Abstract
Background
Considering the high reoperation rate in degenerative lumbar spondylolisthesis (DLS) patients undergoing lumbar surgeries and controversial results on the risk factors for the reoperation, we performed a systematic review and meta-analysis to explore the reoperation rate and risk factors for the reoperation in DLS patients undergoing lumbar surgeries.
Methods
Literature search was conducted from inception to October 28, 2022 in Pubmed, Embase, Cochrane Library, and Web of Science. Odds ratio (OR) was used as the effect index for the categorical data, and effect size was expressed as 95% confidence interval (CI). Heterogeneity test was performed for each outcome effect size, and subgroup analysis was performed based on study design, patients, surgery types, follow-up time, and quality of studies to explore the source of heterogeneity. Results of all outcomes were examined by sensitivity analysis. Publication bias was assessed using Begg test, and adjusted using trim-and-fill analysis.
Results
A total of 39 cohort studies (27 retrospective cohort studies and 12 prospective cohort studies) were finally included in this systematic review and meta-analysis. The overall results showed a 10% (95%CI: 8%-12%) of reoperation rate in DLS patients undergoing lumbar surgeries. In surgery types subgroup, the reoperation rate was 11% (95%CI: 9%-13%) for decompression, 10% (95%CI: 7%-12%) for fusion, and 9% (95%CI: 5%-13%) for decompression and fusion. An increased risk of reoperation was found in patients with obesity (OR = 1.91, 95%CI: 1.04–3.51), diabetes (OR = 2.01, 95%CI: 1.43–2.82), and smoking (OR = 1.51, 95%CI: 1.23–1.84).
Conclusions
We found a 10% of reoperation rate in DLS patients after lumbar surgeries. Obesity, diabetes, and smoking were risk factors for the reoperation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-023-02082-8.
Keywords: Degenerative lumbar spondylolisthesis, Reoperation, Risk factors, Meta-analysis
Background
Degenerative lumbar spondylolisthesis (DLS) refers to anterolisthesis of one vertebral body over another vertebral body secondary to osteoarthritic degeneration, leading to spinal canal stenosis [1]. DLS is an aging-related disease, and its incidence is increasing under the background of the global population aging [2]. Each year, 39 million individuals are diagnosed with DLS, accounting for a global prevalence of 0.53% [3]. DLS may be accompanied with low back pain, radiculopathy, or neurogenic claudication [2].
Surgeries have been regarded as the standard treatment modality for intractable cases [4]. The proportion of lumbar surgeries increases by more than two-fold not only because of elevated prevalence of degenerative lumbar spine disease but also because of improved surgical techniques, good outcomes, and increased hospitals and surgeons [5, 6]. However, due to complications (such as fusion failure, persistent pain, and infection), progressive degenerative changes-related diseases, or an unrelated previous surgeries, some patients require reoperation [7]. Despite improvements in surgical skills and techniques, the reoperation rate is still unimproved, with a 10-year reoperation rate of about 15% [7]. Given the high prevalence and chronicity of DLS, understanding the risk factors for reoperation is important [8]. Park et al. have revealed the longitudinal trends in the lumbar reoperation rate, and the reoperation was associated with demographics, comorbidities, primary surgery type, and preoperative spinal pathology [9]. Noh et al. haven found lifestyle-related factors, such as smoking, drinking, and exercise, were associated with the higher rate of reoperation [10]. However, results of studies on the risk factors for reoperation of DLS patients remain controversial. Rabah et al., have reported that diabetes was related to greater risk of reoperation [11], while Khan et al. reported no significant association between diabetes and reoperation [12]. In the study performed by Zhong et al., obesity was found to be associated with a higher incidence of unplanned reoperations [8]. Nevertheless, Kuo et al. found that obesity was not significantly associated with the reoperation [13].
Considering the controversial results, we aimed to perform a systematic review and meta-analysis to evaluate the incidence and risk factors of reoperation in DLS patients for the purpose of improving surgical outcomes and prognosis.
Methods
Literature search strategy
This study was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14]. Two researchers (YZC and YZ) conducted the literature search from September 28, 2022 to October 28, 2022 in Pubmed, Embase, Cochrane Library, and Web of Science. Consensus was reached by discussion; if consensus cannot be reached by discussion, a third researcher (XHF) was consulted. Search terms were “degenerative spinal diseases” OR “degenerative spondylolisthesis” OR “degenerative lumbar spondylolisthesis” OR “degenerative cervical spondylolisthesis” AND “spinal surgery” OR “fusion” OR “reoperation” OR “repeat surgery” OR “risk factor”.
Selection criteria
Studies meeting the following inclusion criteria were selected: (1) population: DLS patients; (2) patients undergoing lumbar surgeries, including decompression surgeries and fusion surgeries; (3) outcome: reoperation rate and risk factors; and (4) studies: cohort studies. The population included DLS patients and DLS patients with lumbar spinal stenosis (LSS). Fusion surgeries included posterolateral lumbar fusion (PLF) and lumbar interbody fusion (LIF). Reoperation was defined as the secondary lumbar surgeries due to progression of lumbar degenerative changes or postoperative instability [8]. Risk factors included body mass index (BMI), sex, age, diabetes, smoking, and more bleeding.
Studies were excluded by meeting one of the following criteria: (1) animal studies; (2) other degenerative spinal diseases including lumbar spine stenosis, degenerative disc disease, and degenerative cervical spondylosis; (3) not English articles; (4) unable to extract data; (5) case reports, conference abstracts, letters, reviews, and meta-analysis.
Data extraction and critical appraisal
The following data were extracted: the first author, year of publication, country, study design, patients, definition of spondylolisthesis, sample size, age, sex, BMI, disease duration, surgery types, follow-up time, number of reoperations, reasons for reoperation, reoperation methods, and risk factors of reoperation. The Newcastle–Ottawa Scale (NOS) was applied to evaluate the quality of cohort studies [15]. This scale consisted of three items: selection, comparability, and outcome. This scale was scored a total of 9 points, and divided into low quality (0–3 points), fair quality (4–6 points), and high quality (7–9 points) [15].
Statistical analysis
All statistical analyses were performed using Stata15.1 software (Stata Corporation, College Station, TX, USA). Rate was used as the effect index in the analysis of reoperation rate. Odds ratio (OR) was used as the effect index for categorical data, and effect size was represented as 95% confidence interval (CI). Heterogeneity test was performed for each outcome effect size, and results were quantified as I-squared (I2). Random-effect model was used for analysis if I2 ≥ 50%, and fixed-effect model was used if I2 < 50%. For the high heterogeneity (I2 ≥ 50%), subgroup analysis was conducted based on study design, patients, surgery types, follow-up time, and quality of studies to explore the source of heterogeneity. Sensitivity analysis was carried out for all outcomes. Begg test was used to assess publication bias for the outcome included more than 10 articles. Trim-and-fill analysis was used to adjust the publication bias. P < 0.05 was considered statistically significant.
