo and Anne Albert Charitable Foundation Research Scholar Grant (RSG-16-117-01-LIB). A. Sarnaik was supported by NCI-5K23CA178083. J. Mullinax was supported by NIH-NCI (K08CA252642). M. Hall was supported by NCI-F31CA250320. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number F31CA250320. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported in part by the Cell Therapies Core, Tissue Core, Flow Cytometry Core, and Molecular Genomics Core Facilities at the Moffitt Cancer Center. This work was also supported in part by the Cancer Center Support Grant P30 CA076292 from the National Cancer Institute. The authors would like to thank Autumn Joerger, Patrick Verdugo, Shayna Smeltzer, Zachary Sannasardo, Brittany Bunch, PhD, and Nermin Gerges, PhD, for their assistance with this work.
CONFLICT OF INTEREST STATEMENT
Moffitt Cancer Center has licensed Intellectual Property (IP) related to the proliferation and expansion of tumor infiltrating lymphocytes (TILs) to Iovance Biotherapeutics. MH, JEM, SP-T, and AAS are co-inventors on such Intellectual Property.
AAS and SP-T are co-inventors on a patent application with Provectus Biopharmaceuticals.
MH and AMH report common stock holdings in AbbVie, Inc., Amgen, Inc., BioHaven Pharmaceuticals, and Bristol Myers Squibb.
JEM participates in sponsored research agreements with Iovance Biotherapeutics, Intellia Therapeutics, and SQZ Biotech that are not related to this research. JEM has received research support that is not related to this research from the following entities: Ocala Royal Dames and V Foundation. JEM has received ad hoc consulting fees from Merit Medical.
JKT participates in a sponsored research agreement with Turnstone Biologics that is not related to this research.
NIK reports personal consulting fees from Astra-Zeneca, Bristol Myers-Squibb, Incyte, Iovance, Merck, Novartis, Nektar, Replimune, Regeneron, Jounce, Castle Biosciences and Instill Bio; receives research funding (all to institute) from Bristol Myers-Squibb, Merck, Novartis, Replimune, Celgene, Regeneron, HUYA Biopharmaceuticals and GlaxoSmithKline; and common stock holdings in Bellicum Pharmaceuticals, Amarin Corp. and Asensus Surgical.
JSW receives consulting fees from and serves on ad boards for Merck, Genentech, Astra Zeneca, Pfizer, Regeneron, GSK, Alkermes, Novartis, Celldex, Incyte, and EMD Serono, and Bristol Myers Squibb; he holds equity in Instil Bio, Biond, Neximmune, OncoC4, and Evaxion. He serves on scientific advisory boards for OncoC4, Instil Bio, Incyte, Biond, Neximmune, ImCheck and Sellas. Moffitt Cancer Center filed a patent on an IPILIMUMAB biomarker and a TIL growth method that JSW is named on, as well as on a PD-1 biomarker patent by Biodesix.
JJM is Associate Center Director at Moffitt Cancer Center and founder of Piranha Oncology, Inc., has ownership interest in Aleta Biotherapeutics, Inc., CG Oncology, Inc., Turnstone Biologics, Inc., AffyImmune, Inc., Aleta BioTherapeutics, Inc., Ankyra Therapeutics, and is a paid consultant/paid advisory board member for ONCoPEP, Inc., CG Oncology, Inc., Mersana Therapeutics, Inc., Turnstone Biologics, Inc., Aleta BioTherapeutics, Inc., Iovance Biotherapeutics, Vault Pharma, Inc., ORI Capital, UbiVac, LLC, Vycellix, Inc., AffyImmune, Inc., and Ankyra, Inc.
VKS is a compensated consultant for Alkermes, Bristol Myers Squibb, Iovance and Merck, and receives research funding from Neogene Therapeutics and Turnstone.
Moffitt has also licensed IP to Tuhura Biopharma. SP-T is an inventor on such Intellectual Property. SP-T participates in sponsored research agreements with Provectus Biopharmaceuticals, Intellia Therapeutics, Dyve Biosciences, and Turnstone Biologics that are not related to this research. SP-T has received consulting fees from Seagen Inc. and KSQ Therapeutics.
