Abstract
Infections in critically-ill patients caused by extensively-drug-resistant (XDR)-Pseudomonas aeruginosa are challenging to manage due to paucity of effective treatment options. Cefepime/zidebactam, which is currently in global Phase 3 clinical development (Clinical Trials Identifier: NCT04979806, registered on July 28, 2021) is a novel mechanism of action based β-lactam/ β-lactam-enhancer combination with a promising activity against a broad-range of Gram-negative pathogens including XDR P. aeruginosa. We present a case report of an intra-abdominal infection-induced sepsis patient infected with XDR P. aeruginosa and successfully treated with cefepime/zidebactam under compassionate use. The 50 year old female patient with past-history of bariatric surgery and recent elective abdominoplasty and liposuction developed secondary pneumonia and failed a prolonged course of polymyxins. The organism repeatedly isolated from the patient was a New-Delhi metallo β-lactamase-producing XDR P. aeruginosa resistant to ceftazidime/avibactam, imipenem/relebactam and ceftolozane/tazobactam, susceptible only to cefepime/zidebactam. As polymyxins failed to rescue the patient, cefepime/zidebactam was administered under compassionate grounds leading to discharge of patient in stable condition. The present case highlights the prevailing precarious scenario of antimicrobial resistance and the need for novel antibiotics to tackle infections caused by XDR phenotype pathogens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12941-023-00606-x.
Keywords: Cefepime/zidebactam, β-lactam-enhancer, Pseudomonas, New-Delhi metallo-β-lactamase, Extensively-drug-resistant
Main text
Extensively-drug-resistant (XDR) Pseudomonas aeruginosa (non-susceptible to at least one agent in all but one or two anti-pseudomonal antibiotic classes) are usually related to certain sequence-types (ST) that have disseminated across the world and termed “high-risk” clones [1] The worldwide top 10 P. aeruginosa high-risk clones include ST235, ST111, ST233, ST244, ST357, ST308, ST175, ST277, ST654 and ST298 [2]. Infections of XDR P. aeruginosa are associated with higher mortality rate, prolonged hospitalization and an increased treatment cost compared with infections caused by antibiotic-susceptible P. aeruginosa [3]. In recent years, few new anti-pseudomonal antibiotics (ceftolozane/tazobactam, ceftazidime/avibactam, imipenem/relebactam and cefiderocol) have been introduced. While they are active against certain XDR isolates, yet are riddled with spectrum gaps. For instance, against metallo-β-lactamase (MBL)-expressing organisms, none of the new β-lactam/β-lactamase inhibitors exhibit any meaningful activity and cefiderocol demonstrates elevated MICs against such resistotype [4, 5].
Cefepime/zidebactam (WCK 5222) is a novel β-lactam enhancer mechanism based combination currently being studied in a global Phase 3 trial in adult patients with complicated urinary tract infection or acute pyelonephritis (ClinicalTrials.gov identifier: NCT04979806). Zidebactam is distinguished from newer β-lactamase inhibitors such as avibactam and taniborbactam by means of an additional function; selective and high-affinity binding to penicillin-binding protein (PBP) 2. When combined with cefepime that targets PBP3, zidebactam synergistically enhances the bactericidal activity of cefepime, thus functioning as a “β-lactam-enhancer” [6, 7]. In vitro and in vivo studies conducted on cefepime/zidebactam have established its broad-spectrum activity against Gram-negative organisms that include both serine-carbapenemases and MBL- expressing isolates [8–11].
