Abstract
In the past 25 years, the prevalence of Parkinson’s disease (PD) has nearly doubled. Age remains the primary risk factor for PD and as the global aging population increases this trend is predicted to continue. Even when treated with levodopa, the gold standard dopamine (DA) replacement therapy, individuals with PD frequently develop therapeutic side effects. Levodopa-induced dyskinesia (LID), a common side effect of long-term levodopa use, represents a significant unmet clinical need in the treatment of PD. Previously, in young adult (3-month-old) male parkinsonian rats, we demonstrated that the silencing of CaV1.3 (Cacan1d) L-type voltage-gated calcium channels via striatal delivery of rAAV-CaV1.3-shRNA provides uniform protection against the induction of LID, and significant reduction of established severe LID. With the goal of more closely replicating a clinical demographic, the current study examined the effects of CaV1.3-targeted gene therapy on LID escalation in male and female parkinsonian rats of advanced age (18-month-old at study completion). We tested the hypothesis that silencing aberrant CaV1.3 channel activity in the parkinsonian striatum would prevent moderate to severe dyskinesia with levodopa dose escalation. To test this hypothesis, 15-month-old male and female F344 rats were rendered unilaterally parkinsonian and primed with low-dose (3–4 mg/kg) levodopa. Following the establishment of stable, mild dyskinesias, rats received an intrastriatal injection of either the Cacna1d-specific rAAV-CaV1.3-shRNA vector (CAV-shRNA), or the scramble control rAAV-SCR-shRNA vector (SCR-shRNA). Daily (M-Fr) low-dose levodopa was maintained for 4 weeks during the vector transduction and gene silencing window followed by escalation to 6 mg/kg, then to 12 mg/kg levodopa. SCR-shRNA-shRNA rats showed stable LID expression with low-dose levodopa and the predicted escalation of LID severity with increased levodopa doses. Conversely, complex behavioral responses were observed in aged rats receiving CAV-shRNA, with approximately half of the male and female subjects—therapeutic ‘Responders’—demonstrating protection against LID escalation, while the remaining half—therapeutic ‘Non-Responders’—showed LID escalation similar to SCR-shRNA rats. Post-mortem histological analyses revealed individual variability in the detection of Cacna1d regulation in the DA-depleted striatum of aged rats. However, taken together, male and female therapeutic ‘Responder’ rats receiving CAV-shRNA had significantly less striatal Cacna1d in their vector-injected striatum relative to contralateral striatum than those with SCR-shRNA. The current data suggest that mRNA-level silencing of striatal CaV1.3 channels maintains potency in a clinically relevant in vivo scenario by preventing dose-dependent dyskinesia escalation in rats of advanced age. As compared to the uniform response previously reported in young male rats, there was notable variability between individual aged rats, particularly females, in the current study. Future investigations are needed to derive the sex-specific and age-related mechanisms which underlie variable responses to gene therapy and to elucidate factors which determine the therapeutic efficacy of treatment for PD.
Keywords: Parkinson’s disease, CaV1.3 channels, Gene therapy, Levodopa-induced dyskinesia, Striatum, Adeno-associated virus (AAV), Viral vectors, Aging
1. Introduction
The management of levodopa-induced dyskinesia (LID) remains a significant unmet need in the treatment of Parkinson’s disease (PD) (Huot et al., 2022; Bastide et al., 2015; Bove and Calabresi, 2022; Huot et al., 2013). Levodopa, the biosynthetic precursor to dopamine (DA), has been the predominant symptomatic treatment for PD for more than five decades (Bastide et al., 2015; Rascol et al., 2022; Tanner et al., 2020; Belujon et al., 2010). Though the dyskinetic side effects of long-term levodopa use are well-documented, effective therapies for LID remain elusive (for review (Huot et al., 2022; Bastide et al., 2015)). Currently, extended-release amantadine is the only FDA-approved drug available to individuals with PD suffering from LID, which, though partially effective, is not without its own side effects (Rascol et al., 2022; Tanner et al., 2020).
Essential to the development of LID is aberrant synaptic plasticity. This irregular circuitry primarily involves medium spiny projection neurons (SPNs), the striatal cells which receive afferent input from substantia nigra (SN) DA neurons (e.g. (Bastide et al., 2015; Belujon et al., 2010; Fieblinger et al., 2023; Picconi et al., 2003; Zhang et al., 2013; Shen et al., 2022; Calabrese et al., 2020; Picconi et al., 2018; Sellnow et al., 2020)). In PD, the loss of these SN DA neurons and the subsequent depletion of DA in the parkinsonian striatum results in the dysregulation of intraspinous L-type voltage-gated calcium channels (i. e., CaV1.3), which in turn leads to the retraction of dendritic spines and synaptic pruning of SPNs (Bastide et al., 2015; Zhang et al., 2013; Day et al., 2006; Ingham et al., 1989; McNeill et al., 1988; Schuster et al., 2009; Soderstrom et al., 2010; Zaja-Milatovic et al., 2005). These losses are recovered by levodopa treatment and the reestablishment of striatal DA; however, the restoration of dendritic spines and afferent input on SPNs occurs with abnormal patterns of “mis-wiring” (Zhang et al., 2013). This aberrant circuit restoration and the multifarious changes in spine densities on striatopallidal and striatonigral SPNs (for review (Bastide et al., 2015)) are associated with LID, and related in part to alterations in CaV1.3-associated calcium flux. The relationship between dysregulation of intraspinous CaV1.3 channels and aberrant SPN plasticity led to interest in CaV1.3 antagonism as a novel antidyskinetic therapy (Schuster et al., 2009; Soderstrom et al., 2010; Steece-Collier et al., 2019).
Initial studies attempted pharmacological antagonism of striatal CaV1.3 channels using the antihypertensive dihydropyridine drugs isradipine and nimodipine; however, presumed inadequate target engagement led to partial or transient efficacy of these therapies (Schuster et al., 2009; Soderstrom et al., 2010). Using a recombinant adeno-associated virus (rAAV)-mediated short hairpin RNA (shRNA) to provide continuous, high-potency, target-selective mRNA-level silencing of striatal CaV1.3 channels, we recently provided the first unequivocal evidence of the ability of striatal CaV1.3 silencing to provide lasting functional protection against LID (Steece-Collier et al., 2019), without the pharmacological limitations of earlier studies (Schuster et al., 2009; Soderstrom et al., 2010). Specifically, in young adult (3-month-old) male parkinsonian rats, we observed that mRNA-level silencing of striatal CaV1.3 prior to the introduction of levodopa could completely and uniformly prevent the induction of LID in response to a daily (M-Fr) levodopa dose escalation paradigm (6, 9, 12, and 18 mg/kg; 2 weeks per dose) (Steece-Collier et al., 2019). We also reported that, in young male parkinsonian rats with established severe LID and high-dose levodopa administration (12 mg/kg), striatal CaV1.3 silencing could significantly, albeit partially (~60%), reduce LID severity (Steece-Collier et al., 2019).
Amelioration or stabilization of existing LID holds immediate clinical relevance: approximately 90% of levodopa-treated individuals with PD will develop LID within 10 years of diagnosis (e.g. (Huot et al., 2022; Huot et al., 2013)). Acknowledging this, together with the prevalence of PD in subjects of advanced age in both sexes, we have undertaken a second phase of studies using 15-month-old (at start of study) male and female F344 rats to examine the ability of CaV1.3 gene therapy to ameliorate LID. In the current study we tested the hypothesis that mRNA-level silencing of dysfunctional CaV1.3 channels in the parkinsonian striatum of male and female rats with mild LID would prevent the subsequent escalation of LID severity with levodopa dose escalation.
We present here the first functional data in unilaterally parkinsonian rats of advanced age that: 1) directly compares LID profiles between male and female sexes, 2) confirms that CaV1.3 gene therapy can be effective at preventing dose-dependent escalation of LID despite advancing age, 3) demonstrates that individual variability in the effectiveness of this therapy is driven by inconsistent striatal regulation of the target gene Cacna1d in the aged brain. Notably, the prevention of LID escalation in 15-month-old parkinsonian rats occurred only in a subpopulation of subjects—CAV-shRNA ‘Responders’—with a slightly higher percentage of success in the male cohort and more variability in the female cohort. This contrasts our previous findings in 3-month-old male parkinsonian rats, where all subjects showed a significant reduction of established, severe LID and uniform prevention of dose-related LID escalation (Steece-Collier et al., 2019). Quantification of mRNA in the post-mortem striatum demonstrates that while there appears to be complex CaV1.3 regulation in the aged, levodopa-primed brain, reduced Cacna1d expression in rats receiving rAAV-CaV1.3-shRNA correlates with functional benefit in both males and females—CAV-shRNA Responders.
2. Materials and methods
2.1. Experimental subjects
All procedures were performed on 15-month-old (at the time of lesioning) male (n = 15) and female (n = 13) Fischer (F344) rats (National Institute on Aging, Division of Aging Biology, Bethesda MD) in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International guidelines and following approval by the Michigan State University Institutional Animal Care and Use Committee.
2.2. Vector design and production
Cacna1d-specific short-hairpin RNA (shRNA) (CaV1.3 (5’-GAAGAGGCGCGGCCAAGAC-3’) (n = 8 male, 7 female) and scrambled control shRNA (5’CAACAAGATGAAGAGCACC3’) (n = 7 male, 6 female) were designed using standard methods and inserted into a recombinant AAV genome under the control of an H1 promoter downstream of a the a GFP reporter cassette (Steece-Collier et al., 2019). Genomes were packaged into rAAV9 using HEK293 cells, particles purified using an iodixanol step gradient, and concentrated using buffer exchange. Virus titers were determined using digital droplet PCR and normalized to 1 × 1013 vector genomes (vg)/mL using a balanced salt solution as previously described (Benskey et al., 2016; Benskey and Manfredsson, 2016; Sandoval et al., 2019).
2.3. Levodopa administration
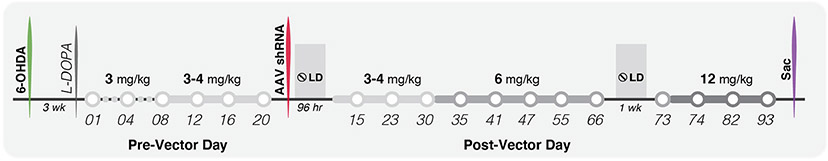
Levodopa was administered subcutaneously (s.c.) to allow assessment of LID behaviors. The goal of the pre-vector levodopa treatment was to induce consistent LID of mild or mild-to-moderate severity in all experimental subjects using a ‘low’ levodopa dose. A dose of 3 mg/kg levodopa was administered initially 3× per week from day 1 to day 8. Because some subjects, predominantly females, appeared refractory to LID induction (Supp Fig. 1B, C), dosing frequency was increased to 5× per week for all subjects (Monday-Friday; days 9–20). In subjects that continued to maintain LID severity below the group mean, levodopa was increased to a slightly higher dose on day 12 (4 mg/kg, M-Fr). Low-dose levodopa (3–4 mg/kg) was administered for 21 days pre-vector and maintained for another 30 days post-vector (Fig. 1A). Levodopa was subsequently increased to a ‘moderate’ dose (6 mg/kg; M-Fr) for approximately one month, including a one-week withdrawal, followed by escalation to a final ‘high’ dose (12 mg/kg; M-Fr) for an additional 2 weeks (Fig. 1A).