Results
Identification of studies and characteristics of patients
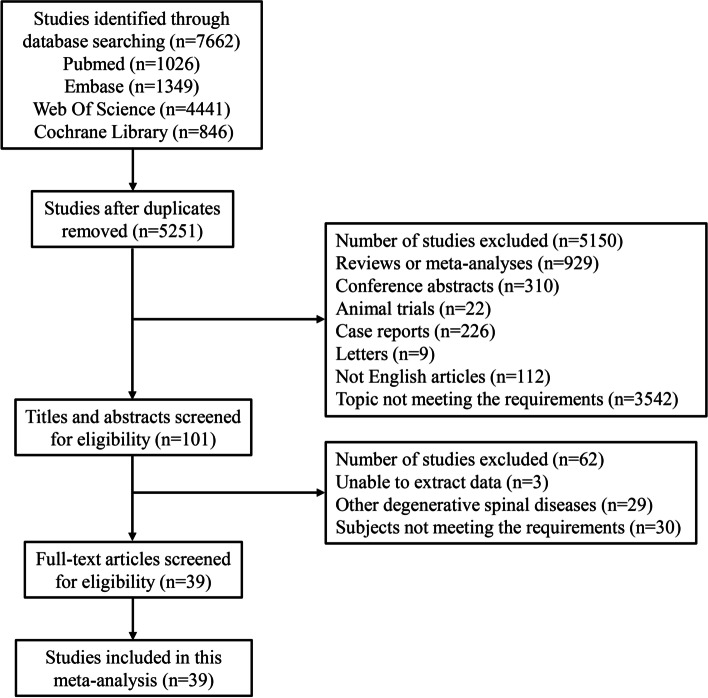
A total of 7,662 articles were searched from Pubmed (n = 1026), Embase (n = 1349), Web of Science (n = 4441), and Cochrane Library (n = 846). After removing the duplicates, 5,251 articles remained. Further, 5,150 articles were excluded due to publishing as reviews or meta-analyses (n = 929), conference abstracts (n = 310), animal trials (n = 22), case reports (n = 226), and letters (n = 9), not English articles (n = 112), and topic not meeting the requirements (n = 3542). In the remaining 101 articles, we further excluded 3 articles unable to extract data, 29 articles reporting other degenerative spinal diseases, and 30 articles with topic not meeting the requirements. Finally, 39 cohort studies were retained in this meta-analysis [7, 8, 10–13, 16–48], with 27 retrospective cohort studies and 12 prospective cohort studies. The flow diagram of our searching was displayed in Fig. 1. In the included studies, 28 studies were assessed as fair quality, and 11 studies were assessed as high quality. Characteristics of the included studies were presented in Table 1.
Fig. 1.
Flowchart of identifying studies
Table 1.
Characteristics of included patients
| Author | Year | Country | Study design | Patients | Definition of spondylolisthesis | Sample size | Age (years) | Male/female |
|---|---|---|---|---|---|---|---|---|
| Salimi | 2022 | Japan | Retrospective cohort | DLS with LSS | ≥ 3 mm anterior slippage | 50 | 69.4 ± 9.5 | 23/27 |
| Noh | 2022 | Korea | Retrospective cohort | DLS with or without LSS | NA | DLS with LSS: 3840; DLS without LSS: 255 | 60.5 ± 9.1 | NA |
| Moayeri | 2022 | Canada | Retrospective cohort | DLS with LSS | grade 1 | 140 | 68.0 ± 10.1 | 64/76 |
| Liang | 2022 | China | Retrospective cohort | DLS with LSS | grade 1 | 104 (PMTD: 53; MIS TLIF: 51) | 62.06 ± 13.6; 59.94 ± 8.3 | 27/26; 17/34 |
| Joelson | 2022 | Sweden | Prospective cohort | DLS with LSS | slip > 3 mm | 372 (decompression and fusion: 228; decompression only: 144) | 63.9 ± 8.9; 69.4 ± 9.4 | 79/149; 47/97 |
| Georgiou | 2022 | USA | Retrospective cohort | DLS | single-level DLS | 51619 (anterior fusion: 14971; posterior fusion: 36648) | ≥ 75: 1229; 2514 | 5629/9342; 12904/23744 |
| Chan | 2021 | USA | Retrospective cohort | DLS | NA | 15658 | 62.5 | 5762/9896 |
| Takaoka | 2021 | Japan | Retrospective cohort | DLS with LSS | NA | 145 (OLIF: 66; TLIF: 79) | 66 ± 12; 71 ± 9 | 28/38; 37/42 |
| Sugiura | 2021 | Japan | Retrospective cohort | DLS | slippage at L3 or L4 of > 3% | 202 (BPL: 51; PLIF: 106) | 70.4 ± 8.4;69.1 ± 7.7 | 21/30; 38/68 |
| Rabah | 2021 | USA | Retrospective cohort | DLS | NA | 6260 | 60.27 ± 12.49 | 2341/3919 |
| Mimura | 2021 | Japan | Retrospective cohort | DLS | grade 1 and 2 | 41 | 69.8 ± 7.1 | 19/22 |
| Katuch | 2021 | Slovakia | Prospective cohort | DLS | NA | 333 (TLIF: 119; PLIF:214) | 55.21 ± 9.22; 56.51 ± 10.71 | 52/67; 95/119 |
| Joelson | 2021 | Sweden | Prospective cohort | DLS with LSS | slip > 3 mm on preoperative radiographs | 1935 (Decompression and fusion: 1338; decompression only: 597) | 65 ± 9.1; 69 ± 9.9 | NA |
| Badhiwala | 2021 | Canada | Retrospective cohort | DLS | NA | 1804 (laminectomy alone: 802; laminectomy plus fusion: 1002) | 64.4 ± 11.6; 62.7 ± 12.1 | 305/497; 348/654 |
| Zhong | 2020 | China | Retrospective cohort | DLS | one-level or two-level DLS |
1100 (posterolateral fusion: 650; intervertebral fusion: 450) |
72.80 ± 11.7; 71.90 ± 10.7 | NA |
| Nyström | 2020 | Sweden | Retrospective cohort | DLS | slip > 3 mm | 200 | 64.8 | 92/108 |
| Lee | 2020 | Korea | Prospective cohort | DLS | NA | 620 (decompressive laminectomy: 383; PLIF: 171; ALIF: 66) | NA | NA |
| Khan | 2020 | USA | Retrospective cohort | DLS | grade 1 and 2 | 850 | 58.35 ± 13.78 | 397/453 |
| Khan | 2019 | USA | Retrospective cohort | DLS | grade 1 and 2 | 141 | 62.28 ± 10.7 | 65/76 |
| Karsy | 2020 | USA | Prospective cohort | DLS | grade 1 | 608 | < 60: 239; 60–70: 209; 71–80: 128; > 80: 32 | 350/258 |
| Chan | 2020 | USA | Prospective cohort | DLS | grade 1 | 297 (MIS-TLIF: 72; Open TLIF: 225) | 62.1 ± 10.6; 59.5 ± 11.7 | 32/40; 82/143 |
| Bisson | 2020 | USA | Retrospective cohort | DLS | grade 1 | 140 (MIS decompression: 71; Open decompression: 69) | 72.264 ± 9.662; 66.913 ± 12.578 | 74/66; 32/39 |
| Minamide | 2019 | USA | Prospective cohort | DLS with LSS | single-level DLS at L3/L4 or L4/L5 | 218 | 69.7 (47–88) | 96/122 |
| Kuo | 2019 | USA | Retrospective cohort | DLS with LSS | NA | 601 (ULBD: 164; fusion: 437) | 68.5 ± 9.6; 69.2 ± 9.6 | 59/105; 125/312 |
| Kelly | 2019 | USA | Retrospective cohort | DLS | grade 1 | 119 (PLF: 49; PLF + TLIF: 70) | 68 ± 10; 65 ± 10 | 22/27; 24/46 |
| Chan | 2019 | USA | Retrospective cohort | DLS | grade 1 | 426 (decompression alone: 84; fusion: 342) | 69.9 ± 10.5; 60.7 ± 11.0 | 43/41; 131/211 |
| Chan (1) | 2019 | USA | Retrospective cohort | DLS | grade 1 | 143 (MIS TLIF: 72; MIS decompression: 71) | 62.1 ± 10.6; 72.3 ± 9.7 | 32/40; 32/39 |
| Vorhies | 2018 | USA | Retrospective cohort | DLS | NA | 75024 (decompression: 6712; fusion: 68312) | 69; 61 | 2776/3936; 25,645/42667 |
| Veresciagina | 2018 | Switzerland | Prospective cohort | DLS | grade 1 and 2 | 36 | 66.53 (47–80) | 6/30 |
| Irmola | 2018 | Finland | Prospective cohort | DLS | NA | 189 | 62 (24–87) | NA |