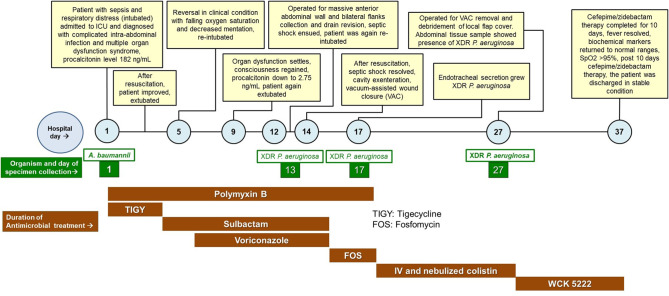
During the month of August, 2022, a critically-ill patient with a complex XDR P. aeruginosa infection was admitted to our tertiary care hospital. With last line antibiotics failing to eradicate the pathogen, the patient was successfully treated with cefepime/zidebactam under the compassionate use. This is the first case of compassionate use of cefepime/zidebactam and the clinical events surrounding the patient from the day of hospitalization to discharge are described below (Fig. 1).
Fig. 1.
Events during the hospital stay of the patient
Case report
A 50-year-old woman from Nepal with a past-history of bariatric surgery (2013) was presented to a speciality hospital in Lucknow, India for an elective abdominoplasty and liposuction. Post-surgery, she developed intra-abdominal sepsis and septic shock with features of severe hypoxemic respiratory failure. She required invasive mechanical ventilation and was subsequently shifted to the critical care unit of Medanta Hospital, Lucknow, India for further management. On admission, the patient was diagnosed with multi-organ failure with high SOFA (Sequential Organ Failure Assessment) score and serum procalcitonin level was 182 ng/mL. In view of suspected Gram-negative-implicated sepsis, an empiric antibiotic regimen consisting of polymyxin B and tigecycline was initiated. Over the next 48–72 h, patient’s condition improved with a significant reduction in the requirement of vasopressors and a drop in the serum procalcitonin level to 32.8 ng/mL. Subsequently, she was extubated after successful spontaneous breathing trial. However, by hospital-day 4, patient experienced respiratory distress and deteriorating clinical condition warranting reintubation, which was performed on hospital-day 5.
Meanwhile, the endotracheal aspirate collected on day 1 showed a growth of Acinetobacter baumannii with susceptibility to polymyxins only (EUCAST criteria). Noting this, tigecycline was discontinued and high-dose sulbactam was added with in vitro-active polymyxin B on day 4. Moreover, in view of persistent fever and vasopressor requirement, the antimicrobial regime was further augmented by addition of anti-fungal, voriconazole on day 6 [12].
The patient remained febrile without clinical improvement and continued to be under mechanical ventilation. The ensuing endotracheal sample (day 13) didn’t show A. baumannii, rather a new organism, P. aeruginosa (ISOLATE 1) with susceptibility only to polymyxins (EUCAST criteria). The isolate was resistant to ceftazidime/avibactam, ceftolozane/tazobactam, and imipenem/relebactam (Table 1). Based on the susceptibility to fosfomycin as reported by VITEK® 2 (later turned to be resistant in reference MIC method), intravenous fosfomycin was added to on-going polymyxin B while sulbactam and voriconazole were discontinued (day 15). On day 17, a 3rd endotracheal sample was collected which once more revealed the presence of P. aeruginosa (ISOLATE 2) with susceptibility to polymyxins (EUCAST criteria) but resistant to fosfomycin (VITEK® 2 & reference MIC > 128 mg/L). With no improvement in the clinical condition of the patient, fosfomycin and polymyxin B were withdrawn, replaced with intravenous and nebulized colistin (day 19).
Table 1.