Fig. 1.
Experimental timeline. 15-month-old male and female F344 rats were unilaterally lesioned with the DA neurotoxin 6-hydroxydopamine (6-OHDA). Three weeks after 6-OHDA, rats began receiving subcutaneous (s.c.) injections of low-dose (3 mg/kg) levodopa, administered initially 3× per week from day 1 to day 8. On day 9, because several subjects appeared refractory to developing mild LID, the dosing frequency was increased to 5× per week (Monday-Friday; days 9–20) in all subjects, followed with a slight dose increase (4 mg/kg) in those remaining refractory (details above). After 21 days of low-dose (3–4 mg/kg) levodopa treatment, intrastriatal viral vector was administered: rAAV-CaV1.3-shRNA (CAV-shRNA) or rAAV-SCR-shRNA (SCR-shRNA). Levodopa dosing was withheld for 96 h post vector surgery, with low-dose (3 or 4 mg/kg; M-Fr) delivery subsequently resumed for another 30 days. Levodopa was increased to a ‘moderate’ dose (6 mg/kg; M-Fr) for approximately one month (days 31–73) with a brief withdrawal from levodopa (days 66–73), and then subsequently escalated to a final ‘high’ dose (12 mg/kg; M-Fr) for an additional 2 weeks (day 74–93). The study concluded after a total of 93 days of post-vector levodopa treatment.
2.4. Dyskinesia ratings
We use the term LID here to refer to abnormal involuntary behaviors including dystonia, hyperkinesia, and/or stereotypies noted in the presence of levodopa in parkinsonian rats. Behaviors were scored according to an abnormal involuntary movement LID severity rating scale for rats developed in our lab with a clinical movement disorders specialist (Steece-Collier et al., 2003) based on specific criteria reflective of the nature and occurrence of multiple behavioral attributes of dyskinesia as previously detailed (Steece-Collier et al., 2003; Maries et al., 2006; Caulfield et al., 2021). LID behaviors were rated for 1 min per subject every 50 min beginning 20 mins after injection and continuing through 180 mins. Rats were randomized for rating and rated by the same blinded investigator throughout the experiment. Each rat was given a ‘total’ LID severity score for each day assessed (Steece-Collier et al., 2003; Maries et al., 2006; Caulfield et al., 2021).
2.5. 6-OHDA unilateral nigral lesioning surgery
Stereotaxic unilateral lesioning surgeries were performed as previously detailed (Steece-Collier et al., 2019). Briefly, rats were anesthetized with inhalant isoflurane (1.5–2%) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). A 10 μL Hamilton syringe attached to a 26-gauge needle was used to administer 2 μLs of 6-hydroxydopamine (6-OHDA; 5 μg/μL; Sigma) at two midbrain sites (medial forebrain bundle (MFB; AP, −4.3 mm; ML, + 1.8 mm; DV, − 8.4 mm) and substantia nigra (SN; AP, −4.8 mm; ML, + 2.0 mm; DV, − 8.0 mm)) for a total volume of 4 μL (0.5 μL/min). Immediately following surgery, rats were administered 5 mg/kg carprofen at 1 mL/kg, placed in clean cages, and monitored for a post-operative period of 10 days.
2.6. Vector administration
Rats were randomly placed into groups to allow for equivalent LID severity between vector groups. This was based on the average ‘Total LID’ (over the 180 min rating period) for last 2 days of treatment prior to vector surgeries. Subjects then received unilateral stereotaxic intrastriatal infusion of the rAAV9 (1 × 1013 vg/mL) expressing the CaV1.3 specific (CAV-shRNA) or scrambled (SCR-shRNA) control vector. Vectors were infused with a glass syringe into two dorsolateral sites within the left, DA-depleted striatum (2 μLs/site, 0.5 μL/min) for a total of 4 μLs per subject (AP, 0.0, ML, +3.0, DV, −5.2; and AP, +1.6, ML, +2.7, DV, −4.9). All rats received an intraperitoneal injection of 20% d-mannitol 20 mins prior to surgery to facilitate vector distribution (Steece-Collier et al., 2019; Burger et al., 2005; Samaranch et al., 2014; Konradi et al., 2004).
2.7. Euthanasia and post-mortem tissue collection
Twenty-four hours after the last LID evaluation (day 93), rats were administered a final dose of levodopa (12 mg/kg), rated and euthanized 60–70 min post injection, (Zhang et al., 2013; Steece-Collier et al., 2019). Rats were deeply anesthetized with pentobarbital (250 mg/kg i. p.; Beuthanasia-D Special, VetOne, Boise, ID, USA) then perfused intracardially with 200 mL room temperature heparinized 0.9% saline followed by 200 mL ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were removed and post-fixed in 4% paraformaldehyde for 24 h at 4 °C, submersed in 30% sucrose solution, and stored at 4 °C until time of sectioning. Brains were sectioned coronally at 40 μm thickness using a sliding microtome and stored in a cryoprotectant solution at −20 °C.
2.8. TH + GFP immunohistochemistry
All postmortem analyses were done by investigators blinded to experimental conditions. Individual series of tissue sections (1-in-6; 240 μm spacing) were processed for tyrosine hydroxylase (TH) (Millipore-AB152b rabbit anti-TH, 1:4000) and green fluorescent protein (GFP) (Millipore-Ab290 rabbit anti-GFP, 1:20,000) immunohistochemistry (IHC) per our previously reported methods (Steece-Collier et al., 2019; Benskey et al., 2016). The degree of nigral DA neuron depletion in each animal was confirmed by total enumeration of TH-positive neurons (Gombash et al., 2014). Nigral TH neuron loss ≥95% in the lesioned hemisphere compared to the non-lesioned hemisphere was used as an inclusion criterion; this magnitude of SN DA neuron depletion is required in this model to produce reliable LID (e.g. (Steece-Collier et al., 2019; Maries et al., 2006; Collier et al., 2015; Lee et al., 2003; Putterman et al., 2007)). The striatal volume of vector transduction in GFP immunostained sections was determined using Stereo Investigator® software (MBF Bioscience, Williston, VT). Briefly, contours were defined for both the full striatum and striatal region of GFP-immunoreactivity. The outlines of each structure were traced at 1× magnification in 6 coronal sections along the rostral-caudal extent of the striatum. The Cavalieri Estimator function was used to determine the volume of the striatum and the volume of vector transduction (GFP-ir).
To determine any effect of aberrant brain morphology on therapeutic outcomes, ImageJ was used to measure the comparative sizes of both hemispheres of the striatum and lateral ventricle in each animal. Brightfield images of GFP immunostained striatal sections were acquired using a Zeiss Axio Scan Z1 slide scanner at 20× magnification. Images were imported into ImageJ processing software (v. 1.53 t, National Institutes of Health), and contours were drawn around the full striatum and lateral ventricle in both hemispheres (two representative coronal section scans of the striatum at approximately the level of the anterior commissure per animal). The degree of striatal distortion in the injected hemisphere is expressed as both the percent change in striatal size (ipsi− / contralateral striatal area) and as the percent change in lateral ventricle size (ipsi − / contralateral ventricle area).
2.9. Cell-specific Cacna1d knockdown analyses
For cell-specific transcript quantification, Cacna1d (CaV1.3) mRNA was fluorescently labeled utilizing RNAscope® in situ hybridization (ISH) (ACD, Newark, CA) (RNAscope® LS 2.5 Probe- Rn-Cacna1d; REF 409361; NM_017298.1, nucleotides 5401–6474). RNAscope ISH is a commercially available method that employs a branched or “tree” method to obtain ultrasensitive, single transcript in situ detection with exceptional precision when considering related transcripts (e.g no off-target detection of CaV1.2 (Cacna1c)) (Putterman et al., 2007). RNAscope-processed sections were then co-stained using IHC methods for GFP (Millipore-Ab290 rabbit anti-GFP, 1:20,000) and the neuronal proteins HuC/D (ThermoFisher Scientific-A21271 mouse anti-HuC/HuD, 1:2000) for identification of transduced vs. non-transduced neurons. For each animal, confocal z-stacks (2 μm steps) were acquired in the dorsolateral curvature of the striatum (both hemispheres) using the 20× objective on a Nikon Eclipse Ni microscope and NIS elements software, maintaining identical settings across all images. Confocal z-stacks were imported into the image visualization and analysis software, Imaris® (v. 9.8.0, Oxford Instruments). The “Surface” function was used to reconstruct GFP and/or HuC/D immunoreactive cells in a three-dimensional (3-D) field of view, and the “Spots” function was used to mark and quantify individual Cacna1d mRNA puncta (Grabinski et al., 2015). Cacna1d mRNA transcripts were reported as the number of puncta per fluorescent cell in GFP+, HuC/D+, or double positive cells (total number of ipsilateral cells used in analysis: 2644 SCR-shRNA GFP+ cells, 2838 CAV-shRNA-shRNA GFP+ cells, 1292 SCR-shRNA HuC/D+ cells, 1115 CAV-shRNA-shRNA HuC/D+). Cacna1d silencing is expressed as relative level of transcript in the vector-injected striatum compared to the non-injected striatum. In vector-transduced cells, Cacna1d fold change is expressed as the average number of Cacna1d puncta per GFP+ (transduced) cell in the ipsilateral striatum normalized to the average number of Cacna1d puncta per HuC/D+ (non-transduced) cell in the contralateral striatum (total number of contralateral HuC/D+ cells used in analysis: 1292 SCR-shRNA cells, 1114 CAV-shRNA-shRNA GFP+ cells). To represent Cacna1d regulation in neighboring, non-transduced cells in the vector-injected striatum, Cacna1d fold change is expressed as the average number of Cacna1d puncta per HuC/D+, GFP- cell in the ipsilateral injected striatum normalized to the average number of Cacna1d puncta per HuC/D+ cell in the contralateral non-injected striatum.
2.10. Total striatal Cacna1d knockdown analyses
For tissue-level, whole striatal (e.g., cell soma and dendrites; transfected and non-transfected cells) transcript quantification per (Steece-Collier et al., 2019), Cacna1d mRNAs were labeled utilizing RNAscope® ISH (ACD, Newark, CA) (RNAscope® LS 2.5 Probe- Rn-Cacna1d; REF 409361; NM_017298.1, nucleotides 5401–6474), this time processed with 3,3’-diaminobenzidine (DAB) chromogenic enzyme rather than a fluorescent chromophore. The brown pigment DAB was employed for this full striatal analysis to minimize complications due to lipofuscin, an autofluorescent material that accumulates with age. Brightfield microscopy was used to obtain z-stacks (2 μm steps) of the dorsolateral striatum of each animal (both hemispheres, 3 z-stacks per hemisphere) using the 20× objective on a Nikon Eclipse 80i microscope and Stereo Investigator® software (MBF Bioscience, Williston, VT), maintaining identical camera settings across all images. These z-stacks were imported as minimum z-projection images in ImageJ software (v. 1.53 t, National Institutes of Health). Using the “Threshold” function, the DAB-positive area was quantified for each image as a quantitative representation of Cacna1d mRNA expression. The quantity of total striatal Cacna1d mRNA is expressed as a ratio of DAB-labeled transcript area in the ipsilateral to the contralateral striatum.