| Hayashi | 2018 | Japan | Retrospective cohort | DLS | grade 1 and 2 | 50 (CBT-PLIF: 20; MEL: 30) | 69.3; 71.2 | 5/15; 7/23 |
| Kato | 2017 | Japan | Prospective cohort | DLS | grade ≤ 1 | 51 | 70.8 ± 7.9 | 20/31 |
| Gerling | 2017 | USA | Prospective cohort | DLS | NA | 406 (292 underwent instrumented fusion, 85 non-instrumented fusion, and 29 decompression alone) | 65.3 | 128/278 |
| Cheung | 2016 | China | Retrospective cohort | DLS | grade 1 | 64 | 69.1 ± 8.7 | 29/36 |
| Sato | 2015 | Japan | Retrospective cohort | DLS | NA | 163 (decompression alone:74; decompression and fusion: 89) | 65.8 ± 8.9 | 73/90 |
| Macki | 2015 | USA | Retrospective cohort | DLS | grade 1 and 2 | 103 (PLF:58; PLF + PLIF/TLIF:45) | 59.27 ± 11.35; 55.44 ± 10.18 | 26/32; 14/31 |
| Blumenthal | 2013 | USA | Prospective cohort | DLS with LSS | grade 1 | 40 | 68.2 ± 7.7 | 10/30 |
| Rihn | 2012 | USA | Retrospective cohort | DLS | NA | 601 | 66 | 189/412 |
| Booth | 1999 | USA | Retrospective cohort | DLS | NA | 41 | 66.7 (52.2–78.7) | 12/29 |
| Author | BMI (kg/m2) | Disease duration (m) | Surgery types | Follow-up (years) | Number of reoperations | Reasons for reoperation | Reoperation methods | Risk factors of reoperation |
|---|---|---|---|---|---|---|---|---|
| Salimi | 24.3 ± 3.9 | 36.2 ± 39.6 | bilateral decompression | 5 | 2 | progression of lumbar degeneration or postoperative instability | NA | NA |
| Noh | NA | NA | OD followed by spinal fusion, laminectomy, and PELD | 10 | 549; 33 | NA | NA | NA |
| Moayeri | 27.7 ± 4.2 | NA | a unilateral laminotomy for ipsilateral decompression | 8.2 | 22 | NA | decompression (12), fusion (10) | NA |
| Liang | 23.70 ± 3.5; 24.30 ± 2.9 | < 6 mos:10; > 6 mos: 94 | PMTD; MIS TLIF | 2 | 3; 1 | NA | NA | NA |
| Joelson | 27.8 ± 5.3; 26.7 ± 4.0 | NA | decompression and fusion; decompression only | 7.6 | 2; 6 | spinal stenosis or disk herniation at the index level (L3-4), the first cranial adjacent level (L2-3), and the first caudal adjacent level (L4-5) | NA | NA |
| Georgiou | NA | NA | anterior fusion; posterior fusion | 2 | 2536; 8101 | NA | NA | NA |
| Chan | 30.7 | NA | posterior lumbar spine decompression with or without single level posterior instrumented fusion | NA | 438 | NA | NA | age, sex, BMI, smoking, addition of fusion |
| Takaoka | NA | NA | single-level OLIF and single-level TLIF | 3 | 3; 2 | NA | NA | NA |
| Sugiura | NA | NA | BPL and PLIF | 3 | 4; 4 | root radiculopathy (4); ASD (3), left L5/S foraminal stenosis (1) | PLIF (3), BPL (1); PLIF (3), herniotomy (1) | NA |
| Rabah | 30.85 ± 6.48 | NA | posterior/transforaminal lumbar interbody fusion (P/TLIF) | NA | NA | NA | NA | operative time, obesity class III, non-insulin-dependent diabetes, smoking |
| Mimura | NA | NA | single-level unistrumented PLF | 5 | 1 | ASD | NA | NA |
| Katuch | 25.56 ± 5.12; 23.78 ± 4.78 | NA | TLIF and PLIF | 3 | 2; 15 | wound infection and dural tear | NA | NA |
| Joelson | 27 ± 4.5; 27 ± 4.1 | NA | decompression and fusion; decompression only | 7.8 | 216; 80 | spinal stenosis with or without concomitant DLS or disk herniation | NA | NA |
| Badhiwala | NA | NA | laminectomy alone; laminectomy plus fusion | 5 | 18; 28 | related to the principal procedure | NA | NA |
| Zhong | NA | NA | Posterolateral fusion; intervertebral fusion | 4.2 | 24; 9 | NA | NA | BMI, sex, diabetes mellitus, age, fusion method, more bleeding |
| Nyström | NA | NA | bilateral laminotomy | 6.8 | 29 | NA | decompression (24); fusion (5) | NA |
| Lee | NA | NA | decompressive laminectomy; PLIF; ALIF | 10 | 52; 13; 1 | occurrence of any lumbar spinal surgery with a disease code | NA | NA |
| Khan | 30.96 ± 6.21 | 39.64 | elective open posterior lumbar spinal fusion | 1.9 | 31 | NA | NA | diabetes |
| Khan | 30.95 ± 5.82 | NA | an open posterior lumbar fusion | 1.3 | 7 | NA | NA | NA |
| Karsy | NA | NA | percutaneous screw placement, interbody placement, or decompression | 3 | 38 | NA | NA | NA |
| Chan | 29.5 ± 5.1; 31.3 ± 7.0 | NA | minimally invasive or open TLIF | 2 | 1; 16 | ASD; surgical site infection; implant removal/revision; pseudoarthrosis | NA | NA |
| Bisson | 28.735 ± 5.380; 28.190 ± 4.688 | NA | MIS decompression or open decompression | 3 | 10; 3 | re-emergence of symptoms; persistent symptoms; recurrence of rt-sided pain | NA | NA |
| Minamide | NA | NA | micro-endoscopic decompression | 2.3 | 17 | NA | fusion (9); decompression (8) | NA |
| Kuo | NA | NA | ULBD or fusion | 3.3 | 17; 75 | NA | fusion; decompression; incision and drainage, revision instrumentation, instrumentation removal | fusion as index operation, BMI, any perioperative complication, estimated blood loss |
| Kelly | 30.6 ± 5.9; 31.5 ± 6.7 | NA | PLF or PLF + TLIF | 2 | 8; 11 | NA | NA | NA |
| Chan | 28.4 ± 5.2; 31.0 ± 6.7 | < 3 mos: 11; > 3 mos: 401 | decompression alone or fusion | 1 | 5; 15 | wound revisions and/or incision and drainage, adjacent-segment disease, screw reposition/hardware failures, bony fracture within the fusion construct, and evacuation of hematoma | NA | NA |
| Chan (1) | 29.5 ± 5.1; 28.2 ± 4.7 | < 3 mos: 4; > 3 mos: 133 | MIS TLIF or MIS decompression | 2 | 1; 10 | ASD | fusion (5); decompression (6) | NA |
| Vorhies | NA | NA | decompression or fusion | 5 | 686; 8469 | recurrent stenosis or progressive instability | fusion; decompression | NA |
| Veresciagina | NA | NA | laminotomy | 10.78 | 5 | NA | decompression | NA |