Antibiotic susceptibility of three XDR P. aeruginosa collected from the patient during the clinical course
| Antibiotics | MICs (mg/L) | ||||
|---|---|---|---|---|---|
| Endotracheal secretion samples | Abdominal tissue sample | ||||
| hospital day 13 | hospital day 17 | hospital day 27 |
|||
| ISOLATE 1 | ISOLATE 2 | ISOLATE 3 | |||
| Cefepime | > 64 | > 64 | > 64 | ||
| Cefepime/zidebactam | 16 | 16 | 16 | ||
| Ceftazidime | > 64 | > 64 | > 64 | ||
| Ceftazidime/avibactam | > 64 | > 64 | > 64 | ||
| Imipenem | > 64 | > 64 | > 64 | ||
| Imipenem/relebactam | > 64 | > 64 | > 64 | ||
| Ceftolozane/tazobactam | > 64 | > 64 | > 64 | ||
| Piperacillin/tazobactam | > 64 | > 64 | > 64 | ||
| Meropenem | > 64 | > 64 | > 64 | ||
| Amikacin | > 64 | > 64 | > 64 | ||
| Levofloxacin | > 64 | > 64 | > 64 | ||
| Fosfomycin | > 256 | > 256 | > 256 | ||
| Colistin | 0.25 | 0.25 | 0.25 | ||
| Polymyxin B | 0.12 | 0.25 | 0.25 | ||
| Imipenem + EDTA | 16 | 16 | 16 | ||
| Ceftazidime/avibactam + EDTA | 16 | 16 | 16 | ||
Cefepime/zidebactam MICs were determined in 1:1 ratio;
For all the β-lactamase inhibitor based combination, inhibitor was at fixed 4 mg/L
EDTA: Ethylene diamine tetraacetic acid at fixed 200 mg/L
For fosfomycin, agar MIC method was employed by supplementing the medium with glucose-6-phosphate at 25 mg/L
Compassionate use of cefepime/zidebactam
Amidst the continued ICU stay of the patient with sepsis-multiple organ dysfunction syndromes (MODS), repeated recovery of an XDR P. aeruginosa and dwindling antibiotic choices, susceptibility of an investigational antibiotic cefepime/zidebactam was requested after obtaining requisite permissions from Drugs Controller General of India. Cefepime/zidebactam MIC was determined as per Clinical and Laboratory Standards Institute (CLSI M100 Ed 32 USA) guidelines and was noted as 16 mg/L, below its PK/PD breakpoint of ≤ 32 mg/L [8, 13]. With data supporting susceptibility, cefepime/zidebactam monotherapy was initiated on day 28 (replacing intravenous and nebulized colistin) under compassionate use, as per the manufacturer’s dosing instructions. Meanwhile, on day 27 (prior to initiation of cefepime/zidebactam), the abdominal tissue sample was submitted for microbiological examination, which showed the presence of P. aeruginosa (ISOLATE 3) with susceptibility profile identical to that of ISOLATE 2. To note, previous to cefepime/zidebactam administration, abdominal wound management was undertaken with debridement on day 13, 21 and 27. The vacuum-assisted closure of wound was performed on day 14.
Post administration of cefepime/zidebactam, the patient showed gradual improvement in the clinical condition and within three days of therapy, fever was resolved. With continuous administration of cefepime/zidebactam, a significant improvement in respiratory and hemodynamic parameters was noticed, and the patient was shifted to general ward for further care. As patient was out of intubation and with resolution of abdominal drain discharge, no specimen for bacterial culture could be collected. Meanwhile, to generate an evidence of clearance of pathogen, on day 7 of cefepime/zidebactam treatment, blood was collected which yielded no culture growth. After receiving 10 days of cefepime/zidebactam therapy with no reported adverse drug events, the patient was discharged in stable condition on hospital day 37. Two follow-up visits on 11 and 36 days after the discharge confirmed the complete recovery paving way for her return to the native country.
Antimicrobial resistance profile of isolates
For all three P. aeruginosa isolates, MICs of carbapenems (> 64 mg/L) in the presence of EDTA was lowered by > 8 times, which phenotypically indicates the presence of MBL. This was confirmed by the detection of blaNDM−1 in whole genome sequence analysis (Supplementary Table 1). Importantly, all three isolates were clonally identical and belonged to an international high-risk clone of P. aeruginosa, ST357. In the single nucleotide polymorphism analysis, mutations in efflux proteins (e.g., MexB, MexC, MexEF), OprD, and β-lactam target (ftsI gene; PBP3), that are known to cause high level of β-lactam resistance were observed [14, 15] Further, the isolates showed presence of acquired genes and mutations linked with resistance to aminoglycosides, fluoroquinolones, and tetracyclines (Supplementary Table 1).