2.11. Data and statistical analyses
Since LID data are created using ordinal rating scales for assigning values to dyskinesia severity and/or duration, all LID behavioral data were analyzed with non-parametric statistics. Mann-Whitney U tests were used to compare two individual groups (e.g., CAV-shRNA vs. SCR-shRNA, Responder vs. Non-Responder, Female vs. Male). The Kruskal-Wallis test, an alternative non-parametric one-way ANOVA, was used for between-subject comparisons (two or more independent groups). The Friedman test, a non-parametric alternative to a one-way ANOVA with repeated measures (within subjects’ comparisons) were used to compare three or more individual groups, as there are no non-parametric two-way ANOVA tests. Post-hoc Dunn or Dunn-Bonferroni tests were used following a significant Kruskal-Wallis or Friedman test.
The degree of LID Fold Change between treatments was calculated as total LID severity on three post-vector rating days (23, 55, 82; one day per levodopa dose paradigm) compared to day 20 pre-vector. LID Fold Change = (Total LID Post-Vector Day ## / Total LID Pre-Vector Day ##) – 1. A fold change of 0 corresponds to no change in LID severity; < 0 = decreased total LID, > 0 = increased total LID. CAV-shRNA subjects were classified as ‘Responders’ or ‘Non-Responders’ based on LID Fold Change values: CAV-shRNA Responders were characterized as having a reduction in total LID severity indicated by a LID Fold Change <0 on at least 2 of the 3 calculated post-vector days. Non-Responders were characterized as having no change or an increase in total LID severity indicated by a LID Fold Change of ≥0 on at least 2 of the 3 calculated days. LID Fold Change differences between CAV-shRNA and SCR-shRNA cohorts were analyzed with non-parametric, one-tailed Mann-Whitney U tests for each calculated post-vector day. LID Fold Change values used in XY plots represent the LID Fold Change calculated at post-vector day 55 (as compared to pre-vector day 20).
All CAV-shRNA vs. SCR-shRNA comparisons of Cacna1d fold change data, estimated TH-positive cells, percent transduction values, percent change in striatal size, and percent increase in ventricle size were analyzed using unpaired, two-tailed t-tests. Two-tailed Spearman’s correlation tests were used to analyze all correlations with LID Fold Change. All analyses were performed with Prism GraphPad.v9.4.1 for MacOSX.
3. Results
3.1. Intrastriatal rAAV-CaV1.3-shRNA administration in parkinsonian rats of advanced age is protective against LID escalation with increasing levodopa dose
Throughout the pre-vector time course of this study there were no significant differences in the mild LID severity established between the CAV-shRNA receiving animals and the SCR-shRNA (Fig. 2A; p > 0.05 for pre-vector days 1 through 20). Generally, the timeframe required for optimal rAAV-shRNA vector transduction and target engagement is 2–6 weeks following vector administration (for details on rAAV vector production and function see (Sandoval et al., 2019; Au et al., 2021; Berns and Muzyczka, 2017; He et al., 2021; Kanaan et al., 2017)). Not surprisingly, then, we did not observe reversal of established mild LID immediately post-vector injection (Fig. 2A; Suppl Fig. 1; Suppl Fig. 2). Specifically, low-level LID were maintained at each experimental timepoint with no significant difference between CAV-shRNA and SCR-shRNA vector groups for the first 30 days following vector delivery (Fig. 2A; one-tailed Mann-Whitney U test, p > 0.05 for post-vector days 1 through 30). However, upon subsequent escalation of levodopa dose to 6 mg/kg and 12 mg/kg, subjects receiving rAAV-CaV1.3-shRNA demonstrate protection against an increase in LID severity (Fig. 2A; one-tailed Mann-Whitney U test, p < 0.05 for post-vector days 35, 47 through 93). In keeping with our hypothesis, we report here that vector-mediated downregulation of striatal Cacna1d prevents the escalation of LID severity with successive increase of levodopa doses in rats of advanced age. While the cumulative data suggest that there is antidyskinetic benefit of CaV1.3-targeted gene therapy in both male and female subjects, the data are complex. As such, we present here several data analysis approaches to provide a comprehensive evaluation of the impact of sex and advancing age on CaV1.3 gene therapy outcomes in parkinsonian subjects with established dyskinesia.
Fig. 2.
Intrastriatal rAAV-CaV1.3-shRNA in male and female parkinsonian rats of advanced age results in protection against escalation of LID severity with increasing doses of levodopa. (A) In response to 3–4 mg/kg levodopa, there is no difference in LID severity between SCR (n = 13) and CAV (n = 15) groups (days 1–20 pre-vector, days 15–30 post-vector, p > 0.05). At increased doses of levodopa (6 mg/kg, 12 mg/kg), there is a significant escalation of LID severity in SCR rats but an inhibition of LID escalation in CAV rats (statistics presented in graph). (B) When total dyskinesia severity is examined separately in male and female subjects, total LID severity in female subjects is more variable, showing significant difference between CAV (n = 7) and SCR (n = 6) groups at a single timepoint (Day 47 post-vector, 6 mg/kg, p = 0.037). (D) Male CAV subjects showed significantly lower LID severity than male SCR subjects at three timepoints with 6 mg/kg and at all times poits with 12 mg/kg time point (stats presented in graph). For both male and female subjects, SCR (n = 7) v. CAV (n = 8) groups were compared at each rating day via Mann-Whitney U tests; lines represent group mean ± SEM. (C,E) A modified bootstrapping method was used to estimate population variation/consistency to specific doses of levodopa. Within each dose paradigm, both male and female CAV subjects show diminished LID severity compared to their SCR counterparts; however, female subjects show less robust protection at the 12 mg/kg dose (33.8% LID reduction) than their male counterparts (63.8% LID reduction). Each symbol represents a ‘total LID severity’ score for a single day of the LID rating at the dose indicated (e.g., Pre-Vector each symbol represents Day 4, 8, 12, 16, & 20). Treatment paradigms were compared at each rating day via one-tailed Mann-Whitney U tests; bars represent mean ± SEM.
3.2. Protection against LID escalation in parkinsonian rats of advanced age is not uniform among all subjects
When total dyskinesia severity data from male and female subjects are combined as in Fig. 2A, parkinsonian rats receiving SCR-shRNA show the expected elevation of LID severity over time with increasing doses of levodopa, whereas those receiving the CAV-shRNA show significantly less severe LID (Fig. 2A; groups compared at each rating day using one-tailed Mann-Whitney U tests, p < 0.05 for all 6 mg/kg and 12 mg/kg post-vector days, except day 41). These LID data were initially combined after no significant difference in LID severity between male and female subjects was observed in the majority of pre-vector and all post-vector 3-4 mg/kg days (Suppl Figs. 1 and 2; Kruskal-Wallis with Dunn’s post-hoc multiple comparison test, p > 0.05 for all pre- and post-vector days 35, 47 through 93).
The impact of sex on the ability of CaV1.3-channel modulation to affect therapeutic outcomes for PD holds strong clinical relevance (Venuto et al., 2021); therefore, male and female behavioral data were also analyzed as separate cohorts (Fig. 2B, C, D, E; Fig. 3D; Suppl Fig. 2). Despite a similar antidyskinetic trend between male and female rats, heightened variability within the females resulted in a lack of statistical significance at most timepoints during the dose escalation phase between SCR-shRNA and CAV-shRNA subjects (Fig. 2B; one-tailed Mann-Whitney U test, p = 0.037 for 6 mg/kg day 47, p > 0.05 for all other post-vector days). In contrast, male SCR-shRNA subjects had statistically significant increases in LID severity scores as compared to male CAV-shRNA subjects at all but one mid-to-high dose rating day (Fig. 2D; one-tailed Mann-Whitney U test, p < 0.05 for post-vector days 47 through 66, 74 through 93).
Fig. 3.
Intrastriatal rAAV-CaV1.3-shRNA results in protection against escalation of LID severity in a subpopulation of parkinsonian rats: ‘Responders’. (A) LID fold change was calculated as total LID severity on three days post-vector 23, 55, 82 (one representative day per levodopa dose) compared to day 20 pre-vector (LID Fold Change = (Total LID Post-Vector Day X / Total LID Pre-Vector Day 20) – 1). A fold change of 0 corresponds to no change in LID severity; < 0 = decreased total LID post-vector, > 0 = increased total LID post-vector. SCR (n = 13) v. CAV (n = 15) groups were compared via one-tailed Mann-Whitney U tests; p < 0.05 at day 55 and 82 post-vector. (B) Total dyskinesia scores for each rating day over the experimental time course for rats receiving rAAV-CaV1.3-shRNA (CAV) are separated into CAV-shRNA Responders (‘CAV Res’ (n = 7)) and Non-Responders (‘CAV Non-Res’ (n = 7)). Groups were compared at each rating day via one-tailed Mann-Whitney U tests; lines represent group mean ± SEM. (C) Total dyskinesia scores for individual CAV (i) and SCR (ii) rats at four representative time points: pre-vector (D20), and at low (D23), moderate (D55), and high (D82) doses of levodopa. (D) Breakdown of male (i) and female (ii) ‘Res’ and ‘Non-Res’ LID severity at the representative time points for individual CAV-shRNA rats. Lines represent total LID severity scores for each individual subjects. CAV ‘Res’ in each sex group are represented by colored lines.
As an additional approach for examining potential sex-related behavioral differences, we employed a modified bootstrapping method to combine the extensive temporal data (3–6 rating days) for each dose group (Fig. 2C, E). This approach involves the inclusion of re-sampled data (e.g., repeated testing for outcome confirmation) to estimate population variation or consistency. In our case, this method examines the response of males vs. females injected with either SCR-shRNA or CAV-shRNA vectors to a given dose of levodopa (Fig. 2C, E; Total LID Pre-Vector days 4, 8, 12, 16, 20 (4–20) low-dose levodopa (3–4 mg/kg), Female SCR-shRNA vs. CAV-shRNA p > 0.05, Male SCR-shRNA vs. CAV-shRNA p > 0.05; Post-Vector days 15, 23, 30 (15–30) low-dose levodopa (3–4 mg/kg) Female SCR-shRNA vs. CAV-shRNA p > 0.05, Male SCR-shRNA vs. CAV-shRNA p > 0.05; Post-Vector days 35, 41, 47, 55, 66, 73 (35–73) moderate dose levodopa (6 mg/kg) Female SCR-shRNA vs. CAV-shRNA p = 0.001, Male SCR-shRNA vs. CAV-shRNA p = 0.001; Post-Vector days 74, 82, 93 (74–93) high-dose levodopa (12 mg/kg), Female SCR-shRNA vs. CAV-shRNA p = 0.05, Male SCR-shRNA vs. CAV-shRNA p = 0.05). These data support the idea that while there appears to be an antidyskinetic benefit of CaV1.3 modulation in both male and female subjects, the antidyskinetic efficacy at higher doses of levodopa (12 mg/kg) in female subjects is less robust compared to their male counterparts—reduction of LID severity (SCR-shRNA vs. CAV-shRNA) in male subjects = 63.77%; reduction of LID severity in female subjects = 33.76% (Fig. 2C, E).