| Irmola | 28.2 ± 4.3 | NA | instrumented lumbar spine fusion | 3.9 | 30 | acute complication; early failure; adjacent segment pathology; late failure | NA | NA |
| Hayashi | 23.7; 23.1 | 39.7; 41.5 | CBT-PLIF or MEL | 2 | 3; 5 | a hematoma perioperatively; a vertebral fracture in the adjacent level; same-segment disease; insufficient decompression | kyphoplasty; adjacent_x005f segment decompression; additional decompression and fusion | NA |
| Kato | NA | NA | microsurgical bilateral decompression via a unilateral approach | 2 | 2 | exacerbation of disc degeneration | NA | NA |
| Gerling | 29.2 | at least 12 weeks | instrumented fusion, non-instrumented fusion, and decompression alone | 8 | 60; 20; 9 | progressive spondylolisthesis, recurrent stenosis, complications, or other | NA | age, gender, moderate or severe stenotic levels, predominant back pain, physical therapy, neurogenic claudication, leg pain bothersomeness scale |
| Cheung | NA | NA | decompression only | 7.1 | 9 | mechanical back pain; residual leg pain | further decompression(2); Fusion(6); epidural steroid injection(1) | NA |
| Sato | 23.1 ± 2.6 | NA | decompression alone or decompression and fusion | 5 | 25; 13 | adjacent segmental disease; same segmental disease; infection; implant related; hematoma | decompression and fusion (25); decompression-only (8); others (5) | NA |
| Macki | NA | NA | NA | 5.5 | 17; 4 | degenerative disease progression | NA | NA |
| Blumenthal | NA | NA | laminectomy | 3.6 | 15 | mechanical low back pain | NA | NA |
| Rihn | 29.2 | at least 12 weeks | posterior decompressive laminectomy | 4 | 59 | NA | NA | obesity |
| Booth | NA | NA | decompression, autogenous iliac crest bone grafting, intertransverse process fusion, and segmental (pedicle screw) instrumentation | 6.5 | 5 |
recurrent stenosis at adjacent levels with transition syndrome |
NA | NA |
Abbreviation: LSS lumbar spinal stenosis, DLS degenerative lumbar spondylolisthesis, PMTD paraspinal mini-tubular lumbar decompression, MIS TLIF minimally invasive transforaminal lumbar interbody fusion, OLIF oblique lateral interbody fusion, TLIF transforaminal lumbar interbody fusion, BPL bilateral partial laminectomy, PLIF posterior lumbar interbody fusion, ALIF anterior lumbar interbody fusion, PLF posterolateral lumbar fusion, CBT-PLIF posterior lumbar interbody fusion with cortical bone trajectory, MEL microendoscopic laminotomy, OD open discectomy, PELD percutaneous endoscopic lumbar discectomy, PMTD paraspinal mini-tubular lumbar decompression, ULBD unilateral laminotomy for bilateral decompression, ASD adjacent segment disease, BMI body mass index, NA not available
Overall results and subgroup analysis results of reoperation rate
This meta-analysis showed a 10% (95%CI: 8%-12%) of reoperation rate in DLS patients (Fig. 2). A high heterogeneity was observed in the results (I2 = 99.3%). To explore the source of heterogeneity, subgroup analyses were performed. In study design subgroup, 10% of reoperation rate was found in both prospective cohort study (95%CI: 7%-13%) and retrospective cohort study (95%CI: 8%-13%). In patients subgroup, DLS patients showed 11% of reoperation rate (95%CI: 8%-13%) and DLS patients with LSS showed 10% of reoperation rate (95%CI: 6%-13%). In surgery types subgroup, the reoperation rate was 11% (95%CI: 9%-13%) in patients undergoing decompression, 10% (95%CI: 7%-12%) in patients undergoing fusion, 9% (95%CI: 5%-13%) in patients undergoing decompression and fusion, and 7% (95%CI: 3%-11%) in patients undergoing other surgeries. In follow-up time subgroup, the reoperation rate was 9% (95%CI: 6%-12%), 12% (95%CI: 9%-14%), and 10% (95%CI: 6%-15%) at follow-up time < 5 years, between 5 to 10 years, and ≥ 10 years, respectively. In study quality subgroup, there was 11% (95%CI: 9%-13%) of reoperation rate in studies with fair quality and 7% (95%CI: 5%-10%) of reoperation rate in studies with high quality. The overall and subgroup analysis results were shown in Table 2.
Fig. 2.
Forest plot regarding to reoperation rate
Table 2.
Meta analysis of reoperation rate
| Outcomes | Number of studies | Rate (95%CI) | I2 |
|---|---|---|---|
| Reoperation rate | 37 | 0.10 (0.08–0.12) | 99.3 |
| Sensitivity analysis | 0.10 (0.08–0.12) | ||
| Publication bias | Z = 2.91 | P = 0.004 | |
| Study design | |||
| Prospective cohort | 12 | 0.10 (0.07–0.13) | 94.5 |
| Retrospective cohort | 25 | 0.10 (0.08–0.13) | 99.5 |
| Patients | |||
| DLS | 27 | 0.11 (0.08–0.13) | 99.4 |
| DLS with LSS | 10 | 0.10 (0.06–0.13) | 96.5 |
| Surgery types | |||
| Decompression | 21 | 0.11 (0.09–0.13) | 91.9 |
| Fusion | 8 | 0.10 (0.07–0.12) | 98.9 |
| Decompression and fusion | 6 | 0.09 (0.05–0.13) | 99.0 |
| Others | 2 | 0.07 (0.03–0.11) | 23.4 |
| Follow-up (years) | |||
| < 5 | 20 | 0.09 (0.06–0.12) | 98.5 |
| 5–10 | 13 | 0.12 (0.09–0.14) | 98.1 |
| ≥ 10 | 3 | 0.10 (0.06–0.15) | 92.8 |
| Quality | |||
| Fair | 26 | 0.11 (0.09–0.13) | 98.6 |
| High | 11 | 0.07 (0.05–0.10) | 96.7 |
Abbreviation: CI confidence interval, I2 I-squared, DLS degenerative lumbar spondylolisthesis, LSS lumbar spinal stenosis
Meta-analysis of risk factors for reoperation
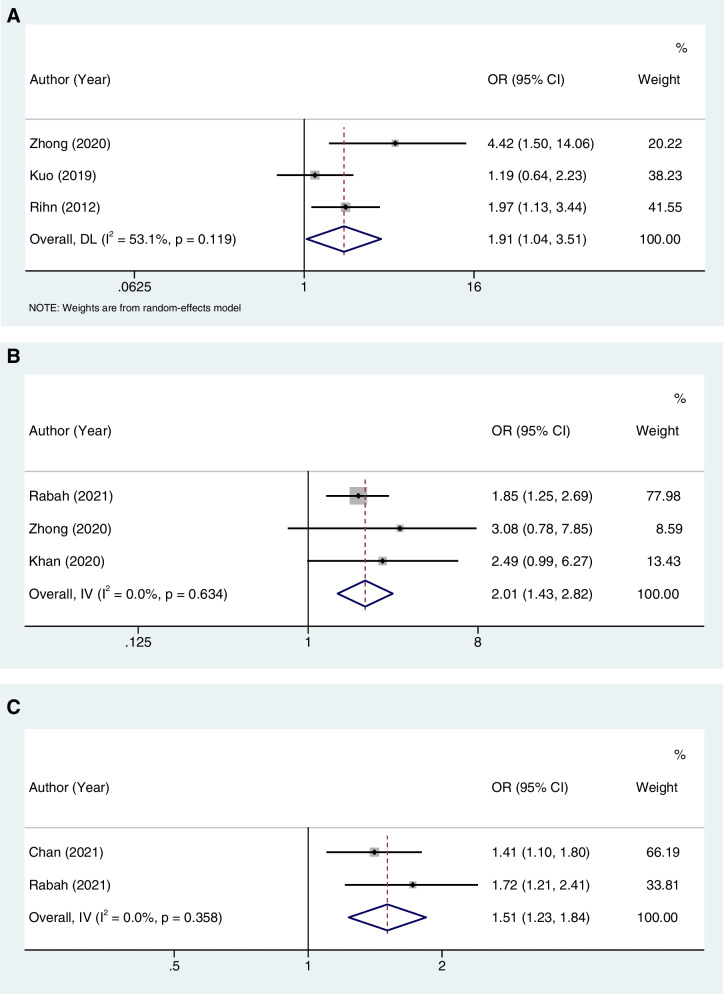