In vitro time-kill activity
Time-kill studies were undertaken to assess the bactericidal activity of cefepime/zidebactam against P. aeruginosa isolated from the patient. Standalone cefepime was ineffective in restricting the growth of organisms even at higher concentrations. On the other hand, at 2x MIC, cefepime/zidebactam showed a time-dependent bactericidal action (~ 1.5-3-log10). Although colistin showed rapid killing initially (2 h), however a bacterial regrowth was observed at later time-points (6–8 h) suggesting selection and proliferations of tolerant population (supplementary Fig. 1).
Discussion
In contrast to Enterobacterales, multi-drug resistance in P. aeruginosa is principally mediated through non-enzymatic resistance mechanisms such as down regulated porins or/and hyper-expression of efflux pumps. From therapeutic perspectives, for such resistotypes, newer anti-pseudomonal drugs show about 60–80% coverage [16, 17], however, they are ineffective against MBL-expressing P. aeruginosa commonly prevalent in Asia, particularly in India. These isolates are often co-resistant to aminoglycosides and fluoroquinolones leaving polymyxins as the only treatment option. However, in the present case, polymyxins were unable to eradicate the pathogen despite their prolonged use. This is not unexpected in view of their significant inter-patient PK variability and PK/PD insufficiency in body sites such as lung and intra-peritoneal fluid [18]. Moreover, the treatment of serious carbapenem-resistant Gram-negative infections with colistin-based therapies has been associated with 40% mortality and about 50% acute kidney injury. All three isolates were also resistant to intravenous fosfomycin (MIC > 256 mg/L). In this context, compassionate use of an investigational drug cefepime/zidebactam helped eradicate the pathogen leading to substantial improvement in the clinical condition of the patient. Moreover, no drug-linked adverse effects were noticed. Previous in vitro studies have shown promising activity of this combination against XDR P. aeruginosa including those producing MBLs [8]. Translational in vivo studies in neutropenic mice lung or thigh models also showed efficacy of cefepime/zidebactam against MBL-expressing P. aeruginosa at exposures mimicking human exposures [10, 19]. Thus, non-clinical studies support the potential of cefepime/zidebactam for the treatment of infections caused by XDR P. aeruginosa.
Albeit the favourable outcome in the end, the present case highlights the dearth of safe and effective antibiotics to deal with the infections caused by XDR P. aeruginosa. Importantly, given the epidemiological diversity in the resistance mechanisms, there is a pressing unmet need for the novel antibiotics that comprehensively addresses all the resistance mechanisms associated with XDR P. aeruginosa.
Conclusion
Serious infections caused by XDR-phenotype Gram-negative organisms are associated with poor clinical outcome mainly due to non-availability of effective & safe antibiotics. Specifically, infections caused by MBL-expressing P. aeruginosa pose severe therapeutic challenges as none of the newer β-lactam/β-lactamase inhibitors are effective against such pathogens. Novel β-lactam enhancer mechanism based cefepime/zidebactam is being developed targeting XDR Gram-negatives including MBL- producers. In this present case, compassionate use of cefepime/zidebactam was opted as a salvage therapy as no other therapeutic options were effective.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Drugs Controller General of India for the permission to use cefepime/zidebactam under compassionate use. We thank Wockhardt Bio AG for timely supply of cefepime/zidebactam vials at no cost and susceptibility testing of isolates.
Abbreviations
- XDR
Extensively-drug-resistant
- SOFA
Sequential organ failure assessment
- PBP
Penicillin binding protein
- MIC
Minimum inhibitory concentration
- MLST
Multi-locus sequence type
- ST
Sequence type
- MBL
Metallo-β-lactamase
- MODS
Multiple organ dysfunction syndrome
- CLSI
Clinical and Laboratory Standards Institute
Authors’ contributions
Patient care: DD, MR, TS, NB, VK, SM, PS, MD and AI; microbial investigation: BJ and YB; data analysis: AV and YB; writing—original draft preparation: DD and BV; writing-review and editing: DD and BV. All authors have read and agreed to publish this version of the manuscript.