When we further examined the LID severity as a fold change at three post-vector timepoints (day 23, 3–4 mg/kg; day 55, 6 mg/kg, day 82, 12 mg/kg) compared to the last rating day pre-vector (day 20, 3–4 mg/kg), it became apparent that the CAV-shRNA subjects stratified into two groups: those with a fold change <0, indicating protection from LID escalation, and those with a fold change >1, indicating a lack of protection (Fig. 3; one-tailed Mann-Whitney U tests, p < 0.05 for day 55 and 82.). We categorized those that showed protection against LID escalation as ‘Responders’ and those that did not display significant protection as ‘Non-Responders’ (Fig. 3; one-tailed Mann-Whitney U tests, p < 0.05 for post-vector days 35–93; Fig. 4). This contrasts the highly uniform antidyskinetic effect we saw in our previously reported cohort of young male rats (Steece-Collier et al., 2019) (Fig. 7, reproduced with permission). In certain individual subjects in both male and female cohorts, vector intervention led to a near complete reversal of dyskinesia severity (Fig. 3C, D; Suppl Fig. 2A), while other CAV-shRNA rats demonstrate LID escalation on par with the SCR-shRNA group. Specifically, about half of the parkinsonian rats of advanced age receiving intrastriatal rAAV-CaV1.3-shRNA demonstrated significant protection from LID with dose escalation, while the other half showed LID escalation (Fig. 3B, C).
Fig. 4.
Soma-localized Cacna1d is downregulated in the vector-injected striatum of CAV-shRNA subjects as compared to SCR-shRNA. (A) In CAV-shRNA-transduced cells, Cacna1d transcript fold change was significantly less than in in SCR-shRNA-transduced cells (p = 0.006, unpaired, two-tailed t-test; lines represent group mean ± SEM). (n = 11 SCR, n = 13 CAV)) (B) In neighboring non-transduced cells (HuC/D+, GFP−), the ipsilateral-to-contralateral Cacna1d mRNA fold change was significantly elevated in SCR compared to CAV striatum (p = 0.029; unpaired, two-tailed t-test; lines represent group mean ± SEM. (C) Representative confocal images showing vector-transduced cells (GFP+, green) and Cacna1d transcripts (cyan puncta) in SCR (left) and CAV (right) striata. Enlarged inserts show Imaris™ construction of Cacna1d mRNA (white puncta) in GFP+ cells—GFP fluorescence “off” in enlargements—demonstrating reduced transcript in CAV compared to SCR cells. (D) Total striatal (e.g., somatic and dendritic; transduced and non-transduced cells) Cacna1d defined using DAB-conjugated ISH, calculated as fold change between ipsilateral and contralateral DAB+ area, shows no difference between CAV (n = 13) and SCR (n = 11) (p > 0.05; two-tailed t-test; lines represent group mean ± SEM). (E) XY plot shows a significant correlation between Cacna1d fold change in the full striatum and LID fold change for CAV-shRNA subjects (Spearman’s correlation tests; statistics in graph). (F) Z-stacks of Cacna1d transcripts (brown) in the dorsolateral striatum in ipsi− (left) and contralateral (right) hemispheres; rectangles in cartoon coronal section show representative imaging area. Quantification of total area of DAB+ Cacna1d puncta was done using ImageJ software “Threshold” function; enlarged insert shows the red threshold constrained to defined DAB+ puncta.
Fig. 7.
Striatal rAAV-CaV1.3-shRNA in young parkinsonian rats provides uniform reversal of severe LID, contrasting variable antidyskinetic benefit in rats of advanced age. (A) Modification of data from our previously reported cohort of young adult male rats (Steece-Collier et al., 2019), demonstrating reduction of LID severity in individual CAV-shRNA subjects (i) over time compared to maintenance of high-level LID severity in SCR-shRNA (ii) rats at four representative experimental time points (pre-vector day 16 and post-vector days 20, 31, 46) in response to chronic daily levodopa (12 mg/kg). Lines represent total LID severity scores for individual subjects. (B) Fold change calculated as total LID severity on days post-vector 20, 31 and 40 compared to day 16 pre-vector. At days 31 and 40 post-vector, CAV-shRNA subjects had significantly lower LID severity, represented as fold change, than SCR-shRNA subjects (one-tailed Mann-Whitney U test, p = 0.0001 and p < 0.0001, respectively). Bars represent group mean ± SEM. (C) There are significantly fewer Cacna1d transcripts in the striatum of CAV-shRNA animals (n = 10) (compared to SCR-shRNA (n = 10), measured as fold change in total DAB+ Cacna1d area (unpaired, two-tailed t-test, p = 0.0002). Bars represent group mean ± SEM.
When considering CAV-shRNA subjects, we observed Responders and Non-Responders in both sex cohorts. In the male subjects, only 2 of 7 males show complete non-responsiveness (Fig. 3Di), with the other 5 showing some degree of therapeutic response at one or both elevated levodopa doses. Of note, there was a single male rat that showed antidyskinetic benefit early on (up to day 55 post-vector) but demonstrated a sharp increase in LID severity upon escalation to the final high dose (12 mg/kg) and was thus categorized as a Non-Responder. Similarly, in the female cohort, 3 of 7 rats show complete non-responsiveness (Fig. 3Dii), with 1 female showing a trend toward LID reversal from day 20 to 23, but then experiencing LID ‘breakthrough’ at the higher levodopa doses (Fig. 3Dii), leading to 4 of 7 females being categorized as Non-Responders. This finding is consistent with a recent clinical trial which suggests that female individuals with PD are more refractory than males to pharmacological CaV1.3 antagonism for neuroprotection of nigral DA neurons with isradipine (Venuto et al., 2021).
3.3. Reduction in striatal Cacna1d expression after rAAV-CaV1.3-shRNA administration correlates with antidyskinetic benefit
While CaV1.3 protein and mRNA (Cacna1d) transcripts localize mainly to neuronal cell bodies, they can also be found in proximal dendrites and synaptic regions including within dendritic spines (Zhang et al., 2013; Olson et al., 2005; Zhang et al., 2006). With this in mind, we present quantification of both cell-specific, soma-localized Cacna1d transcripts as well as an analysis of Cacna1d throughout the parenchyma of the striatum. To examine the impact of rAAV-CaV1.3-shRNA on cell body Cacna1d transcript levels, we employed fluorescent RNAScope® ISH in conjunction with IHC methods for GFP and HuC/D to label vector-transduced cells and non-transduced neurons, respectively (Fig. 4C). Using the contralateral hemisphere as an intra-individual control for each animal, the fold change in Cacna1d transcripts was calculated in vector-transduced (GFP+) and non-transduced (GFP-HuC/D+) cells.
In vector-transduced cells, the Cacna1d fold change (Cacan1d transcripts in GFP+ cells of the ipsilateral striatum / Cacna1d transcripts in GFP−, HuC/D+ cells of the contralateral striatum) showed significantly greater downregulation of Cacna1d in CAV-shRNA subjects as compared to SCR-shRNA subjects (Fig. 4A; unpaired, two-tailed t-test CAV-shRNA vs. SCR-shRNA, p = 0.006). Interestingly, in non-transduced cells, Cacna1d fold change analysis (Cacan1d transcripts in ipsilateral GFP−, HuC/D+ cells / Cacna1d transcripts in contralateral GFP−, HuC/D+ cells) indicated higher levels of Cacan1d transcripts in cells from the SCR-shRNA-injected as compared to the CAV-shRNA-injected striatum (Fig. 4B; unpaired, two-tailed t-test CAV-shRNA vs. SCR-shRNA, p = 0.029). This result suggests a ‘bystander effect,’ where neighboring, non-transduced cells in the CAV-shRNA-injected striatum also demonstrate a downregulation of Cacna1d. These data in both vector-transduced and non-transduced cell bodies are in keeping with the functional outcome of reduced LID severity in CAV-shRNA compared to SCR-shRNA subjects (Fig. 2, Fig. 3).
To determine the effect of rAAV-CaV1.3-shRNA on the entire striatum (e.g., somatic and dendritic; transduced and non-transduced cells), we employed a DAB-based ISH method to visualize Cacna1d transcripts. In ImageJ, brightfield z-stacks from the lateral crescent of the dorsolateral striatum of each animal were used to quantify Cacna1d (DAB-positive area) in both striatal hemispheres (Fig. 4F). This analysis revealed that in all SCR-shRNA receiving rats, Cacna1d was upregulated in the vector-injected, ipsilateral striatal parenchyma relative to the vector-naïve, contralateral striatum. In CAV-shRNA rats, approximately half maintained an increase (fold change >0), while half presented with decreased Cacna1d transcript levels in the ipsilateral hemisphere (fold change <0). Cumulatively, there was no statistical significance between CAV-shRNA and SCR-shRNA subjects (Fig. 4E; unpaired, two-tailed t-test, p > 0.05).
These results suggest that the downregulation of Cacna1d in rAAV-CaV1.3-shRNA-transduced cells is maintained into advanced age, and that the expression of Cacna1d in neighboring striatal cells are impacted by the vector-injected environment. Despite complexities in striatal Cacna1d expression—driven by the interplay of DA-depletion, levodopa treatment, and vector injections—the current findings provide compelling evidence that even modest reductions in striatal Cacna1d transcript levels can be associated with significant antidyskinetic functional benefit.
3.4. Cacna1d is downregulated in striatal parenchyma of therapeutic ‘Responder’ subgroup
As introduced above, parkinsonian rats of advanced age receiving CaV1.3-shRNA stratified into two distinct groups with respect to behavioral outcomes: Responders and Non-Responders (Fig. 3B, C, D, Fig. 5A; Mann-Whitney U test, p = 0.006). Both cohorts include male and female rats with equivalent numbers of DA neurons in both the intact contralateral and lesioned ipsilateral substantia nigra, confirming no discrepancies in lesion status (Fig. 5B; unpaired, two-tailed t-tests, p > 0.05). Additionally, both Responders and Non-Responders show fully transduced ipsilateral striatal hemispheres (Fig. 5C; unpaired, two-tailed t-tests, p > 0.05).
Fig. 5.
Despite equivalent SN DA neuron depletion and striatal vector transduction, CAV Responders demonstrate decreased parenchymal Cacna1d when compared to Non-Responders. (A) CAV Responders (Res (n = 7)) show a significantly more negative LID fold change than Non-Responders (NonR (n = 7)) (Mann-Whitney U test, p = 0.006). The LID fold change shown was defined at the moderate levodopa dose, 6 mg/kg (pre-vector D20 vs. post-vector D55). (B) The number of estimated DA (TH+) neurons in the rat SN did not differ in either the intact contralateral or lesioned ipsilateral hemisphere between Res and NonR (unpaired, two-tailed t-tests, p > 0.05). All animals were unilaterally lesioned, with >95% loss of TH+ neurons on the ipsilateral side. (C) Percent striatal transduction did not differ between Res and NonR (unpaired, two-tailed t-test, p > 0.05). (D) Cacna1d fold change in GFP+ vector-transduced cells (normalized to contralateral HuC/D+ cells) did not differ between Responders and Non-Responders (unpaired, two-tailed t-test, p > 0.05). (E) Cacna1d fold change in HuC/D+ non-transduced cells did not differ between Responders and Non-Responders (unpaired, two-tailed t-test, p > 0.05). (F) CAV Responders compared to Non-Responders showed significantly greater downregulation of total striatal Cacna1d as measured by DAB+ transcript area (unpaired, two-tailed t-tests, p = 0.049). All bars represent group mean; error bars show ± SEM.