The meta-analysis showed that obesity (OR = 1.91, 95%CI: 1.04–3.51, I2 = 53.1%), diabetes (OR = 2.01, 95%CI: 1.43–2.82, I2 = 0%), and smoking (OR = 1.51, 95%CI: 1.23–1.84, I2 = 0%) were associated with an increased risk of reoperation. Age (OR = 0.99, 95%CI: 0.95–1.03, I2 = 78.4%), sex (OR = 1.31, 95%CI: 0.83–2.05, I2 = 60.4%), and more bleeding (OR = 0.86, 95%CI: 0.07–10.22, I2 = 87.5%) were not associated with the reoperation. The overall results were demonstrated in Table 3. Forest plots regarding to obesity, diabetes, and smoking were demonstrated in Fig. 3A, B, and C, respectively.
Table 3.
Risk factors for the reoperation of DLS patients after surgeries
| Risk factors | Number of studies | OR (95%CI) | P | I2 |
|---|---|---|---|---|
| Age | 3 | 0.99 (0.95–1.03) | 0.535 | 78.4 |
| Sensitivity analysis | 0.99 (0.95–1.03) | |||
| Sex | 3 | 1.31 (0.83–2.05) | 0.243 | 60.4 |
| Sensitivity analysis | 1.31 (0.83–2.05) | |||
| Obesity | 3 | 1.91 (1.04–3.51) | 0.037 | 53.1 |
| Sensitivity analysis | 1.91 (1.04–3.51) | |||
| Diabetes | 3 | 2.01 (1.43–2.82) | < 0.001 | 0.0 |
| Sensitivity analysis | 2.01 (1.43–2.82) | |||
| Smoking | 2 | 1.51 (1.23–1.84) | < 0.001 | 0.0 |
| Sensitivity analysis | 1.51 (1.23–1.84) | |||
| More bleeding | 2 | 0.86 (0.07–10.22) | 0.903 | 87.5 |
| Sensitivity analysis | 0.86 (0.07–10.22) |
Abbreviation: DLS degenerative lumbar spondylolisthesis, OR odds ratio, CI confidence interval, I2 I-squared
Fig. 3.
Forest plots regarding to obesity (A), diabetes (B), and smoking (C)
Systematic review of risk factors for reoperation
This systematic review examined two literatures about the obesity. Chan et al. carried out a retrospective cohort study of obesity and reoperation after lumbar surgery [23]. As expected, significant higher risk of reoperation was found in patients who were obese [23]. Similar evidence was supported by Rabah et al. that an increase of one unit in BMI was associated with 4% increased risk of reoperation [11]. Moreover, study of Chan et al. showed addition of fusion was associated with higher risk of reoperation [23]. Rabah et al. found operative time > 5 h to be associated with an increased risk of reoperation [11]. A study consisted of 5-year follow-up indicated that having an index of fusion operation and perioperative complications was associated with the increased odds of reoperation [13]. Compared to intervertebral fusion, patients undergoing posterolateral fusion had 4.02-times risk of reoperation [8]. In addition, Gerling et al. have reported that patients with 2/3 moderate or severe stenotic levels, predominant back pain, no physical therapy, and greater leg pain score at baseline indicated higher reoperation rate [26].
Assessment of publication bias and sensitivity analysis
Sensitivity analysis was performed by sequentially removing the study to assess the robustness of overall results. All results of sensitivity analysis were consistent with those of the main analysis (Tables 2 and 3). By funnel plot, we detected an evidence of publication biases (Z = 2.91, P = 0.004) (Table 2, Supplementary Fig. 1A). Therefore, a trim-and-fill method was utilized to fill the missing data to eliminate the impact of publication bias. Funnel plot with missing data filled was demonstrated in Supplementary Fig. 1B. Before filled, reoperation rate was 10% (95%CI: 8%-12%). After filled, reoperation rate was 11% (95%CI: 9%-13%).
Discussion
The reoperation rate of DLS patients undergoing lumbar surgeries remains high in spite of improved surgical skills and techniques; therefore, exploring risk factors of reoperation is important [7, 8]. Considering the controversial results in the risk factors [8, 11–13], we performed a systematic review and meta-analysis based on currently available studies to analyze the reoperation rate and risk factors. In this study, we found a 10% of reoperation rate in DLS patients after lumbar surgeries. Obesity, diabetes, and smoking were identified as risk factors for the reoperation.
Several previous studies have reported the reoperation rate after lumbar surgeries in DLS patients [7, 49–52]. The reoperation rate was reported as 12.4% from 1990 to 1993 and 14.0% from 1997 to 2000 [51]. Ghogawala et al. proved that the reoperation rate was 15% at 1 year after the surgery in DLS patients only undergoing decompression [52]. In the present studies, the reoperation rate was found nearly the same as that reported in previous studies [7]. The reoperation rate in DLS patients was 15.7% at the mean follow-up of 8.2 years [7]. For patients undergoing fusion procedures, the cumulative reoperation rate was 14% [49]. Another report demonstrated that the reoperation rate ranged from 5.8% to 16.3% according to the type of surgeries [50]. Similar to the studies mentioned above, in our study, the reoperation rate of DLS was 10%, ranging from 8 to 12%. Our results may be useful for clinicians to evaluate the reoperation rate.
Identifying risk factors of reoperation for patients after lumbar surgeries is of clinical interest. In this study, obesity, diabetes, and smoking were found to be associated with higher risk of reoperation. Rabah et al. and Chan et al. have confirmed that smoking status was associated with greater risk of reoperation [11, 23]. Also, there were several studies reporting the positive association between obesity and reoperation of patients undergoing lumbar surgeries [8, 23]. This can be explained by that obese patients were more likely to be frail [53, 54], and frail patients had 56% increased odds of reoperation after lumbar surgery [23].