Funding
No external funding was received to conduct any of the studies.
Data Availability
The whole genome sequence datasets generated and/or analysed during the current study are available on the NCBI Link: https://www.ncbi.nlm.nih.gov/nuccore?term=900707%5BBioProject%5D.
Pseudomonas isolate no. Accession number:
Isolate 1 (LRK01) JAPHVT000000000.1.
Isolate 2 (LRK02) JAPHVU000000000.1.
Isolate 3 (LRK03) JAPHVV000000000.1.
Declarations
Ethics approval and consent to participate
Ethics committee approval and consent for publication was obtained prior to the administration of the study drug under compassionate use.
Consent for publication
Consent for publication was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dilip Dubey, Email: drdilipdubey2d@gmail.com.
Balaji Veeraraghavan, Email: vbalaji@cmcvellore.ac.in.
References
- 1.Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, Benito N, Grau S. Epidemiology and treatment of Multidrug-Resistant and extensively drug-resistant Pseudomonas aeruginosa Infections. Clin Microbiol Rev 2019, 32(4). [DOI] [PMC free article] [PubMed]
- 2.Del Barrio-Tofiño E, López-Causapé C, Oliver A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired β-lactamases: 2020 update. Int J Antimicrob Agents. 2020;56(6):106196. doi: 10.1016/j.ijantimicag.2020.106196. [DOI] [PubMed] [Google Scholar]
- 3.Nathwani D, Raman G, Sulham K, Gavaghan M, Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2014;3(1):32. doi: 10.1186/2047-2994-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Losito AR, Raffaelli F, Del Giacomo P, Tumbarello M. New drugs for the treatment of Pseudomonas aeruginosa Infections with Limited Treatment Options: a narrative review. Antibiot (Basel Switzerland) 2022, 11(5). [DOI] [PMC free article] [PubMed]
- 5.Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob Agents Chemother 2020, 64(12). [DOI] [PMC free article] [PubMed]
- 6.Moya B, Bhagwat S, Cabot G, Bou G, Patel M, Oliver A. Effective inhibition of PBPs by cefepime and zidebactam in the presence of VIM-1 drives potent bactericidal activity against MBL-expressing Pseudomonas aeruginosa. J Antimicrob Chemother. 2020;75(6):1474–8. doi: 10.1093/jac/dkaa036. [DOI] [PubMed] [Google Scholar]
- 7.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. WCK 5107 (Zidebactam) and WCK 5153 Are Novel Inhibitors of PBP2 Showing Potent “β-Lactam Enhancer” Activity against Pseudomonas aeruginosa, Including Multidrug-Resistant Metallo-β-Lactamase-Producing High-Risk Clones. Antimicrob Agents Chemother 2017, 61(6). [DOI] [PMC free article] [PubMed]
- 8.Karlowsky JA, Hackel MA, Bouchillon SK, Sahm DF. In Vitro Activity of WCK 5222 (Cefepime-Zidebactam) against Worldwide Collected Gram-Negative Bacilli not susceptible to Carbapenems. Antimicrob Agents Chemother 2020, 64(12). [DOI] [PMC free article] [PubMed]
- 9.Avery LM, Abdelraouf K, Nicolau DP. Assessment of the in vivo efficacy of WCK 5222 (Cefepime-Zidebactam) against Carbapenem-Resistant Acinetobacter baumannii in the neutropenic murine lung infection model. Antimicrob Agents Chemother 2018, 62(11). [DOI] [PMC free article] [PubMed]
- 10.Kidd JM, Abdelraouf K, Nicolau DP. Efficacy of human-simulated bronchopulmonary exposures of cefepime, zidebactam and the combination (WCK 5222) against MDR Pseudomonas aeruginosa in a neutropenic murine pneumonia model. J Antimicrob Chemother. 2020;75(1):149–55. doi: 10.1093/jac/dkz414. [DOI] [PubMed] [Google Scholar]