Despite different behavioral phenotypes, there appears to be equal reduction in vector-transduced, soma-localized Cacna1d transcript levels between Non-Responders and Responders (Fig. 5D; unpaired, two-tailed t-test, p > 0.05). Similarly, there was no difference in Cacna1d expression in non-transduced neuronal cells between groups (Fig. 5E; unpaired, two-tailed t-test, p > 0.05). However, in our analysis of parenchymal Cacna1d expression—including neuronal and non-neuronal cell bodies and synaptic regions in the full striatum—Cacna1d downregulation did correlate with antidyskinetic benefit, with the Responder subgroup having significantly less striatal Cacna1d than the Non-Responder, as represented by fold change in DAB-positive area (Fig. 5F; unpaired, two-tailed t-test, p = 0.049). These data suggest that the non-cell autonomous effects originating from neighboring structures and glial cells within the striatal microenvironment may contribute to the efficacy of CaV1.3-targeted gene therapy in the aged parkinsonian brain.
3.5. Striatal vector transduction volume and morphological changes observed in aged rat striatum do not correlate with degree of antidyskinetic benefit
As previously reported in young male rats (Steece-Collier et al., 2019), the CAV-shRNA vector achieves marginally greater and more consistent volume of striatal transduction than the SCR-shRNA vector in parkinsonian rats of advanced age (Fig. 6A; Mann-Whitney U test, p < 0.0001). There is no difference in the extent of striatal vector transduction between male and female rats (data not shown). Further, there is no correlation between the volume of striatal vector transduction—indicated by percent of GFP immunoreactivity—and LID fold change in either SCR-shRNA or CAV-shRNA animals (Fig. 6B; two-tailed Spearman’s correlation test; SCR-shRNA: p = 0.7805, r = −0.0902; CAV-shRNA: p = 0.3243, r = −0.2845) suggesting that variations in vector transduction do not account for differential behavioral outcomes.
Fig. 6.
The area of striatal vector transduction and size of the injected striatum differs between CAV and SCR animals. (A) The percent of striatal transduction volume was significantly less in SCR (n = 13) subjects than in CAV (n = 15) (unpaired, two-tailed t-test, p < 0.0001). (B) XY plot showing no correlation between the percent vector transduction and fold change in LID severity in either SCR (two-tailed Spearman’s correlation test; p = 0.7805, r = −0.0902) or CAV animals (two-tailed Spearman’s correlation test; p = 0.3243, r = −0.2845). (C) Quantitation of the size of the ipsilateral striatum (normalized to contralateral) reveals significantly greater decrease in CAV compared to SCR injected striata (unpaired, two-tailed t-test, p = 0.005). (D) XY plot show no correlation between the percent change in ipsilateral striatal size relative to fold change in LID severity in either SCR (two-tailed Spearman’s correlation test; p = 0.7683, r = —0.0949) or CAV p = 0.7683, r = −0.0949) or CAV animals (two-tailed Spearman’s correlation test; p = 0.6285, r = −0.1419). (E) Quantitation of the percent increase in ipsilateral lateral ventricle size (normalized to contralateral) in CAV and SCR injected animals shows no significant difference between groups (unpaired, two-tailed t-test, p > 0.05). All lines represent group mean ± SEM. (F) XY plot shows no correlation between the percent increase in ipsilateral lateral ventricle size and fold change in LID severity in either SCR (two-tailed Spearman’s correlation test; p = 0.2935, r = −0.3486) or CAV animals (two-tailed Spearman’s correlation test; p = 0.6581, r = 0.1299). (G) Digital tracings of representative coronal rat brain sections at the level of precommissural striatum showing morphological variations in the vector-injected striatum.
We observed morphological changes in the brains of rats of advanced age that were not present in young male rats (unpublished observation; (Steece-Collier et al., 2019)). Specifically, there was an increase in the size of the lateral ventricle and a decrease in the size of the striatum in the ipsilateral, vector-injected striatum (Fig. 6C, D, E, F, G). Quantification of the size of the ipsilateral striatum (normalized to the contralateral) demonstrated a small but significant difference between CAV-shRNA and SCR-shRNA subjects (Fig. 5C; unpaired, two-tailed t-test, p = 0.0050; CAV-shRNA group average 10.83% (± 3.035 SEM) decrease, SCR-shRNA group average 3.142% (± 3.364 SEM) increase). Despite this difference between groups, there was no correlation between change in striatal size and LID fold change in either CAV-shRNA or SCR-shRNA animals (Fig. 4D; two-tailed Spearman’s correlation test; SCR-shRNA: p = 0.7693, r = −0.0949; CAV-shRNA: p = 0.6285, r = −0.1419).
An additional morphological analysis of lateral ventricle area revealed that, in aged rats, both SCR-shRNA and CAV-shRNA groups displayed a notable increase in ventricular size ipsilateral to the injection (Fig. 4E; unpaired, two-tailed t-test, p > 0.05; CAV-shRNA group average 308.1% (± 58.07 SEM) increase, SCR-shRNA group average 277.0% (± 108.4 SEM) increase). There was no correlation between ventricular size increase and LID fold change in either CAV-shRNA or SCR-shRNA animals (Fig. 4F; two-tailed Spearman’s correlation test; SCR-shRNA: p = 0.2935, r = −0.3486; CAV-shRNA: p = 0.6581, r = 0.1299). These data suggests that there are no apparent LID behavior-related consequences associated with changes in striatal or ventricular area in aged parkinsonian rats.
3.6. Young male parkinsonian rats demonstrate a more uniform response to CaV1.3 gene therapy than rats of advanced age
In our previous experiments (Steece-Collier et al., 2019) we used 3-month-old male rats for the first proof-of-principle studies aimed at examining the capacity and limitations of CaV1.3-shRNA gene therapy for the amelioration of moderate-to-severe LID established with high dose levodopa (12 mg/kg). We provide here re-analysis of a portion of this data (with permission MDS, License No. 5425451459738) to compare it with the current study in 15-month-old male and female rats with mild-to-moderate LID.
In young adult male rats, rAAV-CaV1.3-shRNA vector was administered at the same titer and using identical surgical parameters as in the current study. By day 31 post-vector, there was a decrease in LID severity in all CAV-shRNA subjects as compared to the moderate-to-severe LID established pre-vector (Fig. 7Ai). This continued to the end of the study (day 40 post-vector), demonstrating consistent therapeutic success in young male subjects for vector-mediated reversal of LID. The SCR-shRNA cohort of young parkinsonian subjects maintained moderate-to-severe LID or experienced LID escalation with continued 12 mg/kg levodopa (Fig. 7Aii). Examining measurements of LID fold change in this original study—days 20, 31, and 40 post-vector compared to day 16 pre-vector—all CAV-shRNA subjects showed a decrease in LID (fold change <0) at 31- and 40-days post-vector, differing significantly from SCR-shRNA subjects (Fig. 7B; one-tailed Mann-Whitney U test, day 31: p = 0.0001, day 40: p < 0.0001). As in aged animals, this antidyskinetic effect was associated with a decrease in striatal Cacna1d expression in CAV-shRNA vs SCR-shRNA groups (Fig. 7C; unpaired, two-tailed t-test, p = 0.0002).
4. Discussion
Gene therapy is currently one of the most innovative areas of biotechnology, with significant potential to impact a wide variety of human diseases including PD (Bulaklak and Gersbach, 2020; Szarowicz et al., 2022). However, as recently reviewed (Polinski et al., 2016), while delivery of therapeutic agents using viral vectors has shown promise in preclinical models of PD, this has not consistently led to success in clinical trials. The suboptimal translation of preclinical, animal-model-based findings into effective clinical therapeutics is partially due to the neglect of advancing age—the primary risk factor for PD—as a covariate in animal studies (Collier et al., 2015; Polinski et al., 2016; Klaestrup et al., 2022). Additionally, despite the National Institutes of Health policy on the inclusion of women in NIH-funded trials (https://orwh.od.nih.gov/sex-gender/nih-policy-sex-biological-variable), direct comparison between male and female sexes is infrequent in preclinical research and sex bias remains in clinical trials (reviewed in (Burra et al., 2022; Prakash et al., 2018)). The incidence of PD is estimated at a 1.5:1 male-to-female ratio, yet the specific therapeutic needs of females with PD have been historically disregarded.
The current study indicates that gene therapy via rAAV-CaV1.3-shRNA in aged parkinsonian rats is effective in preventing the escalation of LID severity with successive increases in levodopa dose in approximately 50% of CAV-shRNA vector subjects. These data also confirm that the antidyskinetic benefit of CAV-shRNA in aged rats, like that previously reported in young subjects, is linked to downregulation of striatal Canca1d expression. Specifically, striatal Canca1d expression in parkinsonian rats of advanced age —evaluated in this study both at the soma level and throughout the striatal parenchyma—was decreased significantly in CAV-shRNA subjects, with decreased parenchymal levels correlating with antidyskinetic efficacy in CAV-shRNA ‘Responders’. This is similar to what we previously reported for parenchymal Canca1d in young parkinsonian rats (Steece-Collier et al., 2019), albeit with much larger variability (standard deviation (SD)) in aged CAV-shRNA and SCR-shRNA groups (CAV-shRNA SD 2.789; SCR-shRNA SD 2.240) compared to the young (CAV-shRNA SD 0.7535; SCR-shRNA SD 1.399). This suggests that the aged brain is potentially less permissive to intervention on an individual-to-individual basis. Finally—consistent with sex differences reported in clinical trials employing the CaV1.2/1.3 antagonist isradipine (Venuto et al., 2021), where a mild degree of neuroprotection was noted in male but not female individuals with PD—our data also demonstrate that CaV1.3-mediated protection against LID is more variable in female parkinsonian rats compared to their male counterparts. Cumulatively, these data and existing literature underscore the importance of age and sex as preclinical variables when considering CaV1.3-targeted therapeutics in PD.
There are multiple barriers to the inclusion of aged animals in preclinical studies. Aged rats, mice, and non-human primates incur additional cost due to extended housing requirements, and including both female and male sexes in an experimental design will double the cost of a study. Additionally, aged subjects are often difficult to acquire, and studies with older animals are plagued with higher rates of morbidity and mortality. Despite the challenges and expense of aged, sex-matched subjects, inclusion of these covariates is critical in creating animal models of PD that better represents the current clinical demographic, and in increasing the translational success of preclinical findings to subsequent clinical studies (Klaestrup et al., 2022).
As we expanded our exploration of striatal CaV1.3 gene therapy to include covariates of advanced age and sex, the data suggest a more complex therapeutic outcome not uncommon to PD clinical trials (e.g. (Manfredsson et al., 2020; Surmeier et al., 2022; Freed et al., 2001)). The current second-phase preclinical study provides novel insight that the advancing age and biological sex of a subject impose challenges to uniform therapeutic efficacy in the rat model employed, with subjects demonstrating variable behavioral responses to mRNA-level CaV1.3 channel silencing. In the following paragraphs we discuss the principal findings from this study, and ideas toward elucidating possible factors underlying the complexity of the current data.