AAS has received Ad hoc consulting fees from Iovance Biotherapeutics, Guidepoint, Defined Health, Huron Consulting Group, KeyQuest Health Inc, Istari, and Gerson Lehrman Group. AAS has received speaker fees from Physicians’ Educational Resource (PER) LLC, Medscape and Medstar Health. The clinical trial included in this report was funded by Iovance Biotherapeutics.
References
- 1.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. Epub 20110415. doi: 10.1158/1078-0432.CCR-11-0116. PubMed PMID: 21498393; PMCID: PMC3131487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, Kroeger J, Eysmans C, Sarnaik AA, Chen YA. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–8. Epub 20131021. doi: 10.1200/JCO.2013.51.4802. PubMed PMID: 24145345; PMCID: PMC3837092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chacon JA, Sarnaik AA, Chen JQ, Creasy C, Kale C, Robinson J, Weber J, Hwu P, Pilon-Thomas S, Radvanyi L. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res. 2015;21(3):611–21. Epub 20141203. doi: 10.1158/1078-0432.CCR-14-1934. PubMed PMID: 25472998; PMCID: PMC4315752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, Yan L, Targan S, Solomon J, Nichol G, Yellin M, Weber JS. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17(4):896–906. Epub 20101124. doi: 10.1158/1078-0432.CCR-10-2463. PubMed PMID: 21106722; PMCID: PMC3041838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber J, Atkins M, Hwu P, Radvanyi L, Sznol M, Yee C, Immunotherapy Task Force of the NCIIDSC. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin Cancer Res. 2011;17(7):1664–73. Epub 20110215. doi: 10.1158/1078-0432.CCR-10-2272. PubMed PMID: 21325070. [DOI] [PubMed] [Google Scholar]
- 6.Chang AE, Rosenberg SA. Overview of interleukin-2 as an immunotherapeutic agent. Semin Surg Oncol. 1989;5(6):385–90. doi: 10.1002/ssu.2980050604. PubMed PMID: 2688029. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA. Treatment of 283 Consecutive Patients With Metastatic Melanoma or Renal Cell Cancer Using High-Dose Bolus Interleukin 2. JAMA: The Journal of the American Medical Association. 1994;271(12). doi: 10.1001/jama.1994.03510360033032; PMCID: 8120958. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210(4):474–84; discussion 84–5. doi: 10.1097/00000658-198910000-00008. PubMed PMID: 2679456; PMCID: PMC1357927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85(8):622–32. doi: 10.1093/jnci/85.8.622. PubMed PMID: 8468720. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. PubMed PMID: 18354418; PMCID: PMC2553205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal RE, Steinberg SM, Krouse RS, Heywood G, White DE, Hwu P, Marincola FM, Parkinson DR, Schwartzentruber DJ, Topalian SL, Yang JC, Rosenberg SA. Correlates of response to IL-2 therapy in patients treated for metastatic renal cancer and melanoma. Cancer J Sci Am. 1996;2(2):91–8. PubMed PMID: 9166506; PMCID: 9166506. [PubMed] [Google Scholar]
- 12.Innamarato P, Asby S, Morse J, Mackay A, Hall M, Kidd S, Nagle L, Sarnaik AA, Pilon-Thomas S. Intratumoral Activation of 41BB Costimulatory Signals Enhances CD8 T Cell Expansion and Modulates Tumor-Infiltrating Myeloid Cells. J Immunol. 2020;205(10):2893–904. Epub 20201005. doi: 10.4049/jimmunol.2000759. PubMed PMID: 33020146; PMCID: PMC7741883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gros A, Tran E, Parkhurst MR, Ilyas S, Pasetto A, Groh EM, Robbins PF, Yossef R, Garcia-Garijo A, Fajardo CA, Prickett TD, Jia L, Gartner JJ, Ray S, Ngo L, Wunderllich JR, Yang JC, Rosenberg SA. Recognition of human gastrointestinal cancer neoantigens by circulating PD-1+ lymphocytes. J Clin Invest. 