- 11.Lasko MJ, Abdelraouf K, Nicolau DP. Comparative in vivo activity of human-simulated plasma and epithelial lining fluid exposures of WCK 5222 (cefepime/zidebactam) against KPC- and OXA-48-like-producing Klebsiella pneumoniae in the neutropenic murine pneumonia model. J Antimicrob Chemother. 2021;76(9):2310–6. doi: 10.1093/jac/dkab183. [DOI] [PubMed] [Google Scholar]
- 12.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, et al. Clinical practice Guideline for the management of Candidiasis: 2016 update by the infectious Diseases Society of America. Clin Infect Dis. 2015;62(4):e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sader HS, Mendes RE, Duncan LR, Carvalhaes CG, Castanheria M. Antimicrobial activity of cefepime/zidebactam (WCK 5222), a β-lactam/β-lactam enhancer combination, against clinical isolates of Gram-negative bacteria collected worldwide (2018–19) J Antimicrob Chemother. 2022;77(10):2642–9. doi: 10.1093/jac/dkac233. [DOI] [PubMed] [Google Scholar]
- 14.Wardell SJT, Rehman A, Martin LW, Winstanley C, Patrick WM, Lamont IL. A large-scale whole-genome comparison shows that experimental evolution in response to antibiotics predicts changes in naturally evolved clinical Pseudomonas aeruginosa. Antimicrob Agents Chemother 2019, 63(12). [DOI] [PMC free article] [PubMed]
- 15.Horna G, López M, Guerra H, Saénz Y, Ruiz J. Interplay between MexAB-OprM and MexEF-OprN in clinical isolates of Pseudomonas aeruginosa. Sci Rep. 2018;8(1):16463. doi: 10.1038/s41598-018-34694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirza HC, Hortaç E, Koçak AA, Demirkaya MH, Yayla B, Güçlü A, Başustaoğlu A. In vitro activity of ceftolozane–tazobactam and ceftazidime–avibactam against clinical isolates of meropenem-non-susceptible Pseudomonas aeruginosa: a two-centre study. J Global Antimicrob Resist. 2020;20:334–8. doi: 10.1016/j.jgar.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Karlowsky JA, Lob SH, DeRyke CA, Hilbert DW, Wong MT, Young K, Siddiqui F, Motyl MR, Sahm DF. In Vitro Activity of Ceftolozane-Tazobactam, Imipenem-Relebactam, Ceftazidime-Avibactam, and comparators against Pseudomonas aeruginosa isolates collected in United States Hospitals according to results from the SMART Surveillance Program, 2018 to 2020. Antimicrob Agents Chemother. 2022;66(5):e0018922. doi: 10.1128/aac.00189-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, et al. International Consensus Guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), infectious Diseases Society of America (IDSA), International Society for anti-infective pharmacology (ISAP), society of critical Care Medicine (SCCM), and Society of Infectious Diseases pharmacists (SIDP) Pharmacotherapy. 2019;39(1):10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monogue ML, Tabor-Rennie J, Abdelraouf K, Nicolau DP. In vivo efficacy of WCK 5222 (Cefepime-Zidebactam) against Multidrug-Resistant Pseudomonas aeruginosa in the neutropenic murine thigh infection model. Antimicrob Agents Chemother 2019, 63(7). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The whole genome sequence datasets generated and/or analysed during the current study are available on the NCBI Link: https://www.ncbi.nlm.nih.gov/nuccore?term=900707%5BBioProject%5D.
Pseudomonas isolate no. Accession number:
Isolate 1 (LRK01) JAPHVT000000000.1.
Isolate 2 (LRK02) JAPHVU000000000.1.
Isolate 3 (LRK03) JAPHVV000000000.1.