4.1. Viral vectors: impact of sex
A systematic evaluation of the influence of sex on transduction by recombinant adeno-associated viral vector (rAAV) reveals contrasting reports. There are reports of higher levels of transgene expression in male mice compared with female mice (Davidoff et al., 2003) (Scott et al., 2002) as well as opposing findings (Maguire et al., 2013). For example, when Lonning and colleagues (Scott et al., 2002) intravenously administered rAAV vectors encapsidated by AAV1, AAV2, and AAV5 to multiple mouse strains of both male and female sexes they found that male subjects, regardless of strain, had significantly higher transgene expression compared to their female counterparts. These findings contrast those made by Maguire and colleagues, who reported higher transgene expression in the brain of female compared with male mice (nude and C57BL/6 strains) following intravenous injection of single-stranded AAV9 encoding the reporter genes firefly luciferase and GFP (Maguire et al., 2013). Taken together, such studies propose a complex interplay between sex-specific steroid hormones and transduction events related to AAV-mediated transgene expression, the details of which are not well understood.
Our data suggest that in parkinsonian rats of advanced age, there is no difference in viral vector transduction volume between sexes as indicated by GFP immunoreactivity in the striatum (data not shown). It is possible that individual female variations in response to our gene therapy may be dependent on the biology of striatal calcium homeostasis within a sex-specific environment, rather than differential vector transduction and silencing. The age of the female rats used in this study suggested they should largely be acyclic, and as such, we did not examine estrous cycle or hormonal status in this particular population of rats. However, in a separate, subsequent study involving female rats of the same advanced age used in the current study, we observed that approximately 70% had a positive proestrus vaginal smear from a 3-day consecutive sampling suggesting they were still undergoing estrus, although the degree of cycle regularity was unclear. These data suggest that the female rats used in the current study likely had not reached a complete anestrous stage ((Cruz et al., 2017), unpublished observation). As such, it is possible that varying cycle status of the female rats in this study may account for the variable outcomes of LID protection. A direct connection between cycling status and LID protection via regulation of CaV1.3 function merits future investigation.
4.2. Viral vectors: impact of age
Advancing age results in numerous changes in brain plasticity and cellular stress responses, as well as alterations in the molecular processes utilized for viral vector transduction (for review (Polinski et al., 2018)). Indeed, we have previously reported that age-related transgene expression deficiencies exist and are dependent on rAAV serotypes and the brain region of vector injection (Polinski et al., 2016). While there is reduced transduction of rAAV 2/5 and expression of GFP associated with rAAV 2/2 and 2/5 serotypes in 20-month-old compared to 3-month-old rats, rAAV serotype 2/9 (used in the current study), showed equivalent cellular transduction yet reduced GFP protein levels in aged rats as compared to young (Polinski et al., 2016). It is possible that this reduction in GFP expression results from the breakdown of the protein life cycle, an age-associated process which includes increased transcription errors, reduced speed of translation, and defunct protein control (Anisimova et al., 2018; Schimanski and Barnes, 2010; Woodward and Shirokikh, 2021). Dysfunction in these basic cellular processes are well-documented consequences of aging and are drivers of age-related neurodegenerative disorders such as PD and AD (reviewed in (Taylor and Dillin, 2011; Eldeeb et al., 2022; Klaips et al., 2018; Kaushik and Cuervo, 2015; Korovila et al., 2017)). Though the current study does not directly quantify GFP protein expression, anatomical observations indicated that the morphology of the aged rat striatum was more negatively affected by the combination of vector injection, vector transduction, lesioning, and/or LID status than the young brain.
An additional challenge presented by the aged brain is lipofuscin, a lipid-containing, electron-dense, auto-fluorescent material that accumulates progressively over time in lysosomes of postmitotic cells, such as neurons (Terman and Brunk, 1998; Oenzil et al., 1994). Lipofuscin accumulation has an inverse relationship with longevity, and is associated with increased sensitivity to oxidative stress, lysosomal instability, and interference with the recycling of cellular components (Brunk and Terman, 2002). These undegradable polymeric substances pose a challenge for the aging cellular machinery to work effectively in the context of vector transduction and gene silencing. For example, in the aged SCR-shRNA animals, there was an overall increase in Cacna1d transcripts in the ipsilateral, vector-injected striatum: on average, ~2-fold increase (Fig. 4D). The CAV-shRNA Responders demonstrated a relatively consistent stabilization or reduction in ipsilateral striatal Cacna1d levels, whereas the CAV-shRNA Non-Responder animals showed a wide range of Cacna1d quantities, up to 6-fold higher over the contralateral striatum (Fig. 5F). These variable readouts of Cacna1d in the aged striatum suggest that the age-associated breakdown in homeostatic cellular processes of protein degradation and the accumulation of lipofuscin may render some subjects unable to effectively regulate Cacna1d transcription in the presence of viral vector.
4.3. Age-related and sex-specific alterations in CaV1.3 channels
Ca2+ elicits wide-ranging effects as an intracellular messenger and metabolic regulator, and disruptions in neuronal Ca2+ homeostasis are associated with various age-related neuronal pathologies including PD, AD, and dementia (e.g. (Ge et al., 2022; Ortner and Striessnig, 2016; Chan et al., 2009)). There is a widespread assumption that increased intracellular Ca2+ is a general feature of neuronal aging, mediated by enhanced Ca2+ influx and/or reduced Ca2+ buffering (reviewed in (Griffith et al., 2000; Marambaud et al., 2009; Bezprozvanny, 2009)). There is an abundance of evidence indicating that neuronal Ca2+ channels contribute to cell loss and the progression of PD. Specifically, CaV1.3 channels in the substantia nigra are major contributors to the pacemaking activity of DA neurons, where high levels of channel activity result in abundant Ca2+ influx in cells with limited Ca2+ buffering capacity. Maintaining physiological ion gradients associated with autonomous excitability of tonically active DA neurons with large, complex axonal arbors results in a significant energetic burden on these neurons, which has been implicated in their vulnerability to neurodegeneration in PD (Surmeier et al., 2017a; Surmeier et al., 2017b; Kang et al., 2013; Bolam and Pissadaki, 2012). With advanced age, these neurons increase their reliance on L-type calcium channel activity to regulate pacemaker activity, likely increasing their vulnerability to neuronal loss ((Chan et al., 2007), reviewed in (Kumar et al., 2009)).
There is little information on changes in CaV1.3 in the aged brain, and to the best of our knowledge, no available data on changes in intraspinous CaV1.3 channels in the striatal medium spiny projection neurons directly relevant to LID. While we did not quantify Cacna1d transcripts in naive young versus aged rats, there appears to be an appreciable decrease in striatal Cacna1d transcript in our naive rats of advanced age (unpublished observation). In the hippocampus of aged rats (30–32 month-old), Núñez-Santana et al. report an overall reduction in CaV1.2 and CaV1.3 compared to young rats (3–4 month old.), though this total protein expression did not correlate to surface/total ratio of each Ca2+ channel—in the CA3 hippocampal subregion, there was increased CaV1.3 on the cell surface in the older rat cohort (Nunez-Santana et al., 2014). The significance of reduced baseline striatal Cacna1d transcripts in aging subjects was not apparent from the current studies and is a matter of future investigation.
Similarly, sex-related differences in CaV1.3-specific activity and expression have not been extensively documented, with most studies focused on L-type Ca2+ channels in general. In the hippocampus, the expression of L-type Ca2+ channels is thought to be regulated by the sex steroid estrogen, with increased expression associated with the decline of the hormone during aging (reviewed in (Foster, 2005)). In the striatum, classic experiments of the 1980’s first demonstrated spontaneous action potential firing in vivo increased in ovariectomized female rats exogenously exposed to estradiol compared to vehicle-exposed females and males (Arnauld et al., 1981). Indeed, electrophysiologic characteristics of SPNs change with the estrous cycle (reviewed in (Krentzel and Meitzen, 2018)). It is now appreciated that membrane estrogen receptors are expressed on SPN axon terminals, somas and dendritic spines (Almey et al., 2014; Almey et al., 2012; Almey et al., 2015; Almey et al., 2022). Further, estradiol acting through ERα, ERβ, and metabotropic glutamate receptors rapidly decreases L-type Ca2+ currents (Mermelstein et al., 1996; Grove-Strawser et al., 2010). Due to the complex nature of the rat estrous cycle and transition into an acyclic phase with age (Cruz et al., 2017) and the impact of sex hormones on L-type Ca2+ currents, future studies are warranted to identify estrogen-derived mechanisms driving variability in gene therapy outcomes targeted to CaV1.3 channels.
4.4. The global striatal environment
Theoretically, vector-mediated silencing of Cacna1d transcription should occur in only transduced striatal cells, with little direct impact on neighboring non-transduced cells. Our data demonstrate that this is not necessarily the case. In the current study, Cacna1d was lower in the transduced (GFP+) cells of CAV-shRNA-injected animals, but expression was also downregulated in non-transduced neuronal cells (GFP−, HuC/D+) in the CAV-shRNA cohort. Specifically, in SCR-shRNA-injected striata, there was an increase in Cacna1d in the ipsilateral hemisphere; in CAV-shRNA-injected striata, levels of Cacna1d between injected, ipsilateral and non-injected, contralateral hemispheres were relatively equal. The resulting Cacna1d levels between SCR-shRNA and CAV-shRNA, ipsi− and contralateral hemispheres is best described as a “relative downregulation,” with CAV-shRNA subjects demonstrating protection from the increase in Cacna1d measured in SCR-shRNA subjects.
The quantification of total striatal Cacna1d—a method which accounts for all striatal transcripts, regardless of cellular location (e.g., soma, dendrite)—showed strong significance between Responder and Non-Responder CAV-shRNA animals. Considering the ability of the CAV-shRNA vector to influence Cacna1d expression in both transduced and neighboring non-transduced cells, these data underscore the importance of future studies to understand mechanistic events in the global striatal environment including the impact on SPNs that express D1 DA receptors (“direct pathway”) as well as those that express D2 DA receptors (“indirect pathway”). D1 and D2 receptors have a variety of targets in SPNs including L-type Ca2+ channels (i.e.; CaV 1.2 and 1.3). As reviewed in (Day et al., 2006), these DA receptors regulate Ca2+ channel activity in opposing ways with D2 receptors impacting only CaV1.3 channels, suppressing the opening of these channels. In the presence of DA depletion and reduced inhibitory action of D2 receptors, CaV1.3 channels are “disinhibited”, promoting increases in intraspinous Ca2+ through an increase in Ca2+ transients mediated by readily opened CaV1.3 channels resulting in spine retraction (Day et al., 2006). There remains controversy as to whether striatal DA depletion results in spine loss specifically in SPNs of D2 receptor containing indirect pathway or whether it occurs in SPNs of both indirect and direct pathway (for review (Bastide and Bezard, 2015; Caulfield et al., 2023)). Silencing CaV1.3 channel activity, either pharmacologically (Schuster et al., 2009; Soderstrom et al., 2010) or with shRNA gene therapy (Steece-Collier et al., 2019) results in amelioration of LID, seemingly related to protection against D2 mediated mechanisms of spine loss and associated aberrant synaptic plasticity. However, there is strong evidence to support a significant role of the D1 receptor signaling in dyskinesia (reviewed in (Guigoni et al., 2005)), despite controversy on whether changes in the density of D1 receptor-immunoreactive spines occurs in the presence of DA depletion. Accordingly, it is possible that manipulation of CaV1.3 in our model dampens D1 receptor hypersensitivity in direct pathway SPNs via spine-autonomous mechanisms, with a combination of D1 and D2 SPN mechanisms playing a role in protection against LID escalation. The specific role of direct vs indirect pathway SPNs in the antidyskinetic mechanism of CAV-shRNA gene therapy warrants future investigation.