Animal studies have long recognized the close association between diabetes and lumbar spine disorders [55–57]. Diabetic models have revealed some harmful changes, such as increase of toxic end products of glycation, expression of matrix metalloproteinases 2 related to extracellular matrix degradation, and hyperglycemia-induced intervertebral disc inflammation, promoting intervertebral disc degeneration process [58–60]. Studies have revealed that diabetes was closely associated with degenerative lumbar spine disorders [61, 62]. Park et al. have found the influence of diabetes on the prevalence of lumbar spine surgeries, indicating that diabetes may be a factor aggravating lumbar spine disorders [62]. In Park et al. study, patients with diabetes underwent more lumbar surgeries than those without diabetes [62]. Their finding suggested that diabetes was significantly associated with the increased number of lumbar spine surgeries, and this finding is of critical importance because it revealed that diabetes may be an incentive for the increase of the severity of lumbar spine disorders, which ultimately led to the necessity of surgeries [62]. In this meta-analysis, diabetes was identified as a risk factor for the reoperation of DLS patients undergoing lumbar surgeries. This was consistent with the findings from Zhong et al. [8] Our findings suggested that when treating DLS patients with diabetes, physicians should pay more attention to glycemic control for the purpose of decreasing the risk of reoperation.
This meta-analysis explores the reoperation rate and risk factors for the reoperation. Results show that there is 10% of reoperation after lumbar surgeries, and obesity, diabetes, and smoking are found to increase the risk of reoperation. Our findings suggest that DLS patients should control glycemic level and weight, and reduce smoking to decrease the risk of reoperation. There are some limitations in this study. First, all fusion techniques (PLF and LIF) were put together. Due to the limitations of the included studies, it is unable to further analyze the reoperation rate in DLS patients undergoing the single fusion technique. Second, the number of studies reporting the risk factors of reoperation is relatively small, and some outcomes can only be qualitatively described, which may affect the stability of the results. Third, the risk of reoperation may be different according to the severity of lumbar spondylolisthesis and the first surgical methods; however, data provided in the currently available studies are insufficient to further analyze. Future meta-analysis including more relevant studies are needed to verify our findings and to explore the effect of lumbar spondylolisthesis severity and the first surgical methods on the risk of reoperation.
Conclusion
Our meta-analysis found 10% of reoperation rate in DLS patients undergoing lumbar surgeries, and identified obesity, diabetes, and smoking as risk factors for the reoperation. Our findings suggested that patients should improve glycemic level and weight, and quit smoking to reduce the reoperation after lumbar surgery.
Supplementary Information
Additional file 1: Supplementary figure 1. Funnel plot for publication bias before and after trim-and-fillanalysis.
Acknowledgements
Not applicable.
Abbreviations
- DLS
Degenerative lumbar spondylolisthesis
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- BMI
Body mass index
- NOS
Newcastle-Ottawa Scale
- OR
Odds ratio
- CI
Confidence interval
Authors’ contributions
YC and XF designed the study. YC wrote the manuscript. YZ, JC, YL and YW collected, analyzed and interpreted the data. XF critically reviewed, edited and approved the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Sichuan Province Key Research and Development Project (2022YFS0418).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bydon M, Alvi MA, Goyal A. Degenerative lumbar spondylolisthesis: definition, natural history, conservative management, and surgical treatment. Neurosurg Clin N Am. 2019;30:299–304. doi: 10.1016/j.nec.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: a systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2017;11:39–52. doi: 10.1016/j.jot.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravindra VM, Senglaub SS, Rattani A, Dewan MC, Härtl R, Bisson E, et al. Degenerative lumbar spine disease: estimating global incidence and worldwide volume. Global spine journal. 2018;8:784–794. doi: 10.1177/2192568218770769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karsy M, Bisson EF. Surgical versus nonsurgical treatment of lumbar spondylolisthesis. Neurosurg Clin N Am. 2019;30:333–340. doi: 10.1016/j.nec.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Kim CH, Chung CK, Choi Y, Kim MJ, Kim MJ, Shin S, et al. Increased proportion of fusion surgery for degenerative lumbar spondylolisthesis and changes in reoperation rate: a nationwide cohort study with a minimum 5-year follow-up. Spine. 2019;44:346–354. doi: 10.1097/BRS.0000000000002805. [DOI] [PubMed] [Google Scholar]
- 6.Gaderer C, Schaumann A, Schulz M, Thomale UW. Neuroendoscopic lavage for the treatment of CSF infection with hydrocephalus in children. Child's Nerv Syst. 2018;34:1893–1903. doi: 10.1007/s00381-018-3894-7. [DOI] [PubMed] [Google Scholar]
- 7.Moayeri N, Rampersaud YR. Revision surgery following minimally invasive decompression for lumbar spinal stenosis with and without stable degenerative spondylolisthesis: a 5- to 15-year reoperation survival analysis. J Neurosurg Spine. 2021;36:1–7. [DOI] [PubMed]
- 8.Zhong W, Liang X, Luo X, Huang T, Quan Z. Complications rate of and risk factors for the unplanned reoperation of degenerative lumbar spondylolisthesis in elderly patients: a retrospective single-Centre cohort study of 33 patients. BMC Geriatr. 2020;20:301. doi: 10.1186/s12877-020-01717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park MS, Ju YS, Moon SH, Kim TH, Oh JK, Sung PS, et al. Reoperation rates after posterior lumbar spinal fusion surgery according to preoperative diagnoses: a national population-based cohort study. Clin Neurol Neurosurg. 2019;184:105408. doi: 10.1016/j.clineuro.2019.105408. [DOI] [PubMed] [Google Scholar]
- 10.Noh SH, Cho PG, Kim KN, Lee B, Lee JK, Kim SH. Risk factors for reoperation after lumbar spine surgery in a 10-year Korean national health insurance service health examinee cohort. Sci Rep. 2022;12:4606. doi: 10.1038/s41598-022-08376-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabah NM, Khan HA, Shost M, Beckett J, Mroz TE, Steinmetz MP. Predictors of operative duration and complications in single-level posterior interbody fusions for degenerative spondylolisthesis. World neurosurgery. 2021;151:e317–e323. doi: 10.1016/j.wneu.2021.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Khan JM, Michalski J, Basques BA, Louie PK, Chen O, Hayani Z, et al. Do Clinical outcomes and sagittal parameters differ between diabetics and nondiabetics for degenerative spondylolisthesis undergoing lumbar fusion? Global spine journal. 2020;10:286–293. doi: 10.1177/2192568219850090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo CC, Merchant M, Kardile MP, Yacob A, Majid K, Bains RS. In Degenerative Spondylolisthesis, Unilateral Laminotomy for Bilateral Decompression Leads to Less Reoperations at 5 Years When Compared to Posterior Decompression With Instrumented Fusion: a propensity-matched retrospective analysis. Spine. 2019;44:1530–1537. doi: 10.1097/BRS.0000000000003121. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(264–9):w64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Xue X, Lu CL, Jin XY, Liu XH, Yang M, Wang XQ, et al. Relationship between serum uric acid, all-cause mortality and cardiovascular mortality in peritoneal dialysis patients: systematic review and meta-analysis of cohort studies. BMJ Open. 2021;11:e052274. doi: 10.1136/bmjopen-2021-052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badhiwala JH, Leung SN, Jiang F, Wilson JRF, Akbar MA, Nassiri F, et al. In-hospital course and complications of laminectomy alone versus laminectomy plus instrumented posterolateral fusion for lumbar degenerative spondylolisthesis: a retrospective analysis of 1804 patients from the NSQIP Database. Spine. 2021;46:617–623. doi: 10.1097/BRS.0000000000003858. [DOI] [PubMed] [Google Scholar]