2019;129(11):4992–5004. doi: 10.1172/JCI127967. PubMed PMID: 31609250; PMCID: PMC6819109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inozume T, Hanada K, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33(9):956–64. doi: 10.1097/CJI.0b013e3181fad2b0. PubMed PMID: 20948441; PMCID: PMC2980947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–59. Epub 20140325. doi: 10.1172/JCI73639. PubMed PMID: 24667641; PMCID: PMC4001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yossef R, Tran E, Deniger DC, Gros A, Pasetto A, Parkhurst MR, Gartner JJ, Prickett TD, Cafri G, Robbins PF, Rosenberg SA. Enhanced detection of neoantigen-reactive T cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight. 2018;3(19). Epub 20181004. doi: 10.1172/jci.insight.122467. PubMed PMID: 30282837; PMCID: PMC6237474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. Epub 20020624. doi: 10.1038/nm730. PubMed PMID: 12091876. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12. Epub 20120109. doi: 10.1016/j.coi.2011.12.009. PubMed PMID: 22236695; PMCID: PMC3319479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. PubMed PMID: 11015443; PMCID: PMC2193311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7. Epub 20020906. doi: 10.1073/pnas.192461099. PubMed PMID: 12218188; PMCID: PMC129438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K-. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. Epub 20150419. doi: 10.1056/NEJMoa1503093. PubMed PMID: 25891173. [DOI] [PubMed] [Google Scholar]
- 22.Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, Gupta RK, Curtis C, Bucktrout SL, Davis MM, Chang ALS, Chang HY. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. 2019;25(8):1251–9. Epub 20190729. doi: 10.1038/s41591-019-0522-3. PubMed PMID: 31359002; PMCID: PMC6689255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullinax JE, Hall M, Prabhakaran S, Weber J, Khushalani N, Eroglu Z, Brohl AS, Markowitz J, Royster E, Richards A, Stark V, Zager JS, Kelley L, Cox C, Sondak VK, Mule JJ, Pilon-Thomas S, Sarnaik AA. Combination of Ipilimumab and Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes for Patients with Metastatic Melanoma. Front Oncol. 2018;8:44. Epub 20180302. doi: 10.3389/fonc.2018.00044. PubMed PMID: 29552542; PMCID: PMC5840208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, Landin AM, Mullinax JE, Saller JJ, Saltos AN, Noyes DR, Montoya LB, Curry W, Pilon-Thomas SA, Chiappori AA, Tanvetyanon T, Kaye FJ, Thompson ZJ, Yoder SJ, Fang B, Koomen JM, Sarnaik AA, Chen DT, Conejo-Garcia JR, Haura EB, Antonia SJ. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27(8):1410–8. Epub 20210812. doi: 10.1038/s41591-021-01462-y. PubMed PMID: 34385708; PMCID: PMC8509078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, Kudchadkar R, Zager J, Gibney G, Sondak VK, Weber J, Mule JJ, Sarnaik AA. Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother. 2012;35(8):615–20. doi: 10.1097/CJI.0b013e31826e8f5f. PubMed PMID: 22996367; PMCID: PMC4467830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarnaik A, Hall M, Mullinax J, Royster E, Richards A, Crago G, Zager J, Vernon S, Weber J, Pilon-Thomas S. Clinical results of combined vemurafenib and tumor-infiltrating lymphocyte therapy for metastatic melanoma. Journal for ImmunoTherapy of Cancer. 2015;3(S2). doi: 10.1186/2051-1426-3-s2-p49. [DOI] [Google Scholar]
- 27.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, Klebanoff CA, Hughes MS, Restifo NP, Langhan MM, Shelton TE, Lu L, Kwong ML, Ilyas S, Klemen ND, Payabyab EC, Morton KE, Toomey MA, Steinberg SM, White DE, Rosenberg SA. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol. 2016;34(20):2389–97. Epub 20160523. doi: 10.1200/JCO.2016.66.7220. PubMed PMID: 27217459; PMCID: PMC4981979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, Dronca R, Mitchell TC, Patnaik A, Zarour HM, Joshua AM, Zhao Q, Jensen E, Ahsan S, Ibrahim N, Ribas A. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–8. doi: 10.1093/annonc/mdz011. PubMed PMID: 30715153; PMCID: PMC6503622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarnaik AA, Hamid O, Khushalani NI, Lewis KD, Medina T, Kluger HM, Thomas SS, Domingo-Musibay E, Pavlick AC, Whitman ED, Martin-Algarra S, Corrie P, Curti BD, Olah J, Lutzky J, Weber JS, Larkin JMG, Shi W, Takamura T, Jagasia M, Qin H, Wu X, Chartier C, Graf Finckenstein F, Fardis M, Kirkwood JM, Chesney JA. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. J Clin Oncol. 2021;39(24):2656–66. Epub 20210512. doi: 10.1200/JCO.21.00612. PubMed PMID: 33979178; PMCID: PMC8376325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–80. Epub 20100412. doi: 10.1073/pnas.1003345107. PubMed PMID: 20385810; PMCID: PMC2867907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125(5):2046–58. Epub 20150413. doi: 10.1172/JCI80445. PubMed PMID: 25866972; PMCID: PMC4463210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923–37. Epub 20141126. doi: 10.1016/j.ccell.2014.10.018. PubMed PMID: 25465800. [DOI] [PubMed] [Google Scholar]
- 33.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutierrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ, Dalle S, Arance A, Grob JJ, Srivastava S, Abaskharoun M, Hamilton M, Keidel S, Simonsen KL, Sobiesk AM, Li B, Hodi FS, Long GV, Investigators R-. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022;386(1):24–34. doi: 10.1056/NEJMoa2109970. PubMed PMID: 34986285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, Robbins PF. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28(3):258–67. doi: 10.1097/01.cji.0000158855.92792.7a. PubMed PMID: 15838383; PMCID: PMC2174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, Kubi A, Shoshani N, Zikich D, Ohayon Y, Ohayon D, Shalmon B, Markel G, Yerushalmi R, Apter S, Ben-Nun A, Ben-Ami E, Shimoni A, Nagler A, Schachter J. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19(17):4792–800. Epub 20130520. doi: 10.1158/1078-0432.CCR-13-0380. PubMed PMID: 23690483. [DOI] [PubMed] [Google Scholar]
- 36.Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ, Jr. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res. 2014;20(1):44–55. Epub 20130917. doi: 10.1158/1078-0432.CCR-13-0945. PubMed PMID: 24045181; PMCID: PMC3947326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110(1):201–10. Epub 20070319. doi: 10.1182/blood-2006-11-056168. PubMed PMID: 17371945; PMCID: PMC1896114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, Palaskas N, Rodriguez GA, Parisi G, Azhdam A, Chmielowski B, Cherry G, Seja E, Berent-Maoz B, Shintaku IP, Le DT, Pardoll DM, Diaz LA, Jr., Tumeh PC, Graeber TG, Lo RS, Comin-Anduix B, Ribas A. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017;7(2):188–201. Epub 20161130. doi: 10.1158/2159-8290.CD-16-1223. PubMed PMID: 27903500; PMCID: PMC5296316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819–29. Epub 20160713. doi: 10.1056/NEJMoa1604958. PubMed PMID: 27433843; PMCID: PMC5007206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. Epub 20160217. doi: 10.1038/ncomms10501. PubMed PMID: 26883990; PMCID: PMC4757784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, Merchant AS, Mehta GU, Chichura A, Shalem O, Tran E, Eil R, Sukumar M, Guijarro EP, Day CP, Robbins P, Feldman S, Merlino G, Zhang F, Restifo NP. Identification of essential genes for cancer immunotherapy. Nature. 2017;548(7669):537–42. Epub 20170807. doi: 10.1038/nature23477. PubMed PMID: 28783722; PMCID: PMC5870757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Effern M, Glodde N, Braun M, Liebing J, Boll HN, Yong M, Bawden E, Hinze D, van den Boorn-Konijnenberg D, Daoud M, Aymans P, Landsberg J, Smyth MJ, Flatz L, Tuting T, Bald T, Gebhardt T, Holzel M. Adoptive T Cell Therapy Targeting Different Gene Products Reveals Diverse and Context-Dependent Immune Evasion in Melanoma. Immunity. 2020;53(3):564–80 e9. Epub 20200803. doi: 10.1016/j.immuni.2020.07.007. PubMed PMID: 32750334. [DOI] [PubMed] [Google Scholar]
- 43.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–23. doi: 10.1016/j.cell.2017.01.017. PubMed PMID: 28187290; PMCID: PMC5391692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this report are available within this manuscript and its supplement or available upon reasonable request from the corresponding author.