Considering the global striatal environment also includes elucidating the role of neurons and surrounding glia in mediating the gene therapy response. While our viral vector is not driven by a cell-type-specific promotor, the AAV2/9 serotype and optimized capsid design efficiently transfects neurons with greater volumetric spread than other serotypes in the CNS (Kanaan et al., 2017; Watakabe et al., 2015; Haery et al., 2019; Lukashchuk et al., 2016; Jackson et al., 2015), though it is likely that some transduced cells are glia (Kanaan et al., 2017). Astrocytes are of particular interest when considering the striatal milieu, as they can express CaV1.2 and/or CaV1.3 ((Latour et al., 2003; Barres et al., 1989); unpublished observation) and are generally amenable to transduction by AAV vectors under astrocyte-specific promoters (e.g., glial fibrillary acidic protein (GFAP) or cytomegalovirus (CMV) (reviewed in (O’Carroll et al., 2020)). Calcium signaling in astrocytes plays an important role in facilitating the bidirectional communication between neurons and astrocytes at the synapse (reviewed in (Broadhead and Miles, 2020; Allen and Eroglu, 2017)). Astrocyte Ca2+ transients are diverse and vary under different conditions, potentially mediating microdomain events that influence synapse modulation, brain circuitry, and behavior (reviewed in (McNeill et al., 2021; Ahmadpour et al., 2021)). There is increasing experimental evidence suggesting that astrocytic dysfunction is a key element in LID (Del-Bel et al., 2016). Additionally, astrocytes play a significant role in the maintenance of the blood brain barrier and in directing neuroinflammation, both of which are thought to be involved with mechanisms of LID (Del-Bel et al., 2016). Of relevance, the inhibition of voltage-gated Ca2+ channels in astrocytes during demyelination in a mouse model of multiple sclerosis has been shown to reduce brain inflammation and promotes myelin regeneration in mice (Zamora et al., 2020).
As summarized here, astrocytes play an important role in Ca2+ homeostasis and neuronal plasticity in the brain, and their expression of L-type voltage-gated Ca2+ channels highlight them as a non-neuronal target of interest in CaV1.3-targeted gene therapy. Considering the interplay between astrocytes and neurons in the context of Ca2+ homeostasis, as well as the role of Ca2+ dysfunction in aging and neurodegeneration, we posit that the entire striatal environment may impact the efficacy of gene therapy interventions for LID, and individual variations in the aging brain may underlie treatment success or failure.
5. Conclusion and future directions
Gene therapy-mediated regulation of CaV1.3 in the parkinsonian striatum is a powerful antidyskinetic tool, capable of providing protection against LID escalation in the presence of chronic, high-dose levodopa. The superior CaV1.3 target engagement provided by a spatially directed gene therapy approach remains an essential characteristic of successful intervention. In STEADY-PD III, a clinical trial employing isradipine in a PD neuroprotection paradigm, it is speculated that a lack of adequate CaV1.3 target engagement with clinically tolerated doses of isradipine may be responsible for the negative trial outcome (Grp, 2020; Maiti and Perlmutter, 2020). Additionally, pharmacological dosing of isradipine (Schuster et al., 2009) and nimodipine (Soderstrom et al., 2010) produced only modest and/or transient protection against LID in parkinsonian rats. As CaV1.3 channel modulation is considered for clinical applications, including neuroprotection and levodopa side-effect management, we posit that a gene therapy approach provides superior target engagement and circumvention of potential off-target side effects associated with dihydropyridines. Gene therapy-mediated regulation of striatal CaV1.3 provides some of the most profound antidyskinetic benefit reported to date (Steece-Collier et al., 2019), though additional studies are needed to understand why some aged subjects respond positively to this approach and others do not. Inclusion of advanced age and sex as covariates in the current preclinical study resulted in greater variability in therapeutic outcomes, a challenge that is a hallmark of clinical PD pharmacotherapies (e.g. (Hauser et al., 2009; Fischer et al., 2020; Sortwell et al., 2022)) and experimental therapeutics (Collier et al., 2019). As the next step in understanding the capacity and limitations of CaV1.3 gene therapy for the abatement of LID, translational studies incorporating non-human primates are warranted.
Supplementary Material
Acknowledgements
We would like to acknowledge the surgical assistance of Dr. Marcus Davidsson and Brian Daley in these studies.
Funding
This study was supported in part by the National Institute of Neurological Disorders and Stroke NS110398 (to KSC, FPM, TJC, CES), NS090107 (to KSC, FPM), and the Parkinson’s disease Foundation International Research Grants Program, now the Parkinson Foundation (to KSC, FPM).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbd.2023.106111.
Data availability
Data will be made available on request.
References
- Ahmadpour N, Kantroo M, Stobart JL, 2021. Extracellular calcium influx pathways in astrocyte calcium microdomain physiology. Biomolecules 11 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Eroglu C, 2017. Cell biology of astrocyte-synapse interactions. Neuron 96 (3), 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A., et al. , 2012. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology 153 (11), 5373–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A., et al. , 2014. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology 155 (11), 4422–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG, 2015. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav 74, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG, 2022. Estrogen receptors observed at extranuclear neuronal sites and in glia in the nucleus accumbens core and shell of the female rat: evidence for localization to catecholaminergic and GABAergic neurons. J. Comp. Neurol 530 (11), 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova AS, et al. , 2018. Protein synthesis and quality control in aging. Aging (Albany NY) 10 (12), 4269–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnauld E., et al. , 1981. Effects of estrogens on the responses of caudate neurons to microiontophoretically applied dopamine. Neurosci. Lett 21 (3), 325–331. [DOI] [PubMed] [Google Scholar]
- Au HKE, Isalan M, Mielcarek M, 2021. Gene therapy advances: a Meta-analysis of AAV usage in clinical settings. Front. Med. (Lausanne) 8, 809118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Chun LL, Corey DP, 1989. Calcium current in cortical astrocytes: induction by cAMP and neurotransmitters and permissive effect of serum factors. J. Neurosci 9 (9), 3169–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastide MF, Bezard E, 2015. L-dopa induced dyskinesia in Parkinson’s disease. Bull. Acad. Natl Med 199 (2–3), 201–212. [PubMed] [Google Scholar]
- Bastide MF, et al. , 2015. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog. Neurobiol 132, 96–168. [DOI] [PubMed] [Google Scholar]
- Belujon P, Lodge DJ, Grace AA, 2010. Aberrant striatal plasticity is specifically associated with dyskinesia following levodopa treatment. Mov. Disord 25 (11), 1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey MJ, Manfredsson FP, 2016. Intraparenchymal stereotaxic delivery of rAAV and special considerations in vector handling. Methods Mol. Biol 1382, 199–215. [DOI] [PubMed] [Google Scholar]
- Benskey MJ, Sandoval IM, Manfredsson FP, 2016. Continuous collection of adeno-associated virus from producer cell medium significantly increases Total viral yield. Hum. Gene. Ther. Meth 27 (1), 32–45. [DOI] [PubMed] [Google Scholar]
- Berns KI, Muzyczka N, 2017. AAV: an overview of unanswered questions. Hum. Gene Ther 28 (4), 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., 2009. Calcium signaling and neurodegenerative diseases. Trends Mol. Med 15 (3), 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Pissadaki EK, 2012. Living on the edge with too many mouths to feed: why dopamine neurons die. Mov. Disord 27 (12), 1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove F, Calabresi P, 2022. Plasticity, genetics, and epigenetics in l-dopa-induced dyskinesias. Handb. Clin. Neurol 184, 167–184. [DOI] [PubMed] [Google Scholar]
- Broadhead MJ, Miles GB, 2020. Bi-directional communication between neurons and astrocytes modulates spinal motor circuits. Front. Cell. Neurosci 14, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk UT, Terman A, 2002. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med 33 (5), 611–619. [DOI] [PubMed] [Google Scholar]
- Bulaklak K, Gersbach CA, 2020. The once and future gene therapy. Nat. Commun 11 (1), 5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C., et al. , 2005. Systemic mannitol-induced hyperosmolality amplifies rAAV2-mediated striatal transduction to a greater extent than local co-infusion. Mol. Ther 11 (2), 327–331. [DOI] [PubMed] [Google Scholar]
- Burra P, Zanetto A, Germani G, 2022. Sex bias in clinical trials in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol 19 (7), 413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V., et al. , 2020. Rapamycin, by inhibiting mTORC1 signaling, prevents the loss of striatal bidirectional synaptic plasticity in a rat model of L-DOPA-induced dyskinesia. Front. Aging Neurosci 12, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield ME, Stancati JA, Steece-Collier K, 2021. Induction and assessment of levodopa-induced Dyskinesias in a rat model of Parkinson’s disease. JoVE 176, e62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield ME, Manfredsson FP, Steece-Collier K, 2023. Jan. The role of striatal Cav1.3 calcium channels in therapeutics for Parkinson’s disease. Handb. Exp. Pharmacol 1–31. 10.1007/164_2022_629. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Chan CS, et al. , 2007. Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447 (7148), 1081–1086. [DOI] [PubMed] [Google Scholar]
- Chan CS, Gertler TS, Surmeier DJ, 2009. Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 32 (5), 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, et al. , 2015. Interrogating the aged striatum: robust survival of grafted dopamine neurons in aging rats produces inferior behavioral recovery and evidence of impaired integration. Neurobiol. Dis 77, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, et al. , 2019. Cell therapy for Parkinson’s disease: why it doesn’t work every time. Mov. Disord 34 (8), 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz G, Fernandois D, Paredes AH, 2017. Ovarian function and reproductive senescence in the rat: role of ovarian sympathetic innervation. Reproduction 153 (2), R59–R68. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, et al. , 2003. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood 102 (2), 480–488. [DOI] [PubMed] [Google Scholar]
- Day M., et al. , 2006. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci 9 (2), 251–259. [DOI] [PubMed] [Google Scholar]
- Del-Bel E., et al. , 2016. L-DOPA-induced dyskinesia in Parkinson’s disease: are neuroinflammation and astrocytes key elements? Synapse 70 (12), 479–500. [DOI] [PubMed] [Google Scholar]
- Eldeeb MA, et al. , 2022. Mitochondrial quality control in health and in Parkinson’s disease. Physiol. Rev 102 (4), 1721–1755. [DOI] [PubMed] [Google Scholar]
- Fieblinger TGS, Sebel LE, Alcacer C, Plotkin JL, Gertler TS, Chan CS, Heiman M, Greengard P, Cenci MA, Surmeier DJ, 2023. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat. Commun 5, 5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DL, et al. , 2020. BDNF rs6265 variant alters outcomes with levodopa in early-stage Parkinson’s disease. Neurotherapeutics 17 (4), 1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, 2005. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the life span. Front. Neuroendocrinol 26 (2), 51–64. [DOI] [PubMed] [Google Scholar]
- Freed CR, et al. , 2001. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med 344 (10), 710–719. [DOI] [PubMed] [Google Scholar]