- 17.Bisson EF, Mummaneni PV, Virk MS, Knightly J, Alvi MA, Goyal A, et al. Open versus minimally invasive decompression for low-grade spondylolisthesis: analysis from the Quality Outcomes Database. J neurosurg Spine. 33(3):349-59. [DOI] [PubMed]
- 18.Blumenthal C, Curran J, Benzel EC, Potter R, Magge SN, Harrington JF, Jr, et al. Radiographic predictors of delayed instability following decompression without fusion for degenerative grade I lumbar spondylolisthesis. J Neurosurg Spine. 2013;18:340–346. doi: 10.3171/2013.1.SPINE12537. [DOI] [PubMed] [Google Scholar]
- 19.Booth KC, Bridwell KH, Eisenberg BA, Baldus CR, Lenke LG. Minimum 5-year results of degenerative spondylolisthesis treated with decompression and instrumented posterior fusion. Spine. 1999;24:1721–1727. doi: 10.1097/00007632-199908150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Chan AK, Bisson EF, Bydon M, Glassman SD, Foley KT, Potts EA, et al. A comparison of minimally invasive transforaminal lumbar interbody fusion and decompression alone for degenerative lumbar spondylolisthesis. Neurosurg Focus. 2019;46:E13. doi: 10.3171/2019.2.FOCUS18722. [DOI] [PubMed] [Google Scholar]
- 21.Chan AK, Bisson EF, Bydon M, Glassman SD, Foley KT, Potts EA, et al. Laminectomy alone versus fusion for grade 1 lumbar spondylolisthesis in 426 patients from the prospective Quality Outcomes Database. J Neurosurg Spine. 2018;30:234–241. doi: 10.3171/2018.8.SPINE17913. [DOI] [PubMed] [Google Scholar]
- 22.Chan AK, Bisson EF, Bydon M, Foley KT, Glassman SD, Shaffrey CI, et al. A Comparison of Minimally Invasive and Open Transforaminal Lumbar Interbody Fusion for Grade 1 Degenerative Lumbar Spondylolisthesis: An Analysis of the Prospective Quality Outcomes Database. Neurosurgery. 2020;87:555–562. doi: 10.1093/neuros/nyaa097. [DOI] [PubMed] [Google Scholar]
- 23.Chan V, Witiw CD, Wilson JRF, Wilson JR, Coyte P, Fehlings MG. Frailty is an important predictor of 30-day morbidity in patients treated for lumbar spondylolisthesis using a posterior surgical approach. Spine J. 2022;22:286–295. doi: 10.1016/j.spinee.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Cheung JP, Cheung PW, Cheung KM, Luk KD. Decompression without Fusion for Low-Grade Degenerative Spondylolisthesis. Asian Spine J. 2016;10:75–84. doi: 10.4184/asj.2016.10.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgiou S, Saggi S, Wu HH, Metz L. Comparison of 90-day complications and two-year reoperation rates between anterior and posterior interbody fusion for single-level degenerative spondylolisthesis. N Am Spine Soc J. 2022;10:100127. doi: 10.1016/j.xnsj.2022.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerling MC, Leven D, Passias PG, Lafage V, Bianco K, Lee A, et al. Risk Factors for Reoperation in Patients Treated Surgically for Degenerative Spondylolisthesis: a subanalysis of the 8-year data from the SPORT Trial. Spine. 2017;42:1559–1569. doi: 10.1097/BRS.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi K, Toyoda H, Terai H, Hoshino M, Suzuki A, Takahashi S, et al. Comparison of minimally invasive decompression and combined minimally invasive decompression and fusion in patients with degenerative spondylolisthesis with instability. J Clin Neurosci. 2018;57:79–85. doi: 10.1016/j.jocn.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Irmola TM, Häkkinen A, Järvenpää S, Marttinen I, Vihtonen K, Neva M. Reoperation Rates Following Instrumented Lumbar Spine Fusion. Spine. 2018;43:295–301. doi: 10.1097/BRS.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 29.Joelson A, Nerelius F, Holy M, Sigmundsson FG. Reoperations after decompression with or without fusion for L4–5 spinal stenosis with or without degenerative spondylolisthesis: a study of 6,532 patients in Swespine, the national Swedish spine register. Acta Orthop. 2021;92:264–268. doi: 10.1080/17453674.2021.1879505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joelson A, Nerelius F, Holy M, Sigmundsson FG. Reoperations After Decompression With or Without Fusion for L3–4 Spinal Stenosis With Degenerative Spondylolisthesis: A Study of 372 Patients in Swespine, the National Swedish Spine Register. Clini Spine Surg. 2022;35:E389–E393. doi: 10.1097/BSD.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 31.Karsy M, Chan AK, Mummaneni PV, Virk MS, Bydon M, Glassman SD, et al. Outcomes and complications with age in spondylolisthesis: an evaluation of the elderly from the quality outcomes database. Spine. 2020;45:1000–1008. doi: 10.1097/BRS.0000000000003441. [DOI] [PubMed] [Google Scholar]
- 32.Kato M, Namikawa T, Matsumura A, Konishi S, Nakamura H. Radiographic risk factors of reoperation following minimally invasive decompression for lumbar canal stenosis associated with degenerative scoliosis and spondylolisthesis. Global Spine J. 2017;7:498–505. doi: 10.1177/2192568217699192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katuch V, Grega R, Knorovsky K, Banoci J, Katuchova J, Sasala M, et al. Comparison between posterior lumbar interbody fusion and transforaminal lumbar interbody fusion in the management of lumbar spondylolisthesis. Bratisl Lek Listy. 2021;122:653–656. doi: 10.4149/BLL_2021_105. [DOI] [PubMed] [Google Scholar]
- 34.Kelly JP, Alcala-Marquez C, Dawson JM, Mehbod AA, Pinto MR. Treatment of degenerative spondylolisthesis by instrumented posterolateral versus instrumented posterolateral with transforaminal lumbar interbody single-level fusion. J Spine Surg (Hong Kong) 2019;5:351–357. doi: 10.21037/jss.2019.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan JM, Harada GK, Basques BA, Nolte MT, Louie PK, Iloanya M, et al. Patients with predominantly back pain at the time of lumbar fusion for low-grade spondylolisthesis experience similar clinical improvement to patients with predominantly leg pain: mid-term results. Spine J. 2020;20:276–282. doi: 10.1016/j.spinee.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Lee CH, Kim CH, Chung CK, Choi Y, Kim MJ, Yim D, et al. Long-term effect of diabetes on reoperation after lumbar spinal surgery: a nationwide population-based sample cohort study. World Neurosurg. 2020;139:e439–e448. doi: 10.1016/j.wneu.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Liang Z, Xu X, Rao J, Chen Y, Wang R, Chen C. Clinical evaluation of Paraspinal mini-tubular lumbar decompression and minimally invasive Transforaminal lumbar interbody fusion for lumbar spondylolisthesis grade i with lumbar spinal stenosis: a cohort study. Front Surg. 2022;9:906289. doi: 10.3389/fsurg.2022.906289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macki M, Bydon M, Weingart R, Sciubba D, Wolinsky JP, Gokaslan ZL, et al. Posterolateral fusion with interbody for lumbar spondylolisthesis is associated with less repeat surgery than posterolateral fusion alone. Clin Neurol Neurosurg. 