- Ge M., et al. , 2022. Role of calcium homeostasis in Alzheimer’s disease. Neuropsychiatr. Dis. Treat 18, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombash SE, et al. , 2014. Neuroprotective potential of pleiotrophin overexpression in the striatonigral pathway compared with overexpression in both the striatonigral and nigrostriatal pathways. Gene Ther. 21 (7), 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabinski TM, et al. , 2015. A method for combining RNAscope in situ hybridization with immunohistochemistry in thick free-floating brain sections and primary neuronal cultures. PLoS One 10 (3), e0120120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith WH, et al. , 2000. Modification of ion channels and calcium homeostasis of basal forebrain neurons during aging. Behav. Brain Res 115 (2), 219–233. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG, 2010. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170 (4), 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grp PS, 2020. Isradipine versus placebo in early Parkinson disease a randomized trial the Parkinson study group STEADY-PD III investigators. Ann. Intern. Med 172 (9), 591-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigoni C., et al. , 2005. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat. Disord 11 (Suppl. 1), S25–S29. [DOI] [PubMed] [Google Scholar]
- Haery L., et al. , 2019. Adeno-associated virus technologies and methods for targeted neuronal manipulation. Front. Neuroanat 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RA, Auinger P, Oakes D, 2009. Levodopa response in early Parkinson’s disease. Mov. Disord 24 (16), 2328–2336. [DOI] [PubMed] [Google Scholar]
- He X., et al. , 2021. Evolving AAV-delivered therapeutics towards ultimate cures. J. Mol. Med. (Berl) 99 (5), 593–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P., et al. , 2013. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol. Rev 65 (1), 171–222. [DOI] [PubMed] [Google Scholar]
- Huot P., et al. , 2022. Levodopa-induced dyskinesia: a brief review of the ongoing clinical trials. Neurodegener. Dis. Manag 12 (2), 51–55. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Arbuthnott GW, 1989. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 503 (2), 334–338. [DOI] [PubMed] [Google Scholar]
- Jackson KL, Dayton RD, Klein RL, 2015. AAV9 supports wide-scale transduction of the CNS and TDP-43 disease modeling in adult rats. Mol. Ther. Meth. Clin. Dev 2, 15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, et al. , 2017. Rationally engineered AAV capsids improve transduction and volumetric spread in the CNS. Mol. Ther. Nucleic. Acids 8, 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., et al. , 2013. Antagonism of L-type Ca2+ channels CaV1.3 and CaV1.2 by 1,4-dihydropyrimidines and 4H-pyrans as dihydropyridine mimics. Bioorg. Med. Chem 21 (14), 4365–4373. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM, 2015. Proteostasis and aging. Nat. Med 21 (12), 1406–1415. [DOI] [PubMed] [Google Scholar]
- Klaestrup IH, et al. , 2022. Impact of aging on animal models of Parkinson’s disease. Front. Aging Neurosci 14, 909273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaips CL, Jayaraj GG, Hartl FU, 2018. Pathways of cellular proteostasis in aging and disease. J. Cell Biol 217 (1), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C., et al. , 2004. Transcriptome analysis in a rat model of L-DOPA-induced dyskinesia. Neurobiol. Dis 17 (2), 219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korovila I., et al. , 2017. Proteostasis, oxidative stress and aging. Redox Biol. 13, 550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Meitzen J, 2018. Biological sex, estradiol and striatal medium spiny neuron physiology: a Mini-review. Front. Cell. Neurosci 12, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bodhinathan K, Foster TC, 2009. Susceptibility to calcium dysregulation during brain aging. Front. Aging Neurosci 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour I., et al. , 2003. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia 41 (4), 347–353. [DOI] [PubMed] [Google Scholar]
- Lee EA, et al. , 2003. The effects of chronic L-DOPA therapy on pharmacodynamic parameters in a rat model of motor response fluctuations. Exp. Neurol 184 (1), 304–312. [DOI] [PubMed] [Google Scholar]
- Lukashchuk V., et al. , 2016. AAV9-mediated central nervous system-targeted gene delivery via cisterna magna route in mice. Mol. Ther. Meth. Clin. Dev 3, 15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire CA, et al. , 2013. Mouse gender influences brain transduction by intravascularly administered AAV9. Mol. Ther 21 (8), 1470–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti B, Perlmutter JS, 2020. A clinical trial of Isradipine: what went wrong? Ann. Intern. Med 172 (9), 625-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson FP, et al. , 2020. The future of GDNF in Parkinson’s disease. Front. Aging Neurosci 12, 593572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Dreses-Werringloer U, Vingtdeux V, 2009. Calcium signaling in neurodegeneration. Mol. Neurodegener 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maries E., et al. , 2006. Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol. Dis 21 (1), 165–180. [DOI] [PubMed] [Google Scholar]
- McNeill TH, et al. , 1988. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res. 455 (1), 148–152. [DOI] [PubMed] [Google Scholar]
- McNeill J., et al. , 2021. Ion channels and electrophysiological properties of astrocytes: implications for emergent stimulation technologies. Front. Cell. Neurosci 15, 644126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ, 1996. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci 16 (2), 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Santana FL, et al. , 2014. Surface L-type Ca2+ channel expression levels are increased in aged hippocampus. Aging Cell 13 (1), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll SJ, Cook WH, Young D, 2020. AAV targeting of glial cell types in the central and peripheral nervous system and relevance to human gene therapy. Front. Mol. Neurosci 13, 618020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oenzil F., et al. , 1994. Age-related accumulation of lipofuscin in three different regions of rat brain. Mech. Ageing Dev 76 (2–3), 157–163. [DOI] [PubMed] [Google Scholar]
- Olson PA, et al. , 2005. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a shank-binding domain. J. Neurosci 25 (5), 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner NJ, Striessnig J, 2016. L-type calcium channels as drug targets in CNS disorders. Channels (Austin) 10 (1), 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B., et al. , 2003. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat. Neurosci 6 (5), 501–506. [DOI] [PubMed] [Google Scholar]
- Picconi B, De Leonibus E, Calabresi P, 2018. Synaptic plasticity and levodopa-induced dyskinesia: electrophysiological and structural abnormalities. J. Neural Transm. (Vienna) 125 (8), 1263–1271. [DOI] [PubMed] [Google Scholar]
- Polinski NK, et al. , 2016. Impact of age and vector construct on striatal and nigral transgene expression. Mol. Ther. Meth. Clin. Dev 3, 16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polinski NK, et al. , 2018. Impact of the Aged Brain Environment on Gene Therapy for Parkinson’s Disease, 2nd edition. Conn’s Handbook of Models for Human Aging, pp. 647–657. [Google Scholar]
- Prakash VS, et al. , 2018. Sex Bias in interventional clinical trials. J. Women’s Health (Larchmt) 27 (11), 1342–1348. [DOI] [PubMed] [Google Scholar]
- Putterman DB, et al. , 2007. Evaluation of levodopa dose and magnitude of dopamine depletion as risk factors for levodopa-induced dyskinesia in a rat model of Parkinson’s disease. J. Pharmacol. Exp. Ther 323 (1), 277–284. [DOI] [PubMed] [Google Scholar]
- Rascol O., et al. , 2022. Immediate-release/extended-release amantadine (OS320) to treat Parkinson’s disease with levodopa-induced dyskinesia: analysis of the randomized, controlled ALLAY-LID studies. Parkinsonism Relat. Disord 96, 65–73. [DOI] [PubMed] [Google Scholar]
- Samaranch L., et al. , 2014. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol. Ther 22 (2), 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval IM, Kuhn NM, Manfredsson FP, 2019. Multimodal production of adeno-associated virus. Methods Mol. Biol 1937, 101–124. [DOI] [PubMed] [Google Scholar]
- Schimanski LA, Barnes CA, 2010. Neural protein synthesis during aging: effects on plasticity and memory. Front. Aging Neurosci 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S., et al. , 2009. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol. Psychiatry 65 (6), 518–526. [DOI] [PubMed] [Google Scholar]
- Scott M, Lonning CAF, et al. , 2002. Influence of gender-specific hormones on adeno-associated virus vector gene transfer and expression. Mol. Ther 5 (5), S2. [Google Scholar]
- Sellnow RC, et al. , 2020. Striatal Nurr1 facilitates the Dyskinetic state and exacerbates levodopa-induced dyskinesia in a rat model of Parkinson’s disease. J. Neurosci 40 (18), 3675–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Zhai S, Surmeier DJ, 2022. Striatal synaptic adaptations in Parkinson’s disease. Neurobiol. Dis 167, 105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom KE, et al. , 2010. Impact of dendritic spine preservation in medium spiny neurons on dopamine graft efficacy and the expression of dyskinesias in parkinsonian rats. Eur. J. Neurosci 31 (3), 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sortwell CE, et al. , 2022. BDNF rs6265 genotype influences outcomes of pharmacotherapy and subthalamic nucleus deep brain stimulation in early-stage Parkinson’s disease. Neuromodulation 25 (6), 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steece-Collier K., et al. , 2003. Embryonic mesencephalic grafts increase levodopa-induced forelimb hyperkinesia in parkinsonian rats. Mov. Disord 18 (12), 1442–1454. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K., et al. , 2019. Genetic silencing of striatal CaV1.3 prevents and ameliorates levodopa dyskinesia. Mov. Disord 34 (5), 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, et al. , 2017a. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun 483 (4), 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Halliday GM, Simuni T, 2017b. Calcium, mitochondrial dysfunction and slowing the progression of Parkinson’s disease. Exp. Neurol 298 (Pt B), 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, et al. , 2022. Re-analysis of the STEADY-PD II trial-evidence for slowing the progression of Parkinson’s disease. Mov. Disord 37 (2), 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarowicz CA, Steece-Collier K, Caulfield ME, 2022. New Frontiers in neurodegeneration and regeneration associated with brain-derived neurotrophic factor and the rs6265 single nucleotide polymorphism. Int. J. Mol. Sci 23 (14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, et al. , 2020. EASE LID 2: a 2-year open-label trial of Gocovri (amantadine) extended release for dyskinesia in Parkinson’s disease. J. Parkinsons Dis 10 (2), 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Dillin A, 2011. Aging as an event of proteostasis collapse. Cold Spring Harb. Perspect. Biol vol. 3 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A, Brunk UT, 1998. Lipofuscin: mechanisms of formation and increase with age. APMIS 106 (2), 265–276. [DOI] [PubMed] [Google Scholar]
- Venuto CS, et al. , 2021. Isradipine plasma pharmacokinetics and exposure-response in early Parkinson’s disease. Ann. Clin. Transl. Neurol 8 (3), 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A., et al. , 2015. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci. Res 93, 144–157. [DOI] [PubMed] [Google Scholar]
- Woodward K, Shirokikh NE, 2021. Translational control in cell ageing: an update. Biochem. Soc. Trans 49 (6), 2853–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S., et al. , 2005. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology 64 (3), 545–547. [DOI] [PubMed] [Google Scholar]
- Zamora NN, et al. , 2020. Deletion of voltage-gated calcium channels in astrocytes during demyelination reduces brain inflammation and promotes myelin regeneration in mice. J. Neurosci 40 (17), 3332–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., et al. , 2006. Ca1.2 and CaV1.3 neuronal L-type calcium channels: differential targeting and signaling to pCREB. Eur. J. Neurosci 23 (9), 2297–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., et al. , 2013. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J. Neurosci 33 (28), 11655–11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.