2015;138:117–123. doi: 10.1016/j.clineuro.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Mimura T, Tsutsumimoto T, Yui M, Misawa H. Does fusion status following posterolateral lumbar fusion in the treatment for stable lumbar degenerative spondylolisthesis affect the long-term surgical outcomes? A propensity score-weighted analysis of consecutive patients. J Orthop Sci. 2022;27:990–994. doi: 10.1016/j.jos.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Minamide A, Simpson AK, Okada M, Enyo Y, Nakagawa Y, Iwasaki H, et al. Microendoscopic Decompression for Lumbar Spinal Stenosis With Degenerative Spondylolisthesis: The Influence of Spondylolisthesis Stage (Disc Height and Static and Dynamic Translation) on Clinical Outcomes. Clin Spine Surg. 2019;32:E20–E26. doi: 10.1097/BSD.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 41.Nyström B, Jin S, Schillberg B, Moström U, Lundin P, Taube A. Are degenerative spondylolisthesis and further slippage postoperatively really issues in spinal stenosis surgery? Scand J Pain. 2020;20:307–317. doi: 10.1515/sjpain-2019-0113. [DOI] [PubMed] [Google Scholar]
- 42.Rihn JA, Radcliff K, Hilibrand AS, Anderson DT, Zhao W, Lurie J, et al. Does obesity affect outcomes of treatment for lumbar stenosis and degenerative spondylolisthesis? Analysis of the Spine Patient Outcomes Research Trial (SPORT) Spine. 2012;37:1933–1946. doi: 10.1097/BRS.0b013e31825e21b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salimi H, Toyoda H, Terai H, Yamada K, Hoshino M, Suzuki A, et al. Mid-term changes in spinopelvic sagittal alignment in lumbar spinal stenosis with coexisting degenerative spondylolisthesis or scoliosis after minimally invasive lumbar decompression surgery: minimum five-year follow-up. Spine J. 2022;22:819–826. doi: 10.1016/j.spinee.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Sato S, Yagi M, Machida M, Yasuda A, Konomi T, Miyake A, et al. Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: minimum 5-year follow-up. Spine J. 2015;15:1536–1544. doi: 10.1016/j.spinee.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Sugiura T, Okuda S, Takenaka S, Nagamoto Y, Matsumoto T, Takahashi Y, et al. Comparing Investigation Between Bilateral Partial Laminectomy and Posterior Lumbar Interbody Fusion for Mild Degenerative Spondylolisthesis. Clin Spine Surg. 2021;34:E403–E409. doi: 10.1097/BSD.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 46.Takaoka H, Inage K, Eguchi Y, Shiga Y, Furuya T, Maki S, et al. Comparison between intervertebral oblique lumbar interbody fusion and transforaminal lumbar interbody fusion: a multicenter study. Sci Rep. 2021;11:16673. doi: 10.1038/s41598-021-95774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veresciagina K, Mehrkens A, Schären S, Jeanneret B. Minimum ten-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. J Spine Surg (Hong Kong) 2018;4:93–101. doi: 10.21037/jss.2018.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vorhies JS, Hernandez-Boussard T, Alamin T. Treatment of Degenerative Lumbar Spondylolisthesis With Fusion or Decompression Alone Results in Similar Rates of Reoperation at 5 Years. Clin Spine Surg. 2018;31:E74–E79. doi: 10.1097/BSD.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 49.Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, et al. Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. N Engl J Med. 2016;374:1424–1434. doi: 10.1056/NEJMoa1508788. [DOI] [PubMed] [Google Scholar]
- 50.Schöller K, Alimi M, Cong GT, Christos P, Härtl R. Lumbar Spinal Stenosis Associated With Degenerative Lumbar Spondylolisthesis: A Systematic Review and Meta-analysis of Secondary Fusion Rates Following Open vs Minimally Invasive Decompression. Neurosurgery. 2017;80:355–367. doi: 10.1093/neuros/nyw091. [DOI] [PubMed] [Google Scholar]
- 51.Mardjetko SM, Connolly PJ, Shott S. Degenerative lumbar spondylolisthesis. A meta-analysis of literature 1970–1993. Spine. 1994;19:2256s–65s. doi: 10.1097/00007632-199410151-00002. [DOI] [PubMed] [Google Scholar]
- 52.Ghogawala Z, Benzel EC, Amin-Hanjani S, Barker FG, 2nd, Harrington JF, Magge SN, et al. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative Grade I spondylolisthesis. J Neurosurg Spine. 2004;1:267–272. doi: 10.3171/spi.2004.1.3.0267. [DOI] [PubMed] [Google Scholar]
- 53.Liao Q, Zheng Z, Xiu S, Chan P. Waist circumference is a better predictor of risk for frailty than BMI in the community-dwelling elderly in Beijing. Aging Clin Exp Res. 2018;30:1319–1325. doi: 10.1007/s40520-018-0933-x. [DOI] [PubMed] [Google Scholar]
- 54.Rietman ML, van der AD, van Oostrom SH, Picavet HSJ, Dollé MET, van Steeg H, et al. The Association between BMI and Different Frailty Domains: A U-Shaped Curve? J Nutr Health Aging. 2018;22:8–15. doi: 10.1007/s12603-016-0854-3. [DOI] [PubMed] [Google Scholar]
- 55.Fields AJ, Berg-Johansen B, Metz LN, Miller S, La B, Liebenberg EC, et al. Alterations in intervertebral disc composition, matrix homeostasis and biomechanical behavior in the UCD-T2DM rat model of type 2 diabetes. J Orthop Res. 2015;33:738–746. doi: 10.1002/jor.22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Illien-Junger S, Grosjean F, Laudier DM, Vlassara H, Striker GE, Iatridis JC. Combined anti-inflammatory and anti-AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PLoS One. 2013;8:e64302. doi: 10.1371/journal.pone.0064302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Illien-Jünger S, Lu Y, Qureshi SA, Hecht AC, Cai W, Vlassara H, et al. Chronic ingestion of advanced glycation end products induces degenerative spinal changes and hypertrophy in aging pre-diabetic mice. PLoS One. 2015;10:e0116625. doi: 10.1371/journal.pone.0116625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng X, Ni B, Zhang Z, Liu Q, Wang L, Ding Y, et al. Polyol pathway mediates enhanced degradation of extracellular matrix via p38 MAPK activation in intervertebral disc of diabetic rats. Connect Tissue Res. 2013;54:118–122. doi: 10.3109/03008207.2012.754886. [DOI] [PubMed] [Google Scholar]
- 59.Chen S, Liao M, Li J, Peng H, Xiong M. The correlation between microvessel pathological changes of the endplate and degeneration of the intervertebral disc in diabetic rats. Exp Ther Med. 2013;5:711–717. doi: 10.3892/etm.2012.868. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Won HY, Park JB, Park EY, Riew KD. Effect of hyperglycemia on apoptosis of notochordal cells and intervertebral disc degeneration in diabetic rats. J Neurosurg Spine. 2009;11:741–748. doi: 10.3171/2009.6.SPINE09198. [DOI] [PubMed] [Google Scholar]
- 61.Agius R, Galea R, Fava S. Bone mineral density and intervertebral disc height in type 2 diabetes. J Diabetes Complications. 2016;30:644–650. doi: 10.1016/j.jdiacomp.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 62.Park CH, Min KB, Min JY, Kim DH, Seo KM, Kim DK. Strong association of type 2 diabetes with degenerative lumbar spine disorders. Sci Rep. 2021;11:16472. doi: 10.1038/s41598-021-95626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary figure 1. Funnel plot for publication bias before and after trim-and-fillanalysis.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.